- 1Department of Epidemiology and Biostatistics, School of Public Health, Addis Ababa University, Addis Ababa, Ethiopia
- 2African Population and Health Research Center, Nairobi, Kenya
- 3Department of Internal Medicine, School of Medicine, Addis Ababa University, Addis Ababa, Ethiopia
Background: For successful glycemic control, diabetes control requires a comprehensive management plan in which patients are educated and supported to make informed decisions about diet, exercise, weight control, blood glucose monitoring, taking medication, and regular screening for complications. Current evidence on the effectiveness of diabetes self-management education and support (D-SMES) interventions on blood glucose control is mixed, with some studies pointing to significant glycemic control benefits, whereas others have shown no significant benefits.
Objective: This systematic review and meta-analysis (SRMA) was conducted to evaluate the effectiveness of D-SMES interventions compared with usual care in controlling blood glucose levels among people living with type 2 diabetes (T2DM) in the World Health Organization (WHO) Africa Region and to describe the core components of D-SMES interventions.
Methods: We performed a SRMA of D-SMES interventions for managing T2DM in the WHO Africa Region. We searched PubMed, CINAHL, the Cochrane Central Register of Controlled Trials (CCRCT), and Google Scholar from inception to May 5, 2025, for studies that were randomized control trials that reported glycated hemoglobin (HbA1c) or fasting blood sugar (FBS) as outcome measures and were delivered to adults with T2DM. The methodological quality of the included studies was assessed via the Cochrane risk of bias tool (RoB2). Random effects model meta-analysis was used to estimate the population average pooled standard mean difference (Hedges’ g) for HbA1c with 95% CIs.
Results: We screened the title/abstract records of 350 studies, of which 19 studies with a total of 3759 participants (1866 in the D-SMES group and 1893 in the usual care group) were included in the meta-analysis of HbA1c. The meta-analysis revealed a significant overall effect of D-SMES interventions on HbA1c among people living with T2DM in the WHO African Region (SMD = -0.468 with a 95% CI of -0.658 to -0.279, I2 = 85.5%). nine of the nineteen included studies reported significant effects. We would expect that in some 95% of all populations comparable to those in the analysis, the true effect size would fall between -1.27 and 0.34 (prediction interval). Of the 19 included studies, 15 had a low risk of bias, two had high risk, and two raised some concerns based on the Cochrane RoB 2 tool.
Conclusions: Diabetes self-management education and support interventions are moderately effective in controlling blood glucose levels in T2DM patients within the WHO African region.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42022375732.
Background
Diabetes mellitus (DM) is a chronic metabolic condition characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both, can leads to severe damage to the heart, blood vessels, eyes, kidneys, and nerves over time, if not treated properly (1, 2).
Type 2 diabetes mellitus (T2DM) is the most common type of diabetes, accounting for approximately 90% of all diabetes cases. It is generally characterized by insulin resistance, where the body does not fully respond to insulin. T2DM is associated with a family history of diabetes, overweight or obesity, an unhealthy diet, physical inactivity, increased age, and high blood pressure (1).
In the IDF Africa Region, one in 20 (25 million) adults aged 20-79 years were living with diabetes in 2024. This number is predicted to increase by 142% to 60 million by 2050. Four in Five (73%) people living with diabetes are undiagnosed. Diabetes was responsible for 216,000 deaths in 2024 in Africa (1).
The progression of diabetes and its complications can be prevented through strict glycemic control. Epidemiological analysis of the UK Prospective Diabetes Study (UKPDS) data revealed that for every 1% reduction in HbA1c, the relative risk for microvascular complications decreased by 37%, that for diabetes-related deaths decreased by 21%, and that for myocardial infarction decreased by 14% (3).
To achieve or maintain the target of glycemic control, diabetes requires a comprehensive management plan in which patients are educated and supported to make informed decisions about diet, exercise, weight control, blood glucose monitoring, medication adherence, and regular screening for complications. In this context, the WHO Global Action Plan on Control of NCDs (4) aims to reduce the burden of NCDs by promoting healthy lifestyles; reducing common risk factors; providing integrated evidence-based, innovative, and cost-effective public health and clinical interventions; and suggesting strategic interventions for decentralizing and integrating NCD services and preventing NCD risk factors into primary health care (PHC) through task shifting.
Self-management is the set of tasks individuals undertake to help them live with one or more long-term conditions (such as eating healthily, being more physically active, and controlling their blood glucose to manage their diabetes). D-SMES interventions are aimed at improving self-care behaviors. A useful framework for defining the scope of self-management interventions is provided by the taxonomy proposed in the Practical Systematic Reviews in Self-Management Support (PRISMS) (5). The PRISMS taxonomy comprises 14 distinct components that may be delivered directly to people with long-term conditions (LTCs) and/or their caregivers to support self-management. These include: 1) information about conditions or management; 2) information about available resources; 3) provision of or agreement on specific clinical action plans and/or rescue medication; 4) regular clinical review; 5) monitoring of conditions with feedback; 6) practical support with taking medication or doing recommended behaviors); 7) provision of equipment; 8) provision of easy access to advise or support when needed; 9) training or rehearsal to communicate with health care professionals; 10) training or rehearsal for everyday activities; 11) training or rehearsal for practical self-management activities; 12) training or rehearsal for psychological strategies; 13) social support; and 14) lifestyle advice and support. Self-management support is typically multifaceted, and the expectation is that several (although not necessarily all) of the PRISMS components may be present in interventions. Several studies, mainly conducted in high-income countries, provide considerable evidence supporting D-SMES interventions as cost-effective and clinically effective in the prevention and management of T2DM by reducing body weight and improving glucose control (6–10).
Current evidence on the effects of D-SMES interventions on blood glucose control is mixed, with some studies pointing to significant glycemic benefits (11–16), whereas others have shown no significant benefits (17–19).
According to Dube and colleagues, D-SMES interventions in most African countries are limited in scope, content, and consistency, and it is unclear how patients from Sub-Saharan Africa (SSA) manage their diabetes (20). Although a systematic review conducted in 2018 among people living with T2DM in SSA revealed that the provision of structured D-SMES was effective in improving patients’ behaviors and health outcomes (21), the finding was based on limited data (only six out of the 43 reviewed studies were based on D-SMES interventions). One recent scoping review on D-SMES in the WHO African Region (22) is available, but it includes different research designs, such as randomized controlled trials, quasi-experimental studies, mixed methods, and observational cohort studies.
On the other hand, we did not find any ongoing SRMA considered to investigate the effectiveness of D-SMES interventions on glycemic levels among peoples living with T2DM.
Therefore, the aim of this SRMA is to determine the effect of D-SMES interventions on glycemic levels in adults living with T2DM in the WHO African Region.
In our systematic review and meta-analysis, we aimed to answer the following two questions (1): Are D-SMES interventions, compared with usual care, effective in improving blood glucose levels among adult patients with T2DM in the WHO African Region? (2) What are the core components of D-SMES interventions, specifically in relation to intervention characteristics for the management of T2DM (method, context of delivery, provider, strategy, intervention duration, and intensity)?
Methods
This SRMA was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (23). The PRISMA 2020 checklist for reporting systematic reviews and meta-analyses is presented in Additional File 1. This review was registered prospectively on the International Prospective Register of Systematic Reviews (PROSPERO 2022: CRD42022375732).
PICO eligibility criteria
Population
This SRMA considered studies carried out among T2DM patients living in the WHO African Region.
Interventions
We included studies assessing any D-SMES interventions for T2DM that matched at least one of the fourteen categories of the Practical Reviews in Self-Management Support (PRISMS) taxonomy (5). There were no inclusion limits on the frequency, duration, or delivery mode of the intervention. No restrictions were applied regarding the year of publication.
Comparators
Studies comparing D-SMES interventions with standard or usual diabetes care were included in this SRMA. Standard or usual diabetes care includes routine medical consultation and follow-up from healthcare providers on the basis of the lifestyle and self-care treatment algorithms recommended by the country’s NCD management guidelines.
Outcome
Studies that assessed HbA1c as an outcome measure were included. When average blood glucose (ABG) levels were reported, we used a formula proposed by Nathan DM et al. (24) to convert ABG into HbA1c.
Types of studies
Only randomized controlled trials (RCTs) at community or outpatient health facility settings were included in this SRMA. Cluster RCTs were included if the unit of analysis was at the patient level.
Exclusion criteria
We excluded studies with the following characteristics: type 1 diabetes, gestational diabetes, studies outside of the WHO African region, study reports written in languages other than English, studies in which the outcome was not reported (either HbA1c or ABG), review protocols, review articles, SRMAs, editorials, qualitative, mixed methods, quasi-experimental, pre-post, and observational studies.
Search strategy
A systematic electronic literature search was conducted to retrieve eligible studies from PubMed (PubMed Central, MEDLINE), CINAHL, CCRCT, and Google Scholar. In addition, we further searched the reference lists of all the included papers and previous reviews. The search was conducted from inception until May 5, 2025.
A combination of search terms was used. The search strategy was developed via the Yale Mesh Analyser on the basis of the PubMed identification (PMID) number of the ten initially identified articles. Accordingly, we developed and constructed the following combined search terms for each PICO criterion (Table 1).

Table 1. Combined search terms based on the PICO criterion to evaluate the effect of D-SMES interventions on HbA1c among T2DM patients in the WHO African Region.
Study selection
All identified citations were exported to EndNote reference management software to manage duplications, and then two independent reviewers (YS and EG) searched and screened the titles and abstracts of the remaining articles against the inclusion criteria. We subsequently searched for the full texts of the eligible articles.
Risk of bias (quality) assessment
To assess how thoroughly studies addressed potential bias in their design, conduct, and analysis, two independent reviewers (YS and EG) evaluated the methodological quality of the selected articles via Version 2 of the Cochrane risk-of-bias tool for RCTs (RoB 2) (25). Each article was assessed across the five domains of the RoB 2 tool: Domain 1 (risk of bias from randomization), Domain 2 (risk of bias from deviations in intended interventions), Domain 3 (missing outcome data), Domain 4 (risk of bias in outcome measurement), and Domain 5 (risk of bias in selection of reported results). The articles were rated as ‘Low’ or ‘High’ risk of bias or ‘Some concerns’ for each domain. The overall risk-of-bias judgment was defined as follows: a low risk of bias indicated that the study was assessed as having low risk in all domains for that result; some concerns indicated that at least one domain raised concerns, but the study was not at high risk in any domain; a high risk of bias indicated that the study had high risk in at least one domain or had concerns in multiple domains that substantially reduced confidence in the result. After the quality assessments were completed, the reviewers met to discuss and resolve any discrepancies. The findings from this evaluation were then used to guide the synthesis and interpretation of the study results.
Data extraction
A data extraction tool, Microsoft Excel, was used to extract data, including the characteristics of the study, characteristics of D-SMES interventions, and effect size data. Two reviewers (YSY and EGK) independently extracted the data to ensure data reliability and trustworthiness. When differences occurred, a conclusion was reached through consensus. We present the data extracted from the included studies in Additional File 2.
Assessment of heterogeneity
We assessed heterogeneity by reviewing the characteristics of the included studies. We also reviewed the forest plot of the included studies to determine whether the confidence intervals for the results of individual studies had poor overlap. In addition, Cochran’s Q test was used to determine whether there were differences between studies or if the variation observed was due to chance. A low P value of <0.10 in the Q test was considered to provide evidence of variation in effect estimates beyond chance. To determine what proportion of observed variance was real and the variance of true effect sizes, we used I2 statistics and the Tau square, respectively. To determine how much the true effect varies, we estimated the prediction interval. The true effect size is the effect we would see if we could enroll the entire population of the study. Furthermore, we explored the source of heterogeneity by conducting sensitivity analysis, subgroup analysis, and meta-regression.
Sensitivity analysis was conducted to ensure that the results were not overly influenced by any study. Subgroup analyses were conducted via mixed effects analysis (a random effects model was used to combine studies within each subgroup, and a fixed effect model was used to combine subgroups) to explore whether intervention characteristics such as setting, intervention modality, intervention content, intervention implementation strategy, application of behavior change theory, duration of intervention might explain some of the variation.
Meta-regression was conducted to explore the effects of multiple factors (characteristics of studies) simultaneously on the pooled effect estimate and to discuss the proportion of variance explained by each factor. The regression coefficient obtained from the meta-regression analysis was used to describe how the SMD changes with a unit increase in the explanatory variable.
Data synthesis
First, we described the characteristics of the included studies in terms of the different study and intervention characteristics and the risks of bias. A meta-analysis was subsequently conducted for the outcome (HbA1c) via Comprehensive Meta-analysis Software Version 3. The postintervention SMD (Hedges’ g) of HbA1c was pooled via random effects models. Cohen suggested that SMDs of 0.2, 0.5, and 0.8 are considered small, medium, and large effect sizes, respectively (26). We chose the random effects model for three reasons. First, it allowed us to take into account the study’s variance when assigning weights to each study. Second, it allowed us to assess the dispersion in effect size across studies (assess the study variance). Third, this model could allow us to generalize to comparable studies from the studies included in the analysis. Furthermore, the random effects model was intended to adjust for both explained and unexplained heterogeneity.
Assessment of publication bias
We tested the presence of small-study effects via one of the regression-based tests: the Egger test and the Begg rank correlation test and performed a trim-and-fill analysis. The main idea behind these tests was to determine whether there was a statistically significant association between the effect sizes and their measures of precision.
Results
Literature selection
Among the 3189 search results, 2824 records were marked as ineligible by automation tools (advanced search options, including age group, sex, place, article type, language etc.). A total of 365 articles were exported to EndNote, and 15 duplicate articles were excluded. Following review by title and abstract, 83 articles progressed to full-text review. Among these studies, 64 were excluded for not meeting the inclusion criteria, including 32 non-RCTs, 9 studies on type 1 diabetes and GDM, 21 studies with no reported outcomes, and 2 nonrandomized studies. The remaining 19 trials were included in this review. The detailed process is illustrated in Figure 1.
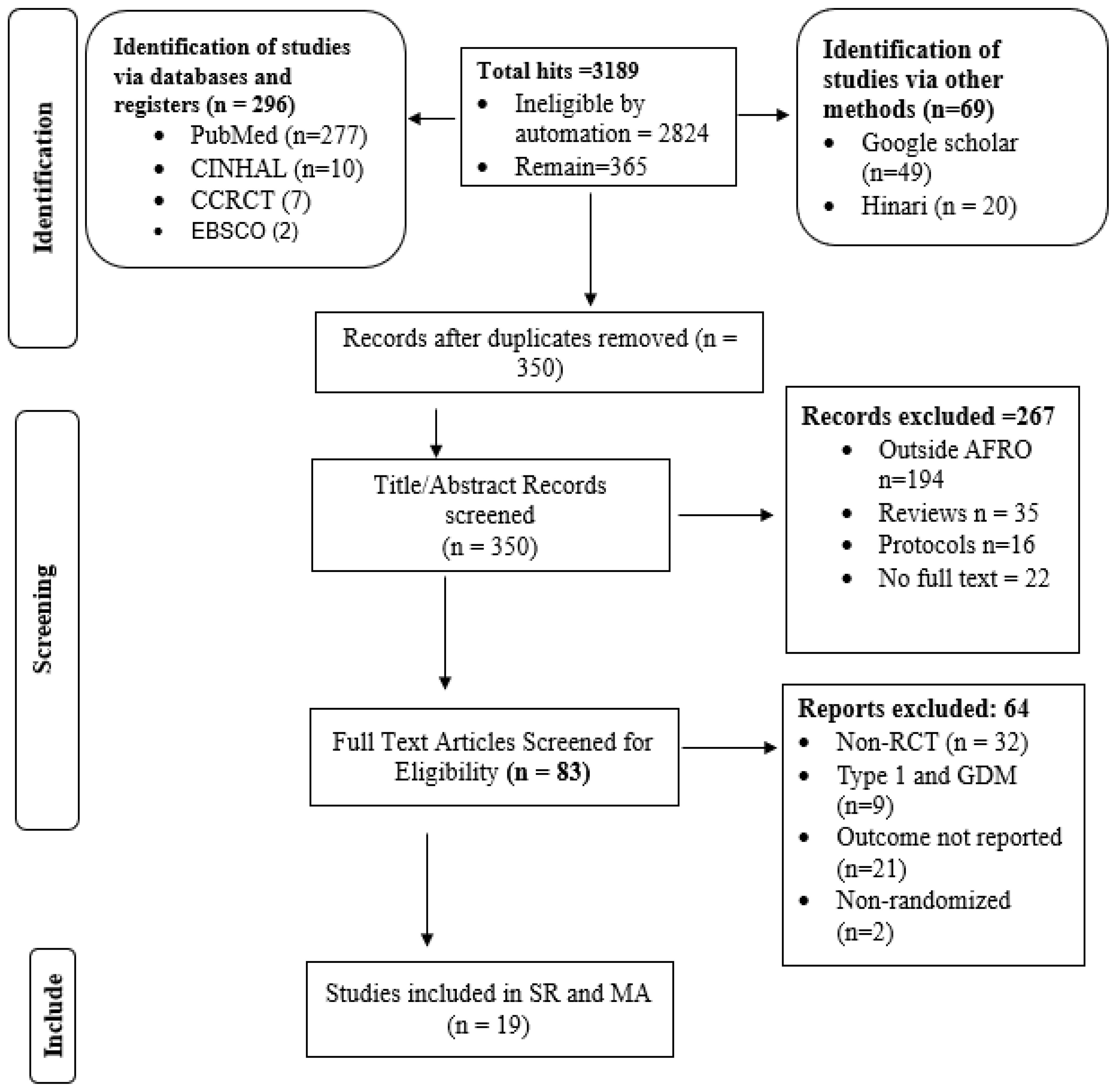
Figure 1. PRISMA 2020 study flow diagram for selecting studies to evaluate the effect of D-SMES interventions on HbA1c among people living with T2DM in the WHO African Region.
Study characteristics
In terms of where the interventions were delivered, ten of the 19 included trials were conducted at hospital outpatient settings (11, 13–15, 27–32), five were conducted at primary health care facilities (12, 17, 18, 33, 34), and the remaining four interventions were community-based (16, 35–37). Five of the studies were conducted in South Africa (12, 18, 28, 29, 37), three in Nigeria (14, 15, 30) and others in different countries of Africa. The total sample size was 3759 (1866 in the D-SMES group and 1893 in the control group), with individual study participants ranging from 41 (33) to 1022 (37). The average duration of follow-up in the included studies was 7.5 months, with a minimum of two months and a maximum of 12 months.
The most commonly investigated outcome measures were HbA1c (n = 18 studies), and only one study (14) reported average blood glucose (ABG) values. Table 2 provides a detailed account of all the study characteristics.
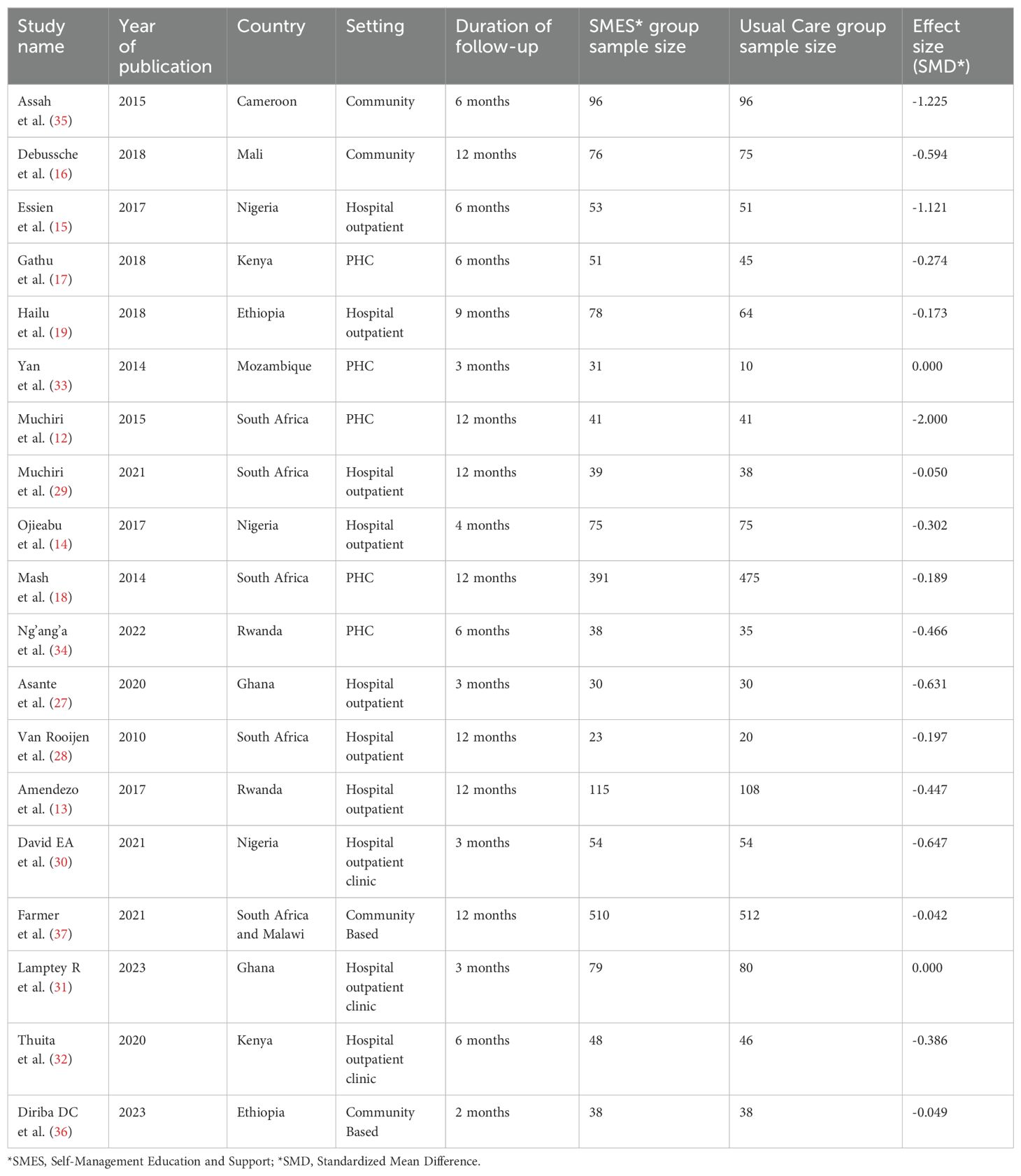
Table 2. Characteristics of the included studies evaluating the effect of D-SMES interventions on HbA1c among patients with T2DM in the WHO Africa Region.
D-SMES intervention characteristics
The majority of the included studies (n = 14) were group-based D-SMES interventions; the remaining five studies were individual-based. In five studies, interventions were delivered by health care providers (11, 13, 15, 31, 34), peer educators/supporters (n = 3) (16, 27, 35), dieticians (n = 2) (12, 29), research teams (n = 6) (28, 30, 32, 33, 36, 37), diabetes educators (n = 1) (17), pharmacists (n = 1) (14), and health promoters (n = 1) (18).
In terms of intervention content, the majority of the included studies (n = 15) focused on multiple components of the D-SMES intervention, including diabetes education and counselling, dietary intervention, physical exercise, and blood glucose monitoring. Two studies focused only on dietary interventions (12, 29), one study focused on physical exercise (33), and one study focused on self-management of blood glucose (34).
The majority of the included studies (n = 16) used multifaceted intervention strategies, including two or more of the following: education, counselling, goal setting, problem solving, experience sharing, reminders, follow-up and supervision, and educational and diagnostic material provision. The remaining three studies used a discrete type of implementation strategy, such as supervised exercise (33), mobile phone follow-up (27), and diabetes education (14). Seven of the 19 studies used theoretical models to bring about the desired behavior change, including social–cognitive theory (16, 29, 36), empowerment theory (17, 28), and motivational interviewing principles (18, 37). Additional file 3 provides an overview of D-SMES intervention characteristics.
Risk of bias (quality) assessment
Out of the 19 studies included, 15 were assessed as having a “low risk” of bias across all domains according to the Cochrane RoB 2 tool. Two studies were judged to have a “high risk” of bias (14, 35), while the remaining two raised “some concerns.” (18, 33). The randomization method was described adequately in 15 trials. A major source of bias identified across all trials was that the participants and implementation providers were not blinded. The detailed results of the quality assessment based on the Cochrane RoB 2 tool are presented in Additional File 4.
Effects of D-SMES interventions on blood glucose levels (HbA1c)
The mean effect size
The analysis is based on 19 studies. The effect size index is the standardized difference in means (Hedges’ g). On average, in populations that are comparable to those in the analysis, the intervention decreased HbA1c by approximately 0.468 standard deviations (the mean SMD is -0.468 with a 95% CI of -0.658 to -0.279), with a prediction interval ranging from -1.27 to 0.34. Figure 2 shows the effect of D-SMES interventions on HbA1c.
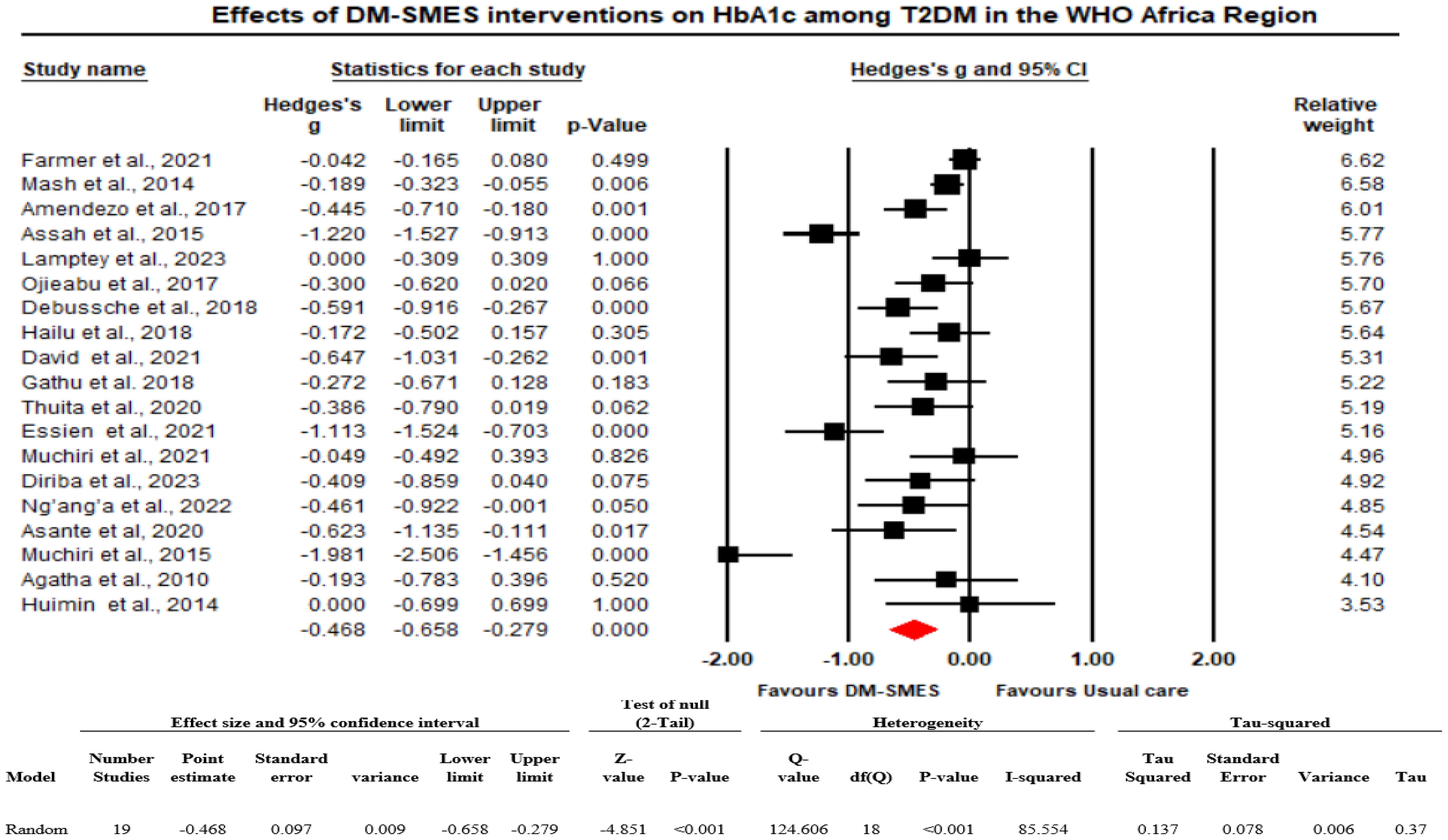
Figure 2. Effects of D-SMES interventions on HbA1c among people living with T2DM in the WHO Africa Region.
How much does the effect size vary across studies?
The Q statistic provides a test of the null hypothesis that all studies in the analysis shared a common effect size. The Q value was 124.606 with 18 degrees of freedom, and the p value was <0.001. Using a criterion of 0.10, we rejected the null hypothesis that the true effect size was the same in all those studies and concluded that the true effect size varies across studies. The I2 statistic (proportion of real variance) is 85.5%, which tells us that some 85.5% of the variance in observed effects reflects variance in true effects rather than sampling error. Tau squared (the variance of true effect sizes) is 0.137, and Tau, the standard deviation (SD) of true effect sizes, is 0.370. If we assume that the true effects are normally distributed, we can estimate that the prediction interval is -1.27 to 0.34. We would expect that in some 95% of all populations comparable to those in the analysis, the true effect size would fall between -1.27 and 0.34 (Figure 2).
Sensitivity analysis
To ensure that the results were not overly influenced by an outlier (i.e., how much of an impact each study has on the analysis). First, we checked how much weight was assigned to each study. Accordingly, the relative weight of each study was not more than 7%, and we also observed that almost every study had at least 3% weight, which shows that no one study dominated the analysis. Second, we sorted the studies by effect size and carried out the analysis with only one study removed. Accordingly, the mean effect size never moves to the right or to the left, and the p values are <0.001, which shows that the basic conclusion remains unchanged when any one study is removed.
Publication bias/small-study effect
To assess publication bias, we conducted both Egger’s regression test and Begg’s rank correlation test. Egger’s test indicated possible small-study effects (p = 0.024), while Begg’s test, a more conservative method, showed no evidence of bias (p = 0.29). Given this discrepancy, we performed a trim-and-fill analysis, which suggested no studies were missing (zero studies imputed), and the adjusted pooled effect size was identical to the original estimate. Therefore, we concluded that there is no substantial evidence of publication bias or small-study effects in our meta-analysis.
Source of heterogeneity
To identify factors associated with the size of the pooled effect for HbA1c and the amount of heterogeneity explained by some factors, we conducted subgroup analysis and meta-regression.
Subgroup analysis
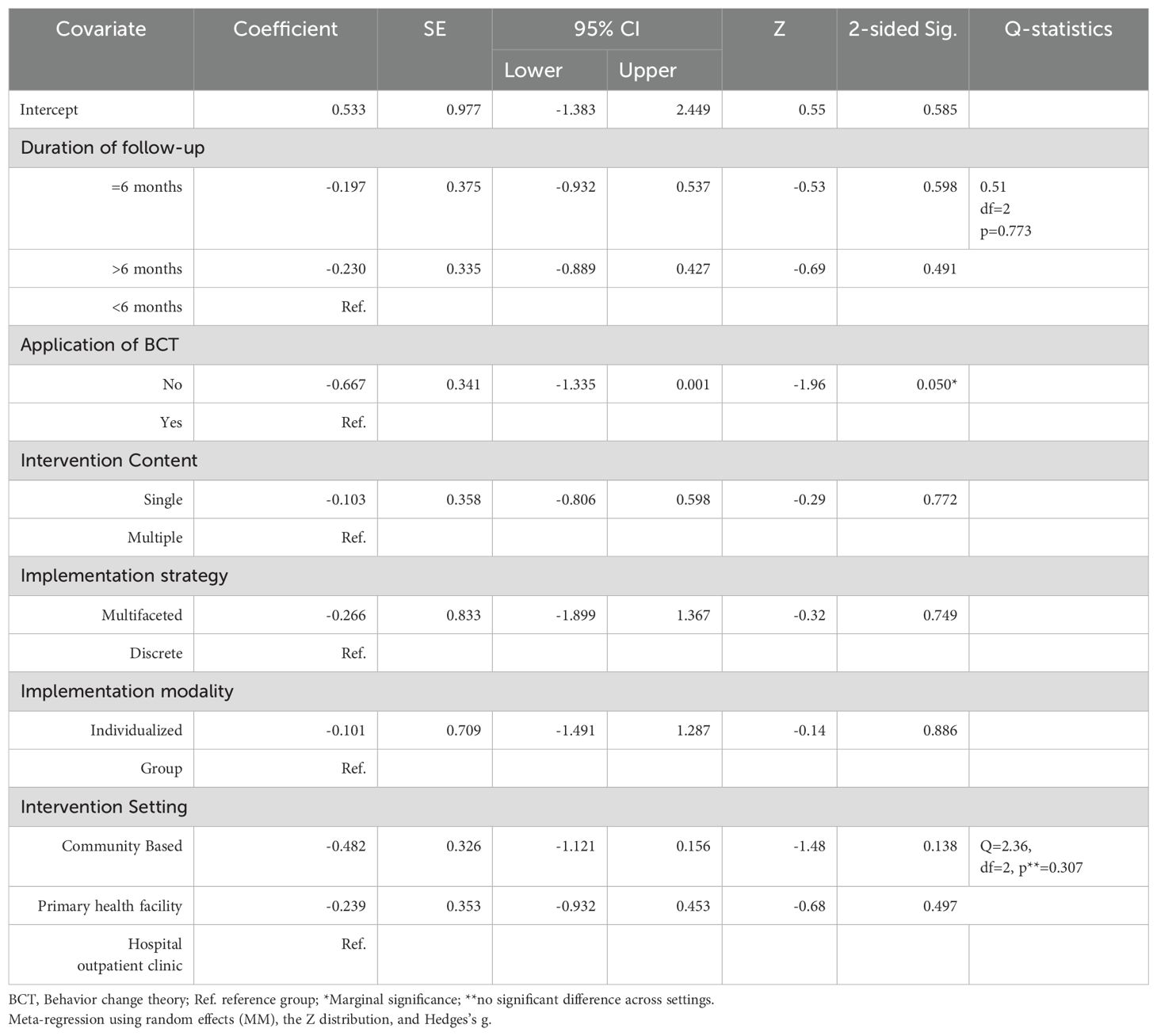
The mean effect size did not substantially vary in terms of setting, intervention modality, intervention content, strategy or duration of intervention. However, the application of behavior change theory resulted in a significant variation in the mean effect size (Q value of 5.568, df. 1, and p value 0.018) at the 0.05 level of significance. Table 3 shows the details of the subgroup analysis.
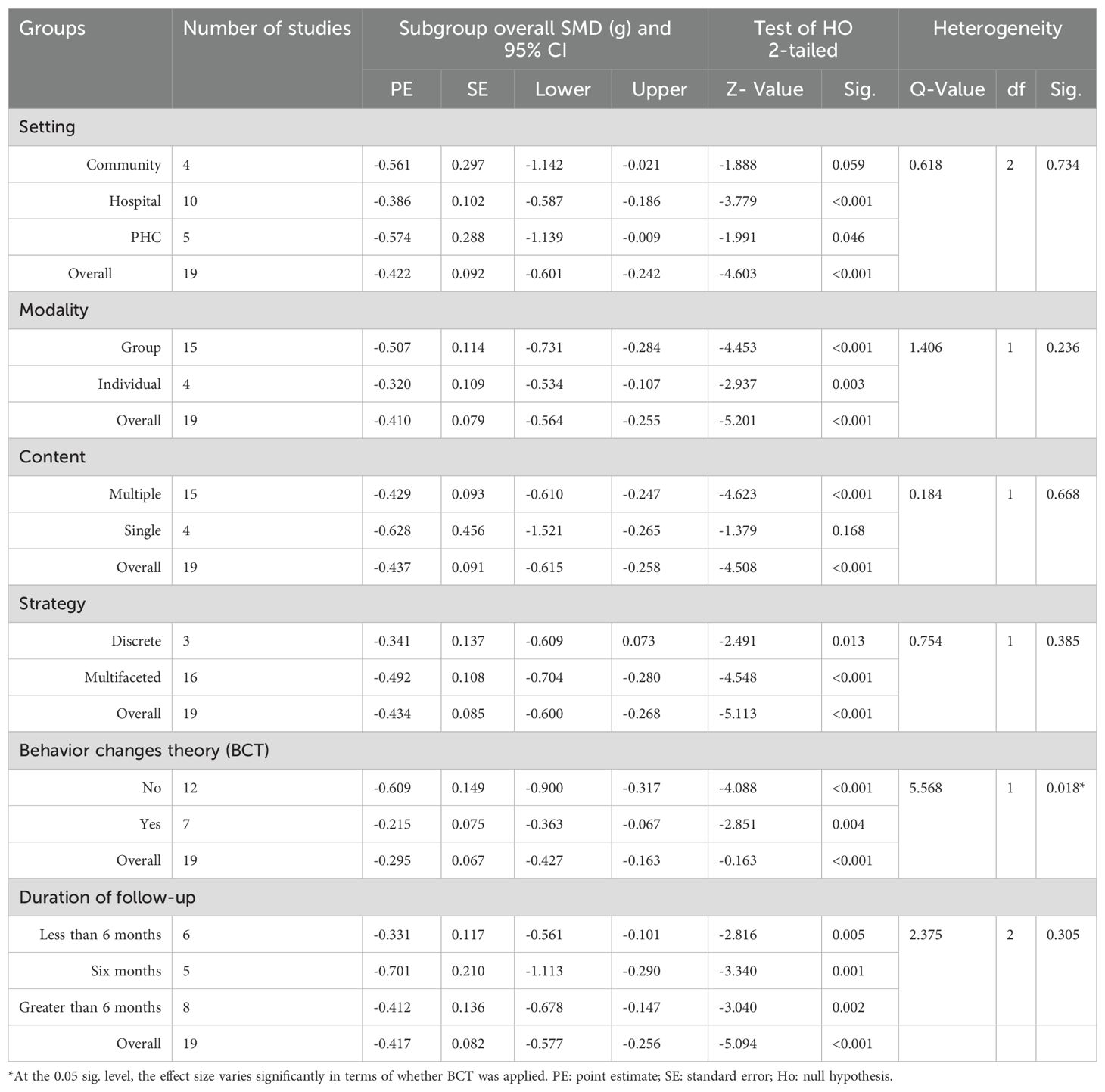
Table 3. Factors associated with the size of the pooled effect of D-SMES interventions on HbA1c, a subgroup analysis using a mixed effects model (analysis).
Meta-regression
Meta-regression was conducted to explore the effects of multiple factors (characteristics of studies) simultaneously on the pooled effect estimate and to discuss the proportion of variance explained by each factor. Adjusting for other covariates, only the application of behavior change theory showed a marginally significant association with the SMD in HbA1c (b= -0.667, 95% CI = -1.335 to 0.001, p=0.050). Accordingly, a better SMD for HbA1c was associated with D-SMES interventions not guided by BCT. Table 4 shows the details of the meta-regression analysis. The Q statistics for the test of the model (a simultaneous test in which all coefficients, excluding intercepts, are zero) revealed that the SMD for HbA1c is not significantly explained or predicted by these covariates (Q = 7.49, df = 8, p = 0.4843). The goodness-of-fit test (where the unexplained variance is zero) shows that 17.2% of the variance is explained by the model (Tau² = 0.1724, Tau = 0.4152, I² = 84.06%, Q = 62.75, df = 10, p = <0.001).
Discussion
For successful target glucose levels, diabetes requires a comprehensive management plan in which patients are educated and supported to make informed decisions about diet, exercise, weight control, blood glucose monitoring, taking medication, and regular screening for complications. Current evidence on the effects of diabetes self-management education and support (D-SMES) interventions on blood glucose levels is mixed, with some studies pointing to significant glycemic benefits (11–16), whereas others have shown no significant benefits (17–19). This systematic review and meta-analysis was conducted to evaluate the effects of D-SMES interventions compared with those of usual care in improving blood glucose levels among adult patients with T2DM in the WHO African Region and to describe the core components of D-SMES interventions.
This meta-analysis revealed a significant overall effect of D-SMES interventions on HbA1c among people living with type 2 diabetes in the WHO Africa Region (SMD = -0.468 with a 95% CI of -0.658 to -0.279). The improvement in glycemic levels is considered to be clinically meaningful, as suggested by Cohen (38). The improvement in glycemic levels reported in this study is consistent with the effects reported in a previous systematic review and meta-analysis of lifestyle interventions in LMICs (39). However, this finding contrasts with the nonsignificant and inconclusive effect on HbA1c observed in a systematic review and meta-analysis of diabetes self-management interventions in Africa, where the pooled effect on HbA1c was not provided (40). This discrepancy may be partly explained by the inclusion of both type 1 and type 2 diabetes cases in (40), as well as the inclusion of countries such as Egypt, which are outside the WHO African Region. Furthermore, only two studies in that review reported a significant improvement in HbA1c.
A greater effect of D-SMES interventions on HbA1c was reported in (41), and a much weaker effect was reported in (42) were empowerment was only a measuring instrument for D-SMES interventions. The difference may be due to the difference in the number of studies included and the difference in setting. Our study is restricted to only the WHO African Region, were others were worldwide and from high income countries.
This finding has a substantial degree of heterogeneity (I2 = 85.55%, Tau squared = 0.137), which tells us that some 85.5% of the variance reflects variance in true effects rather than sampling error. If we assume that the true effects are normally distributed, we would expect that in some 95% of all populations comparable to those in the analysis, the true effect size will fall in the range of -1.27 to 0.34 (prediction interval), which shows that in some populations, D-SMES intervention has a large clinical effect, whereas in others, the effect is small. The results were not overly influenced by any one study, since the basic conclusion remained unchanged, with any one study removed from the sensitivity analysis. No small study effect was shown in this meta-analysis; this may be due to the authors of randomized trials, who are likely to want to see RCTs published even if the result is negative because of the effort involved.
In the current meta-analysis, a subgroup analysis was conducted on the basis of the setting, intervention modality, intervention content, intervention strategy, application of behavior change theory, and duration of follow-up. Concerning the setting where the intervention was conducted, this meta-analysis showed a significant pooled estimate in all settings (community, hospital outpatient clinic, or primary healthcare facilities), but the SMD did not substantially vary across settings. This may be due to the small number of studies that were included in each setting. However, a review conducted in LMICs (39) showed that lifestyle interventions delivered by healthcare professionals in hospital or clinic settings were deemed most effective. Concerning the intervention modality, we found that the majority of the interventions were delivered in group settings. Group-based education has been found to be significantly more effective than individualized educational interventions. However, the variation was not significant between the group based and individual-based interventions. This is consistent with the finding that group-based education has become the preferred format for delivering self-management education (43). However, intervention modalities should be tailored to individual preferences and learning styles since people with diabetes have different learning needs (44).
With respect to the content of interventions, there was no substantial difference between interventions with multiple or single contents. One possible explanation could be that multiple behavioral interventions can be burdensome and complex for patients and that long-term interventions are needed to become habitual. Inconsistent findings have been reported (39), where those that included multiple education components (e.g., diet, physical activity, taking medication, smoking cessation) were deemed most effective.
With respect to the type of implementation strategy, multifaceted interventions were found to have a substantial effect on HbA1c compared with interventions with discrete implementation strategies. However, the overall pooled estimate does not vary based on the type of implementation strategy.
Interventions designed to influence diabetes self-management behavior are more likely to be beneficial when they are grounded in theories. However, the current meta-analysis indicated that interventions grounded in theory had a nonsignificant effect on HbAlc compared with interventions not guided by behavior change theories. This may be related to only seven out of the 19 studies in this review that mentioned the name of behavioral change theories. This might also be related to the fact that other studies used a theoretical model but did not report that; it also raises the question about the usefulness of such models.
Even though the effect was not significant according to the duration of follow-up, average durations of interventions (six months) were more likely to have better effect on reduction of HbA1c levels. In contrast, other reviews (41, 45) have shown that short educational interventions (less than 6 months) are better than longer interventions. One possible explanation may be associated with the initial motivation of the participant to be empowered to obtain positive results in a short period of time. The duration of contact hours between the intervention provider and patient may have contributed to this difference. Another explanation is the difference in the quality (fidelity) of interventions. In addition, relapses in behavior are expected among some of the participants.
The strengths of this review include the use of a registered protocol and a comprehensive search strategy in multiple databases; only RCTs were included, and the methodological quality of the majority of the included studies was high. This study also has limitations. First, studies published in the English language were only considered for this systematic review. Second, significant heterogeneity was observed across studies. Third, the inclusion of only 19 studies in the review is an indication that the conclusions drawn are based on limited data. Despite these limitations, we believe that this SRMA provides useful information that may inform the implementation of D-SMES interventions in Africa and other developing countries.
Conclusion
Diabetes self-management education and support interventions are moderately effective in controlling blood glucose levels in T2DM patients within the WHO African Region. The majority of the interventions had statistically significant positive effects on HbA1c. Few studies on D-SMES have been conducted in the WHO African Region. Therefore, the need to scale up interventional studies on D-SMES in the region is of paramount importance. Moreover, the usefulness and appropriate use of behavior change theories should be investigated in D-SMES interventions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AAd: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing, Validation. EK: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – review & editing, Formal analysis, Visualization. AR: Conceptualization, Supervision, Validation, Writing – review & editing, Methodology, Resources. AAb: Conceptualization, Supervision, Validation, Writing – review & editing, Methodology, Resources. AAh: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank all the authors of the primary studies included in this systematic review and meta-analysis and the study participants who were willing to support their research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2025.1554524/full#supplementary-material
Additional File 1 | PRISMA 2020 Checklist.
Additional File 2 | Data extracted from the included studies.
Additional file 3 | Characteristics of D-SMES interventions of the included studies.
Additional File 4 | RoB 2 quality assessment of the included studies.
Abbreviations
ABG, average blood glucose; BCT, behavior change theory; CCRCT, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature; df, degrees of freedom; D-SMES, Diabetes Self-Management Education and Support; FMoH, Federal Ministry of Health; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus; HbA1c, glycated hemoglobin; IDF, International Diabetes Federation; NCDs, noncommunicable disease; PHC, primary health care; PICOs, population intervention comparator study design; PMID, PubMed ID; PRISMA, practical, systematic reviews in self-management support; RCT, randomized controlled trial; RoB2, risk of bias; SMBG, self-monitoring of blood glucose; SMD, standardized mean difference; SRMA, systematic review and meta-analysis; SSA, Sub-Saharan Africa; T2DM, type 2 diabetes mellitus; UKPDS, UK Prospective Diabetes Study; WHO, World Health Organization.
References
1. International Dibetes Federation (IDF). Diabetes Atlas, 11th Edition. Brussels: International Diabetes Federation (IDF) (2025).
2. Ministry of Health-Ethiopia (MoH-E). National non-communicable diseases management protocols. Addis Ababa: Ministry of Health-Ethiopia (2021).
3. Group UPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. (9131) 1998:837–53:352. doi: 10.1016/S0140-6736(98)07019-6
4. World Health Organization (WHO). . Global action plan for the prevention and control of noncommunicable diseases 2013-2020. Geneva: World Health Organization (2013).
5. Pearce G, Parke HL, Pinnock H, Epiphaniou E, Bourne CL, Sheikh A, et al. The PRISMS taxonomy of self-management support: derivation of a novel taxonomy and initial testing of its utility. J Health Serv Res Policy. (2016) 21:73–82. doi: 10.1177/1355819615602725
6. Abraham AM, Sudhir PM, Philip M, and Bantwal G. Efficacy of a brief self-management intervention in type 2 diabetes mellitus: a randomized controlled trial from India. Indian J Psychol Med. (2020) 42:540–8. doi: 10.1177/0253717620932250
7. Chapman A, Browning CJ, Enticott JC, Yang H, Liu S, Zhang T, et al. Effect of a health coach intervention for the management of individuals with type 2 diabetes mellitus in China: a pragmatic cluster randomized controlled trial. Front Public Health. (2018) 6:252. doi: 10.3389/fpubh.2018.00252
8. Zheng F, Liu S, Liu Y, and Deng L. Effects of an outpatient diabetes self-management education on patients with type 2 diabetes in China: a randomized controlled trial. J Diabetes Res. (2019) 2019:1–7. doi: 10.1155/2019/1073131
9. Azami G, Soh KL, Sazlina SG, Salmiah M, Aazami S, Mozafari M, et al. Effect of a nurse-led diabetes self-management education program on glycosylated hemoglobin among adults with type 2 diabetes. J Diabetes Res. (2018) 2018:1–12. doi: 10.1155/2018/4930157
10. Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. (2011) 34:262–7. doi: 10.2337/dc10-1732
11. Hailu FB, Moen A, and Hjortdahl P. Diabetes self-management education (DSME)–Effect on knowledge, self-care behavior, and self-efficacy among type 2 diabetes patients in Ethiopia: A controlled clinical trial. Diabetes Metab Syndr Obes: Targets Ther. (2019) 12:2489. doi: 10.2147/DMSO.S223123
12. Muchiri JW, Gericke GJ, and Rheeder P. Effect of a nutrition education program on clinical status and dietary behaviors of adults with type 2 diabetes in a resource-limited setting in South Africa: a randomized controlled trial. Public Health Nutrit. (2016) 19:142–55. doi: 10.1017/S1368980015000956
13. Amendezo E, Timothy DW, Karamuka V, Robinson B, Kavabushi P, Ntirenganya C, et al. Effects of a lifestyle education program on glycemic control among patients with diabetes at Kigali University Hospital, Rwanda: a randomized controlled trial. Diabetes Res Clin Pract. (2017) 126:129–37. doi: 10.1016/j.diabres.2017.02.001
14. Ojieabu WA, Bello SI, and Arute JE. Evaluation of pharmacists’ educational and counselling impact on patients’ clinical outcomes in a diabetic setting. J Diabetol. (2017) 8:7. doi: 10.4103/jod.jod_5_17
15. Essien O, Otu A, Umoh V, Enang O, Hicks JP, and Walley J. Intensive patient education improves glycemic control in diabetes compared to conventional education: a randomized controlled trial in a Nigerian tertiary care hospital. PLoS One. (2017) 12:e0168835. doi: 10.1371/journal.pone.0168835
16. Debussche X, Besançon S, Balcou-Debussche M, Ferdynus C, Delisle H, Huiart L, et al. Structured peer-led diabetes self-management and support in a low-income country: The ST2EP randomized controlled trial in Mali. PLoS One. (2018) 13:e0191262. doi: 10.1371/journal.pone.0191262
17. Gathu CW, Shabani J, Kunyiha N, and Ratansi R. Effect of diabetes self-management education on glycemic control among type 2 diabetic patients at a family medicine clinic in Kenya: A randomized controlled trial. Afr J Prim Health Care Family Med. (2018) 10:1–9. doi: 10.4102/phcfm.v10i1.1762
18. Mash RJ, Rhode H, Zwarenstein M, Rollnick S, Lombard C, Steyn K, et al. Effectiveness of a group diabetes education program in under-served communities in South Africa: a pragmatic cluster randomized controlled trial. Diabet Med. (2014) 31:987–93. doi: 10.1111/dme.2014.31.issue-8
19. Hailu FB, Hjortdahl P, and Moen A. Nurse-led diabetes self-management education improves clinical parameters in Ethiopia. Front Public Health. (2018) 6:302. doi: 10.3389/fpubh.2018.00302
20. Dube L, Van den Broucke S, Housiaux M, Dhoore W, and Rendall-Mkosi K. Type 2 diabetes self-management education programs in high and low mortality developing countries: a systematic review. Diabetes Educ. (2015) 41:69–85. doi: 10.1177/0145721714558305
21. Stephani V, Opoku D, and Beran D. Self-management of diabetes in Sub-Saharan Africa: a systematic review. BMC Public Health. (2018) 18:1–11. doi: 10.1186/s12889-018-6050-0
22. Kumah E, Otchere G, Ankomah SE, Fusheini A, Kokuro C, Aduo-Adjei K, et al. Diabetes self-management education interventions in the WHO African Region: A scoping review. PLoS One. (2021) 16:e0256123. doi: 10.1371/journal.pone.0256123
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. System Rev. (2021) 10:1–11. doi: 10.1186/s13643-021-01626-4
24. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. (2008) 31:1473–8. doi: 10.2337/dc08-0545
25. Higgins JP, Savović J, Page MJ, and Sterne JA. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). RoB2 Development Group. London: BMJ Publishing Group (2019).
26. Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, New Jersey, United States: Lawrence Erlbaum Associates, Inc. (1988).
27. Asante E, Bam V, Diji AK-A, Lomotey AY, Owusu Boateng A, Sarfo-Kantanka O, et al. Pilot mobile phone intervention in promoting type 2 diabetes management in an urban area in Ghana: a randomized controlled trial. Diabetes Educ. (2020) 46:455–64. doi: 10.1177/0145721720954070
28. Van Rooijen AJ, Viviers CM, and Becker PJ. A daily physical activity and diet intervention for individuals with type 2 diabetes mellitus: a randomized controlled trial. S Afr J Physiother. (2010) 66:9–16. doi: 10.4102/sajp.v66i2.62
29. Muchiri JW, Gericke GJ, and Rheeder P. Effectiveness of an adapted diabetes nutrition education program on clinical status, dietary behaviors and behavior mediators in adults with type 2 diabetes: a randomized controlled trial. J Diabetes Metab Disord. (2021) 20:293–306. doi: 10.1007/s40200-021-00744-z
30. David EA, Soremekun RO, Abah IO, and Aderemi-Williams RI. Impact of pharmacist-led care on glycemic control of patients with uncontrolled type 2 diabetes: a randomized controlled trial in Nigeria. Pharm Pract (Granada). (2021) 19. doi: 10.18549/pharmpract.2021.3.2402
31. Lamptey R, Amoakoh-Coleman M, Barker MM, Iddi S, Hadjiconstantinou M, Davies M, et al. Change in glycemic control with structured diabetes self-management education in urban low-resource settings: multicenter randomized trial of effectiveness. BMC Health Serv Res. (2023) 23:199. doi: 10.1186/s12913-023-09188-y
32. Thuita AW, Kiage BN, Onyango AN, and Makokha AO. Effect of a nutrition education program on the metabolic syndrome in type 2 diabetes mellitus patients at a level 5 Hospital in Kenya:”a randomized controlled trial. BMC Nutrit. (2020) 6:1–14. doi: 10.1186/s40795-020-00355-6
33. Yan H, Prista A, Ranadive SM, Damasceno A, Caupers P, Kanaley JA, et al. Effect of aerobic training on glucose control and blood pressure in T2DDM East African males. Int Scholar Res Notices. (2014) 2014:864897. doi: 10.1155/2014/864897
34. Ng’ang’a L, Ngoga G, Dusabeyezu S, Hedt-Gauthier BL, Harerimana E, Niyonsenga SP, et al. Feasibility and effectiveness of self-monitoring of blood glucose among insulin-dependent patients with type 2 diabetes: open randomized control trial in three rural districts in Rwanda. BMC Endocr Disord. (2022) 22:1–9. doi: 10.1186/s12902-022-01162-9
35. Assah F, Atanga E, Enoru S, Sobngwi E, and Mbanya J. Community-based peer support significantly improves metabolic control in people with type 2 diabetes in Yaoundé, Cameroon. Diabet Med. (2015) 32:886–9. doi: 10.1111/dme.2015.32.issue-7
36. Diriba DC, Suen LK, and Leung DY. Effects of a culturally tailored, family-supported, community-based self-management education and support program on clinical outcomes among adults with type 2 diabetes in Western Ethiopia: A pilot randomized controlled trial. Diabet Med. (2023) 40:e15094. doi: 10.1111/dme.15094
37. Farmer A, Bobrow K, Leon N, Williams N, Phiri E, Namadingo H, et al. Digital messaging to support control for type 2 diabetes (StAR2D): a multicenter randomized controlled trial. BMC Public Health. (2021) 21:1–14. doi: 10.1186/s12889-021-11874-7
38. Cohen J. Statistical Power Analysis for the Behavioral Sciences. (2nd ed.) New York: Routledge (2013).
39. O’Donoghue G, O’Sullivan C, Corridan I, Daly J, Finn R, Melvin K, et al. Lifestyle interventions to improve glycemic control in adults with type 2 diabetes living in low-and-middle income countries: A systematic review and meta-analysis of randomized controlled trials (RCTs). Int J Environ Res Public Health. (2021) 18:6273. doi: 10.3390/ijerph18126273
40. Diriba DC, Leung DY, and Suen LK. The effects of diabetes self-management interventions on physiological outcomes in people living with diabetes in Africa: a systematic review and meta-analysis. Diabet Med. (2021) 38:e14501. doi: 10.1111/dme.14501
41. Shiferaw WS, Akalu TY, Desta M, Kassie AM, Petrucka PM, and Aynalem YA. Effect of educational interventions on knowledge of the disease and glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. BMJ Open. (2021) 11:e049806. doi: 10.1136/bmjopen-2021-049806
42. Chen Y, Tian Y, Sun X, Wang B, and Huang X. Effectiveness of empowerment-based intervention on HbA1c and self-efficacy among cases with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicine. (2021) 100. doi: 10.1097/MD.0000000000027353
43. World Health Organization (WHO). Package of essential noncommunicable (PEN) disease interventions for primary health care. Geneva: World Health Organization (2020).
44. Chatterjee S, Davies MJ, Heller S, Speight J, Snoek FJ, and Khunti K. Diabetes structured self-management education programs: a narrative review and current innovations. Lancet Diabetes Endocrinol. (2018) 6:130–42. doi: 10.1016/S2213-8587(17)30239-5
45. Caro-Bautista J, Kaknani-Uttumchandani S, García-Mayor S, Villa-Estrada F, Morilla-Herrera JC, León-Campos Á, et al. Impact of self-care programs in type 2 diabetes mellitus population in primary health care: Systematic review and meta-analysis. J Clin Nurs. (2020) 29:1457–76. doi: 10.1111/jocn.15186
Keywords: type 2 diabetes, diabetes self-management education and support, WHO Africa Region, systematic review, meta-analysis
Citation: Yimer YS, Addissie A, Kidane EG, Reja A, Abdela AA and Ahmed AA (2025) Effectiveness of diabetes self-management education and support interventions on glycemic levels among people living with type 2 diabetes in the WHO African Region: a Systematic Review and meta-analysis. Front. Clin. Diabetes Healthc. 6:1554524. doi: 10.3389/fcdhc.2025.1554524
Received: 02 January 2025; Accepted: 08 May 2025;
Published: 03 June 2025.
Edited by:
Andreas Schmitt, Diabetes Zentrum Mergentheim, GermanyReviewed by:
Mohammad Mobashir, Norwegian University of Science and Technology (NTNU), NorwayNeha Saboo, RUHS College of Medical Sciences, India
Copyright © 2025 Yimer, Addissie, Kidane, Reja, Abdela and Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimer Seid Yimer, eWltZXIwNTA1QGdtYWlsLmNvbQ==; eWltZXIuc2VpZEBhYXUuZWR1LmV0
†ORCID: Yimer Seid Yimer, orcid.org/0000-0001-7824-4361
 Yimer Seid Yimer
Yimer Seid Yimer Adamu Addissie
Adamu Addissie Eshetu Girma Kidane
Eshetu Girma Kidane Ahmed Reja
Ahmed Reja Abdurezak Ahmed Abdela3
Abdurezak Ahmed Abdela3