- Department of Obstetrics and Gynecology, The Second Norman Bethune Hospital of Jilin University, Changchun, Jilin, China
Vaginal microecology serves as a crucial defense mechanism in women’s reproductive health. It encompasses vaginal anatomy, microbial flora, endocrine regulation, and immune responses. Lactobacillus species dominate this ecosystem, maintaining a dynamic balance essential for vaginal health. Studies have highlighted a strong association between vaginal microecology, human papillomavirus (HPV) infection, and cervical lesions. A well-balanced vaginal microenvironment enhances mucosal barriers and immune function, aiding in HPV prevention and clearance. Conversely, disruptions in vaginal microecology compromise these defenses, increasing susceptibility to HPV infection. Persistent high-risk HPV (HR-HPV) infections are key contributors to cervical lesions and may further destabilize the vaginal microbiota(VMB). Additionally, cervical lesion progression is influenced by local immune responses, with HPV infection potentially accelerating disease development by suppressing cervical immunity. This review explores the intricate association between vaginal microecology, HPV infection, and cervical lesions, offering insights into early diagnosis, prevention, and treatment strategies.
1 Introduction
Cervical cancer (CC) is a common gynecological malignancy, ranking fourth among female cancer-related deaths worldwide (Pimple and Mishra, 2022). Latest data from the World Health Organization show that in 2022, approximately 660,000 new CC cases and 350,000 related deaths occurred globally (Organization W. H. Cervical cancer). China remains one of the countries with the highest CC incidence and mortality (Singh et al., 2023), with data from 2020 indicating approximately 110,000 new cases annually, accounting for 18.2% of global cases. In addition, approximately 59,000 women die from this disease every year, accounting for 17.3% of total global deaths (Zou et al., 2020), posing a substantial threat to women’s health. Unlike other malignancies, CC has a well-established etiology (Bornstein et al., 1995). Persistent infection with human papillomavirus (HPV), particularly high-risk HPV (HR-HPV), has been identified as the primary causative agent of CC and squamous intraepithelial lesions (SIL) (Rahangdale et al., 2022).
The mucosal immune system of the female genital tract, vaginal microbiota(VMB), and other host factors influence the persistence or clearance of HPV, thus concerning the risk of CC (Schellekens et al., 2025). In a healthy vaginal environment, microbial diversity is relatively low, with Lactobacillus being the predominant bacteria (Leon-Gomez and Romero, 2024). Lactobacilli maintain the vaginal microecological balance and produce lactic acid, hydrogen peroxide (H2O2), and bacteriocins, which effectively inhibit the overgrowth of pathogenic bacteria and strengthen the vaginal mucosal barrier. This protective mechanism reduces the likelihood of viral and bacterial infections and enhances local antimicrobial and anti-tumor defense capabilities (Xu et al., 2025). HPV infection disrupts the original acidic environment of the vagina, potentially triggering mucosal immune responses and genital inflammation, which, in turn, alters the VMB (Gardella et al., 2022). Vaginal microecological imbalance facilitates HPV adhesion, impairs cervical immune defenses, and promotes the invasion and colonization of pathogenic bacteria. This vicious cycle elevates vaginal pH and shifts the microbial community away from Lactobacillus dominance, leading to chronic inflammation, persistent HPV infection, and disease progression, finally increasing CC risk (Lin et al., 2022).
Despite the growing evidence of the association among vaginal microbiota, host immune response, and HPV infection, the underlying mechanism of their interaction remains elusive. Further investigation into the roles of immune regulation and vaginal microecology in HPV infection is essential for elucidating viral persistence and developing more effective prevention and therapeutic strategies. This review looks into the role of vaginal microecology in the development of HPV-related cervical diseases. It provides theoretical support for early diagnosis, future interventions, and microbiota-targeted prevention measures.
2 Vaginal microecology
2.1 Definition and composition
The female vagina is a dynamic yet relatively stable ecosystem encompassing the VMB, host endocrine system, vaginal anatomy, and local immune defenses (Qing et al., 2024). The vaginal microbiota constitutes the core of this microecosystem. As an open cavity, the vagina is colonized by various microorganisms, such as bacteria, fungi, and viruses (Ye and Qi, 2024). These microorganisms primarily reside in the vaginal mucosal epithelium and form biofilms through hierarchical and structured colonization. The microbial composition within these biofilms undergoes constant succession in response to physiological and environmental changes (Usyk et al., 2020). The vaginal flora forms a symbiotic relationship with the host, supporting normal physiological functions and ensuring reproductive health.
2.2 Functions of vaginal microecology
In women of reproductive age, the VMB is both vast and complex. It is estimated that the total bacterial load usually ranges from 10¹0 to 10¹¹. Among these, Lactobacillus is the predominant genus, accounting for over 70% of the total bacteria. Lactobacillus plays a crucial role in maintaining vaginal microbiota balance, curbing the reproduction of pathogenic microorganisms, strengthening local immune defenses, and providing anti-tumor protection (Shen et al., 2024). The key mechanisms by which these beneficial bacteria contribute to health include:
① Lactobacilli produce lactic acid to maintain vaginal acidity. They derive energy from the carbohydrates released by vaginal mucosal epithelial cells. Lactobacilli metabolize glycogen into lactic acid, which creates an acidic environment (pH < 4.5) that significantly inhibits the adhesion, colonization, and proliferation of pathogenic bacteria (Shen et al., 2024).② Lactobacilli secrete H2O2, bacteriocins, and other compounds with antibacterial efficacy. H2O2 increases cell membrane permeability by generating highly reactive hydroxyl radicals, thereby preventing the invasion of pathogens into cervical epithelial cells (Frąszczak et al., 2022). Additionally, Lactobacilli secrete bacteriocins and biological surfactants, which are antimicrobial peptides or proteins that disrupt epithelial cells and form a frontline defense against pathogen adhesion (Borgogna et al., 2020; Nieves-Ramírez et al., 2021). ③ Lactobacilli prevents pathogenic microorganisms from adhering to vaginal epithelium by competitively binding to mucosal epithelial receptors. Furthermore, it secretes peptidoglycans and extracellular polypeptidoglycans (EPS) to form a biofilm with physical barrier functions to reduce pathogen colonization (Kalia et al., 2020). Different Lactobacillus species secrete distinct protective substances (Wang et al., 2019a). For example, Lactobacillus crispatus (L. crispatus), a predominant vaginal species, produces adhesion factors that facilitate mucosal colonization and inhibit Gardnerella vaginalis adhesion. In contrast, Lactobacillus iners(L. iners) lacks this protective effect (Łaniewski et al., 2019). Women with L. iners-dominant microbiota experience a three to five times higher risk of HPV infection and a two to three times greater likelihood of HR-HPV progression, cervical dysplasia, or cancer than women with L. crispatus-dominant microbiota (Palma et al., 2018). This highlights the superior role of L. crispatus in vaginal defense. ④ Lactobacilli enhance immune function by activating T-cell proliferation and differentiation, ameliorating the immunological recognition and proliferation of B cells (Frąszczak et al., 2022). Lactic acid suppresses toll-like receptor agonists, lowering pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor (TNF), RANTES, IL-8, and macrophage inflammatory protein 3α (MIP3α). This anti-inflammatory effect helps shield genital tract epithelial cells from infections and damage (Hearps et al., 2017).
2.3 Classification of vaginal microbiota
Currently, most domestic and international scholars agree that the VMB can be classified into five community state types (CSTs), with dominant bacterial groups identified for each type (McClymont et al., 2022; Peremykina et al., 2024). L. crispatus, Lactobacillus gasseri(L. gasseri), L. iners, and Lactobacillus jensenii(L. jensenii) prevail in CSTs I, II, III, and V, respectively, whereas CST IV is characterized by increased microbial diversity, marked by reduced Lactobacillus abundance and a higher prevalence of anaerobic bacteria. CST IV is further subdivided into: CST IV-A (comprising the Anaerococcus, Peptoniphilus, Corynebacterium, Prevotella, Finegoldia, Streptococcus); CST IV-B (including the Atopobium, Fannyhessea, Gardnerella, Sneathia, Mobiluncus, Megasphaera); and CST IV-C, which features other diverse anaerobic species (France et al., 2020). Notably, differences in vaginal microenvironments across CSTs may directly affect HPV susceptibility and persistence.
3 Abnormal vaginal flora composition is associated with cervical HPV infection and cervical lesions
A balanced VMB plays a critical role in preventing infections of the female reproductive tract. Disruptions in microbiota composition are closely related to the development of cervical lesions (Žukienė et al., 2025). Collectively, vaginal dysbiosis may act as a cofactor for HPV infection. Investigating the interaction between VMB and HPV may enhance the understanding of HPV pathogenesis and facilitate the development of novel approaches for preventing cervical lesions. Common infections of the female urogenital tract include bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), aerobic vaginitis (AV), and sexually transmitted infections(STIs).
Table 1 summarizes the clinical research on VMB, HPV infection, and cervical lesions.
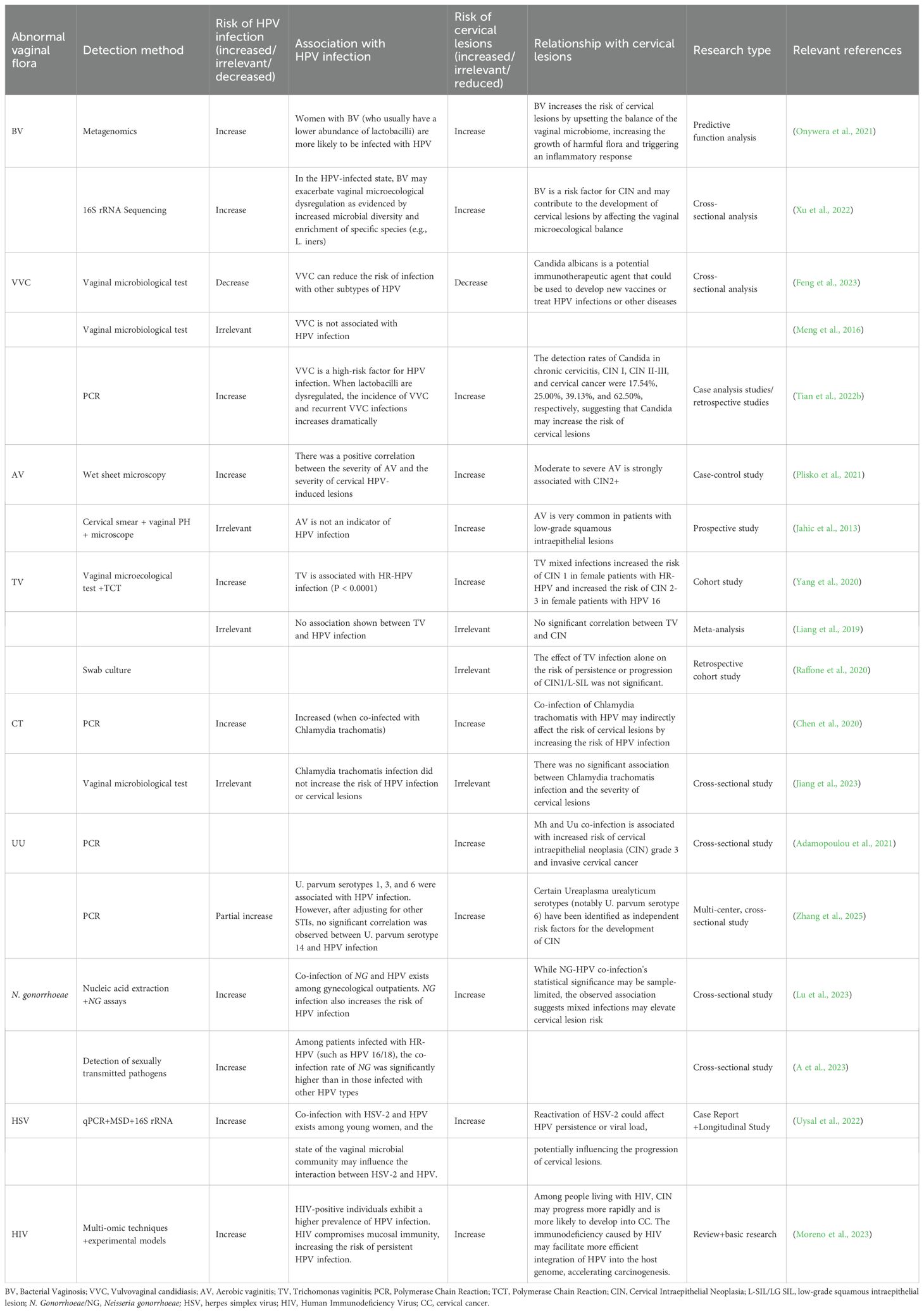
Table 1. Relationships between abnormal vaginal flora findings, HPV infection, and cervical lesions.
3.1 Association of BV with HPV infection and cervical lesions
Numerous studies have demonstrated a strong correlation between BV and HPV infection, with BV recognized as an independent risk factor for HPV acquisition and cervical lesions (Xu et al., 2022; Paul et al., 2023). CST III-B, IV-A, and IV-B are prevalent in patients with BV (Dong et al., 2024). BV is commonly caused by pathogens, including Gardnerella, Prevotella, Campylobacter, Bacteroides, Atopobium vaginae, and Sneathia. A meta-analysis encompassing six studies further confirmed the positive association between BV and cervical HPV infections (Martins et al., 2023). Similarly, another study used 16S rRNA gene sequencing to analyze the association between BV, HPV infections, and cervical lesions (Wei et al., 2020). HR-HPV-positive individuals exhibited decreased levels of Lactobacillus and elevated proportions of BV-associated bacteria, such as Gardnerella, Prevotella, Fusobacterium, Actinomyces, Peptococcus, Anaerococcus, Peptostreptococcus, Streptococcus, and Ureaplasma urealyticum. These results underscore a strong association between BV and HR-HPV infection. Dong et al. conducted a 2-year longitudinal study involving reproductive-aged women, demonstrating that BV-positive individuals showed significantly higher rates of persistent HR-HPV infection than BV-negative individuals (Dong et al., 2022). Through combined 16S rRNA sequencing and quantitative reverse transcription polymerase chain reaction analysis of vaginal secretions and cervical cells, vaginal Prevotella overgrowth was found to activate the NF-κB/C-Myc signaling pathway, facilitating HR-HPV persistence and cervical lesion progression. This effect may be further amplified by sialidase secretion. Microbial infection-induced NF-κB activation stimulates C-Myc expression, which in turn upregulates hTERT to drive malignant transformation (Papanikolaou et al., 2011; Ghareghomi et al., 2021). Lam et al. proposed that intratumoral microbiota may contribute to cervical carcinogenesis through immune modulation. They specifically suggested that Prevotella bivia(P. bivia) upregulate the human cancer driver lysosome-associated membrane protein 3 (LAMP3), which promotes metastasis and may help eliminate episomal HPV. This process can lead to overexpression of the E6 and E7 HPV oncogenes, thereby accelerating cervical disease progression (Lam et al., 2018).
BV may provide a biological rationale for HPV infection and invasion. However, Mao et al. identified a temporal sequence between HPV and BV infections, with HPV infection generally preceding BV. This may be attributed to the imbalance in the vaginal microenvironment caused by HPV infection, which increases the likelihood of BV (Mao et al., 2003). Therefore, the direct association between BV and cervical HPV infection, whether BV infection disrupts vaginal microecology and increases the prevalence of HPV infection and cervical lesions, whether HPV infection induces changes in the vaginal microecology that lead to BV infection, or whether these conditions are interdependent and promote simultaneous infections remains unclear. A substantial number of epidemiological and molecular studies are required to further explore the association between HPV infection and cervical lesions. Additionally, further research on the interaction between HPV infection and BV may facilitate the use of simple vaginal microecology tests, such as pH measurement, Gram staining for Nugent scoring, or molecular assays (e.g., quantitative PCR or 16S rRNA sequencing) targeting key bacteria (e.g., Lactobacillus spp., Gardnerella vaginalis, and Atopobium vaginae). These tests may help assess vaginal dysbiosis and predict HPV susceptibility. For instance, a low Lactobacillus dominance combined with a high anaerobic bacterial load may serve as a practical biomarker for increased HPV risk. Such approaches, if validated, might be integrated into routine gynecological screening to improve early detection and prevention strategies.
3.2 Association of VVC with HPV infection and cervical lesions
VVC is a common infectious disease of the lower genital tract caused by Candida albicans, which is a conditionally pathogenic fungus that causes disease only when the local immune capacity of the body or vagina declines (Sobel and Vempati, 2024).
The correlation between VVC, HPV infection, and cervical lesions remains controversial. Some researchers pose that VVC increases susceptibility to HPV and hinders HPV clearance (Wang et al., 2020b; Wu and Xue, 2020). This may result from pathogen-secreted proteolytic enzymes that activate the complement cascade, generating anaphylatoxins and chemokines. These factors cause local vasodilation, increased permeability, and an inflammatory response, finally inhibiting chemotaxis and the activation of neutrophils and lymphocytes (Ghosh et al., 2016). Additionally, VVC produces invasive enzymes that can damage genital epithelial cells, potentially facilitating HPV adhesion and persistence by creating a favorable microenvironment for viral replication (Wang et al., 2024). However, VVC does not raise the risk of HPV infection, and having both VVC and HPV does not lead to more severe cytological abnormalities (Wang et al., 2020b; Long et al., 2023). Furthermore, most women with VVC have a vaginal pH below 4.5; this acidic environment enhances vaginal defense by suppressing pathogen survival (Kwon and Lee, 2022). The low pH further bolsters immune responses by promoting the production of antimicrobial peptides (e.g., defensins) and lactic acid, which inhibit viral replication and maintain epithelial barrier integrity (Czechowicz et al., 2022). Consequently, VVC may confer a protective effect against persistent HPV infection, potentially reducing the risk of cervical intraepithelial lesions. Smalley et al. found that VVC may lower the risk of infection from non-16/18 HPV subtypes. Moreover, VVC functions as a possible booster for HPV vaccines because it may stimulate T-cell activity and improve immune function (Smalley Rumfield et al., 2020). This presents new avenues for vaccine and immunotherapy development. While numerous clinical studies have investigated the association between VVC and HPV infection/cervical lesions, substantial heterogeneity exists across study populations, including both general and high-risk groups. For instance, some studies enrolled balanced cohorts of premenopausal and postmenopausal women, whereas others specifically focused on HPV-vaccinated individuals. These demographic variations (e.g., age, immune status, and geographic distribution) may account for the inconsistent conclusions regarding the VVC-HPV association. Future investigations should utilize stratified analyses controlling for these covariates to elucidate potential confounding effects.
3.3 Association of AV with HPV infection and cervical lesions
In 2002, Donders et al. introduced the concept of AV based on its bacteriological, immunological, and clinical characteristics (Donders et al., 2002). Similar to BV, AV is characterized by a reduction in H2O2-producing Lactobacillus species or a decrease in Lactobacillus activity within the vaginal microenvironment. However, unlike BV, AV is associated with an overgrowth of aerobic bacteria, primarily Streptococcus, Staphylococcus, and Escherichia coli, which are compositionally aligned with CST IV (Zeng et al., 2023). Because of the relatively recent clinical recognition of AV, studies investigating its association with HPV infection and CC remain limited. Jahic et al. conducted a prospective study and reported that AV was significantly more prevalent in women with cervical intraepithelial lesions than in those with healthy cervical cytology (Jahic et al., 2013). Furthermore, AV treatment appeared to promote the regression of cervical precancerous lesions. The proposed mechanism suggests that AV disrupts vaginal microecology by reducing Lactobacillus populations, thereby increasing the vaginal pH. The loss of Lactobacillus, the dominant protective bacterium, weakens the defense against external pathogens, leading to leukocytosis and enhanced interstitial invasion of cervical tissue by inflammatory cells, particularly through leukocyte esterase activity (Donders, 2007; Wang et al., 2020a). Vieira-Baptista et al. reported that moderate-to-severe AV was independently associated with an increased risk of cervical cellular abnormalities, despite no direct correlation with cervical HPV infection (Vieira-Baptista et al., 2016). Considering the limited national and international research on AV and HPV, further large-scale studies are required to elucidate their association.
3.4 STIs
3.4.1 TV
TV is a lower genital tract infection caused by Trichomonas vaginalis, a prevalent sexually transmitted pathogen. The parasite secretes proteases, consumes or phagocytoses glycogen from vaginal epithelial cells, and inhibits lactic acid production, increasing the vaginal pH. Additionally, it consumes oxygen, creating an anaerobic environment that favors the proliferation of anaerobic bacteria (Li et al., 2022).
There are inconsistent findings about the association between TV and HPV infection and cervical lesions. Belfort et al. reported that TV is associated with an increased risk of HR-HPV infection, with TV-positive patients exhibiting a higher risk of HR-HPV infection than TV-negative patients (Belfort et al., 2021). This may be attributed to the depletion of Lactobacillus populations and subsequent reduction in lactic acid secretion in patients with TV, leading to vaginal microecological imbalances, increased inflammatory factor secretion, and reduced local cervical immunity (Mei et al., 2023). Yang et al. concluded that TV is significantly associated with HPV infection, proposing that flagellated protozoa attach to epithelial cells and induce toxic reactions, thereby increasing HPV infection risk. Moreover, TV induces a sustained inflammatory response in the cervix and vagina, damaging the cervical epithelium and accelerating the erosive effects of HPV on the cervix (Yang et al., 2020). However, Li et al. suggested that HPV infection may prevent TV infection (Li et al., 2022). HPV infection activates the immune response, triggering the release of immune cells and factors that provide localized immunity against TV. Additionally, Feng et al. examined 25,054 women and reported that although TV-positive women had a higher risk of HR-HPV infection, they exhibited a decreased risk of developing cervical intraepithelial neoplasia grade 2 or higher (CIN2+) (Feng et al., 2018). However, other studies report no strong association between TV and HPV. For example, Liang et al. found no association between these two infections (Liang et al., 2019). Similarly, Raffone et al. observed that TV infection alone did not significantly affect HPV rates (Raffone et al., 2020). These inconsistent findings may stem from differences in study populations and sample sizes. This necessitates large-scale clinical studies to clarify the association between trichomoniasis, HPV infection, and cervical lesions.
3.4.2 CT and UU
Chlamydia trachomatis (CT) and Ureaplasma urealyticum (UU) infections represent clinically prevalent urogenital diseases transmitted primarily through sexual contact (Liu et al., 2024). The association of CT and UU infections with the progression of HPV infection and cervical lesions remains debatable. A meta-analysis by Liang et al. suggested that CT infection raises the likelihood of HPV infection. One possible explanation is that CT attaches to the genital mucosa, disrupts lysosomal activity in host cells, and causes microdamage and localized inflammation. This compromises immune defenses of the cervix and vagina, thus increasing susceptibility to HPV and potentially accelerating CIN and CC development (Liang et al., 2019). In contrast, Wang et al. reported no significant correlation between CT and HR-HPV or cervical lesions, despite a moderately higher prevalence of CT infection in HPV-positive cases (Wang et al., 2019b). Similarly, Abreu et al. suggested that CT positivity does not increase the risk of CC but may be associated with LSIL and HSIL (de Abreu et al., 2012). Conversely, other studies found no significant association between HPV infection and CT (Meng et al., 2016).
Researchers have demonstrated significantly higher UU prevalence in HPV-positive groups, establishing a significant association between UU and HPV infections (Lu et al., 2023). UU may trigger viral persistence and cellular abnormalities, acting as a cofactor in HPV-induced precancerous cervical lesions and CC (Plummer et al., 2021). One possible explanation is that mycoplasma infection induces the release of pro-inflammatory cytokines from cervical macrophages, disrupting the mucosal barrier of the cervix. This results in localized congestion, epithelial cell degeneration, necrosis, and periungual inflammatory infiltration of the mucosa, submucosal tissues, and glands (Lv et al., 2019; Liu et al., 2021). Additionally, UU can adhere to host cells and produce phospholipases that degrade host cell membranes, altering cellular functions. UU breaks down urea, releasing toxic ammonia that damages cells, whereas its immunoglobulin A (IgA) proteases degrade mucosal IgA, impairing immune defenses and facilitating HPV invasion and colonization (Chen et al., 2014; Adebamowo et al., 2017). However, other studies have reported no significant correlation between UU infection and HR-HPV infection (Zhang et al., 2017). Hence, larger sample sizes and long-term follow-up studies are necessary to clarify the association and underlying mechanisms.
3.4.3 N. gonorrhoeae and HSV
Neisseria gonorrhoeae (N. gonorrhoeae) and herpes simplex virus (HSV) are common sexually transmitted pathogens. Epidemiological studies indicate a high co-infection rate of N. gonorrhoeae, HSV, and HPV among sexually active populations, likely associated with high-risk sexual behaviors (e.g., unprotected intercourse, multiple partners). Moreover, these pathogens may act synergistically to significantly increase the risk of malignancies, such as cervical and anal cancers (Klein et al., 2024). Co-infections involving HR-HPV and non-HPV STIs (e.g., N. gonorrhoeae, HSV-2) have been related to HPV persistence, cervical dysplasia, and neoplastic progression (Ma et al., 2022). N. gonorrhoeae-HPV co-infection may elevate CC risk, necessitating enhanced clinical surveillance and prevention of STIs like N. gonorrhoeae (Latorre-Millán et al., 2025). Notably, HSV-2 is significantly more prevalent among HPV/HR-HPV-positive women (Klein et al., 2024). However, other research has reported a higher HSV-1 seropositivity rate in HPV-positive women than in HPV-negative individuals, suggesting a possible synergistic role of HSV-1 with HPV in increasing the risk of CIN, whereas the impact of HSV-2 remains unclear (Finan et al., 2006). The underlying mechanisms may involve genital mucosal inflammation and local immune suppression induced by N. gonorrhoeae and HSV, facilitating poly-microbial co-infections and prolonged pathogen persistence (Quillin and Seifert, 2018). Furthermore, treatments for N. gonorrhoeae or HSV (e.g., antibiotics/antivirals) may alter vaginal/cervical microbiota, thus indirectly influencing HPV infection outcomes (Sausen et al., 2023).
3.4.4 HIV
Human Immunodeficiency Virus (HIV) and HPV are both sexually transmitted pathogens and share a complex epidemiological association and biological interaction. A meta-analysis of HPV infection among HIV-infected individuals in China reported an HPV infection rate of 52.54% (Yuan et al., 2023). A systematic review indicated that the infection rate of high-risk HPV (HPV16, HPV18) in HIV-positive individuals was significantly higher than in HIV-negative individuals, and this co-infection status accelerated the progression of CIN to CC (Swase et al., 2025). Cambrea et al. examined HIV-positive women in southeastern Romania and suggested that HPV types 31 and 56 were more prevalent (Cambrea et al., 2022). Pavone et al. stated that HIV infection reduces helper T (CD4+ T) cells, weakening the immune response against HPV. The impaired function of dendritic cells (DCs) during co-infection further affects antigen presentation and T-cell activation, thus promoting persistent HPV infection. HIV-induced immunosuppression enhances the carcinogenic effects of HPV oncoproteins, such as E5, E6, and E7, which interfere with cell cycle regulation, promote cell proliferation, and inhibit apoptosis, thereby accelerating the malignant transformation of cervical epithelial cells. Additionally, HIV infection induces epithelial-mesenchymal transition (EMT) in cervical epithelial cells through the actions of gp120 and Tat proteins, promoting tumor cell invasion and metastasis. The EMT process involves the activation of multiple signaling pathways, such as mitogen-activated protein kinase and transforming growth factor-beta (TGF-β), which are closely related to the carcinogenic effects of both HIV and HPV (Pavone et al., 2024). Additionally, HPV infection may increase the risk of HIV acquisition through multiple mechanisms. First, HPV-induced inflammation leads to elevated levels of cytokines (such as IL-1, IL-6, IL-8, and TNF-α) and chemokines (such as MCP-1 and IP-10) in the genital tract. These mediators recruit more immune cells to the genital mucosa and may also disrupt the mucosal barrier, facilitating HIV entry. Because CD4+ T cells are the primary targets of HIV, their increased numbers directly elevate the risk of HIV infection. HPV infection may modulate immune responses by affecting the Toll-like receptor (TLR) signaling pathway. For example, the HPV E7 protein can recruit histone-modifying enzymes to suppress TLR9 transcription, weakening antiviral immune responses and facilitating HIV infection. Furthermore, HPV infection may alter the composition of the genital microbiota, characterized by a reduction in beneficial bacteria and an increase in harmful bacteria. This microbial imbalance may further exacerbate inflammation and increase the risk of HIV acquisition (Zayats et al., 2022; Swase et al., 2025).
Regarding HPV-HIV co-infection, researchers have proposed targeted prevention and treatment strategies (Arnold et al., 2022; Yuan et al., 2023). For example, strengthening HPV screening and preventive vaccination can reduce HPV infection rates, thereby lowering the risk of HIV acquisition and CC incidence. Meanwhile, for HIV-infected individuals, the early initiation of antiretroviral therapy helps restore immune function and reduces the risk and persistence of HPV infection.
4 Vaginal microecological functions and the role of HPV infection in cervix-associated diseases
The interaction between HPV and vaginal microecology is a prominent research focus in gynecology. An imbalance in vaginal microecology-particularly a reduction in Lactobacillus populations-may elevate the risk of HPV infection. An altered VMB may contribute to the persistence of HPV infection and its progression to malignancy. Possible mechanisms include changes in the local immune response, microbial metabolite activity, and disruption of the epithelial barrier (Figure 1). Therefore, maintaining a balanced vaginal microenvironment may facilitate preventing HPV infection and its associated diseases.
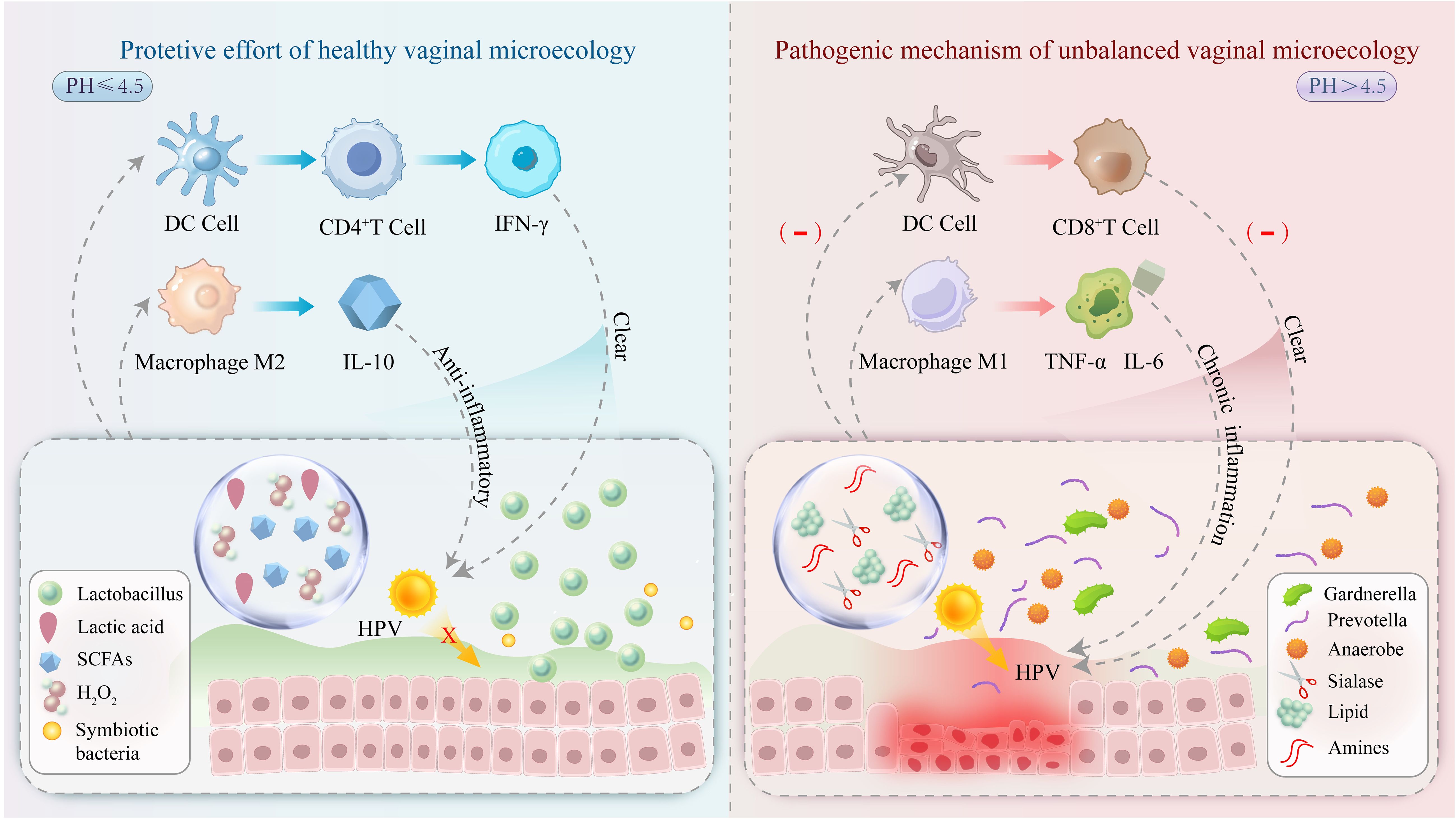
Figure 1. A healthy vaginal microecological niche (left) is dominated by Lactobacillus species, which maintain an acidic environment (pH ≤ 4.5) by secreting lactic acid, inhibiting HPV virus adsorption, and enhancing CD4+ T-cell activity to promote viral clearance. In the imbalanced microecological niche (right), pathogenic bacteria proliferate, and their metabolites (e.g., salivary acid lyase) disrupt the epithelial barrier and promote HPV invasion. In addition, DC cell function is inhibited, CD8+ T cell activity is reduced, and macrophage M1 polarization occurs with chronic inflammation leading to abnormal cell proliferation and promoting cervical lesion progression.
4.1 Impact of cervicovaginal microecological dysregulation on mucosal barrier disruption
The mucosal barrier serves as the first line of immune defense against HPV infection, protecting against harmful environmental factors, including pathogens, while permitting symbiosis with mucosal microorganisms. The vaginal mucosal layer consists of a non-keratinized stratified squamous epithelium with numerous transverse folds. This physiological and anatomical structure functions as a natural defense barrier for the female reproductive system. HPV infection may colonize these vaginal wall folds, making viral clearance more challenging. Additionally, the epithelium contains innate immune cells that express Fc receptors, which bind to the Fc region of immunoglobulins, facilitating antibody-dependent protective functions. Particularly, macrophages and neutrophils express Fc receptor common γ-chain and Fc receptor common α-chain, respectively, allowing them to phagocytose pathogens coated with IgG and IgA (Anderson, 2022). However, when the vaginal mucosa is ruptured, particularly in the squamous-columnar junction zone (transformation zone) of the cervix, which is a preferred site for HPV because of its epithelial characteristics, the virus is more likely to invade to basal cells and integrate into their nuclei. This results in host cell genome alterations and the development of cervical lesions (Boda et al., 2018). Furthermore, dysbiosis of vaginal microorganisms may disrupt epithelial cell proteins and increase cell death, thereby facilitating HPV entry into cervical transformation zone epithelial cells, where the virus can replicate and progress to CIN (Barros et al., 2018). Lactobacilli help maintain an acidic environment and preserve the mucosal barrier by producing metabolites, such as lactic acid, bacteriocins, and biosurfactants, thereby protecting vaginal health and preventing pathogenic infections (Shen et al., 2024). Secretory leukocyte protease inhibitor (SLPI) is a low-molecular-weight protein with antimicrobial, anti-inflammatory, and anti-protease properties. It is secreted by keratinocytes-key targets of HPV infection-and contributes to cervical mucosal immunity (Zhang et al., 2023). Patients with BV exhibit decreased SLPI levels in vaginal secretions, thereby diminishing HPV inhibition (Miquel et al., 2022). Additionally, alterations in the vaginal microbial community reduce L. crispatus and D-lactic acid levels, allowing other bacteria to proliferate rapidly. This raises microbial diversity, expands anaerobic populations, and increases L-lactic acid. Consequently, the expression of extracellular matrix metalloproteinase inducer is enhanced, which activates extracellular matrix metalloproteinase-8 (MMP-8). MMP-8 breaks down the extracellular matrix and cytoskeletal proteins, weakening epithelial structure and accelerating cell death and desquamation. Upon HPV infection, the virus binds to heparan sulfate proteoglycans on basal keratinocytes via its L1 protein, entering through endocytosis before reaching the nucleus in vesicles (Rebolj et al., 2019). In patients with BV, elevated anaerobes and their metabolites, such as porotoxins and sialidase, heighten the activity of mucin-degrading enzymes. This enzymatic activity degrades the protective cervical mucus layer, thereby compromising vaginal epithelial integrity and enhancing viral adhesion, invasion, and genome integration. These effects finally increase cervical susceptibility to HPV (Muzny et al., 2020; Liu et al., 2023). Moreover, clinical proteomic and transcriptional studies have demonstrated that vaginal microbiome alterations lead to significant proteomic changes. These include cytoskeletal modifications (elevated actin histamine, reduced keratin, and keratinized envelope proteins), increased pro-inflammatory cytokine expression, enhanced proteolysis, decreased IgG1/2 levels, antimicrobial peptide imbalances, and altered mucous composition (Borgdorff et al., 2016). Upregulation of cytokine expression strongly correlates with reduced levels of neutrophil proteases (MMP-8 and MMP-9), decreased antiprotease levels, and disruptions in cytoskeletal organization, epithelial differentiation, and keratinization pathways (Mohammadi et al., 2022).
4.2 Localized cervicovaginal immunity and HPV infection
Most women are able to clear HPV infections through immune surveillance and defense mechanisms, thus preventing persistent infection. The immune system consists of two major components, namely innate immunity and adaptive immunity, both of which coordinate and function together to defend against and clear HPV (Gu et al., 2024).
4.2.1 Innate immunity
Innate immune cells, including neutrophils, monocytes, macrophages, eosinophils, mast cells, and DCs, recognize and respond to invading pathogens through pattern-recognition receptors, such as TLRs, nucleotide oligomerization domain-like receptors (NLRs), and retinoic acid-inducible gene-like receptors (Lo Cigno et al., 2024). BV and its associated pathogens, such as Prevotella and Gardnerella, have been related to the expression of TLRs and NLRs, particularly TLR2 (Dong et al., 2022; Gerson et al., 2022). BV-related bacteria can induce immune responses in cervical cells through the TLR2-activated signaling pathway (Anton et al., 2022).
DCs are the most powerful antigen-presenting cells (APCs), and Langerhans cells (LCs) represent a key subset of DCs. LCs directly engage with HPV proteins in epithelial cells (Vine et al., 2024). HPV16 infection can reduce E-cadherin expression in infected keratinocytes, resulting in the depletion of LCs, thereby impairing the initiation of an effective immune response, which promotes persistent viral infection (Jackson et al., 2019). Additionally, macrophages play varied roles in immunity, influenced by their polarization into either M1 or M2 phenotypes (Zhou et al., 2020; Yan and Wan, 2021). The M1 phenotype, associated with classical activation, exerts pro-inflammatory effects, whereas the M2 phenotype primarily exerts protumor effects (Zhou et al., 2020). Specifically, M1 macrophages produce reactive oxygen species (ROS), reactive nitrogenous substances, and pro-inflammatory cytokines, such as TNF-α, IL-12, and IL-6. These substances stimulate Th1 immune reactions and improve the ability of CD8+ T cells to eliminate HPV-infected cells. In contrast, M2 macrophages inhibit CD8+ T-cell function by secreting IL-10 and TGF-β, promote regulatory T-cell (Treg) expansion, and create an immunosuppressive environment (Lin et al., 2019; Huang et al., 2021). Notably, macrophage polarization is a dynamic and complex process. Single-cell sequencing technology has suggested that the phenotypic landscape of macrophages within the microenvironment exhibits significant heterogeneity, extending beyond the simplistic binary classification of M1/M2. The distinction between M1 and M2 macrophages oversimplifies the intricate polarization process, which involves dynamic interactions between multiple cytokines, chemokines, and neighboring cells (Boutilier and Elsawa, 2021). Nevertheless, there is a paucity of research investigating the subtypes of macrophages under physiological or pathological conditions. This review focuses primarily on studies related to the M1 and M2 macrophage types. Natural killer (NK) cells defend against HPV infection. When activated, they produce perforin and granzymes, which induce apoptosis in infected cells, or secrete substantial amounts of inflammatory cytokines, such as interferon-γ and TNF-α. These cytokines inhibit viral replication and recruit other immune cells, including T cells and DCs, thus contributing to the development of HPV-specific adaptive immunity and improving viral elimination (Gutiérrez-Hoya and Soto-Cruz, 2021).
HPV uses multiple mechanisms to evade immune response and allow it to establish a persistent infection. Although the details of immune evasion are unclear, HPV proteins and certain cytokines are possibly involved (Westrich et al., 2017). The HPV16 E6 and E7 proteins inhibit immune cell function in the epithelium by decreasing macrophage-associated cytokines, such as TNF-α and macrophage inflammatory protein(MIP-3α), which blocks macrophage activation (Stern et al., 2000; Hacke et al., 2010; Bashaw et al., 2017). HPV infection impairs the antigen-presenting capacity of DCs by inhibiting monocyte differentiation into mature DCs (Lo Cigno et al., 2020). Another key mechanism involves the HPV E5 protein, which weakens NK cell responses by lowering CD1d expression in HPV16-infected cells, thus allowing them to evade immune detection and destruction. HR-HPV genotypes, such as HPV-16 and HPV-18, further suppress the host immune response by inhibiting type I IFN responses, which reduce immune cell activation (Doorbar et al., 2015; Lo Cigno et al., 2020). The E6 and E7 proteins of HPV16 and HPV18 can interfere with interferon regulatory factor function, leading to decreased IFN production. Additionally, these proteins disrupt the janus kinase-signal transducer and activator of transcription signaling pathway, which is crucial for interferon-mediated immune responses. This mechanism enables high-risk HPV types to evade innate immune surveillance and clearance (Woodby et al., 2018).
4.2.2 Adaptive immunity
Upon infection, APCs process viral antigens and upregulate the expression of major histocompatibility complex molecules. These processed antigens are internalized by DCs via phagocytosis, after which DCs migrate to lymphoid tissues to activate adaptive immunity by secreting inflammatory cytokines, such as IL-1α, IL-1β, IL-6, TNF-α, and IL-12 (Chi et al., 2024). T cells can be further classified into helper T cells (Th), Tregs, and cytotoxic T cells. Th cells are subdivided into Th1, Th2, and Th17 subsets (Bordignon et al., 2017). Th1 cells produce IL-2, a key cytokine involved in protective immune responses, whereas Th2 cells produce IL-10, which may contribute to disease progression (Johansson and Lycke, 2003). The ratio of IL-2 to IL-10 reflects the Th1/Th2 immune response balance. Typically, a Th1-dominant state supports effective immunity; however, a shift toward Th2 dominance may lead to immunosuppression (Zheng et al., 2019). In B cell-mediated humoral immunity, secretory IgA (SIgA) and IgG are the principal effector molecules. SIgA is particularly important for mucosal defense, helping block pathogen entry in the reproductive tract (Dinesh et al., 2020). Meanwhile, T cell-mediated immunity is crucial for combating HPV. CD4+ T cells function as helper T cells, whereas CD8+ T cells function as cytotoxic or suppressor T cells. Patients with HR-HPV infections and cervical lesions exhibit reduced CD4+/CD8+ T cell ratios. Notably, CD4+ T cell levels are significantly higher in patients with CIN I than those with CIN II or III (Walch-Rückheim et al., 2015). Furthermore, the cervical microenvironment shows progressive changes with disease advancement: IL-2 concentrations decrease, whereas IL-10 production rises. This increase in IL-10 correlates with HPV infection severity, likely because of HPV proteins E2, E6, and E7 enhancing IL-10 gene transcription. Such elevated IL-10 expression may promote viral persistence and epithelial cell transformation, establishing a vicious cycle that supports carcinogenesis (Berti et al., 2017; Min et al., 2018). Furthermore, IL-10 may enhance the proliferation and cytotoxic function of HPV-specific CD8+ T lymphocytes induced by IL-2, potentially facilitating HPV clearance and protecting against cervical neoplasia (Farzaneh et al., 2006). Additionally, studies have reported increased IL-6 concentrations in HPV-positive individuals, with levels rising alongside cervical lesion severity. The proposed mechanism involves HPV E6/E7 proteins activating the IL-6/STAT3 signaling pathway, which mediates STAT3 phosphorylation in infected cells. This, in turn, enhances HPV E6/E7 protein expression, thereby promoting cervical tumor progression (Hao et al., 2020; Bonin-Jacob et al., 2021). Additionally, specific bacterial species within the microbiota may influence local immune responses and thereby potentially affect the progression of HPV-related diseases (Sims et al., 2021). VMB characterized by Lactobacillus depletion, elevated pH, and dysbiosis show increased levels of pro-inflammatory cytokines, such as IL-1β, IL-15, and TNF-α, as well as regulatory cytokines IL-12 and growth factor FGF2. These markers may mediate immune responses and chronic inflammation (Łaniewski et al., 2024). Lactobacillus in the vagina is negatively correlated with the expression of IL5/IL13 and TNFα but positively correlated with the expression of IL2 and IL12, which may mediate CC onset and progression (Yang et al., 2024b). Elevated levels of TLR7 and TLR9 have been detected in the cervical cells of BV-positive women infected with HPV, leading to the production of IFN and inflammatory cytokines, thereby causing tissue damage (Fracella et al., 2022).
Immunoglobulins are synthesized by B lymphocytes after antigen-stimulated proliferation and differentiation into plasma cells, which subsequently bind to specific antigens. Among them, IgA controls humoral immunity, whereas large amounts of IgG have been detected in the vagina in cases of persistent HPV infection (Dinesh et al., 2020). SIgA is the key effector molecule of the mucosal immune system. SIgA-mediated agglutination offers improved trapping potency, compared with IgG (Chen et al., 2015). Furthermore, it is normally expressed at low levels in the vagina. However, when the vaginal flora is dysbiotic, changes in bacterial metabolites can reduce SIgA degradation. Contrarily, immune responses triggered by pathogenic bacteria can increase local SIgA synthesis (Dinesh et al., 2020). SIgA secretion increases during mild vaginal infections but decreases in severe infections (Agarwal et al., 2010). Zheng et al. hypothesized that SIgA prevents pathogens from adhering to the cell surface in early-stage lesions, binds to microorganisms on mucosal surfaces, neutralizes viruses, and inactivates them by altering their conformation or blocking binding sites. This results in anti-infective effects and a reduction in SIgA concentration in the early stages of disease. In advanced stages, characterized by persistent HPV infection alongside severe vaginal flora imbalance, H2O2-producing Lactobacilli disappear and IgA protease secretion decreases (Li et al., 2024). This prevents the dissociation of disulfide bonds in the SIgA hinge region, resulting in elevated SIgA levels (Zheng et al., 2019).
4.3 Impact of vaginal microecological dysregulation on gene integration and transcription
The relationship between vaginal microecology and HPV infection involves intricate biological processes, particularly viral gene integration and transcription. Shifts in vaginal microecology may affect HPV infection development, particularly by playing a key role in viral gene integration and transcriptional regulation (Tian et al., 2022a).
Upon HPV entry into the host cell, its gene integration and transcription processes begin silently. The HPV genome consists of early (E) and late (L) gene regions. During gene integration, HPV DNA fragments are randomly inserted into the host genome, and their location often determines subsequent cellular transformation. When key oncogenes, such as p53 and Rb, serve as integration sites, HPV-derived transcripts may impair their normal activities, disrupting cell cycle regulation and apoptosis (Doorbar et al., 2012; Templeton and Laimins, 2023). The transcription of the E6 and E7 genes produces the corresponding E6 and E7 proteins, which bind to p53 and Rb, respectively. This binding leads to protein degradation, rendering cells more susceptible to uncontrolled proliferation (Xing et al., 2024). After HPV gene integration, viral gene transcription is regulated by host cell transcription factors. An imbalanced vaginal microecology may cause chronic inflammation, stimulating cytokine production. These cytokines activate signaling pathways that indirectly influence HPV promoter regions. This activation upregulates the transcription of key genes, such as NF-κB and AP-1, increasing viral protein synthesis and the risk of cellular lesions (Cruz-Gregorio and Aranda-Rivera, 2021). Furthermore, vaginal microecological disruption induces high levels of oxidative stress, generating ROS that cause double-stranded breaks in both the host genome and viral DNA. This process facilitates viral integration into host cells for replication and transformation. Through this mechanism, the HPV E6 protein suppresses the expression of E1 and E2 proteins, leading to dysregulated E6 and E7 transcription, unchecked viral proliferation, and a significant reduction in apoptosis (Adnane et al., 2018; Szymonowicz and Chen, 2020; Łaniewski and Herbst-Kralovetz, 2021).
In summary, vaginal microecology and HPV infection are intricately linked at the levels of gene integration and transcription. A deeper understanding of these mechanisms may provide novel avenues for the prevention, diagnosis, and treatment of HPV-related diseases.
4.4 Impact of cervicovaginal microbial metabolites
Metabolic dysregulation is an emerging hallmark of cancer, and metabolomics is increasingly being explored to identify specific biomarkers. Metabolomic analysis enables the rapid and precise detection of metabolites, making it highly valuable for studying cervical lesions and CC pathogenesis (Yang et al., 2024a).
Lactic acid, a metabolite produced by Lactobacillus plays a crucial role in HPV infection. It enhances cervical mucus’s ability to capture viral particles and inhibits HPV entry into basal cells (Pawar and Aranha, 2022). However, the antibacterial and anticancer effects of lactic acid depend on its type (D-lactic acid vs. L-lactic acid). CST I and II are typically dominated by D-lactic acid-producing L. crispatus or L. gasseri, forming a stable acidic environment. In contrast, CST III is primarily characterized by L-lactic acid-producing L. iners, resulting in an unstable acidic environment prone to dysbiosis. Additionally, the lack of other antimicrobial molecules (such as H202) further diminishes the defensive function of the vaginal microenvironment. This state is strongly associated with persistent HPV infection and recurrent BV. During CST IV, the microbiota becomes dysregulated, with an increase in anaerobic bacteria and a significant rise in vaginal pH (>4.5). The decrease in D-lactic acid concentration further weakens antiviral capacity (Borgogna et al., 2020; Dong et al., 2024). H2O2 impedes the progression of cervical lesions by selectively inducing apoptosis in malignant cells and denaturing bacterial proteins (Krüger and Bauer, 2017; Denys et al., 2019). The vulvovaginal metabolic profiles of HPV-infected women differ significantly from those of healthy controls in terms of lipid metabolism and amino acid metabolism, based on calculated metabolomic scores (Ilhan et al., 2019). Lipid metabolism is strongly related to genital inflammation and cervical lesions, with notably higher lipid accumulation in patients with high-grade CIN and CC because of its role in promoting cell proliferation and membrane synthesis via oncogene activation (Alvarez-Sieiro et al., 2016). Patients with HSIL show significantly elevated levels of acetylated phospholipids, sphingomyelins, phosphatidylcholine, and long-chain polyunsaturated fats. Similarly, 3-hydroxybutyrate, eicosapentaenoic acid esters, and oleic acid esters are markedly increased in patients with CC (Ilhan et al., 2019). Acetylated phospholipids and long-chain polyunsaturated fatty acids act as precursors to inflammatory mediators and may induce abnormal gene expression in cervical cells (Bokulich et al., 2022). Short-chain fatty acids (SCFAs), which are key microbial metabolites in the vaginal environment, regulate local immune responses by modulating vaginal epithelial cell function. SCFA concentrations are elevated in the vaginal tract of patients with BV. High SCFA levels may induce vaginal epithelial cells to secrete pro-inflammatory cytokines, impairing normal antiviral immune function. Additionally, they may disrupt the integrity of the vaginal barrier by affecting tight junction proteins in vaginal epithelial cells, thereby increasing susceptibility to HPV (Mirzaei et al., 2023). Changes in VMB and pH are influenced by amino acid metabolism. HPV infection is associated with reduced levels of key metabolites, including nicotinamide, succinate, and dipeptides (e.g., cysteinylglycine and cysteinyl) (McKenzie et al., 2021) as well as both oxidized and reduced glutathione (Borgogna et al., 2020). The total depletion of glutathione may contribute to oxidative stress, leading to irreversible cervical cell damage and promoting HPV persistence and carcinogenesis (Lebeau et al., 2022). Furthermore, ammonia produced by anaerobic bacterial metabolism and carcinogenic amyl nitrite have been detected in the vaginal environment of patients with BV. These compounds can stimulate the release of inflammatory cytokines, such as IL-1β and IL-8, which may interact with HPV and other factors to induce pathological changes in cervical epithelial cells. This process weakens immune defenses against HPV infection, whereas carcinogenic nitrosamines increase the likelihood of DNA damage (Wang et al., 2020b). Lactic acid produced by Lactobacillus lactis not only regulates vaginal pH but also indirectly affects nucleotide metabolism. The acidic environment can inhibit certain phosphatases involved in nucleotide phosphorylation and modification, affecting deoxynucleotide triphosphate (dNTP) production. This limitation in dNTP availability may restrict HPV replication (Ilhan et al., 2019; Fan et al., 2024).
Future research should further explore the association between vaginal microbial metabolites, HPV infection, and cervical carcinogenesis. Investigating targeted interventions in specific metabolic pathways may highlight novel approaches for disease prevention and management.
5 VMB-based diagnosis and treatment of HPV-related cervical diseases
Currently, effective solutions for HPV infection and low-grade cervical lesions are lacking. Surgical resection, radiotherapy, and chemotherapy are commonly utilized for high-grade lesions; however, these methods have drawbacks, such as fertility impairment and severe adverse effects (Kusakabe et al., 2023). Considering the close association between VMB and HPV infection as well as cervical lesions, VMB modulation has become a growing focus of research in recent years. In terms of early diagnosis, dynamic changes in vaginal microbial diversity may serve as potential biomarkers. High-throughput sequencing-based dynamic monitoring of vaginal microbiota, combined with HPV genotyping or metabolomic analysis (e.g., detection of lactic acid and SCFA levels), can assess the degree of microbial imbalance and aid in identifying high-risk populations (Kudela et al., 2021).
The core of prevention and treatment strategies lies in maintaining or restoring vaginal microecological homeostasis. The efficacy of L1 protein virus-like particle-based vaccines has been well-documented (Mlynarczyk-Bonikowska and Rudnicka, 2024); nonetheless, HPV vaccines do not protect against all HPV types that may develop into CC. Therefore, even vaccinated individuals must undergo regular cervical screenings (Williamson, 2023). Two key therapeutic strategies modulate the vaginal microbiota: probiotics and vaginal microbiome transplantation (VMT) (Zhang et al., 2024). The topical application of probiotics or prebiotics can enhance the vaginal acidic environment, inhibit pathogen colonization, and strengthen mucosal immune barrier function, thereby reducing HPV infection risk. Lactobacillus is the most commonly used probiotic for microbial modulation, followed by Bifidobacterium (Huang et al., 2024). Both oral or vaginal administration of probiotics, including L. paracasei and L. rhamnosus, can significantly increase HPV clearance rates (Huang et al., 2022). Chen et al. demonstrated that a multi-strain Lactobacillus probiotic combination significantly reduced pro-inflammatory cytokine levels (IL-1β and TNF-α) and immune infiltration (neutrophils, lymphocytes, and monocytes) in rat uteri. Hence, the anti-inflammatory properties of probiotics may partially explain their ability to aid HPV clearance (Chen et al., 2021). Bifidobacteria may further enhance anti-tumor immunity and the efficacy of immunotherapy (Kudela et al., 2021). In vitro experiments showed that co-culturing HPV-16-infected SiHa cells with Bifidobacteria reduced HPV E6/E7 mRNA levels (Curty et al., 2019). VMT involves transplanting healthy microbiota from a donor’s vagina into a patient’s vagina and holds promise for VMB improvement (Ma et al., 2019). However, current research on VMT remains limited. Some studies suggest that VMT requires specific vaginal environmental conditions in recipients as well as stringent donor microbiota health criteria, such as the absence of drug-resistant microbes or hidden pathogens in the donor’s microbiome (Gargiulo Isacco et al., 2023). Thus, further research is needed to determine its efficacy and potential adverse effects.
Additionally, multiple novel HPV therapies are currently under investigation. These include inhibitors targeting E1, E5, E6, and E7 proteins, L1 protein-based drugs, plant-derived medications, and therapeutic vaccines. These approaches aim to provide more effective treatment options by either directly inhibiting viral proteins or enhancing the host immune response (Mlynarczyk-Bonikowska and Rudnicka, 2024).
6 Summary
The vaginal microecosystem is a dynamic and balanced system, and alterations in this environment are closely associated with HPV infection. An imbalance in the VMB not only increases HPV infection risk but also impedes viral clearance, creating a vicious cycle. Restoring microbiome balance may improve HPV clearance rates and reduce the incidence of cervical lesions and cancer. Advancements in high-throughput sequencing and bioinformatics are progressively uncovering the mechanisms underlying the association between VMB and HPV clearance. Additionally, the development and clinical application of microbiota-based therapeutics for vaginal infections may provide novel treatment strategies for gynecological conditions, such as HPV infection. In conclusion, studying the VMB enhances the understanding of infections in the female reproductive tract and presents novel opportunities for CC prevention and management. Future large-scale prospective studies are essential to elucidate the composition and role of the vaginal microbiome in cervical lesion progression. As research continues to evolve in this field, further breakthroughs are expected.
Author contributions
MC: Writing – original draft, Writing – review & editing. YW: Writing – original draft, Writing – review & editing. ZL: Writing – review & editing. YL: Writing – review & editing. LF: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Science and Technology Development Program Project of Jilin Province (YDZJ202201ZYTS242).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
A, D., Li, J., Zhang, D., Xiao, B., and Bi, H. (2023). Status of common sexually transmitted infection in population referred for colposcopy and correlation with human papillomavirus infection. BMC Womens Health 23 (1), 579. doi: 10.1186/s12905-023-02693-6
Adamopoulou, M., Avgoustidis, D., Voyiatjaki, C., Beloukas, A., Yapijakis, C., Tsiambas, E., et al. (2021). Impact of combined mycoplasmataceae and HPV co-infection on females with cervical intraepithelial neoplasia and carcinoma. J. buon 26, 1313–1319.
Adebamowo, S. N., Ma, B., Zella, D., Famooto, A., Ravel, J., and Adebamowo, C. (2017). Mycoplasma hominis and Mycoplasma genitalium in the Vaginal Microbiota and Persistent High-Risk Human Papillomavirus Infection. Front. Public Health 5. doi: 10.3389/fpubh.2017.00140
Adnane, M., Meade, K. G., and O’Farrelly, C. (2018). Cervico-vaginal mucus (CVM) - an accessible source of immunologically informative biomolecules. Vet. Res. Commun. 42, 255–263. doi: 10.1007/s11259-018-9734-0
Agarwal, A., Agrawal, U., Verma, S., Mohanty, N. K., and Saxena, S. (2010). Serum Th1 and Th2 cytokine balance in patients of superficial transitional cell carcinoma of bladder pre- and post-intravesical combination immunotherapy. Immunopharmacol. Immunotoxicol. 32, 348–356. doi: 10.3109/08923970903300151
Alvarez-Sieiro, P., Montalbán-López, M., Mu, D., and Kuipers, O. P. (2016). Bacteriocins of lactic acid bacteria: extending the family. Appl. Microbiol. Biotechnol. 100, 2939–2951. doi: 10.1007/s00253-016-7343-9
Anderson, D. J. (2022). Passive immunization of the human vagina. Hum. Vaccin Immunother. 18, 1965423. doi: 10.1080/21645515.2021.1965423
Anton, L., Ferguson, B., Friedman, E. S., Gerson, K. D., Brown, A. G., and Elovitz, M. A. (2022). Gardnerella vaginalis alters cervicovaginal epithelial cell function through microbe-specific immune responses. Microbiome 10, 119. doi: 10.1186/s40168-022-01317-9
Arnold, E. M., Bridges, S. K., Goldbeck, C., Norwood, P., Swendeman, D., Rotheram-Borus, M. J., et al. (2022). HPV Vaccination among Sexual and Gender Minority Youth Living with or at High-Risk for HIV. Vaccines (Basel) 10 (5), 815. doi: 10.3390/vaccines10050815
Barros, M. R., Jr., de Melo, C. M. L., Barros, M., de Cássia Pereira de Lima, R., de Freitas, A. C., and Venuti, A. (2018). Activities of stromal and immune cells in HPV-related cancers. J. Exp. Clin. Cancer Res. 37, 137. doi: 10.1186/s13046-018-0802-7
Bashaw, A. A., Leggatt, G. R., Chandra, J., Tuong, Z. K., and Frazer, I. H. (2017). Modulation of antigen presenting cell functions during chronic HPV infection. Papillomavirus Res. 4, 58–65. doi: 10.1016/j.pvr.2017.08.002
Belfort, I. K. P., Cunha, A. P. A., Mendes, F. P. B., Galvão-Moreira, L. V., Lemos, R. G., de Lima Costa, L. H., et al. (2021). Trichomonas vaginalis as a risk factor for human papillomavirus: a study with women undergoing cervical cancer screening in a northeast region of Brazil. BMC Womens Health 21, 174. doi: 10.1186/s12905-021-01320-6
Berti, F. C. B., Pereira, A. P. L., Cebinelli, G. C. M., Trugilo, K. P., and Brajão de Oliveira, K. (2017). The role of interleukin 10 in human papilloma virus infection and progression to cervical carcinoma. Cytokine Growth Factor Rev. 34, 1–13. doi: 10.1016/j.cytogfr.2017.03.002
Boda, D., Docea, A. O., Calina, D., Ilie, M. A., Caruntu, C., Zurac, S., et al. (2018). Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int. J. Oncol. 52, 637–655. doi: 10.3892/ijo.2018.4256
Bokulich, N. A., Łaniewski, P., Adamov, A., Chase, D. M., Caporaso, J. G., and Herbst-Kralovetz, M. M. (2022). Multi-omics data integration reveals metabolome as the top predictor of the cervicovaginal microenvironment. PloS Comput. Biol. 18, e1009876. doi: 10.1371/journal.pcbi.1009876
Bonin-Jacob, C. M., Almeida-Lugo, L. Z., Puga, M. A. M., MaChado, A. P., Padovani, C. T. J., Noceti, M. C., et al. (2021). IL-6 and IL-10 in the serum and exfoliated cervical cells of patients infected with high-risk human papillomavirus. PloS One 16, e0248639. doi: 10.1371/journal.pone.0248639
Bordignon, V., Di Domenico, E. G., Trento, E., D’Agosto, G., Cavallo, I., Pontone, M., et al. (2017). How human papillomavirus replication and immune evasion strategies take advantage of the host DNA damage repair machinery. Viruses 9 (12), 390. doi: 10.3390/v9120390
Borgdorff, H., Gautam, R., Armstrong, S. D., Xia, D., Ndayisaba, G. F., van Teijlingen, N. H., et al. (2016). Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 9, 621–633. doi: 10.1038/mi.2015.86
Borgogna, J. C., Shardell, M. D., Santori, E. K., Nelson, T. M., Rath, J. M., Glover, E. D., et al. (2020). The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: a cross-sectional analysis. Bjog 127, 182–192. doi: 10.1111/1471-0528.15981
Bornstein, J., Rahat, M. A., and Abramovici, H. (1995). Etiology of cervical cancer: current concepts. Obstet. Gynecol. Surv. 50, 146–154. doi: 10.1097/00006254-199502000-00027
Boutilier, A. J. and Elsawa, S. F. (2021). Macrophage polarization states in the tumor microenvironment. Int. J. Mol. Sci. 22 (13), 6695. doi: 10.3390/ijms22136995
Cambrea, S. C., Aschie, M., Resul, G., Mitroi, A. F., Chisoi, A., Nicolau, A. A., et al. (2022). HPV and HIV coinfection in women from a southeast region of Romania-PICOPIV study. Medicina (Kaunas) 58 (6), 760. doi: 10.3390/medicina58060760
Chen, S., Cai, C., and Li, S. (2014). Study of the relationship between human papilloma virus and Ureaplasma urealyticum infection in women. Int. J. Lab. Med. 35, 1433–1434. doi: 10.3969/j.issn.1673-4130.2014.11.024
Chen, H., Luo, L., Wen, Y., He, B., Ling, H., Shui, J., et al. (2020). Chlamydia trachomatis and human papillomavirus infection in women from Southern Hunan Province in China: A large observational study. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00827
Chen, A., McKinley, S. A., Shi, F., Wang, S., Mucha, P. J., Harit, D., et al. (2015). Modeling of virion collisions in cervicovaginal mucus reveals limits on agglutination as the protective mechanism of secretory immunoglobulin A. PloS One 10, e0131351. doi: 10.1371/journal.pone.0131351
Chen, T., Xia, C., Hu, H., Wang, H., Tan, B., Tian, P., et al. (2021). Dysbiosis of the rat vagina is efficiently rescued by vaginal microbiota transplantation or probiotic combination. Int. J. Antimicrob. Agents 57, 106277. doi: 10.1016/j.ijantimicag.2021.106277
Chi, H., Pepper, M., and Thomas, P. G. (2024). Principles and therapeutic applications of adaptive immunity. Cell 187, 2052–2078. doi: 10.1016/j.cell.2024.03.037
Cruz-Gregorio, A. and Aranda-Rivera, A. K. (2021). Redox-sensitive signalling pathways regulated by human papillomavirus in HPV-related cancers. Rev. Med. Virol. 31, e2230. doi: 10.1002/rmv.2230
Curty, G., de Carvalho, P. S., and Soares, M. A. (2019). The role of the cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int. J. Mol. Sci. 21 (1), 222. doi: 10.3390/ijms21010222
Czechowicz, P., Nowicka, J., and Gościniak, G. (2022). Virulence factors of candida spp. and host immune response important in the pathogenesis of Vulvovaginal candidiasis. Int. J. Mol. Sci. 23 (11), 5895. doi: 10.3390/ijms23115895
de Abreu, A. L., Nogara, P. R., Souza, R. P., da Silva, M. C., Uchimura, N. S., Zanko, R. L., et al. (2012). Molecular detection of HPV and Chlamydia trachomatis infections in Brazilian women with abnormal cervical cytology. Am. J. Trop. Med. Hyg 87, 1149–1151. doi: 10.4269/ajtmh.2012.12-0287
Denys, G. A., Devoe, N. C., Gudis, P., May, M., Allen, R. C., and Stephens, J. T., Jr. (2019). Mechanism of microbicidal action of E-101 solution, a myeloperoxidase-mediated antimicrobial, and its oxidative products. Infect. Immun. 87 (7), e00261-19. doi: 10.1128/iai.00261-19
Dinesh, D. C., Tamilarasan, S., Rajaram, K., and Bouřa, E. (2020). Antiviral drug targets of single-stranded RNA viruses causing chronic human diseases. Curr. Drug Targets 21, 105–124. doi: 10.2174/1389450119666190920153247
Donders, G. G. (2007). Definition and classification of abnormal vaginal flora. Best Pract. Res. Clin. Obstet. Gynaecol. 21, 355–373. doi: 10.1016/j.bpobgyn.2007.01.002
Donders, G. G., Vereecken, A., Bosmans, E., Dekeersmaecker, A., Salembier, G., and Spitz, B. (2002). Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Bjog 109, 34–43. doi: 10.1111/j.1471-0528.2002.00432.x
Dong, B., Huang, Y., Cai, H., Chen, Y., Li, Y., Zou, H., et al. (2022). Prevotella as the hub of the cervicovaginal microbiota affects the occurrence of persistent human papillomavirus infection and cervical lesions in women of childbearing age via host NF-κB/C-myc. J. Med. Virol. 94, 5519–5534. doi: 10.1002/jmv.28001
Dong, W., Wang, S., Wang, X., Xu, G., Liu, Q., Li, Z., et al. (2024). Characteristics of vaginal microbiota of women of reproductive age with infections. Microorganisms 12 (5), 1030. doi: 10.3390/microorganisms12051030
Doorbar, J., Egawa, N., Griffin, H., Kranjec, C., and Murakami, I. (2015). Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 25 Suppl 1, 2–23. doi: 10.1002/rmv.1822
Doorbar, J., Quint, W., Banks, L., Bravo, I. G., Stoler, M., Broker, T. R., et al. (2012). The biology and life-cycle of human papillomaviruses. Vaccine 30 Suppl 5, F55–F70. doi: 10.1016/j.vaccine.2012.06.083
Fan, Z., Han, D., Fan, X., Zeng, Y., and Zhao, L. (2024). Analysis of the correlation between cervical HPV infection, cervical lesions and vaginal microecology. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1405789
Farzaneh, F., Roberts, S., Mandal, D., Ollier, B., Winters, U., Kitchener, H. C., et al. (2006). The IL-10 -1082G polymorphism is associated with clearance of HPV infection. Bjog 113, 961–964. doi: 10.1111/j.1471-0528.2006.00956.x
Feng, F., Hou, Y. M., Zhang, Y., Wang, L. Y., Li, P. P., Guo, Y., et al. (2023). Correlation analysis of vaginal microecology and different types of human papillomavirus infection: a study conducted at a hospital in northwest China. Front. Med. (Lausanne) 10. doi: 10.3389/fmed.2023.1138507
Feng, R. M., Wang, M. Z., Smith, J. S., Dong, L., Chen, F., Pan, Q. J., et al. (2018). Risk of high-risk human papillomavirus infection and cervical precancerous lesions with past or current trichomonas infection: a pooled analysis of 25,054 women in rural China. J. Clin. Virol. 99-100, 84–90. doi: 10.1016/j.jcv.2017.12.015
Finan, R. R., Musharrafieh, U., and Almawi, W. Y. (2006). Detection of Chlamydia trachomatis and herpes simplex virus type 1 or 2 in cervical samples in human papilloma virus (HPV)-positive and HPV-negative women. Clin. Microbiol. Infect. 12, 927–930. doi: 10.1111/j.1469-0691.2006.01479.x
Fracella, M., Oliveto, G., Sorrentino, L., Roberto, P., Cinti, L., Viscido, A., et al. (2022). Common microbial genital infections and their impact on the innate immune response to HPV in cervical cells. Pathogens 11 (11), 1361. doi: 10.3390/pathogens11111361
France, M. T., Ma, B., Gajer, P., Brown, S., Humphrys, M. S., Holm, J. B., et al. (2020). VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8, 166. doi: 10.1186/s40168-020-00934-6
Frąszczak, K., Barczyński, B., and Kondracka, A. (2022). Does lactobacillus exert a protective effect on the development of cervical and endometrial cancer in women? Cancers (Basel) 14 (19), 4909. doi: 10.3390/cancers14194909
Gardella, B., Pasquali, M. F., La Verde, M., Cianci, S., Torella, M., and Dominoni, M. (2022). The complex interplay between vaginal microbiota, HPV infection, and immunological microenvironment in cervical intraepithelial neoplasia: A literature review. Int. J. Mol. Sci. 23 (13), 7174. doi: 10.3390/ijms23137174
Gargiulo Isacco, C., Balzanelli, M. G., Garzone, S., Lorusso, M., Inchingolo, F., Nguyen, K. C. D., et al. (2023). Alterations of vaginal microbiota and chlamydia trachomatis as crucial co-causative factors in cervical cancer genesis procured by HPV. Microorganisms 11, 662. doi: 10.3390/microorganisms11030662
Gerson, K. D., Anton, L., Ferguson, B., Ravel, J., Burris, H. H., and Elovitz, M. A. (2022). Gardnerella vaginalis induces matrix metalloproteinases in the cervicovaginal epithelium through TLR-2 activation. J. Reprod. Immunol. 152, 103648. doi: 10.1016/j.jri.2022.103648
Ghareghomi, S., Ahmadian, S., Zarghami, N., and Kahroba, H. (2021). Fundamental insights into the interaction between telomerase/TERT and intracellular signaling pathways. Biochimie 181, 12–24. doi: 10.1016/j.biochi.2020.11.015
Ghosh, I., Mandal, R., Kundu, P., and Biswas, J. (2016). Association of genital infections other than human papillomavirus with pre-invasive and invasive cervical neoplasia. J. Clin. Diagn. Res. 10, Xe01–xe06. doi: 10.7860/jcdr/2016/15305.7173
Gu, Y., Li, T., Zhang, M., Chen, J., Shen, F., Ding, J., et al. (2024). The display between HPV infection and host immunity in cervical cancer. Front. Biosci. (Landmark Ed) 29, 426. doi: 10.31083/j.fbl2912426
Gutiérrez-Hoya, A. and Soto-Cruz, I. (2021). NK cell regulation in cervical cancer and strategies for immunotherapy. Cells 10 (11), 3104. doi: 10.3390/cells10113104
Hacke, K., Rincon-Orozco, B., Buchwalter, G., Siehler, S. Y., Wasylyk, B., Wiesmüller, L., et al. (2010). Regulation of MCP-1 chemokine transcription by p53. Mol. Cancer 9, 82. doi: 10.1186/1476-4598-9-82
Hao, Y., Yan, Z., Zhang, A., Hu, S., Wang, N., Luo, X. G., et al. (2020). IL-6/STAT3 mediates the HPV18 E6/E7 stimulated upregulation of MALAT1 gene in cervical cancer HeLa cells. Virus Res. 281, 197907. doi: 10.1016/j.virusres.2020.197907
Hearps, A. C., Tyssen, D., Srbinovski, D., Bayigga, L., Diaz, D. J. D., Aldunate, M., et al. (2017). Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 10, 1480–1490. doi: 10.1038/mi.2017.27
Huang, Q., Liang, X., Ren, T., Huang, Y., Zhang, H., Yu, Y., et al. (2021). The role of tumor-associated macrophages in osteosarcoma progression - therapeutic implications. Cell Oncol. (Dordr) 44, 525–539. doi: 10.1007/s13402-021-00598-w
Huang, R., Liu, Z., Sun, T., and Zhu, L. (2024). Cervicovaginal microbiome, high-risk HPV infection and cervical cancer: Mechanisms and therapeutic potential. Microbiol. Res. 287, 127857. doi: 10.1016/j.micres.2024.127857
Huang, R., Wu, F., Zhou, Q., Wei, W., Yue, J., Xiao, B., et al. (2022). Lactobacillus and intestinal diseases: Mechanisms of action and clinical applications. Microbiol. Res. 260, 127019. doi: 10.1016/j.micres.2022.127019
Ilhan, Z. E., Łaniewski, P., Thomas, N., Roe, D. J., Chase, D. M., and Herbst-Kralovetz, M. M. (2019). Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 44, 675–690. doi: 10.1016/j.ebiom.2019.04.028
Jackson, R., Eade, S., and Zehbe, I. (2019). An epithelial organoid model with Langerhans cells for assessing virus-host interactions. Philos. Trans. R Soc. Lond. B Biol. Sci. 374, 20180288. doi: 10.1098/rstb.2018.0288
Jahic, M., Mulavdic, M., Hadzimehmedovic, A., and Jahic, E. (2013). Association between aerobic vaginitis, bacterial vaginosis and squamous intraepithelial lesion of low grade. Med. Arch. 67, 94–96. doi: 10.5455/medarh.2013.67.94-96
Jiang, M., Ding, H., He, L., Xu, D., Jiang, P., Tang, H., et al. (2023). Association between co-infection with Chlamydia trachomatis or Mycoplasma genitalium and cervical lesions in HPV-positive population in Hunan, China: a cross-sectional study. Infect. Agent Cancer 18, 76. doi: 10.1186/s13027-023-00544-5
Johansson, M. and Lycke, N. Y. (2003). Immunology of the human genital tract. Curr. Opin. Infect. Dis. 16, 43–49. doi: 10.1097/00001432-200302000-00008
Kalia, N., Singh, J., and Kaur, M. (2020). Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann. Clin. Microbiol. Antimicrob. 19, 5. doi: 10.1186/s12941-020-0347-4
Klein, J. M. A., Runge, I., Pannen, A. K., Wakuma, T., Abera, S. F., Adissie, A., et al. (2024). Prevalence of bacterial vaginosis, sexually transmitted infections and their association with HPV infections in asymptomatic women attending antenatal care in Ethiopia. Ecancermedicalscience 18, 1783. doi: 10.3332/ecancer.2024.1783
Krüger, H. and Bauer, G. (2017). Lactobacilli enhance reactive oxygen species-dependent apoptosis-inducing signaling. Redox Biol. 11, 715–724. doi: 10.1016/j.redox.2017.01.015
Kudela, E., Liskova, A., Samec, M., Koklesova, L., Holubekova, V., Rokos, T., et al. (2021). The interplay between the vaginal microbiome and innate immunity in the focus of predictive, preventive, and personalized medical approach to combat HPV-induced cervical cancer. Epma J. 12, 199–220. doi: 10.1007/s13167-021-00244-3
Kusakabe, M., Taguchi, A., Sone, K., Mori, M., and Osuga, Y. (2023). Carcinogenesis and management of human papillomavirus-associated cervical cancer. Int. J. Clin. Oncol. 28, 965–974. doi: 10.1007/s10147-023-02337-7
Kwon, M. S. and Lee, H. K. (2022). Host and microbiome interplay shapes the vaginal microenvironment. Front. Immunol. 13. doi: 10.3389/fimmu.2022.919728
Lam, K. C., Vyshenska, D., Hu, J., Rodrigues, R. R., Nilsen, A., Zielke, R. A., et al. (2018). Transkingdom network reveals bacterial players associated with cervical cancer gene expression program. PeerJ 6, e5590. doi: 10.7717/peerj.5590
Łaniewski, P., Cui, H., Roe, D. J., Barnes, D., Goulder, A., Monk, B. J., et al. (2019). Features of the cervicovaginal microenvironment drive cancer biomarker signatures in patients across cervical carcinogenesis. Sci. Rep. 9, 7333. doi: 10.1038/s41598-019-43849-5
Łaniewski, P. and Herbst-Kralovetz, M. M. (2021). Bacterial vaginosis and health-associated bacteria modulate the immunometabolic landscape in 3D model of human cervix. NPJ Biofilms Microbiomes 7, 88. doi: 10.1038/s41522-021-00259-8
Łaniewski, P., Joe, T. R., Jimenez, N. R., Eddie, T. L., Bordeaux, S. J., Quiroz, V., et al. (2024). Viewing native American cervical cancer disparities through the lens of the vaginal microbiome: A pilot study. Cancer Prev. Res. (Phila) 17, 525–538. doi: 10.1158/1940-6207.Capr-24-0286
Latorre-Millán, M., Tristancho-Baró, A., Burillo, N., Ariza, M., Milagro, A. M., Abad, P., et al. (2025). HPV-associated sexually transmitted infections in cervical cancer screening: A prospective cohort study. Viruses 17 (2), 247. doi: 10.3390/v17020247
Lebeau, A., Bruyere, D., Roncarati, P., Peixoto, P., Hervouet, E., Cobraiville, G., et al. (2022). HPV infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by Lactobacilli as amino acid sources. Nat. Commun. 13, 1076. doi: 10.1038/s41467-022-28724-8
Leon-Gomez, P. and Romero, V. I. (2024). Human papillomavirus, vaginal microbiota and metagenomics: the interplay between development and progression of cervical cancer. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1515258
Li, J., Jin, H., Sun, Y., Wang, C., Chen, H., Gong, S., et al. (2024). Reconnoitering correlation between human papillomavirus infection-induced vaginal microecological abnormality and squamous intraepithelial lesion (SIL) progression. BMC Womens Health 24, 5. doi: 10.1186/s12905-023-02824-z
Li, Q., Li, Y., Bai, Y., Zhang, H., and Zhao, W. (2022). Development and validation of a predictive model for the risk of developing trichomonas vaginitis in women. Sci. Rep. 12, 20182. doi: 10.1038/s41598-022-24396-y
Liang, Y., Chen, M., Qin, L., Wan, B., and Wang, H. (2019). A meta-analysis of the relationship between vaginal microecology, human papillomavirus infection and cervical intraepithelial neoplasia. Infect. Agent Cancer 14, 29. doi: 10.1186/s13027-019-0243-8
Lin, Y., Xu, J., and Lan, H. (2019). Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol. 12, 76. doi: 10.1186/s13045-019-0760-3
Lin, W., Zhang, Q., Chen, Y., Dong, B., Xue, H., Lei, H., et al. (2022). Changes of the vaginal microbiota in HPV infection and cervical intraepithelial neoplasia: a cross-sectional analysis. Sci. Rep. 12, 2812. doi: 10.1038/s41598-022-06731-5
Liu, Y., Li, T., Guo, R., Chen, T., Wang, S., Wu, D., et al. (2023). The vaginal microbiota among the different status of human papillomavirus infection and bacterial vaginosis. J. Med. Virol. 95, e28595. doi: 10.1002/jmv.28595
Liu, J., Liang, S., Chen, K., Zhou, J., Jiang, W., and Zhang, G. (2021). Correlation analysis between UU typing and HPV infection. Int. J. Lab. Med. 42, 2321–2323+2328. doi: 10.3969/j.issn.1673-4130.2021.19.005
Liu, S., Ouyang, Y., Tang, Q., Mei, B., and Li, C. (2024). Prevalence of Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum among outpatients in central China: A retrospective study. Diagn. Microbiol. Infect. Dis. 110, 116394. doi: 10.1016/j.diagmicrobio.2024.116394
Lo Cigno, I., Calati, F., Albertini, S., and Gariglio, M. (2020). Subversion of host innate immunity by human papillomavirus oncoproteins. Pathogens 9 (4), 292. doi: 10.3390/pathogens9040292
Lo Cigno, I., Calati, F., Girone, C., Catozzo, M., and Gariglio, M. (2024). High-risk HPV oncoproteins E6 and E7 and their interplay with the innate immune response: Uncovering mechanisms of immune evasion and therapeutic prospects. J. Med. Virol. 96, e29685. doi: 10.1002/jmv.29685
Long, T., Zhang, C., He, G., Hu, Y., Lin, Z., and Long, L. (2023). Bacterial vaginosis decreases the risk of cervical cytological abnormalities. Cancer Prev. Res. (Phila) 16, 109–117. doi: 10.1158/1940-6207.Capr-22-0288
Lu, Z., Zhao, P., Lu, H., and Xiao, M. (2023). Analyses of human papillomavirus, Chlamydia trachomatis, Ureaplasma urealyticum, Neisseria gonorrhoeae, and co-infections in a gynecology outpatient clinic in Haikou area, China. BMC Womens Health 23, 117. doi: 10.1186/s12905-023-02259-6
Lv, P., Zhao, F., Xu, X., Xu, J., Wang, Q., and Zhao, Z. (2019). Correlation between common lower genital tract microbes and high-risk human papillomavirus infection. Can. J. Infect. Dis. Med. Microbiol. 2019, 9678104. doi: 10.1155/2019/9678104
Ma, D., Chen, Y., and Chen, T. (2019). Vaginal microbiota transplantation for the treatment of bacterial vaginosis: a conceptual analysis. FEMS Microbiol. Lett. 366 (4), fnz025. doi: 10.1093/femsle/fnz025
Ma, Z., Gharizadeh, B., Cai, X., Li, M., Fellner, M. D., Basiletti, J. A., et al. (2022). A comprehensive HPV-STI NGS assay for detection of 29 HPV types and 14 non-HPV sexually transmitted infections. Infect. Agent Cancer 17, 9. doi: 10.1186/s13027-022-00420-8
Mao, C., Hughes, J. P., Kiviat, N., Kuypers, J., Lee, S. K., Adam, D. E., et al. (2003). Clinical findings among young women with genital human papillomavirus infection. Am. J. Obstet. Gynecol. 188, 677–684. doi: 10.1067/mob.2003.164
Martins, B. C. T., Guimarães, R. A., Alves, R. R. F., and Saddi, V. A. (2023). Bacterial vaginosis and cervical human papillomavirus infection in young and adult women: a systematic review and meta-analysis. Rev. Saude Publica 56, 113. doi: 10.11606/s1518-8787.2022056004412
McClymont, E., Albert, A. Y., Wang, C., Dos Santos, S. J., Coutlée, F., Lee, M., et al. (2022). Vaginal microbiota associated with oncogenic HPV in a cohort of HPV-vaccinated women living with HIV. Int. J. STD AIDS 33, 847–855. doi: 10.1177/09564624221109686
McKenzie, R., Maarsingh, J. D., Łaniewski, P., and Herbst-Kralovetz, M. M. (2021). Immunometabolic Analysis of Mobiluncus mulieris and Eggerthella sp. Reveals Novel Insights Into Their Pathogenic Contributions to the Hallmarks of Bacterial Vaginosis. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.759697
Mei, X., Zhang, R., Li, D., Xie, X., Yao, Y., Gao, M., et al. (2023). Association between the infections of Trichomonas vaginalis and uterine cervical human papillomavirus: a meta-analysis. J. Obstet. Gynaecol. 43, 2194986. doi: 10.1080/01443615.2023.2194986
Meng, L. T., Xue, Y., Yue, T., Yang, L., Gao, L., and An, R.F.J.Z.F.C.K.Z.Z. (2016). Relationship of HPV infection and BV, VVC, TV: a clinical study based on 1 261 cases of gynecologic outpatients. Chin. J. Obstet. Gynecol. 51, 730–733. doi: 10.3760/cma.j.issn.0529-567x.2016.10.004
Min, Z., Pu, X., and Gu, Z. (2018). Correlative analysis of the expression of IL-10 and Ki-67 in human cervical cancer and cervical intraepithelial neoplasias and human papillomavirus infection. Oncol. Lett. 16, 7189–7194. doi: 10.3892/ol.2018.9520
Miquel, S., Verlaguet, J., Garcin, S., Bertran, T., Evrard, B., Forestier, C., et al. (2022). Lacticaseibacillus rhamnosus Lcr35 Stimulates Epithelial Vaginal Defenses upon Gardnerella vaginalis Infection. Infect. Immun. 90, e0030922. doi: 10.1128/iai.00309-22
Mirzaei, R., Kavyani, B., Nabizadeh, E., Kadkhoda, H., Asghari Ozma, M., and Abdi, M. (2023). Microbiota metabolites in the female reproductive system: Focused on the short-chain fatty acids. Heliyon 9, e14562. doi: 10.1016/j.heliyon.2023.e14562
Mlynarczyk-Bonikowska, B. and Rudnicka, L. (2024). HPV infections-classification, pathogenesis, and potential new therapies. Int. J. Mol. Sci. 25 (14), 7616. doi: 10.3390/ijms25147616
Mohammadi, A., Bagherichimeh, S., Choi, Y., Fazel, A., Tevlin, E., Huibner, S., et al. (2022). Immune parameters of HIV susceptibility in the female genital tract before and after penile-vaginal sex. Commun. Med. (Lond.) 2, 60. doi: 10.1038/s43856-022-00122-7
Moreno, E., Ron, R., and Serrano-Villar, S. (2023). The microbiota as a modulator of mucosal inflammation and HIV/HPV pathogenesis: From association to causation. Front. Immunol. 14, 1072655. doi: 10.3389/fimmu.2023.1072655
Muzny, C. A., Łaniewski, P., Schwebke, J. R., and Herbst-Kralovetz, M. M. (2020). Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 33, 59–65. doi: 10.1097/qco.0000000000000620
Nieves-Ramírez, M. E., Partida-Rodríguez, O., Moran, P., Serrano-Vázquez, A., Pérez-Juárez, H., Pérez-Rodríguez, M. E., et al. (2021). Cervical squamous intraepithelial lesions are associated with differences in the vaginal microbiota of Mexican women. Microbiol. Spectr. 9, e0014321. doi: 10.1128/Spectrum.00143-21
Onywera, H., Anejo-Okopi, J., Mwapagha, L. M., Okendo, J., and Williamson, A. L. (2021). Predictive functional analysis reveals inferred features unique to cervicovaginal microbiota of African women with bacterial vaginosis and high-risk human papillomavirus infection. PloS One 16, e0253218. doi: 10.1371/journal.pone.0253218
Organization, W. H. Cervical cancer. Available online at: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (Accessed November 16, 2024).
Palma, E., Recine, N., Domenici, L., Giorgini, M., Pierangeli, A., and Panici, P. B. (2018). Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect. Dis. 18, 13. doi: 10.1186/s12879-017-2938-z
Papanikolaou, V., Athanassiou, E., Dubos, S., Dimou, I., Papathanasiou, I., Kitsiou-Tzeli, S., et al. (2011). hTERT regulation by NF-κB and c-myc in irradiated HER2-positive breast cancer cells. Int. J. Radiat. Biol. 87, 609–621. doi: 10.3109/09553002.2011.572112
Paul, A., Sarker, S., Banik, B. C., Paul, A., Paul, S. K., Nasreen, S. A., et al. (2023). Detection of oncogenic human papillomavirus (HPV-16 and HPV-18) from bacterial vaginosis positive patient attending at tertiary care hospital in Mymensingh. Mymensingh Med. J. 32, 959–967.
Pavone, G., Marino, A., Fisicaro, V., Motta, L., Spata, A., Martorana, F., et al. (2024). Entangled connections: HIV and HPV interplay in cervical cancer-A comprehensive review. Int. J. Mol. Sci. 25 (19), 10358. doi: 10.3390/ijms251910358
Pawar, K. and Aranha, C. (2022). Lactobacilli metabolites restore E-cadherin and suppress MMP9 in cervical cancer cells. Curr. Res. Toxicol. 3, 100088. doi: 10.1016/j.crtox.2022.100088
Peremykina, A., Cheranev, V., Krivoy, A., Andreev, A. O., Repinskaia, Z., Asaturova, A. V., et al. (2024). Microbiome markers in HPV-positive and HPV-negative women of reproductive age with ASCUS and SIL determined by V4 region of 16S rRNA gene sequencing. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1334502
Pimple, S. and Mishra, G. (2022). Cancer cervix: Epidemiology and disease burden. Cytojournal 19, 21. doi: 10.25259/cmas_03_02_2021
Plisko, O., Zodzika, J., Jermakova, I., Pcolkina, K., Prusakevica, A., Liepniece-Karele, I., et al. (2021). Aerobic vaginitis-underestimated risk factor for cervical intraepithelial neoplasia. Diagnost. (Basel) 11 (1), 97. doi: 10.3390/diagnostics11010097
Plummer, E. L., Vodstrcil, L. A., Bodiyabadu, K., Murray, G. L., Doyle, M., Latimer, R. L., et al. (2021). Are Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum Associated With Specific Genital Symptoms and Clinical Signs in Nonpregnant Women? Clin. Infect. Dis. 73, 659–668. doi: 10.1093/cid/ciab061
Qing, X., Xie, M., Liu, P., Feng, O., Leng, H., Guo, H., et al. (2024). Correlation between dysbiosis of vaginal microecology and endometriosis: A systematic review and meta-analysis. PloS One 19, e0306780. doi: 10.1371/journal.pone.0306780
Quillin, S. J. and Seifert, H. S. (2018). Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 16, 226–240. doi: 10.1038/nrmicro.2017.169
Raffone, A., Travaglino, A., Angelino, A., Esposito, R., Pontillo, M., Mollo, A., et al. (2020). Gardnerella vaginalis and Trichomonas vaginalis infections and the risk of persistence or progression of low-grade cervical intraepithelial neoplasia. Pathol. Res. Pract. 216, 153234. doi: 10.1016/j.prp.2020.153234
Rahangdale, L., Mungo, C., O’Connor, S., Chibwesha, C. J., and Brewer, N. T. (2022). Human papillomavirus vaccination and cervical cancer risk. Bmj 379, e070115. doi: 10.1136/bmj-2022-070115
Rebolj, M., Rimmer, J., Denton, K., Tidy, J., Mathews, C., Ellis, K., et al. (2019). Primary cervical screening with high risk human papillomavirus testing: observational study. Bmj 364, l240. doi: 10.1136/bmj.l240
Sausen, D. G., Shechter, O., Gallo, E. S., Dahari, H., and Borenstein, R. (2023). Herpes simplex virus, human papillomavirus, and cervical cancer: overview, relationship, and treatment implications. Cancers (Basel) 15 (14), 3692. doi: 10.3390/cancers15143692
Schellekens, H. C. J., Schmidt, L. M. S., Morré, S. A., van Esch, E. M. G., and de Vos van Steenwijk, P. J. (2025). Vaginal microbiota and local immunity in HPV-induced high-grade cervical dysplasia: A narrative review. Int. J. Mol. Sci. 26 (9), 3954. doi: 10.3390/ijms26093954
Shen, J., Sun, H., Chu, J., Gong, X., and Liu, X. (2024). Cervicovaginal microbiota: a promising direction for prevention and treatment in cervical cancer. Infect. Agent Cancer 19, 13. doi: 10.1186/s13027-024-00573-8
Sims, T. T., Colbert, L. E., and Klopp, A. H. (2021). The role of the cervicovaginal and gut microbiome in cervical intraepithelial neoplasia and cervical cancer. J. Immunother. Precis Oncol. 4, 72–78. doi: 10.36401/jipo-20-17
Singh, D., Vignat, J., Lorenzoni, V., Eslahi, M., Ginsburg, O., Lauby-Secretan, B., et al. (2023). Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 11, e197–e206. doi: 10.1016/s2214-109x(22)00501-0
Smalley Rumfield, C., Roller, N., Pellom, S. T., Schlom, J., and Jochems, C. (2020). Therapeutic vaccines for HPV-associated Malignancies. Immunotargets Ther. 9, 167–200. doi: 10.2147/itt.S273327
Sobel, J. D. and Vempati, Y. S. (2024). Bacterial vaginosis and vulvovaginal candidiasis pathophysiologic interrelationship. Microorganisms 12 (1), 108. doi: 10.3390/microorganisms12010108
Stern, P. L., Brown, M., Stacey, S. N., Kitchener, H. C., Hampson, I., Abdel-Hady, E. S., et al. (2000). Natural HPV immunity and vaccination strategies. J. Clin. Virol. 19, 57–66. doi: 10.1016/s1386-6532(00)00128-1
Swase, T. D., Fasogbon, I. V., Eseoghene, I. J., Etukudo, E. M., Mbina, S. A., Joan, C., et al. (2025). The impact of HPV/HIV co-infection on immunosuppression, HPV genotype, and cervical cancer biomarkers. BMC Cancer 25, 202. doi: 10.1186/s12885-025-13516-2
Szymonowicz, K. A. and Chen, J. (2020). Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 17, 864–878. doi: 10.20892/j.issn.2095-3941.2020.0370
Templeton, C. W. and Laimins, L. A. (2023). p53-dependent R-loop formation and HPV pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 120, e2305907120. doi: 10.1073/pnas.2305907120
Tian, R., Liu, J., Fan, W., Li, R., Cui, Z., Jin, Z., et al. (2022a). Gene knock-out chain reaction enables high disruption efficiency of HPV18 E6/E7 genes in cervical cancer cells. Mol. Ther. Oncolytics 24, 171–179. doi: 10.1016/j.omto.2021.12.011
Tian, Y., Pan, Y., and Cao, M. (2022b). Analysis of vaginal microecological diversity,HPV infection subtypes and their correlation with cervical lesions in 100 women. Chin. J. Hum. Sex 31, 67–71. doi: 10.3969/j.issn.1672-1993.2022.07.018
Usyk, M., Zolnik, C. P., Castle, P. E., Porras, C., Herrero, R., Gradissimo, A., et al. (2020). Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PloS Pathog. 16, e1008376. doi: 10.1371/journal.ppat.1008376
Uysal, I. B., Boué, V., Murall, C. L., Graf, C., Selinger, C., Hirtz, C., et al. (2022). Concomitant and productive genital infections by HSV-2 and HPV in two young women: A case report. IDCases 30, e01604. doi: 10.1016/j.idcr.2022.e01604
Vieira-Baptista, P., Lima-Silva, J., Pinto, C., Saldanha, C., Beires, J., Martinez-de-Oliveira, J., et al. (2016). Bacterial vaginosis, aerobic vaginitis, vaginal inflammation and major Pap smear abnormalities. Eur. J. Clin. Microbiol. Infect. Dis. 35, 657–664. doi: 10.1007/s10096-016-2584-1
Vine, E. E., Austin, P. J., O’Neil, T. R., Nasr, N., Bertram, K. M., Cunningham, A. L., et al. (2024). Epithelial dendritic cells vs. Langerhans cells: Implications for mucosal vaccines. Cell Rep. 43, 113977. doi: 10.1016/j.celrep.2024.113977
Walch-Rückheim, B., Mavrova, R., Henning, M., Vicinus, B., Kim, Y. J., Bohle, R. M., et al. (2015). Stromal Fibroblasts Induce CCL20 through IL6/C/EBPβ to Support the Recruitment of Th17 Cells during Cervical Cancer Progression. Cancer Res. 75, 5248–5259. doi: 10.1158/0008-5472.Can-15-0732
Wang, C., Fan, A., Li, H., Yan, Y., Qi, W., Wang, Y., et al. (2020a). Vaginal bacterial profiles of aerobic vaginitis: a case-control study. Diagn. Microbiol. Infect. Dis. 96, 114981. doi: 10.1016/j.diagmicrobio.2019.114981
Wang, Y., Liu, Z., and Chen, T. (2024). Vaginal microbiota: Potential targets for vulvovaginal candidiasis infection. Heliyon 10, e27239. doi: 10.1016/j.heliyon.2024.e27239
Wang, H., Ma, Y., Li, R., Chen, X., Wan, L., and Zhao, W. (2019a). Associations of cervicovaginal lactobacilli with high-risk human papillomavirus infection, cervical intraepithelial neoplasia, and cancer: A systematic review and meta-analysis. J. Infect. Dis. 220, 1243–1254. doi: 10.1093/infdis/jiz325
Wang, W., Zhang, X. H., Li, M., Hao, C. H., and Liang, H. P. (2020b). Association between vaginal infections and the types and viral loads of human papillomavirus: A clinical study based on 4,449 cases of gynecologic outpatients. Can. J. Infect. Dis. Med. Microbiol. 2020, 9172908. doi: 10.1155/2020/9172908
Wang, L., Zhu, L., Li, H., Ma, N., Huang, H., Zhang, X., et al. (2019b). Association between asymptomatic sexually transmitted infections and high-risk human papillomavirus in cervical lesions. J. Int. Med. Res. 47, 5548–5559. doi: 10.1177/0300060519865633
Wei, Z. T., Chen, H. L., Wang, C. F., Yang, G. L., Han, S. M., and Zhang, S. L. (2020). Depiction of vaginal microbiota in women with high-risk human papillomavirus infection. Front. Public Health 8. doi: 10.3389/fpubh.2020.587298
Westrich, J. A., Warren, C. J., and Pyeon, D. (2017). Evasion of host immune defenses by human papillomavirus. Virus Res. 231, 21–33. doi: 10.1016/j.virusres.2016.11.023
Williamson, A. L. (2023). Recent developments in human papillomavirus (HPV) vaccinology. Viruses 15 (7), 1440. doi: 10.3390/v15071440
Woodby, B. L., Songock, W. K., Scott, M. L., Raikhy, G., and Bodily, J. M. (2018). Induction of interferon kappa in human papillomavirus 16 infection by transforming growth factor beta-induced promoter demethylation. J. Virol. 92 (8), e01714-17. doi: 10.1128/jvi.01714-17
Wu, H. and Xue, F. (2020). Study on the correlation between persistent infection of high-risk cervical HPV and bacterial vaginosis and vaginal microecology. Maternal Child Health Care China 35, 797–800. doi: 10.19829/j.zgfybj.issn.1001-4411.2020.05.005
Xing, Z., Danhua, S., and Xiaobo, Z. (2024). Diagnostic validity of p16, E-cadherin, cyclin D1, p53, and HPV E6/E7 mRNA in CIN 3-like squamous cell carcinoma of the cervix. Front. Oncol. 14. doi: 10.3389/fonc.2024.1354838
Xu, X., Zhang, Y., Yu, L., Shi, X., Min, M., Xiong, L., et al. (2022). A cross-sectional analysis about bacterial vaginosis, high-risk human papillomavirus infection, and cervical intraepithelial neoplasia in Chinese women. Sci. Rep. 12, 6609. doi: 10.1038/s41598-022-10532-1
Xu, H., Zhang, S., Zhang, B., Jiang, N., Xu, Y., Chen, X., et al. (2025). Vaginal colonization of Lactobacilli: Mechanism and function. Microb. Pathog. 198, 107141. doi: 10.1016/j.micpath.2024.107141
Yan, S. and Wan, G. (2021). Tumor-associated macrophages in immunotherapy. FEBS J. 288, 6174–6186. doi: 10.1111/febs.15726
Yang, M., Li, L., Jiang, C., Qin, X., Zhou, M., Mao, X., et al. (2020). Co-infection with trichomonas vaginalis increases the risk of cervical intraepithelial neoplasia grade 2-3 among HPV16 positive female: a large population-based study. BMC Infect. Dis. 20, 642. doi: 10.1186/s12879-020-05349-0
Yang, X., Shui, Y., and Qian, Y. (2024a). A Crosstalk Analysis of high-risk human papillomavirus, microbiota and vaginal metabolome in cervicovaginal microenvironment. Microb. Pathog. 194, 106826. doi: 10.1016/j.micpath.2024.106826
Yang, Y., Zhu, J., Feng, R., Han, M., Chen, F., and Hu, Y. (2024b). Altered vaginal cervical microbiota diversity contributes to HPV-induced cervical cancer via inflammation regulation. PeerJ 12, e17415. doi: 10.7717/peerj.17415
Ye, J. and Qi, X. (2024). Vaginal microecology and its role in human papillomavirus infection and human papillomavirus associated cervical lesions. Apmis 132, 928–947. doi: 10.1111/apm.13356
Yuan, D., Liu, S., Liu, Y., Ouyang, F., Ai, W., Shi, L., et al. (2023). HPV Infection Profiles among People Living with HIV and HPV Vaccine Acceptance among Individuals with Different HIV Infection Statuses in China: A Systematic Meta-Analysis. Vaccines (Basel) 11 (10), 1614. doi: 10.3390/vaccines11101614
Zayats, R., Murooka, T. T., and McKinnon, L. R. (2022). HPV and the risk of HIV acquisition in women. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.814948
Zeng, Z., Wang, N., Sui, L., Zhang, R., Zhang, Q., Wang, Y., et al. (2023). Characteristics and potential diagnostic ability of vaginal microflora in patients with aerobic vaginitis using 16S Ribosomal RNA sequencing. Diagn. Microbiol. Infect. Dis. 105, 115806. doi: 10.1016/j.diagmicrobio.2022.115806
Zhang, Y., Chen, R., Zhou, Z., Qing, W., Qi, C., Ou, J., et al. (2025). Ureaplasma parvum serovar 6 may be a novel element in the progression of HPV infection to CIN: A cross-sectional study of 7058 women. J. Infect. 90, 106397. doi: 10.1016/j.jinf.2024.106397
Zhang, D., Li, T., Chen, L., Zhang, X., Zhao, G., and Liu, Z. (2017). Epidemiological investigation of the relationship between common lower genital tract infections and high-risk human papillomavirus infections among women in Beijing, China. PloS One 12, e0178033. doi: 10.1371/journal.pone.0178033
Zhang, X., Liu, S. S., Ma, J., and Qu, W. (2023). Secretory leukocyte protease inhibitor (SLPI) in cancer pathophysiology: Mechanisms of action and clinical implications. Pathol. Res. Pract. 248, 154633. doi: 10.1016/j.prp.2023.154633
Zhang, Z., Ma, Q., Zhang, L., Ma, L., Wang, D., Yang, Y., et al. (2024). Human papillomavirus and cervical cancer in the microbial world: exploring the vaginal microecology. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1325500
Zheng, J. J., Song, J. H., Yu, C. X., Wang, F., Wang, P. C., and Meng, J. W. (2019). Difference in vaginal microecology, local immunity and HPV infection among childbearing-age women with different degrees of cervical lesions in Inner Mongolia. BMC Womens Health 19, 109. doi: 10.1186/s12905-019-0806-2
Zhou, J., Tang, Z., Gao, S., Li, C., Feng, Y., and Zhou, X. (2020). Tumor-associated macrophages: recent insights and therapies. Front. Oncol. 10. doi: 10.3389/fonc.2020.00188
Zou, Z., Fairley, C. K., Ong, J. J., Hocking, J., Canfell, K., Ma, X., et al. (2020). Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis. Lancet Glob. Health 8, e1335–e1344. doi: 10.1016/s2214-109x(20)30277-1
Keywords: vagina, microecology, HPV, cervical lesions, cytokines
Citation: Cui M, Wu Y, Liu Z, Liu Y and Fan L (2025) Advances in the interrelated nature of vaginal microecology, HPV infection, and cervical lesions. Front. Cell. Infect. Microbiol. 15:1608195. doi: 10.3389/fcimb.2025.1608195
Received: 08 April 2025; Accepted: 28 May 2025;
Published: 19 June 2025.
Edited by:
António Machado, University of the Azores, PortugalReviewed by:
Anne Loonen, Fontys University of Applied Sciences, NetherlandsBhagyashri Patil-Takbhate, D.Y. Patil Vidyapeeth, India
Copyright © 2025 Cui, Wu, Liu, Liu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limei Fan, ZmFubG1Aamx1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Mingyu Cui
Mingyu Cui Yishi Wu†
Yishi Wu† Yunfei Liu
Yunfei Liu