- 1Department of Palaeobiology, Swedish Museum of Natural History, Stockholm, Sweden
- 2ISEM, Univ Montpellier, CNRS, EPHE, IRD, Montpellier, France
- 3IMBE, Aix Marseille Univ, Avignon Univ, CNRS, IRD, Marseille, France
- 4Department of Plant and Environmental Protection Sciences, Entomology Section, The University of Hawaii, Honolulu, HI, United States
- 5Department of Forest Botany, Faculty of Forestry, Istanbul University-Cerrahpaşa, Istanbul, Türkiye
- 6Forestry Studies Research Center, Istanbul University-Cerrahpaşa, Istanbul, Türkiye
Introduction: Evidence of insect herbivory on fossilized leaves is widely used to ascertain the evolution of feeding strategies, and trophic changes in response to phenomena such as climate change. However, leaves can decompose somewhat before fossilization, and the extent to which decomposition may bias estimates of insect herbivory in deep time is far from fully understood. There are many points at which evidence may become obscured as a leaf travels from its parent tree into the depositional environment where it fossilizes.
Materials & methods: Here, we compare evidence of plant–insect interactions on live leaves and in leaf litter collected directly beneath the same trees to provide an initial glimpse into the first stage at which decomposition may lead to eventual bias in paleontological studies. We measure the frequency and richness of insect damage types on the leaves of Fagaceae in four Mediterranean localities in Turkey and France.
Results & discussion: We observed variations in insect damage on litter leaves compared to those on trees, with some localities showing reduced damage richness, lower damage frequency, or both. This observation was particularly pronounced for external damage types. Galls stood out due to their relatively consistent preservation in leaf litter, suggesting their utility as a more dependable indicator for interpreting paleoecological conditions. Our study builds upon existing methods in paleoecology, highlighting their value in detecting environmental signals and advocating for further refinements to capture the ecological dynamics of the past more comprehensively.
Introduction
Paleoecological data have been used to characterize long-term ecological shifts that occur in response to climate change (e.g., Wingard et al., 2017; Buma et al., 2019; Barouillet et al., 2022). This line of inquiry has taken on increasing urgency as anthropogenic activity continues to increase pCO2. Within this context, the study of plant–insect interactions in the fossil record has become increasingly standardized since the early 2000s (Wilf and Labandeira, 1999; Wilf et al., 2001; Labandeira, 2002; Labandeira et al., 2002). This allows for the reconstruction of past trophic relationships in paleoforests. Plant–insect interactions illuminate ecological changes over timescales of thousands or millions of years (Wilf, 2008; Prevec and Bordy, 2009; Müller et al., 2017; Adroit et al., 2018, 2021, McLoughlin et al., 2021; Labandeira and Wappler, 2023), vastly exceeding the timescales that can be observed in modern habitats or in laboratory studies.
Reconstructing ancient plant–insect interactions is crucial for understanding the ecological structure and function of past ecosystems, as well as for detecting long-term biotic responses to climate fluctuations and mass extinction events. These interactions also offer a unique window into co-adaptive dynamics, especially in the absence of direct observational data. Fossil plant–insect interactions have been used to investigate ecological resilience and turnover across biogeographic regions (Adroit et al., 2018; Cenci and Horodyski, 2022; Giraldo et al., 2025), to track evolutionary dynamics of herbivore guilds through time (e.g., Labandeira, 2002; Labandeira and Wappler, 2023), and to assess the impact of environmental stressors during key transitions in Earth history (e.g., Wappler and Denk, 2011; Carvalho et al., 2014; Müller et al., 2017).
Distinct forms of insect damage on fossilized leaves underlie the study of plant–insect interactions in the fossil record, as they can often be distinguished based on their characteristic morphologies—such as circular holes, marginal excisions, or abnormal growths—each corresponding to different feeding behaviors (Labandeira et al., 2007). This allows researchers to characterize the richness and frequency of plant–insect interactions on fossil leaves and to compare these interactions among different fossil assemblages. However, despite the popularity of this approach and the insights it continues to provide, —such as documenting biotic turnover after mass extinction events (e.g., Wilf et al., 2006), revealing changes in insect feeding guilds under past climatic regimes (e.g., Schatz et al., 2017; Labandeira and Wappler, 2023), or assessing the resilience of plant–insect associations across time (e.g., Donovan et al., 2016; Adroit et al., 2020; Hazra et al., 2023)— two fundamental issues still need to be addressed regarding comparisons of herbivory on modern and fossil leaves. First is the difference in how data are collected to measure levels of modern herbivory versus herbivory on fossil leaves. Second is the possibility that the amount of herbivory observable on a given leaf may depend on whether the leaf is still alive, is in the process of transport to a depositional environment or has been preserved as a fossil.
In modern ecology, researchers use diverse methods including direct observation, experimentation (e.g., Grodzinski et al., 1999; Lemoine et al., 2014, 2017), direct observation through citizen science (Castagneyrol et al., 2020) and modeling (e.g., Braga et al., 2021), to disentangle the complexities of trophic interactions in current ecosystems. For example, some studies have collected leaves directly from trees (e.g., Yarnes and Boecklen, 2005; Leckey et al., 2014; Ximenes Pinho et al., 2017; Sohn et al., 2017), while others have quantified and qualified herbivory by studying herbivorous insects rather than directly measuring herbivory on plants (e.g., Gaston and Williams, 1996; Blanche and Ludwig, 2001; Cuevas-Reyes et al., 2003). These observations are typically made in different parts of the forest structure, either in the canopy (via direct leaf sampling) or on the ground, where fallen litter accumulates. The focus on either stratum often depends on the ecological context and biome: tropical systems favor canopy-based sampling due to their vertical complexity, while temperate and Mediterranean ecosystems often rely on litter-based methods, given easier access and more seasonal leaf fall. Additional studies have focused on herbivory on plant organs other than leaves (e.g., Onodera et al., 2014; Nakamura et al., 2021). The disparity in methodologies is further illustrated by studies of specialized insect behaviors such as galling. Different teams have collected galls from many different plant organs, such as branches (e.g., Boaventura et al., 2018; Coutinho et al., 2019; Melo Júnior et al., 2019; Knuff et al., 2019), leaves (Leite et al., 2009; Cenci and Adami-Rodrigues, 2017), flowers (Bhansali, 2012; Fazan et al., 2023) and even roots (Brown et al., 1991; Ruiz-Ferrer et al., 2018). As with herbivory, some studies have focused on the insects that produce galls rather than the galls themselves (Fernandes and Price, 1992; Price et al., 1998; Cuevas-Reyes et al., 2003; Espírito-Santo and Fernandes, 2007; Fernandes et al., 2010). These disparate forms of data are by no means problematic; researchers adjust their methodology so that their data are best-suited to the specific ecological question they hope to answer. However, problems can arise when data collected for one purpose are reanalyzed by other groups, with the aim of identifying changes in herbivory across space and time. For example when Coley and Barone (1996) contrasted herbivory levels across twenty forests, they emphasized that the use of different protocols for measuring plant–insect interactions caused a large margin of uncertainty. These uncertainties are exemplified by differences in environmental conditions between canopy and understory (i.e. light and temperature), which shape distinct herbivore communities and feeding patterns (Schön et al., 2024). In addition, sampling protocols often target different guilds (i.e. resident vs. transient herbivores), and comparisons are further complicated by the age and condition of leaves—young, actively growing in the canopy vs. senescent or degraded in the litter—while only a handful of studies have systematically compared these strata, yielding inconsistent results (e.g., Santos and Fernandes, 2021; De La Fuente et al., 2024; Schön et al., 2024).
This uncertainty is amplified further when attempting to compare herbivory among modern forests with fossil plant assemblages. Whereas modern ecological studies are able to meticulously control their sampling and measurement of living plants, fossil leaf assemblages consist of fallen material that has undergone millions of years of preservation-related uncertainties. Consequently, the extent to which plant–insect interactions observed on fossil leaves faithfully reflect the trophic structure of the original past community remains unclear. Some studies have already attempted such comparisons, particularly evaluating the ecological impact of events like the Paleocene–Eocene Thermal Maximum or the K-Pg boundary (Labandeira and Wappler, 2023), but the fidelity of the fossil signal remains debated due to the many taphonomic filters affecting preservation (Purnell et al., 2018).
Paleoecological studies rely upon a close link between fossil leaves and leaves from the leaf litter, as fossil leaf assemblages primarily consist of fallen leaves from the trees (Spicer, 1991; Ferguson, 1996). However, the insect damage seen on fossilized leaves might not be entirely representative of the insect damage that would have been observed when the leaf was alive. Many authors have stated that they only record insect damage on fossil leaves if they can state with a high level of certainty that the damage occurred while the leaf was alive (e.g., Wappler, 2010; Wappler and Denk, 2011; Carvalho et al., 2014; Adroit et al., 2018; Donovan et al., 2023). It is possible that this determination becomes increasingly fraught once a leaf is no longer on a tree, due to breakage that a leaf suffers during transport, feeding by detritivores, biodegradation, and fungal interactions that may obscure features such as reaction rims which indicate that a leaf was damaged while it was still alive (Labandeira, 2002). These two stages—herbivory on live foliage and post-abscission alteration—reflect distinct ecological processes. The former informs trophic interactions and coevolution in past ecosystems, while the latter involves detritivore activity and taphonomic modification. This distinction highlights the importance of carefully classifying fossil damage, often relying on features such as reaction rims. Moreover, the dominance-diversity structure of a plant community often varies markedly between the living forest and the allochthonous fossil assemblage in which it was preserved (Burnham et al., 1992; Burnham, 1993; Steart et al., 2002; Ricardi-Branco et al., 2009).
The present study is the first to test whether the signals of herbivory observed on living leaves are faithfully retained in the surrounding leaf litter — the initial stage of the fossilization pathway. This question is central to evaluating the reliability of paleoecological inferences based on fossil leaves. When contemplating the extent to which fossil leaves reflect plant–insect interactions in the forests they represent, the first step is to compare living leaves with the surrounding litter. Although previous studies have investigated whether the richness or diversity of trees in a forest is accurately reflected in the litter layer (Burnham, 1989, 1994, Burnham et al., 1992, 2005, Steart et al., 2006) no study has addressed whether herbivory patterns are similarly retained. To fill this gap, our study focuses on Fagaceae (Quercus and Fagus spp.) across Mediterranean localities. Specifically, we seek to:
1. Quantify differences in herbivory between live and litter leaves, to assess the potential taphonomic bias introduced during early decomposition;
2. Identify which functional feeding groups (FFGs) are underrepresented in litter leaves relative to live ones;
3. Assess the spatial consistency of interaction patterns within and among localities;
4. Explore how environmental variables correlate with herbivory patterns, and whether these relationships persist in litter-derived data.
Material and methods
Study locations
The study was carried out at four distinct localities, located within the Mediterranean basin, and chosen for their comparable Mediterranean climate. Each locality included four replicate sites, labelled Alpha, Beta, Gamma, and Delta situated within a radius of approximately 2–3 km. These sites were considered replicates for each locality. All study localities have similar Mediterranean climate conditions (Peel et al., 2007) characterized by hot, and dry summers and relatively mild, and wet winters (Supplementary Figure S1). Our collection method follows the criteria outlined by Kozlov et al. (2015), which state that the area covered by natural vegetation should be at least 1 ha in size, with no highway within 100 m and no large industrial enterprise within 2 km of the selected location.
Three of the study localities are in the province of Bursa in northwestern Turkey, which was further divided into three districts: Karacabey, Inegöl and Orhaneli. These localities have been maintained with the support of the General Directorate of Forestry of Turkey (Orman Genel Müdürlüğü, OGM), which has been responsible for the management and preservation of natural forests in the country since 1839, ensuring their sustainability and natural state. All three Turkish localities are characterized by leptosols, which are stony or rocky soils (Kük and Burgess, 2010). The plant species sampled were Quercus petraea (Matt.) Liebl., Q. cerris L., Q. frainetto Ten. and Fagus orientalis Lipsky, all belonging to Fagaceae (Table 1). In addition to our quantitative analysis, we also conducted a qualitative assessment of the neighboring species in the different sites. Sampling on the three Turkish localities was done during the 18th, 19th and 20th of October 2022.
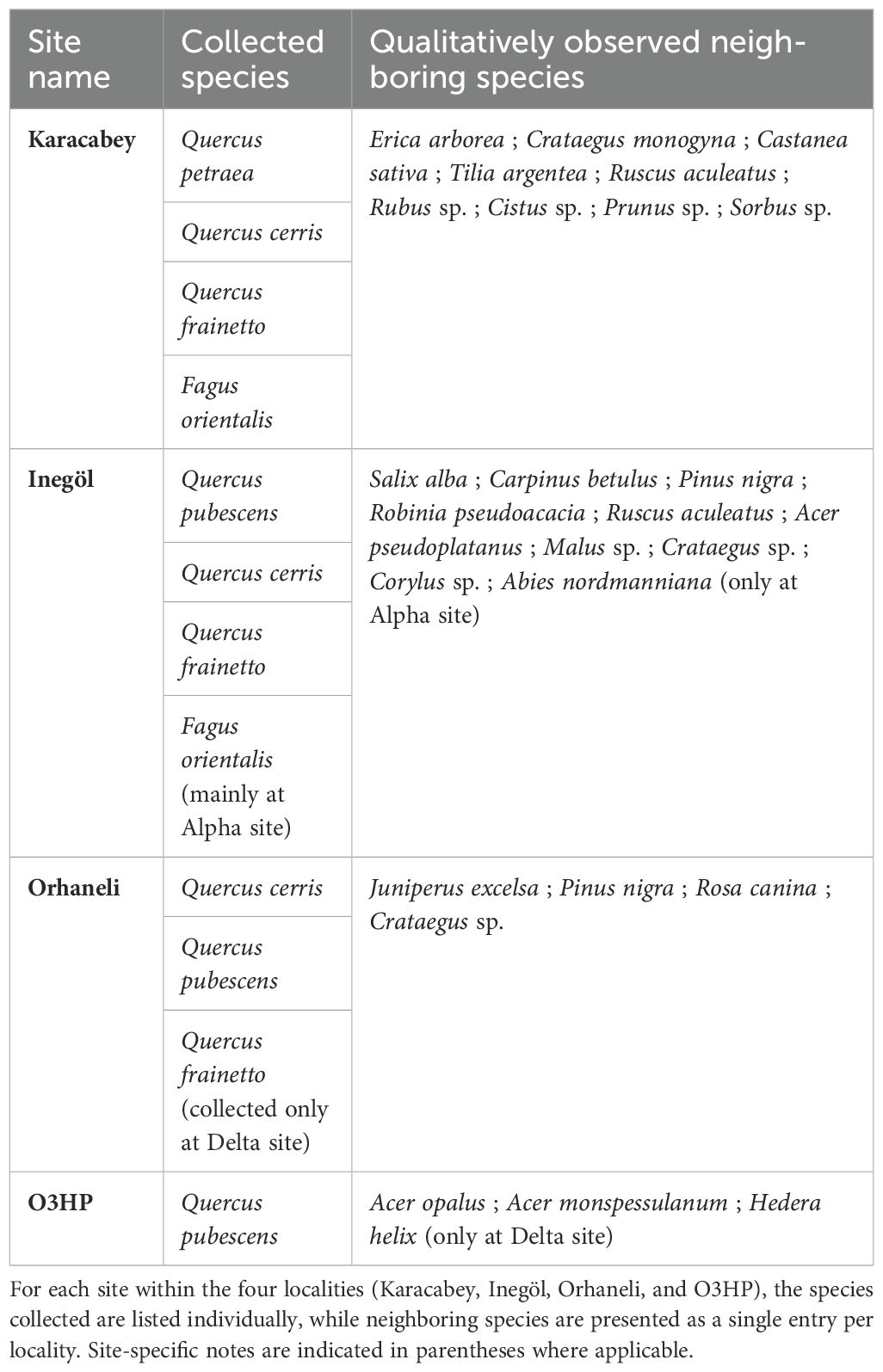
Table 1. Overview of plant species collected for herbivory analysis and qualitatively observed neighboring woody species at each study locality.
The locality in Karacabey district (Figure 1) was the closest to the sea and had a homogenous altitude of approximately 400 m asl. Although the area is mostly known for its floodplain forest, our sampling was carried out in the hills, which made the soil characteristics comparable to the other localities For Karacabey, our observations revealed a relatively consistent presence of several tree and shrub species across the four replicate sites, including Erica arborea L. (Ericaceae), Crataegus monogyna Jacq. (Rosaceae), Castanea sativa Mill. (Fagaceae), Tilia argentea Desf. (Malvaceae), Ruscus aculeatus L. (Asparagaceae), Rubus sp. (Rosaceae), Cistus sp. (Cistaceae), Prunus sp. (Rosaceae), and Sorbus sp. (Rosaceae).
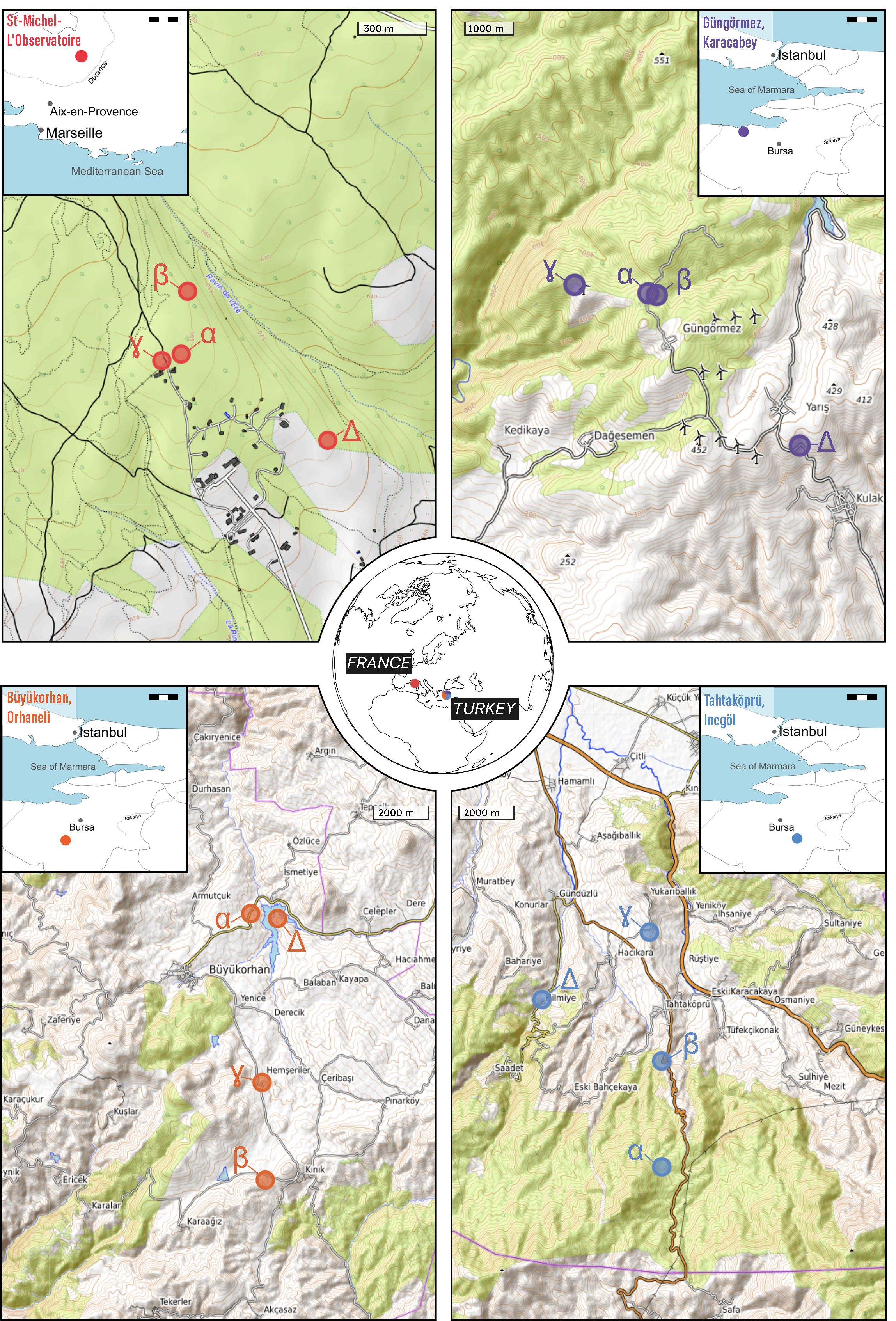
Figure 1. Geographic overview of the four study localities and their respective replicates (i.e. sites), represented by Greek letters α (Alpha), β (Beta), γ (Gamma), and Δ (Delta). The maps detail the locations of Saint-Michel-l’Observatoire, corresponding to the O3HP locality; the village of Gungörmez for the Karacabey locality; the district center of Büyükorhan in the province of Bursa for the Orhaneli locality; and the district of Tahtaköprü in the city of Inegöl for the Inegöl locality. Each map highlights the precise sites where extensive field research and data collection were conducted as part of this study, providing a spatial context for the observed plant–insect interactions within the Mediterranean basin.
The locality in Inegöl district (Figure 1) contains the most heterogenous study sites in terms of altitude, with sampled elevations ranging from approximately 500 m a.s.l. (site Delta) to ca. 1200 m asl (site Alpha). This locality is a sub-temperate alluvial forest, with Alpha predominantly occupied by F. orientalis, and the other sites dominated by Quercus pubescens Willd, Q. cerris and Q. frainetto. Additional neighboring woody plant species observed include Salix alba L. (Salicaceae), Abies nordmanniana ssp. equi-trojani (Steven) Spach (Pinaceae) at the higher-altitude Alpha site, and Carpinus betulus L. (Betulaceae), Pinus nigra Arnold (Pinaceae), Robinia pseudoacacia L. (Fabaceae), R. aculeatus (Asparagaceae), Acer pseudoplatanus L. (Sapindaceae), Malus sp. (Rosaceae), Crataegus sp. (Rosaceae), and Corylus sp. (Betulaceae) at the other sites, which are at lower altitudes.
The locality in the Orhaneli district (Figure 1) is situated in the hinterland and characterized by a consistent altitude of approximately 600 m a.s.l. The dominant species at this location are Q. cerris and Q. pubescens, while Q. frainetto was largely absent throughout the locality, occurring only at the Delta site. We observed the same neighboring plant species in the four sites such as, Juniperus excelsa M.Bieb. (Cupressaceae), Pinus nigra (Pinaceae), Rosa canina L. (Rosaceae), and Crataegus sp. (Rosaceae).
The final study locality is in the southeast of France, at Saint-Michel-L’Observatoire in the department of Alpes-de-Haute-Provence and is labeled as “O3HP” related to the abbreviation of an experimental study called Oak Observatory at Observatoire Haute Provence (Figure 1). Sampling was done the 9th of September. This locality is dominated by Q. pubescens, which is characteristic of the sub-Mediterranean bioclimate (Damesin and Rambal, 1995). Although O3HP is classified as sub-Mediterranean, its climatic parameters—particularly in terms of temperature and precipitation—fall within the range of the other Mediterranean sites, justifying its inclusion in the study. The sites at O3HP exhibit a certain heterogeneity in stand structure, with a mix of younger coppices and older Quercus individuals, and our sampling provides a representative picture of this variability. Acer opalus Mill. and A. monspessulanum L. (Sapindaceae) are the predominant neighboring species, except for the Delta site, where Hedera helix L. (Araliaceae) is also common. The ecological stages of the sites vary significantly, with Alpha and Beta sites predominantly characterized by middle-aged oak coppices, while Gamma site has a very young oak coppice with smaller, even juvenile trees. In contrast, the Delta site has a very old oak stand with considerably taller trees, which also explains the presence of the liana H. helix in this site. This forest has not been subject to logging since the end of World War II, and is considered a ‘subnatural forest’ (Gauquelin et al., 2011), providing a well-characterized historical context suitable for our comparative approach. Together, the four sites constitute one locality. A detailed breakdown of sampled species and co-occurring neighboring species across sites and localities is available in Table 1.
Leaf sampling and metrics
Our leaf collection strategy sampled four replicate sites within each study locality to provide a comprehensive representation of local vegetation and to account for potential heterogeneity within the locality. Differences in topography and accessibility sometimes led to variation in the distances between replicates; however, to minimize heterogeneity in vegetation type and structure, we consistently selected trees belonging to similar strata and canopy positions, within areas exhibiting comparable plant community composition and density. In the Turkish localities, replicates were generally spaced within a 2–3 km radius, while at the French site (O3HP), replicates were situated closer together, within a 1–2 km radius (Figure 1).
Once a first site was defined, a 1-meter square was drawn on the ground, and two to three handfuls of leaves were randomly collected within this square to fill a standard-sized envelope (110 mm x 220 mm). This constituted the sampling of litter leaves from one site within the locality.
From this central point, 5 trees per species sampled were selected from which leaves were collected. To obtain a representative sample from each tree, leaves were collected from the bottom (referred to as “bottom” leaves), middle (referred to as “mid” leaves), and as high as possible (referred to as “top” leaves). These strata were defined using standardized collection procedures involving telescopic pruners and consistent height approximations across sites. Full methodological details, including height estimates and collection tools, are provided in the Supporting Material (Supplementary Figure S2).
Each group of bottom, mid, and top leaves were stored in standard-sized envelopes. For each group of leaves, three random branches were taken for sampling. This sampling protocol was repeated for each sampled species at each site.
In total, for each site, one envelope was filled with litter leaves, and for each sampled species, three envelopes contained bottom, mid, and top leaves. Within each study locality, this protocol was applied across several nearby sub-sites (replicates), ensuring spatial coverage while maintaining comparable vegetation structure.
This sampling strategy resulted in a total of over 4000 leaves collected for this study. Specifically, 1624 leaves were collected in O3HP, 761 in Karacabey, 722 in Inegöl, and 969 in Orhaneli. The variation in leaf counts across sites primarily reflects our method of collecting a specific number of branches rather than leaves, influenced by species diversity and leaf size differences, such as the smaller leaves of Q. pubescens compared to Q. frainetto. This difference in litter degradation could influence the detectability of insect damage, with better-preserved litter at drier sites (e.g., Saint-Michel-L’Observatoire) potentially retaining more visible interaction traces. Conversely, more advanced degradation at Turkish sites might result in the loss of subtle damage types, introducing variability in damage frequency estimates between localities. We aimed to collect approximately 60 leaves per tree, ensuring an even distribution among the bottom, middle, and top canopy layers. The exact number could vary slightly depending on foliage density and accessibility, but this target was generally maintained across sampled trees. No sampling guidelines have yet been proposed for sampling leaves for insect damage, but this sampling strategy exceeds the guidelines suggested by Desmond et al. (2021), who found that 10 to 11 leaves per tree is sufficient for detecting meaningful amounts of leaf morphological variation.
Six environmental variables used in the distance-based redundancy analysis were latitude, longitude, minimum and maximum monthly temperature, and minimum and maximum monthly precipitation. Environmental data were derived from nearby meteorological stations and ERA5-Land climate reanalysis datasets for the years 2019–2022. Full details on data sources, processing methods, and the rationale for temporal selection are provided in the Supplementary Figure S1.
Identification of plant–insect interactions
Once the fieldwork was completed for each site—after one day at the O3HP locality and on each of the three consecutive days at the Turkish sites—we immediately preserved the collected leaf material on a daily basis (Supplementary Figure S2). We scanned all the leaves using an Epson Expression 12000XL scanner at a resolution of 2.400 DPI (ppp) x 4.800 DPI (ppp), creating images with a size of 20,640px by 14,639px. This high resolution allowed us to capture every detail of the leaves, to such an extent that the scanned images revealed more details than could be observed with the naked eye (Figure 2). The abaxial and adaxial surfaces of the leaves were scanned to enable a comprehensive analysis of plant–insect interactions and to determine whether any interactions were exclusive to one side of the leaf.
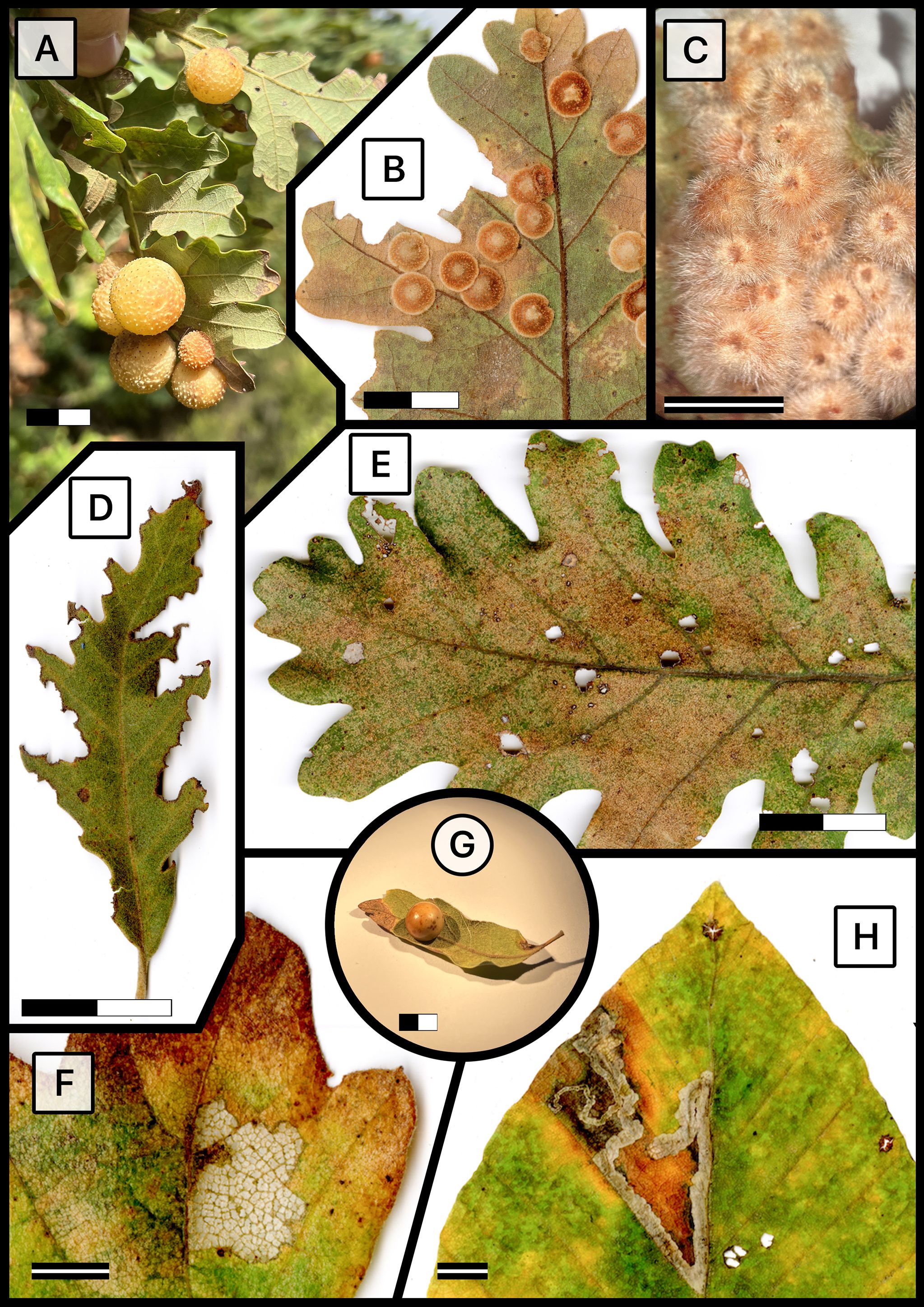
Figure 2. Representative foliar damage types on different tree species across study localities. (A) Cynips quercusfolii gall on Q. frainetto in Karacabey; (B) Neuroterus quercusbaccarum gall on Q. frainetto in Inegöl; (C) Cerroneuroterus lanuginosus gall on Quercus cerris in Orhaneli; (D) Margin Feeding on Q. pubescens in Orhaneli; (E) Hole feeding on Q. frainetto in Karacabey; (F) Skeletonization on Q. cerris in Inegöl; (G) Andricus kollari, commonly known as the Oak Marble Gall, on Q. frainetto in Karacabey; (H) Leaf mine on Fagus orientalis in Inegöl. The scales provided on the images are 2 cm for black and white bars and 0.5 cm for the striped center bars, indicating the actual size of the foliar damage depicted.
Damage types are grouped into functional feeding groups (FFGs), which correspond to what are often referred to as damage guilds. Hole feeding and margin feeding involve the removal of the entire thickness of a contiguous section of the leaf blade or margin (Johnson and Lyon, 1991; Labandeira et al., 2007). Skeletonization is consumption of the leaf blade that leaves the venation intact (Johnson and Lyon, 1991; Labandeira et al., 2007). Surface feeding involves the removal of tissue on one side of the leaf blade (Fiene et al., 2013). Piercing & sucking is performed by insects that pierce the leaf blade and then suck out fluid with their straw-like mouthparts (Garzo et al., 2020). Leaf mines are trails created by larval insects that live within the inner tissue layers of a leaf (Sinclair and Hughes, 2010). Galls are tumor-like growths that occur when heterotrophs hijack a plant’s metabolism, reprogramming plant cells to produce specialized structures that serve as both habitat and food source (Raman et al., 2005). In this study, galls were identified and recorded following the criteria described in Labandeira et al. (2007), and when applicable, based on their known morphological characteristics and literature-based identification (Supplementary Figure S3).
Statistical evaluation of plant–insect interaction in tree and litter leaves
In our study, we primarily focused on contrasting plant–insect interactions in litter leaves with those in tree leaves, assessing the richness and frequency of damage types at each site. In our analysis of plant–insect interactions, we distinguish between two key metrics: frequency, defined as the proportion of leaves affected by a given damage type or functional feeding group; and occurrence, which refers to the richness (i.e., diversity) of damage types observed on individual leaves. To compare patterns at different levels of resolution, we produced boxplots using both detailed data levels (i.e., frequencies calculated per tree and replicate) and aggregated levels (i.e., frequencies pooled across all replicates within each locality), as described in the Results section. Our aim here is not to replicate the full complexity of fossil taphonomic processes, but rather to isolate and characterize one of the very first stages in the loss of ecological signal — the transition from live foliage to leaf litter. This approach allowed us to assess how data resolution may affect interpretation of ecological patterns. To evaluate the significance of observed differences in both interaction frequency and damage richness between litter and tree leaves, we first assessed data normality using the Shapiro–Wilk test and variance equality with the Fisher test. Based on these preliminary analyses, we applied either parametric t-tests or non-parametric Welch tests for each comparison.
Our main hypothesis is that plant–insect interactions recorded in leaf litter differ significantly from those observed in living foliage. We tested this by comparing interaction frequency and richness between litter and tree leaves using exploratory and inferential statistical approaches. All analyses were conducted in R (R core Development Team) (Jupke and Schäfer, 2020). A rarefaction procedure was used to visualize DT richness at each locality, comparing leaf litter (lumped by replicate) to live leaves. Leaf litter was compared to live leaves at each of the four sites per locality (alpha, beta, delta, gamma) lumped by position on the tree, and to each position on the tree (top, middle, and bottom) lumped by replicate. Rarefaction curves were calculated by interpolation only and were not extrapolated beyond the observed sample sizes. Rarefaction analyses were performed using the iNEXT()function in the iNEXT package for R (Hsieh et al., 2022), applying both: (1) the traditional size-based method, and (2) the coverage-based approach (Chao and Jost, 2012). This framework follows recent applications to fossil herbivory datasets by various research teams (Romero-Lebron et al., 2022; Cenci and Horodyski, 2022). One set of hypothesis tests concerns pairwise differences in damage frequency among localities. For example, when we compare these metrics between Karacabey and Inegöl, do our results depend on whether the data come from live leaves or litter? To address this, we performed pairwise chi-squared tests for all four localities. Each test compared the frequency of each functional feeding group (including external foliage feeding) between two localities, using data from either tree or litter leaves. The input for each comparison consisted of a 2×2 matrix detailing the number of damaged and undamaged leaves from the respective localities.
Each pairwise comparison could result in one of the following five outcomes:
i. True positive: A significant difference between localities appears in both live leaves and litter, in the same direction.
ii. True negative: No significant difference is detected in either dataset.
iii. False negative: A significant difference is detected in live leaves but not in litter.
iv. False positive: A significant difference appears in litter but not in live leaves.
v. Opposite polarity: Significant differences are found in both datasets, but with opposing directional trends.
We were not able to conduct a similarly straightforward test when evaluating whether comparisons of damage type (DT) richness among localities yield the same result when conducted with live leaves vs. litter, because damage frequency is an unbiased estimator whereas DT richness is not (Schachat et al., 2023). This is because damage frequency is a binary measure—presence or absence of damage per leaf—and is less sensitive to sample size. In contrast, DT richness is inherently dependent on the number of leaves sampled: the more leaves examined, the more rare damage types are likely to be recorded. Therefore, comparisons of DT richness between assemblages with unequal sampling effort may reflect sampling artifacts rather than true biological differences. We conducted analyses of similarity (ANOSIM) with the anosim()function in the R package vegan (Oksanen et al., 2022) to test whether position (litter vs. tree) or geography (locality, area) is a better predictor of emergent patterns of herbivory. The percentage of leaves with evidence of each functional feeding group was calculated for live leaves and litter at each replicate of each site. To visualize the distance data that underly the ANOSIM analysis, we created a nonmetric multidimensional scaling (NMDS) plot using the vegan function metamds(). We used a Bray-Curtis disssimilarity index for NMDS and ANOSIM. Because NMDS is initialized with a random value, we repeated the analysis ten times, using the base-R function set.seed() to vary the seed from 1 to 10. Because the relationship between herbivory and climate is a major theme of research into the fossil record of plant–insect interactions, we examined whether the relationship between the four sites and climatic variables varies according to whether live leaves or litter are examined. We conducted this analysis using distance-based redundancy analysis, with the capscale() function in the vegan package and a Bray-Curtis dissimilarity index. The percentage of leaves with evidence of each functional feeding group was calculated for live leaves and litter at each replicate of each site.
Whereas NMDS is typically used in analyses of herbivory on fossil leaves (Xu et al., 2018; Santos et al., 2022; Ma et al., 2023), we also performed distance-based redundancy analysis here. We made this decision because NMDS is a form of “indirect gradient analysis,” in which environmental data can only be considered after the ordination has occurred, whereas distance-based redundancy analysis is a form of “direct gradient analysis” that includes environmental data when performing the ordination (Palmer, 2019). Our goal in comparing leaf litter to live leaves was to see whether the relationship between environmental variables and herbivory is less discernible when measured from the leaf litter, requiring a direct gradient analysis. Another advantage of distance-based redundancy analysis is that it quantifies the proportion of variance explained by the environmental variables.
Results
Lower damage type richness in litter leaves compared to tree leaves
The rarefaction curves (Figure 3) illustrate the richness of various damage types in both litter and tree leaves across our four localities. A variation in damage type richness between litter and tree leaves is noted, with the degree of difference varying across localities when considering all damage types combined (Figure 3, Supplementary Figure S4). We see the same result for external foliage feeding: at the localities of Orhaneli and O3HP, litter leaves exhibit a lower richness compared to the tree leaves, but Karacabey and Inegöl show minimal differences in the richness between the two phases of leaves (Figures 3C, D). Regarding galling, Karacabey and Orhaneli stand out with a significant decrease in richness in the litter samples, characterized by the absence of DT127, DT146, DT190, DT213, and DT217, which are present in the tree samples (Supplementary Figure S5). On the other hand, Inegöl and O3HP maintain a comparable level of richness between the litter and tree leaves (Figures 3E, F). Regarding mining, there is a consistent pattern of reduced richness in the litter compared to the tree leaves across all localities except Karacabey (Figures 3G, H). In summary, we observe a general trend of lower damage richness in litter leaves compared to tree leaves.
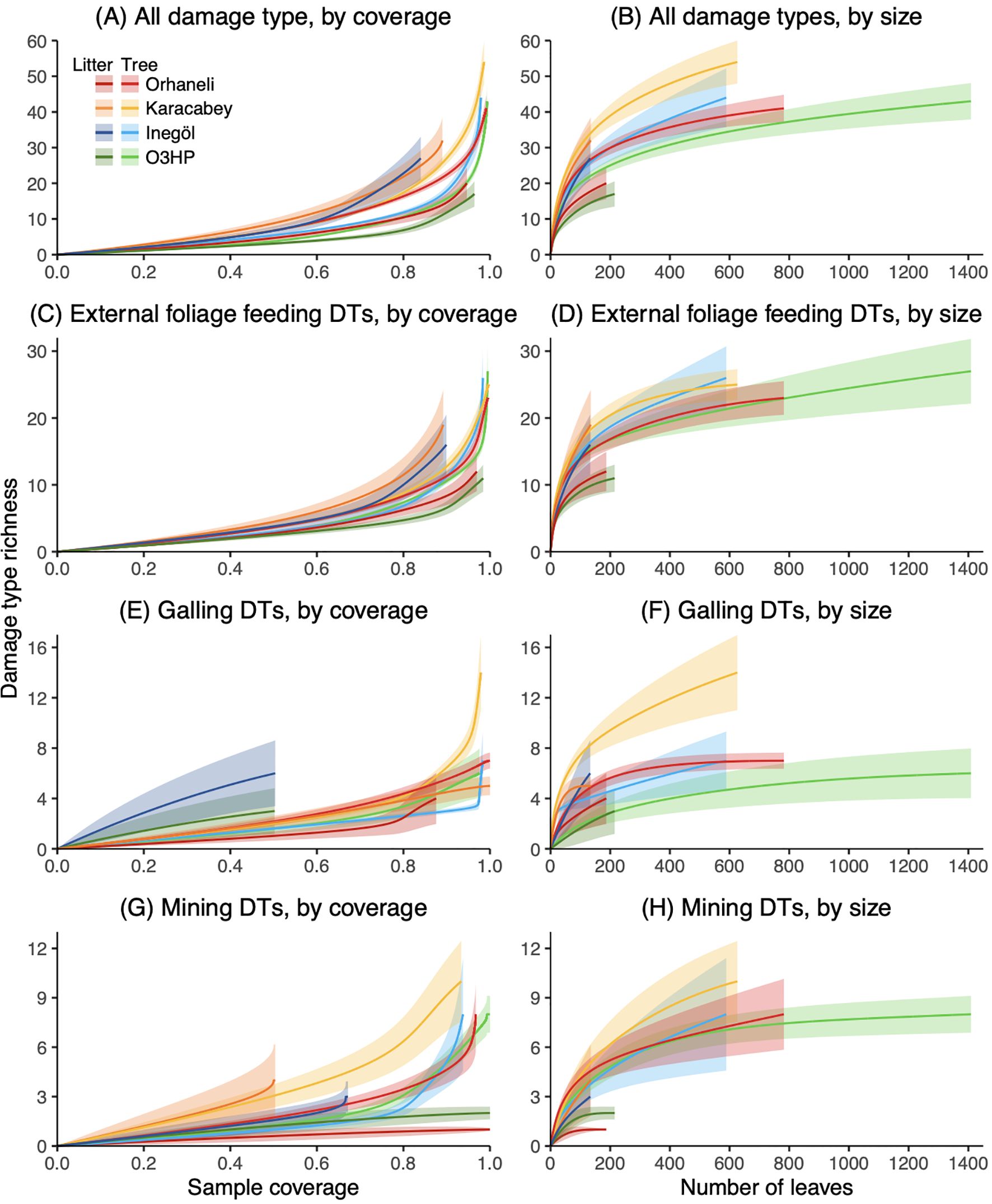
Figure 3. Size-based and coverage-based rarefaction curves comparing plant–insect interaction richness across litter (dark colors) and tree leaves (light colors) from our four Mediterranean sites: O3HP, Orhaneli, Inegöl, and Karacabey. The figure presents four sets of curves: (A, B) ‘All Damage Types’ which encompasses all interaction types; (C, D) ‘EFF Damage Types’ representing external foliar feeding such as hole feeding, margin feeding, surface feeding and skeletonization; and groups for (E, F) ‘Galling’ and (G, H) ‘Mining’ specialized damage types, respectively.
Interaction quantity: a comparative analysis of frequency and occurrence
A general trend emerges from the frequency data, indicating a higher proportion of damaged leaves on trees compared to the litter, consistent across all localities and damage types (Figure 4, Supplementary Figure S6). This finding is corroborated by t-test comparisons, revealing significant differences in all localities: Karacabey (p=0.016), Inegöl (p<0.0001), Orhaneli (p=0.010), and O3HP (p<0.0001). When viewed through the lens of occurrence data, this trend holds, illustrating a consistent underrepresentation of interactions in the litter, although with variations in the degree of difference based on the precision of occurrence data considered (Figures 4, 5). Examining the data through the lens of some specific functional feeding groups reveals that interactions involving external feeding foliage (EFF) are consistently less represented in the litter across all localities. This trend is substantiated by both frequency and occurrence data (Supplementary Figure S6). In Karacabey, a major difference observed in the frequency data, indicating higher richness of galling DTs on tree leaves compared to the litter. In the other localities (i.e., Orhaneli, Inegöl and O3HP), this trend is seen for occurrence data, which reveals far higher damage richness on tree leaves (Supplementary Figure S6). For mining, differences among tree and litter leaves again vary across locations. In Orhaneli and O3HP, both frequency and occurrence data reveal a substantial difference, indicating a higher incidence of mining damage on tree leaves. In Inegöl and partially in Karacabey, this trend is discernible only through the occurrence data, with more mining damage on tree leaves than litter (Supplementary Figure S6). Overall, both frequency and occurrence analyses highlight a pronounced underrepresentation of plant–insect interactions in the litter, with a consistent trend of higher interaction quantities recorded on tree leaves across all localities and damage types.
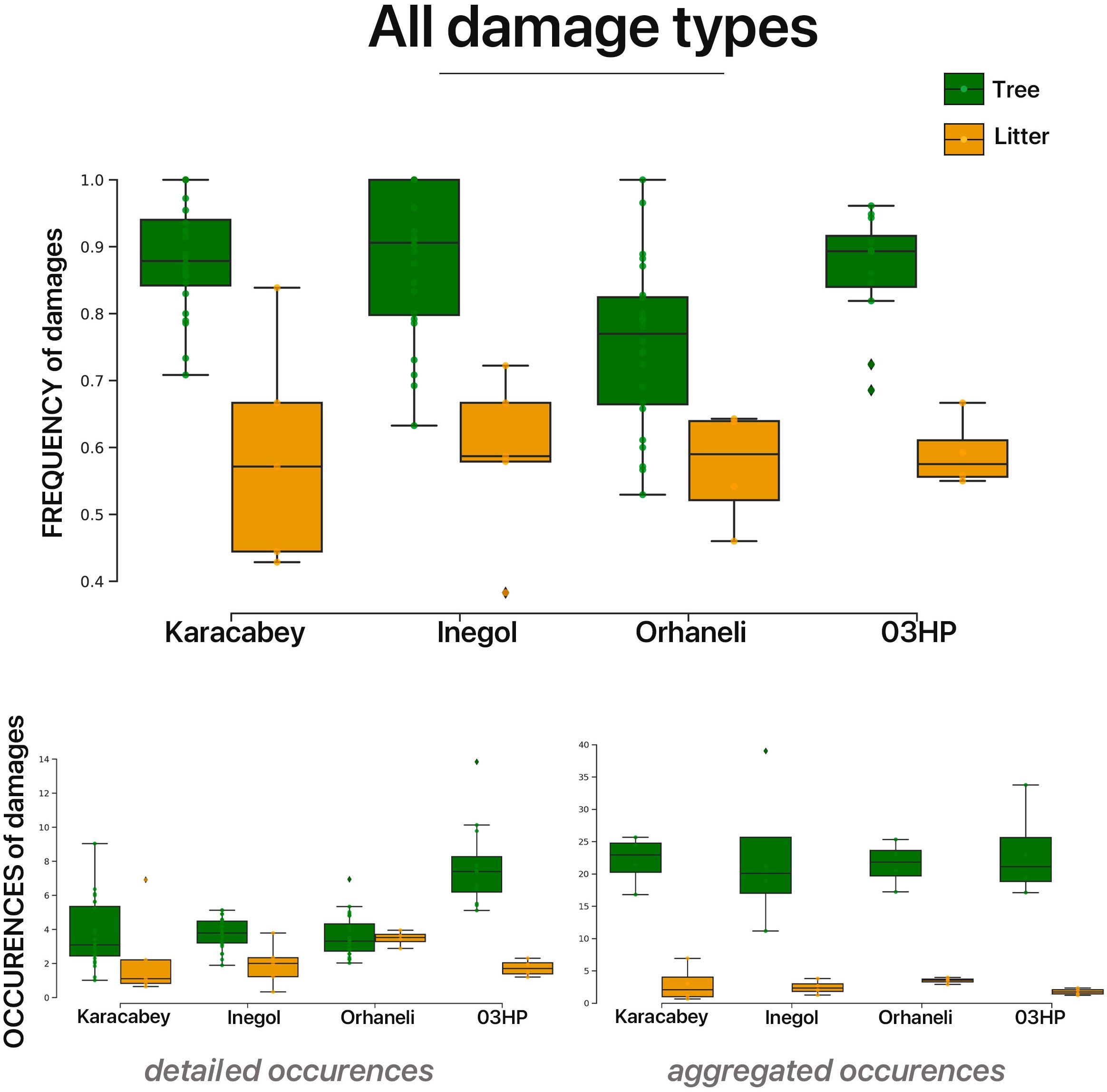
Figure 4. Boxplots compare plant–insect interaction frequencies on tree (green) and litter leaves (orange) across four Mediterranean localities: Karacabey, Inegöl, Orhaneli, and O3HP. The top panel presents interaction frequencies, while the bottom contrasts detailed occurrences with aggregated ones. Detailed occurrences account for each tree and replicate at each locality, showing a granular data view. Aggregated occurrences combine locality data without replicates, reflecting the coarse resolution typical of fossil records. This method simulates the generalized data from fossil layers, where precise replication is absent.
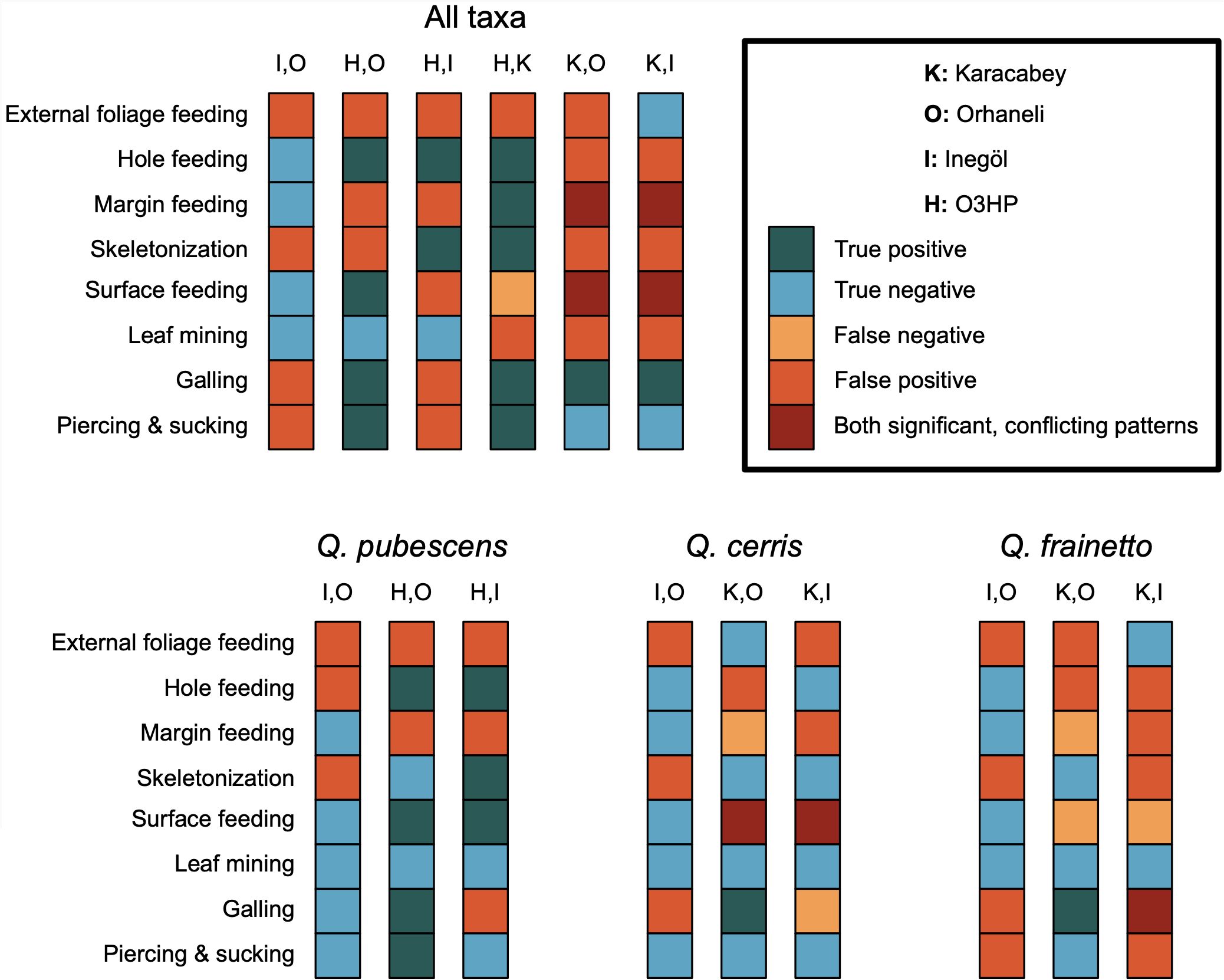
Figure 5. Statistical outcomes of pairwise comparisons for functional feeding groups across four study localities: Karacabey (K), Orhaneli (O), Inegöl (I), and O3HP (H). Each bar represents the result of a chi-squared test conducted in R, comparing the prevalence of damage in damaged versus undamaged leaves for live and litter categories. The color coding indicates five possible outcomes for each functional group: true positive (green), true negative (blue), false negative (yellow), false positive (orange), and both significant with conflicting patterns (red). These results are shown for all taxa collectively and individually for Q. pubescens, Q. cerris, and Q. frainetto, encompassing a range of damage types such as hole feeding, margin feeding, skeletonization, surface feeding, leaf mining, galling, and piercing & sucking. We did not conduct these comparisons for oviposition because too few damage types were observed.
Assessing the impact of litter versus tree leaves
Out of the 48 chi-squared tests conducted for all species, slightly fewer than half (22/48; 45.83%) returned consistent results between tree leaves and litter (Figure 5). This half discrepancy rate is notably high, especially given the close geographical proximity of the locality, their climatic similarities, and the shared plant species under study. A primary observation indicates a variable representation of external foliage feeding across the samples, specifically within the functional feeding groups of hole feeding, margin feeding, skeletonization, and surface feeding. Similarly, leaf mining displays variability in its representation. Conversely, interactions such as galling and piercing & sucking exhibit more uniform representation (Figure 5). Comparing interactions among identical species revealed fewer inconsistencies in plant–insect interactions for two of the three species for which we had sufficient data (Figure 5). When focusing exclusively on Q. pubescens, 16/24 comparisons (66.67%) yielded consistent results for litter and tree leaves, with no comparisons yielding opposing significant results. When focusing exclusively on Q. cerris, 14/24 comparisons (58.33%) yielded consistent results, with only one comparison yielding opposing significant results. However, Q. frainetto yielded consistent results for only 10/24 comparisons (41.67%). Detailed results of the chi-squared comparisons supporting Figure 5, including test statistics, degrees of freedom, and group means with standard deviations, are available in the Supplementary Material (Supplementary Figure S7).
Ordination analysis reveals distinct clustering patterns among the localities
Our first ordination, the indirect gradient method NMDS, shows noticeable separation among both tree and litter leaves, and the French and Turkish localities (Figure 6). The ten iterations of the NMDS procedure all yielded the same result (whose stress value is 0.1308). Tree leaves have more negative scores along the first NMDS axis, and samples from France have more positive scores along the second NMDS axis. The separation observed in the NMDS plot is confirmed by the ANOSIM analysis. ANOSIM revealed significant clustering by locality (R = 0.2963, p = 0.001), with even stronger clustering by substrate type (R = 0.5038, p = 0.001). Clustering by substrate type (litter vs. tree leaves) showed an even more positive R value of 0.5038, with a p-value of 0.001. The ordinations for tree and litter leaves yield broadly similar results (Figure 7). In both ordinations, herbivory at Inegöl is best explained by longitude, herbivory at Karacabey is best explained by low temperatures whereas herbivory at Orhaneli most certainly is not, and herbivory at O3HP is best explained by the remaining four predictors, especially latitude and maximum precipitation. In both ordinations, the data from France are very clearly separated from the Turkish data. The data for functional feeding groups are also consistent among the two ordinations, with the important caveat that four of the eight (oviposition, mining, surface feeding, and piercing and sucking) occur infrequently enough that they have scores close to zero in both axes for the ordination of tree leaves. In both ordinations, we see a close association between galling and Karacabey, between skeletonization and Inegöl, and between hole feeding and O3HP, with the association between margin feeding and O3HP noticeably weaker for litter leaves.
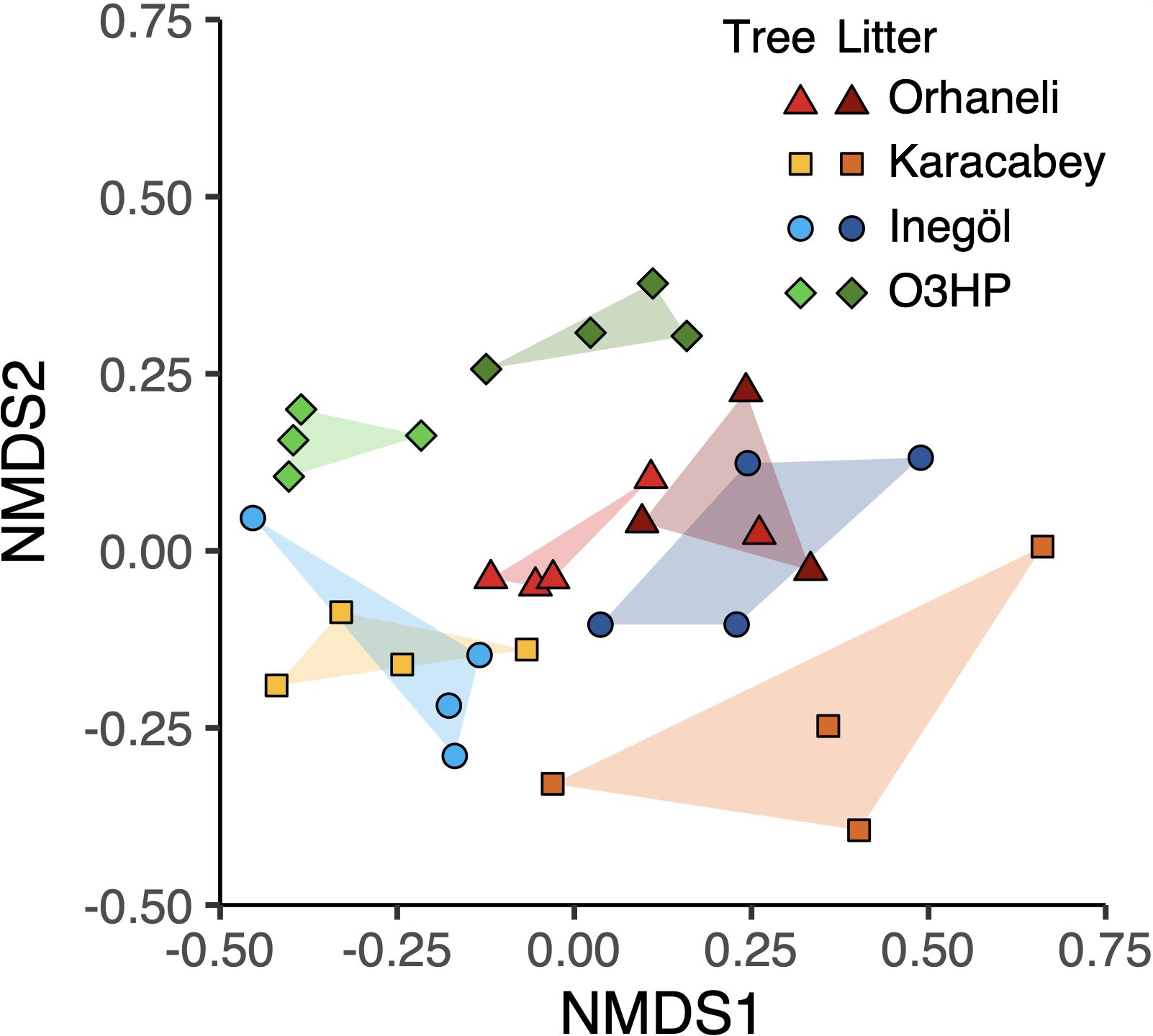
Figure 6. A non-metric multidimensional scaling ordination showing data for litter and live leaves from all four sites at each locality. Each point represents a site within a locality, and convex hulls encompass all sites within each locality for either litter or live leaves.
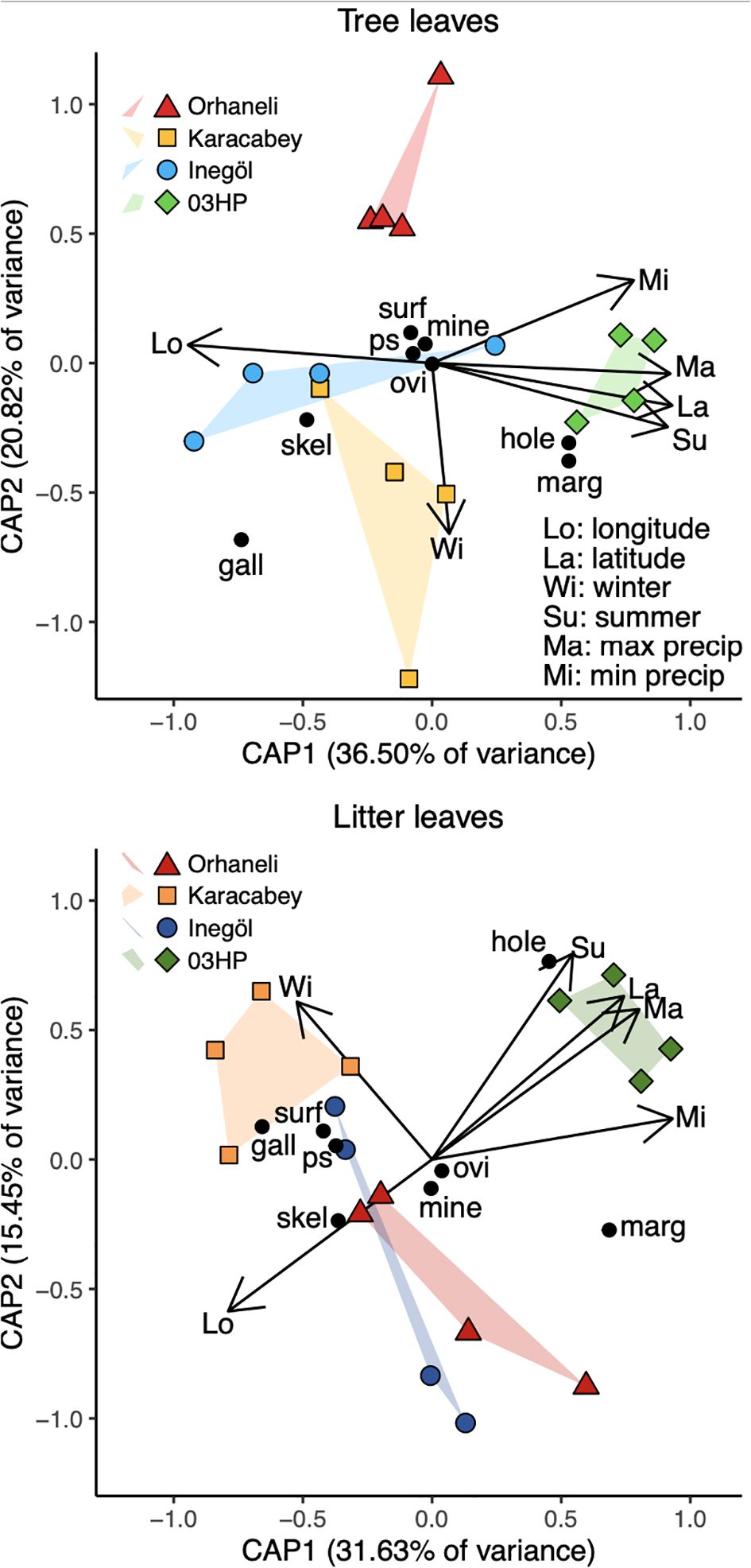
Figure 7. Distance-based redundancy analysis showing variability in herbivory that can be attributed to environmental variables. As in Figure 6, each point represents a site within a locality, and convex hulls encompass all sites within each locality for either litter or live leaves. “Winter” is minimum temperature (averaged by hour for each month), “summer” is maximum temperature (averaged by hour for each month), “max precip” is maximum precipitation per month, and “min precip” is minimum precipitation per month.
As shown in the output available in Supplementary Figure S8, the first two axes—those illustrated in Figure 7—are the only significant axes for both ordinations. Latitude is by far the most significant predictor, with a p-value of 0.001 for both models, followed by longitude, which yields p-values of 0.002 and 0.005 for the tree and litter data, respectively. The environmental variables explained 71.05% of the total variance for tree leaves, and 63.04% of the variance for litter leaves.
Discussion
Our study provides a comprehensive view of plant–insect interactions, highlighting the complex preservation patterns in leaf litter. We emphasize the case of galls—better preserved and easily observed in the field—as a promising proxy for future paleoecological reconstructions. Some interaction types—most notably galls— proved consistently represented in both live leaves and litter. Our results revealed that in over half of all pairwise site comparisons, herbivory patterns differed depending on whether litter or live foliage was considered. These inconsistencies were notably reduced when comparisons were made within the same host species, indicating that taxonomic consistency strengthens interpretive reliability. Despite signal loss, our results showed that meaningful ecological patterns linked to environmental variables could still be retrieved from litter samples, reinforcing the potential of such data when handled cautiously.
This consistent representation of galls in both live leaves and litter may also be influenced by biological processes such as induced abscission triggered by the galling insect itself (Yukawa and Tokuda, 2021). In such cases, galled leaves may fall earlier or more frequently than healthy leaves, potentially biasing their representation in the litter layer. Yet, our observations suggest that the overall signal of galling interactions remains comparable between live and litter leaves. Acknowledging that leaf litter underrepresents the diversity of most of the plant–insect interaction seen in forest trees, our findings challenge the direct extrapolation of current ecological interactions to the fossil context. This discrepancy underscores the need to reexamine how fossil data is interpreted, ensuring that paleoecological inferences cautiously reflect the complexity of ancient ecosystems.
Examining variability in plant–insect interactions: lessons from current localities for fossil comparisons
Considering the subtleties identified earlier and the complexities of comparing fossil data directly with current ecological observations, we investigated the feasibility of making comparisons solely within the fossil domain. The idea was to assess whether inherent limitations in data could allow for relatively coherent comparisons.
Our analysis revealed incomplete or even contradictory information when comparing data for a single functional feeding group among localities (Figure 5). Further study is needed to determine whether these conflicting results are the product of genuinely different signals emerging from the leaf litter, or the limited sampling of the dataset presented here—especially for litter. Although we aimed to account for interannual variability, particularly by ensuring that recent years were climatically stable without exceptional conditions (Supplementary Figure S1), this inherent variability highlights an additional layer of uncertainty. This reinforces our initial question about the reliability of quantitative plant–insect interaction analyses within the fossil record.
On a positive note, our study indicates that galls, as a distinct interaction type, exhibit around 75% accuracy in comparative analysis between trees and litter across different localities (Figure 5). This pattern appears particularly robust for angiosperm leaves, for which gall-inducing interactions are well-documented and may follow different preservation pathways than other plant groups (Shorthouse et al., 2005; Labandeira, 2021). This accuracy could improve by recognizing the uneven presence of galls in certain locations like O3HP, where their rarity might impact the statistical reliability.
Furthermore, our investigation reveals that comparing the same plant species across different localities offers more reliable insights into plant–arthropod interactions (Figure 5). And logically so, as comparing similar plant species across localities enables a more precise analysis of interaction variability, clearly attributing differences to environmental factors without the confounding effect of plant diversity overall (Díaz et al., 1998; Macel et al., 2007; The Herbivory Variability Network, 2023).
In our case, we compared the exact same species—primarily Fagaceae—with consistent leaf morphology across sites, allowing us to minimize the influence of plant identity and morphological variation on observed patterns of herbivory. This species-level consistency offers a complementary perspective to fossil studies, where host identity is often inferred from leaf morphotypes alone. Unlike fossil studies, where plant identity is inferred solely from leaf morphology—often across large temporal and spatial gaps (Burnham, 1993; Ellis et al., 2009)—our design compares unequivocally identified species, sampled at the same time and under similar ecological conditions, thereby capturing not only morphological but also evolutionary and physiological consistency. This strengthens the reliability of our herbivory comparisons beyond what is typically accessible in paleobotanical contexts. Our study shows clear interaction disparities in similar ecosystems existing in close proximity (Figure 1, Supplementary Figure S1). The observed differences among identical plant species support the notion, already recognized by numerous research teams (Adams and Zhang, 2009; The Herbivory Variability Network, 2023), that broad conclusions drawn from fossil studies, which typically encompass a variety of species and environmental settings, may carry inherent biases.
Ultimately, our findings could raise the question of whether plant–insect interaction measures are reliable paleoecological indicators. The observed consistency among sites within the same locality suggests the influence of underlying ecological patterns. Our results, particularly the clear distinction between O3HP and Turkish localities, indicates the influence of unique local factors on these plant–insect interactions. Interestingly, the degree of differentiation observed between O3HP and the Turkish sites may approximate the level of ecological contrast that could be detected in fossil assemblages, offering a tentative reference point for evaluating the resolution of plant–insect interaction patterns in deep time. Moreover, such measurements highlight the importance of biogeographic history (Stam et al., 2014; Adroit et al., 2020, 2021) and local abiotic factors, such as soil composition or microclimatic variations, in determining leaf–insect interaction patterns (Pineda et al., 2013; Pinto and Ongaratto, 2019; Moreira et al., 2021). Some of the inconsistencies described here—including the slightly lower amount of variance attributable to environmental factors in the distance-based redundancy analysis—may stem from the lower sampling completeness of litter leaves, though this pattern is less pronounced for certain interaction types such as external foliage feeding, which shows coverage levels close to the overall dataset. In any case, the ordinations presented here show that leaf litter captures genuine biotic patterns when all functional feeding groups are considered simultaneously, reaffirming the use of paleobotanical data for the study of both herbivory and the influence of environmental factors on ancient ecosystems — while reminding us that such environmental interpretations should always be approached with due caution.
Factors affecting decomposition rates in leaf litter
The rate and efficiency of decomposition are primarily influenced by factors such as nutrient availability, temperature, and water availability, as well as the quality of the litter itself (Pérez-Harguindeguy et al., 2000; Hättenschwiler and Jørgensen, 2010; Joly et al., 2023). Notably, leaves vary considerably among plant species in terms of their physical features and chemical structure (Pérez-Harguindeguy et al., 2000), leading to variable decomposition rates and thus different dominance-diversity patterns in standing vegetation versus leaf litter.
The stoichiometric balance of leaf composition, including elements like carbon, nitrogen, or potassium, is pivotal for leaf preservation in litter. This balance, particularly influenced by the quality of carbon, significantly impacts the leaf material’s durability in the litter layer, as preservation is a result of the stoichiometry’s complex interplay (Hättenschwiler and Jørgensen, 2010; Konan et al., 2021).
Richer nutrient content in soil, along with high organic matter content, play an important role in accelerating litter decomposition by enhancing microbial activity (Pérez-Harguindeguy et al., 2000). Soil pH also influences decomposition, with an optimal pH enhancing microbial activity and thus speeding up litter breakdown (Pérez-Harguindeguy et al., 2000).
Temperature, by affecting soil moisture levels, significantly influences decomposition, as higher temperatures increase soil moisture, thus accelerating the decomposition process (Hättenschwiler and Jørgensen, 2010; Gießelmann et al., 2011). In addition, seasonal variations further affect decomposition rates, with slower decomposition in winter and faster rates during rainy seasons (Devi and Yadava, 2007; Tripathi and Pandey, 2009).
Additionally, soil texture contributes to decomposition efficiency, where good aeration and drainage properties further accelerate the process (Pérez-Harguindeguy et al., 2000). Furthermore, the rate of litter decomposition in forests is closely linked to the forest’s age. Changes in microclimate, soil composition, and vegetation, which occur as the forest matures, directly affect litter decomposition. Decomposition is generally faster in mid-aged forests due to better environmental balance, but tends to slow down in older forests with denser canopy and changes in plant community composition, such as shifts in dominant species or structural heterogeneity caused by natural disturbances (e.g., wind, water, fire) (Li et al., 2023).
These factors, in their collective capacity, appear to affect leaf litter preservation and may contribute to the apparent under-representation of plant–insect interactions in litter leaves (see Figures 3, 5, Supplementary Figure S6), while potentially correlating with other elements that influence the preservation of damaged leaves. In particular, EFF—including hole feeding, margin feeding, skeletonization, and surface feeding—are all less frequently recorded in the litter compared to live leaves, regardless of their specific morphology. This consistent pattern supports the notion of selective loss of superficial feeding traces during early decomposition. Although our study does not isolate the effects of each environmental variable on herbivory, future experimental designs could help clarify how factors such as forest age, canopy openness, or ground humidity influence both the occurrence of herbivory and the likelihood of interaction traces being preserved.
Impact of transport agents and decomposers on damaged leaf litter
In addition to these broader factors, there are specific parameters that directly influence the preservation of already-damaged leaves within the litter. Decomposer diversity is known to expedite litter decomposition through interspecies facilitation and niche complementarity (Tonin et al., 2018). Some detritivores exhibit selective behavior, such as woodlice that prefer leaves decomposing rapidly over those that do not (Vos et al., 2011), influencing overall decomposition rates. Herbivorized leaves may decompose faster due to damaged areas providing easier access to detritivores of all sizes, from microbes to animals (Cárdenas and Dangles, 2012). This accelerated decomposition of already damaged leaves has been notably observed in oak populations, as demonstrated by the study of Łukowski et al. (2021).
Furthermore, transport agents such as wind and water, are also fundamental in modulating leaf litter decomposition. They can inflict mechanical damage, particularly on pre-weakened leaves and then favor rapid decomposition. Specifically, in seasonally dry systems, like those in our study (Supplementary Figure S1), wind and water are key in horizontally shifting litter, with wind facilitating surface creeping and saltation while water primarily transports litter via surface flow (Throop and Belnap, 2019).
While all fallen leaves experience varying degrees of biotic and abiotic deterioration, those preserved in the fossil record, especially those from lacustrine sediments—a major component of fossil leaf outcrops (Locatelli, 2014)—have almost certainly been subject to considerable aquatic influence. Our leaves, already indicating an underestimation of herbivory traces compared to tree leaves, likely further underestimate these differences, as they have not undergone various types of biotic and abiotic deterioration in aquatic deposits – a significant factor preceding leaf fossilization. Additionally, our analysis does not account for the detritivory occurring at the bottom of lakes or watercourses, which is as significant, if not more, as the detritivory in litter layers (DeGasparro et al., 2020; Swan et al., 2021; Becu and Richardson, 2023).
Preservation of gall-affected leaves in litter: inputs from field observations and paleoecological implications
Unlike conventional herbivory, which involves tissue removal and direct feeding damage, galls are the result of a complex interaction that involves biochemical signaling between the insect and the host plant (Stone and Schönrogge, 2003; Yukawa and Tokuda, 2021). These interactions are shaped by the intrinsic ecological and physiological pressures specific to gall-inducing insects, making gall formation and prevalence responsive to a variety of environmental and biological factors.
Galls, for instance, often occur with high host specificity (Shorthouse et al., 2005), which enhances their value as paleoecological indicators, especially when host plant identity can be confidently inferred, as is frequently the case in fossil leaf assemblages. In our study, galls emerge as a unique case. While occasionally leading to some information loss (Supplementary Figure S6), they generally preserve damage well. This finding is supported by previous work showing that gall tissue is often structurally reinforced, less palatable, and more resistant to decay due to lower nitrogen content (Stone and Schönrogge, 2003; Künkler et al., 2013; Labandeira, 2021). These physical characteristics make galls particularly well-suited to fossilization in compression–impression deposits, the most common type of preservation context for fossil leaves (Labandeira, 2021). They have been recorded in a wide variety of paleoenvironments—deltaic, lacustrine, fluvial, and palustrine—spanning geological periods from the Paleozoic to the Pliocene (Labandeira and Currano, 2013; Adroit et al., 2024). In our field observations—particularly at Orhaneli and Karacabey—we repeatedly noted that large, dense galls (e.g., Figure 3G) were more often found detached on litter leaves than on live foliage. While not formally quantified, this consistent pattern suggests gall detachment may affect their litter representation. While gravity may contribute to gall detachment, another plausible explanation is that the presence of galls triggers premature leaf abscission as a plant-induced response (Williams and Whitham, 1986; Fernandes et al., 2008). This hypothesis is supported by studies suggesting that gall presence can lead to induced abscission as a defense mechanism, increasing the likelihood of gall preservation in the litter—and by extension, in the fossil record.
In terms of practical application, galls are integrated into the FFG–DT (Functional Feeding Group – Damage Type) classification system, which underpins quantitative analyses of fossil herbivory. This same framework was applied in our current study and has previously been employed in over 165 publications to track patterns in plant–arthropod interactions across time and space (Labandeira and Wappler, 2023). Accordingly Mertz et al. (2022) emphasized that “a well-organized gall collection has great potential to inform research in taxonomy and systematics, as well as ecology, environmental science, and even paleobiology.”. Moreover, gall morphotypes are commonly used as reliable proxies for insect diversity, particularly in tropical or high-diversity regions where many insect-gall makers remain undescribed (Cuevas-Reyes et al., 2004). While this approach has limitations—morphologies may be altered by inquilines or misidentified as separate entities (Fernandes and Santos, 2014)—the use of gall morphotypes remains a standard and effective practice when handled cautiously. Taken together, the preservation, visibility, and ecological relevance of galls—combined with their frequency in both modern and fossil assemblages—make them powerful indicators for reconstructing trophic dynamics in ancient ecosystems. One promising avenue to strengthen the ecological signal of fossil galls would be to explore their preserved chemical traits, which may offer a complementary means of identification when morphological structures are altered or lost through fossilization. Future work could also test whether galling patterns observed across environmental gradients—such as those described by Fernandes and Price (1992)—are reproducible within the FFG-DT framework.
Conclusion
Our multifaceted exploration of plant–insect interactions within leaf litter provides new insights into their preservation and the consequent implications for paleoecological reconstructions. Galls, with their distinctive preservation patterns, emerge as a potential proxy for more accurate paleoecological interpretations, offering a window into the ecological dynamics of past environments. However, the underrepresentation of external interactions in leaf litter compared to trees cautions against the straightforward application of current ecological observations to the fossil record. Our study calls for a refined approach to paleoecological methods, emphasizing the need for future research to account for the wide array of plant–insect interactions and their unique characteristics within ancient ecosystems. As we continue to bridge the gap between paleontological data and contemporary ecological understanding, it is clear that plant–insect interaction measures can be reliable indicators — at least within well-constrained local and spatial environmental contexts.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: FigShare, https://figshare.com/s/16479e1ddd076774cbee.
Author contributions
BA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. SS: Formal analysis, Writing – review & editing. TG: Data curation, Methodology, Writing – review & editing. J-PO: Data curation, Methodology, Writing – review & editing. TD: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. BA and TD acknowledge support from a Carl Tryggers Stiftelse postdoctoral grant (project no. CTS21: 1585) and a Swedish Research Council grant (grant number 2021–05849 to TD).
Acknowledgments
We are grateful to AnaEE-France and AnaEE-ERIC (https://www.anaee-france.fr/service/experimentation-in-natura/ecosystemes-forestier/ecosystemes-forestiers-mediterraneens/o3hp), the SCDE (Common Service of Experimental Facilities) of IMBE, ECCOREV and Elena Ormeno (scientific director of O3HP). Thank you to AnaEE-France and more particularly the PIA3 (“Plan d’Investissements d’avenir”) through the ANR (ANR-11-INBS-0001 AnaEE France »). We are grateful to the General Directorate of Forestry of Turkey in Bursa (Orman Genel Müdürlüğü, OGM) for their support and authorization to collect samples in their district. Special thanks to Morteza Djamali (IMBE), the General Directorate of Forestry and the Department of Foreign Relations, Education and Research (permission no-date 18.10.2022-512541), the Bursa Regional Directorate of Forestry, The Fighting Forest Pests Branch Directorate, throughout the director Hüseyin Koz, the forest engineer Meryem Bulut and the technician Muhterem Çiçek for their precious help to collect our samples among the different Mediterranean localities in France and Turkey. Thank you to Aurélie Riandet (Méteo-France), Antoine Guiguet (Naturalis Biodiversity Center) and Gilles Escarguel (LEHNA, Université Lyon 1) for their rich discussion in order to conduct this study properly. We extend our heartfelt thanks to the enthusiastic and knowledgeable gall community on iNaturalist (https://www.inaturalist.org/). Their active involvement and invaluable insights have greatly enhanced our understanding and identification of galls. We warmly thank both reviewers for their insightful and constructive comments, which greatly helped to improve the clarity and overall quality of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1549315/full#supplementary-material
Supplementary Figure 1 | Comparison of Ombrothermic Diagrams (2020–2022) between Dauphin (Provence-Alpes-Côte d’Azur, France) and Bursa Weather Stations (Turkey): This figure illustrates the comparison of ombrothermic diagrams for the period 2020-2022, between the Dauphin weather station, located less than 10km from the O3HP locality, and the Bursa station, which covers the Karacabey, Orhaneli, and Inegöl localities. Meteorological data for Dauphin were provided by Météo France, while data for Bursa were sourced from the National Oceanic and Atmospheric Administration (NOAA). Key Observations: The data clearly indicate that the climates at both locations are similar over a year. However, a notable difference is the 2–3 month lag in precipitation patterns between Dauphin and Bursa. In Dauphin, significant precipitation occurs in the last quarter of the year, whereas in Bursa, it is more prominent in the first quarter. Cautionary Note on Precipitation Scales: The precipitation scale for Bursa is not the same as that for Dauphin. This is particularly evident in 2021, where January in Bursa experienced exceptionally high precipitation, offsetting the lower rainfall in November and December. As a result, the Y-axis scale for precipitation in Bursa is larger than that for Dauphin. Additional methodological details: Meteorological records for the years 2020–2022 were used to calculate average monthly minimum and maximum temperatures and precipitation. In addition to these station-based data, temperature and precipitation data were downloaded from ERA5-Land (Muñoz Sabater, 2019) for the period 2019–2022. Near-surface air temperature at 2 meters above ground (‘2m temperature’) was downloaded by hour, and monthly precipitation was obtained for each month. Minimum temperature was calculated from the monthly averages of 04:00–07:00 during the winter months (Nov–Mar), and maximum temperature from 16:00–19:00 during the summer months (May–Sep). Data from 2023 were excluded, as they postdate leaf collection, and data from 2019 were included to account for uncertainty in the exact timing of leaf fall in the litter layer.
Supplementary Figure 2 | Fieldwork terrains across various Mediterranean study sites. Each image corresponding to a specific locality within our research area; (a) Orhaneli locality at Delta site; (b) O3HP locality at Delta site; (c) Karacabey locality at Alpha site; (d) Orhaneli locality at Beta site; (e) Karacabey locality at Gamma site; (f) Orhaneli locality at Alpha site; (g) Inegöl locality at Alpha site; (h) Aerial drone view of the O3HP locality encompassing Alpha, Beta, and Gamma sites; (i) Schematic representation of the theoretical sampling process at a site, showing the stratified collection approach from tree canopy to litter layer. The plate illustrates the diverse environments and methodologies involved in our field sampling for plant–insect interactions studies. The plate illustrates the diverse environments and methodologies involved in our field sampling for plant–insect interaction studies. Leaf collection protocol (schematic detail in panel i): Bottom leaves were collected at chest height (~1.4 m). Mid leaves were collected using a 2.4 m telescopic pruner, combined with arm reach (~2.5 m), reaching ~4.9 m in height. Top leaves were collected with a fully extended 4 m pruner, reaching ~7.4 m with arm extension. When tree height exceeded reach, top leaves were sampled at the outermost sun-exposed branches to best approximate canopy foliage.
Supplementary Figure 3 | Morphological descriptions of oak leaf galls induced by various cynipid wasps. This supplementary data document provides detailed morphological descriptions of galls induced by different species of cynipid wasps on oak leaves. The described galls include those formed by Cerroneuroterus lanuginosus, Dryomyia circinans, Neuroterus quercusbaccarum, Cynips quercusfolii, and Andricus kollari. Each description includes information on the shape, size, color, texture, and typical location of the galls on the oak leaves, offering insights into their distinctive characteristics and developmental patterns.
Supplementary Figure 4 | Rarefaction curves showcasing plant–insect interaction richness for each study locality (O3HP, Orhaneli, Inegöl, and Karacabey) and across all localities combined. For individual localities: (A) represents richness by site, with coverage-based calculations; (B) shows richness by vertical position on the tree, also coverage-based; (C) depicts richness by site, calculated size-based; (D) indicates richness by position, size-based. The combined locality graph presents (A) tree leaf richness and (B) litter richness, both coverage-based, alongside (C) tree leaf richness and (D) litter richness, both size-based. These metrics collectively provide a comprehensive view of interaction diversity within and across the localities, without distinguishing between interaction types.
Supplementary Figure 5 | Raw data of plant-insect interaction identified, used in the study. The file contains four worksheets with the complete raw datasets from the respective localities of O3HP, Karacabey, Inegöl, and Orhaneli. Each worksheet provides the unprocessed data collected at the corresponding location, which served as the basis for the analyses presented in this article.
Supplementary Figure 6 | Boxplot comparisons illustrating interaction frequencies (top panel) and occurrences (bottom panel) for all interaction types, galls, mines, and external feeding foliage (EFF) across four study localities. Each locality is represented by a color pair, with lighter shades indicating tree leaves and darker shades for litter leaves: Karacabey (light and dark yellow/orange), Inegöl (light and dark blue), Orhaneli (light and dark red), and O3HP (light and dark green).
Supplementary Figure 7 | Summary table of chi-squared test results comparing functional feeding group (FFG) frequencies between litter and tree leaf samples across all sites and species. For each pairwise site comparison and FFG, test statistics are provided separately for litter and tree samples, including chi-squared values, degrees of freedom (Df), and p-values. These values support the visualization presented in Figure 5 and allow for interpretation of the significance and consistency of interaction patterns across datasets.
Supplementary Figure 8 | Code and detailed output for the distance-based redundancy analysis. This includes tests of the significance of axes and environmental variables, as well as the amount of information captured by each axis.
References
Adams J. M. and Zhang Y. (2009). Is there more insect folivory in warmer temperate climates? A latitudinal comparison of insect folivory in eastern North America. J. Ecol. 97, 933–940. doi: 10.1111/j.1365-2745.2009.01523.x
Adroit B., Girard V., Kunzmann L., Terral J.-F., and Wappler T. (2018). Plant-insect interactions patterns in three European paleoforests of the late-Neogene-early-Quaternary. PeerJ 6, 24. doi: 10.7717/peerj.5075, PMID: 29942705
Adroit B., Hazra T., Denk T., Kumar Sarkar S., and Khan M. A. (2024). Rich specialized insect damage on Pliocene leaves from the Mahuadanr Valley (India) growing under a warm climate with weak seasonality. Ecol. Evol. 14, e11114. doi: 10.1002/ece3.11114, PMID: 38469042
Adroit B., Teodoridis V., Güner T. H., and Denk T. (2021). Patterns of insect damage types reflect complex environmental signal in Miocene forest biomes of Central Europe and the Mediterranean. Global Planetary Change 199, 12. doi: 10.1016/j.gloplacha.2021.103451
Adroit B., Zhuang X., Wappler T., Terral J.-F., and Wang B. (2020). A case of long-term herbivory: specialized feeding trace on Parrotia (Hamamelidaceae) plant species. R. Soc. Open Sci. 7, 14. doi: 10.1098/rsos.201449, PMID: 33204482
Barouillet C., Vasselon V., Keck F., Millet L., Etienne D., Galop D., et al. (2022). Paleoreconstructions of ciliate communities reveal long-term ecological changes in temperate lakes. Sci. Rep. 12, 7899. doi: 10.1038/s41598-022-12041-7, PMID: 35551223
Becu M. H. J. and Richardson J. S. (2023). Leaf litter decomposition rates in freshwaters differ by ecosystem. Aquat. Sci. 85, 101. doi: 10.1007/s00027-023-00998-0
Bhansali R. R. (2012). Development of flower galls in Prosopis cineraria trees of Rajasthan. J. Plant Prot. Sci 4, 52–56. doi: 10.5555/20133186858
Blanche K. R. and Ludwig J. A. (2001). Species richness of gall-inducing insects and host plants along an altitudinal gradient in Big Bend National Park, Texas. Am. Midland Nat. 145, 219–232. doi: 10.1674/0003-0031(2001)145[0219:SROGII]2.0.CO;2
Boaventura M. G., Pereira C. C., and Cornelissen T. (2018). Plant architecture influences gall abundance in a tropical montane plant species. Acta Botanica Brasilica 32, 670–674. doi: 10.1590/0102-33062018abb0038
Braga M. P., Janz N., Nylin S., Ronquist F., and Landis M. J. (2021). Phylogenetic reconstruction of ancestral ecological networks through time for pierid butterflies and their host plants. Ecol. Lett. 24, 2134–2145. doi: 10.1111/ele.v24.10, PMID: 34297474
Brown M. W., Glenn D. M., and Wisniewski M. E. (1991). Functional and anatomical disruption of apple roots by the woolly apple aphid (Homoptera: aphididae). J. Economic Entomology 84, 1823–1826. doi: 10.1093/jee/84.6.1823
Buma B., Harvey B. J., Gavin D. G., Kelly R., Loboda T., McNeil B. E., et al. (2019). The value of linking paleoecological and neoecological perspectives to understand spatially-explicit ecosystem resilience. Landscape Ecol. 34, 17–33. doi: 10.1007/s10980-018-0754-5
Burnham R. J. (1989). Relationships between standing vegetation and leaf litter in a paratropical forest: Implications for paleobotany. Rev. Palaeobotany Palynology 58, 5–32. doi: 10.1016/0034-6667(89)90054-7
Burnham R. J. (1993). Reconstructing richness in the plant fossil record. Palaios 8, 376–384. doi: 10.2307/3515267
Burnham R. (1994). Patterns in tropical leaf litter and implications for angiosperm paleobotany. Rev. Palaeobotany Palynology 81, 99–113. doi: 10.1016/0034-6667(94)90129-5
Burnham R. J., Ellis B., and Johnson K. R. (2005). Modern tropical forest taphonomy: does high biodiversity affect paleoclimatic interpretations? PALAIOS 20, 439–451. doi: 10.2110/palo.2004.P04-60
Burnham R. J., Wing S. L., and Parker G. G. (1992). The reflection of deciduous forest communities in leaf litter: implications for autochthonous litter assemblages from the fossil record. Paleobiology 18, 30–49. doi: 10.1017/S0094837300012203
Cárdenas R. E. and Dangles O. (2012). Do canopy herbivores mechanically facilitate subsequent litter decomposition in soil? A pilot study Neotropical cloud forest. Ecol. Res. 27, 975–981. doi: 10.1007/s11284-012-0979-8
Carvalho M. R., Wilf P., Barrios H., Windsor D. M., Currano E. D., Labandeira C. C., et al. (2014). Insect leaf-chewing damage tracks herbivore richness in modern and ancient forests. PloS One 9, e94950. doi: 10.1371/journal.pone.0094950, PMID: 24788720
Castagneyrol B., Valdés-Correcher E., Bourdin A., Barbaro L., Bouriaud O., Branco M., et al. (2020). Can school children support ecological research? Lessons from the oak bodyguard citizen science project. Citizen Science: Theory Pract. 5, 10. doi: 10.5334/cstp.267
Cenci R. and Adami-Rodrigues K. (2017). Record of gall abundance as a possible episode of radiation and speciation of galling insects, Triassic, Southern Brazil. Rev. Bras. Paleontologia 20, 279–286. doi: 10.4072/rbp.2017.3.01
Cenci R. and Horodyski R. (2022). Fern-arthropod interactions from the modern upland southeast atlantic rainforest reveals arthropod damage insights to fossil plant-insect interactions. PALAIOS 37, 349–367. doi: 10.2110/palo.2021.002
Chao A. and Jost L. (2012). Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93, 2533–2547. doi: 10.1890/11-1952.1, PMID: 23431585
Coley P. D. and Barone J. A. (1996). Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Systematics 27, 305–335. doi: 10.1146/annurev.ecolsys.27.1.305
Coutinho R. D., Cuevas-Reyes P., Fernandes G. W., and Fagundes M. (2019). Community structure of gall-inducing insects associated with a tropical shrub: regional, local and individual patterns. Trop. Ecol 14, 74–82. doi: 10.1007/s42965-019-00010-7
Cuevas-Reyes P., Quesada M., Hanson P., Dirzo R., and Oyama K. (2004). Diversity of gall-inducing insects in a Mexican tropical dry forest: the importance of plant species richness, life-forms, host plant age and plant density. J. Ecol. 92, 707–716. doi: 10.1111/j.0022-0477.2004.00896.x
Cuevas-Reyes P., Siebe C., Martínez-Ramos M., and Oyama K. (2003). Species richness of gall-forming insects in a tropical rain forest: correlations with plant diversity and soil fertility. Biodiversity Conserv. 12, 411–422. doi: 10.1023/A:1022415907109
Damesin C. and Rambal S. (1995). Field study of leaf photosynthetic performance by a Mediterranean deciduous oak tree (Quercus pubescens) during a severe summer drought. New Phytol. 131, 159–167. doi: 10.1111/j.1469-8137.1995.tb05717.x
DeGasparro S. L., Beresford D. V., Prater C., and Frost P. C. (2020). Leaf litter decomposition in boreal lakes: variable mass loss and nutrient release ratios across a geographic gradient. Hydrobiologia 847, 819–830. doi: 10.1007/s10750-019-04140-w
De La Fuente A., Youngentob K. N., Marsh K. J., Krockenberger A. K., Williams S. E., and Cernusak L. A. (2024). Relationships between abiotic factors, foliage chemistry and herbivory in a tropical montane ecosystem. Oecologia 206, 293–304. doi: 10.1007/s00442-024-05630-y, PMID: 39453448
Desmond S. C., Garner M., Flannery S., Whittemore A. T., and Hipp A. L. (2021). Leaf shape and size variation in bur oaks: an empirical study and simulation of sampling strategies. Am. J. Bot. 108, 1540–1554. doi: 10.1002/ajb2.v108.8, PMID: 34387858
Devi A. S. and Yadava P. (2007). Wood and leaf litter decomposition of Dipterocarpus tuberculatus Roxb. in a tropical deciduous forest of Manipur, Northeast India. Curr. Sci. 93, 243–246.
Díaz S., Cabido M., and Casanoves F. (1998). Plant functional traits and environmental filters at a regional scale. J. Vegetation Sci. 9, 113–122. doi: 10.2307/3237229
Donovan M. P., Iglesias A., Wilf P., Labandeira C. C., and Cúneo N. R. (2016). Rapid recovery of Patagonian plant–insect associations after the end-Cretaceous extinction. Nat. Ecol. Evol. 1, 0012., PMID: 28812553
Donovan M. P., Schachat S. R., and Monarrez P. M. (2023). Ecological and evolutionary responses of terrestrial arthropods to Middle–Late Pennsylvanian environmental change. Geological Soc. 535, SP535-2022–209. doi: 10.1038/s41559-016-0012, PMID: 28812553
Ellis B., Daly D. C., Hickey L. J., Johnson K. R., Mitchell J. D., Wilf P., et al. (2009). Manual of leaf architecture (Ithaca: Cornell University Press).
Espírito-Santo M. M. and Fernandes G. W. (2007). How many species of gall-inducing insects are there on Earth, and where are they? Ann. Entomol Soc. Am. 100, 95–99. doi: 10.1603/0013-8746(2007)100[95:HMSOGI]2.0.CO;2
Fazan L., Dorchin N., Giriens S., Pasta S., Garfì G., Remoundou I., et al. (2023). A new species of Contarinia (Diptera: Cecidomyiidae) from flower galls on the relict tree Zelkova abelicea (Ulmaceae) endemic to Crete (Greece). Zootaxa 5301, 257–268. doi: 10.11646/zootaxa.5301.2.6, PMID: 37518562
Ferguson D. K. (1996). Actuopalaeobotany - a taphonomic peep-show? - Summary of workshop discussions. Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen 202, 149–158. doi: 10.1127/njgpa/202/1996/149
Fernandes G. W., Almada E. D., and Carneiro M. A. A. (2010). Gall-inducing insect species richness as indicators of forest age and health. Environ. Entomology 39, 1134–1140. doi: 10.1603/EN09199, PMID: 22127163
Fernandes G. W., De Marco Júnior P., and Schönrogge K. (2008). Plant organ abscission and the green island effect caused by gallmidges (Cecidomyiidae) on tropical trees. Arthropod-Plant Interact. 2, 93–99. doi: 10.1007/s11829-008-9031-x
Fernandes G. W. and Price P. W. (1992). The adaptive significance of insect gall distribution: survivorship of species in xeric and mesic habitats. Oecologia 90, 14–20. doi: 10.1007/BF00317803, PMID: 28312265
Fernandes G. W. and Santos J. C. (Eds.) (2014). Neotropical Insect Galls (Dordrecht: Springer Netherlands).
Fiene J., Kalns L., Nansen C., Bernal J., Harris M., and Sword G. A. (2013). Foraging on individual leaves by an intracellular feeding insect is not associated with leaf biomechanical properties or leaf orientation. PloS One 8, e80911. doi: 10.1371/journal.pone.0080911, PMID: 24260510
Garzo E., Moreno A., Plaza M., and Fereres A. (2020). Feeding behavior and virus-transmission ability of insect vectors exposed to systemic insecticides. Plants 9, 895. doi: 10.3390/plants9070895, PMID: 32679858
Gaston K. J. and Williams P. H. (1996). Spatial patterns in taxonomic diversity. Pages 202–229 Biodiversity. Biology of Numbers and Difference. Ed. Gaston K. J. (Oxford: Blackwell Science).
Gauquelin T., Boer M., Baldy V., Fernardez C., Montes N., Santonja M., et al. (2011). L’O3HP (Oak Observatory at OHP) : un site expérimental pour l’étude du fonctionnement et de la biodiversité de la Chênaie pubescente face aux changements climatiques (Marseille: Forêt Méditerranéenne), 127.
Gießelmann U. C., Martins K. G., Brändle M., Schädler M., Marques R., and Brandl R. (2011). Lack of home-field advantage in the decomposition of leaf litter in the Atlantic Rainforest of Brazil. Appl. Soil Ecol. 49, 5–10. doi: 10.1016/j.apsoil.2011.07.010
Giraldo L. A., Wilf P., Donovan M. P., Kooyman R. M., and Gandolfo M. A. (2025). Fossil insect-feeding traces indicate unrecognized evolutionary history and biodiversity on Australia’s iconic Eucalyptus. New Phytol. 245, 1762–1773. doi: 10.1111/nph.v245.4, PMID: 39605238
Grodzinski B., Schmidt J. M., Watts B., Taylor J., Bates S., Dixon M. A., et al. (1999). Regulating plant/insect interactions using CO2 enrichment in model ecosystems. Adv. Space Res. 24, 281–291. doi: 10.1016/S0273-1177(99)00315-4, PMID: 11542535
Hättenschwiler S. and Jørgensen H. B. (2010). Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 98, 754–763. doi: 10.1111/j.1365-2745.2010.01671.x
Hazra T., Adroit B., Denk T., Wappler T., Sarkar S. K., Bera S., et al. (2023). Marginal leaf galls on Pliocene leaves from India indicate mutualistic behavior between Ipomoea plants and Eriophyidae mites. Sci. Rep. 13, 1–10. doi: 10.1038/s41598-023-31393-2, PMID: 37029134
Hsieh T. C., Ma K. H., and Chao A. (2022). iNEXT: Interpolation and Extrapolation for Species Diversity. R package version 3.0.0. Vienna: Comprehensive R Archive Network (CRAN).
Johnson W. T. and Lyon H. H. (1991). Insects that Feed on Trees and Shrubs (University of Minnesota: Comstock Pub. Associates).
Joly F.-X., Scherer-Lorenzen M., and Hättenschwiler S. (2023). Resolving the intricate role of climate in litter decomposition. Nat. Ecol. Evol. 7, 214–223. doi: 10.1038/s41559-022-01948-z, PMID: 36624177
Jupke J. F. and Schäfer R. B. (2020). Should ecologists prefer model- over distance-based multivariate methods? Ecol. Evol. 10, 2417–2435. doi: 10.1002/ece3.6059, PMID: 32184990
Knuff A. K., Staab M., Frey J., Helbach J., and Klein A. (2019). Plant composition, not richness, drives occurrence of specialist herbivores. Ecol. Entomology 44, 833–843. doi: 10.1111/een.12767
Konan N. L., N’Guessan D., Koné A., Yapo G., Sall S., Hien E., et al. (2021). Qualité et vitesse de décomposition des litières des principaux arbustes natifs de la savane humide au centre de la Côte d’Ivoire. Agronomie Africaine 33, 191–201. https://www.researchgate.net/publication/355023295_Qualite_et_vitesse_de_decomposition_des_litieres_des_principaux_arbustes_natifs_de_la_savane_humide_au_centre_de_la_Cote_d'Ivoire
Kozlov M. V., Lanta V., Zverev V., and Zvereva E. L. (2015). Global patterns in background losses of woody plant foliage to insects. Global Ecol. Biogeogr. 24, 1126–1135. doi: 10.1111/geb.2015.24.issue-10
Kük M. and Burgess P. (2010). The Pressures on, and the Responses to, the State of Soil and Water Resources of Turkey (Ankara: Ankara Üniversitesi Çevrebilimleri Dergisi).
Künkler N., Brandl R., and Brändle M. (2013). Changes in clonal poplar leaf chemistry caused by stem galls alter herbivory and leaf litter decomposition. PloS One 8, e79994. doi: 10.1371/journal.pone.0079994, PMID: 24260333
Labandeira C. C. (2002). The history of associations between plants and animals. Pages 26–74 Plant–Animal Interactions: An Evolutionary Approach. Eds. Herrera C. and Pellmyr O. (Oxford, U.K: Blackwell Science).
Labandeira C. C. (2021). Ecology and evolution of gall-inducing arthropods: the pattern from the terrestrial fossil record. Front. Ecol. Evol. 9, 632449. doi: 10.3389/fevo.2021.632449
Labandeira C. C. and Currano E. D. (2013). The fossil record of plant-insect dynamics. Annu. Rev. Earth Planetary Sci. 41, 287–311. doi: 10.1146/annurev-earth-050212-124139
Labandeira C. C., Johnson K. R., and Wilf P. (2002). Impact of the terminal Cretaceous event on plant–insect associations. Proc. Natl. Acad. Sci. 99, 2061–2066. doi: 10.1073/pnas.042492999, PMID: 11854501
Labandeira C. C. and Wappler T. (2023). Arthropod and pathogen damage on fossil and modern plants: exploring the origins and evolution of herbivory on land. Annu. Rev. Entomology 68, 341–361. doi: 10.1146/annurev-ento-120120-102849, PMID: 36689301
Labandeira C. C., Wilf P., Johnson K. R., and Marsh F. (2007). Guide to insect (and other) damage types on compressed plant fossils (Washington, DC: Smithsonian Institution, National Museum of Natural History, Department of Paleobiology).
Leckey E. H., Smith D. M., Nufio C. R., and Fornash K. F. (2014). Oak-insect herbivore interactions along a temperature and precipitation gradient. Acta Oecologica 61, 1–8. doi: 10.1016/j.actao.2014.08.001
Leite G. L. D., Guanabens R. E. M., and Fernandes G. W. (2009). Within tree distribution of a gall-inducing Eurytoma (Hymenoptera, Eurytomidae) on Caryocar brasiliense (Caryocaraceae). Rev. Bras. Entomologia 53, 643–648. doi: 10.1590/S0085-56262009000400015
Lemoine N. P., Burkepile D. E., and Parker J. D. (2014). Variable effects of temperature on insect herbivory. PeerJ 2, e376. doi: 10.7717/peerj.376, PMID: 24860701
Lemoine N. P., Burkepile D. E., and Parker J. D. (2017). Insect herbivores increase mortality and reduce tree seedling growth of some species in temperate forest canopy gaps. PeerJ 5, e3102. doi: 10.7717/peerj.3102, PMID: 28344904
Li S., Xu Z., Yu Z., Fu Y., Su X., Zou B., et al. (2023). Litter decomposition and nutrient release are faster under secondary forests than under Chinese fir plantations with forest development. Sci. Rep. 13, 16805. doi: 10.1038/s41598-023-44042-5, PMID: 37798470
Locatelli E. R. (2014). The exceptional preservation of plant fossils: A review of taphonomic pathways and biases in the fossil record. Paleontological Soc. Papers 20, 237–258. doi: 10.1017/S1089332600002874
Łukowski A., Giertych M. J., Żmuda M., Mąderek E., Adamczyk D., and Karolewski P. (2021). Decomposition of herbivore-damaged leaves of understory species growing in oak and pine stands. Forests 12, 304. doi: 10.3390/f12030304
Ma F.-J., Luo D.-D., Liu S., Zhang C.-W., Wang Q.-J., Li B.-X., et al. (2023). Local provincialism of late Permian plant–arthropod associations in South Cathaysia: Evidence of arthropod-mediated damages in a Wuchiapingian assemblage of South China. J. Asian Earth Sci. 254, 105729. doi: 10.1016/j.jseaes.2023.105729
Macel M., Lawson C. S., Mortimer S. R., Šmilauerova M., Bischoff A., Crémieux L., et al. (2007). Climate vs. Soil factors in local adaptation of two common plant species. Ecology 88, 424–433. doi: 10.1890/0012-9658(2007)88[424:CVSFIL]2.0.CO;2, PMID: 17479760
McLoughlin S., Prevec R., and Slater B. (2021). Arthropod interactions with the Permian Glossopteris flora. J. Palaeosciences 70, 43–133. doi: 10.54991/jop.2021.11
Melo Júnior J. C. F., Boeger M. R. T., Isaias R. M. D. S., Arriola I. A., Lorenzi L., da S. Mouga D. M. D., et al. (2019). Positive relationship between soil fertility, plant diversity, and gall richness. Acta Scientiarum. Biol. Sci. 41, 39283. doi: 10.4025/actascibiolsci.v41i1.39283
Mertz A.-K., Awad J., Wendt I., Dalitz C., and Krogmann L. (2022). Curation and digitization of insect galls in the collection of the State Museum of Natural History Stuttgart. Integr. Systematics: Stuttgart Contributions to Natural History 5, 193–202. doi: 10.18476/2022.491606
Moreira X., Pérez-Ramos I. M., Matías L., Francisco M., García-González A., Martins-Noguerol R., et al. (2021). Effects of soil abiotic factors and plant chemical defences on seed predation on sea fennel (Crithmum maritimum). Plant Soil 465, 289–300. doi: 10.1007/s11104-021-04994-x
Müller C., Wappler T., and Kunzmann L. (2017). Insect herbivory patterns in late Eocene coastal lowland riparian associations from central Germany. Palaeogeography Palaeoclimatology Palaeoecol 491, 170–184. doi: 10.1016/j.palaeo.2017.12.006
Muñoz Sabater J. (2019). ERA5-Land monthly averaged data from 1950 to present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS).
Nakamura M., Minoshima M., Terada C., Takagi K., Makoto K., Shibata H., et al. (2021). Response of background herbivory in mature birch trees to global warming. Front. Forests Global Change 4, 675401. doi: 10.3389/ffgc.2021.675401
Oksanen J., Simpson G. L., Blanchet F. G., Kindt R., Legendre P., Minchin P. R., et al. (2022). October 11. vegan: Community Ecology Package. Vienna: Comprehensive R Archive Network (CRAN).
Onodera H., Oguro M., and Sakai S. (2014). Effects of nutrient contents and defense compounds on herbivory in reproductive organs and leaves of Iris gracilipes. Plant Ecol. 215, 1025–1035. doi: 10.1007/s11258-014-0359-2
Palmer M. W. (2019). Gradient Analysis of Ecological Communities (Ordination). Page Handbook of Environmental and Ecological Statistics (New-York: Chapman and Hall/CRC).
Peel M. C., Finlayson B. L., and McMahon T. A. (2007). Updated world map of the K¨oppen-Geiger climate classification. Hydrol. Earth Syst. Sci 11 (5), 1633-1644. doi: 10.5194/hess-11-1633-2007
Pérez-Harguindeguy N., Díaz S., Cornelissen J. H. C., Vendramini F., Cabido M., and Castellanos A. (2000). Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218, 21–30. doi: 10.1023/A:1014981715532
Pineda A., Dicke M., Pieterse C. M. J., and Pozo M. J. (2013). Beneficial microbes in a changing environment: are they always helping plants to deal with insects? Funct. Ecol. 27, 574–586. doi: 10.1111/fec.2013.27.issue-3
Pinto C. P. G. and Ongaratto S. (2019). Influence of abiotic factors on the resistance of plants to insects. EntomoBrasilis 12, 01–05. doi: 10.12741/ebrasilis.v12i1.839
Prevec R. and Bordy E. M. (2009). Plant-Arthropod Interactions in the Early Angiosperm History - Evidence from the Cretaceous of Israel Vol. 17. Eds. Krassilov V., Silantieva N., Lewy Z., Anisyutkin L. N., Grachev V. G., Ponomarenko A. G., Rasnitsyn A. P., Vrsansky P., Krassilov V., and Rasnitsyn A. (Sofia & Leiden: African Entomology), 120–121.
Price P. W., Fernandes G. W., Lara A. C. F., Braw J., Barrios H., Wright M. G., et al. (1998). Global patterns in local number of insect galling species. J. Biogeography 25, 581–591. doi: 10.1046/j.1365-2699.1998.2530581.x
Purnell M. A., Donoghue P. J. C., Gabbott S. E., McNamara M. E., Murdock D. J. E., and Sansom R. S. (2018). Experimental analysis of soft-tissue fossilization: opening the black box. Palaeontology 61, 317–323. doi: 10.1111/pala.2018.61.issue-3
Raman A., Schafer C., and Withers T. (2005). “Galls and gall-inducing arthropods: an overview of their biology, ecology, and evolution,” in Biology, Ecology, and Evolution of Gall-inducing Arthropods. Ed. Withers T. M. W. T. M. M (Enfield, New Hampshire, USA: Science Publishers, Inc).
Ricardi-Branco F., Branco F. C., Garcia R. J. F., Faria R. S., Pereira S. Y., Portugal R., et al. (2009). Plant accumulations along the Itanhaem River Basin, southern coast of Sao Paulo State, Brazil. PALAIOS 24, 416–424. doi: 10.2110/palo.2008.p08-079r
Romero-Lebron E., Robledo M., Delclòs X., Petrulevičius J., and Gleiser R. (2022). Endophytic insect oviposition traces in deep time. Palaeogeography Palaeoclimatology Palaeoecol. 590, 110855. doi: 10.1016/j.palaeo.2022.110855
Ruiz-Ferrer V., Cabrera J., Martinez-Argudo I., Artaza H., Fenoll C., and Escobar C. (2018). Silenced retrotransposons are major rasiRNAs targets in Arabidopsis galls induced by Meloidogyne javanica. Mol. Plant Pathol. 19, 2431–2445. doi: 10.1111/mpp.2018.19.issue-11, PMID: 30011119
Santos J. C. and Fernandes G. W. (2021). Measuring Arthropod Biodiversity: A Handbook of Sampling Methods (Cham, Switzerland: Springer International Publishing).
Santos A. A., Xiao L., Labandeira C. C., Néraudeau D., Dépré É., Moreau J.-D., et al. (2022). Plant–insect interactions from the mid-Cretaceous at Puy-Puy (Aquitaine Basin, western France) indicates preferential herbivory for angiosperms amid a forest of ferns, gymnosperms, and angiosperms. Bot. Lett. 169, 1–20. doi: 10.1080/23818107.2022.2092772
Schachat S. R., Payne J. L., and Boyce C. K. (2023). Linking host plants to damage types in the fossil record of insect herbivory. Paleobiology 49, 232–258. doi: 10.1017/pab.2022.35
Schatz B., Sauvion N., Kjellberg F., and Nel A. (2017). “Chapter One - Plant–Insect Interactions: A Palaeontological and an Evolutionary Perspective,” in Advances in Botanical Research. Eds. Sauvion N., Thiéry D., and Calatayud P.-A. (Academic Press).
Schön J., Wurz A., Inclan D., Farwig N., and Brandl R. (2024). December 12. Global patterns of insect herbivory across forest canopies and understories: Insights from a tropical case study and a global comparison. Life Sci. doi: 10.32942/X20S5F
Shorthouse J. D., Wool D., and Raman A. (2005). Gall-inducing insects – Nature’s most sophisticated herbivores. Basic Appl. Ecol. 6, 407–411. doi: 10.1016/j.baae.2005.07.001
Sinclair R. J. and Hughes L. (2010). Leaf miners: The hidden herbivores. Austral Ecol. 35, 300–313. doi: 10.1111/j.1442-9993.2009.02039.x
Sohn J.-C., Kim N.-H., and Choi S.-W. (2017). Morphological and functional diversity of foliar damage on Quercus mongolica Fisch. ex Ledeb. (Fagaceae) by herbivorous insects and pathogenic fungi. J. Asia-Pacific Biodiversity 10, 489–508. doi: 10.1016/j.japb.2017.08.001
Stam J. M., Kroes A., Li Y., Gols R., van Loon J. J. A., Poelman E. H., et al. (2014). Plant interactions with multiple insect herbivores: from community to genes. Annu. Rev. Plant Biol. 65, 689–713. doi: 10.1146/annurev-arplant-050213-035937, PMID: 24313843
Steart D., Boon P., and Greenwood D. (2006). Overland transport of leaves in two forest types in southern Victoria, Australia and its implications for palaeobotanical studies. Proc. R. Soc. Victoria 118, 65–74.
Steart D., Boon P., Greenwood D., and Diamond N. (2002). Transport of leaf litter in upland streams of Eucalyptus and Nothofagus forests in south-eastern Australia. Archiv für Hydrobiologie 156, 43–61. doi: 10.1127/0003-9136/2002/0156-0043
Stone G. N. and Schönrogge K. (2003). The adaptive significance of insect gall morphology. Trends Ecol. Evol. 18, 512–522. doi: 10.1016/S0169-5347(03)00247-7
Swan C. M., Boyero L., and Canhoto C. (2021). “The Ecology of Plant Litter Decomposition in Stream Ecosystems: An Overview,” in he Ecology of Plant Litter Decomposition in Stream Ecosystems. Eds. Swan C. M., Boyero L., and Canhoto C. (Springer International Publishing, Cham).
The Herbivory Variability Network (2023). Plant size, latitude, and phylogeny explain within-population variability in herbivory. Science 382, 679–683. doi: 10.1126/science.adh8830, PMID: 37943897
Throop H. L. and Belnap J. (2019). Connectivity Dynamics in dryland litter cycles: moving decomposition beyond spatial stasis. BioScience 69, 602–614. doi: 10.1093/biosci/biz061
Tonin A. M., Pozo J., Monroy S., Basaguren A., Pérez J., Gonçalves J. F. Jr., et al. (2018). Interactions between large and small detritivores influence how biodiversity impacts litter decomposition. J. Anim. Ecol. 87, 1465–1474. doi: 10.1111/jane.2018.87.issue-5, PMID: 29928758
Tripathi O. and Pandey H. (2009). Litter production, decomposition and physico-chemical properties of soil in 3 developed agroforestry systems of Meghalaya, Northeast India. Afr. J. Plant Sci. 3, 160–167.
Vos V. C. A., van Ruijven J., Berg M. P., Peeters E. T. H. M., and Berendse F. (2011). Macro-detritivore identity drives leaf litter diversity effects. Oikos 120, 1092–1098. doi: 10.1111/j.1600-0706.2010.18650.x
Wappler T. (2010). Insect herbivory close to the Oligocene–Miocene transition — A quantitative analysis. Palaeogeography Palaeoclimatology Palaeoecol. 292, 540–550. doi: 10.1016/j.palaeo.2010.04.029
Wappler T. and Denk T. (2011). Herbivory in early Tertiary Arctic forests. Palaeogeography Palaeoclimatology Palaeoecol. 310, 283–295. doi: 10.1016/j.palaeo.2011.07.020
Wilf P. (2008). Insect-damaged fossil leaves record food web response to ancient climate change and extinction. New Phytol. 178, 486–502. doi: 10.1111/j.1469-8137.2008.02395.x, PMID: 18331425
Wilf P. and Labandeira C. C. (1999). Response of plant-insect associations to paleocene-eocene warming. Science 284, 2153–2156. doi: 10.1126/science.284.5423.2153, PMID: 10381875
Wilf P., Labandeira C. C., Johnson K. R., Coley P. D., and Cutter A. D. (2001). Insect herbivory, plant defense, and early Cenozoic climate change. Proc. Natl. Acad. Sci. 98, 6221–6226. doi: 10.1073/pnas.111069498, PMID: 11353840
Wilf P., Labandeira C. C., Johnson K. R., and Ellis B. (2006). Decoupled plant and insect diversity after the end-cretaceous extinction. Science 313, 1112–1114. doi: 10.1126/science.1129569, PMID: 16931760
Williams A. G. and Whitham T. G. (1986). Premature leaf abscission: an induced plant defense against gall aphids. Ecology 67, 1619–1627. doi: 10.2307/1939093
Wingard G. L., Bernhardt C. E., and Wachnicka A. H. (2017). The role of paleoecology in restoration and resource management—The past as a guide to future decision-making: review and example from the greater everglades ecosystem, U.S.A. Front. Ecol. Evol. 5. doi: 10.3389/fevo.2017.00011
Ximenes Pinho B., Dáttilo W., and Leal I. R. (2017). Structural breakdown of specialized plant-herbivore interaction networks in tropical forest edges. Global Ecol. Conserv. 12, 1–8. doi: 10.1016/j.gecco.2017.08.007
Xu Q., Jin J., and Labandeira C. C. (2018). Williamson Drive: Herbivory from a north-central Texas flora of latest Pennsylvanian age shows discrete component community structure, expansion of piercing and sucking, and plant counterdefenses. Rev. Palaeobotany Palynology 251, 28–72. doi: 10.1016/j.revpalbo.2018.01.002
Yarnes C. T. and Boecklen W. J. (2005). Abiotic factors promote plant heterogeneity and influence herbivore performance and mortality in Gambel’s oak (Quercus gambelii). Entomologia Experimentalis Applicata 114, 87–95. doi: 10.1111/j.1570-7458.2005.00226.x
Keywords: gall, herbivory, Mediterranean ecosystems, taphonomy, paleoecology
Citation: Adroit B, Schachat SR, Güner HT, Orts J-P and Denk T (2025) Litter leaves misrepresent plant–insect interactions in standing vegetation. Front. Ecol. Evol. 13:1549315. doi: 10.3389/fevo.2025.1549315
Received: 20 December 2024; Accepted: 22 August 2025;
Published: 18 September 2025.
Edited by:
Jerome Reynard, University of the Witwatersrand, South AfricaReviewed by:
Romulo Cenci, Unisinos University, BrazilLiana Chesini Rossi, Rio de Janeiro Botanical Garden, Brazil
Copyright © 2025 Adroit, Schachat, Güner, Orts and Denk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Adroit, YmVuamFtaW4uYWRyb2l0QG5ybS5zZQ==; YmVuamFtaW4uYWRyb2l0QGdtYWlsLmNvbQ==
 Benjamin Adroit
Benjamin Adroit Sandra R. Schachat
Sandra R. Schachat H. Tuncay Güner5,6
H. Tuncay Güner5,6 Thomas Denk
Thomas Denk