- 1AgroBiosciences, Plant and Soil Microbiome Subprogram, Mohammed VI Polytechnic University, Ben Guerir, Morocco
- 2Agrobiotechnology & Bioengineering Center, Reasearch Unit CNRST labeled, Faculty of Sciences & Techniques, Cadi Ayyad University, Marrakech, Morocco
- 3African Sustainable Agriculture Research Institute, Laayoune, Mohammed VI Polytechnic University, Ben Guerir, Morocco
- 4Anhalt University of Applied Sciences, Bernburg, Germany
- 5Situation Innovation-OCP Group, El Jadida, Morocco
Our study aimed to assess the role of inoculation of faba bean/wheat intercrops with selected rhizobacterial consortia (composed of one rhizobium and two P solubilizing bacteria “PSB”) to alleviate the effects of combined water deficit and P limitation on faba bean/wheat intercropping vs. monocropping under greenhouse conditions. One Vicia faba L (Aguadulce) and one Triticum durum L. variety (Karim) were grown as a sole crop or were intercropped in pots containing a sterilized substrate (sand:peat 4:1 v/v) with either rock phosphate (RP) (unavailable P) or KH2PO4 in the nutrient solution (available P). Plant inoculation was performed using the rhizobacterial consortia C1 (Rhizobium laguerreae, Kocuria sp., and Pseudomonas sp.) and C2 (R. laguerreae, Rahnella sp., and Kocuria sp.). Two weeks after inoculation, the plants were subjected to water deficit with 40% substrate water holding capacity (WHC) vs. 80% WHC for the well-watered plants. The trial was assessed at the flowering stage, and the results showed that inoculation with both consortia (C1 and C2) improved faba bean biomass in terms of shoot, root, and nodules dry weight compared to inoculation with rhizobia alone. C2 improved these parameters by 19.03, 78.99, and 72.73%, respectively. The relative leaf water content decreased under combined stress, especially in response to C1 conferring significant improvement of this parameter in wheat intercrops. In faba bean under P limitation, inoculation with C2 increased stomatal conductance (gs), phosphatase, and phytase activity by 35.73, 166.94, and 26.16%, respectively, compared to plants inoculated with rhizobia alone. Furthermore, C2 also improved membrane stability under P deficit by 44.33 vs. 16.16% for C1 as compared to inoculation with rhizobia alone. In sole-cropped faba bean, inoculation with both consortia improved N accumulation compared to single inoculation with an increase of 70.75% under P limitation. Moreover, under combined stress, inoculation with C2 improved biomass and N content (112.98%) in intercropped wheat compared to the sole crop. Our findings revealed that consortium C2 might offer an agronomic advantage under water and P deficit and could serve as a useful inoculum for enhancing faba bean and wheat production in monocropping and intercropping systems.
1. Introduction
By the middle of the 21st century, the human population will pass the nine billion mark and thus put high pressure on food security (Gerland et al., 2014). Nitrogen (N) and phosphorus (P) are essential nutrients in crop production systems. They control plant growth, development, and yield due to their important role in many physiological processes such as signal transduction, respiration, photosynthesis, and energy transduction (Turuko and Mohammed, 2014; Meena et al., 2017). For legumes, P is an essential macronutrient for efficient nodulation and biological N fixation (BNF). Indeed, almost 20% of the P taken up by plants is allocated to nodules to ensure optimal N fixation (Mandri et al., 2012). It was also reported that part of the P applied as fertilizer is either assimilated by soil microbes or complexed by cations such as aluminum (Al), iron (Fe), calcium (Ca), or magnesium (Mg), and only 15–30% of the fertilizer is used by the plants, which reduces the use efficiency of P fertilizers (Sharma et al., 2013). Unfortunately, Morocco has suffered from water limitation in the last decade (Benabdelouahab et al., 2019), and many soil types in dry areas present low available P, which have caused double stress in crops combining water deficit and P limitation.
Furthermore, Morocco is known for its large reservoirs of rock phosphate (RP), which represents an important source of mineral phosphate fertilizers. A main research goal is the optimization of P solubilization to make it more available to plants (Hamdali et al., 2008; Chang and Yang, 2009; Park et al., 2011). The application of RP seems to be an affordable and environment-friendly solution to replace chemical P fertilizers. Additionally, the application of organic fertilizers often did not show the same positive effects as mineral fertilizers (Abbasi and Manzoor, 2018). The utilization of phosphate-solubilizing bacteria (PSB) in combination with RP could increase the level of plant-available P in soils (Wahid et al., 2016).
One solution to enhance plant growth under stressful conditions is to use beneficial plant growth-promoting rhizobacteria (PGPR) capable of mobilizing different forms of P in soils (Shilev, 2020), particularly when plants are co-inoculated with synergistic consortia of strains (Kumar et al., 2017). Furthermore, the cropping system could also affect fertilizer efficiency. Intercropping is defined as growing two or more crops on the same piece of land at the same time (Nasar et al., 2020) to maximize the use of nutrient resources and enhance plant production with rational nutrient inputs (Bargaz et al., 2017). The positive effect of intercropping is generally related to below-ground complementarity or facilitation phenomena between two crop species (Li et al., 2014). Complementarity leads to lowering plant species competition, which may be related to the use of different pools of nutrients unavailable to the associated crop, or to different root architectures that can explore different soil horizons (Bechtaoui et al., 2019; Chamkhi et al., 2022). In addition to the complementarity, the facilitation effect is manifested when a plant makes an unavailable resource available to the other intercrop plant. In this context, the legume facilitation effect toward cereals can be regarded as the partial supply of symbiotically fixed N and available P by the production of protons, organic acids, or phosphatases (Hinsinger, 2001; Li et al., 2008; Betencourt et al., 2012).
The effects of the most prevailing abiotic stresses on legume crops in Morocco, such as drought and P limitation (Bargaz et al., 2012; Mouradi et al., 2015, 2018; Kabbadj et al., 2017), and on intercropping legume/cereal systems (Bargaz et al., 2017; Mouradi et al., 2018) were studied separately. However, only a few studies have considered inoculation with PGPR consortia including rhizobia to enhance legume/cereal growth and production under stressful conditions. This could be an environment-friendly alternative to enhance plant nutrition under abiotic stress. Our research work was based on the hypothesis that inoculation of faba bean/wheat with rhizobia-containing PSB consortia enhances the tolerance of these crops to drought and P limitation and intercropping will improve wheat performance under these conditions. Our research approach included the assessment of the impact of inoculation with rhizobacterial consortia containing multifunctional species, notably rhizobia (for BNF) and two rhizobacteria (for P solubilization and other PGP traits), expecting that this biotechnological measure will allow for plant–microbe and plant–plant beneficial interactions to alleviate combined stress on inter- and sole crops.
2. Material and methods
2.1. Biological material
A randomized block design was carried out using Vicia faba L. variety Aguadulce (Ag) characterized as tolerant to water deficit (Kabbadj et al., 2017), and Triticum durum variety Karim (K). These varieties are commonly grown by farmers in the Haouz area (Hadria et al., 2007; Oukaltouma et al., 2021).
The bacterial inocula consisted of Rhizobium laguerreae and other rhizobacteria (Kocuria sp., Pseudomonas sp., and Rahnella sp.) presenting high P solubilization capacity and at least one PGP trait. These rhizobacteria were isolated from the nodules of faba bean collected from two different sites of Marrakech-Al Haouz region Rahnella sp. from Sidi Ghiat (latitude 31°28′20.0″N, longitude 7°46′31.9″W) and Rhizobium laguerreae., Kocuria sp., and Pseudomonas sp. from Souihla (latitude 31°40′30.1″N, longitude 8°12′17.8″W). These strains were characterized as tolerant to a wide range of pH and temperature and high salinity levels and were endowed with a high capacity to solubilize tricalcium phosphate. They were also characterized for not being antagonistic to each other, which allowed for the composition of two consortia, C1 (R. sp., Kocuria sp., Pseudomonas sp.) and C2 (R. laguerreae, Rahnella sp., and Kocuria sp.), for plant inoculation under greenhouse conditions.
2.2. Bacterial inoculum production and seeds germination
The inoculum was produced separately in a liquid yeast extract mannitol (YEM) for each bacterial species after incubation for 3 days at 28°C under agitation. The bacterial cultures were then centrifuged at 13,000 rpm for 10 min, and the inoculum was prepared by equally mixing the three bacteria for each consortium.
Faba bean and wheat seeds were first surface-disinfected by immersion in 6% sodium hypochlorite for 10 min before they were rinsed five times with sterile distilled water. Faba bean seeds were germinated in sterilized sand for 5 days. The inoculation was performed by soaking seedling roots in the inoculum solution for 20 min and transplanting them into pots (diameter: 16.5 cm; height: 20 cm) presenting in their bottom two draining holes and containing 2.2 kg of substrate consisting of a mixture of sterilized sand and peat at a ratio of 4:1. The sand was sterilized for 3 h at 180°C for three cycles, and peat was autoclaved 1 h at 121°C and 2 bar of pressure three times. The substrate was supplemented with 800 mg/kg of ground rock phosphate as the only source of mineral P. To ensure proper inoculation of faba bean plants, each one received another 5 ml of inoculum at transplantation. Sterilized wheat seeds were soaked in inoculum solution for 20 min and were sown on the same substrate, and 3 ml of inoculum solution was added per seed. A second inoculation was applied by drenching the seedlings 1 week after transplantation. For the sole crop, two plants of faba bean and six plants of wheat were grown per pot. For intercropping, one faba bean plant and three wheat seedlings were grown in the same pot.
The experiment was performed in a greenhouse with a day/night temperature of 25/20°C, an approximate relative humidity of 60–70%, and a 16-h photoperiod with a light intensity of 11.3 Klux.
At 2 weeks after sowing, water deficit was applied by maintaining the pot substrate at 40% WHC for the stressed plants vs. 80% WHC for non-water-stressed (Kabbadj et al., 2017; Oukaltouma et al., 2021). For P limitation, RP was used as the only mineral P source at a rate of 800 mg/Kg substrate. For positive control, plants were irrigated with Hoagland nutrient solution containing 125 μmol/l of KH2PO4 and 46 mg/l of NKO3, and for the negative control (Rh + RP), the substrate was supplemented with RP and plants were inoculated only with rhizobia instead of using KNO3. For the P limitation treatment (RP, 80% WHC), the substrate containing RP was maintained at 80% WHC and for the stress combining P limitation and water deficit (RP, 40% WHC), the substrate was maintained at 40% WHC. The plants were irrigated once a week with an N-free Hoagland nutrient solution. The plants were stressed for 40 days during which physiological parameters were assessed in situ. Afterward, plants were harvested for growth and biochemical assessment. Five replicated pots per treatment were considered.
2.3. Dry biomass measurement
At the flowering stage of faba bean and the appearance of wheat spikes, which corresponded to 60 days after sowing, plants were harvested. Shoots were separated from the roots including rhizosphere soil and nodules were carefully detached from the roots. The three plant parts were washed and dried at 70°C for 72 h, and the dry weights (DW) were determined by weighing the plant tissues for each treatment.
2.4. Stomatal conductance
Stomatal conductance (gs) was measured on the second fully expanded and healthy leaf. The measures were taken at noon under 28 ± 2°C and 60 ± 4% of relative humidity with a porometer (SC1 Model, Decagon Devices, version 2012).
2.5. Leaf relative water content
According to Ghoulam et al. (2002), relative water content (RWC) was determined in well-developed leaves (flag leaves) from three plants per treatment and plant species. Fresh foliar disks of faba bean and wheat were sampled and weighed to determine their fresh weight (FW) and then immersed in distilled water for 6 h to reach full turgidity. Turgid weight (TW) was determined after wiping the surface of the leaf disks. Then, the samples were dried for 24 h at 70°C and their dry weights (DW) were determined. RWC was defined as follows:
2.6. Leaf water potential
Leaf water potential (LWP) was measured at noon on leaves of the same level using a pressure chamber (PMS Instrument Co, Model 600, USA). This measurement was repeated three times per treatment.
2.7. Leaf area
At the flowering stage of the faba bean, the development of wheat spike leaf area (cm2) was determined on three plants per treatment and three leaves per plant using the “Mesurim version 3.4.4.0” software.
2.8. Proline content
The determination of the plant proline content was carried out following the method of Bates et al. (1973), based on the interaction of proline with ninhydrin, which forms a colored complex. Samples of 100 mg of fresh material (faba bean or wheat leaves) were ground in 2 ml of 40% methanol and centrifuged at 5,000 rpm for 20 min. To 1 ml of the supernatant, 1 ml of a mixture of glacial acetic acid and 6 M orthophosphoric acid (3:2 v/v) and 25 mg of ninhydrin were added. Subsequently, the tubes were incubated in a water bath for 1 h at 100°C to allow the formation of the colored complex that was extracted by adding 3 ml of toluene. The solution was stirred for 5 min. The optical density was measured at 520 nm. Proline contents were determined using a standard curve established with known concentrations of proline.
2.9. Chlorophyll “a” fluorescence
Measurement of chlorophyll “a” fluorescence was performed by using a portable fluorometer (plant efficiency analyzer, Hansatech Instruments Ltd.). Before the in situ measurement, leaves were covered with black leaf clips to mimic at least 15 min under dark conditions. The maximum quantum yield (Fv/Fm) was used as the chlorophyll “a” fluorescence-derived parameter. The differential curves were obtained by subtracting the curve of samples of the control plants from the curve of samples of plants that received different treatments.
2.10. Electrolyte leakage
To determine the electrolyte leakage (EL), the method described by Ghoulam et al. (2002) was used. Samples of five disks of 1 cm diameter from faba bean leaves and 50 mg from wheat leaves were rinsed three times with distilled water to remove minerals from the surfaces of the disks. Afterward, the samples were collected in tubes containing 10 ml of deionized water and incubated for 24 h under shaking at 25°C. Subsequently, the initial electrical conductivity (L0) of each sample was determined at 25°C with a conductometer. After autoclaving the samples for 20 min at 120°C followed by cooling for 30 min at 25°C under agitation, the total electrical conductivity (Lt) of the samples was determined. The electrolyte leakage was determined by the formula:
2.11. Acid phosphatase activity in nodules
Nodule APase activity was determined according to the method described by Araújo et al. (2008). Fresh nodules (100 mg) were homogenized in 500 μL of sodium acetate buffer (0.1 M, pH 5.5) containing 2.2% of polyvinylpyrrolidinone (PVP) and 5 μL of beta-mercaptoethanol. After 15 min of centrifugation at 12,000 ×g, 100 μL of the obtained supernatant was added to 200 μL of p-nitro-phenyl phosphate (pNPP) and the mixture was incubated for 30 min at 38°C. The reaction was stopped by adding 1 mL of 1 N NaOH and the OD was recorded at 410 nm. The p-nitrophenol (enzyme substrate) concentration was determined by reference to the standard curve.
2.12. Phytase activity in nodules
Phytase activity was determined by mixing 200 μL of 10 mM phytic acid with 100 μL of nodule enzymatic extracts according to Eeckhout and Paepe (1994). The reaction was maintained at 37°C and stopped after 90 min by adding 1 mL of 10% TCA (trichloroacetic acid). For each sample, a control was prepared by immediately adding 1 mL of 10% TCA to the reaction medium containing phytic acid at t0. The reaction media were centrifuged at 12,000 × g for 5 min. The concentration of Pi in the extract was determined by colorimetry using sodium molybdate and hydrazine sulfate. The phytase activity was defined as the difference between the Pi in the extract and its corresponding blank sample and expressed in μmol Pi min−1 g−1 FM.
2.13. Determination of shoot nutrient contents
Dried wheat and faba bean plants (80°C for 3 days) were finely ground for total N, P, and K contents analyses. The wheat and faba bean plant powders were digested using nitric acid and analyzed for P and K contents using inductively coupled plasma optical emission spectrometry (Agilent 5110 ICP-OES, USA). The total N content was determined by the Kjeldahl method (KjelMaster K-375, Netherlands).
2.14. Statistical analysis
The statistical analysis was carried out using IBM® SPSS® Statistics V. 20 software. A multivariate analysis of variance was used followed by the Tukey post-hoc test to determine the significant difference between the means of the treatments at the p < 0.05 significance level. All tested parameters and their correlation with treatments were subjected to principal component analyses (PCAs) using the same software.
3. Results
3.1. Plant growth and nodulation
Inoculation with either consortium (C1 or C2) and intercropping significantly (Supplementary Table S1) affected shoot dry weight (SDW), root dry weight (RDW), and nodule dry weight (NDW) under combined stress. The application of both consortia improved faba bean shoot dry weights for both cropping systems. In particular, plants inoculated with C2 and supplied with RP showed the highest increase rate of 53.59 vs. 30.56% C1 compared to inoculation with rhizobia alone (Table 1). Plants inoculated with C2 under the same condition showed the highest SDW (2.89 g plant−1) for the sole crop. Intercropping reduced legume SDW no matter which inoculant was applied (Rh, C1, or C2) with the highest reduction rate of 40.48%, when plants were inoculated with C2 under combined stress (Table 1). In wheat under combined stress, inoculation with both consortia and intercropping significantly affected SDW and RDW (p < 0.001) (Supplementary Table S2). Under P limitation, the highest value for SDW (2.48 g plant−1) was recorded in plants inoculated with C2 with an increase of 51.22% relative to the corresponding sole crop treatment under the same conditions (Table 1). We observed that under combined stress and sole crop, the application of C2 improved wheat SDW by 16.83% compared to plants inoculated with C1.
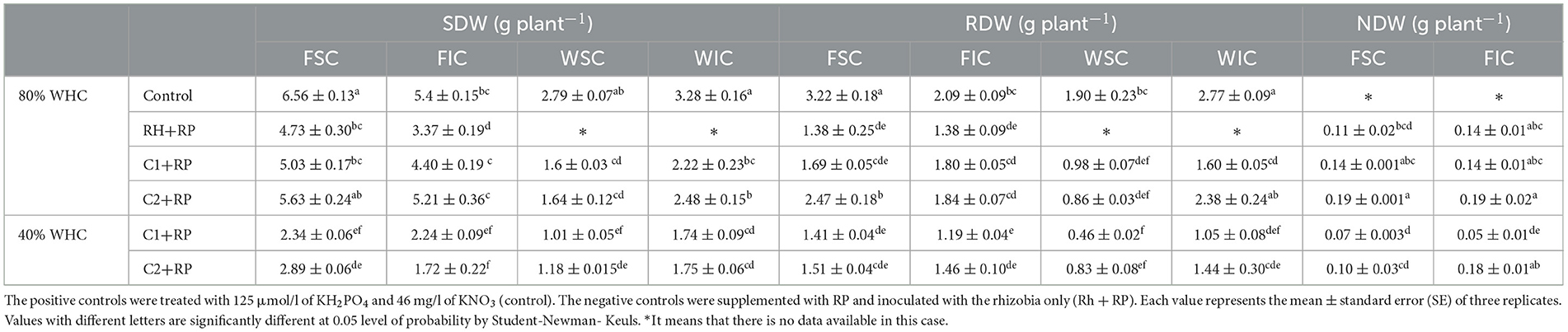
Table 1. Faba bean and wheat dry biomasses (SDW, shoot dry weight; RDW, root dry weight; NDW, nodule dry weight) grown as a sole crop or intercropped (FSC, Faba bean sole crop; FIC, faba bean intercropped; WSC, wheat sole crop; WIC, wheat intercropped) under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) inoculated with two rhizobacterial consortia C1 and C2.
C2 significantly (Supplementary Table S1) improved faba bean RDW under P deficiency compared to the application of rhizobia alone, with an increase of 78.99% (Table 1). Combined stress reduced RDW in both cropping systems, particularly in plants inoculated with C1 compared to P limitation. P deficiency reduced RDW in wheat and combined stress reduced this parameter for all treatments compared to the controls, while intercropping increased RDW in wheat compared to the sole crop for both consortia under both stresses. This improvement was noticeable under the P limitation with an increase of 176.74% for plants inoculated with C2 compared to their corresponding plants grown as a sole crop (Table 1).
Inoculation with C2 significantly improved faba bean NDW by 36% in the intercropping treatments and by 72.73% in the sole crop (Supplementary Table S1), compared to P limitation and inoculation with rhizobia alone (Table 1). Moreover, intercropping and inoculation with C2 improved NDW by 80% compared to sole crops under combined stress.
3.2. Stomatal conductance
Among all treatments subjected to water deficit and P limitation, inoculation with both consortia and cropping system significantly (p < 0.001) (Supplementary Table S1) affected faba bean stomatal conductance. Figure 1A shows that under P limitation, faba bean “gs” was improved by inoculation with both consortia compared to inoculation with rhizobia alone, either for sole crop or intercropping. Particularly, faba bean inoculated with C2 presented a “gs” increase of 35.73% compared to plants under P limitation inoculated with rhizobia alone. Furthermore, plants inoculated with C2 presented the highest value of conductance of 139.02 mmol H2O m−2 s−1. Combined stress significantly reduced “gs” in plants inoculated with both consortia and those inoculated with C2 presented the highest “gs” value of 86.73 mmol H2O m−2 s−1. Intercropping reduced the “gs” of faba bean compared to sole crop in all treatments except for plants inoculated with C2 and the controls.
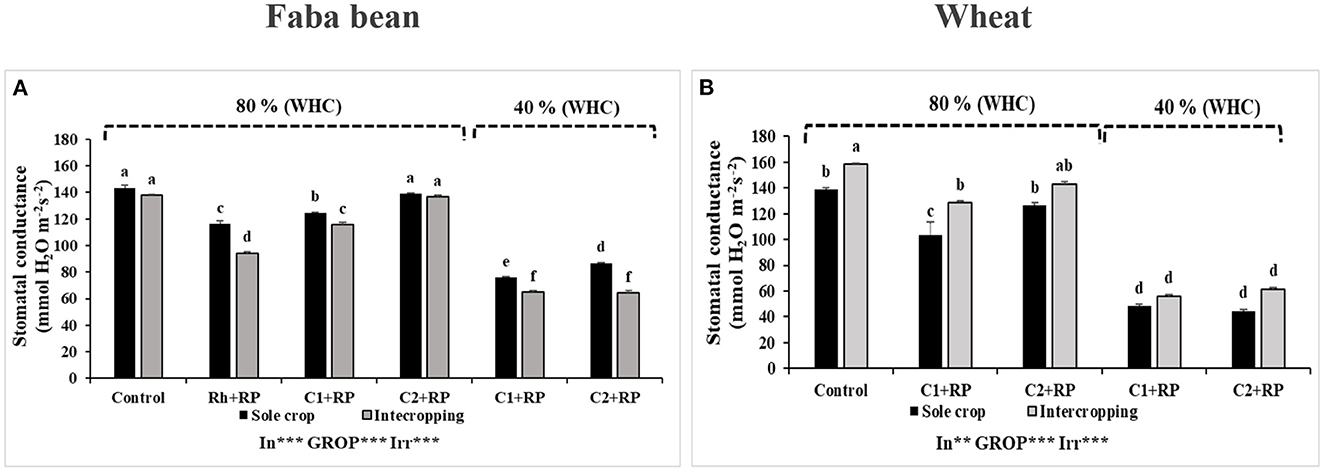
Figure 1. Stomatal conductance of faba bean (A) and wheat (B) grown as a sole crop or intercropped under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
Statistical analyses revealed that combined stress in wheat and inoculation with both consortia, as well as intercropping, significantly affected “gs” (p < 0.001) (Supplementary Table S2). Intercropping improved “gs” under P limitation but did not affect it under combined stress, no matter which inoculant was (C1 or C2) used (Figure 1B).
3.3. Electrolyte leakage
Measurement of electrolyte leakage (EL) revealed a significant effect (p < 0.001) in response to combined stress, inoculation with both consortia, and in the intercropping system (p < 0.05). For the faba bean (Figure 2A), EL was reduced when plants were inoculated with both consortia compared to inoculation with rhizobia alone. This reduction was more pronounced when plants were inoculated with C2 in the sole crop, with a reduction of 44.33% compared to 16.16% when plants were inoculated with C1, relative to inoculation with rhizobia alone under P limitation. The combined stress highly increased the EL, with the highest value detected in plants inoculated with C1 (53.10%).
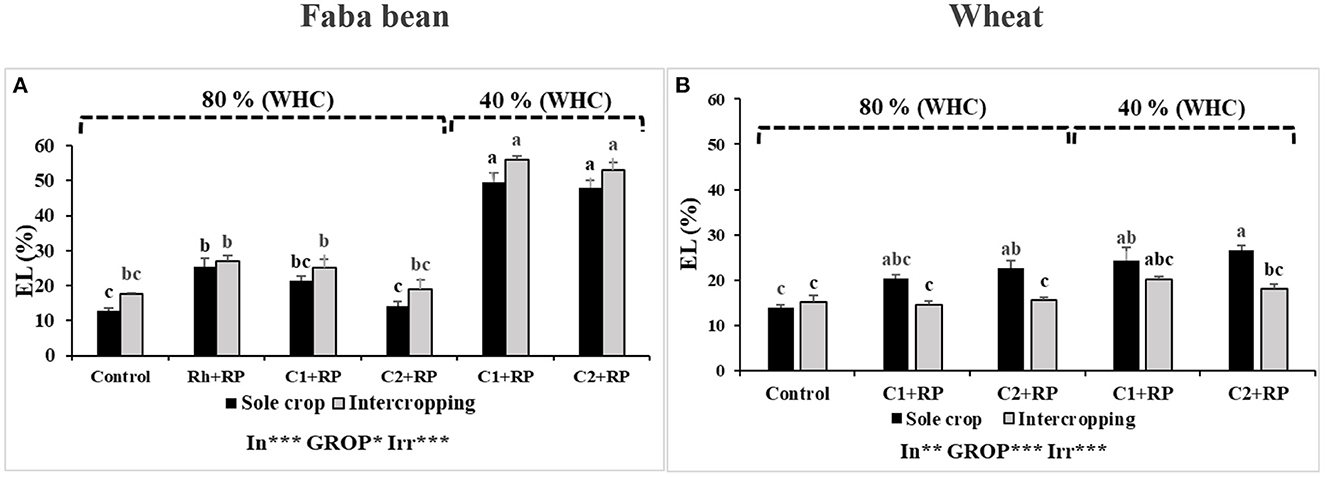
Figure 2. Electrolyte leakage in faba bean (A) and wheat (B) grown as a sole crop or intercropped under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
For water deficit and P limitation in wheat, inoculation with both consortia and intercropping significantly affected EL (p < 0.05). Figure 2B shows that P deficiency increased the EL values, compared to the controls, no matter which consortium was used (C1 or C2). The combined stress did not induce any additional increase in EL, compared to P deficiency alone. Intercropping reduced EL significantly, particularly when plants were inoculated with C2, as compared with the sole crop.
3.4. Relative water content
Combined stress, inoculation, and cropping system showed significant effects (p < 0.001) on RWC under P limitation. The combined stress reduced RWC in sole-cropped plants inoculated with C1 or C2, relative to their corresponding plants under P limitation. The decrease was more pronounced in plants inoculated with C1 (11.81 and 8.6%, respectively) (Figure 3A). In general, intercropping with wheat decreased RWC for faba bean with the lowest value recorded for plants inoculated with C1 under combined stress (54.26%).
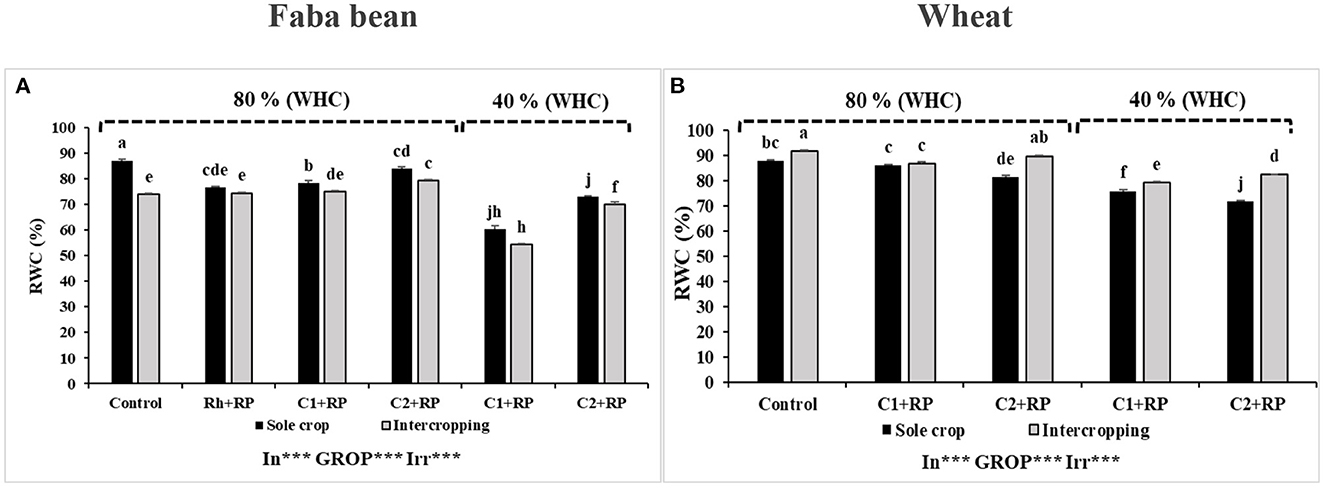
Figure 3. Relative water content in faba bean (A) and wheat (B) grown as a sole crop or intercropped under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
Considering wheat, statistical analyses revealed significant effects (p < 0.05) of combined stress, inoculation, and cropping system on RWC. For sole-cropped wheat plants, the combined stress induced a decrease of RWC that was more evident in plants inoculated with C2 compared to the control (Figure 3B). The intercropping increased RWC for most treatments, and the highest increase was achieved with plants inoculated with C2 (15.06%) compared to sole-cropped plants under combined stress.
3.5. Leaf water potential
Under the combined stress and intercropping system, inoculation with both consortia significantly affected LWP (p < 0.001) in faba bean plants (Figure 4). P limitation induced a decrease of LWP in sole-cropped faba bean inoculated with C1 or C2, compared to those inoculated with rhizobia only. The decrease amounted to 133.33% compared to inoculation with rhizobia alone or when plants were inoculated with C1 (Figure 5). The combined stress together with intercropping decreased LWP, no matter which inoculum was used (C1 or C2). The plants inoculated with C1 showed the lowest value of −7.65 MPa.
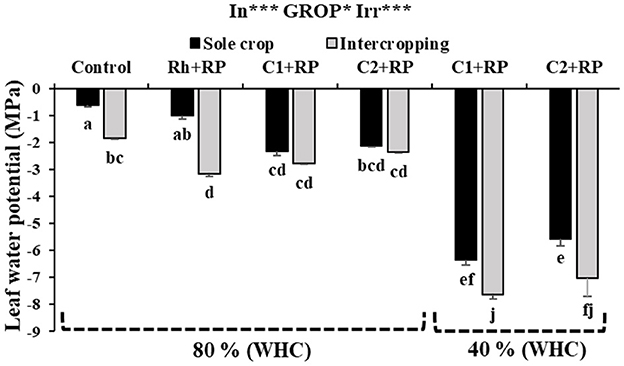
Figure 4. Leaf water potential in faba bean grown as a sole crop or intercropped with wheat under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
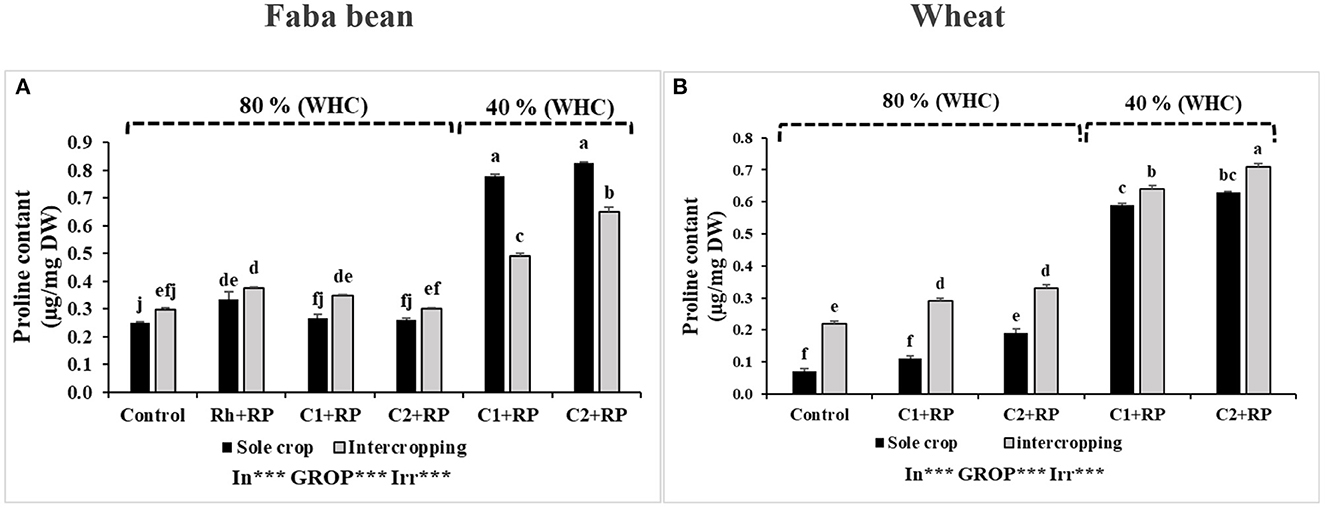
Figure 5. Proline content in faba bean (A) and wheat (B) grown as a sole crop or intercropped under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
3.6. Proline content
Statistical analysis detected significant effects (p < 0.001) of combined stress, inoculation, and intercropping on the plant proline content. The result shown in Figure 5A documents the accumulation of proline in faba bean plants. We noticed that proline was more enriched under combined stress than under P limitation alone, for both inocula (C1 or C2). Under combined stress, the highest proline value was observed in faba bean inoculated with C2 (0.83 μg/mg DW). Intercropping reduced proline accumulation under combined stress and the reduction become obvious when plants were inoculated with C1, as compared to C2, with reduced rates of 37.18 and 21.69%, respectively, compared to the sole crop.
Statistical analyses discovered significant effects of the combined stress on proline content (p < 0.001) in wheat, as well as in inoculation and cropping systems. Under P limitation, inoculation with C2 induced an increase of proline in both cropping systems, compared to inoculation with C1 and the positive control, with the highest value (0.33 μg/mg DW) observed in intercropped plants inoculated with C2 (Figure 5B). Under combined stress, we noticed a high accumulation of proline in plants inoculated with both consortia. Moreover, intercropped wheat plants showed an additional increase compared to their corresponding sole-cropped plants, and the highest value was recorded in plants inoculated with C2 (0.71 μg/mg DW).
3.7. Leaf area
Combined stress and inoculation significantly (p < 0.01) affected the leaf area of faba bean plants. The highest value was recorded for the positive control in the sole crop treatments (25.82 cm2). However, under P limitation, it was significantly reduced, compared to the controls, and when plants were inoculated with rhizobia alone or C1, sole-cropped plants inoculated with C2 did not show any significant reduction (Table 2). Combined stress highly reduced the leaf area compared to the control and P limitation treatments with the highest value recorded for plants inoculated with C2 (14.80 cm2). Intercropping did not significantly affect this parameter, no matter which stress or consortium was applied.
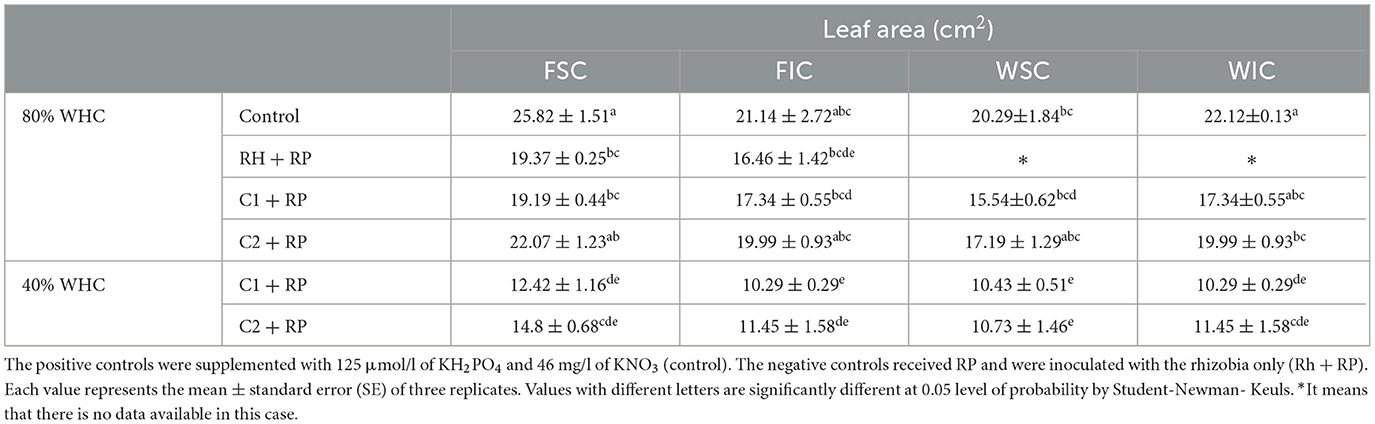
Table 2. Leaf area of faba bean and wheat grown as a sole crop or intercropped (FSC, Faba bean sole crop; FIC, faba bean intercropped; WSC, wheat sole crop; WIC, wheat intercropped) under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs.
Combined stress and inoculation with both consortia significantly affected (p < 0.05) the leaf area in wheat (Table 2), except for the control, which showed an increase in leaf area. Intercropping did not show a significant variation in this parameter for all remaining treatments.
3.8. Chlorophyll “a” fluorescence
The combined effect of P limitation, water deficit, and inoculation with both consortia was studied through the analysis of the dark recovery kinetics curves of the Chl “a” fluorescent transient (OJIP-transient). All treatment curves showed a normal distribution of OJIP transients, which refers to the reduction phase of the electron chain transporters. For the sole faba bean (Figure 6A), the results of Chl “a” fluorescence presented a difference in the shape of all transition states (O, J, I, and P). In general, we observed that all treatments under sole crop showed differences in all transition states compared to the control treatments. The J-step and P-step showed the highest amplitude by inoculation with C1 compared to inoculation with C2 and rhizobia alone. However, for the I-step, inoculation with C2 presented the highest amplitude compared to all other treatments. For intercropped plants, the highest amplitude of all transition states was detected in inoculation with C2 compared to the other treatments (Figure 6B). In sole-cropped wheat (Figure 6C), inoculation with C1 presented the highest amplitude compared to the other treatments, while intercropped plants inoculated with C2 presented the highest amplitude between all steps when compared to inoculation with rhizobia alone and C1. This difference was most pronounced in the I-step (Figure 6D).
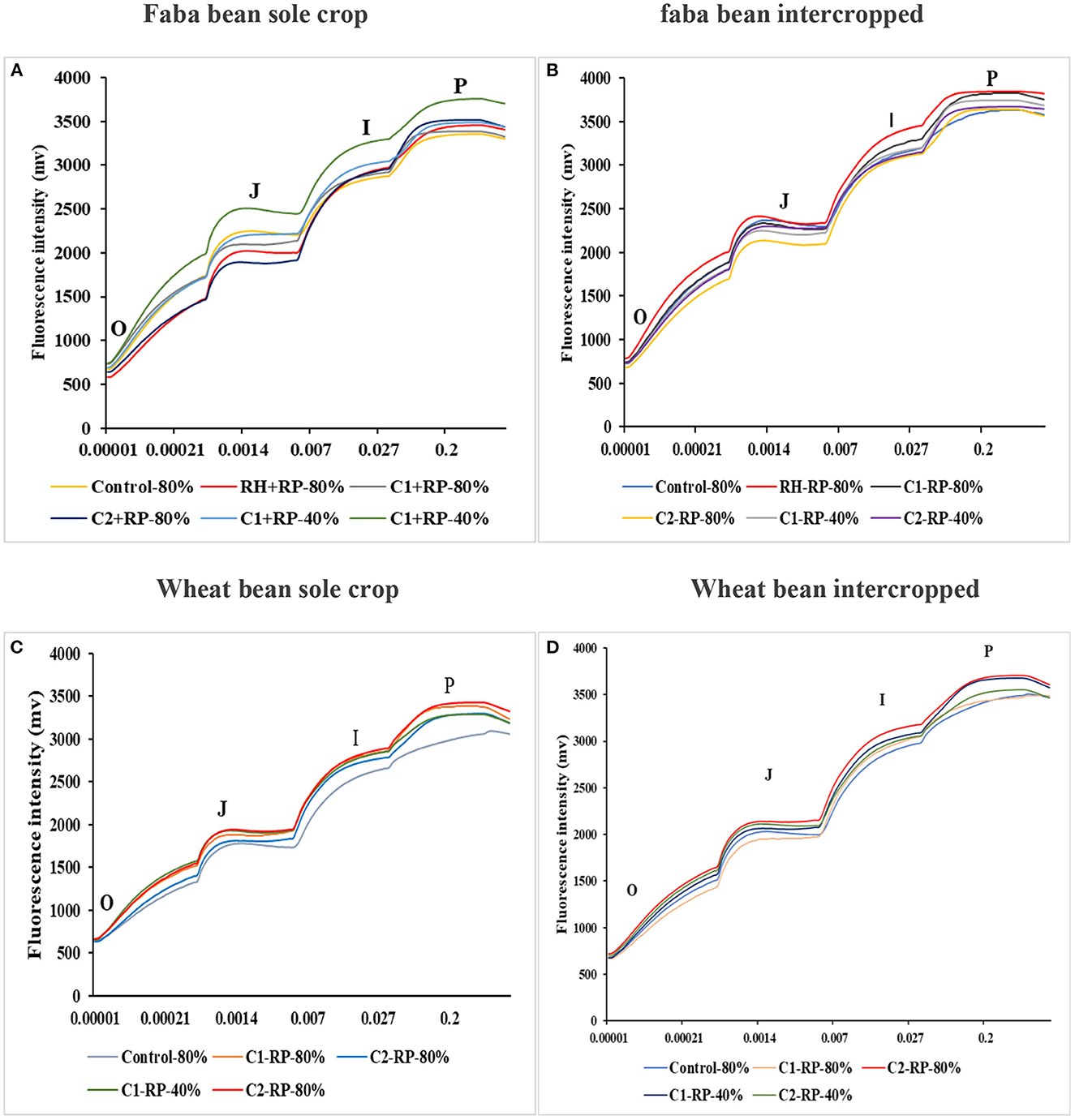
Figure 6. Faba bean sole crop chlorophyll “a” polyphasic fluorescence OJIP (A), Faba bean intercropped chlorophyll “a” polyphasic fluorescence OJIP (B), wheat sole crop chlorophyll “a” polyphasic fluorescence OJIP (C), wheat intercropped chlorophyll “a” polyphasic fluorescence OJIP (D), under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP), and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates. Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
3.9. Acid phosphatase and phytase activities in nodules
Statistical analyses revealed significant effects of combined stress and inoculation on APase and phytase activities in the nodules of faba bean roots (p < 0.001). The cropping system did not affect these two parameters significantly. For APase, under P limitation, the inoculation with consortium C2 improved APase activity compared to inoculation with consortium C1 and inoculation with rhizobia alone (Figure 7A). This improvement was significant under sole crop with the highest increase of 66.92% compared to inoculation with rhizobia alone. Under combined stress, inoculation with C2 improved APase activity with the highest value of (158.43 μmol pNP min−1 g FM−1) which was observed for faba bean in the sole crop inoculated with C2. Under P limitation, intercropping did not affect this activity but reduced it under combined stress for plants inoculated with C2.
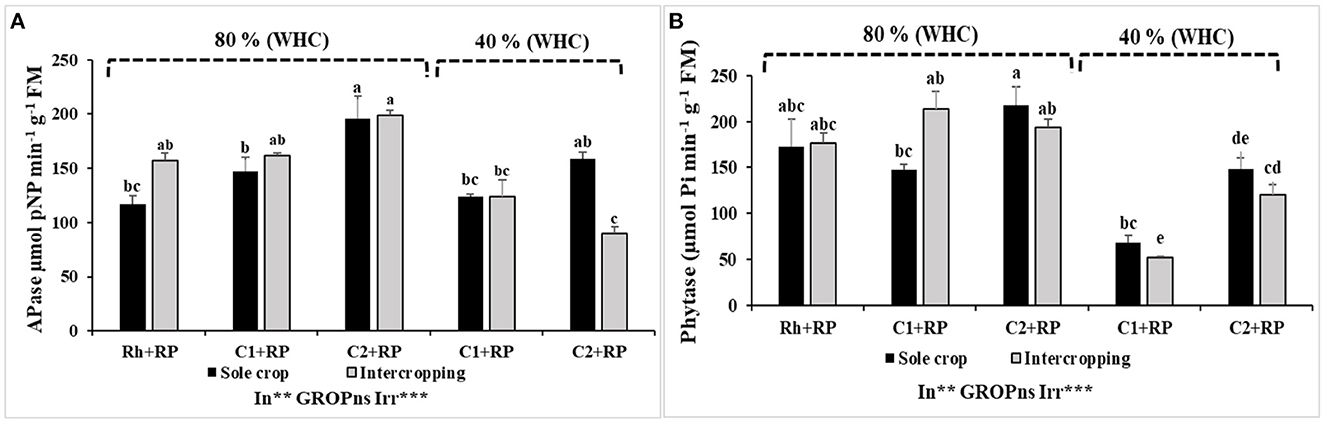
Figure 7. Faba bean nodules APase activity (A) and nodules phytase activity (B). Plants were grown as a sole crop or intercropped under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh+RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
The results revealed that P limitation and inoculation with both consortia did not affect phytase activity for sole crops. Plants inoculated with C2 presented the highest phytase activity of 217.98 μmol Pi min−1 g FM−1 (Figure 7B). Combined stress reduced phytase activity in nodules of plants inoculated with C1 or C2 compared to those inoculated with rhizobia alone. This reduction was more pronounced in plants inoculated with C1.
3.10. Major nutrient contents in faba bean and wheat
3.10.1. N content
Combined stress, inoculation, and intercropping induced a significant effect on N accumulation in faba bean and wheat (p < 0.001). In faba bean, inoculation with both consortia highly improved N accumulation, compared to the inoculation with rhizobia alone (Figure 8A). This improvement was significant for sole-cropped plants under P limitation inoculated with C2, compared to inoculation with C1, with improvement rates of 70.75 and 28.24%, respectively. The combined stress of water deficit and P limitation reduced N accumulation in sole cropping and the reduction was more pronounced for plants inoculated with C1.
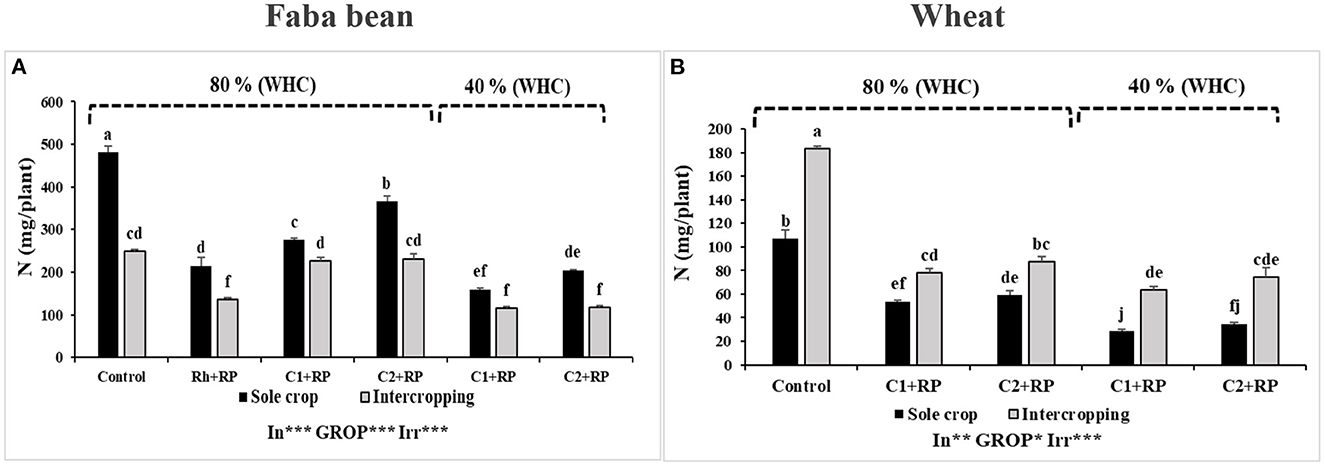
Figure 8. Faba bean (A) and wheat (B) N contents. Plants were grown as a sole crop or intercropped under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
In wheat, intercropping improved N accumulation compared to the sole crop, either under P limitation or under combined stress, where intercropped plants inoculated with C2 showed the highest increase (112.98%) compared to the sole crop (Figure 8B).
3.10.2. P content
ANOVA testing revealed a significant effect of combined stress, inoculation, and intercropping on P accumulation in faba bean and wheat (p < 0.001). Inoculation with both consortia improved P accumulation compared to inoculation with rhizobia alone in faba bean under P limitation (Figure 9A). Under combined stress, plants showed a severe decrease in P content with both inocula (C1 or C2), compared to their corresponding plants under P limitation. Intercropping induced a decrease of P content in plants inoculated with rhizobia alone or with C1, but other treatments did not affect this parameter.

Figure 9. Faba bean (A) and wheat (B) P contents. Plants were grown as a sole crop or intercropped under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
In wheat, combined stress with inoculation (C1 or C2) induced a decrease in P content, compared to the corresponding results under P limitation alone (Figure 9B). Intercropping increased P accumulation compared to sole crop in all considered treatments and the increase was more evident in plants under combined stress inoculated with C1 (122.32%) relative to sole-cropped plants.
3.10.3. K+ content
Faba bean plants under P limitation inoculated with C2 showed improvement of K+ content (245.19 mg plant−1) in sole-cropped plants, but inoculation with C1 did not affect this parameter when compared to inoculation with rhizobia alone (Figure 10A). Combined stress reduced the K+ content in plants inoculated with C1 but showed no effect in plants inoculated with C2 compared to the corresponding treatment under the P limitation. In general, the intercropping practice reduced the K+ content in faba bean, except for the variants inoculated with C1 under P limitation.
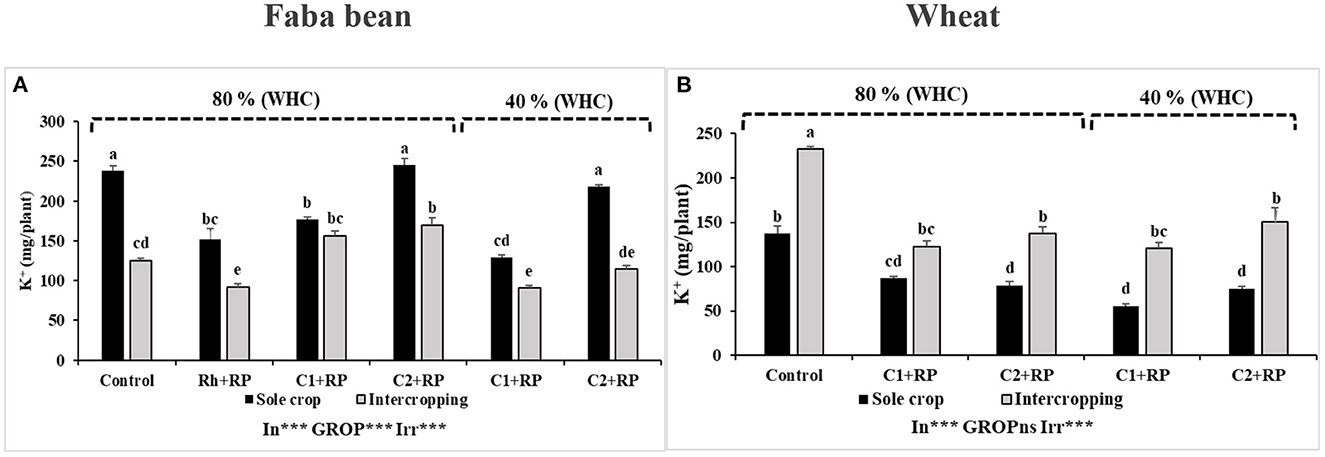
Figure 10. Faba bean (A) and wheat (B) K contents. Plants were grown as a sole crop or intercropped under water sufficiency and P limitation (80% WHC, RP) vs. combined water deficit and P limitation (40% WHC, RP) and inoculated with two rhizobacterial consortia (C1 and C2) containing rhizobia and two PGPRs. The positive controls were supplemented with 125 μmol/l of KH2PO4 and 46 mg/l of KNO3 (control). The negative controls received RP and were inoculated with the rhizobia only (Rh + RP). Each value represents the mean ± standard error (SE) of three replicates (In, inoculation; GROP, type of cropping; Irr, irrigation). Values with different letters are significantly different at 0.05 level of probability by Student-Newman-Keuls. *Represent the significance level of treatments.
Intercropping induced a significant increase of K+ accumulation in wheat, compared to the sole-cropped plants in all the considered treatments (Figure 10B). The highest increase rate of 99.65% was achieved in plants under the combined stress inoculated with C2 compared to sole-cropped.
3.10.4. Principal component analysis
The results of the pot experiments were analyzed using principal component analysis (PCA), which showed that the accounted proportion of variance for the first two axes was 65.15 and 20.64% (eigenvalues), respectively. For faba bean under P limitation inoculated with C1 and C2 (Figure 11A), PCA analyses showed that the treatments with the higher yield, nutrient accumulation, La, RWC, gs, APase, and Phy activities were correlated to intercropping faba bean and inoculation with C2, while RWC and Ph were correlated to sole plants inoculated with C1.
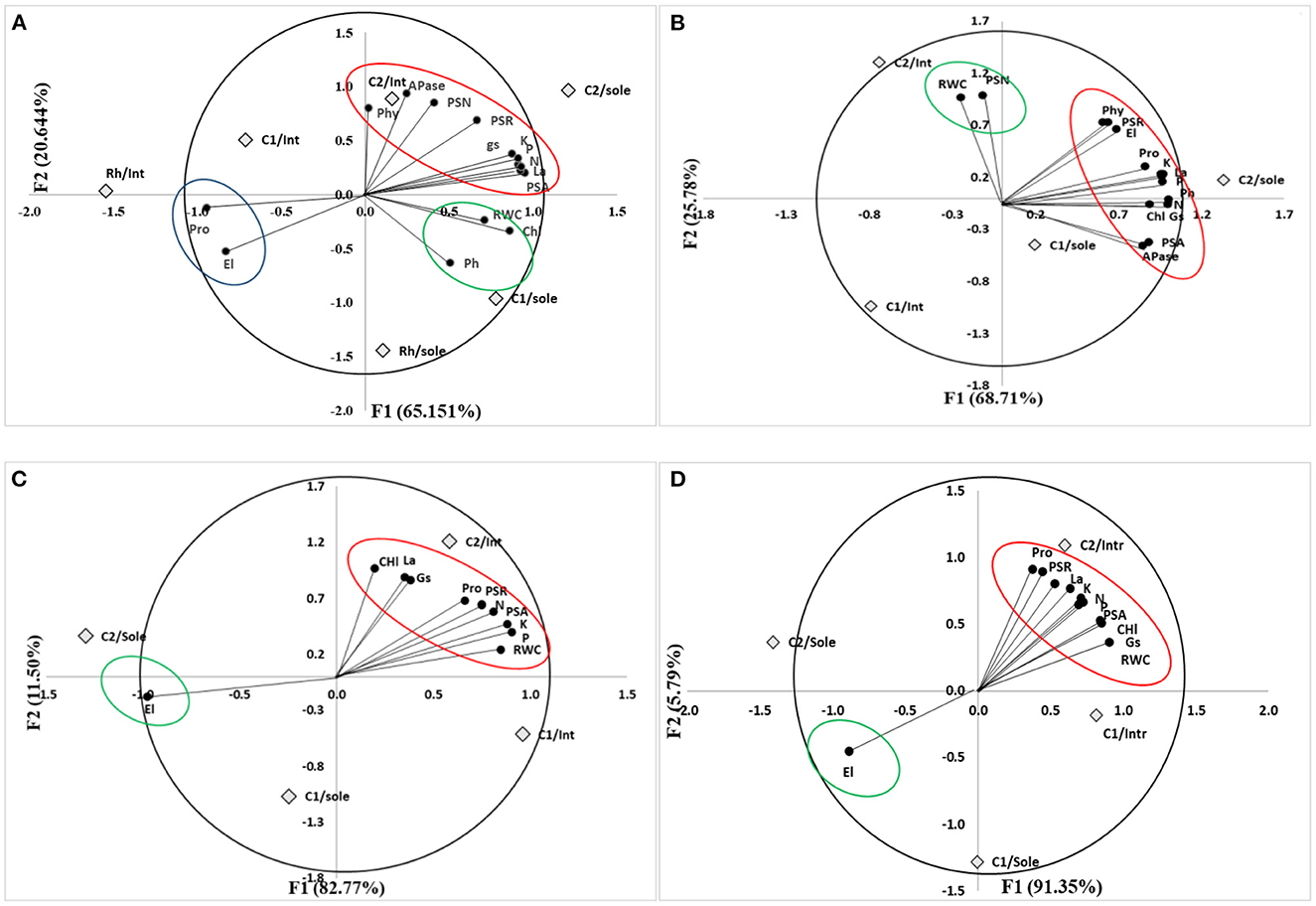
Figure 11. Principal component analyses (PCA) of faba bean inoculated with C1 and C2 under P limitation (A) and under water deficit and P limitation (B) and wheat inoculated with C1 and C2 under P limitation (C) and under water deficit and P limitation (D). Faba bean sole-cropped (Sole): inoculated with rhizobia only; Rh. Faba bean intercropped (Int): Int, wheat sole-cropped; (Sole). wheat intercropped; (Int). PSA, shoot dry weight; PSR, root dry weight; PSN, nodule dry weight; gs, stomatal conductance; La, leaf area; RWC, relative water content; EL, electrolyte leakage; Ph, leaf water potential; Pro, proline content; aPase, phosphatase activity, Phyt, phytase activity; N, nitrogen content, K+, potassium content; P, phosphorus content.
For faba bean grown under water deficit and P limitation, PCA showed that the accounted proportion of variance for the first two axes was 68.71 and 25.78% (eigenvalues), respectively (Figure 11B). This analysis revealed that the treatments with the higher yield, nutrient content, APase, Phy, Pro, gs, and Ph were related to sole-cropped plants inoculated with C2. However, PSN and RWC were correlated to intercropped plants inoculated with C2.
For wheat plants under P limitation, PCA showed that the accounted proportion of variance for the first two axes was 82.77 and 11.50% (eigenvalues), respectively (Figure 11C). PCA showed that nutrient content, higher yield, RWC, Pro, gs, and La were highly expressed in intercropped plants inoculated with C2.
For wheat under combined stress, PCA showed that the accounted proportion of variance for the first two axes was 91.35 and 5.79% (eigenvalues), respectively (Figure 11D). The PCA analyses showed that yield, nutrient content, La, Pro, gs, and RWC were highly expressed in intercropped plants inoculated with C2.
4. Discussion
The present study aimed to evaluate the effects of inoculation with rhizobacterial consortia and intercropping of faba bean/wheat on plant growth, performance, and physiology under combined stress of water deficit and low P availability. Our research addressed the hypothesis that inoculation with rhizobacterial consortia, including one Rhizobium sp. and two P solubilizing bacteria, could increase the nitrogen-fixing potential of faba bean—-rhizobia symbiosis and transfer this benefit to associated wheat plants in the intercropping system under stressful conditions.
The key findings of this study revealed that combined stress, P limitation, and water deficit reduced growth in both crop species and for both cropping systems (sole crop and intercropping). This decrease could be due to a reduction of some physiological properties determining plant growth and performance, e.g., cell water status, membrane stability, photosynthesis, and the activity of enzymes involved in plant nutrition. Our results showed that the combined stress induced a decrease in water potential, RWC, leaf area, and phytase and phosphatase activity, which are key enzymes in plant P nutrition. We observed increases in proline content and electrolyte leakage under stressful conditions in comparison to the untreated controls. Such changes would suggest that membrane stability is affected and that these structures would no longer function properly. Another study conducted by Abbasi and Manzoor (2018) showed that the RWC of wheat plants decreased under P deficiency and salt stress. For the same species, the combination of water and salt stress highly affected the genes that are responsible and related to growth and different trait indicators of nitrogen metabolism (nitrogen content, stable nitrogen isotope composition, glutamine synthetase, and nitrate reductase activities) and photosynthetic carbon metabolism (Yousfi et al., 2016).
Several other studies on wheat have shown that water and salt stress affect the activity of key enzymes involved in nitrogen metabolisms, such as nitrate reductase (NR) and glutamine synthetase (Munns, 2005; Munns and Tester, 2008). Bargaz et al. (2012) demonstrated the negative effect of P limitation on membrane stability. In addition, Farissi et al. (2013) reported that the highest electrolyte leakage levels were observed under severe water stress in alfalfa plants. Furthermore, Oukaltouma et al. (2021) reported that the highest level of malondialdehyde, a product of phospholipid peroxidation, was observed under combined stress of water deficit and P limitation in faba bean, indicating a loss of membrane stability that was reflected in our study by high electrolyte leakage. Our results showed that the effectiveness of Photosystem II (PSII) decreased under the combined stress. These results corresponded to Mouradi et al. (2015), who observed a decrease in this parameter under water deficit in alfalfa plants. This decrease could be associated with a downregulated performance of PSII, linked to the degradation of chlorophyll and, therefore, photosynthetic inactivation (Blackburn, 2007).
Inoculation with the two different rhizobia-containing consortiums significantly improved most of the analyzed parameters compared to inoculation with Rhizobium alone. The improvement was particularly evident when plants were inoculated with consortium C2. This high performance of C2 could be related to the presence of Rahnella sp. in this consortium, compared to Pseudomonas sp. in consortium C1. Indeed, Magallon-Servín et al. (2020) proved that Rahnella sp. presented the highest P-solubilizing activity in solid media followed by A. lannensis with more effective production of indol acetic acid (IAA), siderophore, biofilm, and acid phosphatase, compared to Pseudomonas sp. In our study, inoculation with C2 and intercropping increased P and N accumulation under P limitation and under the combined stress of P limitation and water deficit compared to inoculation with rhizobia alone. This accumulation could be attributed to the effects of the rock phosphate-solubilizing activity of PSB and the fixation of atmospheric nitrogen by rhizobia. The solubilized P would be available for faba bean–rhizobia symbiosis to enhance nodulation and symbiotic nitrogen fixation, and consequently, N nutrition (Hinsinger, 2001; Maazaoui et al., 2016). Indeed, we noticed a higher nodulation density in faba bean roots inoculated with the consortium C2, which could be explained by the sufficiently available P supply related to the PSB, particularly Rhahnella sp. Similarly, Benjelloun et al. (2021) showed that the combined inoculation of chickpeas with Mesorhizobium sp. and PSB was equivalent to the effect of the combined application of N and P fertilizers on P-deficient soil.
The tested rhizobacteria present a potential for K solubilization ability (unpublished data) and contributed to the enhancement of K+ nutrition in inoculated plants (Figure 10). Such adequate mineral nutrition, based on the major elements N, P, and K, could be the reason for the improvement of plant growth (shoot and root biomasses) noticed in plants inoculated with the consortia (with C2 being the more effective inoculum) compared to those inoculated with rhizobia alone (Table 1). This trend corroborates well with the first part of our hypothesis. Moreover, Iqbal et al. (2022) showed that the application of Enterobacter sp. and Bacillus sp. together with the fungus Piriformospora indica significantly increased plant growth, physiological parameters, nutrient uptake, and soil microbiological functions in canola. Furthermore, Govindasamy et al. (2020) proved that physiological stress responses, such as relative water content (RWC) and the cell membrane stability index, showed significant improvement in seedlings inoculated with rhizobacterial endophytes under drought conditions. The maintenance of plant water balance under combined water deficit and P limitation was recorded in plants inoculated with C2 and indicated by relatively higher water potential and water content. This could have contributed to plant growth improvement under these constraints. However, added K+ supply could act as a mineral osmoregulation compound besides organic osmotica (e.g., proline), which accumulates under combined stress and alleviate osmotic stress imposed by water deficit. Such association of inorganic (K+) and organic compounds (e.g., glycine betaine) against combined stress of P limitation and water deficit has been proven for faba bean (Oukaltouma et al., 2021). Proline is a compatible osmolyte involved in the protection of cell membranes and proteins against these disturbing stresses. However, our results did not support the osmo-protecting role of proline, since the plants accumulating proline exhibited high electrolyte leakage, which suggests membrane damage under combined stress (Figures 2, 5).
The intercropping of wheat and faba bean enhanced wheat growth either under P limitation or under combined stress. This enhancement appeared to be at the expense of associated faba bean plants since we observed faba bean growth reduction in intercropping. These results agree with a report by Khalid et al. (2021), who confirmed the improvement of cereal growth in the intercropping system with faba bean, even under water stress. The association was beneficial for the cereal crop, which was most probably due to the legume facilitation effect. It seems worthwhile to highlight that this legume–cereal association represents a successful model for intercropping since the two crops display a complementary root system allowing for the exploration of different soil horizons and hence avoiding competition for resources (Li et al., 2006; Chamkhi et al., 2022). Intercropping increased the RWC and membrane stability of wheat plants indicating an improvement of the plant water status that could be advantageous for metabolism and growth. Under combined stress, intercropping increased nodulation in faba bean inoculated with C2. This nodulation increase has been reported before in intercropped faba bean and wheat (Bargaz et al., 2017). Our results did not show any enhancement of nodular enzyme activities involved in P availability, e.g., acid phosphatase or phytase, in intercropped faba bean. However, previous studies reported that intercropping reduced APase activity in the faba bean rhizosphere, while it increased in the associated barley rhizosphere (Mouradi et al., 2018). This variation in enzyme activity could prove one of the facilitation actions through the release of enzymes by legume nodules into the rhizospheric soil of associated crops, in which we did not assess this activity. The intercropping system increased the major nutrient contents (N, P, and K) in wheat plants intercropped with faba bean compared to sole-cropped plants. These results confirm the importance of legumes and their microbiome for improving the performance of intercropped wheat, even under stressful conditions, which confirms the second part of our research hypothesis. The facilitation effect of legumes in nutrient mobilization and release into the rhizosphere and the high ability of the cereal root system in taking up these nutrients from the shared rhizospheric space substantiate the benefit of the legume–cereal model, despite the trade-off at the expense of the legume crop. The intercropping benefit involved in phosphorus solubilization by the secretion of legume organic acids, protons, and enzymes, such as phosphatase and phytase, improved P nutrition of wheat under stressful conditions and alleviated the impact of combined stress (Betencourt et al., 2012; Oukaltouma et al., 2021). The inoculation with consortia containing PSB improved plant P nutrition based on rock phosphate solubilization to make it available for plant particularly under the intercropping system.
5. Conclusion
The present study demonstrated that P deficiency decreased plant growth and nodulation under both cropping systems, and this reduction was more pronounced under the combined stress of water deficit and P limitation in faba bean and wheat grown under greenhouse conditions. This reduction resulted from an adverse impact on physiological water parameters and plant mineral nutrition (N, P, and K). The inoculation with rhizobia–PSB-containing consortia alleviated the impact of P limitation alone and the combined stress compared to inoculation with rhizobia alone. Consortium C2 was more effective than C1, and it was retained for future confirmation under field conditions. Intercropping faba bean and wheat improved wheat growth at the expense of faba bean through enhancement of their water parameters and major nutrient acquisition. Our findings showed that combining inoculation with rhizobia–PSB consortia and intercropping is a promising agroecological practice to alleviate drought and P limitation by improving plant nutrition and soil fertility, mainly in low-input agrosystems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SC and KO: performed the greenhouse and laboratory experiments and wrote the original draft. IC, AI, and BB: contributed to microbiology work. AQ: supplied methodology and resources. LK: funding acquisition and project coordination. JG: project administration and review and editing. YZ: funding acquisition and project administration. AB: planning, investigation, review, and project management. CG: conceptualization, project administration, funding acquisition, supervision, review, and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by OCP-Innovation represented by YZ, within the framework of the AS1-Anhalt-UM6P project.
Acknowledgments
This work was encouraged by the development strategy in the Morocco Green Generation program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2023.1147939/full#supplementary-material
References
Abbasi, M. K., and Manzoor, M. (2018). Biosolubilization of phosphorus from rock phosphate and other P fertilizers in response to phosphate solubilizing bacteria and poultry manure in a silt loam calcareous soil. J. Plant Nutr. Soil Sci. 181, 345–356. doi: 10.1002/jpln.201800012
Araújo, A. P., Plassard, C., and Drevon, J. J. (2008). Phosphatase and phytase activities in nodules of common bean genotypes at different levels of phosphorus supply. Plant Soil 312, 129–138. doi: 10.1007/s11104-008-9595-3
Bargaz, A., Faghire, M., Abdi, N., Farissi, M., Sifi, B., Drevon, J. J., et al. (2012). Low soil phosphorus availability increases acid phosphatases activities and affects p partitioning in nodules, seeds and rhizosphere of Phaseolus vulgaris. Agriculture 2, 139–153. doi: 10.3390/agriculture2020139
Bargaz, A., Noyce, G. L., Fulthorpe, R., Carlsson, G., Furze, J. R., Jensen, E. S., et al. (2017). Species interactions enhance root allocation, microbial diversity and P acquisition in intercropped wheat and soybean under P deficiency. Appl. Soil Ecol. 120, 179–188. doi: 10.1016/j.apsoil.2017.08.011
Bates, L. S., Waldren, R. P., and Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060
Bechtaoui, N., el Alaoui, A., Raklami, A., Benidire, L., Tahiri, A. I., and Oufdou, K. (2019). Impact of intercropping and co-inoculation with strains of plant growth-promoting rhizobacteria on phosphorus and nitrogen concentrations and yield of durum wheat (Triticum durum) and faba bean (Vicia faba). Crop Past. Sci. 70, 649–658. doi: 10.1071/CP19067
Benabdelouahab, S., Salhi, A., Himi, M., Messari, J. E. S., and Ponsati, A. C. (2019). Geoelectrical investigations for aquifer characterization and geoenvironmental assessment in northern Morocco. Environ. Earth Sci. ‘78, 1–16. doi: 10.1007/s12665-019-8221-4
Benjelloun, I., Alami, I. T., Khadir, M., Douira, A., and Udupa, S. M. (2021). Co-inoculation of Mesorhizobium ciceri with either Bacillus sp. or Enterobacter aerogenes on chickpea improves growth and productivity in phosphate-deficient soils in dry areas of a Mediterranean region. Plants 10, 1–15. doi: 10.3390/plants10030571
Betencourt, E., Duputel, M., Colomb, B., Desclaux, D., and Hinsinger, P. (2012). Intercropping promotes the ability of durum wheat and chickpea to increase rhizosphere phosphorus availability in a low P soil. Soil Biol. Biochem. 46, 181–190. doi: 10.1016/j.soilbio.2011.11.015
Blackburn, G. A. (2007). Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 58, 855–867. doi: 10.1093/jxb/erl123
Chamkhi, I., Cheto, S., Geistlinger, J., Zeroual, Y., Kouisni, L., Bargaz, A., et al. (2022). Legume-based intercropping systems promote beneficial rhizobacterial community and crop yield under stressing conditions. Ind. Crops Prod, 183, 114958. doi: 10.1016/j.indcrop.2022.114958
Chang, C. H., and Yang, S. S. (2009). Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour. Technol. 100, 1648–1658. doi: 10.1016/j.biortech.2008.09.009
Eeckhout, W., and Paepe, M. de. (1994). Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Technol. 47, 19–29. doi: 10.1016/0377-8401(94)90156-2
Farissi, M., Bouizgaren, A., Faghire, M., Bargaz, A., and Ghoulam, C. (2013). Agrophysiological and biochemical properties associated with adaptation of Medicago sativa populations to water deficit. Turk. J. Bot. 37, 1166–1175. doi: 10.3906/bot-1211-16
Gerland, P., Raftery, A. E., Ševčíková, H., Li, N., Gu, D., Spoorenberg, T., et al. (2014). World population stabilization unliskely this century. Science 346, 234–237. doi: 10.1126/science.1257469
Ghoulam, C., Foursy, A., and Fares, K. (2002). Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 47, 39–50. doi: 10.1016/S0098-8472(01)00109-5
Govindasamy, V., George, P., Kumar, M., Aher, L., Raina, S. K., Rane, J., et al. (2020). Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum [Sorghum bicolor (L.) Moench]. 3 Biotech 10, 1–14. doi: 10.1007/s13205-019-2001-4
Hadria, R., Khabba, S., Lahrouni, A., Duchemin, B., Chehbouni, G., Carriou, J., et al. (2007). Calibration and validation of the STICS crop model for managing wheat irrigation in the semi-arid Marrakech/Al Haouz Plain. Arab. J. Sci. Eng. 32, 87–101.
Hamdali, H., Hafidi, M., Virolle, M. J., and Ouhdouch, Y. (2008). Growth promotion and protection against damping-off of wheat by two rock phosphate solubilizing actinomycetes in a P-deficient soil under greenhouse conditions. Appl. Soil Ecol. 40, 510–517. doi: 10.1016/j.apsoil.2008.08.001
Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237, 173–195. doi: 10.1023/A:1013351617532
Iqbal, M., Naveed, M., Sanaullah, M., Brtnicky, M., Hussain, M. I., Kucerik, J., et al. (2022). Plant microbe mediated enhancement in growth and yield of canola (Brassica napus L.) plant through auxin production and increased nutrient acquisition. J. Soils Sedim. 23, 1–17. doi: 10.1007/s11368-022-03386-7
Kabbadj, A., Makoudi, B., Mouradi, M., Pauly, N., Frendo, P., and Ghoulam, C. (2017). Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS ONE 12, e0190284. doi: 10.1371/journal.pone.0190284
Khalid, S., Khalil, F., Elshikh, M. S., Alwahibi, M. S., and Alkahtani, J. (2021). Growth and dry matter partitioning response in cereal-legume intercropping under full and limited irrigation regimes. Sci. Rep. 11, 12585. doi: 10.1038/s41598-021-92022-4
Kumar, A., Maurya, B. R., Raghuwanshi, R., Meena, V. S., and Islam, M. T. (2017). Co-inoculation with Enterobacter and rhizobacteria on yield and nutrient uptake by wheat (Triticum aestivum L.) in the alluvial soil under indo-gangetic plain of India. J. Plant Growth Regul, 36, 608–617. doi: 10.1007/s00344-016-9663-5
Li, H., Shen, J., Zhang, F., Clairotte, M., Drevon, J. J., Cadre, E., et al. (2008). Dynamics of phosphorus fractions in the rhizosphere of common bean (Phaseolus vulgaris L.) and durum wheat (Triticum turgidum durum L.) grown in monocropping and intercropping systems. Plant Soil 312, 139–150. doi: 10.1007/s11104-007-9512-1
Li, L., Sun, J., Zhang, F., Guo, T., Bao, X., Smith, F. A., et al. (2006). Root distribution and interactions between intercropped species. Oecologia 147, 280–290. doi: 10.1007/s00442-005-0256-4
Li, L., Tilman, D., Lambers, H., and Zhang, F. S. (2014). Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol. 203, 63–69. doi: 10.1111/nph.12778
Maazaoui, H., Drevon, J. J., and Sifi, B. (2016). Improvement of Faba bean (Vicia faba L. var. minor) phosphorus uptake and nitrogen fixation in a Tunisian multi local field test. J New Sci. 31.
Magallon-Servín, P., Antoun, H., Taktek, S., Bashan, Y., and de-Bashan, L. (2020). The maize mycorrhizosphere as a source for isolation of arbuscular mycorrhizae-compatible phosphate rock-solubilizing bacteria. Plant Soil 451, 169–186. doi: 10.1007/s11104-019-04226-3
Mandri, B., Drevon, J. J., Bargaz, A., Oufdou, K., Faghire, M., Plassard, C., et al. (2012). Interactions between common bean genotypes and rhizobia strains isolated from Moroccan soils for growth, phosphatase and phytase activities under phosphorus deficiency conditions. J. Plant Nutr. 35, 1477–1490. doi: 10.1080/01904167.2012.689908
Meena, R. S., Vijayakumar, V., Yadav, G. S., and Mitran, T. (2017). Response and interaction of Bradyrhizobium japonicum and arbuscular mycorrhizal fungi in the soybean rhizosphere. Plant Growth Regul. 84, 207–223. doi: 10.1007/s10725-017-0334-8
Mouradi, M., Farissi, M., Bouizgaren, A., Makoudi, B., Kabbadj, A., Very, A. A., et al. (2015). Effects of water deficit on growth, nodulation and physiological and biochemical processes in Medicago sativa-rhizobia symbiotic association. Arid Land Res. Manag. 30, 193–208. doi: 10.1080/15324982.2015.1073194
Mouradi, M., Farissi, M., Makoudi, B., Bouizgaren, A., and Ghoulam, C. (2018). Effect of faba bean (Vicia faba L.) rhizobia symbiosis on barley's growth, phosphorus uptake and acid phosphatase activity in the intercropping system. Ann. Agrar. Sci. 16, 297–303. doi: 10.1016/j.aasci.2018.05.003
Munns, R. (2005). Genes and salt tolerance: bringing them together. New Phytol. 167, 645–663. doi: 10.1111/j.1469-8137.2005.01487.x
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nasar, J., Shao, Z., Arshad, A., Jones, F. G., Liu, S., Li, C., et al. (2020). The effect of maize–alfalfa intercropping on the physiological characteristics, nitrogen uptake and yield of maize. Plant Biol. 22, 1140–1149. doi: 10.1111/plb.13157
Oukaltouma, K., Moukhtari, A., Lahrizi, Y., Mouradi, M., Farissi, M., Willems, A., et al. (2021). Phosphorus deficiency enhances water deficit impact on some morphological and physiological traits in four faba bean (Vicia faba L.) varieties. Ital. J. Agron. 16, 1–13. doi: 10.4081/ija.2020.1662
Park, J. H., Bolan, N., Megharaj, M., and Naidu, R. (2011). Concomitant rock phosphate dissolution and lead immobilization by phosphate solubilizing bacteria (Enterobacter sp.). J. Environ. Manage. 92, 1115–1120. doi: 10.1016/j.jenvman.2010.11.031
Sharma, S. B., Sayyed, R. Z., Trivedi, M. H., and Gobi, T. A. (2013). Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2, 1–14. doi: 10.1186/2193-1801-2-587
Shilev, S. (2020). Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Appl. Sci. 10, 7326. doi: 10.3390/app10207326
Turuko, M., and Mohammed, A. (2014). Effect of different phosphorus fertilizer rates on growth, dry matter yield and yield components of common Bean (Phaseolus vulgaris L.). World J. Agric. Res. 2, 88–92. doi: 10.12691/wjar-2-3-1
Wahid, F., Sharif, M., Steinkellner, S., Khan, M. A., Marwat, K. B., and Khan, S. A. (2016). Inoculation of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria in the presence of rock phosphate improves phosphorus uptake and growth of maize. Pak. J. Bot. 48, 739–747.
Keywords: drought, phosphorus, cropping, PBS, rhizobia, Triticum durum, Vicia faba
Citation: Cheto S, Oukaltouma K, Chamkhi I, Ibn Yasser A, Benmrid B, Qaddoury A, Kouisni L, Geistlinger J, Zeroual Y, Bargaz A and Ghoulam C (2023) Inoculation with rhizobacterial consortia alleviates combined water and phosphorus deficit stress in intercropped faba bean and wheat. Front. Sustain. Food Syst. 7:1147939. doi: 10.3389/fsufs.2023.1147939
Received: 19 January 2023; Accepted: 20 March 2023;
Published: 17 April 2023.
Edited by:
Marouane Baslam, Niigata University, JapanReviewed by:
Bakha Mohamed, Université Sultan Moulay Slimane, MoroccoArafat Abdel Hamed Abdel Latef, South Valley University, Egypt
Yousef Sohrabi, University of Kurdistan, Iran
Copyright © 2023 Cheto, Oukaltouma, Chamkhi, Ibn Yasser, Benmrid, Qaddoury, Kouisni, Geistlinger, Zeroual, Bargaz and Ghoulam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cherki Ghoulam, Yy5naG91bGFtQHVjYS5tYQ==; Said Cheto, c2FpZC5jaGV0b0B1bTZwLm1h
 Said Cheto1,2*
Said Cheto1,2* Khawla Oukaltouma
Khawla Oukaltouma Imane Chamkhi
Imane Chamkhi Lamfeddal Kouisni
Lamfeddal Kouisni Adnane Bargaz
Adnane Bargaz Cherki Ghoulam
Cherki Ghoulam