- 1College of Animal Science, Fujian Agriculture and Forestry University, Fuzhou, China
- 2University Key Laboratory for Integrated Chinese Traditional and Western Veterinary Medicine and Animal Healthcare in Fujian Province/Fujian Key Laboratory of Traditional Chinese Veterinary Medicine and Animal Health, Fujian Agriculture and Forestry University, Fuzhou, China
- 3Institute of Animal Husbandry and Veterinary Medicine, Fujian Academy of Agricultural Sciences, Fuzhou, China
Introduction: Oxidative stress is closely linked to various diseases in chickens, representing an urgent concern that needs to be addressed in the poultry industry. Curcumin (CUR) and selenium (Se) are both recognized for their great antioxidant effects, however, the combination use of them in broilers has not been reported. This study aims to demonstrate the synergistic antioxidant effects of CUR and Se in vitro and in vivo, and to explore the underlying antioxidant mechanism.
Methods: The experiments were conducted on the DF-1 cell line and 400 healthy male White-feathered Broilers, day old, weighing 43.89 ± 0.70 g. Hydrogen peroxide (H₂O₂) and dexamethasone (Dex) were used to conduct oxidative stress model.
Results: The results demonstrated that CUR and Se synergistically enhanced the antioxidant capacity of DF-1 cells, with a combination index (CI) less than 1; next, CUR and Se increased the total antioxidant capacity and superoxide dismutase (SOD) activity, decreased lactate dehydrogenase (LDH) and malondialdehyde (MDA) levels in broiler liver and heart tissues, alleviated Dex-induced liver and heart injury and liver cell apoptosis in broilers; moreover, the protein expression of IGF-1, PI3K-p110β, phosphorylated AKT and phosphorylated mTOR in liver and heart tissues were increased after the combination treatment.
Discussion: In conclusion, CUR and Se alleviate oxidative stress in White-feathered Broilers synergistically, and the synergistic antioxidant effects are related to IGF-1/PI3K/AKT/mTOR pathway.
Introduction
Oxidative stress is a status of imbalance between oxidation and reduction, which is closely associated with a variety of diseases in broilers (1–4). Oxidative stress induces metabolite accumulation in poultry, this condition further leads to higher mortality rates, reduced egg production, diminished meat and egg quality, and compromised overall performance (5–8). There are several factors that can induce oxidative stress, such as abnormal temperature (9, 10), high density (11), long-distance transport (12), etc. In modern feeding management, different strategies have been used to mitigate the effects of oxidative stress in poultry, such as genetic selection for better breeding (13), enhancing management strategies to mitigate stress within the production environment (14), antioxidant supplementation (15) and nutritional interventions (16) to improve poultry resistance.
The combined use of drugs for the prevention and treatment of diseases has emerged as a new trend (17). The advantages of drug combination include: synergistic effects of drugs leading to enhanced therapeutic outcomes; a reduction in the dosage of individual drugs, thereby mitigating drug side effects; and minimizing or slowing down the development of drug resistance, among others (18).
Curcumin (CUR) is a phenolic compound extracted from Curcuma longa L, the multiple medical value of CUR has been explored for over 30 years (19). Studies have proved that CUR can enhance the immunity and antioxidant capacity of poultry, improve lipid metabolism and intestinal health (20–23), simultaneously, CUR can ameliorate ochratoxin A induced oxidative stress through kelchlike ECH-Associating protein 1 (Keap1)-nuclear factor erythroid-2 related factor 2 (Nrf2)-antioxidant response element (ARE) and aryl hydrocarbon receptor (AHR) pathways (24). Other studies have shown that CUR can activate the mitogen-activated protein kinase (MAPK)-Nrf2 signaling pathway of chicken embryonic fibroblast cells to relieve heat-induced oxidative stress (25). Despite its exceptional antioxidant capabilities, the clinical application of CUR is limited by its low solubility and bioavailability (26). Consequently, numerous innovative strategies have been proposed to ameliorate these limitations, such as the use of biocarriers (27), the construction of structural analogs (28), and combination therapies (29), etc.
Selenium (Se) is one of the essential trace elements, and is crucial for maintaining fundamental life processes. Studies have demonstrated that Se possesses antioxidant properties and serves as the active site in antioxidant enzymes, including glutathione peroxidase (GPX) (30–33). Researchers have elucidated that Se can mitigate the spleen toxicity induced by aflatoxin B1 in broilers through the inhibition of oxidative stress and the attenuation of excessive apoptosis (34). In addition to the application alone, Se can also be used in combination with other agents to produce synergistic effects. For example, dietary supplementation of sodium selenite and vitamin E (VE) in broilers under summer heat stress can significantly increase the activities of catalase (CAT), superoxide dismutase (SOD), and GPX compared with Se or VE alone (35).
The Insulin-like growth factor 1 (IGF-1)/Phosphatidylinositol-3-kinase (PI3K)/Protein kinase B (AKT)/Mammalian target of rapamycin (mTOR) signaling pathway is critical in various vital life functions such as antioxidation (36, 37), cell growth (38), proliferation (39), differentiation (40) and apoptosis (41, 42). Previous studies have shown that activation of IGF-1 can activate PI3K, AKT and mTOR, which then activate the antioxidant function of cells to resist oxidative stress (43–45).
In the present study, the DF-1 cell line and White-feathered Broilers were used to test the hypothesis that CUR and Se exert synergistic anti-oxidative effects in White-feathered Broilers in vitro and in vivo, and the underlying mechanism is related to the IGF-1/PI3K/AKT/mTOR pathway. The findings support the use of CUR and Se as a potential antioxidant therapy in poultry industry.
Materials and methods
Cell maintenance and drug treatments
The chicken embryo fibroblast cell line DF-1 (Cell bank of the Chinese Academy of Science, Beijing, China) was cultured in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies Inc., Carlsbad, CA, United States) supplemented with 10% fetal bovine serum (FBS, Life Technologies Inc., CA, United States), penicillin (100 units/mL) and streptomycin (0.1 mg/mL; Life Technologies Inc., CA, United States) at 37°C in an atmosphere containing 5% CO2.
CUR was purchased from MedChemExpress Inc. (HY-N0005, Monmouth Junction, NJ, United States), Sodium Selenite was purchased from Sigma-Aldrich, Inc. (214,485, ST Louis, Missouri, United States). DF-1 cells were treated with 1–35 μM CUR, 0.1–3.5 μM Se or the combination of CUR and Se for 24 h, then the drugs were removed, 100 μM H₂O₂ was added to the fresh culture medium and cells were treated for 2 h before conducting subsequent experiments.
Animals and treatments
The animal experiment was reviewed and approved by the Animal Ethics Committee of Fujian Agriculture and Forestry University (approval code: PZCASFAFU24029), according to the guidelines for Laboratory Animal Use and Care from the Chinese Center for Disease Control and Prevention and the Rules for Medical Laboratory Animals (1998) from the Chinese Ministry of Health.
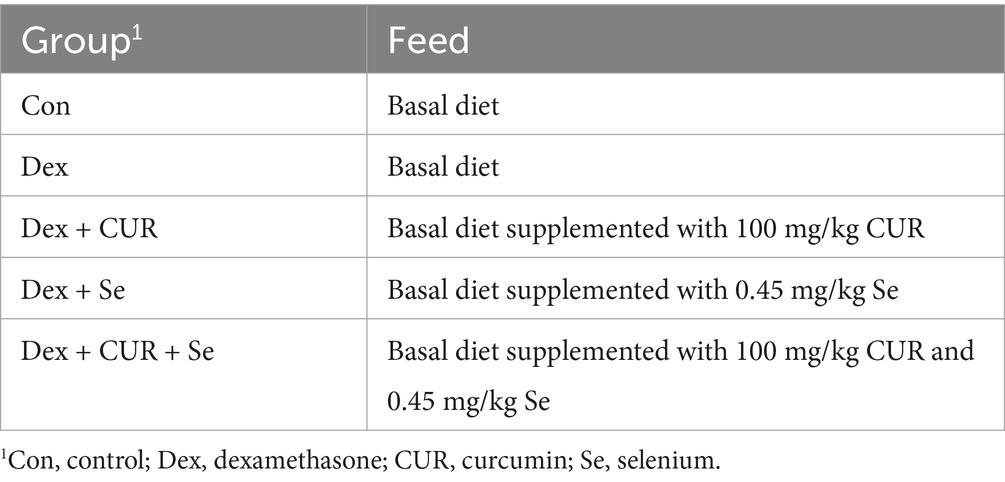
A total of 400 healthy male White-feathered Broilers, which were day old and had similar body weight (43.89 ± 0.70 g) were acquired from Fujian Shengnong Inc. (Fujian, China) and randomly divided into 5 groups with 10 replicates per group and 8 broilers per replicate. Broilers were, respectively, fed diets supplemented with 100 mg/kg CUR, 0.45 mg/kg Se, or the combination of 100 mg/kg CUR and 0.45 mg/kg Se from day old to day 42. Broilers that needed to be sacrificed were anesthetized and euthanized on day 42 using Thiopental Sodium injection (0.7 mL/kg). Grouping and treatment protocols were presented in Table 1. Broilers were given free access to water and feed. The basal diet was formulated according to the National standard of the People’s Republic of China “Compound Feed for Laying Hens and Broiler” (GB/T 5916–2020). The composition and nutritional level of the basal diet were shown in Supplementary Table S1.
On the 35 d, 37 d and 39 d of the experiment, 3 mg/kg Dexamethasone (Dex) was injected intramuscularly into chickens in all groups except the control group, and the chickens in the control group was injected with the same amount of normal saline (46–50). The average daily feed intake (ADFI) and average daily weight gain (ADG) of broilers were recorded, with the feed conversion ratio (FCR) calculated using the formula: FCR = ADFI/ADG.
Serum samples were collected at 35 d to detect serum liver and kidney biochemical indices, while liver and heart tissues were collected at 42 d to perform pathological section with hematoxylin and eosin (H&E) staining and subsequent experiments.
Antioxidant capability assays
Ferric Ion Reducing Antioxidant Power (FRAP) Assay Kit, Total Antioxidant Capacity Assay Kit with ABTS method (ABTS), SOD Activity Assay Kit, Malondialdehyde (MDA) Assay Kit (Beyotime Biotechnology Inc., Shanghai, China) and Lactate Dehydrogenase (LDH) Assay Kit (Nanjing jiancheng bioengineering Inc., Jiangsu, China) were used for detecting antioxidant capability of cells or tissues following the manufacturer’s instructions. Briefly, cells or tissues were homogenized using an ultrasonic grinder, after which the supernatant was collected into a 96-well plate and the appropriate reagents were added. The results were read using a microplate reader (Molecular Devices, 22,202-SANGMSMA1, Shanghai, China). The experiments were carried out in triplicate.
Reactive oxygen species assay
Intracellular ROS levels were detected by DCFH-DA probe (Beyotime Biotechnology Inc., Shanghai, China) following the manufacturer’s instructions. Briefly, 1 × 106 cells were plated in 6-well plates, the probe was diluted with a serum-free medium (1: 1,000), then cells were mixed with the probe and incubated at 37°C for 25 min in dark. The experiments were carried out in triplicate and the images were captured with a fluorescence microscope (IRX50, Sunny Optical Technology Inc., Jiangsu, China). Fluorescence intensity was measured using the Image J (Version 1.8.0, National Institutes of Health, United States) software.
Serum liver and kidney biochemical indices assay
Serum liver and kidney biochemical indexes were determined by Shanghai Zhucai Biological Inc. (Shanghai, China). The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (CREA) and blood urea nitrogen (BUN) in serum were detected by automatic biochemical analyzer (Chemray240, Shenzhen Leidu Life Technology Inc., Shenzhen, China). The experiments were carried out in triplicate.
Deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
The TUNEL assay was performed to detect cell apoptosis. Briefly, liver samples were fixated in 4% paraformaldehyde for 1 h, and then were stained with TUNEL Apoptosis Assay Kit (abs50047, Absin, Shanghai, China) following the manufacturer’s instructions. Then the slides were mounted using a DAPI-containing medium (P0131, Beyotime Biotechnology Inc., Shanghai, China). Images were captured with a confocal laser scanning microscope (STELLARIS 5, Leica Inc., Wetzlar, Germany), six photographs were taken for each group. The number of apoptotic cells and the total number of cells in the visual field were counted by the ImageJ (Version 1.8.0, National Institutes of Health, United States) software, respectively, and then the apoptosis ratio was calculated using the equation: number of apoptotic cells/numbers of total cells × 100%.
Transcriptome sequencing assay
The transcriptome sequencing assay was done by Majorbio Inc. (Shanghai, China). Total RNA was extracted from the tissue using TRIzol® reagent according the manufacturer’s instructions. RNA purification, reverse transcription, library construction and sequencing were performed at Shanghai Majorbio Bio-pharm Biotechnology Inc. (Shanghai, China) according to the manufacturer’s instructions (Illumina, San Diego, CA). To identify differential expression genes (DEGs) between two different samples, the expression level of each transcript was calculated according to the transcripts per million reads (TPM) method. Sequencing was performed on NovaSeqXPlus platform. The experiments were carried out in triplicate. The transcriptome sequencing data reported in this paper have been submitted to sequence read archive (SRA) database of NCBI and have been assigned the accession number PRJNA1192045.
Immunohistochemistry assay
The tissue samples were fixed in 4% formaldehyde at pH 7.4 and 4°C for 48 h. After dehydration, paraffin wax was embedded and the tissues were sliced at a thickness of 4 μm. After deparaffination and antigen retrieval with EDTA antigen repair solution (MVS-0099, Maixin Biotechnology Inc., Fuzhou, China), sections were incubated with primary antibodies: IGF-1 (28530-1-AP, Proteintech Inc., Wuhan, China, 1: 200), PI3K-p110β (67121-1-Ig, Proteintech Group Inc., Wuhan, China, 1: 300 for liver, 1: 800 for heart), Phospho-AKT (66444-1-Ig, Proteintech Inc., Wuhan, China, 1: 200 for liver, 1: 800 for heart), and Phospho-mTOR (67778-1-Ig, Proteintech Inc., Wuhan, China, 1: 50) at 4°C overnight, followed by incubation of biotinylated secondary antibodies at 37°C for 1 h. Sections were stained with diaminobenzidine (DAB-1031, Maixin Biotechnology Inc., Fuzhou, China) and counterstained with hematoxylin (G1004, Servicebio Inc., Wuhan, China). Images were captured with a bright field digital microscope. The experiments were carried out in triplicate.
Statistical analysis
The combination index (CI) was calculated to examine the interaction between CUR and Se by Compusyn software (version 1.0, Inc., Paramus, NJ, United States). CI values 1, <1, and >1 indicated an additive effect, synergism, or antagonism, respectively.
Statistical analysis was performed using GraphPad Prism10 software (version 10, GraphPad Software Inc., San Diego, CA, United States) and SPSS 26.0 software (version 26.0, IBM Inc., NY, United States). A two-tailed unpaired t-test with Welch’s correction was applied when the variances of two groups were proven equal by the F test. A p value of 0.05 or less indicated statistical significance.
Results
CUR and Se emerge synergistical antioxidant effect on DF-1 cells
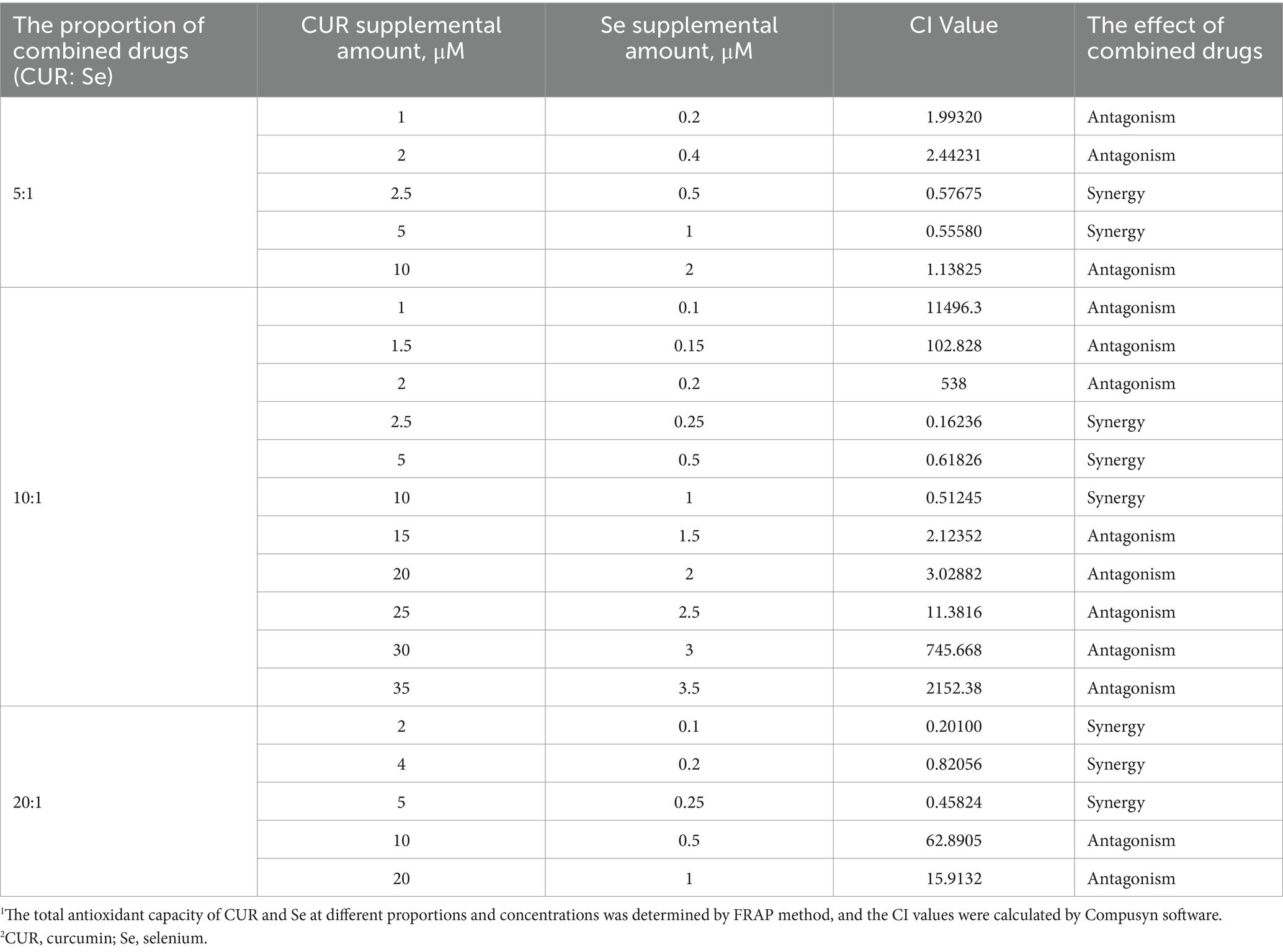
In order to assess the combined effect of CUR and Se, different doses and ratio of CUR and Se were applied on DF-1 cells, and FRAP assays were performed, then the CI value was calculated based on the antioxidant results (Table 2). As the results shown, CUR and Se achieved synergistic effects at specific concentrations and varying ratios, the CI value reached its minimum at CUR 2.5 μM and Se 0.25 μM, indicating a better ratio of CUR to Se is 10:1.
Then, the FRAP, ABTS and ROS assays of DF-1 cells under these doses of CUR and Se were conducted to confirm the synergistic antioxidant effects (Figure 1). As the results shown, compared to the control group, H2O2 significantly decreased the cell total antioxidant ability, and increased the cellular ROS level (p < 0.001); CUR or Se alone treatments rescued the cells from the oxidative stress to a certain extent (p < 0.05), nevertheless, the combination treatment of CUR and Se significantly improved the antioxidant capability of DF-1 cells and reduced the production of ROS, compared with H2O2, CUR or Se groups (p < 0.05). These results demonstrated that CUR and Se emerged synergistic antioxidant effects in vitro.
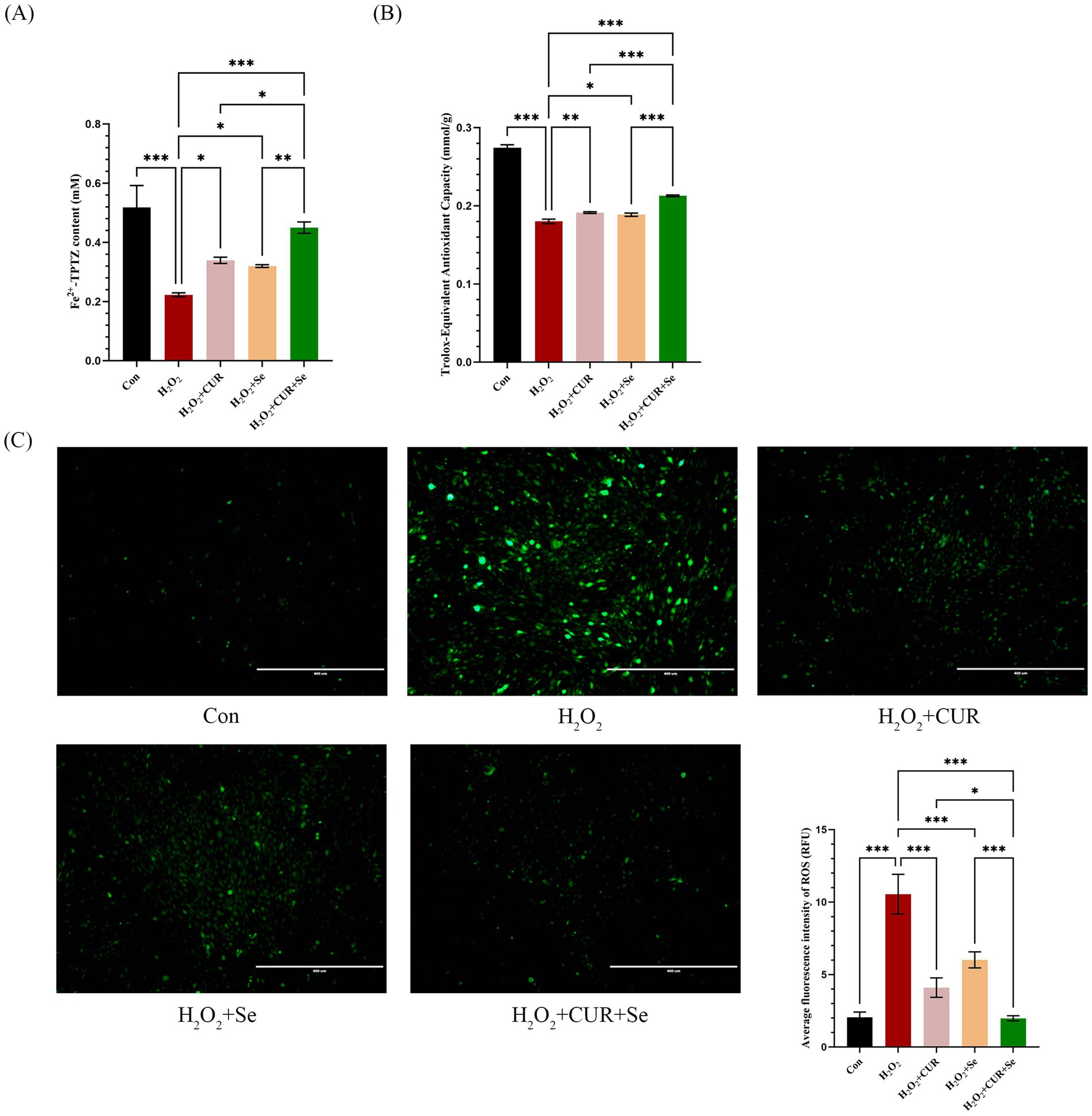
Figure 1. Cur and Se emerge antioxidant effect on DF-1 cells synergistically. DF-1 cells were plated in six-well plates and treated with 2.5 μM CUR and 0.25 μM Se in the medium for 24 h, then drugs were removed, 100 μM H₂O₂ was added to the culture medium for 2 h, after that, the FRAP (A), ABTS (B) and ROS (C) assays were conducted following the manufacturer’s instructions. Data are representative of three independent experiments. Bar: 400 μm. *p < 0.05; ***p < 0.001. Con, control; Dex, dexamethasone; CUR, curcumin; Se, selenium.
CUR and Se alleviate Dex induced hepatic and cardiac damage in broilers
To investigate the combined antioxidant effects of CUR and Se in vivo, a feeding trial with broilers was conducted, and an in vivo model of oxidative stress was established. There were no significant differences in FCR, ADG, ADFI or serum biochemical indices among all the groups (Supplementary Table S2; Supplementary Figure S1), indicating that CUR and Se did not have a notable impact on the growth performance or biochemical indices of broilers.
The liver and heart tissue sections were subjected to H&E staining (Figure 2). Compared with the control group, the liver of the Dex-treated group exhibited a substantial increase in the presence of vacuolar degeneration cells. This was predominantly characterized by the formation of transparent vacuoles within the cellular matrix, resulting in the displacement of cytoplasm to the periphery of the affected cells. Simultaneously, the number of vacuoles decreased in the drug-treated groups, especially in the combination treatment group. In addition, compared with the control group, the Dex-treated group also showed obvious hepatic cord disorder, resulting in no clear hepatic cord route, but the combination of drugs ameliorated this phenomenon. The results indicated that CUR and Se alleviated oxidative stress-induced liver damage.
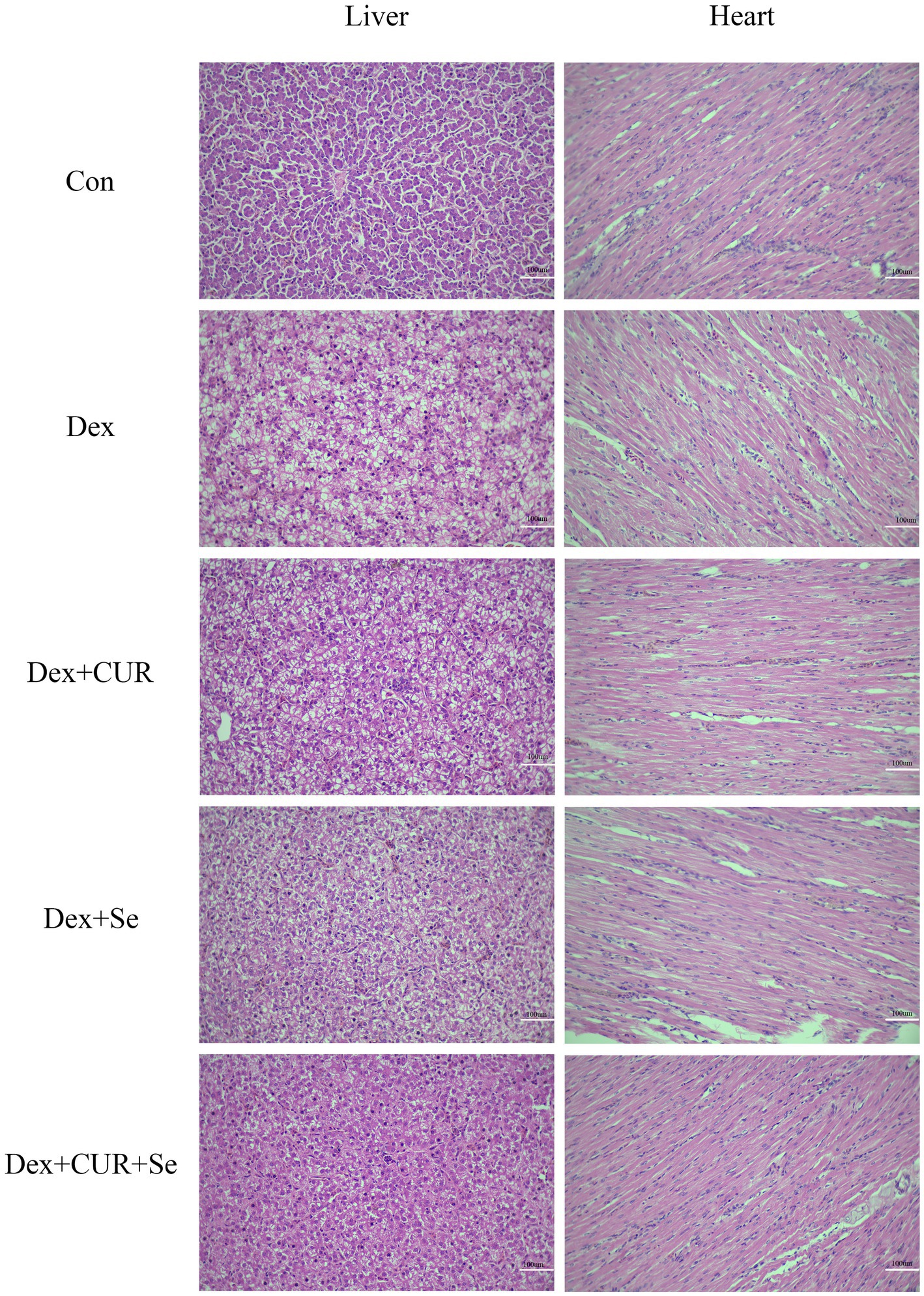
Figure 2. CUR and Se alleviate Dex induced hepatic and cardiac damage in broilers. Sections of liver and heart tissues from 42-day-old broilers, embedded in paraffin, were stained with hematoxylin and eosin (H&E) and examined under an optical microscope. Bar: 100 μm. Con, control; Dex, dexamethasone; CUR, curcumin; Se, selenium.
A similar phenomenon was also observed in the heart tissues. Compared to the control group, the myocardial fibers in the Dex-treated group exhibited disarray and fragmentation, with an increase in intercellular spaces. Treatment with CUR or Se alone ameliorated this damage, but the combination of CUR and Se demonstrated a more pronounced beneficial effect.
CUR and Se significantly ameliorate Dex-induced apoptosis in broilers’ liver tissues
A TUNEL assay was performed to evaluate apoptosis level of the liver tissues. As shown in Figure 3, compared with the control group, the ratio of apoptotic cells in the Dex group increased significantly (p < 0.001), and compared with the Dex group, the ratio of apoptotic cells in all treatment groups was significantly reduced (p < 0.05). The level of apoptosis in the combination group is even lower compared to the monotherapy group (p < 0.05). These results indicated that combination of CUR and Se alleviated liver apoptosis under oxidative stress, thereby protecting the liver of broilers.
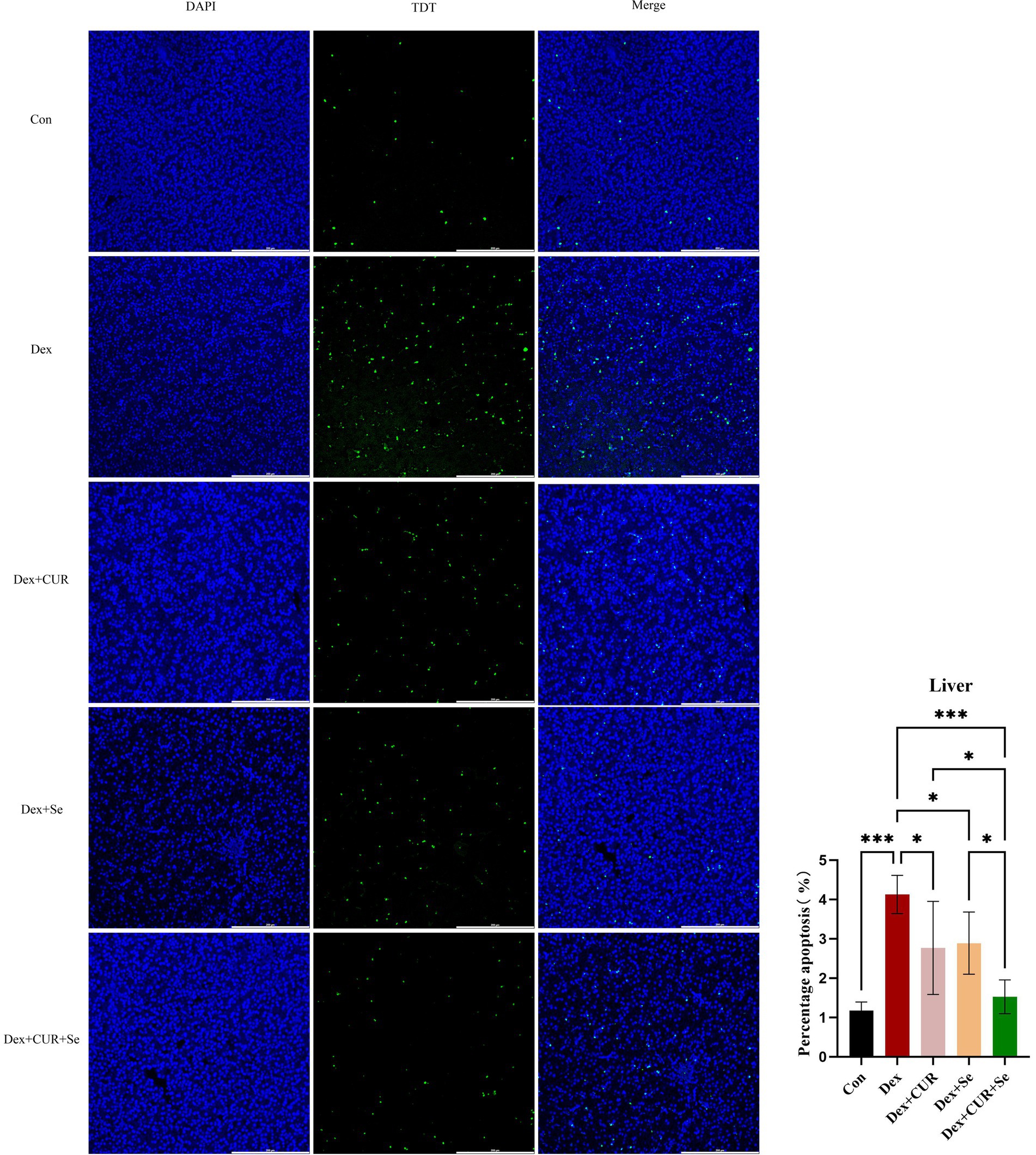
Figure 3. CUR and Se significantly ameliorate Dex-induced apoptosis in broilers’ liver tissues. Liver tissue sections were stained using TUNEL kits, the number of apoptotic cells and the total number of cells in the visual field were counted, respectively, and then the apoptosis ratio was calculated using the equation: number of apoptotic cells/numbers of total cells × 100%. Bar: 200 μm. Data are representative of six independent experiments. *p < 0.05; ***p < 0.001. Con, control; Dex, dexamethasone; CUR, curcumin; Se, selenium.
CUR and Se enhance the antioxidant capacity of broilers’ liver and heart tissues
In order to investigate the synergistic antioxidant effect of CUR combined with Se in vivo, various assays were conducted in the liver and heart tissues. In the context of liver tissues (Figure 4A), when compared to the control group, the Dex group exhibited a significant reduction in total antioxidant capacity and SOD enzyme activity, along with a significant elevation in MDA content (p < 0.05); CUR and Se alone protected the liver form Dex to a certain extent (p < 0.05); while combined treatment emerged a better effect compared with that in the monotherapy groups (p < 0.05).
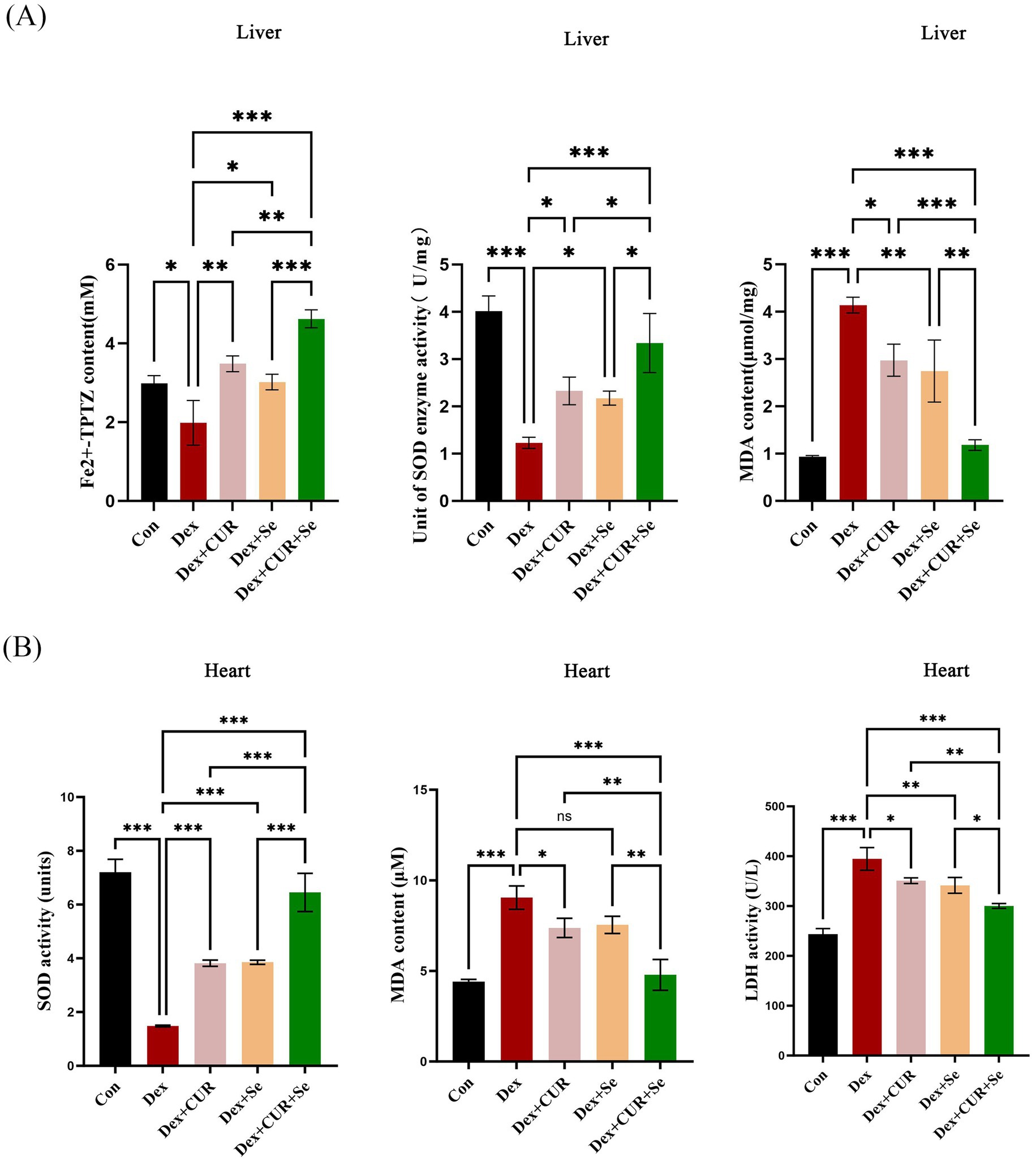
Figure 4. CUR and Se enhance the antioxidant capacity of broilers’ liver and heart tissues. The tissues were collected at 42 d, then FRAP, SOD and MDA assays were performed for the liver (A), SOD, MDA and LDH assays were conducted for the heart. (B) Data are representative of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. Con, control; Dex, dexamethasone; CUR, curcumin; Se, selenium.
For the heart tissues (Figure 4B), compared with the control group, the activity of SOD in the Dex group was significantly decreased, while the MDA and LDH levels were significantly increased (p < 0.001). CUR and Se alone improved these indicators to a certain extent (p < 0.05), while combined treatment significantly relieved the damage caused by Dex compared with CUR or Se alone (p < 0.05). These results demonstrated that CUR and Se had synergistic antioxidant effects in vivo.
The antioxidant mechanism of CUR and Se relates to IGF-1/PI3K/AKT/mTOR pathway
To explore the underlying mechanism involved in the synergistical antioxidant effect of CUR and Se, a transcriptome sequencing assay of liver tissues were conducted (Figure 5). A total of 30,108 genes were detected, there were 582 genes up-regulated and 396 genes down-regulated in the Dex group compared with the control group, while when compared with the combination group, 293 genes were up-regulated and 92 genes were down-regulated in the Dex group (Figure 5A). Then, one hundred differentially expressed genes between the Dex group and the combination group were analyzed (Figure 5B). Meanwhile, the GO gene enrichment analysis revealed that the altered genes were enriched in multiple signaling pathways related to oxidative stress (Figure 5C). Among these differentially expressed genes, we noticed that IGF-1 was significantly down-regulated in the Dex group compared with that in the control group (p < 0.01), and up-regulated in the combination group compared with that in the Dex group (p < 0.001), as shown in Figure 5D.
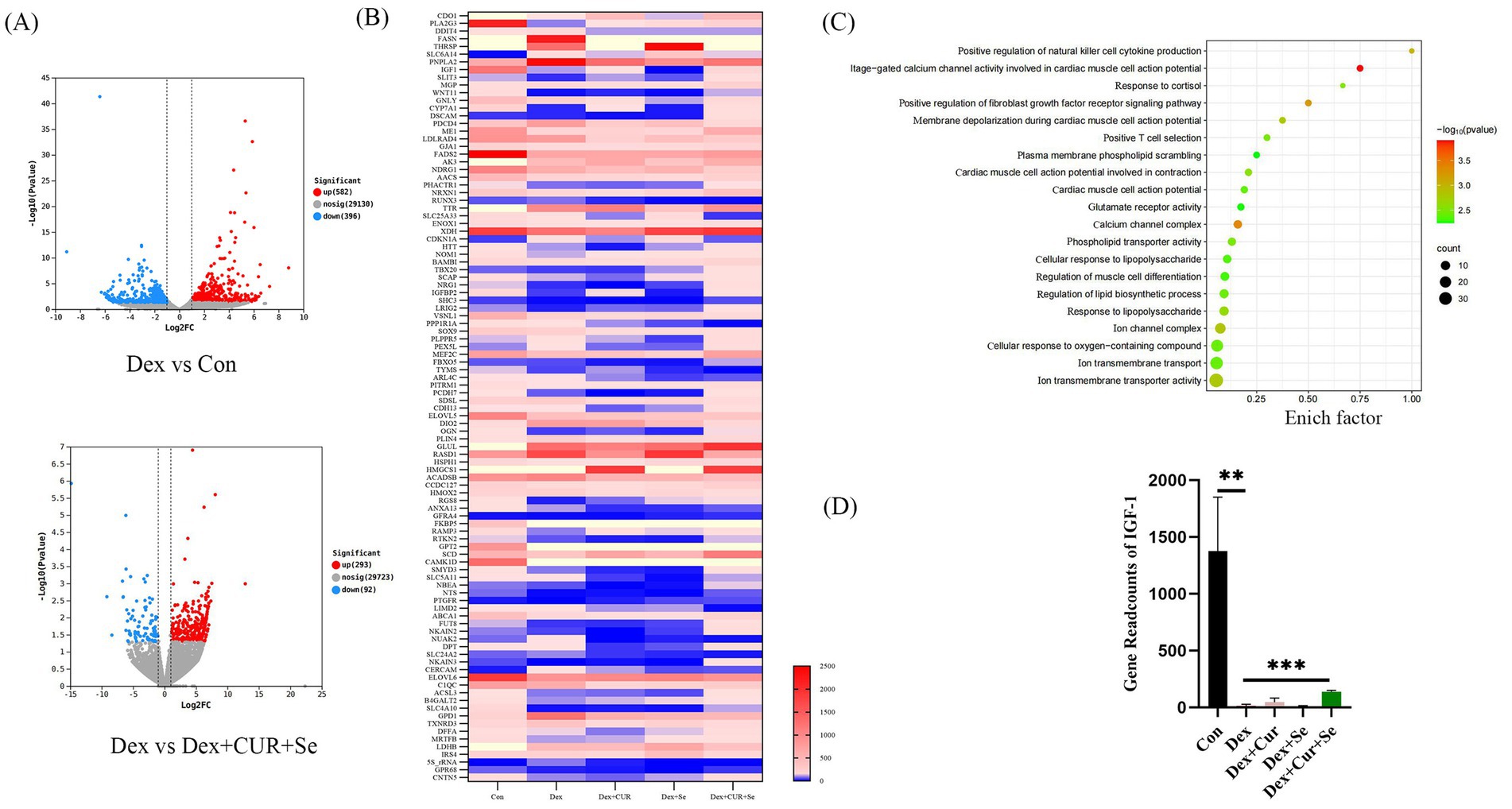
Figure 5. CUR and Se regulate signaling pathways associated with oxidative stress in broilers. (A) Gene expression differences among Control group, Dex group, Dex + CUR + Se group. (B) One hundred differentially expressed genes between the Dex group and the Dex + CUR + Se group. (C) The GO gene enrichment analysis results. (D) The gene readcounts of IGF-1 among groups. Data are representative of three independent experiments. **p < 0.01; ***p < 0.001. Con, control; Dex, dexamethasone; CUR, curcumin; Se, selenium.
Next, to further confirm the transcriptome sequencing results, the expression of IGF-1 related proteins in liver and heart tissues were detected by IHC. Consequently, the hepatic expression levels of IGF-1, PI3K-p110β, p-AKT, and p-mTOR were highest in the control group, followed by the combination group, with the Dex group exhibiting the lowest levels (Figure 6). In cardiac tissues, a comparable trend of alterations was observed (Figure 7).
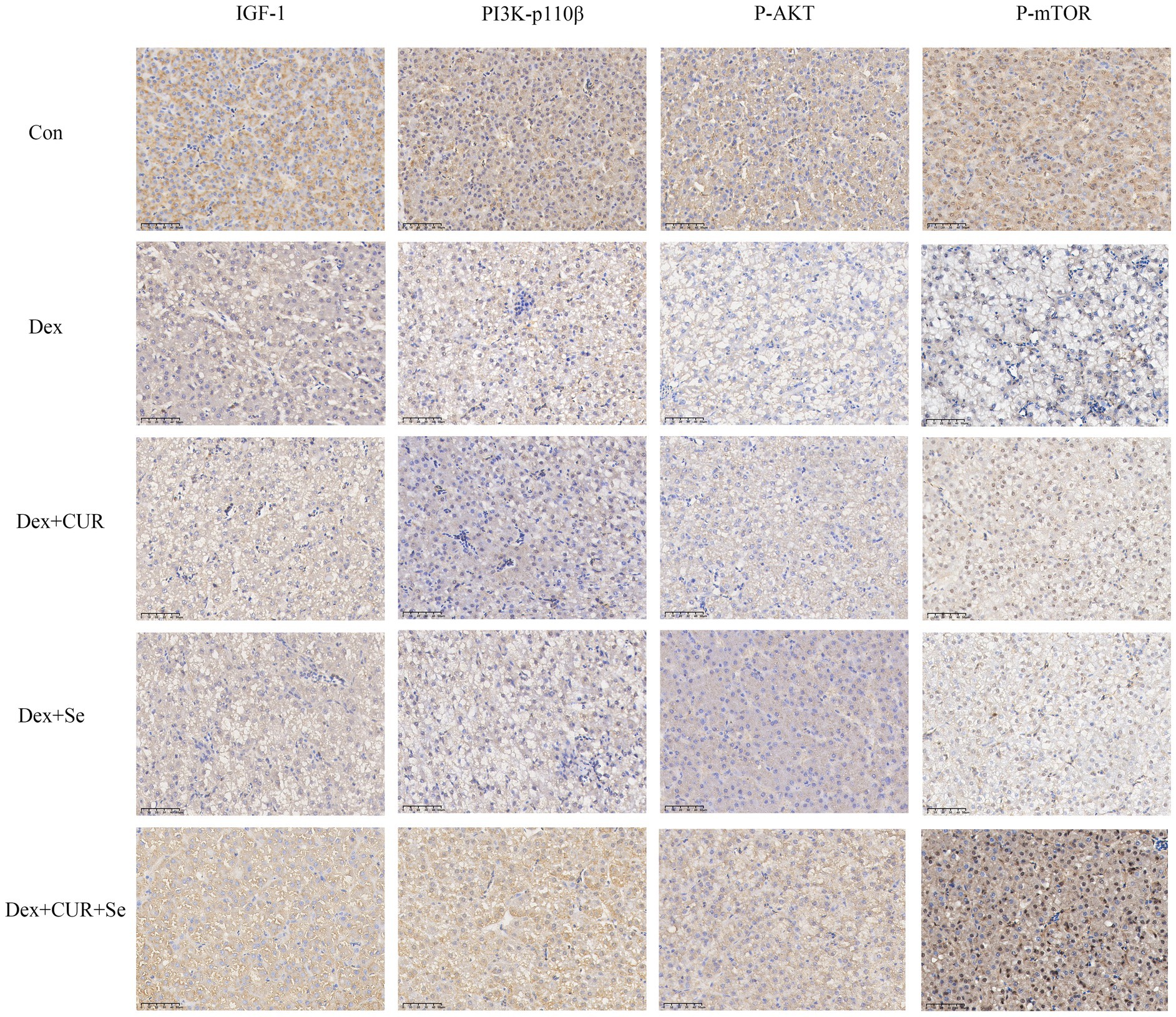
Figure 6. The IHC results indicated that IGF-1/PI3K/AKT/mTOR signaling pathway in broilers’ liver is activated by CUR and Se. Bar: 50 μm. Data are representative of six independent experiments. Con, control; Dex, dexamethasone; CUR, curcumin; Se, selenium.
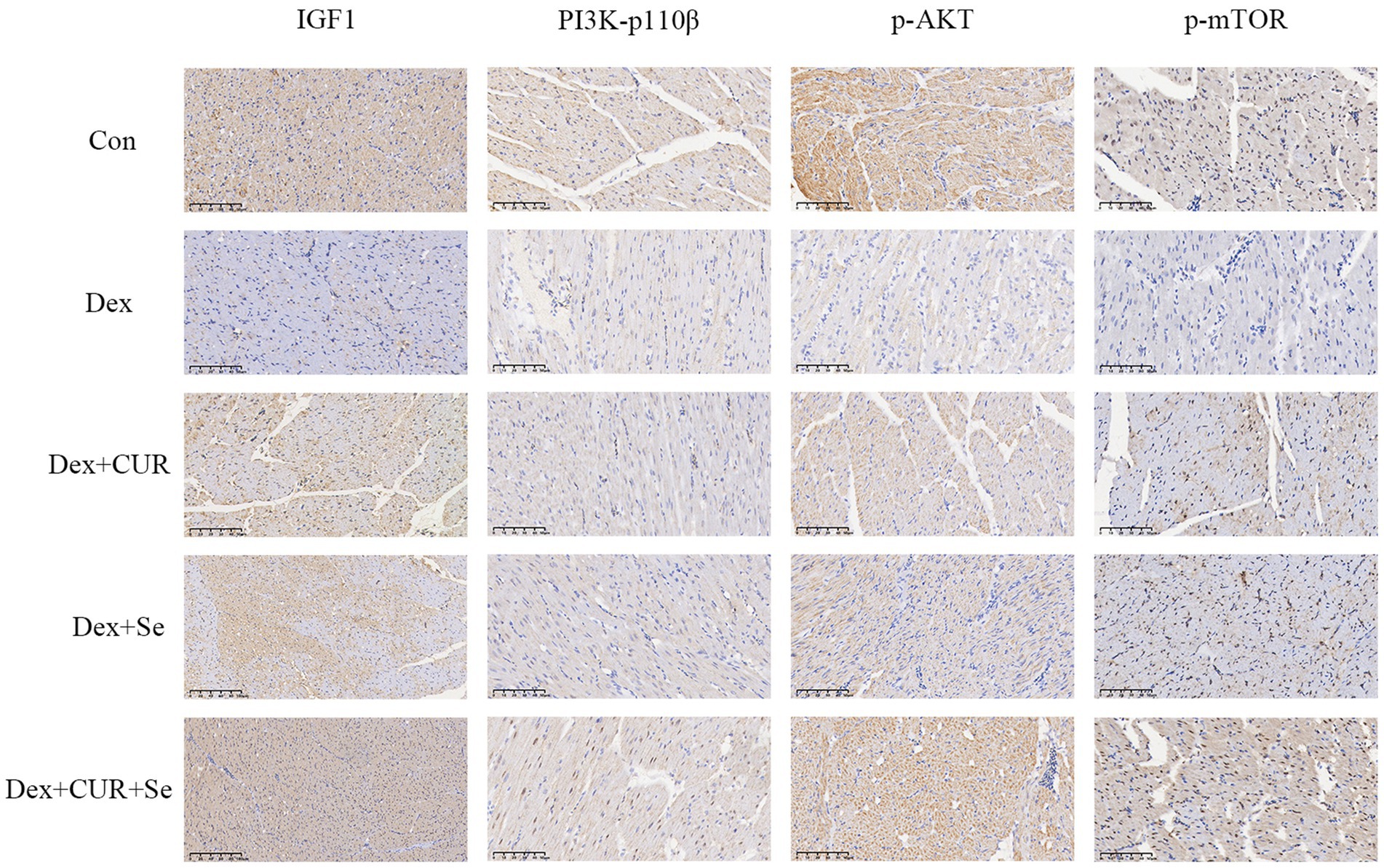
Figure 7. The IHC results indicated that IGF-1/PI3K/AKT/mTOR signaling pathway in broilers’ heart is activated by CUR and Se. Bar: 50 μm. Data are representative of six independent experiments. Con, control; Dex, dexamethasone; CUR, curcumin; Se, selenium.
Discussion
Oxidative stress is closely associated with a variety of diseases in poultry, such as ascites syndrome in broilers (51), temperature stress (52), and egg-laying stress in laying hens (53), etc. The disease process is accompanied by oxidative stress, and the state of oxidative stress can further promote the progression of the disease (54). In the present study, H2O2 and Dex were selected as the inducer of oxidative stress, which is consistent with some previous studies (47, 48, 55). However, other studies have employed different types of agents to establish oxidative stress models in poultry, such as Diquat (56) or Decabromodiphenylether (57). These various inducers may simulate the oxidative stress status of chickens under real farming conditions in different aspects. Future studies could comprehensively evaluate the synergistic antioxidant effects of CUR and Se by employing different methods of inducing oxidative stress.
A previous study has showed that dietary supplementation of 50, 100 and 150 mg/kg curcumin can alleviate Diquat-induced oxidative stress, and the recommended dosage of curcumin in diets of broilers is in the range of 100 ~ 150 mg/kg (56). Another study applied a combination of vitamin E and organic selenium in the basal diet of broilers, the drugs alleviated the negative effects of heat stress by improving antioxidant status, regulating cytokine response, and altering the ileal flora and its function (58). In the present study, a therapeutic strategy combining CUR and Se was proposed to counteract oxidative stress in poultry, and the synergistic effects of these two reagents were discovered. Then, antioxidant assays have confirmed that the combination of CUR and Se exerts a stronger antioxidant effect compared to their individual use in vitro and in vivo.
One of the advantages of drug combination therapy is the potential to reduce the dosage of individual drugs, thereby decreasing the toxicity associated with their use. Numerous studies have demonstrated that the biological activity and toxic dose range of selenium are relatively narrow (59–61). Therefore, the antioxidant function of Se may be limited by its dosage application. In this study, a drug combination approach was employed to enhance the antioxidant properties of CUR or Se while avoiding potential toxicity. Additionally, some studies have indicated that the safety profile of organic Se or nano Se is superior to that of inorganic Se (62, 63). Hence, in future studies, the use of inorganic Se or nano Se in combination with CUR may lead to more favorable outcomes.
Oxidative stress is considered to be a key mediator leading to liver injury and progression of pathological liver disease (64). In the present study, the liver transcriptome sequencing results showed that the underlying antioxidant mechanism of CUR and Se was related to multiple antioxidant-associated pathways. The activation of IGF-1 can activate PI3K, AKT and mTOR, thereby activating the antioxidant function of cells to resist oxidative stress and reduce cell apoptosis (43–45). Studies have shown that IGF-1/PI3K/AKT/mTOR signaling pathway is the main pathway for ROS to mediate oxidative stress, regulating oxidative stress response in an active form (65, 66). The results of this study also confirmed that CUR and Se significantly enhanced the expression of IGF-1/PI3K/p-AKT/p-mTOR in liver and heart tissue. These results suggested that the underlying antioxidant mechanism of CUR and Se was related to IGF-1/PI3K/AKT/mTOR pathway. Nevertheless, the current study has only investigated the protein expression at the IHC level. Future research should extend to include Western blotting analysis and gene knockout models to provide a more comprehensive understanding of the results.
In conclusion, this study demonstrated that CUR and Se have a synergistic effect in combating oxidative stress in broilers, and their antioxidant mechanism is associated with the IGF-1/PI3K/AKT/mTOR signaling pathway. Further research in this area is warranted.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA1192045.
Ethics statement
The animal study was approved by Animal Ethics Committee of Fujian Agriculture and Forestry University (approval code: PZCASFAFU24029). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZH: Writing – original draft, Data curation, Investigation, Writing – review & editing. ZL: Writing – review & editing, Conceptualization, Project administration, Writing – original draft. YY: Conceptualization, Methodology, Software, Writing – original draft, Writing – review & editing. JW: Conceptualization, Software, Writing – review & editing. SZ: Methodology, Writing – review & editing. BZ: Methodology, Writing – review & editing. XH: Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China [grant numbers 32402954, 2024]; the Fujian Provincial Department of Education, Fujian, China [grant number JAT220073, 2022]; and the Fujian Provincial Department of Finance, Fujian, China [grant number 2130199, 2022].
Acknowledgments
The authors thank Analysis and Testing Center of Fujian Agriculture and Forestry University for their help in the experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fvets.2025.1650011.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1600466/full#supplementary-material
References
1. Forman, HJ, and Zhang, H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. (2021) 20:689–709. doi: 10.1038/s41573-021-00233-1
2. Mao, Y, Kong, X, Liang, Z, Yang, C, Wang, S, Fan, H, et al. Viola yedoensis Makino alleviates heat stress-induced inflammation, oxidative stress, and cell apoptosis in the spleen and thymus of broilers. J Ethnopharmacol. (2024) 319:117350. doi: 10.1016/j.jep.2023.117350
3. Yang, F, Pei, R, Zhang, Z, Liao, J, Yu, W, Qiao, N, et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol in Vitro. (2019) 54:310–6. doi: 10.1016/j.tiv.2018.10.017
4. Yang, Y, Wang, X, Zhang, H, Li, J, Chen, J, Yu, M, et al. Oxidative stress and ferroptosis involved in 2-ethylhexyl diphenyl phosphate-induced hepatotoxicity in chicken. Chem Biol Interact. (2022) 368:110216. doi: 10.1016/j.cbi.2022.110216
5. Chen, Z, Xing, T, Li, J, Zhang, L, Jiang, Y, and Gao, F. Oxidative stress induced by hydrogen peroxide promotes glycolysis by activating CaMKK/LKB1/AMPK pathway in broiler breast muscle. Poult Sci. (2022) 101:101681. doi: 10.1016/j.psj.2021.101681
6. Estévez, M. Oxidative damage to poultry: from farm to fork. Poult Sci. (2015) 94:1368–78. doi: 10.3382/ps/pev094
7. Wang, J, Jia, R, Gong, H, Celi, P, Zhuo, Y, Ding, X, et al. The effect of oxidative stress on the chicken ovary: involvement of microbiota and melatonin interventions. Antioxidants (Basel). (2021) 10:1422. doi: 10.3390/antiox10091422
8. Wasti, S, Sah, N, and Mishra, B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals (Basel). (2020) 10:1266. doi: 10.3390/ani10081266
9. Jing, J, Zeng, H, Shao, Q, Tang, J, Wang, L, Jia, G, et al. Selenomethionine alleviates environmental heat stress induced hepatic lipid accumulation and glycogen infiltration of broilers via maintaining mitochondrial and endoplasmic reticulum homeostasis. Redox Biol. (2023) 67:102912. doi: 10.1016/j.redox.2023.102912
10. Mujahid, A, and Furuse, M. Oxidative damage in different tissues of neonatal chicks exposed to low environmental temperature. Comp Biochem Physiol A Mol Integr Physiol. (2009) 152:604–8. doi: 10.1016/j.cbpa.2009.01.011
11. Nasr, MAF, Alkhedaide, AQ, Ramadan, AAI, Hafez, ASE, and Hussein, MA. Potential impact of stocking density on growth, carcass traits, indicators of biochemical and oxidative stress and meat quality of different broiler breeds. Poult Sci. (2021) 100:101442. doi: 10.1016/j.psj.2021.101442
12. Pan, L, Ma, XK, Zhao, PF, Shang, QH, Long, SF, Wu, Y, et al. Forsythia suspensa extract attenuates breast muscle oxidative injury induced by transport stress in broilers. Poult Sci. (2018) 97:1554–63. doi: 10.3382/ps/pey012
13. Meteyake, HT, Collin, A, Bilalissi, A, Dassidi, N, Assion, MEP, and Tona, K. Naked neck gene and intermittent thermal manipulations during embryogenesis improve posthatch performance and thermotolerance in slow-growing chickens under tropical climates. Poult Sci. (2023) 102:102912. doi: 10.1016/j.psj.2023.102912
14. Onagbesan, OM, Uyanga, VA, Oso, O, Tona, K, and Oke, OE. Alleviating heat stress effects in poultry: updates on methods and mechanisms of actions. Front Vet Sci. (2023) 10:1255520. doi: 10.3389/fvets.2023.1255520
15. Oni, AI, Abiona, JA, Fafiolu, AO, and Oke, OE. Early-age thermal manipulation and supplemental antioxidants on physiological, biochemical and productive performance of broiler chickens in hot-tropical environments. Stress. (2024) 27:2319803. doi: 10.1080/10253890.2024.2319803
16. Zeitz, JO, Fleischmann, A, Ehbrecht, T, Most, E, Friedrichs, S, Whelan, R, et al. Effects of supplementation of DL-methionine on tissue and plasma antioxidant status during heat-induced oxidative stress in broilers. Poult Sci. (2020) 99:6837–47. doi: 10.1016/j.psj.2020.08.082
17. van Hasselt, JGC, and Iyengar, R. Systems pharmacology: defining the interactions of drug combinations. Annu Rev Pharmacol Toxicol. (2019) 59:21–40. doi: 10.1146/annurev-pharmtox-010818-021511
18. Chou, TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. (2006) 58:621–81. doi: 10.1124/pr.58.3.10
19. Marton, LT, Pescinini, ESLM, Camargo, MEC, Barbalho, SM, Haber, J, Sinatora, RV, et al. The effects of curcumin on diabetes mellitus: a systematic review. Front Endocrinol (Lausanne). (2021) 12:669448. doi: 10.3389/fendo.2021.669448
20. Li, S, Liu, R, Xia, S, Wei, G, Ishfaq, M, Zhang, Y, et al. Protective role of curcumin on aflatoxin B1-induced TLR4/RIPK pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol Environ Saf. (2022) 233:113319. doi: 10.1016/j.ecoenv.2022.113319
21. Liu, M, Lu, Y, Gao, P, Xie, X, Li, D, Yu, D, et al. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens. Poult Sci. (2020) 99:2196–202. doi: 10.1016/j.psj.2019.12.001
22. Ruan, D, Wu, S, Fouad, AM, Zhu, Y, Huang, W, Chen, Z, et al. Curcumin alleviates LPS-induced intestinal homeostatic imbalance through reshaping gut microbiota structure and regulating group 3 innate lymphoid cells in chickens. Food Funct. (2022) 13:11811–24. doi: 10.1039/D2FO02598A
23. Xie, Z, Shen, G, Wang, Y, and Wu, C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult Sci. (2019) 98:422–9. doi: 10.3382/ps/pey315
24. Kövesi, B, Worlanyo, AP, Kulcsár, S, Ancsin, Z, Erdélyi, M, Zándoki, E, et al. Curcumin mitigates ochratoxin A-induced oxidative stress and alters gene expression in broiler chicken liver and kidney. Acta Vet Hung. (2024) 72:41–50. doi: 10.1556/004.2024.01016
25. Wu, J, Ibtisham, F, Niu, YF, Wang, Z, Li, GH, Zhao, Y, et al. Curcumin inhibits heat-induced oxidative stress by activating the MAPK-Nrf2/ARE signaling pathway in chicken fibroblasts cells. J Therm Biol. (2019) 79:112–9. doi: 10.1016/j.jtherbio.2018.12.004
26. Hegde, M, Girisa, S, Bharathwaj Chetty, B, Vishwa, R, and Kunnumakkara, AB. Curcumin formulations for better bioavailability: what we learned from clinical trials thus far? ACS Omega. (2023) 8:10713–46. doi: 10.1021/acsomega.2c07326
27. Jafari, Y, Sabahi, H, and Rahaie, M. Stability and loading properties of curcumin encapsulated in Chlorella vulgaris. Food Chem. (2016) 211:700–6. doi: 10.1016/j.foodchem.2016.05.115
28. Zhang, MJ, Shi, M, Yu, Y, Wang, H, Ou, R, and Ge, RS. CP41, a novel curcumin analogue, induces apoptosis in endometrial cancer cells by activating the H3F3A/proteasome-MAPK signaling pathway and enhancing oxidative stress. Life Sci. (2024) 338:122406. doi: 10.1016/j.lfs.2023.122406
29. Hasan Khudhair, D, Al-Gareeb, AI, Al-Kuraishy, HM, El-Kadem, AH, Elekhnawy, E, Negm, WA, et al. Combination of vitamin C and curcumin safeguards against methotrexate-induced acute liver injury in mice by synergistic antioxidant effects. Front Med (Lausanne). (2022) 9:866343. doi: 10.3389/fmed.2022.866343
30. Ahmad, H, Tian, J, Wang, J, Khan, MA, Wang, Y, Zhang, L, et al. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J Agric Food Chem. (2012) 60:7111–20. doi: 10.1021/jf3017207
31. Kiełczykowska, M, Kocot, J, Paździor, M, and Musik, I. Selenium - a fascinating antioxidant of protective properties. Adv Clin Exp Med. (2018) 27:245–55. doi: 10.17219/acem/67222
32. Shen, Y, Huang, H, Wang, Y, Yang, R, and Ke, X. Antioxidant effects of se-glutathione peroxidase in alcoholic liver disease. J Trace Elem Med Biol. (2022) 74:127048. doi: 10.1016/j.jtemb.2022.127048
33. Sun, LH, Huang, JQ, Deng, J, and Lei, XG. Avian selenogenome: response to dietary se and vitamin E deficiency and supplementation. Poult Sci. (2019) 98:4247–54. doi: 10.3382/ps/pey408
34. Wang, F, Shu, G, Peng, X, Fang, J, Chen, K, Cui, H, et al. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int J Environ Res Public Health. (2013) 10:2834–44. doi: 10.3390/ijerph10072834
35. Kumbhar, S, Khan, AZ, Parveen, F, Nizamani, ZA, Siyal, FA, El-Hack, MEA, et al. Impacts of selenium and vitamin E supplementation on mRNA of heat shock proteins, selenoproteins and antioxidants in broilers exposed to high temperature. AMB Express. (2018) 8:112. doi: 10.1186/s13568-018-0641-0
36. Li, W, Yin, X, Yan, Y, Liu, C, and Li, G. Kurarinone attenuates hydrogen peroxide-induced oxidative stress and apoptosis through activating the PI3K/Akt signaling by upregulating IGF1 expression in human ovarian granulosa cells. Environ Toxicol. (2023) 38:28–38. doi: 10.1002/tox.23659
37. Orellana, AM, Mazucanti, CH, Dos Anjos, LP, de Sá Lima, L, Kawamoto, EM, and Scavone, C. Klotho increases antioxidant defenses in astrocytes and ubiquitin-proteasome activity in neurons. Sci Rep. (2023) 13:15080. doi: 10.1038/s41598-023-41166-6
38. Zhang, SJ, Zhang, YF, Bai, XH, Zhou, MQ, Zhang, ZY, Zhang, SX, et al. Integrated network pharmacology analysis and experimental validation to elucidate the mechanism of Acteoside in treating diabetic kidney disease. Drug Des Devel Ther. (2024) 18:1439–57. doi: 10.2147/DDDT.S445254
39. Mandal, AK, Leask, MP, Sumpter, NA, Choi, HK, Merriman, TR, and Mount, DB. Genetic and physiological effects of insulin-like growth Factor-1 (IGF-1) on human urate homeostasis. J Am Soc Nephrol. (2023) 34:451–66. doi: 10.1681/ASN.0000000000000054
40. Tong, C, Wu, Y, Zhang, L, and Yu, Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: association with PI3K signaling pathway. Front Endocrinol (Lausanne). (2022) 13:1091147. doi: 10.3389/fendo.2022.1091147
41. Hu, Q, Zheng, J, Xu, XN, Gu, C, and Li, W. Uranium induces kidney cells apoptosis via reactive oxygen species generation, endoplasmic reticulum stress and inhibition of PI3K/AKT/mTOR signaling in culture. Environ Toxicol. (2022) 37:899–909. doi: 10.1002/tox.23453
42. Liu, Y, Gong, S, Li, K, Wu, G, Zheng, X, Zheng, J, et al. Coptisine protects against hyperuricemic nephropathy through alleviating inflammation, oxidative stress and mitochondrial apoptosis via PI3K/Akt signaling pathway. Biomed Pharmacother. (2022) 156:113941. doi: 10.1016/j.biopha.2022.113941
43. Feng, L, Li, B, Xi, Y, Cai, M, and Tian, Z. Aerobic exercise and resistance exercise alleviate skeletal muscle atrophy through IGF-1/IGF-1R-PI3K/Akt pathway in mice with myocardial infarction. Am J Phys Cell Phys. (2022) 322:C164–c176. doi: 10.1152/ajpcell.00344.2021
44. Ma, RJ, Tan, YQ, and Zhou, G. Aberrant IGF1-PI3K/AKT/MTOR signaling pathway regulates the local immunity of oral lichen planus. Immunobiology. (2019) 224:455–61. doi: 10.1016/j.imbio.2019.01.004
45. Yoshida, T, and Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells. (2020) 9:1970. doi: 10.3390/cells9091970
46. Fathi, M, Saeedyan, S, and Kaoosi, M. Gamma-amino butyric acid (GABA) supplementation alleviates dexamethasone treatment-induced oxidative stress and inflammation response in broiler chickens. Stress. (2023) 26:2185861. doi: 10.1080/10253890.2023.2185861
47. Gao, J, Lin, H, Wang, XJ, Song, ZG, and Jiao, HC. Vitamin E supplementation alleviates the oxidative stress induced by dexamethasone treatment and improves meat quality in broiler chickens. Poult Sci. (2010) 89:318–27. doi: 10.3382/ps.2009-00216
48. Liu, H, Li, X, Shi, S, Zhou, Y, Zhang, K, Wang, Y, et al. Chlorogenic acid improves growth performance and intestinal health through autophagy-mediated nuclear factor erythroid 2-related factor 2 pathway in oxidatively stressed broilers induced by dexamethasone. Poult Sci. (2022) 101:102036. doi: 10.1016/j.psj.2022.102036
49. Min, Y, Sun, T, Niu, Z, and Liu, F. Vitamin C and vitamin E supplementation alleviates oxidative stress induced by dexamethasone and improves fertility of breeder roosters. Anim Reprod Sci. (2016) 171:1–6. doi: 10.1016/j.anireprosci.2016.04.005
50. Zhao, J, Zhao, F, Li, X, Yuan, J, Zhang, K, Liu, H, et al. Multi-omics reveals the mechanisms underlying Lactiplantibacillus plantarum P8-mediated attenuation of oxidative stress in broilers challenged with dexamethasone. Anim Nutr. (2023) 14:281–302. doi: 10.1016/j.aninu.2023.06.002
51. Díaz-Cruz, A, Nava, C, Villanueva, R, Serret, M, Guinzberg, R, and Piña, E. Hepatic and cardiac oxidative stress and other metabolic changes in broilers with the ascites syndrome. Poult Sci. (1996) 75:900–3.
52. Giannenas, I, Sakkas, P, Papadopoulos, GA, Mitsopoulos, I, Stylianaki, I, Dokou, S, et al. The association of Curcuma and Scutellaria plant extracts improves laying hen thermal tolerance and egg oxidative stability and quality under heat stress conditions. Front Vet Sci. (2022) 9:957847. doi: 10.3389/fvets.2022.957847
53. Ding, X, Cai, C, Jia, R, Bai, S, Zeng, Q, Mao, X, et al. Dietary resveratrol improved production performance, egg quality, and intestinal health of laying hens under oxidative stress. Poult Sci. (2022) 101:101886. doi: 10.1016/j.psj.2022.101886
54. Oke, OE, Akosile, OA, Oni, AI, Opowoye, IO, Ishola, CA, Adebiyi, JO, et al. Oxidative stress in poultry production. Poult Sci. (2024) 103:104003. doi: 10.1016/j.psj.2024.104003
55. El-Senousey, HK, Chen, B, Wang, JY, Atta, AM, Mohamed, FR, and Nie, QH. Effects of dietary vitamin C, vitamin E, and alpha-lipoic acid supplementation on the antioxidant defense system and immune-related gene expression in broilers exposed to oxidative stress by dexamethasone. Poult Sci. (2018) 97:30–8. doi: 10.3382/ps/pex298
56. Wu, F, Yang, X, Wang, F, Liu, Y, Han, S, Liu, S, et al. Dietary curcumin supplementation alleviates diquat-induced oxidative stress in the liver of broilers. Poult Sci. (2023) 102:103132. doi: 10.1016/j.psj.2023.103132
57. Dong, B, Jiang, Y, Shi, B, Zhang, Z, and Zhang, Z. Selenomethionine alleviates decabromodiphenyl ether-induced oxidative stress and ferroptosis via the NRF2/GPX4 pathway in the chicken brain. J Hazard Mater. (2024) 465:133307. doi: 10.1016/j.jhazmat.2023.133307
58. Calik, A, Emami, NK, Schyns, G, White, MB, Walsh, MC, Romero, LF, et al. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, part II: oxidative stress, immune response, gut integrity, and intestinal microbiota. Poult Sci. (2022) 101:101858. doi: 10.1016/j.psj.2022.101858
59. Dey, S, and Raychaudhuri, SS. Selenium biofortification improves bioactive composition and antioxidant status in Plantago ovata Forsk., a medicinal plant. Genes Environ. (2023) 45:38. doi: 10.1186/s41021-023-00293-2
60. Hadrup, N, and Ravn-Haren, G. Acute human toxicity and mortality after selenium ingestion: a review. J Trace Elem Med Biol. (2020) 58:126435. doi: 10.1016/j.jtemb.2019.126435
61. Zhou, J, Liu, Y, Hu, Y, Zhang, D, Xu, W, Chen, L, et al. Selenium nanoparticles synergistically stabilized by starch microgel and EGCG: synthesis, characterization, and bioactivity. Food Secur. (2022) 12:13. doi: 10.3390/foods12010013
62. Hadrup, N, and Ravn-Haren, G. Toxicity of repeated oral intake of organic selenium, inorganic selenium, and selenium nanoparticles: a review. J Trace Elem Med Biol. (2023) 79:127235. doi: 10.1016/j.jtemb.2023.127235
63. Zainudin, NN, Hemly, NIM, Muhammad, AI, Nayan, N, and Samsudin, AA. Effects of supplementing organic-and inorganic-based selenium with vitamin E on intestinal histomorphology, caecal bacterial proliferation, and short-chain fatty acid profile in layer hens. Trop Anim Health Prod. (2023) 55:90. doi: 10.1007/s11250-023-03482-x
64. Zhong, G, Li, Y, Li, L, Huo, Y, Zhang, W, Li, T, et al. Mitochondrial miR-12294-5p regulated copper-induced mitochondrial oxidative stress and mitochondrial quality control imbalance by targeted inhibition of CISD1 in chicken livers. J Hazard Mater. (2023) 458:131908. doi: 10.1016/j.jhazmat.2023.131908
65. Liu, S, Meng, F, Zhang, D, Shi, D, Zhou, J, Guo, S, et al. Lonicera caerulea berry polyphenols extract alleviates exercise fatigue in mice by reducing oxidative stress, inflammation, skeletal muscle cell apoptosis, and by increasing cell proliferation. Front Nutr. (2022) 9:853225. doi: 10.3389/fnut.2022.853225
Keywords: oxidative stress, curcumin, selenium, drug combination, IGF-1/PI3K/AKT/mTOR pathway
Citation: He Z, Lin Z, Yan Y, Wang J, Zhang S, Zheng B and Huang X (2025) Curcumin and selenium synergistically mitigate oxidative stress in white-feathered broilers. Front. Vet. Sci. 12:1600466. doi: 10.3389/fvets.2025.1600466
Edited by:
Monsuru Oladimeji Abioja, Federal University of Agriculture, NigeriaReviewed by:
Bukola Majekodunmi, Federal University of Agriculture, NigeriaOlalekan Shittu, Federal University of Agriculture, Nigeria
Copyright © 2025 He, Lin, Yan, Wang, Zhang, Zheng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Huang, aHVhbmd4aWFvaG9uZ0BmYWZ1LmVkdS5jbg==
†These authors have contributed equally to this work
 Zixuan He
Zixuan He Zhaoyan Lin
Zhaoyan Lin Ye Yan1,2
Ye Yan1,2