- 1Department of Medicinal Chemistry & amp, Quality Control, National Institute for Pharmaceutical Research and Development (NIPRD), Abuja, Nigeria
- 2Department of Community Medicine, Kaduna State University, Kaduna, Nigeria
- 3Department of Biosciences and Biotechnology, University of Medical Sciences, Ondo, Nigeria
- 4Department of Medical Laboratory Science, McPherson University, Seriki Sotayo, Ogun State, Nigeria
- 5Department of Computer Science, University of Benin, Benin, Nigeria
- 6Department of Physiology, Faculty of Basic Medical Sciences Baze University, Abuja, Nigeria
- 7Department of Biochemistry and Forensic Science, Faculty of Natural and Applied Sciences, Abiola Ajimobi Technical University, Ibadan, Nigeria
- 8Department of Human Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, Federal University Wukari, Wukari, Taraba, Nigeria
- 9Department of Epidemiology and Health Statistics, Nanjing Medical University, Nanjing, China
- 10Center for Reproduction and Population Health Studies, Nigerian Institute of Medical Research, Lagos, Nigeria
- 11Clinical Sciences Department, Nigerian Institute of Medical Research, Lagos, Nigeria
- 12Nigerian Institute of Medical Research Foundation, Lagos, Nigeria
Background: Diabetes mellitus (DM) is a major cause of morbidity and mortality globally as it is associated with long-term health complications which affect the quality of life. Several plants are used in traditional medicine to manage diabetes, with claims of efficacy from traditional healers. One such plant is Balanites aegyptiaca (L.) Delile commonly called Desert Date. This systematic review examines the therapeutic effect of B.aegyptiaca on diabetes mellitus.
Methods: The protocol for the systematic review was registered with PROSPERO (CRD42024587444). Four databases were searched for articles from 1986 to 1st August 2024. Keywords related to “therapeutic effect”, “Balanites aegyptiaca” and “diabetes mellitus” were used. Studies included were all animal models. Each article was critically appraised by two independent reviewers for their methodological quality using the Joanna Briggs Institute Case Control Checklist. The Cochrane SYRICLE Risk of bias tool was used for risk of bias assessment in these animal intervention studies. The animal experiments were conducted mainly in Alloxan- and streptozotocin-induced rat/mice diabetes and a control of non-diabetes induced rats.
Result: A total of 32 articles were included. All the studies were appraised for blood glucose levels, and a reduction in blood glucose was reported in all in vivo studies, regardless of the plant part used. Significant decrease in blood glucose level was recorded in Alloxan- and streptozotocin-induced rat/mice diabetes. All the studies reported reduced blood glucose, reduced levels of lipids, reduced weight and increased insulin production. B. aegyptiaca mitigated hyperglycaemia irrespective of the presentation form, which includes extract and meal supplementation in rodents, oral capsule intake, and tea or fruit consumption in humans. Various mechanisms, including modulation of glucose metabolizing enzymes, were reported to underlie the B. aegyptiaca antidiabetic effect.
Conclusions: Repeated administration of different parts of B. aegyptiaca in different presentation forms controlled hyperglycaemia in animal-models. A full-phase clinical trial is needed to determine the therapeutic effects of B. aegyptiaca in humans.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024587444.
1 Introduction
Diabetes mellitus (DM) is a metabolic disorder resulting either from the lack of insulin, impaired insulin action or both, eventually resulting in hyperglycemia (1). Globally, one in every 10 adults has diabetes (2). DM can be classified into type 1, type 2, gestational, hybrid and other specific types (3), with type 2 accounting for more than 90% of DM (4). It is a chronic debilitating disease associated with poor quality of life and high mortality (2, 5, 6).
The management of DM includes lifestyle modification such as exercise, dietary modification and drug therapy (7). Oral synthetic hypoglycemic drugs are effective in controlling hyperglycaemia but are associated with adverse effects such as hepatic toxicity, weight gain, abdomen enlargement and gastrointestinal discomfort (8–10). Alternative therapies, including medicinal plants, have proven efficacy with minimal side effects (11).
Several plants, especially in developing countries have been used by traditional healers for the management of different ailments. One of such plants; Balanites aegyptiaca DEL, commonly called Desert date has been used to manage patients with diabetes mellitus (12, 13). It is a deep-rooted, evergreen, semi-deciduous tree up to 12 meters high, widely distributed in Africa and across the Sahel Savannah (12, 13). It is used traditionally to manage several diseases and has been reported to exhibit several pharmacological activities, including antidiabetes, anticancer, anti-inflammatory, antioxidant, antiangiogenic and antimicrobial effects (14, 15). There have been many studies on the antidiabetic effects of the different parts of B. aegyptiaca (16–40). The aim of this systematic review is to critically appraise the available evidence on the efficacy of Balanites aegyptiaca for managing type 2 DM.
2 Methods
2.1 Protocol registration
The proposed study was registered as a systematic review with the Prospero Protocol Registration ID Number CRD42024587444 and reported according to PRISMA 2020 checklist (41), pg. 4-5]. (Supplementary 1).
2.2 Research questions
1. What proportions of previous studies reported hypoglycemic effects when Balanites aegyptiaca DEL were used in experimental animals to induce type 2 diabetes mellitus?
2. What were the other medicinal effects when Balanites aegyptiaca DEL were used in experimental animals?
2.3 Main study outcome
2.3.1 Primary outcome
Level of blood glucose post administration of the Balanites aegyptiaca DEL on experimental animals.
2.3.2 Secondary outcome
This includes other ancillary outcomes such blood insulin level, lipid profile and liver function test post-administration of the Balanites aegyptiaca DEL on experimental animals.
2.4 Search strategy
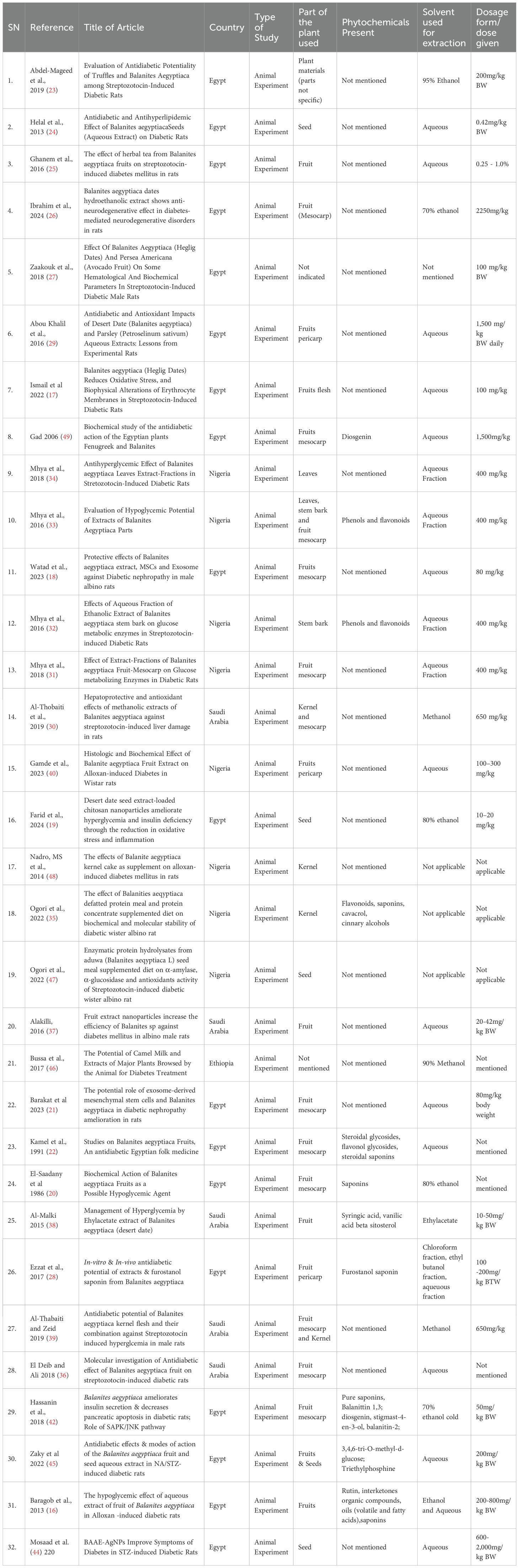
We used the population, intervention, comparison and outcome (PICO) framework to guide the search for relevant article for this systematic review (Table 1). Three databases (Web of Science, Scopus, PubMed) and Google Scholar were searched for articles from 1986 to 1st August 2024. The PubMed search strategy is presented in the Supplementary materials (Supplementary File 2). We used medical subject headings (MeSH) keywords and free text for the PubMed search. The two were combined with Boolean operators “AND” and “Or”. Keywords related to “therapeutic effect”, “Balanites aegyptiaca”, and “diabetes mellitus” were also used. Search terms include “Therapeutic effect” OR “Medicinal effect” OR “Benefits” OR “Biological activity” OR “Side effect” OR “Therapeutic biological activity” OR “Antidiabetic”, AND “Balanites aegyptiaca” OR “Desert date” AND “Diabetes mellitus” OR “Type 2 diabetes” OR “Type 1 diabetes” OR “Non-insulin-dependent diabetes” OR “Diabet*”.
2.5 Inclusion criteria
All animal studies that reported therapeutic effects of Balanites aegyptiaca DEL with experimental evidence carried out using any parts of Balanites aegyptiaca DEL with hypoglycemic indices such as reduction in plasma glucose level or hypoglycaemia were included. Research on cells lines or human subjects were not included in this systematic review.
2.6 Exclusion criteria
Studies were excluded if they were abstracts only or review articles. The titles and abstracts were used to screen the articles. The full texts that lacked primary data, well-defined methodology, duplication, missing information, or similar studies were excluded from the study. The exclusion criteria included review studies, studies that combined B. aegyptiaca with other plants, studies that examined the effects of Balanites aegyptiaca on conditions other than diabetes mellitus, studies with incomplete or missing information and duplicate publications.
2.7 Study selection and screening
We downloaded all titles and abstracts retrieved by electronic searching (Web of Science, Pubmed, Google Scholar) to the reference management software. Duplicates were removed using the Rayyan tool, and three authors (IO, AO and OA) independently screened the titles and abstracts of the articles.
We obtained the full text of potentially relevant studies. The eligible articles were retrieved and independently screened by three (3) authors (IO, AO and OA). Cohen’s Kappa screening (Excel sheet designed to screen the selected articles) was used to evaluate the inter-rater agreement between two data extractors by two authors (IO & AO) to ensure the data was void of bias and accurate. At each phase of the screening, it was ensured that there was an agreement between the three authors on the selected articles that will be included in the study, and cases of conflict were resolved by a third author (AO). We strictly followed the article selection process to answer the research questions, and to achieve the study objectives.
2.8 Data extraction
Microsoft Excel spreadsheet was used for data extraction. An Excel spreadsheet was created to organize and store the extracted data. A column was set up for each data point or variable for extraction. These include the reference, citation, journal name, title of the journal, first author, year, country, study setting, type of study, age, sex, species, number of specimens used, therapeutic effects of Balanites aegyptiaca, part of the plant used, bioactive phytochemicals, mechanism of action, dosage, primary outcome, test statistics used, study limitations/future work. Data were extracted from the full text of the included articles using the Proforma (the excel data extraction sheet) and entered into the corresponding columns. The extracted data were cross-checked to ensure consistency and accuracy.
2.9 Data cleaning
Data cleaning was done to delete any errors or duplications. We used Excel features like sorting, filtering, and grouping to manage and analyze the extracted data. We also used multiple sheets within the spreadsheet to separate data extraction, analysis, and results. Five authors (GA, IO, OO, AO and OA) participated in screening the selected full-text articles and cross-checking the extracted data.
2.10 Quality assessment
The quality of the papers included in the study were assessed by two authors (AO, OA) using the Joanna Briggs Institute Case Control Checklist. The checklist assesses the methodological quality of case-control studies based on ten questions (S3 File). Possible responses were ‘yes’, ‘no’, or ‘maybe’. We assigned a maximum score of 1 to each question, with a potential minimum score of 0 and a maximum score of 10 for each article. However, before the quality assessment, we decided not to exclude any study based on the quality rating only.
3 Results
3.1 Selection of studies
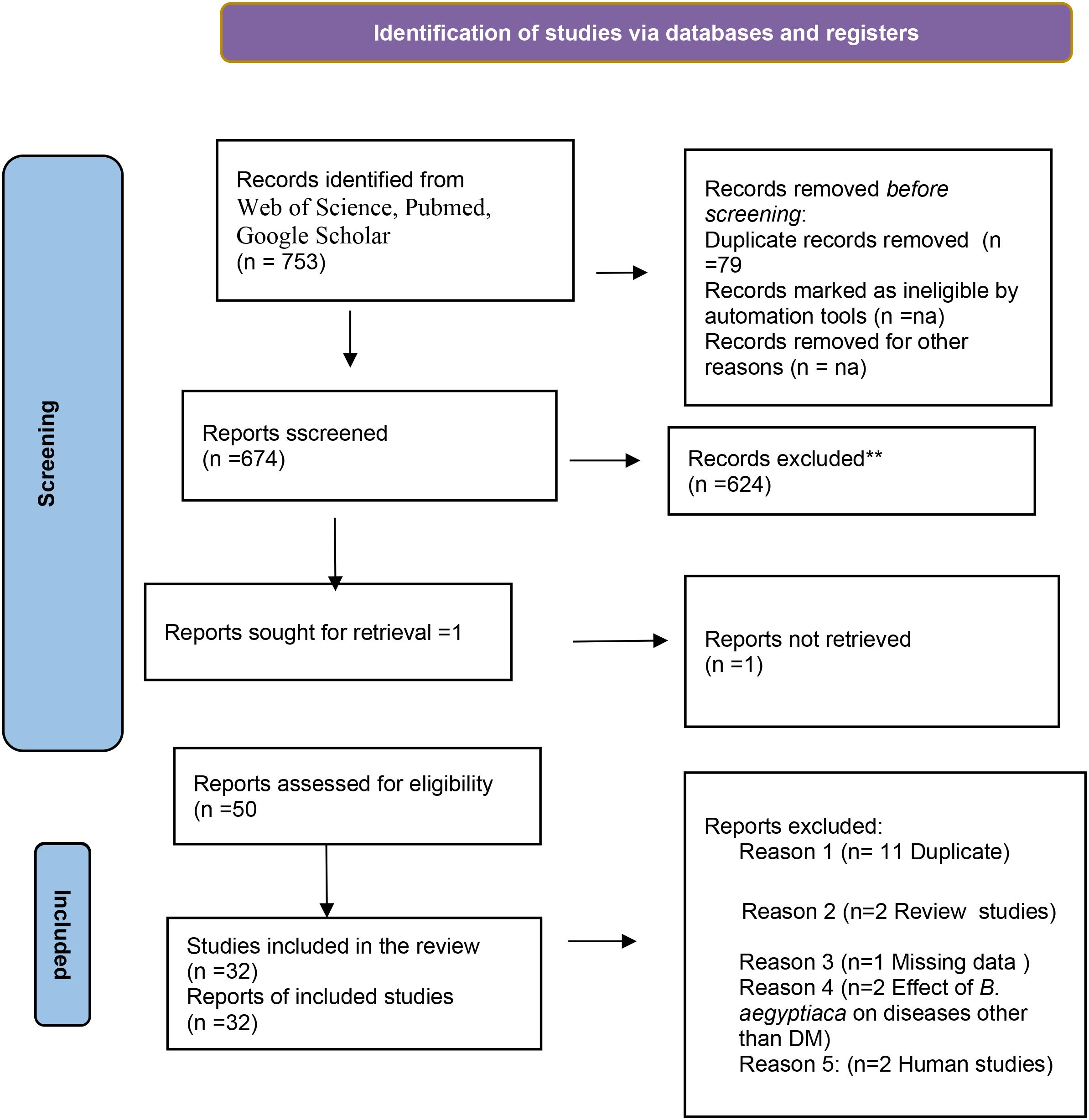
We identified 753 articles through three databases (Web of Science, PubMed and Google Scholar). After removing seventy nine (79) duplicates studies, the remaining 674 articles were screened using their abstracts and titles and 624 articles were excluded. The full text manuscript of the remaining 50 articles were obtained for full text evaluation. Eighteen of the full text manuscript were further excluded because they were either duplicates, review studies, non-retrievable manuscript, Human studies or were researches with outcome different from the study objectives. Only thirty two (32) articles were found eligible and were included in the study (Figure 1).
The PRISMA flow chart for our study is presented in Figure 1.
3.2 Narrative synthesis
All the 32 studies in this review were animal experiment, mostly involved Streptozotocin-Induced diabetic Rats and non-diabetic induced controls. The included studies were conducted across various countries in Africa and the Middle East. Eighteen studies (56.3%) from Egypt (16–29, 42–45), one (3.1%) from Ethiopia (46), eight studies (25%) from Nigeria (31–35, 40, 47, 48) and five studies (15.6%) from Saudi Arabia (30, 36–39).
In over 20 years, from 1986 to 2015, only six (18.8%) of these experimental studies on rats were conducted (16, 20, 22, 24, 38, 49). A significant rise in research activity occurred between 2016 and August 2024, during which 26 studies were published. Various parts of the Balanites aegyptiaca tree were utilized in these studies, including leaves, bark, fruits, seeds, and kernels. However, the fruit mesocarp was the most frequently used, accounting for 35% of the studies.
The predominant extraction method was aqueous extraction, although other solvents such as hydroethanol, hydromethanol, and non-polar solvents (e.g., ethyl acetate, chloroform, and hexane) were also used. Numerous phytochemicals were identified in the plant, and a few studies advanced further to isolate and characterize the active compounds responsible for the plant’s hypoglycemic effects.
In these animal studies, the dosage used to mitigate hyperglycaemia ranges from 10 - 1,500 mg/kg body weight. These were presented as capsules, raw fruit mesocarp, or infusions. For most of the studies, hypoglycemia was achieved from the 14th day of treatment, while a few observed a decrease in hyperglycaemia a few hours after the first dosage was administered. The summary of the characteristics of the included studies is presented in Table 2.
3.3 Study outcome
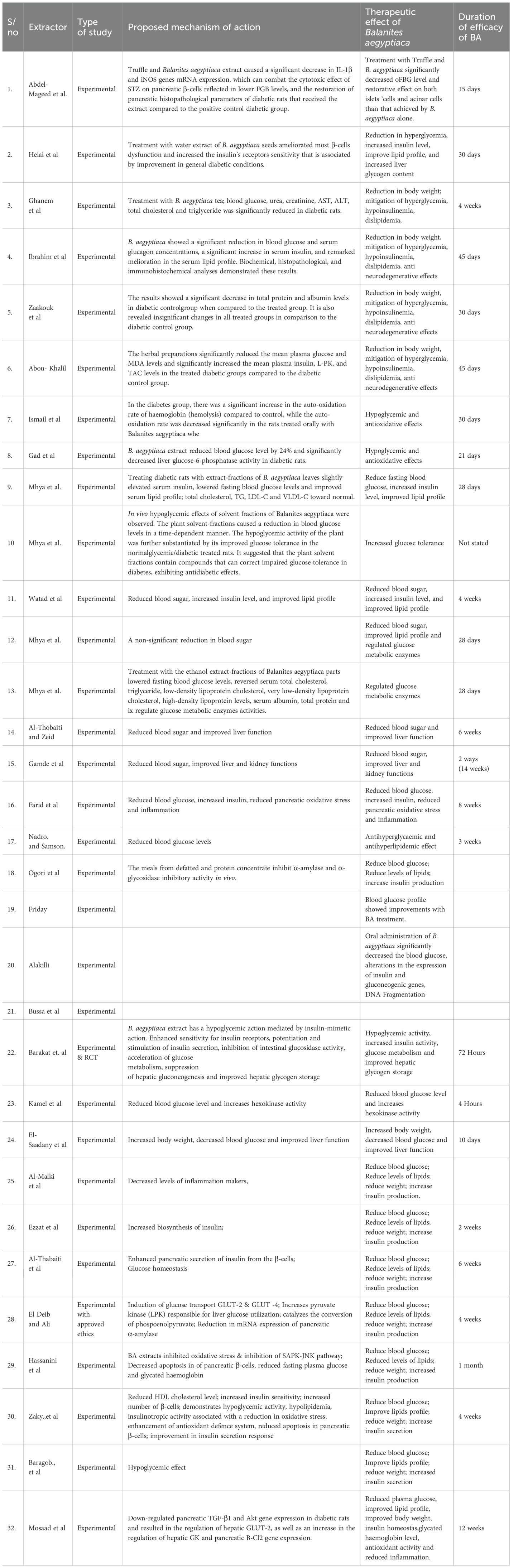
The summary of the study outcome is presented in Table 3. Treatment with B. aegyptiaca showed a significant decrease in blood glucose levels of the experimental rats induced with streptozotocin (STZ) or alloxan-induced diabetes. Aside from regulating blood glucose, other outcomes observed include improved lipid profile, increased body weight, and insulin production. These all buttressed the antidiabetic effects of B. aegyptiaca. Some studies also demonstrated that treatment with B. aegyptiaca ameliorated most of the toxic effects of Alloxan and STZ used to induce diabetes in experimental rats (42, 49, 50). Some studies showed that B. aegyptiaca fruit mitigated hyperglycaemia via inhibition of oxidative stress demonstrated by inhibition of lipid peroxidation in diabetic rats (17, 19). Three of the studies further revealed that B. aegyptiaca not only had a hypoglycemic effect in diabetic rats but also improved liver and kidney functions, which are frequently impaired in diabetes (24, 30, 40).
Three studies (36,37,39) also reported neurodegenerative effect when diabetes induced rats were treated with the B. aegyptiaca extra.
3.4 SRYCLE risk of bias assessment –
We use the Systematic Review Centre for laboratory animal experiment (SRYCLE) risk of bias assessment for the risk of bias assessment in this study.
The SYRCLE Risk of Bias (RoB) is a comprehensive framework used in the assessment of methodological quality of animal studies (51). The assessment for this study has ten domains which assessed either selection, performance, detection, attrition or a reporting bias (Table 4).
The SYRCLE RoB would adjudge low-risk for a particular domain if the answer to the domain question was “Yes”. A ‘high score’ would be allocated if the answer was “NO” The scoring would be ‘unclear risk’ if there were no sufficient information to make judgement (51).
The assessment revealed that in twenty-nine (90.6%) of the study, the allocation sequence was adequately generated and applied. Baseline Characteristics of the experimental rats were similar in all (100%) cases. Allocation concealment was reported in only 2 (6.25%) studies (38, 53). Only twenty-one (65.6%) studies reported random housing.
No study reported blinding of the investigators and random outcome assessment and blinding of the outcome assessors. There was no attrition bias, no selective outcome reporting. All the studies were apparently free of other problems that could result in high risk of bias (Figure 2).
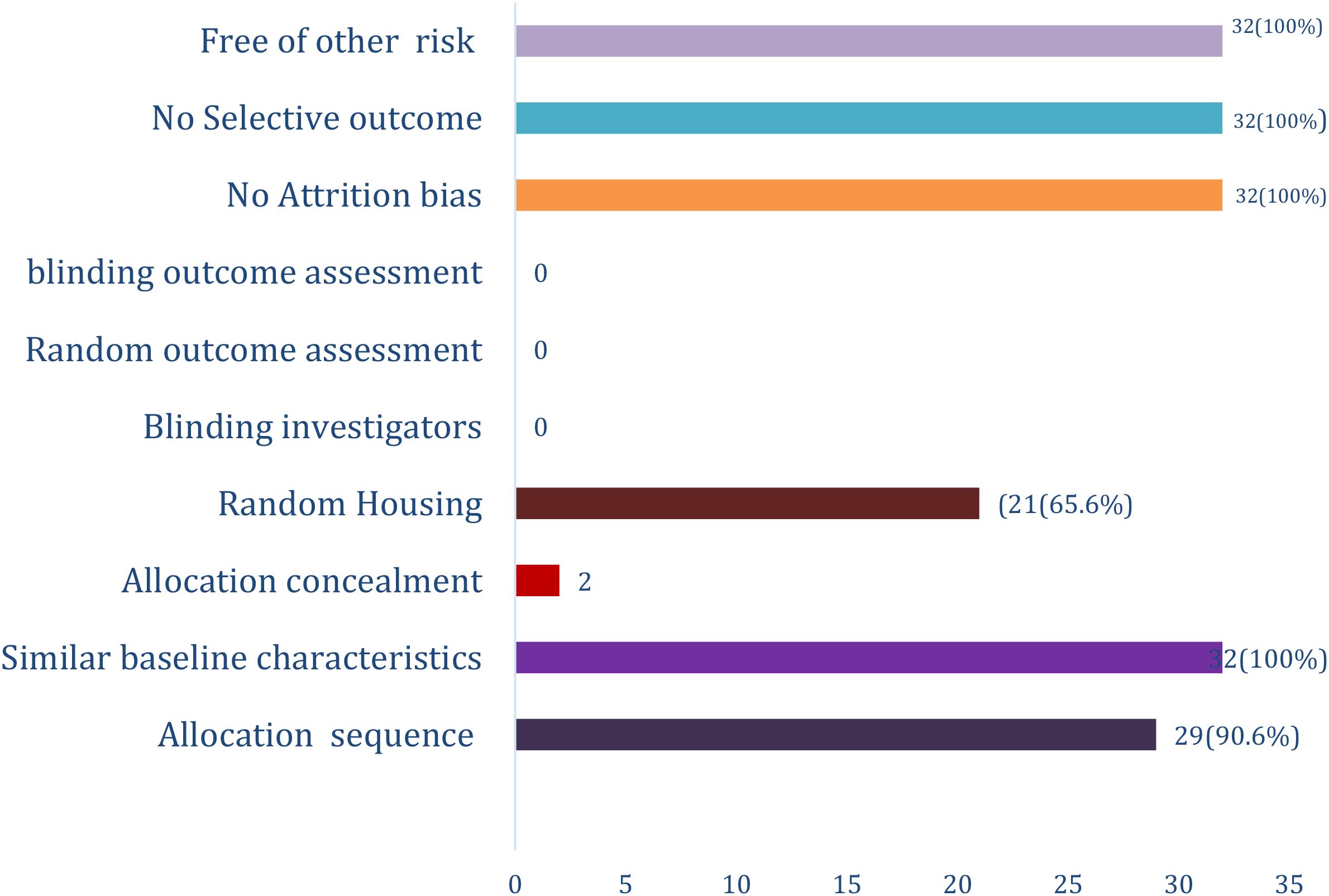
Figure 2. SYRCLE risk of bias assessment for Therapeutic Effects of Balanites aegyptiaca DEL Extract on Diabetes Mellitus: A Systematic Review.
4 Discussion
Balanites aegyptiaca, a plant widely distributed in Africa, is used in traditional African medicine to manage various diseases, including diabetes. The use of B. aegyptiaca has recently attracted significant attention from scientists, leading to an increase in the number of studies investigating its antidiabetic effects in the last test years. This systematic review shows that the use of the Balanites aegyptiaca plants for animals induced diabetes produced antidiabetic effects in all the animal studies. This preclinical study therefore showed the potential of B. aegyptiaca to mitigate diabetes mellitus in human subjects.
Over the ages, human beings have relied on plants as a source of therapeutic agents, and this has attracted scientific attention. Hence, much effort has been directed toward studying the medicinal effects of these plants and investigating the underlying mechanisms and bioactive phytochemicals responsible for the observed medicinal. Our findings shows that a significant hypoglycemic effect was recorded in both Alloxan and STZ-induced diabetes. Other antidiabetic-related effects observed with B. aegyptiaca include improved lipid profile (24–29, 32–35, 39, 45), increased insulin production (16, 19, 21, 22, 24, 38, 39, 42, 44), changes in serum protein and increased glycogen content (21). Also of interest is that virtually all parts of the plants- the seed kernel, fruit mesocarp, leaf, and stem barks from the tree showed a hypoglycemic effect. However, a study comparing the activity of stem bark, leaf, and fruit mesocarp reported higher activity for the leaf and fruit mesocarp than the stem (33).
The medicinal effects of plants have been attributed to their phytochemical constituents, which can modulate different targets and pathways (52, 53–55). Various phytochemicals were identified in this plant using different spectrometry methods, which have identified various phytochemicals, showing that the plant is rich in phytochemicals (50). Specific flavonoids and saponin compounds were isolated from the plant. The flavonoids were inactive, while the saponins showed antidiabetic effects. The saponin compounds namely; 26-O-beta-D-glucopyranosyl-(25R)-furost-5-ene-377beta,22,26-triol 3-O-[alpha-L-rhamnopyranosyl-(1—2)]-[beta-D-xylopyranosyl-(1—3)]-[alpha-L-rhamnopyranosyl-(1—4)]-beta-D-glucopyranoside, 26-O-beta-D-glucopyranosyl-(25R)-furost-5-ene-beta,22,26-triol 3-O-(2,4-di-O-alpha-L-rhamnopyranosyl)-beta-D-glucopyranoside, 26-(O-β-d-glucopyranosyl)-22-O-methylfurost-5-ene-3β,26-diol-3-O-β-d-glucopyranosyl-(1 → 4)-[α-l-rhamnopyranosyl-(1 → 2)]-β-d-glucopyranoside individually exhibited lower antidiabetic effect compared to the active fraction (22, 42). However, when the saponin compounds were combined, the combined saponin showed a greater antidiabetic effect (22). This implies that the pooled saponin in the plant is responsible for its antidiabetic effect as the individual saponins either work synergistically or additively to produce the antidiabetic effect.
Studies have also investigated the mechanisms underlying the antidiabetic effects of B aegyptiaca. Reported mechanisms include antioxidant (17, 21, 22, 25, 29, 35, 38, 44, 47, 50), anti-inflammatory (22, 23, 38, 44), regulation of glucose/carbohydrate metabolism (32, 33, 49, 56–58), mitigation of insulin resistance (50), inhibition of pancreatic α-amylase (35, 36, 47) and inhibition intestinal α-glycosidase (35, 47), modulation of glucose transporter (36, 44) and apoptosis (44).
We observed in this study that there was low risk of bias for allocation sequence, similarity of the animals at baseline, attrition bias, random housing, selective outcome and other risk of bias There were however High risk of bias noted for blinding and random outcome assessment and blinding of the investigators. This risk of bias assessment tool gave a fair critical appraisal of evidence from this animal studies (45, 59).
4.1 Strengths of the study
This systematic review utilized an extensive search strategy of the database sources.
The inclusion and exclusion criteria were also clearly pre- defined. The PRISMA flow diagram reveals the rigorous process for the article selection, it shows that the methodology were reproducible, ensures standardization, transparency and clarity of the data generation process. The SYRCLE risk of bias specifically applies to animal studies, helps to improve study validity and the research quality.
4.2 Limitation of the study
Geographical spread of the study was limited; they were skewed to few countries in Africa and Asia.
5 Conclusion
The study found that B. agyptiaca had antidiabetic effects in the experimental animals. All parts of the plants showed antidiabetic effect, but the leaves and fruit showed superior activity. The plant worked by modulating different targets and pathways. Therefore, clinical studies on the antidiabetic effect of B. aegyptiaca in humans are recommended to determine the antidiabetic effects and the usefulness of the B. agyptiaca as a therapeutic agent in human with diabetes mellitus.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
OO: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. AO: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing, Formal analysis, Investigation, Supervision, Validation. IO: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AO: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. GA: Data curation, Formal analysis, Visualization, Writing – review & editing. GA-G: Formal analysis, Methodology, Visualization, Writing – review & editing. PA: Formal analysis, Methodology, Validation, Writing – review & editing. LA: Formal analysis, Methodology, Validation, Visualization, Writing – review & editing. OA: Formal analysis, Methodology, Project administration, Supervision, Writing – review & editing. FA: Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. OS: Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding support for the publication of this systematic review was from the Nigerian Institute of Medical Research Foundation (Grant Number NF-GMTP-24-152809).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2025.1651789/full#supplementary-material
References
1. Chen H, Nie Q, Hu J, Huang X, Huang W, and Nie S. Metabolism amelioration of Dendrobium officinale polysaccharide on type II diabetic rats. Food Hydrocoll. (2020) 102:105582. doi: 10.1016/J.FOODHYD.2019.105582
2. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. (2022) 183:109119. doi: 10.1016/J.DIABRES.2021.109119
3. Antar SA, Ashour NA, Sharaky M, Khattab M, Ashour NA, Zaid RT, et al. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. BioMed. Pharmacother. (2023) 168:115734. doi: 10.1016/J.BIOPHA.2023.115734
4. Green A, Hede SM, Patterson CC, Wild SH, Imperatore G, Roglic G, et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia. (2021) 64:2741–50. doi: 10.1007/S00125-021-05571-8/FIGURES/3
5. Kumar A, Gangwar R, Ahmad Zargar A, Kumar R, and Sharma A. Prevalence of diabetes in India: A review of IDF diabetes atlas 10th edition. Curr. Diabetes Rev. (2023) 20(1):105–14. doi: 10.2174/1573399819666230413094200
6. Oster H and Chaves I. Effects of healthy lifestyles on chronic diseases: diet, sleep and exercise. Nutr. (2023) 15:4627. doi: 10.3390/NU15214627
7. Association AD. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. (2019) 42:S46–60. doi: 10.2337/DC19-S005
8. Akhter MS and Uppal P. Toxicity of metformin and hypoglycemic therapies. Adv. Chronic Kidney Dis. (2020) 27:18–30. doi: 10.1053/J.ACKD.2019.08.004
9. Rowden AK and Fasano CJ. Emergency management of oral hypoglycemic drug toxicity. Emerg. Med. Clin. North Am. (2007) 25:347–56. doi: 10.1016/J.EMC.2007.02.010
10. Aldobeaban S, Mzahim B, and Alshehri AA. Recurrent hypoglycemia secondary to metformin toxicity in the absence of co-ingestions: A case report. J. Med. Case Rep. (2018) 12:1–5. doi: 10.1186/S13256-018-1758-0/FIGURES/1
11. Mohammed A. Hypoglycemic potential of african medicinal plants in diabetic and non-diabetic human subjects: A review. Clin. Complement Med. Pharmacol. (2023) 3:100081. doi: 10.1016/J.CCMP.2023.100081
12. Mint Abdelaziz S, Medraoui L, Alami M, Pakhrou O, Makkaoui M, Ould Mohamed Salem Boukhary A, et al. Inter simple sequence repeat markers to assess genetic diversity of the desert date (Balanites aEgyptiaca Del.) for Sahelian ecosystem restoration. Sci. Rep. (2020) 10:1–8. doi: 10.1038/s41598-020-71835-9
13. El-Banhawy AA, Ellmouni FY, Al-Juhani W, Hamada FA, El-Banhawy A, Ellmouni FY, et al. Comparative taxonomic study of balanites aEgyptiaca (L.) delile (Zygophyllaceae). Biol. Life Sci. Forum. (2021) 11:72. doi: 10.3390/IECPS2021-12060
14. Gautam SK, Paul RK, Sawant DM, Sarwal A, and Raza K. Critical review on balanites aEgyptiaca delile: phytoconstituents, pharmacological properties and nanointerventions. Chin. J. Integr. Med. (2024) 30:653–63. doi: 10.1007/S11655-023-3563-X/METRICS
15. Zein N, Yassin F G, and Ayoub H. An overview of the phytochemical compon ents, pharmacological action, and therapeutic applications of Balanites aEgyptiaca Del (desert date). Biochem. Lett. (2024) 0:0–0. doi: 10.21608/BLJ.2024.268748.1054
16. Baragob AE, Almalki W, Shahid I, Bakhdhar F, Bafhaid H, and Eldeen OM. The hypoglycemic effect of the aqueous extract of the fruits of Balanites aEgypticea in Alloxan-induced diabetic rats. Pharmacognosy Res. (2014) 6:1. doi: 10.4103/0974-8490.122909
17. Ismail NM. Balanites aEgyptiaca (Heglig dates) reduces oxidative stress and biophysical alterations of erythrocyte membranes in streptozotocin-induced diabetic rats. J. Adv. Pharm. Res. (2022) 6:78–83. doi: 10.21608/APRH.2022.125054.1151
18. Watad SH, Barakat N, Nassr A, and Zahran F. Protective effects of Balanites aEgyptiaca extract, MSCs and Exosome against Diabetic nephropathy induced in male albino rats. Biochem. Lett. (2023) 19:64–77. doi: 10.21608/BLJ.2023.314430
19. Farid A, Ahmed A, Alaa O, and Safwat G. Desert date seed extract-loaded chitosan nanoparticles ameliorate hyperglycemia and insulin deficiency through the reduction in oxidative stress and inflammation. Sci. Rep. (2024) 141:2024. doi: 10.1038/s41598-024-56352-3
20. El-Saadany SS, Abdel-Rahim EA, and Wasif MM. Biochemical action of Balanites aEgyptiaca fruits as a possible hypoglycemic agent. Food Chem. (1986) 19:307–15. doi: 10.1016/0308-8146(86)90054-3
21. Barakat N, Ali M, Nassr A, and Zahran F. The potential role of exosome-derived mesenchymal stem cells and Balanites aEgyptiaca in diabetic nephropathy amelioration in rats. Cell Mol. Biol. (Noisy-le-grand). (2023) 69:37–44. doi: 10.14715/CMB/2023.69.2.7
22. Kamel MS, Ali AA, Assaf MH, El-Shanawany MA, Ohtani K, Kurokawa T, et al. Studies on Balanites aEgyptiaca fruits, an antidiabetic Egyptian folk medicine. Chem. Pharm. Bull. (Tokyo). (1991) 39:1229–33. doi: 10.1248/CPB.39.1229
23. Abdel-Mageed AM, Osman AKE, Awad NS, and Abdein MA. Evaluation of Antidiabetic Potentiality of Truffles and Balanites AEgyptiaca among Streptozotocin Induced Diabetic Rats. Int. J. Pharm. Res. Allied Sci. (2019) 8:36–44. Available online at: https://www.researchgate.net/publication/337856579_Evaluation_of_Antidiabetic_Potentiality_of_Truffles_and_Balanites_Aegyptiaca_among_Streptozotocin_Induced_Diabetic_Rats (Accessed August 24, 2024).
24. Helal EGE, Abd El-Wahab SM, El Refaey H, and Mohammad AA. Antidiabetic and antihyperlipidemic effect of balanites aEgyptiaca seeds (Aqueous extract) on diabetic rats. Egypt J. Hosp Med. (2013) 52:725–39. doi: 10.12816/0000610
25. Ghanem KZ, Ghanem HZ, Ramadan M, and Mabrok J. The effect of herbal tea from Balanites aEgyptiaca fruits on streptozotocin-induced diabetes mellitus in rats. Int. J. PharmTech Res. (2015) 9:8–15. Available online at: https://www.researchgate.net/publication/311594988_The_effect_of_herbal_tea_from_Balanites_aegyptiaca_fruits_on_streptozotocin-induced_diabetes_mellitus_in_rats (Accessed August 24, 2024).
26. Ibrahim AY, Shaffie NM, and El-Newary SA. Balanites aEgyptiaca dates hydroethanolic extract shows anti-neurodegenerative effect in diabetes-mediated neurodegenerative disorders in rats. Egypt J. Chem. (2024) 67:559–76. doi: 10.21608/EJCHEM.2023.231893.8501
27. Zaakouk SA, Rasheid HGA el, Belal A, and Elfeky KS. Effect of balanites aEgyptiaca (Heglig dates) and persea americana (Avocado fruit) on some hematological and biochemical parameters in streptozotocin induced diabetic male rats. Al-Azhar Bull. Sci. (2018) 29:49–59. doi: 10.21608/ABSB.2018.33818
28. Ezzat SM, Motaal AA, and El Awdan SAW. In vitro and in vivo antidiabetic potential of extracts and a furostanol saponin from Balanites aEgyptiaca. Pharm. Biol. (2017) 55:1931–6. doi: 10.1080/13880209.2017.1343358
29. Abou Khalil NS, Abou-Elhamd AS, Wasfy SIA, El Mileegy IMH, Hamed MY, and Ageely HM. Antidiabetic and Antioxidant Impacts of Desert Date (Balanites aEgyptiaca) and Parsley (Petroselinum sativum) Aqueous Extracts: Lessons from Experimental Rats. J. Diabetes Res. (2016) 2016. doi: 10.1155/2016/8408326
30. Al-Thobaiti SA and Zeid IMA. Hepatoprotective and antioxidant effects of methanolic extracts of Balanites aEgyptiaca against streptozotocin-induced liver damage in rats. J. Appl. Sci. Res. (2019) 15:13–27. doi: 10.22587/JASR.2019.15.6.2
31. Mhya D, Alegbejo J, Anigo K, and Umar I. Effect of extract-fractions of balanites aEgyptiaca fruit-mesocarp on glucose metabolizing enzymes in diabetic rats. World Scientific News 2018 (2018) 108:180–94.
32. Mhya DH, Anigo KM, Umar IA, and Alegbejo JO. Effects of aqueous fraction of ethanolic extract of balanites aEgyptiaca stem-bark on glucose metabolic enzymes in streptozotocin-induced diabetic rats. Int. J. Biochem. Res. Rev. (2016) 13:1–12. doi: 10.9734/IJBCRR/2016/27598
33. Anigo MD, Umar K, and Alegbejo N. Evaluation of hypoglycemic potential of extracts of balanites aEgyptiaca parts(2016). Available online at: www.ijiras.com (Accessed August 24, 2024).
34. Mhya DH, Anigo KM, Umar IA, and Alegbejo JO. Antihyperglycemic effect of balanites aEgyptiaca leaves extract-fractions in streptozotocin-induced diabetic rats. J. Complement Altern. Med. Res. (2018) 6:1–12. doi: 10.9734/JOCAMR/2018/41997
35. Ogori AF, Abraham GT, Eke MO, Abu JO, Adeniyi AS, and Famuwagun AA. The effect of balanities aeqyptiaca defatted protein meal and protein concentrate supplemented diet on biochemical and molecular stability of diabetic wister albino rat. BioMed. Pharmacother. (2022) 153:113510. doi: 10.1016/J.BIOPHA.2022.113510
36. El Deib MM and Ali HA. Molecular investigation of antidiabetic effect of balanites aEgyptiaca fruits in streptozotocin-induced diabetic rats. Slov Vet. Res. (2018) 55:137–45. doi: 10.26873/SVR-638-2018
37. Alakilli SYM. Fruit extract nanoparticles increase the efficiency of Balanites sp against diabetes mellitus in albino male rats. Int. J. Adv. Res. (2016) 4:1493–506. Available online at: https://www.academia.edu/22836814/Fruit_extract_nanoparticles_increase_the_efficiency_of_Balanites_sp_against_diabetes_mellitus_in_albino_male_rats (Accessed August 24, 2024).
38. Al-Malki AL, Barbour EK, Abulnaja KO, and Moselhy SS. Management of hyperglycaemia by ethyl acetate extract of balanites aEgyptiaca (Desert date). Molecules. (2015) 20:14425–34. doi: 10.3390/MOLECULES200814425
39. Al-Thobaiti SA and Zeid IMA. Antidiabetic potential of Balanites AEgyptiaca kernel, flesh and their combination against streptozotocin-induced hyperglycemia in male rats. Trop. J. Pharm. Res. (2019) 18:263–71. Available online at: https://www.researchgate.net/publication/331738250_Antidiabetic_potential_of_Balanites_Aegyptiaca_kernel_flesh_and_their_combination_against_streptozotocin-induced_hyperglycemia_in_male_rats (Accessed August 24, 2024).
40. Gamde SM, Ugwah-Oguejiofor CJ, Garba A, Avwioro GO, Moronkeji A, and Jimoh AA. Histologic and biochemical effect of balanite aEgyptiaca fruit extract on alloxan-induced diabetes in wistar rats. Ethiop J. Health Sci. (2023) 33:441. doi: 10.4314/EJHS.V33I3.7
41. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. bmj. (2021) 372. doi: 10.1136/bmj.n71
42. Hassanin KMA, Mahmoud MO, Hassan HM, Abdel-Razik ARH, Aziz LN, and Rateb ME. Balanites aEgyptiaca ameliorates insulin secretion and decreases pancreatic apoptosis in diabetic rats: Role of SAPK/JNK pathway. BioMed. Pharmacother. (2018) 102:1084–91. doi: 10.1016/J.BIOPHA.2018.03.167
43. Al-Ashaal HAAHS. Antidiabetic, cytotoxic, antioxidant and antitremato dal medicinal efficacy of polar and non-polar phytochemicals of Balanites aEgyptiaca Del. Asian Pacific J. Trop. Dis. (2009) 7:797–805. doi: 10.12980/APJTD.7.2017D7-128
44. Mosaad YO, Hussein MA, Ateyya H, Hassan SA, Wink M, Gobba NAEK, et al. BAAE-agNPs improve symptoms of diabetes in STZ-induced diabetic rats. Curr. Pharm. Biotechnol. (2023) 24:1812–26. doi: 10.2174/1389201024666230313105049
45. Zhang W, Jiang Y, Shang Z, Zhang N, Tao G, Zhang T, et al. The methodological quality of animal studies: A cross-sectional study based on the SYRCLE’s risk of bias tool. bioRxiv. (2019) 12:701110. doi: 10.1101/701110
46. Bussa N, Belayneh A, and Deressa M. The potential of camel milk and extracts of major plants browsed by the animal for diabetes treatment. East Afr. J. Sci. (2017) 11:129–38. Available online at: https://www.ajol.info/index.php/eajsci/article/view/183089 (Accessed August 24, 2024).
47. Ogori FA, Girgih TA, Eke MO, Abu JO, Famuwagun AA, and Adefegah SA. Enzymatic protein hydrolysates from aduwa (Balanities Aeqyptiaca. Afr. J. Food Sci. Technol. (2022) 13. Available online at: https://www.interesjournals.org/articles/enzymatic-protein-hydrolysates-from-aduwa-balanities-aeqyptiaca-l-seed-meal-supplemented-diet-on-alphaamylase-alphaglucosidase-and-88945.html.
48. Nadro MS and Samson FP. The effects of Balanite aEgyptiaca kernel cake as supplement on alloxan-induced diabetes mellitus in rats. J. Appl. Pharm. Sci. (2014) 4:: 058–061. doi: 10.7324/JAPS.2014.401011
49. Gad MZ, El-Sawalhi MM, Ismail MF, and El-Tanbouly ND. Biochemical study Of the antidiabetic action of the Egyptian plants fenugreek and balan ites. Mol. Cell Biochem. (2006) 281:173–83. doi: 10.1007/S11010-006-0996-4/METRICS
50. Zaky AS, Kandeil M, Abdel-Gabbar M, Fahmy EM, Almehmadi MM, Ali TM, et al. The antidiabetic effects and modes of action of the balanites aEgyptiaca fruit and seed aqueous extracts in NA/STZ-induced diabetic rats. Pharmaceutics. (2022) 14(2):263. doi: 10.3390/PHARMACEUTICS14020263
51. Hooijmans CR, Rovers MM, De Vries RB, Leenaars M, Ritskes-Hoitinga M, and Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. methodology. (2014) 14:43. doi: 10.1186/1471-2288-14-43
52. Oyeyemi IT, Adewole KE, and Gyebi GA. In silico prediction of the possible antidiabetic and anti-inflammatory targets of Nymphaea lotus-derived phytochemicals and mechanistic insights by molecular dynamics simulations. J. Biomol Struct. Dyn. (2023) 41:12225–41. doi: 10.1080/07391102.2023.2166591
53. Gaber KE, Singhal U, and Daowd O. Hypoglycemic and hypolipidaemic effects of some common plants extract in type 2 diabetic patients at Eldabba area (North Sudan). IOSR J. Pharm. Biol. Sci. (2013) 8:38–43. Available online at: www.iosrjournals.org (Accessed August 24, 2024).
54. Abubakar HA. Impact of Balanites aEgyptiaca (Desert Date) fruit consumption on metabolic syndrome markers in diabetic patients attending Murtala Muhammad Specialist Hospital Kano, Nigeria. BJMLS. (2017). Available online at: https://www.academia.edu/104839032/IMPACT_OF_BALANITES_AEGYPTIACA_DESERT_DATE_FRUITCONSUMPTION_ON_METABOLIC_SYNDROME_MARKERS_IN_DIABETIC_PATIENTS_ATTENDING_MURTALA_MUHAMMAD_SPECIALIST_HOSPITAL_KANO_NIGERIA.
55. Abdelmalek B, Abdelhakim E, and Boussaid M. République Algérienne Démocratique et populaire Ministère de l’enseignement supérieur et de la recherche scientifique تأثير األشعة الكهروضىئية الخاليا تدهىر على الكىنية Etude de l’effet des rayons cosmiques sur la dégradation des cellu (2018). Available online at: https://www.researchgate.net/publication/375520561_Republique_Algerienne_Democratique_et_populaire_Ministere_de_l%27enseignement_superieur_et_de_la_recherche_scientifique_tathyr_aalsht_alkhrwdyyyt_alkhalya_tdhyr_ly_alkynyt_Etude_de_l%27effet_des_rayons_co (Accessed August 24, 2024).
56. Karole R, Tsatsop T, Djiobie Tchienou G, Rodrigue Talla E, Martial S, Chendjou S, et al. Spray Drying of Fruits Juice Formulations of Ananas cosmosus L. and Balanites aEgyptiaca: Antihyperglycemic Activity. Curr. J. Appl. Sci. Technol. (2024) 43:61–8. doi: 10.9734/cjast/2024/v43i74406
57. Motaal AA, Shaker S, and Haddad PS. Antidiabetic Activity of Standardized Extracts of Balanites aEgyptiaca Fruits using Cell-based Bioassays. Pharmacogn J. (2012) 4:20–4. doi: 10.5530/PJ.2012.30.4
58. Bhardwaj M, Yadav P, Yadav M, Chahal J, Dalal S, and Kataria SK. Phytochemical screening and antidiabetic efficacy of balanites aEgyptiaca seed extract and their silver nanoparticles on muscle and pancreatic cell lines. ACS omega. (2024) 9:22660–76. doi: 10.1021/ACSOMEGA.4C00327
Keywords: Balanites aegyptiaca, diabetes mellitus, therapeutics effects, systematic review, animal experiment
Citation: Odeniran OA, Oyefabi AM, Oyeyemi IT, Oke AA, Aziken G, Adebayo-Gege G, Adegbola PI, Adedayo LD, Abodunrin OR, Akinsolu FT and Sobande OO (2025) Therapeutic effects of Balanites aegyptiaca DEL extract on diabetes mellitus: a systematic review. Front. Clin. Diabetes Healthc. 6:1651789. doi: 10.3389/fcdhc.2025.1651789
Received: 22 June 2025; Accepted: 15 August 2025;
Published: 02 September 2025.
Edited by:
Dimitrios Patoulias, Aristotle University of Thessaloniki, GreeceReviewed by:
Himansu Bhusan Samal, Centurion University, IndiaMarcos Roberto Brasil, Centro Universitário Guairacá - UNIGUAIRACÁ, Brazil
Copyright © 2025 Odeniran, Oyefabi, Oyeyemi, Oke, Aziken, Adebayo-Gege, Adegbola, Adedayo, Abodunrin, Akinsolu and Sobande. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adegboyega Moses Oyefabi, YWRlZ2JveWVnYS5veWVmYWJpQGthc3UuZWR1Lm5n
†ORCID: Olubukola Adebisi Odeniran, orcid.org/0000-0003-4565-3681
Grace Aziken, orcid.org/0000-0002-8771-4991
Lawrence Dayo Adedayo, orcid.org/0000-0002-2686-9009
 Olubukola Adebisi Odeniran
Olubukola Adebisi Odeniran Adegboyega Moses Oyefabi
Adegboyega Moses Oyefabi Ifeoluwa Temitayo Oyeyemi
Ifeoluwa Temitayo Oyeyemi Adewale Adegboyega Oke
Adewale Adegboyega Oke Grace Aziken
Grace Aziken Grace Adebayo-Gege
Grace Adebayo-Gege Peter Ifeoluwa Adegbola
Peter Ifeoluwa Adegbola Lawrence Dayo Adedayo
Lawrence Dayo Adedayo Olunike Rebecca Abodunrin
Olunike Rebecca Abodunrin Folahanmi Tomiwa Akinsolu
Folahanmi Tomiwa Akinsolu Olajide Odunayo Sobande
Olajide Odunayo Sobande