- 1Department of Microbiology, Institute of Home Economics, University of Delhi, New Delhi, India
- 2Department of Food and Nutrition and Food Technology, Institute of Home Economics, University of Delhi, New Delhi, India
- 3Department of Physiology and Promotive Health, Institute of Home Economics, University of Delhi, New Delhi, India
The microbiome innovation has resulted in an umbrella term, postbiotics, which refers to non-viable microbial cells, metabolic byproducts and their microbial components released after lysis. Postbiotics, modulate immune response, gene expression, inhibit pathogen binding, maintain intestinal barriers, help in controlling carcinogenesis and pathogen infections. Postbiotics have antimicrobial, antioxidant, and immunomodulatory properties with favorable physiological, immunological, neuro-hormonal, regulatory and metabolic reactions. Consumption of postbiotics relieves symptoms of various diseases and viral infections such as SARS-CoV-2. Postbiotics can act as alternatives for pre-probiotic specially in immunosuppressed patients, children and premature neonates. Postbiotics are used to preserve and enhance nutritional properties of food, elimination of biofilms and skin conditioning in cosmetics. Postbiotics have numerous advantages over live bacteria with no risk of bacterial translocation from the gut to blood, acquisition of antibiotic resistance genes. The process of extraction, standardization, transport, and storage of postbiotic is more natural. Bioengineering techniques such as fermentation technology, high pressure etc., may be used for the synthesis of different postbiotics. Safety assessment and quality assurance of postbiotic is important as they may induce stomach discomfort, sepsis and/or toxic shock. Postbiotics are still in their infancy compared to pre- and pro- biotics but future research in this field may contribute to improved physiological functions and host health. The current review comprehensively summarizes new frontiers of research in postbiotics.
Introduction
According to microbiome researchers, every individual has a distinctive set of microbiota, determined by their DNA and environment. During delivery and lactation, the infant is first exposed to microorganisms (Ursell et al., 2012). The species of microbiome found in the maternal GI flora decides the type of microbiome exposure to the infant. Various factors e.g., genetics, environment, type of delivery, medication and food plays an important role in determining the microbiota inhabiting the colon. These exposures create a unique microbiome milieu varying from one individual to another individual. Later on, these exposures can change microbiome species which may be either beneficial or detrimental to health (den Besten et al., 2013). Several researchers have suggested various health-promoting effects of gut microbiomes which are dependent on the viability of bacteria. These processes lead to alteration of the gut microbiota, attachment to mucosa and epithelium host cells, improved epithelial function and immune-modulation (Sanders, 2009; Bermudez-Brito et al., 2012; Vyas and Ranganathan, 2012). However, recent research shows that neither all mechanisms nor clinical benefits are directly related to bacterial viability. Thus, viability of microbiome is not essential to attain the health-promoting effects of pre-probiotics. This led to the further research, development and use of non-viable components of microbiome for health benefits. Therefore, current evolving terminologies being added to probiotic scientific literature are postbiotics, ghost probiotics, synbiotics, nutribiotics, para probiotics and pharmabiotics. This paper reviews postbiotics which are non-viable intact microorganisms, their subcellular fractions and metabolic products secreted by live bacteria during their growth or released after bacterial lysis which add nutritional and health advantage to the host wellbeing (Salminen et al., 2021). Diverse antimicrobial compounds are produced that fall into two categories of low molecular weight (i.e., hydrogen peroxide, carbon dioxide, and di-acetylene) and high molecular weight (i.e., bacteriocins and bacteriocins-like substances) compounds which are known as postbiotics (Šušković et al., 2010; de Almeida Júnior et al., 2015). Lactobacillus is the most researched bacteria among the various LAB (Lactic acid bacteria) which is used for production of postbiotics.
Another major aspect in postbiotics research is their inherent stability, both during industrial processes and storage. As postbiotics are not able to replicate, thay are safer than probiotics. Thus, they are unable to cause probiotic associated risks like bacteraemia, fungaemia etc. (Yelin et al., 2019). Postbiotics are characterized by the microorganisms used as the starting point for its production and also the inactivation procedure or technique used for its production, as each procedure affects the quality and quantity of the final postbiotic produced & results in a different postbiotic with varied effects. However, for wider acceptance of postbiotics, an expert consensus is needed on the exact definition of postbiotics. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement unlocked the mystery of postbiotic action by five mechanisms: modulation of the resident microbiota; enhancement of epithelial barrier functions; modulation of local and systemic immune responses; modulation of systemic metabolic responses; and systemic signaling via the nervous system (Salminen et al., 2021). Therefore, postbiotics research in humans and validation of clinical & non clinical benefits of these bioactive molecules is the need of the hour. Novel postbiotics formulation may open a new horizon resulting in new therapeutic and preventive clinical approaches in diseases like diabetes mellitus, wound healing, adjunctive therapeutic drug as well as in food biopreservation, food packaging, biofilm control, functional food, food supplements, pharma food etc. To summarize, postbiotics research unfolded the concept of microbial metabolites in the sustenance of health.
Concept of postbiotics
In 2019, ISAPP convened a panel of experts specializing in nutrition, microbial physiology, gastroenterology, pediatrics, food science and microbiology to review the definition and scope of postbiotics (Yelin et al., 2019; Salminen et al., 2021). The panel defined a postbiotic as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host”. Effective postbiotics must contain inactivated microbial cells or cell components, with or without metabolites, that contribute to observed health benefits (Yelin et al., 2019). Their beneficial effect can be judged or confirmed in humans, animals, and other target organs and should not be limited only to the intestine. Further, the panel concluded that postbiotics are deliberately inactivated microbes that may contain metabolites or cell components exhibiting health benefits. Furthermore, there must be confirmed local as well as systemic health benefits, on the target population which can include humans, companion animals, livestock and other targets. Excluding injections, postbiotics can be administered anywhere at a host surface provided they have a safe profile. However, purified microbial metabolites and vaccines were excluded from postbiotics definition. Postbiotic may not necessarily be derived from a probiotic.
Various researchers reported beneficial effects of postbiotics such as immunomodulatory, antimicrobial and anti-cancer activity. It causes reduction in inflammation, oxidative stress, proliferative properties, reduction in blood pressure, cholesterol level and body weight. Postbiotics are well-tolerated in healthy people. However, certain groups of people should avoid increasing their level of postbiotics through eating probiotic rich foods e.g., people who have recently had surgery, have structural heart disorders, with digestive tract disorders, pregnant females and children. These groups tend to have compromised immune systems and may therefore be at an increased risk of an adverse reaction.
Certain foods may improve postbiotics in the gut e.g., buttermilk, cottage cheese, fermented pickles, yogurt, high-fiber foods like oats, flaxseed and garlic. Various researchers reported that internal and external factors may affect the performance of postbiotics. The interaction between active metabolites of postbiotic and internal factors like the resident microbiota, enzymes, and various food components can inhibit metabolites functions (Rad et al., 2020). Proteolytic enzymes are associated with postbiotic dysfunctions (Izuddin et al., 2020; Humam et al., 2021). The most important proteolytic enzymes are pepsin, trypsin, chymotrypsin etc., which have been shown to interfere with the activity of postbiotic compounds. External food factors like hydrogen ion concentration of food alters the antimicrobial activity of postbiotics, the optimal activity of postbiotics being reported between pH 4 and 9. Out of the food types used in postbiotics research, pasteurized milk and ground meat have an optimal pH, therefore they cause no disturbance in the function of postbiotics (Moradi et al., 2020). Another external factor affecting the antimicrobial activity of postbiotics is temperature variation. Heat at 30°C for 30 min or at 121°C for 15 min, decreases the antimicrobial effect of postbiotics (Mirnejad et al., 2013). Therefore, temperature and pH control is critical for the production of postbiotics.
The concept of postbiotics is fairly recent in comparison to prebiotics and probiotics. Postbiotic supplements are not as extensively available yet but postbiotics are superior to probiotics because of their purity, ease of preparation, long shelf life, mass production capability, precise action and more targeted responses by specific ligand-receptor interactions (Nataraj et al., 2020). Therefore, researchers are using postbiotics to modulate microbial signatures of health, nutrition and disease status.
Postbiotic components: Production and characterization
Over trillions of microbes carrying more than 3 million genes are estimated to inhabit the human gastrointestinal tract (GI), the main site being the large intestine that carry the most diverse and metabolically active microbial community (Belizário and Napolitano, 2015). The microbial colonization of the fetus seems to start in the uterus through ingestion of amniotic fluid and continues even after birth constituting the human microbiota (microbiome) unique to an individual (Tanaka and Nakayama, 2017; Toda et al., 2019). It includes more than 1,000 microbial species and is crucial for human development.
The gut microbiome of an individual shows family inheritance and comprises both beneficial and harmful microbes. A very delicate balance exists between the two and any disturbance in this normal microflora (dysbiosis) not only affects the GI tract but also negatively impacts the functions of other organs. This increases the risk of various infections and chronic diseases like colon dysfunction, gastroenteritis, irritable bowel syndrome, obesity, autism, psychiatric disturbances and other metabolic problems (Carding et al., 2015). It has been shown by a number of studies that this balance can be restored through use of postbiotics which in comparison to pre- and probiotics pose less risk (De Marco et al., 2018).
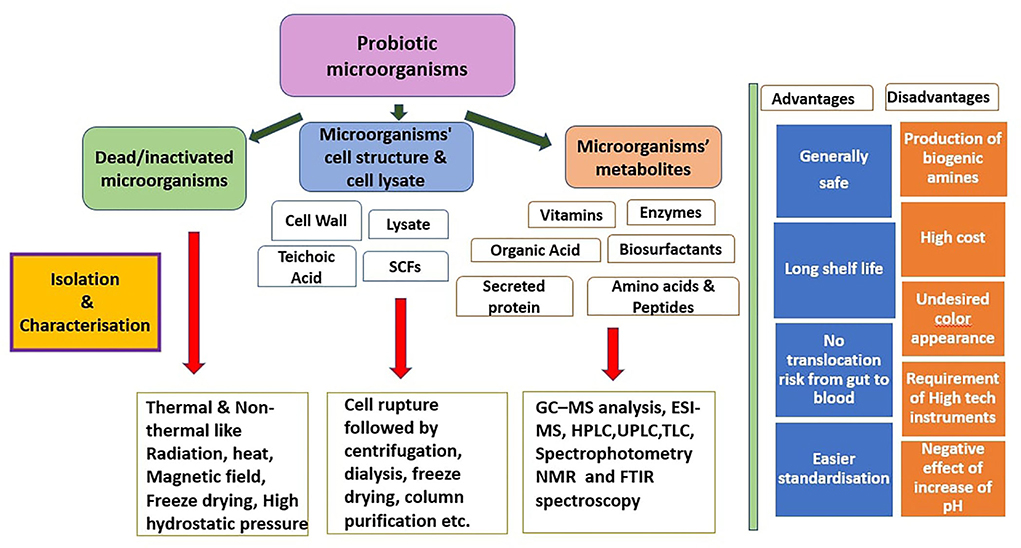
There are a number of investigations suggesting that the beneficial effects of probiotics in health may not necessarily be related to microorganisms' viability. Many of the health benefits of pre-, pro- and syn-biotics appeared to be mediated by various metabolic products, cellular and subcellular structural components and intact or ruptured dead microbes. Postbiotics are the structural and metabolic microbial products that include cell free supernatants, bacterial lysates, cell wall fragments, teichoic acid, short chain fatty acids, vitamins, enzymes, exopolysaccharide, various peptides, amino acids, and fermentation by-products (Wegh et al., 2019). These postbiotics components are produced by probiotics on feeding on prebiotics, during their prolonged storage or processing like pasteurization, baking or during metabolic processes. Besides these can also be produced in the lab by using methods such as radiations (UV/ionizing), high pressure, high temperature, sonication and inactivation by formalin.
Number of yeasts and bacteria used as probiotic but their use as postbiotic is underexploited. As postbiotics provide a safe alternative to live probiotics, the focus in recent time has shifted on postbiotics studies. Probiotic yeast, Saccharomyces cerevisiae var. boulardii has been used in many functional foods including dairy foods, grains, coffee, fruit juices etc. However, researchers need to develop postbiotic based functional foods preparations of S. boulardii, which may possibly improve shelf stability, sensorial properties, safety, and health benefits (Chan and Liu, 2022). Researches are being conducted to isolate and characterize different postbiotic components employing advanced technologies and to investigate their bioactivities for futuristic therapeutic use in medicine (Pyclik et al., 2020). Metabolomic methods are nowadays used to estimate the metabolic products of microbiota in stool and serum (Zhao et al., 2017). Many commercial pharmaceutical products like Nyaditum resea (inactivated Mycobacterium), Lacteol (inactivated Lactobacillus sp.), Cytoflora (cw components of Lactobacillus and Bifidobacterium sp.) are already approved for their use as supplements, immunomodulators and controlling risk of infections (Barros et al., 2020).
The storage and transportation of viable probiotic products requires cold chains whereas postbiotics are more stable and do not require cold chains for their industrial use. There is no interaction of postbiotics with the food matrix, no risk of acquiring antibiotic resistance genes and also no undesirable changes in taste and aroma. So postbiotics are a safe substitute in individuals with immune deficiencies, with transplantation or for newborns. Postbiotics act both locally and/or systematically on other organs through the communication axis between gut and target organs like Gut brain axis, Gut lung axis, gut-liver axis (Nataraj et al., 2020). The main postbiotic components as shown in Figure 1.
Inactivated and dead probiotics (non-viable probiotics)
Heat is the most commonly used method to produce non-viable preparation though other methods like gamma or ultraviolet radiations, tyndallization, sonication and chemical treatment can also be used. The cellular composition and biological activities differ with the inactivation process (Taverniti and Guglielmetti, 2011). Studies conducted on experimental models revealed that the non-viable cells retain the biological properties of their viable counterparts like scavenging oxygen radicals, decreasing inflammatory markers and modulating host physiology (Sugahara et al., 2017). Both the live and heat killed cells of eight Lactobacillus reuteri strains tested were themselves adherent to caco 2 cell culture, and inhibit the adherence of entero pathogens viz E. coli, Salmonella typhi, Listeria monocytogenes and enterococcus faecalis to these cells (Singh et al., 2017).
Cell free suspensions
Various biologically active metabolites are produced during microbial growth that can be separated from microbial cells by centrifugation and found to be rich in antioxidants, phenolics, flavonoid, antimicrobics and anticancer compounds (Hamad et al., 2020). These are reported to increase the expression of anti-inflammatory cytokines e.g., IL-10 and decrease the pro-inflammatory e.g., TNFα, IL-1β.
Lee et al. (2020) explored the anticancer property of the cell free culture filtrate from Lactobacillus fermentum and noted its anti-proliferative effects on 3D spheroid cultures of colorectal cancer cells. In another study cell free supernatant from Lactobacillus reuten, probably containing carbohydrates and fatty acid metabolites showed the anti-bacterial activity against dental pathogenic bacteria and seems to have potential in prevention and treatment of dental caries and periodontal diseases (Yang et al., 2021). Antimicrobial potential was also noted in culture suspensions from four probiotic strains viz Lactobacillus rhamnosus, L. fermentum, L. delbrueckii subsp. lactis and Pediococcus acidilactici against Clostridium perfringens present in poultry meat, indicating future applications of CFS in controlling clostridium infections (Hamad et al., 2020).
A measurable amount of lactic acid, hydrogen peroxide, protein and diacetyl were detected in Lactobacillus and Pediococcus species culture filtrate that are inhibitory to Staphylococcus aureus, Escherichia coli, Aspergillus niger and Aspergillus flavus (George-Okafor et al., 2020). The inhibition seems to occur by formation of pores in cell membranes and cell lysis due to lactic acid followed by action of diacetyl and bacteriocins (Oscáriz and Pisabarro, 2001). The culture suspension of oral care probiotic Weissella cibaria strain CMU found to have hydrogen peroxide, fatty acids, secreted proteins and organic acids. Antibacterial activity against periodontal pathogens was demonstrated by organic acid, secreted proteins and hydrogen peroxide (Lim et al., 2018) through disrupting cell membrane, decline in cytosol pH, production of hydroxyl radical, and interfering with cellular metabolic function (Konings et al., 1989).
In comparison to purified bio molecules, CFS appears to have better biological effects on host health as different biomolecules work in collaboration (Hartmann et al., 2011). On characterization, CSF from the various investigated species of lactobacilli using GC-MS was found to contain Pyrrolo [1,2-a] pyrazine-1,4-dione. Other compounds including organic acid like butyric acid, benzoic acid, biosurfactants (laurostearic acid), various peptides, fatty acid, ethanol, phenol, cyclopentanes, esters, and aldehyde are also present in strain specific manner. Many of these compounds show antioxidant activity, biofilm removal and antagonistic ability against L. monocytogenes, indicating their potential use as food additive especially of L. salivarius (Moradi et al., 2020). The antibacterial activity of Enterococcus faecalis CFS against foodborne pathogens was thermostable and maximum at neutral pH (7), indicating its application in food preservation (de Las M Cardoso et al., 2012).
Exo polysaccharides and extracellular vesicles
Different types of homo- and hetero-polysaccharides like kefricin, β glucans, uronic acid etc. are produced by Lactobacilli and other microbes. These are as a group termed as exopolysaccharides that are released either extracellularly or remain attached firmly as a capsule or loosely as a slime layer to the microbial cell surface (Caggianiello et al., 2016).
These macromolecules not only provide protection to microorganisms from phages, phagocytes and toxic compounds but also influences hosts' immunity, physiological mechanisms, lipid metabolism, and pathogen colonization. Dinić et al. (2018) showed the reduction in proinflammatory (IL-Iβ, TNFα, iNOS) and simultaneous increase in anti-inflammatory (IL-6, IL-10) cytokines, thus reducing the inflammation in rats on using EPS from Lactobacillus paraplantarum BGCG11. They were the first to show the counter hyperalgesia effect of an EPS. The EPS obtained from probiotics Lactobacillus fermentum and Paenibacillus polymyxa cultures showed antioxidant activity and thus may have health benefits in diseases like diabetes, arthrosclerosis and rheumatoid arthritis (Liu et al., 2010; Wang et al., 2020). Moreover, EPS isolated from lactic acid bacilli exhibited activity against tumor progress and inflammation as well as inhibited biofilm formation by pathogenic E.coli and S. aureus (Wang et al., 2020).
EPS from certain bacteria, particularly gut commensals, also show their expanded applications in bioremediation, pharmaceutical, food, and textiles industry (Angelin and Kavitha, 2020). Few exopolysaccharides already in use as food additives or in pharma industries include dextran, xanthan, alginate, gellen, and pullulan (Moscovici, 2015).
EPS extraction from LAB culture is a multi step process involving centrifugation followed by protein removal by acid, precipitation by cold ethanol, filtration to remove small molecules, finally dialysis and lyophilization (Jurášková et al., 2022). EPS preparations vary in their structure and composition depending on the source, thus impacting its bioactive functionalities.
EVs are lipid bilayer membrane bound spherical structures released by commensal bacteria like E.coli, Akkermansia muciniphila. These enclose diverse compounds like proteins, DNA, RNA, glycolipids, polysaccharides, enzymes, etc. and are involved in horizontal transfer of genetic material among bacterial species. These compounds are reported to play a role in controlling gut barrier permeability, signaling pathways, maintaining intestinal homeostasis, improving lipid profile and communication between gut and brain (Ahmadi Badi et al., 2017; Chelakkot et al., 2018).
Cell envelope components and pili
Bacterial cell wall consists of two macromolecules; lipopolysaccharide (in Gram negatives) and peptidoglycan (both in Gram positives and Gram negatives) that are released continuously during bacterial cell growth and death. Peptidoglycan is a rigid complex structure made of sugar and amino acid (Schaefer et al., 2018) and constitutes 30–70% of the Gram-positive cell wall. It consists of teichoic acids, polysaccharides and proteins. Teichoic acids are negatively charged molecules present only in Gram positives and are grouped into two types; (i) wall teichoic acid and (ii) lipo teichoic acids on the basis of their anchoring to peptidoglycan and cell membranes, respectively (Swoboda et al., 2009). Bacterial cell wall components are involved in host signaling as well in adhesion (Pyclik et al., 2020). These are excellent effector macromolecule and involved in activating immune system and release of various cytokines including IL-1, IL-6, IL-8, IL-12, TNFα, and IFNα; hyper release of which may result in pathophysiological reactions like septic shock, multiple organ failure, etc. (Hamann et al., 1998). Various peptidoglycan fragments released by action of endogenous peptidoglycan hydrolases are involved in modulation of host response. Two peptidoglycan hydrolases; proteins p75 and p40 from probiotic bacterium Lactobacillus rhamnosus were demonstrated to maintain the integrity of gut lining and suppress programmed cell death (Yan et al., 2007).
Several investigations indicated the boost in innate immunity, mast cell stimulation and immune regulatory mechanisms by LTA showing its immunogenicity. By suppressing regulatory activity of CD 25+ regulatory T cells and increasing cell mediated immunity, teichoic acid (LTA) of Bifidobacterium along with 5-fluorouracil regresses the proliferation of hepatoma cells indicating its anticancer property (Guo et al., 2015). The increase in T regulatory cells Foxp3+ was noted with B. bifidum cell wall polysaccharides (Verma et al., 2018). Hickey et al. (2021) observed a rise in the level of proinflammatory cytokines TNF-α, IFN-γ, IL-12, when B. breve lacked the EPS and suggested the involvement of EPS in establishing mutual relationship between host and B. breve. Bifidobacterium sp. EPS demonstrated to increase the level of IL-10 (α inflammatory) but decline INFα (pro-inflammatory) cytokines.
The investigators have also studied the role of pili as postbiotics which are long extracellular extensions on the cell surface and play a role in cell adhesion to intestinal wall or aggregation of bacteria. Bacterial pili is also involved in the development of the immune system in infants by modulating pro-inflammatory cytokines (Bach, 2002).
Bacterial lysates
Bacterial lysates are the soluble factors liberated by bacterial cell lysis. These can be prepared by sonication and mimic the presence of bacteria. These lysates pose multiple health benefits and are reported to decrease the inflammatory conditions like ulcerative colitis. The development of inflammatory bowel diseases like colitis and Crohn's disease is prevented by cell lysate from Lactobacillus caseii & other postbiotics including short chain fatty acids & tryptophan; thus providing a complementary treatment for inflammatory diseases. This probably results by correcting the gut microbiome, improving intestinal barrier integrity, modulating local & systemic immune response, regulating immune cells functions, reducing inflammation, and inhibiting the growth of pathogens (Zakostelska et al., 2011; Russo et al., 2019). Mi et al. (2022) observed the immune enhancing effect of cell lysate from Bacillus velezensis (Kh 2-2) derived from Korean fermented food Squid Jeotgal through in vivo, in vitro, and ex vivo studies. The lysate was reported to increase the cytokine production, promote specific and non-specific immune responses and activate macrophages.
The study done on rats using the supernatant and cell lysate from five probiotic strains i.e., Lactobacillus acidophilus, L. reuteri, L. casei, Bifidobacterium longum and B. coagulans were found to increase the bone mineral density at multiple bone sites in species dependent manner. The use of lysate appear to be an alternative for maintaining bone health and treating osteoporosis in elderly and women post menopause (Montazeri-Najafabady et al., 2021).
Bifidobacterium longum lysate was found to improve the reactive skin by controlling vasodilatation, oedema, degranulation of mast cells and other inflammation parameters (Guéniche et al., 2009). Moreover, use of both live and lysate of probiotic strain Lactobacillus reuter DSM 17938 in ex vivo skin models also illustrated the decline in proinflammatory cytokines (IL-6 and IL-8) and thus may give a new approach for managing skin inflammation and keeping skin healthy. Therefore, numerous skin care brands are also incorporating bacterial lysates in their formulations (Khmaladze et al., 2019).
Suppression of colorectal tumor cell proliferation, cell necrosis and invasion was reported on using cell lysate of two Lactobacillus species i.e., L. acidophilus and L. casei (Dallal et al., 2015). Heat shock culture lysate of L. plantarum appeared more potent inducer of immune response than normal cell lysate in cancer patients. Increased expression of heat shock proteins (HSPs) in heat shocked lysate may be associated with enhancement of cytokine expression and cytotoxic T cells activation (Sanaei et al., 2021).
Bioactive metabolites
The number of low and high molecular weight substances are produced by beneficial microbes in the gut or during fermentation in culture. There is a need to optimize the fermentation conditions for maximal yield of various metabolites. Amiri et al. (2021) optimized the conditions for simultaneous production of three metabolites viz. conjugated linoleic acid (CLA), exopolysaccharides (EPSs), and bacteriocins (BACs) by Bifidobacterium lactis using cheese whey and permeates, otherwise a waste, as the culture medium. As reviewed by Moradi et al. (2020), the chemical composition can be analyzed qualitatively or quantitatively employing various new techniques including two dimensional gel electrophoresis (2D-PAGE), gas chromatography (GC), high-performance liquid chromatography (HPLC), ultra-performance liquid chromatography (UPLC), thin layer chromatography (TLC), spectrophotometric technique, nuclear magnetic resonance (NMR) spectroscopy, fourier transform infrared (FTIR) spectroscopy, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), electrospray ionization mass spectrometry (ESI-MS). The composition and functional attributes depend on the microbial strains, culture conditions and processing conditions. The major ones include:
Vitamins
Humans require exogenous sources of vitamins as they lack the biochemical pathways for their synthesis. These vitamins are required in various physiological processes like folate in DNA replication, repair and methylation, vitamin K as cofactor of gamma carboxylase activity in blood clotting, bone health, neural functions, and riboflavin in redox reactions as hydrogen carrier. Gut commensal especially lactic acid bacteria and Bifidobacterium sp. supply many of these essential vitamins that include vitamin K and many B group vitamins like folate, riboflavin, cobalamin, pyridoxine, thymine, niacin, nicotinic acid (Hill, 1997; LeBlanc et al., 2013). A variety of fermented foods like fermented milk, yogurt, and cheese are rich sources supplying these vitamins. While the vitamins produced by the gut commensals are mainly absorbed in the colon, the main absorption site for dietary vitamins is the small intestine.
Neurotransmitters
Neurotransmitters like serotonins, dopamines, norepinephrine, catecholamines, acetylcholines are produced in gut lumen by commensals like Bifidobacterium, Lactobacillus plantarum, Lactobacillus brevis, Bacillus subtilis, etc. These impact the brain function via gut brain axis through modulation of enteric nerves signaling. Serotonin derived from amino acid tryptophan is involved in elevating the mood. While catecholamines and acetylcholine play a critical role in CNS functions like memory and learning processes, emotion, motor control, gamma amino butyric acid acts as neurotransmission inhibitor and its dysfunction results in anxiety and depression. These compounds seem to have potential as antidepressants and psychiatric related disorders can be taken care of by microbiota management (Patterson et al., 2014).
Biosurfactants
Biosurfactants are amphiphilic compounds excreted generally at the end of the exponential phase by microbes either extracellularly or remain bound to the cell surface. The major ones include glycolipids, glycoproteins, phospholipids, fatty acids, polysaccharides and glycopeptides (Cameotra and Makkar, 2010). These surface-active macromolecules possess anti-bacterial and anti-film properties. By preventing the establishment of biofilm or removing the existing one, biosurfactants help in preventing the colonization by the enteropathogens (Satpute et al., 2016). These also disrupt the cell membrane integrity and outflow of cytoplasmic content of pathogens. Bio surfactants can be obtained and characterized by centrifugation, acidification, extraction, membrane filtration, dialysis, gel filtration, freeze drying, FTIR, TLC, NMR, MS.
Short chain fatty acids
These are produced in the colon from indigestible carbohydrates, oligo- and poly- saccharides, mainly dietary fibers by Bacteroides and Firmicutes. As evidenced, SCFs have multiple health benefits. Besides improving the colon's function and lowering the pH, these enhance the proliferation of epithelial cells and the blood flow in the colon (Topping, 1996). SCFs also play an important role in lowering the incidence of colo-rectal diseases (Bird et al., 2010). Major fatty acids produced by colonic bacteria on fermentation of undigested carbohydrates are acetate, propionate and butyrate, with ratios varying usually from 3:1:1 to 10:2:1. Acetate is used by other microbes as a growth factor and also involved in cholesterol management. Propionate and butyrate are involved in gluconeogenesis and are the key supplier of energy for colonocytes and epithelial cells (Rowland et al., 2017) and also promote apoptosis of colon cancerous cells. Some amounts of lactate, succinate and fumarate may also be present as fermentation products. SCFs can play a crucial role in clinical trials and more holistic approaches can help to study the interactions between postbiotics, host and gut microbiota.
Bacteriocins
Bacteriocins comprise a large heterogeneous group of more than hundreds ribosomally synthesized small peptides or proteins produced both by lactic acid (LAB) and other eubacteria and archaebacteria that can kill or inhibit the growth of other bacteria. Examples include nisin, subtilosin, lactococcin G&Q, enterocin, lactocyclicin, bovicin, plantaricin, lacticin etc. (Perez et al., 2014). These vary in their molecular weight, structure and activity and have been classified into three classes: heat stable Class I Bacteriocins/Lantibiotics (e.g., nisin, mersacidin, lacticin) that are modified highly after translation; heat stable Class II-bacteriocins (lactacin F, lactococcin G, pediocin PA-1, sakacin A) that are not modified much posttranslationally; and heat-labile Class III bacteriocins (colicins, megacins, enterolysin, helveticin I) (Negash and Tsehai, 2020). Bacteriocins show their potential in food preservation. Nisin was the first bacteriocin that was given approval by regulatory authorities like European Food Safety Authority (EFSA), FDA, Health Canada for its commercial use as food preservative and now it is being used in more than 80 countries as a food additive. Bacteriocins inhibit growth of pathogens in GIT through pore formation in cell membranes, inhibiting correct cell wall formation and inhibiting enzyme activity and protein functions. Belguesmia et al. (2020) and Kim et al. (2020) isolated multi-bacteriocinogenic strain of Lactobacillus paracasei and Lactobacillus taiwanensis that demonstrated antimicrobial activity against E. coli and Salmonella gallinarum & enteropathogenic E. coli, respectively. Moreover, they exhibit narrow to broad spectrum inhibitory activity against bacterial growth, thus drawing attention for their therapeutic use as next generation antimicrobials in controlling global threat of drug resistant infectious organisms (Soltani et al., 2020). Besides, bacteriocins are also reported to possess anticancer activity and antiviral activity. Despite their positive characteristics like odorless, colorless, biodegradability, their widespread use in medicine, cancer therapy, food, cosmetics, veterinary use, require more research on their safety, toxicity, and immunogenicity.
Hydrogen peroxide
It is another postbiotic produced by many bacteria including LAB that has antimicrobial effect and helps in improving inflammatory bowel disease (IBD) conditions marked by disintegrated tight junctions and increased intestinal permeability. Hydrogen peroxide synthesized by eight different L. johnsonii strains and L. gasseri is observed to kill the Salmonella enterica serovar Typhimurium (Pridmore et al., 2008). It is found to provide protection against a number of viruses by inducing antibody and T cell response. It is therefore proposed that gargling and nasal spray of H2O2 may be effective against SARS-2 COVID through increase in localized innate immune response (Caruso et al., 2020). Singh et al. (2018) reported in their study that increasing H2O2 production by L. johnsonii within the physiological range enhances the recovery from colitis.
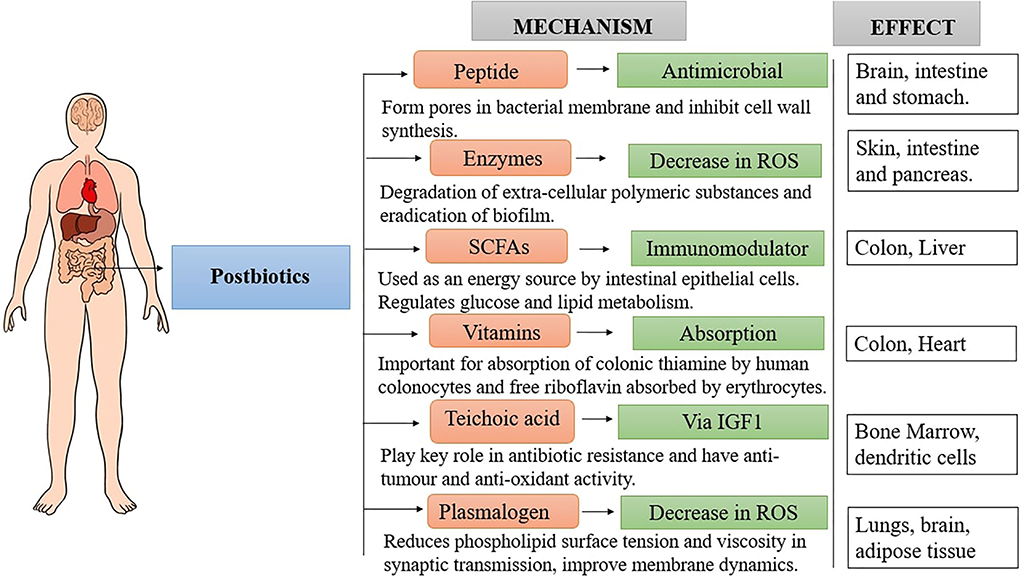
Possible signaling mechanisms
The signaling mechanism of postbiotics depends on the interaction of host and microbial products through MAMPs (microbe-associated molecular patterns) of commensals with specific PRRs (pattern recognition receptors) on host cells (Figure 2). These MAMPs include various surface molecules like teichoic acids, polysaccharides, cell wall proteins, nucleic acid etc. and the main PRRs involved are TLRs (Toll-Like Receptors), NLRs (Nucleotide-Binding Oligomerization Domain-Like Receptors), CTLRs (C-Type Lectin-Like Receptors) and GPCRs (G-Protein-Coupled Receptors) (Teame et al., 2020). This interaction activate the host immune system and eventually lead to anti-inflammatory response (immunomodulation), and have health promoting effects like anti-carcinogenic, hypolipidemic and hypertensive properties (Fang et al., 2014; Aguilar-Toalá et al., 2019; Engevik et al., 2021; Yeşilyurt et al., 2021).
Although the precise mechanisms have not been fully elucidated, postbiotics like SCFs bind to the specific receptors (GPCRs) present on the intestinal epithelium and obstruct Treg (Regulatory T cells) cell suppression, pro-inflammatory cytokine production, and NF-κB (Nuclear factor kappa B) pathway of neutrophils and macrophages which hinder the inflammatory response and anti-inflammatory effect is generated (Park et al., 2007; Vinolo et al., 2011). Similarly EPS, peptidoglycan and lipoteichoic acid binds to specific TLRs and through inhibition of enteropathogen mediated NF-κB and MAPK (Mitogen-activated protein kinases) signal pathways downregulate inflammatory cytokines and modulate inflammatory conditions in gut inflammation (Lebeer et al., 2011; Wachi et al., 2014). Peptidoglycan peptides via binding to NLRs (intracellular proteins e.g., NOD1 & NOD2) upregulate proinflammatory cytokines and antimicrobial activity by regulating NF-κB, MAPK, and caspase-1 signals (Franchi et al., 2009). Immunomodulation also occurs on up- and down- regulation of several signaling pathways. Postbiotics seems to promote the secretion of IL-10 and in turn stimulate Foxp3+ T regulatory cells and suppress the release of IL-6, IL-8 and monocyte chemoattractant protein (MCP-1) (Teame et al., 2020). Polysaccharide A (PSA) from Bacterioides fragilis, also enhances IL-10 and T cell mediated regulation and inhibits IL-17 production, thus managing inflammation progression (Peluzio et al., 2021).
In some cases, it is observed that heat-killed microbes can trigger the transcription of toll-like receptors and expression of proinflammatory cytokines (Wang et al., 2013). Cytokine IL-8 plays an important role in inflammatory response by recruiting the immune cells. While some strains of heat-killed bacteria obstruct IL-8 secretion in intestinal cells by secreting soluble anti-inflammatory factors which can initiate cellular immune and anti-inflammatory response (Kamiya et al., 2006; Imaoka et al., 2008). It is hypothesized that postbiotics protect hosts from pathobionts by immunomodulatory boosting the levels of Th1-associated cytokines and suppress the Th2- associated cytokines (de Almada et al., 2016). Immunosenescence with age particularly in elders under nutritional control results in decreased efficacy of the vaccination. Akatsu (2021) reviewed the interventions by gut modulation on the elders' immunity and as evidenced by some studies administering pre-, pro- and post- biotic raised the NK cells & phagocytic activity, increase the antibody titer & cytokine production, thus highlighting the potential adjuvant function in vaccination. However, more clinical studies on a larger group of subjects is required.
Postbiotics are emerging anticancer agents due to their non-hydrolytic stable chemical structure, longer shelf life and nontoxicity (Nataraj et al., 2020). Postbiotics are able to modulate immune response by inhibiting mutagenesis and bacterial translocation and triggering pro-apoptotic pathways, apoptosis and autophagy. By inducing apoptosis and altering expression of ZO-1 and MMP-9 (responsible for maintaining & degrading tight junctions, respectively), SCFs prevent cancer progression (Zółkiewicz et al., 2020). Abnormalities in gut microbiota can be a cause of host's tumorigenesis, especially colorectal cancer. Growth of pathogens can affect the immune system by secreting toxins which cause mutation in host's DNA hence inducing tumor development (Mager, 2006).
Postbiotics also have antiatheroschlerotic effects and help in lipid metabolism by lowering the risk of various cardiovascular diseases. Some examples include Lactobacillus bacteria which boost the production of HDL cholesterol and lowers the level of LDL cholesterol and triglycerides. SCFA also leads to statin-like effects by obstructing the condensation of the cholesterol precursors (Olle, 2013).
However, more advanced genomic approaches including metabolomics, proteomics, transcriptomics, need to be employed for better understanding of complex host- postbiotic interactions and their benefits (Wegh et al., 2019).
Health and nutritional benefits of postbiotics through the life cycle
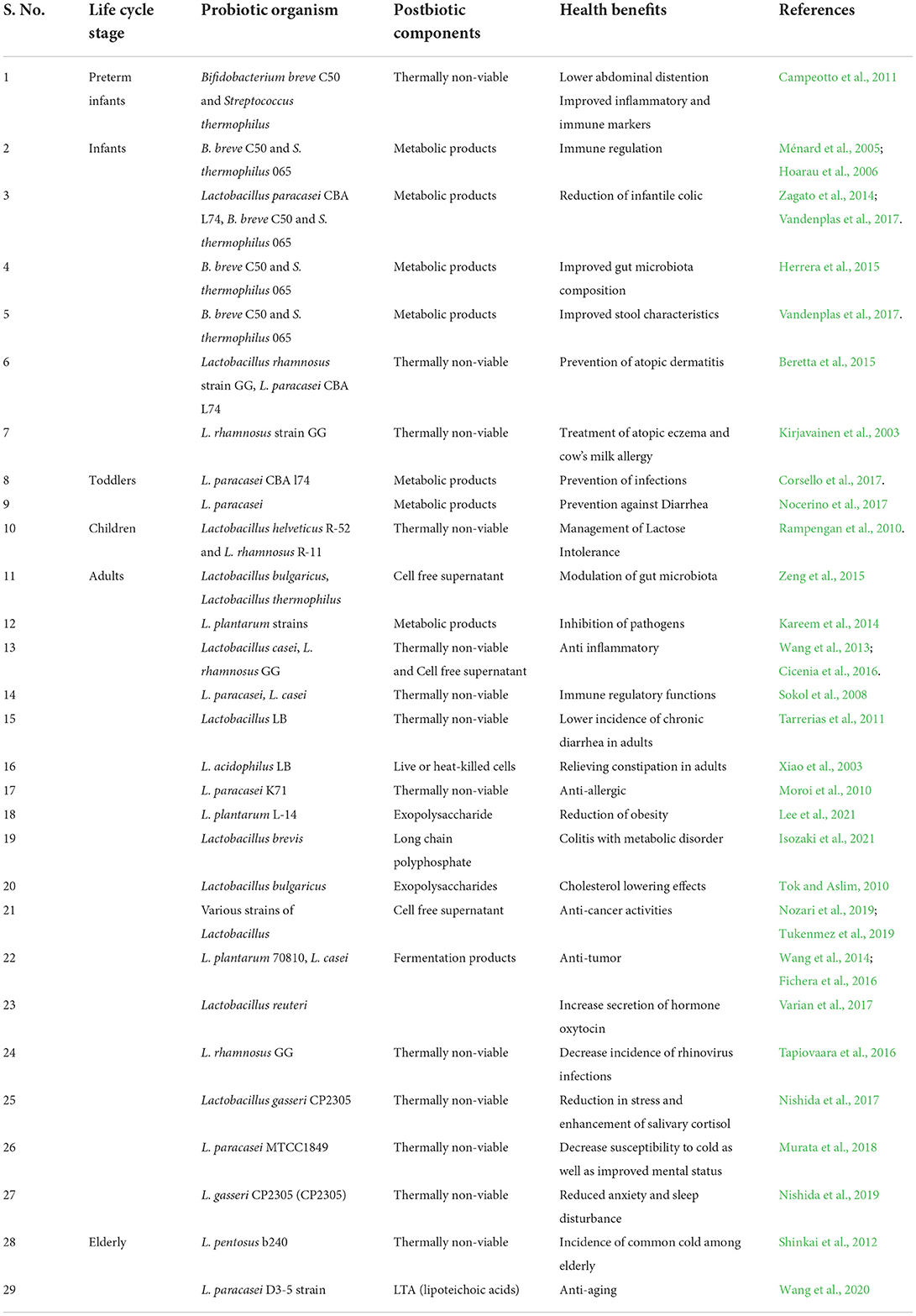
There is no dearth of evidence highlighting health benefits of consuming prebiotics and probiotics. However, advances in research have indicated that many of these health benefits actually come from the production of postbiotics. The microbial metabolites and other molecules that are generated in the fermented foods are responsible for the health benefits of postbiotics. The composition of the postbiotic product depends on the various processing methods involved. Postbiotics have been found to influence nearly every physiological function in the human body. The health promoting and disease preventive benefits of postbiotics throughout the life-cycle are summarized in Table 1.
The spectrum of health and nutritional benefits of postbiotics is growing with advancing research and many distinct features are still emerging. However, it is also important to note that some adverse concerns have been reported after consumption of postbiotics in specific groups of people which warrants a need to further investigate safe use of postbiotics.
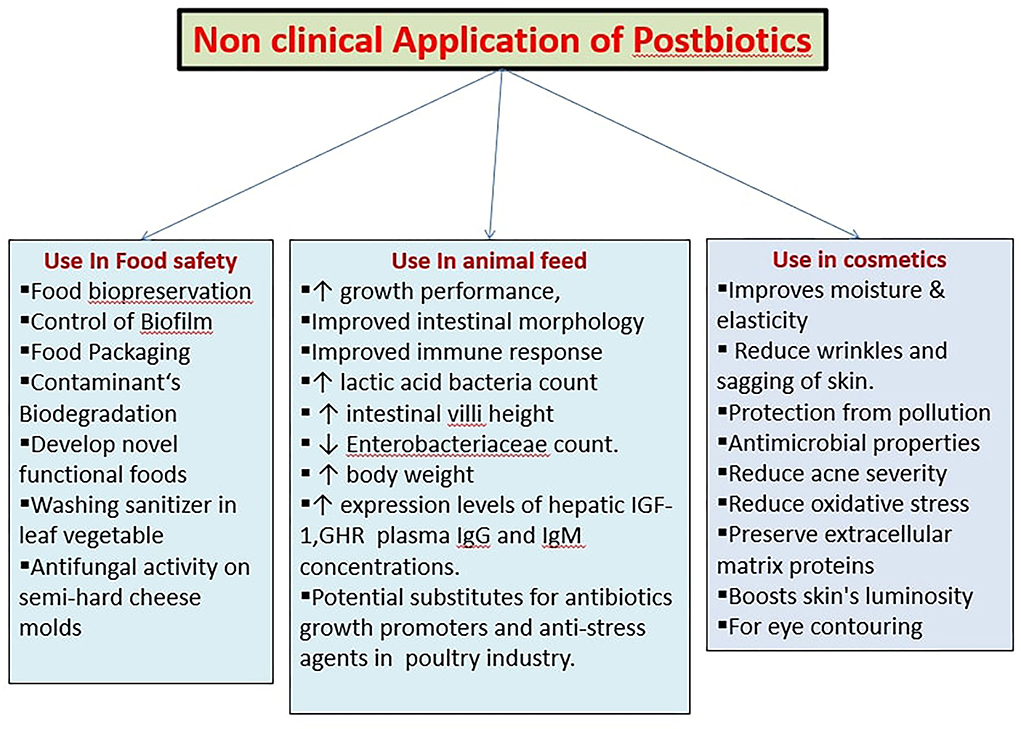
Perspectives for non-clinical application
Food applications
A notable use of postbiotics can be found in the food safety profile for example biopreservation and packaging, removal of biofilm, degradation of toxic contaminants.
Biopreservation
In biopreservation, specific microorganisms and their metabolites are used as postbiotics to prevent the microbial spoilage of food and to enhance their shelf life. Postbiotics exhibit anti-microbial activity on both pathogenic and spoilage microorganisms by multiple mechanisms including creating cavities in CM, affecting cell wall proteins and decreasing pH of bacteria cytoplasm, thus proving to be of considerable significance in the food sector (Homayouni Rad et al., 2021). Non-vegetarian food can be preserved by directly applying the postbiotic coating (for example fish filet and meat slices) or spraying (for example ground fish and meat), depending upon the nature of the postbiotic and type of the meat to protect their nutritional properties and organoleptic changes. Postbiotics containing flavonoids and phenolics from Pediococcus acidilactici & Latilactobacillus sakei/Staphylococcus xylosus reduce the number of Salmonella typhimurium in chicken drumstick (İncili et al., 2022). Dietary inclusion of Saccharomyces cerevisiae fermentation products may be a potential intervention to control Salmonella enterica in Poultry foods (Chaney et al., 2022). Postbiotic-containing preservatives were found to be equally effective as commonly used commercial preservatives in preserving vacuum-packaged cooked sausages, thus providing natural preservation technologies (de Lima et al., 2022). Although, these studies need to be substantiated with more robust in vivo trials.
Similarly, more extensive studies are required to elucidate the bactericidal effect of various LAB strains in the food sector. Postbiotics also aids in the biopreservation of dairy products since the viability of probiotic strains can be affected by extrinsic or intrinsic factors. Postbiotics can be used as a biocontrol alternative for making dairy products, fruits and vegetables safe. For instance, for controlling cheese blowing defects the role of different bacteriocins and bacteriocin-producing LAB is reported. Postbiotics can also be used as sanitizers in food industries (Moradi et al., 2020).
Functional food preparation
Host immune stimulation can be achieved primarily through the functional foods prepared using postbiotics. Milk fortified using postbiotics derived from B. breve and S. thermophilus reduces food intolerance and/or respiratory allergic reactions in early childhood (Scarpellini et al., 2021).
Food packaging
To improve the shelf-life of food, an active packaging approach is used which involves an interaction between food, packaging material and surrounding environment. This active postbiotic packaging system involves preservation and prevents microbial deterioration during delivery to the consumer. To increase the stability, the postbiotic can be applied in various ways such as thin coating or by covalent linkage for immobilization or by adding in the packaging matrix or by lamination on the polymer (Hosseini et al., 2021). Various types of postbiotic packaging are based on organic acids, peptides and bacteriocins. Organic acids are key candidates of postbiotics and are suitable anti-microbial factors. Lactic acid, citric acid and acetic acid disturbs the growth of pathogens by decreasing the cytoplasmic pH and membrane function. Through their pleiotropic action mechanisms, peptides and bacteriocins degrade the cell membrane, prevent synthesis of macromolecules and block microbial growth (Hosseini et al., 2021).
Sensory acceptability of postbiotics
Presence of postbiotics may change the sensory characteristics of foods, necessitating the need to evaluate different postbiotics containing foods for their sensory acceptability. Sensory evaluation of Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.) containing postbiotics produced by E. coli (Darwish et al., 2022) indicated consumer acceptance was significantly higher for most sensory aspects like appearance, smoothness, sourness, mouthfeel, and overall acceptance. The only aspect which was undesirable in comparison to the control sample was the color of the product. However, there was a significant decline in sensory properties of all yogurt samples with progressing storage periods (Darwish et al., 2022). In another study, sensory parameters of the lamb meat slices preserved using edible coating containing postbiotics of Lactobacillus paracasei ATCC 55544 (PLP) were assessed during cold storage. The results reveal that the sensory acceptability of samples preserved using edible coating were greater than the uncoated samples and there were no significant changes in color, appearance, and overall acceptance of the coated lamb meat slices throughout the storage period (Ozma et al., 2022). Similar results were demonstrated in a study done by Kamble et al. (2017), in which it was reported that application of postbiotics by spray method reduced the bacterial population on cold beef carcass surfaces without affecting the sensory specifications of meat. Garnier et al. (2019), investigated the sensory aspects of sour cream and semi-hard cheese to which postbiotics from LAB were added primarily to inhibit fungal spoilage. The study concluded that postbiotics significantly reduced the fungal population of cheese without affecting sensory acceptance, whereas postbiotics at 2 and 5% (w/w) concentrations had negative effects on the sensory attributes in sour cream. A review on postbiotics produced by lactic acid bacteria concluded that the postbiotics mixture or their purified metabolites are effective as packaging material or surface coatings which have the ability to prolong the shelf life of perishable foods without altering their sensory characteristics (Moradi et al., 2020). Given the health and nutritional benefits of postbiotics and minimal evidence of adverse effect on sensory characteristics of food, use of postbiotics should be promoted by food technologists and scientists.
Removal of biofilms
Biofilms are made of microbial polysaccharide or protein matrix. Listeria monocytogenes, Yersinia enterocolitica, Campylobacter jejuni, Staphylococcus aureus, and Bacillus cereus are biofilm-forming bacteria in the food sector. Biofilms are difficult to remove by conventional cleaning methods and disinfection. Postbiotics have antimicrobial properties and can destroy biofilms formed by bacteria. A study shows that Lactobacillus acidophilus LA5, Lactobacillus casei 431, and Lactobacillus salivarius demonstrates antimicrobial effect on a biofilm formed by L. monocytogenes on the polystyrene surfaces. It further demonstrated that biofilm reduction of L. monocytogene is brought about by bacteriocin and organic acid-based postbiotics. Therefore, postbiotics are utilized as an agent for the reduction of biofilm formation by pathogens in the food sector (Homayouni Rad et al., 2021). The Non-clinical application of Posbiotics are summarized in Figure 3.
Cosmetic applications
The human skin provides the first-line defense against external agents and acts as a physical and immunological barrier. Aggravation of skin diseases causes dysbiosis of the microbiome on the skin. In patients with acne there is a change in microbiota, which is dominated by C. acnes and S. epidermis. Scientific evidence indicates that use of topical probiotics reduces the number of acne causing bacteria like S. epidermidis, S. aureus, S. pyogenes and C. acnes (Lee et al., 2019). Bacteriocin from E. faecalis SL-5 containing lotion was found to reduce the inflammation in acne lesions. At acidic pH conditions, LactoSporin, an antimicrobial, heat stable peptide derived from Bacillus coagulans is suggested to be highly effective as a topical formulation (Majeed et al., 2020).
Biological doping
Biological doping is the new area for postbiotic application. Evidence suggests that overall physical performance of mice is increased by conversion of lactic acid to propionate, by the intestinal Veillonella species. The run-to exhaustion time increased in mice after administration of propionate, thereby showing a positive relationship with physical performance (Fernández-Sanjurjo et al., 2020). The Non-clinical use of Posbiotics are summarized in Table 2.
Future prospects
Biotics research shows that postbiotics are better alternatives to probiotics, due to their similar beneficial health effects and minimal risk of introducing live microbes. This is particularly beneficial for preterm infants and people with compromised defense systems. Although the microorganisms metabolites are being explored as a therapeutic option for a devastating intestinal disease called necrotizing enterocolitis (NEC) which affects premature or very low birth weight infants. Postbiotics can also be helpful in treating diseases like Alzheimer's disease or multiple sclerosis, whose effective therapies are still not found (Goswami et al., 2018).
Precision postbiotics
Another futuristic direction of postbiotic research is development of model “precision postbiotics” for effective therapeutic and preventive medicine. Precision medicine emphasizes on the medical treatment customized according to the patient instead of one drug that fits all models. So development of precision postbiotics for specific illness in specific subgroups of patients is an interesting proposition (Goswami et al., 2018; Veiga et al., 2020).
Nanotechnology
Nanotechnology's use in nutraceuticals has exploded in recent years, thanks to its capacity to improve the bioavailability of concentrated active components, leading to better therapeutic/nutraceutical results and necessitating the creation of nano nutraceuticals from nutritious elements such as antioxidants, vitamins, fatty acids etc. Natural chemical substances of medicinal importance include bioactive micro and macromolecules (postbiotics) produced by gut beneficial microorganisms. Currently, a novel treatment technique has been created that focuses on the microbiome's tiny molecular weight biomolecules, which provide the host with a variety of physiological health advantages. Scientific literature authenticates that active targeting systems based on nanoparticles can be used as an innovative and convenient approach for controlled delivery of postbiotics in-vivo. Administration of postbiotics is still in its early stages, and if it is to be widely used as a therapeutic strategy, it will require substantial research and randomized double-blind clinical studies (Nile et al., 2020).
Biomarkers for viral infections
According to the latest research, postbiotics structure and metabolic activity can be used as biomarkers for predicting viral diseases such as the coronavirus disease, and hence they can be helpful in the controlling COVID pandemic (Gou et al., 2020). CSFs from Lactobacillus plantarum Probio-88 found to contain the spread of coronavirus indicating microbiome modification approach may be used along with other therapies including vaccines against highly infectious pathogens (Rather et al., 2021).
Despite extensive research in postbiotics, there is still a lack of knowledge in the mechanistic actions to validate their health claims. Future research in postbiotics can pave the way toward turning microorganisms into functional ingredients. Postbiotics research can lead to an intersection of various fields like food, microbiology, health and personalized treatment.
Conclusion
To summarize, this review provides an overview of postbiotics as an emerging concept within the “-biotics” family, though the exact definition of postbiotics is yet to be accepted as functional food with clinical and non-clinical benefits. Postbiotics can be produced naturally from probiotics species most commonly from Lactobacillus species. Various laboratory methods like radiations (UV/ionizing), high pressure, high temperature, sonication and inactivation by formalin, can also be used to produce postbiotics. Postbiotics are less challenging than probiotics in terms of preparation, long shelf life as they do not require cold chains, precise action, etc. but their use is still in the nascent stage.
The paper highlights the main postbiotic components, such as inactivated and dead probiotics, microbial cells, metabolites, CFS, EPS, and their antimicrobial, antioxidant, and immunomodulatory properties with favorable physiological, immunological, neuro-hormonal, biological, regulatory and metabolic reactions.
There are many health and nutritional benefits of postbiotics which can treat a range of diseases and improve the general health status. Postbiotics have many non-clinical applications. They are able to increase food shelf life using an active packaging approach. These have cosmetic applications to reduce inflammation and acne. As positive biological responses to postbiotics have been observed in animal models & cell cultures, more human clinical trials are needed to assess their safety profile. A novel emerging area of postbiotic application is biological doping to study and enhance the physical performance of organisms.
In conclusion, postbiotics can be beneficial for the host's health even though the exact mechanisms are still under research. Advanced research on the biological response of the metabolites and host postbiotic interactions using different - omic approaches can reveal more applications of postbiotics in the clinical as well as non-clinical sector.
Author contributions
The conceptualization and design was contributed by SA, MS, and VS. Definition of intellectual content, literature search, manuscript preparation, manuscript editing, and manuscript review was equally contributed by all authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguilar-Toalá, J. E., Hall, F. G., Urbizo-Reyes, U. C., Garcia, H. S., Vallejo-Cordoba, B., González-Córdova, A. F., et al. (2019). In silico prediction and in vitro assessment of multifunctional properties of postbiotics obtained from two probiotic bacteria. Probiotics Antimicrob. Proteins 12, 608–622. doi: 10.1007/s12602-019-09568-z
Ahmadi Badi, S., Moshiri, A., Fateh, A., Rahimi Jamnani, F., Sarshar, M., Vaziri, F., et al. (2017). Microbiota-derived extracellular vesicles as new systemic regulators. Front. Microbiol. 8, 1610. doi: 10.3389/fmicb.2017.01610
Akatsu, H. (2021). Exploring the effect of probiotics, prebiotics, and postbiotics in strengthening immune activity in the elderly. Vaccines 9, 136. doi: 10.3390/vaccines9020136
Amiri, S., Rezazadeh-Bari, M., Alizadeh-Khaledabad, M., Rezaei-Mokarram, R., and Sowti-Khiabani, M. (2021). Fermentation optimization for co-production of postbiotics by Bifidobacterium lactis BB12 in cheese whey. Waste Biomass Valoriza. 12, 5869–5884. doi: 10.1007/s12649-021-01429-7
Angelin, J., and Kavitha, M. (2020). Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 162, 853–865. doi: 10.1016/j.ijbiomac.2020.06.190
Bach, J.-F. (2002). The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920. doi: 10.1056/NEJMra020100
Barros, C. P., Guimarães, J. T., Esmerino, E. A., Duarte, M. C. K., Silva, M. C., Silva, R., et al. (2020). Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr. Opin. Food Sci. 32, 1–8. doi: 10.1016/j.cofs.2019.12.003
Belguesmia, Y., Bendjeddou, K., Kempf, I., Boukherroub, R., and Drider, D. (2020). Heterologous biosynthesis of five new class II bacteriocins from Lactobacillus paracasei CNCM I-5369 with antagonistic activity against pathogenic Escherichia coli strains. Front. Microbiol. 11, 1198. doi: 10.3389/fmicb.2020.01198
Belizário, J. E., and Napolitano, M. (2015). Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 6, 1050. doi: 10.3389/fmicb.2015.01050
Beretta, S., Fabiano, V., Petruzzi, M., Budelli, A., and Zuccotti, G. V. (2015). Fermented rice flour in pediatric atopic dermatitis. Dermatitis 26, 104–106. doi: 10.1097/DER.0000000000000103
Beristain-Bauza, S., del, C., Mani-López, E., Palou, E., and López-Malo, A. (2017). Antimicrobial activity of whey protein films supplemented with Lactobacillus sakei cell-free supernatant on fresh beef. Food Microbiol. 62, 207–211. doi: 10.1016/j.fm.2016.10.024
Bermudez-Brito M. Plaza-Díaz J. Muñoz-Quezada S. Gómez-Llorente C. Gil A. (2012) Probiotic mechanisms of action. Ann. Nutr. Metabol. 61, 160–174. doi: 10.1159/000342079
Bird, A., Conlon, M., Christophersen, C., and Topping, D. (2010). Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef. Microbes 1, 423–431. doi: 10.3920/BM2010.0041
Caggianiello, G., Kleerebezem, M., and Spano, G. (2016). Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 100, 3877–3886. doi: 10.1007/s00253-016-7471-2
Cameotra, S. S., and Makkar, R. S. (2010). Biosurfactant-enhanced bioremediation of hydrophobic pollutants. Pure Appl. Chem. 82, 97–116. doi: 10.1351/PAC-CON-09-02-10
Campeotto, F., Suau, A., Kapel, N., Magne, F., Viallon, V., Ferraris, L., et al. (2011). A fermented formula in pre-term infants: clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 105, 1843–1851. doi: 10.1017/S0007114510005702
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M., and Owen, L. J. (2015). Dysbiosis of the gut microbiota in disease. Microbial Ecol. Health Dis. 26. doi: 10.3402/mehd.v26.26191
Caruso, A. A., Del Prete, A., and Lazzarino, A. I. (2020). Hydrogen peroxide and viral infections: a literature review with research hypothesis definition in relation to the current covid-19 pandemic. Med. Hypotheses 144, 109910. doi: 10.1016/j.mehy.2020.109910
Chan, M. Z. A., and Liu, S.-Q. (2022). Fortifying foods with synbiotic and postbiotic preparations of the probiotic yeast, Saccharomyces boulardii. Curr. Opin. Food Sci. 43, 216–224. doi: 10.1016/j.cofs.2021.12.009
Chaney, W. E., Naqvi, S. A., Gutierrez, M., Gernat, A., Johnson, T. J., and Petry, D. (2022). Dietary inclusion of a saccharomyces cerevisiae-derived postbiotic is associated with lower salmonella enterica burden in broiler chickens on a commercial farm in honduras. Microorganisms 10, 544. doi: 10.3390/microorganisms10030544
Chelakkot, C., Choi, Y., Kim, D.-K., Park, H. T., Ghim, J., Kwon, Y., et al. (2018). Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 50, e450–e450. doi: 10.1038/emm.2017.282
Cicenia, A., Santangelo, F., Gambardella, L., Pallotta, L., Iebba, V., Scirocco, A., et al. (2016). Protective role of postbiotic mediators secreted by Lactobacillus rhamnosus GG versus lipopolysaccharide-induced damage in human colonic smooth muscle cells. J. Clin. Gastroenterol. 50, S140–S144. doi: 10.1097/MCG.0000000000000681
Corsello, G., Carta, M., Marinello, R., Picca, M., De Marco, G., Micillo, M., et al. (2017). Preventive effect of cow's milk fermented with Lactobacillus paracasei CBA L74 on common infectious diseases in children: a multicenter randomized controlled trial. Nutrients 9, 669. doi: 10.3390/nu9070669
Dallal, M. M. S., Mojarrad, M., Baghbani, F., Raoofian, R., Mardaneh, J., and Salehipour, Z. (2015). Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on colorectal tumor cells activity (CaCo-2). Arch. Iran. Med. 18, 167–172.
Darwish, M. S., Qiu, L., Taher, M. A., Zaki, A. A., Abou-Zeid, N. A., Dawood, D. H., et al. (2022). Health benefits of postbiotics produced by E. coli nissle 1917 in functional yogurt enriched with cape gooseberry (Physalis peruviana L.). Fermentation, 8, 128. doi: 10.3390/fermentation8030128
de Almada, C. N., Almada, C. N., Martinez, R. C. R., and Sant'Ana, A. S. (2016). Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 58, 96–114. doi: 10.1016/j.tifs.2016.09.011
de Almeida Júnior, W. L. G., Ferrari, Í., da, S., de Souza, J. V., da Silva, C. D. A., da Costa, M. M., et al. (2015). Characterization and evaluation of lactic acid bacteria isolated from goat milk. Food Control 53, 96–103. doi: 10.1016/j.foodcont.2015.01.013
de Las M Cardoso, M., Manzo, R.M., Tonarelli, G.G., and Simonetta, A.C. (2012). Characterisation of a cell-free supernatant obtained from cultures ofEnterococcus faecalisDBFIQ E24 with antagonistic activity against bacteria, yeasts and moulds. Int. J. Dairy Technol. 65, 568–577. doi: 10.1111/j.1471-0307.2012.00852.x
de Lima, A. L., Guerra, C. A., Costa, L. M., de Oliveira, V. S., Lemos Junior, W. J. F., Luchese, R. H., et al. (2022). A natural technology for vacuum-packaged cooked sausage preservation with potentially postbiotic-containing preservative. Fermentation 8, 106. doi: 10.3390/fermentation8030106
De Marco, S., Sichetti, M., Muradyan, D., Piccioni, M., Traina, G., Pagiotti, R., et al. (2018). Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid. Based Comp. Altern. Med. 2018, 1–12. doi: 10.1155/2018/1756308
den Besten, G., van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D.-J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Dinić, M., Pecikoza, U., Djokić, J., Stepanović-Petrović, R., Milenković, M., Stevanović, M., et al (2018) Exopolysaccharide produced by probiotic strain lactobacillus paraplantarum bgcg11 reduces inflammatory hyperalgesia in rats. Front. Pharmacol. 9, 1. doi: 10.3389/fphar.2018.00001.
Engevik, M. A., Ruan, W., Esparza, M., Fultz, R., Shi, Z., Engevik, K. A., et al. (2021). Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites. Physiol. Rep. 9. doi: 10.14814/phy2.14719
Fang, S.-B., Shih, H.-Y., Huang, C.-H., Li, L.-T., Chen, C.-C., and Fang, H.-W. (2014). Live and heat-killed Lactobacillus rhamnosus GG upregulate gene expression of pro-inflammatory cytokines in 5-fluorouracil-pretreated Caco-2 cells. Support. Care Cancer 22, 1647–1654. doi: 10.1007/s00520-014-2137-z
Fernández-Sanjurjo, M., Fernández, J., Tomás-Zapico, C., Fernández-García, B., Villar, C. J., Lomb,ó, F., et al. (2020). Is physical performance (in mice) increased by Veillonella atypica or decreased by Lactobacillus bulgaricus? J. Sport Health Sci. 9, 197–200. doi: 10.1016/j.jshs.2020.02.005
Fichera, G. A., Fichera, M., and Milone, G. (2016). Antitumoural activity of a cytotoxic peptide of Lactobacillus casei peptidoglycan and its interaction with mitochondrial-bound hexokinase. Anticancer. Drugs 27, 609–619. doi: 10.1097/CAD.0000000000000367
Franchi, L., Warner, N., Viani, K., and Nuñez, G. (2009). Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 227, 106–128. doi: 10.1111/j.1600-065X.2008.00734.x
Garnier, L., Mounier, J., Lê, S., Pawtowski, A., Pinon, N., Camier, B., et al. (2019). Development of antifungal ingredients for dairy products: from in vitro screening to pilot scale application. Food Microbiol. 81, 97–107. doi: 10.1016/j.fm.2018.11.003
George-Okafor, U., Ozoani, U., Tasie, F., and Mba-Omeje, K. (2020). The efficacy of cell-free supernatants from Lactobacillus plantarum Cs and Lactobacillus acidophilus ATCC 314 for the preservation of home-processed tomato-paste. Sci. Afr. 8, e00395. doi: 10.1016/j.sciaf.2020.e00395
Gómez-Sala, B., Herranz, C., Díaz-Freitas, B., Hernández, P. E., Sala, A., and Cintas, L. M. (2016). Strategies to increase the hygienic and economic value of fresh fish: Biopreservation using lactic acid bacteria of marine origin. Int. J. Food Microbiol. 223, 41–49. doi: 10.1016/j.ijfoodmicro.2016.02.005
Goswami, C., Iwasaki, Y., and Yada, T. (2018). Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 57, 130–135. doi: 10.1016/j.jnutbio.2018.03.009
Gou, W., Fu, Y., Yue, L., Chen, G., Cai, X., Shuai, M., et al. (2020). Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv. doi: 10.1101/2020.04.22.20076091
Guéniche, A., Bastien, P., Ovigne, J. M., Kermici, M., Courchay, G., Chevalier, V., et al. (2009). Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp. Dermatol. 19, e1–e8. doi: 10.1111/j.1600-0625.2009.00932.x
Guo, B., Xie, N., and Wang, Y. (2015). Cooperative effect of Bifidobacteria lipoteichoic acid combined with 5-fluorouracil on hepatoma-22 cells growth and apoptosis. Bull. Cancer 102, 204–212. doi: 10.1016/j.bulcan.2014.09.003
Hamad, G. M., Abdelmotilib, N. M., Darwish, A. M. G., and Zeitoun, A. M. (2020). Commercial probiotic cell-free supernatants for inhibition of Clostridium perfringens poultry meat infection in Egypt. Anaerobe 62, 102181. doi: 10.1016/j.anaerobe.2020.102181
Hamad, G. M., Botros, W. A., and Hafez, E. E. (2017). Combination of probiotic filtrates as antibacterial agent against selected some pathogenic bacteria in milk and cheese. Int. J. Dairy Sci. 12, 368–376. doi: 10.3923/ijds.2017.368.376
Hamann, L. E. L.-, Samalouti, V., Ulmer, A. J., Flad, H.-D., and Rietschel, E.T. (1998). Components of gut bacteria as immunomodulators. Int. J. Food Microbiol. 41, 141–154. doi: 10.1016/S0168-1605(98)00047-6
Hartmann, H. A., Wilke, T., and Erdmann, R. (2011). Efficacy of bacteriocin-containing cell-free culture supernatants from lactic acid bacteria to control Listeria monocytogenes in food. Int. J. Food Microbiol. 146, 192–199. doi: 10.1016/j.ijfoodmicro.2011.02.031
Herrera, A. R., Ludwig, T., Bouritius, H., Rubio, R. P., Muñoz, A., Agosti, M., et al. (2015). OP-18 the combination of SCGOS/LCFOS and fermented infant formula softens stools of infants compared to unfermented infant formula without SCGOS/LCFOS. J. Pediatr. Gastroenterol. Nutr. 61, 516–517. doi: 10.1097/01.mpg.0000472222.09292.b9
Hickey, A., Stamou, P., Udayan, S., Ramón-Vázquez, A., Esteban-Torres, M., Bottacini, F., et al. (2021). Bifidobacterium breve exopolysaccharide blocks dendritic cell maturation and activation of CD4+ T cells. Front. Microbiol. 12, 653587. doi: 10.3389/fmicb.2021.653587
Hill, M. J. (1997). Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 6, S43–S45. doi: 10.1097/00008469-199703001-00009
Hoarau, C., Lagaraine, C., Martin, L., Velge-Roussel, F., and Lebranchu, Y. (2006). Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 117, 696–702. doi: 10.1016/j.jaci.2005.10.043
Homayouni Rad, A., Aghebati-Maleki, L., Samadi Kafil, H., Gilani, N., Abbasi, A., and Khani, N. (2021). Postbiotics, as dynamic biomolecules, and their promising role in promoting food safety. Biointerface Res. Appl. Chem. 11, 14529–14544. doi: 10.33263/BRIAC116.1452914544
Hosseini, S. A., Abbasi, A., Sabahi, S., and Khani, N. (2021). Application of postbiotics produced by lactic acid bacteria in the development of active food packaging. Biointerface Res. Appl. Chem. 12, 6164–6183. doi: 10.33263/BRIAC125.61646183
Humam, A. M., Loh, T. C., Foo, H. L., Izuddin, W. I., Zulkifli, I., Samsudin, A. A., et al. (2021). Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci. 100, 100908. doi: 10.1016/j.psj.2020.12.011
Imaoka, A., Shima, T., Kato, K., Mizuno, S., Uehara, T., Matsumoto, S., et al. (2008). Anti-inflammatory activity of probiotic Bifidobacterium: enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 14, 2511. doi: 10.3748/wjg.14.2511
İncili, G. K., Karatepe, P., Akgöl, M., Güngören, A., Koluman, A., Ilhak, O. I., et al. (2022). Characterization of lactic acid bacteria postbiotics, evaluation in-vitro antibacterial effect, microbial and chemical quality on chicken drumsticks. Food Microbiol. 104, 104001. doi: 10.1016/j.fm.2022.104001
Isozaki, S., Konishi, H., Fujiya, M., Tanaka, H., Murakami, Y., Kashima, S., et al. (2021). Probiotic-derived polyphosphate accelerates intestinal epithelia wound healing through inducing platelet-derived mediators. Mediat. Inflamm. 11, 1–14. doi: 10.1155/2021/5582943
Izuddin, W. I., Humam, A. M., Loh, T. C., Foo, H. L., and Samsudin, A. A. (2020). Dietary postbiotic Lactobacillus plantarum improves serum and ruminal antioxidant activity and upregulates hepatic antioxidant enzymes and ruminal barrier function in post-weaning lambs. Antioxidants 9, 250. doi: 10.3390/antiox9030250
Jonkuviene, D., Vaičiulyte-Funk, L., Šalomskiene, J., Alenčikiene, G., and MieŽeliene, A. (2016). Potential of Lactobacillus reuteri from spontaneous sourdough as a starter additive for improving quality parameters of bread. Food Technol. Biotechnol. 54, 342–350. doi: 10.17113/ftb.54.03.16.4143
Jurášková, D., Ribeiro, S. C., and Silva, C. C. G. (2022). Exopolysaccharides produced by lactic acid bacteria: from biosynthesis to health-promoting properties. Foods 11, 156. doi: 10.3390/foods11020156
Kamble, P. K., Rao, V. A., Abraham, R. J. J., and Dhanalakshmi, B. (2017). Effect of pediocin NCDC252 as cell free supernatant produced from Pediococus acidilactici NCDC252 with EDTA on total viable count and sensory evaluation of chicken carcasses stored at refrigeration temperature. Int. J. Curr. Microbiol. Appl. Sci. 6, 2269–2276. doi: 10.20546/ijcmas.2017.607.327
Kamiya, T., Wang, L., Forsythe, P., Goettsche, G., Mao, Y., Wang, Y., et al. (2006). Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 55, 191–196. doi: 10.1136/gut.2005.070987
Kareem, K., Hooi Ling, F., Teck Chwen, L., May Foong, O., and Anjas Asmara, S. (2014). Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 6, 23. doi: 10.1186/1757-4749-6-23
Khmaladze, I., Butler, É., Fabre, S., and Gillbro, J. M. (2019). Lactobacillus reuteri DSM 17938—A comparative study on the effect of probiotics and lysates on human skin. Exp. Dermatol. 28, 822–828. doi: 10.1111/exd.13950
Kim, S. W., Ha, Y. J., Bang, K. H., Lee, S., Yeo, J.-H., Yang, H.-S., et al. (2020). Potential of bacteriocins from Lactobacillus taiwanensis for producing bacterial ghosts as a next generation vaccine. Toxins 12, 432. doi: 10.3390/toxins12070432
Kirjavainen, P. V., Salminen, S. J., and Isolauri, E. (2003). Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J. Pediatr. Gastroenterol. Nutr. 36, 223–227. doi: 10.1097/00005176-200302000-00012
Konings, W. N., Poolman, B., Driessen, A. J. M., and Maloney, P. C. (1989). Bioenergetics and solute transport in lactococci. CRC Crit. Rev. Microbiol. 16, 419–476. doi: 10.3109/10408418909104474
Lebeer, S., Claes, I., Tytgat, H. L. P., Verhoeven, T. L. A., Marien, E., von Ossowski, I., et al. (2011). Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 78, 185–193. doi: 10.1128/AEM.06192-11
LeBlanc, J. G., Milani, C., de Giori, G. S., Sesma, F., van Sinderen, D., and Ventura, M. (2013). Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168. doi: 10.1016/j.copbio.2012.08.005
Lee, J., Lee, J.-E., Kim, S., Kang, D., and Yoo, H. M. (2020). Evaluating cell death using cell-free supernatant of probiotics in three-dimensional spheroid cultures of colorectal cancer cells. J. Vis. Exp. doi: 10.3791/61285
Lee, J., Park, S., Oh, N., Park, J., Kwon, M., Seo, J., et al. (2021). Oral intake of Lactobacillus plantarum L-14 extract alleviates TLR2- and AMPK-mediated obesity-associated disorders in high-fat-diet-induced obese C57BL/6J mice. Cell Prolif. 54. doi: 10.1111/cpr.13039
Lee, K. J., Park, H. W., Choi, E. J., and Chun, H. H. (2016). Effects of CFSs produced by lactic acid bacteria in combination with grape seed extract on the microbial quality of ready-to-eat baby leaf vegetables. Cogent Food Agric. 2. doi: 10.1080/23311932.2016.1268742
Lee, Y. B., Byun, E. J., and Kim, H. S. (2019). Potential role of the microbiome in acne: a comprehensive review. J. Clin. Med. 8, 987. doi: 10.3390/jcm8070987
Lim, H.-S., Yeu, J.-E., Hong, S.-P., and Kang, M.-S. (2018). Characterization of antibacterial cell-free supernatant from oral care probiotic Weissella cibaria, CMU. Molecules 23, 1984. doi: 10.3390/molecules23081984
Liu, J., Luo, J., Ye, H., Sun, Y., Lu, Z., and Zeng, X. (2010). In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 82, 1278–1283. doi: 10.1016/j.carbpol.2010.07.008
Mager, D. (2006). Bacteria and cancer: cause, coincidence or cure? A review. J. Transl. Med. 4. doi: 10.1186/1479-5876-4-14
Majeed, M., Majeed, S., Nagabhushanam, K., Mundkur, L., Rajalakshmi, H. R., Shah, K., et al. (2020). Novel topical application of a postbiotic, LactoSporin®, in mild to moderate acne: a randomized, comparative clinical study to evaluate its efficacy, tolerability and safety. Cosmetics 7, 70. doi: 10.3390/cosmetics7030070
Ménard, S., Laharie, D., Asensio, C., Vidal-Martinez, T., Candalh, C., Rullier, A., et al. (2005). Bifidobacterium breve and Streptococcus thermophilus secretion products enhance T helper 1 immune response and intestinal barrier in mice. Exp. Biol. Med. 230, 749–756. doi: 10.1177/153537020523001008
Mi, X.-J., Tran, T. H. M., Park, H.-R., Xu, X. Y., Subramaniyam, S., Choi, H. S., et al. (2022). Immune-enhancing effects of postbiotic produced by Bacillus velezensis Kh2-2 isolated from Korea Foods. Food Res. Int. 152, 110911. doi: 10.1016/j.foodres.2021.110911
Mirnejad, R., Vahdati, A. R., Rashidiani, J., Erfani, M., and Piranfar, V. (2013). The antimicrobial effect of Lactobacillus casei culture supernatant against multiple drug resistant clinical isolates of shigella sonnei and shigella flexneri in vitro. Iran. Red Crescent Med. J. 15, 122–126. doi: 10.5812/ircmj.7454
Montazeri-Najafabady, N., Ghasemi, Y., Dabbaghmanesh, M. H., Ashoori, Y., Talezadeh, P., Koohpeyma, F., et al. (2021). Exploring the bone sparing effects of postbiotics in the post-menopausal rat model. BMC Compl. Med. Ther. 21. doi: 10.1186/s12906-021-03327-w
Moradi, M., Kousheh, S. A., Almasi, H., Alizadeh, A., Guimarães, J. T., Yilmaz, N., et al. (2020). Postbiotics produced by lactic acid bacteria: the next frontier in food safety. Compr. Rev. Food Sci. Food Safety. 19, 3390–3415. doi: 10.1111/1541-4337.12613
Moroi, M., Uchi, S., Nakamura, K., Sato, S., Shimizu, N., et al. (2010). Beneficial effect of a diet containing heat-killed Lactobacillus paracasei K71 on adult type atopic dermatitis. J. Dermatol. 38, 131–139. doi: 10.1111/j.1346-8138.2010.00939.x
Moscovici, M. (2015). Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 6, 1012. doi: 10.3389/fmicb.2015.01012
Murata, M., Kondo, J., Iwabuchi, N., Takahashi, S., Yamauchi, K., Abe, F., et al. (2018). Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef. Microbes 9, 855–864. doi: 10.3920/BM2017.0197
Nataraj, B. H., Ali, S. A., Behare, P. V., and Yadav, H. (2020). Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 19. doi: 10.1186/s12934-020-01426-w
Negash, A. W., and Tsehai, B. A. (2020). Current applications of bacteriocin. Int. J. Microbiol. 2020, 1–7. doi: 10.1155/2020/4374891
Nile, S. H., Baskar, V., Selvaraj, D., Nile, A., Xiao, J., and Kai, G. (2020). Nanotechnologies in food science: applications, recent trends, and future perspectives. Nano Micro Lett. 12. doi: 10.1007/s40820-020-0383-9
Nishida, K., Sawada, D., Kuwano, Y., Tanaka, H., and Rokutan, K. (2019). Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: a randomized, double-blind, placebo-controlled study. Nutrients 11, 1859. doi: 10.3390/nu11081859
Nishida, K., Sawada, D., Kuwano, Y., Tanaka, H., Sugawara, T., Aoki, Y., et al. (2017). Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 36, 112–121. doi: 10.1016/j.jff.2017.06.031
Nocerino, R., Paparo, L., Terrin, G., Pezzella, V., Amoroso, A., Cosenza, L., et al. (2017). Cow's milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: a randomized controlled trial. Clin. Nutr. 36, 118–125. doi: 10.1016/j.clnu.2015.12.004
Nozari, S., Faridvand, Y., Etesami, A., Ahmad Khan Beiki, M., Miresmaeili Mazrakhondi, S. A., and Abdolalizadeh, J. (2019). Potential anticancer effects of cell wall protein fractions from Lactobacillus paracasei on human intestinal Caco-2 cell line. Lett. Appl. Microbiol. 69, 148–154. doi: 10.1111/lam.13198
Oscáriz, J. C., and Pisabarro, A. G. (2001). Classification and mode of action of membrane-active bacteriocins produced by gram-positive bacteria. Int. Microbiol. 4, 13–19. doi: 10.1007/s101230100003
Ozma, M. A., Abbasi, A., and Sabahi, S. (2022). Characterization of postbiotics derived from Lactobacillus paracasei ATCC 55544 and its application in malva sylvestris seed mucilage edible coating to the improvement of the microbiological, and sensory properties of lamb meat during storage. Biointer. Res. Appl. Chem. 13. doi: 10.33263/BRIAC133.267
Park, J.-S., Lee, E.-J., Lee, J.-C., Kim, W.-K., and Kim, H.-S. (2007). Anti-inflammatory effects of short chain fatty acids in IFN-γ-stimulated RAW 264.7 murine macrophage cells: involvement of NF-κB and ERK signaling pathways. Int. Immunopharmacol. 7, 70–77. doi: 10.1016/j.intimp.2006.08.015
Patterson, E., Cryan, J. F., Fitzgerald, G. F., Ross, R. P., Dinan, T. G., and Stanton, C. (2014). Gut microbiota, the pharmabiotics they produce and host health. Proc. Nutr. Soc. 73, 477–489. doi: 10.1017/S0029665114001426
Peluzio, M., do, C. G., Martinez, J. A., and Milagro, F.I. (2021). Postbiotics: metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 108, 11–26. doi: 10.1016/j.tifs.2020.12.004
Perez, R. H., Zendo, T., and Sonomoto, K. (2014). Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb. Cell Fact. 13, S3. doi: 10.1186/1475-2859-13-S1-S3
Pridmore, R. D., Pittet, A.-C., Praplan, F., and Cavadini, C. (2008). Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol. Lett. 283, 210–215. doi: 10.1111/j.1574-6968.2008.01176.x
Pyclik, M., Srutkova, D., Schwarzer, M., and Górska, S. (2020). Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins – their chemical structure and biological attributes. Int. J. Biol. Macromol. 147, 333–349. doi: 10.1016/j.ijbiomac.2019.12.227
Rad, A. H., Abbasi, A., Kafil, H. S., and Ganbarov, K. (2020). Potential pharmaceutical and food applications of postbiotics: a review. Curr. Pharm. Biotechnol. 21, 1576–1587. doi: 10.2174/1389201021666200516154833
Rampengan, N. H., Manoppo, J., and Warouw, S. M. (2010). Comparison of efficacies between live and killed probiotics in children with lactose malabsorption. Southeast Asian J. Trop. Med. Public Health 41, 474–481 .
Rather, I. A., Choi, S.-B., Kamli, M. R., Hakeem, K. R., Sabir, J. S. M., Park, Y.-H., et al. (2021). Potential adjuvant therapeutic effect of Lactobacillus plantarum probio-88 postbiotics against SARS-CoV-2. Vaccines 9, 1067. doi: 10.3390/vaccines9101067
Rowland, I., Gibson, G., Heinken, A., Scott, K., Swann, J., Thiele, I., et al. (2017). Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 57, 1–24. doi: 10.1007/s00394-017-1445-8
Russo, E., Giudici, F., Fiorindi, C., Ficari, F., Scaringi, S., and Amedei, A. (2019). Immunomodulating activity and therapeutic effects of short chain fatty acids and tryptophan post-biotics in inflammatory bowel disease. Front. Immunol. 10, 2754. doi: 10.3389/fimmu.2019.02754
Salminen, S., Collado, M. C., Endo, A., Hill, C., Lebeer, S., Quigley, E. M. M., et al. (2021). The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667. doi: 10.1038/s41575-021-00481-x
Sanaei, M., Mahdavi, M., Setayesh, N., Shahverdi, A. R., Sepehrizadeh, Z., and Yazdi, M. H. (2021). Comparison of cytokine expression in human PBMCs stimulated with normal and heat-shocked lactobacillus plantarum cell lysate. Probiotics Antimicrob. Proteins 13, 1539–1545. doi: 10.1007/s12602-021-09785-5
Sanders, M. (2009). How do we know when something called “probiotic” is really a probiotic? a guideline for consumers and health care professionals. Funct. Food Rev. 1, 3–12.
Satpute, S. K., Kulkarni, G. R., Banpurkar, A. G., Banat, I. M., Mone, N. S., Patil, R. H., et al. (2016). Biosurfactant/s from Lactobacilli species: properties, challenges and potential biomedical applications. J. Basic Microbiol. 56, 1140–1158. doi: 10.1002/jobm.201600143
Scarpellini, E., Rinninella, E., Basilico, M., Colomier, E., Rasetti, C., Larussa, T., et al. (2021). From pre- and probiotics to post-biotics: a narrative review. Int. J. Environ. Res. Public Health 19, 37. doi: 10.3390/ijerph19010037
Schaefer, A. K., Melnyk, J. E., He, Z., Del Rosario, F., and Grimes, C. L. (2018). Pathogen- and microbial- associated molecular patterns (PAMPs/MAMPs) and the innate immune response in crohn's disease. Immun. Inflamm. Health Dis. 11, 175–187. doi: 10.1016/B978-0-12-805417-8.00014-7
Shinkai, S., Toba, M., Saito, T., Sato, I., Tsubouchi, M., Taira, K., et al. (2012). Immunoprotective effects of oral intake of heat-killed Lactobacillus pentosus strain b240 in elderly adults: a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 109, 1856–1865. doi: 10.1017/S0007114512003753
Singh, A. K., Hertzberger, R. Y., and Knaus, U. G. (2018). Hydrogen peroxide production by lactobacilli promotes epithelial restitution during colitis. Redox Biol. 16, 11–20. doi: 10.1016/j.redox.2018.02.003
Singh, T. P., Kaur, G., Kapila, S., and Malik, R. K. (2017). Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 8, 486. doi: 10.3389/fmicb.2017.00486
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermudez-Humaran, L. G., Gratadoux, J.-J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Nat. Acad. Sci. U. S. A. 105, 16731–16736. doi: 10.1073/pnas.0804812105
Soltani, S., Hammami, R., Cotter, P. D., Rebuffat, S., Said, L. B., Gaudreau, H., et al. (2020). Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol. Rev. 45. doi: 10.1093/femsre/fuaa039
Sugahara, H., Yao, R., Odamaki, T., and Xiao, J. Z. (2017). Differences between live and heat-killed bifidobacteria in the regulation of immune function and the intestinal environment. Benef. Microbes 8, 463–472. doi: 10.3920/BM2016.0158
Šušković, J., Kos, B., Beganović, J., Leboš Pavunc, A., Habjanič, K., and Matošić, S. (2010). Antimicrobial activity – the most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 48, 296–307.
Swoboda, J. G., Campbell, J., Meredith, T. C., and Walker, S. (2009). Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 11, 35–45. doi: 10.1002/cbic.200900557
Tanaka, M., and Nakayama, J. (2017). Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 66, 515–522. doi: 10.1016/j.alit.2017.07.010
Tapiovaara, L., Kumpu, M., Mäkivuokko, H., Waris, M., Korpela, R., Pitkäranta, A., et al. (2016). Human rhinovirus in experimental infection after peroral Lactobacillus rhamnosus GG consumption, a pilot study. Int. Forum Allergy Rhinol. 6, 848–853. doi: 10.1002/alr.21748
Tarrerias, A. L., Costil, V., Vicari, F., Létard, J. C., Adenis-Lamarre, P., Aisène, A., et al. (2011). The effect of inactivated Lactobacillus LB fermented culture medium on symptom severity: observational investigation in 297 patients with diarrhea-predominant irritable bowel syndrome. Digest. Dis. 29, 588–591. doi: 10.1159/000332987
Taverniti, V., and Guglielmetti, S. (2011). The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. 6, 261–274. doi: 10.1007/s12263-011-0218-x
Teame, T., Wang, A., Xie, M., Zhang, Z., Yang, Y., Ding, Q., et al. (2020). Paraprobiotics and postbiotics of probiotic lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 7, 570344. doi: 10.3389/fnut.2020.570344
Toda, K., Hisata, K., Satoh, T., Katsumata, N., Odamaki, T., Mitsuyama, E., et al. (2019). Neonatal oral fluid as a transmission route for bifidobacteria to the infant gut immediately after birth. Sci. Rep. 9. doi: 10.1038/s41598-019-45198-9
Tok, E., and Aslim, B. (2010). Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol. Immunol. 54, 257–64. doi: 10.1111/j.1348-0421.2010.00219.x
Topping, D. L. (1996). Short-chain fatty acids produced by intestinal bacteria. Asia Pac J Clin Nutr. 5, 15–19.
Tukenmez, U., Aktas, B., Aslim, B., and Yavuz, S. (2019). The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 9. doi: 10.1038/s41598-019-44753-8
Ursell, L. K., Metcalf, J. L., Parfrey, L. W., and Knight, R. (2012). Defining the human microbiome. Nutr. Rev. 70, S38–S44. doi: 10.1111/j.1753-4887.2012.00493.x
Vandenplas, Y., Ludwig, T., Bouritius, H., Alliet, P., Forde, D., Peeters, S., et al. (2017). Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides reduces the incidence of infantile colic. Acta Paediatr. 106, 1150–1158. doi: 10.1111/apa.13844
Varian, B. J., Poutahidis, T., DiBenedictis, B. T., Levkovich, T., Ibrahim, Y., Didyk, E., et al. (2017). Microbial lysate upregulates host oxytocin. Brain Behav. Immun. 61, 36–49. doi: 10.1016/j.bbi.2016.11.002
Veiga, P., Suez, J., Derrien, M., and Elinav, E. (2020). Moving from probiotics to precision probiotics. Nat. Microbiol. 5, 878–880. doi: 10.1038/s41564-020-0721-1
Verma, R., Lee, C., Jeun, E.-J., Yi, J., Kim, K. S., Ghosh, A., et al. (2018). Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3 + regulatory T cells. Sci. Immunol. 3. doi: 10.1126/sciimmunol.aat6975
Vinolo, M. A. R., Rodrigues, H. G., Hatanaka, E., Sato, F. T., Sampaio, S. C., and Curi, R. (2011). Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 22, 849–855. doi: 10.1016/j.jnutbio.2010.07.009
Vyas, U., and Ranganathan, N. (2012). Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterol. Res. Pract. 2012, 1–16. doi: 10.1155/2012/872716
Wachi, S., Kanmani, P., Tomosada, Y., Kobayashi, H., Yuri, T., Egusa, S., et al. (2014). Lactobacillus delbrueckii TUA4408L and its extracellular polysaccharides attenuate enterotoxigenic Escherichia coli- induced inflammatory response in porcine intestinal epitheliocytes via Toll-like receptor-2 and 4. Mol. Nutr. Food Res. 58, 2080–2093. doi: 10.1002/mnfr.201400218
Wang, K., Li, W., Rui, X., Chen, X., Jiang, M., and Dong, M. (2014). Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 63, 133–139. doi: 10.1016/j.ijbiomac.2013.10.036
Wang, K., Niu, M., Song, D., Song, X., Zhao, J., Wu, Y., et al. (2020). Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 129, 206–214. doi: 10.1016/j.jbiosc.2019.07.009
Wang, Y., Xie, J., Wang, N., Li, Y., Sun, X., Zhang, Y., et al. (2013). Lactobacillus caseiZhang modulate cytokine and Toll-like receptor expression and beneficially regulate poly I:C-induced immune responses in RAW264.7 macrophages. Microbiol. Immunol. 57, 54–62. doi: 10.1111/j.1348-0421.516.x
Wegh, C. A., Geerlings, S. Y., Knol, J., Roeselers, G., and Belzer, C. (2019). Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 20, 4673. doi: 10.3390/ijms20194673
Xiao, S.-D., De Zhang, Z., Lu, H., Jiang, S. H., Liu, H. Y., Wang, G. S., et al. (2003). Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Adv. Ther. 20, 253–260. doi: 10.1007/BF02849854
Yan, F., Cao, H., Cover, T. L., Whitehead, R., Washington, M. K., and Polk, D. B. (2007). Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132, 562–575. doi: 10.1053/j.gastro.2006.11.022
Yang, K. M., Kim, J.-S., Kim, H.-S., Kim, Y.-Y., Oh, J.-K., Jung, H.-W., et al. (2021). Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci. Rep. 11. doi: 10.1038/s41598-020-80921-x
Yelin, I., Flett, K. B., Merakou, C., Mehrotra, P., Stam, J., Snesrud, E., et al. (2019). Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 25, 1728–1732. doi: 10.1038/s41591-019-0626-9
Yeşilyurt, N., Yilmaz, B., Agagündüz, D., and Capasso, R. (2021). Involvement of probiotics and postbiotics in the immune system modulation. Biologics 1, 89–110. doi: 10.3390/biologics1020006
Zagato, E., Mileti, E., Massimiliano, L., Fasano, F., Budelli, A., Penna, G., et al. (2014). Lactobacillus paracasei CBA L74 metabolic products and fermented milk for infant formula have anti-inflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLoS ONE 9, e87615. doi: 10.1371/journal.pone.0087615
Zakostelska, Z., Kverka, M., Klimesova, K., Rossmann, P., Mrazek, J., Kopecny, J., et al. (2011). Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE 6, e27961. doi: 10.1371/journal.pone.0027961
Zeng, J., Jiang, J., Zhu, W., and Chu, Y. (2015). Heat-killed yogurt-containing lactic acid bacteria prevent cytokine-induced barrier disruption in human intestinal Caco-2 cells. Ann. Microbiol. 66, 171–178. doi: 10.1007/s13213-015-1093-2
Zhao, L., Ni, Y., Su, M., Li, H., Dong, F., Chen, W., et al. (2017). High throughput and quantitative measurement of microbial metabolome by gas chromatography/mass spectrometry using automated alkyl chloroformate derivatization. Anal. Chem. 89, 5565–5577. doi: 10.1021/acs.analchem.7b00660
Keywords: postbiotics, microbiome, cell free suspensions (CFS), bacterial lysate, Lactobacillus, nutritional benefits
Citation: Aggarwal S, Sabharwal V, Kaushik P, Joshi A, Aayushi A and Suri M (2022) Postbiotics: From emerging concept to application. Front. Sustain. Food Syst. 6:887642. doi: 10.3389/fsufs.2022.887642
Received: 01 March 2022; Accepted: 01 September 2022;
Published: 21 September 2022.
Edited by:
Anil Panghal, Chaudhary Charan Singh Haryana Agricultural University, IndiaReviewed by:
Jayanta Kumar Patra, Dongguk University Seoul, South KoreaAdriano Gomes Cruz, Adriano Gomes da Cruz, Brazil
Copyright © 2022 Aggarwal, Sabharwal, Kaushik, Joshi, Aayushi and Suri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manjula Suri, bWFuanVsYS5zdXJpQGloZS5kdS5hYy5pbg==
†These authors share first authorship
 Sunita Aggarwal
Sunita Aggarwal Vandana Sabharwal2†
Vandana Sabharwal2† Pragya Kaushik
Pragya Kaushik Anushka Joshi
Anushka Joshi