- 1Department of Occupational Health, Gifu University Graduate School of Medicine, Gifu, Japan
- 2Department of Diabetes, Endocrinology and Metabolism Gifu University Graduate School of Medicine, Gifu, Japan
- 3Department of Rheumatology and Clinical Immunology, Gifu University Graduate School of Medicine, Gifu, Japan
- 4Innovative and Clinical Research Promotion Center, Gifu University Hospital, Gifu, Japan
- 5Department of Ophthalmology, Gifu University Graduate School of Medicine, Gifu, Japan
- 6Center for One Medicine Innovative Translational Research, Gifu University Institute of Advanced Studies, Gifu, Japan
Aims/introduction: Diabetic retinopathy (DR) often remains asymptomatic until it reaches advanced stages, when delayed treatment can lead to irreversible visual impairment. To promote timely ophthalmology visits, this study investigated the utility of a simple nerve conduction device, DPNCheck®, as a predictor of DR severity. Previous research has established a relationship between diabetic neuropathy (assessed by conventional nerve conduction studies) and DR progression; however, the specialized equipment and expertise required limit its practicality. In contrast, DPNCheck® is a simpler alternative that quantifies neuropathy severity through the severity of the estimated modified Baba classification (eMBC).
Materials and methods: Using electronic medical records (EHRs), we identified individuals with diabetes who underwent DPNCheck® and subsequent ophthalmologic assessment for DR. Based on age and sural nerve conduction data, an eMBC was calculated. Meanwhile, DR severity was scored using a modified Davis classification, defining four stages (DR severity scores 0–3).
Results: Of 181 individuals extracted from our hospital’s EHRs, 146 were eligible for analysis. Ordinal logistic regression showed that eMBC was significantly associated with DR stage, independent of diabetes duration and HbA1c. Receiver operating characteristic (ROC) curve analyses yielded eMBC cut-off values of 1.11, 1.51, and 1.51 to predict DR severity scores of ≥1, ≥2, and ≥3, respectively. Sensitivities ranged from 0.67 to 0.78, and specificities from 0.66 to 0.81. An eMBC of 1.51 or above was strongly associated with preproliferative or proliferative DR, indicating a need for urgent ophthalmology referral.
Conclusions: DPNCheck®, a simple nerve conduction measurement device, may help predict DR severity and facilitate timely ophthalmologic care.
Introduction
In 2021, approximately 537 million people worldwide were living with diabetes, and this number is predicted to rise to 783 million by 2045—a substantial global burden in terms of health outcomes, welfare, and healthcare costs (1). Poor glycemic control in diabetes can lead to complications such as diabetic retinopathy (DR), diabetic nephropathy (DN), and diabetic polyneuropathy(DPN), all of which severely affect patients’ quality of life (QOL). Of these complications, DR results from chronic hyperglycemia-induced microvascular and vitreous damage in the retina, causing a range of pathological changes. In the United States, DR is responsible for 5-14% of blindness (2). A meta-analysis involving 22,896 individuals with diabetes across 35 countries reported a global DR prevalence of 35.4% (3). Given the growing number of diabetes cases, there is concern about a parallel increase in DR incidence. In Japan, DR accounts for 10.2% of visual impairment cases (4). Among Japanese adults with type 2 diabetes, the reported annual DR incidence is 3.83%, and the progression rate is 2.11% (5). DR is also recognized as a risk factor for DN, DPN and macrovascular complications (6–8). Therefore, timely detection and accurate assessment of DR severity are vital not only for preserving vision but also for improving long-term survival.
Despite its potential severity, DR often remains asymptomatic until advanced stages. Individuals with diabetes may only notice visual impairment after macular edema, vitreous hemorrhage, or tractional retinal detachment develops, by which time urgent therapeutic interventions are required. Elevated HbA1c levels and a diabetes duration exceeding five years are known to increase the risk of DR (5). In Japan, people with diabetes are advised to undergo at least one fundus examination per year; more frequent exams are recommended if DR is already present, glycemic control is inadequate, or diabetes duration exceeds 10 years (9, 10). Despite these recommendations, only 40-50% of people with diabetes receive regular ophthalmologic care (11). Strengthening systems that encourage ophthalmology visits is thus critical for preventing DR progression.
According to the Japanese Clinical Practice Guideline for Diabetes 2024, individuals with mild DPN should undergo nerve conduction studies (NCS) every 6 months to a year; those with more advanced DPN require more frequent testing to assess progression of DPN more precisely (12). Based on this guideline, we hypothesized that if DR severity could be inferred from routinely monitored DPN severity, it might be possible to prioritize ophthalmology referrals for high-risk individuals. Indeed, studies have reported correlations between DR severity and DPN severity when assessed by the Baba classification (13, 14). The Baba classification is a widely utilized method in Japan for evaluating the severity of diabetic neuropathy, particularly distal symmetric polyneuropathy (12). This system relies on the results of nerve conduction studies (NCS) to provide an objective and highly reproducible assessment of neuropathy progression in individuals with diabetes, categorized into four stages. However, the Baba classification requires NCS, which depends on specialized equipment and trained healthcare professionals with technical expertise (12). As a result, its implementation can be challenging in medical facilities lacking the necessary resources, limiting its widespread adoption. Recently, the simpler and more accessible DPNCheck® device, used in the estimated modified Baba classification (eMBC), has shown good correlation with DPN severity (15). We reasoned that this finding could be leveraged to create a powerful referral tool for ophthalmology.
We further speculated that if DR progression could be anticipated using eMBC values derived from DPNCheck®, it might prompt more frequent and timely ophthalmology visits among individuals with diabetes who receive their routine diabetes care from general practitioners rather than diabetes specialists. This could facilitate earlier detection of DR and help prevent severe vision loss. Accordingly, this study aimed to validate a prediction score for DR staging using eMBC values derived from DPNCheck®.
Materials and methods
Study Design and Population. In this single-center retrospective observational study, we included individuals aged 16 years or older with diabetes who underwent DPNCheck® testing while attending our departments at Gifu University Hospital, and whose DR was evaluated by ophthalmologists using the modified Davis classification. The DR evaluation needed to occur within three months before or after DPNCheck® between January 1, 2019, and September 30, 2023.
We excluded those who did not have a DR evaluation within the specified window, those whose nerve conduction velocity (NCV) or amplitude could not be measured by DPNCheck®, those who with peripheral neuropathy other than DPN, and those who opted out of study participation. The Gifu University Graduate School of Medicine’s ethics review board approved this study (Approval no. 2021-A057), which was conducted in accordance with the principles of the Declaration of Helsinki, with opt-out informed consent.
Data Collection. We collected baseline characteristics (e.g., age, sex, body mass index [BMI], diabetes duration, smoking status), comorbidities, laboratory data, and medication history from Gifu University Hospital’s electronic medical records (EMRs) for analysis. Macrovascular complications were defined as a history of stroke, coronary artery disease, or peripheral vascular disease. Dyslipidemia and hypertension were defined by the presence of pertinent medication use or relevant physician diagnoses in the EMRs. Laboratory data (e.g., HbA1c, urinary albumin-to-creatinine ratio [ACR], 24-hour urinary albumin, and estimated glomerular filtration rate [eGFR]) obtained within one month before or after DPNCheck® were used. Data on resting CVR-R which was measured within three months before or after DPNCheck® were used. DPNCheck® was used to measure sural NCV and amplitude bilaterally, with the average of these measurements included in the analysis. Instead of the Baba classification, which relies on NCS requiring expensive equipment and specialized technicians, we utilized the eMBC. This alternative demonstrates a strong correlation with the Baba classification and can be easily calculated using sural nerve conduction data obtained via DPNCheck® as follows (15):
Statistical Analysis. Baseline characteristics were summarized as median (interquartile range) for continuous variables and frequencies for categorical variables. The Kruskal-Wallis’s test was used to compare continuous variables across RSS categories, and the chi-square test was used for categorical variables. An ordinal logistic regression model was employed to assess the association between eMBC and RSS, adjusting for potential confounders (diabetes duration and HbA1c). Subsequently, binary logistic regression models with the same covariates were run to investigate the relationship between eMBC and each binary outcome (RSS ≥1, RSS ≥2, and RSS ≥3). Receiver operating characteristics (ROC) curve was generated to assess predictive performance and to identify eMBC cut-off values for each DR severity category, using the Youden Index to determine the optimal cut-offs. Sensitivities and specificities were calculated for each. All statistical analyses were conducted using R version 4.4.1 (https://www.r-project.org/), with two-sided p-values <0.05 considered significant.
Results
Among 181 individuals with diabetes who underwent DPNCheck® and received a DR evaluation at Gifu University Hospital, 29 were excluded because the DR evaluation did not occur within the three-month window before or after DPNCheck®, 5 were excluded because NCV or amplitude could not be measured, and 1 was excluded because his diabetes diagnosis could not be confirmed from the EMR (Figure 1). Ultimately, 146 individuals with complete data were included in the analysis (Table 1). Their median age was 69 years; 95 were men, and 51 were women. The median BMI was 24.6 kg/m², and the median diabetes duration was 10 years. Macrovascular complications were present in 25.3% of the individuals analyzed, and approximately half had dyslipidemia or hypertension. The median HbA1c was 8.0%. The median NCV was 50.5 m/s, amplitude was 9.5 µV, and resting CVR-R was 2.3%. The median ACR was 11.3 mg/g, and the median eGFR was 72.2 mL/min/1.73m². Biguanides (43.2%) were the most used glucose-lowering agents, followed by DPP-4 inhibitors (35.6%), insulin (32.2%), and SGLT2 inhibitors (31.5%). Among the 6 patients who received anti-VEGF therapy, the underlying conditions were age-related macular degeneration in 1 patient (RSS 1 point), glaucoma in 1 patient (RSS 0 points), and diabetic macular edema in 4 patients (1 with RSS 2 points and 3 with RSS 3 points). Duration of diabetes, CVR-R, ACR, insulin use, GLP-1 receptor agonist use, NCV, amplitude and eMBC differed significantly across RSS groups.
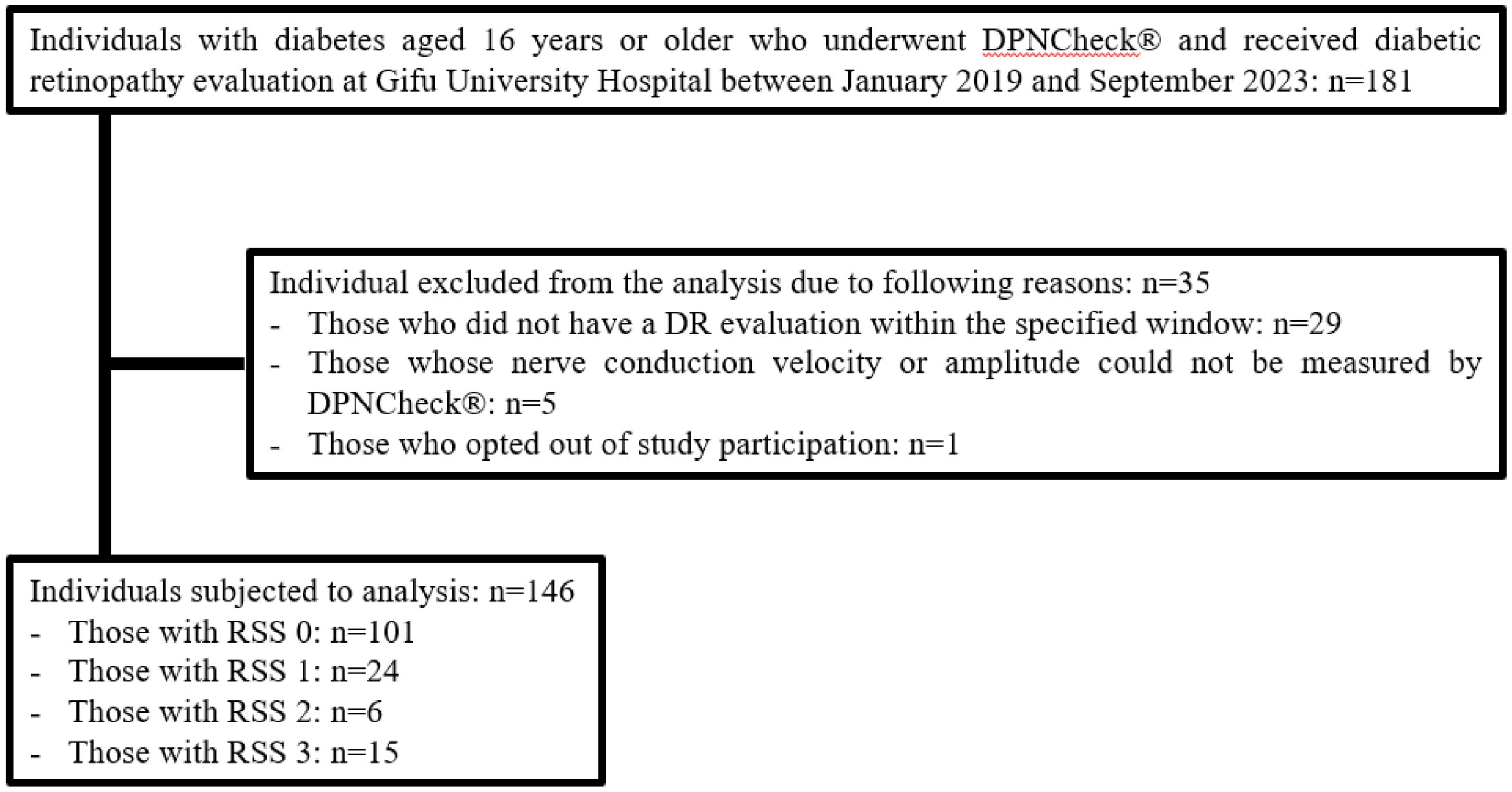
Figure 1. Flowchart of the participants and exclusions in this study. Among 181 individuals aged 16 and older with diabetes who underwent both DPNCheck® and eye examinations at Gifu University Hospital between January 2019 and September 2023, 35 were excluded based on exclusion criteria. The breakdown of excluded individuals is as follows: 29 individuals did not undergo retinopathy evaluation within 3 months before or after the DPNCheck®, 5 individuals could not be detected by the DPNCheck®, and 1 individual had an unknown duration of diabetes. The final analysis included 146 individuals, categorized as follows: 101 individuals with an RSS of 0 points, 24 with 1 point, 6 with 2 points, and 15 with 3 points.
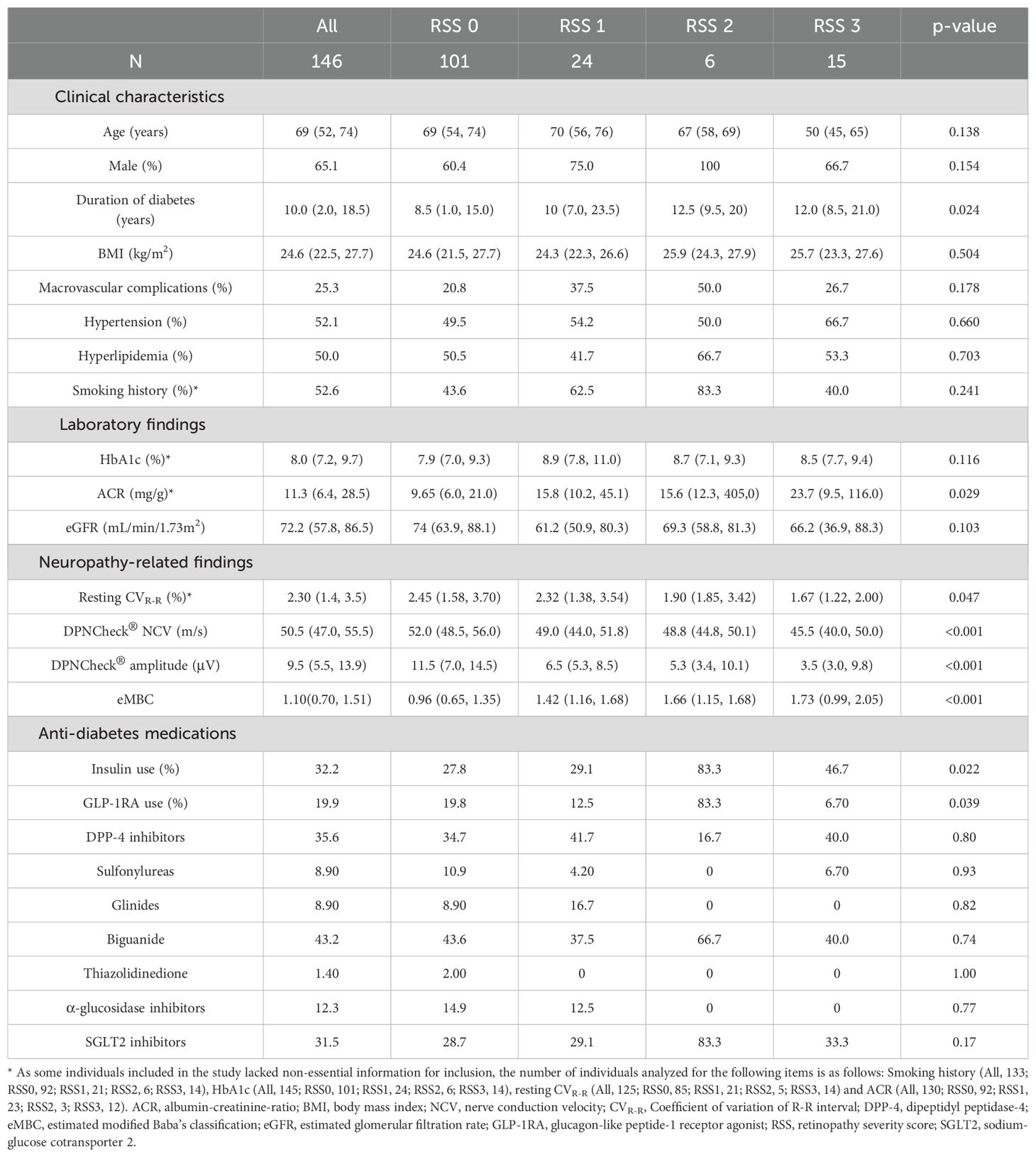
Table 1. Clinical characteristics, laboratory results, and neuropathy-related findings of individuals with diabetes included in the current study.
Ordinal logistic regression was performed to explore the relationship between eMBC and RSS, adjusting for diabetes duration and HbA1c, as initially hypothesized. Even after accounting for these factors, eMBC remained significantly associated with DR severity (Table 2).
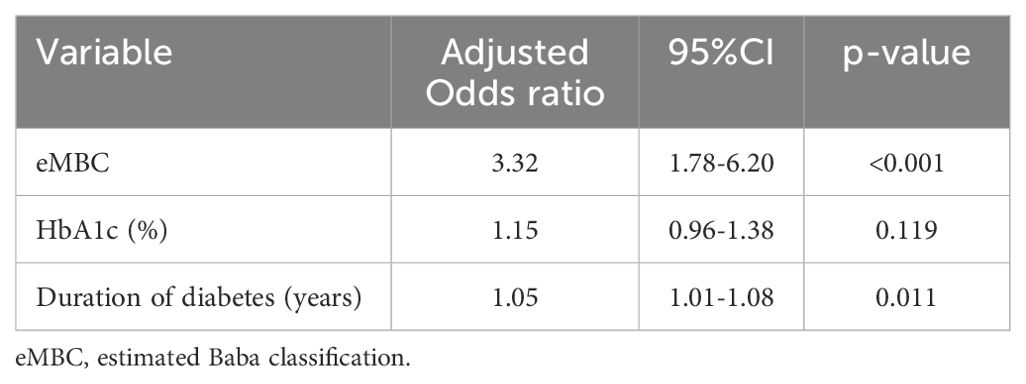
Table 2. Ordinal logistic regression analysis of the association between retinopathy severity scores and the estimated modified Baba classification.
Next, binary logistic regression models (adjusted for diabetes duration and HbA1c) were used to analyze whether eMBC was significantly associated with each binary outcome (RSS ≥1, ≥2, and ≥3) (Table 3). We then plotted ROC curves to estimate eMBC cut-off values for each DR severity category (Figure 2). For RSS ≥1, ≥2, and ≥3, the optimal eMBC cut-offs were 1.11, 1.51, and 1.51, respectively (with ROC-AUCs of 0.75, 0.72, 0.72). The sensitivity for these respective thresholds was 0.78, 0.67, and 0.67, while specificity was 0.66, 0.81, and 0.79.
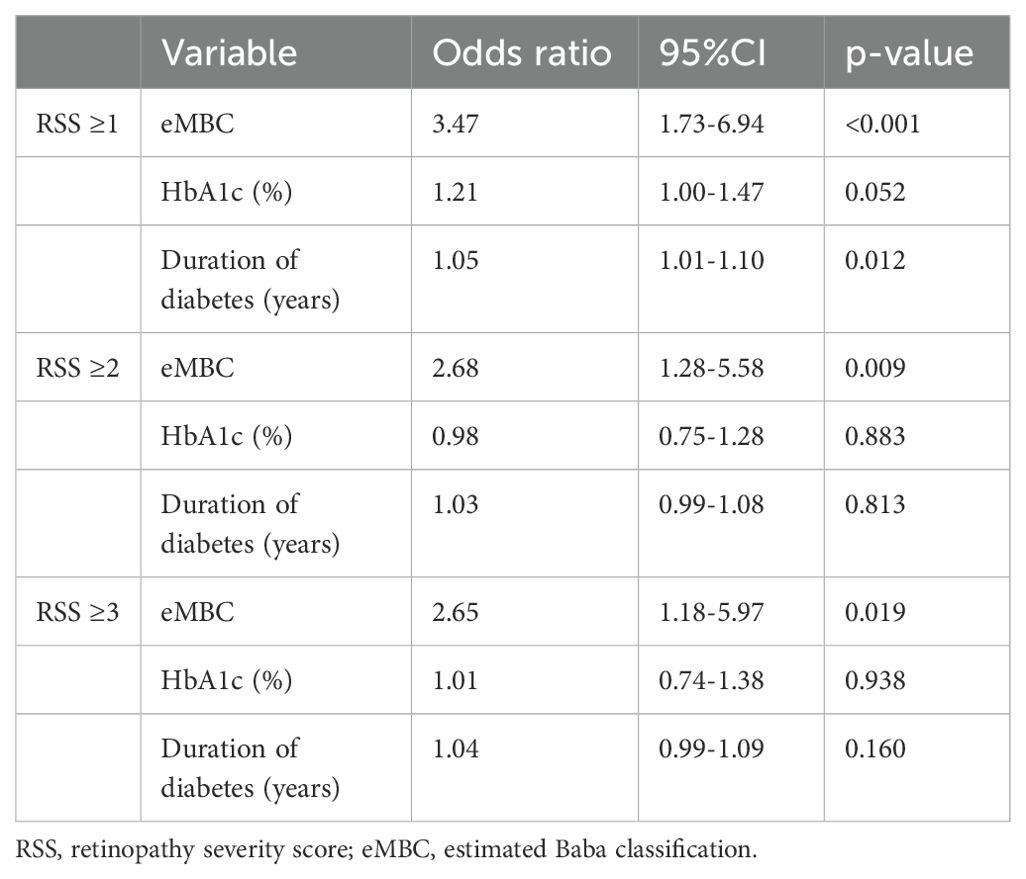
Table 3. Binary logistic regression analysis of the association between retinopathy severity scores and the estimated modified Baba classification.
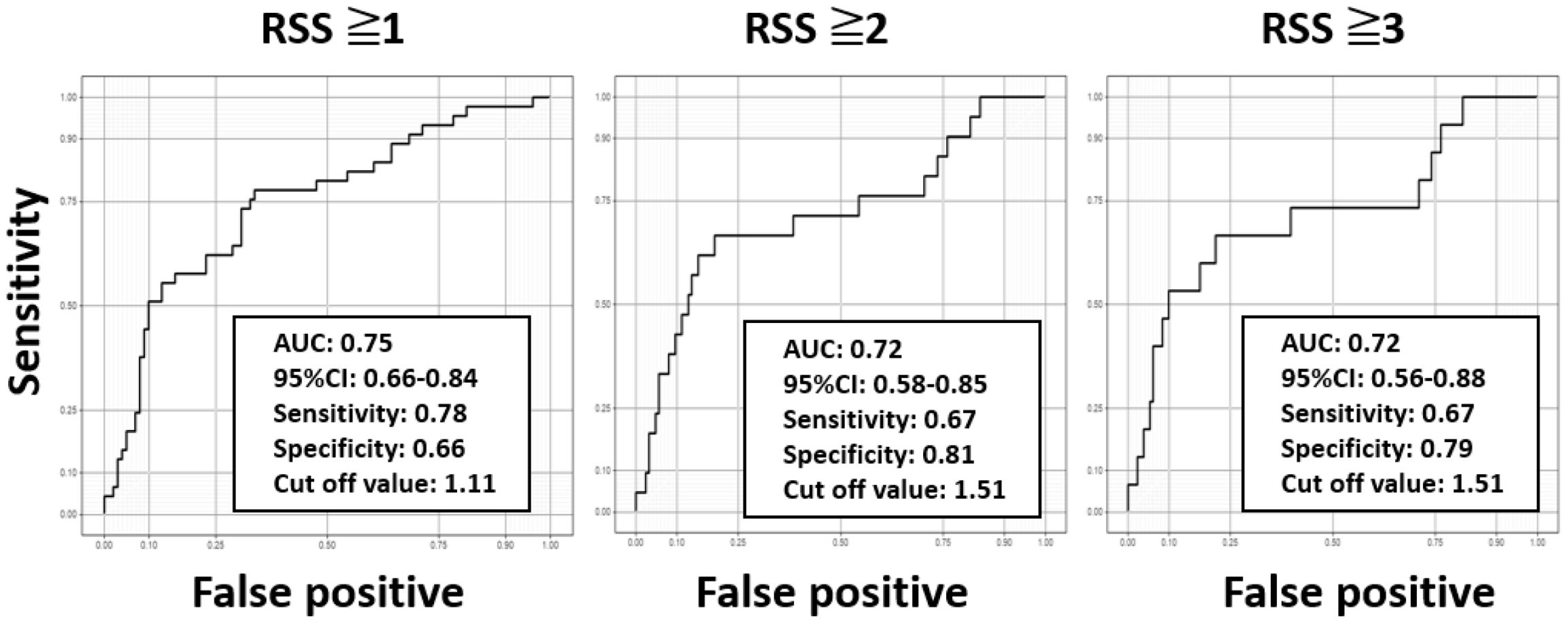
Figure 2. Receiver operating characteristic curves for determining cut-off values for predicting diabetic retinopathy stages using the estimated modified Baba classification with DPNCheck®. Using the receiver operating characteristic curves, the optimal estimated modified Baba classification (eMBC) cutoff values for retinopathy severity score (RSS) ≥1, ≥2, and ≥3 were calculated as 1.11, 1.51, and 1.51, respectively. The area under the curve (AUC), 95% confidence interval (CI), sensitivity, and specificity for each cutoff value are indicated in the graphs.
Discussion
Our findings indicate that eMBC—calculated from DPNCheck® measurements—can help predict DR severity. This aligns with earlier reports showing a correlation between DR severity and DN severity, as assessed by the Baba classification (13, 14). Although traditional risk factors for DR include longer diabetes duration, higher HbA1c, hypertension, and microalbuminuria (6, 16–19), our study highlighted significant differences in duration of diabetes, eMBC, CVR-R, and ACR across DR severity categories, whereas differences in HbA1c and hypertension were not significant. Large-scale clinical trials have found considerable variation in the onset and progression of DR, suggesting that factors beyond established risks (e.g., glycemic variability and genetic predisposition) may influence DR progression (16, 20, 21). Further research is needed to determine how these additional factors affect DR onset and progression. Notably, individuals with high eMBC values showed the progression of DR, regardless of HbA1c levels or the duration of diabetes. This suggests that high eMBC values may be an indicator of the progression of not only DPN due to chronic hyperglycemia, but also DR. Chronic hyperglycemia–induced cellular metabolic abnormalities are currently hypothesized to represent a shared pathophysiological mechanism underlying both peripheral nerve damage and retinal vascular pathology. These abnormalities include activation of the polyol pathway, increased activity of protein kinase C, enhanced flux through the hexosamine pathway, elevated oxidative stress, and upregulation of the advanced glycation end product (AGE) pathway (22). As a consequence, capillary basement membrane thickening and endothelial cell hyperplasia occur in both neural and vascular tissues. These histological changes contribute to the development of diabetic microvascular complications, such as axonal degeneration and demyelination in peripheral nerves, and DR in the retina (23). eMBC has been reported to be a useful indicator of diabetic polyneuropathy (15, 24, 25). In the present study, significant differences in eMBC and ACR were also observed across DR severity classifications, suggesting that eMBC may additionally serve as an indirect marker of microvascular complications such as retinopathy and nephropathy. Although the importance of regular ophthalmologic follow-up and prompt DR treatment is broadly recognized, many people with diabetes do not visit ophthalmologists regularly because of the need for pharmacological mydriasis and the associated time-consuming tests. Delayed DR treatment can lead to severe, sometimes irreversible, visual impairment. In Japan, discontinuation or failure to attend ophthalmic evaluations remains a key obstacle to effective DR management (26). Individuals with diabetes who receive care from non-diabetes specialists are particularly susceptible to missing or skipping ophthalmology visits due to less awareness of DR risks (26). Conversely, those who have lived with diabetes for a longer time may better understand DR risks and thus adhere more consistently to follow-up schedules. This underscores the need for targeted education on DR risks among those with shorter disease duration, including younger people with diabetes.
In this study, eMBC cut-off values for predicting DR severity were identified. An eMBC ≥1.11 signifies a heightened likelihood of simple DR or worse, indicating that such individuals should be strongly encouraged to seek ophthalmologic assessment. More crucially, an eMBC ≥1.51 points to a high risk of severe, vision-threatening DR, highlighting the need for timely treatments such as panretinal photocoagulation and/or intravitreal anti-VEGF therapy. Predicting DR severity based on DPNCheck® readings could serve as a powerful trigger for more urgent ophthalmologic consultations, particularly for individuals who otherwise might not prioritize regular ophthalmologic follow-up.
Innovations in DR detection technology are progressing rapidly; for instance, ultra-widefield scanning laser ophthalmoscopy, which does not require pharmacological mydriasis, can capture detailed images of peripheral retinal regions quickly (27). However, patients must visit an ophthalmology clinic to benefit from this technology. By contrast, DPNCheck® is relatively affordable, easy to administer by non-specialists, and well-suited for primary care settings. These characteristics make it an attractive tool for identifying individuals with diabetes who might be at higher risk of DR and in need of more specialized ophthalmic evaluation. Notably, the Japanese Clinical Practice Guideline for Diabetes 2024 recommends conducting NCS every 6 to 12 months (12); thus, regular use of DPNCheck® at similar intervals is strongly encouraged.
Our study has several limitations. This study has several limitations. First, as the study was conducted at a single center in Japan, the generalizability of the findings to other countries and ethnic groups is limited. Indeed, previous reports have indicated that DPNCheck® measurements may vary by ethnicity (28). Therefore, multicenter and international validation studies are needed to confirm and refine the cutoff values for use in non-Japanese populations. This study examined only the cross-sectional correlation between eMBC and RSS and did not assess the potential of eMBC to predict DR progression or clinical outcomes. We believe that a longitudinal study is warranted to evaluate whether changes in eMBC are associated with the progression of DR. Second, because DPNCheck® can deliver a maximum stimulus of 70 mA, individuals with foot edema or significantly thickened skin due to obesity may have undetectable amplitudes—potentially reflecting more advanced DPN and DR. Third, the cut-off values for RSS ≥2 and RSS ≥3 were both 1.51 in this study, reflecting the limited number of individuals in these more severe stages. Nevertheless, identifying RSS ≥2 remains clinically critical as it is a threshold for earlier ophthalmic intervention. Fourth, standard nerve conduction studies (NCS) were not performed concurrently with DPNCheck® measurements in this study, primarily due to limitations related to insurance coverage. As a result, we were unable to evaluate the correlation between NCS findings and DR severity. Future studies should include direct comparisons between NCS and DPNCheck® to further validate the utility of eMBC in this context. Fifth, in this study, only 6 out of 146 individuals received anti-VEGF therapy, which limited our ability to evaluate its effects on eMBC values. Regarding the impact of anti-VEGF agents on neural tissue, both neuroprotective effects (29) and potential adverse effects (30) have been reported, and a clear consensus has yet to be reached. Further longitudinal studies comparing DPNCheck® results before and after anti-VEGF therapy are warranted to clarify its influence on peripheral nerve function. A key strength of our investigation is that it showcases the potential of a straightforward, cost-effective system using DPNCheck® to approximate DR severity, presenting a practical strategy to address barriers to regular fundus examinations.
In conclusion, DPNCheck®, a simple nerve conduction measurement device, may help predict DR severity and facilitate timely ophthalmologic care. Integrating DPNCheck® into routine diabetes care could be instrumental in preventing the progression of DR, ultimately preserving vision and improving long-term outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Gifu University Graduate School of Medicine’s ethics review board . The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because the study used retrospectively collected data.
Author contributions
MS: Data curation, Writing – original draft, Writing – review & editing. TK: Data curation, Writing – original draft, Writing – review & editing. TaI: Formal Analysis, Writing – original draft, Writing – review & editing. KT: Writing – review & editing. ToH: Writing – review & editing. SK: Writing – review & editing. SK-O: Writing – review & editing. ToI: Writing – review & editing. YT: Writing – review & editing. MM: Writing – review & editing. TaH: Writing – review & editing. YH: Writing – review & editing. HS: Writing – review & editing. ST: Writing – review & editing. DY: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank M. Yato and Y. Ogiso for their valuable secretarial support. The current affiliations and addresses are as follows: HS is with the Department of Ophthalmology and Visual Science, Hiroshima University Graduate School of Biomedical and Health Sciences (1-2–3 Kasumi, Minami-ku, Hiroshima City, Hiroshima 734-8551, Japan), and TI and DY are with the Department of Diabetes, Endocrinology, and Nutrition, Kyoto University Graduate School of Medicine (54 Shogoin-kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan).
Conflict of interest
DY received clinically commissioned/joint research grants from Novo Nordisk, Ono Pharmaceutical, Taisho Pharmaceutical, Terumo, and Arklay. DY also received consulting or speaker fees from Sumitomo Dainippon Pharma, Boehringer Ingelheim, Astellas Pharma, MSD, Novo Nordisk, Ono Pharmaceutical, Eli Lilly, and Takeda Pharmaceutical. HS also received speaker fees from Santen Pharmaceutical Co., Ltd, Senjyu Pharmaceutical Co., Ltd, Kowa company, Chugai Co., Ltd, and Alcon Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2025.1590407/full#supplementary-material
References
1. Magliano DJ, Boyko EJ, and IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS. 10th ed. Brussels: International Diabetes Federation (2021).
2. Congdon N, O’Colmain B, Klaver CC, Klein R, Muñozet B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. (2004) 122:477–85. doi: 10.1001/archopht.122.4.477
3. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. doi: 10.2337/dc11-1909
4. Matoba R, Morimoto N, Kawasaki R, Fujiwara M, Kanenaga K, Yamashita H, et al. A nationwide survey of newly certified visually impaired individuals in Japan for the fiscal year 2019: impact of the revision of criteria for visual impairment certification. Jpn J. Ophthalmol. (2023) 67:346–52. doi: 10.1007/s10384-023-00986-9
5. Kawasaki R, Tanaka S, Tanaka S, Yamamoto T, Ohashi Y, Akanuma Y, et al. Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8-year follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia. (2011) 54:2288–94. doi: 10.1007/s00125-011-2199-0
6. Chen H, Zheng Z, Huang Y, Guo K, Lu J, Zhang L, et al. A microalbuminuria threshold to predict the risk for the development of diabetic retinopathy in type 2 diabetes mellitus patients. PloS One. (2012) 7:e36718. doi: 10.1371/journal.pone.0036718
7. El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, Al-Amro SA, Moharram OA, Kangave D, et al. Retinopathy as a predictor of other diabetic complications. Int. Ophthalmol. (2001) 24:1–11. doi: 10.1023/a:1014409829614
8. Liu X, Xu Y, An M, et al. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PloS One. (2019) 14:e0212574. doi: 10.1371/journal.pone.0212574
9. Misra A, Bachmann MO, Greenwood RH, Jenkins C, Shaw A, Barakat O, et al. Trends in yield and effects of screening intervals during 17 years of a large UK community-based diabetic retinopathy screening programme. Diabetes Med. (2009) 26:1040–7. doi: 10.1111/j.1464-5491.2009.02820.x
10. Nathan DM, Bebu I, Hainsworth D, Klein R, Tamborlane W, Lorenzi G, et al. Frequency of evidence-based screening for retinopathy in type 1 diabetes. N Engl. J. Med. (2017) 376:1507–16. doi: 10.1056/NEJMoa1612836
11. Tanaka H, Sugiyama T, Ihana-Sugiyama N, Ueki K, Kobayashi Y, and Ohsugi M. Changes in the quality of diabetes care in Japan between 2007 and 2015: a repeated cross-sectional study using claims data. Diabetes Res. Clin. Pract. (2019) 149:188–99. doi: 10.1016/j.diabres.2019.02.001
12. Japan Diabetes Society. Japanese Clinical Practice Guideline for Diabetes 2024 Vol. 207. Tokyo: Japan Diabetes Society (2024).
13. Himeno T, Kamiya H, and Nakamura J. Lumos for the long trail: strategies for clinical diagnosis and severity staging for diabetic polyneuropathy and future directions. J. Diabetes Investig. (2020) 11:5–16. doi: 10.1111/jdi.13173
14. Iwamoto Y, Nakanishi S, Itoh T, Nakao E, Sugisaki T, Kusano T, et al. Correlation of Baba’s diabetic neuropathy classification with various diabetes-related complications. Front. Endocrinol. (Lausanne). (2022) 13:1054934. doi: 10.3389/fendo.2022.1054934
15. Kamiya H, Shibata Y, Himeno T, Tani H, Nakayama T, Murotani K, et al. Point-of-care nerve conduction device predicts the severity of diabetic polyneuropathy: a quantitative, but easy-to-use, prediction model. J. Diabetes Investig. (2021) 12:583–91. doi: 10.1111/jdi.13386
16. Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, Zinman B, and Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial-revisited. Diabetes. (2008) 57:995–1001. doi: 10.2337/db07-1618
17. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. (1998) 352:837–53. doi: 10.1016/S0140-6736(98)07019-6
18. Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. (2014) 121:2443–51. doi: 10.1016/j.ophtha.2014.07.019
19. Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group and the Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) follow-on study. Diabetes Care. (2016) 39:1089–100. doi: 10.2337/dc16-0024
20. Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. (2018) 41:2370–6. doi: 10.2337/dc18-1131
21. Monti MC, Lonsdale JT, Montomoli C, Montross R, Schlag E, and Greenberg DA. Familial risk factors for microvascular complications and differential male-female risk in a large cohort of American families with type 1 diabetes. J. Clin. Endocrinol. Metab. (2007) 92:4650–5. doi: 10.1210/jc.2007-1185
22. Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. (2005) 54:1615–25. doi: 10.2337/diabetes.54.6.1615
23. Strand N, Anderson MA, Attanti S, Gill B, Wie C, Dawodu A, et al. Diabetic neuropathy: pathophysiology review. Curr. Pain Headache Rep. (2024) 28:481–7. doi: 10.1007/s11916-024-01243-5
24. Hayashi Y, Himeno T, Shibata Y, Hirai N, Asada-Yamada Y, Sasajima S, et al. Simplified electrophysiological approach combining a point-of-care nerve conduction device and an electrocardiogram produces an accurate diagnosis of diabetic polyneuropathy. J. Diabetes Investig. (2024) 15:736–42. doi: 10.1111/jdi.14174
25. Morita M, Sada K, Hidaka S, Ogawa M, and Shibata H. Glycemic variability is associated with sural nerve conduction velocity in outpatients with type 2 diabetes: Usefulness of a new point-of-care device for nerve conduction studies. J. Diabetes Investig. (2024) 15:1075–83. doi: 10.1111/jdi.14211
26. Fujita Y and Haneda M. Clinical practice of diabetic foot, nephropathy, and retinopathy in Japan: cross-sectional study using local and nationwide questionnaire surveys. Diabetol. Int. (2021) 13:493–502. doi: 10.1007/s13340-021-00559-6
27. Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, and Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. (2013) 120:2587–95. doi: 10.1016/j.ophtha.2013.05.004
28. Hirayasu K, Sasaki H, Kishimoto S, Kurisu S, Noda K, Ogawa K, et al. Difference in normal limit values of nerve conduction parameters between Westerners and Japanese people might need to be considered when diagnosing diabetic polyneuropathy using a Point-of-Care Sural Nerve Conduction Device (NC-stat®/DPNCheck™). J. Diabetes Investig. (2018) 9:1173–81. doi: 10.1111/jdi.12818
29. Taiana MM, Lombardi R, Porretta-Serapiglia C, Ciusani E, Oggioni N, Sassone J, et al. Neutralization of schwann cell-secreted VEGF is protective to in vitro and in vivo experimental diabetic neuropathy. PloS One. (2014) 9:e108403. doi: 10.1371/journal.pone.0108403
Keywords: diabetic retinopathy, diabetic neuropathy, DPNCheck®, modified Baba classification, retrospective observational study
Citation: Sakai M, Kato T, Ishihara T, Takao K, Hirose T, Kubota S, Kubota-Okamoto S, Imaizumi T, Takahashi Y, Mizuno M, Hirota T, Horikawa Y, Sakaguchi H, Tsunekawa S and Yabe D (2025) Predicting diabetic retinopathy stages using a simple nerve conduction measuring device, DPNCheck®: a retrospective observational study. Front. Clin. Diabetes Healthc. 6:1590407. doi: 10.3389/fcdhc.2025.1590407
Received: 09 March 2025; Accepted: 26 June 2025;
Published: 16 July 2025.
Edited by:
Bhim Bahadur Rai, Australian National University, AustraliaReviewed by:
Ankit P. Laddha, University of Connecticut, United StatesBabu Dhanendra Chaurasiya, University of Southern California, United States
Copyright © 2025 Sakai, Kato, Ishihara, Takao, Hirose, Kubota, Kubota-Okamoto, Imaizumi, Takahashi, Mizuno, Hirota, Horikawa, Sakaguchi, Tsunekawa and Yabe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takehiro Kato, a2F0by50YWtlaGlyby52NkBmLmdpZnUtdS5hYy5qcA==
 Mayu Sakai1,2,3
Mayu Sakai1,2,3 Takehiro Kato
Takehiro Kato Daisuke Yabe
Daisuke Yabe