- 1Department of Pharmacy, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Institute of Maternal and Child Health, Wuhan Children's Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University and Technology, Wuhan, Hubei, China
Objective: Published studies suggest that the effects of curcumin on blood lipids in adults are controversial, and it is unclear whether there is a dose response to lipid changes following curcumin supplementation. Therefore, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the effects of curcumin on triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL) in the Asian populations with metabolic diseases.
Methods: We systematically searched four electronic databases, including Web of Science, PubMed, Google Scholar, and Cochrane Library, for randomized controlled trials (RCTs) of the effects of curcumin on TG, TC, LDL, and HDL in the Asian populations with metabolic diseases. Mean difference (MD) indicates effect size with combined 95% confidence interval (95% CI). Heterogeneity among studies was assessed by I2. Subgroup analyses were performed to explore potential sources of heterogeneity.
Results: Evidence from 23 RCTs for TG, 21 RCTs for TC and LDL, and 22 RCTs for HDL showed that curcumin supplementation significantly reduced TG (MD: −18.07 mg/dL, 95% CI: −30.30, −5.85, P < 0. 01), TC (MD: −13.29 mg/dL, 95% CI: −20.43, −6.16, P < 0.01), and LDL (MD: −10.44 mg/dL, 95% CI: −16.87, −4.00, P < 0.01), but no effect on HDL (MD: 1.66 mg/dL, 95% CI: −0.13, 3.44, P = 0.07). In the non-linear dose-response analysis, we observed a significant effect of curcumin supplementation dose on TG levels (P-non-linearity = 0.022).
Conclusion: In conclusion, curcumin may be beneficial in reducing TG, TC, and LDL levels in the Asian populations with metabolic diseases. The dose of curcumin intervention may be an underlying factor influencing TG levels.
Introduction
Dyslipidaemia manifests clinically as abnormally high blood lipid concentrations, particularly elevated triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL). In recent years, metabolic diseases have become the largest burden of non-communicable diseases worldwide due to their rapidly increasing prevalence (GBD 2017 Causes of Death Collaborators, 2017). Metabolic diseases include those associated with type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS), hyperlipidaemia, obesity, overweight, and non-alcoholic fatty liver disease (NAFLD) (Rothschild et al., 2014).
Research suggests that patients with metabolic diseases are at increased risk of developing dyslipidaemia; for example, the prevalence of dyslipidaemia is higher in patients with T2DM than in the healthy population, and children and adolescents who are overweight or obese are more likely to develop dyslipidaemia and MetS (Rašlová, 2016; Reuter et al., 2016). In addition, dyslipidaemia is a major risk factor for cardiovascular disease (Haghighatdoost and Hariri, 2018; Cao et al., 2022b). Due to the side effects of chemical drugs, many patients prefer to use foods with medicinal value for prevention and treatment (Houston et al., 2009). Since ancient times, traditional medicinal foods have played an important role in the prevention and treatment of metabolic diseases. Turmeric, a natural spice that can be made into condiments such as curry paste and mustard, is widely used in cooking and is particularly popular among Asian people (Kochhar, 2008). Curcumin (diferuloylmethane), an antioxidant polyphenol with anti-inflammatory properties, is extracted from the rhizome of turmeric (Curcuma longa). Numerous studies have shown that curcumin has a variety of pharmacological activities, including antibacterial, antitumour, anti-inflammatory, antioxidant, anticoagulant, and is particularly prominent in improving metabolic diseases (Asai and Miyazawa, 2001; Ejaz et al., 2009).
Curcumin has been reported to promote weight loss, improve hyperlipidaemia, and reduce hepatic steatosis (Menon and Sudheer, 2007; Ak and Gülçin, 2008). In addition, curcumin has been shown to have therapeutic effects on various diabetic complications such as nephropathy and cardiovascular disease in patients with type 2 diabetes (Jeenger et al., 2015). A meta-analysis showed that curcumin supplementation also significantly reduced levels of inflammatory markers and biomarkers of oxidative stress in patients with Mets (Sun et al., 2022). Second, the potential mechanisms by which curcumin improves obesity and lowers blood lipids have received increasing attention. For example, curcumin blocks preadipocyte differentiation in vitro (Sakuma et al., 2017) and inhibits the expression of inflammatory cytokines in TNFα-stimulated adipocytes (Gonzales and Orlando, 2008). Curcumin also inhibits 3T3-L1 adipocyte differentiation and promotes preadipocyte apoptosis (Wu et al., 2019).
Although many studies have reported the positive effects of curcumin on lipid levels, the results have been inconsistent. Curcumin has been reported to significantly affect TG, TC, LDL, and HDL levels (Anand et al., 2007; Chong et al., 2014; Lopresti et al., 2015). However, some studies have reported an insignificant hypolipidaemic effect of curcumin (Shehzad et al., 2011; Rivera-Mancía et al., 2015). Two existing meta-analyses have reached different conclusions regarding the effects of curcumin on blood lipids (Sahebkar, 2014; Qin et al., 2017). A systematic review of seven randomized trials of turmeric and curcumin in patients with cardiovascular disease found a beneficial effect on TG and LDL levels, but no effect on TC and HDL (Qin et al., 2017). Sahebkar et al. pooled five trials and found no significant improvement in TC, LDL, TG, and HDL levels with curcumin supplementation (Sahebkar, 2014). Furthermore, the relationship between the dose of curcumin supplementation and blood lipids is unclear.
Given that turmeric is a popular spice in Asia and that its major constituent, curcumin, has the potential to modulate dyslipidemia, we synthesized and updated studies from the past 10 years to assess the effects of curcumin on dyslipidemia in Asian populations with metabolic disorders and added a dose-response relationship to provide a more comprehensive view of the effects of curcumin on lipid modulation in Asian populations.
Methods
The study was designed in accordance with the guidelines for preferred reporting methods in the PRISMA (Prevention of Systematic Evaluation and Meta-analysis) statement for 2020.
Literature search strategy
We identified articles published between 30 October 2011 and 1 April 2023 by systematically searching four electronic databases: Web of Science, PubMed, Google Scholar, and the Cochrane Library. The language was restricted to English. The literature was searched using the terms in the title or abstract and MeSH. The representative search strategy was as follows: “(curcumin OR curcuminoid OR curcuminoids OR Curcuma OR Curcuma longa OR turmeric OR C. longa OR Curcuma domestica) AND (hyperlipidemia OR lipid metabolism OR dyslipidemia OR hypercholesterolemia OR hypertriglyceridemia OR triglycerides OR TG OR total cholesterol OR TC OR low-density lipoprotein cholesterol OR LDL OR high-density lipoprotein cholesterol OR HDL) AND (Clinical Trial [ptyp] AND Humans [MeSH] AND Clinical Trial [ptyp] Sort by: Best Match).”
Second, we also identified any studies that might have been missed by hand searching, and also reviewing the reference lists of each systematic review and meta-analysis. If original data were not available, the corresponding author was contacted by e-mail to obtain. The protocol for this review was pre-registered in the PROSPERO database (International Prospective Register of Systematic Reviews; registration number: CRD42022377635; www.crd.york.ac.uk/PROSPERO).
Inclusion criteria
After extensive reading, studies were selected if they met the following inclusion criteria. Inclusion criteria included: (1) The study was conducted in the Asian population; (2) The study design was a randomized controlled trial; (3) Curcumin was the only intervention, no other food or supplement was used in the intervention and placebo groups, and the duration of intervention was ≥4 weeks; (4) All subjects were adults (≥18 years) with metabolic disorders (e.g., T2DM, dyslipidaemia, overweight, obesity, Mets, or NAFLD), no primary malignancy, and no primary systemic disease (e.g., lupus erythematosus); (5) All data are available from original study, including baseline and endpoint values with mean, SD or SE, number of participants (n) in the intervention and placebo groups; baseline values are not significantly different between the intervention and placebo groups. All studies reported at least one of the outcomes TG, TC, LDL, HDL.
Original studies were excluded if they met one or more of the following criteria: single-arm, open-label or other studies of uncontrolled quality; use of an intervention with a control group; no placebo group; presence of other lifestyle changes such as physical activity; studies on animals or cells; insufficient data; or duplicate published studies.
Data collection
The original study screening and data extraction were performed independently by two authors (ZD and KY), and any disputes were resolved in consultation with a third author (XC). The following raw data were extracted and recorded, including study characteristics, sample characteristics, intervention type, and biochemical indicators. Detailed information included: (1) Study characteristics: first author, year of publication, country, and study design; (2) Sample characteristics: subject age, male/female ratio, sample size per group; (3) Intervention dose (mg/d) and intervention duration (weeks); (4) Baseline and endpoint values for TG, TC, LDL, and HDL in the intervention and placebo groups, expressed as mean ± SD. According to the Cochrane Handbook for Systematic Reviews, if a trial included different intervention doses and durations, we split it into separate trials with the same placebo group (Higgins and Green, 2023). If the trial included different intervention groups, we extracted information only for curcumin intervention group. If a single trial reported multiple intervention assessments at different intervention times, only the results for the longest intervention duration were included in this meta-analysis.
Risk of bias and certainty assessment
Risk of bias (RoB 2.0 tool) was used to evaluate the quality of included literature. We rated the overall certainty of evidence across RCTs according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group guidelines. Based on the evaluation criteria, the quality of evidence was classified into four categories: high, medium, low, and very low. High quality: very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: moderately confident in the effect estimate, such that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: confidence in the effect estimate is limited, and the true effect may be substantially different from the estimate of the effect. Very low quality: very little confidence in the effect estimates, and the true effect is likely to be substantially different from the estimate of the effect.
Data analysis
The different units of the lipid indicators were uniformly converted to mg/dL (for TG, 1 mmol/L = 88.5 mg/dL; for TC, LDL, and HDL, 1 mmol/L = 38.7 mg/dL). Mean changes (mean ± SD) in outcome indicators (TG, TC, LDL, HDL) were used to calculate overall effect sizes. We recorded sample size n, baseline and endpoint means and SDs for the intervention and placebo groups. Where studies did not directly report change in mean and SD, they were calculated using different conversion formulas. The formulas were as follows:
mean change = mean endpoint–mean baseline;
SD = SE × square root n (n: number of participants);
SD = square root [(SD pre-treatment)2 + (SD post-treatment)2 - (2R × SD pre-treatment × SD post-treatment)], correlation coefficient R = 0.5; (Cao et al., 2022a; Higgins et al., 2022).
In addition, heterogeneity between studies was assessed by I2, with I2 = 50% being the cut-off point for low and high heterogeneity. When I2 < 50%, a fixed-effects model was used to calculate the overall effect size, whereas when heterogeneity was I2 ≥ 50%, a random-effects model was used. Subgroup analyses were performed according to intervention dose, intervention duration, and population status. Sensitivity analyses were used to assess the effect of individual studies on the overall results. Sensitivity analyses were performed by removing the original studies one at a time to calculate the effect of each original study on the overall effect size. When at least ten data points were available, we performed funnel plots and Egger's regression test to assess publication bias (Sterne et al., 2000); otherwise, funnel plots could not be used to test for publication bias. We also used fractional polynomial modeling (polynomials) to explore potential non-linear effects of curcumin dose (mg) and intervention duration (weeks) (Zhang, 2016). R software (v 4.0.3, R Core Team) and was used for all analyses in this study. A two-tailed P-value < 0.05 was considered as statistically significant.
Results
Literature search process
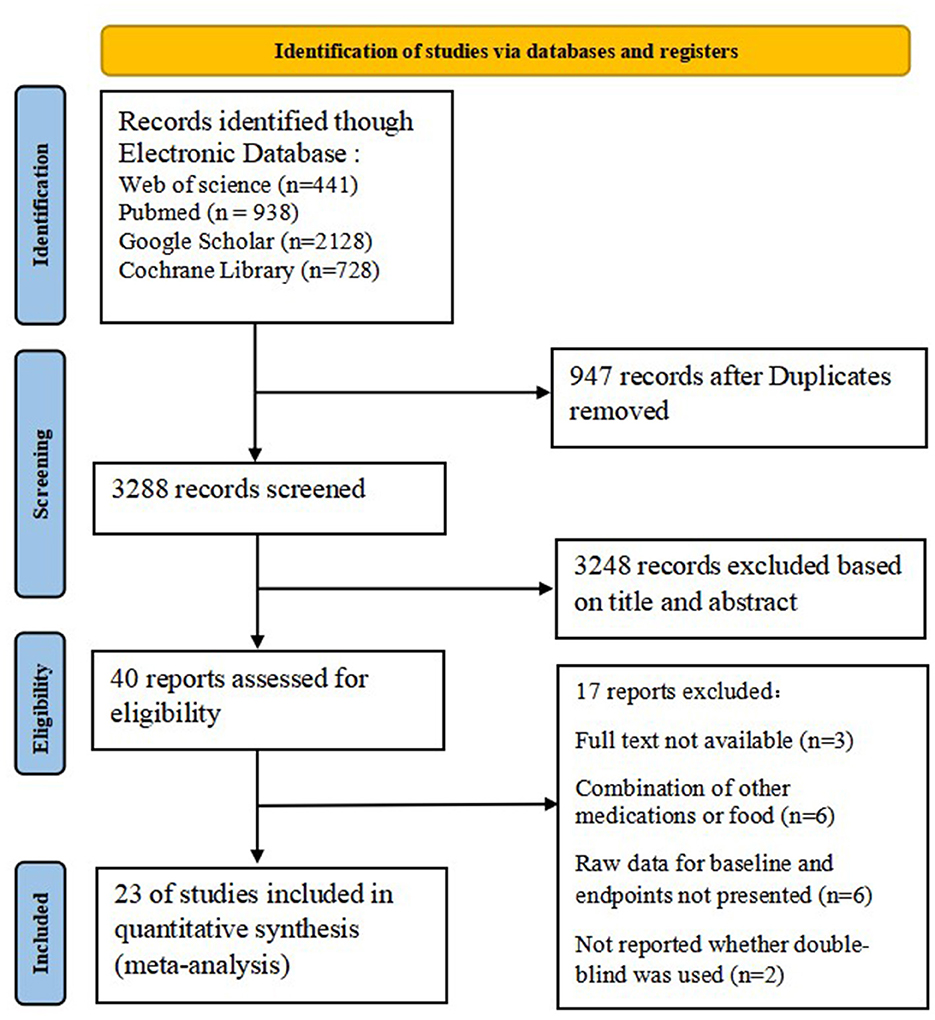
A total of 4,235 studies were retrieved from four electronic databases. After we removed 947 duplicate studies and 3,288 irrelevant studies based on title and abstract, the remaining 40 trials were included for further evaluation. An additional 17 records were excluded for the following reasons: full text was not available, combination with other drug treatments, raw data on baseline and endpoints were not presented, and whether double-blinding was used was not reported. Twenty-three studies that met the inclusion criteria were entered into the final meta-analysis. The selection process for the included studies is detailed in Figure 1.
The characteristics of the included studies
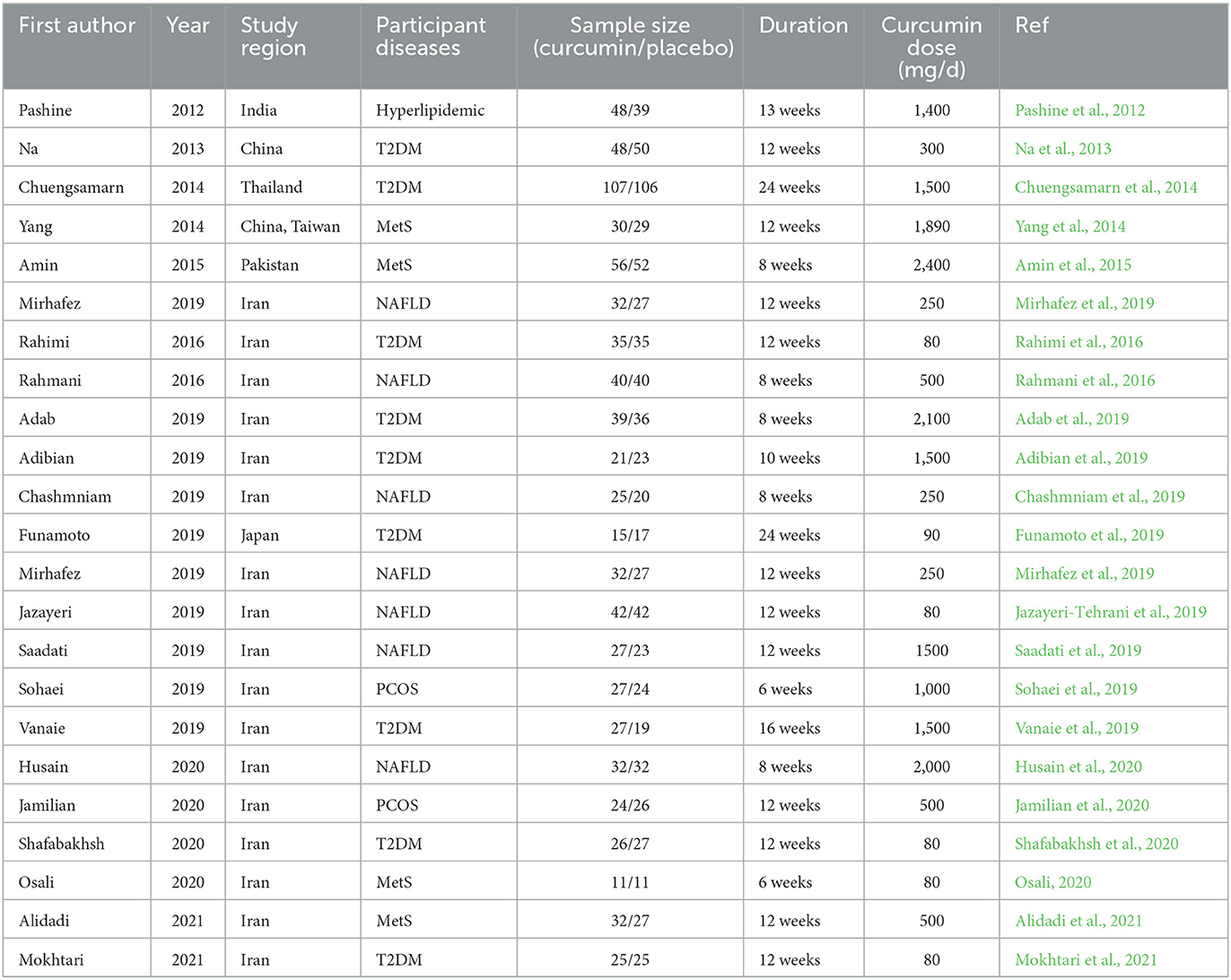
The characteristics of all included studies are summarized in Table 1. A total of 1,532 subjects were included in 23 studies published between 2011 and 2023, including 681 patients with T2DM, 248 with Mets, 382 with NAFLD, 87 with hyperlipidaemia, 101 with obese polycystic ovarian syndrome, and 33 with obesity complicated by coronary artery disease. There were 23 TG trials, 21 TC and LDL trials, and 22 HDL cholesterol trials. In the trial by Rahimi et al. (2016) the raw data for TG were presented using median values, suggesting that the distribution of baseline values may be abnormal. Secondly, five trials used nanocurcumin for intervention (Rahmani et al., 2016; Jazayeri-Tehrani et al., 2019; Osali, 2020; Shafabakhsh et al., 2020; Mokhtari et al., 2021), three trials used other highly bioavailable curcumin preparations (Mirzabeigi et al., 2015; Funamoto et al., 2019; Mirhafez et al., 2019) and 15 trials used low bioavailable curcumin preparations (e.g., homemade curcumin capsules, turmeric root powder, etc.) (Pashine et al., 2012; Na et al., 2013; Chuengsamarn et al., 2014; Yang et al., 2014; Amin et al., 2015; Rahmani et al., 2016; Adab et al., 2019; Adibian et al., 2019; Chashmniam et al., 2019; Saadati et al., 2019; Sohaei et al., 2019; Vanaie et al., 2019; Husain et al., 2020; Jamilian et al., 2020; Alidadi et al., 2021). In addition, the duration of the intervention ranged from 6 weeks to 24 weeks and the dose ranged from 80 mg/d to 2400 mg/d. The distribution of curcumin formulations, intervention doses, durations and sample sizes for all included trials is shown in Figure 2.
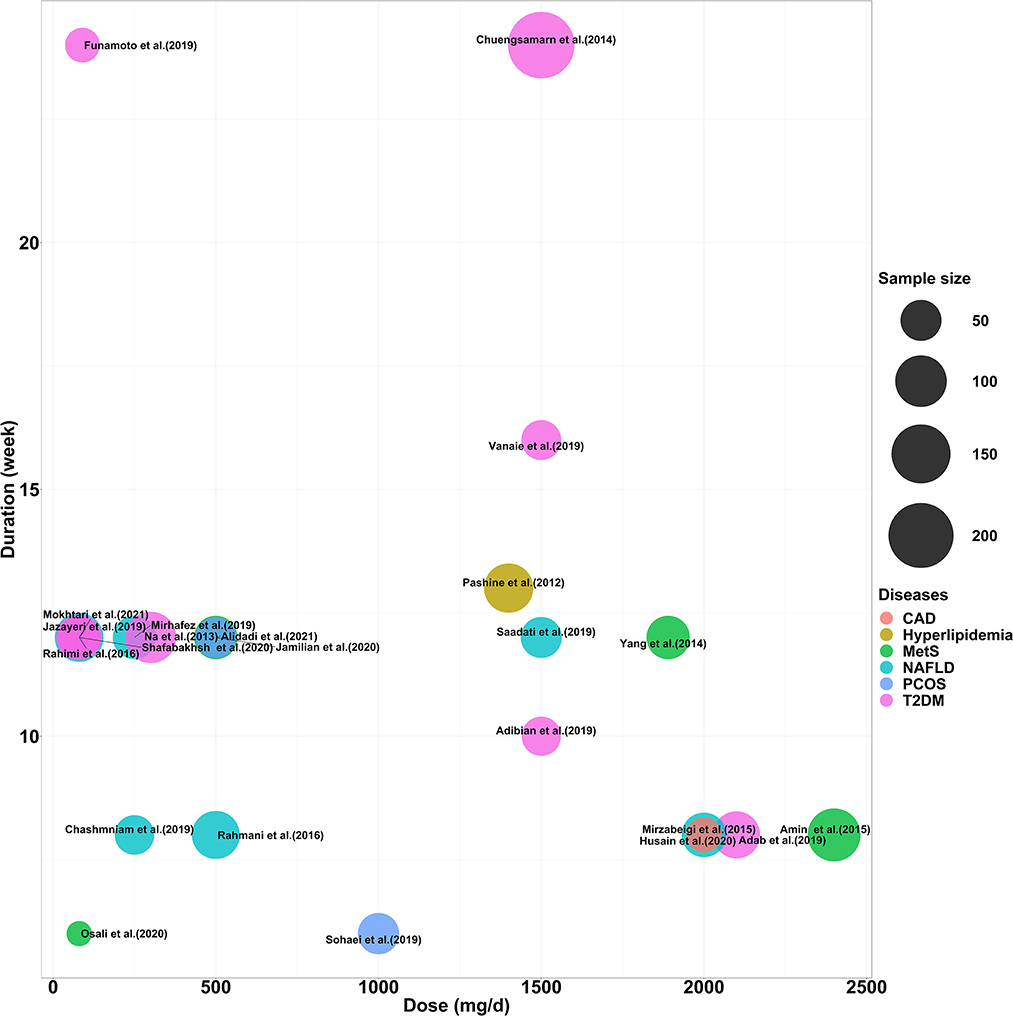
Figure 2. Distribution of intervention dose, duration and number of participants included in the study.
Assessment of quality
Fourteen studies were judged to be at low risk of bias while additional five studies were classified as having some concerns, and four studies were categorized as at high risk of bias (Figures 3A, B). To assess the quality of evidence for the outcomes, the GRADE framework was implemented, determining that the evidence for TC, LDL, and HDL was downgraded to moderate, while the evidence regarding TG was determined to be low (Figure 3C).
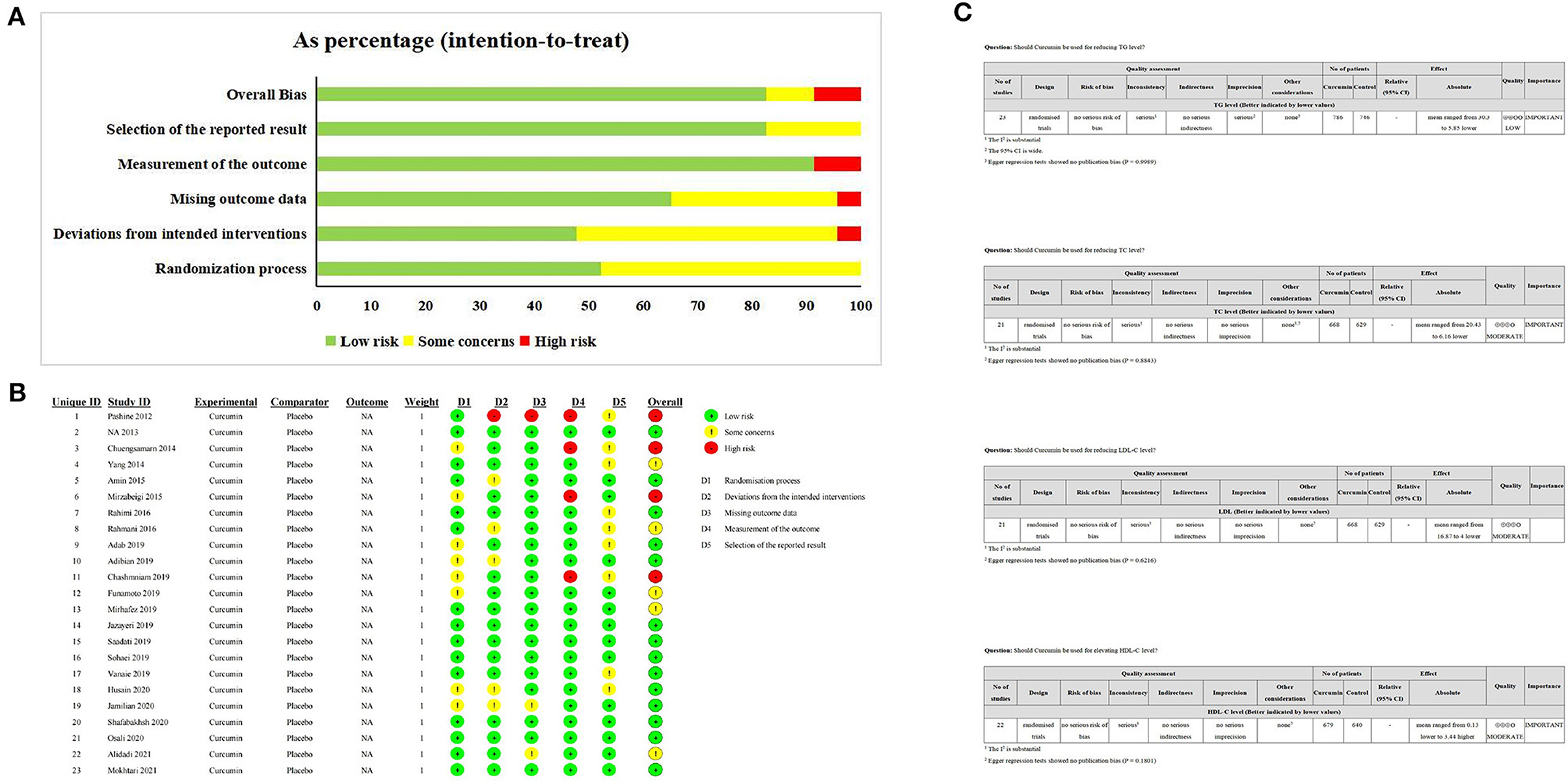
Figure 3. The Risk of bias and certainty assessment. (A, B) ROBII assessmeng of included studies; (C) GRADE assessment of included study.
Effect of curcumin supplementation on TG
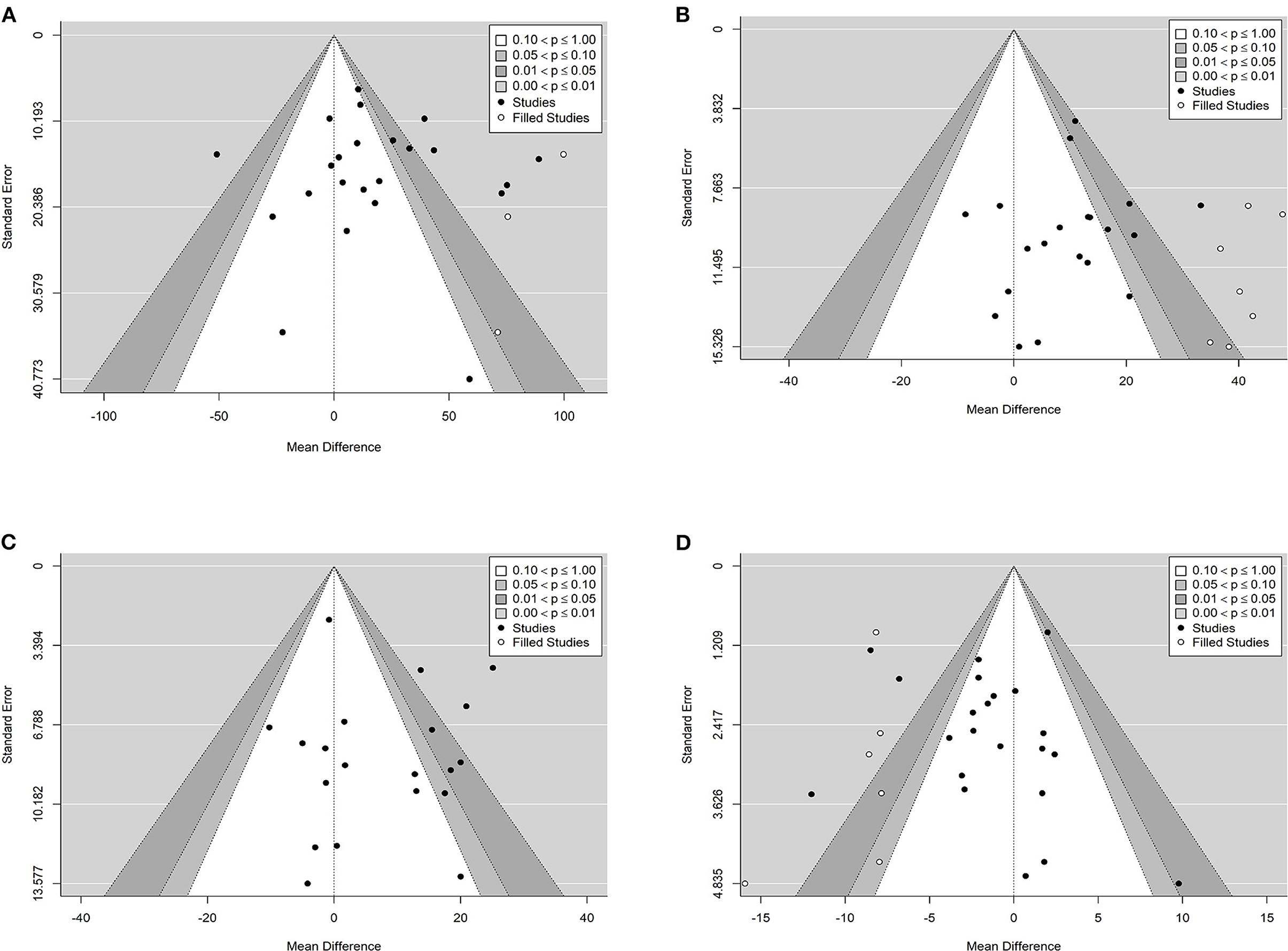
The effect of curcumin on TG was reported in all 23 studies (Figure 4A). According to a random effects model (I2 = 77.00%, P < 0.01), the mean change in TG concentration between the intervention and placebo groups differed by −18.07 mg/dL (MD: −18.07 mg/dL, 95% CI: −30.30, −5.85, P < 0.01), suggesting that curcumin significantly reduced TG concentration. As the heterogeneity exceeded 50%, subgroup analysis was performed to determine the source of heterogeneity (Table 2). The results showed that TG levels were lower in the curcumin group than in the placebo group for different intervention durations and pooled data for different intervention doses. In addition, curcumin reduced TG levels in subjects with different status, including T2DM, NAFLD, Mets and PCOS. Furthermore, both observed TG levels were significantly changed in studies with >1,000 mg/d curcumin (MD: −36.64 mg/dL, 95% CI: −57.51–15.76, P < 0.01) and in studies with an intervention duration of <8 weeks (MD: −21.58 mg/dL, 95% CI: −38.28–4.89, P = 0.01). However, the heterogeneity of RCTs in each subgroup remained high. After removing each original study individually using sensitivity analyses, we found no change in the combined effect size (Figure 5A). Egger regression test showed no publication bias (P = 0.9989) (Figure 6A). According to the fractional polynomial model, curcumin above 80 mg/kg had a significant reduction in TG (P-non-linearity = 0.02, Figure 7A), but no effect of intervention duration on TG was observed (P-non-linearity = 0.69, Figure 8A).
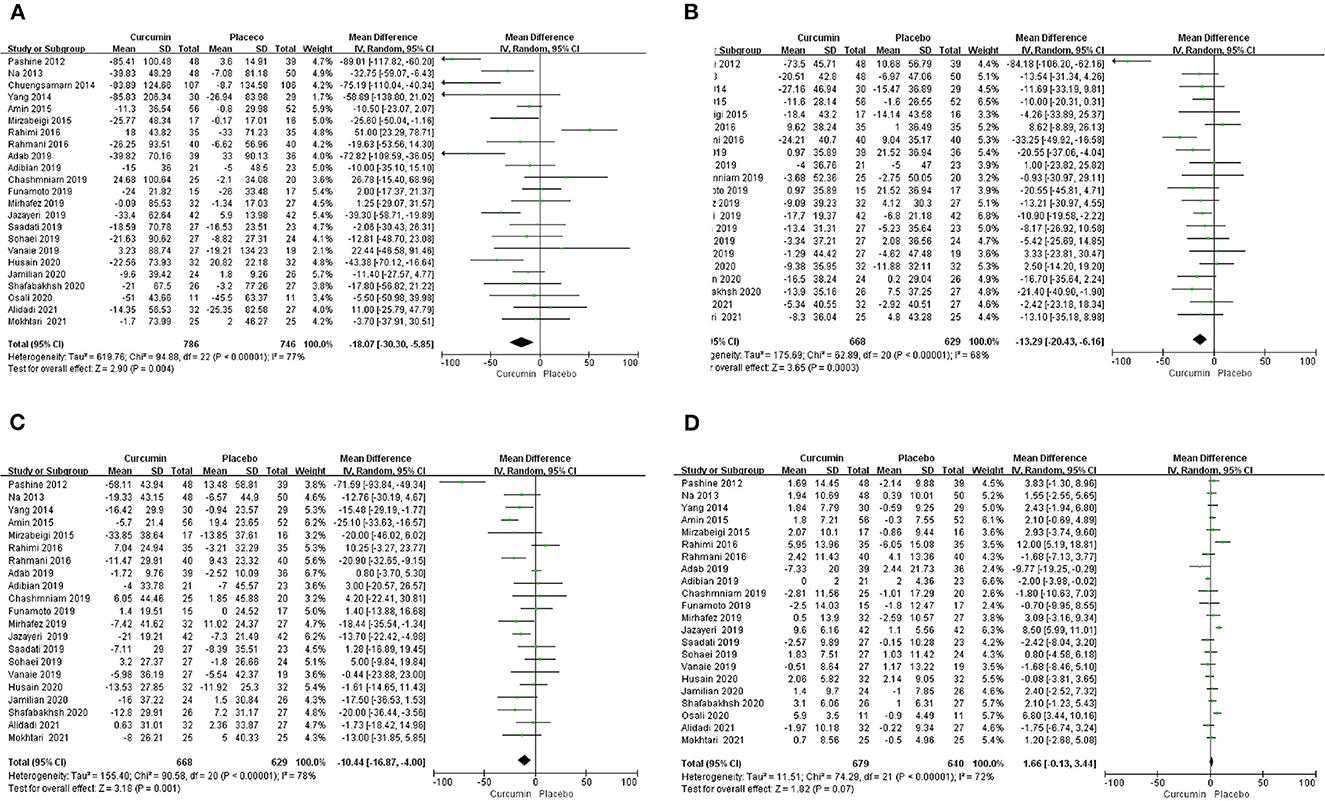
Figure 4. Forest plots of weighted mean difference (WMD, 95% CI) of (A) TG, (B) TC, (C) LDL, and (D) HDL change by curcumin supplementation across all included studies.
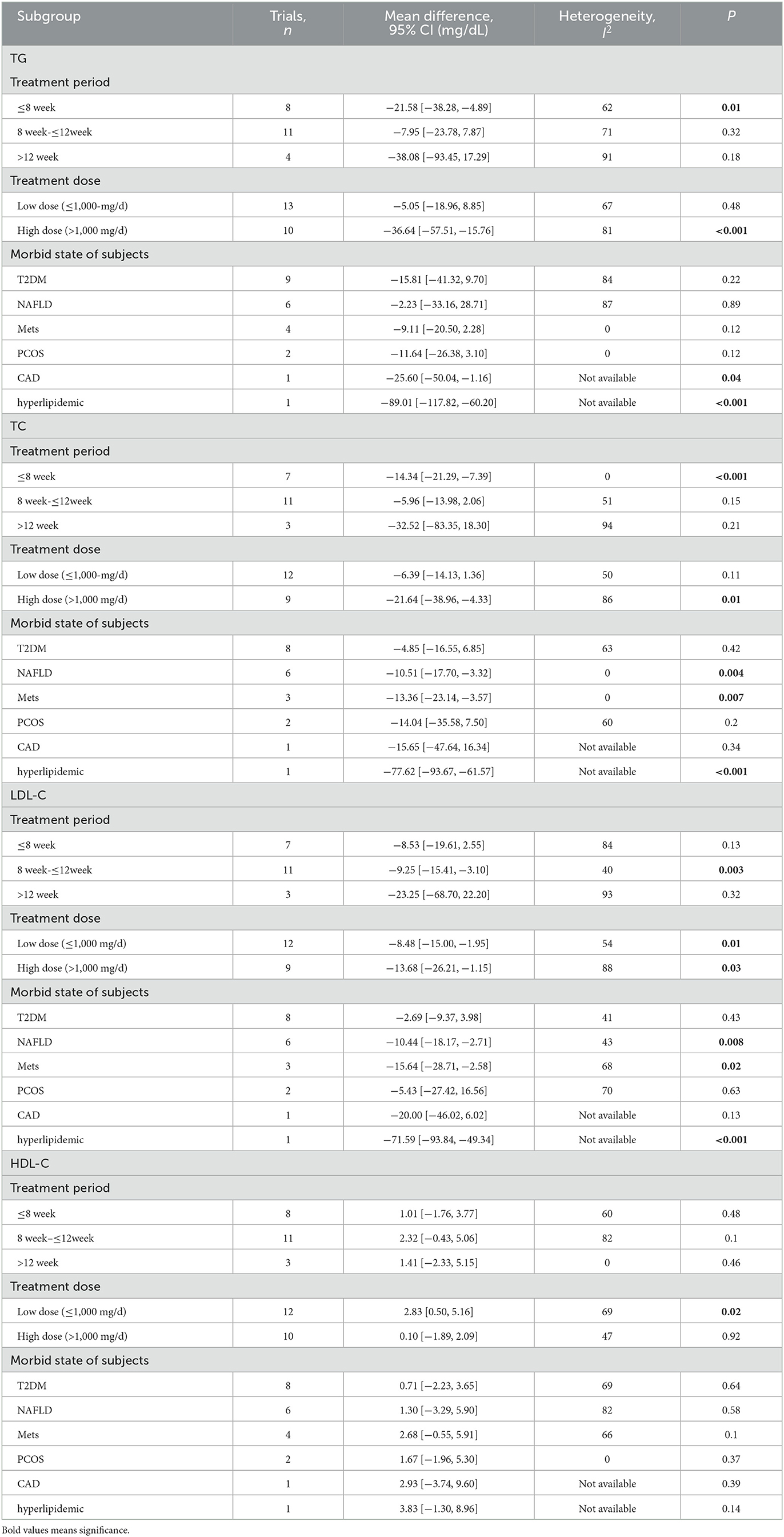
Table 2. Subgroup analysis about treatment period, treatment dose, and diseases for TG, TC, LDL-C, and HDL-C.
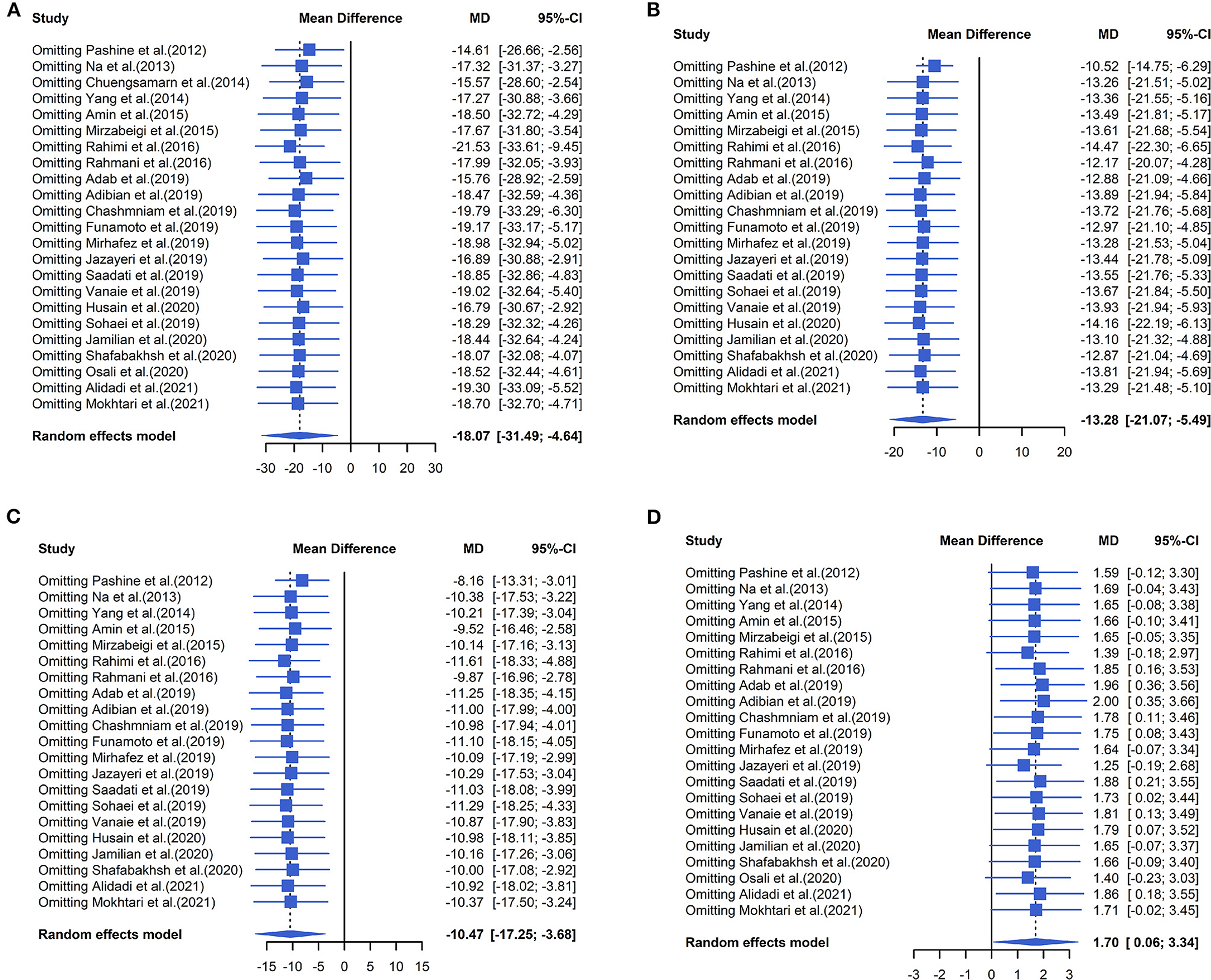
Figure 5. Forest plots of sensitivity analysis of (A) TG, (B) TC, (C) LDL, and (D) HDL to curcumin supplementation.
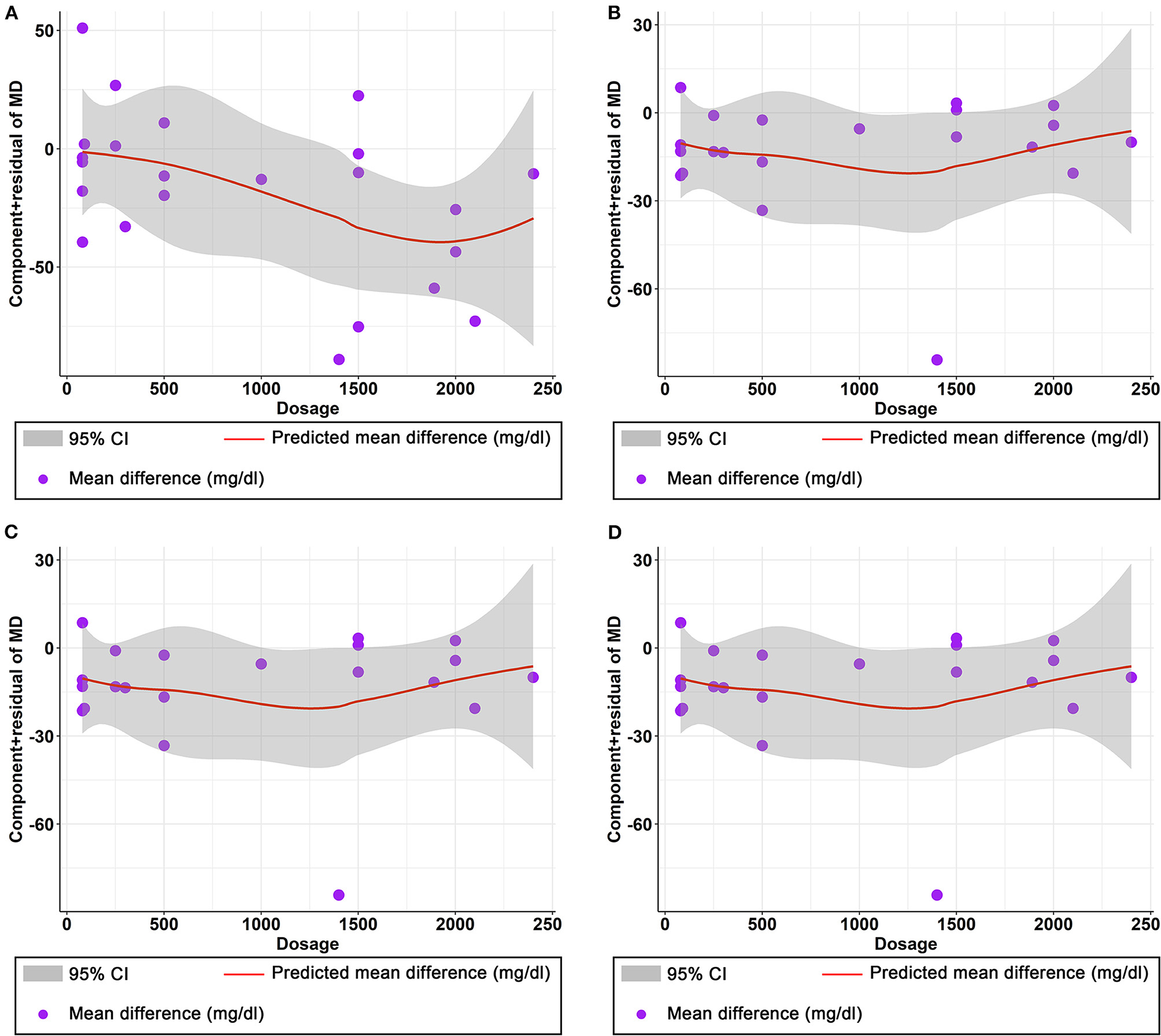
Figure 7. Nonlinear dose–response relations between curcumin dosage (mg) and unstandardized mean difference (mg/dL) in (A) TG, (B) TC, (C) LDL, and (D) HDL.
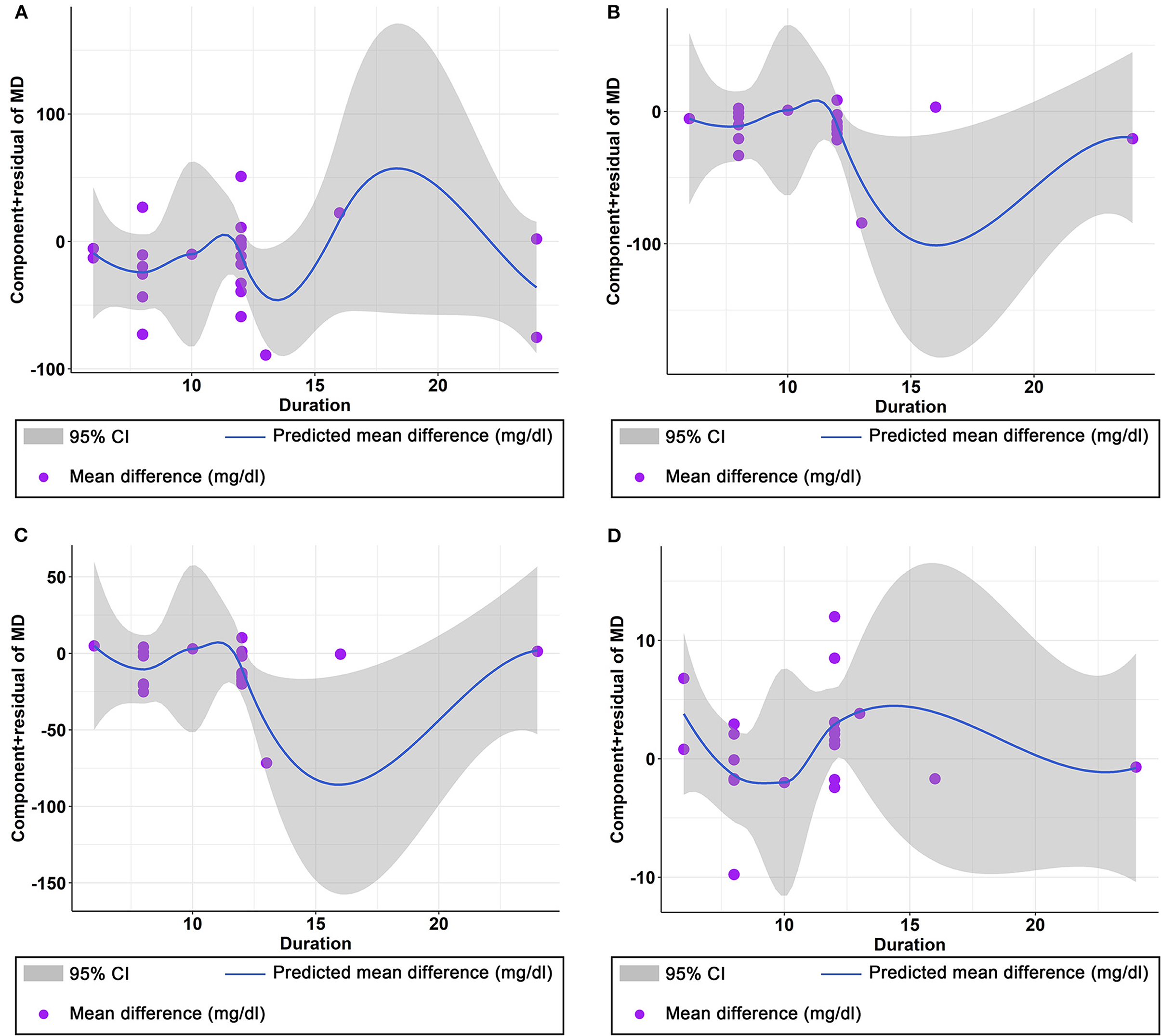
Figure 8. Non-linear dose–response relations between curcumin duration (weeks) and unstandardized mean difference (mg/dL) in (A) TG, (B) TC, (C) LDL, and (D) HDL.
Effect of curcumin on TC
The combined results of 21 studies showed that curcumin intervention reduced TC concentrations in subjects with metabolic disorders (MD: −13.29 mg/dL, 95% CI: −20.43, −6.16, P < 0.01). There was significant heterogeneity among the included studies (I2 = 68.00%, P < 0.01) (Figure 4B). The results of subgroup analysis showed that the heterogeneity between studies remained high with respect to treatment dose, intervention duration, and subject population grouping. However, the use of high doses (>1,000 mg/d) (MD: −21.64 mg/dL, 95% CI: −38.96 to −4.33, P = 0.01) of curcumin with an intervention duration of <8 weeks (MD: −14.34 mg/dL, 95% CI: −21.29 to −7.39, P < 0.01) all significantly reduced TC level. Significant downregulation of TC was observed in subjects with NAFLD (MD: −10.51 mg/dL, 95% CI: −17.70, −3.32, P < 0.01) and Mets (MD: −13.36 mg/dL, 95% CI: −23.14, −3.57, P < 0.01) (Table 2). Second, the results of sensitivity analysis showed that the overall effect size was stable (Figure 5B). The publication bias test showed that there was probably no publication bias (P = 0.8843) (Figure 6B). Furthermore, there was no significant non-linear relationship between dose (P-non-linearity = 0.99, Figure 7B) and intervention duration (P-non-linearity = 0.51, Figure 8B) and TC.
The effect of curcumin on LDL
A total of 21 trials reported the effect of curcumin on LDL. According to the random effects model (I2 = 78.00%, P < 0.01), curcumin significantly reduced LDL level (MD: −10.44 mg/dL, 95% CI: −16.87, −4.00; P < 0.01) (Figure 4C). Subgroup analysis showed that TC levels were significantly lower in the pool of participants taking both high (>1,000 mg/d) (MD: −13.68 mg/dL, 95% CI: −26.21, −1.15, P = 0.03) and low doses (≤1,000 mg/d) of curcumin (MD: −8.48 mg/dL, 95% CI: −15.00, −1.95, P = 0.01).
The LDL-lowering effect of curcumin was more pronounced in subjects with NAFLD (MD: −10.44 mg/dL, 95% CI: −18.17, −2.71, P < 0.01) and Mets (MD: −15.64 mg/dL, 95% CI: −28.71, −2.58, P = 0.02). Sensitivity analyses showed that combined OR and 95% CI did not change significantly when any of the studies were removed, suggesting the stability of the results of current analysis (Figure 5C). Egger regression testing showed no difference between the observed and estimated values (p = 0.6216) (Figure 6C). Regarding the non-linear dose-response analysis, there was no significant effect of supplementation dose (P-non-linearity = 0.60, Figure 7C) and intervention duration (P-non-linearity = 0.99, Figure 8C) on LDL levels.
Effect of curcumin on HDL
The combined effect of 22 trials showed no effect of curcumin intake on HDL level (MD: 1.66 mg/dL, 95% CI: −0.13, 3.44; P = 0.07). Subgroup analysis showed that low doses (≤1,000 mg/d) of curcumin may increase HDL levels (MD: 2.83 mg/dL, 95% CI: 0.50, 5.16, P = 0.02) (Figure 4D). The effect sizes did not change after the removal of individual studies in the sensitivity analysis (Figure 5D). Publication bias analysis showed no differences between observed and estimated values (P = 0.1801) (Figure 6D). Furthermore, the effects of curcumin dose (P-non-linearity = 0.58, Figure 7D) and intervention duration (P-non-linearity = 0.93, Figure 8D) on HDL levels were not significant.
Discussion
Studies have shown that lifestyle modification and calorie control are effective treatments for metabolic disorders, but many patients are unable to complete them due to poor adherence and persistence (Narayan et al., 2002; Maruthur et al., 2013). In addition, the use of nutritional products to correct the lipid profile of patients with metabolic disorders has received increasing attention due to the high cost and side effects of chemical drugs (Houston, 2012). Evidence from our analysis of 23 RCTs showed that curcumin supplementation significantly reduced TG, TC, and LDL levels, but not HDL, in patients with metabolic disorders in the Asian population. When analyzing more homogeneous trials based on the underlying disease of subjects, beneficial effects of curcumin on TG, TC, and LDL levels were observed in subjects with NAFLD and Mets; however, such beneficial effects on HDL levels were not observed in subjects with T2DM, NAFLD, Mets, PCOS, and CAD. In addition, there was a more beneficial effect on TG and TC when the curcumin intervention was administered at high doses (>1,000 mg/d) for < 8 weeks. In a non-linear dose-response analysis, we observed a significant effect of curcumin supplementation dose on TG levels. In addition, larger studies in patients with metabolic disorders may be needed to investigate the effect of curcumin on lowering lipid concentrations.
There were some similar and discrepant results in our meta-analysis compared to the three available meta-analyses: Qin et al. (2017) reported that curcumin significantly reduced LDL and TG levels, but did not significantly improve HDL levels. This is similar to our findings. However, Yuan et al. (2019) reported that curcumin reduced TG, TC, LDL and increased HDL. Sahebkar et al. (2017) and Saeedi et al. (2022) found no effect of curcumin on TG, TC, LDL, and HDL levels. The results of our analysis showed that curcumin significantly reduced TG, TC, LDL, and had no significant effect on HDL. We can suggest several explanations as to why their results are contrary to those analyzed in the present study. First, the trials included different ethnic groups, and our review only included trials in the Asian populations. Second, the studies we included were all randomized, double-blind, placebo-controlled trials; studies from parallel and cross-over randomized trials were not included because these studies may have affected the final results. Thirdly, the study by Yuan and Sahebkar included trials in which subjects took curcumin in combination with other therapeutic measures in addition to curcumin (Khajehdehi et al., 2011; Yang et al., 2014; Panahi et al., 2017), whereas the subjects included in this meta-analysis took curcumin only. Finally, the disease status of the included population may also partially explain this difference. The present meta-analysis only included patients with various chronic metabolic diseases, whereas the studies by Sahebkar et al. (2017) and Saeedi et al. (2022) also included healthy subjects, and these differences may have contributed to the different results. In addition, the evidence from our study is likely to be stronger because of the larger number of trials and more stringent inclusion criteria.
A number of recent findings have revealed mechanisms by which curcumin may improve hyperlipidaemia. First, curcumin may affect cellular transduction pathways associated with obesity and metabolic disease. Curcumin can alter transduction signaling (Akt, AMPK), balance gene expression of specific transcription factors (Foxo1/3a, Nrf2, Srebp1/2, CREB, CREBH, PPARγ, and LXRα) and factors related to lipogenesis (aP2/FABP4, CD36, HMG-CoA reductase and carnitine palmitoyltransferase-I) (Zingg et al., 2013); Curcumin also activates Wnt/β-catenin signaling, inhibits preadipocyte differentiation, macrophage expansion and infiltration into white adipose tissue, thereby reducing adipocyte numbers and inhibiting inflammatory adipokine secretion (Ahn et al., 2010). In addition, curcumin may modulate lipid metabolic pathways affected by statins and exert an anti-obesity effect (Urasaki and Le, 2022). Overall, a variety of mechanisms may be responsible for the hypolipidaemic effects of curcumin.
In this meta-analysis, we also performed a dose-response analysis and found a non-linear relationship between curcumin dose and TG levels, suggesting that the effect of curcumin on TG is dependent on the intervention dose. The results showed that just over 80 mg/d of curcumin reduced TG. In the subgroup analysis, we also found that >1,000 mg/d curcumin showed a significant change in TG levels, which is consistent with the upward trend we observed in the dose-response plots. The results of this analysis suggest that dose may be a key factor in curcumin supplementation.
It must be acknowledged that the current meta-analysis still has some limitations. Firstly, the manufacturing process of curcumin, the dose of the intervention and the duration of treatment varied between the included trials; secondly, most of the results had a high degree of heterogeneity that could not be resolved by appropriate subgroup analysis. Data from some of the trials were based on formulas from the Cochrane Handbook of Systematic Reviews, which may have been a source of heterogeneity; thirdly, the large proportion of randomized controlled trials from the Middle East affected the applicability of the results. Due to the apparent heterogeneity and the limited number of RCTs, this meta-analysis may not lead to precise conclusions. Therefore, more homogeneous and high-quality randomized controlled trials should be conducted to demonstrate the effects of curcumin on blood lipids.
In conclusion, this meta-analysis suggests that curcumin supplementation may significantly affect TG, TC, and LDL levels, but not HDL levels. Furthermore, the dose of curcumin intervention is an important factor that should be considered with caution. The results of this meta-analysis may be useful in making recommendations for the use of curcumin as a dietary supplement. Given the limitations of this meta-analysis, more and higher quality RCTs are needed to confirm these results.
Author contributions
WG designed the idea for the systematic review and meta-analysis. HX revised the original manuscript. ZD, KY, XC, and HM were involved in obtaining and analyzing the data. ZD drafted the manuscript. All authors read and agreed to the published version of the manuscript.
Funding
This project was supported by Wuhan Yellow Crane Talent-Outstanding Young Talent Project (WG and ZD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Triglyceride, TG; Total cholesterol, TC; Low-density lipoprotein cholesterol, LDL; High-density lipoprotein cholesterol, HDL; Randomized controlled trials, RCTs; Mean difference, MD; Metabolic syndrome, Mets; Type 2 diabetes mellitus, T2DM; Non-alcoholic fatty liver disease, NAFLD.
References
Adab, Z., Eghtesadi, S., Vafa, M. R., Heydari, I., Shojaii, A., Haqqani, H., et al. (2019). Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytotherapy Res. 33, 1173–81. doi: 10.1002/ptr.6312
Adibian, M., Hodaei, H., Nikpayam, O., Sohrab, G., Hekmatdoost, A., Hedayati, M., et al. (2019). The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Phytotherapy Res. 33, 1374–83. doi: 10.1002/ptr.6328
Ahn, J., Lee, H., Kim, S., and Ha, T. (2010). Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol,. Cell Physiol. 298, C1510–C1516. doi: 10.1152/ajpcell.00369.2009
Ak, T., and Gülçin, I. (2008). Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 174, 27–37. doi: 10.1016/j.cbi.2008.05.003
Alidadi, M., Sahebkar, A., and Eslami, S. (2021). The effect of curcumin supplementation on pulse wave velocity in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Adv. Exp. Med. Biol. 1308, 1–11. doi: 10.1007/978-3-030-64872-5_1
Amin, F., Islam, N., Anila, N., and Gilani, A. H. (2015). Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome–A double blind randomized controlled trial–TAK-MetS trial. Complement. Therap. Med. 1) 23, 165–74. doi: 10.1016/j.ctim.2015.01.008
Anand, P., Kunnumakkara, A. B., Newman, R. A., and Aggarwal, B. B. (2007). Bioavailability of curcumin: problems and promises. Mol. Pharm. 4, 807–818. doi: 10.1021/mp700113r
Asai, A., and Miyazawa, T. (2001). Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J. Nutr. 131, 2932–5. doi: 10.1093/jn/131.11.2932
Cao, X., Liao, W., Xia, H., Wang, S., and Sun, G. (2022a). The effect of resveratrol on blood lipid profile: a dose-response meta-analysis of randomized controlled trials. Nutrients 14, 3755. doi: 10.3390/nu14183755
Cao, X., Xia, J., Zhou, Y., Wang, Y., Xia, H., Wang, S., et al. (2022b). The The effect of MUFA-rich food on lipid profile: a meta-analysis of randomized and controlled-feeding trials. Foods 11, 1982. doi: 10.3390/foods11131982
Chashmniam, S., Mirhafez, S. R., Dehabeh, M., Hariri, M., Azimi Nezhad, M., Gh, N. M., et al. (2019). Pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutri. 73, 1224–35. doi: 10.1038/s41430-018-0386-5
Chong, L., Zhang, W., Nie, Y., Yu, G., Liu, L., Lin, L., et al. (2014). Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation 37, 1476–1485. doi: 10.1007/s10753-014-9873-6
Chuengsamarn, S., Rattanamongkolgul, S., Phonrat, B., Tungtrongchitr, R., and Jirawatnotai, S. (2014). Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J. Nutr. Biochem. 25, 144–150. doi: 10.1016/j.jnutbio.2013.09.013
Ejaz, A., Wu, D., Kwan, P., and Meydani, M. (2009). Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 139, 919–25. doi: 10.3945/jn.108.100966
Funamoto, M., Shimizu, K., Sunagawa, Y., Katanasaka, Y., Miyazaki, Y., Kakeya, H., et al. (2019). Effects of highly absorbable curcumin in patients with impaired glucose tolerance and non-insulin-dependent diabetes mellitus. J. Diab. Res. 2019, 8237. doi: 10.1155/2019/8208237
GBD 2017 Causes of Death Collaborators (2017). Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study. Lancet. 392, 1736–1788.
Gonzales, A. M., and Orlando, R. A. (2008). Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 5, 17. doi: 10.1186/1743-7075-5-17
Haghighatdoost, F., and Hariri, M. (2018). Effect of resveratrol on lipid profile: an updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 129, 141–150. doi: 10.1016/j.phrs.2017.12.033
Higgins, J. P, Thomas, J, Chandler, J, Cumpston, M, Li, T, and Page, M. J editors. (2022). Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. London, UK: Version 6, 3. (Updated February 2022) Available online at: http://www.training.cochrane.org/handbook (accessed 27 November 2017).
Higgins, P. T. J., and Green, S. (2023). “General procedures for risk-of-bias assessment,” in Cochrane Handbook for Systematic Reviews of Interventions: the Cochrane Collaboration. Version 6.3. Available online at: https://training.cochrane.org/handbook/current/chapter-07
Houston, M. (2012). The role of nutraceutical supplements in the treatment of dyslipidemia. J. Clin. Hypertens. 14, 121–32. doi: 10.1111/j.1751-7176.2011.00576.x
Houston, M. C., Fazio, S., and Chilton, F. H. (2009). Non-pharmacologic treatment of dyslipidemia. Prog. Cardiovasc. Dis. 52, 61–94. doi: 10.1016/j.pcad.2009.02.002
Husain, D., Jarahzadeh, M., Alavinejad, P., Rezazadeh, A., and Haghighizadeh, M. (2020). Turmeric supplementation in non-alcoholic fatty liver disease. Plant. Arch. 20, 3640–7.
Jamilian, M., Foroozanfard, F., Kavossian, E., Aghadavod, E., Shafabakhsh, R., Hoseini, A., et al. (2020). Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin. Nutri. ESPEN. 36, 128–33. doi: 10.1016/j.clnesp.2020.01.005
Jazayeri-Tehrani, S. A., Rezayat, S. M., Mansouri, S., Qorbani, M., Alavian, S. M., Daneshi-Maskooni, M., et al. (2019). Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutri. Metabol. 16, 1–3. doi: 10.1186/s12986-019-0331-1
Jeenger, M. K., Shrivastava, S., Yerra, V. G., Naidu, V. G., Ramakrishna, S., Kumar, A., et al. (2015). Curcumin: a pleiotropic phytonutrient in diabetic complications. Nutrition 31, 276–82. doi: 10.1016/j.nut.2014.06.015
Khajehdehi, P., Pakfetrat, M., Javidnia, K., Azad, F., Malekmakan, L., Nasab, M. H., et al. (2011). Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 45, 365–370. doi: 10.3109/00365599.2011.585622
Kochhar, K. P. (2008). Dietary spices in health and diseases (II). Indian J. Physiol. Pharmacol. 52, 327–54.
Lopresti, A. L., Maes, M., Meddens, M. J., Maker, G. L., Arnoldussen, E., Drummond, P. D., et al. (2015). Curcumin and major depression: a randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur. Neuropsychopharmacol. 25, 38–50. doi: 10.1016/j.euroneuro.2014.11.015
Maruthur, N. M., Ma, Y., Delahanty, L. M., Nelson, J. A., Aroda, V., White, N. H., et al. (2013). Diabetes prevention program research group. Early response to preventive strategies in the diabetes prevention program. J. Gen. Intern. Med. 28, 1629–36. doi: 10.1007/s11606-013-2548-4
Menon, V. P., and Sudheer, A. R. (2007). Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 595, 105–125. doi: 10.1007/978-0-387-46401-5_3
Mirhafez, S. R., Farimani, A. R., Dehhabe, M., Bidkhori, M., Hariri, M., Ghouchani, B. F., et al. (2019). Effect of phytosomal curcumin on circulating levels of adiponectin and leptin in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled clinical trial. J. Gastroint. Liver Dis. 28, 179. doi: 10.15403/jgld-179
Mirzabeigi, P., Mohammadpour, A. H., Salarifar, M., Gholami, K., Mojtahedzadeh, M., Javadi, M. R., et al. (2015). The effect of curcumin on some of traditional and non-traditional cardiovascular risk factors: a pilot randomized, double-blind, placebo-controlled trial. Iran. J Pharmaceut Res IJPR. 14, 479.
Mokhtari, M., Razzaghi, R., and Momen-Heravi, M. (2021). The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 35, 2099–2107. doi: 10.1002/ptr.6957
Na, L. X., Li, Y., Pan, H. Z., Zhou, X. L., Sun, D. J., Meng, M., et al. (2013). Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 57, 1569–1577. doi: 10.1002/mnfr.201200131
Narayan, K. M., Imperatore, G., Benjamin, S. M., and Engelgau, M. M. (2002). Targeting people with pre-diabetes. BMJ 325, 403–4. doi: 10.1136/bmj.325.7361.403
Osali, A. (2020). Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol. Metab. Syndr. 12, 26. doi: 10.1186/s13098-020-00532-4
Panahi, Y., Khalili, N., Sahebi, E., Namazi, S., Reiner, Ž., Majeed, M., et al. (2017). Curcuminoids modify lipid profile in type 2 diabetes mellitus:a randomized controlled trial. Complement. Ther. Med. 33, 1–5. doi: 10.1016/j.ctim.2017.05.006
Pashine, L., Singh, J. V., and Vaish, A. K. (2012). Effect of turmeric (Curcuma longa) on overweight hyperlipidemic subjects: double blind study. Indian J Commun. Health 24, 113–7.
Qin, S., Huang, L., Gong, J., Shen, S., Huang, J., Ren, H., et al. (2017). Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr. J. 16, 68. doi: 10.1186/s12937-017-0293-y
Rahimi, H. R., Mohammadpour, A. H., Dastani, M., Jaafari, M. R., Abnous, K., Mobarhan, M. G., et al. (2016). The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avic. J. Phytomed. 6, 567.
Rahmani, S., Asgary, S., Askari, G., Keshvari, M., Hatamipour, M., Feizi, A., et al. (2016). Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytoth. Res. 30, 1540–8. doi: 10.1002/ptr.5659
Rašlová, K. (2016). Diabetes a dyslipidémia: prečo majú k sebe tak blízko? [Diabetes and dyslipidemia: why are they so closely related? Vnitr. Lek. 62, 908–911.
Reuter, C. P., Silva, P. T. D., Renner, J. D. P., Mello, E. D. D., Valim, A. R. D. M., Pasa, L., et al. (2016). Dyslipidemia is associated with unfit and overweight-obese children and adolescents. Arq. Bras. Cardiol. 106, 188–193. doi: 10.5935/abc.20160025
Rivera-Mancía, S., Lozada-García, M. C., and Pedraza-Chaverri, J. (2015). Experimental evidence for curcumin and its analogs for management of diabetes mellitus and its associated complications. Eur. J. Pharmacol. 756, 30–37. doi: 10.1016/j.ejphar.2015.02.045
Rothschild, J., Hoddy, K. K., Jambazian, P., and Varady, K. A. (2014). Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr. Rev. 72, 308–318. doi: 10.1111/nure.12104
Saadati, S., Hatami, B., Yari, Z., Shahrbaf, M. A., Eghtesad, S., Mansour, A., et al. (2019). The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin.Nutri. 73, 441–9. doi: 10.1038/s41430-018-0382-9
Saeedi, F., Farkhondeh, T., Roshanravan, B., Amirabadizadeh, A., Ashrafizadeh, M., Samarghandian, S., et al. (2022). Curcumin and blood lipid levels: an updated systematic review and meta-analysis of randomised clinical trials. Arch. Physiol Biochem. 128, 1493–1502. doi: 10.1080/13813455.2020.1779309
Sahebkar, A., Saboni, N., Pirro, M., and Banach, M. (2017). Curcumin:An effective adjunct in patients with statin-associated muscle symptoms?. J. Cachexia Sarcopenia Muscle. 8, 19–24. doi: 10.1002/jcsm.12140
Sahebkar, A. A. (2014). systematic review and meta-analysis of randomized controlled trials investigating the effects of curcumin on blood lipid levels. Clin. Nutr. 33, 406–414. doi: 10.1016/j.clnu.2013.09.012
Sakuma, S., Sumida, M., Endoh, Y., Kurita, A., Yamaguchi, A., Watanabe, T., et al. (2017). Curcumin inhibits adipogenesis induced by benzyl butyl phthalate in 3T3-L1 cells. Toxicol. Appl. Pharmacol. 329, 158–164. doi: 10.1016/j.taap.2017.05.036
Shafabakhsh, R., Asemi, Z., Reiner, Z., Soleimani, A., Aghadavod, E., Bahmani, F., et al. (2020). The effects of nano-curcumin on metabolic status in patients with diabetes on hemodialysis, a randomized, double blind, placebo-controlled trial. Iran. J. Kidney Dis. 14, 290–299.
Shehzad, A., Ha, T., Subhan, F., and Lee, Y. S. (2011). New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 50, 151–161. doi: 10.1007/s00394-011-0188-1
Sohaei, S., Amani, R., Tarrahi, M. J., and Ghasemi-Tehrani, H. (2019). The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Complement. Therap. Med. 47, 102201. doi: 10.1016/j.ctim.2019.102201
Sterne, J. A., Gavaghan, D., and Egger, M. (2000). Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 53, 1119–1129. doi: 10.1016/s0895-4356(00)00242-0
Sun, Z., Wei, X., Bai, J., Li, W., Yang, J., Deng, Z., et al. (2022). The effects of curcumin on anthropometric and cardiometabolic parameters of patients with metabolic related diseases: a systematic review and dose-effect meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 1-17. doi: 10.1080/10408398.2022.2067826
Urasaki, Y., and Le, T. T. (2022). Functional complementation of anti-adipogenic phytonutrients for obesity prevention and management. Nutrients 14, 4325. doi: 10.3390/nu14204325
Vanaie, A., Shahidi, S., Iraj, B., Siadat, Z. D., Kabirzade, M., Shakiba, F., et al. (2019). Curcumin as a major active component of turmeric attenuates proteinuria in patients with overt diabetic nephropathy. J. Isfahan Univ. Med Sci. 24. doi: 10.4103/jrms.JRMS_1055_18
Wu, L. Y., Chen, C. W., Chen, L. K., Chou, H. Y., Chang, C. L., Juan, C. C., et al. (2019). Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients 11, 2307. doi: 10.3390/nu11102307
Yang, Y. S., Su, Y. F., Yang, H. W., Lee, Y. H., Chou, J. I., Ueng, K. C., et al. (2014). Lipid-lowering effects of curcumin in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Phytother. Res. 28, 1770–1777. doi: 10.1002/ptr.5197
Yuan, F., Dong, H., Gong, J., et al. (2019). A systematic review and meta-analysis of randomized controlled trials on the effects of turmeric and curcuminoids on blood lipids in adults with metabolic diseases. Adv. Nutr. 10, 791–802. doi: 10.1093/advances/nmz021
Zhang, Z. (2016). Multivariable fractional polynomial method for regression model. Ann. Trans.l Med. 4, 174. doi: 10.21037/atm.2016.05.01
Keywords: blood lipid, curcumin, dose-response analysis, metabolic related diseases, meta-analysis
Citation: Deng Z, Yang K, Cai X, Mei H, Xiao H and Gao W (2023) The beneficial effects of curcumin supplementation on blood lipid levels among patients with metabolic related diseases in Asia area: a systematic review and meta-analysis of randomized controlled trials. Front. Sustain. Food Syst. 7:1167913. doi: 10.3389/fsufs.2023.1167913
Received: 17 February 2023; Accepted: 02 May 2023;
Published: 02 June 2023.
Edited by:
Smail Aazza, Sidi Mohamed Ben Abdellah University, MoroccoReviewed by:
Fatemeh Naeini, Tehran University of Medical Sciences, IranBaskaran N., Indian Institute of Food Processing Technology, India
Copyright © 2023 Deng, Yang, Cai, Mei, Xiao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenqi Gao, Z3dxMTEwM0BjdGd1LmVkdS5jbg==; Han Xiao, dGp4aWFvaGFuQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work
 Zhifang Deng1†
Zhifang Deng1† Wenqi Gao
Wenqi Gao