- 1Department of Recovery Ecology, Institute for Conservation Research, San Diego Zoo Global, Escondido, CA, United States
- 2Department of Environmental Science and Policy, University of California, Davis, Davis, CA, United States
- 3Evolutionsbiologie, Universität Bielefeld, Bielefeld, Germany
- 4Cognitive and Cultural Ecology Group, Max Plank Institute of Animal Behavior, Radolfzell, Germany
- 5Department of Human Behavior, Ecology, and Culture, Max Plank Institute for Evolutionary Anthropology, Leipzig, Germany
Many animals respond well behaviorally to stimuli associated with human-induced rapid environmental change (HIREC), such as novel predators or food sources. Yet others make errors and succumb to evolutionary traps: approaching or even preferring low quality, dangerous or toxic options, avoiding beneficial stimuli, or wasting resources responding to stimuli with neutral payoffs. A common expectation is that learning should help animals adjust to HIREC; however, learning is not always expected or even favored in many scenarios that expose animals to ecological and evolutionary traps. We propose a conceptual framework that aims to explain variation in when learning can help animals avoid and escape traps caused by HIREC. We first clarify why learning to correct two main types of errors (avoiding beneficial options and approaching detrimental options) might be difficult (limited by constraints). We then identify and discuss several key behavioral mechanisms (adaptive sampling, generalization, habituation, reversal learning) that can be targeted to help animals learn to avoid traps. Finally, we discuss how individual differences in neophobia/neophilia and personality relate to learning in the context of HIREC traps, and offer some general guidance for disarming traps. Given how devastating traps can be for animal populations, any breakthrough in mitigating trap outcomes via learning could make the difference in developing effective solutions.
Introduction
By altering food, predators and habitat, human-induced rapid environmental change (HIREC) presents organisms with new, survival-relevant decisions (Candolin and Wong, 2012; Sih, 2013; Wong and Candolin, 2015). On their first encounter with altered or novel situations (e.g., novel resources, habitats, or predators), animals often respond using their previously adaptive cue-response systems; e.g., respond to the smell of food by attacking, but respond to the smell or sight of danger by fleeing. One potential problem is that these previously adaptive systems may not continue to be adaptive post-HIREC. When previously adaptive cue-response pairings are mismatched with post-HIREC outcomes, animals can get drawn into ecological traps via maladaptive habitat preferences or range shifts (Battin, 2004; Hale et al., 2016), or commit themselves to evolutionary traps by mis-categorizing cues associated with novel food or predators (Robertson et al., 2013). The errors that cause traps can go “both ways.” They include the underuse of good habitat or resources (Gilroy and Sutherland, 2007), and the overuse of poor habitat (Robertson et al., 2013) or toxic “foods” (e.g., cane toads, Shine, 2010); as well as the under-avoidance of novel predators (Sih et al., 2010; Miles et al., 2013), and the over-avoidance of situations and habitats that are safe, but appear dangerous (Hale and Swearer, 2017; Trimmer et al., 2017).
While ecological traps are habitat based, evolutionary traps involve a wider context of errors (Schlaepfer et al., 2002; Robertson et al., 2013). Both share the common feature of driving animals toward population decline due to maladaptive behavioral choices (Schlaepfer et al., 2002; Robertson et al., 2013; Hale and Swearer, 2016). However, not all animals get drawn into traps; some immediately respond adaptively to novel circumstances (Sih, 2013), others escape via phenotypic plasticity (i.e., plastic rescue; Snell-Rood et al., 2018). Key questions are thus: what explains the variation in response to traps caused by HIREC (Sih et al., 2011, 2016; Sih, 2013), and can they be disarmed either by animals themselves or by human intervention?
As a major form of phenotypic plasticity, learning gives animals flexibility to respond to changes in their environment. Indeed, learning can be an important precursor and facilitator of future evolutionary change (West-Eberhard, 2003, 2005; Brown, 2013; Dukas and Dukas, 2017). Learning can allow animals to escape ecological and evolutionary traps (Schlaepfer et al., 2002; Greggor et al., 2014). Operationally, learning is defined as a change in behavior as a result of experience, excluding changes that can be attributed to physiological adaptation or reflexes (Shettleworth, 2010). Almost all animals have an ability to learn (Shettleworth, 2010). Thus, many ecologists and conservation biologists might start with the a priori expectation that if animals initially respond poorly, they ought to learn to exhibit more appropriate behavioral responses to novel situations. Yet, many species (Ellenberg et al., 2007) or individuals (Ellenberg et al., 2009) often do not learn to adjust behavior after responding sub-optimally to an altered or novel cue, despite having the capacity to learn (Berger-Tal and Saltz, 2016).
We turn the a priori expectation on its head and argue that the main types of errors that animals make can be inherently difficult to correct by learning. Our argument draws on existing concepts from animal cognition, evolutionary theory and behavioral ecology to provide a conceptual framework for explaining variation in learning outcomes. We first acknowledge areas where learning may not be necessary to respond to HIREC and then focus on situations in which learning could improve outcomes. We classify these situations based on how animals should ideally respond, and examine the errors animals can make initially before they have the opportunity to learn. Taking a cognitive perspective of these errors reveals potential barriers to learning that arise due to processes such as the spatio-temporal structure of cues, costs of learning, and constraints of the types of associations animals make. This backdrop of learning barriers serves as the foundation for our framework that explains how organisms might learn in the face of traps: e.g., via adaptive sampling or generalizing. We then draw on literature using a cost-benefit approach to generate general predictions on how an organism's evolutionary or developmental history might explain variation in behaviors relevant for escaping traps—for example, the tendency to sample options that previously adaptive cue-response systems suggest are poor options, but after HIREC, are now beneficial. We also discuss the role of individual differences in behavioral tendencies, in neophobia/neophilia and in personality that might explain variation in the ability to learn to cope with the traps HIREC presents.
The principles we draw on are not restricted to novel situations that cause traps. They apply whenever animals mis-assess situations and make suboptimal or maladaptive decisions. However, these mis-assessments are often of critical importance following HIREC because the pace of change can be drastically faster than would have occurred over evolutionary time, and even minor increases in the rate of change can tip animals toward extinction (Botero et al., 2015). Also, we refrain from providing detailed, specific recommendations for managers. Providing workable management advice will require expert knowledge on specific situations. Instead we provide applied examples where learning, or the lack thereof, has influenced the success of a species, and we create a framework for how conservationists might think about learning in their systems, in the hope of encouraging the future development of specific learning-focused interventions.
Prelude: Learning and Learning Ability Are Not Always the Key
Learning is not always necessary for organisms to avoid traps, and can even be deleterious. In some cases, animals' pre-existing, cue-response systems immediately produce an adaptive behavioral response (and thus little need for learning). For example, the “cue similarity” hypothesis (Sih et al., 2010; Carthey and Banks, 2016) notes that prey often immediately respond adaptively to exotic predators when those novel predators resemble familiar ones. Learning can even lead to traps when it predisposes animals to suboptimal behavior or human-wildlife conflict (Donaldson et al., 2012; Costa et al., 2016; Morehouse et al., 2016). For example, when seabirds first forage on discarded bycatch near fishing vessels, it may initially be a good choice since it allows them to gain food with little flight cost. However, when this becomes a learned cue—as has been documented for gannets (Morus capensis) and other seabirds—tracking fishing boats changes movement patterns (Oro et al., 2013), and can lead to an over-use of a lower quality food which is unsuitable for their chicks (Grémillet et al., 2008). Despite these costs, birds would be unlikely to change their behavior because it would require learning to avoid a seemingly rewarding stimulus, hence they are trapped. Similar patterns of learning around people emerge in other species such as bears (Mazur and Seher, 2008), suggesting that in many situations learning itself should not be considered a default survival tool.
Even if learning is not useful for all traps, how do we predict when and where learning could be beneficial? The breadth and flexibility in what organisms can learn clearly differs at both broad and narrower taxonomic levels, which makes predicting learning to escape traps based on species' learning ability a tempting prospect. For instance, primates can learn to flexibly respond to nuanced aspects of HIREC (Hockings et al., 2015) (e.g., deactivating snares) (Ohashi and Matsuzawa, 2011) in ways that are unlikely for snails. Meanwhile other species have more developmentally or contextually constrained learning abilities, which may only be effective in very well-defined contexts (e.g., flatworms' rapid anti-predator learning) (Wisenden and Millard, 2001). However, there can be variation in learning propensity even within narrower taxonomic groupings (e.g., among birds, amphibians, or primates), and surprising convergence between others (Emery and Clayton, 2004). Additionally, despite variation in the use of learning, it is not always the most cognitively flexible species (population or individuals) which succeed in using learning to avoid traps (e.g., even humans are susceptible to evolutionary traps, such as our insatiable attraction to sugar and fat) (Pijl, 2011). Therefore, learning ability alone can be a poor predictor of post-trap adjustment. Instead, we focus on the matches or mismatches between the types of traps HIREC produces and how likely animals are to perceive and respond to them. By taking a cognitive perspective on animals' responses, we can examine how evolutionarily-shaped learning and information gathering biases are likely to influence learning and trap outcomes.
We should note that although social learning is an equally valid means through which animals can gain information about escaping traps (see Barrett et al., 2019, this issue), this paper focuses on learning through individual experience. Social learning involves similar learning mechanisms to individual learning, but animals' use of social cues is subject to a different suite of biases than individual cues (Heyes, 1994, 2012). Therefore, we focus on individual learning to provide a simple foundation for learning to avoid traps, although we acknowledge some areas where social learning is likely highly relevant.
The Conceptual Framework: Breaking Down the Steps Required for Learning
By definition, traps result from a mismatch between the cues HIREC produces and animals' resultant behavior (Schlaepfer et al., 2002). To figure out whether learning can play a role in escaping maladaptive behavior, we need to break down the learning process that can occur between the HIREC cues and animals' responses. In this context, learning involves several stages: (1) encountering and perceiving cues; (2) responding to them; (3) experiencing an outcome; and (4) adjusting behavior based on that outcome. Cues can involve single or multiple stimuli that animals use for decision making, e.g., a novel food type, or several markers of habitat quality.
How an animal responds the first time it encounters a cue post-HIREC is critical for determining not only whether it survives the experience, but also what it learns about that experience (Figure 1). If animals do not perceive the novel or altered cues, they cannot use the cue to respond or to learn. HIREC can alter the perception of cues, which can lead to ecological and evolutionary traps that give little opportunity for learning. For example, when HIREC interferes with perception by increasing turbidity, fathead minnows (Pimephales promelas) fail to perceive visual cues from novel predators well enough to learn to respond to them (Ferrari et al., 2010).
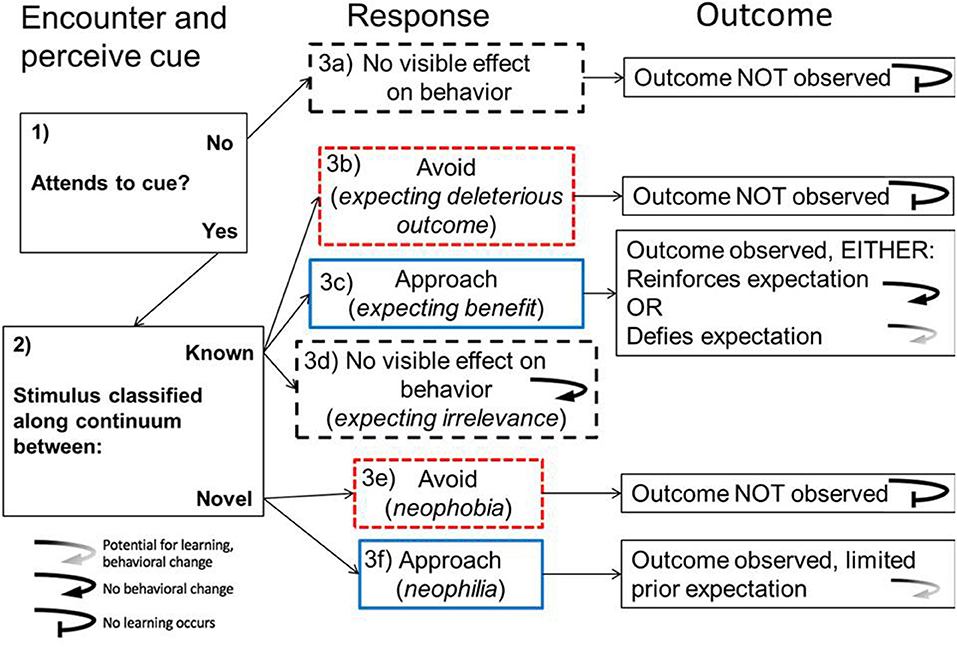
Figure 1. Stages of responses to HIREC and potential for learning. Different underlying motivations can promote approach, avoidance and apparent neutrality toward a stimulus. The outcome of the animal's behavior will direct future responses to that same stimulus. Even when an animal makes the wrong choice, if the animal is not able to observe the outcome or does not perceive the outcome to be relevant to the preceding behavior, then it will not learn about it. Straight arrows imply the flow of response. Curved arrows symbolize the different responses animals will have on subsequent encounters. The greater the extent that an experience defines expectations, the higher likelihood there will be learning. In cases where there is no behavioral change, learning may not be overtly observed, but animals could still gain information that reinforces details of their response or associated outcome.
Assuming that the stimulus is perceived, signal detection theory (Green and Swets, 1966) has been used to make predictions about how animals initially respond when encountering known and unknown cues (Wickens, 2001; Trimmer et al., 2017), partly based on how closely those cues match evolutionary or experienced norms (Sih et al., 2011; Robertson et al., 2013). For example, native Australian bush rats (Rattus fuscipes) exhibit a stronger anti-predator response to novel predators that are more closely related to known predators (Carthey and Banks, 2016). Other factors that affect whether animals correctly categorize novel stimuli include the specificity of the cues, and the asymmetry of costs associated with over vs. under-responding (Macmillan and Creelman, 2005; Ehlman et al., 2019). For example, prey are more likely to correctly respond to novel predators (e.g., flee) without the need for learning if the prey evolved in environments with a broad diversity of predators (the “multiple predator hypothesis”) (Blumstein, 2006), and if, in the past, the cost of under-responding was high (e.g., familiar predators were very dangerous), but the cost of over-responding (e.g., to non-predators that look like predators) was low. In such cases where animals respond correctly in their initial interactions, learning may not be needed for immediate survival, but could still be useful to fine tune their responses.
If animals perceive and attend to cues detected during initial encounters with novel or altered stimuli, they can respond in three basic ways: (1) avoid, (2) approach, or (3) ignore (no visible effect on behavior). As a generality, animals should have evolved to avoid bad options, approach or utilize good options, and ignore neutral ones. In Figure 2 we outline a 3 × 3 matrix which plots the potential outcomes when animals respond (i.e., avoid, approach, or ignore) to stimuli that are defined by their fitness values (beneficial, i.e., good; neutral, or; costly, i.e., bad). This simple categorization allows us to organize HIREC scenarios to predict the experiences and type of learning (e.g., reinforcement learning, reversal learning, habituation) required for behavioral change when errors occur. As a rule, whether animals learn about the cues presented depends on the likelihood that animal's experiences with said cues yield the relevant, perceivable and useable information required for learning. The different types of learning required tap into ideas about optimal sampling regimes, generalization, reversal learning, habituation, neophobia, and personality for predicting learning and survival amid HIREC. In the following sections, we discuss each of these ideas and concepts in detail, with a focus on testable predictions.
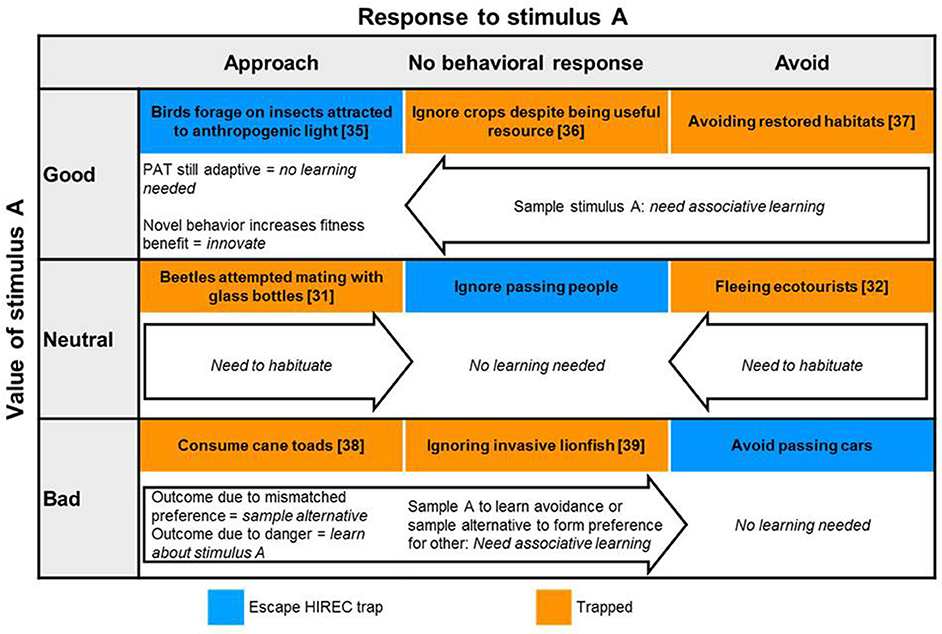
Figure 2. Categories and example consequences for responding to stimuli post-HIREC. Adaptive responses in blue, potential traps in orange. Learning routes for behavioral adjustment listed in arrows. Learning can only occur if potential traps offer multiple opportunities for response. Sampling can involve gathering individual information or social cues.
In our simple matrix of responses (approach, avoid, ignore) and fitness values (good, bad, neutral), there are three main types of errors (orange boxes, Figure 2) that animals can make on their initial encounter with a novel or altered stimulus. Each of these errors can lead to detrimental traps if they are repeated on subsequent encounters or on single occasions by multiple individuals.
• Avoid or ignore beneficial options (e.g., avoid high quality restored habitats, Hale and Swearer, 2017; or novel foods, Pearse et al., 2013);
• Approach or fail to avoid stimuli with negative fitness outcomes (e.g., consume novel toxic foods, Crossland et al., 2008; oviposit on invasive plants, Keeler and Chew, 2008; or allow close contact with novel predators, Miles et al., 2013; or pathogens, Bouwman and Hawley, 2010);
• Fail to ignore neutral stimuli (e.g., avoid or be stressed by passing tourists unnecessarily, Ellenberg et al., 2007).
Upon an animal's first interaction with a stimulus, the animal does not know if it has encountered something that is good, neutral, or bad. Avoiding good stimuli or approaching bad stimuli both have obvious sub-optimal fitness consequences. Importantly, the mis-categorization of neutral stimuli may also carry significant opportunity and energy costs (Gwynne and Rentz, 1983; Ydenberg and Dill, 1986; Trimmer et al., 2017), many of which are only recently being realized (Geffroy et al., 2015). Although intuition suggests learning is beneficial for correcting errors and escaping traps, theory in animal learning suggests various constraints might limit learning.
Whether learning can help animals respond appropriately also depends on the type of trap. Some traps only offer a single opportunity for animals to respond in their lifetime, because an error is fatal, or the trap involves a choice they only make once, such as spawning. In these cases individual learning cannot occur and the initial choice alone determines whether they are trapped. In other cases, traps that offer multiple opportunities to respond have the potential to allow for learning, but can fail to offer animals the experiences they need to learn. When faced with multiple opportunity traps, the learning type and behaviors necessary for escaping errors can differ by error type (as listed in the arrows, Figure 2), and by the stimulus type in question. In the next section, we discuss general evolved constraints, or limits, on learning. Later, we distinguish solutions that are most relevant for the different types of errors listed above.
Evolved Constraints on Learning: Why Experience May NOT Result in Learning
Learning hinges on experiencing salient and reliable cues that predict a relevant outcome, which can indicate a change in the rewards or dangers of a given situation. Even if an animal can perceive cue changes post-HIREC, the changes may lack salience (and thus be ignored) because of historical correlations between that cue and its outcomes. First, a particular cue (that is now meaningful) may have been unreliable in predicting fitness outcomes in the past. For example, species for which winter temperature did not predict spring conditions ignore warmer temperatures in the winter, and rely instead on photoperiod to time the onset of spring breeding (Dawson et al., 2001). Second, animals that evolved in conditions where the best behavior was highly certain did not need cues to guide their behavior (e.g., island animals that evolved in predator-free environments did not need and thus often ignore predator-relevant cues). Dunlap and Stephens “flag model” predicts that organisms should use cues to guide behavior primarily when cue reliability is high and the certainty of the best behavior (without using reliable cues) is relatively low (e.g., Dunlap and Stephens, 2009). Over evolutionary time, cue reliability was also influenced by the rate it changed relative to the lifespan of an animal. A very slow change would select for tendencies toward fixed genetic traits, but changes within the lifespan of an animal could select for phenotypic plasticity and learning potential (Botero et al., 2015). As a result, animal's evolutionary history may render certain types of HIREC cues irrelevant to them, regardless of the consequences. Research into how quickly cue biases disappear in the absence of selective pressure is highly relevant to predicting which species may be ill-equipped to recognize novel HIREC cues (see Carthey and Blumstein, 2017 for relevant discussion relating to predatory cue responses).
When cues are relevant to survival, evolution shapes animals' cognitive biases to increase the salience of the cue-response relationship, and reduce the number of cue presentations necessary for learning (Shettleworth, 2010). For example, during a sensitive period of development salmonid fish rapidly imprint on the chemical signature of their home stream to help them return for breeding since it has historically been a reliable indicator of stream location, which they only experience during a set time period of their life (Dittman and Quinn, 1996). While evolutionary advantageous, dependence on olfactory cues makes salmonids particularly susceptible to chemical pollutants (Tierney et al., 2010), and thus makes them unlikely to learn to adjust to this interference. Switching to different cues for homing, such as visual ones, would require overcoming a highly-ingrained cue bias.
Even when HIREC-altered or introduced cues are salient, salience may not promote optimal learning if the cues historically triggered a fixed response. For instance, animals may not learn that a high intensity sound predicts the appearance of food if the loud sound is overly salient and causes a fixed startle response, which can lead to sensitization (increased response with repeated exposure, the opposite of habituation), and continued avoidance (Blumstein, 2016). Therefore, there may be certain types of intense HIREC stimuli, such as the abrupt crack of a firearm that animals never learn to ignore or to use as a cue.
Learning can also be limited if animals do not have the opportunity to assess the outcome of their response (Schakner and Blumstein, 2016), i.e., their experience is temporally disconnected from their initial choice. For example, frogs often leave after laying eggs in a pond. Even if climate change results in ponds drying sooner and mass tadpole mortality, female frogs might not have access to the consequences of their action and thus would be unlikely to learn to choose deeper or differently positioned pools. Conversely, if the outcome occurs too quickly after the cue, animals may not have time to respond or learn from their experience. For instance, approaching traffic or trains may not allow enough time between perception and consequence to elicit appropriate avoidance (Cassady et al., 2019) (however, when traffic can be perceived in time to escape, habituation can be a separate issue, Lima et al., 2015). Finally, even if the animal can perceive the outcome, the number of cue-outcome pairings needed for learning to occur depends upon the evolved strength of that association. Some associations have evolved to be learned quickly to avoid deadly outcomes (e.g., fear conditioning and taste aversion, Garcia et al., 1974; Griffin, 2004), but others like spatial foraging preferences may take longer to change because the cues are noisier or have fewer immediate fitness consequences.
Predicting Post-HIREC Learning Despite Constraints
When faced with multiple-opportunity traps, reinforced, associative learning can help animals adjust to the errors outlined in Figure 2, assuming the outcome is salient. Different challenges to learning arise and thus different solutions are relevant, depending on whether the animals mistakenly avoid vs. approach the cue. We first address ways that animals might solve the mistake of avoiding novel beneficial options, and then move on to the problem of approaching or utilizing costly ones.
Solving the Problem of Avoiding or Ignoring Beneficial Options by Promoting Adaptive Sampling
When an organism's initial response is to avoid or ignore the novel or HIREC-altered stimulus, opportunities to learn are limited. If, however, animals sample novel foods or habitat, or approach novel organisms (that have not been identified as non-predators), this permits learning and potentially corrects initial errors. Thus, understanding when animals should sample (or not) is a key issue for predicting whether animals will gather information that allows them to escape traps associated with undervalued resources. Sampling rate is a previously adaptive trait shaped by past costs and benefits. Sampling benefits come in the form of additional information that can result in better future decisions, and costs include exposure to risks and wasted time and/or energy (i.e., opportunity costs).
Numerous models (Stephens, 1987, 2007; Dall et al., 1999; Eliassen et al., 2007) have explored simple scenarios where organisms may reduce uncertainty by sampling the environment. One such scenario gives animals the choice to stick with a known, mediocre (KM) option or sample a variable option (V) that is sometimes good, but sometimes bad. Even if the mean value of V is lower than the mean value of KM, frequent sampling is favored if the payoff of V in its “good state” is high enough relative to the payoff from KM, particularly if the cost of sampling is not too large. Thus, we predict animals to be more likely to sample and learn to use favorable options that they initially avoided if, in their evolutionary history, variable or unknown options were often exceptionally good relative to familiar, commonly utilized options. However, even if the “good” state of V is very good, highly stochastic reward schedules reduce useful information and are less likely to favor sampling. We also expect animals to not sample and thus remain ignorant about novel options if in the past, the cost of sampling was high; e.g., if it exposed animals to substantial risks of mortality or predation (Sih, 1992). In practice, for example, this theory would predict that species which live in environments with many poisonous potential prey items would be unlikely to sample a perfectly edible, invasive prey species.
Adaptive sampling also depends heavily on the rate of change in the value of the variable option. If V changes very rapidly, the organism does not have enough time to reap benefits before the option again becomes “bad”; there is no point in trying to track a rapidly fluctuating environment. If it changes very infrequently, sampling can be favored, but only occasionally. Thus, organisms should most readily sample and learn post-HIREC if they evolved in past conditions with a moderate rate of change, particularly when costs of sampling were low. Research that maps these change rates onto specific HIREC problems will be able to tap into a rich theoretical sampling literature. For example, there is evidence that urban populations sample more before switching foraging preferences, potentially due to living in a more variable environment (Griffin et al., 2016; Federspiel et al., 2017). Theory also predicts more sampling when organisms have a long lifetime to use information (Eliassen et al., 2007), when sampling reduces variation in fitness (Stephens, 1991) and when sampling substantially increases cue reliability (Abbott and Sherratt, 2013). Via these theories, we might logically predict that long-lived species with prior selection to learn about the type of HIREC-altered cue in question are going to be more likely to approach beneficial options that they initially avoided or ignored. In cases where gathering information individually is time-consuming or risky, social cues can also serve as a sampling mechanism (Rendell et al., 2011). Through this route animals can avoid having to personally sample potentially toxic foods (Thorogood et al., 2018), or interact with unknown predators (Griffin, 2004). Additionally, as a management technique, providing alternative cues that advertise the benefits of a given option, such as artificial social cues (e.g., Andrews et al., 2015), could help animals gain the experiences they need to stop avoiding or ignoring beneficial options.
Solving the Problem of Mistakenly Approaching Dangerous Options
Conversely, there are several reasons why animals that mistakenly approach dangerous options would have trouble learning to avoid these low fitness situations. First, they cannot learn to avoid single-opportunity traps if approaching the poor option kills them either immediately or via unrecoverable injury (e.g., Crossland et al., 2008), or they only make one choice in their lifetime (e.g., oviposition site choice in animals without parental care, Keeler and Chew, 2008). Second, they are unlikely to learn about multiple-opportunity traps if they cannot obtain information on the poor payoff of their choice, because they are not present, or because it is difficult to associate their behavior with the outcome (e.g., contracting an illness after interacting with a conspecific, Bouwman and Hawley, 2010). Finally, animals should be prone to approaching stimuli that HIREC has changed from good to bad if those options had historically been highly variable in short-term rewards, but stable in yielding good average returns; this is known as the partial reinforcement effect (Mackintosh, 1974; Houston et al., 1982). In that case, a run of poor payoffs could be viewed by the animal as simply a run of bad luck, and not an indication that the option has changed value. For example, many mammalian herbivores that commonly consume plants with varying levels of secondary compounds sample frequently (Freeland and Janzen, 1974), and thus may be more likely to continue sampling an unpalatable novel plant.
One way that animals might be less likely to “over-accept” poor options is if they become aware of highly attractive, beneficial, alternative options via sampling. This theory is used in practice when management action purposefully draws animals' attention toward alternative, beneficial options, such as encouraging settlement away from habitat sinks by broadcasting attractive cues in better areas (Patten and Kelly, 2010; Hale and Swearer, 2017). As discussed above, sampling rates should depend on the species' evolutionary history of costs and benefits, and individual differences (Pintor and Byers, 2015). In all cases, however, the effectiveness of sampling for producing optimal behavior depends on how much individuals generalize their sampling experience. For example, if an animal survives a negative experience eating a small, unpalatable, but not fatally-toxic cane toad, will it generalize to avoid a large cane toad carrying a fatal amount of toxin as the northern quoll (Dasyurus hallucatus) do (Kelly and Phillips, 2017)? How organisms categorize stimuli (e.g., safe vs. dangerous) is an important issue in cognitive ecology that can be critical for understanding how they respond to potential traps. Here, we summarize basic ideas on how a cost-benefit approach can be used to analyze adaptive generalizing.
Will Animals Generalize When Stimuli Change?
An organism's evolutionary history shapes its ability and tendency to discriminate cues and generalize from experiences (Shettleworth, 2010). Animals must generalize to some extent every time they encounter a cue or suite of cues—even known cues will differ slightly (in rotational appearance, intensity, etc.). The degree of similarity needed for an encountered cue to be generalized depends on the costs of under- vs. over-generalizing. Generalizing broadly is expected to be favored in contexts of danger; e.g., horses will quickly generalize their fear responses toward unknown objects, startling even toward known objects if presented from a different spatial perspective (Hanggi, 2005). In contrast, there are situations where generalizing would be unfavorable. For example in birds distinguishing their own eggs from brood parasites', overgeneralizing is very costly—thus birds may notice and respond very differently to small details in egg size or shell patterns (Spottiswoode and Stevens, 2011). In most cases, however, generalization depends upon the degree of novelty of a new cue. For instance, if the difference between known and novel is large, animals are less likely to respond adaptively and need more cue presentations before learning adaptive behavior (Ferrari et al., 2007, 2016).
These ancestral differences in the costs/benefits of generalizing influence both the neural wiring of the brain and how those synaptic connections change with experience. Psychological and computer science fields have a rich literature addressing the statistical bases and learning mechanisms of adaptive generalization (Shettleworth, 2010). One approach to understand the neural processes underlying generalization utilizes neural networks, consisting of sets of linked input and output nodes (similar, in principle, to clusters of neurons and their connections; Mitchell, 1997) to determine optimal categorization responses. If animals' experiences (inputs) are costly (in time, energy, or risk), their neural networks should be constrained to use salient features of stimuli. For instance, foraging can be a costly endeavor, and therefore animals can often focus on a narrow set of cues for making foraging decisions that can easily be over-generalized. However, if salient features are no longer the most relevant post-HIREC, then animals may be slow to generalize, or not learn to distinguish the novelty they encounter. For example, the narrowness of foraging cues becomes an issue when seabirds encounter ocean plastic because it emits dimethyl sulfide, a potent foraging cue (Savoca et al., 2016), and therefore many species are prone to errors of over-generalization based on a single cue error.
Responding appropriately to HIREC may require a change in the pattern of generalization. Whether that happens depends upon the cue type and learning type. For example, mis-categorizing predators as non-predators is costly. When an animal habituates to a predator-like cue, they habituate only to a precise cue presentation, which should not generalize to other predator-like stimuli (Hemmi and Merkle, 2009). In contrast, animals may readily generalize after a set of rewarding experiences. Chicks that experience numerous palatable novel foods are more likely to generalize about the palatability of a new food, reducing dietary wariness (Marples et al., 2007). A better understanding of generalization is important in scenarios ranging from the carryover of habituation from humans to natural predators (Geffroy et al., 2015), to the lethal mis-categorization of invasive species as native ones (Llewelyn et al., 2010). Experiments that assess to what extent the speed and breadth of category formation (as often measured in the lab) predicts accurate category formation around HIREC stimuli will be an important step in addressing these HIREC problems. For instance, the costs of overgeneralizing do not always map well onto the breadth of generalization tendencies as predicted (e.g., woodfrogs generalize to a similar extent in predatory and non-threatening contexts, despite the higher potential costs of generalizing around predators, Ferrari and Chivers, 2011).
Solving the Problem of Over-Responding to Irrelevant Stimuli
The third error type involves failing to recognize and learn the irrelevance of a stimulus; i.e., persistent over-responding (either avoiding or approaching) to options that are neither beneficial nor costly, and should be ignored. In essence, this is a problem of lack of habituation. Habituation is taxonomically widespread and in the strict psychological sense, it involves a reduced reaction to a specific, repeated stimulus through a simple form of learning (Rankin et al., 2009). Animals should habituate to irrelevant HIREC stimuli after repeated, predictable cues yield outcomes of little or no importance (Greggor et al., 2014). However, if animals always avoid novel stimuli (e.g., human habitats, human-generated noise, ecotourists), they will not experience the outcomes necessary for learning about irrelevance, and will not readily habituate to human activities. In addition, species' cognitive biases may make habituation toward some types and contexts of stimuli easier, even those that occur with equal frequency and strength (e.g., pigeons are less likely to habituate at night than during the day, Valentinuzzia and Ferraria, 1997). Compared to other learning types, research on habituation in the wild is in its early stages, and it is still unknown how many of the well-studied psychological habituation mechanisms apply in HIREC contexts (Nowacek et al., 2007; Blumstein, 2016; Schakner and Blumstein, 2016). Additionally it is unclear how habituation toward novel anthropogenic stimuli generalizes in a dangerous way to reduce wariness toward genuine threats such as predators (Geffroy et al., 2015; Trimmer et al., 2017). Testing differences in habituation speed/propensity between sympatric species that respond differently to HIREC (e.g., Blumstein, 2014) can help illuminate the extent to which habituation plays a role in their success and avoidance of traps. Meanwhile, testing the theory that increasing the regularity and reliability of harmless, but disturbing, cues should improve HIREC outcomes (Greggor et al., 2014), could guide management action that addresses this trap (Hale and Swearer, 2016).
Reversal Learning and Innovation
Apart from considering the error type animals make, how the cue-reward relationship has changed due to HIREC may also influence how easily animals will learn to adjust. Are the same cues available, but new reward contingencies present, or does the animal need to respond to a novel cue with a novel behavior to access a reward? If reward contingencies are swapped, i.e., if previously unrewarding stimuli become beneficial or previously rewarding stimuli no longer carry benefits, then animals face the challenge of reversal learning. Such a scenario could occur, for instance, if previously palatable crops are routinely sprayed with dangerous pesticides. Although species ranging from honey bees to primates have demonstrated reversal learning (Komischke, 2002), how often and easily an animal will reverse an association is related to their level of inhibitory control, memory retention (Gonzalez et al., 1967), and sampling rate (Dunlap and Stephens, 2012). Inhibitory control allows animals to suppress a habitual or well-learned response—thus providing opportunities to gather information about alternative responses or stimuli—and has been shown to correlate positively with reversal learning abilities (Bond et al., 2007; Shaw et al., 2015), despite stemming from different brain regions (Aron et al., 2014). Meanwhile, higher levels of memory retention allow animals to remember prior change rates, i.e., that associations may have swapped in the past. Finally, a higher propensity for sampling, also makes it likely that species will occasionally try the previously unrewarded option (Dunlap and Stephens, 2012), which makes them more likely to discover when reward contingencies have swapped.
Alternatively, if a different cue set needs to be learned to predict a known outcome (e.g., shifting from daylight cues to temperature cues to determine the seasonal onset of spring), then animals face a more difficult problem of set shifting (Roberts et al., 1988). Set shifting involves different brain regions than reversal learning, at least in several mammal species (McAlonan and Brown, 2003), and can decline due to age because it incurs substantial attentional costs (Barense, 2002). Little is known about how often set shifting occurs in the wild, or across species that vary in the number of cues they use, but it could be the only escape route for many HIREC traps.
Beyond learning whether an option is good or bad, animals often also need to learn what behavior they should perform once they have approached or avoided a stimulus. Even if the animal makes the correct response (e.g., approach a beneficial food item), they may still fail to behave optimally (e.g., exhibit an inappropriate attack strategy) after approaching the stimulus. In some cases, when faced with novel situations, animals may need to exhibit a novel behavior or devise a novel solution to a known problem (e.g., a behavioral innovation, Reader and Laland, 2003; Ramsey et al., 2007; Tebbich et al., 2016; Dukas and Dukas, 2017). Not all innovations are equally as challenging to develop, which is why the magnitude of the innovation needed for an animal to escape a HIREC trap may determine how likely the animal is to adjust their behavior optimally (Arbilly and Laland, 2017). Low magnitude innovations that rely on employing an existing behavior in a new context (e.g., exploiting a new foraging patch of known food), could occur via sampling a novel cue and generalizing a known behavioral action. In contrast, a high magnitude innovation involves the creation of an entirely novel behavior. For example, a new foraging technique, such as opening milk bottles (Hinde and Fisher, 1951), may require a more extensive set of trial and error learning steps and a wider behavioral repertoire (Arbilly and Laland, 2017). Predicting innovations requires an understanding of how animals interact with, and learn about, novelty and is also conditional upon the properties of innovators and behavioral context of plausible innovations (Perry et al., 2017).
Learning About Novelty
Thus, far we have considered responses to altered or novel cues without explicitly considering how animal reactions might depend on their relationship with novelty itself. Although neophobia and neophilia are often thought of as ends of one spectrum, experimental work suggests that they are distinct psychological phenomena driven by different evolutionary pressures which influence repulsion or attraction to novel cues, respectively, both based on the historical costs and benefits of interacting with novelty (reviewed in: Greenberg and Mettke-Hofmann, 2001; Mettke-Hofmann, 2014; Greggor et al., 2015). In creating a fear response, neophobia would trigger avoidance, which may look similar to fear around known threatening stimuli (Figure 1, Greggor et al., 2015). Meanwhile, neophilic reactions would initially look similar to attraction to known beneficial stimuli. Neophobic individuals should be more likely than neophilic individuals to correctly avoid novel bad options, but less likely to adopt novel good options. In general, the underlying motivation for avoidance or approach would influence how the animal's response would change over time.
Although neophobia and neophilia have been suggested as important for predicting species' responses to changing environments (Sol et al., 2011), and to serve as potential conservation tools (Greggor et al., 2014), the extent to which neophobia/philia influence responses to ecological and evolutionary traps remains unknown. In theory, the influence of neophobia/neophilia could be critical in determining responses toward single-opportunity traps, but their effect on multiple-opportunity traps is less clear. Effects of novelty wear off with subsequent encounters; thus, both the initial levels of neophobia or neophilia and the rate at which attraction or repulsion toward novelty decays likely influence long-term responses. Additionally, the effect of neophobia on learning may depend on the learning type in question (Griffin and Guez, 2014). Innovative problem solving that requires persistence appears to be inhibited by neophobia, while learning that relies on inhibiting initial interactions, such as reversal learning could benefit from neophobia (Mathieu et al., 2012; Griffin and Guez, 2014; Guillette et al., 2014; Bebus et al., 2016). In contrast, neophilia can also influence learning if it increases sampling rate, but it can also increase the likelihood of an animal approaching a deleterious, novel cue. Additionally, whether animals have enough encounters to adequately learn adaptive choices once novelty is no longer the most salient cue depends upon the stimulus, memory retention, and lifespan of the animal. Even if animals make beneficial choices once novelty dissipates, there can still be opportunity costs in delaying their choices.
Animal Personalities and Learning in Response to HIREC
Finally, we consider how individual differences in “trapability” might also depend on the animal's personality or behavioral type (BT) as shaped by past selection and experience. Individuals within and between populations often differ consistently in their behavioral tendencies (e.g., aggressiveness, boldness, exploratory tendency) across time and ecological contexts (Sih et al., 2004; Réale et al., 2007). Although boldness, for example, clearly varies depending on ecological and social conditions, some individuals are consistently bolder and others consistently more fearful (shy, cautious) than others. In the context of HIREC, the animal's BT likely impacts each step in the formation of traps. As a broad generality, bold, exploratory animals are exposed more often to novel stimuli than shy, unexploratory ones (Cote et al., 2010; Spiegel et al., 2017). Therefore, bold, aggressive, or exploratory individuals should be more likely to approach and less likely to avoid novel cues. These effects of personality likely influence the relative success of individuals or populations in the face of traps, depending on how cues have changed. When HIREC produces novel dangers, the bolder species and individuals would be more likely to commit an error in approaching human-influenced stimuli, as can be the case for the individuals within populations that contribute to heightened human-wildlife conflict (Swan et al., 2017). In contrast, where HIREC produces novel, beneficial cues, such as access to new habitats, the less bold species and individuals would be more likely to commit avoidance errors. For example, invasive populations of cane toads, which are benefitting from approaching novel habitats, contain more bolder, exploratory phenotypes than native ones do (Candler and Bernal, 2015). In this way, even if traps do not lead to precipitous species or population decline, they may exert strong selection pressures based on personality phenotypes (e.g., recreational hunters can be more likely to catch bolder individuals, Ciuti et al., 2012; Madden and Whiteside, 2014).
With regard to subsequent learning, Sih and Del Giudice (2012) suggest and present evidence supporting the general hypothesis that high risk, high reward BTs might tend to be quicker to learn new activity-based tasks (and thus adopt novel resources or habitats that cautious individuals avoid), but tend to be slower to learn novel avoidance tasks, and slower to exhibit reversal learning. The animal's BT might directly affect learning tendencies, or both BT and learning might be associated with individual differences in hormonal stress response systems along a proactive-reactive axis (Koolhaas et al., 1999). Reactive animals tend to be more fearful (and thus less likely to explore novel situations), but more sensitive to (i.e., more likely to notice and learn about) environmental changes. Although these ideas seem intuitively plausible, they are probably oversimplified. A recent critique of the field connecting personality and cognition emphasizes that relationships are likely to be complex and both context and task- dependent (Griffin et al., 2015); nonetheless, a better understanding of within-species, individual differences in learning to better respond to HIREC should be insightful.
Disarming Traps
Since there are many scenarios where animals will be unlikely to escape traps on their own, knowing where and when learning should be targeted could help disarm or prevent traps more effectively. In general it has been suggested that evolutionary traps can be disarmed by: reducing the attractiveness of poor resources, increasing the fitness value of these resources, or a combination of the two (Robertson et al., 2013). These suggestions can be made more specific when behavioral decisions and cognitive theory is considered in the process of disarming traps (Greggor et al., 2014; Hale and Swearer, 2016; Hale et al., 2018; Cassady et al., 2019). Throughout this paper we have identified areas of future research (Table 1), and illustrated a number of potential techniques. Although most of the techniques come down to the basics of attracting and repelling animals, we present them with the caveat that the most effective techniques for manipulating attraction and repulsion are still unknown (Greggor et al., 2016). Additionally, since the mechanism underlying an ecological trap can be a challenge to identify (Hale and Swearer, 2017), the relevant cues and experiences for a given trap may not be immediately apparent. Finally, the relationship between good and bad cues can be complex in the real world. HIREC changes can result in good cues (e.g., novel food resources) being presented alongside bad cues (e.g., new roads), and understanding how animals navigate these minefields of changes can require thinking about systems holistically. That being said, there are some guidelines that may be useful for thinking through solutions to traps.
Different approaches are likely necessary for disarming or preventing traps depending on whether they are single-opportunity or multiple-opportunity traps. Single-opportunity traps allow no space for individual learning. Therefore, animals must either be discouraged from interacting with the trapping cues initially by using deterrents (e.g., keeping marine mammals away from fishing nets, reviewed in Schakner and Blumstein, 2013), or drawing them to alternatives with rewards or social cues (Andrews et al., 2015). If traps are single opportunity traps because their cues occur too quickly for animals to respond (e.g., oncoming trains), then offering animals an additional warning cue may allow them to make associations which they otherwise would have been incapable of (e.g., giving bears a warning system that allows them to learn to avoid trains, Cassady et al., 2019). If none of the above is possible, single-opportunity traps need to be removed from the environment (e.g., changing the wavelength of light to prevent attractiveness for moths, or birds, Jones and Francis, 2003; van Langevelde et al., 2011).
Some multiple-opportunity traps may still be difficult to disarm with learning. For example, traps that result from interference with existing cues, rather than changes to the cue themselves (e.g., electromagnetic noise disrupting migrating birds' magnetic compass, Engels et al., 2014) are only likely to be alleviated by removal of the interference, since it can be a challenge for animals to set-shift to a new set of predicative cues. In contrast, there are a number of potential options for disarming multiple-opportunity traps when cue perception remains intact post-HIREC. However, their effectiveness will depend upon what type of error animals make. Encouraging animals to sample alternative options may help when animals approach bad options, or avoid good options. How one goes about encouraging sampling will depend upon the reason why it was not sampled in the first place, and whether we have any power to change the cues available. If animals have not sampled the beneficial option because they have not interacted with it spatially, then encouraging sampling by adding attractive cues to the beneficial option and repulsive cues to the detrimental option could help. In contrast, if animals approach bad options because those options themselves offer the most alluring cues, the only way to disarm the bad options is to remove the cues it offers. Finally, fixing errors relating to habituation may involve changing the repetition or predictability of cues to either encourage animals to ignore stimuli or facilitate their attention toward it. In all cases, understanding the mechanism underlying the trap will help determine if learning applies and target the correct learning ability if necessary.
Conclusions
There are many instances where learning can help animals avoid or escape potential traps caused by HIREC. In some situations, however, animals will not learn, or would do better by not learning. The error types we identify and the potential routes for learning they generate help highlight the circumstances where learning (or not) should be important for survival post-HIREC. While learning outcomes are challenging to predict, we are closer than ever to understanding the processes involved. Examining mismatches between the cognitive specializations that animals possess, and specific changes to cues may help explain why certain species commit errors post-HIREC. However, without greater attention toward patterns of sampling, generalization and individual differences in neophobia and personality, we will not understand when, or why, individuals or species escape their errors (or fail to do so). By focusing on the evolved constraints surrounding these processes we should better predict which animals will adjust to specific HIREC changes or need our help in disarming the traps we lay.
Author Contributions
All authors contributed to the ideas and writing of this review. All authors approved of the final submitted version.
Funding
PT was supported by the NSF (IOS 1456724 grant to AS) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), project number 316099922 - TRR 212. AG was supported by a Clark-Endowed postdoctoral fellowship to San Diego Zoo Global. We acknowledge the financial support of the Max Plank Institute for the article processing charge.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dave Stephens, Phil Crowley, and the entire Sih lab for their input and discussion, and Bryce Masuda for feedback on the figures.
References
Abbott, K. R., and Sherratt, T. (2013). Optimal sampling and signal detection: unifying models of attention and speed-accuracy tradeoffs. Behav. Ecol. 24, 605–616. doi: 10.1093/beheco/art001
Andrews, J. E., Brawn, J. D., and Ward, M. P. (2015). When to use social cues: conspecific attraction at newly created grasslands. Condor 117, 297–305. doi: 10.1650/CONDOR-14-172.1
Arbilly, M., and Laland, K. N. (2017). The magnitude of innovation and its evolution in social animals. Proc. R. Soc. B Biol. Sci. 284:20162385. doi: 10.1098/rspb.2016.2385
Aron, A., Robbins, T. W., and Poldrack, R. A. (2014). Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci. 18, 177–185. doi: 10.1016/j.tics.2013.12.003
Barense, M. D. (2002). Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn. Mem. 9, 191–201. doi: 10.1101/lm.48602
Barrett, B. J., Zepeda, E., Pollack, L., Munson, A., and Sih, A. (2019). Counter-culture: does social learning help or hinder adaptive response to human-induced rapid environmental change. Front. Ecol. Evol. 7:183. doi: 10.3389/fevo.2019.00183
Battin, J. (2004). When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv. Biol. 18, 1482–1491. doi: 10.1111/j.1523-1739.2004.00417.x
Bebus, S. E., Small, T. W., Jones, B. C., Elderbrock, E. K., and Schoech, S. J. (2016). Associative learning is inversely related to reversal learning and varies with nestling corticosterone exposure. Anim. Behav. 111, 251–260. doi: 10.1016/j.anbehav.2015.10.027
Berger-Tal, O., and Saltz, D. (2016). “Behavioral rigidity in the face of anthropogenic change,” in Conservation Behavior, eds O. Berger-Tal and D. Saltz (Cambridge: Cambridge University Press), 95–120.
Blumstein, D. T. (2006). The multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethology 112, 209–217. doi: 10.1111/j.1439-0310.2006.01209.x
Blumstein, D. T. (2014). “Attention, habituation, and antipredator behaviour: implications for urban birds,” in Avian Urban Ecology: Behavioural and Physiological Adaptations, eds D. Gill and H. Brumm (Oxford: Oxford University Press), 41–53.
Blumstein, D. T. (2016). Habituation and sensitization: new thoughts about old ideas. Anim. Behav. 120, 255–262. doi: 10.1016/j.anbehav.2016.05.012
Bond, A. B., Kamil, A. C., and Balda, R. P. (2007). Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica). J. Comp. Psychol. 121, 372–379. doi: 10.1037/0735-7036.121.4.372
Botero, C. A., Weissing, F. J., Wright, J., and Rubenstein, D. R. (2015). Evolutionary tipping points in the capacity to adapt to environmental change. PNAS 112, 184–189. doi: 10.1073/pnas.1408589111
Bouwman, K. M., and Hawley, D. M. (2010). Sickness behaviour acting as an evolutionary trap? Male house finches preferentially feed near diseased conspecifics. Biol. Lett. 6, 462–465. doi: 10.1098/rsbl.2010.0020
Brown, R. L. (2013). Learning, evolvability and exploratory behaviour: extending the evolutionary reach of learning. Biol. Philos. 28, 933–955. doi: 10.1007/s10539-013-9396-9
Candler, S., and Bernal, X. E. (2015). Differences in neophobia between cane toads from introduced and native populations. Behav. Ecol. 26, 97–104. doi: 10.1093/beheco/aru162
Candolin, U., and Wong, B. B. M. (eds.). (2012). Behavioural Responses to a Changing World; Mechanisms and Consequences. Oxford: Oxford University Press.
Carthey, A. J. R., and Banks, P. B. (2016). Naiveté is not forever: responses of a vulnerable native rodent to its long term alien predators. Oikos 125, 918–926. doi: 10.1111/oik.02723
Carthey, A. J. R., and Blumstein, D. T. (2017). Predicting predator recognition in a changing world. Trends Ecol. Evol. 33, 106–115. doi: 10.1016/j.tree.2017.10.009
Cassady, C., Clair, S., Backs, J., Friesen, A., Gangadharan, A., Gilhooly, P., et al. (2019). Animal learning may contribute to both problems and solutions for wildlife – train collisions. Philos. Trans. R. Soc. B Biol. Sci. 374:20180050. doi: 10.1098/rstb.2018.0050
Ciuti, S., Muhly, T. B., Paton, D. G., McDevitt, A. D., Musiani, M., and Boyce, M. S. (2012). Human selection of elk behavioural traits in a landscape of fear. Proc. Biol. Sci. 279, 4407–4416. doi: 10.1098/rspb.2012.1483
Costa, T. M., Hebets, E. A., Melo, D., and Willemart, R. H. (2016). Costly learning: preference for familiar food persists despite negative impact on survival. Biol. Lett. 12:20160256. doi: 10.1098/rsbl.2016.0256
Cote, J., Clobert, J., Brodin, T., Fogarty, S., and Sih, A. (2010). Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos. Trans. R. Soc. B Biol. Sci. 365, 4065–4076. doi: 10.1098/rstb.2010.0176
Crossland, M. R., Brown, G. P., Anstis, M., Shilton, C. M., and Shine, R. (2008). Mass mortality of native anuran tadpoles in tropical Australia due to the invasive cane toad (Bufo marinus). Biol. Conserv. 141, 2387–2394. doi: 10.1016/j.biocon.2008.07.005
Dall, S. R., McNamara, J. M., and Cuthill, I. C. (1999). Interruptions to foraging and learning in a changing environment. Anim. Behav. 57, 233–241. doi: 10.1006/anbe.1998.0944
Dawson, A., King, V. M., Bentley, G. E., and Ball, G. F. (2001). Photoperiodic control of seasonality in birds. J. Biol. Rythms 16, 365–380. doi: 10.1177/074873001129002079
Dittman, A., and Quinn, T. (1996). Homing in Pacific salmon: mechanisms and ecological basis. J. Exp. Biol. 199, 83–91.
Donaldson, R., Finn, H., Bejder, L., Lusseau, D., and Calver, M. (2012). Social learning of risky behaviour: importance for impact assessments, conservation and management of human-wildlife interactions. Anim. Conserv. 15, 442–444. doi: 10.1111/j.1469-1795.2012.00601.x
Dukas, R., and Dukas, R. (2017). Cognitive innovations and the evolutionary biology of expertise. Philos. Trans. R. Soc. B Biol. Sci. 372:20160427. doi: 10.1098/rstb.2016.0427
Dunlap, A. S., and Stephens, D. W. (2009). Components of change in the evolution of learning and unlearned preference. Proc. Biol. Sci. 276, 3201–3208. doi: 10.1098/rspb.2009.0602
Dunlap, A. S., and Stephens, D. W. (2012). Tracking a changing environment: optimal sampling, adaptive memory and overnight effects. Behav. Processes 89, 86–94. doi: 10.1016/j.beproc.2011.10.005
Ehlman, S. M., Trimmer, P. C., and Sih, A. (2019). Prey responses to exotic predators: effects of old risk and new cues. Am. Nat. 193, 575–587. doi: 10.1086/702252
Eliassen, S., Jorgensen, C., Mangel, M., and Giske, J. (2007). Exploration or exploitation: life expectancy changes the value of learning in foraging strategies. Oikos 116, 513–523. doi: 10.1111/j.2006.0030-1299.15462.x
Ellenberg, U., Mattern, T., and Seddon, P. J. (2009). Habituation potential of yellow-eyed penguins depends on sex, character and previous experience with humans. Anim. Behav. 77, 289–296. doi: 10.1016/j.anbehav.2008.09.021
Ellenberg, U., Setiawan, A. N., Cree, A., Houston, D. M., and Seddon, P. J. (2007). Elevated hormonal stress response and reduced reproductive output in Yellow-eyed penguins exposed to unregulated tourism. Gen. Comp. Endocrinol. 152, 54–63. doi: 10.1016/j.ygcen.2007.02.022
Emery, N. J., and Clayton, N. S. (2004). The mentality of crows: convergent evolution of intelligence in corvids and apes. Science 306, 1903–1907. doi: 10.1126/science.1098410
Engels, S., Schneider, N.-L., Lefeldt, N., Hein, C. M., Zapka, M., Michalik, A., et al. (2014). Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature 509, 353–356. doi: 10.1038/nature13290
Federspiel, I. G., Garland, A., Guez, D., Bugynar, T., Healy, S. D., Güntürkün, O., et al. (2017). Adjusting foraging strategies: a comparison of rural and urban common mynas (Acridotheres tristis). Anim. Cogn. 20, 65–74. doi: 10.1007/s10071-016-1045-7
Ferrari, M. C. O., and Chivers, D. P. (2011). Learning about non-predators and safe places: the forgotten elements of risk assessment. Anim. Cogn. 14, 309–316. doi: 10.1007/s10071-010-0363-4
Ferrari, M. C. O., Crane, A. L., and Chivers, D. P. (2016). Certainty and the cognitive ecology of generalization of predator recognition. Anim. Behav. 111, 207–211. doi: 10.1016/j.anbehav.2015.10.026
Ferrari, M. C. O., Gonzalo, A., Messier, F., and Chivers, D. P. (2007). Generalization of learned predator recognition: an experimental test and framework for future studies. Proc. R. Soc. B Biol. Sci. 274, 1853–1859. doi: 10.1098/rspb.2007.0297
Ferrari, M. C. O., Lysak, K. R., and Chivers, D. P. (2010). Turbidity as an ecological constraint on learned predator recognition and generalization in a prey fish. Anim. Behav. 79, 515–519. doi: 10.1016/j.anbehav.2009.12.006
Freeland, W. J., and Janzen, D. H. (1974). Strategies in herbivory by mammals: the fole of plant secondary compounds. Am. Nat. 108, 296–289. doi: 10.1086/282907
Garcia, J., Hankins, W. G., and Rusiniak, K. W. (1974). Behavioral regulation of the milieu interne in man and rat. Science 185, 824–831. doi: 10.1126/science.185.4154.824
Geffroy, B., Samia, D. S. M., Bessa, E., and Blumstein, D. T. (2015). How nature-based tourism might increase prey vulnerability to predators. Trends Ecol. Evol. 30, 755–765. doi: 10.1016/j.tree.2015.09.010
Gilroy, J. J., and Sutherland, W. J. (2007). Beyond ecological traps: perceptual errors and undervalued resources. Trends Ecol. Evol. 22, 351–356. doi: 10.1016/j.tree.2007.03.014
Gonzalez, R. C., Behrend, E. R., and Bitterman, M. E. (1967). Reversal learning and forgetting in bird and fish. Science 158, 519–521. doi: 10.1126/science.158.3800.519
Greenberg, R., and Mettke-Hofmann, C. (2001). “Ecological aspects of neophobia and neophilia in birds,” in Current Ornithology, eds V. Nolan Jr. and C. F. Thompson, 119–178.
Greggor, A. L., Berger-Tal, O., Blumstein, D. T., Angeloni, L., Bessa-Gomes, C., Blackwell, B. F., et al. (2016). Research priorities from animal behaviour for maximising conservation progress. Trends Ecol. Evol. 31, 953–964. doi: 10.1016/j.tree.2016.09.001
Greggor, A. L., Clayton, N. S., Phalan, B., and Thornton, A. (2014). Comparative cognition for conservationists. Trends Ecol. Evol. 29, 489–495. doi: 10.1016/j.tree.2014.06.004
Greggor, A. L., Thornton, A., and Clayton, N. S. (2015). Neophobia is not only avoidance: improving neophobia tests by combining cognition and ecology. Curr. Opin. Behav. Sci. 6, 82–89. doi: 10.1016/j.cobeha.2015.10.007
Grémillet, D., Pichegru, L., Kuntz, G., Woakes, A. G., Wilkinson, S., Crawford, R. J. M., et al. (2008). A junk-food hypothesis for gannets feeding on fishery waste. Proc. Biol. Sci. 275, 1149–1156. doi: 10.1098/rspb.2007.1763
Griffin, A. S. (2004). Social learning about predators: a review and prospectus. Learn. Behav. 32, 131–140. doi: 10.3758/BF03196014
Griffin, A. S., and Guez, D. (2014). Innovation and problem solving: a review of common mechanisms. Behav. Processes 109(Pt. B), 121–134. doi: 10.1016/j.beproc.2014.08.027
Griffin, A. S., Guillette, L. M., and Healy, S. D. (2015). Cognition and personality: an analysis of an emerging field. Trends Ecol. Evol. 30, 207–214. doi: 10.1016/j.tree.2015.01.012
Griffin, A. S., Tebbich, S., and Bugynar, T. (2016). Animal cognition in a human-dominated world. Anim. Cogn. 20, 1–6. doi: 10.1007/s10071-016-1051-9
Guillette, L. M., Hahn, A. H., Hoeschele, M., Przyslupski, A. M., and Sturdy, C. B. (2014). Individual differences in learning speed, performance accuracy and exploratory behaviour in black-capped chickadees. Anim. Cogn. 18, 165–178. doi: 10.1007/s10071-014-0787-3
Gwynne, D. T., and Rentz, D. C. F. (1983). Beetles on the bottle: male buprestids mistake stubbies for females (Coleoptera). J. Aust. Entomol. Soc. 22, 79–80. doi: 10.1111/j.1440-6055.1983.tb01846.x
Hale, R., Coleman, R., Sievers, M., Brown, T. R., and Swearer, S. E. (2018). Using conservation behavior to manage ecological traps for a threatened freshwater fish. Ecosphere 9:e02381. doi: 10.1002/ecs2.2381
Hale, R., Morrongiello, J. R., and Swearer, S. E. (2016). Evolutionary traps and range shifts in a rapidly changing world. Biol. Lett. 12:20160003. doi: 10.1098/rsbl.2016.0003
Hale, R., and Swearer, S. E. (2016). Ecological traps: current evidence and future directions. Proc. R. Soc. Lond. B Biol. Sci. 283:20152647. doi: 10.1098/rspb.2015.2647
Hale, R., and Swearer, S. E. (2017). When good animals love bad restored habitats: how maladaptive habitat selection can constrain restoration. J. Appl. Ecol. 54, 1478–1486. doi: 10.1111/1365-2664.12829
Hanggi, E. B. (2005). “The thinking horse: cognition and perception reviewed,” in American Association of Equine Practitioners 51st Annual Convention Proceedings (Seattle, WA), 246–255.
Hemmi, J. M., and Merkle, T. (2009). High stimulus specificity characterizes anti-predator habituation under natural conditions. Proc. R. Soc. B Biol. Sci. 276, 4381–4388. doi: 10.1098/rspb.2009.1452
Heyes, C. (2012). Simple minds: a qualified defence of associative learning. Philos. Trans. R. Soc. B Biol. Sci. 367, 2695–2703. doi: 10.1098/rstb.2012.0217
Heyes, C. M. (1994). Social learning in animals: categories and mechanisms. Biol. Rev. Camb. Philos. Soc. 69, 207–231. doi: 10.1111/j.1469-185X.1994.tb01506.x
Hinde, R. A., and Fisher, J. (1951). Further observations on the opening of milk bottles by birds. Br. Birds 44, 393–396.
Hockings, K. J., McLennan, M. R., Carvalho, S., Ancrenaz, M., Bobe, R., Byrne, R. W., et al. (2015). Apes in the Anthropocene: flexibility and survival. Trends Ecol. Evol. 30, 215–222. doi: 10.1016/j.tree.2015.02.002
Houston, A. I., Kacelnik, A., and McNamara, J. M. (1982). “Some learning rules for acquiring information,” in Functional Ecology, ed D. J. McFarland (London: Pitman), 140–191.
Jones, J., and Francis, C. M. (2003). The effects of light characteristics on avian mortality at lighthouses. J. Avian Biol. 34, 328–333. doi: 10.1111/j.0908-8857.2003.03183.x
Keeler, M. S., and Chew, F. S. (2008). Escaping an evolutionary trap: preference and performance of a native insect on an exotic invasive host. Oecologia 156, 559–568. doi: 10.1007/s00442-008-1005-2
Kelly, E., and Phillips, B. L. (2017). Get smart: native mammal develops toad-smart behavior in response to a toxic invader. Behav. Ecol. 28, 854–858. doi: 10.1093/beheco/arx045
Komischke, B. (2002). Successive olfactory reversal learning in honeybees. Learn. Mem. 9, 122–129. doi: 10.1101/lm.44602
Koolhaas, J. M., Korte, S. M., de Boer, S. F., Van Der Vegt, B. J., Van Reenen, C. G., Hopster, H., et al. (1999). Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. doi: 10.1016/S0149-7634(99)00026-3
Lima, S. L., Blackwell, B. F., Devault, T. L., and Fernández-Juricic, E. (2015). Animal reactions to oncoming vehicles: a conceptual review. Biol. Rev. 90, 60–76. doi: 10.1111/brv.12093
Llewelyn, J., Webb, J. K., Schwarzkopf, L., Alford, R., and Shine, R. (2010). Behavioural responses of carnivorous marsupials (Planigale maculata) to toxic invasive cane toads (Bufo marinus). Aust. Ecol. 35, 560–567. doi: 10.1111/j.1442-9993.2009.02067.x
Macmillan, N. A., and Creelman, C. D. (2005). Detection Theory: A User's Guide, 2nd Edn. Mahway, NJ: Lawrence Erlbaum.
Madden, J. R., and Whiteside, M. A. (2014). Selection on behavioural traits during “unselective” harvesting means that shy pheasants better survive a hunting season. Anim. Behav. 87, 129–135. doi: 10.1016/j.anbehav.2013.10.021
Marples, N. M., Quinlan, M., Thomas, R. J., and Kelly, D. J. (2007). Deactivation of dietary wariness through experience of novel food. Behav. Ecol. 18, 803–810. doi: 10.1093/beheco/arm053
Mathieu, A., van Oers, K., and Naguib, M. (2012). Worms under cover: relationships between performance in learning tasks and personality in great tits (Parus major). Anim. Cogn. 15, 763–770. doi: 10.1007/s10071-012-0500-3
Mazur, R., and Seher, V. (2008). Socially learned foraging behaviour in wild black bears, Ursus americanus. Anim. Behav. 75, 1503–1508. doi: 10.1016/j.anbehav.2007.10.027
McAlonan, K., and Brown, V. J. (2003). Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 146, 97–103. doi: 10.1016/j.bbr.2003.09.019
Mettke-Hofmann, C. (2014). Cognitive ecology: ecological factors, life-styles, and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 5, 345–360. doi: 10.1002/wcs.1289
Miles, W. T. S., Parsons, M., Close, A. J., Luxmoore, R., and Furness, R. W. (2013). Predator-avoidance behaviour in a nocturnal petrel exposed to a novel predator. IBIS 155, 16–31. doi: 10.1111/ibi.12007
Morehouse, A. T., Graves, T. A., Mikle, N., and Boyce, M. S. (2016). Nature vs nurture: evidence for social learning of conflict behaviour in Grizzly Bears. PLoS Biol. 11:e0165425. doi: 10.1371/journal.pone.0165425
Nowacek, D. P., Thorne, L. H., Johnston, D. W., and Tyack, P. L. (2007). Responses of cetaceans to anthropogenic noise. Mamm. Rev. 37, 81–115. doi: 10.1111/j.1365-2907.2007.00104.x
Ohashi, G., and Matsuzawa, T. (2011). Deactivation of snares by wild chimpanzees. Primates 52, 1–5. doi: 10.1007/s10329-010-0212-8
Oro, D., Genovart, M., Tavecchia, G., Fowler, M. S., and Martínez-Abraín, A. (2013). Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 16, 1501–1514. doi: 10.1111/ele.12187
Patten, M. A., and Kelly, J. F. (2010). Habitat selection and the perceptual trap. Ecol. Appl. 20, 2148–2156. doi: 10.1890/09-2370.1
Pearse, I. S., Harris, D. J., Karban, R., and Sih, A. (2013). Predicting novel herbivore – plant interactions. Oikos 122, 1554–1564. doi: 10.1111/j.1600-0706.2013.00527.x
Perry, S. E., Barrett, B. J., and Godoy, I. (2017). Older, sociable capuchins (Cebus capucinus) invent more social behaviors, but younger monkeys innovate more in other contexts. Proc. Natl. Acad. Sci. U.S.A. 114, 7806–7813. doi: 10.1073/pnas.1620739114
Pijl, H. (2011). Obesity: evolution of a symptom of affluence. How food has shaped our existence. Neth. J. Med. 69, 159–166.
Pintor, L. M., and Byers, J. E. (2015). Individual variation in predator behavior and demographics affects consumption of non-native prey. Behav. Ecol. 26, 797–804. doi: 10.1093/beheco/arv013
Ramsey, G., Bastian, M. L., and van Schaik, C. (2007). Animal innovation defined and operationalized. Behav. Brain Sci. 30, 393–407. doi: 10.1017/S0140525X07002373
Rankin, C. H., Abrams, T., Barry, R. J., Bhatnagar, S., Clayton, D. F., Colombo, J., et al. (2009). Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 92, 135–138. doi: 10.1016/j.nlm.2008.09.012
Reader, S. M., and Laland, K. N. (eds.). (2003). “Animal innovation: an introduction,” in Animal Innovation (Oxford: Oxford University Press), 3–38.
Réale, D., Reader, S. M., Sol, D., McDougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Philos. Soc. 82, 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Rendell, L., Fogarty, L., Hoppitt, W. J. E., Morgan, T. J. H., Webster, M. M., and Laland, K. N. (2011). Cognitive culture: theoretical and empirical insights into social learning strategies. Trends Cogn. Sci. 15, 68–76. doi: 10.1016/j.tics.2010.12.002
Roberts, A., Robbins, T. W., and Everitt, B. (1988). The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q. J. Exp. Psychol. 40, 321–341.
Robertson, B. A., Rehage, J. S., and Sih, A. (2013). Ecological novelty and the emergence of evolutionary traps. Trends Ecol. Evol. 28, 552–560. doi: 10.1016/j.tree.2013.04.004
Savoca, M. S., Wohlfeil, M. E., Ebeler, S. E., and Nevitt, G. A. (2016). Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Sci. Adv. 2:e1600395. doi: 10.1126/sciadv.1600395
Schakner, Z. A., and Blumstein, D. T. (2013). Behavioral biology of marine mammal deterrents: a review and prospectus. Biol. Conserv. 167, 380–389. doi: 10.1016/j.biocon.2013.08.024
Schakner, Z. A., and Blumstein, D. T. (2016). “Learning and conservation behavior: an introduction and overview,” in Conservation Behavior, eds O. Berger-Tal and D. Saltz (Cambridge: Cambridge University Press), 66–91.
Schlaepfer, M. A., Runge, M. C., and Sherman, P. W. (2002). Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480. doi: 10.1016/S0169-5347(02)02580-6
Shaw, R. C., Boogert, N. J., Clayton, N. S., and Burns, K. C. (2015). Wild psychometrics: evidence for “general” cognitive performance in wild New Zealand robins, Petroica longipes. Anim. Behav. 109, 101–111. doi: 10.1016/j.anbehav.2015.08.001
Shettleworth, S. (2010). Cognition, Evolution, and Behaviour. New York, NY: Oxford University Press.
Shine, R. (2010). The ecological impact of invasive cane toads (Bufo Marinus) in Australia. Q. Rev. Biol. 3, 253–291. doi: 10.1086/655116
Sih, A. (1992). Prey uncertainty and the balancing of antipredator and feeding needs. Am. Nat. 139, 1052–1069. doi: 10.1086/285372
Sih, A. (2013). Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077–1088. doi: 10.1016/j.anbehav.2013.02.017
Sih, A., Bell, A., and Johnson, J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Sih, A., Bolnick, D. I., Luttbeg, B., Orrock, J. L., Peacor, S. D., Pintor, L. M., et al. (2010). Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119, 610–621. doi: 10.1111/j.1600-0706.2009.18039.x
Sih, A., and Del Giudice, M. (2012). Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos. Trans. R. Soc. B Biol. Sci. 367, 2762–2772. doi: 10.1098/rstb.2012.0216
Sih, A., Ferrari, M. C. O., and Harris, D. J. (2011). Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. doi: 10.1111/j.1752-4571.2010.00166.x
Sih, A., Trimmer, P. C., and Ehlman, S. M. (2016). A conceptual framework for understanding behavioral responses to HIREC. Curr. Opin. Behav. Sci. 12, 109–114. doi: 10.1016/j.cobeha.2016.09.014
Snell-Rood, E. C., Kobiela, M. E., Sikkink, K. L., and Shephard, A. M. (2018). Mechanisms of plastic rescue in novel environments. Annu. Rev. Ecol. Evol. Syst. 49, 331–354. doi: 10.1146/annurev-ecolsys-110617-062622
Sol, D., Griffin, A. S., Bartomeus, I., and Boyce, H. (2011). Exploring or avoiding novel food resources? The novelty conflict in an invasive bird. PLoS ONE 6:e19535. doi: 10.1371/journal.pone.0019535
Spiegel, O., Leu, S. T., Bull, C. M., and Sih, A. (2017). What's your move? Movement as a link between personality and spatial dynamics in animal populations. Ecol. Lett. 20, 3–18. doi: 10.1111/ele.12708
Spottiswoode, C. N., and Stevens, M. (2011). How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc. R. Soc. B Biol. Sci. 278, 1–8. doi: 10.1098/rspb.2011.0401
Stephens, D. W. (1987). On economically tracking a variable environment. Theor. Popul. Biol. 32, 15–25. doi: 10.1016/0040-5809(87)90036-0
Stephens, D. W. (1991). Change, regularity, and value in the evolution of animal learning. Behav. Ecol. 2, 77–89. doi: 10.1093/beheco/2.1.77
Stephens, D. W. (2007). “Models of information use,” in Foraging. Behavioral Ecology, eds D. W. Stephens and J. S. Brown (Chicago, IL: University of Chicago Press), 31–58.
Swan, G. J. F., Redpath, S. M., Bearhop, S., and Mcdonald, R. A. (2017). Ecology of problem individuals and the efficacy of selective wildlife management. Trends Ecol. Evol. 32, 518–530. doi: 10.1016/j.tree.2017.03.011
Tebbich, S., Griffin, A. S., Peschl, M., and Sterelny, K. (2016). From mechanisms to function: an integrated framwork of animal innovation. Philos. Trans. R. Soc. B Biol. Sci. 371:20150195. doi: 10.1098/rstb.2015.0195
Thorogood, R., Kokko, H., and Mappes, J. (2018). Social transmission of avoidance among predators facilitates the spread of novel prey. Nat. Ecol. Evol. 2, 254–261. doi: 10.1038/s41559-017-0418-x
Tierney, K. B., Baldwin, D. H., Hara, T. J., Ross, P. S., Scholz, N. L., and Kennedy, C. J. (2010). Olfactory toxicity in fishes. Aquat. Toxicol. 96, 2–26. doi: 10.1016/j.aquatox.2009.09.019
Trimmer, P. C., Ehlman, S. M., and Sih, A. (2017). Predicting behavioural responses to novel organisms: state-dependent detection theory. Proc. R. Soc. B Biol. Sci. 284:20162108. doi: 10.1098/rspb.2016.2108
Valentinuzzia, V. S., and Ferraria, E. A. (1997). Habituation to sound during morning and night sessions in pigeons (Columba livia). Physiol. Behav. 62, 1203–1209. doi: 10.1016/S0031-9384(97)00009-7
van Langevelde, F., Ettema, J. A., Donners, M., WallisDeVries, M. F., and Groenendijk, D. (2011). Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 144, 2274–2281. doi: 10.1016/j.biocon.2011.06.004
West-Eberhard, M. J. (2005). Phenotypic accomodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. 304, 610–618. doi: 10.1002/jez.b.21071
Wisenden, B. D., and Millard, M. C. (2001). Aquatic flatworms use chemical cues from injured conspecifics to assess predation risk and to associate risk with novel cues. Anim. Behav. 62, 761–766. doi: 10.1006/anbe.2001.1797
Wong, B. B. M., and Candolin, U. (2015). Behavioral responses to changing environments. Behav. Ecol. 26, 665–673. doi: 10.1093/beheco/aru183
Keywords: environmental change, learning, optimal sampling, stimulus-response contingencies, novelty, neophobia, set-shift
Citation: Greggor AL, Trimmer PC, Barrett BJ and Sih A (2019) Challenges of Learning to Escape Evolutionary Traps. Front. Ecol. Evol. 7:408. doi: 10.3389/fevo.2019.00408
Received: 21 July 2019; Accepted: 09 October 2019;
Published: 25 October 2019.
Edited by:
Laure Cauchard, University of Aberdeen, United KingdomReviewed by:
Simon Ducatez, Ecological and Forestry Applications Research Center (CREAF), SpainAlex Taylor, The University of Auckland, New Zealand
Copyright © 2019 Greggor, Trimmer, Barrett and Sih. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison L. Greggor, YWdyZWdnb3JAc2FuZGllZ296b28ub3Jn; Brendan J. Barrett, YmJhcnJldHRAYWIubXBnLmRl
 Alison L. Greggor
Alison L. Greggor Pete C. Trimmer
Pete C. Trimmer Brendan J. Barrett
Brendan J. Barrett Andrew Sih
Andrew Sih