- 1School of Traditional Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2School of Traditional Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Diabetes mellitus has emerged as a global public health crisis, with over half of patients experiencing gastrointestinal (GI) symptoms that exacerbate glucose fluctuations and impair quality of life. While prior research on the pathophysiology of diabetic gastroenteropathy (DGE) focused primarily on autonomic neuropathy, particularly involving the vagus nerve, recent studies have shifted toward the impairment of the enteric nervous system (ENS). As the largest autonomous neural network governing GI motility independent of central control, structural and functional abnormalities of the ENS constitute the fundamental pathological basis for DGE. This review first delineates gut microbial alterations in diabetes and mechanisms by which dysbiosis compromises the integrity of the ENS. Second, we analyze how microbiota-derived metabolites (short-chain fatty acids, bile acids, tryptophan), gut hormones (glucagon-like peptide-1, ghrelin), and neurotransmitters (acetylcholine, vasoactive intestinal peptide, nitric oxide) multitarget the ENS—collectively establishing the “microbiota-ENS axis” as the central hub for GI sensorimotor control. Finally, we provide an overview of preclinical and clinical evidence for microbiome-targeted therapies (probiotics, prebiotics, fecal microbiota transplantation) in alleviating DGE symptoms and repairing ENS while outlining translational challenges and future research priorities.
1 Introduction
Diabetes has become a global public health crisis, with epidemiological data revealing a prevalence of 10.5% among individuals aged 20–70 years, projected to climb to 12.2% by 2045 (Sun et al., 2021). Diabetic gastroenteropathy (DGE), a prevalent complication of diabetes, impacts the gastrointestinal (GI) tract from the esophagus to the colon, presenting clinical symptoms including esophageal motility disorders, gastroparesis, constipation, diarrhea, and fecal incontinence. In patients with diabetes mellitus, the incidence of GI symptoms is significantly higher than in those without diabetes, especially in women with poor glycemic control, long-standing diabetes, or other diabetic complications, although the reported prevalence of DGE varies widely by region (Du et al., 2018). Research indicates that 70% to 75% of diabetic individuals experience at least one GI symptom (Krishnasamy and Abell, 2018), with dysphagia (63%), gastroesophageal reflux (41%), early satiety or nausea (10%–20%), constipation (60%), and diarrhea (20%) being especially prominent (Concepción Zavaleta et al., 2021). These symptoms contribute to malnutrition, impaired drug absorption, decreased treatment adherence, and reduced quality of life. Despite the widespread use of validated tools, such as the Gastrointestinal Symptom Rating Scale (GSRS) and Gastroparesis Cardinal Symptom Index (GCSI), the diagnosis of DGE remains hindered by the lack of specific biomarkers and the reliance on exclusion criteria, resulting in high rates of underdiagnosis and clinical oversight (Revicki et al., 2003; Kulich et al., 2008). Nuclear gastric emptying scintigraphy is the gold standard for diagnosing gastroparesis, yet its practical use is limited due to radiation exposure and time-consuming procedures. Current treatments, such as prokinetics, antiemetics, and laxatives, aim to alleviate symptoms. Unfortunately, metoclopramide is the sole medication that has received Food and Drug Administration (FDA) approval for the treatment of gastroparesis. The utilization of domperidone, erythromycin, and mosapride (a 5-hydroxy tryptamine 4 agonist) is limited owing to safety concerns (Camilleri and Sanders, 2022). Novel approaches, including ghrelin receptor agonists, pyloric botulinum toxin injections, and surgical pyloromyotomy, show promise but necessitate thorough evaluation (Fleming et al., 2020; Schol et al., 2021). Therefore, clarifying the pathophysiology of DGE and discovering novel therapeutic targets are imperative.
Gastrointestinal function is coordinately regulated by two principal neural systems: the extrinsic autonomic nervous system and the intrinsic enteric nervous system. Prior investigations into the pathophysiology of DGE predominantly focused on autonomic dysfunction, particularly damage to the vagus nerve. Sustained hyperglycemia and its sequelae, including oxidative stress and inflammatory cascades, induce segmental demyelination and axonal degeneration in vagal fibers. These pathological changes disrupt bidirectional gut-brain communication and impair neuromodulation of gastrointestinal smooth muscle. However, contemporary research has progressively shifted toward elucidating impairment of the enteric nervous system (ENS). Termed the “second brain” of the gut, the ENS constitutes the largest autonomously functioning neural network within the GI tract. It orchestrates motility independent of the central nervous system (CNS) while maintaining anatomical and functional connectivity with the CNS via vagal pathways. First described by Albert von Haller in 1755 (Gershon, 1998), this system exhibits persistent GI motility even after intestinal disconnection from the brain. Analogous to the CNS, it integrates sensory neurons, interneurons, and motor neurons to form autonomous sensory-motor reflex arcs, which are the essential framework for the autonomous regulation of digestive tract functions (Niesler et al., 2021). Originating from vagal neural crest progenitors during embryogenesis, the ENS comprises millions of neurons and glial cells organized into myenteric (Auerbach’s) and submucosal (Meissner’s) plexuses. The myenteric plexus, spanning the GI tract, coordinates smooth muscle contractions to propel luminal contents, whereas the submucosal plexus, localized to the small and large intestines, modulates secretion, absorption, and responses to chemical and mechanical stimuli (Pawolski and Schmidt, 2020). Thus, key gastrointestinal functions including motility, sensation, and secretion are all regulated by the ENS, with its impairment contributing to a spectrum of gastrointestinal neuropathies. As observed in disease models of gastroparesis (Tseng et al., 2022), chronic constipation (Wang et al., 2023), functional dyspepsia (Tait and Sayuk, 2021), and irritable bowel syndrome (IBS) (Mayer et al., 2023), there is a notable loss of enteric neurons and ICCs, along with a reduction in the size and quantity of ganglia, underscoring the critical role of the ENS in maintaining GI function and motility. Nonetheless, the ENS, along with enteric neurons, enteric glial cells (EGCs), and ICCs, may be damaged from several diabetes-related variables, including chronic hyperglycemia, advanced glycation end products, oxidative stress, gut dysbiosis, and inflammation (Abdalla, 2024; Uppaluri et al., 2024). Undoubtedly, glycemic control remains the cornerstone for preventing and delaying the progression of DGE, while the search for targets to repair and regulate the ENS continues to be a major focus of current research.
Emerging evidence suggests bidirectional interactions between the ENS and gut microbiome (Sharkey and Mawe, 2023). The gut microbiome, the most complex microecosystem in the digestive tract, plays a pivotal role in various physiological processes, such as nutrient absorption, glucose and lipid metabolism, immune regulation, and GI motility (Del Chierico et al., 2022). Recent studies have demonstrated that the development, maturation, and integrity of the ENS are profoundly influenced by the gut microbiome. Research (Sajdel-Sulkowska, 2023) shows that the development of fetal ENS could be influenced by maternal gut dysbiosis during pregnancy. In adulthood, antibiotic-induced germ-free mice also exhibit a significant reduction in the number of enteric neurons, glial cells, and vagal afferent neurons, leading to extended small intestinal and prolonged GI transit time (Hung et al., 2020). However, these traits were reversible upon microbial colonization or supplementation with Bacteroides species or short-chain fatty acids (SCFAs), along with restoring cholinergic neuronal activity and promoting GI motility (Aktar et al., 2020). Thus, the gut microbiota may modulate the function and homeostasis of the ENS through its metabolites and signaling molecules. These findings suggest that microbiome-based therapies may represent an innovative approach to improve GI dysfunction (Simon et al., 2021). Nevertheless, despite these encouraging findings, existing research on the specific pathways by which gut microbiota modulate the ENS to ameliorate GI issues in patients with diabetes remains insufficient. There exists a notable gap in identifying viable treatment targets.
This review aims to provide an update on the mechanisms and therapeutic potential of the “microbiome-ENS axis” in DGE, with a particular emphasis on the interactions between the gut microbiome and the ENS (Figure 1). We first elaborate on the impact of gut dysbiosis on the ENS and GI motility. Subsequently, we analyze the mechanisms by which microbiota-derived metabolites, gut hormones, and neurotransmitters regulate the ENS and GI function and provide a focused discussion on their contributions. Finally, we evaluate the translational potential of microbiome-based therapies, including probiotics, prebiotics, and fecal microbiota transplantation (FMT), to lay the theoretical groundwork for developing precision treatments of DGE and improve patients’ quality of life.
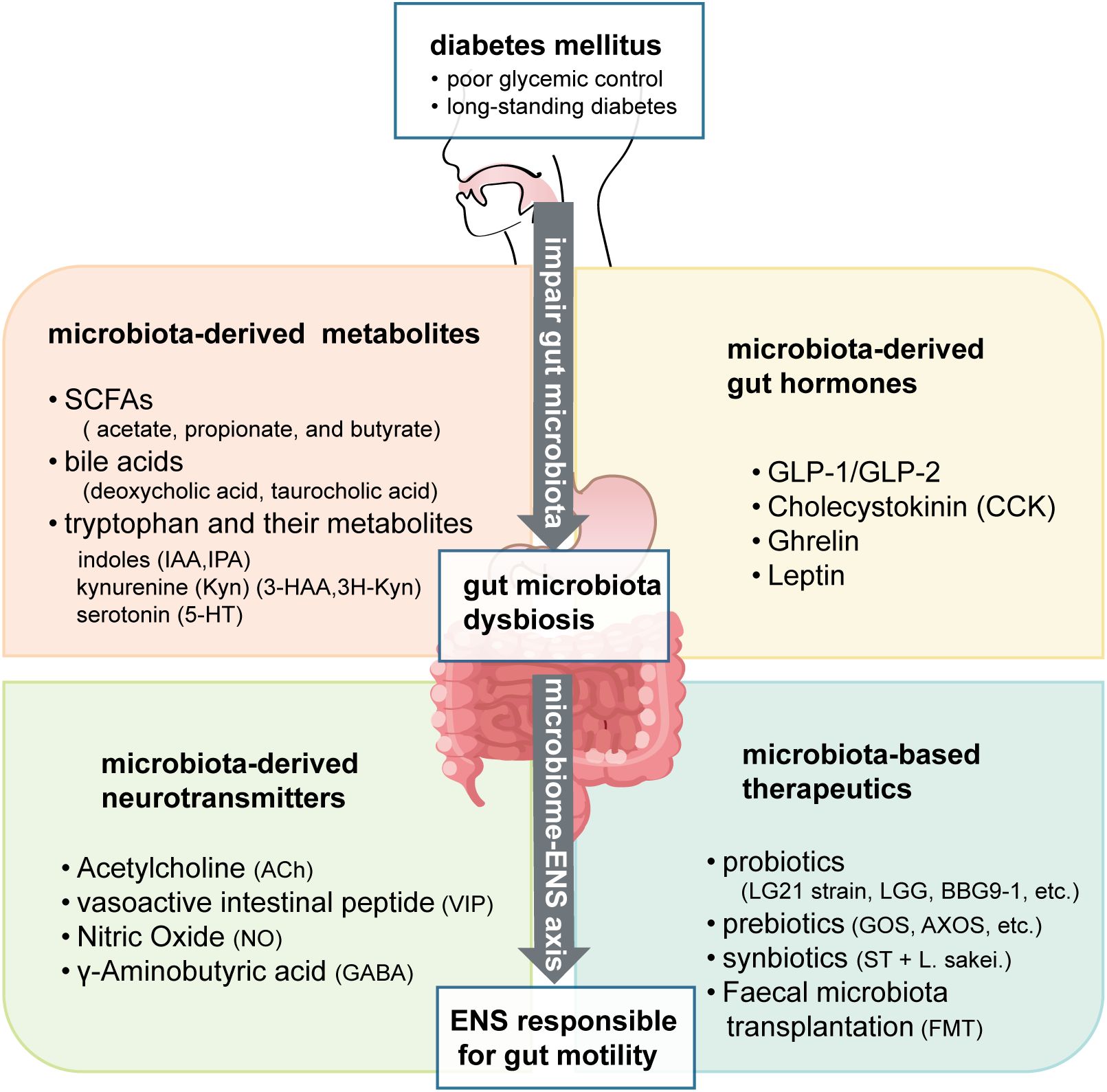
Figure 1. Mechanistic insights into diabetic gastroenteropathy: bidirectional interactions between diabetes and gut microbiota impacting the enteric nervous system.
2 Mechanisms and consequences of diabetes-induced gut microbiota dysbiosis
2.1 Mechanisms underlying gut dysbiosis-induced diabetes
Although the gut microbiota is initially established at birth, it evolves dynamically throughout life, modulated by host age, dietary patterns, and physical activity (Rinninella et al., 2019). Dietary patterns differentially influence the pathogenesis of diabetes, potentially mediated by gut microbiota alterations. For instance, high-sugar or high-fat diets increase Akkermansia, Proteobacteria, and endotoxemia while elevating the Firmicutes/Bacteroidetes ratio and reducing bifidobacteria abundance (Sen et al., 2017). Protein sources differentially modulate the composition of gut microbiota: poultry and fish consumption increase Actinobacteria, beef consumption elevates Bacteroidetes, and soy protein enhances probiotics like Lactobacillus and Bifidobacterium and reduces Bacteroides (Wu et al., 2022a). These microbial shifts increase intestinal permeability, thereby promoting bacterial translocation and endotoxemia. Moreover, dysbiosis alters microbial metabolites, including short-chain fatty acids (SCFAs), trimethylamine N-oxide (TMAO), and indoles, which disrupts endocrine signaling (PYY, GLP-1/2, adiponectin, and resistin), impairs insulin pathways, and promotes lipogenesis, ultimately driving obesity and diabetes (Moszak et al., 2020). Conversely, meta-analyses demonstrate that high-fiber diets significantly increase the abundance of Bifidobacterium and Lactobacillus along with SCFAs, while suppressing enteropathogens (Shigella, Escherichia coli, Klebsiella) and improving glucose and lipids. Subgroup analyses reveal that specific prebiotics (fructans and galacto-oligosaccharides) further elevate the abundance of Bifidobacterium and Lactobacillus (So et al., 2018). Vegetable/fruit-rich diets reduce the risk of gestational diabetes by modulating Lachnospiraceae, Blautia, and Ruminococcus (Shan et al., 2024). Sedentary lifestyles, established obesity risk factors, may induce insulin resistance through altered Firmicutes/Bacteroidetes ratios (Sikalidis and Maykish, 2020). Regular exercise, conversely, promotes beneficial bacteria proliferation, enhances intestinal barrier integrity, and maintains metabolic homeostasis (Campaniello et al., 2022). Thus, dietary modification and physical activity represent pragmatic approaches for correcting metabolic dysregulation in diabetes. Interventions incorporating fiber, plant-based foods, probiotics, and prebiotics demonstrate efficacy in reversing metabolic syndrome through microbial remodeling (Upadhyaya and Banerjee, 2015).
2.2 Mechanisms underlying diabetes-driven microbiota remodeling
Notably, fecal microbiota transplantation (FMT) from obese type 2 diabetic donors into germ-free mice induces weight gain and glucose intolerance, underscoring the pivotal role of gut microbiota dysbiosis in the pathogenesis of diabetes (Pearson et al., 2022). However, the alterations of gut microbiota not only contribute to diabetes but are reciprocally reshaped by diabetes. As evidenced in db/db and ob/ob mouse models (Thaiss et al., 2018), sustained hyperglycemia activates intestinal epithelial glucose transporter 2 (GLUT2) and downregulates the expression of zonula occludens-1 (ZO-1), increasing intestinal permeability. This compromised barrier facilitates the translocation of harmful microorganisms, such as Escherichia coli, from the gut lumen into systemic circulation, thereby exacerbating systemic inflammation (Darra et al., 2023). Furthermore, significant reductions in butyrate producers (Pseudoflavonifractor, Clostridium, Alistipes, Faecalibacterium, Oscillibacter) and secondary bile acid producers (Eubacterium rectale, Clostridium scindens, Bacteroides fragilis) are observed in prediabetic and type 2 diabetic individuals. These microbial deficits disrupt intestinal barrier homeostasis, further promoting microbial translocation and perpetuating dysbiosis. Diabetes-associated chronic inflammation, characterized by elevated serum TNF-α and IL-6, further compromises gut immune equilibrium, amplifying barrier dysfunction and dysbiotic cascades.
2.3 Characteristic of diabetes-associated gut microbiota dysbiosis
A growing body of evidence (Wu et al., 2020; Zhao et al., 2020b; Zhou et al., 2022) reveals markedly altered abundance and diversity of gut microbiota in patients with diabetes compared to healthy individuals, characterized by low-diversity dysbiosis and an overgrowth of opportunistic pathogens. Bacteroides and Bifidobacterium, frequently implicated in type 2 diabetes, exhibit an inverse correlation with disease severity (Gurung et al., 2020). While an elevated Firmicutes/Bacteroidetes ratio is often associated with obesity (Koliada et al., 2017), numerous studies (Ahmad et al., 2023; Karačić et al., 2024) report non-significant or inverse correlations with obesity. Magne et al. (2020). attribute these discrepancies to methodological heterogeneity, including variations in sample size, participant characteristics, and sequencing approaches (16S rRNA vs. metagenomics). Notably, a systematic review (Zhou et al., 2020) indicated a decreased F/B ratio in fecal samples from type 1 diabetes (T1DM) mellitus patients analyzed via 16S rRNA sequencing, whereas duodenal biopsies revealed an increased ratio in Italian cohorts (Pellegrini et al., 2017, p. 1). This spatial heterogeneity underscores the necessity for segmental gut sampling (e.g., gastric mucosa, jejunal contents) coupled with spatial microbiome analysis to elucidate microbiota-host interactions.
Type 2 diabetes is associated with increased abundance of Aspergillus, Micrococcus, and Actinomycetes, alongside opportunistic pathogens (Streptococcus, Clostridium, Escherichia-Shigella, Enterococcus, Klebsiella) that contribute to metabolic endotoxemia (Zhou et al., 2022). Although reduction of Akkermansia muciniphila typically correlates with intestinal hyperpermeability, facilitating pathogen translocation and metabolic disease pathogenesis, paradoxical increases have been reported in some T2DM studies (Zhao et al., 2020b). This anomaly may arise from confounding factors such as metformin exposure or consumption of polyphenol-rich green tea, though mechanistic validation is pending (de la Cuesta-Zuluaga et al., 2017; Gurley et al., 2019). Such confounders necessitate stratified analyses, particularly given metformin’s documented stimulation of SCFA-producing taxa (Butyrivibrio, Bifidobacterium bifidum, Megasphaera, Prevotella, Escherichia coli), which may obscure true microbiota-disease relationships (de la Cuesta-Zuluaga et al., 2017).
3 Impacts of gut microbiota dysbiosis on gastrointestinal nerves and function
In the diabetic state, gut microbiota dysbiosis further exacerbates GI motility dysfunction and impairs the ENS. Alterations in microbiota observed in both clinical and animal models of DGE underscore the association between these microbial communities and GI motility. Enrichment of Proteobacteria represents one of the most prominent microbiota features in DGE, with increased abundance positively correlating with the progression of DGE (Du et al., 2022). A small-scale Chinese clinical study (Lin et al., 2024) reported an increased abundance of Proteobacteria, including Pseudomonas and Alkalibacterium, in patients with DGE. A cohort study (Du et al., 2022) on diabetic autonomic neuropathy revealed a significant increase in taxa within Proteobacteria, including Escherichia-Shigella, Escherichia coli, and Megasphaera. Actinobacteria, Proteobacteria, and Firmicutes are dominant in DGE and show a significant positive correlation with impaired gastrointestinal motility (Huang et al., 2024; Zheng et al., 2024). Crucially, this diabetes-induced gut microbiota dysbiosis may profoundly adversely affect the ENS and impede GI motility, as evidenced by recent mechanistic investigations (Figure 2).
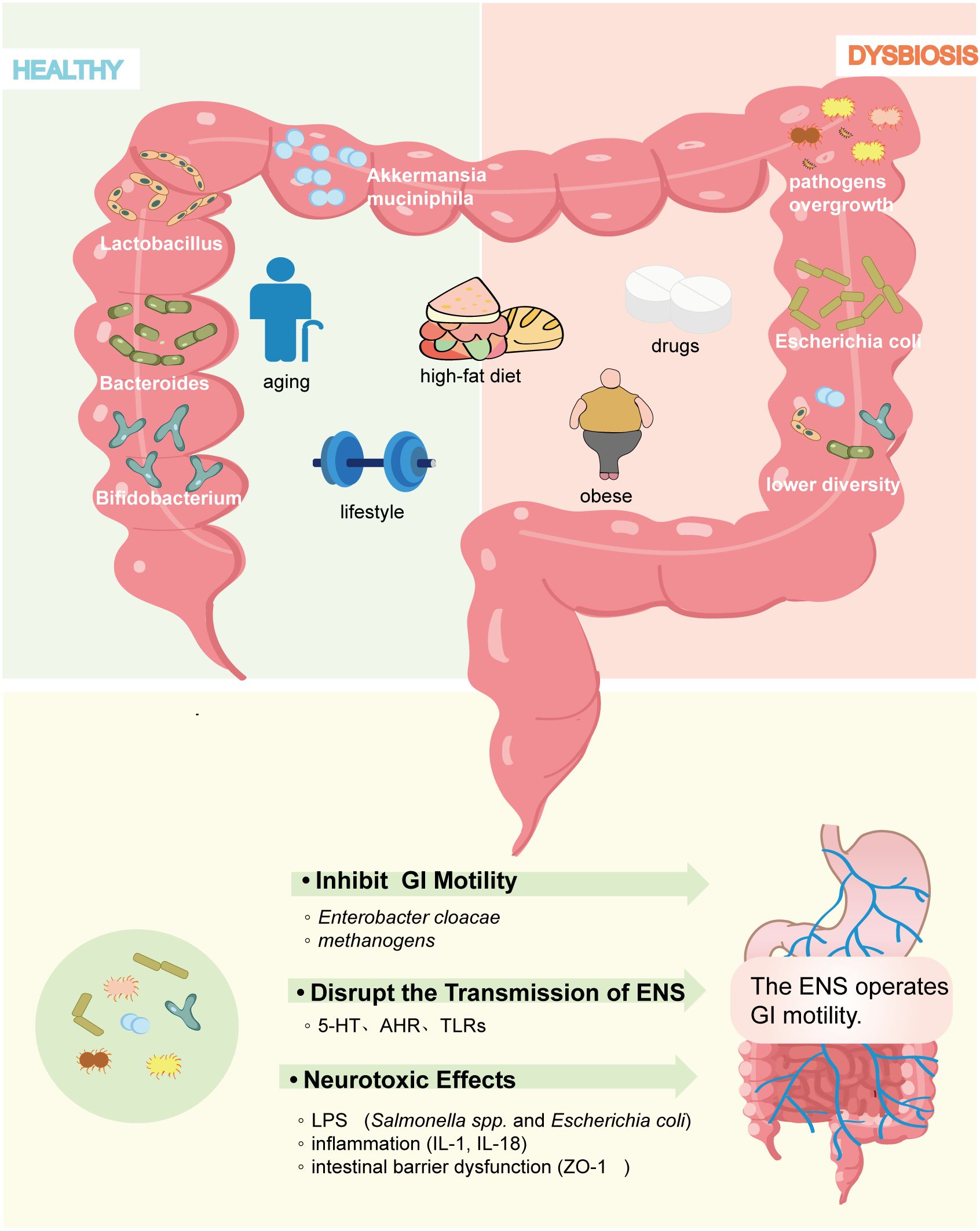
Figure 2. Factors influencing gut microbiome composition in diabetes models. Multiple variables, including age, dietary patterns, physical activity, hyperglycemia, obesity, and glucose-lowering agents, alter the abundance and composition of gut microbiota and contribute to diabetic gastrointestinal dysfunction.
3.1 Inhibition of gastrointestinal motility
Studies indicate that GI motility can be directly impacted by the abundance of specific bacterial populations. For instance, an increase in certain specific microbiota has been found to be associated with reduced GI motility in patients with constipation, including potentially pathogenic bacteria such as methanogenic bacteria, Desulfovibrionaceae, Escherichia coli, and Staphylococcus aureus (Pan et al., 2022). A notable increase in the abundance of Enterobacter cloacae and methanogens is observed in patients with diabetes, which are the main sources of methane and hydrogen sulfide (Tian et al., 2022). It is reported that they are inversely related to colonic transit speed (Attaluri et al., 2010; Tang et al., 2018), and the mechanism involves the alteration of ion channel mechanisms. Clinical cohort studies (Feng and Li, 2022) have demonstrated that patients with diabetes have a 2.91-fold higher risk of developing small intestinal bacterial overgrowth (SIBO) compared to non-diabetic individuals. The delayed small intestinal transit time is closely associated with a positive lactulose breath test, also known as the methane-hydrogen test, further confirming that the overgrowth of hydrogen-producing and methanogenic bacteria can directly impede GI motility (Talamantes et al., 2024).
3.2 Disruption of the transmission of the ENS signaling
Research conducted by McVey Neufeld et al. (2015) revealed that germ-free mice exhibit lower excitability of intrinsic primary afferent neurons (IPANs) and mesenteric neurons in comparison to healthy controls. Although the precise mechanisms remain elusive, gut microbiota indirectly modulate the ENS signaling through multiple pathways, including interference with neurotransmitter biosynthesis and activation of aryl hydrocarbon receptors (AHR) and Toll-like receptors (TLRs). Serotonin (5-hydroxytryptamine, 5-HT), a critical neurotransmitter and paracrine signaling molecule, regulates diverse gastrointestinal functions by acting on neurons, smooth muscle cells, and immune cells (De Vadder et al., 2018). Beyond its production by enterochromaffin cells (ECCs), 5-HT is generated through tryptophan metabolism by Bacteroides, Lactococcus, and Klebsiella species, while its reuptake via the serotonin transporter (SERT) is inhibited by Escherichia coli (Esmaili et al., 2009). Although diminished 5-HT levels correlate with dysmotility in patients with diabetic gastroparesis, direct evidence linking microbiota dysbiosis to 5-HT reduction in this condition remains limited (Bharucha et al., 2019). Microbiota-derived 5-HT and its metabolites additionally serve as AHR agonists. Functioning as a ligand-dependent transcription factor, AHR bridges gut microbiota-ENS crosstalk by detecting microbial alterations that influence colonic motility development. Notably, both dysbiosis and 5-HT deficiency impair AHR-mediated ENS regulation (Obata et al., 2020). Additionally, TLRs recognize pathogen-associated molecular patterns and interfere with ENS signal transmission by detecting pathogen-associated molecular patterns (PAMPs) such as LPS and lipoproteins (Li and Wu, 2021). Anitha (Anitha et al., 2012), Yarandi (Yarandi et al., 2020), and their colleagues noticed that TLR2- and TLR4-deficient mice align with the mechanism responsible for gut dysmotility in germ-free mice, characterized by a diminished quantity of nitrergic inhibitory neurons. Marked decreases in glial cell line-derived neurotrophic factor (GDNF) were also observed in TLR2-deficient and microbiota-depleted mice, whereas supplementation with GDNF or TLR2 agonist restored the ENS function, indicating that both are prerequisites for ensuring the integrity of ENS (Brun et al., 2013).
3.3 Neurotoxic effects
Clinical studies (Gomes et al., 2017) indicate that serum lipopolysaccharide (LPS) levels in patients with T2DM are 1.66-fold higher than in healthy individuals. Hyperglycemia- and obesity-associated free fatty acid (FFA) and bile acid dysmetabolism selectively suppress SCFA-producing bacteria, including Bifidobacterium, Faecalibacterium prausnitzii, and Roseburia, while promoting opportunistic pathogens such as Clostridium and Streptococcus (Pinart et al., 2021). This dysbiosis compromises the synthesis of 5-HT and promotes endotoxemia. Furthermore, hyperglycemia induces GLUT2-dependent tight junction impairment, increasing intestinal permeability and establishing leaky gut (Di Vincenzo et al., 2024). This compromised barrier facilitates translocation of Escherichia coli and Salmonella, driving endotoxemia and low-grade chronic inflammation. Although low-dose LPS is essential for the survival of the ENS, chronic exposure to high-dose LPS triggers neurotoxicity and impairs viability through the activation of the TLR4/nuclear factor kappa-B (NF-κB) pathway (Anitha et al., 2012). Despite insufficient data demonstrating a direct correlation between the impaired ENS and higher LPS levels in diabetes models, studies on LPS-induced endotoxemia indicate that high doses of LPS lead to delayed gastric emptying and weakened contractile activity in the cecum and colon (De Winter and De Man, 2010). Beyond that, LPS activates NOD-like receptor thermal protein domain-associated protein 3 (NLRP3) inflammasomes in intestinal epithelial cells and stimulates dendritic cells and macrophages, leading to the release of various inflammatory factors, including interleukin-1β (IL-1β), IL-18, and TNF-α (Han et al., 2021; Chen et al., 2023). In mouse models, IL-1β (Lefèvre et al., 2023) and macrophage-derived (especially CD45+/CD11b+/F4/80+) inflammatory factors (Cipriani et al., 2018) have shown strong correlations with prolonged colonic transit and delayed gastric emptying. It also alters the structure and signaling of the ENS by inducing myenteric plexitis, neuronal hyperplasia, and neuropeptide dysregulation (Bubeck et al., 2023). Furthermore, LPS may downregulate ZO-1, leading to intestinal barrier impairment and exacerbating inflammatory responses (Wang et al., 2022c). Zogg et al. (2023). demonstrated that GI dysfunction can be ameliorated by restoring intestinal barrier integrity and mitigating inflammation-induced neurotoxicity. This research provides compelling evidence for the link between intestinal barrier impairment and gastrointestinal dysfunction. Collectively, these alterations disrupt GI motility via impaired ENS electrophysiology, neurotoxic damage, and dysregulated contractility. Nevertheless, future investigations must establish direct evidence linking specific microbial disruptions to ENS pathology in DGE models or clinical cohorts.
4 The gut microbiome-ENS axis in diabetic gastroenteropathy
Given the emerging evidence linking alterations in gut microbiota with ENS impairment, further exploration of these interactions could unveil novel strategies for mitigating the impact of diabetes on digestive health. In this section, we will focus on gut metabolites, as well as gut hormones and neurotransmitters regulated by the gut microbiota, elucidate their mechanisms of action in communicating with the ENS to influence diabetic gastrointestinal motility, while discussing the limitations of current research and potential future breakthroughs based on existing evidence. Given that distinct microbial derivatives act through unique molecular targets and pathways to regulate the enteric nervous system (ENS), this review adopts a categorical approach to elucidate the mechanisms underlying their crosstalk more clearly within the gut microbiota-ENS axis (Figure 3).
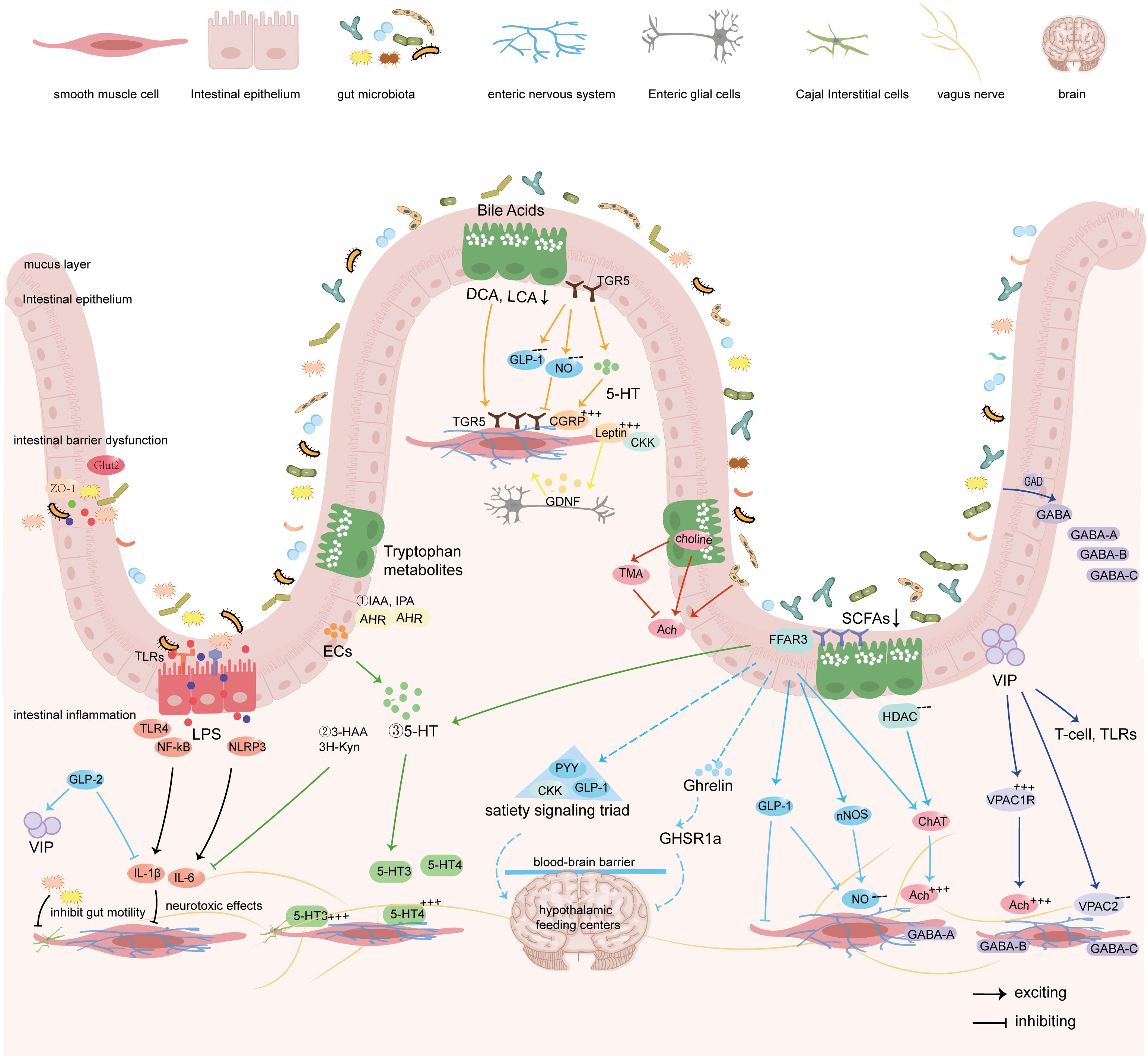
Figure 3. Gut microbiota regulates the sensation and motility of the gastrointestinal tract by modulating the ENS via its metabolites, gut hormones, and synthesized neurotransmitters.
4.1 Microbiota-metabolites-ENS interactions
SCFAs, bile acids, and tryptophan along with its metabolites are primary metabolic products of the gut microbiota. In addition to providing energy to the host, these metabolites are essential for regulating GI physiological functions. This section provides an update on how these metabolites influence the ENS through complex signaling networks.
4.1.1 Short-chain fatty acids
Short-chain fatty acids (SCFAs), the primary end products of anaerobic bacterial fermentation of dietary fibers in the mammalian colon, can reach concentrations of 50–200 mM, with acetate (C2), propionate (C3), and butyrate (C4) accounting for approximately 95% of total SCFAs (Louis and Flint, 2017). Bacteroidetes and Firmicutes serve as predominant producers of SCFAs: Bacteroidetes primarily generates acetate and propionate, whereas Firmicutes predominantly produces butyrate. Elevated butyrate associates with improved islet function, while increased propionate correlates with higher risk of type 2 diabetes (Sanna et al., 2019). Nevertheless, both butyrate and propionate modulate the ENS through three principal mechanisms:
Firstly, neurotransmitter modulation: SCFAs balance excitatory and inhibitory signals in the ENS by modulating certain neurotransmitters. As a potent histone deacetylase (HDAC) inhibitor, butyrate significantly increases the proportion of choline acetyltransferase (ChAT)-immunoreactive myenteric neurons, thereby enhancing cholinergic-mediated colonic circular muscle contractility (Soret et al., 2010). Concurrently, SCFAs activate enterochromaffin cells (ECCs) to upregulate tryptophan hydroxylase 1 (TPH1) transcription—the rate-limiting enzyme for 5-HT biosynthesis from tryptophan (Reigstad et al., 2015). 5-HT exerts its biological effects through specific receptors, including 5-HT1, 5-HT2, 5-HT3, 5-HT4, and 5-HT7, with SCFAs modulating both serotonin transporter (SERT) activity and receptor expression profiles (Buey et al., 2023). Notably, 5-HT4 receptor activation initiates cholinergic motor neuron-dependent circular muscle contraction. Critically, SCFAs activate free fatty acid receptor 3 (FFAR3/GPR41) on submucosal neurons, myenteric plexuses, and vagal ganglia, thereby remodeling GI motility reflexes through coordinated modification of nitrergic and cholinergic neurotransmission (Nøhr et al., 2015). Secondly, neuroprotective: SCFAs attenuate antibiotic-induced neuronal loss and regulate the survival of enteric neurons (Vicentini et al., 2021). Butyrate additionally improves GI motility and prevents ICC depletion in chronic constipation via AKT/NF-κB signaling (He et al., 2020). Thirdly, hormone regulation: Acting via vagal afferents or free fatty acid receptor 2/3 (FFAR2/FFAR3) activation on enteroendocrine cells, SCFAs stimulate secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), suppressing appetite and delaying gastric emptying (Goswami et al., 2018).
Notably, SCFAs exert concentration-dependent effects on GI motility. Dass et al. (2007) demonstrated that low concentrations (1 mmol/L) exert negligible effects, while moderate-to-high levels (10–100 mmol/L) dose-dependently reduce peristaltic pressure thresholds, shorten contraction intervals, and diminish wave amplitudes, collectively impairing propulsive activity. Supratherapeutic concentrations (300 mmol/L) will disrupt intrinsic motility rhythms. Furthermore, a physiological SCFA concentration gradient exists along the gut: high cecal concentrations (>115 mmol/L) inhibit intestinal peristalsis, whereas lower concentrations in the distal colon, terminal ileum, and ileum (<10 mmol/L) primarily suppress longitudinal muscle high-frequency contractions via the ENS, promoting intestinal content propulsion (Ono et al., 2004). In DGE, hyperglycemia-induced dysbiosis likely disrupts this physiological concentration gradient, impairing ENS rhythmicity and precipitating dysmotility. Future investigations must establish optimal SCFAs therapeutic windows while developing strategies to mitigate high-concentration toxicity. Crucially, direct evidence demonstrating the efficacy of exogenous SCFA supplementation in restoring ENS function under diabetic conditions remains lacking, necessitating rigorous translational models to evaluate SCFA-mediated neuromodulation in DGE pathogenesis.
4.1.2 Bile acid metabolism
Bile acids (BAs) serve as pivotal mediators in microbiota-host crosstalk, with their biotransformation from primary to secondary forms critically dependent on gut microbiota. Primary BAs are synthesized predominantly via hepatic 12α-hydroxylation pathways involving CYP7A1 and CYP8B1, while secondary BAs derive from non-12α-hydroxylated routes catalyzed by bacterial 7α-dehydroxylase. Given the absence of endogenous 7α-dehydroxylase in humans, conversion to secondary BAs depends on commensal bacteria, including Clostridium and Bacteroides, establishing gut microbiota as indispensable for BA metabolism (Jia et al., 2021). Secondary BAs, such as deoxycholic acid (DCA) and lithocholic acid (LCA), regulate GI motility by activating farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5). In diabetes, diminished abundance of Akkermansia, Bacteroides, Bifidobacterium, Faecalibacterium, and Roseburia reduces β-glucuronidase and 7α-dehydroxylase activities, thereby blocking the conversion of primary BAs to secondary BAs and consequently decreasing the synthesis of DCA and LCA (Li et al., 2021b). This microbiota-driven BAs dysmetabolism compromises intestinal barrier integrity and disrupts the ENS signaling via TGR5 receptors on ECCs and enteric neurons.
Notably, the widespread expression of TGR5 in enteric neurons, ECCs, and enteroendocrine cells (EECs) results in regional effects of BAs on GI motility. Studies (Ferrell and Chiang, 2019) have shown that intragastric administration of BAs impedes gastric emptying and small intestine transit through TGR5-mediated secretion of GLP-1. Bile acids also stimulate TGR5 on motor neurons, producing nitric oxide (NO) and inhibiting spontaneous contractions in the ileum (Poole et al., 2010). On the contrary, in the colon, DCA and taurocholic acid could activate TGR5 on ECs and enteric neurons to stimulate the release of TPH1 and 5-HT, thus enhancing colonic motility (Bunnett, 2014). The TGR5 receptor is a critical mediator of BAs in the GI tract. The deficiency of the TGR5 receptor results in constipation by delaying GI transit, reducing bowel movement frequency, and decreasing fecal water content (Zheng et al., 2022). Conversely, the overexpression of TGR5 results in diarrhea due to accelerating colonic transit in mice (Zhao et al., 2020a). Thus, given the region-specific effects of BAs and the concentration dependence of TGR5, future studies should further investigate how gut microbiota synergize with BAs and TGR5-mediated 5-HT release to regulate GI motility.
4.1.3 Tryptophan and its metabolites
Tryptophan, an essential amino acid, exhibits intimate associations with gut microbiota functions through its metabolic pathways, particularly the synthesis of 5-HT that plays a pivotal role in regulating the ENS (Agus et al., 2018). Approximately 90% of intestinal 5-HT originates from ECCs, with gut microbiota directly modulating its synthesis and release via ECC interactions. Commensals, including Lactococcus, Streptococcus, Escherichia coli, and Akkermansia muciniphila, activate TPH1 in ECCs through SCFAs, catalyzing the conversion of tryptophan to 5-HT (Reigstad et al., 2015). And reduced abundance of Bifidobacterium bifidum similarly downregulates TPH1 and SERT expression, impairing 5-HT synthesis (Taverniti et al., 2021).
Within ENS signaling, 5-HT coordinates GI motility through ascending/descending interneuronal activation and vago-vagal reflexes by targeting multiple receptor targets (Keating and Spencer, 2019). Prokinetic receptors 5-HT3 and 5-HT4, densely localized to myenteric plexuses and autonomic terminals, mediate distinct effects: 5-HT4 activation evokes acetylcholine release from cholinergic neurons, enhancing smooth muscle contraction (Pauwelyn and Lefebvre, 2017), whereas 5-HT3 sensitization of afferent nerves regulates motility rhythms and secretory (Touhara et al., 2025). Experimental evidence (Israelyan et al., 2019; Spencer and Keating, 2022) confirms that mice lacking the 5-HT3 receptor exhibit slowed colorectal motility and prolonged gastrointestinal transit time due to a reduction in the number of enteric neurons, particularly dopaminergic and GABAergic neurons, underscoring the 5-HT–ENS axis’s necessity for GI motility. Paradoxically, microbiota-driven 5-HT excess induces hypermotility via 5-HT3 overactivation, underscoring the delicate equilibrium between microbiota and 5-HT.
Clinically, 5-HT3 receptor antagonists (e.g., alosetron) (Savarino et al., 2022) and 5-HT4 receptor agonists (e.g., prucalopride) (Barbara et al., 2023) treat diarrhea-predominant and constipation-predominant irritable bowel syndrome, respectively, reflecting differential microbiota effects on 5-HT signaling. In DGE, however, dysbiosis-induced cholinergic neuronal damage necessitates multimodal neuromodulatory strategies beyond single-receptor targeting. Future research should prioritize interventions coordinating 5-HT-producing microbiota with complementary pathways (like SCFAs or BAs metabolism) to achieve comprehensive repairment of the ENS.
4.2 Microbiota-hormones-ENS crosstalk
The GI tract functions as a significant endocrine organ, primarily due to specialized epithelial cells known as EECs. EECs, located in the mucosal lining from the stomach to the rectum, secrete over 20 hormones, including GLP-1, PYY, and cholecystokinin (CCK) (Adriaenssens et al., 2018). These hormones coordinate nutrient absorption, GI motility, and metabolic homeostasis through a dual mechanism: paracrine (acting on enteric neurons) and neuroendocrine (via vagal transmission) (Bany Bakar et al., 2023). Meanwhile, the release of gut hormones by EECs is influenced by gut microbiota and its metabolites, including SCFAs, bile acids, indoles, which changes GI homeostasis (Masse and Lu, 2023). In this section, we review several key hormones that play central roles in regulating the ENS, with a particular focus on GLP-1, CCK, ghrelin, and leptin.
4.2.1 GLP-1 and GLP-2
As core components of the incretin system, glucagon-like peptide-1 (GLP-1) secreted by intestinal L-cells not only regulates glucose metabolism through glucose-dependent insulin secretion enhancement and glucagon suppression but also induces weight loss via gastric emptying delay and appetite inhibition. These dual actions establish GLP-1 receptor agonists (GLP-1RAs) as cornerstone therapies for type 2 diabetes and obesity (Nogueiras et al., 2023). Both GLP-1 and its homolog GLP-2 concentration-dependently enhance enteric neuronal survival, synergistically preserving the integrity of the ENS structure. GLP-2 further inhibits NF-κB signaling to reduce pro-inflammatory cytokines, such as TNF-α and IL-6, prevents inflammation-induced submucosal ganglion neuron loss, and increases vasoactive intestinal peptide-positive (VIP+) neuron proportions, collectively repairing neuroinflammatory damage (Abdalqadir and Adeli, 2022). However, the weight-loss-associated gastric emptying delay induced by GLP-1 remains contentious regarding potential exacerbation of diabetic gastroparesis and perioperative dysmotility (Camilleri and Lupianez-Merly, 2024). A recent systematic review (Hiramoto et al., 2024) indicates GLP-1RAs delay solid-phase gastric emptying by approximately 36 minutes, yet without statistically significant effects on liquid-phase emptying. Grasset et al. (2017). further demonstrated GLP-1-associated upregulation of nitric oxide synthase (NOS) expression in enteric neurons and vagal pathways. NOS catalyzes nitric oxide—an inhibitory neurotransmitter critical for ENS function. Nonetheless, the evidence remains insufficient to suggest that GLP-1RAs exacerbate GI motility; clinical vigilance and further high-quality studies are warranted.
Gut microbiota orchestrates GLP-1 secretion through metabolite-mediated mechanisms: firstly, SCFAs promote GLP-1 release via dual pathways: one is to activate the cell signaling pathway by binding to the FFAR2/FFAR3 receptor, which is impaired in FFAR2-deficient mice and shows decreased GLP-1 levels and glucose tolerance abnormalities (Tolhurst et al., 2012); the other is to increase the number of jejunal L-cells by inhibiting HDAC. Secondly, BAs synergistically enhance GLP-1 synthesis through TGR5/FXR co-activation on L-cells (Brighton et al., 2015). In type 2 diabetes, dysbiosis reduces SCFA, secondary BAs, and indole production, impairing jejunal L/K-cell activity and diminishing GLP-1/GIP secretion. This ultimately attenuates cholinergic neuron and vagal afferent activation efficiency within the ENS (Lee et al., 2012). Germ-free murine models (Yang et al., 2017) confirm that microbiota deprivation increases GLP-1R+ cells and prolongs gastrointestinal transit, whereas fecal microbiota transplantation normalizes GLP-1 secretion and motility, underscoring microbial indispensability in sustaining the GLP-1-ENS signaling axis.
4.2.2 Cholecystokinin
CCK, a gut-brain peptide hormone predominantly secreted by duodenal and jejunal I-cells in response to lipids and proteins, is a key “satiety signaling triad” member alongside GLP-1 and PYY (Andermann and Lowell, 2017). CCK exhibits region-specific effects through its CCK1 receptor: In the gastric antrum and pylorus, CCK activates vago-vagal reflexes via CCK1 receptors, inhibiting antral contractions while enhancing pyloric sphincter tone to delay solid-phase gastric emptying, a mechanism validated by Lorenz and Goldman (Lorenz and Goldman, 1982). Direct antral smooth muscle hyperpolarization reduces action potential firing, further suppressing peristalsis. Within the gallbladder, CCK1 receptor stimulation induces smooth muscle contraction for bile expulsion. In the distal small intestine, CCK1 activation mobilizes intracellular calcium in smooth muscle cells and coordinates intersegmental peristalsis via vagal afferent fibers to facilitate chyme propulsion (Doong et al., 1998).
These CCK1-mediated effects remain unaltered by CCK2 receptor antagonists. Early CCK1 antagonists (e.g., loxiglumide) showed therapeutic potential for functional dyspepsia and gastroparesis (Chua et al., 1994), but clinical utility is limited by off-target effects, including abdominal pain and mood disturbances via action on anxiety-related brain regions and somatic nociceptive pathways (Li et al., 2024). Recent structural insights reveal that CCK1 receptor conformational plasticity enables selective Gs/Gi/Gq protein coupling, providing a molecular basis for developing gut-selective modulators (Zhang et al., 2021b). Gut microbiota regulates CCK secretion through interactions between metabolites and jejunal I-cells: SCFAs and bile acids enhance CCK synthesis by activating FFAR2 and TGR5 receptors on jejunal I-cells. Prebiotics such as inulin indirectly upregulate CCK expression by enriching SCFA-producing microbiota (Avirineni et al., 2022); however, whether SCFA-promoted CCK release in the stomach and intestines exacerbates GI motility disorders remains unclear.
4.2.3 Leptin
Leptin, an adipocyte-derived endocrine hormone, regulates GI motility through binding leptin receptors (Ob-R) widely distributed on gastric vagal afferents and EECs. Notably, although leptin excites submucosal and myenteric neurons, it does not directly stimulate GI muscular activity but instead potentiates CCK-mediated intestinal propulsion (Reichardt et al., 2011). Mechanistically, leptin induces synthesis of glial cell line-derived neurotrophic factor (GDNF) within the ENS, preserving myenteric cholinergic neuron activity via GDNF-mediated neuroprotection. This effect counteracts high-fat diet-induced ENS damage in Western diet obesity models (Baudry et al., 2012). Leptin-deficient mice exhibit significantly delayed intestinal transit and barrier dysfunction, confirming its essential role in maintaining ENS structural and functional integrity (Tsai et al., 2020).
Gut microbiota modulates this process through leptin sensitivity regulation: probiotics predominantly comprising Lactobacillus and Bifidobacterium species significantly reduce serum leptin levels by mitigating endotoxemia and improving adipocyte secretory profiles (López-Moreno et al., 2020). In diabetic gastroenteropathy, dysbiosis-induced leptin resistance may exacerbate cholinergic neuronal injury and hypomotility by impairing the GDNF-ENS axis. As a negative regulator of insulin sensitivity, microbiota-dependent leptin modulation offers novel therapeutic targets for ameliorating diabetes-associated gastrointestinal symptoms.
4.2.4 Ghrelin
Ghrelin, the sole orexigenic GI hormone, is produced by gastric X/A-like cells with secretion regulated by nutritional status, exhibiting fasting-induced elevation and postprandial suppression. As a pivotal modulator of ENS and metabolic homeostasis, ghrelin activates growth hormone secretagogue receptor 1a (GHSR1a) to exert dual regulatory effects (Rouault et al., 2020). Centrally, it stimulates neuropeptide Y (NPY) neurons and vagal afferents to coordinate feeding behavior and glucose metabolism (Nunez-Salces et al., 2021). Peripherally, ghrelin enhances cholinergic neuron density and activity in gastric myenteric plexuses and vagal pathways, augmenting contraction frequency and amplitude to accelerate solid-phase gastric emptying. This central-peripheral synergy underscores its therapeutic potential for DGE. Phase 2B study demonstrates that the ghrelin receptor agonist relamorelin shortens gastric emptying time in patients with DGE and alleviates vomiting via central antiemetic effects (Camilleri et al., 2017). The novel agonist HM01 increases myenteric cholinergic neurons by 50%, effectively ameliorating dysmotility associated with abdominal surgery or Parkinson’s disease (Yuan et al., 2023).
However, clinical translation is limited by metabolic sequelae: Ghrelin inhibits pancreatic β-cell KATP channels to reduce insulin secretion, potentially exacerbating postprandial glycemia—a critical concern in DGE. A primary underlying cause is the disruption of the ghrelin signaling axis by gut microbiota dysbiosis: in diabetic states, an elevated Firmicutes/Bacteroidetes ratio will induce ghrelin resistance (Ahmed et al., 2024), and specific bacterial groups (e.g., Clostridium, Ruminococcus) show positive correlations with ghrelin levels, whereas Bacteroides and Bifidobacterium show bidirectional relationships contingent on host metabolism (Leeuwendaal et al., 2021). Microbial metabolites also participate in regulating ghrelin levels through multiple mechanisms: LPS and the gaseous signaling molecule hydrogen sulfide (H2S) can interfere with intracellular ghrelin signaling (Slade et al., 2018); SCFAs act in a concentration-dependent manner, with low concentrations inhibiting ghrelin secretion by antagonizing GHSR1a, whereas excessive acetate activates the parasympathetic nervous system to promote its release. Therefore, future studies should focus on the precise regulation of the ghrelin-ENS pathway by specific functional microbiota, providing a basis for developing DGE treatment strategies that balance motility improvement and glycemic stability.
4.3 Microbiota-neurotransmitters-ENS pathways
Emerging evidence suggests that the gut microbiota plays a key role in neurological disorders by directly synthesizing neurotransmitters and regulating host neurotransmitter metabolism. It has been proved that gut microbiota possesses genes that encode neurotransmitter-synthesizing enzymes like glutamate decarboxylase and tryptophan hydroxylase (Banerjee et al., 2021; Otaru et al., 2021). Furthermore, microbial metabolites such as SCFAs could regulate neurotransmitter production in enteric neurons through epigenetic modifications (He et al., 2024b). Neurotransmitters such as acetylcholine (ACh), 5-HT, γ-aminobutyric acid (GABA), and nitric oxide (NO) are pivotal in regulating the ENS and GI motility. In this section, we systematically analyze the microbiome-neurotransmitter interaction network and explore its potential as a therapeutic target for DGE.
4.3.1 Acetylcholine
Acetylcholine (ACh), the primary excitatory neurotransmitter of ENS cholinergic neurons, is synthesized by choline acetyltransferase (ChAT) and modulates GI functions through muscarinic receptors (M2/M3). M3 receptors mediate smooth muscle contraction and glandular secretion, predominantly driving motility, while M2 receptors indirectly regulate tone via inhibitory G-protein pathways (Foong et al., 2015). Gut microbiota orchestrates ACh homeostasis through multifactorial mechanisms: specific strains (e.g., Lactobacillus plantarum Lsi) secrete ACh-like compounds, directly enhancing intestinal contractions (Fujita et al., 2024); conversely, trimethylamine (TMA)-producing Firmicutes and Proteobacteria metabolize dietary choline, depleting ACh synthesis substrates (Eslami et al., 2024). In diabetic mice models, this metabolic shift correlates with downregulated expression of ACh receptors, kinases, and substrate, potentially impairing ENS cholinergic signaling (Tanase et al., 2020; Xu et al., 2020).
Microbiota-driven ACh dysregulation is validated in inflammatory bowel disease (IBD) models: Dysbiosis-induced choline deficiency is reversed by cytidine diphosphate (CDP)-choline supplementation, which upregulates choline transporter (ChT1), acetylcholinesterase (AChE), and α7 nicotinic acetylcholine receptor (α7nAChR) expression to restore neurotransmission (Guo et al., 2023a). In primary dysmotility or diabetic autonomic neuropathy, nAChR autoantibodies block ganglionic transmission, exacerbating dysmotility (Vernino et al., 2000). Butyrate counteracts this by inhibiting HDAC to promote regulatory T-cell (Treg) differentiation, reducing autoantibody production, and indirectly preserving cholinergic signaling (He et al., 2024a). Furthermore, SCFAs activate FFAR2/FFAR3 receptors to directly release cecal ACh, further reinforcing excitatory drive within the ENS (Ballout et al., 2021). Collectively, these findings delineate the core role of the “microbiota-SCFA-ACh” axis in sustaining ENS cholinergic activity.
4.3.2 Vasoactive intestinal peptide
Vasoactive intestinal peptide (VIP), predominantly secreted by neurons in the myenteric and submucosal plexuses of the ENS, serves as a pivotal neurotransmitter coordinating gastrointestinal functions through dual VPAC1/VPAC2 receptor signaling. VPAC2 receptors enriched in gastrointestinal smooth muscle mediate VIP-induced relaxation of gastric fundus circular muscle (Robberecht et al., 1998). Conversely, VPAC1 receptors highly expressed in colonic mucosa regulate epithelial ion transport, mucus secretion, and barrier integrity, while their co-localization with ChAT on enteric neurons facilitates acetylcholine release to enhance longitudinal muscle contraction (Fung et al., 2014). Beyond neuromodulation, VIP maintains intestinal immune homeostasis by regulating T-cell responses and TLR signaling, demonstrating therapeutic potential in neuroimmune disorders like T1DM and irritable bowel syndrome (Iwasaki et al., 2019).
VIP is critical for preserving ENS function: VIP-deficient mice exhibit attenuated jejunal motility (Lelievre et al., 2007), and downregulated VIP expression correlates with severe constipation, indicating attenuated VIP signaling as a common pathological feature in dysmotility (Bai et al., 2023). Gut microbiota regulates VIP with striking strain specificity: VIP gene expression is significantly reduced in germ-free animal models, while colonization with Escherichia coli (but not Lactobacillus) restores its levels, suggesting specific microbial metabolites modulate VIP synthesis (Bai et al., 2023). Nevertheless, modulation of VIP activity by gut microbiota remains relatively limited. How gut dysbiosis in diabetes affects VIP activity remains unclear, as does whether this activity can be altered by regulating microbial species and abundance. Future studies should prioritize elucidating microbiota-VIP crosstalk in ENS regulation to advance microbiome-based therapeutics for GI motility disorders.
4.3.3 Nitric oxide
Nitric oxide (NO), a key inhibitory neurotransmitter, is synthesized by NOS via the catalysis of L-arginine. Among its three isoforms (endothelial eNOS, neuronal nNOS, and inducible iNOS), nNOS-positive neurons predominantly regulate smooth muscle tone via the NO-cGMP pathway, mediating relaxation of the lower esophageal sphincter, pyloric sphincter, and Oddi sphincter, as well as coordinating gastrointestinal peristaltic rhythms (Groneberg et al., 2016; Idrizaj et al., 2021). In patients with diabetic gastroparesis, typical features include apoptosis of nitrergic neurons, downregulated nNOS and NO levels, accompanied by disrupted connections between nNOS+ neurons and SIP syncytia, leading to rhythmic disturbances in the jejunum, ileum, and colon, which is closely linked to gut microbiota dysbiosis (Bódi et al., 2019; Sanders and Ward, 2019; Camilleri and Sanders, 2022). Gut microbiota influence NO-mediated ENS effects through both direct synthesis and indirect regulation: certain Lactobacillus strains (e.g., Lactobacillus lactis, Lactobacillus plantarum) can directly secrete NO to induce ileal smooth muscle relaxation (Yarullina et al., 2016). Further evidence from antibiotic intervention experiments (Yarandi et al., 2020) shows that ampicillin treatment, which reduces Gram-positive bacteria and enriches Gram-negative bacteria, significantly decreases colonic nNOS enzymatic activity and nitrergic neuron count in mice, impairing colonic motility; notably, microbial recovery after antibiotic withdrawal reverses this phenotype, highlighting the critical role of microbiota structural stability in maintaining the NO-ENS axis.
4.3.4 Gamma-aminobutyric acid
Gamma-aminobutyric acid (GABA), a dual-function neurotransmitter with both excitatory and inhibitory properties in the ENS, regulates gastrointestinal functions via ionotropic (GABA_A/C) and metabotropic (GABA_B) receptors, exhibiting segment-specific and receptor subtype-specific effects: in the stomach, GABA_A receptor activation mediates smooth muscle relaxation through NO release, while GABA_B receptors promote contraction by enhancing cholinergic signaling; in the duodenum, GABA_A receptors are involved in releasing ACh, and GABA_C receptors are associated with releasing NO (Tonini et al., 1989; Zizzo et al., 2007; Rotondo et al., 2010; Auteri et al., 2014, 2015). This regional specificity arises from differences in receptor distribution between ENS neurons and smooth muscle cells, which will significantly limit the clinical translation of effects targeting DGE. Gut microbiota represents an important source of GABA; genera such as Bacteroides, Lactobacillus, and Levilactobacillus brevis directly secrete GABA by encoding glutamate decarboxylase (GAD), a key enzyme in GABA synthesis (Banerjee et al., 2021; Otaru et al., 2021). In diabetic states, reduced abundance of these genera leads to weakened GABAergic signaling, impairing ENS regulation of intestinal motility and mucosal secretion (Strandwitz et al., 2019; Konstanti et al., 2024). However, the causal relationship between decreased GABA levels and gut dysbiosis in diabetes remains unclear: whether microbiota dysbiosis causes reduced GABA or hyperglycemia directly inhibits GAD activity requires further experimental verification.
5 Therapeutic strategies targeting the gut microbiome-ENS axis
Gut microbiota and their metabolites influence the ENS and GI function through multiple pathways and targets. Leveraging this advantage to develop systematic therapeutic approaches could represent a breakthrough in addressing DGE. At the 2011 annual meeting of the International Scientific Association for Probiotics and Prebiotics (ISAPP), experts reviewed the role of these microbial agents in neurogastroenterology, providing evidence for the interaction between the microbiome and the ENS (Saulnier et al., 2013). Probiotics, prebiotics, and synbiotics have been shown in extensive clinical studies (Markowiak and Śliżewska, 2017; Quigley, 2019; Mokkala et al., 2021; Wang et al., 2022a) to effectively treat gastrointestinal disorders, like functional dyspepsia, IBS, and chronic constipation, as well as metabolic diseases such as obesity, insulin resistance, and T2DM. Based on the “microbiome-ENS axis,” we therefore provide a summary of the preclinical and clinical data on the role of microbiota in regulating the ENS and improving GI motility.
5.1 Probiotics, prebiotics, and synbiotics
Probiotics, prebiotics, and synbiotics, as core interventions targeting the gut microbiota-ENS axis, exhibit tremendous potential in repairing neural damage and improving motility disorders in DGE by reshaping microbial structure and metabolic profiles (as detailed in Tables 1, 2). As live non-pathogenic microorganisms that confer beneficial effects when consumed in adequate amounts, probiotics primarily include genera such as Lactobacillus, Bifidobacterium, Bacillus, and Saccharomyces, which exist in forms like fermented foods and enteric-coated capsules (Liu et al., 2018). Their core mechanism involves regulating the composition of gut microbiota to increase concentrations of SCFAs and secondary BAs, thereby activating ECCs to release 5-HT, enhancing ACh secretion from cholinergic neurons, and optimizing ENS inhibitory neural signals via the neuronal nNOS pathway, ultimately promoting gastric emptying and regulating intestinal transit rhythms (Huang et al., 2023; Liu et al., 2023). Prebiotics, as indigestible dietary fibers that resist direct digestion by the host gastrointestinal tract but selectively promote the proliferation of beneficial bacteria, such as inulin, fructooligosaccharides (FOS), galactooligosaccharides (GOS), lactulose, and resistant starch (Hutkins et al., 2016), indirectly enhance the function of the ENS by enriching SCFA-producing microbiota while synergistically regulating gastrointestinal hormone release to accelerate GI motility (Lee et al., 2024). Synbiotics further amplify these effects through the synergistic action of probiotics and prebiotics; for example, stachyose combined with Latilactobacillus sakei repairs ICC networks by increasing 5-HT and substance P (SP) levels, inhibiting expression of VIP and NOS, with significantly greater efficacy in promoting GI peristalsis than single-agent interventions (Guo et al., 2023b).
Notably, alterations in specific microbiota abundances correlate with ENS restoration and improved GI motility. Studies have shown that increased abundance of Bifidobacterium, Bacteroides, Prevotella, and Akkermansia muciniphila, or decreased levels of Dubosiella, Erysipelatoclostridium, Alistipes, and Enterococcus faecalis, may directly contribute to the repairment of the ENS, inhibiting intestinal inflammation and enhancing neurotransmitter synthesis. Kang et al. (2021); Cheng et al., 2023; Lai et al., 2023; Wu et al., 2024; Zhou et al., 2024; Wang et al., 2025) demonstrated that Lactobacillales and Synergistales exhibit negative and positive correlations with colonic transit time, respectively; however, the regulation of the Firmicutes/Bacteroidetes ratio remains highly contentious. Hata et al. (2022) found that an increased Firmicutes/Bacteroidetes ratio following Bifidobacterium BBG9–1 intervention in patients with diabetic gastrointestinal dysfunction was associated with improved gastrointestinal symptoms, whereas Lee et al. (2024)) reported that reducing this ratio in patients with functional constipation enhanced intestinal peristalsis and shortened colonic transit time. This discrepancy may stem from differences in study populations, and both studies are limited by small sample sizes, making it difficult to exclude confounding effects of baseline microbiota composition, disease duration, and intervention duration. Therefore, larger-scale studies focusing on DGE populations are warranted to further validate the true association between this ratio and GI motility.
Furthermore, current research has significant limitations: mechanistically, although some animal models indicate that microbial therapies can regulate neurotransmitters such as 5-HT and ACh, as well as hormone levels like motilin (MTL), clinical studies show substantial heterogeneity. For example, randomized controlled trials (RCTs) on Bifidobacterium animalis subsp. lactis HN019 (referred to as B. animalis subsp. lactis HN019) show dose-dependent differences in its effects on colonic transit time: in a triple-blind trial by Waller et al (Waller et al., 2011), high-dose B. animalis subsp. lactis HN019 shortened colonic transit time by over 50% in patients with functional constipation (from 49 ± 30 hours to 21 ± 32 hours), while the low-dose group showed only about 30% reduction (from 60 ± 33 hours to 41 ± 39 hours). Similarly, Lai et al. (2023) observed increased defecation frequency and improved straining in chronic constipation patients receiving B. animalis subsp. lactis HN019 combined with Lacticaseibacillus rhamnosus HN001, though colonic transit time was not assessed. In contrast, a randomized double-blind trial by Ibarra et al. (2018) involving 228 participants found no statistically significant difference in colonic transit time. Both Waller’s and Ibarra’s studies used abdominal X-rays to calculate colonic transit time, ensuring the reliability of their results. In addition, studies in healthy populations (Russo et al., 2011; Tulk et al., 2013) found no evidence that prebiotics or synbiotics promote gastrointestinal peristalsis; some even reported delayed gastric emptying, contradicting findings in models of gastrointestinal dysfunction or diabetes (Lim et al., 2018; Ohtsu et al., 2021). These inconsistencies may arise from strain specificity, dosage variations, treatment duration, and baseline characteristics of participants. Notably, high-quality clinical evidence directly targeting DGE is scarce, with most studies conducted in models of chronic constipation, gastrointestinal motility disorders, or irritable bowel syndrome (IBS). To date, only two double-blind RCTs have demonstrated that Lactobacillus gasseri OLL2716 (Ohtsu et al., 2021) and Bifidobacterium bifidum G9-1 (Hata et al., 2022) improve delayed gastric emptying and GI symptoms in patients with DGE, but both had small sample sizes, posing significant challenges for clinical translation. Additionally, there is a lack of replicative studies on the same probiotics or prebiotics, as well as limited clinical application. Therefore, future research requires large-scale, multicenter, more replicative RCTs to further validate efficacy, safety, and stability.
At the clinical application level, microbial therapies still face multiple challenges (Suez et al., 2019). Firstly, bioavailability issues: probiotics may experience possible diminution or inactivation as they navigate the hostile milieu in the GI tract, marked by low pH stomach acid and diverse digesting enzymes. Additionally, probiotics exhibit insufficient colonization capacity on gastrointestinal mucosal surfaces, which is further influenced by host microbiota composition and intestinal transit rate. Currently, several innovative strategies are being investigated, including the use of biofilms and nanocoating for “nano armor” (Xu et al., 2022), single-cell technology-based “armored probiotics” (Zhao et al., 2024), targeted delivery systems (Li and Zhang, 2024), and encapsulating probiotics into microcapsules and microspheres using prilling or vibration techniques (D’Amico et al., 2024). These strategies aim to address key issues in the therapeutic use of probiotics by stabilizing probiotic activity, enhancing intestinal colonization, and improving bioavailability. Secondly, safety and long-term effects remain unclear: despite the relative safety of microbial therapies with rare adverse reactions, existing studies suffer from limitations like small sample sizes and short observation periods. Long-term supplementation of single strains may reduce native microbiota diversity and exacerbate intestinal dysbiosis (Elshaghabee et al., 2017). Immunocompromised patients using Lactobacillus or Bacillus probiotics may develop bacteremia or even septic shock due to sepsis (Corredor-Rengifo et al., 2024). A meta-analysis (Zeng et al., 2024) evaluating 4 RCTs suggested that probiotic supplementation improves glycemic control in T1DM patients without severe adverse events; however, the limited number of RCTs precludes definitive conclusions on the efficacy or safety of microbiome-based therapies, necessitating more RCTs for validation. Furthermore, current interventions predominantly rely on “one-size-fits-all” formulations, neglecting individual variability among patients with DGE. Future efforts should focus on developing personalized regimens integrating host metabolic status and gut microbiota characteristics.
5.2 Fecal microbiota transplantation
Fecal microbiota transplantation (FMT), which reestablishes microbial homeostasis by colonizing functional microbiota from healthy donors in the host gut, has demonstrated therapeutic potential in various gastrointestinal diseases (Ooijevaar et al., 2019). In Clostridioides difficile infection (CDI), FMT achieves a clinical symptom resolution rate of 92% (Hvas et al., 2019; Rokkas et al., 2019); in inflammatory bowel disease, systematic reviews report clinical remission rates of 47.5% for Crohn’s disease and 39.6% for ulcerative colitis (Lai et al., 2019); its long-term ameliorative effects on chronic constipation have also been validated, suggesting unique value in repairing intestinal motility disorders (Zhang et al., 2018). More importantly, FMT can modulate the ENS via the “microbiota-neuron” axis: for instance, FMT lowers serum 5-HT and GABA levels and increases dopaminergic neurotransmission to help children with autism who experience digestive issues such as diarrhea, constipation, dyspepsia, and abdominal pain (Li et al., 2021a); in a Parkinson’s mouse model, FMT from healthy donors also improves GI motility by preventing TLR4/TNF-α signaling and neuroinflammation (Sun et al., 2018). These findings support FMT as a potential intervention for neurogenic gastrointestinal motility disorders. In metabolic diseases, FMT regulates 15 key metabolites, including indole-3-propionic acid, reduces leptin levels, and improves insulin sensitivity in patients with T2DM (Zhang et al., 2020; Wu et al., 2022b), indirectly indicating its potential in DGE. However, FMT application in DGE lacks direct clinical evidence, with minimal research addressing core DGE pathologies. The mechanisms by which FMT directly restores the ENS via microbial metabolites also remain unelucidated.
As a special population, diabetic populations face unique risks and challenges in the application of FMT. First, amplified infection risks. Long-term hyperglycemia in diabetes causes immune dysfunction, potentially increasing infection risk with FMT (Campbell et al., 2023). Second, distinct adverse reactions. Beyond common FMT-related symptoms (abdominal pain, diarrhea, nausea) (Aroniadis et al., 2019), patients with diabetes may experience exacerbated intestinal bacterial translocation due to impaired intestinal barrier, triggering systemic inflammation, elevated C-reactive protein, or even bacteremia (Quera et al., 2014). Third, standardization barriers: Unoptimized protocols for stool processing, preservation, and delivery combined with diabetic-specific gut microenvironment alterations compromise donor microbiota engraftment efficiency. Fourth, therapeutic heterogeneity: Zhang et al., 2021a demonstrated that baseline gut microbiota diversity critically determines efficacy, with lower diversity correlating with superior outcomes. Several RCTs have shown that FMT from healthy donors can reverse insulin resistance in diabetes and even preserve islet function in new-onset T1DM (de Groot et al., 2021; Ng et al., 2022; Wu et al., 2022b). However, in mouse models, administration of Parabacteroides distasonis accelerated progression of diabetes, primarily due to aberrant immune cross-reactivity and reduced Foxp3+ CD4+ Treg cells. Thus, given these uncertainties, large-scale multicenter randomized controlled trials are imperative to confirm its long-term efficacy and safety (Zecheng et al., 2023). Addressing these core challenges is essential for translating FMT from theoretical promise to clinically effective therapy for diabetes and DGE.
6 Conclusion and perspectives
The dynamic regulatory network formed by gut microbiota and the ENS constitutes a central regulator of intestinal homeostasis, with its dysfunction playing a pivotal role in the pathogenesis and progression of DGE. This review systematically elucidates how the microbiota-ENS axis modulates the function of the ENS through multilevel signaling transduction involving microbial metabolites (SCFAs, BAs), intestinal hormones (GLP-1, CCK, leptin, ghrelin), and neurotransmitters (5-HT, Ach, VIP, NO, GABA), thereby regulating gastrointestinal motility. This mechanism provides a robust theoretical framework for microbiota-targeted therapies in DGE. Current evidence confirms that probiotics, prebiotics, and synbiotics can reshape the structure of gut microbiota and enhance neuroprotection, demonstrating potential to alleviate gastroparesis and improve motility in animal models and limited clinical trials. Although FMT shows advantages in microbiota reconstitution for refractory gastrointestinal diseases, direct evidence for its application in DGE remains insufficient; in particular, the specific association between donor microbiota composition and ENS restoration in patients with DGE requires clarification.
However, clinical translation of microbiome-based therapies still faces multiple challenges. Firstly, causal ambiguity: specific microbiota associated with GI motility in DGE remain unidentified, as most studies are limited to pan-microbial community analyses at the genus or species level. Notably, functional differences between strains of the same species are substantial, and causal validation of the “microbiota-metabolite-nerve” axis is lacking. Secondly, therapeutic heterogeneity: clinical trials exhibit marked heterogeneity, such as the efficacy of probiotics or prebiotics being influenced by the baseline microbiota of host, diabetic duration, and glycemic control, while the scarcity of large-scale, multicenter RCT data hinders the establishment of uniform treatment standards. Thirdly, immune dysfunction may amplify infection risks from probiotics, hyperglycemia-altered microenvironments may reduce the efficiency of microbial colonization, and long-term safety of prolonged interventions lacks longitudinal follow-up data. Fourthly, technical standardization: there is a lack of consensus on viable probiotic delivery, FMT donor screening, and outcome evaluation.
Future research could focus on interdisciplinary breakthroughs (Bober et al., 2018; Wang et al., 2022b): applying spatial transcriptomics and single-cell sequencing to map spatial localization of microbiota-ENS interactions, and decipher interaction sites between specific strains and ENS neuron subsets; engineering probiotics via synthetic biology to achieve precise regulation of neurotransmitters and targeted ENS; establishing a DGE microbiota typing system based on multi-omics integration (genomics, metabolomics, neuroelectrophysiology) to guide personalized interventions; exploring combined strategies of microbial therapies with hypoglycemic agents to balance glycemic control and gastrointestinal motility improvement.
Through refinement of mechanism, personalization of intervention strategies, and scaling of clinical evidence, microbiome research is expected to advance from laboratory to clinical practice, ultimately providing DGE patients with novel therapeutic regimens that integrate neural repair, metabolic regulation, safety, and tolerability, thereby tangibly improving their gastrointestinal function and quality of life.
Author contributions
WT: Conceptualization, Writing – original draft, Writing – review & editing. YY: Conceptualization, Investigation, Writing – review & editing. DT: Methodology, Software, Writing – review & editing. XH: Data curation, Formal analysis, Investigation, Writing – review & editing. JH: Formal analysis, Methodology, Visualization, Writing – review & editing. CL: Software, Writing – review & editing. RY: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the vital support project of the Regional Innovation and Development Joint Fund of the National Natural Science Foundation of China (U21A20411), the National Natural Science Foundation of China (82330078, NO.T2341009), and Hunan Provincial Natural Science Foundation Project for Innovative Research Groups (2024JJ1007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
DGE: Diabetic gastroenteropathy
GI: gastrointestinal
T2DM: type 2 diabetes mellitus
GSRS: Gastrointestinal Symptom Rating Scale
GCSI: Gastroparesis Cardinal Symptom Index
FDA: Food and Drug Administration
ENS: enteric nervous system
CNS: central nervous system
ICCs: interstitial cells of Cajal
ECCs: enterochromaffin cells
EGCs: enteric glial cells
EECs: enteroendocrine cells
IBS: irritable bowel syndrome
IBS-D: diarrhea-predominant
IBS-C: constipation-predominant
IBD: inflammatory bowel disease
SCFAs: short-chain fatty acids
FMT: fecal microbiota transplantation
AHR: aryl hydrocarbon receptors
TLRs: Toll-like receptors
GDNF: glial cell line-derived neurotrophic factor
LPS: lipopolysaccharides
NF-κB: nuclear factor kappa-B
HDAC: inhibiting histone deacetylase
TMA: trimethylamine
SERT: serotonin transporter
PYY: peptide YY
Kyn: kynurenine
DCA: deoxycholic acid
LCA: lithocholic acid
NO: nitric oxide
CGRP: calcitonin gene-related peptide
IBAT: ileal bile acid transporter
CCK: cholecystokinin
GIP: glucose-dependent insulinotropic polypeptide
TNF-α: tumor necrosis factor-α
GHSR1a: growth hormone secretagogue receptor 1a;Ach:acetylcholine
GABA: γ-aminobutyric acid
VIP: Vasoactive Intestinal Peptide
ChAT: choline acetyltransferase
NOS: nitric oxide synthase
nNOS: neuronal NOS
GAD: glutamate decarboxylase
SS: somatostatin
MTL: motilin
SP: substance P
FOS: fructooligosaccharides
GOS: galactooligosaccharides
WGTT: whole-gut transit time.
References
Abdalla, M. M. I. (2024). Enteric neuropathy in diabetes: Implications for gastrointestinal function. World J. Gastroenterol. 30, 2852–2865. doi: 10.3748/wjg.v30.i22.2852
Abdalqadir, N. and Adeli, K. (2022). GLP-1 and GLP-2 orchestrate intestine integrity, gut microbiota, and immune system crosstalk. Microorganisms 10, 2061. doi: 10.3390/microorganisms10102061
Adriaenssens, A. E., Reimann, F., and Gribble, F. M. (2018). Distribution and stimulus secretion coupling of enteroendocrine cells along the intestinal tract. Compr. Physiol. 8, 1603–1638. doi: 10.1002/cphy.c170047
Agus, A., Planchais, J., and Sokol, H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724. doi: 10.1016/j.chom.2018.05.003
Ahmad, M. A., Karavetian, M., Moubareck, C. A., Wazz, G., Mahdy, T., and Venema, K. (2023). Association of the gut microbiota with clinical variables in obese and lean Emirati subjects. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1182460
Ahmed, K., Choi, H.-N., Cho, S.-R., and Yim, J.-E. (2024). Association of firmicutes/bacteroidetes ratio with body mass index in korean type 2 diabetes mellitus patients. Metabolites 14, 518. doi: 10.3390/metabo14100518
Aktar, R., Parkar, N., Stentz, R., Baumard, L., Parker, A., Goldson, A., et al. (2020). Human resident gut microbe Bacteroides thetaiotaomicron regulates colonic neuronal innervation and neurogenic function. Gut Microbes 11, 1745–1757. doi: 10.1080/19490976.2020.1766936
Andermann, M. L. and Lowell, B. B. (2017). Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778. doi: 10.1016/j.neuron.2017.06.014
Anitha, M., Vijay-Kumar, M., Sitaraman, S. V., Gewirtz, A. T., and Srinivasan, S. (2012). Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 143, 1006–1016.e4. doi: 10.1053/j.gastro.2012.06.034
Aroniadis, O. C., Brandt, L. J., Oneto, C., Feuerstadt, P., Sherman, A., Wolkoff, A. W., et al. (2019). Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 4, 675–685. doi: 10.1016/S2468-1253(19)30198-0
Attaluri, A., Jackson, M., Paulson, J., and Rao, S. S. (2010). Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am. J. Gastroenterol. 105, 10.1038/ajg.2009.655. doi: 10.1038/ajg.2009.655
Auteri, M., Zizzo, M. G., Mastropaolo, M., and Serio, R. (2014). Opposite role played by GABAA and GABAB receptors in the modulation of peristaltic activity in mouse distal colon. Eur. J. Pharmacol. 731, 93–99. doi: 10.1016/j.ejphar.2014.03.003
Auteri, M., Zizzo, M. G., and Serio, R. (2015). GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol. Res. 93, 11–21. doi: 10.1016/j.phrs.2014.12.001
Avirineni, B. S., Singh, A., Zapata, R. C., Phillips, C. D., and Chelikani, P. K. (2022). Dietary whey and egg proteins interact with inulin fiber to modulate energy balance and gut microbiota in obese rats. J. Nutr. Biochem. 99, 108860. doi: 10.1016/j.jnutbio.2021.108860
Bai, X., De Palma, G., Boschetti, E., Nishiharo, Y., Lu, J., Shimbori, C., et al. (2023). Vasoactive intestinal polypeptide plays a key role in the microbial-neuroimmune control of intestinal motility. Cell Mol. Gastroenterol. Hepatol. 17, 383–398. doi: 10.1016/j.jcmgh.2023.11.012
Ballout, J., Akiba, Y., Kaunitz, J. D., and Diener, M. (2021). Short-chain fatty acid receptors involved in epithelial acetylcholine release in rat caecum. Eur. J. Pharmacol. 906, 174292. doi: 10.1016/j.ejphar.2021.174292
Banerjee, S., Poore, M., Gerdes, S., Nedveck, D., Lauridsen, L., Kristensen, H. T., et al. (2021). Transcriptomics reveal different metabolic strategies for acid resistance and gamma-aminobutyric acid (GABA) production in select Levilactobacillus brevis strains. Microb. Cell Fact 20, 173. doi: 10.1186/s12934-021-01658-4
Bany Bakar, R., Reimann, F., and Gribble, F. M. (2023). The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat. Rev. Gastroenterol. Hepatol. 20, 784–796. doi: 10.1038/s41575-023-00830-y
Barbara, G., Cremon, C., Bellini, M., Corsetti, M., Di Nardo, G., Falangone, F., et al. (2023). Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig Liver Dis. 55, 187–207. doi: 10.1016/j.dld.2022.11.015
Baudry, C., Reichardt, F., Marchix, J., Bado, A., Schemann, M., des Varannes, S. B., et al. (2012). Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. J. Physiol. 590, 533–544. doi: 10.1113/jphysiol.2011.219717
Bharucha, A. E., Kudva, Y. C., and Prichard, D. O. (2019). Diabetic gastroparesis. Endocr. Rev. 40, 1318–1352. doi: 10.1210/er.2018-00161
Bober, J. R., Beisei, C. L., and Nair, N. U. (2018). Synthetic biology approaches to engineer probiotics and members of the human microbiota for biomedical applications. Annu. Rev. BioMed. Eng. 20, 277–300. doi: 10.1146/annurev-bioeng-062117-121019
Bódi, N., Szalai, Z., and Bagyánszki, M. (2019). Nitrergic enteric neurons in health and disease—Focus on animal models. Int. J. Mol. Sci. 20, 2003. doi: 10.3390/ijms20082003
Brighton, C. A., Rievaj, J., Kuhre, R. E., Glass, L. L., Schoonjans, K., Holst, J. J., et al. (2015). Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology 156, 3961–3970. doi: 10.1210/en.2015-1321
Brun, P., Giron, M. C., Qesari, M., Porzionato, A., Caputi, V., Zoppellaro, C., et al. (2013). Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 145, 1323–1333. doi: 10.1053/j.gastro.2013.08.047
Brun, P., Scarpa, M., Marchiori, C., Sarasin, G., Caputi, V., Porzionato, A., et al. (2017). Saccharomyces boulardii CNCM I-745 supplementation reduces gastrointestinal dysfunction in an animal model of IBS. PLoS One 12, e0181863. doi: 10.1371/journal.pone.0181863
Bubeck, M., Becker, C., and Patankar, J. V. (2023). Guardians of the gut: influence of the enteric nervous system on the intestinal epithelial barrier. Front. Med. (Lausanne) 10. doi: 10.3389/fmed.2023.1228938
Buey, B., Forcén, A., Grasa, L., Layunta, E., Mesonero, J. E., and Latorre, E. (2023). Gut microbiota-derived short-chain fatty acids: novel regulators of intestinal serotonin transporter. Life (Basel) 13, 1085. doi: 10.3390/life13051085
Bunnett, N. W. (2014). Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J. Physiol. 592, 2943–2950. doi: 10.1113/jphysiol.2014.271155
Camilleri, M. and Lupianez-Merly, C. (2024). Effects of GLP-1 and other gut hormone receptors on the gastrointestinal tract and implications in clinical practice. Am. J. Gastroenterol. 119, 1028–1037. doi: 10.14309/ajg.0000000000002519
Camilleri, M., McCallum, R. W., Tack, J., Spence, S. C., Gottesdiener, K., and Fiedorek, F. T. (2017). Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: A randomized, placebo-controlled study. Gastroenterology 153, 1240–1250.e2. doi: 10.1053/j.gastro.2017.07.035
Camilleri, M. and Sanders, K. M. (2022). Gastroparesis. Gastroenterology 162, 68–87.e1. doi: 10.1053/j.gastro.2021.10.028
Campaniello, D., Corbo, M. R., Sinigaglia, M., Speranza, B., Racioppo, A., Altieri, C., et al. (2022). How diet and physical activity modulate gut microbiota: evidence, and perspectives. Nutrients 14, 2456. doi: 10.3390/nu14122456
Campbell, C., Kandalgaonkar, M. R., Golonka, R. M., Yeoh, B. S., Vijay-Kumar, M., and Saha, P. (2023). Crosstalk between gut microbiota and host immunity: impact on inflammation and immunotherapy. Biomedicines 11, 294. doi: 10.3390/biomedicines11020294
Chandrasekharan, B., Saeedi, B. J., Alam, A., Houser, M., Srinivasan, S., Tansey, M., et al. (2019). Interactions between commensal bacteria and enteric neurons, via FPR1 induction of ROS, increase gastrointestinal motility in mice. Gastroenterology 157, 179–192.e2. doi: 10.1053/j.gastro.2019.03.045
Chen, X.-H., Liu, H.-Q., Nie, Q., Wang, H., and Xiang, T. (2023). Causal relationship between type 1 diabetes mellitus and six high-frequency infectious diseases: A two-sample mendelian randomization study. Front. Endocrinol. (Lausanne) 14. doi: 10.3389/fendo.2023.1135726
Cheng, S., Li, H., Ding, Y., Huo, J., Zheng, Y., Jiang, Y., et al. (2023). The Probiotic Combination of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 Alleviates Gastrointestinal Motility Disorder via Improving Gut Microbiota. Nutrients 15, 839. doi: 10.3390/nu15040839
Chua, A. S., Bekkering, M., Rovati, L. C., and Keeling, P. W. (1994). Clinical efficacy and prokinetic effect of the CCK-A antagonist loxiglumide in nonulcer dyspepsia. Ann. N Y Acad. Sci. 713, 451–453. doi: 10.1111/j.1749-6632.1994.tb44124.x
Cipriani, G., Gibbons, S. J., Miller, K. E., Yang, D. S., Terhaar, M. L., Eisenman, S. T., et al. (2018). Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 154, 2122–2136.e12. doi: 10.1053/j.gastro.2018.02.027
Concepción Zavaleta, M. J., Gonzáles Yovera, J. G., Moreno Marreros, D. M., Rafael Robles, L., del, P., Palomino Taype, K. R., et al. (2021). Diabetic gastroenteropathy: An underdiagnosed complication. World J. Diabetes 12, 794–809. doi: 10.4239/wjd.v12.i6.794
Corredor-Rengifo, D., Tello-Cajiao, M. E., García-Molina, F. A., Montero-Riascos, L. F., Segura-Cheng, J. D., Corredor-Rengifo, D., et al. (2024). Bacillus clausii bacteremia following probiotic use: A report of two cases. Cureus 16, e57853. doi: 10.7759/cureus.57853
D’Amico, V., Lopalco, A., Iacobazzi, R. M., Vacca, M., Siragusa, S., De Angelis, M., et al. (2024). Multistimuli responsive microcapsules produced by the prilling/vibration technique for targeted colonic delivery of probiotics. Int. J. Pharm. 658, 124223. doi: 10.1016/j.ijpharm.2024.124223
Darra, A., Singh, V., Jena, A., Popli, P., Nada, R., Gupta, P., et al. (2023). Hyperglycemia is associated with duodenal dysbiosis and altered duodenal microenvironment. Sci. Rep. 13, 11038. doi: 10.1038/s41598-023-37720-x
Dass, N. B., John, A. K., Bassil, A. K., Crumbley, C. W., Shehee, W. R., Maurio, F. P., et al. (2007). The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol. Motil. 19, 66–74. doi: 10.1111/j.1365-2982.2006.00853.x
de Groot, P., Nikolic, T., Pellegrini, S., Sordi, V., Imangaliyev, S., Rampanelli, E., et al. (2021). Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 70, 92–105. doi: 10.1136/gutjnl-2020-322630
de la Cuesta-Zuluaga, J., Mueller, N. T., Corrales-Agudelo, V., Velásquez-Mejía, E. P., Carmona, J. A., Abad, J. M., et al. (2017). Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 40, 54–62. doi: 10.2337/dc16-1324
Del Chierico, F., Rapini, N., Deodati, A., Matteoli, M. C., Cianfarani, S., and Putignani, L. (2022). Pathophysiology of type 1 diabetes and gut microbiota role. Int. J. Mol. Sci. 23, 14650. doi: 10.3390/ijms232314650
De Vadder, F., Grasset, E., Mannerås Holm, L., Karsenty, G., Macpherson, A. J., Olofsson, L. E., et al. (2018). Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. U. S. A. 115, 6458–6463. doi: 10.1073/pnas.1720017115
De Winter, B. Y. and De Man, J. G. (2010). Interplay between inflammation, immune system and neuronal pathways: Effect on gastrointestinal motility. World J. Gastroenterol. 16, 5523–5535. doi: 10.3748/wjg.v16.i44.5523
Di Vincenzo, F., Del Gaudio, A., Petito, V., Lopetuso, L. R., and Scaldaferri, F. (2024). Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern. Emerg. Med. 19, 275–293. doi: 10.1007/s11739-023-03374-w
Dixon, S. A., Mishra, S., Dietsche, K. B., Jain, S., Mabundo, L., Stagliano, M., et al. (2023). The effects of prebiotics on gastrointestinal side effects of metformin in youth: A pilot randomized control trial in youth-onset type 2 diabetes. Front. Endocrinol. (Lausanne) 14. doi: 10.3389/fendo.2023.1125187
Doong, M. L., Lu, C. C., Kau, M. M., Tsai, S. C., Chiao, Y. C., Chen, J. J., et al. (1998). Inhibition of gastric emptying and intestinal transit by amphetamine through a mechanism involving an increased secretion of CCK in male rats. Br. J. Pharmacol. 124, 1123–1130. doi: 10.1038/sj.bjp.0701937
Du, Y., Neng, Q., Li, Y., Kang, Y., Guo, L., Huang, X., et al. (2022). Gastrointestinal autonomic neuropathy exacerbates gut microbiota dysbiosis in adult patients with type 2 diabetes mellitus. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.804733
Du, Y. T., Rayner, C. K., Jones, K. L., Talley, N. J., and Horowitz, M. (2018). Gastrointestinal symptoms in diabetes: prevalence, assessment, pathogenesis, and management. Diabetes Care 41, 627–637. doi: 10.2337/dc17-1536
Elshaghabee, F. M. F., Rokana, N., Gulhane, R. D., Sharma, C., and Panwar, H. (2017). Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01490
Eslami, M., Alibabaei, F., Babaeizad, A., Banihashemian, S. Z., Mazandarani, M., Hoseini, A., et al. (2024). The importance of gut microbiota on choline metabolism in neurodegenerative diseases. Biomolecules 14, 1345. doi: 10.3390/biom14111345
Esmaili, A., Nazir, S. F., Borthakur, A., Yu, D., Turner, J. R., Saksena, S., et al. (2009). Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 137, 2074–2083. doi: 10.1053/j.gastro.2009.09.002
Fan, Q., Gao, Y., Zhou, Y., Wu, J., Wang, H., Dong, Y., et al. (2025). Weizmannia coagulans BC99 relieves constipation symptoms by regulating inflammatory, neurotransmitter, and lipid metabolic pathways: A randomized, double-blind, placebo-controlled trial. Foods 14, 654. doi: 10.3390/foods14040654
Feng, X. and Li, X.-Q. (2022). The prevalence of small intestinal bacterial overgrowth in diabetes mellitus: a systematic review and meta-analysis. Aging (Albany NY) 14, 975–988. doi: 10.18632/aging.203854
Ferrell, J. M. and Chiang, J. Y. L. (2019). Understanding bile acid signaling in diabetes: from pathophysiology to therapeutic targets. Diabetes Metab. J. 43, 257–272. doi: 10.4093/dmj.2019.0043
Fleming, M. A., Ehsan, L., Moore, S. R., and Levin, D. E. (2020). The enteric nervous system and its emerging role as a therapeutic target. Gastroenterol. Res. Pract. 2020, 8024171. doi: 10.1155/2020/8024171
Foong, J. P. P., Hirst, C. S., Hao, M. M., McKeown, S. J., Boesmans, W., Young, H. M., et al. (2015). Changes in nicotinic neurotransmission during enteric nervous system development. J. Neurosci. 35, 7106–7115. doi: 10.1523/JNEUROSCI.4175-14.2015
Fujita, Y., Kosakamoto, H., and Obata, F. (2024). Microbiota-derived acetylcholine can promote gut motility in Drosophila melanogaster. Philos. Trans. R. Soc. B 379, 20230075. doi: 10.1098/rstb.2023.0075
Fung, C., Unterweger, P., Parry, L. J., Bornstein, J. C., and Foong, J. P. P. (2014). VPAC1 receptors regulate intestinal secretion and muscle contractility by activating cholinergic neurons in Guinea pig jejunum. Am. J. Physiol. Gastrointest Liver Physiol. 306, G748–G758. doi: 10.1152/ajpgi.00416.2013
Gershon, M. (1998). The Second Brain : The Scientific Basis of Gut Instinct and a Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestines. 1st (New York, NY: Harper).
Gomes, J. M. G., Costa, J., de, A., and R. de, C. G. (2017). Metabolic endotoxemia and diabetes mellitus: A systematic review. Metabolism 68, 133–144. doi: 10.1016/j.metabol.2016.12.009
Gomi, A., Yamaji, K., Watanabe, O., Yoshioka, M., Miyazaki, K., Iwama, Y., et al. (2018). Bifidobacterium bifidum YIT 10347 fermented milk exerts beneficial effects on gastrointestinal discomfort and symptoms in healthy adults: A double-blind, randomized, placebo-controlled study. J. Dairy Sci. 101, 4830–4841. doi: 10.3168/jds.2017-13803
Goswami, C., Iwasaki, Y., and Yada, T. (2018). Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J. Nutr. Biochem. 57, 130–135. doi: 10.1016/j.jnutbio.2018.03.009
Grasset, E., Puel, A., Charpentier, J., Collet, X., Christensen, J. E., Tercé, F., et al. (2017). A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab. 25, 1075–1090.e5. doi: 10.1016/j.cmet.2017.04.013
Groneberg, D., Voussen, B., and Friebe, A. (2016). Integrative control of gastrointestinal motility by nitric oxide. Curr. Med. Chem. 23, 2715–2735. doi: 10.2174/0929867323666160812150907
Guo, L., Chen, Q., Gao, Y., Jiang, H., Zhou, F., Zhang, F., et al. (2023a). CDP-choline modulates cholinergic signaling and gut microbiota to alleviate DSS-induced inflammatory bowel disease. Biochem. Pharmacol. 217, 115845. doi: 10.1016/j.bcp.2023.115845
Guo, Y., Song, L., Huang, Y., Li, X., Xiao, Y., Wang, Z., et al. (2023b). Latilactobacillus sakei Furu2019 and stachyose as probiotics, prebiotics, and synbiotics alleviate constipation in mice. Front. Nutr. 9. doi: 10.3389/fnut.2022.1039403
Gurley, B. J., Miousse, I. R., Nookaew, I., Ewing, L. E., Skinner, C. M., Jenjaroenpun, P., et al. (2019). Decaffeinated green tea extract does not elicit hepatotoxic effects and modulates the gut microbiome in lean B6C3F1 mice. Nutrients 11, 776. doi: 10.3390/nu11040776
Gurung, M., Li, Z., You, H., Rodrigues, R., Jump, D. B., Morgun, A., et al. (2020). Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51, 102590. doi: 10.1016/j.ebiom.2019.11.051
Han, C., Guo, N., Bu, Y., Peng, Y., Li, X., Ma, X., et al. (2021). Intestinal microbiota and antibiotic-associated acute gastrointestinal injury in sepsis mice. Aging (Albany NY) 13, 10099–10111. doi: 10.18632/aging.202768
Hata, S., Nakajima, H., Hashimoto, Y., Miyoshi, T., Hosomi, Y., Okamura, T., et al. (2022). Effects of probiotic Bifidobacterium bifidum G9-1 on the gastrointestinal symptoms of patients with type 2 diabetes mellitus treated with metformin: An open-label, single-arm, exploratory research trial. J. Diabetes Investig. 13, 489–500. doi: 10.1111/jdi.13698
He, Q., Han, C., Huang, L., Yang, H., Hu, J., Chen, H., et al. (2020). Astragaloside IV alleviates mouse slow transit constipation by modulating gut microbiota profile and promoting butyric acid generation. J. Cell Mol. Med. 24, 9349–9361. doi: 10.1111/jcmm.15586
He, Y., Wang, K., Su, N., Yuan, C., Zhang, N., Hu, X., et al. (2024b). Microbiota–gut–brain axis in health and neurological disease: Interactions between gut microbiota and the nervous system. J. Cell Mol. Med. 28, e70099. doi: 10.1111/jcmm.70099
He, L., Zhong, Z., Wen, S., Li, P., Jiang, Q., and Liu, F. (2024a). Gut microbiota-derived butyrate restores impaired regulatory T cells in patients with AChR myasthenia gravis via mTOR-mediated autophagy. Cell Commun. Signal 22, 215. doi: 10.1186/s12964-024-01588-9
Hiramoto, B., McCarty, T. R., Lodhia, N. A., Jenkins, A., Elnaiem, A., Muftah, M., et al. (2024). Quantified metrics of gastric emptying delay by glucagon-like peptide-1 agonists: A systematic review and meta-analysis with insights for periprocedural management. Am. J. Gastroenterol. 119, 1126–1140. doi: 10.14309/ajg.0000000000002820
Huaman, J.-W., Mego, M., Manichanh, C., Cañellas, N., Cañueto, D., Segurola, H., et al. (2018). Effects of prebiotics vs a diet low in FODMAPs in patients with functional gut disorders. Gastroenterology 155, 1004–1007. doi: 10.1053/j.gastro.2018.06.045
Huang, Y., Guo, Y., Li, X., Xiao, Y., Wang, Z., Song, L., et al. (2023). Effects of lactiplantibacillus plantarum GUANKE on diphenoxylate-induced slow transit constipation and gut microbiota in mice. Nutrients 15, 3741. doi: 10.3390/nu15173741
Huang, J., Song, Y., Cheng, S., and Yang, X. (2024). Mechanism of action of FoxiangSan in diabetic gastroparesis: Gut microbiota and cAMP/PKA pathway. Heliyon 10, e35558. doi: 10.1016/j.heliyon.2024.e35558
Hung, L. Y., Parathan, P., Boonma, P., Wu, Q., Wang, Y., Haag, A., et al. (2020). Antibiotic exposure postweaning disrupts the neurochemistry and function of enteric neurons mediating colonic motor activity. Am. J. Physiol. Gastrointest Liver Physiol. 318, G1042–G1053. doi: 10.1152/ajpgi.00088.2020
Hutkins, R. W., Krumbeck, J. A., Bindels, L. B., Cani, P. D., Fahey, G., Goh, Y. J., et al. (2016). Prebiotics: why definitions matter. Curr. Opin. Biotechnol. 37, 1–7. doi: 10.1016/j.copbio.2015.09.001
Hvas, C. L., Dahl Jørgensen, S. M., Jørgensen, S. P., Storgaard, M., Lemming, L., Hansen, M. M., et al. (2019). Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology 156, 1324–1332.e3. doi: 10.1053/j.gastro.2018.12.019
Ibarra, A., Latreille-Barbier, M., Donazzolo, Y., Pelletier, X., and Ouwehand, A. C. (2018). Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: A double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes 9, 236–251. doi: 10.1080/19490976.2017.1412908
Ibrahim, A., Ali, R. A. R., Manaf, M. R. A., Ahmad, N., Tajurruddin, F. W., Qin, W. Z., et al. (2020). Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS One 15, e0244680. doi: 10.1371/journal.pone.0244680
Idrizaj, E., Traini, C., Vannucchi, M. G., and Baccari, M. C. (2021). Nitric oxide: from gastric motility to gastric dysmotility. Int. J. Mol. Sci. 22, 9990. doi: 10.3390/ijms22189990
Israelyan, N., Del Colle, A., Li, Z., Park, Y., Xing, A., Jacobsen, J. P. R., et al. (2019). Effects of serotonin and slow-release 5-hydroxytryptophan on gastrointestinal motility in a mouse model of depression. Gastroenterology 157, 507–521.e4. doi: 10.1053/j.gastro.2019.04.022
Iwasaki, M., Akiba, Y., and Kaunitz, J. D. (2019). Recent advances in vasoactive intestinal peptide physiology and pathophysiology: focus on the gastrointestinal system. F1000Res 8, F1000 Faculty Rev–1629. doi: 10.12688/f1000research.18039.1
Jia, W., Wei, M., Rajani, C., and Zheng, X. (2021). Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell 12, 411–425. doi: 10.1007/s13238-020-00804-9
Kang, S., Park, M. Y., Brooks, I., Lee, J., Kim, S. H., Kim, J. Y., et al. (2021). Spore-forming Bacillus coagulans SNZ 1969 improved intestinal motility and constipation perception mediated by microbial alterations in healthy adults with mild intermittent constipation: A randomized controlled trial. Food Res. Int. 146, 110428. doi: 10.1016/j.foodres.2021.110428
Karačić, A., Renko, I., Krznarić, Ž., Klobučar, S., and Liberati Pršo, A.-M. (2024). The association between the firmicutes/bacteroidetes ratio and body mass among european population with the highest proportion of adults with obesity: an observational follow-up study from Croatia. Biomedicines 12, 2263. doi: 10.3390/biomedicines12102263
Keating, D. J. and Spencer, N. J. (2019). What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacol. Res. 140, 50–55. doi: 10.1016/j.phrs.2018.06.017
Koliada, A., Syzenko, G., Moseiko, V., Budovska, L., Puchkov, K., Perederiy, V., et al. (2017). Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 17, 120. doi: 10.1186/s12866-017-1027-1
Konstanti, P., Ligthart, K., Fryganas, C., Constantinos, P., Smidt, H., de Vos, W. M., et al. (2024). Physiology of γ-aminobutyric acid production by Akkermansia muciniphila. Appl. Environ. Microbiol. 90, e01121–e01123. doi: 10.1128/aem.01121-23
Krishnasamy, S. and Abell, T. L. (2018). Diabetic gastroparesis: principles and current trends in management. Diabetes Ther. 9, 1–42. doi: 10.1007/s13300-018-0454-9
Kulich, K. R., Madisch, A., Pacini, F., Piqué, J. M., Regula, J., Van Rensburg, C. J., et al. (2008). Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study. Health Qual Life Outcomes 6, 12. doi: 10.1186/1477-7525-6-12
Lai, H., Li, Y., He, Y., Chen, F., Mi, B., Li, J., et al. (2023). Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: a double-blinded randomized placebo trial. Gut Microbes 15, 2197837. doi: 10.1080/19490976.2023.2197837
Lai, C. Y., Sung, J., Cheng, F., Tang, W., Wong, S. H., Chan, P. K. S., et al. (2019). Systematic review with meta-analysis: review of donor features, procedures and outcomes in 168 clinical studies of faecal microbiota transplantation. Aliment Pharmacol. Ther. 49, 354–363. doi: 10.1111/apt.15116
Lee, J., Cummings, B. P., Martin, E., Sharp, J. W., Graham, J. L., Stanhope, K. L., et al. (2012). Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R657–R666. doi: 10.1152/ajpregu.00345.2011
Lee, J.-H., Kim, G.-B., Han, K., Jung, E.-J., Suh, H. J., and Jo, K. (2024). Efficacy and safety of galacto-oligosaccharide in the treatment of functional constipation: randomized clinical trial. Food Funct. 15, 6374–6382. doi: 10.1039/d4fo00999a
Leeuwendaal, N. K., Cryan, J. F., and Schellekens, H. (2021). Gut peptides and the microbiome: focus on ghrelin. Curr. Opin. Endocrinol. Diabetes Obes. 28, 243–252. doi: 10.1097/MED.0000000000000616
Lefèvre, C., Le Roy, C., Bessard, A., Le Berre-Scoul, C., Marchix, J., Coron, E., et al. (2023). Region-specific remodeling of the enteric nervous system and enteroendocrine cells in the colon of spinal cord injury patients. Sci. Rep. 13, 16902. doi: 10.1038/s41598-023-44057-y
Lelievre, V., Favrais, G., Abad, C., Adle-Biassette, H., Lu, Y., Germano, P. M., et al. (2007). Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung’s disease. Peptides 28, 1688–1699. doi: 10.1016/j.peptides.2007.05.006
Li, R., Andreu-Sánchez, S., Kuipers, F., and Fu, J. (2021b). Gut microbiome and bile acids in obesity-related diseases. Best Pract. Res. Clin. Endocrinol. Metab. 35, 101493. doi: 10.1016/j.beem.2021.101493
Li, N., Chen, H., Cheng, Y., Xu, F., Ruan, G., Ying, S., et al. (2021a). Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.759435
Li, B., Li, M., Luo, Y., Li, R., Li, W., and Liu, Z. (2022). Engineered 5-HT producing gut probiotic improves gastrointestinal motility and behavior disorder. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.1013952
Li, D. and Wu, M. (2021). Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 6, 291. doi: 10.1038/s41392-021-00687-0
Li, S. and Zhang, Y.-X. (2024). Sensitive delivery systems and novel encapsulation technologies for live biotherapeutic products and probiotics. Crit. Rev. Microbiol. 50, 371–384. doi: 10.1080/1040841X.2023.2202237
Li, J.-H., Zhao, S.-J., Guo, Y., Chen, F., Traub, R. J., Wei, F., et al. (2024). Chronic stress induces wide-spread hyperalgesia: The involvement of spinal CCK1 receptors. Neuropharmacology 258, 110067. doi: 10.1016/j.neuropharm.2024.110067
Lim, Y. J., Jamaluddin, R., Hazizi, A. S., and Chieng, J. Y. (2018). Effects of synbiotics among constipated adults in serdang, selangor, Malaysia—A randomised, double-blind, placebo-controlled trial. Nutrients 10, 824. doi: 10.3390/nu10070824
Lin, W.-J., Zheng, S., Yang, X.-Q., Lu, L.-H., Liu, W.-W., and Chen, C.-Y. (2024). Clinical efficacy observation of electroacupuncture based on the “ascending lucidity and descending turbidity” theory: a study of gut microbiota in patients with diabetic gastrointestinal dysfunction. Zhen Ci Yan Jiu 49, 1084–1091. doi: 10.13702/j.1000-0607.20230690
Liu, T.-H., Chen, G.-L., Lin, C.-H., Tsai, T.-Y., and Cheng, M.-C. (2025). Lactobacillus plantarum TWK10 relieves loperamide-induced constipation in rats fed a high-fat diet via modulating enteric neurotransmitters, short-chain fatty acids and gut microbiota. Food Funct. 16, 181–194. doi: 10.1039/d4fo02270j
Liu, Y., Tran, D. Q., and Rhoads, J. M. (2018). Probiotics in disease prevention and treatment. J. Clin. Pharmacol. 58, S164–S179. doi: 10.1002/jcph.1121
Liu, J., Wang, S., Yi, R., Long, X., Luo, G., Zhao, X., et al. (2023). LimosiLactobacillus pentosus isolated from mustard relieves drug-induced constipation in mice fed a high-fat diet by modulating enteric neurotransmitter function. Probiot Antimicro Prot. 15, 1371–1381. doi: 10.1007/s12602-022-09991-9
López-Moreno, A., Suárez, A., Avanzi, C., Monteoliva-Sánchez, M., and Aguilera, M. (2020). Probiotic strains and intervention total doses for modulating obesity-related microbiota dysbiosis: A systematic review and meta-analysis. Nutrients 12, 1921. doi: 10.3390/nu12071921
Lorenz, D. N. and Goldman, S. A. (1982). Vagal mediation of the cholecystokinin satiety effect in rats. Physiol. Behav. 29, 599–604. doi: 10.1016/0031-9384(82)90226-8
Louis, P. and Flint, H. J. (2017). Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41. doi: 10.1111/1462-2920.13589
Magne, F., Gotteland, M., Gauthier, L., Zazueta, A., Pesoa, S., Navarrete, P., et al. (2020). The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 12, 1474. doi: 10.3390/nu12051474
Markowiak, P. and Śliżewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9, 1021. doi: 10.3390/nu9091021
Masse, K. E. and Lu, V. B. (2023). Short-chain fatty acids, secondary bile acids and indoles: gut microbial metabolites with effects on enteroendocrine cell function and their potential as therapies for metabolic disease. Front. Endocrinol. (Lausanne) 14. doi: 10.3389/fendo.2023.1169624
Mayer, E. A., Ryu, H. J., and Bhatt, R. R. (2023). The neurobiology of irritable bowel syndrome. Mol. Psychiatry 28, 1451–1465. doi: 10.1038/s41380-023-01972-w
McVey Neufeld, K. A., Perez-Burgos, A., Mao, Y. K., Bienenstock, J., and Kunze, W. A. (2015). The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil. 27, 627–636. doi: 10.1111/nmo.12534
Mokkala, K., Paulin, N., Houttu, N., Koivuniemi, E., Pellonperä, O., Khan, S., et al. (2021). Metagenomics analysis of gut microbiota in response to diet intervention and gestational diabetes in overweight and obese women: a randomised, double-blind, placebo-controlled clinical trial. Gut 70, 309–318. doi: 10.1136/gutjnl-2020-321643
Moszak, M., Szulińska, M., and Bogdański, P. (2020). You are what you eat—The relationship between diet, microbiota, and metabolic disorders—A review. Nutrients 12, 1096. doi: 10.3390/nu12041096
Müller, M., Hermes, G. D. A., Emanuel E., C., Holst, J. J., Zoetendal, E. G., Smidt, H., et al. (2020). Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: a randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes 12, 1704141. doi: 10.1080/19490976.2019.1704141
Ng, S. C., Xu, Z., Mak, J. W. Y., Yang, K., Liu, Q., Zuo, T., et al. (2022). Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomised controlled trial. Gut 71, 716–723. doi: 10.1136/gutjnl-2020-323617
Niesler, B., Kuerten, S., Demir, I. E., and Schäfer, K.-H. (2021). Disorders of the enteric nervous system - a holistic view. Nat. Rev. Gastroenterol. Hepatol. 18, 393–410. doi: 10.1038/s41575-020-00385-2
Nogueiras, R., Nauck, M. A., and Tschöp, M. H. (2023). Gut hormone co-agonists for the treatment of obesity: from bench to bedside. Nat. Metab. 5, 933–944. doi: 10.1038/s42255-023-00812-z
Nøhr, M. K., Egerod, K. L., Christiansen, S. H., Gille, A., Offermanns, S., Schwartz, T. W., et al. (2015). Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 290, 126–137. doi: 10.1016/j.neuroscience.2015.01.040
Nunez-Salces, M., Li, H., Feinle-Bisset, C., Young, R. L., and Page, A. J. (2021). The regulation of gastric ghrelin secretion. Acta Physiol 231, e13588. doi: 10.1111/apha.13588
Obata, Y., Castaño, Á., Boeing, S., Bon-Frauches, A. C., Fung, C., Fallesen, T., et al. (2020). Neuronal programming by microbiota regulates intestinal physiology. Nature 578, 284–289. doi: 10.1038/s41586-020-1975-8
Ohtsu, T., Haruma, K., Ide, Y., and Takagi, A. (2021). The effect of continuous intake of lactobacillus gasseri OLL2716 on mild to moderate delayed gastric emptying: A randomized controlled study. Nutrients 13, 1852. doi: 10.3390/nu13061852
Ono, S., Karaki, S., and Kuwahara, A. (2004). Short-chain fatty acids decrease the frequency of spontaneous contractions of longitudinal muscle via enteric nerves in rat distal colon. Jpn J. Physiol. 54, 483–493. doi: 10.2170/jjphysiol.54.483
Ooijevaar, R. E., Terveer, E. M., Verspaget, H. W., Kuijper, E. J., and Keller, J. J. (2019). Clinical application and potential of fecal microbiota transplantation. Annu. Rev. Med. 70, 335–351. doi: 10.1146/annurev-med-111717-122956
Otaru, N., Ye, K., Mujezinovic, D., Berchtold, L., Constancias, F., Cornejo, F. A., et al. (2021). GABA production by human intestinal bacteroides spp.: prevalence, regulation, and role in acid stress tolerance. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.656895
Pan, R., Wang, L., Xu, X., Chen, Y., Wang, H., Wang, G., et al. (2022). Crosstalk between the gut microbiome and colonic motility in chronic constipation: potential mechanisms and microbiota modulation. Nutrients 14, 3704. doi: 10.3390/nu14183704
Pauwelyn, V. and Lefebvre, R. A. (2017). 5-HT4 receptors facilitate cholinergic neurotransmission throughout the murine gastrointestinal tract. Neurogastroenterol Motil. 29, e13064. doi: 10.1111/nmo.13064
Pawolski, V. and Schmidt, M. H. H. (2020). Neuron-glia interaction in the developing and adult enteric nervous system. Cells 10, 47. doi: 10.3390/cells10010047
Pearson, J. A., Ding, H., Hu, C., Peng, J., Galuppo, B., Wong, F. S., et al. (2022). IgM-associated gut bacteria in obesity and type 2 diabetes in C57BL/6 mice and humans. Diabetologia 65, 1398–1411. doi: 10.1007/s00125-022-05711-8
Pellegrini, S., Sordi, V., Bolla, A. M., Saita, D., Ferrarese, R., Canducci, F., et al. (2017). Duodenal mucosa of patients with type 1 diabetes shows distinctive inflammatory profile and microbiota. J. Clin. Endocrinol. Metab. 102, 1468–1477. doi: 10.1210/jc.2016-3222
Pinart, M., Dötsch, A., Schlicht, K., Laudes, M., Bouwman, J., Forslund, S. K., et al. (2021). Gut microbiome composition in obese and non-obese persons: A systematic review and meta-analysis. Nutrients 14, 12. doi: 10.3390/nu14010012
Poole, D. P., Godfrey, C., Cattaruzza, F., Cottrell, G. S., Kirkland, J. G., Pelayo, J. C., et al. (2010). Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 22814–825, e227–e228. doi: 10.1111/j.1365-2982.2010.01487.x
Quera, R., Espinoza, R., Estay, C., and Rivera, D. (2014). Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J. Crohns Colitis 8, 252–253. doi: 10.1016/j.crohns.2013.10.002
Quigley, E. M. M. (2019). Prebiotics and probiotics in digestive health. Clin. Gastroenterol. Hepatol. 17, 333–344. doi: 10.1016/j.cgh.2018.09.028
Reichardt, F., Krueger, D., and Schemann, M. (2011). Leptin excites enteric neurons of Guinea-pig submucous and myenteric plexus. Neurogastroenterol. Motil. 23, e165–e170. doi: 10.1111/j.1365-2982.2010.01665.x
Reigstad, C. S., Salmonson, C. E., Rainey, J. F., Szurszewski, J. H., Linden, D. R., Sonnenburg, J. L., et al. (2015). Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29, 1395–1403. doi: 10.1096/fj.14-259598
Revicki, D. A., Rentz, A. M., Dubois, D., Kahrilas, P., Stanghellini, V., Talley, N. J., et al. (2003). Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol. Ther. 18, 141–150. doi: 10.1046/j.1365-2036.2003.01612.x
Rinninella, E., Raoul, P., Cintoni, M., Franceschi, F., Miggiano, G. A. D., Gasbarrini, A., et al. (2019). What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7, 14. doi: 10.3390/microorganisms7010014
Robberecht, P., De Neef, P., and Lefebvre, R. A. (1998). Influence of selective VIP receptor agonists in the rat gastric fundus. Eur. J. Pharmacol. 359, 77–80. doi: 10.1016/s0014-2999(98)00662-1
Rokkas, T., Gisbert, J. P., Gasbarrini, A., Hold, G. L., Tilg, H., Malfertheiner, P., et al. (2019). A network meta-analysis of randomized controlled trials exploring the role of fecal microbiota transplantation in recurrent Clostridium difficile infection. United Eur. Gastroenterol. J. 7, 1051–1063. doi: 10.1177/2050640619854587
Rotondo, A., Serio, R., and Mulè, F. (2010). Functional evidence for different roles of GABAA and GABAB receptors in modulating mouse gastric tone. Neuropharmacology 58, 1033–1037. doi: 10.1016/j.neuropharm.2010.01.004
Rouault, A. A. J., Rosselli-Murai, L. K., Hernandez, C. C., Gimenez, L. E., Tall, G. G., and Sebag, J. A. (2020). The GPCR accessory protein MRAP2 regulates both biased signaling and constitutive activity of the ghrelin receptor GHSR1a. Sci. Signal 13, eaax4569. doi: 10.1126/scisignal.aax4569
Russo, F., Clemente, C., Linsalata, M., Chiloiro, M., Orlando, A., Marconi, E., et al. (2011). Effects of a diet with inulin-enriched pasta on gut peptides and gastric emptying rates in healthy young volunteers. Eur. J. Nutr. 50, 271–277. doi: 10.1007/s00394-010-0135-6
Sajdel-Sulkowska, E. M. (2023). The impact of maternal gut microbiota during pregnancy on fetal gut–brain axis development and life-long health outcomes. Microorganisms 11, 2199. doi: 10.3390/microorganisms11092199
Sanders, K. M. and Ward, S. M. (2019). Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 176, 212–227. doi: 10.1111/bph.14459
Sanna, S., van Zuydam, N. R., Mahajan, A., Kurilshikov, A., Vich Vila, A., Võsa, U., et al. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605. doi: 10.1038/s41588-019-0350-x
Saulnier, D. M., Ringel, Y., Heyman, M. B., Foster, J. A., Bercik, P., Shulman, R. J., et al. (2013). The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 4, 17–27. doi: 10.4161/gmic.22973
Savarino, E., Zingone, F., Barberio, B., Marasco, G., Akyuz, F., Akpinar, H., et al. (2022). Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 10, 556–584. doi: 10.1002/ueg2.12259
Schol, J., Wauters, L., Dickman, R., Drug, V., Mulak, A., Serra, J., et al. (2021). United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on gastroparesis. United Eur. Gastroenterol. J. 9, 287–306. doi: 10.1002/ueg2.12060
Sen, T., Cawthon, C. R., Ihde, B. T., Hajnal, A., DiLorenzo, P. M., de la Serre, C. B., et al. (2017). Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 173, 305–317. doi: 10.1016/j.physbeh.2017.02.027
Shan, X., Peng, C., Zou, H., Pan, Y., Wu, M., Xie, Q., et al. (2024). Association of vegetables-fruits dietary patterns with gestational diabetes mellitus: mediating effects of gut microbiota. Nutrients 16, 2300. doi: 10.3390/nu16142300
Sharkey, K. A. and Mawe, G. M. (2023). The enteric nervous system. Physiol. Rev. 103, 1487–1564. doi: 10.1152/physrev.00018.2022
Sikalidis, A. K. and Maykish, A. (2020). The gut microbiome and type 2 diabetes mellitus: discussing A complex relationship. Biomedicines 8, 8. doi: 10.3390/biomedicines8010008
Simon, E., Călinoiu, L. F., Mitrea, L., and Vodnar, D. C. (2021). Probiotics, prebiotics, and synbiotics: implications and beneficial effects against irritable bowel syndrome. Nutrients 13, 2112. doi: 10.3390/nu13062112
Slade, E., Williams, L., and Gagnon, J. (2018). Hydrogen sulfide suppresses ghrelin secretion in vitro and delays postprandial ghrelin secretion while reducing appetite in mice. Physiol. Rep. 6, e13870. doi: 10.14814/phy2.13870
So, D., Whelan, K., Rossi, M., Morrison, M., Holtmann, G., Kelly, J. T., et al. (2018). Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 107, 965–983. doi: 10.1093/ajcn/nqy041
Soret, R., Chevalier, J., De Coppet, P., Poupeau, G., Derkinderen, P., Segain, J. P., et al. (2010). Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 138, 1772–1782. doi: 10.1053/j.gastro.2010.01.053
Spencer, N. J. and Keating, D. J. (2022). Role of 5-HT in the enteric nervous system and enteroendocrine cells. Br. J. Pharmacol. 182, 471–483. doi: 10.1111/bph.15930
Strandwitz, P., Kim, K. H., Terekhova, D., Liu, J. K., Sharma, A., Levering, J., et al. (2019). GABA modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403. doi: 10.1038/s41564-018-0307-3
Suez, J., Zmora, N., Segal, E., and Elinav, E. (2019). The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729. doi: 10.1038/s41591-019-0439-x
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2021). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi: 10.1016/j.diabres.2021.109119
Sun, M.-F., Zhu, Y.-L., Zhou, Z.-L., Jia, X.-B., Xu, Y.-D., Yang, Q., et al. (2018). Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 70, 48–60. doi: 10.1016/j.bbi.2018.02.005
Tait, C. and Sayuk, G. S. (2021). The Brain-Gut-Microbiotal Axis: A framework for understanding functional GI illness and their therapeutic interventions. Eur. J. Intern. Med. 84, 1–9. doi: 10.1016/j.ejim.2020.12.023
Talamantes, S., Steiner, F., Spencer, S., Neshatian, L., and Sonu, I. (2024). Intestinal methanogen overgrowth (IMO) is associated with delayed small bowel and colonic transit time (TT) on the wireless motility capsule (WMC). Dig Dis. Sci. 69, 3361–3368. doi: 10.1007/s10620-024-08563-x
Tanase, D. M., Gosav, E. M., Neculae, E., Costea, C. F., Ciocoiu, M., Hurjui, L. L., et al. (2020). Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients 12, 3719. doi: 10.3390/nu12123719
Tang, Q., Quan, X., Yan, L., Ren, H., Chen, W., Xia, H., et al. (2018). Mechanism of sodium hydrosulfide modulation of L-type calcium channels in rat colonic smooth muscle cells. Eur. J. Pharmacol. 818, 356–363. doi: 10.1016/j.ejphar.2017.11.002
Tang, N., Yu, Q., Mei, C., Wang, J., Wang, L., Wang, G., et al. (2023). Bifidobacterium bifidum CCFM1163 alleviated cathartic colon by regulating the intestinal barrier and restoring enteric nerves. Nutrients 15, 1146. doi: 10.3390/nu15051146
Taverniti, V., Cesari, V., Gargari, G., Rossi, U., Biddau, C., Lecchi, C., et al. (2021). Probiotics modulate mouse gut microbiota and influence intestinal immune and serotonergic gene expression in a site-specific fashion. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.706135
Thaiss, C. A., Levy, M., Grosheva, I., Zheng, D., Soffer, E., Blacher, E., et al. (2018). Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359, 1376–1383. doi: 10.1126/science.aar3318
Tian, H., Ye, C., Yang, B., Cui, J., Zheng, Z., Wu, C., et al. (2022). Gut metagenome as a potential diagnostic and predictive biomarker in slow transit constipation. Front. Med. (Lausanne) 8. doi: 10.3389/fmed.2021.777961
Tolhurst, G., Heffron, H., Lam, Y. S., Parker, H. E., Habib, A. M., Diakogiannaki, E., et al. (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371. doi: 10.2337/db11-1019
Tonini, M., Crema, A., Frigo, G. M., Rizzi, C. A., Manzo, L., Candura, S. M., et al. (1989). An in vitro study of the relationship between GABA receptor function and propulsive motility in the distal colon of the rabbit. Br. J. Pharmacol. 98, 1109–1118. doi: 10.1111/j.1476-5381.1989.tb12654.x
Touhara, K. K., Rossen, N. D., Deng, F., Castro, J., Harrington, A. M., Chu, T., et al. (2025). Topological segregation of stress sensors along the gut crypt–villus axis. Nature 640, 732–742. doi: 10.1038/s41586-024-08581-9
Tsai, Y.-T., Ruan, J.-W., Chang, C.-S., Ko, M.-L., Chou, H.-C., Lin, C.-C., et al. (2020). Antrodia cinnamomea confers obesity resistance and restores intestinal barrier integrity in leptin-deficient obese mice. Nutrients 12, 726. doi: 10.3390/nu12030726
Tseng, P.-H., Chao, C.-C., Cheng, Y.-Y., Chen, C.-C., Yang, P.-H., Yang, W.-K., et al. (2022). Diabetic visceral neuropathy of gastroparesis: Gastric mucosal innervation and clinical significance. Eur. J. Neurol. 29, 2097–2108. doi: 10.1111/ene.15333
Tulk, H. M. F., Blonski, D. C., Murch, L. A., Duncan, A. M., and Wright, A. J. (2013). Daily consumption of a synbiotic yogurt decreases energy intake but does not improve gastrointestinal transit time: a double-blind, randomized, crossover study in healthy adults. Nutr. J. 12, 87. doi: 10.1186/1475-2891-12-87
Upadhyaya, S. and Banerjee, G. (2015). Type 2 diabetes and gut microbiome: at the intersection of known and unknown. Gut Microbes 6, 85–92. doi: 10.1080/19490976.2015.1024918
Uppaluri, S., Jain, M. A., Ali, H., Shingala, J., Amin, D., Ajwani, T., et al. (2024). Pathogenesis and management of diabetic gastroparesis: An updated clinically oriented review. Diabetes Metab. Syndrome: Clin. Res. Rev. 18, 102994. doi: 10.1016/j.dsx.2024.102994
Vernino, S., Low, P. A., Fealey, R. D., Stewart, J. D., Farrugia, G., and Lennon, V. A. (2000). Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl. J. Med. 343, 847–855. doi: 10.1056/NEJM200009213431204
Vicentini, F. A., Keenan, C. M., Wallace, L. E., Woods, C., Cavin, J.-B., Flockton, A. R., et al. (2021). Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 9, 210. doi: 10.1186/s40168-021-01165-z
Waller, P. A., Gopal, P. K., Leyer, G. J., Ouwehand, A. C., Reifer, C., Stewart, M. E., et al. (2011). Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 46, 1057–1064. doi: 10.3109/00365521.2011.584895
Wang, L., Cao, L., Yu, Q., Liang, M., Yang, Z., Wang, G., et al. (2025). Bifidobacterium bifidum CCFM1359 alleviates intestinal motility disorders through the BDNF-TrkB pathway. Food Funct. 16, 437–451. doi: 10.1039/d4fo03710c
Wang, L., Li, F., Gu, B., Qu, P., Liu, Q., Wang, J., et al. (2022b). Metaomics in clinical laboratory: potential driving force for innovative disease diagnosis. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.883734
Wang, Y., Lin, J., Cheng, Z., Wang, T., Chen, J., and Long, M. (2022c). Bacillus coagulans TL3 inhibits LPS-induced caecum damage in rat by regulating the TLR4/myD88/NF-κB and nrf2 signal pathways and modulating intestinal microflora. Oxid. Med. Cell Longev 2022, 5463290. doi: 10.1155/2022/5463290
Wang, L., Lv, W.-Q., Yang, J.-T., Lin, X., Liu, H.-M., Tan, H.-J., et al. (2023). Enteric nervous system damage caused by abnormal intestinal butyrate metabolism may lead to functional constipation. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1117905
Wang, C.-H., Yen, H.-R., Lu, W.-L., Ho, H.-H., Lin, W.-Y., Kuo, Y.-W., et al. (2022a). Adjuvant Probiotics of Lactobacillus salivarius subsp. salicinius AP-32, L. johnsonii MH-68, and Bifidobacterium animalis subsp. lactis CP-9 Attenuate Glycemic Levels and Inflammatory Cytokines in Patients With Type 1 Diabetes Mellitus. Front. Endocrinol. (Lausanne) 13. doi: 10.3389/fendo.2022.754401
Wu, S., Bhat, Z. F., Gounder, R. S., Mohamed Ahmed, I. A., Al-Juhaimi, F. Y., Ding, Y., et al. (2022a). Effect of dietary protein and processing on gut microbiota-A systematic review. Nutrients 14, 453. doi: 10.3390/nu14030453
Wu, Y., Li, S., Lv, L., Jiang, S., Xu, L., Chen, H., et al. (2024). Protective effect of Pediococcus pentosaceus Li05 on diarrhea-predominant irritable bowel syndrome in rats. Food Funct. 15, 3692–3708. doi: 10.1039/d3fo04904c
Wu, H., Tremaroli, V., Schmidt, C., Lundqvist, A., Olsson, L. M., Krämer, M., et al. (2020). The gut microbiota in prediabetes and diabetes: A population-based cross-sectional study. Cell Metab. 32, 379–390.e3. doi: 10.1016/j.cmet.2020.06.011
Wu, Z., Zhang, B., Chen, F., Xia, R., Zhu, D., Chen, B., et al. (2022b). Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: A randomized, controlled, prospective study. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.1089991
Xia, P., Liu, X., Hou, T., Zhan, F., Geng, F., Zhang, Z., et al. (2022). Evaluation of the effect of prebiotic sesame candies on loperamide-induced constipation in mice. Food Funct. 13, 5690–5700. doi: 10.1039/d2fo00067a
Xu, C., Ban, Q., Wang, W., Hou, J., and Jiang, Z. (2022). Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems. J. Control Release 349, 184–205. doi: 10.1016/j.jconrel.2022.06.061
Xu, Y., Cao, K., Guo, B., Xiang, J., Dong, Y.-T., Qi, X.-L., et al. (2020). Lowered levels of nicotinic acetylcholine receptors and elevated apoptosis in the hippocampus of brains from patients with type 2 diabetes mellitus and db/db mice. Aging (Albany NY) 12, 14205–14218. doi: 10.18632/aging.103435
Yang, M., Fukui, H., Eda, H., Xu, X., Kitayama, Y., Hara, K., et al. (2017). Involvement of gut microbiota in association between GLP-1/GLP-1 receptor expression and gastrointestinal motility. Am. J. Physiol. Gastrointest Liver Physiol. 312, G367–G373. doi: 10.1152/ajpgi.00232.2016
Yarandi, S. S., Kulkarni, S., Saha, M., Sylvia, K. E., Sears, C. L., and Pasricha, P. J. (2020). Intestinal bacteria maintain adult enteric nervous system and nitrergic neurons via toll-like receptor 2-induced neurogenesis in mice. Gastroenterology 159, 200–213.e8. doi: 10.1053/j.gastro.2020.03.050
Yarullina, D. R., Mikheeva, R. O., Sabirullina, G. I., Zelenikhin, P. V., Ilinskaya, O. N., and Sitdikova, G. F. (2016). Role of nitric oxide produced by lactobacilli in relaxation of intestinal smooth muscles. Bull. Exp. Biol. Med. 160, 343–346. doi: 10.1007/s10517-016-3166-z
Yuan, P.-Q., Wu, S. V., Wang, L., and Taché, Y. (2023). The ghrelin agonist, HM01 activates central vagal and enteric cholinergic neurons and reverses gastric inflammatory and ileus responses in rats. Neurogastroenterol Motil. 35, e14561. doi: 10.1111/nmo.14561
Zecheng, L., Donghai, L., Runchuan, G., Yuan, Q., Qi, J., Yijia, Z., et al. (2023). Fecal microbiota transplantation in obesity metabolism: A meta analysis and systematic review. Diabetes Res. Clin. Pract. 202, 110803. doi: 10.1016/j.diabres.2023.110803
Zeng, L., Yang, K., He, Q., Zhu, X., Long, Z., Wu, Y., et al. (2024). Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials. BMC Med. 22, 110. doi: 10.1186/s12916-024-03303-4
Zhang, L., Chu, J., Hao, W., Zhang, J., Li, H., Yang, C., et al. (2021a). Gut microbiota and type 2 diabetes mellitus: association, mechanism, and translational applications. Mediators Inflammation 2021, 5110276. doi: 10.1155/2021/5110276
Zhang, X., He, C., Wang, M., Zhou, Q., Yang, D., Zhu, Y., et al. (2021b). Structures of the human cholecystokinin receptors bound to agonists and antagonists. Nat. Chem. Biol. 17, 1230–1237. doi: 10.1038/s41589-021-00866-8
Zhang, X., Tian, H., Gu, L., Nie, Y., Ding, C., Ge, X., et al. (2018). Long-term follow-up of the effects of fecal microbiota transplantation in combination with soluble dietary fiber as a therapeutic regimen in slow transit constipation. Sci. China Life Sci. 61, 779–786. doi: 10.1007/s11427-017-9229-1
Zhang, L., Zhou, W., Zhan, L., Hou, S., Zhao, C., Bi, T., et al. (2020). Fecal microbiota transplantation alters the susceptibility of obese rats to type 2 diabetes mellitus. Aging (Albany NY) 12, 17480–17502. doi: 10.18632/aging.103756
Zhao, L., Yang, W., Chen, Y., Huang, F., Lu, L., Lin, C., et al. (2020a). A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J. Clin. Invest. 130, 438–450. doi: 10.1172/JCI130976
Zhao, R., Yu, T., Li, J., Niu, R., Liu, D., and Wang, W. (2024). Single-cell encapsulation systems for probiotic delivery: Armor probiotics. Adv. Colloid Interface Sci. 332, 103270. doi: 10.1016/j.cis.2024.103270
Zhao, X., Zhang, Y., Guo, R., Yu, W., Zhang, F., Wu, F., et al. (2020b). The alteration in composition and function of gut microbiome in patients with type 2 diabetes. J. Diabetes Res. 2020, 8842651. doi: 10.1155/2020/8842651
Zheng, Z., Tang, J., Hu, Y., and Zhang, W. (2022). Role of gut microbiota-derived signals in the regulation of gastrointestinal motility. Front. Med. (Lausanne) 9. doi: 10.3389/fmed.2022.961703
Zheng, X., Zhang, Y., Tan, Y., Li, Y., Xue, Q., Li, H., et al. (2024). Alpinia officinarum Hance extract ameliorates diabetic gastroparesis by regulating SCF/c-kit signaling pathway and rebalancing gut microbiota. Fitoterapia 172, 105730. doi: 10.1016/j.fitote.2023.105730
Zhou, L., Song, W., Liu, T., Yan, T., He, Z., He, W., et al. (2024). Multi-omics insights into anti-colitis benefits of the synbiotic and postbiotic derived from wheat bran arabinoxylan and Limosilactobacillus reuteri. Int. J. Biol. Macromol 278, 134860. doi: 10.1016/j.ijbiomac.2024.134860
Zhou, Z., Sun, B., Yu, D., and Zhu, C. (2022). Gut microbiota: an important player in type 2 diabetes mellitus. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.834485
Zhou, H., Zhao, X., Sun, L., Liu, Y., Lv, Y., Gang, X., et al. (2020). Gut microbiota profile in patients with type 1 diabetes based on 16S rRNA gene sequencing: A systematic review. Dis. Markers 2020, 3936247. doi: 10.1155/2020/3936247
Zizzo, M. G., Mulè, F., and Serio, R. (2007). Functional evidence for GABA as modulator of the contractility of the longitudinal muscle in mouse duodenum: role of GABA(A) and GABA(C) receptors. Neuropharmacology 52, 1685–1690. doi: 10.1016/j.neuropharm.2007.03.016
Keywords: diabetic gastroenteropathy, the enteric nervous system, gut microbiota, microbiota-ENS axis, microbial metabolites, gut hormones, neurotransmitters, microbiome-based therapies
Citation: Tao W, Yu Y, Tan D, Huang X, Huang J, Lin C and Yu R (2025) Microbiota and enteric nervous system crosstalk in diabetic gastroenteropathy: bridging mechanistic insights to microbiome-based therapies. Front. Cell. Infect. Microbiol. 15:1603442. doi: 10.3389/fcimb.2025.1603442
Received: 02 April 2025; Accepted: 21 July 2025;
Published: 11 August 2025.
Edited by:
Dinakaran Vasudevan, SKAN Research Trust, IndiaReviewed by:
Ieshita Pan, Saveetha University, IndiaZhaoxiang Wang, First People’s Hospital of Kunshan, China
Copyright © 2025 Tao, Yu, Tan, Huang, Huang, Lin and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Yu, eXVyb25nMTk2OTA1QDE2My5jb20=
†These authors have contributed equally to this work
 Wang Tao
Wang Tao Yunfeng Yu
Yunfeng Yu Danni Tan
Danni Tan Xiangning Huang
Xiangning Huang Jiawang Huang1
Jiawang Huang1 Rong Yu
Rong Yu