Abstract
Insect protein production is considered a sustainable alternative to livestock protein which furthermore utilizes waste streams. Its production can have positive but also potentially negative environmental effects, which require evaluation. Frass, the byproduct of insect production, is regarded an efficient organic fertilizer or soil amendment. However, several studies report negative frass effects on plant growth and nitrogen (N) cycling. Therefore, a pot trial was carried out which sought to understand N release from frass and subsequent growth and nutrient uptake of Italian ryegrass. Mealworm frass (MWF) or buffalo worm frass (BFW) was applied at two rates (1.5 and 3% w/w) to a soil-sand mix. To evaluate N release processes, frass was applied alone, with a nitrification inhibitor (NI), a urease inhibitor (UI), or both (NI+UI). Plant N, nutrient uptake and soil inorganic N were measured at the experiment's end. To gauge whether altered N fluxes induced changes in the microbial community, soil microbial biomass, bacterial/archaeal abundances and ergosterol content as a fungal biomarker, were determined. Both frass types and application rates stimulated microbial growth and N mineralization. The 3% rate inhibited seed germination, possibly due to salinity or ammonia toxicity. At the 1.5% rate, both frass types were effective fertilizers. MWF led to higher biomass and nutrient uptake, owing to its higher extractable nutrient concentrations. The 3% rate caused nitrite accumulation in the absence of NI. NI improved plant biomass, nutrient uptake, stimulated archaeal and bacterial abundances and prevented nitrite accumulation. UI reduced N mineralization, showing that a substantial fraction of frass organic N is ureic. UI enhanced fungal contribution to the microbial biomass, revealing the importance of bacteria in frass N mineralization processes when UI is not applied. NI and UI combined, induced greater N release from frass than UI or NI alone. Our study demonstrated the usefulness of NI and UI in studying N release from frass. NI can improve plant N uptake and minimize N losses following frass application, reducing its potentially negative effects. UI can retard N release from frass, allowing its application as a slow-release fertilizer, but should not be used concurrently with NI.
Introduction
Global demand for protein such as meat and dairy products is increasing, but with growing environmental awareness fueling demand for food with lower environmental footprints. Animal husbandry is characterized by relatively low nutrient use efficiency, with a large part of the nitrogen (N) and phosphorus (P) in the feed not being converted into animal based protein and thus being applied as manure to agricultural land in excess of plant demand. As a result, eutrophication of groundwater and surface water bodies became a problem in Europe such as in parts of France, Belgium, the Netherlands and Germany (van der Wiel et al., 2020). To this end, insect rearing, which has a more efficient feed to protein conversion rate and can potentially utilize agricultural waste or side-products, may provide a technological solution to nutrient excess and be an alternative protein source for feed and food (Smetana et al., 2019; Schmitt and de Vries, 2020). With lower emissions per unit of protein and contribution to a regional nutrient circularity it may reduce negative consequences for the environment. However, this needs to be verified for the whole production chain and starts with evaluating the different components from feed provision, insect rearing, processing to waste management.
Two insect larvae commercially produced include those of mealworm (Tenebrio molitor L.) and buffalo worm/lesser mealworm (Alphitobius diaperinus). Both can be reared using by-products from potato processing, bakeries, breweries and bioethanol production (Van Broekhoven et al., 2015). Mealworm production has been reported to be of superior N efficiency than that of conventional livestock animals (Oonincx et al., 2015). After the insects have been harvested, a mixture of feed residues, feces and exoskeleton remains, which is commonly known as frass. This by-product is rich in nutrients and organic carbon (C) and is therefore considered a viable organic fertilizer and soil amendment suitable for use in sustainable agriculture (Poveda, 2021).
Frass, particularly that of black soldier fly (Hermetia illucens) has been demonstrated to be an effective substitute for conventional fertilizers in field-grown cabbage (Choi et al., 2009) and maize (Quilliam et al., 2020), and in pot trials monitoring growth of perennial ryegrass (Klammsteiner et al., 2020; Menino et al., 2021). While, to our knowledge, no studies have focussed on the fertilization potential of buffalo worm frass (BWF), mealworm frass (MWF) has been proven in pot trials to induce yield gains comparable to those of inorganically fertilized controls in chard (Poveda et al., 2019) and barley (Houben et al., 2020).
For a full environmental evaluation of the insect industry, potentially negative effects of frass must also be studied. High application rates of black soldier fly frass (BSFF) have been shown to negatively impact growth of maize (Alattar et al., 2016) and Japanese mustard spinach (Kawasaki et al., 2020), respectively surmised to be due to induced nutrient deficiency and high salinity. Moreover, Rummel et al. (2021) demonstrated considerable greenhouse gas emissions after BSFF application to soil, which was driven by the high available C and N content. A further negative consequence of frass application to soil was reported by Watson et al. (2021), who incubated soil with 2.5% of MWF or BWF and observed accumulation of nitrite, which is potentially harmful to microbial activity and has been demonstrated to inhibit root growth (Nabel et al., 2018). This nitrite accumulation was accompanied by a relative increase of the archaeal gene copy numbers and thus was thought to be an indicator of enhanced ammonia oxidation through AOA (ammonia oxidizing archaea) while nitrite oxidation was inhibited. Therefore, insect frass is thought to influence the soil microbial community with consequences for N cycling. This observation warrants further investigation to better understand how N is released from frass, to optimize its use as fertilizer and to reduce potential negative effects on plants, soil microbes and the environment.
Inorganic N extractable from frass is dominated by ammonium ()-N (Lovett and Ruesink, 1995; Kagata and Ohgushi, 2012a; Kawasaki et al., 2020; Table 1), of which ammonification of organic N is a major source. The organic N can be present in the forms of undigested protein (Fielding et al., 2013), chitin derived from insect exoskeletons (Jacquiod et al., 2013), while a considerable fraction will be ureic. Larval N waste precipitates rectally as uric acid, which is broken down into allantoin and subsequently urea (Green and Popa, 2012).
Table 1
| Buffalo worm frass | Mealworm worm frass | |||
|---|---|---|---|---|
| pH | 6.25 (0.01) b | 6.61 (0.01) a | ||
| Electrical conductivity (S/m) | 0.32 (0.01) b | 0.43 (0.01) a | ||
| Total nitrogen (%) | 4.42 (0.11) a | 4.13 (0.23) b | ||
| Total organic C (%) | 39.24 (0.79) a | 37.36 (0.80) b | ||
| C:N ratio | 8.89 (0.36) | 9.15 (0.25) | ||
| Total S (%) | 0.51 (0.03) | 0.47 (0.09) | ||
| -N (g kg−1) in 0.01 M CaCl2 | 0.14 (0.01) | 0.14 (0.01) | ||
| -N (g kg−1) in 0.01 M CaCl2 | 1.44 (0.09) | 1.31 (0.15) | ||
| Extractable P (g kg−1) in Mehlich-3 | 9.89 (2.11) b | 14.86 (2.47) a | ||
| Extractable K (g kg−1) in Mehlich-3 | 16.37 (3.95) b | 29.66 (5.68) a | ||
| Extractable Ca (g kg−1) in Mehlich-3 | 9.74 (1.40) | 10.71 (1.69) | ||
| Extractable Mg (g kg−1) in Mehlich-3 | 5.38 (1.21) b | 7.30 (1.22) a | ||
| Extractable Na (g kg−1) in Mehlich-3 | 2.70 (0.55) b | 4.64 (0.75) a | ||
| Extractable Fe (mg kg−1) in Mehlich-3 | 242 (48) b | 340 (57) a | ||
| 1.5% | 3.0% | 1.5% | 3.0% | |
| N addition (g kg−1) | 0.66 | 1.33 | 0.62 | 1.24 |
| C addition (g kg−1) | 5.89 | 11.77 | 5.60 | 11.21 |
Chemical composition of frass types and added amounts of N and C (mean ± standard deviation for n = 5).
Within a row, means without a letter in common are significantly different (p <0.05).
Urea hydrolysis causes accumulation and soil microsites featuring high pH, inducing NH3 volatilization (Cantarella et al., 2018). Urease inhibitors are therefore utilized in agriculture where high loads of urea are applied to reduce this volatilization. Nitrification inhibitors are applied to soils to prevent conversion of to nitrate () and possible subsequent loss via the pathways of leaching or denitrification. Nitrification and urease inhibitors can be concurrently applied with the goal of preventing N losses by all three mechanisms (volatilization, leaching, denitrification), through slowing down the processes of ammonification and nitrification.
As well as allowing N flux management in agricultural soils, inhibitors can also be used to differentiate between release pathways from organic amendments. Therefore, this study aimed to improve our understanding of N ammonification and nitrification processes from insect frass. To this end we sought to establish how frass application to soil led to better N utilization by Italian ryegrass (Lolium multiflorum Lam. ssp. italicum cv. Fabio) plants, and if the utilization was affected by the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP), the urease inhibitor N-(n-butyl) thiophosphoric triamide (NBPT), or both. We investigated the effects of these treatments on soil N mineralization and nitrification and the abundances of soil bacteria, archaea and fungi.
We hypothesized that (I) a substantial proportion of frass total N mineralization is facilitated by N cleavage through urease (II) nitrification inhibition will prevent soil nitrite accumulation and potentially enhance ryegrass yield, and (III) that inhibiting both urea hydrolysis and nitrification will lower the available inorganic N which will be reflected by lower plant growth and N uptake.
We tested these hypotheses in a plant growth pot experiment, where a soil-sand mixture was amended at two rates (1.5 and 3%) with frass derived from two insect species and the nitrification inhibitor (NI) DMPP, the urease inhibitor (UI) NBPT or both (NI+UI) were applied.
Materials and Methods
Growth Medium/Substrate
The substrate used in the pot trial comprised a 1:1 mixture of quartz sand (0.1–0.5 mm, RKW, Falkenstein, Germany) and air-dried (sieved <4 mm) soil, creating a carbon and nutrient poor, yet biologically active, substrate comparable to a soil. The soil used was the B horizon of a Stagnic Luvisol grassland (textural class silt loam with 10% sand, 80% silt and 10% clay, pH 5.5 in 0.01 M CaCl2, 0.29% TOC, <0.05% N) from Neulouisendorf, Germany.
Frass
Two types of frass with comparable C/N ratio were used in the experiment. They originated from mealworm (Tenebrio molitor L., MWF) and buffalo worm (Alphitobius diaperinus, BWF) and were provided by Vivara Natuurbeschermingsproducten, Vierlingsbeek, Netherlands. Larvae were reared on Insectus Mealworm Grow (Mijten nv, Bekkevoort, Belgium) which contains 20% protein, 4% fat, 1.01% Ca, 0.76% P and 0.23% Mg, with carrots supplementing the feed.
Frass samples were air dried for 3 days and sieved (<1 mm) and then analyzed for organic matter content, total N, total S, pH in deionised water (1:25 amendment:water ratio), electrical conductivity (1:25), extractable and in 0.01 M CaCl2 (1:25 frass:extractant ratio) and P, K, Ca, Mg, Na, and Fe in Mehlich 3 (1:25 frass:extractant ratio). A more extensive suite of analyses of the same frass types was reported by Watson et al. (2021). Total C, N and S were determined using gas chromatography after combustion (Carlo Erba NA 1500 CNS Analyzer, Thermo Scientific) in subsamples of dried and milled frass samples. Elemental nutrient concentrations were determined using an Optima 8000 inductively coupled plasma optical emission spectrometer (ICP-OES, PerkinElmer, Baesweiler, Germany). Nitrate and were measured using an AA3 HR Nutrient Autoanalyzer (SEAL Analytical GmbH, Norderstedt, Germany). No nitrite () was detectable.
Both dried frass types were applied at two application rates: 1.5 or 3.0% (w/w), high application rates broadly comparable with those used by Kagata and Ohgushi (2012b), Poveda et al. (2019) and Kawasaki et al. (2020). MWF addition resulted in higher application rates for all elements with the exception of total S, N and extractable -N (Table 1).
Pot Experiment
The pot trial was conducted in 2019–2020 over 16 weeks under controlled conditions in a greenhouse at the Rhine-Waal University in Kleve, Germany providing respective night and day temperatures of 16–21 and 20–23°C and a photoperiod of 16 h. All pots were arranged randomly in two blocks. Frass from either mealworm (MWF) or buffalo worm (BWF) was mixed into the substrate at two application rates [1.5 and 3.0% (w/w)]. Then, 1 kg of substrate was placed into 1 L pots and brought to 70% of its water holding capacity (WHC). Each pot was amended with water only (control, C), water with the nitrification inhibitor (NI) 3,4-dimethylpyrazole phosphate (DMPP) at 2 mg kg−1 soil, water providing urease inhibitor (UI) N-(n-butyl) thiophosphoric triamide (NBPT) at 1 mg kg−1 soil, or a combination of both inhibitors (NI+UI) to investigate N release and nitrification from frass (Figure 1). Watson et al. (2019) applied 20 mg kg−1 of the nitrification inhibitor dicyandiamide (DCD) to the same substrate in a separate experiment. DMPP application rates used in the current study were based on the assumption that one order of magnitude less DMPP than DCD is required (Wissemeier et al., 2001). After water application, 0.5 g of Italian ryegrass (Lolium multiflorum Lam. ssp. italicum cv. Fabio) seeds were added to each pot. Substrates were gravimetrically raised to 70% WHC twice weekly. The following provides an overview of the 16 treatments, which were carried out in replicates of five:
1.5% buffalo worm frass control (BWF1.5C)
1.5% buffalo worm frass + nitrification inhibitor (BWF1.5N)
1.5% buffalo worm frass + urease inhibitor (BWF1.5U)
1.5% buffalo worm frass + urease inhibitor + nitrification inhibitor (BWF1.5UN)
1.5% mealworm frass control (MWF1.5C)
1.5% mealworm frass + nitrification inhibitor (MWF1.5N)
1.5% mealworm frass + urease inhibitor (MWF1.5U)
1.5% mealworm frass + urease inhibitor + nitrification inhibitor (MWF1.5UN)
3% buffalo worm frass control (BWF3C)
3% buffalo worm frass + nitrification inhibitor (BWF3N)
3% buffalo worm frass + urease inhibitor (BWF3U)
3% buffalo worm frass + urease inhibitor + nitrification inhibitor (BWF3UN)
3% mealworm frass control (MWF3C)
3% mealworm frass + nitrification inhibitor (MWF3N)
3% mealworm frass + urease inhibitor (MWF3U)
3% mealworm frass + urease inhibitor + nitrification inhibitor (MWF3UN)
Figure 1
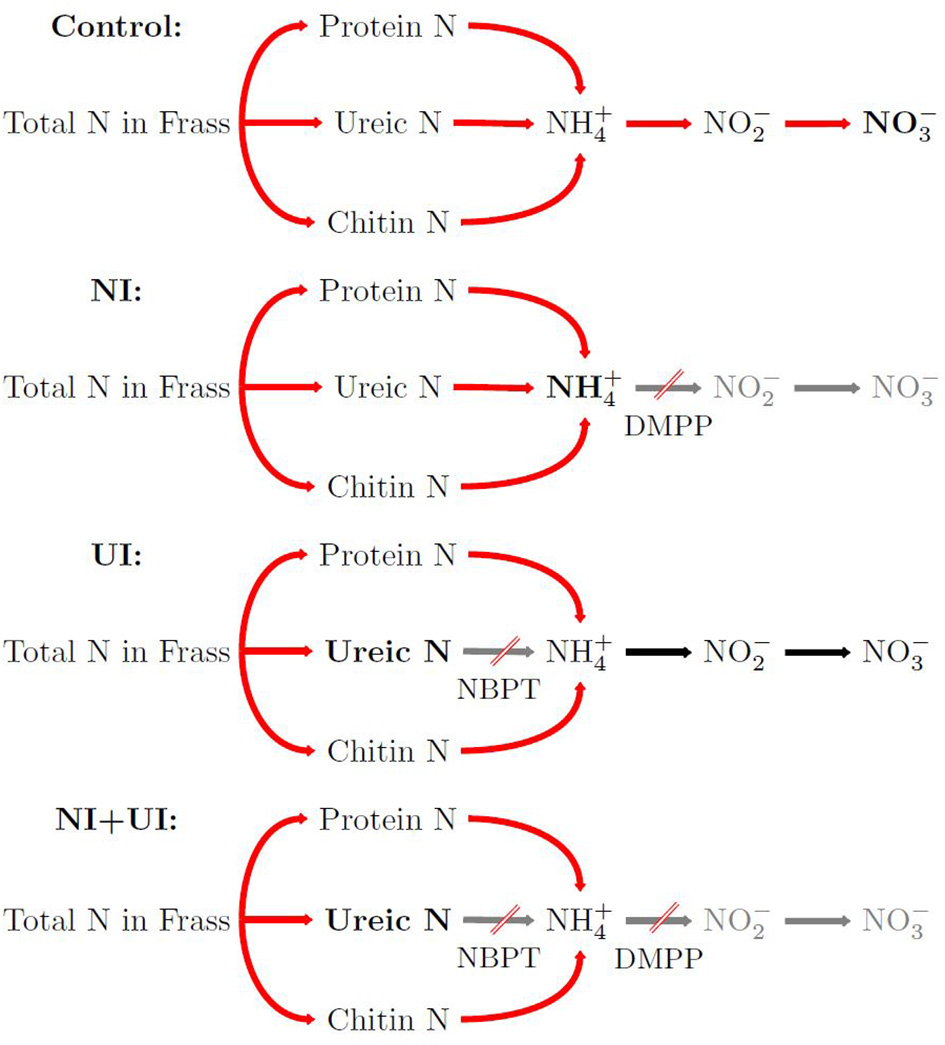
Simplified visualization of nitrogen mineralization processes targeted in the different experimental treatments: control shows normal N release, nitrification inhibitor (NI) slows ammonium oxidation, urease inhibitor (UI) slows N release from ureic organic N, combined inhibitor application (NI + UI) slows N release from ureic organic N, and ammonium oxidation as well. DMPP, 3,4-dimethylpyrazole phosphate (NI); NBPT, N-(n-butyl) thiophosphoric triamide (UI).
Shoots from all pots were harvested 2 cm above the soil surface after 28, 61, 84 and 112 days. Following each harvest, shoot weights were determined after drying overnight at 70°C. Following grinding, total C, N and S were determined using gas chromatography after combustion (Carlo Erba NA 1500 CNS Analyzer, Thermo Scientific), while all other nutrient element concentrations (Ca, Mg, K, P and Fe) were determined after microwave digestion in concentrated nitric acid via an Optima 8000 ICP-OES (Perkin Elmer, Baesweiler, Germany).
Frass application at 3.0% of MWF and BWF inhibited germination of Italian ryegrass seeds almost completely and thus inhibited plant growth, possibly caused by ammonia toxicity or high salinity (see Table 1). Consequently, statistical comparison between 1.5 and 3.0% frass is omitted. Nevertheless, samples were taken from every pot at the end of the pot trial for a suite of analyses which provided information on the various treatments' effects on N fluxes and microbial properties (see Substrate Analyses and Soil Microbial Properties sections).
Substrate Analyses
At the end of the experiment, the substrate from each pot was homogenized and sieved (<2 mm) and subsamples were taken to determine pH and electrical conductivity (EC) in deionised water (1:2.5 w/v). Furthermore, inorganic N was measured in each subsample. Briefly, 10 g of moist soil was extracted for 30 min by oscillating shaking at 200 rev min−1 with 40 mL 0.5 M K2SO4 and subsequently analyzed for , , and using an AA3 HR Nutrient Autoanalyzer (SEAL Analytical GmbH). In the same extract, extractable organic C (EOC) was measured as CO2 by infrared absorption and total N by chemoluminescence after combustion at 850°C using a multi N/C® 2100 S Analyzer (Analytik Jena). Extractable organic N (EON) was defined as N after subtracting inorganic N from total N in the extract. Extractable organic N is further given as a percentage of total extractable N. Soil water content and soil dry weight (DW) was determined by oven drying at 105°C for 24 h.
Soil Microbial Properties
Moist subsamples of the homogenized substrate at the end of the pot trial were used to determine microbial biomass C (MBC) and N (MBN), extractable DNA, the microbial domains bacteria and archaea, and ergosterol as an indicator of saprotrophic fungi.
MBC and MBN were determined by chloroform fumigation extraction (Brookes et al., 1985; Vance et al., 1987) from a subsample of fresh 2 mm-sieved substrate. Briefly, 10 g of fresh substrate was fumigated for 24 h at 25°C with ethanol-free chloroform (CHCl3). After removal of CHCl3, the fumigated sample was extracted with 40 ml 0.5 M K2SO4 horizontally for 30 min at 200 rev. min−1 and filtered (VWR 305, particle retention: 2–3 μm). An identical non-fumigated 10 g sample was extracted similarly. Organic C and N in the extracts were measured using a multi N/C® 2100S Analyzer (Analytik Jena AG). MBC was calculated as EC/kEC, where EC = (organic C extracted from fumigated soils) – (organic C extracted from non-fumigated soils) and kEC, the correction factor for MBC = 0.45 (Wu et al., 1990). MBN was calculated as EN/kEN, where EN = (organic N extracted from fumigated soils) – (organic N extracted from non-fumigated soils) and kEN, the correction factor for MBN = 0.54 (Brookes et al., 1985).
Total genomic DNA was extracted from 0.5 g of fresh soil using a FastDNATM SPIN Kit for Soil and FastPrep®-24 bead-based homogeniser (both MP Bio, Santa Ana, CA) according to the manufacturer's instructions as modified by Hemkemeyer et al. (2014). Quantitative PCR of archaeal and bacterial 16S rRNA genes was conducted in a LightCycler® 480 II (Roche, Mannheim, Germany). PCR reactions were carried out in a 20 μl total volume which contained 10 μl of a 2 × concentrated master mix (LightCycler® 480 Probes Master, Roche), 0.5 μM of each primer, 0.2 μM probe and 2 μl of template DNA. Bacterial 16S rRNA amplication was done using the primers BAC338F and BAC805R (0.5 μM each) in combination with the probe BAC516F (0.2 μM), for archaeal 16S rRNA the primers ARC787F and ARC1059R (0.5 μM each) and the probe ARC915F (0.2 μM) were used (Yu et al., 2005). In order to check for inhibitory effects, reactions of each sample were run in duplicate, with one half being supplied with 10-fold and the other half with 50-fold dilutions of template DNA. Reaction conditions started with 95°C for 10 min. followed by 45 cycles of 95°C for 15 s, 60°C for 50 s, and 72°C for 1 s. DNA fragments of Methanobacterium oryzae and Bacillus subtilis cloned into pGEM®-T vector (Vector System II Kit, Promega Corporation, Madison, USA), utilizing Escherichia coli JM109 High Efficiency Competent Cells, served as standards ranging from 101 to 107 (archaea) and 102 to 108 (bacteria) copies μl−1 template, respectively. Data were pre-processed using “Abs Quant/2nd Derivative Max” -analysis of the instrument accompanying software (1.5.0 SP4) and obtained crossing threshold (CT) values were further processed in Microsoft Excel 2010.
Fungi were quantified by measuring the ergosterol content since melting curve analysis of a SYBR Green-based qPCR indicated that fungal ITS1 sequences differed too strongly between the soil treatments, prohibiting their quantitative comparison. Ergosterol content (an indicator of saprotrophic fungi) was measured according to Djajakirana et al. (1996). Briefly, 2 g moist soil was extracted with 100 mL ethanol for 30 min. by oscillating shaking at 250 rev. min−1 and then filtered (Whatman GF/A, 1.6 μm). Ergosterol determination was carried out via reversed-phase HPLC analysis at 26°C, using a 125 ×4 mm Sphereclone 5 μm ODS II column with a Phenomenex guard column (4 ×3 mm). Chromatography was performed isocratically with methanol (100%) and a resolution of detection of 282 nm (Dionex UVD 170 S).
Calculations and Statistics
Data presented in the tables and graphs are arithmetic means and based on oven-dry weight of substrate (24 h, 105°C). All statistical calculations were performed using R (R Core Team, 2019) with the packages agricolae, MASS, emmeans tidyverse and multcomp. Means of each frass treatment were tested for statistically significant differences using ANOVA. The prerequisites for the ANOVA were tested with a Levene-Test from the car package for homoscedasticity and the normality of residuals was tested visually with a QQ-Plot. Where presumptions were not fulfilled Box & Cox power transformations were carried out. When treatments were significantly different at p < 0.05, a post hoc Tukey HSD-test was conducted or estimated means test, if the sample sizes were unequal.
Results
Plant Nutrient Uptake
Frass addition at 1.5% resulted in higher plant biomass compared to the unfertilized control for both frass types (data not shown) revealing the fertilizer effect of the nutrient-rich frass. Comparing the two frass types, cumulated biomass after MWF application was consistently higher than following BWF amendment (Table 2). This observation could be related to the MWF featuring significantly higher extractable concentrations of P, K and Mg (Table 1); indeed, the cumulated uptake of all three were consistently greater in the MWF-fertilized treatments. Additionally, the higher cumulated biomass of MWF plants was accompanied by slightly higher uptake of N (Table 2), despite MWF's lower total N content than BWF and similar concentrations of extractable inorganic N (Table 1).
Table 2
| Treatment | Biomass (g) | N (mg) | S (mg) | Ca (mg) | K (mg) | Mg (mg) | P (mg) | Fe (μg) |
|---|---|---|---|---|---|---|---|---|
| BWF 1.5% | 2.81 (1.25) b | 147 (70) b | 14.4 (5.65) | 30.1 (13.2) | 1089 (46) b | 12.1 (5.88) | 12.7 (5.33) b | 839 (243) b |
| BWF 1.5% + NI | 3.43 (0.50) b | 200 (27) ab | 14.3 (2.04) | 39.1 (7.97) | 121 (16) b | 13.9 (2.70) | 12.6 (1.73) b | 1,174 (323) ab |
| BWF 1.5% + UI | 2.62 (1.38) b | 144 (56) b | 19.1 (5.89) | 25.3 (13.0) | 96 (50) b | 10.8 (6.32) | 11.1 (6.06) b | 1,066 (599) ab |
| BWF 1.5% + NI + UI | 3.40 (1.54) ab | 186 (82) ab | 17.8 (4.23) | 32.5 (12.1) | 118 (47) b | 13.1 (6.13) | 13.2 (6.39) b | 1,000 (490) ab |
| MWF 1.5% | 3.95 (0.92) ab | 194 (34) ab | 15.7 (4.35) | 35.3 (7.73) | 136 (21) b | 15.4 (3.17) | 17.8 (4.81) b | 1,114 (166) ab |
| MWF 1.5% + NI | 4.94 (0.29) a | 240 (5) a | 20.8 (1.45) | 36.3 (1.66) | 191 (6) a | 16.8 (0.57) | 28.5 (1.80) a | 1,655 (148) a |
| MWF 1.5% + UI | 3.65 (0.37) ab | 175 (14) ab | 15.0 (2.30) | 32.2 (3.60) | 133 (13) b | 14.6 (1.41) | 17.8 (3.28) b | 872 (189) b |
| MWF 1.5% + NI + UI | 3.76 (0.61) ab | 177 (17) ab | 14.1 (2.93) | 32.8 (3.68) | 143 (18) b | 15.1 (1.38) | 15.6 (2.61) b | 1,007 (73) ab |
Cumulated Italian ryegrass shoot dry weight and nutrient uptake in pots amended with 1.5% (w/w) buffalo worm frass, BWF or mealworm frass, MWF (mean ± standard deviation for n = 5).
Pots were amended with frass alone, or concurrently with nitrification inhibitor (NI), urease inhibitor (UI), or both. Within a column, means without a letter in common are significantly different (p <0.05).
Of the inhibitor treatments (Table 2), NI showed a consistent trend of the highest values for cumulated biomass and take-up of N, Ca, Mg, K and Fe. In MWF-fertilized plants, cumulated P and K uptake were significantly higher when NI was applied than in all other treatments. Interestingly, when both, NI and a urease inhibitor (UI) were added (NI+UI) these effects were not observed and values were in the range of the control.
Nitrogen uptake was substantially higher in the second and third harvest for both frass types and all treatments (Figure 2). The higher N uptake in the second harvest was more pronounced in MWF as compared to BWF and revealed higher N uptake in the NI treatment than in the UI and NI+UI treatments. N uptake of the first and second harvest in the MWF NI treatment was markedly higher than in the NI+UI treatment, which showed the lowest N uptake. This was reversed in the fourth harvest, where the NI+UI treatment showed a higher N uptake than did NI and UI. In BWF-fertilized plants, this trend of N uptake as affected by NI was not observed. Total N uptake from both frass types was the lowest in the UI treatments without being significantly different from the other treatments (Table 2).
Figure 2
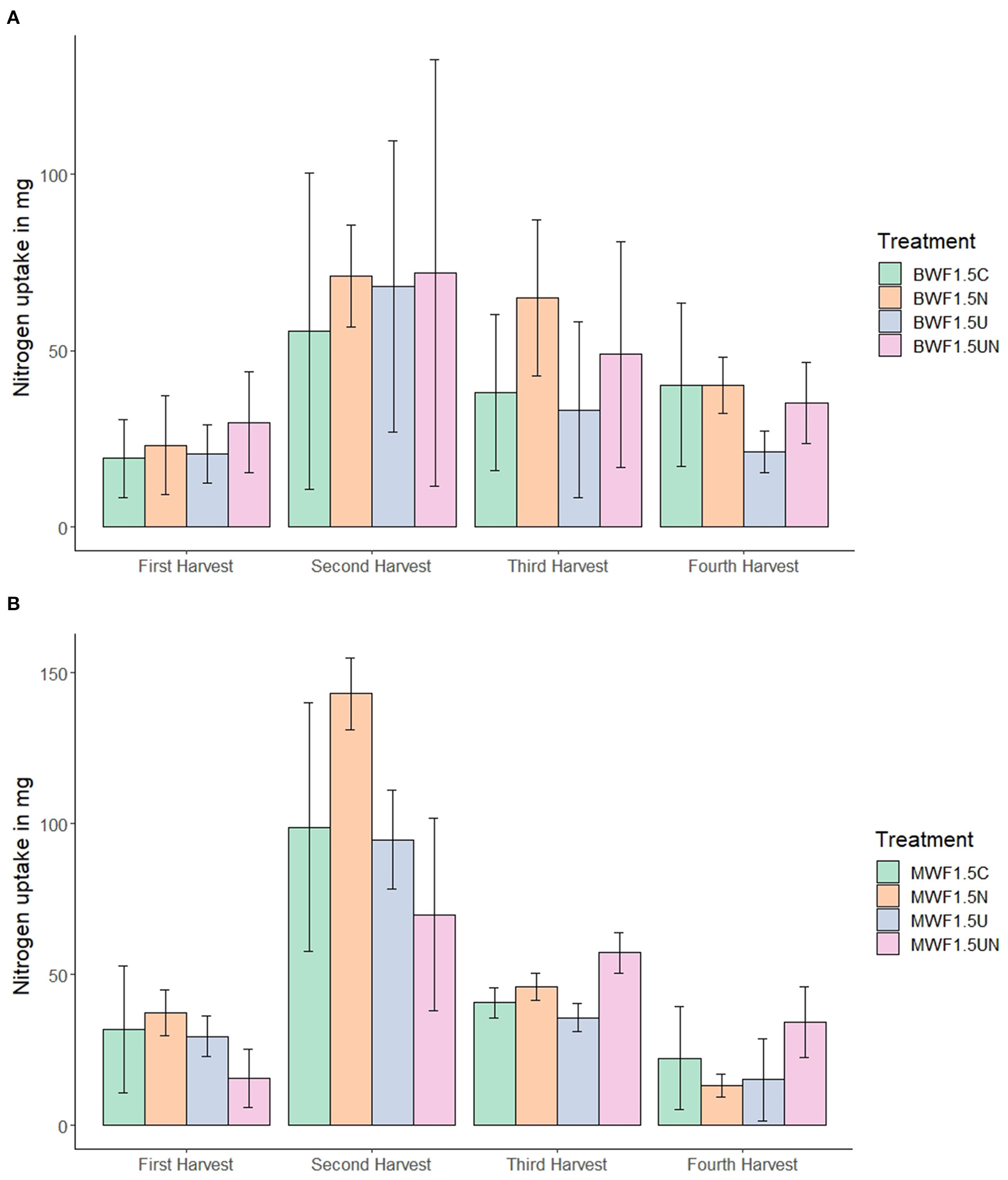
N uptake in Italian ryegrass shoot biomass at four different harvest dates (mean ± 95% confidence interval for n = 5) in replicates fertilized with 1.5% (w/w) buffalo worm frass, BWF (A) or mealworm frass, MWF (B). Pots were amended with frass alone, or concurrently with nitrification inhibitor (NI), urease inhibitor (UI), or both.
Soil Nitrogen Dynamics
As expected, inorganic N levels at the end of the experiment were lower in the 1.5% frass-amended pots, where plants had grown for 16 weeks and application rates were lower (Tables 3, 4). Total inorganic N was higher when BWF as compared to MWF was applied. This contrast was particularly marked in the 1.5% frass treatments (Table 3). This observation is likely to be explained by the superior plant biomass and N uptake of MWF-fertilized plants (Table 2). The substantially higher inorganic N in BWF at 3% frass addition compared to MWF (pots in which no plants grew), reveals a higher N release from BWF frass during the experiment (Table 4). The more marked mineralization of frass in the 3%-amended pots, with resultant release of organic acids, can potentially explain the lower pH in pots amended with 3% of either frass type as compared to the 1.5%-amended pots (Tables 3, 4).
Table 3
| Treatment | pH | -N (mg kg−1) | -N (mg kg−1) | -N (mg kg−1) | Total inorganic N (mg kg−1) | EOC (mg kg−1) | EON (mg kg−1) | EON as %ge of total extractable N |
|---|---|---|---|---|---|---|---|---|
| BWF 1.5% | 6.64 (0.25) c | 1.54 (0.67) ab | 0.01 (0.01) n = 2 | 19.9 (12.4) a | 21.4 (12.2) a | 90.7 (27.4) | 118 (87.3) a | 82.7 (3.70) b |
| BWF 1.5% + NI | 6.53 (0.15) c | 2.51 (0.51) a | 0.01 (0.01) n = 2 | 9.04 (2.24) ab | 11.6 (2.43) a | 79.5 (16.6) | 47.0 (11.6) ab | 80.0 (1.73) b |
| BWF 1.5% + UI | 6.94 (0.37) bc | 1.15 (0.48) b | 0.004 (0.01) n = 2 | 7.12 (9.05) ab | 8.28 (9.19) ab | 91.1 (20.3) | 46.1 (43.4) ab | 87.7 (4.34) ab |
| BWF 1.5% + NI + UI | 6.45 (0.34) c | 1.81 (0.58) ab | 0.002 n = 1 | 16.5 (13.6) ab | 18.3 (13.3) a | 84.2 (22.1) | 81.0 (63.6) ab | 80.2 (3.08) b |
| MWF 1.5% | 7.28 (0.22) ab | 1.17 (0.98) b | 0.01 (0.02) n = 3 | 5.87 (7.83) ab | 7.06 (8.79) ab | 75.5 (12.5) | 41.7 (47.1) ab | 87.9 (3.31) ab |
| MWF 1.5% + NI | 7.36 (0.03) a | 0.92 (0.53) b | 0.01 (0.01) n = 3 | 0.37 (0.32) b | 1.30 (0.71) b | 84.9 (39.1) | 6.47 (1.71) b | 82.6 (10.9) b |
| MWF 1.5% + UI | 7.27 (0.10) ab | 0.68 (0.24) b | 0.01 (0.01) n = 2 | 0.40 (0.28) b | 1.08 (0.49) b | 113 (14.4) | 13.2 (2.42) b | 92.6 (1.79) a |
| MWF 1.5% + NI + UI | 7.16 (0.21) ab | 1.16 (0.39) b | 0.01 (0.02) n = 2 | 2.68 (4.06) b | 3.85 (4.43) ab | 79.7 (33.9) | 17.1 (16.3) b | 83.9 (3.56) b |
Substrate pH, ammonium (-N), nitrite (NO2-N), nitrate (-N), total inorganic N, extractable organic C (EOC) and N (EON) and EON as a percentage of total extractable N, at the end of the experiment (mean ± standard deviation for n = 5 except where indicated).
Pots were amended with 1.5% (w/w) buffalo worm frass (BWF) or mealworm frass (MWF) alone, or concurrently with nitrification inhibitor (NI), urease inhibitor (UI), or both. Within a column, means without a letter in common are significantly different (p <0.05).
Table 4
| Treatment | pH | -N (mg kg−1) | -N (mg kg−1) | -N (mg kg−1) | Total inorganic N (mg kg−1) | EOC (mg kg−1) | EON (mg kg−1) | EON as %ge of total extractable N |
|---|---|---|---|---|---|---|---|---|
| BWF 3% | 6.21 (0.07) cd | 3.61 (1.33) | 3.30 (3.66) | 92.3 (13.1) a | 99.2 (11.6) a | 207 (75.2) | 552 (120) a | 84.6 (1.51) ab |
| BWF 3% + NI | 6.12 (0.09) d | 20.3 (21.0) | 0.01 (0.03) n = 2 | 64.6 (16.9) ab | 84.9 (23.2) ab | 106 (51.0) | 358 (93) ab | 80.4 (5.62) b |
| BWF 3% + UI | 6.40 (0.09) bc | 1.14 (0.62) | 2.20 (2.36) n = 4 | 54.4 (22.4) abc | 57.7 (23.9) abc | 170 (48.6) | 333 (108) ab | 85.5 (1.83) ab |
| BWF 3% + NI + UI | 6.32 (0.20) cd | 23.8 (25.1) | 0.10 (0.17) | 72.1 (18.6) ab | 96.0 (28.8) a | 150 (65.8) | 401 (157) ab | 79.6 (5.57) b |
| MWF 3% | 6.59 (0.08) ab | 1.98 (0.71) | 3.57 (6.01) | 69.8 (31.7) ab | 75.4 (36.5) ab | 231 (108) | 411 (160) ab | 84.7 (3.43) ab |
| MWF 3% + NI | 6.64 (0.06) a | 8.88 (3.01) | 0.07 (0.11) | 34.4 (5.53) bc | 43.4 (4.61) bc | 159 (34.1) | 279 (54) b | 86.4 (1.44) ab |
| MWF 3% + UI | 6.75 (0.09) a | 0.93 (0.21) | 0.84 (1.83) n = 4 | 25.5 (10.3) c | 27.3 (11.1) c | 188 (26.9) | 185 (69) b | 87.3 (0.66) a |
| MWF 3% + NI + UI | 6.62 (0.09) a | 4.83 (1.89) | 0.01 (0.01) n = 2 | 43.7 (19.6) bc | 48.5 (20.5) bc | 193 (49.5) | 346 (73) ab | 88.0 (2.41) a |
Substrate pH, ammonium (-N), nitrite (-N), nitrate (-N), total inorganic N, extractable organic C (EOC) and N (EON) and EON as a percentage of total extractable N, at the end of the experiment (mean ± standard deviation for n = 5 except where indicated).
Pots were amended with 3% (w/w) buffalo worm frass (BWF) or mealworm frass (MWF) alone, or concurrently with nitrification inhibitor (NI), urease inhibitor (UI), or both. Within a column, means without a letter in common are significantly different (p <0.05).
At the end of the experiment total inorganic N was highest in the treatments without inhibitors following 1.5 or 3% frass amendment, with the largest fraction being in the form of nitrate (Tables 3, 4). Whereas nitrite was almost absent in the 1.5% treatments, 3% frass addition resulted in substantial nitrite accumulation in the treatments without inhibitors. This nitrite build up was hugely reduced in the presence of NI, but only partially by UI (Table 4).
Of the inhibitors, UI resulted in a consistent trend of inducing the lowest extractable total inorganic N for both frass types at both application rates (Tables 3, 4). Due to this, the percentages of total extractable N in the UI treatments which was organic N were consistently higher than where NI was applied, significantly so in the 1.5% MWF regime (Tables 3, 4).
At both frass application rates, NI led to lower extractable -N than where no inhibitor was applied, although not significantly due to the high data variability. In the 3% frass-amended pots where NI was applied, with or without UI, -N concentrations were the highest (Table 4). NI led to the highest -N concentrations in the 1.5% BWF frass-amended pots (Table 3). This was not the case with MWF, possibly due to higher uptake of N by MWF-fertilized plants (Table 2).
Microbial Biomass and Community Composition
MBC and MBN increased after frass addition in both treatments (1.5 and 3%) relative to an unfertilized control (data not shown). In the replicates receiving inhibitors, there was a consistent trend at both frass application rates of NI yielding the highest MBC, UI the lowest, with intermediate values for NI + UI (Tables 5, 6). This was reflected by the bacterial and archaeal 16S rRNA gene copy numbers, which were higher in the NI and NI + UI treatments than in the UI treatment, often significantly (Figures 3A,B). These effects of the NI were more pronounced in BWF as compared to MWF. In contrast to the treatments containing NI, the UI treatment led to archaeal and gene copy numbers that were never significantly higher than those of the treatments receiving no inhibitor (Figures 3A,B). The UI treatment consistently led to the lowest MBN (Tables 5, 6).
Table 5
| Treatment | MBC (mg kg−1) | MBN (mg kg−1) | Ergosterol (mg kg−1) | Conductivity (μS/cm) |
|---|---|---|---|---|
| BWF 1.5% | 88 (51) ab | 22 (10) ab n = 4 | 0.17 (0.03) n = 3 | 319 (199) a |
| BWF 1.5% + NI | 156 (52) a | 12 (6) b | 0.18 (0.04) n = 4 | 147 (43) ab |
| BWF 1.5% + UI | 83 (24) b | 8 (3) b | 0.18 (0.03) | 166 (171) ab |
| BWF 1.5% + NI + UI | 124 (13) ab | 40 (32) a | 0.18 (0.03) | 175 (184) ab |
| MWF 1.5% | 102 (49) ab | 10 (5) b n = 4 | 0.19 (0.06) | 130 (90) ab |
| MWF 1.5% + NI | 112 (20) ab | 11 (3) b | 0.17 n = 1 | 51 (5) b |
| MWF 1.5% + UI | 101 (23) ab | 9 (6) b | 0.28 (0.08) n = 3 | 78 (12) ab |
| MWF 1.5% + NI + UI | 102 (27) ab | 12 (5) b | 0.19 (0.003) n = 2 | 90 (58) ab |
Microbial biomass C (MBC), N (MBN), ergosterol content and conductivity at the end of the experiment (mean ± standard deviation for n = 5 except where indicated).
Pots were amended with 1.5% (w/w) buffalo worm frass (BWF) or mealworm frass (MWF) alone, or concurrently with nitrification inhibitor (NI), urease inhibitor (UI), or both. Within a column, means without a letter in common are significantly different (p <0.05).
Table 6
| Treatment | MBC (mg kg−1) | MBN (mg kg−1) | Ergosterol (mg kg−1) | Conductivity (μS/cm) |
|---|---|---|---|---|
| BWF 3% | 121 (31) | 92 (122) n = 2 | 0.45 (0.11) bc | 1,456 (203) a |
| BWF 3% + NI | 176 (83) | 120 (107) | 0.22 (0.02) c n = 3 | 1,189 (238) ab |
| BWF 3% + UI | 99 (15) | 10 (12) n = 3 | 0.62 (0.15) ab | 910 (246) bc |
| BWF 3% + NI + UI | 155 (73) | 186 (120) n = 4 | 0.23 (0.11) c | 1,264 (300) ab |
| MWF 3% | 116 (19) | 71 (99) n = 4 | 0.31 (0.11) c | 1,140 (368) abc |
| MWF 3% + NI | 198 (72) | 50 (38) n = 3 | 0.37 (0.12) c | 825 (65) bc |
| MWF 3% + UI | 152 (67) | 16 (20) | 0.75 (0.13) a | 641 (189) c |
| MWF 3% + NI + UI | 159 (112) | 107 (147) n = 4 | 0.24 (0.04) c | 1,089 (226) abc |
Microbial biomass C (MBC), N (MBN), ergosterol content and conductivity, at the end of the experiment (mean ± standard deviation for n = 5 except where indicated).
Pots were amended with 3% (w/w) buffalo worm frass (BWF) or mealworm frass (MWF) alone, or concurrently with nitrification inhibitor (NI), urease inhibitor (UI), or both. Within a column, means without a letter in common are significantly different (p <0.05).
Figure 3
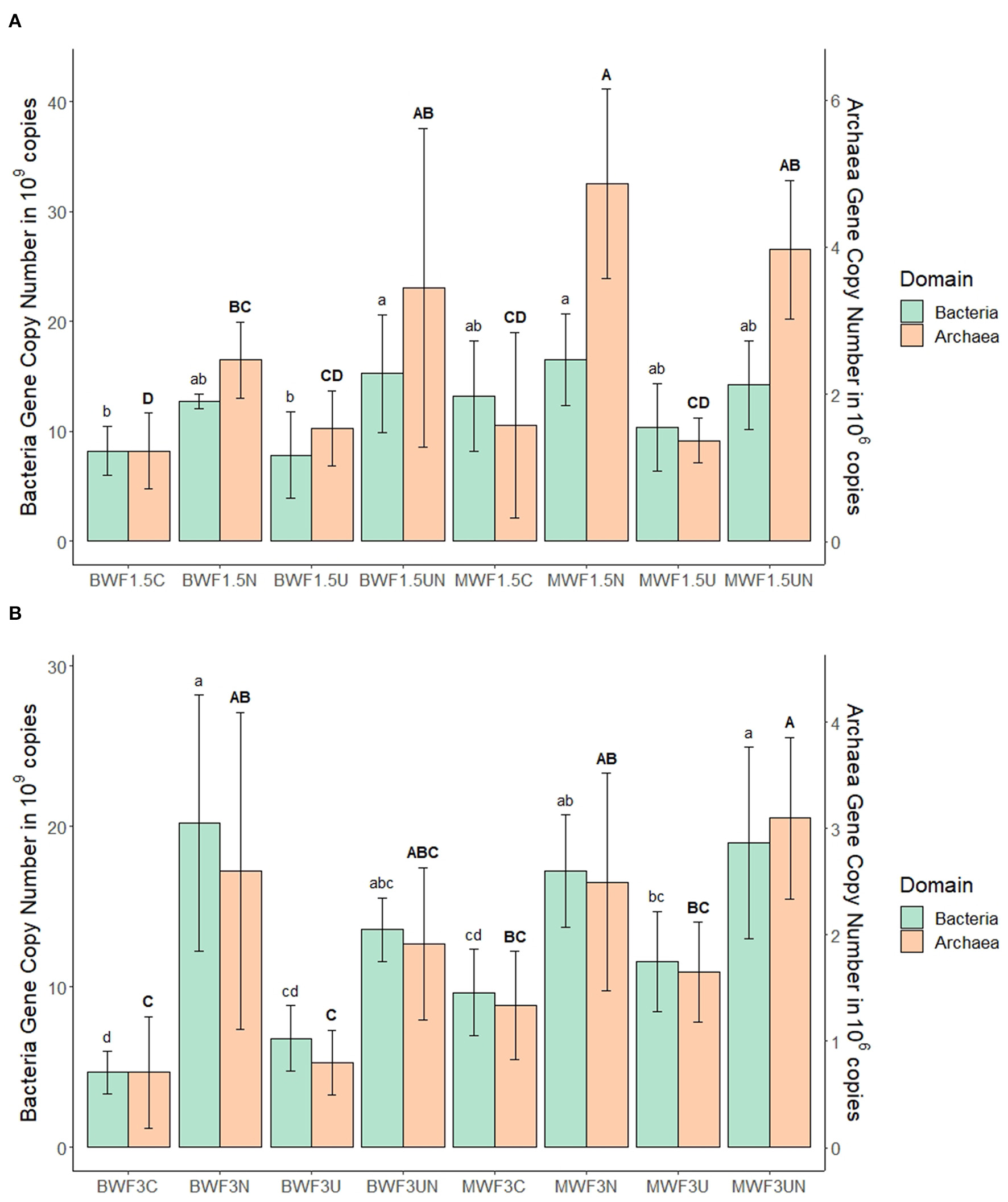
Mean 16S rRNA gene copy numbers of bacteria (standard letters) and archaea (capital letters) per gram of soil at the end of the experiment after (A) 1.5% frass addition with plants and (B) 3.0% frass addition without plants (mean ± 95% confidence interval for n = 5). Pots were amended with frass alone, or concurrently with nitrification inhibitor (NI), urease inhibitor (UI), or both. For each microbial domain, different letters indicate significant differences between the means of the treatments (p < 0.05).
The ergosterol results of the 1.5% treatments were marred by high variability but nevertheless show a stimulatory effect of the UI treatment on the fungal biomass where MWF was applied (Table 5). This became more evident when relating the ergosterol content to MBC (Figure 4A), which displays the UI-induced increases in the fungal portion of the microbial biomass. This trend was much more evident in the 3% frass treatments: ergosterol concentrations were significantly higher where UI was applied than in the other treatments (Table 6), while the ergosterol/MBC quotients were markedly higher in the UI treatments (Figure 4B).
Figure 4
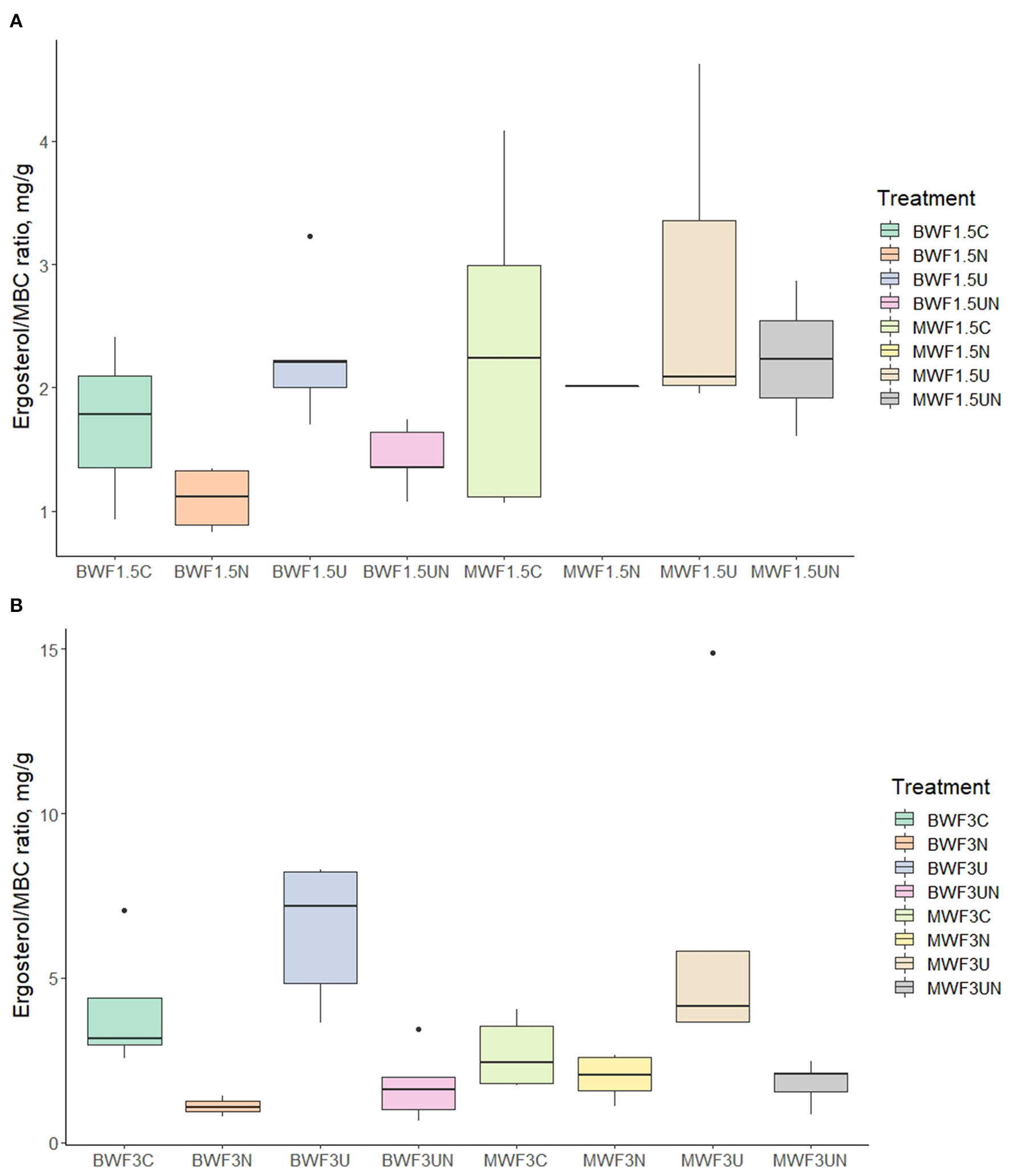
Ergosterol/microbial biomass carbon ratio (mg/g) following (A) 1.5% frass addition with plants and (B) 3.0% frass addition without plants (mean ± 95% confidence interval). Pots were amended with frass alone, or concurrently with nitrification inhibitor (NI), urease inhibitor (UI), or both.
Discussion
Frass Quantity and Quality Can Benefit or Harm Plants
Application of 3% frass almost completely inhibited germination of ryegrass in our experiment. One reason for this may be a higher salt load. Extractable Na concentrations were considerable (Table 1), yet electrical conductivity values, although elevated, were not extremely high (Table 6) and osmotic stress was potentially limited as pots were kept moist throughout the experiment. Kawasaki et al. (2020) surmised inhibition of Japanese mustard spinach germination in BSF frass-amended soil to be salinity induced, albeit at the much higher application rate of 10%. Another possibility is that wetting of the 3% frass-amended soil caused locally high NH3 concentrations, which can be inhibitory to seed germination (Cantarella et al., 2018). The observed negative effect of high frass loads on plant germination warrant further mechanistic investigation to be able to exclude negative effects of frass on plant growth when used as fertilizer or soil amendment.
Application of 1.5% frass on the other hand resulted in rigorous plant growth. This was expected as both MWF and BWF frass provided easily available nutrients (Table 1). Successful fertilization using frass has been demonstrated in previous studies. For example, Klammsteiner et al. (2020) reported BSF frass to be a rapid provider of N to perennial ryegrass, while Houben et al. (2020) described MWF as an effective fertilizer to barley plants as it is rapidly mineralized. Interestingly, of the frass types used in this study, BWF contains more total N than MWF and has similar extractable inorganic N concentrations (Table 1) and yet yielded lower biomass and lower N uptake in ryegrass (Table 2). One reason for this may be the higher extractable concentrations of other primary, secondary and micro nutrients in MWF (Table 1) which could become increasingly important if the plant growth was not N-limited. This indicates that a stoichiometric evaluation of frass as a fertilizer requires more attention in future studies to allow for a better understanding how frass derived N can be used by plants most efficiently. A further benefit of this evaluation would be understanding of how to avoid N losses shown in a recent study (Rummel et al., 2021) which are an undesired environmental disadvantage of insect protein production.
The frass used in our study originated from two different insect species fed with the same diet. Diet, in particular N content of feed influences the N content of the frass (Fielding et al., 2013), which strongly influences its C/N ratio. The C/N ratio of frass further affects its mineralization and subsequent N release (Rummel et al., 2021). However, our study shows that frass originating from two species fed with the same diet with very similar C/N ratios can differently affect plant growth and N uptake. The reason for this may lie in the aforementioned higher extractable nutrient content of MWF (Table 1) allowing a more efficient N uptake and use by plants. The application of NI is likely to have led to increased -N uptake by ryegrass and the higher biomass depicted in Table 2. Although Griffith and Streeter (1994) demonstrated higher biomass in hydroponically-grown Italian ryegrass fertilized with compared to , Chalk and Smith (2020) argue that plant response to N supply is fully plastic, and improved biomass or N uptake following NI application could be a result of reduced loss to leaching or denitrification. Despite the biomass increase in the present study not being statistically significant, it led to the sometimes significantly greater uptake of N, Ca, Mg, K, P and Fe. Correspondingly, -N provided by the ammonification of frass ureic N would have been reduced by the UI treatment, leading to the lowest average biomass and uptake of N, Ca, K, and Mg for both frass types (Table 2).
Nitrogen Release From Frass: Ammonification and Nitrification
Most of the inorganic N extractable from the frass used in the current study is -N (Table 1), while degradation of organic compounds containing N will ultimately release more . Addition of high amounts of -N to a soil can reduce N mineralization and even microbial biomass N if there is a deficiency of labile C (Zaman et al., 1999). Our results clearly show that each treatment's considerable extractable EOC (Tables 3, 4) provided adequate labile C for N mineralization to take place in this C-poor substrate, as evidenced by the substantial extractable -N concentrations where 3% frass was applied (Table 4). Total inorganic N extractable in 3% treatments with inhibitors display reductions relative to the inhibitor-free controls, which represent the original N mineralization pattern of frass. These reductions were most marked in the UI treatments, where total inorganic N amounted to respectively 36 and 58% of the MWF and BWF inhibitor-free controls (Table 4). Such substantial reductions in the presence of a urease inhibitor point to ureic N as being a considerable fraction of the organic N in frass that becomes mineralized. The remaining organic N pools that could via ammonification supply -N in these treatments are chitin and protein (Figure 1). Given chitin's recalcitrance caused by its crystallinity and heterogeneic composition (Jacquiod et al., 2013), protein could be considered a more mineralizable organic N source in frass, as extracellular proteases are commonly produced by bacteria and fungi (Geisseler et al., 2010).
In aerated soils, -N concentrations are typically low due to root uptake and nitrification (Geisseler et al., 2010). This was the case in the 1.5% treatments in the present study, particularly where MWF was applied and ryegrass uptake and biomass production was greatest. The soils in these treatments were mildly alkaline (Table 3) and so might have additionally lost more N via NH3 volatilization. What cannot be overlooked is the substantial nitrate production even when NI was applied. Of the 1.5 and 3% frass treatments with NI application, nitrate is the largest fraction of the total inorganic N, with the exception of the MWF 1.5% NI regime (Tables 3, 4). This could be explained by the small but significant fraction of inorganic N in both frass types that was originally -N (Table 1), and by a likely degradation of DMPP over the course of the experiment resulting in some nitrification. While only slight mineralization of the inhibitor was reported months after its field application by Weiske et al. (2001), its breakdown will be much more rapid in the optimal temperatures of a pot trial. DMPP has been reported to be effective for 40 days at 20°C (Zerulla et al., 2001), whereas Gong et al. (2013) postulated it to have decomposed halfway through a pot trial of similar duration to ours.
The expected additive effect of applying NI and UI simultaneously did not materialize: total inorganic N for NI+UI was consistently greater than for NI or UI alone (Tables 3, 4). Lasisi et al. (2020) reported the presence of DMPP to decrease the effectiveness of NBPT by an average of over 20% in a variety of soils, thought to be due to a combination of ammonium persistence and increased urea hydrolysis. While we can only speculate as to whether urease inhibitors in turn reduce the effectiveness of nitrification inhibitors, the supposition in our experiment is that NI partially reduced the effectiveness of UI, allowing greater concentrations of to be nitrified upon DMPP breakdown.
The nitrification inhibitors were effective in the prevention of nitrite accumulation in the 3% frass treatments (Table 4). Nitrite-N concentrations were minimal where NI was applied in comparison to the UI treatments or inhibitor-free controls. In treatments in the present study without a nitrification inhibitor hindering ammonium-oxidizing microorganisms, extractable -N reached similar or even higher concentrations to those of -N. A 2.5% application rate of MWF and BWF to a sandy loam was observed in a 28-day incubation experiment to cause profound nitrite accumulation (Watson et al., 2021). Nitrite can accumulate following N fertilization as the rates of and oxidation are not interdependent; the oxidation rate of can be higher than that of as it is a better energy source (Taylor et al., 2019). It is possible that the nitrite build-up was caused by its oxidation being inhibited by ammonia or nitrous acid. Analyses of these would be relevant to future studies of frass-amended soil.
Effect of Frass on the Microbial Biomass and Community
As discussed in Frass Quantity and Quality Can Benefit or Harm Plants section, the 3% frass application rate prohibited ryegrass germination, possibly due to salinity. Microbial properties can be negatively affected by high salt loads (Wichern et al., 2006; Rath and Rousk, 2015). However, in our experiment microbial biomass was not negatively affected, with 3% frass addition leading to slightly higher microbial biomass C than in the 1.5% frass treatments (Tables 5, 6) which can be explained by the higher C addition. The negative impacts of the frass salinity which prevented plant growth possibly did not adversely affect the microbial biomass due to frass organic matter affording amelioration (Iqbal et al., 2016; Wichern et al., 2020).
Our study confirms earlier observations that frass amendment of soil increases microbial growth (Frost and Hunter, 2004; Gebremikael et al., 2020; Watson et al., 2021). This is not remarkable given that the frass provided easily available C, N and other nutrients (Table 1) required for microbial functions (Hemkemeyer et al., 2021). The use of nitrification and urease inhibitors allowed a differentiation of N release pathways as outlined in Figure 1. Additionally, the inhibitors also revealed pronounced interactions between the altered N transformation and abundance of the microbial domains archaea, bacteria and fungi.
Inhibition of nitrification at both frass application rates resulted in accumulation of -N, the highest MBC (Tables 3, 4) and a preferential growth of bacteria and archaea: treatments featuring NI led to the consistently highest 16S rRNA gene copy numbers of these domains (Figure 3). Preferential immobilization of to by bacteria when both are available, in which ammonium inhibits nitrate uptake, was first observed by Recous et al. (1990). Ammonium uptake is thought to be optimal between pH 6 and 7 as was the case in the majority of pots in our study (Tables 3, 4) and preserves microbial energy as it can be taken up directly whereas must be reduced by two energy-costing enzymes (Geisseler et al., 2010).
Frass application in our experiment was stimulatory to fungal growth, as observed in previous studies (Lovett and Ruesink, 1995; Fielding et al., 2013; Rummel et al., 2021; Watson et al., 2021). While fungal preference for has also been reported (Geisseler et al., 2010), the contribution of fungi to the microbial biomass, as revealed by the ergosterol concentrations (Tables 5, 6) and ergosterol/MBC ratios (Figure 4), was markedly increased when ammonification of ureic N was hindered by the urease inhibitor. In these treatments, the -N concentrations were reduced and the relative proportion of the extractable N that was organic, rose (Tables 3, 4). Saprotrophic fungi may contain suites of enzymes better suitable to degrade the organic N derived from frass and thus proliferate when urease activity is inhibited and content lowered. Gebremikael et al. (2020) reported BSF frass to induce increases in saprotrophic fungi and related this to fungal biomass growth typical of the later stages of organic matter decomposition.
Watson et al. (2021) incubated MWF and BWF in a sandy loam at application rates of 2.5 and 5%. The 2.5% treatment led to nitrite accumulation which was accompanied by, relative to the 5% treatment, an increase in the archaeal 16S rRNA gene copy numbers. In the present study, nitrite accumulation was observed at a similar application rate of 3% (Table 6). However, there was no higher application rate with which to ascertain whether there was a relative archaeal enrichment, and microbial comparisons between the 3 and 1.5% treatments are complicated by the presence of a sizeable rhizosphere in the latter. Nevertheless, the mechanisms of this nitrite build-up should be elucidated in future studies with the help of a more detailed analysis of the ammonia-oxidizing bacteria and archaea and the presence of relevant functional genes.
Conclusions
Our results show the high potential of insect frass, both as an amendment which can benefit the soil microbial biomass and as an organic fertilizer which can provide adequate nutrients to allow plant growth in a substrate of low fertility. Nevertheless, three aspects of soil amendment with frass must be systematically evaluated before it becomes an accepted norm. Firstly, (i) the nutrient content and availability of frass to plants depend heavily on several factors including larval species, feeding regime, processing and storage. Predictions of plant response to various frass types will become easier once these factors are thoroughly understood. Secondly, (ii) optimal frass application rates and timing in relation to crop demand need to be ascertained. Other studies using different frass types have demonstrated considerably higher application rates than the one used in this study to be successful, while we observed a 3% application rate of mealworm or buffalo worm frass to inhibit ryegrass seed germination. It has not been established whether ammonia toxicity or salinity was the cause of this, nor whether such an application rate would affect other plants similarly. There could be a specificity of crop response to frass type yet to be elucidated. Thirdly, (iii) to allow for a more environmentally friendly insect protein production, optimal use of frass with reduced nutrient losses need to be guaranteed, in particular those of C and N. Accordingly, this study sought to evaluate N release processes from frass applied to soil at relatively high rates, via the use of inhibitors typically applied with fertilizers. We demonstrated that the ammonification of ureic substances is an important release of N from frass, confirming our first hypothesis. More importantly, we established that urease inhibitors can be co-applied with frass where the goal is to utilize frass as a slow-release N fertilizer. A yield penalty was not effected in ryegrass over a 16-week pot trial when NBPT was added, presumably because the ammonification of proteinaceous and chitinaceous N provided sufficient N for plant growth. Our second hypothesis was corroborated by our results indicating that the co-application of nitrification inhibitors with frass could induce yield gains and is effective in preventing soil nitrite accumulation and any consequent impacts on nitrite-sensitive crops. A combination of both is not recommended as soil extractable N concentrations were consistently higher than when the inhibitors were used separately, an observation which did not uphold our third hypothesis.
Funding
We are grateful for EU German-Dutch INTERREG V A-funding of this work as part of the Food Pro.tec.ts project.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CW and FW designed the experiment and CW carried it out. CW carried out microbial and soil analyses. CW and TP processed data. CW, TP, and FW interpreted the results and prepared the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We acknowledge the support of Michael Hemkemeyer, Bernd Hetjens, Sabrina Meisen, Sanja Schwalb, and a huge number of student assistants in the soil and plant analyses.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
AlattarM. A.AlattarF. N.PopaR. (2016). Effects of microaerobic fermentation and black soldier fly larvae food scrap processing residues on the growth of corn plants (Zea mays). Plant Sci. Today3, 57–62. 10.14719/pst.2016.3.1.179
2
BrookesP. C.LandmanA.PrudenG.JenkinsonD. S. (1985). Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem.17, 837–842. 10.1016/0038-0717(85)90144-0
3
CantarellaH.OttoR.SoaresJ. R.de Brito SilvaA. G. (2018). Agronomic efficiency of NBPT as a urease inhibitor: a review. J. Adv. Res.13, 19–27. 10.1016/j.jare.2018.05.008
4
ChalkP.SmithC. (2020). On inorganic N uptake by vascular plants: Can 15N tracer techniques resolve the NH4+ versus NO3− “preference” conundrum?Eur. J. Soil Sci.72, 1762–1779. 10.1111/ejss.13069
5
ChoiY.ChoiJ.KimJ.KimM.KimW.ParkK.et al. (2009). Potential usage of food waste as a natural fertilizer after digestion by Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol.19, 171–174.
6
DjajakiranaG.JoergensenR. G.MeyerB. (1996). Ergosterol and microbial biomass relationship in soil. Biol. Fertil. Soils22, 299–304. 10.1007/BF00334573
7
FieldingD. J.TrainorE.ZhangM. (2013). Diet influences rates of carbon and nitrogen mineralization from decomposing grasshopper frass and cadavers. Biol. Fertil. Soils49, 537–544. 10.1007/s00374-012-0702-5
8
FrostC. J.HunterM. D. (2004). Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology85, 3335–3347. 10.1890/04-0003
9
GebremikaelM. T.RanasingheA.HosseiniP. S.LaboanB.SonneveldE.PipanM.et al. (2020). How do novel and conventional agri-food wastes, co-products and by-products improve soil functions and soil quality?Waste Manage.113, 132–144. 10.1016/j.wasman.2020.05.040
10
GeisselerD.HorwathW. R.JoergensenR. G.LudwigB. (2010). Pathways of nitrogen utilization by soil microorganisms—a review. Soil Biol. Biochem.42, 2058–2067. 10.1016/j.soilbio.2010.08.021
11
GongP.ZhangL. L.WuZ. J.ChenZ. H. CChenL.J. (2013). Responses of ammonia-oxidizing bacteria and archaea in two agricultural soils to nitrification inhibitors DCD and DMPP: a pot experiment. Pedosphere23, 729–739. 10.1016/S1002-0160(13)60065-X
12
GreenT. R.PopaR. (2012). Enhanced ammonia content in compost leachate processed by black soldier fly larvae. Appl. Biochem. Biotechnol.166, 1381–1387. 10.1007/s12010-011-9530-6
13
GriffithS. M.StreeterD. J. (1994). Nitrate and ammonium nutrition in ryegrass: changes in growth and chemical composition under hydroponic conditions. J. Plant Nutr.17, 71–81. 10.1080/01904169409364710
14
HemkemeyerM.PronkG. J.HeisterK.Kögel-KnabnerI.MartensR.TebbeC. C. (2014). Artificial soil studies reveal domain-specific preferences of microorganisms for the colonisation of different soil minerals and particle size fractions. FEMS Microbiol Ecol. 90, 770–782. 10.1111/1574-6941.12436
15
HemkemeyerM.SchwalbS. A.HeinzeS.JoergensenR. G.WichernF. (2021): Functions of elements in soil microorganisms. Microbiol. Res. 252:126832. 10.1016/j.micres.2021.126832
16
HoubenD.DaoulasG.FauconM. P.DulaurentA. M. (2020). Potential use of mealworm frass as a fertilizer: impact on crop growth and soil properties. Sci. Rep.10, 1–9. 10.1038/s41598-020-61765-x
17
IqbalM. T.JoergensenR. G.KnoblauchC.LucassenR.SinghY.WatsonC.et al. (2016). Rice straw addition does not substantially alter microbial properties under hypersaline soil conditions. Biol. Fertil. Soils52, 867–877. 10.1007/s00374-016-1126-4
18
JacquiodS.FranquevilleL.CecillonS. MVogelT.SimonetP. (2013). Soil bacterial community shifts after chitin enrichment: an integrative metagenomic approach. PLoS ONE8:e79699. 10.1371/journal.pone.0079699
19
KagataH.OhgushiT. (2012a). Non-additive effects of leaf litter and insect frass mixture on decomposition processes. Ecol. Res.27, 69–75. 10.1007/s11284-011-0868-6
20
KagataH.OhgushiT. (2012b). Positive and negative impacts of insect frass quality on soil nitrogen availability and plant growth. Popul. Ecol.54, 75–82. 10.1007/s10144-011-0281-6
21
KawasakiK.KawasakiT.HirayasuH.MatsumotoY.FujitaniY. (2020). Evaluation of fertilizer value of residues obtained after processing household organic waste with black soldier fly larvae (Hermetia illucens). Sustainability 12:4920. 10.3390/su12124920
22
KlammsteinerT.TuranV.JuárezM. F. D.ObereggerS.InsamH. (2020). Suitability of black soldier fly frass as soil amendment and implication for organic waste hygienization. Agronomy10:1578. 10.3390/agronomy10101578
23
LasisiA. A.AkinremiO. O.KumaragamageD. (2020). Nitrification inhibitor reduces the inhibitory effect of N-(n-butyl) thiophosphoric triamide (NBPT) on the hydrolysis of urea. Soil Sci. Soc. Am. J.84, 1782–1794. 10.1002/saj2.20122
24
LovettG. M.RuesinkA. E. (1995). Carbon and nitrogen mineralization from decomposing gypsy moth frass. Oecologia104, 133–138. 10.1007/BF00328577
25
MeninoR.FelizesF.Castelo-BrancoM. A.FareleiraP.MoreiraO.NunesR.et al. (2021). Agricultural value of Black Soldier Fly larvae frass as organic fertilizer on ryegrass. Heliyon7:e05855. 10.1016/j.heliyon.2020.e05855
26
NabelM.SchreyS. D.PoorterH.KollerR.NagelK. A.TempertonV. M.et al. (2018). Coming late for dinner: Localized digestate depot fertilization for extensive cultivation of marginal soil with Sida hermaphrodita. Front. Plant Sci.9:1095. 10.3389/fpls.2018.01095
27
OonincxD. G.Van BroekhovenS.Van HuisA.van LoonJ. J. (2015). Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE10:e0144601. 10.1371/journal.pone.0144601
28
PovedaJ. (2021). Insect frass in the development of sustainable agriculture. A review. Agron. Sustain. Dev.41, 1–10. 10.1007/s13593-020-00656-x
29
PovedaJ.Jiménez-GómezA.Saati-SantamaríaZ.Usategui-MartínR.RivasR.García-FraileP. (2019). Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol.142, 110–122. 10.1016/j.apsoil.2019.04.016
30
QuilliamR. S.Nuku-AdekuC.MaquartP.LittleD.NewtonR.MurrayF. (2020). Integrating insect frass biofertilisers into sustainable peri-urban agro-food systems. J. Insects Food Feed6, 315–322. 10.3920/JIFF2019.0049
31
R Core Team (2019). R: A Language and Environment for Statistical Computing. Available online at: https://www.r-project.org/ (accessed June 06, 2021).
32
RathK. M.RouskJ. (2015). Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: a review. Soil Biol. Biochem.81, 108–123. 10.1016/j.soilbio,.2014.11.001
33
RecousS.MaryB.FaurieG. (1990). Microbial immobilization of ammonium and nitrate in cultivated soils. Soil Biol. Biochem.22, 913–922. 10.1016/0038-0717(90)90129-N
34
RummelP. S.BeuleL.HemkemeyerM.SchwalbS.WichernF. (2021). Black soldier fly diet impacts soil greenhouse gas emissions from frass applied as fertilizer. Front. Sustain. Food Syst.5:709993. 10.3389/fsufs.2021.709993
35
SchmittE.de VriesW. (2020). Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 25:100335. 10.1016/j.cogsc.2020.03.005
36
SmetanaS.SchmittE.MathysA. (2019). Sustainable use of Hermetia illucens insect biomass for feed and food: attributional and consequential life cycle assessment. Resour. Conserv. Recycl.144, 285–296. 10.1016/j.resconrec.2019.01.042
37
TaylorA. E.MyroldD. D.BottomleyP. J. (2019). Temperature affects the kinetics of nitrite oxidation and nitrification coupling in four agricultural soils. Soil Biol. Biochem. 136:107523. 10.1016/j.soilbio.2019.107523
38
Van BroekhovenS.OonincxD. G.Van HuisA.Van LoonJ. J. (2015). Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol.73, 1–10. 10.1016/j.jinsphys.2014.12.005
39
van der WielB. Z.WeijmaJ.van MiddelaarC. E.KleinkeM.BuismanC. J. N.WichernF. (2020). Restoring nutrient circularity: a review of nutrient stock and flow analyses of local agro-food-waste systems. Resour. Conserv. Recycl.160:104901. 10.1016/j.resconrec.2020.104901
40
VanceE. D.BrookesP. C.JenkinsonD. S. (1987). An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 19, 703–707. 10.1016/0038-0717(87)90052-6
41
WatsonC.ClemensJ.WichernF. (2019). Plant availability of magnesium and phosphorus from struvite with concurrent nitrification inhibitor application. Soil Use Manage.35, 675–682. 10.1111/sum.12527
42
WatsonC.SchlösserC.VögerlJ.WichernF.Excellent excrement? Frass impacts on a soil's microbial community processes metal bioavailability. (2021). Appl. Soil Ecol.168:104110. 10.1016/j.apsoil.2021.104110
43
WeiskeA.BenckiserG.HerbertT.OttowJ. (2001). Influence of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol. Fertil. Soils34, 109–117. 10.1007/s003740100386
44
WichernF.IslamM.HemkemeyerM.WatsonC.JoergensenR. G. (2020). Organic amendments alleviate salinity effects on soil microorganisms and mineralisation processes in aerobic and anaerobic paddy rice soils. Front. Sustain. Food Syst.4:30. 10.3389/fsufs.2020.00030
45
WichernJ.WichernF.JoergensenR. G. (2006). Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma137, 100–108. 10.1016/j.geoderma.2006.08.001
46
WissemeierA. H.LinzmeierW.GutserR.WeigeltW.SchmidhalterU. (2001). “The new nitrification inhibitor DMPP (ENTEC®)—comparisons with DCD in model studies and field applications,” in Plant Nutrition (Dordrecht: Springer), 702–703. 10.1007/0-306-47624-X_340
47
WuJ.JoergensenR. G.PommereningB.ChaussodR.BrookesP. C. (1990). Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol. Biochem. 22, 1167–1169. 10.1016/0038-0717(90)90046-3
48
YuY.LeeC.KimJ.HwangS. (2005). Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng.89, 670–679. 10.1002/bit.20347
49
ZamanM. D. H. J.DiH. J.CameronK. C.FramptonC. M. (1999). Gross nitrogen mineralization and nitrification rates and their relationships to enzyme activities and the soil microbial biomass in soils treated with dairy shed effluent and ammonium fertilizer at different water potentials. Biol. Fertil. Soils29, 178–186. 10.1007/s003740050542
50
ZerullaW.BarthT.DresselJ.ErhardtK.von LocquenghienK. H.PasdaG.et al. (2001). 3, 4-Dimethylpyrazole phosphate (DMPP)—a new nitrification inhibitor for agriculture and horticulture. Biol. Fertil. Soils34, 79–84. 10.1007/s003740100380
Summary
Keywords
ammonification, microbial community, nitrogen mineralization, nutrient recycling, organic fertilizer, soil amendment, buffalo worm, mealworm
Citation
Watson C, Preißing T and Wichern F (2021) Plant Nitrogen Uptake From Insect Frass Is Affected by the Nitrification Rate as Revealed by Urease and Nitrification Inhibitors. Front. Sustain. Food Syst. 5:721840. doi: 10.3389/fsufs.2021.721840
Received
07 June 2021
Accepted
28 September 2021
Published
27 October 2021
Volume
5 - 2021
Edited by
Henrique Manuel da Fonseca Trindade, University of Trás-os-Montes and Alto Douro, Portugal
Reviewed by
Peter Groot Koerkamp, Wageningen University and Research, Netherlands; Paolo Mantovi, Centro Ricerche Produzioni Animali, Italy
Updates
Copyright
© 2021 Watson, Preißing and Wichern.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Conor Watson conor.watson@hsrw.eu
This article was submitted to Waste Management in Agroecosystems, a section of the journal Frontiers in Sustainable Food Systems
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.