- 1Department of Biology, Central Michigan University, Mount Pleasant, MI, United States
- 2Neuroscience Program, Central Michigan University, Mount Pleasant, MI, United States
- 3Institute for Great Lakes Research, Central Michigan University, Mount Pleasant, MI, United States
- 4Division of Aquatic Ecology and Evolution, Institute of Ecology and Evolution, University of Bern, Bern, Switzerland
- 5Department of Fish Ecology and Evolution, Centre of Ecology, Evolution and Biogeochemistry (CEEB), Eawag, Swiss Federal Institute of Aquatic Science and Technology, Kastanienbaum, Switzerland
Male-male competition and female-female competition can play important roles in the origin and maintenance of phenotypic polymorphism and speciation. If territory owners bias aggression towards others of their own phenotype, rare male phenotypes will be involved in fewer costly fights, facilitating the evolution of diversity, and stabilizing the coexistence of distinct phenotypes or species. However, the mechanisms that regulate aggression biases have received little attention. We discuss how learning and plasticity in behavioral biases may dramatically influence how aggression biases evolve, which in turn may have important consequences for clarifying the role of intrasexual competition in the process of speciation. We then present data from a field study of two cichlid species in Lake Victoria and illustrate how the social environment could modulate aggression biases of territorial males towards specific intruder phenotypes. Specifically, in Pundamilia nyererei (males are red) and P. ‘pink anal’ (males are blue), blue territory holders showed a tendency to shift their aggression bias more towards red intruder (stimulus) males relative to blue intruder males when these territory holders had more red territorial neighbors. By contrast, red territory holders tended to reduce aggression towards red intruder males relative to blue intruder males when they were surrounded by more red territorial neighbors. Although sample sizes are small, our data suggest that social context may shape aggression biases in the Pundamilia species complex and that these effects may vary between species. We conclude that considering the social environment and experience in shaping aggression biases may advance our understanding of how mate competition shapes evolutionary patterns of phenotypic diversification.
Introduction
Sexual selection via mate choice plays an important role in speciation (Safran et al., 2013). For example, sexual selection can cause reproductive isolation between diverging populations when sexually selected traits are an indicator of local adaptation (van Doorn et al., 2009; Servedio and Boughman, 2017). While classically most attention has been given to mate choice, it is now increasingly recognized that intrasexual disputes for mates or territories plays a critical role in several models of evolutionary diversification (Weissing et al., 2011; McCullough et al., 2016; Grether et al., 2017; Tinghitella et al., 2018). Specifically, male-male competition can generate negative frequency dependent selection if territory owners bias aggression towards intruders that phenotypically resemble themselves (‘own-type’ aggression bias, which is adaptive because similar phenotypes likely belong to the same species and compete for the same pool of females) or toward common phenotypes (‘common-type’ aggression bias) (Seehausen and Schluter, 2004). In these situations, rare phenotypes spend less time and energy on costly territorial disputes. Such a ‘rare male advantage’ would help rare phenotypes invade the population and stabilize the coexistence of distinct phenotypes and sister species (van Doorn et al., 2004; Dijkstra and Border, 2018). Previous studies have tested for own-type aggression biases using territorial intrusion tests in several animal species (Bolnick et al., 2016; Yang et al., 2018). Some studies have reported that animals preferentially attack competitors that resemble their own phenotypes (Anderson and Grether, 2010; Lehtonen, 2014; Bolnick et al., 2016; Moran et al., 2017; Scali et al., 2021). Others found that territorial aggression was biased towards specific phenotypes (Dijkstra et al., 2006; Tinghitella et al., 2015; Drury et al., 2020), such as phenotypes that are more common in the local environment [but see (Bieri et al., 2024)]. Despite the considerable attention that has been paid to aggression biases in the context of evolutionary diversification, few studies have considered the mechanisms that generate and maintain aggression biases.
Selection is expected to favor own-type aggression biases, because similar (conspecific) rivals compete for the same pool of mates. However, gene flow would break up combinations of alleles that are responsible for own-type aggression biases unless one-allele mechanisms, such as learning, induce a link between behavioral biases and (own) phenotype (Yeaman and Whitlock, 2011; Flaxman et al., 2014). Prior studies in, for example, cichlid fish and poison dart frogs indicate that early social experiences may increase own-type aggression biases (Dijkstra et al., 2008; Verzijden et al., 2008; Yang et al., 2019). Such plasticity is important as it could almost instantly create (within one generation) and maintain own-type aggression biases despite gene flow (Dijkstra and Border, 2018). Although there is a significant interest in learning and cognitive processes in studies on conflict behavior and aggression (Reichert and Quinn, 2017), we know very little about how the local environment shapes aggression biases based on early and recent social experience. Nevertheless, there is a large body of literature on rival recognition and adjustments in territorial responses to neighbors versus strangers based on recent social context (Christensen and Radford, 2018). Territory owners may exhibit weaker responses to familiar territorial neighbors versus strangers as neighbors are more likely to respect territorial boundaries while the intentions of strangers are often unclear [the ‘dear enemy effect’ (Temeles, 1994)] or the opposite [the ‘nasty-neighbor effect’ (Müller and Manser, 2007)]. Territorial male bullfrogs (Rana catesbeiana) exhibited diminished responses with repeated synthetic bullfrog calls in field playback experiments, suggesting that habituation to rival-specific stimuli could explain the dear enemy effect in this species (Bee, 2003). This suggests that habituation to specific recognition cues may also result in adjusted responses to rival phenotypes that resemble familiar neighbors. Habituation to familiar male phenotypes would actually diminish or even eliminate rare male advantages since territory owners are more likely to escalate fights when encountering rivals with an unfamiliar/rare phenotype (Carazo et al., 2008; delBarco-Trillo et al., 2009; Humfeld et al., 2009). By contrast, ‘nasty neighbor’ effects could provide rapid means for creating rare male advantages if males learn to bias aggression towards their own or the more abundant phenotype that poses a greater threat to territory owners (Hyman and Hughes, 2006; Akçay et al., 2009). If ‘dear enemy’ effects diminish aggression biases towards common phenotypes but increase it towards unfamiliar, rare phenotypes, then male-male competition would hinder speciation. By contrast, in the case of ‘nasty neighbor’ effects favoring the expression of own-type or common-type aggression biases, male-male competition would facilitate the emergence of new phenotypes and stabilize the speciation process by promoting coexistence of divergent phenotypes or sister species (Figure 1).
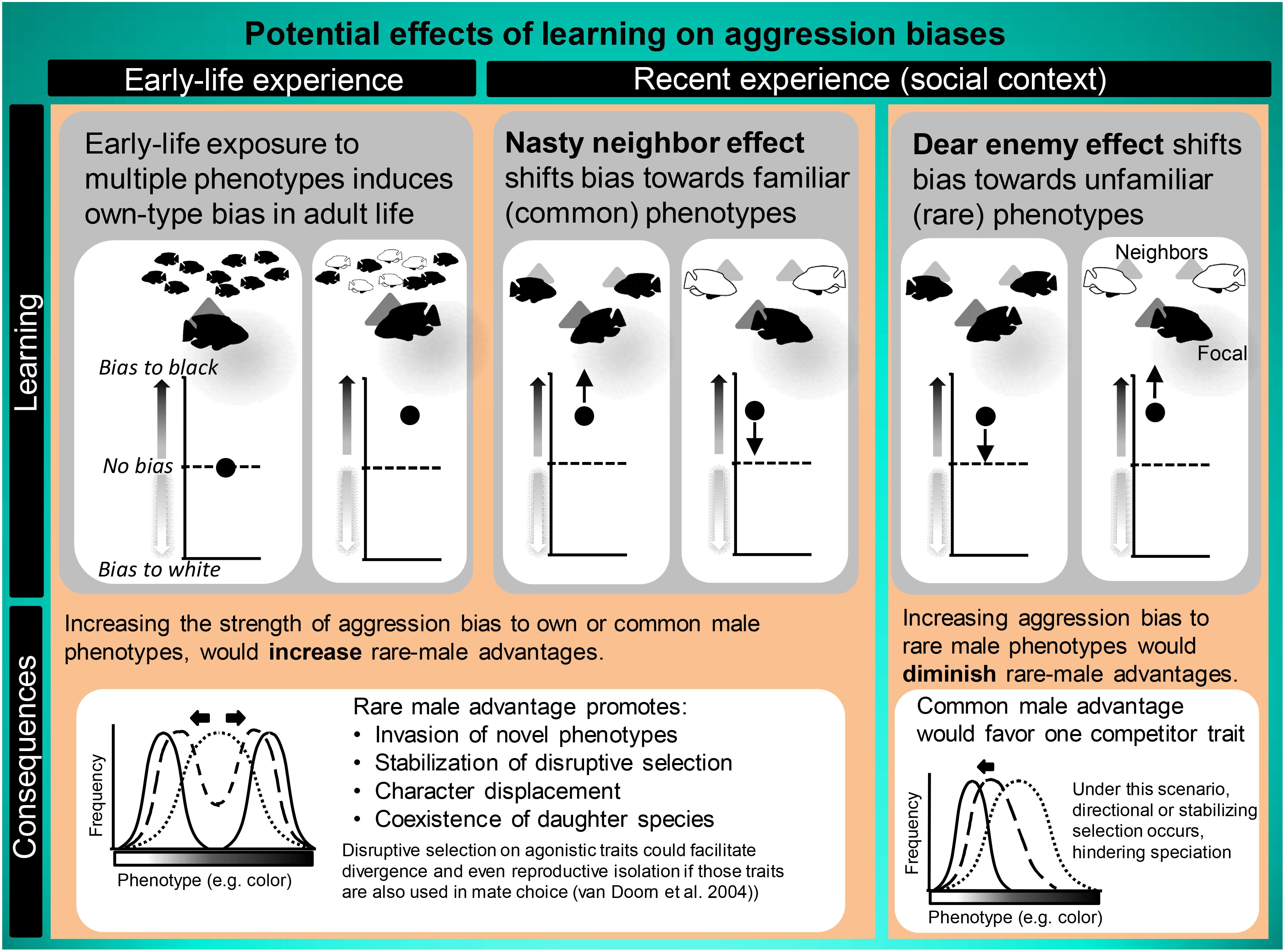
Figure 1. Schematic overview of the role of learning and plasticity in modulating aggression biases and potential consequences for speciation and divergence. Experience with heterospecific competitors early in life is known to facilitate own-type aggression biases (Dijkstra et al., 2008; Dijkstra unpublished data). In addition, more recent interactions with immediate territorial neighbors may lead to plasticity in agonistic behavior (expected shifts in biases are indicated by arrows), either enhancing, diminishing, or even abolishing rare-male advantages.
Several studies have suggested that exposure to competitors in local neighborhoods in early life may allow individuals to recognize direct competitors for mates and/or territories and lead to aggression biases towards the more abundant phenotype (Verzijden et al., 2008; Bolnick et al., 2016) or own phenotype (Dijkstra et al., 2008; Verzijden et al., 2009; Yang et al., 2019). While social learning early in life may facilitate the evolution of rare male advantages (Dijkstra et al., 2008; Verzijden et al., 2009; Yang et al., 2019) (Figure 1), less attention has been given to the effects of recent social experience on aggression biases. Here we use a dataset collected in the field of two sympatric species of Lake Victoria cichlid fish to illustrate how the local environment and recent social experiences could shape aggression biases (note that we were not able to study the effect of early experience in our study). The East African cichlids are an iconic and powerful model system for evolutionary and behavioral research, showing incredible variation in traits related to ecology and behavior (Albertson et al., 2003; Fanouraki et al., 2007; Brawand et al., 2014; York et al., 2018; Baran and Streelman, 2020). Males of some species, such as those confined to rocky reefs, are highly territorial and territory ownership is key to their reproductive success (Maan et al., 2004). Females take care of the offspring in the form of mouthbrooding of eggs and larvae (Renn and Schumer, 2013; Maruska et al., 2020). Sexual dimorphism is pronounced, and male nuptial coloration is used as a communication cue in both mate choice and competitive interactions (Seehausen and Van Alphen, 1998; Dijkstra et al., 2005). The Lake Victoria rocky reef dwelling cichlid fishes of the genus Pundamilia include species and morphs with red and blue male nuptial coloration with varying degrees of reproductive isolation (Seehausen et al., 2008; Svensson et al., 2017; Van Rijssel et al., 2018). Using simulated intrusion choice tests, we have previously found that blue and red males of reproductively isolated species bias aggression towards conspecifics, hence to their own nuptial phenotype (Dijkstra et al., 2006, 2007). In many cichlid species, territories can be found in aggregations with males engaging in aggressive interactions with surrounding territorial neighbors (Maan et al., 2004) to establish and maintain territorial boundaries (Dijkstra et al., 2022). It is possible that these social interactions as well as eavesdropping on neighbor’s interactions with third parties (Oliveira et al., 1998) modulate aggression biases in territorial cichlid males. In Pundamilia nyererei (males have a crimson red dorsum, referred to as ‘red’) and P. ‘pink anal’ (males are dark blue, referred to as ‘blue’), we have previously shown that males bias aggression towards their own species using simulated intruder choice tests with a red and a blue ‘stimulus’ (or ‘intruder’) male each enclosed in a glass jar (Dijkstra et al., 2006). Here we used the same field data to explore how aggression biases are correlated with the species composition in the social environment. To this end, we recorded the number of red and blue immediate neighbor(s) of each focal male and assessed how species composition of immediate territorial neighbor(s) was associated with the measured aggression biases towards red and blue stimulus males. If males learn to bias aggression towards the more abundant phenotype that is frequently encountered, we predict that their aggression preference will be tilted towards the more abundant phenotype, such that males with more red neighbors will exhibit a stronger aggression bias towards red stimuli compared to males from mixed-species neighborhoods (Figure 1). We have also previously shown that across different Pundamilia populations, blue phenotypes tend to exhibit more variable aggression biases compared to red phenotypes, which display strong own-type aggression biases (Dijkstra et al., 2007; Dijkstra and Groothuis, 2011). We therefore predicted that the effect of social context on aggression biases will be stronger in the blue species than in the red species.
Methods
Species
This study focuses on two species from Makobe island. Males of Pundamilia nyererei are crimson dorsally, yellow on their flanks, and have a crimson dorsal fin (referred to as ‘red’). Males of P. ‘pink anal’ are dark metallic blue and have a characteristic pink anal fin and pink lappets on the dorsal fin (referred to as ‘blue’) (Figure 2). Both species occur sympatrically and syntopically around Makobe Island [for details see (Seehausen, 1996; Seehausen and Bouton, 1997)]. The data was collected in the field between Dec. 22nd, 2002, and July 23rd, 2003, and observations took place in the mornings between 9 am and 12:00 pm.
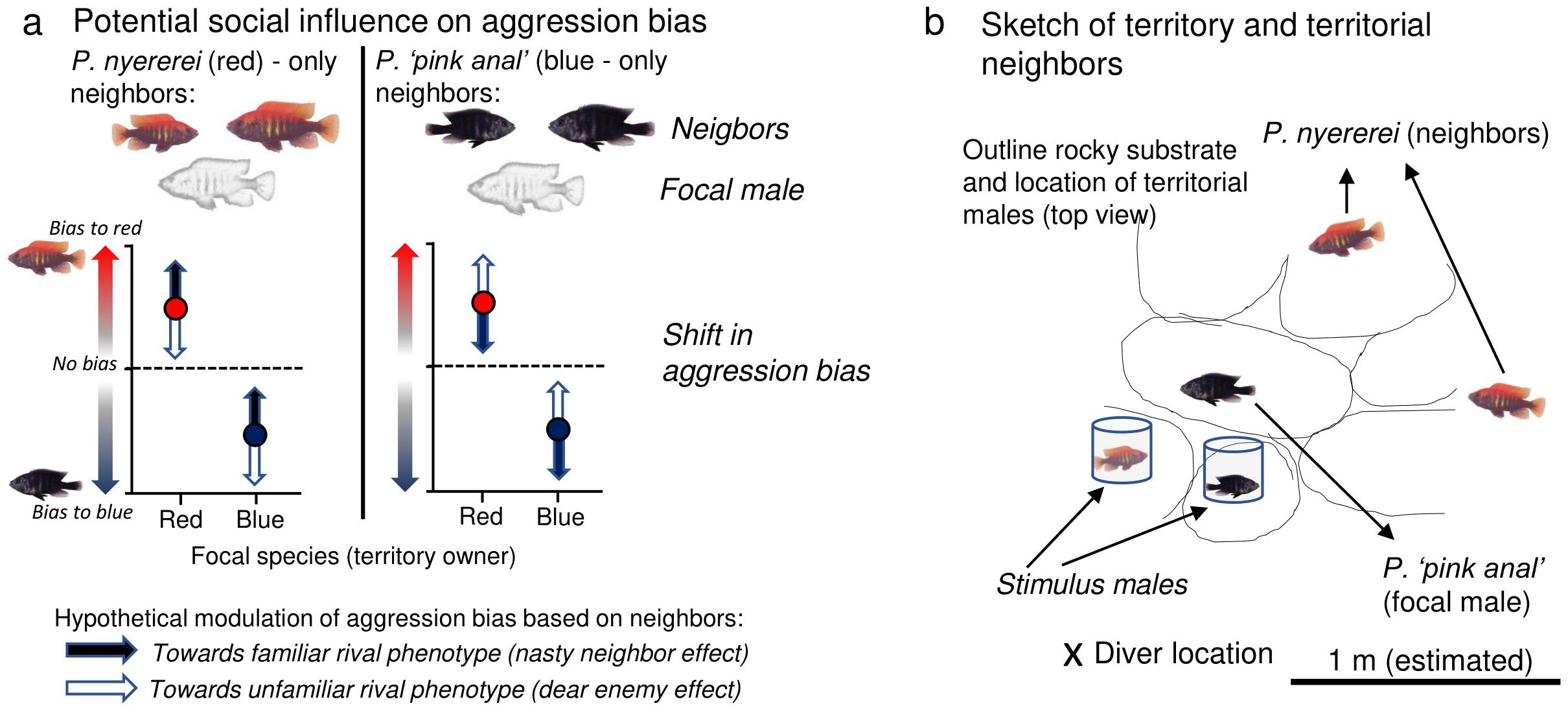
Figure 2. (a) Aggression biases were measured in red (Pundamilia nyererei) and blue males (P. ‘pink anal’) using 10-min. simulated intruder choice tests in the field. Stimulus males were enclosed in clear glass jars. Shown is the predicted direction in which the neighbor’s phenotype can modulate aggression biases in red (red circles) and blue focal males (blue circles). The baselines response (circles) assumes that males exhibit own-type aggression biases if there is not effect of experience. (b) Sketches were made of territory characteristics of territory owners. Shown is a representative territory of a blue males (P. ‘pink anal’) surrounded by two red territorial neighbors. Picture credit (P. nyererei): Martine Maan.
Fieldwork
Details about the experimental procedures are provided elsewhere (Maan et al., 2004; Dijkstra et al., 2006). In brief, fieldwork was conducted by scuba diving (PDD was the diver). Territory holders of both species were located along a transect line at a depth of six to nine meters. Males occupy mutually exclusive territories. Red males defend a rocky crevice in a rocky area while blue males usually defend a rocky crevice surrounded by a sandy bottom (Seehausen, 1996). Each male territory was marked with a coded tile placed between the rocks. Although this was not measured for the focal males in this study, territory size likely varied between 2 and 4 m2 as was reportedly previously for P. nyererei territory holders in the same study site (Maan et al., 2004). For each focal male (except for three P. nyererei males and one P. ‘pink anal’ male), a diver recorded the species of each neighboring territorial male that occupied a territory immediately adjacent to that of the focal male. Neighboring males shared territorial boundaries with the focal male and consequently the focal male was able to interact at close range with the neighboring male without either male leaving their own territory. Neighboring males consisted of red (P. nyererei) and/or blue (P. ‘pink anal’) males; one red male and one blue male had a Lithochromis sp. “yellow chin” territorial neighbor. The average number of immediate territorial neighbors was 1.7 ± 0.3 (standard error) for blue focal males and 1.5 ± 0.3 for red focal males (range both species 0 – 4 neighbors). A representative drawing of a focal male’s territory is shown in Figure 2 and an overview of all the males with information on immediate territorial neighbors is shown in Supplementary Table 1. After this, a diver positioned a red and a blue stimulus male (size-matched, standard length asymmetry < 6%), each individually confined in a watertight transparent glass jar, into the center of the focal male’s territory, approximately 15 cm from the opening of a crevice in which the territorial male was hiding. Stimulus males were obtained by gillnetting or angling at nearby locations around Makobe Island. We successfully measured aggression biases in 14 blue and 13 red males. We had neighborhood data for 13 blue and 10 red males (Supplementary Table 1). Within each species, we used unique red-blue stimulus pairs (i.e. each stimulus pair was presented once to one blue territory holder and one red territory holder), except for four stimulus pairs which were each presented to two different blue territory holders due to a lack of stimulus fish. Each trial lasted 10 minutes, and halfway through the trial we switched the left right positions of the stimulus males. For each trial, we recorded the frequencies of lateral display, frontal display, and attack behavior (Dijkstra et al., 2006). Lateral and frontal display are covert aggressive acts whereby the male positions itself laterally or in front of the stimulus male, ending with a change in posture. An attack is defined as biting and butting at the walls of the tubes containing the stimulus males. An attack bout was defined as a series of rapid bite attempts with <1 second time intervals between the bites. The observer (PDD) was not blind with respect to focal species or the species identity of neighboring males. However, when the data was collected in the field, the observer had not yet developed hypotheses relative to the potential effect of neighboring territorial males on aggression biases.
Statistical analysis
The statistical software R v3.6.1 (2019) (R Core Team 2012) was used for all statistical analysis of the data. We calculated two measures of own-species aggression biases. The first is a previously used response ratio based on the sum of aggressive behaviors (lateral display, frontal display, and attack bouts). It was calculated as sum of behavior towards one stimulus species divided by the sum of behavior to both species. Response ratios are commonly used in studies on aggression biases (Dijkstra et al., 2006; Verzijden et al., 2009; Yang et al., 2019) and we have reported the response ratio data of the current study in a previous report showing that males bias aggression towards their own species (Dijkstra et al., 2006). The second measure of aggression bias is the bite intensity when interacting with a particular stimulus male. The bite intensity was calculated as the total number of bites divided by the number of attack bouts directed at a specific stimulus male. Higher values correspond to more bites delivered at each attack bout, reflecting more intense aggression to a specific stimulus male. One red male was excluded from this latter analysis because he only showed display behavior.
To test if the species composition of neighboring males influenced aggression biases (response ratio and bite intensity), we fitted models with ‘neighborhood type’ and species identity of the territory holder (focal species) as independent variables. Focal males that were classified as ‘red-only neighborhood’ were males that had one or multiple red territorial neighbors and no blue territorial neighbors. If a male was alone or had at least one blue neighbor, it was categorized as ‘other neighborhood’ (Table 1). We also ran models to test if the proportion of red neighbors relative to blue neighbors (number of red neighbors/total number of neighbors) was correlated with aggression biases (in these models we did not include neighborhood type). One red and one blue male were excluded from this analysis because they did not have immediate territorial neighbors. We also examined the effect of ‘blue-only neighborhood’ (defined as shown in Table 1) but we do not present the statistical findings due to limited samples sizes (two males in some categories) (Supplementary Figure S1). Since the response ratio is proportional data, we fitted beta regression models using the betareg package (Cribari-Neto and Zeileis, 2010). For the bite intensity analysis, we used linear mixed models (LMMs), using fish identity as random effect since each fish had data for bite intensity towards red and blue stimulus males. In the initial model, we included as independent variables the species identity of the territory holder (focal species) as well as species identity of the stimulus males. Since some stimulus pairs were used more than once, we also fitted models using stimulus pair ID as a random effect. However, the random effect for stimulus pair could only be estimated for very few cases leading to poorly fitted or overfitted models. We therefore report the findings of models without stimulus pair as a random effect. For all models we retained (and examined) interaction effects if p < 0.1. Model assumptions were verified by plotting residuals against fitted values. For all effects we reported estimates and standard error.

Table 1. Shown are the neighborhood type categories used in our models to assess the effect of having only red territorial neighbors versus other neighborhood types as well as having only blue territorial neighbors versus other neighborhood types on aggression biases.
Results
Own-type aggression bias in bite intensity
As shown in a previous report (Dijkstra et al., 2006), males bias aggression towards their own species and this effect was stronger in red males. Here we show the same data and include some additional data points that were not reported in the previous publication (Figure 3). We tested if an own-type aggression bias is also found in bite intensity. In the overall model, we found that bite intensity towards conspecific versus heterospecific stimulus males was not influenced by focal species (LMM, stimulus species* focal species: -0.138 ± 1.335, t24 = -0.104, p = 0.9). Males exhibited a higher bite intensity to conspecific stimuli than heterospecific stimuli (Figure 3, LMM, stimulus species: 1.947 ± 0.652, t23 = 2.986, p = 0.007). The model also retained a significant focal species effect (LMM, focal species: -3.526 ± 1.265, t27 = -2.788, p = 0.01) as blue males exhibited a bite intensity that was almost double of that observed in red males (bite intensity, blue male: 6.46 ± 0.87, red males: 3.31 ± 0.48 bites/attack bout). Below we describe how experience with certain neighbor phenotypes could modulate these own-species aggression biases.
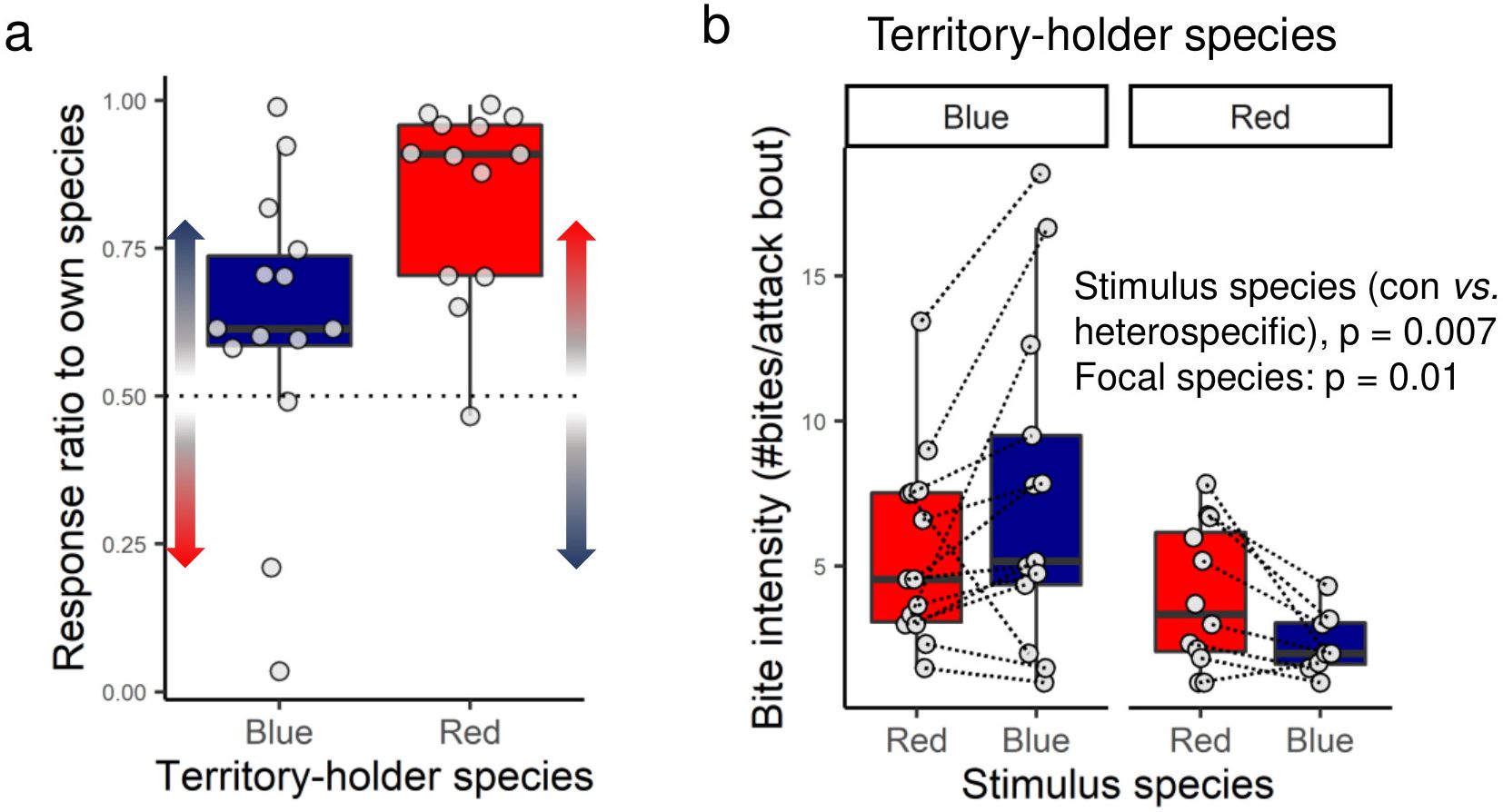
Figure 3. Own-type aggression bias in blue and red Pundamilia territory holders. (a) The response ratio to own species (both species bias aggression towards their own species; for statistics, see Dijkstra et al., 2006). (b) Shown is the bite intensity towards red and blue stimulus males. Indicated are p values based on linear mixed models. Boxes enclose 25th to 75th percentiles. Error bars enclose data range, excluding outliers. Dots are data points. Dashed lines connect stimulus males within pairs.
The effect of having only red neighbors on aggression biases
To test if the presence of specific territorial neighbors influences aggression biases, we coded focal males as having red territorial neighbors only (red-only neighborhood) or other types of neighbors (both red and blue neighbors, only blue neighbors, or alone). We found a significant interaction between neighborhood type and focal species on response ratio towards red males (Beta regression, neighborhood type x focal species: -2.172 ± 0.838, z = -2.593, p = 0.01). This interaction effect suggests that the effect of having only red neighbors on aggression biases differed between red and blue males (Figure 4a). Blue territory owners in red-only neighborhoods exhibited a stronger aggression bias towards red stimuli compared to blue territory owners with other types of neighbors (Beta regression, neighborhood type: 1.550 ± 0.552, z = 2.798, p = 0.005). Within red males, there was no significant effect of neighbor type on the response ratio towards red males (Beta regression, neighborhood type: -0.618 ± 0.623, z = -0.992, p = 0.32).
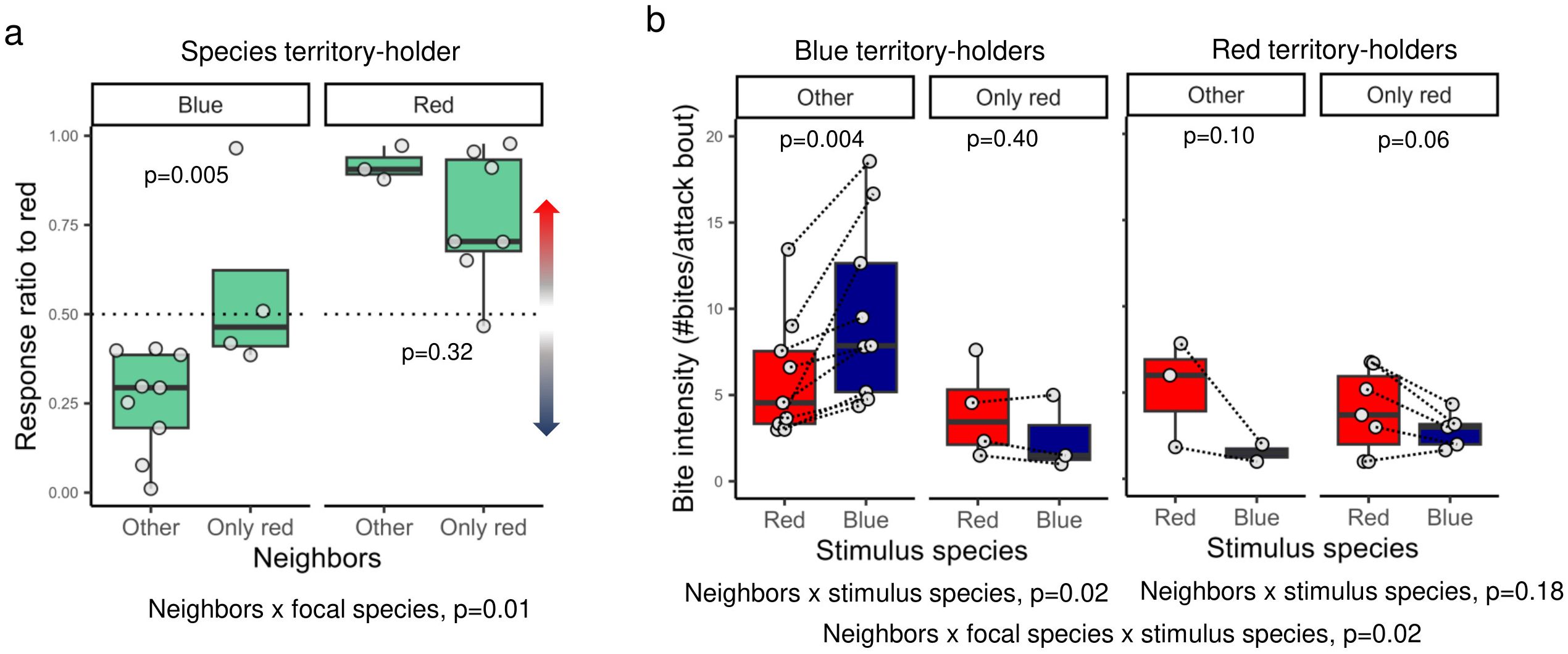
Figure 4. The effect of having only red territorial neighbors versus other types of neighborhoods on aggression biases towards red versus blue stimulus males for red and blue Pundamilia territory holders. (a) Shown is the response ratio towards red males (#aggressive acts towards red/total #aggressive acts). (b) Bite intensity towards red and blue males is the number of sequential bites within a given attack bout. Territory holders that had only red neighbor(s) were labeled as ‘only red’. ‘Other’ refers to males that had at least one blue neighbor, both blue and red neighbors or who were alone (Table 1). Boxes enclose 25th to 75th percentiles. Error bars enclose data range, excluding outliers. Dots are data points. Dashed lines connect stimulus males within pairs.
There was also a species-specific pattern in the effect of neighbors on bite intensity to red versus blue stimulus males (Figure 4b), as indicated by a significant three-way interaction effect between, neighborhood type (red-only or other neighborhoods), territory-holder (focal) species, and stimulus species (LMM: 6.209 ± 2.360, t21 = 2.631, p = 0.02). Within blue males, there was a significant interaction between neighborhood type and stimulus species (LMM, neighborhood type x stimulus species: -4.248 ± 1.638, t12 = -2.594, p = 0.02), suggesting that there was an effect of neighborhood type on aggression biases in terms of bite intensity. More specifically, in blue males who were not surrounded by only red males (i.e., territory owners coded as ‘other’, Table 1), there was a significantly higher bite intensity towards blue stimulus males than towards red stimulus males (LMM, stimulus species: 3.688 ± 0.949, t9 = 3.888, p = 0.004). By contrast, blue males with only red neighbors showed no significant aggression bias in terms of bite intensity (LMM, stimulus species: -0.324 ± 0.315, t3 = -1.029, p = 0.4). In red males, neighborhood type (red only versus other) was not linked to differences in bite intensity towards red or blue stimulus males (LMM, neighborhood type x stimulus species: 2.115 ± 1.457, t8 = 1.452, p = 0.18).
The effect of proportion of red neighbors on aggression biases
To test whether the relative abundance of red relative to blue territorial neighbors influenced aggression biases, we examined the effect of proportion of red neighbors on response ratios and bite intensity (Figure 5). For response ratios towards red, there was no significant effect of proportion of red neighbors (with or without focal species, p values > 0.26, Figure 5a). For bite intensity, the model retained a significant three-way interaction effect between stimulus species (blue or red), proportion of red neighbors, and focal species (LMM, 7.439 ± 3.142, t18 = 2.368, p = 0.03, Figure 5b). This finding is consistent with the ‘red-only neighborhood’ analysis, in that the neighborhood effect on aggression biases varies by species. In blue males, bite intensity towards red or blue stimulus males did not significantly depend on the proportion of red males (LMM, proportion of red neighbors x stimulus species: -3.443 ± 2.190, t10 = -1.573, p = 0.15). However, in red males the aggression bias in terms of bite intensity depended significantly on the proportion of red neighbors, with red males exhibiting lower bite intensity towards red stimulus males relative to blue stimulus males with increasing proportion of red males (LMM, proportion of red neighbors x stimulus species: 4.051 ± 1.402, t7 = 2.889, p = 0.025). Within red males, bite intensity towards red stimuli tended to decline somewhat with a higher proportion of red neighbors (LM, proportion of red neighbors: -3.694 ± 1.660, t = -2.225, p = 0.06) but there was no such effect in bite intensity towards blue stimuli (0.519 ± 1.163, t = 0.446, p = 0.68).
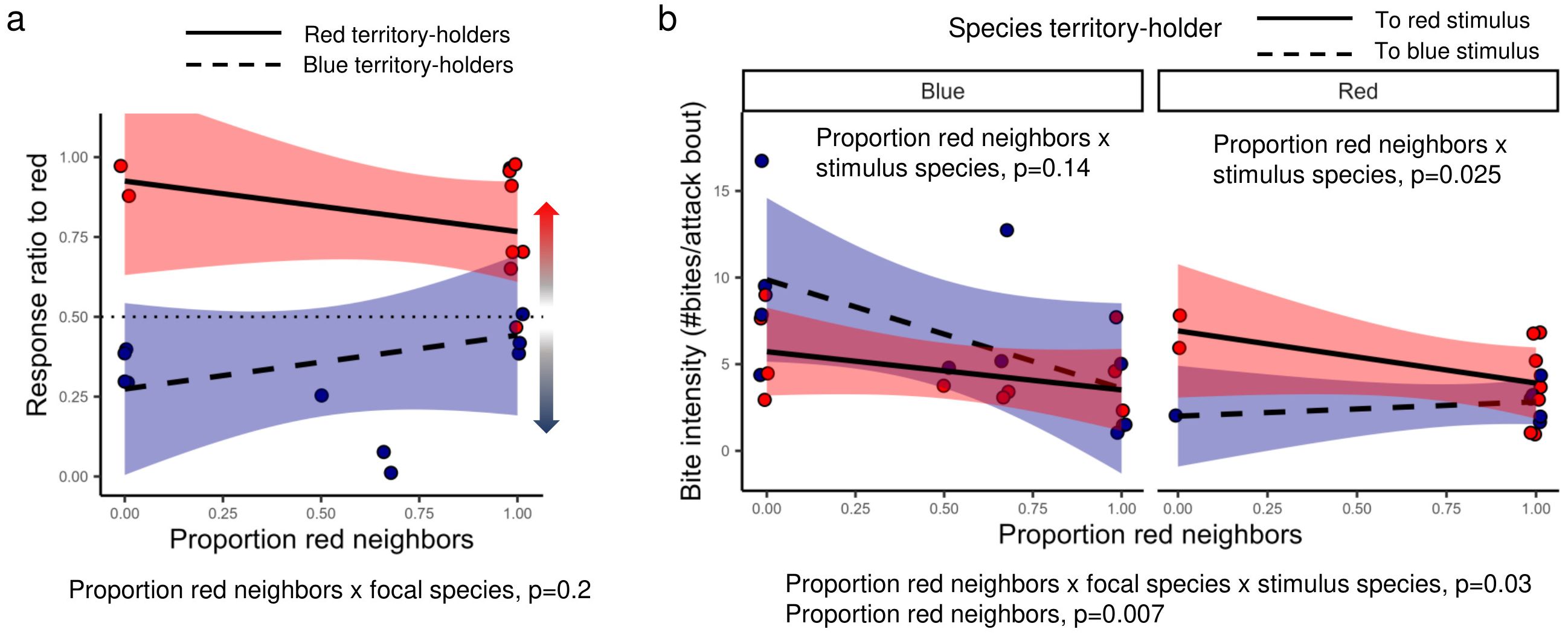
Figure 5. The effect of proportion of red territorial neighbors on aggression biases for red and blue Pundamilia territory holders. (a) Shown is the response ratio towards red males (#aggressive acts towards red/total #aggressive acts). (b) Shown is the bite intensity towards red and blue stimulus males. Bite intensity is the number of sequential bites within a given attack bout. Lines are model-specific trend lines, and shaded area are 95% confidence intervals.
Overall, there was a significant decline in overall bite intensity (i.e. to either stimulus species) with proportion of red neighbors (LMM, proportion of red neighbors: -3.588 ± 1.199, t20 = -2.993, p = 0.007, Figure 5b) and this model was not dependent on focal species (p values > 0.37). This suggests that territory holders with a higher proportion of red neighbors showed reduced bite intensity.
Discussion
In the current study, we confirmed that territorial red (P. nyererei) and blue (P. ‘pink anal’) Pundamilia males bias aggression towards males of their own species. Such own-type aggression biases are thought to facilitate invasion of rare phenotypes and favor coexistence of divergent phenotypes both during and after speciation (Dijkstra and Border, 2018). However, our data also suggest that aggression biases are modulated by the social neighborhood. Blue males with only red neighbors directed a higher proportion of aggression (response ratio) to red stimulus males compared to blue territory owners with other neighborhoods. These findings suggest that blue territory owners shift their aggression preferences towards the red phenotype if red is more abundant and territory owners are more likely to be surrounded by only red neighbors. This effect of social context would enhance the rare male advantage to blue males, potentially promoting co-existence and preventing red males from taking over the population. By contrast, we found that red males tended to reduce their aggression bias in terms of bite intensity towards red stimulus males when they were surrounded by more red territorial neighbors. Our findings suggest that in red males, social context may diminish the rare male advantage to blue males since more red males in the local environment (and higher likelihood of red-only neighborhoods) tended to shift aggression preferences away from red rivals. Since the effect of neighborhood on aggression biases was stronger in blue males, our results suggest the social context may especially shape aggression biases in a way that disadvantages red males when they are abundant, aiding in keeping red males in check and favoring blue males when they are rare. This finding is in line with the distribution of different Pundamilia phenotypes across different localities in Lake Victoria, with red Pundamilia phenotypes always co-existing with blue Pundamilia phenotypes while entirely blue Pundamilia populations are not uncommon (Seehausen et al., 1997; Seehausen and Van Alphen, 1999; Dijkstra et al., 2007). We clarify our results in more detail and discuss alternative explanations that can explain the correlation between neighborhood type and aggression biases.
Our findings suggest that species composition of local neighborhoods can influence aggression biases in the territory holders. Here we specifically tested whether increased exposure to a certain phenotype (i.e. red territorial neighbors) increased aggressive responses towards males of that specific, more familiar phenotype. Support for this prediction was found in blue males but not in red males. We hypothesize that recent social interactions with red territorial males primed blue territory owners to increase aggressive responses to red intruders, consistent with the ‘nasty neighbor effect’ (Müller and Manser, 2007). Red males, on the other hand, may reduce responses to red intruders when surrounded by more red neighbors. This would be consistent with a ‘dear enemy effect’ in the red species, which suggests that individuals habituate to specific stimuli of neighbors leading to a generalized reduced response to red phenotypes relative to blue phenotypes. These findings in red males are consistent with previous studies in other haplochromine cichlids showing that males reduce territorial aggression towards familiar neighbors (Aires et al., 2015; Weitekamp and Hofmann, 2017). The species differences in modulation of aggressive behavior based on social neighborhood is also supported in the analysis where we split territory holders into having ‘only blue’ territorial neighbors versus other types of neighborhoods as defined in Table 1 (Supplementary Figure S1). The pattern is visually similar for the response ratio (Supplementary Figure S1a), with blue territory holders shifting bias towards blue stimuli when only surrounded by blue neighbors, while red territory holders tilting it away from blue stimuli when more familiar with blue. The observed species difference in modulation of aggressive behavior based on social neighborhood is difficult to explain, but it could be related to differences in how red and blue males perceive the relative threat level of each male phenotype based on interspecific differences in motivation to expand territories or willingness to engage in physical confrontations. For example, the blue species is less abundant at our study site than the red species, but blue males are considerably more aggressive than red males. However, how these species differences in local abundance and aggressiveness are linked to species-specific adjustment of behavior according to species composition of neighborhoods is unclear and requires further studies. We also note that ‘nasty neighbor effects’, and ‘dear enemy effects’ are not mutually exclusive mechanisms shaping aggression biases.
Given the previously reported more variable aggression biases in blue Pundamilia phenotypes and extremely robust own-type aggression biases in red Pundamilia phenotypes (Dijkstra et al., 2007; Dijkstra and Groothuis, 2011), we predicted that the effect of social context on aggression biases was stronger in blue males than in red males. As discussed above, this prediction was correct for the proportion of aggression (response ratio) to red stimulus males, with an effect detected in blue males but not in red males. However, the observation that the proportion of red males influences biases in bite intensity in red males was unexpected. Our findings suggest that social context may be more important in shaping aggression biases than previously thought.
Although behavioral adjustment based on recent social interactions among neighboring territorial males could influence aggression biases, alternative explanations are also possible. Specifically, it is possible that intrinsic behavioral differences among males of the two species explain the correlation between aggression biases and neighborhood characteristics if those characteristics influence how males sort themselves into territorial neighborhoods (Lehtonen and Helanterä, 2024). For example, red males that are inherently less aggressive to red intruders may tolerate more red and fewer blue neighbors around their territories. Past studies in cichlid fish have shown that own-type aggression biases predict the distribution and spacing of territories occupied by species of specific phenotypes, such that males are more likely to have territorial neighbors that are dissimilar to themselves (Kohda, 1998; Seehausen and Schluter, 2004; Tyers et al., 2021). Experimental tests are required to examine the causal relationship between neighborhood characteristics and aggression biases.
One limitation of the current study is the lack of statistical power to detect differences among males with varying social neighborhoods. We began examining the reported neighborhood effects after the data had been collected in the field. Findings were therefore serendipitous. Future studies should not only increase sample sizes but also use experimental manipulations of neighborhoods and additional behavioral observations on focal males and their neighbors, including the frequency and nature of interactions between territory owners and neighboring territorial males of different species or phenotypes. Territory holders may acquire threat-sensitive information linked to previous encounters with rivals of specific phenotype and adjust behavior accordingly. For example, territory owners may learn to associate rival phenotypes with personality characteristics, tendency to escalate conflicts, or intrusion rates (Olendorf et al., 2004; Hyman and Hughes, 2006; Akçay et al., 2009). This type of information gathering could also occur in ‘bystanders’ through witnessing behavioral interactions between males of specific phenotypes in local neighborhoods (Oliveira et al., 1998; Clotfelter and Paolino, 2003). Finally, we placed stimulus males close to the core of a male’s territory. One could argue that this is a somewhat artificial approach and that in more realistic scenarios, territory owners can see approaching opponents, and will likely evict (potential) intruders in the border region. Establishing how males approach such rivals under more realistic scenarios is important (Lehtonen et al., 2015), especially since experience-dependent defensive responses are modulated by encounter location (Bolyard and Rowland, 2000; Carazo et al., 2008; Sogawa and Kohda, 2018).
The current study suggests that intrinsic own-type aggression biases are modulated by learning and social context. However, it is unknown how territory owners integrate rival cues with early and recent social experiences, which ultimately determines the aggressive response to specific phenotypes. Aggression biases are important for theoretical models of evolutionary diversification (Tinghitella et al., 2018; Yang et al., 2019) and the outcome of species interactions (Drury et al., 2020; Grether et al., 2020). Although others have suggested that cognitive processes are important for understanding aggression biases (Grether, 2011) and contest behavior more generally (Hsu et al., 2006; Reichert and Quinn, 2017), more studies are needed to examine how social environment and learning shape aggressive behavior (Roleira et al., 2017; Friesen et al., 2022). This research can draw from studies on the cognitive and neural mechanisms of species discrimination and rival recognition in birds and other vertebrates (Mateo, 2004; Hoke et al., 2008; Lynch et al., 2017). Insights from these fields may influence our understanding of the role of male-male competition in speciation quite significantly as discussed in the introduction (Figure 1).
We described how the social environment could modulate aggression biases using a case study of two cichlid species. We found that aggression biases of territory holders towards red and blue intruders depended on their own phenotype but also on the presence or absence of red territorial neighbors. These neighbor effects on modulation of aggression biases were also different for blue and red males. Territory owners in a range of species are known to show large plasticity in behavioral responses based on the social context and past social encounters, but such plasticity has rarely been studied in the context of aggression biases. Our case study focused on recent social experience and the effect of social context. Future studies should study how early life and recent social experiences interact to shape aggression biases using experimental approaches in the lab and the field. Studying plasticity in aggression biases may advance our understanding of how male-male competition and competition more generally shape evolutionary patterns of phenotypic diversification (Winkelmann et al., 2014; Drury et al., 2020; Grether et al., 2020).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Animal experiment license (DEC 2812) from Groningen University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. OS: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was financed by an NWO (SLW) grant 810.64.013 and publication was made possible by a grant from the National Institute of General Medical Sciences (NIGMS, R15GM150286).
Acknowledgments
We thank the Tanzanian Commission for Science and Technology for research permission and the Tanzanian Fisheries Research Institute (Philip Bwathondi, Egid Katunzi, S.B. Mahongo) for hospitality and assistance. We thank John Mrosso, Martine Maan, Kees Hofker, Machteld Verzijden, Inke van der Sluijs and Marcel Haesler for their help in the field. Mhoja Kayeba and Mohamed Haluna are greatly acknowledged for their expertise in the field. Ton Groothuis, Serge Daan, Bernd Riedstra, Machteld Verzijden, Hans Hofmann, Ryan Wong, and Topi Lehtonen are acknowledged for stimulating discussions. The research was carried out with an animal experiment license (DEC 2812) from Groningen University and complied with current laws in The Netherlands.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1568964/full#supplementary-material
References
Aires R. F., Oliveira G. A., Oliveira T. F., Ros A. F. H., Oliveira R. F. (2015). Dear enemies elicit lower androgen responses to territorial challenges than unfamiliar intruders in a cichlid fish. PloS One 10, 1–11. doi: 10.1371/journal.pone.0137705
Akçay Ç., Wood W. E., Searcy W. A., Templeton C. N., Campbell S. E., Beecher M. D. (2009). Good neighbour, bad neighbour: song sparrows retaliate against aggressive rivals. Anim. Behav. 78, 97–102. doi: 10.1016/j.anbehav.2009.03.023
Albertson R. C., Streelman J. T., Kocher T. D. (2003). Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc. Natl. Acad. Sci. U. S. A. 100, 5252–5257. doi: 10.1073/pnas.0930235100
Anderson C. N., Grether G. F. (2010). Interspecific aggression and character displacement of competitor recognition in Hetaerina damselflies. Proc. Biol. Sci. 277, 549–555. doi: 10.1098/rspb.2009.1371
Baran N. M., Streelman J. T. (2020). Ecotype differences in aggression, neural activity and behaviorally relevant gene expression in cichlid fish. Genes. Brain Behav. 19, 1–15. doi: 10.1111/gbb.12657
Bee M. A. (2003). Experience-based plasticity of acoustically evoked aggression in a territorial frog. J. Comp. Physiol. A. Neuroethol. Sensory. Neural. Behav. Physiol. 189, 485–496. doi: 10.1007/s00359-003-0420-4
Bieri E., Rubio A. O., Summers K. (2024). Beyond color and pattern: elucidating the factors associated with intraspecific aggression in the mimic poison frog (Ranitomeya imitator). Evol. Ecol. 38, 621–638. doi: 10.1007/s10682-023-10285-x
Bolnick D. I., Hendrix K., Jordan L. A., Veen T., Brock C. D. (2016). Intruder colour and light environment jointly determine how nesting male stickleback respond to simulated territorial intrusions. Biol. Lett. 12, 10–13. doi: 10.1098/rsbl.2016.0467
Bolyard K. J., Rowland W. J. (2000). The effects of spatial context and social experience on the territorial aggression of male threespine stickleback. Behaviour 137, 845–864. doi: 10.1163/156853900502493
Brawand D., Wagner C. E., Li Y. I., Malinsky M., Keller I., Fan S., et al. (2014). The genomic substrate for adaptive radiation in African cichlid fish. Nature 513, 375–381. doi: 10.1038/nature13726
Carazo P., Font E., Desfilis E. (2008). Beyond “nasty neighbours” and “dear enemies”? Individual recognition by scent marks in a lizard (Podarcis hispanica). Anim. Behav. 76, 1953–1963. doi: 10.1016/j.anbehav.2008.08.018
Christensen C., Radford A. N. (2018). Dear enemies or nasty neighbors? Causes and consequences of variation in the responses of group-living species to territorial intrusions. Behav. Ecol. 29, 1004–1013. doi: 10.1093/beheco/ary010
Clotfelter E. D., Paolino A. D. (2003). Bystanders to contests between conspecifics are primed for increased aggression in male fighting fish. Anim. Behav. 66, 343–347. doi: 10.1006/anbe.2003.2227
Cribari-Neto F., Zeileis A. (2010). Beta regression in R. J. Stat. Software 34, 1–24. doi: 10.18637/jss.v034.i02
delBarco-Trillo J., McPhee M. E., Johnston R. E. (2009). Nonagonistic familiarity decreases aggression in male Turkish hamsters, Mesocricetus brandti. Anim. Behav. 77, 389–393. doi: 10.1016/j.anbehav.2008.10.012
Dijkstra P. D., Border S. E. (2018). How does male–male competition generate negative frequency-dependent selection and disruptive selection during speciation? Curr. Zool. 64, 89–99. doi: 10.1093/cz/zox079
Dijkstra P. D., Groothuis T. G. G. (2011). Male-male competition as a force in evolutionary diversification: evidence in haplochromine cichlid fish. Int. J. Evol. Biol. 2011, 689254. doi: 10.4061/2011/689254
Dijkstra P. D., Piefke T. J., Bonnell T. R. (2022). Behavioral changes during social ascent and descent in replicate social networks of an African cichlid fish. Hydrobiologia 850, 2405–2423. doi: 10.1007/s10750-022-04980-z
Dijkstra P. D., Seehausen O., Fraterman R. E., Groothuis T. G. G. (2008). Learned aggression biases in males of Lake Victoria cichlid fish. Anim. Behav. 76, 649–655. doi: 10.1016/j.anbehav.2008.03.013
Dijkstra P. D., Seehausen O., Gricar B., Maan M. E., Groothuis T. G. G. (2006). Can male-male competition stabilize speciation? A test in Lake Victoria haplochromine cichlid fish. Behav. Ecol. Sociobiol. 59, 704–713. doi: 10.1007/s00265-005-0100-1
Dijkstra P. D., Seehausen O., Groothuis T. G. G. (2005). Direct male-male competition can facilitate invasion of new colour types in Lake Victoria cichlids. Behav. Ecol. Sociobiol. 58, 136–143. doi: 10.1007/s00265-005-0919-5
Dijkstra P. D., Seehausen O., Pierotti M. E. R., Groothuis T. G. G. (2007). Male-male competition and speciation: Aggression bias towards differently coloured rivals varies between stages of speciation in a Lake Victoria cichlid species complex. J. Evol. Biol. 20, 496–502. doi: 10.1111/j.1420-9101.2006.01266.x
Drury J. P., Cowen M. C., Grether G. F. (2020). Competition and hybridization drive interspecific territoriality in birds. Proc. Natl. Acad. Sci. U. S. A. 117, 12923–12930. doi: 10.1073/pnas.1921380117
Fanouraki E., Laitinen J. T., Divanach P., Pavlidis M. (2007). Endocrine regulation of skin blanching in red porgy, Pagrus pagrus. Ann. Zool. Fennici. 44, 241–248.
Flaxman S. M., Wacholder A. C., Feder J. L., Nosil P. (2014). Theoretical models of the influence of genomic architecture on the dynamics of speciation. Mol. Ecol. 23, 4074–4088. doi: 10.1111/mec.12750
Friesen C. N., Maclaine K. D., Hofmann H. A. (2022). Social status mediates behavioral, endocrine, and neural responses to an intruder challenge in a social cichlid, Astatotilapia burtoni. Horm. Behav. 145, 105241. doi: 10.1016/j.yhbeh.2022.105241
Grether G. F. (2011). The neuroecology of competitor recognition. Integr. Comp. Biol. 51, 807–818. doi: 10.1093/ICB/ICR060
Grether G. F., Drury J. P., Okamoto K. W., McEachin S., Anderson C. N. (2020). Predicting evolutionary responses to interspecific interference in the wild. Ecol. Lett. 23, 221–230. doi: 10.1111/ele.13395
Grether G. F., Peiman K. S., Tobias J. A., Robinson B. W. (2017). Causes and consequences of behavioral interference between species. Trends Ecol. Evol. 32, 760–772. doi: 10.1016/j.tree.2017.07.004
Hoke K. L., Ryan M. J., Wilczynski W. (2008). Candidate neural locus for sex differences in reproductive decisions. Biol. Lett. 4, 518–521. doi: 10.1098/RSBL.2008.0192
Hsu Y., Earley R. L., Wolf L. L. (2006). Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. Camb. Philos. Soc 81, 33–74. doi: 10.1017/S146479310500686X
Humfeld S. C., Marshall V. T., Bee M. A. (2009). Context-dependent plasticity of aggressive signalling in a dynamic social environment. Anim. Behav. 78, 915–924. doi: 10.1016/j.anbehav.2009.06.028
Hyman J., Hughes M. (2006). Territory owners discriminate between aggressive and nonaggressive neighbours. Anim. Behav. 72, 209–215. doi: 10.1016/j.anbehav.2006.01.007
Kohda M. (1998). Coexistence of permanently territorial cichlids of the genus Petrochromis through male-mating attack. Environ. Biol. Fishes. 52, 231–242. doi: 10.1023/A:1007381217885
Lehtonen T. K. (2014). Colour biases in territorial aggression in a Neotropical cichlid fish. Oecologia 175, 85–93. doi: 10.1007/s00442-013-2879-1
Lehtonen T. K., Helanterä H. (2024). The role of neighbours in aggressive defence of territories in mixed-species breeding aggregations of cichlid fish. Hydrobiologia. doi: 10.1007/s10750-024-05749-2
Lehtonen T. K., Sowersby W., Gagnon K., Wong B. B. M. (2015). Cichlid fish use coloration as a cue to assess the threat status of heterospecific intruders. Am. Nat. 186, 547–552. doi: 10.1086/682711
Lynch K. S., Gaglio A., Tyler E., Coculo J., Louder M. I. M., Hauber M. E. (2017). A neural basis for password-based species recognition in an avian brood parasite. J. Exp. Biol. 220, 2345–2353. doi: 10.1242/jeb.158600
Maan M. E., Seehausen O., Söderberg L., Johnson L., Ripmeester E. A. P., Mrosso H. D. J., et al. (2004). Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proc. Biol. Sci. 271, 2445–2452. doi: 10.1098/rspb.2004.2911
Maruska K. P., Butler J. M., Field K. E., Forester C., Augustus A. (2020). Neural activation patterns associated with maternal mouthbrooding and energetic state in an African cichlid fish. Neuroscience 446, 199–212. doi: 10.1016/j.neuroscience.2020.07.025
Mateo J. M. (2004). Recognition systems and biological organization: The perception component of social recognition. Ann. Zool. Fennici. 41, 729–745.
McCullough E. L., Miller C. W., Emlen D. J. (2016). Why sexually selected weapons are not ornaments. Trends Ecol. Evol. 31, 742–751. doi: 10.1016/j.tree.2016.07.004
Moran R. L., Zhou M., Catchen J. M., Fuller R. C. (2017). Male and female contributions to behavioral isolation in darters as a function of genetic distance and color distance. Evol. (N. Y). 71, 2428–2444. doi: 10.1111/evo.13321
Müller C. A., Manser M. B. (2007). Nasty neighbours” rather than “dear enemies” in a social carnivore. Proc. R. Soc B. Biol. Sci. 274, 959–965. doi: 10.1098/rspb.2006.0222
Olendorf R., Getty T., Scribner K., Robinson S. K. (2004). Male red-winged blackbirds distrust unreliable and sexually attractive neighbours. Proc. R. Soc B. Biol. Sci. 271, 1033–1038. doi: 10.1098/rspb.2004.2687
Oliveira R. F., McGregor P. K., Latruffe C. (1998). Know thine enemy: Fighting fish gather information from observing conspecific interactions. Proc. R. Soc B. Biol. Sci. 265, 1045–1049. doi: 10.1098/rspb.1998.0397
R Development Core Team (2012). R: A Language and Environment for Statistical Computing. Vienna: R foundation for Statistical Computing. Available at: http://www.R-project.org/.
Reichert M. S., Quinn J. L. (2017). Cognition in contests: mechanisms, ecology, and evolution. Trends Ecol. Evol. 32, 773–785. doi: 10.1016/j.tree.2017.07.003
Renn S. C. P., Schumer M. E. (2013). Genetic accommodation and behavioural evolution: insights from genomic studies. Anim. Behav. 85, 1012–1022. doi: 10.1016/j.anbehav.2013.02.012
Roleira A., Oliveira G. A., Lopes J. S., Oliveira R. F. (2017). Audience effects in territorial defense of male cichlid fish are associated with differential patterns of activation of the brain social decision-making network. Front. Behav. Neurosci. 11. doi: 10.3389/fnbeh.2017.00105
Safran R. J., Scordato E. S. C., Symes L. B., Rodríguez R. L., Mendelson T. C. (2013). Contributions of natural and sexual selection to the evolution of premating reproductive isolation: a research agenda. Trends Ecol. Evol. 28, 643–650. doi: 10.1016/j.tree.2013.08.004
Scali S., Mangiacotti M., Sacchi R., Coladonato A. J., Falaschi M., Saviano L., et al. (2021). Close encounters of the three morphs: Does color affect aggression in a polymorphic lizard? Aggress. Behav. 47, 430–438. doi: 10.1002/ab.21961
Seehausen O. (1996). Lake Victoria Rock Cichlids. Taxonomy, Ecology and Distribution (Zevenhuizen, NL: Cichlid Press).
Seehausen O., Bouton N. (1997). Microdistribution and fluctuations in niche overlap in a rocky shore cichlid community in Lake Victoria. Ecol. Freshw. Fish. 6, 161–173. doi: 10.1111/J.1600-0633.1997.TB00159.X
Seehausen O., Schluter D. (2004). Male-male competition and nuptial-colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proc. R. Soc B. Biol. Sci. 271, 1345–1353. doi: 10.1098/rspb.2004.2737
Seehausen O., Terai Y., Magalhaes I. S., Carleton K. L., Mrosso H. D. J., Miyagi R., et al. (2008). Speciation through sensory drive in cichlid fish. Nature 455, 620–626. doi: 10.1038/nature07285
Seehausen O., Van Alphen J. J. M. (1998). The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav. Ecol. Sociobiol. 42, 1–8. doi: 10.1007/s002650050405
Seehausen O., Van Alphen J. J. M. (1999). Can sympatric speciation by disruptive sexual selection explain rapid evolution of cichlid diversity in Lake Victoria? Ecol. Lett. 2, 262–271. doi: 10.1046/j.1461-0248.1999.00082.x
Seehausen O., Van Alphen J. J. M., Witte F. (1997). Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277, 1808–1811. doi: 10.1126/science.277.5333.1808
Servedio M. R., Boughman J. W. (2017). The role of sexual selection in local adaptation and speciation. Annu. Rev. Ecol. Evol. Syst. 48, 85–109. doi: 10.1146/annurev-ecolsys-110316-022905
Sogawa S., Kohda M. (2018). Tit for tat in the dear enemy relationship between territorial females of a cichlid fish. Front. Ecol. Evol. 6. doi: 10.3389/fevo.2018.00044
Svensson O., Woodhouse K., Van Oosterhout C., Smith A., Turner G. F., Seehausen O. (2017). The genetics of mate preferences in hybrids between two young and sympatric Lake Victoria cichlid species. Proc. R. Soc B. Biol. Sci. 284, 20162332. doi: 10.1098/rspb.2016.2332
Temeles E. J. (1994). The role of neighbours in territorial systems: when are they “dear enemies”? Anim. Behav. 47, 339–350. doi: 10.1006/ANBE.1994.1047
Tinghitella R. M., Lackey A. C. R., Martin M., Dijkstra P. D., Drury J. P., Heathcote R., et al. (2018). On the role of male competition in speciation: A review and research agenda. Behav. Ecol. 29, 783–797. doi: 10.1093/beheco/arx151
Tinghitella R. M., Lehto W. R., Minter R. (2015). The evolutionary loss of a badge of status alters male competition in three-spine stickleback. Behav. Ecol. 26, 609–616. doi: 10.1093/beheco/aru242
Tyers A. M., Cooke G. M., Turner G. F. (2021). Rare morph Lake Malawi mbuna cichlids benefit from reduced aggression from con- and hetero-specifics. J. Evol. Biol. 34, 1678–1690. doi: 10.1111/jeb.13929
van Doorn G. S., Dieckmann U., Weissing F. J. (2004). Sympatric speciation by sexual selection: a critical reevaluation. Am. Nat. 163, 709–725. doi: 10.1086/383619
van Doorn G. S., Edelaar P., Weissing F. J. (2009). On the origin of species by natural and sexual selection. Science 326, 1704–1707. doi: 10.1126/science.1181661
Van Rijssel J. C., Moser F. N., Frei D., Seehausen O. (2018). Prevalence of disruptive selection predicts extent of species differentiation in lake victoria cichlids. Proc. R. Soc B. Biol. Sci. 285, 20172630. doi: 10.1098/rspb.2017.2630
Verzijden M. N., Korthof R. E. M., ten Cate C. (2008). Females learn from mothers and males learn from others. The effect of mother and siblings on the development of female mate preferences and male aggression biases in Lake Victoria cichlids, genus Mbipia. Behav. Ecol. Sociobiol. 62, 1359–1368. doi: 10.1007/s00265-008-0564-x
Verzijden M. N., Zwinkels J., ten Cate C. (2009). Cross-fostering does not influence the mate preferences and territorial behaviour of males in lake victoria cichlids. Ethology 115, 39–48. doi: 10.1111/j.1439-0310.2008.01582.x
Weissing F. J., Edelaar P., van Doorn G. S. (2011). Adaptive speciation theory: a conceptual review. Behav. Ecol. Sociobiol. 65, 461–480. doi: 10.1007/s00265-010-1125-7
Weitekamp C. A., Hofmann H. A. (2017). Neuromolecular correlates of cooperation and conflict during territory defense in a cichlid fish. Horm. Behav. 89, 145–156. doi: 10.1016/j.yhbeh.2017.01.001
Winkelmann K., Genner M. J., Takahashi T., Ru L. (2014). Competition-driven speciation in cichlid fish. Nat. Commun. 5, 3412. doi: 10.1038/ncomms4412
Yang Y., Dugas M. B., Sudekum H. J., Murphy S. N., Richards-Zawacki (2018). Male – male aggression is unlikely to stabilize a poison frog polymorphism. J Evol Biol. 31, 457–468. doi: 10.1111/jeb.13243
Yang Y., Servedio M. R., Richards-Zawacki C. L. (2019). Imprinting sets the stage for speciation. Nature 574, 99–102. doi: 10.1038/s41586-019-1599-z
Yeaman S., Whitlock M. C. (2011). The genetic architecture of adaptation under migration-selection balance. Evol. (N. Y). 65, 1897–1911. doi: 10.1111/j.1558-5646.2011.01269.x
Keywords: sexual selection & conflicts, speciation, fish, cichlid, territoriality, learning, aggression
Citation: Dijkstra PD and Seehausen O (2025) The effect of social context and learning on aggression biases: consequences for the role of male-male competition in speciation and a field study in cichlid fish. Front. Ecol. Evol. 13:1568964. doi: 10.3389/fevo.2025.1568964
Received: 31 January 2025; Accepted: 05 March 2025;
Published: 08 April 2025.
Edited by:
Alejandro Cantarero, Complutense University of Madrid, SpainReviewed by:
Ingo Schlupp, University of Oklahoma, United StatesAlexa Guerrera, Michigan State University, United States
Copyright © 2025 Dijkstra and Seehausen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter D. Dijkstra, ZGlqa3MxcEBjbWljaC5lZHU=
 Peter D. Dijkstra
Peter D. Dijkstra Ole Seehausen
Ole Seehausen