- 1Laboratory of Genetic Breeding, Reproduction and Precision Livestock Farming, School of Animal Science and Nutritional Engineering, Wuhan Polytechnic University, Wuhan, China
- 2Hubei Provincial Center of Technology Innovation for Domestic Animal Breeding, Wuhan Polytechnic University, Wuhan, China
- 3Institute of Animal Science and Veterinary Medicine, Hubei Academy of Agricultural Sciences, Wuhan, China
Glaesserella parasuis (GPS) infection causes severe inflammatory disorder, resulting in lung injury. SIRT7 is an NAD+-dependent deacetylase known to regulate inflammatory responses, but its role in GPS infection remains unclear. Here we found that GPS infection increased SIRT7 expression and induced inflammatory responses. Deficiency of SIRT7 by CRISPR/Cas9 technology significantly inhibited GPS-induced cytopathic effects and inflammatory responses. In addition, RNA-seq analysis showed that differentially expressed genes(DEGs) induced by SIRT7 deficiency were enriched in biological processes such as cell proliferation, actin cytoskeleton formation, lipid synthesis, protein kinase activation regulation, and GTPase activity regulation. Functional enrichment analysis further indicated the involvement of these DEGs in tight junction pathway, PI3K-Akt signaling pathway, actin cytoskeleton regulation, cGMP-PKG signaling pathway, Hippo signaling pathway, and TNF signaling pathway. Finally, we identified some hub genes (GNAI3, GNAI1, JAK1, NDUFS8, CYC1) related to oxidative phosphorylation. In summary, our results demonstrate that SIRT7 is pivotal for GPS-induced inflammatory responses, which represents a promising target resistant to GPS infection.
Introduction
The sirtuin family is a class of evolutionarily conserved NAD+ (nicotinamide adenine dinucleotide)-dependent deacetylases, with multifunctional roles (Wu et al., 2022). Sirtuins regulate diverse biological processes, such as metabolic regulation, epigenetic modifications, cellular aging, and inflammatory responses (Ji et al., 2022). Among the seven mammalian sirtuins (SIRT1-SIRT7), SIRT1 is predominantly unclear but minor distribution can also localize to the cytosol under certain conditions (Tanno et al., 2007). It is involved in various biological processes, including oxidative stress (Vancura et al., 2018), lipid metabolism (Qiang et al., 2011), apoptosis (Chen et al., 2021), cellular aging (You and Liang, 2023), and inflammation (Yang et al., 2022). SIRT2 is the only predominantly cytoplasmic sirtuin, with minor presence in the nucleus and mitochondria. It plays a crucial physiological role in mammals, being pivotal in aging (Zhang et al., 2023), differentiation (Fang et al., 2019), inflammation (Sola-Sevilla et al., 2023), cancer (Chen et al., 2020), and neurodegenerative diseases (Lu et al., 2023). SIRT3, SIRT4, and SIRT5 localize to mitochondria and are collectively termed mitochondrial sirtuins, key regulators of cellular metabolism (Ji et al., 2022). SIRT6 and SIRT7 are nuclear-localized sirtuins, with SIRT6 enriched in chromatin and SIRT7 residing primarily in the nucleolus (Wu et al., 2022). SIRT6 plays a critical role in regulating cellular energy sensing and homeostasis, linking to cellular aging, metabolism, inflammation, and cardiovascular diseases (Chang et al., 2020; Guo et al., 2022). SIRT7, a recently identified member of the sirtuin family, is involved in maintaining genomic integrity, physiological homeostasis, and anti-aging. SIRT7 deficiency disrupts metabolic homeostasis, accelerates aging, and predisposes to inflammatory disorders, cancer, and cardiovascular diseases (Raza et al., 2024).
The SIRT7 gene is located on chromosome 12 in the porcine genome and exhibits ubiquitous expression across multiple tissues, including the heart, kidneys, liver, lungs, subcutaneous fat, spleen, and muscles. Its expression is most abundant in the lungs, spleen, and adipose tissue. Studies have revealed that SIRT7 plays a crucial role in pathogen-host interactions. In HBV-infected cell models, SIRT7 overexpression significantly suppresses HBV RNAs expression, whereas silencing SIRT7 enhances HBV transcription and replication (Yu, 2019). In oral cancer, SIRT7 modulates tumor cell activity by polarizing macrophage phenotypes-suppressing M2 macrophages while activating M1 macrophages, thereby exerting antitumor effects (Gu et al., 2023). Furthermore, the study reveals that SIRT7 plays a protective role in Mycobacterium tuberculosis (Mtb) infection. SIRT7 functions in combating Mtb infection by regulating macrophage nitric oxide (NO) production and apoptosis pathway (Liu, 2020). Recent studies indicate that SIRT7 has an anti-inflammatory role. In lipopolysaccharide (LPS)-stimulated bovine mammary epithelial cells, SIRT7 downregulation exacerbates NF-κB p65 and nuclear translocation, augmenting inflammatory cytokine secretion (Chen et al., 2019). Conversely, SIRT7 silencing attenuates LPS-induced inflammation in lung endothelial cells and reduces renal ischemia-reperfusion injury by suppressing pro-inflammatory cytokines (Wyman et al., 2020; Sánchez-Navarro et al., 2022). Murine colitis models further demonstrate elevated SIRT7 levels in inflamed colonic mucosa, where its knockdown ameliorates inflammation (Kim et al., 2022). Notably, the regulatory function of SIRT7 during Glaesserella parasuis (GPS) infection remains unexplored, warranting further investigation.
GPS, a Gram-negative bacterium exhibiting pleomorphic characteristics (including elongated rods, filaments, and coccobacilli), is a common pathogen in swine upper respiratory tracts and the causative agent of Glässer’s disease (Zhang, 2024). It features a capsule, pili, and outer membrane proteins. Based on agar gel diffusion typing, GPS isolates are classified into 15 serotypes, with approximately 20% remaining untypable. Among these, serotypes 1, 5, 10, 12, 13, and 14 demonstrate high pathogenicity, capable of inducing systemic inflammation in piglets. Epidemiological data indicate serotypes 4 and 5 predominate in China, followed by 13, 14, and 12 (Cai et al., 2005). The virulence factors of GPS are numerous, and its pathogenic mechanisms are complex. The specific virulence factors and detailed mechanisms of pathogenicity remain not fully understood (Ye et al., 2021).
RNA sequencing (RNA-seq) is a high-throughput sequencing technique used to sequence and analyze the transcriptome, including both mRNA and noncoding RNAs, from specific cells or tissues. This approach provides unprecedented insights into transcriptional regulation, offering distinct advantages including high sensitivity, broad dynamic range, and strand-specific information. RNA-seq has become indispensable for elucidating molecular mechanisms underlying disease resistance and reproductive traits (Xiu et al., 2023). In this study, to investigate SIRT7’s regulatory role during GPS infection, we first constructed SIRT7 deficiency 3D4/21 cell lines using CRISPR/Cas9 gene editing technology. RNA-seq analysis comparing wild-type (WT) and SIRT7-knockout (KO) cells enabled systematic identification of differentially expressed genes (DEGs) and associated signaling pathway.
Materials and methods
Cell culture and SIRT7-knockout 3D4/21 cell lines construction
The Cas9-3D4/21 WT cells and SIRT7-KO cell lines were cultured in RPMI 1640 medium (Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco) at 37°C, 5% CO2 atmosphere. Cells were passaged every 2–3 days at 90% confluence using 0.25% trypsin-EDTA (Gibco).
SIRT7-KO cell lines were generated by using CRISPR/Cas9 gene editing technology. Targeting sites exon 2 and exon 3 of the porcine SIRT7 gene (ENSSSCG00000034695) were identified using the Ensembl (https://www.ensembl.org/index.html). Two high-score sgRNAs were selected (Table 1), and the BbsI restriction endonuclease sticky ends sequences of the pb-U6-puro-BFP vector were added to both ends. PCR primers were designed at both ends of the sgRNA-targeted exons to amplify the DNA sequence of this region. The sgRNA primers (forward and reverse) were then annealed. The vector pb-U6-puro-BFP was digested with the BbsI (NEB, USA). The digested products were recovered using the TIANquick Midi Purification Kit (TianGen, China). The annealed sgRNA duplexes were ligated into BbsI-digested vectors using T4 DNA Ligase (Transgen Biotech, China). The ligation mixture was transformed into competent DH5α cells (TianGen, China) and screened on ampicillin-resistant plates. Single colonies were picked and cultured in LB liquid medium. After expanding the culture, plasmid DNA was extracted using the EndoFree Mini Plasmid Kit II (TianGen, China) and sequenced. The pb-U6-SIRT7-sgRNA-puro-BFP plasmid was transfected into the WT cell lines. After 48 h post-transfection, cells were selected with 1.5 μg/mL puromycin (Beyotime, China) for 7 days until complete death of untransfected controls. When all WT cells died, the surviving cells in the treatment group were further cultured. Surviving single-cell colonies were picked and seeded into 96-well plates for culture. Single-cell colonies were expanded for genotyping by PCR (GoTaq polymerase, Promega) and sequencing. The PCR primers used are listed in Table 1.
Bacteria culture
GPS serotype 5 strain (SH0165) was provided by the Laboratory of Genetic Breeding Reproduction and Precision Farming, School of Animal Science and Nutrition Engineering, Wuhan Polytechnic University. Frozen stock cultures were rapidly thawed at 37°C and inoculated into Tryptic Soy Broth (TSB; BD, USA) supplemented with 10% FBS and 0.01% NAD (Beyotime), and cultured at 37°C with 180 rpm shaking for 10–12 h. The medium was then checked for turbidity. Subsequently, the culture was streaked onto TSA (BD) plates and incubated at 37°C for 36 h.
Genomic identification of the SIRT7
Genomic DNA was isolated from SIRT7-KO cell lines using the Universal Genomic DNA Kit (CWBIO, China) and amplified by PCR and sequenced. The PCR products were analyzed by agarose gel electrophoresis. Based on the size of the electrophoresis bands, gene deletions or insertions were assessed. PCR products were subjected to gel extraction (TIANGEN, China) and sent to Sangon Biotech for sequencing. The sequencing results were compared with WT cell sequences to further verify if fragment deletions occurred between the designed sgRNA target sites.
Quantitative real-time PCR
Cells were collected at different time points post-infection, and total RNA was extracted using RNAsimple Total RNA Kit (TIANGEN) according to the manufacturer’s instructions. Following RNA quality assessment, RNA samples were reverse-transcribed into cDNA using HiScript II Q RT SuperMix (Vazyme, China). The relative mRNA levels were quantified by qRT-PCR and calculated via the 2-ΔΔCT method with normalization to the internal control gene GAPDH. The amplification protocol consisted of 95°C for 30 s, 40 cycles of 95°C for 15 s and 60°C for 1 min. Primer sequences are listed in Table 1.
Western blotting
Proteins were extracted from GPS-infected cells at different time points, and the protein concentrations were determined using BCA Protein Quantification Kit (Vazyme, China). Protein samples were separated by SDS-PAGE electrophoresis and transferred to PVDF membranes. The membranes were blocked with 5% non-fat milk and subsequently incubated with the primary antibodies [GAPDH (Proteintech, USA) and SIRT7 (FineTest, China)] overnight at 4°C followed by incubation with the secondary antibody at room temperature for 1 h. After three washes with TBST buffer, protein signals were detected using SuperPico ECL Chemiluminescence Kit (Vazyme) with a chemiluminescence imaging system.
RNA-seq analysis, DEGs identification and functional enrichment analysis
RNA-Seq technology was performed to identify the DEGs in SIRT7-KO cells. Total RNA was extracted using the TRIzol method for RNA-seq (Zhenyue Biotechnology, China) with the MGI DNBseq-T7 platform (BGI, Hong Kong) with 150 bp paired-end reads. Raw reads from RNA-seq libraries were filtered to obtain clean reads, which were then aligned to Sus scrofa reference genome (Sscrofa11.1) using HISAT2 with default parameters. DEGs were identified using DESeq2 (v1.38.3) with the thresholds of P < 0.05 and |log2 Foldchange| > 1. Functional enrichment analysis was conducted through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses using the clusterProfiler R package. Protein-protein interaction (PPI) networks were constructed using the STRING database, and hub genes were identified via the cytoHubba plugin (v0.1) in Cytoscape software.
Results
SIRT7 expression was upregulated in GPS-infected porcine alveolar macrophages
To analyze the role of SIRT7 in GPS infection, we first detected the expression of SIRT7 in 3D4/21 cells (WT cells) with GPS infection at MOI of 10. QRT-PCR analysis revealed significant upregulation of pro-inflammatory cytokines (IL-6, IL-8 and TNF-α) in GPS-infected cells compared to uninfected controls, demonstrating that the inflammatory model of GPS-infected cells has been successfully established (Figure 1A). Western blot analysis found that the protein expression of SIRT7 was significantly upregulated during GPS infection (Figures 1B, C), indicating that SIRT7 may participate in GPS-induced inflammatory response.
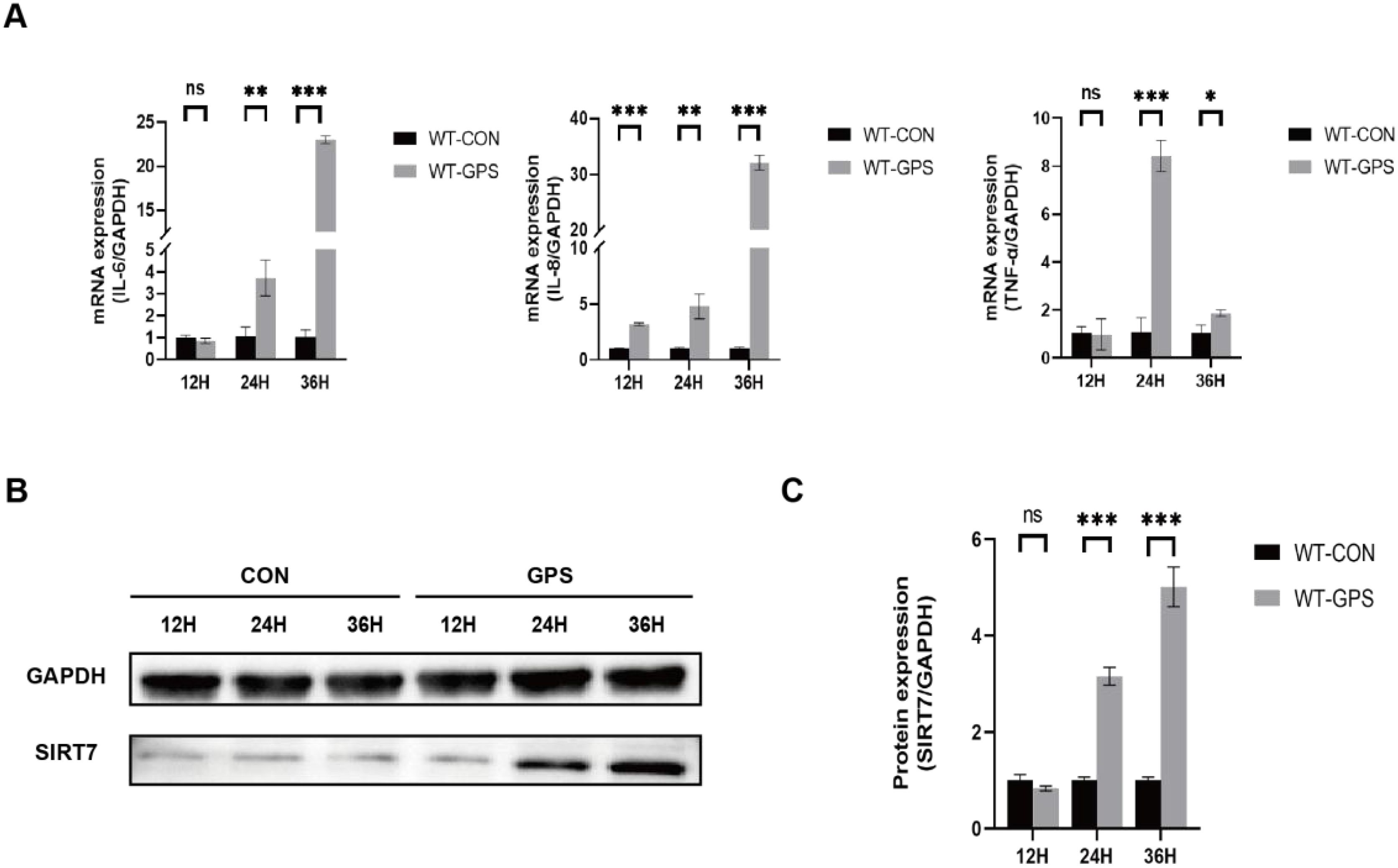
Figure 1. SIRT7 is involved in GPS-induced inflammatory response. (A) Relative expression levels of inflammatory factor genes after GPS infection in WT cells. (B) Expression levels of SIRT7 protein after GPS infection in WT cells. (C) Quantitative analysis of SIRT7/GAPDH gray values. Statistical significance versus control: *P < 0.05, **P < 0.01, ***P < 0.001.
Construction of SIRT7-KO cell line
SIRT7-KO cell line was established by CRISPR/Cas9 system (Figure 2A). After picking the single cell colonies, DNA was extracted and amplified by PCR. Based on the electrophoresis and sequencing results, we found that clone 2 amplified a 1408 bp band and had a mutation with a 925-bp deletion near the protospacer adjacent motif (PAM) sequence, while the WT sample had a 2333 bp band (Figures 2B, C). Western blot analysis showed that SIRT7 protein expression was significantly reduced in KO cell line compared with WT cells (Figure 2D).
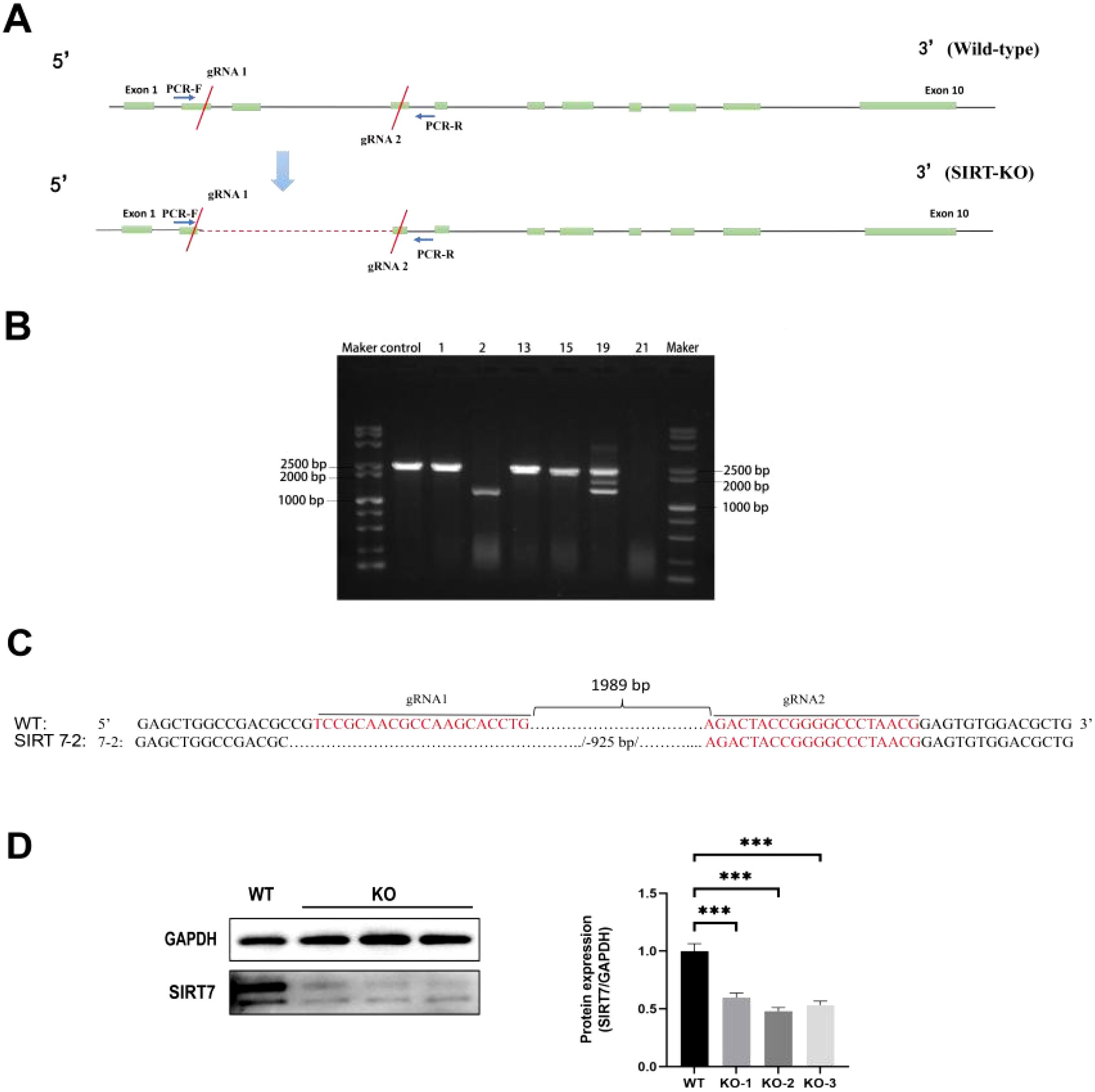
Figure 2. Construction of SIRT7-KO cell line. (A) SIRT7 gene structure and gRNA targeting sites. (B) PCR analysis (M: DL100 DNA Marker; Control: WT cells; 1, 2, 13, 15, 19, 21: Monoclonal cell lines). (C) The sequencing results of SIRT7-KO. (D) SIRT7 protein expression assessment in the KO cell line (internal control: GAPDH) included three biological replicates: KO-1, KO-1, KO-3. Data represent mean ± standard deviation (n = 3). Statistical significance versus control: ***P < 0.001.
SIRT7 deficiency reduced GPS-induced cell damage and inflammatory responses
To analyze the function of SIRT7 in GPS infection, WT and SIRT7-KO cells were infected with GPS at MOI=10. The results indicated that SIRT7 deficiency significantly ameliorated GPS-induced cytopathic effects compared to the WT cells (Figure 3A). In addition, pro-inflammatory cytokine mRNA expression (IL-6, IL-8, TNF-α) were significantly downregulated in SIRT7-KO cells compared to the WT cells after GPS treatment (Figure 3B). These results indicated that the SIRT7 might plays a pivotal role in mediating inflammatory responses triggered by GPS infection.
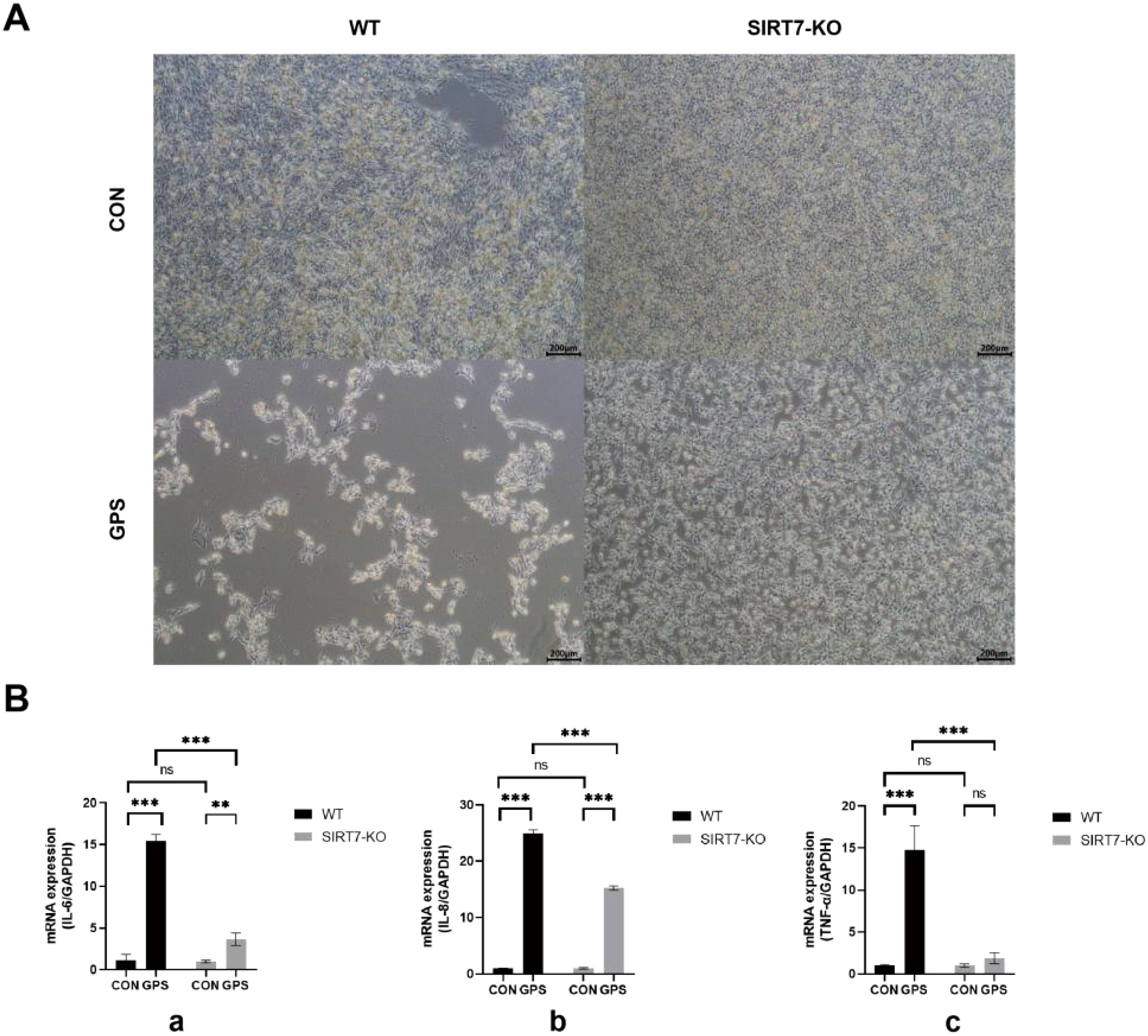
Figure 3. (A) SIRT7 deficiency inhibits GPS-induced cellular damage. (B) SIRT7 deficiency inhibits the inflammatory responses induced by GPS infection. (a: IL-6 expression levels; b: IL-8 expression levels; c: TNF-α expression levels). Data represent mean ± standard deviation (n = 3). Statistical significance versus control: *P < 0.05, **P < 0.01, ***P < 0.001.
DEGs identification and function enrichment analysis in SIRT7-KO cells
To elucidate the molecular mechanisms by which SIRT7 regulates GPS infection, RNA-Seq was used to detect the mRNA expression profiles of SIRT7-KO cells and WT cells with or without GPS infection. 1,588 upregulated and 897 downregulated DEGs were identified in SIRT7-KO cells compared to their expressions without GPS infection using the selection criteria: |log2Foldchange| ≥ 1 and P < 0.05 (Figure 4A, and Supplementary Table S1), While 543 genes were significantly upregulated, and 504 genes were significantly downregulated in SIRT7-KO cells compared to their expressions in WT cells infected with GPS (Figure 4B, and Supplementary Table S2). Furthermore, functional enrichment analysis showed that protein processing associated terms were significantly enriched with or without GPS infection, but some of those DEGs without infection were mainly associated with regulation of GTPase activity, regulation of cytoskeleton organization, while the biological process of some of those DEGs with infection was enriched in angiogenesis, ossification and Wnt signaling pathway (Figures 4C, D). In addition, we observed enriched immune response related terms such as cGMP-PKG signaling pathway, TNF signaling pathway and IL-17 signaling pathway, as well as human papillomavirus infection in SIRT7-KO cells with or without GPS infection. Notably, PI3K-Akt signaling and tight junction pathway showed specific enrichment in SIRT7-KO cells without infection compared to WT cells (Figures 4E, F).
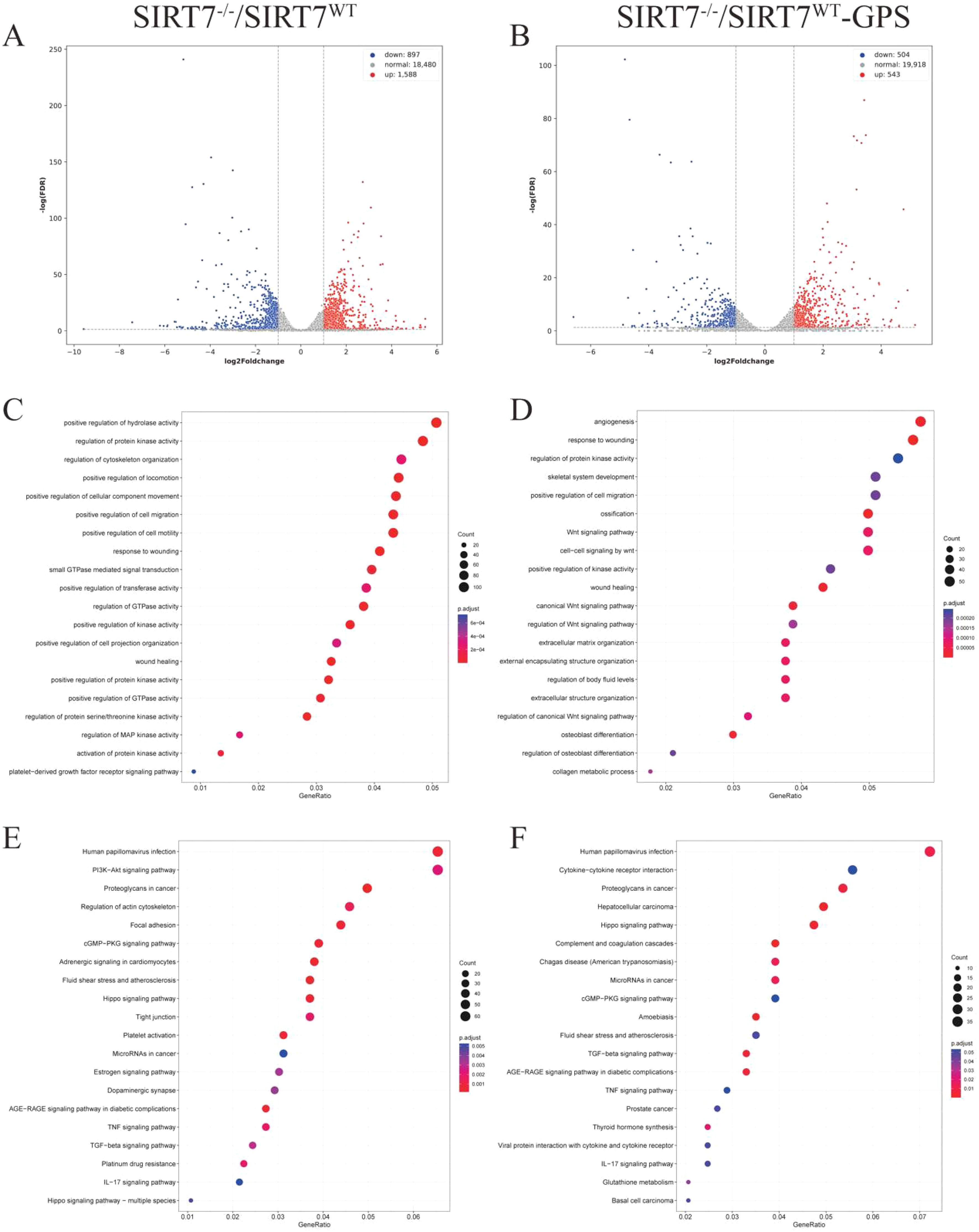
Figure 4. SIRT7 deficiency affects the transcriptome regulation. (A) Volcano plots of DEGs in SIRT7-KO cells without GPS infection. (B) Volcano plots of DEGs in SIRT7-KO cells with GPS infection. (C, D) GO enrichment analysis. (E, F) KEGG function enrichment analysis.
Identification of key gene clusters regulated by SIRT7 deficiency in response to GPS infection
To systematically characterize SIRT7-regulated key gene clusters during GPS infection, we identified overlapping DEGs between SIRT7-KO and WT cells under both basal and infected conditions (Figures 5A, B; Supplementary Table S3). We then performed k-means clustering on the upregulated and downregulated DEGs, respectively.
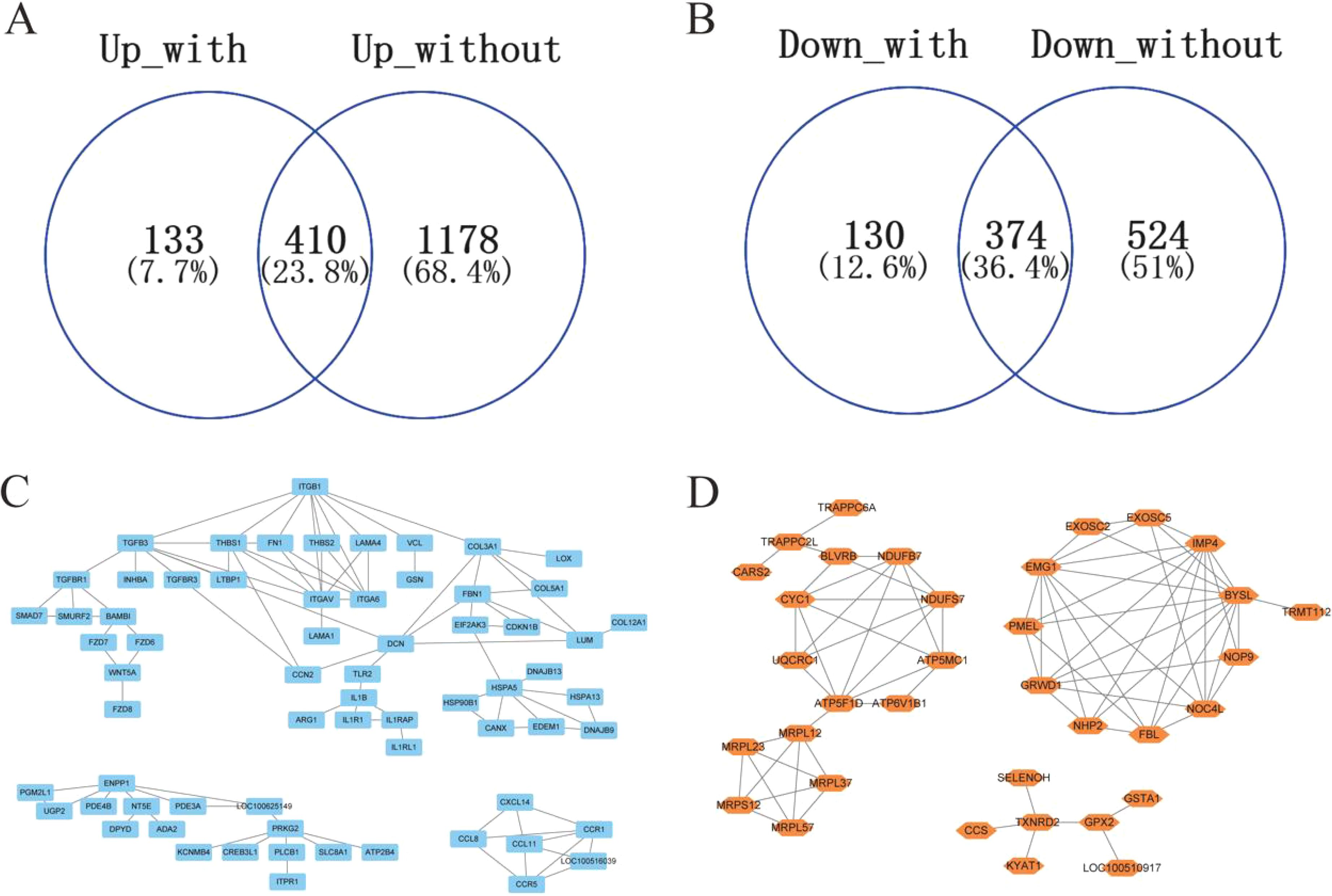
Figure 5. Identification of key gene clusters regulated by SIRT7 deficiency. (A) The overlapping upregulated DEGs in SIRT7 deficiency between control and GPS infection. (B) The overlapping upregulated DEGs in SIRT7 deficiency between control and GPS infection. (C, D) The k-means clustering of the overlapping DEGs in SIRT7 deficiency.
We found that the upregulated common DEGs were divided into three clusters based on their expression patterns, which were mainly associated with extracellular matrix organization, cGMP-PKG signaling pathway, inflammation and immune response (Figure 5C), while three clusters were also identified in the downregulated common DEGs and were enriched in functions associated with oxidative phosphorylation, ribosome biogenesis, glutathione metabolism (Figure 5D). In addition, we used the cytohubba plugin in Cytoscape software to identify the upregulated hub genes (ITGB1, ITGAV, ITGA6, THBS1, FN1, TGFB3, CCR1, FBN1, COL3A1, HSPA5) and the downregulated hub genes (NDUFS7, NDUFB7, BYSL, IMP4, EMG1, GRWD1, NOC4L, FBL, ATP5F1D, CYC1), which might coregulate GPS infection with SIRT7.
Discussion
GPS is a non-motile, encapsulated, facultatively anaerobic Gram-negative bacterium that lacks spore-forming capacity and hemolytic activity (Brockmeier et al., 2014). GPS is the etiological agent of Glässer’s disease, characterized by multiple fibrinous pleuritis, arthritis, and meningitis (Sun, 2024). As a commensal-turned-pathogen, GPS colonizes the porcine upper respiratory tract and becomes invasive under immunosuppressive conditions, such as viral co-infections (e.g., PRRSV) or stress-induced immunocompromise. With the intensification of pig farming and widespread antibiotic misuse, the prevalence of GPS has been rising in China, resulting in significant economic losses to the pig industry (Dai et al., 2024). Research indicates that GPS infection induced cellular inflammation and damage (Zeng et al., 2022). In this study, we observed significant upregulation of IL-6, IL-8, and TNF-α mRNA levels, confirming the successful establishment of a GPS infection model in 3D4/21 cells.
As a nuclear-localized member of the NAD+-dependent sirtuin deacetylase family, SIRT7 has emerged as a key epigenetic regulator in host-pathogen interactions. SIRT7 decreases IRF3/IRF7 phosphorylation to block the interaction between tbk1 and IRF3/IRF7, resulting in the suppression of antiviral responses, disruption of SIRT7 increases the survival rate of carp during virus infection (Liao et al., 2021). Newcastle disease virus infection induced SIRT7 expression, which in turn enhances cellular proteins deacetylation causing high virus replication (Shokeen and Kumar, 2024).However, the regulatory mechanism of the SIRT7 gene in GPS infection has not yet been reported. Our results showed that GPS infection upregulates SIRT7 expression in macrophages suggests its potential involvement in antibacterial defense mechanisms.
This study established a model by creating SIRT7-KO cell lines with CRISPR/Cas9 technology. It was found that SIRT7-KO cells showed less cell damage infection, and significantly reduced expression of pro-inflammatory cytokines (IL-6, IL-8, TNF-α) upon GPS infection, suggesting that SIRT7 deficiency can suppress the occurrence of inflammation induced by GPS infection. Previous studies reported that SIRT7 played a key role in regulating the inflammatory responses, and silencing SIRT7 can inhibit LPS-induced pro-inflammatory responses and NF-κB signaling (Miyasato et al., 2018; Wyman et al., 2020; Mizumoto et al., 2022; Mizutani et al., 2022; Sánchez-Navarro et al., 2022). RNA-seq results revealed that the DEGs induced by SIRT7 deficiency were mainly enriched in biological processes such as cell proliferation, actin cytoskeleton organization, lipid synthesis, regulation of protein kinase activity, and GTPase activity. KEGG pathway analysis identified enrichment in pathways related to viral infection, cancer, tight junctions, PI3K-Akt signaling, actin cytoskeleton regulation, cGMP-PKG signaling, Hippo signaling, and TNF signaling, suggesting that SIRT7 may regulate GPS infection via these signaling pathways. The Hippo signaling pathway plays a crucial role in regulating many biological processes, such as cell proliferation, differentiation, and stem cell self-renewal (Kwon et al., 2013). Dysregulation of the Hippo signaling pathway can lead to various diseases (Fu et al., 2022). The Hippo signaling pathway regulates lung inflammation, with increased activity of type II alveolar epithelial cells associated with elevated nuclear expression of Hippo signaling mediators Yap and Taz. The absence of Yap/Taz in mice affects the repair and regeneration of alveolar epithelial cells in bacterial pneumonia, while downregulation of YAP1 alleviates LPS-induced lung injury by promoting M2 macrophage polarization (Lacanna et al., 2019; Liang et al., 2023). Additionally, the SIRT7 gene activates the Hippo/YAP signaling pathway by directly binding to MST1 and deacetylating it, leading to MST1 ubiquitination and degradation (Gu et al., 2024).
The PI3K/Akt axis plays a crucial role in recruiting and activating innate immune cells, such as macrophages, while exhibiting dual regulatory effects on inflammatory factors production (Hawkins and Stephens, 2015). Studies indicate that under chronic overnutrition conditions, elecated pro-inflammatory factors activate mTORC1, which subsequently suppresses the PI3K/Akt signaling pathway through Ser/Thr residue phosphorylation (Saxton and Sabatini, 2017). Notably, extensive research shows that the sirtuin family can enhance autophagy by inhibiting the PI3K/Akt signaling pathway (Yang et al., 2019; Mishra et al., 2022; Zhang et al., 2022; Zhou et al., 2023). We hypothesize that SIRT7 may enhance autophagy by inhibiting the PI3K/Akt pathway, thereby exacerbating inflammatory responses.
The tight junction signaling pathway maintains tissue homeostasis and integrity by regulating paracellular permeability and cell polarity. Tight junction proteins play a crucial role in regulating cell proliferation, migration, and differentiation (Nehme et al., 2023). Research indicates that overexpression of SIRT6 can restore the expression of tight junction proteins and alleviate cell apoptosis and inflammatory responses (Liu et al., 2023). SIRT1-activated by quercetin upregulates tight junction proteins to mitigate LPS-induced alveolar damage (Deng et al., 2023). Additionally, curcumin activates the AMPK/SIRT3/SOD2/mtROS axis, significantly downregulating inflammatory factors expression levels and increasing tight junction proteins expression (Xiao et al., 2023). We propose that SIRT7 deficiency activates tight-junction signaling, thereby attenuating GPS-induced inflammation. The k-means clustering analysis further revealed the expression pattern shift of key gene clusters regulating GPS infection is due to SIRT7 deficiency. All of the upregulated hub genes (ITGB1, ITGAV, ITGA6, THBS1, FN1, TGFB3, CCR1, FBN1, COL3A1, HSPA5) regulate inflammation. Overexpression of ITGB1 can reduce LPS-induced inflammation and oxidative stress (Qi et al., 2024). Knockdown of THBS1 inhibits autophagy and promotes NLRP3 inflammasome formation (Chiu et al., 2024). HSPA5 can be deacetylated by inhibition of SIRT1/2 to trigger the pro-survival autophagy, suggesting that SIRT7 may also interact with HSPA5 to regulate GPS-induced inflammation response (Mu et al., 2019). Moreover, BYSL, EMG1, GRWD1, FBL and NOC4L were identified as the downregulated hub genes associated with ribosome biogenesis, a process known to suppress immune response (Gratenstein et al., 2005; Adachi et al., 2007; Schilling et al., 2012; Bianco and Mohr, 2019; El Hassouni et al., 2019; Zhu et al., 2019). This suggests that SIRT7 may interact with these hub genes to regulate inflammation response or ribosome biogenesis for affecting GPS infection.
Conclusion
In our study, SIRT7 deficiency significantly suppressed cytopathy and inflammation induced by GPS infection. Combining with RNA-seq, we found that SIRT7 deficiency alters the expression pattern of gene related to tight junctions, ribosome biogenesis, cGMP-PKG signaling pathway, TNF signaling pathway and IL-17 signaling pathway, regulating GPS-induced inflammatory response. Our results provide possible implications of SIRT7 inhibition as a therapeutic strategy against GPS infection.
Data availability statement
All data utilized in this manuscript are available online from their respective databases. The raw data has been deposited in https://doi.org/10.57760/sciencedb.26878.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
HZ: Writing – original draft, Writing – review & editing. BW: Writing – original draft, Writing – review & editing. XD: Writing – original draft, Writing – review & editing. JW: Writing – original draft, Writing – review & editing. LS: Writing – original draft, Writing – review & editing. JZ: Writing – original draft, Writing – review & editing. HC: Writing – original draft, Writing – review & editing. AZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Natural Science Foundation of Hubei Province of China (2025AFB534), National Key Research and Development Program (2023YFD1300200), Natural Science Foundation of Hubei Province of China (2023AFB946) and Wuhan Polytechnic University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1589199/full#supplementary-material
References
Adachi, K., Soeta-Saneyoshi, C., Sagara, H., and Iwakura, Y. (2007). Crucial role of Bysl in mammalian preimplantation development as an integral factor for 40S ribosome biogenesis. Mol. Cell Biol. 27, 2202–2214. doi: 10.1128/MCB.01908-06
Bianco, C. and Mohr, I. (2019). Ribosome biogenesis restricts innate immune responses to virus infection and DNA. Elife 8, e49551. doi: 10.7554/eLife.49551.sa2
Brockmeier, S. L., Register, K. B., Kuehn, J. S., Nicholson, T. L., Loving, C. L., Bayles, D. O., et al. (2014). Virulence and draft genome sequence overview of multiple strains of the swine pathogen Haemophilus parasuis. PloS One 9, e103787. doi: 10.1371/journal.pone.0103787
Cai, X., Chen, H., Blackall, P. J., Yin, Z., Wang, L., Liu, Z., et al. (2005). Serological characterization of Haemophilus parasuis isolates from China. Veterinary Microbiol. 111, 231–236. doi: 10.1016/j.vetmic.2005.07.007
Chang, A. R., Ferrer, C. M., and Mostoslavsky, R. (2020). SIRT6, a mammalian deacylase with multitasking abilities. Physiol. Rev. 100, 145–169. doi: 10.1152/physrev.00030.2018
Chen, G., Huang, P., and Hu, C. (2020). The role of SIRT2 in cancer: A novel therapeutic target. Int. J. Cancer 147, 3297–3304. doi: 10.1002/ijc.v147.12
Chen, K. L., Li, L., Li, C. M., Wang, Y. R., Yang, F. X., Kuang, M. Q., et al. (2019). SIRT7 regulates lipopolysaccharide-induced inflammatory injury by suppressing the NF-κB signaling pathway. Oxid. Med. Cell. Longevity 2019, 3187972. doi: 10.1155/2019/3187972
Chen, L., Li, S., Zhu, J., You, A., Huang, X., Yi, X., et al. (2021). Mangiferin prevents myocardial infarction-induced apoptosis and heart failure in mice by activating the Sirt1/FoxO3a pathway. J. Cell. Mol. Med. 25, 2944–2955. doi: 10.1111/jcmm.16329
Chiu, H. W., Chou, C. L., Lee, K. T., Shih, C. C., Huang, T. H., and Sung, L. C. (2024). Nattokinase attenuates endothelial inflammation through the activation of SRF and THBS1. Int. J. Biol. Macromol. 268, 131779. doi: 10.1016/j.ijbiomac.2024.131779
Dai, L., Zhang, Q., Wan, J. J., Xie, T. T., Jia, Y. Z., Zhang, R., et al. (2024). Isolation, identification and biological characteristics analysis of a strain of haemophilus parvovirus serotype 10. Chin. J. Anim. Sci. 51, 1671–1685. doi: 10.16431/j.cnki.1671-7236.2024.04.035
Deng, S., Li, J., Li, L., Lin, S., Yang, Y., Liu, T., et al. (2023). Quercetin alleviates lipopolysaccharide−induced acute lung injury by inhibiting ferroptosis via the Sirt1/Nrf2/Gpx4 pathway. Int. J. Mol. Med. 52, 118. doi: 10.3892/ijmm.2023.5321
El Hassouni, B., Sarkisjan, D., Vos, J. C., Giovannetti, E., and Peters, G. J. (2019). Targeting the ribosome biogenesis key molecule fibrillarin to avoid chemoresistance. Curr. Med. Chem. 26, 6020–6032. doi: 10.2174/0929867326666181203133332
Fang, N., Cheng, J., Zhang, C., Chen, K., Zhang, C., Hu, Z., et al. (2019). Sirt2 epigenetically down-regulates PDGFRα expression and promotes CG4 cell differentiation. Cell Cycle (Georgetown Tex) 18, 1095–1109. doi: 10.1080/15384101.2019.1609818
Fu, M., Hu, Y., Lan, T., Guan, K. L., Luo, T., and Luo, M. (2022). The Hippo signalling pathway and its implications in human health and diseases. Signal transduction targeted Ther. 7, 376. doi: 10.1038/s41392-022-01191-9
Gratenstein, K., Heggestad, A. D., Fortun, J., Notterpek, L., Pestov, D. G., and Fletcher, B. S. (2005). The WD-repeat protein GRWD1: potential roles in myeloid differentiation and ribosome biogenesis. Genomics 85, 762–773. doi: 10.1016/j.ygeno.2005.02.010
Gu, Y., Ding, C., Yu, T., Liu, B., Tang, W., Wang, Z., et al. (2024). SIRT7 promotes Hippo/YAP activation and cancer cell proliferation in hepatocellular carcinoma via suppressing MST1. Cancer Sci. 115, 1209–1223. doi: 10.1111/cas.v115.4
Gu, C., Zhang, H. M., Tong, Y. Y., Sun, H. L., and He, X. P. (2023). Study on the mechanism of SIRT7 gene on autophagy protein and macrophage polarization of oral cancer cell line based on NF-κB signal pathway. J. Clin. Stomatology 39, 712–717. doi: 10.3969/j.issn.1003-1634.2023.12.003
Guo, Z., Li, P., Ge, J., and Li, H. (2022). SIRT6 in aging, metabolism, inflammation and cardiovascular diseases. Aging Dis. 13, 1787–1822. doi: 10.14336/AD.2022.0413
Hawkins, P. T. and Stephens, L. R. (2015). PI3K signalling in inflammation. Biochim. Biophys. Acta 1851, 882–897. doi: 10.1016/j.bbalip.2014.12.006
Ji, Z., Liu, G. H., and Qu, J. (2022). Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genomics = Yi Chuan xue bao 49, 287–298. doi: 10.1016/j.jgg.2021.11.005
Kim, S., Byun, J., Jung, S., Kim, B., Lee, K., Jeon, H., et al. (2022). Sirtuin 7 inhibitor attenuates colonic mucosal immune activation in mice-potential therapeutic target in inflammatory bowel disease. Biomedicines 10, 2693. doi: 10.3390/biomedicines10112693
Kwon, Y., Vinayagam, A., Sun, X., Dephoure, N., Gygi, S. P., Hong, P., et al. (2013). The Hippo signaling pathway interactome. Sci. (New York NY) 342, 737–740. doi: 10.1126/science.1243971
Lacanna, R., Liccardo, D., Zhang, P., Tragesser, L., Wang, Y., Cao, T., et al. (2019). Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Invest. 129, 2107–2122. doi: 10.1172/JCI125014
Liang, L., Xu, W., Shen, A., Fu, X., Cen, H., Wang, S., et al. (2023). Inhibition of YAP1 activity ameliorates acute lung injury through promotion of M2 macrophage polarization. MedComm 4, e293. doi: 10.1002/mco2.v4.3
Liao, Q., Ouyang, G., Zhu, J., Cai, X., Yu, G., Zhou, Z., et al. (2021). Zebrafish sirt7 Negatively Regulates Antiviral Responses by Attenuating Phosphorylation of irf3 and irf7 Independent of Its Enzymatic Activity. J. Immunol. (Baltimore Md: 1950) 207, 3050–3059. doi: 10.4049/jimmunol.2100318
Liu, Y. Y. (2020). SIRT7 inhibits the infection of mycobacterium tuberculosis on macrophages by regulating nitric oxide and apoptosis (University of South China, Hengyang).
Liu, H., Wang, S., Gong, L., Shen, Y., Xu, F., Wang, Y., et al. (2023). SIRT6 ameliorates LPS-induced apoptosis and tight junction injury in ARDS through the ERK1/2 pathway and autophagy. Int. J. Med. Sci. 20, 581–594. doi: 10.7150/ijms.80920
Lu, W., Ji, H., and Wu, D. (2023). SIRT2 plays complex roles in neuroinflammation neuroimmunology-associated disorders. Front. Immunol. 14, 1174180. doi: 10.3389/fimmu.2023.1174180
Mishra, S., Cosentino, C., Tamta, A. K., Khan, D., Srinivasan, S., Ravi, V., et al. (2022). Sirtuin 6 inhibition protects against glucocorticoid-induced skeletal muscle atrophy by regulating IGF/PI3K/AKT signaling. Nat. Commun. 13, 5415. doi: 10.1038/s41467-022-32905-w
Miyasato, Y., Yoshizawa, T., Sato, Y., Nakagawa, T., Miyasato, Y., Kakizoe, Y., et al. (2018). Sirtuin 7 deficiency ameliorates cisplatin-induced acute kidney injury through regulation of the inflammatory response. Sci. Rep. 8, 5927. doi: 10.1038/s41598-018-24257-7
Mizumoto, T., Yoshizawa, T., Sato, Y., Ito, T., Tsuyama, T., Satoh, A., et al. (2022). SIRT7 deficiency protects against aging-associated glucose intolerance and extends lifespan in male mice. Cells. 11, 3609. doi: 10.3390/cells11223609
Mizutani, H., Sato, Y., Yamazaki, M., Yoshizawa, T., Ando, Y., Ueda, M., et al. (2022). SIRT7 Deficiency Protects against Aβ(42)-Induced Apoptosis through the Regulation of NOX4-Derived Reactive Oxygen Species Production in SH-SY5Y Cells. Int. J. Mol. Sci. 23, 9027. doi: 10.3390/ijms23169027
Mu, N., Lei, Y., Wang, Y., Wang, Y., Duan, Q., Ma, G., et al. (2019). Inhibition of SIRT1/2 upregulates HSPA5 acetylation and induces pro-survival autophagy via ATF4-DDIT4-mTORC1 axis in human lung cancer cells. Apoptosis 24, 798–811. doi: 10.1007/s10495-019-01559-3
Nehme, Z., Roehlen, N., Dhawan, P., and Baumert, T. F. (2023). Tight junction protein signaling and cancer biology. Cells 12, 243. doi: 10.3390/cells12020243
Qi, R., Wang, Y., Yan, F., and Zhong, J. (2024). Exosomes derived from ITGB1 modified Telocytes alleviates LPS-induced inflammation and oxidative stress through YAP1/ROS axis. Heliyon 10, e27086. doi: 10.1016/j.heliyon.2024.e27086
Qiang, L., Lin, H. V., Kim-Muller, J. Y., Welch, C. L., Gu, W., and Accili, D. (2011). Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through Creb deacetylation. Cell Metab. 14, 758–767. doi: 10.1016/j.cmet.2011.10.007
Raza, U., Tang, X., Liu, Z., and Liu, B. (2024). SIRT7: the seventh key to unlocking the mystery of aging. Physiol. Rev. 104, 253–280. doi: 10.1152/physrev.00044.2022
Sánchez-Navarro, A., Martínez-Rojas, M., Albarrán-Godinez, A., Pérez-Villalva, R., Auwerx, J., De La Cruz, A., et al. (2022). Sirtuin 7 deficiency reduces inflammation and tubular damage induced by an episode of acute kidney injury. Int. J. Mol. Sci. 23, 2573. doi: 10.3390/ijms23052573
Saxton, R. A. and Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371. doi: 10.1016/j.cell.2017.03.035
Schilling, V., Peifer, C., Buchhaupt, M., Lamberth, S., Lioutikov, A., Rietschel, B., et al. (2012). Genetic interactions of yeast NEP1 (EMG1), encoding an essential factor in ribosome biogenesis. Yeast 29, 167–183. doi: 10.1002/yea.v29.5
Shokeen, K. and Kumar, S. (2024). Newcastle disease virus regulates its replication by instigating oxidative stress-driven Sirtuin 7 production. J. Gen. Virol. 105, 001961. doi: 10.1099/jgv.0.001961
Sola-Sevilla, N., Mesa-Lombardo, A., Aleixo, M., Expósito, S., Diaz-Perdigón, T., Azqueta, A., et al. (2023). SIRT2 inhibition rescues neurodegenerative pathology but increases systemic inflammation in a transgenic mouse model of alzheimer's disease. J. neuroimmune pharmacology: Off. J. Soc. NeuroImmune Pharmacol. 18, 529–550. doi: 10.1007/s11481-023-10084-9
Sun, M. T. (2024). Advances in diagnostic methods for Haemophilus parvovirus disease. Today Anim. Husbandry Veterinary Med. 40, 80–82. doi: 10.3969/j.issn.1673-4092.2024.03.028
Tanno, M., Sakamoto, J., Miura, T., Shimamoto, K., and Horio, Y. (2007). Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 282, 6823–6832. doi: 10.1074/jbc.M609554200
Vancura, A., Nagar, S., Kaur, P., Bu, P., Bhagwat, M., and Vancurova, I. (2018). Reciprocal regulation of AMPK/SNF1 and protein acetylation. Int. J. Mol. Sci., 19 (11), 3314. doi: 10.3390/ijms19113314
Wu, Q. J., Zhang, T. N., Chen, H. H., Yu, X. F., Lv, J. L., Liu, Y. Y., et al. (2022). The sirtuin family in health and disease. Signal transduction targeted Ther. 7, 402. doi: 10.1038/s41392-022-01257-8
Wyman, A. E., Nguyen, T. T. T., Karki, P., Tulapurkar, M. E., Zhang, C. O., Kim, J., et al. (2020). SIRT7 deficiency suppresses inflammation, induces EndoMT, and increases vascular permeability in primary pulmonary endothelial cells. Sci. Rep. 10, 12497. doi: 10.1038/s41598-020-69236-z
Xiao, L., Fang, Z., Wang, Q., Sheng, X., Qi, X., Xing, K., et al. (2023). Curcumin ameliorates age-induced tight junction impaired in porcine sertoli cells by inactivating the NLRP3 inflammasome through the AMPK/SIRT3/SOD2/mtROS signaling pathway. Oxid. Med. Cell. Longevity 2023, 1708251. doi: 10.1155/2023/1708251
Xie, J. H., Chen, X. F., Wang, G. G., Li, R., Tan, D. J., Ma, J., et al. (2023). Development of transcriptome sequencing technology and its application in livestock and poultry farming. Chin. J. Anim. Sci. 59, 103–110. doi: 10.19556/j.0258-7033.20230504-02
Yang, X., Jiang, T., Wang, Y., and Guo, L. (2019). The role and mechanism of SIRT1 in resveratrol-regulated osteoblast autophagy in osteoporosis rats. Sci. Rep. 9, 18424. doi: 10.1038/s41598-019-44766-3
Yang, Y., Liu, Y., Wang, Y., Chao, Y., Zhang, J., Jia, Y., et al. (2022). Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 13, 831168. doi: 10.3389/fimmu.2022.831168
Ye, J. X., Liu, Y. F., Feng, X. N., and Chen, R. A. (2021). Progress on virulence genes and pathogenesis of haemophilus parasuis. Prog. Veterinary Med. 42, 90–94. doi: 10.3969/j.issn.1007-5038.2021.11.017
You, Y. and Liang, W. (2023). SIRT1 and SIRT6: The role in aging-related diseases. Biochim. Biophys. Acta Mol. basis Dis. 1869, 166815. doi: 10.1016/j.bbadis.2023.166815
Yu, H. B. (2019). The function of SIRT7 in hepatitis B virus transcription and replication (Chongqing Medical University, Chongqing).
Zeng, Z., Zhang, H. Q., and Gui, G. B. (2022). The effect of Macleaya cordata extract on the infIammation and signaling pathways induced by Glaesserella parasuis in porcine alveolar macrophages. Chin. J. Preventive Veterinary Med. 44, 875–880. doi: 10.3969/j.issn.1008-0589.202203008
Zhang, J. (2024). Control of Haemophilus parvovirus disease. China Anim. Health 26, 38–39. doi: 10.3969/j.issn.1008-4754.2024.01.019
Zhang, C. Y., Tan, X. H., Yang, H. H., Jin, L., Hong, J. R., Zhou, Y., et al. (2022). COX-2/sEH dual inhibitor alleviates hepatocyte senescence in NAFLD mice by restoring autophagy through sirt1/PI3K/AKT/mTOR. Int. J. Mol. Sci. 23, 8267. doi: 10.3390/ijms23158267
Zhang, Y., Wang, X., Li, X. K., Lv, S. J., Wang, H. P., Liu, Y., et al. (2023). Sirtuin 2 deficiency aggravates ageing-induced vascular remodelling in humans and mice. Eur. Heart J. 44, 2746–2759. doi: 10.1093/eurheartj/ehad381
Zhou, B., Yang, Y., Pang, X., Shi, J., Jiang, T., and Zheng, X. (2023). Quercetin inhibits DNA damage responses to induce apoptosis via SIRT5/PI3K/AKT pathway in non-small cell lung cancer. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie 165, 115071. doi: 10.1016/j.biopha.2023.115071
Keywords: SIRT7, Glaesserella parasuis, inflammatory responses, CRISPR/Cas9, disease-resistant breeding
Citation: Zheng H, Wang B, Dong X, Wu J, Shi L, Zhang J, Chen H and Zhou A (2025) SIRT7 deletion inhibits Glaesserella parasuis-mediated inflammatory responses in porcine alveolar macrophages. Front. Cell. Infect. Microbiol. 15:1589199. doi: 10.3389/fcimb.2025.1589199
Received: 07 March 2025; Accepted: 29 April 2025;
Published: 25 June 2025.
Edited by:
Qiang Zhang, Huazhong Agricultural University, ChinaReviewed by:
Cong Yin, South-Central University for Nationalities, ChinaHairui Fan, Yangzhou University, China
Copyright © 2025 Zheng, Wang, Dong, Wu, Shi, Zhang, Chen and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ao Zhou, emhvdWFvMjAwOEBhbGl5dW4uY29t
 Hao Zheng
Hao Zheng Baoxin Wang1,2
Baoxin Wang1,2 Liangyu Shi
Liangyu Shi Jing Zhang
Jing Zhang Ao Zhou
Ao Zhou