- Evolutionary Ecology and Conservation Genomics, Ulm University, Ulm, Baden-Württemberg, Germany
Urbanization is a highly disperse process, resulting in urban sprawl across landscapes. Within such landscapes, structural heterogeneity may be an important factor for maintaining biodiversity. We investigated the importance of habitat heterogeneity on bats in villages across the Schwäbische Alb, Germany, a progressively urbanized region. Bat activity and diversity were assessed using acoustic monitoring. We characterized habitat composition at the local and neighborhood scale and assessed environmental characteristics of urban density, vegetation cover and architectural features, combining satellite and ground-based measures. Our results revealed that the extent of urban areas determines the occurrence of different bat species, while local spatial, structural, and architectonic parameters at recording sites affected bat activity, feeding activity and social encounters. Larger urban areas with increased proportion of impervious surfaces and newly constructed housing areas were associated with fewer bat species and lower bat activity. Bat activity and feeding were highest in housing areas constructed between 1950-2000 and increased with higher proportions of older, rather openly structured vegetation. Our results clearly show a combined importance of environmental parameters across spatial scales, affecting habitat suitability and quality of rural urban areas for bats. This highlights that strategies for biodiversity inclusion in rural urban planning need to consider both local and neighborhood conditions to support bat diversity and vital bat activity. In particular, it exemplifies future challenges to maintain biodiversity within progressively urbanized rural landscapes, as this needs support by municipalities for maintaining space for nature in areas designated for urban development and also the consciousness by local residents for biodiversity-friendly modernizations.
1. Introduction
Globally, biodiversity faces unprecedented pressures from human actions (Newbold et al., 2015; Joppa et al., 2016), including climatic changes, intensification and industrialization of land use practices in agricultural areas (Sala et al., 2000; Foley et al., 2005) and forests, as well as increased urbanization (Liu et al., 2020). Especially the past 30 years revealed an unprecedented rate of global urbanization and gain in the spatial extend of urban land cover (Liu et al., 2020), with high human population density in cities (Antrop, 2004; Lambin and Geist, 2008). However, in Europe, urbanization is rather characterized by urban sprawl than by large metropolitan areas, and smaller towns and villages within agricultural areas shape large parts of the landscape. This is also true for Germany, where differences in demographic population trends, especially between the north-east and the south of the country, determine the extent of urban sprawl into rural areas and the heterogeneity in land cover. Especially southern regions of the country are currently experiencing an increased population growth (Hollbach-Grömig et al., 2012) and urban development in rural areas, resulting in more urbanized and fragmented rural landscapes.
Regions that have undergone the process of urbanization are characterized by an increased amount of impervious surfaces as the dominating landcover (McKinney, 2006) due to buildings, industrial areas and increased need for infrastructure. In turn, vegetation is mostly limited to smaller parks, gardens and roadside vegetation (McKinney, 2008). These structural changes result in increased air and noise pollution and habitat loss for wildlife, compared to non-urban habitats (Grimm et al., 2008; McDonnell et al., 2009; Niemelä et al., 2011). Increasingly urbanized rural regions however may remain rather heterogenous landscapes, as impervious surfaces within urban settlements directly neighbors agricultural and semi-natural environments. In addition, villages and smaller towns are a mosaic of old and newly constructed housing areas and homesteads with different modernization standards. They provide a multitude of structures (e.g., old tiled rooves and building crevices) that offer potential habitat for wildlife including many bird (Rosin et al., 2016) and bat species. However, the increasing re-migration trend towards rural areas in the highly populated South of Germany results in increased renovation of older buildings, and growing villages and towns. While modernizations mostly include the renewal of roofs and improved insulation to meet climate-friendly standards, newly developed housing areas are characterized by very similar and well-insulated buildings (Antrop, 2004) with smaller properties, gardens and immature woody vegetation, and thus provide less architectural and structural heterogeneity within entire neighborhoods.
Urbanization has complex effects not only on the structural composition of the environment, but also on the occurrence and behavior of wildlife (e.g., McKinney, 2006; Rivkin et al., 2019), which may inhabit or visits urban areas for different reasons, including additional roosting opportunities and food sources (e.g., Blair, 2001; Kühn et al., 2004; Clergeau et al., 2006; Strohbach et al., 2009). Several wildlife species are known to strive urban areas. In particular, in moderate developed urban areas, suburbs, smaller towns, and villages seem to provide new roosting and foraging grounds. While several studies investigated the effect of increased urban density in larger, often metropolitan areas (e.g., Lumsden and Bennett, 2005; Threlfall et al., 2011; Hale et al., 2012; Straka et al., 2019), very few studies so far focused on the dispersed urbanization process in rural areas (but see Gili et al., 2020). However, it is here, where urbanization processes are changing landscapes most drastically and where humans and wildlife of nearby agricultural and forested areas can more likely come in closer contact.
One of the most species richest group of mammals that occur in urban areas are bats (Jung and Threlfall, 2018). By eating up to 80–100% of their own body mass in insects per night (Puig-Montserrat et al., 2015), they provide an essential ecosystem function as insect and pest control. Among a broad variety of prey insects, they also consume mosquitos, an important vector of infectious diseases (Gonsalves et al., 2013). Maintaining diverse bat populations in urbanized areas of rural regions, is thus not only in the interest of wildlife conservation, but also to sustainable management of nearby agricultural areas and production forests, but it is also important for human health.
Previous studies on bats along gradients of urbanization generally revealed a decline in species richness and bat activity with increasing urbanization (e.g., Duchamp et al., 2004; Jung and Kalko, 2011; Threlfall et al., 2011; Luck et al., 2013; Jung and Threlfall, 2016, 2018). In particular, urban density and continuous impervious surfaces have been generally linked to lower bat species richness and activity (Threlfall et al., 2011; Dixon, 2012; Caryl et al., 2016). Positive effects on bats have been attributed to vegetation and forest cover in the urban area (Kalda et al., 2015; Moretto et al., 2019; Gili et al., 2020; Straka et al., 2021) and the surrounding landscapes (Duchamp and Swihart, 2008; Treitler et al., 2016). This suggests that vegetation within urban areas is a crucial prerequisite to increase habitat suitability for bats (Lumsden and Bennett, 2005; Caryl et al., 2016) also when foraging on insect accumulations around street lights (Avila-Flores and Fenton, 2005; Kerbiriou et al., 2020; Straka et al., 2021). Tolerance to urbanization is further known to differ between bat species and has been linked to species traits (Jung and Threlfall, 2018), namely lower echolocation call frequency, higher mobility, and flexible roosting strategies. Thus, especially fast flying and rather flexible and opportunistic species are likely to dominate urban assemblages (Luck et al., 2013; Jung and Threlfall, 2018). However, the effect of urbanization differs due to the extent of urban development (Jung and Threlfall, 2016; Gili et al., 2020). Therefore, rural villages likely provide suitable habitat also for less urban tolerant bat species. Especially in the light of the ongoing biodiversity crisis, climatic changes, and the emergence of zoonotic diseases, it is crucial to understand how the alteration of rural regions due to increased urbanization, affects occurrence, behavior and species composition of bats, and how can rural regions uphold biodiverse landscapes and safeguard regional bat diversity to assure vital ecosystem services within urbanized rural regions.
Here, we investigated whether and how urban development in rural landscapes affects bats. We specifically ask how spatial, structural, and architectonic environmental features of rural urban areas, locally and at the neighborhood scale, affect bat species occurrence and behavior to derive environmental parameters of the urban environments that may support bat species diversity and vital bat activity. Such knowledge is of high relevance for urban planners and conservation ecologists to target strategies for biodiversity inclusion within increasingly urbanized rural landscapes.
2. Materials and methods
2.1. Study sites
This study was conducted in the mid mountain range of the Schwäbische Alb plateau (Baden-Württemberg, Figure 1), which is part of the Biodiversity Exploratories1 for functional biodiversity research in Germany. The Exploratory Schwäbische Alb (420 km2) includes submontane and montane landscapes. A large extend of the Exploratory is part of the UNESCO Biosphere Reserve Schwäbische Alb (850 km2); a diverse and heterogenous landscape including differently managed forest and grassland, agricultural areas and urban settlements. Mean annual temperature ranges from 6.0–7.0°C and the mean annual precipitation from 750 to 1,000 mm (see Footnote 1). The Southern and Western regions of Germany are generally very densely populated, and especially rural regions have experienced a significant increase in population during the past decades (Demographieportal, 2016). Economic development in commuting distance to the Schwäbische Alb region promoted an increased urban development also on the Alb plateau.
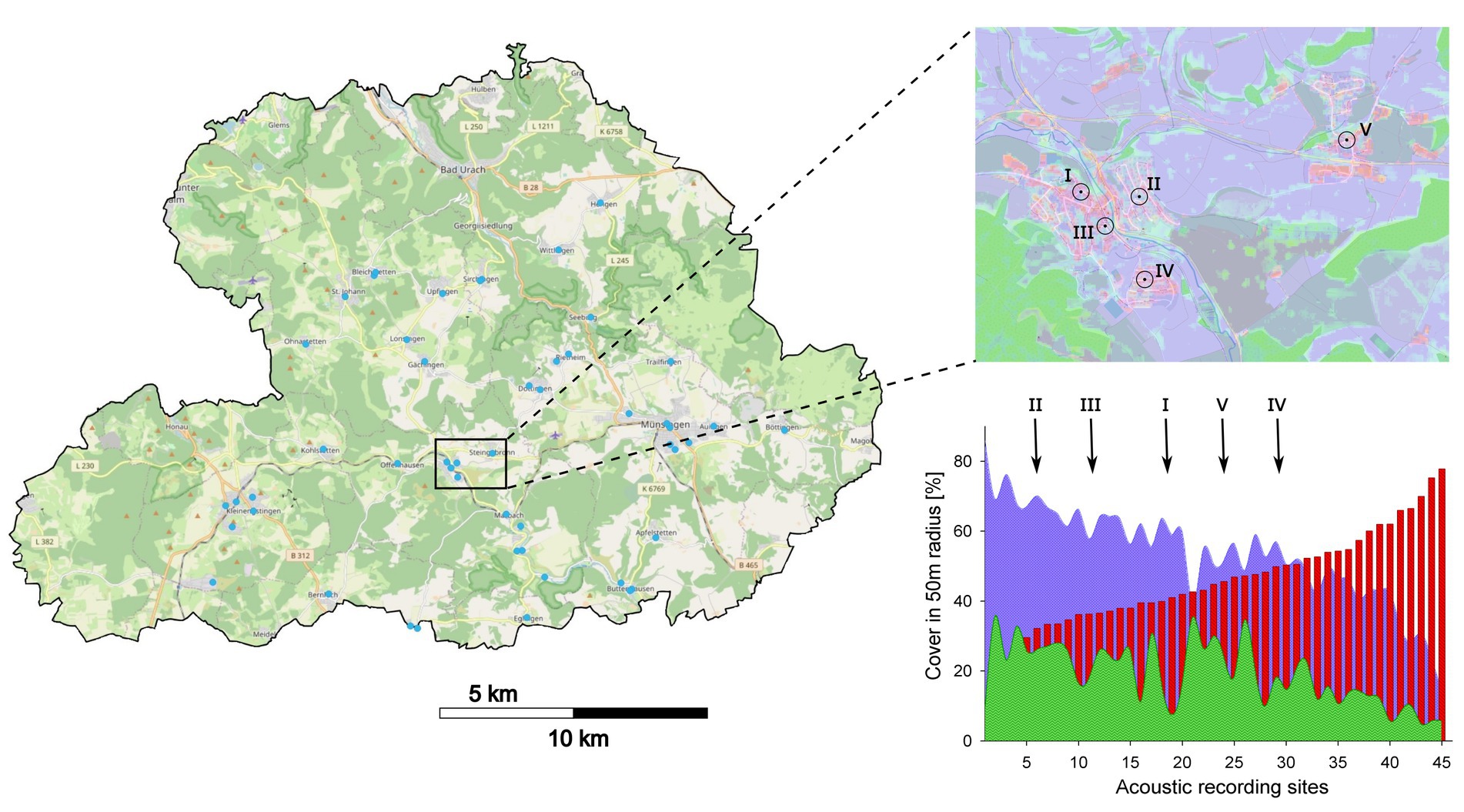
Figure 1. Open street map of the study region Schwäbische Alb in southern Germany (left). Forests are mapped in green, open grassland and agricultural areas in light yellow. Urban areas are marked in grey and acoustic recording sites within urban areas are depicted with blue dots. Right upper panel: open street map containing the Sentinental-2 Satellite data layer (10 m resolution) of woody vegetation (green) non-woody vegetation (purple), and urban areas (red), provided and published by Schug et al. (2020). Fife recording sites and the respective 50 m buffer representing the local scale are shown and their position along the urban gradient (lower right panel) across the 45 recording sites are marked with arrows.
2.2. Environmental data
Urban recording sites (N = 45) were located in different villages and urban environmental settings, ranging from new constructed housing areas to old village centers and industrial areas. Sites differed in spatial, structural and architectonic composition, reflecting the heterogeneity of urban settlements, including parklands, gardens and build-up infrastructure. Because the combination of environmental features may also vary due to the urban extent, the construction history and architecture of distinctive village neighborhoods, we gathered data on human population per village and also chose two scales for which we extracted environmental features: a 50 m local scale which approximately matches the maximum detection range of our acoustic recording devices for bat echolocation calls, and a surrounding neighborhood scale. Neighborhoods were characterized by relatively similar construction age and history, and were generally delimited by major streets or natural features such as smaller streams, village meadows or slopes (see Figure 1). Due to urban development size of these neighborhoods differed.
For both scales we extracted spatial environmental features (Table 1) of built-up surfaces and infrastructure (impervious surfaces), woody vegetation and non-woody vegetation from a map based on Sentinental-2 Satellite data (10 m resolution) provided and published by Schug et al. (2020). This dataset is based on spectral-temporal metrics derived from Sentinel-2 reflectance time series using all available Sentinel-2 data from 2017 and 2018 (< 70% cloud cover) and can be accessed online.2 Extracted features for the neighborhood scale were then standardized by neighborhood size.
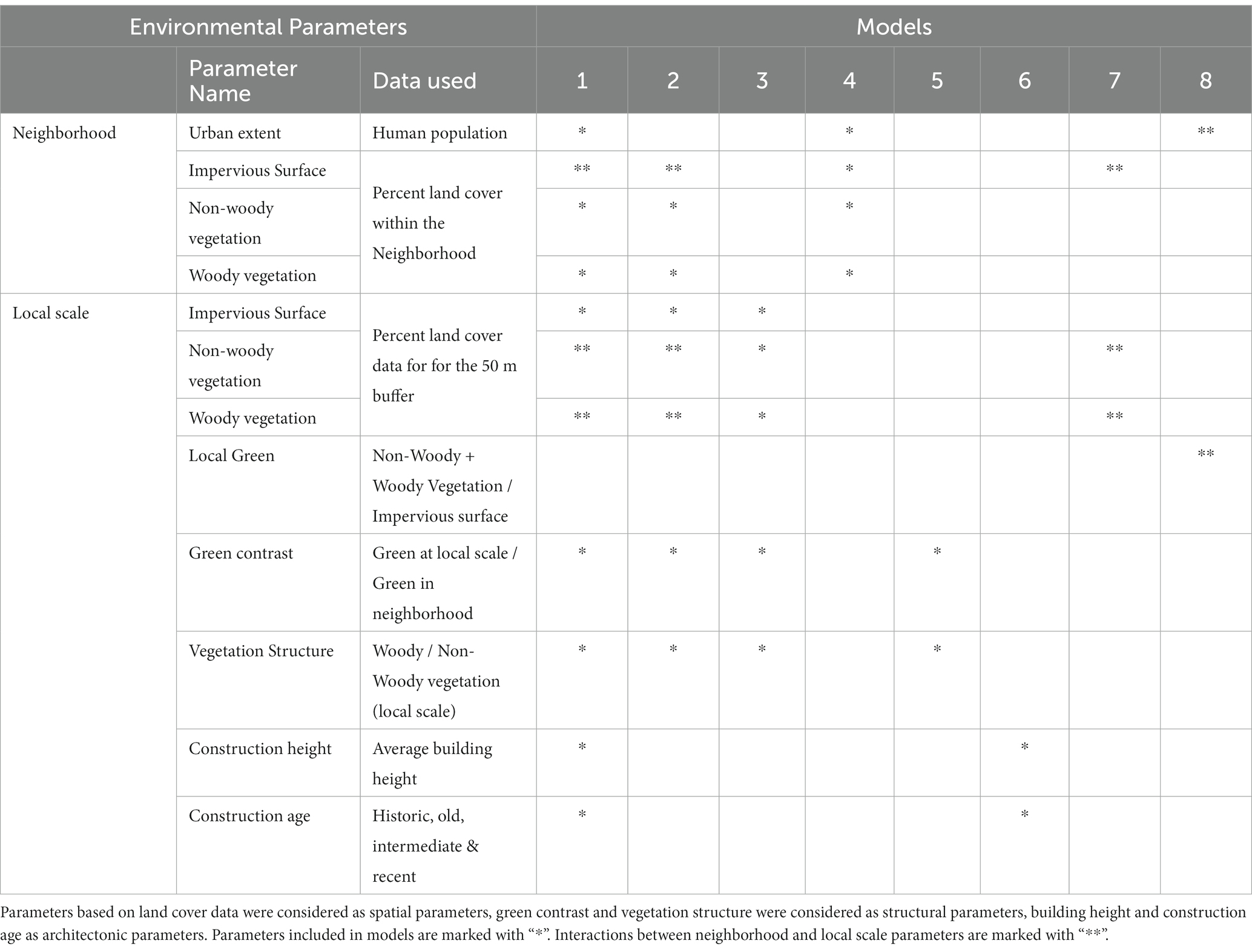
Table 1. Environmental parameters used to describe the heterogeneity of urban recordings sites across scales, the original data used to derive each parameter and the combination of parameters for statistical modelling.
To reflect structural setting of the local scale within the neighborhood context we calculated the ratio of vegetated versus impervious land for both scales and calculated the relative greenness of the local scale compared to the surrounding neighborhood scale (hereafter “Green contrast”). Higher values of green contrast would suggest locally green islands within relatively impervious neighborhoods and vice versa. For the local scale only, we additionally calculated the ratio of woody per non-woody vegetation to reflect the local vegetation structure (higher values = higher structural heterogeneity) and gathered data on architectonic features including average building height and information regarding the construction age of buildings from local administrations. Due to a high range (1510–2010) and the very skewed distribution of this data, further analysis however required a classification of building age into the categories: build before 1900, build between 1900–1950 and 1950–2000 and build past 2000 based on the majority of buildings within the 50 m radius.
2.3. Acoustic monitoring and species identification of bats
We recorded echolocation calls of bats (sampling rate of 500 kHz, 16 bit), using stationary autonomous ultrasound recorders (Batcorder, EcoObs GmbH, Nürnberg, Germany). Recorders were set up in the center of respective village neighborhoods within the public domain and were triggered by sound intensities higher than −36 dB SPL. Each study site was repeatedly visited for one night in June, July and the beginning of September 2021. Batcorders were installed on street posts, and bare tree trunks in about 2,5–3,0 m heights to avoid disturbing ground echoes and theft of devices. Microphones were directed towards potential flight ways and open space to avoid that nearby vegetation or buildings would block the recording of echolocating bats. Recordings were identified to species level by pre-classifying bat calls using the automatic acoustic identification software bcIdent (Version 1.5.1, EcoObs GmbH,) integrated in BCAdmin 4 (Version 1.1.10). We manually inspected all recordings for errors in species assignation using published literature (Skiba, 2003; Barataud, 2015). In addition, we manually checked sequences for feeding buzzes and social calls to evaluate feeding activity and the occurrence of social interactions. Sonograms were generated by a Hamming window, FFT length of 512 points and an overlap of 93.75%. We unambiguously identified echolocation sequences of Pipistrellus pipistrellus, Pipistrellus nathusii, Pipistrellus pygmeaus, Nyctalus noctula, Eptesicus nilssonii, Myotis myotis and Myotis nattereri. Due to high similarity in echolocation call features, we did not distinguish between Plecotus auritus and P. austriacus and grouped them to Plecotus spec. Also M. daubentonii, M. mystacinus and M. bechsteinii overlap substantially in echolocation call parameters and were thus grouped into the Sonotype Myotis. Only in some cases could we identify Eptesicus serotinus, Vespertilio murinis and Nyctalus leislerli unambiguously. If this was not the case species were assigned into the sonotype Nyctaloid.
2.4. Statistical data analysis
To evaluate different aspects of bat distributions and activity patterns in urban areas we used bat activity (the number of recorded files per night), indicating the intensity in habitat use and bat occurrence (accumulated number of different species occurrences from 1 h before sunset to 1 h after sunrise). This is a more conservative measure, indicating the relative abundance of different bat species and can be seen as a measure of relative bat diversity. We additionally assessed feeding activity, based on the number of sequences including feeding buzzes of bats, and social activity, based as the number of files with social calls.
To assess the relative importance of spatial environmental variables and scales we used random forest [(Liaw and Wiener, 2002), package randomForest]. Random forest, is a useful method to evaluate variable importance (function: randomForest, 10,000 randomizations), especially in datasets with high multicollinearity as it is typical for land cover metrics which naturally are non-independent. This analysis suggested that that importance of environmental parameters varied for the different aspects of bat distribution and activity patterns (Supplementary Figure S1). We thus used an information theoretic approach and build a candidate sets of generalized linear mixed effect models (see Table 1), to assess which scale and set of parameters may best explain the different aspects of bat distribution and activity patterns (Table 1). Models were fit with the function glmmTMB using a negative binomial distribution with a quadratic parameterization due to overdispersion (package: glmmTMB). Environmental factors were fit as fixed factors and sampling sites included as random factors, due to repetitive sampling. All models were conducted separately for bat activity, bat occurrence, feeding and social activity. Candidate sets included 1) a global mode including all environmental factors and interactions between an impervious neighborhood and local vegetation 2) a spatial and structural model across scales, 3) a neighborhood model containing spatial parameters for this scale only 4) a local model containing spatial and structural and architectonic parameters of the 50 m local scale and additional local model subsets containing 5) structural and 6) architectonic information and 7/8) two models specifically asking whether local green space could buffer the effect of an increased impervious neighborhood or village size. We then derived a confidence set of the best approximating models (see Table 2) using an information-theoretic approach based on Akaike’s Information Criterion adjusted for small samples (AICc; Burnham and Anderson, 2002, package: AICcmodavg).
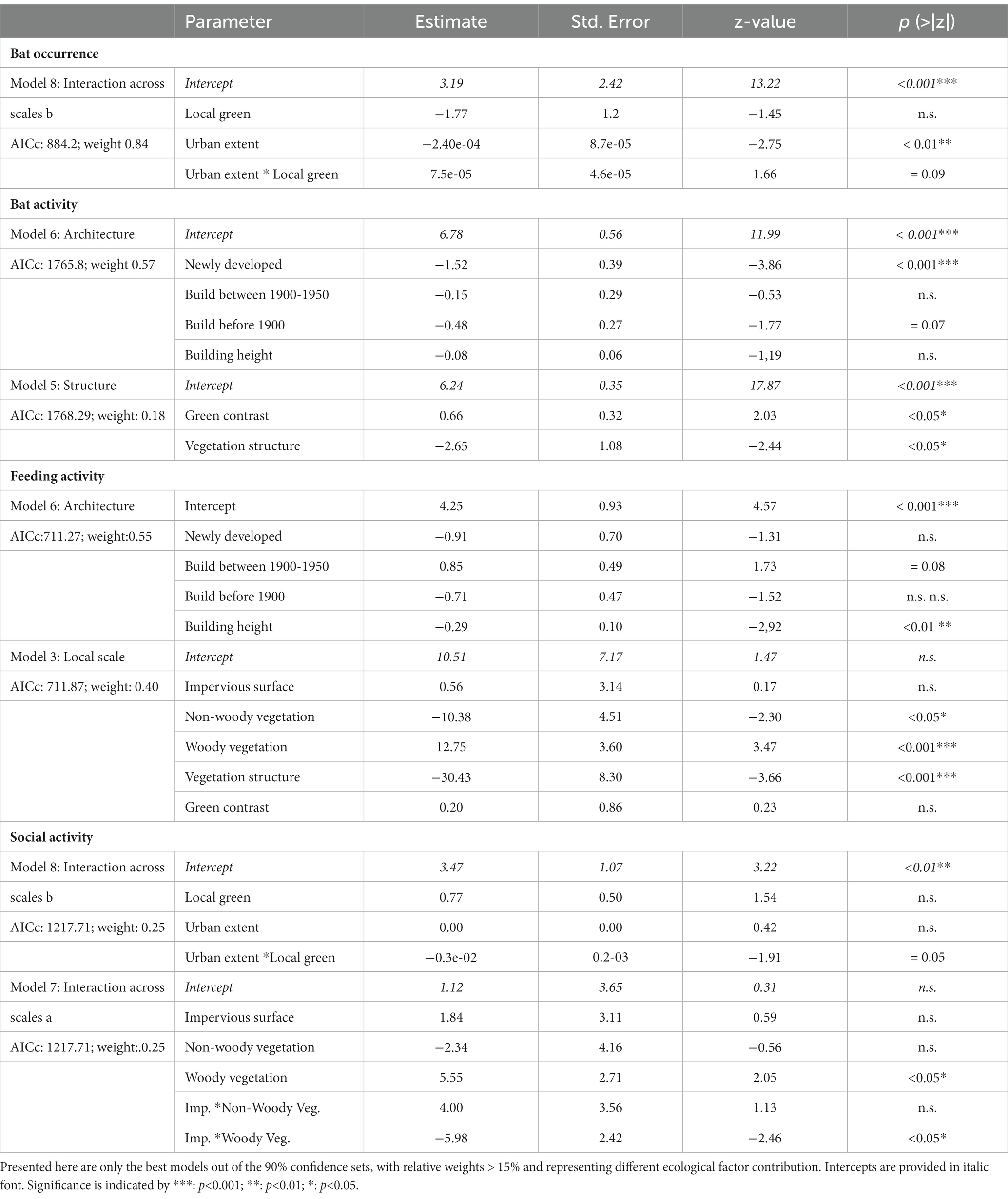
Table 2. Approximate models explain bat occurrence and activity of bats in villages and small urban towns across the Schwäbische Alb.
Differences in species composition between urban sampling sites were further assessed by performing non-metric multidimensional scaling (Oksanen et al., 2020, NMDS, with 10,000 permutations) based on Bray–Curtis dissimilarity and weighted by species activity. We further used environmental fitting (Oksanen et al., 2020, function: envfit) and permutational multivariate analysis of variance (function: adonis) with 10,000 randomizations to evaluate the importance of environmental parameters for differences in bat species composition between individual recording sites. Both analyses were conducted using the package vegan 2.6–4.
All statistical analysis was conducted in R (Version 4.21) using the RStudio environment (Version 2022.02.3).
3. Results
Our standardized acoustic monitoring resulted in a total of 53,208 recordings of bats in villages and towns of the Schwäbische Alb. Of those, 2.1% (N = 1,097) contained feeding buzzes and 13% (N = 6,952) included social calls. In total, we identified 13 species or sonotypes, with P. pipistrellus as the most frequent recorded species (Supplementary Table S2).
3.1. Importance of urban heterogeneity for bats
Model averaging revealed that urban extent and the composition of the neighborhood scale played an important role for different bat species occurrence, while local conditions of spatial, structural, and architectonic parameters mainly affected bat activity and feeding activity. Differences in the occurrence of social interactions were best explained by differences in vegetation cover at different spatial scales (Table 2; Supplementary Table S1).
In particular occurrence of different bat species decreased significantly with the extent of urban area (e.g., village size). With increasing urban extent, however an increased proportion of local green space revealed increasing importance and showed a tentatively positive effect on different bat species occurrences (p = 0.09).
Bat activity, as well as feeding activity were mainly affected by local architectonic features and structural parameters of the vegetation (see Table 2). Bat activity was highest in residential areas constructed between 1950 and 2000. Especially newly developed housing areas constructed after 2000, but also historic residential areas with buildings mainly constructed before 1900 revealed significantly lower bat activity. Feeding activity also significantly deceased with increasing building height (Table 2).
Bat activity also increased significantly with increasing green contrast of the local scale compared to the surrounding neighborhood, but decreased with higher structural heterogeneity of the vegetation, suggesting that rather openly structured orchards and well-grown, park-like garden areas within the villages, were more frequently used by bats, also for feeding. Higher cover of vegetation (green space), in particular woody vegetation cover also benefitted social encounters of bats (Table 2).
Overall, these results indicate that mainly additive effects and the combination of local and neighborhood conditions affects habitat suitability for different bat species and highlights the relevance of local conditions explaining differences in the intensity of habitat use and the quality of urban feeding grounds.
3.2. Bat assemblages within urban areas of rural landscapes
Urban recording sites differed in species composition of bats (NMDS: final stress = 0.16, linear fit R2 = 0.86). Predominantly urban extent (R2 = 0.17, p < 0.05), impervious surfaces at the neighborhood scale (R2 = 0.13, p = 0,05) and the amount of non-woody vegetation within the local buffer (R2 = 0.14, p < 0,05) correlated significantly with the NMDS axis 1 and 2 (Figure 2). Permutational multivariate analysis of variance based on distance matrices further indicated a change in species composition with increased complexity of the vegetation (F = 6.48, p < 0.01). In particular a greater amount of grassland (e.g., gardens, orchards), supported the abundance of gleaning bats such as Myotis spec. and Plecotus spec, while N. leisleri and both Eptesicus species were more associated with an increased complexity of the vegetation (vegetation structure). In contrast, both Pipistrelles (P. pipistrellus and P. nathusii) were more tolerant towards impervious surfaces and frequently occurred at sites with higher building density and little vegetation.
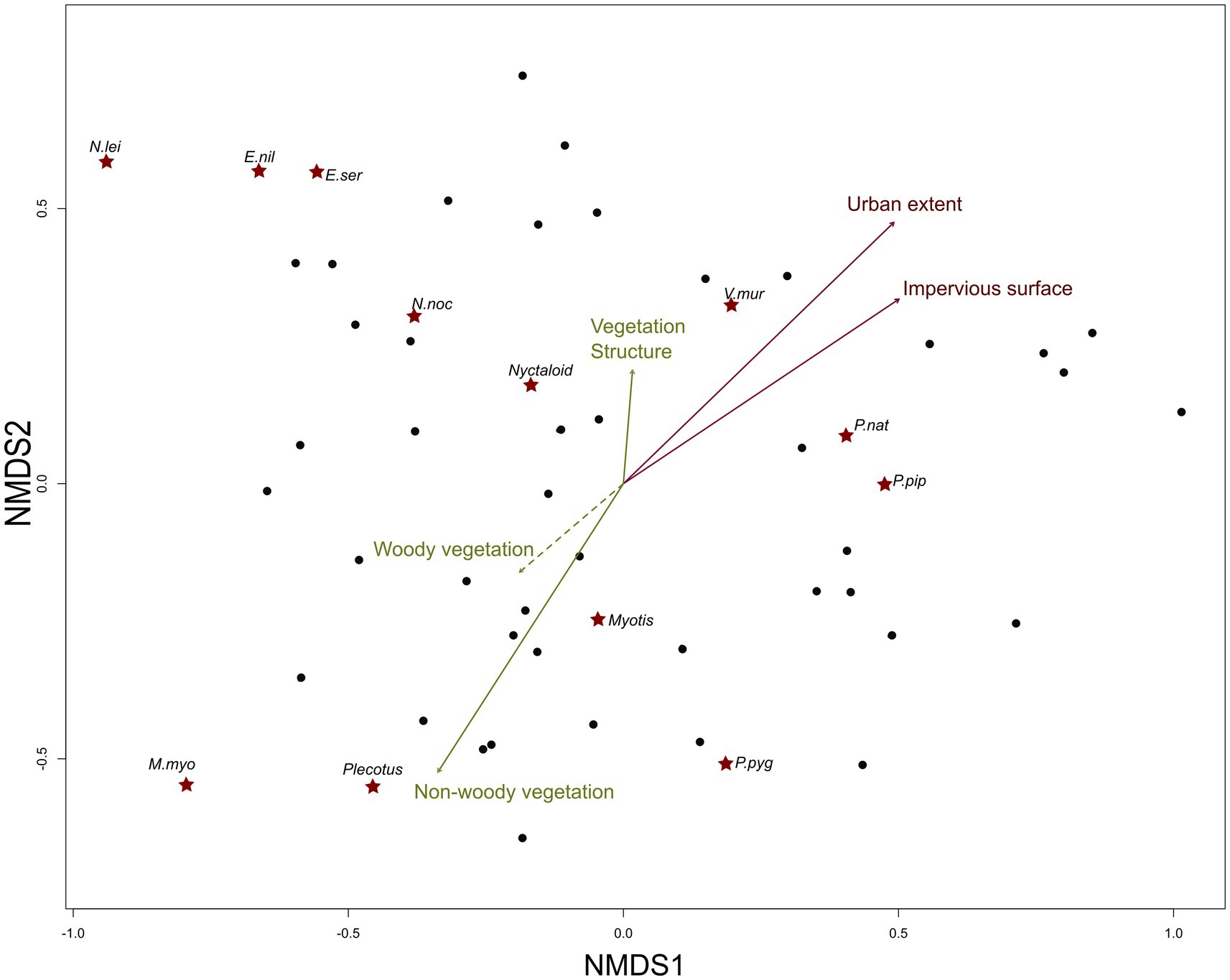
Figure 2. Ordination (nmds) of urban recording sites based on Bray–Curtis dissimilarity of bat species composition weighted by their activity. Black dots represent the placement of acoustic recording sites, red stars the placement of species within multidimensional space. Environmental factors are represented as green and red arrows. The non-significant effect of woody vegetation, indicated by the dashed green arrow, is included as it contributes to the parameter vegetation structure. Relative importance of environmental factors is indicated by the length of the arrows. Species names are abbreviated: E.nil = Eptesicus nilssonii; E.ser = Eptesicus serotinus; N.lei = Nyctalus leisleri; N.noc = Nyctalus noctula; V.mur = Vespertilio murinus; P.nat = Pipistrellus nathusii; P.pip = Pipistrellus pipistrellus; P.pyg = Pipistrellus pygmaeus; M.myo = Myotis myotis. Plecotus, Myotis and Nyctaloid refer to species grouped in acoustic sonotypes. As M. nattereri only occurred one time we omitted it from this graph.
4. Discussion
Urbanization is a strong environmental driver (Piano et al., 2019). It can be seen as the most drastic form of land use change (McKinney, 2006; Shochat et al., 2006) and is no longer limited to the expansion and densification of metropolitan areas but rather extends far beyond city boundaries into rural areas (McDonald, 2008). We investigated whether and how urban heterogeneity of villages in rural landscapes affects activity of bats. Such knowledge is crucial for a guided management and conservation of bats in rural areas (Racey and Entwistle, 2003), where land use intensification in the surrounding agricultural landscapes causes habitat loss (Rosin et al., 2016) and dramatic declines in insect prey (Hallmann et al., 2017; Seibold et al., 2019) while climatic changes favor the spread of insect pests such as the Tiger mosquitoes, a vector for various disease.
Our results revealed that the combination of local and neighborhood conditions in rural urban areas affects the occurrence of different bat species. Species occurrence significantly decreased with increasing urban extent, confirming that even very restricted urban development in rural areas restructures biotic communities of bats (see, e.g., Jung and Threlfall, 2016). However, bat occurrence and activity increased with local greenspace, in particular openly structured woody vegetation as it is typical for parklands, orchards and older village gardens. This also highlighted the potential of local conditions to support diverse bat assemblages and vital bat activity in rural urban areas. Our results are in agreement with previous studies in urban (Moretto et al., 2019; Gili et al., 2020) and in agricultural landscapes (Lumsden and Bennett, 2005; Heim et al., 2015; Kalda et al., 2015; Treitler et al., 2016), where woody vegetation and tree cover are considered key components for higher diversity and abundance of bats. Moretto et al. (2019), even showed that the positive effect of single trees in urban areas extends into the neighborhood. Hereby in particular, the retention of large and old trees has previously been shown to be critical for urban biodiversity conservation (Threlfall et al., 2012) and even buffers negative effects of light pollution (Straka et al., 2019). Nevertheless, our results also indicated the importance of non-woody vegetated areas, such as traditionally managed meadows or orchards, which are important habitat especially for gleaning Myotis and Plecotus species. Overall, this emphasizes the positive effects of even small, differently structures vegetated areas, such as for example residential gardens, single trees, and orchards even within structurally rather poor urban areas and supports the idea that very local decisions on management can help to support the occurrence of different bat species in urbanized rural landscapes.
Bat activity and feeding activity were mainly affected by local environmental parameters, including architectonic features as a key parameter explaining varying bat activity between different urban sites. We found highest bat activity in residential areas with 1–2 story houses. This is consistent with Pearce and Walters (2012), who reported a negative association between roof height and bat activity. Buildings with 1–2 stories are most often family residentials, surrounded by gardens, resulting in a mosaic of impervious surfaces and vegetation patches locally. In addition, many householders are implementing practices to enhance wildlife, such as limiting the use of pesticides and planting a variety of different plants (Good, 2000), potentially resulting in a higher insect diversity compared to nearby agricultural areas (Seibold et al., 2019). We however found notable differences between residential areas of different construction ages. Our results indicated higher bat activity in residential areas built between 1950–2000, suggesting that those are currently better habitats for bats. Such residential areas are often characterized by less well-insulated and not yet renovated houses, large and well-grown gardens, small ponds or rain barrels and are thus structurally more diverse. Housing areas with historic buildings constructed prior to 1900 showed less bat activity. This is likely due to renovation and modernization of these historic buildings. Especially newly constructed neighborhoods and buildings (constructed after 2000) however revealed lowest bat activity. New buildings are well insulated to meet climate-friendly standards, but no longer provide crevices under roofs or facades. Additionally, new developed building areas generally lack old growth trees, and are often surrounded by rather young vegetation and smaller premises, and thus provide only limited foraging or roosting grounds for bats.
Several bat species, in particular flexible and opportunistic species are known to persist in and might even benefit from additional resources in urban areas (Russo and Ancillotto, 2015; Jung and Threlfall, 2016). However, several studies have cautioned that the response towards urbanization is species-specific and trait-dependent (e.g., Jung and Threlfall, 2016), and urbanization likely functions as a strong trait-based environmental filter (Jung and Threlfall, 2018), resulting in altered species assemblages (e.g., Grimm et al., 2008). Our results showed that bat assemblages in villages were dominated by P. pipistrellus, which together with the group Nyctaloid and P. nathusii comprised the vast majority of our recordings. P. pipistrellus is an edge foraging bat, with a very broad distribution range, tolerating also rough climate conditions typical for lower mountain areas as the Schwäbische Alb plateau where our study took place. All other species were much less recorded in villages, although long term data obtained in this area from 2008–2012 (Jung and Tschapka, 2022) shows, that those are present in surrounding forest and grassland areas. This is in accordance with prior studies, that indicated that species richness may be generally low in urban areas, but total abundance of bats increases due to the occurrence of a few dominating urban-tolerant species (Avila-Flores and Fenton, 2005; Francis and Chadwick, 2012; Krauel and LeBuhn, 2016). Pipistrelle bats can be frequently observed within urban areas (Gaisler et al., 1998), are known to roost in buildings (Dietz et al., 2009), and show opportunistic foraging behavior by hunting regularly around street-lights (Rydell and Racey, 1995). Thus, pipistrelle bats seem to be rather resource generalists that benefit from additional roosting and foraging sites provided by urban structures. In contrast, Myotis species showed lower activity rates as the total impervious surface increased. This is consistent with a prior study (Jung and Threlfall, 2018), and particularly gleaning bats, such as Myotis or Plecotus, are less likely to forage in urban areas. Myotis and Plecotus species, specialized to listen for prey generated noises, and known to collect ground-dwelling arthropods (e.g., carabids) from the ground, were also almost exclusively detected in villages with high amount of non-woody vegetation, e.g., grassland or orchards. Similar results have been reported by Uhrin et al. (2017), who showed a positive association of grassland landcover with the occurrence of P. auritus. It is however important to note, that Myotis and Plecotus may very well roost in village barns or church attics but rather leave the urban setting for foraging in nearby grassland or forest habitats, if the urban neighborhood provides enough green and connective elements for a safe commute.
Our study provides important insight on how the process of increased urbanization in rural areas affects bat species occurrence and behavior. Our results indicate that a lack of connectivity in the neighborhood due to limited greenness and vegetation likely restricts the accessibility of different urban sites while local conditions may rather determine the quality of urban habitats for roosting, and foraging. Thus, the retention of green areas, such as village meadows, old and large trees in village centers and farmer’s gardens, which have been an essential part of the local culture, are key to maintain biodiversity in rural areas. Altogether, these green elements can serve as a green infrastructure, connecting villages with habitats in the surrounding landscape matrix and assuring movement of wildlife. This includes bats as well as the provision of valuable ecosystem services (e.g., the consumption of mosquitos) within rural urbanized landscapes. In addition, within increasingly urbanized rural landscapes, strategies are needed to decrease negative impacts on biodiversity by urban expansion and modernizations. Particularly, new constructed housing areas and modernized historic buildings revealed lower bat diversity and activity, which is likely due to the lack of potential roosting sites in well-insulated buildings and roof structures and gardens with predominantly young vegetation. It may be a matter of natural development that those areas can support biodiversity in the future, but it is also and foremost a matter of integrating the space for natural development in urban planning and the consciousness for biodiversity inclusion in the cause of modernizations by local residents.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: This work is based on data (ID: 31295) elaborated by the project “birds&bats” of the Biodiversity Exploratories program (DFG Priority Program 1374). The dataset will be available publicly in the Biodiversity Exploratories Information System (http://doi.org/10.17616/R32P9Q). However, as this data is part of further analysis at the national level it underlies an embargo period of three years from the end of data collection/data assembly. After this period this dataset will be made publicly available via the same data repository: KJ and LP (2021): Bat activity urban sites Exploratory ALB summer 2021. Biodiversity Exploratories Information System. Dataset https://www.bexis.uni-jena.de/. Dataset id: 31295.
Ethics statement
Ethical review and approval was not required for the animal study because we used passive acoustic monitoring as a method, which in a non-invasive technique to gather information about bat activity and behavior. We did not disturb animals in any way.
Author contributions
This study has been conceived and designed by KJ. Data collection and analysis were performed by LP. The first draft of the manuscript was written by LP jointly edited by LP and KJ. All authors contributed to the article and approved the submitted version.
Funding
This work has been funded by the DFG Priority Program 1374 "Biodiversity-Exploratories" (JU 2752/2-1 granted to KJ).
Acknowledgments
The authors are very thankful to the municipalities of the Schwäbische Alb, Münsingen, Gomadingen, Engstingen, Bad Urach, Sonnenbühl, St. Johann and Hohenstein, who granting permission to record bats in public areas. The authors are especially thankful for their help with informing the public and providing information regarding construction age of buildings near our recording sites. The authors also thank Paul Magdon for his advice concerning the evaluation of the remote sensing data. The authors thank the management team and the manager of the Schwäbische Alb Exploratory Julia Bass, and all former managers for their work in maintaining the project infrastructure; Victoria Grießmeier for giving support through the central office, Andreas Ostrowski for managing the central data base, and Markus Fischer, Eduard Linsenmair, Dominik Hessenmöller, Daniel Prati, Ingo Schöning, François Buscot, Ernst-Detlef Schulze, Wolfgang W. Weisser and the late Elisabeth Kalko for their role in setting up the Biodiversity Exploratories project. The authors thank the administration of the UNESCO Biosphere Reserve Swabian Alb as well as the communities for the excellent collaboration. The authors cordially thank Prof. Dr. Tschapka and Prof. Dr. Ayasse, for their comments to previous versions of this manuscript and their constant support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1194670/full#supplementary-material
Footnotes
References
Antrop, M. (2004). Landscape change and the urbanization process in Europe. Landsc. Urban Plan. 67, 9–26. doi: 10.1016/S0169-2046(03)00026-4
Avila-Flores, R., and Fenton, M. B. (2005). Use of spatial features by foraging insectivorous bats in a large urban landscape. J. Mammal. 86, 1193–1204. doi: 10.1644/04-MAMM-A-085R1.1
Azam, C., Kerbiriou, C., Vernet, A., Julien, J.-F., Bas, Y., Plichard, L., et al. (2015). Is part-night lighting an effective measure to limit the impacts of artificial lighting on bats? Glob. Chang. Biol. 21, 4333–4341. doi: 10.1111/gcb.13036
Barataud, M., (2015). Acoustic Ecology of European Bats. Species Identification and Studies of Their Habitats and Foraging Behaviour. Biotope Editions, Mèze.
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Blair, R. B. (2001). “Creating a homogeneous avifauna” in Avian Ecology and Conservation in an Urbanizing World. eds. J. M. Marzluff, R. Bowman, and R. Donnelly (Boston, MA: Springer US), 459–486.
Burnham, K. P., and Anderson, D. R. (2002). Model selection and multimodel inference: a practical information-theoretic approach, second edition. Springer Verlag. New York, USA.
Caryl, F. M., Lumsden, L. F., Ree, R., and Wintle, B. A. (2016). Functional responses of insectivorous bats to increasing housing density support ‘land-sparing’ rather than ‘land-sharing’ urban growth strategies. J. Appl. Ecol. 53, 191–201. doi: 10.1111/1365-2664.12549
Clergeau, P., Jokimaki, J., and Snep, R. (2006). Using hierarchical levels for urban ecology. Trends Ecol. Evol. 21, 660–661. doi: 10.1016/j.tree.2006.09.006
Coleman, J. L., and Barclay, R. M. R. (2012). Urbanization and the abundance and diversity of prairie bats. Urban Ecosyst. 15, 87–102. doi: 10.1007/s11252-011-0181-8
Demographieportal (2016) in Bundesinstitut für Bevölkerungsforschung. ed. C. K. Spieß (Nürnberg: Informationstechnikzentrum Bund)
Dietz, C., Nill, D., and von Helversen, O., (2009). Bats of Britain, Europe and Northwest Africa. A and C Black. London
Dixon, M. D. (2012). Relationship between land cover and insectivorous bat activity in an urban landscape. Urban Ecosyst. 15, 683–695. doi: 10.1007/s11252-011-0219-y
Duchamp, J. E., Sparks, D. W., and Whitaker, J. O. Jr. (2004). Foraging-habitat selection by bats at an urban–rural interface: comparison between a successful and a less successful species. Can. J. Zool. 82, 1157–1164. doi: 10.1139/z04-095
Duchamp, J. E., and Swihart, R. K. (2008). Shifts in bat community structure related to evolved traits and features of human-altered landscapes. Landsc. Ecol. 23, 849–860. doi: 10.1007/s10980-008-9241-8
Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., et al. (2005). Global consequences of land use. Science 309, 570–574. doi: 10.1126/science.1111772
Francis, R. A., and Chadwick, M. A. (2012). What makes a species synurbic? Appl. Geogr. 32, 514–521. doi: 10.1016/j.apgeog.2011.06.013
Frantz, D., Schug, F., Okujeni, A., Navacchi, C., Wagner, W., van der Linden, S., et al. (2021). National-scale mapping of building height using Sentinel-1 and Sentinel-2 time series. Remote Sens. Environ. 252:112128. doi: 10.1016/j.rse.2020.112128
Gaisler, J., Zukal, J., Rehak, Z., and Homolka, M. (1998). Habitat preference and flight activity of bats in a city. J. Zool. 244, 439–445. doi: 10.1111/j.1469-7998.1998.tb00048.x
Gili, F., Newson, S. E., Gillings, S., Chamberlain, D. E., and Border, J. A. (2020). Bats in urbanising landscapes: habitat selection and recommendations for a sustainable future. Biol. Conserv. 241:108343. doi: 10.1016/j.biocon.2019.108343
Gonsalves, L., Bicknell, B., Law, B., Webb, C., and Monamy, V. (2013). Mosquito consumption by insectivorous bats: does size matter? PLoS One 8:e77183. doi: 10.1371/journal.pone.0077183
Good, R. (2000). The value of gardening for wildlife. What contribution does it make to conservation? Brit. Wildlife 12, 77–84.
Grimm, N. B., Foster, D., Groffman, P., Grove, J. M., Hopkinson, C. S., Nadelhoffer, K. J., et al. (2008). The changing landscape: ecosystem responses to urbanization and pollution across climatic and societal gradients. Front. Ecol. Environ. 6, 264–272. doi: 10.1890/070147
Hale, J. D., Fairbrass, A. J., Matthews, T. J., and Sadler, J. P. (2012). Habitat composition and connectivity predicts bat presence and activity at foraging sites in a large UK conurbation. PLoS One 7:e33300. doi: 10.1371/journal.pone.0033300
Hallmann, C. A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., et al. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12:e0185809. doi: 10.1371/journal.pone.0185809
Heim, O., Schröder, A., Eccard, J., Jung, K., and Voigt, C. C. (2016). Seasonal activity patterns of European bats above intensively used farmland. Agric. Ecosyst. Environ. 233, 130–139. doi: 10.1016/j.agee.2016.09.002
Heim, O., Treitler, J. T., Tschapka, M., Knörnschild, M., and Jung, K. (2015). The importance of landscape elements for bat activity and species richness in agricultural areas. PLoS One 10:e0134443. doi: 10.1371/journal.pone.0134443
Hollbach-Grömig, B., Langel, N., Göll, E., and Henseling, C., (2012). Demografischer Wandel – Herausforderungen und Handlungsempfehlungen für Umwelt- und Naturschutz. Im Auftrag des Dessau-Roßlau. Umweltbundesamtes.
Hourigan, C. L., Catterall, C. P., Jones, D., and Rhodes, M. (2010). The diversity of insectivorous bat assemblages among habitats within a subtropical urban landscape: bat diversity in a subtropical city. Austral Ecol. 35, 849–857. doi: 10.1111/j.1442-9993.2009.02086.x
Joppa, L. N., O'Connor, B., Visconti, P., Smith, C., Geldmann, J., Hoffmann, M., et al. (2016). Filling in biodiversity threat gaps. Science 352, 416–418. doi: 10.1126/science.aaf3565
Jung, K., Kaiser, S., Böhm, S., Nieschulze, J., and Kalko, E. K. V. (2012). Moving in three dimensions: effects of structural complexity on occurrence and activity of insectivorous bats in managed forest stands: bats and 3D forest structure. J. Appl. Ecol. 49, 523–531. doi: 10.1111/j.1365-2664.2012.02116.x
Jung, K., and Kalko, E. K. V. (2010). Where forest meets urbanization: foraging plasticity of aerial insectivorous bats in an anthropogenically altered environment. J. Mammal. 91, 144–153. doi: 10.1644/08-MAMM-A-313R.1
Jung, K., and Kalko, E. K. V. (2011). Adaptability and vulnerability of high flying neotropical aerial insectivorous bats to urbanization: responses of insectivorous bats to urbanization. Divers. Distrib. 17, 262–274. doi: 10.1111/j.1472-4642.2010.00738.x
Jung, K., and Threlfall, C. G. (2016). “Urbanisation and its effects on bats—a global Meta-analysis” in Bats in the Anthropocene: Conservation of bats in a changing world. eds. C. C. Voigt and T. Kingston (Cham: Springer International Publishing), 13–33.
Jung, K., and Threlfall, C. G. (2018). Trait-dependent tolerance of bats to urbanization: a global meta-analysis. Proc. R. Soc. B Biol. Sci. 285:20181222. doi: 10.1098/rspb.2018.1222
Jung, K., and Tschapka, M. (2022). Bat activity in all Exploratories, summer 2012, using acoustic monitoring. Version 3. Biodiversity Exploratories Information System. Available at: https://www.bexis.uni-jena.deddm/data/Showdata/19852?version=3
Kalda, R., Kalda, O., Lõhmus, K., and Liira, J. (2015). Multi-scale ecology of woodland bat the role of species pool, landscape complexity and stand structure. Biodivers. Conserv. 24, 337–353. doi: 10.1007/s10531-014-0811-6
Kerbiriou, C., Barré, K., Mariton, L., Pauwels, J., Zissis, G., Robert, A., et al. (2020). Switching LPS to LED streetlight may dramatically reduce activity and foraging of bats. Diversity 12:165. doi: 10.3390/d12040165
Krauel, J. J., and LeBuhn, G. (2016). Patterns of bat distribution and foraging activity in a highly urbanized temperate environment. PLoS One 11:e0168927. doi: 10.1371/journal.pone.0168927
Kühn, I., Brandl, R., and Klotz, S. (2004). The flora of German cities is naturally species rich. Evol. Ecol. Res. 6, 749–764.
Lambin, E. F., and Geist, H. J., (2008). Land-Use and Land-Cover Change: Local Processes and Global Impacts. Springer Science and Business Media. Berlin
Lintott, P. R., Barlow, K., Bunnefeld, N., Briggs, P., Gajas Roig, C., and Park, K. J. (2016). Differential responses of cryptic bat species to the urban landscape. Ecol. Evol. 6, 2044–2052. doi: 10.1002/ece3.1996
Liu, X., Huang, Y., Xu, X., Li, X., Li, X., Ciais, P., et al. (2020). High-spatiotemporal-resolution mapping of global urban change from 1985 to 2015. Nat. Sustain 3, 564–570. doi: 10.1038/s41893-020-0521-x
Luck, G. W., Smallbone, L., Threlfall, C., and Law, B. (2013). Patterns in bat functional guilds across multiple urban centres in South-Eastern Australia. Landsc. Ecol. 28, 455–469. doi: 10.1007/s10980-012-9842-0
Lumsden, L. F., and Bennett, A. F. (2005). Scattered trees in rural landscapes: foraging habitat for insectivorous bats in South-Eastern Australia. Biol. Conserv. 122, 205–222. doi: 10.1016/j.biocon.2004.07.006
McDonald, R. I. (2008). Global urbanization: can ecologists identify a sustainable way forward? Front. Ecol. Environ. 6, 99–104. doi: 10.1890/070038
McDonnell, M. J., Hahs, A. K., and Breuste, J. H., (2009). Ecology of Cities and Towns: A Comparative Approach. Cambridge University Press. Cambridge
McKinney, M. L. (2006). Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260. doi: 10.1016/j.biocon.2005.09.005
McKinney, M. L. (2008). Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst. 11, 161–176. doi: 10.1007/s11252-007-0045-4
Moretto, L., Fahrig, L., Smith, A. C., and Francis, C. M. (2019). A small-scale response of urban bat activity to tree cover. Urban Ecosyst. 22, 795–805. doi: 10.1007/s11252-019-00846-w
Newbold, T., Hudson, L. N., Hill, S. L., Contu, S., Lysenko, I., Senior, R. A., et al. (2015). Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50. doi: 10.1038/nature14324
Niemelä, J., Breuste, J. H., Guntenspergen, G., McIntyre, N. E., Elmqvist, T., and James, P., (2011). Urban ecology: Patterns, processes, and applications. OUP Oxford, Oxford.
Oksanen, J., Guillaume Blanchet, F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2020). Vegan: community ecology package. R package version 2, 5–7. Available at: https://CRAN.R-project.org/package=vegan
Pearce, H., and Walters, C. L. (2012). Do green roofs provide habitat for bats in urban areas? Acta Chiropt. 14, 469–478. doi: 10.3161/150811012X661774
Piano, E., Souffreau, C., Merckx, T., Baardsen, L. F., Backeljau, T., Bonte, D., et al. (2019). Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob. Chang. Biol. 26, 1196–1211. doi: 10.1111/gcb.14934
Puig-Montserrat, X., Torre, I., López-Baucells, A., Guerrieri, E., Monti, M. M., Ràfols-García, R., et al. (2015). Pest control service provided by bats in Mediterranean rice paddies: linking agroecosystems structure to ecological functions. Mamm. Biol. 80, 237–245. doi: 10.1016/j.mambio.2015.03.008
Racey, P. A., and Entwistle, A. C. (2003). “Conservation ecology of bats” in Bat Ecology. eds. T. H. Kunz and Y. M. B. Fenton (Chicago: University of Chicago Press), 680–743.
Rey, V., and Bachvarov, M. (1998). Rural settlements in transition – agricultural and countryside crisis in the Central-Eastern Europe. Geo. J. 44, 345–353. doi: 10.1023/A:1006850525893
Rich, C., and Longcore, T., (2013). Ecological Consequences of Artificial Night Lighting. Island Press. Washington, DC.
Rivkin, L. R., Santangelo, J. S., Alberti, M., Aronson, M. F. J., de Keyzer, C. W., Diamond, S. E., et al. (2019). A roadmap for urban evolutionary ecology. Evol. Appl. 12, 384–398. doi: 10.1111/eva.12734
Rosin, Z. M., Skórka, P., Pärt, T., Żmihorski, M., Ekner-Grzyb, A., Kwieciński, Z., et al. (2016). Villages and their old farmsteads are hot spots of bird diversity in agricultural landscapes. J. Appl. Ecol. 53, 1363–1372. doi: 10.1111/1365-2664.12715
Russo, D., and Ancillotto, L. (2015). Sensitivity of bats to urbanization: a review. Mamm. Biol. 80, 205–212. doi: 10.1016/j.mambio.2014.10.003
Rydell, J., and Racey, P. (1995). Exploitation of insects around streetlamps by bats in Sweden. Funct. Ecol. 6:744. doi: 10.2307/2389972
Sala, O. E., Stuart Chapin, F. I. I. I., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., et al. (2000). Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. doi: 10.1126/science.287.5459.1770
Schug, F., Frantz, D., Okujeni, A., van der Linden, S., and Hostert, P. (2020). Mapping urban-rural gradients of settlements and vegetation at national scale using Sentinel-2 spectral-temporal metrics and regression-based unmixing with synthetic training data. Remote Sens. Environ. 246:111810. doi: 10.1016/j.rse.2020.111810
Seibold, S., Gossner, M. M., Simons, N. K., Blüthgen, N., Müller, J., Ambarlı, D., et al. (2019). Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574, 671–674. doi: 10.1038/s41586-019-1684-3
Shochat, E., Warren, P., Faeth, S., Mcintyre, N., and Hope, D. (2006). From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. doi: 10.1016/j.tree.2005.11.019
Stone, E. L., Harris, S., and Jones, G. (2015). Impacts of artificial lighting on bats: a review of challenges and solutions. Mamm. Biol. 80, 213–219. doi: 10.1016/j.mambio.2015.02.004
Straka, T. M., von der Lippe, M., Voigt, C. C., Gandy, M., Kowarik, I., and Buchholz, S. (2021). Light pollution impairs urban nocturnal pollinators but less so in areas with high tree cover. Sci. Total Environ. 778:146244. doi: 10.1016/j.scitotenv.2021.146244
Straka, T. M., Wolf, M., Gras, P., Buchholz, S., and Voigt, C. C. (2019). Tree cover mediates the effect of artificial light on urban bats. Front. Ecol. Evol. 7:91. doi: 10.3389/fevo.2019.00091
Strohbach, M. W., Haase, D., and Kabisch, N. (2009). Birds and the City: urban biodiversity, land use, and socioeconomics. Ecol. Soc. 14:31. doi: 10.5751/ES-03141-140231
Threlfall, C. G., Law, B., and Banks, P. B. (2012). Influence of landscape structure and human modifications on insect biomass and bat foraging activity in an urban landscape. PLoS One 7:e38800. doi: 10.1371/journal.pone.0038800
Threlfall, C., Law, B., Penman, T., and Banks, P. B. (2011). Ecological processes in urban landscapes: mechanisms influencing the distribution and activity of insectivorous bats. Ecography 34, 814–826. doi: 10.1111/j.1600-0587.2010.06939.x
Treitler, J. T., Heim, O., Tschapka, M., and Jung, K. (2016). The effect of local land use and loss of forests on bats and nocturnal insects. Ecol. Evol. 6, 4289–4297. doi: 10.1002/ece3.2160
Keywords: urbanization, bats (chiroptera), spatial heterogeneity, rural landscapes, villages, agricultural landscapes
Citation: Printz L and Jung K (2023) Urban areas in rural landscapes – the importance of green space and local architecture for bat conservation. Front. Ecol. Evol. 11:1194670. doi: 10.3389/fevo.2023.1194670
Edited by:
Zoltan Elek, University of Szeged, HungaryReviewed by:
Jana Růžičková, ELKH-ELTE-MTM Integrative Ecology Research Group, HungaryLenka Harmackova, Charles University, Czechia
Copyright © 2023 Printz and Jung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten Jung, a2lyc3Rlbi5qdW5nQHVuaS11bG0uZGU=
 Lisa Printz
Lisa Printz Kirsten Jung
Kirsten Jung