- 1Desenvolvimento Sustentável, Instituto Tecnológico Vale, Belém, Pará, Brazil
- 2Diretoria de Licenciamento Ambiental/Gerência de Estudos Técnicos de Longo Prazo, Vale S.A., Parauapebas, Pará, Brazil
- 3Ecology Department, Biosciences Institute, São Paulo University, São Paulo, Brazil
- 4Oak Park Crops Research Centre, Teagasc, Carlow, Ireland
Current studies of animal–plant mutualistic interaction networks and species climate change resilience call for redesigning biodiversity conservation management toward preventing species coextinction cascades and using interspecific hybridization as a species conservation tool. The upgrade of conservation management is urgent for narrow endemic plant species highly vulnerable to habitat destruction and defaunation. Ipomoea cavalcantei is a red-flowered, self-incompatible, narrow endemic morning glory confined to Amazon savanna-like ecosystems known as canga. Mining cangas reduces I. cavalcantei range, population sizes, and standing phenotypic variation. Here, we advance our understanding of the pollinator network that sustains I. cavalcantei reproductive success and interspecific gene flow. We show that ello sphinx, Erinnyis ello, is a new flower visitor in our model foraging nectar on I. cavalcantei and sister species Ipomoea marabaensis in cangas. We describe legitimate visiting of I. marabaensis flowers by the long-billed starthroat hummingbird, Heliomaster longirostris. On artificial flower displays, hawkmoths and hummingbirds readily foraged on the magenta-colored flowers of I. cavalcantei × I. marabaensis natural hybrids. Thus, a new pollinator, the ello sphinx, and previously unknown Ipomoea–hummingbird interactions may sustain interspecific gene flow that could enhance the species’ adaptive potential and be considered a conservation tool. Our results suggest that the overall reproductive success of I. cavalcantei is likely dependent on the long-billed hummingbird species. To avoid functional extinction, e.g., reduced genetic diversity due to pollinator loss, conservation must include assessing and monitoring the abundance and richness of hummingbird species at fragments of the remaining historical range and new introduction sites.
1 Introduction
Global biodiversity is in decline (Butchart et al., 2010; Pimm et al., 2014), suggesting that the world has entered a sixth mass extinction event (Barnosky et al., 2011; Cowie et al., 2022), which represents the most serious environmental threat (Ceballos et al., 2020). Assessment of census extinctions, meaning where no individuals survive (Cronk, 2016), indicated that approximately 600 plant species have become extinct at a rate surpassing the background extinction rate (Humphreys et al., 2019). Meta-analyses estimated that extinction threatens approximately 39% of all vascular plant species (Lughadha et al., 2020), and many may be functionally extinct (Cronk, 2016). Among the drivers of species extinction, habitat destruction, particularly in humid tropical forests, is one of the primary causes (Pimm and Raven, 2000; Le Roux et al., 2019). Narrow endemic plant species are especially vulnerable to habitat destruction (Lavergne et al., 2004; Médail and Baumel, 2018).
The mutualistic interactions between plants and their animal pollinators and seed dispersers underpin much of Earth’s biodiversity (Bascompte and Jordano, 2007). These interactions create complex networks with a well-defined structure that contribute to the persistence of biodiversity (Bascompte et al., 2006; Gonzalez et al., 2011). Network analysis indicates that phylogenetic effects on interaction patterns could trigger coextinction cascades among related species (Rezende et al., 2007). Coextinction cascades from plants to animals can amplify the impacts of climate change (Schleuning et al., 2016). The ability of plants to adapt to climate change through range shifts facilitated by seed dispersal is compromised by 60% due to defaunation of mammals and birds (Fricke et al., 2022). Defaunation also diminishes pollen dispersal, thereby decreasing the genetic diversity of plant populations (Wessinger, 2021). This threat is more significant in bird-pollinated plants than in those pollinated by insects (Krauss et al., 2017). Thus, shifting the focus from species census to interaction networks is necessary to achieve pressing conservation management and restoration ecology goals for conserving biodiversity (Harvey et al., 2017).
Interspecific hybridization is a natural process with contrasting roles in evolution. On the one hand, hybridization can lead to species extinction by genetic and demographic swamping (Levin et al., 1996; Todesco et al., 2016). On the other hand, hybridization can generate novel intraspecific phenotypic variation (Schmickl et al, 2017), give rise to new species (Yakimowski and Rieseberg, 2014), facilitate genetic rescue and demographic recovery (Whiteley et al., 2015), and underpin adaptive introgression (Arnold and Kunte, 2017; Schmickl et al., 2017; Bock et al., 2018; Oziolor et al., 2019). The two side effects created a controversy in setting appropriate conservation policies to treat hybridization and introgression (Allendorf et al., 2001). The current loss of biodiversity raises the question of whether organisms will adapt in time to survive the current era of rapid environmental change. Today’s conservation biology, therefore, must consider hybridization as a conservation management tool that may enhance the adaptive potential and survival of the species (Chan et al., 2019; Quilodrán et al., 2020).
The most noticeable endemic plant in the Amazon canga ecosystems is Ipomoea cavalcantei, which belongs to the morning glory family Convolvulaceae (Figure 1A; Austin, 1981). I. cavalcantei is exclusively found in Brazil’s Carajás National Forest on five northern canga islands (Figure 1I; Babiychuk et al., 2017). Ipomoea marabaensis is a sister species inhabiting cangas with a broader distribution (Figures 1C, I; Austin and Secco, 1988). Although the two species primarily exhibit allopatric distribution, sympatry has been found in the N4 and N5 cangas (Figure 1I; Babiychuk et al., 2019). Both species display significant phenotypic variation and molecular diversity, suggesting that current populations are near the species’ center of origin (Figure 1B; Babiychuk et al., 2019). The economically valuable high-grade iron ore deposits beneath cangas drive mining operations in the Carajás National Forest. On the North Ridge of the Carajás National Forest, where the N4 and N5 mines are located, 45.6% of the canga vegetation was lost between 1973 and 2016 (Souza-Filho et al., 2019), whereas the allopatric cangas N1, N2, and N3 remain intact (Figures 1H–J; Supplementary Table S1). I. cavalcantei and I. marabaensis are both self-incompatible species with flowers that provide significant rewards for pollinators, averaging 64 ± 19 and 75 ± 13 μL of nectar, respectively. Therefore, their reproductive success and the maintenance of genetic variation depend entirely on pollen dispersal by pollinators. Additionally, I. cavalcantei and I. marabaensis readily hybridize, producing fertile F1 hybrids with magenta-colored flowers (Figure 1B). However, the pollinators of the magenta-colored hybrid flowers remain poorly understood (Babiychuk et al., 2019). Thus, canga conservation management must focus on a more thorough characterization and understanding of the plant–animal interaction network’s composition and functioning, shifting toward preserving the essential network properties that underpin biodiversity persistence and climate change resilience.

Figure 1. Plant species structuring plant–hummingbird interaction networks in canga and Ipomoea cavalcantei range. The red-colored I. cavalcantei (A) and pale lavender-colored Ipomoea marabaensis (C) are predominant flower types in canga ecosystems. (B) The flowers were harvested from plants rescued for ex situ collection that comprised white, pink, purple, and magenta colors, with the last flower type from natural interspecies hybrids. Cuphea annulata is a densely branched perennial shrub (D) that produces large masses of red-orange-colored, tubular flowers (E). A bromelia Dyckia duckei developed orange-colored inflorescence stalks (F) bearing orange-red tubular flowers (G). (I) The map illustrates the study locations. Dark green color is due to the rain forest that covers eroding Carajás Mountain range, the part of which is preserved within the Carajás National Forest. Canga savannas evolved on iron lateritic rocks of the mountain plateaus that are false-colored in Adobe Photoshop CS6 to emphasize the morning glory species distribution. The allopatric I. cavalcantei populations are found in cangas N1, N2, and N3, which are in red, according to the predominant flower color of the species. Lavender-colored cangas N6 to N9, Morro 1 (M1) and Morro 2 (M2), Tarzan (T), and S11 plateau (S11) host I. marabaensis allopatric populations. Magenta color of the cangas N4 and N5 fragments signifies species co-occurrence, i.e., sympatric cangas. Sossego (SO) is a granitic inselberg populated by I. marabaensis, where the species grows along the boundaries of exposed granitic bedrocks and the forest. The yellow dot corresponds to the location of the ex situ collection. Cangas are named in accordance with the geological survey maps. The geographic map was generated using the software QGIS version 2.18 (http://qgis.org) based on satellite imagery source (https://mt1.google.com/vt/lyrs%3Ds%26×%3D%7Bx%7D%26y%3D%7By%7D%26z%3D7Bz%7D&zmax=20&zmin=0) from Google (Google Maps satellite Carajás, Pará, Brazil; retrieved December 16, 2018). (H, J) Close-up satellite images downloaded from Google Earth Pro to compare mining exploration of cangas N4 and N5 in 1985 and 2024, respectively. The red bar is 1 km.
Contrasting flower trait suites, such as red versus pale lavender flower color and exerted versus inserted stamens and styles, indicated that I. cavalcantei is an ornithophile, a species pollinated by birds, while I. marabaensis is bee-pollinated, known as a mellitophile (Fenster et al., 2004). The initial analysis of flower visitor assemblages showed that several native animal species accessed I. cavalcantei flowers legitimately, i.e., through the corolla tube opening, suggesting a potential functional role as pollinators (Babiychuk et al., 2019). Stingless Trigona spp. bees were common, displaying two types of behavior: nectar robbing by chewing through sepals and corolla (illegitimate visiting) and destructive behavior within the flower tube (legitimate visiting), which damaged the flower’s reproductive organs, often leading to the complete absence of stamens and styles. This behavior suggested that the contribution of stingless bees to the reproductive success of I. cavalcantei was likely negative. Long-tongued orchid bees, Eulaema cingulata and Eulaema bombiformis, also collected nectar from I. cavalcantei flowers, accounting for ca. 2% of total legitimate visits when excluding Trigona bee visitations. Thus, preliminary data showed that several species of hummingbirds were the most frequent legitimate visitors of I. cavalcantei, comprising ca. 98% of visits. In this study, we aimed to better understand which hummingbird species could be the most important for the reproductive success of I. cavalcantei. Additionally, the native species of flower visitors that could facilitate the formation of interspecific I. cavalcantei × I. marabaensis hybrids and the recruitment of hybrids into the interspecific gene flow remained largely unknown. A more comprehensive understanding of the I. cavalcantei × I. marabaensis hybrid flower visitor network was necessary due to its implications for the conservation management of I. cavalcantei. Thus, we questioned whether our model included undiscovered plant–pollinator interactions that could underpin the process of interspecific hybridization and gene flow.
2 Results
2.1 Balancing nectar robbing and legitimate foraging could influence hummingbird pollinator services
In canga ecosystems, six hummingbird species were recorded foraging for nectar on ornithophile species I. cavalcantei, Cuphea annulata (Figures 1D, E), and Dyckia duckei (Figures 1F, G), including black-throated mango (Anthracothorax nigricollis; Greeney et al., 2020); grey-breasted sabrewing (Campylopterus largipennis; Züchner et al., 2021); long-billed starthroat (Heliomaster longirostris; Stiles and Boesman, 2020); long-tailed hermit (Phaethornis superciliosus; Hinkelmann et al., 2020); glittering-throated emerald (Chionomesa fimbriata; Weller et al., 2021); and fork-tailed woodnymph (Thalurania furcata; Stiles et al., 2020), see Figure 2. In our sampling, the occurrence of the hummingbird species varied between locations (Supplementary Table S2). Only glittering-throated emerald and fork-tailed woodnymph were observed in allopatric I. marabaensis cangas N6 and N8. Other hummingbird species were found in cangas with the presence of I. cavalcantei. The hummingbird species with the longest bills (ranging from 38.8 ± 2.1 to 25.5 ± 0.8 mm) exhibited legitimate feeding behavior (Supplementary Table S3) on I. cavalcantei flowers, which have narrow flower tubes with a mean length of 38 ± 4.2 mm (Babiychuk et al., 2019). These hummingbird species did not forage on C. annulata or D. duckei. The hummingbird species with shorter bills, namely, fork-tailed woodnymph and glittering-throated emerald, were facultative nectar robbers with 28% and 68% legitimate visits, respectively (Supplementary Table S3; Supplementary Figures S1F, G). Additionally, fork-tailed woodnymph and glittering-throated emerald legitimately foraged on C. annulata and D. duckei, accounting for 67% and 69% of combined flower visitations among the three plant species, respectively (Supplementary Table S3). During the dry season, very few plant species flower in canga. The most notable was evergreen Norantea guianensis, which was found in all recognized canga microhabitats, including rocky outcrops, “terra firme”, and low forests. The black-throated mango and fork-tailed woodnymph were feeding on Norantea flower inflorescences (Figure 2; Supplementary Table S3), indicating that these hummingbird species could be (semi)permanent canga residents.
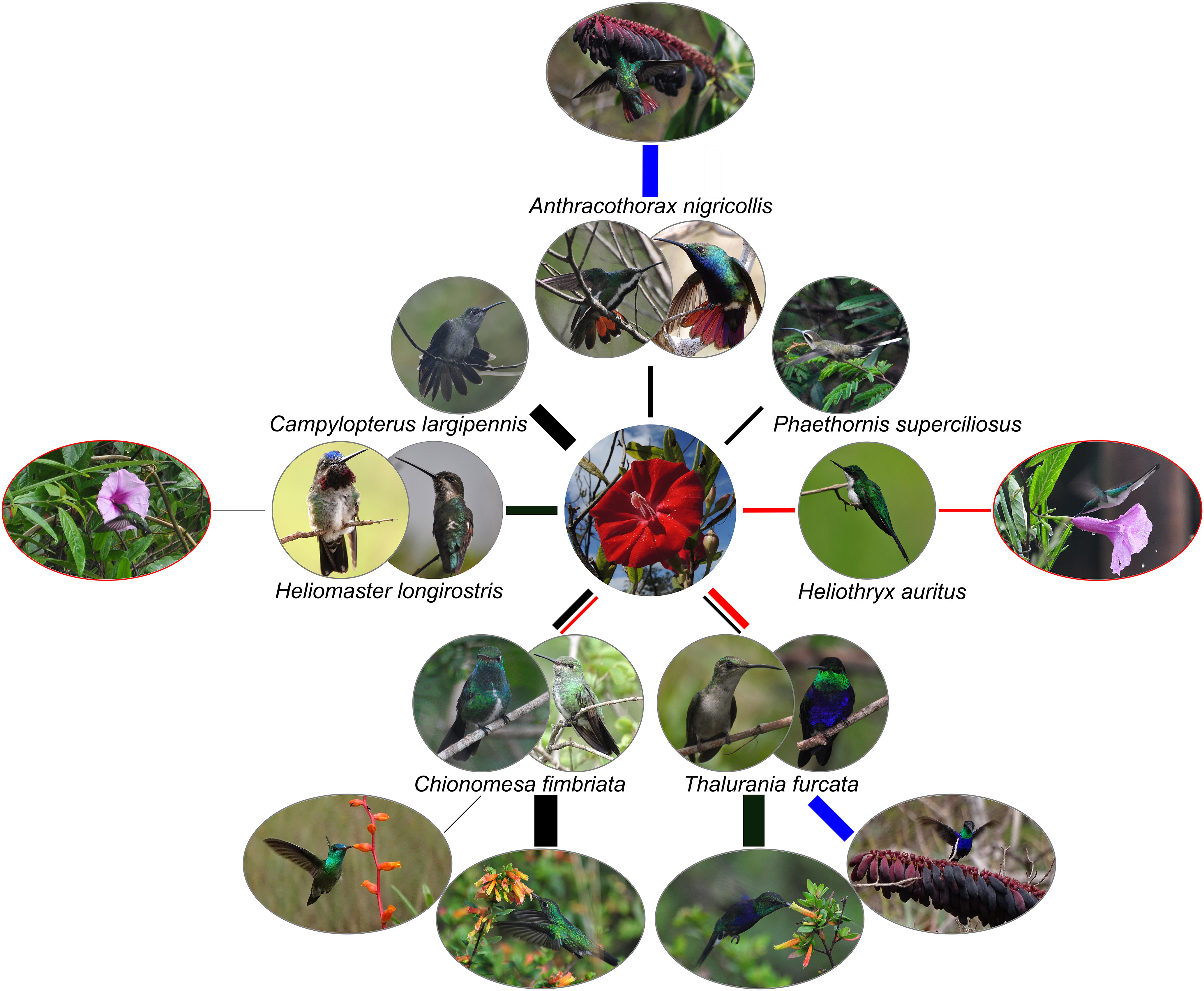
Figure 2. Summary of hummingbird species foraging on canga plant species. The plant–hummingbird interaction network is centered on the red-colored Ipomoea cavalcantei flower. Other plant species are represented by the lavender-colored Ipomoea marabaensis, orange flowers of Cuphea annulata and Dyckia duckei, and purple extrafloral cup-shaped nectaries of Norantea guianensis. Hummingbird species are identified by their Latin names. Sexual dimorphism is illustrated, permitting image availability. Flower visitations of canga plant species are indicated by the connecting lines (edges). The thickness of the edge reflects bird–plant interaction frequencies; see Supplementary Table S3 for observed interactions. Legitimate nectar feeding and nectar robbing behaviors are shown by the edge color, black and red, respectively. Connectors to N. guianensis that flower during the dry season are colored in blue. Images encircled by red lines are illustrations of a hummingbird foraging on I. marabaensis flowers as observed in ex situ collection.
Hummingbirds can be primary or secondary nectar robbers (Irwin et al., 2010). Within three distinct components of flower function, i.e., attraction, reward, and filtering mechanisms, the nectar chamber likely plays a role in filtering among flower visitors and is typically associated with hummingbird pollination (Stiles, 1981; Gill, 1987; Gonzalez et al., 2021). The nectar produced by I. cavalcantei and I. marabaensis flowers was primarily contained in the nectar chamber (Supplementary Figures S1A–D). The centrally located style and the abundant epidermal hairs on the stamens, particularly in I. cavalcantei (Supplementary Figure S1C), obstruct access to the nectar chamber through five narrow passages. In cangas, nectar robbing by large carpenter bees, Xylocopa spp., and stingless Trigona spp. bees was prevalent (Babiychuk et al., 2019). Unable to enter the narrow flower tubes of I. cavalcantei, carpenter bees use their maxillae to make slits in the flower corollas (Supplementary Figure S1E). We examined 47 flowers from three I. cavalcantei individuals in canga N1 to capture a ≥95% confidence interval in a nectar-robbing pattern. All flower corollas exhibited slit-like perforations. The examination of flowers robbed by glittering-throated emerald and fork-tailed woodnymph revealed longitudinal slit perforations, a typical feature of carpenter bee robbing. This observation indicates that those hummingbirds may be secondary nectar robbers utilizing the flower tube perforations created by carpenter bees. In an ex situ collection, the black-eared fairy hummingbird (Heliothryx auritus; Schuchmann et al., 2020) was identified as a primary, likely obligatory nectar robber on both I. cavalcantei and I. marabaensis (Supplementary Tables S3, Supplementary Figures S1H, I). Unlike carpenter bees, the hummingbird created puncture-like perforations in the sepal-covered proximal corolla tube section, accessing the nectar chamber directly (Supplementary Figures S1J, K).
2.2 Long-billed starthroat hummingbird was a legitimate visitor of I. marabaensis flowers
Previously, we did not obtain evidence of hummingbirds foraging for nectar on I. marabaensis (Babiychuk et al., 2019). This result was surprising because i) the flowers of this species produced more nectar than those of I. cavalcantei; ii) the passage to the nectar chamber appeared less restrictive (Supplementary Figure S1B versus S1C); and iii) mean nectar offer is thought to be the only parameter related to hummingbird visitation frequency, regardless of flower color or pollination syndrome (Waser et al., 1996; Maruyama et al., 2013). However, while monitoring plants in an ex situ collection that simulated sympatry, i.e., the co-occurrence of two Ipomoea species, we documented five instances of legitimate visits to I. marabaensis flowers by the long-billed starthroat (Figure 2). To validate this observation in canga settings and to characterize the flower visitors of the I. cavalcantei × I. marabaensis hybrids, we presented hummingbirds with artificial flower displays prepared as described in the Materials and Methods. We tested 52 displays, one per day per location, at six sites: cangas N1, N2, N3, N4, N8, and an ex situ collection (Supplementary Table S4). Hummingbirds did not visit displays in cangas N1 (10 displays, 35 videos each with a 20-minute duration), N2 (four displays, 12 videos), and N3 (15 displays, 34 videos). At the boundary with the forest in the canga N4 fragment 1 (Supplementary Table S1), two hummingbird species, the long-billed starthroat and the long-tailed hermit, visited three and four displays out of the 11 presented, respectively (Supplementary Figure S2; Supplementary Table S5). Out of 10 displays at the ex situ collection, the long-billed starthroat also foraged on three displays, and additionally, the black-eared fairy robbed nectar from both Ipomoea species and their hybrids on four different displays in which flowers were not placed in water-filled plastic tubes (Supplementary Table S5; Supplementary Figure S2). The Kruskal–Wallis tests revealed significant differences in visitation rates among flower types for the long-billed starthroat: H(6) = 74.0, p < 0.0001, and the long-tailed hermit: H(5) = 29.0, p < 0.0001. Post-hoc pairwise Dunn’s tests with Bonferroni correction identified specific differences among flower colors. H. longirostris showed highly significant differences in visitation among flower types on artificial displays (Figure 3A). For example, there were notable contrasts between magenta and red (p = 1.1 × 10−4), pink and purple (p = 5.2 × 10−5), and lavender and white (p = 0.0188). This species clearly distinguishes between different flower types and may prefer certain color groups, such as frequently visiting lavender and magenta-colored flowers. Despite this biological difference, the rank-based Dunn’s test did not find substantial differences between some groups. This could be due to the many tied ranks and the similarly low visitation numbers among certain floral types. The long-tailed hermit, P. superciliosus, showed fewer meaningful differences in flower visitation (Figure 3B). Notably, lavender vs. red (p = 0.00025) and pink vs. red (p = 0.0043) were significant, indicating a preference for red flowers over paler colors like lavender and pink. The nectar robber black-eared fairy foraged on floral displays at the ex situ site, showing differences in visitation rates in the Kruskal–Wallis test H(3) = 31.0, p < 0.0001. Dunn’s tests revealed that the species robbed lavender flowers significantly more than red (p = 9.0 × 10−7) and magenta (p = 0.0146), indicating a tendency to forage more on pale morphs (Figure 3C). Analysis of the video footage also highlighted the noteworthy features of I. marabaensis visitation by the long-billed starthroat. In six of the nine recorded visits, hummingbirds grasped the limb of the I. marabaensis flower while feeding (Supplementary Figure S2D). Additionally, the corolla limbs of I. marabaensis began to flutter upon the bird’s close approach. The corolla limbs sometimes flipped upward, enfolding the bird and touching its wings.
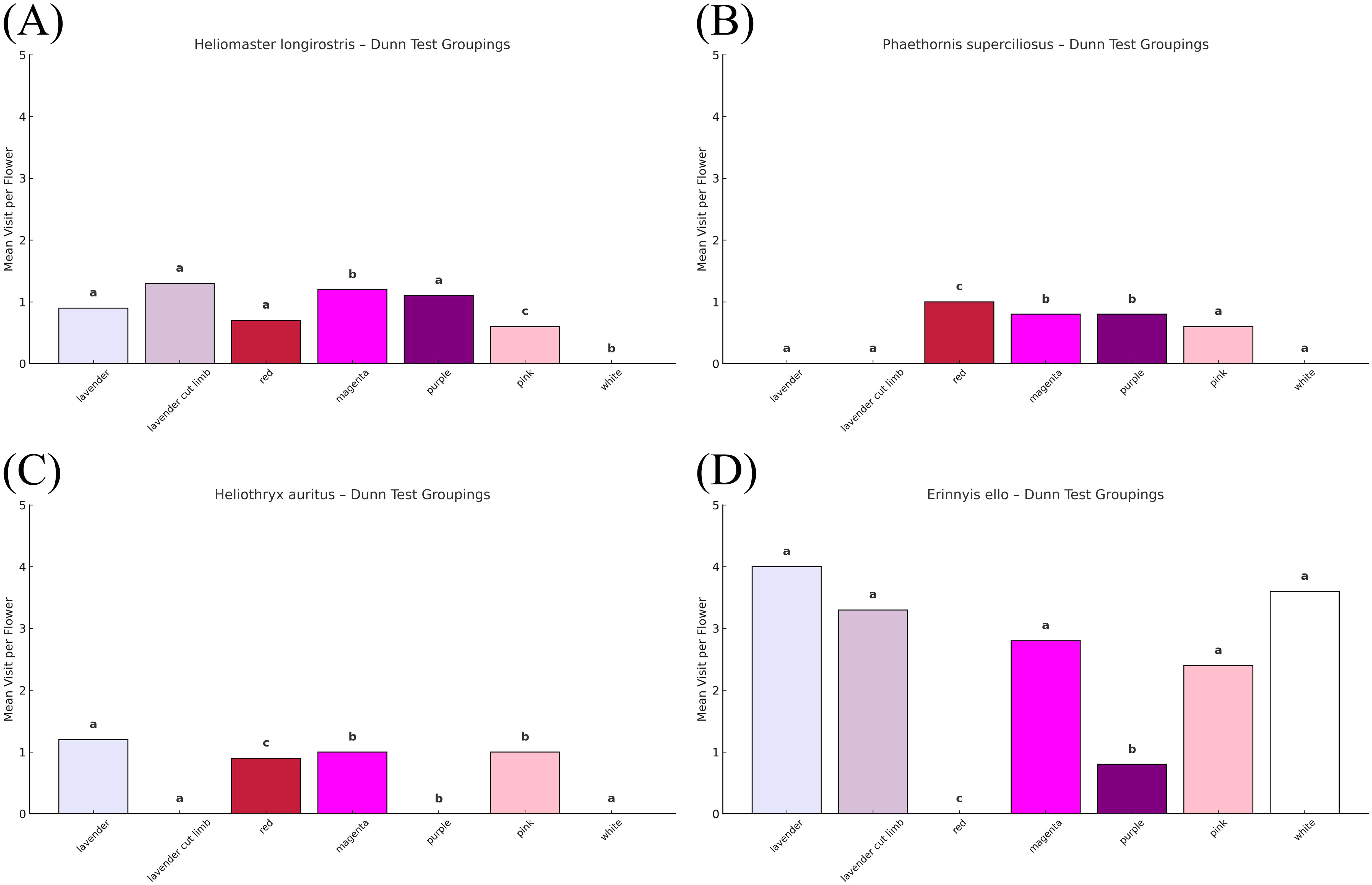
Figure 3. Flower visitor preferences on artificial floral displays. Bar plots show mean visitation rates per flower for each floral type. Bars are colored to match natural flower coloration. Letters above bars indicate statistical groupings based on pairwise post-hoc Dunn’s tests with Bonferroni correction (p < 0.05). Latin names of visitor species are at the bar plot titles: (A) Heliomaster longirostris, the long-billed starthroat; (B) Phaethornis superciliosus, the long-tailed hermit; (C) Heliothryx auritus, the black-eared fairy; and (D) Erinnyis ello, the ello sphinx.
2.3 Hawkmoth foraged on I. cavalcantei and I. marabaensis flowers
To determine whether other groups of plant pollinators were also visiting morning glory flowers, we began fieldwork in 2020 at the start of the Ipomoea flowering period. We found that on each day between February 20 and 28, individuals of a single hawkmoth species were foraging for nectar on both I. marabaensis and I. cavalcantei, at sympatric canga N4; allopatric cangas N1, N2, and N3 (I. cavalcantei); and allopatric cangas N6 and N8 (I. marabaensis) (Figures 4A, B). We recorded 86 visitations of I. cavalcantei and 81 visitations of I. marabaensis. The earliest hawkmoth visitations occurred at 8:46 am and the latest at 1:36 pm. In addition to morning glory flower visitations, we noted 110 instances of ello sphinxes foraging on the flowers of Bauhinia longicuspis from the Fabaceae family (Figure 4C), indicating generalist behavior. Hawkmoths disappeared abruptly and were not seen again until the end of the field trip, from March 1 to 11. Identification from the acquired digital imagery strongly suggested that the hawkmoth species was ello sphinx, Erinnyis ello. As shown in Figure 4B, ello sphinxes landed on the corolla limbs and were able to enter the flower tubes of I. marabaensis, which were broad enough to accommodate the large insect’s body. The flower tubes of I. cavalcantei were too narrow for ello sphinxes to enter. These animals landed on the flower limbs such that the hawkmoth’s head was near the exerted anthers of I. cavalcantei (Figure 4A). Ello sphinxes also foraged on floral displays in allopatric canga N3 and a remaining fragment of sympatric canga N4 (Figures 4D–F; Supplementary Tables S4, S5). The Kruskal–Wallis test revealed significant differences in ello sphinx visitation rates among flower types on artificial floral displays: H (6) = 33.0, p < 0.0001. Dunn’s tests revealed significant pairwise differences, such as lavender vs. purple (p = 0.0118), lavender vs. red (p = 0.0001), and red vs. white (p = 0.0008), indicating that the moth preferred pale-colored flowers like lavender and white and avoided red flowers (Figures 3D, 4D, E). Ello sphinx foraged on magenta hybrid flowers at frequencies not significantly different from lavender or white flowers (Figure 4F).

Figure 4. Ello sphinx flower visitation in the wild and on artificial floral displays. In canga, Erinnyis ello was feeding on flowers of Ipomoea cavalcantei (A), Ipomoea marabaensis (B), and Bauhinia pulchella (C). The hawkmoth foraging on flower displays, white I. cavalcantei flowers (D), flower limb trimmed I. marabaensis (E), and magenta-colored interspecies hybrids (F). The black arrow in panel (A) indicates the exerted anthers of the I. cavalcantei flower.
3 Discussion
Here, we demonstrate that at least two species of hummingbirds foraged nectar from the pale lavender-colored flowers of I. marabaensis, which exhibit characteristics of melittophily, or pollination by bees. The long-billed starthroat, H. longirostris, and the black-eared fairy, H. auritus, were identified as new potential pollinator and nectar robber of I. marabaensis, respectively. Hummingbird foraging behaviors on artificial floral displays indicated contrasting preferences by the long-billed starthroat and the long-tailed hermit, P. superciliosus, which are legitimate visitors. The long-tailed hermit favored I. cavalcantei and the magenta-colored I. cavalcantei × I. marabaensis hybrids but did not visit I. marabaensis lavender flowers. It is well known that hummingbirds visit different plant species at varying rates (Colwell, 1973; Feinsinger, 1976). Five community roles linking hummingbird foraging strategies to the spatial distribution and defensibility of nectar-bearing floral resources were proposed (Feinsinger and Colwell, 1978; Leimberger et al., 2022). In this classification, both the hermit P. superciliosus and the non-hermit H. longirostris are considered as high-reward trapliners that repeatedly visit a sequence of spatially dispersed, nectar-rich flowers (Feinsinger and Colwell, 1978). The long-tailed hermit has a longer bill than the long-billed starthroat, measuring 38.8 ± 2.1 compared to 35.0 ± 2.8 mm, which argues against the trait-matching hypothesis as an explanation for the differing visitation rates to I. marabaensis flowers. We also report on the movement of I. marabaensis flower limbs during hummingbird visits. The biomechanical properties of flowers may influence hummingbird foraging preferences.
We found that the short-billed species, glittering-throated emerald, C. fimbriata, and fork-tailed woodnymph, T. furcata, were legitimate nectar foragers on ornithophiles with short corolla tubes, facultative nectar robbers on I. cavalcantei, and, as previously tested (Babiychuk et al., 2019), were not attracted to nectar-rich I. marabaensis tubular flowers. This structuring of pairwise interactions in canga aligns with the known roles of trait-matching and spatiotemporal co-occurrence of interaction partners as primary reasons why hummingbirds visit some plants more frequently than others (Dalsgaard et al., 2021). However, unlike black-eared fairy, short-billed hummingbirds did not rob I. marabaensis, although carpenter bees robbed many flowers in allopatric cangas N6 and N8, which created flower tube perforations that hummingbirds can use to access the nectar chamber, as observed in canga N1 on I. cavalcantei flowers. The results suggest that the primary nectar larceny of I. cavalcantei by carpenter bees positively correlates with the secondary nectar robber behavior of the short-billed species, reducing the role of glittering-throated emerald and the fork-tailed woodnymph hummingbirds in I. cavalcantei reproductive success despite their relatively high abundance in wild canga ecosystems, accounting for 32% and 34% of all plant species visits, respectively (Supplementary Table S3). The absence of the single visit pollination effectiveness (SVE) data is a significant limitation of our work (Page et al., 2021). However, pollination effectiveness and visitation frequencies are often correlated (Page et al., 2021). Additionally, hummingbirds could be more effective pollinators than insects (Leimberger et al., 2022). Current data suggest that in our model, the long-billed hummingbird species, which account for 76% of total legitimate visits, are likely primary pollinators, largely determining I. cavalcantei reproductive success in canga.
We show that the long-billed starthroat, H. longirostris, hummingbird and ello sphinx hawkmoth, E. ello, foraged on both I. cavalcantei and I. marabaensis flowers. Thus, in addition to the native orchid bees, Eulaema spp., and the alien honeybees, Apis mellifera (Babiychuk et al., 2019), species from two other distinct pollinator groups could mediate the natural formation of the I. cavalcantei × I. marabaensis F1 hybrids. The ello sphinx appeared as an ephemeral pollinator and abruptly disappeared by the end of February; thus, their role in the overall reproductive success of morning glories during the flowering period lasting until May needs to be assessed more thoroughly. Furthermore, in the Amazon regions, the peak of ello sphinx activity was reported to occur at 5–6 am, i.e., before local sunrise. By that time, I. cavalcantei and I. marabaensis flowers are open, as found in this study, suggesting that reported visitation frequencies are underestimated because fieldwork started at 8 am at the earliest. On floral displays, ello sphinxes favored the pale lavender-colored flowers of I. marabaensis and the white flowers of I. cavalcantei. E. ello is thought to be a nocturnal or crepuscular, short-tongued hawkmoth (Amorim et al., 2013). Many nocturnal hawkmoth-pollinated flowers are white or cream-colored, which offers a contrast to the surrounding environment. Differences in flower visibility between I. cavalcantei flower color variants could explain the observed high visitation rates of the white flowers on artificial flower displays. In contrast to data on foraging in natural settings, we did not detect a single feeding attempt on six tested, red-colored I. cavalcantei flowers on artificial flower displays. The discrepancy between visitation differences on natural displays of flowers in canga versus artificial flower displays indicated that in distinct plant communities, i.e., I. cavalcantei allopatric cangas N1, N2, and N3, the ello sphinx showed the so-called reward economics behavior, e.g., nectar quantity/quality trade-offs influencing pollinator foraging choices. We also need to consider methodological biases caused by limitations of artificial flower displays, such as the absence of scent, foliage cues, and the rigid angle at which flowers are held in tubes, which may skew pollinator preferences.
We demonstrate that the long-billed starthroat, long-tailed hermit, and ello sphinx legitimately foraged on magenta-colored I. cavalcantei × I. marabaensis flowers. The evolutionary significance of interspecific hybridization relies on hybrid fertility. Our prior manual pollination experiments at ex situ collection indicated that I. cavalcantei × I. marabaensis hybrids were both male and female fertile. In the wild, all examined magenta-flowered hybrids in N4 and N5 canga fragments produced seeds that germinated into fully viable F2 progeny plants, confirming natural female fertility (Babiychuk et al., 2019). The hybrid fertility in our model can support interspecies gene flow, generating phenotypic variation for natural selection. For instance, variation at the Intensity locus I/i, which encodes the R2R3-Myb protein in Phlox drummondii, results in the co-occurrence of plants with light red, dark red, light blue, and dark blue flowers, influencing visitation frequencies by pollinators (Hopkins and Rausher, 2011). A similar intensity locus may determine the intense red, dominant I allele, and pale lavender, recessive i, in the common flower color types of I. cavalcantei and I. marabaensis, respectively. Such genetic control could account for pink flowers in sympatric cangas and I. marabaensis individuals with intensely colored flowers in canga N6 (Babiychuk et al., 2017). A limitation of our study is that we infer hybrid and color variant flower visitation frequencies from artificial flower displays. Several constraints influenced the use of an artificial flower display experimental design. At the beginning of our studies, we observed hummingbirds foraging on hybrids and color variants found in fragments of cangas N4 and N5. However, the plants of interest were growing far apart, which made direct comparisons difficult, and only a few hours of fieldwork at those sites were feasible. In the following years, we were unable to access intensely mined sympatric sites. Growing plants with contrasting flower phenotypes adjacent to each other is a common approach reported in several comparative studies of plant–pollinator interactions. Rescuing unique genotypes from the mining-driven local extinction and recreating a sympatry site where all color variants grow together, enabling further experimental work, were key objectives in establishing the ex situ collection. However, the pollinator species composition at the ex situ site differed from that of the cangas; for example, we have not sighted black-throated mango, grey-breasted sabrewing, long-tailed hermit, glittering-throated emerald, or ello sphinx hawkmoth at the ex situ site indicated by a yellow dot in Figure 1I. Nevertheless, tracking animal visitors through direct observation in the ex situ collection demonstrated that orchid bees (data not shown) and long-billed starthroat hummingbirds foraged on hybrid plants with magenta flowers. Our data suggest that native species of hummingbirds, hawkmoths, and orchid bees are likely candidates for mediating interspecific hybridization and gene flow, resulting in both F1 and F2 progeny in the wild.
Human civilization needs iron. In the coming years, the range of I. cavalcantei could be further diminished (Figure 1; Supplementary Table S1). The species persistence can only be ensured at protected fragments of the historical range, the so-called refugees, or through an introduction to new habitats, which are not a guarantee against functional extinction and genetic bottlenecks (Cronk, 2016). Our previous and new results show that to avoid functional extinction, to preserve the species’ adaptability, and to avoid genetic bottlenecks, it will be critical to conserve standing phenotypic diversity by systematic collection of seeds from as many individuals as feasible and through the entire remaining species range, followed by seed stock deposition in curated seed banks with seed long-term storage capabilities. At the expanding iron ore mines’ edges, an additional rescue effort must be carried out by replanting living plants for ex situ collection and/or refugees, focusing on phenotypic variants. Interspecies hybrids were common in sympatry on cangas N4 and N5 (Babiychuk et al., 2019). The evolutionary role of interspecific hybrids can have different consequences. As a positive effect, it can facilitate genetic rescue and demographic recovery (Whiteley et al., 2015) or underpin the introgression of favorable traits, the so-called adaptive introgression, which explains recent adaptations to the changing environment (Arnold and Kunte, 2017). The downside of interspecific hybridization is a risk of species extinction by genetic and demographic swamping, for example, of a rare Eucalyptus tetrapleura (Rutherford et al., 2019; reviewed Allendorf et al., 2001; Todesco et al., 2016). We do not know if sympatry existed before iron ore mining at Carajás was initiated in the 1980s. The identification of the I. cavalcantei individual, a likely migrant, in canga N8, can be due to a geological exploration road connecting N4 to N8 through the surrounding rainforest (Babiychuk et al., 2019), suggesting that mining-associated traffic may have altered historical species distribution, creating new sympatry zones. Interspecific hybrids are often limited to disturbed sites, endangering rare plant species, for example, Eucalyptus benthami trees (Butcher et al., 2005) and the endemic shrub Kunzea sinclairii (de Lange and Norton, 2004). Given the uncertainty about the history of sympatric zones and the possibility of local population extinctions through genetic and demographic swamping, i) I. cavalcantei refugee sites must be controlled against I. marabaensis migrants, and ii) the new introduction sites must be differentiated as sites with and without I. marabaensis. The interspecies hybridization zone, as we knew it in cangas N4 and N5, can be reconstructed in I. marabaensis-populated cangas outside of the protected Carajás National Forest. Other introduction sites must be i) I. marabaensis-free; ii) at several kilometers from I. marabaensis populations, considering the foraging ranges of described pollinators; iii) controlled by genetic monitoring every 5 years; and iv) sun-exposed but near the forest. Most hummingbird species prefer forest habitats (Leimberger et al., 2022). In fragmented tropical forest landscapes, hummingbird visitation rates showed significant and substantial decay with increasing distance to the forest of 10–40 m (Kormann et al., 2016). In the Carajás geographic area, the boundaries between native forest and agricultural land resulting from deforestation appear to be appropriate sites for I. cavalcantei introduction. Using I. cavalcantei in such natural biodiversity management borders on farms could also enhance hummingbird species abundance, hummingbird species richness and potentially benefit the yields of crops that depend on effective pollinators, e.g., coffee plantations. At public and private parks and gardens, the design and establishment of “hummingbird gardens” using local, native ornithophiles, such as I. cavalcantei, C. annulata, and D. duckei, is strongly recommended.
4 Materials and methods
4.1 Study sites
Fieldwork was carried out in the Carajás National Forest (Pará, Brazil), which comprises 13 Canga islands (Babiychuk et al., 2019). Amazon savanna-like ecosystems known as cangas evolved on iron laterite rock outcrops at similar elevations of ca. 700 m above sea level in the Carajás Mountain range. A mountainous rainforest encircles cangas, indicating the insular type of geographic isolation (Babiychuk et al., 2017). Canga soils are shallow and edaphically restrictive (Schaefer et al., 2016). Dry–wet seasons are partitioned by rain precipitation that varies between <60 and 1,900 mm/month; thus, most canga plants flower during periods of the wet season, November–May. The openness, heat, low nutrients, drought susceptibility, and toxic metal-rich conditions in combination with the insular isolation resulted in highly specialized canga plant communities composed of more than 800 plant species (Mota et al., 2018). The focus species I. cavalcantei was restricted to the Northern cangas N1, N2, N3, N4, and N5. Sister species I. marabaensis was common in N6, N7, N8, N9, Morro 1, Morro 2, S11 plateau, and Tarzan and had localized occurrence in N4 and N5. Therefore, we distinguished cangas N4 and N5 as “sympatric”; other cangas were designated as “allopatric”. The areas of cangas and canga fragments, as shown in Supplementary Table S1, were measured using the polygon tool in Google Earth Pro. Work was carried out with permissions per authorization #48272–3 and #63324–1 by the SISBIO (https://www.gov.br/icmbio/pt-br), Chico Mendes Institute for Biodiversity Conservation (ICMBio), and Brazilian Ministry of Environment (MMA). The Supplementary Table S1 footnotes detail the additional authorizations and accessibility limitations for work at study sites.
4.2 Morning glory anthesis and field work time frames
To characterize morning glory flower anthesis, we set a Bushnell camera to acquire images every 5 minutes at the ex situ collection. Time-lapse tracking of 15 pre-anthesis flower buds showed that I. cavalcantei flowers began to open between 2:45 and 3:15 am and were fully expanded at 5:30–5:40 am, i.e., just before sunrise. I. marabaensis flowers (n = 45) began to unfold at 2:50–3:30 am and were fully open at 5:30–6:15 am. Morning glory flowers were short-lived and began to show senescence at midday and late afternoon among I. marabaensis and I. cavalcantei individuals, respectively. As explained in the Supplementary Table S1 footnotes, we were only able to observe the activity of diurnal animal species in the field starting at 8 am at the earliest and ending at 5 pm at the latest, a time frame that excluded nocturnal pollinator groups such as nectarivorous bats and species-rich moths. At the ex situ collection, the pollinator visitation of morning glory individuals in BioParque Vale Amazônia (https://vale.com/pt/bioparque-vale-amazonia) could be followed at dawn, approximately 5–6 am, or after 5 pm. In the wild, we conducted 64 days of observation, spanning the wet season months of January through May and during the dry season in August. We conducted daily surveys between 8 am and 5 pm when visiting cangas N1, N2, and N3. Data collection time was limited to 3–4 hours when visiting more difficult-to-access cangas N4, N5, N6, and N8, which depended on unpredictable waiting time for scout cars mandatory for a passage through mining areas and, occasionally, longer driving time when the geological survey dirt track road required machete clearing of the fallen trees.
4.3 Canga ornithophile plant species, flower visitation tracking, and species identification
In addition to I. cavalcantei, two canga co-flowering plant species had conspicuous hummingbird pollination flower trait suites: C. annulata, family Lythraceae (Cavalcanti et al., 2016), and D. duckei, family Bromeliaceae (Monteiro and Forzza, 2016). C. annulata plants grew as densely branched perennial shrubs that were 30–150 cm tall. Plants produced large masses of long-lived, red-orange-colored, 15–21-mm-long– tubular flowers (Figures 1D, E). C. annulata was common in most cangas. In some parts of cangas N1, N3, N4, N5, and N8, the species were very abundant, covering the ground. The bromeliad with rosette growth habit, D. duckei, is a succulent that mainly grows on open rocky outcrops. Plants developed 30–55-cm-long, orange-colored inflorescence stalks bearing orange-red tubular flowers measuring 10–13 mm in length (Figures 1F, G). We dedicated approximately 250 person-hours of field studies to observations of hummingbirds foraging on I. cavalcantei, I. marabaensis, C. annulata, and D. duckei and the acquisition of still images and digital video recordings. We found that the videos recorded from a stationary camera had limited usefulness because i) hummingbirds would forage on several nearby plants of I. cavalcantei, D. duckei, and C. annulata; thus, the birds were often out of the camera frame. ii) In addition, the orientation of flowers in relation to the camera made it difficult to distinguish between legitimate and illegitimate foraging. Still images were less sensitive to the shortcomings of the video recording and were produced using a handheld Nikon D90 camera equipped with a 300-mm zoom lens. We filtered 7,917 still images and 102 videos to ensure that we report unambiguous records of legitimate or illegitimate feeding and bird species identification. Hummingbirds spend less than a second visiting Cuphea flowers. Consequently, our results may underestimate the accurate visitation rates of that species. To identify animal species, we used digital images. We captured no hawkmoths or hummingbirds for specimen depositions in museum collections.
4.4 Ex situ collection and flower displays
I. cavalcantei and I. marabaensis are perennial morning glories with woody stems and enlarged storage roots. Therefore, it was possible to maintain unique genotypes by vegetative propagation. Representative wild types and color variants from continuously expanding canga-mine boundaries were rescued for ex situ collection by excavating plant storage roots and replanting them in BioParque Vale Amazônia (Parauapebas, Pará, Brazil). I. cavalcantei is listed on the National Red List as a critically endangered species. The ex situ collection establishment followed The International Union for Conservation of Nature (IUCN, https://iucn.org/) guidelines for endangered species. The soils at BioParque Vale Amazônia, located within a mountainous rain forest, were distinct from canga soils; nevertheless, morning glories grew well, which was consistent with our common garden experiments (Babiychuk et al., 2017) and showed that in a greenhouse, I. cavalcantei and I. marabaensis grew the best on agricultural grade soil mixes. However, maximal exposure to the sun was critical to ensure compact growth and abundant flowering. Thus, plants were planted next to and climbed over a wire fence that was exposed to the sun from approximately 9 am to 5 pm. The flower colors, shapes, and sizes recorded in canga kept true in the ex situ collection, ruling out the possibility that the local native environment, e.g., soils and water availability, was the key cause of color, size, and shape variation. The ex situ collection comprised individuals of i) I. cavalcantei with intense red “wild type” (n = 3), pink (n = 6), purple (n = 3), and white (n = 2) flowers; ii) I. marabaensis with “wild type” pale lavender (n = 2), white (n = 3), and intense pink (n = 2) flowers; and iii) I. cavalcantei × I. marabaensis hybrids with magenta-colored flowers (n = 5). I. cavalcantei flower color/shape variants and I. cavalcantei × I. marabaensis hybrids occurred in fragments of sympatric cangas N4 and N5. It was important to characterize the flower visitor assemblages of the color variants and hybrids. Producing respective datasets in fragments of cangas N4 and N5 was impractical due to study site access difficulties, i.e., ongoing mining operations. We could follow the visitation of color variants and hybrids at the ex situ collection, in which we reconstructed, to some extent, the sympatry as found in the wild. However, the pollinator species composition differed from cangas; e.g., we have not sighted black-throated mango, grey-breasted sabrewing, long-tailed hermit, and glittering-throated emerald, as well as ello sphinx hawkmoth at the ex situ site. To address those technical problems, we decided to offer pollinators in canga the entire range of flowers preserved at ex situ collection using artificial flower displays. To prepare displays with flowers from the ex situ collection, we used a 15-mL laboratory plastic tube filled with water to prevent flower wilting. We placed tubes in tube racks at random. Available tube racks were orange in color. On some displays, we covered racks with green paper. We did not notice a difference between “green” and “orange” racks regarding visitation by foraging animals. At dawn, we detached freshly opened flowers from a plant and placed them in water for transportation to canga. In the wild, we hung tube racks on branches of shrubs, which I. cavalcantei climbs at ca. 1.5 m above the ground, i.e., at the level with surrounding I. cavalcantei flowers, or placed on rocks at the height of ornithophilous C. annulata bushes favored by the short-billed hummingbird species. We presented displays to pollinators in cangas N1, N2, N3, N4, N6, and N8 and at ex situ collection (Supplementary Table S4). In canga N4 fragment 1, it was only possible to present displays at the canga-forest boundary. At other sites, we tested displays in more open canga areas. We directed a Nikon COOLPIX P7800 camera, fixed on a tripod, at a flower display, and we recorded 20-min-long videos in a continuous series. Displays comprised i) “wild types”, i.e., intense red I. cavalcantei and pale lavender I. marabaensis flowers; ii) flower color variants of both species; and iii) magenta-colored flowers of natural interspecies hybrids. One striking interspecies difference between I. cavalcantei and I. marabaensis flowers is the structural strength of the corolla limbs. Smaller flowers of I. cavalcantei are very rigid, presumably due to high cellular turgor pressure. In large I. marabaensis flowers, the corolla limb is weak and floppy, e.g., wind and rain easily distort flower shapes and damage corolla tissues. We hypothesized that such flower biomechanical properties could interfere with the hummingbird hovering flight that generates an air wake (Wolf et al., 2013), which could induce corolla limb flapping, affecting plant–hummingbird pairwise interactions. To check the working hypothesis, we also included I. marabaensis flowers with surgically trimmed corolla limbs (“cut limb” flowers).
4.5 Statistical analysis
To determine whether visitation rates differ among flower color types, we calculated the number of visits per flower and performed non-parametric statistical tests for each of the four main floral visitors (H. longirostris, P. superciliosus, H. auritus, and E. ello). We conducted a Kruskal–Wallis rank-sum test for each visitor to evaluate whether visitation rates varied significantly across flower colors. When significant differences were identified (p < 0.05), we carried out pairwise comparisons using Dunn’s test with Bonferroni correction for multiple testing, implemented through the DunnTest() function in the R package FSA. We separately analyzed each floral visitor by subsetting the dataset, and we performed the statistical tests iteratively within a loop. We assigned letters to flower colors based on the adjusted p-values: flower colors that did not differ significantly (p ≥ 0.05) shared the same letter, while those with significant differences (p < 0.05) received different letters. We performed all analyses in R version 4.3.2 using the FSA, dplyr, and readr packages.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
EB: Investigation, Writing – review & editing, Project administration, Funding acquisition, Writing – original draft, Resources, Visualization, Conceptualization, Formal analysis, Methodology. JT: Methodology, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. LT: Methodology, Writing – review & editing, Investigation, Resources. VI-F: Writing – review & editing, Conceptualization, Project administration, Funding acquisition, Formal analysis, Data curation. SK: Methodology, Data curation, Conceptualization, Supervision, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Vale S.A. supported the research at Instituto Tecnológico Vale (ITV), and the study received funding from Vale S.A. The funder provided access to the study sites.
Acknowledgments
We thank Delmo Fonseca da Silva and Alexandre Castilho for continuous support and contributions to the fieldwork studies, Dr. Frederico Lencioni (Museu De História Natural De Taubaté Doutor Herculano Alvarenga, Sao Paulo, Brazil) for help in the identification of hummingbird species, and Andre Luis Acosta for the advice on statistical data analysis. S.K. is grateful to Marcel Regis M. da C. Machado (Director of the Carajás National Forest, ICMBio) for permission to enter the protected areas.
Conflict of interest
LT is an employee of the multinational mining company Vale S.A. Vale S.A. did not influence the study design, data analysis, or the interpretation of the results.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1594599/full#supplementary-material
References
Allendorf F. W., Leary R. F., Spruell P., and Wenburg J. K. (2001). The problems with hybrids: setting conservation guidelines. Trends Ecol. Evol. 16, 613–622. doi: 10.1016/S0169-5347(01)02290-X
Amorim F. W., Galetto L., and Sazima M. (2013). Beyond the pollination syndrome: nectar ecology and the role of diurnal and nocturnal pollinators in the reproductive success of Inga sessilis (Fabaceae). Plant Biol. 15, 317–327. doi: 10.1111/j.1438-8677.2012.00643.x
Arnold M. L. and Kunte K. (2017). Adaptive genetic exchange: a tangled history of admixture and evolutionary innovation. Trends Ecol. Evol. 32, 601–611. doi: 10.1016/j.tree.2017.05.007
Austin D. F. (1981). Novidades nas Convolvulaceae da flora Amazonica. Acta Amazonica 11, 291–295. doi: 10.1590/1809-43921981112291
Austin D. F. and Secco R. D. S. (1988). Ipomoea marabaensis, nova Convolvulaceae da Serra dos Carajás (PA). Boletim Museu Paraense Emilio Goeldi sér. Bot. 4, 187–194. Available online at: http://repositorio.museu-goeldi.br/handle/123456789/857 (Accessed November 11, 2014).
Babiychuk E., Kushnir S., Vasconcelos S., Dias M. C., Carvalho-Filho N., Nunes G. L, et al. (2017). Natural history of the narrow endemics Ipomoea cavalcantei and I. marabaensis from Amazon Canga savannahs. Sci. Rep. 7, 7493. doi: 10.1038/s41598-017-07398-z
Babiychuk E., Teixeira J. G., Tyski L., Guimaraes J. T. F., Romeiro L. A., da Silva E. F., et al. (2019). Geography is essential for reproductive isolation between florally diversified morning glory species from Amazon canga savannahs. Sci. Rep. 9, 18052. doi: 10.1038/s41598-019-53853-4
Barnosky A. D., Matzke N., Tomiya S., Wogan G. O. U., Swartz B., Quental T. B., et al. (2011). Has the Earth’s sixth mass extinction already arrived? Nature 471, 51–57. doi: 10.1038/nature09678
Bascompte J., Jordano P., and Olesen J. M. (2006). Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312, 431–433. doi: 10.1126/science.1123412
Bascompte J. and Jordano P. (2007). Plant-animal mutualistic networks: the architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 38, 567–593. doi: 10.1146/annurev.ecolsys.38.091206.095818
Bock D. G., Kantar M. B., Caseys C., Matthey-Doret R., and Rieseberg L. H. (2018). Evolution of invasiveness by genetic accommodation. Nat. Ecol. Evol. 2, 991. doi: 10.1038/s41559-018-0553-z
Butchart S. H. M., Walpole M., Collen B., Van Strien A., Scharlemann J.P.W., Almond R.E.A., et al. (2010). Global biodiversity: indicators of recent declines. Science 328, 1164–1168. doi: 10.1126/science.1187512
Butcher P. A., Skinner A. K., and Gardiner C. A. (2005). Increased inbreeding and inter-species gene flow in remnant populations of the rare Eucalyptus benthamii. Conserv. Genet. 6, 213–226. doi: 10.1007/s10592-004-7830-x
Cavalcanti T. B., Facco M. G., and Brauner L. D. M. (2016). Flora das cangas da Serra dos Carajás, Pará, Brasil: lythraceae. Rodriguésia 67, 1411–1415. doi: 10.1590/2175-7860201667539
Ceballos G., Ehrlich P. R., and Raven P. H. (2020). Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. U.S.A. 117, 13596–13602. doi: 10.1073/pnas.1922686117
Chan W. Y., Hoffmann A. A., and van Oppen M. J. (2019). Hybridization as a conservation management tool. Conserv. Lett. 12, e12652. doi: 10.1111/conl.12652
Colwell R. K. (1973). Competition and coexistence in a simple tropical community. Am. Nat. 107, 737–760. doi: 10.1086/282872
Cowie R. H., Bouchet P., and Fontaine B. (2022). The Sixth Mass Extinction: fact, fiction or speculation? Biol. Rev. 97, 640–663. doi: 10.1111/brv.12816
Dalsgaard B., Maruyama P. K., Sonne J., Hansen K., Zanata T. B., Abrahamczyk S., et al. (2021). The influence of biogeographical and evolutionary histories on morphological trait-matching and resource specialization in mutualistic hummingbird–plant networks. Funct. Ecol. 35, 1120–1133. doi: 10.1111/1365-2435.13784
de Lange P. J. and Norton D. A. (2004). The ecology and conservation of Kunzea sinclairii (Myrtaceae), a naturally rare plant of rhyolitic rock outcrops. Biol. Conserv. 117, 49–59. doi: 10.1016/S0006-3207(03)00262-3
Feinsinger P. (1976). Organization of a tropical guild of nectarivorous birds. Ecol. Monogr. 46, 257–291. doi: 10.2307/1942255
Feinsinger P. and Colwell R. K. (1978). Community organization among Neotropical nectar-feeding birds. Am. Zoologist 18, 779–795. doi: 10.1093/icb/18.4.779
Fenster C. B., Armbruster W. S., Wilson P., Dudash M. R., and Thomson J. D. (2004). Pollination syndromes and floral specialization. Ann. Rev. Ecol. Evol. Syst. 35, 375–403. doi: 10.1146/annurev.ecolsys.34.011802.132347
Fricke E. C., Ordonez A., and Rogers H. S. (2022). The effects of defaunation on plants’ capacity to track climate change. Science 375, 210–214. doi: 10.1126/science.abk3510
Gill F. B. (1987). Ecological fitting: use of floral nectar in Heliconia stilesii Daniels by three species of hermit hummingbirds. Condor 89, 779–787. doi: 10.2307/1368525
Gonzalez V. V., Gorostiague P., Ortega-Baes P., Galati B. G., and Ferrucci M. S. (2021). Nectary structure is not related to pollination system in Trichocereeae cactus from Northwest Argentina. Anais da Academia Bras. Ciências 93, e20201401, 1-20. doi: 10.1590/0001-3765202120201401
Gonzalez A., Rayfield B., and Lindo Z. (2011). The disentangled bank: how loss of habitat fragments and disassembles ecological networks. Am. J. Bot. 98, 503–516. doi: 10.3732/ajb.1000424
Greeney H. F., Schuchmann K. L., and Kirwan G. M. (2020). “Black-throated Mango (Anthracothorax nigricollis), version 1.0,” in Birds of the world. Eds. Billerman S. M., Keeney B. K., Rodewald P. G., and Schulenberg T. S. (Ithaca, NY, USA: Cornell Lab of Ornithology). doi: 10.2173/bow.bltman1.01
Harvey E., Gounand I., Ward C. L., and Altermatt F. (2017). Bridging ecology and conservation: from ecological networks to ecosystem function. J. Appl. Ecol. 54, 371–379. doi: 10.1111/1365-2664.12769
Hinkelmann C., Kirwan G. M., and Boesman P. F. D. (2020). “Long-tailed Hermit (Phaethornis superciliosus), version 1.0,” in Birds of the world. Eds. del Hoyo J., Elliott A., Sargatal J., Christie D. A., and de Juana E. (Ithaca, NY, USA: Cornell Lab of Ornithology). doi: 10.2173/bow.lother1.01
Hopkins R. and Rausher M. D. (2011). Identification of two genes causing reinforcement in the Texas wildflower Phlox drummondii. Nature 469, 411–414. doi: 10.1038/nature09641
Humphreys A. M., Govaerts R., Ficinski S. Z., Lughadha E. N., and Vorontsova M. S. (2019). Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 3, 1043–1047. doi: 10.1038/s41559-019-0906-2
Irwin R. E., Bronstein J. L., Manson J. S., and Richardson L. (2010). Nectar robbing: ecological and evolutionary perspectives. Ann. Rev. Ecol. Evol. Syst. 41, 271–292. doi: 10.1146/annurev.ecolsys.110308.120330
Kormann U., Scherber C., Tscharntke T., Klein N., Larbig M., Valente J. J., et al. (2016). Corridors restore animal-mediated pollination in fragmented tropical forest landscapes. Proc. R. Soc Biol. Sci. 283, 20152347. doi: 10.1098/rspb.2015.2347
Krauss S. L., Phillips R. D., Karron J. D., Johnson S. D., Roberts D. G., and Hopper S. D. (2017). Novel consequences of bird pollination for plant mating. Trends Plant Sci. 22, 395–410. doi: 10.1016/j.tplants.2017.03.005
Lavergne S., Thompson J. D., Garnier E., and Debussche M. (2004). The biology and ecology of narrow endemic and widespread plants: a comparative study of trait variation in 20 congeneric pairs. Oikos 107, 505–518. doi: 10.1111/j.0030-1299.2004.13423.x
Leimberger K. G., Dalsgaard B., Tobias J. A., Wolf C., and Betts M. G. (2022). The evolution, ecology, and conservation of hummingbirds and their interactions with flowering plants. Biol. Rev. 97, 923–959. doi: 10.1111/brv.12828
Le Roux J. J., Hui C., Castillo M. L., Iriondo J. M., Keet J.-H., Khapugin A. A., et al. (2019). Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 29, 2912–2918. doi: 10.1016/j.cub.2019.07.063
Levin D. A., Francisco-Ortega J., and Jansen R. K. (1996). Hybridization and the extinction of rare plant species. Conserv. Biol. 10, 10–16. doi: 10.1046/j.1523-1739.1996.10010010.x
Lughadha N., Bachman S. P., Leão T. C. C., Forest F., Halley J. M., Moat J., et al. (2020). Extinction risk and threats to plants and fungi. Plants People Planet 2, 389–408. doi: 10.1002/ppp3.10146
Maruyama P. K., Oliveira G. M., Ferreira C., Dalsgaard B., and Oliveira P. E. (2013). Pollination syndromes ignored: importance of non-ornithophilous flowers to Neotropical savanna hummingbirds. Naturwissenschaften 100, 1061–1068. doi: 10.1007/s00114-013-1111-9
Médail F. and Baumel A. (2018). Using phylogeography to define conservation priorities: The case of narrow endemic plants in the Mediterranean Basin hotspot. Biol. Conserv. 224, 258–266. doi: 10.1016/j.biocon.2018.05.028
Monteiro R. F. and Forzza R. C. (2016). Flora das cangas da Serra dos Carajás, Pará, Brasil: Bromeliaceae. Rodriguésia 67, 1253–1265. doi: 10.1590/2175-7860201667523
Mota N. F. D. O., Watanabe M. T. C., Zappi D. C., Hiura A. L., Pallos J., Viveros R. S., et al. (2018). Amazon canga: the unique vegetation of Carajás revealed by the list of seed plants. Rodriguésia 69, 1435–1488. doi: 10.1590/2175-7860201869336
Oziolor E. M., Reid N. M., Yair S., Lee K. M., VerPloeg S. G., Bruns P. C., et al. (2019). Adaptive introgression enables evolutionary rescue from extreme environmental pollution. Science 364, 455–457. doi: 10.1126/science.aav4155
Page M. L., Nicholson C. C., Brennan R. M., Britzman A. T., Greer J., Hembergeret J., et al. (2021). A meta-analysis of single visit pollination effectiveness comparing honeybees and other floral visitors. Am. J. Bot. 108, 2196–2207. doi: 10.1002/ajb2.1764
Pimm S. L., Jenkins C. N., Abell R., Brooks T. M., Gittleman J. L., Joppa L. N., et al. (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752. doi: 10.1126/science.1246752
Quilodrán C. S., Montoya-Burgos J. I., and Currat M. (2020). Harmonizing hybridization dissonance in conservation. Commun. Biol. 3, 391. doi: 10.1038/s42003-020-1116-9
Rezende E. L., Lavabre J. E., Guimarães P. R., Jordano P., and Bascompte J. (2007). Non-random coextinctions in phylogenetically structured mutualistic networks. Nature 448, 925–928. doi: 10.1038/nature05956
Rutherford S., van der Merwe M., Wilson P. G., and Kooyman R. M. (2019). Managing the risk of genetic swamping of a rare and restricted tree. Conserv. Genet. 20, 1113–1131. doi: 10.5061/dryad.76t6j77
Schaefer C. E., Cândido H. G., Corrêa G. R., Nunes J. A., and Arruda D. M. (2016). “Soils associated with rupestrian grasslands,” in Ecology and conservation of mountaintop grasslands in Brazil (Switzerland: Springer International Publishing), 55–69. doi: 10.1007/978-3-319-29808-5_3
Schleuning M., Fründ J., Schweiger O., Welk E., Albrecht J., Albrecht M., et al. (2016). Ecological networks are more sensitive to plant than to animal extinction under climate change. Nat. Commun. 7, 13965. doi: 10.1038/ncomms13965
Schmickl R., Marburger S., Bray S., and Yant L. (2017). Hybrids and horizontal transfer: introgression allows adaptive allele discovery. J. Exp. Bot. 68, 5453–5470. doi: 10.1093/jxb/erx297
Schuchmann K. L., Kirwan G. M., and Boesman P. F. D. (2020). “Black-eared Fairy (Heliothryx auritus), version 1.0,” in Birds of the world. Eds. del Hoyo J., Elliott A., Sargatal J., Christie D. A., and de Juana E. (Ithaca, NY, USA: Cornell Lab of Ornithology). doi: 10.2173/bow.bkefai1.01
Souza-Filho P. W. M., Giannin T. C., Jaffé R., Giulietti A. M., Santos D. C., Wilson R., et al (2020). Mapping and quantification of ferruginous outcrop savannas in the Brazilian Amazon: A challenge for biodiversity conservation. PLoS One. 14, e0211095. doi: 10.1371/journal.pone.0211095
Stiles F. G. (1981). Geographical aspects of bird-flower coevolution, with particular reference to Central America. Ann. Missouri Bot. Garden 68, 323–351. doi: 10.2307/2398801
Stiles F. G. and Boesman P. F. D. (2020). “Long-billed Starthroat (Heliomaster longirostris), version 1.0,” in Birds of the world. Eds. del Hoyo J., Elliott A., Sargatal J., Christie D. A., and de Juana E. (Ithaca, NY, USA: Cornell Lab of Ornithology). doi: 10.2173/bow.lobsta1.01
Stiles F. G., Kirwan G. M., and Boesman P. F. D. (2020). “Fork-tailed Woodnymph (Thalurania furcata), version 1.0,” in Birds of the world. Eds. del Hoyo J., Elliott A., Sargatal J., Christie D. A., and de Juana E. (Ithaca, NY, USA: Cornell Lab of Ornitholog). doi: 10.2173/bow.fotwoo1.01
Todesco M., Pascual M. A., Owens G. L., Ostevik K. L., Moyers B. T., Hübner S., et al. (2016). Hybridization and extinction. Evol. Appl. 9, 892–908. doi: 10.1111/eva.12367
Waser N. M., Chittka L., Price M. V., Williams N. M., and Ollerton J. (1996). Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060. doi: 10.2307/2265575
Weller A. A., Kirwan M., and Boesman P. F. D. (2021). “Glittering-throated Emerald (Chionomesa fimbriata), version 1.1,” in Birds of the world. Eds. del Hoyo J., Elliott A., Sargatal J., Christie D. A., and de Juana E. (Ithaca, NY, USA: Cornell Lab of Ornithology). doi: 10.2173/bow.glteme1.01.1
Wessinger C. A. (2021). From pollen dispersal to plant diversification: genetic consequences of pollination mode. New Phytol. 229, 3125–3132. doi: 10.1111/nph.17073
Whiteley A. R., Fitzpatrick S. W., Funk W. C., and Tallmon D. A. (2015). Genetic rescue to the rescue. Trends Ecol. Evol. 30, 42–49. doi: 10.1016/j.tree.2014.10.009
Wolf M., Ortega-Jimenez V. M., and Dudley R. (2013). Structure of the vortex wake in hovering Anna’s hummingbirds (Calypte anna). Proc. R. Soc Biol. Sci. 280, 20132391. doi: 10.1098/rspb.2013.2391
Yakimowski S. B. and Rieseberg L. H. (2014). The role of homoploid hybridization in evolution: a century of studies synthesizing genetics and ecology. Am. J. Bot. 101, 1247–1258. doi: 10.3732/ajb.1400201
Keywords: endemic, hummingbird, hybrids, Ipomoea, conservation, Amazon
Citation: Babiychuk E, Teixeira JG, Tyski L, Imperatriz-Fonseca VL and Kushnir S (2025) Conservation of animal–plant mutualistic networks is essential to prevent functional extinction of the narrow endemic morning glory Ipomoea cavalcantei in Amazon canga ecosystems. Front. Ecol. Evol. 13:1594599. doi: 10.3389/fevo.2025.1594599
Received: 16 March 2025; Accepted: 25 July 2025;
Published: 23 September 2025.
Edited by:
Emilio Badalamenti, University of Palermo, ItalyReviewed by:
Ning Li, Nanjing Xiaozhuang University, ChinaRafael Silveira Bueno, University of Palermo, Italy
Copyright © 2025 Babiychuk, Teixeira, Tyski, Imperatriz-Fonseca and Kushnir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Babiychuk, YmFiaXljaHVrLmVsZW5hQGdtYWlsLmNvbQ==
 Elena Babiychuk
Elena Babiychuk Juliana Galaschi Teixeira
Juliana Galaschi Teixeira Lourival Tyski
Lourival Tyski Vera L. Imperatriz-Fonseca1,3
Vera L. Imperatriz-Fonseca1,3 Sergei Kushnir
Sergei Kushnir