- Reef Fish Ecology and Conservation Lab, Universidade Federal Fluminense, Niterói, RJ, Brazil
The greenbeak parrotfish, Scarus trispinosus, is the largest southwestern Atlantic parrotfish, endemic to Brazil and targeted by reef-associated fisheries along the coast. After a sharp population decline, S. trispinosus is now considered one of the most endangered parrotfish worldwide. The lack of basic biological information has hindered the development of appropriate management policies, until recently. I compiled data on abundance, biomass, size class distribution, ecology, demography, and fisheries of S. trispinosus along the Brazilian coast to explore how Marine Protected Areas (MPAs) and newly implemented management strategies could support the species’ recovery and protection. I found that the Brazilian equatorial margin is nearly as important as the Abrolhos Bank in terms of S. trispinosus abundance. Shallower inshore reefs serve as nurseries, while larger individuals often occupy deeper offshore reefs. The estimated size and age of first maturity (39.2 cm in total length; 4.2 years) indicate that S. trispinosus is relatively long-lived and reaches sexual maturity later than other parrotfishes in the Caribbean and Pacific, highlighting its vulnerability. Most small-scale fishing targets immature individuals in inshore reefs, while recreational spearfishing primarily targets significantly larger individuals. While fishing is significant, the value chain of S. trispinosus is poorly understood, hindering the development of new tools to regulate its fishing. Brazilian MPAs protect some critical areas, but the species’ ongoing decline suggests that MPAs alone are insufficient without proper fisheries management. Although the most effective management strategy would likely be a fishing moratorium, as adopted in Belize, social challenges and the lack of political context in Brazil hinder its implementation. Alternatively, the Brazilian government has released the National Recovery Plan for Endangered Species, which restricts S. trispinosus fishing to multiple-use MPAs with proper regulatory measures, while prohibiting fishing for this species elsewhere on the coast. The development and adaptation of this novel strategy were made possible by advances in research over the past 10 years. However, some key areas still lack implementation, and it may be too early to determine whether the plan is effectively working. If effectively implemented and successful, it could serve as a model for managing parrotfishes elsewhere.
Introduction
Parrotfishes (Labridae: Scarinae) are ubiquitous components of reef environments worldwide, but parrotfish species richness is not evenly distributed across the globe. Among the 100 species currently recognized (Cowman et al., 2009; Parenti and Randall, 2011), only ten species within four genera occur in the southwestern Atlantic, seven of which are endemic to Brazil (Hoey et al., 2018a; Pinheiro et al., 2018). Brazilian endemic parrotfishes are separated from their Caribbean counterparts by the Amazon River plume (Moura et al., 2001; Rocha, 2003; Choat et al., 2012; Araujo et al., 2022). This includes the greenbeak parrotfish, Scarus trispinosus (Valenciennes, 1840), the largest Brazilian parrotfish, which can reach up to 91 cm in total length (TL; Freitas et al., 2019).
Scarus tripinosus occurs along almost the entire Brazilian coast, from Maranhão (MA; 0°S) to Santa Catarina state (SC; 27°S), although the species is more commonly found in tropical waters (from 0°S to 18°S; Ferreira et al., 2004). Given its larger size compared to other Brazilian parrotfishes and the distinctive morphology of its jaw and pharyngeal apparatus, S. trispinosus may act as an excavating bioeroder after reaching 40 cm TL (Lellys et al., 2019). These features, combined with its conspicuous presence along the Brazilian coast, make the species the primary excavating parrotfish of the southwestern Atlantic.
Parrotfishes are widely recognized as an ecologically important group in reef ecosystems due to their ability to scrape surfaces, particularly influencing the early succession dynamics of benthic communities, and to excavate carbonates, making them significant producers and transporters of sediments (Bonaldo et al., 2014; Mallela and Fox, 2018; Molina-Hernández and Álvarez-Filip, 2024). These structural processes are linked to their targeting of protein-rich epilithic, endolithic, and epiphytic microscopic phototrophs, mainly cyanobacteria (Clements et al., 2017). The contribution of parrotfishes in enhancing corals’ resilience amid the impacts of climate change has been debated (Harborne and Mumby, 2018; Bruno et al., 2019). In fact, the functional importance of parrotfishes to the reef resilience and/or resistance to changes may be context-dependent and relies on many factors, including the local parrotfish diversity, the frequency of thermal anomalies, the productivity potential of the benthos, and the macroalgae-coral cover ratio on the reef (Burkepile and Hay, 2008; Hughes et al., 2017; Harborne and Mumby, 2018; Hoey et al., 2018b; Bruno et al., 2019). For instance, experimental exclusion of large parrotfishes has led to substantial increases in algal cover in the Caribbean, where macroalgal cover is generally higher than on Indo-Pacific reefs (Steneck et al., 2014; Shantz et al., 2020). Even low harvest rates of parrotfishes have been shown to cause significant negative effects on reef ecosystems (Bozec et al., 2016).
Brazilian reefs are characterized by a higher cover of epilithic algal matrices and macroalgae, and a lower cover of scleractinian corals (ranging from 4% to 12% on average) compared to the Caribbean and Indo-Pacific (Aued et al., 2018). The contribution of parrotfishes to the functioning of Brazilian reef ecosystems requires further investigation (Hoey et al., 2018a), but evidence suggests that they may help prevent the overgrowth of algae, particularly filamentous algae (Feitosa et al., 2023). In the context of global warming, it remains unclear whether recent mass mortalities of Brazilian corals (see Ferreira et al., 2021; Braz et al., 2022; Corazza et al., 2024) have led to bottom-up increases in parrotfish biomass through cyanobacterial blooms, as observed elsewhere (Russ et al., 2015; Taylor et al., 2020). The detection of such phenomena through methods like underwater visual censuses may be hindered by the constant fishing pressure to which Brazilian parrotfishes have been subjected (Bender et al., 2014; Roos et al., 2016, 2020a, b; Roos and Longo, 2021).
Parrotfishes are important for subsistence and commercial reef fisheries worldwide, especially in developing countries (Edwards et al., 2014), accounting for significant amounts of reef-associated catches in tropical regions of the Pacific (Rhodes et al., 2008; Houk et al., 2012; Taylor and Choat, 2014), western Indian (Thyresson et al., 2011), Caribbean (Harms-Tuohy, 2021), and southwestern Atlantic (Francini-Filho and Moura, 2008; Bender et al., 2014; Roos et al., 2016; Pereira et al., 2021). However, the increasing fishing pressure on this group has been leading populations to decline (Hawkins and Roberts, 2004; Aswani and Sabetian, 2010; Comeros-Raynal et al., 2012; Bender et al., 2014; Hamilton et al., 2016). For parrotfishes, particularly large-bodied species, susceptibility to overfishing arises from several traits, including protogynous hermaphroditism, slow growth, long lifespans, their behavior of sleeping in easily accessible spots, and conspicuous occurrence in shallow depths (Choat and Robertson, 1975; Taylor et al., 2014). Despite this, large-bodied species are the main fishing targets among parrotfishes, leading to a disproportionate impact on the ecological roles they perform (Bellwood et al., 2012; Edwards et al., 2014; Taylor et al., 2014). Although no-take marine protected areas (MPAs) are generally important tools for the conservation of parrotfishes (Francini-Filho and Moura, 2008; Questel and Russ, 2018; Pereira et al., 2022), fishing bans and regulations have been implemented in many countries as an additional conservation effort, especially in the Caribbean (Harms-Tuohy, 2021). Managing fisheries in developing countries, however, is particularly challenging because vulnerable socioeconomic contexts may influence fisher’s compliance with restrictions (Lopes et al., 2013).
In Brazil, parrotfishes’ fishing is multifaceted, involving various methods to target different species for distinct purposes (Queiroz-Véras et al., 2023). Smaller species like Sparisoma axillare and Sp. frondosum are targeted along the Northeast coast by small-scale fisheries using hook and line, nets, or traps (Roos et al., 2016; Queiroz-Véras et al., 2025), and by semi-industrial fisheries using traps, mainly for international export (Cunha et al., 2012; Queiroz-Véras et al., 2025). Scarus tripinosus is targeted by small-scale fisheries with nets and spearguns (Francini-Filho and Moura, 2008; Roos et al., 2016; Pereira et al., 2021; Queiroz-Véras et al., 2025), mainly for subsistence and regional markets. Additionally, recreational fishers, particularly along the Northeast and Southeast coasts, use spearguns to target large-bodied parrotfishes such as S. trispinosus and Sp. amplum (Nunes et al., 2012; Giglio et al., 2020; Roos and Longo, 2021).
Since the late 1980s, increasing fishing pressure on S. trispinosus has led to a population decline of at least 50% (Padovani-Ferreira et al., 2012), as observed in many regions across Brazil (Bender et al., 2014; Roos et al., 2020b; Pereira et al., 2021; Eggertsen et al., 2024; Queiroz-Véras et al., 2025), consequently making it one of the most endangered parrotfish species worldwide (Comeros-Raynal et al., 2012). Scarus trispinosus is listed as Endangered by the International Union for Conservation of Nature since 2012 (Padovani-Ferreira et al., 2012) and by the Brazilian Red List of Endangered Species since 2014 (Decree n° 445, Brazil’s Red List, 2014). In 2014, however, limited information was available to support conservation efforts for the species, including landing and demographic data, both of which are essential for fisheries management. Although significant knowledge gaps remain (Queiroz-Véras et al., 2023), progress has been made over the past decade. In addition to advances in understanding the species, the Brazilian National Recovery Plan for endangered species, which includes S. trispinosus among its targets, was published in 2018 (Decree No. 59-B/2018). However, its implementation only began in some regions of Brazil in 2021. Here, I summarize the current understanding of S. trispinosus and discuss potential pathways and challenges for its effective conservation in Brazil.
Current understanding of distribution and connectivity along the Brazilian coast
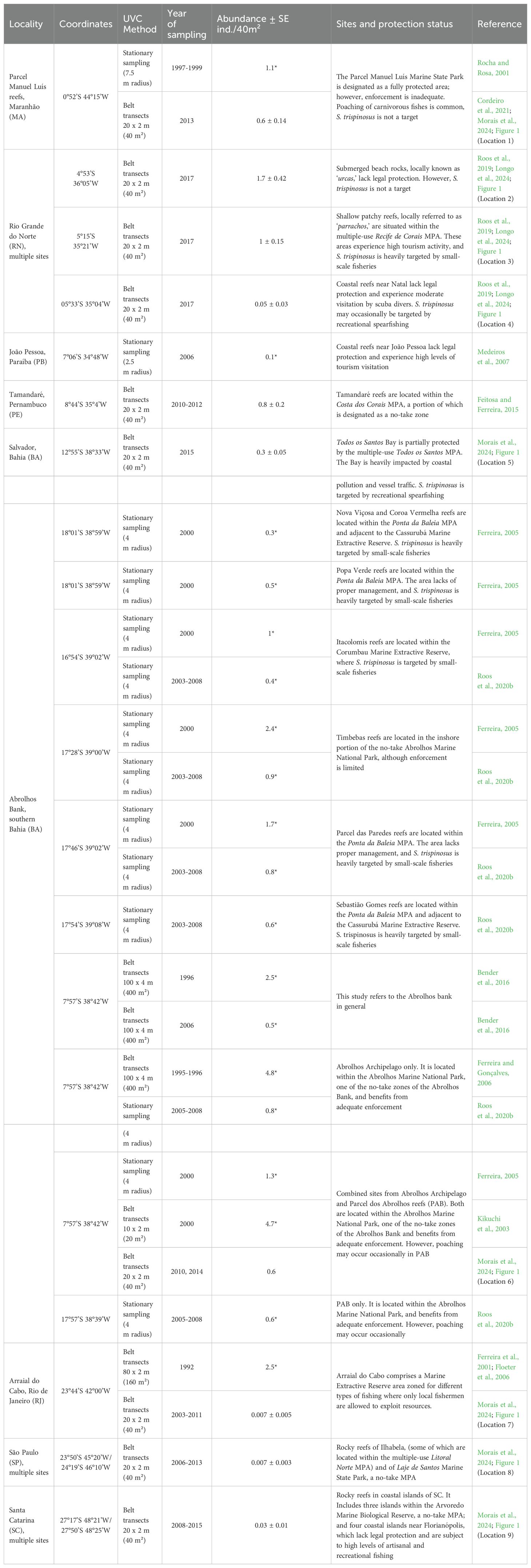
I obtained standardized data on the abundance, biomass and size class distribution of S. trispinosus across the Brazilian coast from datasets on Brazilian reef diversity (Luza et al., 2024). Specifically, I used data from Longo et al. (2024), which included 300 visual censuses conducted across the coast of Rio Grande do Norte state (RN) (04°52’6.58’’S–36°39’9.80’’W/06°22’44.3’’S–4°58’13.4’’W) between 2016 and 2017. Parrotfish data from these surveys were previously published in Roos et al. (2019). Additionally, I used data from Morais et al. (2024), which included 4,570 visual censuses conducted between 2001 and 2017 over 137 sites in 20 locations, spanning from 0° to 27°S along the Brazilian coast and oceanic islands. Biomass data on fish assemblages from these surveys were previously published in Morais et al. (2017). Both datasets were collected using the same visual census methodology, specifically, belt transects measuring 40 m² (20 × 2 m). The species S. trispinosus was recorded in nine locations along the Brazilian coast in these datasets. Differences in the species abundance among locations with varying fishing pressure and protection status were assessed using a pairwise permutational test, which does not require normality or homogeneity of variances as estimates are obtained from permutation. The test was implemented using the rcompanion package (pairwisePermutationTest function; Mangiafico, 2023) in the R software (R Core Team, 2024). Locations were categorized into three groups: (1) not targeted/protected area, (2) not targeted/unprotected area (i.e., areas without legal protection where S. trispinosus is not commonly fished), and (3) targeted/partially protected or unprotected area (i.e., multiple-use MPAs and unprotected areas where S. trispinosus is fished). Locations with historically low natural abundances of S. trispinosus (i.e., São Paulo [SP] and Santa Catarina [SC] states) were excluded from the analysis to avoid bias. Additionally, I compiled information on the abundance of S. trispinosus from several published studies that used various visual census methodologies or transects of differing dimensions to report findings across different locations and time periods.
Although the greater abundance of S. trispinosus in tropical waters is well-documented, with the Abrolhos Bank recognized as one of its most critical habitats, the Brazilian equatorial margin (particularly from 0°S–44°W to 5°S–35°W) has also proven to be equally significant (Figure 1a). However, this region has historically received less scientific attention compared to other parts of the Brazilian coast. Considering only the standardized datasets from Longo et al. (2024) and Morais et al. (2024), the highest abundances were recorded in the Brazilian equatorial margin, including the offshore submerged beach rocks, followed by the shallow patchy reefs, both located in RN state, and Parcel de Manuel Luís reefs, Maranhão state (MA; Figure 1a). The species showed similar abundance in the Abrolhos Archipelago and slightly lower abundance in Todos os Santos Bay, both located in Bahia state (BA). Other recorded but lower abundances included coastal reefs near Natal, RN; the coastal islands of SC; rocky reefs in Ilhabela and Laje de Santos, SP; and rocky reefs in Arraial do Cabo, Rio de Janeiro state (RJ) (Figure 1a).

Figure 1. Patterns of Scarus trispinosus’ distribution across nine locations on the Brazilian coast, including its mean (a) abundance (ind. 40 m−2), (b) biomass (kg 40 m−2) and (c) size class distribution (densities). Data were obtained from standardized datasets on Brazilian reef diversity (Longo et al., 2024; Morais et al., 2024). The areas of the circles are proportional to the log-scaled mean abundance and biomass values.
The biomass of S. trispinosus was similar between the Abrolhos Archipelago and the equatorial margin (Figure 1b), despite the larger size of individuals observed in the Abrolhos Bank (Figure 1c). Maximum sizes at higher latitudes are generally expected to be larger, as the age-based demography of reef fishes is influenced by sea temperature (e.g., Choat et al., 2003; Robertson et al., 2005; Taylor and Pardee, 2017). Considering only the standardized datasets from Longo et al. (2024) and Morais et al. (2024), the highest biomass was recorded in the offshore submerged beach rocks, RN (~4600 ± 1170 g 40m-2); followed by the Abrolhos Archipelago, BA (~766 ± 156 g 40m-2); Parcel de Manuel Luís reefs, MA (~577 ± 148 g 40m-2); shallow patchy reefs, RN (~525 ± 157 g 40m-2); Todos os Santos Bay, BA (~92 ± 19 g 40m-2); coastal reefs near Natal, RN (~90 ± 64 g 40m-2); rocky reefs in Ilhabela and Laje de Santos, SP (~5.8 ± 4.2 g 40m-2); rocky reefs in Arraial do Cabo, RJ (~2.7 ± 2 g 40m-2); and the coastal islands of SC (~0.1 ± 0.05 g 40m-2). Overall, outer-shelf reefs supported larger individuals and higher biomasses, particularly the offshore submerged beach rocks, RN, and the Abrolhos Archipelago, BA. In contrast, coastal shallower reefs were characterized by higher abundances of juveniles, especially the patchy reefs in RN, and Todos os Santos Bay, BA (Figure 1c).
Locations where S. trispinosus is not targeted, either due to no-take MPAs or because it is not traditionally fished, have significantly higher abundances compared to locations where the species is fished (Table 1). Since most of these data were collected over a decade ago, this result should be reevaluated soon to: (1) assess whether no-take MPAs continue to protect S. trispinosus; (2) determine if the species has become a target and declined in previously unfished, unprotected reefs; and (3) evaluate whether it has recovered in areas where fishing persisted, particularly after the Recovery Plan’s implementation.
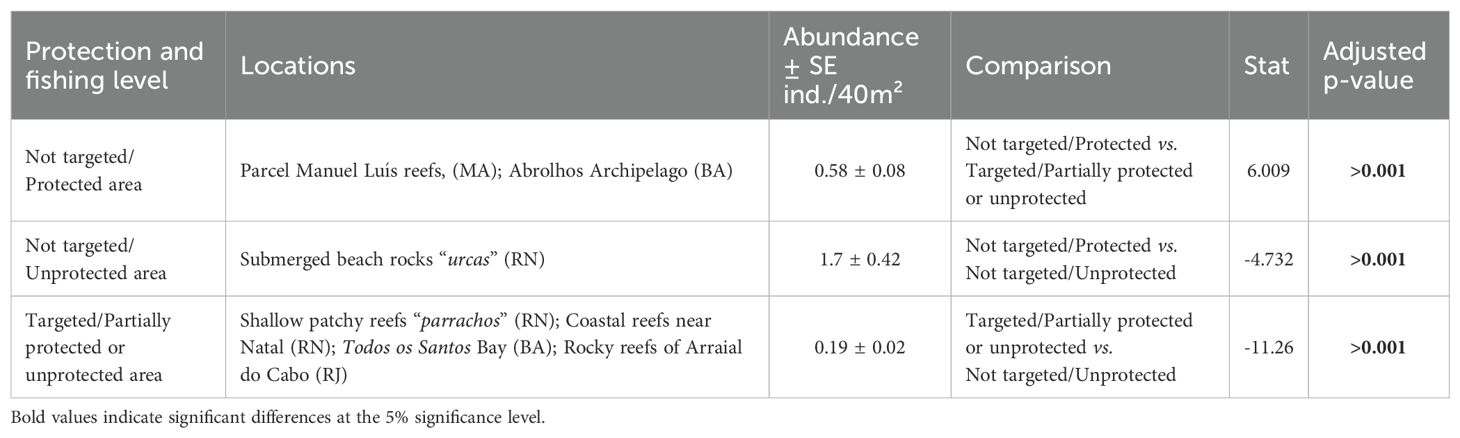
Table 1. Differences in Scarus trispinosus abundance (ind. 40 m–2) among locations with different fishing pressure and protection status, with the summary of results from the pairwise permutation test.
Based on the compiled studies, S. trispinosus occurs in at least two locations not covered or not detected by Morais et al. (2024) and Longo et al. (2024), and which are outside the Abrolhos Bank: coastal reefs near João Pessoa, Paraíba state (PB; Medeiros et al., 2007), and Tamandaré reefs, located within the Costa dos Corais MPA, Pernambuco state (PE; Feitosa and Ferreira, 2015; Table 2). The species’ abundance was considerably higher in the Abrolhos Archipelago and in Arraial do Cabo, RJ, particularly before the 2000s (Table 2). The decline in abundance across the entire Abrolhos Bank, even within the no-take MPAs, has been described (see Roos et al., 2020b), as well as in Arraial do Cabo, RJ (see Bender et al., 2014).
Although the genus Scarus does not typically occur in the Brazilian oceanic islands, few vagrant individuals of S. trispinosus were spotted at Fernando de Noronha Archipelago (3°52′17″S–32°28′02″W) in 2013, and at Rocas Atoll (3°51′15″S–33°49′04″W) in 2014, 2015 and 2017 (Mazzei et al., 2019). Scarus trispinosus exhibited slight but significant genetic substructuring between the northern population, specifically from RN and Pernambuco (PE) states, and the population from southern BA, with the latter exhibiting higher levels of genetic diversity (Bezerra et al., 2018). This pattern may be linked to factors such as habitat availability and heterogeneity, and the presence of a no-take MPA in the southern BA, and intense fishing pressure on the northeast coast (Bezerra et al., 2018). Genetic studies covering the entire range of the species’ distribution would provide further insights into the levels of genetic structure of S. trispinosus along the coast.
Although fishing pressure may be a key factor contributing to the decreasing size of parrotfish in shallow inshore habitats, current evidence on the cross-shelf distribution of S. trispinosus suggests that coastal, shallower reefs function as nursery areas, while deeper reefs, where larger and older individuals are found, may also play an important role in the species’ life cycle (Freitas et al., 2019; Roos et al., 2020b). However, visual censuses below 20 m deep are generally scarce, and the distribution patterns of S. trispinosus in mesophotic reefs remain poorly understood. For instance, large individuals, ranging from 70 to 80 cm in total length, were spotted at ~70 m depth in mesophotic reefs of RN (pers. comm. Longo, GO; Feitoza et al., 2005). Exploring deeper cross-shelf distributions along the coast in future research could provide new insights to the understanding of S. trispinosus’ connectivity among reefs, habitat use, and priority areas for conservation.
Current understanding of ecology
Habitat preferences of S. trispinosus include structurally complex reef environments with higher covers of calcareous substrates, such as calcareous algae and stone corals (Roos et al., 2019), which may better support a species reliant on substratum excavation. Individuals larger than 40 cm TL were functionally classified as excavators due to their robust premaxilla, jaws with simple joints, and the high proportion of their bites leaving pronounced marks on the substratum (Lellys et al., 2019). It was estimated that individuals larger than 30 cm of TL take around 5k bites d-1, with an estimated erosion rate of 207 cm3 d-1, and an annual erosion rate of 75,534 cm3 (Lellys et al., 2019). The feeding rate of S. trispinosus decreases with increasing body size, with individuals smaller than 20 cm TL taking ~8.5 bites min−1, while those larger than 60 cm TL take ~1.5 bites min−1 (Lellys et al., 2019). Although larger parrotfishes are known for their bioerosion abilities, smaller individuals act primarily as scrapers and often exhibit higher bite rates (Bellwood and Choat, 1990). This helps maintain epilithic algal matrices (EAM) in an early successional stage, limiting macroalgae establishment and sediment accumulation (Bonaldo et al., 2014). The functional diversity across size classes highlights the importance of preserving population size structure, as both small and large individuals contribute in distinct but complementary ways to reef processes (Bonaldo et al., 2014).
Scarus trispinosus feeds predominantly on EAM and crustose coralline algae (CCA; Francini-Filho et al., 2010; Lellys et al., 2019), with less than 1% of bites taken on live corals (Francini-Filho et al., 2008). Half of the ingested content of S. trispinosus consists of detritus (54.5%), while 43.5% comprises algae, predominantly filamentous algae (54%), followed by CCA (29%) and foliose algae (15%; Ferreira and Gonçalves, 2006). All findings on erosion rates and feeding ecology have been conducted in the Abrolhos Archipelago or other sites within the Abrolhos Bank. Although bioerosion is a key function linked to the structural dynamics of biogenic reefs, such as those in the Abrolhos Bank, the role of S. trispinosus in the structural dynamics of other Brazilian reefs requires investigation.
Protein-rich microorganisms found on or within calcareous substrata like CCA and corals, as well as on macroalgae and EAM, are recognized as key nutritional sources for parrotfishes (Clements et al., 2017). While the specific microscopic dietary targets of S. trispinosus and their potential latitudinal variation also remain unknown, the species has been shown to contribute to seaweed dispersion (macroalgae and cyanobacteria) after consumption. At least one cyanobacterium and 16 macroalgal taxa were reported to survive passage through the gut of S. trispinosus in both laboratory and field experiments (see Tâmega et al., 2016).
Current understanding of demography
Managing reef fisheries requires an understanding of several demographic processes identified through age-based studies (Choat and Robertson, 2002). However, knowledge gaps persist, particularly in tropical developing countries like Brazil (Queiroz-Véras et al., 2023), where hundreds of thousands of people depend on reef-associated fisheries for income and protein, yet limited infrastructure and funding hinder scientific research.
The first published age-based study on S. trispinosus was conducted in the Abrolhos Bank (see Freitas et al., 2019), and the second was conducted in RN state (see Roos et al., 2020a). Both studies estimated similar values for most life-history traits investigated, but found differences in maximum age and size between locations (Table 3).
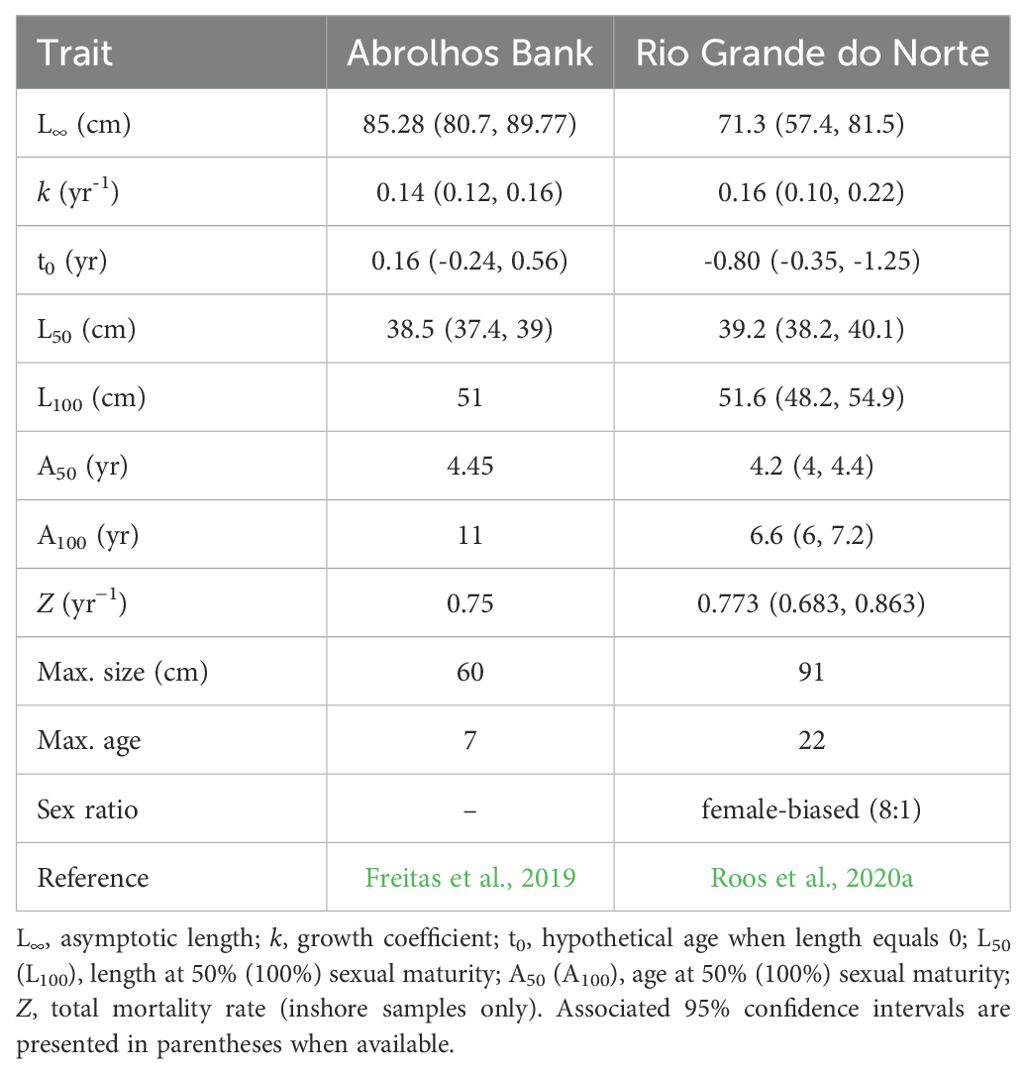
Table 3. Summary of life-history trait estimates for Scarus trispinosus from the Abrolhos Bank (Freitas et al., 2019), and Rio Grande do Norte state (Roos et al., 2020a).
Both studies indicate that S. trispinosus undergoes an ontogenetic habitat shift, with smaller, younger individuals predominantly inhabiting shallow inshore reefs, while larger, older individuals, including most males, tend to occupy deeper offshore reefs (Freitas et al., 2019; Roos et al., 2020a). Although these studies have shed light on the species’ demography, similar information is still lacking for other locations along the extensive Brazilian coast, particularly the northern region.
Current understanding of fisheries
The increasing catch of fish from lower trophic levels over the past decades is a global trend (Pauly et al., 1998). Combined with the lack of consistent monitoring of fishing landings and statistics, this poses a major challenge to fish stock assessments. Despite being heavily exploited worldwide, few studies provide detailed information on parrotfish catch quantities, species-specific information, size distributions, fishing grounds, gear used, and catch per unit effort (CPUE), and Brazil is no exception in such trend.
The northern area where the fishing of S. trispinosus has occurred consistently over the years is Rio do Fogo, RN (5°15’S–35°22’W), within the multiple-use Recifes de Corais MPA (Roos et al., 2016). The species became a common fishery resource in the area in the late 1970’s, targeted mainly by a small-scale fishery with the use of nets and spearguns (Queiroz-Véras et al., 2025). Fishers from this location use both spearguns and nets to catch S. trispinosus at shallow patchy reefs close to the coast (Roos et al., 2016; Queiroz-Véras et al., 2025). The total recorded landings in this area, based on a monthly two-day sampling between 2013 and 2014, were 1.2 t, with an estimated annual catch of 9.4 t, and a mean CPUE of 2 (± 0.83) kg hour-1 crew size-1 using spearguns, and of 4.2 (± 0.41) and 2.8 (± 1) kg hour-1 crew size-1 using nets of 50 mm and 60 mm of mesh size, respectively (Roos et al., 2016). However, recent evidence based on local ecological knowledge from 53 fishers, who were asked about their individual fishing activities (e.g., gear used, number of trips per week, and trip duration), suggests that catches of S. trispinosus in this area were actually much higher, with an estimated weekly catch of 7.5 t between 2010 and 2021 (Queiroz-Véras et al., 2025). Landings of S. trispinosus in Rio do Fogo predominantly comprised small, immature individuals (Figure 2a), especially those caught with nets. These ranged from 22 to 60 cm TL, with a higher frequency of individuals around 38 cm TL (Roos et al., 2016) and ages between 2 and 4 years (Roos et al., 2020a).
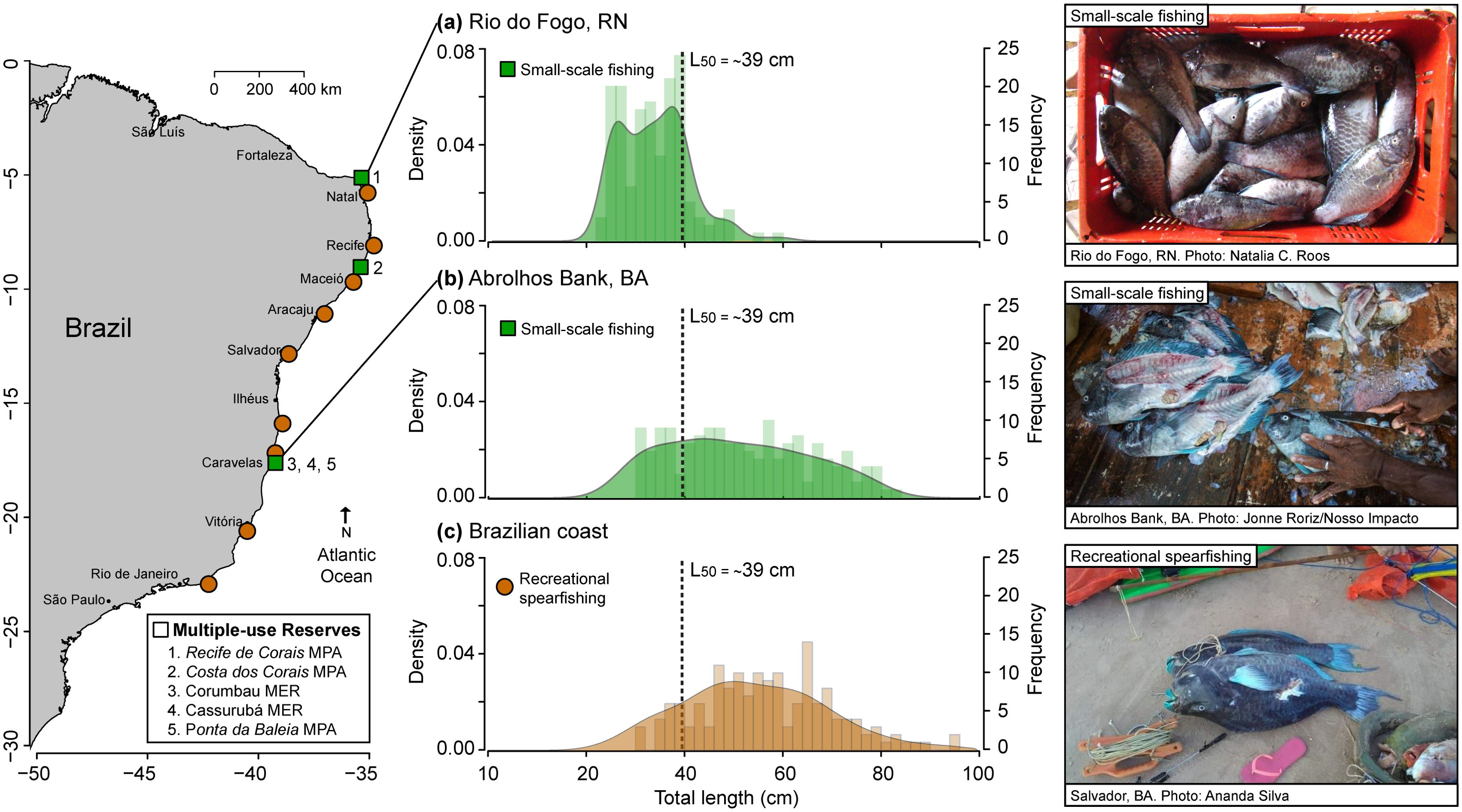
Figure 2. Size class distribution (density and frequency) of Scarus trispinosus individuals caught in different locations across the Brazilian coast. Different colors indicate different types of fishing: (a) small-scale fishing in Rio do Fogo, RN (n = 160; Roos et al., 2016, 2020a); (b) small-scale fishing in the Abrolhos Bank (n = 160; Freitas et al., 2019); recreational spearfishing (n = 158; Roos and Longo, 2021); dashed line indicates the species’ size at first maturity (L50). MPA, Marine Protected Area; MER, Marine Extractive Reserve.
In the Abrolhos Bank, BA, S. trispinosus became a common target after recreational spearfishing was introduced in the region in the early 1980s. However, the catch of the species considerably increased after spearfishing became a commercial activity in the 1990s. In the 2000’s, the species became one of the most important fishery resources, commonly sold in the local and regional markets as fillets for a very low price (Francini-Filho and Moura, 2008; Queiroz-Véras et al., 2025). A Status Review Report published by NOAA in 2015 (Salz, 2015), provided the first information on catch quantities of S. trispinosus in the Abrolhos Bank, initially described in Previero’s (2014) thesis dissertation. The data were collected at the main ports of the region between 2010 and 2012 through the program “Participatory Fisheries Monitoring in the Marine Extractive Reserves of Corumbau, Canavieiras, and Cassurubá, and in the Buffer Zone of the Abrolhos Marine National Park”. The program was implemented through a partnership between the Brazilian Ministry of Fisheries and Aquaculture and the non-governmental organizations “Coastal and Marine Studies Association” (ECOMAR) and Conservation International Brazil (CI-Brazil). Landings were recorded from November 2010 to June 2012 in Alcobaça (17°31’S–39°11’W), and from November 2010 to November 2011 in Caravelas (17°44’S–39°11’W) and Corumbau (16°55’S–39°08’). Results showed that fishers from Alcobaça use spearguns and air compressors, which is forbidden under the Brazilian law, and tended to go further from the coast, to deeper fishing grounds (up to 40 m) on the Abrolhos Bank and Royal Charlotte Bank, over longer fishing trips (~11 days). Landings of S. trispinosus in Alcobaça were characterized by larger mature individuals, ranging from 35 to 91 cm TL (Figure 2b), with a higher frequency of individuals around 60 cm TL and ages between 8 and 9 years. The total recorded landings in Alcobaça were 9.21 t (averaging 1.31 t per month), but it was not possible to calculate the CPUE in this area. Conversely, fishers from Caravelas and Corumbau use spearguns and nets during single-day trips to shallower fishing grounds (up to 15 m deep) closer to the coast. Landings of S. trispinosus in these areas were characterized by smaller individuals, ranging from 30 to 63 cm of TL (shown together with the size distribution from Alcobaça in Figure 2b), with a higher frequency of individuals around 42 cm TL and ages between 4 and 5 years. The total recorded landings in Caravelas were 24.80 t (averaging 1.90 t per month), and a CPUE ranging from 0.91 to 1.92 kg fisherman-1hour-1day-1; while in Corumbau were 1.93 t (averaging 0.27 t per month) and a CPUE ranging from 0.65 to 1.25 kg fisherman-1hour-1day-1. According to interviewed fishers, the largest recorded catch of S. trispinosus from the Abrolhos Bank during a single fishing trip was 700 kg in 2012 (Previero, 2014; Salz, 2015). Most of these fishing events occur within or near multiple-use reserves, including the Marine Extractive Reserves (MER) of Corumbau and Cassurubá, and the Ponta da Baleia MPA, as well as near or occasionally within the no-take Abrolhos Marine National Park, all situated within the Abrolhos Bank region.
Scarus trispinosus has also been a fishing target at coastal reefs between PE and Alagoas (AL) states, within the multiple-use Costa dos Corais MPA (8°53’S–35°08’W to 9°25’S–35°30’W) (Figure 2), where the species sharply declined over the last four decades (Pereira et al., 2021). Based on fishers’ empirical knowledge, the number of individuals caught decreased 64% and the weight of the largest individual ever caught decreased 67% over time from 1980s to 2010s (Pereira et al., 2021). Currently, the species is considered rare in this region, with an abundance of 0.80 ± 0.22 ind. 40m-2 recorded in Tamandaré, located in the northern portion of Costa dos Corais MPA (Feitosa & Ferreira, 2015).
Similarly, both fishers’ knowledge and scientific data indicate a sharp population decline of S. trispinosus in the subtropical rocky reefs of Arraial do Cabo, RJ (Bender et al., 2014). While large individuals weighing up to 20 kg were caught in this area until the 1990s, the species was absent from visual censuses conducted between 1997 and 2012 (Bender et al., 2014). Due to its current rarity, S. trispinosus is considered ecologically extinct in Arraial do Cabo, RJ (Bender et al., 2014). The vanishing of this species in this region can be attributed to fishing pressure, coupled with other factors such as the narrow continental shelf, which limits the availability of refuges, and the presence of cold waters from upwelling events, creating an environment unfavorable for recolonization.
Recreational spearfishing poses an additional threat to S. trispinosus, exacerbated by the lack of scientific attention due to the challenges of monitoring this activity. For example, spearfishing championships organized by the Brazilian Confederation of Spearfishing (Confederação Brasileira de Caça Submarina—CBCS) are a frequent activity in Salvador, BA. Between 2006 and 2008, S. trispinosus was the most commonly caught herbivorous fish in Bahia state through recreational spearfishing, with Salvador accounting for 63% of the catches (Nunes et al., 2012). In the Abrolhos Bank, a study using social media data identified S. trispinosus as the fourth most caught fish by recreational spearfishers (Giglio et al., 2020). Similarly, another social media-based study revealed that S. trispinosus is caught by recreational spearfishing across most of the Brazilian coast and at larger sizes when compared to artisanal fisheries, revealing complementary fishing pressures operating on different life stages of this species (Figure 2c; Roos and Longo, 2021).
In 2021, a reconstruction of Brazilian marine commercial landings from 1950 to 2015, encompassing both industrial and artisanal fisheries was published (see Freire et al., 2021). The states of RN, PE, and BA together accounted for ~95% of the total parrotfish catches (multiple species; Freire et al., 2021). This study also provided information on S. trispinosus landings in RN and Espírito Santo (ES) states, with the reconstruction based on data derived from unpublished documents issued by federal agencies, institutions, and universities. In RN, the reported catch of S. trispinosus ranged from 0.034 t in 1950 to 6.7 t in 2015, peaking at 35.52 t in 2008. For ES, the available data covered a shorter timeframe, with reported catches ranging from 0.096 t in 2011 to 0.076 t in 2015. Notably, most of the S. trispinosus landed in ES were actually caught in the Abrolhos Bank.
Current understanding of conservation
Driven by concerns over the intense fishing pressure on S. trispinosus in the Abrolhos Bank, researchers began investigating the effects of MPAs with different protection categories in the early 2000s (Francini-Filho and Moura, 2008). Their findings revealed that the biomass of the species increased significantly shortly after the fishing ban for S. trispinosus was implemented in the Corumbau MER in 2001. However, this recovery was followed by a decline from 2003 onward, coinciding with increased poaching and the reopening of the parrotfish fishery (Francini-Filho and Moura, 2008). Still, the abundance and biomass of S. trispinosus have been considerably higher within fully protected areas, such as the Abrolhos Marine National Park (Ferreira, 2005; Francini-Filho and Moura, 2008; Roos et al., 2020b; Table 1).
Despite their critical role, no-take MPAs alone are sometimes insufficient to mitigate critical impacts occurring outside their boundaries (Lester et al., 2009), a reality in several countries, including Belize (Cox et al., 2017), New Zealand (LaScala-Gruenewald et al., 2021), and Brazil (Roos et al., 2020b; Boelter et al., 2024). In the case of S. trispinosus, despite important nursery habitats being located within no-take MPAs in the Abrolhos Bank, both juvenile and adult populations showed declining trends between 2003 and 2008, even within these no-take areas (Roos et al., 2020b). This suggests that excessive removal of adult individuals outside no-take MPAs and within multiple-use reserves may be reducing the generation of new recruits. These findings highlight that while MPAs are essential, they alone cannot ensure the conservation of species targeted by fisheries. Complementary conservation policies, alongside with well-implemented MPAs, must be adopted to ensure effective protection for S. trispinosus (Roos et al., 2020b; Pereira et al., 2022), and for parrotfishes elsewhere.
In 2012, S. trispinosus was listed as Endangered by the International Union for Conservation of Nature (IUCN; Padovani-Ferreira et al., 2012). Following this, a status review report for the species was warranted by the U.S. National Marine Fisheries Service (NMFS/NOAA Fisheries) in early 2014. This document was the first to compile comprehensive information about S. trispinosus and specialists concluded that the species was at risk of extinction due to overfishing and the absence of regulatory measures (see Salz, 2015). Before S. trispinosus was listed as Endangered by the Brazilian Red List of Endangered Species, in December 18th 2014 (Decree n° 445, Brazil’s Red List, 2014), conservation efforts for the species were already underway in Brazil through a partnership between Conservation International (CI-Brazil), researchers, Federal Universities and the Brazilian Federal Environmental Agency (ICMBio). After discussions based on data mostly collected in the Abrolhos Bank, a proposal for the management of Brazilian herbivorous fish was released (Freitas, 2016). This document was later used to develop the Brazilian National Recovery Plan for endangered species (Decree N° 59-B/2018), published in 2018. This decree regulated S. trispinosus fishing under restrict rules, which included the ban on recreational fishing and fishing nets, night fishing, determined spearguns as the only fishing gear allowed and a slot-size limit for catches between 39 and 63 cm total length (aiming to protect both immature and older mature individuals, including most males), and that fishing would only be allowed within multiple-use MPAs by authorized artisanal fishers, with continuous monitoring, and prohibited across the Brazilian coast. This Recovery Plan was later discussed by other scientists, who called it “An Inverted Management Strategy” (see Pinheiro et al., 2021). Although parrotfish fishing has never been prohibited within multiple-use MPAs, the authors highlighted the innovative approach of prohibiting fishing for S. trispinosus in unprotected/unmanaged areas (i.e., most of the Brazilian coast). They emphasize that this strategy can be effective if proper enforcement is in place and suggest it could yield even better results if implemented after a period of fishing ban, allowing populations to first partially recover from current overfishing (Pinheiro et al., 2021).
The Abrolhos bank seems to be a suitable region to enforce these measures, due to the presence of multiple-use reserves, particularly the Corumbau and Cassurubá MER, and the fact that most of the artisanal catches are within the proposed slot size limit (Freitas et al., 2019). In other regions, such as Rio do Fogo (RN), the Recovery Plan should be adapted or implemented with additional restrictions, as most of the individuals caught there are below the proposed slot size limit (Roos et al., 2016). For instance, if the Recovery Plan were properly implemented and enforced in Rio do Fogo, the use of nets to catch S. trispinosus would be prohibited, an action that alone would increase the average size of individuals landed. Nevertheless, most of the individuals caught with spearguns would still be close to the minimum size limit (Roos et al., 2016). Therefore, introducing a maximum daily catch limit could serve as an additional regulation within the local management plan for this area.
Discussion
Given the unique ecological roles performed by certain parrotfish species and the threats they face, conservation efforts should prioritize key species. This is the case of the largest endemic Brazilian parrotfish, S. trispinosus, whose 4,000 km distribution is confined to a single country’s jurisdiction. While a fishing ban would ideally be the most effective conservation measure for this species, similar to those implemented in the Caribbean (Harms-Tuohy, 2021), the socio-economic context of Brazil poses significant challenges to adopting such an approach. For instance, in 2022, over 33 million people (15% of the population) in Brazil faced hunger (Kakaei et al., 2022), while approximately one million individuals relied on artisanal fishing for their livelihoods (Silveira and Padovani-Ferreira, 2024). Top-down regulations have proven costly and often ineffective due to low compliance among resource users. In contrast, stakeholder involvement in the decision-making process has been shown to reduce costs and conflicts while increasing the likelihood of successful management outcomes (McClanahan et al., 2016; McCay and Jones, 2011; Lopes et al., 2013). Considering these challenges and the current knowledge of S. trispinosus, I summarize key pathways and highlight critical gaps that must be addressed for the effective conservation of this species.
Investigating age-based demography through both space and time
While numerous studies focus on parrotfishes’ feeding behavior, monitoring abundance, catch rates, and changes in demographic traits lacks attention despite being essential for stock assessments and fisheries management. This includes evaluating recruitment rates, age and size at maturation, size structures, and mortality rates in areas where the species is fished (Taylor et al., 2018). To date, only two demographic studies on S. trispinosus have been published, one of which includes data collected over a decade ago (Freitas et al., 2019; Roos et al., 2020a). The lack of updated information on potential changes in the species’ demographic traits is currently a critical gap for its assessment.
Since 2021, the Brazilian Parrotfish Project (Projeto Budiões, sponsored by the Petrobras Socio-Environmental Program) has been monitoring parrotfishes population abundances and catch rates along the Brazilian coast. While monitoring legal fish landings through local participatory approaches, and the species populations through visual censuses marks significant progress in S. trispinosus conservation, demographic studies should also be prioritized. Reevaluating life-history traits, particularly in the Abrolhos Bank, is especially important given the ongoing fishing activities in the region. This will be essential for assessing whether the Recovery Plan is effectively maintaining the species’ demographic structure.
Implementing and enforcing the recovery plan in key areas through participatory approaches
Although the Brazilian National Recovery Plan for endangered species, which included S. trispinosus (Decree N° 59-B/2018), has been released since 2018, its implementation was hindered by the government at the time and later by the COVID-19 pandemic. Recently, steps have been taken to implement the plan. The idea behind the Recovery Plan is to properly manage and monitor S. trispinosus fisheries where it represents an important income source for small-scale fishers, restricting it to specific multiple-use MPAs where enforcement would be more feasible, while banning other non-essential fishing for the species along most of the coast. However, to balance the needs of small-scale fishers with the species conservation, the plan must be effectively implemented, regularly monitored, evaluated, and adapted to ensure its effectiveness. Participatory approaches on its implementation and effective surveillance are crucial to ensure compliance and achieve positive outcomes (Cinner et al., 2012). If successful, it could serve as a model for managing parrotfish populations in other developing countries. For instance, despite existing regulations, parrotfish fishing persists in 73% of 37 Caribbean countries (Harms-Tuohy, 2021). In some of them, such as Jamaica, Haiti, and Grenada, parrotfishes remain a staple food resource and are caught without any regulation (Harms-Tuohy, 2021). Identifying key areas where local regulations could be enforced while banning non-essential fishing would be a crucial first step toward the conservation of Caribbean parrotfish in these nations.
Currently, the Brazilian Federal Environmental Agency (ICMBio) has been working with the help of Projeto Budiões, local fishers, and other entities to implement the Recovery Plan. So far, Corumbau and Cassurubá MER have published local management plans (Decrees N° 284 and 285/2021) based on the guidelines of the National Recovery Plan. The local plans include not only S. trispinosus, but other endemic parrotfishes listed as Vulnerable, such as S. zelindae, Sp. frondosum, and Sp. aillare, and adds an extra rule which establishes a catch limit of 20 individuals of S. trispinosus per fisher per day. The creation of local management plans in Corumbau and Cassurubá MER is a significant step toward the effective management in these areas where S. trispinosus has been fished for decades.
In addition to the Abrolhos Bank, the multiple-use Recife de Corais MPA in Rio do Fogo, RN, has supported persistent fishing of S. trispinosus over the past decades and should also be prioritized. However, no efforts in implementing the Recovery Plan have been made in this area to date. Still, if implemented, the plan would require adaptation for this area, as most of the S. trispinosus caught are below the minimum slot-size limit (Roos et al., 2016). Enforcing a ban on net usage, for example, would be an important step, along with establishing a daily catch limit of individuals, similar to the regulations implemented in Corumbau and Cassurubá MER.
The multiple-use Costa dos Corais MPA, which comprises PE and AL states, may also benefit from local management plans for S. trispinosus, as suggested by Pereira et al. (2021). However, due to the species’ rarity in this area, its catch by small-scale fishers appears to be more opportunistic. In this case, a complete ban on S. trispinosus fishing might be more appropriate, as fishers may not rely on this resource for income.
Enforcing S. trispinosus fishing ban and monitoring illegal fishing across the Brazilian coast
As established by the Brazilian National Recovery Plan for endangered species, S. trispinosus fishing is prohibited in unmanaged/unprotected areas, i.e., most of the Brazilian coast. However, effective implementation remains hindered by insufficient public awareness of the ban and inadequate surveillance efforts. For instance, Alcobaça port in southern BA remains a critical location due to non-compliance by local fishers who use illegal methods, such as air compressors, to catch S. trispinosus on deep offshore reefs. Another significant challenge is enforcing the ban on S. trispinosus recreational spearfishing, which occurs along nearly the entire Brazilian coast but is particularly prevalent in Salvador and southern Bahia (Roos and Longo, 2021). As ‘trophy fishes’ are usually the main targets, this type of fishing is particularly detrimental to larger, older individuals with greater reproductive capacity (McClenachan, 2009). This poses a serious issue for a protogynous hermaphrodite species like S. trispinosus, where females transition to males, since males, which are typically larger and less abundant than females (Freitas et al., 2019), are more likely to be removed from the population. A decreasing number of males can disrupt population dynamics in several ways, including a reduction in the mean length and age at maturation and sex change, an increasingly female-biased sex ratio, lower fecundity and fertilization rates, and disruption of social structure (Rowe and Hutchings, 2003; Hawkins and Roberts, 2004; Taylor et al., 2018).
Spearfishing, often regarded as a leisure activity, frequently involves sharing fishing accomplishments on social media (Giglio et al., 2020; Roos and Longo, 2021). Social media has thus become a rapid and cost-effective tool for gathering nationwide fisheries data, particularly for recreational fishing, which typically lacks formal monitoring. This approach can help identify illegal fishing, areas where spearfishing is most intense, and the life stages most targeted. Such insights can guide the enforcement of the S. trispinosus fishing ban along the Brazilian coast, as well as aid in monitoring recreational spearfishing in countries where the activity lacks specific regulations, such as Puerto Rico, or where species-specific regulations exist, such as Saint-Martin (Harms-Tuohy, 2021).
Tracking the value chain and restraining the distribution of S. trispinosus
A major challenge in small-scale fisheries policy-making, particularly in developing countries, is the lack of reliable data on several facets of the sector, which includes not only biological data of targeted species, but information on fishers and all users of the fish value chain (Thyresson et al., 2013; Damasio et al., 2020). Smaller Brazilian parrotfishes such as Sp. axillare and Sp. frondosum are primarily targeted for international export (Cunha et al., 2012). Although their export is now prohibited (Decrees No 59-B and 63/2018), illegal trade persists. Similarly, while S. trispinosus is predominantly sold as fillets in local and regional markets, it is also traded in larger cities like Vitória, Porto Seguro (Francini-Filho and Moura, 2008), and Natal (pers. obs.), and even exported abroad (Francini-Filho and Moura, 2008). The expansion of the S. trispinosus trade must be urgently controlled, however, the lack of understanding about its trade remains a critical gap that hinders the effective application of current laws. From a global perspective, gaining insight into parrotfish value chains could help establish fiscal and sanitary barriers to better regulate parrotfish fishing worldwide.
Outreach and dissemination of information to the general public
Conserving an endangered species may depend on public awareness, particularly those used as food resources. Outreach and information dissemination can significantly enhance conservation efforts by raising awareness of the species’ importance, promoting compliance through better understanding of regulations, and supporting enforcement by encouraging public reporting of illegal activities to authorities. In the case of an endangered fishing target like S. trispinosus, it is fundamental to inform both fishers and the general citizens about fishing regulations and bans, discourage the consumption of the species, and raise awareness about the ecological consequences of overfishing. For example, several Caribbean nations have incorporated parrotfish conservation into their environmental education and outreach efforts, particularly in the contexts of sustainable fisheries and community-based conservation (Harms-Tuohy, 2021). In Brazil, Projeto Budiões has been conducting outreach since 2021. While public awareness of Brazilian parrotfishes is growing, the socioeconomic challenges that hinder their conservation persist.
The future of parrotfish research and conservation in Brazil
Conserving one of the world’s most endangered parrotfishes along thousands of kilometers of coastline in a developing country facing socioeconomic problems presents a significant conservation challenge. Nevertheless, efforts to conserve S. trispinosus began before 2014, even when knowledge of the species was limited. While progress has been slower than desired, substantial advances have been made over the past decade. We now have a better understanding of S. trispinosus, including its distribution patterns, demographic traits, and areas of intense exploitation along the Brazilian coast. The publication of local management plans in the marine extractive reserves of the Abrolhos Bank, alongside scientific research and outreach initiatives from some Brazilian projects, marks a major step forward in the species’ conservation. However, these efforts must be continuously monitored and evaluated. Future research and conservation efforts should prioritize key knowledge gaps, including a broader understanding of its distribution in mesophotic reefs, life-history traits, and value chain, along with expanding public awareness through outreach and education. Additionally, improving communication between scientists, fishery managers, and fishers is crucial for effective conservation. Equally important, socioeconomic improvements supported by effective public policies are essential, as they can enhance compliance by reducing fishers’ reliance on fishing, particularly illegal fishing, through the provision of alternative sources of income. Such improvements and efforts to fill knowledge gaps may benefit not only S. trispinosus but also other endangered species, both in Brazil and in other countries facing similar socioeconomic and conservation challenges.
Author contributions
NR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. I was supported by Fundação deAmparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) with afellowship (#200.569/2025).
Acknowledgments
I am grateful to the many mentors from whom I have learned about Scarus trispinosus, experts who were researching and working toward the conservation of this species long before my own involvement: B. Padovani-Ferreira, C. E. L. Ferreira, S. R. Floeter, L. A. Rocha, R. L. Moura, J. L. Gasparini, R. M. Bonaldo, R. B. Francini-Filho, M. O. Freitas, C. W. Hackradt, F. C. F. Hackradt, M. Previero, G. O. Longo, among others. I would like to thank everyone who granted permission to use their photographs of Scarus trispinosus in this work: Léo Francini/Bio Teia Estudos Ambientais, Bruno C. S. Antunes, Roberto C. Pinto, Jonne Roriz/Nosso Impacto, and Ananda Silva.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Araujo G. S., Rocha L. A., Lastrucci N. S., Luiz O. J., Di Dario F., and Floeter S. R. (2022). The Amazon-Orinoco Barrier as a driver of reef-fish speciation in the Western Atlantic through time. J. Biogeogr. 49, 1407–1419. doi: 10.1111/jbi.14398
Aswani S. and Sabetian A. (2010). Implications of urbanization for artisanal parrotfish fisheries in the Western Solomon Islands. Conserv. Biol. 24, 520–530. doi: 10.1111/j.1523-1739.2009.01377.x
Aued A. W., Smith F., Quimbayo J. P., Candido D. V., Longo G. O., Ferreira C. E., et al. (2018). Large-scale patterns of benthic marine communities in the Brazilian Province. PloS One 13, e0198452. doi: 10.1371/journal.pone.0198452
Bellwood D. R. and Choat J. H. (1990). “A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications,” in Alternative life-history styles of fishes. Developments in environmental biology of fishes, vol. 10 . Ed. Bruton M. N. (Springer, Dordrecht). doi: 10.1007/978-94-009-2065-1_11
Bellwood D. R., Hoey A. S., and Hughes T. P. (2012). Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proc. R. Soc B 279, 1621–1629. doi: 10.1098/rspb.2011.1906
Bender M. G., Ferreira C. E. L., Hanazaki N., Zapelini C., and Giglio V. J. (2016). “Como eram os recifes brasileiros? Mudanças na percepção individual do ambiente e da diversidade marinha,” in Conhecendo os Recifes Brasileiros: Rede de Pesquisas Coral Vivo. Eds. Zilberberg C., Abrantes D. P., Marques J. A., MaChado L. F., and Marangoni L. F. B. (Museu Nacional, UFRJ, Brazil, Rio de Janeiro).
Bender M. G., MaChado G. R., Silva P. J. A., Floeter S. R., Monteiro-Netto C., Luiz O. J., et al. (2014). Local ecological knowledge and scientific data reveal overexploitation by multigear artisanal fisheries in the Southwestern Atlantic. PloS One 9, e0110332. doi: 10.1371/journal.pone.0110332
Bezerra I. M., Gramacho K. P., Barreto M. A., Hackradt C. W., Feitosa J. L.L., Torres R. A., et al. (2018). Genetic diversity and gene flow of the threatened Brazilian endemic parrotfish Scarus trispinosus (Valenciennes 1840). Mar. Environ. Res. 142, 155–162. doi: 10.1016/j.marenvres.2018.10.004
Boelter J. P., Silva F. C., Quimbayo J. P., and Floeter S. R. (2024). Broken expectations: Population decline of a key grouper species within a 30-year-old no-take MPA in the Southwestern Atlantic. Ocean Coast. Manage. 257, 107318. doi: 10.1016/j.ocecoaman.2024.107318
Bonaldo R. M., Hoey A. S., and Bellwood D. R. (2014). The ecosystem roles of parrotfishes on tropical reefs. Oceanogr. Mar. Biol. Annu. Rev. 52, 81–132.
Bozec Y. M., O’Farrell S., Bruggemann J. H., Luckhurst B. E., and Mumby P. J. (2016). Tradeoffs between fisheries harvest and the resilience of coral reefs. PNAS 113, 4536–4541. doi: 10.1073/pnas.1601529113
Braz G. B., Lacerda C. H., Evangelista H., Güth A. Z., Rumbelsperger A. M., Capel K. C., et al (2022). Unprecedented erosion of Mussismilia harttii, a major reef-building species in the Southwestern Atlantic, after the 2019 bleaching event. Coral Reefs 41, 1537–1548. doi: 10.1007/s00338-022-02303-1
Bruno J. F., Côté I. M., and Toth L. T. (2019). Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Annu. Rev. Mar. Sci. 11, 307–334. doi: 10.1146/annurev-marine-010318-095300
Burkepile D. E. and Hay M. E. (2008). Herbivore richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl. Acad. Sci. U.S.A. 105, 16201–16206. doi: 10.1073/pnas.0801946105
Choat J. H., Klanten O. S., van Herwerden L., Robertson D. R., and Clements K. D. (2012). Patterns and processes in the evolutionary history of the parrotfishes (Family Labridae). Biol. J. Linn. Soc 107, 529–557. doi: 10.1111/j.1095-8312.2012.01959.x
Choat J. H. and Robertson D. R. (1975). “Protogynous hermaphroditism in fishes of the family Scaridae,” in Intersexuality in the Animal Kingdom. Ed. Reinboth R. (Springer Berlin Heidelberg, Berlin, Heidelberg), 263–283.
Choat J. H. and Robertson D. R. (2002). “Age-based studies,” in Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem. Ed. Sale P. F. (Academic Press, San Diego), 57–80.
Choat J. H., Robertson D. R., Ackerman J. L., and Posada J. M. (2003). An age-based demographic analysis of the Caribbean stoplight parrotfish Sparisoma viride. Mar. Ecol. Prog. Ser. 246, 265–277. doi: 10.3354/meps246265
Cinner J. E., McClanahan T. R., MacNeil M. A., Graham N. A. J., Daw T. M., Mukminin A., et al. (2012). Comanagement of coral reef social-ecological systems. Proc. Natl. Acad. Sci. U.S.A. 109, 5219–5222. doi: 10.1073/pnas.1121215109
Clements K. D., German D. P., Piché J., Tribollet A., and Choat J. H. (2017). Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol. J. Linn. Soc 120, 729–751. doi: 10.1111/bij.12914
Comeros-Raynal M. T., Choat J. H., Polidoro B. A., Clements K. D., Abesamis R., Craig M. T., et al. (2012). The likelihood of extinction of iconic and dominant herbivores and detritivores of coral reefs: the parrotfishes and surgeonfishes. PloS One 7, e39825. doi: 10.1371/journal.pone.0039825
Corazza B. M., Lacerda C. H., Güth A. Z., Marcançoli R. K., Bianchini A., Calderon E. N., et al. (2024). No coral recovery three years after a major bleaching event in reefs in the Southwestern Atlantic refugium. Mar. Biol. 171, 1–14. doi: 10.1007/s00227-024-04432-3
Cordeiro C. A. M. M., Quimbayo J. P., Nunes J. A. C. C., Nunes L. T., Sissini M. N., Sampaio C. L. S., et al. (2021). Conservation status of the southernmost reef of the Amazon Reef System: the Parcel de Manuel Luís. Coral Reefs 40, 165–185. doi: 10.1007/s00338-020-02026-1
Cowman P. F., Bellwood D. R., and van Herwerden L. (2009). Dating the evolutionary origins of wrasse lineages (Labridae) and the rise of trophic novelty on coral reefs. Mol. Phylogenet. Evol. 52, 621–631. doi: 10.1016/j.ympev.2009.05.015
Cox C., Valdivia A., McField M., Castillo K., and Bruno J. F. (2017). Establishment of marine protected areas alone does not restore coral reef communities in Belize. Mar. Ecol. Prog. Ser. 563, 65–79. doi: 10.3354/meps11984
Cunha F. E. A., Carvalho R. A. A., and Araújo M. E. (2012). Exportation of reef fish for human consumption: long-term analysis using data from Rio Grande do Norte, Brazil. Bol. Inst. Pesca 38, 369–378.
Damasio L. M., Peninno M. G., and Lopes P. F. (2020). Small changes, big impacts: geographic expansion in small-scale fisheries. Fish. Res. 226, 105533. doi: 10.1016/j.fishres.2020.105533
Edwards C. B., Friedlander A. M., Green A. G., Hardt M. J., Sala E., Sweatman H. P., et al. (2014). Global assessment of the status of coral reef herbivorous fishes: evidence for fishing effects. Proc. R. Soc B 281, 20131835. doi: 10.1098/rspb.2013.1835
Eggertsen L., Luza A. L., Cordeiro C. A., Dambros C., Ferreira C. E., Floeter S. R., et al. (2024). Complexities of reef fisheries in Brazil: a retrospective and functional approach. Rev. Fish Biol. Fish. 34, 511–538. doi: 10.1007/s11160-023-09826-y
Feitosa J. L. L. and Ferreira B. P. (2015). Distribution and feeding patterns of juvenile parrotfish on algal-dominated coral reefs. Mar. Ecol. 36, 462–474. doi: 10.1111/maec.12154
Feitosa J. L. L., Queiroz-Véras L. V., Maida M., and Ferreira B. P. (2023). Going further on herbivore fishing: the removal of smaller fishes from algal-dominated reefs. Mar. Ecol. Prog. Ser. 713, 117–132. doi: 10.3354/meps14335
Feitoza M. F., Rosa R. S., and Rocha L. A. (2005). Ecology and zoogeography of deep reef fishes in northeastern Brazil. Bull. Mar. Sci. 76, 725–742.
Ferreira C. E. L. (2005). “The status of target reef fishes,” in A Rapid Marine Biodiversity Assessment of the Abrolhos Bank, Bahia, Brazil, vol. 38 . Eds. Dutra G. F., Allen G. R., Werner T., and McKenna A. S. (Conservation International, Washington, DC).
Ferreira C. E. L., Floeter S. R., Gasparini J. L., Ferreira B. P., and Joyeux J. C. (2004). Trophic structure patterns of Brazilian reef fishes: A latitudinal comparison. J. Biogeogr. 31, 1093–1106. doi: 10.1111/j.1365-2699.2004.01044.x
Ferreira C. E. L. and Gonçalves J. E. A. (2006). Community structure and diet of roving herbivorous reef fishes in the Abrolhos Archipelago, southwestern Atlantic. J. Fish Biol. 69, 1533–1551. doi: 10.1111/j.1095-8649.2006.01220.x
Ferreira C. E., Gonçalves J. E., and Coutinho R. (2001). Community structure of fishes and habitat complexity on a tropical rocky shore. Environ. Biol. Fishes 61, 353–369. doi: 10.1023/A:1011609617330
Ferreira L.C.L., Grillo A.C., Repinaldo Filho F.P.M., Souza F.N.R., and Longo G. O. (2001). Different responses of massive and branching corals to a major heatwave at the largest and richest reef complex in South Atlantic. Mar. Biol. 168 (5), 54. doi: 10.1007/s00227-021-03863-6
Floeter S. R., Halpern B. S., and Ferreira C. E. L. (2006). Effects of fishing and protection on Brazilian reef fishes. Biol. Conserv. 128, 391–402. doi: 10.1016/j.biocon.2005.10.005
Francini-Filho R. B., Ferreira C. M., Coni E. O. C., Moura R. L., and Kaufman L. (2010). Foraging activity of roving herbivorous reef fish (Acanthuridae and Scaridae) in eastern Brazil: influence of resource availability and interference competition. J. Mar. Biol. Assoc. U.K. 90, 481–492. doi: 10.1017/S0025315409991147
Francini-Filho R. B. and Moura R. L. (2008). Dynamics of fish assemblages on coral reefs subjected to different management regimes in the Abrolhos Bank, eastern Brazil. Aquat. Conserv. 18, 1166–1179. doi: 10.1002/aqc.966
Francini-Filho R. B., Moura R. L., Ferreira C. M., and Coni E. O. C. (2008). Live coral predation by parrotfishes (Perciformes: Scaridae) in the Abrolhos Bank, eastern Brazil, with comments on the classification of species into functional groups. Neotrop. Ichthyol. 6, 191–200. doi: 10.1590/S1679-62252008000200006
Freire K. M. F., Almeida Z. S., Amador J. R. E. T., Aragão J. A., Araújo A. R.R., Ávila-da-Silva A. O., et al. (2021). Reconstruction of marine commercial landings for the Brazilian industrial and artisanal fisheries from 1950 to 2015. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.659110
Freitas M. O. (2016). Plano de Recuperação para o budião-azul (Scarus trispinosus), peixe-papagaio-banana (Scarus zelindae) e peixes-papagaio-cinza (Sparisoma axillare e Sparisoma frondosum). (Curitiba, Brazil, Ministério do Meio Ambiente).
Freitas M. O., Previero M., Leite J. R., Francini-Filho R. B., Minte-Vera C. V., and Moura R. L. (2019). Age, growth, reproduction and management of Southwestern Atlantic’s largest and endangered herbivorous reef fish, Scarus trispinosus Valenciennes 1840. PeerJ 7, e7459. doi: 10.7717/peerj.7459
Giglio V. J., Suhett A. C., Zapelini C. S., Ramiro A. S., and Quimbayo J. P. (2020). Assessing captures of recreational spearfishing in Abrolhos reefs, Brazil, through social media. Reg. Stud. Mar. Sci. 34, 100995. doi: 10.1016/j.rsma.2019.100995
Hamilton R. J., Almany G. R., Stevens D., Bode M., Pita J., Peterson N. A., et al. (2016). Hyperstability masks declines in bumphead parrotfish (Bolbometopon muricatum) populations. Coral Reefs 35, 751–776. doi: 10.1007/s00338-016-1441-0
Harborne A. R. and Mumby P. J. (2018). “FAQs about Caribbean parrotfish management and their role in reef resilience,” in Biology of Parrotfishes (CRC Press), 383–406.
Harms-Tuohy C. A. (2021). Parrotfishes in the Caribbean: a regional review with recommendations for management Vol. 1240 (Food & Agriculture Organization).
Hawkins J. P. and Roberts C. M. (2004). Effects of artisanal fishing on Caribbean coral reefs. Conserv. Biol. 18, 215–226. doi: 10.1111/j.1523-1739.2004.00328.x
Hoey A. S., Berumen M. L., Bonaldo R. M., Burt J. A., Feary D. A., Ferreira C. E., et al. (2018a). “The ecology of parrotfishes in marginal reef systems,” in Biology of Parrotfishes (CRC Press), 276–301.
Hoey A. S., Taylor B. M., Hoey J., and Fox R. J. (2018b). “Parrotfishes, are we still scraping the surface? Emerging topics and future research directions,” in Biology of Parrotfishes (CRC Press), 407–416.
Houk P., Rhodes K., Cuetos-Bueno J., Lindfield S., Fread V., and McIlwain J. L. (2012). Commercial coral-reef fisheries across Micronesia: a need for improving management. Coral Reefs 31, 13–26. doi: 10.1007/s00338-011-0826-3
Hughes T. P., Barnes M. L., Bellwood D. R., Cinner J. E., Cumming G. S., et al. (2017). Coral reefs in the anthropocene. Nature 546, 82–90. doi: 10.1038/nature22901
Kakaei H., Nourmoradi H., Bakhtiyari S., Jalilian M., and Mirzaei A. (2022). “Effect of COVID-19 on food security, hunger, and food crisis,” in COVID-19 and the Sustainable Development Goals (Elsevier), 3–29. doi: 10.1016/B978-0-323-91307-2.00005-5
Kikuchi R. K. P., Leão Z. M. A. N., Sampaio C. L., and Telles M. D. (2003). “Rapid assessment of the Abrolhos reefs, eastern Brazil (Part 2: Fish communities),” in Status of Coral Reefs in the Western Atlantic: Results of Initial Surveys, Atlantic and Gulf Rapid Reef Assessment (AGRRA) Program, vol. 496 . Ed. Lang J. C. (Washington, DC, USA, Atlantic and Gulf Rapid Reef Assessment (AGRRA) program), 188–203.
LaScala-Gruenewald D. E., Grace R. V., Haggitt T. R., Hanns B. J., Kelly S., MacDiarmid A., et al. (2021). Small marine reserves do not provide a safeguard against overfishing. Conserv. Sci. Pract. 3, e362. doi: 10.1111/csp2.362
Lellys N. T., Moura R. L., Bonaldo R. M., Francini-Filho R. B., and Gibran F. Z. (2019). Parrotfish functional morphology and bioerosion on SW Atlantic reefs. Mar. Ecol. Prog. Ser. 629, 149–163. doi: 10.3354/meps13102
Lester S. E., Halpern B. S., Grorud-Colvert K., Lubchenco J., Ruttenberg B. I., Gaines S. D., et al. (2009). Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Prog. Ser. 384, 33–46. doi: 10.3354/meps08029
Longo G. O., Roos N. C., Bleuel J., and Luza A. L. (2024). ReefSYN | Standardized datasets of Brazilian reef diversity in space and time - Dataset XI: Rio Grande do Norte monitoring. Version 2.1. Tropical and Subtropical Western South Atlantic OBIS. Sampling event dataset. Earth Syst. Sci. Data Discuss. doi: 10.25607/2doybv
Lopes P. F. M., Rosa E. M., Salyvonchyk S., Nora V., and Begossi A. (2013). Suggestions for fixing top-down coastal fisheries management through participatory approaches. Mar. Policy 40, 100–110. doi: 10.1016/j.marpol.2012.12.033
Luza A., Cordeiro C., Aued A., Barneche D., Bleuel J., Ferreira C., et al. (2024). Standardized datasets of Brazilian reef diversity in space and time. Earth Syst. Sci. Data Discuss. doi: 10.5194/essd-2024-244
Mallela J. and Fox R. J. (2018). “The role of parrotfishes in the destruction and construction of coral reefs,” in Biology of Parrotfishes (CRC Press), 161–196.
Mangiafico S. S. (2023). rcompanion: Functions to Support Extension Education Program Evaluation. version 2.4.30 (New Jersey: Rutgers Cooperative Extension. New Brunswick). Available at: https://CRAN.R-project.org/package=rcompanion (Accessed January 4, 2025).
Mazzei E. F., Pinheiro H. T., Morais R. A., Floeter S. R., Veras D. P., Queiroz L. V., et al. (2019). Parrotfishes of the genus Scarus in southwestern Atlantic oceanic reef environments: occasional pulse or initial colonization? Mar. Biodivers. 49, 555–561. doi: 10.1007/s12526-017-0827-8
McCay B. J. and Jones P. J. (2011). Marine protected areas and the governance of marine ecosystems and fisheries. Conserv. Biol. 25, 1130–1133. doi: 10.1111/j.1523-1739.2011.01771.x
McClanahan T. R., Marnane M. J., Cinner J., and Kiene W. E. (2016). A comparison of marine protected areas and alternative approaches to coral-reef management. Curr. Biol. 16, 1408–1413. doi: 10.1016/j.cub.2006.05.062
McClenachan L. (2009). Documenting loss of large trophy fish from the Florida Keys with historical photographs. Conserv. Biol. 23, 636–643. doi: 10.1111/j.1523-1739.2008.01152.x
Medeiros P. R., Grempel R. G., Souza A. T., and Ilarri M. I. (2007). Effects of recreational activities on the fish assemblage structure in a northeastern Brazilian reef. Pan-Am. J. Aquat. Sci. 2, 288–300.
Molina-Hernández A. and Álvarez-Filip L. (2024). Incorporating parrotfish bioerosion into the herbivory paradigm of coral reef resilience. Conserv. Lett. 17, e13058. doi: 10.1111/conl.13058
Morais R. A., Ferreira C. E. L., and Floeter S. R. (2017). Spatial patterns of fish standing biomass across Brazilian reefs. J. Fish Biol. 91, 1642–1667. doi: 10.1111/jfb.13482
Morais R., Ferreira C. E. L., Floeter S. R., Quimbayo J. P., Mendes T. C., Longo G. O., et al. (2024). ReefSYN | Standardized datasets of Brazilian reef diversity in space and time - Dataset I: Fish communities from the Brazilian province. Version 3.3. Tropical and Subtropical Western South Atlantic OBIS. Sampling event dataset. Earth Syst. Sci. Data Discuss. doi: 10.25607/7nxv5v
Moura R. L., Figueiredo J. L., and Sazima I. (2001). A new parrotfish (Scaridae) from Brazil, and revalidation of Sparisoma amplum (Ranzani 1842), Sparisoma frondosum (Agassiz 1831), Sparisoma axillare (Steindachner 1878), and Scarus trispinosus Valenciennes 1840. Bull. Mar. Sci. 68, 505–524.
Nunes J. A. C. C., Medeiros D. V., Reis-Filho J. A., Sampaio C. L. S., and Barros F. (2012). Reef fishes captured by recreational spearfishing on reefs of Bahia State, northeast Brazil. Biota Neotrop. 12, 179–185. doi: 10.1590/S1676-06032012000100014
Padovani-Ferreira B., Floeter S., Rocha L. A., Ferreira C. E. L., Francini-Filho R., Moura R., et al. (2012). Scarus trispinosus (The IUCN red list of threatened species 2012). doi: 10.2305/IUCN.UK.2012.RLTS.T190748A17786694.en
Parenti P. and Randall J. E. (2011). Checklist of the species of the families Labridae and Scaridae: an update. Smithiana Bull. 13, 29–44.
Pauly D., Christensen V., Dalsgaard J., Froese R., and Torres F. (1998). Fishing down marine food webs. Science 279, 860–863. doi: 10.1126/science.279.5352.860
Pereira P. H., Araujo J. C., Lima G. V., Côrtes L. G., Gomes E., and Magris R. A. (2022). Effectiveness of management zones for recovering parrotfish species within the largest coastal marine protected area in Brazil. Sci. Rep. 12, 12232. doi: 10.1038/s41598-022-15990-1
Pereira P. H. C., Ternes M. L. F., Nunes J. A. C., and Giglio V. J. (2021). Overexploitation and behavioral changes of the largest South Atlantic parrotfish (Scarus trispinosus): Evidence from fishers’ knowledge. Biol. Conserv. 254, 108940. doi: 10.1016/j.biocon.2020.108940
Pinheiro H. T., Nunes J. A. C. C., Coni E. O. C., Almeida E. C.G., Sampaio C. L.S., Ferreira C. E., et al. (2021). An inverted management strategy for the fishery of endangered marine species. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.604108
Pinheiro H. T., Rocha L. A., Macieira R. M., Carvalho-Filho A., Anderson A. B., Bender M. G., et al. (2018). South-western Atlantic reef fishes: zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic Ocean. Divers. Distrib. 24, 951–965. doi: 10.1111/ddi.12729
Previero M. (2014). A pesca do budião-azul (Scarus trispinosus Valenciennes 1840) no maior complexo coralíneo do Atlântico Sul (Universidade Federal de Maringá).
Queiroz-Véras L. V. M. V., Ferreira B. P., Freitas M., and Feitosa J. L. L. (2023). A critical review and knowledge gaps to assess and manage threatened parrotfishes’ stocks in Brazil. Aquat. Sci. 85, 44. doi: 10.1007/s00027-023-00939-x
Queiroz-Véras L. V. M. V., Ferreira B. P., Oliveira T. C. T., Véras D. P., Silveira C. B. L., Roos N. C., et al. (2025). Unveiling the fishing history of threatened Brazilian parrotfishes through Local Ecological Knowledge. Rev. Fish Biol. Fish. doi: 10.1007/s11160-025-09931-0
Questel S. L. A. and Russ G. R. (2018). “No-take marine reserve effects on parrotfish and parrotfish-benthos interactions,” in Biology of Parrotfishes (CRC Press), 329–354.
R Core Team (2024). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/. (Accessed January 4, 2025).
Rhodes K. L., Tupper M. H., and Wichilmel C. B. (2008). Characterization and management of the commercial sector of the Pohnpei coral reef fishery, Micronesia. Coral Reefs 27, 443–454. doi: 10.1007/s00338-007-0331-x
Robertson D. R., Choat J. H., Posada J. M., Pitt J., and Ackerman J. L. (2005). Ocean surgeonfish Acanthurus bahianus. II. Fishing effects on longevity, size and abundance? Mar. Ecol. Prog. Ser. 295, 245–256. doi: 10.3354/meps295245
Rocha L. A. (2003). Patterns of distribution and processes of speciation in Brazilian reef fishes. J. Biogeogr. 30, 1161–1171. doi: 10.1046/j.1365-2699.2003.00900.x
Rocha L. A. and Rosa I. L. (2001). Baseline assessment of reef fish assemblages of Parcel Manuel Luiz Marine State Park, Maranhão, northeast Brazil. J. Fish Biol. 58, 985–998. doi: 10.1111/j.1095-8649.2001.tb00549.x
Roos N. C. and Longo G. O. (2021). Critical information for fisheries monitoring may be available in social media. Aquat. Conserv. Mar. Freshw. Ecosyst. 31(9), 2420-2428doi: 10.1002/aqc.3655
Roos N. C., Longo G. O., Pennino M. G., Francini-Filho R. B., and Carvalho A. R. (2020a). Protecting nursery areas without fisheries management is not enough to conserve the most endangered parrotfish of the Atlantic Ocean. Sci. Rep. 10, 1–10. doi: 10.1038/s41598-020-76207-x
Roos N. C., Pennino M. G., Carvalho A. R., and Longo G. O. (2019). Drivers of abundance and biomass of Brazilian parrotfishes. Mar. Ecol. Prog. Ser. 623, 117–130. doi: 10.3354/meps13005
Roos N. C., Pennino M. G., Lopes P. F. M., and Carvalho A. R. (2016). Multiple management strategies to control selectivity on parrotfishes harvesting. Ocean Coast. Manage. 134, 20–29. doi: 10.1016/j.ocecoaman.2016.09.029
Roos N. C., Taylor B. M., Carvalho A. R., and Longo G. O. (2020b). Demography of the largest and most endangered Brazilian parrotfish, Scarus trispinosus, reveals overfishing. Endanger. Species Res. 41, 319–327. doi: 10.3354/esr01024
owe S. and Hutchings J. A. (2003). Mating systems and the conservation of commercially exploited marine fish. Trends Ecol. Evol. 18, 567–572. doi: 10.1016/j.tree.2003.09.004
Russ G. R., Questel S. L. A., Rizzari J. R., and Alcala A. C. (2015). The parrotfish–coral relationship: refuting the ubiquity of a prevailing paradigm. Mar. Biol. 162, 2029–2045. doi: 10.1007/s00227-015-2728-3
Salz R. J. (2015). Greenback parrotfish (Scarus trispinosus) status review report (Report to National Marine Fisheries Service).
Shantz A. A., Ladd M. C., and Burkepile D. E. (2020). Overfishing and the ecological impacts of extirpating large parrotfish from Caribbean coral reefs. Ecol. Monogr. 90, e01403. doi: 10.1002/ecm.1403
Silveira M. F. and Padovani-Ferreira B. (2024). Temporal changes in a small-scale artisanal reef fishery in Brazil: Coastal development and its impacts. Mar. Policy 165, 106186. doi: 10.1016/j.marpol.2024.106186
Steneck R. S., Arnold S. N., and Mumby P. J. (2014). Experiment mimics fishing on parrotfish: insights on coral reef recovery and alternative attractors. Mar. Ecol. Prog. Ser. 506, 115–127. doi: 10.3354/meps10764
Tâmega F. T. S., Figueiredo M. A. O., Ferreira C. E. L., and Bonaldo R. M. (2016). Seaweed survival after consumption by the greenbeak parrotfish, Scarus trispinosus. Coral Reefs 35, 329–334. doi: 10.1007/s00338-015-1373-0
Taylor B. M., Benkwitt C. E., Choat H., Clements K. D., Graham N. A., and Meekan M. G. (2020). Synchronous biological feedbacks in parrotfishes associated with pantropical coral bleaching. Global Change Biol. 26, 1285–1294. doi: 10.1111/gcb.14909
Taylor B. M. and Choat J. H. (2014). Comparative demography of commercially important parrotfish species from Micronesia. J. Fish Biol. 84, 383–402. doi: 10.1111/jfb.12294
Taylor B. M., Houk P., Russ G. R., and Choat J. H. (2014). Life histories predict vulnerability to overexploitation in parrotfishes. Coral Reefs 33, 869–878. doi: 10.1007/s00338-014-1187-5
Taylor B. M. and Pardee C. (2017). Growth and maturation of the redlip parrotfish Scarus rubroviolaceus. J. Fish Biol. 90, 2452–2461. doi: 10.1111/jfb.13309
Taylor B. M., Trip E. D., and Choat J. H. (2018). “Dynamic demography: Investigations of life-history variation in the parrotfishes,” in Biology of Parrotfishes (CRC Press), 69–98.
Thyresson M., Crona B., Nyström M., de la Torre-Castro M., and Jiddawi N. (2013). Tracing value chains to understand effects of trade on coral reef fish in Zanzibar, Tanzania, Mar. Policy 38, 246–256. doi: 10.1016/j.marpol.2012.05.041
Keywords: management, reef fish, southwestern Atlantic, small-scale fishing, Brazil
Citation: Roos NC (2025) Challenges and pathways for parrotfish conservation in developing countries: lessons from the endemic and endangered greenbeak parrotfish Scarus trispinosus. Front. Ecol. Evol. 13:1557132. doi: 10.3389/fevo.2025.1557132
Received: 08 January 2025; Accepted: 16 April 2025;
Published: 23 May 2025.
Edited by:
Lynne Beaty, Penn State Erie, The Behrend College, United StatesReviewed by:
Sukran Yalcin Ozdilek, Çanakkale Onsekiz Mart University, TürkiyeHayley R. Nessia, EnviroStrat Ltd, New Zealand
Copyright © 2025 Roos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalia C. Roos, bmF0YWxpYXJvb3NAZ21haWwuY29t
 Natalia C. Roos
Natalia C. Roos