- 1Indian Institute of Public Health-Delhi, Public Health Foundation of India, Gurgaon, India
- 2Department of Food and Nutrition, Lady Irwin College, University of Delhi, New Delhi, India
India is endowed with several indigenous foods (IFs), that hold special cultural significance among local and ethnic caommunities, yet no attempts have been made till date to systematically compile their nutritive values. As per FAO's recent mandate on creation of “Global-Hub on Indigenous Food Systems,” IFs have received renewed global recognition for their potential to contribute to improved food security while enhancing biodiversity across the world. Hence, the useful properties of wild IFs require proper study and documentation in order to bridge the gap between scientific evidence generation and indigenous peoples' ancestral knowledge. For this purpose, we conducted a literature search in two scientific databases: PubMed and Google Scholar, between July 2020 and December 2021, to identify studies reporting nutritive values and/or antinutrient content of IFs (not included in Indian food composition database), consumed by Indian indigenous communities. A total of 52 Indian research articles were included, from which data was selected and extracted, to create a compendium on nutrient (n = 508) and antinutrient (n = 123) content of IFs, followed by computation of antinutrient-to-mineral molar ratios for 98 IFs to predict their mineral bioavailability. Maximum nutritive values were available for green leafy vegetables (n = 154), followed by other vegetables (n = 98), fruits (n = 66), cereals (n = 63), roots & tubers (n = 51) and nuts and legumes (n = 36). Several IFs seen to have better nutritional content than conventional foods and were found to be rich (i.e., >20% Indian recommended dietary allowances per reference food serve) in iron (54%), calcium (35%), protein (30%), vitamin C (27%), vitamin A (18%), zinc (14%) and folate (13%). Some IFs displayed high levels of antinutrients, however, anti-nutrient-to-mineral molar ratios were found to be low (for mainly leafy vegetables, other vegetables, and roots and tubers), thus indicating high mineral bioavailability. Hence, efforts are desirable to encourage the inclusion of these nutritionally superior IFs into the usual diets of indigenous communities. The IF database collated in our review can serve as a resource for researchers and policymakers to better understand the nutritional properties of region-specific IFs and promote them through contextual food-based interventions for improved dietary quality and nutrition outcomes in indigenous population of India.
Introduction
Traditional food systems, which include all the foods available from local natural resources, are unprocessed, small-scale and low-technology human managed biophysical systems, with short farm-to-plate value chains (Kuhnlein et al., 2006; Burlingame et al., 2012; Dubé et al., 2014). These food systems consist of harvesting, foraging, hunting, fishing and gathering of plant and animal foods, and are often shaped by diverse eating practices, ecological features, geographical variations and socio-cultural as well as historical experiences (Kuhnlein et al., 2013). Indigenous peoples who form distinct social and cultural groups, and have strong links with their territories and surrounding natural resources, are often the sole custodians of traditional food systems and the ancestral ecological knowledge associated with them (Kuhnlein, 2009). The food systems of indigenous people have sustained them for thousands of generations, generating food in harmony while preserving the local biodiversity. Indigenous foods or IFs, an integral part of traditional food systems, are central to indigenous people's culture and identity and contribute to their physical, mental, spiritual and economic wellbeing. These foods, include wild, domesticated or cultivated plant, animal and/or fungi species, that are derived from surrounding natural environment, and act as a crucial source of nourishment, and material subsistence for people belonging to the indigenous communities (Kuhnlein et al., 2013; Settee and Shukla, 2020).
During the past decade, IFs have received renowned global recognition for their potential to contribute to improved food security while enhancing biodiversity across the world. They are known to be of high nutritive value and their potential contribution to improved health and nutrition is significant (Kuhnlein et al., 2019). Research in Sub-Saharan African and South American countries have reported better intake of protein, fiber, vitamin A, iron, calcium and riboflavin among people consuming IFs (Akinola et al., 2020; Lugo-Morin, 2020). Further, ample evidence from the literature suggests that indigenous crops have a critical role in addressing malnutrition and hunger (Shackleton et al., 1998; Steyn et al., 2001; Yiridoe and Anchirinah, 2005; Modi et al., 2006) and may contribute to more diverse diets at both household and individual levels (Trichopoulou et al., 2006; Frison et al., 2011; Powell et al., 2011; Burlingame et al., 2012). Realizing the extreme potential of IFs in contributing to optimal health and nutrition, Food and Agriculture Organization (FAO) in 2018, collaborated with other member organizations, to create a “Global-Hub on Indigenous Food Systems,” which aims to preserve and promote the indigenous peoples' food systems, through creation of knowledge bearer's platform, online database, technical advice and creation of synergies. This platform further proposes to bring together universities, research centers, indigenous organizations and FAO Members around a common platform for exchange of evidence-based knowledge to inform better nutrition and sustainable practices and policies worldwide (FAO, 2018).
India, a country of 1.2 billion people, is home to 705 indigenous communities (Census, 2011), who have their distinct food systems with several IFs adding to dietary diversity (Misra et al., 2008; Ghosh-Jerath et al., 2020a). These communities are locally recognized as scheduled tribes and live in environments characterized by defined regions with specific food habits, dialects, cultural homogeneity, and a unified social organization (Ministry of Tribal Affairs, Government of India, 2020). Many of these indigenous communities often reside in areas surrounded by lush green and dense forests, hills and rivers, and are hence exposed to a rich biodiversity, which they manage and utilize for food, livelihood and income generation (MoHF and Ministry of Tribal Affairs, 2019). They produce and collect local IFs from several food sources in the form of edible greens (leaves, stems, shoots, including marine algae); root vegetables (including true roots and underground storage organs like bulbs, corms, tubers and rhizomes); fleshy fruits (berries, pomes, drupes); edible wild mushrooms, grains, seeds, and nuts, as well as wild-harvested fish, insects and game (Agrahar-Murugkar and Subbulakshmi, 2005; Chyne et al., 2019; Ghosh-Jerath et al., 2020a, 2021). Other Indian indigenous communities residing in deserts and barren areas also skillfully manage their natural resources, and utilize a wide range of edible herbs, shrubs and trees that are well-adapted to some of the most extreme weather conditions of the hot desert (Mukhopadhyay, 2008; Bhansali, 2011). Many of the IFs accessed by indigenous communities, are sourced from the natural environment, i.e., wild (forests, disturbed habitats, open pastures, ponds and rivers) and cultivated food environment (fields, orchards and backyard gardens), are deemed to be nutritious, palatable and easily harvested, and are often adapted to unique climatic and environmental conditions (Agrahar-Murugkar and Subbulakshmi, 2005; Ghosh-Jerath et al., 2020a, 2021).
However, despite having access to rich dietary diversity from these sources, indigenous communities in India consume poor quality diets and display high levels of malnutrition. Almost one-third of the indigenous women in India are underweight while 4.7 million indigenous children under 5 years of age suffer from chronic nutrition deprivation, with 43% children stunted, 27% wasted and 45.3% underweight (NFHS-4, 2015). More than half the women and children are anemic, and about 40% children are folate deficient while almost one-fifth children suffer from vitamin A, zinc and vitamin B12 deficiencies (NFHS-4, 2015). Furthermore, with urbanization and ongoing nutrition transition, the agricultural and natural resource management practices of indigenous communities, i.e., their traditional ecological knowledge (TEK) is becoming lost, a trend that may be responsible for reduced IF consumption and poorer nutrition (Kuhnlein et al., 2004; Ghosh-Jerath et al., 2021). Hence, there exists an urgent need to document this TEK, explore the nutrient potential of community specific IFs and promote their consumption which can potentially alleviate malnutrition and hunger in these vulnerable indigenous communities.
Although many studies have listed several IFs of indigenous communities in India, but to date no attempts have been made to systematically compile the nutritive values of these foods. The useful properties of wild IFs requires proper study and documentation in order to bridge the gap between scientific evidence generation and indigenous peoples' ancestral knowledge (Alexander et al., 2019). Moreover, there is limited information available on anti-nutrient content of IFs, which may have an impact on the nutrient bioavailability of these foods. Therefore, the objective of this review is to compile the available information on different IFs of indigenous communities across different states in India, and create a compendium with specific information on their (a) macro- and micro-nutrient contents (b) content of anti-nutritional components and (c) mineral bioavailability.
Methods
Literature Search
The data sources for this review included published research articles and reports that were selected through two phases. In the first phase, a literature search was conducted between July, 2020 and February 2021 in well-known databases such as PubMed and Google Scholar. Searches were performed using various combinations of keywords like “nutritive value,” “nutrition,” “anti-nutritional factors,” “indigenous foods,” “traditional foods,” “wild foods,” “tribal communities,” and “India” to identify relevant studies. Articles were scanned and checked against the inclusion criteria, duplicate citations were removed and those that met the inclusion criteria were read and assessed for inclusion. In the second phase, by utilizing snowballing technique, additional relevant studies were identified from the reference lists of all included papers identified in the initial search. Figure 1 presents the step-wise methodological approach followed in selection of articles for the review.
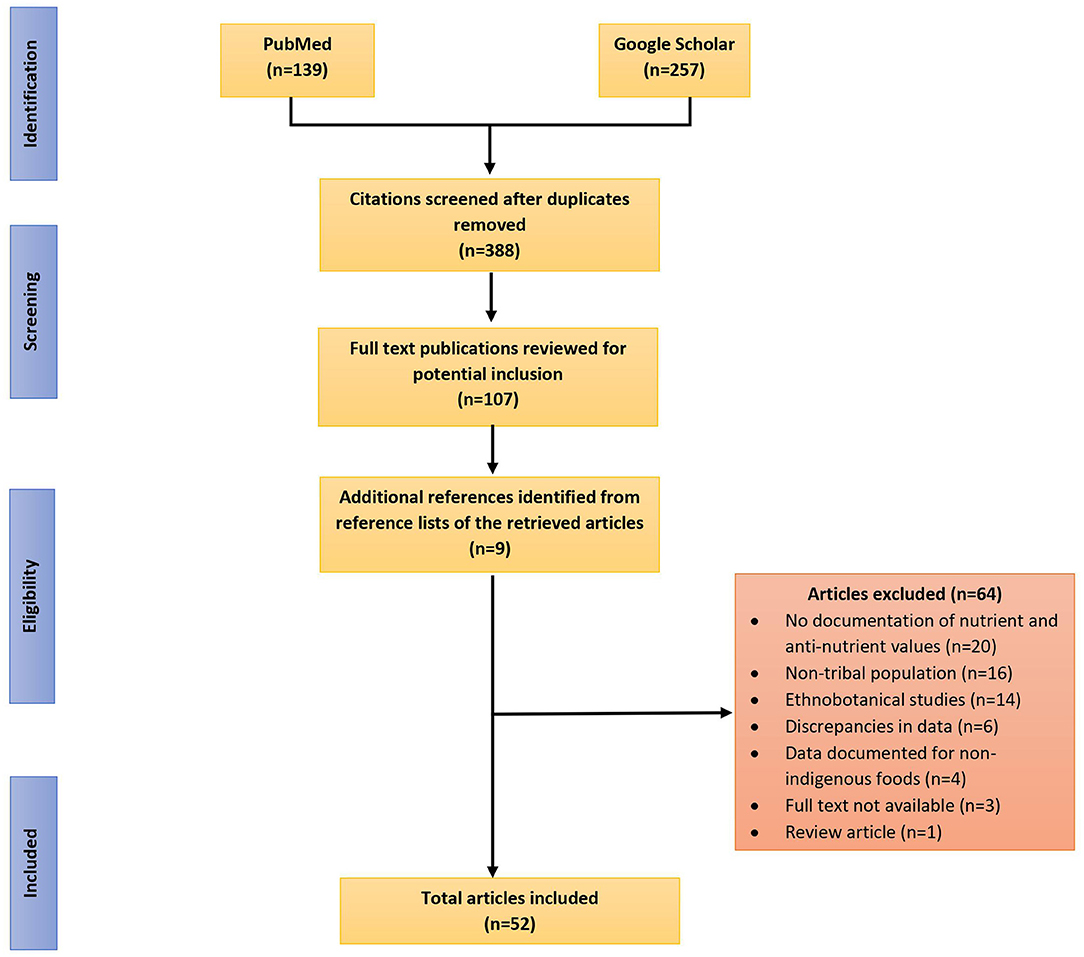
Figure 1. Flow diagram reporting the screening and selection process used in identification of research studies reporting nutritive values and antinutritional content of indigenous foods consumed by and known to indigenous communities of India.
Selection Criteria
In the present review, we have used the term “Indigenous foods or IFs” to denote foods that are native or have been introduced in the region a long time back, and are accessed from the natural food environment (i.e., either wild or cultivated food environment). Considering the globalization of current food systems, these foods may also be purchased from informal built food environment, i.e., local markets or small shops (Kuhnlein, 2009; Kasimba et al., 2019; Settee and Shukla, 2020). Based on this definition, we selected articles following these eligibility criteria: (i) original full-text articles in English, published till 1st February, 2021, (ii) research studies conducted in Indian states and/or Union Territories (UT), and (iii) research studies reporting nutritive values and/or anti-nutrient content of IFs consumed and known to indigenous communities [scheduled tribes as per Article 342 of Indian constitution (Ministry of Tribal Affairs, Government of India)]. We defined nutrient composition data as inclusive of macronutrients (energy, protein, fat) and/or micronutrients (vitamins A, B1, B2, B3, B6, B9, C, iron, calcium, and zinc) while, anti-nutrient data was defined as inclusive of different anti-nutritional factors (tannins, saponins, phytates, oxalates, total free phenols, trypsin inhibitor, amylase inhibitor and/or hydrogen cyanide).
Data Extraction and Preparation
After reviewing different scientific sources based on the selection criteria, a final inventory of 52 articles were prepared. The data and units were extracted from each study for all macronutrients, micronutrients and anti-nutrients, critically reviewed and arranged systematically according to the food groups. Indian food composition table (IFCT) prepared by National Institute of Nutrition (Longvah et al., 2017) was also referred to document additional nutrient and anti-nutrient composition data (if available) on IFs that were mentioned in the included research articles. All information on IFs such as their scientific names, vernacular as well as common names were gathered and compiled. The scientific names of the foods were verified from The Plant List (2020). The foods with nutrient content (protein and micronutrients) higher than 20% of Recommended dietary allowances (RDA) for moderately active Indian adult (per reference amount) (ICMR-NIN, 2020) were categorized as “rich” sources of nutrients (United States Food and Drug Administration, 2019). The nutrient and anti-nutrient data from included research studies were further utilized to compute the anti-nutrient-to-mineral molar ratio indices. For this purpose, molar ratio indices of phytate-to-minerals and oxalate-to-minerals [i.e., phytate/calcium (Phy/Ca), phytate/iron (Phy/Fe), phytate/zinc (Phy/Zn), phytate x calcium/zinc (Phy x Ca/Zn), oxalate/calcium (Ox/Ca)] were obtained after dividing the mole of phytate and oxalate with the mole of minerals (Morris and Ellis, 1989; Norhaizan and Nor Faizadatul Ain, 2009). Standard critical values of molar ratio indices defined by existing literature were used [i.e., > 0.24 for Phy/Ca (Morris and Ellis, 1985), >1 for Phy/Fe (Hallberg et al., 1989), >15 for Phy/Zn (Turnlund et al., 1984; Sandberg et al., 1987; Morris and Ellis, 1989), >200 for Phy x Ca/Zn (Mills et al., 1985; Bindra et al., 1986) and > 2.5 for Ox/Ca (Mills et al., 1985; Bindra et al., 1986)] to predict low mineral bioavailability in IFs.
Results
Description of Studies
Out of 52 studies included in the review, majority of the studies were carried out in North-east India (n = 25), followed by southern (n = 11), eastern (n = 10), western (n = 3), central (n = 2) and northern (n = 1) regions of the country. Estimation of macronutrients and/or micronutrients was reported in all the studies while only 11 studies reported the estimation of anti-nutritional factors (ANFs) in IFs. Out of 52 studies, 48 studies reported the methodology of food sample collection; the IFs were mostly collected from areas of natural vegetation (such as farms, forests, near river banks etc.), were cleaned/washed to remove dirt and packed for further analysis. The methodology of taxonomic identification was available in 40 studies; food samples were either prepared as herbariums and sent to botanical/zoological laboratories for identification or identified by a taxonomist at the field sites and/or with help of photographs. All the studies followed standard analytical procedures for determination of proximate compositions, micronutrients, as well as anti-nutrient components (details in Supplementary Table 1).
Nutritive Values of Indigenous Foods of India
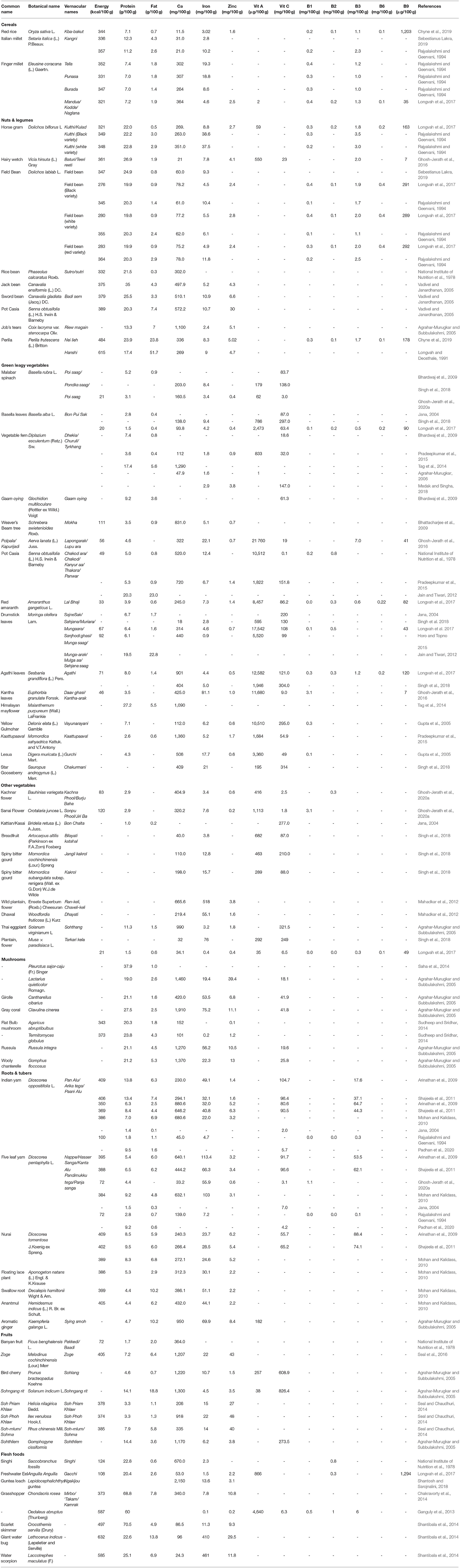
In the present review, we have compiled the nutritive values of 508 IFs, consumed by indigenous communities across 15 states and 1 UT of India. Majority of IFs with their nutritive values estimated, were documented from Jharkhand (28%), followed by Meghalaya (15%) and Kerala (11%). These IFs have been classified into the following categories: (a) 63 cereals (b) 36 nuts and legumes, (c) 154 green leafy vegetables (GLVs), (d) 98 other vegetables (including mushrooms), (e) 51 roots & tubers, (f) 66 fruits, (g) 40 flesh foods (including livestock and wild game). Supplementary Data Sheet 1 presents the common names of these IFs, with additional information on their local and scientific names, edible part(s) consumed, indigenous communities accessing the foods and their state of residence. The nutritive values of IFs have been compiled food group wise in Supplementary Table 2. Upon review, several IFs are seen to be rich in iron (54%) and calcium (35%), and nearly one-third of them are protein-rich (30%). Some IFs are also rich in other micronutrients like vitamin C (27%), vitamin A (18%), zinc (14%) and folate (13%). Nutritive values of some nutrient rich IFs are provided in Table 1. Despite their rich nutritional content, about one-third of the studies (Gupta et al., 2005; Vadivel and Janardhanan, 2005; Agrahar-Murugkar, 2006; Arinathan et al., 2009; Shajeela et al., 2011; Jain and Tiwari, 2012; Das et al., 2015; Ghosh-Jerath et al., 2015, 2016, 2020a, 2021; Horo and Topno, 2015; Pradeepkumar et al., 2015; Terangpi and Teron, 2015; Singh et al., 2018; Chyne et al., 2019; Longvah et al., 2020) (33%) have reported under-utilization of most IFs in the indigenous communities.
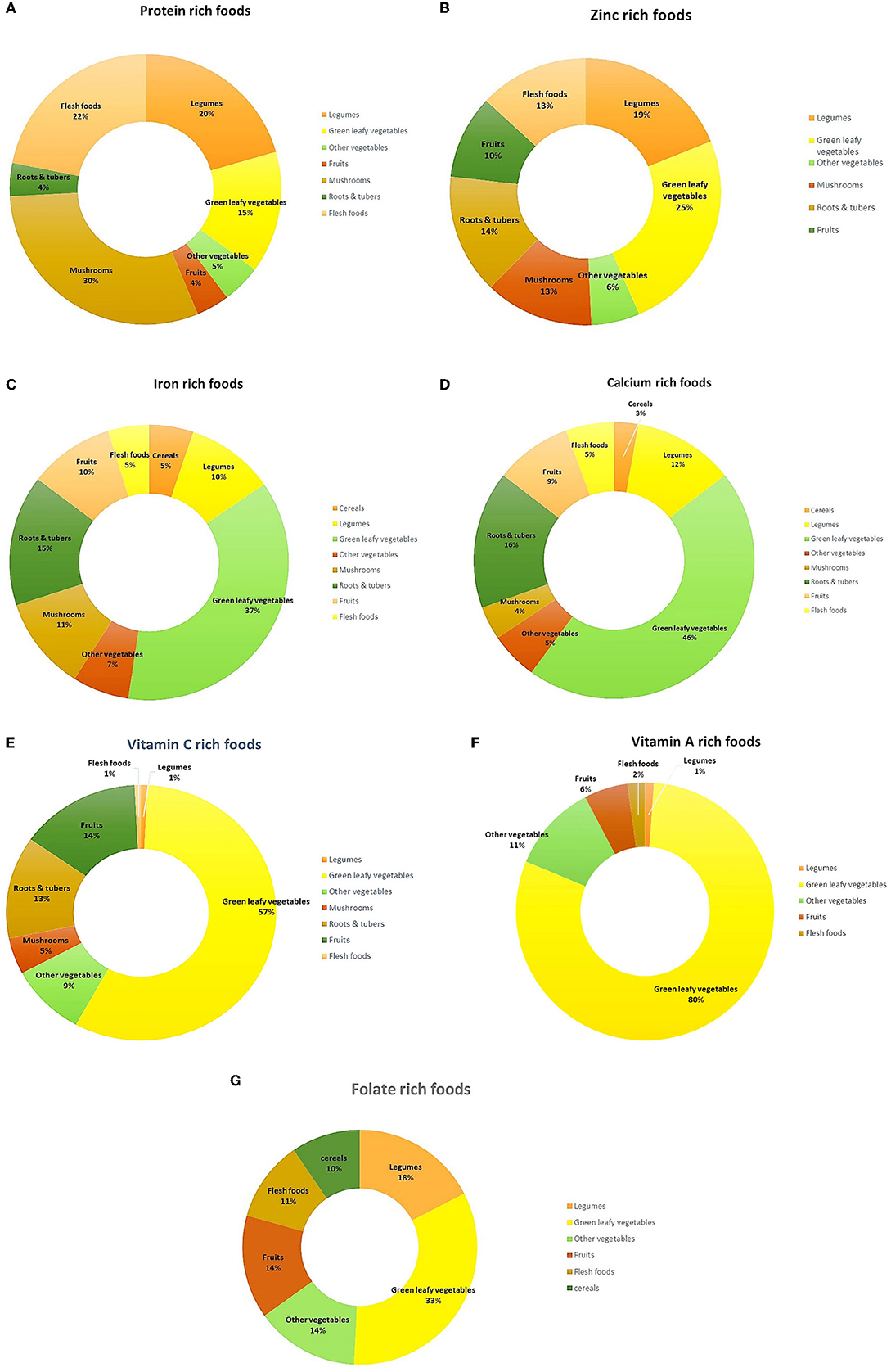
Several micronutrient-rich foods belong to the GLV (28%) food group, followed by other vegetables (13%) and wild roots & tubers (8%) (Figure 2). Nonetheless, other food groups have also been found to contain rich amounts of several nutrients. Among cereals, nutritive values of 49 local rice landraces have been documented, which are cultivated and consumed by Santhals, Sauria Paharias, Oraons and Mundas (Jharkhand), Savera, Jatapu, Gadabe and Kondadora (Andhra Pradesh), Garo and Khasi (Meghalaya) and indigenous communities of Himachal Pradesh. A high iron (3.1–6.1 mg/100 g) and folate content (65–1,203 μg/100 g) was observed in some varieties of red rice (Kba-baswoit, Ladhan, Kba-bakut, Kba-Iwai, Kba-stem) and sticky rice (Kba-shulia), that are consumed in North-east India. and folate. Many millet varieties also showed high content of iron (3.9–19.3 mg/100 g) and calcium (264–364 mg/100 g). These include, Indian goosegrass (Eleusine indica), Japanese millet (Echinochloa frumentacea), Salharkutki [scientific name not available (NA)], Italian millet/Kangni (Setaria italica), Little millet (Panicum antidotale) and indigenous varieties of Finger millet (Eleusine coracana).
In case of indigenous legumes, the usual protein content varies in the range of 18.3–35 g/100 g, with the highest content in Jack bean (Canavalia ensiformis), a wild legume consumed by indigenous communities of Kerala. Several legumes reported from Jharkhand, Kerala and Andhra Pradesh have also been found to be rich sources of calcium (260–945.4 mg/100 g), iron (3.6–38.6 mg/100 g) and zinc (3.4–7.7 mg/100 g), respectively. Among indigenous nuts, exceptionally high levels of calcium (1,020–1,540 mg/100 g) are reported in varieties consumed by Khasi tribe of Meghalaya, with the highest content in Indian Chestnut (Castanopsis indica). Perilla (Perilla frutescens), an important oilseed of Meghalaya and Manipur, has been found to have high levels of iron (8.3–9 mg/100 g) and zinc (4.7–5.02 mg/100 g).
Majority of the indigenous GLVs are found to be rich sources of iron (n = 101), calcium (n = 82), vitamin C (n = 75), vitamin A (n = 73), folate (n = 21) and zinc (n = 17). Most of these micronutrient rich leafy vegetables are reported from north-eastern part of India, in states of Assam, Manipur, Arunachal Pradesh and Meghalaya. Many common GLVs consumed by different indigenous communities across the country, have also high content of several essential micronutrients. For instance, Basella leaves (Basella alba), an indigenous GLV consumed by Lodha tribe of West Bengal and Nicobari tribe of Andaman & Nicobar Islands, is found to be rich in iron (4.2–9.4 mg/100 g), vitamin A (2,473 μg/100 g) and vitamin C (63.4–297 mg/100 g). Gogu leaves (Hibiscus sabdariffa), a common leafy vegetable consumed by indigenous communnities of Maharashtra, Jharkhand, Meghalaya and Andaman and Nicobar Islands, is an excellent source of zinc (15.8 mg/100 g). Similarly, Drumstick leaves (Moringa oleifera), a popular leafy vegetable across various indigenous communities, is a rich source of calcium (range: 314–440 mg/100 g), vitamin A (595–17,542 μg/100 g) and vitamin C (range: 991–30 mg/100 g), respectively. Different varieties of Amaranth leaves such as red Amaranth leaves (Amaranthus gangeticus), green leaves of Amaranth spinosus (Amaranthus spinosus), Chinese spinach (Amaranthus tricolor) and Purple Amaranth (Amaranthus lividus) which are consumed by the indigenous communities of Jharkhand, Andaman and Nicobar Islands, and Kerala, are specifically found to be rich sources of vitamin C (range: 77.3–308 mg/100 g).
Many varieties of other vegetables like Kattian/Kasai (Bridelia retusa), Hairy-fruited eggplant (Solanum lasiocarpum), Thai eggplant (Solanum virginianum), and indigenous Bittergourd (Momordica charantia) are found to be rich sources of vitamin C (range 277–826.4 mg/100 g), which are consumed by indigenous communities in Meghalaya and West Bengal, while high folate levels (106–413 μg/100 g) are reported for indigenous vegetables like Bitter tomato (Solanum aethiopicum), Tree tomato (Cyphomandra betacea), Wild bean (Canavalia cathartica), Broad bean (Vicia faba), Fejia (Wendlandia glabrata) and Wild banana stem (Musa acuminate), that are consumed by indigenous communities of Manipur. Flowers of many wild plants, particularly flowers of wild plantain (Ensete Superbum) and Dhawal (Woodfordia fruticose), consumed by indigenous communities of Maharashtra, are rich in both iron (range 55.1–518 mg/100 g) and calcium (range 219.4–665.5 mg/100 g), respectively. Apart from wild vegetables, indigenous communities of India also consume a wide variety of mushrooms, usually during the monsoon period. In our review, we have compiled nutritive values of 50 indigenous mushrooms of India, most of which (n = 46) are rich sources of protein (range: 15.3–39.1 g/100 g), with the maximum content in Vellathazan Kumizh (Pleurotus sajor caju), a wild edible mushroom consumed by Kaani community of Tamil Nadu. Most of the iron rich mushrooms (range 14.4–47.6 mg/100 g) are reported from West Bengal where they are consumed by Santhal and Lodha communities while, all mushroom varieties documented for Khasi communnity of Meghalaya are rich in calcium (range 420–1,910 mg/100 g), zinc (6.8–39.4 mg/100 g) and vitamin C (range 14.9–41.9 mg/100 g), respectively.
Several indigenous roots and tubers documented in our review display high levels of micronutrients; about 29 varieties of indigenous roots and tubers are rich sources of calcium (range 218.1–784.2 mg/100 g), 34 are rich in iron (range: 4.7–124.3 mg/100 g) and 17 varieties are rich in vitamin C (range: 18.1–250 mg/100 g). Most of these tubers (n = 13) belong to Dioscorea species, which are consumed across different regions in the country, including states of Tamil Nadu, Kerala, Andhra Pradesh, Jharkhand, West Bengal and Andaman and Nicobar Islands. High levels of niacin (19.9–88.4 mg/100 g) are reported in roots & tubers consumed by Palliyar communtiy of Tamil Nadu, with the highest content in Nurai (Dioscorea tomentosa). Some of the wild root vegetables known to the indigenous communities of Tamil Nadu (Palliyars, Kanikkars, and Valiyans), West Bengal (Lodhas) and Kerala (Saveras, Jatapus, Gadabes and Kondadoras) are found to have rich amounts of macro- as well as micronutrients. These include, Indian yam (Dioscorea oppositifolia), Mou Alu (Dioscorea wallichii) and Kedrostis foetidissima, which are rich in protein (range 10.8–13.8 g/100 g), calcium (230–748.3 mg/100 g), and iron (20.1–40.1 mg/100 g), respectively. Among indigenous fruits, many varieties are rich in iron (n = 26), vitamin C (n = 19) and calcium (n = 16). Several indigenous fruits consumed by Lodha community of West Bengal are reported to have rich amounts of vitamin C (96–600 mg/100 g), while many seasonal wild fruits consumed by Khasi communnity of Meghalaya, are rich in calcium (range 206–1,176 mg/100 g). Few indigenous fruits of Arunachal Pradesh, such as Machilus robusta, Ocotea lancifolia and Autumn olive (Elaeagnus umbellate), which are consumed by indigenous communities like Aka, Bugun, Miji, Monpa, Sherdukpen, Memba and Khamba, have been found to contain high protein content in the range of 12.7–15.2 g/100 g.
All the indigenous flesh foods (meat, poultry and fish) are found to be protein-rich (range-13.1–68.8 g/100 g). High calcium levels (370–690 mg/100 g) are observed in several small local fishes, consumed by indigenous communities of Jharkhand and Manipur. Entomophagy or insect consumption is reported among indigenous communities of North-east India (Manipur, Assam and Arunachal Pradesh) (Chakravorty et al., 2011, 2014; Ganguly et al., 2013). These insects are extremely nutritious, and are found to be rich in protein (range 10.6–65.7 g/100 g), iron (range 7.3–461 mg/100 g) and zinc (range 5.8–29.5 mg/100 g). Grasshopper (Chondacris rosea), in particular, has exceptionally high amount of protein (69.8 g/100 g), calcium (340 mg/100 g), iron (7.8 mg/100 g) and zinc (10.8 mg/100 g).
Anti-nutritional Content in Indigenous Foods of India
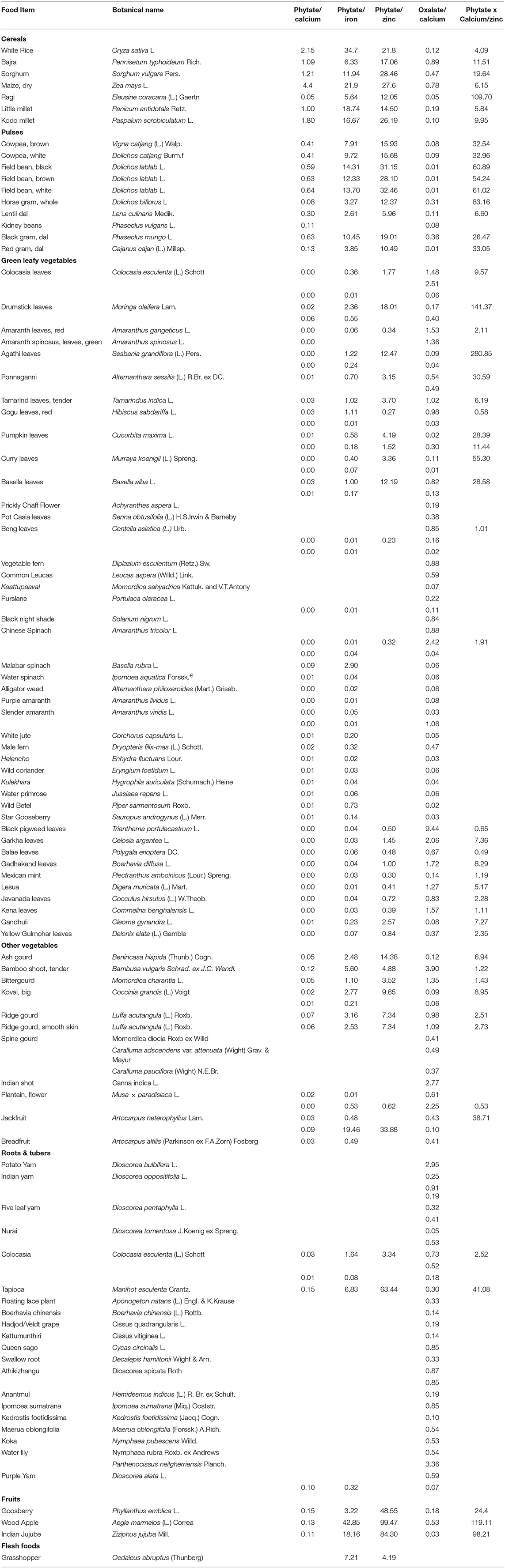
ANFs have been documented for 123 IFs (7 cereals, 17 legumes, 47 GLVs, 13 other vegetables, 30 roots and tubers, three fruits and six flesh foods) (Supplementary Table 3). Among cereals, most of the millets have high levels of phytate, with the highest content in Sorghum (549 mg/100 g). Total oxalate levels in cereals are mostly low, with particularly low content observed in Rice (1.9 mg/100 g), Kodo millet (3.5 mg/100 g) and Little millet (6.7 mg/100 g). In case of legumes, all the varieties with documented ANFs have phytate content >300 mg, with the highest in field bean varieties (791–799 mg/100 g). Indigenous varieities of Horse gram has the highest total oxalate level (181 mg/100 g) while, Red gram and Field beans (black, white and red) have markedly lower levels (1.2–1.4 mg/100 g). Least trypsin inhibitor activity is found in Pot Cassia (Senna obtusifolia) (13.5 TIU/mg protein) while highest is observed in Mucuna monosperma (65.4 TIU/mg protein).
Among GLVs, Ponnaginni (Alternanthera sessilis) and Agathi leaves (Sesbania grandiflora) have the highest saponin levels (880 mg/100 g) while Malabar spinach (Basella rubra) has the highest phytate content (287 mg/100 g). Although, there are about 34 GLVs with low phytate levels (<50 mg/100 g). such as, Colocasia leaves (Colocasia esculenta) (1–18 mg/100 g), Chinese spinach (2–18 mg/100 g), Beng leaves (Centella asiatica) (2.1–17 mg/100 g), Kena leaves (Commelina benghalensis) (2.4 mg/100 g), Lesua leaves (Digera muricata) (2.5 mg/100 g), Balae leaves (Polygala erioptera) (3.4 mg/100 g) and Red amaranth leaves (4.9 mg/100 g). Total oxalates are low in most GLVs, with few exceptions such as Lesua (1,410 mg/100 g), Chinese spinach (1,270 mg/100 g), Gadhakand leaves (Boerhavia diffusa) (1,250 mg/100 g) and Black pigweed leaves (Trianthema portulacastrum) (1,080 mg/100 g). Tannins ranged between 15 and 1,330 mg/100 g, with highest content in Yellow Gulmohar leaves (Delonix elata) and lowest content in Mexican mint leaves (Plectranthus amboinicus). Among vegetables, relatively lower phytate levels are reported, with the highest content of 68.8 mg/100 g in Jackfruit. Saponin levels are documented for only 5 vegetable varieties, with the highest content of 237 mg/100 g in Plantain flowers (Musa × paradisiaca). Indian shot (Canna indica), in particular, has high content of anti-nutrients, with total oxalate levels of 940 mg/100 g, tannin content of 1,830 mg/100 g and total free phenols of 1,470 mg/100 g. On the other hand, vegetables like Ash gourd (Benincasa hispida), Kovai (Coccinia grandis), bamboo stems and Field beans/ (Lablab purpureus) have reported low content of anti-nutrients.
About 10 indigenous roots and tubers are found to contain high levels of total oxalates (>400 mg), with maximum levels observed in Parthenocissus neilgherriensis (2,750 mg/100 g). Potato yam (Dioscorea bulbifera), particularly, has high content of total oxalates (780 mg/100 g), total free phenols (220–24,802 mg/100 g), amylase inhibitor activity (400 mg AIU 100 g) and tannins (70–2,550 mg/100 g). High levels of tannins (245–528.6 mg/100 g) and total free phenols (141–268.6 mg/100 g) are present in various indigenous insects. No substantial levels of anti-nutrients are reported in fruits.
Mineral Bioavailability in Indigenous Foods of India
The mineral bioavailability in IFs was predicted using phytate-to-mineral (calcium, iron and zinc) and oxalate-to-mineral (calcium) molar ratios. Secondary data on both mineral (calcium, iron and zinc) as well as anti-nutrient content (phytate and oxalate) was available for only 98 IFs (seven cereals, 10 legumes, 43 GLVs, 13 other vegetables, 21 roots and tubers, three fruits and one flesh food), which were utilized for computing molar ratios of Phy/Ca, Phy/Fe, Phy/Zn, Phy x Ca/Zn, and Ox/Ca, respectively (Table 2). Through our review, we found that most indigenous cereals (n = 7) have phytate-to-mineral molar ratios higher than the critical values while, values of Ox/Ca molar ratio are lower in all varieties. Finger millet emerged as one of the exceptions, which despite having a high phytate content of 306 mg/100 g, reported a low Phy/Ca molar ratio of 0.05.
Among legumes, high Phy/Fe molar ratios are observed in nine varieties, followed by high Phy/Ca and Phy/Zn molar ratios in six varieties. High bioavailability of calcium was reported in Horse-gram, a calcium-rich (260 mg/100 g) source, with a low Phy/Ca molar ratio of 0.08. Many micronutrient-rich GLVs are found to have exceptionally low molar ratios for Phy/Ca, Phy/Fe, Phy/Zn, Ox/Ca and/or Phy x Ca/Zn. Some of these GLVs include Colocasia leaves (Colocasia esculenta), Red amaranth leaves, Amaranth spinosus, Ponnagini (Alternanthera sessilis), Pumpkin leaves (Cucurbita maxima), Beng leaves (Centella asiatica), Basella leaves, Malabar spinach and many more. High mineral bioavailability is also observed in other indigenous vegetables. Some of these varieties are particularly rich sources of minerals; for instance, iron-rich sources like Breadfruit and Kovai have a low Phy/Fe molar ratio in the range of 0.21–0.49 while, Spinegourd, a calcium-rich source, has a molar Ox/Ca molar ratio of 0.41, respectively. For most calcium and iron-rich roots and tubers, phytate content was not available and hence, phytate-to-mineral molar ratios could not be calculated. However, Ox/Ca molar ratios were documented for 23 varieties, out of which most roots and tubers (n = 19) reported low values. Molar ratio indices in fruits could be documented for only 3 varieties, among which molar ratios of Phy/Ca and Phy x Ca/Zn are seen to be lower than the critical values. Although none of these varieties are rich sources of calcium, iron or zinc.
Discussion
India is endowed with a rich reservoir of wild and cultivated IFs, which are adapted to the local natural ecosystems and hold special cultural significance among various indigenous communities. For generations, the indigenous communities have utilized these food sources for their food and livelihood. In the present review, we have made an attempt to create a repository of nutritive values of the IFs consumed by indigenous communities of India, along with information on their anti-nutrient content and mineral bioavailability. For this purpose, 52 Indian research studies were reviewed, from which a total of 508 IFs were documented with nutrient values, consumed by different indigenous communities across India. Out of 508 IFs, anti-nutritional values were documented for 123 IFs and anti-nutrient-to-mineral molar ratios were computed for 98 IFs.
In our review, we found that many IFs are a rich storehouse of both macro- as well as micronutrients. The traditional rice varieties, consumed by indigenous communities of Jharkhand, Meghalaya, Himachal Pradesh and Arunachal Pradesh, were found to have better nutritional content than conventional varieties. For instance, local varieties of white rice (Pundi Goda), red rice (Lalhat (desi) and Laldhan), brown rice (Gopalbhok) and sticky rice (Minil-michudari) display calcium content in the range of 14–21 mg/100 g, which is much higher than the calcium content of raw milled rice variety (7.49 mg/100 g) reported in IFCT (Longvah et al., 2017). Most of the Indian traditional rice varieties have protein levels comparable to local rice varieties of Africa and Thailand (Ebuehi and Oyewole, 2007), although the calorific value and iron content are higher in Indian varieties. Apart from superior nutritional qualities, the colored traditional rice varieties (such as Red rice, Brown rice and Black rice) also contain high levels of anthoxanthin and anthocyanin, and are thus known for their anti-oxidant, anti-inflammatory and anti-carcinogenic effects (Rathna Priya et al., 2019). Studies from Assam, Chhattisgarh, Meghalaya and Jharkhand have further documented that indigenous rice varieties are drought tolerant, disease resistant and require less labor and farm inputs and hence provide stable grain yield as compared to high yielding hybrid rice varieties (Das and Das, 2014; Dwivedi et al., 2017; Borah et al., 2018; Nahar et al., 2018; Ghosh-Jerath et al., 2020a, 2021). Despite the favorable nutritional and agro-ecological attributes associated with indigenous rice varieties, their cultivation is on decline due to preference for agronomically improved varieties for higher yield. Hence, efforts must be taken to reinforce the cultivation of local varieties, through conservation of TEK associated with traditional agro-ecosystems and production practices in the community.
Our review also suggests superior mineral profile in indigenous millets as compared to rice and other cereals. Existing literature indicates that while millets are consumed by different indigenous communities across India, their overall consumption (both frequency and amount) is mostly low, especially in communities where rice production and consumption has preceded dominance over other coarse cereals (DeFries et al., 2018). Current scientific evidence has established a link between reduced consumption levels of coarse cereals and the low iron intake in the Indian population (DeFries et al., 2018). Hence, this trend may not only result in poor diet quality, but may also have implications on the overall micronutrient profile of the vulnerable communities. From a production perspective, millets offer a range of benefits for the farmers-they require less water, are more adaptable to climate variation, and thus provide assured yield even in case of climate stresses (Davis et al., 2018; DeFries et al., 2018). Thus, measures must be directed toward scaling up of millet cultivation, which will help revive their consumption in the habitual diets of the people. We also found that most millets contain high phytate levels, and have high molar ratios indices of phytate-to-mineral, thus indicating the possible inhibitory effect of phytate on mineral bioavailability in these foods. Therefore, optimal food processing methods must be followed to increase the nutrient bioavailability in millets.
Indigenous legumes were not only found to be protein rich, but also were rich sources of folate, calcium and iron. In fact, some of the under-utilized legumes consumed by indigenous communities in Kerala (like Jack bean, Velvet bean, and Mucuna monosperma) were found to contain higher protein, iron, calcium and zinc content than staple legumes (Red gram, Green gram, Lentils) (Longvah et al., 2017). The levels of trypsin inhibitors documented for wild legumes (13.5–65.4 TIU/mg proteins) were found to be lower than the trypsin inhibitor activity in conventional legumes like pigeon pea (67.1–71.3 TIU/mg proteins) and kidney bean (79.7–87.6 TIU/mg proteins) (Singh and Eggum, 1984; Vadivel and Janardhanan, 2005). Several indigenous GLVs were found to be rich in different micronutrients, especially in comparison to the popular GLVs like Spinach, Mustard leaves and Fenugreek leaves consumed across the country (Longvah et al., 2017). Among these, many GLVs have similar or better nutritional qualities in comparison to indigenous leafy vegetables consumed in other parts of the world. For instance, comparable micronutrient levels are reported in indigenous GLVs (like Drumstick leaves, Pot cassia, Pumpkin leaves and Amaranth leaves) of Sub-Saharan Africa, while micronutrient levels (calcium, iron, zinc and/or beta-carotene) of some Indian Indigenous GLVs (Sweet potato leaves, Gogu leaves, Garkha leaves, Colocasia leaves and Purslane) are seen to be higher as compared to their African counterparts (Nutritional Contributions of Important African Indigenous Vegetables, 2009; Uusiku et al., 2010; Schönfeldt and Pretorius, 2011; Traore et al., 2018; Catarino et al., 2019). Through our review, we also found that most indigenous GLVs contain ANFs in low amounts and have phytate and oxalate-to-mineral molar ratios lower than critical values, thus indicating low inhibitory effect of phytate and oxalate on mineral bioavailability.
Different varieties of indigenous vegetables were seen to be rich in vitamin C, iron and calcium. Wild bean, an indigenous variety consumed in Manipur, was found to have a folate content of 193 μg/100 g, which is much higher than the folate content of French beans (47 μg/100 g), Field beans (127 μg/100 g) and Broad beans (20.46 μg/100 g), respectively (Longvah et al., 2017). Comparable levels of micronutrients are reported in few vegetables (Bitter gourd, Tree bean and Bitter tomato) of Thailand and Sub-Saharan Africa (Kongkachuichai et al., 2015). We also observed exceptionally high protein and calcium content in indigenous mushrooms of India, some of which (Straw mushroom, White rot fungus mushroom and Termitomyces microcopus) have either better or equivalent nutrient content in comparison to African varieties (Mshandete and Cuff, 2007; Nakalembe et al., 2015). It was interesting to note that the protein, calcium, zinc and vitamin C content in all indigenous mushrooms were seen to be particularly higher than Button mushrooms (Longvah et al., 2017), a common variety consumed in urban regions of India. Many indigenous fruits documented in our review were found to be micronutrient-rich, although some varieties (like Monkey Jack, Java Plum and Indian gooseberry) contained relatively lower micronutrient content as compared to native varieties in other South-Asian countries (Tariqul Islam Shajib et al., 2013).
The calcium and iron content of most insect varieties documented in our review were found to be comparable to conventional meats such as poultry, beef, pork etc., while some of the insects such as Grasshopper, Scarlet skimmer and mole cricket, had protein content higher than most flesh foods. Since most indigenous communities (specially in North-eastern and eastern parts of India), do not consume dairy products (Malhotra and Sailo, 2016), consumption of these wild insects can hence be recommended and promoted to combat the problem of malnutrition, particularly protein deficiency (Ganguly et al., 2013). Apart from insects, other bush meats such as indigenous fishes and wild meats were also found to be rich in different micronutrients and contained nutrient levels comparable to other similar varieties consumed in different countries (Kuhnlein et al., 2002; Fanzo et al., 2013).
It is also important to mention that few variations were observed in the nutritional content of similar foods consumed across different regions in India. This could perhaps be due to the different protocols followed for nutrient analysis by different laboratories as well as due to the actual variances in chemical properties of the food samples (owing to genetic factors, soil mineral content and related properties) (Oko et al., 2012). Despite their nutritional benefits and cultural importance, majority of the IFs remain underutilized in the usual diets of the indigenous communities. Studies included in our review have reported a large set of factors leading to underutilization, such as habitat destruction due to industrial activities, agricultural expansion, changing social values and lack of awareness among the younger genaration (Bhattacharjee et al., 2009; Ghosh-Jerath et al., 2020a). The consumption of indigenous and traditional crops in many communities worldwide has particularly been affected by the general perception of these foods as “poverty foods,” especially by the younger generations (Cloete and Idsardi, 2013). Moreover, due to the high opportunity cost in terms of time and effort required for sourcing wild foods and non- participation of younger generation in collection and processing of IF resources, the TEK relating to IFs is fast eroding, thus threatening their use and consumption (Ghosh-Jerath et al., 2020a). The fast growing urbanization is further leading to easy access of market foods, which has rendered most ethnic communities with altered food habits and overdependence on cheap quality energy dense foods (Mungreiphy and Kapoor, 2010; Ghosh-Jerath et al., 2020b, 2021). As documented in our review, many conventional foods are not as nutritious as IFs, and thus contribute to inferior diet quality. Additionally, the current national programmes (such as Targeted Public Distribution System) and agricultural policies are promoting the production and consumption of conventional energy dense cereals (wheat and rice) among vulnerable section of the population, which is leading to overall reduced dietary diversity (Welthungerlife, 2016). In this context, a detailed and focused research is needed to establish the differences in the nutritional and anti-nutritional content of IFs vs. the conventional staple foods, in order to mainstream the IF consumption in day-to-day diets.
Efforts are desirable to promote and integrate the IFs back into the diets of the indigenous communities. Since most indigenous communites in India are smallholder subsistence farmers (MoHF and Ministry of Tribal Affairs, 2019), domestic cultivation of indigenous varieties can be the most useful strategy for IF promotion. Collection, preservation and use of indigenous seeds in agriculture must be encouraged as much as possible among the indigenous communities, who are gradually shifting to high yielding hybrid seeds (Singh et al., 2018; Ghosh-Jerath et al., 2020a, 2021). Under Horticulture Mission [Mission for Integrated Development of Horticulture (MIDH), 2021], a centrally sponsored scheme by Indian Government, homestead production of local fruits, vegetables, mushrooms and roots and tubers can further be reinforced for encouraging IF production. There is an also an important need to make people aware about the nutritional and ecological importance of including wild IFs in local diets, through effective information, education and behavior change communication activities (Bhattacharjee et al., 2009). With Indian Government's recent initiative under Poshan Atlas (2019), that aims to map nutritionally superior crops and food grains across different regions of India, many additional IF varieties can be identified in near future. The use of ancestral TEK among the elders, could further be leveraged for utilization of these foods from surrounding areas. In addition to this, cooking demonstrations could be conducted to give knowledge about various recipes and food preservation techniques using diverse list of IFs as well as to educate about processing techniques for removal of anti-nutrients. Most of the toxic and anti-nutritional factors could be removed by several processing methods (such as soaking, germination, blanching, fermentation and many others) but extensive research is still required to discover elimination methods for heat stable anti-nutrients and their impact on nutrient retention.
Study Limitations
Due to the narrative nature of the present review, no criteria were established to evaluate the methodological quality of the studies included in the review. Although we have reported the methodology descriptions of the included studies, with specific information on the analytical procedures followed for nutritional and antinutriitonal analysis of the foods.
Conclusion
A diverse variety of nutrient-dense IFs are available in India which have the potential to alleviate the persisting problem of hunger and malnutrition in Indian indigenous communities. However, until recently, these foods did not receive the desirable attention among policymakers and programme practitioners. The IF database collated in our review can thus serve as a resource for nutrition researchers, experts and practitioners to better understand the diversity of region-specific IFs and to advocate their incorporation in food based nutrition programmes. This review further highlights the need for a more detailed research on the nutrient and anti-nutrient values of IFs and its potential role in alleviating malnutrition, especially among vulnerable segments of the population. Although the consumption of IFs among indigenous communities has eroded over time, it could be resurrected with contextual food-based interventions through proper framework, policy, and information dissemination. Documenting and popularizing the nutritional benfits of IFs will be crucial for enhancing their consumption, and improving their demand in the global food systems. At the national level, holistic government policies in India like POSHAN Atlas and Horticulture Mission, which are aimed at identification and production of region specific foods, will be imperative to shift our focus from a small subset of foods to a diverse food base contextualized to regional, cultural and traditional food habits that may potentially enrich the dietary quality of the vulnerable population. We therefore, advocate the recognition of these foods and highlight the need for researchers, universities and government agencies to implement coordinated efforts to maximize the nutritional potential of IFs in terms of production, post-harvest and processing, marketing, and consumption, while understanding the ecological, social, cultural and economic context of IFs for their sustainable utilization and consumption Globally, a right step in this direction could lead to the diversification of existing global food systems, that may potentially reward us with diets that provide better nutrition, have low environmental impacts, are culturally acceptable and economically accessible to all.
Author Contributions
RK, MS, and SG-J formulated the research question and designed the review. RK conducted the literature search, screened the articles, extracted the data, and drafted the manuscript. MS and SG-J critiqued and provided comments on subsequent manuscript drafts. All authors approved the final version of the manuscript.
Funding
This work was supported by the DBT/Wellcome Trust India Alliance Fellowship (https://www.indiaalliance.org/) [IA/CPHI/16/1/502639] awarded to SG-J.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.696228/full#supplementary-material
References
Agrahar-Murugkar, D. (2006). Interventions using wild edibles to improve the nutritional status of khasi tribal women. Hum. Ecol. 2006, 83−88.
Agrahar-Murugkar, D., and Subbulakshmi, G. (2005). Nutritive values of wild edible fruits, berries, nuts, roots and spices consumed by the Khasi tribes of India. Ecol. Food Nutr. 44, 207–223. doi: 10.1080/03670240590953025
Akinola, R., Pereira, L. M., Mabhaudhi, T., de Bruin, F.-M., and Rusch, L. (2020). A review of indigenous food crops in africa and the implications for more sustainable and healthy food systems. Sustainability 12:3493. doi: 10.3390/su12083493
Alexander, S. M., Provencher, J. F., Henri, D. A., Taylor, J. J., Lloren, J. I., Nanayakkara, L., et al. (2019). Bridging Indigenous and science-based knowledge in coastal and marine research, monitoring, and management in Canada. Environ. Evid. 8:36. doi: 10.1186/s13750-019-0181-3
Arinathan, V., Mohan, V. R., and Maruthupandian, A. (2009). Nutritional and anti-nutritional attributes of some under utilized tubers. Trop. Subtrop. Agroecosyst. 10, 273–278.
Bhardwaj, R., Singh, R. K., Sureja, A. K., Tag, H., and Singh, A. (2009). Nutritionally rich wild vegetables of tribal communities of Northeast India: gaining insights on valuable traditional biocultural resources. Acta Hortic. 806, 243–248. doi: 10.17660/ActaHortic.2009.806.30
Bhattacharjee, L., Kothari, G., Priya, V., and Nandi, B. K. (2009). “The bhil food system: links to food security, nutrition and health,” in Indigenous Peoples' Food Systems: The Many Dimensions of Culture, Diversity and Environment for Nutrition and Health, eds H. V. Kuhnlein, B. Erasmus, and D. Spigelski (Rome: Food and Agriculture Organization of the United Nations Centre for Indigenous Peoples' Nutrition and Environment), 209–229.
Bindra, G. S., Gibson, R. S., and Thompson, L. U. (1986). [Phytate][calcium]/[zinc] ratios in Asian immigrant lacto-ovo vegetarian diets and their relationship to zinc nutriture. Nutr. Res. 6, 475–483. doi: 10.1016/S0271-5317(86)80101-4
Borah, N., Athokpam, F. D., and Semwal, R. (2018). Chakhao (Black Rice; Oryza sativa L.): A culturally important and stress tolerant traditional rice variety of Manipur. Trad. Knowled. Syst. Clim. Change Adaptation Mitigation 17:6.
Burlingame, B., Dernini, S., and Nutrition Consumer Protection Division, FAO (2012). Sustainable diets and biodiversity - Directions and solutions for policy research and action Proceedings of the International Scientific Symposium Biodiversity and Sustainable Diets United Against Hunger. Rome: Food and Agriculture Organization.
Catarino, L., Romeiras, M. M., Bancessi, Q., Duarte, D., Faria, D., Monteiro, F., et al. (2019). Edible leafy vegetables from West Africa (Guinea-Bissau): consumption, trade and food potential. Foods 8:493. doi: 10.3390/foods8100493
Census (2011). Census of India Website : Office of the Registrar General & Census Commissioner, India. Available online at: http://censusindia.gov.in/ (accessed September 29, 2019).
Chakravorty, J., Ghosh, S., Jung, C., and Meyer-Rochow, V. B. (2014). Nutritional composition of Chondacris rosea and Brachytrupes orientalis: Two common insects used as food by tribes of Arunachal Pradesh, India. J. Asia Pac. Entomol. 17, 407–415. doi: 10.1016/j.aspen.2014.03.007
Chakravorty, J., Ghosh, S., and Meyer-Rochow, V. B. (2011). Chemical composition of Aspongopus nepalensis Westwood 1837 (Hemiptera; Pentatomidae), a common food insect of tribal people in Arunachal Pradesh (India). Int. J. Vitam. Nutr. Res. 81, 49–56. doi: 10.1024/0300-9831/a000050
Chyne, D. A. L., Ananthan, R., and Longvah, T. (2019). Food compositional analysis of Indigenous foods consumed by the Khasi of Meghalaya, North-East India. J. Food Composit. Anal. 77, 91–100. doi: 10.1016/j.jfca.2019.01.008
Cloete, P. C., and Idsardi, E. F. (2013). Consumption of indigenous and traditional food crops: perceptions and realities from South Africa. Agroecol. Sustain. Food Syst. 37, 902–914. doi: 10.1080/21683565.2013.805179
Das, S. K., Mandal, A., Datta, A. K., Das, D., Paul, R., Saha, A., et al. (2015). Identification of wild edible mushrooms from tropical dry deciduous forest of Eastern Chota Nagpur Plateau, West Bengal, India. Proc. Natl. Acad. Sci. India, Sect. B Biol. Sci. 85, 219–232. doi: 10.1007/s40011-014-0330-y
Das, T., and Das, A. K. (2014). Inventory of the traditional rice varieties in farming system of southern Assam: A case study. Ind. J. Tradit. Knowl. 13, 157–163. doi: 10.4236/ojst.2014.47050
Davis, K. F., Chiarelli, D. D., Rulli, M. C., Chhatre, A., Richter, B., Singh, D., et al. (2018). Alternative cereals can improve water use and nutrient supply in India. Sci. Adv. 4:eaao1108. doi: 10.1126/sciadv.aao1108
DeFries, R., Chhatre, A., Davis, K. F., Dutta, A., Fanzo, J., Ghosh-Jerath, S., et al. (2018). Impact of historical changes in coarse cereals consumption in India on Micronutrient Intake and Anemia Prevalence. Food Nutr. Bull. 39, 377–392. doi: 10.1177/0379572118783492
Dubé, L., Webb, P., Arora, N. K., and Pingali, P. (2014). Agriculture, health, and wealth convergence: bridging traditional food systems and modern agribusiness solutions. Ann. N. Y. Acad. Sci. 1331, 1–14. doi: 10.1111/nyas.12602
Dwivedi, A. P., Chand, P., Mishra, A., Singh, S. R. K., and Athare, T. (2017). Identification of traditional rice varieties in Chhattisgarh: An institutional arrangement. Ecol. Environ. Conserv. Paper 23, 313–320.
Ebuehi, O. T., and Oyewole, A. C. (2007). Effect of cooking and soaking on physical characteristics, nutrient composition and sensory evaluation of indigenous and foreign rice varieties in Nigeria. Afr. J. Biotechnol. 38, 15–21. doi: 10.1108/00346650810847972
Fanzo, J., Hunter, D., Borelli, T., and Mattei. (2013). Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health. 1st ed. New York, NY: Routledge doi: 10.4324/9780203127261
FAO (2018). The Global Hub on Indigenous Food Systems. Available online at: http://www.fao.org/indigenous-peoples/global-hub/en/ (accessed March 17, 2021).
Frison, E. A., Cherfas, J., and Hodgkin, T. (2011). Agricultural biodiversity is essential for a sustainable improvement in food and nutrition security. Sustainability 3, 238–253. doi: 10.3390/su3010238
Ganguly, A., Chakravorty, R., Das, M., Gupta, M., Mandal, D. K., Haldar, P., et al. (2013). A preliminary study on the estimation of nutrients and anti-nutrients in Oedaleus abruptus (Thunberg) (Orthoptera: Acrididae). Int. J. Nutr. Metab. 5, 50–65. doi: 10.5897/IJNAM12.022
Ghosh-Jerath, S., Kapoor, R., Barman, S., Singh, G., Singh, A., Downs, S., et al. (2021). Traditional food environment and factors affecting indigenous food consumption in munda tribal community of Jharkhand, India. Front. Nutr. 7, e600470. doi: 10.3389/fnut.2020.600470
Ghosh-Jerath, S., Kapoor, R., Singh, A., Downs, S., Barman, S., and Fanzo, J. (2020a). Leveraging traditional ecological knowledge and access to nutrient-rich indigenous foods to help achieve SDG 2: An analysis of the indigenous foods of sauria paharias, a vulnerable tribal community in Jharkhand, India. Front. Nutr. 7, 61. doi: 10.3389/fnut.2020.00061
Ghosh-Jerath, S., Kapoor, R., Singh, A., Nilima, Downs, S., Goldberg, G., et al. (2020b). Agroforestry diversity, indigenous food consumption and nutritional outcomes in Sauria Paharia tribal women of Jharkhand, India. Matern Child Nutr. 2020:e13052. doi: 10.1111/mcn.13052
Ghosh-Jerath, S., Singh, A., Kamboj, P., Goldberg, G., and Magsumbol, M. S. (2015). Traditional knowledge and nutritive value of indigenous foods in the oraon tribal community of Jharkhand: An exploratory cross-sectional study. Ecol. Food Nutr. 54, 493–519. doi: 10.1080/03670244.2015.1017758
Ghosh-Jerath, S., Singh, A., Magsumbol, M. S., Kamboj, P., and Goldberg, G. (2016). Exploring the potential of indigenous foods to address hidden hunger: nutritive value of indigenous foods of santhal tribal community of Jharkhand, India. J. Hunger Environ. Nutr. 11, 548–568. doi: 10.1080/19320248.2016.1157545
Gupta, S., Jyothi Lakshmi, A., Manjunath, M. N., and Prakash, J. (2005). Analysis of nutrient and antinutrient content of underutilized green leafy vegetables. LWT - Food Sci. Technol. 38, 339–345. doi: 10.1016/j.lwt.2004.06.012
Hallberg, L., Brune, M., and Rossander, L. (1989). Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am. J. Clin. Nutr. 49, 140–144. doi: 10.1093/ajcn/49.1.140
Horo, S., and Topno, S. (2015). Study and analysis of nutritional value of some wild and semi wild edible plants consumed by “HO” tribes of W. Singhbhum district, Jharkhand, India. Int. J. Herbal Med. 3, 25–32.
ICMR-NIN (2020). Nutrient Requirements for Indians: A Report of the Expert Group, 2020. Hyderabad: ICMR-National Institute of Nutrition, Indian Council of Medical Research, Department of Health Research, Minsitry of Health and Family Welfare, Government of India.
Jain, A. K., and Tiwari, P. (2012). Nutritional value of some traditional edible plants used by tribal communities during emergency with reference to Central India. Ind. J. Tradit. Knowled. 11: 7. Available online at: http://hdl.handle.net/123456789/13423
Jana, S. (2004). Dietary Practices of the Lodhas and the Nutrient Content of Unconventional Foods Consumed: An Anthropological Study. Vanyajati, New Delhi XLXII.
Kasimba, S., Covic, N., Motswagole, B., Laubscher, R., and Claasen, N. (2019). Consumption of traditional and indigenous foods and their contribution to nutrient intake among children and women in Botswana. Ecol. Food Nutr. 58, 281–298. doi: 10.1080/03670244.2019.1598980
Kongkachuichai, R., Charoensiri, R., Yakoh, K., Kringkasemsee, A., and Insung, P. (2015). Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem. 173, 838–846. doi: 10.1016/j.foodchem.2014.10.123
Kuhnlein, H. (2009). Why are indigenous peoples' food systems important and why do they need documentation? Indigen. People Food Syst. 2009, 1–4.
Kuhnlein, H., Eme, P., and Larrinoa, Y. F. de (2019). “Indigenous food systems: contributions to sustainable food systems and sustainable diets,” in Sustainable Diets: Linking Nutrition and Food Systems, eds B. Burlingame and S. Dernini (Wallingford: CABI) 64–78. doi: 10.1079/9781786392848.0064
Kuhnlein, H. V., Chan, H. M., Leggee, D., and Barthet, V. (2002). Macronutrient, mineral and fatty acid composition of Canadian arctic traditional food. J. Food Composit. Analy. 15, 545–566. doi: 10.1016/S0889-1575(02)91066-5
Kuhnlein, H. V. Food Agriculture Organization of the United Nations McGill University. (2013). Indigenous Peoples' Food Systems & Well-Being: Interventions & Policies for Healthy Communities. Rome: Ste. Anne de Bellevue, Quebec: Food and Agriculture Organization of the United Nations; Centre for Indigenous Peoples' Nutrition and Environment.
Kuhnlein, H. V., Receveur, O., Soueida, R., and Egeland, G. M. (2004). Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J. Nutr. 134, 1447–1453. doi: 10.1093/jn/134.6.1447
Kuhnlein, H. V., Yesudas, S., Bhattacharjee, L., Dan, L., and Ahmed, S. (2006). Documenting traditional food systems of indigenous peoples: international case studies. Centre Indigen. People Nutr. Environ. McGill University 2006, 121.
Longvah, T., Ananthan, R., Bhaskarachary, K., and Venkaiah, K. (2017). Indian Food Composition Tables. Hyderabad: National Institute of Nutrition, Indian Council of Medical Research.
Longvah, T., and Deosthale, Y. G. (1991). Chemical and nutritional studies on hanshi (perilla frutescens), a traditional oilseed from northeast india. J. Am. Oil Chem. Soc. 68, 781–784. doi: 10.1007/BF02662172
Longvah, T., Vooradi Sathya Sai, P., Rajendran, A., Kharkhonger, G. C., and Rangad, C. (2020). In situ nutrient variability in rice landraces from garo Hills, meghalaya in North East India. J. Food Composit. Analy. 92:103543. doi: 10.1016/j.jfca.2020.103543
Lugo-Morin, D. R. (2020). Indigenous communities and their food systems: a contribution to the current debate. J. Ethn. Food 7:6. doi: 10.1186/s42779-019-0043-1
Mahadkar, S., Valvi, S., and Rathod, V. (2012). Nutritional assessment of some selected wild edible plants as a good source of mineral. Asian J. Plant Sci. Res. 2, 468–472.
Malhotra, R., and Sailo, L. (2016). Production and consumption pattern of milk and meat in north eastern region of India. Agri. Rural Dev. 3, 15–16.
Medak, B., and Singha, L. (2018). Trace elements and antioxidant activity of six wild edible plants that are widely consumed by ethnic tribes of Arunachal Pradesh, India. Ind. J. Agri. Res. 52, 85–88. doi: 10.18805/IJARe.A-4755
Mills, C. F., Bremner, I., and Chester, J. K. (1985). Trace Elements in Man and Animals – TEMA 5: Proceedings of the Fifth International Symposium on Trace Elements in Man and Animals. Farnham Royal: Oxford University Press.
Ministry of Tribal Affairs Government of India. (2020). Ministry of Tribal Affairs, Government of India. Available online at: https://tribal.nic.in/ (accessed June 14, 2020).
Misra, S., Maikhuri, R. K., Kala, C. P., Rao, K. S., and Saxena, K. G. (2008). Wild leafy vegetables: a study of their subsistence dietetic support to the inhabitants of Nanda Devi Biosphere Reserve, India. J. Ethnobiol. Ethnomed. 4:15. doi: 10.1186/1746-4269-4-15
Mission for Integrated Development of Horticulture (MIDH) (2021). Mission for Integrated Development of Horticulture (MIDH). Available online at: https://midh.gov.in/ (accessed February 24, 2021).
Modi, M., Modi, A., and Hendriks, S. (2006). Potential role for wild vegetables in household food security: A preliminary case study in KwaZulu-Natal, South Africa. Afr. J. Food Agri. Nutr. Dev. 6:19167. doi: 10.4314/ajfand.v6i1.19167
Mohan, V. R., and Kalidass, C. (2010). Nutritional and antinutritional evaluation of some unconventional wild edible plants. Trop. Subtrop. Agroecosyst. 12, 495–506.
MoHF, W., and Ministry of Tribal Affairs (2019). Tribal Health in India: Bridging the gap and a Roadmap for the Future. Ministry of Health and Family Welfare & Minsitry of Tribal Affairs, Government of India. Available at: https://www.nhm.gov.in/nhm_components/tribal_report/Executive_Summary.pdf (accessed June 15, 2020).
Morris, E. R., and Ellis, R. (1985). “Bioavailability of Dietary Calcium,” in Nutritional Bioavailability of Calcium ACS Symposium Series (American Chemical Society). doi: 10.1021/bk-1985-0275.ch006
Morris, E. R., and Ellis, R. (1989). Usefulness of the dietary phytic acid/zinc molar ratio as an index of zinc bioavailability to rats and humans. Biol. Trace Elem. Res. 19, 107–117. doi: 10.1007/BF02925452
Mshandete, A. M., and Cuff, J. (2007). Proximate and nutrient composition of three types of indigenous edible wild mushrooms grown in Tanzania and their utilization prospects. Afr. J. Food Agri. Nutr. Dev. 7:2615. doi: 10.18697/ajfand.17.2615
Mukhopadhyay, D. (2008). “Indigenous knowledge and sustainable natural resource management in the Indian desert,” in The Future of Drylands, eds C. Lee and T. Schaaf (Dordrecht: Springer Netherlands). doi: 10.1007/978-1-4020-6970-3_21
Mungreiphy, N. K., and Kapoor, S. (2010). Socioeconomic changes as covariates of overweight and obesity among Tangkhul Naga tribal women of Manipur, north-east India. J. Biosoc. Sci. 42, 289–305. doi: 10.1017/S0021932009990587
Nahar, S., Vemireddy, L. R., Sahoo, L., and Tanti, B. (2018). Antioxidant Protection Mechanisms Reveal Significant Response in Drought-Induced Oxidative Stress in Some Traditional Rice of Assam, India. Rice Sci. 25, 185–196. doi: 10.1016/j.rsci.2018.06.002
Nakalembe, I., Kabasa, J. D., and Olila, D. (2015). Comparative nutrient composition of selected wild edible mushrooms from two agro-ecological zones, Uganda. Springerplus 4:433. doi: 10.1186/s40064-015-1188-z
National Institute of Nutrition, Gopalan, C., Rama Sastri, B. V., and Balasubramanian, S. C. (1978). Nutritive Value of Indian Foods. Hyderabad, India: National Institute of Nutrition, Indian Council of Medical Research.
NFHS-4 (2015). Available at: http://rchiips.org/nfhs/pdf/NFHS4/India.pdf (accessed September 25, 2019).
Norhaizan, M. E., and Nor Faizadatul Ain, A. W. (2009). Determination of phytate, iron, zinc, calcium contents and their molar ratios in commonly consumed raw and prepared food in malaysia. Malays. J. Nutr. 15, 213–222.
Nutritional Contributions of Important African Indigenous Vegetables (2009). Nutritional Contributions of Important African Indigenous Vegetables. Routledge
Oko, A., Ubi, B., Efisue, A., and Nahemiah, D. (2012). Comparative analysis of the chemical nutrient composition of selected local and newly introduced rice varieties grown in Ebonyi state of Nigeria. Int. J. Agri. Forest. 2, 16–23. doi: 10.5923/j.ijaf.20120202.04
Padhan, B., Biswas, M., and Panda, D. (2020). Nutritional, anti-nutritional and physico-functional properties of wild edible yam (Dioscorea spp.) tubers from Koraput, India. Food Biosci. 34:100527. doi: 10.1016/j.fbio.2020.100527
Poshan Atlas (2019). India Research Center. Available online at: https://www.hsph.harvard.edu/india-center/research/poshan-atlas/ (accessed March 6, 2021).
Powell, B., Hall, J., and Johns, T. (2011). Forest cover, use and dietary intake in the East Usambara Mountains, Tanzania. Commonwealth Forest. Assoc. 13, 305–317. doi: 10.1505/146554811798293944
Pradeepkumar, T., Indira, V., and Sankar, M. (2015). Nutritional evaluation of wild leafy vegetables consumed by Tribals in the Wayanad District of Kerala. Proc. Natl. Acad. Sci. India, Sect. B Biol. Sci. 85, 93–99. doi: 10.1007/s40011-013-0271-x
Rajyalakshmi, P., and Geervani, P. (1994). Nutritive value of the foods cultivated and consumed by the tribals of south India. Plant Foods Hum. Nutr. 46, 53–61. doi: 10.1007/BF01088461
Rathna Priya, T. S., Eliazer Nelson, A. R. L., Ravichandran, K., and Antony, U. (2019). Nutritional and functional properties of coloured rice varieties of South India: a review. J. Ethnic Foods 6:11. doi: 10.1186/s42779-019-0017-3
Saha, D., Sundriyal, M., and Sundriyal, R. C. (2014). Diversity of food composition and nutritive analysis of edible wild plants in a multi-ethnic tribal land, Northeast India: an important facet for food supply. IJTK 13, 698–705. Available online at: http://hdl.handle.net/123456789/29521
Sandberg, A. S., Andersson, H., Carlsson, N. G., and Sandström, B. (1987). Degradation products of bran phytate formed during digestion in the human small intestine: effect of extrusion cooking on digestibility. J. Nutr. 117, 2061–2065. doi: 10.1093/jn/117.12.2061
Schönfeldt, H. C., and Pretorius, B. (2011). The nutrient content of five traditional South African dark green leafy vegetables—A preliminary study. J. Food Composit. Anal. 24, 1141–1146. doi: 10.1016/j.jfca.2011.04.004
Seal, T., and Chaudhuri, K. (2014). Ethnobotanical importance and nutritional potential of wild edible fruits of Meghalaya state in India. J. Chem. Pharm. Res. 6, 680–684.
Seal, T., Pillai, B., and Chaudhuri, K. (2016). Evaluation of nutritional potential of five unexplored wild edible plants consumed by the tribal people of Arunachal Pradesh State in India. J. Food Nutr. Res. 5, 1–5. doi: 10.12691/jfnr-5-1-1
Sebestianus Lakra, S. J. (2019). Sustainable resource management through indigenous knowledge and practices – a case of food security among the Baiga Tribe in India. EJSD 8, 233–233. doi: 10.14207/ejsd.2019.v8n4p233
Settee, P., and Shukla, S. (2020). Indigenous Food Systems: Concepts, Cases, and Conversations. Canadian Scholars.
Shackleton, S. E., Dzerefos, C. M., Shackleton, C. M., and Mathabela, F. R. (1998). Use and trading of wild edible herbs in the central lowveld savanna region, South Africa. Econ. Bot. 52, 251–259. doi: 10.1007/BF02862142
Shajeela, P. S., Mohan, V. R., Jesudas, L. L., and Soris, P. T. (2011). Nutritional and antinutritional evaluation of wild Yam (Dioscorea spp.). Trop. Subtrop. Agroecosyst. 14, 723–730.
Shantibala, T., Lokeshwari, R. K., and Debaraj, H. (2014). Nutritional and antinutritional composition of the five species of aquatic edible insects consumed in Manipur, India. J. Insect Sci. 14:14. doi: 10.1093/jis/14.1.14
Shantosh, M., and Sarojnalini, C. (2018). Nutritional quality of three cobitid fishes of Manipur, India: with special reference to essential mineral elements. Int. J. Sci. Res. Biol. Sci. 5, 24–33. doi: 10.26438/ijsrbs/v5i2.2433
Singh, S., Singh, L. B., Singh, D. R., Chand, S., Zamir Ahmed, S. K., Singh, V. N., et al. (2018). Indigenous underutilized vegetables for food and nutritional security in an island ecosystem. Food Sec. 10, 1173–1189. doi: 10.1007/s12571-018-0840-1
Singh, U., and Eggum, B. O. (1984). Factors affecting the protein quality of pigeonpea (Cajanus cajan L.). Plant Food Hum. Nutr. 34, 273–283. doi: 10.1007/BF01126556
Steyn, N. P., Olivier, J., Winter, P., Burger, S., and Nesamvuni, C. (2001). A survey of wild, green, leafy vegetables and their potential in combating micronutrient deficiencies in rural populations: research in action. S. Afr. J. Sci. 97, 276–278. Available online at: https://hdl.handle.net/10520/EJC97355
Sudheep, N. M., and Sridhar, K. R. (2014). Nutritional composition of two wild mushrooms consumed by the tribals of the Western Ghats of India. Mycology 5, 64–72. doi: 10.1080/21501203.2014.917733
Tag, H., Tsering, J., Hui, P. K., Gogoi, B. J., and Veer, V. (2014). Nutritional potential and traditional uses of high altitude wild edible plants in Eastern Himalayas, India. Int. J. Nutr. Food Eng. 8, 238–243. doi: 10.5281/zenodo.1091426
Tariqul Islam Shajib, M., Kawser, M., Nuruddin, M., and Begum, P. (2013). Nutritional composition of minor indigenous fruits: Cheapest nutritional source for the rural people of Bangladesh. Food Chem. 140, 466–470. doi: 10.1016/j.foodchem.2012.11.035
Terangpi, R., and Teron, R. (2015). Nutritional consideration of three important emergency food plants studied among Karbi Tribe of North East India. J. Sci. Innovative Res. 4, 138–141.
The Plant List (2020). Available online at: http://www.plantlist.org/ (accessed November 3, 2020).
Traore, K., Parkouda, C., dbGuissou, A., Kamga, R., and Savadogo, A. (2018). Production, consumption and nutritional content of five neglected vegetables in two secondary cities of Burkina Faso. Int. J. Adv. Res. 6, 1144–1155. Available online at: https://www.journalijar.com/uploads/550_IJAR-25345.pdf
Trichopoulou, A., Vasilopoulou, E., Georga, K., Soukara, S., and Dilis, V. (2006). Traditional foods: Why and how to sustain them. Trends Food Sci. Technol. 17, 498–504. doi: 10.1016/j.tifs.2006.03.005
Turnlund, J. R., King, J. C., Keyes, W. R., Gong, B., and Michel, M. C. (1984). A stable isotope study of zinc absorption in young men: effects of phytate and alpha-cellulose. Am. J. Clin. Nutr. 40, 1071–1077. doi: 10.1093/ajcn/40.5.1071
United States Food Drug Administration (2019). Code of Federal Regulations Title 21. Food and Drugs. Available online at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=101 (accessed November 3, 2020).
Uusiku, N. P., Oelofse, A., Duodu, K. G., Bester, M. J., and Faber, M. (2010). Nutritional value of leafy vegetables of sub-Saharan Africa and their potential contribution to human health: A review. J. Food Composit. Anal. 23, 499–509. doi: 10.1016/j.jfca.2010.05.002
Vadivel, V., and Janardhanan, K. (2005). Nutritional and antinutritional characteristics of seven South Indian wild legumes. Plant Foods Hum. Nutr. 60, 69–75. doi: 10.1007/s11130-005-5102-y
Welthungerlife (2016). India: Making Food a Right for All. Global Hunger Index - Peer-Reviewed Annual Publication Designed to Comprehensively Measure and Track Hunger at the Global, Regional, and Country Levels. Available online at: https://www.globalhungerindex.org/case-studies/2016-india.html (accessed September 28 2019).
Keywords: indigenous food, nutrient composition, antinutrient components, mineral bioavailability, Indian tribes, molar ratio, traditional foods
Citation: Kapoor R, Sabharwal M and Ghosh-Jerath S (2022) Indigenous Foods of India: A Comprehensive Narrative Review of Nutritive Values, Antinutrient Content and Mineral Bioavailability of Traditional Foods Consumed by Indigenous Communities of India. Front. Sustain. Food Syst. 6:696228. doi: 10.3389/fsufs.2022.696228
Received: 16 April 2021; Accepted: 25 February 2022;
Published: 28 April 2022.
Edited by:
Maria M. Romeiras, University of Lisbon, PortugalReviewed by:
Francis Kweku Amagloh, University for Development Studies, GhanaSumit Chakravarty, Uttar Banga Krishi Viswavidyalaya, India
Copyright © 2022 Kapoor, Sabharwal and Ghosh-Jerath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suparna Ghosh-Jerath, c3VwYXJuYS5naG9zaGpAaWlwaGQub3Jn
 Ridhima Kapoor
Ridhima Kapoor Manisha Sabharwal
Manisha Sabharwal Suparna Ghosh-Jerath
Suparna Ghosh-Jerath