- 1Department of Biotechnology, Hemvati Nandan Bahuguna Garhwal University, Srinagar, India
- 2High Altitude Plant Physiology Research Centre, Hemvati Nandan Bahuguna Garhwal University, Srinagar, India
Colors are added to food items to make them more attractive and appealing. Food colorants therefore, have an impressive market due to the requirements of food industries. A variety of synthetic coloring agents approved as food additives are available and being used in different types of food prepared or manufactured worldwide. However, there is a growing concern that the use of synthetic colors may exert a negative impact on human health and environment in the long run. The natural pigments obtained from animals, plants, and microorganisms are a promising alternative to synthetic food colorants. Compared to animal and plant sources, microorganisms offer many advantages such as no seasonal impact on the quality and quantity of the pigment, ease of handling and genetic manipulation, amenability to large scale production with little or no impact on biodiversity etc. Among the microorganisms algae, fungi and bacteria are being used to produce pigments as food colorants. This review describes the types of microbial food pigments in use, their benefits, production strategies, and associated challenges.
Introduction
The world cannot be imagined without colors and this is equally true for the food that we eat. Some food materials such as fruits, vegetables etc. have striking natural shades and hues and therefore, do not require any further coloration. However, for many food items addition of food colorants is an integral part of their recipe before final packaging or serving. Food colorants enhance the visual appeal and grant unique identity to the food so that it could look more attractive and seem enjoyable to eat. Many times, food color is also associated with the flavor, safety and nutritional value (Sigurdson et al., 2017). The market of food colorants was estimated to be USD 3.88 billion in 2018 and it is estimated to reach USD 5.12 billion by 2023 with a compound annual growth rate (CAGR) of 5.7% [Food Colors Market by Type (Natural, Synthetic, Nature-Identical), Application (Beverages, Processed Food, Bakery & Confectionery Products, Oils & Fats, Dairy Products, Meat, Poultry, Seafood), Form, Solubility, and Region—Global Forecast to 20231]. Food coloring has been known to be in practice as early as 1,500 BC (Burrows, 2009). Earlier, all the colorants used were of natural origin such as saffron, paprika, turmeric, various flowers etc. (Burrows, 2009). In the midst of nineteenth century, synthetic colors were started to be produced and owing to their low production cost, high tinctorial strength, and chemical stability they became popular as food colorants (Sigurdson et al., 2017). However, in later years several health issues were realized with the use of many potentially hazardous synthetic chemicals as food colorants which led to the banning of various such food color additives e.g., Quinoline Yellow, Yellow 2G, Ponceau SX, Brilliant Black B etc.2. At present, although strict regulations are in practice in different countries toward approval of a synthetic colorant for intended use as food additive, people with the growing awareness about personal health and environment are now more inclined toward their substitute obtained from natural sources. However, there is still a substantial share of synthetic colors in the market of food colorants. Mono- and di-azo dyes are the most commonly used synthetic food colorants approved by FDA and EU. The other approved food grade colorants include Triarylmethane derivatives, xanthenes dyes, quinophthalones, and indigoid compounds (Corradini, 2018).
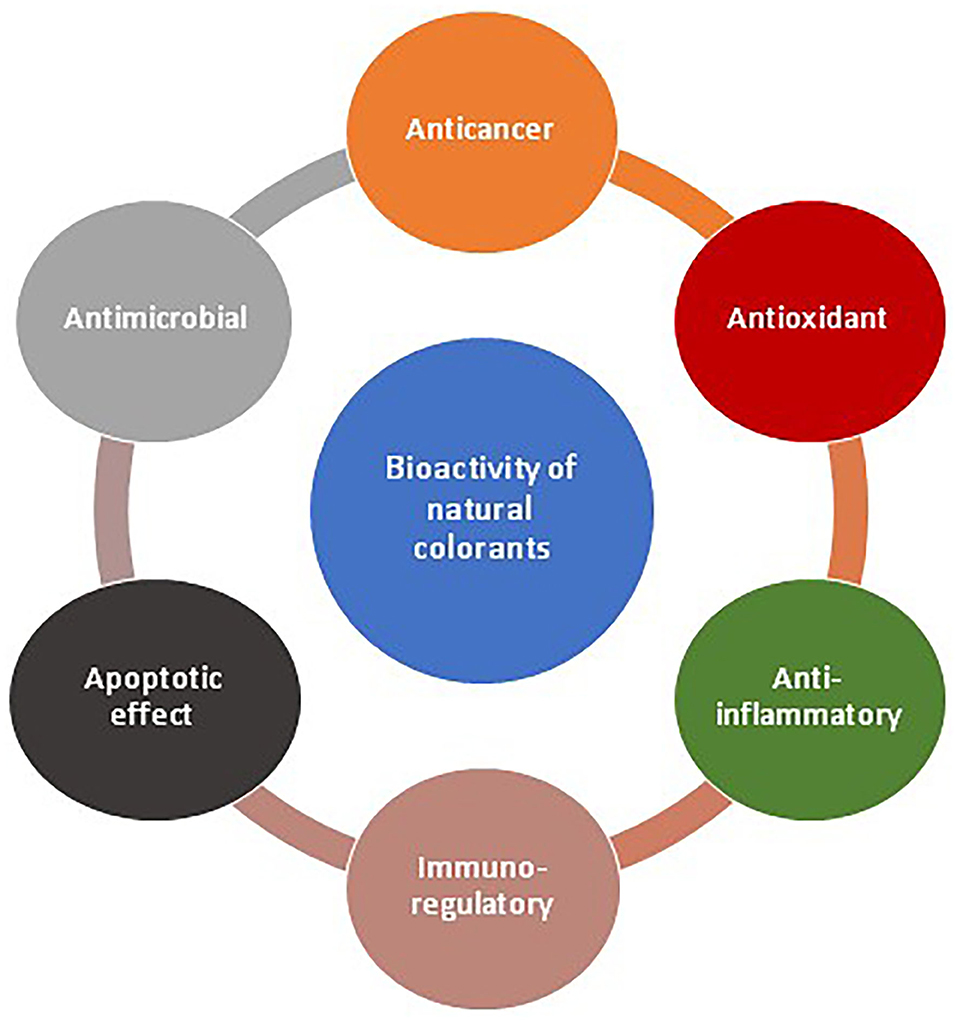
Natural pigments, the colored compounds synthesized by plants, animal, microorganisms or derived from mineral ores are a promising alternative to the synthetic food colorants (Corradini, 2018). Titanium dioxide (E171), calcium carbonate (E170), iron oxides (E172) are some examples of mineral pigments approved as food colorants by FDA3. Although for similar shades the cost of synthetic colors is on lower side for most cases in comparison to natural colors but the mass production of natural colors may fill this gap. Unlike synthetic colorants, they have nutritional values and associated with cytotoxic, antioxidant, antimicrobial, antimalarial, anticancer, antitumor, and antifouling activities (Ramesh et al., 2019) (Figure 1). Not only the natural colorants/pigments but their identical compounds made by chemical processes are also exempted from the certification process before use as food additive (Sen et al., 2019). Although plants are a major source of natural pigments, pigments obtained from microbial sources offer special advantages. Compared to plants, microbial pigments are more stable, cost-effective, uninfluenced by seasonal variations, amenable to yield improvement, and smoothly extractable (Nigam and Luke, 2016). Also, the excessive use of microbial culture for pigment production is not likely to harm the biodiversity and environment. Currently, a variety of different food color additives produced through fermentative processes are available in the market. Monascus pigments, Astaxanthin from Xanthophyllomyces dendrorhous, Arpink Red from Penicillium oxalicum, Riboflavin from Ashbya gossypii, and carotene from Blakeslea trisporatrispora (Venil et al., 2013) are the examples of some microbial food grade pigments.
Pigment production is one of the strategies of bacteria to escape from adverse effect of UV radiations. The photo-protective pigments help bacteria to cope up with prolonged exposure to UV radiation (Wynn-Williams et al., 2002). Some of these pigments are also well-known for their ability to provide protection against oxidative damage which helps in stimulation of immune response and cancer inhibition (Krinsky and Johnson, 2005). Symbiotic pigmented bacteria are known to protect their host from other pathogens (Egan et al., 2002). Fungi also produce pigments as a protection strategy against abiotic stresses like UV radiation and desiccation (Issac, 1994). Endophytic fungi have been reported to protect the host plant from insects or other microorganisms by producing the pigment Anthraquinones (Gessler et al., 2013). In microalgae, pigments are known to play light harvesting, photo protective and structural roles and they are also involved in carbon and energy storage (Mulders et al., 2014). Microbial pigments are thus more than simple coloring compounds because of the associated biological activities that can be of potential human benefit in case of their use as food additives. This article comprises the up to date details about the types, advantages and challenges related to the production and use of microbial food pigments.
Types of Microbial Pigments as Food Colorants
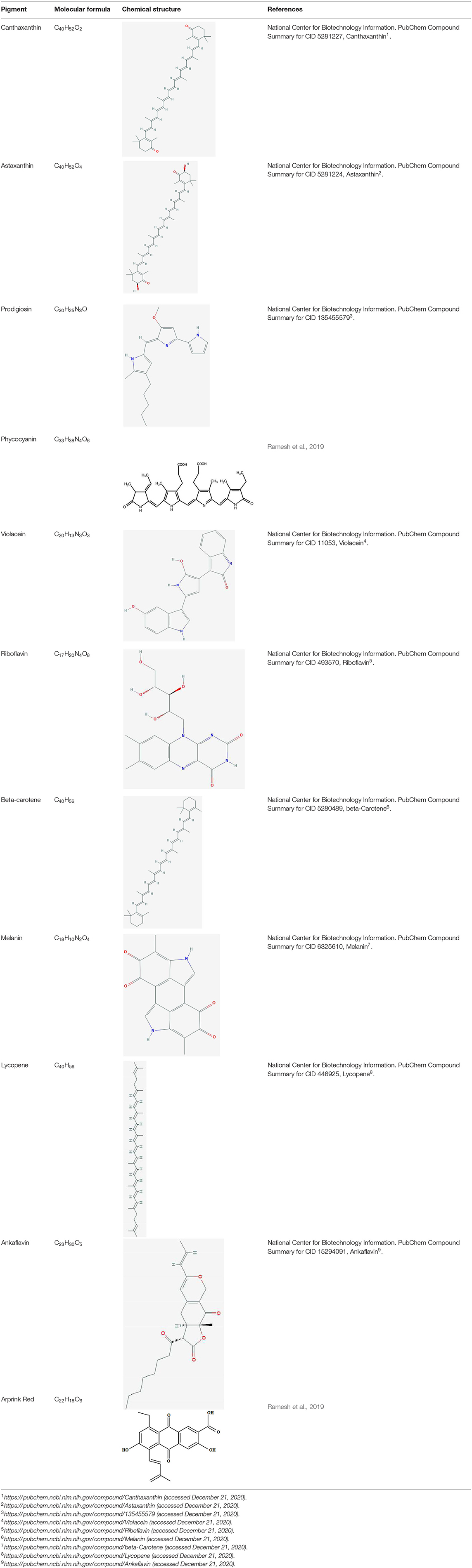
Among microorganisms, fungi, bacteria, and microalgae are well-known to produce a range of natural pigmented substances having marked variation in chemical compositions, function, stability and solubility. These naturally occurring pigments are reflection of the secondary metabolites with great commercial value in food & dairy, cosmetics, pharmaceutical, textile, and dyeing industry (Narsing Rao et al., 2017). They belong to distinct categories based on their chemical composition, functional activities and natural occurrence such as derivatives of flavonoids, pyrroles, carotenoids, etc. Riboflavin, Beta-carotene, Canthaxanthin, Prodigiosin, Phycocyanin, Melanin, Violacein, Astaxanthin, and Lycopene are the major pigments reported from microbial sources having application as food colorants (Sen et al., 2019). The important natural food pigments reported to be produced by microorganisms and their advantages are discussed below and their details are also summarized in Tables 1, 2.
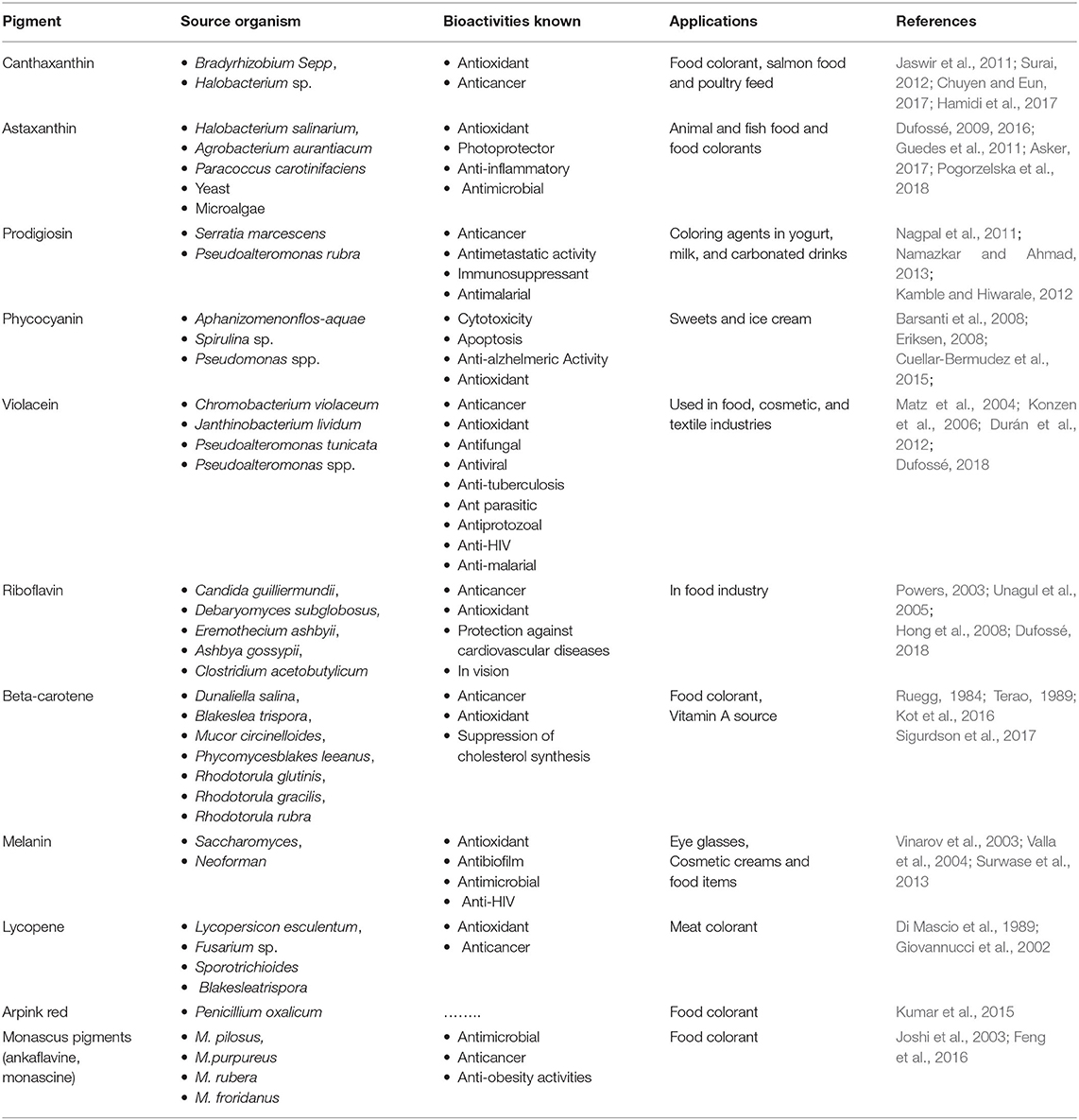
1. Riboflavin, also known as B2 vitamin, is a water soluble pigment of yellow color having applications as a dietary supplement and food additive in dairy products, sauces, baby foods, fruit, and energy juices. It helps body break the dietary polymeric compounds such as carbohydrates, proteins, and fats to generate energy and use oxygen. Microorganisms such as Candida guilliermundii, Debaryomyces subglobosus, Eremothecium ashbyii, Ashbya gossypi, Clostridium acetobutylicum have been reported to produce it (Unagul et al., 2005; Hong et al., 2008; Nigam and Luke, 2016; Dufossé, 2018).
2. Beta-carotene, a red-orange water insoluble organic pigment, is a very good source of vitamin A for human body that boosts immunity, prevents aging and helps in night vision issues (Eroglu et al., 2012). Several microorganisms such as Dunaliella salina, Blakeslea trispora, Mucor circinelloides, Phycomyces blakesleeanus, Rhodotorula glutinis, Rhodotorula gracilis, Rhodotorula rubra are known to produce it (Ruegg, 1984; Nigam and Luke, 2016; Sigurdson et al., 2017; Dufossé, 2018). Some natural colorants belonging to carotenoid family obtained from Haematococcus pluvialis and Phaffia rhodozyma are extensively used as food additives for animals and fish as well as in pharmaceuticals and aquaculture fields (Stafsnes et al., 2010).
3. Canthaxanthin is orange to dark pink colored, keto-carotenoid and lipid soluble pigment. There are reports of its production by Bacteriochlorophyll containing microbes such as Bradyrhizobium sp. and Halobacterium sp. (Jaswir et al., 2011; Surai, 2012; Chuyen and Eun, 2017). The production of canthaxanthin from microalgae like Nannochloropsis gaditana (Millao and Uquiche, 2016) and Chlorella zofingiensis (Li et al., 2006) has also been reported. Canthaxanthins are effective antioxidants and inhibit the oxidation of lipids in liposomes (Woodall et al., 1997).
4. Prodigiosin, a red colored multipurpose pigment, is reported to be produced by Serratia marcescens, Vibrio psychoerythrus, Rugamonas rubra, Streptoverticillium rubrireticuli, and other eubacteria (Nagpal et al., 2011). It is used in yogurt, milk and carbonated drinks (Namazkar and Ahmad, 2013). Prodigiosin has been shown to have insecticidal, antifungal, antibacterial, anticancer, and anti-malarial activities (Kavitha et al., 2010; Kamble and Hiwarale, 2012).
5. Phycocyanin is a blue colored photosynthetic pigment produced by blue-green algae that contain chlorophyll A (Sen et al., 2019). It is water soluble and an accessory pigment to chlorophyll. It is found in Aphanizomenon flos-aquae and Spirulina sp. (Barsanti et al., 2008; Cuellar-Bermudez et al., 2015). It is used in sweets, ice creams and also as a dietary supplement rich in proteins. Pyocyanin also acts as bio-control agent that have anti-bacterial, anti-fungal and anti-alzhelmeric activity (Jayaseelan et al., 2014).
6. Melanin is a natural pigment which is known to be produced by a wide variety of microorganisms such as Colletotrichum lagenarium, Aspergillus fumigates Vibrio cholerae, Shewanella colwelliana, Alteromonas nigrifaciens (Soliev et al., 2011). This pigment is also present in animals and plants. Besides several other uses such as in eye glasses, cosmetic creams, pharmaceuticals, they are also added in food items (Sen et al., 2019). The pigment is also reported to be associated with anti-HIV activity (Surwase et al., 2013).
7. Violacein is a versatile purple colored pigment that possess numerous biological activities. Various bacteria like Chromobacterium violaceum, Pseudoalteromonas, Collimonas, Janthinobacterium, Microbulbifer are known to produce this pigment (Choi et al., 2015). It is highly demanded at large scale in cosmetics, food, medicine and textiles (Dufossé, 2018). The pigment is associated with several useful bioactivity including antibacterial, anticancer, antiviral, enzyme modulation, antiulcerogenic, and anti-leishmanial (Soliev et al., 2011).
8. Astaxanthin is a lipid soluble orange-red pigment present in yeast, microalgae, marine organisms, and in feather of some birds (Sen et al., 2019). It is also reported to be produced by various bacteria such as Halobacterium salinarium, Agrobacterium aurantiacum, Paracoccus carotinifaciens (Guedes et al., 2011; Asker, 2017; Zuluaga et al., 2017; Pogorzelska et al., 2018). It is associated with anti-aging and memory improving activities and used as coloring agent in animal and fish foods (Capelli and Cysewski, 2013).
9. Lycopene is an approved meat coloring agent in several countries. It is a water insoluble biopigment belonging to carotene. It is present in tomato and other red fruits and vegetables (Di Mascio et al., 1989; Giovannucci et al., 2002) and can also be chemically synthesized. However, microbial production of lycopene is comparatively more economical and sustainable and has been produced in microbial hosts like Blakeslea trispora, E. coli, and yeasts by genetic engineering methods (Chen et al., 2016).
10. Arprink red is a red colored extracellular metabolite of the anthraquinone class produced by Penicillium oxalicum. It is also suggested to have anticancer effects when used as food supplements (Sardaryan, 2002). It is used as food colorant in various food products in different amounts as recommended by Codex Alimentarius Commission (Kumar et al., 2015).
11. Monascus pigments are a group of fungal secondary metabolites called azaphilones produced by filamentous fungi belonging to the genus Monascus of Ascomycetes group (Chung et al., 2008). They are red (monascorubramine and rubropunctamine), yellow (ankaflavin and monascin) and orange (rubropunctatin and monascorubrine) colored pigments (Vendruscolo et al., 2013). These pigments are extracted from various species of this fungi i.e., M. pilosus, M. purpureus, M. ruberand, M. froridanus, etc. and being used as food colorants for many years in red wines, yogurt, sausages, hams, and meats (Dufossé et al., 2005). They are also known to exhibit antimicrobial, anticancer, anti-obesity, and antioxidant activities (Vendruscolo et al., 2013).
Fermentation Conditions for Microbial Pigment Production
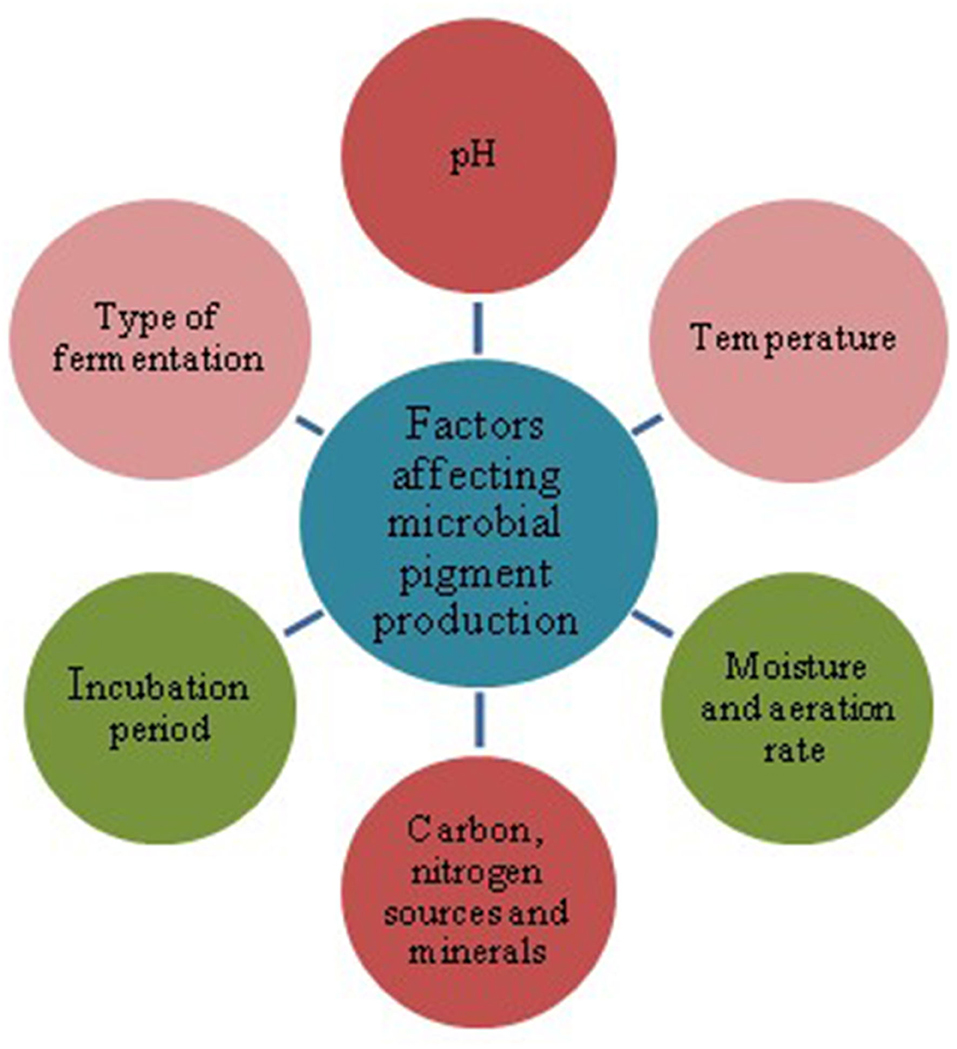
Microbial pigments intended to be used as food additive or colorants are being commercially obtained from bacteria, fungi, and algae (Nigam and Luke, 2016). For industrial production of pigments the desired microorganism should possess properties like acceptability of wide range of carbon and nitrogen sources and tolerance of process pH and temperature (Kirti et al., 2014; Kumar et al., 2015). In addition, the yield should be sufficiently high enough to make it a cost-effective affair. The microorganisms capable to produce promising pigments can be isolated and screened through bioprospecting programs in different environment. Alternatively, the known pigment producing microorganisms can be subjected to strain improvement techniques for the desired yield and properties. A combination of the aforesaid two approaches can also be applied. Various factors mainly the type of fermentation, media components (carbon, nitrogen sources, and minerals), pH, temperature, time of incubation, moisture content and aeration rate (Figure 2) affect the growth and yield of pigments through microbial fermentation. Solid state and submerged fermentation approaches are used for the production of microbial pigments (Tuli et al., 2015). Solid state fermentation offers the advantages of higher yield and productivity as well as the direct applicability of the fermented product as a colorant without isolating the product (Babitha, 2009). SSF is particularly suitable for the growth of fungi and the use of this technique also leads to savings in wastewater and yield of the metabolites (Tuli et al., 2015). The optimum value of various factors affecting the fermentation conditions vary with the microorganism used for pigment production. For instance, optimum temperature range for pigment production by Monascus spp. is 25–28°C whereas Pseudomonas sp. prefers the temperature of 35–36°C (Kumar et al., 2015). The maximum growth and production of carotenoid from Sarcina sp after an incubation period of 72 h was reported by Joshi et al. (2011). The same incubation period has been found as the optimum for pigment production by Rhodototrula and Micrococcus (Attri and Joshi, 2005; Joshi and Attri, 2006). On the other hand, an incubation period of 48 hr has been reported as optimum for Chromobacter for pigment production by Attri and Joshi (2006).
The pH can affect the pigment production and its shade. Monascus sp. produces pigments optimally at pH between 5.5 and 6.5 whereas Rhodotorula does it as pH 4.0–4.5. Lycopene formation occurs at neutral to slightly alkaline pH whereas β-carotene formation occurs at acidic pH (Joshi et al., 2003). Carbon sources have impact on the microbial growth and shade of the microbial pigment. Depending on the species used monosaccharide or their polymers can be the optimum carbon source choice for pigment production (Joshi et al., 2003). A range of inorganic and organic nitrogenous compounds may be preferred by different microbes for maximum pigment production. In addition, various minerals have also been documented to affect microbial pigment production (Joshi et al., 2003).
Although synthetic media can be used for the microbial production of pigments but use of agrochemical waste is suggested to be much better in terms of reducing the overall production cost (Panesar et al., 2015). Hamano and Kilikian (2006) demonstrated the use of corn steep liquor favorable for the production of pigment by Monascus ruber. A waste stream cellulose culture medium was utilized and optimized for pigment production by Penicillum sp. (Sopandi et al., 2013). Tinoi et al. (2005) produced carotenoid pigment by culturing Rhodotorula glutinis on hydrolyzed mung bean waste flour. Whey and soya protein have also been successfully used as raw material for the production of microbial pigments (Kaur et al., 2008; Panesar et al., 2015). Various food and vegetable waste products have also been utilized for microbial pigment production (Nigam and Luke, 2016).
Technical Advancement Toward Microbial Pigment Production
Numerous technical advancements have been reported in recent years toward successful production of microbial pigments intended for various industrial applications. The progress in various aspects related to fermentative production of pigments is discussed below.
Strain Improvement
Microbial pigments of desired characteristics with increased yield can be obtained through the use of established strain improvement techniques. Often a wild microbial strain is associated with limited production of pigment which negatively affects the economy of the process. The routinely used method of random mutagenesis and selection has resulted in the increase of pigment yield. Exposure to UV light and other mutagens such as 1-methyl-3-nitro-1-nitrosoguanidine, Ethyl methyl sulfonate is known to cause several fold increase in microbial pigment production (Nigam and Luke, 2016). Fakorede et al. (2019) have reported 5 fold increase in pigment production by Serratia marcescens (GBB151) after mutagenic treatment with Ethidium bromide. Techniques of genetic engineering have also been successfully employed for enhancing the microbial pigment yield and alter its molecular structure and color (Sen et al., 2019). Although information on the complete blueprint of the biochemical synthetic pathways and the intermediates is generally a prerequisite for all such genetic manipulations targeting the rate limiting step for enhancing production. Bartel et al. (1990) and McDaniel et al. (1993) have reported such genetic alteration for blue pigment Actinorhodin, produced by Streptomyces coelicolor. Increased production of Zeaxanthin and other pigments by genetic engineering of Synechocystis sp. Strain PCC 6803 has been reported by Lagarde et al. (2000). There are reports of the development of cell factories using heterologous expression for the production of microbial pigments (Nielsen and Nielsen, 2017; Sankari et al., 2018). Technique of transposon mutagenesis applied on Pseudomonas fluorescens revealed 8 genes involved in blue pigment production and antioxidant protection (Andreani et al., 2019).
Optimization of Fermentation Conditions and Downstream Processing
The optimization of fermentation conditions and development of economic downstream processing can lead to the cost- effective production of microbial pigments. Media optimization includes variation in fermentation conditions like temperature, pH, incubation time, nutritional sources, aeration, and agitation rate etc. for selection of conditions that provide best yield. The technique of Response surface methodology (RSM) has many advantages over classical methods used for media optimization. Fewer experiments are required to derive an optimum combination of all the variable factors under investigation. Optimization of fermentation conditions thus require less time and efforts leading to reduction in the overall cost. Hamidi et al. (2017) determined the optimum values of temperature, pH and saline concentration and the effect of light on total carotenoid production by Halorubrum sp. TBZ126 using response surface methodology. Optimization of culture medium for yellow pigments production with Monascus anka mutant using response surface methodology has been reported by Zhou et al. (2009). Artificial neural network (ANN) is another technique that can be used to study the impact of fermentation conditions as well as their optimization for microbial pigment production. Singh et al. (2015) have investigated the application of Artificial Neural Network (ANN) in modeling a Liquid State Fermentation (LSF) for red pigment production by Monascus purpureus MTCC 369 and reported that ANN model can be used to predict the effects of fermentation parameters on red pigment production with a high correlation.
The conventional method of organic solvent extraction of pigments from fermentation broth is a complicated and time-consuming process with disadvantages of high cost, low yield and possible solvent leftover in the purified product as contaminants (Sen et al., 2019). Sen et al. (2019) have also mentioned that use of non-ionic resin due to their high loading ability is particularly suitable for large scale recovery of pigments and the technique also offer the advantage of direct absorption of compounds form the culture broth, thereby reducing the overall cost. Wang et al. (2004) have described the use of a non-ionic adsorbent (X-5) resin in the presence of Tween 80 for direct recovery of prodigiosin from the culture broth of S. marcescens. A novel approach of perstraction for recovery of intracellular pigments through submerged fermentation of Talaromyces spp. in a surfactant rich media has been described by Morales-Oyervides et al. (2017).
Pigment Stabilization
Stability against light, pH, temperature, UV radiation, and food matrices is an important issue with regard to the suitability of a microbial pigment for industrial applications and strategies like microencapsulation, nanoemulsion, and nanoformulations have been suggested for this purpose (Sen et al., 2019). In encapsulation solids, liquids, or gaseous materials are packaged in matrices (encapsulants) which sustain and release their contents under specific conditions (de Boer et al., 2019). The technique offer multiple advantages such as protection against light, moisture, or heat and also increase the brightness of the natural colorants and enhance their stability for many industrial applications (de Boer et al., 2019). The commonly used techniques for encapsulating colorants are spray-drying, electrospraying and anti-solvent precipitation (de Boer et al., 2019). A number of reports are available on the microencapsulation of microbial pigments and their enhanced applicabilities. Lycopin and carotenoids have been reported to be encapsulated by the methods of spray drying and lyophilization, respectively (Rocha et al., 2012; Nogueira et al., 2017). The strategy of nano-encapsulation or nano-emulsions which employs the droplet sizes of 100 nm or less with water, soil and emulsifier can be used for encapsulating microbial pigments which offers further advantages of stronger kinetic stability and resistance to chemical and physical changes because of larger surface area per unit (Gupta et al., 2016; Sen et al., 2019). There are various studies about the positive impact of nanoemulsion on stability of β carotene (Yi et al., 2014; Sen et al., 2019). Although various nanostructures are known to confer stability to carotenoid pigments, encapsulated polymeric nanocapsules are most utilized due to its stability during storage, high efficiency to encapsulate and to control the release of the carotenoid (Dos Santos et al., 2018). Bazana et al. (2015) have explored some nanoencapsulation techniques such as emulsification, coacervation, inclusion complexation and nanoprecipitation for lycopene. Other strategies for enhancing pigment stability that has been worked upon are like additions of copigment compounds, such as polymers, phenolic compounds, and metals as well as the exclusion of O2 during processing (Cortez et al., 2017). Various patents have been filed for novel methods of pigment stabilization such as the hard candy coating for various colors (Cortez et al., 2017). The technique of genetic engineering has also been applied for enhancing the natural pigment stability. A novel method of gene-encoded acyltransferase of aromatic acyl groups has been filed for patent by Tanaka et al. (2011).
Analysis and Detection of Microbial Pigments
Various analytical techniques developed over the years are in use to detect and analyze microbial pigments. TLC, UV–VIS spectrophotometry, FTIR, NMR, HPLC are the techniques in routine use for identification and characterization of microbial pigments. A handheld Raman spectrometer, working on the principle of excitation laser, has been employed for the detection of microbial pigments in various environments (Jehlicka and Oren, 2013; Kumar et al., 2015). Mass spectrometry coupled with electrospray ionization can be used for classifying the pigments producing fungi (Smedsgaard and Frisvad, 1996).
Major Challenges Associated With the Use of Microbial Pigments as Food Colors
Both extracellular and intracellular production of pigments is known in microorganisms. Although commercial production of food pigments from microorganism offers special advantages, the species under use must be amenable to culture with a fast growth rate and productivity in limited space and time (Ramesh et al., 2019). In addition, it must be non-toxigenic, non-pathogenic, and able to grow on a wider range of cheaper raw materials with stability under harsh physical and chemical process conditions (Ramesh et al., 2019). Due to various factors natural colors are more expensive as compared to their synthetic counterparts. In confectionary items biopigments can be 20 times more expensive as synthetic pigments (Sigurdson et al., 2017). Microbial pigments may also be associated with the tendency to react with the other food components and may generate unwanted odors and flavors (Sen et al., 2019). Extraction and purification of microbial pigments from fermentation broth is a time consuming, low yielding and costly affair (Nigam and Luke, 2016) and use of organic solvent may itself overcome the idea of obtaining natural pigments (Hicketier and Buchholz, 2002).
The use of synthetic media for microbial production can overprice the production cost although cheap agro-industrial residues such as coconut residue, soybean meal, corn syrup, starch, cheese whey, rice water, jackfruit seed extract, mustard waste, sugar beet molasses, etc. are promising alternative media substitutes for pigment production (Venil et al., 2014). However, the availability of such byproducts throughout the year at many places may be difficult. The another issue with natural pigments is their stability and sensitivity toward light, pH, UV, temperature, oxygen, heat, and other environmental conditions that may lead to color loss and a reduced shelf life (Sen et al., 2019). Various encapsulation strategies as well as genetic engineering methods have been developed to address this issue. In future the development of novel techniques like the combination and evaluation of new pigment stabilizing material will further enhance their prospects to be used as value-added natural food pigments (Cortez et al., 2017). Since all food additives are under very strict legislation and approval mechanism, it is of paramount importance that microbial pigment production and its purification process must not allow any unwanted toxic or allergic metabolite in the final product (Gao et al., 2003).
Conclusion
Given the growing public perception and concern over the use of safe food ingradients, the industrial demand of natural pigments is expected to increase in future by many folds. Microbial pigments are attractive alternative to synthetic food colorants not only because of their natural origin but also due to their several proven health benefits. Although a plethora of microorganisms have been reported to produce food grade pigments at laboratory level, large scale production and purification of the products from many of them is still a challenge. More studies are required with respect to media and fermentation condition optimization for sufficient production and easy recovery of microbial pigments. In addition, classical strain improvement methods as well as the advance techniques of genetic or metabolic engineering can be used for sustainable production of microbial pigments of high use. The strain improvement methods can also be preceded by bioprospecting programs to screen and identify novel pigment producing microbial strains from different environments in adherence to the Nagoya protocol and other applicable state rules. Exploration of traditional fermentative food in isolated or tribal region can also lead to the identification of promising pigment producing isolates. Although only non-pathogenic microbes are acceptable for food grade pigments, co-production of toxic or undesirable compound by so called “safe” organism is also possible and therefore, appropriate cost effective purification strategies are to be devised.
Author Contributions
BR, MB, and BP prepared the first draft of the manuscript. GJ critically evaluated the same and prepared the final version with the help of all of them and MA. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^https://www.marketsandmarkets.com/Market-Reports/food-colors-market-36725323.html
2. ^http://importedfoods.afdo.org/food-color-additives-banned-in-the-usa.html
References
Andreani, N. A., Carraro, L., Zhang, L., Vos, M., and Cardazzo, B. (2019). Transposon mutagenesis in Pseudomonas fluorescens reveals genes involved in blue pigment production and antioxidant protection. Food Microbiol. 82, 497–503. doi: 10.1016/j.fm.2019.03.028
Asker, D. (2017). Isolation and characterization of a novel, highly selective astaxanthin-producing marine bacterium. J. Agri. Food Chem. 65, 9101–9109. doi: 10.1021/acs.jafc.7b03556
Attri, D., and Joshi, V. K. (2005). Optimization of apple pomace based medium and fermentation conditions for pigment production by Micrococcus species. J. Sci. Ind. Res. 64, 598-601.
Attri, D., and Joshi, V. K. (2006). Optimization of apple pomace based medium and fermentation condition for pigment production by Chromobacter species. J. Food Sci. Technol. 43, 515–520.
Babitha, S. (2009). “Microbial pigments,” in Biotechnology for Agro Industrial Residues Utilization, eds. N. Singh, P.S. Nigam, and A. Pandey (Dordrecht: Springer), 147–162. doi: 10.1007/978-1-4020-9942-7_8
Barsanti, L., Coltelli, P., Evangelista, V., Frassanito, A. M., Passarelli, V., Vesentini, N., et al. (2008). “Oddities and curiosities in the algal world,” in Algal toxins: nature, occurrence, effect and detection (Dordrecht: Springer), 353–391. doi: 10.1007/978-1-4020-8480-5_17
Bartel, P. L., Zhu, C. B., Lampel, J. S., Dosch, D. C., Connors, N. C., Strohl, W. R., et al. (1990). Biosynthesis of anthraquinones by interspecies cloning of actinorhodin biosynthesis genes in streptomycetes: clarification of actinorhodin gene functions. J. Bacteriol. 17, 4816–4826. doi: 10.1128/JB.172.9.4816-4826.1990
Bazana, M. T., Codevilla, C. F., da Silva, C. D. B., and de Menezes, C. R. (2015). Lycopene nanoencapsulation in food. Ciência Natura 37, 38–48. doi: 10.5902/2179460X19713
Burrows, J. D. A. (2009). Palette of our palates: a brief history of food coloring and its regulation. Com. Rev. Food Sci. Food Saf. 8, 394–408. doi: 10.1111/j.1541-4337.2009.00089.x
Capelli, G. C., and Cysewski, G. (2013). The Worlds' Best Kept Health Secret Natural Astaxanthin. Kailua-Kona, HI: Cyanotech Corporation.
Chen, Y., Xiao, W., Wang, Y., Liu, H., Li, X., and Yuan, Y. (2016). Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Micro.Cell Fact. 15, 1–13. doi: 10.1186/s12934-016-0509-4
Choi, S. Y., Yoon, K. H., Lee, J. I., and Mitchell, R. J. (2015). Violacein: properties and production of a versatile bacterial pigment. BioMed Res. Int. 2015, 1–8. doi: 10.1155/2015/465056
Chung, C. C., Chen, H. H., and Hsieh, P. C. (2008). Optimization of the Monascus purpureus fermentation process based on multiple performance characteristics. J. Grey System 11, 85−96.
Chuyen, H. V., and Eun, J. B. (2017). Marine carotenoids: Bioactivities and potential benefits to human health. Com. Rev. Food Sci. Food Saf. 57, 2600–2610. doi: 10.1080/10408398.2015.1063477
Corradini, M. G. (2018). “Synthetic Food Colors” in Encyclopedia of Food Chemistry, eds P. Verelis, L. Melton, and F. Shahidi (Cambridge, MA: Elsevier), 291–296. doi: 10.1016/B978-0-08-100596-5.21606-5
Cortez, R., Luna-Vital, D. A., Margulis, D., and Gonzalez de Mejia, E. (2017). Natural pigments: stabilization methods of anthocyanins for food applications. Com. Rev. Food Sci. Food Saf. 16, 180–198. doi: 10.1111/1541-4337.12244
Cuellar-Bermudez, S. P., Aguilar-Hernandez, I., Cardenas-Chavez, D. L., Ornelas-Soto, N., Romero-Ogawa, M. A., and Parra-Saldivar, R. (2015). Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microbial Biotech. 8, 190–209. doi: 10.1111/1751-7915.12167
de Boer, F. Y., Imhof, A., and Velikov, K. P. (2019). Encapsulation of colorants by natural polymers for food applications. Coloration Technol. 135, 183–194. doi: 10.1111/cote.12393
Di Mascio, P., Kaiser, S., and Sies, H. (1989). Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 274, 532–538. doi: 10.1016/0003-9861(89)90467-0
Dos Santos, P. P., de Aguiar Andrade, L., Flôres, S. H., and de Oliveira Rios, A. (2018). Nanoencapsulation of carotenoids: a focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 55, 3851–3860. doi: 10.1007/s13197-018-3316-6
Dufossé, L. (2009). “Pigments, microbial” in Encyclopedia of Microbiology, ed. M. Schaechter (New York, NY: Elsevier/Academic Press), 457–71. doi: 10.1016/B978-012373944-5.00155-3
Dufossé, L. (2016). “Current and potential natural pigments from microorganisms (bacteria, yeasts, fungi, microalgae),” in Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color, eds R. Carle and R. Ralf Schweiggert (Cambridge: Woodhead Publishing), 337–352. doi: 10.1016/B978-0-08-100371-8.00016-6
Dufossé, L. (2018). “Microbial pigments from bacteria, yeasts, fungi, and microalgae for the food and feed industries,” in Handbook of Food Bioengineering: Natural and Artificial Flavoring Agents and Food Dyes, Vol. 7, eds A. M. Grumezescu and A. M. Holban (Amsterdam: Academic Press), 113–132. doi: 10.1016/B978-0-12-811518-3.00004-1
Dufossé, L., Galaup, P., Yaron, A., Arad, S. M., Blanc, P., Murthy, K. N. C., et al. (2005). Microorganisms and microalgae as sources of pigments for food use: a scientific oddity or an industrial reality. Trends in Food Sci. Technol. 16, 389–406. doi: 10.1016/j.tifs.2005.02.006
Durán, M., Ponezi, A. N., Faljoni-Alario, A., Teixeira, M. F., Justo, G. Z., and Durán, N. (2012). Potential applications of violacein: a microbial pigment. Med. Chem. Res. 21, 1524–1532. doi: 10.1007/s00044-011-9654-9
Egan, S., James, S., Holmström, C., and Kjelleberg, S. (2002). Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata. Environ. Microbiol. 4, 433–442. doi: 10.1046/j.1462-2920.2002.00322.x
Eriksen, N. T. (2008). Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbial. Biotechnol. 80, 1–14. doi: 10.1007/s00253-008-1542-y
Eroglu, A., Hruszkewycz, D. P., dela Sena, C., Narayanasamy, S., Riedl, K. M., Kopec, R. E., et al. (2012). Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287, 15886–15895. doi: 10.1074/jbc.M111.325142
Fakorede, C. N., Itakorode, B. O., Odeyemi, O., and Babalola, G. (2019). Enhancement of pigment production potential of Serratia marcescens (GBB151) through mutation and random amplified polymorphic deoxyribonucleic acid analysis of its mutants. J. Appl. Biol. Biotechnol. 7, 1–6. doi: 10.7324/JABB.2019.70401
Feng, Y., Shao, Y., Zhou, Y., Chen, W., and Chen, F. (2016). “Monascus pigments” in Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants, eds E. J. Vandamme, J. L. Revuelta (Weinheim: Wiley-VCH), 497–526. doi: 10.1002/9783527681754.ch18
Gao, L. Y., Groger, R., Cox, J. S., Beverley, S. M., Lawson, E. H., and Brown, E. J. (2003). Transposon mutagenesis of Mycobacterium marinum identifies a locus linking pigmentation and intracellular survival. Inf. Immun. 71, 922–929. doi: 10.1128/IAI.71.2.922-929.2003
Gessler, N. N., Egorova, A. S., and Belozerskaya, T. A. (2013). Fungal anthraquinones. Appl. Biochem. Microbiol. 49, 85–99. doi: 10.1134/S000368381302004X
Giovannucci, E., Rimm, E. B., Liu, Y., Stampfer, M. J., and Willett, W. C. (2002). A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 94, 391–398. doi: 10.1093/jnci/94.5.391
Guedes, A. C., Amaro, H. M., and Malcata, F. X. (2011). Microalgae as sources of carotenoids. Marine Drugs 9, 625–644. doi: 10.3390/md9040625
Gupta, A., Eral, H. B., Hatton, T. A., and Doyle, P. S. (2016). Nanoemulsions: formation, properties and applications. Soft Matter 12, 2826–2841. doi: 10.1039/C5SM02958A
Hamano, P. S., and Kilikian, B. V. (2006). Production of red pigments by Monascus ruber in culture media containing corn steep liquor. Braz. J. Chem. Eng. 23, 443–449. doi: 10.1590/S0104-66322006000400002
Hamidi, M., Hejazi, M. S., Nazemyieh, H., Hejazi, M. A., and Naziri, D. (2017). Halorubrum Sp. TBZ112, an extremely halophilic carotenoid-producing archaeon isolated from Urmia Lake. Pharm. Sci. 23, 150–158. doi: 10.15171/PS.2017.22
Hicketier, M., and Buchholz, K. (2002). Fluidized bed adsorption of Cephalosporin C. J. Biotechnol. 93, 253–268. doi: 10.1016/S0168-1656(01)00408-4
Hong, M. Y., Seeram, N. P., Zhang, Y., and Heber, D. (2008). Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J. Nut. Biochem. 19, 448–458. doi: 10.1016/j.jnutbio.2007.05.012
Issac, S. (1994). Many fungi are brightly coloured; does pigmentation provide an advantages to those species. Mycol. Ans. 8,178–179.
Jaswir, I., Noviendri, D., Hasrini, R. F., and Octaviatin, F. (2011). Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 5, 7119–7131. doi: 10.5897/JMPRX11.011
Jayaseelan, S., Ramaswamy, D., and Dharmaraj, S. (2014). Pyocyanin: production, applications, challenges and new insights. World J. Microbiol. Biotechnol. 30, 1159–1168. doi: 10.1007/s11274-013-1552-5
Jehlicka, J., and Oren, A. (2013). Raman spectroscopy in halophile research. Front. Microbiol. 4:380. doi: 10.3389/fmicb.2013.00380
Joshi, V. K., and Attri, D. (2006). Optimization of apple pomace based medium and fermentation conditions for pigment production by Rhodotorula species. Proc. Nat. Acad. Sci. India 76, 171–177.
Joshi, V. K., Attri, D., Bala, A., and Bhushan, S. (2003). Microbial pigments. Ind. J. Biotechnol. 2, 362–369.
Joshi, V. K., Attri, D., and Rana, N. S. (2011). Optimization of apple pomace based medium and 492 fermentation conditions for pigment production by Sarcina sp. I. J. Nat. Prod. Res. 2, 421–427.
Kamble, K. D., and Hiwarale, V. D. (2012). Prodigiosin production from Serratia marcescens strains obtained from farm soil. Int. J. Environ. Sci. 3, 631–638.
Kaur, B., Chakraborty, D., and Kaur, H. (2008). Production and stability analysis of yellowish pink pigments from Rhodotorula rubra MTCC 1446. Internet J. Microbiol 7:243–5. doi: 10.5580/245b
Kavitha, R., Aiswariya, S., and Ratnavali, C. M. (2010). Anticancer activity of red pigment from Serratia marcescens in human cervix carcinoma. Int. J. Pharm.Tech. Res. 2, 784–787.
Kirti, K., Amita, S., Priti, S., Mukesh Kumar, A., and Jyoti, S. (2014). Colorful world of microbes: carotenoids and their applications. Adv. Bio. 2014, 1–13. doi: 10.1155/2014/837891
Konzen, M., De Marco, D., Cordova, C. A., Vieira, T. O., Antonio, R. V., and Creczynski-Pasa, T. B. (2006). Antioxidant properties of violacein: possible relation on its biological function. Bioorg. Med. Chem. 14, 8307–8313. doi: 10.1016/j.bmc.2006.09.013
Kot, A. M., Błazejak, S., Kurcz, A., Gientka, I., and Kieliszek, M. (2016). Rhodotorula glutinis—potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbial. Biotechnol. 100, 6103–6117. doi: 10.1007/s00253-016-7611-8
Krinsky, N. I., and Johnson, E. J. (2005). Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 26, 459–516. doi: 10.1016/j.mam.2005.10.001
Kumar, A., Vishwakarma, H. S., Singh, J., Dwivedi, S., and Kumar, M. (2015). Microbial pigments: production and their applications in various industries. Int. J. Pharm. Chem. Biol. Sci. 5, 203–212.
Lagarde, D., Beuf, L., and Vermaas, W. (2000). Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 66, 64–72. doi: 10.1128/AEM.66.1.64-72.2000
Li, H. B., Fan, K. W., and Chen, F. (2006). Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high-speed counter-current chromatography. J. Separation Sci. 29, 699–703. doi: 10.1002/jssc.200500365
Matz, C., Deines, P., Boenigk, J., Arndt, H., Eberl, L., Kjelleberg, S., et al. (2004). Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl. Environ. Microbiol. 70, 1593–1599. doi: 10.1128/AEM.70.3.1593-1599.2004
McDaniel, R., Ebert-Khosla, S., Hopwood, D. A., and Khosla, C. (1993). Engineered biosynthesis of novel polyketides. Science 2620, 1546–1550. doi: 10.1126/science.8248802
Millao, S., and Uquiche, E. (2016). Extraction of oil and carotenoids from pelletized microalgae using supercritical carbon dioxide. J. Supercrit. Fluids 116, 223–231. doi: 10.1016/j.supflu.2016.05.049
Morales-Oyervides, L., Oliveira, J., Sousa-Gallagher, M., Méndez-Zavala, A., and Montañez, J. C. (2017). Perstraction of intracellular pigments through submerged fermentation of Talaromyces spp. in a surfactant rich media: A novel approach for enhanced pigment recovery. J. Fungi 3:33. doi: 10.3390/jof3030033
Mulders, K. J., Lamers, P. P., Martens, D. E., and Wijffels, R. H. (2014). Phototrophic pigment production with microalgae: biological constraints and opportunities. J. Phycol. 50, 229–242. doi: 10.1111/jpy.12173
Nagpal, N., Munjal, N., and Chatterjee, S. (2011). Microbial pigments with health benefits-a mini review. Trends Biosci. 4, 157–160.
Namazkar, S., and Ahmad, W. A. (2013). Spray-dried prodigiosin from Serratia marcescens as a colorant. Biosciences Biotechnol. Res. Asia 10, 69–76. doi: 10.13005/bbra/1094
Narsing Rao, M. P., Xiao, M., and Li, W. J. (2017). Fungal and bacterial pigments: secondary metabolites with wide applications. Front. Microbiol. 8:1113. doi: 10.3389/fmicb.2017.01113
Nielsen, J. C., and Nielsen, J. (2017). Development of fungal cell factories for the production of secondary metabolites: linking genomics and metabolism. Synth. Syst. Biotechnol. 2, 5–12. doi: 10.1016/j.synbio.2017.02.002
Nigam, P. S., and Luke, J. S. (2016). Food additives: production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 7, 93–100. doi: 10.1016/j.cofs.2016.02.004
Nogueira, M. B., Prestes, C. F., and de Medeiros BURKERT, JF. (2017). Microencapsulation by lyophilization of carotenoids produced by Phaffia rhodozyma with soy protein as the encapsulating agent. Food Sci. Technol. 37, 1–4. doi: 10.1590/1678-457x.05417
Panesar, R., Kaur, S., and Panesar, P. S. (2015). Production of microbial pigments utilizing agro-industrial waste: a review. Curr. Opin. Food Sci. 1, 70–76. doi: 10.1016/j.cofs.2014.12.002
Pogorzelska, E., Godziszewska, J., Brodowska, M., and Wierzbicka, A. (2018). Antioxidant potential of Haematococcus pluvialis extract rich in astaxanthin on colour and oxidative stability of raw ground pork meat during refrigerated storage. Meat Sci. 135, 54–61. doi: 10.1016/j.meatsci.2017.09.002
Powers, H. J. (2003). Riboflavin (vitamin B-2) and health. Am. J. Clin. Nut. 77, 1352–1360. doi: 10.1093/ajcn/77.6.1352
Ramesh, C., Vinithkumar, N. V., Kirubagaran, R., Venil, C. K., and Dufossé, L. (2019). Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorg 7:186. doi: 10.3390/microorganisms7070186
Rocha, G. A., Carmen, S. F. T., and Carlos, R. F. G. (2012). Microencapsulation of lycopene by spray drying: characterization, stability and application of microcapsules. Food Bioprod. Process. 90, 37–42. doi: 10.1016/j.fbp.2011.01.001
Ruegg, R. (1984). Extraction Process for Beta-Carotene. U.S. Patent No. 4,439,629. Washington, DC: U.S. Patent and Trademark Office.
Sankari, M., Rao, P. R., Hemachandran, H., Pullela, P. K., Tayubi, I. A., Subramanian, B., et al. (2018). Prospects and progress in the production of valuable carotenoids: Insights from metabolic engineering, synthetic biology, and computational approaches. J. Biotechnol. 266, 89–101. doi: 10.1016/j.jbiotec.2017.12.010
Sardaryan, E. (2002). Strain of the Microorganism Penicillium oxalicum var. Armeniaca and Its Application. U.S. Patent No. 6,340,586. Washington, DC: U.S. Patent and Trademark Office.
Sen, T., Barrow, C. J., and Deshmukh, S. K. (2019). Microbial pigments in the food industry-challenges and the way forward. Front. Nutr. 6, 1–14. doi: 10.3389/fnut.2019.00007
Sigurdson, G. T., Tang, P., and Giusti, M. M. (2017). Natural colorants: Food colorants from natural sources. Ann. Rev. Food Sci. Technol. 8, 261–280. doi: 10.1146/annurev-food-030216-025923
Singh, N., Goel, G., Singh, N., Pathak, B. K., and Kaushik, D. (2015). Modeling the red pigment production by Monascus purpureus MTCC 369 by Artificial Neural Network using rice water based medium. Food Biosci. 11, 17–22. doi: 10.1016/j.fbio.2015.04.001
Smedsgaard, J., and Frisvad, J. C. (1996). Using direct electrospray mass spectrometry in taxonomy and secondary metabolite profiling of crude fungal extracts. J. Microbiol. Methods, 25, 5–17. doi: 10.1016/0167-7012(95)00073-9
Soliev, A. B., Hosokawa, K., and Enomoto, K. (2011). Bioactive pigments from marine bacteria: applications and physiological roles. Evid Compl Alter. Med. 2011, 1–17. doi: 10.1155/2011/670349
Sopandi, T., Wardah, A., Surtiningsih, T., Suwandi, A., and Smith, J. J. (2013). Utilization and optimization of a waste stream cellulose culture medium for pigment production by Penicillium spp. J. Appl. Microbiol. 114, 733–745. doi: 10.1111/jam.12110
Stafsnes, M. H., Josefsen, K. D., Kildahl-Andersen, G., Valla, S., Ellingsen, T. E., and Bruheim, P. (2010). Isolation and characterization of marine pigmented bacteria from Norwegian coastal waters and screening for carotenoids with UVA-blue light absorbing properties. J. Microbiol. 48, 16–23. doi: 10.1007/s12275-009-0118-6
Surai, P. F. (2012). The antioxidant properties of canthaxanthin and its potential effects in the poultry eggs and on embryonic development of the chick. Part 1. World's Poultry Sci. J. 68, 465–476. doi: 10.1017/S0043933912000578
Surwase, S. N., Jadhav, S. B., Phugare, S. S., and Jadhav, J. P. (2013). Optimization of melanin production by Brevundimonas sp. SGJ using response surface methodology. 3 Biotech. 3, 187–194. doi: 10.1007/s13205-012-0082-4
Tanaka, Y., Katsumoto, Y., Mizutani, M., Fukui, Y., and Togami, J. (2011). Stabilization and Blueing of Anthocyanin Pigments Using Gene Encoding Aromatic Acyltransferase Capable of Transferring an Aromatic Acyl Group to the 3-Position of Anthocyanin. U.S. Patent No. 8,053,634. Washington, DC: U.S. Patent and Trademark Office.
Terao, J. (1989). Antioxidant activity of β-carotene-related carotenoids in solution. Lipids 24, 659–661. doi: 10.1007/BF02535085
Tinoi, J., Rakariyatham, N., and Deming, R. L. (2005). Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem. 40, 2551–2557. doi: 10.1016/j.procbio.2004.11.005
Tuli, H. S., Chaudhary, P., Beniwal, V., and Sharma, A. K. (2015). Microbial pigments as natural color sources: current trends and future perspectives. J. Food Sci. Technol. 52, 4669–4678. doi: 10.1007/s13197-014-1601-6
Unagul, P., Wongsa, P., Kittakoop, P., Intamas, S., Srikitikulchai, P., and Tanticharoen, M. (2005). Production of red pigments by the insect pathogenic fungus Cordyceps unilateralis BCC 1869. J. Indust. Microbiol. Biotechnol. 32, 135–140. doi: 10.1007/s10295-005-0213-6
Valla, A., Cartier, D., Valla, B., Le Guillou, R., Andriamialisoa, Z., Breithaupt, D., et al. (2004). “New synthesis of natural carotenoid isoreinieratene,” in Peer-Reviewed Proceedings of the 3rd International Conference on Pigments in Food (Quimper: Pigments Publishing, Université de Bretagne Occidentale), 67–69.
Vendruscolo, F., Müller, B. L., Moritz, D. E., de Oliveira, D., Schmidell, W., and Ninow, J. L. (2013). Thermal stability of natural pigments produced by Monascus ruber in submerged fermentation. Biocatalysis Agri. Biotechnol. 2, 278–284. doi: 10.1016/j.bcab.2013.03.008
Venil, C. K., Aruldass, C. A., Dufossé, L., Zakaria, Z. A., and Ahmad, W. A. (2014). Current perspective on bacterial pigments: emerging sustainable compounds with coloring and biological properties for the industry–an incisive evaluation. RSC Adv. 4, 39523–39529. doi: 10.1039/C4RA06162D
Venil, C. K., Zakaria, Z. A., and Ahmad, W. A. (2013). Bacterial pigments and their applications. Process Biochem. 48, 1065–1079. doi: 10.1016/j.procbio.2013.06.006
Vinarov, A., Robucheva, Z., Sidorenko, T., and Dirina, E. (2003). Microbial biosynthesis and making of pigment melanin. Commun. Agri. Appl. Biolog. Sci. 68, 325–326.
Wang, X., Tao, J., Wei, D., Shen, Y., and Tong, W. (2004). Development of an adsorption procedure for the direct separation and purification of prodigiosin from culture broth. Biotechnol. Appl. Biochem. 40, 277–280. doi: 10.1042/BA20030210
Woodall, A. A., Britton, G., and Jackson, M. J. (1997). Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochimica et Biophysica Acta (BBA)-General Subjects 1336, 575–586. doi: 10.1016/S0304-4165(97)00007-X
Wynn-Williams, D. D., Edwards, H. G. M., Newton, E. M., and Holder, J. M. (2002). Pigmentation as a survival strategy for ancient and modern photosynthetic microbes under high ultraviolet stress on planetary surfaces. Int. J. Astrobiol. 1, 39–49. doi: 10.1017/S1473550402001039
Yi, J., Lam, T. I., Yokoyama, W., Cheng, L. W., and Zhong, F. (2014). Controlled release of β-carotene in β-lactoglobulin–dextran-conjugated nanoparticles' in vitro digestion and transport with Caco-2 monolayers. J. Agri. Food Chem. 62, 8900–8907. doi: 10.1021/jf502639k
Zhou, B., Wang, J., Pu, Y., Zhu, M., Liu, S., and Liang, S. (2009). Optimization of culture medium for yellow pigments production with Monascus anka mutant using response surface methodology. Europ. Food Res. Technol. 228, 895–901. doi: 10.1007/s00217-008-1002-z
Keywords: microbial pigment, food, fermentation, bio-colorant, market
Citation: Rana B, Bhattacharyya M, Patni B, Arya M and Joshi GK (2021) The Realm of Microbial Pigments in the Food Color Market. Front. Sustain. Food Syst. 5:603892. doi: 10.3389/fsufs.2021.603892
Received: 08 September 2020; Accepted: 08 February 2021;
Published: 22 March 2021.
Edited by:
Lourdes Morales-Oyervides, Universidad Autónoma de Coahuila, MexicoReviewed by:
Pablo Fuciños, International Iberian Nanotechnology Laboratory (INL), PortugalRobert David Hall, Wageningen University and Research, Netherlands
Copyright © 2021 Rana, Bhattacharyya, Patni, Arya and Joshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gopal K. Joshi, Z2tqb3NoaUByZWRpZmZtYWlsLmNvbQ==
 Babita Rana1
Babita Rana1 Malini Bhattacharyya
Malini Bhattacharyya Babita Patni
Babita Patni Gopal K. Joshi
Gopal K. Joshi