Abstract
Anthracnose disease on chili pepper has been known to seriously interfere with the plant growth and obviously reduce the yield. The disease is caused by Colletotrichum spp. In Bali, Indonesia, six species of Colletotrichum have been identified: Colletotrichum scovillei, C. acutatum, C. nymphaeae, C. gloeosporioides, C. truncatum, and C. fructicola. However, among them the C. scovillei was found to be the most prevalent cause of anthracnose on chili pepper in Bali. Two species of antagonist against C. scovillei, namely Paenibacillus polymyxa C1 and Bacillus siamensis C7B, have been identified. In this study the effectiveness of P. polymyxa C1 formulation was evaluated under greenhouse condition on chili pepper cultivars Cabe Besar. Application of formulation was conducted by a mini hand sprayer once to five times with a week interval. Results of the study showed that treatment with five applications significantly (p < 0.05) reduced the disease incidence, disease intensity, and the yield loss of chili pepper cultivar Cabe Besar. Alose relationship was observed between the number of applications with disease intensity, with coefficient of determination (R2) at 0.929. These results revealed that the formulation of P. polymyxa C1 effectively control the anthracnose disease on chili pepper, particularly on chili pepper cultivar Cabe Besar, and thus can be recommended for field testing to confirm its stability under field conditions.
Introduction
Cultivation of chili pepper in Bali, Indonesia, is frequently affected by anthracnose disease, caused by Colletotrichum spp. The incidence of the disease in this region was reported at 84% and disease intensity varied from 22 to 78% (Khalimi et al., 2019). A study done by Khalimi et al. (2019) identified six species of Colletotrichum as the cause of anthracnose disease on chili pepper in Bali: Colletotrichum scovillei, C. acutatum, C. nymphaeae, C. gloesporioides, C. truncatum, and C. fructicola. Among them, C. scovillei was the most prevalent, with rates of 55%. The disease causes 10 to 80% of yield losses depending on cultivation areas (Poonpolgul and Kumphai, 2007; Asare-Badiako et al., 2015; Diao et al., 2017).
Development of a resistant chili cultivar is expected to be effective in controlling the disease, however the resistance against Colletotrichum spp. is often broken down under field conditions (Park, 2007). Synthetic fungicides are generally used by farmers to control anthracnose disease; however, this measure is not always effective to reduce the disease incidence. Other alternative measures are needed to overcome the problem of anthracnose disease on chili pepper, while remaining safe for the consumers and environment.
Because of the increased preference of consumers for organic agricultural products in Indonesia, organic farming systems are becoming popular among farmers and consumers (Shiotsu et al., 2015). To develop organic farming, it is important to provide bio-control agents to manage plant diseases. The use of bio-agents to control plant fungal diseases may reduce the use of synthetic chemical fungicides and hence reduce the adverse effect to the environment. Several bio-agents have been found and reported to possess inhibitory activities against plant pathogens. Piriformospora indica, Trichoderma viride, Acremonium lolii, and Colletotrichum lindemuthianum protected against leaf rust of wheat caused by Puccinia recondite (Anwaar et al., 2019), Bacillus cereus SSB1 effectively controlled Pectobacterium infection in potato (Sarfraz et al., 2019) and Bacillus amyloliquesfaciens BA-16-8 inhibited the growth of Penicillium expansum, which is the cause of postharvest decay in apple (Fu et al., 2020).
One type of bio-agent that could potentially be used for plant fungal disease control is rhizobacteria. Rhizobacteria are bacteria that colonize the plant root and give beneficial effect to the plant through growth promotion (Saharan and Nehra, 2011). Enterobacter cloacae KtB3 isolated from the rhizosphere of groundnut were proven to effectively control the damping-off disease on soybean caused by Sclerotium rolfsii (Mahartha and Suprapta, 2018). Rhizobacteria are able to produce phyto-hormones such as indole acetic acid (IAA), ACC deaminase to fix atmospheric nitrogen and act as antagonistic microbes against plant pathogen through the production of siderophores, and β-1,3-glucanase, chitinase, cellulase, and antibiotics, which are able to dissolve phosphate and other nutrients in the soil (Soesanto, 2008; Guo et al., 2015; Saleem et al., 2015). For example, Pseudomonas fluorescens produced 2, 4-diacetyl phloroglucinol that effectively suppressed plant fungal pathogens (Nowak-Thompson et al., 1994). Pseudomonas stutzeri produced extracellular chitinase enzyme and laminarinase that were able to decompose the mycelia of Fusarium solani (Mauch et al., 1988). Our previous study showed that two species of antagonist, namely Paenibacillus polymyxa C1 and Bacillus siamensis C7B, were proven to possess strong antifungal activities against C. scovillei (Darmadi et al., 2020).
Based on the above-mentioned consideration, this study was done to evaluate the effectiveness of the formulation of P. polymyxa C1 to reduce the disease incidence and intensity of anthracnose on chili pepper as well as to reduce the yield losses of chili cultivar Cabe Besar.
Materials and Methods
Preparation of Formulation
Paenibacillus polymyxa isolate C1 was used to develop the formulation. In general the formulation contained the following components: potato broth 3–5% (v/v), dextrose 1–3% (w/v), yeast extract 0.2–0.4% (w/v), extract of green bean sprouts 2–5% (v/v), spore's suspension of P. polymyxa C1 (106 CFU/ml) 0.2–0.5% (v/v), and distilled water to bring the total volume to one liter. Seven formulations were prepared with the components as shown in Table 1.
Table 1
| Components | Formulations | ||||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
| Potato dextrose (%, v/v) | 3 | 3 | 3 | 4 | 4 | 4 | 5 |
| Dextrose (%, w/v) | 1 | 1 | 2 | 2 | 3 | 3 | 3 |
| Yeast extract (%, w/v) | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 |
| Green bean sprout (%, v/v) | 2 | 3 | 3 | 4 | 5 | 5 | 5 |
| Suspension of P. polymyxa C1 (%, v/v) | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 |
| Distilled water (%, v/v) | 93.7 | 92.7 | 91.5 | 89.5 | 87.5 | 87.3 | 86.3 |
Detail components of formulation of P. polymyxa C1.
All components of the formulation (except for suspension of P. polymyxa C1) were put into 1.000 ml Erlenmeyer, covered with aluminum foil, and autoclaved for 20 min at 121°C. After the formulation reached room temperature, suspension of P. polymyxa C1 was added aseptically in a laminar air flow. Each formulation was made in four replications (four L). These cultures were incubated for 72 h in the dark at room temperature (28 ± 2°C. Population of bacteria in each formulation was counted. A serial of dilution (10−1−10−9) was made using sterile distilled water. Each 0.2 ml of respective dilution was grown in NA medium on Petri dishes and incubated in the dark under room temperature for 48 h. The number of colonies were counted and compared among formulations. The formulation with the highest number of colonies was used for further tests.
Application of Formulation on Chili Pepper Fruit
Formulation F6 was selected and used for testing on fruits of chili pepper cultivar Cabe Besar. This experiment was done to know the optimum concentration to control anthracnose disease. Six levels of concentration (v/v) were tested: Control (0%, with distilled water only) (K0), concentration 1% (K1), 3% (K3), 5% (K5), 7% (K7), and 9% (K9). Each concentration was replicated four times. Thus, there are 24 experimental units consisting of 10 chili pepper fruits placed on plastic trays.
Inoculum Preparation
Colletotrichum scovillei SGCR was provided by the Laboratory of Biopesticide Faculty of Agriculture Udayana University, Bali that previously was identified based on 18S rRNA gene analysis (Khalimi et al., 2019). The fungus was transferred onto slant PDA medium for 7 days and the spores were harvested using sterile distilled water by wire loop. The suspension was then sieved with No. 1 Whatman filter paper to separate the mycelia and hyphae. The spore's density was determined on hemocytometer under light microscope. The spore's density was adjusted into 106 spores/ml by adding sterile distilled water.
Preparation of Chili Pepper Fruits
The mature fruits of chili pepper cultivar Cabe Besar obtained directly from the farmers with similar size and maturity were selected; they were then washed with tap water to remove the surface contaminants and then were washed with sterile distilled water and wiped with sterile tissue paper. All fruits were then surface sterilized using alcohol 70% for about 2 min, then rinsed in sterile distilled water, and wiped with tissue paper. Fruits were divided into 10 fruits then put in a plastic tray (17.5 x 22.5 cm in size) with wet towel tissue on the bottom.
Application of Formulation and Inoculation
The chili pepper fruits were pierced with needles on the upper surface at three sites. Application of formulation was done by spraying the formulation at six levels of concentration: 0% (K0), 1% (K1), 3% (K3), 5% (K5), 7% (K7), and 9% (K9). Each concentration was replicated four times, thus there are 24 experimental units consisting of plastic tray with chili pepper fruits. Thirty minutes after formulation application, all fruit were inoculated with spore's suspension of C. scovillei with 107 spores/ml. Inoculation was done over the upper surface of chili pepper fruit using a mini hand sprayer. After inoculation the trays were covered with cling plastic wrap to maintain the humidity. All trays were incubated in the dark under room temperature (28 ± 2°C) for 5 days. Number of inoculation sites developed into anthracnose lesions were counted and compared among treatments.
Green House Experiment
As the best concentration was determined based on in vivo experiment of chili pepper fruits to be 5%, in the greenhouse experiment the frequency of application was evaluated. Frequencies of application varied from 0 to 5 times, namely 0 (F0 = Control), once (F1), twice (F2), three times (F3), four times (F4), and five times (F5). Each frequency was replicated four times, thus, there are 24 experimental units consisting of 10 polybags of chili pepper plants. All treatment was allocated based on randomized block design (CRD).
Parameters observed in this experiment included disease incidence, disease intensity, and weight of consumable fruits per plant.
Observation of disease intensity on chili pepper fruit was done three days after formulation application according to formula developed by Sinaga (2006), as follows:
Description:
The score for each category is determined as follows:
-
0 = no Symptom Observed
-
1 = Very Light Symptom (0–10% Fruit Surface Destroyed)
-
2 = Light Symptom (10–30% Fruit Surface Destroyed)
-
3 = Medium Symptom (30–50% Fruit Surface Destroyed)
-
4 = Severe Symptom (50–75% Fruit Surface Destroyed)
-
5 = Very Severe Symptom (75–100% Fruit Surface Destroyed)
Data Analysis
All data obtained in this experiment were subjected to the analysis of variance (ANOVA) and followed by Duncan's Multiple Range Test (DMRT) at 5% to determine significant difference. This statistical analysis was done using software SPSS for windows version 17.0 year 2009.
Results
Among seven formulations tested, formulation F6 showed the highest number of P. polymyxa C1 colonies. This formulation containing 4% (v/v) potato broth, 3% (w/v) dextrose, 0.3% (w/v) yeast extract, 5% (v/v) green bean sprout extract, 0.4% (v/v) suspension of P. polymyxa C1, and 87.3% (v/v) distilled water resulted in 26.25 x 107 CFU/ml superior among others, as presented in Table 2.
Table 2
| No. | Formulation |
Density ± Sd
(x 10 7 CFU/ml) |
|---|---|---|
| 1 | F1 | 10.98 ± 2,23* |
| 2 | F2 | 11.77 ± 1,78 |
| 3 | F3 | 12.89 ± 1,07 |
| 4 | F4 | 16.47 ± 2,08 |
| 5 | F5 | 20.55 ± 1,98 |
| 6 | F6 | 26.25 ± 2,02 |
| 7 | F7 | 17.87± 2,37 |
Population of Paenibacillus polymyxa C1 in respective formulation.
Sd, Standard deviation. *Average of 5 (five) replications.
Effectiveness of F6 to Control Anthracnose Symptom on Chili Pepper Fruit
Six levels of concentration of formulation F6 were evaluated for their effectiveness to suppress anthracnose symptom in vivo on fruits of chili pepper cultivar Cabe Besar. Results of this experiment showed that concentration at 5, 7, and 9% (v/v) totally inhibited the development of anthracnose symptom on Cabe Besar as presented in Table 3. This result suggested that formulation F6 at a concentration of 5% (v/v) is enough to suppress the growth of C. scovillei and the development of anthracnose disease.
Table 3
| No. | Treatment | Number of anthracnose lesions ± Sd* | Percentage of inhibitory activity against K0 (Control) |
|---|---|---|---|
| 1 | K0 | 11.75d**± 2.87 | - |
| 2 | K1 | 8.50c ± 2.03 | 27.66 |
| 3 | K3 | 1.75b ± 0.87 | 85.11 |
| 4 | K5 | 0a ± 0.00 | 100 |
| 5 | K7 | 0a ± 0.00 | 100 |
| 6 | K9 | 0a ± 0.00 | 100 |
Effects of concentration of formulation F6 on the number of anthracnose lesions on chili pepper fruit cultivar Cabe Besar in vivo.
Sd, Standard deviation.
*Average of 4 (four) replications.
**Values followed by the same letter did not significantly different according to Duncan's Multiple Range Test at 5% level.
Effectiveness of Formulation in a Green House
Result of the experiment under greenhouse condition showed that treatment with formulation F6 significantly (p < 0.05) reduced the disease incidence, disease intensity, and consumable chili pepper fruits per plant. Treatment of five times application (F5) showed the disease incidence to be only 1.88% with disease intensity at 2.12%, but it is not significantly different from the treatment of F4 (four applications). While in the Control (F0) the disease incidence and intensity were 70.68 and 69.80% respectively (Tables 4, 5). The frequency of application also significantly (p < 0.05) affected the weight of healthy fruits per plant. The weight of healthy fruits on Control (F0) was only 43.93 g/plant while for that of F5 was 476.98 g/plant (Table 6). This result suggested that the formulation of P. polyxyxa C1 effectively reduced the disease incidence and intensity on the one hand, and on the other obviously avoided yield losses.
Table 4
| No. | Treatment | Disease incidence (%) + Sd* | Inhibitory activity compared to F0 (%) |
|---|---|---|---|
| 1 | F0 | 70.68a**± 12.24 | - |
| 2 | F1 | 49.20b ± 10.76 | 30.38 |
| 3 | F2 | 31.28c ± 7.89 | 55.73 |
| 4 | F3 | 18.43d ± 3.45 | 68.70 |
| 5 | F4 | 4.96e ± 1.98 | 92.98 |
| 6 | F5 | 1.88e ± 0.87 | 97.34 |
Effect of frequency of application of F6 to the incidence of anthracnose disease on chili pepper cultivar Cabe Besar.
Sd, Standard deviation.
*Average of 4 (four) replications.
**Values followed by the same letter did not significantly different according to Duncan's Multiple Range Test at 5% level.
Table 5
| No. | Treatment | Disease intensity (%) ± Sd* | Inhibitory activity compared to F0 (%) |
|---|---|---|---|
| 1 | F0 | 69.80a**± 18.17 | - |
| 2 | F1 | 45.85b ± 16.78 | 34.31 |
| 3 | F2 | 29.49c ± 8.65 | 57.75 |
| 4 | F3 | 12.49d ± 5.42 | 82.11 |
| 5 | F4 | 3.11e ± 2.74 | 95.54 |
| 6 | F5 | 2.12e ± 1.88 | 96.96 |
Effect of frequency of application of F6 to the disease intensity on chili pepper cultivar Cabe Besar.
Sd, Standard deviation.
*Average of 4 (four) replications.
**Values followed by the same letter did not significantly different according to Duncan's Multiple Range Test at 5% level.
Table 6
| No. | Treatment | Weight of healthy fruits (g/plant) ± Sd* | Estimated yield (ton/ha)** |
|---|---|---|---|
| 1 | F0 | 43.93a***± 9.26 | 1.05 |
| 2 | F1 | 107.15b ± 24.47 | 2.55 |
| 3 | F2 | 210.10c ± 23.78 | 5.00 |
| 4 | F3 | 283.92d ± 32.46 | 6.76 |
| 5 | F4 | 447.56e ± 52.78 | 10.66 |
| 6 | F5 | 476.98e ± 48.93 | 11.36 |
Effect of frequency of application of F6 to the weight of healthy fruits/plant on chili pepper cultivar Cabe Besar.
Sd = Standard deviation.
*Average of 4 (four) replications.
**Planting spacing was 60 x 70 cm, with estimated number of plants 23,809/ha.
***Values followed by the same letter did not significantly different according to Duncan's Multiple Range Test at 5% level.
There was a close relationship between the frequency of application and disease intensity, and the disease intensity with the weight of healthy fruits per plant. The coefficient determination for those relationships were 0.929 and 0.905 respectively (Figures 1, 2).
Figure 1
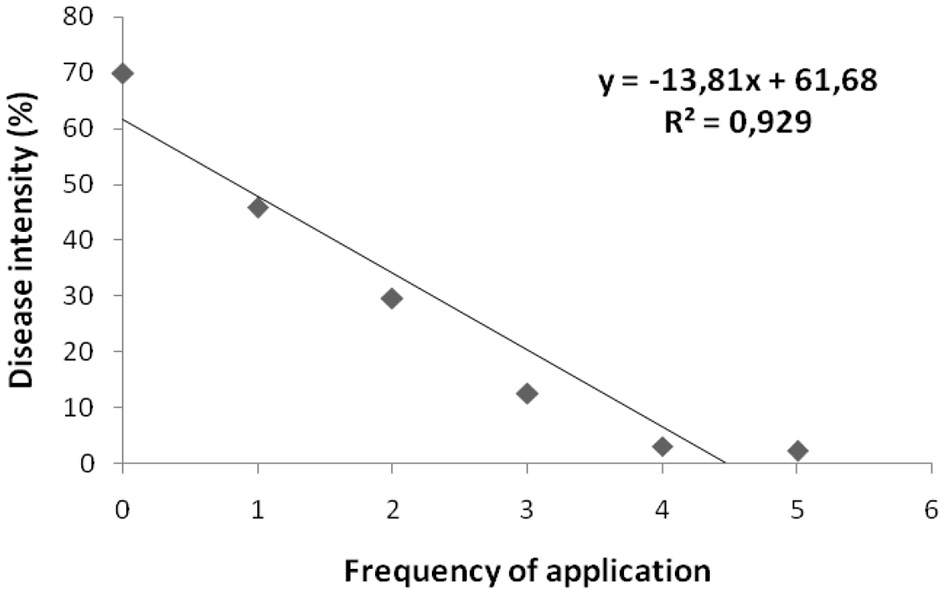
Relationship between frequency of application of formulation F6 with the disease intensity on chili pepper cultivar Cabe Besar.
Figure 2
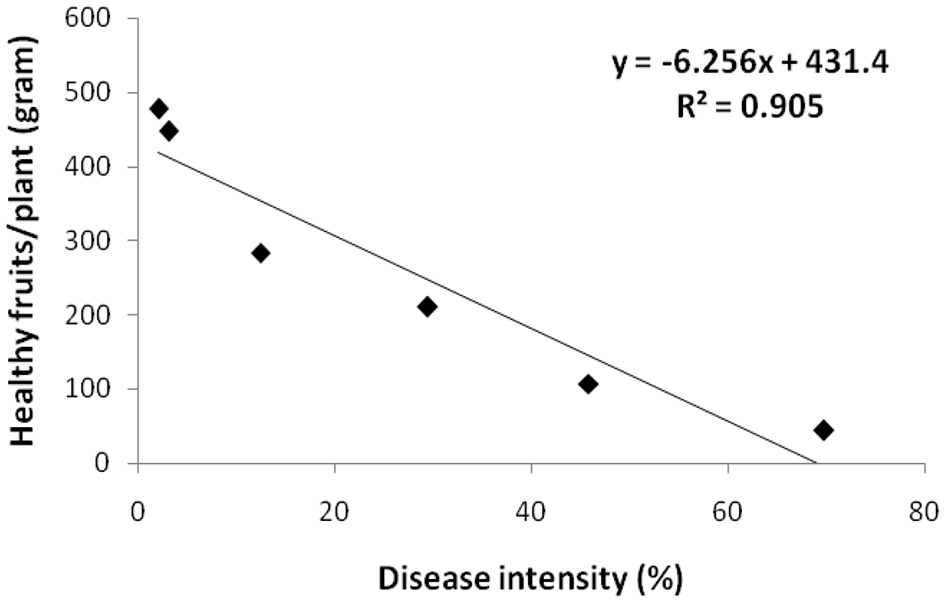
Relationship between disease intensity with the weight of healthy fruits per plant on chili pepper cultivar Cabe Besar.
Discussion
Paenibacillus polymyxa C1 was proven to be a potential bioagent against C. scovillei, the prominent cause of anthracnose disease on chili pepper in Bali, Indonesia (Khalimi et al., 2019). In the present study the effectiveness of a formulation containing suspension of P. polymyxa C1 was tested in a greenhouse to control anthracnose disease on chili pepper cultivar Cabe Besar. Some species of microbes have been proven to possess potential antagonistic activities against plant fungal pathogens. Several of them have been successfully used as biocontrol agents against plant fungal diseases (Suprapta, 2012; Widnyana et al., 2013; Parwati et al., 2014; Suprapta et al., 2014; Guo et al., 2015; Hudge, 2015; Adame-Garcia et al., 2016).
In our previous study we detected three compounds in the cell-free filtrate of P. polymixa C1: 3-hydroxy-2-butanone, 2, 3-Butanediol, and 2, 3-Butanediol (Darmadi et al., 2020). Other researchers proved that 3-hydroxy-2-butanone is a volatile compound with antifungal activity (Lim et al., 2017). Arrebola et al. (2010) also proved that 3-hydroxy-2-butanone is a volatile compound with antifungal activity against Penicillium crustosum. A compound 2,3-butanediol extracted from a culture of P. polymyxa was proven to induce systemic acquired resistance against Phytophthora parasitica var. nicotianae (Park et al., 2018). Scanning electron microscopy showed that serious damage on the mycelia of C. scovillei was observed. Wrinkles were observed on the mycelia of C. scovillei grown side by side with P. polymyxa C1, whereas no such wrinkle was observed on C. scovillei grown alone (Darmadi et al., 2020). A study done by Shahbazi et al. (2014) showed that antagonistic Streptomyces rochei strain P14 caused hyphal tip lyses, folding back, stunted mycelium, disintegrated hyphae, and curling of hyphae on Colletotrichum, the cause of anthracnose on chili pepper. This strain, P14, produced chitinase (Shahbazi et al., 2014).
In this study the best formulation was F6 containing 4% (v/v) potato broth, 3% (w/v) dextrose, 0.3% (w/v) yeast extract, 5% (v/v) green bean sprout extract, 0.4% (v/v) suspension of P. polymyxa C1, and 87.3% (v/v) distilled water. This formulation resulted in the highest population of bacteria. In a greenhouse test on chili pepper cultivars Cabe Besar, treatment with formulation containing suspension of P. polymyxa C1 effectively reduced the disease incidence and intensity, as well as increased the weight of healthy fruit per plant. A close relationship was observed between frequency of application (1 to 5 times) and the disease intensity of anthracnose on chili fruit with coefficient of determination (R2) = 0.929, suggesting that the higher frequency of application resulted in less disease intensity. A close relationship was also observed between disease intensity with number of healthy fruits per plant, suggesting that disease intensity is the main factor influencing number of healthy fruits per plant. Although the test was done under greenhouse conditions, our present results revealed that formulation F6 is promising for controlling the anthracnose disease on chili pepper. This result is similar to the result obtained by Shahbazi et al. (2014) where Streptomyces rochei strain P14 significantly decreased disease severity and increased fresh fruit weight of chili when compared with control (treatment with Colletotrichum only). The average disease severity reduction from 75% (on control) to 10% (treatment with strain P14). Raghunandan et al. (2019) tested the efficacy of antagonistic yeasts (Pichia guilliesmondii Y-12, Hanseniaspora uvarum Y-73), fungus (Trichoderma asperellum Th-3), and bacterium (Pseudomonas fluorescens Pf-1) to control fruit rot/anthracnose disease on chili under field conditions over two seasons. All bio-agents significantly reduced the disease intensity and increased the fruit yield per hectare. The disease intensities on treated plants varied from 13.42 to 16.38%, while fruits yield obtained on plants treated with bio-agents varied from 7.36 to 80.89 ton/ha, while for that of control was only 4.35 ton/ha.
Several other bioagents have been studied in relation to their antifungal activity against Colletotrichum as well as their efficacy to control anthracnose disease on chili pepper. The use of Trichoderma viridae and Pseudomonas fluorescens individually or in combination significantly lowers the anthracnose disease incidence (Reddy et al., 2011). An antagonistic yeast, Pichia guilliermondii strain R13, was reported to reduce the disease incidence of anthracnose in chili pepper incited by C. truncatum as low as 6.5% (Chanchaichaovivat et al., 2008). Kim and Yun (2011) reported that under greenhouse conditions, treatment with Myxococcus sp. KYC 1126 obviously lowered the incidence of anthracnose in hot pepper. The incidence of anthracnose in control seedlings was 74%, while for treated seedlings was only 29%. Prihatiningsing et al. (2019) proved that microencapsulates containing Bacillus subtilis B298 reduced anthracnose disease intensity by 48%. This microencapsulated B. Subtilis B298 is considered to induce systemic resistance on chili as total phenol in the treated plants increased.
In the present study it is proven that a formulation containing suspension of P. polymyxa C1 significantly reduced both disease incidence and disease intensity on chili pepper, and thus can be considered an environmentally friendly measure to protect chili pepper from anthracnose disease.
Conclusion
This study confirmed that treatment with a formulation containing suspension of P. polymyxa C1 effectively reduced the disease incidence, disease intensity, and the yield loss of chili pepper cultivar Cabe Besar. Alose relationship was observed between the number of applications with disease intensity, and the disease intensity with the number of healthy fruits. These results suggested that the formulation of P. polymyxa C1 effectively controlled the anthracnose disease on chili pepper cultivar Cabe Besar, and can be recommended for field testing before it is used widely in the field.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Acknowledgments
I wish to express a high appreciation to the Institute of Research and Community Services, Udayana University for providing the research grant in the fiscal year 2020 under the scheme of Penelitian Percepatan Guru Besar (P2GB).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Adame-Garcia J. Luna-Rodriguez M. Iglesias-Andreu L. G. (2016). Vanilla rhizobacteria as antagonists against Fusarium oxysforum f.sp. vanillae. Int. J. Agric. Biol. 18, 23–30. 10.17957/IJAB/15.0053
2
Anwaar H. A. Ali S. Sahi S. T. Siddiqui M. T. (2019). Evaluating the antagonistic role of fungal endophytes against leaf rust of wheat caused by Puccinia recondita. Int. J. Agric. Biol. 21, 333–337. 10.17957/IJAB/15.0898
3
Arrebola E. Sivakumar D. Korsten L. (2010). Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol. Control. 53, 122–128. 10.1016/j.biocontrol.2009.11.010
4
Asare-Badiako E. Addo-Quaye A. Boakye B. Sarbah J. M. Asante P. Dorm E. (2015). Incidence and severity of viral and fungal diseases of Chili pepper (Capsicum frutescens) in some districts in Chana. Int. J. Plant and Soil Sci. 1, 147–159. 10.9734/IJPSS/2015/16830
5
Chanchaichaovivat A. Panijpan B. Ruenwongsa P. (2008). Putative modes of action of Pichia guilliermondii strain R13 in controlling chili anthracnose after harvest. Biol. Control. 47, 207–215. 10.1016/j.biocontrol.2008.07.018
6
Darmadi A. A. K. Suprapta D. N. Khalimi K. (2020). Potential antagonistic rhizobacteria to control Colletotrichum scovillei, the cause of anthracnose disease in chili pepper. Biodiversitas21, 2727–2734. 10.13057/biodiv/d210648
7
Diao Y. Z. Zhang C. Liu F. Wang W. Z. Liu L. Cai L. et al . (2017). Colletotrichum species causing anthracnose disease of chili in China. Persoonia38, 20–37. 10.3767/003158517X692788
8
Fu R. Zhang H. Chang H. Zhang F. Chen W. (2020). Evaluation of antifungal mechanism of Bacillus amyloliquefaciens BA-16-8. Int. J. Agric. Biol.23, 405–408. 10.17957/IJAB/15.1302
9
Guo J. H. Jiang C. H. Xie P. Huang Z. Y. Fa Z. H. (2015). The plant healthy and safety guards plant growth promoting rhizobacteria (PGPR). Transcriptomics3:109. 10.4172/2329-8936.1000109
10
Hudge B. V.. (2015). Management of damping-off disease on soybean caused by Pythium ultimum Trow. Int. J. Curr. Microbiol. Appl. Sci. 4, 799–808.
11
Khalimi K. Darmadi A. A. K. Suprapta D. N. (2019). First report on the prevalence of Colletotrichum scovillei associated with anthracnose on chili pepper in Bali, Indonesia. Int. J. Agric. Biol. 22, 363–368. 10.17957/IJAB/15.1072
12
Kim S. T. Yun S. C. (2011). Biocontrol with Myxococcus sp. KYC 1126 against anthracnose in hot pepper. Plant Pathol. J. 27, 156-163. 10.5423/PPJ.2011.27.2.156
13
Lim S. M. Yoon M. Y. Choi G. J. Choi Y. H. Jang K. S. Shin T. S. et al . (2017). Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus vezelensis G341 against various phytopathogenic fungi. Plant Pathol. J. 33, 488–498. 10.5423/PPJ.OA.04.2017.0073
14
Mahartha K. A. Suprapta D. N. (2018). Efficacy of Enterobacter cloacae KtB3 to control damping-off disease on soybean caused by Sclerotium rolfsii. Int. J. Agric. Biol. 20, 871–876. 10.17957/IJAB/15.0578
15
Mauch F. Mauch-Mani B. Boller T. (1988). Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and 3-1,3 glucanase. Plant Physiol.88, 936–942. 10.1104/pp.88.3.936
16
Nowak-Thompson B. Gould S. J. Kraus J. Loper J. E. (1994). Production of 2,4-diacetylphloroglucinol by the biocontrol agent Pseudomonas fluorescens Pf-5. Can. J. Microbiol. 40, 1064–1066. 10.1139/m94-168
17
Park H. G.. (2007). Problems of anthracnose in pepper and prospects for its management, in Abstracts of the First International Symposium on Chilli Anthracnose, eds D. G. Oh, K. T. Kim (Seoul: National Horticultural Research Institute, Rural Development of Administration), 19.
18
Park K. Y. Seo S. Y. Oh B. R. Seo J. W. Kim Y. J. (2018). 2, 3-butanediol induces systemic acquired resistance in the plant immune response. J. Plant Biol. 61, 424–434. 10.1007/s12374-018-0421-z
19
Parwati G. A. K. C. Khalimi K. Adiartayasa W. (2014). Efficacy test of Pantoea agglomerans GTA24 formulation in controlling damping off disease on soybean caused by Sclerotium rolfsii. Agroekoteknologi Tropika3, 218–229 (in Indonesian language).
20
Poonpolgul S. Kumphai S. (2007). Chilli pepper anthracnose in Thailand. Country report, in Abstracts of the First International Symposium on Chili Anthracnose, eds D. G. Oh, K. T. Kim (Suwon: National Horticultural Research Institute, Rural Development of Administration, Republic of Korea), 23.
21
Prihatiningsing N. Djatmiko H. A. Erminawati W. (2019). Bio-management of anthracnose disease in chili with microencapsulates containing Bacillus subtilis B298. IOP Conf. Series: Earth Environ. Sci.250:012041. 10.1088/1755-1315/250/1/012041
22
Raghunandan B. L. Patel M. V. M. N Patel M. N. Mehta D.M. (2019). Bio-efficacy of different biological agents for management of chili fruit rot/anthracnose disease. J. Biol. Control.33, 163–168. 10.18311/jbc/2019/22716
23
Reddy Y. N. P. Jakhar S. S. Dahiya O. S. (2011). Efficiency of bio-fungicides (Trichoderma spp. and Pseudomonas fluorescens) on seedling emergence, vigor and health of infected chili seeds (Capsicum annuum) by anthracnose fungus Colletotrichum gloeosporioides (Penz.) of chili. Arch. Phytopathol. Plant Prot.44, 287–297.
24
Saharan B. S. Nehra V. (2011). Plant growth promoting rhizobacteria: a critical review. LSMR.21, 1–30.
25
Saleem A. R. Bangash N. Mahmood T. Khalid A. Centritto M. Siddique M. T. (2015). Rhizobacteria capable of producing ACC deaminase promote growth of velvet bean (Mucuna pruriens) under water stress condition. Int. J. Agric. Biol. 17, 663–667. 10.17957/IJAB/17.3.14.788
26
Sarfraz S. Sahi S. T. Ali M. A. Khan S. H. Faure D. (2019). Antagonistic potential of N-acyl-homoserine lactone degrading Bacillus species for controlling Pectobacterium based infections in potato. Int. J. Agric. Biol. 22, 639–646. 10.17957/IJAB/15.1110
27
Shahbazi P. M. Y Musa M. Y. Tan G. Y. A. Avin F. A. Teo W. F. A. et al . (2014). In vitro and in vivo evaluation of Streptomyces suppression against anthracnose in chili caused by Colletotrichum. Sains Malaysiana43, 697–705.
28
Shiotsu F. Sakagami N. Asagi N. Suprapta D. N. N Agustiani N. et al . (2015). Initiation and dissemination of organic rice cultivation in Bali, Indonesia. Sustainability.7, 5171–5181. 10.3390/su7055171
29
Sinaga M. S.. (2006). Fundamental of Plant Diseases. Bogor: Penebar Swadaya Publisher. Indonesia (in Indonesian language).
30
Soesanto L.. (2008). Introduction to Biological Control of Plant Diseases. Jakarta: Raja Grafindo Persada.
31
Suprapta D. N.. (2012). Potential of microbial antagonists as biocontrol agents against plant fungal pathogens. J. ISSAAS. 18, 1–18.
32
Suprapta D. N. Quintao V. Khalimi K. (2014). Effectiveness of rhizobacteria to reduce rice blast disease intensity. J. Biol. Agric. Healthcare4, 35–41.
33
Widnyana I. K. Suprapta D. N. Sudana I. M. Temaja I. G. R. M. (2013). Pseudomonas alcaligenes, potential antagonist against Fusarium oxysforum f.sp. lycopersici the cause of Fusarium wilt disease on tomato. J. Biol. Agric. and Healthcare. 3, 163–169.
Summary
Keywords
biocontrol, anthracnose disease, chili pepper, formulation, Paenibacillus polymyxa C1
Citation
Suprapta DN (2022) Biocontrol of Anthracnose Disease on Chili Pepper Using a Formulation Containing Paenibacillus polymyxa C1. Front. Sustain. Food Syst. 5:782425. doi: 10.3389/fsufs.2021.782425
Received
24 September 2021
Accepted
08 December 2021
Published
10 January 2022
Volume
5 - 2021
Edited by
Xingyuan Men, Shandong Academy of Agricultural Sciences, China
Reviewed by
Yong Liu, Hunan Academy of Agricultural Sciences (CAAS), China; Durgesh Kumar Jaiswal, Savitribai Phule Pune University, India
Updates
Copyright
© 2022 Suprapta.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dewa Ngurah Suprapta ngurahsuprapta@unud.ac.id
This article was submitted to Agroecology and Ecosystem Services, a section of the journal Frontiers in Sustainable Food Systems
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.