- Graduate School of Environment and Information Sciences, Yokohama National University, Yokohama, Japan
Introduction: Parasitism by infectious diseases and insect pests significantly shapes wild plant communities by stabilizing them through suppressing dominant species and destabilizing them by suppressing minor species. However, the dynamics of parasitism in wild ecosystems remain understudied. This study aimed to determine whether parasites infect a wide range of host species or are plant-specific, assess the stabilizing and destabilizing effects of parasitism on plant community structure, and determine the influence of environmental and seasonal factors on parasitism.
Methods: We conducted field surveys in herbaceous plant communities within a 1 km² area in the Tokyo metropolitan region, focusing on fungal diseases (rust-like and powdery mildew-like symptoms) and leaf-eating insect pests. Using zero-inflated binomial regression, we evaluated the symptom prevalence and intensity of parasitism across species, seasons, and environmental variables.
Results: The results indicated that a few plant species were highly susceptible to parasitism, with rust-like infections tending to predominantly affect dominant species and leaf-eating insects targeting minor species.
Conclusion: These findings highlight the contrasting roles of parasites in stabilizing and destabilizing plant communities and that both environmental and seasonal factors influence parasitism similar to cultivated ecosystems.
1 Introduction
Parasitism in plants, including fungal pathogens that reproduce on the host and cause disease, as well as insect pests that feed on plants, plays a crucial role in shaping plant community structure (Erizal and Koike, 2007; Konno and Seiwa, 2011; Wang et al., 2019; Halliday et al., 2020; Liu et al., 2020; Rohr et al., 2020). Fungal pathogens and insect pests are smaller than their host plants, and many individuals depend on a single host. Defensive host evolution has led to species-specific host-parasite relationships (Dobler et al., 1996; Ferreira et al., 2007; Kolmer et al., 2001), and plant species affect fungal community on leaves (Liu et al., 2021). In this study, both fungal pathogens and insect pests are considered forms of parasitism. Since insects often exhibit species preferences (Bernays and Chapman, 1994), and fungal pathogens are species-specific (Gilbert and Webb, 2007), it is unclear whether parasitism affects all plant species in a community equally or only impacts a subset of species. This suggests that parasitic effects may vary across communities, potentially influencing their composition and species diversity (Hatcher et al., 2006). Parasitism has a dual effect on plant communities (Gilbert and Webb, 2007; Gilbert et al., 2012; Forister et al., 2015; Fordyce et al., 2016), stabilizing wild plant communities through intensive infection of dominant species and destabilizing them by suppressing minor species (Mordecai, 2011). Although both infectious fungal diseases and insect pests are host-specific, they often differ in dispersal methods. Fungi disperse their spores passively via wind and water droplets (Lacey, 1996; Madden, 1997), whereas adult insects actively seek specific host plants to lay eggs (Mayhew, 1997). This suggests that insect pests may be more effective at spreading to rare hosts than fungi.
To understand the effect of parasitism on plant community assembly, two areas should be studied. First, we need to examine parasitism across the entire plant community, including its occurrence, intensity, host susceptibility, and environmental effects. Next, we must assess the damage parasitism causes to plant populations and species interactions represented as community-matrix (Kawatsu and Kondoh, 2018). This study focuses on the first step, with damage evaluation to be addressed in future studies.
This study examines the presence of visible symptoms associated with parasitism. The absence of such symptoms may indicate either the absence of parasites or conditions that are not conducive to symptom development. Plants previously not considered hosts can be infected without showing symptoms, and some hosts may exhibit symptoms only under certain conditions (Bacon and Hill, 1996).
The epidemiological triangle (pathogen occurrence, host plant susceptibility, and environmental effects) posits that infection occurrence and intensity depend on interactions between the parasite, host, and environment (Stevens, 1960). Although we analyzed visible symptoms, the epidemiological triangle can serve as a framework to investigate the local occurrence of symptoms, host plant susceptibility, and the effects of the local environment. These factors are key drivers of outbreaks and play a crucial role in shaping plant community assembly through parasitism.
We investigated wild plant parasitism (specifically fungal infections and insect infestations) in herb layer plant communities across a 1 km2 area. The main objectives of this study were to (1) determine whether parasites infect all plant host species evenly or target a few species, (2) assess the stabilization and destabilization effects by testing positive or negative correlations between plant dominance and parasitism, and (3) examine the effects of environmental and seasonal factors on parasitism. This study formulated the epidemiological triangle concept (disease occurrence, host plant susceptibility, and environmental effects) using a zero-inflated binomial regression and evaluated these epidemiological factors in wild plant communities.
2 Materials and methods
2.1 Research site
The study was conducted in a suburban 1 × 1 km landscape (latitude 35.473°, longitude 139.589°, and altitude 50 m) in Yokohama, Tokyo metropolitan area, Japan (Figure 1). Yokohama has a mean annual temperature of 16.3°C and mean annual precipitation of 1687.5 mm (Japan Meteorological Agency, https://www.data.jma.go.jp), and falls within a warm-temperate moist forest biome (Miyawaki, 1986). The examined vegetation included seminatural grasslands, little-managed lawns, roadside verges, and forest floor vegetation of abandoned coppices and evergreen broad-leaved forests. Since vegetation types were continuous and difficult to classify by plant species composition, we used a principal component axis that represented the grassland-forest vegetation continuum. Fungicides, pesticides, and herbicides were not applied to any of the vegetation types studied under the guidelines by Yokohama City and Yokohama National University.
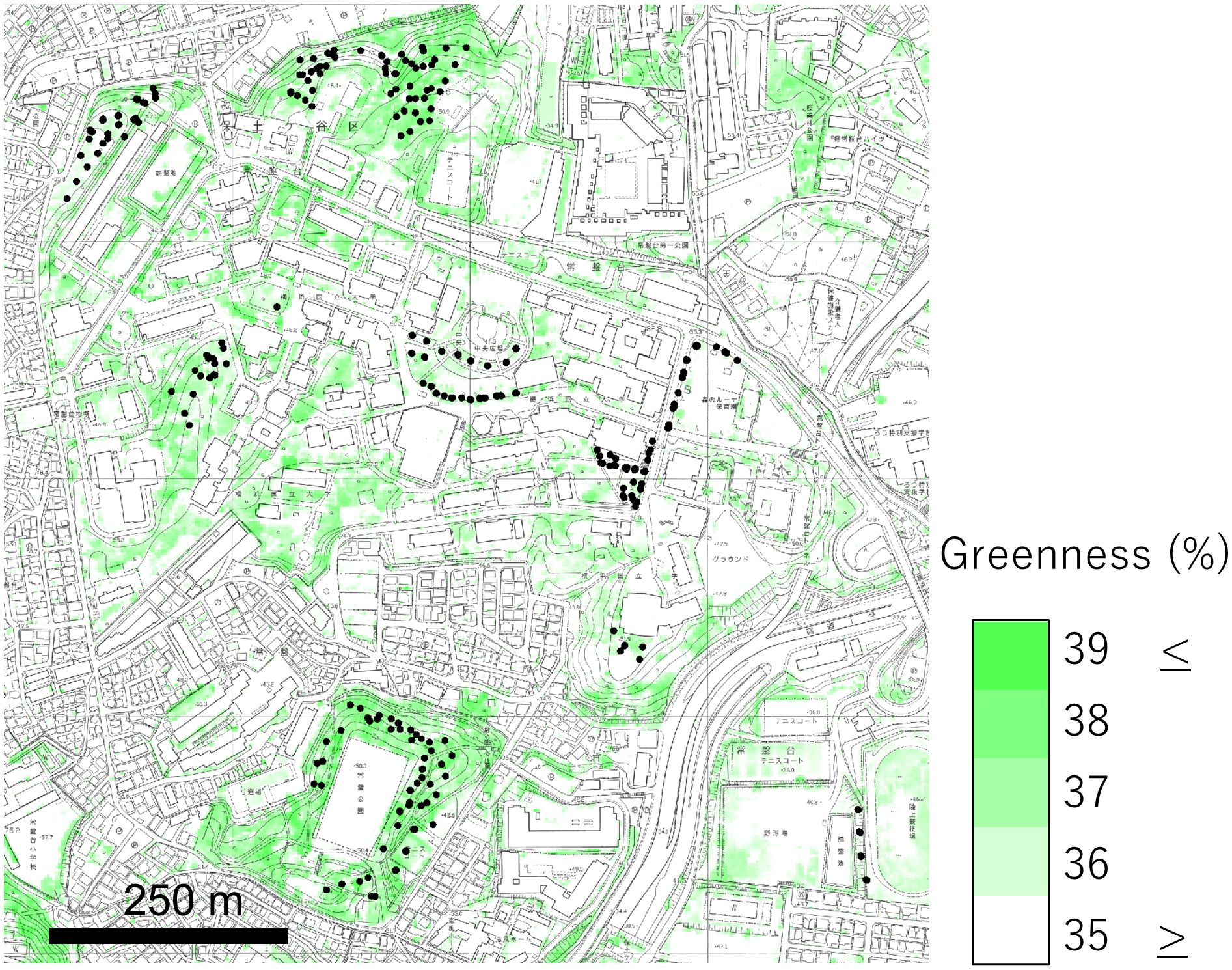
Figure 1. Map of the research site. The depth of the green color represents the greenness of the aerial photograph, green/(red + green + blue). The black dots represent research plots.
2.2 Symptoms and pests
We visually assessed symptom types according to Rottstock et al. (2014), focusing on easily observed powdery mildew-like visible external mycelia and rust-like visible external sporulation structures (Supporting Information 1). Plant diseases that cause spots, lots, and breechings are sometimes difficult to distinguish from mineral deficiency, mineral toxicity, sunburn, or leaf aging without detailed information on each plant species. Several fungal taxa occur even in a disease spot (Tao et al., 2021) and molecular identification of symptoms is difficult. Consequently, in this study, to avoid including non-infectious symptoms, we excluded these and did not use molecular identification, as it was impractical to analyze all leaves this way.
During the examination of plant pests, we focused on insect pests, including leafminers (larvae of moths, sawflies, and flies), sap-sucking insects (aphids, whiteflies, and scale insects), and leaf-eating insects (mainly lepidopteran larvae and leaf beetles). These insects are major plant pests that affect young plant shoots. We did not record leaf scars unless we found the corresponding insect species on or near affected plants, due to the difficulty of identifying insects based on scars.
2.3 Epidemic surveys
We established 365 circular plots, each 2 m in diameter, within the vegetated areas (Figure 1) during the growing seasons of spring (April–June), summer (July–August), and autumn (October–November) from October 2019 to November 2021. The surveys were conducted on sunny days between 9:00 and 14:00 to ensure sufficient lighting. The plots were spaced at least 20 m apart. The same vegetation was examined multiple times across different seasons.
All seed plants less than 2 m in height were examined. The height and plant coverage (%) of each plant species were measured. Coverage was estimated as the percentage of ground area within each 2m diameter circular plot occupied by the vertical projection of the foliage of each plant species. Specifically, within each plot, we visually estimated the foliage-covered area of each species and calculated its proportion relative to the total plot area. We carefully examined the fungal diseases and insect pests of each plant species within the plot, ensuring a spatial precision of at least 1% of the plot. A portable optical microscope with 60–120×magnification (Kenko, STV-120M) was used in the field to observe leaf lesions. When diseases or pests were found, we examined individual leaves randomly and recorded the number of damaged or infected leaves. We attempted to examine as many leaves as possible to ensure more accurate results, but in some cases, we stopped at 100 leaves because the work was labor-intensive. We did not count the leaves if parasitism was not detected because counting healthy leaves is labor-intensive. Hemispherical photographs were taken from the plot center to measure the upper canopy cover above the herb layer in summer.
2.4 Analysis
We estimated epidemic triangle parameters using a zero-inflated regression based on the results of our epidemic survey and environments and conditions (Figure 2). We distinguished between endemic occurrence (in the epidemiological sense rather than biogeography) and outbreak intensity (Figure 3). Endemic occurrence (Q) refers to the probability of parasitism occurring in a plot where the host species exists. This probability can be influenced by the environment and might not necessarily affect all leaves within the plot. In contrast, outbreak intensity (S) refers to the probability that leaf is parasitized, and this probability is similarly influenced by the environment. In a real landscape, there might be a plot with an environment unsuitable for the disease (Figure 3). If the environment of a plot is highly suitable, but no symptoms are found on highly susceptible plants, this suggests that the pathogen is absent. We can evaluate such a low endemic occurrence (Q) in a plot by comparing the observed deficit of symptoms with the expected level based on the environment and plant susceptibility.
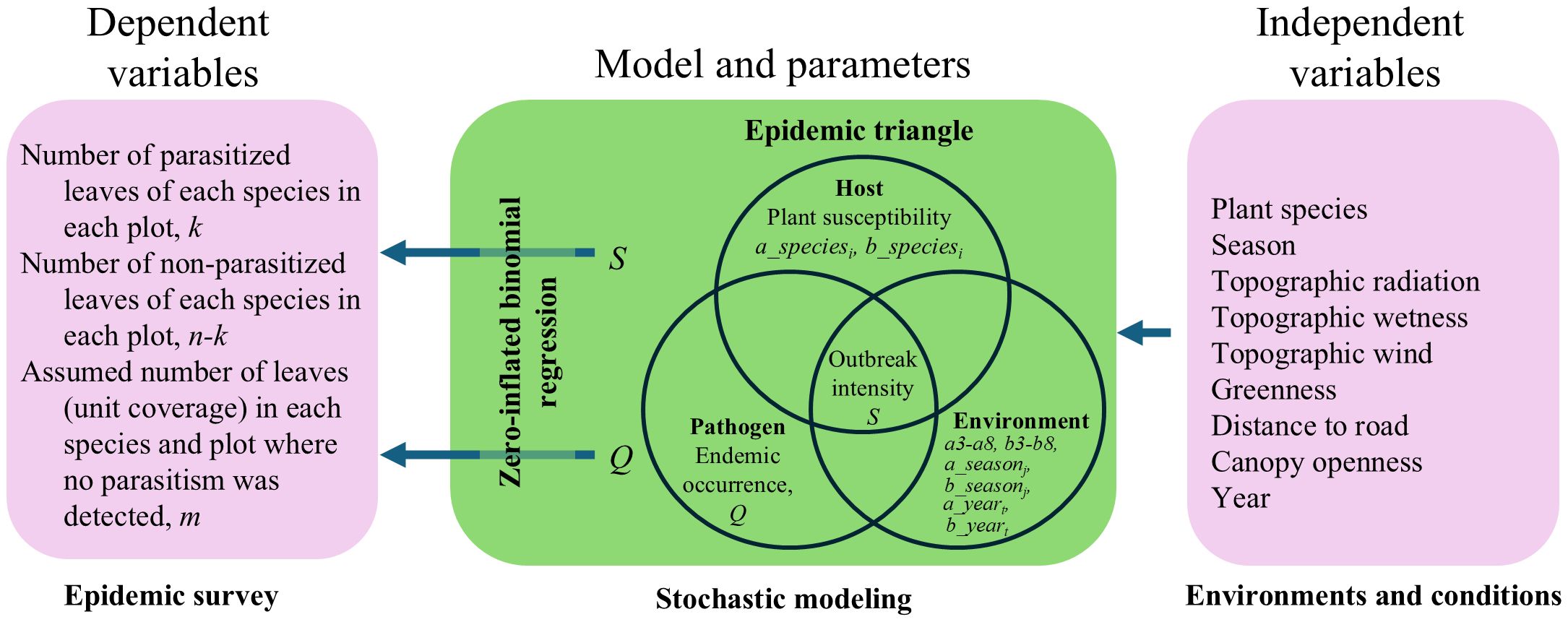
Figure 2. A diagram representing the outline of our analysis. A zero-inflated binomial regression model was constructed based on the epidemic triangle, incorporating measured environments and conditions. The model parameters were estimated to fit the epidemic survey data. Italicized symbols are in Equations 1–4.
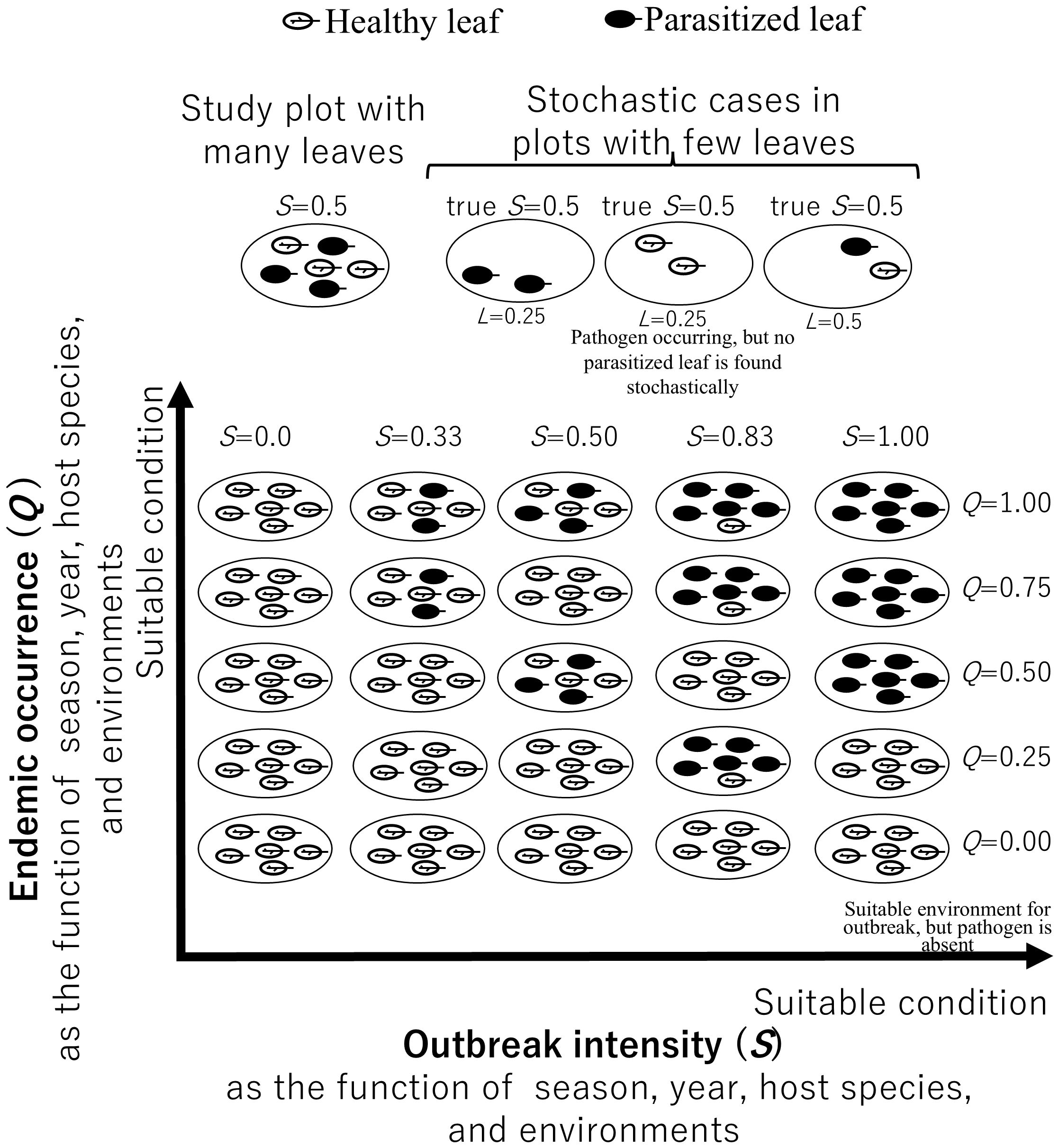
Figure 3. Simplified visual representation of endemic occurrence and outbreak intensity. Endemic occurrence (Q) represents the true probability of parasitism occurring in a plot, and outbreak intensity (S) represents the true probability that a leaf in the plot is parasitized. The upper diagram shows examples of sample plots with the same outbreak intensity but varying numbers of leaves. When there are few leaves in a plot, parasitism may go undetected due to stochastic reasons, even if parasites are actually present. The probability of each case (i.e. likelihood, L) is shown. The lower diagram shows sample plots with varying levels of endemic occurrence and outbreak intensity, both of which depend on season, year, host species, and environmental conditions. A typical stochastic case for a given combination of Q and S is illustrated.
We used zero-inflated binomial regression to analyze the effects of three major factors on outbreak intensity (S): the endemic occurrence of parasitism on host plants (Q), the susceptibility of host plants to parasitism, and environmental factors (Supporting Information 2). The zero-inflated binomial regression can model excess occurrence of unparasitized leaves under suitable conditions due to the absence of pathogen, thus, it is quite suitable for modeling the epidemic triangle. The susceptibility of host plants and the effects of the environment are estimated as regression coefficients in the model. The suitability of environment, susceptibility of plants, and outbreak intensity S and endemic occurrence Q are analyzed simultaneously, by iterative adjustment of parameters in Markov Chain Monte Carlo in the stochastic modeling system (RStan by the Stan Development Team, 2023). The basic concept of stochastic modeling based on likelihood distribution is in McElreath (2020).
We denote Q and S for the h-th sample as Qh and Sh respectively. Each sample corresponds to a unique combination of plot, species, season, and year. Outbreak intensity was evaluated as the probability Sh of parasitism (visible symptoms) on a leaf (Figure 3). Given at least one positive leaf is detected when inspecting n leaves and identifying k positive leaves, the probability followed a binomial distribution with the number of trials n, successes k, and success probability Sh:
where the logit of Sh can be modeled as the linear combination of environment and outbreak susceptibility of plants as logit(Sh) = ln(Sh/(1-Sh)),
where b0 is the intercept; b3 to b8 are regression coefficients; and b_speciesi, b_seasonj, and b_yeart are categorical variables. The b_speciesi represents the outbreak susceptibility of i-th species. Similary, b_seasonj captures the effect of j-th season on outbreak intensity, and b_yeart is the effect of the t-th year. The levels of each categorical variable are modeled to follow a normal distribution with mean zero, the mean is the reference value of each variable (McElreath, 2020). This is a kind of mixed effect model. All numerical environmental variables were specific to each plot and labeled with the suffix “g” for the g-th plot. They were standardized before analysis using the overall mean and standard deviation of all plots. The intercept, b0, represents the expected value of logit (Sh) when all independent variables (three categorical and six quantitative environmental variables) are at their reference levels (zero).
The endemic occurrence of disease and parasitic insects in h-th sample is Qh (Figure 3). The calculation is not straightforward, as there is a possibility that the amount of foliage in the plot is too little for at least one positive leaf to be present in the plot due to stochastic reasons (Figure 3 upper diagram). There are many causes of leaf-to-leaf variation within a plot. For example, spores or insect eggs may fall or be laid stochastically, resulting in some leaves being parasitized while others are not. Insects may not feed on all leaves simply because their number is insufficient. Additionally, variation in leaf age, cuticle thickness, or nutrient content may cause differences in susceptibility among leaves of the same species within a plot. If the true outbreak intensity in a plot is S = 0.5 and endemic occurrence Q=1.0, there is a 25% chance (likelihood, L, probability of 0.25) of failing to detect parasitism when only two leaves exist (pseudo negative for Q). However, if there are more than 11 leaves, the chance drops to less than 5%. The bias caused by such pseudo negatives is corrected. The observed probability that parasitism was not detected (Rh) in field survey is the sum of the probability that parasitism was truly absent (1−Qh), and that parasitism existed but was undetected on each of the m examined foliage, Qh ×(1 - Sh)m:
where m is the assumed number of examined foliage, with the assumption that the occurrence of parasitism was examined at a precision of 1% plant cover (with the 10 cm radius circle of foliage being the unit coverage). If we had enough labor, we would count all healthy leaves for m in the plots without parasitism. However, due to the labor-intensive nature of the work, we were unable to count them all. We used the ratio of foliage cover to unit coverage as a proxy for leaf number. When the unit coverage is large, m decreases, and plots with no observed parasitism tend to be interpreted as indicating that “parasitism exists in the plot but is undetected,” which may lead to an overestimation of Qh. If the unit coverage is extremely small, it is less likely that “parasitism exists in the plot but is undetected,” and Qh will approach the observed probability that parasitism was detected (1 - Rh) causing underestimation of Qh. The used unit coverage, 10 cm radius, was roughly equal to the leaf size. The logit of Qh can be modeled as the linear combination of environment and endemic susceptibility of plants, as logit(Qh)=ln(Qh/(1- Qh)):
where a0 is the intercept; a3 to a8 are regression coefficients; and a_speciesi, a_seasonj, and a_yeart are categorical variables. The a_speciesi represents the endemic susceptibility of i-th species, a_seasonj represents the effect of j-th season on endemic occurrence, and a_yeart is the effect of t-the year. The levels of each categorical variable are modeled to follow a normal distribution with mean zero to avoid redundancy with the intercept a0 (McElreath, 2020). All environmental variables are the same as those in Equation 2.
If no infection is found in any plot of the i-th species, then we cannot distinguish whether the endemic occurrence (Qh) is zero, the outbreak intensity (Sh) is zero, or both (Figure 3). In these cases, one of the two parameters, a_speciesi or b_speciesi, or both, should be sufficiently small, while the other value remains indeterminate. To address this, we assumed that b_speciesi and a_speciesi to be close by modeling their difference, a_speciesi - b_speciesi, as follows a normal distribution with a mean of zero. For example, when Qh is close to zero, the indeterminate Sh will also be close to zero because of the small value of b_speciesi (Equation 2). Please refer to a textbook, such as McElreath (2020), that explains this type of treatment.
Using this zero-inflated binomial regression (Equations 2–4), we evaluate the outbreak intensity in natural ecosystems (Sh), endemic occurrence (Qh), host plant susceptibility (a_speciesi and b_speciesi), and environmental effects (a_seasonj, a3, a4, etc.). This is an application of epidemiological triangle concept of disease occurrence, host plant susceptibility, and environmental effects (Figure 2).
The environmental factors studied included season (categorical variables such as spring, summer, and autumn representing seasonal differences in temperature, humidity, radiation, and rainfall), and plot specific environments as distance from roads (using digital national land information https://nlftp.mlit.go.jp/ksj/gmlold/datalist/gmlold_KsjTmplt-N01.html and QGIS Ver.3.30, QGIS Development Team, 2023), canopy openness (measured using hemispherical photographs), greenness (pixel brightness of green/red + green + blue) on a 3 m resolution aerial photograph taken during the growing season), and plot specific environments as topographical environments (slope radiation, wetness as a log-specific catchment area, and wind by the width of unobstructed direction calculated, from a digital elevation model with 5 m horizontal resolution, https://fgd.gsi.go.jp/download/menu.php , following Koike, 2022). All topographical environments were averaged over a 10 m radius. The survey year was considered to eliminate the effect of year-to-year differences. Highly correlated environmental variables were excluded from the analysis to avoid multicollinearity.
To determine whether parasites infected all plant host species evenly or only a few limited species within a community, the frequency distribution of susceptibility among host plants was tested based on the skewness of endemic susceptibility a_speciesi and outbreak susceptibility b_speciesi using RStan.
To assess the stabilization and destabilization effects of parasitism on plant communities, we examined the positive or negative correlations between plant dominance and parasitism. Pearson’s correlation coefficients were calculated for plant species susceptibility and plant host abundance. Plant occurrence (number of plots where the plant was found divided by all plots) and log- transformed plant species cover (average log-transformed cover in detected plots) were considered measures of plant host abundance.
Finally, the relation between susceptibility and plant position along the forest-grassland vegetation gradient was examined using principal component analysis (PCA) based on plot plant composition.
3 Results
The 1654 rows in Supporting Information 3 correspond to a unique combination of plot, host species, season, and year. Each row has survey results on fungal diseases and insect pests. The number of samples in the zero-inflated binomial regression was 1654 in every disease and pest. As shown in Supporting Information 3, we identified 169 plant species in 365 plots and focused our analysis on 49 species present in more than nine plots. Among them, seven forest-community species and three grassland-community species, as classified by Miyawaki (1986), were present in more than 50 plots. The forest-community species included Aphananthe aspera (Thunb.) Planch., Aucuba japonica Thunb. var. japonica, Machilus thunbergii Siebold et Zucc., Ligustrum lucidum Aiton, Trachycarpus fortunei (Hook.) H.Wendl., Quercus myrsinifolia Blume, and Houttuynia cordata Thunb. The grassland-community species included Pleioblastus chino (Franch. et Sav.) Makino., Causonis japonica (Thunb.) Raf, and Erigeron philadelphicus L. In the PCA of plot plant composition, forest plants were positively associated with the first principal component, whereas grassland plants were generally negatively associated (Table 1, Supporting Information 4). The environmental variable “topographic wind” was removed from the analysis because of a high correlation with “topographic wetness” and “greenness.” After removing “topographic wind,” mutual correlation among analyzed environmental variables was r2 < 0.14. Zero-inflated binomial regression did not provide a converged solution for leaf miners and sap-sucking insects because of their low total occurrences; therefore, it was excluded from the subsequent results.
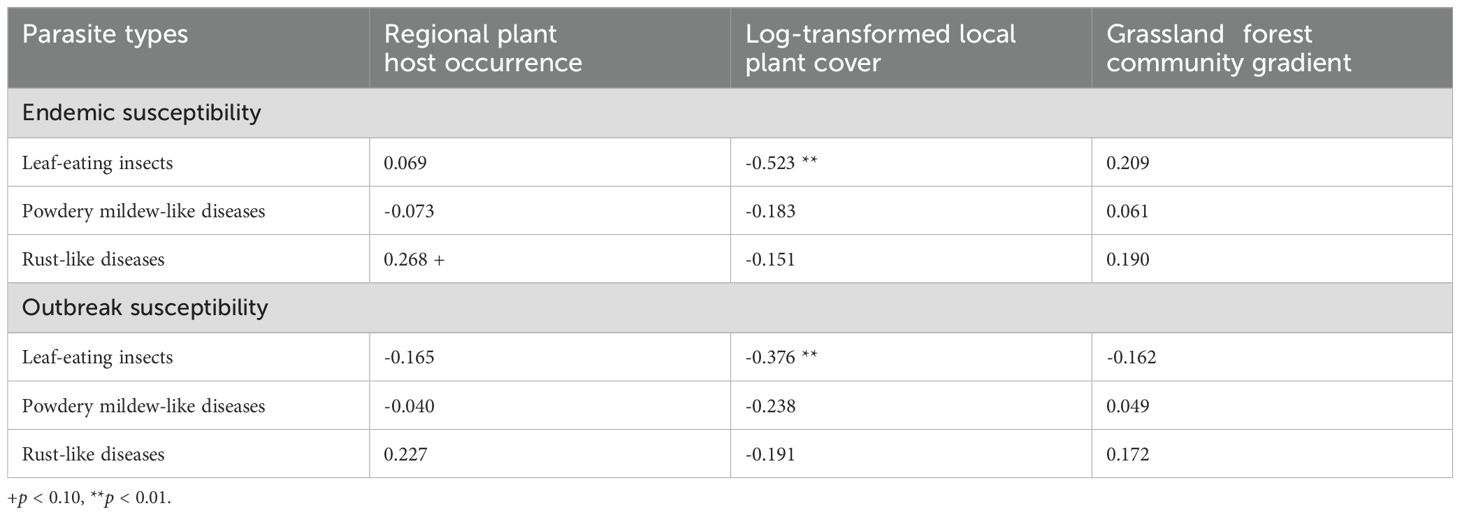
Table 1. Pearson’s correlation coefficient between plant host dominance (occurrence and log-transformed cover), preference in a grassland–forest gradient (plant community PCA), and susceptibility of 49 host plants.
3.1 Susceptibility of host plant species
The endemic and outbreak susceptibilities, and taxonomical and ecological properties of plant species are in Supporting Information 4. The plants most susceptible to endemic occurrence in our study were Plantago asiatica L. to leaf-eating insects, Celtis sinensis Pers. to powdery mildew, and Quercus myrsinifolia Blume to rust. The skewness of endemic (0.65, p=0.023) and outbreak (0.91, p=0.007) susceptibilities was significantly positive in rust-like diseases. Plants that are susceptible to rust-like diseases were limited (Figure 4). The skewness values were 0.43 (p=0.077) for endemic and 0.89 (p=0.067) for outbreak susceptibilities, which was positive in powdery mildew-like diseases with limited susceptible plants (Figure 4). Endemic (-0.16, p=0.73) and outbreak (-0.11, p=0.62) susceptibilities for leaf-eating insects were not positively skewed.
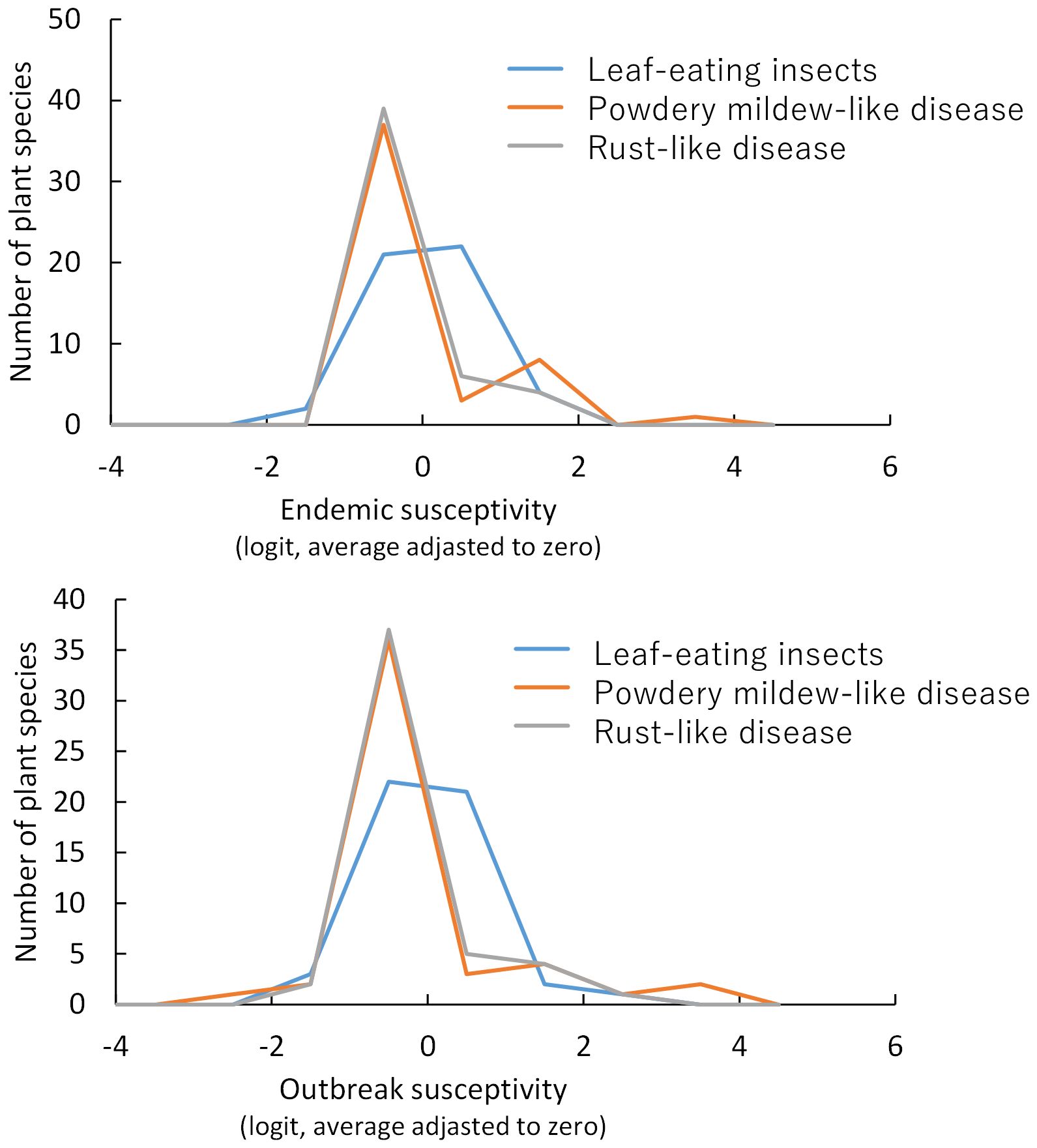
Figure 4. Effect size of host plant species on endemic parasitism occurrence (Q) and outbreak intensity (S), based on zero-inflated binomial regression.
3.2 Host abundance and susceptibility
Host plant occurrence (the ratio of plots where a host plant was found) showed a weak positive correlation with endemic susceptibility to rust-like diseases, although the significance level was low (Table 1, Supporting Information 1). This suggests that commonly found plant hosts may be more frequently infected. In contrast, plant species with greater cover were less susceptible to leaf-eating insects in terms of both endemic and outbreak susceptibilities (Table 1), indicating that locally dominant plant hosts were both less frequently and less intensively infected. The plant susceptibilities did not correlate with plant position along the forest-grassland gradient (Table 1).
3.3 Seasonality and plot environments
Parasitism by leaf-eating insects was frequent (endemism) and intense (outbreak) in spring (Figure 5). Powdery mildew-like disease was intensely parasitic in spring and autumn, and rust-like disease in Autumn. In general, the studied insects parasitized in spring, whereas the studied diseases were more prevalent in autumn although some occurred in spring. Both insects and fungal diseases were not active in summer.
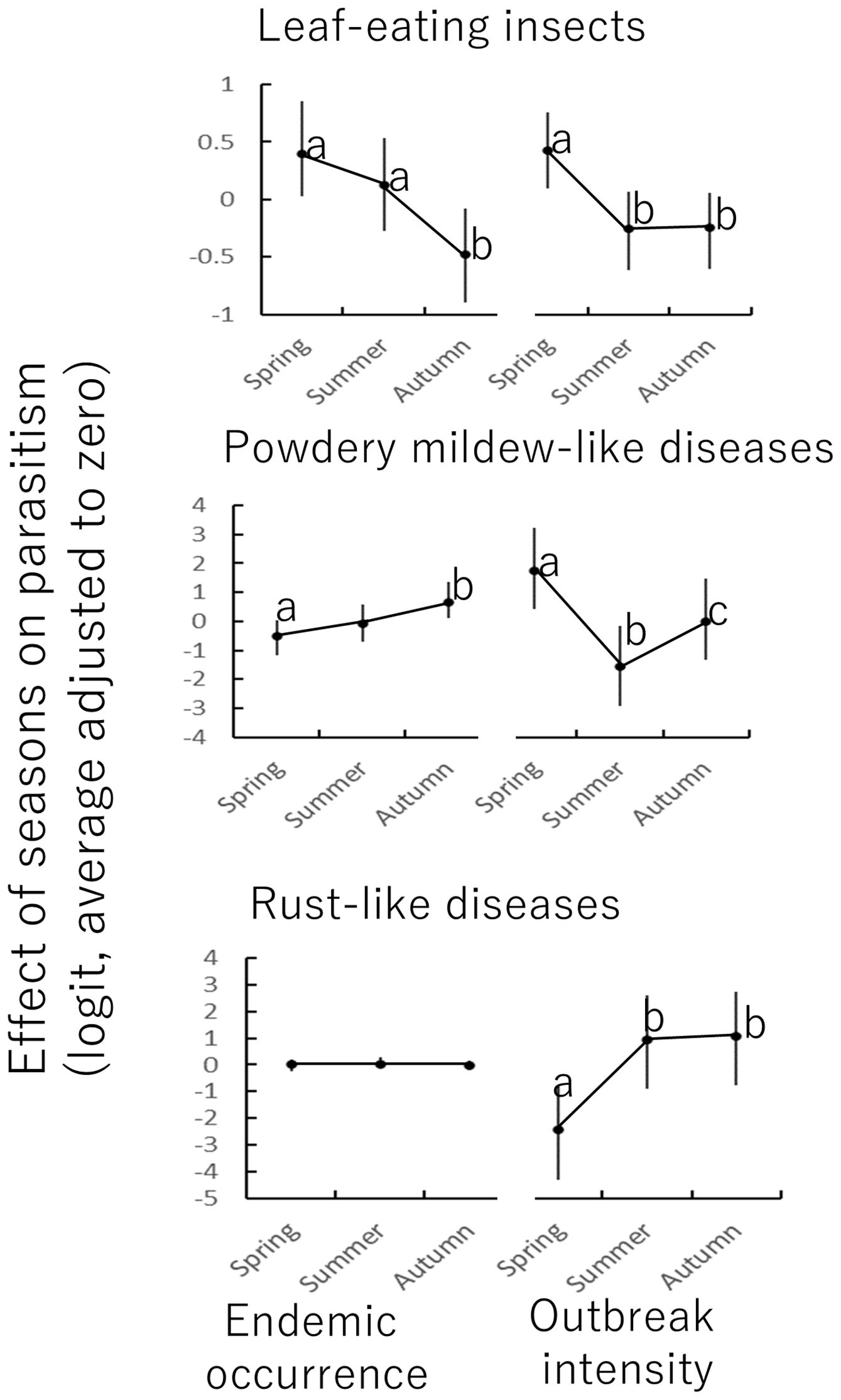
Figure 5. Effect sizes of seasons (categorical variable) based on zero-inflated binomial regression (Equations 2–4). Different lowercase letters represent statistically significant differences (p < 0.05).
Leaf-eating insects preferred topographically wet sites that were distant from the road (Figure 6). Powdery mildew-like diseases favored areas with strong radiation, wet topography, and proximity to roads. Rust-like diseases thrived in well-vegetative areas (aerial photograph greenness) with locally open canopy. Environmental effects on endemism were generally weak (Supporting Information 5).
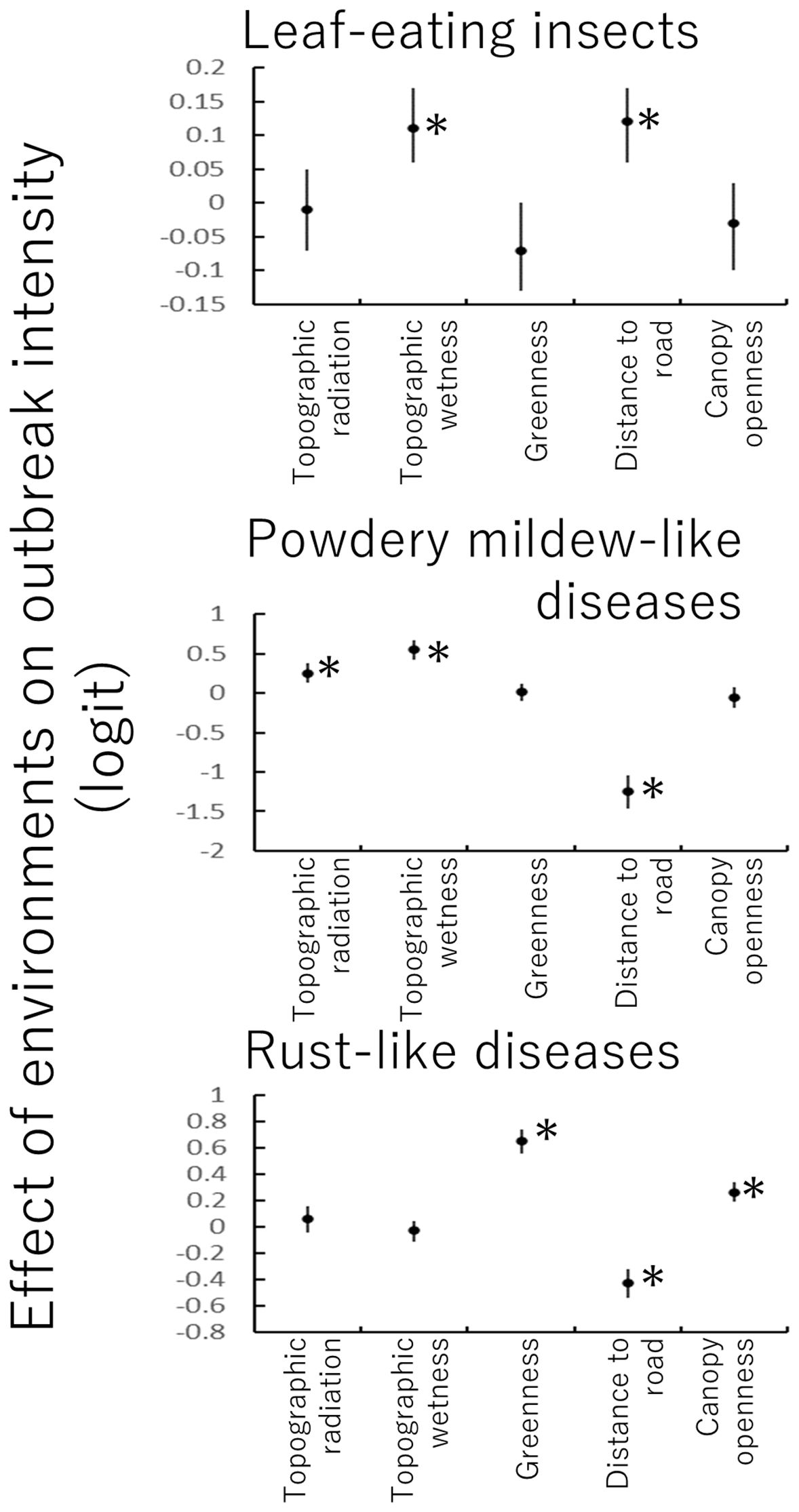
Figure 6. Effect sizes of environmental variables of research plots on parasitism intensity (outbreak) based on zero-inflated binomial regression (Equation 4). Environmental variables were standardized before analysis. *p < 0.05.
4 Discussion
4.1 Implications on plant community assembly
The presence/absence of symptoms reflects both the existence of the pathogen and the conditions that lead to symptom development, while an outbreak signifies potential widespread damage. Some pathogen-host combinations show symptoms without significant damage, whereas others correlate strongly with damage. However, the exact nature of these interactions remains unclear. The results showed that only a limited number of species from the regional plant pool experienced damage from fungal diseases, suggesting that parasites might suppress plants unevenly with regard to community assembly processes, at least in fungal diseases.
Selective infection of minor hosts causes a destabilization effect, with minor host species experiencing more suppression than dominant host species because of positive feedback (Mordecai, 2011). In this study, damage by leaf-eating insects was higher in minor host species than in dominant host species. This suggests that leaf-eating insects may contribute to the destabilization of plant communities.
Plant leaf consumption by leaf-eating insects increases with the growth of individual caterpillars in the case of lepidopterans. Therefore, a small plant patch searched out by a mother may be completely consumed by a few large caterpillar individuals, whereas large plant patches remain unconsumed. Notably, strong density-controlling mechanisms for caterpillars driven by higher-trophic level predators and parasites (insects, microorganisms, and viruses) prevent caterpillars from causing widespread damage to dominant plant species (Myers and Cory, 2016), except in the case of some alien caterpillars.
In contrast, intensive parasitism of dominant plants can establish a negative feedback loop in host dominance, resulting in a community-stabilizing effect (Mordecai, 2011). We observed a weak stabilizing mechanism by rust-like diseases (Table 1), with regionally common hosts being susceptible to the parasites, as reported by Mitchell et al. (2002). Plant hosts are considered habitat patches for parasites and are a type of metapopulation (Thrall and Burdon, 1997). Habitat density (host plant) is crucial for the persistence and spread of focal parasitic species and involves a threshold habitat (host) density (Komuro and Koike, 2005).
4.2 Season and landscape environment
The effects of seasons and environment factors observed in this study generally align with well-known phenomena in cultivated crops in the research area (Yamaoka, 2014; Takamatsu and Miyamoto, 2019). Leaf-eating insect pest outbreaks were observed in spring. This can be attributed to active plant shoot growth during spring in temperate climates that provide soft tissues rich in nutrients for insects (Awmack and Leather, 2002). In contrast, fungal leaf disease outbreaks were generally observed in autumn (powdery mildew-like and rust-like diseases) and spring (powdery mildew-like diseases), which is consistent with the seasonality patterns observed in agricultural crops in the study area (Yamaoka, 2014; Takamatsu and Miyamoto, 2019).
Topographic soil wetness, such as valleys, caused parasitic outbreaks, except for rust-like diseases. Large canopy openness enhanced the intensity of rust-like diseases. Typically, sites with active plant growth and humid conditions promote parasite outbreaks (Awmack and Leather, 2002; Yamaoka, 2014; Takamatsu and Miyamoto, 2019). Topographic radiation, as experienced by south-facing slopes, creates a dry air environment if there is solar radiation and is a positive factor for powdery mildew-like diseases (Takamatsu and Miyamoto, 2019). The roads act as a wind corridor in urban landscapes (Cao et al., 2015), likely facilitating wind dispersal of fungal spores, leading to the outbreak of two fungal diseases at sites close to the road. However, proximity to roads suppressed leaf-eating insects.
The findings of this study (Table 1) are not consistent with those of Dobson and Crawley (1994), who reported higher susceptibility in early successional vegetation. The discrepancy is likely attributed to the fact that, while the studied grasslands were secondary vegetation, they represented stable traditional seminatural grasslands rather than rapidly successional grasslands where early species are suppressed by late-successional species.
4.3 Limitation of the study
This is the first step of the study to understand the effect of parasitism on plant community assembly, and we examined parasitism across the entire plant community, including its occurrence, intensity, host susceptibility, and environmental effects.
We analyzed the correlation between dominance and plant susceptibility. However, in the next study, we must assess the damage to plant population parameters and species interactions represented as community-matrices (Kawatsu and Kondoh, 2018) to clarify the quantitative effect on plant community assembly.
We surveyed visible symptoms. Molecular approaches will make exact identification of symptoms and pathogen existence possible. In our research, we had 2011 plot-by-host samples for fungal occurrence analysis and 3465 leaves with symptoms for disease identification (Supporting Information 3). Further development of cost-effective methods can enhance molecular studies of plant and fungal communities. However, our three objectives: (1) testing the uneven effect on the plant community, (2) assessing the stabilization and destabilization effects, and (3) evaluating environmental and seasonal factors, could be examined using our approach.
When estimating Qh, we evaluated instances where “parasitism exists in the plot but is undetected,” we used the ratio of foliage cover to the area of a 10 cm radius circle as a proxy for leaf number to remove the pseudo negative bias (Figure 3 upper diagram). Considering leaf size and leaf overlap will improve leaf number estimation based on coverage. We adopted a normal distribution for a_speciesi - b_speciesi as a means of parameter estimation to address indeterminate cases. This type of modeling may help address similar issues involving other explanatory variables.
This study focused on suburban landscapes in a warm-temperate climate. Expanding the research to include diverse climates, ranging from tropical to boreal, across various landscapes from wilderness areas of primary vegetation to urban landscapes, and conducting surveys over several years with different weather trends would enhance the generalizability of the findings on community assembly mechanisms.
The research approach outlined in this paper can be applied in a wide range of community ecology. It can also be applied in agriculture to design feasible landscape-scale farming systems (Tscharntke et al., 2021) that optimize crop selection and field placement by examining symptom occurrence, host plant susceptibility, and environmental effects.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
XW: Writing – original draft, Writing – review & editing. KH: Writing – original draft, Writing – review & editing. FK: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Project C of the Graduate School of Environment and Information Sciences, Yokohama National University, and JSPS KAKENHI Grant Number 25K02041.
Acknowledgments
We sincerely thank Professor Sasaki Takehiro for his guidance and support throughout this research and to Professor Kagami Maiko for her detailed advice on plant disease epidemiology. We also appreciate the valuable input and support from faculty and colleagues at Yokohama National University. Additionally, the financial support provided by the Takayama International Education Foundation was crucial to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1498915/full#supplementary-material
References
Awmack C. S. and Leather S. (2002). Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844. doi: 10.1146/annurev.ento.47.091201.145300, PMID: 11729092
Bacon C. W. and Hill N. S. (1996). “Symptomless grass endophytes: products of coevolutionary symbioses and their role in the ecological adaptations of grasses,” in Endophytic fungi in grasses and woody plants. Eds. Redlin S. C. and Carris L. M. (St Paul: APS Press), 155–178.
Bernays E. A. and Chapman R. E. (1994). “Behavior: the impact of ecology and physiology,” in Host-plant selection by phytophagous insects. (Boston: Springer). doi: 10.1007/978-0-585-30455-7_6
Cao A., Li Q., and Meng Q. (2015). Effects of orientation of urban roads on the local thermal environment in Guangzhou City. Proc. Engineering. 121, 2075–2082. doi: 10.1016/j.proeng.2015.09.209
Dobler S., Mardulyn P., Pasteels J. M., and Rowell-Rahier M. (1996). Host-plant switches and the evolution of chemical defense and life history in the leaf beetle genus Oreina. Evolution. 50, 2373–2386. doi: 10.2307/2410706, PMID: 28565678
Dobson A. and Crawley M. (1994). Pathogens and the structure of plant communities. Trends Ecol. Evol. 9, 393–398. doi: 10.1016/0169-5347(94)90062-0, PMID: 21236900
Erizal M. and Koike F. (2007). Dispersal and survival of juveniles of dominant tree species in a tropical rain forest of West Sumatra. Tropics. 16, 205–214. doi: 10.3759/tropics.16.205
Ferreira R. B., Monteiro S., Freitas R., Santos C. N., Chen Z., Batista L. M., et al. (2007). Fungal pathogens: the battle for plant infection. Crit. Rev. Plant Sci. 25, 505–524. doi: 10.1080/07352680601054610
Fordyce J. A., Nice C. C., Hamm C. A., and Forister M. L. (2016). Quantifying diet breadth through ordination of host association. Ecology. 97, 842–849. doi: 10.1890/15-0093.1, PMID: 27220201
Forister M. L., Novotny V., Panorska A. K., Baje L., Basset Y., Butterill P. T., et al. (2015). The global distribution of diet breadth in insect herbivores. Proc. Natl. Acad. Sci. U S A. 12, 442–447. doi: 10.1073/pnas.1423042112, PMID: 25548168
Gilbert G. S., Magarey R., Suiter K., and Webb C. O. (2012). Evolutionary tools for phytosanitary risk analysis: phylogenetic signal as a predictor of host range of plant pests and pathogens. Evol. Appl. 5, 869–878. doi: 10.1111/j.1752-4571.2012.00265.x, PMID: 23346231
Gilbert G. S. and Webb C. O. (2007). Phylogenetic signal in plant pathogen–host range. Proc. Natl. Acad. Sci. U S A. 104, 4979–4983. doi: 10.1073/pnas.0607968104, PMID: 17360396
Halliday F. W., Rohr J. R., and Laine A. L. (2020). Biodiversity loss underlies the dilution effect of biodiversity. Ecol. Lett. 23, 1611–1622. doi: 10.1111/ele.13590, PMID: 32808427
Hatcher M. J., Dick J. T. A., and Dunn A. M. (2006). How parasites affect interactions between competitors and predators. Ecol. Lett. 9:1253–1271. doi: 10.1111/j.1461-0248.2006.00964.x, PMID: 17040328
Kawatsu K. and Kondoh M. (2018). Density-dependent interspecific interactions and the complexity–stability relationship. Proc. R. Soc B. 285, 20180698. doi: 10.1098/rspb.2018.0698, PMID: 29794052
Koike F. (2022). Minna de GIS: spatial information processing system for education, research, and environmental assessment by citizens. Available online at: https://www.minnagis.com (Accessed October 2, 2023).
Kolmer J. A., Ordonez M. E., and Groth J. V. (2001). The rust fungi (Hoboken, NJ: John Wiley & Sons). doi: 10.1002/9780470015902.a0021264
Komuro T. and Koike F. (2005). Colonization by woody plants in fragmented habitats of a suburban landscape. Ecol. Appl. 15, 662–673. doi: 10.1890/03-5232
Konno M. and Seiwa K. (2011). The validity of the Janzen-Connell model in the maintenance of tree species diversity. Jpn. J. Ecol. 61, 319–328. doi: 10.18960/seitai.61.3_319
Lacey J. (1996). Spore dispersal — its role in ecology and disease: the British contribution to fungal aerobiology. Mycol Res. 100, 641–660. doi: 10.1016/S0953-7562(96)80194-8
Liu X., Chen L., Liu M., García-Guzmán G., Gilbert G. S., and Zhou S. (2020). Dilution effect of plant diversity on infectious diseases: latitudinal trend and biological context dependence. Oikos. 129, 457–465. doi: 10.1111/oik.07027
Liu X., Jia P., Cadotte M. W., Chen Z., Xingfeng S., Yunquan W., et al. (2021). Host plant environmental filtering drives foliar fungal community assembly in symptomatic leaves. Oecologia. 195, 737–749. doi: 10.1007/s00442-021-04849-3, PMID: 33582871
Madden L. V. (1997). Effects of rain on splash dispersal of fungal pathogens. Can. J. Plant Pathol. 19, 225–230. doi: 10.1080/07060669709500557
Mayhew P. J. (1997). Adaptive patterns of host-plant selection by phytophagous insects. Oikos. 79, 417–428. doi: 10.2307/3546884
McElreath R. (2020). Statistical rethinking: A Bayesian course with examples in R and STAN second edition (Boca Raton: Chapman & Hall/CRC).
Mitchell C. E., Tilman D., and Groth J. V. (2002). Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology. 83, 1713–1726. doi: 10.1890/0012-9658(2002)083[1713:EOGPSD]2.0.CO;2
Mordecai E. A. (2011). Pathogen impacts on plant communities: unifying theory, concepts, and empirical work. Ecol. Monogr. 81, 429–441. doi: 10.1890/10-2241.1
Myers J. H. and Cory J. S. (2016). Ecology and evolution of pathogens in natural populations of Lepidoptera. Evol. Appl. 9, 231–247. doi: 10.1111/eva.12328, PMID: 27087850
QGIS Development Team (2023). “QGIS geographic information system,” in Open source geospatial foundation project. Available at: https://qgis.osgeo.org.
Rohr J. R., Civitello D. J., Halliday F. W., Hudson P. J., Lafferty K. D., Wood C. L., et al. (2020). Towards common ground in the biodiversity–disease debate. Nat. Ecol. Evol. 4, 24–33. doi: 10.1038/s41559-019-1060-6, PMID: 31819238
Rottstock T., Joshi J., Kummer V., and Fischer M. (2014). Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology. 95, 1907–1917. doi: 10.1890/13-2317.1, PMID: 25163123
Stan Development Team (2023). “RStan: the R interface to Stan,” in R package version 2.21.8. Available at: https://mc-stan.org/.
Stevens R. B. (1960). “Cultural practices in disease control,” in Plant pathology, an advanced treatise, vol. III. Eds. Horsfall J. G. and Dimond A. E. (Academic Press, New York, NY, USA), 357–429.
Takamatsu S. and Miyamoto T. (2019). Ecology and control of plant diseases caused by powdery mildew fungi. Plant Protection. 73, 53–58.
Tao J., Cao P., Xiao Y., Wang Z., Huang Z., Jin J., et al. (2021). Distribution of the potential pathogenic Alternaria on plant leaves determines foliar fungal communities around the disease spot. Environ. Res. 200, 111715. doi: 10.1016/j.envres.2021.111715, PMID: 34297933
Thrall P. H. and Burdon J. J. (1997). Host-pathogen dynamics in a metapopulation context: the ecological and evolutionary consequences of being spatial. J. Ecol. 85, 743–753. doi: 10.2307/2960598
Tscharntke T., Grass I., Wanger T. C., Westphal C., and Batáry P. (2021). Beyond organic farming – harnessing biodiversity-friendly landscapes. Trends Ecol. Evol. 36, 919–930. doi: 10.1016/j.tree.2021.06.010, PMID: 34362590
Wang Y. X. G., Matson K. D., Prins H. H. T., Gort G., Awada L., Huang Z. Y. X., et al. (2019). Phylogenetic structure of wildlife assemblages shapes patterns of infectious livestock diseases in Africa. Funct. Ecol. 33, 1332–1341. doi: 10.1111/1365-2435.13311
Keywords: plant community process, fungal disease, insect pest, host susceptibility, zero-inflated binomial regression, plant-parasite dynamics, suburban landscape, epidemiological triangle
Citation: Wang X, Hiratsuka K and Koike F (2025) Prevalence of leaf parasitism by insects and fungi in wild plant communities: implications for community assembly. Front. Ecol. Evol. 13:1498915. doi: 10.3389/fevo.2025.1498915
Received: 19 September 2024; Accepted: 09 June 2025;
Published: 07 July 2025.
Edited by:
Etsuko Nonaka, University of Helsinki, FinlandReviewed by:
Gabor Pozsgai, University of the Azores, PortugalJustin Bastow, Eastern Washington University, United States
Matthew Michalska-Smith, University of Minnesota Twin Cities, United States
Mia Miranti, Padjadjaran University, Indonesia
Copyright © 2025 Wang, Hiratsuka and Koike. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Wang, d2FuZy14aS13a0B5bnUuanA=
 Xi Wang
Xi Wang Kazuyuki Hiratsuka
Kazuyuki Hiratsuka Fumito Koike
Fumito Koike