- 1Department of Oceanography & Coastal Sciences, Louisiana State University, Baton Rouge, LA, United States
- 2Coastal Studies Institute, Louisiana State University, Baton Rouge, LA, United States
- 3U.S. Army Corps of Engineers, Engineer Research and Development Center, Vicksburg, MS, United States
The intentional thin layer placement (TLP) of dredged sediment is an increasingly popular approach to maintaining marsh elevation and restoring degraded marshes, which can improve conditions for vegetation establishment. Prior TLP restoration projects, assessed shortly after construction, evaluated soil, hydrology, plant, and faunal responses. However, few long-term studies (>3 yrs) investigate TLP-induced shifts in soil properties and especially properties related to biogeochemical cycling. In response, this study revisited a salt marsh 6+ years after TLP restoration and determined both soil physiochemical and microbial properties related to plant growth (nitrogen (N) mineralization) and water quality improvement (denitrification). Data were compared with samples collected before TLP project implementation and 0.5 years after project completion. Bulk density increased to 342% of the control 0.5 years after project completion and was 272% of the control after 6+ years, suggesting significant sediment retention in the marsh over time. Microbial biomass declined to 7.6% of the control following TLP, then rebounded to 29.4% of control after 6+ years. The N mineralization rate increased from 22% to 31% of control after 0.5 years and 6+ years, respectively. Notably, live root density was 3x higher in the TLP marsh compared with the control, suggesting that the restored marsh likely responded to reduced nutrient availability (approximately 1/3) by generating additional belowground biomass. TLP marsh denitrification rates were not significantly different from the control suggesting the water quality improvement ecosystem services recovers more quickly than other soil properties. While TLP soil properties appear to be trending more similar to controls over time, longer-term studies are needed to inform the ecological trajectories of sediment amended marshes.
1 Introduction
Coastal marshes provide important ecosystem functions. Marshes have some of the highest rates of primary productivity of any ecosystem (Reddy and DeLaune, 2008), making them an essential component of global biogeochemical cycles. However, coastal marshes worldwide are threatened by an increasing multitude of stressors, including sea level rise (SLR), sediment starvation, subsidence, coastal edge erosion (Turner and Cahoon, 1987; DeLaune et al., 1978; Day et al., 2024). Sea level has been rising for over 18,000 yrs since the Holocene Era (Smith et al., 2011). However, modern rates of SLR are trending higher when compared to recent historical rates by a few mm y-1 (Church and White, 2011). This poses challenges to communities and critical infrastructure in the coastal zone where human populations, military installations, and economic drivers (i.e., transportation hubs) are disproportionately concentrated. Generally, coastal marsh elevations keep pace with eustatic SLR by maintaining elevation via soil organic matter accretion and mineral sedimentation (Day et al., 1999). However, when SLR exceeds a marsh’s ability to accrete material, the marsh may drown and convert to open water or mud flats (Orson et al., 1985). The degradation of marshes reduces the extent of habitat for a variety of species and increases the vulnerability of coastal communities and infrastructure to storm flooding.
A variety of factors influence a coastal marsh’s ability to effectively build elevation. For example, coastal marshes receive sediment primarily through the deposition of material carried by rivers or channels from inland areas as well as from coastal waters (Roberts, 1997). When the major source of sediment is disconnected from the coastal marshes, the marshes experience sediment starvation, and accretion capacity is reduced (Day et al., 2007). Coastal wetlands around the globe are undergoing sediment starvation due to urban encroachment and the historical construction of dams and levees that have decreased sediment delivery by over 50% in some areas (Peteet et al., 2018; Yang et al., 2017; Torab and Azab, 2007). Subsidence consists of soil compaction and subsequent settling of the ground surface resulting in a decrease in surface elevation. Globally, subsidence can result from withdrawal of groundwater and hydrocarbons, or increased vegetation waterlogging and drowning (Walker et al., 1987). In large deltaic systems (i.e., the Mississippi River Delta), much of the subsidence is due to interstitial moisture that is forced out of the sediment structure due to the weight of overlying sediments leading to compaction (Day et al., 2024). Marsh edge erosion is driven primarily by wind and storm waves in many regions (Sapkota and White, 2019; Sapkota et al., 2023; Zhou et al., 2024). Subsidence and erosion combined with SLR increases the stress on marsh vegetation leading to open water ponding (DeLaune et al., 1994: Haywood et al., 2020). This results in a coastal landscape in critical need of rehabilitation, without which, the many coastal marsh ecosystem services delivered to communities in the region are diminished (Vaccare et al., 2019).
Globally, coastal wetlands offer trillions of United States dollars’ worth of ecosystem services per year (Costanza et al., 1997). Livelihoods and economies alike are increasingly threatened by wetland loss and coastal degradation (Williams et al., 1997). Resource managers are continuing to pursue restoration and rehabilitation techniques to compensate for wetland loss. As such, there are many management practices being implemented on coastlines worldwide in efforts to protect and help maintain the remaining coastal marsh. One emerging restoration management technique is the thin layer placement (TLP) of dredged sediments, which serves to nourish marsh soil with mineral material to build elevation capital and offset subsiding soils. TLP is increasingly carried out by the US Army Corps of Engineers and other entities and has been defined as the “purposeful placement of thin layers of sediment (e.g., dredged sediment) in an environmentally acceptable manner to achieve a target elevation or thickness”. Thin layer placement projects may include efforts to support infrastructure and/or create, maintain, enhance, or restore ecological function” (Berkowitz et al., 2019). The TLP projects can raise marsh elevation and increase marsh resilience (Raposa et al., 2023). Thin layer placement can also improve vegetation health in previously degraded marshes via increases of elevation and bulk density, providing macrophytes with stable substrates while mitigating waterlogging (Reimold et al., 1978; Wilber, 1992; Ford et al., 1999; Davis et al., 2022). Coastal marshes with higher bulk density often erode more slowly, and re-establishing plants in sparsely vegetated or unvegetated areas improved soil shear strength (Sapkota and White, 2019). Additionally, when TLP is implemented without exceeding ecological thickness thresholds (e.g., conversion to uplands), macrofauna and infauna populations can fully recover shortly after placement (Croft et al., 2006; Wilber et al., 2007), often in <3 months. TLP can also serve to restore macroinvertebrate communities (Tong et al., 2013; Raposa et al., 2023). The expediency of recovery for plant growth and fauna is one advantage of TLP which preserves seed/rhizome sources and nutrients in the rooting zone, whereas approaches such as sediment diversion and marsh creation may take years or decades to manifest suitable habitat and function.
Most TLP projects completed to date include very limited and essentially no long-term monitoring beyond 3 years, particularly for microbial properties. Thus, longer-term trajectories of TLP outcomes are poorly understood in the research and management community, limiting the ability of practitioners and resource managers to predict or model the environmental implications over decadal timespans. In particular, very little is known about the effects of TLP on soil microbial communities beyond very short term (<1yr) monitoring periods. Microbial populations drive biogeochemical cycling, and their activity delivers wetland functions (e.g., denitrification) and associated ecosystem services (e.g., water quality improvement). Nutrient cycling and N availability, vegetation productivity, and C sequestration are all dependent on microbial-driven biogeochemical processes. Consequently, longer-term data that includes microbial properties are needed to fully understand the implications of TLP techniques for ecosystem restoration trajectories. Therefore, the goal of this study was to extend the monitoring of select soil physiochemical and microbial properties out to 6+ years at a coastal marsh TLP in Avalon, New Jersey, USA. Our approach was to compliment measurements made just before the TLP occurred and within the first year.
2 Materials and methods
2.1 Study site
In 2016, the US Army Corps of Engineers and project partners implemented a TLP restoration project at a rapidly degrading coastal marsh near Avalon, NJ (Figure 1). Vegetated areas of the marsh were dominated by Spartina alterniflora occupying >90% absolute areal cover of the soil surface, with small amounts of Distichlis spicata and other trace species (e.g., Salicornia Sp.) occupying <5% of the soil surface marsh. The marsh soils consisted of Typic Sulfihemists mapped as the very frequently flooded Appoquinimink-Transquaking-Mispillion complex on 0 to 1 percent slopes (Soil Survey Staff, 2025).
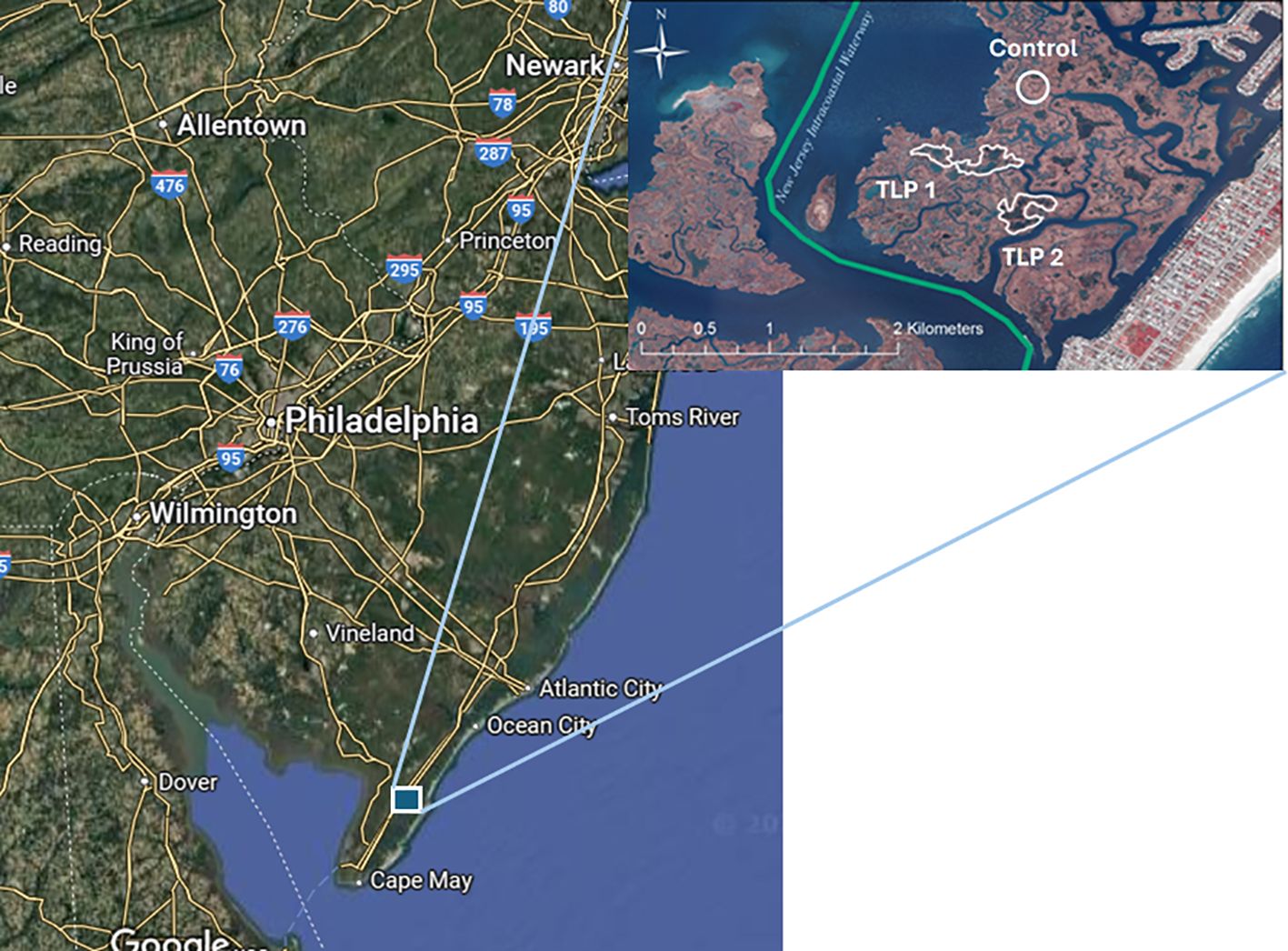
Figure 1. Location of the Avalon, New Jersey, USA thin-layer placement sediment project. Map inset identifies the locations of TLP locations 1 and 2 (irregular polygons), and the control marsh location (circle).
Degradation of the back bay marsh resulted in the interspersion of vegetated areas with expanding unvegetated deepwater pools and shallow water pannes, interior marsh erosion and collapse, and other rapid marsh platform destabilization. This impaired marsh has experienced increasing inundation during which has resulted in dieback of vegetation and weaking of the soil structure via root system decay (Schepers et al., 2017; DeLaune et al., 1994). Following open water area expansion, vegetation can no longer re-establish for a number of reasons. First, the bulk density is reduced past the threshold required for vegetation colonization as the elevation decreases (DeLaune et al., 2016; Day et al., 2011). Second, water depth increased until it prevents re-establishment of vegetation. Finally, wind waves led to expansion of open water by inducing edge erosion (Sapkota and White, 2021). Thus, efforts were initiated to supplement the marsh elevation with dredged sediment to increase bulk density and help stabilize the marsh platform.
TLP was performed via high-pressure spraying of a sediment slurry (~20% sediment, 80% water) in a practice known as “rainbowing,” in which the dredge material is dispersed from a nozzle in an arc-shape, and by moving the dredged sediment outlet pipe into target locations surrounded by coconut fiber roll containment features (Figure 2). There are two assessments of the Avalon, NJ TLP project published in the literature: one assessing biogeochemical properties prior to the sediment placement (Berkowitz et al., 2018) and another assessing biogeochemical properties 0.5 years after the placement (VanZomeren et al., 2018). This study extends the monitoring timeframe, now 6+ years after the TLP project initiation, to investigate the longer-term effects of TLP on wetland soil physical and microbial characteristics. In doing so, the authors of the current study compare data from all three discrete sampling intervals, evaluating the trajectory of changes in soil biogeochemical properties. The same TLP sampling stations were revisited from the two previous studies to ensure that locations were consistent across sampling temporal intervals. Sampling stations included vegetated areas that received TLP and vegetated areas that did not receive TLP to serve as unamended sediment controls (Figure 1).

Figure 2. High-pressure slurry marsh amendment technique for thin-layer placement (TLP) in the Avalon, New Jersey, USA. marsh (source: USACE).
2.2 Field sampling
In August 2023, soil samples (0–5 cm) were collected at two vegetated TLP sites and 1 control site. Three stations were sampled at each site in duplicate using 7 cm diameter acrylic coring tubes for a total of 18 characterization samples (3 sites x 3 stations x duplicates). Samples were placed into labeled Ziploc bags and shipped overnight in a cooler on ice to the laboratory. In addition to the soil characterization samples, quadruplicate intact 20 cm long soil cores were taken from two vegetated TLP sites and one vegetated control site for a total of 12 cores for a N cycling incubation study. Cores were sealed with rubber stoppers and immediately shipped overnight on ice to the laboratory for an assessment of N mineralization and denitrification. For the original TLP, project target elevations ranged from 0.73 to 0.91m NAVD88 (VanZomeren et al., 2018).
2.3 Soil characterization
Soil characterization followed the same methods utilized by Berkowitz et al. (2018) and VanZomeren et al. (2018) to minimize variance between datasets. At the lab, duplicate soil samples were homogenized, and live root matter with diameter >1 mm was removed. Roots were dried and weighed to determine live root mass on a dry weight basis. The 9 soil samples were analyzed for moisture content, soil dry weight bulk density, weight % organic matter, total C, N and P, extractable nutrients, extractable dissolved organic C and N, microbial biomass N (MBN), and potentially mineralizable N (PMN) rate. Briefly, moisture content was determined from weight difference of wet soil subsamples before and after placing them in a drying oven at 60°C until constant weight (White and Reddy, 2000). Soil bulk density was determined by dividing the total dry weight of the sample by the volume of the core. Soil weight % organic matter (SOM) was determined by loss on ignition (LOI), which consists of ashing dried ground subsamples at 550°C and recording the loss of mass (Andersen, 1976). Total P was determined via the TP ashing-acid digestion method (Andersen, 1976). Total C and total N were determined from dry ground subsamples analyzed on a Costech Analytical Technologies Elemental Combustion System (Berkowitz et al., 2018). Soil extractable nutrients were determined using a 2 M KCl extraction followed by filtration through a 0.45 μm membrane filter and acidifying samples at a pH of <2 (VanZomeren et al., 2018). Samples were analyzed on a SEAL Analytical AQ300 discrete Analyzer (Mequon, WI) using USEPA methods (U.S. EPA, 1993). Dissolved organic C (DOC) and N (DON) were determined from a 2 M K2SO4 extraction (White and Reddy, 2000). Microbial biomass N (MBN) was determined via chloroform fumigation-extraction method, in which non-fumigate sample N value is subtracted from fumigate samples N value to determine the amount of N contained within lysed cells (Brookes et al., 1985) with modification by White and Reddy (2000). Extracted liquid samples for DOC, DON and MBN were analyzed on a Shimadzu Scientific Instrument TOC-VCSN (Columbia, MD). Potentially mineralizable nitrogen (PMN) rate was determined from the change of NH4-N production from soil under anaerobic conditions at 40°C over a period of 10 d (White and Reddy, 2000). Sample were extracted @ day 0, 2, 5, and 10 of the incubation. The 2 M KCL extracted samples were analyzed on a SEAL Analytical AQ300 (U.S. EPA, 1993) and values were plotted over time to calculate PMN rate from linear regression (White and Reddy, 2000).
2.4 Water column incubation: nitrate and ammonium flux
Twelve intact cores, four replicates each from two different TLP areas, and four replicates from an adjacent non-TLP control area, underwent incubation to determine the ammonium flux rates and nitrate removal capacity of the wetland soil. The NH4 production rate is linked to supporting plant growth and NO3 reduction is linked to water quality improvement. Cores were flooded to create a 20 cm water column with salt water adjusted to a salinity of 30 to mimic site conditions using Instant Ocean®. The cores were placed into a temperature-controlled water bath at 20°C and each core was aerated with room air to ensure aerobic conditions within the water column. The cores were spiked to produce a water column concentration of 1 mg L-1 NO3-N. Seven mL water samples were collected each day for a period of 8 days, filtered through a 0.45-μm membrane syringe filter and acidified with H2SO4 to a pH <2 (Cheng and White, 2022). Water samples were refrigerated at 4°C until analysis (within 2 weeks of collection) on a SEAL Analytical AQ300 colorimetric analyzer for NO3-N concentration using method 353.2 (U.S. EPA, 1993). Upon completion of aerobic incubation, the water column was removed and replaced, and the experiment was repeated under anaerobic water column conditions. Cores were sealed with rubber stoppers which contained inlet and outlet glass tubes and constantly bubbled with 99.99% N2 gas to ensure anaerobic conditions (Bowes et al., 2022). Water samples were collected 2 times each day for a period of 3 days. Both NO3-N and NH4-N were measured using a SEAL Analytical AQ300 colorimetric analyzer, methods 353.2 and 350.1, respectively (U.S. EPA, 1993). Values for NO3-N and NH4-N concentrations were plotted over time on an areal basis to determine anaerobic and aerobic nitrate reduction rates and anaerobic ammonification rates, respectively.
2.5 Statistical analyses and data comparison
Statistics were run using R in RStudio (Version 4.3.1; Vienna, Austria). One-way ANOVA testing was used, for which a p-value of <0.05 was considered significant for differences between control and TLP values as well as between time points. Shapiro-Wilk test for normality and Levene’s test for equal variance were used to ensure that ANOVA assumptions were met. A Tukey-HSD post-hoc test was used if assumptions were met, and a Games-Howell post-hoc test was used if Tukey-HSD was violated.
3 Results
3.1 TLP impacts on soil properties
The TLP-treated marsh sites had significantly higher mean bulk density than the control sites at 0.66 g cm-3 and 0.24 g cm-3, respectively. The inverse was the case for gravimetric moisture content at 49.1% vs 75.8% for the TLP and control sites, respectively (Table 1). These differences in bulk density and moisture content are expected, even after 6+ years, due to the addition of dense, mineral sediment and reduced soil pore space of the TLP treatment relative to the degraded, organic soil conditions of the control (VanZomeren et al., 2018). The SOM, total C, and total N were significantly lower (~30%) in the TLP soil than the control (Table 1). Soil organic matter (LOI), total C, and total N were all strongly correlated (R>0.9). Total P was not significantly different between TLP and control. Microbial biomass N and PMN rates in the TLP treatment soils were ~1/3 of the control marsh soils. Extractable NH4-N values were low in both the TLP and control plots, likely a result of ammonium uptake and subsequent porewater depletion during the latter part of growing season when sampling occurred (Feder and White, 2024). Live root mass was significantly higher (3x) in the TLP marsh compared to the control marsh (Table 1). This difference is likely due to variations in soil properties and associate vegetation responses to nutrient availability, which are explored further in the discussion below.
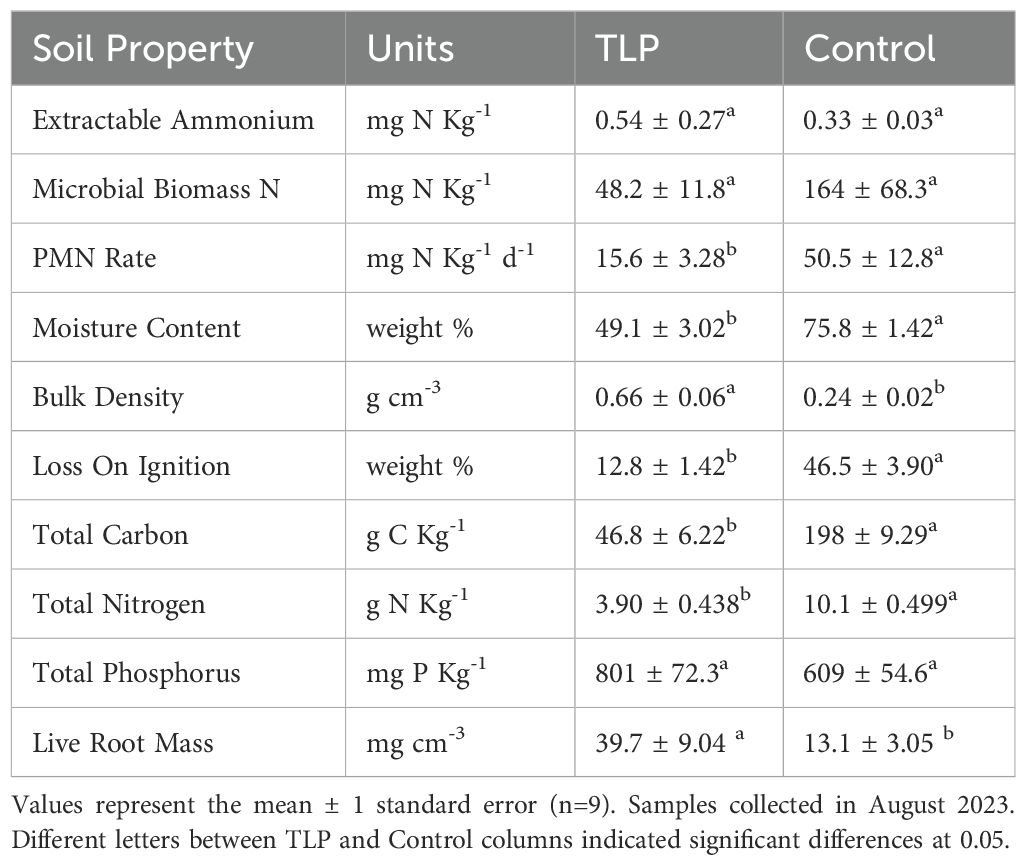
Table 1. Marsh soil properties of control and thin-layer placement (TLP) treatment after 6+ years (t2).
3.2 TLP impacts on N cycling
There was no significant difference between the NO3- reduction rates of the TLP cores and the control cores under aerobic (Figure 3A) or anaerobic (Figure 3B) water column conditions. Nitrate reduction rates under aerobic water column conditions for the control averaged 45.3 ± 9.01 mg m-2 d-1 while mean rates for the TLP averaged 53.3 ± 4.49 mg m-2 d-1. Nitrate reduction rates under anaerobic water columns were statistically higher, averaging 255 ± 27.7 mg m-2 d-1 for the control and 205 ± 15.8 mg m-2 d-1 for the TLP. Notably, the N-reduction related water quality improvement potential of these TLP marsh soils was not significantly different than the control marsh, despite the observed differences in soil properties.
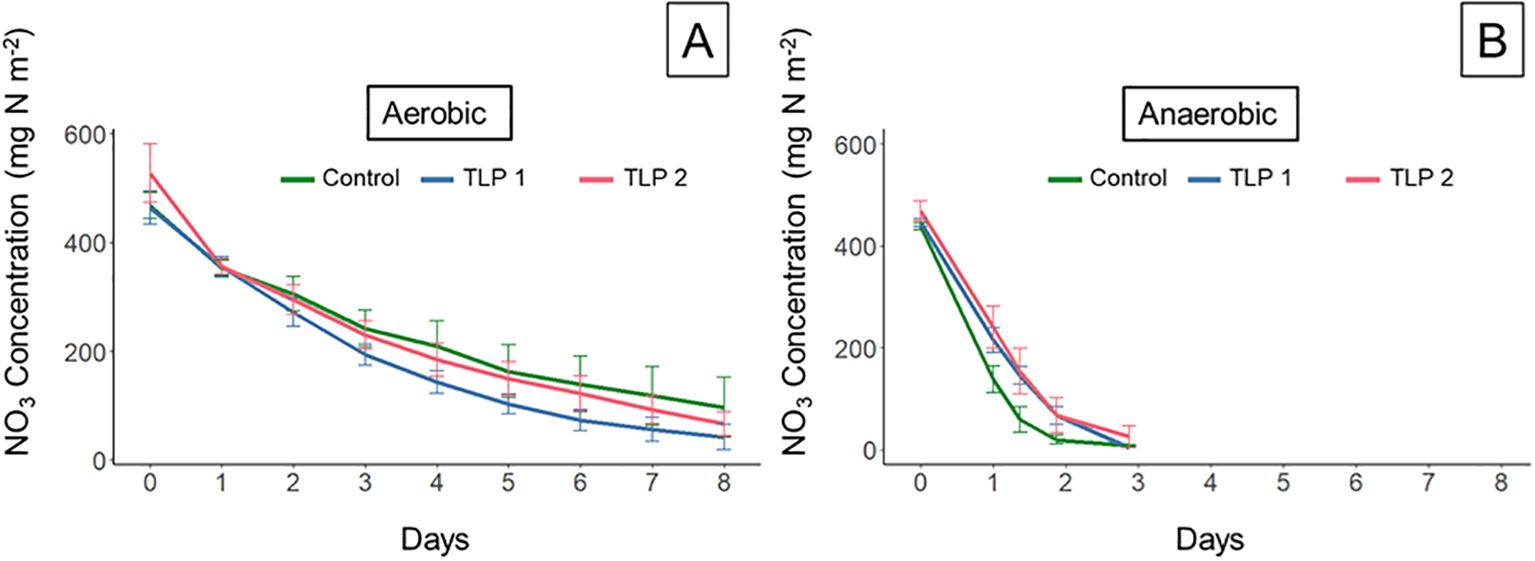
Figure 3. Water column incubation nitrate concentrations on an areal basis over time for both (A) aerobic and (B) anaerobic water column conditions. Data represent mean ± 1 standard error (n = 4).
The mean anaerobic NH4+ flux rate was 44.4 ± 24.14 mg N kg−1 d−1 in both control and TLP marsh soils under anaerobic water column conditions, indicating similar N availability in both sites (Figure 4). This ammonium flux from heterotrophic soil bacterial activity, reflects the soils’ capacity to supply N to the root zone for macrophyte uptake (White and Reddy, 2001).
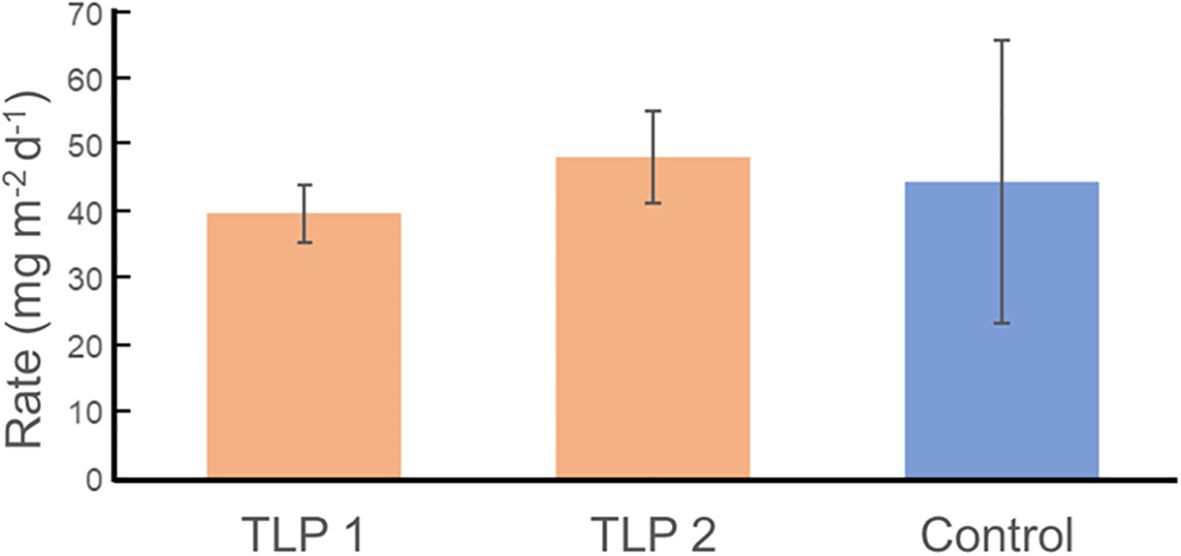
Figure 4. Mean ± 1 standard error (n=4) intact core, anaerobic ammonium flux rates for two thin-layer placement (TLP) marsh sites and one control site.
There was a range of changes of soil parameters and biogeochemical measures across the time points found in this study when compared with the two previous studies covering 6+ years (Table 2). These differences over time are explored in more detail in the following Discussion section.
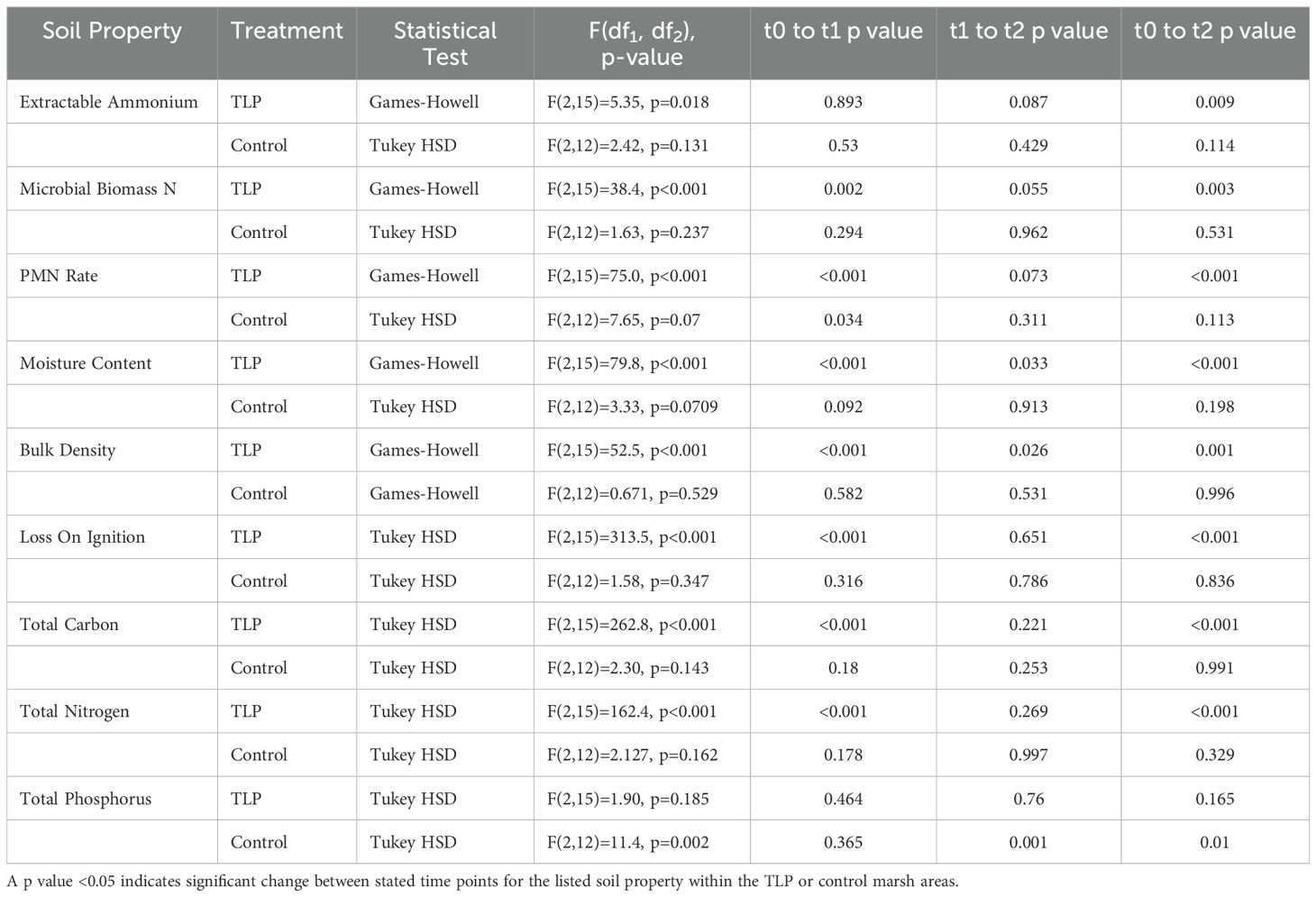
Table 2. Change in soil property over time for the Avalon, NJ marsh thin-layer placement (TLP) project (November 2016 (t0) (Berkowitz et al., 2018); June 2017 (t1) (VanZomeren et al., 2018); August 2023 (t2) (this study).
4 Discussion
4.1 TLP soil property trajectory
This study integrated data from the two aforementioned previous studies: Berkowitz et al. (2018), collected in November 2016 (t0) (Supplementary Table S1), and VanZomeren et al. (2018) collected 0.5 years post-TLP in June 2017 (t1; Supplementary Table S2) with our data 6+ years after project implementation (t2) to analyze the trajectory of soil properties and wetland functions following project implementation. These data represent the longest investigation of soil N biogeochemical changes in TLP treated wetlands currently available in the literature that the authors are aware.
In November 2015 (t0), before the initiation of the project, the bulk density of the vegetated TLP marsh soil (Supplementary Table S1; Figure 5A) was not significantly different from the control. However, by June 2016 (t1), approximately 0.5 years (t1) after TLP application, the bulk density in the TLP area was 342% of the control. By August 2023 (t2), the bulk density remained relatively high, at 272% of the control, which suggests long-term sediment retention related to marsh stability, important as Sapkota and White (2019) found that coastal marsh with higher soil bulk density eroded more slowly. The observed decrease in bulk density over time could reflect root colonization and bioturbation of the TLP sediment, along with increases in soil organic matter. Moisture content increased from 52.4% of the control at t1 to 64.8% at t2 (Figure 5B), showing an inverse relationship with bulk density.
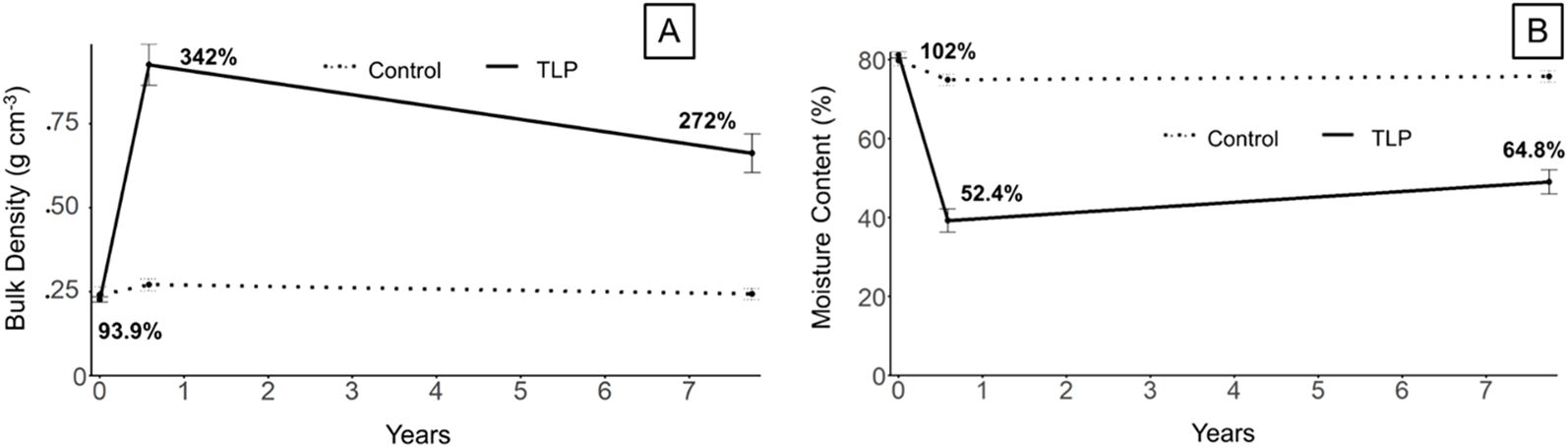
Figure 5. Trajectory of (A) bulk density and (B) moisture content of thin-layer placement (TLP) Marsh vs. Control sites over time. Data represent mean ± standard error (n = 6), except for the 6+ yr control time point, for which (n=3). Bold percentage represent % of control for each soil property for each time point.
Although soil organic matter (SOM), total C, and total N as percentages of control increased on average from t1 to t2, the changes were not statistically significant (Figures 6A-C; Table 2). This lack of difference is also likely related to the fact that the density of organic matter remains very low compared to mineral sediment and therefore requires large changes in organic matter content to detect density differences. However, this trend is consistent with the expected gradual accumulation of organic matter in the TLP marsh. Total P increased from 84.5% of the control in t1 to 131% in t2 (Table 1; Figure 6D). Again, the mineral fraction will dominate total P in the sediment amended marsh soils. One recent coastal marsh study separated total P into total organic P and total inorganic P to assess the mineral-bound P. When mineral river sediment was deposited in the marsh, the overall total P did not change but the relative distribution of majority total organic P shifted to total inorganic P (Spera et al., 2020).
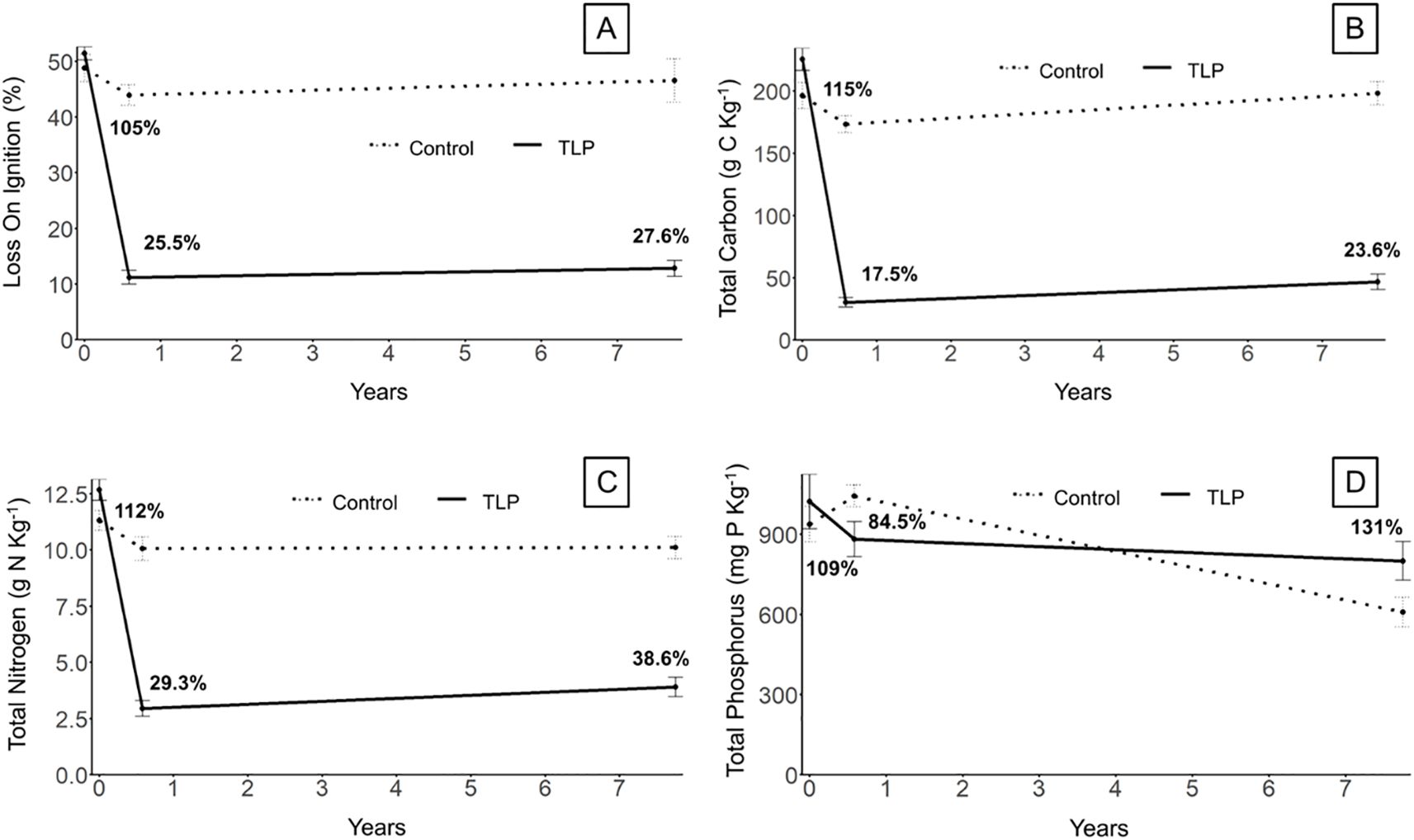
Figure 6. Soil organic matter properties of thin-layer placement (TLP) marsh vs. control sites over time for (A) Loss on ignition (organic matter content), (B) Total soil carbon, (C) total soil nitrogen, (D) total soil phosphorus. Data represent mean ± standard error (n=6), except for the 6+ yr control time point, for which (n=3). Bold percentage represent % of control for each soil property for each time point.
Extractable ammonium of the TLP sites (Figure 7A) did not differ significantly from the controls at any time point. Ammonium levels for all sites were notably low in t2, likely due to plant uptake. The microbial-mediated process of PMN rose from 22.9% of the control in t1 to 30.9% in t2 (Figure 7B), while MBN increased from 7.58% to 29.4% of the control over the same period (Figure 7C). These increases of PMN and MBN as a % of the control indicate increasing N cycling rates which are typically associated with higher organic matter soils (White and Reddy, 2000). While the soil trajectory of the sediment amended soils appears to be trending closer to the control, the N measures remain well below pre-project site conditions, and available data suggest that the restored wetland soil properties continue to develop along trajectories that differ from both the control and the original site conditions.
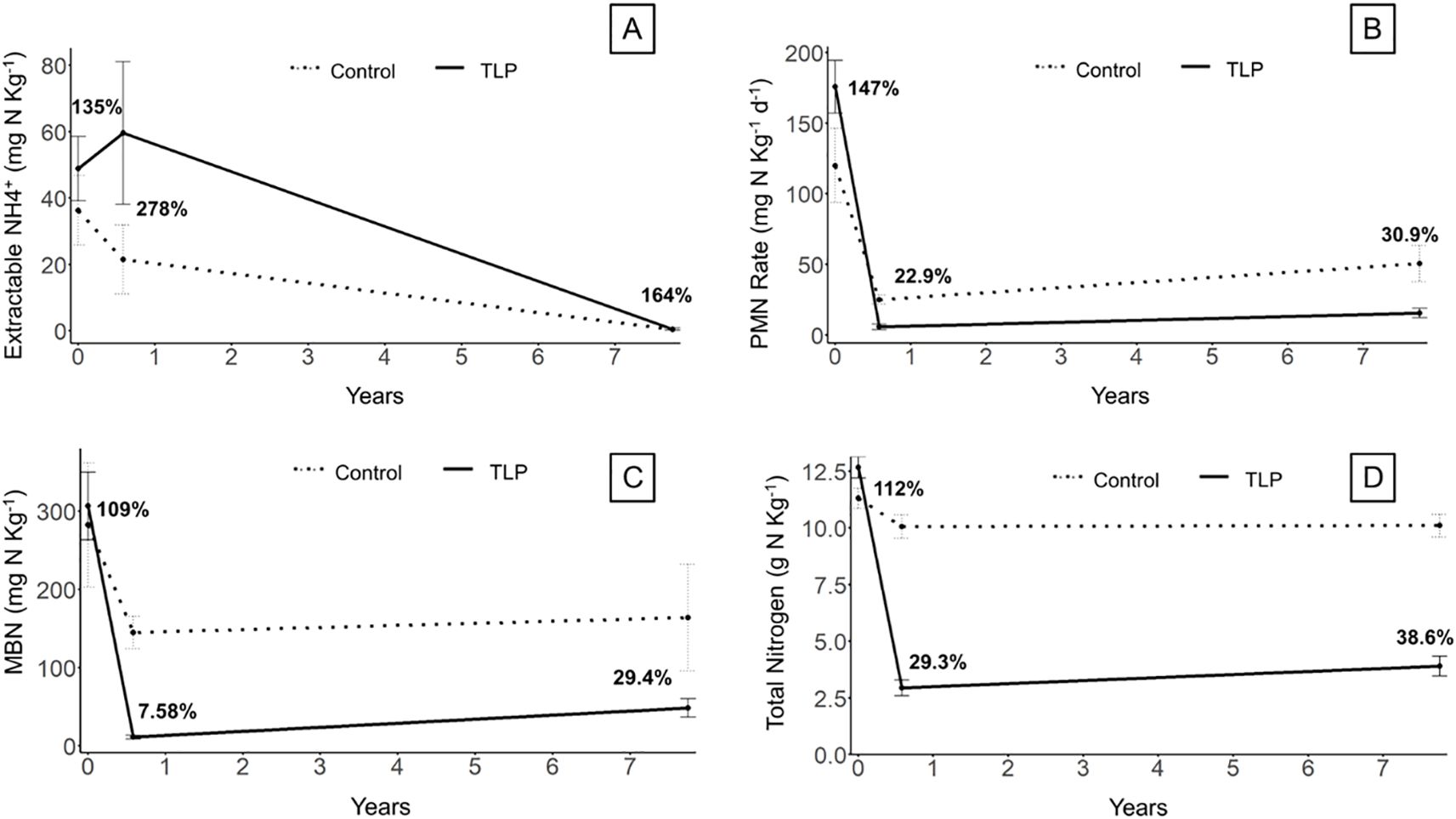
Figure 7. Soil N cycling properties of thin-layer placement (TLP) Marsh vs. Control sites over time for (A) Extractable NH4-N, (B) Potentially Mineralizable N rates, (C) Microbial biomass N, and (D) Total N. Data represent mean ± standard error (n=6), except for the 6+ yr control time point, for which (n=3). Bold percentage represent % of control for each soil property for each time point.
Low marsh elevation and bulk density are primary contributors to marsh degradation (Day et al., 2011). The TLP adds mineral material to the marsh soil, increasing elevation and bulk density. The restoration of these two physical factors allows for the re-establishment of vegetation and prevents further ponding and degradation. Additionally, by t1, the re-vegetation of the TLP area was already taking place with plant pushing up through the dredge material and the restored marsh was visually indistinguishable from naturally vegetated areas in the region by t2 (Harris et al., 2025).
Examining the trajectories of the percentage difference of biogeochemical properties reveals that most soil characteristics linked to microbial activity in the TLP soil are moving towards conditions observed at control sites. However, some properties, including bulk density and organic matter content, are likely to follow distinct patterns due to the addition of mineral sediments, which instantaneously impact soil structure and composition (Berkowitz et al., 2022) (Figures 5A, 6A). The organic matter and total C and N each display slight, but non-significant average increases over time. In properties related to N cycling, such as total N, PMN and MBN, there is a similar, non-significant increase over time, suggesting these characteristics may ultimately converge toward control marsh levels over long time scales (Figures 6A–D; Table 2). As plants re-establish and soil organic material accumulates, further increases in organic matter and N are anticipated over time (Craft et al., 2003). Longer-term data are needed to accurately define the progression of these changes and the slope of the restoration trajectory curve. Additional data is also required to inform the application of control (or ‘reference’) conditions as milestones for determining restoration project success, or if alternative process-based measures of ecological function and service delivery yield more desirable outcomes.
Raising marsh elevation reduces the duration of soil inundation, leading to increased oxidation at the soil surface. In contrast, prolonged flooding creates more anaerobic conditions in surface soil (Reddy and DeLaune, 2008). This shift toward oxidation with higher elevation may initially accelerate the decomposition of newly deposited detrital material, potentially lowering C storage from detritus. Increased decomposition from higher elevation will affect soil properties such as PMN, MBN, TN, and TC. However, shorter inundation periods also reduce flooding stress on plants. Snedden et al. (2015) found that increased elevation led to higher plant primary proactivity and biomass, with notable growth observed at elevations up to 19 cm above mean high water—the maximum elevation in their study. Other studies extending the soil surface elevation up to 30 and 40 cm showed that intermediate elevations best supported plant growth (Kirwan and Guntenspergen, 2012; Morris et al., 2013). Thus, balancing plant productivity with decomposition is crucial to optimizing ecological outcomes when implementing marsh restoration projects using TLP, where elevation gradient and target tolerances are often smaller than other coastal restoration/creation approaches.
4.2 Root density and carbon sequestration
As mentioned previously, the live root density in the TLP marsh soil is 3x higher than the vegetated control per unit volume of soil. One explanation for this difference is that the plants responded to a lower pool of N availability and increased root biomass to compensate. In the 2023 (t2) TLP marsh dataset, the soil contained approximately 1/3 of the TN, 1/3 of the MBN, and 1/3 of the PMN rate of the control, which are inversely proportional to the 3x increased root matter found in the TLP soils. This result suggests that the plants in the TLP marsh increased root mass in order to compensate for lower nutrient availability (Deegan et al., 2012). Payne et al. (2021) also reported higher belowground biomass in TLP marshes, approximately 50% greater root density, 3 years after the TLP project. Ford et al. (1999) reported that root biomass had reached or exceeded that of the control just 1 year after a TLP in another TLP project. Another possible explanation for the increased root growth is the absence of prior root occupation in the soil. Roots and rhizomes naturally expand in the subsurface, invading any viable nearby soil (Cahoon et al., 2021; Ford et al., 1999). Consequently, the unused soil space following TLP allows for the rapid expansion of root mass into previously unoccupied areas, as there is minimal belowground vegetation. This enables plant roots to quickly establish themselves in the dredged sediment, effectively filling the unclaimed “territory”.
Whether root growth is driven by physical or nutrient dynamics or a combination of factors, the increase in plant roots and rhizomes has the potential to enhance marsh sediment retention and resilience (Stagg and Mendelssohn, 2011). Increased root biomass strengthens soil shear resistance and reduces erosion (Graham and Mendelssohn, 2014; Sasser et al., 2018). The TLP is likely to contribute to coastal plant community resilience by providing new soil for roots and rhizomes to colonize. Consequently, the combined effects of increased root biomass and the greater bulk density of TLP-amended soils suggest that TLP enhances the marsh’s capacity for storm protection and erosion resilience.
Thin-layer placement has the potential to be an effective method to build elevation capital and C storage strategy to enhance biogeochemical nutrient cycling. In addition to burying existing organic rich soil (Bartolucci and Fulweiler, 2024), TLP has been shown to stimulate increased growth of belowground biomass. Notably, primary production in marsh plants is typically higher in belowground vs aboveground biomass. Stagg et al. (2017) reported that belowground biomass in coastal marshes can be up to five times greater than aboveground biomass. Further research is needed on the longer-term biogeochemical cycling capacity and trajectory of sites restored using TLP and other techniques.
5 Conclusions
The difference in soil properties, particularly the higher bulk density in the TLP, appear to have improved marsh stability and reduced edge erosion through sediment retention over time. This study could not detect any significant difference in water quality improvement (N cycling) between the TLP marsh and control areas after 6+ years after TLP project, despite the observed differences in soil physical and microbial properties. Soil physiochemical properties are trending more similar to the control marsh sites over time but are likely to remain on different trajectories depending on soil property. Further studies are needed to track these changes over longer periods of time to reduce the uncertainty in the slope of the restoration trajectory curve. The wetland function of denitrification and nutrient supply for vegetation (ammonium flux) was not statistically different between the control and TLP sites. The PMN and total N concentrations in the TLP was approximately 1/3 of the control, which may explain the threefold increase in root biomass as macrophytes seek out additional resources. After 6+ years, the restored marsh has achieved several of the functional measures or project success (e.g., denitrification rates, vegetation colonization), while some measures (e.g., SOM) continue to slowly advance toward but fail to reach control conditions. Overall, results suggest that degraded marshes can be successfully restored using TLP and that project benefits are sustained over near-decadal timescales.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JC: Investigation, Writing – original draft, Methodology. JB: Conceptualization, Methodology, Supervision, Funding acquisition, Writing – review & editing. JW: Resources, Conceptualization, Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was provided by the Technical Director for Military Environmental Engineering and Sciences, US Army Engineer Research and Development Center.
Acknowledgments
This research was developed as part of the Developing Engineering Practices for Ecosystem Design Solutions (DEEDS) project supervised by Dr. Yoko Slowey. Lee Potter is acknowledged for his assistance in field sampling and Lenore Tedesco from the Wetlands Institute acknowledged for planning and field day logistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1605785/full#supplementary-material
References
Andersen J. M. (1976). An ignition method for determination of total phosphorus in lake sediments. Water Res. 10, 329–331. doi: 10.1016/0043-1354(76)90175-5
Bartolucci N. N. and Fulweiler R. W. (2024). Sediment addition leads to variable responses in temperate saltmarsh greenhouse gas fluxes during the growing season. J. Geophysical Res.: Biogeosci. 129, e2023JG007756. doi: 10.1029/2023JG007756
Berkowitz J. F., Beane N. R., Hurst N. R., Jung J. F., and Philley K. D. (2022). A multidecadal assessment of dredged sediment beneficial use outcomes Part 1: Ecological Outcomes. J. Dredg. 20, 54–75. Available online at: https://www.westerndredging.org/phocadownload/WEDA_Journal_of_Dredging_Vol_20_No_1.pdf (Accessed July 30, 2025).
Berkowitz J. F., Piercy C., Welp T., and VanZomeren C. M. (2019). Thin layer placement: technical definition for US army corps of engineers applications. ERDC Vicksburg, MS. United States. Tech. Rep. Available online at: https://apps.dtic.mil/sti/citations/AD1067526 (Accessed July 30, 2025).
Berkowitz J. F., VanZomeren C. M., Piercy C. D., and White J. R. (2018). Evaluation of coastal wetland soil properties in a degrading marsh. Estuar. Coast. Shelf Sci. 212, 311–317. doi: 10.1016/j.ecss.2018.07.021
Bowes K., White J. R., Maiti K., and Meselhe E. (2022). Surface water temperature impacts on denitrification: implications for river reconnection. Sci. Total Environ. 828, 154397. doi: 10.2139/ssrn.3989505
Brookes P. C., Landman A., Pruden G., and Jenkinson D. S. (1985). Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17, 837–842. doi: 10.1016/0038-0717(85)90144-0
Cahoon D. R., McKee K. L., and Morris J. T. (2021). How plants influence resilience of salt marsh and mangrove wetlands to sea-level rise. Estuar. Coast. 44, 883–898. doi: 10.1007/s12237-020-00834-w
Cheng J. Z. and White J. R. (2022). Dredge-material created coastal marshes are more effective at improving water quality than natural marshes in early-stage development. Ecol. Eng. 185, 106814. doi: 10.1016/j.ecoleng.2022.106814
Church J. A. and White N. J. (2011). Sea-level rise from the late 19th to the early 21st century. Surv. Geophys. 32, 585–602. doi: 10.1007/s10712-011-9119-1
Costanza R., d’Arge R., De Groot R., Farber S., Grasso M., and Van Den Belt M. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260. doi: 10.1038/387253a0
Craft C., Megonigal P., Broome S., Stevenson J., Freese R., and Cornell J. (2003). The pace of ecosystem development of constructed Spartina alterniflora marshes. Ecol. App. 13, 1417–1432. doi: 10.1890/02-5086
Croft A. L., Leonard L. A., Alphin T. D., Cahoon L. B., and Posey M. H. (2006). The effects of thin layer sand renourishment on tidal marsh processes: Masonboro Island, North Carolina. Estuar. Coast. 29, 737–750. doi: 10.1007/BF02786525
Davis J., Currin C., and Mushegian N. (2022). Effective use of thin layer sediment application in Spartina alterniflora marshes is guided by elevation-biomass relationship. Ecol. Eng. 177, 106566. doi: 10.1016/j.ecoleng.2022.106566
Day J. W., Anthony E., Corstanza R., Edmonds D., Gunn J., Hopkinson C., et al. (2024). A review of major issues related to coastal wetlands in the anthropocene. Annu. Rev. Environ. Resour. 49, 20.1–20.31. doi: 10.1146/annurev-environ-121922-041109
Day J. W., Boesch D. F., Clairain E. J., Kemp G. P., Laska S. B., Mitsch W.J., et al. (2007). Restoration of the Mississippi Delta: lessons from hurricanes Katrina and Rita. Science 315, 1679–1684. doi: 10.1126/science.1137030
Day J. W., Kemp G. P., Reed D. J., Cahoon D. R., Boumans R. M., Suhayda J. M., et al. (2011). Vegetation death and rapid loss of surface elevation in two contrasting Mississippi delta salt marshes: The role of sedimentation, autocompaction and sea-level rise. Ecol. Eng. 37, 229–240. doi: 10.1016/j.ecoleng.2010.11.021
Day J. W., Rybczyk J., Scarton F., Rismondo A., Are D., and Cecconi G. (1999). Soil accretionary dynamics, sea-level rise and the survival of wetlands in Venice Lagoon: a field and modelling approach. Estuar. Coast. Shelf Sci. 49, 607–628. doi: 10.1006/ecss.1999.0522
Deegan L. A., Johnson D. S., Warren R. S., Peterson B. J., Fleeger J. W., and Fagherazzi S. (2012). Coastal eutrophication as a driver of salt marsh loss. Nature 490, 388–392. doi: 10.1038/nature11533
DeLaune R. D., Nyman J. A., and Patrick (1994). Peat collapse, ponding and wetland loss in a rapidly submerging coastal marsh. J. Coast. Res. 10, 1021–1030. Available online at: https://www.jstor.org/stable/4298293.
DeLaune R. D., Patrick W. H. Jr., and Buresh R. J. (1978). Sedimentation rates determined by 137Cs dating in a rapidly accreting salt marsh. Nature 275, 532–533. doi: 10.1038/275532A0
DeLaune R. D., Sasser C. E., Evers-Hebert E., White J. R., and Roberts H. H. (2016). Influence of the wax lake sediment diversion on aboveground plant productivity and carbon storage in deltaic island and mainland coastal marshes. Estuar. Coast. Shelf Sci. 177, 83–89. doi: 10.1016/j.ecss.2016.05.010
Feder R. F. and White J. R. (2024). Impact of freshwater river reconnection on porewater salinity and ammonium availability in coastal brackish marsh soils. Sci. Total Environ. 926, 172131. doi: 10.1016/j.scitotenv.2024.172131
Ford M. A., Cahoon D. R., and Lynch J. C. (1999). Restoring marsh elevation in a rapidly subsiding salt marsh by thin-layer deposition of dredged material. Ecol. Eng. 12, 189–205. doi: 10.1016/S0925-8574(98)00061-5
Graham S. A. and Mendelssohn I. A. (2014). Coastal wetland stability maintained through counterbalancing accretionary responses to chronic nutrient enrichment. Ecology 95, 3271–3283. doi: 10.1890/14-0196.1
Harris B. D., Ostojic A., Tedesco L. P., VanDerSys K., Bailey S., and Shawler J. L. (2025). Wetland elevation change following beneficial use of dredged material nourishment. Front. Ecol. Evol. 13. doi: 10.3389/fevo.2025.1518759
Haywood B. J., Hayes M. P., White J. R., and Cook R. L. (2020). Potential fate of wetland soil carbon in a deltaic coastal wetland subjected to high relative sea level rise. Sci. Total Environ. 711, 135185. doi: 10.1016/j.scitotenv.2019.135185
Kirwan M. L. and Guntenspergen G. R. (2012). Feedbacks between inundation, root production, and shoot growth in a rapidly submerging brackish marsh. J. Ecol. 100, 764–770. doi: 10.2307/41496125
Morris J. T., Sundberg K., and Hopkinson C. S. (2013). Salt marsh primary production and its responses to relative sea level and nutrients in estuaries at Plum Island, Massachusetts, and North Inlet, South Carolina, USA. Oceanography 26, 78–84. doi: 10.5670/oceanog.2013.48
Orson R., Panageotou W., and Leatherman S. P. (1985). Response of tidal salt marshes of the US Atlantic and Gulf coasts to rising sea levels. J. Coast. Res. 1, 29–37. Available online at: https://www.jstor.org/stable/4297007.
Payne A. R., Burdick D. M., Moore G. E., and Wigand C. (2021). Short-term effects of thin-layer sand placement on salt marsh grasses: A marsh organ field experiment. J. Coast. Res. 37, 771–778. doi: 10.2112/JCOASTRES-D-20-00072.1
Peteet D. M., Nichols J., Kenna T., Chang C., Browne J., and Reza M. (2018). Sediment starvation destroys New York City marshes’ resistance to sea level rise. Proc. Natl. Acad. Sci. U.S.A. 115, 10281–10286. doi: 10.1073/pnas.1715392115
Raposa K. B., Woolfolk A., Endris C. A., Fountain M. C., Moore G., and Tyrrell M. (2023). Evaluating thin-layer sediment placement as a tool for enhancing tidal marsh resilience: a coordinated experiment across eight US national estuarine research reserves. Estuar. Coast. 46, 595–615. doi: 10.1007/s12237-022-01161-y
Reddy K. R. and DeLaune R. D. (2008). Biogeochemistry of Wetlands: Science and Applications. 1st ed (Boca Raton, FL: CRC Press) 46:595–615. doi: 10.1201/9780203491454
Reimold R. J., Hardisky M. A., and Adams P. C. (1978). The Effects of Smothering a ‘Spartina alterniflora’ Salt Marsh with Dredged Material. Georgia Univ Brunswick Marine Extension Service. University of GA, Brunswick, GA. Tech. Rep. D. Available online at: https://usace.contentdm.oclc.org/digital/collection/p266001coll1/id/5615/ (Accessed July 30, 2025).
Roberts H. H. (1997). Dynamic changes of the Holocene Mississippi River delta plain: the delta cycle. J. Coast. Res. 13, 605–627. Available online at: https://journals.flvc.org/jcr/article/view/80176.
Sapkota Y. and White J. R. (2019). Marsh edge erosion and associated carbon dynamics in coastal Louisiana: A proxy for future wetland-dominated coastlines world-wide. Estuar. Coast. Shelf Sci. 226, 106289. doi: 10.1016/j.ecss.2019.106289
Sapkota Y. and White J. R. (2021). Long term fate of rapidly eroding carbon stock soil profiles in coastal wetlands. Sci. Total Environ. 753, 141913. doi: 10.1016/j.scitotenv.2020.141913
Sapkota Y., Xu K., Maiti K., Inglett P. W., and White J. R. (2023). Temporal variability in soil organic matter accretion rates in coastal deltaic wetlands under changing depositional environments. Soil Sci. Soc Am. J. 87, 390–403. doi: 10.1002/saj2.20515
Sasser C. E., Evers-Hebert E., Holm G. O., Milan B., Sasser J. B., and Peterson E. F. (2018). Relationships of marsh soil strength to belowground vegetation biomass in Louisiana coastal marshes. Wetlands 38, 401–409. doi: 10.1007/s13157-017-0977-2
Schepers L., Kirwan M., Guntenspergen G., and Temmerman S. (2017). Spatio-temporal development of vegetation die-off in a submerging coastal marsh. Limnol. Oceanogr. 62, 137–150. doi: 10.1002/lno.10381
Smith D. E., Harrison S., Firth C. R., and Jordan J. T. (2011). The early Holocene sea level rise. Quat. Sci. Rev. 30, 1846–1860. doi: 10.1016/j.quascirev.2011.04.019
Snedden G. A., Cretini K., and Patton B. (2015). Inundation and salinity impacts to above-and belowground productivity in Spartina patens and Spartina alterniflora in the Mississippi River deltaic plain: Implications for using river diversions as restoration tools. Ecol. Eng. 81, 133–139. doi: 10.1016/j.ecoleng.2015.04.035
Soil Survey Staff (2025). Natural resources conservation service, United States department of agriculture. Web Soil Survey. Available online at: https://websoilsurvey.nrcs.usda.gov/app/ (June 28 2025).
Spera A. C., White J. R., and Corstanje R. (2020). Spatial and temporal changes to a hydrologically-reconnected coastal wetland: Implications for restoration. Estuar. Coast. Shelf Sci. 238, 106728. doi: 10.1016/j.ecss.2020.106728
Stagg C. L. and Mendelssohn I. A. (2011). Controls on resilience and stability in a sediment-subsidized salt marsh. Ecol. App. 21, 1731–1744. doi: 10.2307/23023113
Stagg C. L., Schoolmaster D. R., Piazza S. C., Snedden G., Steyer G. D., and Fischenich C. J. (2017). A landscape-scale assessment of above-and belowground primary production in coastal wetlands: implications for climate change-induced community shifts. Estuar. Coast. 40, 856–879. doi: 10.1007/s12237-016-0177-y
Tong C., Baustian J. J., Graham S. A., and Mendelssohn I. A. (2013). Salt marsh restoration with sediment-slurry application: effects on benthic macroinvertebrates and associated soil–plant variables. Ecol. Eng. 51, 151–160. doi: 10.1016/j.ecoleng.2012.12.010
Torab M. and Azab M. (2007). Modern shoreline changes along the Nile Delta coast as an impact of construction of the Aswan High Dam. Geogr. Tech. 2, 69–76. Available online at: https://technicalgeography.org/pdf/2_2007/gt_2_2007.pdf. (Accessed July 30, 2025).
Turner R. E. and Cahoon D. R. (1987). Causes of wetland loss in the coastal central Gulf of Mexico. Vol II: Technical Narrative. Final Report submitted to the Minerals Management Service, New Orleans, LA. Contract No.14-12-0001-30252. OCS Study/MMS 87-0120. Available online at: https://espis.boem.gov/final%20reports/3779.pdf (Accessed July 30, 2025).
U.S. EPA (1993). Methods for Determination of Inorganic Substances in Environmental Samples. EPA/600/R-93/100 (Washington D.C). Available online at: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=30002U3P.TXT (Accessed July 30, 2025).
Vaccare J., White J. R., and Meselhe E. (2019). The denitrification potential of eroding wetlands in Barataria Bay, LA USA: implications for river reconnection. Sci. Total Environ. 686, 529–537. doi: 10.1016/j.ecss.2019.04.027
VanZomeren C. M., Berkowitz J. F., Piercy C. D., and White J. R. (2018). Restoring a degraded marsh using thin layer sediment placement: Short term effects on soil physical and biogeochemical properties. Ecol. Eng. 120, 61–67. doi: 10.1016/j.ecoleng.2018.05.012
Walker H. J., Coleman J. M., Roberts H. H., and Tye R. S. (1987). Wetland loss in louisiana. Geografiska Annaler. Ser. A. Phys. Geog. 69 (1), 189–200. doi: 10.1080/04353676.1987.11880207
White J. R. and Reddy K. R. (2000). Influence of phosphorus loading on organic nitrogen mineralization of Everglades soils. Soil Sci. Soc Am. J. 64, 1525–1534. doi: 10.2136/sssaj2000.6441525x
White J. R. and Reddy K. R. (2001). Effect of select inorganic electron acceptors on organic nitrogen mineralization in northern Everglades soils. Soil Sci. Soc Am. J. 65, 941–948. doi: 10.2136/sssaj2001.653941x
Wilber P. (1992). “Case studies of the thin-layer disposal of dredged material – Gull Rock, North Carolina,” in Environmental Effects of Dredging, vol. D-92-3. (U.S. Army Engineer Waterways Experiment Station, Vicksburg, MS), 1–6.
Wilber D. H., Clarke D. G., and Rees S. I. (2007). Responses of benthic macroinvertebrates to thin-layer disposal of dredged material in Mississippi Sound, USA. Mar. pollut. Bull. 54, 42–52. doi: 10.1016/j.marpolbul.2006.08.042
Williams S. J., Stone G. W., and Burruss A. E. (1997). A perspective on the Louisiana wetland loss and coastal erosion problem. J. Coast. Res. 13 (3), 593–594. Available online at: http://www.jstor.org/stable/4298657.
Yang H. F., Yang S. L., Xu K. H., Wu H., Shi B. W., and Zhu Q. (2017). Erosion potential of the Yangtze Delta under sediment starvation and climate change. Sci. Rep. 7, 10535. doi: 10.1038/s41598-017-10958-y
Keywords: wetland, sediment, marsh resilience, soil properties, restoration
Citation: Cheng JZ, Berkowitz JF and White JR (2025) Trajectory of coastal wetland soil physical and microbial properties 6+ years after thin layer placement sediment amendment. Front. Ecol. Evol. 13:1605785. doi: 10.3389/fevo.2025.1605785
Received: 04 April 2025; Accepted: 02 July 2025;
Published: 08 August 2025.
Edited by:
Mariko Polk, North Carolina Sea Grant (NOAA), United StatesReviewed by:
Gina Marie Wimp, Georgetown University, United StatesNia Bartolucci, United States Environmental Protection Agency (EPA), United States
Ansley Levine, Texas A&M University at Galveston, United States
Copyright © 2025 Cheng, Berkowitz and White. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John R. White, anJ3aGl0ZUBsc3UuZWR1
 Jacob Z. Cheng1,2
Jacob Z. Cheng1,2 Jacob F. Berkowitz
Jacob F. Berkowitz John R. White
John R. White