- 1Georgetown University School of Medicine, Washington, DC, United States
- 2Precision Vaccines Program, Department of Pediatrics, Boston Children’s Hospital, Boston, MA, United States
- 3Harvard Medical School, Boston, MA, United States
Type 1 diabetes (T1D) is not only a disorder of insulin production from beta cell destruction, but also a progressive condition that brings about life-threatening complications such as diabetic nephropathy, impaired wound recovery, and cardiovascular disease. Mesenchymal stromal cell (MSC) use has recently become an encouraging new way to treat these complications and can result in better health outcomes for T1D patients. Some research has shown that MSC injections into mice and rat models have resulted in reduced mesangial cell thickening, inflammatory mediator recruitment, proteinuria, and fibrosis normally seen in diabetic nephropathy. Other studies have demonstrated that MSCs aid wound healing by increasing anti-inflammatory M2 macrophage differentiation, stimulating angiogenesis and collagen synthesis, and signaling the proliferation and migration of dermal fibroblasts toward injury sites. Additionally, there is evidence that MSCs are capable of activating the PI3K pathway and exhibiting antioxidant effects in murine models experiencing diabetic-related heart disease. However, given these efforts, further research is needed to establish the prolonged safety and efficacy of MSC use in humans to treat T1D.
1 Introduction
Type 1 Diabetes (T1D), a chronic disease affecting an estimated 2 million Americans, is characterized by the autoimmune destruction of the body’s pancreatic beta cells (1). This results in a loss of endogenous insulin production, leading to insulin deficiency and dysregulation of blood glucose levels with potentially fatal consequences (2). Current treatments for T1D include replacement therapy of insulin in the form of injections or a pump, regular blood glucose monitoring, dietary control, and exercise regimens aimed at preventing both acute and chronic health complications (3). Despite these treatment options, complications such as cardiovascular disease, nephropathy, and poorly healing cutaneous wounds, amongst others, can still occur with inadequate management (4–7).
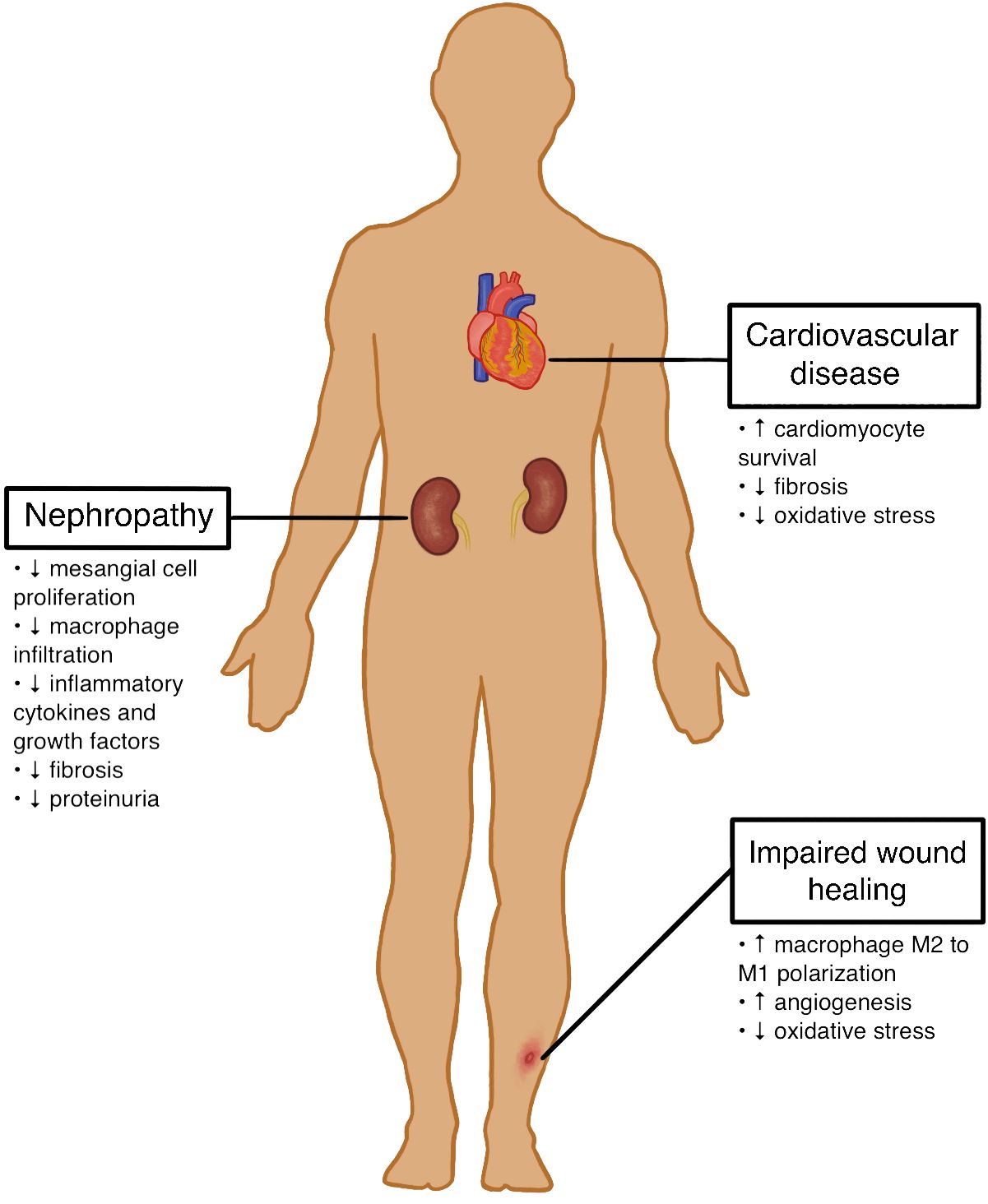
Given the persistent life-threatening complications in patients with T1D, developing alternative therapies is an important next step toward improving diabetic care. Mesenchymal stromal cells (MSCs) have recently become a promising novel treatment for complications related to T1D. MSCs are defined as multipotent cells with the ability to differentiate into a variety of mesenchymal cell lineages such as adipocytes, osteoblasts, and chondroblasts in vitro (8). Human MSCs are capable of differentiating into insulin-producing cells, protecting engineered pancreatic islet beta cells from hypoxia, and promoting the regeneration of pancreatic islet beta cells (9–13). These characteristics highlight the potential of these cells in regenerative medicine, particularly for treating various T1D complications such as cardiovascular disease, nephropathy, and impaired wound healing. The potential effects of MSC therapy on these T1D complications are outlined in Figures 1 and 2.
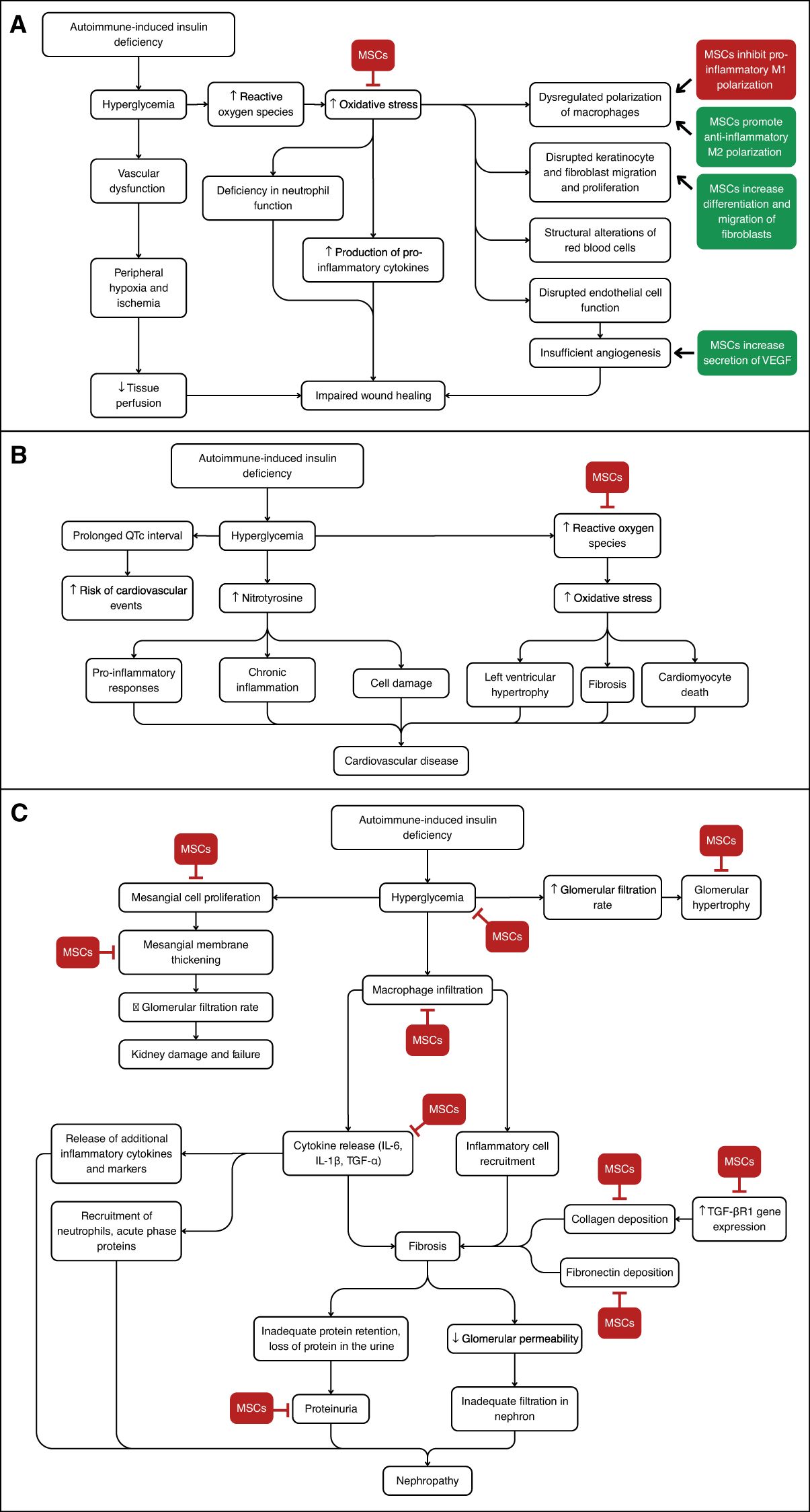
Figure 2. Overview of the Signaling Pathways Discussed and Key Points Where MSC Therapy May Intervene. (A) Development of diabetic impaired wound healing. MSC therapy can help decrease oxidative stress and inhibit macrophage M1 polarization, while increasing macrophage M2 polarization and fibroblast differentiation. (B) Development of diabetic cardiovascular disease. MSC therapy may decrease oxidative stress. (C) Development of diabetic nephropathy. MSC therapy can inhibit several steps including mesangial cell proliferation, collagen deposition, and inflammatory cytokine release, among others.
The use of MSCs presents an important opportunity for the future of T1D treatments. Although further research is needed to determine the long-term safety and efficacy of MSC therapies, this review aims to analyze the current evidence and provide insights for future research and clinical applications. We will delve into the effects of MSCs on the T1D complications of diabetic nephropathy, impaired wound healing, and cardiovascular disease. We will also address important challenges surrounding MSC use to treat T1D in humans and why, despite these challenges, MSC therapy is still a worthwhile treatment avenue to further explore.
1.1 MSC use in diabetic nephropathy
T1D can lead to hemodynamic and metabolic dysregulation, which can progress to chronic renal failure. MSC therapy has been a promising avenue in several pre-clinical in vivo and in vitro studies to repair nephron damage and other profound effects of diabetic nephropathy (4–7). Table 1 shows results from MSC therapy in diabetic mice and rat models.
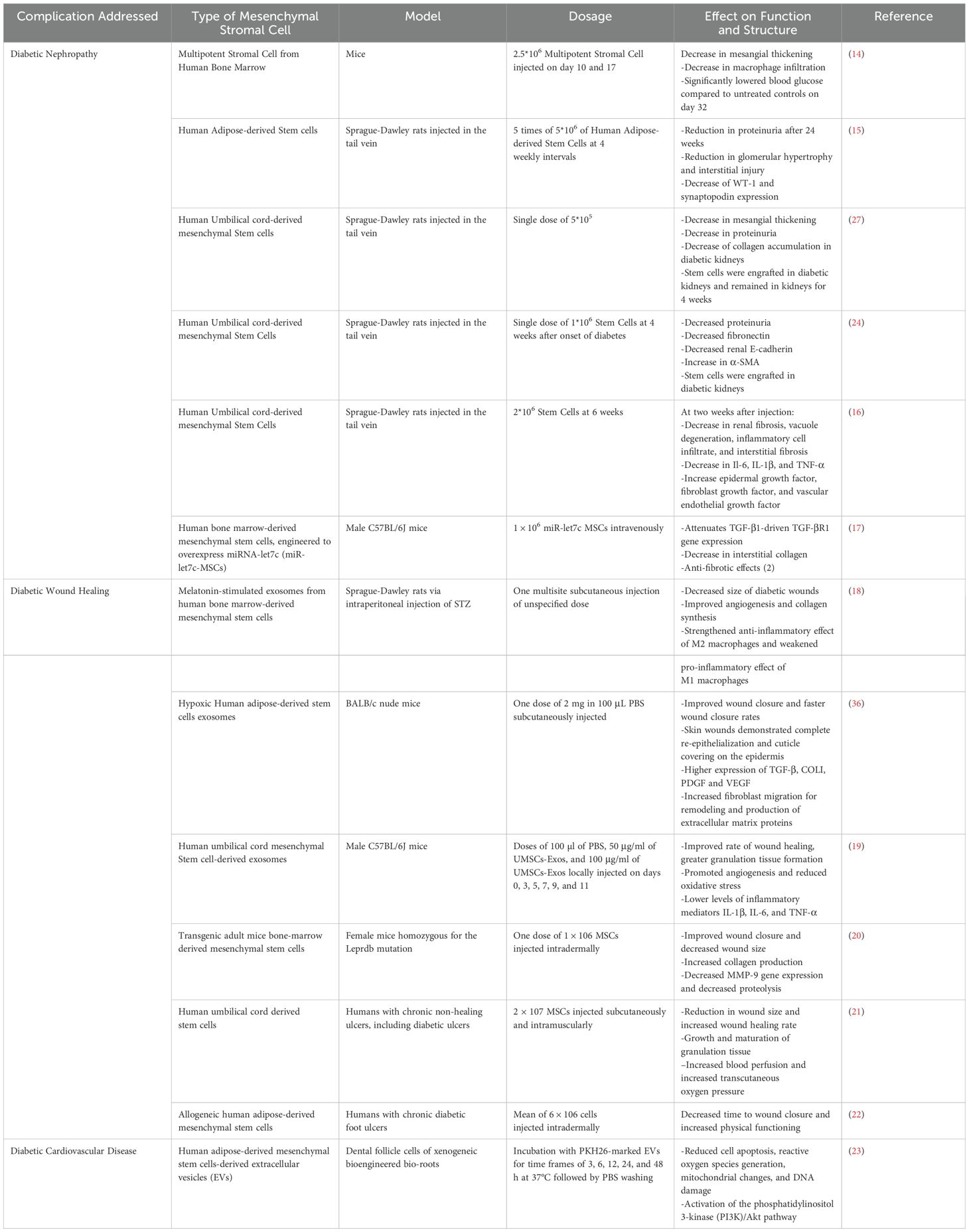
Table 1. Mesenchymal stromal cell therapy in mouse and rat models demonstrating the effect on diabetic nephropathy, diabetic wound healing, and cardiovascular disease.
One such complication of diabetic nephropathy is mesangial cell proliferation caused by hyperglycemia. Mesangial cells are involved in several renal functions including phagocytosis and cell-to-cell signaling, and the proliferation of these cells has been a contributing factor to chronic renal failure in T1D. MSC therapy addresses these renal complications in mice and rat models with significant reductions in mesangial cell proliferation. For instance, one study showed that multipotent stromal cells from human bone marrow in mice led to significantly lowered blood glucose after 32 days compared to untreated controls. Simultaneously, there was also the appearance of human cells that differentiated into glomeruli and a decrease in mesangial thickening (14). Similarly, when human umbilical cord blood-derived mesenchymal were engrafted in the kidneys of Sprague-Dawley rats, mesangial membrane thickening was reduced).
MSC therapy may not only attenuate mesangial cell proliferation, but it may also decrease inflammation from inflammatory cells and signaling molecules that exacerbate nephron damage. MSCs derived from human bone marrow injected into mice showed significantly lower macrophage infiltration. Since macrophages typically release cytokines that recruit inflammatory cells to the nephron, which may increase fibrosis and scarring, lower macrophage infiltration can consequently mitigate the damage caused by inflammation of the kidneys (14). Human umbilical cord-derived MSCs decreased levels of interleukin (IL)-6, IL-1β, and transforming growth factor (TGF)-α (16). IL-6, IL-1β, and TGF-α are pro-inflammatory cytokines involved in both acute and chronic inflammation and are actors that recruit inflammatory cells and mediators such as neutrophils, acute phase proteins, and release of other inflammatory cytokines and markers (16). Thus, a reduction in these cytokines may prevent the damaging effects of inflammation of the nephron in diabetic nephropathy by decreasing the recruitment of mediators that promote hyper-inflammation.
The degree of renal fibrosis also contributes to the progression of chronic renal failure as a greater degree of fibrosis decreases the permeability of the glomerulus, resulting in inadequate filtration from the nephron. In examining the effects of MSCs on fibrosis, delayed administration of human umbilical cord blood-derived MSCs attenuated the progression of kidney injury by decreasing deposition of collagen, a structural extracellular matrix protein, and fibronectin, a recruiter of extracellular matrix components (24). Likewise, when male C57BL/6J mice engineered to overexpress miRNA-let7c were injected with human bone marrow-derived MSCs, TGF-β1-driven TGF-βR1 gene expression, which significantly increased collagen expression, was attenuated (17). Subsequently, the deposition of extracellular matrix components contributes to fibrosis, and by attenuating the deposition of these components, MSCs may lead to anti-fibrotic effects, mitigating the filtration impairment mediated by T1D.
A marker for glomerular damage caused by diabetic nephropathy is proteinuria, as fibrosis and nephron damage lead to inadequate retention of proteins in the blood and into the urine. Human adipose-derived MSCs injected into Sprague-Dawley rats led to significantly decreased proteinuria after 24 weeks of injection, while reducing glomerular hypertrophy (15). Moreover, human umbilical cord-derived MSCs injected into Sprague-Dawley rats also led to marked reductions in proteinuria (24). Since proteinuria is a marker for kidney damage from fibrosis and glomerular damage, MSC injections may show promise in reversing the nephrogenic effects of fibrosis and glomerular damage in diabetic nephropathy (25, 26).
The injection of these MSCs was performed in animal models. Additional research is needed to show the translational effects in humans in a safe and efficacious manner. However, the injection of MSCs from different lineages shows promise in reducing mesangial cell thickening, inflammatory mediator recruitment, fibrosis, and proteinuria seen in diabetic nephropathy that contribute to chronic renal failure (14–17, 24–27).
1.2 MSC use in diabetic wound healing
For T1D patients, cutaneous diabetic wounds, such as diabetic foot ulcers, are serious complications that can arise from diabetes with neuropathic abnormalities or diabetes with peripheral artery disease of the lower extremities (28, 29). Wounds in diabetic patients can progress more rapidly due to certain risk factors including vascular dysfunction with peripheral hypoxia and ischemia, chronic inflammation, peripheral neuropathy, and hyperglycemia (30, 31). Barriers and dysfunction in the healing of diabetic wounds are commonly attributed to poor glycemic control and the resulting hyperglycemia in patients. Hyperglycemic states and their associated states of oxidative stress lead to dysregulated polarization and modulation of cells, such as macrophages, neutrophils, keratinocytes, and endothelial cells, that otherwise promote wound healing (31). Deficiencies in neutrophil function, keratinocyte and fibroblast migration and proliferation, continuous production of pro-inflammatory cytokines, and structural alterations to red blood cells all contribute to delays in wound healing (31). Currently, treatments for diabetic wounds include debridement supplemented with pharmacological therapy, using growth factors and skin substitutes, and applying negative pressure wound therapy (28, 29). However, the use of MSCs in treating diabetic wounds holds promise as it can improve nearly all stages of wound healing between inflammation regulation, angiogenesis, and tissue remodeling (32–34).
One of the benefits of MSCs in modulating inflammatory processes to improve diabetic wound healing involves their ability to polarize classically activated, pro-inflammatory macrophages known as M1 macrophages to M2 macrophages which are conditionally activated and possess anti-inflammatory properties. In diabetic rat models, melatonin-stimulated exosomes from bone-marrow derived MSCs increased the ratio of M2 to M1 polarization of macrophages through respective promotion and inhibition with increases in the M2 macrophages, leading to shortening of the inflammatory periods that hinder healing for diabetic wounds (18). M2 macrophage-driven decreases in inflammation additionally encourage improvements in subsequent wound healing stages by promoting angiogenesis as well as collagen synthesis involved in tissue remodeling (18). The anti-inflammatory mechanisms of MSCs allow for amelioration of diabetic wounds across several stages of healing and prevent further diabetic wound pathogenesis (18, 32–35).
MSCs have been reported to improve angiogenesis of diabetic wounds by reducing oxidative stress and eliciting secretion of associated growth factors. Hypoxic exosomes from adipose-derived MSCs transplanted into diabetic wound nude mouse models found that transplanted MSCs could accelerate high-quality healing of diabetic wounds by secreting higher levels of vascular endothelial growth factor (VEGF) (36). Higher levels of VEGF stimulated differentiation of progenitor cells into endothelial cells and extracellular matrix progenitors as well as angiogenesis through the PI3K/threonine kinase signaling pathway, overall increasing synthesis of capillaries, granulation tissues, and epithelium (36). MSCs promote environments conducive to angiogenesis by regulating oxidative stress damage occurring in diabetic cutaneous wounds (19). Administration of human umbilical cord MSCs in mouse models with diabetic cutaneous wounds found that exosomes derived from the administered cells accelerate diabetic cutaneous wound healing via amelioration of oxidative stress and enhancement of angiogenesis, counteracting the pathogenic effects of hyperglycemia on chronic wounds (19).
Further effects of MSCs in diabetic wound healing have been demonstrated with their capabilities in cell differentiation, cell signaling, and their regulation of key enzymes to improve tissue remodeling. In addition to studies showing the use of human bone-marrow derived MSCs in promoting angiogenesis and possessing the ability to differentiate in keratinocytes and endotheliocytes responsible for tissue remodeling, adipose MSCs transplanted into diabetic wound nude mice models signaled for early proliferation and dermal fibroblast migration towards remodeling (18, 19, 37). MSCs within in vivo murine diabetic wound healing models have been shown to correct dysregulation of matrix metalloproteinase-9 levels in impaired diabetic wound healing where dermal extracellular matrix protein profiles favor proteolysis and further pathogenesis (20).
Animal models further demonstrate how MSCs could behave synergistically to improve diabetic wounds, such as the action of MSCs on the PI3K/threonine kinase signaling pathway. In addition to MSC-derived exosomes promoting angiogenesis through this pathway, the PI3K/threonine kinase pathway is upregulated by MSCs through an upstream PTEN enzyme target to suppress inflammation and remediate diabetic wound environments (18, 36). By impacting multiple stages of wound healing and targeting upstream actors in cell proliferation, polarization, and differentiation, MSCs can arguably offer wider and better therapeutic potential compared to existing diabetic wound treatments.
1.3 MSC use in diabetic cardiovascular disease
Cardiovascular diseases remain the leading cause of death in individuals with diabetes (1). Extensive research has shown that acute hyperglycemic states, as seen in diabetic patients, lead to elevated levels of nitrotyrosine, a reactive nitrogen species indicative of cell damage and inflammatory responses (38). Chronic hyperglycemia can result in prolonged inflammation and exacerbate the pathogenesis of cardiovascular diseases. Patients with T1D experienced prolonged QTc intervals compared to healthy controls and therefore were at greater risk for cardiovascular events (39). Understanding the intricate molecular mechanisms governing this relationship is imperative in creating targeted therapeutics aimed at mitigating the cardiovascular complications associated with diabetes.
One pivotal pathway under investigation is the phosphatidylinositol-3-kinase (PI3K) pathway. Phosphatidylinositol-3-kinases are a family of G-protein coupled receptors involved in cell proliferation, glucose transport, and inflammation (40). Specifically, the PI3K pathway is linked to the activation of lymphocytes and is therefore implicated in many inflammatory and autoimmune diseases (41).
Given the link between PI3K, inflammation, and diabetes, there is growing curiosity about how PI3K modulation could offer potential therapeutic benefits in addressing inflammatory-mediated diabetic heart disease. It has been demonstrated that diminished expression of the P110α isoform of PI3K (PI3K(p110 α)) correlates with heightened oxidative damage and worsened development of diabetic cardiomyopathy in murine models of T1D (42). Additionally, inhibition of PI3K(p110 α) impedes insulin signaling in the liver, resulting in diminished glucose uptake, impaired glucose metabolism, and overall increased hyperglycemia (43). Mice with T1D treated with a single-dose of PI3K(p110 α) experienced lower levels of oxidative reactants (43). These studies not only suggest that a deficiency in PI3K(p110 α) contributes to the pathogenesis of diabetic-associated heart disease, but also that PI3K(p110 α) is a potential therapeutic target in treating diabetic cardiovascular complications.
In targeting the PI3K pathway, MSCs hold immense therapeutic potential. MSCs can activate the PI3K pathway and exhibit antioxidant effects (44). For a diabetic patient, this holds potential to not only prevent the exacerbation of cardiovascular disease but possibly exhibit cardioprotective effects as well. Using MSCs as a method for enhancing PI3K(p110α) activity within the diabetic heart presents a promising strategy to counteract the adverse remodeling and dysfunction characteristic of diabetic heart disease. Through targeted delivery, MSCs could enhance PI3K(p110α) signaling specifically within cardiac tissue, promoting cardiomyocyte survival, attenuating fibrosis, and mitigating oxidative stress.
2 Discussion
The use of MSCs for complications of T1D is a promising avenue for the future. Through preclinical studies in animal models, MSCs have been shown to reduce the presence of pro-inflammatory cytokines and alleviate the potential for fibrosis and mesangial cell thickening often associated with diabetic nephropathy. They have demonstrated promise in enhancing tissue remodeling and angiogenesis in diabetic wounds. MSCs could additionally protect against diabetes-induced cardiovascular diseases by potentiating the PI3K pathway.
MSCs can address multiple diabetic issues through similar pathways and actors, highlighting the potential for combinatory effects. Figure 2 demonstrates the pathways involved in the development of the various complications discussed here, and highlights where MSCs may modulate these pathways. In treating diabetic wounds, the effects of MSCs on the PI3K/threonine pathway are implicated in improving inflammatory stages and promoting angiogenesis. Similarly, in diabetic cardiac issues, the inflammatory pathway involving PI3K can be improved with MSC therapy. Likewise, the effect of MSCs in lowering inflammation by altering the activity of macrophages is seen in the prevention of nephron damage where MSCs lower macrophage infiltration, and in diabetic wound healing where MSCs polarize inflammatory M1 macrophages to anti-inflammatory M2 macrophages. Overall, MSC therapy could revolutionize the management of T1D complications through anti-inflammatory regulation, tissue regeneration, and pathway modulation.
While research on animal models has demonstrated benefits for diabetic wound treatment, clinical work has highlighted the practical therapeutic potential of MSCs while also raising concerns for adverse effects. Umbilical cord MSCs can increase and renew vasculature and improve the completion of diabetic wound healing through intravascular and intralesional injections (23). Similarly, it was demonstrated in a cohort of 59 patients that adipose-derived MSCs can quicken the rate of wound healing and the time it takes for a lesion to close (21). While this clinical work highlights the immense benefit of MSCs towards diabetic wounds, adverse clinical outcomes including febrile neutropenia, alopecia, gastrointestinal reactions, and relapse of diabetic injury in patients with T1D introduce controversy as to how and if MSC therapy for diabetic wound should be applied (22, 45). Investigating how MSCs can be combined gradually with existing wound treatment techniques and preventative measures against their anticipated adverse effects may provide safer pathways to applying MSC therapy without ignoring the transformative potential MSCs provide to diabetic individuals’ quality of life.
In the case of diabetic cardiovascular diseases, its application of the PI3K pathway in the context of T1D remains relatively unexplored. Most current research on PI3K modulation focuses on type 2 diabetes (T2D), where insulin resistance plays a central role in the pathophysiology of the disease. However, T1D, characterized by autoimmune destruction of pancreatic beta cells and insulin deficiency, presents distinct challenges and mechanisms. One complication of this discrepancy is that the potential therapeutic benefits of PI3K using MSCs may not be fully applicable to T1D. The underlying pathogenesis and disease mechanisms differ significantly between T1D and T2D, potentially affecting the efficacy of treatment strategies (1). Moreover, the limited knowledge and current research available on PI3K modulation of T1D further complicates efforts to translate findings from T2D to T1D.
The appeal of MSC therapy, however, lies in its potential advantages over conventional treatments for diabetes (46). Currently, insulin-based remedies remain the mainstay for management in T1D patients (1). If a patient presents with diabetic complications, additional symptom-based treatments are necessary as well. Over time, these therapies become financially demanding, resulting in many patients forgoing essential medications. While the initial investment in MSC research and therapy development may be high, the long-term benefits of an upfront MSC therapy could outweigh the expenses associated with ongoing treatments.
Moving forward, additional research is needed to bridge the gap between preclinical studies and clinical trials. While producing functioning beta cells in vitro and in animal models has been successful, similar success has been promising but rare in human patients (47). Table 2 offers a synopsis of active, completed, and terminated clinical trials investigating the use of MSCs in the treatment of T1D. While the number of ongoing studies and growing interest in this field is exciting, no clinical trial has yet reached Phase III or further, and none have demonstrated sustained effects in human participants so far. The relative lack of treatment success in humans to date highlights the need for further studies to better understand the discrepancy between the responses seen in animal models versus humans. Critical questions also remain unanswered regarding the long-term effects of MSC treatments, including the possibility of adverse immune reactions, tumorigenesis, and unintended consequences depending on individual physiology. Moreover, the variability in MSC sources, methods of isolation, and delivery techniques further emphasizes the need for standardized protocols and comprehensive safety assessments.
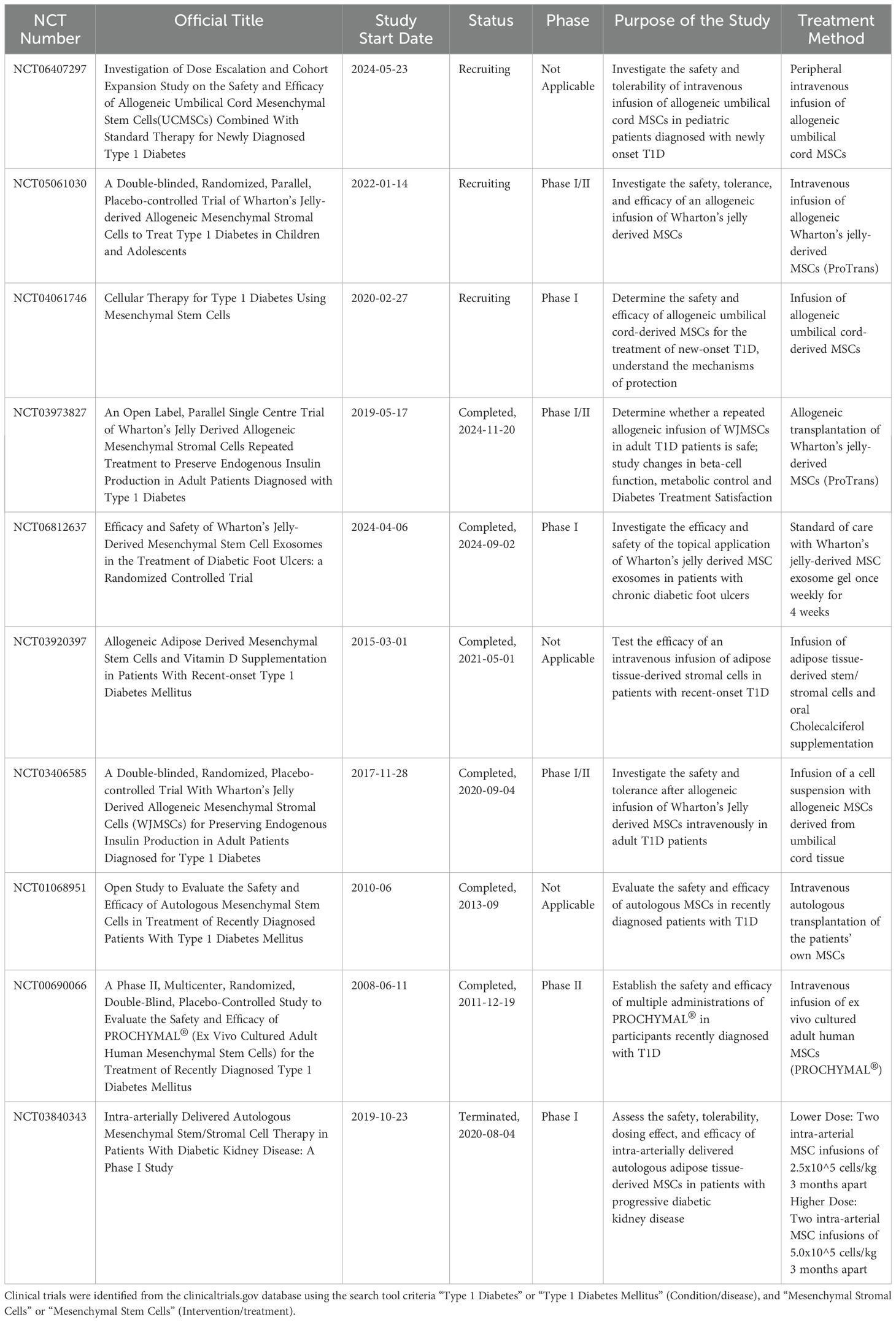
Table 2. Summary of active, completed, and terminated clinical trials investigating MSCs for the treatment of T1D.
Questions also remain regarding personalized treatment approaches, considering T1D may present in a variety of ways. Further research must also be done on the long-term implications of prolonged MSC therapy, and strategies to mitigate potential adverse reactions. Despite these remaining challenges, MSC therapy holds potential for improved patient outcomes through an upstream model for T1D complications that could enhance patient accessibility and treatment adherence. Currently, conventional insulin treatments require daily injections and lifelong monitoring. The less frequent use of MSC treatments could improve treatment adherence and relieve the mental fatigue that comes with daily treatments. Considering the success of MSC therapy in other medical domains such as cancer treatment, there is promising potential to advance MSC therapy for diabetes with ongoing research and innovation.
Author contributions
DB: Writing – original draft, Writing – review & editing. SB: Writing – original draft, Writing – review & editing. AN: Writing – original draft, Writing – review & editing. MY: Writing – original draft, Writing – review & editing. KS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the Georgetown University School of Medicine faculty and peers for their support of the conception, design, and writing of this paper. We also thank the Boston Children’s Hospital’s Precision Vaccine Program for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Diabetes Association. Statistics about diabetes. American Diabetes Association (2023). Available at: https://diabetes.org/about-diabetes/statistics/about-diabetes (Accessed April 17, 2024).
2. Brereton MF, Iberl M, Shimomura K, Zhang Q, Adriaenssens AE, Proks P, et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat. Commun. (2014) 5. doi: 10.1038/ncomms5639
3. Pathak V, Pathak NM, O’Neill CL, Guduric-Fuchs J, and Medina RJ. Therapies for type 1 diabetes: current scenario and future perspectives. Clin. Med. Insights Endocrinol. Diabetes. (2019) 12:1179551419844521. doi: 10.1177/1179551419844521
4. Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson AM, Miftaraj M, et al. Range of risk factor levels: control, mortality, and cardiovascular outcomes in type 1 diabetes mellitus. Circ. (2017) 135:1522–31. doi: 10.1161/CIRCULATIONAHA.116.025961
5. Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, and Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. (2015) 38:316–22. doi: 10.2337/dc14-0920
6. Bell S, Fletcher EH, Brady I, Looker HC, Levin D, Joss N, et al. End-stage renal disease and survival in people with diabetes: a national database linkage study. QJM. (2014) 108:127–34. doi: 10.1093/qjmed/hcu170
7. Rastogi A, Goyal G, Kesavan R, Bal A, Kumar H, Mangalanadanam, et al. Long term outcomes after incident diabetic foot ulcer: Multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study. Diabetes Res. Clin. Pract. (2020) 162:108113. doi: 10.1016/j.diabres.2020.108113
8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
9. Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, et al. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. (2007) 17:333–44. doi: 10.1038/cr.2007.28
10. Robert T, De Mesmaeker I, Stangé GM, Suenens KG, Ling Z, Kroon EJ, et al. Functional beta cell mass from device-encapsulated hESC-derived pancreatic endoderm achieving metabolic control. Stem Cell Rep. (2018) 10:739–50. doi: 10.1016/j.stemcr.2018.01.040
11. Wu LF, Wang NN, Liu YS, and Wei X. Differentiation of wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng Part A. (2009) 15:2865–73. doi: 10.1089/ten.TEA.2008.0579
12. Chandravanshi B and Bhonde RR. Shielding engineered islets with mesenchymal stem cells enhance survival under hypoxia. J. Cell. Biochem. (2017) 118:2672–83. doi: 10.1002/jcb.25885
13. Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, et al. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes. (2012) 61:1616–25. doi: 10.2337/db11-1141
14. Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. (2006) 103:17438–43. doi: 10.1073/pnas.0608249103
15. Zhang L, Li K, Liu X, Li D, Luo C, Fu B, et al. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. (2013) 22:3074–86. doi: 10.1089/scd.2013.0142
16. Xiang E, Han B, Zhang Q, Rao W, Wang Z, Chang C, et al. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res. Ther. (2020) 11:336. doi: 10.1186/s13287-020-01852-y
17. Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, et al. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. (2016) 24:1290–301. doi: 10.1038/mt.2016.90
18. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. (2020) 11:259. doi: 10.1186/s13287-020-01756-x
19. Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y, et al. Human umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing via ameliorating oxidative stress and promoting angiogenesis. Front. Bioeng Biotechnol. (2022) 10:829868. doi: 10.3389/fbioe.2022.829868
20. Xu J, Zgheib C, Hodges MM, Caskey RC, Hu J, and Liechty KW. Mesenchymal stem cells correct impaired diabetic wound healing by decreasing ECM proteolysis. Physiol. Genomics. (2017) 49:541–8. doi: 10.1152/physiolgenomics.00090.2016
21. Suzdaltseva Y, Zhidkih S, Kiselev SL, and Stupin V. Locally delivered umbilical cord mesenchymal stromal cells reduce chronic inflammation in long-term nonhealing wounds: A randomized study. Stem Cells Int. (2020) 2020:1–11. doi: 10.1155/2020/5308609
22. Uzun E, Güney A, Gönen ZB, Özkul Y, Kafadar İH, Günay M, et al. Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: Phase I/2 safety study. Foot Ankle Surg. (2021) 27:636–42. doi: 10.1016/j.fas.2020.08.002
23. Fu H, Sen L, Zhang F, Liu S, Wang M, Mi H, et al. Mesenchymal stem cells-derived extracellular vesicles protect against oxidative stress-induced xenogeneic biological root injury via adaptive regulation of the PI3K/Akt/NRF2 pathway. J. Nanobiotechnol. (2023) 21:466. doi: 10.1186/s12951-023-02214-5
24. Park JH, Park J, Hwang SH, Han H, and Ha H. Delayed treatment with human umbilical cord blood-derived stem cells attenuates diabetic renal injury. Transplant. Proc. (2012) 44:1123–6. doi: 10.1016/j.transproceed.2012.03.044
25. Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G, et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. (2019) 9. doi: 10.1038/s41598-019-41100-9
26. Hsiao PJ, Kao WY, Sung LC, Lin CY, Tsou LLA, Kao YH, et al. The role of mesenchymal stem cells in treating diabetic kidney disease: Immunomodulatory effects and kidney regeneration. Int. J. Med. Sci. (2025) 22:1720–35. doi: 10.7150/ijms.103806
27. Park JH, Hwang I, Hwang SH, Han H, and Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res. Clin. Pract. (2012) 98:465–73. doi: 10.1016/j.diabres.2012.09.034
28. El-Mesallamy HO, Hamdy NM, Ezzat OA, and Reda AM. Levels of soluble advanced glycation end product-receptors and other soluble serum markers as indicators of diabetic neuropathy in the foot. J. Investig. Med. (2011) 59:1233–8. doi: 10.2130/JIM.0b013e318231db64
29. Lin C, Tian J, Zhang Z, Zheng C, and Liu J. Risk factors associated with the recurrence of diabetic foot ulcers: A meta-analysis. PloS One. (2025) 20:e0318216–6. doi: 10.1371/journal.pone.0318216
30. Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. (1998) 47:457–63. doi: 10.2337/diabetes.47.3.457
31. Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, and D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. (2007) 170:1178–91. doi: 10.2353/ajpath.2007.060018
32. Chinnici CM, Amico G, Gallo A, Iannolo G, Cuscino N, Vella S, et al. Small extracellular vesicles from human fetal dermal cells and their microRNA cargo: KEGG signaling pathways associated with angiogenesis and wound healing. Stem Cells Int. (2020) 2020:1–18. doi: 10.1155/2020/8889379
33. Palumbo FS, Calligaris M, Calzà L, Fiorica C, Baldassarro VA, Carreca AP, et al. Topical application of a hyaluronic acid-based hydrogel integrated with secretome of human mesenchymal stromal cells for diabetic ulcer repair. Regen Ther. (2024) 26:520–32. doi: 10.1016/j.reth.2024.07.008
34. Wan J, Xia L, Liang W, Liu Y, and Cai Q. Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J. Diabetes Res. (2013) 2013:1–11. doi: 10.1155/2013/647107
35. Lee KB, Choi J, Cho SB, Chung JY, Moon ES, Kim NS, et al. Topical embryonic stem cells enhance wound healing in diabetic rats. J. Orthop. Res. (2011) 29:1554–62. doi: 10.1002/jor.21385
36. Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y, et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobiotechnol. (2021) 19:202. doi: 10.1186/s12951-021-00942-0
37. Wu Y, Chen L, Scott PG, and Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. (2007) 25:2648–59. doi: 10.1634/stemcells.2007-0226
38. Marfella R, Quagliaro L, Nappo F, Ceriello A, and Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J. Clin. Invest. (2001) 108:635–6. doi: 10.1172/JCI13727
39. Gordin D, Forsblom C, Rönnback M, and Groop PH. Acute hyperglycaemia disturbs cardiac repolarization in Type 1 diabetes. Diabet Med. (2008) 25:101–5. doi: 10.1111/j.1464-5491.2007.02322.x
40. Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, and Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. (1994) 14:4902–11. doi: 10.1128/mcb.14.7.4902-4911.1994
41. Winkler David G, Faia Kerrie L, DiNitto Jonathan P, Ali Janid A, White Kerry F, Brophy Erin E, et al. PI3K-δ and PI3K-γ Inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem. Biol. (2013) 21:1364–74. doi: 10.1016/j.chembiol.2013.09.017
42. Ritchie RH, Love JE, Huynh K, Bernardo BC, Henstridge DC, Kiriazis H, et al. Enhanced phosphoinositide 3-kinase(p110α) activity prevents diabetes-induced cardiomyopathy and superoxide generation in a mouse model of diabetes. Diabetologia. (2012) 55:3369–81. doi: 10.1007/s00125-012-2720-0
43. Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. (2018) 560:499–503. doi: 10.1038/s41586-018-0343-4
44. Prakoso D, De Blasio MJ, Qin C, Rosli S, Kiriazis H, Qian H, et al. Phosphoinositide 3-kinase (p110α) gene delivery limits diabetes-induced cardiac NADPH oxidase and cardiomyopathy in a mouse model with established diastolic dysfunction. Clin. Sci. (2017) 131:1345–60. doi: 10.1042/CS20170063
45. Madani S, Amanzadi M, Aghayan HR, Setudeh A, Rezaei N, Rouhifard M, et al. Investigating the safety and efficacy of hematopoietic and mesenchymal stem cell transplantation for treatment of T1DM: a systematic review and meta-analysis. Syst. Rev. (2022) 11. doi: 10.1186/s13643-022-01950-3
46. Gu B, Miao H, Zhang J, Hu J, Zhou W, Gu W, et al. Clinical benefits of autologous haematopoietic stem cell transplantation in type 1 diabetes patients. Diabetes Metab. (2018) 44:341–5. doi: 10.1016/j.diabet.2017.12.006
Keywords: type I diabetes, stem cells, diabetic nephropathy, diabetic wound healing, diabetic cardiovascular disease, mesenchymal stromal cells
Citation: Bejugam D, Bu S, Nguyen AN, Yaltaghian M and Smolen KK (2025) New frontiers in type I diabetes treatment: the impact of mesenchymal stromal cells on long-term complications. Front. Clin. Diabetes Healthc. 6:1586061. doi: 10.3389/fcdhc.2025.1586061
Received: 01 March 2025; Accepted: 28 April 2025;
Published: 19 May 2025.
Edited by:
Alessando Mattina, IRRCS ISMETT/UPMC Italy, ItalyReviewed by:
Federica Bellone, Department of Clinical and Experimental Medicine, ItalyBraulio Marfil-Garza, Instituto Tecnologico Y De Estudios Superiores De Monterrey, Mexico
Gioacchin Iannolo, Mediterranean Institute for Transplantation and Highly Specialized Therapies (ISMETT), Italy
Copyright © 2025 Bejugam, Bu, Nguyen, Yaltaghian and Smolen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Athena N. Nguyen, YW45ODRAZ2VvcmdldG93bi5lZHU=; Kinga K. Smolen, S2luZ2EuU21vbGVuQGNoaWxkcmVucy5oYXJ2YXJkLmVkdQ==
†These authors share first authorship
 Deeptha Bejugam
Deeptha Bejugam Sarah Bu
Sarah Bu Athena N. Nguyen
Athena N. Nguyen Mariam Yaltaghian
Mariam Yaltaghian Kinga K. Smolen
Kinga K. Smolen