- 1Institute of Arid Agroecology, State Key Laboratory of Grassland Agro-Ecosystem, and College of Ecology, Lanzhou University, Lanzhou, Gansu, China
- 2Ministry of Education (MOE) Key Laboratory of Western China’s Environmental System, College of Earth and Environmental Sciences, Lanzhou University, Lanzhou, Gansu, China
Introduction: Climate change and extreme rainfall events pose significant challenges to water use strategies for forest species in subtropical regions. Increased degrees of drought and significant seasonal precipitation differences in recent decades in Yunnan Province, China, have exposed forests to high mortality rates. Therefore, there is an urgent need to understand the water use strategies of plants to help develop relevant forest conservation measures in this region.
Methods: In this study, we selected three co-occurring woody species (Pinus yunnanensis, Keteleeria evelyniana, and Castanopsis delavayi). We continuously monitored their sap flow and water potential, as well as environmental factors, to reveal plant water use strategies and to determine how water use strategies relate to environmental factors and vegetation traits.
Results: The results of this study revealed that seasonal water use strategies of plants were significantly different (P<0.01), with Js lower in the dry season than in the wet season, while the Js/Js,n was significantly higher in the dry season. Plant water use responded to seasonal environmental factors similarly. SWC was the main limiting factor for Jsin the dry season, and there was a positive correlation between Js,n and VPD; when SWC was sufficient in the wet season, VPD and PAR were the main factors on Js, and there was a negative correlation between Js,n and VPD. In addition, Js,n during the dry season consisted of En and Re, and En accounted for a high percentage (more than 60%). Finally, there are differences in the water use strategies of different species, with Pinus having less tight stomatal control in the dry season, possibly related to its deeper roots and relatively smaller leaf area.
Discussion: These findings on the water use strategies and environmental responses of different species complement our knowledge of survival strategies in subtropical forests and provide valuable advice for forest management.
1 Introduction
Against the background of global climate change, interannual or seasonal variability strongly impacts the processes of ecosystem water cycling. Previous studies have highlighted that the variability in precipitation amount, frequency and seasonal distributions has significantly changed during the past several decades (Domingo et al., 2011; Jia et al., 2016; Tarin et al., 2020). This variability could induce soil moisture fluctuations, and thus affect ecosystem processes and functions (Robertson et al., 2008; Niu et al., 2011; Verduzco et al., 2015). Transpiration, the principal pathway for plant water loss, closely couples terrestrial water cycling and energy balance (Ungar et al., 2013; Kumagai et al., 2014) and plays a critical role in the water budget of forest ecosystems (Siegert and Levia, 2011). Although several studies have quantified and compared tree transpiration amongst a diverse range of species at different sites (Yan et al., 2018; Lyu et al., 2020; Kassahun and Renninger, 2021), studies on the differences in transpiration among co-occurring tree species in subtropical forests are rare.
Sap flow is largely influenced by environmental factors, such as air temperature (Ta), photosynthetically active radiation (PAR), relative humidity (RH), water vapour pressure deficit (VPD), soil water moisture (SWC) and wind speed (WS) (Asbjornsen et al., 2007; Loranty et al., 2008; Chen et al., 2018; Hayat et al., 2020). The stomatal conductance is ultimately controlled by these factors. Usually, low or decreasing light, decreasing external humidity and different abiotic stresses, including low temperature and drought stress with reduced cellular water availability, will promote stomatal closure (Zhang et al., 2021; Yang and Qin, 2023). By contrast, high or increasing light, higher humidity and temperature can promote stomatal opening (Zhang et al., 2024a).
In addition, different species may exhibit different stomatal regulation strategies in changing environments (Kannenberg et al., 2021; Wright et al., 2024), with some species showing stronger stomatal control being described as isohydric plants, which reduce plant transpiration at the expense of reduced carbon gain. Other species with less stomatal control are described as anisohydric plants, which regulate transpiration more loosely and are therefore at higher risk of hydraulic damage (Jones and Raynal, 1986; Tardieu and Simonneau, 1998; McDowell et al., 2008). Strong correlations between iso-anisohydric stomatal behaviour and traits, such as specific leaf area (SLA), embolism resistance, woody density and root water uptake depth (Chen et al., 2021; Kaproth et al., 2023). SLA is an important leaf trait (White and Scott, 2006; Hulshof et al., 2013) and is intimately linked to water use strategies (Boucher et al., 2017). It is often applied to evaluate the performance of plants under drought conditions. Strategies with smaller SLA can improve stress resistance and competitive ability in poor environments (Westoby et al., 2002; Long et al., 2011). Root water uptake depth is an important part of plant water adaptation strategies, it is generally accepted that the different plant water uptake depth is associated with leaf water potential for their water use strategies (Liu et al., 2021). The species with shallower water uptake depth usually exhibited the larger diurnal ranges of leaf water potentials for their transpiration (Ding et al., 2020).
Nocturnal sap flow density (Js,n) has contributed to improved tree growth (Caird et al., 2007) and avoidance of tree mortality due to hydraulic failure (Klein et al., 2018; Zeppel et al., 2019). Js,n is influenced by the interaction among several environmental factors, including temperature (T), VPD, wind speed (WS), and soil water content (SWC) (Zeppel et al., 2014; Siddiq and Cao, 2018; Hayat et al., 2021). The temperature can directly affect the rate of Js,n, whereas the VPD indirectly regulates Js,n, mainly by affecting plant leaf transpiration (Wang et al., 2016). The WS not only affects the rate of evaporation from the plant leaf surface (Schymanski and Or, 2016) but also affects sap flow density by the generation of mechanical stress (Zhao and Fan, 2024). The soil water content determines the effective amount of soil water that can be absorbed by trees (Di et al., 2019). Js,n comprises nocturnal transpiration (En) and stem refilling activities (Re) following daytime water depletion, either occurring alone or concurrently (Daley and Phillips, 2006; Caird et al., 2007; Forster, 2014). Different effects of environmental factors on En and Re also lead to different responses of Jn,s to environmental factors. Therefore, we must combine these factors and consider the differences between species (Phillips et al., 2010; Zeppel et al., 2010) to more accurately reveal the formation pattern of Js,n.
Despite the increasing research documenting plant transpiration and its relationship with environmental factors, studies on the plant transpiration of the subtropical forest in Yunnan Province of China were rare. This region has a typical “southern subtropical dry and hot valley” climate, with a clear distinction of wet and dry, and is continuously subjected to up to half a year of drought (Luo et al., 2016; Li et al., 2019), which allows for a deeper study of the response of plant transpiration to environmental changes. In addition, this region is a popular area for global biodiversity research with very rich forest types (Pu et al., 2007; Qian et al., 2020), providing more possibilities to better understand the differences in transpiration responses of different species. Under such a situation, we chose the mixed forest formed by Pinus yunnanensis with other conifers and broadleaf trees as the research object to study the survival strategy of different species in typical seasonal drought. We investigated the water potential and sap flow changes and along with concurrent observations of climatic variables and the soil water content (SWC) in an experimental forest stand. The objectives of this study were to (1) differentiate the water use strategies in response to seasonal drought among different woody species; (2) identify the distinct meteorological drivers influencing Js in different species during both the wet and dry seasons; and (3) quantify the composition and use pattern of Js,n of the different tree species during both the wet and dry seasons.
2 Materials and methods
2.1 Site description
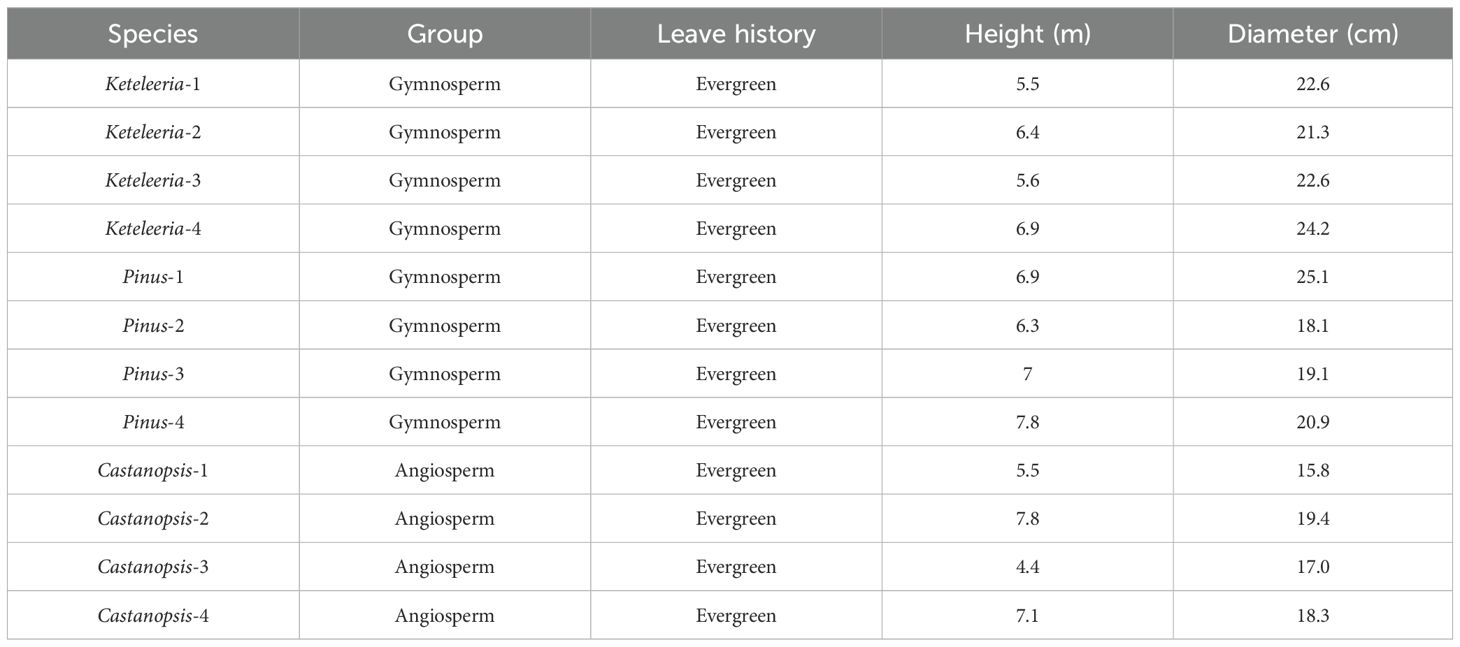
The study site is located at the top of Shasongpo in Yongren County, Chuxiong Yi Autonomous Prefecture, northern Yunnan Province (101°43′26″E, 26°5′53″N) (Figure 1), with a southern subtropical monsoon climate. The average rainfall in the study site was 840 mm, the average temperature was 22.6°C, the extremely high temperature on record was 43°C, and the average annual sunshine was 2534h (Zhang et al., 2024b). The site is dominated by the Pinus yunnanensis forest, which, in addition to Pinus yunnanensis, are accompanied by three species of trees, Keteleeria evelyniana, Castanopsis delavayi, and Quercus franchetii. The Quercus in the site were shrubs with numerous tillers, so three other trees were selected for the monitoring study (Table 1).
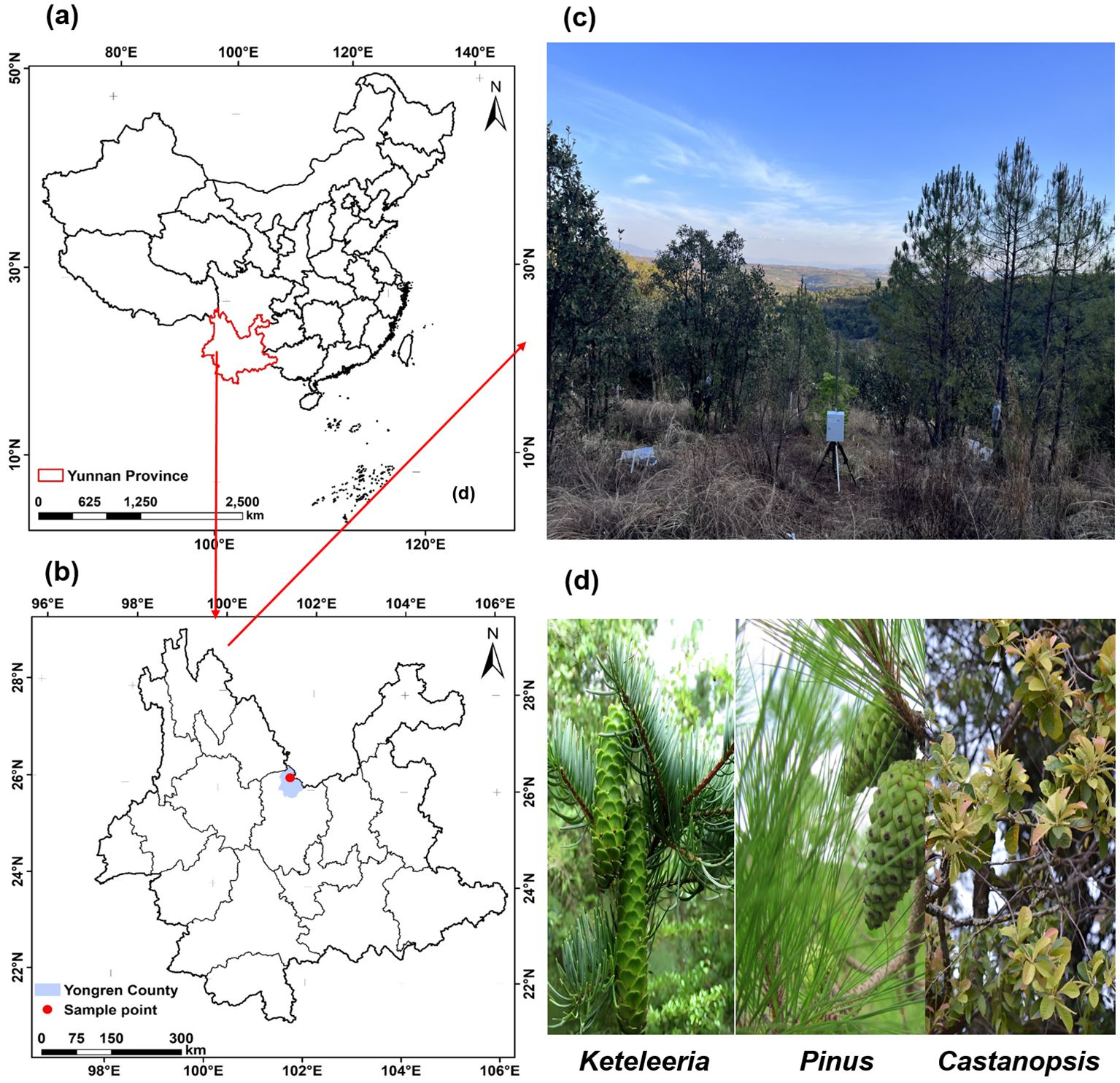
Figure 1. Location information for the research area (a, b), the current status of the sample plots (c), and the three researched co-occurring woody plants (d).
2.2 Environmental parameters
An integrated ATMOS 41 weather station (METER Group Inc., USA) was mounted on a forestry building at a distance of 100 m from the sample plot. The weather station was programmed to capture meteorological data once every 30 minutes. The observation parameters included solar radiation (Ra, W·m-2), precipitation (P, mm), air temperature (Ta, °C), WS (m·s-1), photosynthetically active radiation (PAR) (µmol·m-2·s-1), and VPD (kPa).
The soil volumetric water content was measured every 30 min at depths of 10 and 30 cm in this site via a Hydra Probe II device (Stevens Water Monitoring System, Inc., Portland, OR, USA). The sensors were inserted horizontally into the soil. Measurements were obtained every 10 s, and the 30-min averages were recorded via CR1000 data loggers (Campbell Scientific Inc., Logan, UT, USA). The SWC values (m3·m-3) analysed in this study were the average measurements at the depths of 10 and 30 cm.
2.3 Xylem water potential
We examined the xylem water potential of the three species (Pinus, Keteleeria, and Castanopsis). Measurements were conducted from December 2022 to May 2023 (dry season) and from July 2023 to November 2023 (wet season). 3–5 trees of each species were measured with a pressure chamber (PMS, Albany, OR, USA). The predawn water potential (Ψpd) measurements were taken before sunrise (between 05:30 and 06:30), and midday water potential (Ψmd) measurements were taken between 12:00 and 14:00 on the same day. We measured water potential in samples of branches with leaves (Keteleeria, Castanopsis: because the petiole of leaves is not long enough to fit in the sample holder of the pressure chamber) or leaves (Pinus). We used plastic bags and aluminum foil to wrap branches after sunset on the day before measurements; such operations reduced water loss by transpiration and thus equalised the water potential between leaves and branches. Samples were cut and water potential measurements were taken at the site within 15 minutes.
2.4 Sap flow measurements
The sap flow density (Js) was measured via heat dissipation probes (Granier, 1987), and four individuals of the three species selected in the study site were instrumented with two thermal dissipation probes (TDPs) mounted at the height of 1.3 m, with a distance of 10 cm between them. To avoid direct sunlight, the sensors were positioned on the shaded side of the trees and covered with radiation protection film. The instantaneous temperature difference between the probes was converted into a voltage value that was recorded via a data logger (Campbell Scientific Inc., Logan, UT, USA). The sap flow data were measured automatically at 30-minute intervals from January 2023 to December 2023.
As a measure of water exchange, sap flow density (g H2O·m-2 sapwood area ·s-1) was calculated based on an empirical calibration equation (Granier, 1985). Granier found that:
where Js is sap flow density (m3·m-2·s-1) and K is related to the temperature difference between the two probes:
where K is the sap flow index, ΔT is the temperature difference between heated and reference probe and ΔTmax is the temperature when there is no sap flow density (Js= 0).
According to the empirical relationship between sap flow density and the temperature difference between probes established by Grainer and revalidated by other researchers (Granier, 1985, 1987; Du et al., 2011), the sap flow density (Js, g·m-2·s-1) was calculated as follows:
where ΔT (°C) is the temperature difference between the heated and reference probes, and ΔTmax is the maximal temperature difference between the two probes, which was determined as the maximum value of daily ΔTmax over a 7–10 day period to avoid the underestimation of the nighttime sap flow (Lu et al., 2004).
2.5 Partitioning of nocturnal sap flow density refilling and nighttime transpiration
Js was separated into daytime sap flow density (Js,d) and nocturnal sap flow density (Js,n) components according to solar radiation (Ra) value greater than or less than 5 W·m-2 (Daley and Phillips, 2006). To distinguish the contributions of stem refilling (Re) and nighttime transpiration (En) to the nocturnal sap flow density, the ‘forecasted refilling’ approach was adopted in this study (Fisher et al., 2007; Yu et al., 2018). This is a time separation method where the declining portion of the diurnal course of Js is extrapolated forward in time until it reaches zero flow, as expected if there was no nocturnal water loss, thus the area below this forecasted curve is an estimate of refilling (Re) (Fisher et al., 2007). In turn, the area above the forecasted curve and all Js,n observed after this curve has reached zero, indicates nocturnal water loss (En) (Fisher et al., 2007). The proportion attributable to En was then calculated as the area above the forecasted refilling curve divided by the total Js,n.
To construct the forecasted curve, we fitted an exponential decay based on the trend of Js,n over time on a typical sunny day in winter, where t = 0 was 2 h before sunset. Since SWC is sufficient in winter, when nighttime VPD is minimal, all of the Js,n can be considered to be caused by Re. When nighttime VPD is high during the dry season, changes in Js,n caused by Re should follow the same slope and timing pattern as in winter (Fisher et al., 2007).
2.6 Statistical analysis
The different responses of Js for three co-occurring woody plants to meteorological factors in the dry and wet seasons were fitted by nonlinear functions. We employed analysis of variance (ANOVA) to compare their interactions for the variables Ψ, Js, Js,n, and Js,n/Js. We calculated the contribution of meteorological factors to Js,n by performing multiple linear regressions. All the statistical analyses and plotting were performed via SPSS 13.0 (SPSS, Chicago, IL, USA) and Origin 8.0 (OriginLab, USA), respectively. All the above analyses were performed based on daily average data.
3 Results
3.1 Climate and soil water content
There were significant differences in meteorological factors and soil water content between dry and wet seasons in the research area (Table 2). The precipitation mainly between June to November (wet season) and more than 95% of the total rainfall was concentrated during the wet seasons (Figure 2). The VPD during the dry season (1.25 Kpa) was significantly greater than that during the wet season (0.66Kpa) (Figure 2, Table 2). The dry season exhibits high atmospheric evaporative demand and water scarcity. The SWC is highly consistent with rainfall and differs between the wet and dry seasons. The SWC in the 10-cm soil layer was low during the dry season (SWC< 0.05 m3·m-3) and recovered during the rainy season (SWC > 0.1 m3·m-3). The SWC in the 10-cm layer was more sensitive to the timing of short rainfall events than that in the 30-cm layer.
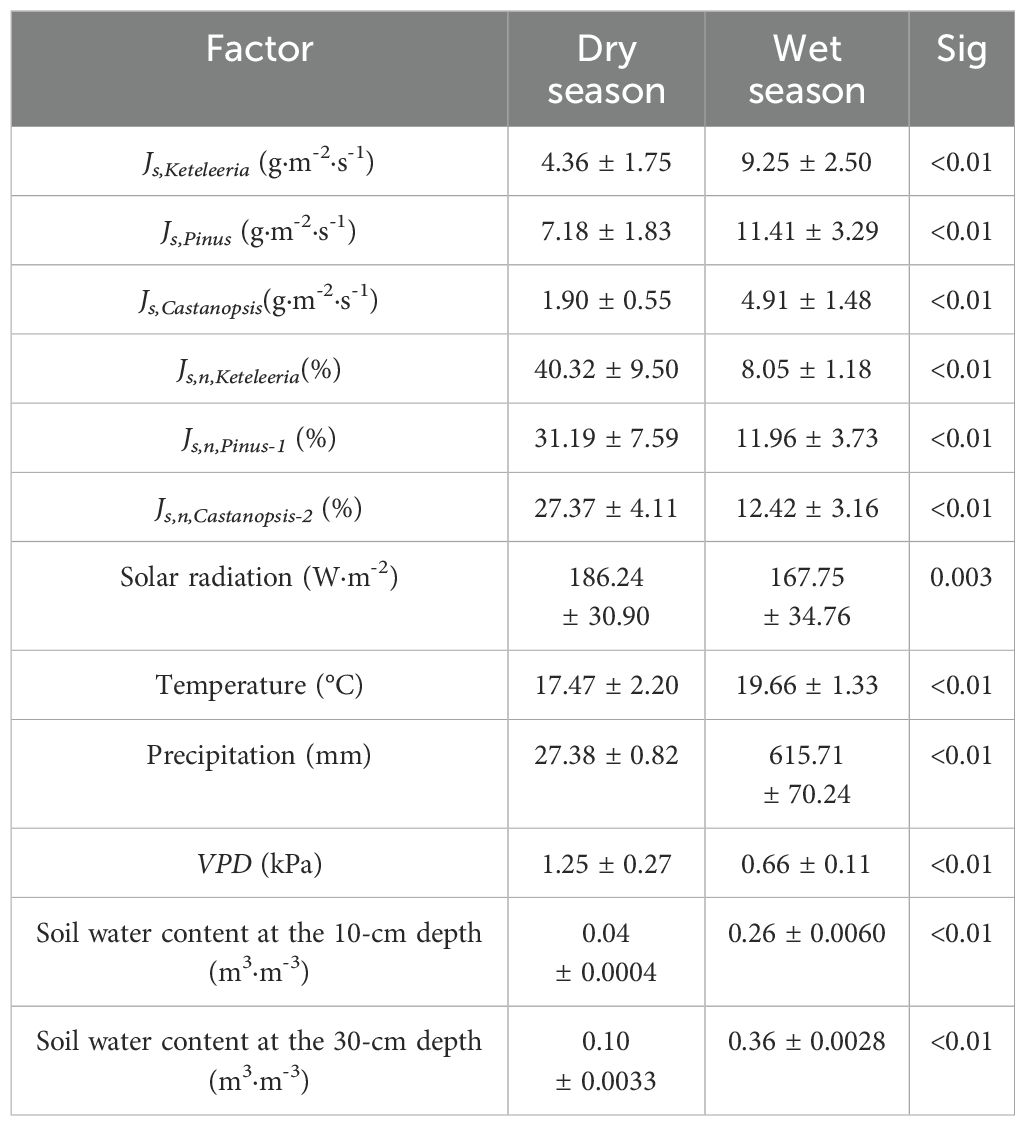
Table 2. Seasonal differences in sap flow (Js), the proportion of the total sap flow (Js, n/Js) and meteorological factors.
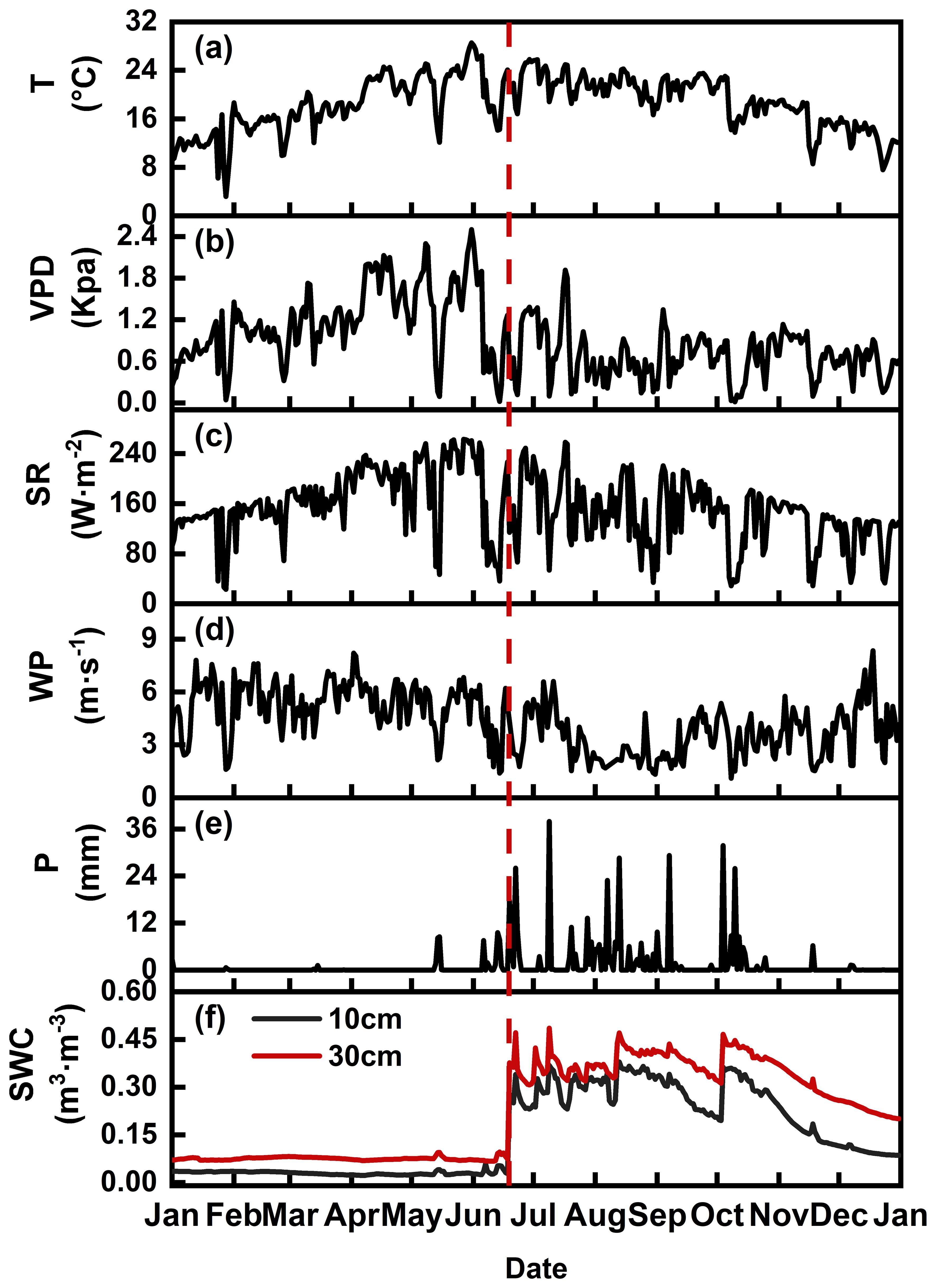
Figure 2. Monthly average temperature (T) (a), vapour pressure deficit (VPD) (b), solar radiation (SR) (c), wind speed (WS) (d), precipitation (P) (e) and soil water content (SWC) (f) at a soil depth of 10 cm in the research stand over the study period. The red dotted line represents the first effective rainfall of the year.
3.2 Seasonal variations in xylem water potential
The xylem water potential of the three woody plants during the wet and dry seasons were significantly different, and Ψpd and Ψmd were significantly more negative during the dry season than during the wet season for all three woody plants (Figure 3). During the dry season, the Ψpd values of Keteleeria and Pinus were greater than that of Castanopsis, indicating that these two conifers may access more water for refilling at night. However, there was no significant difference between the three woody plants in terms of either Ψpd or Ψmd during the wet season.
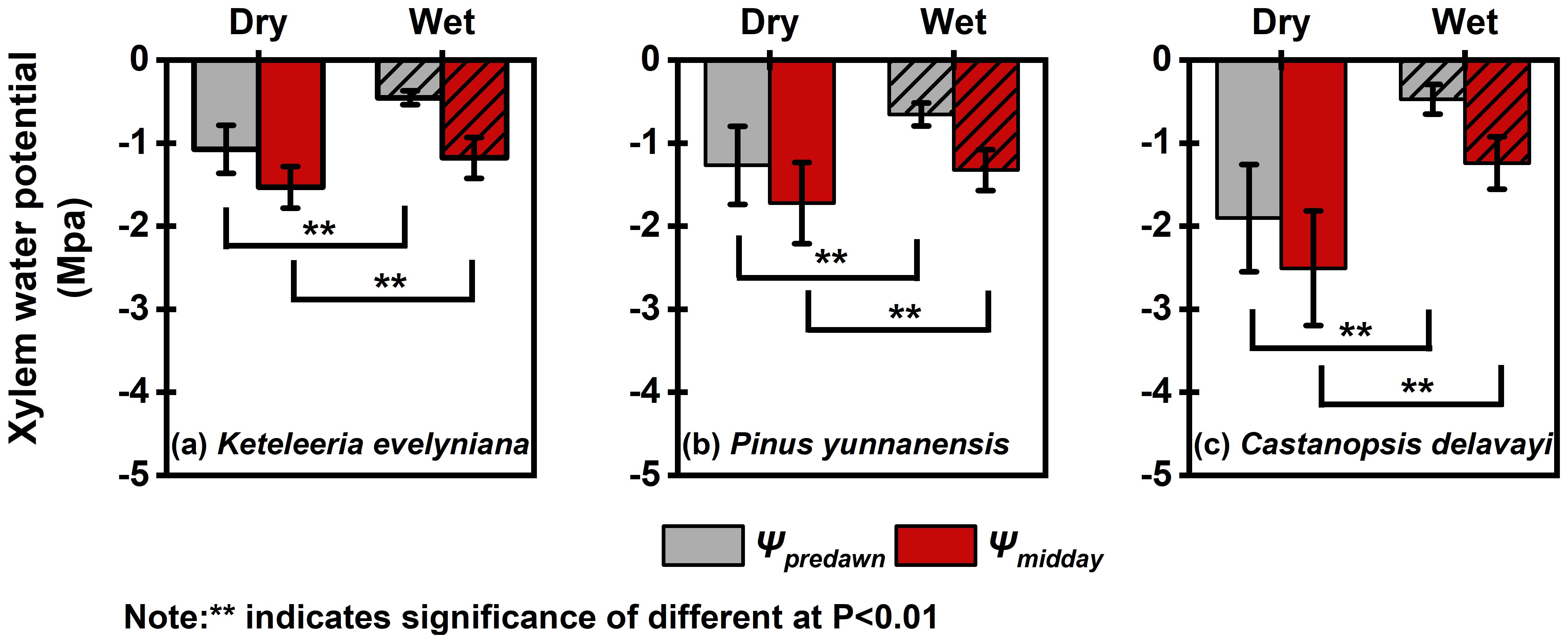
Figure 3. Seasonal changes in the predawn (Ψpredawn) and midday (Ψmidday) xylem water potentials of the three tree species: Keteleeria evelyniana (a), Pinus yunnanensis (b), and Castanopsis delavayi (c). The filled shading (diagonal lines) in the figure is used to distinguish between dry and wet seasons. The values are means ± standard errors (SEs). “**” indicates significance of difference at P < 0.01.
3.3 Seasonal variations in Js and controlling factors
There species were significant differences in the sap flow density (Js) of the three plants between the dry and wet seasons (Figure 4). The Js of the Pinus, Keteleeria, and Castanopsis were 4.36 g·m-2·s-1, 7.18 g·m-2·s-1 and 1.90 g·m-2·s-1 in the dry season while 9.25 g·m-2·s-1, 11.41 g·m-2·s-1 and 4.91 g·m-2·s-1 in the wet season, respectively (Table 2).
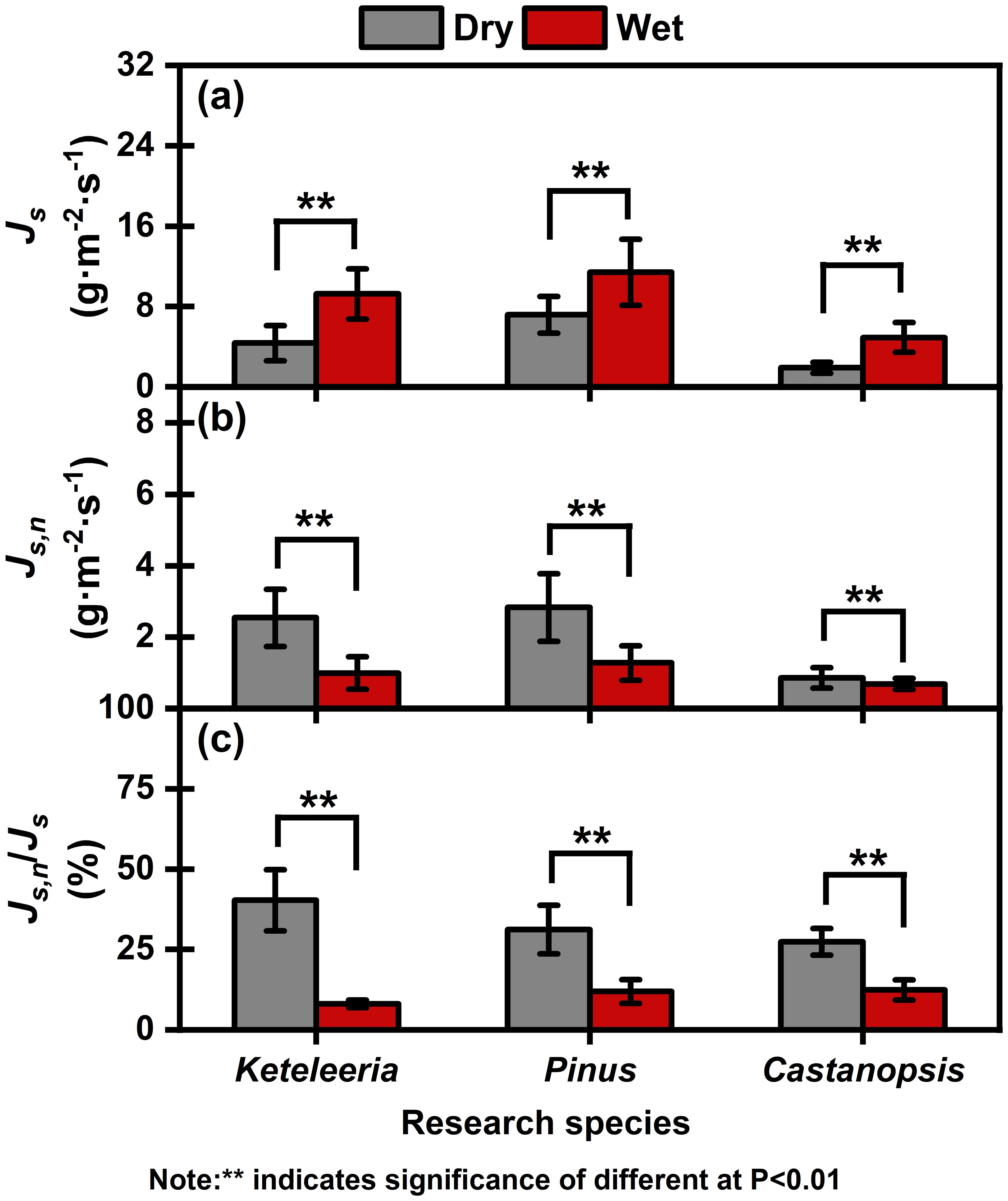
Figure 4. Seasonal mean sap flow density (Js) (a), nocturnal sap flow density (Js, n) (b), and percentage of the nocturnal sap flow density (Js, n/Js) (c) for Keteleeria, Pinus, and Castanopsis during the dry and wet seasons. Different filled colours are used to distinguish between dry and wet seasons. The values are means ± standard errors (SEs). “**” indicates significance of difference at P < 0.01.
The effects of environmental factors on Js significantly differed between the dry and wet seasons (Figure 5). During the dry season, the soil water content was the main factor influencing Js, and Js increased with increasing soil water content (Keteleeria, R2 = 0.76; Pinus, R2 = 0.74; Castanopsis, R2 = 0.74; all P values< 0.01). An increase in the VPD at this time resulted in a decrease in Js, but this negative correlation was not significant. During the wet season, the VPD, however, was the main factor influencing Js. Unlike during the dry season, at this time, there was a positive correlation between the VPD and Js, and Js increased with increasing VPD (Keteleeria, R2 = 0.76; Pinus, R2 = 0.73; Castanopsis, R2 = 0.32; all p values< 0.01). Additionally, solar radiation attained a significant positive correlation with Js during the wet season.
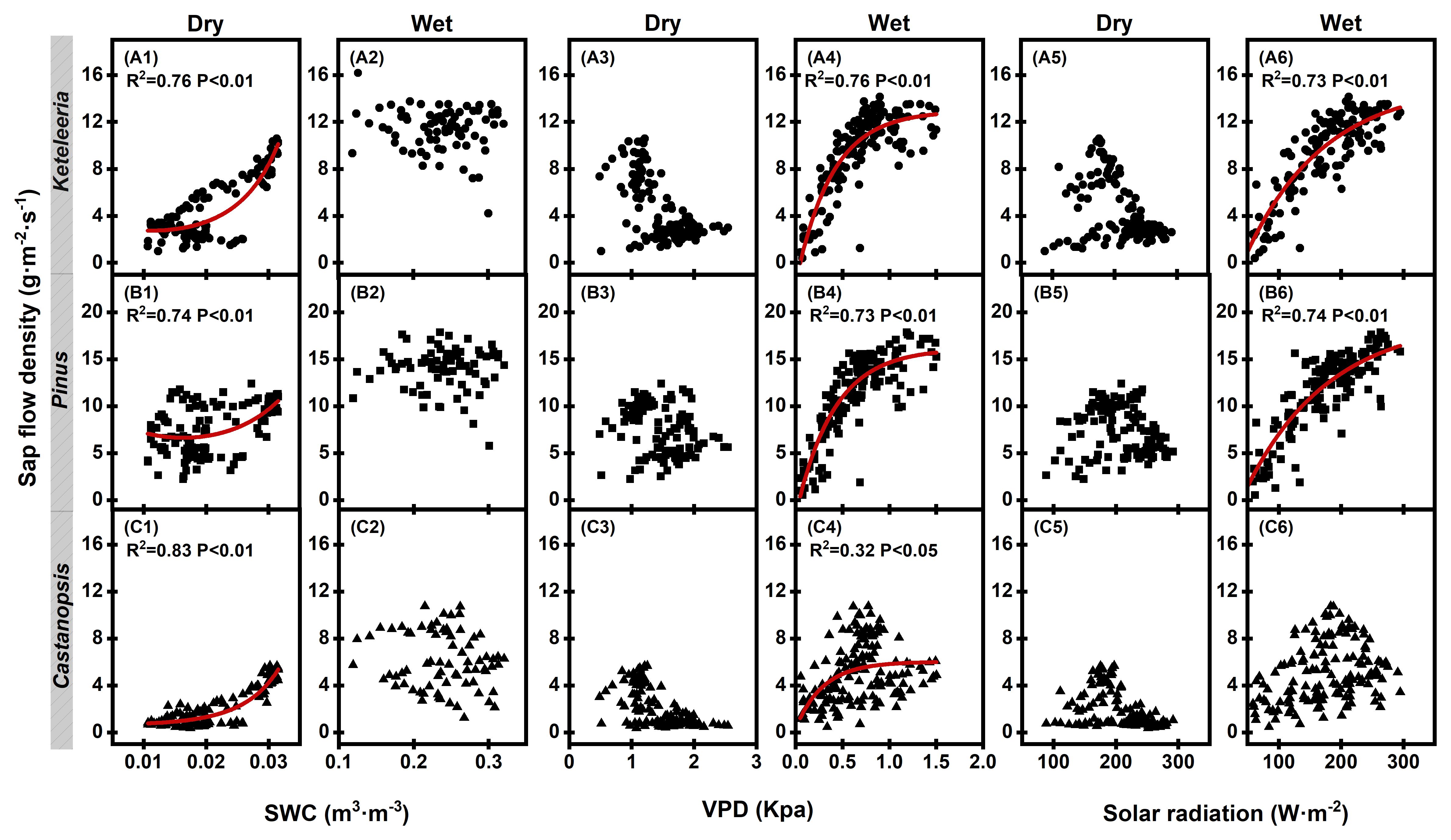
Figure 5. Results of nonlinear fitting of plant sap flow density (Js) to meteorological factors in dry and wet seasons. Relationships between the Js and the soil water content (SWC), vapour pressure deficit (VPD), and solar radiation (Ra) for Keteleeria (A1–A6), Pinus (B1–B6), and Castanopsis (C1–C6) during the dry and wet seasons. A single point represents the mean of the four sample trees monitored for each tree species. The symbol shapes of circles, squares and triangles represent different species of Keteleeria, Pinus, Castanopsis, respectively. The red curve is the result of nonlinear fitting.
3.4 Daily variations in Js and controlling factors
The three woody plants differed in their daily course of the sap flow density (Js) on clear days with comparable climatic conditions (Figure 6). On typical sunny days, Keteleeria and Castanopsis showed opposite trends to changes in VPD after sunrise, while Pinus remained roughly consistent with changes in VPD. However, Pinus showed a midday depression of Js, which is a typical physiological response to high VPD in the dry season.
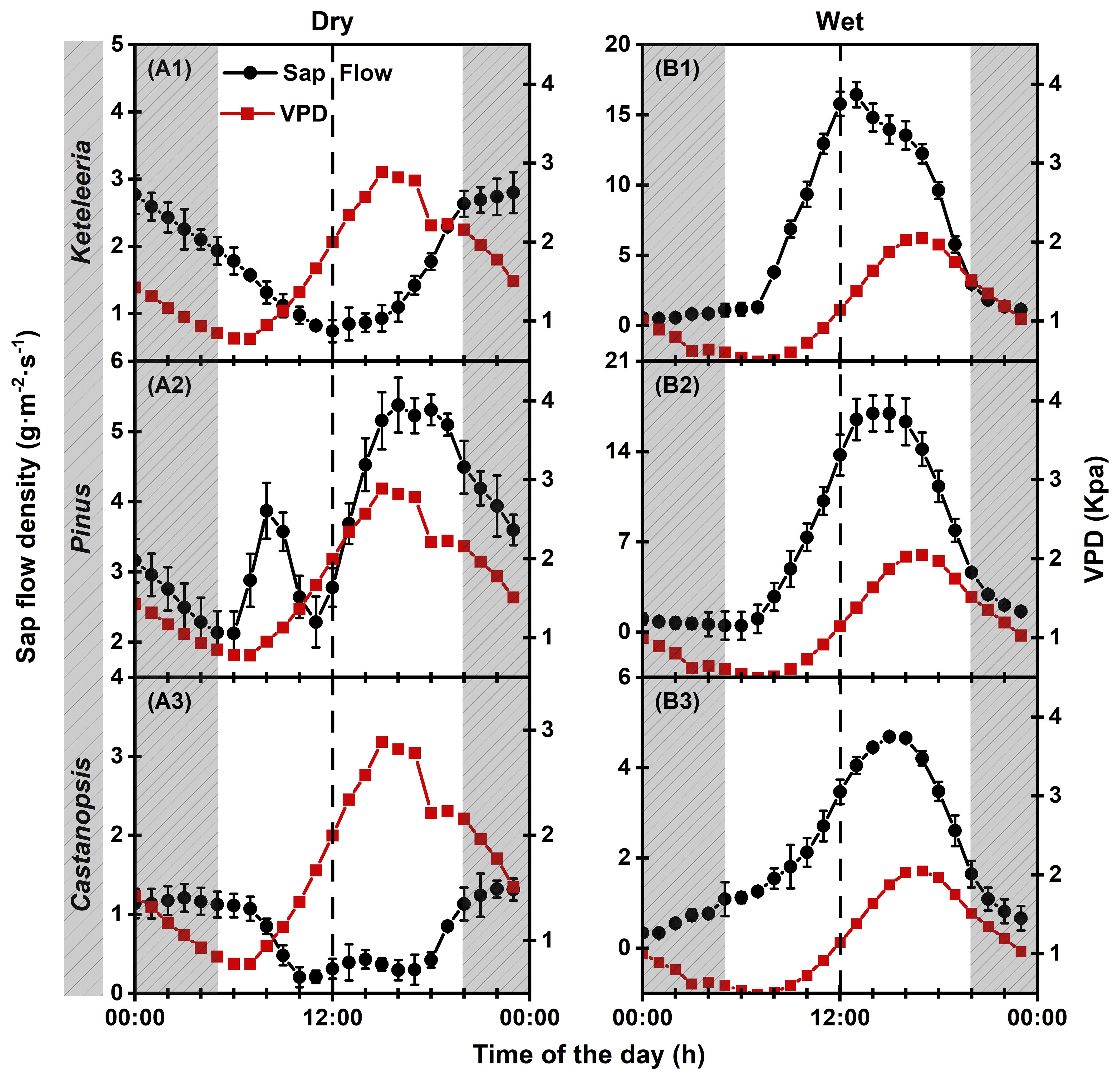
Figure 6. Diurnal courses of the VPD and the sap flow density (Js) for Keteleeria, Pinus, and Castanopsis during the dry (A1–A3) and wet (B1–B3) seasons. The values are the averages of 5–10 representative clear days for each individual. The shaded areas denote nighttime values. The straight line where the circle is located represents plant sap flow. The straight line where the square is located represents VPD. The error bars are the SEs of the repeated samples for each species.
3.5 Seasonal variations in Js,n/Js and controlling factors
The daily percentage of the nocturnal sap flow density (Js,n/Js) in the three co-occurring tree plants increased consistently during the dry season (with increases of five times for Keteleeria, three times for Pinus, and more than two times for Castanopsis) (Figure 4; Table 2). The Js,n/Js were significantly higher in Keteleeria and Pinus than in Castanopsis (Figure 4).
The response of Js,n to meteorological factors differed between the dry and wet seasons (Table 3). During the dry season, the SWC was negatively correlated with Js,n/Js, whereas the temperature (Ta), VPD and WS were positively correlated with Js,n/Js (Table 3). During the wet season, when the relationship between the SWC and Js,n/Js was not significant, the WS and VPD were negatively correlated with the percentage of the nocturnal sap flow density, whereas the temperature (Ta) was positively correlated with Js,n/Js. The contribution of environmental factors to Js,n/Js was different among species in the dry season (Figure 7). The contribution of SWC was the largest to the Js,n/Js for Castanopsis and Pinus, yet the contribution of VPD was the largest to the Js,n/Js for Keteleeria. During the wet season, it was VPD that contributed the most to the Js,n/Js for all three species.
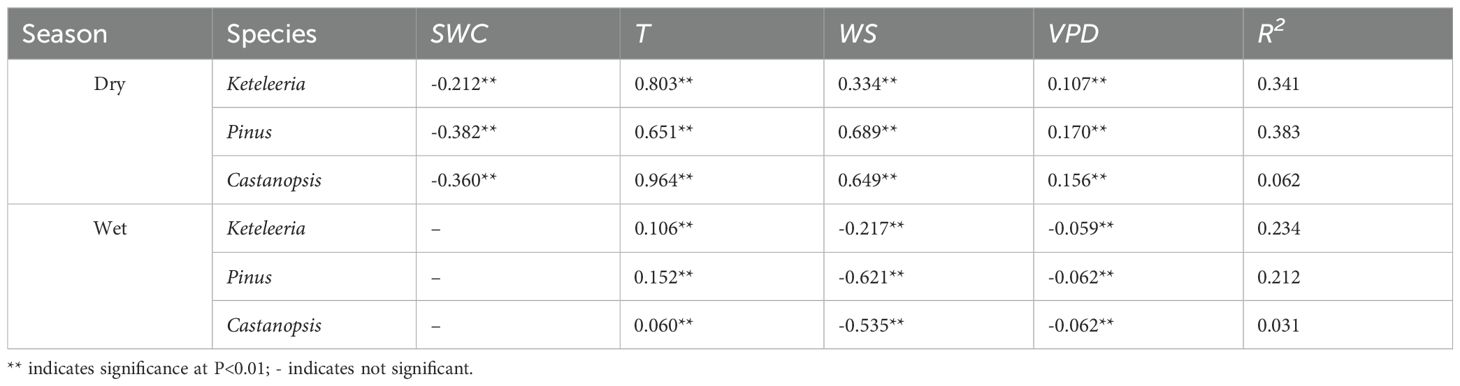
Table 3. Standardised regression coefficients between the percentage of nocturnal sap flow and meteorological factors.
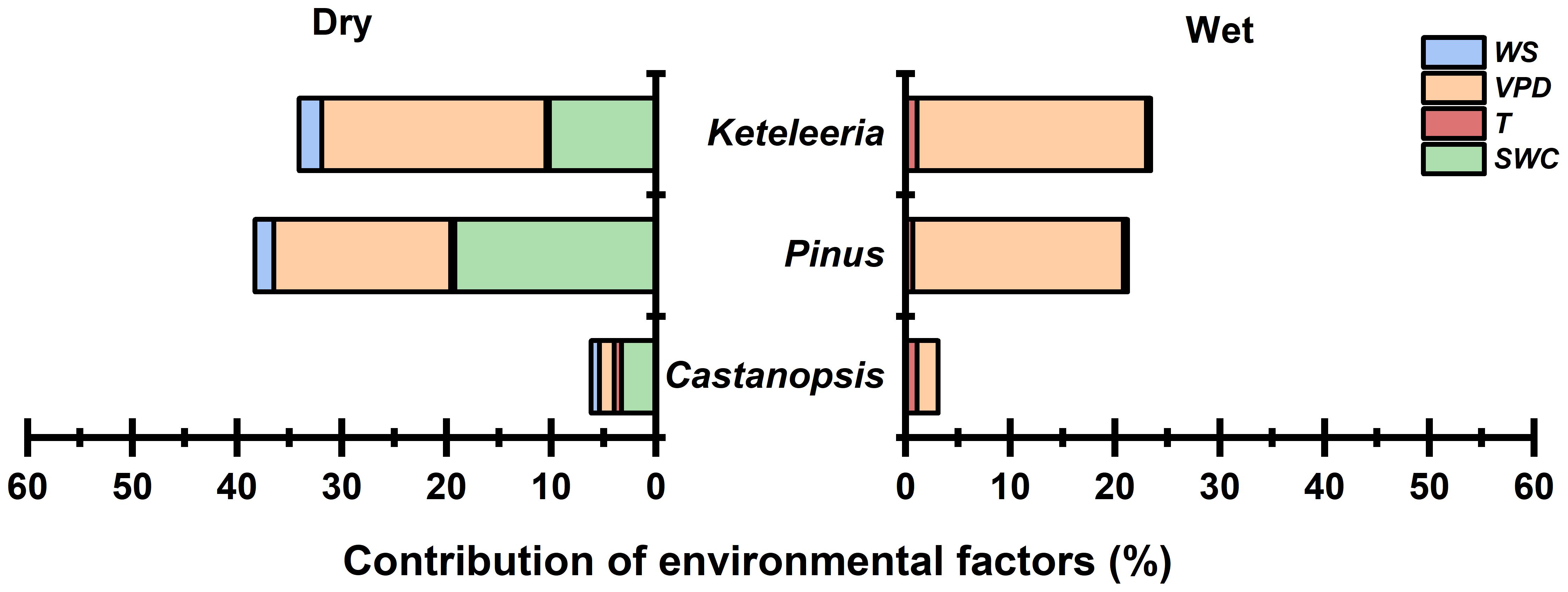
Figure 7. Contribution of environmental factors (wind speed, vapour pressure deficit, air temperature, and soil water content) to the nocturnal sap flow density of the three tree species (Keteleeria, Pinus, and Castanopsis) during the dry and wet seasons, obtained from multiple linear regression equations (contribution expressed as R2 values).
3.6 Daily variations in Js,n and the composition
The composition of Js,n differed between the wet and dry seasons. Notably, Js,n during the dry season did not decrease gradually to zero with the decline in Ra (Figure 8). The Js,n comprised a combination of nocturnal transpiration (En) and refilling activities (Re), and a higher proportion of the En at night (Keteleeria=64.90%; Pinus=79.70%; Castanopsis=73.01%), whereas during the wet season, it encompassed Re alone (Figures 8, 9). The percentage of En in Js,n was higher in Pinus than that in Keteleeria and Castanopsis.
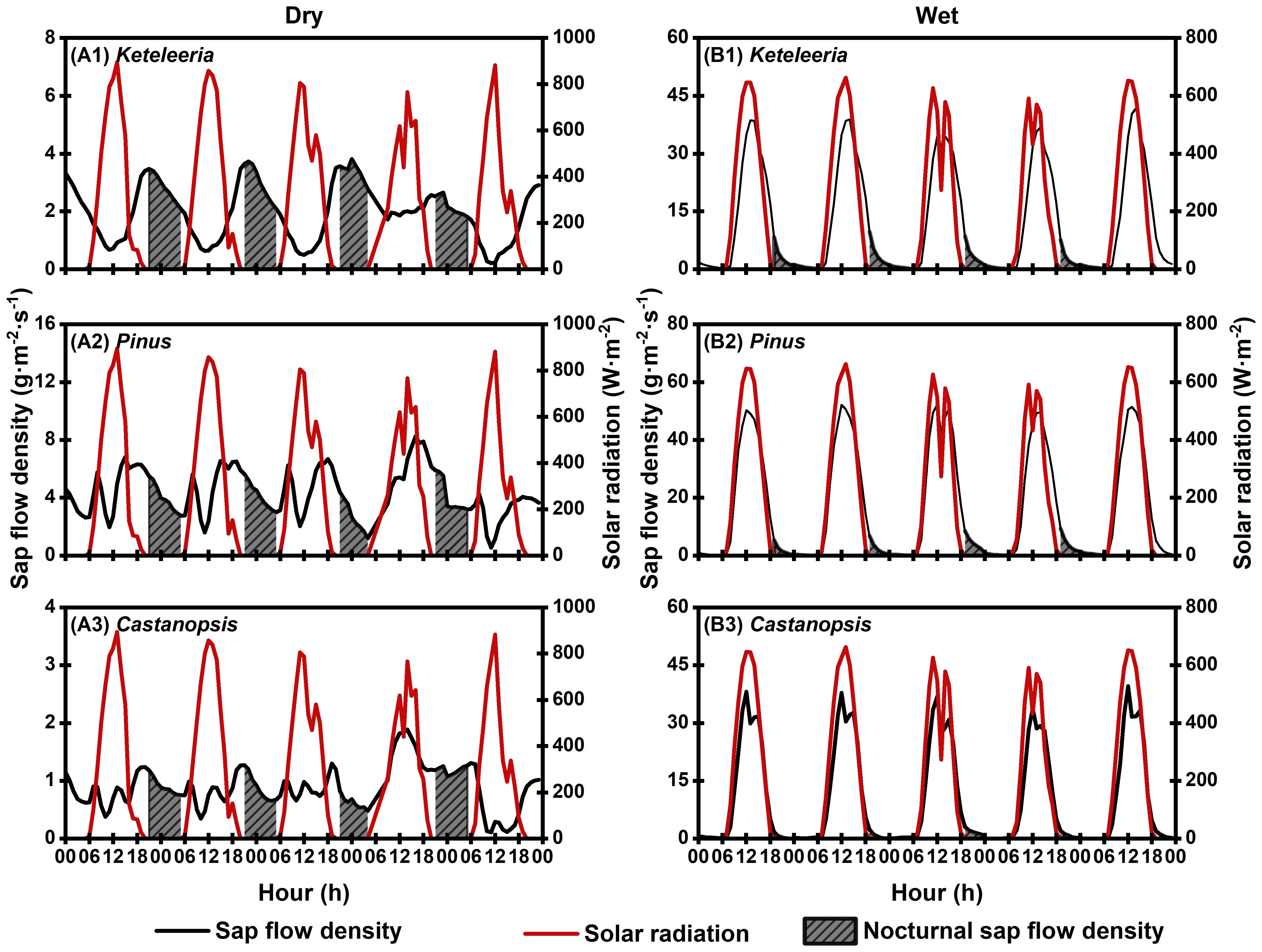
Figure 8. Continuous changes in the nocturnal sap flow density of the three species (Keteleeria, Pinus, and Castanopsis) on five typical sunny days during the dry (A1–A3) and wet seasons (B1–B3). The solid black line is plant sap flow. The solid red line is solar radiation. The shaded area is the time period when nocturnal sap flow density occurs.
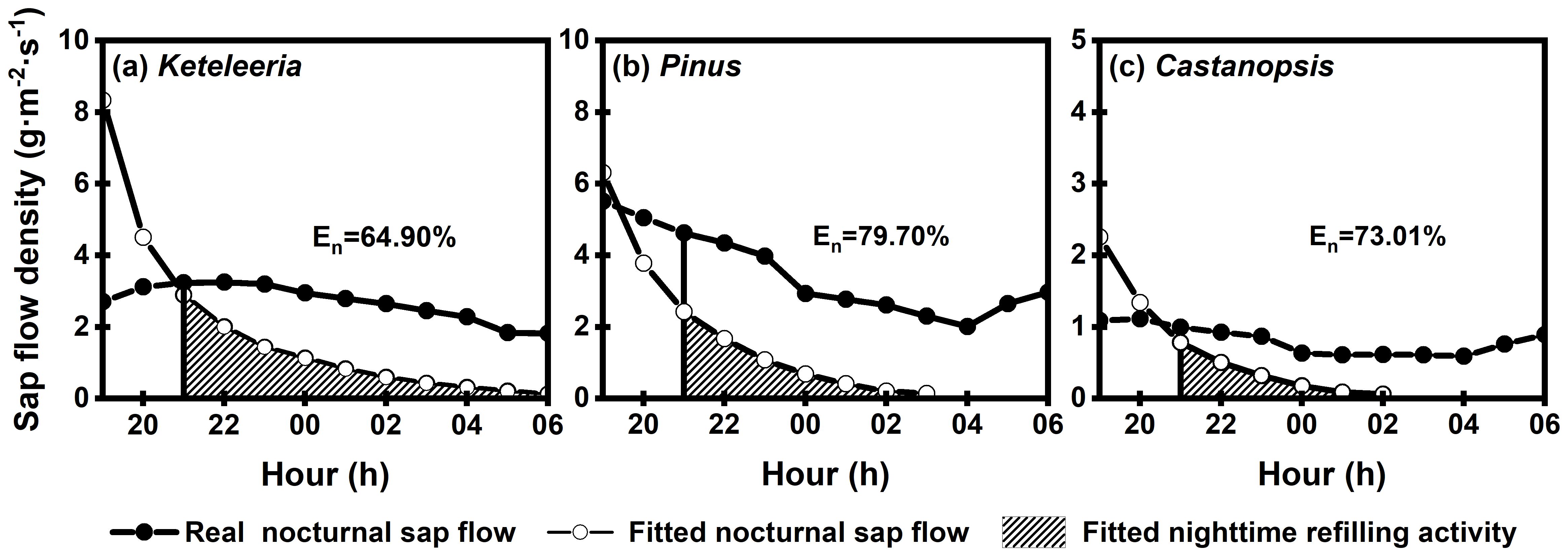
Figure 9. Composition of nocturnal sap flow in the three tree species (Keteleeria (a), Pinus (b), and Castanopsis (c)) during the dry season. The solid circular straight line is the real nocturnal sap flow; the dashed circular straight line is the nocturnal sap flow obtained by fitting. The shaded area is the area below the fitted curve and represents nighttime refilling activity.
4 Discussion
Three co-occurring woody plants showed the same seasonal variation in Js, the mean Js is lower in the dry season than in the wet season (Figure 4). Our results were consistent with those of Lu et al. (2013), who studied variations in whole-tree transpiration of poplar during the wet and dry seasons. They found that the whole-tree transpiration during the dry season was much lower than in the wet season (only 10-20%). Additionally, the Js,n/Js of three co-occurring woody plants were higher in the dry season than in the wet season (Figure 4). We suggest that increasing Js,n in the dry season is a long-term adaptive strategy for plants in this region. Js,n has been proven to occur in a range of woody plants and has important effects on the water balance between soil and plants (Daley and Phillips, 2006; Zeppel et al., 2010; Forster, 2014).
The Js of three co-occurring woody plants responded similarly to environmental factors in the wet and dry seasons. The SWC was the most significant factor influencing Js during the dry season and the PAR and VPD were the major factors influencing Js during the wet season in our study (Figure 5). This similar pattern is consistent with previous results from mixed tropical forests (Meinzer et al., 1995, 1997; O’Brien et al., 2004). More than 85% of the precipitation occurred in wet season during the experimental period, and our study site is second only to Tibet in terms of solar radiation (Figure 2; Table 2). During the dry season, less precipitation and strong light intensity decrease available moisture in soil and/or atmosphere, thus increasing water stress in plants (Wang et al., 2017b). During the wet season, when SWC increased, VPD and PAR were the main environmental factors driving transpiration. Several studies have shown that high VPD and PAR are critical driving variables for transpiration in numerous ecosystems (Wang et al., 2017a; Oogathoo et al., 2020; Ghimire et al., 2022). Ochoa and Abdallah (2023) also showed that VPD is the major environmental factor driving transpiration under higher SWC, and that increased transpiration is enhanced by increased sensitivity of transpiration to PAR.
In our study, SWC affected the response of Js,n to meteorological factors. VPD was positively correlated with Js,n in the dry season, while it was negatively correlated in the wet season (Table 3). Rosado et al. (2012) showed the same results: a positive correlation exists between Js,n and VPD in tropical rainforest plants during the dry season. But Chen et al. (2020) observed a significant negative effect between nocturnal VPD and Js,n in forests with dry soils, while there was no correlation between nocturnal VPD and Js,n under wetter soil conditions. Low soil moisture and atmospheric drought trigger stomatal closure (Cavender-Bares et al., 2007; Zeppel et al., 2010; Cirelli et al., 2015). VPD is a major transpiration driver that increases transpiration even when light is absent, thus facilitating Js,n in previous studies (Zeppel et al., 2010; Pfautsch et al., 2011). Thus, in the mixed forests of the study area, the physical promotion of VPD on stomatal water loss overwhelmed the inhibition of stomatal opening by soil drought and atmospheric drought. Whereas, in the wet season, sufficient soil moisture avoided plant stomatal opening at night.
Daily variations in water use strategies differed among species unlike the similar seasonal water use for the three co-occurring plants. The changes in hourly Js with VPD and solar radiation over the day differed for the three woody plants on a typical sunny day in the dry season (Figure 6; Figure 10). This suggests that responses to drought depend on species. The Js for Castanopsis and Keteleeria were smaller in daytime than at nighttime, and Js decreased with increasing VPD after sunrise on typical sunny days in the dry season (Figure 6). This reflects relatively strong stomatal control of Castanopsis and Keteleeria and is considered isohydric plants. In contrast, the Js in Pinus was greater in daytime than at nighttime, and there was a significant positive correlation between Js and VPD (Figures 6, 10), which reflects relatively weak stomatal control and is considered an isohydric plant. Previous studies have shown that the relevant traits that determine water strategy are acquisition efficiency (root characteristics) and utilisation efficiency (leaf and stem characteristics) (Wright et al., 2004; Moreno-Gutierrez et al., 2012). In this study, Castanopsis, the only broadleaf tree among the three species. Broadleaf species are less resistant to drought-induced cavitation than coniferous species and have less hydraulic safety thresholds (Choat et al., 2012). This may be due to the coupling differences between leaf and wood phenology, cell (vessel/tracheid) density, or anatomical structure (Vitasse et al., 2019; Tan et al., 2020). Castanopsis has higher SLA than Keteleeria and Pinus (Tang 2025; under review), and higher SLA accelerate water loss (Reich and Cornelissen, 2014; Künzi et al., 2025). However, the findings of this study demonstrate that the transpiration of Castanopsis was still smaller than that of the other two conifers during the wet season. Therefore, it can be hypothesised that other environmental or morphological factors may have contributed to the result of lower transpiration of Castanopsis. We will continue to explore the plant functional traits of the three species in the hope of explaining in more detail the differences in survival strategies of the three species. The Keteleeria is a conifer species, but a previous study showed that Pinus could use deeper soil water, and the leaf δ13C of Pinus was higher than that of Keteleeria, which had a higher water utilisation efficiency in the dry season (He, 2022). These characteristics enhance the drought resistance of Pinus, which has a relatively constant stomatal conductance, ensuring transpiration and making their survival less limited.
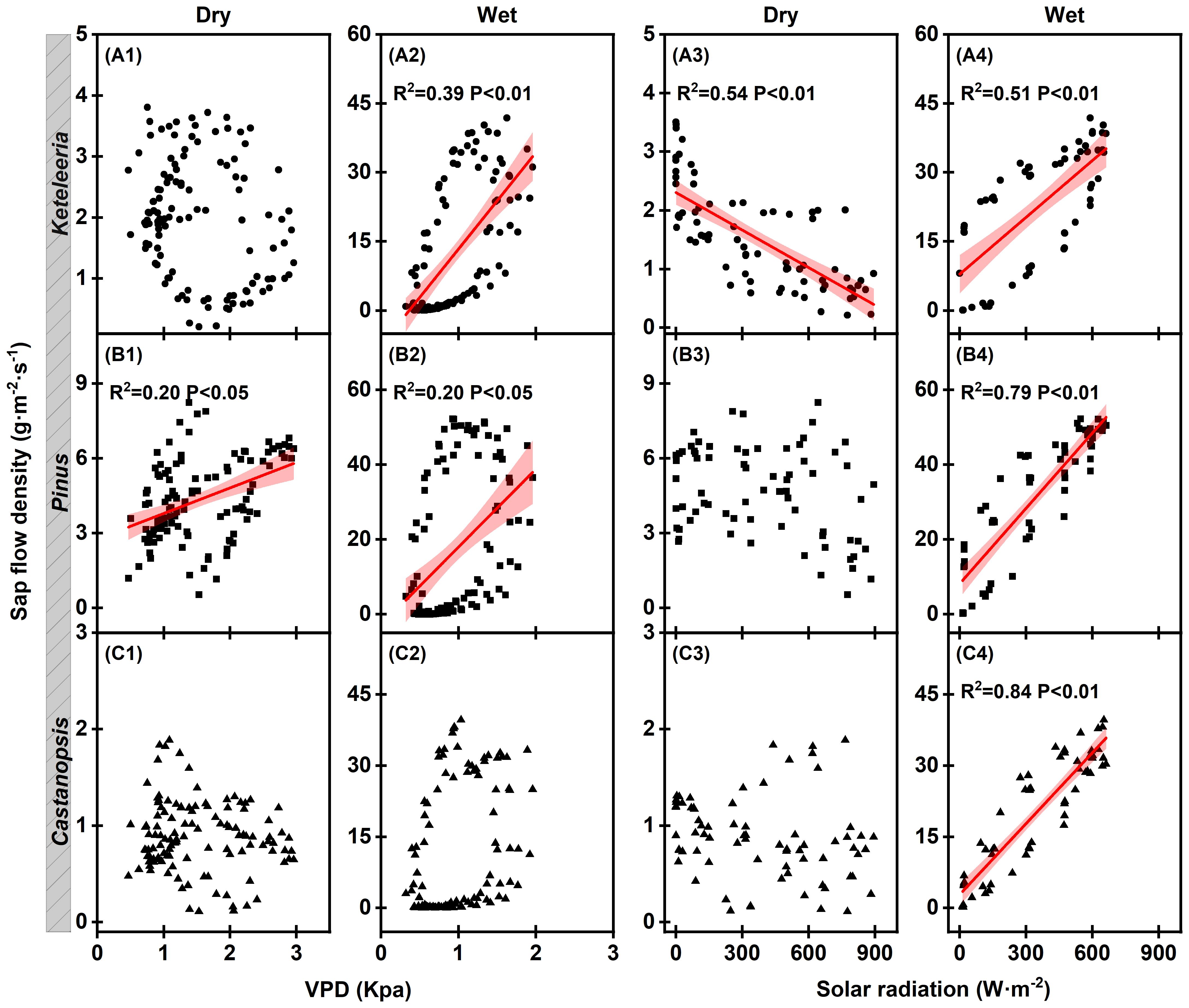
Figure 10. Result of linear fitting of hourly sap flow density to VPD and Solar radiation for five typical sunny days, Keteleeria (A1–A4), Pinus (B1–B4), and Castanopsis (C1-C4) in the dry and wet seasons. A single point represents the mean of the four sample trees monitored for each tree species. The solid red line is the result of a linear fitting. Shaded areas represent 95% confidence intervals.
Significant differences in the Js,n/Js among the three species during the dry season (Figure 4), with a higher Js,n/Js in Keteleeria and Pinus, and a lower Js,n/Js in Castanopsis, which may be related to their use of different water use strategies. The low Js,n/Js may reflect low water loss at night due to strict stomatal control (Zeppel et al., 2010). Thus, Keteleeria and Pinus had less tight stomatal control. The Js,n for three co-occurring woody plants in the dry season was composed of En and Re, and the proportion of En was more than 60% in our study (Figure 9). En is closely linked to stomatal activity, higher En may exacerbate water loss and expose plants to higher hydraulic stress. Pinus has the highest percentage of En and therefore may be most stressed under future climate change.
5 Conclusions
There were seasonal and species differences in sap flow and water potential of three co-occurring woody species (Keteleeria evelyniana, Pinus yunnanensis, and Castanopsis delavayi) in subtropical forests of Yunnan Province. Seasonal differences in the water use strategies of the three species were characterised by significantly smaller sap flow in the dry season than in the wet season and a significantly larger percentage of nocturnal sap flow in the dry season. The seasonal response of the sap flow to environmental factors was shown that SWC was the main influence factor in the dry season, and nocturnal sap flow was negatively correlated with VPD; while in the wet season, when there was sufficient soil moisture, VPD and PAR were the main influence factors, and nocturnal sap flow was negatively correlated with VPD. In addition, Pinus, which has less tight control of stomata conductance, may suffer higher hydraulic stress during the dry season. The sap flow of Pinus increased with increasing VPD on typical sunny days during the dry season and had the highest percentage of nocturnal sap flow. The results of the fitted exponential decay equations showed that the Pinus had the highest percentage of nocturnal transpiration. Our results provide greater insights into the water use strategies of plants in this region, highlighting the physiological and ecological significance of plant water use strategies and nocturnal sap flow during the different seasons. The results could help to more accurately predict plant feedback to the environment and changes in survival strategies, especially within the context of climate change, with increasing nighttime temperatures and more frequent droughts.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author contributions
JT: Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. ZD: Investigation, Methodology, Writing – review & editing. GW: Investigation, Methodology, Writing – review & editing. YW: Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by the National Natural Science Foundation of China (No. 42271048).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Js, sap flow density; Js,n, nocturnal sap flow density; En, nocturnal transpiration; Re, stems refilling activities; Ψleaf, leaf water potential; Ψpredawn, predawn water potential; Ψmidday, midday water potential; T, temperature; VPD, vapour pressure deficit; Ra, solar radiation; P, precipitation; WS, wind speed; SWC, soil water content; DBH, diameter at breast height.
References
Asbjornsen H., Tomer M. D., Gomez-Cardenas M., Brudvig L. A., Greenan C. M., and Schilling K. (2007). Tree and stand transpiration in a Midwestern bur oak savanna after elm encroachment and restoration thinning. For. Ecol. Manage. 247, 209–219. doi: 10.1016/j.foreco.2007.04.043
Boucher F. C., Verboom G. A., Musker S., and Ellis A. G. (2017). Plant size: a key determinant of diversification? New Phytol. 216, 24–31. doi: 10.1111/nph.14697
Caird M. A., Richards J. H., and Donovan L. A. (2007). Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 143, 4–10. doi: 10.1104/pp.106.092940
Cavender-Bares J., Sack L., and Savage J. (2007). Atmospheric and soil drought reduce nocturnal conductance in live oaks. Tree Physiol. 27, 611–620. doi: 10.1093/treephys/27.4.611
Chen Z., Zhang Z., Sun G., Chen L., Xu H., and Chen S. (2020). Biophysical controls on nocturnal sap flow in plantation forests in a semi-arid region of northern China. Agric. For. Meteorol. 284, 107904–107915. doi: 10.1016/j.agrformet.2020.107904
Chen Z., Zhang Y., Yuan W., Zhu S., Pan R., Wan X., et al. (2021). Coordinated variation in stem and leaf functional traits of temperate broadleaf tree species in the isohydric–anisohydric spectrum. Tree Physiol. 41, 1601–1610. doi: 10.1093/treephys/tpab028
Chen X., Zhao P., Hu Y., Zhao X., Ouyang L., Zhu L., et al. (2018). The sap flow-based assessment of atmospheric trace gas uptake by three forest types in subtropical China on different timescales. Environ. Sci. pollut. Res. 25, 28431–28444. doi: 10.1007/s11356-018-2891-4
Choat B., Jansen S., Brodribb T. J., Cochard H., Delzon S., Bhaskar R., et al. (2012). Global convergence in the vulnerability of forests to drought. Nature 491, 752–755. doi: 10.1038/nature11688
Cirelli D., Equiza M. A., Lieffers V. J., Tyree M. T., and Tognetti R. (2015). Populusspecies from diverse habitats maintain high night-time conductance under drought. Tree Physiol. 36, 229–242. doi: 10.1093/treephys/tpv092
Daley M. J. and Phillips N. G. (2006). Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiol. 26, 411–419. doi: 10.1093/treephys/26.4.411
Di N., Xi B., Clothier B., Wang Y., Li G., and Jia L. (2019). Diurnal and nocturnal transpiration behaviors and their responses to groundwater-table fluctuations and meteorological factors of Populus tomentosa in the North China Plain. For. Ecol. Manage. 448, 445–456. doi: 10.1016/j.foreco.2019.06.009
Ding Y., Nie Y., Chen H., Wang K., and Querejeta J. I. (2020). Water uptake depth is coordinated with leaf water potential, watertuse efficiency and drought vulnerability in karst vegetation. New Phytol. 229, 1339–1353. doi: 10.1111/nph.16971
Domingo F., Serrano-Ortiz P., Were A., Villagarcía L., García M., Ramírez D. A., et al. (2011). Carbon and water exchange in semiarid ecosystems in SE Spain. J. Arid Environments 75, 1271–1281. doi: 10.1016/j.jaridenv.2011.06.018
Du S., Wang Y.-L., Kume T., Zhang J.-G., Otsuki K., Yamanaka N., et al. (2011). Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agric. For. Meteorol. 151, 1–10. doi: 10.1016/j.agrformet.2010.08.011
Fisher J. B., Baldocchi D. D., Mission L., Dawson T. E., and Goldstein A. H. (2007). What the towers don’t see at night, nocturnal sap flow in trees and shrubs at two AmeriFlux sites in California. Tree Physiol. 27, 597–610. doi: 10.1093/treephys/27.4.597
Forster M. A. (2014). How significant is nocturnal sap flow? Tree Physiol. 34, 757–765. doi: 10.1093/treephys/tpu051
Ghimire C. P., van Meerveld H. J., Zwartendijk B. W., Bruijnzeel L. A., Ravelona M., Lahitiana J., et al. (2022). Vapour pressure deficit and solar radiation are the major drivers of transpiration in montane tropical secondary forests in eastern Madagascar. Agric. For. Meteorol. 326, 109159–109177. doi: 10.1016/j.agrformet.2022.109159
Granier A. (1985). A new method of sap flow measurement in tree stems. Annales Des. Sci. Forestieres 42, 193–200. doi: 10.1051/forest:19850204
Granier A. (1987). Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 3, 309–320. doi: 10.1093/treephys/3.4.309
Hayat M., Iqbal S., Zha T., Jia X., Qian D., Bourque C. P. A., et al. (2021). Biophysical control on nighttime sap flow in Salix psammophila in a semiarid shrubland ecosystem. Agric. For. Meteorol. 300, 108329–108341. doi: 10.1016/j.agrformet.2021.108329
Hayat M., Zha T., Jia X., Iqbal S., Qian D., Bourque C. P. A., et al. (2020). A multiple-temporal scale analysis of biophysical control of sap flow in Salix psammophila growing in a semiarid shrubland ecosystem of northwest China. Agric. For. Meteorol. 288, 288–289. doi: 10.1016/j.agrformet.2020.107985
He T. (2022). Water use strategies of Pinus yunnanensis under different restoration patterns in Haifeng Karst slopes (East Yunnan: Yunnan Mormal University).
Hulshof C. M., Violle C., Spasojevic M. J., McGill B., Damschen E., Harrison S., et al. (2013). Intra)t,cn/j. and inter)t,cn/j. variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude. J. Vegetation Sci. 24, 921–931. doi: 10.1111/jvs.12041
Jia X., Zha T., Gong J., Wang B., Zhang Y., Wu B., et al. (2016). Carbon and water exchange over a temperate semi-arid shrubland during three years of contrasting precipitation and soil moisture patterns. Agric. For. Meteorol. 228, 120–129. doi: 10.1016/j.agrformet.2016.07.007
Jones R. H. and Raynal D. J. (1986). Spatial distribution and development of root sprouts in fagus grandifolia (Fagaceae). Amer. J. B 73, 1723–1731. doi: 10.1002/j.1537-2197.1986.tb09703.x
Kannenberg S. A., Guo J. S., Novick K. A., Anderegg W. R. L., Feng X., Kennedy D., et al. (2021). Opportunities, challenges and pitfalls in characterizing plant water-use strategies. Funct. Ecol. 36, 24–37. doi: 10.1111/1365-2435.13945
Kaproth M. A., Fredericksen B. W., González-Rodríguez A., Hipp A. L., and Cavender-Bares J. (2023). Drought response strategies are coupled with leaf habit in 35 evergreen and deciduous oak (Quercus) species across a climatic gradient in the Americas. New Phytol. 239, 888–904. doi: 10.1111/nph.19019
Kassahun Z. and Renninger H. J. (2021). Effects of drought on water use of seven tree species from four genera growing in a bottomland hardwood forest. Agric. For. Meteorol. 301, 108353–108362. doi: 10.1016/j.agrformet.2021.108353
Klein T., Zeppel M. J. B., Anderegg W. R. L., Bloemen J., De Kauwe M. G., Hudson P., et al. (2018). Xylem embolism refilling and resilience against droughtnce6/j.a mortality in woody plants: processes and tradessese6. Ecol. Res. 33, 839–855. doi: 10.1007/s11284-018-1588-y
Kumagai T.o., Tateishi M., Miyazawa Y., Kobayashi M., Yoshifuji N., Komatsu H., et al. (2014). Estimation of annual forest evapotranspiration from a coniferous plantation watershed in Japan (1): Water use components in Japanese cedar stands. J. Hydrol. 508, 66–76. doi: 10.1016/j.jhydrol.2013.10.047
Künzi Y., Zeiter M., Fischer M., and Stampfli A. (2025). Rooting depth and specific leaf area modify the impact of experimental drought duration on temperate grassland species. J. Ecol. 113, 445–458. doi: 10.1111/1365-2745.14468
Li Y., Wang Z., Zhang Y., Li X., and Huang W. (2019). Drought variability at various timescales over Yunnan Province, China: 1961–2015. Theor. Appl. Climatol. 138, 743–757. doi: 10.1007/s00704-019-02859-z
Liu W., Chen H., Zou Q., and Nie Y. (2021). Divergent root water uptake depth and coordinated hydraulic traits among typical karst plantations of subtropical China: Implication for plant water adaptation under precipitation changes. Agric. Water Manage. 249, 106798–106807. doi: 10.1016/j.agwat.2021.106798
Long W., Zang R., Schamp B. S., and Ding Y. (2011). Within and among-species variation in specific leaf area drive community assembly in a tropical cloud forest. Oecologia 167, 1103–1113. doi: 10.1007/s00442-011-2050-9
Loranty M. M., Mackay D. S., Ewers B. E., Adelman J. D., and Kruger E. L. (2008). Environmental drivers of spatial variation in whole-tree transpiration in an aspen-dominated upland-to-wetland forest gradient. Water Resour. Res. 44, 6272–6286. doi: 10.1029/2007wr006272
Lu S., Chen B., Li S., Pan Q., Zhang Y., and Yang X. (2013). Variations of whole-tree transpiration of poplar with different diameters in the wet and dry seasons. J. Food Agric. Environ. 11, 1262–1267.
Lu P., Urban L., and Zhao P. (2004). Granier’s thermal dissipation probe (TDP) method for measuring sap flow in trees theory and practice. Acta Botanica Sin. 46, 631–646.
Luo Z., Guan H., Zhang X., Zhang C., Liu N., and Li G. (2016). Responses of plant water use to a severe summer drought for two subtropical tree species in the central southern China. J. Hydrol.: Regional Stud. 8, 1–9. doi: 10.1016/j.ejrh.2016.08.001
Lyu J., He Q.-Y., Yang J., Chen Q.-W., Cheng R.-R., Yan M.-J., et al. (2020). Sap flow characteristics in growing and non-growing seasons in three tree species in the semiarid Loess Plateau region of China. Trees 34, 943–955. doi: 10.1007/s00468-020-01972-1
McDowell N., Pockman W. T., Allen C. D., Breshears D. D., Cobb N., Kolb T., et al. (2008). Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 178, 719–739. doi: 10.1111/j.1469-8137.2008.02436.x
Meinzer F. C., Andrade J. L., Goldstein G., Holbrook N. M., Cavelier J., and Jackson P. (1997). Control of transpiration from the upper canopy of a tropical forest: the role of stomatal, boundary layer and hydraulic architecture components. Plant Cell Environ. 20, 1242–1252. doi: 10.1046/j.1365-3040.1997.d01-26.x
Meinzer K., Goldstein G., Jackson R., Holbrook N. M., Guti6rrez M. V., and Cavelier J. (1995). Environmental and physiological regulation of transpiation in tropical forest gap species the influence of boundary layer and hydraulic properties. Oecologia 101, 514–522. doi: 10.1007/BF00329432
Moreno-Gutierrez C., Dawson T. E., Nicolás E., and Querejeta J. I. (2012). Isotopes reveal contrasting water use strategies among coexisting plant species in a Mediterranean ecosystem. New Phytol. 196, 489–496. doi: 10.1111/j.1469-8137.2012.04276.x
Niu S., Luo Y., Fei S., Montagnani L., Bohrer G. I. L., Janssens I. A., et al. (2011). Seasonal hysteresis of net ecosystem exchange in response to temperature change: patterns and causes. Global Change Biol. 17, 3102–3114. doi: 10.1111/j.1365-2486.2011.02459.x
O’Brien J. J., Oberbauer S. F., and Clark D. B. (2004). Whole tree xylem sap flow responses to multiple environmental variables in a wet tropical forest. Plant Cell Environ. 27, 551–567. doi: 10.1111/j.1365-3040.2003.01160.x
Ochoa C. G. and Abdallah M. A. B. (2023). The seasonal variability and environmental factors influencing the transpiration of western juniper (Juniperus occidentalis) saplings. Hydrology 10, 232–244. doi: 10.3390/hydrology10120232
Oogathoo S., Houle D., Duchesne L., and Kneeshaw D. (2020). Vapour pressure deficit and solar radiation are the major drivers of transpiration of balsam fir and black spruce tree species in humid boreal regions, even during a short-term drought. Agric. For. Meteorol. 291, 108063–108073. doi: 10.1016/j.agrformet.2020.108063
Pfautsch S., Keitel C., Turnbull T. L., Braimbridge M. J., Wright T. E., Simpson R. R., et al. (2011). Diurnal patterns of water use in Eucalyptus victrix indicate pronounced desiccation-rehydration cycles despite unlimited water supply. Tree Physiol. 31, 1041–1051. doi: 10.1093/treephys/tpr082
Phillips N. G., Lewis J. D., A.Logan B., and T.Tissue D. (2010). Inter- and intra-specific variation in nocturnal water transport in Eucalyptus. Tree Physiol. 30, 586–596. doi: 10.1093/treephys/tpq005
Pu Y.-s., Zhang Z.-y., and Pu L.-n. (2007). Strategic studies on the biodiversity sustainability in Yunnan Province, Southwest China. Forestry Stud. China 9, 225–237. doi: 10.1007/s11632-007-0037-8
Qian L.-S., Chen J.-H., Deng T., and Sun H. (2020). Plant diversity in Yunnan: Current status and future directions. Plant Diversity 42, 281–291. doi: 10.1016/j.pld.2020.07.006
Reich P. B. and Cornelissen H. (2014). The world)isse ‘orld)issen plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301. doi: 10.1111/1365-2745.12211
Robertson T. R., Bell C. W., Zak J. C., and Tissue D. T. (2008). Precipitation timing and magnitude differentially affect aboveground annual net primary productivity in three perennial species in a Chihuahuan Desert grassland. New Phytol. 181, 230–242. doi: 10.1111/j.1469-8137.2008.02643.x
Rosado B. H. P., Oliveira R. S., Joly C. A., Aidar M. P. M., and Burgess S. S. O. (2012). Diversity in nighttime transpiration behavior of woody species of the Atlantic Rain Forest, Brazil. Agric. For. Meteorol. 158-159, 13–20. doi: 10.1016/j.agrformet.2012.02.002
Schymanski S. J. and Or D. (2016). Wind increases leaf water use efficiency. Plant Cell Environ. 39, 1448–1459. doi: 10.1111/pce.12700
Siddiq Z. and Cao K.-F. (2018). Nocturnal transpiration in 18 broadleaf timber species under a tropical seasonal climate. For. Ecol. Manage. 418, 47–54. doi: 10.1016/j.foreco.2017.12.043
Siegert C. M. and Levia D. F. (2011). Stomatal conductance and transpiration of co-occurring seedlings with varying shade tolerance. Trees 25, 1091–1102. doi: 10.1007/s00468-011-0584-4
Tan F., Song H., Fu P., Chen Y., Siddiq Z., Cao K., et al. (2020). Hydraulic safety margins of co-occurring woody plants in a tropical karst forest experiencing frequent extreme droughts. Agric. For. Meteorol. 292-293, 108107–108115. doi: 10.1016/j.agrformet.2020.108107
Tardieu F.o. and Simonneau T. (1998). Variability among species of stomatal control under fluctuating soil water status and evaporative demand modelling isohydric and anisohydric behavious. J. Exp. Bot. 49, 419–432. doi: 10.1093/jxb/49.Special_Issue.419
Tarin T., Nolan R. H., Eamus D., and Cleverly J. (2020). Carbon and water fluxes in two adjacent Australian semi-arid ecosystems. Agric. For. Meteorol. 281, 107853–107866. doi: 10.1016/j.agrformet.2019.107853
Ungar E. D., Rotenberg E., Raz-Yaseef N., Cohen S., Yakir D., and Schiller G. (2013). Transpiration and annual water balance of Aleppo pine in a semiarid region: Implications for forest management. For. Ecol. Manage. 298, 39–51. doi: 10.1016/j.foreco.2013.03.003
Verduzco V. S., Garatuza J., Yepez E. A., Watts C. J., Rodriguez J. C., RobleszMorua A., et al. (2015). Variations of net ecosystem production due to seasonal precipitation differences in a tropical dry forest of northwest Mexico. J. Geophysical Research: Biogeosciences 120, 2081–2094. doi: 10.1002/2015jg003119
Vitasse Y., Bottero A., Cailleret M., Bigler C., Fonti P., Gessler A., et al. (2019). Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Global Change Biol. 25, 3781–3792. doi: 10.1111/gcb.14803
Wang X., Liu J., Sun Y., Li K., and Zhang C. (2016). RETRACTED ARTICLE: Sap flow characteristics of three afforestation species during the wet and dry seasons in a dry–hot valley in Southwest China. J. Forestry Res. 34, 575–575. doi: 10.1007/s11676-016-0273-7
Wang X., Liu J., Sun Y., Li K., and Zhang C. (2017b). Sap flow characteristics of three afforestation species during the wet and dry seasons in a dry-hot valley in Southwest China. J. Forestry Res. 28, 51–62. doi: 10.1007/s11676-016-0276-4
Wang H., Tetzlaff D., Dick J. J., and Soulsby C. (2017a). Assessing the environmental controls on Scots pine transpiration and the implications for water partitioning in a boreal headwater catchment. Agric. For. Meteorol. 240-241, 58–66. doi: 10.1016/j.agrformet.2017.04.002
Westoby M., Falster D. S., Moles A. T., Vesk P. A., and Wright I. J. (2002). Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Systematics 33, 125–159. doi: 10.1146/annurev.ecolsys.33.010802.150452
White J. D. and Scott N. A. (2006). Specific leaf area and nitrogen distribution in New Zealand forests: Species independently respond to intercepted light. For. Ecol. Manage. 226, 319–329. doi: 10.1016/j.foreco.2006.02.001
Wright I. J., Reich P. B., Westoby M., Ackerly D. D., Baruch Z., Bongers F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/nature02403
Wright C. L., West J. B., de Lima A. L. A., Souza E. S., Medeiros M., Wilcox B. P., et al. (2024). Contrasting water-use strategies revealed by species-specific transpiration dynamics in the Caatinga dry forest. Tree Physiol. 44, 137–151. doi: 10.1093/treephys/tpad137
Yan C., Wang B., Zhang Y., Zhang X., Takeuchi S., and Qiu G. (2018). Responses of sap flow of deciduous and conifer trees to soil drying in a subalpine forest. Forests 9, 32–47. doi: 10.3390/f9010032
Yang Z. and Qin F. (2023). The battle of crops against drought: Genetic dissection and improvement. J. Integr. Plant Biol. 65, 496–525. doi: 10.1111/jipb.13451
Yu T., Feng Q., Si J., Mitchell P. J., Forster M. A., Zhang X., et al. (2018). Depressed hydraulic redistribution of roots more by stem refilling than by nocturnal transpiration for Populus euphratica Oliv. in situ measurement. Ecol. Evol. 8, 2607–2616. doi: 10.1002/ece3.3875
Zeppel M. J. B., Anderegg W. R. L., Adams H. D., Hudson P., Cook A., Rumman R., et al. (2019). Embolism recovery strategies and nocturnal water loss across species influenced by biogeographic origin. Ecol. Evol. 9, 5348–5361. doi: 10.1002/ece3.5126
Zeppel M. J. B., Lewis J. D., Phillips N. G., Tissue D. T., and Steppe K. (2014). Consequences of nocturnal water loss: a synthesis of regulating factors and implications for capacitance, embolism and use in models. Tree Physiol. 34, 1047–1055. doi: 10.1093/treephys/tpu089
Zeppel M., Tissue D., Taylor D., Macinnis-Ng C., and Eamus D. (2010). Rates of nocturnal transpiration in two evergreen temperate woodland species with differing water-use strategies. Tree Physiol. 30, 988–1000. doi: 10.1093/treephys/tpq053
Zhang J., Chen X., Song Y., and Gong Z. (2024a). Integrative regulatory mechanisms of stomatal movements under changing climate. J. Integr. Plant Biol. 66, 368–393. doi: 10.1111/jipb.13611
Zhang M., Zhu S., Duan Q., and Yang Y. (2024b). Evaluation of influencing factors of soil shear performance of typical vegetation in Dry-Hot Valley Area. Res. Soil Water Conserv. 31, 153–162. doi: 10.13869/j.cnki.rswc.2024.04.019
Zhang H., Zhu J., Gong Z., and Zhu J.-K. (2021). Abiotic stress responses in plants. Nat. Rev. Genet. 23, 104–119. doi: 10.1038/s41576-021-00413-0
Keywords: subtropical monsoon climate, seasonal drought, sap flow density, water regulation strategies, soil water moisture, vapour pressure deficit
Citation: Tang J, Ding Z, Wang G and Wang Y (2025) Sap flow dynamics of co-occurring trees in response to seasonal droughts in a subtropical climate. Front. Ecol. Evol. 13:1550290. doi: 10.3389/fevo.2025.1550290
Received: 23 December 2024; Accepted: 23 May 2025;
Published: 03 July 2025.
Edited by:
Giovanbattista Domenico de Dato, Council for Agricultural Research and Agricultural Economy Analysis (CREA), ItalyReviewed by:
Dario Liberati, University of Tuscia, ItalyMohamed Abdallah, Oregon State University, United States
Copyright © 2025 Tang, Ding, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfang Wang, d2FuZ3lmMjAyMEBsenUuZWR1LmNu
 Jiaqi Tang
Jiaqi Tang Zhiqiang Ding2
Zhiqiang Ding2 Yanfang Wang
Yanfang Wang