- 1Behaviour and Biomechanics, Department of Biology, University of Oxford, Oxford, United Kingdom
- 2Primate Models for Behavioural Evolution Lab, School of Anthropology and Museum Ethnography, University of Oxford, Oxford, United Kingdom
- 3Interdisciplinary Centre for Archaeology and the Evolution of Human Behaviour (ICArEHB), Universidade do Algarve Pav, Faro, Portugal
- 4Department of Science, Gorongosa National Park, Sofala, Mozambique
- 5Centro de Investigação em Biodiversidade e Recursos Genéticos (CIBIO/InBIO), Universidade do Porto, Porto, Portugal
- 6Department of Brain and Cognitive Sciences, University of Rochester, Rochester, NY, United States
Introduction: Route-based navigation is a common movement strategy for a variety of taxa, wherein animals repeatedly re-use familiar paths during travel. However, this type of navigation is understudied in wild animals that experience regular displacement, raising questions about the robustness and longevity of such routes and route memories. The seasonal flooding of Gorongosa National Park, Mozambique, provides an opportunity to test multiple facets of route-based navigation in wild primates, due to its high seasonality and annual flooding.
Methods: Data was collected from GPS collars placed on four chacma baboons in two troops in Gorongosa National Park. Using GPS points taken every 15 minutes, we use nearest-neighbour analysis to compare daily paths across the year, to identify high-use paths. We then look at the identified high-use paths to see if they are used across the entire study period, with a focus on areas that were vacated for more than two months of the study period.
Results: We find that the baboons do have vacated areas, but return to the same areas after displacement. We did not find high-use routes in these areas used both before and after displacement, although high-use routes did exist that were used across the study period in different areas.
Discussion: Our results indicate that routes may not be maintained in long-term memory spanning several months, or that route reuse is in part dependant on seasonal resources or navigational aids. Although the study period did not span a full year, this study presents a replicable method of analysing route reuse and identifying high-use routes without traditional methods of manually overlaying and analysing daily paths.
Introduction
Animals moving through their environments are limited in where they can travel by a number of factors, including predation risk (Laundré et al., 2010), and access to food (Altmann, 1974; Amato and Garber, 2014; Caillaud et al., 2010; Di Fiore, 2003), shelter (Markham et al., 2016; Samson et al., 2018), and areas to rest and regulate temperature (Cain et al., 2008; Hill, 2006; Pochron, 2000). When these variables change, through seasonality, migrations, or other major natural or anthropogenic events, animals may rely on information acquired during previous analogous situations to navigate and make decisions (Crystal and Wilson, 2015; Griffiths et al., 1999; Janson, 2016). Beyond environmental variables, the animal’s mode of locomotion, navigational strategies, and knowledge of its environment also impact the animal’s daily travel (Nathan et al., 2008). Animals may make movement decisions based on previously acquired knowledge about the environment, move randomly through their environment, or combine both strategies in order to meet their basic daily needs (Boyer and Walsh, 2010). Although it is generally accepted that many animals move non-randomly (Alavi et al., 2022; Janson and Byrne, 2007), understanding how they make movement decisions, and the limits of their spatial memory, remains key questions.
One oft-studied navigational strategy in animals involves the use of routes, or repeatedly used pathways (Di Fiore and Suarez, 2007). These routes often emerge because they are more efficient than most alternatives; that is, they minimize travel distance or travel time, require less energy both to physically traverse and to navigate, maximize the gain-to-cost or resource-to-travel ratio, or some combination of these metrics (Janson, 2000). For instance, chimpanzees use landscape features to mitigate movement costs, preferring to use human-made trails to travel, as well as ridge tops, both of which may be energetically more effective to travel on than through dense forest (Green et al., 2020). Route-based navigation is generally associated with a topographic mental map, or a mental representation of space based on how specific landmarks are connected. Other types of mental maps, such as Euclidean maps, may contain, but don’t rely on, route networks, given that the individual can mentally represent their entire space and should be able to navigate between two points, using prior knowledge of an object’s location in space, whether or not they have done so before (Mueller and Fagan, 2008; Poucet, 1993).
In understanding how and why animals use habitual routes, various experimental and observational approaches have been used. Some species lend themselves well to the study of route-based navigation. Over smaller spatial scales, navigating individuals, such as insects, can be directly observed traversing over a fine-scale route repeatedly in experimental or natural conditions, either through following pheromones or using memorized landmarks (M. Collett, 2010; Collett et al., 2006; Sasaki et al., 2020). Larger animals that make annual migrations have also been shown to reuse habitual routes, with some ungulates (mule deer, Odocoileus hemionus) following the same route annually; within individuals, the routes they use strongly predict their survival rate, although the factors responsible for this trend are unknown (Sawyer et al., 2019a, b). Until relatively recently, fine-scale data collection of moderate-scale movements, e.g., an animal moving daily through its home range over an extended period, was difficult to collect, particularly in wild animals, due to constraints on biologger size, battery life, and memory capacity (Joo et al., 2022). However, as biologgers become a more accessible research tool for studies of wild animals, fine-scale longitudinal data sets have become increasingly common, allowing for more in-depth analysis of movement than through observation alone (Nathan et al., 2022).
In wild, group living animals, route-based navigation has been studied with a combination of observation and GPS devices. Both group-living animals such as elephants (Loxodonta africana) and solitary animals such as the lynx (Lynx canadensis) have been found to use routes to navigate within their territories (Presotto et al., 2019; Squires et al., 2013). However, few studies have looked at route fidelity, or the habitual reuse of routes over long periods, in populations where routes are not used continuously over time. In experimental studies in a natural environment, homing pigeons (Columba livia) were found to remain loyal to their own navigation routes when released from the same site to navigate home multiple times, to return to these routes even when released off-route, and to partially remember their previous routes after a hiatus of 3–4 years (Biro et al., 2004; Collet et al., 2021). However, different pigeons released from the same site do not follow the same routes, suggesting that multiple alternative routes exist rather than there being one globally optimal one (Biro et al., 2004). These findings indicate that even animals with limited terrain constraints reuse routes, in these cases based on visual landmarks. Often in terrestrial or arboreal animals, routes are associated with stopping points in the form of a feeding patch, rest area or sleep site (Di Fiore, 2003; Hopkins, 2011; Porter and Garber, 2013). However, even without stopping to utilize a resource patch, pigeons may follow previously used paths because they are “tried and tested”, i.e. known to lead safely to the correct end point, because of the high cognitive cost of computing a new path, or a combination of both, and this reasoning may be transferred to other animals that are more topographically constrained.
How routes are identified, to what extent a route network is mapped, and how reuse is defined varies between studies. A recent study of four arboreal animals in Barro-Colorado Island, Panama, proposed a route detection framework using machine learning to analyze path directedness, density, and directionality to identify routes. While the researchers detected routes in all four species studied, modelling showed that this analysis might not be suitable for coarser-resolution data, i.e., GPS fixes more than every 10 minutes (Alavi et al., 2022). However, other studies have similarly found routes with lower resolution data and by visual analysis (Bebko, 2021; De Guinea et al., 2019; de Raad, 2012; Di Fiore, 2003). Many such studies used manual recording or handheld GPS devices to track their subjects, and all visually identified routes by plotting each track and categorizing routes as paths with similar directionality that fall within a certain buffer, usually 10–50 meters.
Another more recently developed method of identifying route networks is line density analysis. This method allows researchers to effectively measure grid cells superimposed on the landscape, counting how often a grid cell is passed through, generally with the aid of spatial analyst software; however, it does not account for the direction in which the paths cross the cell (Alavi et al., 2022; Green et al., 2020; Gregory et al., 2014).
Wild primates are exceptionally well suited for studies of movement, given their often-large daily ranges and ability to wear GPS collars. Among primates, multiple instances of route-based navigation have been found (Janson and Byrne, 2007). Arboreal primates in particular are frequently found to use routes, as their movement is limited by treetop “highways”. Bearded saki (Chiropotes sagulatus) used ridge tops and slopes as travel routes, either to avoid canyon-preferring predators, to monitor fruit trees, or a combination of both (Gregory et al., 2014). Orangutans (Pongo pygmaeus morio) were found to use routes, with multiple individuals using the same route corridors near resources (Bebko, 2021). Similarly, spider monkeys (Ateles belzebuth) and woolly monkeys (Lagothrix poeppigii) not only used the same routes, but entered into a cycle where the routes they used became better arboreal travel routes as they dispersed seeds along them, allowing the routes to continue to be used in subsequent years (Di Fiore and Suarez, 2007). In a comparison of terrestrial chimpanzees (Pan troglodytes verus) and arboreal saddleback tamarins (Leontocebus weddelli), two primates that rely on fruit for a large portion of their diet, tamarins were found to use routes, but reuse only 14% of their total identified routes between study years, while chimpanzees’ reuse of routes varied from 0-59% (Porter, 2021). Chimpanzees further used terrain features to their advantage, using higher ground to travel when approaching another group’s area but not leaving it, allowing them to have a safer approach (Lemoine et al., 2023).
Amongst primates, considerable work has been done on the movement drivers of baboons (genus Papio, Coleman and Hill, 2014; Hill et al., 2004; Marshall et al., 2013; Stelzner, 1988; Strandburg-Peshkin et al., 2015), but less on their navigation strategies. In chacma baboons, Papio ursinus, evidence for out-of-sight memory was shown, as well as evidence of route-based navigation, based on observational data and GPS analysis of movement patterns (Noser and Byrne, 2007b, a). A study of a different population also found evidence of a dense route network in chacma baboons, with enough routes to mimic Euclidean mental maps but lacking approaches to resources from all directions, implying topological mental mapping. Interestingly, this study also found similar travel patterns and equally dense route networks in both the core and periphery of the study troop’s home range (de Raad and Hill, 2019).
Widely studied across sub-Saharan Africa, baboons are group-living generalist omnivores, able to occupy a wide range of habitats and ecological conditions (Altmann, 1974; Fuchs et al., 2018; Stone et al., 2013). While their range encompasses many areas of high seasonality, marked by periods of intense rain juxtaposed with drought throughout the year, baboons have shown a remarkable ability to adapt to water shortages, floods, and wide temperature ranges (Cheney and Seyfarth, 2008; Stone et al., 2013). However, there have been no studies on navigation in areas where these seasonal events disrupt their ranging patterns in such a way that they are forced to vacate an area for an extended period of time.
Here we present the first study of route preferences and route reuse in the baboons (Papio ursinus griseipes) of Gorongosa National Park, Mozambique (GNP). This unique study site presents an opportunity to study how seasonality affects ranging patterns, and whether or not baboons are able to remember and reuse routes in areas that are inaccessible or simply not used by them for part of the year. A mosaic habitat, GNP exhibits a defined rainy season and dry season annually (Herrero et al., 2020; Stalmans and Beilfuss, 2008; Tinley, 1977). During the rainy season, GNP’s central Lake Urema floods, inundating the floodplain, including a large portion of the road network. During the dry season, the lake and its rivers recede, turning from deep rivers to oxbow lakes to pans to dry riverbeds over the course of months. This causes a major shift in wildlife as animals are forced to move to higher ground, although they return to their normal areas as flooding recedes (Beardmore-Herd et al., 2025; Walker et al., 2023). At the time of the study, GNP also had an unusually high density of baboon troops, but a low density of predators, following the effects of the Mozambique Civil War, which led to extensive poaching of all fauna in GNP, but especially carnivores (Bouley et al., 2018; Correia et al., 2017; Gaynor et al., 2020; Stalmans et al., 2018).
Two baboon troops ranging in the southern portion of GNP have been studied since 2017 and 2018, the first in this unique environment. The two troops, while having overlapping home ranges, exist in dramatically different environments. The Woodland Troop (WT) lives primarily in a woodland in the central part GNP’s road network, navigating through dense vegetation, frequently resting on termite mounds and relying primarily on transient pans for water. The Floodplain Troop (FT) occupies the southern portion of GNP’s alluvial floodplain for a portion of the year, sleeping most often in fever trees (Vachellia xanthophloea) and navigating on flat, open grassland with fewer roads but two major, seasonal rivers in their home range. For the other part of the year, seasonal flooding forces them into the southern half of their home range, where they occupy fever trees and Vachellia robusta woodland until they return annually to their floodplain range.
Using similar methods to previous studies of pigeon navigation to identify habitually used routes (e.g., Collet et al., 2021), and analyzing data to quantify the extent to which seasonal changes affect baboons ranging areas, we are able to evaluate the effects of a natural phenomenon to help understand whether baboon navigation strategies in a highly seasonal environment match those of other study sites, and whether baboons are able to retain memory of these habitual routes when they are unable to use them for more than two months at a time.
Methods
Study site and subjects
Data were collected in Gorongosa National Park, a 4,000km2 park located in central Mozambique. GNP is home to a number of vertebrate and invertebrate taxa, including an exceptional density of baboons (Stalmans et al., 2018; Lewis-Bevan et al., 2024). The two troops in this study, FT and WT, ranged in the southern portion of GNP within the main road network.
This study utilized GPS collar data from four chacma baboons (Papio ursinus griseipes) from two study troops: Eve (FT), Abacaxi (FT), Acacia (WT) and Kigalia (WT).
At the time of study, FT had approximately 30 individuals, including all age classes. Their full home range was approximately 15 km2, and their diet consisted mainly of underground corms from Cyperaceae sedges, grasses, and Vachellia xanthophloea and Chrozophora plicata seeds. They were also frequently observed hunting snails, river mussels, birds, infant warthogs and infant ungulates. The troop travelled approximately 3500m (± 1200m) daily (Lewis-Bevan et al., 2025). FT ranged directly north of WT, with the southern portion of their home range overlapping the northernmost section of the WT home range. Their home range surrounded a large loop formed by two roads across the floodplain, and was bisected by the Mussicadzi River in the west and the Tsungue River in the east. Both rivers are seasonal, overflowing in the wet season and drying to puddles or dirt in the peak of the dry season. FT’s habitats consisted of open flood plain made up of grasses and sedges, dense elephant grass patches, riparian woodland along the Mussicadzi, and low-vegetation fever tree woodland. There was sparse Vachellia woodland in the southern portion of their home range. Most of their habitat was open, with very low-density ground vegetation and visibility at baboon eye-level for several hundred meters; the exception to this was in dense riparian areas, where tall understory plants and high grass limited visibility to a few meters.
WT consisted of over 90 individuals at the time of the study, of all ages. Their full home range was approximately 7 km2, comprising moderate to dense Vachellia-Combretum savannah woodland, including areas of dense Vachellia robusta, numerous termite mounds, and two large, seasonal pans, as well as a small section of the Mussicadzi River utilized only during the dry period. They often travelled through dense understory, with roads and pans being the predominant open areas, making it difficult to see the entire troop at once. They foraged on Vachellia seed pods, and engaged in bark stripping of Vachellia robusta, as well as eating numerous plant species in the woodland and occasionally hunting small vertebrates. This troop travelled approximately 2700m (± 700m) daily (Hammond et al., 2021; Biro et al., 2025; Lewis-Bevan et al., 2025).
At the time of the study, lions (Panthera leo) and painted wolves (Lycaon pictus) ranged within both troops’ home ranges, but were not known to predate on baboons in GNP (Atkins et al., 2019; Bouley et al., 2018). One of the main predators of baboons, leopards, was not known to routinely inhabit GNP, but one was occasionally sighted by camera trap (Gaynor et al., 2020).
Collar data
Individual baboons were captured either using a baited cage with a manual door release (n = 1), or through free darting from a vehicle (n = 3). Once sedated by a licensed veterinarian, the baboons were then fitted with collars (model 1D, e-obs Digital Telemetry, Grünwald, Germany) equipped with GPS and tri-axial accelerometers, placed in a shaded recovery cage, and observed until they had recovered enough to be released.
Collaring took place between 28 July 2019 and 16 August 2019, and spatial data were collected from 30 July, 2019 to 24 June, 2020. The days of active data collection varied across monkeys due to the timing of collar placement and the end of the collar’s battery life (min = 232, max = 311, mean = 282). Collars were designed with a fabric strap connecting the ends of the collar, which would wear down over a period of at least two years and ideally after no more than four to five years, and were not intended to be retrieved.
GPS data cleaning
Data were cleaned and processed in R version 1.3.159 and QGIS 3.14.10-Pi (QGIS Development Team, 2020; R Core Team, 2020).
All four GPS units were programmed to sample baboon location once a second for five seconds every 15 minutes (i.e., a burst of up to 10 fixes at 1Hz at 15-minute intervals). GPS data were downloaded from the collars monthly from August 2019-November 2019, then again in February 2020, May 2020, and June 2020, using the e-obs BaseStation II connected to a Yagiantenna (868 MHz 10E), at a distance of between 10-50m. Downloaded data were then processed using e-obs decoder_v10s1.
During analysis to identify oft-used paths in the environment, only the final fix out of each 10-second burst was used from each collar. First, points were filtered to only days with a 95% or above fix success rate; that is, of 96 scheduled fixes in a day, at least 90 must have been successful. These points were then filtered to remove any points within 50m of the sleep site used at the start or end of the day, in order to minimize bias from being close to the site as well as the monkeys staying in the same place for extended periods.
Daily route use over extended periods
To compare route similarity on a daily level across the study period, each day-long trajectory, from departing the morning sleep site to entering the evening sleep site, was isolated. We created a “dissimilarity score” between pairs of days, which represented the average nearest neighbor distance between two daily trajectories. A higher dissimilarity score indicates the daily routes were on average further apart.
The analysis compared pairs of days, with one daily trajectory assigned as the reference day, and the other as the comparison day. The distance between each GPS point in the reference day was measured to each point in the comparison day to create a list of distances. The smallest of these distances, or the nearest neighbor distance, was taken for each point in the reference day. The average of the nearest neighbor distances was taken twice for each pair, with each daily trajectory respectively being the comparison and reference day. The average of these two averages was taken as the dissimilarity distance for that pair of days.
Identification of high-use paths
To identify routes that were reused by the baboons, points were compared using a rolling-window approach.
After data cleaning, each of the remaining points were grouped into rolling groups of four sequential points (“tracklets”), with the first point of the group (r1) being the reference point and the next three points being the rest of the reference track (r2, r3, r4). The tracklet was evaluated to ensure that (a) the fixes occurred consecutively at 15-minute intervals, i.e., no fixes were missed, and (b) each point was at least 50m distance from the previous point, to ensure the baboons were moving at the time of the fix as opposed to stationary. Although this criteria ensured the baboons were moving during the 15 minutes between fixes, it did not account for linearity or directionality of movement.
If the tracklet met the criteria of both (a) and (b), then it was considered a valid reference tracklet. This was repeated for all possible consecutive sets of four points, resulting in a set of multiple temporally overlapping tracklets. Once all valid reference tracks were identified, they were also considered to be valid comparison tracklets.
Each reference tracklet was converted from a set of four points to a line feature, assuming straight line travel between each consecutive point. The distance between this line and the first point of each comparison tracklet (c1) was then measured. If c1 was greater than 50m away from the reference tracklet, then no further comparison was made between the two features. If c1 was within 50m of the reference tracklet, then the same comparison was made for all points in the comparison tracklet. In order for the comparison tracklet (c) to count as a reuse of the reference tracklet (r), it had to meet a number of conditions: (1) all points in c must fall within 50m of the r; (2) r must not be made up of points from c, i.e., be the same tracklet; (3) r and c must have been travelled at least 30 minutes apart, or with a difference of two GPS fixes; (4) c must not have been travelled within 30 minutes of another comparison tracklet that already reused r (Figure 1). The criteria for reuse was based on observation of the primates moving within and between patches in 2018 and 2019 (Hammond et al., 2021; Lewis-Bevan, 2024). Based on these observations, group spread was often 80–200 meters apart, with movement within foraging patches tending to be less than 50m, and movement between foraging patches tending to be more than 100m. We further examined the possibility of other criteria based on visual examination of routes and comparison to continues GPS tracks from observers following the baboons, and chose these criteria the least-likely to create false negatives.
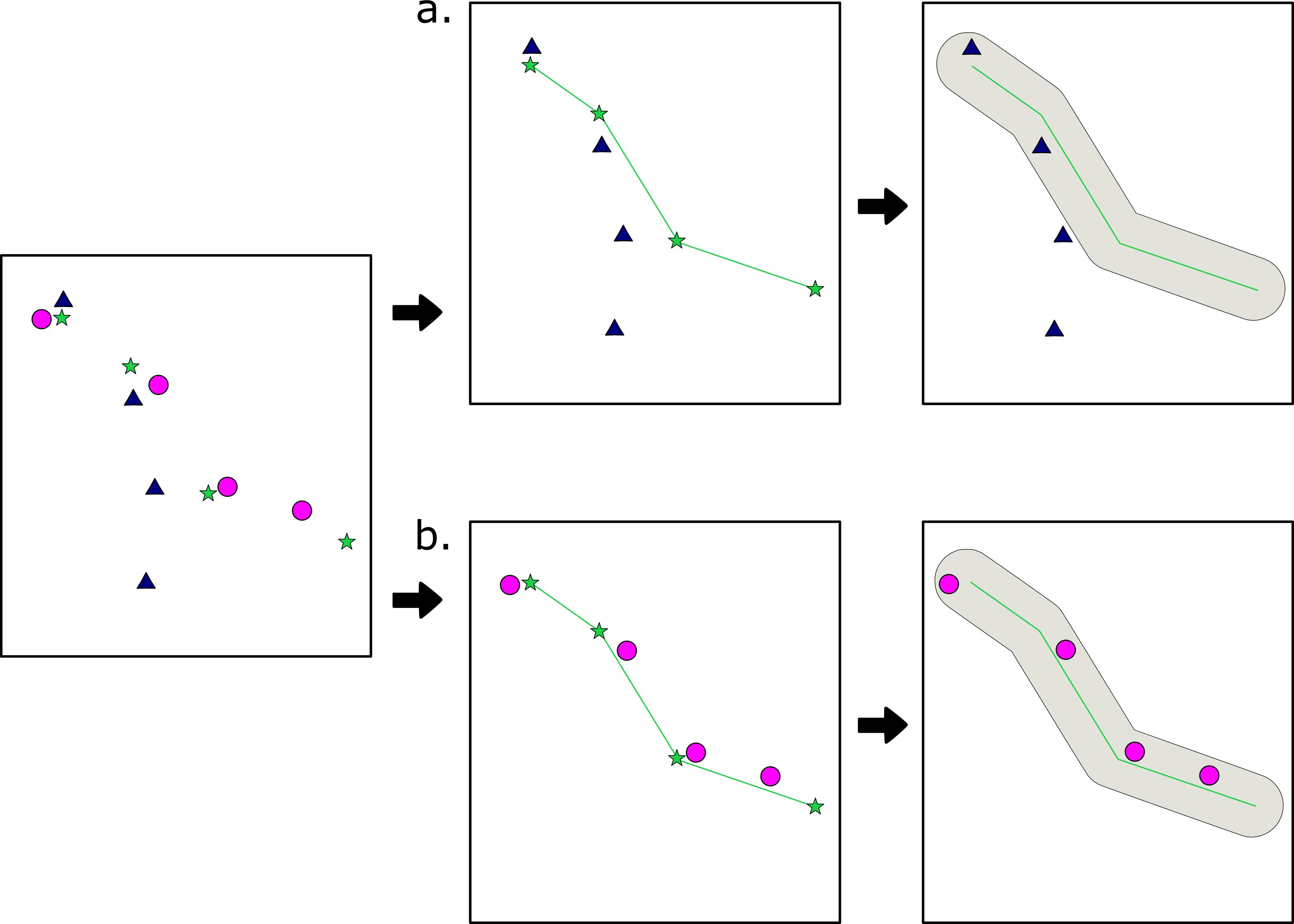
Figure 1. Method of identifying reused routes. Each route started by identifying tracklets, shown in the first panel. (a) identifies a reference tracklet, converts it into a line, and adds a buffer; because all points of the comparison tracklet (triangles) do not fall within the buffer, this is not a valid reuse. (b) follows the same methods, but shows an example of a valid “reuse”.
If a comparison tracklet met all four conditions, it was considered a reuse of the reference tracklet. To account for differences in speed of travel, each comparison tracklet was also analyzed as a reference tracklet, although reuse was only counted once for each pair. Despite the conditions imposed, preliminary analysis showed a high margin of error in identifying tracks that were only reused two times, as in other studies (de Raad, 2012; Di Fiore, 2003), due to the fact that this analysis does not explicitly account for the linearity and directionality of each tracklet. To mitigate this, only routes in the top quartile of all reuses were considered reused routes for this analysis, given that the more times a route was reused the less likely it was to be a false match, based on visual analysis.
Route re-use following displacement
In order to identify periods and areas of displacement, full GPS data for each monkey was plotted and visually examined both weekly and monthly. Areas that were identified as being vacated for more than two months (vacation period), and subsequently reoccupied, were considered vacated areas (Figure 2).
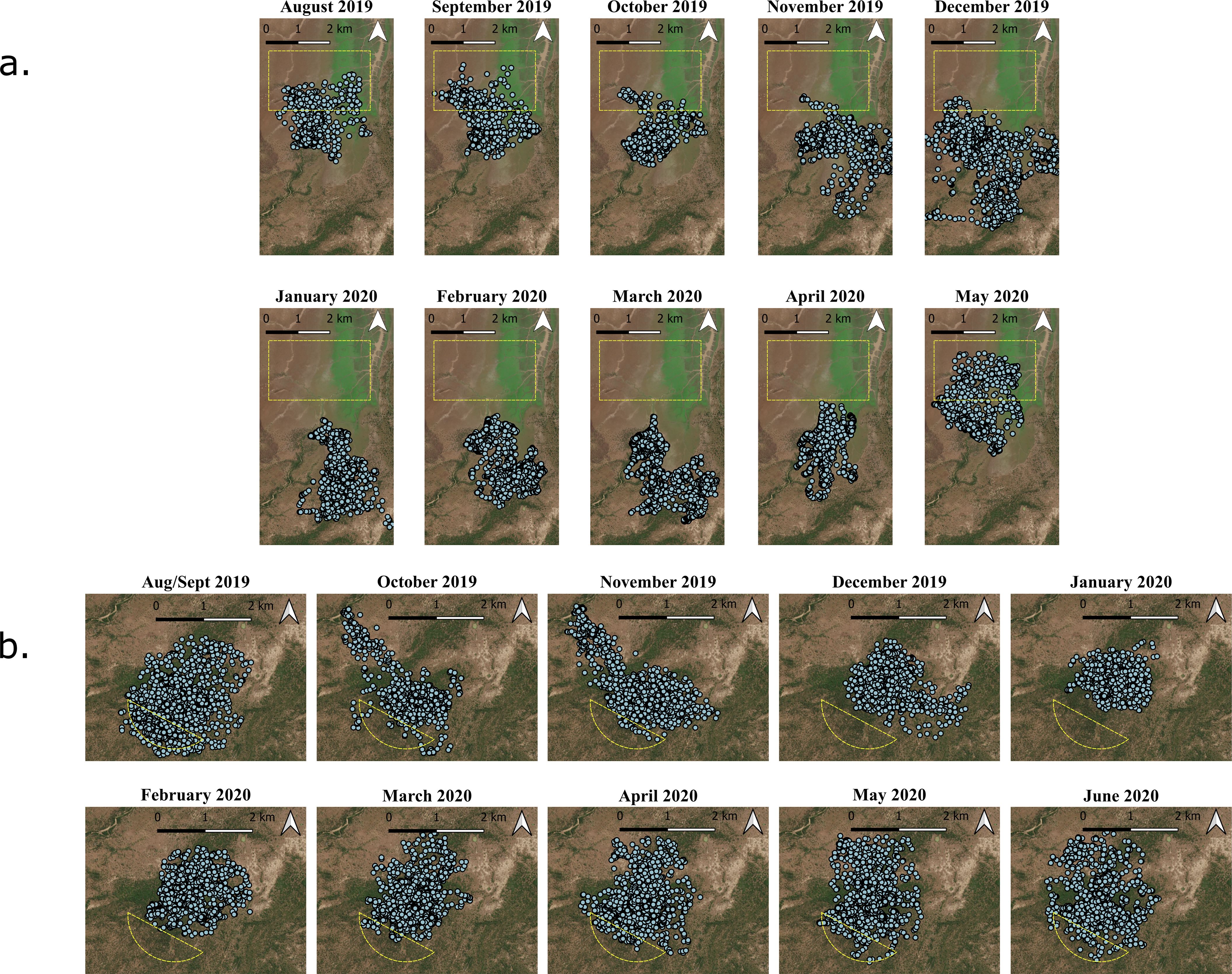
Figure 2. Monthly mapping of daily ranging data for FT (a) and WT (b). Identified vacated areas are shown in yellow. For analysis, these were transformed to match the edge of the home range.
To analyze whether routes were reused in vacated areas, all identified routes that were majority inside the vacated areas (3/4 fixes) were isolated in QGIS (QGIS Development Team, 2020). The isolated routes and all records of their reuse were then compared to see if they were used both before and after the area was vacated.
Ethics statement
This work was carried out with ethical clearance from Oxford University (APA/1/5/ACER/23Jan2018) and from the Ministry of Tourism and the Gorongosa Restoration Project in Mozambique (permit numbers PNG/DSCi/C114/2018, PNG/DSCi/C93/2018, PNG/DSCi/C147/2019 and PNG/DSCi/C142/2019). All handling of the study subjects was performed by professional staff, and following collar deployment all subsequent data collection was completed remotely and in the animals’ natural habitat.
Results
Similarity of daily routes
When comparing route dissimilarity over time, we found that the two troops showed different patterns consistent with the known patterns of flooding in GNP. WT remained almost consistently dissimilar throughout the study period, particularly for days that were more than two weeks apart; that is, there was little variation in how different their daily paths were, despite visual differences in their ranging patterns, implying that the majority of their movement occurred in the core of their home range (in this case defined as the central 50% of the total area around the centroid) throughout the year, and that changes to the periphery of their home range seasonally (Figure 3) did not strongly affect route dissimilarity.
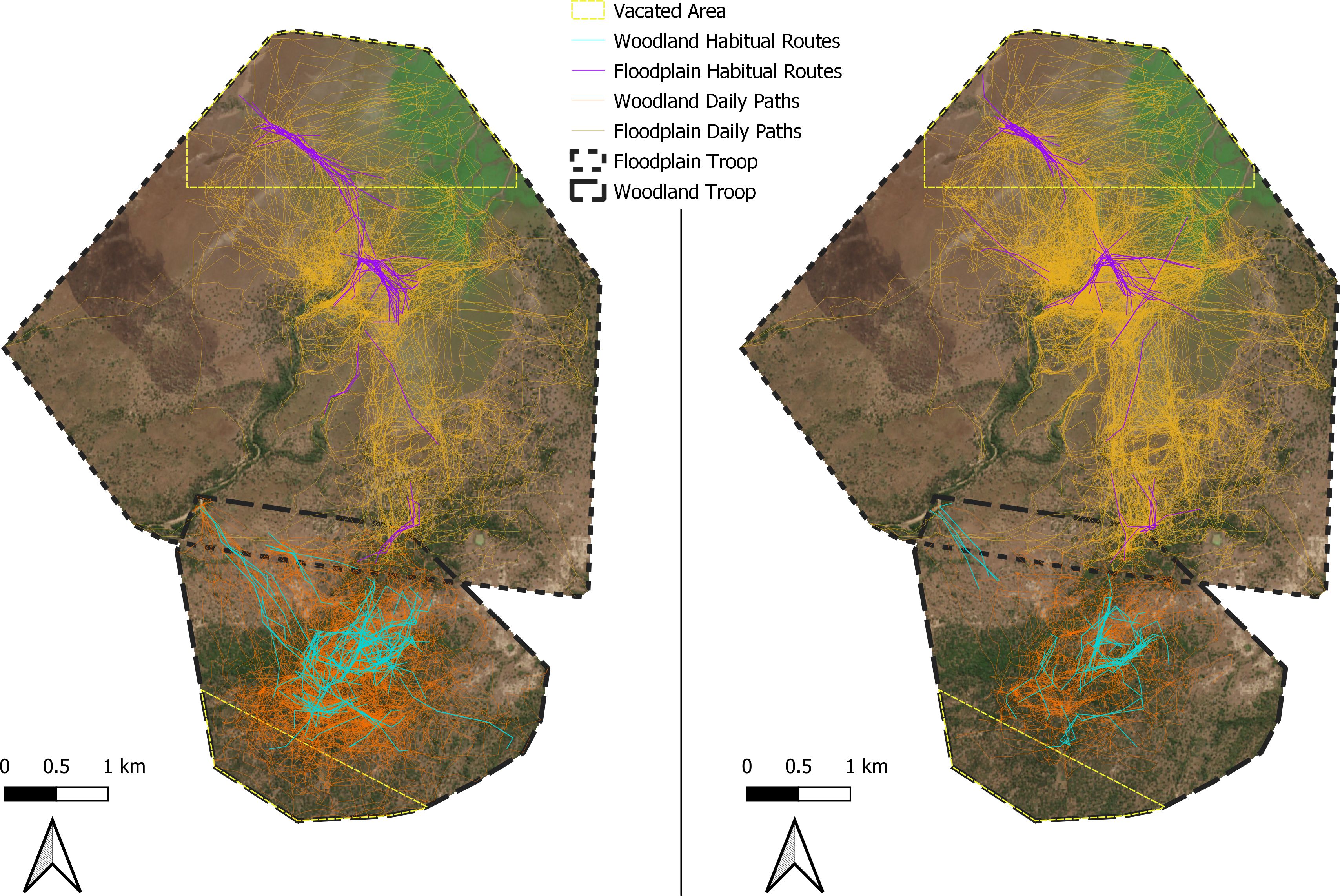
Figure 3. Baboon home ranges with full daily paths (yellow for FT and orange for WT) as well as identified routes (purple and blue, respectively).The map on the left shows the routes of Eve (FT) and Kigalia (WT) while the map on the right shows the routes of Abacaxi (FT) and Acacia (WT).
However, FT was cyclically dissimilar. In pairs of days that were temporally close together (<50 days), or very far apart (>250 days), their daily paths were more similar. In other pairs, pairs became more and more dissimilar until about 125 days apart, when they began gradually becoming more similar. This follows the pattern of displacement throughout the year, with periods before and after displacement due to flooding being more similar than the periods during and not during the flooding, indicating that the baboons had smaller ranging areas at the periphery of their home ranges that they used seasonally, rather than a central core as in WT (Figure 4).
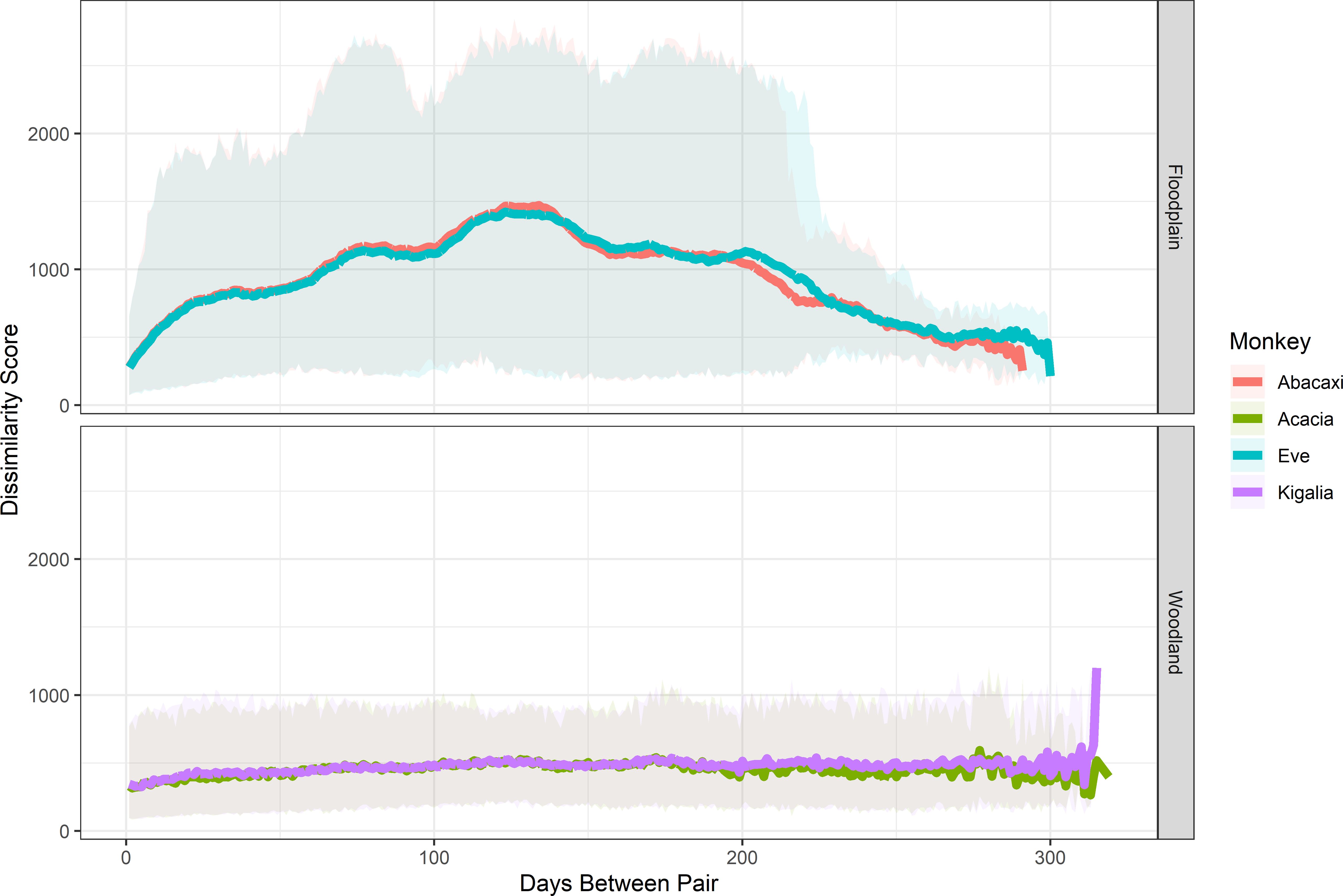
Figure 4. Dissimilarity scores, indicative of average meters between compared daily routes, between baboon daily tracks across the year. The y-axis indicates how dissimilar each pair of days was, while the x-axis indicates how many days occurred between each pair. The more dissimilar the scores are, the further apart two days’ daily tracks were.
Occurrence of route reuse
For each collar, between 378–1137 tracklets were identified. From these, between 69–179 tracklets were found to have been reused more than the upper quartile, which ranged from 4–8 reuses. Tracklets that were reused more than the upper quartile are hereafter simply referred to as “routes.”
The most any route was reused was 30 times, with the average length of the route ranging from 631–836 meters. Further information can be found in Table 1 and the route networks are visualized in Figure 3.
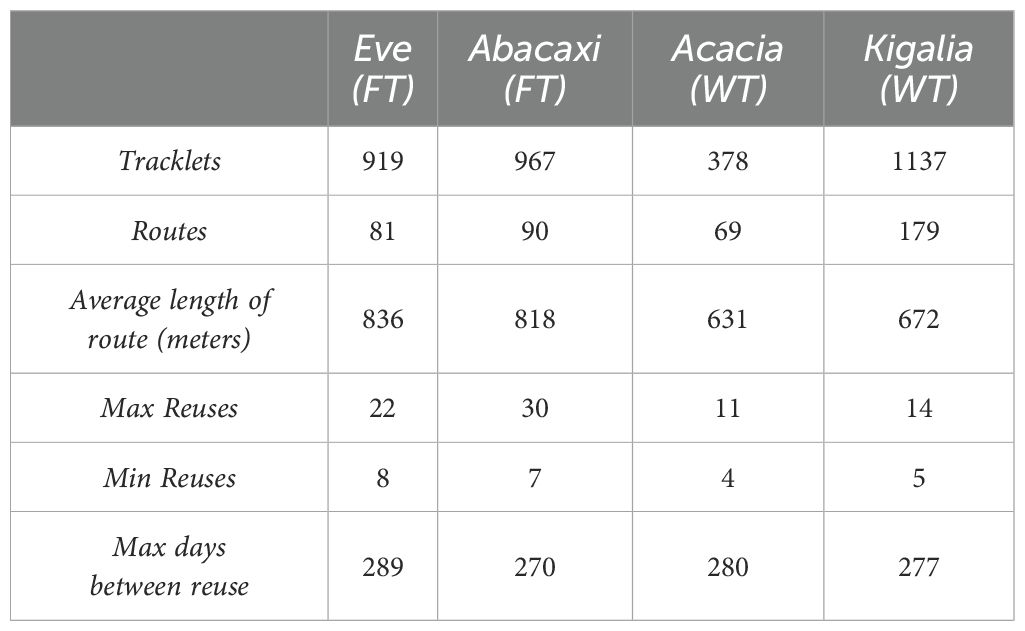
Table 1. Summary of analyzable tracklets, or sequence of four points meeting defined criteria, and routes, or tracklets used more times than the upper quartile for that individual.
Route reuse in vacated areas
Vacated areas were identified on the periphery of each group’s home range. WT occupied the same core home range for the entirety of the year, while only the peripheries were vacated for extended periods. FT displayed similar patterns; however, one area near the center of their home range was vacated for a four-week period at the peak of flooding. Because it was vacated for less than the two-month displacement chosen to test memory and route robustness, route reuse in this area was not directly examined.
In WT, only one route occurred partially inside the vacated area, despite multiple tracklets. This route began close to the core home range, and was only used over a period from the end of September to early January when there was no vacation of the area, and so was not included in any further analysis.
In FT, several routes occurred within the vacated area. However, these routes were only used for a portion of the year from October to November, despite the troop ranging in the vacated area outside of these months. Only one route from after the displacement period occurred partially within the vacated area, and as the majority occurred outside of the area, we were unable to analyze route reuse in FT’s vacated area (Figure 3).
Discussion
Besides the Okavango Delta, where baboons have adapted to cope with the annual flooding by wading and swimming between islands (Cheney and Seyfarth, 2008), GNP is the only baboon study site that faces extensive annual flooding. Unlike the Okavango, baboons in GNP are rarely seen to enter the water, and as a result must take considerable measures in order to cross rivers and streams, such as jumping from treetop to treetop or crossing on sandbanks by sunning crocodiles. In this study, we examined to what extent this seasonal disruption affects baboons’ daily ranging areas, whether or not they have habitually used routes despite these disruptions, and whether or not those routes are robust to disruptions, i.e. whether baboons return to previously established routes after areas of their home range are vacated seasonally.
We found that the two troops showed different levels of annual variation, or dissimilarity, in their daily movement routes. High dissimilarity could indicate a complete displacement where the baboons (and hence their routes) are in two physically different locations, or it could indicate high variability where baboons move through the same environment but follow very different paths (Hammond et al., 2025). Previous analysis showed that WT experienced a general shrinking of their home range during the wet season, while FT shifted south; however, both troops maintained a core home range for most of the year. For FT, this area was simply the overlap of their wet and dry season home ranges, although it was inaccessible for FT for around four weeks at the height of the flooding.
Given this, it is unsurprising that WT maintained a stable dissimilarity score across the year, as they maintained the same core home range and variation on the peripheries occurred only for relatively short time periods. However, despite continuously occupying a core home range, FT’s dissimilarity showed variation across the year, with days in opposite seasons being more dissimilar than days close together or in the same season almost a year apart. In addition, even days close together showed more dissimilarity than the same pairs of days in WT. This indicates that they may have used the periphery of their home range more than WT, and at different parts of the year.
This difference between troops could be related to a number of factors. First, FT relies seasonally on various food sources that each exhibit a different growth cycle, often spending entire days utilizing a single resource patch, and travelling further than WT on days where they utilize multiple resource patches, implying more scarce or transient resource patches that require longer travel distances to exploit. FT also utilizes more spread-out, low-density food types than WT, and are one of the smallest troops in their area (LLB, unpublished data). These long travel distances between seasonal patches, and a high potential for displacement from patches due to intergroup encounters, could cause more route dissimilarity even between days close together in time.
In addition, habitat type could alter route similarity. We found that Kigalia (WT) exhibited a higher number of reused routes, but a lower number of reuses per route compared to FT. Acacia (WT) also had a low habitual-route-number-to-reuse ratio; however, Acacia’s collar often failed to record GPS fixes, giving less overall analyzable movement data than any other monkey. Nevertheless, this indicates that WT has a larger, dense route network, with each route being used less often, while the FT baboons exhibit a smaller route network that is used more frequently. This is supported by visual examination of the data (Figure 3). In an earlier study, baboons in a mosaic habitat that primarily foraged in woodland areas in Limpopo, South Africa, displayed a similarly dense route network to WT, extending to the edges of their home range and remaining dense even in the periphery (de Raad and Hill, 2019). The similarity in habitat type may indicate that baboons are topographically constrained in woodland areas, where tree and undergrowth density create significant energetic barriers, and repeated routes could carve paths that are easier to travel, as shown in arboreal primates (Di Fiore, 2003). Observational data collected on these troops in 2018 indicated this pattern was similar to the paths of WT, and FT in denser areas, but not in open floodplain areas. Other inhabitants of the woodland of GNP, such as elephants and antelope, may similarly contribute to a more easily traversable route network. In addition, baboons in densely treed areas may be exhibiting patch to patch travel networks, utilizing discrete food items as in a trapline, but using the dense route network to travel between food patches in different orders as depletion and seasonality vary (Noser and Byrne, 2009, 2015). One of the denser parts of the route network in WT’s peripheral home range led to the Mussicadzi river; similar tracks were also recorded by handheld GPS in 2018, when baboons had to travel daily to the one remaining water source in their home range, indicating that this resource also led to the formation of a habitual route.
On the other hand, baboons ranging in open areas may not need to utilize routes so frequently. The main topographic barriers in FT are the Mussicadzi River to the east, the Tsungue river to the west and north, and a large, dense patch of elephant grass in the core of the home range, in which lions, buffalo, and elephants are frequently sighted. Interestingly, the most central part of FT’s route reuse network occurs in the area that traverses the edge of the elephant grass through a patch of palm trees where the baboons often rest. Travel through this area mimics travel through the woodland in terms of low visibility and difficult terrain. The second densest part of their habitual route network extends to the northeast, following the Mussicadzi river seasonally when large swaths of Chrozophora plicata, a preferred food source for FT baboons, grows in the drying riverbed. These observations indicate that topographic barriers may promote route reuse rather than routes being a preferred form of navigation, and that baboons may not use routes when they are able to easily visually orient themselves, or that these routes are more general orientations based on landmarks rather than distinct movements along a 50-metre-wide path. These observations were furthered by on-the-ground troop follows of FT, where baboons could predictably disappear into dense grasses at the same location daily, and be expected to appear on the other side at a known location after crossing. A single attempt to cross the dense grass showed that there was in fact a narrow path leading from the entrance to the exit, crossing a patch of palm trees where the baboons foraged, and that buffalo also used this path.
This study also faced limitations in identifying route reuse due to its narrow criteria of “reuse”. While the criterion of 50m of movement between fixes reduced the likelihood of oft-used patches being identified as routes, it also potentially precluded routes travelled slowly over the course of a day, where the baboons grazed as they travelled rather than stopping to forage. Similarly, the narrow overlapping distance used excluded times when the baboons were moving 50m within a patch, but meant oft-used paths with a wider spread were excluded. Potential paths were also excluded because of the decision to only use the top quartile of reuses. This decision was made based on visual examination of the data, with the impact of this threshold varying; for Kigalia (WT), the threshold for reuse remained steady in the upper quartiles, going from 6 reuses at 50% to 7 at 75%; for Eve (FT) it moved from 10 to 13.
Because of the low resolution of the GPS data, stricter criteria were used to avoid false-positive identification of routes, at the cost of creating false-negatives. The low resolution also meant that routes were lost when GPS fixes were not successful, since these sections of data were automatically excluded, and differences in fix success rate made data less comparable within the same troop. Longer-term studies or studies with higher GPS resolution should consider methods that balance these by identifying known false-positives and true positives. Where that is not possible due to a lack of known false positives, further studies should incorporate both linearity of routes and vector angles from one point to another, rather than relying on fixed width, to better identify more spread-out routes in open habitats.
Despite finding route reuse in line with other study sites, this study failed to identify route reuse in vacated areas, primarily due to the lack of routes in these areas. This is may not be reflective of a true lack of routes or memory of them, however. The baboons used the peripheries of their home ranges less often, and the edges of their home ranges changed seasonally, meaning there were less overall tracklets in these areas. Further, because the data did not cover a full year, seasonal resources that might have led to the formation of routes in these areas may not have been available at both ends of the study period. This, combined with the relatively high number of reuses we used as the criterion for classifying routes as “habitually” used, may indicate that it is a lack of data limiting the identification of routes reused after vacation, rather than a lack of those routes themselves. What effect varying this strict threshold has on the nature and density of baboon route networks identified should be revisited in future studies.
Nonetheless, this study did find that baboons returned to vacated areas, and FT did have some routes in those areas. Because of battery failure, the GPS points from this study do not cover an entire year. This meant that routes that were only used in certain conditions may have been reused without being recorded, especially for routes that are highly seasonal. Without a full year of data, it is impossible to say whether or not baboons did reuse the routes after the collar batteries died. Future work should look at routes over a full calendar year or multiple years as a better indication of the role memory plays in repetition of routes. In addition, factors besides flooding that cause vacations from areas should be considered in future studies. Temperatures on the Floodplain consistently reach above 40C, which might influence the baboons’ ability to travel to the vacated area in their home range; in 2020, temperatures reached 50C on the floodplain (SC, unpublished data). However, observational data from 2018 showed baboons repeatedly crossing the floodplain in high temperatures due to the need for water that was only available in the northwestern section of their home range (Lewis-Bevan et al., 2019), showing that a balance between shade, food and water availability might drive route use. The change in both availability and location of resources depending on climate variables could lead to routes being unused for whole seasons or years; daily follows of the baboons showed that they reused some routes only when external conditions called for it, such as water or food scarcity. Replications of this study over multiple years would help to further identify factors causing route reuse.
Another limitation of this study is the resolution of the GPS points taken. Our positional fixes were taken at 15-min intervals, and we interpolated the routes connecting these locations as straight lines - this represents an overall route estimate that is almost certainly shorter and less tortuous than the actual paths taken by the baboons. This also reduces our ability to analyze route complexity and memory, since it reduces the resolution of any routes taken, and excludes smaller sections of routes that may have been reused more often. In the future, more frequent GPS fixes would allow for higher-resolution reconstructions of routes, and hence for a more robust nearest neighbor analysis both at the daily-route level and at the tracklet-level. While this would come at the expense of battery life, we expect that technological advances in biologging will soon enable researchers to collect the necessary long-term datasets at sufficient resolution to explore our questions with added precision.
Despite its drawbacks, this study presents a tractable analysis of route reuse that is both faster and more replicable than visual examination, but does not necessarily require high-resolution data. Further improvements on this method should involve accounting for the angle of travel in each point in the tracklet, similar to change-point analysis (Byrne et al., 2009), and a measure of the parallelism of both the reference and corresponding tracks. Although this study focuses on baboon movement, the method presented could be applied to any subject where traditional methods of overlaying GPS data are not possible, or to test parameters of what each species considers a route. This could be especially helpful for animals traversing smaller areas, where GPS points are often clustered and appear random, or where subjects cannot be directly observed. This method could also help identify areas of large data-sets to focus on, particularly for animals with extensive home ranges. This study provides methodology to help understand how baboons navigate in a changing environment, and will hopefully be followed up by further studies monitoring animal movement, environmental knowledge and memory in a rapidly changing world.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This work was carried out with ethical clearance from Oxford University (APA/1/5/ACER/23Jan2018) and from the Ministry of Tourism and the Gorongosa Restoration Project in Mozambique (permit numbers PNG/DSCi/C114/2018, PNG/DSCi/C93/2018, PNG/DSCi/C147/2019 and PNG/DSCi/C142/2019). All handling of the study subjects was performed by professional staff, and following collar deployment all subsequent data collection was completed remotely and in the animals’ natural habitat.
Author contributions
LL-B: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Visualization, Writing - original draft. PH: Investigation, Methodology, Project administration, Writing - review & editing. SC: Funding acquisition, Methodology, Supervision, Writing – review & editing. DB: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Gorongosa Restoration Project, the Leverhulme Trust (PLP-2016-114 to SC), University of Oxford (John Fell Fund to SC), St Hugh’s College (Jackie Lambert Fund to SC) and St John’s College (Oxford). Open Access to this article is financed by FCT – Fundação para a Ciência e a Tecnologia, within the scope of the project UID/04211: Centro Interdisciplinar de Arqueologia e Evolução do Comportamento Humano (ICArEHB). The authors are very grateful to FCT for allowing this article to be openly accessible.
Acknowledgments
We thank the staff of Gorongosa National Park for assisting with the collaring of baboons used in this study, and for facilitating our field work. In particular, we are grateful to M. Stalmans, J. Denlinger, M. Mutemba, P. Muagura, L. van Wyk, A. Paulo, M. Marchington, and P. Bouley, as well as the park rangers who joined us in the field, including Gonçalves António Veronica, Nhampoca Dauce, Ernesto Xavier, Salvador Simão, Daniel Maveneco, Albano Vasco, Herculano Beca, Sérgio João Amaral, and Inoque Nicodimo Chai. We also thank Julien Collet for his input in planning the analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alavi S. E., Vining A. Q., Caillaud D., Hirsch B. T., Havmøller R. W., Havmøller L. W., et al. (2022). A quantitative framework for identifying patterns of route-use in animal movement data. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.743014
Altmann S. A. (1974). Baboons, space, time, and energy. Am. Zoologist 14, 221–248. doi: 10.1093/icb/14.1.221
Amato K. R. and Garber P. A. (2014). Nutrition and foraging strategies of the black howler monkey (Alouatta pigra) in palenque national park, Mexico. Am. J. Primatology 76, 774–787. doi: 10.1002/ajp.22268
Atkins J. L., Long R. A., Pansu J., Daskin J. H., Potter A. B., Stalmans M. E., et al. (2019). Cascading impacts of large-carnivore extirpation in an African ecosystem. Science 364, 173–177. doi: 10.1126/science.aau3561
Beardmore-Herd M., Gaynor K. M., Palmer M. S., and Carvalho S. (2025). Effects of an extreme weather event on primate populations. Am. J. Biol. Anthropology 186, e25049. doi: 10.1002/ajpa.25049
Bebko A. O. (2021). “Determining the Presence of Habitual Travel Route Networks in Orangutans (Pongo pygmaeus morio) in Kutai National Park, Borneo,” in Dolins F.L., Shaffer C.A., Porter L.M., Hickey J.R., Nibbelink N.P., et al. Spatial Analysis in Field Primatology: Applying GIS at Varying Scales. (Cambridge University Press), 204–224.
Biro D., Meade J., and Guilford T. (2004). Familiar route loyalty implies visual pilotage in the homing pigeon. Proc. Natl. Acad. Sci. U.S.A 101, 17440–17443. doi: 10.1073/pnas.0406984101
Biro D., Muschinski J., Hammond P., Bobe R., Bamford M. K., Capelli C., et al. (2025). West Side Story: Regional inter-troop variation in baboon bark-stripping at Gorongosa National Park, Mozambique. Am J Biol Anthropol. doi: 10.1002/ajpa.70057
Bouley P., Poulos M., Branco R., and Carter N. H. (2018). Post-war recovery of the African lion in response to large-scale ecosystem restoration. Biol. Conserv. 227, 233–242. doi: 10.1016/j.biocon.2018.08.024
Boyer D. and Walsh P. D. (2010). Modelling the mobility of living organisms in heterogeneous landscapes: Does memory improve foraging success? Philos. Trans. R. Soc. A: Mathematical Phys. Eng. Sci. 368, 5645–5659. doi: 10.1098/rsta.2010.0275
Byrne R. W., Noser R., Bates L. A., and Jupp P. E. (2009). How did they get here from there? Detecting changes of direction in terrestrial ranging. Anim. Behav. 77, 619–631. doi: 10.1016/j.anbehav.2008.11.014
Caillaud D., Crofoot M. C., Scarpino S. V., Jansen P. A., Garzon-Lopez C. X., Winkelhagen A. J. S., et al. (2010). Modeling the spatial distribution and fruiting pattern of a key tree species in a neotropical forest: Methodology and potential applications. PLoS One 5:e15002. doi: 10.1371/journal.pone.0015002
Cain J. W., Jansen B. D., Wilson R. R., and Krausman P. R. (2008). Potential thermoregulatory advantages of shade use by desert bighorn sheep. J. Arid Environments 72, 1518–1525. doi: 10.1016/j.jaridenv.2008.02.010
Cheney D. L. and Seyfarth R. M. (2008). Baboon Metaphysics: The Evolution of a Social Mind. 2008th ed (Chicago: The University of Chicago Press).
Coleman B. T. and Hill R. A. (2014). Living in a landscape of fear: The impact of predation, resource availability and habitat structure on primate range use. Anim. Behav. 88, 165–173. doi: 10.1016/j.anbehav.2013.11.027
Collet J., Sasaki T., and Biro D. (2021). Pigeons retain partial memories of homing paths years after learning them individually, collectively or culturally. Proc. R. Soc. B: Biol. Sci. 288:2110. doi: 10.1098/rspb.2021.2110
Collett M. (2010). How desert ants use a visual landmark for guidance along a habitual route. Proc. Natl. Acad. Sci. U.S.A 107, 11638–11643. doi: 10.1073/pnas.1001401107
Collett T. S., Graham P., Harris R. A., and Hempel-de-Ibarra N. (2006). Navigational memories in ants and bees: memory retrieval when selecting and following routes. Advances in the Study of Behavior, Academic Press. 36, 123–172. doi: 10.1016/S0065-3454(06)36003-2
Correia M., Timóteo S., Rodríguez-Echeverría S., Mazars-Simon A., and Heleno R. (2017). Refaunation and the reinstatement of the seed-dispersal function in Gorongosa National Park. Conserv. Biol. 31, 76–85. doi: 10.1111/cobi.12782
Crystal J. D. and Wilson A. G. (2015). Prospective memory: A comparative perspective. Behav. Processes 112, 88–99. doi: 10.1016/j.beproc.2014.07.016
De Guinea M., Estrada A., Nekaris K. A. I., and Van Belle S. (2019). Arboreal route navigation in a Neotropical mammal: Energetic implications associated with tree monitoring and landscape attributes. Movement Ecol. 7, 1–12. doi: 10.1186/s40462-019-0187-z
de Raad A. (2012). Travel routes and spatial abilities in wild chacma baboons (Papio ursinus). (Durham, UK:University of Durham). Available at: http://etheses.dur.ac.uk/3554/.
de Raad A. and Hill R. A. (2019). Topological spatial representation in wild chacma baboons (Papio ursinus). Anim. Cogn. 22, 397–412. doi: 10.1007/s10071-019-01253-6
Di Fiore A. (2003). Ranging behavior and foraging ecology of lowland woolly monkeys (Lagothrix lagotricha poeppigii) in Yasuní National Park, Ecuador. Am. J. Primatology 59, 47–66. doi: 10.1002/ajp.10065
Di Fiore A. and Suarez S. A. (2007). Route-based travel and shared routes in sympatric spider and woolly monkeys: Cognitive and evolutionary implications. Anim. Cogn. 10, 317–329. doi: 10.1007/s10071-006-0067-y
Fuchs A. J., Gilbert C. C., and Kamilar J. M. (2018). Ecological niche modeling of the genus Papio. Am. J. Phys. Anthropology 166, 812–823. doi: 10.1002/ajpa.23470
Gaynor K. M., Daskin J. H., Rich L. N., and Brashares J. S. (2020). Postwar wildlife recovery in an African savanna: evaluating patterns and drivers of species occupancy and richness. Anim. Conserv. 24, 510–522. doi: 10.1111/acv.12661
Green S. J., Boruff B. J., and Grueter C. C. (2020). From ridge tops to ravines: landscape drivers of chimpanzee ranging patterns. Anim. Behav. 163, 51–60. doi: 10.1016/j.anbehav.2020.02.016
Gregory T., Mullett A., and Norconk M. A. (2014). Strategies for navigating large areas: A GIS spatial ecology analysis of the bearded Saki monkey, Chiropotes sagulatus, in Suriname. Am. J. Primatology 75, 586–595. doi: 10.1002/ajp.22251
Griffiths D., Dickinson A., and Clayton N. (1999). Episodic memory: what can animals remember about their past? Trends Cogn. Sci. 3, 74–80. doi: 10.1016/S1364-6613(98)01272-8
Hammond P., Lewis-Bevan L., Biro D., and Carvalho S. (2021). Risk perception and terrestriality in primates: A quasi-experiment through habituation of chacma baboons (Papio ursinus) in Gorongosa National Park, Mozambique. Am. J. Biol. Anthropology, 179:48–59. doi: 10.1002/ajpa.24567
Hammond P., Gaynor K., Easter T., Biro D., and Carvalho S. (2025). Landscape-scale effects of season and risk on the terrestrial activity patterns of chacma baboons (Papio ursinus). Am J Biol Anthropol. doi: 10.1002/ajpa.70052
Herrero H., Waylen P., Southworth J., Khatami R., Yang D., and Child B. (2020). A healthy park needs healthy vegetation: The story of Gorongosa National Park in the 21st century. Remote Sens. 12:476. doi: 10.3390/rs12030476
Hill R. A. (2006). Thermal constraints on activity scheduling and habitat choice in baboons. Am. J. Phys. Anthropology 129, 242–249. doi: 10.1002/ajpa.20264
Hill R. A., Weingrill T., Barrett L., and Henzi S. P. (2004). Indices of environmental temperatures for primates in open habitats. Primates 45, 7–13. doi: 10.1007/s10329-003-0054-8
Hopkins M. E. (2011). Mantled howler (Alouatta palliata) arboreal pathway networks: relative impacts of resource availability and forest structure. Int. J. Primatology 32, 238–258. doi: 10.1007/s10764-010-9464-9
Janson C. H. (2000). “Spatial movement strategies: theory, evidence, and challenges,” in On the Move: How and Why Animals Travel in Groups. Eds. Boinski S. and Garber P. A. (Chicago University Press), 165–203.
Janson C. H. (2016). Capuchins, space, time and memory: an experimental test of what-where-when memory in wild monkeys. Proc. R. Soc. B: Biol. Sci. 283:1432. doi: 10.1098/rspb.2016.1432
Janson C. H. and Byrne R. (2007). What wild primates know about resources: Opening up the black box. Anim. Cogn. 10, 357–367. doi: 10.1007/s10071-007-0080-9
Joo R., Picardi S., Boone M. E., Clay T. A., Patrick S. C., Romero-Romero V. S., et al. (2022). Recent trends in movement ecology of animals and human mobility. Movement Ecol. 10, 1–20. doi: 10.1186/s40462-022-00322-9
Laundré J. W., Hernandez L., and Ripple W. J. (2010). The landscape of fear: ecological implications of being afraid. Open Ecol. J. 3, 1–7. doi: 10.2174/1874213001003030001
Lemoine S. R. T., Samuni L., Crockford C., and Wittig R. M. (2023). Chimpanzees make tactical use of high elevation in territorial contexts. PLoS Biol. 21, 1–26. doi: 10.1371/journal.pbio.3002350
Lewis-Bevan L., Biro D., and Carvalho S. (2019). Baboon habitat uuse in a complex environment, Gorongosa National Park, Mozambique. 16th Conf. Gesellschaft Fur Primatologie 42.
Lewis-Bevan L. (2024). From the Floodplain to the Woodland (and Back): Baboon Movement in the Highly Seasonal and Heterogeneous Ecosystem of Gorongosa National Park. University of Oxford, Doctoral Dissertation.
Lewis-Bevan L., Hammond P., Carvalho S., and Biro D. (2025). The Travelling Salesbaboon: Chacma baboon route efficiency in multi-stop daily travel routes. WILD. doi: 10.3390/wild2020018doi: 10.3390/wild2020018
Markham A. C., Alberts S. C., and Altmann J. (2016). Haven for the night: Sleeping site selection in a wild primate. Behav. Ecol. 27, 29–35. doi: 10.1093/beheco/arv118
Marshall H. H., Carter A. J., Ashford A., Rowcliffe J. M., and Cowlishaw G. (2013). How do foragers decide when to leave a patch? A test of alternative models under natural and experimental conditions. J. Anim. Ecol. 82, 894–902. doi: 10.1111/1365-2656.12089
Mueller T. and Fagan W. F. (2008). Search and navigation in dynamic environments – from individual behaviors to population distributions. Oikos 117, 654–664. doi: 10.1111/j.0030-1299.2008.16291.x
Nathan R., Getz W. M., Revilla E., Holyoak M., Kadmon R., Saltz D., et al. (2008). A movement ecology paradigm for unifying organismal movement research. PNAS 105, 19052–19059. doi: 10.1021/i360006a005
Nathan R., Monk C. T., Arlinghaus R., Adam T., Alós J., Assaf M., et al. (2022). Big-data approaches lead to an increased understanding of the ecology of animal movement. Science 375:eabg1780. doi: 10.1126/science.abg1780
Noser R. and Byrne R. W. (2007a). Mental maps in chacma baboons (Papio ursinus): Using inter-group encounters as a natural experiment. Anim. Cogn. 10, 331–340. doi: 10.1007/s10071-006-0068-x
Noser R. and Byrne R. W. (2007b). Travel routes and planning of visits to out-of-sight resources in wild chacma baboons, Papio ursinus. Anim. Behav. 73, 257–266. doi: 10.1016/j.anbehav.2006.04.012
Noser R. and Byrne R. W. (2009). How do wild baboons (Papio ursinus) plan their routes? Travel among multiple high-quality food sources with inter-group competition. Anim. Cogn 13, 145–55. doi: 10.1007/s10071-009-0254-8
Noser R. and Byrne R. W. (2015). Wild chacma baboons (Papio ursinus) remember single foraging episodes. Anim. Cogn. 18:921–29. doi: 10.1007/s10071-015-0862-4
Pochron S. T. (2000). Sun avoidance in the yellow baboons (Papio cynocephalus cynocephalus) of Ruaha National Park, Tanzania. Variations with season, behavior and weather. Int. J. Biometeorology 44, 141–147. doi: 10.1007/s004840000058
Porter L. M. (2021). “Finding fruit in a tropical rainforest: A comparison of the foraging patterns of two distinct fruit-eating primates across years,” in Spatial Analysis in Field Primatology. Eds. Dolins F., Shaffer C. A., Porter L. M., Hickey J. R., and Nibbelink N. P. (Cambridge University Press), 225–246. doi: 10.1017/9781107449824.013
Porter L. M. and Garber P. A. (2013). Foraging and spatial memory in wild weddell’s saddleback tamarins (Saguinus fuscicollis weddelli) when moving between distant and out-of-sight goals. Int J Primatol. 34, 30–48. doi: 10.1007/s10764-012-9644-x
Poucet B. (1993). Spatial cognitive maps in animals: new hypotheses on their structure and neural mechanisms. psychol. Rev. 100, 163–182. doi: 10.1037/0033-295X.100.2.163
Presotto A., Fayrer-Hosken R., Curry C., and Madden M. (2019). Spatial mapping shows that some African elephants use cognitive maps to navigate the core but not the periphery of their home ranges. Anim. Cogn. 22, 251–263. doi: 10.1007/s10071-019-01242-9
QGIS Development Team (2020). QGIS Geographic Information System (Open Source Geospatial Foundation).
R Core Team (2020). R: A Language and Environment for Statistical Computing. Available online at: https://www.r-project.org/ (Accessed June 30, 2024).
Samson D. R., Bray J., and Nunn C. L. (2018). The cost of deep sleep: Environmental influences on sleep regulation are greater for diurnal lemurs. Am. J. Phys. Anthropology 166, 578–589. doi: 10.1002/ajpa.23455
Sasaki T., Danczak L., Thompson B., Morshed T., and Pratt S. C. (2020). Route learning during tandem running in the rock ant Temnothorax albipennis. J. Exp. Biol. 223:jeb221408. doi: 10.1242/jeb.221408
Sawyer H., LeBeau C. W., McDonald T. L., Xu W., and Middleton A. D. (2019a). All routes are not created equal: An ungulate’s choice of migration route can influence its survival. J. Appl. Ecol. 56, 1860–1869. doi: 10.1111/1365-2664.13445
Sawyer H., Merkle J. A., Middleton A. D., Dwinnell S. P. H., and Monteith K. L. (2019b). Migratory plasticity is not ubiquitous among large herbivores. J. Anim. Ecol. 88, 450–460. doi: 10.1111/1365-2656.12926
Squires J. R., DeCesare N. J., Olson L. E., Kolbe J. A., Hebblewhite M., and Parks S. A. (2013). Combining resource selection and movement behavior to predict corridors for Canada lynx at their southern range periphery. Biol. Conserv. 157, 187–195. doi: 10.1016/j.biocon.2012.07.018
Stalmans M. and Beilfuss R. (2008). “Landscapes of the gorongosa national park,” in Landscapes of the Gorongosa National Park. Technical Report. Available at: https://www.researchgate.net/publication/314878798_Landscapes_of_the_Gorongosa_National_Park (Accessed June 30, 2024).
Stalmans M., Peel M., and Goncalves D. (2018). Aerial wildlife count of the Parque Nacional da Gorongosa. https://gorongosa.org/wp-content/uploads/2020/08/gorongosaaerialwildlifecount2018_report_december2018.pdf (Accessed June 30, 2024).
Stelzner J. K. (1988). Thermal effects on movement patterns of yellow baboons. Primates 29, 91–105. doi: 10.1007/BF02380852
Stone O. M., Laffan S. W., Curnoe D., and Herries A. I. (2013). The spatial distribution of Chacma baboon (Papio ursinus) habitat based on an environmental envelope model. Int. J. Primatology 34, 407–422. doi: 10.1007/s10764-013-9669-9
Strandburg-Peshkin A., Farine D. R., Couzin I. D., and Crofoot M. C. (2015). Shared decision-making drives collective movement in wild baboons. Science 348, 1358–1361. doi: 10.1126/science.aaa5099
Tinley K. (1977). Framework of the Gorongosa Ecosystem (University of Pretoria:Pretoria). Available at: http://hdl.handle.net/2263/24526.
Keywords: route reuse, GIS analysis, chacma baboon, movement, memory
Citation: Lewis-Bevan L, Hammond P, Carvalho S and Biro D (2025) Baboon route repetition in a seasonal environment. Front. Ecol. Evol. 13:1571302. doi: 10.3389/fevo.2025.1571302
Received: 05 February 2025; Accepted: 27 May 2025;
Published: 09 July 2025.
Edited by:
Antoine Souron, UMR5199 De la Prehistoire a l’Actuel: Culture, Environnement et Anthropologie (PACEA), FranceReviewed by:
Anshuman Swain, University of Michigan, United StatesJuliana M. Berbert, Federal University of ABC, Brazil
Copyright © 2025 Lewis-Bevan, Hammond, Carvalho and Biro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lynn Lewis-Bevan, bHlubmxld2lzYmV2YW5AZ21haWwuY29t
†These authors have contributed equally to this work and share senior authorship
 Lynn Lewis-Bevan
Lynn Lewis-Bevan Philippa Hammond
Philippa Hammond Susana Carvalho
Susana Carvalho Dora Biro
Dora Biro