- 1Center for Infectious Diseases, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Intensive Care Unit, People’s Hospital of Dafang, Bijie, Guizhou, China
Caspase recruitment domain containing protein 9 (CARD9) deficiency is an autosomal-recessive primary immunodeficiency disorder, undermines the body’s capacity to combat fungal infections. In recent years, the number of reported cases of fungal infections associated with CARD9 deficiency has been increasing. This study undertook a systematic review of case reports, incorporating 89 patients with CARD9 deficiency complicated by fungal infections. The findings demonstrated that the patient population predominantly consisted of young and middle-aged individuals (33.43 ± 19.12 years, range: 1-91), and the majority (52 patients, 58.43%) developed the disease during childhood or adolescence. Significant geographical variations were observed in the distribution of gene mutations. Specifically, the c.820dupG mutation was predominantly found in East Asia, while the c.865C>T mutation was primarily found North Africa. Regarding the clinical manifestations, the most frequently affected sites were the skin, central nervous system, and lymph nodes, and the principal fungal pathogens identified were Trichophyton and Candida. Correlation analysis indicated that c.883C>T increased the likelihood of Candida infection (p=0.008, OR=10.421, 95% CI 1.849-58.748), c.865C>T increased the probability of Trichophyton infection (p=0.038, OR=5.760, 95% CI 1.098-30.217) and dematiaceous fungi infection (p=0.005, OR=9.653, 95% CI 2.019-46.153). According to the types of mutations, nonsense mutation increased the risk of dematiaceous fungi infection (p=0.014, OR=6.212, 95% CI 1.453-26.556). Notably, a proportion of patients succumbed to the disease, and this was predominantly associated with infections of the central nervous system, blood system, and viscera. This underscores the importance of adequate antifungal therapy and long-term follow-up for patients with CARD9 deficiency-related fungal infections.
Introduction
CARD9 is a crucial adaptor protein in the innate immune response against fungal infections and its Online Mendelian Inheritance in Man (OMIM) number is 607212. Autosomal recessive CARD9 deficiency was first documented in 2009 within a consanguineous Iranian pedigree presenting with chronic mucocutaneous candidiasis (CMC) and dermatophytosis (Glocker et al., 2009). When the immune system detects fungal pathogens, CARD9 plays a pivotal role in the activated signaling pathways (Yazdi et al., 2023). Mutations in the CARD9 gene (NM_052813) result in CARD9 deficiency, which substantially compromises the body’s capacity to elicit an effective antifungal immune response. This disruption targets mechanisms primarily mediated by the C-type lectin receptor (CLR) and Toll-like receptor (TLR) families, which initiate defense responses against fungal pathogens (Drummond et al., 2018; Doron et al., 2021). In recent years, the number of reported cases of fungal infections associated with CARD9 deficiency has been gradually increasing. These infections present diverse clinical manifestations and can affect multiple organs and systems in the human body. Understanding the clinical features of patients with CARD9 deficiency-related fungal infections is of great significance for early diagnosis, appropriate treatment, and improving patient prognosis. However, due to the relatively rare study of CARD9 deficiency and the wide variety of fungal pathogens involved, the current comprehensive understanding of its clinical characteristics remains limited. Previous studies have been fragmented, and it is necessary to conduct a systematic review of case reports to summarize and analyze the existing data. This review aims to provide more perspectives by collecting and analyzing case reports from around the world. By systematically examining the clinical features, gene mutations, treatment strategies, and prognoses of patients with CARD9 deficiency-related fungal infections, we hope to provide valuable insights for clinicians and researchers in the fields of infectious diseases and immunology, facilitating better management of these complex cases.
Materials and methods
Literature search
The review process entailed a comprehensive exploration of all extant published literature on reported cases of fungal infections attributable to CARD9 deficiency. In the pursuit of relevant published works, a systematic search was conducted across the PubMed and China National Knowledge Infrastructure (CNKI) databases. The search terms employed were “CARD9”, “caspase recruitment domain deficiency” and “caspase recruitment domain containing protein 9”. Subsequently, the references of the initially selected papers underwent meticulous examination and screening. Articles of a review nature, those lacking detailed clinical data, and reports concerning patients without fungal infections were meticulously excluded from the analysis.
Data extraction
The following data were extracted: publication year, first author, age of the patient at the time of reporting, age of onset of the patient, patient’s gender, site of infection, fungal culture results, mutation sites, treatment regimens, treatment outcomes, whether the patient died of the disease, and patient origin. According to Melanized Fungi in Human Disease (Revankar and Sutton, 2010), the dematiaceous fungi category was extracted. According to Fungal Infection: Diagnosis and Management, Fourth Edition (Fsbath, 2012), superficial fungal infections are defined as only infections confined to the outermost layers of the skin, nails, hair, and mucous membranes. Deep fungal infections include the subcutaneous mycoses and the systemic mycoses, defined as infections of the dermis, subcutaneous tissues, and adjacent bones, as well as infections involving internal organs and vital structures. Define invasive fungal infection according to the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (Donnelly et al., 2020). We distinguish the types of gene mutations through https://www.ncbi.nlm.nih.gov/clinvar.
Regarding the treatment outcomes, a subjective classification was employed, categorizing them into five distinct groups. The “not reported” category encompassed cases where treatment outcome information was unavailable. The “ineffective” category denoted cases in which, following systematic treatment, the patient’s general condition and the results of auxiliary examinations exhibited no signs of improvement. The “slightly improved” category referred to cases showing some degree of improvement, yet with a low likelihood of achieving complete clinical remission. The “partially improved” category applied to cases demonstrating improvement and a relatively high probability of attaining complete clinical remission. Finally, the “complete clinical remission” category signified cases where the patient’s fungal infection was eradicated, and organ functions were essentially restored.
Statistical analysis
The data extracted from the study were analyzed by the SPSS 27.0 software. The Mantel-Haenszel test was used to analyze the association between different factors, with sex as the stratification factor. When the sample size (n) is≥40 and all the theoretical count under the null hypothesis (T) are≥5, choose the Pearson chi-square test. When n≥40 and at least one theoretical count meets 1≤T<5, use the continuity-corrected chi-square test (Yates’ correction). When n<40 or T<1, select Fisher’s exact test. To explore further correlations, univariate and multivariate binary logistic regression analysis were conducted. In the multivariate regression analysis, we included age, gender, and different pathogens to eliminate confounding. The outcomes of this analysis were presented in terms of odds ratios (ORs) and their corresponding 95% confidence intervals (CIs).
Results
Patient basic information
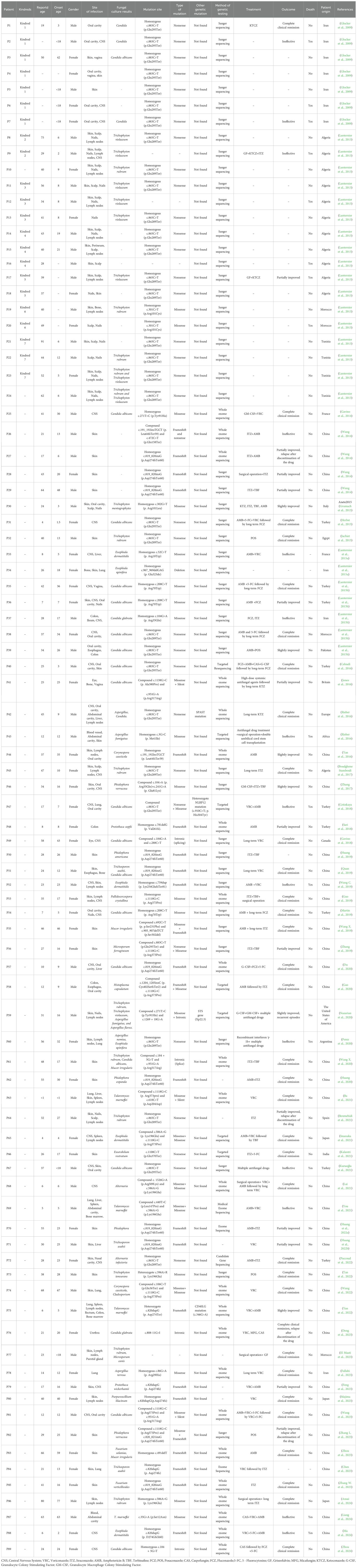
In this study, a total of 58 articles were comprehensively incorporated, involving 89 patients with CARD9 deficiency, as detailed in Table 1. Among them, 48 patients were male (56.18%). The reported average age was 33.82 ± 18.90 years (range: 1-91), and 52 patients (58.43%) whose age of onset was less than 18 years old. The patients in this study originated from 17 distinct countries. As depicted in Figure 1, the countries with the highest 3 number of cases were China (34 cases, 38.20%), Algeria (12 cases, 13.48%), and Iran (10 cases, 11.24%).
Gene variation distribution
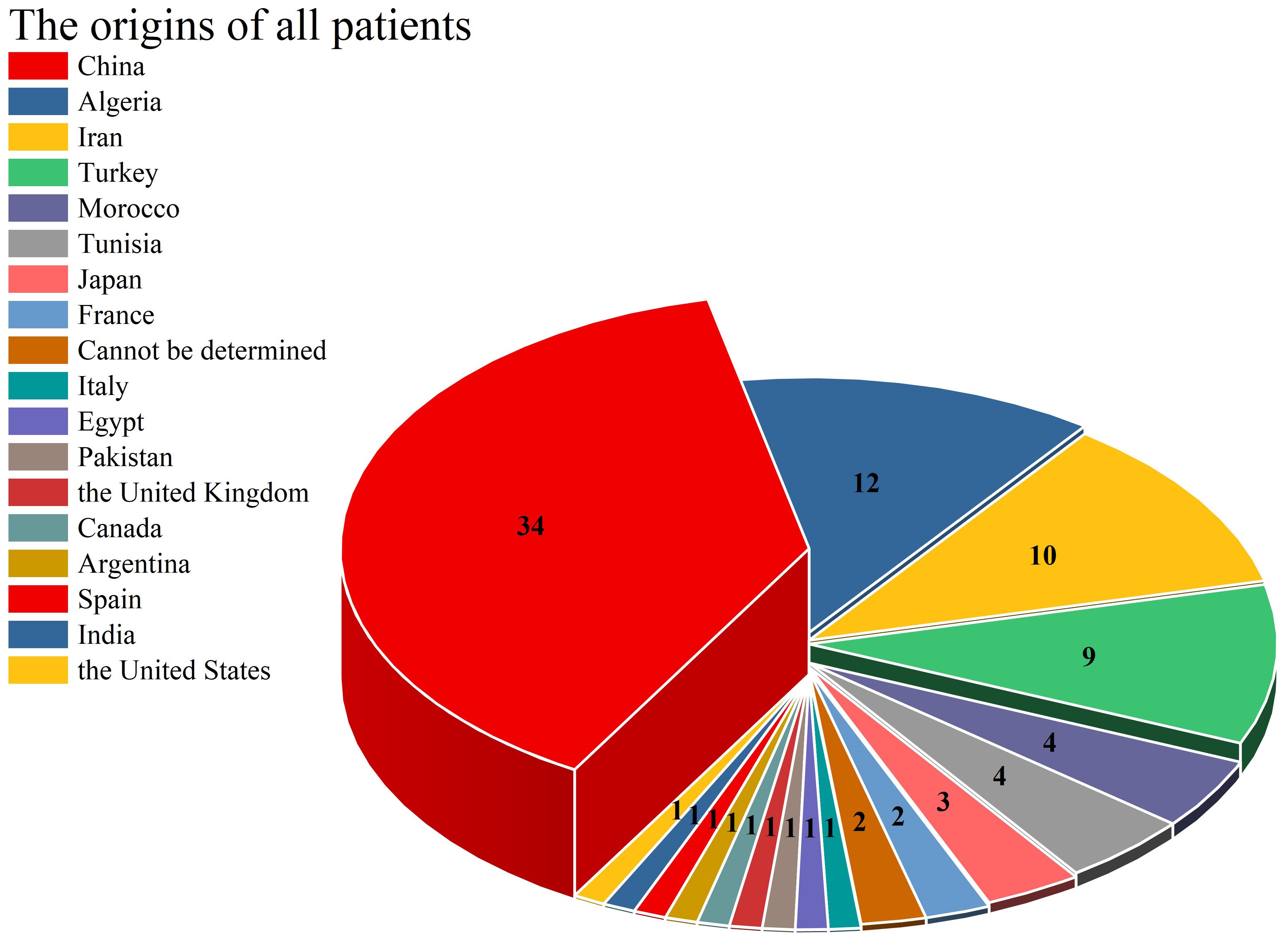
As illustrated in Figure 2, this article comprehensively encompasses a total of 38 CARD9 gene mutations. The 5 most frequently occurring mutations are as follows: c.865C>T (18 cases), c.883C>T (14 cases), c.819-820insG (12 cases), c.1118G>C (9 cases) and c.820dupG (5 cases). The “others” segment in Figure 2 encompasses 27 distinct gene mutations, each with a frequency of only one instance. These mutations are c.472C>T, c.302G>T, c.52C>T, c.967_969delGAG,c.1138G>C,c.3G>C,c.241G>A,c.781delG,c.184G>A,c.288C>T,c.759dup,c.692C>T,c.905_907delTCT,c.1204_1205insC,c.1269 + 18G>A,c.610C>T, c.1108C>T, c.1526G>A, c.440T>C, c.596A>R, c.106C>T, c.808-11G>I, c.86G>A, c.491delT, and c.35G>A. The CARD9 gene and related gene mutations are shown in Figure 3. There are 6 types of gene mutations: nonsense (30 cases), missense (29 cases), frameshift (23 cases), deletion (1 cases), silent (2 cases), and intronic (6 cases) mutation.
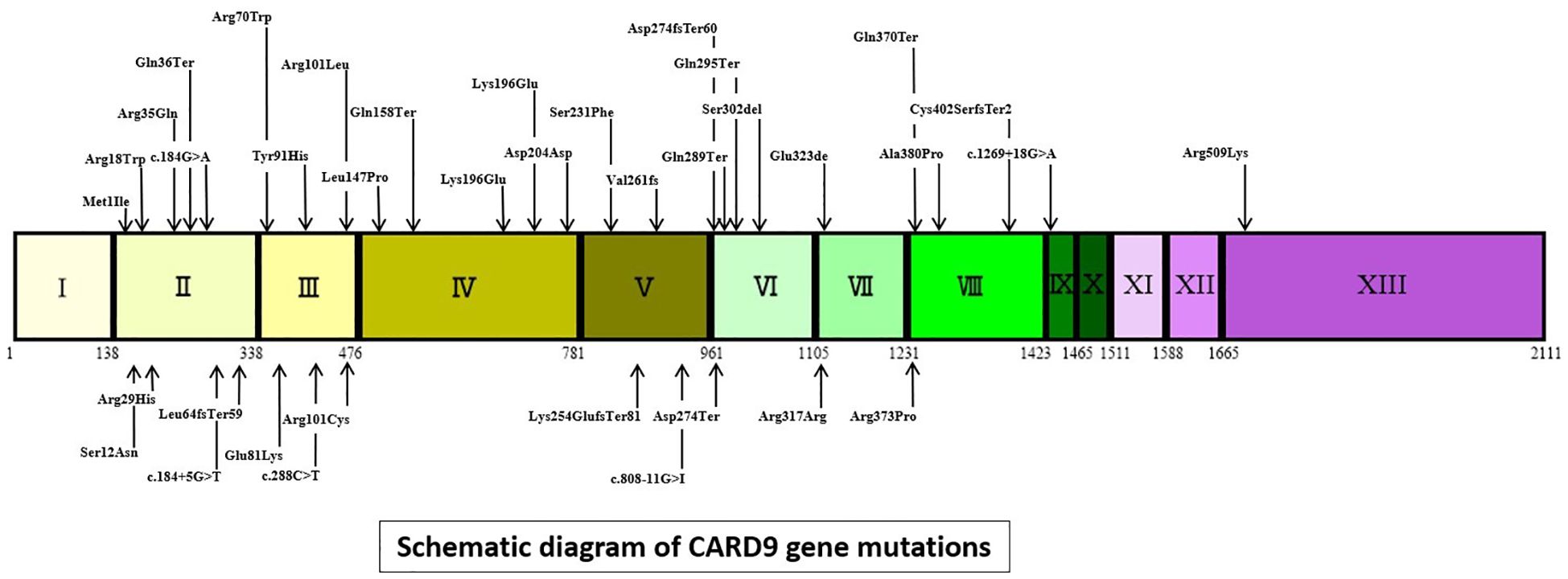
Figure 3. Schematic diagram of CARD9 gene mutations (intronic mutations represented by gene changes, other mutations denoted by amino acid changes. I to XIII = exons of CARD9, Coding DNA Sequence:155-1765).
Clinical features
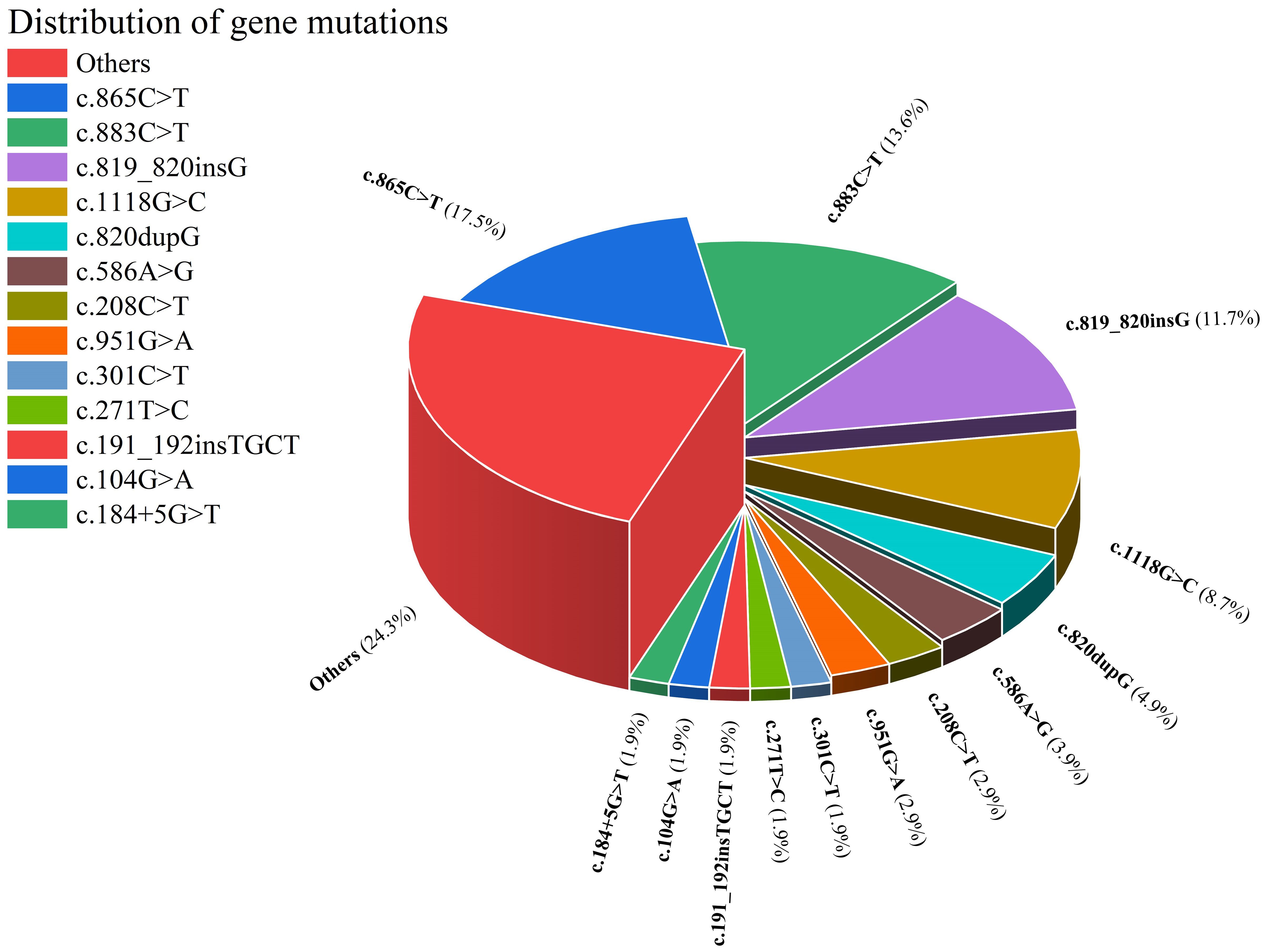
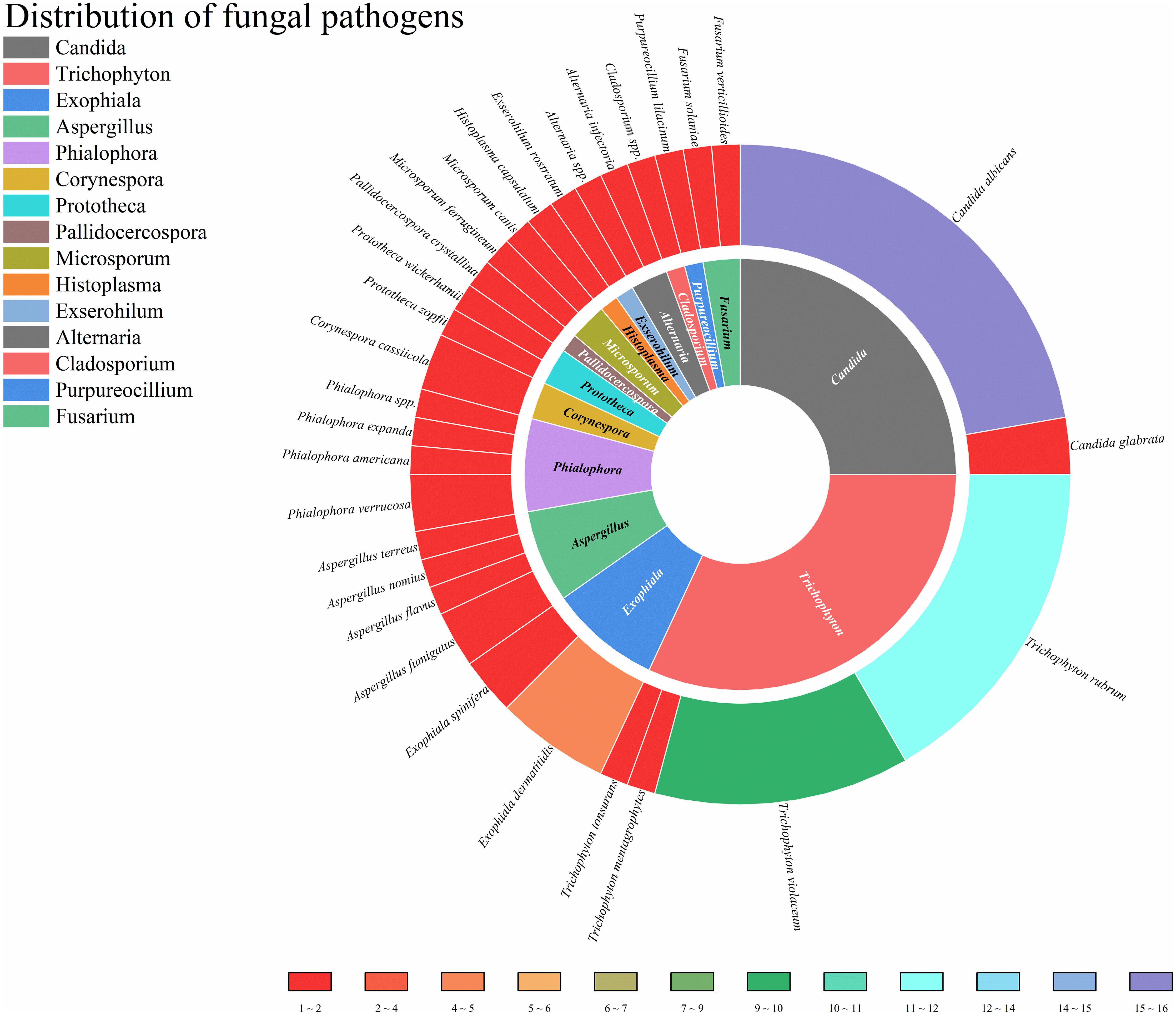
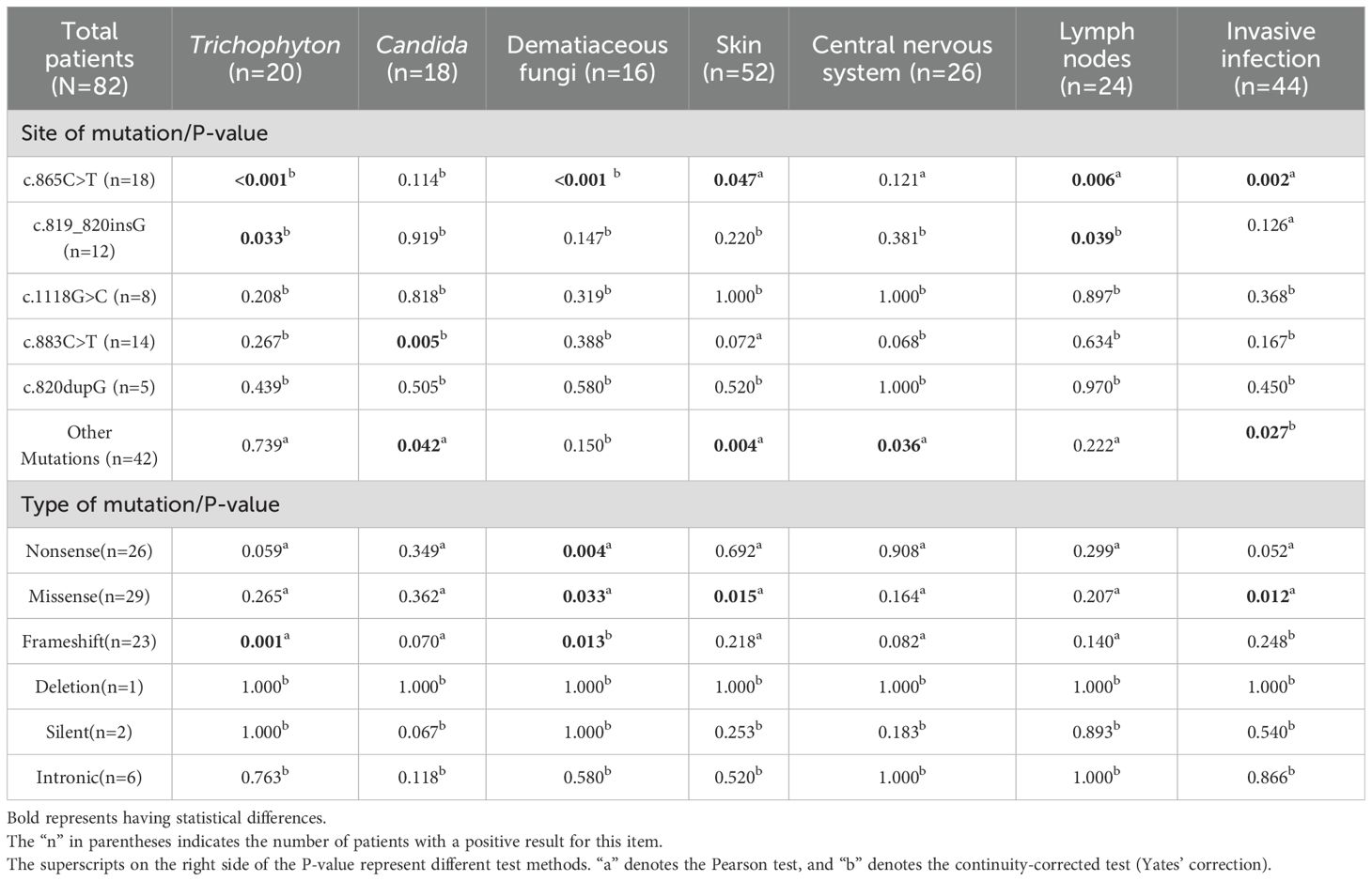
This study enrolled patients with fungal infections involving 18 distinct anatomical sites, as depicted in Figure 4. All patients had deep infections. Among them, 32.82% were invasive infections and 67.18% were non-invasive infections. The 3 most commonly affected sites were the skin, central nervous system, and lymph nodes. In terms of taxonomic classification at the genus level, Trichophyton and Candida were the 2 most prevalent pathogens, as illustrated in Figure 5. Dematiaceous fungi (16 cases) including: Exophiala, Phialophora, Corynespora, Exserohilum, Alternaria, and Cladosporium. In addition to standard antifungal pharmacotherapy, diverse treatment modalities were employed. Colony-stimulating factor (CSF) was administered to 5 patients (P18, P33, P39, P50, P52), surgical interventions were performed on 6 patients (P21, P36, P46, P61, P70, P79), and 1 patient (P53) received recombinant interferon γ-1b treatment. According to the clinical outcomes, they were classified into the following 5 categories: not reported (22 cases, 24.71%), ineffective (14 cases, 15.73%), slightly improved (6 cases, 6.74%), partially improved (13 cases, 14.61%), and complete clinical remission (34 cases,38.20%). Unfortunately, 16 patients (17.98%) succumbed to the disease.
The relationship among genes, fungal pathogens and infection sites
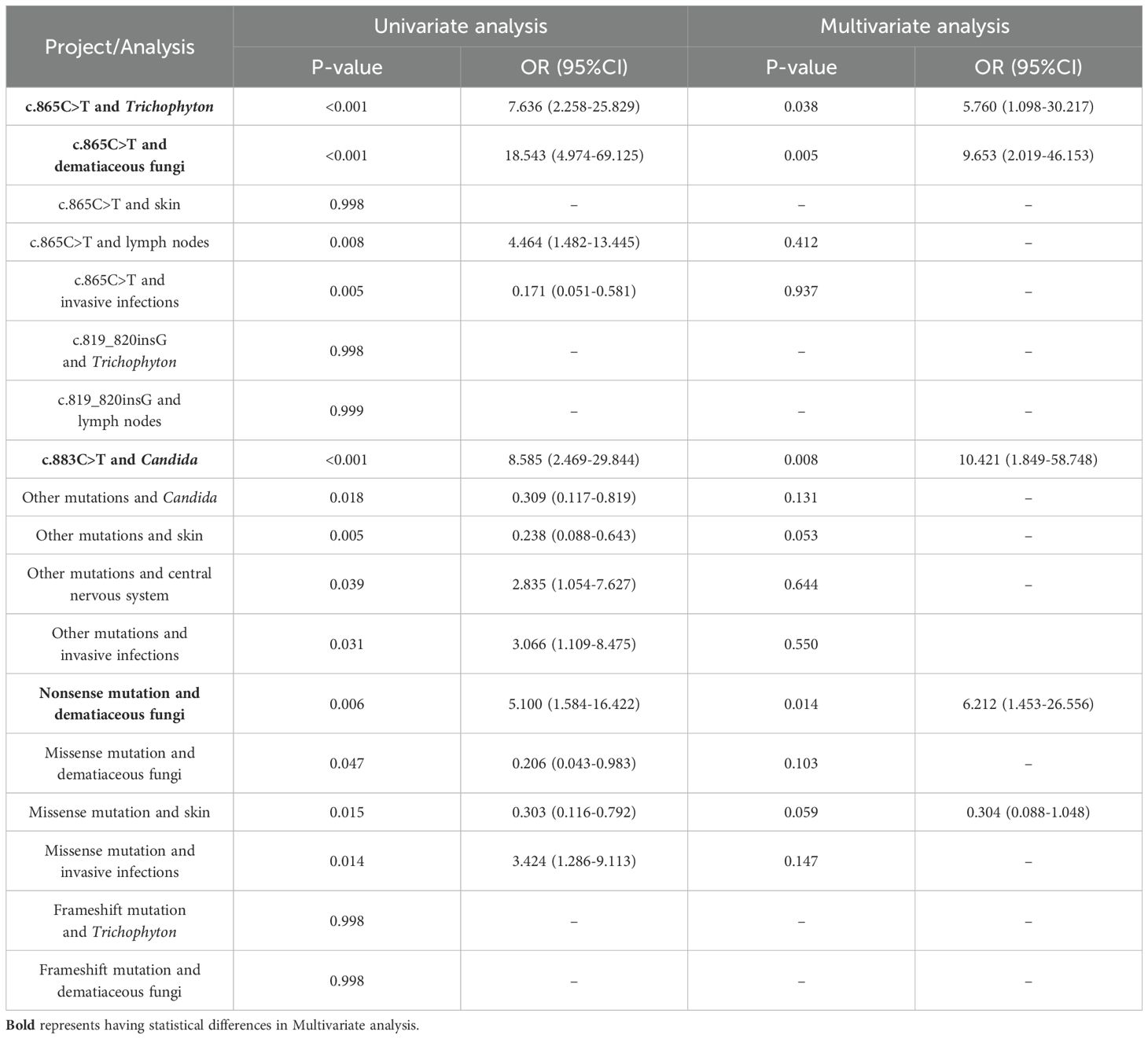
To explore the relationships among various factors, we included the top 5 most frequent gene mutations (c.865C>T, c.819_820insG, c.1118G>C, c.883C>T, c.820dupG), gene mutations not in the top 5 (other mutations), Trichophyton, Candida, dematiaceous fungi, the top 3 most frequent anatomical sites (skin, CNS, lymph nodes), as well as invasive infections in the data analysis. Initially, the Mantel-Haenszel test was employed to assess the relationships between these factors. This statistical approach identified 18 significant associations, as detailed in Table 2: c.865C>T and Trichophyton, c.865C>T and dematiaceous fungi, c.865C>T and skin, c.865C>T and lymph nodes, c.865C>T and invasive infections, c.819_820insG and Trichophyton, c.819_820insG and lymph nodes, c.883C>T and Candida, other mutations and Candida, other mutations and skin, other mutations and central nervous system, other mutations and invasive infections, nonsense mutation and dematiaceous fungi, missense mutation and dematiaceous fungi, missense mutation and skin, missense mutation and invasive infections, frameshift mutation and Trichophyton, frameshift mutation and dematiaceous fungi. Subsequently, binary logistic regression analysis was carried out on these 19 identified associations to further quantify the relationships and estimate the strength of the associations, as presented in Table 3. The results indicated that c.883C>T increased the likelihood of Candida infections(p=0.008, OR=10.421, 95% CI 1.849-58.748), c.865C>T increased the probability of Trichophyton infections (p=0.038, OR=5.760, 95% CI 1.098-30.217) and dematiaceous fungi (p=0.005, OR=9.653, 95% CI 2.019-46.153). According to the types of mutation, nonsense mutation increased the risk of dematiaceous fungi infections (p=0.014, OR=6.212, 95% CI 1.453-26.556).
Discussion
CARD9, a pivotal downstream component of pattern recognition receptors (PRRs), plays a central role in mediating a cascade of inflammatory responses against invasive fungi, bacteria, viruses, and parasites. Mutations in the CARD9 gene, which lead to reduced expression and functional impairment, are associated with an autosomal recessive primary immunodeficiency disorder. This genetic defect renders affected individuals highly susceptible to microbial infections. The PRRs/Syk/CARD9 signaling pathway, situated downstream of PRRs, is one of the most well-characterized and fundamental signaling cascades in the immune response (Hu et al., 2022). CARD9-related C-type lectin receptors (CLRs) primarily include Dectin-1, Dectin-2, Dectin-3, and Mincle. Upon recognition of carbohydrate agonists, these CLRs recruit the tyrosine kinase Syk following Src kinase-mediated tyrosine phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM)-like motifs (hem-ITAMs) or canonical ITAMs within their cytoplasmic tails (Rogers et al., 2005; Drummond et al., 2011). Syk serves as a pivotal signaling mediator, coupling activated immunoreceptors to downstream pathways in immune cells. Following recruitment, Syk undergoes phosphorylation, triggering the activation of protein kinase Cδ (PKCδ). This, in turn, facilitates the recruitment and phosphorylation of CARD9 at Thr231, initiating downstream signaling cascades (Wang Y. et al., 2020).Animals with a genetic deletion of Card9 are susceptible to challenge with a variety of fungal species, including Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans, and some rarer dematiaceous fungi (Drummond et al., 2018).
The demographic profile of patients with CARD9-deficiency-associated fungal infections predominantly comprises young and middle-aged individuals. A significant proportion, specifically 57.32% (47 cases) of the patients, experience disease onset during childhood or adolescence. Notably, there are distinct geographical variations in the distribution of CARD9 gene mutations. For instance, the c.820dupG mutation is predominantly observed in East Asia, a finding that aligns with previous research by Tomomasa et al. (Tomomasa et al., 2024). Additionally, our study identified that the c.819-820insG and c.1118G>C mutations are uniquely present in the East Asian region, with 819-820insG being reported exclusively in China. In the case series presented by Lanternier et al. (Lanternier et al., 2013), all 12 patients with the c. 865C>T mutation were from Algeria, Morocco, and Tunisia. Over the past 12 years, 6 additional cases of this mutation have been reported, of which only 3 were from Spain, Turkey and Argentina, and the rest were from the above-mentioned North African countries, indicating that c.865C>T is mainly distributed in North Africa.
Fungal infections associated with CARD9 deficiency exhibit remarkable heterogeneity. The present study documented involvement of 18 distinct anatomical sites and identified 19 different genera of fungal pathogens. Among them, Candida and Trichophyton were the most isolated fungi. Meanwhile, fungal infections in CARD9-deficient patients showed a tendency toward severe invasiveness. According to the classification criteria of Classification and Nomenclature of Fungi, Fungal diseases (Fsbath, 2012), all patients met the criteria for deep infection (involving at least the dermis and subcutaneous tissues). According to the definition of invasive fungal infection (Donnelly et al., 2020), 32.82% of patients had definite invasive infections. Through correlation analysis, we found that the c.883C>T mutation significantly increased the likelihood of Candida infection, consistent with the analysis by Vaezi (Vaezi et al., 2018) and Dantas (Dantas et al., 2024). Moreover, the c.865C>T mutation was associated with an elevated probability of Trichophyton and dematiaceous fungi infection. A previous study (Vaezi et al., 2018) reported an association between c.819-820insG and disseminated phaeohyphomycosis (OR=2.42, 95%CI 1.84–3.2, p<0.001), and we did not find similar results.
The c.883C>T mutation in the CARD9 gene results from the substitution of cytosine (C) with thymine (T) at nucleotide position 883, leading to the premature formation of a stop codon. This reduces the short-term killing ability of CARD9-deficient neutrophils against unopsonized Candida albicans conidia (Gazendam et al., 2014; Corvilain et al., 2018). The c.865C>T mutation, where the cytosine (C) at nucleotide position 865 is replaced by thymine (T), results in a premature stop codon. This mutation inhibits the release of inflammatory cytokines such as IL-6, IL-1β, and IL-17A, potentially serving as the underlying mechanism for Trichophyton infections (Lanternier et al., 2013; Tan et al., 2022). This may explain the different pathogen susceptibilities associated with the two gene mutations. Dematiaceous fungi have been reported to cause subcutaneous and invasive infections, including chromoblastomycosis, phaeohyphomycosis, and mycetoma (McGinnis, 1983). A study investigating the response to pathogenic dematiaceous fungi in Card9-knockout mice found that the inability to control these fungi was associated with a lack of Th17 differentiation and reduced levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-17A in footpad homogenates (Wu et al., 2016). Previous research has not explored the relationship between mutation types and pathogens. We found that nonsense mutations increased the risk of dematiaceous fungi infections, yet the c.883C>T mutation, a relatively frequent nonsense mutation, did not exhibit this association. This discrepancy may be related to epidemiological differences. Although there is limited epidemiological data on dematiaceous fungi in Africa, a study on chromoblastomycosis prevalence, showed that Africa has the second-highest incidence after South America, while the c.883C>T mutation is absent in both regions.
Among the 82 patients included in this study, 13 succumbed to the disease. The majority of these fatal cases were associated with infections of the central nervous system, blood system, and/or viscera. This poor prognosis can be attributed, at least in part, to the reduced effectiveness to antifungal medications, which is a consequence of genetic defects in these patients. The prognosis of CARD9 patients is associated with co-existing mutations in other genes, some of which may exhibit synergistic effects. For example, co-mutations in the DOCK8 gene can lead to severe fungal infections (El Hawary et al., 2022). The genetic heterogeneity of inborn errors of immunity and diagnostic delays in atypical cases lead to significant morbidity and mortality. Establishing a definitive genetic diagnosis is crucial for patient management (Ripen et al., 2021). Among the patients included in this study, 28.05% (23/82) of the patients underwent whole exome sequencing. Only 4 cases were found to have mutations in other genes: P35 (SPAST mutation) (Rieber et al., 2016), P40 (NLRP12 mutation) (Cetinkaya et al., 2018), P52 (STS gene mutation) (Nazarian et al., 2020), and P68 (CD40LG mutation) (Yan et al., 2022). The latter 3 gene mutations are associated with infections, and in these 3 patients, the disease is more severe and the treatment is more difficult. Granulocyte colony stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF) exert pleiotropic effects on the innate immune system by enhancing the function of human neutrophils (Lin et al., 2024). While their efficacy has been demonstrated in individual case reports (Gavino et al., 2014; Du et al., 2020), large-scale clinical trials are still lacking. Nevertheless, they represent valuable salvage treatment options for patients who do not respond adequately to conventional antifungal therapy.
In conclusion, CARD9 deficiency should be considered in the differential diagnosis of patients presenting with progressive fungal infections of unknown etiology. Early initiation of antifungal treatment is crucial for improving patient outcomes, and long-term prophylactic treatment and regular follow-up are essential components of comprehensive management strategies.
Limitations
1. Our judgment of the patients’ clinical outcomes was subjective and only represented their conditions at that time, which might lead to a certain degree of bias.
2. There was no subjective classification of anatomical sites, such as the scalp and skin. However, for the integrity of the data, we directly extracted the sites stated in the articles. This might have some impact on the results.
3. Limited by the low prevalence of CARD9 deficiency, the statistical results may not reflect the true situation, especially for the interpretation of OR values.
4. This study did not include all CARD9 patients. It only included case reports and case series, and excluded patients without detailed clinical data and those with non-fungal infections.
Conclusion
In the contemporary landscape of medical research, there has been a burgeoning focus on non-HIV-associated opportunistic infections, which has emerged as a crucial area of investigation due to their increasing prevalence and clinical significance. This study retrospectively analyzed 82 patients with CARD9 deficiency complicated by fungal infections and found significant differences in clinical symptoms, fungal pathogens, and gene mutation sites. It provides potential relationships between gene mutations, pathogens, infection sites, and regional distributions, aiming to enhance the understanding of this disease.
Author contributions
CT: Methodology, Writing – original draft, Data curation, Software. YL: Software, Writing – original draft, Data curation. JL: Investigation, Writing – review & editing. XL: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ansai, O., Hayashi, R., Nakamura, A., Sasaki, J., Hasegawa, A., Deguchi, T., et al. (2024). Deep dermatophytosis caused by Trichophyton rubrum in an elderly patient with CARD9 deficiency: A case report and literature review. J. Dermatol. 51, 294–300. doi: 10.1111/1346-8138.16995
Ba, H., Peng, H., Cheng, L., Lin, Y., Li, X., He, X., et al. (2021). Case report: talaromyces marneffei infection in a chinese child with a complex heterozygous CARD9 mutation. Front. Immunol. 12, 685546. doi: 10.3389/fimmu.2021.685546
Benmehidi, N., Maatouk, I., Puel, A., Boussaid, R., and Belkacem, F. A. (2021). A new case of deep dermatophytic disease with inherited CARD9 deficiency. Int. J. Dermatol. 60, e15–ee6. doi: 10.1111/ijd.15294
Boudghene Stambouli, O., Amrani, N., Boudghéne Stambouli, K., and Bouali, F. (2017). Dermatophytic disease with deficit in CARD9: A new case with a brain impairment. J. Mycol. Med. 27, 250–253. doi: 10.1016/j.mycmed.2017.01.001
Celmeli, F., Oztoprak, N., Turkkahraman, D., Seyman, D., Mutlu, E., Frede, N., et al. (2016). Successful granulocyte colony-stimulating factor treatment of relapsing candida albicans meningoencephalitis caused by CARD9 deficiency. Pediatr. Infect. Dis. J. 35, 428–431. doi: 10.1097/INF.0000000000001028
Cetinkaya, P. G., Ayvaz, D. C., Karaatmaca, B., Gocmen, R., Söylemezoğlu, F., Bainter, W., et al. (2018). A young girl with severe cerebral fungal infection due to card 9 deficiency. Clin. Immunol. 191, 21–26. doi: 10.1016/j.clim.2018.01.002
Chen, W., Li, S., and Xu, H. (Eds.) (2023). “A case of cutaneous trichosporon asahii infection associated with CARD9 deficiency,” in 2023 National Conference on Integrated Traditional and Western Medicine in Dermatology and Venereology(Kunming, Yunnan, China).
Corvilain, E., Casanova, J. L., and Puel, A. (2018). Inherited CARD9 deficiency: invasive disease caused by ascomycete fungi in previously healthy children and adults. J. Clin. Immunol. 38, 656–693. doi: 10.1007/s10875-018-0539-2
Dantas, M. D. S., Cintra, M. E. C., Lucini, F., Venturini, J., de Souza, G. H. A., and Rossato, L. (2024). CARD9 mutations in patients with fungal infections: An update from the last 5 years. Mycoses 67, e13712. doi: 10.1111/myc.13712
Deng, R., Meng, X., Li, R., Wang, A., and Song, Y. (2023). Asymptomatic Candida glabrata urinary tract infection in an immunocompetent young female: A case report. Med. (Baltimore). 102, e33798. doi: 10.1097/MD.0000000000033798
Donnelly, J. P., Chen, S. C., Kauffman, C. A., Steinbach, W. J., Baddley, J. W., Verweij, P. E., et al. (2020). Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 71, 1367–1376. doi: 10.1093/cid/ciz1008
Doron, I., Leonardi, I., Li, X. V., Fiers, W. D., Semon, A., Bialt-DeCelie, M., et al. (2021). Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 184, 1017–31.e14. doi: 10.1016/j.cell.2021.01.016
Drummond, R. A., Franco, L. M., and Lionakis, M. S. (2018). Human CARD9: A critical molecule of fungal immune surveillance. Front. Immunol. 9, 1836. doi: 10.3389/fimmu.2018.01836
Drummond, R. A., Saijo, S., Iwakura, Y., and Brown, G. D. (2011). The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur. J. Immunol. 41, 276–281. doi: 10.1002/eji.201041252
Du, B., Shen, N., Hu, J., Tao, Y., Mo, X., and Cao, Q. (2020). Complete clinical remission of invasive Candida infection with CARD9 deficiency after G-CSF treatment. Comp. Immunol. Microbiol. Infect. Dis. 70, 101417. doi: 10.1016/j.cimid.2020.101417
El Hawary, R. E., Meshaal, S. S., Abd Elaziz, D. S., Alkady, R., Lotfy, S., Eldash, A., et al. (2022). Genetic testing in Egyptian patients with inborn errors of immunity: a single-center experience. J. Clin. Immunol. 42, 1051–1070. doi: 10.1007/s10875-022-01272-y
El Maati, M., Berjaou, Z., Hammouch, Z., Khachani, K., Moustakbal, I., Meziane, M., et al. (2023). Surgical management of deep dermatophytosis in a patient with CARD9 deficiency. J. Surg. Case Rep. 2023, rjad388. doi: 10.1093/jscr/rjad388
Fallahi, M., Mahdaviani, S. A., Shafiei, M., Ghadimi, S., Rezaei, N., Klein, C., et al. (2023). CARD9 deficiency with allergic bronchopulmonary aspergillosis (ABPA)-like presentation: a case report. Oxf. Med. Case Rep. 2023, omad103. doi: 10.1093/omcr/omad103
Feng, Y., Xiong, X., Su, Z., Su, T., Zhang, M., Yin, Z., et al. (2023). Disseminated Prototheca wickerhamii infection in a patient with CARD9 gene mutation. J. Dermatol. 50, e342–e3e3. doi: 10.1111/1346-8138.16852
Fsbath, M. D. R. (2012). Introduction. Fungal Infection: Diagnosis and Management. 4th ed. (John Wiley & Sons Ltd, Chichester, UK), 1–11.
Gao, T. J., Zhang, H. H., Wu, H. Y., Liu, H. Y., and Fang, Y. (2020). CARD9 gene mutation in a patient with intestinal histoplasmosis. Zhonghua. Er. Ke. Za. Zhi. 58, 600–601. doi: 10.3760/cma.j.cn112140‑20191231‑00843
Gavino, C., Cotter, A., Lichtenstein, D., Lejtenyi, D., Fortin, C., Legault, C., et al. (2014). CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin. Infect. Dis. 59, 81–84. doi: 10.1093/cid/ciu215
Gavino, C., Mellinghoff, S., Cornely, O. A., Landekic, M., Le, C., Langelier, M., et al. (2018). Novel bi-allelic splice mutations in CARD9 causing adult-onset Candida endophthalmitis. Mycoses 61, 61–65. doi: 10.1111/myc.12701
Gazendam, R. P., van Hamme, J. L., Tool, A. T., van Houdt, M., Verkuijlen, P. J., Herbst, M., et al. (2014). Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood 124, 590–597. doi: 10.1182/blood-2014-01-551473
Glocker, E. O., Hennigs, A., Nabavi, M., Schäffer, A. A., Woellner, C., Salzer, U., et al. (2009). A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735. doi: 10.1056/NEJMoa0810719
Grumach, A. S., de Queiroz-Telles, F., Migaud, M., Lanternier, F., Filho, N. R., Palma, S. M., et al. (2015). A homozygous CARD9 mutation in a Brazilian patient with deep dermatophytosis. J. Clin. Immunol. 35, 486–490. doi: 10.1007/s10875-015-0170-4
Guo, Y., Zhu, Z., Gao, J., Zhang, C., Zhang, X., Dang, E., et al. (2019). The phytopathogenic fungus pallidocercospora crystallina-caused localized subcutaneous phaeohyphomycosis in a patient with a homozygous missense CARD9 mutation. J. Clin. Immunol. 39, 713–725. doi: 10.1007/s10875-019-00679-4
Herbst, M., Gazendam, R., Reimnitz, D., Sawalle-Belohradsky, J., Groll, A., Schlegel, P. G., et al. (2015). Chronic candida albicans meningitis in a 4-year-old girl with a homozygous mutation in the CARD9 gene (Q295X). Pediatr. Infect. Dis. J. 34, 999–1002. doi: 10.1097/INF.0000000000000736
Hu, A., Hu, Z., Zou, H., Zhang, J., Zhang, D., Wang, H., et al. (2022). CARD9 in host immunity to fungal, bacterial, viral, and parasitic infections: An update. Front. Microbiol. 13, 1021837. doi: 10.3389/fmicb.2022.1021837
Huang, C., Dan, W., and An, K. (2020). First report of Phialophora expanda causing chromoblastomycosis with CARD9 deficiency: a case report. Chin. J. Mycol. 15, 230–232.
Huang, C., Deng, W., Zhang, Y., Zhang, K., Ma, Y., Song, Y., et al. (2022a). CARD9 deficiency predisposing chromoblastomycosis: A case report and comparative transcriptome study. Front. Immunol. 13, 984093. doi: 10.3389/fimmu.2022.984093
Huang, C., Liu, B., Zhang, Y., Song, Y., Liu, X., Wan, Z., et al. (2022b). Disseminated trichosporosis in a young patient with CARD9 deficiency. Clin. Microbiol. Infect. 28, 681–683. doi: 10.1016/j.cmi.2021.06.012
Huang, C., Zhang, Y., Song, Y., Wan, Z., Wang, X., and Li, R. (2019). Phaeohyphomycosis caused by Phialophora americana with CARD9 mutation and 20-year literature review in China. Mycoses 62, 908–919. doi: 10.1111/myc.12962
Imanaka, Y., Taniguchi, M., Doi, T., Tsumura, M., Nagaoka, R., Shimomura, M., et al. (2021). Inherited CARD9 Deficiency in a Child with Invasive Disease Due to Exophiala dermatitidis and Two Older but Asymptomatic Siblings. J. Clin. Immunol. 41, 975–986. doi: 10.1007/s10875-021-00988-7
Jachiet, M., Lanternier, F., Rybojad, M., Bagot, M., Ibrahim, L., Casanova, J. L., et al. (2015). Posaconazole treatment of extensive skin and nail dermatophytosis due to autosomal recessive deficiency of CARD9. JAMA Dermatol. 151, 192–194. doi: 10.1001/jamadermatol.2014.2154
Jones, N., Garcez, T., Newman, W., and Denning, D. (2016). Endogenous Candida endophthalmitis and osteomyelitis associated with CARD9 deficiency. BMJ Case Rep. 2016. doi: 10.1136/bcr-2015-214117
Kalantri, M., Khopkar, U., Shah, A., Bargir, U. A., Hule, G., and Madkaikar, M. (2021). A case of disseminated subcutaneous phaeohyphomycosis caused by Exserohilum rostratum with CARD9 mutation. Indian J. Dermatol. Venereol. Leprol. 88, 59–61. doi: 10.25259/IJDVL_293_19
Kuruoğlu, T., Çelik, M., Çelmeli, F., and Tanyel, E. (2021). CARD9 mutation in a patient with candida albicans meningoencephalitis; A case report. Mikrobiyol. Bul. 55, 656–664. doi: 10.5578/mb.20219717
Lai, S. H. Y., Duque, J. S. R., Chung, B. H., Chung, T. W., Leung, D., Ho, R. S., et al. (2021). Invasive cerebral phaeohyphomycosis in a Chinese boy with CARD9 deficiency and showing unique radiological features, managed with surgical excision and antifungal treatment. Int. J. Infect. Dis. 107, 59–61. doi: 10.1016/j.ijid.2021.04.052
Lanternier, F., Barbati, E., Meinzer, U., Liu, L., Pedergnana, V., Migaud, M., et al. (2015a). Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J. Infect. Dis. 211, 1241–1250. doi: 10.1093/infdis/jiu412
Lanternier, F., Mahdaviani, S. A., Barbati, E., Chaussade, H., Koumar, Y., Levy, R., et al. (2015b). Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J. Allergy Clin. Immunol. 135, 1558–68.e2. doi: 10.1016/j.jaci.2014.12.1930
Lanternier, F., Pathan, S., Vincent, Q. B., Liu, L., Cypowyj, S., Prando, C., et al. (2013). Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 369, 1704–1714. doi: 10.1056/NEJMoa1208487
Liang, H., Duan, X., Li, T., Hu, L., and Guo, J. (2024). Disseminated Combined Talaromyces marneffei and Enterococcus faecium Bloodstream Infection Presenting as Gastrointestinal Perforation in a Patient with CARD9 Gene Mutation. Infect. Drug Resist. 17, 4783–4790. doi: 10.2147/IDR.S479629
Lin, S., Moreinos, D., Mavridou, A. M., Novak, R., Rotstein, I., and Abbott, P. V. (2024). The role of infection in signalling root resorption: A narrative review. Int. Endod. J. 57, 1727–1744. doi: 10.1111/iej.14132
Ma, N., Zhao, Y., Tang, M., Xia, H., Li, D., and Lu, G. (2024). Concurrent infection of Exophiala dermatitidis and Angiostrongylus cantonensis in central nervous system of a child with inherited CARD9 deficiency: A case report and literature review. J. Mycol. Med. 34, 101455. doi: 10.1016/j.mycmed.2023.101455
Majima, H., Inoue, Y., Otsuka, Y., Yaguchi, T., Watanabe, A., and Kamei, K. (2023). Lymphadenitis caused by Purpureocillium lilacinum in a patient with CARD9 deficiency. Med. Mycol. Case Rep. 42, 100609. doi: 10.1016/j.mmcr.2023.100609
Martin, S., Balligand, E., Peeters, J., Nassogne, M. C., Mondovits, B., Loop, M., et al. (2019). A 7-year-old child with headaches and prolonged fever associated with oral and nail lesions. Open Forum Infect. Dis. 6, ofz229. doi: 10.1093/ofid/ofz229
McGinnis, M. R. (1983). Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J. Am. Acad. Dermatol. 8, 1–16. doi: 10.1016/S0190-9622(83)70001-0
Nazarian, R. M., Lilly, E., Gavino, C., Hamilos, D. L., Felsenstein, D., Vinh, D. C., et al. (2020). Novel CARD9 mutation in a patient with chronic invasive dermatophyte infection (tinea profunda). J. Cutan. Pathol. 47, 166–170. doi: 10.1111/cup.13574
Paccoud, O., Vignier, N., Boui, M., Migaud, M., Vironneau, P., Kania, R., et al. (2022). Invasive rhinosinusitis caused by alternaria infectoria in a patient with autosomal recessive CARD9 deficiency and a review of the literature. J. Fungi. (Basel). 8. doi: 10.3390/jof8050446
Perez, L., Messina, F., Negroni, R., Arechavala, A., Bustamante, J., Oleastro, M., et al. (2020). Inherited CARD9 Deficiency in a Patient with Both Exophiala spinifera and Aspergillus nomius Severe Infections. J. Clin. Immunol. 40, 359–366. doi: 10.1007/s10875-019-00740-2
Quan, C., Li, X., Shi, R. F., Zhao, X. Q., Xu, H., Wang, B., et al. (2019). Recurrent fungal infections in a Chinese patient with CARD9 deficiency and a review of 48 cases. Br. J. Dermatol. 180, 1221–1225. doi: 10.1111/bjd.17092
Revankar, S. G. and Sutton, D. A. (2010). Melanized fungi in human disease. Clin. Microbiol. Rev. 23, 884–928. doi: 10.1128/CMR.00019-10
Rieber, N., Gazendam, R. P., Freeman, A. F., Hsu, A. P., Collar, A. L., Sugui, J. A., et al. (2016). Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI Insight 1, e89890. doi: 10.1172/jci.insight.89890
Ripen, A. M., Chear, C. T., Baharin, M. F., Nallusamy, R., Chan, K. C., Kassim, A., et al. (2021). A single-center pilot study in Malaysia on the clinical utility of whole-exome sequencing for inborn errors of immunity. Clin. Exp. Immunol. 206, 119–128. doi: 10.1111/cei.13626
Rogers, N. C., Slack, E. C., Edwards, A. D., Nolte, M. A., Schulz, O., Schweighoffer, E., et al. (2005). Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517. doi: 10.1016/j.immuni.2005.03.004
Sari, S., Dalgic, B., Muehlenbachs, A., DeLeon-Carnes, M., Goldsmith, C. S., Ekinci, O., et al. (2018). Prototheca zopfii colitis in inherited CARD9 deficiency. J. Infect. Dis. 218, 485–489. doi: 10.1093/infdis/jiy198
Tan, J., Yu, Q., Gao, Z., Yang, H., Chen, Q., and Yang, L. (2022). Case report: Severe deep ulcer on the left abdomen mimicking mycosis fungoides caused by Trichophyton tonsurans in a patient with novel CARD9 mutation. Front. Immunol. 13, 1015000. doi: 10.3389/fimmu.2022.1015000
Tomomasa, D., Lee, B. H., Hirata, Y., Inoue, Y., Majima, H., Imanaka, Y., et al. (2024). Inherited CARD9 deficiency due to a founder effect in east asia. J. Clin. Immunol. 44, 121. doi: 10.1007/s10875-024-01724-7
Vaezi, A., Fakhim, H., Abtahian, Z., Khodavaisy, S., Geramishoar, M., Alizadeh, A., et al. (2018). Frequency and geographic distribution of CARD9 mutations in patients with severe fungal infections. Front. Microbiol. 9, 2434. doi: 10.3389/fmicb.2018.02434
Wang, J., An, Y., and Zhang, G. (2023). A case of infectious encephalomyelitis caused by CARD9 gene-associated candida albicans infection. Zhonghua. Er. Ke. Za. Zhi. 61, 737–739. doi: 10.3760/cma.j.cn112140-20230104-00005
Wang, X., Ding, H., Chen, Z., Zeng, X., Sun, J., Chen, H., et al. (2020). CARD9 deficiency in a chinese man with cutaneous mucormycosis, recurrent deep dermatophytosis and a review of the literature. Mycopathologia 185, 1041–1050. doi: 10.1007/s11046-020-00487-0
Wang, W. Y., Luo, H. B., Hu, J. Q., and Hong, H. H. (2022). Pulmonary Cladosporium infection coexisting with subcutaneous Corynespora cassiicola infection in a patient: A case report. World J. Clin. Cases. 10, 3490–3495. doi: 10.12998/wjcc.v10.i11.3490
Wang, X., Wang, W., Lin, Z., Wang, X., Li, T., Yu, J., et al. (2014). CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J. Allergy Clin. Immunol. 133, 905–8.e3. doi: 10.1016/j.jaci.2013.09.033
Wang, X., Wang, A., Wang, X., Li, R., and Yu, J. (2019). Cutaneous mucormycosis caused by Mucor irregularis in a patient with CARD9 deficiency. Br. J. Dermatol. 180, 213–214. doi: 10.1111/bjd.17144
Wang, C., Xing, H., Jiang, X., Zeng, J., Liu, Z., Chen, J., et al. (2019). Cerebral phaeohyphomycosis caused by exophiala dermatitidis in a chinese CARD9-deficient patient: A case report and literature review. Front. Neurol. 10, 938. doi: 10.3389/fneur.2019.00938
Wang, Y., Zhang, D., Hou, Y., Shen, S., and Wang, T. (2020). The adaptor protein CARD9, from fungal immunity to tumorigenesis. Am. J. Cancer Res. 10, 2203–2225.
Wu, W., Zhang, R., Wang, X., Song, Y., Liu, Z., Han, W., et al. (2016). Impairment of immune response against dematiaceous fungi in card9 knockout mice. Mycopathologia 181, 631–642. doi: 10.1007/s11046-016-0029-0
Yan, H., Mo, Y., Liu, S., Luo, X., Liu, L., Zhou, L., et al. (2022). Case report: Hemophagocytic lymphohistiocytosis in a child with primary immunodeficiency infected with Talaromyces marneffei. Front. Immunol. 13, 1038354. doi: 10.3389/fimmu.2022.1038354
Yan, X. X., Yu, C. P., Fu, X. A., Bao, F. F., Du, D. H., Wang, C., et al. (2016). CARD9 mutation linked to Corynespora cassiicola infection in a Chinese patient. Br. J. Dermatol. 174, 176–179. doi: 10.1111/bjd.14082
Yazdi, M., Behnaminia, N., Nafari, A., and Sepahvand, A. (2023). Genetic susceptibility to fungal infections. Adv. BioMed. Res. 12, 248. doi: 10.4103/abr.abr_259_22
You, C. Y., Hu, F., Lu, S. W., Pi, D. D., Xu, F., Liu, C. J., et al. (2021). Talaromyces marneffei infection in an HIV-negative child with a CARD9 mutation in China: A case report and review of the literature. Mycopathologia 186, 553–561. doi: 10.1007/s11046-021-00576-8
Zhang, L., Zhang, Y., Ma, Y., Wang, Z., Wan, Z., Song, Y., et al. (2023). Challenges towards management of CARD9-deficient patients with phaeohyphomycosis: A case report and case series study. Mycoses 66, 317–330. doi: 10.1111/myc.13556
Zhang, R., Wang, X., Wan, Z., and Li, R. (2017). CARD9 mutations and related immunological research of one case with disseminated phaeohyphomycosis. J. Microbes Infect. 12, 14–23.
Zhang, W., Shun, L., and Bao, F. (2023). Facial cutaneous infection caused by Fusarium verticillioides in a Chinese patient with CARD9 deficiency: a case report. Chin. J. Leprosy. Skin. Dis. 39, 485–492. doi: 10.12144/zgmfskin202307485
Zhang, Y., Mijiti, J., Huang, C., Song, Y., Wan, Z., Li, R., et al. (2019). Deep dermatophytosis caused by Microsporum ferrugineum in a patient with CARD9 mutations. Br. J. Dermatol. 181, 1093–1095. doi: 10.1111/bjd.18146
Zhou, L. H., Qiu, W. J., Que, C. X., Cheng, J. H., Zhu, R. S., Huang, J. T., et al. (2024). A novel inherited CARD9 deficiency in an otherwise healthy woman with CNS candidiasis. Clin. Immunol. 265, 110293. doi: 10.1016/j.clim.2024.110293
Keywords: CARD9 deficiency, fungal infection, gene mutation, clinical features, review
Citation: Tang C, Liu Y, Long J and Lv X (2025) Clinical features of patients with fungal infections caused by CARD9 deficiency: a literature review of case reports. Front. Cell. Infect. Microbiol. 15:1615929. doi: 10.3389/fcimb.2025.1615929
Received: 07 May 2025; Accepted: 30 June 2025;
Published: 16 July 2025.
Edited by:
Andrew L. Snow, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Anne Puel, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceRabab Elsayed El Hawary, Cairo University, Egypt
Donald C. Vinh, McGill University Health Centre, Canada
Copyright © 2025 Tang, Liu, Long and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoju Lv, bHZ4akBzY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Congchen Tang
Congchen Tang Yalan Liu
Yalan Liu Jiangchao Long
Jiangchao Long Xiaoju Lv
Xiaoju Lv