- 1Leibniz Institute for Zoo and Wildlife Research (IZW), Department of Ecological Dynamics, Berlin, Germany
- 2Technische Universität Berlin, Institute of Ecology, Chair of Applied Animal Ecology, Berlin, Germany
- 3Estación Biológica de Doñana (EBD), Spanish National Research Council (CSIC), Seville, Spain
In urban areas, wildlife has to adapt to human presence and novel predators such as pet species, including the altered conditions of the environment. In such novel settings, the timing of activity is crucial to minimize the risk of mortality. To do so, species may reduce total activity time by increasing activity peaks at specific moments or shifting activity times. We analyzed camera trap data from a citizen science project over four project phases, including spring and autumn before and during the SARS-CoV-2 lockdown, to understand the effects of human, pet (cat, dog), and predator (marten) presence on the activity patterns of urban red squirrels (Sciurus vulgaris; hereafter ‘squirrel’). We examined squirrel activity at seasonal and hourly resolutions in relation to human, garden, urban, and predator factors. We considered human presence as both a direct effect of lockdown and an indirect disturbance measured through urban variables. Results show that direct human presence during lockdown increased squirrel activity intensity in both seasonal and hourly patterns without reducing total activity time. Predator presence affected timing of activity, decreasing total daily activity. Pets, like cats, decreased activity at both resolutions, while martens had a limited effect detected only at the hourly resolution. During lockdown, squirrels may have increased their activity in gardens due to more anthropogenic resources (food or nesting material), but constant threats from pets force them to avoid certain areas despite the benefits. This highlights the delicate balance squirrels must maintain in adapting to human-altered environments while managing predation risks.
1 Introduction
A species’ temporal niche represents a balance between maximizing resource use and minimizing risk (Gilbert et al., 2023). However, in changing environments, this niche is subject to plastic behavioral adjustments (Sol et al., 2013; Ikeda et al., 2016; Gilbert et al., 2023). Urban areas are challenging environments, characterized by high levels of human disturbance (Pickett et al., 2001; Lundholm and Richardson, 2010), limited and fragmented habitats (Miller and Hobbs, 2002; Parsons et al., 2022), altered predator communities (e.g. due to increased pet density; Lenth et al., 2008; Plaza et al., 2019), and a lack of natural resources but an overabundance of anthropogenic food (human food, pet food, trash) and shelter (Bateman and Fleming, 2012; Lowry et al., 2013; Sol et al., 2013). To survive in cities, species must rapidly adjust to changing environmental conditions, often involving trade-offs (Smith et al., 2018) that affect their diet composition (Santini et al., 2019; Scholz et al., 2020), movement behavior (Prange et al., 2004; Stillfried et al., 2017; Thomas et al., 2018; Rast et al., 2019), or activity patterns (Thomas et al., 2018; Gilbert et al., 2022; Louvrier et al., 2022; Cox and Gaston, 2024).
Species modify their activity times to minimize exposure to perceived risks – termed risk allocation hypothesis (Lima and Bednekoff, 1999) – while maintaining necessary resources (Baker et al., 2007; Dowding et al., 2010; Thomas et al., 2018). Species can deal with risk allocation in two ways (Figure 1): Reducing total time while increasing activity intensity (Potash et al., 2023; Tobajas et al., 2024) or shifting the temporal niche to times of lower risk (Louvrier et al., 2022). Changes in the temporal niche can have different fitness consequences, they can reduce stress factors such as warming, but they can also have negative effects, for example if predators shift their activity to times when their prey is less active, or if the shift conflicts with the species’ genetic predispositions (Spoelstra et al., 2016; Levy et al., 2019; Gilbert et al., 2023). Moreover, as most species avoid human presence, the available time for shifting their temporal niche is reduced (Moll et al., 2018; Gilbert et al., 2022), forcing animals to occupy smaller temporal niches, in which to meet their metabolic demands.
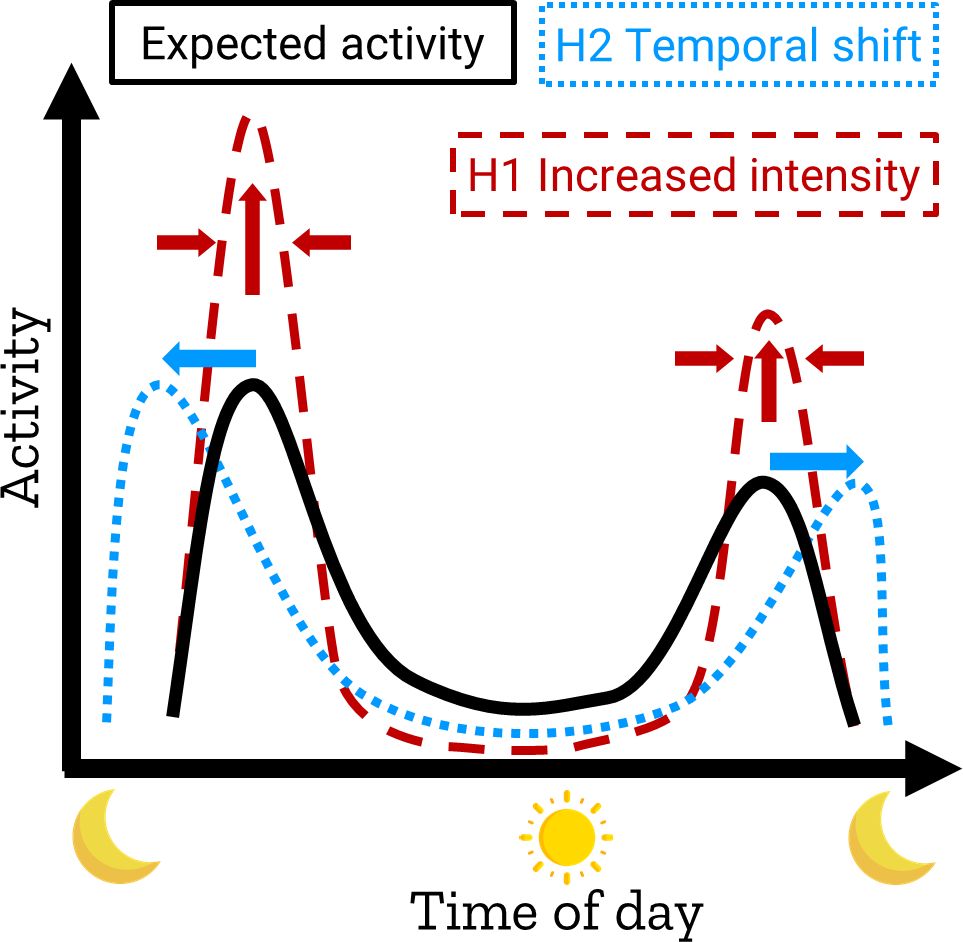
Figure 1. Scheme of risk allocation hypothesis, showing examples of increased intensity (red, dashed line) and temporal shift (blue, dotted line) of activity response compared to the expected activity (black line).
Species respond to the presence of predators in various ways to ensure their survival. For instance, a low attack ratio of the least weasel (Mustela nivalis nivalis) lead to higher foraging effort of bank voles (Clethrionomys glareolus) (Sundell et al., 2004). Fox squirrels (Sciurus niger) increased their foraging in areas with less terrestrial predation but react to the calls of other animals (Potash et al., 2023). Rabbits (Oryctolagus cuniculus) adapt their behavior by increasing their vigilance when cats (Felis catus) are likely to be present at night or by avoiding these areas entirely (Blanchard et al., 2018). Similarly, rats (Rattus spp.) shift their activity to times with less cat activity (Parsons et al., 2018). Therefore, in the landscape of fear created by predators’ prey species react by modifying their behavior.
Human presence and the introduction of novel predators like pets significantly affect the behavior of various species, including generalists (Stillfried et al., 2017). One example is the Eurasian red squirrel (Sciurus vulgaris, hereafter: squirrel) commonly found in urban areas (Shar et al., 2016; Fingland et al., 2022), where they prefer environments with low imperviousness (refers to sealed surfaces like concrete, asphalt etc.) and access to green spaces and mature trees (Grabow et al., 2022). Squirrels are diurnal and adapt their activity patterns to local conditions (Ikeda et al., 2016; Thomas et al., 2018; Beliniak et al., 2021). In natural conditions, squirrels have a peak of activity in the morning and sometimes a second, smaller peak of activity in the afternoon and they show an increase in ground activity during autumn due to food stashing (Ikeda et al., 2016; Beliniak et al., 2021).
However, urban squirrels also face new challenges, including ubiquitous human presence (e.g. direct disturbance, noise, trash, altering landscapes), habitat fragmentation, road mortality and the need to avoid numerous natural predators like birds of prey, crows, foxes, martens, raccoons, and pets like dogs and cats (Lurz et al., 2005; Ikeda et al., 2016; Selonen et al., 2016; Jokimäki et al., 2017; Fingland et al., 2022; Grabow et al., 2022). Urban squirrels have smaller home ranges and higher population densities (Babińska-Werka and Zółw, 2008; Kopij, 2014; Thomas et al., 2018; Fingland et al., 2022). They exhibit bolder behaviors and reduced flight responses compared to their rural counterparts (Uchida et al., 2016, 2019). Squirrels must balance resource acquisition with avoiding humans and predators (Moll et al., 2017, 2018; Uchida et al., 2019; Fingland et al., 2022). Their activity patterns reflect predator avoidance behaviors, with the intensity indicating the level of perceived risk from various predators.
We investigated squirrel activity patterns using camera trap data from a city-wide Citizen Science project developed in Berlin, Germany (WTimpact; www.wtimpact.de; for details see Louvrier et al., 2022). The project ran from autumn 2018 to autumn 2020, coinciding with the first year of the COVID-19 pandemic and its lockdowns. This provided a unique opportunity to quantify the effects of human activity along an urbanization gradient on wildlife during the global anthropause (Rutz et al., 2020). During this time, many people stayed at home, cared more for their gardens became more interested in wildlife and set up feeding stations. In this study, we focus on squirrel and predator activity.
Here, we examined how squirrels structure their ground activity time in relation to risk and resource availability at two hierarchical temporal scales: a broad seasonal scale (i.e. spring and autumn) and a detailed hourly scale. Specifically, we analyzed: I) How environmental characteristics, predator presence, and lockdown effects influenced seasonal activity. II) How season, lockdown effects, environmental characteristics, and predators influenced hourly activity.
We expect that: a) Squirrel activity will be higher in autumn, during lockdown, and in areas with low imperviousness, as squirrels benefit from an increase in feeding stations in gardens (resource availability). b) Squirrels will display a daily activity pattern that reduces overlap with predator or human activity times by increasing intensity and shifting their temporal niche (risk allocation). Understanding these behavioral adjustments is essential for successful urban wildlife planning and conservation.
2 Materials and methods
2.1 Study area
We conducted the study in the city-state of Berlin, Germany (Figure 2A). Berlin covers an area of 891 km2 with a population of almost 4 million people (Statistics Office Berlin-Brandenburg, 2023a). The city is characterized by the abundance of green spaces, from allotments close to the city center to parks, private gardens and suburban forests. Impervious surfaces – settlement and transport areas – cover almost 60% of the surface, green areas 11,6%, forests 17.7%, water bodies 6.6%, and agriculture 4% (Statistics Office Berlin-Brandenburg, 2023b).

Figure 2. (A) Study area in Berlin, Germany, and locations of the camera traps (stations) in each of the project phases included in the study. (B) Comparison of the activity of cats and squirrels during all project phases. (C) Comparison of the activity of martens and squirrels during all project phases. (D) Comparison of the activity of squirrels in spring with and without lockdown. (E) Comparison of the activity of squirrels in autumn with and without lockdown. Shaded areas represent activity overlap.
2.2 Multi-season citizen science project
We used camera-trap data collected by the semi-structured Citizen Science project “Wildtierforscher Berlin” as part of “Verbundprojekt WTimpact” for our study (www.wtimpact.de; for details see Louvrier et al., 2022). The volunteer citizen scientists set up camera traps in their gardens for 4 consecutive weeks over five project phases: autumn 2018, spring and autumn 2019, and spring and autumn 2020 during the SARS-CoV-2 lockdown periods. To locate the cameras, the city was divided into 2x2 km grids and, in each project phase, around 200 volunteers were selected based on their location to cover as much of the city as possible (Figure 2A). The citizens received training and clear instructions on the use and installation of the camera traps before each project phase to ensure the comparability of the results. All camera traps (named “stations” hereafter) were installed in private gardens at about 50 cm height with a clear sight across the garden. After the sampling period, the citizens uploaded all the mammal pictures, including cats, to the project website. An expert from the project confirmed the correct identification of the species detected. Citizens removed pictures of humans and dogs to respect privacy, but the information whether garden owners had a dog or a cat was noted for each station. In addition, cat pictures were kept, as they usually roam without humans. To have a balanced, full-factorial design, we omitted the data from the first project phase from autumn 2018, the test phase, and only used the data from spring 2019 to autumn 2020 for the analyses, yielding 2 spring and autumn seasons, one of each with lockdown. Supplementary Material 1 provides a summary of project phase 1. No station was used in more than one project phase.
2.3 Species data
For each project phase, we extracted all pictures of squirrels and their potential predators in the area: marten species (Martes foina or in rare cases also Martes martes) as natural predators, and cats (Felis catus) as novel predators. We pooled both species of marten together due to the difficulties to identify the pictures reliable at species level.
For the seasonal analyses, we calculated an index of activity per station for squirrels by adding all detections at the same station across each project phase. To avoid detections of the same individual multiple times, we removed duplicates from the same burst of photos. It is important to note that the camera traps were aiming at open areas, thus the pictures show squirrels on the ground. 346 out of 516 (67%) stations did not detect any squirrels. These non-detections were denoted with zero. Similarly, we treated predator detections as binary variables, with a value of one if the species was detected at a station or zero when it was not detected. We used this approach due to the low number of predator detections in the stations with squirrels.
For the hourly activity analyses, we restricted the dataset to those stations with at least one squirrel detection and obtained an index of activity per hour per station at specific hours across the project phase. We restricted the hourly activity index to those hours where stations detected squirrels at least once (5am-5pm). For the analyses of the predator effects, the dataset was further restricted to stations that detected both squirrel and the predator of interest (cats: 63 stations (37% of squirrel stations), martens: 15 stations (9% of squirrel stations)). In this case, we defined daily predator detection – non-detection, indicating whether a predator was detected at the station on the same day and before the first detection of a squirrel.
2.4 Environmental data
We extracted environmental information at two spatial hierarchical levels, the local garden level where the station was installed and the urban level where the gardens were located:
1. Garden data: The citizen scientists provided us with garden data with information about the specific characteristics of the gardens: garden size, presence of compost, fence type, whether they own a dog (Canis lupus familiaris) or cat. From this set of variables, we only used garden size and the presence of dogs for our analysis, as we considered all the fences to be permeable to squirrels and compost to have no effect on squirrels. We obtained cat information from the camera trap pictures.
2. Urban data: We obtained the urban data from remote sensing data freely accessible on online databases. These contained information on urban variables such as imperviousness, human population density, tree cover, light pollution, and noise levels. These remote sensing data are freely accessible via Berlin’s geoportal (https://fbinter.stadt-berlin.de/fb/index.jsp) or Copernicus (https://land.copernicus.eu/), the Earth observation program of the EU (Supplementary Material 2.1). All raster data had a spatial resolution of 10x10m. When the raw data was provided as vector data, we rasterized it to the same resolution. Then we extracted the urban data using the focal mean centered at each station covering an area of 100 x 100m. Additionally, we computed the distance from the station to the administrative city border, as a measure of urbanization (see Supplementary Material 2.2).
We assessed collinearity between variables of both the garden and urban datasets using Pearson’s moment correlation coefficient (r) (Supplementary Material 2.3). We considered all variables with | r | > 0.7 to be highly correlated and therefore excluded one of the variables from the correlated pair from the analyses. In these cases, we kept the variables that we expected to better represent squirrel activity or the urban environment. For the final analyses, we used the variables imperviousness, human population density, noise level, and distance to the city border.
2.5 Data analysis
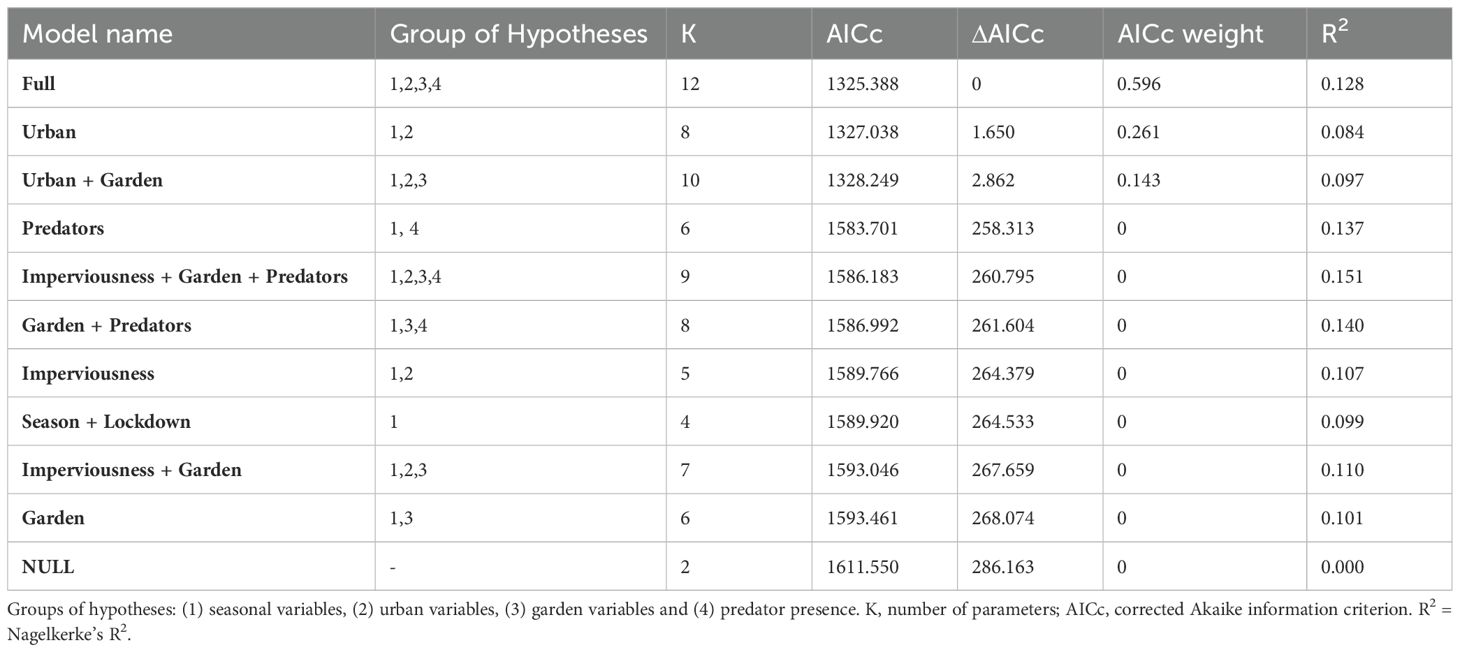
We analyzed seasonal activity patterns using generalized linear models (GLM) with negative binomial distributions and log-link functions. As response variable, we used the activity index of squirrels per station. To assess the relevance of the different variables in determining squirrel activity, we designed 11 competing models based on four groups of hypotheses: (1) seasonal variables (season, lockdown), (2) urban variables (imperviousness, noise level, population density, distance to city border), (3) garden variables (garden size, dogs yes/no) and (4) predator presence (cats (‘cats on cams’), martens (‘martens on cams’)). We implemented season as a control variable in all models, because we expected the activity to be mainly driven by season and wanted to reduce confounding effects. Climatic factors can also have an influence on the activity patterns of animals, for example by changing resource availability or the living conditions. However, we assume that the differences from one year to the next in Berlin are too small. For comparison, we also included a full and a null model. We used a model selection approach using Akaike’s information criterion (AIC) with a difference of at least Δ7 to the next best model (Burnham et al., 2011).
We compared the hourly activity patterns of squirrels in gardens with and without the presence of predators – dogs, cats, and martens – across different project phases. In our analysis, we only considered whether the predators were observed at least once per project phase. For dogs, we had only information on whether they were kept as pets in the gardens, no pictures. For martens and cats, we similarly recorded whether they were sighted at least once per project phase. For the analysis, we used the hourly number of squirrel images to create overlap plots and calculated the overlap estimator ‘Dhat4’ based on kernel density estimates of the original data (Ridout and Linkie, 2009).
To further analyses changes in the hourly activity patterns of squirrels, we used generalized linear mixed models (GLMMs). We only used stations that detected squirrels at least once and we calculated the total number of pictures per station per hour of the day. Hours without detections received the value zero. This number served as response variable in the GLMMs, which we ran with negative binomial distributions, log-link functions and the station IDs as random intercepts. We used a model selection approach based on AIC, with three groups of hypotheses: seasonal, urban (only imperviousness) or garden (only dogs) effects. Additionally, we included the hour of the day as a polynomial of order four to represent the activity patterns detected in the overlap plots, and the interaction between the hour of the day and the other explanatory variables to detect changes in the activity pattern caused by them.
Finally, we analyzed the effects of the predators cats and martens on squirrel hourly activity patterns, using a similar approach as above. For this, we ran similar GLMMs, comparing the activity patterns of squirrels on days with and without predator detections. We used the hour of the day as a polynomial of order four to match the pattern in squirrel activity and the possible interactions with seasonal effects and the predator presence as response variables. We thus formulated four models per predator. Because of the fourth degree polynomial term, we tested the significance of the variable effects and the interaction using an anova approach on the model.
Lastly, while overparameterization of our GLMMs was a concern, we included the variables we deemed important and tested various combinations. Despite the number of explanatory variables, models incorporating all of them showed the highest AIC and R² values, suggesting these models were the most appropriate.
We performed all analyses in R (version 4.3.0; R Core Team, 2023), using packages “MASS” (Venables and Ripley, 2002) and “lme4” (Bates et al., 2015) for GLMMs, “ggeffects” (Lüdecke, 2018) to compute marginal means and “overlap” (Ridout and Linkie, 2009) to compute the hourly overlap analyses and obtain the overlap estimator.
3 Results
After retaining only independent events, we obtained a total of 1335 pictures of squirrels, 14087 pictures of cats and 715 pictures of martens (Figure 3), collected via 346 stations. Squirrels were detected every day in at least one camera, except one day in project phase 4, to a maximum of 42 times per day. They were active between 5am and 5pm. There were 56 gardens with dogs. Cats were detected at least 48 times and a maximum of 229 times per day during each project phase. Martens were not detected on 3 days during project phase 2 and on one day during project phase 5. Otherwise, they were detected at least once and a maximum of 22 times per day.

Figure 3. Summary of camera trap data per project phase per species. Bars represent the total number of pictures. The green line indicates the percentage of days on which the species was detected in each project phase, while the red line shows the percentage of stations where the species was detected during each project phase.
3.1 Seasonal activity
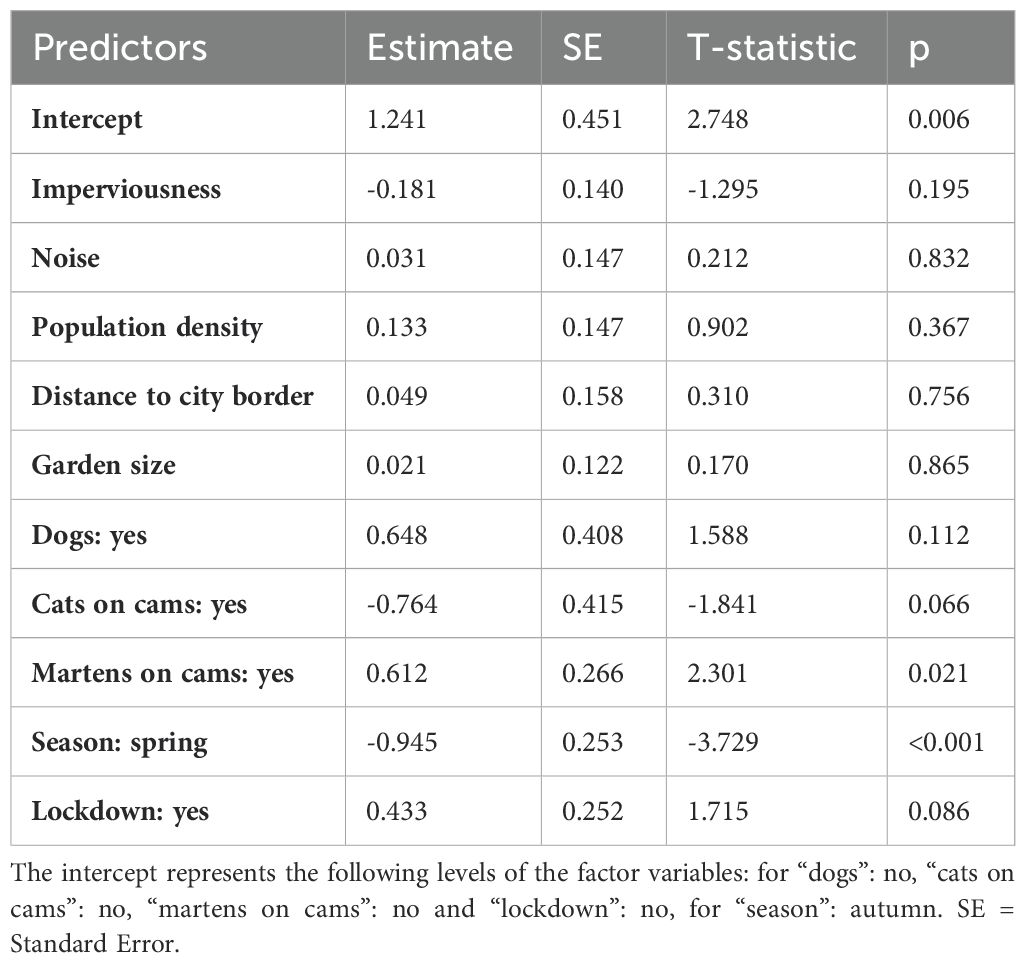
In the analysis of the seasonal patterns, the best model was the full model, which explained 13% of the variance, followed closely by the urban model and the urban and garden model (ΔAICc 1.65 and 2.86; Table 1). However, R2 is higher for the full model than for the other two models, making it a better model. All three models included seasonal and urban variables. The full model (Table 2; Supplementary Material 3) showed significantly lower activity in spring (GLM, β = -0.945 ± 0.253, p = <0.001), but higher activity in gardens with presence of martens (GLM, β = 0.612 ± 0.266, p = 0.021). There was a trend to lower squirrel activity in gardens with cats (GLM, β = -0.765 ± 0.415, p = 0.066) and without lockdown (GLM, β = 0.433 ± 0.252, p = 0.086). The other two models show similar effects for season and lockdown (Supplementary Material 3). A comparison between the season and lockdown (Figures 2B, C) shows that the activity peaks are lower during the lockdown, but the activity spreads more evenly throughout the day.
3.1.1 Activity overlap
Squirrels are diurnal, while cats are active throughout the day (Figure 2D) and martens are primarily crepuscular and nocturnal (Figure 2E). In spring, the overlap between squirrel activity with and without predators is generally lower than in autumn, regardless of whether there was a lockdown or not (Figure 4; Supplementary Material 4.1). The presence of dogs leads to the biggest difference in squirrel activity patterns, or the smallest overlap. Overlap of squirrel activity in gardens with vs without dogs ranges between 0.360 (in spring during lockdown, 95% CI [0.258, 0.461]) and 0.700 (in autumn during lockdown, 95% CI [0.621, 0.781]). Only in spring without lockdown did cats lead to a higher difference in squirrel activity, with an overlap of 0.373 (95% CI [0.247, 0.499]). The highest overlap of squirrel activity in gardens with and without cats was in autumn during lockdown with 0.746 (95% CI [0.682, 0.809]). Presence of martens always has the least influence on squirrel activity. The overlap was here between 0.519 (in spring during lockdown, 95% CI [0.423, 0.615]) and 0.772 (in autumn during lockdown, 95% CI [0.718, 0.825]).
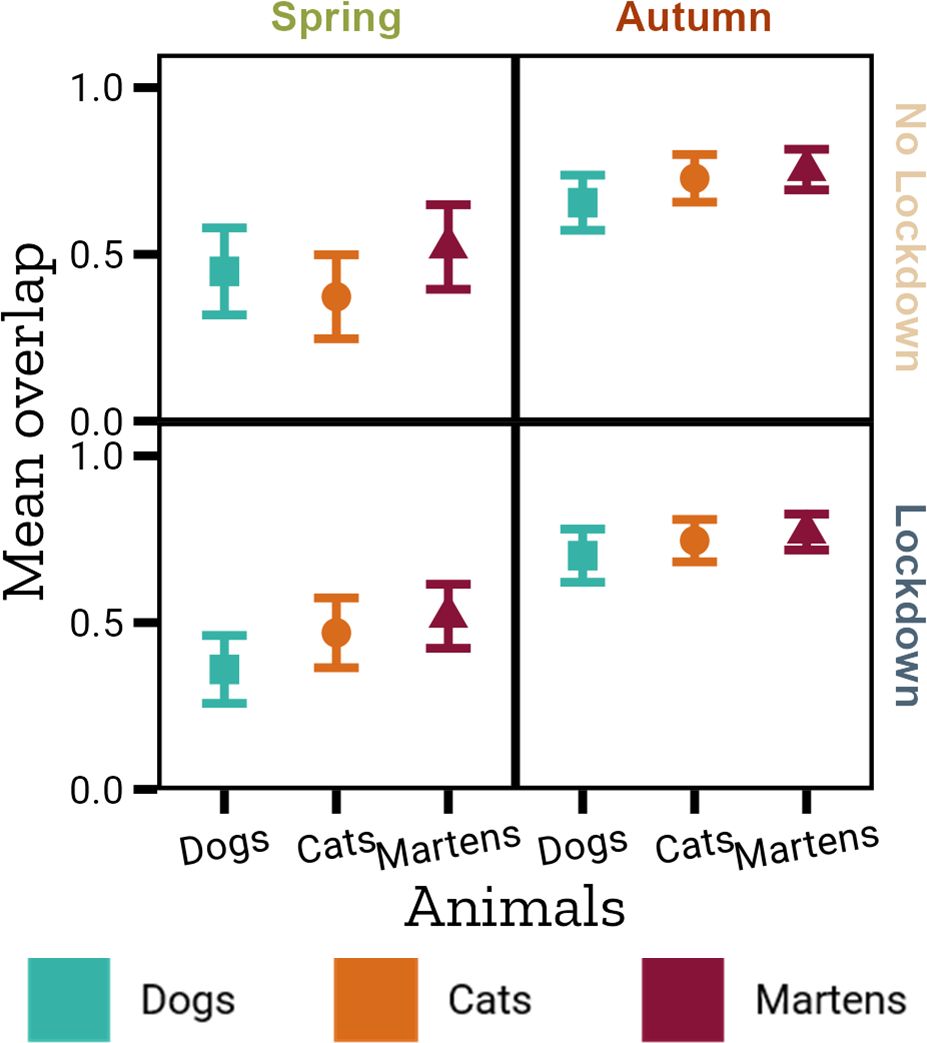
Figure 4. Mean overlap of squirrel activity with different predators (x-axis), divided by season (column-wise) and lockdown (row-wise).
3.2 Hourly activity
3.2.1 Hourly activity in relation to environmental factors
We used all 1335 pictures of squirrels from 170 stations, ranging from 0 pictures to 19 pictures per hour (taken at 11 am). The best model was the full model, including all interactions (Table 3A, see Supplementary Material 4.2 for anova and model summary). Squirrel activity was lower in spring than in autumn (GLMM, β = -0.362 ± 0.215, p = 0.09) with two activity peaks in autumn and only one in spring (Figure 5A). Lockdown significantly increased activity (GLMM, β = 0.556 ± 0.201, p = 0.006, Figure 5B). Imperviousness modified the activity pattern from one pronounced peak in the morning hours in areas with low impervious surface to a lower morning peak and increased afternoon activity in areas with high imperviousness (GLMM, β = -0.151 ± 0.102, p = 0.139, Figure 5C). We did not detect an effect of dogs on the hourly activity (GLMM, β = 0.135 ± 0.344, p = 0.695, Figure 5D).

Table 3. Model selection table for the hourly GLMMs in relation to (A) environmental factors, (B) presence of cats, (C) presence of martens.
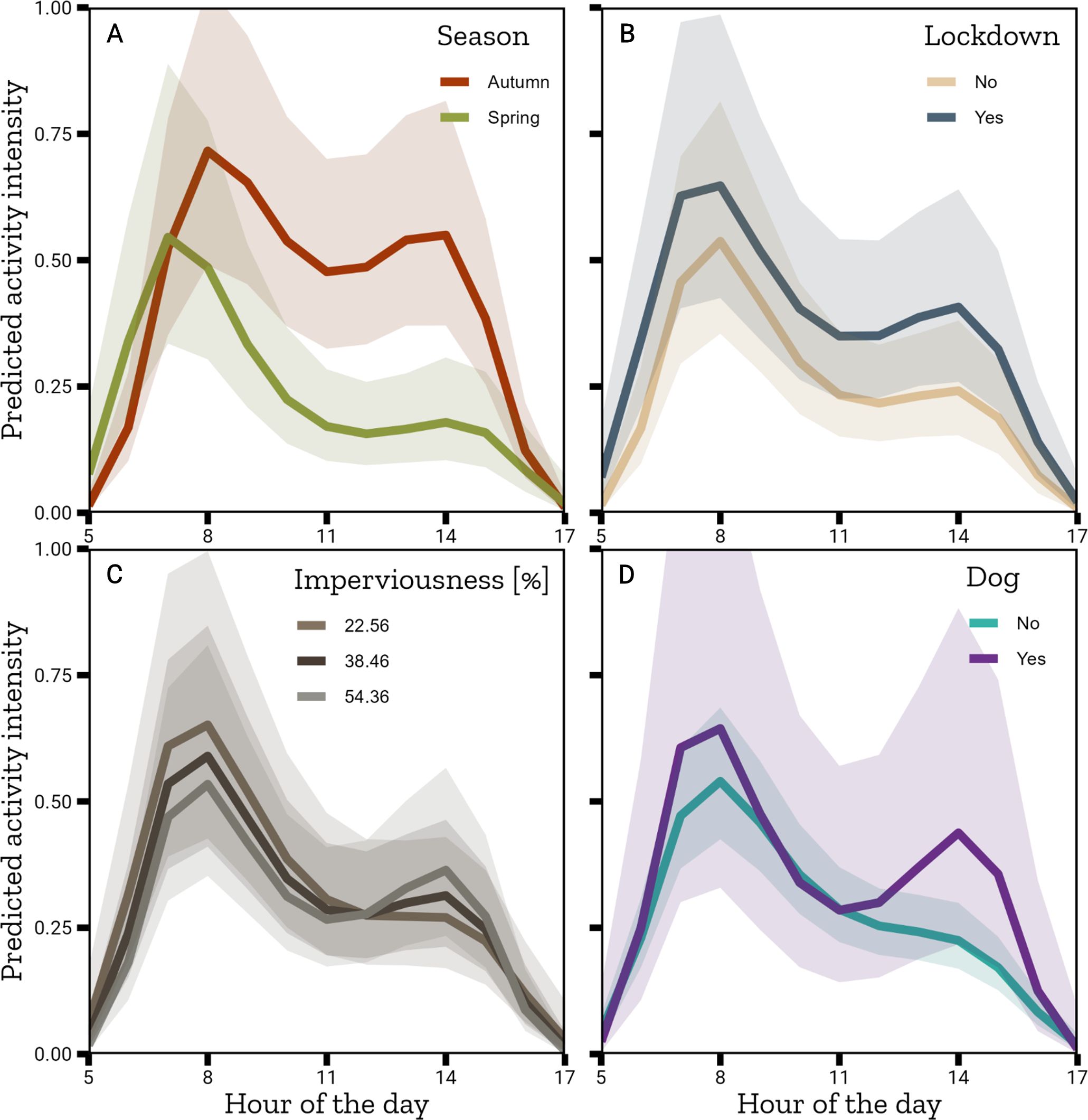
Figure 5. Marginal effects plots of the best model for hourly ground activity of squirrels regarding environmental factors using the average values across all predictors. Interaction of the hour of the day with (A) Season, (B) Lockdown, (C) Imperviousness, (D) Dogs.
3.2.2 Hourly activity in relation to predators
For the analysis of the effect of cats on squirrel activity, we used 662 pictures of squirrels from 63 stations (project phase 2: 8, project phase 3: 22, project phase 4: 13, project phase 5: 20). Stations detected cats on a minimum of one day and a maximum of 19, with an average of 3.11 ± 3.35 days per station. The best model included all interactions of the hour of the day with season, lockdown and the presence of cats (Table 3B, see Supplementary Material 4.3 for anova and model summary). Squirrel activity was higher in autumn (GLMM, β = -0.51 ± 0.323, p = 0.114) with two activity peaks (Figure 6A). Lockdown significantly increased the hourly squirrel activity (GLMM, β = 1.178 ± 0.332, p < 0.001, Figure 6B). Whereas the presence of cats significantly decreased squirrel activity (GLMM, β = -0.552 ± 0.151, p < 0.001, Figure 6C). The presence of cats leads to two peaks of activity, with the peak in the morning (around 8 am) being higher than the peak in the afternoon (around 2 pm).
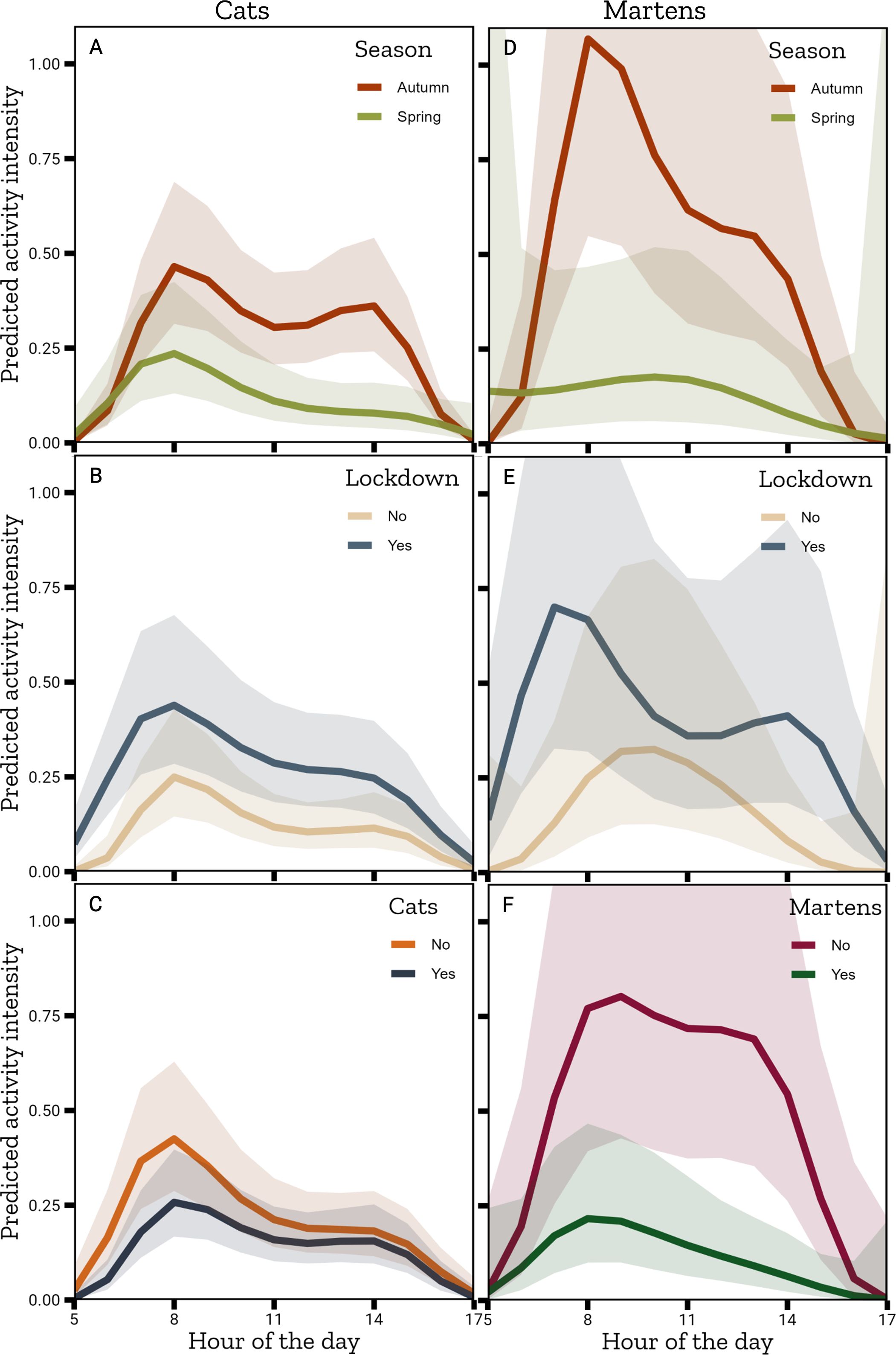
Figure 6. Marginal effects plots of the best GLMM for hourly ground activity of squirrels regarding cats (CATS03) and martens (MARTENS03) using the average values across all predictors. 1st column cats: Interaction of the hour of the day with (A) Season, (B) Lockdown, (C) Presence of cats. 2nd column martens: Interaction of the hour of the day with (D) Season, (E) Lockdown, (F) Presence of martens.
For the analysis of the effect of martens on squirrel activity, we used 312 pictures of squirrels from 15 stations (project phase 2: 1, project phase 3: 6, project phase 4: 4, project phase 5: 4). Stations detected martens on a minimum of one day and a maximum of 7 days, with an average of 2.20 ± 1.82 days per station. Again, the best model included all interactions of the hour of the day with season, lockdown and the presence of martens (Table 3C, see Supplementary Material 4.4 for anova and model summary). As above, squirrels were more active in autumn than in spring (GLMM, β = -0.532 ± 0.593, p = 0.369, Figure 6D) and they were more active during lockdown (GLMM, β = 1.821 ± 0.784, p = 0.02, Figure 6E). Martens significantly decreased squirrel activity (GLMM, β = -1.359 ± 0.243, p < 0.001, Figure 6F) and there is only one activity peak in the morning (around 8am).
4 Discussion
We analyzed how urban and garden conditions, human activity, predation risk, and season affected the activity patterns of urban red squirrels. Squirrels were generally more active during Covid lockdowns. Our study demonstrates that squirrels primarily alter their activity patterns to avoid predators rather than human presence. They exhibit clear risk allocation behavior and temporal niche shifting, increasing activity on days without predators to maximize resource acquisition and decreasing it when predators are present to minimize risk. Furthermore, we found a seasonal effect, as responses to these variables were stronger during spring.
During the Covid lockdowns, squirrel activity in gardens increased. During this time, most humans were staying at home and some used the opportunity to make their gardens more wildlife-friendly by adding suitable plants or installing bird and squirrel feeders (Brock et al., 2021; Vimal, 2022). The additional food sources might have encouraged squirrels to move closer to gardens with feeders (Babińska-Werka and Zółw, 2008; Reher et al., 2016; Jokimäki et al., 2017; Starkey and delBarco-Trillo, 2019). Hansen et al. (2020) found that gardens with feeders, fences and pets attract more wildlife than those without. Gardens with regular human activity offer protection from birds of prey, which can reach high densities in cities (Kettel et al., 2018).
Another dimension of human impact on wildlife is at the landscape level, for example through imperviousness or a higher human density in urban areas. At a small-scale level, people have an influence, for example, through the garden design, choice of plant species or whether they feed animals. Imperviousness, connectivity of green spaces, and human population density all have significant effects on squirrel abundance and occupancy (Jokimäki et al., 2017; Grabow et al., 2022). However, our study did not find significant effects of broader urban landscape factors, such as imperviousness, noise level, population density, distance to the city border or garden size on squirrel activity timing. While the interaction between the hour of the day and imperviousness was statistically significant according to the Wald chi-square test, it did not show significance in the overall model. These findings suggest that while certain urban factors may influence the likelihood of squirrels being present in an area, they do not appear to affect their activity patterns. It is likely that these characteristics are too broad to influence squirrel activity directly, and more specific factors, such as the presence of predators, have a stronger impact on activity patterns. Additionally, squirrels may exhibit behavioral plasticity, allowing them to adapt to urban environments in ways that mitigate the influence of these broad factors. Other untested variables, such as traffic volume, may also play a more critical role in shaping squirrel activity.
In contrast, some urban wildlife species, like red foxes (Vulpes vulpes) and hedgehogs (Erinaceus europaeus), shift their activity to more nocturnal times to reduce the risk from humans, particularly traffic (Baker et al., 2007; Dowding et al., 2010). Wild boars (Sus scrofa) exhibit habituation to human activity and have shorter flight distances compared to their rural counterparts (Stillfried et al., 2017).
Dogs, often seen as a proxy for human activity, as people walk their dogs or the dogs are often only allowed in the garden when their humans are at home (at least in the case of Berlin), did not show a significant effect in our seasonal or hourly models. This output should be interpreted cautiously, as data for dogs was only provided at the seasonal scale – whether dogs live in house with the garden or not – and not obtained from pictures due to data protection issues. However, in the overlap analysis, dogs had the strongest effect on reducing squirrel activity, suggesting that dogs may have a more substantial impact than our data could fully capture. This effect might have been heightened during the lockdown when people and their dogs were more frequently at home, as noted in other studies (Lenth et al., 2008; Kays and Parsons, 2014; Hansen et al., 2020).
Our study also highlights the impact of domestic pets, particularly cats, on squirrel behavior. Cats, which roam freely and are active throughout the day, pose a significant threat to squirrels, requiring them to remain vigilant. This is consistent with previous studies showing the negative effects of cats on squirrels (Jokimäki et al., 2017) and other wildlife (Loss et al., 2013; Parsons et al., 2018; Hansen et al., 2020; Louvrier et al., 2022). Unlike martens, whose infrequent presence allows squirrels to resume normal activity in their absence, the constant presence of cats forces squirrels to adapt continuously.
Interestingly, the positive association between marten presence and squirrel activity at the seasonal level (Table 2) suggests that both species might share similar habitats (Twining et al., 2022), a pattern also observed by Ikeda et al. (2016) during autumn but not for other seasons. However, at the hourly scale (Figure 6F), squirrels clearly avoid martens by decreasing their activity in gardens where martens are present, demonstrating a risk allocation strategy.
Cats and martens significantly reduced squirrel activity, with no observed compensatory mechanisms. Although human activities, especially during the lockdowns, influenced squirrel behavior, it seems that squirrels can partially buffer the effects of urban landscape changes. The increased activity during lockdowns suggests that feeding may have a positive effect on squirrel behavior, potentially supported by reduced predator activity in gardens. Wildlife species must adapt to both direct and indirect human disturbances to survive, making it essential to understand their responses to humans in various contexts (Burton et al., 2024).
Squirrels avoided predators by reducing their activity during times when threats were present, a behavior amplified in spring. This seasonal variation might be explained by the increased need to gather food in autumn, which could outweigh the fear of predators. Additionally, the importance of parental care during the reproductive season in spring may lead to greater caution. Our results confirm previous findings that squirrels are more active in autumn, likely due to their need to collect and hide nuts (Ikeda et al., 2016; Beliniak et al., 2021). Potash et al. (2023) found that eastern fox squirrels (Sciurus niger) increased foraging in areas free from terrestrial predators.
One limitation of our study is the use of ground-targeted cameras, which may underestimate the overall activity of squirrels, as they are more likely to be active in trees. Despite this, we assume our findings accurately reflect known squirrel activity patterns. Additionally, the lack of data on direct human presence in the gardens prevents us from assessing its impact on squirrel behavior, leaving this aspect unexplored.
Our findings show that squirrels primarily adjust their behavior to avoid predators rather than human activity. They demonstrate risk allocation behavior and temporal niche shifting to enhance resource acquisition and minimize predation risk across seasons. The hourly analysis provided detailed insights into how small-scale activity shifts help squirrels survive in urban areas. Overall, this study underscores the complexity of urban impacts on squirrel activity and highlights the diverse strategies squirrels use to navigate these challenges. Understanding wildlife responses to disturbances is crucial for their conservation in increasingly urbanized landscapes.
Data availability statement
The original contributions presented in the study are publicly available. The data and scripts can be found in the Github repository of the Department of Ecological Dynamics of the Leibniz Institute for Zoo and Wildlife Research: https://github.com/EcoDynIZW/Drenske_2024_FrontEcolEvol.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because it is a noninvasive camera trap study.
Author contributions
SD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CL: Project administration, Supervision, Writing – original draft, Writing – review & editing. SK-S: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. SD has funding from the Elsa-Neumann foundation. The German Federal Ministry of Education and Research BMBF supported WTImpact (funding number 01|O1725). SD, MG and SK-S are associated with the DFG Research Training Group ‘BioMove’ (DFG-GRK 2118/2).
Acknowledgments
We deeply thank Miriam Brandt for initiating and leading WTImpact. We thank Moritz Wenzler for providing environmental layers. We are thankful for all participating citizen scientists in and around Berlin who spent numerous hours in maintaining the camera traps and uploading and classifying camera trap photos in the WTImpact project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2024.1455142/full#supplementary-material
References
Babińska-Werka J., Zółw M. (2008). Urban populations of the red squirrel (Sciurus vulgaris) in Warsaw. Annales Zoologici Fennici. 45 (4), 270–276. doi: 10.5735/086.045.0405
Baker P. J., Dowding C. V., Molony S. E., White P. C. L., Harris S. (2007). Activity patterns of urban red foxes (Vulpes vulpes) reduce the risk of traffic-induced mortality. Behav. Ecol. 18, 716–724. doi: 10.1093/beheco/arm035
Bateman P. W., Fleming P. A. (2012). Big city life: Carnivores in urban environments. J. Zool. 287, 1–23. doi: 10.1111/j.1469-7998.2011.00887.x
Bates D., Mächler M., Bolker B. M., Walker S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Beliniak A., Krauze-Gryz D., Jasińska K., Jankowska K., Gryz J. (2021). Contrast in daily activity patterns of red squirrels inhabiting urban park and urban forest. Hystrix 31, 159–164. doi: 10.4404/hystrix-00476-2021
Blanchard P., Lauzeral C., Chamaillé-Jammes S., Brunet C., Lec’hvien A., Péron G., et al. (2018). Coping with change in predation risk across space and time through complementary behavioral responses. BMC Ecol. 18, 1–10. doi: 10.1186/s12898-018-0215-7
Brock M., Doremus J., Li L. (2021). Birds of a feather lockdown together: Mutual bird-human benefits during a global pandemic. Ecol. Econ. 189, 1–9. doi: 10.1016/j.ecolecon.2021.107174
Burnham K. P., Anderson D. R., Huyvaert K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35. doi: 10.1007/s00265-010-1029-6
Burton A. C., Beirne C., Gaynor K. M., Sun C., Granados A., Allen M. L., et al. (2024). Mammal responses to global changes in human activity vary by trophic group and landscape. Nat. Ecol. Evol. 8, 924–935. doi: 10.1038/s41559-024-02363-2
Cox D. T. C., Gaston K. J. (2024). Ecosystem functioning across the diel cycle in the Anthropocene. Trends Ecol. Evol. 39, 31–40. doi: 10.1016/j.tree.2023.08.013
Dowding C. V., Harris S., Poulton S., Baker P. J. (2010). Nocturnal ranging behavior of urban hedgehogs, Erinaceus europaeus, in relation to risk and reward. Anim. Behav. 80, 13–21. doi: 10.1016/j.anbehav.2010.04.007
Fingland K., Ward S. J., Bates A. J., Bremner-Harrison S. (2022). A systematic review into the suitability of urban refugia for the Eurasian red squirrel Sciurus vulgaris. Mammal. Rev. 52, 26–38. doi: 10.1111/mam.12264
Gilbert N. A., McGinn K. A., Nunes L. A., Shipley A. A., Bernath-Plaisted J., Clare J. D. J., et al. (2023). Daily activity timing in the Anthropocene. Trends Ecol. Evol. 38, 324–336. doi: 10.1016/j.tree.2022.10.008
Gilbert N. A., Stenglein J. L., Pauli J. N., Zuckerberg B. (2022). Human disturbance compresses the spatiotemporal niche. Proc. Natl. Acad. Sci. United States America 119, 1–9. doi: 10.1073/pnas.2206339119
Grabow M., Louvrier J. L. P., Planillo A., Kiefer S., Drenske S., Börner K., et al. (2022). Data-integration of opportunistic species observations into hierarchical modeling frameworks improves spatial predictions for urban red squirrels. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.881247
Hansen C. P., Parsons A. W., Kays R., Millspaugh J. J. (2020). Does use of backyard resources explain the abundance of urban wildlife? Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.570771
Ikeda T., Uchida K., Matsuura Y., Takahashi H., Yoshida T., Kaji K., et al. (2016). Seasonal and diel activity patterns of eight sympatric mammals in northern Japan revealed by an intensive camera-trap survey. PloS One 11, 1–16. doi: 10.1371/journal.pone.0163602
Jokimäki J., Selonen V., Lehikoinen A., Kaisanlahti-Jokimäki M. L. (2017). The role of urban habitats in the abundance of red squirrels (Sciurus vulgaris, L.) in Finland. Urban Forest. Urban Green. 27, 100–108. doi: 10.1016/j.ufug.2017.06.021
Kays R., Parsons A. W. (2014). Mammals in and around suburban yards, and the attraction of chicken coops. Urban Ecosyst. 17, 691–705. doi: 10.1007/s11252-014-0347-2
Kettel E. F., Gentle L. K., Quinn J. L., Yarnell R. W. (2018). The breeding performance of raptors in urban landscapes: a review and meta-analysis. J. Ornithol. 159, 1–18. doi: 10.1007/s10336-017-1497-9
Kopij G. (2014). Distribution and abundance of the Red Squirrel Sciurus vulgaris in an urbanized environment. Acta Musei Silesiae Scientiae Naturales 63, 255–262. doi: 10.2478/cszma-2014-0022
Lenth B. E., Knight R. L., Brennan M. E. (2008). The effects of dogs on wildlife communities. Natural Areas J. 28, 218–227. doi: 10.3375/0885-8608(2008)28[218:TEODOW]2.0.CO;2
Levy O., Dayan T., Porter W. P., Kronfeld-Schor N. (2019). Time and ecological resilience: can diurnal animals compensate for climate change by shifting to nocturnal activity? Ecol. Monogr. 89, 1–22. doi: 10.1002/ecm.1334
Lima S. L., Bednekoff P. A. (1999). Temporal variation in danger drives antipredator behavior: thePredation risk allocation hypothesis. Am. Nat. 153, 649–659. doi: 10.1086/303202
Loss S. R., Will T., Marra P. P. (2013). The impact of free-ranging domestic cats on wildlife of the United States. Nat. Commun. 4, 1–7. doi: 10.1038/ncomms2380
Louvrier J. L. P., Planillo A., Stillfried M., Hagen R., Börner K., Kimmig S., et al. (2022). Spatiotemporal interactions of a novel mesocarnivore community in an urban environment before and during SARS-CoV-2 lockdown. J. Anim. Ecol. 91, 367–380. doi: 10.1111/1365-2656.13635
Lowry H., Lill A., Wong B. B. M. (2013). Behavioral responses of wildlife to urban environments. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
Lüdecke D. (2018). ggeffects: tidy data frames of marginal effects from regression models. J. Open Source Softw. 3, 772. doi: 10.21105/joss.00772
Lundholm J. T., Richardson P. J. (2010). Habitat analogues for reconciliation ecology in urban and industrial environments. J. Appl. Ecol. 47, 966–975. doi: 10.1111/j.1365-2664.2010.01857.x
Lurz P. W. W., Gurnell J., Magris L. (2005). Sciurus vulgaris. Mamm. Species 769, 1–10. doi: 10.1644/1545-1410(2005)769[0001:sv]2.0.co;2
Miller J. R., Hobbs R. J. (2002). Conservation where people live and work. Conserv. Biol. 16, 330–337. doi: 10.1046/j.1523-1739.2002.00420.x
Moll R. J., Redilla K. M., Mudumba T., Muneza A. B., Gray S. M., Abade L., et al. (2017). The many faces of fear: a synthesis of the methodological variation in characterizing predation risk. J. Anim. Ecol. 86, 749–765. doi: 10.1111/1365-2656.12680
Moll R. J., Cepek J. D., Lorch P. D., Dennis P. M., Robison T., Millspaugh J. J., et al. (2018). Humans and urban development mediate the sympatry of competing carnivores. Urban Ecosyst. 21, 765–778. doi: 10.1007/s11252-018-0758-6
Parsons M. H., Banks P. B., Deutsch M. A., Munshi-South J. (2018). Temporal and space-use changes by rats in response to predation by feral cats in an urban ecosystem. Front. Ecol. Evol. 6, 1–13. doi: 10.3389/fevo.2018.00146
Parsons A. W., Kellner K. F., Rota C. T., Schuttler S. G., Millspaugh J. J., Kays R. W., et al. (2022). The effect of urbanization on spatiotemporal interactions between gray foxes and coyotes. Ecosphere 13. doi: 10.1002/ecs2.3993
Pickett S. T. A., Cadenasso M. L., Grove J. M., Nilon C. H., Pouyat R. V., Zipperer W. C., et al. (2001). Urban ecological systems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu. Rev. Ecol. Syst. 32, 127–157. doi: 10.1146/annurev.ecolsys.32.081501.114012
Plaza P. I., Speziale K. L., Zamora-Nasca L. B., Lambertucci S. A. (2019). Dogs and cats put wildlife at risk. J. Wildlife Manage. 83, 767–768. doi: 10.1002/jwmg.21637
Potash A. D., Conner L. M., Clinchy M., Zanette L .Y., McCleery R. A. (2023). Prey species increase activity in refugia free of terrestrial predators. Oecologia 201, 661–671. doi: 10.1007/s00442-023-05350-9
Prange S., Gehrt S. D., Wiggers E. P. (2004). Influences of anthropogenic resources on raccoon (Procyon, lotor) movements and spatial distribution. J. Mammal. 85, 483–490. doi: 10.1644/BOS-121
Rast W., Barthel L. M. F., Berger A. (2019). Music festival makes Hedgehogs move: How individuals cope behaviorally in response to human-induced stressors. Animals 9, 1–19. doi: 10.3390/ani9070455
R Core Team (2023). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.r-project.org/ (Accessed 16 June 2024).
Reher S., Dausmann K. H., Warnecke L., Turner J. M. (2016). Food availability affects habitat use of Eurasian red squirrels (Sciurus vulgaris) in a semi-urban environment. J. Mammal. 97, 1543–1554. doi: 10.1093/jmammal/gyw105
Ridout M. S., Linkie M. (2009). Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat 14, 322–337. doi: 10.1198/jabes.2009.08038
Rutz C., Loretto M. C., Bates A. E., Davidson S. C., Duarte C. M., Jetz W., et al. (2020). COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nat. Ecol. Evol., 1156–1159. doi: 10.1038/s41559-020-1237-z
Santini L., González-Suárez M., Russo D., Gonzalez-Voyer A., von Hardenberg A., Ancillotto L. (2019). One strategy does not fit all: determinants of urban adaptation in mammals. Ecol. Lett. 22, 365–376. doi: 10.1111/ele.13199
Scholz C., Firozpoor J., Kramer-Schadt S., Gras P., Schulze C., Kimmig S. E., et al. (2020). Individual dietary specialization in a generalist predator: A stable isotope analysis of urban and rural red foxes. Ecol. Evol. 10, 8855–8870. doi: 10.1002/ece3.6584
Selonen V., Varjonen R., Korpimäki E. (2016). Predator Presence, but not Food Supplementation, Affects Forest Red Squirrels in Winter. Annales Zoologici Fennici 53, 183–193. doi: 10.5735/086.053.0407
Shar S., Skhagvasuren D., Bertolino S., Henttonen H., Kryštufek B., Meinig H. (2016). “Eurasian red squirrel,” in The IUCN Red List of Threatened Species, vol. 2016, 8235. Available at: http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T20025A22245887.en
Smith J. A., Thomas A. C., Levi T., Wang Y., Wilmers C. C. (2018). Human activity reduces niche partitioning among three widespread mesocarnivores. Oikos 127, 890–901. doi: 10.1111/oik.04592
Sol D., Lapiedra O., González-Lagos C. (2013). Behavioral adjustments for a life in the city. Anim. Behav. 85, 1101–1112. doi: 10.1016/j.anbehav.2013.01.023
Spoelstra K., Wikelski M., Daan S., Loudon A. S. I., Hau M. (2016). Natural selection against a circadian clock gene mutation in mice. Proc. Natl. Acad. Sci. United States America 113, 686–691. doi: 10.1073/pnas.1516442113
Starkey A., delBarco-Trillo J. (2019). Supplementary feeding can attract red squirrels (Sciurus vulgaris) to optimal environments. Mamm. Biol. 94, 134–139. doi: 10.1016/j.mambio.2018.05.004
Statistics Office Berlin-Brandenburg (2023a). Einwohnerinnen und Einwohner in Berlin. Available online at: https://www.statistik-berlin-brandenburg.de/a-i-5-hj (Accessed 23 June 2024).
Statistics Office Berlin-Brandenburg (2023b). Katasterflächen 2022 nach Art der tatsächlichen Nutzung (Potsdam). Available online at: https://www.statistik-berlin-brandenburg.de/a-v-3-j (Accessed 23 June 2024).
Stillfried M., Gras P., Börner K., Göritz F., Painer J., Röllig K., et al. (2017). Secrets of success in a landscape of fear: Urban wild boar adjust risk perception and tolerate disturbance. Front. Ecol. Evol. 5. doi: 10.3389/fevo.2017.00157
Sundell J., Dudek D., Klemme I., Koivisto E., Pusenius J., Ylönen H. (2004). Variation in predation risk and vole feeding behavior: A field test of the risk allocation hypothesis. Oecologia 139, 157–162. doi: 10.1007/s00442-004-1490-x
Thomas L. S., Teich E., Dausmann K. H., Reher S., Turner J. M. (2018). Degree of urbanization affects Eurasian red squirrel activity patterns. Hystrix 29, 175–180. doi: 10.4404/hystrix-00065-2018
Tobajas J. C., Ferreira C., Delibes-Mateos M., Villafuerte R., Rouco Zufiaurre C. (2024). Adaptive anti-predatory responses of European rabbits exposed to different predation pressure. Mamm. Biol. 104, 185–192. doi: 10.1007/s42991-024-00398-3
Twining J. P., Sutherland C., Reid N., Tosh D. G. (2022). Habitat mediates coevolved but not novel species interactions. Proc. R. Soc. B: Biol. Sci. 289, 1–9. doi: 10.1098/rspb.2021.2338
Uchida K., Suzuki K., Shimamoto T., Yanagawa H., Koizumi I. (2016). Seasonal variation of flight initiation distance in Eurasian red squirrels in urban versus rural habitat. J. Zool. 298, 225–231. doi: 10.1111/jzo.12306
Uchida K., Suzuki K. K., Shimamoto T., Yanagawa H., Koizumi I. (2019). Decreased vigilance or habituation to humans? Mechanisms on increased boldness in urban animals. Behav. Ecol. 30, 1583–1590. doi: 10.1093/beheco/arz117
Venables W. N., Ripley B. D. (2002). Modern Applied Statistics with S (New York: Springer). Available online at: https://www.stats.ox.ac.uk/pub/MASS4/ (Accessed 16 June 2024).
Keywords: Sciurus vulgaris, urban ecology, activity patterns, camera trap, SARS-CoV-2 lockdown effect, species interactions, anthropogenic disturbance, predation risk
Citation: Drenske S, Louvrier J, Grabow M, Landgraf C, Kramer-Schadt S and Planillo A (2024) Human and predator presence shape diel activity of urban red squirrels. Front. Ecol. Evol. 12:1455142. doi: 10.3389/fevo.2024.1455142
Received: 26 June 2024; Accepted: 09 October 2024;
Published: 13 November 2024.
Edited by:
Anindita Bhadra, Indian Institute of Science Education and Research Kolkata, IndiaReviewed by:
Thomas Göttert, Eberswalde University for Sustainable Development, GermanyDominik Werner Melville, University of Ulm, Germany
Copyright © 2024 Drenske, Louvrier, Grabow, Landgraf, Kramer-Schadt and Planillo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sinah Drenske, ZHJlbnNrZUBpenctYmVybGluLmRl
 Sinah Drenske
Sinah Drenske Julie Louvrier1
Julie Louvrier1 Marius Grabow
Marius Grabow Stephanie Kramer-Schadt
Stephanie Kramer-Schadt Aimara Planillo
Aimara Planillo