- 1Department of Biosciences and Territory, University of Molise, Termoli, CB, Italy
- 2National Biodiversity Future Center (NBFC), Palermo, PA, Italy
- 3Department of Agriculture, Environment and Food Sciences, University of Molise, Campobasso, CB, Italy
Introduction
Coastal dunes are dynamic ecosystems influenced by both natural and anthropogenic processes that shape vegetation zonation and faunal assemblages within dunes (Acosta et al., 2007; Bessa et al., 2013; Delgado-Fernandez et al., 2019). They are included in the European Habitat Directive (European Council Directive 92/43/EEC) as ecosystems of conservation interest, and they are largely considered in a bad (or inadequate) conservation status (Genovesi et al., 2014; Prisco et al., 2020). The maintenance of these environments is also essential for the ecosystem services they provide (Drius et al., 2019), such as climate regulation (Drius et al., 2016), protection from wind and aerosol (Bonari et al., 2017), control of coastal erosion (Drius et al., 2013), as reservoirs and shelter of biodiversity (Maiorano et al., 2015; Drius et al., 2016; Pellissier et al., 2020; Rasino et al., 2024), as well as recreation and tourist resources (Mastronardi et al., 2015; Petrosillo et al., 2007).
However, wildlife may cause direct or indirect impacts due to consumption or trampling of vegetation and may alter the composition/structure of soil (Enquist et al., 2020; Warner and Cushman, 2002). Consequently, native plant communities may change as nitrophilous species and/or new generalist or alien species take advantage (Amori et al., 2016; Forbes et al., 2019; Kristensen et al., 2022; Mori et al., 2021a). Coastal dune habitats are considered as transitional ecosystems, harboring important refugia for wildlife in human-dominated landscapes, but they are still understudied concerning their role as habitat for wildlife (Kissling et al., 2024; Rendall et al., 2019).
Wildlife monitoring and inventory pose more challenges than vegetation monitoring due to animals’ continuous movements and varying activity periods (Simo et al., 2023). The use of camera traps has become an excellent alternative to traditional field surveys of medium and large-size mammal species, and their use is increasing over time thanks to their effectiveness and non-invasiveness (Buxton et al., 2018; Kissling et al., 2024; Kleiven et al., 2023; van Meurs et al., 2024). Camera traps consist of automatically triggered cameras that allow collecting photographic records of species at a specific location (Rovero and Zimmermann, 2016; O’Connell et al., 2011). They represent a good alternative for detecting and monitoring animals in remote areas (van Meurs et al., 2024), and invasive and problematic species (Di Cerbo and Biancardi, 2013; Ferretti et al., 2021; Piscopo et al., 2023), and improving knowledge of species ecology and behavior (Wong and Kachel, 2024).
The aim of our work is to describe the CAM-COAST database, which contains bird and mammal image records detected in coastal dune habitats of an Italian Natura 2000 Network site (N2K), across different seasons and daily phases using photo-trapping techniques. The study also provides a preliminary descriptive analysis of the collected data to outline the seasonal and daily distribution of the observed fauna, and determine the potential pressures and threats that may affect the conservation of EU habitats in the area.
This study was part of the global research project called LIFEPLAN – A Planetary Inventory of Life, funded by the European Research Council (ERC) and coordinated by Helsinki University (Rogers et al., 2023), with around 200 globally distributed research sites, aiming at improving knowledge of biodiversity worldwide and using datasets for predictions and future scenarios (Rogers et al., 2023). LIFEPLAN is both running a global sampling biodiversity program and developing advanced bioinformatics and statistical approaches to make the best use of these data (Hardwick et al., 2024). Camera-trapping activity is still being conducted to implement international standards for data collection and analysis and promote collaborative, open data networks aimed at enhancing wildlife monitoring.
Site selection
The sampling site (Figure 1) is located in the N2K SAC IT7228221 Foce Trigno-Marina di Petacciato (Italy), also included in the eLTER (integrated European Long-Term Ecosystem, critical zone and socio-ecological Research) network (Wohner et al., 2019), established to facilitate high-impact research and to catalyze new insights about the compounded impacts of climate change, biodiversity loss, soil degradation, pollution, and unsustainable resource use in terrestrial, freshwater, and transitional water ecosystems (Stoll et al., 2015; Mazzocchi et al., 2019). It is characterized by a natural vegetation zonation of sand coastal dunes (Prisco et al., 2016) of a great naturalistic value, with 11 habitats of European conservation concern (EU) (Stanisci et al., 2014; Prisco et al., 2015; Di Paola et al., 2022). The habitat zonation (following the sea-inland gradient) consists of embryonic and shifting dune habitats (EU habitats 1210, 2110, 2120), transition dunes with annual grassland (EU habitats 2230), well-preserved low Mediterranean maquis (EU habitats 2260), and back dunes with pine forest (EU habitat 2270*) (Del Vecchio et al., 2015) (Figure 1). In dune habitats of this N2K site the following bird species are known (EEA; GBIF.org, 2025; Compagnone et al., 2023): Cettia cetti (Temminck, 1820), Buteo buteo (Linnaeus, 1758), Sturnus vulgaris (Linnaeus, 1758), Passer italiae (Vieillot, 1817), Hirundo rustica (Linnaeus, 1758) Corvus monedula (Linnaeus, 1758) Corvus cornix (Linnaeus, 1758), Cisticola juncidis (Rafinesque, 1810), Hirundo rustica (Linnaeus, 1758), Galerida cristata (Linnaeus, 1758),; Emberiza calandra (Linnaeus, 1758), and Pica pica (Linnaeus, 1758). These last three species are also included in our database while no mammal species was reported for this area before our study.
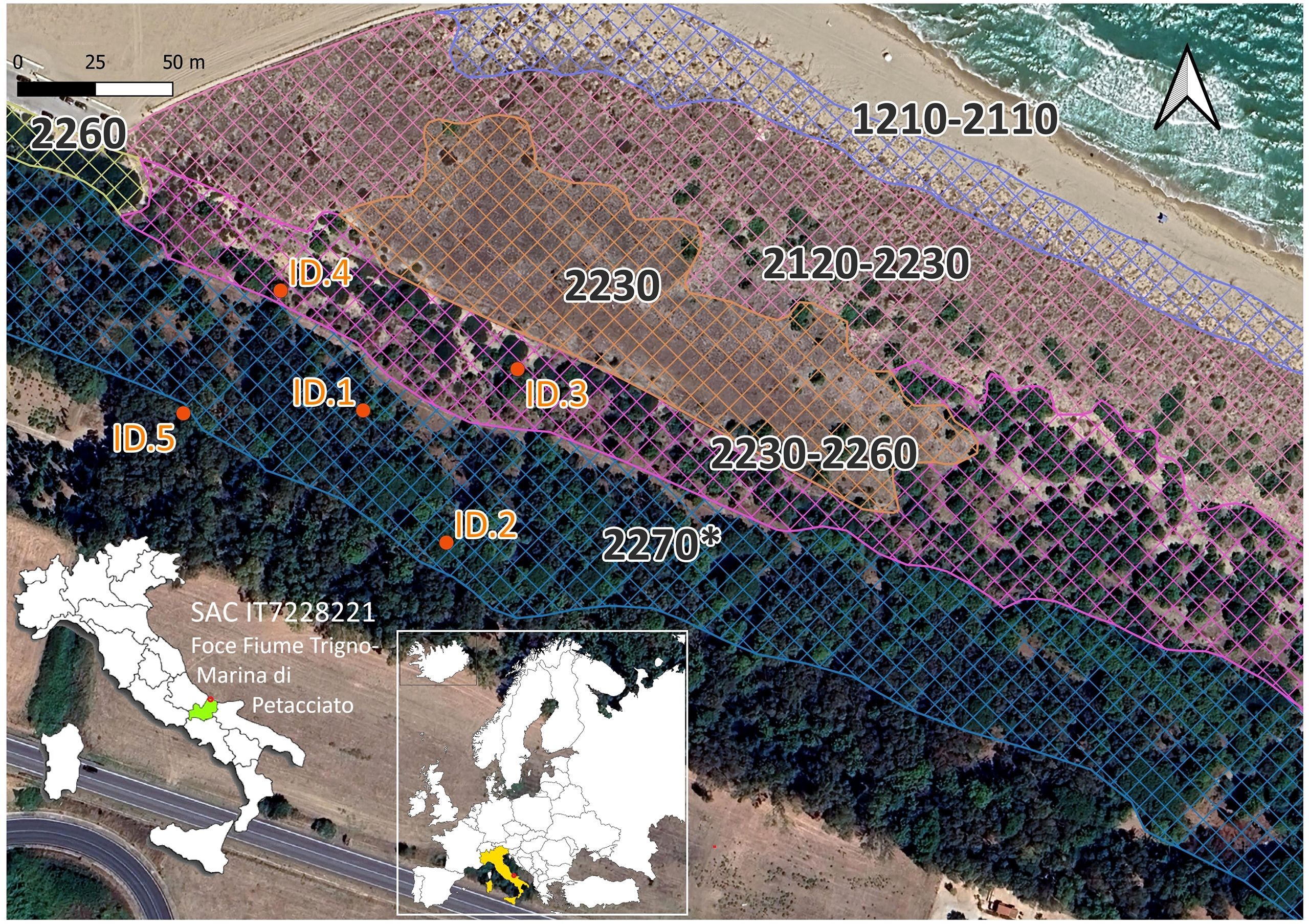
Figure 1. Localization of LIFEPLAN Research Site (orange dots referred to five camera traps with corresponding IDs). The image shows the following EU habitats: 1210 – Annual vegetation of drift lines; 2110 – Embryonic shifting dunes; 2120 – Shifting dunes along the shoreline with Ammophila arenaria (white dunes); 2230 – Malcolmietalia dune grassland; 2260 – Cisto-Lavanduletalia dune sclerophyllous scrub; 2270* – Wooded dunes with Pinus pinea and/or Pinus pinaster. The geographical coordinates of each camera trap are reported in Supplementary Table S1.
Data collection and description
Five camera traps (model 4.0C from Wildlife Monitoring Solutions) were placed within a one-hectare natural area, according to the sampling design of the LIFEPLAN project (Hardwick et al., 2024), as shown in Figure 1. Here, the coastal landscape is characterized by three habitats of European conservation concern (EU habitats 2230, 2260, and 2270*), close to embryonic and mobile habitats 1210, 2110, and 2120. (Habitat Directive 92/43/CEE) (Supplementary Table S1).
Each camera trap was fixed on a tree at 0.5 m from the ground and oriented downward and was motion activated by a passive infrared sensor, taking a series of 5 photographs approximately 1 s apart (5-megapixel color CMOS) with 2560x1920 effective pixels and IR range of 20 m. Following the LIFEPLAN protocol, they were equipped with an access code to minimize the risks of tampering (Rogers et al., 2023) and were active for 233 days from February to December 2021. Camera trapping captured images of mammals and some occasional birds.
Image records were previously analyzed through visual inspection image by image to eliminate repetitive images of the same specimen separated by fractions of a second both photographed by the same camera trap and by nearby camera traps, considering that the space separating the 5 camera traps is too small to identify them as completely independent from each other (Bowkett et al., 2008; Rovero and Zimmermann, 2016). A thorough examination of each individual photo sequence was conducted to identify separate photo- capture events. Subsequent images of the same species individuals captured within 30 minutes of each other were considered single events if the individuals could not be clearly recognized by particular signs related to sex, age group or body markings. Subsequently, images were analyzed with the aid of specialist literature and taxonomic manuals to identify the species (Amori and Nappi, 2011; Corbet and Ovenden, 2012; Paolucci and Mauro, 2022).
Camera traps produced a total of 2932 wildlife pictures in 233 days for a total of 766 occurrences (Supplementary Table S2).
CAM-COAST database included for each recorded image the following attributes, as requested by Darwin Core guidelines of GBIF repository: a) the ID number of image record; b) the number of occurrences; b) the taxonomic description at species and subspecies level with scientific name and authors (Avibase; Mammal Diversity); c) English common name (Avibase; Mammal Diversity); d) date and time of records; e) daily phases (as for Desk Aeronautico; Time and Date AS, 1995–2025) and seasons (identification refers to ISTAT) of records; f) number of individuals per each camera image; g) total occurrences after aggregation of repetitive images. Moreover, the European Conservation Status of species listed in the CAM-COAST is reported in Supplementary Table S3 (Supplementary Materials).
Methods applied for exploratory analyses
We performed exploratory analyses to examine the frequency of species observations, we calculated the Relative Abundance Index (RAI), using the following formula (Lim et al., 2023; Palmer et al., 2018):
where Di represents the number of days with observations for a specific species and N the total number of observation days for all the camera traps.
To analyze the seasonal and daily activity patterns of the recorded species, we employed two complementary abundance indices: The Monthly Relative Abundance Index (MRAI) and the Daily Phase Relative Abundance Index (DRAI) (Liu et al., 2013). MRAI was calculated as:
where DM is the number of days in which a species was recorded within a given month, and N is the total number of sampling days for all species. This index allowed us to quantify relative abundance across months and to group these monthly values into seasonal categories (spring, summer, autumn, winter). To explore diel activity, we used the DRAI, defined as:
where DD represents the number of days a given species was detected during a specific phase of the day (sunrise, daylight, sunset, or night), and N is the total number of observations per daily phase across all species.
We then visualized these indices using boxplots, to display the data distribution with minimum and maximum scores and median, and barplots, to illustrate the relationship between a numeric and a qualitative variable, by season (spring, summer, autumn, winter) and daily phases (sunshine, daylight, sunset, night). These graphical approaches were chosen to summarize the variation in species activity patterns over time. For consistency in temporal reference, the 15th day of each month was used as the standard midpoint for monthly grouping.
In addition, we calculated the RAI for each species separately for EU habitat 2270* and for EU habitat mosaic 2230/2260.
The identification of daily phases (sunrise, daylight, sunset, and night) for 2021 was based on a calendar specific to the study area (Desk Aeronautico; Time and Date AS, 1995–2025). We used the timings of sunrise and sunset to calculate the duration of each daily phase on a weekly basis. Each camera trap photograph is time-stamped, allowing us to classify occurrences into their respective daily phases.
Descriptive analyses were conducted using R Statistical Software (v4.3.0; R Core Team, 2023), using the “ggplot2” package for donut chart, bar chart, and boxplot; “dplyr”, “networkD3” (Gandrud et al., 2017) package for Sankey diagram.
Database structure and application
We collected a total of 2932 records of images, with 2191 records of Mammalia class (74.7% of the total) and 741 records of Aves class (25.3% of the total) in 233 days (Figure 2A); after aggregation of repetitive images of the same individual, we detected a total of 766 individual occurrences, 586 of mammals (76.5% of the total) and 180 occurrences of birds (23.5% of the total) (Figure 2), corresponding to7 mammal species and 13 bird species (Supplementary Table S2).
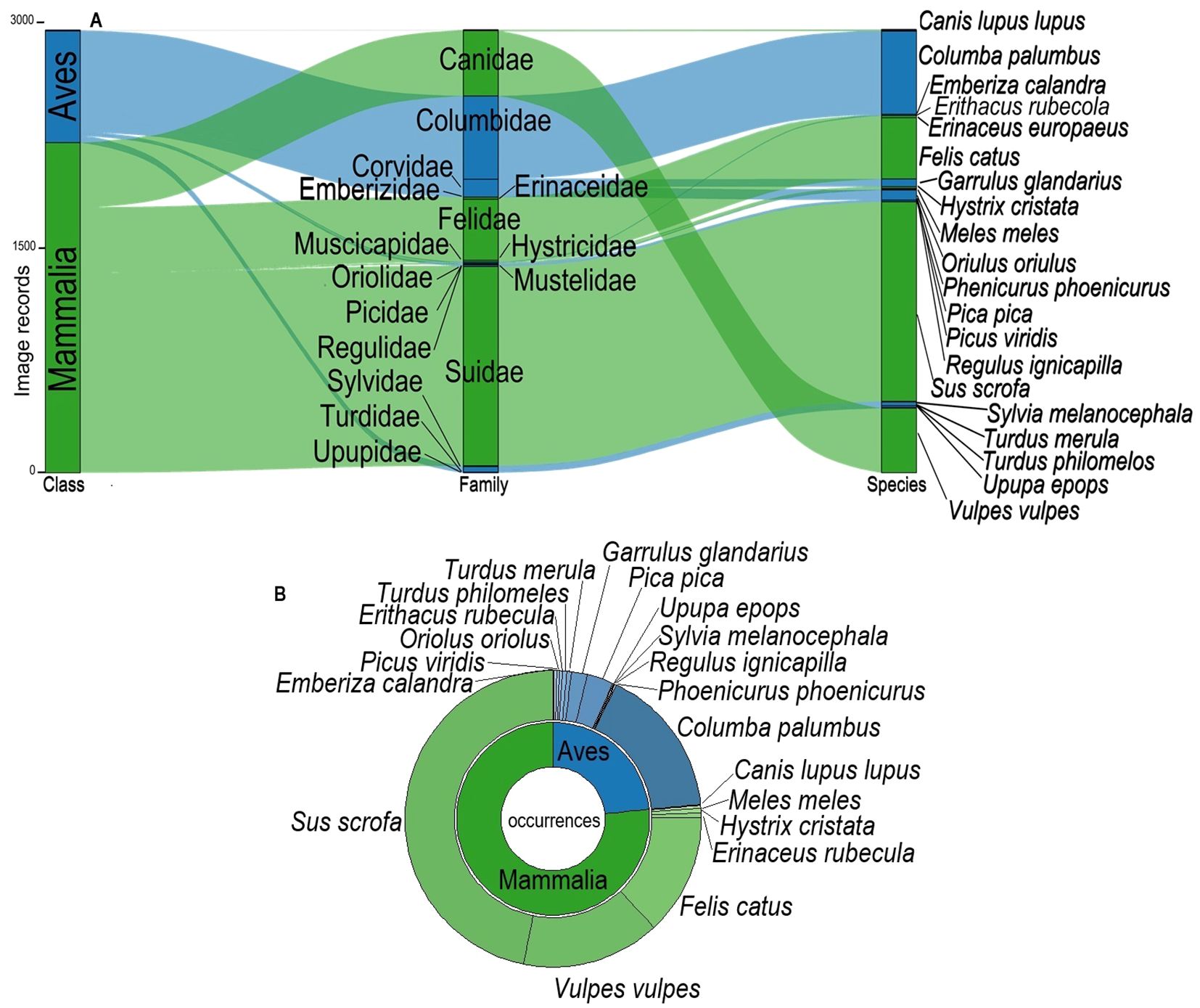
Figure 2. Sankey diagram, (A) represents the distribution of the image records into families and species; (B) shows the distribution of occurrences at species level of mammals and birds. In green the mammals and in blue the birds.
Most records and occurrences (as shown in Figure 2) refer to mammals rather than birds, and in particular to the families Suidae (Sus scrofa, Linnaeus, 1758), Canidae (Vulpes vulpes, Linnaeus, 1758) and Felidae (Felis catus, Linnaeus, 1758); among birds, most records and occurrences concern the family Columbidae (Columba palumbus, Linnaeus, 1758).
CAM-COAST provided new fauna data for the Adriatic coast, and updated data on rare species of conservation interest, as well as problematic mammal species, for which specific conservation and management measures should be implemented within the N2K (Rovero and Zimmermann, 2016).
CAM-COAST represents the first organized database related to a coastal N2K site in Italy and despite some critical gaps, such as the short time span of data collection and the small sampling area, we think that it is a first step to be implemented in the future with other data. Results may provide some important assessments for the management and conservation of EU coastal habitats.
Furthermore, the database shows the potential for studying, researching, and managing species and habitats that are of conservation importance (Kissling et al., 2024).
Possible applications of image records and occurrence data in CAM-COAST:
1. Biodiversity Monitoring.
a. An occurrence database enriched with images enables spatial and temporal monitoring of coastal species, including rare, invasive, or threatened taxa.
b. It supports the identification of trends and changes in species distribution and abundance over time (daily phases and seasons).
2. Data Validation.
a. The inclusion of images and repeated records of the same individual enhances the reliability of taxonomic identifications, which is particularly valuable in citizen science and historical datasets.
b. The availability of multiple images allows for retrospective validation by taxonomic experts and the broader scientific community.
3. Conservation Planning.
a. Records of rare or conservation-priority species contribute to the development or revision of local and national Red Lists.
b. Spatially and temporally explicit data facilitate the planning of protected areas and ecological corridors.
4. Applications in Artificial Intelligence and Computer Vision.
The photographic dataset can be used to train automated species recognition algorithms (e.g., convolutional neural networks) and facilitate their application in automated biodiversity monitoring, such as through camera traps or drone surveys.
The dataset fills a gap in mammal-bird co-occurrence patterns and temporal activity rhythms in a coastal Mediterranean habitat, which are underrepresented in current ecological datasets. We emphasize its value for long-term ecological monitoring, conservation management within N2K and eLTER networks, and as a baseline for evaluating anthropogenic pressures and climate-driven changes in wildlife.
Data accessibility
CAM-COAST ensures standardized data organization and promotes open access, providing free download of the database and metadata through the GBIF repository [https://cloud.gbif.org/eca/resource?r=cam-coast], and image resources via Zenodo [https://zenodo.org/records/15622278].
Exploratory analysis utilizing the relative abundance index
RAI values calculated for all species (Supplementary Table S4) showed a high relative abundance of mammal species. The most frequent was wild boar (Sus scrofa Linnaeus, 1758) (44.2% RAI), followed by red fox (Vulpes vulpes Linnaeus, 1758) (33.5% RAI), and domestic cat (Felis catus Linnaeus, 1758) (28.7% RAI). Other species detected at lower frequency were the Western European hedgehog (Erinaceus europaeus Linnaeus, 1758) and the European badger (Meles meles Linnaeus, 1758).
Bird species with good representativeness in the study site are common wood pigeon Columba palumbus (14.6% RAI), the Eurasian magpie Pica pica (6.0% RAI) and Eurasian jay Garrulus glandarius (3.4% RAI); RAI values of these species were significantly higher than RAI of other bird species.
Although with minimal relative abundances, two mammal species of EU conservation importance, the gray wolf (Canis lupus lupus Linnaeus, 1758) and the crested porcupine (Hystrix cristata Linnaeus, 1758), were also recorded, respectively with one and four occurrences. The distribution of mammals’ total occurrences by seasons and daily phases are reported in Supplementary Table S5 and Supplementary Figure S6 (Supplementary Materials). In terms of mammal occurrences by season, the highest rate was observed in spring (35.1%), followed by autumn, while winter had the lowest value (4.9%) (Supplementary Figure S6A). Mammals detected in all seasons are wild boar, common red fox, and domestic cat (Supplementary Figure S6A). Wild boar was the most observed species in spring and summer (17.9%). The red fox was more frequent in autumn (15.1%), while the domestic cat mainly visited the target site in spring and autumn (13.2%).
All the following descriptive data analysis was conducted exclusively for mammals. Seasonal RAI calculated per EU habitats mosaic 2230–2260 and 2270* (Supplementary Table S5) showed statistical differences only for autumn season, with a preference for the habitats with annual vegetation and maquis (EU habitats 2230/2260) (Supplementary Figure S7).
Concerning the daily phases, mammal occurrence was highest during the night, and subordinately in the daylight phase, while the lowest occurrence was recorded at sunrise (Supplementary Table S5; Supplementary Figures S6B, S8).
DRAI values significantly differed among seasons (Supplementary Figure S9). In spring, daylight had the highest value, while sunrise had the lowest. During summer, DRAI values were generally low, with a significant increase at sunset. In autumn, DRAI values were significantly higher during both daylight and night. In winter, the values remained very low, with a slight increase during daylight and night.
The high number of wild boar and domestic cat occurrences may represent a threat for biodiversity conservation in the analyzed N2K site, as recorded in other natural areas (Chadwick et al., 2022).
Two mammal species of EU conservation interest are to be highlighted, the gray wolf (priority species of II and IV Annexes – HD92/43/EEC) and the crested porcupine (priority species of IV Annex – HD92/43/EEC), even if they are rare. The gray wolf trophic niche is centered on available wild/domestic ungulates and Central Italy hosts one of the highest densities of wild ungulates in Europe (Apollonio et al., 2010), especially roe deer (Capreolus capreolus Linnaeus, 1758) and wild boar (Cerri et al., 2023). This latter was the most abundant species in our study area, likely attracting the wolf in this most unsuitable area (Mattioli et al., 1995; Nores et al., 2008). According to Ercole et al. (2021), the study area is located outside of the traditional Italian range of the gray wolf. However, in the last decade, an extension of the gray wolf’s range toward the coastal habitats was detected (La Morgia et al., 2022).
The crested porcupine is experiencing a range expansion in various Italian territories, but it was rarely detected in the coastal area (Mori et al., 2013). Climate change and the abandonment of agricultural practices have favored the colonization of residual coastal woody areas, where new records have been collected in recent years (Mori et al., 2021b).
As far as the most recorded species, the wild boar, it worth to note that it has been listed among the “World’s Worst Invaders”, because it may cause a loss of plant diversity, the damage to crops and forestry, disease transmission to livestock, and habitat changes for other species (Carpio et al., 2021; Valente et al., 2020).
In Italy, in the first half of the last century, wild boars were reduced to some fragmented and small populations; conversely, in the last decades, the Italian range of the species has rapidly increased (Boitani et al., 1994; Massei et al., 1997; Meriggi and Sacchi, 2001; Merli and Meriggi, 2006), with a high presence in the Molise Region due to the abandonment of agricultural activities, renaturalization of vast areas, and increase in protected areas off-limits to hunting activity. These protected areas act as a refuge for the species, using these spaces as daytime shelters and/or breeding areas (Miraglia and Brita, 2022). The wild boar has strong negative effects on the surface layers of the soil due to its rooting behavior (Mori et al., 2020), causing widespread trampling (Mori et al., 2020; Mori and Lazzeri, 2021). This behavior causes the mix of soil horizons and the alteration of the nutrient retention rate, which also enhances the erosion processes, alters plant succession, favors exotic plant species invasion, and may affect wildlife communities and threatened and endangered species (Calosi et al., 2024; Mack and D’Antonio, 1998; McClure et al., 2018; Rosell et al., 2001).
Still, in rural environment, which are common in sub-coastal areas, wild boars damage croplands by consuming crops, rooting in search of bulbs, invertebrates or tubers, removing seeds, trampling and damaging agricultural infrastructures (Cappa et al., 2019; Fattorini and Ferretti, 2020). Moreover, the continuous movement across different habitats makes the wild boar a vector of seeds or spores of alien and ruderal plant species, which grow quickly in altered environments (Chandru et al., 2020; Dovrat et al., 2012). A significant decline in plant species diversity and plant species of conservation concern was observed after 20 years of stabilizing a wild boar population in a Mediterranean forest (Todini and Crosti, 2020). Other studies evidenced that the presence of wild boar determines great differences in the composition and abundance of species in the herbaceous layer of Mediterranean woods (Burrascano et al., 2015a, b) and in coastal prairie in Northern California (Cushman et al., 2004).
Finally, in our study area, the domestic cat was widely present. It is an opportunistic and generalist predator (Loyd et al., 2013; Thomas et al., 2012) and is considered among the main threats to global biodiversity (Doherty et al., 2016, 2017; Loss et al., 2022; Nogales et al., 2004). In urban and semi-natural areas, domestic cats have a great impact on local wildlife, especially on birds and small mammals (Hanmer et al., 2017). Impacts include competition, predation, ecological disturbance, disease transmission, and environmental contamination (Baker et al., 2008; Thomas et al., 2012). Due to the above effects, the presence of this mammal in the area could be detrimental to the biodiversity that hosts this N2K site.
Despite the recognized ecological value of the site, comprehensive faunistic surveys, particularly for mammals and birds, are limited. The CAM-COAST database provides essential data for improving our understanding of how Mediterranean coastal dunes serve as habitats for wildlife and the threats facing native biodiversity.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
MF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CH: Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. MR: Formal Analysis, Investigation, Software, Visualization, Writing – review & editing. ASc: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. ASt: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was partially funded by University of Helsinki (LIFEPLAN project grant agreement No 856506 European Research Council under the European Union’s Horizon 2020 research and innovation program). The research was also partially supported by the University of Tuscia and the Institute of Research on Terrestrial Ecosystems (IRET) of the National Research Council of Italy (CNR) in the framework of the ITINERIS project - Italian Integrated Environmental Research Infrastructures System and by the e-LTER (European Long Term Ecosystem, critical zone, and socio-ecological Research). The research also received the support of National Recovery and Resilience Plan (NRRP)-Spoke 5 Urban Biodiversity. Funder: Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment. 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU. Award Number: Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP, H43C22000530001 Project title “National Biodiversity Future Center - NBFC”.
Acknowledgments
The authors thank Mr. Antonio Del Vecchio and Mr. Francesco Ricci of the Vivaio Regionale Le Marinelle of Petacciato Marina (ARSARP - Regional Agency of the Molise Region) for their support in data collection. We are also thankful to Dr. Simone Angelucci of Wildlife Research Center of Maiella National Park (Italy), Dr. Tania Travaglini, Dr. Marco Colacci, and Dr. Giulia Gagliardi for their help in data collection and identification.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1603295/full#supplementary-material
References
Acosta A. T. R., Ercole S., Stanisci A., De Patta Pillar V., and Blasi C. (2007). Coastal vegetation zonation and dune morphology in some Mediterranean ecosystems. J. Coast. Res. 236, 1518–1524. doi: 10.2112/05-0589.1
Amori G., Luiselli L., Milana G., and Casula P. (2016). Negative effect of the wild boar (Sus scrofa) on the population size of the wood mouse (Apodemus sylvaticus) in forest habitats of Sardinia. Mammalia 80, 463–467. doi: 10.1515/mammalia-2015-0023
Amori G. and Nappi A. (2011). Guida dei mammiferi d’Europa, dell’Africa del Nord e del vicino Oriente (Florence, Italy: Emmebi Edizioni), 272 pp.
Apollonio M., Andersen R., and Putman R. (2010). European ungulates and their management in the 21st century (United Kingdom: Cambridge University Press), 398 pp.
Avibase. Available online at: https://avibase.bsc-eoc.org/ (Accessed 04/03/2025).
Baker P. J., Molony S. E., Stone E., Cuthill I. C., and Harris S. (2008). Cats about town: is predation by free-ranging pet cats Felis catus likely to affect urban bird populations? Ibis 150, 86–99. doi: 10.1111/j.1474-919X.2008.00836.x
Bessa F., Cunha D., Correia Goncalves S., and Carlos Marques J. (2013). Sandy beach macrofaunal assemblages as indicators of anthropogenic impacts on coastal dunes. Ecol. Indic. 30, 196–204. doi: 10.1016/j.ecolind.2013.02.022
Boitani L., Mattei L., Nonis D., and Corsi F. (1994). Spatial and activity patterns of wild boar in Tuscany, Italy. J. Mammal. 75, 600–612. doi: 10.2307/1382507
Bonari G., Acosta A. T. R., and Angiolini C. (2017). Mediterranean coastal pine forest stands: understorey distinctiveness or not? For. Ecol. Manage. 391, 19–28. doi: 10.1016/j.foreco.2017.02.002
Bowkett A. E., Rovero F., and Marshall A. R. (2008). The use of camera-trap data to model habitat use by antelopespecies in the Udzungwa Mountain forests, Tanzania. Afr. J. Ecol. 46, 479–487. doi: 10.1111/j.1365-2028.2007.00881.x
Burrascano S., Copiz R., Del Vico E., Fagiani S., Giarrizzo E., Mei M., et al. (2015a). Wild boar rooting intensity determines shifts in understorey composition and functional traits. Community Ecol. 16, 244–253. doi: 10.1556/168.2015.16.2.12
Burrascano S., Giarrizzo E., Bonacquisti S., Copiz R., Del Vico E., Fagiani S., et al. (2015b). Quantifying Sus scrofa rooting effects on the understorey of the deciduous broadleaf forests in Castelporziano Estate (Italy). Rendiconti. Lincei. 26, 317–324. doi: 10.1007/s12210-014-0350-9
Buxton R. T., Lendrum P. E., Crooks K. R., and Wittemyer G. (2018). Pairing camera traps and acoustic recorders to monitor the ecological impact of human disturbance. GECCO 16, e00493. doi: 10.1016/j.gecco.2018.e00493
Calosi M., Gabbrielli C., Lazzeri L., Fattorini N., Cesaretti G., Burrini L., et al. (2024). Seasonal and Ecological determinant of Wild Boar rooting o priority protected grassland. Environ. Manag. 74, 268–281. doi: 10.1007/s00267-024-01952-y
Cappa F., Lombardini M., and Meriggi A. (2019). Influence of seasonality, environmental and anthropic factors on crop damage by wild boar Sus scrofa. Folia Zool. 68, 261–268. doi: 10.25225/fozo.015.2019
Carpio A. J., Apollonio M., and Acevedo P. (2021). Wild ungulate overabundance in Europe: contexts, causes, monitoring and management recommendations. Mamm. Rev. 51, 95–108. doi: 10.1111/mam.12221
Cerri J., Musto C., Stefanini F. M., di Nicola U., Riganelli N., Fontana M. C., et al. (2023). A human-neutral large carnivore? No patterns in the body mass of gray wolves across a gradient of anthropization. PloS One 18, e0282232. doi: 10.1371/journal.pone.0282232
Chadwick A., Weston M. A., Burns T., Randall G., Radvan M., and Rendall A. R. (2022). Natural and anthropogenic processes influence the occurrence of vertebrate fauna in coastal dunes. Estuar. Coast. Shelf. Sci. 276, 108025. doi: 10.1016/j.ecss.2022.108025
Chandru G., Pandiyan J., Durga V., Govindarajan M., Alharbi N. S., Kadaikunnan S., et al. (2020). Seed dispersal by ungulates in the point calimere wildlife sanctuary: A scientific and perspective analysis. Saudi. J. Biol. Sci. 27, 2790–2797. doi: 10.1016/j.sjbs.2020.06.042
Compagnone F., Varricchione M., Innangi M., Di Febbraro M., Loy A., Stanisci A., et al. (2023). Coastal biodiversity assessment aided by citizen science volunteers: A look at the italian central adriatic. Land 12, 2023. doi: 10.3390/land12112023
Corbet G. and Ovenden D. (2012). Guida dei mammiferi d’Europa. Ed. Muzzio F. (Roma, Italy: Franco Muzzio Publishing). doi: 10.5066/F7KH0KBK
Cushman J. H., Tierney T. A., and Hinds J. M. (2004). Variable effects of feral pig disturbances on native and exotic plants in a California grassland. Ecol. Appl. 14, 1746–1756. doi: 10.1890/03-5142
Delgado-Fernandez I., O’Keeffe N., and Davidson-Arnott R. G. D. (2019). Natural and human controls on dune vegetation cover and disturbance. STOTEN 672, 643–656. doi: 10.1016/j.scitotenv.2019.03.494
Del Vecchio S., Prisco I., Acosta A. T. R., and Stanisci A. (2015). Changes in plant species composition of coastal dune habitats over a 20-year period. AoB. Plants 7, plv018. doi: 10.1093/aobpla/plv018
Desk Aeronautico. Available online at: https://www.deskaeronautico.it/calcolo-effemeridi/ (Accessed 06/03/2024).
Di Cerbo A. R. and Biancardi C. M. (2013). Monitoring small and arboreal mammals by camera traps: effectiveness and applications. Acta Theriol. 58, 279–283. doi: 10.1007/s13364-012-0122-9
Di Paola G., Minervino Amodio A., Dilauro G., Rodriguez G., and Rosskopf C. M. (2022). Shoreline evolution and erosion vulnerability assessment along the central adriatic coast with the contribution of UAV beach monitoring. Geosciences 12, 353. doi: 10.3390/geosciences12100353
Doherty T. S., Dickman C. R., Johnson C. N., Legge S. M., Ritchie E. G., and Woinarski J. C. Z. (2017). Impacts and management of feral cats Felis catus in Australia. Mamm. Rev. 47, 83–97. doi: 10.1111/mam.12080
Doherty T. S., Glen A. S., Nimmo D. G., Ritchie E. G., and Dickman C. R. (2016). Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. 113, 11261–11265. doi: 10.1073/pnas.1602480113
Dovrat G., Perevolotsky A., and Ne’Eman G. (2012). Wild boars as seed dispersal agents of exotic plants from agricultural lands to conservation areas. J. Arid. Environ. 78, 49–54. doi: 10.1016/j.jaridenv.2011.11.011
Drius M., Carranza M. L., Stanisci A., and Jones L. (2016). The role of Italian coastal dunes as carbon sinks and diversity sources. A multi-service perspective. Appl. Geogr. 75, 127–136. doi: 10.1016/j.apgeog.2016.08.007
Drius M., Jones L., Marzialetti F., de Francesco M. C., Stanisci A., and Carranza M. L. (2019). Not just a sandy beach. The multiservice value of Mediterranean coastal dunes. Sci. Total. Environ. doi: 10.1016/j.scitotenv.2019.02.364
Drius M., Malavasi M., Acosta A. T. R., Ricotta C., and Carranza M. L. (2013). Boundary-based analysis for the assessment of coastal dune landscape integrity over time. Appl. Geogr. 45, 41–48. doi: 10.1016/j.apgeog.2013.08.003
Enquist B. J., Abraham A. J., Harfoot M. B. J., Malhi Y., and Doughty C. E. (2020). The megabiota are disproportionately important for biosphere functioning. Nat. Commun. 11, 699. doi: 10.1038/s41467-020-14369-y
Ercole S., Angelini P., Carnevali L., Casella L., Giacanelli V., Grignetti A., et al. (2021). Rapporti Direttive Natura (2013-2018). Sintesi dello stato di conservazione delle specie e degli habitat di interesse comunitario e delle azioni di contrasto alle specie esotiche di rilevanza unionale in Italia. ISPRA, Serie Rapporti 349/2021. (Roma, Italy: ISPRA Publishing).
Fattorini N. and Ferretti F. (2020). Estimating wild boar density and rooting activity in a Mediterranean protected area. Mamm. Biol. 100, 241–251. doi: 10.1007/s42991-020-00030-0
Ferretti F., Lazzeri L., Burrini L., and Fattorini N. (2021). Habitat correlates of wild boar density and rooting along an environmental gradient. J. Mammal. 102, 1536–1547. doi: 10.1093/jmammal/gyab095
Forbes E. S., Cushman J. H., Burkepile D. E., Young T. P., Klope M., and Young H. S. (2019). Synthesizing the effect of large, wild herbivores exclusion on eco system function. Funct. Ecol. 33, 1597–1610. doi: 10.1111/1365-4612435.13376
Gandrud C., Allaire J. J., Kenton R., and Yetman C. J. (2017). networkD3: D3 JavaScript Network Graphs from R. R package version 0.4. Available online at: https://CRAN.R-project.org/package=networkD3 (Accessed February 21, 2025).
Genovesi P., Angelini P., Bianchi E., Dupré E., Ercole S., Giacanelli V., et al. (2014). Specie e habitat di interesse comunitario in Italia: distribuzione, stato di conservazione e trend. ISPRA Serie Rapporti 194/2014. (Roma, Italy: ISPRA Publishing).
Hanmer H. J., Thomas R. L., and Fellowes M. D. E. (2017). Urbanisation influences range size of the domestic cat (Felis catus): consequences for conservation. J. Urban. Ecol. 3 (1), 1–11. doi: 10.1093/jue/jux014
Hardwick B., Kerdraon D., Rogers H. M. K., Raharinjanahary D., Rajoelison E. T., Mononen T., et al. (2024). LIFEPLAN: A worldwide biodiversity sampling design. PloS One. 19 (12). doi: 10.1371/journal.pone.0313353
ISTAT. Available online at: https://www.istat.it/ (Accessed 14/05/2024).
Kissling W. D., Schneider L. C., Evans J. C., Chalmers C., Zilber R., Fergus P., et al. (2024). Development of a cost-efficient automated wildlife camera network in a European Natura 2000 site. Basic. Appl. Ecol. 79, 141–152. doi: 10.1016/j.baae.2024.06.006
Kleiven E. F., Nicolau P. G., Sørbye S. H., Aars J., Yoccoz N. G., and Ims R. A. (2023). Using camera traps to monitor cyclic vole populations. Remote Sens. Ecol. Conserv. 9, 390–403. doi: 10.1002/rse2.317
Kristensen J. A., Svenning J.-C., Georgiou K., and Malhi Y. (2022). Can large herbivores enhance ecosystem carbon persistence. TREE 37, 117–128. doi: 10.1016/j.tree.2021.09.006
La Morgia V., Marucco F., Aragno P., Salvatori V., Gervasi V., De Angelis D., et al. (2022). Stima della Distribuzione e Consistenza del lupo a Scala Nazionale 2020/2021 (Rome, Italy: Relazione tecnica realizzata nell’ambito della convenzione ISPRA-Ministero della Transizione Ecologica “Attività di monitoraggio nazionale nell’ambito del Piano di Azione del lupo. Relazione Tecnica. ISPRA Istituto Superiore per la Protezione e la Ricerca Ambientale).
Lim S. J., Han S. H., Kim K. Y., Hong S., and Park Y. C. (2023). Relative abundance of mammals and estimation of minimum trapping effort using camera traps in Jangsudae, Seoraksan National Park. Mamm. Study 48 (3), 171–179. doi: 10.3106/ms2022-0035
Liu X., Wuc P., Songer M., Cai Q., Hef X., Zhue Y., et al. (2013). Monitoring wildlife abundance and diversity with infra-red camera traps in Guanyinshan Nature Reserve of Shaanxi Province, China. Ecol. Indic. 33, 121–128. doi: 10.1016/j.ecolind.2012.09.022
Loss S. R., Boughton B., Cady S. M., Londe D. W., McKinney C., O’Connell T. J., et al. (2022). Review and synthesis of the global literature on domestic cat impacts on wildlife. J. Anim. Ecol. 91, 1361–1372. doi: 10.1111/1365-2656.13745
Loyd K. A. T., Hernandez S. M., Carroll J. P., Abernathy K. J., and Marshall G. J. (2013). Quantifying free-roaming domestic cat predation using animal-borne video cameras. Biol. Conserv. 160, 183–189. doi: 10.1016/j.biocon.2013.01.008
Mack M. C. and D’Antonio C. M. (1998). Impacts of biological invasions on disturbance regimes. Trends Ecol. Evol. 13, 195–198. doi: 10.1016/S0169-5347(97)01286-X
Maiorano L., Amori G., Montemaggiori A., Rondinini C., Santini L., Saura S., et al. (2015). On how much biodiversity is covered in Europe by national protected areas and by the Natura 2000 network: insights from terrestrial vertebrates. Conserv. Biol. 0, 1–10. doi: 10.1111/cobi.12535
Mammal Diversity. Available online at: https://www.mammaldiversity.org/ (Accessed 11/03/2025).
Massei G., Genov P., Staines B. W., and Gorman M. L. (1997). Factors influencing home range and activity of wild boar (Sus scrofa) in a Mediterranean coastal area. J. Zool. 242, 411–423. doi: 10.1111/j.1469-7998.1997.tb03845.x
Mastronardi L., de Francesco M. C., Giannelli A., and Stanisci A. (2015). Biodiversity and tourism in the teatina coastal area (Italy): impact or convergence. Geotema 49, 131–136.
Mattioli L., Apollonio M., Lovari C., Siemoni N., and Crudele G. (1995). Wild boar as the main prey of wolf in an area of Northern Apennines (Italy). Ibex 3, 212.
Mazzocchi M. G., Capotondi L., Freppaz M., Lugliè A., and Campanaro A. (2019). Italian Long-Term Ecological Research for understanding ecosystem diversity and functioning. Case studies from aquatic, terrestrial and transitional domains. Nat. Conserv. 34, 1–8. doi: 10.3897/natureconservation.34.35517
McClure M. L., Burdett C. L., Farnsworth M. L., Sweeney S. J., and Miller R. S. (2018). A globally-distributed alien invasive species poses risks to United States imperiled species. Sci. Rep. 8, 5331. doi: 10.1038/s41598-018-23657-z
Meriggi A. and Sacchi O. (2001). Habitat requirements of wild boar in the Northern Apennines (N Italy): a multi- - level approach. Ital. J. Zool. 68, 47–55. doi: 10.1080/11250000109356382
Merli E. and Meriggi A. (2006). Using harvest data to predict habitat-population relationship of the wild boar Sus scrofa in Northern Italy. Acta Theriol. 51, 383–394. doi: 10.1007/BF03195185
Miraglia N. and Brita A. D. (2022). Behavior of wildlife species in urban areas to changing conditions during COVID-19 lockdowns: A review. J. Appl. Anim. Welf. Sci. 25, 119–125. doi: 10.1080/10888705.2022.2047682
Mori E., Ferretti F., Lagrotteria A., La Greca L., Solano E., and Fattorini N. (2020). Impact of wild boar rooting on small forest-dwelling rodents. Ecol. Res. 35, 675–681. doi: 10.1111/1440-1703.12113
Mori E., Ficetola G. F., Bartolomei R., Capobianco G., Varuzza P., and Falaschi M. (2021b). How the South was won: current and potential range expansion of the crested porcupine in Southern Italy. Mamm. Biol. 101, 11–19. doi: 10.1007/s42991-020-00058-2
Mori E. and Lazzeri L. (2021). Does wild boar rooting affect spatial distribution of active burrows of meadow- dwelling voles? Biologia 76, 981–986. doi: 10.2478/s11756-020-00622-8
Mori E., Lazzeri L., Ferretti F., Gordigiani L., and Rubolini D. (2021a). The wild boar Sus scrofa as a threat to ground-nesting bird species: an artificial nest experiment. J. Zool. 314, 311–320. doi: 10.1111/jzo.12887
Mori E., Sforzi A., and Di Febbraro M. (2013). From the Apennines to the Alps: recent range expansion of the crested porcupine Hystrix cristata L. 1758 (Mammalia: rodentia: hystricidae) in Italy. Ital. J. Zool. 80, 469–480. doi: 10.1080/11250003.2013.857729
Nogales M., Martín A., Tershy B. R., Donlan C. J., Veitch D., Puerta N., et al. (2004). A review of feral cat eradication on islands. Conserv. Biol. 18, 310–319. doi: 10.1111/j.1523-1739.2004.00442.x
Nores C., Llaneza L., and Alvarez M. A. (2008). Wild boar Sus scrofa mortality by hunting and wolf Canis lupus predation: an example in northern Spain. Wildl. Biol. 14, 44–51. doi: 10.1007/s10344-014-0807-2
O’Connell A. F., Nichols J. D., and Karanth K. U. (2011). “Camera traps in animal ecology: methods and analyses,” in Habitat selection: General theory and applications to human behavior. The evolution of human social behavior. Eds. O’Connell A. F., Nichols J. D., and Karanth K. U. (Springer, Orians, New York), 1–271. doi: 10.1007/978-4-431-99495-4
Palmer M. S., Swanson A., Kosmala M., Arnold T., and Packer C. (2018). Evaluating relative abundance indices for terrestrial herbivores from large-scale camera trap surveys. Afr. J. Ecol. 56, 791–803. doi: 10.1111/aje.12566
Paolucci P. and Bon M. (2022). Mammiferi terrestri d'Italia, Riconoscimento, ecologia e tricologia. (Verona, Italy: Publishing WBA PROJECT SRL).
Pellissier V., Schmucki R., Pe’er G., Aunins A., Brereton T. M., Brotons L., et al. (2020). Effects of Natura 2000 on nontarget bird and butterfly species based on citizen science data. Conserv. Biol. 34, 666–676. doi: 10.1111/cobi.13434
Petrosillo I., Zurlini G., Corlianò M. E., Zaccarelli N., and Dadamo M. (2007). Tourist perception of recreational environment and management in a marine protected area. Landscape Urban. Plan. 79, 29–37. doi: 10.1016/j.landurbplan.2006.02.017
Piscopo N., Tamburis O., Bonavolontà F., Verde M. T., Manno M., Mancusi M., et al. (2023). Assessing wild boar presence and activity in a monitoring specific area of Campania region using camera traps. Acta Imeko. 12, 1–5. doi: 10.21014/actaimeko.v12i4.1617
Prisco I., Angiolini C., Assini S., Buffa G., Gigante D., Marcenò C., et al. (2020). Conservation status of Italian coastal dune habitats in the light of the 4th Monitoring Report (92/43/EEC Habitats Directive). Plant Sociol. 57, 55–64. doi: 10.3897/pls2020571/05
Prisco I., Stanisci A., and Acosta A. T. R. (2015). Temporal changes in Adriatic coastal dunes: results from a short term vegetation monitoring. Plant Sociol. 52, 95–100. doi: 10.7338/pls2015522/05
Prisco I., Stanisci A., and Acosta A. T. R. (2016). Mediterranean dunes on the go: evidence from a short term study on coastal herbaceous vegetation. Estuar. Coast. Shelf. Sci. 182, 40–46. doi: 10.1016/j.ecss.2016.09.012
Rasino M. D. V., Fattorini S., Sciarretta A., Colacci M., Stanisci A., and Carranza M. L. (2024). Cross-taxon analysis in the highly threatened Mediterranean dunes reveals consistent diversity patterns in butterfly and plant communities. Biodivers. Conserv. 33, 3643–3661. doi: 10.1007/s10531-024-02914-w
R Core Team (2023). R: A language and Environment for Statistical Computing. Available online at: https://www.R-project.org/ (Accessed November 20, 2024).
Rendall A. R., Cooke R., White J. G., and Weston M. A. (2019). Zonation of a small mammal community within coastal dunes. Estuar. Coast. Shelf. Sci. 217, 206–210. doi: 10.1016/j.ecss.2018.11.023
Rogers H. M. K., Banelyte G. G., Farrell A. M., Hardwick B., Mononen T., and Kerdraon D. (2023). Lifeplan Camera Trapping Protocol V.3. (Berkeley, USA: protocols.io Publishing). doi: 10.17504/protocols.io.q26g7pxp1gwz/v3
Rosell C., Fernandez-Llario P., and Herrero J. (2001). El jabalı´ (Sus scrofa Linnaeus 1758). Galemys 13, 1–25.
Rovero F. and Zimmermann F. (2016). Camera Trapping for Wildlife Research. Exeter (Exeter, United Kingdom: Pelagic Publishing), 320 pp.
Simo F. T., Difouo G. F., Kekeunou S., Ichu I. G., Olson D., Deere N. J., et al. (2023). Adapting camera-trap placement based on animal behavior for rapid detection: A focus on the Endangered, white-bellied pangolin (Phataginus tricuspis). Ecol. Evol. 13, 1–10. doi: 10.1002/ece3.10064
Stanisci A., Acosta A. T. R., Carranza M. L., De Chiro M., Del Vecchio S., Di Martino L., et al. (2014). EU habitats monitoring along the coastal dunes of the LTER sites of Abruzzo and Molise (Italy). Plant Sociol. 51, 51–56.
Stoll S., Frenzel M., Burkhard B., Adamescu M., Augustaitis A., Baeßler C., et al. (2015). Assessment of ecosystem integrity and service gradients across Europe using the LTER Europe network. Ecol. Model. 295, 75–87. doi: 10.1016/j.ecolmodel.2014.06.019
Thomas R. L., Fellowes M. D., and Baker P. J. (2012). Spatio-temporal variation in predation by urban domestic cats (Felis catus) and the acceptability of possible management actions in the UK. PloS One 7, e49369. doi: 10.1371/journal.pone.0049369
Time and Date AS (1995–2025). Available online at: https://www.timeanddate.com/ (Accessed February 2024).
Todini A. and Crosti R. (2020). Il cinghiale (Sus scrofa) come determinante di cambiamenti di vegetazione in una foresta urbana mediterranea: impatto sulla biodiversità di un’area protetta. Forest@ 17, 71–77. doi: 10.3832/efor3284-017
Valente A. M., Acevedo P., Figueiredo A. M., Fonseca C., and Torres R. T. (2020). Overabundant wild ungulate populations in Europe: management with consideration of socio-ecological consequences. Mamm. Rev. 50, 353–366. doi: 10.1111/mam.12202
van Meurs E., Moland E., Bjørge A., and Freitas C. (2024). Haulout patterns of harbour seal colonies in the norwegian skagerrak, as monitored through time-lapse camera surveys. Diversity 16, 38. doi: 10.3390/d16010038
Warner P. J. and Cushman J. H. (2002). Influence of herbivores on a perennial plant: variation with life history stage and herbivore species. Oecologia 132, 77–85. doi: 10.1007/s00442-002-0955-z
Wohner C., Peterseil J., Poursanidis D., Kliment T., Wilson M., Mirtl M., et al. (2019). DEIMS-SDR – A web portal to document research sites and their associated data. Ecol. Inf. 51, 15–24. doi: 10.1016/j.ecoinf.2019.01.005
Keywords: GBIF repository, camera trapping, coastal dunes, wildlife, Natura 2000 network, eLTER network
Citation: de Francesco MC, Huamaní Cahuas CF, Rasino MdV, Sciarretta A and Stanisci A (2025) CAM-COAST: the database of bird and mammal fauna collected through camera traps in a Natura 2000 coastal site in Italy. Front. Ecol. Evol. 13:1603295. doi: 10.3389/fevo.2025.1603295
Received: 31 March 2025; Accepted: 04 August 2025;
Published: 25 August 2025.
Edited by:
Carolina Puerta-Pinero, Joint Research Centre (JRC), ItalyReviewed by:
Ferdinando Urbano, Joint Research Centre, ItalyAntonio Jesús Pérez-Luque, Instituto Nacional de Investigación y Tecnología Agroalimentaria (INIA), Spain
Copyright © 2025 de Francesco, Huamaní Cahuas, Rasino, Sciarretta and Stanisci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Micaela del Valle Rasino, bS5yYXNpbm9Ac3R1ZGVudGkudW5pbW9sLml0
 Maria Carla de Francesco
Maria Carla de Francesco Claudia Fiorella Huamaní Cahuas
Claudia Fiorella Huamaní Cahuas Micaela del Valle Rasino
Micaela del Valle Rasino Andrea Sciarretta
Andrea Sciarretta Angela Stanisci
Angela Stanisci