- 1Department of Diabetology and Endocrinology, National Hospital Organization (NHO) Okayama Medical Center, Okayama, Japan
- 2Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Faculty of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
- 3Department of Obstetrics and Gynecology, Okayama University Faculty of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
Introduction: The prevalence of gestational diabetes mellitus (GDM) is significantly increasing. Hyperglycaemia and dyslipidaemia have been demonstrated to contribute to endothelial dysfunction linked to foetal–placental circulation. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) is crucial for the lipolytic processing of TG-rich lipoproteins through the anchoring of lipoprotein lipase (LPL). In this study, circulating GPIHBP1 levels during pregnancy were evaluated, and their associations with hypertriglyceridaemia and the perinatal outcomes of GDM were evaluated.
Methods: This study included 12 pregnant women with GDM and 21 pregnant women with normal glucose tolerance (NGT).
Results: No significant differences in obstetrical outcomes were detected between the two groups. In participants with NGT, circulating GPIHBP1 levels were markedly lower in the 3rd trimester than in the 2nd trimester and at delivery. In women with GDM, circulating GPIHBP1 levels were unchanged during the 3rd trimester, and circulating GPIHBP1 levels throughout the 3rd trimester were negatively correlated with neonatal birth weight percentile and umbilical venous pO2 (ρ=-0.636, p=0.026; ρ=-0.657, p=0.020).
Discussion: Our findings suggest a possible association between circulating GPIHBP1 levels and perinatal outcomes in patients with GDM.
Introduction
The current prevalence of gestational diabetes mellitus (GDM), which poses a significantly increased risk for perinatal complications, is notably high at 14% (1). Prepregnancy overweight or obesity and advanced maternal age have been identified as significant risk factors for GDM (2, 3). Foetal growth is influenced by maternal factors, including GDM, prepregnancy BMI, maternal age, and parity (4–6), and GDM is a well-established risk factor for large for gestational age (LGA) neonates, as affected women exhibit a 2.83-fold greater risk than those with normal glucose tolerance (7). Recent findings indicate that maternal glycaemic levels are not the sole risk factor for foetal overgrowth in cases of obesity and GDM (8). Certain studies have revealed an association between maternal blood triglyceride (TG) levels and neonatal weight, although no such correlation has been observed with maternal plasma glucose levels (9). In GDM, maternal blood TG levels are increased in early pregnancy compared with those in mothers without GDM and remain elevated throughout gestation, contributing to increased foetal subcutaneous fat mass and adiposity; maternal blood TG levels are also linked to foetal overgrowth (10, 11). Hyperglycaemia and dyslipidaemia have also been shown to contribute to the endothelial dysfunction associated with foetal–placental circulation (12–14).
Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1) is essential for the lipolytic processing of TG-rich lipoproteins (TRLs) because it anchors lipoprotein lipase (LPL) to the abluminal surface of blood capillaries, thereby stabilising its structure and facilitating its transport to the capillary lumen. GPIHBP1-anchored LPLs are crucial for the margination of TRLs within capillaries, which facilitates the process of lipolysis (15). Mutations in GPIHBP1 have been associated with severe hypertriglyceridaemia, which results in an increased risk of acute pancreatitis, underscoring the importance of GPIHBP1 in intravascular TG processing (16). During pregnancy, women with GPIHBP1 mutations exhibit high TG levels, particularly in the third trimester, which leads to severe pancreatitis and postnatal problems, including foetal distress (17, 18). Nonetheless, the relationships among circulating GPIHBP1 levels, dyslipidaemia, and maternal and foetal complications during GDM remain largely unexplored. This study assessed circulating GPIHBP1 levels during pregnancy and investigated their associations with hypertriglyceridaemia and perinatal outcomes in cases of GDM.
Materials and methods
Study participants
This prospective study included a cohort of 33 pregnant women recruited from the 26th of November, 2019, to the 31st of March, 2023. Participants were recruited from Okayama University Hospital, and GDM (n=12) was diagnosed using the 75 g oral glucose tolerance test (75 g OGTT) in accordance with the diagnostic criteria established by the International Association of Diabetes and Pregnancy Study Group (IADPSG) (19). Individuals with normal glucose tolerance (NGT, n=21) are defined as those who exhibit postprandial glucose levels below 100 mg/dL during screening tests or those who do not meet the diagnostic criteria for GDM after a 75 g OGTT. The exclusion criteria were as follows (1): multiple pregnancies (2); overt diabetes during pregnancy; and (3) preexisting type 1 or type 2 diabetes mellitus. Written informed consent was obtained from all participants. The study protocol received approval from the Ethics Committee of Okayama University (1910–015) and was executed in compliance with the Declaration of Helsinki.
Data collection
Blood samples were collected after a 12-hour fast. Serum TG, HbA1c and glucose levels were quantified within one hour after blood collection by conventional methods using an automated clinical chemistry analyser (JCA-BM8040G; JEOL, Ltd., Tokyo, Japan). Serum samples were promptly frozen and stored at the Okayama University Hospital Biobank (Okadai Biobank) prior to the assessment of the other parameters using a GPIHBP1 (Immuno-Biological Laboratories [IBL]) enzyme-linked immunosorbent assay (ELISA) kits, as previously described (20).
Body mass index (BMI) was calculated using the following formula: body weight (kg)/height2 (m2). Systolic blood pressure was the median blood pressure recorded during the patient’s 5-day post-partum hospital stay. Medical history and current prescription information were extracted from each patient’s medical records.
Histopathological examination of the placenta
The placenta was histologically examined by an experienced perinatal pathologist. Placental tissue samples were sliced into blocks of four μm, fixed in formalin, embedded in paraffin and stained with haematoxylin and eosin.
Statistical analysis
Continuous variables are presented as the median (interquartile range: IQR), while categorical variables are expressed as absolute numbers or percentages. Differences between two groups in each separate experiment were analysed using Student’s t test, the nonparametric Mann–Whitney test, or the χ2 test. The Wilcoxon signed-rank test was employed to assess disparities between paired datasets. Spearman’s rank correlation was used to determine correlation coefficients. All the statistical analyses were conducted with SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA). P values < 0.05 were considered to indicate statistical significance.
Post hoc power analysis
To assess the reliability of the correlations in the GDM group (n = 12), post hoc power was calculated using G*Power 3.1.9.7 (Bivariate normal model, Exact test). In terms of the correlation between circulating GPIHBP1 levels and the neonatal birthweight percentile (ρ = -0.636) and between circulating GPIHBP1 levels and the umbilical venous pO2 level (ρ = -0.657), the observed power was approximately 0.65 and 0.70, respectively.
Results
Baseline characteristics and comparisons between study groups
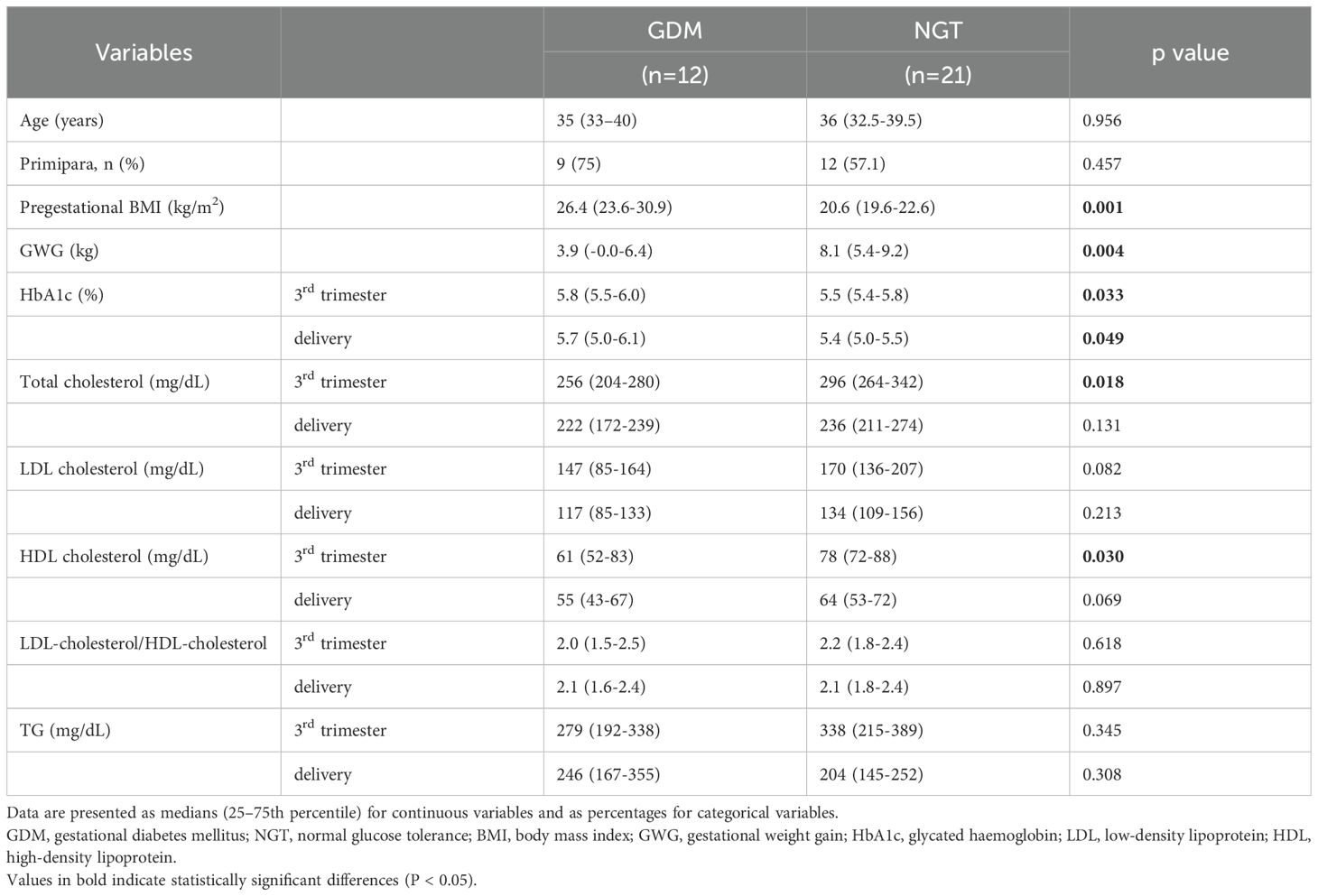
In all, 12 women with GDM and 21 participants with NGT, all of whom were Japanese, were included in this study. Blood samples were obtained during the 2nd trimester at 25 to 26 weeks of gestation, during the 3rd trimester at 35 to 36 weeks and within 3 days after delivery. Table 1 describes the baseline characteristics and comparisons between the study groups. Statistical analysis indicated that compared with participants with NGT, women with GDM had significantly higher pregestational BMI and HbA1c levels (in the 3rd trimester and at delivery). Women with GDM had significantly lower gestational weight gain (GWG), total cholesterol (3rd trimester), and HDL (3rd trimester) levels than did participants with NGT. Although women with GDM received dietary counselling only once at the initial visit, they exhibited lower gestational weight gain than did women with NGT, which likely reflects dietary glycaemic management and efforts to control weight. Nonetheless, no statistically significant difference was observed between the two groups in terms of age, proportion of primiparous women, total cholesterol (at delivery), LDL cholesterol (3rd trimester and at delivery), HDL cholesterol (at delivery), LDL-cholesterol/HDL-cholesterol (3rd trimester and at delivery), or TG levels (3rd trimester and at delivery).
Comparisons of obstetrical outcomes and neonatal characteristics between study groups
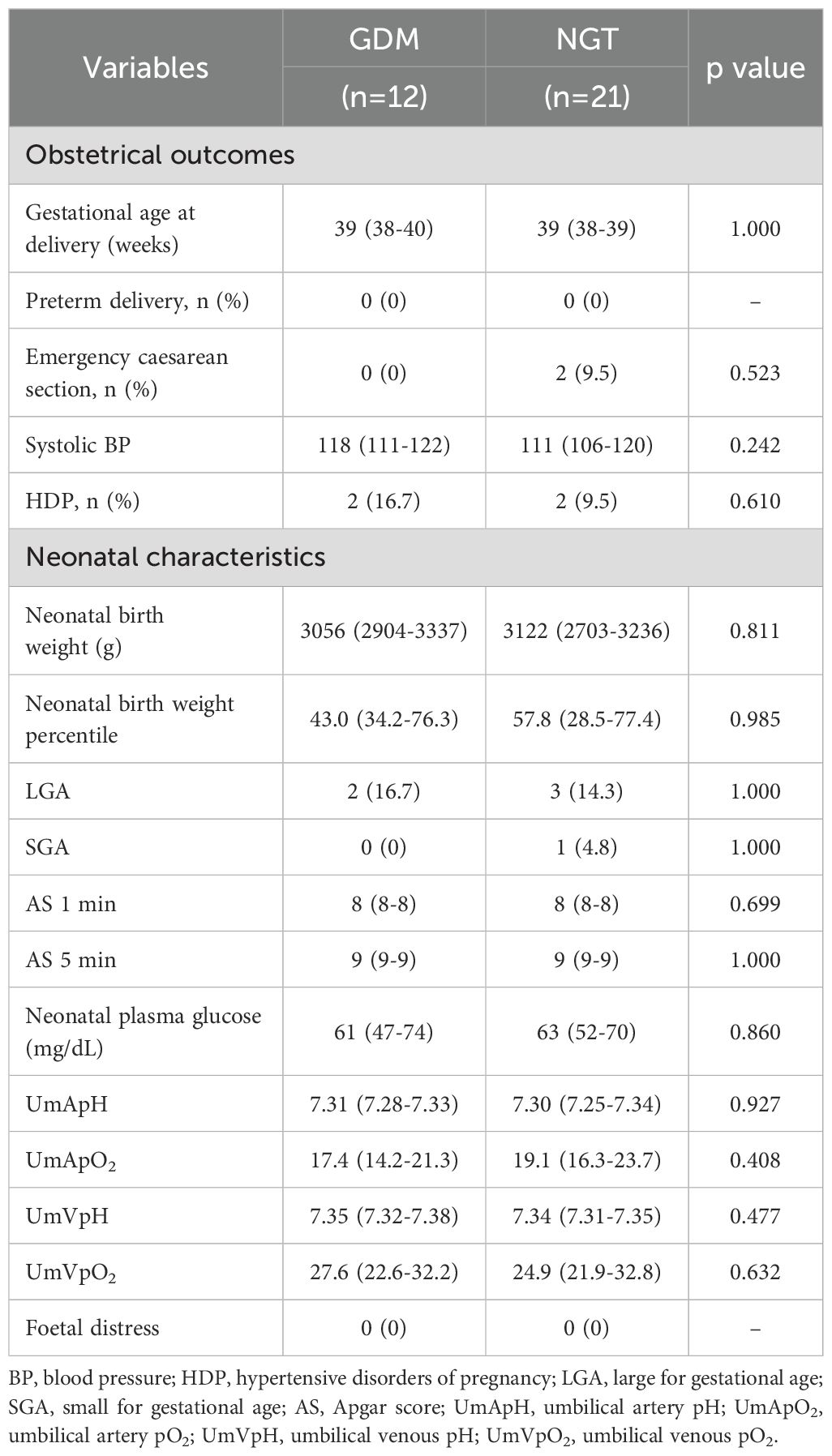
With respect to obstetrical outcomes, no significant differences were observed between the two groups in terms of gestational age at delivery and in the incidences of preterm delivery, emergency caesarean section, or hypertensive disorders of pregnancy (HDP) (Table 2). In terms of neonatal characteristics, the two groups did not significantly differ in terms of neonatal birth weight, LGA, small for gestational age (SGA), Apgar score (AS), neonatal plasma glucose, umbilical blood gas analysis, or incidence of foetal distress (Table 2).
Changes in circulating GPIHBP1 levels during pregnancy
Next, we evaluated circulating GPIHBP1 levels during pregnancy in participants with NGT. Circulating GPIHBP1 levels were markedly lower in the 3rd trimester than in the 2nd trimester and at delivery (Figure 1A). Conversely, serum TG levels had markedly increased in the 3rd trimester compared with the 2nd trimester and at delivery (Figure 1B). In women with GDM, circulating GPIHBP1 levels and serum TG levels were unchanged during the 3rd trimester (Figure 1). circulating GPIHBP1 levels during the third trimester were not significantly correlated with serum TG levels. Circulating GPIHBP1 levels throughout the 3rd trimester were not significantly correlated with serum TG levels in both the NGT and GDM groups.
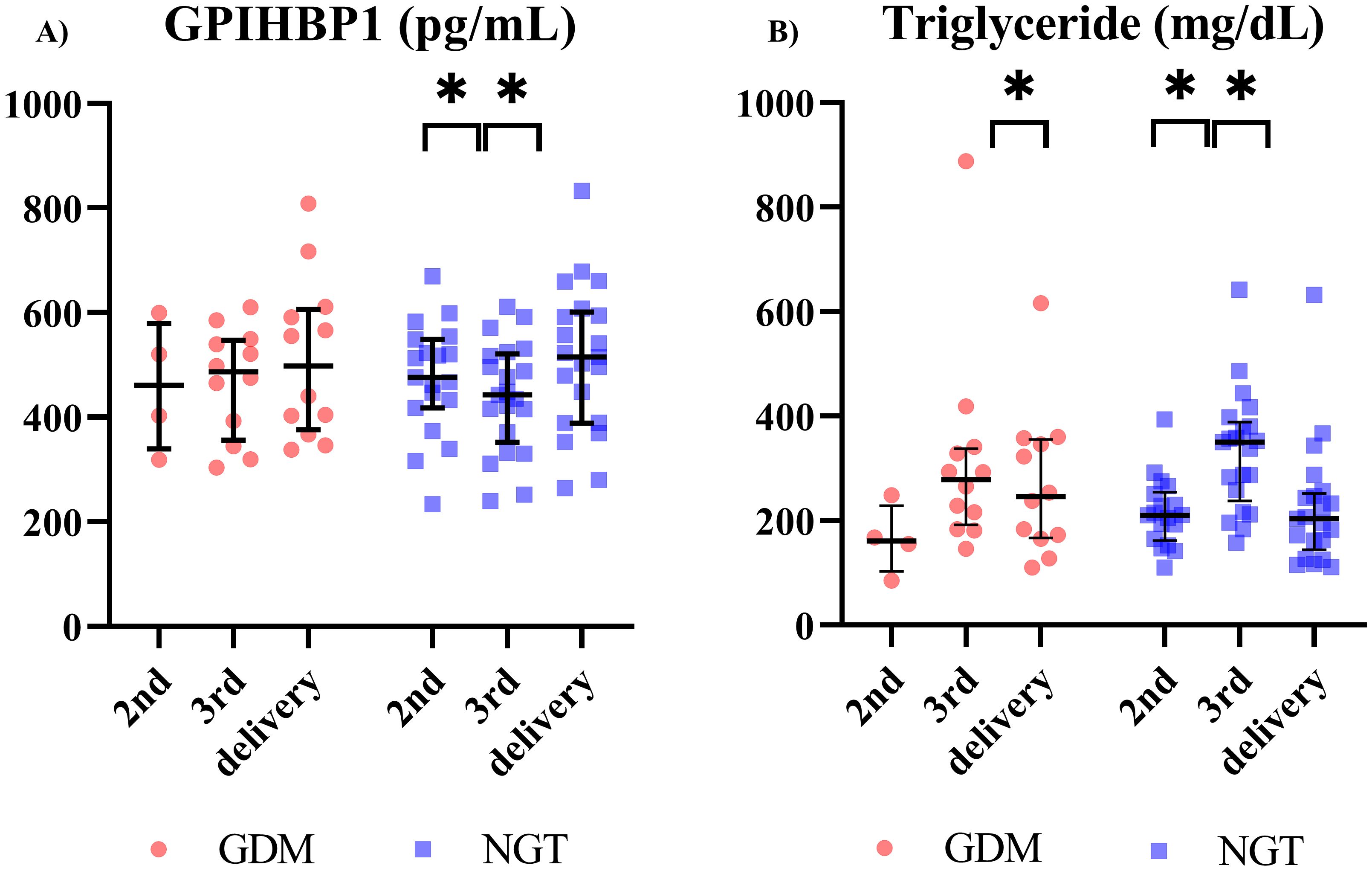
Figure 1. Longitudinal changes in circulating GPIHBP1 and triglyceride (TG) levels during pregnancy in women with gestational diabetes mellitus (GDM) and participants with normal glucose tolerance (NGT) (GDM; n=12; NGT; n=21). (A) Circulating GPIHBP1 levels. (B) Maternal TG levels. Red circles indicate women with GDM, and blue squares indicate women with NGT. The data are presented as individual values. Wilcoxon signed-rank test; *p < 0.05.
Correlation between circulating GPIHBP1 levels and perinatal outcomes of patients with GDM
Given the variability in circulating GPIHBP1 levels during the 3rd trimester, we investigated the correlation between circulating GPIHBP1 levels and perinatal complications during the 3rd trimester. In the 3rd trimester, circulating GPIHBP1 levels were negatively correlated with neonatal birth weight (BW) percentile(p=-0.636, p=0.026) (Figure 2A). Furthermore, circulating GPIHBP1 levels throughout the 3rd trimester were negatively correlated with umbilical venous pO2 levels (ρ=-0.657; p=0.020) (Figure 2B). After the Bonferroni correction was applied for multiple testing (α = 0.025), only the correlation with umbilical venous pO2 remained statistically significant. Notably, TG levels throughout the 3rd trimester were positively correlated with maternal age and prepregnancy BMI (maternal age: ρ=0.647, p=0.023; prepregnancy BMI: ρ=0.629, p=0.028); however, no association was observed with neonatal outcome.
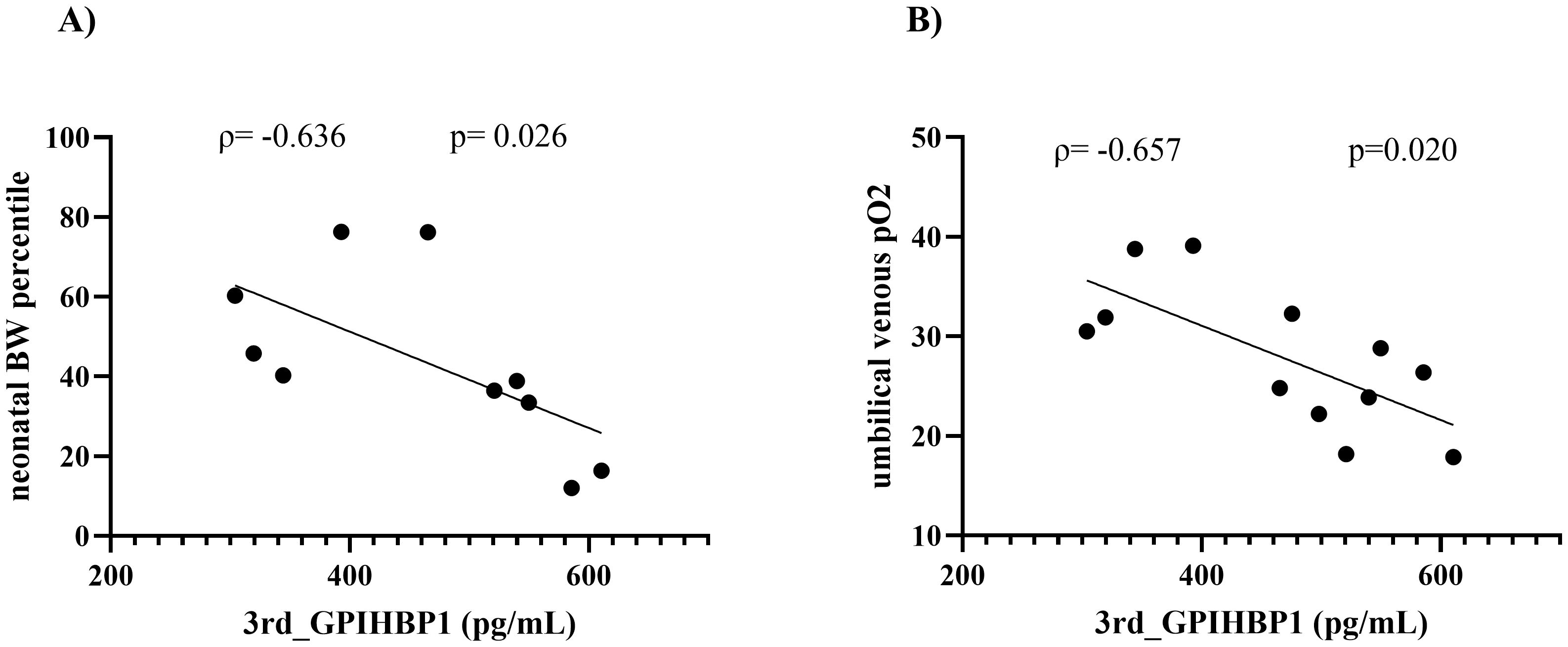
Figure 2. Correlations between circulating GPIHBP1 levels and neonatal outcomes in women with GDM. Spearman’s rank correlation coefficients (ρ) and corresponding p values are indicated for each relationship. (A) Correlations between circulating GPIHBP1 levels in the 3rd trimester and neonatal birth weight (BW) percentiles. (B) Correlations between circulating GPIHBP1 levels in the 3rd trimester and umbilical venous pO2 levels.
In this study, among six women with placental pathology, circulating GPIHBP1 levels throughout the 3rd trimester were elevated in those with placental infarction (n=2) and in those with chorangiosis (n=1) compared with those without these conditions (n=3). However, these observations are exploratory given the very limited sample size (Supplementary Figures 1, 2).
Additionally, maternal age, maternal BMI, GWG, HbA1c (3rd trimester), LDL-C (3rd trimester) and HDL-C (3rd trimester) were not significantly associated with GPIHBP1 levels, neonatal birth weight percentiles or umbilical venous pO2 in this cohort. Furthermore, no significant differences in neonatal BW percentile or umbilical venous pO2 levels were observed between the groups in patients who met one positive criterion and in those who met two or 3 positive criteria on the 75 g OGTT (neonatal BW percentile: p=1.000; umbilical venous pO2 level: p=0.527).
Discussion
In this study, we demonstrated that circulating GPIHBP1 levels were markedly lower in the 3rd trimester than in the 2nd trimester and at delivery. Notably, circulating GPIHBP1 levels in women with GDM were negatively correlated with neonatal BW percentiles (ρ = -0.636, p = 0.026) and umbilical venous pO2 (ρ = -0.657, p = 0.020). In contrast, maternal TG levels throughout the 3rd trimester were not associated with neonatal outcomes. This work represents a novel contribution, as it is the first to suggest a potential link between circulating GPIHBP1 levels and perinatal outcomes.
Previous studies have indicated that GDM is associated with an increased risk of perinatal complications such as macrosomia, preterm birth, polyhydramnios, and preeclampsia (3, 7) and have reported that pregnant women with GDM or obesity typically exhibit higher TG levels than do women of normal weight (10). However, in our study, TG levels in women with GDM did not differ significantly from those in women with NGT, and perinatal outcomes also did not differ markedly between the groups. Previous studies have indicated that elevated serum TG levels during pregnancy are associated with an increased risk of higher birth weight (21, 22), which results in excessive fat accumulation in the foetus (23). Early maternal obesity has been reported to be a risk factor for neonatal adiposity (10, 24). Even in well-managed GDM pregnancies, maternal TG levels remain strong predictors of foetal lipid profiles and foetal growth (25). Despite these findings, in the present study, maternal TG levels throughout the 3rd trimester were not associated with the neonatal BW percentile. In healthy pregnant women, total cholesterol, TG, and HDL-cholesterol levels typically increase during pregnancy, while the atherogenic index (LDL-cholesterol/HDL-cholesterol) remains unchanged. In contrast, women with GDM exhibit elevated TG levels, altered cholesterol and lipoprotein levels, and reduced HDL levels (9). In the present study, women with GDM exhibited lower HDL-C levels, whereas TG levels and the atherogenic index did not differ significantly from those in women with NGT, which suggests that lipid metabolic alterations in women with GDM in the present cohort may have been less pronounced than those reported in previous studies. The women with GDM in this cohort had a median prepregnancy BMI of 26.4 kg/m²; these women were categorised as overweight, and their median GWG was only 3.9 kg, which is below the recommended range of 6.8–11.3 kg for this population (26). Insufficient GWG has been linked to an increased risk of SGA (27), which may partly explain the lack of association between maternal TG levels and neonatal birthweight percentiles observed in this study.
Currently, no findings have been published on GPIHBP1 or its role in placental function, and the regulatory mechanisms underlying circulating GPIHBP1 levels during pregnancy remain to be elucidated. In this study, circulating GPIHBP1 levels in women with GDM tended to be negatively correlated with neonatal birth weight percentiles, regardless of maternal TG levels. Although GDM is typically linked to excessive foetal growth and consequently higher rates of LGA and macrosomia, associations with SGA have also been reported, which suggests that GDM affects foetal growth through a variety of mechanisms (28). GPIHBP1 is essential for TG hydrolysis because it binds LPL and facilitates its transit from the extravascular space to the lumen (15). GPIHBP1 is expressed predominantly in adipose tissue, but its expression has also been documented in the placenta (29). LPL mRNA is expressed on both the maternal and foetal sides of the human placenta, and the LPL protein is also detectable in foetal endothelial cells. In other tissues, such as adipose tissue and skeletal muscle, parenchymal adipocytes and myocytes produce LPL, which is subsequently transported to the luminal surface of the vascular endothelium. LPL hydrolyses TG-rich lipoproteins to generate free fatty acids for uptake by local tissues. LPL in placental endothelial cells may facilitate the uptake of lipids from both the maternal circulation and the foetal circulation, thereby contributing to the foetal nutrient supply (30). In pregnant rats, LPL activity decreases in adipose tissue and the liver during late pregnancy but increases in the placenta. In the placenta, TG of maternal origin is hydrolysed into free fatty acids, which are supplied to the foetus (31). These observations highlight the substantial alterations in lipid metabolism that occur during the 3rd trimester. In this context of marked metabolic changes, LPL anchored by GPIHBP1 may be modulated, which may affect circulating GPIHBP1 levels. Notably, even within a range of TG changes that did not differ significantly from those in women with NGT, circulating GPIHBP1 levels tended to be negatively correlated with neonatal birth weight percentiles, which suggests that circulating GPIHBP1 may reflect aspects of foetal growth regardless of maternal TG levels.
In the present study, circulating GPIHBP1 levels tended to be negatively correlated with umbilical venous pO2 in women with GDM. Umbilical vein blood gas analysis primarily indicates placental metabolism (32). Placental dysfunction is associated with complications, including low birth weight in infants (33, 34). Our prior work indicated that circulating GPIHBP1 levels are associated with the incidence of microvascular complications in women with type 2 diabetes, irrespective of TG levels (35). Comparable results were reported in a study that investigated the association between vascular disorders and circulating GPIHBP1 levels (20). Circulating GPIHBP1 levels may be associated with vascular damage irrespective of serum TG levels. Previous studies have also suggested an association between GDM and SGA, which may reflect vascular or endothelial dysfunction and placental insufficiency, suggesting that multiple mechanisms may underlie the influence of GDM on foetal growth (28). In pregnancies complicated by GDM and/or maternal overweight or obesity, fetoplacental endothelial dysfunction appears to arise from dysfunction in the regulation of several critical pathways, including epigenetic modifications, inflammatory signalling, nitric oxide-mediated vascular signalling, mitochondrial function, and alterations in the L-arginine/nitric oxide and insulin/adenosine signalling axes (36, 37). GPIHBP1 is localised to endothelial cells, and fetoplacental endothelial dysfunction could be related to its expression and possibly to its levels in the circulation. Although the sample size was limited, circulating GPIHBP1 levels tended to be elevated in patients with placental infarction and in those with chorangiosis. Previous studies have suggested that placental infarction, a marker of maternal vascular insufficiency in placental pathology, is associated with foetal growth failure (38), whereas chorangiosis, a vascular alteration affecting the terminal villi of the placenta, arises from moderate hypoxia and is linked to intrauterine growth restriction (39). Taken together, the observed negative correlation between circulating GPIHBP1 levels and umbilical venous pO2 in GDM pregnancies suggests that circulating GPIHBP1 may serve as an indicator of placental vascular function, particularly given the multifaceted involvement of GDM and prepregnancy overweight or obesity in vascular dysfunction. Several biomarkers have already been proposed to reflect placental dysfunction (40, 41); our findings indicate that circulating GPIHBP1 may also partially reflect placental function, which highlights its potential as a novel biomarker.
Despite our novel findings, this study has several limitations. First, the small sample size inherent to this pilot study constitutes a major limitation. The limited cohort reduces statistical power and considerably restricts the generalisability of the findings; therefore, the results should be interpreted with caution. Confirmation in larger, independent cohorts is essential. Second, heterogeneity was observed in the interventions: the GDM group received a single session of dietary counselling, while the NGT group received no intervention. Given that the intervention was limited to one session, its direct impact was likely minimal. The observed differences may instead reflect heightened individual attention to gestational weight control in the GDM group, which could not be quantified. This represents an additional important limitation when interpreting the results. Third, multiple correlations were conducted without formal adjustment for multiple testing. After the Bonferroni correction was applied, only the correlation between circulating GPIHBP1 levels and umbilical venous pO2 remained statistically significant. Given the limited number of cases, these findings should be interpreted with caution, as the correction may have been overly conservative. Although maternal BMI, maternal age, and parity were not significantly associated with GPIHBP1 levels, birth weight percentile, umbilical venous pO2, neonatal blood glucose, or umbilical arterial pO2 in our exploratory analyses, the small sample size limits the statistical power to exclude residual confounding. Finally, the impact of GPIHBP1 on placental function remains underexplored, and thus additional comprehensive pathological and molecular biological investigations are needed. Taken together, these limitations should be carefully considered when interpreting the results.
In summary, our study suggests that in women with GDM, higher circulating GPIHBP1 levels may be associated with lower birth weight percentiles and lower umbilical venous pO2 levels. These observations may indicate a potential association between circulating GPIHBP1 levels and placental function; however, this notion remains preliminary and requires confirmation in future studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and Okayama University Hospital, Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MW: Data curation, Conceptualization, Investigation, Methodology, Writing – review & editing, Writing – original draft, Formal analysis. JE: Supervision, Writing – review & editing, Writing – original draft. NK: Supervision, Data curation, Writing – review & editing. EE: Supervision, Data curation, Writing – review & editing. HM: Supervision, Data curation, Writing – review & editing. JW: Writing – original draft, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by a Grant-in-Aid for Young Scientists (19K17984), a Grant-in-Aid for Scientific Research (B) (19H03675), and the Japan Agency for Medical Research and Development (AMED, grant no: 17ek0210095h0001, 20ek0109445h0001).
Acknowledgments
The authors sincerely thank all the participants for their cooperation in this study.
Conflict of interest
JW receives speaker honoraria from Astra Zeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Kyowa Kirin, Novo Nordisk, and Mitsubishi Tanabe and receives grant support from Abbott, Bayer, Chugai, Kyowa Kirin, Otsuka, Shionogi, Sumitomo, and Mitsubishi Tanabe.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fcdhc.2025.1724699.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2025.1682012/full#supplementary-material
References
1. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res. Clin. practice. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
2. Kahveci B, Melekoglu R, Evruke IC, and Cetin C. The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth. (2018) 18:343. doi: 10.1186/s12884-018-1984-x
3. Sweeting A, Hannah W, Backman H, Catalano P, Feghali M, Herman WH, et al. Epidemiology and management of gestational diabetes. Lancet. (2024) 404:175–92. doi: 10.1016/S0140-6736(24)00825-0
4. Yang G, Wen J, Si L, Wang N, and Zhao Y. Establishment of a risk prediction model for large-for-gestational-age infants among Chinese women with gestational diabetes mellitus. Sci. Rep. (2025) 15:15164. doi: 10.1038/s41598-025-98447-5
5. Glick I, Kadish E, and Rottenstreich M. Management of pregnancy in women of advanced maternal age: improving outcomes for mother and baby. Int. J. Womens Health. (2021) 13:751–9. doi: 10.2147/IJWH.S283216
6. Wijesiriwardhana P, Habtewold TD, Wang G, Gleason JL, Wapner RJ, Grantz KL, et al. Maternal parity modifies the association of birthweight polygenic score with fetal growth. Sci. Rep. (2025) 15:27915. doi: 10.1038/s41598-025-10415-1
7. Karkia R, Giacchino T, Hii F, Bradshaw C, Ramadan G, and Akolekar R. Gestational diabetes mellitus: relationship of adverse outcomes with severity of disease. J. Matern Fetal Neonatal Med. (2024) 37:2356031. doi: 10.1080/14767058.2024.2356031
8. Hivert MF, Backman H, Benhalima K, Catalano P, Desoye G, Immanuel J, et al. Pathophysiology from preconception, during pregnancy, and beyond. Lancet. (2024) 404:158–74. doi: 10.1016/S0140-6736(24)00827-4
9. Cibickova L, Schovanek J, and Karasek D. Changes in serum lipid levels during pregnancy in women with gestational diabetes. A narrative review. BioMed. Pap Med. Fac Univ Palacky Olomouc Czech Repub. (2021) 165:8–12. doi: 10.5507/bp.2021.009
10. Barbour LA and Hernandez TL. Maternal lipids and fetal overgrowth: making fat from fat. Clin. Ther. (2018) 40:1638–47. doi: 10.1016/j.clinthera.2018.08.007
11. Barbour LA and Hernandez TL. Maternal non-glycemic contributors to fetal growth in obesity and gestational diabetes: spotlight on lipids. Curr. Diabetes Rep. (2018) 18:37. doi: 10.1007/s11892-018-1008-2
12. Sobrevia L, Abarzua F, Nien JK, Salomon C, Westermeier F, Puebla C, et al. Review: Differential placental macrovascular and microvascular endothelial dysfunction in gestational diabetes. Placenta. (2011) 32 Suppl 2:S159–64. doi: 10.1016/j.placenta.2010.12.011
13. Rao R, Sen S, Han B, Ramadoss S, and Chaudhuri G. Gestational diabetes, preeclampsia and cytokine release: similarities and differences in endothelial cell function. Adv. Exp. Med. Biol. (2014) 814:69–75. doi: 10.1007/978-1-4939-1031-1_6
14. Zhou M, Feng Y, Zhang C, Tian X, Li M, and Zheng Y. Relationship between glucose/lipid metabolism and placental biomarkers in gestational diabetes and preeclampsia. Diabetes Metab. Syndr. Obes. (2025) 18:691–702. doi: 10.2147/DMSO.S504653
15. Davies BS, Beigneux AP, Barnes RH 2nd, Tu Y, Gin P, Weinstein MM, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. (2010) 12:42–52. doi: 10.1016/j.cmet.2010.04.016
16. Olivecrona G, Ehrenborg E, Semb H, Makoveichuk E, Lindberg A, Hayden MR, et al. Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia. J. Lipid Res. (2010) 51:1535–45. doi: 10.1194/jlr.M002717
17. Lin MH, Tian XH, Hao XL, Fei H, Yin JL, Yan DD, et al. Management of a pregnant patient with chylomicronemia from a novel mutation in GPIHBP1: a case report. BMC Pregnancy Childbirth. (2020) 20:272. doi: 10.1186/s12884-020-02965-1
18. Xie SL, Chen TZ, Huang XL, Chen C, Jin R, Huang ZM, et al. Genetic variants associated with gestational hypertriglyceridemia and pancreatitis. PloS One. (2015) 10:e0129488. doi: 10.1371/journal.pone.0129488
19. International Association of D, Pregnancy Study Groups Consensus P, Metzger BE, Gabbe SG, Persson B, Buchanan TA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848
20. Miyashita K, Fukamachi I, Nagao M, Ishida T, Kobayashi J, Machida T, et al. An enzyme-linked immunosorbent assay for measuring GPIHBP1 levels in human plasma or serum. J. Clin. Lipidol. (2018) 12:203–10 e1. doi: 10.1016/j.jacl.2017.10.022
21. Peng J, Zhang L, Jin J, Miao H, Liu G, and Guo Y. Impact of maternal lipid profiles on offspring birth size in late pregnancy among women with and without gestational diabetes. Lipids Health Dis. (2025) 24:43. doi: 10.1186/s12944-025-02458-0
22. Wang J, Moore D, Subramanian A, Cheng KK, Toulis KA, Qiu X, et al. Gestational dyslipidaemia and adverse birthweight outcomes: a systematic review and meta-analysis. Obes. Rev. (2018) 19:1256–68. doi: 10.1111/obr.12693
23. Desoye G, Gauster M, and Wadsack C. Placental transport in pregnancy pathologies. Am. J. Clin. Nutr. (2011) 94:1896S–902S. doi: 10.3945/ajcn.110.000851
24. Herrera E and Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm. Mol. Biol. Clin. Investig. (2016) 26:109–27. doi: 10.1515/hmbci-2015-0025
25. Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. (2008) 31:1858–63. doi: 10.2337/dc08-0039
26. American Diabetes Association Professional Practice C. Management of diabetes in pregnancy: standards of care in diabetes-2025. Diabetes Care. (2025) 48:S306–S20. doi: 10.2337/dc25-S015
27. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA. (2017) 317:2207–25. doi: 10.1001/jama.2017.3635
28. Fota A and Petca A. Gestational diabetes mellitus: the dual risk of small and large for gestational age: A narrative review. Med. Sci. (Basel). (2025) 13:144. doi: 10.3390/medsci13030144
29. Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteomics. (2014) 13:397–406. doi: 10.1074/mcp.M113.035600
30. Lindegaard ML, Olivecrona G, Christoffersen C, Kratky D, Hannibal J, Petersen BL, et al. Endothelial and lipoprotein lipases in human and mouse placenta. J. Lipid Res. (2005) 46:2339–46. doi: 10.1194/jlr.M500277-JLR200
31. Herrera E, Lasuncion MA, Gomez-Coronado D, Aranda P, Lopez-Luna P, and Maier I. Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. Am J Obstet Gynecol. (1988) 158:1575–83. doi: 10.1016/0002-9378(88)90193-7
33. Rana S, Lemoine E, Granger JP, and Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ. Res. (2019) 124:1094–112. doi: 10.1161/CIRCRESAHA.118.313276
34. Melamed N, Baschat A, Yinon Y, Athanasiadis A, Mecacci F, Figueras F, et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol Obstet. (2021) 152 Suppl 1:3–57. doi: 10.1002/ijgo.13522
35. Kurooka N, Eguchi J, Murakami K, Kamei S, Kikutsuji T, Sasaki S, et al. Circulating GPIHBP1 levels and microvascular complications in patients with type 2 diabetes: A cross-sectional study. J. Clin. Lipidol. (2022) 16:237–45. doi: 10.1016/j.jacl.2022.01.006
36. Cornejo M, Fuentes G, Valero P, Vega S, Grismaldo A, Toledo F, et al. Gestational diabesity and foetoplacental vascular dysfunction. Acta Physiol. (Oxf). (2021) 232:e13671. doi: 10.1111/apha.13671
37. Diniz MS, Hiden U, Falcao-Pires I, Oliveira PJ, Sobrevia L, and Pereira SP. Fetoplacental endothelial dysfunction in gestational diabetes mellitus and maternal obesity: A potential threat for programming cardiovascular disease. Biochim. Biophys. Acta Mol. Basis Dis. (2023) 1869:166834. doi: 10.1016/j.bbadis.2023.166834
38. Bujorescu DL, Ratiu AC, Motoc AGM, Citu IC, Sas I, Gorun IF, et al. Placental pathology in early-onset fetal growth restriction: insights into fetal growth restriction mechanisms. Rom J. Morphol Embryol. (2023) 64:215–24. doi: 10.47162/RJME.64.2.12
39. Vafaei H, Karimi Z, Akbarzadeh-Jahromi M, and Asadian F. Association of placental chorangiosis with pregnancy complication and prenatal outcome: a case-control study. BMC Pregnancy Childbirth. (2021) 21:99. doi: 10.1186/s12884-021-03576-0
40. Schofield LG, Zhao J, Wang Y, Delforce SJ, Endacott SK, Lumbers ER, et al. Unravelling soluble (pro)renin receptor-mediated endothelial dysfunction. Eur. J. Pharmacol. (2025) 996:177601. doi: 10.1016/j.ejphar.2025.177601
Keywords: glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1), gestational diabetes mellitus (GDM), perinatal outcomes, placenta, triglyceride (TG)
Citation: Watanabe M, Eguchi J, Kurooka N, Eto E, Masuyama H and Wada J (2025) Maternal circulating GPIHBP1 levels and neonatal outcomes in patients with gestational diabetes mellitus: a pilot study. Front. Clin. Diabetes Healthc. 6:1682012. doi: 10.3389/fcdhc.2025.1682012
Received: 08 August 2025; Accepted: 25 September 2025;
Published: 10 October 2025; Corrected: 27 October 2025.
Edited by:
Giovanni Tossetta, Marche Polytechnic University, ItalyReviewed by:
Eyal Sheiner, Ben-Gurion University of the Negev, IsraelBo Sun, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2025 Watanabe, Eguchi, Kurooka, Eto, Masuyama and Wada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mayu Watanabe, bWF5dXcyOTRAZ21haWwuY29t
 Mayu Watanabe
Mayu Watanabe Jun Eguchi
Jun Eguchi Naoko Kurooka2
Naoko Kurooka2 Jun Wada
Jun Wada