- 1Department of Burns and Plastic Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2The 2011 Collaborative Innovation Center of Tissue Damage Repair and Regeneration Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 3The Collaborative Innovation Center of Tissue Damage Repair and Regeneration Medicine, Zunyi Medical University, Zunyi, China
Background: Diabetic wounds are a serious complication for diabetic patients, characterized by refractoriness, high recurrence rates, and susceptibility to infection. Although current guidelines recommend evidence-based treatment strategies, clinical outcomes remain suboptimal. This paper reviews the current research status and development trends in diabetic wound treatment.
Methods: Articles on diabetic wound treatment published between 2014 and 2023 were identified using the Web of Science Core Collection database, resulting in a total of 9,099 articles. Bibliometric methods were employed to analyze authors, institutions, countries, journals, keywords and references using CiteSpace and VOSviewer.
Results: China has published the most articles in the field, followed by the United States. Shanghai Jiao Tong University is the leading institution in diabetic wound treatment research, and David G. Armstrong from the United States has made significant contributions to this field. “Wound Repair and Regeneration” was identified as the most influential journal. Cluster analysis of keywords revealed four main categories: (1) mechanisms of diabetic wound healing, (2) prognosis, (3) treatment, and (4) management.
Conclusion: This paper systematically reviews the research on diabetic wound treatment from 2014 to 2023, outlining and forecasting global research hotspots and trends. Future research is expected to focus on treatment strategies for diabetic wounds, while interdisciplinary collaboration and advancements in intelligent management technologies have the potential to improve patient outcomes.
1 Introduction
With the ongoing development of the global economy, the number of people with diabetes is steadily increasing. Currently, over 500 million people worldwide are affected by diabetes, and this number is projected to exceed 750 million by 2045 (1). Diabetic wounds are a severe complication associated with diabetes, with diabetic foot ulcers being among the most critical. Data indicate that between 7.2%-15% of diabetic patients will develop diabetic foot ulcers. Of those, approximately 7.7%-44% will experience recurrence of the ulcers after they have healed. Furthermore, 50%-60% of these ulcers are prone to secondary infections and, in severe cases, may necessitate amputation. This situation significantly increases both the personal burden on patients and their risk of mortality (2).
The updated International Working Group on the Diabetic Foot (IWGDF) guidelines outline several treatment strategies for diabetic wounds, particularly diabetic foot ulcers. These strategies include antimicrobial treatments, necrotic tissue debridement, enhancement of tissue perfusion, wound dressing, negative pressure therapy, oxygen therapy, pressure offloading, nutritional support, and psychological counseling (3, 4). Despite these comprehensive approaches, treatment outcomes for diabetic wounds remain unsatisfactory. The underlying reasons for this lack of success are unclear—whether they stem from patient compliance issues or variations in medical practices. In an era marked by advanced science and technology, finding effective ways to manage diabetic wounds and alleviate the burden on patients and society is a critical and ongoing challenge.
In this study, we investigate the century-old challenge of treating diabetic wounds by examining research conducted over the past decade. Our goal is to summarize the current trends in diabetic wound treatment research and to analyze and forecast future research hotspots.
2 Materials and methods
2.1 Database and search strategy
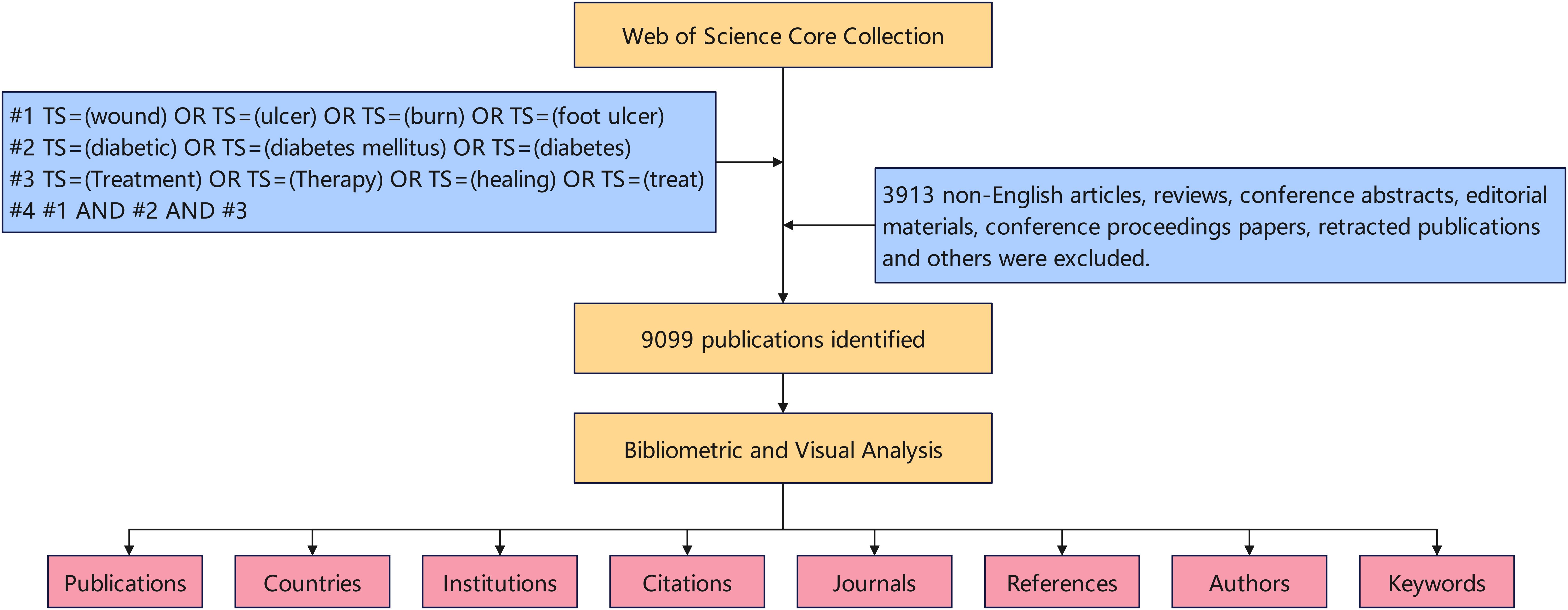
We conducted a search by TS (Topic) in the Web of Science Core Collection (WOSCC) and collected all the publications of this study, which included more than 12,000 highly influential high-quality scientific journals. Figure 1 provides a detailed overview of the data retrieval procedures and inclusion criteria employed in this research. The search strategies are also outlined in Figure 1. Only English-language articles published between January 1, 2014, and December 31, 2023.
2.2 Data analysis
The authors, title, publication year, institution, country/region, keywords, citations, abstracts, and references were obtained from the WOSCC database in plain text format. To calculate the impact factor, Journal Citation Reports (JCR) 2023 was utilized. Data qualifying for inclusion in this study were extracted using the VOSviewer 1.6.19 program and subsequently imported into CiteSpace 6.2.R6 and Microsoft Excel 2021. VOSviewer, a traditional bibliometric analysis tool, was employed for visual analysis in bibliometric research (5). CiteSpace was used to analyze co-occurrence and centrality within collaborative networks among countries, authors, and institutions (6). Detailed usage instructions for VOSviewer are available at https://www.vosviewer.com/getting-started, while CiteSpace can be accessed and downloaded from https://citespace.podia.com/, which offers text and video tutorials. Differences in publication volume between countries were presented using https://amcharts.com/editor/map/.
3 Results
3.1 Analysis of the number of publications and citations
From 2014 to 2023, there were 9099 papers on treatment of diabetes wound, according to comprehensive manual screening. The numbers of publications and citations are shown in Figure 2. We observed that the number of papers published and citations continued to increase from the year 2014 onward.
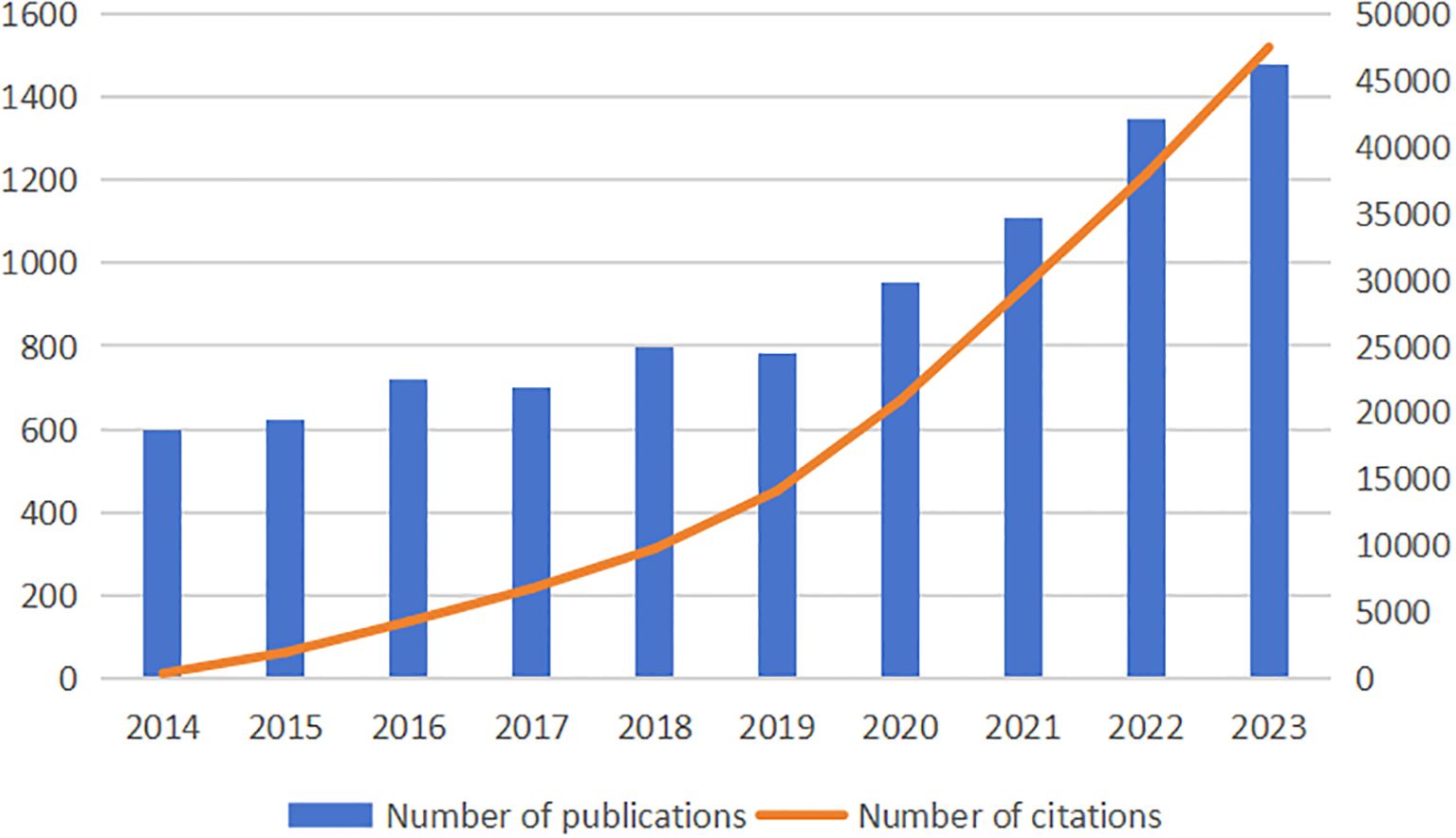
Figure 2. The annual number of publications and citations on treatment of diabetes wound from 2014 to 2023.
3.2 Analysis of contributions of prolific authors and co-cited authors
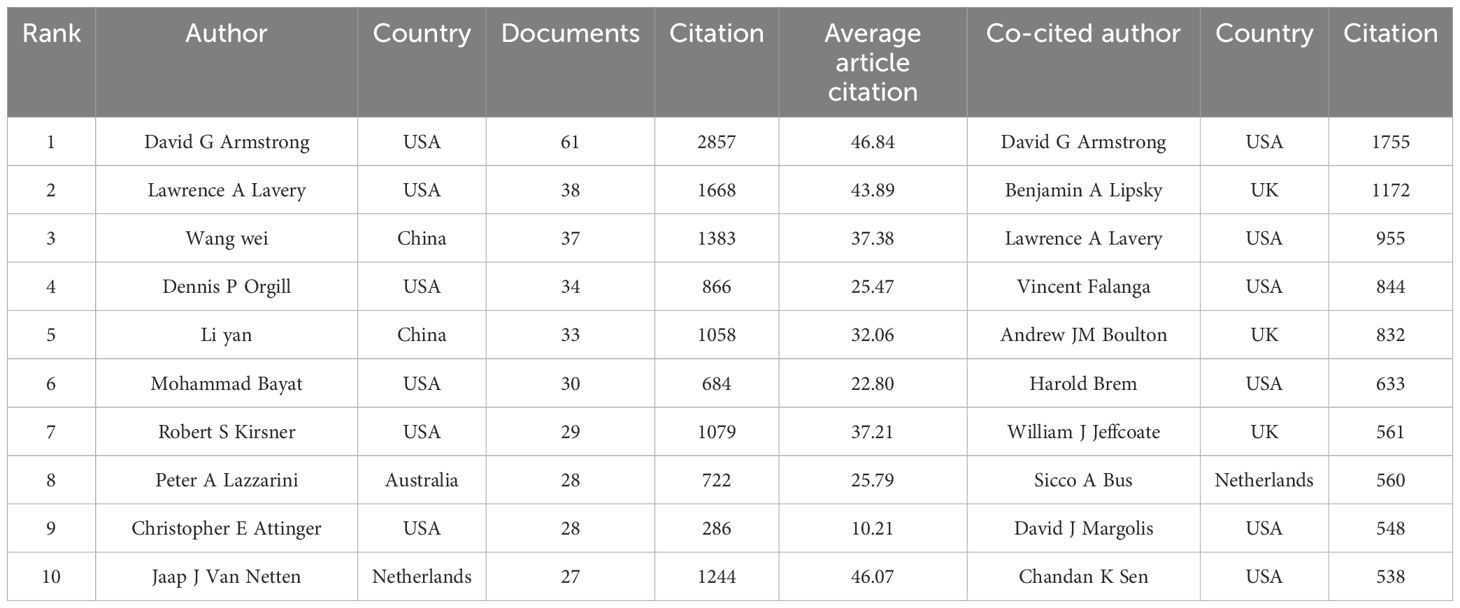
As shown in Table 1, among the top 10 most prolific authors in this discipline, only two authors are from China, while the majority are from the United States. Furthermore, most of the top 10 co-cited authors originate from the USA, with the remaining few from the UK and the Netherlands.
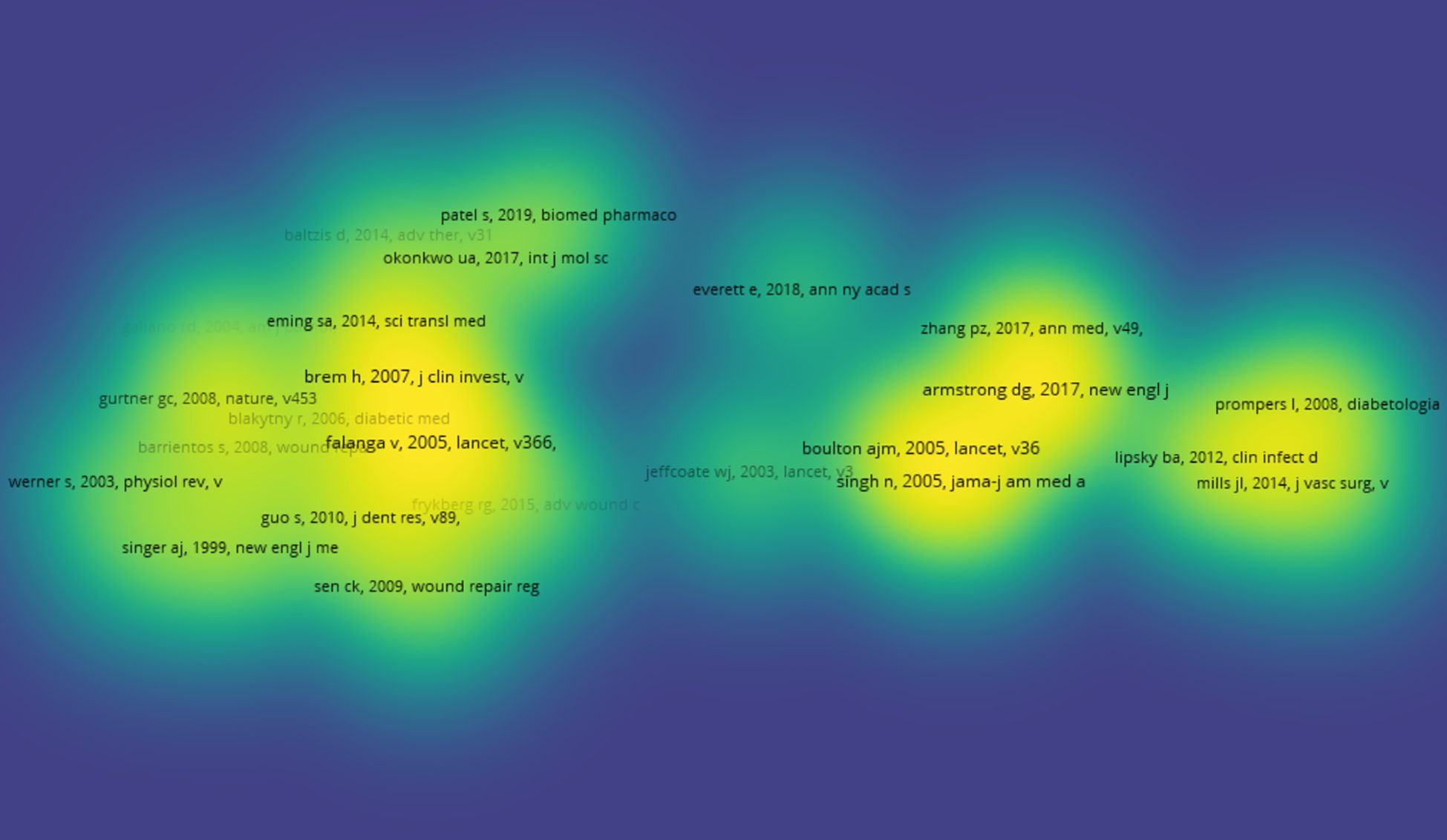
Figure 3 presents a collaborative network of authors with more than 10 publications. The network visualization map (Figure 3A) displays 31 clusters. David G Armstrong stands out as the most connected author, with a total of 79 links. The overlay map indicates the formation of new research teams led by Chinese scholars Chen Jiang and Wang Cheng (Figure 3B). The density visualization map illustrates that Chinese researchers have been notably active in diabetic wound research in recent years (Figure 3C). Finally, Figure 3D depicts the relationships among the top 11 authors, including their countries and affiliated institutions.
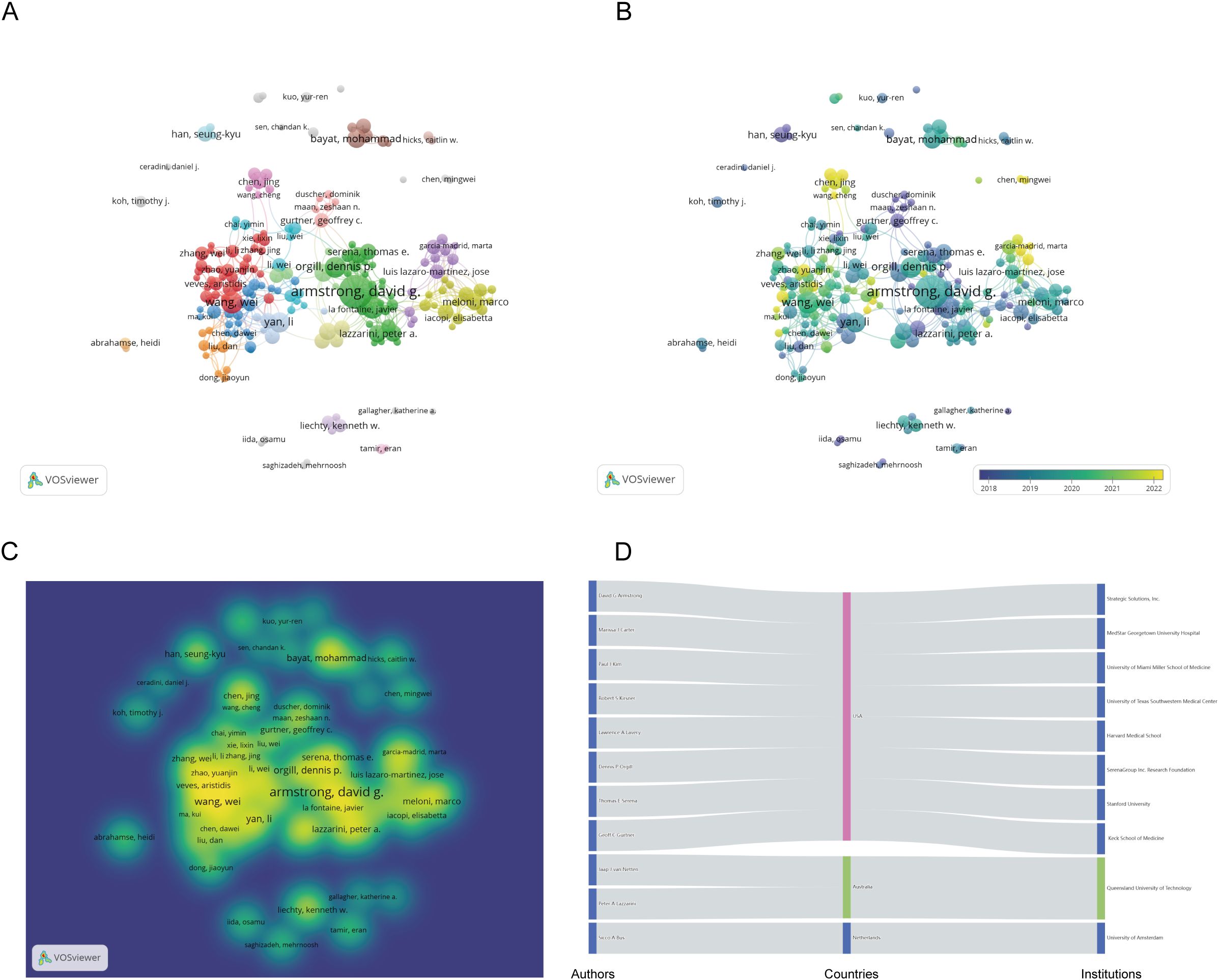
Figure 3. Co-authorship analysis of the excellent authors in the field of diabetes wound. (A) A network visualization map. (B) An overlay visualization map. (C) A density visualization map. (D) Countries and institutions of these distinguished authors.
3.3 Analysis of the contributions of journals
To illustrate the distribution of citing and cited journals, we used a dual-map overlay atlas. In Figure 4, the geographic area on the left represents the citing journals, while the map on the right represents the cited journals. Different disciplines are indicated by varying line colors. The citing journals primarily focus on molecular biology, immunology, genetics, nursing, surgery, medicine, clinical studies, and chemistry. In contrast, the cited journals mainly cover chemistry, environmental science, genetics, molecular biology, medicine, health, nursing, biology, dermatology, surgery, nutrition, and toxicology.
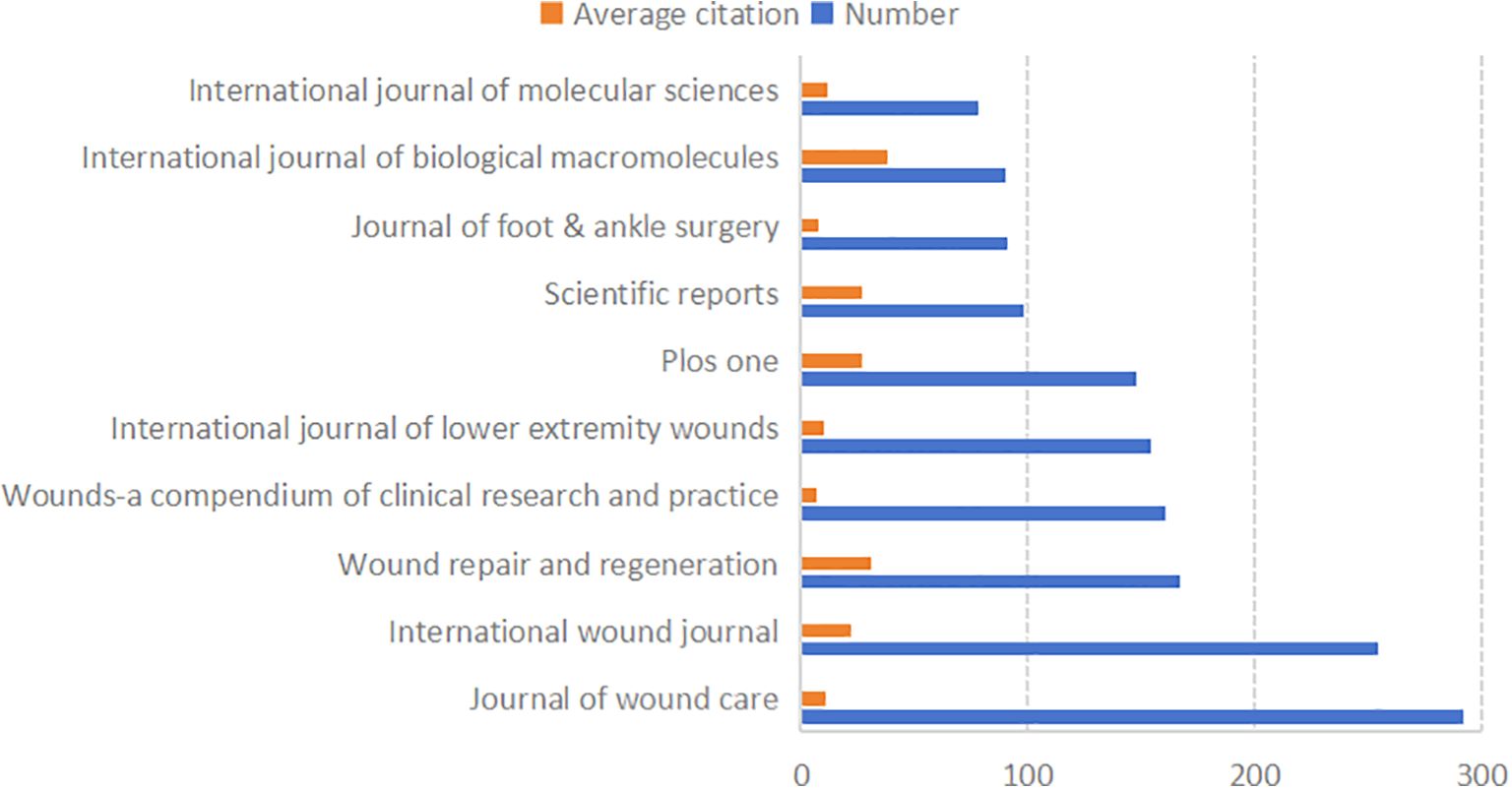
A total of 1,729 journals have published articles in this field. Figure 5 lists the 10 most prolific journals, along with their average citation rates and total publication numbers in this domain. Most of these top 10 journals are classified as JCR Q1 and Q3.
3.4 Analysis of the contributions by institutions
Figure 6A illustrates the top 10 most productive institutions, with eight of them located in China. The analysis focused on institutions with at least 40 publications, using VOSviewer to identify 29 institutions involved in co-authorship. Figure 6B depicts the institutional co-authorship network, which includes 29 institutions and is organized into 5 clusters. Recent trends indicate that Chinese institutions are increasingly influential in the field of diabetic wound research, as demonstrated by the overlay map in Figure 6C, which shows the historical trend of article production.
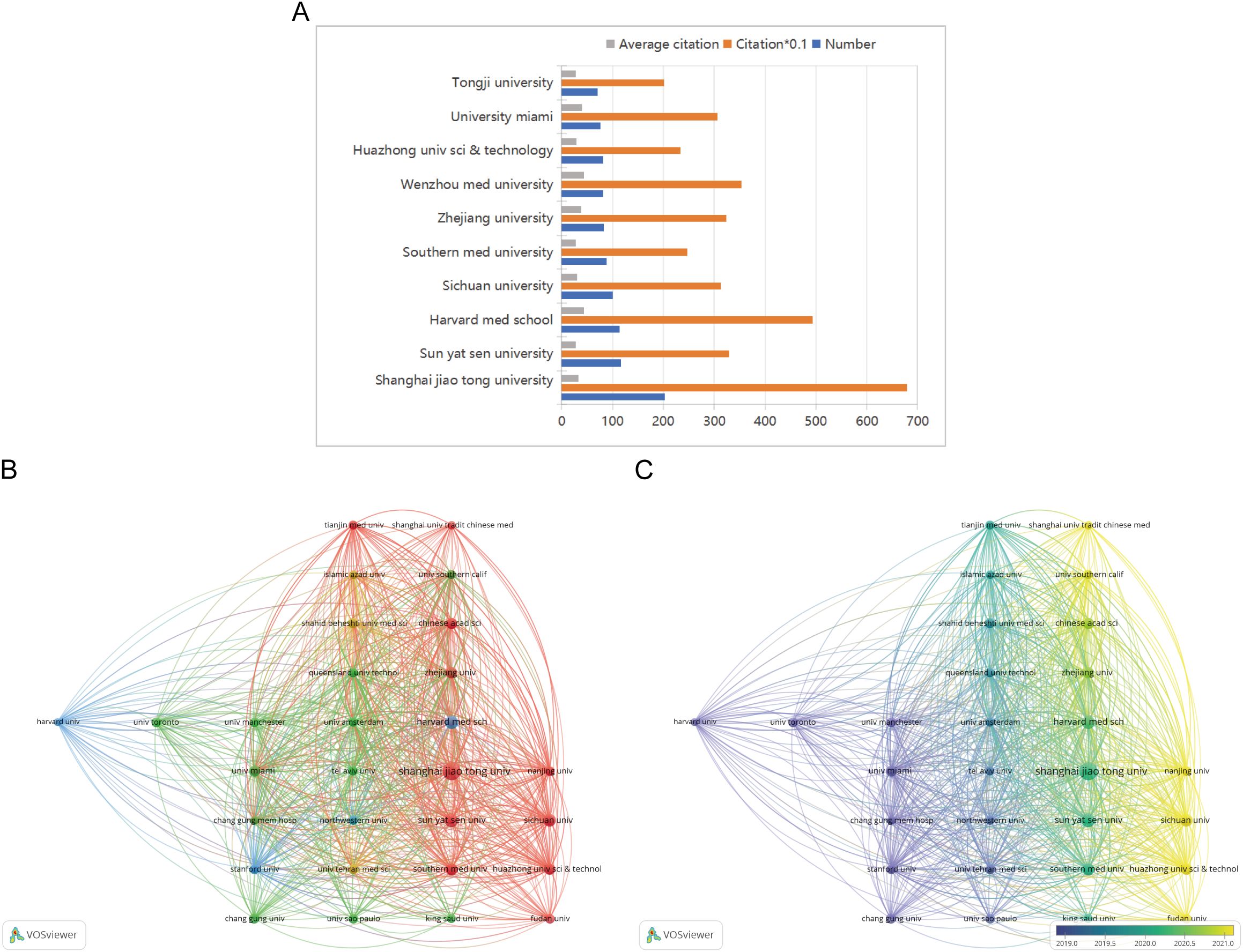
Figure 6. (A) The total number of publications, the average number of citations and total number of citations of the top 10 institutions. (B) A network visualization map. (C) An overlay visualization map.
3.5 Analysis of the contributions of countries
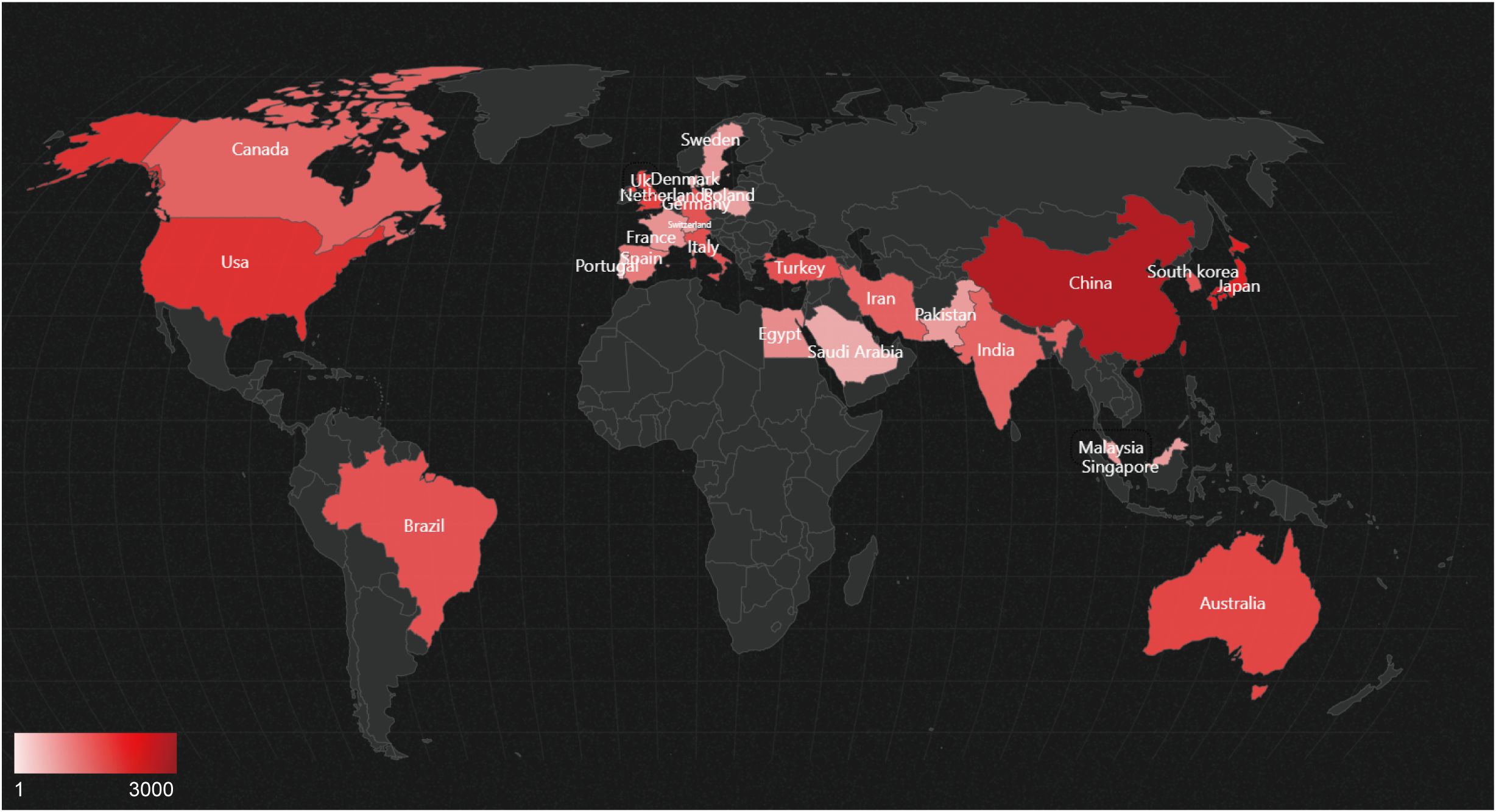
Figure 7 displays the geographical distribution of global publications in the field of diabetic wounds across 27 countries and regions. Figure 8A provides a comparison of publication proportions among these countries. Figure 8B examines the co-authorship network between these 27 countries using VOSviewer. As shown in overlay visualization map (Figure 8C), China has recently actively involved in substantial research collaborations.
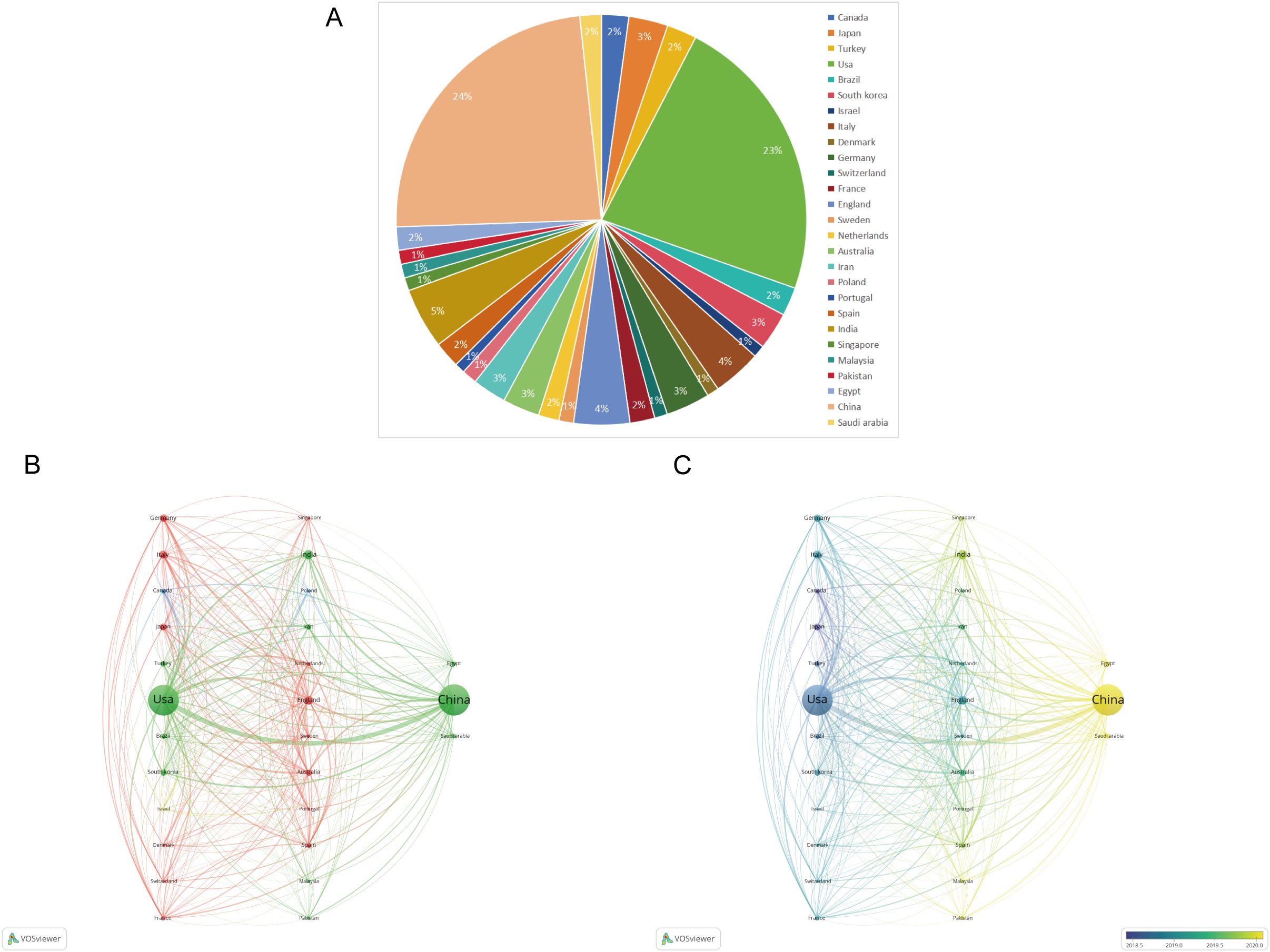
Figure 8. (A) Pie chart of the 27 productive countries/regions. (B) Network visualization map of the top 27 countries’ collaboration. (C) An overlay visualization map.
3.6 Analysis of a highly cited study
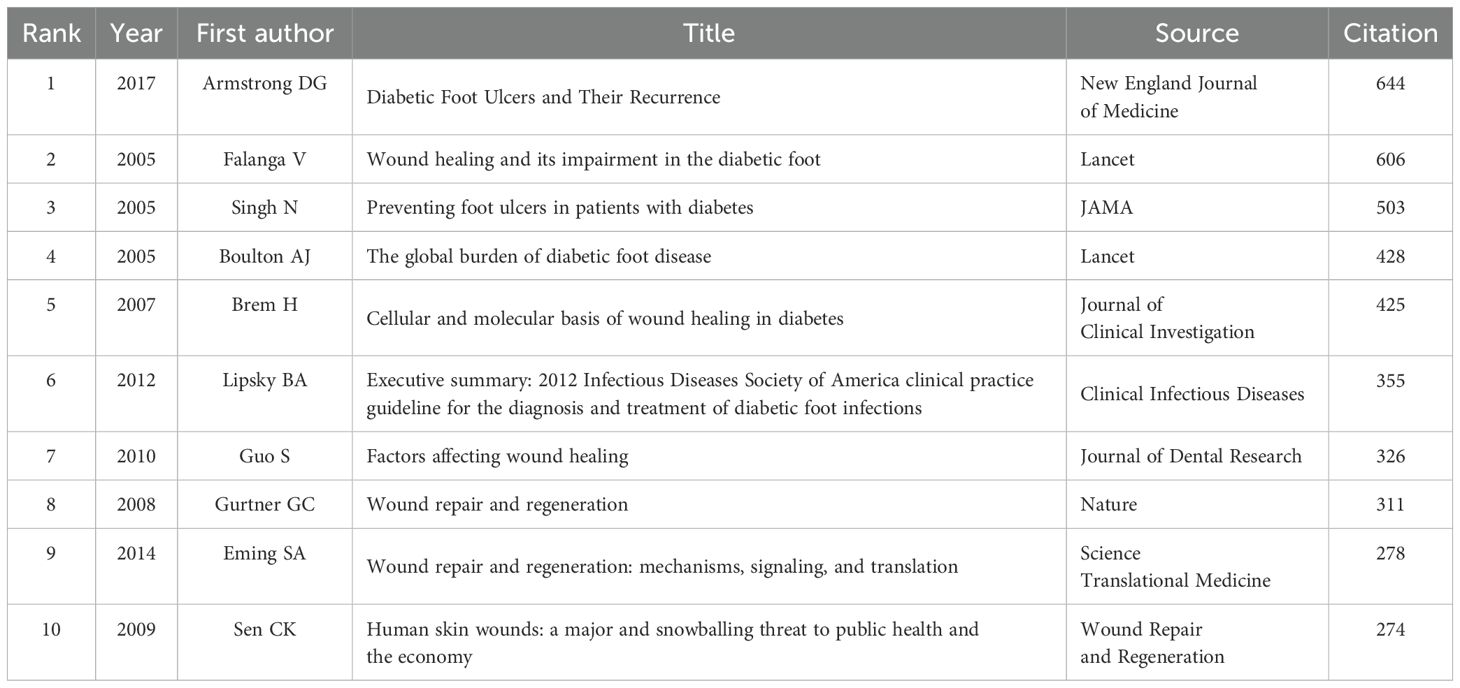
The top 10 articles with the highest citation frequencies are listed in Table 2. The article titled ‘Diabetic Foot Ulcers and Their Recurrence’ published in the New England Journal of Medicine in 2017 was cited the most. Out of 232823 references 27, articles were cited more than 150 times are shown in Figure 9.
3.7 Analysis of keywords
Figure 10A shows keywords with a frequency of higher than 58. In total, 200 high-frequency keywords were selected from 9099 studies, and divided into four clusters. Cluster 1 (red cluster): mechanism of diabetic wound, including gene expression, cell migration and proliferation, oxidative stress, growth-factor, inflammation, and angiogenesis; cluster 2 (blue cluster): prognosis, including efficacy, safety, complications; cluster 3 (yellow cluster): treatment, include antioxidant, scaffolds, antimicrobial, silver nanoparticles, delivery, curcumin; cluster 4 (green cluster): management, include pain, risk factors, diabetes mellitus, pressure, health, glycemic control, prevention of infection. As shown in Figure 10B, the top five keywords with the highest occurrences across the four major clusters. The timeline picture can show the period and time trend of different research keywords. According to Figure 10C, angiogenesis, diabetic foot, and diabetic wound healing are the longest-lasting hotspots, which lasted from 2014 to the present. Burst word analysis can reveal hot research in a research field during a specific period. We show the top 25 keywords with the strongest citation bursts in Figure 10D.
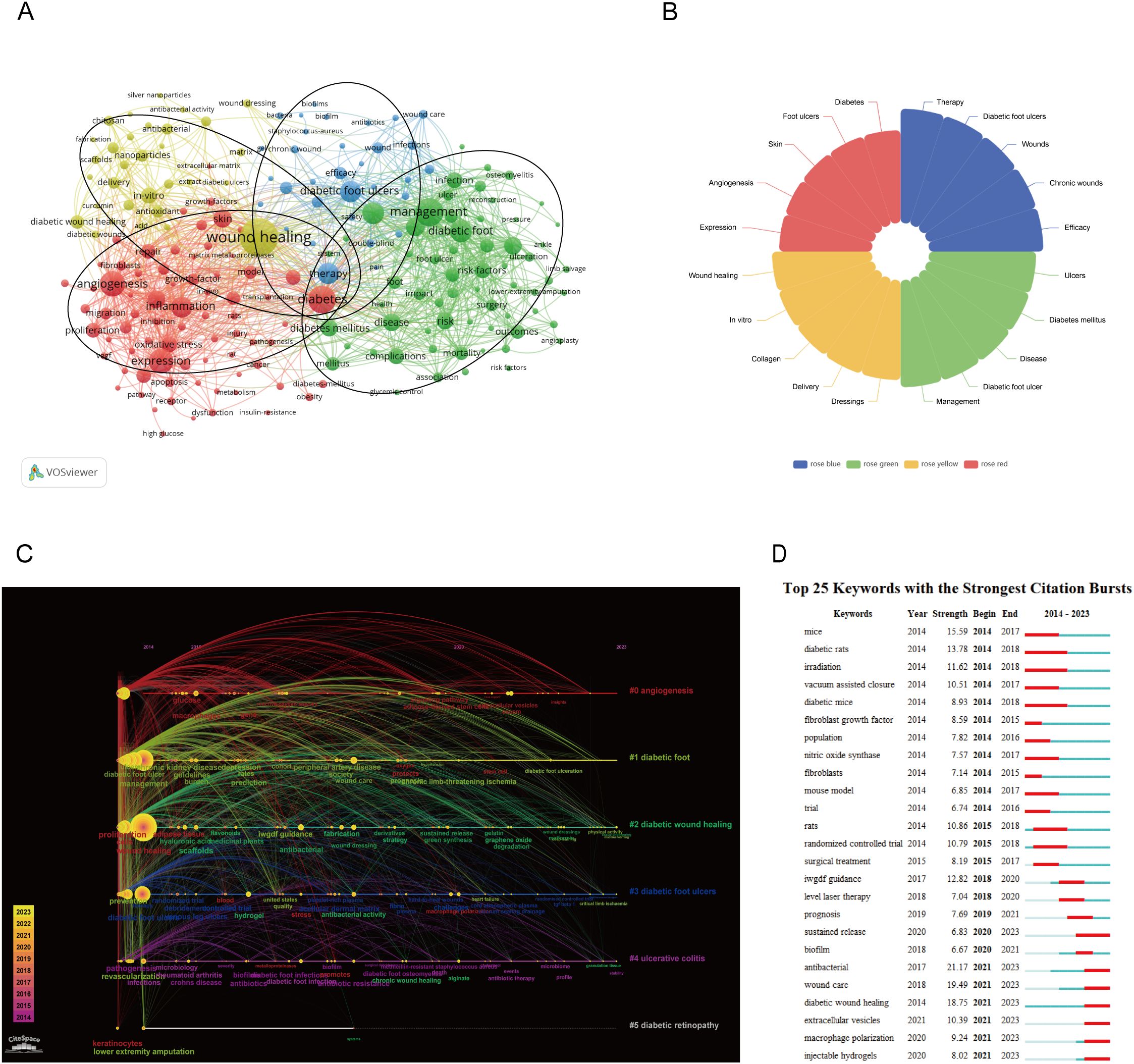
Figure 10. (A) A network visualization map. (B) Keywords that are particularly important in the four groups. (C) A timeline and keyword clustering display for the diabetics wound. (D) The top 25 terms with the most significant diabetics wound citation bursts.
4 Discussion
4.1 Global research status and trends
According to an analysis of national publication data, China and the United States have made substantial contributions, with a total of 4,688 articles, accounting for 51.52% of the overall literature. Additionally, data on author collaboration strength indicate that the top three authors are from China and the United States, suggesting a closely connected and influential group of researchers from these countries. From the perspective of journal publications and citations, “Wound Repair and Regeneration” stands out as a significant journal in the field of diabetic wound research, offering highly valuable and relevant papers.
In our analysis of the top 10 institutions, we found that eight are based in China. The top three institutions are Shanghai Jiao Tong University, Sun Yat-sen University, and Harvard Medical School.
China and the United States have significantly contributed to the literature on diabetic wounds, with their leading positions attributable to national strategic orientations and disciplinary integration models. China benefits from strong policy-driven initiatives and collaborative disciplines, whereas the United States primarily emphasizes research innovation mechanisms and the connections among industry, academia, and research institutions. This will further promote the output of their research results.
4.2 Research keyword analysis
Through cluster analysis of 200 keywords with frequencies exceeding 58, these keywords were categorized into four main clusters: Cluster 1 (red cluster) focuses on the mechanism of diabetic wounds healing; Cluster 2 (blue cluster) covers prognosis; Cluster 3 (yellow cluster) relates to treatment; and Cluster 4 (green cluster) pertains to management. These keywords highlight the primary research directions and topics of interest within the co-occurrence analysis.
By examining the timeline of keywords and the top 20 emerging terms, a high frequency of keywords related to mechanisms and treatments from 2014-2023 indicates that these areas have been research focal points. The recent emergence of terms such as “antimicrobial,” “wound care,” “diabetic wound healing,” “extracellular vesicles,” “macrophage polarization,” and “injectable hydrogels” suggests that these are recent research hotspots and may also be future areas of interest. Furthermore, studies related to management have also gained prominence, often intertwining with prognosis, in line with the results of our cluster analysis. By analyzing these four categories, we can explore the current research hotspots and directions in the field of diabetic wound therapy.
4.3 Red cluster: mechanism of diabetic wounds healing
The complexity of wound healing arises from the intricate coordination between various tissues and cells, involving processes such as cell migration, proliferation, matrix deposition and remodeling, inflammatory response, and angiogenesis (7). However, these processes are often significantly disrupted in diabetic patients due to the effects of prolonged hyperglycemia, leading to impaired wound healing (8). Recent studies have highlighted the impact of aberrant cell signaling pathways on wound healing under high glucose conditions, particularly emphasizing the role of dysregulated macrophage polarization and impaired angiogenesis (9, 10).
Macrophages are crucial immune cells that regulate the inflammatory process and can be activated and polarized into different phenotypes. M1 macrophages exhibit pro-inflammatory properties, while M2 macrophages possess anti-inflammatory capabilities that aid in wound healing by promoting vascular regeneration, cell proliferation, and inhibiting inflammation. During the inflammatory phase of diabetic wounds, macrophages release various inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, which impede the progression of the healing process into the proliferative phase (7). Moreover, sustained activation of the NF-κB signaling pathway in high glucose conditions triggers a chronic inflammatory response, further delaying the wound healing process (11).
Impaired angiogenesis is a significant factor contributing to delayed wound healing in diabetes. Vascular endothelial cells (ECs) are crucial for forming new blood vessels, but their growth and function are often compromised, leading to difficulties in vessel formation and wound repair (12). In hyperglycemic conditions, endothelial cell damage is caused by several factors. Chronic high glucose levels result in direct damage to endothelial cells through the formation of advanced glycation end products (AGEs). AGEs modify cellular proteins and DNA via non-enzymatic glycosylation, disrupting normal cellular functions (13, 14). Additionally, hyperglycemia induces mitochondrial dysfunction, leading to the production of excessive reactive oxygen species (ROS). This damages the mitochondrial membrane potential and further impairs wound healing (15).
Oxidative stress from elevated free radicals and oxidative substances exacerbates endothelial cell damage, activates inflammatory pathways, and intensifies the inflammatory response. The chronic low-grade inflammation in diabetes promotes the release of inflammatory mediators such as IL-6 and TNF-α, which not only worsen endothelial cell damage but also increase blood vessel permeability and impede angiogenesis (16, 17). Furthermore, reduced angiogenesis in a hyperglycemic environment affects endothelial cell proliferation and migration, leading to insufficient new vessel formation, inadequate nutrient supply, and a hypoxic environment. This, in turn, triggers and worsens inflammation, further exacerbating endothelial cell damage (18, 19).
4.4 Blue cluster: prognosis
Diabetic foot ulcers and amputations significantly increase the risk of mortality (2). Research indicates that the 5-year mortality rate for individuals with a diabetic foot ulcer is approximately 30%, exceeding 70% for those with a major amputation (2). Consequently, researchers are focusing on early identification and intervention for diabetic wounds. A cohort study showed that by using statistics and machine learning techniques on patient data, it was demonstrated that genetic information has predictive potential in diabetic wound healing. This data includes clinical factors, circulating endothelial precursor cells (CEPCs), and fine predictive sequencing of the NOS1AP gene (20). Additionally, in patients with diabetic peripheral neuropathy, enhanced sensory testing using a tuning fork has shown that vibration sensitivity of the distal phalanx is highly accurate and clinically relevant for predicting the development of calluses and skin ulcers (21).
In addition to research on early prediction, many studies have concentrated on effectively assessing prognosis and improving long-term survival in patients with diabetic wounds (2). For instance, Jia et al. investigated the granulation tissue in negative pressure wound therapy (NPWT) using label-free quantitative mass spectrometry. Their analysis revealed that NPWT significantly altered protein expression in granulation tissue, particularly in processes related to detoxification, antioxidant activity, inflammatory regulation, and extracellular matrix modification. Notably, changes in proteins such as PRDX2, PROS1, CTSS, and ITIH4 offer valuable insights into potential biomarkers for assessing responses to diabetic wound treatments in the future (22). Bender et al. examined characteristics of diabetic wounds, including necrosis, wound size, and patient age, to develop a simple predictive model based on bedside wound characteristics. This model effectively predicts the healing outcome of diabetic foot ulcers, whether the wound size is likely to increase or decrease (23). Artificial intelligence, as a cutting-edge technology, holds significant promise for integration into diabetic wound treatment (24). Pereira MG et al. employed machine learning and decision tree algorithms to evaluate the risk of ulcer healing in diabetic feet. They found that factors such as disease perception, ulcer duration, and biochemical markers (e.g., IL-6, microRNA-146a-5p, PECAM-1, and angiopoietin-2) had substantial predictive value for healing outcomes (25). The high recurrence rate of diabetic foot ulcers post-healing is another critical issue. To address this, Wang et al. developed and validated three prediction models to assess the risk of ulcer recurrence. Model 1 uses social and clinical indicators; Model 2 adds factors such as medication, blood pressure, and body mass index; and Model 3 further incorporates laboratory indicators. Model 3 demonstrated the highest performance in predicting the risk of diabetic foot ulcer recurrence, with an AUC value of 0.899 and the greatest sensitivity and specificity. While Models 1 and 2 are effective for general screening, Model 3 provides a more accurate screening tool (26).
4.5 Yellow cluster: treatment
The treatment strategies recommended by the IWGDF guidelines for diabetic wound care include antimicrobial therapy, necrotic tissue resection, enhanced tissue perfusion, wound dressing, negative pressure therapy, oxygen therapy, and decompression (3, 4). Although these evidence-based approaches have proven effective, clinical management of diabetic wounds often remains suboptimal. The bacteria causing wound infections in diabetes are mainly Gram-positive and Gram-negative. Among them, the most common ones are Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae. In fungal infections, Candida albicans is also relatively common, especially in patients with low immunity. Furthermore, the prevalence rate of multiple microbial infections is 22.8% (27, 28). In addition to using conventional antibiotics, researchers are exploring novel treatments such as stem cells, extracellular vesicles, miRNAs, nanoenzymes, hydrogels, and microneedles (28–32).
Stem cells can accelerate diabetic wound healing by promoting cell regeneration, regulating the microenvironment, and reducing inflammation (33). However, their application is constrained by ethical issues and potential immunogenicity. Recent studies have highlighted extracellular vesicles, such as stem cell-derived exosomes, as superior therapeutic carriers compared to synthetic nanoparticles. These vesicles offer extended circulatory half-life, low immunogenicity, biocompatibility, ease of modification, and stable payload (34). Additionally, the miRNAs contained in exosomes have garnered significant attention. miRNAs are non-coding RNAs that regulate protein expression and associated pathways post-transcriptionally, and they have been extensively studied in diabetic wound management (35). Notably, encapsulating therapeutic miRNAs in exosomes protects them from ribonuclease degradation, increases their concentration in wound tissue or cells, and enhances wound healing. Tao et al (36). utilized gene overexpression technology to generate exosomes overexpressing miR-126-3p from synovial mesenchymal stem cells (SMSCs), demonstrating accelerated wound reepithelialization, angiogenesis, and collagen maturation in diabetic mice. Similarly, Yan et al (37) used miR-31-5p loaded in ex vivo milk. However, the clinical application of engineered exosomes faces challenges, including stability and persistence in wounds. Nanoenzymes also encounter similar issues (38).
Hydrogels and microneedles are innovative biomaterials playing a crucial role in the treatment of diabetic wounds. Hydrogels create a moist healing environment, promote wound repair, reduce infection risks, and facilitate drug or growth factor release to accelerate healing (10). On the other hand, microneedles can painlessly penetrate the skin, directly delivering drugs or therapeutic agents to deeper tissues, thereby enhancing the therapeutic effect and minimizing the dosage of drugs as well as the pain and discomfort associated with traditional injections. They can also inhibit pathogenic bacteria such as Escherichia coli and Staphylococcus aureus, exerting an antibacterial effect and promoting the healing of diabetic wounds (39, 40). Recent research indicates that microneedles made from hydrogels offer these combined advantages (41). Furthermore, the use of hydrogels or microneedles has been shown to significantly improve diabetic wound healing (10, 41).
Given the persistent and multifactorial nature of diabetic wound treatment, researchers have addressed the limitations of other therapeutic agents—such as exosomes, miRNA, or nanoenzymes—by integrating them into multifunctional hydrogels or microneedles, they not only exhibit sustained release properties but also possess antimicrobial, anti-inflammatory, antioxidant, and angiogenic effects (38, 39, 42, 43). Moreover, recent studies have combined phototherapy and electrotherapy with these hydrogels or microneedles, providing multiple options for personalized and precise diabetic wound treatment (30, 44, 45). These combined therapies using combined materials may become breakthrough points and provide more research directions for the treatment of diabetic wounds. It is also noteworthy that the application of AI in diabetic wound care can enable real-time dynamic monitoring, representing a promising strategy for future advancements (46, 47).
4.6 Green cluster: management
The management of diabetic wounds is undergoing significant transformation, with innovations in management approaches standing out. Remote monitoring technology and intelligent management platforms now enable medical teams to track patients’ wound conditions and blood glucose levels in real time. This capability allows for personalized treatment recommendations and ensures centralized management and intelligent data analysis (47, 48). Additionally, interdisciplinary collaboration has become crucial in enhancing treatment outcomes. Experts in endocrinology, surgery, nursing, and nutrition work together to provide comprehensive care through blood glucose control, specialized wound management, meticulous care, and nutritional support (49–51). This integrated approach not only accelerates wound healing but also optimizes the overall health of patients and significantly improves their quality of life.
4.7 Limitations
Our bibliometric study has several limitations. Firstly, it only covers literature published in English, leaving out significant works in other languages. Secondly, high-quality articles published recently may not have garnered adequate attention due to their brief publication period and lower citation counts.Ultimately, we only retrieved WOSCC, which may result in the omission of some studies.
5 Conclusion
In conclusion, China and the United States are at the forefront of global research on diabetic wounds, making significant contributions and fostering collaborations. The journal “Wound Repair and Regeneration” is a prominent publication in this field. Recent research has concentrated on various aspects of wound healing, including mechanisms, prognosis, treatment, and management. Current research hotspots emphasize therapeutic approaches such as antimicrobial methods, advanced wound care, extracellular vesicles, macrophage polarization, and injectable hydrogels. The growing recognition of prognostic analysis by scholars highlights the importance of integrating mechanistic and prognostic studies to enhance individualized patient evaluations. The more effective treatment strategies and intelligent clinical management techniques, offering more personalized and precise treatment for diabetic wound healing. These trends underscore the dynamic and evolving nature of research in diabetic wound therapy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YW: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft. KN: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank CiteSpace, VOSviewer and Amcharts for providing the platform for figure production.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Armstrong DG, Tan TW, Boulton A, and Bus SA. Diabetic foot ulcers: A review. JAMA. (2023) 330:62–75. doi: 10.1001/jama.2023.10578
3. Chen P, Vilorio NC, Dhatariya K, Jeffcoate W, Lobmann R, McIntosh C, et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. (2024) 40:e3644. doi: 10.1002/dmrr.v40.3
4. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Fitridge R, Game F, et al. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab. Res. Rev. (2024) 40:e3657. doi: 10.1002/dmrr.v40.3
5. Thompson DF and Walker CK. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy. (2015) 35:551–9. doi: 10.1002/phar.2015.35.issue-6
6. Wang C, Zhang Y, Zhang Y, and Li B. A bibliometric analysis of gastric cancer liver metastases: advances in mechanisms of occurrence and treatment options. Int. J. Surg. (2024) 110:2288–99. doi: 10.1097/JS9.0000000000001068
7. Peña OA and Martin P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. (2024) 25:599–616. doi: 10.1038/s41580-024-00715-1
8. Guo L, Xiao D, Xing H, Yang G, and Yang X. Engineered exosomes as a prospective therapy for diabetic foot ulcers. Burns Trauma. (2024) 12:tkae023. doi: 10.1093/burnst/tkae023
9. Qian Y, Zheng Y, Jin J, Wu X, Xu K, Dai M, et al. Immunoregulation in diabetic wound repair with a photoenhanced glycyrrhizic acid hydrogel scaffold. Adv. Mater. (2022) 34:e2200521. doi: 10.1002/adma.202200521
10. Xiang T, Guo Q, Jia L, Yin T, Huang W, Zhang X, et al. Multifunctional hydrogels for the healing of diabetic wounds. Adv. Healthc Mater. (2024) 13:e2301885. doi: 10.1002/adhm.202301885
11. Cai F, Chen W, Zhao R, and Liu Y. Mechanisms of Nrf2 and NF-κB pathways in diabetic wound and potential treatment strategies. Mol. Biol. Rep. (2023) 50:5355–67. doi: 10.1007/s11033-023-08392-7
12. Okonkwo UA, Chen L, Ma D, Haywood VA, Barakat M, Urao N, et al. Compromised angiogenesis and vascular Integrity in impaired diabetic wound healing. PLoS One. (2020) 15:e0231962. doi: 10.1371/journal.pone.0231962
13. Friedman EA. Advanced glycation end-products in diabetic nephropathy. Nephrol. Dial Transpl. (1999) 14 Suppl 3:1–9. doi: 10.1093/ndt/14.suppl_3.1
14. Barman PK and Koh TJ. Macrophage dysregulation and impaired skin wound healing in diabetes. Front. Cell Dev. Biol. (2020) 8:528. doi: 10.3389/fcell.2020.00528
15. Jiang G, Jiang T, Chen J, Yao H, Mao R, Yang X, et al. Mitochondrial dysfunction and oxidative stress in diabetic wound. J. Biochem. Mol. Toxicol. (2023) 37:e23407. doi: 10.1002/jbt.23407
16. Navarro JF and Mora-Fernández C. The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev. (2006) 17:441–50. doi: 10.1016/j.cytogfr.2006.09.011
17. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. (2020) 11:259. doi: 10.1186/s13287-020-01756-x
18. Bitar MS and Al-Mulla F. Upregulation of CREM/ICER suppresses wound endothelial CRE-HIF-1α-VEGF-dependent signaling and impairs angiogenesis in type 2 diabetes. Dis. Model. Mech. (2015) 8:65–80. doi: 10.1242/dmm.017145
19. Wang X, Li R, and Zhao H. Enhancing angiogenesis: Innovative drug delivery systems to facilitate diabetic wound healing. BioMed. Pharmacother. (2024) 170:116035. doi: 10.1016/j.biopha.2023.116035
20. Hettinger G, Mitra N, Thom SR, and Margolis DJ. An improved clinical and genetics-based prediction model for diabetic foot ulcer healing. Adv. Wound Care (New Rochelle). (2024) 13:281–90. doi: 10.1089/wound.2023.0194
21. Yoshikawa Y, Maeshige N, Uemura M, Tanaka M, Kawabe N, Yamaguchi A, et al. Prediction of callus and ulcer development in patients with diabetic peripheral neuropathy by isosceles triangle-forming tuning fork. SAGE Open Med. (2022) 10:20503121221085097. doi: 10.1177/20503121221085097
22. Jia Z, Liu L, Zhang S, Zhao X, Luo L, Tang Y, et al. Proteomics changes after negative pressure wound therapy in diabetic foot ulcers. Mol. Med. Rep. (2021) 24:834. doi: 10.3892/mmr.2021.12474
23. Bender C, Cichosz SL, Pape-Haugaard L, Hartun Jensen M, Bermark S, Laursen AC, et al. Assessment of simple bedside wound characteristics for a prediction model for diabetic foot ulcer outcomes. J. Diabetes Sci. Technol. (2021) 15:1161–7. doi: 10.1177/1932296820942307
24. Xue Y, Chen C, Tan R, Zhang J, Fang Q, Jin R, et al. Artificial intelligence-assisted bioinformatics, microneedle, and diabetic wound healing: A “New deal” of an old drug. ACS Appl. Mater Interfaces. (2022) 14:37396–409. doi: 10.1021/acsami.2c08994
25. Pereira MG, Vilaça M, Braga D, Madureira A, Da Silva J, Santos D, et al. Healing profiles in patients with a chronic diabetic foot ulcer: An exploratory study with machine learning. Wound Repair Regen. (2023) 31:793–803. doi: 10.1111/wrr.13141
26. Wang M, Chen D, Fu H, Xu H, Lin S, Ge T, et al. Development and validation of a risk prediction model for the recurrence of foot ulcer in type 2 diabetes in China: A longitudinal cohort study based on a systematic review and meta-analysis. Diabetes Metab. Res. Rev. (2023) 39:e3616. doi: 10.1002/dmrr.v39.4
27. Du F, Ma J, Gong H, Bista R, Zha P, Ren Y, et al. Microbial infection and antibiotic susceptibility of diabetic foot ulcer in China: literature review. Front. Endocrinol. (Lausanne). (2022) 13:881659. doi: 10.3389/fendo.2022.881659
28. Li X, Jing X, Yu Z, and Huang Y. Diverse antibacterial treatments beyond antibiotics for diabetic foot ulcer therapy. Adv. Healthc Mater. (2023) 12:e2300375. doi: 10.1002/adhm.202300375
29. Chen F, Qin J, Wu P, Gao W, and Sun G. Glucose-responsive antioxidant hydrogel accelerates diabetic wound healing. Adv. Healthc Mater. (2023) 12:e2300074. doi: 10.1002/adhm.202300074
30. Guo Y, Zhang C, Xie B, Xu W, Rao Z, Zhou P, et al. Multifunctional microneedle patch based on metal-phenolic network with photothermal antimicrobial, ROS scavenging, immunomodulatory, and angiogenesis for programmed treatment of diabetic wound healing. ACS Appl. Mater Interfaces. (2024) 16:33205–22. doi: 10.1021/acsami.4c07091
31. Liu L and Liu D. Bioengineered mesenchymal stem cell-derived exosomes: emerging strategies for diabetic wound healing. Burns Trauma. (2024) 12:tkae030. doi: 10.1093/burnst/tkae030
32. Shang L, Yu Y, Jiang Y, Liu X, Sui N, Yang D, et al. Ultrasound-augmented multienzyme-like nanozyme hydrogel spray for promoting diabetic wound healing. ACS Nano. (2023) 17:15962–77. doi: 10.1021/acsnano.3c04134
33. Yu X, Liu P, Li Z, and Zhang Z. Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front. Endocrinol. (Lausanne). (2023) 14:1099310. doi: 10.3389/fendo.2023.1099310
34. van Niel G, D’Angelo G, and Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. (2018) 19:213–28. doi: 10.1038/nrm.2017.125
35. Cui Y, Qi Y, Ding L, Ding S, Han Z, Wang Y, et al. miRNA dosage control in development and human disease. Trends Cell Biol. (2024) 34:31–47. doi: 10.1016/j.tcb.2023.05.009
36. Tao SC, Guo SC, Li M, Ke QF, Guo YP, and Zhang CQ. Chitosan wound dressings incorporating exosomes derived from microRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl. Med. (2017) 6:736–47. doi: 10.5966/sctm.2016-0275
37. Yan C, Chen J, Wang C, Yuan M, Kang Y, Wu Z, et al. Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv. (2022) 29:214–28. doi: 10.1080/10717544.2021.2023699
38. Sun C, Zhou X, Liu C, Deng S, Song Y, Yang J, et al. An integrated therapeutic and preventive nanozyme-based microneedle for biofilm-infected diabetic wound healing. Adv. Healthc Mater. (2023) 12:e2301474. doi: 10.1002/adhm.202301474
39. Gao S, Zhang W, Zhai X, Zhao X, Wang J, Weng J, et al. An antibacterial and proangiogenic double-layer drug-loaded microneedle patch for accelerating diabetic wound healing. Biomater Sci. (2023) 11:533–41. doi: 10.1039/D2BM01588A
40. Liu W, Zhai X, Zhao X, Cai Y, Zhang X, Xu K, et al. Multifunctional double-layer and dual drug-loaded microneedle patch promotes diabetic wound healing. Adv. Healthc Mater. (2023) 12:e2300297. doi: 10.1002/adhm.202300297
41. Ji M, Zhan F, Qiu X, Liu H, Liu X, Bu P, et al. Research progress of hydrogel microneedles in wound management. ACS Biomater Sci. Eng. (2024) 10:4771–90. doi: 10.1021/acsbiomaterials.4c00972
42. Li JY, Feng YH, He YT, Hu LF, Liang L, Zhao ZQ, et al. Thermosensitive hydrogel microneedles for controlled transdermal drug delivery. Acta Biomater. (2022) 153:308–19. doi: 10.1016/j.actbio.2022.08.061
43. Wang P, Wu J, Yang H, Liu H, Yao T, Liu C, et al. Intelligent microneedle patch with prolonged local release of hydrogen and magnesium ions for diabetic wound healing. Bioact Mater. (2023) 24:463–76. doi: 10.1016/j.bioactmat.2023.01.001
44. Wang Y, Gao B, and He B. Toward efficient wound management: bioinspired microfluidic and microneedle patch. Small. (2023) 19:e2206270. doi: 10.1002/smll.202206270
45. Zhao C, Wu Z, Pan B, Zhang R, Golestani A, Feng Z, et al. Functional biomacromolecules-based microneedle patch for the treatment of diabetic wound. Int. J. Biol. Macromol. (2024) 267:131650. doi: 10.1016/j.ijbiomac.2024.131650
46. Joorabloo A and Liu T. Smart theranostics for wound monitoring and therapy. Adv. Colloid Interface Sci. (2024) 330:103207. doi: 10.1016/j.cis.2024.103207
47. Pappachan JM, Cassidy B, Fernandez CJ, Chandrabalan V, and Yap MH. The role of artificial intelligence technology in the care of diabetic foot ulcers: the past, the present, and the future. World J. Diabetes. (2022) 13:1131–9. doi: 10.1016/j.cis.2024.103207
48. Keegan AC, Bose S, McDermott KM, Starks White MP, Stonko DP, Jeddah D, et al. Implementation of a patient-centered remote wound monitoring system for management of diabetic foot ulcers. Front. Endocrinol. (Lausanne). (2023) 14:1157518. doi: 10.3389/fendo.2023.1157518
49. Donnelly HR, Clarke ED, Collins CE, and Tehan PE. ‘Nutrition has everything to do with wound healing’-health professionals’ perceptions of assessment and management of nutrition in individuals with diabetes-related foot ulceration. Int. Wound J. (2024) 21:e14898. doi: 10.1111/iwj.14898
50. Patry J, Tourigny A, Mercier MP, and Dionne CE. Outcomes and prognosis of diabetic foot ulcers treated by an interdisciplinary team in Canada. Int. Wound J. (2021) 18:134–46. doi: 10.1111/iwj.13505
Keywords: bibliometric, diabetic wounds, treatment, prognosis, management
Citation: Wen Y and Nie K (2025) A bibliometric analysis of global research hotspots and development trends in diabetic wound treatment. Front. Clin. Diabetes Healthc. 6:1603206. doi: 10.3389/fcdhc.2025.1603206
Received: 31 March 2025; Accepted: 04 June 2025;
Published: 20 June 2025.
Edited by:
Calvin Omolo, United States International University - Africa, KenyaReviewed by:
David Nyamu, University of Nairobi, KenyaZimbwe Kauke Bakari, University of Antwerp, Belgium
Copyright © 2025 Wen and Nie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaiyu Nie, MTE0NzkwMDUyOUBxcS5jb20=
 Yin Wen
Yin Wen Kaiyu Nie
Kaiyu Nie