- Key Laboratory of Subtropical Medicinal Edible Resources Development and Utilization in Yunnan Province, College of Biology and Chemistry, Pu'er University, Pu'er, Yunnan, China
Introduction: This study aims to explore the reproductive characteristics and invasiveness of Crassocephalum rubens and Crassocephalum crepidioides, offering valuable insights for the future management and control of invasive plant species.
Methods: A series of experiments were conducted on the invasive plants C. rubens and C. crepidioides, including pollen viability determination, flowering dynamics observation, stigma receptivity tests, pollen-ovule ratio analysis, artificial bagging experiments, and seed germination tests.
Results: The results indicated that: (1) C. rubens had significantly more florets per inflorescence (246.1 ± 70) compared to C. crepidioides (154.6 ± 16.3), and a longer floral longevity (11.3 ± 0.9 days vs. 8.5 ± 1.6 days); (2) The P/O ratio of C. rubens was 1020.8 ± 398.57, indicating a facultative outcrossing system, while that of C. crepidioides was 240 ± 69.28, suggesting a facultative selfing system; (3) The pollen viability of C. rubens remained above 80% (84.45%-59.85%) between days 3 and 5 of flowering, whereas C. crepidioides reached 80.59% only on day 5, with a shorter duration; (4) Both species exhibited high seed set rates under natural conditions (C. rubens: 92.97%, C. crepidioides: 95.53%), with reproductive modes including autogamy and self-compatibility; (5) Germination rates were 93.9% for C. rubens and 97.9% for C. crepidioides, with C. crepidioides germinating earlier and surpassing 50% germination sooner.
Discussion: Both C. rubens and C. crepidioides possess traits such as self-compatibility, high seed set rates, high germination rates, and year-round growth, which enable them to rapidly establish and spread in new environments. While there is no significant difference in seed set or germination rates between the two species, C. rubens shows advantages in floral longevity and seed production. These factors may provide C. rubens with a stronger competitive edge in natural environments, potentially facilitating its dominance over C. crepidioides in certain conditions.
1 Introduction
Biological invasions have become an increasingly significant environmental issue in the 21st century. They directly impact the survival and development of native species and can have profound effects on ecosystems. Invasive species often disrupt ecological balance, threaten agriculture, forestry, and fisheries, and contribute to the loss of biodiversity (Diagne et al., 2021). Extensive research has been conducted on the biological characteristics, invasion mechanisms, and control strategies of invasive plants to monitor, manage, and prevent the spread of alien species (Kumar et al., 2024; Lorenzo and Morais, 2023). Reproduction plays a critical role in plant life history, directly influencing population growth and demography as well as ecosystem functioning, and can significantly affect the invasion process and successful establishment of alien plant species of alien plants (Daehler, 2003; Jiang et al., 2022; Pysek and Richardson, 2007). Therefore, studying the reproductive systems of invasive plants provides essential theoretical foundations for understanding the mechanisms behind plant invasions.
Numerous studies in China have investigated the reproductive characteristics of invasive plants in the Asteraceae family, including Xanthium spinosum, Bidens alba, Impatiens glandulifera, Bidens pilosa, and Tithonia diversifolia (Tao et al., 2022; Kato-Noguchi and Kurniadie, 2024; Lin et al., 2023; Ajao and Moteetee, 2017). Research has shown that the ability to reproduce both sexually and asexually, combined with a flexible mating system involving cross-pollination and self-pollination, are common features of successful invasive plants. This reproductive flexibility enables them to survive and propagate in harsh environments (Wang et al., 2022). Asteraceae plants often produce numerous small seeds, many with a pappus, which aids in their dispersal. These traits align with the reproductive characteristics of invasive plants, making Asteraceae species some of the most significant ecological threats (Sun et al., 2022).
Crassocephalum rubens and Crassocephalum crepidioides are both alien species for China in the Asteraceae family, belonging to the genus Crassocephalum. They primarily grow in barren hillsides and along rural roadsides (Rojas-Sandoval and Acevedo-Rodríguez, 2022; Gurung et al., 2022). A prior survey in Pu’er City revealed that both species share similar ecological preferences and survival conditions, often co-occurring, with C. rubens having a larger population than C. crepidioides. As invasive species, it is important to explore whether differences exist in their reproductive traits and invasiveness. This study focuses on C. rubens and C. crepidioides found along the roadsides of barren fields in Simao District, Pu’er City. A series of experiments, including flowering dynamics observation (Wan et al., 2024), pollen-to-ovule ratio determination (Nepal et al., 2023), pollen viability testing (Nepal et al., 2023), stigma receptivity assessment (Wang et al., 2012), controlled bagging experiments (Koltunnow, 1993), and germination tests (Reed et al., 2022), were conducted to understand their reproductive characteristics and analyze their relationship with invasiveness. The aim of this experiment is to explore the differences in reproductive traits between these two closely related invasive plant species. By analyzing these reproductive characteristics, we aim to assess their invasive potential and predict the likely competitive dynamics and future trends between the two species.
2 Materials and methods
2.1 Overview of the study area and materials
The experiment was conducted at the edge of abandoned tea plantations around Xima Lake Wetland Park in Nanping Town, Simao District, Pu’er City, Yunnan Province. The study area is located at an altitude of 1,298–1,315 meters, with geographic coordinates of 22°46’12.28″N and 101°00’64″E. The region experiences an annual average temperature ranging from 15°C to 20°C, with approximately 315 frost-free days each year. The experiment was conducted from October to December 2021. The study focused on C. rubens and C. crepidioides from the same community. Fieldwork included controlled bagging and flowering dynamics observations, while lab analyses covered pollen-to-ovule ratios, pollen viability, and stigma receptivity. This area has a typical subtropical climate, providing favorable conditions for plant reproduction and ecological studies.
2.2 Experimental methods
2.2.1 Observation of flowering dynamics
The method for observing flowering dynamics follows the definition outlined by Burtt (1961). Flowering onset is marked by the unfolding of the involucral bracts and the opening of the first row of florets on the periphery of the inflorescence. The flowering period concludes when the central florets of the inflorescence fully open. This approach allows for accurate recording of flowering duration, the number of florets, and other relevant parameters. Five 5m × 5m plots were set up, each containing both plant species. Within each plot, two individuals of C. rubens and two individuals of C. crepidioides were randomly chosen, resulting in a total of 10 C. rubens and 10 C. crepidioides plants selected for the study. From each plant, six inflorescences were randomly chosen for observation, and flowering periods, along with other associated parameters, were recorded.
2.2.2 Pollen-to-ovule ratio
The pollen-to-ovule ratio (P/O) was determined using the classical pollen count method, following the procedure outlined by Nepal et al. (2023). Twenty inflorescences from 10 plants of C. rubens were randomly selected, collected, and fixed with FAA. Ten florets with unopened anthers were chosen, and the anthers were extracted and processed using a centrifuge. Pollen grains were observed under a microscope using a glycerol-lactic acid solution (3:1). To determine the ovule count, unopened inflorescences were randomly selected from different plants. All florets except one were removed from each capitulum using forceps, and the number of seeds produced by the remaining floret was recorded. The pollen and ovule counts were then used to calculate the P/O ratio, which was used to infer reproductive systems according to Cruden (1976):
● P/O of 2.7–5.4: Cleistogamous
● P/O of 18.1–39.0: Obligate selfing
● P/O of 39.1–396.0: Facultative selfing
● P/O of 244.7–2588.0: Facultative outcrossing
● P/O of 2108.0–195525.0: Obligate outcrossing
The method for determining the P/O ratio in C. crepidioides was identical to that used for C. rubens.
2.2.3 Pollen viability
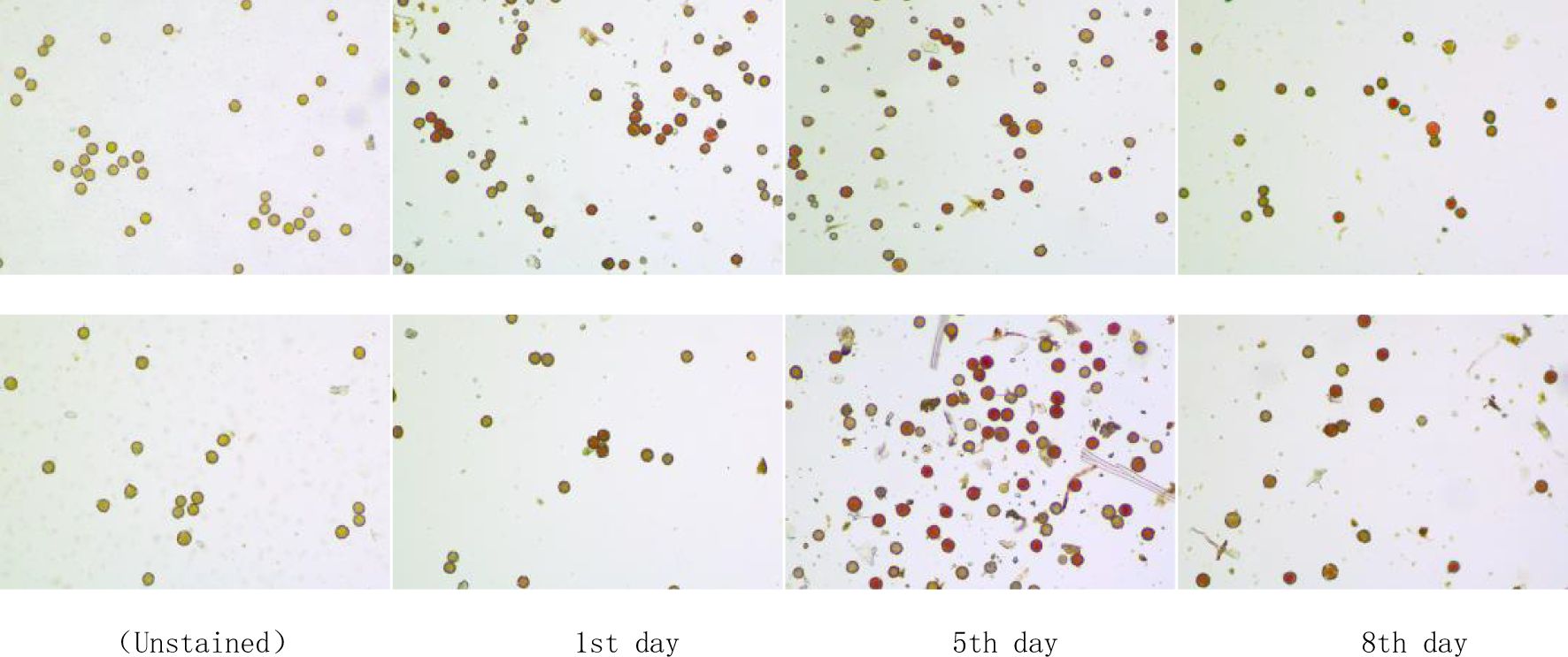
Pollen viability was assessed using the TTC(Triphenyl Tetrazolium Chloride) staining method, which is widely used for evaluating pollen viability (Luo et al., 2020). The TTC solution reduces inactive pollen to red-stained pollen, with the intensity of staining being proportional to pollen viability. Forty unopened inflorescences were randomly selected from 10 plants of C. rubens and C. crepidioides. From days 1 to 8 post-flowering, five inflorescences from each species were collected daily. Pollen smears were prepared on slides, and 0.5% TTC solution was applied. The slides were then incubated at 37°C for 30–60 minutes. The staining rate (%, the ratio of red-stained pollen grains to the total pollen count in the field of view.) was observed and recorded under a microscope, with the pollen staining rate representing viability. Each inflorescence was set up with three replicates, ensuring that the number of pollen grains counted per replicate was no fewer than 200 (except when pollen grain dispersal was low during the late flowering stage) (Breygina et al., 2021).
2.2.4 Stigma receptivity
Stigma receptivity was assessed using the decolorized aniline blue method, which effectively measures pollen tube germination ability. Forty-eight unopened inflorescences were randomly selected and marked with sulfuric acid paper bags. During the experiment, six inflorescences were collected daily and fixed in FAA. Each inflorescence was softened with 5 mol·L−1 NaOH, rinsed with distilled water, and separated into individual florets. Stigmas from 20–30 florets were isolated and stained with decolorized aniline blue. The number of germinated pollen tubes was then observed and recorded under a microscope (Wang et al., 2012).
2.2.5 Artificial controlled bagging experiment
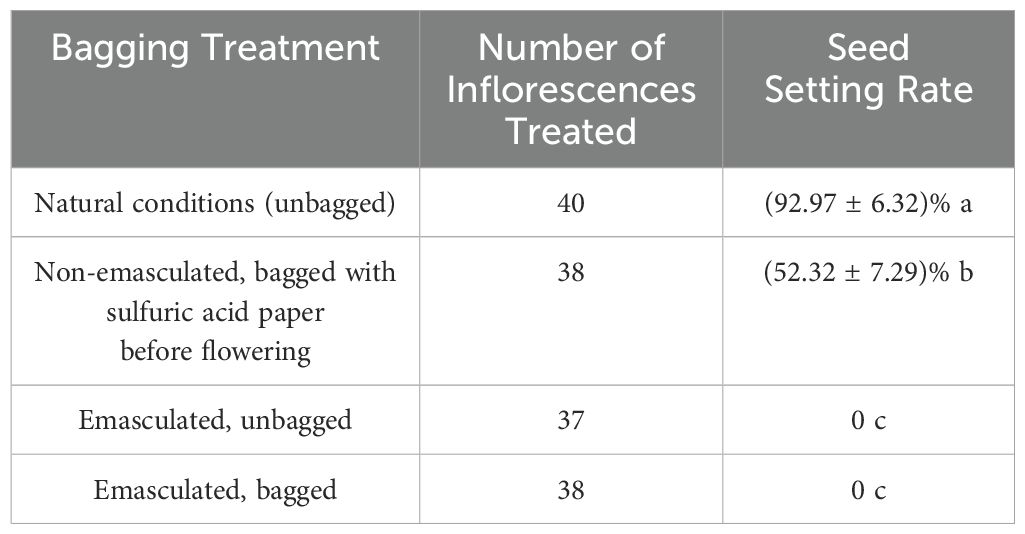
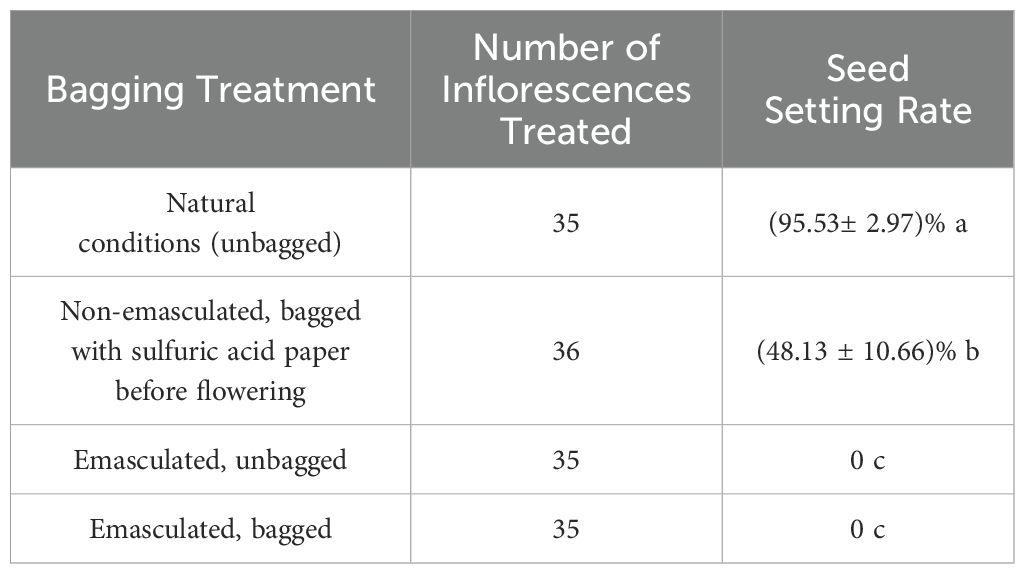
Ten plants of each species were randomly selected, and four uniformly developed inflorescences per plant were chosen, marked, and grouped into 10 replicates (40 inflorescences in total). The treatments were as follows:
1. Natural opening (control).
2. Unemasculated, bagged before flowering to test for self-compatibility.
3. Emasculated, unbagged to test for cross-compatibility.
4. Emasculated and bagged to test for apomixis.
Emasculation followed the method of Koltunnow (1993), where all anthers and stigmas were removed before flowering, leaving only some ovaries and styles. Mature seeds were collected, counted, and used to calculate seed set rates.
2.2.6 Seed collection and germination experiment
Seeds from each species were collected from at least 20-30 plants, cleaned of impurities, and thoroughly mixed to reduce individual variations. They were then stored in envelopes for future use. A random sample of 100 dry seeds was weighed to the nearest 0.0001 g, with three replicates, and the average was calculated as the hundred-seed weight. The seed germination experiment followed the standard protocol outlined by Reed et al. (2022). The experiment was conducted under alternating temperatures of 18/25°C with 12-hour photo-period. Each treatment consisted of three replicates, each containing 50 seeds, evenly placed on two layers of filter paper in 100 mm Petri dishes. The dishes were placed in an incubator, with distilled water added daily to maintain moisture on the filter paper. Germination was recorded every 24 hours, and seeds protruded radicle were being counted and removed. The main germination index is the germination rate, which refers to the total germination percentage (%) at the end of the germination process.
2.3 Statistical methods
To ensure compliance with the assumption of normality, the number of florets per inflorescence was log-transformed. Data on pollen viability, pollen germination rate, seed set rate, and seed weight were confirmed to follow a normal distribution. The Least Significant Difference (LSD) test was employed to evaluate the significance of differences among species across various treatment conditions. All statistical analyses were conducted using SPSS 16.0. Different lowercase letters indicate significant differences between groups (P < 0.05), while the same letters indicate no significant differences (P > 0.05).
3 Results and analysis
3.1 Flowering dynamics observation
Both C. rubens and C. crepidioides were observed to blossom and bear fruit throughout the year in the study area.
● C. rubens: Flowers are blue, with capitulum inflorescences containing 11 ± 3 whorls and 246.1 ± 70 florets per inflorescence. The outermost whorl of florets blooms first, with 1–2 whorls blooming daily until all florets bloom by the 7th day. From the 9th day, peripheral flowers start wilting, and by the 12th day, all florets have wilted and fallen. The flowering period lasts approximately 11.3 ± 0.9 days.
● C. crepidioides: Flowers are brick-red, with capitulum inflorescences containing 9 ± 2 whorls and 154.6 ± 16.3 florets per inflorescence. Blooming follows a similar pattern, with 1–2 whorls blooming daily. Full blooming occurs by the 4th day, peripheral flowers start wilting on the 7th day, and all florets wilt and fall by the 10th day. The flowering period lasts about 8.5 ± 1.6 days.
In summary, the number of florets per inflorescence in C. rubens (246.1 ± 70) was significantly higher than that in C. crepidioides (154.6 ± 16.3)(P<0.001), and its flowering period (11.3 ± 0.9 days) was also longer than that of C. crepidioides (8.5 ± 1.6 days).
3.2 Pollen-ovule ratio
Significant differences in pollen count and mating system were observed between the two species:
● C. rubens: Each floret contains 625–1750 pollen grains, averaging 1020.8 ± 398.57 grains per floret. With only one ovule per floret, the P/O ratio is 1020.8 ± 398.57, classifying it as a facultative outcrossing species according to Cruden’s standard.
● C. crepidioides: Each floret contains 120–360 pollen grains, averaging 240 ± 69.28 grains per floret. With one ovule per floret, the P/O ratio is 240 ± 69.28, classifying it as a facultative selfing species according to Cruden’s standard.
3.3 Pollen viability
The pollen viability of the two species showed distinct patterns over their flowering periods:
● C. rubens: The pollen viability of C. rubens exhibited a dynamic pattern throughout the flowering period. On the first day of flowering, pollen viability was relatively low at 46.37%. However, it increased significantly to 77.27% by the second day and reached its peak at 89.85% on the third day. From the third to the fifth day, pollen viability remained consistently high, exceeding 84%. Starting on the sixth day, a gradual decline in viability was observed, with values decreasing to 66.14%, 33.52%, and 28.64% on the sixth, seventh, and eighth days, respectively (Figures 1, 2).
● C. crepidioides: Pollen viability was only 8.39% on the first day of flowering, with little increase initially. On the second and third days, viability remained low, at 20.95% and 24.87%, respectively. A significant increase occurred on the fourth day, reaching 67.27%, and it peaked at 80.59% on the fifth day. However, viability declined sharply thereafter, dropping to 37.58% by the eighth day (Figures 1, 2).
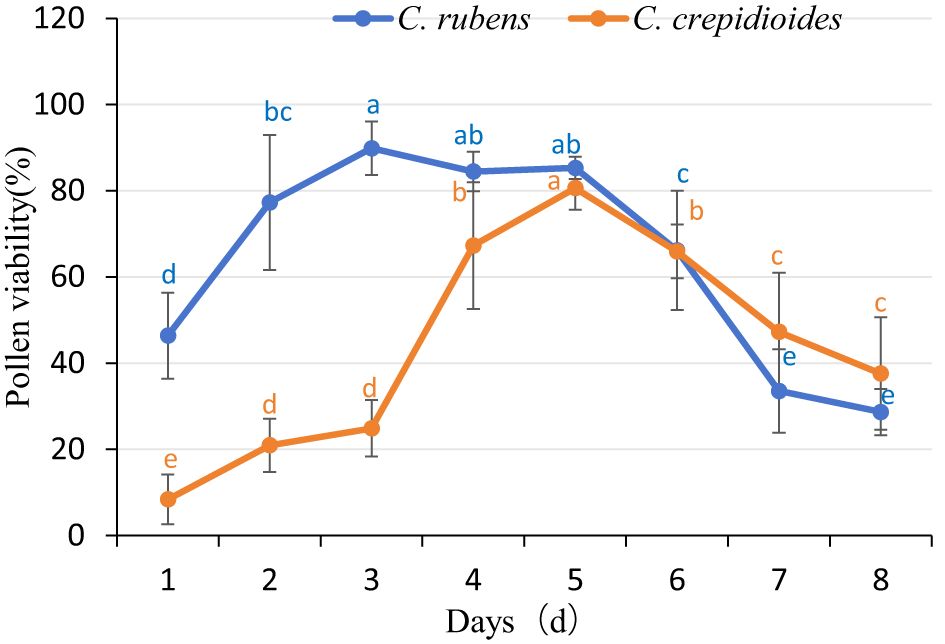
Figure 1. Pollen viability of C. rubens and C. crepidioides. Intra-species comparison of different treatment times: Different lowercase letters denote a significant difference between groups (P < 0.05), while the same letter indicates no significant difference (P > 0.05), the same applies below.
Overall, the first-day pollen viability of C. rubens was significantly higher than that of C. crepidioides, and the duration of pollen viability exceeding 80% was longer in C. rubens.
3.4 Stigma receptivity
Stigma receptivity tests revealed differences between the two species:
● C. rubens: The highest number of germinated pollen grains on the stigma’s receptive area was observed on the second day (13 grains), indicating peak receptivity. Between the third and sixth days, the number remained around 4 grains, but by the seventh and eighth days, it decreased to 1 grain (Figure 3).
● C. crepidioides: Receptivity was relatively weaker, peaking on the third day, and began declining from the fourth day. The number of germinated pollen grains remained at approximately 1 grain between the fourth and eighth days (Figure 3).
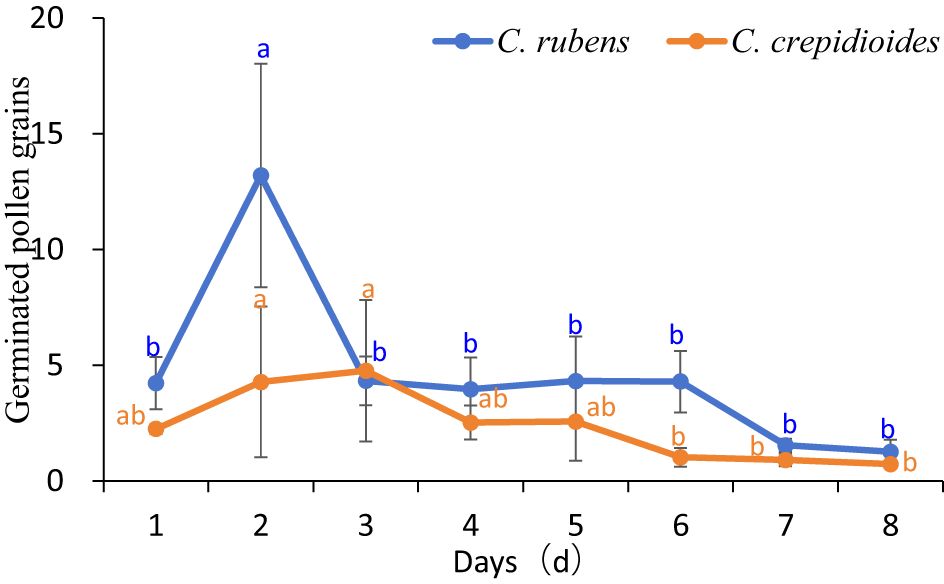
Figure 3. Change of germinated pollen numbers on the receptive area of stigma of C. rubens and C. crepidioides.
3.5 Controlled pollination bagging experiment
Under natural conditions, the fruit set rates of C. rubens and C. crepidioides were 92.97% and 95.53%, respectively, significantly higher than those observed in the non-emasculated, bagged treatment prior to flowering (52.32% and 48.13%, respectively; P < 0.001). In contrast, both the emasculated, unbagged and emasculated, bagged treatments resulted in a fruit set rate of 0%, demonstrating the absence of spontaneous apomixis in C. rubens and C. crepidioides. These findings indicate that both species are self-compatible and partially rely on self-fertilization for reproductive success (Tables 1, 2).
3.6 Germination characteristics
● Seed weight: The seeds of C. rubens (0.263 ± 0.009 mg/seed) were significantly heavier than those of C. crepidioides (0.195 ± 0.006 mg/seed)(P< 0.001).
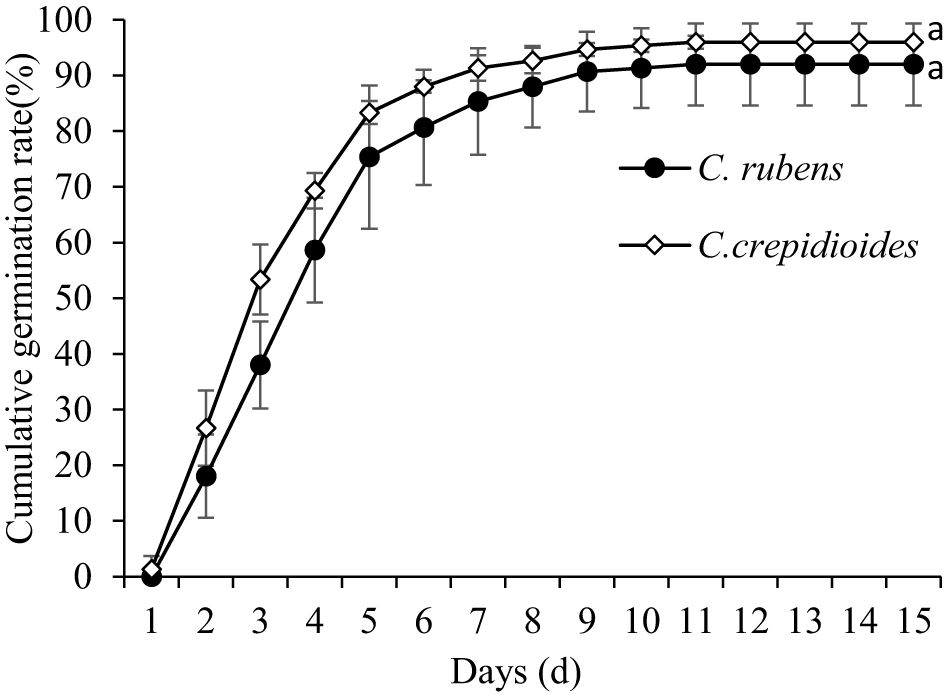
● Germination rate and process: C. rubens had a germination rate of 93.9%, starting on the second day, exceeding 50% by the fifth day, and completing germination in 11 days. C. crepidioides had a slightly higher germination rate of 97.9%, starting on the first day, exceeding 50% by the third day, and completing germination in 12 days (Figure 4).
Overall, C. crepidioides exhibited a slightly higher cumulative germination rate compared to C. rubens at the same time point (p > 0.05). Additionally, C. crepidioides not only initiated germination earlier but also reached 50% germination more rapidly than C. rubens.
4 Discussion
The reproductive traits of invasive plants play a critical role in determining their colonization and establishment in novel environments. This study investigates and compares the reproductive characteristics and invasive potential of C. rubens and C. crepidioides, elucidating the differences between these two species in terms of mating systems, seed production, and ecological adaptability, thereby uncovering the biological mechanisms underlying their successful invasion.
4.1 Flowering dynamics and adaptive advantages of reproductive strategies
C. rubens has significantly more florets per inflorescence (246.1 ± 70 vs. 154.6 ± 16.3) and a longer flowering period (11.3 ± 0.9 d vs. 8.5 ± 1.6 d), giving it a reproductive advantage. A large body of research indicates that longer flowering periods can effectively increase pollination chances and gene flow between populations (Elzinga et al., 2007; Kehrberger and Holzschuh, 2019; He et al., 2020). The extension of flowering time emerges as a critical adaptive response in environments characterized by unstable pollinator populations or resource constraints, as it directly addresses pollen limitation challenges and promotes ecological adaptation in heterogeneous settings (Kehrberger and Holzschuh, 2019; Pan et al., 2017). Furthermore, the synchronization of C. rubens flowering dynamics with pollinator activity patterns may further enhance its reproductive success, a mechanism widely observed in many invasive species (Rodríguez-Pérez and Traveset, 2016).
4.2 Pollen-to-ovule ratio and flexibility of mating systems
The higher P/O ratio of C. rubens (1020.8 ± 398.57) indicates that its mating system is biased toward facultative outcrossing, while the lower P/O ratio of C. crepidioides (240 ± 69.28) suggests a predominantly facultative selfing system. This difference reflects significant reproductive strategy variation between the two species:
● C. rubens: Facultative outcrossing provides greater genetic diversity, which is important for adapting to environmental changes and competition (Pujolar et al., 2023; Schierenbeck and Ellstran, 2008). This characteristic enhances its spread potential in areas with high environmental pressures, such as resource-scarce or highly competitive ecosystems.
● C. crepidioides: Facultative selfing helps ensure reproductive success in the absence of pollinators, a mechanism that facilitates the rapid establishment of populations in novel habitats (Zhang and Bockhoff, 2019; Vega-Polanco et al., 2023). Although long-term selfing provides short-term reproductive assurance under certain conditions, such as pollinator scarcity, it ultimately leads to reduced genetic diversity, decreased adaptability, and increased risks to population dynamics. Therefore, facultative outcrossing, which strikes a balance between selfing and outcrossing, is generally considered a more advantageous reproductive strategy for maintaining genetic diversity and adaptability (Goodwillie et al., 2005).
4.3 Pollen viability and stigma receptivity competitiveness
C. rubens maintains pollen viability above 80% between days 3–5 of flowering, while C. crepidioides maintains high viability for only one day (day 5). This significant difference directly impacts the reproductive success of the two species:
● Longer periods of high pollen viability provide C. rubens with a substantial advantage in outcrossing fertilization, especially when pollinator visitation frequency is low, ensuring fertilization success (Vanbergen et al., 2017; Kehrberger and Holzschuh, 2019).
● Stigma receptivity experiments show that C. rubens reaches peak receptivity on day 2 of flowering, while C. crepidioides peaks on day 3 and rapidly declines. This further underscores the adaptive flexibility of Crassocephalum rubens in its reproductive phenology, enabling more efficient exploitation of pollinators (Helsen et al., 2020).
4.4 Seed set and reproductive advantage of seed traits
Both C. rubens and C. crepidioides exhibit high seed set rates (92.97% and 95.53%, respectively), indicating strong reproductive capability. However, the seed weight of C. rubens is significantly higher than that of C. crepidioides (0.263 ± 0.009 mg/seed vs. 0.195 ± 0.006 mg/seed), suggesting a seed quality advantage that may result in higher germination rates and seedling survival (Zhang et al., 2019). Although C. crepidioides has a slightly higher germination rate (97.9% vs. 93.9%) and its seeds germinate earlier, with 50% germination occurring sooner than in C. rubens, the slower germination of C. rubens may help stabilize the population in competitive conditions over the long term (Shariatinia et al., 2021).
4.5 Comprehensive analysis of invasion potential
Both C. rubens and C. crepidioides exhibit high reproductive adaptability and ecological expansion capacity. Their self-compatibility degree, high seed set rates, and year-round growth provide important support for their rapid spread in novel habitats. However, C. rubens has a clear advantage in resource competition and environmental adaptability due to its longer floral longevity, higher pollen viability, longer receptivity period, and larger seed mass. While their invasion potential may be lower than that of other invasive Asteraceae species like Ambrosia artemisiifolia and Bidens pilosa, C. rubens is clearly a species requiring closer attention and management. In the future, we should further monitor the populations of both plant species, conduct risk assessments and early warnings, strengthen preventive measures, and develop emergency control technologies to implement effective strategies for their containment and eradication.
5 Conclusion
Both C. rubens and C. crepidioides possess traits such as self-compatibility, high seed set rates, high germination rates, and year-round growth, which enable rapid establishment and spread in new environments. Although there is no significant difference in seed set or germination rates between the two, C. rubens exhibits advantages in floral longevity and seed production, potentially giving it a stronger competitive edge in natural environments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
XLC: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. YTL: Methodology, Supervision, Validation, Writing – review & editing. YLL: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China under Grant (number 32360306) and the Yunnan Provincial Department of Science and Technology Local Universities Joint Special Project - General Program (number202401BA070001-125).
Acknowledgments
We thank Xu Linhui and Wan Yu for her valuable contribution to this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We applied AI to translate Chinese into English and made language polishing.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajao A. A., Moteetee A. (2017). Tithonia diversifolia (Hemsl) A Gray (Asteraceae: Heliantheae), an invasive plant of significant ethnopharmacological importance: A review. South Afr. J. Bot. 113, 396–403. doi: 10.1016/j.sajb.2017.09.017
Breygina M., Klimenko E., Schekaleva O. (2021). Pollen germination and pollen tube growth in gymnosperms. Plants 10, 1301. doi: 10.3390/plants10071301
Burtt B. L. (1961). The structure and function of the inflorescence in Asteraceae. Phytomorphology 11, 221–234. doi: 10.1016/B978-0-12-417162-6.00010-9
Cruden R. W. (1976). Intraspecific variation in pollen-ovule ratios and nectar secretion -preliminary evidence of ecotypic variation. Ann. Missouri Botanic Garden 63, 277–289. doi: 10.2307/2395306
Daehler C. (2003). Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 34, 183–211. doi: 10.1146/annurev.ecolsys.34.011802.132403
Diagne C., Leroy B., Vaissière A. C., Gozlan R. E., Roiz D., Jarić I., et al. (2021). High and rising economic costs of biological invasions worldwide. Nature 592, 571–576. doi: 10.1038/s41586-021-03405-6
Elzinga J. A., Atlan A., Biere A., Gigord L. D. B., Weis A. E., Bernasconi G. (2007). Time after time: flowering phenology and biotic interactions. Trends Ecol. Evol. 22, 432–439. doi: 10.1016/j.tree.2007.05.006
Goodwillie C., Kalisz S., Eckert C. G. (2005). The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 36, 47–79. doi: 10.1146/annurev.ecolsys
Gurung S., Poudel P., Adhikari N., Lamichhane G., Thapa R. (2022). "Crassocephalum crepidioides (Benth.) S. Moore: Traditional uses, chemical constituents, and biological activities.", in Medicinal Plants of the Asteraceae Family, eds. H. P. Devkota, and T. Aftab. (Singapore: Springer), 145–152. doi: 10.1007/978-981-19-6080-2_9
He Y. H., Chen T., Zeng X. L. (2020). Genetic and epigenetic understanding of the seasonal timing of flowering. Plant Commun. 1, 100008. doi: 10.1016/j.xplc.2019.100008
Helsen K., Acharya K. P., Graae B. J., Kort H. D., Brunet J., Chabrerie O., et al. (2020). Earlier onset of flowering and increased reproductive allocation of an annual invasive plant in the north of its novel range. Ann. Bot. 126, 1005–1016. doi: 10.1093/aob/mcaa110
Jiang M., Ma M., Lin H., Ma X. (2022). Reproductive strategies of Xanthium italicum differ from those of native Xanthium sibiricum, and they are key to its invasiveness. Plant Ecol. 223, 453–463. doi: 10.1007/s11258-021-01219-0
Kato-Noguchi H., Kurniadie D. (2024). The invasive mechanisms of the noxious alien plant species Bidens pilosa. Plants (Basel) 13, 356. doi: 10.3390/plants13030356
Kehrberger S., Holzschuh A. (2019). How does timing of flowering affect competition for pollinators, flower visitation and seed set in an early spring grassland plant? Sci. Rep. 9, 4742–4742. doi: 10.1038/s41598-019-41185-7
Koltunnow A. M. (1993). Apomixis: Embryo Sacs and Embryos Formed without Meiosis or Fertilization in Ovules. The Plant Cell 5, 1425–1437. doi: 10.1105/tpc.5.10.1425
Kumar A., Singh S., Kumar D., Singh R. K., Gupta A. K., Premkumar K., et al. (2024). Investigating the phenology and interactions of competitive plant species co-occurring with invasive Lantana camara in Indian Himalayan Region. Sci. Rep. 14, 400. doi: 10.1038/s41598-023-50287-x
Lin H. Y., Chen L. X., Li J. M. (2023). Multiple introductions and distinct genetic groups of Canada Goldenrod (Solidago canadensis) in China revealed by genomic single-nucleotide polymorphisms. Plants 12, 1734. doi: 10.3390/plants12091734
Lorenzo P., Morais M. C. (2023). Strategies for the management of aggressive invasive plant species. Plants (Basel Switzerland) 12, 2482. doi: 10.3390/plants12132482
Luo S., Zhang K., Zhong W. P., Chen P., Fan X. M., Yuan D. Y. (2020). Optimization of in vitro pollen germination and pollen viability tests for Castanea mollissima and Castanea henryi. Scientia Hortic. 271, 109481. doi: 10.1016/j.scienta.2020.109481
Nepal S., Trunschke J., Ren Z. X., Burgess K., Wang H. (2023). Community-wide patterns in pollen and ovule production, their ratio (P/O), and other floral traits along an elevation gradient in southwestern China. BMC Plant Biol. 23, 425. doi: 10.1186/s12870-023-04433-2
Pan C. C., Feng Q., Zhao H., Liu L. D., Li Y. L., Li Y. Q., et al. (2017). Earlier flowering did not alter pollen limitation in an early flowering shrub under short-term experimental warming. Sci. Rep. 7, 2795. doi: 10.1038/s41598-017-03037-9
Pujolar J. M., Breitburg D. L., Lee J., Decker M. B., Jaspers C. (2023). Hybridization and adaptive introgression in a marine invasive species in native habitats. iScience 26, 108430. doi: 10.1016/j.isci.2023.108430
Pysek P., Richardson D. M. (2007). “Traits associated with invasiveness in alien plants: Where do we stand?,” in Biological Invasions, Section II. Ed. Nentwig W. (Verlag-Springer, Berlin), 97–125.
Reed R., Bradford K. J., Khanday I. (2022). Seed germination and vigor: ensuring crop sustainability in a changing climate. Heredity 128, 450–459. doi: 10.1038/s41437-022-00497-2
Rodríguez-Pérez J., Traveset A. (2016). Effects of flowering phenology and synchrony on the reproductive success of a long-flowering shrub. AoB Plants 8, plw007. doi: 10.1093/aobpla/plw007
Rojas-Sandoval J., Acevedo-Rodríguez P. (2022). Crassocephalum crepidioides (redflower ragleaf). CABI Compendium. 15870. doi: 10.1079/cabicompendium.15870
Schierenbeck K. A., Ellstran N. C. (2008). Hybridization and the evolution of invasiveness in plants and other organisms. Biol. Invasions 11, 1093–1105. doi: 10.1007/s10530-008-9388-x
Shariatinia F., Azari A., Rahimi A., Panahi B., Madahhosseini S. (2021). Germination, growth, and yield of rocket populations show strong ecotypic variation under NaCl stress. Scientia Hortic. 278, 109841. doi: 10.1016/j.scienta.2020.109841
Sun D. S., Yang X. P., Wang Y., Fan Y., Ding P. C., Song X. E., et al. (2022). Stronger mutualistic interactions with arbuscular mycorrhizal fungi help Asteraceae invaders outcompete the phylogenetically related natives. New Phytol. 236, 1487–1496. doi: 10.1111/nph.18435
Tao Y. Y., Shang T. C., Yan J. J., Hu Y. X., Zhao Y., Liu Y., et al. (2022). Effects of sand burial depth on Xanthium spinosum seed germination and seedling growth. BMC Plant Biol. 22, 43. doi: 10.1186/s12870-022-03424-z
Vanbergen A. J., Espíndola A., Aizen M. A. (2017). Risks to pollinators and pollination from invasive alien species. Nat. Ecol. Evol. 2, 16–25. doi: 10.1038/s41559-017-0412-3
Vega-Polanco M., Solís-Montero L., Vallejo-Marín M., Arévalo-Monterrubio L. D., García- Crisóstomo J. F. (2023). Reproductive strategy of an invasive buzz-pollinated plant (Solanum rostratum). South Afr. J. Bot. 162, 342–352. doi: 10.1016/j.sajb.2023.09.020
Wan X., Sun D., Gao C. (2024). Flower opening dynamics, pollen-ovule ratio, stigma receptivity and stigmatic pollen germination (in-vivo) in Chaenomeles speciosa (Sweet) Nakai. Sci. Rep. 14, 7127. doi: 10.1038/s41598-024-57655-1
Wang X., Wang J., Hu B., Zheng W. L., Li M., Shen Z. X., et al. (2022). Richness, not evenness, of invasive plant species promotes invasion success into native plant communities via selection effects. Oikos 6, e08966. doi: 10.1111/oik.08966
Wang Y. L., Guan Z. Y., Chen F. D., Fang W. M., Teng N. J. (2012). Pollen viability, pistil receptivity, and embryo development in hybridization of Nelumbo nucifera Gaertn. Sci. World J. 2012, 1–8. doi: 10.1100/2012/678706
Zhang B., Bockhoff R. C. (2019). Sex-differential reproduction success and selection on floral traits in gynodioecious Salvia pratensis. BMC Plant Biol. 19, 375. doi: 10.1186/s12870-019-1972-y
Keywords: invasive plants, floral longevity, mating system, germination, pollen vigor
Citation: Cui X, Luo Y and Luo Y (2025) A study on the reproductive characteristics and invasiveness of Crassocephalum rubens and Crassocephalum crepidioides. Front. Ecol. Evol. 13:1571992. doi: 10.3389/fevo.2025.1571992
Received: 06 February 2025; Accepted: 03 April 2025;
Published: 30 April 2025.
Edited by:
Spyros Tsiftsis, Democritus University of Thrace, GreeceReviewed by:
Georgios Varsamis, Democritus University of Thrace, GreeceAnush Nersesyan, The Institute of Botany of the National Academy of Sciences of the Republic of Armenia, Armenia
Copyright © 2025 Cui, Luo and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yating Luo, NDExNzUyMzgxQHFxLmNvbQ==; Yinling Luo, NjcwNTAzNDNAcXEuY29t
 Xianliang Cui
Xianliang Cui Yating Luo*
Yating Luo*