- 1Laboratory for Agricultural Productions Improvement, Biotechnology and Environment, Faculty of Sciences, University Mohammed First, Oujda, Morocco
- 2Laboratory of Sustainable Agriculture Management, Higher School of Technology Sidi Bennour, University Chouaib Doukkali, El Jadida, Morocco
- 3Higher School of Education and Training, Mohammed I University, Oujda, Morocco
- 4Agro-Food Technology and Quality Laboratory, Regional Center of Agricultural Research of Oujda National Institute of Agricultural Research, Rabat, Morocco
- 5Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 6Laboratory of Agro-Industrial Chemistry (LCA), University of Toulouse, National Institute for Agricultural Research, Toulouse, France
- 7Royal Institute of Livestock Fouarat, Kenitra, Morocco
Introduction: Enriching egg yolks with n-3 polyunsaturated fatty acids (n-3 PUFAs) enhances their nutritional value. While phytobiotics like hemp seed, turmeric, and black pepper show potential for this purpose, their optimal dietary inclusion levels in laying hens remain insufficiently studied.
Methods: This study employed a Box-Behnken Design (BBD) to evaluate the effects of dietary supplementation with hemp seed, turmeric, and black pepper on the enrichment of egg yolks in laying hens. A total of 570 hens were divided into 19 treatment groups, 18 according to the BBD and one control group. The primary responses measured included the contents of saturated fatty acids (SFAs), n-3 PUFAs, n-6 PUFAs, the n-6/n-3 ratio, cholesterol, and total tocopherols in egg yolks.
Results: All models showed statistically significant results (p < 0.05), with coefficients of correlation (R2) ranging from 0.80 to 0.90. Response surface analysis and Pareto charts indicated that dietary hemp seed and black pepper significantly influenced all measured parameters, while turmeric primarily affected cholesterol levels in combination with hemp seed. Ridge optimization analysis identified optimal outcomes at 27.05% SFAs, 5.86% n-3 PUFAs, a 6.04 n-6/n-3 ratio, 846.55 μg/g tocopherols, and 7.02 mg/g cholesterol. The best combination was determined to be 30% hemp seed, 3% turmeric, and 0.3% black pepper.
Conclusion: This study demonstrates that BBD and response surface methodology are effective tools for optimizing functional feed ingredient levels. The results strongly encourage the development of enriched eggs with improved quality and nutritional properties, contributing to a sustainable and healthier poultry product.
1 Introduction
A balanced diet is essential for maintaining good health. Among the various food products that provide the body with essential daily nutrients, eggs play an important role as a rich and balanced diet of fats, essential amino acids, vitamins and minerals (Rbah et al., 2024).
As consumer demand for healthier products becomes more and more prevalent, eggs are increasingly being studied to reduce their composition in terms of cholesterol and saturated fatty acids, which could be responsible for coronary heart disease (Chen and Liu, 2020). Since one of the most common methods of improving egg quality is to manipulate the diet of laying hens, there is great promise in modifying both the quality of egg yolks and their nutritional characteristics.
As unsaturated fatty acids (UFA) are important in terms of diet and health, researches are directed to improve the proportion of UFA in egg yolk by the inclusion of a source (animal or vegetable) of polyunsaturated fatty acids (PUFAs) such as fish oil, seaweed, rapeseed, sunflower, soybean, flaxseed and hemp seed (Omri et al., 2019). Plant sources of PUFAs are known to be rich in α-linolenic acid (ALA; C18: 3n3), which are subsequently metabolized into a long chain PUFAs, which are mainly eicosapentaenoic acid (C20: 5n3), docosapentaenoic acid (C22: 5n3) and docosahexaenoic acid (C22: 6n3), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), recognized for its potential health-promoting benefits for prevention of coronary heart disease in addition to inflammatory processes.
Over the past decade, due to various factors (war, COVID-19, drought), oilseed prices have risen. Consequently, selecting an oilseed source rich in PUFAs for feeding hens presents a cost challenge. In 2021, the Moroccan government officially adopted Law No. 13-21, legalizing the cultivation of Cannabis and its use for medical and agro-industrial purposes. This legislation opened avenues of research on the incorporation of hemp seeds, in the formulation of animal and human food.
Cannabis sativa seeds, indigenous to the Rif region of Morocco, have been chosen for trials aimed at producing PUFAs-enriched eggs (particularly omega-3), at an affordable cost for the local population. Studies conducted previously in our laboratory have shown that these seeds contain around 33% oil with a high ALA content and γ-tocopherol (Taaifi et al., 2021; Allay et al., 2024b,a). However, are very highly oxidized. Therefore, the incorporation of PUFAs into the diet of laying hens, through the addition of non-industrial hemp seeds, will increase the susceptibility of eggs lipids to oxidation. To protect fatty acids from oxidation and consequently improve the shelf-life of eggs, natural antioxidant ingredients can be incorporated into laying hen diets, several additives are used successfully in poultry production, especially turmeric, ginger and black pepper.
Turmeric (Curcuma longa L.) and black pepper (Piper nigrum L.) are natural feed additives that offer complementary benefits in poultry (Abou-Elkhair et al., 2014). Turmeric, rich in curcumin, promotes growth, liver health, yolk color, and antioxidant activity, while reducing cholesterol and lipid peroxidation (da Silva et al., 2018). Black pepper offers antioxidant, antimicrobial, and anti-inflammatory properties (Ashokkumar et al., 2021), with piperine boosting curcumin absorption by 2,000% (Shoba et al., 1998). When used together, they improve meat quality, fatty acid profiles, and gut health more effectively than individually (Ashayerizadeh et al., 2023). Studies also show that supplements like hempseed, turmeric, or ginger can enhance egg yolk fatty acid profiles, including increased ω-3 and ω-6 levels. A study on the combination of coriander, turmeric, and black pepper in broiler diets found improvements in weight gain, feed efficiency, serum protein, and immune function, along with reductions in glucose and triglyceride levels (Abou-Elkhair et al., 2014).
The Box-Behnken design (BBD), an alternative response surface design; helps minimize both the number of feed treatments and the number of animals involved in the study (De Leon et al., 2010). In this study, the BBD was used to estimate and optimize the rate of incorporation of hemp seed, with turmeric and black pepper in the diet of laying hens. By exploring the use of plant-based functional food additives to improve the nutritional value of eggs in a sustainable poultry production system, this study makes a significant contribution to the Sustainable Development Goals (SDGs), notably SDG 2 (Zero Hunger), SDG 3 (Good Health and WellBeing), and SDG 12 (Responsible Consumption and Production) (Naim et al., 2024).
As far as we know the optimization of dietary supplementation with hemp seed, turmeric and black pepper on the fatty acid composition, tocopherols, and cholesterol levels in the eggs yolk of laying hens by response surface methodology (RSM), has never been used in laying hens' diet. Therefore, the aim of this study is to investigate the combined effects of these three phytobiotic ingredients inclusion in the laying hen's diet, on the egg's yolk fatty acid composition, cholesterol and tocopherols levels.
2 Materials and methods
2.1 Ethic statement
This study was approved by the Royal Institute for Livestock Research in Kenitra, Morocco, ensuring its compliance with ethical guidelines and animal research regulations (Directive 2010/63/EU). It also complies with European Commission Council Directive 2007/43/EC, which defines minimum welfare standards for laying hens. The study was reported in accordance with ARRIVE guidelines (Percie du Sert et al., 2020), and performed with recommended housing, feeding and animal management behaviors to reduce stress and discomfort during the research.
2.2. Experimental design
A Box-Behnken factorial design was used in this study, employing three factors at three different levels, using the JMP Pro 17 software (SAS Institute Inc., USA), over 18 experimental runs, to investigate the effects of dietary hemp seed (Cannabis sativa) with turmeric (Curcuma longa) and black pepper (Piper nigrum) on fatty acids, health lipid indexes, cholesterol, carotenoids, tocopherols, and color indices in the egg yolk of 245-day-old Lohmann brown laying hens. In accordance with Box et al. (1978), this factorial design featured twelve factorial points with six central points, with five replicates for each run. The BBD may be seen as having three interlocking 22 factorial plans plus one central point. A formula used to calculate the total number of experiments (N = 18) to be run in the BBD is given by this equation: (N = 2x[x1]+Cp). Herein, x is the number of factors while Cp is the number of central point's such that x = 3 and Cp = 6, (Ferreira et al., 2007). In general, the center points are repeated 3–6 times to guarantee both the reliability of the model estimate, and to serves as reference points within the experimental field (Ghanaatparast-Rashti et al., 2018). The experimental factors, along with their corresponding low, medium, and high levels, are listed in Table 1, and the arrangement of the treatments is listed in all results tables. The levels for hemp seed were selected based on a previous study conducted in our laboratory (Taaifi et al., 2023b), which involved feeding hens with non-industrial hemp seed. The levels for turmeric and black pepper were chosen according to existing literature.
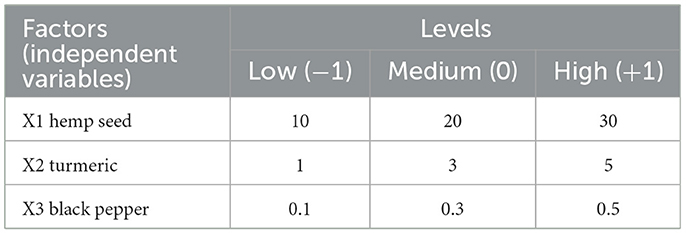
Table 1. Experimental factors and levels used in Box-Behnken design for optimization of the, hemp seed turmeric and black pepper inclusion in the diet for laying hens.
In order to relate the responses to the independent variables, a second-degree polynomial was used based on the equation below:
Where β0 is the constant, β1 and β2 are the linear coefficients, β12 is the interaction coefficient, and β11 and β22 are the quadratic coefficients.
2.3. Animals and housing
Five hundred and seventy Lohmann Brown laying hens (35 weeks old) were used for this study. The hens were weighed and randomly divided into 19 treatments, including a control group that received no hemp seed, turmeric, or black pepper supplementation. The laying hens were raised in an animal house equipped with an automatic drop belt system and enriched pens that comply with Council Directive 1999/74/EC on animal health and welfare for laying hens (European Council Directive, 1999). Each enriched pen provided 750 cm2 of space per bird, as required by EU standards. The pens were equipped with nesting areas, perches, and a litter zone, allowing the birds to express natural behaviors such as scratching, laying, and perching. Stocking densities, along with access to feed and water, adhered strictly to regulatory requirements. Feed and water were supplied ad libitum. Environmental conditions were maintained within a regulated range of 18–22°C temperature, 55–60% humidity, and a light schedule of 16 h of light and 8 h of darkness. After an adaptation period of 10 days, the feeding trial lasted for 42 days. No mortality was observed during the experimental period. The experiment was conducted at the poultry facility of the Royal Livestock Research Institute in Kenitra, Morocco.
2.4. Diet formulation and analysis
The composition of the feed and ingredients are shown in Table 2, concerning the composition of the hemp seeds used in this study has already been reported in our previous work (Rbah et al., 2024). The formulated diets were isocaloric and isonitrogenic, adapted to meet the nutritional requirements of laying hens (National Research Council, 1994). The incorporation levels tested were as follows: hemp seed (HS) at 10%, 20%, and 30%; turmeric (Tr) at 1% (Tr-1), 3% (Tr-3), and 5% (Tr-5); and black pepper (BP) at 0.1% (BP-0.1), 0.3% (BP-0.3), and 0.5% (BP-0.5).
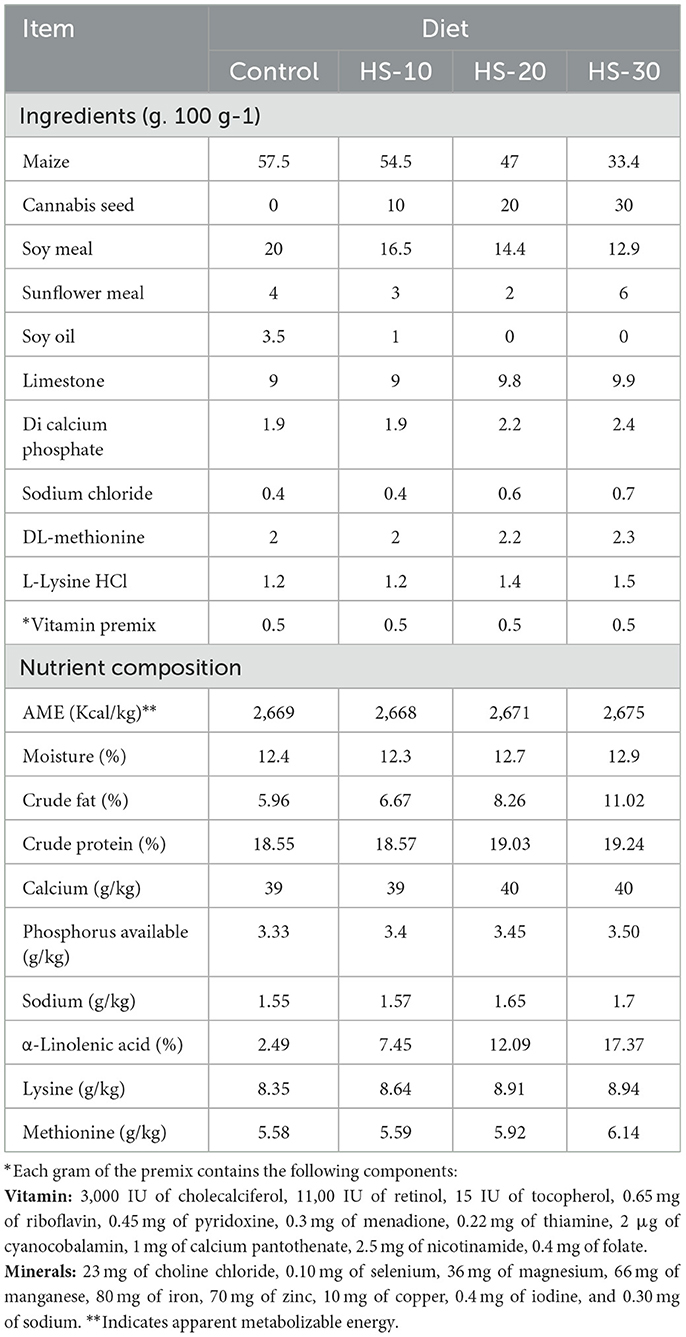
Table 2. Formulation, proximate chemical composition and nutrient profile of laying hen's diets containing hempseed (HS) compared with the control.
A total of 19 dietary treatments (T1–T19) were tested. Treatments T1–T18 included combinations of HS, Tr, and BP, while T19 served as the control diet with no added phytobiotics. All diets were formulated using a mixture primarily composed of soy and corn. The specific compositions for each treatment group are as follows: T1 (10% HS, 5% Tr, 0.1% BP), T2 (10% HS, 1% Tr, 0.3% BP), T3 (10% HS, 3% Tr, 0.1% BP, T4 (10% HS, 5% Tr, 0.3% BP, T5 (10% HS, 3% Tr, 0.5% BP), T6 (20% HS, 1% Tr, 0.5% BP), T7 (20% HS, 1% Tr, 0.1% BP), T8 (20% HS, 5% Tr, 0.5% BP), T9–T14 (20% HS, 3% Tr, 0.3% BP; replicated to ensure statistical robustness), T15 (30% HS, 5% Tr, 0.3% BP), T16 (30% HS, 3% Tr, 0.1% BP), T17 (30% HS, 1% Tr, 0.3% BP), T18 (30% HS, 3% Tr, 0.5% BP), T19 (Control; 0% HS, 0% Tr, 0% BP). The formulated diets were examined for various nutritional constituents including moisture, crude fiber, crude protein, calcium, total and available phosphorus, and lipid content, according to Horwitz (2002).
2.5. Egg yolk analysis
Eggs were collected daily during the last week of the feeding experiment from each replicate of the feeding groups and stored at 4°C until analysis. Various parameters such as color index (L*, a*, b*, Chromaticity, and Hue angle), fatty acids profile, total tocopherols, carotenoids and cholesterol levels, were analyzed in triplicate.
2.5.1 Egg yolk color index
Egg yolk color was measured using the MINOLTA CR410 colorimeter (Konica Minolta CR 410 Sensing, Inc., Osaka, Japan), basing on the CIE Lab color system, which includes parameters for lightness index (L*), redness index (a*) and yellowness index (b*). Each sample was measured in triplicate.
Chromaticity (C*) was calculated according to Belhaj et al. (2021):
Hue angle (H*) was determined according to Taaifi et al. (2023a):
2.5.2 Lipid extraction and fatty acid analysis
The extraction of total lipids from egg yolk and laying hen feed samples was conducted using the Bligh dyer method (Bligh and Dyer, 1959). The samples were homogenized in a chloroform/methanol solution (2:1, v/v), then after being centrifuged, the organic phase was separated, evaporated under vacuum and the fat extracts obtained were stored at −20°C for further analysis.
The transformation of fatty acids to fatty acids methyl esters was carried out according to the AOCS “Ce 1k-09” method (Aocs Official Method, 2007), and the fatty acids analysis realized by gas chromatography coupled with a FID detector (GC-6890, Agilent Technologies) using a BPX70 capillary column (length 60 m, internal diameter 0.32 mm and film thickness 0.25 μm, SGE Europe), with 1 μL of sample was injected in the splitless mode. Helium gas was used as the carrier gas at a flow rate of 1 ml/min. the temperature of the oven started at 50°C and was gradually raised at a rate of 30°C per minute until it reached 170°C. It was then raised at 4°C/min until it was 220°C, where it was kept for 10 min. For the identification of fatty acids, a mixture of FAs standards including 37 FAME (Sigma-Aldrich, Supelco, Bellefonte PA, USA) was used, and the results were expressed as percentages.
2.5.3 Estimation of health-related lipid quality indexes
The fatty acid profile of egg yolk is used to calculate health lipid indexes, including Thrombogenic Index (TI), Atherogenic Index (AI), Hypocholesterolemic/hypercholesterolemic ratio (h/H), the LA/ALA and PUFAs/SFAs ratio. Specific formulas for these indices are described in our previous publication (Rbah et al., 2024).
2.5.4 Tocopherols analysis
Egg yolk tocopherols were analyzed using a Shimadzu HPLC equipped with DAD detection technology. The protocol began by dissolving the samples in hexane (w/w) before separating on a silica Uptisphere NH2 column (length 250 mm, internal diameter 4.6 mm with particle size 5 μm), according to Mansouri et al. (2023). A hexane/isopropanol mixture (99:1, v/v) served as the mobile phase, maintaining an isocratic condition throughout the analysis. The rate of flow is set to 1 ml/min, and compounds were separated at wavelengths of 292, 296 and 298 nm using a UV detector. Quantification was based on external calibration with a standard tocopherol (Sigma-Aldrich, St. Louis, USA).
2.5.5 Cholesterol and carotenoids content in egg yolk
Cholesterol levels in egg yolk were determined using the Liebermann-Burchard (LB) method, as described in our previous work (Rbah et al., 2024); This method involves diluting 0.1 g of fat in chloroform, adding LB reagent to aliquots, and measuring absorbance at 640 nm after a 15-min incubation. A cholesterol standard is also used for calibration.
Carotenoid content was determined by the modified AOAC spectrophotometric method (Latimer, 2007). 2 g of egg yolk was solubilized in 7 ml of acetone for 3 times (sequential extraction), next the mixture was centrifuged at 6,000 rpm for 2 min. Then after filtration through Whatman paper, absorbance was taken at 450 nm with an UV-Visible spectrophotometer (Rayleigh UV-1800), and finally the results were expressed as β-carotene using a calibration curve.
2.6. Statistical analysis
ANOVA was used to analyze the surface responses of the results whether Tukey's test and they were illustrated via a standardized Pareto chart. We then compare the experimental and predicted values of the responses in order to assess the model's validity with regard to a significance level at (p < 0.05).
To compare the means of the results obtained with the control group diet and the predicted results with the optimal combination diet, statistical analysis was used using IBM SPSS software (version 27; IBM Corp., Chicago, IL, USA), applying the student's t-test, and significance was established at the p < 0.05 level.
3 Results and discussion
In this study, we provided laying hens with a diet primarily enriched with hemp seeds, turmeric, and black pepper to assess the impact on omega-3 fatty acid enrichment in eggs. This research is motivated by the fact that table eggs are an inexpensive staple of our daily diet. Furthermore, it is well established that the fatty acid profile of eggs is influenced by the diet, digestive system, and biosynthetic process of laying hens (Popiela et al., 2013; Tang et al., 2015). Thus, PUFAs-enriched diets, including n-3 PUFA-enriched eggs, provide an excellent low-cost approach to increasing human consumption of long-chain n-3 PUFAs such as DHA and EPA. In this regard, many studies have demonstrated successful changes in the fatty acid profile of eggs through modifications in the diet of laying hens (Bruneel et al., 2013; Taaifi et al., 2023b; Sim and Sunwoo, 2002).
Table 3 shows the significant responses from the eighteen experiments used to optimize dietary supplementation of hemp seed, turmeric and black pepper for laying hens, in particular responses concerning fatty acids content [SFAs (Y1), n3 PUFAs (Y2), n6 PUFAs (Y3), and n6 PUFAs/n3 PUFAs (Y4)], total-tocopherols (Y5), and cholesterol levels (Y6), are listed in Table 3. To predict the response models based on the intake rates of hemp seed (X1), turmeric (X2), and black pepper (X3), we utilize the second-order polynomial equations presented in Table 3.
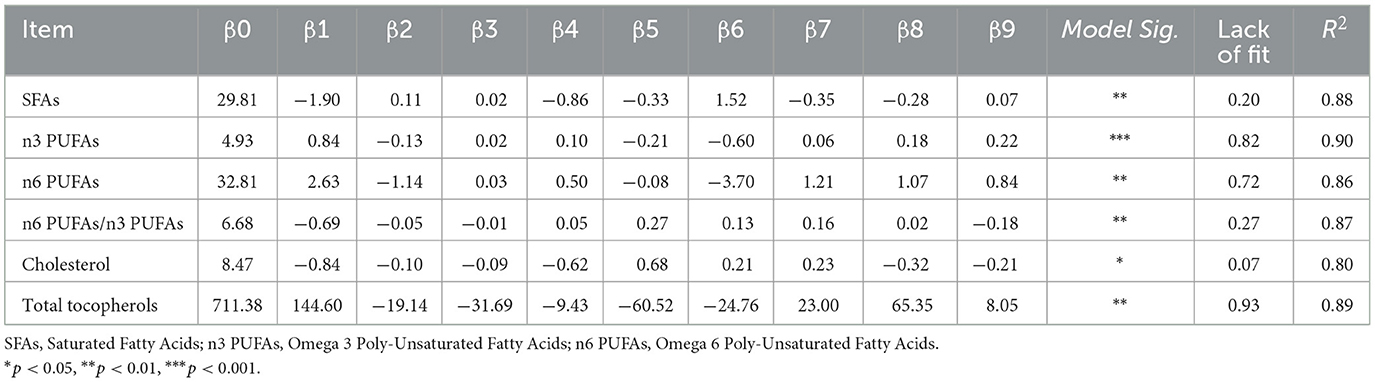
Table 3. Model fitting and regression equation coefficients for Saturated fatty acids (SFAs), Polyunsaturated fatty acids omega-3 (n3 PUFAs), Polyunsaturated fatty acids omega-6 (n6 PUFAs), n6 PUFAs/n3 PUFAs ratio, total tocopherols and cholesterol levels in egg yolk.
The ANOVA results (Table 3) indicated that all six prediction models were statistically significant (p < 0.05), and demonstrated a strong correlation coefficient (R2). Ranging from 0.80 to 0.90. This ensures that the regression equations are consistent with the experimental data. Based on these correlation coefficients, regression models can explain 88%, 90%, 90%, 87%, 86%, and 80% of the response variation in SFAs, n3 PUFAs, n6 PUFAs, n6/n3 ratio, total-tocopherols and cholesterol level, respectively. In addition, the lack-of-fit test was found to be non-significant (p > 0.05), confirming the models' validity.
The Pareto charts (Figures 1, 2) serve as a visual tool to illustrate the varied impacts of supplementing with hemp seed, turmeric, and black pepper on the studied responses. It encompasses linear interaction, and quadratic effects in a standardized format. A bar, whose length proportionally reflects the value of the estimated coefficient, represents each effect.
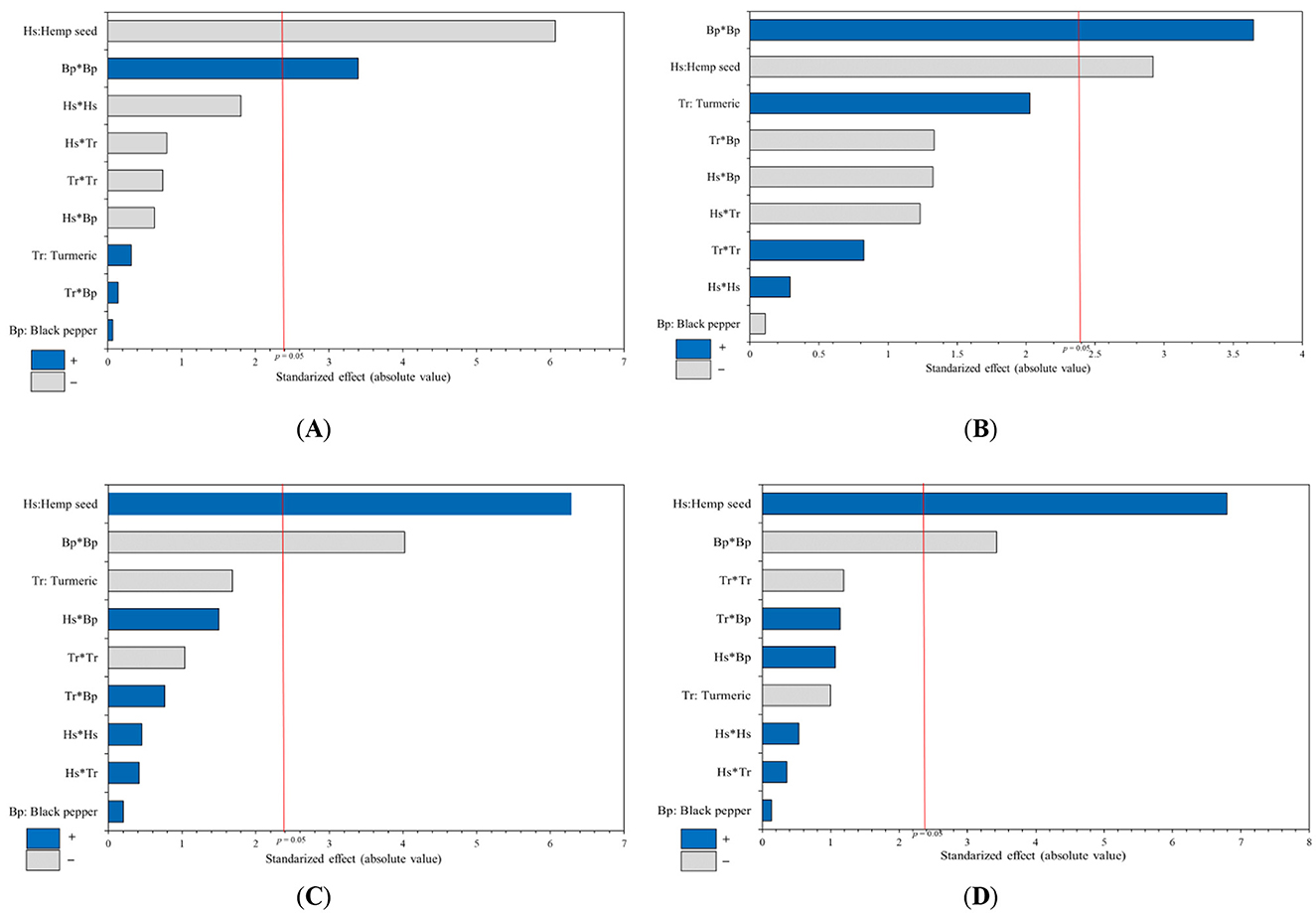
Figure 1. Pareto charts standardized from the optimization study evaluate the impact of dietary supplementation variables for laying hens (hemp seed, turmeric, and black pepper) on the lipid profile of egg yolk. The analyzed parameters include variations in the percentage of: (A) Saturated fatty acids, (B) Mono-Unsaturated fatty acids, (C) α-linolenic acid, and (D) Polyunsaturated fatty acids omega 3 type, in the egg yolk.
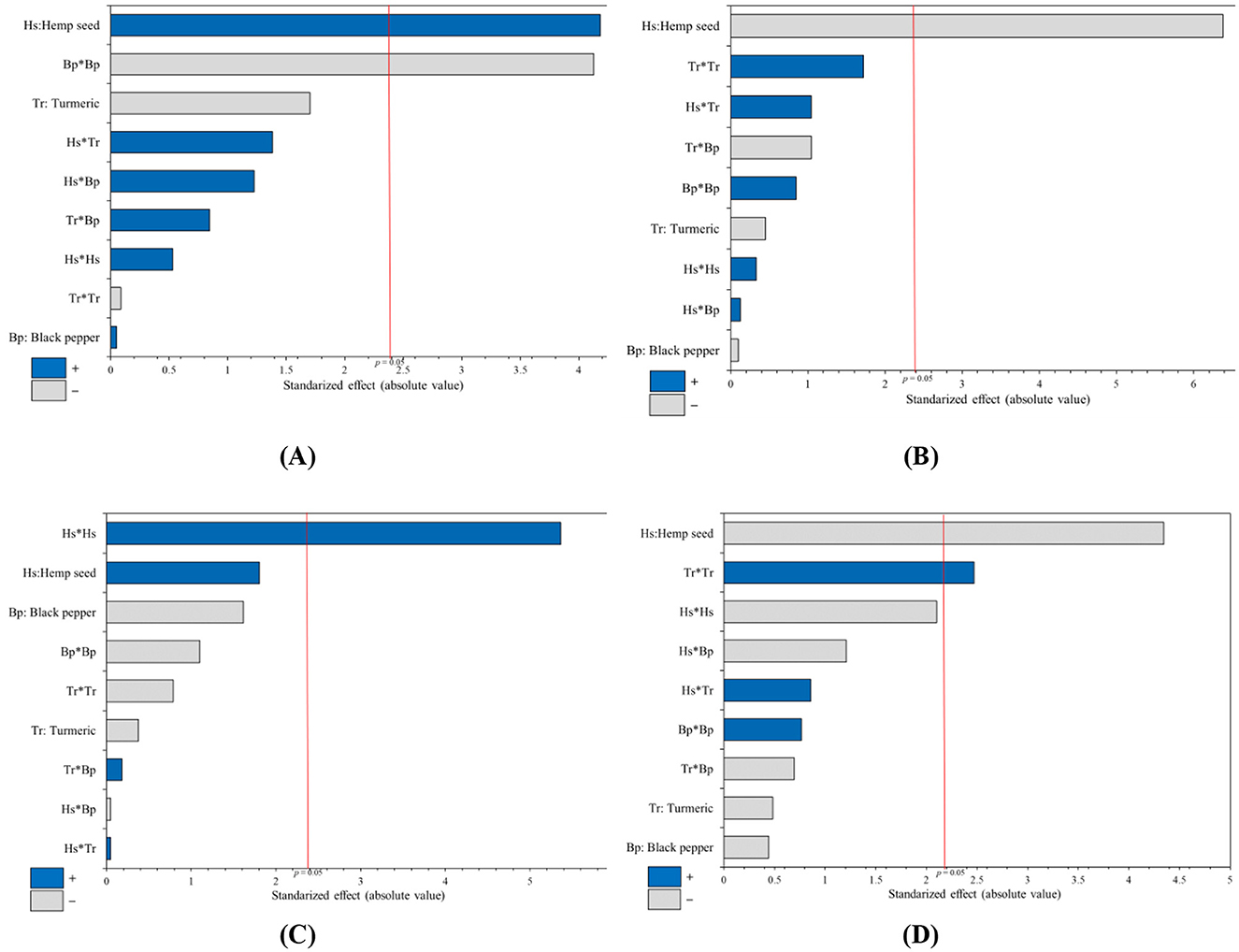
Figure 2. Pareto charts standardized from the optimization study evaluate the impact of dietary supplementation variables for laying hens (hemp seed, turmeric, and black pepper) on the lipid profile of egg yolk. The analyzed parameters include variations in the percentage of (A) Polyunsaturated fatty acids n6, (B) the n6 PUFAs/n3 PUFAs ratio, (C) the levels of tocopherols and (D) the levels of cholesterol in the egg yolk.
3.1 Egg yolk lipid profile and health lipid indexes
3.1.1. Sum of saturated fatty acids
When looking at the sum of SFAs in egg yolk [mainly the myristic (C14:0), palmitic (C16:0), and stearic acids (C18:0)], the Pareto chart shows that the linear effect of hemp seed intake is the variable with most important influence, followed by the quadratic effect of black pepper supplementation; for turmeric, it appears that its inclusion had no significant effect. Hemp seed have a negative impact on the sum of SFAs, leading to a decrease in SFA concentration in egg yolk, while black pepper has a positive influence on SFA content (Figure 1A).
Dietary treatments incorporating hemp seed, turmeric, and black pepper appear to reduce the level of SFAs in the lipid profile of egg yolk. All treatments exhibit a reduction in their levels of SFAs (Table 4) ranging from 31.98% (Traitement-1) to 26.54% (Traitement-17). This significant decrease in SFAs levels is more distinctly showcased when visualized on the 3D-response surface. As shown in Figure 3A, we observed that when hemp seed supplementation is up to 30% and black pepper is added at a rate of 0.3%, the percentage of SFAs becomes around 27% of total fatty acids.
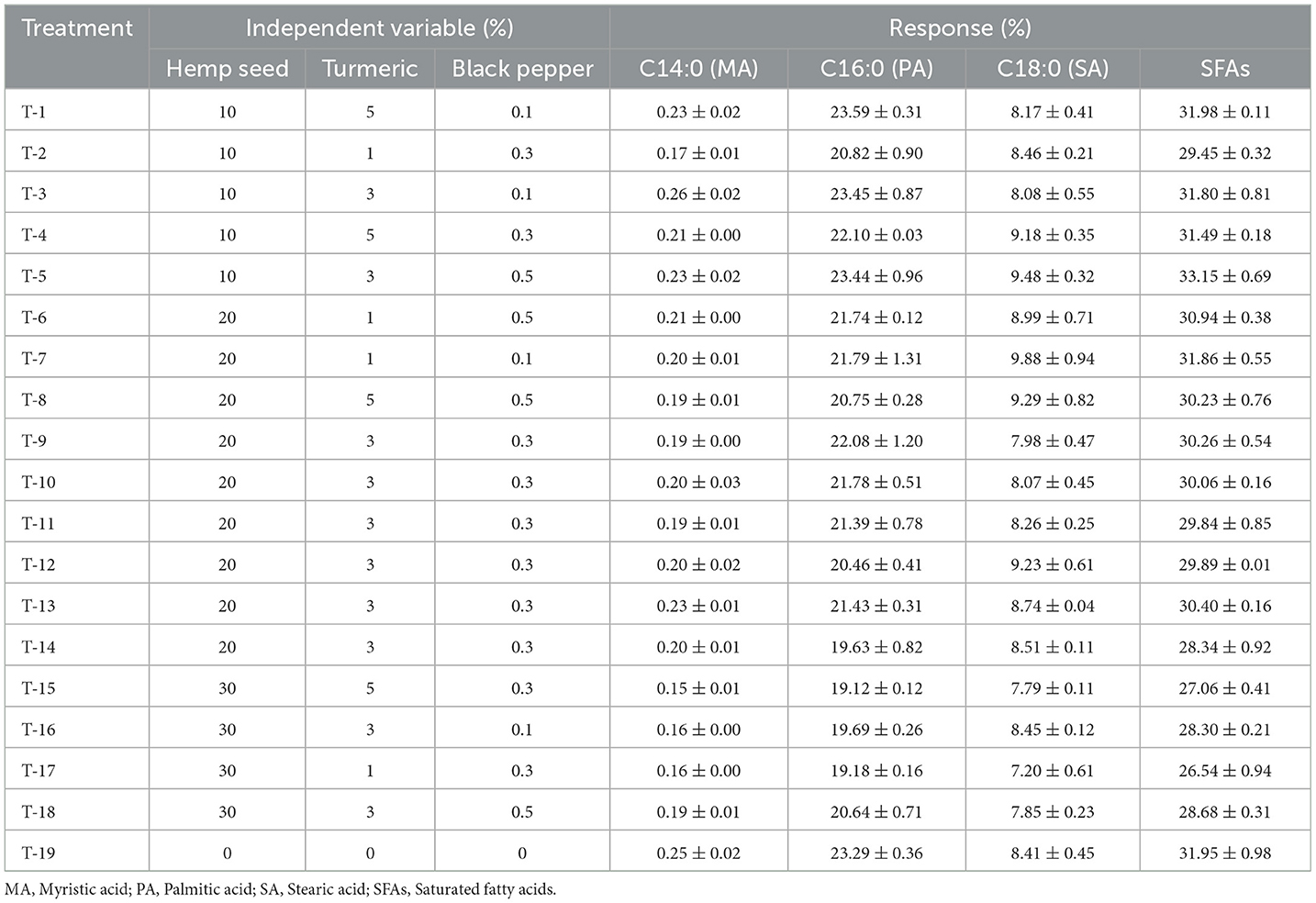
Table 4. The impact of dietary treatments (Hemp seeds, Turmeric, and Black pepper) on the levels of Saturated fatty acids (SFAs %) in the lipid profile of egg yolk.
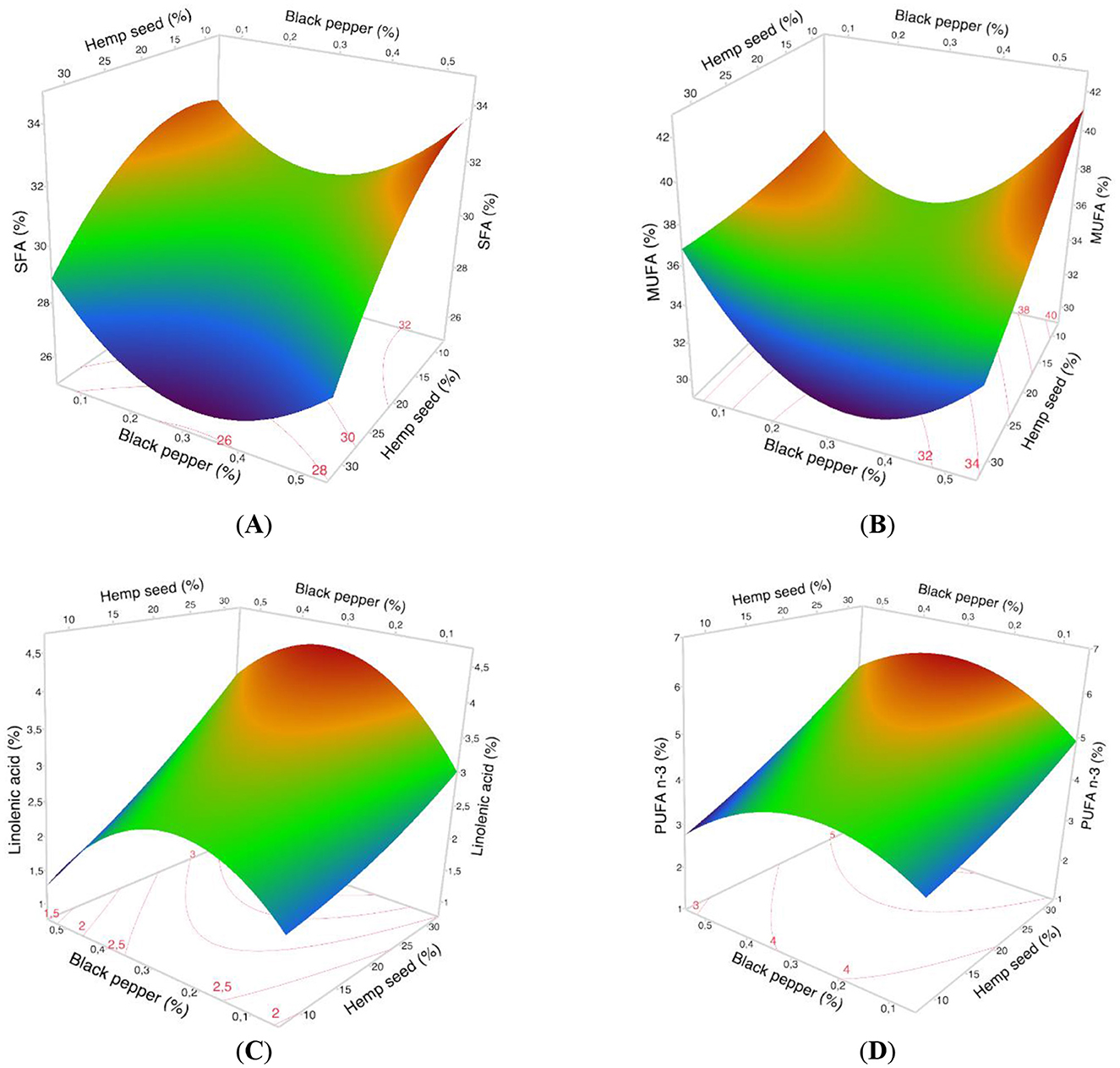
Figure 3. The 3D surface responses from the optimization study of dietary variables (hemp seed, turmeric, and black pepper) in hens' diet for: (A) Saturated fatty acids, (B) Mono-unsaturated fatty acids (C) α-Linoleic Acid, (D) Polyunsaturated fatty acids n 3; in eggs yolk.
This reduction was evident, with levels decreasing from 32% to 27% for regimes at 10% and 30% hemp seed and 0.1% and 0.3% turmeric respectively, mainly attributed to lower concentrations of palmitic acid in the egg yolk. Indeed, as reported by Simopoulos et al. (2000) palmitic acid is particularly sensitive to variations in dietary polyunsaturated fatty acids. In addition, recent studies have demonstrated that hemp seed supplementation helps reduce the SFA fraction in egg yolk (Neijat et al., 2016b; Taaifi et al., 2023b). With regard to the inclusion of black pepper, Ashayerizadeh et al. (2023) incorporated black pepper into the diet of quails and reported a reduction in the proportion of saturated fatty acids in meat. This reduction in SFA levels has potential health benefits, particularly given the negative perception of SFA-rich foods, which is often associated with increased health risks associated with cardiovascular disease (de Souza et al., 2015).
3.1.2. Sum of mono-unsaturated fatty acids
In terms of total Mono-Unsaturated Fatty Acids (MUFAs), primarily comprising oleic and palmitoleic acids, results from the Pareto charts (Figure 1B) indicate that black pepper has a positive quadratic effect by increasing the amount of MUFAs. In contrast, the inclusion of hemp seed in the diet of laying hens has a negative linear effect on MUFAs in egg yolk, while the inclusion of turmeric has no effect on MUFAs percentage. Table 5 illustrates a significant decrease in the level of MUFAs ranging from 39.27% (Treatment-1) to 30.34% (Treatment-14). The 3D-response surface corresponding (Figure 3B) clearly highlights the dramatic decrease in MUFAs in egg yolk with high levels of hemp seed supplementation (30-hemp seed). It also shows an increase in the proportion of MUFAs with the addition of black pepper up to 0.5%.
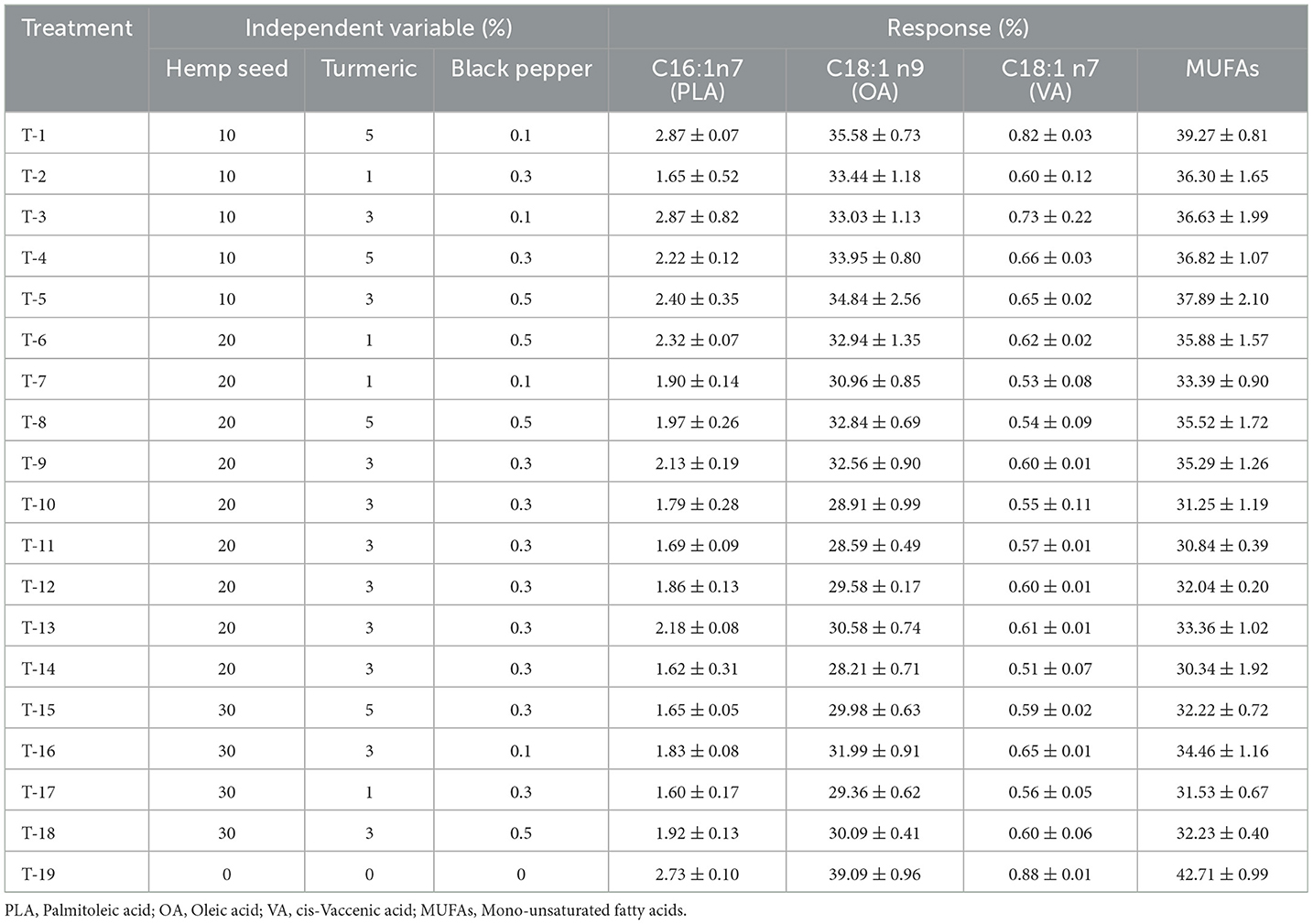
Table 5. Dietary treatments (hemp seed, turmeric and black pepper) and their effects on mono-unsaturated fatty acids (MUFAs) profile (%) of egg yolk.
Typically, modifications in MUFAs are mainly due to changes in oleic acid (C18:1 n9), the most common MUFAs in eggs. Previous research suggesting a depressive impact of PUFA-rich diets on the conversion of palmitic acid (C16:0) to oleic acid (C18:1 n9) by reducing the Δ-9 desaturase activity (Garg et al., 1988; Lee et al., 2019). These findings align with those of Taaifi et al. (2023b), who reported a significant decrease in total MUFAs, particularly the oleic acid, in eggs from hens supplemented with hemp seed. Regarding the inclusion of black pepper, our results align with those reported by Ashayerizadeh et al. (2023), who observed an increase in the percentage of MUFAs in meat with the addition of black pepper.
3.1.3. Sum of poly-unsaturated fatty acids
Polyunsaturated fatty acids (PUFAs) including linoleic acid (LA), γ-linolenic acid (GLA), and arachidonic acid (ARA) are essential n6 PUFAs, while α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are essential n3 PUFAs. These fatty acids are renowned for their anti-inflammatory properties; they are implicated in protecting against various diseases such as cancer, thrombosis, osteoarthritis, and autoimmune disorders. Additionally, they play pivotal roles in diverse cellular activities such as cellular signaling, maintaining cell membrane fluidity, and regulating blood pressure (Minihane and Lovegrove, 2006; Kapoor et al., 2021).
Hens are able to convert the α-LA into very long-chain omega 3 fatty acids (n3 VLCFA), mainly DHA and EPA, and the LA into omega 6 VLCFA, primarily the ARA, through the enzymatic processes of elongation and desaturation, and store them in yolk lipids (Alagawany et al., 2019; Monroig et al., 2022).
Regarding ALA and total n3 PUFAs contents, the results show similar Pareto plots with a significant positive linear effect for hemp seed supplementation, and a negative quadratic effect of black pepper; while the turmeric inclusion has no effect on ALA and total n3 PUFAs percentage (Figures 1C, D). In addition, Table 6 shows that the quantity of ALA and n3 PUFAs increased significantly, from 1.79% (Traitement-1) to 4.23% (Traitement-17) and from 3.28% (Traitement-1) to 5.66% (Traitement-17), respectively. The 3D-response surface showed in Figures 3C, D clearly indicates that supplementation with up to 30% hemp seed and 0.3% black pepper results in the highest levels of both ALA and total n3 PUFAs in egg yolk.
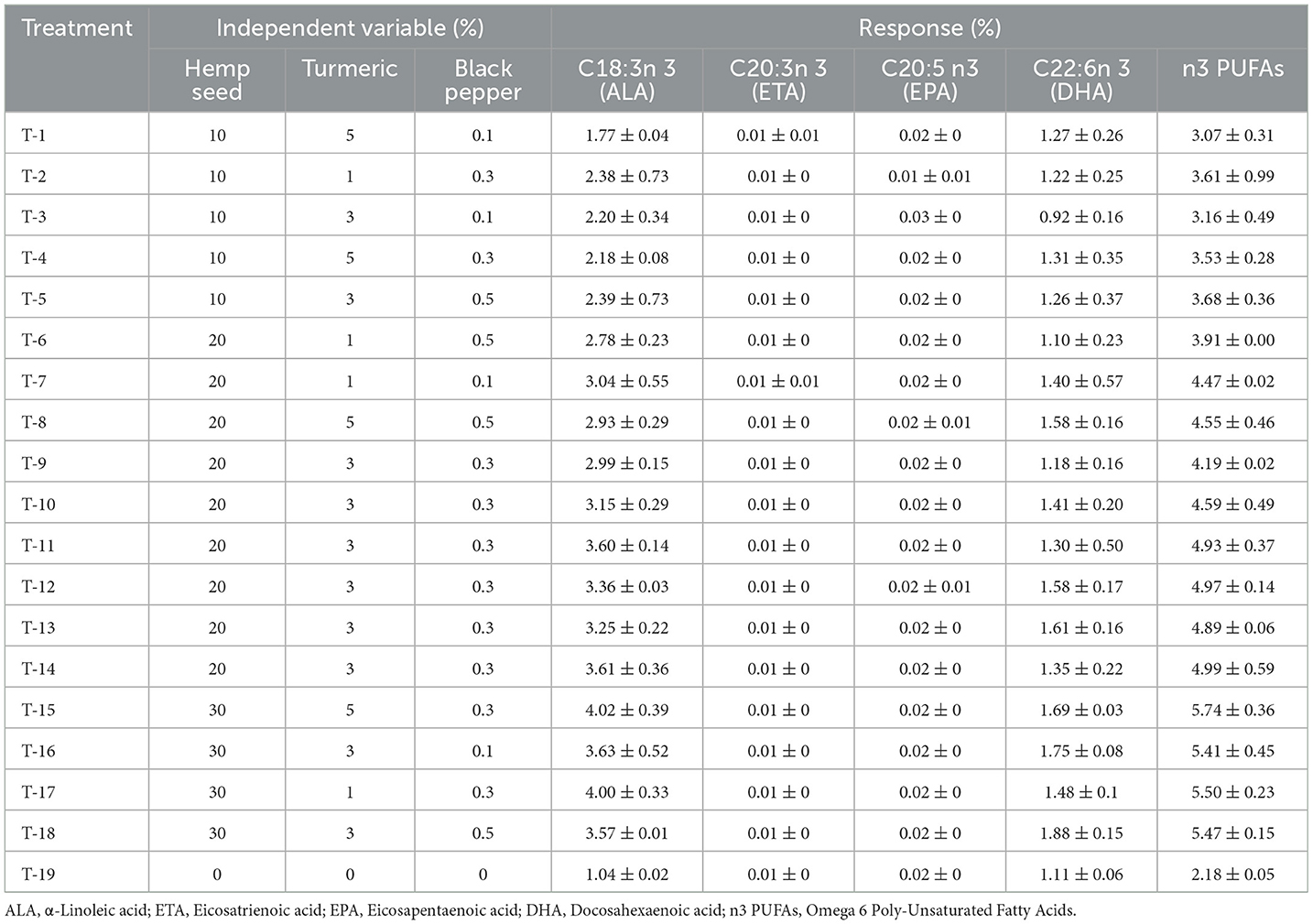
Table 6. Dietary treatments (Hemp seed, Turmeric and Black pepper) and their effects on Poly-Unsaturated Fatty Acids omega 3 type (n3 PUFAs) profile (%) of egg yolk.
Concerning n6 PUFAs, the Pareto chart (Figure 2A) highlights a positive linear effect of hemp seed and a negative quadratic effect of black pepper on the total n6 PUFA content, while there was no effect for turmeric supplementation. Table 7 highlights the change in total n6 PUFAs from 25.16% (Treatment-1) to 36.03% (Treatment-17). The 3D-response surface (Figure 4A) clearly indicates that dietary supplementation with hemp seed and black pepper increases the percentage of n6 PUFAs in egg yolk fatty acids.
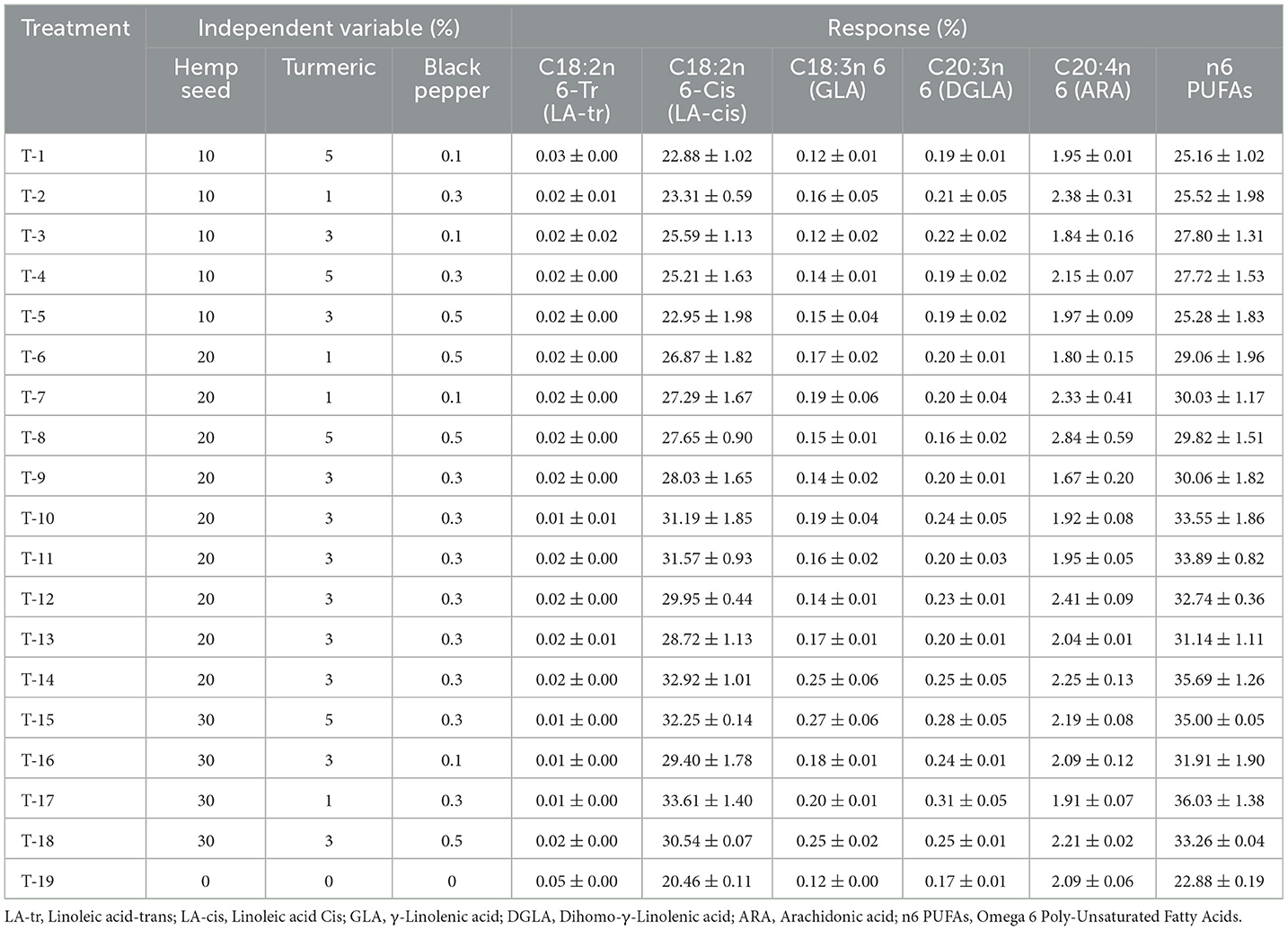
Table 7. Dietary treatments (Hemp seed, Turmeric and Black pepper) and their effects on Poly-Unsaturated Fatty Acids omega 6 type (n6 PUFAs) profile (%) of egg yolk.
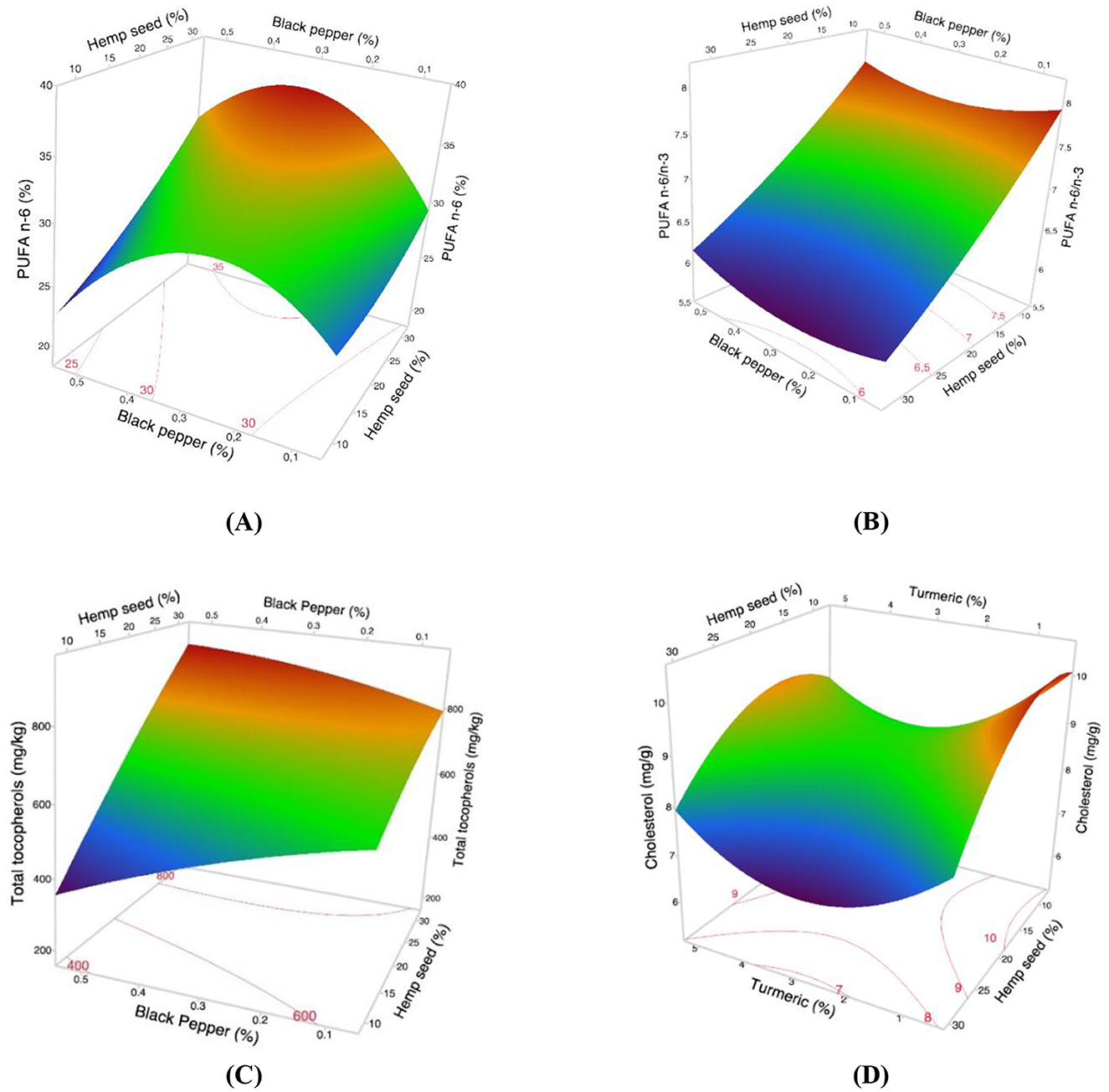
Figure 4. The 3D surface responses from the optimization study of dietary variables (hemp seed, turmeric, and black pepper) in hens' diet for (A) Polyunsaturated fatty acids n6 (B) n6 PUFA/n3 PUFA ratio (C) Total tocopherols levels and (D) Cholesterol levels in eggs yolk.
The augmentation in n3 PUFAs relative to n6 PUFAs in egg yolks leads to a reduction in the n6 PUFAs/n3 PUFAs ratio. This trend is visually depicted in the Pareto diagram and the response surface corresponding to the n6 PUFAs/n3 PUFAs ratio (Figures 2B, 4B), suggesting that only hemp seed supplementation exerts a significant linear negative effect on reducing this ratio in egg yolk. Table 8 provides the results obtained for this ratio, with the lowest value achieved with a 30% hemp seed inclusion (5.96).
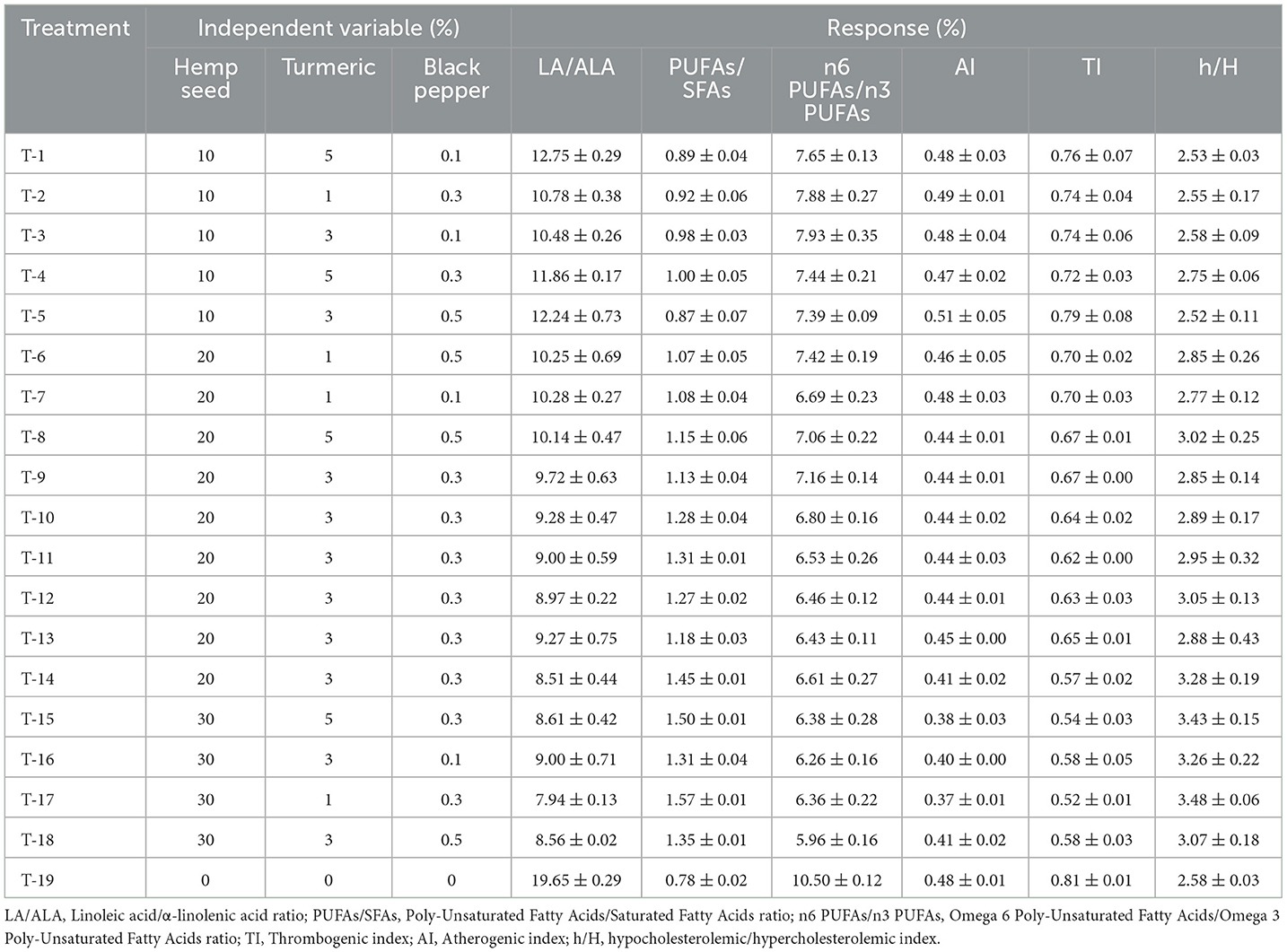
Table 8. Dietary treatments (hemp seed, turmeric and black pepper) and their effects on health-related lipid quality indexes of egg yolk.
The results in Tables 6, 7 clearly illustrate the variations observed in the Pareto chart. ALA-rich diets showed a significant increase (p < 0.01) in total PUFAs content, total n3 PUFAs content (p < 0.01) and total n6 content (p < 0.05) in egg yolk in all experiments. The concentration of the LA shows a significant increase (p < 0.05) in egg yolk with the hemp seed inclusion, with regard to γ-LA and ARA, the results show an in-crease in their concentrations in egg yolk but they are not significant (p > 0.05). The α-LA content shows a significant increase (p < 0.01) with the inclusion of hemp seeds, EPA and DHA depot in yolk were not significantly increased (p > 0.05). This may be due to the limited conversion of ALA and LA to VLC PUFAs in birds (Elkin et al., 2016). Most data indicates that large quantities of VLC PUFA are deposited in the egg yolk When DHA is supplied directly, whether from fish or algae (Neijat et al., 2016a; Elkin and Harvatine, 2023). Conversely, with ALA supplementation, it must undergo biosynthesis in the liver through delta-5 and delta-6 desaturase and elongase enzymes before being exported to deposited in yolk (Rbah et al., 2024). In addition, research into the incorporation of phytogenic feed additives suggests that the addition of black pepper leads to an increase in PUFAs levels. This increase is the result of the reinforcement of the antioxidant defense system, which serves to protect against PUFA oxidation and ultimately promotes the production of stable end products (Mohebodini et al., 2021; Ashayerizadeh et al., 2023).
3.1.4. Health-related lipid quality indexes in egg yolk
Increasing the content of n3 PUFAs in egg yolks led to a significant reduction (p < 0.01) in the n6/n3 ratio. Furthermore, Increasing ALA levels more significantly than LA led to an interesting reduction (p < 0.01) in LA/ALA ratios. Decreasing the n6/n3 and LA/ALA ratios holds significance for human health (Chen and Liu, 2020), as it diminishes the likelihood of chronic diseases like coronary heart disease; obesity and cancer, while enhancing nervous system functionality (Alagawany et al., 2019; Kapoor et al., 2021; Liput et al., 2021).
The PUFAs/SFAs ratio, Hypocholesterolemic/hypercholesterolemic index (h/H), thrombogenic index (TI), and atherogenic index (AI), are established measures used to assess the physiological effects of fats on consumer health. As a general rule, an optimal PUFAs/SFAs ratio >0.45 is desired, while AI and TI values should be < 0.5 and 1, respectively (Chen and Liu, 2020). Highest h/H values are considered to be beneficial to health (Rafieian-Kopaei et al., 2014). Our results, presented in Table 8, revealed a clear increase in PUFAs/SFAs values with increasing hemp seed inclusion in all treatments (p < 0.01) with values above 0.45, the highest value (1.57) was observed in the Treatment-17. With increasing inclusion of hemp seeds, AI and TI values decreased significantly (p < 0.01) below 0.5 and 1 respectively for AI and TI. The lowest values were observed with Treatment-17, reaching 0.369 and 0.521 for AI and TI respectively, this progressive inclusion also led to significantly high values (p < 0.01) in the h/H interval, ranging from 2.52 to 3.47. The highest value was observed with the same dietary treatment (Treatment-17) mentioned above for PUFAs/SFAs ratio, AI and TI indices. Our results for these indices are consistent with findings from researchers who have investigated the impact of hemp seed inclusion on health-related lipid indexes (Taaifi et al., 2023b; Mierlita et al., 2024).
3.1.5. Cholesterol in egg yolk
Consumer concern about the lipid composition of egg yolks, especially their cholesterol content, has long been recognized as an unfavorable nutrient (Myers and Ruxton, 2023). Cholesterol content can be influenced by a variety of factors, including the; hen breed, age, farm management practices and nutritional strategies (Dalle Zotte et al., 2021). As a result, a considerable effort has been made in the field of nutrition to reduce cholesterol levels in egg yolks.
The Pareto chart of cholesterol content (Figure 2C), reveals that supplementation with hemp seed have a significant (p < 0.05) negative linear effect on cholesterol content, whereas turmeric has a significant (p < 0.05) positive quadratic effect. In contrast, black pepper does not significantly (p > 0.05) affect cholesterol levels. The 3D surface response of cholesterol (Figure 4C) indicates that the lowest value for cholesterol was obtained with the diet Treatment-17. This result is consistent with that of Table 9, showing that hemp seed intake significantly reduced (p < 0.01) cholesterol levels in egg yolk with increasing levels of hemp seed and turmeric administered to laying hens, ranging from 10.2 to 7.05 for the Treatment-2 and Treatment-17, respectively. It is likely that this cholesterol-lowering effect stems from the synergistic interaction between several vegetal compounds such as phytosterols, fibers and fatty acids, which appear to influence cholesterol biosynthesis.
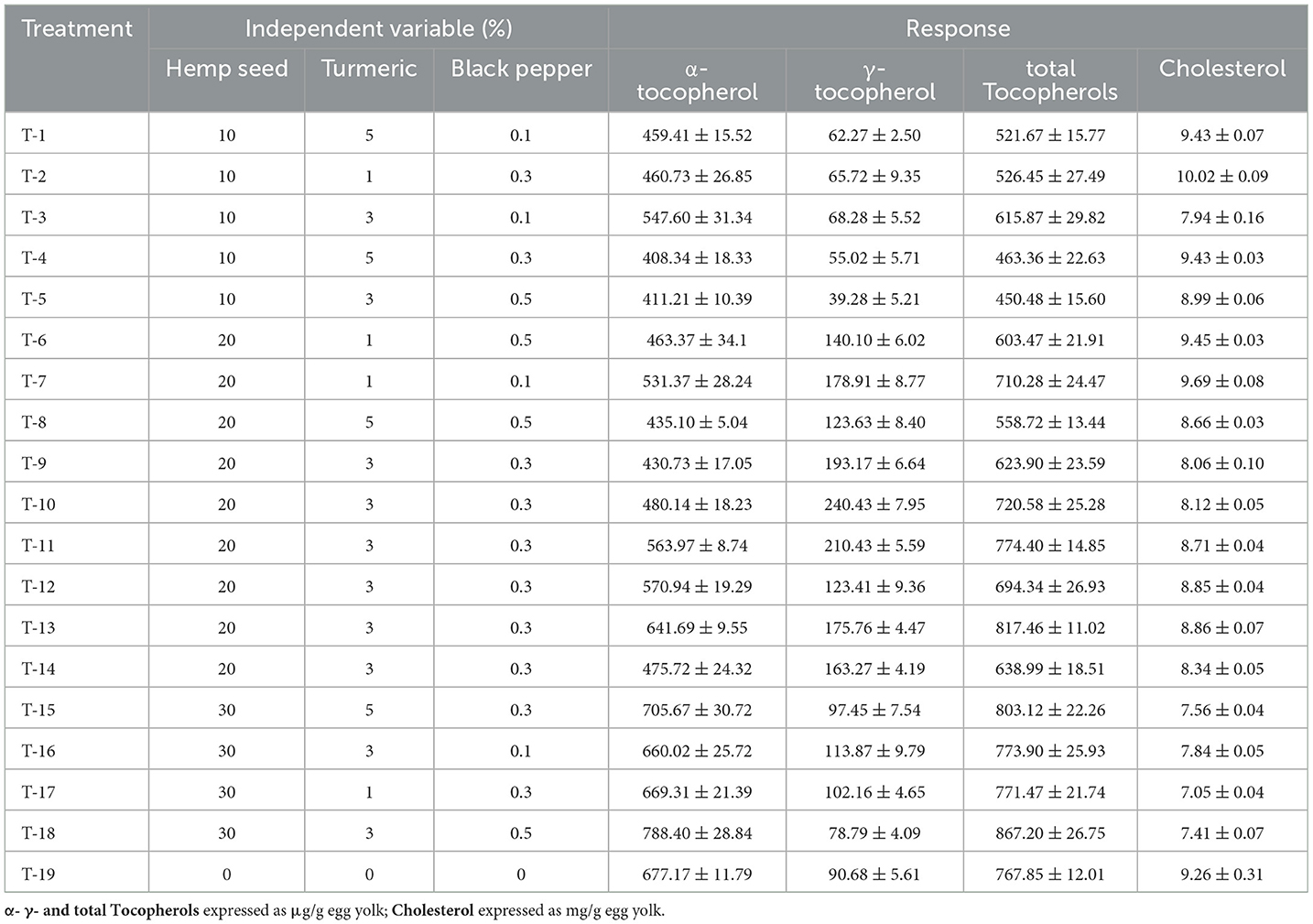
Table 9. Dietary treatments (hemp seed, turmeric and black pepper) and their effects on tocopherols and cholesterol levels in egg yolk.
Phytosterols, present in hemp seed, act to lower hypercholesterolemia by hindering the absorption of cholesterol via both crystallization and co-precipitation, as indicated in the literature (Zhang et al., 2022). Their characteristically lower water-solubility than cholesterol enables them to compete for its place in intestinal micelles, thus lowering its absorption rate (Trautwein et al., 2003; Brufau et al., 2008). In addition, phytosterols can reduce liver biosynthesis of cholesterol by regulating the activity of enzymes like those implicated in the synthesis of terpenes and 3-hydroxy-3-methylglutaryl-coenzyme A reductase, thus limiting cholesterol synthesis (Rbah et al., 2024). Consequently, the addition of hemp seed, rich in phytosterols and unsaturated fatty acids, is likely to reduce the concentration of cholesterol in egg yolk. This hypothesis is corroborated by previous findings (Shahid et al., 2015; Taaifi et al., 2023b). Studies have shown that dietary supplementation with turmeric can lower cholesterol levels, possibly by increasing the expression of cholesterol 7α-hydroxylase, a key enzyme in the formation of bile acids from cholesterol, as suggested by Kim and Kim (2010). Curcumin (the most active substance in turmeric) may also inhibit ATP-citrate lyase, a crucial enzyme in fatty acid synthesis that converts citrate to acetyl-CoA (Xie et al., 2019). Furthermore, it has been suggested that curcumin may also promote cholesterol catabolism and excretion, potentially by binding to cholesterol to facilitate its elimination (Keshavarz, 1976).
3.1.6 Tocopherols in egg yolk
Tocopherols, compounds naturally present in plants, are found in varying quantities in foods, mainly in vegetable oils and certain oilseeds (Shahidi and de Camargo, 2016). Isomers α and γ-tocopherols are the most abundant, due to their high amount in food. However, α-Tocopherol predominates as the “primary” vitamin E form, with the greatest concentration in tissues. Metabolically, α-tocopherol platforms are considered the most active (Szewczyk et al., 2021).
Hemp seeds are well known for their richness in tocopherols (Taaifi et al., 2021) and phenolic compounds (Benkirane et al., 2023). Tocopherols, being lipid-soluble molecules, play a crucial role in preserving membrane integrity. Serving as antioxidants, they shield the body from oxygen toxicity (Munné-Bosch and Alegre, 2002). Furthermore, they safeguard lipids and other membrane constituents by both physically quenching singlet oxygen and chemically reacting with it (Kruk et al., 2005).
The Pareto chart (Figure 2D) indicates that only the dietary variable of hemp seed has a significant positive quadratic effect on tocopherol content. The 3D surface response (Figure 4D) confirms these results, showing a consistent trend of increasing tocopherol levels with the inclusion of more than 30% hemp seed in the diet of laying hens. Table 9 presents total concentrations of tocopherol ranging from 450.48 μg/g egg yolk (Treatment-5) to 867.20 μg/g egg yolk (Treatment-17). This finding is closely aligned with the results reported in many other studies that use hemp seed as feed for laying hens (Skrivan et al., 2019; Taaifi et al., 2023b).
3.1.7. Carotenoids in egg yolk and color indices
Yolk color tops the list in terms of consumer satisfaction, as it is easily visible and greatly affects buyer perception, among other quality traits (Abive-Bortsi et al., 2022). As reported by Saleh et al. (2021), yolk coloration is primarily determined by the hen's dietary intake of carotenoids, rather than intrinsic nutrient density.
Table 10 shows the results of the color indices and carotenoids levels in egg yolk from this study. A significant increase (p < 0.05) in the b* value resulting from hemp seed supplementation was observed in all experiments, with the exception of the experiments with 30-hemp seed inclusion, showing a decrease (p < 0.05) in the b* value and the chromaticity index, and there is no change in the a* value. An increase in the b* value is generally interpreted as a color shift toward yellow. For the hue angle, the results showed a significant increase (p < 0.05) in hue angle values with the inclusion of hemp seed. Taaifi et al. (2023a) report that a large inclusion of hemp seeds reduces b* values in egg yolk.
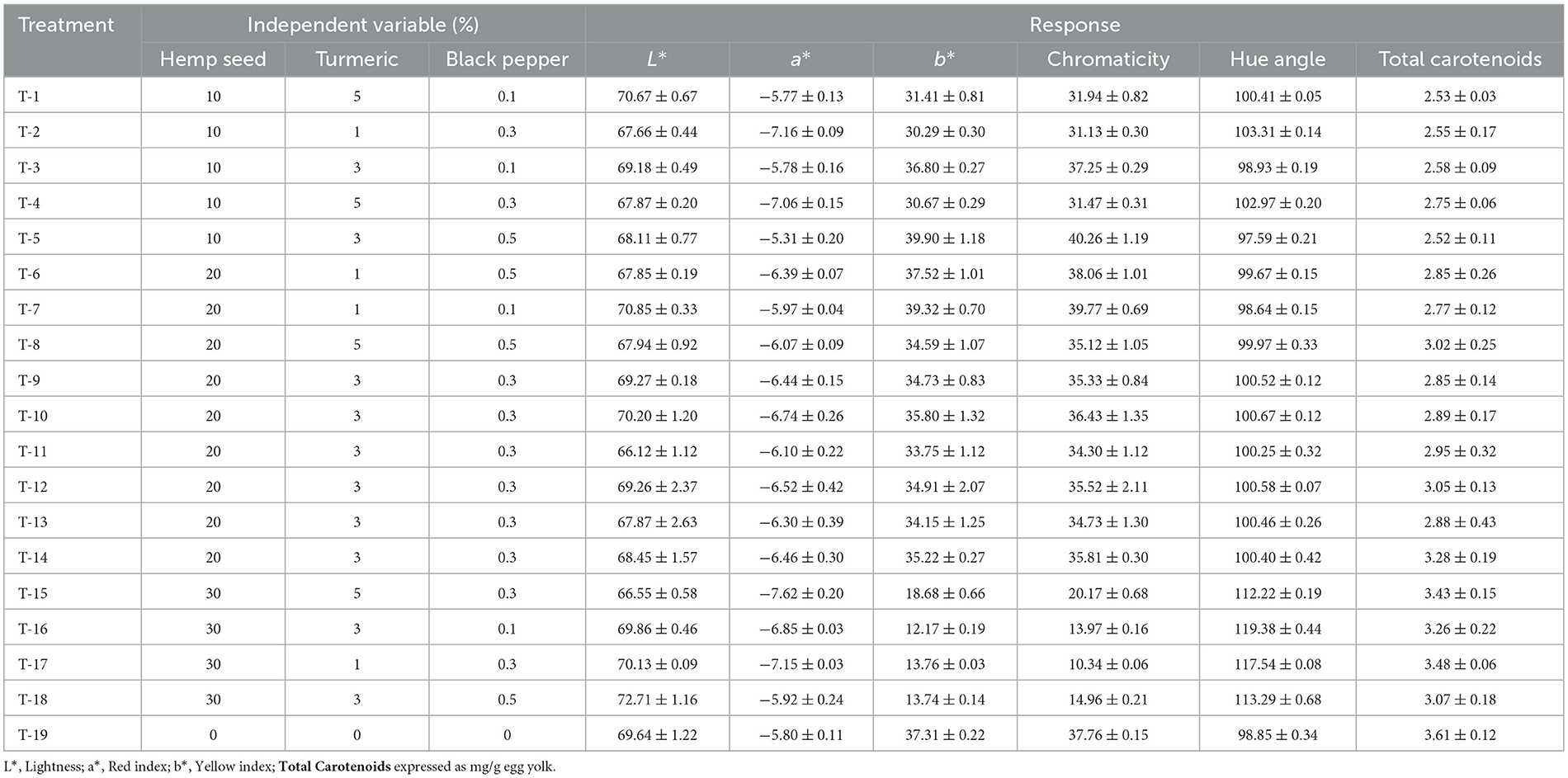
Table 10. Dietary treatments (hemp seed, turmeric and black pepper) and their effects on color parameters and total carotenoids in egg yolk.
For carotenoids, they show the same results for the b* value, a significant increase (p < 0.05) with 10 and 20-hemp seed and a significant decrease (p < 0.05) with 30-hemp seed, probably due to the large substitution of corn (carotenes origin) by hemp seed. Instead Skrivan et al. (2019) shows that hemp seed do not affect carotenoid concentrations in egg yolks up to 30% inclusion.
3.2 Optimal diet combination
Based on the results obtained from egg yolk analyses mainly the fatty acid profile (n3 PUFAs, SFA and n6 PUFAs/n3 PUFAs ratio), tocopherols and cholesterols levels, we generate a 2-D superimposed contour plot for the choice of the optimal combination of the three diets (hemp seed, turmeric and black pepper).
The results shown in Table 11 and Figures 5A–C reveals that with 30% hemp seed, 0.3% black pepper and 3% turmeric of dietary inclusion, we obtained the optimal results which are very suitable, in terms of fatty acid composition, the results show that n3 PUFAs have the highest percentage 5.76% compared to the value of the control diet 2.18% (p < 0.05), the SFAs show a low percentage (p < 0.05) of about 27.05% compared with 31.95% for the control diet, The n6/n3 PUFAs ratio for this dietary combination was significantly enhanced (p < 0.05), reaching ~6.04, which is close to the recommended ratio of below 4. This represents a remarkable improvement compared to the control diet, which had a ratio of 10.5, effectively reducing it by nearly half. With regard to the total tocopherols content in egg yolk, this dietary combination reached a very high level of 844.73 μg/g egg yolk, significantly higher (p < 0.05) than that observed in the control diet (767.85 μg/g egg yolk). This clearly reflects a marked improvement in the total tocopherols content of egg yolk. Cholesterol levels in the egg yolk were substantially reduced, reaching 7.05 mg per gram of egg yolk, compared to the control level of 9.26 mg per gram. This significant decrease highlights the dietary combination's effectiveness in lowering cholesterol content in egg yolk.
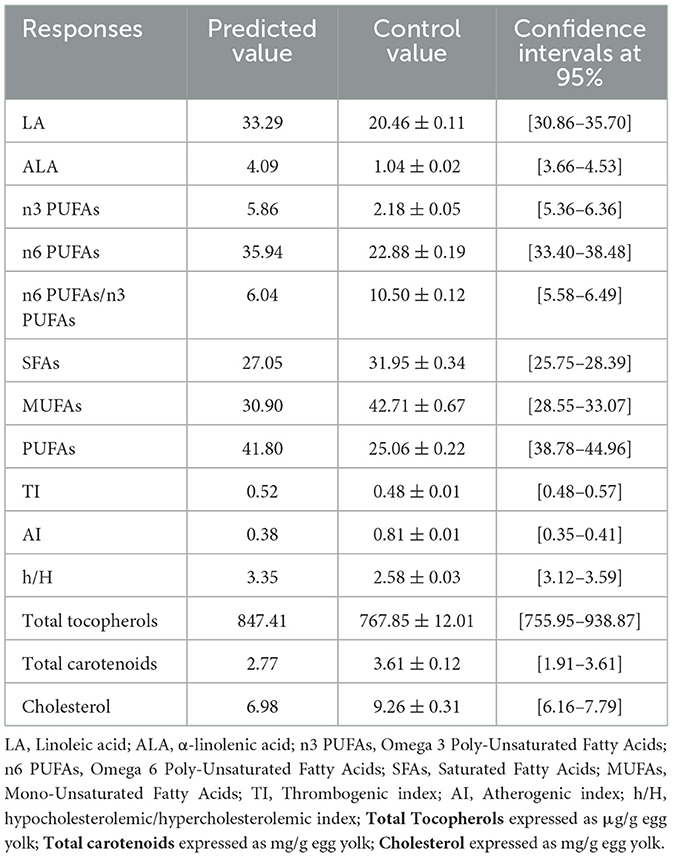
Table 11. Fatty acid profile, health-related lipid quality indexes, cholesterol, Total carotenoids and Total tocopherols levels in egg yolks obtained with the optimal combination (Hemp seed, Turmeric, Black pepper; 30%/3/0.3%) and data validation.
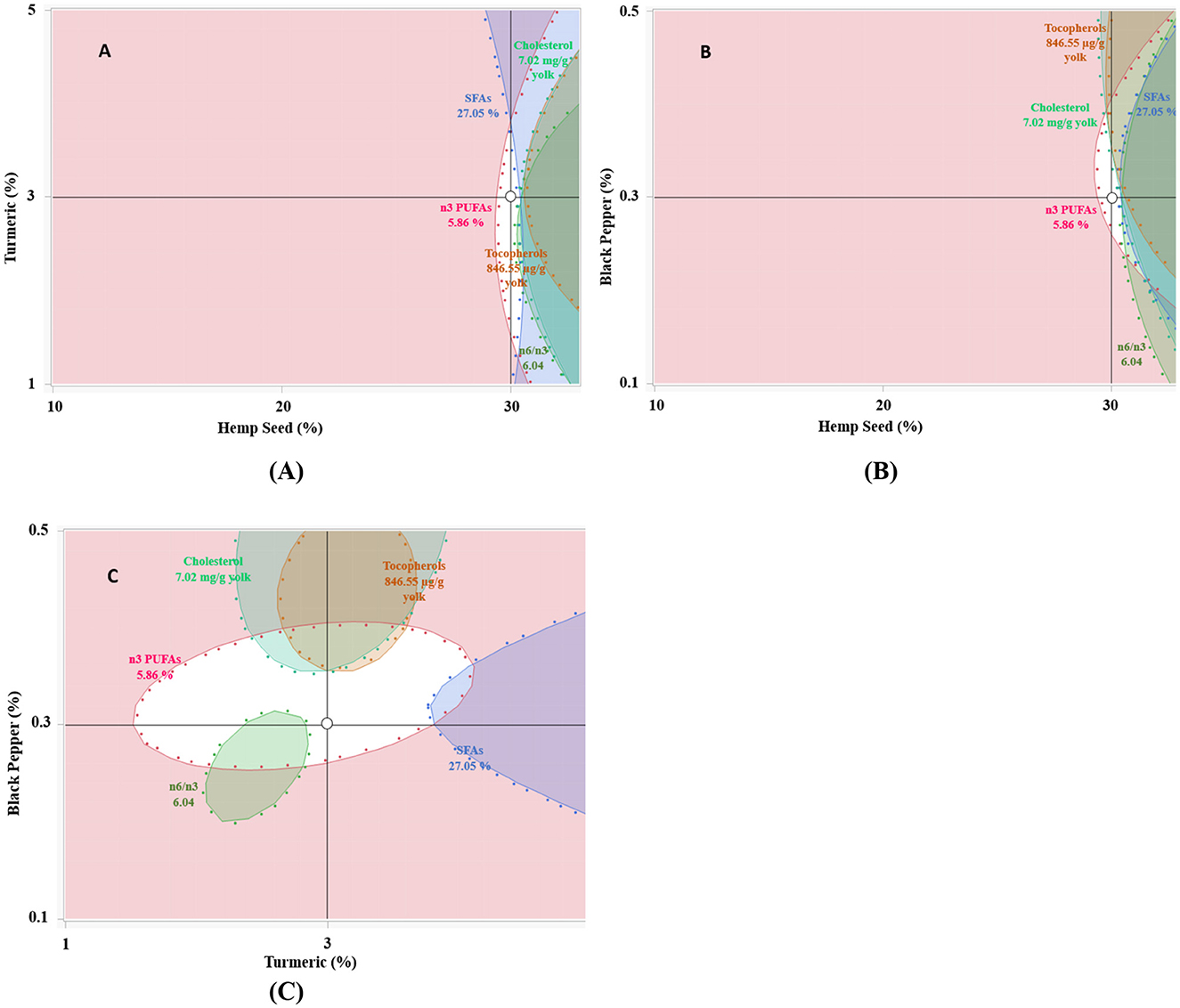
Figure 5. (A–C) represent the overlaid contours of the five responses (SFAs, n3 PUFAs, n6/n3 ratio, tocopherols and cholesterol levels). Pointed area indicates optimal response (27.05% of SFAs, 5.86% of n3 PUFAs, 6.04 of n6 PUFAs/n3 PUFAs ratio, 846.55 μg/g of yolk for tocopherols and 7.02 mg/g of yolk for cholesterol). The combination of 30% hemp seeds, 3% turmeric and 0.3% black pepper was considered suitable for obtaining a high-quality eggs yolk.
4 Conclusion
In the current study, we investigated the effects of incorporating hemp seed, turmeric, and black pepper into the diet of laying hens. Response Surface Methodology was used to determine the optimal levels of saturated fatty acids, n3 PUFAs, n6 PUFAs, n6/n3 ratio, tocopherols and cholesterol levels in egg yolk. Our research findings highlight the effectiveness of RSM and the Box-Behnken statistical method for enriching egg yolk with n3 PUFAs. By introducing hemp seed, turmeric, and black pepper into the laying hens' feed, we successfully enhanced the nutritional quality of eggs. The results show that the combination of 30% hemp seed, 0.3% black pepper and 3% turmeric in the diet of laying hens gives the optimum results in terms of fatty acid profile, cholesterol and tocopherol levels in the egg yolk. Also, this study demonstrates the advantages of employing RSM to evaluate the importance of various variables in egg enrichment research with n3 PUFAs. We consider that these insights, particularly demonstrated through response surface plots, could inspire future research in this area. We are confident that our results will contribute to the continued development of functional foods with excellent health value, in line with global objectives for sustainable food systems. Subsequent research could explore the long-term effects of these phytobiotic combinations on egg laying performance, egg shelf life and consumer acceptance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Royal Institute for Livestock Research in Kenitra, Morocco, ensuring its compliance with ethical guidelines and animal research regulations (Directive 2010/63/EU). It also complies with European Commission Council Directive 1999/74/EC, which defines minimum welfare standards for laying hens. The study was reported in accordance with ARRIVE guidelines [13], and performed with recommended housing, feeding and animal management behaviors to reduce stress and discomfort during the research. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YT: Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing. AA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. FM: Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. KB: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – review & editing. NH: Formal analysis, Methodology, Resources, Software, Writing – review & editing. AS: Funding acquisition, Resources, Validation, Visualization, Writing – review & editing. ON: Funding acquisition, Resources, Validation, Visualization, Writing – review & editing. OM: Resources, Validation, Visualization, Writing – review & editing. EA: Methodology, Resources, Validation, Visualization, Writing – review & editing. RM: Methodology, Supervision, Validation, Visualization, Writing – review & editing. MA: Resources, Validation, Visualization, Writing – review & editing. HS-C: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – review & editing. AE: Conceptualization, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The authors declare that financial support was received for the publication of this article. The research was carried out with institutional support from the Ministry of Higher Education, Scientific Research and Innovation; the National Center for Scientific and Technical Research; in collaboration with the National Agency for Medicinal and Aromatic Plants, and the Royal Institute of Livestock Technicians - Fouarat, as part of the VPMA2/ref 2020/1 project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abive-Bortsi, M., Baidoo, S. T., and Amiteye, S. (2022). Assessment of consumers' perception of chicken eggs consumption and associated health implications in the Volta Region of Ghana. Nutr. Metab. Insights 15:11786388221118872. doi: 10.1177/11786388221118872
Abou-Elkhair, R., Ahmed, H. A., and Selim, S. (2014). Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian-Australas. J. Anim. Sci. 27, 847–854. doi: 10.5713/ajas.2013.13644
Alagawany, M., Elnesr, S. S., Farag, M. R., Abd El-Hack, M. E., Khafaga, A. F., Taha, A. E., et al. (2019). Omega-3 and Omega-6 fatty acids in poultry nutrition: effect on production performance and health. Animals 9:573. doi: 10.3390/ani9080573
Allay, A., Benkirane, C., Ben Moumen, A., Rbah, Y., Fauconnier, M.-L., Caid, H. S., et al. (2024a). Enhancing bioactive compound extractability and antioxidant properties in hemp seed oil using a ternary mixture approach of polar and non-polar solvents. Ind. Crops Prod. 219:119090. doi: 10.1016/j.indcrop.2024.119090
Allay, A., Benkirane, C., Rbah, Y., Moumen, A. B., Taaifi, Y., Belhaj, K., et al. (2024b). Combined effect of protease, hemicellulase and pectinase on the quality of hemp seed oil (Cannabis sativa L.) obtained by aqueous enzymatic extraction as an eco-friendly method. J. Oleo Sci. 73, 963–976. doi: 10.5650/jos.ess24031
Aocs Official Method (2007). Direct Methylation of Lipids in Foods for the Determination of Total Fat, Saturated, Cis Monounsaturated, Cis-Polyunsaturated and Trans Fatty Acids by Gas Chromatography. Rockville, MD: Champaign IL Method Additions and Revisions, AOAC International.
Ashayerizadeh, O., Dastar, B., Shams Shargh, M., Soumeh, A. E., and Jazi, V. (2023). Effects of black pepper and turmeric powder on growth performance, gut health, meat quality, and fatty acid profile of Japanese quail. Front. Physiol. 14:1218850. doi: 10.3389/fphys.2023.1218850
Ashokkumar, K., Murugan, M., Dhanya, M. K., Pandian, A., and Warkentin, T. D. (2021). Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: a review. Clin. Phytosci. 7 :52. doi: 10.1186/s40816-021-00292-2
Belhaj, K., Mansouri, F., Sindic, M., Fauconnier, M.-L., Boukharta, M., Serghini Caid, H., et al. (2021). Effect of rearing season on meat and intramuscular fat quality of Beni-Guil sheep. J. Food Qual. 2021:6615169. doi: 10.1155/2021/6615169
Benkirane, C., Mansouri, F., Ben Moumen, A., Taaifi, Y., Melhaoui, R., Caid, H. S., et al. (2023). Phenolic profiles of non-industrial hemp (Cannabis sativa L.) seed varieties collected from four different Moroccan regions. Int. J. Food Sci. Technol. 58, 1367–1381. doi: 10.1111/ijfs.16298
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/y59-099
Box, G. E. P., Hunter, J. S., and Hunter, W. G. (1978). Statistics for Experimenters: Design, Innovation, and Discovery, 2nd Edition. Hoboken: Wiley.
Brufau, G., Canela, M. A., and Rafecas, M. (2008). Phytosterols: physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr. Res. 28, 217–225. doi: 10.1016/j.nutres.2008.02.003
Bruneel, C., Lemahieu, C., Fraeye, I., Ryckebosch, E., Muylaert, K., Buyse, J., et al. (2013). Impact of microalgal feed supplementation on omega-3 fatty acid enrichment of hen eggs. J. Funct. Foods 5, 897–904. doi: 10.1016/j.jff.2013.01.039
Chen, J., and Liu, H. (2020). Nutritional indices for assessing fatty acids: a mini-review. Int. J. Mol. Sci. 21:5695. doi: 10.3390/ijms21165695
da Silva, W. J., Gouveia, A. B. V. S., de Sousa, F. E., dos Santos, F. R., Minafra-Rezende, C. S., Silva, J. M. S., et al. (2018). Turmeric and sorghum for egg-laying quails. Ital. J. Anim. Sci. 17, 368–376. doi: 10.1080/1828051X.2017.1360160
Dalle Zotte, A., Cullere, M., Pellattiero, E., Sartori, A., Marangon, A., Bondesan, V., et al. (2021). Is the farming method (cage, barn, organic) a relevant factor for marketed egg quality traits? Livest. Sci. 246:104453. doi: 10.1016/j.livsci.2021.104453
De Leon, A. C., Kidd, M. T., and Corzo, A. (2010). Box-Behnken Design: alternative multivariate design in broiler nutrition research. World's Poult. Sci. J. 66, 699–706. doi: 10.1017/S0043933910000668
de Souza, R. J., Mente, A., Maroleanu, A., Cozma, A. I., Ha, V., Kishibe, T., et al. (2015). Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 351:h3978. doi: 10.1136/bmj.h3978
Elkin, R. G., and Harvatine, K. J. (2023). A review of recent studies on the enrichment of eggs and poultry meat with omega-3 polyunsaturated fatty acids: novel findings and unanswered questions. Poult. Sci. 102:102938. doi: 10.1016/j.psj.2023.102938
Elkin, R. G., Ying, Y., Fan, Y., and Harvatine, K. J. (2016). Influence of feeding stearidonic acid (18:4n-3)-enriched soybean oil, as compared to conventional soybean oil, on tissue deposition of very long-chain omega-3 fatty acids in meat-type chickens. Anim. Feed Sci. Technol. 217, 1–12. doi: 10.1016/j.anifeedsci.2016.04.019
European Council Directive (1999). Council Directive 1999/74/EC—Legislation Animal Welfare Farm/Laying-Hens. Available online at: https://food.ec.europa.eu/animals/animal-welfare/eu-animal-welfare-legislation/animal-welfare-farm/laying-hens_en (accessed December 28, 2024).
Ferreira, S. L. C., Bruns, R. E., da Silva, E. G. P., dos Santos, W. N. L., Quintella, C. M., David, J. M., et al. (2007). Statistical designs and response surface techniques for the optimization of chromatographic systems. J. Chromatogr. A 1158, 2–14. doi: 10.1016/j.chroma.2007.03.051
Garg, M. L., Sebokova, E., Wierzbicki, A., Thomson, A. B., and Clandinin, M. T. (1988). Differential effects of dietary linoleic and alpha-linolenic acid on lipid metabolism in rat tissues. Lipids 23, 847–852. doi: 10.1007/BF02536203
Ghanaatparast-Rashti, M., Mottaghitalab, M., and Ahmadi, H. (2018). In ovo feeding of nutrients and its impact on post-hatching water and feed deprivation up to 48 hr, energy status and jejunal morphology of chicks using response surface models. J. Anim. Physiol. Anim. Nutr. 102, e806–e817. doi: 10.1111/jpn.12838
Horwitz, W. (2002). Official Methods of Analysis of AOAC International. Rockville: AOAC International.
Kapoor, B., Kapoor, D., Gautam, S., Singh, R., and Bhardwaj, S. (2021). Dietary polyunsaturated fatty acids (PUFAs): uses and potential health benefits. Curr. Nutr. Rep. 10, 232–242. doi: 10.1007/s13668-021-00363-3
Keshavarz, K. (1976). The influence of turmeric and curcumin on cholesterol concentration of eggs and tissues1. Poult. Sci. 55, 1077–1083. doi: 10.3382/ps.0551077
Kim, M., and Kim, Y. (2010). Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 4, 191–195. doi: 10.4162/nrp.2010.4.3.191
Kruk, J., Holländer-Czytko, H., Oettmeier, W., and Trebst, A. (2005). Tocopherol as singlet oxygen scavenger in photosystem II. J. Plant Physiol. 162, 749–757. doi: 10.1016/j.jplph.2005.04.020
Latimer, G. W. (ed). (2007). “Eggs and egg products,” in Official Methods of Analysis of AOAC International, (AOAC Official Method). Rockville: AOAC International
Lee, S. M., Lee, M. H., Son, Y. K., Kim, S. E., Park, Y., Rha, S. H., et al. (2019). Omega-3 fatty acid decreases oleic acid by decreasing SCD-1 expression in the liver and kidney of a cyclosporine-induced nephropathy rat model. Ren. Fail. 41, 211–219. doi: 10.1080/0886022X.2019.1591996
Liput, K. P., Lepczyński, A., Ogłuszka, M., Nawrocka, A., Poławska, E., Grzesiak, A., et al. (2021). Effects of Dietary n−3 and n−6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 22:6965. doi: 10.3390/ijms22136965
Mansouri, F., Allay, A., Moumen, A. B., Benkirane, C., Taaifi, Y., Belhaj, K., et al. (2023). Laboratory-scale optimization of hemp seed roasting temperature and time for producing a high-quality pressed oil. J. Food Process. Preserv. 2023:8261279. doi: 10.1155/2023/8261279
Mierlita, D., Teusdea, A. C., Matei, M., Pascal, C., Simeanu, D., Pop, I. M., et al. (2024). Effect of dietary incorporation of hemp seeds alone or with dried fruit pomace on laying hens' performance and on lipid composition and oxidation status of egg yolks. Animals 14:750. doi: 10.3390/ani14050750
Minihane, A. M., and Lovegrove, J. A. (2006). “5 - Health benefits of polyunsaturated fatty acids (PUFAs),” in Improving the Fat Content of Foods, eds. C. Williams and J. Buttriss (New Delhi: Woodhead Publishing), 107–140.
Mohebodini, H., Jazi, V., Ashayerizadeh, A., Toghyani, M., and Tellez-Isaias, G. (2021). Productive parameters, cecal microflora, nutrient digestibility, antioxidant status, and thigh muscle fatty acid profile in broiler chickens fed with Eucalyptus globulus essential oil. Poult. Sci. 100:100922. doi: 10.1016/j.psj.2020.12.020
Monroig, Ó., Shu-Chien, A. C., Kabeya, N., Tocher, D. R., and Castro, L. F. C. (2022). Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: from genes to functions. Prog. Lipid Res. 86:101157. doi: 10.1016/j.plipres.2022.101157
Munné-Bosch, S., and Alegre, L. (2002). The function of tocopherols and tocotrienols in plants. CRC. Crit. Rev. Plant Sci. 21, 31–57. doi: 10.1080/0735-260291044179
Myers, M., and Ruxton, C. H. S. (2023). Eggs: healthy or Risky? A review of evidence from high quality studies on hen's eggs. Nutrients 15:2657. doi: 10.3390/nu15122657
Naim, R. M., Mutalib, M. A., Shamsuddin, A. S., Lani, M. N., Ariffin, I. A., Tang, S. G. H., et al. (2024). Navigating the environmental, economic and social impacts of sustainable agriculture and food systems: a review. Front. Agr. Sci. Eng. 11, 652–673. doi: 10.15302/J-FASE-2024550
National Research Council (1994). Nutrient Requirements of Poultry: Ninth Revised Edition, 1994. Washington, D.C.: National Academies Press.
Neijat, M., Ojekudo, O., and House, J. D. (2016a). Effect of flaxseed oil and microalgae DHA on the production performance, fatty acids and total lipids of egg yolk and plasma in laying hens. Prostaglandins, Leukotrienes and Essential Fatty Acids 115, 77–88. doi: 10.1016/j.plefa.2016.10.010
Neijat, M., Suh, M., Neufeld, J., and House, J. D. (2016b). Hempseed products fed to hens effectively increased n-3 polyunsaturated fatty acids in total lipids, triacylglycerol and phospholipid of egg yolk. Lipids 51, 601–614. doi: 10.1007/s11745-015-4088-7
Omri, B., Chalghoumi, R., Izzo, L., Ritieni, A., Lucarini, M., Durazzo, A., et al. (2019). Effect of dietary incorporation of linseed alone or together with tomato-red pepper mix on laying hens' egg yolk fatty acids profile and health lipid indexes. Nutrients 11:813. doi: 10.3390/nu11040813
Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18:e3000410. doi: 10.1371/journal.pbio.3000410
Popiela, E., Króliczewska, B., Zawadzki, W., Opaliński, S., and Skiba, T. (2013). Effect of extruded amaranth grains on performance, egg traits, fatty acids composition, and selected blood characteristics of laying hens. Livest. Sci. 155, 308–315. doi: 10.1016/j.livsci.2013.05.001
Rafieian-Kopaei, M., Setorki, M., Doudi, M., Baradaran, A., and Nasri, H. (2014). Atherosclerosis: process, indicators, risk factors and new hopes. Int. J. Prev. Med. 5, 927–946.
Rbah, Y., Taaifi, Y., Allay, A., Belhaj, K., Melhaoui, R., Houmy, N., et al. (2024). A comprehensive exploration of the fatty acids profile, cholesterol, and tocopherols levels in liver from laying hens fed diets containing nonindustrial hemp seed. Scientifica 2024:e8848436. doi: 10.1155/2024/8848436
Saleh, A. A., Gawish, E., Mahmoud, S. F., Amber, K., Awad, W., Alzawqari, M. H., et al. (2021). Effect of natural and chemical colorant supplementation on performance, egg-quality characteristics, yolk fatty-acid profile, and blood constituents in laying hens. Sustainability 13:4503. doi: 10.3390/su13084503
Shahid, S., Chand, N., Khan, R. U., Suhail, S. M., and Khan, N. A. (2015). Alternations in cholesterol and fatty acids composition in egg yolk of rhode island red x fyoumi hens fed with hemp seeds (Cannabis sativa L.). J. Chem. 2015:362936. doi: 10.1155/2015/362936
Shahidi, F., and de Camargo, A. C. (2016). Tocopherols and tocotrienols in common and emerging dietary sources: occurrence, applications, and health benefits. Int. J. Mol. Sci. 17:1745. doi: 10.3390/ijms17101745
Shoba, G., Joy, D., Joseph, T., Majeed, M., Rajendran, R., Srinivas, P. S., et al. (1998). Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 64, 353–356. doi: 10.1055/s-2006-957450
Sim, J. S., and Sunwoo, H. H. (2002). Designer eggs: nutritional and functional significance. Available online at: https://onlinelibrary.wiley.com/doi/10.1002/9780470376973.ch3 (accessed January 1, 2002).
Simopoulos, A. P., Leaf, A., and Salem, N. (2000). Workshop statement on the essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 63, 119–121. doi: 10.1054/plef.2000.0176
Skrivan, M., Englmaierová, M., Vít, T., and Skrivanová, E. (2019). Hempseed increases gamma-tocopherol in egg yolks and the breaking strength of tibias in laying hens. PLoS ONE 14:e0217509. doi: 10.1371/journal.pone.0217509
Szewczyk, K., Chojnacka, A., and Górnicka, M. (2021). Tocopherols and tocotrienols—bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 22 :6222. doi: 10.3390/ijms22126222
Taaifi, Y., Belhaj, K., Mansouri, F., Rbah, Y., Elbouanani, N., Melhaoui, R., et al. (2023a). Impact of cannabis seed incorporation in layer diet on productive performance and egg quality traits. Scientifica 2023:e5565825. doi: 10.1155/2023/5565825
Taaifi, Y., Belhaj, K., Mansouri, F., Rbah, Y., Melhaoui, R., Houmy, N., et al. (2023b). The effect of feeding laying hens with nonindustrial hemp seed on the fatty acid profile, cholesterol level, and tocopherol composition of egg yolk. Int. J. Food Sci. 2023:1360276. doi: 10.1155/2023/1360276
Taaifi, Y., Benmoumen, A., Belhaj, K., Aazza, S., Abid, M., Azeroual, E., et al. (2021). Seed composition of non-industrial hemp (Cannabis sativa L.) varieties from four regions in northern Morocco. Int. J. Food Sci. Technol. 56, 5931–5947. doi: 10.1111/ijfs.15136
Tang, S. G. H., Sieo, C. C., Kalavathy, R., Saad, W. Z., Yong, S. T., Wong, H. K., et al. (2015). Chemical compositions of egg yolks and egg quality of laying hens fed prebiotic, probiotic, and synbiotic diets. J. Food Sci. 80, C1686–95. doi: 10.1111/1750-3841.12947
Trautwein, E. A., Duchateau, G. S. M. J. E., Lin, Y., Mel'nikov, S. M., Molhuizen, H. O. F., and Ntanios, F. Y. (2003). Proposed mechanisms of cholesterol-lowering action of plant sterols. Eur. J. Lipid Sci. Technol. 105, 171–185. doi: 10.1002/ejlt.200390033
Xie, Z., Shen, G., Wang, Y., and Wu, C. (2019). Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 98, 422–429. doi: 10.3382/ps/pey315
Keywords: egg yolk, Box-Behnken Design, hemp seed, phytobiotics, fatty acids, tocopherols, cholesterol, carotenoids
Citation: Rbah Y, Taaifi Y, Allay A, Mansouri F, Belhaj K, Houmy N, Shahat AA, Noman OM, Merah O, Azeroual E, Melhaoui R, Addi M, Serghini-Caid H and Elamrani A (2025) Optimization of hemp seed supplementation with phytobiotics in laying hen feed to improve egg yolk fatty acids, tocopherols and cholesterol using response surface models. Front. Sustain. Food Syst. 9:1566447. doi: 10.3389/fsufs.2025.1566447
Received: 24 January 2025; Accepted: 18 June 2025;
Published: 11 July 2025.
Edited by:
Arda Sözcü, Bursa Uludağ University, TürkiyeReviewed by:
Gerardo Mendez-Zamora, Autonomous University of Nuevo León, MexicoMuhammad Naim Rosli, Management and Science University, Malaysia
Copyright © 2025 Rbah, Taaifi, Allay, Mansouri, Belhaj, Houmy, Shahat, Noman, Merah, Azeroual, Melhaoui, Addi, Serghini-Caid and Elamrani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youssef Rbah, eXJiYWg1MEBnbWFpbC5jb20=; Othmane Merah, b3RobWFuZS5tZXJhaEBlbnNpYWNldC5mcg==
 Youssef Rbah
Youssef Rbah Yassine Taaifi2
Yassine Taaifi2 Aymane Allay
Aymane Allay Farid Mansouri
Farid Mansouri Abdelaaty A. Shahat
Abdelaaty A. Shahat Omar M. Noman
Omar M. Noman Othmane Merah
Othmane Merah Hana Serghini-Caid
Hana Serghini-Caid