- 1Faculty of Medicine, Bogomolets National Medical University (NMU), Kyiv, Ukraine
- 2Systematic Review and Meta-analysis Expert Group (SRMEG), Universal Scientific Education and Research Network (USERN), Tehran, Iran
- 3Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Reproductive Health Research Center, School of Medicine, Guilan University of Medical Sciences (GUMS), Rasht, Iran
- 5Student Research Committee, School of Medicine, Mazandaran University of Medical Sciences (MazUMS), Sari, Iran
- 6Faculty of Medicine, Istanbul Yeni Yuzyil University, Istanbul, Türkiye
- 7Department of Food Science and Technology, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran
Background: Type 2 diabetes mellitus (T2DM) is a significant global health challenge whose prevalence is projected to increase alarmingly. Recently, due to better safety and fewer adverse effects, herbal medicines have been used to manage T2DM. This study aimed to evaluate the efficacy of boswellia in improving glycemic markers and lipid profiles in T2DM patients.
Methods: A comprehensive search was conducted on the PubMed, Web of Science, and Scopus databases for all relevant studies published up to April 30, 2024. The effects of boswellia supplementation were evaluated using glycemic markers and lipid profiles. The data were extracted and meta-analyzed using Stata software.
Results: This meta-analysis included five studies with a total of 287 patients with T2DM. It was found that boswellia in patients with T2DM compared to the placebo or control group significantly reduced hemoglobin A1C (HbA1C) (SMD: -1.01; 95%CI: -1.55 to -0.46; P=0.00), total cholesterol (TC) (SMD: -0.44; 95%CI: -0.68 to -0.21; P=0.00), Triglycerides (TG) (SMD: -0.42; 95%CI: -0.66 to -0.19); P=0.00) and low-density lipoprotein (LDL) (SMD: -0.43; 95%CI: -0.73 to -0.12); P=0.006) levels, while reduced fasting blood glucose (FBG) but it was not significant (SMD: -1.34, 95%CI: -2.68 to 0.00; P=0.05). Notably, it did not affect high-density lipoprotein (HDL) (SMD: 0.56, 95%CI: -0.14 to -1.26; P=0.118).
Conclusion: In summary, boswellia supplementation has the potential to improve glycemic markers and lipid profiles in patients with T2DM. It may help diabetic patients in addition to a controlled diet and other treatments.
Systematic review registration: crd.york.ac.uk/PROSPERO/display_record.php?RecordID=538347, identifier CRD42024538347.
1 Introduction
Type 2 Diabetes Mellitus (T2DM) is a prevalent chronic endocrine and metabolic disorder globally, characterized by long-term hyperglycemia, insulin resistance, and relative insulin insufficiency (1). The 2021 International Diabetes Federation report reported 536.6 million people diagnosed with T2DM (2). Diabetes prevalence is projected to rise to 642.7 million by 2030 and 783.2 million globally by 2040, with one in two adults unaware of their condition. Modifying risk factors like obesity, smoking, hypertension, dyslipidemia, and cardiovascular diseases can reduce the prevalence and incidence of diabetes, a common condition that increases the likelihood of developing the disease (3, 4) T2DM can also stimulate other diseases, such as cardiovascular diseases, making it a significant risk factor (5, 6).
Despite the widespread use of medications and lifestyle modifications among diabetic patients, many individuals turn to complementary and alternative medicine (CAM) and herbal remedies due to their affordability and potentially fewer side effects (7). In recent years, there has been growing interest in the use of herbal supplements as adjunct therapy for the management of T2DM (8–11). Notably, emerging evidence suggests that some herbals may exert a more comprehensive effect on both glycemic indicators and lipid profiles. For instance a study on okra reported a reduction in fasting blood glucose (FBG) without impacting glycated hemoglobin in T2DM patients (9), boswellia appears to offer broader benefits (12–16).
Boswellia serrata is a traditional medicinal plant used in Ayurveda since ancient times due to its anti-inflammatory and therapeutic properties. Its active components, particularly boswellia acids, have been reported to offer potential benefits by altering pathways leading to diabetes and related complications via inflammation (17–20). To assess the effectiveness of boswellia, several studies in animal populations investigated the anti-diabetic role of Boswellia serrata (8–10). Additionally, a few randomized clinical trials (RCT) evaluated the supplementation of Boswellia serrata extracts in human patients with T2DM and assessed its effects on the glycemic markers and lipid profile (12–16).
The consistency of reported outcomes across studies on boswellia supplementation in improving glycemic markers and lipid profiles in patients with type 2 diabetes remains unclear. A comprehensive evaluation of available evidence is needed to determine its efficacy and safety. This study assesses the effectiveness of boswellia on glycemic indicators and lipid profiles in T2DM patients, aiming to better understand its potential role as a complementary treatment option. This will inform clinical practice and guide future research in this area.
2 Materials and methods
This systematic review and meta-analysis was conducted based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statements (21). The methodology encompasses critical steps to ensure transparency and rigor in the research. The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration #CRD42024538347.
2.1 Search strategy
We performed a comprehensive systematic search across four online databases: PubMed, Scopus, Web of Science, and Embase, covering publications from their inception through April 30, 2024. Additionally, we manually searched Google Scholar to identify relevant gray literature. Our search strategy involved an exhaustive examination of Medical Subject Headings (MeSH) terms in the title and abstract ([title/abstract]) for Boswellia, glycemic markers, and lipid profile.
Our research question was formulated using the Patient, Intervention, Comparison, and Outcome (PICO) framework, which guided the selection of studies and ensured the relevance and specificity of the search strategy (Table 1) (22).
2.2 Inclusion and exclusion criteria
Studies were included if they involved individuals with T2DM of any age and health status, with interventions consisting of boswellia or its extract and comparisons to placebo, no treatment, or alternative interventions, specifically examining changes in lipid profile and glycemic markers. Exclusion criteria encompassed studies involving participants with other types of diabetes (e.g., type 1 diabetes mellitus, gestational diabetes), records including pregnant or breastfeeding women, non-human studies (animal, in vitro, and in vivo studies), non-English language publications, and reviews, editorials, and opinion pieces.
2.3 Study selection
Two independent reviewers (P.R. & M.A.) conducted a multi-step screening process for the identified studies to ensure the inclusion of relevant and high-quality data. Initially, they performed a title and abstract review to eliminate studies that did not meet the predefined criteria quickly. Studies deemed potentially relevant underwent a detailed full-text assessment, where the reviewers evaluated them against specific inclusion and exclusion criteria, such as study design, population characteristics, interventions, and outcomes. Any discrepancies between reviewers were resolved through discussion, and if consensus was not reached, a third reviewer (M.K.) provided an independent evaluation. Throughout the selection process, the reasons for excluding studies were documented to ensure transparency and reproducibility.
The screening process involved three steps: removing duplicates with reference management software, reviewing titles, abstracts, and keywords, followed by full-text assessment based on PICO criteria, and finally, selecting relevant studies for analysis.
2.4 Data extraction
Two individual reviewers (P.R. and M.A.) independently performed data extraction, ensuring accuracy and consistency through a cross-over verification process. In cases of disagreement or conflict, resolution was achieved through discussion and consultation with a third reviewer (M.K.), followed by double-checking the extracted data to confirm accuracy. Data from each included article were systematically compiled across five key categories: general information (such as the first author, publication year, country of origin, and study design), population characteristics (age and gender), intervention features (form, dose, and duration of boswellia administration), comparative analysis (control groups including placebo, no treatment, or alternative interventions), and primary outcomes (glycemic markers and lipid profile). This rigorous and comprehensive approach assured accurate capture and analysis of all relevant data, providing a robust basis for evaluating the effects of boswellia on specified health outcomes.
2.5 Interpretation and recommendations
The results were interpreted in the context of existing literature, highlighting the clinical implications of boswellia on lipid profile and glycemic markers. Recommendations for future research were proposed based on the gaps identified in the current evidence.
2.6 Risk of bias assessment
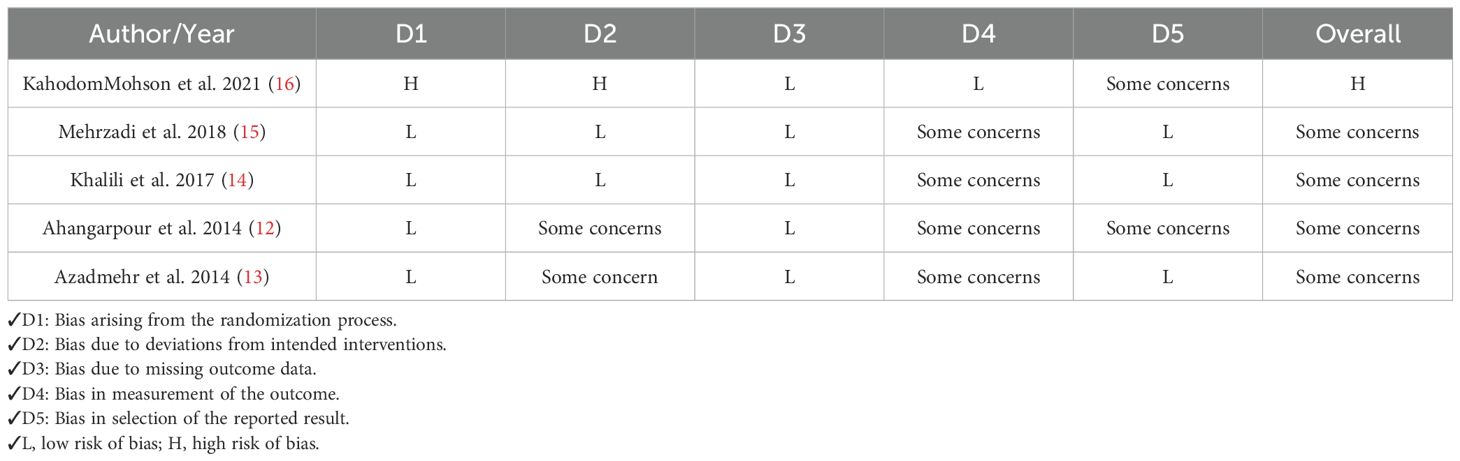
Two researchers (M.K. & K.V.) evaluated the methodological quality of each included study through the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). This tool was built to assess the risk of bias in randomized clinical trial studies (23). The tool consists of 5 items that concern five domains, including selection bias, reporting bias, performance bias, attrition bias, and other sources of bias.
2.7 Meta-analysis
We used Stata version 18 (Stata Corp, College Station, TX, USA) software to calculate the pooled standard mean difference (SMD) [95% confidence interval (CI)] of serum lipid profile levels in diabetic patients receiving boswellia treatments versus those who received placebo or no treatment in both pretreatment and posttreatment. We also performed SMD analysis to compare the pretreatment blood levels of lipid profiles to the posttreatment levels in both groups. In addition, we used the inconsistency index (I2) and Cochrane Q to examine the heterogeneity of the included studies. An I² greater than 50% or a p-value less than 0.05 indicated significant heterogeneity among the studies. Pooled prevalences were estimated using a random-effect model. In addition, we assess the effect of potential factors. The confidence intervals for I² were reported to determine the degree of heterogeneity among the studies (24).
3 Results
3.1 Study selection
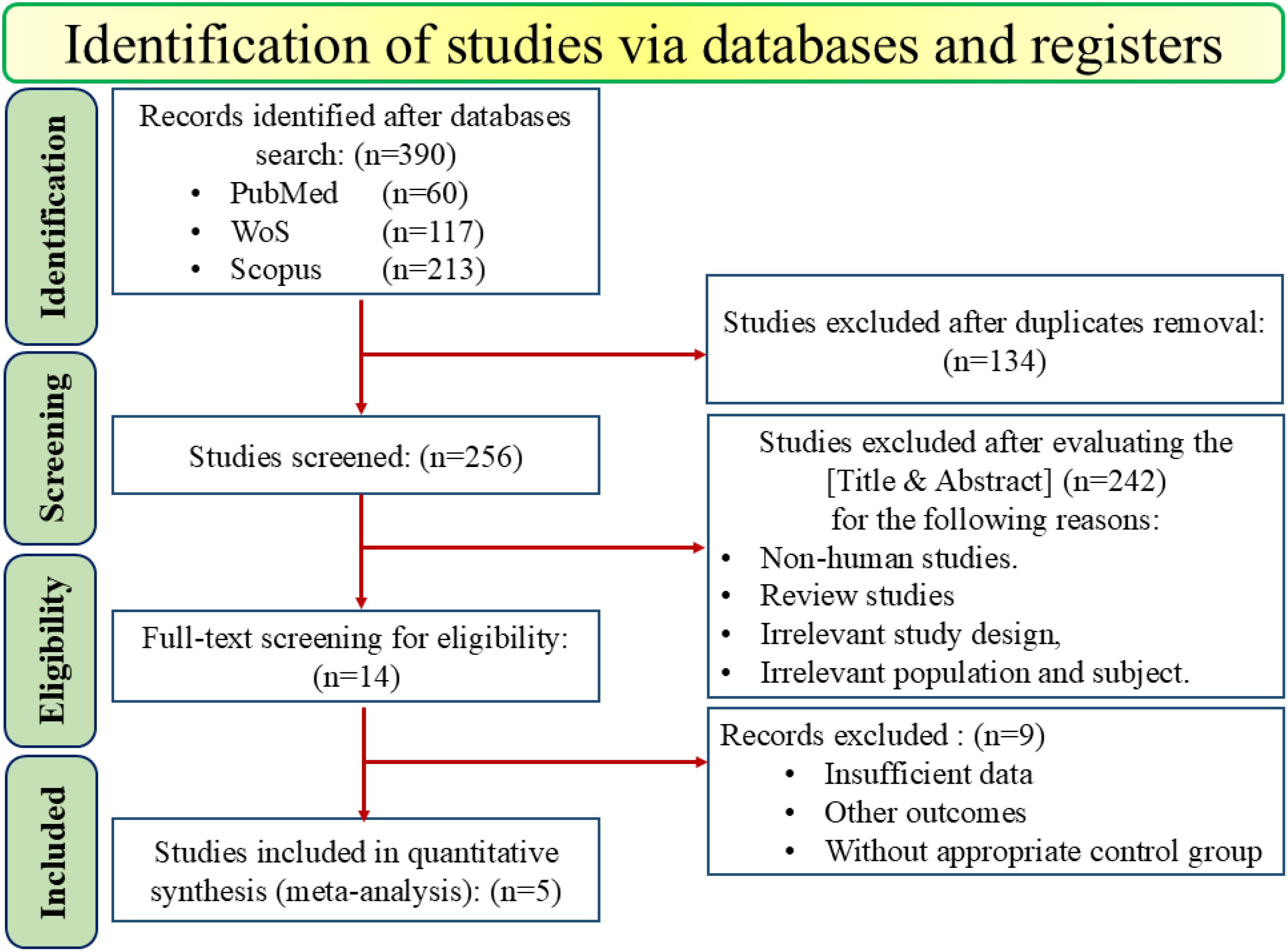
A comprehensive systematic search across online databases, including PubMed (n=60), Web-of-Science (n=117), and Scopus (n=213), initially identified 390 studies. Also, to find any gray literature, Google Scholar was manually searched. After removing 134 duplicates, 256 studies were screened based on the [Titles and Abstract]. During this screening process, 242 additional records were excluded due to non-human studies (in vivo, in vitro, etc.), review studies, irrelevant studies, and population. This left 14 studies for full-text review to assess their eligibility. Of these, 9 studies were excluded primarily due to insufficient data, other outcomes, or without appropriate treatment or control groups. Finally, 5 papers published between 2014 and 2024 were included in the meta-analysis, as shown in Figure 1.
3.2 Study characteristics and demographic data
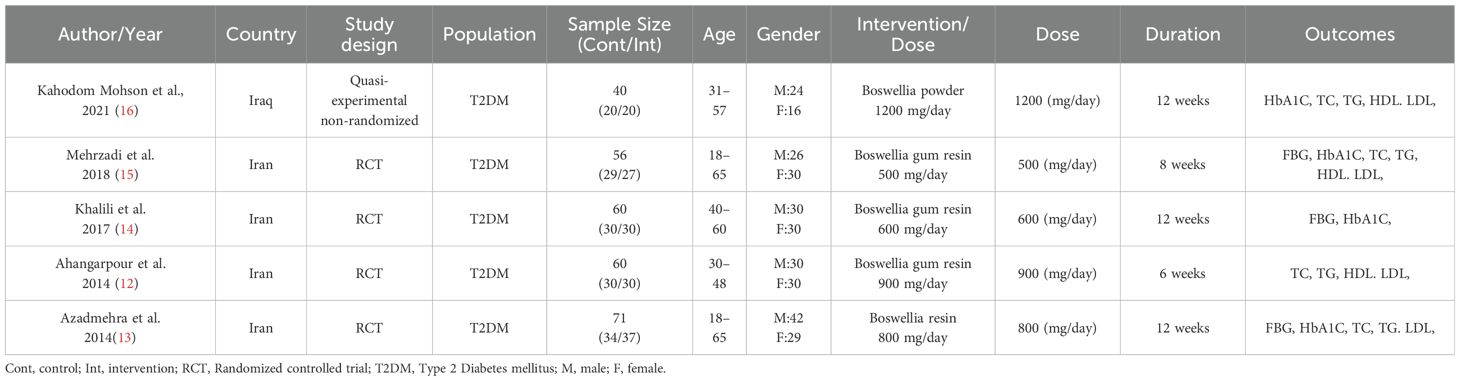
The five studies included in the meta-analysis involved 287 patients with T2DM. A total of 144 individuals received the boswellia supplementation, and 143 patients in the control group received either a placebo or no treatment (in 3 of 5 studies, subjects received a placebo; in the other 2, they received nothing). The dosage regimen for the boswellia supplementation ranged from 500 to 1200 mg per day, with treatment durations spanning 6, 8, or 12 weeks. The form of Boswellia, in one study, was powder (16), and in the remaining 4 studies were resin (12–15) (Table 2).
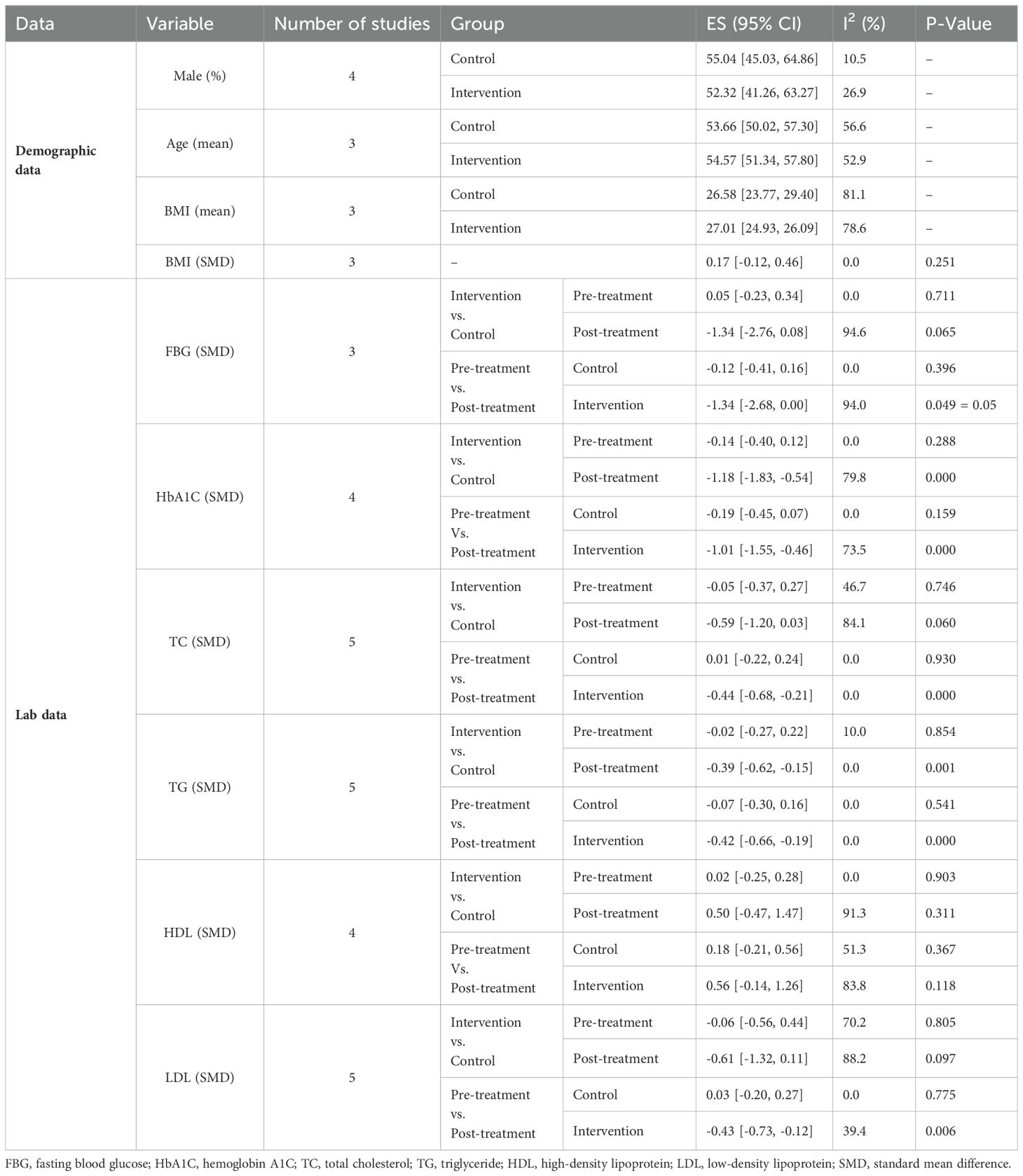
The study population was pretty balanced in terms of gender, with 52.32% (95%CI: 41.26-63.27; I2 = 26.98%) of cases and 55.04% (95%CI: 45.03-64.86; I2 = 10.48%) of controls being men. The age range of all studies varied from 18 to 65 years, with most patients falling in their 30-50th decades of life. In the intervention group, the mean age and BMI were 54.57 (95%CI: 51.34-57.80; I2 = 52.9%) and 27.01 (95%CI: 24.93 – 29.09; I2 = 78.6%), respectively. Controls’ pooled mean age and BMI were 53.66 (95%CI: 50.2 -57.30; I2 = 56.6%) and 26.58 (95%CI: 23.77 -29.40; I2 = 81.1%), respectively. The mean BMI between the two groups showed no significant difference (SMD=0.17; 95%CI: -0.12 to 0.46; I2 = 0.0%, P=0.251) (Table 3).
3.3 Quality assessment
Risk of bias assessment.
Our results showed that four studies had “Some concerns,” suggesting the potential for bias in certain domains, though not significant enough to fully compromise the findings. One study exhibited a “High risk of bias,” indicating serious issues that could affect the reliability of its results. More details regarding quality assessment are mentioned in Table 4.
3.4 Effect of boswellia on FBG & HbA1C
In 3 out of 5 studies, we retrieved the mean and SD of the fasting blood glucose (FBG) level in both intervention and control groups before and after the supplementation. The SMD of before and after supplementation levels of FBG in control and intervention groups was -0.12 (95%CI: -0.41 - 0.16; I2 = 0.0%; P=0.396) (Figure 2A) and -1.34 (95%CI: -2.68 - 0.00; I2 = 94.0%; P=0.049) respectively (Figure 2B). We also conducted an SMD analysis between the treatment and control groups in both time points and retrieved 0.05 (95%CI: -0.23 - 0.34; I2 = 0.0%; P=0.711) (Figure 2C) and -1.34 (95%CI: -2.76 - 0.08; I2 = 94.6%; P=0.065) (Figure 2D) for before and after supplementation levels, respectively.
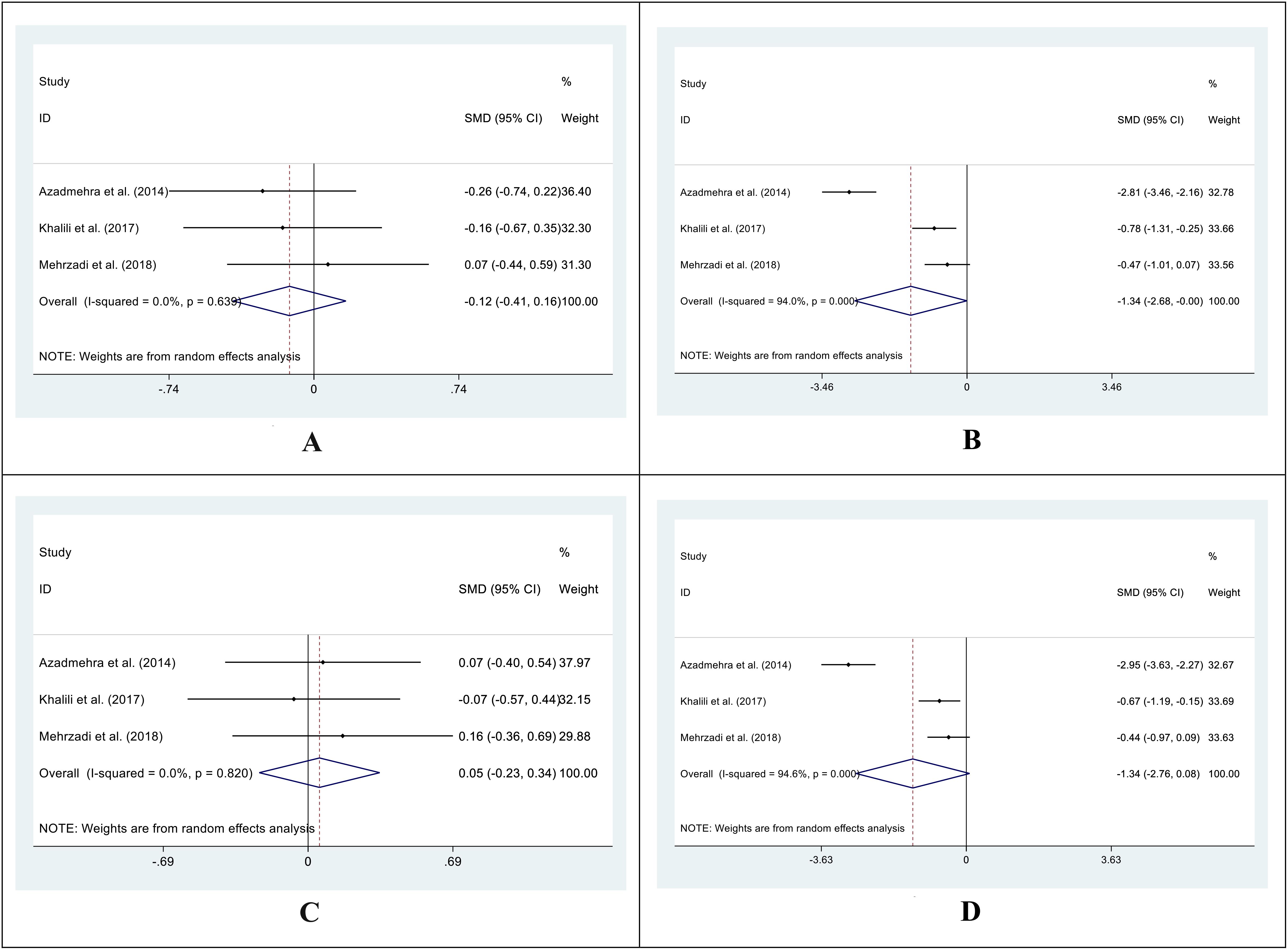
Figure 2. Forest plot of SMD for the FBG (A: before vs. after in the control groups; B: before vs. after in the intervention groups; C: intervention vs. control groups before supplementation; D: intervention vs. control groups after supplementation).
In 4 out of 5 studies, we collected data on the mean and SD of HbA1C levels in both the intervention and control groups before and after supplementation. The SMD for the before versus after supplementation HbA1C levels in the control group was -0.19 (95%CI: -0.45 - 0.07; I2 = 0.0%; P=0.159) (Figure 3A), while in the intervention group, it was -1.01 (95%CI: -1.55-(-0.46); I2 = 73.5%; P=0.000) (Figure 3B). We also compared the SMD between the treatment and control groups at both time points and found values of -0.14 (95%CI: -0.40 - 0.12; I2 = 0.0%; P=0.882) (Figure 3C) and -1.18 (95%CI: -1.83-(-0.54); I2 = 79.8%; P=0.000) (Figure 3D) for the before and after supplementation levels, respectively.
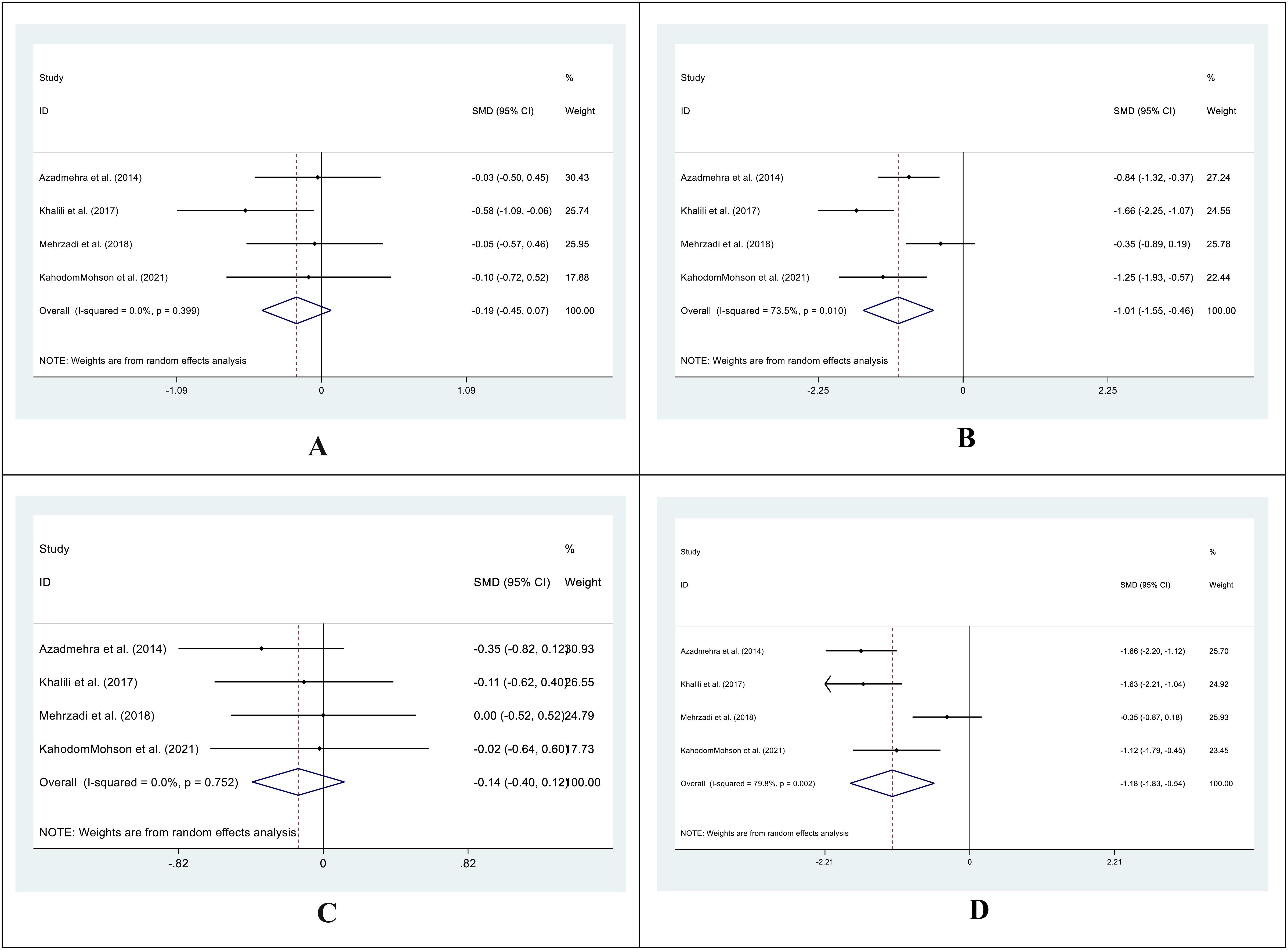
Figure 3. Forest plot of SMD for the HbA1C. (A: before vs. after in the control groups; B: before vs. after in the intervention groups; C: intervention vs. control groups before supplementation; D: intervention vs. control groups after supplementation).
3.5 Effect of boswellia on lipid profile
We obtained data on the mean and SD of all lipid profile values (including TC, TG, LDL, and HDL) in the intervention and control groups before and after supplementation in all 5 included studies, except for the HDL reported in 4 out of 5 studies.
The SMD for the control group’s pre- vs. post-supplementation TC levels was 0.01 (95%CI: -0.22 - 0.24; I2 = 0.0%; P=0.930) (Figure 4A). In the intervention group, the SMD was -0.44 (95%CI: -0.68 – (-0.21); I2 = 0.0%; P=0.00) (Figure 4B). We also compared the SMD between the treatment and control groups at two different time points. The SMD for the pre-treatment level was -0.05 (95%CI: -0.37 to 0.27; I2 = 46.7%; P = 0.746) (Figure 4C), while for the post-treatment level, it was -0.59 (95%CI: -1.20 to 0.03; I2 = 84.1%; P = 0.060) (Figure 4D).
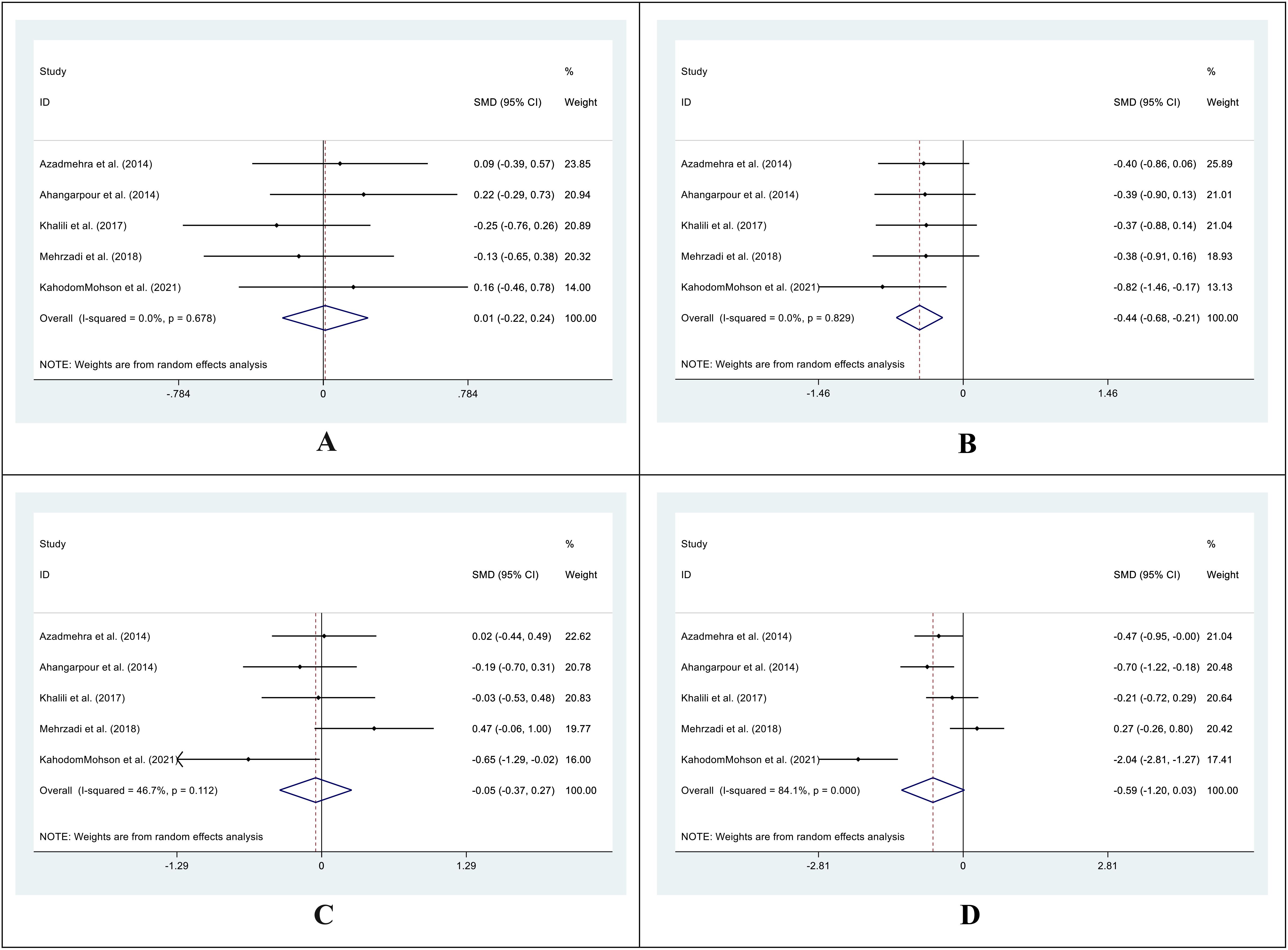
Figure 4. Forest plot of SMD for the TC. (A: before vs. after in the control groups; B: before vs. after in the intervention groups; C: intervention vs. control groups before supplementation; D: intervention vs. control groups after supplementation).
For TG levels, the SMD between the pre- and post-treatment in the control group was -0.07 (95%CI: -0.30-0.16; I2 = 0.0%; P=0.541) (Figure 5A). The SMD in the treatment group was -0.42 (95%CI: -0.66 – (-0.19); I2 = 0.0%; P=0.000) (Figure 5B). We analyzed the SMD between the treatment and control groups at two periods. The SMD for the pre-treatment level was -0.02 (95%CI: -0.27-0.22; I2 = 10.0%; P=0.854) (Figure 5C). For the post-treatment level, the SMD was -0.39 (95%CI: -0.62-(-0.15); I2 = 0.0%; P=0.001) (Figure 5D).

Figure 5. Forest plot of SMD for the TG. (A: before vs. after in the control groups; B: before vs. after in the intervention groups; C: intervention vs. control groups before supplementation; D: intervention vs. control groups after supplementation).
The SMD in HDL levels between the pre- and post-treatment in the control group was 0.18 (95%CI: -0.21 -0.56; I2 = 51.3%; P=0.367) (Figure 6A), while in the treatment group was 0.56 (95%CI: -0.14 -1.26; I2 = 83.8%; P=0.118) (Figure 6B). We also compared the SMD between the treatment and control groups at two-time points. The SMD for the pre-treatment level was 0.02 (95%CI: -0.25 -0.28; I2 = 0.0%; P=0.903) (Figure 6C), while for the post-treatment level was 0.50 (95%CI: -0.47 -1.47; I2 = 91.3%; P=0.311) (Figure 6D).
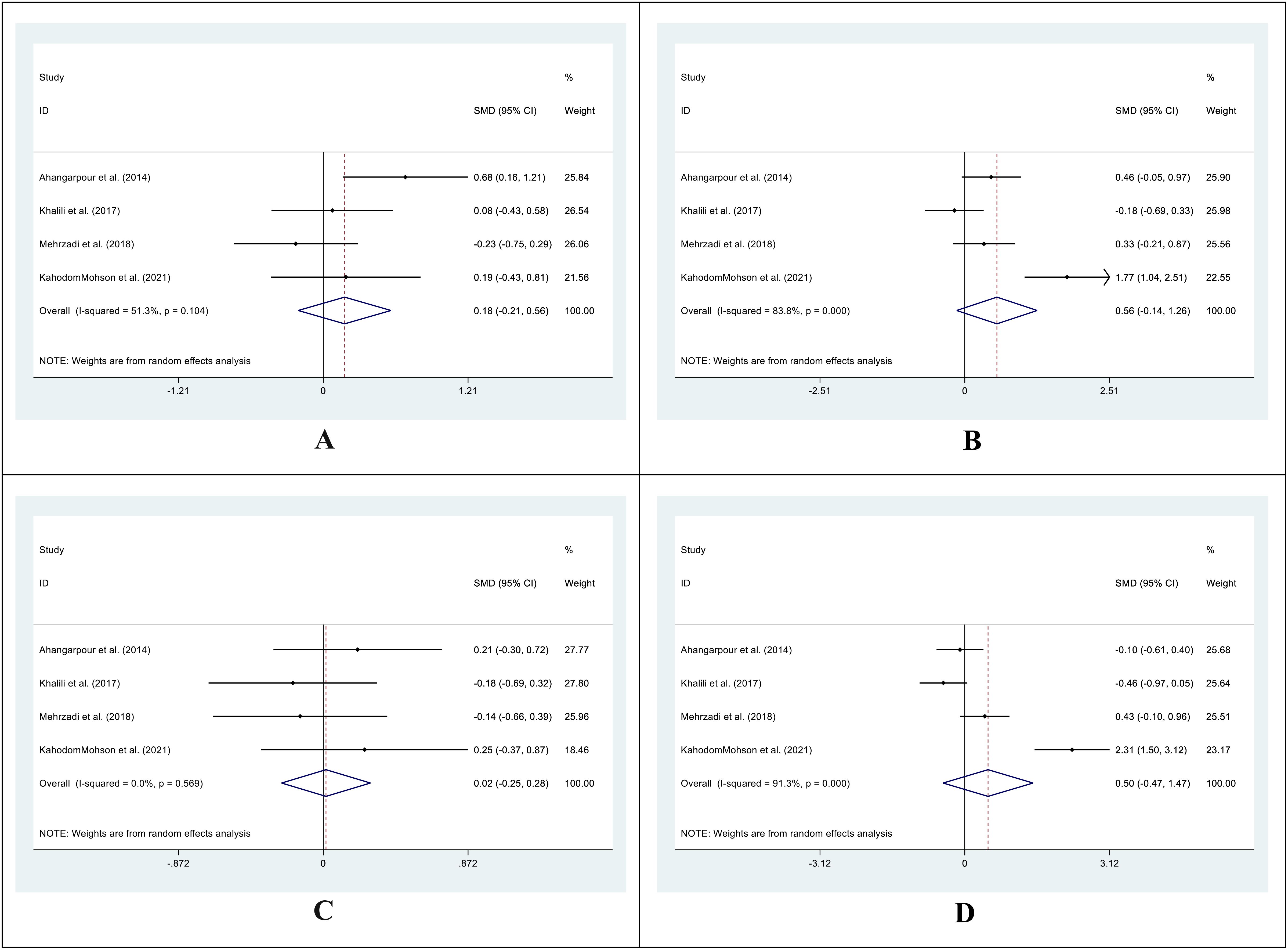
Figure 6. Forest plot of SMD for the HDL. (A: before vs. after in the control groups; B: before vs. after in the intervention groups; C: intervention vs. control groups before supplementation; D: intervention vs. control groups after supplementation).
In the treatment group, the SMD in LDL levels between pre- and post-treatment was -0.43 (95%CI: -0.73 – (-0.12); I2 = 39.4%; P=0.006) (Figure 7A), while in the control group, it was 0.03 (95%CI: -0.20 -0.27; I2 = 0.0%; P=0.775) (Figure 7B). Additionally, we compared the SMD at two different periods between the treatment and control groups. SMD was -0.06 (95%CI: -0.56 -0.44; I2 = 70.2%; P=0.805) (Figure 7C) for the pre-treatment level and -0.61 (95%CI: -1.32 -0.11; I2 = 88.2%, P=0. 0.097) (Figure 7D) for the post-treatment level.
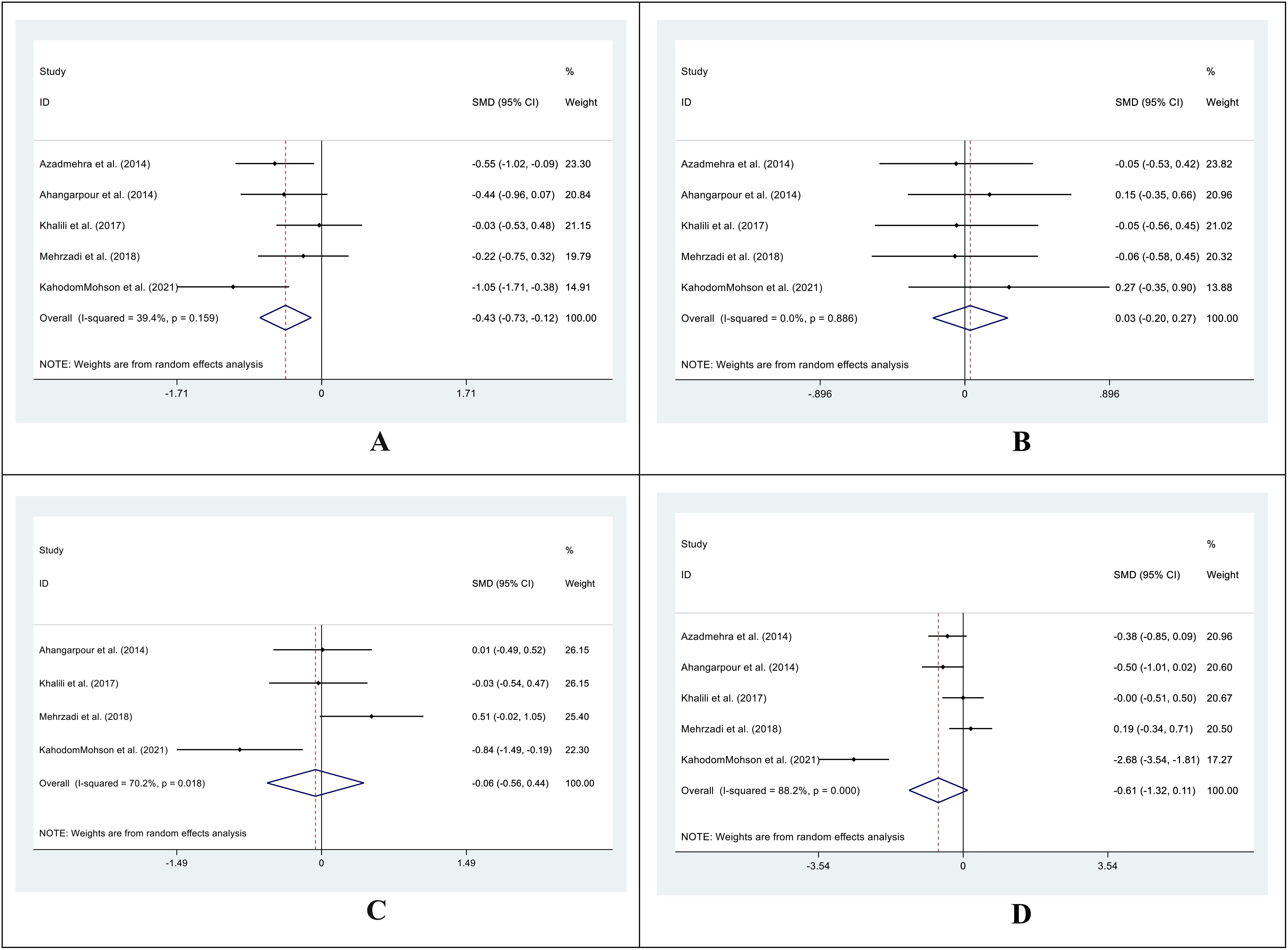
Figure 7. Forest plot of SMD for the LDL. (A: before vs. after in the control groups; B: before vs. after in the intervention groups; C: intervention vs. control groups before supplementation; D: intervention vs. control groups after supplementation).
3.6 Sub-group analysis and meta-regression
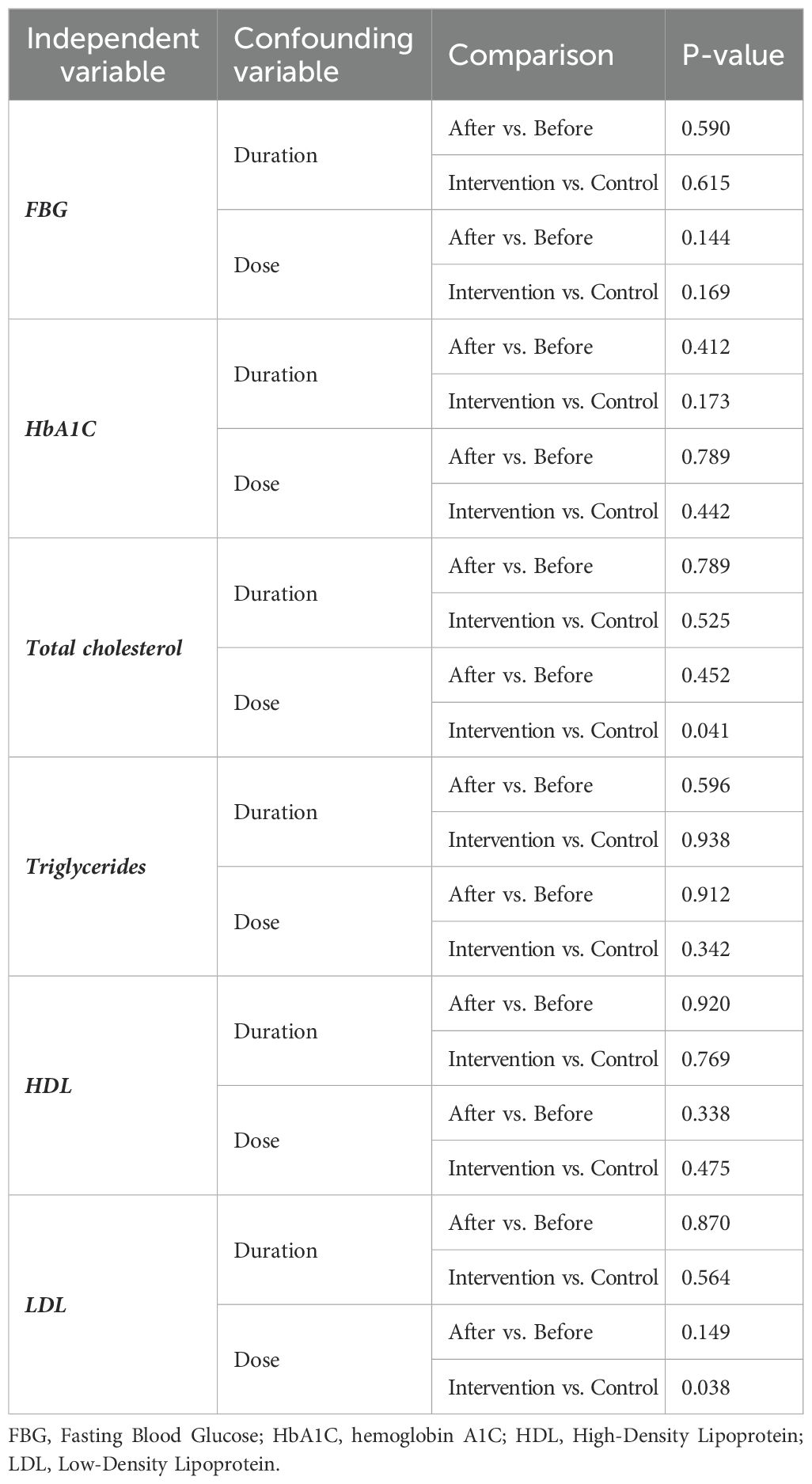
Due to the insufficient number of papers, we did not perform a subgroup analysis. However, we used meta-regression as an alternative to address heterogeneity. The meta-regression results for the effect size (SMD) with the dose and duration of treatment did not show significant results, except for the dose of therapy in post-treatment SMD between intervention and control groups in TC (P=0.041) and LDL (P=0.039) levels (Table 5; Figures 8A, B).

Figure 8. (A) Meta-regression analysis of SMD for the intervention and control in the post-treatment TC levels with a supplement dose. (B) Meta-regression analysis of SMD for the intervention and control in the post-treatment LDL levels with a supplement dose.
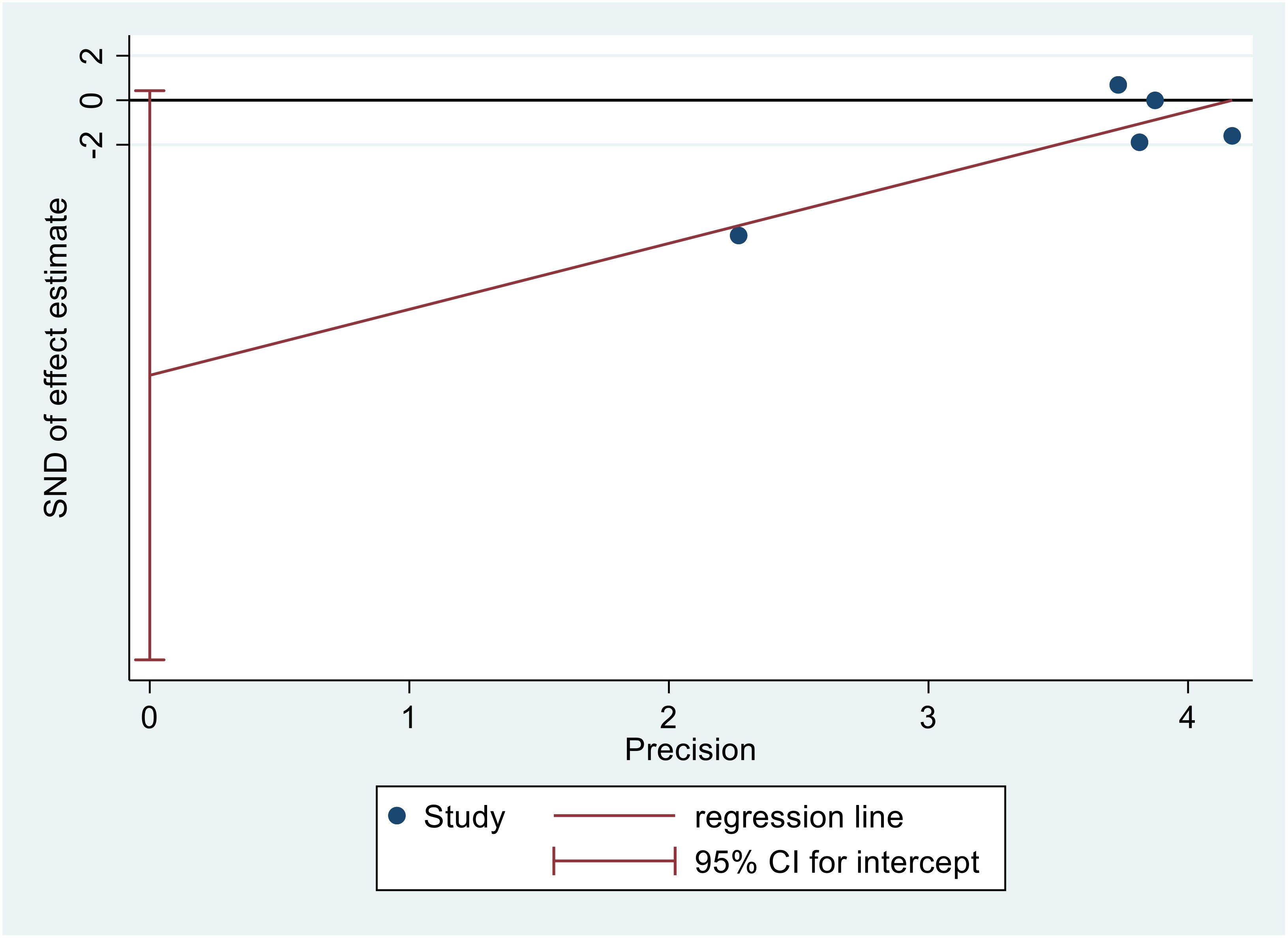
3.7 Publication bias
Figure 9 displays Egger’s publication bias plot for TC. The analysis of this plot indicated the absence of publication bias (p=0.054), indicating that both positive and negative findings have been reported.
4 Discussion
To our knowledge, this study represents the first comprehensive systematic review and meta-analysis evaluating the effects of boswellia supplementation on the glycemic markers and lipid profile in patients with T2DM. The meta-analysis found that boswellia supplementation led to notable improvements in glycemic markers and lipid profiles. HbA1c levels showed significant reductions in the intervention group compared to the control while fasting blood glucose (FBG) improved but was not statistically significant. Additionally, there were significant decreases in total cholesterol (TC), triglycerides (TG), and LDL levels, with a slight but non-significant increase in HDL levels. These findings suggest that boswellia supplementation effectively enhances glycemic control and lipid profiles.
In the clinical practice of medicine, FBG and HbA1C are considered the backbone of DM diagnosis. FBG and HbA1C indicate the relative short-term and long-term glycemic control in the clinical evidence (25).
T2DM, as a multi-organ, metabolic, and chronic disease, is strongly associated with the development of dyslipidemia, contributing to life-threatening cardiovascular complications (26). It is essential to leverage anti-glycemic agents with a favorable impact on lipid metabolism, as they can provide optimal benefits in managing glycemic markers while simultaneously improving lipid indices (27).
Patients diagnosed with non-communicable diseases often experience multimorbidity, polypharmacy, and adverse drug reactions, leading to numerous complications (28). Regarding this matter, the trend toward utilizing natural phytonutrients in managing non-communicable diseases, especially T2DM, has emerged as an era of research interest. Since immemorial, complementary herbal medicine and phytonutrients have attracted considerable attention and yielded promising therapeutic outcomes (29).
Emerging evidence has shown that Boswellia serrata, a potential traditional herbal medicine, possesses favorable anti-inflammatory (30), antitumor (31), antimicrobial (32), and hepatoprotective effects (12). Surprisingly, preclinical research projects determined the antihyperglycemic and anti-hyperlipidemic properties of boswellia in a diabetic rat model induced by streptozotocin (33).
Boswellia serrata, commonly known as Indian frankincense, is a traditional medicinal plant used in Ayurveda since ancient times due to its anti-inflammatory and therapeutic properties. Its active components, particularly boswellia acids, have been reported to offer potential benefits by altering pathways leading to diabetes and related complications via inflammation (17–20).
Evidence has shown that boswellia has a significant regulatory effect on glycemic and lipid metabolism. It achieves this by protecting pancreatic beta cells and playing a crucial role in regulating insulin signaling, which is key in diabetes management. Additionally, it influences gluconeogenesis (34–36).
Pro-inflammatory cytokines play a crucial role in insulin resistance and T2DM development (37). In this regard, numerous studies demonstrated that the protective effect of boswellic acid on diabetes management could be explained by a significant improvement in the concentration of inflammatory markers, possibly via downregulating the Nuclear Kappa B (NF-kB) signaling pathways (38, 39). Moreover, the anti-oxidant effect of boswellia species can modulate the serum lipid level by reducing the Tumor Necrosis Factor α (TNF-α) and Interleukin-1β levels while increasing the adiponectin level (40). In this connection, Pandey et al. demonstrated that the boswellia extracts showed a remarkable decline in TC and a notable increase in HDL in the rat models (41). Interestingly, a computational analysis by Khan A. et al. revealed that boswellia acid extracts might potentially treat diabetes by inhibiting dipeptidyl peptidase-4. Their biochemical analysis suggested that the insulin receptors could be the therapeutic targets of boswellia acid extracts (42).
According to the previously determined favorable effect of boswellia in diabetic animal models, several clinical trials evaluated the antihyperglycemic and anti-hyperlipidemic effects of different doses of boswellia compounds in patients with T2DM (12–16). It is noteworthy to consider some points when interpreting the results of included studies. Mehrzadi et al. showed that eight weeks of treatment with 500 mg boswellia gum resin in T2DM patients did not significantly improve the glycemic and lipid profile compared with the placebo group (15). Another randomized clinical trial found that 1200 mg of boswellia powder for 12 weeks with or without a metformin supplement benefited T2DM patients with a significant reduction in blood glucose and HbA1C (16).
It is important to note that the varying results from the studies may be due to differences in factors such as sample size, dose, duration, mode of intervention, and measurement kit errors. Other confounding factors could impact the glycemic index results, which are worth discussing. Diabetic patients participating in trials may receive oral hypoglycemic agents, which may be a source of heterogeneity based on the particular type of anti-diabetic medications. Physical activity, lifestyle modification, weight loss, and supplementation treatment can significantly induce a lower blood glucose level compared to the alone supplementation therapy. Another important note is that boswellia compounds utilized in different studies may have different percentages of boswellia or other promising nutrients. Importantly, not all included studies used a specific form of boswellia compound, which should be considered. For example, Khalili et al. (14) performed a randomized clinical trial that showed how a mix of herbal compounds (olibanum, silymarin, and nettle) could lower blood glucose in people with T2DM for three months (14). Investigators should consider that concurrent phytonutrients or drugs may cause bias and distorted results during the research project.
The present systematic review and meta-analysis have some limitations. More importantly, our study includes a limited number of eligible studies with a small sample size. The included articles were heterogeneous, concerning the different characteristics of the population, as well as the diverse dose, quality, and duration of intervention. The short follow-up periods in studies limit the capability to evaluate the safety and long-term efficacy of boswellia on glycemic and lipid markers. Besides, most of the recruited studies were conducted in Iran, resulting in a limited generalization of our findings.
Furthermore, due to the limited number of included studies, our analysis needed to incorporate subgroup analysis. To address the heterogeneity precisely, we performed a meta-regression analysis. We observed a significant impact of intervention dose on the effect size for TC and LDL levels, providing a remarkable dose-response relationship in these outcomes. Correspondingly, our systematic review and meta-analysis necessitate the performance of large-scale, well-designed clinical trials with prolonged duration of intervention alone with long-term follow-up to elucidate further the potential therapeutic effect of boswellia on glucose control, lipid profile, safety, and tolerability in T2DM patients. The clinical implication of boswellia in managing glycemic and lipid profiles is crucial in preventing and managing cardiovascular complications in diabetic patients.
5 Conclusion
In conclusion, boswellia and its extract supplementation may positively impact glycemic markers and lipid profiles in patients with T2DM. However, well-designed clinical trials on a larger scale are recommended to validate these findings and fully understand their effects, with extended intervention durations and long-term follow-up. These future studies should aim to confirm the benefits, assess the sustainability of the effects, and explore any potential long-term outcomes or risks associated with boswellia supplementation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KV: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. S-KR-A: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to express our sincere gratitude to all the researchers and healthcare professionals whose work significantly contributed to understanding Type 2 diabetes mellitus. These contributions are invaluable to the ongoing fight against diabetes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2024.1466408/full#supplementary-material
Abbreviations
T2DM, Type 2 Diabetes Mellitus; IDF, International Diabetes Federation; CVD, Cardiovascular diseases; RCTs, Randomized clinical trial; CAM, Complementary and alternative medicine; FBG, Fasting Blood Glucose; HbA1C, Hemoglobin A1C; TC, Total Cholesterol; TG, Triglyceride; HDL, High-Density Lipoprotein; LDL, Low-Density Lipoprotein.
References
1. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21176275
2. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
3. Magliano DJ, Boyko EJ, committee IDFDAtes. IDF Diabetes Atlas. Idf diabetes atlas. Brussels: International Diabetes Federation© International Diabetes Federation (2021).
4. Chakraborty S, Verma A, Garg R, Singh J, Verma H. Cardiometabolic risk factors associated with type 2 diabetes mellitus: A mechanistic insight. Clin Med Insights Endocrinol Diabetes. (2023) 16:11795514231220780. doi: 10.1177/11795514231220780
5. Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. (2019) 26:25–32. doi: 10.1177/2047487319878371
6. Rahimlou M, Mirzaei K, Keshavarz SA, Hossein-Nezhad A. Association of circulating adipokines with metabolic dyslipidemia in obese versus non-obese individuals. Diabetes Metab Syndr. (2016) 10:S60–5. doi: 10.1016/j.dsx.2015.09.015
7. Alfian S, Sukandar H, Arisanti N, Abdulah R. Complementary and alternative medicine use decreases adherence to prescribed medication in diabetes patients. Ann Trop Med Public Health. (2016) 9. doi: 10.4103/1755-6783.179108
8. Yedjou CG, Grigsby J, Mbemi A, Nelson D, Mildort B, Latinwo L, et al. The management of diabetes mellitus using medicinal plants and vitamins. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24109085
9. Mokgalaboni K, Lebelo SL, Modjadji P, Ghaffary S. Okra ameliorates hyperglycaemia in pre-diabetic and type 2 diabetic patients: A systematic review and meta-analysis of the clinical evidence. Front Pharmacol. (2023) 14:1132650. doi: 10.3389/fphar.2023.1132650
10. Ghanavati M, Rahmani J, Clark CCT, Hosseinabadi SM, Rahimlou M. Pistachios and cardiometabolic risk factors: A systematic review and meta-analysis of randomized controlled clinical trials. Complement Ther Med. (2020) 52:102513. doi: 10.1016/j.ctim.2020.102513
11. Morshedzadeh N, Rahimlou M, Shahrokh S, Karimi S, Mirmiran P, Zali MR. The effects of flaxseed supplementation on metabolic syndrome parameters, insulin resistance and inflammation in ulcerative colitis patients: An open-labeled randomized controlled trial. Phytother Res. (2021) 35:3781–91. doi: 10.1002/ptr.v35.7
12. Ahangarpour A, Heidari H, Fatemeh RAA, Pakmehr M, Shahbazian H, Ahmadi I, et al. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type2 diabetic patients. J Diabetes Metab Disord. (2014) 13:1–5. doi: 10.1186/2251-6581-13-29
13. Azadmehr A, Ziaee A, Ghanei L, Fallah Huseini H, Hajiaghaee R, Tavakoli-Far B, et al. A randomized clinical trial study: anti-oxidant, anti-hyperglycemic and anti-hyperlipidemic effects of olibanum gum in type 2 diabetic patients. Iran J Pharm Res. (2014) 13:1003–9.
14. Khalili N, Fereydoonzadeh R, Mohtashami R, Mehrzadi S, Heydari M, Huseini HF. Silymarin, olibanum, and nettle, A mixed herbal formulation in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. J Evid Based Complementary Altern Med. (2017) 22:603–8. doi: 10.1177/2156587217696929
15. Mehrzadi S, Tavakolifar B, Huseini HF, Mosavat SH, Heydari M. The effects of boswellia serrata gum resin on the blood glucose and lipid profile of diabetic patients: A double-blind randomized placebo-controlled clinical trial. J Evid Based Integr Med. (2018) 23:2515690x18772728. doi: 10.1177/2515690X18772728
16. KahodomMohson A, Hamza RAH, Al-Mubarak ZA. Effect of using Boswellia serrata powder on blood glucose level and lipid profile of the patient with diabetes mellitus type II. Ann Romanian Soc Cell Biol. (2021), 16924–31.
17. Siddiqui MZ. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci. (2011) 73(3):255–61. doi: 10.4103/0250-474X.93507
18. Barik MR, Kaur H, Amin T, Tiwari H, Kour G, Goswami A, et al. Network pharmacology and in vitro validation to elucidate the molecular mechanism of Boswellia serrata phytoconstituents on inflammation. Journal of Proteins and Proteomics. (2024) 30:1–7.
19. Verma M, Fatima S, Saeed M, Ansari IA. Anti-proliferative, pro-apoptotic, and chemosensitizing potential of 3-acetyl-11-keto-β-boswellic acid (AKBA) against prostate cancer cells. Mol Biotechnol. (2024). doi: 10.1007/s12033-024-01089-7
20. Rudrappa GH, Murthy M, Saklecha S, Kumar Kare S, Gupta A, Basu I. Fast pain relief in exercise-induced acute musculoskeletal pain by turmeric-boswellia formulation: A randomized placebo-controlled double-blinded multicentre study. Med (Baltimore). (2022) 101:e30144. doi: 10.1097/MD.0000000000030144
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Brown D. A review of the pubMed PICO tool: using evidence-based practice in health education. Health Promot Pract. (2020) 21:496–8. doi: 10.1177/1524839919893361
23. Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. Cochrane Methods Cochrane Database Syst Rev. (2016) 10.
24. Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. Bmj. (2007) 335:914–6. doi: 10.1136/bmj.39343.408449.80
25. Misra A, Garg S. HbA1c and blood glucose for the diagnosis of diabetes. Lancet. (2011) 378:104–6. doi: 10.1016/S0140-6736(11)60789-7
26. Kane JP, Pullinger CR, Goldfine ID, Malloy MJ. Dyslipidemia and diabetes mellitus: Role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. Curr Opin Pharmacol. (2021) 61:21–7. doi: 10.1016/j.coph.2021.08.013
27. Piccirillo F, Mastroberardino S, Nusca A, Frau L, Guarino L, Napoli N, et al. Novel antidiabetic agents and their effects on lipid profile: A single shot for several cardiovascular targets. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241210164
28. Matthias AT, Fernando G, Somathilake B, Prathapan S. Predictors and patterns of polypharmacy in chronic diseases in a middle-income country. Int J Physiol Pathophysiol Pharmacol. (2021) 13:158–65.
29. Keskin M, Thiruvengadam M. Phytochemicals from natural products for the prevention and treatment of non-communicable diseases. Curr Top Med Chem. (2022) 22:1907–8. doi: 10.2174/156802662223221019141622
30. Siddiqui MZ. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci. (2011) 73:255–61. doi: 10.4103/0250-474X.93507
31. Roy NK, Deka A, Bordoloi D, Mishra S, Kumar AP, Sethi G, et al. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. (2016) 377:74–86. doi: 10.1016/j.canlet.2016.04.017
32. Sadhasivam S, Palanivel S, Ghosh S. Synergistic antimicrobial activity of Boswellia serrata Roxb. ex Colebr. (Burseraceae) essential oil with various azoles against pathogens associated with skin, scalp and nail infections. Lett Appl Microbiol. (2016) 63:495–501. doi: 10.1111/lam.12683
33. Shehata AM, Quintanilla-Fend L, Bettio S, Singh CB, Ammon HP. Prevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE). Phytomedicine. (2011) 18:1037–44. doi: 10.1016/j.phymed.2011.06.035
34. Kherouf A, Aouacheri O, Tichati L, Tebboub I, Kherouf M, Saka S. Potential antioxidant properties and anti-diabetic and hepatic/pancreatic protective effects of dietary Boswellia serrata gum resin powder against oxidative damage in streptozotocin-induced diabetic rats. Comp Clin Path. (2021) 30:891–904.
35. Lee J, Noh S, Lim S, Kim B. Plant extracts for type 2 diabetes: from traditional medicine to modern drug discovery. Antioxidants (Basel). (2021) 10. doi: 10.3390/antiox10010081
36. al-Awadi F, Fatania H, Shamte U. The effect of a plants mixture extract on liver gluconeogenesis in streptozotocin induced diabetic rats. Diabetes Res. (1991) 18:163–8.
37. Pirola L, Ferraz JC. Role of pro- and anti-inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World J Biol Chem. (2017) 8:120–8. doi: 10.4331/wjbc.v8.i2.120
38. Cuaz-Pérolin C, Billiet L, Baugé E, Copin C, Scott-Algara D, Genze F, et al. Antiinflammatory and antiatherogenic effects of the NF-κB inhibitor acetyl-11-keto-β-boswellic acid in LPS-challenged apoE–/– mice. Arterioscler Thromb Vasc Biol. (2008) 28:272–7. doi: 10.1161/ATVBAHA.107.15560
39. Eltahir HM, Fawzy MA, Mohamed EM, Alrehany MA, Shehata AM, Abouzied MM. Antioxidant, anti−inflammatory and anti−fibrotic effects of Boswellia serrate gum resin in CCl4−induced hepatotoxicity. Exp Ther Med. (2020) 19:1313–21. doi: 10.3892/etm.2019.8353
40. Gomaa AA, Farghaly HSM, El-Sers DA, Farrag MM, Al-Zokeim NI. Inhibition of adiposity and related metabolic disturbances by polyphenol-rich extract of Boswellia serrata gum through alteration of adipo/cytokine profiles. Inflammopharmacology. (2019) 27:549–59. doi: 10.1007/s10787-018-0519-4
41. Pandey RS, Singh BK, Tripathi YB. Extract of gum resins of Boswellia serrata L. inhibits lipopolysaccharide induced nitric oxide production in rat macrophages along with hypolipidemic property. Indian J Exp Biol. (2005) 43:509–16.
Keywords: boswellia, Kundur, herb, diabetes, anti-diabetic, glycemic markers, lipid profile, meta-analysis
Citation: Karimi M, Vakili K, Rashidian P, Razavi-Amoli S-K, Akhbari M and Kazemi K (2024) Effect of boswellia (Boswellia serrata L.) supplementation on glycemic markers and lipid profile in type 2 diabetic patients: a systematic review and meta-analysis. Front. Clin. Diabetes Healthc. 5:1466408. doi: 10.3389/fcdhc.2024.1466408
Received: 17 July 2024; Accepted: 25 September 2024;
Published: 10 October 2024.
Edited by:
Mehran Rahimlou, Zanjan University of Medical Sciences, IranReviewed by:
Kabelo Mokgalaboni, University of South Africa, South AfricaMohamed Rafiullah, King Saud University, Saudi Arabia
Seyed Ahmad Hosseini, Ahvaz Jundishapur University of Medical Sciences, Iran
Copyright © 2024 Karimi, Vakili, Rashidian, Razavi-Amoli, Akhbari and Kazemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Karimi, a2FyaW1pOTAxMEBnbWFpbC5jb20=; ZHIubWVoZGlpLmthcmltaUBnbWFpbC5jb20=
 Mehdi Karimi
Mehdi Karimi Kimia Vakili
Kimia Vakili Pegah Rashidian4
Pegah Rashidian4 Seyedeh-Kiana Razavi-Amoli
Seyedeh-Kiana Razavi-Amoli Matin Akhbari
Matin Akhbari