- 1Section of Microbiology, Department of Medical and Surgical Sciences (DIMEC), Alma Mater Studiorum - University of Bologna, Bologna, Italy
- 2International PhD College, Collegio Superiore of Alma Mater Studiorum, University of Bologna, Bologna, Italy
- 3Institute of Biomedical Technologies, National Research Council, Segrate, Italy
- 4National Biodiversity Future Center S.c.a.r.l., Palermo, Italy
- 5Department of Medical Sciences, University of Ferrara, Ferrara, Italy
- 6Department of Animal Science, Food and Nutrition (DIANA), Università Cattolica Del Sacro Cuore, Piacenza, Italy
- 7Human Nutrition Unit, Department of Agricultural and Food Sciences (DISTAL), University of Bologna, Cesena, Italy
- 8Interdepartmental Centre for Agri-Food Industrial Research (CIRI Agrifood), University of Bologna, Cesena, Italy
- 9Centre of Foodomics, Department of Agricultural and Food Sciences (DISTAL), University of Bologna, Cesena, Italy
- 10Microbiology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 11Department of Medical and Surgical Sciences (DIMEC), Alma Mater Studiorum - University of Bologna, Bologna, Italy
Introduction: In reproductive-aged women, a vaginal microbiota dominated by several Lactobacillus species is crucial for maintaining vaginal health. Among the various factors affecting the composition of the vaginal ecosystem, the impact of dietary habits has rarely been explored. Thus, in this cross-sectional study, we assessed the role of macronutrient intake on the vaginal microbiota in a cohort of 113 young women, independently from potential confounders.
Methods: For each subject, we characterized (i) the vaginal bacterial community-state type (CST) by 16S rRNA gene profiling, (ii) the vagina lmetabolic profile by 1H-NMR spectroscopy, and (iii) the energy, nutrient and alcohol intake through a validated food frequency questionnaire.
Results: We found that the increase in animal protein intake, mainly derived from red and processed meat, was positively associated with the dysbiotic condition of CST IV and, similarly, alcohol consumption was significantly associated with the levels of Gardnerella spp. and Ureaplasma spp. On the other hand, we noticed a beneficial effect of a-linolenic acid, with its increase inversely associated with CST III, dominated by the ‘less-protective’ species Lactobacillus iners. Moreover, linolenic acid was related to the abundance of Lactobacillus crispatus, in turn related tovarious vaginal metabolites such as 4-hydroxyphenyllactate and several amino acids. Total carbohydrates, vegetable proteins, total fiber, and starch were negatively correlated with Gardnerella spp.
Discussion: We highlighted that specific dietary habits (i.e., reduced consumption of alcohol and animal proteins, higher intake of linolenic acid) can have a beneficial impact on the vaginal environment, through the maintenance of a microbiota mainly dominated by ‘protective’ Lactobacillus species.
1 Introduction
In reproductive-aged women, a protective vaginal microbiota is typically dominated by various species of Lactobacillus. These beneficial bacteria play a key role in maintaining vaginal health by preventing colonization and proliferation of harmful microorganisms through several mechanisms (Severgnini et al., 2022; Morselli et al., 2024).
The depletion of lactobacilli, in parallel with the increase in different species of anaerobic bacteria (e.g., Gardnerella, Fannyhessea, Prevotella, Mobiluncus), results in a dysbiotic condition called bacterial vaginosis (BV) (Ceccarani et al., 2019; Plummer et al., 2024). BV is characterized by marked alterations in the composition of vaginal metabolites (e.g., reduction of lactic acid, higher concentrations of biogenic amines, and short chain fatty acids), and is associated with an increased risk of sexually transmitted infections (STIs), as well as adverse pregnancy outcomes such as preterm birth and low birth weight (Srinivasan et al., 2015; Laghi et al., 2021; Occhipinti et al., 2025).
Molecular characterization of the vaginal microbiota using 16S rRNA gene amplicon sequencing has afforded the high-resolution characterization of the composition and taxonomy of the vaginal microenvironment (Tamarelle et al., 2024). Indeed, vaginal bacterial communities can be clustered into five broad community state types (CSTs) (Ravel et al., 2011; Greenbaum et al., 2019). CST I, II, III, and V are dominated by one species of Lactobacillus, L. crispatus, L. gasseri, L. iners, and L. jensenii, respectively (Ravel et al., 2011). While L. crispatus, L. gasseri, and L. jensenii are well-recognized as hallmarks of vaginal health, L. iners has often been considered a ‘transitional’ species, showing fewer protective capabilities (Raimondi et al., 2021; Novak et al., 2022).
Contrariwise, CST IV, which aligns well with BV, is depleted of Lactobacillus spp. and consists of a diverse array of anaerobes, including Gardnerella spp., Fannyhessea vaginae (Atopobium vaginae), Candidatus Lachnocurva vaginae (formerly known as BVAB1) or Prevotella spp., further classifiable as CST IV-A, CST IV-B or CST-IVC (France et al., 2020).
Longitudinal studies have shown that the composition of the vaginal microbiota is influenced by various local and systemic factors, such as hormonal levels, psychosocial stress, smoking, sexual habits, the use of topical products or antibiotics, and the presence of urogenital infections (Govender and Ghai, 2024; Morsli et al., 2024).
In this context, it has also been shown that dietary habits can affect the bacterial composition of the vaginal environment (Tuddenham et al., 2019; Dall’Asta et al., 2021; Noormohammadi et al., 2022). Indeed, previous studies have demonstrated that increased dietary fat intake, energy intake, and glycemic load are associated with a higher risk of BV, whereas increased intake of folate, vitamin A, calcium, and more in general, diets rich in fiber, seem to be protective factors against this condition (Neggers et al., 2007; Thoma et al., 2011; Shivakoti et al., 2020).
Recently, in a cohort of pregnant Caucasian women, we observed that a lactobacilli-dominated vaginal microbiota was negatively correlated with a higher pre-pregnancy intake of animal-sourced proteins, whereas a higher pre-pregnancy consumption of total carbohydrates and sugars seemed to be a protective factor for vaginal health (Dall’Asta et al., 2021).
Nevertheless, exhaustive data on the impact of dietary macronutrient intake on the composition of the vaginal environment are still lacking, and many aspects remain to be fully elucidated.
Therefore, in this cross-sectional study, we assessed the role of energy, macronutrient, alcohol intake and related dietary sources on the vaginal ecosystem in terms of bacterial composition and vaginal metabolite concentration in a cohort of 113 non-pregnant young women, considering the effect of potential confounders that could perturb the vaginal environment.
To this goal, we characterized for each woman (i) the vaginal bacterial composition by means of 16S rRNA gene profiling, (ii) the vaginal metabolic profile (1H-NMR spectroscopy), and (iii) the dietary nutrient intake using a validated food frequency questionnaire.
2 Materials and methods
2.1 Study setting and population
Between November 2023 and April 2024, 119 sexually active women having sex with men (WSM) of reproductive age were included in the study. The women were volunteers attending degree courses at the University of Bologna, Italy, and were selected from those who expressed an interest in participating in the study. At the time of the enrollment, exclusion criteria were: (i) antibiotic use in the month prior to sampling; (ii) menstruation at the time of sampling; (iii) HIV infection; (iv) presence of chronic conditions (e.g., diabetes, autoimmune diseases, malignancies); (v) pregnancy at enrollment and/or previous pregnancies; (vi) age < 18 years. Moreover, women with urogenital infections due to sexually transmitted pathogens (i.e., Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium) were further excluded when diagnostic tests results were available.
The following data and variables were recorded for each woman: age, education (categorized as high school, university degree, and postgraduate/doctorate), work status (student vs. other), marital status (married/cohabiting vs. single/separated), contraceptive use (yes vs. no), smoking habits (never, former, or current smoker), body mass index (BMI, calculated as weight in kg divided by height in cm squared), and presence/absence of vaginal symptoms or disorders (i.e., dyspareunia, vaginal itching and/or burning, and unusual vaginal discharge) (see Table 1).
As previously reported, marital status was used as a proxy for sexual behavior (Exavery et al., 2015; Duberstein Lindberg and Singh, 2008; Jackson et al., 2019). The Perceived Stress Scale (PSS) was used to assess the level of stress and discomfort perceived by participants during the past month (available for 103 participants out of 113). The scale includes 10 items, and the answers are provided on a 5-point Likert scale (from 0 = Never to 4 = Very often) (Cohen et al., 1983).
Each woman completed a validated food frequency questionnaire to assess dietary habits (see specific paragraph) and underwent two self-collected vaginal samples during the late follicular phase. The first swab (E-swab, Copan, Brescia, Italy) was used for diagnostic tests to exclude the presence of sexually-transmitted infections using nucleic acid amplification tests (Alinity m STI Assay; Abbott Molecular Diagnostics, Des Plaines, IL, USA). The second was collected with a sterile cotton bud, resuspended in 1 mL of sterile saline, and stored at -80°C until use. Frozen vaginal swabs were thawed, vortexed for 1 min, and removed from the liquid. After centrifugation (10000 × g for 15 min), cell-free supernatants were used for metabolomic analysis, whereas bacterial pellets were employed for vaginal microbiota profiling (see paragraphs below).
Written informed consent was obtained from all participants, and the study protocol was approved by the Bioethics Committee of the University of Bologna (protocol number 0122421).
2.2 Vaginal microbiota molecular profiling
Nucleic acids were extracted from vaginal swabs using the DNeasy Blood & Tissue kit (QIAGEN GmbH, Hilden, Germany), following the manufacturer’s protocol.
The V3-V4 hypervariable regions of the bacterial 16S rRNA gene were amplified according to the 16S metagenomic sequencing library preparation protocol (Illumina, San Diego, CA, USA). Final indexed libraries were prepared by equimolar (4 nmol/L) pooling, denaturation, and dilution to 6 pmol/L before loading on one MiSeq flow cell (Illumina) for a 2 × 300 bp paired-end run. Raw sequence data were processed by rebuilding single amplicon fragments from the paired reads using PANDAseq (Masella et al., 2012) and quality-filtering the obtained fragments trimming from the 3’-end stretches of at least three bases with a Phred score < 3; trimmed reads shorter than 75% of the original fragment were discarded. The retained reads were clustered in zero-radius Operational Taxonomic Units (zOTUs) using usearch (version 11.0.667; Edgar, https://www.biorxiv.org/content/10.1101/081257v1) and taxonomic classification was performed using the RDP classifier against SILVA 138 release (Wang et al., 2007; Quast et al., 2013). Depth of sequencing was set to the lowest sequenced sample (n=6,627 reads), in order to compensate for the sequencing unevenness of the samples and to provide a consistent minimum amount for the downstream analysis, carried out through the QIIME pipeline (version 1.9.0; Caporaso et al., 2010). Alpha-diversity evaluation was performed according to several microbial diversity metrics (i.e., Chao1, Shannon Index, Observed Species, and the Faith’s phylogenetic tree diversity metric), Beta-diversity evaluated using unweighted and weighted UniFrac distances (Lozupone et al., 2011) and the principal coordinate analysis (PCoA) to graphically represent the data.
Lactobacillus species-level characterization was performed as in Severgnini et al. (2022) by BLAST-aligning all reads belonging to the Lactobacillaceae family to a custom reference database made up of all available reference sequences in the NIH-NCBI database (https://ftp.ncbi.nlm.nih.gov/genomes/GENOME_REPORTS/prokaryotes.txt) of 17 species commonly found in the vaginal environment and filtering the results in order to obtain an unequivocal classification. In case of multiple matches with the same confidence, the taxonomy was reset to “Unclassified Lactobacillus”. The custom database included a total of 3,392 genomes at different finishing grades.
Community-state types (CST) of the vaginal microbial communities were determined from the taxonomic profiles using VALENCIA, a nearest centroid-based tool that classifies samples into 5 major CST and 13 sub-CST according to the similarity to a set of about 13,000 reference microbial profiles (France et al., 2020).
2.3 Vaginal metabolome assessment
Metabolomic analysis was performed by 1H-NMR spectroscopy: 100 μL of a D2O solution of 3-(trimethylsilyl)-propionic-2,2,3,3-d4 acid sodium salt (TSP) 10 mM set to pH 7.0 was added to 700 µL of the cell-free supernatants of the vaginal swabs. 1H-NMR spectra were recorded at 298 K with an AVANCE III spectrometer (Bruker, Milan, Italy), operating at a frequency of 600.13 MHz, equipped with Topspin software (version 3.5, Bruker) (Foschi et al., 2018). The signals originating from large molecules were suppressed by a CPMG filter of 400 spin-echo periods, generated by 180° pulses of 24 μs separated by 400 μs (Ventrella et al., 2016). To each spectrum, line broadening (0.3 Hz) and phase adjustment were applied by Topspin software, while any further spectra processing, molecules quantification, and data mining steps were performed in R computational language (version 4.0.5) through in-house developed scripts. The spectra were aligned toward the TSP signal, set at −0.017 ppm in agreement with Chenomx software data bank (version 8.3, Chenomx Inc., Edmonton, Alberta, Canada), and then baseline-adjusted by means of peak detection according to the “rolling ball” principle implemented in the “baseline” R package (Liland et al., 2010). The signals were assigned by comparing their chemical shift and multiplicities with the Chenomx software data bank. Molecules were quantified in the first sample acquired by employing the added TSP as an internal standard. To compensate for differences in sample amounts any other sample was then normalized to such sample by means of probabilistic quotient normalization (Dieterle et al., 2006). Integration of the signals was performed for each molecule following rectangular integration.
2.4 Dietary assessment and data processing
Participants’ usual dietary habits over the past one-year period were assessed using a semi-quantitative Food Frequency Questionnaire (FFQ). This FFQ was originally developed for the European Prospective Investigation into Cancer and Nutrition (EPIC) study (Bingham et al., 2001) and later validated in the Italian population (Pala et al., 2003). FFQ represents a suitable tool for evaluating long-term dietary habits, even in the context of the vaginal environment (Noormohammadi et al., 2025). The questionnaire consisted of 248 questions covering 188 different food items. Trained researchers administered the FFQ and collected data on the frequency of consumption and on portion sizes. For each food item, participants indicated their consumption frequency (per day, week, month, or year) and selected portion sizes through pictures showing small, medium, and large portions. Additional quantifiers were available, such as “smaller than the small portion”, and predefined standard portions were used when images were unavailable. Daily intake for each food item was calculated in grams, and energy, macronutrient, and alcohol intakes were derived using the Italian Food Composition Database (Gnagnarella P, Salvini S, Parpinel M. Food Composition Database for Epidemiological Studies in Italy. Version 1.2015. Available from: http://www.bda-ieo.it/).
We screened the data for implausible energy intake (<500 or >3500 kcal/day), although no participants were excluded on this basis. Alcohol intake was converted to alcoholic units (AU), with 1 AU defined as 10 g of alcohol (World Health Organization, 2000). Participants were classified according to their consumption as abstainers, those consuming ≤1 AU/day, or those consuming >1 AU/day, following national guidelines (SINU, 2024; available at: https://sinu.it/larn/). Four participants with missing dietary data were excluded from analysis.
The 188 individual FFQ food items were aggregated into 24 food categories according to their nutrient compositions and food similarities. These categories comprised tubers, vegetables, legumes, fruits, dried fruits and seeds, milk and yogurt, cheese, cereals and derived products (pasta and cereals), bakery products (bread, bread substitutes, breakfast cereals, and biscuits), stuffed pasta, sweets and snacks, red and processed meat, white meat, fish, eggs, olive oil, other plant-derived fats, animal-derived fats, sugar, non-alcoholic beverages, alcoholic beverages, vegetable products (plant-based milk and meat substitutes), salt and spices, and other non-classified foods.
To identify the main food sources of energy, macronutrients and alcohol, we conducted Spearman’s correlations analysis between all food groups and key nutritional components. These components included total proteins (TP), animal-derived protein (AP), plant-derived protein (VP), total fats (TF), total carbohydrates (TC), total dietary fiber (TDF), starch (ST), simple sugars (SS), saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), linoleic acid (LA), alpha-linolenic acid (ALA), other polyunsaturated fatty acids (PUFA), alcohol, and total energy intake.
The Mediterranean diet adherence score (MEDI-LITE; Sofi et al., 2017) was calculated to provide an overall assessment of diet quality. This score ranges from 0 (minimum adherence) to 18 (maximum adherence) and evaluates the nine dietary components. The scoring system considers the consumption of whole grains, legumes, fruits, vegetables, nuts, and olive oil as positive contributors to the score, whereas the consumption of dairy products and red and processed meat are considered negative contributors. Alcohol consumption was scored according to intake levels, with moderate consumption receiving higher scores.
2.5 Dietary data analysis using the CoDA approach
To take into account the compositional nature of dietary data, i.e. that they are parts of a whole and essentially convey relative information, macronutrient intakes were analyzed using the Compositional Data Analysis (CoDA) approach, based on log-ratio transformations (Pawlowsky-Glahn et al., 2015). The application of this methodology to dietary data and the discussion of the interpretative implications of using different log-ratios as explanatory variables in regression models have been extensively studied (Leite and Prinelli, 2017; Corrêa Leite, 2019; Leite, 2020; Corrêa Leite, 2021). Here, we considered a nine-part macronutrient composition: AP, VP, ST, SS, SFA, MUFA, LA, ALA, PUFA.
We were interested in assessing the effect of the proportions of each component in relation to all others (relative dominance). The pivot balance approach is generally used for this purpose (Hron et al., 2012). A pivot balance can capture all relevant information regarding a compositional part. Taking advantage of the fact that the additive log-ratio (ALR) transformation and the simplified pivot balance are explanatory equivalents (Coenders and Pawlowsky-Glahn, 2020), we used this simple log-ratio transformation to obtain the same measures of effects as those provided by the pivot balances. The ALR was calculated by dividing each macronutrient by an arbitrarily chosen component (Corrêa Leite, 2021). In the present work, eight ALRs were calculated by dividing each macronutrient by starch. Each ALR represents new variables that can be included as predictor variables in standard regression models.
Let y be the outcome (CST):
where zi are the control variables.
A B coefficient represents the change in response associated with a one unit change in the corresponding log-ratio, holding all other log-ratios constant. The coefficient B1, for example, represents the expected change in CST when AP increases and ST decreases, while keeping all the other terms in the model constant. This necessarily means that VP, SS, SFA, MUFA, LA, ALA, and other PUFA will decrease as well by the same factor as ST. To estimate the relative dominance of ST, another set of eight ALRs was calculated with AP as the denominator and the regression model was then run a second time (Corrêa Leite, 2021).
2.6 Statistical analysis
Sample characteristics were described using mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables and frequencies and percentages for categorical variables. One-way ANOVA (for normally distributed variables) and Kruskal-Wallis (for non-normally distributed variables) tests were used to compare participant characteristics in relation to the three categories of CST for continuous variables and the chi-squared test was used for categorical variables. Statistical evaluation of alpha-diversity indices was performed by non-parametric Monte Carlo-based tests through the QIIME pipeline. Beta-diversity differences were assessed by a permutation test with pseudo F-ratios using the “adonis” function from R package “vegan” (version 2.0-10, https://cran.r-project.org/package=vegan). Indicator species analysis (Dufrene and Legendre, 1997) was performed in MATLAB (version 2008b, Natick, MA, USA). Macronutrient intake and metabolite concentrations were correlated with bacterial composition by calculating the pairwise Spearman’s correlation coefficients among macronutrients, metabolites and bacterial genera present ≥1% in at least one sample. Correlations were presented using heatmaps and a correlation network created using Cytoscape (version 3.10.2; Shannon et al., 2003).
Multinomial logistic regression models were constructed to estimate the independent association between the nine-parts macronutrient composition (exposure) and the three-level CST (outcome, see below), and the β-coefficient, standard error (SE), and p-value were reported. The univariate regression model included the nine-parts macronutrient composition and total energy, fiber, and alcohol intake. The multivariate model was adjusted for confounders selected on the basis of previous literature and a number of theoretical assumptions regarding their possible influence on vaginal bacterial composition, including age, marital status, BMI, and hormonal contraceptive use (Morsli et al., 2024). In addition, as psychological distress has been reported to influence vaginal microbiota (Song et al., 2020) and dietary intake (Gonçalves et al., 2024), we performed a sensitivity analysis by including the PSS scale (Cohen et al., 1983) in the model to assess whether this factor could influence the associations between macronutrient intake and CST.
For the present analysis, as CST III (L. iners-dominated microbiota) has been associated with vaginal dysbiosis and less protective capabilities (Mikhanoshina and Priputnevich, 2024), we grouped the CSTs into three categories: a) L. crispatus/L. jensenii/L. gasseri-dominated CSTs (I, II, and V); b) L. iners-dominated (CST III); and c) polymicrobial microbiota (CST IV).
All analyses were performed using IBM SPSS Statistics for Windows (version 24.0, IBM Corp., Armonk, NY, USA) and STATA (version 15, StataCorp LLC, College Station, TX). All p-values ≤ 0.05 were considered statistically significant.
2.7 Data availability
Raw sequencing data for this project are available in NCBI Short-Read Archive (SRA) under accession number PRJNA1188525 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1188525). Raw metabolomic and nutritional data are displayed as Supplementary material (see Excel Files S1 and S2).
3 Results
3.1 Vaginal environment composition
A total of 119 participants were initially recruited; excluding those with missing data on dietary exposure (n=4) and those with unavailable vaginal microbiota data (n=2), 113 subjects (mean age ± standard deviation: 21.5 ± 2.5; min-max: 19-30) were finally included in the present analysis (Table 1). The sequencing process generated a total of 9,474,380 raw reads, which led to 4,019,683 (42.1%) high-quality amplicon fragments mapped in the zOTUs. Samples were categorized in Community State Types (CST) according to their microbial composition. Overall, the bacterial composition of the samples resembled the expected for the vaginal environment, as per the Lactobacillus genus species, with L. crispatus being the most abundant species (40.7% on average), followed by L. iners (22.6%), L. gasseri (4.2%), and L. jensenii (3.7%); among the other genera, Gardnerella vaginalis and Prevotella spp. accounted for 6.1% and 6.0% on average, respectively, whereas Streptococcus spp. (2.4%), Atopobium vaginae (1.4%), and Ureaplasma spp. (1.1%) were also consistently present (Supplementary Figure S1).
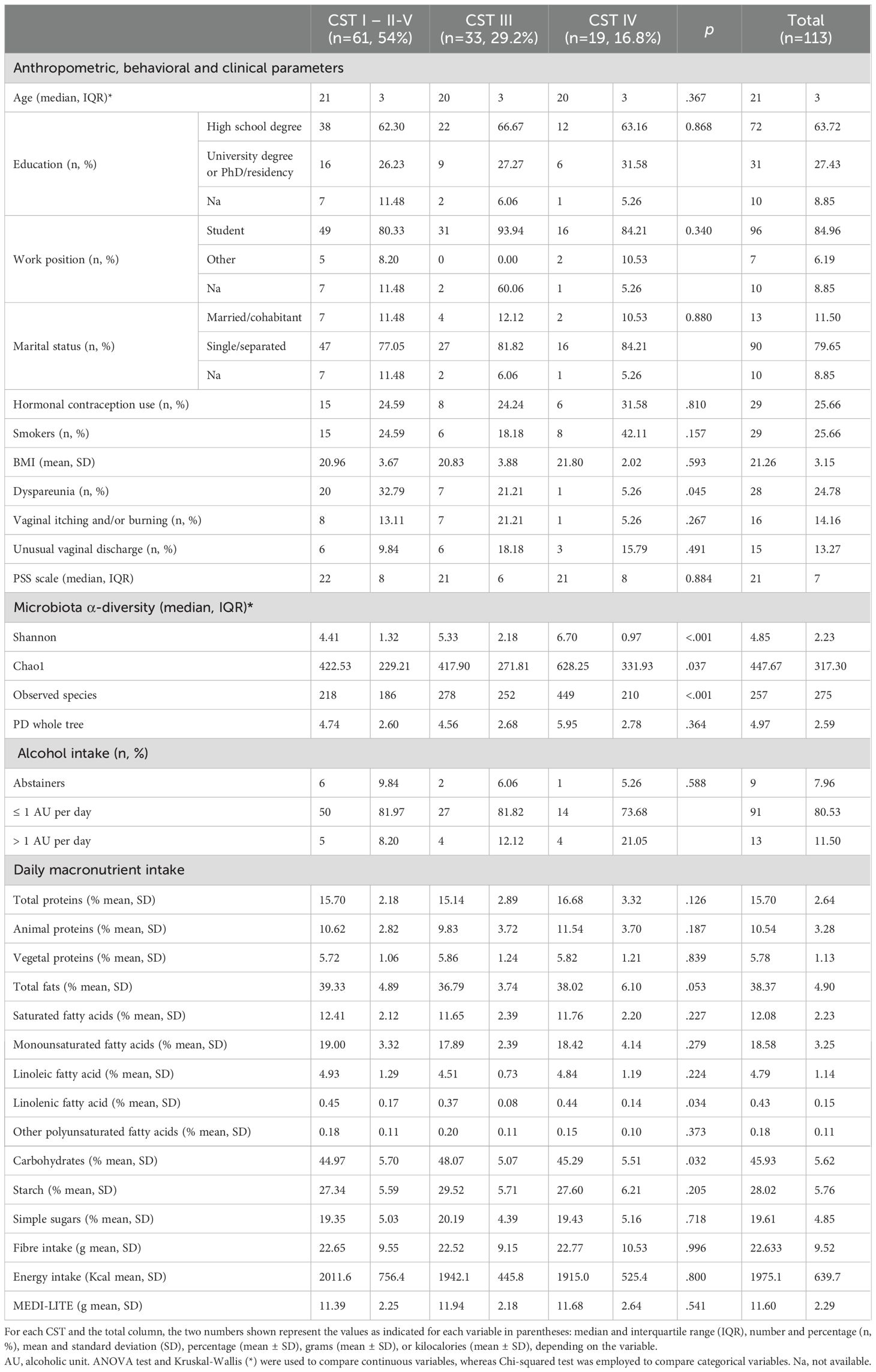
The vaginal microbiota composition was highly different among CST: CST IV had a significantly higher biodiversity compared to CST I, II, V (p=0.003) and, partially, to CST III (p=0.069), whereas no significant differences were evident between CST I, II, V and CST III. At the same time, the microbial profiles were significantly different among all the CST (p=0.001 and p=0.004 for the unweighted and the weighted UniFrac distances, respectively) (Supplementary Figure S2). This was reflected in the average microbial composition of the CSTs: CST I, II, V had a high prevalence of L. crispatus, which, nevertheless, was lower in CST III and almost absent in CST IV (70.3% vs 10.5% vs 0.2% in CST I, II, V, CST III, and CST IV, respectively). Then again, L. iners was a hallmark of CST III (abundance of 0.8%, 71.2% and 6.8% in CST I,II,V, CST III and CST IV, respectively), whereas the high presence of Gardnerella vaginalis, Prevotella spp., Streptococcus spp., Atopobium vaginae, and Sneathia sanguinegens was correlated to CST IV (accounting in total for 8.0%, 8.1%, and 60.5% in CST I,II,V, CST III and CST IV, respectively) (Figure 1).
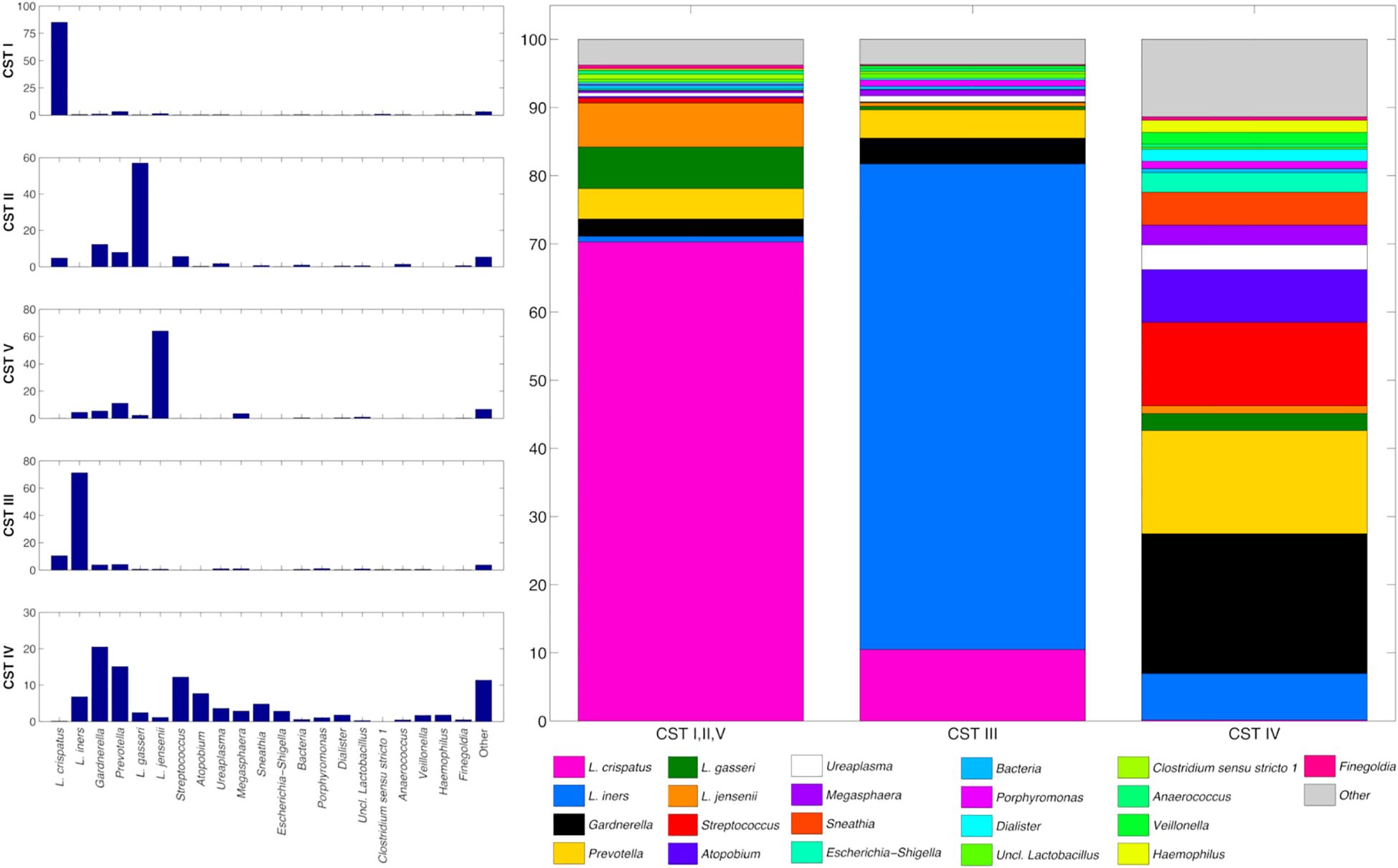
Figure 1. (left) Histograms of the average relative abundance (%) of bacteria over CST. (right) Stacked bars of the average relative abundance (%), grouping together samples classified in CSTs I, II and V. Only the main taxa (average abundance >0.4% over all the samples) are represented here, with Lactobacillus genus further subclassified to species level. Less abundant taxa are grouped in the “Other” category.
Descriptive characteristics of the study population stratified by CST are displayed in Table 1.
3.2 Dietary habits
Spearman’s correlation analysis revealed distinct patterns in how different food groups contributed to macronutrient intake. For instance, for total protein consumption, significant contributors included dairy products (particularly cheese), bakery products, animal fats, and fish. Animal proteins were predominantly derived from red and processed meat, white meat, fish, and animal fats. Regarding total fat intake, the primary sources were cheese and sweets and snacks. The main sources of MUFAs were olive oils and cheese, while vegetable fats and dried fruits were the primary sources of linoleic and linolenic fatty acids, respectively. Starch intake was most closely correlated with bakery products, and total fiber intake with fruits and legumes. Total energy intake was primarily correlated with the consumption of sweets and snacks, cheese, and bakery products. Alcohol consumption showed a straightforward relationship exclusively with alcoholic beverages.
3.3 Association of macronutrient intake with CST
Figure 2 shows the results of the multivariate multinomial regression model of CST in relation to the ALR(s) transformations of macronutrients including age, marital status, BMI, and hormonal contraceptive use, total energy, fiber, and alcohol intake. We first included in the model the eight ALR(s) calculated by dividing each macronutrient by starch and then, to obtain estimates for the latter, in the second run included the ALR(s) with animal protein as the denominator. For example, the β-coefficient of “Linolenic acid vs other macronutrient” represents the negative change in CST III (β-coefficient -5.320, p-value 0.007) when linolenic FA increases and ST decreases, holding all other terms in the model constant.
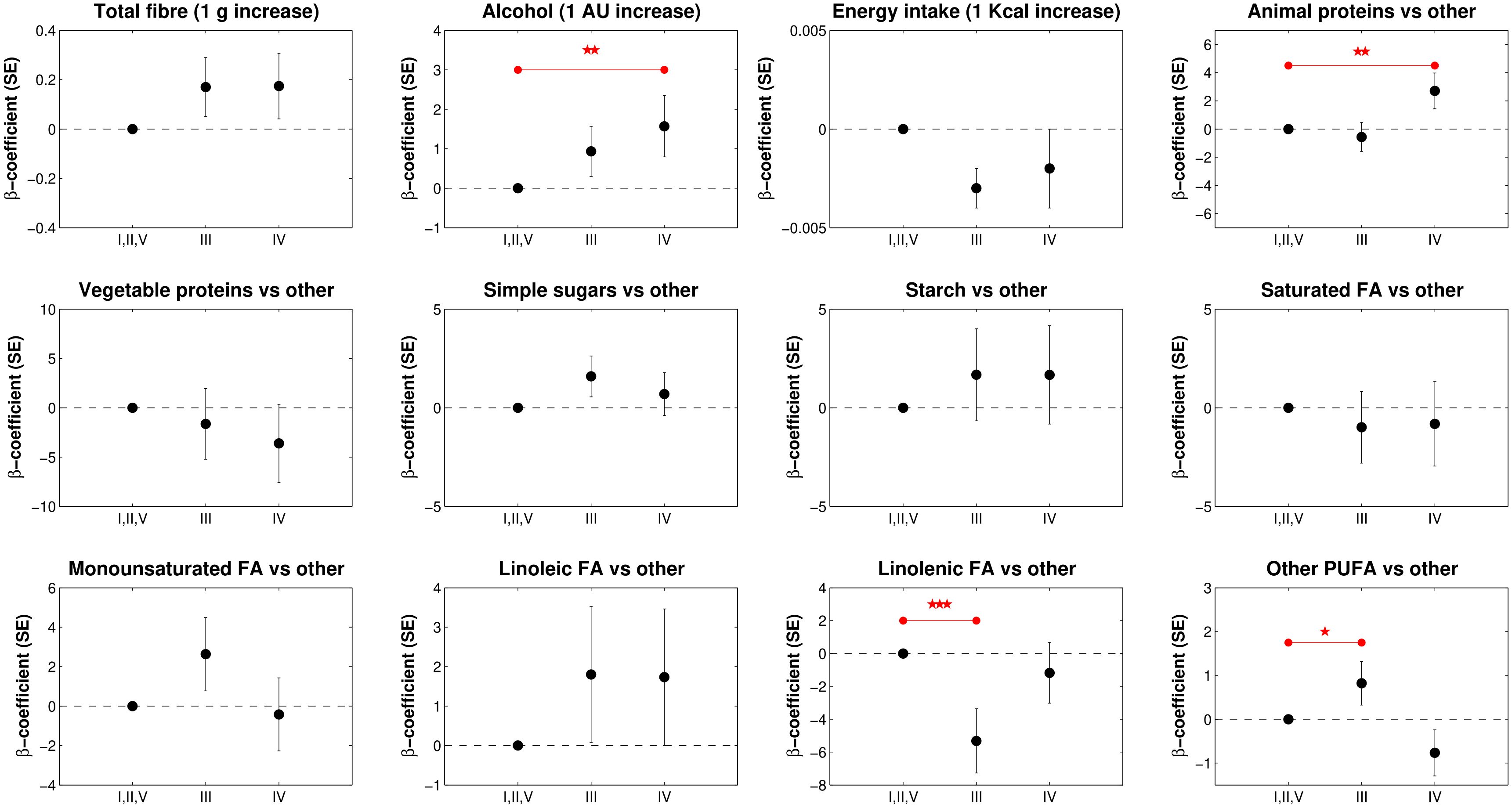
Figure 2. Multinomial logistic regression coefficient (b-coefficient) and standard errors (SE) of CST in relation to the macronutrient balances, total energy, fiber and alcohol intake (n=113). The model also included terms for age, BMI, marital status, and hormonal contraception use. Asterisks (*) indicate statistical significance of the model: * p<0.1; ** p<0.05, *** p<0.01.
In contrast, the β-coefficient of the balance “Animal proteins vs other macronutrient” expresses the positive change in CST IV (β-coefficient 2.702, p-value 0.033) when AP increases and ST decreases, keeping all other terms constant. We also found that an increase in alcohol units was statistically significantly positively associated with CST IV (β-coefficient 1.570, p-value 0.043). No other dietary components were statistically significantly associated with CSTs.
When analyzing MEDI-LITE scores across CST categories, we found no statistically significant associations (CST III β-coefficient 0.170, p-value 0.140; CST IV β-coefficient 0.115, p-value 0.395), suggesting that overall adherence to the Mediterranean diet was not associated with vaginal microbiota composition in our population (see also Supplementary Table S1).
With regard to psychological distress, further adjustment for this factor did not substantially alter the strength of the association between macronutrient intake and CTS (Supplementary Table S2).
Descriptive characteristics of the dietary macronutrients intake of the study population stratified by CST is presented in Table 1.
3.4 Correlations between macronutrients and vaginal ecosystem
A correlation network and three pairwise heatmaps showing the Spearman correlation among bacteria, metabolites, and macronutrients were constructed in order to gain a better understanding of their mutual relationships (Figure 3; Supplementary Figures S3–S5). Bacteria were grouped according to the indicator species for the three CST groups here considered (i.e.: CST I, II, V vs. CST III vs. CST IV), with metabolites and nutrients clustered according to their significant positive correlation to the bacteria within each group.
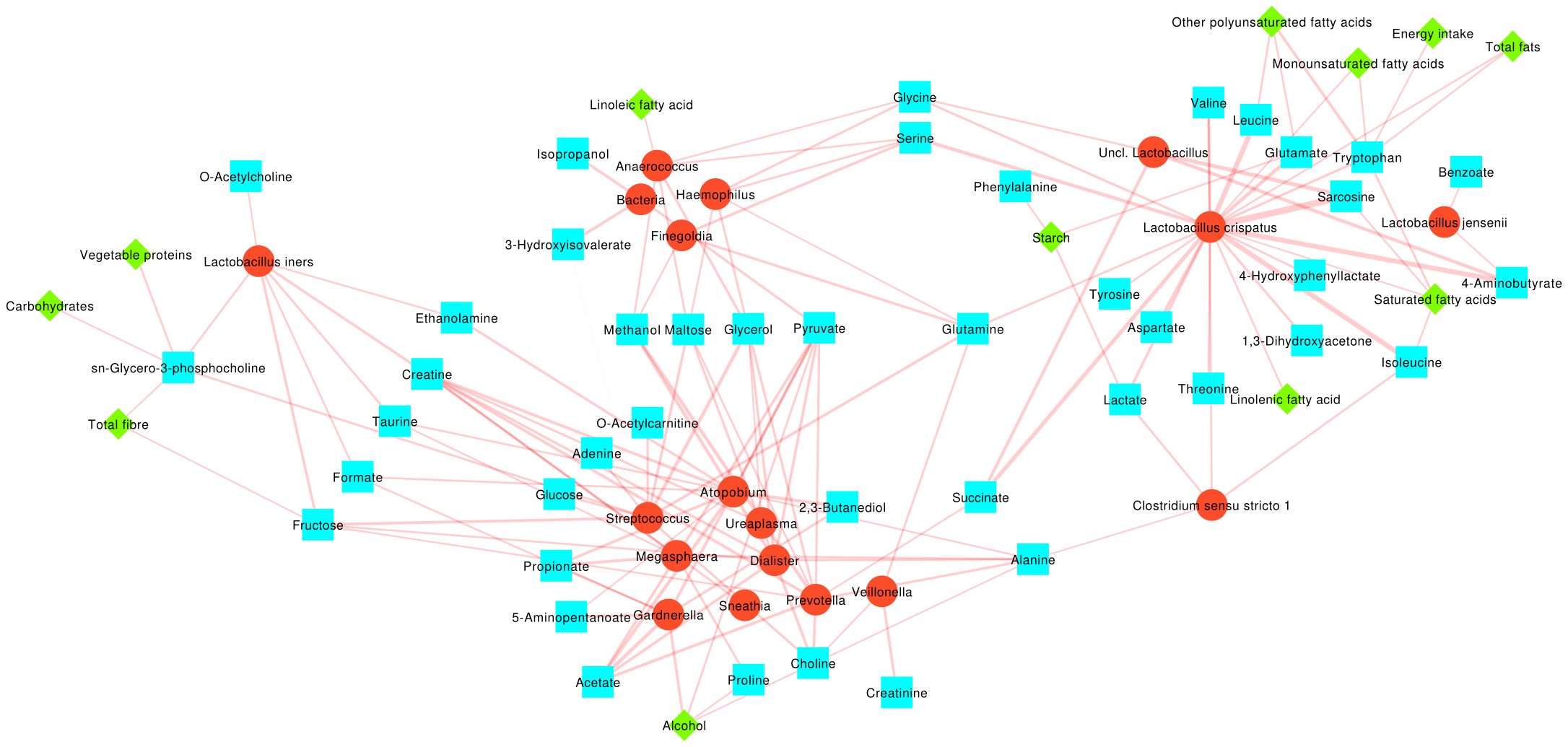
Figure 3. Network representing the Spearman correlation values among bacterial, metabolite and macronutrient abundances. Bacterial taxa are depicted as red circles, metabolites as cyan squares and macronutrients as green diamonds. Edges thickness is proportional to the correlation coefficient. Only significant (p<0.05) correlations are represented. Bacteria, metabolites and macronutrients are clustered according to the indicator species analysis over the three CST groups considered.
A first group (cluster 1) was composed of L. crispatus (indicator species for CST I, II, V), L. jensenii, and other Lactobacillus. Several metabolites were positively associated to this group, including amino acids (i.e.: Glutamate, Leucine, Isoleucine, Valine, Tryptophan, Tyrosine, Aspartate and Threonine), their byproducts (4-Hydroxyphenyllactate, Sarcosine), 4-Aminobutyrate, Lactate, and 1,3-Dihydroxyacetone; among macronutrients, Total fats, Monounsaturated fatty acids, Other polyunsaturated fatty acids, Energy intake, Starch, and Linolenic acid all were directly or indirectly related to these group.
A second group of bacteria (cluster 2) comprised the indicator species for CST IV (e.g.: Gardnerella, Atopobium, Megasphaera, Sneathia, etc.). We observed several correlations to some carboxylic acids (i.e.: Creatinine, Acetate, Propionate), to amino acids (Proline, Alanine), to 2,3-Butanediol, Choline, 5-Aminopentanoate, Glucose, Adenine and O-Acetylcarnitine; interestingly, Alcohol consumption was the only macronutrient correlated to this group.
A third cluster of bacteria (cluster 3) involved those showing no differences among the CSTs (i.e.: Finegoldia, Anaerococcus, Haemophilus, Uncl. Bacteria) which were related to 3-hydroxyisovalerate, isopropanol, and Linoleic fatty acid.
Finally, L. iners formed itself a group (cluster 4), together with O-Acetylcholine and its precursor sn-Glycero-3-posphocholine, Vegetable proteins, Carbohydrates and Total fiber.
At the same time, some metabolites were common among two or more clusters: Ethanolamine, carboxylic acids such as Creatine and Formate, Taurine, and Fructose were shared between clusters 2 and 4; Methanol, carbohydrates such as Maltose and Glycerol and Pyruvate between clusters 2 and 3; amino acids such as Glycine and Serine between clusters 1 and 3; Succinate between 1 and 3; finally, Glutamine was correlated to bacteria in clusters 1, 2 and 3.
4 Discussion
The aim of the study was to assess in detail the relationship between dietary habits, in terms of macronutrient intake, and the taxonomic and metabolomic composition of the vaginal environment in a cohort of non-pregnant young women.
For this purpose, a total of 113 reproductive-aged women were asked to complete a validated food frequency questionnaire, while their vaginal ecosystem was analyzed both in terms of bacterial profiles (16S rRNA sequencing) and metabolic signatures (1H-NMR spectroscopy).
At first, we confirmed the strict association between the composition of vaginal bacterial communities (i.e., CSTs) and the levels of metabolites detected in the vaginal fluids.
Among the beneficial metabolites related to CST I (i.e.: dominated by L. crispatus), 4-hydroxyphenyllactate, a byproduct of amino acid degradation, has already been shown to decrease ROS (reactive oxygen species) production in both mitochondria and neutrophils, acting as a natural antioxidant (Beloborodova et al., 2012). Moreover, Lactobacillus species in a healthy vaginal environment are known producers of branched-chain amino acids, such as leucine, isoleucine, serine, and tryptophan (Marangoni et al., 2021). According to research, Lactobacillus species, including L. crispatus, can metabolize tryptophan into a variety of bioactive compounds, including indole derivatives. These metabolites are known to play roles in immune modulation and inflammation reduction: for instance, the metabolite norharman, produced from tryptophan by Lactobacillus, has been found to inhibit inflammatory responses both in vitro and in vivo (Zhou et al., 2023).
It is well known that L. crispatus produces high quantities of lactate, responsible for the protective acidic pH preventing pathogen proliferation in the vaginal environment, from glucose conversion through lactic fermentation.
In our observation, L. crispatus was negatively correlated to simple sugars (i.e.: glucose, fructose) that, then again, were positively correlated to some taxa typical of CST IV (e.g.: Streptococcus, Dialister, Prevotella): these correlations suggest a possible nutrient competition with Lactobacillus species resulting in a stimulated CST IV elements’ growth favored by the higher pH following the reduced lactic fermentation. Additionally, it was previously reported that the vaginal fluids collected from women with bacterial vaginosis (BV) had a higher concentration of glucose (Vitali et al., 2015). Therefore, the absence of lactobacilli, especially those that are more effective at fermenting glucose, is linked to a higher availability of glucose in the vaginal environment, which can then encourage the growth of undesirable microorganisms, including bacteria linked to a dysbiotic environment (i.e., CST IV) (Navarro et al., 2023).
Interesting findings emerged as well when examining the associations between dietary data and the vaginal microbiota. Regardless of any confounders, we discovered that a higher animal protein balance (mostly derived from red and processed meat) in relation to the other macronutrients was substantially positively correlated with CST IV. To our knowledge, there are no prior studies reporting a positive independent association between increased animal protein intake and vaginal microbiota dysbiosis. However, we found some similarities between our study and previous research. One study showed that higher consumption of animal-sourced proteins was positively correlated with a higher Nugent score (indicating dysbiosis), in a cohort of Caucasian pregnant women, although no control was performed for confounders (Dall’Asta et al., 2021). Another study indicated that, even after adjusting for confounders, BV was linked to unhealthy dietary patterns, including also red and visceral meat (Noormohammadi et al., 2022). On the other hand, in two other studies of reproductive-age women, protein intake was not associated with molecular BV in multivariate analysis (Neggers et al., 2007; Shivakoti et al., 2020).
Although further research is needed to determine the underlying causes of the detrimental effects of a diet high in animal-sourced proteins, we can hypothesize an effect similar to that described for the gut microbiota. For instance, in the gut, a higher intake of plant-sourced protein is associated with greater abundance of “health-related” microorganisms (e.g., Bifidobacterium, Roseburia, Lactobacillus), as opposed to Bacteroides and Clostridia, which are mainly found primarily in the case of animal-sourced proteins (Prokopidis et al., 2020). From a biological point of view, we can speculate that a high consumption of animal proteins, particularly derived from red and processed meat, may increase the levels of inflammatory markers, which, in turn, may alter the balance of the microbiota environment (Bolte et al., 2021). Furthermore, animal protein fermentations may produce potentially toxic metabolites like ammonia and sulfides, that might favor the growth of harmful bacteria through increasing the vaginal pH (Windey et al., 2012).
We also pointed out that alcohol consumption was significantly positively associated with a BV condition (i.e., CST IV) and, in particular, was particularly positively correlated with the levels of Gardnerella spp. and Ureaplasma spp. Despite the limited number of studies in this area, it was observed that alcohol consumption may have a similar effect on the vaginal microbiota as tobacco, thereby increasing the risk of BV (Froehle et al., 2021; Turpin et al., 2021; Morsli et al., 2024). Alcohol could favor infections, due to its association with an alteration of the immune system function in an animal model (Loganantharaj et al., 2014). In a similar way, researchers have underscored a correlation between alcohol use and alterations in the oral microbiota, characterized by a diminished Lactobacillales abundance and an increased presence of Neisseria, Streptococcus, and Prevotella (Rajasekaran et al., 2024). The mechanisms of the negative effects of alcohol on the vaginal microbiota are still unknown; however, we can hypothesize both a direct effect on bacteria and an indirect effect on tissues, leukocyte function, and cytokine production, creating an environment conducive to microbial proliferation. Moreover, alcohol was found positively related to proline, an amino acid involved in collagen synthesis, which was also related to Megasphaera abundance. This is similar to what was reported in a recent study by Li and colleagues showing that, in the vaginal microecology, the high expression of the enzyme prolyl aminopeptidase (specifically releasing the terminal proline residue from a peptide) in BV-associated bacteria could be substantially correlated to the occurrence and the development of vaginal inflammatory diseases (Li et al., 2024).
We also noticed a beneficial effect of α-linolenic acid, one of the essential omega-3 polyunsaturated fatty acids mainly derived from plant sources such as nuts and seeds. Indeed, an increase of linolenic acid balance, relative to the other nutritional components, was inversely associated with CST III, dominated by the transitional species L. iners. The observed positive correlation between linolenic acid and L. crispatus, the hallmark of vaginal eubiosis, went in the same direction.
Although there are no data on the specific effects of omega-3 on the vaginal microbiota composition, numerous studies have shown that omega-3 fatty acids affect the gut microbial composition in different ways, including (i) a direct modulation of the gut microbial communities, (ii) an alteration of the inflammatory mediators, (iii) a regulation of the levels of SCFAs. By the means of these mechanisms, it has been demonstrated that the dietary intake of omega-3 fatty acids leads to the reduction of Enterobacterales, supporting the growth of Bifidobacteria, with an amelioration of intestinal inflammation and a higher protection from intestinal infections (Kumar et al., 2022). Interestingly, omega-3 fatty acid supplementation has already been observed to have beneficial effects on tryptophan neuroprotective metabolites during endurance training (Tomczyk et al., 2024) and, in an animal experimental model, Wang and colleagues observed that plant-derived α-linolenic acid visibly changed the initial proportion of vaginal microbiota bacteria by increasing Lactobacillus, Faecalibacterium, and Parabacteroides, as well as decreasing Streptococcus (Wang et al., 2020).
Moreover, lactic-acid bacteria (LAB) as lactobacilli, are able to transform α-linolenic acid into conjugated linoleic acids, which later get hydrogenated to saturated fatty acids such as stearic acid, thereby reducing the composition of PUFA (Kumar et al., 2022).
We also observed that total carbohydrates, vegetable proteins, total fiber and starch were negatively correlated with Gardnerella spp., possibly implying that their reduced consumption favors pathogens proliferation. We can therefore speculate that diets including a high intake of total carbohydrates and starch may lead to high levels of vaginal glycogen, which, in turn, might create a favorable environment for a lactobacilli-dominated flora with low levels of BV-associated microbes, as Gardnerella spp., as also reported by previous works (Miller et al., 2016; Song et al., 2020). Interestingly, starch was positively correlated to lactate, the main product of lactic fermentation, and to phenylalanine and glutamate, both within the neurotransmitter class.
Finally, even though it has been shown that the adherence to the Mediterranean diet can cause multiple changes in the gut microbiota (Meslier et al., 2020; Dahl et al., 2020), we did not observe a significant effect of this factor on the vaginal environment. Previous studies have shown that higher adherence to the Mediterranean diet (MEDI-LITE score ≥13) is associated with more pronounced beneficial effects, including reduced inflammation markers (Sureda et al., 2018), improved metabolic parameters (Galilea-Zabalza et al., 2018), and more substantial changes in gut microbiota composition (Meslier et al., 2020). Considering that in our population the mean MEDI-LITE score was 11 (range: 6-16) out of a possible maximum of 18, we can speculate that a higher adherence to the Mediterranean diet may be necessary to observe significant effects. Therefore, the moderate adherence observed in our population may explain the limited impact on the vaginal microbiota, suggesting that there may be a threshold effect where a more stringent dietary adherence is required to induce significant changes in vaginal microbial communities.
Overall, our data shed new light on the importance of the interaction between the gastrointestinal-tract and the vagina, the so-called ‘vagina-gut axis’, described for the first time by Ravel and Brotman in 2016 (Ravel and Brotman, 2016). A healthy diet could preserve the vaginal homeostasis by regulating the trafficking of bacterial species across the vagina and gut (bacterial translocation), in turn modulating the level and type of metabolites produced by the microbiota, acting as indirect players of the vagina-gut axis (Takada et al., 2023). In conclusion, we highlighted that specific dietary habits (i.e., reduced consumption of alcohol and animal proteins, higher intake of linolenic acid) can have a beneficial impact on the vaginal environment, through the maintenance of a microbiota mostly dominated by ‘protective’ Lactobacillus species such as L. crispatus, L. gasseri, and L. jensenii.
The main strengths of the present work include (i) the use of a standardized and validated tool to collect dietary data, (ii) a deep analysis of the vaginal ecosystem (i.e., classification of the vaginal microbiota composition in CST, quantification of vaginal metabolites) (iii) the extensive socio-demographic and behavioral data collection from the participants. Finally, to the best of our knowledge, this is the first study to use the CoDA (compositional data analysis) statistical technique to investigate the effect of macronutrient intake on the vaginal microbiota. This statistical method provides fully adjusted isocaloric estimates of the effects of specific nutrient balances on the vaginal microbiota, taking into account the compositional nature of the dietary data itself and being able to capture the interdependent dynamics of dietary components.
We are fully aware of some limitations of this study. The main one is the cross-sectional design, which limits causal inference for the association between diet and CST, and reverse causation cannot be excluded, particularly because the study does not allow us to determine whether dietary behaviors preceded or followed the changes in vaginal bacterial composition. In addition, the voluntary participation of young and highly educated women in the study suffers from an inherent selection bias, which limits the generalizability of our results to older and less educated adult women, because the sample was self-selected and not fully representative of the Italian population. However, this selection bias is likely to affect our results toward the null, thus underestimating the observed associations. Finally, although we controlled for known potential confounders, we cannot rule out the possibility of residual confounding due to unmeasured factors (e.g. physical activity, sexual habits). However, we speculate that our participants were relatively healthy and had a good lifestyle, supported by the fact that they were volunteers, young medical students, mostly non-smokers, and light drinkers (Fry et al., 2017). In terms of sexual behavior, as in previous studies (Duberstein Lindberg and Singh, 2008; Exavery et al., 2015; Jackson et al., 2019), we assumed that single women were more likely to have a higher number of partners than married/cohabiting women. We accounted for this by controlling for marital status, used as a proxy for sexual behavior.
Thus, more research is required to better understand the mechanisms underlying the impact of dietary macronutrients on the vaginal ecosystem as well as the role and activity of saturated and unsaturated fatty acids on the vaginal environment, and how the vaginal inhabitants may contribute to their metabolism and transformation. In this context, even though it is crucial to assess long-term dietary patterns when investigating the potential effects of dietary habits on the microbiome, future studies could benefit from combining the FFQ with either 24h recall or 3-day food diary to provide additional insights into the dietary habits of the enrolled cohort.
In addition, future in vitro experimental studies will be useful to demonstrate a beneficial or detrimental effect of specific dietary metabolites on the inhabitants of the vaginal ecosystem.
Data availability statement
The datasets presented in this study can be found in online repositories or in the Supplementary Material. The names of the repository/repositories and accession number(s) can be found in the article (see section 2.7).
Ethics statement
The studies involving humans were approved by Bioethics Committee of the University of Bologna (protocol number 0122421). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MED: Formal Analysis, Investigation, Writing – original draft. FP: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. TC: Data curation, Investigation, Methodology, Writing – original draft. CCe: Data curation, Investigation, Methodology, Writing – original draft. CCo: Data curation, Investigation, Methodology, Writing – original draft. SC: Data curation, Investigation, Methodology, Writing – original draft. MD: Data curation, Investigation, Methodology, Writing – original draft. FD: Data curation, Investigation, Methodology, Writing – original draft. LL: Data curation, Investigation, Methodology, Writing – original draft. FC: Data curation, Writing – original draft. SM: Data curation, Methodology, Writing – original draft. CF: Conceptualization, Project administration, Supervision, Validation, Writing – original draft. PC: Data curation, Writing – original draft. AM: Conceptualization, Project administration, Resources, Supervision, Writing – original draft. MS: Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Marielle Ezekielle Djusse was supported by the European Union-funded grant - NextGenerationEU from the National Recovery and Resilience Plan (NRRP) Mission 4, Component 1, Investment 3.4 (DM 118/2023) - Digital and Environmental Transitions - CUP: J33C23002390002. The research group acknowledges the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Award Number: Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP: B83C22002930006, Project title “National Biodiversity Future Center - NBFC”, for supporting Camilla Ceccarani’s research grant. Silvia Conti was supported by the European Union-funded grant - NextGenerationEU – Mission 4, Component 2, Investiment 1.1, 2023-PRIN-VS_001 - PRIN 2022 - CUP F53D23007190006; and by the INVAT Fondo Ordinario dell’Ente 2022, National Research Council, CUP B53C22010140001.
Acknowledgments
We would like to thank the Clinical Laboratory Scientists of the of the Microbiology Unit of IRCCS Policlinico Sant’Orsola in Bologna for the assistance during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1582283/full#supplementary-material
References
Beloborodova, N., Bairamov, I., Olenin, A., Shubina, V., Teplova, V., and Fedotcheva, N. (2012). Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. BioMed. Sci. 19, 89. doi: 10.1186/1423-0127-19-89
Bingham, S. A., Welch, A. A., McTaggart, A., Mulligan, A. A., Runswick, S. A., Luben, R., et al. (2001). Nutritional methods in the european prospective investigation of cancer in norfolk. Public Health Nutr. 4, 847–858. doi: 10.1079/phn2000102
Bolte, L. A., Vich Vila, A., Imhann, F., Collij, V., Gacesa, R., Peters, V., et al. (2021). Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 70, 1287–1298. doi: 10.1136/gutjnl-2020-322670
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Ceccarani, C., Foschi, C., Parolin, C., D’Antuono, A., Gaspari, V., Consolandi, C., et al. (2019). Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 9, 14095. doi: 10.1038/s41598-019-50410-x
Coenders, G. and Pawlowsky-Glahn, V. (2020). On interpretations of tests and effect sizes in regression models with a compositional predictor. SORT 44, 201–220. doi: 10.2436/20.8080.02.100
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. doi: 10.2307/2136404
Corrêa Leite, M. L. (2019). Compositional data analysis as an alternative paradigm for nutritional studies. Clin. Nutr. ESPEN. 33, 207–212. doi: 10.1016/j.clnesp.2019.05.011
Corrêa Leite, M. L. (2021). Log-ratio transformations for dietary compositions: numerical and conceptual questions. J. Nutr. Sci. 10, e97. doi: 10.1017/jns.2021.93
Dahl, W. J., Rivero Mendoza, D., and Lambert, J. M. (2020). Diet, nutrients and the microbiome. Prog. Mol. Biol. Transl. Sci. 171, 237–263. doi: 10.1016/bs.pmbts.2020.04.006
Dall’Asta, M., Laghi, L., Morselli, S., Re, M. C., Zagonari, S., Patuelli, G., et al. (2021). Pre-pregnancy diet and vaginal environment in caucasian pregnant women: an exploratory study. Front. Mol. Biosci. 8. doi: 10.3389/fmolb.2021.702370
Dieterle, F., Ross, A., Schlotterbeck, G., and Senn, H. (2006). Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 78, 4281–4290. doi: 10.1021/ac051632c
Duberstein Lindberg, L. and Singh, S. (2008). Sexual behavior of single adult American women. Perspect. Sex. Reprod. Health 40, 27–33. doi: 10.1363/4002708
Dufrene, M. and Legendre, P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67, 345–366. doi: 10.2307/2963459
Edgar, R. C. UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv. doi: 10.1101/081257v1
Exavery, A., Kanté, A. M., Tani, K., Hingora, A., and Phillips, J. F. (2015). Sociodemographic drivers of multiple sexual partnerships among women in three rural districts of Tanzania. HIV AIDS (Auckl). 7, 105–113. doi: 10.2147/HIV.S76694
Foschi, C., Laghi, L., D’Antuono, A., Gaspari, V., Zhu, C., Dellarosa, N., et al. (2018). Urine metabolome in women with Chlamydia trachomatis infection. PloS One 13, e0194827. doi: 10.1371/journal.pone.0194827
France, M. T., Ma, B., Gajer, P., Brown, S., Humphrys, M. S., Holm, J. B., et al. (2020). VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8, 166. doi: 10.1186/s40168-020-00934-6
Froehle, L., Ghanem, K. G., Page, K., Hutton, H. E., Chander, G., Hamill, M. M., et al. (2021). Bacterial vaginosis and alcohol consumption: A cross-sectional retrospective study in baltimore, maryland. Sex. Transm. Dis. 48, 986–990. doi: 10.1097/OLQ.0000000000001495
Fry, A., Littlejohns, T. J., Sudlow, C., Doherty, N., Adamska, L., Sprosen, T., et al. (2017). Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034. doi: 10.1093/aje/kwx246
Galilea-Zabalza, I., Buil-Cosiales, P., Salas-Salvadó, J., Toledo, E., Ortega-Azorín, C., Díez-Espino, J., et al. (2018). Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PloS One 13, e0198974. doi: 10.1371/journal.pone.0198974
Gonçalves, I. D. S. A., Filgueiras, M. S., Moreira, T. R., Thomé, M. S., Paiva, G. L. D., Almeida, G. P., et al. (2024). Interrelation of stress, eating behavior, and body adiposity in women with obesity: do emotions matter? Nutrients 16, 4133. doi: 10.3390/nu16234133
Govender, P. and Ghai, M. (2024). Population-specific differences in the human microbiome: Factors defining the diversity. Gene 933, 148923. doi: 10.1016/j.gene.2024.148923
Greenbaum, S., Greenbaum, G., Moran-Gilad, J., and Weintraub, A. Y. (2019). Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 220, 324–335. doi: 10.1016/j.ajog.2018.11.1089
Hron, K., Filzmoser, P., and Thompson, K. (2012). Linear regression with compositional explanatory variables. J. Appl. Stat 39, 1115–1128. doi: 10.1080/02664763.2011.644268
Jackson, S. E., Yang, L., Veronese, N., Koyanagi, A., López Sánchez, G. F., Grabovac, I., et al. (2019). Sociodemographic and behavioural correlates of lifetime number of sexual partners: findings from the English Longitudinal Study of Ageing. BMJ Sex. Reprod. Health 45, 138–146. doi: 10.1136/bmjsrh-2018-200230
Kumar, M., Pal, N., Sharma, P., Kumawat, M., Sarma, D. K., Nabi, B., et al. (2022). Omega-3 fatty acids and their interaction with the gut microbiome in the prevention and amelioration of type-2 diabetes. Nutrients 14, 1723. doi: 10.3390/nu14091723
Laghi, L., Zagonari, S., Patuelli, G., Zhu, C., Foschi, C., Morselli, S., et al. (2021). Vaginal metabolic profiles during pregnancy: Changes between first and second trimester. PloS One 16, e0249925. doi: 10.1371/journal.pone.0249925
Leite, M. L. C. (2020). Orthonormal balances as a means of characterizing dietary exposure. Nutr. Res. 81, 90–96. doi: 10.1016/j.nutres.2020.06.016
Leite, M. L. C. and Prinelli, F. (2017). A compositional data perspective on studying the associations between macronutrient balances and diseases. Eur. J. Clin. Nutr. 71, 1365–1369. doi: 10.1038/ejcn.2017.126
Li, J., Jiang, L., Wang, C., Meng, J., Wang, H., and Jin, H. (2024). Investigation of the relationship between the changes in vaginal microecological enzymes and human papillomavirus (HPV) infection. Med. (Baltimore). 103, e37068. doi: 10.1097/MD.0000000000037068
Liland, K. H., Almøy, T., and Mevik, B. H. (2010). Optimal choice of baseline correction for multivariate calibration of spectra. Appl. Spectrosc. 64, 1007–1016. doi: 10.1366/000370210792434350
Loganantharaj, N., Nichols, W. A., Bagby, G. J., Volaufova, J., Dufour, J., Martin, D. H., et al. (2014). The effects of chronic binge alcohol on the genital microenvironment of simian immunodeficiency virus-infected female rhesus macaques. AIDS Res. Hum. Retroviruses 30, 783–791. doi: 10.1089/AID.2014.0065
Lozupone, C., Lladser, M. E., Knights, D., Stombaugh, J., and Knight, R. (2011). UniFrac: an effective distance metric for microbial community comparison. ISME. J. 5, 169–172. doi: 10.1038/ismej.2010.133
Marangoni, A., Laghi, L., Zagonari, S., Patuelli, G., Zhu, C., Foschi, C., et al. (2021). New insights into vaginal environment during pregnancy. Front. Mol. Biosci. 8. doi: 10.3389/fmolb.2021.656844
Masella, A. P., Bartram, A. K., Truszkowski, J. M., Brown, D. G., and Neufeld, J. D. (2012). PANDAseq: paired-end assembler for illumina sequences. BMC Bioinf. 13, 31. doi: 10.1186/1471-2105-13-31
Meslier, V., Laiola, M., Roager, H. M., De Filippis, F., Roume, H., Quinquis, B., et al. (2020). Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 69, 1258–1268. doi: 10.1136/gutjnl-2019-320438
Mikhanoshina, N. V. and Priputnevich, T. V. (2024). New aspects in the study of lactobacillus iners. Bull. Exp. Biol. Med. 177, 84–87. doi: 10.1007/s10517-024-06136-6
Miller, E. A., Beasley, D. E., Dunn, R. R., and Archie, E. A. (2016). Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01936
Morselli, S., Ceccarani, C., Djusse, M. E., Laghi, L., Camboni, T., Consolandi, C., et al. (2024). Anti-chlamydial activity of vaginal fluids: new evidence from an in vitro model. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1403782
Morsli, M., Gimenez, E., Magnan, C., Salipante, F., Huberlant, S., Letouzey, V., et al. (2024). The association between lifestyle factors and the composition of the vaginal microbiota: a review. Eur. J. Clin. Microbiol. Infect. Dis. 43, 1869–1881. doi: 10.1007/s10096-024-04915-7
Navarro, S., Abla, H., Delgado, B., Colmer-Hamood, J. A., Ventolini, G., and Hamood, A. N. (2023). Glycogen availability and pH variation in a medium simulating vaginal fluid influence the growth of vaginal Lactobacillus species and Gardnerella vaginalis. BMC Microbiol. 23, 186. doi: 10.1186/s12866-023-02916-8
Neggers, Y. H., Nansel, T. R., Andrews, W. W., Schwebke, J. R., Yu, K. F., Goldenberg, R. L., et al. (2007). Dietary intake of selected nutrients affects bacterial vaginosis in women. J. Nutr. 137, 2128–2133. doi: 10.1093/jn/137.9.2128
Noormohammadi, M., Eslamian, G., Kazemi, S. N., and Rashidkhani, B. (2022). Association between dietary patterns and bacterial vaginosis: a case-control study. Sci. Rep. 12, 12199. doi: 10.1038/s41598-022-16505-8
Noormohammadi, M., Eslamian, G., Kazemi, S. N., Rashidkhani, B., and Jafari Yeganeh, S. (2025). Relationship between dietary inflammatory index, plant-based dietary index, and bacterial vaginosis: A case-control study. Int. J. Gynaecol. Obstet. 168, 551–558. doi: 10.1002/ijgo.15886
Novak, J., Ravel, J., Ma, B., Ferreira, C. S. T., Tristão, A. D. R., Silva, M. G., et al. (2022). Characteristics associated with Lactobacillus iners-dominated vaginal microbiota. Sex. Transm. Infect. 98, 353–359. doi: 10.1136/sextrans-2020-054824
Occhipinti, S., Incognito, G. G., and Palumbo, M. (2025). The influence of the vaginal ecosystem on vaginitis, bacterial vaginosis, and sexually transmitted diseases: an epidemiological study and literature review. Arch Gynecol Obstet. 311(2):347–353. doi: 10.1007/s00404-024-07626-8
Pala, V., Sieri, S., Palli, D., Salvini, S., Berrino, F., Bellegotti, M., et al. (2003). Diet in the Italian EPIC cohorts: presentation of data and methodological issues. Tumori 89, 594–607. doi: 10.1177/030089160308900603
Pawlowsky-Glahn, V., Egozcue, J. J., and Tolosana-Delgado, R. (2015). Modelling and analysis of compositional data. doi: 10.1002/9781119003144
Plummer, E. L., Vodstrcil, L. A., and Bradshaw, C. S. (2024). Unravelling the vaginal microbiome, impact on health and disease. Curr. Opin. Obstet. Gynecol. 36, 338–344. doi: 10.1097/GCO.0000000000000976
Prokopidis, K., Cervo, M. M., Gandham, A., and Scott, D. (2020). Impact of protein intake in older adults with sarcopenia and obesity: A gut microbiota perspective. Nutrients 12, 2285. doi: 10.3390/nu12082285
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Raimondi, S., Candeliere, F., Amaretti, A., Foschi, C., Morselli, S., Gaspari, V., et al. (2021). Vaginal and Anal Microbiome during Chlamydia trachomatis Infections. Pathogens 10, 1347. doi: 10.3390/pathogens10101347
Rajasekaran, J. J., Krishnamurthy, H. K., Bosco, J., Jayaraman, V., Krishna, K., Wang, T., et al. (2024). Oral microbiome: A review of its impact on oral and systemic health. Microorganisms 12, 1797. doi: 10.3390/microorganisms12091797
Ravel, J. and Brotman, R. M. (2016). Translating the vaginal microbiome: gaps and challenges. Genome Med. 8, 35. doi: 10.1186/s13073-016-0291-2
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108 Suppl 1, 4680–4687. doi: 10.1073/pnas.1002611107
Severgnini, M., Morselli, S., Camboni, T., Ceccarani, C., Laghi, L., Zagonari, S., et al. (2022). A deep look at the vaginal environment during pregnancy and puerperium. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.838405
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303
Shivakoti, R., Tuddenham, S., Caulfield, L. E., Murphy, C., Robinson, C., Ravel, J., et al. (2020). Dietary macronutrient intake and molecular-bacterial vaginosis: Role of fiber. Clin. Nutr. 39, 3066–3071. doi: 10.1016/j.clnu.2020.01.011
Sofi, F., Dinu, M., Pagliai, G., Marcucci, R., and Casini, A. (2017). Validation of a literature-based adherence score to Mediterranean diet: the MEDI-LITE score. Int. J. Food Sci. Nutr. 68, 757–762. doi: 10.1080/09637486.2017.1287884
Song, S. D., Acharya, K. D., Zhu, J. E., Deveney, C. M., Walther-Antonio, M. R. S., Tetel, M. J., et al. (2020). Daily vaginal microbiota fluctuations associated with natural hormonal cycle, contraceptives, diet, and exercise. mSphere 5, e00593–e00520. doi: 10.1128/mSphere.00593-20
Srinivasan, S., Morgan, M. T., Fiedler, T. L., Djukovic, D., Hoffman, N. G., Raftery, D., et al. (2015). Metabolic signatures of bacterial vaginosis. mBio 6, e00204–e00215. doi: 10.1128/mBio.00204-15
Sureda, A., Bibiloni, M. D. M., Julibert, A., Bouzas, C., Argelich, E., Llompart, I., et al. (2018). Adherence to the mediterranean diet and inflammatory markers. Nutrients 10, 62. doi: 10.3390/nu10010062
Takada, K., Melnikov, V. G., Kobayashi, R., Komine-Aizawa, S., Tsuji, N. M., and Hayakawa, S. (2023). Female reproductive tract-organ axes. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1110001
Tamarelle, J., Thiébaut, A. C. M., de Barbeyrac, B., Bébéar, C., Bourret, A., Fauconnier, A., et al. (2024). Vaginal microbiota stability over 18 months in young student women in France. Eur. J. Clin. Microbiol. Infect. Dis. 43, 2277–2292. doi: 10.1007/s10096-024-04943-3
Thoma, M. E., Klebanoff, M. A., Rovner, A. J., Nansel, T. R., Neggers, Y., Andrews, W. W., et al. (2011). Bacterial vaginosis is associated with variation in dietary indices. J. Nutr. 141, 1698–1704. doi: 10.3945/jn.111.140541
Tomczyk, M., Bidzan-Wiącek, M., Kortas, J. A., Kochanowicz, M., Jost, Z., Fisk, H. L., et al. (2024). Omega-3 fatty acid supplementation affects tryptophan metabolism during a 12-week endurance training in amateur runners: a randomized controlled trial. Sci. Rep. 14, 4102. doi: 10.1038/s41598-024-54112-x
Tuddenham, S., Ghanem, K. G., Caulfield, L. E., Rovner, A. J., Robinson, C., Shivakoti, R., et al. (2019). Associations between dietary micronutrient intake and molecular-Bacterial Vaginosis. Reprod. Health 16, 151. doi: 10.1186/s12978-019-0814-6
Turpin, R., Tuddenham, S., He, X., Klebanoff, M. A., Ghanem, K. G., and Brotman, R. M. (2021). Bacterial vaginosis and behavioral factors associated with incident pelvic inflammatory disease in the longitudinal study of vaginal flora. J. Infect. Dis. 224, S137–S144. doi: 10.1093/infdis/jiab103
Ventrella, D., Laghi, L., Barone, F., Elmi, A., Romagnoli, N., and Bacci, M. L. (2016). Age-related 1H NMR characterization of cerebrospinal fluid in newborn and young healthy piglets. PloS One 11, e0157623. doi: 10.1371/journal.pone.0157623
Vitali, B., Cruciani, F., Picone, G., Parolin, C., Donders, G., and Laghi, L. (2015). Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 34, 2367–2376. doi: 10.1007/s10096-015-2490-y
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Wang, T., Sha, L., Li, Y., Zhu, L., Wang, Z., Li, K., et al. (2020). Dietary α-linolenic acid-rich flaxseed oil exerts beneficial effects on polycystic ovary syndrome through sex steroid hormones-microbiota-inflammation axis in rats. Front. Endocrinol. (Lausanne). 11. doi: 10.3389/fendo.2020.00284
Windey, K., De Preter, V., and Verbeke, K. (2012). Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 56, 184–196. doi: 10.1002/mnfr.201100542
World Health Organization (2000). International guide for monitoring alcohol consumption and related harm (Geneva: WHO).
Keywords: vaginal microbiota, diet, macronutrients, metabolome, women’s health
Citation: Djusse ME, Prinelli F, Camboni T, Ceccarani C, Consolandi C, Conti S, Dall’Asta M, Danesi F, Laghi L, Curatolo FM, Morselli S, Foschi C, Castellano P, Marangoni A and Severgnini M (2025) Dietary habits and vaginal environment: can a beneficial impact be expected? Front. Cell. Infect. Microbiol. 15:1582283. doi: 10.3389/fcimb.2025.1582283
Received: 25 February 2025; Accepted: 23 May 2025;
Published: 18 June 2025.
Edited by:
Leonardo Mancabelli, University of Parma, ItalyReviewed by:
Andrea Carolina Entrocassi, University of Buenos Aires, ArgentinaMarcelo Rodriguez Fermepin, University of Buenos Aires, Argentina
Copyright © 2025 Djusse, Prinelli, Camboni, Ceccarani, Consolandi, Conti, Dall’Asta, Danesi, Laghi, Curatolo, Morselli, Foschi, Castellano, Marangoni and Severgnini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Foschi, Y2xhdWRpby5mb3NjaGkyQHVuaWJvLml0
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Marielle Ezekielle Djusse
Marielle Ezekielle Djusse Federica Prinelli
Federica Prinelli Tania Camboni
Tania Camboni Camilla Ceccarani
Camilla Ceccarani Clarissa Consolandi
Clarissa Consolandi Silvia Conti
Silvia Conti Margherita Dall’Asta
Margherita Dall’Asta Francesca Danesi
Francesca Danesi Luca Laghi
Luca Laghi Francesco Matteo Curatolo6
Francesco Matteo Curatolo6 Sara Morselli
Sara Morselli Claudio Foschi
Claudio Foschi Paola Castellano
Paola Castellano Antonella Marangoni
Antonella Marangoni Marco Severgnini
Marco Severgnini