- 1Center for Yunnan Plateau Biological Resources Protection and Utilization, College of Biology and Food Engineering, Qujing Normal University, Qujing, Yunnan, China
- 2School of Medical, Molecular and Forensic Sciences, Murdoch University, Perth, WA, Australia
- 3Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, Thailand
- 4College of Biodiversity Conservation, Southwest Forestry University, Kunming, China
The family Ganodermataceae encompasses several genera, including the widely studied Ganoderma, which is prominent in traditional medicine for its therapeutic properties. Species within this family, particularly Ganoderma lucidum, have been valued for centuries in regions such as China, Korea, and Japan for enhancing vitality, longevity, and overall health. However, the taxonomy of Ganodermataceae remains complex, with ongoing debates about species identification and classification. Members of this family are globally distributed, with the Lower Mekong Basin—comprising Laos, Thailand, Cambodia, and Vietnam—offering optimal conditions for their growth due to its warm, humid climate. In the Lower Mekong Basin, the species of Ganodermataceae are significant for their medicinal applications in treating conditions such as bronchitis, hepatitis, diabetes, and cancer. They also hold significant economic value, being used in products like teas, dietary supplements, and cosmetics. Ganoderma lucidum is particularly notable as a high-value market product in this region. Recent research has revealed a rich diversity of Ganodermataceae species in the region, highlighting their ecological roles, medicinal properties, and importance in plant pathology, particularly in addressing diseases in crops such as oil palm. These findings underscore the need for further research into the taxonomy, ecological functions, and potential applications of Ganodermataceae species. Advancing our understanding will support sustainable utilization, conservation efforts, and the maximization of their medicinal and commercial benefits.
1 Introduction
The Ganodermataceae family, encompassing the genus Ganoderma, has long been recognized for its diverse ecological, medicinal, and economic significance (Galappaththi et al., 2024; Karunarathna et al., 2024a). Ganoderma species, particularly G. lucidum, have played a central role in traditional medicine across Asia for centuries, where they are highly esteemed for their purported health benefits, including promoting vitality, longevity, and overall well-being (Karunarathna et al., 2024b; Klaus and Wan, 2024; Wu et al., 2024a). These fungi have been integral to various therapeutic practices in countries such as China, Korea, and Japan, where their use is deeply rooted in cultural and medicinal traditions (Chen et al., 2024; Zhong et al., 2024). The association of Ganoderma with health-enhancing properties, coupled with its economic value, has contributed to its widespread use in modern-day products such as dietary supplements, teas, and cosmetics (Karunarathna et al., 2024c; Wu et al., 2024b; Zheng et al., 2024). However, despite the long history of use and growing commercial importance of Ganoderma, its taxonomy remains complex and evolving, with ongoing debates surrounding species classification, identification, and ecological roles.
Ganoderma species are found worldwide, thriving predominantly in tropical and subtropical climates, where their growth is facilitated by warm and humid conditions (Papp, 2019; Konara et al., 2024; Karunarathna et al., 2024d; Wei et al., 2024). These fungi are saprophytic or parasitic, often growing on decaying wood or living trees, and play a vital role in the decomposition of lignin and cellulose in forest ecosystems (Konara et al., 2022; Asad et al., 2024; Li et al., 2024). While the genus is distributed across a wide geographic range, the Lower Mekong Basin, which encompasses Laos, Thailand, Cambodia, and Vietnam, is a particularly notable region for the growth of Ganoderma species (Hapuarachchi et al., 2019a; Luangharn et al., 2019, 2021; Duong et al., 2022a; Pungpa et al., 2023, Figure 1). The warm, humid climate of the Lower Mekong Basin provides optimal conditions for the proliferation of these fungi, making it a key area for studying their diversity, ecological functions, and potential applications (Nguyen et al., 2023a; Wannasawang et al., 2023; Siriarchawatana et al., 2024).
The medicinal properties of Ganoderma are well documented in scientific literature, with numerous studies confirming its effectiveness in treating a range of ailments, including respiratory conditions such as bronchitis, liver diseases like hepatitis, metabolic disorders like diabetes, and even cancer (Chen et al., 2024; Lau et al., 2024; Rašeta et al., 2024). Bioactive compounds such as triterpenoids, polysaccharides, and peptidoglycans, which are found in various Ganoderma species, have been identified as responsible for many of these therapeutic effects (Rode et al., 2024; Xia et al., 2024). As a result, Ganoderma has become a focal point of research in the fields of pharmacology and natural product development (Azi et al., 2024; Wu et al., 2024b). The medicinal potential of the genus has led to the commercialization of Ganoderma-based products, which are widely consumed in the form of supplements, teas, and even incorporated into cosmetics (Karunarathna et al., 2024c; Thakur et al., 2024).
The economic significance of Ganoderma is particularly evident in the Lower Mekong Basin, where G. lucidum commands a high market value (Mortimer et al., 2014; Hapuarachchi et al., 2018a; Sopov et al., 2022; Nguyen et al., 2023a). In recent years, the demand for Ganoderma-derived products has seen a marked increase, driven by growing consumer interest in natural health solutions (Seethapathy et al., 2023; Ganoderma-Derived Products Market Overview, 2023; Ganoderma Market Trends and Growth Factors, 2024). This commercialization has spurred research into the cultivation, quality control, and standardization of Ganoderma products to meet market demand (Zhang et al., 2023; Wu et al., 2024b). Recent studies in the Lower Mekong Basin have emphasized the remarkable diversity of Ganoderma species and their ecological and medicinal importance (Nghien et al., 2019; Prasopthum et al., 2022; Nguyen et al., 2022, 2023; Wannasawang et al., 2023; Siriarchawatana et al., 2024). Researchers have identified several key species within the region and explored their roles in plant pathology, particularly in relation to diseases affecting economically important crops such as oil palm (Hapuarachchi et al., 2019a; Luangharn et al., 2021; Karunarathna et al., 2024d). These studies have underscored the genus’s dual role as both a beneficial medicinal organism and a potential plant pathogen, further complicating the classification and management of Ganoderma species (Nguyen et al., 2021; Suwannarach et al., 2022; Viet Hung et al., 2022; Thuy et al., 2023; Petwattanapha et al., 2024). The findings highlight the need for continued research into the ecological dynamics, taxonomy, and therapeutic potential of Ganoderma species in this region.
Furthermore, the increasing commercialization of Ganoderma products in the Lower Mekong Basin presents both opportunities and challenges. While the sustainable harvesting and cultivation of Ganoderma could provide economic benefits to local communities, the rising demand for these fungi also raises concerns regarding overexploitation, conservation, and the need for sustainable management practices. As research continues to uncover the complex interactions between Ganoderma species and their environment, it becomes clear that a comprehensive understanding of their taxonomy, ecological functions, and medicinal properties is essential for their sustainable utilization and conservation.
This paper aims to provide a comprehensive overview of the current status of Ganoderma research and development in the Lower Mekong Basin, with a particular focus on the diversity of species, their ecological roles, and their medicinal and economic significance. By examining the ongoing research efforts and highlighting key findings, this work seeks to contribute to the broader understanding of Ganoderma and its potential for future applications in medicine, agriculture, and commercial industries. Ultimately, a deeper understanding of Ganoderma will be essential for maximizing its benefits, ensuring its sustainable use, and addressing the challenges associated with its conservation and commercialization in the Lower Mekong Basin.
2 Taxonomy
The family Ganodermataceae is one of the primary families of polypores, comprising 15 accepted genera: Amauroderma s.str. Y.F. Sun, D.H. Costa, and B.K. Cui, Amaurodermellus Costa-Rezende, Drechsler-Santos & Góes-Neto, Cristataspora Robledo & Costa-Rezende, Foraminispora Robledo, Costa-Rezende & Drechsler-Santos, Furtadoella B.K. Cui & Y.F. Sun, Furtadomyces Leonardo-Silva, Cotrim & Xavier-Santos, Ganoderma P. Karst., Haddowia Steyaert, Humphreya Steyaert, Magoderna Steyaert, Neoganoderma B.K. Cui & Y.F. Sun, Sanguinoderma Y.F. Sun, D.H. Costa & B.K. Cui, Sinoganoderma B.K. Cui, J.H. Xing & Y.F. Sun, Tomophagus Murrill, and Trachydermella B.K. Cui & Y.F. Sun (Costa-Rezende et al., 2020; Sun et al., 2022a; Leonardo-Silva et al., 2022). Most species within the family are classified under the genus Ganoderma.
Ganoderma P. Karst. (Ganodermataceae, Agaricomycetes) was first described by Karsten (1881), based on Polyporus lucidus (Curtis) Fr., to encompass species characterized as laccate and stipitate white rot fungi. It is the most prolific genus within the family, with 498 species recorded in Index Fungorum (2025) (http://www.indexfungorum.org/) and 542 in MycoBank (http://www.mycobank.org/), as of 16 March 2025. The genus Ganoderma is defined by its laccate or non-laccate basidiocarps, sessile to stipitate basidiomata, white to pale yellow margins, and red-brown, truncate, double-walled basidiospores. These spores feature an apical germinal pore, a thin, colorless external wall (exosporium), and brown to dark brown interwall pillars (endosporium) (Hapuarachchi et al., 2019a; Sun et al., 2022a; He et al., 2022, 2024). The double-walled basidiospores with interwall pillars are a key diagnostic feature for the genus (Wei et al., 2024). Species within this genus exhibit diverse characteristics, including variations in the shape and color of the fruiting body, host specificity, and geographical distribution, which aid in species identification (Li et al., 2023; Mardones et al., 2023; Cho et al., 2024; Ndeh et al., 2024). However, the species concept in Ganoderma remains neither widely agreed upon nor clearly defined due to significant morphological variability, even within the same species (Hapuarachchi et al., 2019b; Costa-Rezende et al., 2020; Pristas et al., 2023). Environmental factors, inter-hybridization, and morphological biases further complicate the identification of Ganoderma species (Luangharn et al., 2023). The genus also presents considerable taxonomic challenges, as its morphology varies significantly across environments, while microscopic features remain consistent (Cortina-Escribano et al., 2024). Nearly half of the recorded entries have been identified as synonyms, underscoring the complexity of Ganoderma taxonomy (He et al., 2022; Galappaththi et al., 2024). Nevertheless, robust molecular and phylogenetic analyses have confirmed the existence of 191 valid Ganoderma taxa (Sun et al., 2022b; Cabarroi-Hernández et al., 2023; He et al., 2024). Ganoderma species are also known for causing white rot in woody plants and are further valued for their medicinal properties (Kumari et al., 2024).
Amauroderma s.str. Y.F. Sun, D.H. Costa, and B.K. Cui, the second largest genus within Ganodermataceae, is distinguished by a di-trimitic hyphal system and an endospore wall with solid columnar to semi-reticulated ornamentation (Costa-Rezende et al., 2017; Sun et al., 2020). The genus is distributed mainly in the Neotropics and tropical or subtropical areas of Africa, Asia, and Oceania (Costa-Rezende et al., 2016; Peres et al., 2023). Amauroderma contains sterols, flavonoids, fatty acids, polysaccharides, and triterpenes, offering antioxidant, anti-inflammatory, neuroprotective, and antibacterial effects. Its bioactive components show therapeutic potential, especially for age-related diseases (Hapuarachchi et al., 2018b). Sanguinoderma (Blume and T. Nees) Y.F. Sun, D.H., Costa & B.K. Cui, one of the largest genera in Ganodermataceae after Ganoderma and Amauroderma, is distinguished by annual basidiomata with a corky to woody texture, a central or lateral stipe, and a nearly sessile pileus. The pileus, suborbicular to reniform, may be glabrous or tomentose, with concentric zones or radial furrows. Its pore surface turns blood red when bruised (Sun et al., 2020). Sanguinoderma includes species with notable medicinal and cultural significance. Most species in the genus inhabit soil and are primarily distributed across tropical and subtropical regions, including Africa, Asia, North America, Oceania, and South America (Sun et al., 2020, 2022a; Niu et al., 2024).
3 Diversity of Ganodermataceae in Lower Mekong Basin
The Lower Mekong River Basin is divided into four geographic regions, covering a catchment area of approximately 571,000 km². This extensive basin encompasses much of northeastern Thailand, nearly all of Lao PDR and Cambodia, and the southern tip of Vietnam (Mekong River Commission, accessed 16 March 2025). The Mekong River is essential for over 245 million people in the Lower Mekong Region, supporting agriculture, fisheries, food security, and economic stability. However, hydropower projects threaten the resources of the river by altering water flow, disrupting fish migration, and affecting agriculture, which could jeopardize the region’s economy (International Rivers, 2021). Mushroom growing is increasingly becoming popular in this region as a means to generate income, improve the quality of life for rural people, and promote sustainable development in local communities. Ganoderma lucidum has a 33.0–50.0 US$ market price per 1 kg as a commercial mushroom in Thailand (Tippayawong et al., 2011).
Ganoderma japonicum Imaz is recognized as a medicinal mushroom, known for its potential therapeutic benefits (Chandrasrikul et al., 1978). The disease of oil palm (Elaeis guineensis Jacq.) caused by G. boninense becomes the most important disease in Thailand, Indonesia, and Malaysia. Chandrasrikul et al. (1986) reported these groups of fungi had not been fully investigated and described in Thailand, and some species of Ganoderma and allied genera are considered possible parasites of living trees, found on dead trees, logs, and stumps and known to have some medicinal value. Furthermore, he studied a collection of seventeen specimens, described and identified by comparing morphological characters. They were G. lucidum (Leyss. ex Fr.) Karst. G. colossum (Fr.) Bres. G. applanatum (Fr.) Karst. and Amauroderma rugosum (Nees) Bose. Ganodermataceae family members: Amauroderma rugosum (Fr.) Torr. Ganoderma applanatum (Fr.) Pat., G. australe (Fr.) Pat., and G. lucidum (Fr.) Karst. were found in Northern Thailand (Hjortstam and Ryvarden, 1982).
The diversity of mushrooms surveyed in the Plant Genetic Protection Area of RSPG, Nampung Dam EGAT, Sakhon Nakhon Province in Thailand during the rainy season between July and August discovered Amauroderma rugosum (Blume et Nees) Bres., Ganoderma applanatum (Pers. Ex Wallr.) Pat., Ganoderma chiungchungense X.L.Wu Ganoderma dahlii (Henn.) Aoshima, and G. lucidum (Leys.ex Fr.) Karst (Pitakpong et al., 2013). Ganoderma subresinosum Fr. and two unidentified Ganoderma species were reported from the Pattani watershed in Southern Thailand (Rangpan, 2015); furthermore, he found Amauroderma dubiopansum and Amauroderma rude (B.) Pat from the same location.
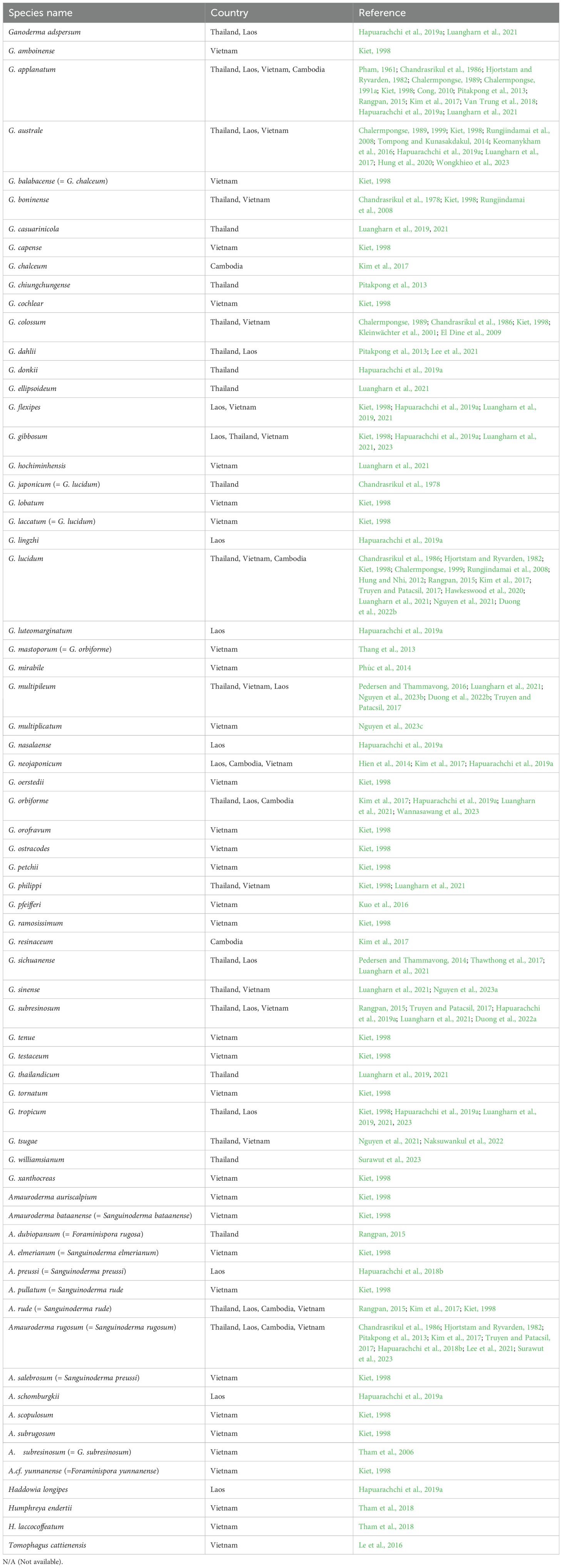
The Ganodermataceae was studied in Son Tra, Danang City, Vietnam, where 38 species across three genera (Ganoderma, Amauroderma, and Haddowia) were identified. Ganoderma had the highest species richness (29 species), followed by Amauroderma (8 species) and Haddowia (1 species) (Phu, 2023). Mushroom surveys in Cambodia identified 1,383 specimens, including Ganoderma species, which accounted for 5.6% of the collected fungi. Specimens were collected from western (Koh Kong forests) and eastern (Seima and Mondulkiri forests) regions, covering elevations from 0 to 750 m. Ganoderma sp. was among the dominant genera in both regions, highlighting its widespread distribution. The biodiversity analysis revealed 238 species across various elevations, with higher diversity observed at 251–500 m. These findings emphasize the significance of Ganoderma in Cambodia’s fungal biodiversity and its potential for ecological and economic applications (Kim et al., 2016; Wijaya et al., 2021). In addition, species of Ganodermataceae found in the Lower Mekong Basin, along with their current legitimate names, are listed in Table 1.
4 Cultivation of Ganoderma and other species from related genera in the Lower Mekong Basin
4.1 Thailand
Thailand plays a vital role in the Lower Mekong Basin, standing out for its advanced agricultural practices and rich biodiversity, which provide ideal conditions for mushroom cultivation. With a long history of mushroom farming, the Thai government has actively promoted this sector to improve rural livelihoods. Initiatives such as the Royal Mushroom Projects and government-supported loan programs have significantly contributed to steady growth in mushroom production. These efforts showcase Thailand’s commitment to sustainable agriculture and rural development, serving as a model for neighboring countries in the region (Kwon and Thatithatgoon, 2004). Ganoderma was cultivated for a long time in Thailand; however, the information for their activities and chemical components was insufficient (Poomsing et al., 2013). Ganoderma applanatum, G. australe, G. curtisii, G. lucidum, G. oregonense, G. orbiforme, G. resinaceum, G. sichuanense, G. sinense, G. tenus, G. tropicum, and G. tsugae are presently cultivated in Thailand (Pothisuwan et al., 2010; Thawthong et al., 2014; Luangharn et al., 2017, 2019; Wannasawang et al., 2023).
Ganoderma australe, identified from Thailand, grew best at 25°C–30°C and pH 7–8, with sorghum and barley as the top grain media for spawn production. Potato Dextrose Agar (PDA) was ideal for mycelial growth. Cultivated on para rubber sawdust with additives, mycelia spread fully after 18 days at 30°C and 60%–75% humidity. Three fruiting cycles yielded decreasing mushroom weights (Luangharn et al., 2017). Thai G. lucidum (G2) has been grown in Thailand as part of the Royal Project since 1988 (Petcharat, 1996). The production of G. lucidum and its spores was studied at the Muang Ngai Special Agricultural Project under the patronage of Her Majesty Queen Sirikit in the fiscal year 2009. The findings indicated the potential for commercial production of these mushrooms and spores. In the Thai markets, dried G. lucidum is priced at 850 baht (USD 25) per kilogram, while spores ranged from 2,000 to 100,000 baht (USD 59 to 2,957) per kilogram. However, if farmers are to adopt Ganoderma cultivation, they must adhere to good agricultural practices to ensure effective production (Pothisuwan et al., 2010). Furthermore, substitution of sawdust with Nash leaves (Vetiveria zizaniodes L.) can reduce the production cost of G. lucidum (Sornprasert and Aroonsrimorakotet, 2014). Sugarcane bagasse was evaluated as a substrate for cultivating G. lucidum in comparison to sawdust. While G. lucidum grew faster in sawdust, sugarcane bagasse resulted in higher cellulase activity and biological efficiency. These findings suggest sugarcane bagasse is a promising alternative substrate for G. lucidum production, supporting improved enzyme activity and yield (Ninluam et al., 2016). Ganoderma lucidum strains (GA1, GA2, and GA3) cultivated in Tam Dao, Vietnam, exhibited similar polysaccharide levels, with GA3 showing the highest lucidenic N acid (0.33 mg/g) and ganoderic acid (2.38 mg/g), while GA1 had the highest ganodermanontriol content (0.3 mg/g) (Nghien et al., 2019). The isolation of strong G. lucidum mycelium is crucial for successful transplantation. Mycelium was cultivated on PDA, brown rice, and grain substrates with varying ratios of rice bran, MgSO4, and CaCO3. Using wheat bran supplemented with 4 g of rice bran and MgSO4 at different concentrations significantly enhanced G. lucidum mycelial growth on Manihot esculenta substrate (Nhung, 2019). Ganoderma lucidum yields the highest polysaccharide content when grown under specific conditions. The optimal growing environment includes temperatures of 25°C–30°C and humidity of 60%–70% for the first 34 days, followed by 22°C–28°C and 80%–90% humidity for the next 33 days, and finishing at 22°C–28°C with 60%–70% humidity. The best extraction conditions involve a 1:40 mushroom/solvent ratio, 80°C temperature, 90-min extraction time, and three extractions. These conditions maximize polysaccharide content for medicinal use (Duyen and Mai, 2023). Wild Ganoderma strains from northern Thailand, including G. sichuanense and G. orbiforme, were studied for optimal growth conditions at 25°C–30°C and pH 4–8. Ganoderma sichuanense thrived on potato sucrose agar and G. orbiforme on oatmeal agar. Both species produced fruiting bodies in bag culture, with G. orbiforme also thriving in field conditions (Wannasawang et al., 2023). The growth and bioactive compound production in G. sichuanense was optimized using various fruit peels as substrates. Durian peel was most effective for mycelial growth on solid media, achieving 9.4 mm/day. In liquid culture, mango, durian, and mangosteen peels resulted in similar mycelial yields (around 11 g/L). Durian peel (0.1% concentration) significantly boosted polysaccharide (74.25 mg/g), phenolic content (57.26 mg GAE/g), and triterpenoid production (21.52 mg/g) after 21 days. Using fruit peels as supplements for cultivation enhances bioactive compound production (Apiwatanapiwat et al., 2024). Hed Nua Yang (G. subresinosum Fr.), an edible medicinal mushroom from Thailand, grows best on PDA at 25°C and pH 5.7. Cultivation on sawdust supplemented with gypsum, rice bran, and magnesium sulfate produced mycelial growth in 15 days, with fruiting beginning after 4 months and yielding 11.4 g per bag in the first flush (Petcharat, 1996).
Ganoderma tropicum is reported for the first time from Chiang Rai Province, Thailand, and its optimal conditions for mycelial growth were found on PDA, MEA, and YPD media at pH 7–8 and temperatures of 25°C–28°C. Although successful growth conditions were identified, fruiting could not be achieved, making this the first report on the mycelial growth of this species (Luangharn et al., 2019). Green mold disease, caused by Trichoderma species, poses a major threat to Ganoderma cultivation. Recently, an outbreak in a Ganoderma farm in Songkhla, Thailand, prompted research to identify the causative Trichoderma species, and it was identified that T. harzianum, T. pleuroticola, and T. reesei. This study marks the first report of T. pleuroticola and T. reesei causing green mold disease in G. lingzhi in Thailand (Ubolsuk and Pornsuriya, 2022).
4.2 Vietnam
The rising demand for Ganoderma sp. in Vietnam highlights the need for better breeding programs hindered by limited genetic knowledge. A study was analyzed nine accessions from southern Vietnam using morphological and molecular methods with 20 ISSR (Inter Simple Sequence Repeat) markers, revealing significant variation in growth, fruiting bodies, and genetic composition. Two main genetic groups were identified, offering insights for classification and breeding improvements (Vo and Le, 2019). The effects of vanadium (V), selenium (Se), and germanium (Ge) on G. lucidum mycelia were examined. Se and V showed high toxicity in pure culture, whereas Ge was non-toxic at tested levels. In cultivation, G. lucidum grown on V-enriched sawdust developed mature fruit bodies with low V bioaccumulation. Se was effectively absorbed and concentrated in the pileus, with depletion via basidiospores. Ge was readily absorbed and transported into fruit bodies (Le Tham et al., 1999). The biological efficiency and bioactive components of three G. lucidum strains (GA1, GA2, and GA3) cultivated in Tam Dao, Vietnam, were evaluated. All strains grew well on rice-bran-supplemented PDA, colonized in 9 days, and yielded 13%–17%, making them suitable for commercial cultivation (Nghien et al., 2019). Optimal conditions for G. lucidum strain GA3 were identified as potato, glucose, and agar (PGA) media supplemented with rice bran, a temperature range of 25°C–30°C for mycelial growth, and a pH range of 4–12. For fruiting body development, the best substrate consisted of 87% sawdust, 4% corn powder, 8% rice bran, and 1% calcium carbonate (CaCO3) (Nguyen et al., 2019). An IoT-based monitoring system for indoor G. lucidum cultivation was developed to track temperature and humidity in real time, optimizing growth conditions. The system proved cost-effective and practical, with results showing it successfully maintained optimal parameters. The produced G. lucidum met Vietnamese regulatory quality standards (Nguyen et al., 2023d). A study found glucose and ammonium sulfate to be the best carbon and nitrogen sources for its mycelial growth (5.52–5.63 mm/day). Ga-TB grows well at pH 5.0–10.0 (optimal at 7.0) and temperatures of 25°C–30°C (6.03–6.16 mm/day) (Nguyen et al., 2023e). Ganoderma sinense was successfully cultivated under optimized conditions in Vietnam. It is identified fructose (15 g/l) and yeast extract (1 g/l) as the best carbon and nitrogen sources, with optimal pH 7 and temperature 25°C–30°C for mycelial growth. The fastest growth occurred with a substrate mix of 69% rice grains, 30% sawdust, and 1% calcium carbonate, while the highest fruiting body yield (2.95% biological efficiency) was achieved with 96% sawdust, 1% wheat bran, and 1% lime. These findings highlight the potential for commercial cultivation of G. sinense strain GA21 (Nguyen et al., 2023). Ganoderma lucidum strain Ga-TB stands out for its high yield in Vietnam.
4.3 Cambodia
Cost-effective local substrates in Cambodia, including rubber tree sawdust, sugarcane bagasse, and acacia sawdust, were tested for thermophilic mushrooms, including Pleurotus sajor-caju, G. lucidum, Auricularia auricula, and Lentinula edodes. Rubber tree sawdust and sugarcane bagasse showed high efficiency (~60%), while acacia sawdust, although less efficient (22.4%), was 6.5 times cheaper, making it a viable option to reduce production costs in rural areas (Chang et al., 2016). Another study on mushroom cultivation in Cambodia highlights the production of G. lucidum and its specific requirements for successful growth. Ganoderma lucidum thrives at 18°C–25°C and 85%–90% humidity, with sawdust substrates yielding higher biological efficiency and fruiting body production compared to log cultures. Contamination rates were lower in bag cultures (13 cases) than in logs (22 cases). These findings emphasize the importance of optimizing substrates and growing conditions for G. lucidum to enhance its production in the mushroom industry in Cambodia (Srey, 2019). Table 2 presents a summary of the important findings regarding the growth and production of bioactive compounds in different Ganoderma species within the Lower Mekong Basin.
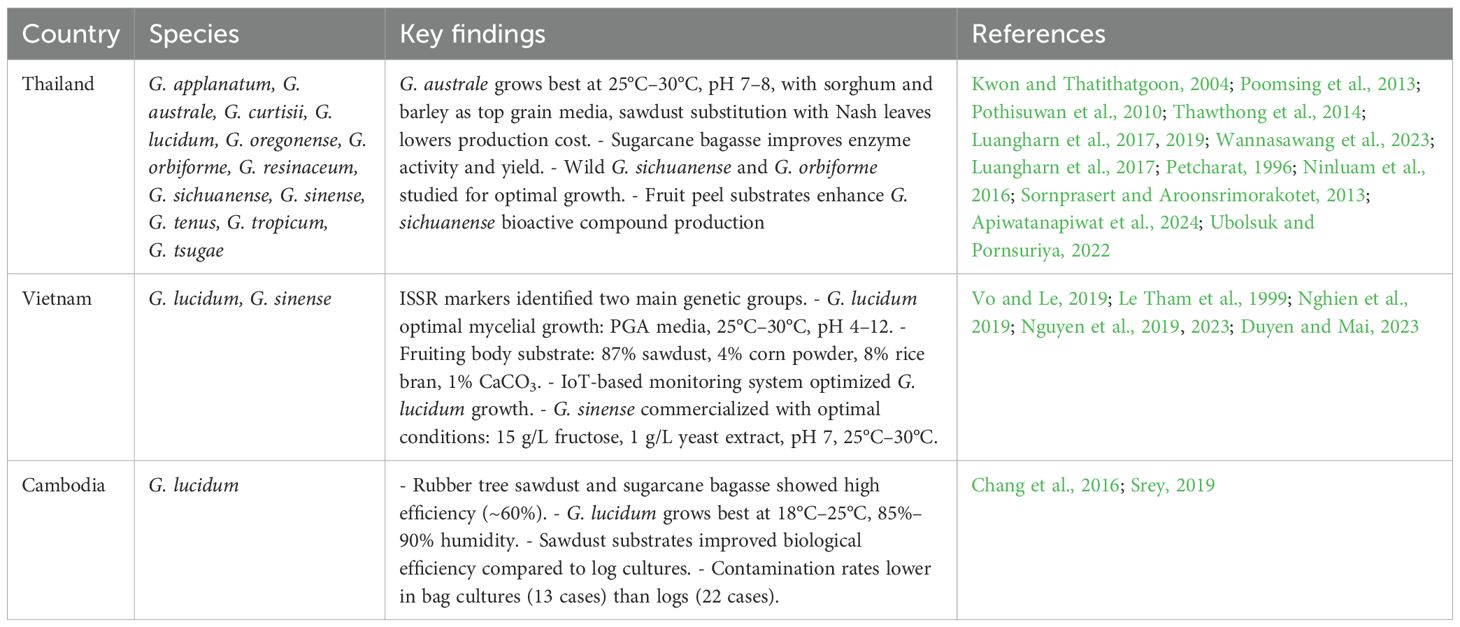
Table 2. Summary of key findings related to the growth and bioactive compound production of various Ganoderma species in Lower Mekong Basin.
5 Ganoderma as a plant pathogen
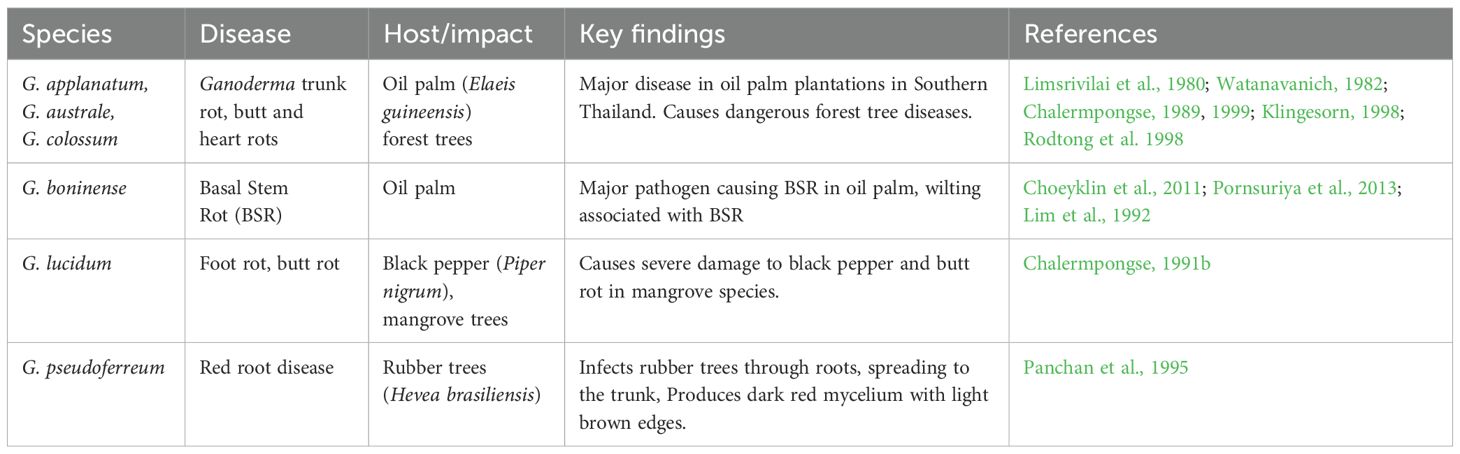
The Lower Mekong Region, comprising Thailand, Laos, Cambodia, and Vietnam, faces significant agricultural challenges due to pathogenic Ganoderma species. Diseases such as basal stem rot (BSR) in oil palms and root and butt rots in economically and ecologically valuable trees are prevalent across the region. In addition to Thailand, where G. boninense, G. applanatum, and G. lucidum have been well documented, similar infections impact crops and forests in neighboring countries. Research on biodiversity and innovative biocontrol strategies is vital for sustainable management in the region.
5.1 Ganoderma-associated diseases and prevention methods
5.1.1 Ganoderma applanatum, G. australe, and G. colossum
Ganoderma trunk rot has been identified as a major disease affecting oil palm (Elaeis guineensis) plantations in the southern provinces of Thailand (Limsrivilai et al., 1980; Watanavanich, 1982). Since 1977, dangerous forest tree diseases in Thailand, such as butt and heart rots, were caused by G. applanatum, G. australe, and G. colossum (Chalermpongse, 1989; Likhitekaraj and Tummakate, 2000). Research conducted between 1993 and 1997 at the Mae Klong Watershed Research Station in Thong Phaphoom District, Kanchanaburi Province, Western Thailand, investigated the biodiversity dynamics of ectomycorrhizal (ECM) and wood-rotting fungi. This study identified G. australe and G. lucidum as a prominent species of Basidiomycota (Chalermpongse, 1999). Four Ganoderma species were documented, including G. australe from Aung-Reu-Nai Wildlife Sanctuary, along with two additional unidentified Ganodermataceae species recorded in the upper Khao Soi Dao Wildlife Sanctuary, in Eastern Thailand (Klingesorn, 1998a, 1998b). In addition, species of Ganoderma have been reported from Nong-rawieng Plant Genetics Forest, Nakhon Ratchasima, Thailand (Rodtong et al., 1998).
5.1.2 Ganoderma boninense
A 2-year study of aphyllophoraceous fungi in Thai forests included Amauroderma parasiticum Corner, Beih., and G. boninense in the checklist compiled by Choeyklin et al. (2011). Ganoderma boninense has been identified as one of the major pathogens responsible for BSR in oil palm plantations in Southern Thailand (Pornsuriya et al., 2013). Wilting associated with BSR is recognized as the most devastating disease affecting oil palm, with the pathogen typically found growing at the basal portion of infected palms (Lim et al., 1992).
5.1.3 Ganoderma lucidum
Ganoderma lucidum was also associated with butt rot in mangrove tree species at different mangrove localities in southern Thailand (Chalermpongse, 1991b).
5.1.4 Ganoderma pseudoferreum
Red root disease caused by G. pseudoferreum was discovered in rubber plantations in the Surat Thani and Chumporn provinces (Panchan et al., 1995). Two root rot diseases impacting rubber plantations in Thailand include white root disease, caused by Rigidoporus lignosus, and red root disease, caused by G. pseudoferreum. Ganoderma pseudoferreum infects trees through the roots, spreading to the trunk and producing dark red mycelium with light brown edges. Preventive treatments with BERET 400 FS (fenpiclonil) and SCORE 250 EC (difenoconazole) were effective when applied twice annually. Curative treatments also controlled the disease effectively, provided infections were under 25% of the trunk circumference (Panchan et al., 1995).
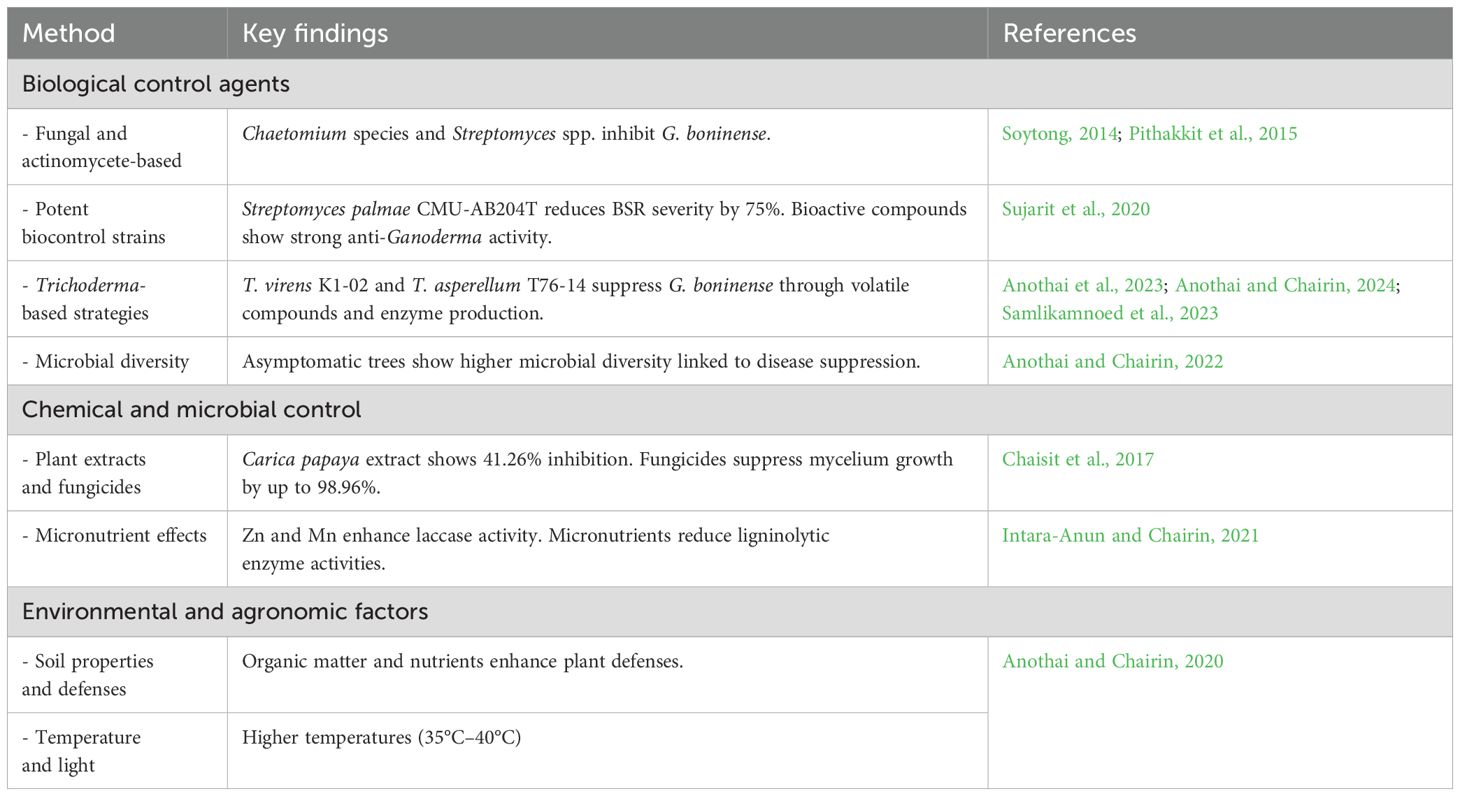
5.2 Disease prevention and management of G. boninense
5.2.1 Biological control agents
5.2.1.1 Fungal and actinomycete-based biocontrol
A biological control agent, Chaetomium species, may release antagonistic substances to suppress G. boninense, a pathogen that causes significant losses in palm oil production (Soytong, 2014). Actinomycetes isolated from the oil palm rhizosphere were tested for their ability to inhibit G. boninense. Three species—Streptomyces abikoensis, Kitasatospora nipponensis, and S. angustmyceticus—were identified as effective inhibitors (Pithakkit et al., 2015).
5.2.1.2 Potent biocontrol strains and their bioactive compounds
Streptomyces palmae CMU-AB204T was identified as a potent biocontrol agent, reducing BSR severity by over 75% and enhancing plant vigor in oil palm seedlings. Bioactive compounds from S. palmae—actinopyrone A, anguinomycin A, and leptomycin A—showed strong anti-Ganoderma activity, highlighting its potential as a protective agent for oil palm (Sujarit et al., 2020). The use of palm oil mill effluent (POME) for producing antifungal compounds by Streptomyces philanthi RM-1-138 demonstrated potential in inhibiting G. boninense, Ceratocystis paradoxa, and Curvularia oryzae. In-vitro tests revealed high inhibition of these fungal pathogens, with POME offering optimal conditions for compound production. The antifungal compounds exhibited strong activity against these oil palm pathogens, suggesting the potential of POME as a sustainable resource for biocontrol in oil palm disease management (Boukaew et al., 2022).
5.2.1.3 Trichoderma-based biocontrol strategies
The biocontrol agent Trichoderma virens K1-02 demonstrated effective suppression of G. boninense through volatile antifungal compounds and enzyme production (Anothai et al., 2023). Greenhouse trials showed treated oil palm roots had higher lignocellulose content, offering a promising strategy for BSR management (Anothai and Chairin, 2024). The effects of G. boninense infection and Trichoderma asperellum T76-14 treatment on oil palm seedlings were investigated. Ganoderma increased phenylalanine ammonia-lyase (PAL) activity early, while T. asperellum enhanced PAL and polyphenol oxidase (PPO) activity. Trichoderma-treated seedlings showed no visible BSR symptoms after 20 weeks and reduced necrosis compared to controls. Morphological traits were largely unaffected, highlighting the potential of T. asperellum as an early-stage biocontrol for BSR (Samlikamnoed et al., 2023).
5.2.1.4 Microbial diversity and disease suppression
Asymptomatic trees in G. boninense-infected oil palm plantations exhibit higher microbial diversity and a greater abundance of beneficial bacteria like Actinobacteria and Firmicutes compared to symptomatic trees. These bacteria are linked to disease suppression and plant health (Anothai and Chairin, 2022).
5.2.2 Chemical and microbial control strategies
Plant extracts, antagonistic microorganisms, and fungicides were evaluated for controlling G. boninense. Among the plant extracts, Carica papaya showed the highest inhibition at 41.26%. Antagonistic bacterial isolates B001, B002, and B003, as well as fungal isolate T003, demonstrated significant efficacy. Fungicides such as prochloraz, kresoxim-methyl, and chlorothalonil showed mycelium growth suppression of up to 98.96% (Chaisit et al., 2017). The effects of micronutrients (Fe, Zn, Mo, and Mn at 1 mM) on ligninolytic enzymes produced by G. boninense on oil palm root substrates were evaluated. Zn and Mn enhanced laccase activity in solid-state cultures, while all tested micronutrients significantly reduced activities of lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase in crude extracts (Intara-Anun and Chairin, 2021).
5.2.3 Environmental and agronomic factors influencing disease control
In Thailand, the relationship between soil properties, fungal enzymes, and plant defense responses in G. boninense–infected oil palm plantations highlight that organic matter and nutrients enhance defenses like PAL and chitinase, while fungal enzymes correlate with organic carbon and low soil pH, collectively influencing BSR disease (Anothai and Chairin, 2020). Research identified key factors, including temperature, potassium, boron, and mancozeb, that inhibit G. boninense growth and lignocellulosic enzyme activity. The effects of temperature and light on G. boninense enzyme activities were studied under laboratory conditions. Higher temperatures (35°C–40°C) and light exposure reduced laccase, lignin peroxidase, and manganese peroxidase activities. These results suggest that temperature and light can be utilized in future strategies for managing BSR disease in oil palm (Intara-Anun and Chairin, 2023).
5.2.3 Genetic and technological advances in disease management
Hyperspectral and multispectral remote sensing effectively detected diseased oil palms in Krabi, Thailand. Healthy leaves exhibited higher visible and near-infrared radiance compared to diseased ones. Using 113 samples, vegetation indices derived from WorldView-2 imagery achieved 85.98% classification accuracy and a Kappa coefficient of 0.71 (Malinee et al., 2021). BSR caused by G. boninense threatens oil palm production, with climate change worsening its impact. Modified oil palms (mOPs) are being developed to resist BSR, although their full deployment will take decades. CLIMEX modeling highlights the significant benefits of mOP in mitigating BSR (Paterson, 2023). A prototype system for early detection of BSR in oil palm trees was developed in Thailand, combining traditional tapping techniques with modern sound analysis. The study utilized machine learning models, including Convolutional Neural Networks (CNN), Support Vector Machine Classifier (SVC), and Multi-Layer Perceptron (MLP). The CNN model achieved the best performance, with 90.73% accuracy in two-class classification (Augsornthip et al., 2024). Tables 3, 4 list Ganoderma-associated diseases reported in the Lower Mekong Basin, detailing their impact on economically important crops such as oil palm, rubber trees, and black pepper, along with effective prevention and management strategies.
6 Analysis of bioactive compounds and therapeutic properties of Ganodermataceae by researchers in the Lower Mekong Basin
Herbal medicines commonly used by chronic disease patients vary across countries. In Laos, popular herbs include Moringa pterygosperma, Curcuma longa, Centella asiatica, and G. lucidum. In Vietnam, herbs such as Curcumin, Gynostemma pentaphyllum, Artichoke, and G. lucidum are prevalent, while in Thailand, frequently used herbs include Zingiber officinale, Andrographis paniculata, Curcuma longa, and G. lucidum with 71 herbal products listed in the National List of Essential Drugs. These reflect the region’s widespread reliance on traditional medicine (Peltzer and Pengpid, 2019). Research on the medicinal properties of Ganodermataceae within the Lower Mekong Basin is growing, with studies focusing on its antimicrobial, anti-inflammatory, and anticancer activities, among others. This analysis examines the current body of research conducted in the region, exploring the pharmacological properties of Ganoderma and its potential applications in traditional and modern medicine. By synthesizing findings from various studies, the next section provides a comprehensive overview of the medicinal value of the species of Ganoderma and allied genera, highlighting their role in the healthcare practices of the Lower Mekong Basin.
6.1 Anticancer and tumor suppression potential
Ganoderma species, particularly G. lucidum have been extensively studied for their medicinal properties. The anticancer and tumor suppression potential of G. lucidum is one of its most prominent benefits. Studies have demonstrated that extracts of G. lucidum can help extend the lifespan of cancer patients, with some patients living 3–6 months longer and experiencing reduced chemotherapy side effects (Thaithatgom, 1995). Other studies have also observed anticancer activities in G. lucidum as part of Thai medicinal teas (Cheeptham and Towers, 2002), while Armassa et al. (2009) found that G. lucidum mycelium extracts reduced the viability of breast cancer cells. In addition, research by Soksawatmakhin and Boonyahotra (2013) and Pesee et al. (2013) found that the use of G. lucidum extracts resulted in positive therapeutic effects in cancer patients, including improved survival rates and better quality of life. A study found that Ganoderma lucidum hot water extract is not mutagenic and shows strong antimutagenic effects in lab tests. It significantly reduced mutagen-induced changes in bacteria and fruit flies, suggesting potential for cancer prevention (Pakdee et al., 2014). Ganoderma lucidum was utilized along with other Vietnamese mushrooms to extract 1–3/1–6 β-glucan for encapsulating curcumin into nanoparticles (NanoGluCur) via nano-precipitation. NanoGluCur significantly enhanced curcumin’s water solubility (180-fold) and demonstrated potent anti-cancer effects against Hep-G2 and LU-1 cell lines, with IC50 values of 6.82 and 15.53 mg/ml, respectively. At 40 mg/ml, NanoGluCur reduced tumor size by 59.93% and density by 40.52%, surpassing the performance of free curcumin. These results emphasize G. lucidum-derived β-glucan’s potential in improving drug delivery and its applications in functional foods and cancer therapies (Le et al., 2016). During the rainy season, 13 medicinal mushroom specimens were collected in four Thai provinces. Ganoderma calidophilum and Amauroderma rugosum showed the highest 1,3-β-glucan content, known to inhibit tumor growth by boosting immune responses. Environmental factors such as vegetation, soil, and microclimate contributed to the bioactive compound levels in these mushrooms (Suwanno and Phutphat, 2018).
Two new lanostane triterpenes, 3α,12β,15α-triacetoxy-5α-lanosta-7,9(11),24-trien-26-oic acid (1) and 5α-lanosta-8,24-diene-26,27-dihydroxy-3,7-dione (2), along with sixteen known compounds, were isolated from G. lucidum. Compound 1 exhibited significant antitumor activity against PC-3 prostate cancer cells (IC50 = 11.5 μM). Ganoderic acid F (17) demonstrated strong anti-angiogenic effects, inhibiting capillary-like formation in human umbilical vein endothelial cells (Nguyen et al., 2015). A new gymnomitrane-type sesquiterpenoid, gymnomitrane-3α,5α,9β,15-tetrol (1), was isolated from the fruiting body of G. lucidum. Its structure was determined using spectroscopic techniques. This compound showed significant inhibition of the growth of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI)–resistant human lung cancer (A549) and human prostate cancer (PC3) cell lines (Binh et al., 2015). In cancer treatment, G. lucidum has shown positive clinical outcomes. Sornprasert and Aroonsrimorakot (2015) demonstrated that the mycelial growth of G. lucidum on various substrates can contribute to its clinical efficacy in cancer treatments. Other studies, such as Suprasert et al. (2014, 2015), also confirmed that water extracts and spores of G. lucidum could help control gynecological cancers, improving immune function with minimal side effects. In addition, Teekachunhatean et al. (2012) provided insights into the pharmacokinetics of ganoderic acids, suggesting that food intake affects the absorption of these compounds, which could have implications for their clinical effectiveness. Seven novel triterpenoid metabolites, named colossolactones (1−7), were isolated from the fruiting body of Ganoderma colossum, from Vietnam, with their structures elucidated using MS and NMR techniques (Kleinwächter et al., 2001). Five compounds, including ergosterol and lanostane derivatives, were isolated from the fruiting body of G. applanatum for the first time in Vietnam. Their structures were identified using advanced spectroscopic techniques. Notably, lanosta-7,9(11),24-triene-3,26-diol was reported as a novel compound in this fungus (Van Trung et al., 2018). The anticancer potential of G. lucidum triterpenoid extract was evaluated on human Hep-G2 liver cancer cells. The extract demonstrated significant activity, with a half-maximal inhibitory concentration (IC50) value of 67.25 ± 0.82 µg/ml. This suggests that triterpenoids extracted from G. lucidum could be considered a promising agent for medicinal treatment, particularly in cancer therapy (Linh et al., 2021). The inhibitory effects of various Thai herbal extracts on the metabolism of anticancer drugs gefitinib, lapatinib, and sorafenib, mediated by the cytochrome P450 enzyme CYP3A, were studied. Ganoderma lucidum exhibited minimal impact on the metabolism of these drugs, with IC50 values greater than 10 μg/ml, indicating weak inhibition. In contrast, Curcuma zedoaria and Murdannia loriformis showed stronger inhibitory effects. The findings suggest potential pharmacokinetic interactions between tyrosine kinase inhibitors and certain herbal extracts, with G. lucidum having a lesser effect than the other herbs (Rodseeda et al., 2022). Ganoderma lucidum broken spores (GLBS) taken at 750 mg/day for 8 weeks in post-chemotherapy patients increased white blood cell and neutrophil counts, improved quality of life, and caused only mild side effects such as dry mouth. GLBS did not affect liver or kidney function, indicating it may safely support immune recovery (Tangkhaphiphat et al., 2022). Three novel lanostane triterpenoids, ganoellipsic acids A–C, along with seven known Ganoderma lanostanoids, were isolated from artificially cultivated Ganoderma ellipsoideum (strain BCC 16634). Structural elucidation was conducted using NMR spectroscopy and mass spectrometry, with the absolute configuration of C-25 in compound 1 determined as 25S via the phenylglycine methyl ester (PGME) method (Sappan et al., 2022).
Five steroids, including stigmasterol, ergosterol peroxide, ganodertriol M, lucidumol B, and kansenone, were isolated from G. australe fruit bodies in Laos. Structural characterization was performed using HR-MS and NMR spectroscopy. Ergosterol peroxide and kansenone demonstrated notable cytotoxicity against four cancer cell lines (KB, MCF7, SK-LU-1, and Hep-G2). This marks the first report of cytotoxic steroids from Ganoderma species in Laos (Keomanykham et al., 2016). Ganoderma sinense fruit bodies cultivated in Laos (GS-LW) had the highest triterpenoid content (538.8 µg/g) among samples from different regions. While water-soluble polysaccharide levels were moderate (1.20%), β-glucan levels were comparable (~16%) across all regions. This study highlights the unique bioactive compound profiles in GS-LW, emphasizing its potential for medicinal use (Liu et al., 2017). Table 5 highlights the anticancer and tumor suppression potential of Ganoderma species found in the Lower Mekong Basin.
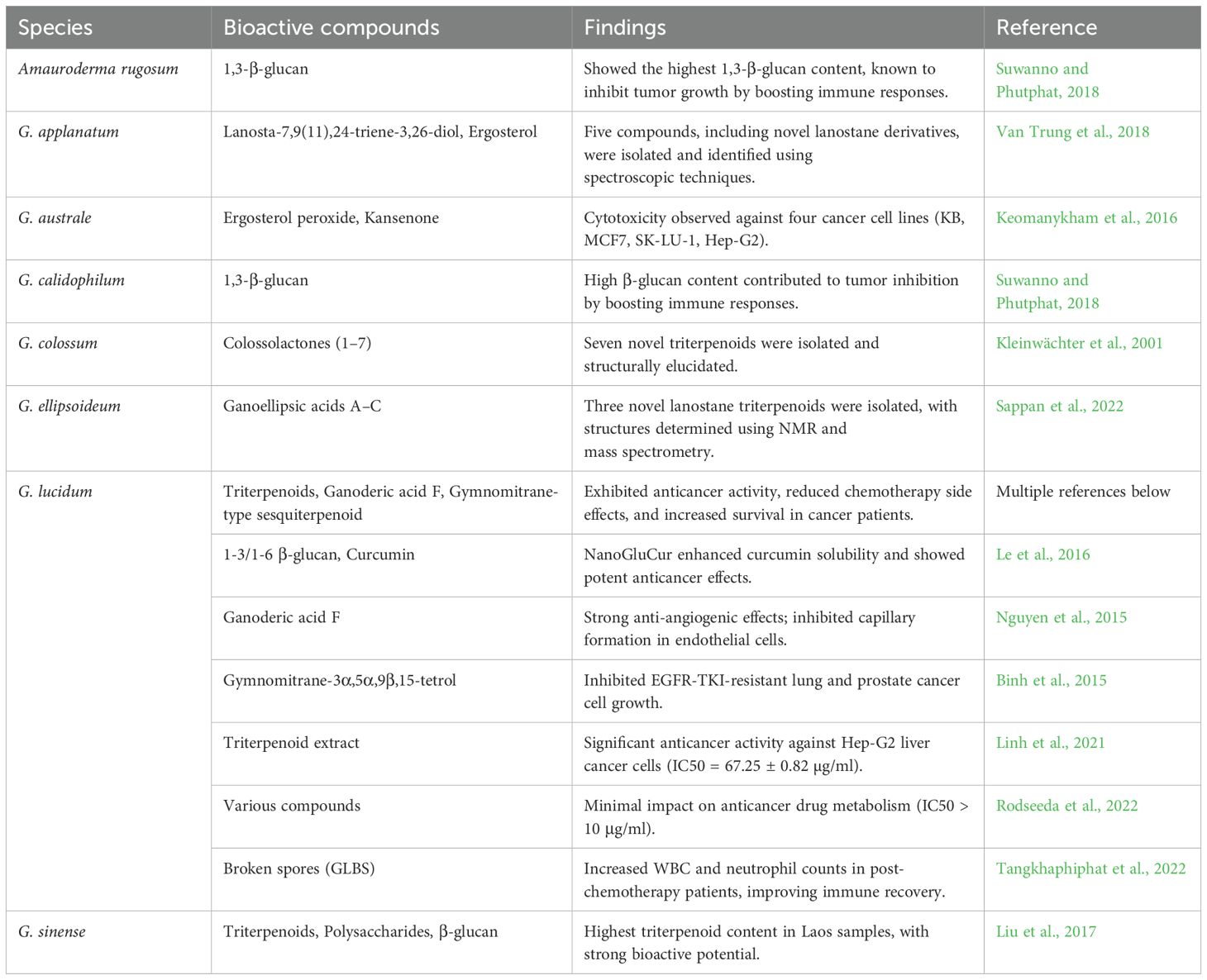
Table 5. Anticancer and tumor suppression potential of Ganoderma species found in Lower Mekong Basin.
6.2 Immunomodulatory effects
Beyond cancer, G. lucidum is known for its immunomodulatory properties. Studies like Mizuno et al. (1997) showed that crude G. lucidum extracts can restore immune function in immunosuppressed individuals, enhancing T-cell activity. Further research by Futrakul et al. (2003) highlighted the ability of G. lucidum to modulate immunocirculatory balance, demonstrating its potential in treating nephrotic syndrome and other immune-related conditions. Sornprasert and Aroonsrimorakot (2015) also observed significant immunomodulatory effects when G. lucidum was cultivated in certain substrates, emphasizing its clinical potential. Chronic fatigue syndrome (CFS) patients in a study received either Ganoderma lucidum extract or a placebo. After 4 weeks, the G. lucidum group showed significantly improved quality of life (p = 0.005) and reduced fatigue (p = 0.010) compared to the placebo. After 12 weeks, serum cortisol levels rose in the G. lucidum group, with higher satisfaction reported by these participants (p < 0.001). Side effects (diarrhea and nausea) were similar in both groups. Findings suggest that G. lucidum extract may be effective in alleviating fatigue and enhancing the quality of life for CFS patients (Soksawatmakhin and Boonyahotra, 2013).
6.3 Neuroprotective and cognitive benefits
The neuroprotective effects of G. lucidum have also been a subject of interest, with studies such as Porntip et al. (2006) showing that G. lucidum extracts promote neuroprotection in neuronal cultures, suggesting benefits in cognitive health. Pinhewa et al. (2008) found that G. lucidum extracts increased the expression of amyloid precursor protein (APP) and promoted sAPPα secretion, both of which are linked to improved cognitive function. In addition, Boonyanuphap and Hansawasdi (2011) showed that G. lucidum contains β-glucans, which are known to support cognitive health, potentially aiding in memory enhancement.
Neural stem cells (NSCs) are promising for treating neurological disorders due to their self-renewal and pluripotency, but exogenous sources are often needed for effective therapy. This study demonstrated that G. lucidum extract at 500 μg/ml significantly enhanced NSC proliferation, with isolated cells forming neurospheres, expressing neural markers, and differentiating into GFAP-positive cells (Dan et al., 2017). Ganoderma lucidum extracts, at doses of 200–400 mg/kg, effectively reduced morphine addiction and improved morphine-induced memory impairments in animal models. Using the conditioned place preference model and memory tests (Y maze, novel recognition, and Morris water maze), the extracts demonstrated the ability to prevent addiction and enhance short-term, visual, and long-term memory. These findings suggest G. lucidum extracts as a potential natural treatment for drug addiction and memory loss (Tran et al., 2021).
6.4 Antioxidant and anti-inflammatory properties
6.4.1 Amauroderma subresinosum
Amauroderma subresinosum (= Ganoderma subresinosum) polysaccharides were extracted and characterized, revealing glucose-rich fractions with strong antioxidant activity. The primary fractions, ASF-1, ASF-3, and ASF-7, demonstrated significant DPPH and ABTS radical scavenging, suggesting their potential as antioxidant-rich functional food ingredients (Nguyen et al., 2023f).
6.4.2 Ganoderma australe
A new lanostane triterpene and three known compounds were isolated from cultivated fruiting bodies of G. australe. NMR and mass spectrometry confirmed their identities, with a revised olefinic geometry of methyl australate from 20(22)Z to 20(22) E. These compounds differ from lanostanes previously found in mycelial cultures of the same strain. Given their known antioxidant and anti-inflammatory properties, these newly identified compounds may contribute to these bioactivities, although further studies are needed (Isaka et al., 2018). An HPLC-DAD method was developed for quality control of G. lucidum (and related species), focusing on 14 triterpene compounds. The method showed good linearity, low detection limits, and recovery rates between 97.09% and 100.79%. Significant differences in triterpene content were found, with wild G. lucidum having higher levels than cultivated samples. G. australe had four times the triterpene content of wild G. lucidum (Ha et al., 2015).
6.4.3 Ganoderma lucidum
In terms of antioxidant and anti-inflammatory properties, G. lucidum is a powerful agent. Studies by Armassa et al. (2009) revealed that extracts from G. lucidum exhibited significant antioxidant activity, neutralizing free radicals and showing promise in reducing oxidative stress. Other studies, such as Sa-ard et al. (2014), confirmed that the antioxidant potential of G. lucidum could play a role in managing oxidative stress-related conditions. Dalodom et al. (2010) found that hot water extracts of G. lucidum exhibited both antioxidant properties and a lack of mutagenic effects, making them suitable for therapeutic use. Thai G.lucidum (G2), cultivated as part of Thailand’s Royal Project since 1988, was evaluated for safety and efficacy. Comparing the fruiting body and mycelium extracts, both showed no mutagenic effects, notable antioxidant activity, and mild iron-chelating properties, with the fruiting body having superior antioxidant capacity. Non-mutagenic doses also displayed cytotoxicity to lung carcinoma cells, providing useful insights for developing safe health-promoting products (Dalodom et al., 2010). The polysaccharide content, antioxidant activity, and cytotoxicity of G. lucidum mycelium extracts were investigated. The water extract exhibited a higher polysaccharide content and stronger antioxidant activity compared to the ethanol extract. In addition, it demonstrated significant cytotoxic effects on HeLa cervical cancer cells, reducing cell viability by 56.30% at 1 mg/ml, whereas the ethanol extract showed cytotoxicity to normal cells. These findings suggest that G. lucidum mycelium extracts possess potential antioxidant and anticancer properties, making them promising candidates for pharmaceutical and functional food applications (Sanmanoch et al., 2024). Crude proteins from G. lucidum mycelia and fruiting bodies showed strong antioxidant and antibacterial activities. The mycelia protein had better antioxidant effects with IC50 values of 2.47 μg/ml (ABTS•+) and 2.5 μg/ml (DPPH•), compared to the fruiting body protein. Both proteins exhibited antibacterial activity, and the mycelia protein also protected DNA from hydroxyl radicals. Partial purification revealed a major protein of 45 kDa. These results suggest that G. lucidum protein extracts have potential as antioxidant and antibacterial agents (Sa-Ard et al., 2015). Ganoderma lucidum, a functional food ingredient, contains hydrolysates with diverse biological activities. This study identified and modified a peptide (VDLPTCKGF), synthesizing seven variants. Among these, three exhibited antioxidant activity, with VDLPTC showing the strongest capacity and intracellular ROS suppression, highlighting its potential for developing novel functional food products (Krobthong and Yingchutrakul, 2020). An HPLC-DAD method was developed for quality control of G. lucidum (and related species), focusing on 14 triterpene compounds. The method showed good linearity, low detection limits, and recovery rates between 97.09% and 100.79%. Significant differences in triterpene content were found, with wild G. lucidum having higher levels than cultivated samples. G. australe had four times the triterpene content of wild G. lucidum (Ha et al., 2015). An extract from G. lucidum and Cordyceps militaris was developed and analyzed for its composition, antioxidant activities, and protective effects. Using LC-QTOF MS, 94 compounds were identified, including ferulic acid, cinnamic acid, ganoderic acid A, adenosine, and cordycepin. The extract demonstrated antioxidant properties, scavenged free radicals, reduced ferric ions, and protected human fibroblasts from oxidative stress, reducing cell death by 21%–22%. These results highlight its potential applications in functional foods and pharmaceuticals (Nguyen et al., 2024a). Methanol extracts of G. mastoporum fruiting bodies from Vietnam yielded eight compounds, including three triterpenoids and five steroids. Among these, ergosta-4,6,8(14),22-tetraen-3-one showed the strongest inhibitory effects on superoxide anion generation and elastase release, with IC50 values of 2.30 ± 0.38 and 1.94 ± 0.50 µg/ml, respectively (Thang et al., 2013).
6.4.4 Ganoderma neo-japonicum, G. pfeifferi and G. tropicum
Hot-water extracts of G. neo-japonicum (GnJ) and G. lucidum (GL) were examined for their functional composition and antioxidant properties. While both species showed antioxidant activity, GnJ exhibited a FRAP value of 181.1. The maximum DPPH and ABTS scavenging rates for GnJ were 27.9% and 76.0%, respectively, at 5 mg/ml concentrations. These values were lower compared to the antioxidant activity observed in G. lucidum (Ayimbila et al., 2023). Three triterpenoids and three steroids were identified from the fruiting bodies of G. pfeifferi collected in Vietnam, and their effects on nitric oxide (NO) production were evaluated. The findings suggest that certain compounds from this fungus could serve as potential leads for the development of new anti-inflammatory drugs (Kuo et al., 2016). A new compound, 3β-acetoxylanosta-7,9(11),24-triene-26-al, along with seven known compounds, was isolated from G. tropicum in Tay Nguyen, Vietnam. Structural identification was achieved using NMR and mass spectrometry. Compounds 2–4 and 6–8 showed dose-dependent enhancement of nitroblue tetrazolium (NBT) reduction in yeast-stimulated RAW 246.7 cells (Nguyen et al., 2020). Table 6 explores the antioxidant and anti-inflammatory properties of Ganoderma species found in the Lower Mekong Basin, emphasizing their bioactive compounds and potential therapeutic applications.
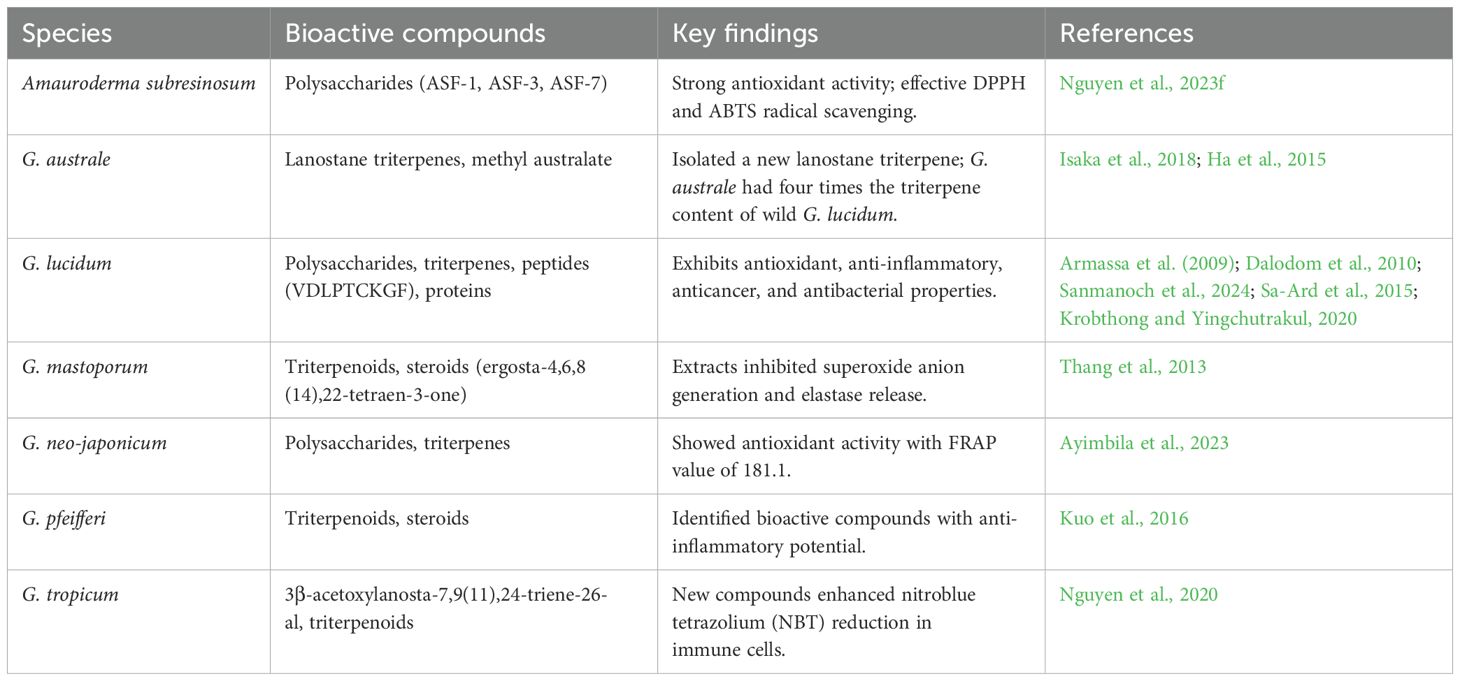
Table 6. Antioxidant and anti-inflammatory properties of Ganoderma species found in Lower Mekong basin.
6.5 Antimicrobial effects
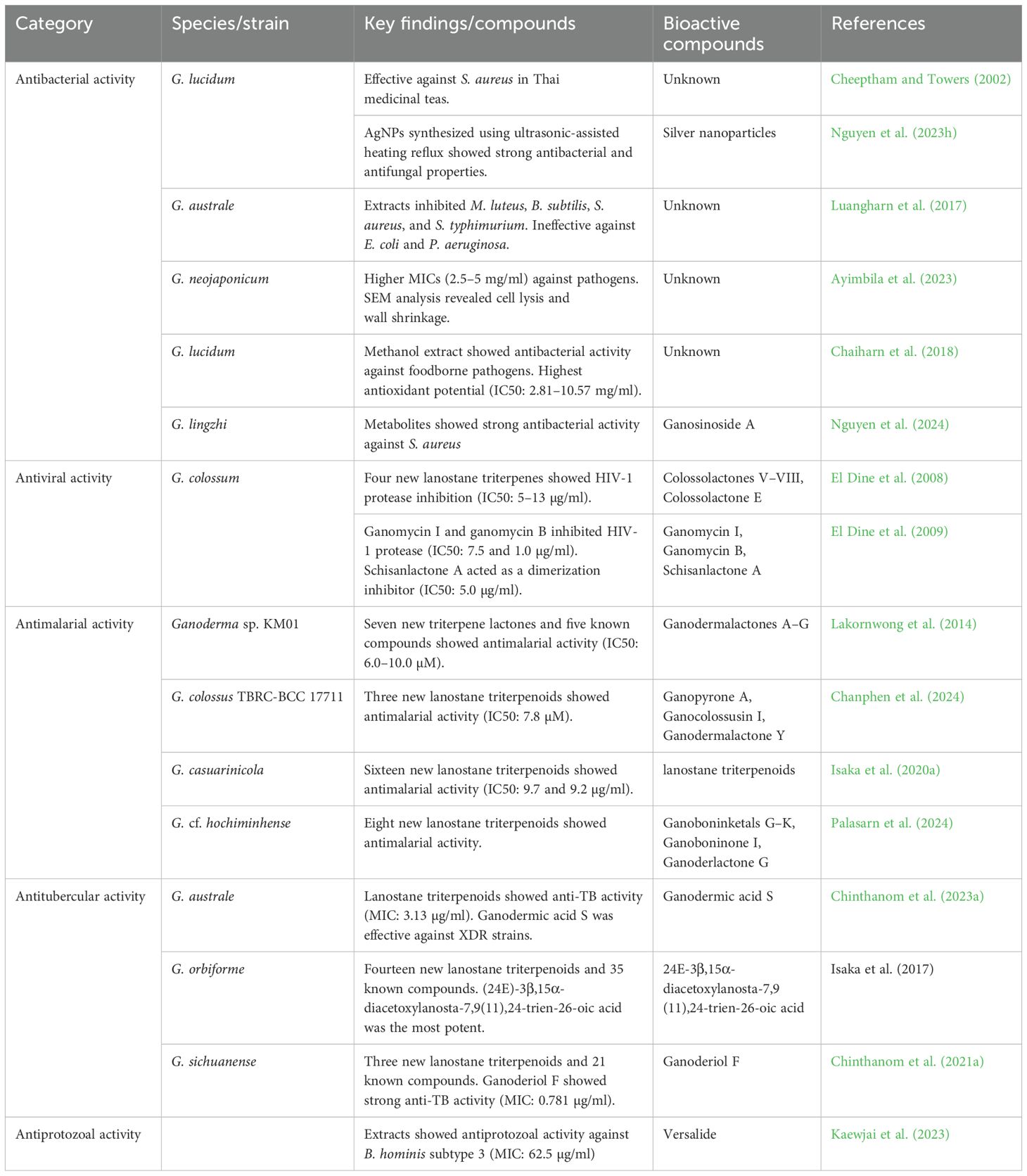
6.5.1 Antiviral activity
Four new lanostane triterpenes (colossolactones V–VIII) and a known compound (colossolactone E) were isolated from G. colossum. Their structures and absolute configurations were identified using spectroscopic techniques. Along with two previously isolated compounds, these were evaluated for HIV-1 protease inhibition, with the most active showing IC50 values of 5–13 µg/ml (El Dine et al., 2008). Ganomycin I and ganomycin B, isolated from G. colossum, inhibited HIV-1 protease with IC50 values of 7.5 and 1.0 μg/ml, respectively. Kinetic studies showed that ganomycin B competitively inhibited the enzyme’s active site, while schisanlactone A, another compound from the same fungus, acted as a dimerization inhibitor (IC50 = 5.0 μg/ml). Virtual docking confirmed these inhibitory mechanisms (El Dine et al., 2009).
6.5.2 Antibacterial activity
Cheeptham and Towers (2002) showed that G. lucidum was effective against Staphylococcus aureus, a common bacterial pathogen, in Thai medicinal teas. G. australe extracts inhibited Micrococcus luteus, Bacillus subtilis, S. aureus, and Salmonella ser. typhimurium but were ineffective against E. coli and Pseudomonas aeruginosa. This is the first report of its antimicrobial activity (Luangharn et al., 2017). Ganoderma neojaponicum demonstrated higher minimum inhibitory concentrations (MICs) ranging from 2.5 to 5 mg/ml against pathogens, compared to G. lucidum. Scanning electron microscope (SEM) analysis further revealed that G. neojaponicum caused cell lysis and wall shrinkage in the pathogens, underscoring its potential antibacterial properties, although it was less potent than G. lucidum (Ayimbila et al., 2023). The methanol extract of G. lucidum exhibited significant antibacterial activity against several foodborne pathogens, including both Gram-positive and Gram-negative bacteria, suggesting its potential as a natural antibacterial agent (Chaiharn et al., 2018). Ganoderma metabolites showed strong antibacterial potential against S. aureus, with ganosinoside A exhibiting the highest affinity for clumping factor A. In addition, G.lingzhi and A. subresinosum strains from Vietnam demonstrated antibacterial activity, suggesting their value as sources for developing antibiotics (Nguyen et al., 2024b). Four new 3,4-seco-27-norlanostane triterpenoids (ganoboninketals E and F, ganoboninones G and H) and two known derivatives, along with a new C30 lanostane and twelve known lanostanes, were isolated from G. orbiforme fruiting bodies. Furthermore, three known meroterpenoids (fornicin A, ganomycin B, and ganomycin I) were identified. The structures of the compounds were determined using NMR, mass spectrometry, and chemical correlations. Biological testing revealed that ganomycin I exhibited moderate activity against Gram-positive bacteria (Li et al., 2018).
6.5.3 Antimalarial activity
A revised structure of colossolactone G and seven new triterpene lactones, ganodermalactones A−G, and five known triterpene lactones and ergosterol were isolated from cultured Ganoderma sp. KM01. Structures were identified using spectroscopic methods, with x-ray analysis confirming configurations for compounds 3, 7, and 8. Compounds 7, 10, and 12 showed antimalarial activity against Plasmodium falciparum with IC50 values between 6.0−10.0 μM (Lakornwong et al., 2014). Three new lanostane triterpenoids—ganopyrone A, ganocolossusin I, and ganodermalactone Y—were isolated from the cultivated fruiting bodies of Ganoderma colossus TBRC-BCC 17711. Ganopyrone A has a unique polycyclic structure with an α-pyrone ring and a C-18/C-23 bond. It demonstrated antimalarial activity against the multidrug-resistant P. falciparum K1 strain (IC50 7.8 μM) with low cytotoxicity on Vero cells (IC50 103 μM) (Chanphen et al., 2024). From the cultivated fruiting bodies of Ganoderma weberianum, two lanostane dimers, ganoweberianones A and B, along with seven new lanostanes (ganoweberianic acids A–G) and three known compounds, were isolated. Ganoweberianone A showed notable antimalarial activity against the multidrug-resistant strain P. falciparum K1 (IC50 = 0.050 μM). A semisynthesis method for ganoweberianone A was also developed using acid-catalyzed transesterification (Isaka et al., 2020a). Sixteen new lanostane-type triterpenoids (1–16) and fourteen known compounds were isolated from the cultivated fruiting bodies of Ganoderma casuarinicola. The structures of these compounds were determined using NMR spectroscopy and mass spectrometry. Two of the compounds, 9 and 10, exhibited antimalarial activity with IC50 values of 9.7 and 9.2 μg/ml, respectively (Isaka et al., 2020b). Eight new highly modified lanostane triterpenoids, ganoboninketals G–K (1–5), ganoboninone I (6), ganoderlactone G (7), and (24E)-3,11-dioxolanosta-8,24-dien-26-oic acid (8), were isolated from the fruiting bodies of Ganoderma cf. hochiminhense. Their structures were determined using NMR spectroscopy and mass spectrometry. Ganoboninketals G (1), H (2), and J (4) showed antimalarial activity against the multidrug-resistant P. falciparum K1 strain, with IC50 values of 17, 16, and 5.1 μM, respectively (Palasarn et al., 2024). Ten new lanostane-type triterpenoids (1–10) and 15 known lanostanes were isolated from cultivated Ganoderma sp. BCC 21329. Their structures were determined using NMR, mass spectrometry, and Mosher’s method. Compounds 1, 3, 5, and 7 exhibited moderate antimalarial activity with IC50 values of 3.8–7.6 μg/ml (Isaka et al., 2020c). Eleven novel lanostane triterpenoids, including a unique chlorinated derivative, were isolated from Ganoderma mbrekobenum. Structural elucidation was performed using NMR, mass spectrometry, and ECD calculations, with chemical derivatization confirming the C-20 configuration in the most abundant compound. Two of the compounds showed moderate antimalarial activity (Yangchum et al., 2022). Eight new lanostane triterpenoids were isolated from G. weberianum TBRC-BCC 60642 cultures, with structural differences observed between mycelial cultures and fruiting bodies. Compounds 2, 3, and 6 exhibited moderate antimalarial activity against P. falciparum K1, with IC50 values of 10–15 μM (Chinthanom et al., 2022a). Colossolactone J, a newly identified lanostane-type triterpenoid, was purified from the fruiting body of Ganoderma colossus via silica gel column chromatography and preparative HPLC. Its molecular structure and absolute configuration were determined using spectroscopic techniques, including NMR and the modified Mosher’s method (Chinthanom et al., 2022b).
6.5.4 Antitubercular activity
Lanostane triterpenoids with strong anti-tuberculosis (anti-TB) activity were isolated from the mycelial cultures of G. australe strain TBRC-BCC 22314. To evaluate its use in anti-TB products, a chemical analysis of autoclaved and non-autoclaved mycelial powders was conducted. Both powders showed the same anti-TB activity (MIC 3.13 μg/ml) against Mycobacterium tuberculosis H37Ra. However, sterilization led to unique chemical conversions of lanostanes. The main active lanostane, ganodermic acid S, was also effective against extensively drug-resistant (XDR) strains of M. tuberculosis (Chinthanom et al., 2023a). Antitubercular research on lanostane triterpenoids from Ganoderma submerged cultures examined three strains: G. orbiforme BCC 22325, Ganoderma sp. BCC 60695, and G. australe BCC 22314. Fourteen new lanostane triterpenoids and 35 known compounds were isolated and tested against M. tuberculosis H37Ra. Structure–activity relationship analysis identified 3β- and 15α-acetoxy groups as essential for antimycobacterial activity, with the most potent compound being (24E)-3β,15α-diacetoxylanosta-7,9(11),24-trien-26-oic acid (Isaka et al., 2017a). Sixteen new lanostane triterpenoids (1–16) and 26 known compounds (17–42) were isolated from Ganoderma sp. BCC 16642. The antitubercular activities of these compounds were tested against M. tuberculosis H37Ra, and structure–activity relationships were proposed based on the results (Isaka et al., 2016). Ganoderma weberianum yielded 11 novel lanostane dimers (ganoweberianones C-H and isoganoweberianones A/B/D/G/H), 6 new ganodermanontriol derivatives, and 5 ganoweberianic acids. A semisynthetic condensation method aided structural characterization. Notably, ganoweberianone D (IC50 0.057 μM) and isoganoweberianone D (IC50 0.035 μM) exhibited potent antiplasmodial activity against multidrug-resistant P. falciparum K1 with minimal cytotoxicity (Vero cell IC50 8.1-19 μM), highlighting their therapeutic potential (Chinthanom et al., 2023b). Seven new lanostane triterpenoids (1–7) were isolated from cultivated Ganoderma wiiroense (strain TBRC-BCC 60613) and identified using NMR and mass spectrometry. The absolute configuration of C-23 in compound 1 was determined as 23S. Among the isolates, compound 7 exhibited antitubercular activity against M. tuberculosis H37Ra, with an MIC of 50 μg/ml (Yangchum et al., 2023). Antitubercular lanostane triterpenoids were isolated from the mycelial cultures of G. australe and structurally modified through semisynthesis. One of the synthetic compounds, GA003 (9), demonstrated greater potency against M. tuberculosis H37Ra than the natural lead compound (1). GA003 also exhibited significant activity against the virulent H37Rv strain and extensively drug-resistant tuberculosis strains (Chinthanom et al., 2021a). Three new lanostane triterpenoids, along with 21 known compounds, were isolated from the fruiting bodies of Ganoderma sichuanense. The absolute configuration at C-25 of ganoderic acid A and its derivatives was determined to be 25R using the phenylglycine methyl ester (PGME) method. Among the isolated compounds, ganoderiol F demonstrated the strongest activity against M. tuberculosis H37Ra, with a MIC value of 0.781 μg/ml (Chinthanom et al., 2021b). A new 3,4-seco-27-norlanostane triterpene, ganoboninketal D (1), and a new lanostane, (24S)-3-oxo-7α,24,25-trihydroxylanosta-8-ene (2), along with six known lanostanes, were isolated from G. orbiforme fruiting bodies. Structures were determined using NMR and mass spectrometry, with compounds 1 and 2 confirmed through chemical comparisons. Only compound 8 showed weak antitubercular activity, while the others were inactive against M. tuberculosis and P. falciparum (Isaka et al., 2017b).
6.5.5 Antiprotozoal activity
Ganoderma lucidum extracts showed potent antiprotozoal activity against Blastocystis hominis subtype 3, with a minimum inhibitory concentration of 62.5 μg/ml. At higher concentrations, the extract inhibited parasite growth by up to 90% within 12h. The key compound identified in G. lucidum was versalide, highlighting its potential for medicinal applications (Kaewjai et al., 2023). Table 7 examines the antimicrobial properties of Ganoderma species found in the lower Mekong region, highlighting their bioactive compounds and effectiveness against pathogenic microbes.
6.6 Metabolic and physiological regulation
Researchers cultivated mycelia from a wild Thai mushroom, G. australe, identified through PCR and morphological analysis. LC-MS/MS revealed bioactive compounds, including lovastatin. The extracts inhibited HMG-CoA reductase and increased HDL production in HepG2 spheroids to 71.35%, compared to 33.26% in controls and 32.13% with lovastatin alone. This study highlights the potential of G. australe as a functional food for hypercholesterolemia prevention (Wongkhieo et al., 2023).
The metabolic and physiological benefits of G. lucidum are also significant. Sornprasert (1995) highlighted its high protein content, which adds to its nutritional value. Studies like Futrakul et al. (2003) demonstrated that G. lucidum could suppress proteinuria and improve vascular function in patients with nephrotic syndrome, underscoring its potential for managing metabolic conditions. In patients with nephrotic syndrome and focal segmental glomerulosclerosis (FSGS) who had persistent proteinuria despite treatment with prednisolone, cyclophosphamide, and vasodilators, G. lucidum was introduced as an additional therapy. Initially, these patients showed increased endothelial cell cytotoxicity and an imbalance in cytokines, with elevated TNF-alpha and low IL-10 levels. Treatment with G. lucidum reduced endothelial cytotoxicity, restored cytokine balance, and successfully decreased proteinuria in all 14 patients (Futrakul et al., 2004). Ganomycin I (GMI) from G. lucidum inhibits RANKL-induced osteoclast formation, bone resorption, and related signaling pathways (MAPKs, c-Fos, NFATc1) without affecting cell viability. It downregulates osteoclast-specific genes, suggesting its potential as an anti-osteoporotic agent (Tran et al., 2019). Ganoderma lucidum was studied as a natural hypolipidemic agent for reducing lipid accumulation. Optimized enzyme and time conditions produced hydrolysates with high yield and hydrolysis, encapsulated in nanoscale liposomes. These liposomes promoted triglyceride breakdown in adipocytes and reduced lipid levels without harming cell viability. Proteomic analysis highlighted key proteins affected by treatment, suggesting the potential for G. lucidum hydrolysates in obesity management (Krobthong et al., 2021). The anti-diabetic potential of G. lucidum methanol-extracted components (1–15) were evaluated using molecular docking simulations. Component 1 (Butyl lucidenate P) showed the best α-glucosidase inhibition (DS -12.8 kcal/mol, RMSD 1.23 Å). QSARIS and ADMET analyses confirmed their biocompatibility and pharmacological suitability. Quantum-based analysis further supported the potential of components 1, 2, 11, and 13 for anti-diabetic applications (Nguyen et al., 2023g). Wild Ganoderma strains from northern Thailand, including G. orbiforme and G. sichuanense were rich in fiber, protein, fat, and carbohydrates. Both showed strong alpha-glucosidase inhibition, outperforming acarbose (Wannasawang et al., 2023).
Natural PPAR ligands were investigated in Vietnamese medicinal plants, fungi, and foods as potential alternatives to synthetic ligands for obesity and metabolic syndrome prevention. The extracts exhibited varying levels of PPAR agonistic activity, with fungi and certain plants showing strong PPARδ activity, indicating their potential as natural resources for preventing metabolic disorders (Ngoc et al., 2019). In Cambodia, a study on TCAM (Traditional, Complementary, and Alternative Medicine) used among patients with chronic diseases found that 27% consulted TCAM providers in the past year, with herbalists (17.3%) being the most common. The use of herbal medicine was reported by 41%, vitamins by 26.5%, and other supplements by 9.7%. The study also highlighted self-help practices such as praying for health (30.1%) and meditation (13.9%). Factors associated with TCAM use in Cambodia included older age, rural residence, and higher formal education. In addition, having two or more chronic conditions was linked to higher use of TCAM providers and products (Peltzer et al., 2016). The prevalence of herbal and dietary supplement (HDS) use among Thai patients with type 2 diabetes mellitus (DM) was found to be 61%, with 28% actively consuming them. Many patients did not inform their physicians, often citing a lack of concern, and 73% were unaware of potential drug-herb interactions. Common HDS included drumstick tree, turmeric, bitter gourd, and Ganoderma, influenced by social media and peer recommendations. These findings emphasize the importance of addressing HDS use in clinical practice to improve safety and glycemic management for Thai DM patients (Prasopthum et al., 2022). Table 8 explores the metabolic and physiological benefits of Ganoderma species found in the Lower Mekong Basin.
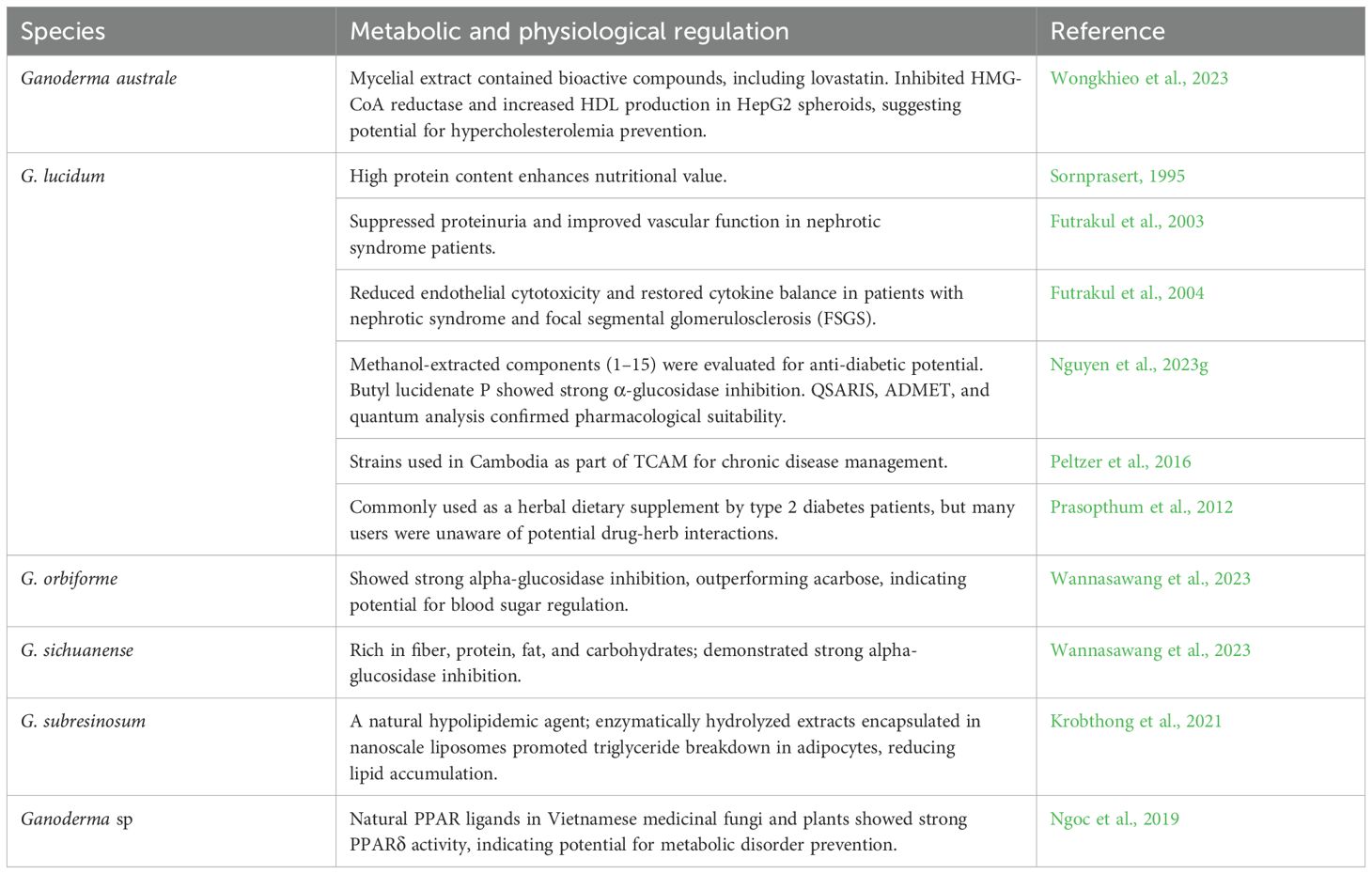
Table 8. Metabolic and physiological benefits of Ganoderma species found in the Lower Mekong basin and their bioactive compounds.
6.7 Toxicity and side effects
While G. lucidum offers numerous health benefits; its toxicity and side effects should not be overlooked. Wanachiwanawin et al. (2006) reported a case of pseudoparasitosis linked to the consumption of G. lucidum spores, while Wanmuang et al. (2007) noted hepatotoxicity in patients consuming G. lucidum powder. These studies indicate that, while generally safe, G. lucidum should be used with caution in individuals with liver problems or those who are particularly sensitive to its effects. A 49-year-old man in Thailand with non-Hodgkin’s lymphoma developed chronic diarrhea, which was linked to his consumption of powdered G. lucidum extract as a dietary supplement. Stool tests showed numerous G. lucidum spores, initially mistaken for parasitic infections. His diarrhea improved when he stopped ingesting the mushroom spores, and no further Ganoderma spores were found. This case highlights the importance of distinguishing fungal spores from parasitic organisms in stool samples to avoid misdiagnosis (Wanachiwanawin et al., 2006). Heavy metal levels and toxicity on G. lucidum were evaluated, showing trace amounts in fruit bodies and substrates. Toxicity to mycelial growth ranked highest for Hg and Cd, with Zn uptake reaching over 60% from substrates, resulting in high accumulation in fruitbodies and basidiospores (Tham et al., 1999). Hepatotoxicity linked to G. lucidum powder was first reported in 2004 with a Hong Kong (China) patient, followed by a fatal case of fulminant hepatitis in 2005. Both patients had previously consumed traditionally boiled G. lucidum without adverse effects. However, after switching to powdered G. lucidum for 1–2 months, they developed hepatotoxicity. The potential risks of G. lucidum powder, particularly when combined with other medications, warrant careful monitoring in the future (Wanmuang et al., 2007). Hepatoprotective effects of G. lucidum from dead ironwood trees in Vietnam’s Central Highlands were evaluated in mice with cyclophosphamide-induced liver toxicity (150 mg/kg, intraperitoneal). Oral administration of the extract (120, 230, and 330 mg/kg body weight) significantly reduced liver malondialdehyde (MDA) levels and restored glutathione (GSH) levels, showing efficacy comparable to silymarin. Histopathological analysis confirmed these findings, suggesting G. lucidum as a potential natural liver-protective agent (Pham et al., 2016). Wall-broken G. lucidum spores were processed via autoclaving and extracted with ethanol at varying concentrations. The 70% ethanol extract showed the highest triterpenoid content, predominantly ganoderic acid A, and the strongest antioxidant activity in the DPPH assay. Safe in mice at a dose of 2,000 mg/kg, it also demonstrated hepatoprotective effects by preventing serum ALT and AST elevation and reducing oxidative stress markers in cyclophosphamide-induced liver injury (Thuy et al., 2022).
6.8 Nutritional and health benefits
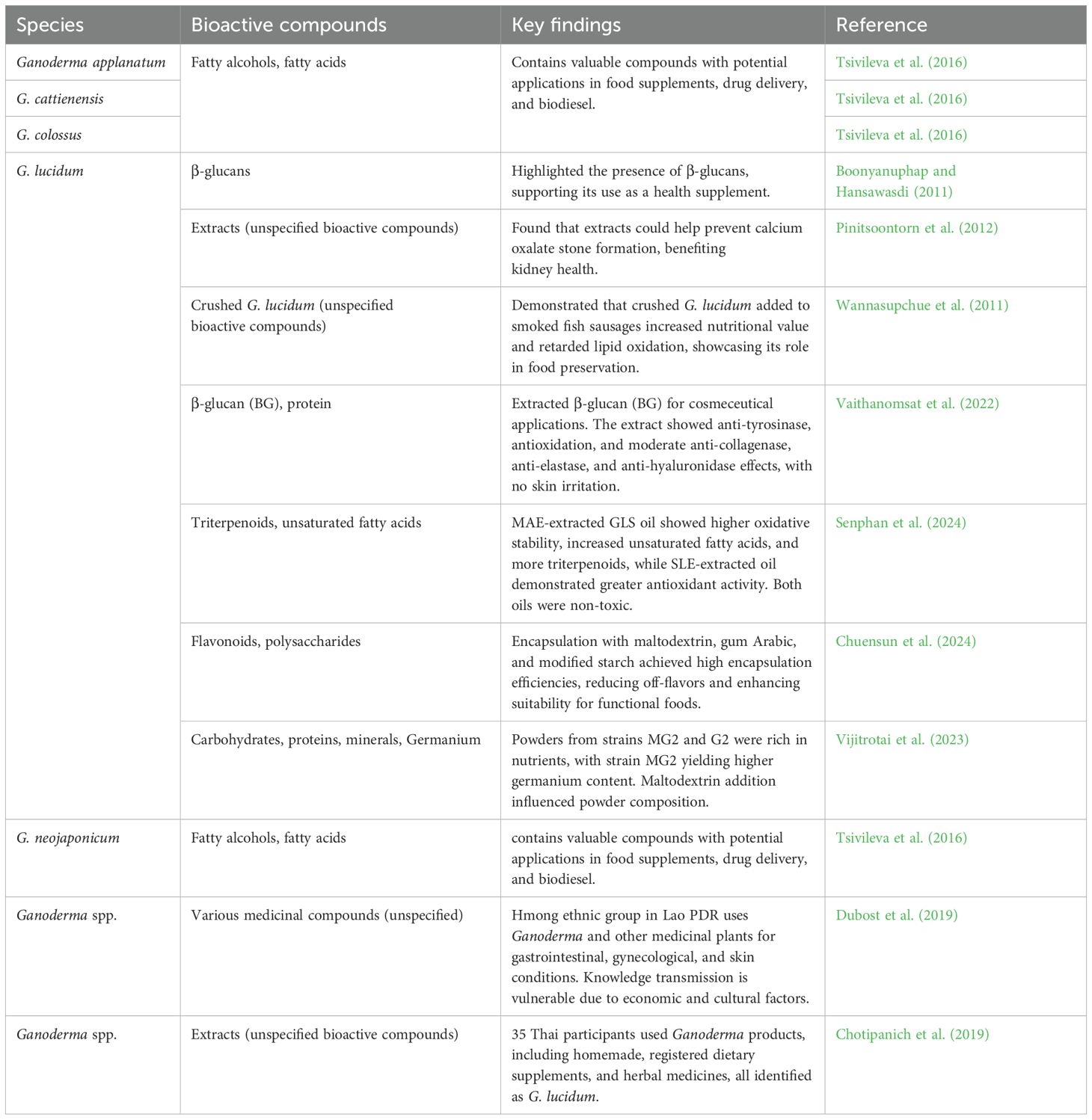
Nutritionally, G. lucidum is an excellent source of health-promoting compounds. Armassa et al. (2005) recognized it as a valuable nutritional food and alternative medicine for promoting longevity and overall well-being. Boonyanuphap and Hansawasdi (2011) highlighted the presence of β-glucans in G. lucidum, supporting its use as a supplement for improving health. Pinitsoontorn et al. (2012) also found that G. lucidum extracts could help prevent the formation of calcium oxalate stones, making it a beneficial supplement for kidney health. Wannasupchue et al. (2011) demonstrated that adding crushed G. lucidum to smoked fish sausages increased nutritional value and helped retard lipid oxidation, showcasing its role in food preservation. The antler-type fruiting body of G. lucidum was used to extract β-glucan (BG) for cosmeceutical applications. The extract, containing 40.57% BG and 7.47% protein, demonstrated anti-tyrosinase and antioxidation activities, suggesting potential for skin whitening. It also showed moderate anti-collagenase, anti-elastase, and anti-hyaluronidase effects. Skin irritation tests were negative, and BG had no significant effect on cell viability. The extract outperformed commercially available BG in oil-binding capacity, indicating its promising potential for the cosmeceutical industry (Vaithanomsat et al., 2022). Ganoderma lucidum G2 spore (GLS) oils extracted by Soxhlet (SLE) and microwave-assisted extraction (MAE) showed distinct properties. MAE-extracted oil had higher oxidative stability, increased unsaturated fatty acids, and more triterpenoids, while SLE-extracted oil demonstrated greater antioxidant activity. Both oils were non-toxic to Caco-2 cells, suggesting MAE-extracted GLS oil as a potential health supplement (Senphan et al., 2024). The sensory profile of G. lucidum was characterized to address challenges of bitterness and undesirable flavors. Fresh, dried, and extract forms were analyzed, showing earthy and mushroomy notes as dominant flavors. The study achieved high encapsulation efficiencies for key components like flavonoids and polysaccharides using encapsulation with 32.75% maltodextrin, 42.25% gum Arabic, and 25% modified starch. Gas chromatography electronic nose (GC-E-Nose) analysis detected ten primary flavor compounds, and encapsulation effectively reduced off-flavors. This method enhances the suitability of G. lucidum for incorporating instant beverages and other functional foods (Chuensun et al., 2024). Ganoderma lucidum (Lingzhi) powders from strains MG2 and G2 were evaluated for nutritional quality. The powders were prepared using water extraction and spray drying, with or without maltodextrin. Both strains provided good energy sources, rich in carbohydrates, proteins, and minerals, with strain MG2 yielding higher germanium content than G2. Maltodextrin addition also influenced the composition of the powders (Vijitrotai et al., 2023).
Five Ganoderma species (G. colossus, G. neojaponicum, G. cattienensis, G. lucidum, and G. applanatum) from Vietnamese National Parks, along with three strains from Europe and Siberia, were analyzed for their morphology, cultural characteristics, and chemical constituents. Valuable compounds such as fatty alcohols and acids, identified through gas chromatography, have potential applications in food supplements, drug delivery, and biodiesel (Tsivileva et al., 2016). The Hmong ethnic group in Lao PDR relies extensively on medicinal plants, including Ganoderma, for primary healthcare, with knowledge passed orally and kept within families. A study documented 333 medicinal species, highlighting their use for gastrointestinal, gynecological, and skin conditions, although knowledge transmission remains vulnerable due to economic and cultural factors (Dubost et al., 2019). In a study, 35 (16.2%) of Thai participants used Ganoderma, with 6 using homemade products, 17 using registered dietary supplements, 5 using registered herbal medicines, and 8 using undetermined products, all of which were identified as G. lucidum (Chotipanich et al., 2019). Table 9 outlines the nutritional and health benefits of Ganoderma species found in the Lower Mekong Basin.
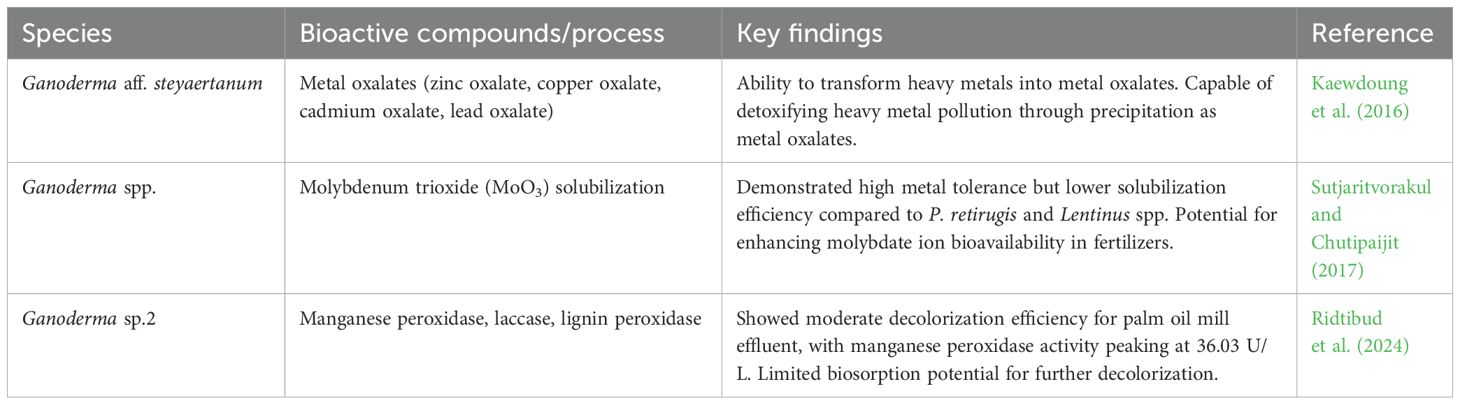
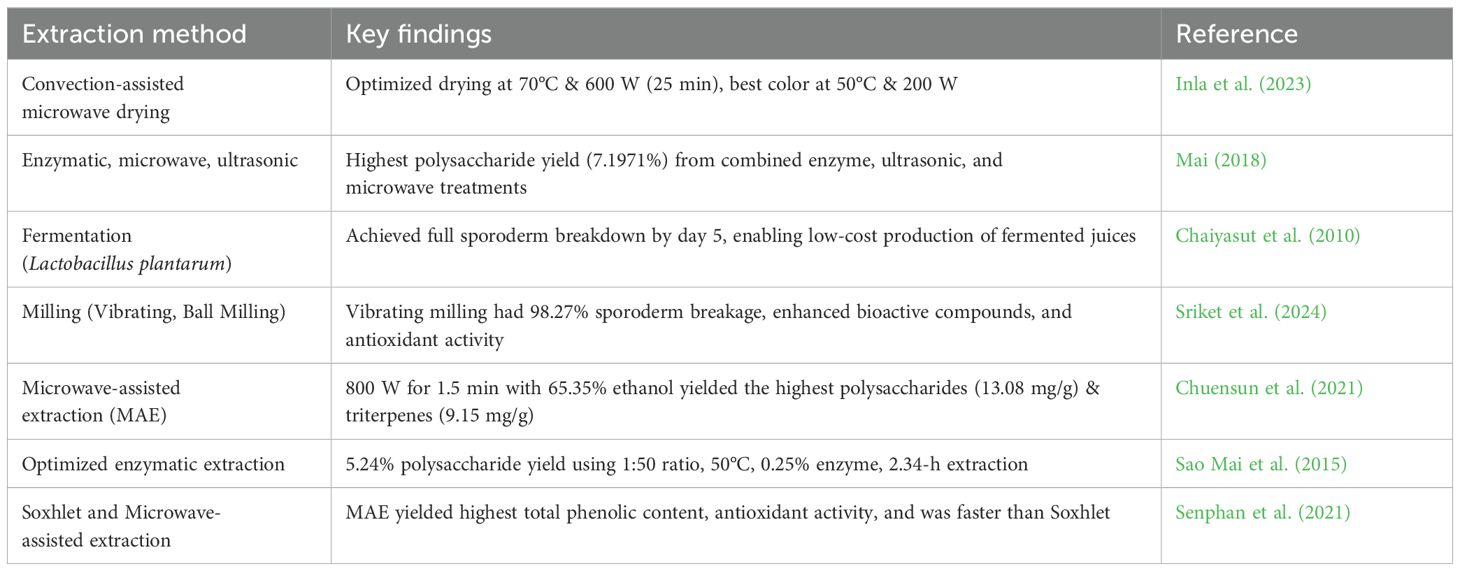
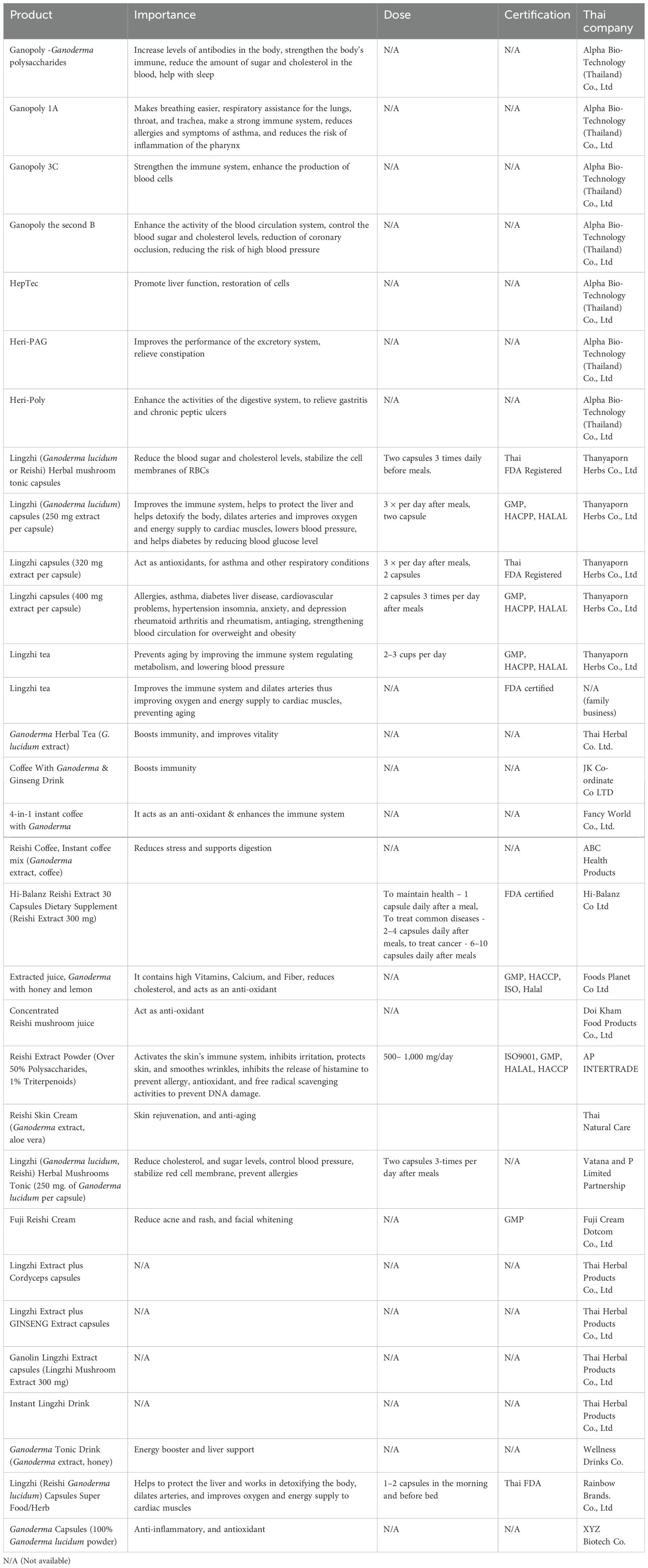
6.9 Enzyme production and industrial applications
Laccase enzymes have diverse industrial applications due to their catalytic oxidation abilities (Asad et al., 2024; Scheibel et al., 2024; Wang et al., 2025). These multicopper oxidases are essential for lignin degradation, textile dye decolorization, bioremediation, and bioactive compound synthesis (Deng et al., 2024; Liu et al., 2024; Tayar et al., 2025). Their utility in wastewater treatment, pulp and paper processing, and pharmaceuticals has been extensively explored (Hanafiah et al., 2024; Hultberg and Golovko, 2024; Pandey and Gupta, 2024). Given their eco-friendly nature and adaptability, laccases serve as valuable biocatalysts in industrial processes.
Ganoderma species also play a significant role in environmental monitoring due to their ability to bioaccumulate heavy metals, radionuclides, and pollutants, making them effective bioindicators of contamination (Vyas and Kulshrestha, 2023; Xu et al., 2024; Song et al., 2025). Studies have demonstrated that G. lucidum can absorb and retain cesium-137, lead, and cadmium, aiding in pollution assessment (Ipeaiyeda et al., 2020; Jin et al., 2023; El-Baz et al., 2024). In addition, their enzymatic activity, particularly laccase production, contributes to biodegradation and bioremediation, further highlighting their ecological significance.
Studies like Chuwech and Rakariyatham (2014) observed that G. australe produced cellulase during fermentation, suggesting potential applications in enzyme production. Khammuang (2009) and Punnapayak et al. (2009) highlighted the laccase activity of G. lucidum, which has applications in both industrial dye decolorization and environmental cleanup efforts, demonstrating its broad industrial utility. Ganoderma lucidum cultivated in Vietnam was found to contain 137Cs at a concentration of approximately 20 Bq/kg fresh weight, likely accumulated directly from the atmosphere rather than the substrata. While this indicates potential environmental exposure, it also suggests that G. lucidum could serve as a bioindicator for monitoring radioactive contamination (Van and Le Duy, 1991). Ganoderma has proven industrial applications, particularly in enzyme production. Laccase from G. lucidum Chaaim-001 BCU effectively degraded 16 polycyclic aromatic hydrocarbons (PAHs). Complete degradation of anthracene occurred with or without a redox mediator. With the mediator, degradation rates for benzo[a]pyrene, fluorene, acenaphthene, acenaphthylene, and benzo[a]anthracene reached up to 100%, 98.6%, 95.4%, 90.1%, and 85.3%, respectively. In the absence of the mediator, the degradation rates decreased to 71.71%, 62.9%, 80.49%, 85.85%, and 9.14%, respectively. Compared to Trametes versicolor, G. lucidum laccase demonstrated higher degradation efficiency, particularly in the absence of the mediator. This could be due to the unique structural and catalytic properties of the laccase enzyme from G. lucidum, which might allow for more efficient interactions with the PAHs without the need for a redox mediator. These findings highlight its potential for bioremediation of PAHs (Punnapayak et al., 2009). Laccase from Ganoderma sp., isolated in northeast Thailand, was purified and characterized for its enzymatic properties. Among three strains tested, Ganoderma sp. 03 showed the highest laccase activity when cultured with rice bran and husk. The enzyme, with a molecular weight of 39.81 kDa, had optimal activity at pH 3.5 and 40–55°C, and showed best stability at 30°C. Laccase activity was strongly inhibited by DTT and p-coumaric acid, and reduced by most metal ions except ZnSO4. These results highlight the enzyme's potential for industrial and environmental applications (Sarnthima et al., 2022). In Vietnam, Ganoderma is valued for medicinal use, but its classification often relies on inaccurate morphological traits. Using ITS-based DNA barcoding, 10 commercial genotypes were analyzed, revealing genetic diversity with distances ranging from 0.000 to 0.047. Seven samples were reclassified as G. lingzhi and three as G. lucidum. ITS barcoding provides a precise and reliable method for species identification, reducing misclassification that could impact product consistency and efficacy. Accurate classification ensures quality control in commercial Ganoderma products, helping to standardize medicinal properties and maintain consumer confidence in the industry (Viết Thế et al., 2019). Ganoderma lucidum offers high nutritional value and health benefits but is highly perishable due to its short shelf life. To enhance its value, a process for canning white G. lucidum was developed, focusing on preserving total phenolic content and antioxidant activity. Optimal conditions were cooking for 4 min at 90°C with 5 g/L of salt. The resulting canned G. lucidum can serve as a functional food (Minh et al., 2019). Quantifying triterpenoids in G. lucidum mycelium is challenging due to low concentrations and matrix complexity. A study developed an HR-QTOF-MRM method to quantify seven target triterpenoids, which outperformed the QQQ-MRM method by providing lower detection limits, better reproducibility, and higher accuracy (84%–99% accuracy vs. 69%–114%). Recovery rates for HR-QTOF-MRM ranged from 80% to 117%. Triterpenoid concentrations ranged from 0.06 to 6.72 mg/g in the fruiting body and 0.0009–0.01 mg/g in the mycelium. The HR-QTOF-MRM method improves sensitivity and precision for trace triterpenoid analysis in complex samples (Kaewnarin et al., 2021). Ganoderma lucidum extract (GE), rich in ergosterol, flavonoids, and triterpenoids, shows strong antioxidant activity but requires intestinal-targeted delivery. This study developed multilayer microcapsules using sodium alginate (SA) as the primary layer and chitosan (CS) to create SA-CS polyelectrolyte layers for GE encapsulation. The SA-CS layers enhanced controlled release in intestinal conditions (82.15 ± 3.99%) and protected GE from acid, bile, trypsin, and heat, extending its shelf life. This microencapsulation system effectively preserves the active antioxidant compounds in GE for improved delivery and stability (Petwattanapha et al., 2024).
Laccase production in Ganoderma sp. KU-Alk4 was influenced by glucose concentration, regulating isozyme expression with molecular masses of 53–112 kDa. A Box–Behnken factorial design optimized culture conditions, increasing laccase activity 12-fold to 240 U/ml. The isozymes exhibited high thermal stability, retaining full activity at 60°C for 1h and optimal activity at pH 3.5. These findings highlight Ganoderma sp. KU-Alk4 as a promising source of laccase for industrial applications (Teerapatsakul et al., 2007a, b). Table 10 explores the enzyme production and industrial applications of Ganoderma species found in the Lower Mekong Basin.
7 Bioremediation
In a study of 18 wood-rotting fungi isolated from Thailand, 5 strains were found to produce mycogenic crystals when grown on media amended with zinc, copper, cadmium, and lead salts. Notably, Ganoderma aff. steyaertanum was identified for the first time for its ability to transform heavy metals into metal oxalates. These fungi were capable of converting zinc sulfate into zinc oxalate, copper sulfate into copper oxalate, cadmium sulfate into cadmium oxalate, and lead nitrate into lead oxalate. The results suggest that wood-rotting fungi, including Ganoderma aff. steyaertanum, can be used for the detoxification of heavy metal pollution through precipitation as metal oxalates (Kaewdoung et al., 2016). Four wood-decaying fungi—Polyporus retirugis, Trametes spp., Lentinus spp., and Ganoderma spp.—were screened for their ability to tolerate and solubilize molybdenum trioxide (MoO3). Polyporus retirugis and Lentinus spp. exhibited the highest solubilization efficiency, producing clear zones over 40 mm on molybdenum-supplemented agar. All strains demonstrated high metal tolerance, although increasing MoO3 concentrations reduced mycelial biomass. These findings suggest that these fungi could be effective agents for enhancing molybdate ion bioavailability in fertilizers containing molybdenum compounds (Sutjaritvorakul and Chutipaijit, 2017). Ganoderma species, including Ganoderma sp. 2, were assessed for decolorizing POME, with moderate efficiency observed. Ganoderma sp. 2 showed notable decolorization but was less effective than Trametes elegans. Manganese peroxidase activity peaked at 36.03 U/L, while laccase and lignin peroxidase had minimal activity. Biosorption tests with mycelial biomass resulted in a 12.5% color reduction, suggesting limited further decolorization potential through biomass. These results underscore the environmental application potential of Ganoderma (Ridtibud et al., 2024). Table 11 examines the bioremediation potential of Ganoderma species in the Lower Mekong Basin.
7.1 Extraction methods
Ganoderma lucidum spores contain abundant bioactive compounds, but their hard sporoderm limits absorption. Traditional spore-breaking methods are costly and inefficient. A study used Lactobacillus plantarum fermentation to break down the sporoderm, achieving full breakdown by day 5. This low-cost method enables the production of beneficial fermented juices with G. lucidum spores, with further research needed on the breakdown mechanism (Chaiyasut et al., 2010). Polysaccharides from Vietnamese G. lucidum spores were extracted using enzymatic, microwave, and ultrasonic methods. The highest yield was achieved with enzymatic extraction using a 2.3% cellulase concentration, 150 min of treatment, and a temperature of 50°C. The complex method, combining preheating, enzyme treatment, ultrasonic, and microwave treatment, resulted in the highest polysaccharide yield of 7.1971% (Mai, 2018) a study optimized ethanol-modified SC-CO₂ extraction for triterpenoids from Ganoderma lucidum. Optimal conditions (380 bar, 7% ethanol, 60°C) yielded 1.49g/100g, outperforming traditional methods. The process followed a second-order kinetic model, confirming SC-CO₂ as an efficient extraction technique (Phan et al., 2023). In Vietnam, G. lucidum grows year-round and is a valuable source of polysaccharides. An optimized enzymatic extraction process, guided by a central composite design, identified ideal conditions: a 1:50 G. lucidum i-to-water ratio, 50°C solvent temperature, 0.25% enzyme concentration, and 2.34-h extraction time, yielding 5.24% polysaccharides. This efficient method is well-suited for industrial application (Sao Mai et al., 2015). Soxhlet and MAE methods yielded the highest extraction rates and total phenolic content from broken G. lucidum spores. However, MAE was faster than Soxhlet extraction. Ethanol extraction using MAE produced the highest total phenolic content and ferric-reducing antioxidant power (FRAP) value. Ethanol and hexane extract also showed significant metal chelating activity. These results highlight the importance of selecting the right extraction method and solvent to obtain extracts with high antioxidant properties (Senphan et al., 2021). Ganoderma lucidum was dried using convection-assisted microwave drying to improve shelf life. Drying rates increased, and drying times decreased with higher air temperatures (50°C–70°C) and microwave powers (200–600 W). Optimal drying (25 min) was achieved at 70°C and 600 W, while the best color retention and rehydration were at 50°C and 200 W. The drying model accurately predicted moisture content (Inla et al., 2023). The impact of different processing techniques on G. lucidum G2 spores (GLS) were evaluated. Vibrating and ball milling increased lipid content and bioactive compounds, with vibrating milling achieving the highest sporoderm breakage (98.27%). Both milling methods enhanced DPPH radical scavenging activity and altered GLS structure, increasing crystallinity. Ball milling led to the highest lipid oxidation. FT-IR and x-ray diffraction analyses revealed key compounds and structural changes. These findings provide valuable insights for utilizing GLS in various applications (Sriket et al., 2024). Ganoderma lucidum was evaluated for nutrient and antioxidant extraction under various drying and extraction conditions. Optimal drying at 80°C for 1h and 37 min yielded 1.17 mg/g flavonoids and 11.49 mg/g triterpenes. MAE at 800 W for 1.5 min with 65.35% ethanol provided the highest yields, with 13.08 mg/g polysaccharides and 9.15 mg/g triterpenes. MAE was more efficient than other methods, offering time, solvent savings, and high extraction efficiency for dried G. lucidum (Chuensun et al., 2021). Table 12 explains the methods used in the extraction of Ganoderma species in the Lower Mekong Basin.
8 Market potential of Ganoderma in Lower Mekong Basin: export and import dynamics
The global Ganoderma extract market was valued at approximately USD 2.1 billion in 2023 and is projected to reach around USD 4.5 billion by 2032, growing at a compound annual growth rate (CAGR) of 9.0%. This growth is driven by increasing consumer awareness of its potential health benefits, such as immune system support, anti-cancer properties, liver protection, and cardiovascular health. (Data Bridge Market Research, 2021; Ganoderma lucidum Extract Market Research Report, DataIntelo 2023). The Ganoderma trade in Southeast Asia, particularly in Vietnam, Cambodia, Laos, and Thailand, is part of a rapidly expanding global market driven by increasing consumer demand for health products derived from medicinal mushrooms. The Ganoderma trade in Southeast Asia, particularly in Vietnam, Cambodia, Laos, and Thailand, is part of a rapidly expanding global market driven by increasing consumer demand for health products derived from medicinal mushrooms (Putthapiban et al., 2017). These countries engage in both exports and imports of Ganoderma, each contributing uniquely to regional trade while facing distinct challenges and opportunities.
Thailand is an emerging exporter of Ganoderma, with raw mushrooms and processed products like powders, extracts, and capsules being the primary exports (Figure 2). The price for raw Ganoderma in Thailand is approximately USD 10–14 per kilogram, while processed products are priced at USD 20–35 per kilogram, depending on quality and packaging. Key export destinations include China, Japan, and Europe, with Thailand focusing on organic Ganoderma to cater to the growing wellness market (Sornchareon and Methiyothin, 2020). Thailand imports finished Ganoderma products, particularly from Vietnam and China. These imports are primarily consumed in the health supplement sector, with increasing demand for natural remedies. Thailand’s agricultural imports in 2023 were significant, with Ganoderma products making up a portion of the wellness products sector (Tan, 2023). Vietnam is a leader in the production and export of Ganoderma products. The country exports raw mushrooms, powders, and capsules primarily to China, Japan, South Korea, the United States, and Europe. Raw Ganoderma is priced at USD 8–15 per kilogram, while processed products such as powder and capsules are priced at USD 20–30 per kilogram. In 2023, Vietnam’s agricultural export value surpassed USD 53 billion, with Ganoderma playing a growing role in this sector (Department of Foreign Trade, Thailand, 2023; General Statistics Office of Vietnam, 2024). Despite its strong production capacity, Vietnam imports Ganoderma from Cambodia and Laos for processing, which it re-exports in value-added forms. The country also imports some finished Ganoderma products from China. Vietnam’s total agricultural imports in 2023 amounted to USD 267 million, which includes raw Ganoderma (KPL News, 2023; The Star, 2024).

Figure 2. Ganoderma-based products (a) Dried Ganoderma lucidum (https://www.mustthai.com/) (b) Instant Lingzhi drink (https://sakasaka.net) (c) Ganoderma lucidum spore powder (https://thethaiday.com/) (d) Ganoderma tea (https://thethaiday.com) (e) Ganoderma tea slices (https://rstspices.com) (f) Ganoderma lucidum spore oil (www.thaiherbalproducts.com) (g) Ganoderma lucidum capsules (https://english.thaicare100.com) (h) Ganoderma lucidum polysaccharides (https://traditionalherbalstore.com).
Cambodia mainly exports raw Ganoderma to Vietnam for further processing. The price for fresh Ganoderma is around USD 10–12 per kilogram. While the overall export of Ganoderma from Cambodia is limited, the country’s agricultural exports increased by 37% in 2023, with Vietnam being a key destination for these niche products (Phnom Penh Post, 2024; The Star, 2024). Cambodia imports processed Ganoderma products like capsules and powdered extracts from Vietnam and China. These products are typically priced higher due to the value-added processing in the exporting countries. Demand for Ganoderma supplements is growing, especially in urban centers like Phnom Penh and Siem Reap (The Star, 2024). Laos exports raw Ganoderma to Vietnam, where they are processed and sold internationally. The price of raw Ganoderma is approximately USD 8–10 per kilogram. In 2023, Laos’ total export value to Vietnam reached USD 565 million, including agricultural goods like Ganoderma (KPL News, 2023). Laos imports value-added Ganoderma products from Vietnam and China, including medicinal capsules and extracts. These products cater to a small but growing domestic market, especially in Vientiane, which is becoming a hub for health-conscious consumers (World Bank, 2023; ASEAN Trade Database, 2024; Data Bridge Market Research, 2022).
The Ganoderma market in Southeast Asia, particularly in Vietnam, Cambodia, Laos, and Thailand, faces common challenges, including the lack of standardized production methods, inconsistent quality control, and limited processing infrastructure. These issues, compounded by taxonomic ambiguities that complicate species identification, hinder market growth. However, there are significant opportunities for expansion. Technological advancements in molecular techniques, coupled with sustainable agricultural practices, could address these challenges and enhance production consistency. Regional collaboration, investment in research and development, and the integration of local knowledge with modern science could unlock further potential for the Ganoderma market. With the rising global demand for Ganoderma-based health products, particularly in immune support and anti-cancer treatments, the traditional significance of this medicinal mushroom adds substantial value to its market potential. By focusing on sustainable cultivation and improving processing capabilities, these countries can strengthen their positions in both regional economies and international trade. Tables 13–15 list Ganoderma-based products in Thailand, Vietnam, Laos, and Cambodia, highlighting their diverse applications in health supplements, functional foods, and traditional medicine.
9 Future research directions
Building on the current understanding of Ganoderma species, future research should explore several critical areas to enhance its medicinal, ecological, and commercial potential. First, there is a need for more comprehensive molecular studies to refine the taxonomy of Ganoderma, addressing current challenges in species identification. Advanced techniques like genome sequencing and multi-locus phylogenetic analysis can provide deeper insights into the genetic diversity and evolutionary relationships within the genus, which is essential for accurate classification and quality control.
Secondly, research into sustainable cultivation methods should be prioritized to increase production efficiency while maintaining the ecological balance. Identifying optimal cultivation substrates, environmental factors, and biotechnological interventions for enhancing bioactive compound yield will be crucial for improving the sustainability and profitability of Ganoderma farming. Moreover, further investigations into the pharmacological mechanisms of Ganoderma bioactive compounds are needed to better understand their therapeutic effects. This includes studying the molecular pathways by which Ganoderma-derived compounds exert their immunomodulatory, anticancer, and anti-inflammatory effects, thus advancing the potential for novel drug development.
In parallel, research into the ecological roles of Ganoderma species, especially their interactions with soil ecosystems and their potential use in bioremediation and agriculture, can provide insights into their broader environmental significance. Specifically, their capacity to degrade pollutants, including polycyclic aromatic hydrocarbons (PAHs), warrants further exploration to assess their applicability in sustainable environmental practices. Finally, with the growing commercial interest in Ganoderma-based products, more emphasis should be placed on standardizing the quality control processes for both cultivation and product development. Establishing uniform criteria for bioactive compound content, consistency in therapeutic effects, and product safety will ensure the reliability of Ganoderma-derived products in the global market. By addressing these areas, future research will contribute to the sustainable development of the Ganoderma industry, maximize its medicinal benefits, and expand its applications in diverse sectors, including healthcare, agriculture, and environmental protection.
10 Conclusion
Ganoderma species, particularly G. lucidum, hold significant medicinal and economic value in the Lower Mekong Basin, where demand for their bioactive compounds is increasing. ITS-based DNA barcoding has improved species identification, addressing taxonomic challenges, while optimization of cultivation methods enhances production efficiency. Despite their therapeutic potential, challenges such as inconsistent cultivation, sustainability concerns, and quality control persist. To maximize their applications in medicine, agriculture, and industry, standardized cultivation practices and regulatory measures are essential. Strengthening research on Ganoderma taxonomy, ecological roles, and commercial viability will position the Lower Mekong Basin as a leader in sustainable Ganoderma production, benefiting both local economies and global healthcare.
Author contributions
SK: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. NP: Methodology, Software, Writing – original draft, Writing – review & editing. TL: Data curation, Writing – original draft, Writing – review & editing. KH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Samantha C. Karunarathna thanks the High-Level Talent Recruitment Plan of Yunnan Province (“High-End Foreign Experts” Program), the National Natural Science Foundation of China (Grant No. 32260004), and the Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River, Qujing Normal University, Qujing, Yunnan 655011, China, for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anothai, J., Chairin, T. (2020). Soil physicochemical properties closely associated with fungal enzymes and plant defense enzymes in Ganoderma-infected oil palm orchards. Plant Soil 456, 99–112. doi: 10.1007/s11104-020-04705-y
Anothai, J., Chairin, T. (2022). Analysis of rhizobacterial community associated with the occurrence of Ganoderma basal stem rot disease in oil palm by Illumina next-generation sequencing. Arch. Microbiol. 204, 31. doi: 10.1007/s00203-021-02670-3
Anothai, J., Chairin, T. (2024). Development of integrated factor modeling: Inhibiting Ganoderma lignocellulosic enzymes while promoting Trichoderma sporulation for enhanced plant disease control. Physiol. Mol. Plant Pathol. 133, 102382. doi: 10.1016/j.pmpp.2024.102382
Anothai, J., Intaraanun, S., Samlikamnoed, P., Chairin, T. (2023). Understanding factors influencing growth and lignocellulosic enzyme of Ganoderma for developing integrated control strategies for basal stem rot disease in oil palm. Trop. Plant Pathol. 48, 154–162. doi: 10.1007/s40858-022-00551-8
Apiwatanapiwat, W., Janchai, P., Vaithanomsat, P., Boondaeng, A., Meelaksana, J., Trakunjae, C., et al. (2024). Polysaccharide, triterpenoid, and phenolic compounds of antler-type Ganoderma sichuanense grown with fruit peels supplementation in liquid culture. Heliyon.
Armassa, N., Thamsirirak, S., Rodtong, S., Sanoamuang, N. (2005). Nutritional and medicinal potential of twenty-three wild mushrooms from Northeast Thailand. Int. J. Med. Mushrooms 7, 374–375. doi: 10.1615/IntJMedMushr.v7.i3.280
Armassa, N., Poungchompu, O., Rayan, S., Leethong, S., Weerapreeyakul, N., Machana, S. (2009). Antioxidant activity and cytotoxicity in breast cancer cells line of mushrooms extracts; Lentinus polychrous Lev. compared to Ganoderma lucidum (Fr.) karst. Isan J. Pharm. Sci. 5 (3), 243–250.
Asad, S., Gu, P., Peng, C., Huang, H., Jiang, F., Patabedige, N., et al. (2024). Biotechnological potential of Ganoderma species: Current progress and future prospects. N. Z. J. Bot., 1–60. doi: 10.1080/0028825X.2024.2376924
ASEAN Trade Database. (2024). HS Code 130219 (Plant Extracts) Trade Flows [Internet]. ASEANstats. Available online at: https://www.aseanstats.org.
Augsornthip, C., Neranon, P., Phukpattaranont, P., Romyen, A. (2024). Sound classification for non-destructive diagnosis of basal stem rot disease based on stem density in oil palm trunks. J. Ad. Res. App. Mech. 124, 19–38. doi: 10.37934/aram.124.1.1938
(2021). Global reishi mushroom market - data bridge market research (Data Bridge Market Research). Available online at: https://www.databridgemarketresearch.com/reports/global-reishi-mushroom-market (Accessed 27 November 2024).
Ayimbila, F., Siriwong, S., Chaiyama, V., Srihanant, N., Keawsompong, S. (2023). Comparative study of bio-functional profile and bioactivities of polysaccharides from Ganoderma lucidum and Ganoderma neo-japonicum. Biocatal. Agric. Biotechnol. 53, 102875. doi: 10.1016/j.bcab.2023.102875
Azi, F., Wang, Z., Chen, W., Lin, D., Xu, P. (2024). Developing Ganoderma lucidum as a next-generation cell factory for food and nutraceuticals. Trends Biotechnol. 42, 197–211. doi: 10.1016/j.tibtech.2023.07.008
Binh, P. T., Descoutures, D., Dang, N. H., Nguyen, N. P., Dat, N. T. (2015). A new cytotoxic gymnomitrane sesquiterpene from Ganoderma lucidum fruiting bodies. Nat. Prod Commun. 10 (11), 1911–1912.
Boonyanuphap, J., Hansawasdi, C. (2011). Spatial distribution of beta glucan containing wild mushroom communities in subtropical dry forest, Thailand. Fungal Diversity 46, 29–42. doi: 10.1007/s13225-010-0067-8
Boukaew, S., Cheirsilp, B., Yossan, S., Khunjan, U., Petlamul, W., Prasertsan, P. (2022). Utilization of palm oil mill effluent as a novel substrate for the production of antifungal compounds by Streptomyces philanthi RM-1-138 and evaluation of its efficacy in suppression of three strains of oil palm pathogen. J. App. Microbiol. 132, 1990–2003. doi: 10.1111/jam.15304
Cabarroi-Hernández, M., Decock, C., Welti, S., Amalfi, M., Rosa Villalobos-Arámbula, A., Aliaga-Ramos, D., et al. (2023). Ganoderma from Cuba: an approach to some species based on morphology and phylogenetic analyses. Biol. J. Linn. Soc 140, 323–357. doi: 10.1093/biolinnean/blad055
Chaiharn, M., Phutdhawong, W. S., Amornlerdpison, D., Phutdhawong, W. (2018). Antibacterial, antioxidant properties and bioactive compounds of Thai cultivated mushroom extracts against food-borne bacterial strains. Chiang Mai J. Sci. 45, 1713–1727. Available at: http://cmuir.cmu.ac.th/jspui/handle/6653943832/64155 (Accessed: April 15, 2025).
Chaisit, P., Wethi, W., Seephueak, P. (2017). Screening for plant extract, antagonistic microorganism and fungicides to control Ganoderma boninense caused stem rot of oil palm in vitro. IJAT 13, 141–147. Available at: https://www.thaiscience.info/journals/Article/IJAT/10985411.pdf (Accessed: April 15, 2025).
Chaiyasut, C., Kruatama, C., Sirilun, S. (2010). Breaking the spores of Ganoderma lucidum by fermentation with Lactobacillus plantarum. Afr. J. Biotechnol. 9, 7379–7382. doi: 10.5897/AJB09.1085
Chalermpongse, A. (1991a). Current potentially dangerous forest tree diseases in Thailand (Indonesia: Biotrop Special Publication).
Chalermpongse, A. (1991b). “Fungal diseases in mangrove ecosystems,” in Proceedings of the 5th silviculture seminar in Thailand (Division of Silviculture, Royal Forest Department, Bangkok, Thailand), 307–338.
Chalermpongse, A. (1999). Biodiversity dynamics of ectomycorrhizal and wood-rotting fungi in forested watershed areas of western Thailand. Thai Journal of Forestry 18, 9–29. Available at: https://www.thaiscience.info/journals/Article/TJOF/10470683.pdf (Accessed: April 15, 2025).
Chandrasrikul, A., Visessung, A., Chr, V. (1978). Medicine from fungi in Thailand. presented at: 16th annual conference on agricultural and biological science (Bangkok, Thailand: Kasetsart University).
Chandrasrikul, A., Choobamrung, W., Po-ngern, K. (1986). Species of Ganoderma and allied genera. In Proceedings of the 24th National Conference: Poster Session, Bangkok, Thailand, 27–29 January 1986 (pp. 193–199). Kasetsart University.
Chang, H. Y., Huh, Y. J., Soeun, P., Lee, S. H., Song, I., Sophatt, R., et al. (2016). Thermophile mushroom cultivation in Cambodia: Spawn production and development of a new substrate, acacia tree sawdust. J. Mushroom 14, 1–5. doi: 10.14480/JM.2016.14.1.1
Chanphen, R., Pruksatrakul, T., Choowong, W., Choeyklin, R., Surawatanawong, P., Isaka, M. (2024). Ganopyrone A, a highly rearranged lanostane triterpenoid with antimalarial activity from artificially cultivated fruiting bodies of Ganoderma colossus. Phytochemistry 224, 114168. doi: 10.1016/j.phytochem.2024.114168
Cheeptham, N., Towers, G. H. (2002). Light-mediated activities of some thai medicinal plant teas. Fitoterapia 73 (7-8), 651–662. doi: 10.1016/S0367-326X(02)00224-1
Chen, X. J., Deng, Z., Zhang, L. L., Pan, Y., Fu, J., Zou, L., et al. (2024). Therapeutic potential of the medicinal mushroom Ganoderma lucidum against Alzheimer’s disease. Biomed. Pharmacother. 172, 116222. doi: 10.1016/j.biopha.2024.116222
Chinthanom, P., Choowong, W., Thummarukcharoen, T., Chen, H. P., Liu, J. K., Isaka, M. (2022a). Lanostane triterpenoids from mycelial cultures of the basidiomycete Ganoderma weberianum. Phytochem. Lett. 51, 12–17. doi: 10.1016/j.phytol.2022.06.009
Chinthanom, P., Dokladda, K., Vichai, V., Choeyklin, R., Thongpanchang, C., Isaka, M. (2023a). Chemical analysis and antitubercular activity evaluation of the dried mycelial powders of the basidiomycete Ganoderma australe TBRC-BCC 22314. Fitoterapia 169, 105597. doi: 10.1016/j.fitote.2023.105597
Chinthanom, P., Sappan, M., Srichomthong, K., Boonpratuang, T., Isaka, M. (2022b). Colossolactone J, a highly modified lanostane triterpenoid from a natural fruiting body of Ganoderma colossus. Nat. Prod Res. 37, 2639–2646. doi: 10.1080/14786419.2022.2124987
Chinthanom, P., Srichomthong, K., Rachtawee, P., Boonpratuang, T., Choeyklin, R., Feng, T., et al. (2021a). Lanostane triterpenoids from cultivated fruiting bodies of Ganoderma sichuanense: Determination of the C-25 absolute configuration of ganoderic acid A and its derivatives using the phenylglycine methyl ester (PGME) method. Phytochemistry 192, 112963. doi: 10.1016/j.phytochem.2021.112963
Chinthanom, P., Vichai, V., Dokladda, K., Sappan, M., Thongpanchang, C., Isaka, M. (2021b). Semisynthetic modifications of antitubercular lanostane triterpenoids from Ganoderma. J. Antibiot. 74, 435–442. doi: 10.1038/s41429-021-00422-5
Chinthanom, P., Vichai, V., Rachtawee, P., Boonpratuang, T., Isaka, M. (2023b). Antimalarial lanostane dimers from artificially cultivated fruiting bodies of Ganoderma weberianum. J. Nat. Prod. 86, 2304–2314. doi: 10.1021/acs.jnatprod.3c00457
Cho, S. E., Lee, S. G., Kim, M. S., Park, S. H., Park, J. B., Kim, N. K., et al. (2024). First report of Ganoderma gibbosum (Ganodermataceae, Basidiomycota), a wood-rotting fungus of urban trees in Korea. J. Asia-Pac. Biodivers. 17, 228–231. doi: 10.1016/j.japb.2023.12.005
Choeyklin, R., Hattori, T., Jones, E. B. G. (2011). A checklist of aphyllophoraceous fungi in Thailand: Part I. New records. Mycosphere 2, 161–177. Available at: https://mycosphere.org/pdf/MC2_2_No7.pdf (Accessed: April 15, 2025).
Chotipanich, A., Sooksrisawat, C., Jittiworapan, B. (2019). Association between complementary and alternative medicine use and prolonged time to conventional treatment among Thai cancer patients in a tertiary–care hospital. PeerJ 7, e7159. doi: 10.7717/peerj.7159
Chuensun, T., Chewonarin, T., Laopajon, W., Kawee-ai, A., Pinpart, P., Utama-ang, N. (2021). Comparative evaluation of physicochemical properties of Lingzhi (Ganoderma lucidum) as affected by drying conditions and extraction methods. Int. J. Food Sci. Technol. 56, 2751–2759. doi: 10.1111/ijfs.14906
Chuensun, T., Chewonarinm, T., Laopajon, W., Samakradhamrongthai, R. S., Chaisan, W., Utama-ang, N. (2024). Evaluation of the phytochemical, bioactive compounds and descriptive sensory of encapsulated Lingzhi (Ganoderma lucidum) extracts with combined wall materials for masking effect on the perception of off-flavour and bitterness. Heliyon 10 (21), e40094. doi: 10.1016/j.heliyon.2024.e40094
Chuwech, M., Rakariyatham, N. (2014). Potential of peanut hulls as substrates for fungal cellulase bioproduction through solid state fermentation. KKU Res. J. 19 (Supplement Issue), 235–243. http://resjournal.kku.ac.th.
Cong, V. T. (2010). Ganoderma spp.-Biology, species and culture in Vietnam and in the Czech Republic (Brno, Czech Republic: Ph.D. Thesis, Mendel University in Brno).
Cortina-Escribano, M., Veteli, P., Wingfield, M. J., Wingfield, B. D., Coetzee, M. P., Vanhanen, H., et al. (2024). Phylogenetic analysis and morphological characteristics of laccate Ganoderma specimens in Finland. Mycologia 116 (6), 1046–1062. doi: 10.1080/00275514.2024.2381424
Costa-Rezende, D. H., Gugliotta, A. M., Goes-Neto, A., Reck, M. A., Robledo, G. L., Drechsler-Santos, E. R. (2016). Amauroderma calcitum sp. nov. and notes on taxonomy and distribution of Amauroderma species (Ganodermataceae). Phytotaxa 244, 101–124. doi: 10.11646/phytotaxa.244.2.1
Costa-Rezende, D. H., Robledo, G. L., Drechsler-Santos, E. R., Glen, M., Gates, G., de Madrignac Bonzi, B. R., et al. (2020). Taxonomy and phylogeny of polypores with ganodermatoid basidiospores (Ganodermataceae). Mycol. Prog. 19, 725–741. doi: 10.1007/s11557-020-01589-1
Costa-Rezende, D. H., Robledo, G. L., Góes-Neto, A., Reck, M. A., Crespo, E., Drechsler-Santos, E. R. (2017). Morphological reassessment and molecular phylogenetic analyses of Amauroderma s. lat. raised new perspectives in the generic classification of the Ganodermataceae family. Persoonia-Molecular Phylogeny Evol. Fungi. 39 (1), 254–269. doi: 10.3767/persoonia.2017.39.10
Dalodom, T., Supavilai, P., Aramphongphan, A. (2010). Antioxidant and antimutagenic of Thai Ganodema lucidum. Thai J. Pharmacol. 32, 307. Available at: https://li01.tci-thaijo.org/index.php/JBAP/article/view/36698/30564 (Accessed: April 15, 2025).
Dan, N. T., Nhung, T. H., Le Minh Dung, N. T., Thanh, L. T., Van Phuc, P. (2017). Evaluation of effects of Lingzhi mushroom (Ganoderma lucidum) on neural stem cells isolated from embryonic mouse brain (Mus musculus var. albino). Asia-Pac. J. Sci. Technol. 19, 181–189. Available at: https://so01.tci-thaijo.org/index.php/APST/article/view/83118 (Accessed: April 15, 2025).
Data Bridge Market Research (2022) Global reishi mushroom market – industry trends and forecast to 2029. Available at: https://www.databridgemarketresearch.com/reports/global-reishi-mushroom-market (Accessed 12 Apr. 2025).
Deng, W., Ge, M., Wang, Z., Weng, C., Yang, Y. (2024). Efficient degradation and detoxification of structurally different dyes and mixed dyes by LAC-4 laccase purified from white-rot fungi Ganoderma lucidum. Ecotoxicology Environ. Saf. 279, 116450. doi: 10.1016/j.ecoenv.2024.116450
Department of Foreign Trade, Thailand. (2023). Herbal Product Import Statistics 2023 [Internet]. Department of Foreign Trade. Available online at: https://www.dft.go.th
Dubost, J. M., Phakeovilay, C., Her, C., Bochaton, A., Elliott, E., Deharo, E., et al. (2019). Hmong herbal medicine and herbalists in Lao PDR: pharmacopeia and knowledge transmission. J. Ethnobiol. Ethnomed. 15, 1–5. doi: 10.1186/s13002-019-0307-2
Duong, M. T., Loi, V. L., Truong, D. H. (2022a). Habitat distribution of fungal species collected at Lung Ngoc Hoang Nature Reserve. Eco. Env. Cons. 28, 621–625.
Duong, T. M., Truong, D. H., Loi, V. L. (2022b). The use values of macro fungi in Lung Ngoc Hoang Nature Reserve, Vietnam. Eco. Env. Cons. 28, 631–634.
Duyen, H. T., Mai, T. N. (2023). Factors affecting the total polysaccharide content in Ganoderma lucidum (Leyss. EX. FR.) KARST, Ganodermataceae. Can. Tho J. Med. 30, 140–148. Available at: https://tapchi.ctump.edu.vn/index.php/ctump/article/view/653 (Accessed: April 15, 2025).
El-Baz, A., Shetaia, Y., Abdelghani, D. Y., Abaza, A. A. (2024). Myco-remediation in industrial wastewater treatment. Trends Biol. Processes Ind. Wastewater Treat 12, 12–33. doi: 10.1088/978-0-7503-5678-7ch12
El Dine, R. S., El Halawany, A. M., Ma, C. M., Hattori, M. (2008). Anti-HIV-1 protease activity of lanostane triterpenes from the Vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 71, 1022–1026. doi: 10.1021/np8001139
El Dine, R. S., El Halawany, A. M., Ma, C. M., Hattori, M. (2009). Inhibition of the dimerization and active site of HIV-1 protease by secondary metabolites from the Vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 72, 2019–2023. doi: 10.1021/np900279u
Futrakul, N., Boonyen, M., Patumraj, S., Siriviriyakul, P., Tosukhowong, P., Futrakul, P. (2003). Treatment of glomerular endothelial dysfunction in steroid-resistant nephrosis with Ganoderma lucidum, vitamins C, E, and vasodilators. Clin. Hemorheol. Microcirc. 29, 205–210. Available at: https://pubmed.ncbi.nlm.nih.gov/14724343/ (Accessed: April 15, 2025).
Futrakul, N., Panichakul, T., Butthep, P., Futrakul, P., Jetanalin, P., Patumraj, S., et al. (2004). Ganoderma lucidum suppresses endothelial cell cytotoxicity and proteinuria in persistent proteinuric focal segmental glomerulosclerosis (FSGS) nephrosis. Clin. Hemorheol. Microcirc. 31, 267–272. Available at: https://pubmed.ncbi.nlm.nih.gov/15567896/ (Accessed: April 15, 2025).
Galappaththi, M. C., Priyashantha, A. K., Patabendige, N. M., Stephenson, S. L., Hapuarachchi, K. K., Karunarathna, S. C. (2024). “Taxonomy, phylogeny, and beneficial uses of Ganoderma (Ganodermataceae, Polyporales),” in Ganoderma: cultivation, chemistry and medicinal applications, vol. 1 . Eds. Acharya, K., Khatua, S. (CRC Press, Boca Raton (FL), 1–18.
Ganoderma-Derived Products Market Overview (2023). Ganoderma market growth, trends, and forecast (GlobeNewswire) (Accessed June 7, 2023).
Ganoderma Market Trends and Growth Factors (2023). Ganoderma lucidum extract market research report (Dataintelo) (Accessed November 16, 2024).
General Statistics Office of Vietnam (2024). Vietnam’s agricultural export value in 2023. Available at: https://hfoods.vn/agricultural-product-exports-in-2023-exceed-the-53-billion-usd-mark/ (Accessed: April 15, 2025).
Ha, D. T., Loan, L. T., Hung, T. M., Han, L. V. N., Khoi, N. M., Dung, L. V., et al. (2015). An improved HPLC-DAD method for quantitative comparisons of triterpenes in Ganoderma lucidum and its five related species originating from Vietnam. Molecules 20, 1059–1077. doi: 10.3390/molecules20011059
Hanafiah, Z. M., Mohtar, W. H., Wan, W. A., Bithi, A. S., Rohani, R., Indarto, A., et al. (2024). Removal of pharmaceutical compounds and toxicology study in wastewater using Malaysian fungal Ganoderma lucidum. Chemosphere 358, 142209. doi: 10.1016/j.chemosphere.2024.142209
Hapuarachchi, K. K., Elkhateeb, W. A., Karunarathna, S. C., Cheng, C. R., Bandara, A. R., Kakumyan, P., et al. (2018a). Current status of global Ganoderma cultivation, products, industry and market. Mycosphere 9, 1025–1052. doi: 10.5943/mycosphere/9/5/6
Hapuarachchi, K. K., Karunarathna, S. C., McKenzie, E. H. C., Wu, X. L., Kakumyan, P., Hyde, K. D., et al. (2019b). High phenotypic plasticity of Ganoderma sinense (Ganodermataceae, Polyporales) in China. Asian J. Mycol. 2, 1–47. doi: 10.5943/ajom/2/1/1
Hapuarachchi, K. K., Karunarathna, S. C., Phengsintham, P., Kakumyan, P., Hyde, K. D., Wen, T. C. (2018b). Amauroderma (Ganodermataceae, Polyporales) – bioactive compounds, beneficial properties and two new records from Laos. Asian J. Mycol. 1, 121–136. doi: 10.5943/ajom/1/1/10
Hapuarachchi, K. K., Karunarathna, S. C., Phengsintham, P., Yang, H. D., Kakumyan, P., Hyde, K. D., et al. (2019a). Ganodermataceae (Polyporales): diversity in greater mekong subregion countries (China, Laos, Myanmar, Thailand and Vietnam). Mycosphere 10, 221–309. doi: 10.5943/mycosphere/10/1/6
Hawkeswood, T. J., Sommung, B., Sommung, A. (2020). First record of the bracket fungus, Ganoderma lucidum (Curtis) P. Karsten, (1881) (Basidiomycota: ganodermataceae) from sisaket province, Thailand. Calodema 714, 1–5. Available at: https://www.researchgate.net/publication/345038562_First_record_of_the_bracket_fungus_Ganoderma_lucidum_Curtis_P_Karsten_1881_Basidiomycota_Ganodermataceae_from_Sisaket_Province_Thailand (Accessed: April 15, 2025).
He, J., Han, X., Luo, Z. L., Xian, L. E., Tang, S. M., Luo, H. M., et al. (2022). Species diversity of Ganoderma (Ganodermataceae, Polyporales) with three new species and a key to Ganoderma in Yunnan Province, China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1035434
He, J., Li, X.-J., Tan, W.-Z., Wu, X.-Q., Wu, D., Luo, Z.-L., et al. (2024). Two new species of Ganoderma (Ganodermataceae, Basidiomycota) from Southwest China. MycoKeys 106, 97–116. doi: 10.3897/mycokeys.106.121526
Hien, B. T. T., Thu, T. N., Tuyen, N. Q., Tham, L. X., Quang, D. N. (2014). Cytotoxic steroids found in Vietnamese Lingzhi. HNUE J. Sci. 59, 25–29. http://stdb.hnue.edu.vn/.
Hjortstam, K., Ryvarden, L. (1982). Aphyllophorales from northern Thailand. Nord. J. Bot. 2, 273–281. doi: 10.1111/j.1756-1051.1982.tb01189.x
Hultberg, M., Golovko, O. (2024). Use of sawdust for production of ligninolytic enzymes by white-rot fungi and pharmaceutical removal. Bioprocess Biosyst. Engineering. 47, 475–482. doi: 10.1007/s00449-024-02976-8
Hung, D. X., Hieu, T. T., Quang, D. N., Thang, T. D. (2020). Triterpenoids from fruit body of Ganoderma australe (Fr.) PAT. VinhUni. J. Sci. 49, 37–42. doi: 10.56824/vujs.2020nt25
Hung, P. V., Nhi, N. N. (2012). Nutritional composition and antioxidant capacity of several edible mushrooms grown in the Southern Vietnam. Int. Food Res. J. 19 (2), 611–615.
Index Fungorum (2025). Available online at: http://www.indexfungorum.org (Accessed March 16, 2025).
Inla, K., Bunchan, S., Krittacom, B., Luampon, R. (2023). Drying behavior, color change and rehydration of lingzhi mushroom (Ganoderma lucidum) under convection-assisted microwave drying. Case Stud. Therm. Eng. 49, 103348. doi: 10.1016/j.csite.2023.103348
Intara-Anun, S., Chairin, T. (2021). Effect of micronutrients on ligninolytic enzyme activities of Ganoderma boninense, a causal pathogen of basal stem rot disease in oil palm. Khon Kaen. Agr. J. 49, 827–831. https://ag2.kku.ac.th/kaj/PDF.cfm?filename=75Pat06_P_Accepted-%E0%B8%88%E0%B8%B1%E0%B8%94%E0%B8%AB%E0%B8%99%E0%B9%89%E0%B8%B2.pdf&id=4421&keeptrack=1.
Intara-Anun, S., Chairin, T. (2023). Effect of temperature and light on ligninolytic enzyme activities of Ganoderma boninense under laboratory condition. Khon Kaen. Agr. J. 51, 623–629. https://ag2.kku.ac.th/kaj/PDF.cfm?filename=56-Pat02_P.pdf&id=4871&keeptrack=2.
International Rivers (2021). The mekong feeds millions: dams threaten southeast asia’s vital lifeline (International Rivers website) (Accessed 10 November 2024).
Ipeaiyeda, A. R., Adenipekun, C. O., Oluwole, O. (2020). Bioremediation potential of Ganoderma lucidum (Curt: Fr) P. Karsten to remove toxic metals from abandoned battery slag dumpsite soil and immobilisation of metal absorbed fungi in bricks. Cogent Environ. Science. 6, 1847400. doi: 10.1080/23311843.2020.1847400
Isaka, M., Chinthanom, P., Mayteeworakoon, S., Laoteng, K., Choowong, W., Choeyklin, R. (2018). Lanostane triterpenoids from cultivated fruiting bodies of the basidiomycete ganoderma australe. Natural Product Res. 32 (9), 1044–1049. doi: 10.1080/14786419.2017.1378208
Isaka, M., Chinthanom, P., Mayteeworakoon, S., Laoteng, K., Choowong, W., Choeyklin, R. (2017a). Lanostane triterpenoids from cultivated fruiting bodies of the basidiomycete Ganoderma australe. Nat. Prod. Res. 32, 1044–1049. doi: 10.1080/14786419.2017.1378208
Isaka, M., Chinthanom, P., Mayteeworakoon, S., Laoteng, K., Suvannakad, R., Choeyklin, R. (2017b). Lanostane triterpenoids from cultivated fruiting bodies of the basidiomycete Ganoderma orbiforme. Phytochem. Lett. 21, 251–255. doi: 10.1016/j.phytol.2017.07.010
Isaka, M., Chinthanom, P., Rachtawee, P., Choowong, W., Choeyklin, R., Thummarukcharoen, T. (2020a). Lanostane triterpenoids from cultivated fruiting bodies of the wood-rot basidiomycete Ganoderma casuarinicola. Phytochem. 170, 112225. doi: 10.1016/j.phytochem.2019.112225
Isaka, M., Chinthanom, P., Sappan, M., Danwisetkanjana, K., Boonpratuang, T., Choeyklin, R. (2016). Antitubercular lanostane triterpenes from cultures of the basidiomycete Ganoderma sp. BCC 16642. J. Nat. Prod. 79, 161–169. doi: 10.1021/acs.jnatprod.5b00826
Isaka, M., Chinthanom, P., Vichai, V., Sommai, S., Choeyklin, R. (2020b). Ganoweberianones A and B, antimalarial lanostane dimers from cultivated fruiting bodies of the basidiomycete Ganoderma weberianum. J. Nat. Prod. 83, 3404–3412. doi: 10.1021/acs.jnatprod.0c00879
Isaka, M., Sappan, M., Choowong, W., Boonpratuang, T., Choeyklin, R., Feng, T., et al. (2020c). Antimalarial lanostane triterpenoids from cultivated fruiting bodies of the basidiomycete Ganoderma sp. J. Antibiot. 73, 702–710. doi: 10.1038/s41429-020-0357-7
Jin, X., Wu, P., Li, P., Xiong, C., Gui, M., Huang, W. (2023). Transcriptome analysis reveals insight into the protective effect of N-acetylcysteine against cadmium toxicity in Ganoderma lucidum (Polyporales: Polyporaceae). Environ. Sci. pollut. Res. 30, 58436–58449. doi: 10.1007/s11356-023-26635-9
Kaewdoung, B., Sutjaritvorakul, T., Gadd, G. M., Whalley, A. J., Sihanonth, P. (2016). Heavy metal tolerance and biotransformation of toxic metal compounds by new isolates of wood-rotting fungi from Thailand. Geomicrobiol. J. 33, 283–288. doi: 10.1080/01490451.2015.1048394
Kaewjai, C., Tonsomboon, A., Pawiwongchai, J., Prommano, A. O. (2023). Antiprotozoal activity of Boesenbergia rotunda (L.) Mansf and Ganoderma lucidum (Fr.) Kart extracts against Blastocystis hominis. Vet. World 16, 187–193. doi: 10.14202/vetworld.
Kaewnarin, K., Limjiasahapong, S., Jariyasopit, N., Anekthanakul, K., Kurilung, A., Wong, S. C., et al. (2021). High-resolution QTOF-MRM for highly accurate identification and quantification of trace levels of triterpenoids in Ganoderma lucidum mycelium. J. Ame. Soc Mass Spectrom. 32, 2451–2462. doi: 10.1021/jasms.1c00175
Karsten, P. A. (1881). Enumeralio boletinearum et polypore arum fennicarum, systemate novodispositarum. Rev. Mycol. 3, 16–19.
Karunarathna, S. C., Ediriweera, A., Prasannath, K., Mingfei, Y., Hapuarachchi, K. K. (2024a). Exploring the health benefits of Ganoderma: Bioactive compounds and mechanisms of action; immunomodulatory, and anti-tumour activities. N. Z. J. Bot., 1–85. doi: 10.1080/0028825X.2024.2375996
Karunarathna, S. C., Lu, W., Patabedige, N., Zhao, C. L., Hapuarachchi, K. K. (2024b). Unlocking the therapeutic potential of edible mushrooms: Ganoderma and their secondary metabolites as novel antiviral agents for combating COVID-19. N. Z. J. Bot., 1–59. doi: 10.1080/0028825X.2024.2384453
Karunarathna, S. C., Patabendige, N. M., Lu, W., Asad, S., Hapuarachchi, K. K. (2024d). An in-depth study of phytopathogenic Ganoderma: Pathogenicity, advanced detection techniques, control strategies, and sustainable management. J. Fungi 10, 414. doi: 10.3390/jof10060414
Karunarathna, S. C., Prasannath, K., Lu, W., Hapuarachchi, K. K. (2024c). Ganoderma: bridging traditional wisdom with modern innovation in medicinal mushroom and dietary supplement industry. N. Z. J. Bot., 1–60. doi: 10.1080/0028825X.2024.2410486
Keomanykham, O., Khamko, V. A., Keorodom, B., Khemmarath, S., Quang, D. N. (2016). Cytotoxic steroids from the mushroom Ganoderma australe collected in Laos. Vietnam J. Chem. 54, 688–688. doi: 10.15625/0866-7144.2016-00388
Khammuang, S., Sarnthima, R. (2009). Laccase activity from fresh fruiting bodies of Ganoderma sp. MK05: purification and Remazol Brilliant Blue R decolorization. J. Biol. Sci. 9, 83–87. doi: 10.3923/jbs.2009.83.87
Kiet, T. T. (1998). Preliminary checklist of macrofungi of Vietnam. Feddes Repert. 109, 257–277. doi: 10.1002/fedr.19981090309
Kim, N. K., Lee, J. H., Jo, J. W., Bunthoeun, R., Ngeth, C., Lee, J. K. (2016). Fungal biodiversity in cardamom protected forests and seima biodiversity conservation area of Cambodia. J. For. Environ. Sci. 32, 158–163. doi: 10.7747/JFES.2016.32.2.158
Kim, N. K., Lee, J. H., Jo, J. W., Lee, J. K. (2017). A checklist of mushrooms of Cambodia. J. For. Environ. Sci. 33, 49–65. doi: 10.7747/JFES.2017.33.1.49
Klaus, A., Wan, W. A. (2024). “Ganoderma in traditional culture,” in Ganoderma: Cultivation, Chemistry, and Medicinal Applications, Volume 1. Eds. Acharya, K., Khatua, S. (Boca Raton, FL, USA: CRC Press), 35–60.
Kleinwächter, P., Anh, N., Kiet, T. T., Schlegel, B., Dahse, H. M., Härtl, A., et al. (2001). Colossolactones, new triterpenoid metabolites from a Vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 64, 236–239. doi: 10.1021/np000437k
Klingesorn, P., Chotikasatiara, T. (1998a). “Biodiversity of macrofungi in aung-Reu-Nai wildlife sanctuary,” in Mahidol University Annual Research Abstracts and Bibliography of Non-Formal Publications, Volume 25 (1997). (Bangkok, Thailand: Mahidol University), p. 291.
Klingesorn, P., Chotikasatiara, T. (1998b). “Biodiversity of macrofungi in upper khao soi dao wildlife sanctuary,” in Mahidol University Annual Research Abstracts and Bibliography of Non-Formal Publications, Volume 25 (1997). (Bangkok, Thailand: Mahidol University), p. 290.
Konara, U. A., Thambugala, K. M., Karunarathna, S. C., Ediriweera, A., Hapuarachchi, K. K. (2024). Unveiling the hidden diversity of Ganoderma (Ganodermataceae, Polyporales) in Sri Lanka: The first report of G. angustisporum, G. ellipsoideum and G. orbiforme. N. Z. J. Bot., 1–25.
Konara, U. A., Thambugala, K. M., Hapuarachchi, K. K. (2022). Ganoderma (Ganodermataceae, Polyporales): Historical perspectives, recent advances, and future research in Sri Lanka. Stud. Fungi. 7 (1), 1–7. doi: 10.1080/0028825X.2024.2415555
KPL News (2023). Vietnam imports Ganoderma from Cambodia and Laos for processing. KPL News Agency, Vientiane, Lao People's Democratic Republic.
Krobthong, S., Yingchutrakul, Y. (2020). Identification and enhancement of antioxidant P1-peptide isolated from Ganoderma lucidum hydrolysate. Food Biotechnol. 34, 338–351. doi: 10.1080/08905436.2020.1844228
Krobthong, S., Yingchutrakul, Y., Visessanguan, W., Mahatnirunkul, T., Samutrtai, P., Chaichana, C., et al. (2021). Study of the lipolysis effect of nanoliposome-encapsulated Ganoderma lucidum protein hydrolysates on adipocyte cells using proteomics approach. Foods 10, 2157. doi: 10.3390/foods10092157
Kumari, A., Tapwal, A., Thakur, N. (2024). Ganoderma lucidum: Insights on host range, diagnosis, and management strategies. J. Basic Microbiol. 64, e202300769. doi: 10.1002/jobm.202300769
Kuo, P. C., Thang, T. D., Huang, G. J., Ngoc, N. T. B., Ngan, N. T., Yang, M. L., et al. (2016). Chemical constituents of Ganoderma pfeifferi and their inhibitory effect on nitric oxide production. Chem. Nat. Comp. 52, 948–950. doi: 10.1007/s10600-016-1829-7
Kwon, H., Thatithatgoon, S. (2004). Mushroom growing in Northern Thailand. In Mushroom Growers’ Handbook 1: Oyster Mushroom Cultivation. MushWorld; Heineart Inc. Seoul,Korea. pp. 30–47.
Lakornwong, W., Kanokmedhakul, K., Kanokmedhakul, S., Kongsaeree, P., Prabpai, S., Sibounnavong, P., et al. (2014). Triterpene lactones from cultures of Ganoderma sp. KM01. J. Nat. Prod. 77, 1545–1553. doi: 10.1021/np400846k
Lau, M. F., Phan, C. W., Sabaratnam, V., Kuppusamy, U. R. (2024). Bibliometric, taxonomic, and medicinal perspectives of Ganoderma neo-japonicum Imazeki: A mini review. Mycology 15 (3), 360–373. doi: 10.1080/21501203.2024.2302028
Le, M. H., Do, H. D., Thi, H. H., Dung, L. V., Nguyen, H. N., Thi, H. N., et al. (2016). The dual effect of curcumin nanoparticles encapsulated by 1-3/1-6 β-glucan from medicinal mushrooms Hericium erinaceus and Ganoderma lucidum. Adv. Natural Sciences: Nanoscience Nanotechnology. 7 (4), 45019. doi: 10.1088/2043-6262/7/4/045019
Le, X. T., Nguyen Le, Q. H., Pham, N. D., Duong, V. H., Dentinger, B. T., Moncalvo, J. M. (2012). Tomophagus cattienensis sp. nov., a new Ganodermataceae species from Vietnam: Evidence from morphology and ITS DNA barcodes. Mycol. Prog. 11, 775–780. doi: 10.1007/s11557-011-0789-3
Lee, J., Kim, D., Nguyen, M. H., Bae, Y. J., Manilak, P. (2021). A checklist of mushrooms of dong hua sao national biodiversity conservation area (DHSNBCA) of Lao-PDR. J. For. Environ. Sci. 37, 163–167. doi: 10.7747/JFES.2021.37.2.163
Leonardo-Silva, L., Cotrim, C. F., Xavier-Santos, S. (2022). Furtadomyces nom. nov. (Ganodermataceae, Basidiomycota) with description of F. sumptuosus, a new species of ganodermatoid fungi from Brazil. Mycol. Prog. 21, 36. doi: 10.1007/s11557-022-01794-0
Le Tham, X., Matsuhashi, S., Kume, T. (1999). Growth and fruitbody formation of Ganoderma lucidum on media supplemented with vanadium, selenium and germanium. Mycoscience 40, 87–92. doi: 10.1007/BF02465679
Li, W., Chinthanom, P., Rachtawee, P., Intereya, K., Feng, T., Liu, J. K., et al. (2018). Isolation of 3, 4-seco-27-norlanostane triterpenoids from cultivated fruiting bodies of Ganoderma orbiforme. Phytochem. Lett. 28, 104–109. doi: 10.1016/j.phytol.2018.09.017
Li, Y., Li, T., Yang, Z., Bau, T., Dai, Y. (2024). “Overview: brief introduction to fungal atlases in China,” in Atlas of chinese macrofungal resources (Springer, Singapore). doi: 10.1007/978-981-99-6315-7_1
Li, G., Yu, D., Zhu, P., Zhao, G., Liu, C., Zhao, H. (2023). Three new cultivars of Ganoderma sinense and Auricularia heimuer from Southern Anhui. Mycosystema 42, 1219–1222. doi: 10.13346/j.mycosystema.220259
Likhitekaraj, S., Tummakate, A. (2000). “Basal stem rot of oil palm in Thailand caused by Ganoderma,” in Ganoderma diseases of perennial crops (CABI, Wallingford UK), 69–70.
Lim, T. K., Chung, G. F., Ko, W. H. (1992). Basal stem rot of oil palm caused by ganoderma boninense. Plant Pathol. Bulletin. 1 (3), 147–152.
Limsrivilai, P., Likitakaraj, S., Surin, P. (1980). “Oil palm diseases (crown diseases; spear rot) in Thailand,” in 2nd Southeast Asian Symposium on Plant Diseases in the Tropics: Program and Abstracts(Bangkok, Thailand: Kasetsart University Press). p. 22.
Linh, N. T., Do Dat, T., Tai, N. T., Linh, N. T., My, P. L., Ngan, N. T., et al. (2021). Response surface optimized extraction of triterpenoids from red Vietnamese Ganoderma lucidum and anticancer evaluation of the extract. Vietnam. J. Sci. Technol. 59, 158–168. doi: 10.15625/2525-2518/59/2/15485
Liu, D., Diao, W., Chen, H., Qi, X., Fang, H., Yu, X., et al. (2024). Heterologous expression and characterization of a dye-decolorizing peroxidase from Ganoderma lucidum, and its application in decolorization and detoxifization of different types of dyes. World J. Microbiol. Biotechnol. 40, 303. doi: 10.1007/s11274-024-04084-x
Liu, Y., Ying, Y., Tang, Q., Han, W., Yang, Y., Zhou, S., et al. (2017). Bioactive component profiles of Ganoderma sinense fruit bodies cultivated in three different regions. Acta Edulis Fungi 24, 72–76. doi: 10.16488/j.cnki.1005-9873.2017.01.013
Luangharn, T., Karunarathna, S. C., Dutta, A. K., Paloi, S., Promputtha, I., Hyde, K. D., et al. (2021). Ganoderma (Ganodermataceae, Basidiomycota) species from the greater Mekong subregion. J. Fungi 7, 819. doi: 10.3390/jof7100819
Luangharn, T., Karunarathna, S. C., Khan, S., Xu, J. C., Mortimer, P. E., Hyde, K. D. (2017). Antibacterial activity, optimal culture conditions and cultivation of the medicinal Ganoderma australe, new to Thailand. Mycosphere 8, 1108–1123. doi: 10.5943/mycosphere/8/8/11
Luangharn, T., Karunarathna, S. C., Mortimer, P. E., Hyde, K. D., Xu, J. (2019). Additions to the knowledge of Ganoderma in Thailand: Ganoderma casuarinicola, a new record; and Ganoderma Thailandicum sp. nov. MycoKeys 59, 47–65. doi: 10.3897/mycokeys.59.36823
Luangharn, T., Salichanh, T., Khyaju, S. (2023). New host records of Ganoderma in Northern Thailand and determination of nutritional contents of selected Ganoderma species. Asian J. Mycol. 6, 48–60. doi: 10.5943/ajom/6/2/4
Mai, D. S. (2018). The effect of cellulase, microwave and ultrasonic methods on crude polysaccharides extraction from the spore of Vietnamese lingzhi (Ganoderma lucidum). Acta Hortic. 1213, 373–378. doi: 10.17660/ActaHortic.2018.1213.54
Malinee, R., Stratoulias, D., Nuthammachot, N. (2021). Detection of oil palm disease in plantations in Krabi Province, Thailand with high spatial resolution satellite imagery. Agriculture 11, 251. doi: 10.3390/agriculture11030251
Mardones, M., Carranza-Velázquez, J., Mata-Hidalgo, M., Amador-Fernández, X., Urbina, H. (2023). Taxonomy and phylogeny of the genus Ganoderma (Polyporales, Basidiomycota) in Costa Rica. MycoKeys 100, 5–47. doi: 10.3897/mycokeys.100.106810
Mekong River Commission Geographic regions of the mekong basin (Mekong River Commission for Sustainable Development) (Accessed 10 November 2024).
Minh, N. P., Nhan, N. P., Le Pha, P. T., Ngoc, N. H., Thao, T. P. (2019). Effect of technical variables on the total phenolic and antioxidant activity in cooking of canned white lingzhi (Ganoderma lucidum) fruit. J. Pharm. Sci. Res. 11, 708–711. Available at:https://www.jpsr.pharmainfo.in/Documents/Volumes/vol11issue03/jpsr11031906.pdf.
Mizuno, T. (1997). “Studies on bioactive substances and medicinal effect of reishi, Ganoderma lucidum in japan,” in Proceedings of the 1st international symposium on Ganoderma lucidum in japan (Tokyo: Toyo-Igaku-sha Co. Ltd.), 121–127.
Mortimer, P. E., Xu, J., Karunarathna, S. C., Hyde, K. D. (2014). Mushrooms for trees and people: A field guide to useful mushrooms of the Mekong region (East Asia, Kunming, China: The World Agroforestry Centre), 125.
Naksuwankul, K., Thongbor, A., Chantharasena, C., Khottawong, W., Parnmen, S., Nooron, N., et al. (2022). Identification by morphological and local wisdom and distribution of Poisonous and edible mushroom in Thailand. Burapha Science J 10, 66–84. Available at: https://scijournal.buu.ac.th/index.php/sci/article/view/3861.
Ndeh, B. J., Tacham, W. N., Katamssadan, T. H., Rosemary Kinge, T. (2024). Morphological diversity of Ganoderma species and its host trees in Mezam Division, Northwest Region, Cameroon. MBJ 9, 1–6. doi: 10.21608/mb.2024.353098
Nghien, N. X., Thuy, N. T., Luyen, N. T., Thu, N. T., Quan, N. D. (2019). Morphological characteristics, yield performance, and medicinal value of some lingzhi mushroom (Ganoderma lucidum) strains cultivated in Tam Dao, Vietnam. Vietnam J. Agric. Sci. 2, 321–331. doi: 10.31817/vjas.2019.2.1.03
Ngoc, L. P., Man, H. Y., Besselink, H., Cam, H. D., Brouwer, A., van der Burg, B. (2019). Identification of PPAR-activating compounds in herbal and edible plants and fungi from Vietnam. Ind. Crop Prod. 129, 195–200. doi: 10.1016/j.indcrop.2018.12.003
Nguyen, T. T., Bui, T. T., Pham, B. P., Nguyen, H. T., Ngo, X. B., La, V. H., et al. (2021). Identification of Ganoderma lucidum (Curtis) p. karst. Species isolated from ironwood (Erythrophleum fordii OLIVER) in Vietnam act through by ITS1 sequence and phylogenetic analysis. Plant Cell. Biotechnol. Mol. Biol. 22, 59–70. Available at: https://ikprress.org/index.php/PCBMB/article/view/6071.
Nguyen, Q. C., Huynh, H. T., Dao, T. S., Kwon, H. (2023d). Application of internet of things based monitoring system for indoor Ganoderma lucidum cultivation. Int. J. Adv. Smart Converg. 12, 153–158.
Nguyen, B. T., Ngo, N. X., Van Le, V., Nguyen, L. T., Kana, R., Nguyen, H. D. (2019). Optimal culture conditions for mycelial growth and fruiting body formation of Ling Zhi mushroom Ganoderma lucidum strain GA3. VJSTE 61, 62–67. doi: 10.31276/VJSTE.vol(number).page
Nguyen, G. V., Nguyen, B. T., Bui, L. H., Nguyen, T. D., Tran, H. T., Nguyen, T. T., et al. (2023e). “. Effects of nutrient sources and culture conditions on the mycelial growth of Ganoderma lucidum strain Ga-TB,” in AIP conference proceedings, vol. 2817. (AIP Publishing).
Nguyen, T. T., Nguyen, H. D., Bui, A. T., Pham, K. H., Van, K. T., Tran, L. T., et al. (2023b). Phylogenetic analysis and morphology of Ganoderma multipileum, a Ganoderma species associated with dieback of the metropolitan woody plant Delonix regia (Boj. ex Hook.) Raf. in Vietnam. Sci. Prog. 106, 00368504231195503. doi: 10.1177/00368504231195503
Nguyen, K. D., Nguyen, C. M., Le, D. A., Huynh, H. T., Tran, M. T., Truong, A. T., et al. (2024a). The mixture of Ganoderma lucidum and Cordyceps militaris: Chemical composition and protective effect against oxidative stress. J. Agric. Food Res. 15, 101045. doi: 10.1016/j.jafr.2024.101045
Nguyen, T. H., Nguyen, V. P., Le, T. H., Tran, T. H. (2023h). Green synthesis of silver nanomaterials using Ganoderma lucidum extract as reducing agent and stabilizer with ultrasonic assistance and application as an antibacterial agent. HUJOS-NS 132, 15–23. doi: 10.26459/hueunijns.v132i1D.7018
Nguyen, T. T. T., Nguyen, T. T. T., Nguyen, H. D., et al. (2023). Integrating in silico and in vitro studies to screen anti-Staphylococcus aureus activity from Vietnamese Ganoderma multiplicatum and Ganoderma sinense. Nat. Prod. Commun. 18. doi: 10.1177/1934578X231167289
Nguyen, T. T., Nguyen, T. T., Nguyen, H. D., Nguyen, T. K., Pham, P. T., Tran, L. T., et al. (2024b). Anti-Staphylococcus aureus potential of compounds from Ganoderma sp.: A comprehensive molecular docking and simulation approaches. Heliyon 10.
Nguyen, T. D., Nguyen, M. K., Phan, N. T., Duong, M. T., Tran, V. H., Do, T. H. (2020). Lanostane triterpenoids from Ganoderma tropicum collected in Vietnam and their nitroblue tetrazolium reductive activity in vitro. Nat. Prod. Sci. 26, 334–339. doi: 10.20307/nps.2020.26.4.334
Nguyen, T. T., Pham, N. A., Tran, Q. T., Nguyen, H. D., Nguyen, D. H., Pham, K. H., et al. (2023f). Antioxidant activities of enriched polysaccharide fractions from mycelia of Amauroderma subresinosum. Trop. J. Nat. Prod. Res. 7.
Nguyen, N. P., Quy, P. T., To, D. C., Bui, T. Q., Phu, N. V., My, T. T., et al. (2023g). Combinatory in silico study on anti-diabetic potential of Ganoderma lucidum compounds against α-Glucosidase. Trop. J. Nat. Prod. Res. 7.
Nguyen, N. T., Tran, T. H., Nguyen, T. N., Nguyen, T. T., Nguyen, T. B., Kuo, P. C., et al. (2022). Secondary metabolites from higher fungi in Vietnam: Discovery, chemodiversity, and bioactivity. VJST 60, 1–20.
Nguyen, V. T., Tung, N. T., Cuong, T. D., Hung, T. M., Kim, J. A., Woo, M. H., et al. (2015). Cytotoxic and anti-angiogenic effects of lanostane triterpenoids from Ganoderma lucidum. Phytochem. Lett. 12, 69–74. doi: 10.1016/j.phytol.2015.02.012
Nguyen, L. T., Van Le, V., Nguyen, B. T., Nguyen, H. T., Tran, A. D., Ngo, N. X. (2023a). Optimization of mycelial growth and cultivation of wild Ganoderma sinense. Biotechnologia 104, 65. doi: 10.5114/bta.2023.125087
Nhung, N. T. (2019). Isolation and mycelium growth of Ganoderma lucidum on Manihot esculent substrate with mineral supplement. J. Adv. Agric. Technol. 6 (2), 123–127. doi: 10.18178/joaat.6.2.123-127
Ninluam, N., Potiprasert, W., Romreun, U., Bangyeekhun, E. (2016). Cultivation of Lingzhi mushroom, Ganoderma lucidum, by using sugarcane bagasse. Veridian E-J. Sci. Technol. Silpakorn Univ. 3, 390–397. Available at: https://ph01.tci-thaijo.org/index.php/VESTSU/article/view/84946.
Niu, K. Y., He, J., Tang, S. M., Su, X. J., Luo, Z. L. (2024). Morphological and phylogenetic analyses reveal three novel species of Sanguinoderma (Ganodermataceae, Basidiomycota) from Yunnan Province, China. J. Fungi. 10, 589. doi: 10.3390/jof10080589
Pakdee, P., Kangsadalampai, K., Suttisansanee, U., Kruawan, K. (2014). “Evaluation of mutagenic and antimutagenic activities of hot water extract from Ganoderma lucidum (Fr.) Karst in Ames test and Drosophila somatic mutation and recombination test (SMART),” in Proceedings of the 16th Food Innovation Asia Conference 2014: Science and Innovation for Quality of Life. Thailand Section of AOAC International, Nonthaburi, Thailand. pp. 216–225.
Palasarn, S., Choowong, W., Wiriyathanawudhiwong, N., Choeyklin, R., Isaka, M. (2024). Highly modified lanostane triterpenoids from natural fruiting bodies of Ganoderma Cf. Hochiminhense. doi: 10.2139/ssrn.4980980
Panchan, B., Leejareon, W., Chantawee, S., Chiangkul, A. (1995). “Major root diseases of rubber and their control,” in Proceedings of the 2nd national plant protection conference (Plant Protection for Environmental Quality; Thailand Weed Science Association, Thailand Entomology and Zoology Association, Thailand Plant Protection Association, Thai Association-Agricultural Chemistry Business, Bangkok, Thailand), 27–34.
Pandey, S., Gupta, S. (2024). Exploring laccase: a sustainable enzymatic solution for the paper recycling domain. Arch. Microbiol. 206, 211. doi: 10.1007/s00203-024-03927-3
Papp, V. (2019). “Global diversity of the genus ganoderma taxonomic uncertainties and challenges,” in Sridhar, K. R., Deshmukh, S. K. eds. Advances in Macrofungi: Diversity, Ecology and Biotechnology (Boca Raton, FL, USA: CRC Press), 10–33.
Paterson, R. R. M. (2023). Future climate effects on basal stem rot of conventional and modified oil palm in Indonesia and Thailand. Forests 14, 1347. doi: 10.3390/f14071347
Pedersen, O. S., Thammavong, K. (2014). Biodiversity: Wild mushrooms – edible and medicinal species; local knowledge and use; pilot survey in Bong, Mixay, Yai, Lethong, Gnordphe, and Poua-Xai villages (Xieng Khouang Province, Lao PDR: Agro-biodiversity Project. Phoukhout District).
Pedersen, O. S., Thammavong, K. (2016). Biodiversity: Wild mushrooms – edible and medicinal species; local knowledge and use; field survey in Bong, Mixay, Yai, Lethong, Gnordphe, and Poua-Xai villages (Xieng Khouang Province, Lao PDR: Agro-biodiversity Project. Phoukout District).
Peltzer, K., Pengpid, S. (2019). The use of herbal medicines among chronic disease patients in Thailand: a cross-sectional survey. J. Multidiscip. Healthc. 12, 573–582. doi: 10.2147/JMDH.S212953
Peltzer, K., Pengpid, S., Puckpinyo, A., Yi, S., Vu Anh, L. (2016). The utilization of traditional, complementary and alternative medicine for non-communicable diseases and mental disorders in health care patients in Cambodia, Thailand and Vietnam. BMC Complement. Altern. Med. 16, 92. doi: 10.1186/s12906-016-1078-0
Peres, R. S., Bittencourt, F., Robledo, G. L., Drechsler-Santos, E. R., Põldmaa, K., Ryvarden, L., et al. (2023). Filling gaps in the phylogeny of Amauroderma s. lat. (Polyporales, Ganodermataceae). S. Afri. J. Bot. 155, 140–153. doi: 10.1016/j.sajb.2023.02.018
Pesee, M., Kirdpon, W., Puapairoj, A., Kirdpon, S., Prathnadi, P. (2013). Palliative treatment of advanced cervical cancer with radiotherapy and thai herbal medicine as supportive remedy-analysis of survival. Asian Pacific J. Cancer Prev. 14 (3), 1593–1596. doi: 10.7314/APJCP.2013.14.3.1593
Petcharat, V. (1996). Cultivation of wild mushroom: VII. Hed Nua Yang (Ganoderma subresinosum Fr.). SJST 18, 267–273. https://agris.fao.org/agris-search/search.do?recordID=TH1996000040.
Petwattanapha, P., Buwjoom, T., Maneewan, B., Rattanang, P., Thuekeaw, S. (2024). Microencapsulation of Ganoderma lucidum extract: Evaluation of functional components, in vitro simulated digestion, and stability as a potential feed antioxidant. ACS Food Sci. Technol. 4, 2199–2208. doi: 10.1021/acsfoodscitech.4c00457
Pham, H. H. (1961). “L’étagement du peuplement littoral sures c’tesrocheuses du Vietnam,” in Proceedings of the 4th international seaweed symposium (Macmillan. Oxford, England; New York, USA: Pergamon Press), pp. 256–258.
Pham, H. N., Hoang, L. S., Phung, V. T. (2016). Hepatoprotective activity of Ganoderma lucidium (Curtis) P. Karst against cyclophosphamide-induced liver injury in mice. Cogent. Biol. 2, 1267421. doi: 10.1080/23312025.2016.1267421
Phan, V. M., Thi, M. S., Tran, D. D. (2023). Optimization and kinetics of the supercritical fluid extraction of triterpenoids from Ganoderma lucidum with CO2 and ethanol as cosolvent. Carpath. J. Food Sci. Technol. 15, 118–132. doi: /10.34302/crpjfst/2023.15.1.10
Phu, T. T. (2023). Building a fungi identification key for the main fungi of the Ganodermataceae (Donk) Donk in Son Tra, Da Nang City. JST-UD 31, 83–89. doi: 10.31130/ud-jst.2023.487DB
Phúc, N. T., Hien, B. T., Hoa, L. T., Quang, D. N. (2014). Anticancer constituents from the Vietnamese Lingzhi Ganoderma mirabile. Vietnam J. Sci. Technol. 52, 308–312. Available at: https://vjs.ac.vn/jst.
Pinitsoontorn, C., Suwantrai, S., Boonsiri, P. (2012). Antioxidant activity and oxalate content of selected Thai herbal teas. KKU Res. J. 17, 162–168. Retrieved from http://resjournal.kku.ac.th.
Pinweha, S., Wanikiat, P., Sanvarinda, Y., Supavilai, P. (2008). The signaling cascades of ganoderma lucidum extracts in stimulating non-amyloidogenic protein secretion in human neuroblastoma SH-SY5Y cell lines. Neurosci. Lett. 448 (1), 62–66. doi: 10.1016/j.neulet.2008.10.028
Pitakpong, A., Suwanwaree, P., Boonyanuphap, J. (2013). “Diversity of mushrooms in the plant genetic protection area of RSPG, Nampung Dam EGAT, Sakhon Nakhon Province,” in The 7th botanical conference of Thailand (Ramkhamhaeng University, Bangkok, Thailand).
Pithakkit, S., Petcharat, V., Chuenchit, S., Pornsuriya, C., Sunpapao, A. (2015). Isolation of antagonistic actinomycetes species from rhizosphere as effective biocontrol against oil palm fungal diseases. WJST 12, 481–490. doi: 10.14456/WJST.2015.81
Poomsing, P., Pattanapanyasat, K., Wongsinkongman, P., Soonthornchareonnon, N. (2013). Research and development of Ganoderma lucidum cultivated in Thailand. MUJPS 40, 1–7. Retrieved from https://pharmacy.mahidol.ac.th/journal/_files/2013-40-3_01-07.pdf.
Pornsuriya, C., Sunpapao, A., Srihanant, N., Worapattammasri, K., Kittimorakul, J., Phithakkit, S., et al. (2013). A survey of diseases and disorders in oil palms of southern thailand. Plant Pathol. J. (Faisalabad) 12 (4), 169–175.
Porntip, S., Sirinthorn, P., Auratai, A., Krongtong, Y., Payong, W., Yukolporn, S., et al. (2006). “Neuroprotective effects of ganoderma lucidum in human neuroblastoma SH-SY5Y cells,” in Proceedings of the 15th International Congress of Pharmacology [In Chinese].
Pothisuwan, R., Detha, A., Intasan, T., Rodpai, N., Wanaratna, L. (2010). Cost analysis of lacquered mushroom (Ganoderma lucidum (Fr.) Karst) production and its spores at Muang Ngai Special Agricultural Project under the patronage of Her Majesty Queen Sirikit, fiscal year 2009. J Tradit Complement Med. 8 (1), 39–46. Retrieved from http://thailand.digitaljournals.org/index.php/JTTAM/article/view/18366.
Prasopthum, A., Insawek, T., Pouyfung, P. (2022). Herbal medicine use in Thai patients with type 2 diabetes mellitus and its association with glycemic control: A cross-sectional evaluation. Heliyon 8 (10), e10790. doi: 10.1016/j.heliyon.2022.e10790
Pristas, P., Beck, T., Nosalova, L., Gaperova, S., Gaper, J. (2023). How different molecular markers estimate the diversity of European species of the Ganoderma genus. J. Fungi 9, 1023. doi: 10.3390/jof9101023
Pungpa, S., Jamklang, M., Musika, J., Leelasakulchai, S., Chumkiew, S. (2023). “Biodiversity of mushroom during the dry season at Suranaree University of Technology,” in SUT International Virtual Conference on Science and Technology. Suranaree University of Technology, Nakhon Ratchasima, Thailand. pp. 335–341.
Punnapayak, H., Prasongsuk, S., Messner, K., Danmek, K., Lotrakul, P. (2009). Polycyclic aromatic hydrocarbons (PAHs) degradation by laccase from a tropical white rot fungus Ganoderma lucidum. Afr. J. Biotechnol. 8 (14), 3195–3202. doi: 10.5897/AJB09.1073
Putthapiban, P., Sukhumthammarat, W., Sriphrapradang, C. (2017). Concealed use of herbal and dietary supplements among Thai patients with type 2 diabetes mellitus. J. Diabetes Metab. Disord. 16, 36. doi: 10.1186/s40200-017-0317-3
Rangpan, V. (2015). “Utilization management of biological diversity in Pattani watershed, South Thailand,” in Proceedings of the 2nd International Conference on Research, Implementation, and Education of Mathematics and Sciences (ICRIEMS 2015) (Yogyakarta, Indonesia: Yogyakarta State University), pp. 530–540.
Rašeta, M., Kebert, M., Mišković, J., Kostić, S., Kaišarević, S., Stilinović, N., et al. (2024). Ganoderma pfeifferi Bres. and Ganoderma resinaceum Boud. as potential therapeutic agents: a comparative study on antiproliferative and lipid-lowering properties. J. Fungi 10, 501.
Ridtibud, S., Suwannasai, N., Sawasdee, A., Champreda, V., Phosri, C., Sarp, S., et al. (2024). Selection of white-rot fungi for decolorization of palm oil mill effluent and evaluation of biodegradation and biosorption processes. Nat. Environ. pollut. Technol. 23 (1), 95–105. doi: 10.46488/NEPT.2024.v23i01.019
Rode, A., Müller, N., Kováč, O., Wurst, K., Magauer, T. (2024). A general entry to Ganoderma meroterpenoids: Synthesis of applanatumol E, H, and I, lingzhilactone B, meroapplanin B, and lingzhiol. Org. Lett. 26, 9017–9021. doi: 10.1021/acs.orglett.4c03192
Rodseeda, C., Yamanont, P., Pinthong, D., Korprasertthaworn, P. (2022). Inhibitory effects of Thai herbal extracts on the cytochrome P450 3A-mediated the metabolism of gefitinib, lapatinib and sorafenib. Toxicol. Rep. 9, 1846–1852. doi: 10.1016/j.toxrep.2022.10.004
Rodtong, S., Teaumroong, N., Chooklay, P. (1998). “A preliminary study on the diversity of macrofungi in nong-rawieng plant genetics forest,” in Proceedings of The Asia-Pacific Mycological Conference on Biodiversity and Biotechnology 1998, Hua Hin, Thailand. 281–283.
Rungjindamai, N., Pinruan, U., Choeyklin, R., Hattori, T., Jones, E. B. (2008). Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis in Thailand. Fungal Divers. 33, 139–162. Retrieved from https://www.fungaldiversity.org/fdp/sfdp/33-8.pdf
Sa-Ard, P., Sarnthima, R., Khammuang, S., Kanchanarach, W. (2015). Antioxidant, antibacterial and DNA protective activities of protein extracts from Ganoderma lucidum. J. Food Sci. Technol. 52, 2966–2973. doi: 10.1007/s13197-014-1343-5
Samlikamnoed, P., Anothai, J., Chairin, T. (2023). Defense-related enzyme production in oil palm seedlings against basal stem rot pathogen Ganoderma boninense and its biological control by Trichoderma asperellum. Physiol. Mol. Plant Pathol. 128, 102154. doi: 10.1016/j.pmpp.2023.102154
Sanmanoch, W., Surapat, W., Phosri, S., Yaraksa, N. (2024). Antioxidant activity and cytotoxicity against the cervical epithelial carcinoma (HeLa) cell line of crude Ganoderma lucidum mycelial extracts. Cre. Sci. 16, 254094. doi: 10.55674/cs.v16i1.254094
Sao Mai, D., Binh, T. T., Xi, T. T., Tram, N. T., Suong, N. K. (2015). Optimizing the polysaccharide extraction from the Vietnamese Lingzhi (Ganoderma lucidum) via enzymatic method. JFNS 3, 111–114. doi: 10.11648/j.jfns.s.2015030102.31
Sappan, M., Rachtawee, P., Srichomthong, K., Boonpratuang, T., Choeyklin, R., Feng, T., et al. (2022). Ganoellipsic acids A–C, lanostane triterpenoids from artificially cultivated fruiting bodies of Ganoderma ellipsoideum. Phytochem. Lett. 49, 27–31. doi: 10.1016/j.phytol.2022.03.001
Sarnthima, R., Kanchanarach, W., Khammuang, S. (2022). Purification and characterization of laccase from Ganoderma sp. 03. Asia-Pacific J Sci Technol. 28 (02), APST-28-02-07. https://www.tci-thaijo.org/index.php/APST/index.
Scheibel, D. M., Gitsov, I. P. I., Gitsov, I. (2024). Enzymes in “Green” Synthetic chemistry: laccase and lipase. Molecules 29, 989. doi: 10.3390/molecules29050989
Seethapathy, P., Sankaralingam, S., Muniraj, I. K., Perumal, M., Pandurangan, N. (2023). “Mass multiplication, economic analysis, and marketing of Ganoderma sp. (Reishi Mushroom),” in Food microbiology based entrepreneurship. Eds. Amaresan, N., Dharumadurai, D., Babalola, O. O. (Springer, Singapore). doi: 10.1007/978-981-19-5041-4_6
Senphan, T., Benjakul, S., Sukketsiri, W., Chotphruethipong, L., Sriket, C. (2024). Comparative studies on characterizations and cytotoxicity of oil extracted from Lingzhi (Ganoderma lucidum) G2 spore using Soxhlet extraction and microwave-assisted extraction. Appl. Food Res. 4, 100483. doi: 10.1016/j.afres.2024.100483
Senphan, T., Takeungwongtrakul, S., Kaewthong, P. (2021). Extraction and antioxidant activities of broken Ganoderma lucidum spore. IJAT 17, 2303–2316. Retrieved from https://www.ijat-aatsea.com/past_v17_n6.html
Siriarchawatana, P., Harnpicharnchai, P., Phithakrotchanakoon, C., Kitikhun, S., Mayteeworakoon, S., Chunhametha, S., et al. (2024). Fungal communities as dual indicators of river biodiversity and water quality assessment. Water Res. 253, 121252. doi: 10.1016/j.watres.2024.121252
Soksawatmakhin, S., Boonyahotra, W. (2013). Preliminary study of the applications of Ganoderma lucidum in chronic fatigue syndrome. J. Appl. Sci. Process 2, 262–268. Available at: https://www.aaspjournal.org/uploads/155/5940_pdf.pdf (Accessed: April 15, 2025).
Song, L., Liu, Y., Xiao, S., Li, Y., Yu, H., Zeng, Y. (2025). Exploring the adsorption potential of different ganoderma lucidum mycelium morphologies for microplastic removal. SSRN. Elsevier Inc., New York, USA. doi: 10.2139/ssrn.5163851
Sopov, M., Huong, T. T., Thao, L. P., Trang, P. T., Quy, B. H., Thu, N. T., et al. (2022). Tam Binh Mushroom and Fresh Vegetable Cooperative, Bac Ninh, Vietnam (Wageningen, Netherlands: Wageningen University & Research). Available at: https://edepot.wur.nl/587205 (Accessed: April 15, 2025).
Sornchareon, C., Methiyothin, S. (2020). Export model of highland lingzhi to china trade market. J. Grad Sch Commer Burapha Rev. 15 (2), 45–59.
Sornprasert, R. (1995). The Comparison of protein and amino acid in mushroom mycelium and fruiting body. Food. 25 (3), 178–184.
Sornprasert, R., Aroonsrimorakot, S. (2014). Utilization of Vetiveria zizaniodes (L.) Nash leaves in Ganoderma lucidum cultivated. APCBEE Proc. 8, 47–52. doi: 10.1016/j.apcbee.2014.01.078
Sornprasert, R., Aroonsrimorakot, S. (2015). Using of vetiver (Vetiveria zizanioides (L.) Nash) leaves in Ling Zhi mushroom (Ganoderma lucidum (Ley. ex Fr.) Kar.) cultivation. J. Agric. Res. Ext. 32, 7–16. Available at: https://search.tci-thailand.org/article.html?b3BlbkFydGljcGUmaWQ9MTY2OTQy (Accessed: April 15, 2025).
Soytong, K. (2014). Biological control of stem rots of oil palms caused by Ganoderma boninense using Chaetomium lucknowense and Chaetomium cochiliodes. J. Agric. Technol. 10, 87–92. Available at: https://www.ijat-aatsea.com/pdf/v10_n1_14_January/8_IJAT_2014_10%281%29_Kasem%20Soytong3-Bio%20Control.pdf (Accessed: April 15, 2025).
Srey, C. (2019). Log and bag cultivation of Oyster (Pleurotus ostreatus) and Lingzhi (Ganoderma lucidum) mushrooms in Cambodia. CJBAR 1, 33–43. doi: 10.61945/cjbar.2019.1.2.2
Sriket, C., Kuimalee, S., Yarnpakdee, S., Benjakul, S., Sriket, P., Kishimura, H., et al. (2024). Elucidating the physicochemical and structural properties of Ganoderma lucidum spores: Comparative analysis of various disruption techniques. Powder Technol. 439, 119731. doi: 10.1016/j.powtec.2024.119731
Sujarit, K., Pathom-aree, W., Mori, M., Dobashi, K., Shiomi, K., Lumyong, S. (2020). Streptomyces palmae CMU-AB204T, an antifungal producing-actinomycete, as a potential biocontrol agent to protect palm oil producing trees from basal stem rot disease fungus, Ganoderma boninense. Biol. Cont. 148, 104307. doi: 10.1016/j.biocontrol.2020.104307
Sun, Y. F., Costa-Rezende, D. H., Xing, J. H., Zhou, J. L., Zhang, B., Gibertoni, T. B., et al. (2020). Multi-gene phylogeny and taxonomy of Amauroderma s. lat. (Ganodermataceae). Pers. Mol. Phylogeny Evol. Fungi 44, 206–239. doi: 10.3767/persoonia.2020.44.08
Sun, Y. F., Fang, Y. X., Cui, B. K. (2022a). Taxonomy and phylogeny of Sanguinoderma rugosum complex with descriptions of a new species and a new combination. Front. Microbiol. 13, 1087212. doi: 10.3389/fmicb.2022.1087212
Sun, Y. F., Lebreton, A., Xing, J. H., Fang, Y. X., Si, J., Morin, E., et al. (2022b). Phylogenomics and comparative genomics highlight specific genetic features in Ganoderma species. J. Fungi 8, 311. doi: 10.3390/jof8030311
Suprasert, P., Apichartpiyakul, C., Sakonwasun, C., Nitisuwanraksa, P., Phuackchantuck, R. (2014). Clinical characteristics of gynecologic cancer patients who respond to salvage treatment with lingzhi. Asian Pac J. Cancer Prev. 15 (10), 4193–4196. doi: 10.7314/apjcp.2014.15.10.4193
Suprasert, P., Apichartpiyakul, C., Sakonwasun, C., Nitisuwanraksa, P., Phuackchantuck, R. (2015). A randomized double blinded study of ganoderma lucidum (Lingzhi) in salvage setting of recurrent gynecologic cancer. Int. J. Cancer Clin. Res. 2 (3), 1–6. doi: 10.23937/2378-3419/2/3/1021
Surawut, S., Kunsook, C., Nak-eiam, S., Khamchatra, N. M., Bhudharak, S., Phontharod, W., et al. (2023). Biodiversity and functional distribution of macrofungi from plant genetic conservation area, Chanthaburi Province, Thailand. Curr. Appl. Sci. Technol. 23, 10–55003. doi: 10.55003/cast.2023.05.23.003
Sutjaritvorakul, T., Chutipaijit, S. (2017). Solubilization and tolerance of molybdenum trioxide (MoO3) by wood-decaying fungi. Khon Kaen Agr. J. 45 (2), 179–188.
Suwannarach, N., Kumla, J., Khuna, S., Wannathes, N., Thongklang, N., Sysouphanthong, P., et al. (2022). History of thai mycology and resolution of taxonomy for thai macrofungi confused with Europe and American names. Chiang Mai J. Sci. 49 (3), 654–683. doi: 10.12982/CMJS.2022.052
Suwanno, S., Phutphat, C. (2018). 1, 3-β-glucan content of local medicinal mushrooms from the southern region of Thailand. WJST 15, 189–200. Available at: https://wjst.wu.ac.th/index.php/wjst/article/view/2751 (Accessed: April 15, 2025).
Tan, L. (2023). Thailand’s Booming Demand for TCM and Mushroom Supplements. NutraIngredients-Asia [Internet]. Available online at: https://www.nutraingredients-asia.com.
Tangkhaphiphat, P., Siritientong, T., Jaruhathai, S., Pipopchaiyasit, N., Ratanajarusiri, T., Aramwit, P. (2022). Immunomodulatory efficacy and safety of Ganoderma lucidum broken spore supplement in patients after chemotherapy. Sci Eng Health Stud 16, 22050017. doi: 10.14456/sehs.2022.41
Tayar, S., Villagra, J., Gaju, N., Martínez-Alonso, M., Beltrán-Flores, E., Sarrà, M. (2025). Ganoderma lucidum immobilized on wood demonstrates high persistence during the removal of OPFRs in a trickle-bed bioreactor. J. Fungi. 11, 85. doi: 10.3390/jof11020085
Teekachunhatean, S., Sadja, S., Ampasavate, C., Chiranthanut, N., Rojanasthien, N., Sangdee, C. (2012). Pharmacokinetics of Ganoderic Acids A and F after oral administration of Ling Zhi preparation in healthy male volunteers. Evid. Based Complementary Altern. Med. 2012, 780892. doi: 10.1155/2012/780892
Teerapatsakul, C., Abe, N., Bucke, C., Kongkathip, N., Jareonkitmongkol, S., Chitradon, L. (2007a). Novel laccases of Ganoderma sp. KU-Alk4, regulated by different glucose concentration in alkaline media. World J. Microbiol. Biotechnol. 23, 1559–1567. doi: 10.1007/s11274-007-9401-z
Teerapatsakul, C., Parra, R., Bucke, C., Chitradon, L. (2007b). Improvement of laccase production from Ganoderma sp. KU-Alk4 by medium engineering. World J. Microbiol. Biotechnol. 23, 1519–1527. doi: 10.1007/s11274-007-9396-5
Thaithatgom, S. (1995). [Study on ganoderma lucidum as an auxiliary treatment for cancer patients] [Unpublished raw data] (Thailand).
Thakur, P., Khanal, S., Tapwal, A., Kumar, D., Verma, R., Chauhan, P., et al. (2024). Exploring Ganoderma lucidum: Morphology, cultivation and market potential. World J. Microbiol. Biotechnol. 40, 1–8. doi: 10.1007/s11274-024-04180-y
Tham, L. X., Hung, N. L., Thiem, B. H., Luong, L., My, N. T., Nhan, L. T., et al. (2018). Current status of Humphreya endertii and a new species (Ganodermataceae) recorded in South Vietnam. Agrica. 7, 102–109. doi: 10.5958/2394-448X.2018.00016.0
Tham, L. X., Matsuhashi, S., Kume, T. (1999). Responses of Ganoderma lucidum to heavy metals. Mycoscience 40, 209–213. doi: 10.1007/BF02464301
Tham, L. X., Trung, B. Q., Thuy, N. T., Quang, D. N. (2006). “The Black Lingzhi fungus newly-recorded from National Park of Cat tien, South Vietnam,” in The Meeting of the Mycological Society of Japan the 50th Anniversary of Annual Meeting for the Mycological Society of Japan. Session ID: 20-B. Tokyo, Japan: Mycological Society of Japan. 20–20. doi: 10.11556/msj7abst.50.0.20.0
Thang, T. D., Kuo, P. C., Hwang, T. L., Yang, M. L., Ngoc, N. T., Han, T. T., et al. (2013). Triterpenoids and steroids from Ganoderma mastoporum and their inhibitory effects on superoxide anion generation and elastase release. Molecules 18, 14285–14292. doi: 10.3390/molecules181114285
Thawthong, A., Hapuarachchi, K. K., Wen, T. C., Raspé, O., Thongklang, N., Kang, J. C., et al. (2017). Ganoderma sichuanense (Ganodermataceae, Polyporales) new to Thailand. MycoKeys 22, 27–43. doi: 10.3897/mycokeys.22.13083
Thawthong, A., Karunarathna, S. C., Thongklang, N., Chukeatirote, E., Kakumyan, P., Chamyuang, S., et al. (2014). Discovering and domesticating wild tropical cultivatable mushrooms. Chiang Mai J. Science. 41 (4), 731–764.
Thuy, N. H. C., Diem, V. T., Thuong, T. T. D., Anh, T. T., Nhut, T. M., Dat, T. V., et al. (2022). In vitro antioxidant activity and in vivo hepatoprotective effects of ethanolic extracts from wall-broken ganoderma lucidum spores. Open Access Maced J Med Sci. 10 (A), 1450–1455. doi: 10.3889/oamjms.2022.10421
Thuy, N. H., Tu, V. L., Giang, T. T., Huyen, D. T., Loc, D. H., Tam, D. N., et al. (2023). Pharmacological activities and safety of Ganoderma lucidum spores: A systematic review. Cureus 15 (10), e44574. doi: 10.7759/cureus.44574
Tippayawong, N., Chaichana, C., Promwungkwa, A., Rerkkriangkrai, S. (2011). Clean energy from gasification of biomass for sterilization of mushroom growing substrates. Int. J. Energy Res. 5 35(5), 391–399. doi: 10.1002/er.1795
Tompong, S., Kunasakdakul, K. (2014). Causal agent, symptoms and environmental factors of root rot disease of organic Assam tea in Mae Taeng district, Chiang Mai province. Int. J. Agric. Technol. 10, 767–777. Available at: http://www.ijat-aatsea.com (Accessed: April 15, 2025).
Tran, P. T., Dat, N. T., Dang, N. H., Van Cuong, P., Lee, S., Hwangbo, C., et al. (2019). Ganomycin I from Ganoderma lucidum attenuates RANKL-mediated osteoclastogenesis by inhibiting MAPKs and NFATc1. Phytomedicine 55, 1–8. doi: 10.1016/j.phymed.2018.10.029
Tran, Y. H., Nguyen, T. T., Nguyen, P. T., Nguyen, K. T., Duong, C. X., Tran, H. M. (2021). Effects of Ganoderma lucidum extract on morphine-induced addiction and memory impairment in mice. Biointerface. Res. Appl. Chem. 12 (1), 1076–1084. doi: 10.33263/BRIAC121.10761084
Truyen, D. M., Patacsil, F. F. (2017). Survey the composition and distribution of fungi species in the natural reserve Wetland Lung Ngoc Hoang, Vietnam. JATER 3, 19–26. doi: 10.20474/jater-3.1.4
Tsivileva, O., Nguyen, T., Vu, L., Yurasov, N., Chernyshova, M., Petrov, A., et al. (2016). Vietnamese Ganoderma: growth, peculiarities, and low-molecular composition compared to European and Siberian strains. Turkish J. Bot. 40, 269–286. doi: 10.3906/bot-1410-15
Ubolsuk, C., Pornsuriya, C. (2022). Trichoderma species associated with green mold disease of Ganoderma lingzhi in Thailand. Songklanakarin J Sci Technol. 44 (1), 1–5.
Vaithanomsat, P., Boonlum, N., Chaiyana, W., Tima, S., Anuchapreeda, S., Trakunjae, C., et al. (2022). Mushroom β-glucan recovered from antler-type fruiting body of Ganoderma lucidum by enzymatic process and its potential biological activities for cosmeceutical applications. Polymers 14, 4202. doi: 10.3390/polym14194202
Van, L. T., Le Duy, T. (1991). Linhchi mushrooms as biological monitors for 137 Cs pollution. J. Radioanal Nucl. Chem. 155, 451–458. doi: 10.1007/BF02163640
Van Trung, H., Thanh, N. T., Tuan, N. N., Dung, D. M., Thang, T. D. (2018). The triterpenoid and steroid from the fruiting body of Ganoderma applanatum (Pers.) Pat. in Viet Nam. Vietnam J. Sci. Technol. 56, 550–556. doi: 10.15625/2525-2518/56/5/12588
Viết Thế, H., Thị Ngọc Hà, V., Ngọc Giàu, L. (2019). Classification of some commercial lingzhi (Ganoderma spp.) accessions in Vietnam by its-based DNA barcode. Hueuni-JNS 128, 163–171. Available at: https://jos.hueuni.edu.vn/index.php/hujos-ns/article/view/5380 (Accessed: April 15, 2025).
Viet Hung, T., Thang, P. N., Hien, H. M., Diep, V. T., Thu, N. T., Tan, D. M., et al. (2022). Cytotoxic activities and fingerprint analysis of triterpenes by HPTLC technique for distinguishing Ganoderma species from Vietnam and other Asian countries. Plants 11, 3397. doi: 10.3390/plants11233397
Vijitrotai, P., Vijitrotai, N., Puttongsiri, T. (2023). Proximate analysis, mineral and germanium of ganoderma lucidum or lingzhi powder by spray dry affecting in different strains and their maltodextrin. Int. J. Agric. Technol. 19 (1), 301–310. https://www.ijat-aatsea.com.
Vo, T. N., Le, N. G. (2019). Developmental, morphological and molecular variation of commercial Ganoderma spp. accessions from southern Vietnam. Biodiversitas 20 (12), 3684–3689. doi: 10.13057/biodiv/d201230
Vyas, M., Kulshrestha, M. (2023). “Removal of radioactive material (Uranium-VI) from low level wastewaters and subsequent disposal of loaded biomass,” in Materials Today: Proceedings. Elsevier Ltd., Amsterdam, Netherlands. 84, 256–258. doi: 10.1016/j.matpr.2023.01.274
Wanachiwanawin, D., Piankijagum, A., Chaiprasert, A., Lertlaituan, P. (2006). Ganoderma lucidum: a cause of pseudoparasitosis. Southeast Asian J. Trop. Med. Public Health 37, 1099–1102. Available at: https://www.proquest.com/openview/b8412c0ac35a3c81c3db5efabebd9791/1?cbl=34824&pq-origsite=gscholar (Accessed: April 15, 2025).
Wang, H., Jin, C., Li, X., Ma, J. X., Ye, Y. F., Tang, L. X., et al. (2025). A green biocatalyst fabricated by fungal laccase immobilized onto Fe3O4@ polyaniline-chitosan nanofibrous composites for the removal of phenolic compounds. Chem. Eng. J. 507, 160486. doi: 10.1016/j.cej.2025.160486
Wanmuang, H., Leopairut, J., Kositchaiwat, C., Wananukul, W., Bunyaratvej, S. (2007). Fatal fulminant hepatitis associated with Ganoderma lucidum (Lingzhi) mushroom powder. J. Med. Assoc. Thai. 90, 179–181. Available at: https://www.researchgate.net/publication/6215821_Fatal_fulminant_hepatitis_associated_with_Ganoderma_lucidum_Lingzhi_mushroom_powder (Accessed: April 15, 2025).
Wannasawang, N., Luangharn, T., Thawthong, A., Charoensup, R., Jaidee, W., Tongdeesoontorn, W., et al. (2023). Study of optimal conditions to grow Thai Ganoderma, fruiting test, proximate, and their alpha glucosidase inhibitory activity. Life 13, 1887. doi: 10.3390/life13091887
Wannasupchue, W., Siriamornpun, S., Huaisan, K., Huaisan, J., Meeso, N. (2011). Effect of adding Ling-zhi (Ganoderma lucidum) on oxidative stability, textural and sensory properties of smoked fish sausage. Thai J. Agric. Sci. 44, 505–512.
Watanavanich, P. (1982). “Oil palm disease [fungal] in Thailand,” in International Conferences on Oil Palm in Agriculture. Kuala Lumpur, Malaysia: The Incorporated Society of Planters, pp. 457–46.
Wei, Q. L., Zheng, H. F., Shao, Y. Y., Rasheed, U., Lin, J. T., Huang, F. C., et al. (2024). A New Species of Ganoderma (Ganodermataceae, Polyporales) from Southern China and optimum condition for mycelia production. Mycobiology 52, 58–67. doi: 10.1080/12298093.2024.2306012
Wijaya, N. H., Savitri, A. D., Wahyuni, A. T., Alhadad, E. S., Edo, N., Shabrina, A., et al. (2021). Wild mushrooms diversity in tropical rainforest. Ecol. Environ. Conserv. 27, 622–627. Available at: https://www.researchgate.net/publication/363579302_Wild_Mushrooms_Diversity_in_Tropical_Rainforest (Accessed: April 15, 2025).
Wongkhieo, S., Tangmesupphaisan, W., Siriwaseree, J., Aramsirirujiwet, Y., Wiriyajitsomboon, P., Kaewgrajang, T., et al. (2023). In vitro cholesterol lowering activity of Ganoderma australe mycelia based on mass spectrometry, synchrotron Fourier-transform infrared analysis and liver-spheroid bioactivity. Sci. Rep. 13, 13619. doi: 10.1038/s41598-023-40861-8
World Bank. (2023). Lao PDR Economic Update: Rising Demand for Health Products [Internet]. World Bank. Available online at: https://www.worldbank.org/en/country/lao.
Wu, S., Zhang, S., Peng, B., Tan, D., Wu, M., Wei, J., et al. (2024a). Ganoderma lucidum: a comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 13, 568–596. doi: 10.26599/FSHW.2022.9250051
Wu, P., Zhang, C., Yin, Y., Zhang, X., Li, Q., Yuan, L., et al. (2024b). Bioactivities and industrial standardization status of Ganoderma lucidum: A comprehensive review. Heliyon 10 (19), e36987. doi: 10.1016/j.heliyon.2024.e36987
Xia, J., He, X., Yang, W., Song, H., Yang, J., Zhang, G., et al. (2024). Unveiling the distribution of chemical constituents at different body parts and maturity stages of Ganoderma lingzhi by combining metabolomics with desorption electrospray ionization mass spectrometry imaging (DESI). Food Chem. 436, 137737. doi: 10.1016/j.foodchem.2023.137737
Xu, M., Meng, Q., Zhu, S., Yu, R., Chen, L., Shi, G., et al. (2024). The performance and evolutionary mechanism of ganoderma lucidum in enhancing selenite tolerance and bioaccumulation. J. Fungi. 10, 415. doi: 10.3390/jof10060415
Yangchum, A., Fujii, R., Choowong, W., Rachtawee, P., Pobkwamsuk, M., Boonpratuang, T., et al. (2022). Lanostane triterpenoids from cultivated fruiting bodies of basidiomycete Ganoderma mbrekobenum. Phytochem. 196, 113075. doi: 10.1016/j.phytochem.2021.113075
Yangchum, A., Srichomthong, K., Thummarukcharoen, T., Sommai, S., Isaka, M. (2023). Lanostane triterpenoids from artificially cultivated fruiting bodies of Ganoderma wiiroense. Phytochem. Lett. 58, 69–75. doi: 10.1016/j.phytol.2023.10.004
Zhang, H., Zhang, J., Liu, Y., Tang, C. (2023). Recent advances in the preparation, structure, and biological activities of β-glucan from Ganoderma species: A review. Foods 12, 2975. doi: 10.3390/foods12152975
Zheng, M., Pi, X., Li, H., Cheng, S., Su, Y., Zhang, Y., et al. (2024). Ganoderma spp. polysaccharides are potential prebiotics: a review. Crit. Rev. Food Sci. Nutr. 64 (4), 909–927. doi: 10.1080/10408398.2022.2110035
Keywords: Cambodia, Ganoderma, Laos, medicinal mushrooms, Thailand, Vietnam
Citation: Karunarathna SC, Patabendige NM, Luangharn T and Hapuarachchi KK (2025) Ganodermataceae—current status, research, and development in Lower Mekong Basin. Front. Cell. Infect. Microbiol. 15:1545135. doi: 10.3389/fcimb.2025.1545135
Received: 14 December 2024; Accepted: 24 March 2025;
Published: 12 May 2025.
Edited by:
István Pócsi, University of Debrecen, HungaryReviewed by:
Sudharshan S. J., University of Tennesse, United StatesDanka Matijašević, University of Belgrade, Serbia
Copyright © 2025 Karunarathna, Patabendige, Luangharn and Hapuarachchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kalani K. Hapuarachchi, a2FsYW5pZmlyc3RAeWFob28uY29t
 Samantha C. Karunarathna
Samantha C. Karunarathna Nimesha M. Patabendige2
Nimesha M. Patabendige2 Kalani K. Hapuarachchi
Kalani K. Hapuarachchi