- Department of Environmental and Sustainability Sciences, Texas Christian University, Fort Worth, TX, United States
The availability and accessibility of water resources are important factors influencing bat presence in urban areas. As bats drink ‘on the wing’, total surface area of a water sources determines overall water availability, but the presence of clutter dictates water accessibility. Understanding how water accessibility influences bat resource use may therefore provide a more accurate measure of water availability in urban environments. To explore this, we assessed how variation in water surface area influenced bat activity and species richness in an urban area. We conducted surveys at six study sites in Tarrant County, Texas, USA in 2023 and 2024 using a thermal camera to measure the total duration of bat presence and the number of drinking events. Additionally, we used an acoustic detector to record the number of species recorded drinking at the sites during each survey. A drone was used to evaluate water surface area metrics, including fundamental and realized surface areas, maximum patch sizes, and the longest stretches of continuous area. Our findings indicated that decreasing the length of available surface was associated with reduced bat activity. Notably, drinking activity, a key indicator of water resource use, declined with increasing clutter due to litter, emergent vegetation, algal blooms, and even fallen trees. These results suggest that the presence of clutter, in particular, may limit and even prevent bats from accessing water. Effective management of urban water sources should prioritize clutter removal to improve water accessibility for bats and support a diverse urban wildlife community.
1 Introduction
Improving resource availability is key to fostering wildlife diversity in urban environments (Ancillotto et al., 2019; Berthon et al., 2021). While there are five fundamental resources that wildlife require, including food, shelter, mating opportunities, movement corridors or pathways, and water sources, the specific resources selected (i.e., type of roost site) are species-dependent (Elbroch and Allen, 2013; Kloskowski et al., 2013; Leveau and Ibáñez, 2022). Therefore, to support a diverse community, urban areas must offer a variety of resources (Felderhoff et al., 2023; Hansen et al., 2020), which should be both available and accessible to wildlife (Buchholtz et al., 2021; Finn et al., 2021). Accessibility refers to the ability of an individual to locate and obtain resources (Tsalyuk et al., 2019). For instance, it can be influenced by landscape features that may impede or facilitate movement (Snyder et al., 2022). In addition, species-specific characteristics can also determine whether a species can access a particular resource (Conan et al., 2023). Urban bat communities are ideal for examining how accessibility influences resource use, as bats drink on the wing (i.e., while flying) and their species-specific maneuverability dictates whether they can access a particular water source (Nystrom and Bennett, 2019). Species with low maneuverability may, therefore, only be able to access water sources that exceed a certain size (Hall et al., 2016). While the total surface area of a water source (referred to here as the fundamental surface area) dictates its overall size, this does not account for the presence of clutter, which determines the actual amount of surface area available (hereafter referred to as the realized surface area; Rodríguez and Sánchez, 2022). Clutter can be created by the presence of vegetation in and surrounding a water source, by rocks and other objects breaching the water surface, or by objects floating on the surface of the water, such as leaves, pollen, and litter (Kataoka and Nihei, 2020). This clutter can fragment the water surface forming smaller isolated patches of water that can also vary in shape from which bats can drink (Greenfeld et al., 2018). Moreover, surface area can vary seasonally due to changes in precipitation and evaporation rates (Konapala et al., 2020). For example, in summer months, high temperatures and reduced precipitation can decrease water volume, subsequently decreasing fundamental surface area and increasing clutter (Feng et al., 2022). In addition, as water sources become shallow, aquatic vegetation can grow more prolifically, further decreasing surface area availability (Zheng et al., 2020). In other words, while water may be present in a water source, it may not be accessible to bats, particularly among those species with less maneuverability. Therefore, a greater understanding of the spatial and temporal variations in water surface area and how such changes impact resource use by bats is needed, especially in urban areas where water availability may already be limited.
To address this need and increase our understanding of water source accessibility, we conducted a study to assess how variation in water surface area influenced bat activity and species diversity at urban water sources in north central Texas, where prolonged hot and dry conditions can limit water availability (Hall and Bennett, 2021). Using unmanned aerial vehicles (UAVs), thermal technology, and acoustic monitoring, we evaluated multiple characteristics of available surface area. We hypothesized that bat activity and species richness would decrease as water surface area became more limited, fragmented, and obstructed during the summer months. Specifically, we predicted that: (1) reduced surface area due to obstruction (e.g., vegetation, debris) would lower both drinking activity and species diversity; (2) smaller, isolated fragments of open water would further constrain bat access; and (3) shorter continuous stretches of open water would limit maneuvering space, particularly for less agile species. By evaluating how cluttered or fragmented water sources influence bat access, our study aims to determine if accessibility, not just availability, shapes bat use of urban water sources. These insights can guide the management of water bodies to better support local bat populations and more broadly the conservation of bats in urban environments.
2 Materials and methods
2.1 Study sites
Our surveys were conducted in Tarrant County within the Cross Timbers and Prairies Ecoregion of north central Texas. Previous studies have confirmed the presence of six bat species in this predominantly urban area, including eastern red (Lasiurus borealis), hoary (Lasiurus cinereus), silver-haired (Lasionycteris noctivagans), tricolored (Perimyotis subflavus), evening (Nycticeius humeralis), and Mexican free-tailed (Tadarida brasiliensis) bats (Agpalo, 2020; Nystrom and Bennett, 2019; Smith, 2019). Water availability for these bat species is thought to be influenced by the local climate (Hall and Bennett, 2021). For instance, during the bat activity season from March to September, this area experiences temperatures that range on average from 2.2°C to 36.1°C and average monthly precipitation ranging from 53 mm to 121 mm (www.weather.gov). More specifically, from June to September temperatures are consistently above 29.4°C during the day and 18.3°C during the night. To explore variability in water availability for bats across their activity season, we selected six water sources in local parks throughout the county, including retention ponds, lakes, and river tributaries (Table 1).

Table 1. Six study sites surveyed in Fort Worth, Texas in 2023 and 2024, including the field-of-view for the thermal camera set up at each site and the aerial view taken by the drone.
2.2 Behavioral surveys
We conducted behavioral observation and acoustic surveys at the six study sites from March to September 2023 and 2024. Each study site was surveyed once every two weeks, avoiding nights when temperatures were <5°C, it was raining, or wind speeds were>24 km/hr (Nystrom and Bennett, 2019). For the behavioral observation surveys, we used Axis Q1942-E 19mm ThermNetCam 30 FPS (Axis Communications, Lund, Sweden) surveillance cameras with ~9,000-14,000 micrometers infrared spectrum range; as recommended in Huzzen et al. (2020). The thermal cameras were set to a resolution of 640 by 480 pixels, the “Ice-and-Fire” false-color scheme setting, and a sampling rate of 30 frames/sec. We positioned the cameras 10 m away from the edge of the water to capture a 10 m by 10 m field-of-view of the surface of the water and airspace above starting from the water’s edge. We conducted 1 hr surveys that began 20 minutes after sunset to incorporate the primary period when local bats are actively searching for and drinking water (i.e., soon after the bats emerged from their roosts; (Nystrom and Bennett, 2019). Once a survey was completed, we used Vosaic video analysis software (version 1.1.3686, Studiocode Business Group, Lincoln, Nebraska) to manually log bats present in the field-of-view within the footage recorded. We also identified one distinct inflight behavior: drinking. This activity was characterized as a bat swooping down to the surface of the water with its body angled head-first facing towards the water before making contact with the surface ≥1 time as it passed over the water (Nystrom and Bennett, 2019; Tuttle et al., 2006).
To ensure the accuracy of our observations, a second technician reviewed 25% of the footage blind (i.e., without access to the primary technician’s log). We then compared the timeline logged by the primary technician with that of the second technician to identify any observer errors made by the initial reviewer. This preliminary analysis indicated that only 1.2% of bats were not observed during the primary review, indicating that the number of bats potentially unrecorded during the analysis of the footage was unlikely to impact our findings. Thus, we proceeded to use 1) the total duration (in seconds) that bats were present and 2) the number of drinking events observed within the field-of-view during each 1-hour survey, as our first two dependent variables. These metrics represent bat activity within the thermal camera’s field-of-view, rather than estimates of abundance, as individual bats could not be tracked or distinguished across time. Furthermore, as thermal footage does not allow for species-level identification, our observations of bat activity and drinking behavior reflect general patterns across the bat community rather than species-specific responses. Instead, acoustic monitoring (described below) was used to assess species richness.
2.3 Acoustic monitoring
We used a Song Meter SM4Bat acoustic detector with an external U2 ultrasonic microphone from Wildlife Acoustics (Maynard, Massachusetts) to record bat echolocation calls at the study sites during the 1-hr behavioral surveys. We placed the detector at the edge of the water with the microphone directed towards the water surface. The detector was set to trigger at frequencies between 16 kHz and 192 kHz to encompass the echolocation frequencies of local bats (Nystrom and Bennett, 2019). The trigger volume was set at 12.0 dB and gain threshold at 12.0 dB. Once surveys were completed, we used SonoBat Scrubber software (Version 4, SonoBat, Arcata, California) to filter out any sound files recorded on the acoustic detectors containing noise. We then used SonoBat bat call analysis software (Version 3.03, SonoBat, Arcata, California) to process the recorded bat calls. In this program, we manually identified echolocation calls to species, where possible. We also identified all calls that contained drinking buzzes, defined as acoustically distinct sequence of echolocation pulses emitted by bats as they approach water to drink (Nystrom and Bennett, 2019). These sequences are characterized by a rapid decrease in call shape, frequency bandwidth, and interval duration, ending abruptly at the moment of contact, often accompanied by an audible splash (Russo et al., 2016). We used the number of species recorded drinking at each site during each survey to represent our third response variable: species richness.
2.4 Surface area surveys
Using UAVs, we recorded surface area metrics at each of the six study sites every two weeks from March to September 2023 and 2024. During these surveys, we used two DJI drones (SZ DJI Technology Co, Ltd; Shenzhen, China) with identical camera specifications to record an aerial view of each survey site (see Table 1 for aerial views). We established and executed predetermined flight paths to consistently generate the same aerial image in each survey for each of our study sites. Note that UAVs recorded video imagery at a resolution of 3840 by 2160 pixels and 30 frames per second.
To process the footage collected during each survey, we used Agisoft Metashape photogrammetry software (Version 2.0.1, Agisoft LLC, St. Petersburg, Russia) to generate a 2D model of each study site. From these models, we extracted four surface area-related variables using the Draw tool: 1) fundamental surface area (m2; total open water), 2) realized surface areas (m2; total open water minus areas obstructed by clutter), 3) maximum fragment size (m2; uninterrupted by clutter) within the site, and 4) longest stretch of continuous area (m) within the site. These four metrics represent our independent variables. While fundamental surface area is a commonly used measure of water availability, it does not consider the influence of clutter on the ability of a bat to access water (Bergey and Getty, 2006). The remaining three variables, realized surface area, maximum fragment size, and longest continuous stretch, incorporate the presence and impact of clutter on surface area availability. In the context of this study, we defined clutter as any physical obstruction visible on or up to 3 m above the water surface that could interfere with bat drinking activity. This included emergent or overhanging vegetation (e.g., reeds, shrubs), floating material (e.g., leaves, litter, algae), rocks, or any other anthropogenic debris breaching the surface of the water. Realized surface area represents the total open surface area available for drinking after clutter is excluded and therefore better reflects functional accessibility (Warren et al., 2000). As clutter has the potential to fragment the water surface (Rodríguez and Sánchez, 2022), we also included fragment size as a variable to assess whether bats are limited by the size of the largest uninterrupted patch of water (Greenfeld et al., 2018). Finally, we included longest continuous stretch to capture additional spatial characteristics known to influence drinking behavior, such as maneuverability constraints and species-specific flight requirements. For example, some bat species require a clear, linear stretch of water to successfully drink. It is these species-specific limitations that not only influence drinking activity, but also species diversity (Greenfeld et al., 2018; Tuttle et al., 2006).
2.5 Analysis
To investigate how each of the four independent variables influenced our three response variables, we employed Generalized Linear Mixed Models (GLMMs; Zuur et al., 2009). This approach was selected to account for the nested structure of the data, specifically the repeated measures collected across multiple nights at each site. By including site as a random effect, the models account for non-independence among observations within the same site, thereby improving the robustness of our inferences. Given that the four surface area metrics (fundamental surface area, realized surface area, maximum fragment size, and longest continuous stretch) were designed to build upon one another by incorporating clutter to refine accessible water, collinearity among them was conceptually expected. Including all four metrics in a single model would obscure their individual effects due to shared variance, but doing so also allows comparison of their relative contributions to bat activity and species richness when considered together. Therefore, we opted to conduct and compare both approaches. First, we ran separate GLMMs for each surface metric to isolate and interpret how each one individually influenced bat activity and species richness. Second, we ran a combined model including all four metrics simultaneously to explore their relative contributions when accounting for collinearity. For both, we conducted separate GLMMs for each dependent variable. For duration bats were present, we used a Gaussian distribution with an identity link function, as this variable was continuous and approximated normality after log transformation. For number of drinking events and number of species detected drinking, we used Poisson distributions with a log link, as these variables represented count data. Fixed effects included each surface area metric, as well as time-related covariates (month and year) to account for seasonal and interannual variation. All analyses were performed using IBM SPSS Statistics (Version 21, IBM Corp., Armonk, New York), with significance set at α = 0.05.
3 Results
We conducted behavioral surveys from 8 March to 27 September 2023 and 4 March to 19 September 2024 for a total of 180 survey nights with 30 surveys at each site. Bats were observed in thermal imagery at all of our study sites for a total of 86 mins and 22 secs, with individual bats in the field-of-view for an average of one second. The maximum length of time a bat was recorded was 15 secs. Over a survey, bats were present in the field-of-view on average 28.6 ± 47.6 SD sec per site per night (ranging from 0 to 393 sec). We noted that bat activity fluctuated across the survey season and between years at all sites (Figure 1). We identified bats drinking on 643 occasions at six of the six sites on 88 survey nights, which averaged 3.6 ± 7.0 SD of drinking events per site per night (ranging from 0 to 45). In the acoustic surveys, we recorded a total of 23,814 bat calls. Bat calls were recorded at six of the six sites, averaging 132.3 ± 252.9 SD per site per night (ranging from 0 to 1,360) for a total of 23,814 calls recorded. From these calls, we identified all six bat species and all six species were recorded drinking (n= 1,432) at our sites, including evening (n=1253), eastern red (n=97), silver-haired (n=22), hoary (n=15), tricolored (n=40) and Mexican free-tailed (n= 1) bats. In the 30 surface area surveys we conducted at each site, we found that all four of our surface area metrics varied across the survey season at all sites, although the extent of this variation differed between each site (Figure 2). In addition, we provide minimum and maximum values of each surface area metric associated with species presence (Figure 3). These values represent the observed range of each metric at sites where each bat species was detected emitting drinking buzzes.

Figure 1. The total duration bats were present in the field of view of our thermal cameras over our survey period from March to September in 2023 and 2024. (A) through (F) represents sites 1 through 6 respectively.
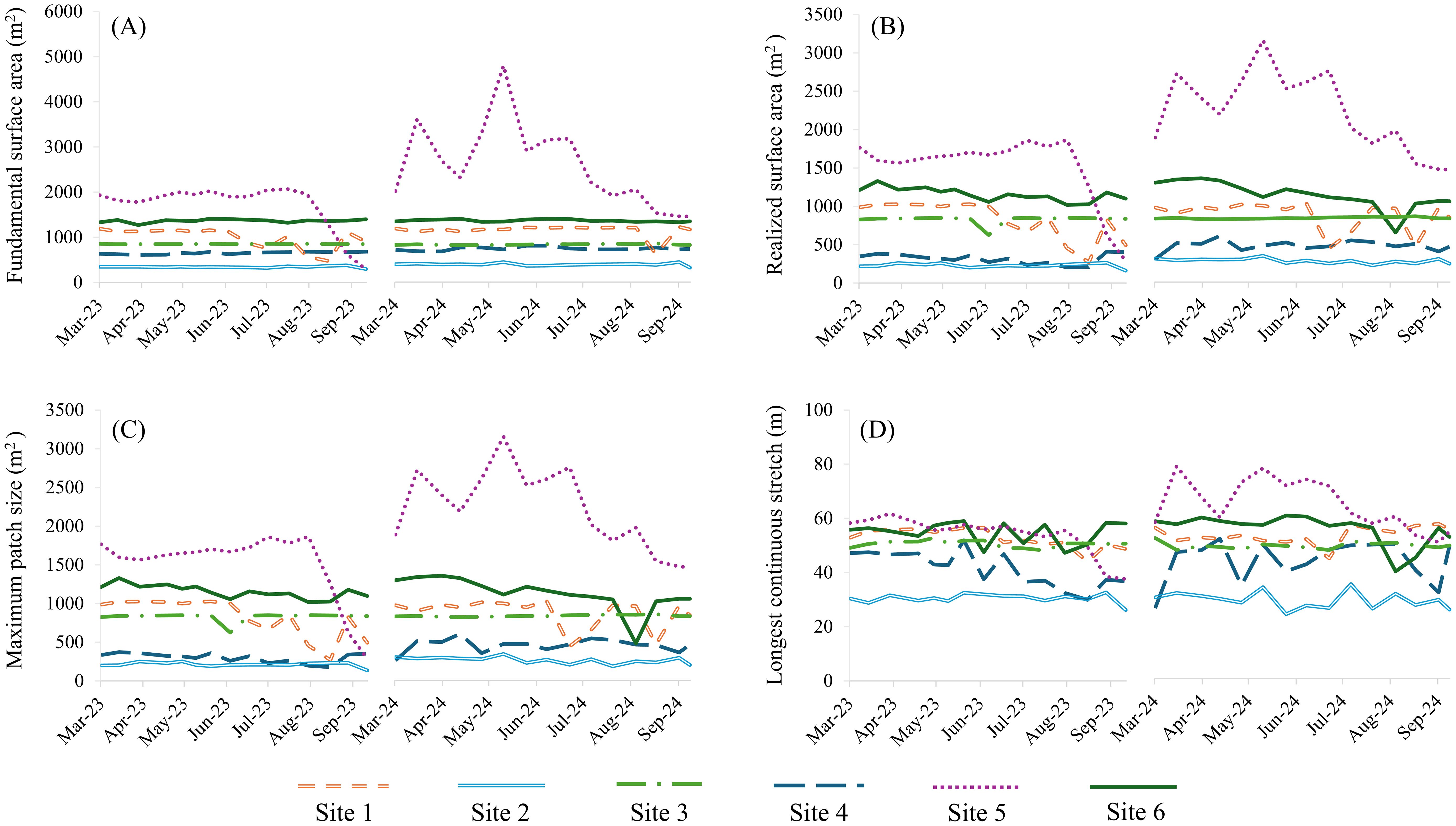
Figure 2. The change in fundamental surface area (A), realized surface area (B), maximum patch size (C), and longest continuous stretch (D) across our study period from March to September 2023 and 2024 for each of our study sites.
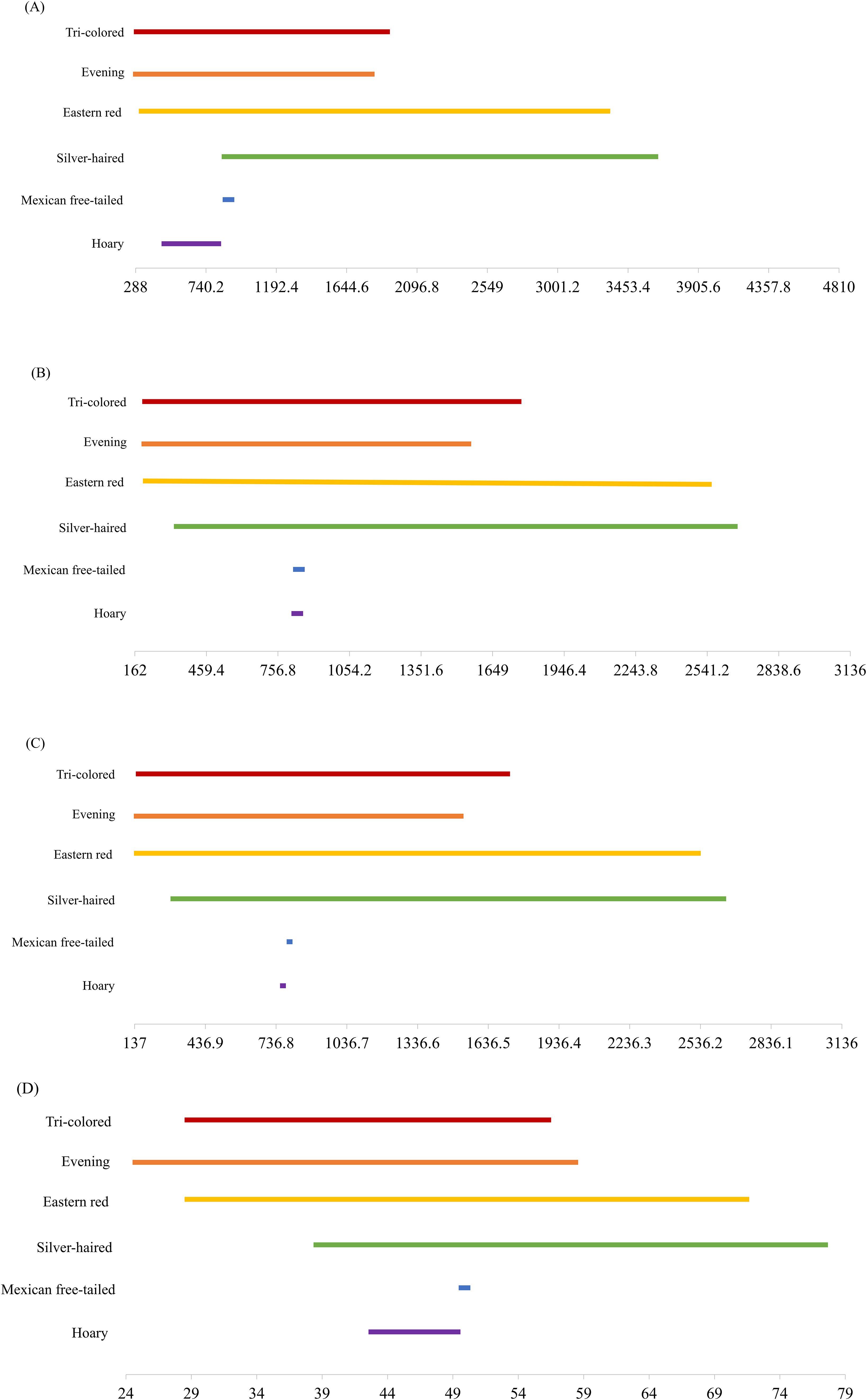
Figure 3. Minimum and maximum values of each surface area metric at sites where each bat species was detected during 1-hour surveys across all study sites. Values are based on the presence of species identified emitting drinking buzzes from acoustic monitoring. Surface area metrics include fundamental surface area (A), realized surface area (B), maximum patch size (C), and longest continuous stretch (D).
Across our GLMMs, we found that the single-variable models did not identify any consistent relationships between surface area metrics and our response variables (Supplementary Data 1). None of the four metrics, fundamental surface area, realized surface area, longest continuous stretch, or maximum patch size, explained variation in bat activity, drinking frequency, or species richness when evaluated independently. In contrast, our grouped models revealed that only one surface area metric influenced bat activity, with effects varying by dependent variable. We found a positive relationship between longest continuous stretch and the duration bats were present within the field of view during our 1-hour surveys across all sites (Table 2). In other words, as the longest stretch increased, the duration bats were present also increased. Similarly for the number of observed drinking events, we only identified a relationship with longest continuous stretch (Table 3). We found that increases in the length of available water led to a corresponding increase in the frequency at which bats drank at our study sites. In comparison, we did not find a relationship between any of our four surface area metrics and species richness (Table 4). Lastly, we found that among all five of these models, monthly variation influenced all three of our response variables, and annual variation affected species richness.
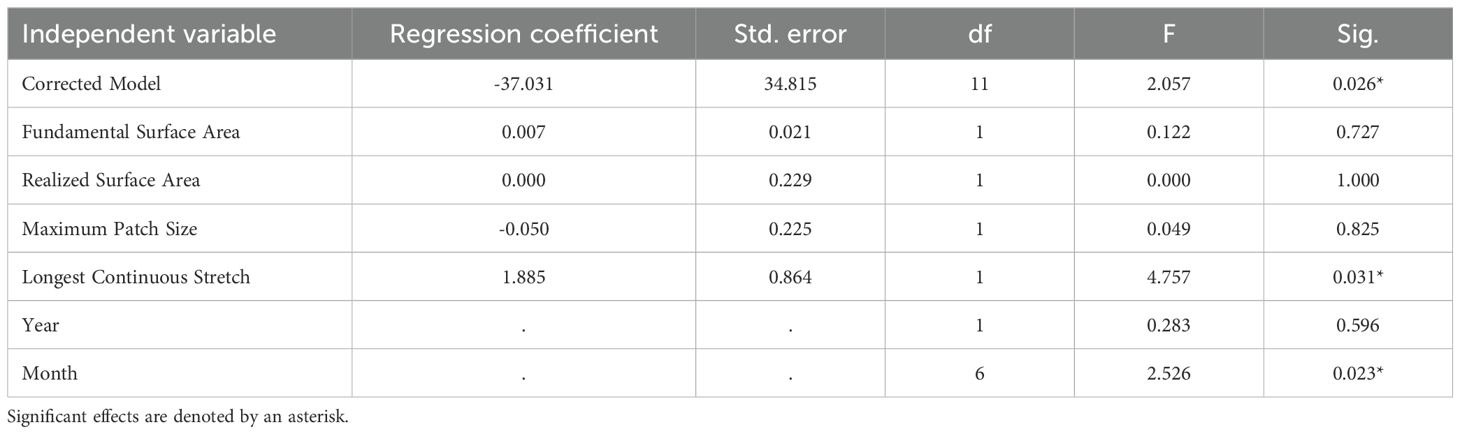
Table 2. Results of General Linear Mixed Model (GLMM) analysis highlighting the effects of four surface area metrics on the total duration bats were observed at six water sources surveyed in Fort Worth, Texas in 2023 and 2024.
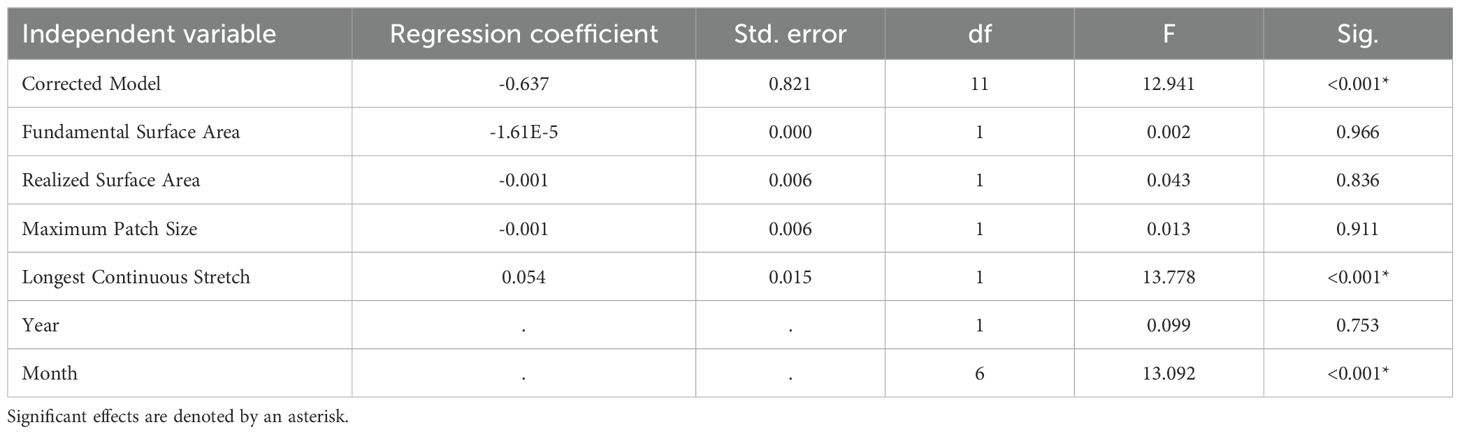
Table 3. Results of General Linear Mixed Model (GLMM) analysis highlighting the effects of four surface area metrics on observed bat drinking events recorded at six water sources surveyed in Fort Worth, Texas in 2023 and 2024.
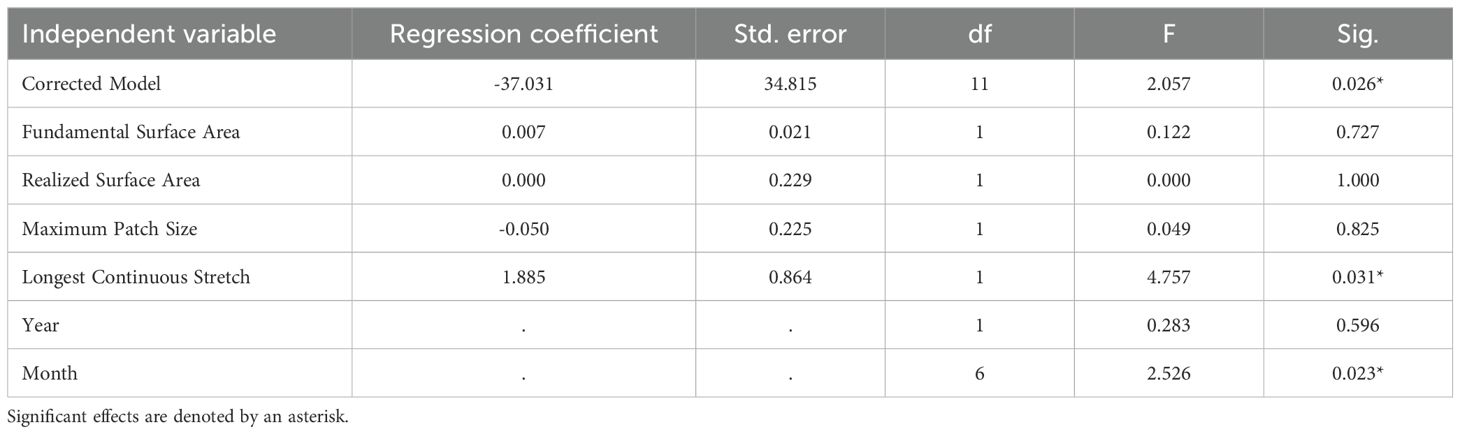
Table 4. Results of General Linear Mixed Model (GLMM) analysis highlighting the effects of four surface area metrics on number of bat species recorded emitting drinking buzzes at six water sources surveyed in Fort Worth, Texas in 2023 and 2024.
4 Discussion
Our study demonstrated that variations in available water surface area can influence the use of urban ponds as drinking resources for bats. More specifically, it confirmed that while water may be present in a given area, it is not necessarily accessible to bats. We found that accessibility was not determined by the overall size of a water source but rather by the shape of unobstructed stretches of surface area that enable bats to drink effectively. Among our other surface area variables, we determined that bat activity increased with the length of an unobstructed water surface. The results of this study also highlight that water accessibility is site-dependent. We observed site-specific differences in the extent to which this variable influenced bat activity, shaped by the composition and configuration of clutter. At Sites 2 and 4, for instance, short-term changes in the presence and distribution of clutter in the form of fallen trees and litter caused frequent fluctuations in bat activity (Figure 4). In addition, we observed monthly variation in bat activity, which appeared to correspond with monthly changes in clutter across sites. Not surprisingly, although water was present throughout the survey period, seasonal conditions influenced accessibility. In particular, during the summer months (when temperatures in our study area were consistently high and rainfall was at its lowest) clutter levels peaked from emergent vegetation, exposed rocks, and algal blooms, and bat activity declined. These temporal fluctuations in clutter, and the resulting shifts in available surface area, highlight that access to water is highly variable over time. As a result, our study demonstrates that even when water is present, it may not always be accessible.

Figure 4. Site 4 with high levels of clutter on 5 September 2023 (A), and with low levels of clutter on 15 September 2023 (B).
At sites where the longest continuous stretch of surface remained constant (such as Sites 1 and 5), bat activity also remained relatively stable, even as fundamental surface area, realized surface area, and maximum patch size fluctuated. This consistency was driven by how the water at these sites evaporated during dry periods: rather than fragmenting into smaller patches, they became thinner, essentially preserving a long continuous stretch of surface area. This finding further underscores the importance of the shape of the water and site-specific nature of water accessibility for bats (Tuttle et al., 2006). Similarly, we found that both Sites 3 and 6 maintained a consistent length of available water surface. Unlike Sites 1 and 5, water levels at these sites did not fluctuate substantially over the survey period. Site 3, a recreational pond in a city park, was kept at full capacity and clutter-free, resulting in minimal variation in bat presence and the highest recorded drinking activity, by far, of any of our survey sites. In contrast, although Site 6 experienced some variation in clutter, this was limited to the edges of the water and the remaining uncluttered area was large enough to consistently provide a long continuous stretch of water. This presence of a consistent stretch of water likely explains the minimal fluctuation in bat activity observed throughout the survey period at this site.
Across all of our study sites, we observed the lowest number of drinking events when the length of the longest continuous stretch of water was less than 30 m. These results align with previous studies that have shown that bats require long, uninterrupted stretches of water for drinking (Razgour et al., 2010; Tuttle et al., 2006). Furthermore, we found that the minimum length required for drinking varied across species. Notably, only evening bats were recorded drinking at sites with stretches shorter than 30 m. As one of the smallest and most agile flyers in the area, it is not surprising that evening bats are less affected by clutter and are known to drink from small water sources less than 10 m in length (Bienz, 2016). In contrast, larger species such as hoary and silver-haired bats were only recorded drinking at sites where the longest unobstructed stretch exceeded 40 m. Even among our other smaller-bodied species, drinking appeared limited by the length of available surface area. Both eastern red and tri-colored bats were only observed at sites with stretches greater than 30 m. Again, this result appears to align with their known level of maneuverability, as edge-space flyers (Norberg and Rayner, 1987). Finally, Mexican free-tailed bats, one of the larger and least maneuverable species in our study (McCracken et al., 2008, 2021), were not only recorded drinking at stretches greater than 50 m, they were rarely recorded drinking in our study. In fact, despite being recorded on acoustic detectors at five of our six survey sites, this species was only recorded drinking at Site 3 on one occasion. Given how highly adapted Mexican free-tailed bats are for fast, open-space flight (Ammerman et al., 2012), it raises the question of whether this species relies on open water as a drinking resource to the same extent as other species and warrants further investigation. Overall, species-specific differences demonstrate that stretches of water over 40 m would allow at least five of the six species present in our study area to access water. Moreover, our findings emphasize the importance of the overall shape of a water source on its accessibility for bats and, in particular, highlight the role of uninterrupted stretches of water in predicting drinking activity and species richness. We also recommend that future research should aim to include a broader range of bat species to better understand how different species interact with varying surface area metrics and clutter. This knowledge would provide further insights into the role of maneuverability and other species-specific factors in water source accessibility for bat communities.
For the other three surface area variables considered in this study, we found that fundamental surface area, realized surface area, and maximum patch size were not effective predictors of bat drinking activity at our sites. These variables did not appear to effectively account for the distribution of clutter, which could vary substantially with little influence on these surface area metrics. Instead, these results demonstrate it is not the size of a water source or its fragments that hinders or encourages bat drinking, but rather the shape of them (i.e., uninterrupted stretches of water), which ultimately determines water accessibility for bats.
In comparison to other studies that have explored the relationship between bat drinking activity and surface area (Jackrel and Matlack, 2010; Laverty and Berger, 2020; Rabe and Rosenstock, 2005; Razgour et al., 2010), our study is the only one that explores the impacts of clutter. In fact, in all three studies cited above, only the overall surface area was considered and their survey sites were maintained to be clutter-free. However, these conditions are not necessarily realistic scenarios, particularly among water sources in urban areas that are subject to high variability due to frequent changes in the presence of clutter caused by litter. We noted that two of our sites (Sites 4 and 6), where bat activity fluctuated more noticeably on a weekly basis, were more prone to litter build-up from the surrounding neighborhoods compared to the other, more stable sites. It is these examples of fluctuations in clutter that further emphasize that access to water is highly variable between sites. We also acknowledge that site-specific differences likely drive bat activity and presence, as each site notably varied in factors beyond surface area, including overall bat activity, the composition of the surrounding landscape, and the availability of other resources.
5 Conclusion
To enhance urban environments for bats, it is essential to evaluate, not only the availability of water sources, but also accessibility. Our findings show that accessibility is predominately shaped by the length of available surface area from which bats can drink, and this is influenced by both seasonal fluctuations in water levels, particularly in areas subject to prolonged dry periods, and physical clutter, such emergent vegetation, algal blooms, litter, and other debris that collects obstructing access. In urban environments, clutter poses a unique challenge, as many available water bodies function as drainage ditches and retention ponds that naturally accumulate litter and debris from surrounding neighborhoods. Their very structure and purpose contribute to the buildup of these materials. Thus, effective management strategies in such areas could focus on maintaining clear, unobstructed stretches of water by removing emergent vegetation, implementing regular community litter clean-up initiatives, clearing accumulations of debris, and even removing algae. Incorporating these approaches into urban water management plans will help ensure that water resources remain accessible to bats year-round and support their long-term survival in urban landscapes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
PH: Formal Analysis, Methodology, Writing – original draft, Investigation, Writing – review & editing. VB: Data curation, Project administration, Methodology, Conceptualization, Supervision, Writing – review & editing, Resources, Writing – original draft, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the City of Fort Worth Parks and Recreation and the Army Corps of Engineers for permitting us to conduct surveys at our study sites. Furthermore, we thank the technicians of the TCU Bat Lab for assisting in data collection including Aleah Appel, Vianca Arias, Kait Beerman, Andrew Campola, Ty Cleveland, Kate Davis, Manuel de Oyarzabal Barba, Riley Ederlein, Lexi Foster, Jackson Galloway, Audrey Haffner, Elizabeth Hargis, Athalia Hite, Nicole Kiczek, Emma Kovalsky, Delanie McClanahan, Kyla Moberly, Kaitlyn Roussel, I’Yanna Scott, Gloria Serrano, Zoey Suasnovar, Elise Skiles, Caroline Waldvogel, Justyn Wallace, Abi Welch.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript.
To check for grammatical errors after a completed manuscript had been written.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1596619/full#supplementary-material
References
Agpalo E. (2020). Improving Urban Habitats for Bats: What Makes a Bat-Friendly Residential Swimming Pool? master’s thesis (Fort Worth (TX: Texas Christian University).
Ammerman L. K., Hice C. L., and Schmidly D. J. (2012). Bats of Texas (College Station: Texas A&M University Press).
Ancillotto L., Bosso L., Salinas-Ramos V., and Russo D. (2019). The importance of ponds for the conservation of bats in urban landscapes. Landsc. Urban. Plan. 190, 103607. doi: 10.1016/j.landurbplan.2019.103607
Bergey E. A. and Getty G. M. (2006). A review of methods for measuring the surface area of stream substrates. Hydrobiologia. 556, 7–16. doi: 10.1007/s10750-005-1042-3
Berthon K., Thomas F., and Bekessy S. (2021). The role of ‘nativeness’ in urban greening to support animal biodiversity. Landsc. Urban. Plan. 205, 103959. doi: 10.1016/j.landurbplan.2020.103959
Bienz C. (2016). Surface texture discrimination by bats: Implications for reducing bat mortality at wind turbines. master’s thesis (Fort Worth (TX: Texas Christian University).
Buchholtz E. K., Spragg S., Songhurst A., Stronza A., McCulloch G., and Fitzgerald L. A. (2021). Anthropogenic impact on wildlife resource use: Spatial and temporal shifts in elephants’ access to water. Afr. J. Ecol. 59, 614–623. doi: 10.1111/aje.12860
Conan A., Le Brishoual M., Garnier L., Fleitz J., Dehaut N., Enstipp M., et al. (2023). Efficacy of permanent wildlife fences as barriers to amphibian movement. Front. Ecol. Evol. 11. doi: 10.3389/fevo.2023.1074072
Elbroch M. L. and Allen M. L. (2013). Prey indices and behaviors at a gray fox den in San Mateo County, California. West. N. Am. Nat. 73, 240–243. doi: 10.3398/064.073.0215
Felderhoff J., Gathof A. K., Buchholz S., and Egerer M. (2023). Vegetation complexity and nesting resource availability predict bee diversity and functional traits in community gardens. Ecol. Appl. 33, e2759. doi: 10.1002/eap.2759
Feng Y., Zhang H., Tao S., Ao Z., Song C., Chave J., et al. (2022). Decadal lake volume changes, (2003-2020) and driving forces at a global scale. Remote Sens. 14, 1032. doi: 10.3390/rs14041032
Finn R. J. R., Chalifour L., Gergel S. E., Hinch S. G., Scott D. C., and Martin T. G. (2021). Quantifying lost and inaccessible habitat for Pacific salmon in Canada’s Lower Fraser River. Ecosphere. 12, e03646. doi: 10.1002/ecs2.3646
Greenfeld A., Saltz D., Kapota D., and Korine C. (2018). Managing anthropogenic driven range expansion behaviorally: Mediterranean bats in desert ecosystems. Eur. J. Wildl. Res. 64, 24. doi: 10.1007/s10344-018-1182-1
Hall E. M. and Bennett V. J. (2021). Seasonal variation in home range size of evening bats (Nycticeius humeralis) in an urban environment. J. Mammal. 102, 1497–1506. doi: 10.1093/jmammal/gyab106
Hall L. K., Lambert C. T., Larsen R. T., Knight R. N., and McMillan B. R. (2016). Will climate change leave some desert bat species thirstier than others? Biol. Conserv. 201, 284–292. doi: 10.1016/j.biocon.2016.07.020
Hansen C. P., Parsons A. W., Kays R., and Millspaugh J. J. (2020). Does use of backyard resources explain the abundance of urban wildlife? Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.570771
Huzzen B. E., Hale A. M., and Bennett V. J. (2020). An effective survey method for studying volant species activity and behavior at tall structures. PeerJ. 8, e8438. doi: 10.7717/peerj.8438
Jackrel S. L. and Matlack R. S. (2010). Influence of surface area, water level and adjacent vegetation on bat use of artificial water sources. Amer. Midl. Naturalist. 164, 74–79. doi: 10.1674/0003-0031-164.1.74
Kataoka T. and Nihei Y. (2020). Quantification of floating riverine macro-debris transport using an image processing approach. Sci. Rep. 10, 2198. doi: 10.1038/s41598-020-59201-1
Kloskowski J., Rechulicz J., and Jarzynowa B. (2013). Resource availability and use by Eurasian otters Lutra lutra in a heavily modified river-canal system. Wildl. Biol. 19, 439–451. doi: 10.2981/12-104
Konapala G., Mishra A. K., Wada Y., and Mann M. E. (2020). Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 11, 3044. doi: 10.1038/s41467-020-16757-w
Laverty T. M. and Berger J. (2020). Do bats seek clean water? A perspective on biodiversity from the Namib Desert. Biol. Conserv. 248, 108686. doi: 10.1016/j.biocon.2020.108686
Leveau L. M. and Ibáñez I. (2022). Nesting site and plumage color are the main traits associated with bird species presence in urban areas. Animals. 12, 1148. doi: 10.3390/ani12091148
McCracken G. F., Gillam E. H., Westbrook J. K., Lee Y. F., Jensen M. L., and Balsley B. B. (2008). Brazilian free-tailed bats (Tadarida brasiliensis: Molossidae, Chiroptera) at high altitude: links to migratory insect populations. Integr. Comp. Biol. 48, 107–118. doi: 10.1093/icb/icn033
McCracken G. F., Lee Y. F., Gillam E. H., Frick W., and Krauel J. (2021). “Bats flying at high altitudes,” in 50 Years of Bat Research. Eds. Lim B. K., Fenton M. B., Brigham R. M., Mistry S., Kurta A., Gillam E. H., Russell A., and Ortega J. (New York: Springer), 189–205.
Norberg U. M. and Rayner J. M. V. (1987). Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philo. Trans. R. Soc B: Biol. Sci. 316, 335–427. doi: 10.1098/rstb.1987.0030
Nystrom G. S. and Bennett V. J. (2019). The importance of residential swimming pools as an urban water source for bats. J. Mammal. 100, 394–400. doi: 10.1093/jmammal/gyz020
Rabe M. J. and Rosenstock S. S. (2005). Influence of water size and type on bat captures in the lower sonoran desert. West. N. Am. Nat. 65, 87–90. Available online at: https://scholarsarchive.byu.edu/wnan/vol65/iss1/10.
Razgour O., Korine C., and Saltz D. (2010). Pond characteristics as determinants of species diversity and community composition in desert bats. Anim. Conserv. 13, 505–513. doi: 10.1111/j.1469-1795.2010.00371.x
Rodríguez J. D. and Sánchez F. (2022). Physical clutter affects the use of artificial ponds by the Lesser Bulldog Bat Noctilio albiventris (Chiroptera: Noctilionidae). Pap. Avulsos. Zool. 62, e202262019. doi: 10.11606/1807-0205/2022.62.019
Russo D., Ancillotto L., Cistrone L., and Korine C. (2016). The buzz of drinking on the wing in echolocating bats. Ethology 122, 226–235. doi: 10.1111/eth.12460
Smith K. (2019). Assessing the Potential Impacts of Radio Transmitters on Bat Flight and Behavior in a Controlled Environment. master’s thesis (Fort Worth (TX: Texas Christian University).
Snyder R., Mausteller E., Matlaga T. J. H., and Miller D. A. W. (2022). How does landscape permeability affect the movement of eastern red-backed salamanders? J. Wildl. Manage. 86, e22132. doi: 10.1002/jwmg.22132
Tsalyuk M., Kilian W., Reineking B., and Getz W. M. (2019). Temporal variation in resource selection of African elephants follows long-term variability in resource availability. Ecol. Monogr. 89, e01348. doi: 10.1002/ecm.1348
Tuttle S. R., Chambers C. L., and Theimer T. C. (2006). Potential effects of livestock water-trough modifications on bats in Northern Arizona. Wildl. Soc Bull. 34, 602–608. doi: 10.2193/0091-7648(2006)34
Warren R. D., Waters D. A., Altringham J. D., and Bullock D. J. (2000). The distribution of Daubenton's bats (Myotis daubentonii) and pipistrelle bats (Pipistrellus pipistrellus) (Vespertilionidae) in relation to small-scale variation in riverine habitat. Biol. Conserv. 92, 85–91. doi: 10.1016/S0006-3207(99)00062-2
Zheng L., Zhan P., Xu J., Xu L., Tan Z., and Wang X. (2020). Aquatic vegetation dynamics in two pit lakes related to interannual water level fluctuation. Hydrol. Process. 34, 2645–2659. doi: 10.1002/hyp.13757
Keywords: Chiroptera, drinking behavior, urban wildlife, resource accessibility, resource availability, unmanned aerial vehicle
Citation: Harper PE and Bennett VJ (2025) Variation in water surface area and its impacts on bat drinking activity in an urban environment. Front. Ecol. Evol. 13:1596619. doi: 10.3389/fevo.2025.1596619
Received: 19 March 2025; Accepted: 30 May 2025;
Published: 23 June 2025.
Edited by:
Rusty Gonser, Indiana State University, United StatesReviewed by:
Laura C. Pereyra, CONICET Instituto de Ecorregiones Andinas (INECOA), ArgentinaVanessa Gorecki, University of Southern Queensland, Australia
Copyright © 2025 Harper and Bennett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victoria J. Bennett, di5iZW5uZXR0QHRjdS5lZHU=
 Peyton E. Harper
Peyton E. Harper Victoria J. Bennett
Victoria J. Bennett