- U. S. Geological Survey, Wetland and Aquatic Research Center, Gainesville, FL, United States
Conservation resources have become increasingly limited and, along with social, cultural and political complexities, this shortfall frequently challenges effectiveness in conservation. Because conservation can be costly, efforts are often only initiated after a species has declined below a critical threshold and/or when statutory protection is mandated. However, implementing conservation proactively, rather than reactively, is predicted to be less costly and to decrease a species' risk of extinction. Despite these benefits, I document that the number of studies that have implemented proactive conservation around the world are far fewer than those that simply acknowledge the need for such action. I provide examples of proactive actions that can ameliorate shortfalls in funding and other assets, thus helping conservation practitioners and managers cope with the constraints that resource limitation imposes. Not all of these options are new; however, the timing of their implementation is critical for effective conservation, and the need for more proactive conservation is increasingly recognized. These actions are (1) strengthening and diversifying stakeholder involvement in conservation projects; (2) complementing time-consuming and labor-intensive demographic studies with alternative approaches of detecting declines and estimating extinction risk; and (3) minimizing future costly conservation and management by proactively keeping common species common. These approaches may not constitute a cure-all for every conservation crisis. However, given escalating rates of species' losses, perhaps a reminder that these proactive actions can reduce conservation costs, save time, and potentially thwart population declines is warranted.
Introduction
Effective conservation is frequently influenced by the availability of critical resources and their allocation among competing needs. Shortfalls in funding and other assets, along with social, political, and cultural challenges, often oblige practitioners and managers to make difficult allocation decisions so that conservation impacts can be maximized. Subsequently, prioritization of conservation needs can leave some goals unmet, management actions delayed or rejected, or at-risk species with inadequate management and protection. Guidelines exist for setting conservation priorities using formal decision theory and return on investment approaches, with demonstrated utility for prioritizing protected sites, management actions, and species (e.g., Joseph et al., 2009; Wilson et al., 2009; Carwardine et al., 2012; Semlitsch et al., 2017). Referred to as “conservation triage,” this approach is currently a highly debated topic in conservation biology. Supporters argue that this approach is simply an efficient, logical use of limited conservation resources, whereas opponents are concerned that the practice of investing in recovery of some species at the expense of others is unethical (e.g., Bottrill et al., 2008; Jachowski and Kesler, 2009; Buckley, 2016; Wilson and Law, 2016; Vucetich et al., 2017).
Going forward, there are ways to reduce the severity of resource limitation impacts on conservation which, in some situations, could limit or even eliminate the need for triage. If population declines are observed and acted on quickly in a proactive manner, basic, cost-effective actions could help prevent species from declining to critical levels, avoid costly species recovery and, therefore, avoid the need for triage. I am not the first to express concerns about the need for more proactive conservation (e.g., Drechsler et al., 2011; Martin et al., 2012). However, the extent to which proactive measures are actually implemented, rather than simply promoted, is not clear. Here I first outline the cost and time savings that proactive conservation affords. I then explore existing literature to assess the frequency with which proactive strategies are actually implemented. Last, I offer a reminder that by proactively strengthening and diversifying interactions with stakeholders, using time-efficient approaches to detect declines and estimate extinction risk, and keeping common species common, conservation could be sustained in the face of limited resources and time constraints.
Proactive Strategies Can Minimize Conservation Costs and Time Delays
The cost of conservation is usually a pivotal consideration for decision makers. Globally, the annual cost to reduce extinction risk of threatened species has been estimated at US $76 billion (McCarthy et al., 2012) and, in the United States, the annual cost to protect endangered species from just two conservation threats (alien species and the disruption of fire regimes) was estimated at US $32–42 million per year (1997 $US; Wilcove and Chen, 1998). Because of cost, in many cases conservation only starts when species are under mandated statutory protection to prevent extinction. Yet, ironically, proactive conservation is predicted to cost less than delayed action. In Germany, for example, the common hamster (Cricetus cricetus) is threatened by habitat loss and agricultural intensification (Drechsler et al., 2011). An exercise in comparing cost functions of a proactive approach (implementing conservation measures once population size is threatened to decline below a critical level), vs. that of the existing hamster conservation policy (conservation actions delayed until population was critically endangered) demonstrated that the proactive approach would have saved between 17.2 and 36.4 million euro ($18.6–$39.2 million) compared to the existing policy of delayed conservation (Drechsler et al., 2011).
Conservation biologists argue that actions need to be initiated before a species becomes endangered and is at risk of extinction (Martin et al., 2012). In reality, however, conservation often only starts when species are already in crisis, with populations having declined to critical levels (Drechsler et al., 2011). Such delays in starting conservation can limit conservation options for managers, increase uncertainty regarding outcomes, and elevate a species' extinction risk and recovery costs (Dresser et al., 2017). When species reach critically low levels, recovery typically requires bringing individuals into captivity to establish captive assurance colonies and breeding programs. This conservation dilemma is exemplified by the now-extinct Christmas Island pipistrelle (Pipistrellus murrayi) and the critically endangered Orange-bellied Parrot (Neophema chrysogaster)—both from Australia (Martin et al., 2012). This dilemma is also well-illustrated by three United States' species listed under the Endangered Species Act as endangered in 1967—the black-footed ferret (Mustela nigripes), California Condor (Gymnogyps californianus), and red wolf (Canis rufus)—all of which continued to decline after listing and currently remain classified as endangered (Lockhart et al., 2006). Even for species less critically endangered, starting conservation efforts before they receive formal protection can accelerate the recovery process and minimize further declines while waiting for legal safeguards to get underway (e.g., island foxes, Urocyon littoralis littoralis; U. l. santacruzae; and U. l. santarosae: Coonan et al., 2014; King et al., 2014; Williams, 2016).
How Often is Proactive Conservation Practiced Worldwide?
Implementation is a critical part of any proactive conservation planning, yet this step is often neglected (e.g., the Christmas Island pipistrelle, Martin et al., 2012; Cook et al., 2014). Using the Thomson Reuters Web of Science™ database, I used the Boolean search string “proactive Near/5 conservation” to compile studies of proactive conservation. I identified 62 studies from 12 different regions of the world in which proactive conservation at the species level was either implemented or, at least, recognized as a need (systems-level actions, such as establishment of protected areas or payment for ecosystem services, were not included in this search). All of these studies acknowledged that proactive conservation was needed, but only 22.6% indicated that such a conservation strategy had actually been implemented (Appendix S1). Nearly one-third of the studies that referred to proactive conservation were conducted in the United States and its territories (32.3%), with the second-highest number of studies conducted in Central and South American countries (14.5%) (Figure 1, Appendix S1). Thus, although the need for proactive conservation is widely recognized, its actual implementation is less common and is geographically biased.
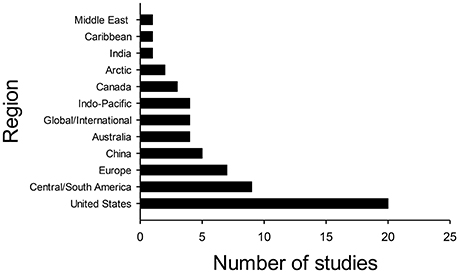
Figure 1. Number of studies in various regions of the world in which proactive conservation was implemented or the need for such a strategy was recognized.
Ways to Improve Conservation Effectiveness Under Resource Limitation
Strengthen and Diversify Stakeholder Involvement
The involvement of stakeholders—defined as “all interested and affected parties, including governmental agencies, non-governmental organizations, the private sector, and the general public” (Burger et al., 2017)—is increasingly necessary to address complex challenges in conservation and management of imperiled species, especially given increasing financial constraints and shortfalls in personnel (Moore et al., 2011; Cheruvelil et al., 2014; Housty et al., 2014; Burger et al., 2017). Several stakeholder types (e.g., state and federal agencies, independent scientists, non-governmental conservation organizations: Burger et al., 2017) can provide personnel to assist with project needs, as well as funding in the form of grants and “in-kind” support. Other stakeholders, such as local communities and the public, in general, can provide access to diverse, volunteer workforces and offer a cost-effective means of accomplishing project goals (Silvertown, 2009; Burger et al., 2017). Thus, involving a wide range of stakeholders in conservation efforts may be an effective means of “accomplishing more with less, while gaining public support” (Burger et al., 2017). Stakeholders can provide additional benefits, such as opportunities to work in locations that may otherwise not be feasible (e.g., private lands and urban environments), as well as a variety of perspectives and experiences from which to develop solutions to conservation and management problems. Although stakeholder involvement is mainstream and widespread, empirical evidence of its effectiveness is either sparse or uncertain, indicating a need to strengthen and improve relationships with stakeholders (Beever et al., 2014; Baylis et al., 2016; Pattberg and Widerberg, 2016). Moreover, the process of engaging stakeholders of various types is likely much more complicated than is represented here.
The involvement of citizen stakeholders in scientific projects—a practice known as citizen science— is not a new phenomenon, although volunteer participation, as well as the projects themselves, are rapidly increasing and diversifying worldwide (Cohn, 2008; Bonney et al., 2014). Advocates of citizen science assert that this practice can facilitate efforts that would otherwise be prohibitive because of the cost, time, and/or geographic scale of the project (Silvertown, 2009). Moreover, citizen science educates participants about environmental issues, leading to better-informed public engagement in government decision-making (McKinley et al., 2017). In some cases, citizen scientists can provide meaningful estimates of population trends, at least for common species whose identification is straightforward (Petrovan and Schmidt, 2016; Dennis et al., 2017). However, not all conservation efforts are amenable to public involvement, and citizen science remains a controversial issue in conservation biology, with concerns expressed about the accuracy and reliability of data collected by volunteers (Cohn, 2008; Bernard et al., 2013; Bonney et al., 2014).
Local communities constitute another low-cost workforce, and including community members in conservation efforts also fosters stewardship, a sense of ownership, and teamwork in finding solutions to environmental issues that impact them. Many conservation efforts with local communities have been effective in protecting habitat and/or imperiled species (e.g., Li et al., 2013; Lambrick et al., 2014; Shanee and Shanee, 2015; Shanee et al., 2015; Aryal et al., 2017). Not all conservation efforts have had positive effects on local communities, however. Since 1900, the establishment of protected conservation areas worldwide has frequently displaced the indigenous peoples that had been living sustainably on their ancestral lands (Dowie, 2011). In Madagascar, the enormous investment into environmental projects by the international donor community and the expansion of a protected areas network has failed to reduce deforestation and poverty among the country's rural poor (Waeber et al., 2016). Thus, sensitivities to local communities, their cultures, and their socio-economic issues are essential to consider at the start of any conservation effort. Overlooking these critical details can also exacerbate the extinction risk of imperiled species, as has been the case for the critically endangered vaquita, Phocoena sinus, of Mexico (Aburto-Oropeza et al., 2017).
Target Data Deficiencies With Time-Efficient, Supplemental Approaches
Data deficiencies exist for most species, and those that are rare, in decline, or newly described are especially lacking (Gallagher et al., 2015; Roberts et al., 2016). Information on population demography and trends, life history, dispersal, spatial and genetic structure, and habitat use is essential for proactively protecting existing populations, setting conservation priorities, and recovery planning (e.g., Zeigler et al., 2013; Peñaranda and Simonetti, 2015; Turkalo et al., 2017). Long-term, continuous monitoring and capture-mark-recapture (CMR) studies are ideal for collecting such data, but these types of studies require time—a scarce commodity for declining species. For example, power analyses suggested that a decade or more may be required to detect moderate trends in local populations of many pond-breeding amphibians (Gibbs et al., 1998). For extremely long-lived species such as forest elephants (Loxodonta cyclotis), recent data suggest that status assessments need to be conducted for 60-year time periods (approximately twice the species' generation time; Turkalo et al., 2017).
In the absence of long-term data that can guide decision-making, managers are challenged to determine how to prioritize limited funding and personnel among management needs (Salatas et al., 2013). In recent years, new applications of existing approaches have emerged that enable researchers to detect subtle effects of environmental stress—presumably a correlate of population declines—on at-risk species (Table 1). Assessments of stress hormone levels could help detect early indications of population decline, thus allowing management and conservation actions to be taken before the risk of extinction escalates (Janin et al., 2011). Additionally, other approaches have emerged that likewise do not rely on long-term population data to predict extinction risk, metapopulation connectivity, changes in threats, and demographic or conservation status (Table 1). My intent is not to encourage researchers to abandon more direct methods of estimating vital rates, which are critical for developing population viability models and recovery planning. Rather, my aim is to illustrate that these various approaches could be a first approximation to population processes and, thus, supplement monitoring and CMR studies. These approaches also can function as conservation tools that may help natural resource managers and conservation biologists identify at-risk populations relatively quickly, especially when potential threats are not readily apparent.
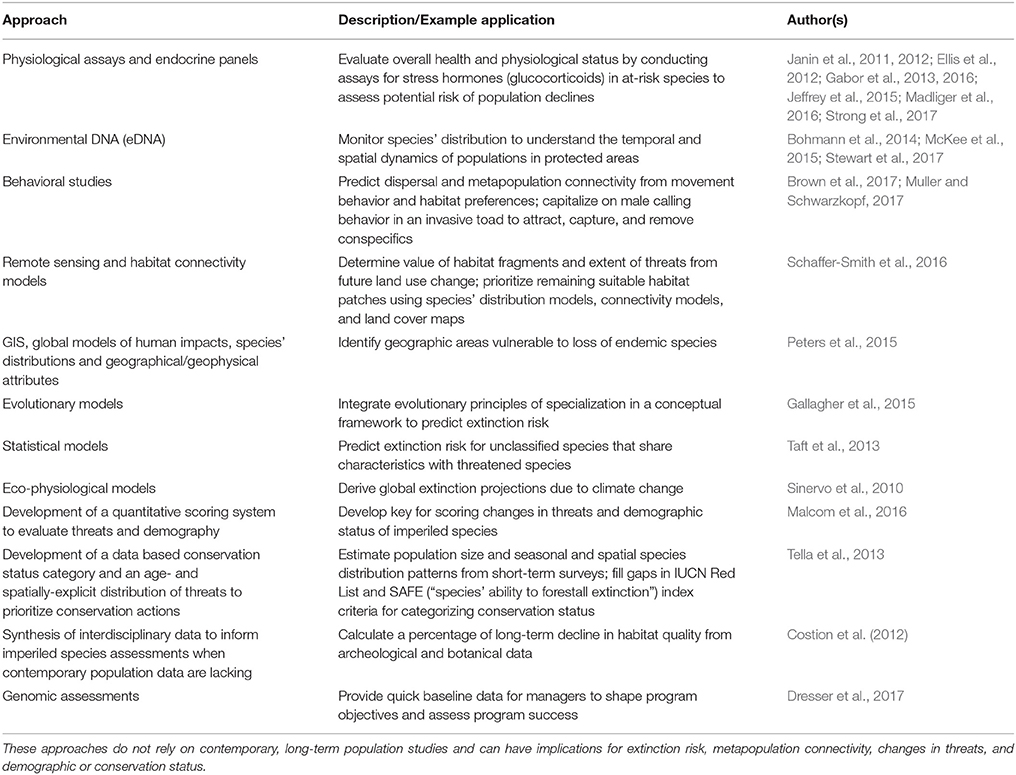
Table 1. Applications of various approaches as conservation tools to help natural resource managers and conservation biologists identify at-risk populations in a time-efficient fashion.
Minimize Future Costly Conservation and Management by Proactively Keeping Common Species Common
Conservation efforts typically target rare species that are at risk of extinction (Lindenmayer et al., 2011; Denoël et al., 2012). However, Inger et al. (2015) discovered that common bird species are declining more than rare ones, and recent evidence demonstrates that the most evolutionarily distinct species are not necessarily the ones that are declining (Morelli and Møller, 2018). Thus, changes in the distribution or abundance of common species can have profound negative consequences for ecosystem structure, function and services (Winfree et al., 2015). Moreover, common species, rather than rare ones, are considered “disproportionately influential in shaping many macroecological patterns” (Gaston and Fuller, 2007), and common species are the most likely to best adapt to rapid climate change (Lindenmayer et al., 2011). Because of their greater numbers, common species comprise the vast majority of biomass in ecosystems (Lindenmayer et al., 2011; Denoël et al., 2012). For example, some common amphibians can reach incredible densities, resulting in extraordinary biomass that can exceed that of large terrestrial mammals such as deer (Gibbons et al., 2006; Semlitsch et al., 2014; Milanovich and Peterman, 2016). Thus, losses of amphibians, even of common species, can disrupt the transfer of energy and nutrients between tropic levels and ecosystems, the regulation of invertebrate prey, and carbon retention in forest ecosystems (Semlitsch et al., 2014; Milanovich and Peterman, 2016). The Mexican axolotl (Ambystoma mexicanum) represents a “conservation paradox”; i.e., its popularity in the pet industry as well as in the laboratory as a biomedical model makes it perhaps the most widely distributed amphibian in the world (Vance, 2017). Yet, this species is almost extinct in its native habitat (Vance, 2017). Proactively implementing conservation of common species helps provide ecosystem stability, minimizes further losses of biodiversity and their scientific contributions and, thus, reduces their need for costly management and recovery.
Conclusions
Existing shortfalls in conservation resources have led to widespread consideration of the controversial tactic of conservation triage, regarded as an extreme practice by some researchers. The need for prioritization signals an alarm that conservation generally is not being implemented soon enough to stem population declines, thus requiring recovery efforts that further deplete limited resources. Although implementing conservation is costly, delayed action costs even more. In an era of budgetary shortfalls, there are several activities that can help conservation practitioners and managers cope with the constraints that resource limitation imposes. Stakeholder involvement can potentially ameliorate the impacts of resource limitations through cost sharing and providing access to other assets, although empirical evidence of its effectiveness is either sparse or uncertain (Beever et al., 2014; Baylis et al., 2016; Pattberg and Widerberg, 2016). Other cost-effective measures, albeit controversial, involve use of volunteer citizen scientists, where feasible, and local-scale conservation initiatives, in which local communities implement conservation efforts themselves or in collaboration with local authorities. Alternative approaches exist that can provide an estimate of extinction risk in natural populations, helping natural resource managers and conservation biologists prioritize competing conservation demands when empirical demographic data are unavailable to guide decision-making. Last, addressing population declines while species are still common can result in a long-term cost savings. Although these approaches may not constitute a cure-all for every conservation crisis, overall, proactive conservation is more cost-effective than delayed conservation actions, protecting species sooner and lowering their risk of extinction.
Despite the potential for these activities to ameliorate the severity of resource limitation impacts on conservation, the availability of conservation resources may still be insufficient to distribute across species. Where possible, one potential option is to pursue a “triage of means” conservation strategy (Mondal et al., 2016). For example, in India, resources (funding) for conservation are diversified among several different government sectors that support activities that benefit biodiversity but for which conservation is not the principal objective. Mondal et al. (2016) advocate that such resources (“means to achieve conservation”) could be leveraged to accomplish desired conservation objectives. In these authors' view, by prioritizing various means of accomplishing conservation, one avoids selectively assisting some species, populations, or localities at the expense of others (Mondal et al., 2016). In extreme cases, however, it may be inevitable that conservation prioritization, perhaps based on some quantification of a species' threat status (e.g., the Evolutionarily Distinct and Globally Endangered [EDGE] index: Washington et al., 2015), is the most viable option.
Globally, progess has been made toward meeting some of the conservation goals to which world leaders committed at the 2002 Convention on Biological Diversity (Butchart et al., 2010). Advancements in conservation biology are being overshadowed, however, by escalating rates of population declines, species' extinctions, and losses of ecological interactions and ecosystem services (Wake and Vredenburg, 2008; Butchart et al., 2010; Barnosky et al., 2011; De Vos et al., 2014; Ceballos et al., 2015, 2017; Valiente-Banuet et al., 2015). The impacts of these losses are further exacerbated by fiscal austerity and shortfalls in conservation funding (McCarthy et al., 2012), along with political polarity and uncertainty in many parts of the world (Allred et al., 2014). Globally, the effectiveness of conservation laws, policies, and programs are being altered (Chapron et al., 2017). In the United States, the Endangered Species Act of 1973—unequivocally one of the most powerful environmental laws in existence—continues to draw criticism, close scrutiny, and calls for reform (Schadegg, 2017). In this era of uncertainty and propensity for a “doom and gloom” outlook among many conservation scientists (Knowlton, 2017), bolstering conservation programs with cost-saving and time-efficient approaches could help stem many population declines, thus averting conservation crises and diminished protection for imperiled species.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer VRG and handling Editor declared their shared affiliation.
Acknowledgments
I thank N. Greenwald, B. R. Hossack, N. Johnson, J. C. Mitchell, E. Nichols, and K. M. O'Donnell for comments on earlier drafts of this manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the United States Government. This work was funded by the U.S. Geological Survey's Amphibian Research and Monitoring Initiative (ARMI) and is ARMI contribution number 612.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00024/full#supplementary-material
References
Aburto-Oropeza, O., López-Sagástegui, C., Moreno-Báez, M., Mascareñas-Osorio, I., Jiménez-Esquivel, V., Frederick Johnson, A., et al. (2017). Endangered species, ecosystem integrity, and human livelihoods. Conserv. Lett. 11:e12358. doi: 10.1111/conl.12358
Allred, B. W., Twidwell, D., and Fuhlendorf, S. D. (2014). The interaction of climate change, land cover, and political representation in the U.S.A. Ecosphere 5:159. doi: 10.1890/ES14-00220.1
Aryal, A., Acharya, K. P., Shrestha, U. B., Dhakal, M., Raubenhiemer, D., and Wright, W. (2017). Global lessons from successful rhinoceros conservation in Nepal. Conserv. Biol. 31, 1494–1497. doi: 10.1111/cobi.12894
Barnosky, A. D., Matzke, N., Tomiya, S., Wogan, G. O., Swartz, B., Quental, T. B., et al. (2011). Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. doi: 10.1038/nature09678
Baylis, K., Honey-Rosés, J., Börner, J., Corbera, E., Ezzine-de-Blas, D., Ferraro, P. J., et al. (2016). Mainstreaming impact evaluation in nature conservation. Conserv. Lett. 9, 58–64. doi: 10.1111/conl.12180
Beever, E. A., Mattsson, B. J., Germino, M. J., Van der Burg, M. P., Bradford, J. B., and Brunson, M. W. (2014). Success and challenges from formation to implementation of eleven broad-extent conservation programs. Conserv. Biol. 28, 302–314. doi: 10.1111/cobi.12233
Bernard, A. T. F., Götz, A., Kerwath, S. E., and Wilke, C. G. (2013). Observer bias and detection probability in underwater visual census of fish assemblages measured with independent double-observers. J. Exp. Mar. Bio. Ecol. 443, 75–84. doi: 10.1016/j.jembe.2013.02.039
Bohmann, K., Evans, A., Gilbert, M. T., Carvalho, G. R., Creer, S., Knapp, M., et al. (2014). Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol. Evol. 29, 358–367. doi: 10.1016/j.tree.2014.04.003
Bonney, R., Shirk, J. L., Phillips, T. B., Wiggins, A., Ballard, H. L., Miller-Rushing, A. J., et al. (2014). Next steps for citizen science. Science 343, 1436–1437. doi: 10.1126/science.1251554
Bottrill, M. C., Joseph, L. N., Carwardine, J., Bode, M., Cook, C., Game, E. T., et al. (2008). Is conservation triage just smart decision making? Trends Ecol. Evol. 12, 649–654. doi: 10.1016/j.tree.2008.07.007
Brown, L. M., Fuda, R. K., Schtickzelle, N., Coffman, H., Jost, A., Kazberouk, A., et al. (2017). Using animal movement behavior to categorize land cover and predict consequences for connectivity and patch residence times. Landscape Ecol. 32, 1657–1670. doi: 10.1007/s10980-017-0533-8
Buckley, R. C. (2016). Triage approaches send adverse political signals for conservation. Front. Ecol. Evol. 4:39. doi: 10.3389/fevo.2016.00039
Burger, J., Gochfeld, M., Zappalorti, R. T., DeVito, E., Jeitner, C., Pittfield, T., et al. (2017). Stakeholder contributions to conservation of threatened Northern Pine Snakes (Pituophis melanoleucus, Daudin, 1803) in the New Jersey Pine Barrens as a case study. Amphib. Reptile Conserv. 11, 17–32.
Butchart, S. H., Walpole, M., Collen, B., van Strien, A., Scharlemann, J. P., Almond, R. E., et al. (2010). Global biodiversity: indicators of recent declines. Science 328, 1164–1168. doi: 10.1126/science.1187512
Carwardine, J., O'Connor, T., Legge, S., Mackey, B., Possingham, H. P., and Martin, T. G. (2012). Prioritizing threat management for biodiversity conservation. Conserv. Lett. 5, 196–204. doi: 10.1111/j.1755-263X.2012.00228.x
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., García, A., Pringle, R. M., and Palmer, T. M. (2015). Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci. Adv. 1:e1400253. doi: 10.1126/sciadv.1400253
Ceballos, G., Ehrlich, P. R., and Dirzo, R. (2017). Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Nat. Acad. Sci. U.S.A. 114, E6089–E6096. doi: 10.1073/pnas.1704949114
Chapron, G., Epstein, Y., Trouwborst, A., and López-Bao, J. V. (2017). Bolster legal boundaries to stay within planetary boundaries. Nat. Ecol. Evol. 1:0086. doi: 10.1038/s41559-017-0086
Cheruvelil, K. S., Soranno, P. A., Weathers, K. C., Hanson, P. C., Goring, S. J., Filstrup, C. T., et al. (2014). Creating and maintaining high-performing collaborative research teams: the importance of diversity and interpersonal skills. Front. Ecol. Environ. 12, 31–38. doi: 10.1890/130001
Cohn, J. P. (2008). Citizen science: can volunteers do real research? BioScience 58, 192–197. doi: 10.1641/B580303
Cook, C. N., Inayatullah, S., Burgman, M. A., Sutherland, W. J., and Wintle, B. A. (2014). Strategic foresight: how planning for the unpredictable can improve environmental decision-making. Trends Ecol. Evol. 29, 531–541. doi: 10.1016/j.tree.2014.07.005
Coonan, T. J., Bakker, V., Hudgens, B., Boser, C. L., Garcelon, D. K., Morrison, S. A., et al. (2014). On the fast track to recovery: island foxes on the northern channel islands. Monogr. West. N. Am. Nat. 7, 373–381. doi: 10.3398/042.007.0128
Costion, C. M., Liston, J., Kitalong, A. H., Iida, A., and Lowe, A. J. (2012). Using the ancient past for establishing current threat in poorly inventoried regions. Biol. Conserv. 147, 153–162. doi: 10.1016/j.biocon.2011.12.026
Dennis, E. B., Morgan, B. J. T., Brereton, T. M., Roy, B. D., and Fox, R. (2017). Using citizen science butterfly counts to predict species population trends. Conserv. Biol. 31, 1350–1361. doi: 10.1111/cobi.12956
Denoël, M., Perez, A., Cornet, Y., and Ficetola, G. F. (2012). Similar local and landscape processes affect both a common and a rare newt species. PLoS ONE 8:e62727. doi: 10.1371/journal.pone.0062727
De Vos, J. M., Joppa, L. N., Gittleman, J. L., Stephens, P. R., and Pimm, S. L. (2014). Estimating the normal background rate of species extinction. Conserv. Biol. 29, 452–462. doi: 10.1111/cobi.12380
Dowie, M. (2011). Conservation Refugees: The Hundred-Year Conflict between Global Conservation and Native Peoples. Cambridge, MA: MIT Press.
Drechsler, M., Eppink, F. V., and Wätzold, F. (2011). Does proactive biodiversity conservation save costs? Biodivers. Conserv. 20, 1045–1055. doi: 10.1007/s10531-011-0013-4
Dresser, C. M., Ogle, R. M., and Fitzpatrick, B. M. (2017). Genome scale assessment of a species translocation program. Conserv. Genet. 18, 1191–1199. doi: 10.1007/s10592-017-0970-6
Ellis, R. D., McWhorter, T. J., and Maron, M. (2012). Integrating landscape ecology and conservation physiology. Landscape Ecol. 27, 1–12. doi: 10.1007/s10980-011-9671-6
Gabor, C. R., Bosch, J., Fries, J. N., and Davis, D. R. (2013). A non-invasive water-borne hormone assay for amphibians. Amphibia Reptilia 34, 151–162. doi: 10.1371/journal.pone.0056054
Gabor, C. R., Zabierek, K. C., Kim, D. S., da Barbiano, L. A., Mondelli, M. J., Bendik, N. F., et al. (2016). A non-invasive water-borne assay of stress hormones in aquatic salamanders. Copeia 104, 172–181. doi: 10.1643/OT-14-207
Gallagher, A. J., Hammerschlag, N., Cooke, S. J., Costa, D. P., and Irschick, D. J. (2015). Evolutionary theory as a tool for predicting extinction risk. Trends Ecol. Evol. 30, 61–65. doi: 10.1016/j.tree.2014.12.001
Gaston, K. J., and Fuller, R. A. (2007). Commonness, population depletion and conservation biology. Trends Ecol. Evol. 23, 14–19. doi: 10.1016/j.tree.2007.11.001
Gibbons, J. W., Winne, C. T., Scott, D. E., Willson, J. D., Glaudas, X., Andrews, K. M., et al. (2006). Remarkable amphibian biomass and abundance in an isolated wetland: implications for wetland conservation. Conserv. Biol. 20, 1457–1465. doi: 10.1111/j.1523-1739.2006.00443.x
Gibbs, J. P., Droege, S., and Eagle, P. (1998). Monitoring populations of plants and animals. Bioscience 48, 935–940. doi: 10.2307/1313297
Housty, W. G., Noson, A., Scoville, G. W., Boulanger, J., Jeo, R. M., Darimont, C. T., et al. (2014). Grizzly bear monitoring by the Heiltsuk people as a crucible for First Nation conservation practice. Ecol. Soc. 19:70. doi: 10.5751/ES-06668-190270
Inger, R., Gregory, R., Duffy, J. P., Stott, I., Voríšek, P., and Gaston, K. J. (2015). Common European birds are declining rapidly while less abundant species' numbers are rising. Ecol. Lett. 18, 28–36. doi: 10.1111/ele.12387
Jachowski, D. S., and Kesler, D. C. (2009). Allowing extinction: should we let species go? Trends Ecol. Evol. 24:180. doi: 10.1016/j.tree.2008.11.006
Janin, A., Léna, J. P., Deblois, S., and Joly, P. (2012). Use of stress-hormone levels and habitat selection to assess functional connectivity of a landscape for an amphibian. Conserv. Biol. 26, 923–931. doi: 10.1111/j.1523-1739.2012.01910.x
Janin, A., Léna, J.-P., and Joly, P. (2011). Beyond occurrence: body condition and stress hormone as integrative indicators of habitat availability and fragmentation in the common toad. Biol. Conserv. 144, 1008–1016. doi: 10.1016/j.biocon.2010.12.009
Jeffrey, J. D., Hasler, C. T., Chapman, J. M., Cooke, S. J., and Suski, C. D. (2015). Linking landscape-scale disturbances to stress and condition of fish: implications for restoration and conservation. Integr. Comp. Biol. 55, 618–630. doi: 10.1093/icb/icv022
Joseph, L. N., Maloney, R. F., and Possingham, H. P. (2009). Optimal allocation of resources among threatened species: a project prioritization protocol. Conserv. Biol. 23, 328–338 doi: 10.1111/j.1523-1739.2008.01124.x
King, J. L., Duncan, C. L., and Garcelon, D. K. (2014). Status of the Santa Catalina Island Fox thirteen years after its decline. Monogr. West. N. Am. Nat. 7, 382–396. doi: 10.3398/042.007.0129
Lambrick, F. H., Brown, N. D., Lawrence, A., and Bebber, D. P. (2014). Effectiveness of community forestry in Prey Long Forest, Cambodia. Conserv. Biol. 28, 372–381. doi: 10.1111/cobi.12217
Li, J., Wang, D., Yin, H., Zhaxi, D., Jiagong, Z., Schaller, G. B., et al. (2013). Role of Tibetan Buddhist monasteries in snow leopard conservation. Conserv. Biol. 28, 87–94. doi: 10.1111/cobi.12135
Lindenmayer, D. B., Wood, J. T., McBurney, L., Youngentob, K., and Banks, S. C. (2011). How to make a common species rare: a case against conservation complacency. Biol. Conserv. 144, 1663–1672. doi: 10.1016/j.biocon.2011.02.022
Lockhart, J. M., Thorne, E. T., and Gober, D. R. (2006). “A historical perspective on recovery of the black-footed ferret and the biological and political challenges affecting its future,” in Recovery of the Black-Footed Ferret—Progress And Continuing Challenges: U.S. Geological Survey Scientific Investigations Report 2005–5293, eds J. E. Roelle, B. J. Miller, J. L. Godbey, and D. E. Biggins, 288, 6–19.
Madliger, C. L., Cooke, S. J., Crespi, E. J., Funk, J. L., Hultine, K. R., Hunt, K. E., et al. (2016). Success stories and emerging themes in conservation physiology. Conserv. Physiol. 4:cov057. doi: 10.1093/conphys/cov057
Malcom, J. W., Webber, W. M., and Li, Y. W. (2016). A simple, sufficient, and consistent method to score the status of threats and demography of imperiled species. PeerJ 4:e2230. doi: 10.7717/peerj.2230
Martin, T. G., Nally, S., Burbidge, A. A., Arnall, S., Garnett, S. T., Hayward, M. W., et al. (2012). Acting fast helps avoid extinction. Conserv. Lett. 5, 274–280. doi: 10.1111/j.1755-263X.2012.00239.x
McCarthy, D. P., Donald, P. F., Scharlemann, J. P., Buchanan, G. M., Balmford, A., Green, J. M., et al. (2012). Financial costs of meeting global biodiversity conservation targets: current spending and unmet needs. Science 338, 946–949. doi: 10.1126/science.1229803
McKee, A. M., Calhoun, D. L., Barichivich, W. J., Spear, S. F., Goldberg, C. S., and Glenn, T. C. (2015). Assessment of environmental DNA for detecting presence of imperiled aquatic amphibian species in isolated wetlands. J. Fish Wild. Manage. 6, 498–510. doi: 10.3996/042014-JFWM-034
McKinley, D. C., Miller-Rushing, A. J., Ballard, H. L., Bonney, R., Brown, H., Cook-Patton, S. C., et al. (2017). Citizen science can improve conservation science, natural resource management, and environmental protection. Biol. Conserv. 208, 15–28. doi: 10.1016/j.biocon.2016.05.015
Milanovich, J. R., and Peterman, W. E. (2016). Revisiting Burton and Likens (1975): nutrient standing stock and biomass of a terrestrial salamander in the midwestern United States. Copeia 104, 165–171. doi: 10.1643/OT-14-180
Mondal, I., Habib, B., Talukdar, G., and Nigam, P. (2016). Triage of means: options for conserving tiger corridors beyond designated protected lands in India. Front. Ecol. Evol. 4:133. doi: 10.3389/fevo.2016.00133
Moore, C. T., Lonsdorf, E. V., Knutson, M. G., Laskowski, H. P., and Lor, S. K. (2011). Adaptive management in the U.S. National Wildlife Refuge System: science-management partnerships for conservation delivery. J. Environ. Manage. 92, 1395–1402. doi: 10.1016/j.jenvman.2010.10.065
Morelli, F., and Møller, A. P. (2018). Pattern of evolutionarily distinct species among four classes of animals and their conservation status: a comparison using evolutionary distinctiveness scores. Biodivers. Conserv. 27, 381–394. doi: 10.1007/s10531-017-1441-6
Muller, B. J., and Schwarzkopf, L. (2017). Success of capture of toads improved by manipulating acoustic characteristics of lures. Pest Manage. Sci. 73, 2372–2378. doi: 10.1002/ps.4629
Pattberg, P., and Widerberg, O. (2016). Transnational multistakeholder partnerships for sustainable development: conditions for success. Ambio 45, 42–51. doi: 10.1007/s13280-015-0684-2
Peñaranda, D. A., and Simonetti, J. A. (2015). Predicting and setting conservation priorities for Bolivian mammals based on biological correlates of the risk of decline. Conserv. Biol. 29, 834–843. doi: 10.1111/cobi.12453
Peters, H., O'Leary, B. C., Hawkins, J. P., and Roberts, C. M. (2015). Identifying species at extinction risk using global models of anthropogenic impact. Glob. Change Biol. 21, 618–628. doi: 10.1111/gcb.12749
Petrovan, S. O., and Schmidt, B. R. (2016). Volunteer conservation action data reveals large-scale and long-term negative population trends of a widespread amphibian, the common toad (Bufo bufo). PLoS ONE 11:e0161943. doi: 10.1371/journal.pone.0161943
Roberts, D. L., Taylor, L., and Joppa, L. N. (2016). Threatened or data deficient: assessing the conservation status of poorly known species. Divers. Distrib. 22, 558–565. doi: 10.1111/ddi.12418
Salatas, J. H., Gard, N. W., Wickwire, T., and Menzie, C. (2013). Stressor analysis approaches for endangered species assessments. Nat. Sci. 5, 27–35. doi: 10.4236/ns.2013.55A004
Schaffer-Smith, D., Swenson, J. J., and Bȯveda-Penalba, A. J. (2016). Rapid conservation assessment for endangered species using habitat connectivity models. Environ. Conserv. 43, 221–230. doi: 10.1017/S0376892915000405
Semlitsch, R. D., O'Donnell, K. M., and Thompson, F. R. III. (2014). Abundance, biomass production, nutrient content, and the possible role of terrestrial salamanders in Missouri Ozark forest ecosystems. Can. J. Zool. 92, 997–1004. doi: 10.1139/cjz-2014-0141
Semlitsch, R. D., Walls, S. C., Barichivich, W. J., and O'Donnell, K. M. (2017). Extinction debt as a driver of amphibian declines: an example with imperiled flatwoods salamanders. J. Herpetol. 51, 12–18. doi: 10.1670/16-090
Shanee, N., Shanee, S., and Horwich, R. H. (2015). Effectiveness of locally run conservation initiatives in north-east Peru. Oryx 49, 239–247. doi: 10.1017/S0030605313001002
Shanee, S., and Shanee, N. (2015). Measuring success in a community conservation project: local population increase in a critically endangered primate, the yellow-tailed woolly monkey (Lagothrix flavicauda) at la Esperanza, northeastern Peru. Trop. Conserv. Sci. 8, 169–186. doi: 10.1177/194008291500800114
Silvertown, J. (2009). A new dawn for citizen science. Trends Ecol. Evol. 24, 467–471. doi: 10.1016/j.tree.2009.03.017
Sinervo, B., Méndez-de-la-Cruz, F., Miles, D. B., Heulin, B., Bastiaans, E., Villagrán-Santa Cruz, M., et al. (2010). Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899. doi: 10.1126/science.1184695
Stewart, K., Ma, H., Zheng, J., and Zhao, J. (2017). Using environmental DNA to assess population-wide spatiotemporal reserve use. Conserv. Biol. 31, 1173–1182. doi: 10.1111/cobi.12910
Strong, R., Martin, F. L., Jones, K. C., Shore, R. F., and Halsall, C. J. (2017). Subtle effects of environmental stress observed in the early life stages of the common frog, Rana temporaria. Sci. Rep. 7:44438. doi: 10.1038/srep44438
Taft, H. R., Roff, D. A., Komonen, A., and Kotiaho, J. S. (2013). A comparison of three statistical methods for analyzing extinction threat status. Environ. Conserv. 41, 37–44. doi: 10.1017/S0376892913000246
Tella, J. L., Rojas, A., Carrete, M., and Hiraldo, F. (2013). Simple assessments of age and spatial population structure can aid conservation of poorly known species. Biol. Conserv. 167, 425–434. doi: 10.1016/j.biocon.2013.08.035
Turkalo, A. K., Wrege, P. H., and Wittemyer, G. (2017). Slow intrinsic growth rate in forest elephants indicates recovery from poaching will require decades. J. Appl. Ecol. 54, 153–159. doi: 10.1111/1365-2664.12764
Valiente-Banuet, A., Aizen, M. A., Alcántara, J. M., Arroyo, J., Cocucci, A., Galetti, M., et al. (2015). Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307. doi: 10.1111/1365-2435.12356
Vance, E. (2017). Biology's beloved amphibian - the axolotl - is racing towards extinction Nature 551, 286–289. doi: 10.1038/d41586-017-05921-w
Vucetich, J. A., Nelson, M. P., and Bruskotter, J. T. (2017). Conservation triage falls short because conservation is not like emergency medicine. Front. Ecol. Evol. 5:45. doi: 10.3389/fevo.2017.00045
Waeber, P. O., Wilmé, L., Mercier, J. R., Camara, C., and Lowry, P. P. II. (2016). How effective have thirty years of internationally driven conservation and development efforts been in Madagascar? PLoS ONE 11:e0161115. doi: 10.1371/journal.pone.0161115
Wake, D. B., and Vredenburg, V. T. (2008). Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Nat. Acad. Sci. U.S.A. 105, 11466–11473. doi: 10.1073/pnas.0801921105
Washington, H., Baillie, J., Waterman, C., and Milner-Gulland, E. J. (2015). A framework for evaluating the effectiveness of conservation attention at the species level. Oryx 49, 481–491. doi: 10.1017/S0030605314000763
Wilcove, D. S., and Chen, L. Y. (1998). Management costs for endangered species. Conserv. Biol. 12, 1405–1407. doi: 10.1111/j.1523-1739.1998.97451.x
Williams, T. (2016). Recovery: America's Dwarf Fox Gets a Second Chance. Available online at: http://blog.nature.org/science/2016/08/15/recovery-americas-dwarf-fox-second-chance-channel-island-endangered/ (Accessed 24 January 2017).
Wilson, K. A., Carwardine, J., and Possingham, H. P. (2009). Setting conservation priorities. Ann. N.Y. Acad. Sci. 1162, 237–264. doi: 10.1111/j.1749-6632.2009.04149.x
Wilson, K. A., and Law, E. A. (2016). Ethics of conservation triage. Front. Ecol. Evol. 4:112. doi: 10.3389/fevo.2016.00112
Winfree, R., Fox, J. W., Williams, N. M., Reilly, J. R., and Cariveau, D. P. (2015). Abundance of common species, not species richness, drives delivery of a real-world ecosystem service. Ecol. Lett. 18, 626–635. doi: 10.1111/ele.12424
Keywords: citizen science, conservation triage, extinction risk, population declines, prioritization, proactive conservation, resource limitation, stakeholder collaboration
Citation: Walls SC (2018) Coping With Constraints: Achieving Effective Conservation With Limited Resources. Front. Ecol. Evol. 6:24. doi: 10.3389/fevo.2018.00024
Received: 29 October 2017; Accepted: 28 February 2018;
Published: 16 March 2018.
Edited by:
Krithi K. Karanth, Wildlife Conservation Society, IndiaReviewed by:
Bilal Habib, Wildlife Institute of India, IndiaBilal Butt, University of Michigan, United States
Varun R. Goswami, Wildlife Conservation Society, India
Copyright © 2018 Walls. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susan C. Walls, c3dhbGxzQHVzZ3MuZ292
 Susan C. Walls
Susan C. Walls