- 1Department of Respiratory Medicine, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 2Department of Critical Care, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 3Department of Anesthesia and Critical Care, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
Objective: It is well recognized that high heterogeneity represents a key driver of the elevated mortality in severe community-acquired pneumonia (sCAP). Precise subtype classification is therefore critical for both treatment strategy formulation and prognostic evaluation in this patient population. This study aimed to develop a predictive model for novel clinical subtypes of sCAP, leveraging microbiome profiles identified via metagenomic next-generation sequencing (mNGS).
Methods: This retrospective multicenter cohort study enrolled adult patients with sCAP who underwent clinical mNGS testing of bronchoalveolar lavage fluid in intensive care units (ICUs) across 17 medical centers in China. Based on mNGS-identified microbiome characteristics, unsupervised machine learning (UML) was employed for clustering analysis of sCAP patients. LASSO regression and random forest (RF) algorithms were applied to screen and identify predictors of novel sCAP subtypes. A predictive model for the new clinical subtypes was constructed according to the screening results, with a nomogram generated. The discriminative ability, calibration, and clinical utility of the model were evaluated using ROC curves, calibration curves, and decision curve analysis, respectively.
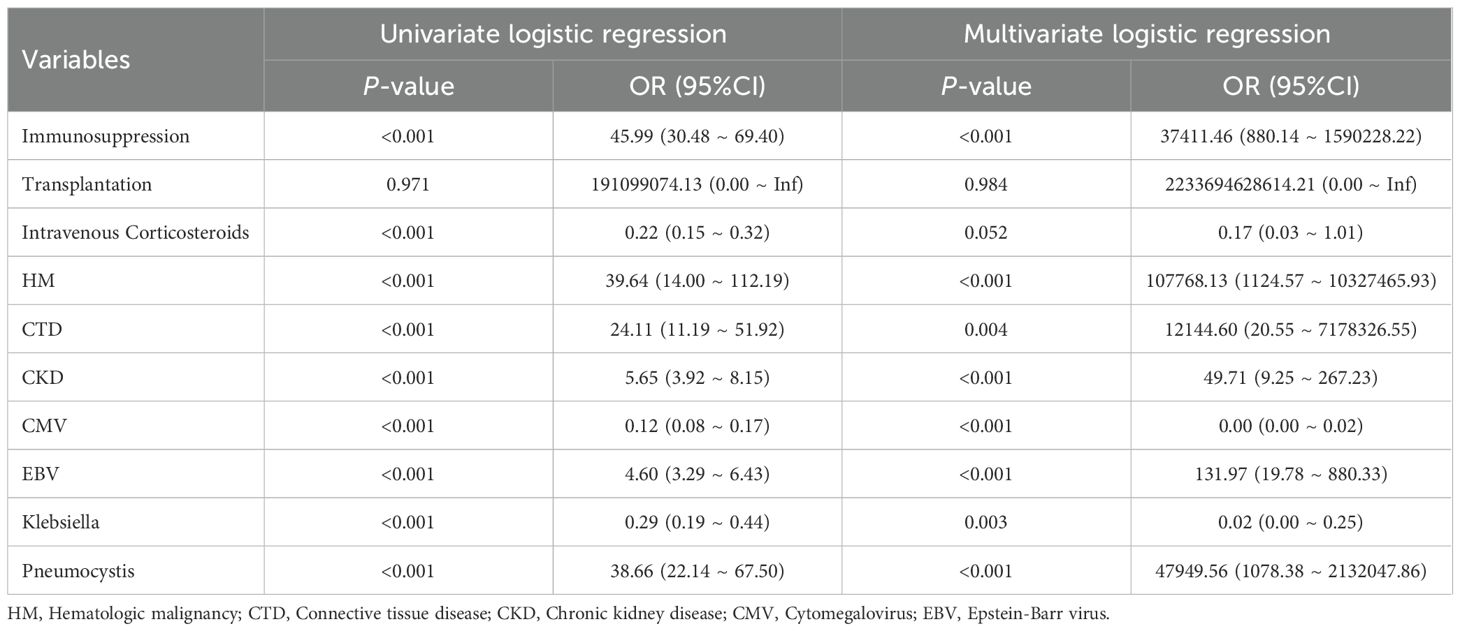
Results: A total of 1,051 sCAP patients were included in the final analysis. The 28-day all-cause mortality rate was 45% (473/1,051). UML clustering identified two distinct sCAP subtypes: the 28-day mortality rate was 42.19% (343/813) in subtype 1 and 54.62% (130/238) in subtype 2. Incorporating clinical and microbial features, a predictive model for the novel sCAP subtypes was developed using the following predictors: immunosuppression (OR = 37,411.46, P < 0.001), connective tissue disease (CTD) (OR = 12,144.60, P = 0.004), hematological malignancy (HM) (OR = 107,768.13, P < 0.001), chronic kidney disease (CKD) (OR = 49.71, P < 0.001), cytomegalovirus (CMV) (OR = 0.00, P < 0.001), Epstein-Barr virus (EBV) (OR = 131.97, P < 0.001), Pneumocystis (OR = 47,949.56, P < 0.001), and Klebsiella (OR = 0.02, P = 0.003). The model demonstrated excellent discriminative ability with an area under the ROC curve (AUC) of 0.992. Calibration curves showed good agreement between predicted and observed outcomes. Decision curve analysis confirmed high clinical utility for predicting novel sCAP subtypes.
Conclusion: This study identified novel clinical subtypes of sCAP based on mNGS-derived microbiome characteristics. This approach exhibits superior performance in identifying high-risk sCAP patients, facilitating precise subtyping.
1 Introduction
Severe community-acquired pneumonia (sCAP) is among the leading causes of death from infectious diseases globally, with its incidence continuing to rise worldwide, especially in elderly individuals, those with comorbidities, and immunocompromised patients (Nair and Niederman, 2021; Huang et al., 2024). Hospital mortality in sCAP is high, ranging from 25% to over 50% (Niederman and Torres, 2022). Traditional classification systems, which rely solely on macro-level clinical indicators, fail to account for the marked heterogeneity and variability in mortality observed in sCAP patients. This indicates that current standards neglect critical sources of heterogeneity, highlighting the need for subtype analysis to identify potentially high-risk subgroups (Xu et al., 2023a; Peetermans et al., 2024; Jiang et al., 2025b). Thus, there is an urgent requirement for precise subtyping of sCAP patients, coupled with the implementation of individualized, stratified diagnosis and treatment strategies, to effectively improve patient outcomes and alleviate the healthcare burden.
In recent years, machine learning (ML) has advanced significantly in the field of pneumonia prognosis prediction, with particular value demonstrated in subtype identification and risk stratification. The XGBoost model by Xu et al. accurately predicts adverse outcomes in sCAP patients through integration of 12 clinical features (Xu et al., 2022). The Qin team further showed that a random forest model constructed using inflammatory markers and immune indicators can predict 30-day mortality and severity classification in CAP patients (Qin et al., 2024). However, tools currently used in clinical practice for predicting sCAP prognosis are primarily derived from routine clinical indicators, such as underlying diseases, inflammatory markers, and imaging features (Fourgeaud et al., 2024). These tools fail to capture the critical role of the pulmonary microbiome in sCAP prognosis, resulting in substantial population bias in their clinical application and limiting their ability to accurately predict outcomes across all patient groups.
Metagenomic next-generation sequencing (mNGS), as a first-line diagnostic tool, significantly enhances pathogen identification rates in sCAP patients while enabling the acquisition of respiratory tract microbiome data (Xu et al., 2023b; Fourgeaud et al., 2024; Wu et al., 2025). In microbiome research, mNGS overcomes the limitation of targeted sequencing methods, which are restricted to studying only bacteria or fungi, allowing for efficient analysis of all microorganisms in the lungs (Miao et al., 2018). More importantly, mNGS provides a comprehensive analysis of the true ecological structure of the pulmonary microbiome, encompassing low-abundance flora and difficult-to-culture pathogens, thereby better capturing the dynamic balance or disordered state of respiratory microorganisms. These microbial characteristics are closely linked to the host’s immune status, with colonization by opportunistic pathogens often indicating impaired immune function (Vidaur et al., 2025). Furthermore, viral co-activation has been confirmed to be associated with poor prognosis (Liu et al., 2025). Thus, establishing subtyping and prognostic tools for pulmonary microbiome analysis based on mNGS can better reflect the mechanisms underlying the occurrence and progression of sCAP, and offer more accurate information for prognostic assessment of sCAP.
Given high heterogeneity is a key factor contributing to elevated mortality in severe Community-Acquired Pneumonia (sCAP), the accurate subtype classification of patients with sCAP is essential for formulating treatment strategies and performing prognostic assessment. However, the specific microbial changes associated with sCAP and their contributions to disease severity remain unclear. To address this gap, this study aims to identify different clinical subtypes of sCAP using metagenomic next-generation sequencing (mNGS) and develop a predictive model based on microbiome characteristics. This approach is expected to provide a more detailed understanding of sCAP heterogeneity and improve the accuracy of prognostic evaluation. We hypothesize that integrating relevant clinical data and microbiome data will reveal novel sCAP subtypes with unique microbiome characteristics, and these subtypes may be associated with disease severity and mortality.
2 Material and methods
2.1 Study design and patient enrollment
This multicenter, retrospective cohort study was conducted in adult intensive care units (ICUs) across 17 medical centers in China, including clinical data and mNGS results of all sCAP patients admitted to the ICU from January 1, 2019, to June 30, 2023. Inclusion criteria were: 1. Age ≥ 18 years, 2. Diagnosis of severe community-acquired pneumonia (sCAP), 3. Undergoing commercial metagenomic next-generation sequencing (mNGS) of bronchoalveolar lavage fluid. The exclusion criterion was: 1. Lost to follow-up or abandonment of treatment within 28 days after ICU admission, 2. Patients with concurrent COVID-19 infection. This study was approved by the ethics committees of all participating hospitals (Approval Number: K20230510). Informed consent was waived due to the use of retrospective data.
2.2 Definition and data collection
The diagnosis of sCAP is based on the criteria for assessing CAP severity established by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS). Diagnosis requires meeting one of two major criteria or three or more minor criteria. Major criteria are: requiring mechanical ventilation via endotracheal intubation, (2) needing vasoactive agents following aggressive fluid resuscitation for septic shock. Minor criteria are: (1) respiratory rate ≥ 30 breaths/min, (2) PaO2/FiO2 ≤ 250 mm Hg, (3) multilobar infiltrates, (4) altered mental status and/or disorientation, (5) blood urea nitrogen ≥ 20 mg/dL. Immunosuppression is defined as previously described: (1) neutropenia (< 0.5 × 109/L for ≥ 10 days post-admission), (2) recent use of immunosuppressants (e.g., tacrolimus, cyclosporine, rituximab), or (3) history of AIDS, or organ transplantation.
All clinical metagenomic tests were performed in accordance with previously reported protocols (Huang et al., 2023), with results available within 36 hours of sample submission. During clinical metagenomic testing, all patients or their families were informed of relevant details and signed informed consent forms for the tests in accordance with Chinese law. For patients who underwent multiple clinical metagenomic tests, only results from the first test were included in the analysis.
Data were collected in a standardized manner via review of electronic medical record systems. Collected data included gender, age, comorbidities, immunosuppressive status, laboratory test results, clinical metagenomic results, and clinical scores (Sequential Organ Failure Assessment [SOFA]) for included patients. Data collection across all centers adhered to uniform standards. The clinical metagenomic laboratory was accredited by the College of American Pathologists or the external quality assessment program of the National Health Commission of China.
2.3 Statistical analysis
Statistical analyses in this study were performed using SPSS 27.0 software. Measurement data with a normal distribution were expressed as mean ± standard deviation; those without a normal distribution were expressed as median (interquartile range, Q1-Q3). Group comparisons for normally distributed data, non-normally distributed data, and categorical data used the Student’s t-test, Mann-Whitney test (for skewed data), and Pearson’s chi-square test (for categorical variables), respectively. The level of statistical significance was set at P < 0.05.
We used R software (v4.1.3) for unsupervised machine learning (UML) analysis. Data from 1051 sCAP patients were normalized using the “factoextra” package in R. Patients were clustered via the K-means algorithm within UML. The “Fpc” package was used to calculate silhouette coefficients for determining the optimal number of clusters, with cluster plots employed to visualize K-means clustering results. Survival analysis was performed using Kaplan-Meier curves.
This study used LASSO regression to screen predictive variables. LASSO regression analysis was performed via importing collected data into R software (using the “glmnet” package) to identify predictive factors for novel sCAP subtypes. Concurrently, the random forest (RF) algorithm was used to identify predictive factors for novel sCAP subtypes, with a 5-time 10-fold cross-validation iterative process to reduce overfitting risk. A predictive model for novel sCAP subtypes was then constructed using the forward selection method in logistic regression analysis, with collinearity testing conducted concurrently. The model was visually presented via a nomogram. Additionally, the “pROC” and “ggplot2” packages in R were used to plot receiver operating characteristic (ROC) curves and evaluate model discriminative ability. The “rmst” package was used to generate calibration curves. The “dcurves” and “rmda” packages were used to plot decision curves for assessing the clinical benefits of the model.
3 Results
3.1 Patient characteristics
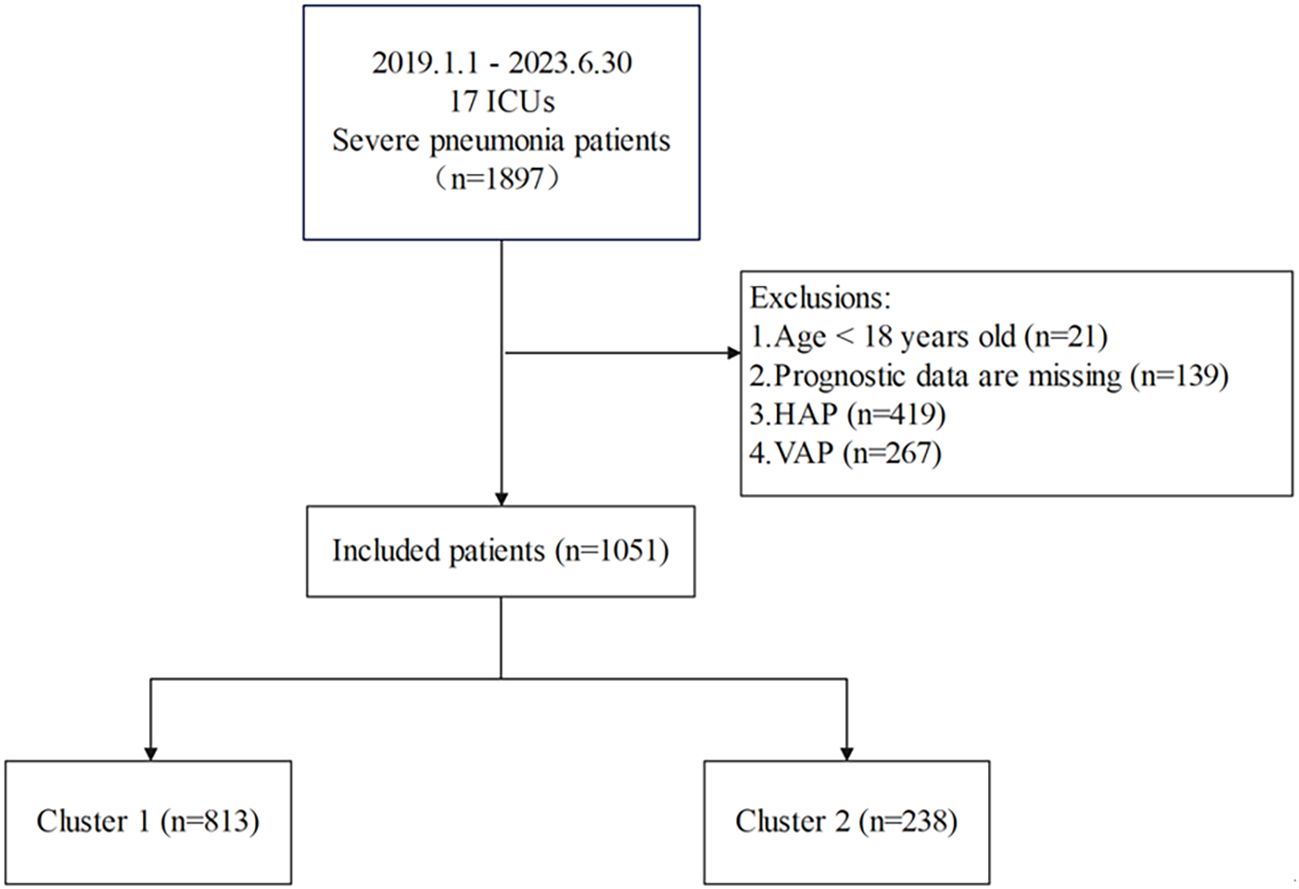
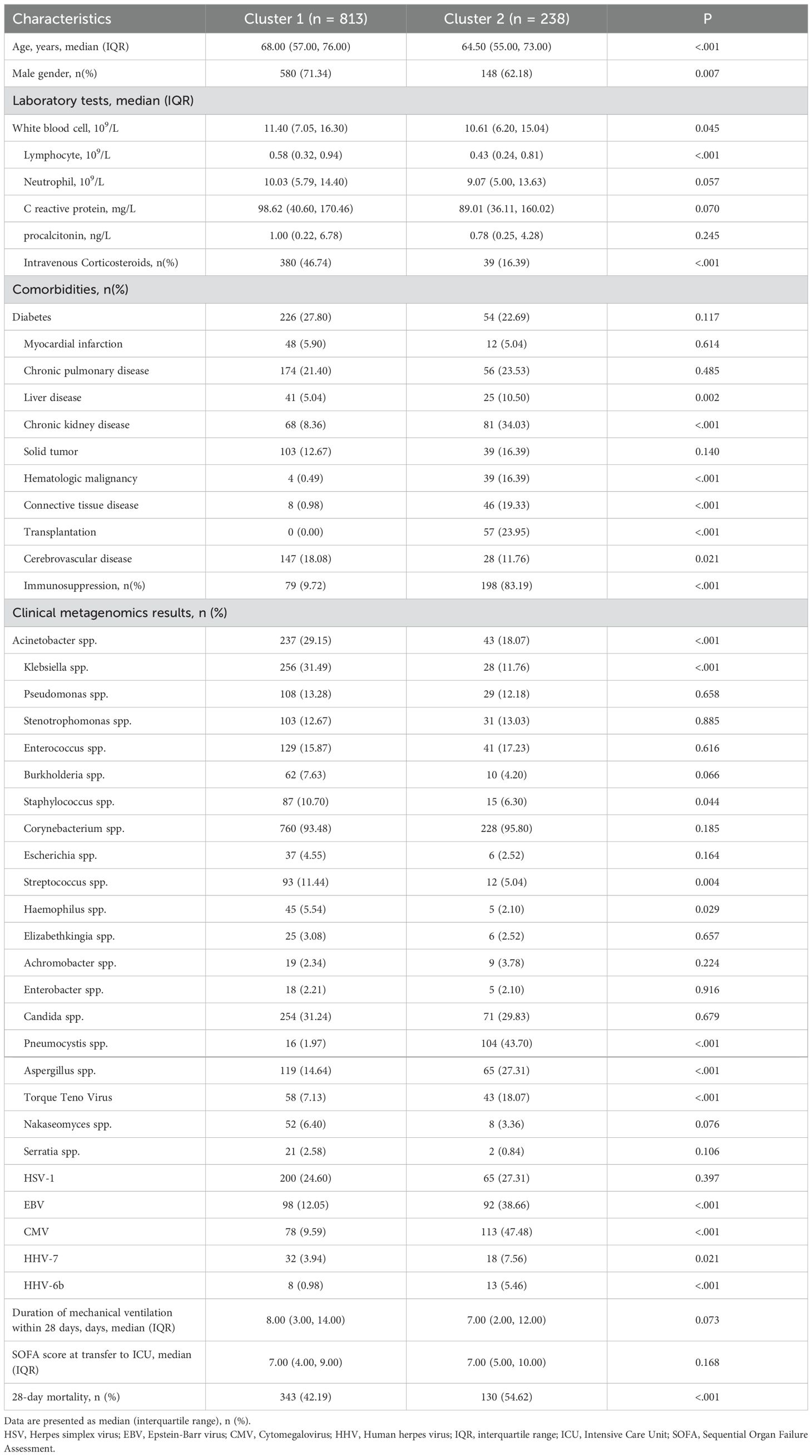
This study used LASSO regression to screen predictive variables: A total of 1,897 patients with severe pneumonia admitted to adult intensive care units were screened, with 1,051 finally included after excluding 21 patients aged < 18 years, 139 with missing prognostic data, 419 with hospital-acquired pneumonia, and 267 with ventilator-associated pneumonia (Figure 1). Patients were divided into 2 clusters via unsupervised machine learning: Cluster 1 (n = 813) and Cluster 2 (n = 238) (Figure 2). Among the 1,051 included patients, 69.27% (728/1,051) were male, with a mean age of 65.06 ± 16.06, and the 28-day mortality rate was 45% (473/1,051). Table 1 presents comparisons of baseline data between the two clusters, including demographics, comorbidities, laboratory indicators, and metagenomic data. The median age of Cluster 1 was 68.00 (57.00, 76.00), with 71.34% male. The median age of Cluster 2 was 64.50 (55.00, 73.00), with 62.18% male. The 28-day mortality rate was 42.19% (343/813) in Cluster 1 and 54.62% (130/238) in Cluster 2, with a statistically significant difference between the two clusters (P < 0.001). Compared with Cluster 1, Cluster 2 had a higher proportion of immunosuppression, as well as higher incidences of liver disease, chronic kidney disease (CKD), hematologic malignancy(HM), connective tissue disease (CTD), and a higher rate of transplantation history (Immunosuppression: Cluster 1/Cluster 2 = 9.72%/83.19%, p < 0.001; Liver Disease: Cluster 1/Cluster 2 = 5.04%/10.5%, p = 0.002; CKD: Cluster 1/Cluster 2 = 8.36%/34.03%, p = 0.002; HM: Cluster 1/Cluster 2 = 0.49%/16.39%, p < 0.001; CTD: Cluster 1/Cluster 2 = 0.98%/19.33%, p < 0.001; Transplantation: Cluster 1/Cluster 2 = 0.00%/23.95%, p < 0.001).
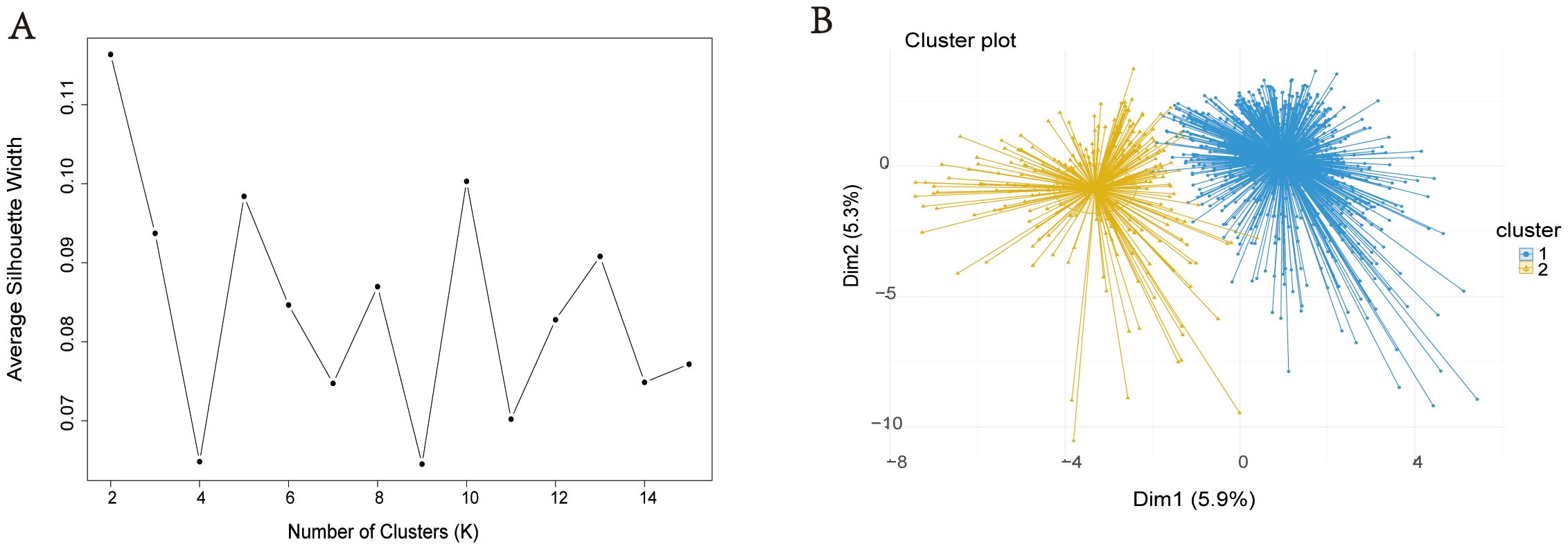
Figure 2. Visualization of unsupervised machine learning. Silhouette Coefficient (SC) of K-means clustering algorithm which was determined the optimal clustering result. Peak of the broken line is the optimal value for Silhouette Coefficient (Y Axis), the optimal clustering results were equal to 2 (X Axis) (A). Scatter plots of 1051 sCAP patients. Each dot in the figure represents a patient. The blue scatter represents cluster 1 and the yellow scatter represents cluster 2 (B).
Additionally, the proportions of infections with Pneumocystis, Aspergillus, Torque Teno Virus, Epstein-Barr virus (EBV), Cytomegalovirus (CMV), human herpesvirus 7 (HHV-7), and human herpesvirus 6b (HHV-6b) were higher (Pneumocystis: Cluster 1/Cluster 2 = 1.97%/43.70%, p < 0.001; Aspergillus: Cluster 1/Cluster 2 = 14.64%/27.31%, p < 0.001; Torque Teno Virus: Cluster 1/Cluster 2 = 18.07%/7.03%, p < 0.001; EBV: Cluster 1/Cluster 2 = 12.05%/38.66%, p < 0.001; CMV: Cluster 1/Cluster 2 = 9.59%/47.48%, p < 0.001; HHV-7: Cluster 1/Cluster 2 = 3.94%/7.56%, p < 0.001; HHV-6b: Cluster 1/Cluster 2 = 5.46%/0.98%, p < 0.001) (Table 2). In contrast, Cluster 1 had higher levels of white blood cells (WBC) and lymphocytes, a higher incidence of cerebrovascular disease (CBD) (WBC: Cluster 1/Cluster 2 = 11.40 (7.05, 16.30)/10.61 (6.20, 15.04), p = 0.045; Lymphocyte: Cluster 1/Cluster 2 = 0.58 (0.32, 0.94)/0.43 (0.24, 0.81), p < 0.001; CBD: Cluster 1/Cluster 2 = 18.08%/11.76%, p < 0.001), as well as higher proportions of infections with Acinetobacter, Klebsiella, Staphylococcus, Streptococcus, and Haemophilus (Acinetobacter: Cluster 1/Cluster 2 = 29.15%/18.07%, p < 0.001; Klebsiella: Cluster 1/Cluster 2 = 31.49%/11.76%, p < 0.001; Staphylococcus: Cluster 1/Cluster 2 = 10.70%/6.30%, p < 0.001; Streptococcus: Cluster 1/Cluster 2 = 11.44%/5.04%, p = 0.004; Haemophilus: Cluster 1/Cluster 2 = 5.54%/2.10%, p = 0.029).
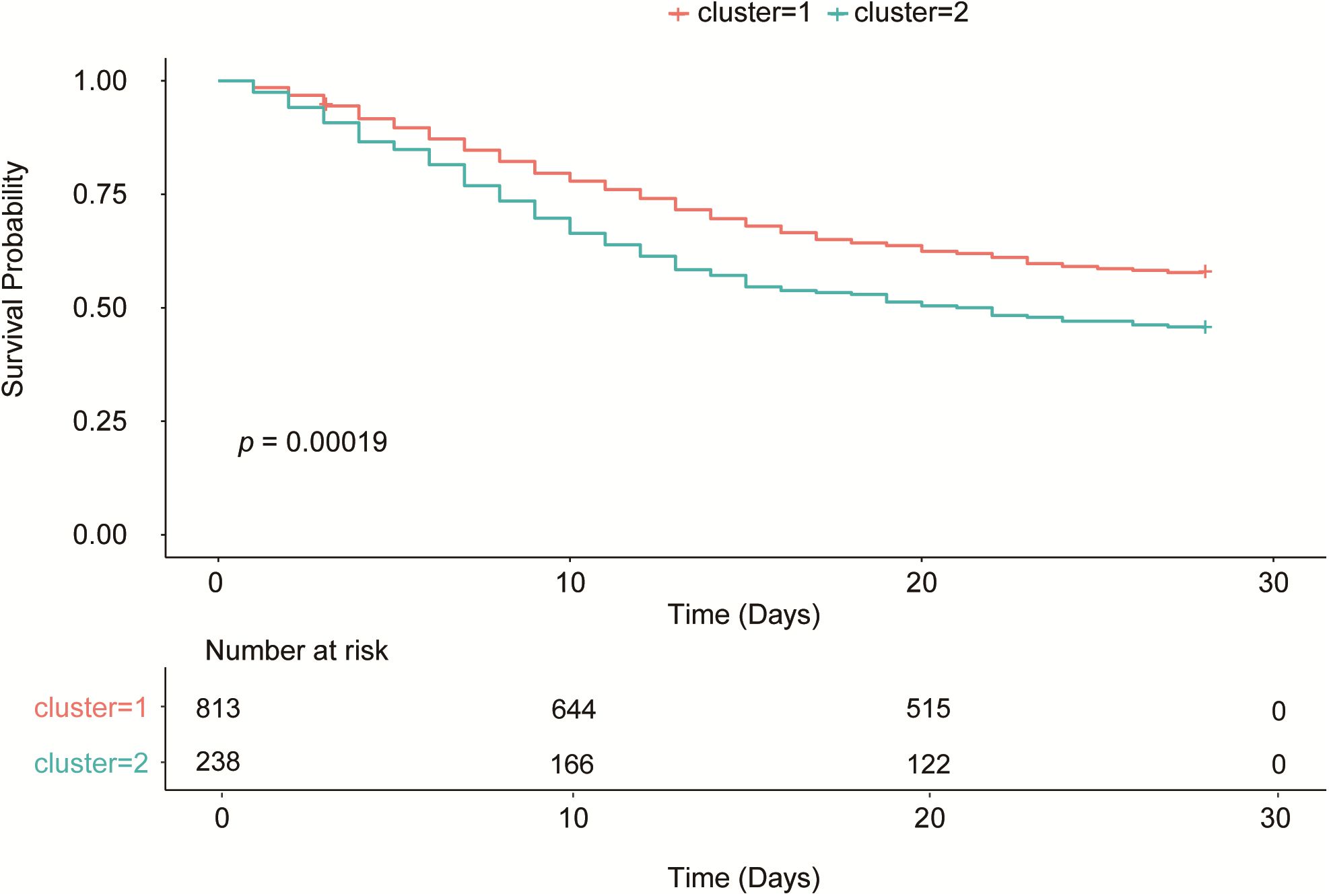
3.2 Survival curve analysis
In the survival analysis, the Kaplan-Meier curve (Figure 3) showed that the survival rate of patients in Cluster 2 was significantly lower than that in Cluster 1 (p = 0.00019). The 28-day mortality rate was 42.19% (343/813) in Cluster 1 and 54.62% (130/238) in Cluster 2, with a statistically significant difference between the two clusters (P < 0.001) (Table 1). Thus, Cluster 1 was defined as SCAPML1 and Cluster 2 as SCAPML2.
3.3 Factors associated WITH SCAPML2 and prediction model
LASSO regression analysis was performed based on clinical data and microbiome results to screen for predictive factors of SCAPML2, with 33 predictive factors finally identified, including Intime SOFA, Solid tumor, HM, CTD, Transplantation, CBD, Neutrophil, and Liver disease (Figures 4A, B). Concurrently, based on 5-time 10-fold cross-validation results (Figure 4C), the RF algorithm was used to screen for predictive factors of SCAPML2 (Figure 4D), identifying 10 key predictive factors: Immunosuppression, HM, CTD, CKD, CMV, Transplantation, EBV, Intravenous Corticosteroids, Klebsiella, and Pneumocystis. Figure 4E shows the intersection of predictive factors screened via LASSO regression and the RF algorithm, namely Immunosuppression, HM, CTD, CKD, CMV, Transplantation, EBV, Intravenous Corticosteroids, Klebsiella, and Pneumocystis.
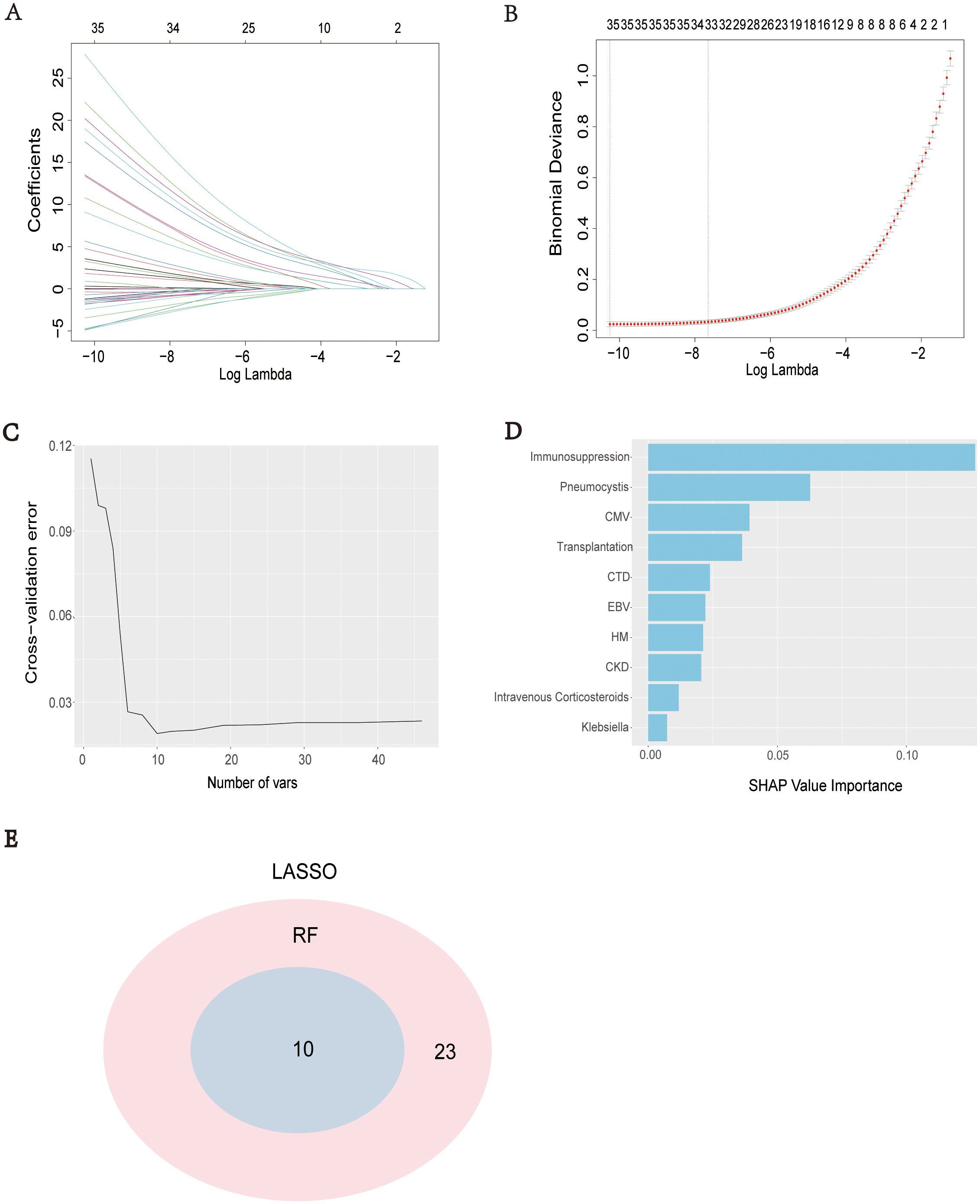
Figure 4. Selection of predictive factors. LASSO coefficient profiles of the predictive factors (A), The results of LASSO regression (B), The optimal regression results can be obtained by retaining the 10 predictive factors after five iterations of 10-fold cross validation (C), 10 predictive factors were screened by IncNodePurity of Ramdon Forest algorithms (D), Intersection of predictive factors screened using LASSO regression and Random Forest algorithm (E).
Univariate and multivariate logistic regression analyses were performed on the 10 screened predictive factors, identifying 8 independent predictive factors: Immunosuppression (OR = 37411.46, P < 0.001), CTD (OR = 12144.60, P = 0.004), HM (OR = 107768.13, P < 0.001), CKD (OR = 49.71, P < 0.001), CMV (OR = 0.00, P < 0.001), EBV (OR = 131.97, P < 0.001), Pneumocystis (OR = 47949.56, P < 0.001), and Klebsiella (OR = 0.02, P = 0.003) (Table 2). Collinearity analysis was applied to assess collinearity among variables, with results showing all included variables had a VIF < 10 and Tolerance > 0.1, indicating no significant collinearity in the model (Supplementary Table S1).
3.4 Model evaluation and validation
A nomogram for predicting SCAPML2 was constructed based on the key factors screened by logistic regression analysis (Figure 5A). The diagnostic efficacy of the predictive model was evaluated using the receiver operating characteristic (ROC) curve, with an area under the curve (AUC) of 0.992 (Figure 5B), indicating good predictive performance. The calibration of the model was assessed via the calibration curve (Bootstrap method, n = 1000) (Figure 5C), and the Hosmer-Lemeshow test showed that the final model had a good fit (χ² = 0.628, P = 1.000). Decision curve analysis (DCA) with 1000 Bootstrap resamplings showed that the model had a high net benefit for predicting novel sCAP subtypes within the threshold probability range of 0.05–0.95 (Figure 5D).
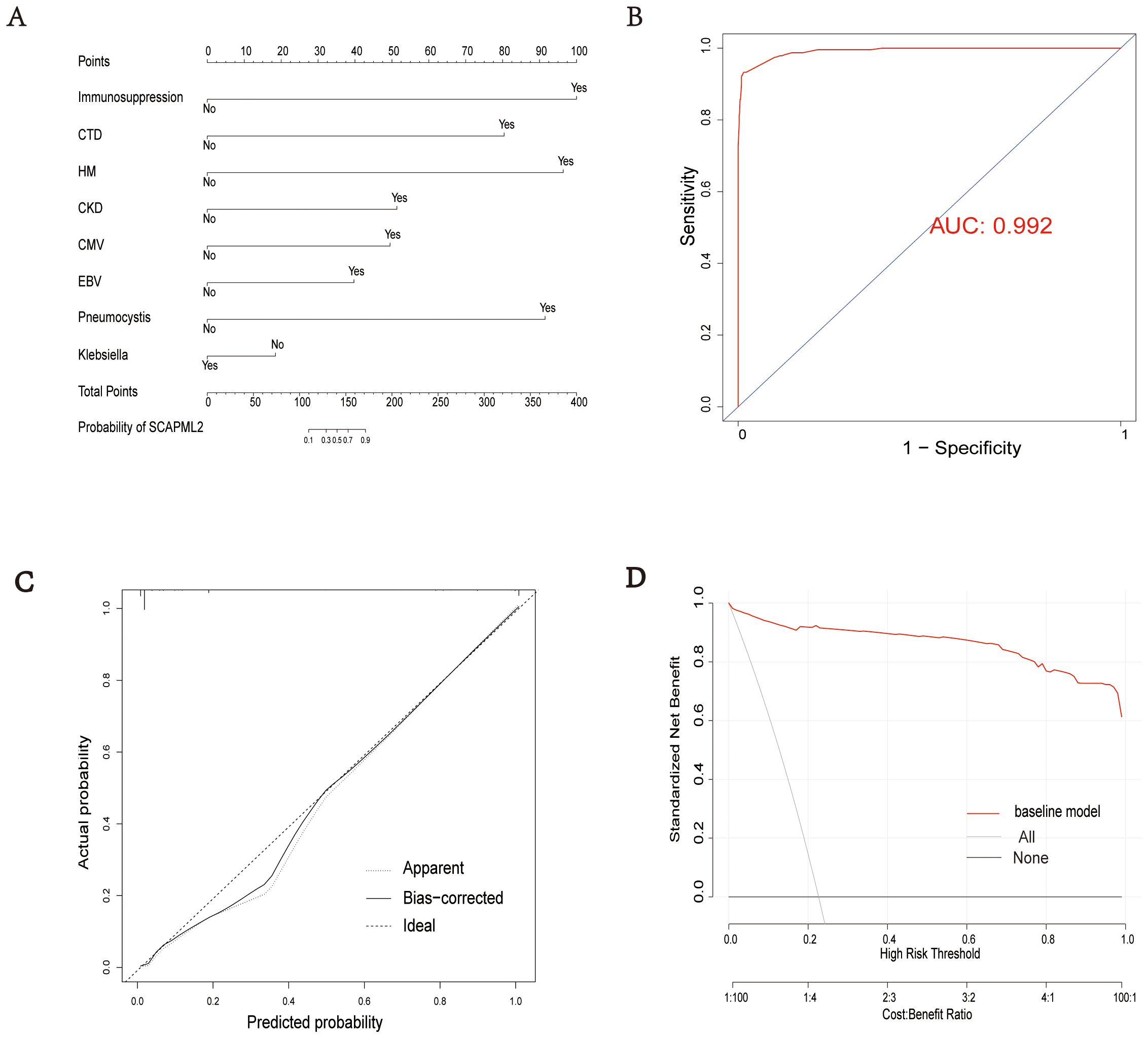
Figure 5. Model evaluation. Establishment of a nomogram for SCAPML2 (A). AUC of the nomogram based on 8 predictive factors (B). Calibration curves for predicting SCAPML2 probability (C). Decision curves for model (D).
4 Discussion
To our knowledge, this study is the first to apply unsupervised machine learning (UML) to the clinical subtyping of patients with severe community-acquired pneumonia (sCAP) by integrating microbiome characteristics derived from metagenomic next-generation sequencing (mNGS). Using this methodology, we successfully delineated a novel subtype (SCAPML2), and identified its independent predictive factors. The overall mortality rate of included sCAP patients was 45%, which is consistent with the mortality rates reported in previous studies (Garnacho-Montero et al., 2018; Martin-Loeches et al., 2025; Reyes et al., 2025). SCAPML2 is characterized by immunosuppression, specific comorbidities, and specific pathogenic infections, with a mortality rate as high as 54.62%, which is significantly higher than the 43% mortality rate of sCAP patients reported in prior research (Reyes et al., 2025). This subtype exhibits superior performance in identifying high-risk sCAP patients, highlighting the elevated risks faced by patients with this subtype.
The predictive model for SCAPML2 established in this study has a clear clinical early warning value. A German cohort study showed that immunosuppressed patients have a higher incidence of CAP and poorer prognosis (Reichel et al., 2024), which is consistent with our findings. Furthermore, research has shown that patients with hematological malignancies(HM) constitute a high-risk group for sCAP, and these patients are often complicated by infections with cytomegalovirus(CMV), Epstein-Barr virus(EBV), and Pneumocystis (Certan et al., 2022; Park et al., 2024; Jiang et al., 2025a). Additionally, literature has proposed that connective tissue disease-associated interstitial lung disease (CTD-ILD) is a common complication of CTD, and such patients typically receive long-term treatment with glucocorticoids or immunosuppressants, which increases the risk of pulmonary infections (Guiot et al., 2024). A study specifically constructed a 90-day mortality prediction model for CTD patients with pneumonia, showing that these patients have a higher risk of mortality (Li et al., 2023). Another study observed that in patients with chronic kidney disease (CKD) who have severe pneumonia, the incidence of acute kidney injury is significantly increased (OR = 2.45) and is associated with a higher risk of death (Xie et al., 2024). Meanwhile, this study identified CMV, EBV, and Pneumocystis as independent risk factors for predicting SCAPML2. Literature clearly indicates that among severe pneumonia patients, CMV, HSV-1, and EBV are the most common reactivated viruses, and their reactivation significantly increases the risk of death, specifically a 2.052-fold increase in 28-day mortality, suggesting a significant association between reactivation of these viruses and elevated death risk (Huang et al., 2023). Moreover, a multicenter prospective study indicated that microbial markers, including Pneumocystis jirovecii and viral markers such as EBV and CMV, correlate with poor prognosis (Song et al., 2025).
Notably, although Klebsiella is a typical pathogen in immunosuppressed patients (Calderón-Parra et al., 2025; Reyes et al., 2025), and tends to cause pneumonia, urinary tract infections, and bacteremia as a risk factor for poor prognosis (Alishvandi et al., 2025), the present study shows that Klebsiella negativity is significantly associated with a high risk of SCAPML2. This reflects the unique characteristics of this subtype, where immunosuppression, as an independent predictive factor, is present in a high proportion (83.19%). Studies have noted that sCAP patients with immunosuppression have multiple comorbidities, high drug resistance rates, and limited available antibiotics, thus supporting early use of potent antibiotics to cover potential high-risk pathogens (Ramirez et al., 2020). We hypothesize that such patients received potent antibiotics before disease progression to sCAP, which would significantly suppress infections with typical bacteria like Klebsiella. Meanwhile, immunosuppressive status represents a major risk factor for fungal and viral infections, as immune system impairment enables viruses and fungi to become dominant pathogens through immune evasion, increasing the risk of such infections (Reichel et al., 2024; Sayson et al., 2024). Taken together, heightened vigilance for the SCAPML2 subtype is needed in sCAP patients with evidence of immunosuppression, fungal or viral infection, and negativity for typical bacteria such as Klebsiella. Additionally, based on the clinical early warning value of the SCAPML2 predictive model, treatment strategies should comprehensively consider patients’ immune status, infecting pathogen types, and potential drug resistance risks to implement individualized therapy.
This study has several notable strengths. First, it is the first to integrate mNGS-based microbiome characteristics. With its unbiased full microbial coverage and high sensitivity, mNGS can efficiently analyze the overall ecological characteristics of the microbiome, thereby overcoming the inherent limitations of traditional detection methods in pathogen coverage, detection timeliness, and ecological perspective analysis (Simner et al., 2018). It also takes the lead in proposing a predictive model for a new subtype of sCAP, which shows good discriminative ability and calibration performance, providing a more comprehensive microbiome perspective for the precise diagnosis and treatment of sCAP. Second, we combined unsupervised machine learning (UML) for typing sCAP patients. This method can accurately classify patients according to the inherent heterogeneity of their clinical and microbiome characteristics, avoiding reliance on predefined labels in traditional supervised learning and thus more truly revealing the complexity of the patient population (Sajda, 2006). Additionally, the independent predictive factors identified and the predictive model constructed in this study provide clinicians with a tool for early identification of high-risk sCAP patient subtypes, facilitating timely adjustment of treatment plans, improvement of treatment effects, and reduction of mortality.
This study has several limitations. First, this study adopted a retrospective design, which inevitably introduces potential biases. On the one hand, data collection relied on the electronic medical record (EMR) system, in which information recording may be incomplete or may lack consistent standardization—both of which can introduce information bias. On the other hand, the retrospective design precludes random assignment of participants, which may give rise to selection bias driven by factors such as the timing of hospital admission and clinical testing conditions. Collectively, these limitations restrict the study’s ability to infer causal relationships. Second, mNGS primarily reflects the relative composition of microbial communities and cannot accurately quantify the absolute abundance of individual species. Additionally, it fails to distinguish between pathogenic infection and colonization. This limitation may obscure the microbiological basis for subtyping and thus necessitates comprehensive assessment in conjunction with clinical symptoms and imaging findings. Third, Third, the present study only conducted internal validation using data from a single-region cohort, and the constructed prediction model has not been validated in an independent external cohort. This shortcoming may reduce the applicability of the prediction model constructed in this study to other populations and limit the generalizability of the results. Future multicenter prospective studies should enroll patients with severe community-acquired pneumonia (sCAP) from diverse regions and populations, with the aims of validating the model externally, further optimizing its parameters, and enhancing its value for clinical translation.
5 Conclusion
This study identified a novel high-risk subtype, SCAPML2, in sCAP patients using unsupervised machine learning. Its independent predictive factors include immunosuppression, hematologic malignancies, and specific pathogen infections. The predictive model exhibits excellent discriminative efficacy, providing an important basis for the precise diagnosis and treatment of sCAP.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Taizhou Hospital Medical Ethics Committee, Zhejiang Province, affiliated with Taizhou Hospital, Zhejiang Province. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Informed consent was waived due to the use of retrospective data.
Author contributions
SC: Writing – original draft. YJ: Funding acquisition, Writing – original draft. DL: Writing – original draft. YZ: Writing – original draft. RZ: Writing – original draft. HD: Writing – original draft. ZW: Writing – original draft. SL: Writing – original draft. RQ: Writing – original draft. HX: Writing – original draft. YY: Writing – original draft. CX: Writing – original draft. XL: Writing – original draft. YX: Funding acquisition, Writing – review & editing. SJ: Writing – review & editing. XW: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The National Key Research and Development Program of Zhejiang Province (2023C03083); The Joint Fund of Zhejiang Provincial Natural Science Foundation of China under Grant No. LKLY25H200001; The Science and Technology Project of Taizhou (23ywa44); The Medicines Health Research Fund of Zhejiang (2024ky1784, 2025ky1821); The Zhejiang basic public welfare research program project fund (LTGY23H010007); The Zhejiang Health science and technology plan (2022K Y445).
Acknowledgments
The authors extend their heartfelt gratitude to seventeen institutions for their invaluable support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1676502/full#supplementary-material
Abbreviations
HSV, Herpes simplex virus; EBV, Epstein-Barr virus; CMV, Cytomegalovirus; HHV, Human herpes virus; ICU, Intensive Care Unit; HM, Hematologic malignancy; CTD, Connective tissue disease; CKD, Chronic kidney disease; WBC, White blood cell; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
References
Alishvandi, A., Barancheshemeh, M., Firuzpour, F., Aram, C., Kamali, M. J., and Keikha, M. (2025). Decoding virulence and resistance in Klebsiella pneumoniae: Pharmacological insights, immunological dynamics, and in silico therapeutic strategies. Microb. Pathog. 205, 107691. doi: 10.1016/j.micpath.2025.107691
Calderón-Parra, J., Carretero-Henriquez, M. T., Escudero, G., Suances-Martin, E., Murga de la Fuente, M., González-Merino, P., et al. (2025). Current epidemiology, risk factors and influence on prognosis of multidrug resistance in Klebsiella spp. bloodstream infection. Insights from a prospective cohort. Eur. J. Intern. Med 139, 106369. doi: 10.1016/j.ejim.2025.05.034
Certan, M., Garcia Garrido, H. M., Wong, G., Heijmans, J., Grobusch, M. P., and Goorhuis, A. (2022). Incidence and predictors of community-acquired pneumonia in patients with hematological cancers between 2016 and 2019. Clin. Infect. Dis. 75, 1046–1053. doi: 10.1093/cid/ciac005
Fourgeaud, J., Regnault, B., Ok, V., Da Rocha, N., Sitterlé, É., Mekouar, M., et al. (2024). Performance of clinical metagenomics in France: a prospective observational study. Lancet Microbe 5, e52–e61. doi: 10.1016/S2666-5247(23)00244-6
Garnacho-Montero, J., Barrero-García, I., Gómez-Prieto, M. D. G., and Martín-Loeches, I. (2018). Severe community-acquired pneumonia: current management and future therapeutic alternatives. Expert Rev. Anti Infect. Ther. 16, 667–677. doi: 10.1080/14787210.2018.1512403
Guiot, J., Miedema, J., Cordeiro, A., De Vries-Bouwstra, J. K., Dimitroulas, T., Søndergaard, K., et al. (2024). Practical guidance for the early recognition and follow-up of patients with connective tissue disease-related interstitial lung disease. Autoimmun. Rev. 23, 103582. doi: 10.1016/j.autrev.2024.103582
Huang, L., Zhang, X., Pang, L., Sheng, P., Wang, Y., Yang, F., et al. (2023). Viral reactivation in the lungs of patients with severe pneumonia is associated with increased mortality, a multicenter, retrospective study. J. Med. Virol. 95, e28337. doi: 10.1002/jmv.28337
Huang, Z., Hu, B., Li, J., Feng, M., Wang, Z., Huang, F., et al. (2024). Metagenomic versus targeted next-generation sequencing for detection of microorganisms in bronchoalveolar lavage fluid among renal transplantation recipients. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1443057
Jiang, Y., Huang, X., Zhou, H., Wang, M., Wang, S., Ren, X., et al. (2025a). Clinical characteristics and prognosis of patients with severe pneumonia with pneumocystis jirovecii colonization. CHEST 167, 54–66. doi: 10.1016/j.chest.2024.07.140
Jiang, Y., Huang, X., Zhou, H., Wang, M., Wang, S., Ren, X., et al. (2025b). Clinical characteristics and prognosis of patients with severe pneumonia with pneumocystis jirovecii colonization: A multicenter, retrospective study. Chest 167, 54–66. doi: 10.1016/j.chest.2024.07.140
Li, D., Ding, L., Luo, J., and Li, Q.-G. (2023). Prediction of mortality in pneumonia patients with connective tissue disease treated with glucocorticoids or/and immunosuppressants by machine learning. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1192369
Liu, F., Zhuang, Y., Huang, X., Papazian, L., Cai, H., Shao, H., et al. (2025). The Landscape of lower respiratory tract herpesviruses in severe pneumonia patients: a multicenter, retrospective study with prospective validation. Crit. Care 29, 254. doi: 10.1186/s13054-025-05496-3
Martin-Loeches, I., Reyes, L. F., and Rodriguez, A. (2025). Severe community-acquired pneumonia (sCAP): advances in management and future directions. Thorax, 80, 222296. doi: 10.1136/thorax-2024-222296
Miao, Q., Ma, Y., Wang, Q., Pan, J., Zhang, Y., Jin, W., et al. (2018). Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin. Infect. Dis. 67, S231–S240. doi: 10.1093/cid/ciy693
Nair, G. B. and Niederman, M. S. (2021). Updates on community acquired pneumonia management in the ICU. Pharmacol. Ther. 217, 107663. doi: 10.1016/j.pharmthera.2020.107663
Niederman, M. S. and Torres, A. (2022). Severe community-acquired pneumonia. Eur. Respir. Rev. 31, 220123. doi: 10.1183/16000617.0123-2022
Park, J. H., Hong, S.-B., Huh, J. W., Jung, J., Kim, M. J., Chong, Y. P., et al. (2024). Severe human parainfluenza virus community- and healthcare-acquired pneumonia in adults at tertiary hospital, Seoul, South Korea 2010–2019. Emerg. Infect. Dis. 30, 1088–1095. doi: 10.3201/eid3006.230670
Peetermans, M., Matheeussen, V., Moerman, C., De Rydt, F., Thieren, S., Pollet, E., et al. (2024). Clinical and molecular epidemiological features of critically ill patients with invasive group A Streptococcus infections: a Belgian multicenter case-series. Ann. Intensive Care 14, 19. doi: 10.1186/s13613-024-01249-7
Qin, Q., Yu, H., Zhao, J., Xu, X., Li, Q., Gu, W., et al. (2024). Machine learning-based derivation and validation of three immune phenotypes for risk stratification and prognosis in community-acquired pneumonia: a retrospective cohort study. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1441838
Ramirez, J. A., Musher, D. M., Evans, S. E., Dela Cruz, C., Crothers, K. A., Hage, C. A., et al. (2020). Treatment of community-acquired pneumonia in immunocompromised adults. Chest 158, 1896–1911. doi: 10.1016/j.chest.2020.05.598
Reichel, F., Tesch, F., Berger, S., Seifert, M., Koschel, D., Schmitt, J., et al. (2024). Epidemiology and risk factors of community-acquired pneumonia in patients with different causes of immunosuppression. Infection 52, 2475–2486. doi: 10.1007/s15010-024-02314-w
Reyes, L. F., Sanabria-Herrera, N., Nseir, S., Ranzani, O. T., Povoa, P., Diaz, E., et al. (2025). Nosocomial lower respiratory tract infections in patients with immunosuppression: a cohort study. Ann. Intensive Care 15. doi: 10.1186/s13613-025-01462-y
Sajda, P. (2006). Machine learning for detection and diagnosis of disease. Annu. Rev. Biomed. Eng 8, 537–565. doi: 10.1146/annurev.bioeng.8.061505.095802
Sayson, S. G., Ashbaugh, A., Porollo, A., Smulian, G., and Cushion, M. T. (2024). Pneumocystis murina promotes inflammasome formation and NETosis during Pneumocystis pneumonia. mBio 15, e01409–e01424. doi: 10.1128/mbio.01409-24
Simner, P. J., Miller, S., and Carroll, K. C. (2018). Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin. Infect. Dis. 66, 778–788. doi: 10.1093/cid/cix881
Song, W., Yang, Q., Lv, H., Lv, Y., Jiang, Y., Qu, J., et al. (2025). Prospective multicenter study identifying prognostic biomarkers and microbial profiles in severe CAP using BALF, blood mNGS, and PBMC transcriptomics. Sci. Rep. 15, 16252. doi: 10.1038/s41598-025-00812-x
Vidaur, L., Guridi, A., Leizaola, O., Marin, J., Rello, J., Sarasqueta, C., et al. (2025). Respiratory dysbiosis as prognostic biomarker of disease severity for adults with community-acquired pneumonia requiring mechanical ventilation. Pneumonia 17, 10. doi: 10.1186/s41479-025-00163-1
Wu, X., Sun, T., He, H., Xing, L., Cheng, Z., Geng, S., et al. (2025). Effect of metagenomic next-generation sequencing on clinical outcomes of patients with severe community-acquired pneumonia in the ICU. CHEST 167, 362–373. doi: 10.1016/j.chest.2024.07.144
Xie, K., Guan, S., Kong, X., Ji, W., Du, C., Jia, M., et al. (2024). Predictors of mortality in severe pneumonia patients: a systematic review and meta-analysis. Syst. Rev. 13, 210. doi: 10.1186/s13643-024-02621-1
Xu, Z., Guo, K., Chu, W., Lou, J., and Chen, C. (2022). Performance of machine learning algorithms for predicting adverse outcomes in community-acquired pneumonia. Front. Bioeng Biotechnol. 10. doi: 10.3389/fbioe.2022.903426
Xu, J., Zhong, L., Shao, H., Wang, Q., Dai, M., Shen, P., et al. (2023a). Incidence and clinical features of HHV-7 detection in lower respiratory tract in patients with severe pneumonia: a multicenter, retrospective study. Crit. Care 27, 248. doi: 10.1186/s13054-023-04530-6
Xu, J., Zhou, P., Liu, J., Zhao, L., Fu, H., Han, Q., et al. (2023b). Utilizing metagenomic next-generation sequencing (mNGS) for rapid pathogen identification and to inform clinical decision-making: results from a large real-world cohort. Infect. Dis. Ther. 12, 1175–1187. doi: 10.1007/s40121-023-00790-5
Keywords: severe community-acquired pneumonia, pulmonary microbiome, metagenomic next-generation sequencing, machine learning, subtype classification
Citation: Chen S, Jiang Y, Lv D, Zheng Y, Zhang R, Dai H, Wang Z, Li S, Qi R, Xu H, Yu Y, Xu C, Lu X, Xu Y, Jin S and Wu X (2025) Identification of subtypes and construction of a predictive model for novel subtypes in severe community-acquired pneumonia based on clinical metagenomics: a multicenter, retrospective cohort study. Front. Cell. Infect. Microbiol. 15:1676502. doi: 10.3389/fcimb.2025.1676502
Received: 30 July 2025; Accepted: 10 November 2025; Revised: 29 October 2025;
Published: 09 December 2025.
Edited by:
Beiwen Zheng, Zhejiang University, ChinaReviewed by:
Miodrag Zivkovic, Singidunum University, SerbiaNeven Papic, University of Zagreb, Croatia
Copyright © 2025 Chen, Jiang, Lv, Zheng, Zhang, Dai, Wang, Li, Qi, Xu, Yu, Xu, Lu, Xu, Jin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghe Xu, eHV5aEBlbnplbWVkLmNvbQ==; Shengwei Jin, amluc2hlbmd3ZWk2OUAxNjMuY29t; Xiaomai Wu, d3V4bUBlbnplbWVkLmNvbQ==
†These authors have contributed equally to this work
 Shiyi Chen1†
Shiyi Chen1† Dongqing Lv
Dongqing Lv Yinghe Xu
Yinghe Xu Shengwei Jin
Shengwei Jin Xiaomai Wu
Xiaomai Wu