- 1Wildlife Division, Sasan-Gir, Junagadh, Gujarat, India
- 2Wildlife Circle, Junagadh, Gujarat, India
- 3Chief Wildlife Warden, Gujarat State, Gandhinagar, Gujarat, India
- 4Department of Landscape Level Planning & Management, Wildlife Institute of India, Dehradun, India
This study provides novel insights into the scent-marking behaviors of free-ranging Asiatic lions (Panthera leo persica) in the dry deciduous forests of Western India, focusing on spatial and temporal patterns as well as the factors influencing the selection of scent-marking sites. Using camera traps, we identified that scent-marking and associated behaviors are predominantly exhibited during crepuscular and nocturnal hours, with peaks at dawn and dusk. Seasonal variation was observed, with increased activity during winter, coinciding with the breeding period. Sniffling was the most frequent behavior observed, followed by scratching and spraying. Adult males were more engaged in these behaviors than females, likely due to territorial defense and reproductive strategies. Our analysis of tree characteristics revealed a preference for trees near forest tracks or trails, especially those with rough bark and aromatic properties, which may enhance the persistence and detection of scent marks. This study is the first comprehensive analysis of scent-marking behaviors in Asiatic lions. It lays the foundation for future research into individual scent-marking patterns using GPS-collaring and camera traps. Understanding these behaviors is important for the better conservation of this endangered species.
1 Introduction
Mammals communicate through various means, including auditory, visually, or chemically (Macdonald, 1995; Eisenberg and Kleiman, 1972). The chemical mode of communication through odor is the most prominent in carnivores. The scent marks carrying the signals can retain in the environment long after their release and are, therefore, ideal for solitary, long-raging territorial species for intra- and interspecific communications (Johnston, 2005). The frequency of scent-marking often positively correlates with the individual’s fitness and increases with social status where dominant individuals have higher frequency of marking than the subordinate individual (Allen et al., 1999; Gosling and Roberts, 2001; Jordan et al., 2013). Sent marks deposited as semiochemicals can be carried through urine, feces, skin, and a range of secretions from different glands across the body (Bradbury and Vehrencamp, 2011; Apps, 2013) through various behaviors such as ground scratching, tree scratching, or rubbing of different body parts on the substrate (Asa et al., 1985; Harmsen et al., 2016; Barja et al., 2005; Bothma and le Richet, 1995), which convey different messages and are used for important functions such as defending territory (Gosling, 1982; Zub et al., 2003), mate selection (Meena, 2010; Allen et al., 2015a), intraspecific communication (Seidensticker et al., 1973; Allen et al., 2014, 2015b), interspecific interaction (Allen et al., 2017, 2015b; King et al., 2017; Li et al., 2013), and landmarks aiding in orientation (Barja et al., 2004; Gorman and Trowbridge, 1989).
In long-ranging and territorial felids, scent-marking is the prominent aspect for social organization and the primary form of communication (Smith et al., 1989; Allen et al., 2014, 2016) including lions (Panthera leo) and has been observed, as well described in detail (Andersen, 1998; Brahmachary and Singh, 2000; Schaller, 1972; Barja and de Miguel, 2010; Gilfillan, 2017). To maintain the social status, attract mates, and hold land tenure or prey rights, lions interact indirectly with conspecifics (Chakrabarti and Jhala, 2019). Major kinds of scent-marking behavior that have been observed in lions are spray marking, clawing, scrape marking, and chin rubbing (Schaller, 1972; Andersen, 1998; Jhala et al., 2009). In Asiatic lions, males are observed to mark more frequently than females and females were observed to mark and advertise their territories only during the estrous period (Jhala et al., 2009; Joslin, 1973), whereas in the Serengeti lions, both male and females are observed to show the similar extent of marking behavior (Schaller, 1972). However, clawing in particular has been observed in all age groups, and without any difference in both males and females (Schaller, 1972; Andersen, 1998). For lions, scratching is a common and natural action that they partake in for various purposes, viz, stretching and exercise, grooming, and shedding claws marking territory (Pageat and Gaultier, 2003; Stanton et al., 2015). Additionally, the foot pads include scent glands that serve as a territorial marking indication. Therefore, scratching serves as both a chemical and visual signal (Harmsen et al., 2016).
Asiatic lions (Panthera leo persica) are found in the Gir Forest and the surrounding landscape in the Saurashtra region of Gujarat, Western India. Within this population, prides predominantly consist of females and their dependent cubs, whereas males are either solitary or form coalitions, typically comprising an average of two adult males (Joslin, 1973; Meena, 2009; Chakrabarti and Jhala, 2017). Unlike African lions, female Asiatic lions do not exhibit estrous synchrony, resulting in fewer mating opportunities for males (Chakrabarti and Jhala, 2019). To prevent infanticide by males, lionesses engage in mating with multiple males, thereby creating paternity confusion among males (Chakrabarti and Jhala, 2019). Most studies on scent-marking in lions provide descriptive accounts of the form and function of scent-marking behaviors (Schaller, 1972), primarily focusing on the chemical communication aspect (Andersen, 1998; Andersen and Vulpius, 1999; Brahmachary and Singh, 2000; Pageat and Gaultier, 2003; Barja and de Miguel, 2010; Poddar-Sarkar et al., 2008). These studies have predominantly been conducted on captive lions, with limited research on free-ranging populations (Gilfillan, 2017). Notably, Joslin (1973) documented various advertisement behaviors in lions, including roaring, spraying, scraping, and defecation, in significant detail. However, these observations were largely anecdotal and lacked the quantitative rigor necessary for drawing robust conclusions. In Asiatic lions, the scent-marking behavior in wild populations remains poorly understood. Currently, there is limited information on the relative importance and ecological roles of different scent-marking behaviors in their natural habitat.
The selection of sites for scent-marking is not random; instead, it is influenced by specific habitat characteristics that enhance the likelihood of detection by conspecifics (Gosling and Roberts, 2001; Mohorović and Krofel, 2020; Ruiz-Olmo et al., 2013). For example, Allen et al. (2017) reported that Eurasian lynx (Lynx lynx) preferentially selected objects similar to their body size, positioned along straight road sections, while avoiding more commonly available object types. Similarly, neotropical felids such as jaguars (Panthera onca) and pumas (Puma concolor) chose sites predominantly covered with leaves, narrow paths, and clean, infrequently used areas for scraping (Palomares et al., 2018). Joslin (1973) observed that Asiatic lions frequently scraped on substrates like small bushes, grass tussocks, and dead teak (Tectona grandis) leaves. Vertical structures, such as trees, are particularly important for scent marking in some carnivores, as they increase the visibility and dispersion of scent marks due to their elevated position. This behavior has been documented in species such as leopards (Panthera pardus), cheetahs (Acinonyx jubatus), and giant pandas (Ailuropoda melanoleuca) (Alberts, 1992; Bothma and le Richet, 1995; Marnewick et al., 2006; Nie et al., 2012). Understanding the spatial and temporal patterns of scent-marking behavior in Asiatic lions is essential for identifying critical areas for their territorial and communication needs. Such insights can inform more effective conservation and management strategies for this endangered species, ensuring the preservation of their habitats and behaviors essential for their ecological success.
Motion-triggered cameras are widely utilized for documenting cryptic behaviors in elusive species that are challenging to observe through conventional field surveys (Caravaggi et al., 2017). In this study, we used infrared motion-detection cameras to record and analyze the activity and scent-marking behavior of Asiatic lions, enabling us to test various hypotheses. Our objectives were (1) to characterize scent-marking and associated behaviors across different age and sex classes and (2) to identify factors influencing site selection for these behaviors. We hypothesized that visitation rates at scent-marking sites would peak during nocturnal and crepuscular hours, aligning with periods of heightened lion activity, and would be higher during the mating season than at other times. Additionally, we expected adult males to exhibit greater involvement in scent-marking behaviors compared with females and predicted that scratching and spray marking would be the most frequent behaviors among adult males and females. Lastly, we hypothesized that lions would prefer trees with broader girths, located near forest tracks or trails in low-elevation areas, and favor tree species with hard bark capable of retaining scent marks for extended durations over those that diffuse scent more quickly.
2 Material and methods
2.1 Study area
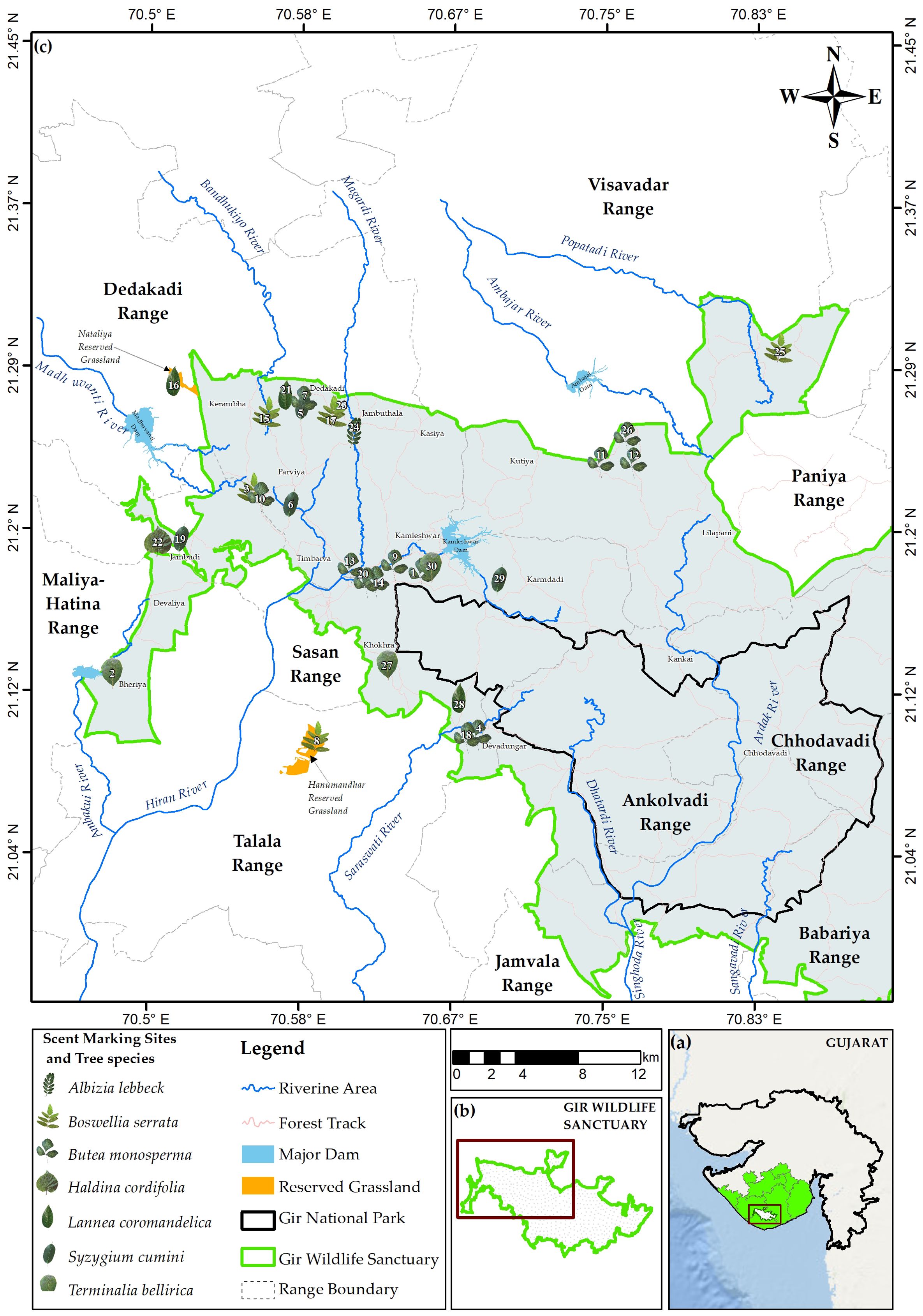
The Gir Forest, situated in the western part of the Saurashtra peninsula in Gujarat, India, spans an area of 1,879.13 km² (Vasavada et al., 2022). It lies within Bio-geographic Zone-4 (semiarid) and Biotic Province 4-B of the Gujarat Rajwara Province (Rodgers and Panwar, 1988; Vasavada et al., 2022) (Figure 1). Renowned for its dry deciduous forest, the largest in Saurashtra, Gir Forest supports a diverse range of wildlife, including approximately 41 mammalian species, 47 reptilian species, and over 338 species of resident and migratory birds. Key prey species contributing significantly to the diet of Asiatic lions include the spotted deer (Axis axis), sambar (Rusa unicolor), wild pig (Sus scrofa), and nilgai (Boselaphus tragocamelus) (Meena, 2009; Vasavada et al., 2022; Ram et al., 2023a). Gir experiences three distinct seasons: the dry winter, dry summer, and monsoon. The dry winter season begins in November and lasts until February, with temperatures dropping to a minimum of 7°C. The dry summer season extends from March to June, with peak temperatures reaching 44°C in May. The monsoon season occurs between July and October, bringing much-needed rainfall to the region (Vasavada et al., 2022). As a critical protected area for biodiversity conservation in India, Gir Forest’s unique climate, vegetation, and landscape play a vital role in maintaining its ecological significance (Vasavada et al., 2022).
2.2 Field methods
The field trackers of Gir Forest, proficient in identifying lions based on their natural markings and daily observations, assisted in locating potential trees frequently marked by lions. To distinguish traditional marking trees from those scratched sporadically or on single occasions, we recorded specific indicators, including punch marks on trees, the presence of hair strands and follicles beneath the trees, and a concentrated pattern of scratch marks. These features clearly identified trees used more frequently than random chance. Lions, like other felids, grow their claws in sheaths, which they shed by scratching trees to remove the worn outer layer (Joslin, 1973). This behavior also facilitates scent marking. After identifying frequently used marking sites, motion-triggered cameras (SPYPOINT SOLAR-DARK Trail Cameras; Model: Force-Pro and Force-Pro-S) were deployed from March 2022 to April 2024. Cameras were positioned to face the scratch-marking site, either attached to a nearby tree or mounted on a metal stake. Only sites meeting the outlined criteria for traditional marking behavior were included for data collection. Fresh scratch marks were identified based on features such as resin oozing from the scratches, exposed fragmented bark, freshly exposed wood, and hair remnants on or near the marks.
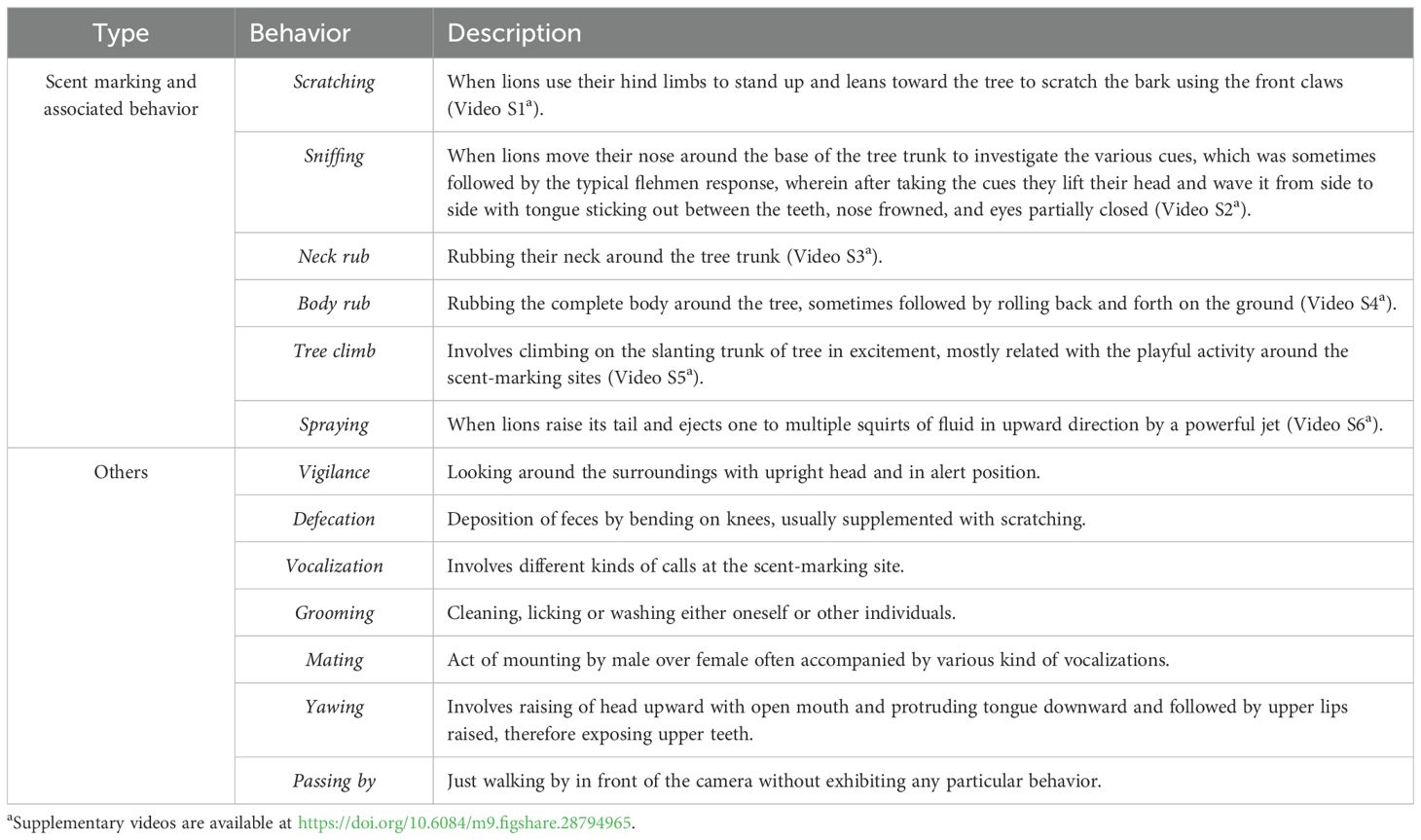
Each camera was programmed to capture one photograph followed by a 30-s video recording, with a 1-s delay before re-triggering. During each visit by a lion, various scent-marking behaviors were documented (Table 1). Additional data collected included tree species, girth at breast height (GBH) measured with a measuring tape (Freemans 30 m), GPS location (Garmin eTrex 30X), and estimated tree height. Other features recorded were the total number of scratch and punch marks, the tallest visible scratch mark from the tree base, the average length of scratch marks, the height of the highest concentration of scratch marks, the number of hair strands and follicles within a 10-m radius of the tree, and the lean angle of the tree measured using trigonometric methods (Avery and Burkhart, 2015).
2.3 Video processing
Each visit to a site was considered a separate event, provided it was separated by at least 30 min from the previous one. Only detections involving lions were included in the analysis, whereas observations of other species were excluded. The data collected were classified into various scent-marking and associated behaviors (Table 1). Additional behaviors such as vigilance, defecation, vocalization, grooming, mating, yawning, and passing by were recorded but excluded from the final analysis due to their low frequency and their tendency to co-occur with primary behaviors. Behavioral data were further categorized by sex (male and female) and age class, with individuals classified as pre-adult (<3 years), adult (3–10 years), or post-adult (>10 years) based on body size, coloration, secondary sexual characteristics, and tooth eruption and wear (Schaller, 1972). Since females typically live in prides with dependent cubs, individual behaviors were categorized separately, regardless of pride composition. Male and female interactions are generally limited to mating or occasional large kills (Chakrabarti and Jhala, 2017), which ensured that individuals were usually captured separately on cameras. Data were further divided by year, month, and hourly intervals for analysis. In a single scent-marking event, multiple behaviors may be exhibited together in no particular sequence (Joslin, 1973). Consequently, each behavior was analyzed separately and included in the overall dataset if it was observed at least once during an event.
2.4 Data analysis
We examined the seasonal variation in lion visitation frequency at scent-marking sites by categorizing months into three seasons: winter (November to February), summer (March to June), and monsoon (July to October). The differences in visitation frequency across seasons and hours of the day were compared using chi-square goodness-of-fit tests. Additionally, we analyzed variations in different scent-marking and associated behaviors across seasons and age–sex classes. In the case of different behaviors performed simultaneously during a single visit, each behavior was considered separately for the analysis. The mean duration of stay for each behavioral category was also assessed. For each chi-square analysis, p ≤ 0.05 was considered as significant. All the chi-square goodness-of-fit test were performed in R v.4.1.2 (R Development Core Team, 2021).
The mean for different scent-marking and associated behaviors was computed using circular statistics for Windows, Program Oriana (Kovach, 2011). Rayleigh’s test for uniformity (Landler et al., 2018) was performed for each behavior to calculate the probability of null hypothesis that the data were uniformly distributed across time intervals, and a probability (P) less than 0.05 indicated that the data showed statistically significant peak periods (Zar, 2010).
The coefficient of overlap (Δ) for the activity pattern of different behaviors between male and female lions was estimated by fitting two kernel density curves (Ridout and Linkie, 2009), which calculates the common area under the two probability densities (Schmid and Schmidt, 2006). The value for Δ ranges from 0 to 1, where 0 indicates no overlap, and 1 indicates complete overlap (Frey et al., 2017). The overlap accuracy and confidence interval were calculated using bootstrap resampling with 10,000 iterations (Ridout and Linkie, 2009). Mardia–Watson–Wheeler test of homogeneity (W) was used to access the null hypothesis of no difference in activity pattern of behaviors between male and female lions (Zar, 2010; Tasdan and Yeniay, 2014).
Finally, to understand the factors that govern the frequency of visits at the scent-marking sites, a generalized linear model (GLM) with number of visits at each site as a response variable was fitted using a Poisson distribution. The independent variables included in the analysis were tree height, GBH, tree species, habitat type, elevation, Terrain Ruggedness Index (TRI), distance to forest tracks/trails, and distance to water. Tree height, GBH, and tree species were obtained from the primary data collected during the field work. Elevation for each pixel in the meter was generated from Digital Elevation Model (DEM) data generated by the ASTER Global Digital Elevation Model Data (NASA LP DAAC, 2018). To assess the TRI for each pixel, v.rast.stats in GRASS (Version 7.4.2. GRASS Development Team, 2018) was used. The habitat was classified into seven habitat types, namely, Thorn, Teak-Acacia-Zizyphus (TAZ), Moist Mixed, Mixed, Scrub, Acacia-Lannea-Boswellia (ALB), and Acacia-Teak-Anogessus (ATA) (Basu, 2013). Availability of roads and water was obtained from the ground validated and digitized data from the Management Plan for Gir Protected Areas (Vasavada et al., 2022). Distance from water and road was then calculated using Euclidean distance from a digitized map using ArcMap (version 10.8.1, ESRI, 2019). Before building the models, Spearman’s rank correlation matrix was performed between the explanatory variables to confirm that none of the variables were intercorrelated using Spearman’s rho (rs)>0.7 as a criterion for exclusion. All the models were fitted using the “glmer” function from the “lme4” package (Bates et al., 2015). We only ran models with no more than three explanatory variables in order to maintain the parsimony and to avoid overfitting. The best model(s) was selected based on the AICc values, and only the model with ΔAICc <2 was selected (Burnham and Anderson, 2002). The effect of variables on the number of scratch marks was assessed by the β-coefficients and associated standard errors (SE) of selected models. All the statistical analysis were performed using the program R v.4.1.2.
3 Results
3.1 Patterns of scent-marking behavior
We monitored 36 sites with the camera traps, out of which 30 sites were recorded with the presence of lions. Out of 15,144 detections, lions were detected 1,542 times (10%) only. Sites were used throughout the year, with maximum visitation occurring during the month of December, followed by February and January. There was a significant difference in visitation frequency between the seasons (χ2 = 735.08, d.f. = 2, p = <0.01). Around 67% of the visits occurred between January to June, and then the other months. Lions visited the sites significantly more during the winters, followed by summers and monsoons. To visualize the data, a kernel density plot was calculated using the overlap package (Meredith and Ridout, 2016), which showed a bimodal peak in lion’s activity during the dusk and dawn (Figure 2). The frequency of visit at the scent-marking sites was significantly more during the early morning between 05:30 am to 07:30 am than the rest of the day (χ2 = 603, d.f. = 23, p <0.01), and the second peak was between 05:00 pm and 06:00 pm. The visitation by the time of the day showed bimodal peaks during winters and only unimodal peaks in summers (Figure 3); that is, lions were more active during the winter season, but there were no significant differences in the activity period between the two seasons (χ2 = 17.49, d.f. = 23, p >0.05). The mean duration of the stay at the site was 22.39 (± 0.25 SE) seconds. Although the mean time spent by lions was more in winters, there were no significant differences among the seasons (χ2 = 1.3, d.f. = 2, p >0.05).
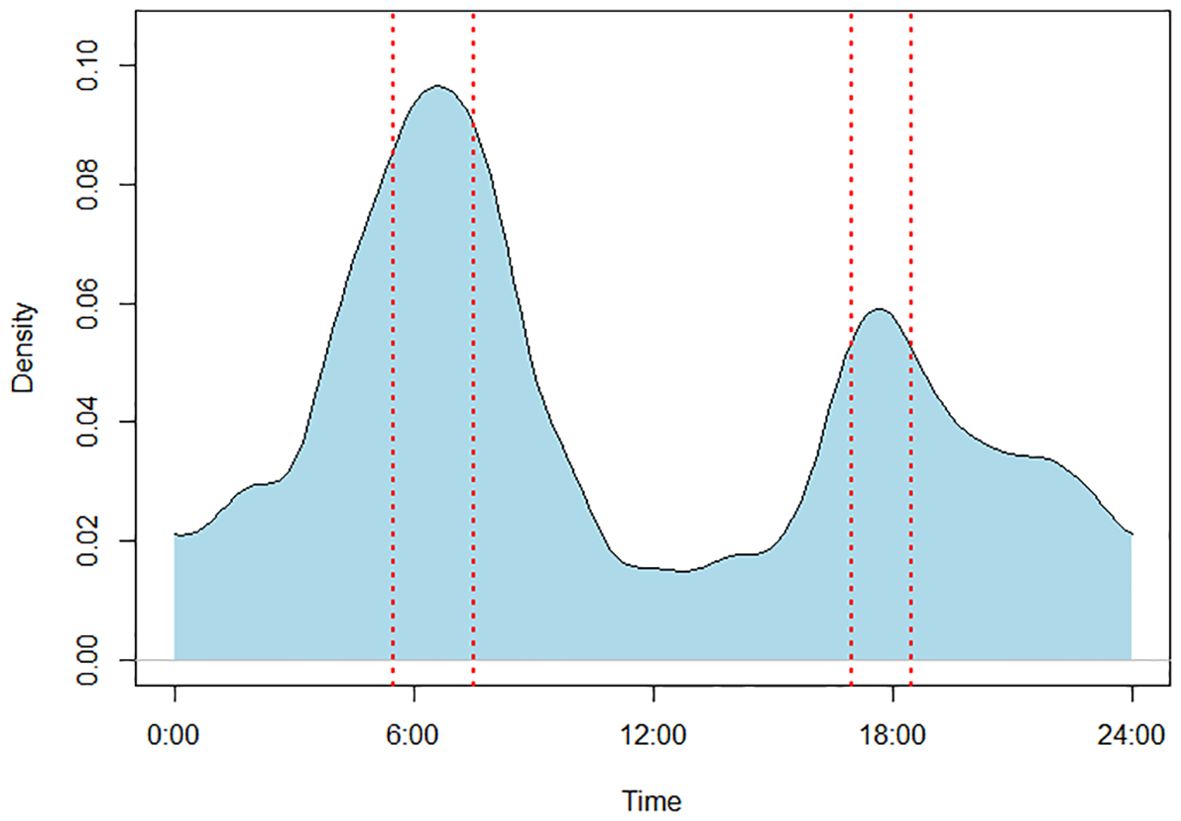
Figure 2. Kernel density plot showing the time of the day that lions visited the scent-marking sites. The dotted red vertical lines denote the peak activity period.
Among all the behaviors, the frequency of sniffing (40%) at least once was exhibited significantly more (χ2 = 726.36, d.f. = 5, p <0.01), followed by scratching (30%) and spraying (12%). Males exhibited these behaviors significantly more than the females (χ2 = 26.87, d.f. = 5, p = <0.01); however, the frequency of body rub, and tree climb particularly, was exhibited more by females than by males. Among both males and females, the frequency of sniffing and scratching was more than other behaviors. Adult lions exhibited significantly more scent-marking and associated behaviors than pre- and post-adult stages of lions (χ2 = 97.33, d.f. = 10, p <0.01); however, the tree climb behavior was exhibited more by the pre-adult stage than the adult stage. All the behaviors were exhibited significantly more in winters than in other seasons (χ2 = 31.46, d.f. = 10, p <0.01), and particularly the frequency of sniffing, scratching, and spraying was exhibited more during winters than the rest of the behaviors. There was not much seasonal difference in the frequency of behavior between males and females. However, the scratching by females was more frequent during the months of January, April, and May than the males.
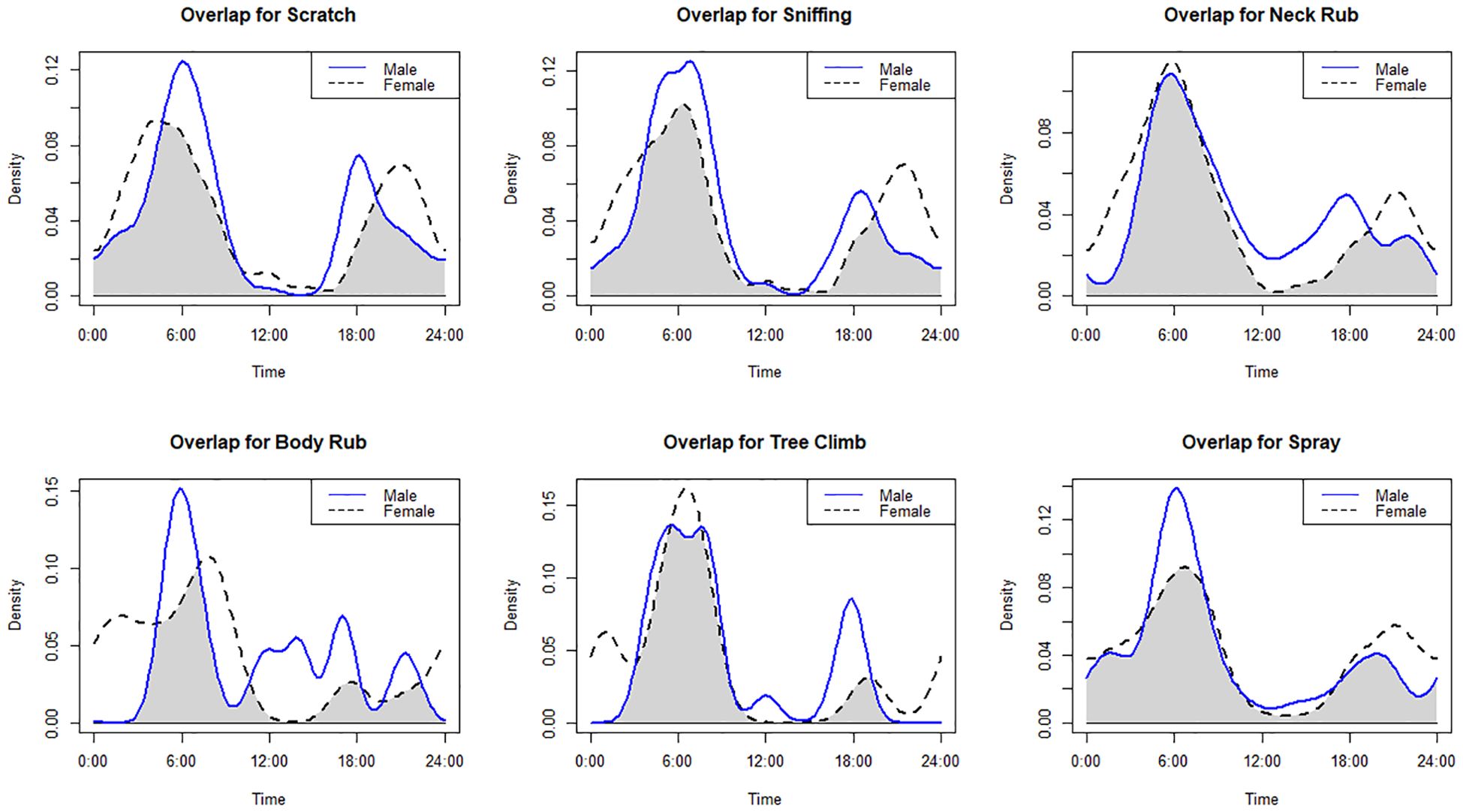
All behaviors showed their peak during the morning hours (Figure 4). Behaviors like scratching, sniffing, and spraying were exhibited during the early morning hours [scratching: mean peak = 03:44 AM (95% CI = 02:54 AM to 04:33 AM), Rayleigh’s Z test (Z) = 39.05, p<0.01; sniffing: mean peak = 04:17 AM (95% CI = 03:41 AM to 04:53 AM), Rayleigh’s Z test (Z) = 71.29, p<0.01; spraying: mean peak = 04:41 AM (95% CI = 03:34 AM to 05:48 AM), Rayleigh’s Z test (Z) = 20.83, p<0.01], whereas neck rub, body rub, and tree climb were exhibited during the late morning hours [neck rub: mean peak = 05:03 AM (95% CI = 03:47 AM to 06:19 AM), Rayleigh’s Z test (Z) = 15.84, p<0.01; body rub: mean peak = 06:47 AM (95% CI = 04:34 AM to 08:59 AM), Rayleigh’s Z test (Z) = 05.46, p<0.01; tree climb: mean peak = 06:23 AM (95% CI = 04:16 AM to 08:31 AM), Rayleigh’s Z test (Z) = 05.80, p<0.01]. The spraying activity has the highest value for the coefficient of overlap (Δ) between the males and females (Δ = 0.80), whereas the body rub has the lowest value (Δ = 0.52). There was a significant difference in the scratching (W = 12.29, p = 0.002) and sniffing (W = 15.84, p = 0.0004) activity between males and female (Table 2; Figure 5).
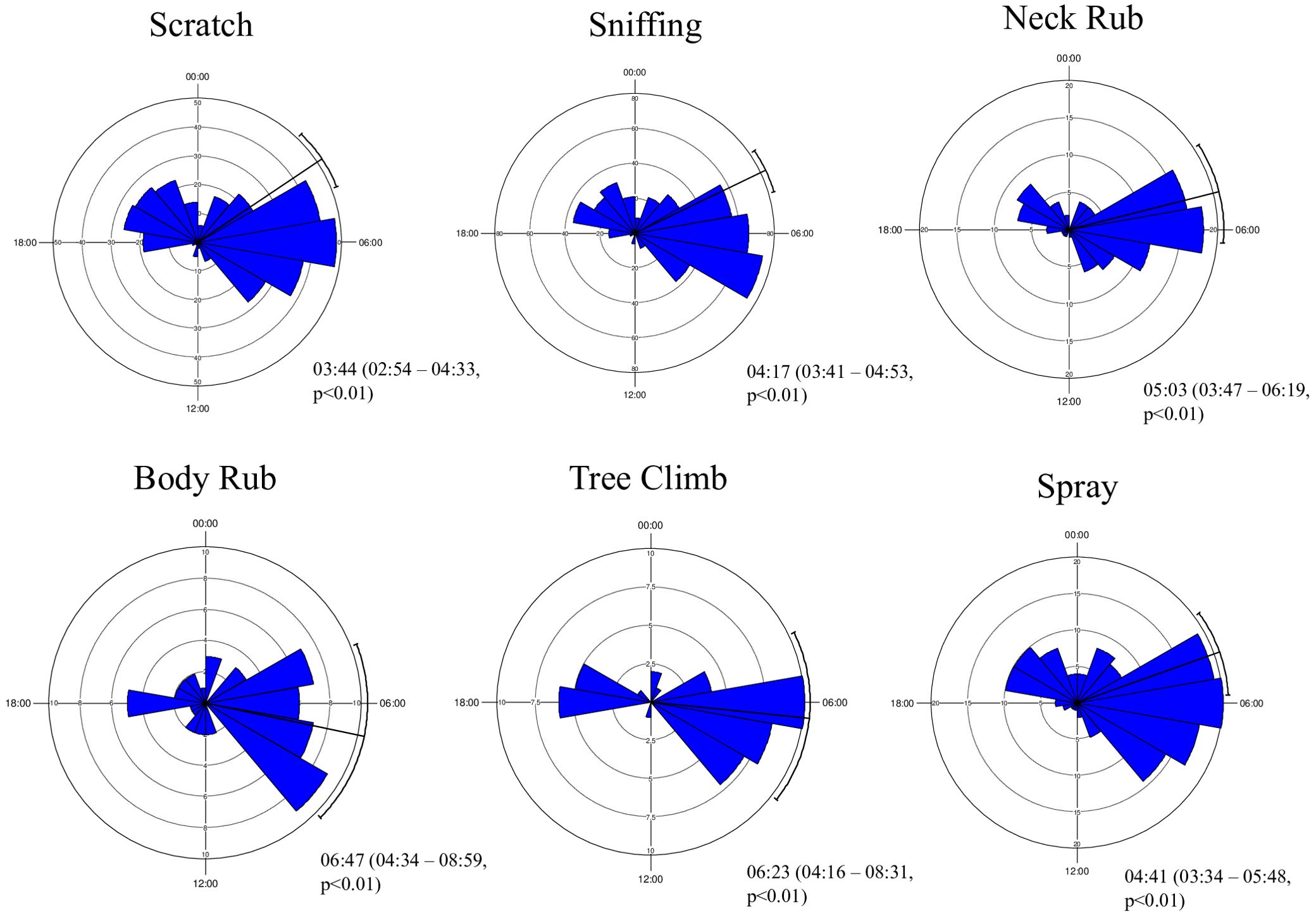
Figure 4. Proportion of time spent by lions (Panthera leo persica) across different marking behaviors during different times of the day and the related activity peaks.
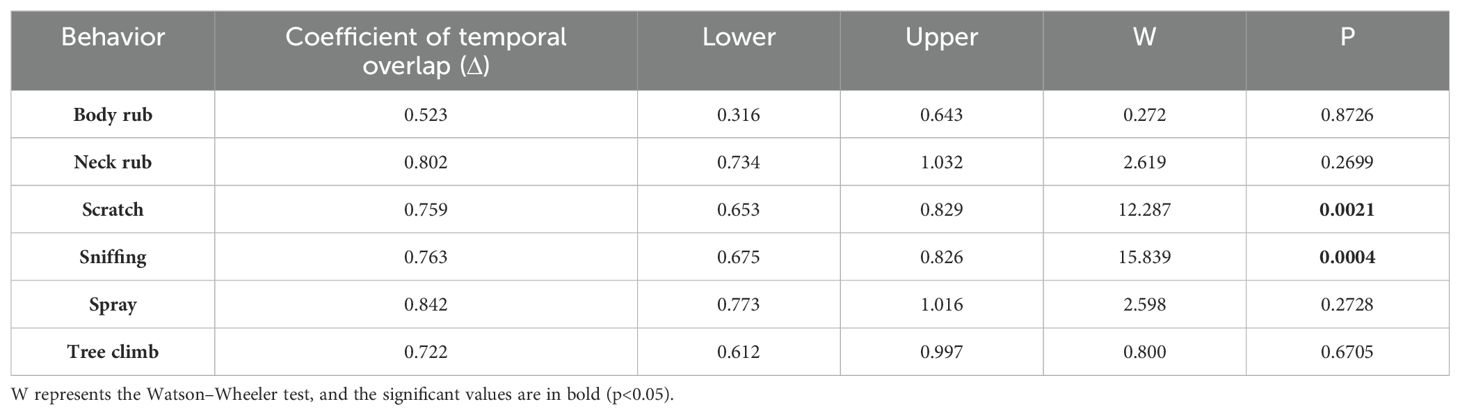
Table 2. Differences in scent-marking behavioral temporal activity pattern between males and females.
3.2 Factors governing the site selection for scent-marking
We monitored 30 scent-mark sites that the lions frequently visited. Most of the trees at the scent marking sites were present within the 5-m radius of the forest tracks (79%, n = 27). Of the trees documented, 11 were Butea monosperma, five Lannea coromandelica, four Syzygium cumini, three Boswellia serrata, four Terminalia bellirica, two Haldina cordifolia, and one Albizia lebbeck (Figure 6). The frequency for selecting sites with Butea monosperma species was significantly higher than the other tree species (n = 11; χ2 = 14.8, d.f. = 6, p <0.05). The mean (± SE) number of frequency of visits at all scent-marking sites was 51.4 (± 17.55), and the mean (± SE) number of scratch marks on all the trees at the scent-marking sites was 182.43 (± 23.36). The mean (± SE) height of scent-marked trees was 8.41 m (± 0.47), and there was no significant difference in the average height of the trees selected across the species (χ2 = 2.79, d.f. = 7, p >0.05). The mean (± SE) GBH of all the trees was 162.93 cm (± 12.97), and the average GBH of Boswellia serrata and Syzgium cumini was significantly higher than the average GBH of other tree species (χ2 = 221.61, d.f. = 6, p <0.01). The mean lean angle (± SE) of all the trees was 51.26° (± 2.24°), and there was no significant difference in the average lean angle of the trees selected across the species (χ2 = 9.41, d.f. = 6, p >0.05).

Figure 6. Different tree species encountered during the deployment of camera traps. The tree species are heavily marked showing characteristic scent-marking behavior of Asiatic lions. The tree species are (A) Albezia lebbeck, (B) Haldina cordifolia, (C) Lannea coromandelica, (D) Syzygium cumini, (E) Boswellia serrata, (F) Butea monosperma, (G) Terminalia bellirica.
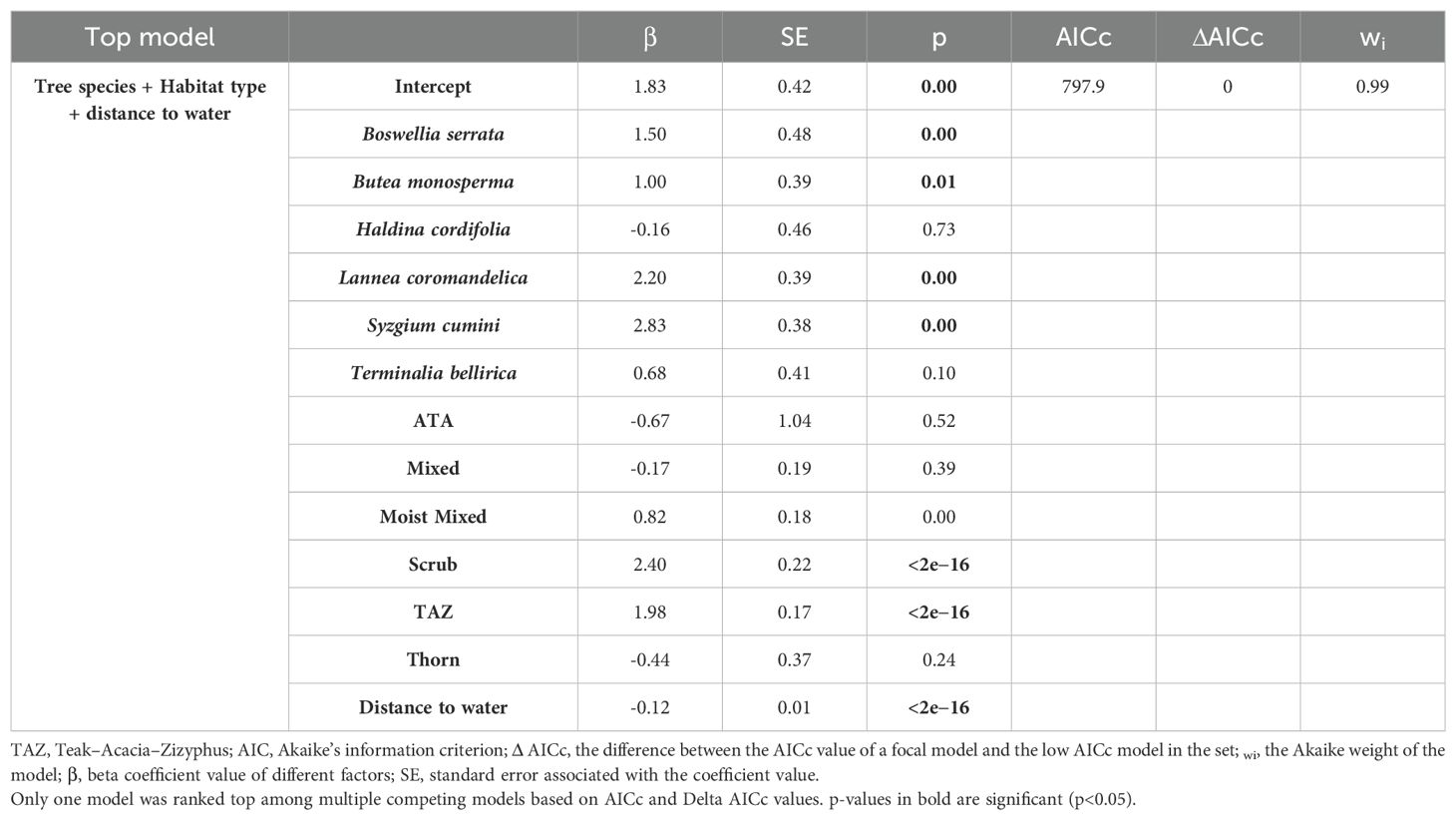
There was no correlation between the variables [i.e., (rs)<0.7]. Multiple GLM models were fitted using a Poisson distribution. A total of 93 candidate models were run through the dredge function, and out of those, only one model was selected as the best model, which had ΔAICc<2 (Bartonń, 2013) (Table 3) with variables habitat, tree species, and distance to water (cumulative wi of 0.99). The distance to water has a significant negative effect on the frequency of visitation at the scent-mark sites (β = −0.12, SE = 0.01, z-value = −15.15, p<0.01); that is, the trees located near the water will have a higher frequency of visitation than the trees located farther away from the water. Among the habitats, the scrub habitat type has a significant positive effect on the frequency of visitation at the scent-mark sites than the other habitat types (β = 2.40, SE = 0.22, z-value = 11.16, p<0.01, Figure 7). Among all the tree species, Syzgium cumini has the highest positive significant effect on the frequency of visitation at the scent-mark sites than the other tree species (β = 2.83, SE = 0.38, z-value = 7.40, p<0.01). Therefore, based on the top selected model, the visitation will be more frequent on Syzgium cumini trees located in scrub habitat near the water source areas of west Gir Wildlife Sanctuary.
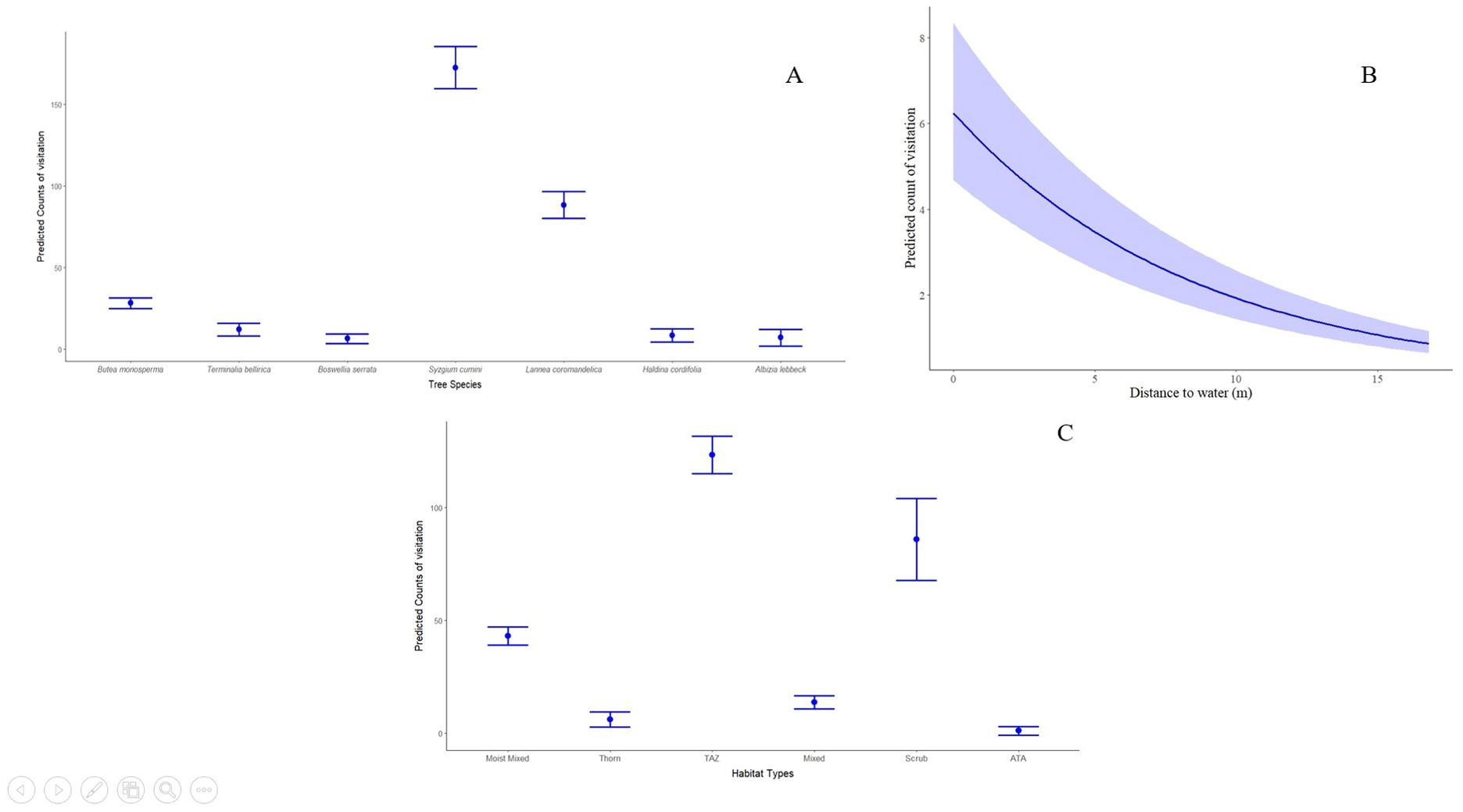
Figure 7. Effect of (A) tree species; (B) distance to water, and (C) habitat type on the predicted count of visitation at the scent-marking sites based on the GLM results.
4 Discussion
Evaluating the spatial patterns and characteristics of scent-marking behavior is fundamental to understanding intra- and interspecific communication in carnivores (Mohorović and Krofel, 2020). Our study provides novel insights into the scent-marking behavior of free-ranging Asiatic lions, identifying key factors influencing marking site selection within the dry deciduous forests of western India. This represents the first quantitative study on scent-marking in free-ranging Asiatic lions, forming a foundation for future research on this critical aspect of their behavior.
The use of camera traps to monitor these elusive behaviors allowed us to quantify the proportional use of marking sites, a task that is otherwise challenging for free-ranging species. Our findings supported the hypothesis that lions are most active at scent-marking sites during crepuscular and nocturnal hours, exhibiting clear bimodal peaks at dawn and dusk. This aligns with Chaudhary et al. (2020), who documented similar activity patterns in lions within the Gir Wildlife Sanctuary. Telemetry studies (Jhala et al., 2009) have also shown that patrolling and territorial behaviors, such as scent-marking and vocalizations, predominantly occur during early morning and late evening hours. Furthermore, earlier observations by Joslin (1973) recorded that advertisement activities such as roaring, spraying, scraping, and defecation are mainly conducted at night, late evening, or early morning. These temporal patterns may help lions avoid heat stress during hot months, concentrating their scent-marking and territorial activities during cooler periods of the day. As hypothesized, our results also demonstrated that scent-marking site visitation peaked during winter, coinciding with the lions’ peak breeding period. While Asiatic lions breed year-round, mating activity tends to increase in winter (Jhala et al., 2009), potentially enhancing reproductive signaling and increasing mating success. The data indicated that the majority of these site were visited between January and June, aligning with previous studies showing a mating peak from January to May (Jhala et al., 2009). Although no statistically significant seasonal differences in temporal activity patterns were observed, which may be linked to the year-round breeding behavior of lions, the higher visitation rates during winter underscore the close link between scent-marking and reproductive behavior.
Contrary to our initial hypothesis, sniffing emerged as the most frequently observed behavior, followed by scratching and spraying. Sniffing appears to be more closely associated with exploratory territorial behavior rather than reproductive signaling and is often accompanied by other behaviors, such as the flehmen response. This pattern aligns with findings from olfactory investigations in solitary felids such as bobcats and clouded leopards (Allen et al., 2015b, 2016). While our focus on heavily marked trees may have resulted in an overestimation of scratching behavior, prior studies indicate that lions often prefer bushes for spray-marking and scraping on small or non-existent objects (Joslin, 1973). In captive lions, spray-marking and scrape/urination are the predominant scent-marking behaviors, whereas clawing and chin rubbing occur less frequently (Andersen, 1998). Similarly, telemetry studies have shown that spraying is the most common method of territorial advertisement, with scraping being a rarer event (Jhala et al., 2009). In contrast, Schaller (1972) observed that scrape/urination occurred three times more frequently than spray marking in African lions. In our study, scraping was recorded in only 0.02% of total observations, likely due to our focus on scratch-marked sites. Clawing and scratch marking are generally considered the most visually conspicuous scent-marking behaviors (Andersen, 1998; Allen et al., 2015b), whereas rubbing behaviors—such as chin, body, and neck rubbing—leave both olfactory and visual cues. In felids, rubbing behaviors have been linked to agonistic and sexual contexts (Reiger, 1979), but their specific role in communication remains unclear and requires further investigation. Additionally, tree climbing, which was predominantly observed in females, may serve as a playful activity and could also play a role in fostering social bonds within the pride. This aspect of social communication warrants further exploration to better understand its implications for lion behavior and interactions.
Adult male lions exhibited a higher frequency of scent-marking behaviors compared with females, a pattern consistent with findings in both captive and wild lions (Joslin, 1973; Andersen, 1998; Jhala et al., 2009). However, studies on Serengeti lions have reported a similar frequency of scent-marking behavior in both sexes (Schaller, 1972). Male lions are more invested in maintaining territorial boundaries, using scent marks to deter competitors. Similarly, in pumas, mature adult males are the primary visitors to scent-marking sites (Allen et al., 2014). Although the sex ratio in Gir Wildlife Sanctuary is more female-biased (Banerjee and Jhala, 2012; Ram et al., 2023b), males dominate scent-marking behaviors due to two key reasons. First, their wide-ranging movements (Ram et al., 2023c) necessitate frequent territorial maintenance to avoid competition from intruders. Second, the polyoestrous nature of female lions prompts males to repeatedly deposit scent marks to gain access to potential females. In contrast, females appear to visit scent-marking sites less frequently, possibly as a strategy to avoid aggressive encounters with males and mitigate the risk of infanticide. Both sexes, however, exhibit increased scent-marking activity during the winter months, coinciding with the peak breeding season (Jhala et al., 2009). Notably, African lions do not demonstrate a seasonal pattern in marking frequency (Schaller, 1972). Females in Gir showed higher sniffing and scratching activity than males during April and May, which may be linked to some females entering estrus following male-inflicted infanticide. Additionally, unlike African lions, Asiatic female lions do not exhibit estrus synchrony, further distinguishing their scent-marking and reproductive behaviors.
By examining the characteristics of trees frequently used for scent marking, we identified notable preferences influenced by ecological and behavioral factors shaping lion communication strategies. This study is the first to investigate the behavioral aspects associated with site selection for scent marking in free-ranging Asiatic lions. Previous studies have primarily focused on descriptive accounts or the chemical composition of scent marks in captivity (Brahmachary et al., 1999; Brahmachary and Singh, 2000; Barja and de Miguel, 2010). Carnivores are known to select marking sites that maximize the likelihood of scent detection and persistence while minimizing the energy required for marking large territories (Allen et al., 2017). Our findings supported the hypothesis that lions preferentially mark trees located near forest tracks or trails. Nearly all marked trees were within a 5-m radius of such paths, suggesting an energy-efficient strategy due to ease of access and a higher probability of conspecific detection of scent marks (Alberts, 1992; Fahrig and Rytwinski, 2009; Stępniak et al., 2020). Similar patterns of marking near roads, game trails, dried riverbeds, and intersections within territories have been observed in other carnivores, including tigers (Sunquist, 1981; Smith et al., 1989), brown bears (Clapham et al., 2013), giant pandas (Schaller et al., 1985), wolves (Peters and Mech, 1978), and foxes (Macdonald, 1979). It is also plausible that lions invest time in scent-marking at locations not visually detectable, which our study may not have captured due to reliance on camera traps at visible marking sites. Future studies could incorporate additional methodologies, such as tracking radio-collared individuals, to identify other potential scent-marking sites and further refine our understanding of scent-marking behavior in free-ranging Asiatic lions.
Moreover, as apex predators in the Gir forest, Asiatic lions are competitively superior to other carnivores, enabling them to select prime habitats for effective intraspecific communication without the fear of intruders or other carnivores. While other carnivores in the landscape might also utilize these areas for interspecific communication (Allen et al., 2017), this aspect requires further study. As per our hypothesis, Syzgium cumini had a higher average girth and had hard and rough bark, which increases the surface area for scent deposition and is also resistant to rainfall (Nie et al., 2012). Moreover, S. cumini has aromatic properties (Kavitha et al., 2012). Therefore, marking on these trees not only acts as an additional attractant for the intended receivers but also helps in the reduction of energetic expenditure (Gosling et al., 2000). Moreover, to make their marks more conspicuous, lions select trees in open dry scrub areas, which increases visibility. Moreover, to prevent scent marks from evaporation, trees are selected that are near the water body, which increases the success for the retention of the scent marks.
Scent-marking behavior in Asiatic lions appears to be more energy-intensive compared with African lions, as previous studies indicate that Asiatic lions allocate more time to marking and patrolling than to feeding or hunting (Jhala et al., 2009). Territorial males, in particular, dedicate a substantial proportion of their active time to patrolling and marking activities. For example, radio-collared males were reported to spend 63% of their active time patrolling, with scent-marking events lasting an average of 2.13 ± 0.9 (SD) hours (Jhala et al., 2009). Although females engage less frequently in scent-marking, their activities often overlap temporally with those of males, suggesting that female scent marking is closely tied to broader patterns of territorial defense and reproduction.
In conclusion, our study provides the first quantitative analysis of scent-marking behaviors in free-ranging Asiatic lions, shedding light on their communication strategies and territorial behaviors. The temporal, behavioral, and spatial patterns identified in this study offer valuable insights into the ecological and social factors that drive scent-marking behavior. By analyzing how these behaviors are linked to reproductive and territorial contexts, we gain a deeper understanding of how Asiatic lions manage their territories and communicate with conspecifics. Future research should focus on investigating individual variations in scent-marking behaviors by integrating GPS-collaring with camera trap monitoring. This combined approach will provide more detailed insights into spatial and temporal patterns of scent marking, allowing for a better understanding of how individual lions engage in this behavior. These efforts are crucial for advancing our knowledge of this endangered species’ behavioral ecology and informing conservation strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because we conducted this study using sensor-based, automatically triggered black-flash camera traps, which cause minimal disturbance to the animals. Additionally, it is the authors’ responsibility to conduct such research to support conservation and management efforts for the species. Therefore, obtaining special permission for this work was not required.
Author contributions
MR: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AS: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. NS: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. PM: Conceptualization, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. SB: Data curation, Formal analysis, Investigation, Writing – review & editing. TD: Data curation, Formal analysis, Investigation, Writing – review & editing. LJ: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was carried out by the Wildlife Division, Sasan-Gir, Gujarat Forest Department, Government of Gujarat, and represents a significant undertaking within the purview of Wildlife Division, Sasan-Gir, Gujarat. Therefore, the research was conducted using the funding received under different heads for conducting such work.
Acknowledgments
We thank the Principal Chief Conservator of Forests & Head of Forest Forces (PCCF&HoFF), Gujarat State, for their support. We thank the foresters, guards, and wildlife trackers of Wildlife Division, Sasan-Gir, for their help in identifying scent-marking trees. We would especially like to thank Mr. Dhyanesh Pattani, Mr. Dhruv Sutaria, Mr. Hanif Sheikh, and Mr. Hamal Bloch for their continuous support in monitoring and data retrieving from the cameras. We thank Mr. Yashpal Zala for map preparation. We also thank the staff of Gir Hi-tech Monitoring Unit, Sasan-Gir, for their support in data shorting and data entry.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alberts A. C. (1992). Constraints on the design of chemical communication systems in terrestrial vertebrates. Am. Nat. 139, S62–S89. doi: 10.1086/285305
Allen J. J., Bekoff M., Crabtree R. L. (1999). An observational study of coyote (Canis latrans) scent-marking and territoriality in Yellowstone National Park. Ethol 105, 289–302. doi: 10.1046/j.1439-0310.1999.00397.x
Allen M. L., Gunther M. S., Wilmers C. C. (2017). The scent of your enemy is my friend? The acquisition of large carnivore scent by a smaller carnivore. J. Ethol. 35, 13–19. doi: 10.1007/s10164-016-0492-6
Allen M. L., Wallace C. F., Wilmers C. C. (2015b). Patterns in bobcat (Lynx rufus) scent marking and communication behaviors. J. Ethol. 33, 9–14. doi: 10.1007/s10164-014-0418-0
Allen M. L., Wittmer H. U., Houghtaling P., Smith J., Elbroch L. M., Wilmers C. C. (2015a). The role of scent marking in mate selection by female pumas (Puma concolor). PloS One 10, e0139087. doi: 10.1371/journal.pone.0139087
Allen M. L., Wittmer H. U., Setiawan E., Jaffe S., Marshall A. J. (2016). Scent marking in Sunda clouded leopards (Neofelis diardi): novel observations close a key gap in understanding felid communication behaviours. Sci. Rep. 6, 35433. doi: 10.1038/srep35433
Allen M. L., Wittmer H. U., Wilmers C. C. (2014). Puma communication behaviours: understanding functional use and variation among sex and age classes. Behav 151, 819–840. doi: 10.1163/1568539X-00003173
Andersen K. F. (1998). Chemocommunication and social behaviour in three Panthera species in captivity, with particular reference to the lion, P. leo. (Doctoral dissertation, University of Cambridge, United Kingdom). doi: 10.17863/CAM.16416
Andersen K. F., Vulpius T. (1999). Urinary volatile constituents of the lion, Panthera leo. Chem. Senses. 24, 179–189. doi: 10.1093/chemse/24.2.179
Apps P. J. (2013). Are mammal olfactory signals hiding right under our noses? Naturwiss 100, 487–506. doi: 10.1007/s00114-013-1054-1
Asa C. S., Mech L. D., Seal U. S. (1985). The use of urine, faeces, and anal-gland secretions in scent-marking by a captive wolf (Canis lupus) pack. Anim. Behav. 33(3), 1034–1036. doi: 10.1016/S0003-3472(85)80043-9
Banerjee K., Jhala Y. V. (2012). Demographic parameters of endangered Asiatic lions (Panthera leo persica) in Gir Forests, India. J. Mamm. 93, 1420–1430. doi: 10.1644/11-MAMM-A-231.1
Barja I., de Miguel F. J. (2010). Chemical communication in large carnivores: urine-marking frequencies in captive tigers and lions. Pol. J. Ecol. 58, 397–400.
Barja I., de Miguel F. J., Bárcena F. (2004). The importance of crossroads in faecal marking behaviour of the wolves (Canis lupus). Naturwiss 91, 489–492. doi: 10.1007/s00114-004-0557-1
Barja I., Miguel F. J., Barcena F. (2005). Faecal marking behaviour of Iberian wolf in different zones of their territory. Folia Zool. 54, 21.
Bartoń K. (2013). Model selection and model averaging based on information criteria (AICc and alike). Compr. R Arch. Netw. 1, 13.
Basu P. (2013). Assessment of landscape pattern for modelling habitat suitability for lions and prey species in gir protected area, Gujarat. (Doctoral Dissertation, Forest Research Institute, Dehradun, India).
Bates D., Maechler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Bothma J. D. P., le Richet E. A. N. (1995). Evidence of the use of rubbing, scent-marking and scratching-posts by Kalahari leopards. J. Arid. Environ. 29, 511–517. doi: 10.1016/S0140-1963(95)80023-9
Bradbury J., Vehrencamp S. (2011). Principles of animal communication, 2nd edn. (Sunderland, MA: Sinauer Associates).
Brahmachary R. L., Singh M. (2000). Behavioral and chemical aspects of scent marking in the Asiatic lion. Curr. Sci. 78, 680–682. Available at: https://www.jstor.org/stable/24103880 (Assessed October 15, 2024).
Brahmachary R. L., Singh M., Rajput M. (1999). Scent marking in the asiatic lion. Curr. Sci. 76, 480–481.
Burnham K. P., Anderson D. R. (2002). Model Selection and Multi- Model Inference: A Practical Information-Theoretic Approach. 2nd ed. (New York: Springer-Verlag), 150.
Caravaggi A., Banks P. B., Burton A. C., Finlay C. M., Haswell P. M., Hayward M. W., et al. (2017). A review of camera trapping for conservation behavior research. Remote Sens. Ecol. Conserv. 3, 109–122. doi: 10.1002/rse2.48
Chakrabarti S., Jhala Y. V. (2017). Selfish partners: resource partitioning in male coalitions of Asiatic lions. Behav. Ecol. 28, 1532–1539. doi: 10.1093/beheco/arx118
Chakrabarti S., Jhala Y. V. (2019). Battle of the sexes: a multi-male mating strategy helps lionesses win the gender war of fitness. Behav. Ecol. 30, 1050–1061. doi: 10.1093/beheco/arz048
Chaudhary R., Zehra N., Musavi A., Khan J. A. (2020). Spatio-temporal partitioning and coexistence between leopard (Panthera pardus fusca) and Asiatic lion (Panthera leo persica) in Gir protected area, Gujarat, India. PloS One 15, e0229045. doi: 10.1371/journal
Clapham M., Nevin O. T., Ramsey A. D., Rosell F. (2013). The function of strategic tree selectivity in the chemical signalling of brown bears. Anim. Behav. 85, 1351–1357. doi: 10.1016/j.anbehav.2013.03.026
Eisenberg J. F., Kleiman D. G. (1972). Olfactory communication in mammals. Annu. Rev. Ecol. Evol. Syst. 3, 1–32. doi: 10.1146/annurev.es.03.110172.000245
Fahrig L., Rytwinski T. (2009). Effects of roads on animal abundance: an empirical review and synthesis. Ecol. Soc 14 (1), 21. Available at: www.ecologyandsociety.org/vol14/iss1/art21/ (Accessed October 15, 2024).
Frey S., Fisher J. T., Burton A. C., Volpe J. P. (2017). Investigating animal activity patterns and temporal niche partitioning using camera-trap data: challenges and opportunities. Remote Sens. Ecol. Conserv. 3, 123–132. doi: 10.1002/rse2.60
Gilfillan G. (2017). An investigation of the olfactory and multi-modal communication of African lions (Panthera leo) in the Okavango Delta, Botswana.. (Doctoral dissertation, University of Sussex, Great Britain)
Gorman M. L., Trowbridge B. J. (1989). “The role of odor in the social lives of carnivores,” in Carnivore behavior, ecology, and evolution (Springer US, Boston, MA), 57–88.
Gosling L. M. (1982). A reassessment of the function of scent marking in territories. Z. Tierpsychol. 60, 89–118. doi: 10.1111/j.1439-0310.1982.tb00492.x
Gosling L. M., Roberts S. C. (2001). Scent-marking by male mammals: cheat-proof signals to competitors and mates. Adv. Study Behav. 30, 169–217. doi: 10.1016/S0065-3454(01)80007-3
Gosling L. M., Roberts S. C., Thornton E. A., Andrew M. J. (2000). Life history costs of olfactory status signalling in mice. Behav. Ecol. Sociobiol. 48, 328e332. doi: 10.1007/s002650000242
GRASS Development Team (2018). Geographic Resources Analysis Support System (GRASS) Software, Version 7.4.2 (Open Source Geospatial Foundation). Available online at: https://grass.osgeo.org/news/2018_10_22_grass_gis_7_4_2_released/ (Accessed 01 May 2024). Electronic document.
Harmsen B. J., Sanchez E. M. M. A., Foster R. J. (2016). Differential marking behavior by sympatric felids in a Neotropical Forest. Cat News 64, 8–12.
Jhala Y. V., Chellam R., Pathak B., Meena V., Banerjee K., Basu P. (2009). Social Organization and Dispersal of Asiatic Lions. Technical Report. Wildlife Institute of India, Dehra Dun, India.
Johnston R. E. (2005). “Communication by mosaic signals: individual recognition and underlying neural mechanisms”. In Chemical Signals in Vertebrates 10. Eds. Mason R. T., LeMaster M. P., Müller- Schwarze D. (Springer, New York, New York), 269–282.
Jordan N. R., Golabek K. A., Apps P. J., Gilfillan G. D., McNutt J. W. (2013). Scent-mark identification and scent-marking behavior in african wild dogs (Lycaon pictus). Ethol 119, 644–652. doi: 10.1111/eth.12105
Joslin P. (1973). The Asiatic lion: a study of ecology and behavior. (Doctoral Dissertation, University of Edinburgh, Edinburgh, United Kingdom).
Kavitha A., Deepthi N., Ganesan R., Joseph G. J. (2012). Common dryland trees of Karnataka: bilingual field guide. (Bangalore: Ashoka Trust for Research in Ecology and the Environment).
King T. W., Salom-Pérez R., Shipley L. A., Quigley H. B., Thornton D. H. (2017). Ocelot latrines: communication centers for Neotropical mammals. J. Mammal. 98, 106–113. doi: 10.1093/jmammal/gyw174
Kovach W. L. (2011). Oriana – circular statistics for Windows, ver. 4 (Wales (UK: Kovach Computing Services).
Landler L., Ruxton G. D., Malkemper. E. P. (2018). Circular data in biology: advice for effectively implementing statistical procedures. Behav. Ecol. Sociobiol. 72, 128. doi: 10.1007/s00265-018-2538-y
Li J., Schaller G. B., McCarthy T. M., Wang D., Jiagong Z., Cai P., et al. (2013). A communal sign post of snow leopards (Panthera uncia) and other species on the Tibetan Plateau, China. Int. J. Biodivers. 2013(1), 1–8. doi: 10.1155/2013/370905
Macdonald D. W. (1979). Some observations and field experiments on the urine marking behavior of the red fox, Vulpes L. Z. Tierpsychol. 51, 1–22. doi: 10.1111/j.1439-0310.1979.tb00667.x
Macdonald D. W. (1995). European mammals. Evolution and behavior (London, United Kingdom: Harper Collins Publisher).
Marnewick K. A., Bothma J. D. P., Verdoorn G. H. (2006). Using camera-trapping to investigate the use of a tree as a scent-marking post by cheetahs in the Thabazimbi district. S. Afr. J. Wildl. Res. 36, 139–145.
Meena V. (2009). Variation in social organisation of lions with particular reference to the Asiatic Lions Panthera leo persica (Carnivora: Felidae) of the Gir forest, India. J. Threat. Taxa. (1), 158–165. doi: 10.11609/JoTT.o2095.158-65
Meena V. (2010). Unique mating behavior of Asiatic lion (Panthera leo persica) in the Gir Forest, Gujarat. Curr. Sci. 281.
Mohorović M., Krofel M. (2020). The scent world of cats: where to place a urine scent mark to increase signal persistence? Anim. Biol. 71, 151–168. doi: 10.1163/15707563-bja10018
NASA LP DAAC (2018). Terra ASTER Global Digital Elevation Model. Version 2 (Sioux Falls, South Dakota: NASA EOSDIS Land Processes DAAC, USGS Earth Resources Observation and Science (EROS) Center). Available online at: https://lpdaac.usgs.gov (Accessed 01 May 2024).
Nie Y., Swaisgood R. R., Zhang Z., Hu Y., Ma Y., Wei F. (2012). Giant panda scent-marking strategies in the wild: role of season, sex and marking surface. Anim. Behav. 84, 39–44. doi: 10.1016/j.anbehav.2012.03.026
Pageat P., Gaultier E. (2003). Current research in canine and feline pheromones. Veterinary Clinics. Small Anim. Pract. 33, 187–211. doi: 10.1016/S0195-5616(02)00128-6
Palomares F., González-Borrajo N., Chávez C., Rubio Y., Verdade L. M., Monsa R. (2018). Scraping marking behavior of the largest Neotropical felids. PeerJ 6, e4983. doi: 10.7717/peerj.4983
Peters R., Mech L. D. (1978). “Scent-marking in wolves,” in Wolf and Man (Cambridge, MA: Academic Press), 133–147.
Poddar-Sarkar M., Chakroborty A., Bhar R., Brahmachary R. L. (2008). “Putative pheromones of lion mane and its ultrastructure,” in Chemical Signals in Vertebrates 11. Eds. Hurst J., Beynon R. J., Roberts S. C., Wyatt T. (Springer New York, New York), 61–67.
Ram M., Sahu A., Srivastava N., Chaudhary R., Jhala L. (2023a). Diet composition of Asiatic lions in protected areas and multi-use land matrix. J. Vertebr. Biol. 72, 1–9. doi: 10.25225/jvb.22065
Ram M., Vasavada D., Tikadar S., Jhala L., Zala Y. (2023b). Population status and distribution of endangered Asiatic lions in Gujarat, India. European. J. Wildl. Res. 69, 87. doi: 10.1007/s10344-023-01720-z
Ram M., Vasavada D., Tikadar S., Jhala L. S., Zala Y., Meena V. (2023c). Movement and activity of endangered Asiatic lions in relation to land-use, season and group characteristics. J. Zool. 319, 23–31. doi: 10.1111/jzo.13025
R Development Core Team (2021). R: a Language and Environment of Statistical Computing. Version 4.1.2. R foundation for Statistical Computing, Vienna, Austria.
Ridout M. S., Linkie M. (2009). Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biolog. Environ. Stat. 14, 322–337. doi: 10.1198/jabes.2009.08038
Rodgers W. A., Panwar S. H. (1988). Biogeographical classification of India (New Forest, Dehra Dun, India).
Ruiz-Olmo J., Such-Sanz A., Piñol C. (2013). Substrate selection for urine spraying in captive wildcats. J. Zool. 290, 143–150. doi: 10.1111/jzo.12025
Schaller G. B. (1972). The Serengeti lion: a study of predator-prey relations (University of Chicago Press, Chicago, Illinois, USA).
Schaller G. B., Jinchu H., Wenshi P., Jing Z. (1985). The Giant Pandas of Wolong (Chicago, Illinois, USA: University of Chicago Press).
Schmid F., Schmidt A. (2006). Nonparametric estimation of the coefficient of overlapping—theory and empirical application. Computational statistics & data analysis 50 (6), 1583–1596. (Accessed October 15, 2024)
Seidensticker J. C., Hornocker M. G., Wiles W. V., Messick J. P. (1973). Mountain lion social organization in the Idaho Primitive Area. Wildl. Monogr. 35), 3–60. Available at: https://www.jstor.org/stable/3830509.
Smith J. L. D., McDougal C., Miquelle D. (1989). Scent marking in free-ranging tigers, Panthera tigris. Anim. Behav. 37, 1–10. doi: 10.1016/0003-3472(89)90001-8
Stępniak K. M., Niedźwiecka N., Szewczyk M., Mysłajek R. W. (2020). Scent marking in wolves Canis lupus inhabiting managed lowland forests in Poland. Mamm. Res. 65, 629–638. doi: 10.1007/s13364-020-00514-x
Stanton L. A., Sullivan M. S., Fazio J. M. (2015). A standardized ethogram for the felidae: A tool for behavioral researchers. App. Anim. Behav. Sci. 173, 3–16. doi: 10.1016/j.applanim.2015.04.001
Sunquist M. E. (1981). The social organization of tigers (Panthera tigris) in Royal Chitawan National Park, Nepal. Smithson. Contrib. Zool. 336.
Tasdan F., Yeniay O. (2014). Power study of circular anova test against nonparametric alternatives. Hacet. J. Math. Stat. 43, 97–115.
Vasavada D. T., Rana V. J., Ram M. (2022). Management Plan for Gir Protected Area Vol. 2 (Gujarat Forest Department, Gujarat, India).
Zub K., Theuerkauf J., Jędrzejewski W., Jędrzejewska B., Schmidt K., Kowalczyk R. (2003). Wolf pack territory marking in the Białowieża Primeval Forest (Poland). Behav 140, 635–648. Available at: https://www.jstor.org/stable/4536049 (Accessed October 15, 2024).
Keywords: Asiatic lion, behavior, camera traps, communication, scent-marking, Gir
Citation: Ram M, Sahu A, Srivastava N, Mahajan P, Baraiya S, Dagur T and Jhala L (2025) Decoding scent-marking behavior of Asiatic lions in Gir Forest, Gujarat, India. Front. Ecol. Evol. 13:1523653. doi: 10.3389/fevo.2025.1523653
Received: 06 November 2024; Accepted: 10 February 2025;
Published: 23 April 2025.
Edited by:
Alejandro Cantarero, Complutense University of Madrid, SpainReviewed by:
Inger Suzanne Prange, Appalachian Wildlife Research Institute, United StatesGalina Nazarova, Institute of Systematics and Ecology of Animals (RAS), Russia
Anindita Bhadra, Indian Institute of Science Education and Research Kolkata, India
Copyright © 2025 Ram, Sahu, Srivastava, Mahajan, Baraiya, Dagur and Jhala. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohan Ram, bXJsZWdoYUBnbWFpbC5jb20=
 Mohan Ram
Mohan Ram Aradhana Sahu2
Aradhana Sahu2 Prashant Mahajan
Prashant Mahajan