- 1Wenzhou Vocational College of Science and Technology (Wenzhou Academy of Agricultural Sciences), Wenzhou, China
- 2School of Ecological and Environmental Sciences, East China Normal University, Shanghai, China
- 3Institute of Soil Sciences, Shanghai Academy of Landscape Architecture Science and Planning, Shanghai, China
- 4School of Design, Shanghai Jiao Tong University, Shanghai, China
- 5Digital Technology Innovation Center for Territorial Optimization & Ecological Governance, Shanghai, China
Coastal land reclamation dramatically alters ecosystem structure and trajectories of ecological succession, yet knowledge about soil faunal colonization and community assembly in reclaimed coastal land is scarce. In this study, we investigated taxonomic and functional shifts in soil nematode communities following reclamation in sites at Hengsha Island, China. Six reclamation stages were identified based on reclamation age and vegetation type, including tidal flat, 1-year bare field, 3-year halotolerant pioneer community, 5-year grassland, 10-year, and 50-year secondary forests. We collected data on the morphological and life history traits of soil nematodes, including body size, cephalic setae, amphids, tail shape, life history strategy, and feeding habit, to assess the functional responses of nematodes to different reclamation stages. We found a significant decrease in both taxonomic and functional diversity as tidal flats were converted to dry land, followed by a gradual recovery that progressed with increasing reclamation age. Significant shifts in the taxonomic and functional composition of soil nematode communities were also observed. Tidal flat reclamation decreased the relative abundance of marine nematodes with cephalic setae, large amphids and clavate/conicocylindrical tails. With increasing reclamation age, bacterivores and r-strategists with conical and long tails were displaced by fungivores, herbivores, and K-strategists with rounded tails. These shifts were driven by changes in soil water content, organic matter, plant communities, and stress factors, such as pH and salinity. Our findings highlight the critical role of morphological and life history traits in understanding how soil nematode communities adapt to human-induced environmental changes, providing valuable insight into the long-term ecological impacts of coastal reclamation on soil biodiversity.
1 Introduction
Human modification of the environment is increasingly recognized as a primary driver of ongoing changes in soil biodiversity (Geisen et al., 2019; Young et al., 2016). Understanding how human activity alters the development of soil faunal communities is essential to accurately predict how soil biodiversity will respond to future changes in land use and its implications for ecosystem functions and services (Eisenhauer et al., 2022). Throughout human history, coastal reclamation has been used to meet the demands of growing populations and to increase food production (Martínantón et al., 2016; Sengupta et al., 2020; Wang et al., 2014). In this practice, embankment and filling are used to transform wetlands and marine areas into terrestrial ecosystems, which significantly alters ecosystem structure and function (Tian et al., 2016). Newly reclaimed land provides space for the development of agriculture, forestry, and industry while also creating new habitats for biotic colonization and turnover (Ma et al., 2019; Xue et al., 2019). Soil chronosequences spanning soils of varying reclamation ages offer unique opportunities to study how soil biota have responded to accelerated ecosystem development during the Anthropocene (Walker et al., 2010; Wang et al., 2020; Xing et al., 2020).
Short-term pedogenesis and the influence of seawater cause the high salinity and low fertility that are frequently characteristic of newly reclaimed coastal lands, both of which restrict plant succession and colonization by terrestrial organisms (Feng et al., 2018). Generally, soil physicochemical qualities improve with increasing reclamation age as a result of natural succession and anthropogenic land management strategies. This improvement is typically characterized by decreasing soil salinity, pH, and bulk density and increasing soil organic matter (Fu et al., 2014; Li et al., 2014; Zhang et al., 2019). Changes in vegetation cover – from halophytic pioneer plants to mesophytes – occur in response to the combined effects of natural succession and human activity (Min and Kim, 2000; Sun et al., 2011). As land availability increases in later stages of reclamation, natural vegetation is often replaced by planted vegetation, which further influences soil properties (Li and Zhang, 2021; Pan et al., 2022). As soil and vegetation characteristics are considered major regulators of the structure of soil biotic communities (De Deyn and van der Putten, 2005; Wardle et al., 2004a), differences in soil development and vegetation succession along a chronosequence inevitably influence assemblages of soil faunal communities and, in turn, enhance succession (De Deyn et al., 2003). Despite the essential roles of soil fauna in ecological processes such as soil formation, decomposition, mineralization, and nutrient cycling (Bardgett and van der Putten, 2014; Maaß et al., 2015; Osler and Martin, 2007), little is known about how soil faunal communities colonize and develop in reclaimed coastal areas, especially from a functional trait perspective.
Traditionally, studies of soil faunal communities tend to focus on taxonomic identities, an approach that is often criticized for neglecting the ecological roles of these organisms (Ellers et al., 2018; Violle et al., 2007). Functional traits integrate the morphological, physiological, phenological, or behavioral properties of organisms that influence their fitness and determine how they respond to changing environmental conditions and their subsequent effects on ecological functioning (Pey et al., 2014; Violle et al., 2007). Some work suggests that trait-based approaches can provide insight into the mechanisms that shape biodiversity along environmental gradients (McGill et al., 2006; Mouillot et al., 2013). Local community assembly is driven by environmental filtering, dispersal limitation, and biotic interactions (Brousseau et al., 2018; McGill et al., 2006). Variation in selection pressure along a soil chronosequence can cause trade-offs in functional traits and shift the ecological strategies employed by biota, thereby resulting in changes in the means (functional composition) and distributions of trait values (functional diversity) within a community (Edwards and Stachowicz, 2010; Ficetola et al., 2021).
Nematodes are the most diverse and abundant metazoans and inhabit both marine and terrestrial ecosystems. They are often early colonizers of newly emerging substrates (Pan et al., 2016; van den Hoogen et al., 2019) and, because they occupy several trophic levels, they play a critical role in soil micro food webs and are involved in key ecological processes, such as decomposition and nutrient cycling (van den Hoogen et al., 2019). They have long been considered ideal indicators of soil quality due to their ubiquitous distribution, ease of sampling, and sensitivity to environmental changes (Bongers and Ferris, 1999; Lu et al., 2020). Several functional traits are thought to influence nematode responses to environmental pressures and their effects on ecosystem functions (Zhang et al., 2024). In particular, nematode feeding habits and life history strategies are widely used in assessments of soil health (Bongers, 1990; Bongers and Bongers, 1998; Yeates et al., 1993). Additionally, nematode body size varies with fertilization regime (Liu et al., 2015), precipitation (Andriuzzi et al., 2020), grazing (Mills and Adl, 2011), and land use and management practices (Mulder and Maas, 2017; Sechi et al., 2018; Zhao et al., 2015). Recent studies increasingly combine morphological traits (e.g., tail shape, amphid, cuticle morphology) to provide comprehensive evaluations of the responses of free-living marine nematodes to environmental changes (Franzo and Del Negro, 2019; Liu et al., 2018; Sroczyńska et al., 2021). These studies demonstrate that nematode morphological traits can respond to environmental factors such as water depth, sediment texture, and hydrodynamic characteristics. However, little is currently known about how nematode morphology and life history traits respond to the marine-to-terrestrial transition resulting from reclamation and subsequent ecosystem development.
In this study, we investigated the taxonomic and functional diversity and composition of soil nematode communities during the reclamation of an estuarine island in the Yangtze River in China. We identified six reclamation stages, which represent plant succession trajectories and land use changes typical of reclaimed land in this region. These stages included tidal flats, 1-year bare fields, 3-year halotolerant pioneer communities, 5-year grasslands, 10-year secondary forests, and 50-year secondary forests. We measured several morphological and life history traits related to soil nematode adaption to local habitats, including body size, tail shape, amphid, cephalic setae, life-history strategy, and feeding habit. We expected that taxonomic and functional diversity would decrease as tidal flats were converted to dry land, primarily due to the loss of marine species and their associated traits — such as large amphids and cephalic setae — which serve as essential sensory organs in aquatic environments (Wu et al., 2002; Zullini and Semprucci, 2020). We hypothesized that taxonomic and functional diversity would increase with increasing reclamation age due to the greater availability of resources and ecological niches provided by higher plant diversity and soil quality. We further hypothesized that functional composition would shift from traits associated with higher dispersal ability and lower resource demands (e.g., lower body size and higher abundances of r-strategists and bacterivores) toward traits associated with greater resource demands and competitive advantages (e.g., higher body size and greater abundances of K-strategists, fungivores, and herbivores) (Guerrieri et al., 2024; Háněl, 2010). We also expected that variation in environmental stress (pH, electrical conductivity) and resource availability (e.g., soil organic matter and plant diversity) would be key factors driving shifts in functional traits.
2 Materials and methods
2.1 Study area
The study was conducted on Hengsha Island, Shanghai, eastern China (31°15′–31°22′N, 121°47′–122°6′E) (Figure 1A). This area is characterized by a subtropical coastal monsoon climate, with an annual average temperature of 15.4°C, an annual average rainfall of 1,100 mm, and annual sunlight exposure of 2,200 hours. Hengsha Island is an alluvial island formed by sediment deposited by the Yangtze River. The island first emerged in 1858 and has undergone reclamation since 1886. Between 1886 and 1948, 44.54 km² of land was reclaimed, followed by an additional 10.99 km² between 1949 and 1985. The largest reclamation project was carried out on the eastern tidal flats between 2003 and 2020 (Figure 1A) and expanded the island’s area by nearly threefold, from 52 km² to 150 km². The project was divided into four phases (III, VI, VII, and VIII), which were completed in 2006, 2015, 2017, and 2020, respectively. According to government plans, Hengsha Island will be developed into a “forest ecological island.” The newly reclaimed area (i.e., the area reclaimed between 2003 and 2020) has remained undeveloped as a reserve, is not open to the public, and experiences minimal human interference. Land in the early stages of reclamation is typically unsuitable for use due to its high salinity and is left to undergo spontaneous succession. At Hengsha Island, reclaimed land was gradually repurposed for afforestation and agricultural production as soil salinity decreased (Cui et al., 2016).
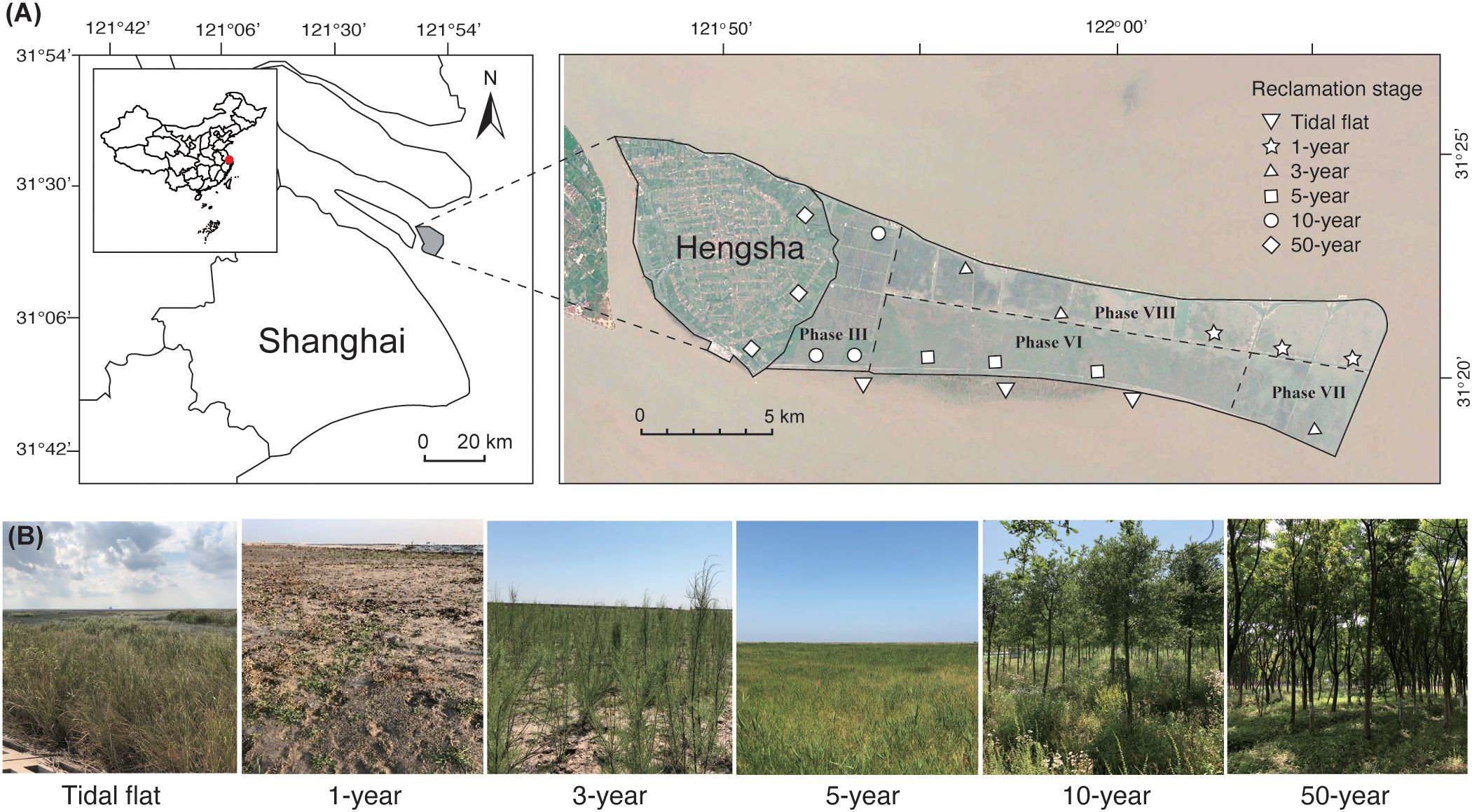
Figure 1. (A) Location of the study area and sampling sites (B) photographs showing changes in vegetation with reclamation stage.
Following a field survey in 2019, we used vegetation type and reclamation age to select six reclamation stages that represented the typical trajectory of plant succession on reclaimed land in this area (Figure 1B). Reclamation ages were verified using satellite images, local records, project plans, and relevant literature (Supplementary Figure S1). Three sites were selected for each reclamation stage, with selected locations uniformly distributed across the entire reclamation area and individual sites separated by at least 1 km (Figure 1A). Plant species and coverage were recorded during the growing season in June 2019 in 10 × 10 m plots for trees and shrubs and in four 1 × 1 m plots for grasses at each site. The characteristics of the six reclamation stages are summarized below:
2.1.1 Tidal flat
Located outside the dammed area and dominated by Phragmites communis, Spartina alterniflora, and × Bolboschoenoplectus mariqueter, with 100% coverage and plant heights of 1.2-2.1 m.
2.1.2 1-year stage
Located east of the Phase VIII project and characterized by predominantly bare soil with sparse salt-tolerant vegetation, including P. communis, Suaeda glauca, and Tamarix chinensis, covering less than 5% of the surface.
2.1.3 3-year stage
Located in the same area as the Phase VII project and west of the Phase VIII project and dominated by halotolerant pioneer communities of T. chinensis, with 60%-75% coverage. Herbaceous plants were sparse and included P. communis, Polypogon fugax, Tripolium vulgare, and S. glauca, with 5%-15% coverage and plant heights of 0.3-0.5 m.
2.1.4 5-year stage
Grassland located in the same area as the Phase VI project and characterized by highly adaptable weed species such as P. fugax, P. communis, Solidago canadensis, Equisetum chinensis, Erigeron annuus, Conyza canadensis, Arthraxon hispidus, Glycine soja, Carex sp., Imperata cylindrica, and Plantago depressa. Coverage ranged from 95% to 100%, with plant heights of 0.4-0.9 m.
2.1.5 10-year stage
Secondary forest located in the same area as the Phase III project, planted with salt-tolerant tree species such as Taxodiomera peizhongii and Quercus virginiana. Tree heights ranged from 4 to 6 m, with diameters at breast height (DBH) of 4.5-6.5 cm. The understory consisted of herbaceous species such as P. fugax, S. canadensis, E. chinensis, E. annuus, P. communis, G. soja, I. cylindrica, Carex sp., Aster subulatus, Lagedium sibiricum, Sonchus arvensis, Geranium carolinianum, Artemisia argyi, Rumex dentatus, Arthraxon hispidus, Trifolium sp., Humulus scandens, and Potentilla supina, with coverage of 95%-100% and plant heights of 0.5-0.8 m.
2.1.6 50-year stage
Secondary forest located within the area reclaimed between 1949 and 1985. These forests were planted with common greening tree species such as Bischofia polycarpa, Sapindus mukorossi, and Ligustrum lucidum. Tree heights ranged from 9 to 11 m, with DBH of 9–20 cm. The understory consisted of herbaceous species such as Cayratia japonica, Cynodon dactylon, Sedum bulbiferum, S. canadensis, Erigeron canadensis, Metaplexis japonica, Alternanthera philoxeroides, Ophiopogon japonicus, Duchesnea indica, Oxalis corymbosa, and Euphorbia humifusa, with 70%-90% coverage and plant heights of 0.3-0.6 m.
2.2 Soil sampling and analyses
Soil sampling was conducted four times: in June, October, and December of 2019 and in April of 2020. We collected four subsamples of soil at randomly selected locations within each site separated from one another by at least 5 m. Subsamples were collected using a stainless steel bucket auger (5 cm diameter, 10 cm height), after which they were combined to form a single composite sample. A fresh subsample was also collected and stored at 4°C for nematode extraction. Two intact soil cores (100 ml) were collected in plastic bags, which were sealed and transported to the laboratory to determine bulk density and soil water content.
Soil water content and bulk density were measured after drying intact soil cores at 105°C. Homogenized composite samples were air-dried and ground, after which an aliquot was passed through a 2 mm sieve for pH and electrical conductivity (EC) analyses and another portion was passed through a 0.150 mm sieve for soil organic matter analysis. Soil pH was measured using an electrode in a 1:2.5 (v:v) soil:water suspension, and EC was measured with a conductivity meter in a 1:5 (v:v) soil:water suspension. Soil organic matter was determined by the oxidation method using K2Cr2O7 at 180°C using an adaptation of the Walkley-Black method.
2.3 Nematode extraction and identification
Nematodes were extracted from 200 g of homogenized soil from each plot using a Baermann funnel over the course of 48 h, after which they were fixed in 4% formaldehyde. Specimens were sorted and counted under a stereomicroscope (SMZ-165, Motic Microscopy, China). Two hundred individuals (when possible) were randomly selected from each sample and mounted on slides for identification at the genus level using a light microscope (BX53, Olympus, Japan). Nematode abundances were expressed as number of individuals per 100 g dry soil.
2.4 Nematode trait determination
The morphological and life history traits of soil nematodes were measured or compiled from literature and databases and included body size, tail shape, amphid, cephalic setae, life-history strategy, and feeding habit. See Supplementary Table S1 for measured or assigned values for each genus.
2.4.1 Body size
Body size was assessed using fresh weight (FW, μg) and calculated based on measured body length (L, μm) and width (W, μm): FM=(L×W^2)/(1.6×106). Body length and width were measured using ImageJ 13.0.6 (National Institutes of Health, USA). Average values were calculated for each genus.
2.4.2 Tail shape
Tail shape was assigned to one of four categories according to Thistle et al. (1995): rounded, long, conical and clavate/conicocylindrical.
2.4.3 Amphid and cephalic setae
Amphids and cephalic setae are chemoreceptors in nematodes’ head region (Zullini and Semprucci, 2020). The amphids in our study were grouped into two categories: small (indistinct or slit-like) or large (round, spiral, or pocket-like), as larger amphid indicate stronger sensing ability (Semprucci et al., 2018). Cephalic setae were identified as either present or absent. We assessed these traits based on descriptions in taxonomic literature and observations of specimens under a light microscope.
2.4.4 Life history strategy
Nematodes were assigned c-p values on a scale from 1 to 5 (r-strategy to K-strategy, respectively) according to Bongers (1990). We grouped nematodes into three life history strategy categories according to the c-p triangles proposed by de Goede et al. (1993): enrichment opportunists (c-p 1), general opportunists (c-p 2) and persisters (c-p 3-5).
2.4.5 Feeding habit
Feeding habits were classified into five categories after Yeates et al. (1993): algivore, bacterivore, fungivore, herbivore, predator, and omnivore.
2.5 Statistical analyses
The soil nematode community was described using abundance (number of individuals per 100 g dry soil) and genus richness (number of genera). The R package UpSetR was used to visualize the number of unique and shared genera across the six reclamation stages. Functional richness (FRic) and functional dispersion (FDis) were calculated to describe the functional diversity of soil nematode communities. Previous research suggests that these two indicators can be used to assess community assembly processes (Gobbi et al., 2017; Mason et al., 2013; Zhang et al., 2023). Functional richness measures how much ecological niche space is occupied by existing species in a community (Mason et al., 2005). Functional dispersion measures the distribution and spread of species within a multidimensional trait space and is calculated as the average distance of an individual species from the community’s trait centroid, weighted by its relative abundance (Laliberté and Legendre, 2010). Community-weighted mean (CWM) was calculated as a measure of functional composition, which reflects the dominant ecological strategies and functional roles that species play in a given environment. We calculated this metric as average trait values weighted by the relative abundance of a species in the community (Garnier et al., 2004).
Linear mixed effects modeling (LMM) was used to test the effects of reclamation stage on soil properties, taxonomic diversity, functional diversity, and functional composition, with sampling time treated as a random effect. Algivores were excluded from this analysis due to their low occurrence. The significance of fixed effects was tested using Type III analysis of variance paired with Satterthwaite’s method. Linear models were used to test the effects of reclamation stage on plan properties. Tukey HSD post hoc test was used to evaluate the significance of differences between reclamation stages. Data were log(x+1) transformed to improve normality and homogeneity when necessary.
Nonmetric multidimensional scaling (NMDS) based on the Bray-Curtis dissimilarity index was used to visualize the taxonomic composition of soil nematodes across reclamation stages. The statistical significance of dissimilarity was evaluated using permutational multivariate analysis of variance (PERMANOVA), and the P values of pairwise comparisons were adjusted using the Bonferroni method.
We used LMM to select the environmental variables that best explained the taxonomic diversity and functional diversity of nematode communities along the chronosequence. Explanatory variables included soil pH, EC, bulk density, water content, organic matter, plant richness, and grass coverage. All explanatory variables were standardized prior to analysis. We constructed candidate models using all possible combinations of the explanatory variables and ranked them according to AICc (Akaike’s information criterion corrected for small sample size). We then applied a model-averaging procedure to calculate weighted average parameter coefficients across all models with ΔAICc < 2 (Symonds and Moussalli, 2011).
Canonical correspondence analysis (CCA) was performed to examine the relationships between functional composition and environmental factors. Environmental factors, including pH, EC, soil organic matter, soil water content, bulk density, plant richness, and grass coverage, were standardized prior to analysis. Model significance was tested using Monte Carlo permutation (9999 permutations).
All statistical analyses were completed using R 4.2.2 (R Core Team, 2022).
3 Results
3.1 Soil and plant properties
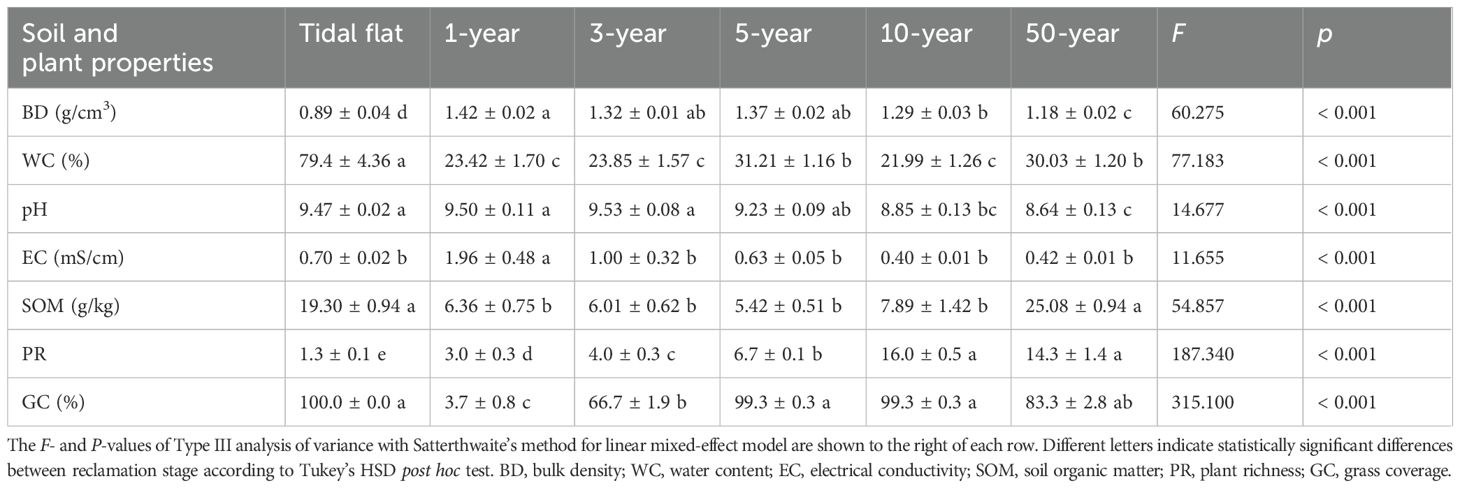
All measured soil and plant properties exhibited significant differences between reclamation stages (Table 1). Soil water content was highest in the tidal flat stage, intermediate in the 5-year and 50-year stages, and lower in the 1-year, 3-year, and 10-year stages. Soil bulk density was lowest in the tidal flat stage, highest in the 1-year stage, and gradually decreased with increasing age. Soil pH was alkaline and remained at similar levels in the tidal flat, 1-year, and 3-year stages and gradually decreased with increasing reclamation age. Soil EC was significantly higher in the 1-year stage than in the other ages. Soil organic matter was significantly lower following reclamation of the tidal flat, declining by 67.0% (19.3 g/kg to 6.4 g/kg) and remaining at similar low levels from the 1-year to the 10-year stage. It was significantly higher (25.1 g/kg) in the 50-year stage. Plant richness increased significantly along the chronosequence. Following tidal flat reclamation, grass coverage initially decreased but increased with increasing reclamation age.
3.2 Taxonomic diversity and composition
A total of 58 genera of soil nematodes were collected in this study (Figure 2A). Eight genera were observed only in the tidal flat stage whereas six were identified only in the 50-year stage (Figure 2B). The tidal flat was primarily dominated by marine nematodes such as Daptonema (23.7%) and Sphaerolaimus (15.8%). In contrast, the 1-year to 10-year stages were dominated by Acrobeloides (9.6–41.4%), Mesodorylaimus (1.0–26.6%), Monhystera (0.9–15.6%), and Panagrolaimus (0.3–18.1%). In the 50-year stage, Helicotylenchus (19.9%) became the dominant taxon (Supplementary Table S2). The taxonomic composition of soil nematode communities was significantly different between reclamation stages (Figure 2C; PERMANOVA, R² = 0.283, P < 0.001). Pairwise comparisons revealed significant differences between stages, except between the 1-year and 3-year stages (Supplementary Table S3; PERMANOVA, R² = 0.092, P-adj = 0.060) and the 3-year and 5-year stages (Supplementary Supplementary Table S3; PERMANOVA, R² = 0.091, P-adj = 0.070).
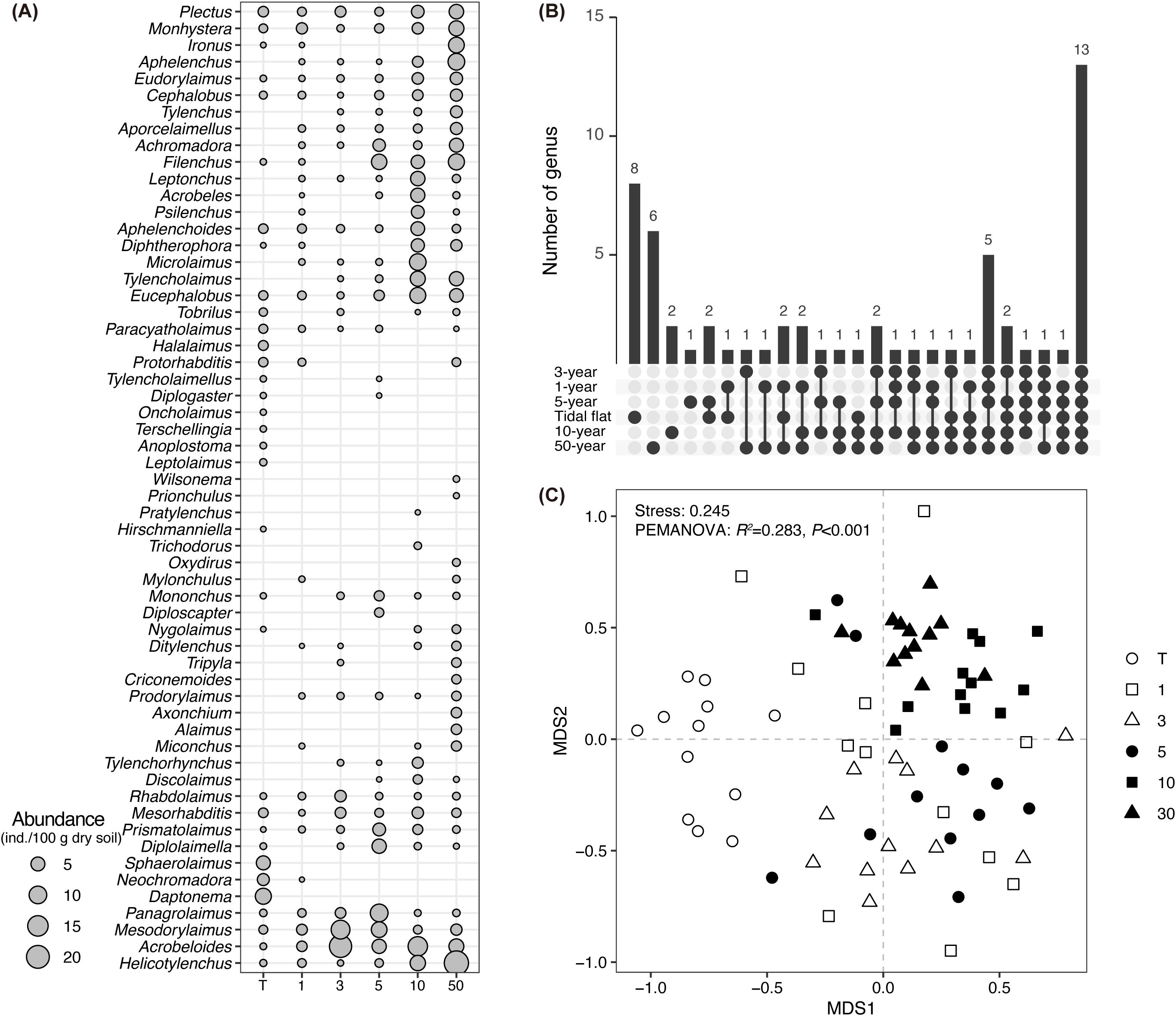
Figure 2. (A) Abundance of each soil nematode genus in different reclamation stages at Hengsha Island. Nematode genera are ordered based on hierarchical clustering of abundance patterns; (B) UpSetR plot showing the number of unique and shared genera across reclamation stages; (C) Nonmetric multidimensional scaling (NMDS) plot of taxonomic composition of soil nematode communities in different reclamation stages. The results of PERMANOVA are shown.
The abundance and taxonomic richness of soil nematode communities were both significantly affected by reclamation age (Figures 3A, B). The total abundance of soil nematodes declined from 32.1 to 15.9 individuals 100 g dry soil-1 following tidal flat reclamation but gradually increased with reclamation age to reach the highest value (116.9 individuals 100 g dry soil-1) in the 50-year stage (Figure 3A). Taxonomic richness exhibited a similar trend across reclamation stages (Figure 3B).
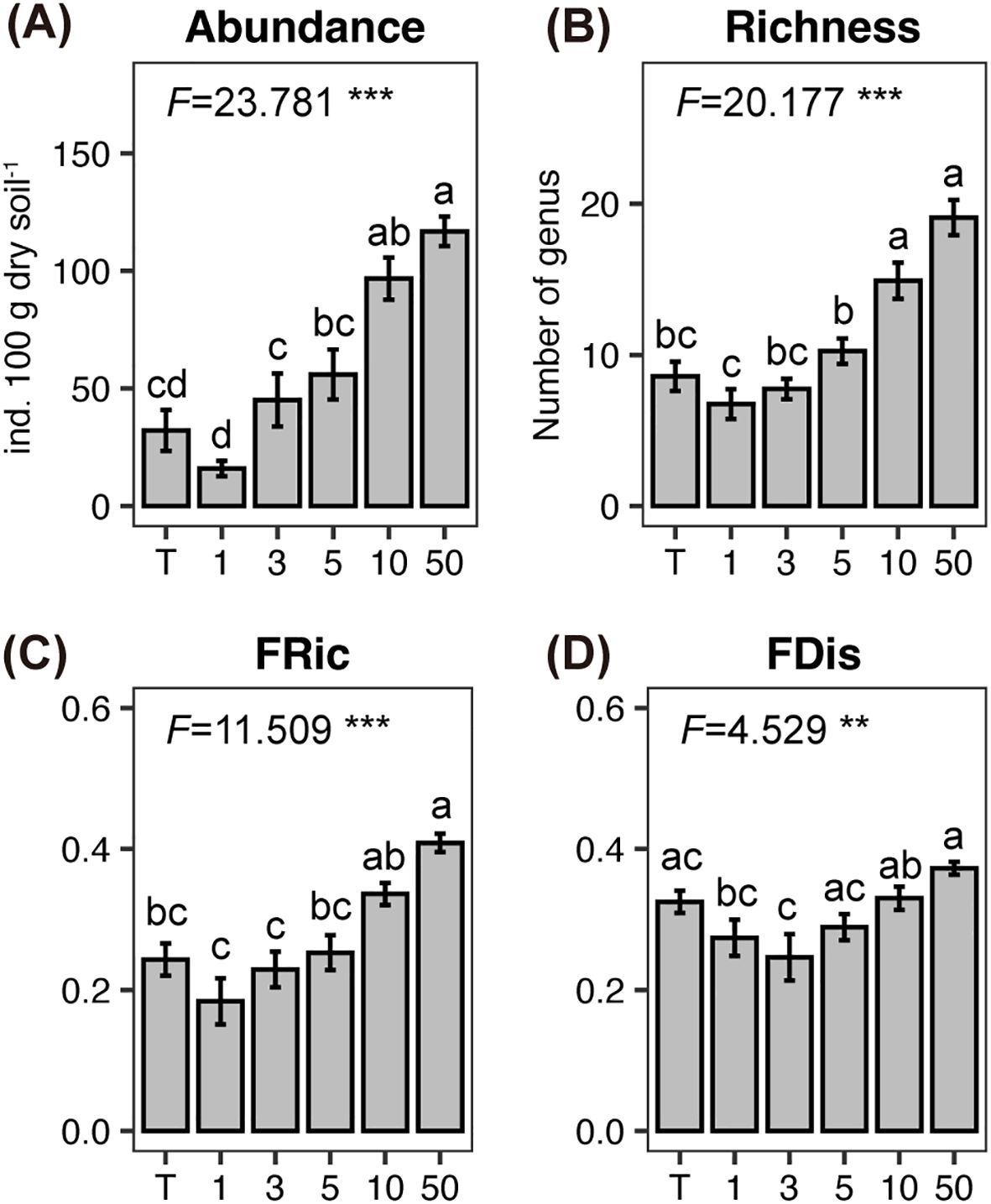
Figure 3. Mean (± SE) abundance (A), richness (B), functional richness (C) and functional dispersion (D) of soil nematode communities in different reclamation stages at Hengsha Island. F-values and significance levels from Type III analysis of variance using Satterthwaite’s method for linear mixed-effect models are presented: ***P < 0.001, **P <0.01. Different letters indicate statistically significant differences between reclamation stages as assessed by Tukey’s HSD post hoc test.
3.3 Functional diversity and composition
Both functional richness and dispersion decreased from the tidal flat to the 1-year stage before subsequent significant increases with increasing reclamation age (Figures 3C, D). The lowest functional richness was observed in the 1-year stage, whereas the lowest functional dispersion was recorded in the 3-year stage (Figures 3C, D).
Regarding functional composition (Figure 4), body mass was not significantly different between reclamation stages. The proportion of nematodes possessing cephalic setae and large amphids was significantly higher in the tidal flat stage compared to other reclamation ages. Tail shape shifted from clavate/conicocylindrical in the tidal flat stage to conical, then long, and finally rounded with increasing age.

Figure 4. Mean (± SE) values of community weighted means (CWM) of functional traits of soil nematode communities in different reclamation stages at Hengsha Island. F-values and significance levels from Type III analysis of variance using Satterthwaite’s method for linear mixed-effect models are presented: ***P < 0.001, **P < 0.01, *P < 0.05, ns: not significant. Different letters indicate statistically significant differences between reclamation stages as assessed by Tukey’s HSD post hoc test.
For life history strategy, the proportion of nematodes classified as enrichment opportunists (c-p 1) was significantly higher in the 5-year stage than in the 10-year or 50-year stages, whereas the proportion of persisters (c-p 3-5) was significantly higher in the 50-year stage than in the 5-year stage. The proportion of nematodes categorized as general opportunists (c-p 2) did not vary significantly with reclamation stage.
Feeding habit also varied along the chronosequence. The proportion of bacterivores was lower in later stages (10-year and 50-year), whereas the proportion of nematodes classified as fungivores or herbivores was higher. Predators were more abundant in the tidal flat stage than in other stages. The relative abundance of omnivores did not vary significantly with reclamation stage.
3.4 Environmental drivers of nematode community characteristics
The best-fitting models suggested that the abundance of soil nematodes increased significantly with increasing soil organic matter, plant richness, and grass coverage (Table 2). Additionally, taxonomic richness, functional richness, and functional dispersion were significantly and positively correlated with plant richness (Table 2).
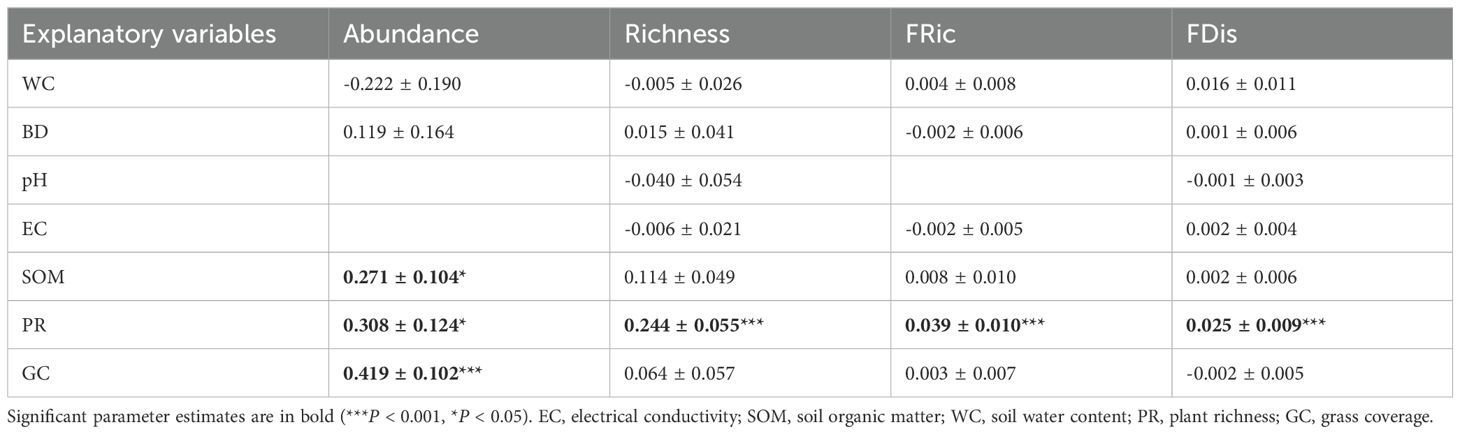
Table 2. Parameter estimates (± SE) of the best models resulting from the model averaging across all candidate models with ΔAICc < 2, relating the abundance, richness, functional richness (FRic) and functional dispersion (FDis) of soil nematode communities to environmental factors.
Functional composition was significantly influenced by soil and plant properties (Figure 5; Permutation, F = 3.832, P < 0.001). The first two axes of the CCA explained 24.8% of total variance. Algivores, predators, and nematodes with large amphids, cephalic setae, and clavate/conicocylindrical tails were positively correlated with soil water content. Bacterivores and enrichment opportunists (cp 1) with conical tails exhibited tolerance to high pH, EC, and bulk density. Herbivores, fungivores, and persisters (cp 3-5) with long and rounded tails were more abundant in habitats with high plant richness, grass coverage, and soil organic matter.
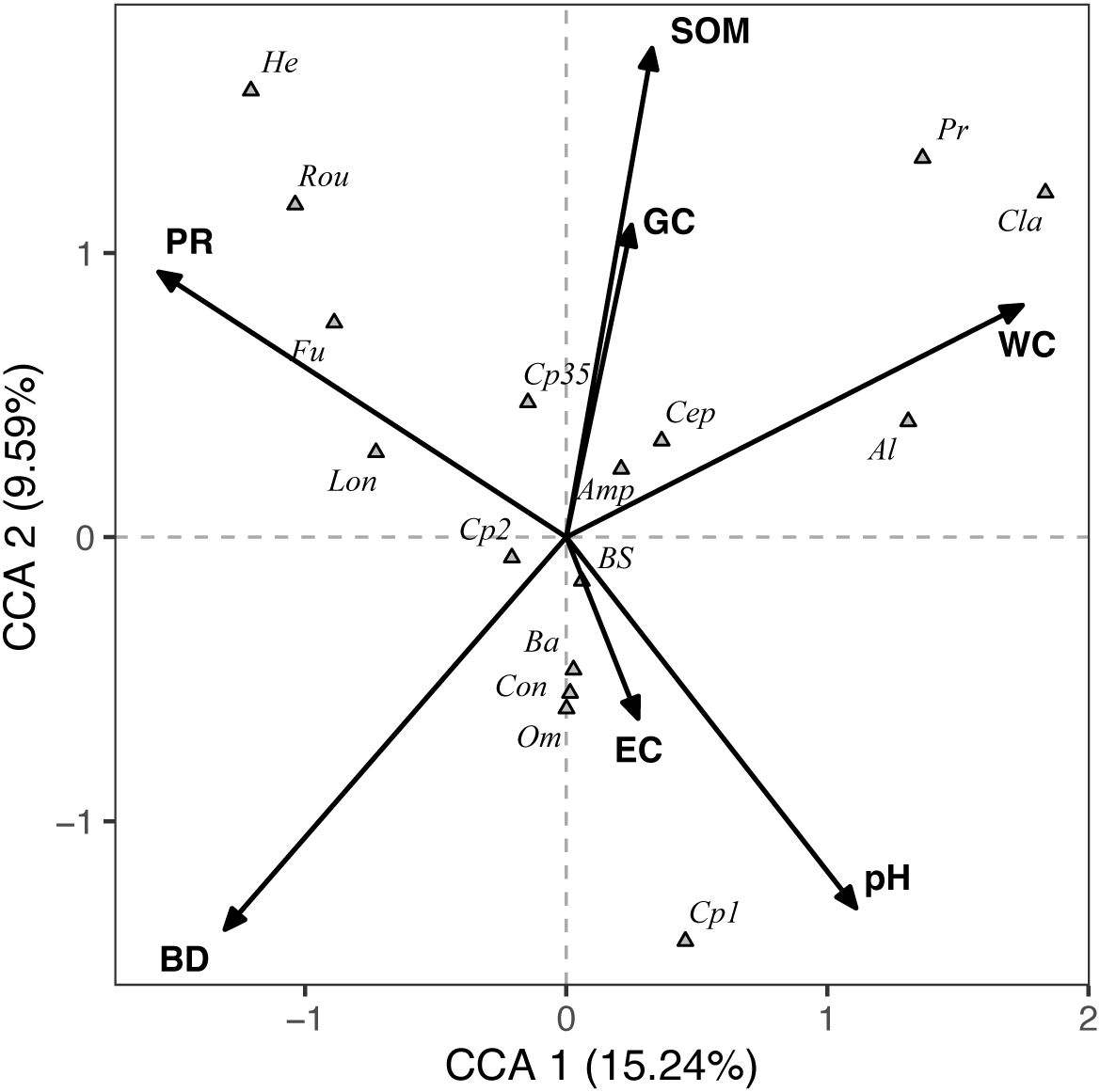
Figure 5. Canonical correspondence analysis (CCA) plot demonstrating the relationship between the functional composition of soil nematode communities and environmental factors across different reclamation stages at Hengsha Island. EC, electrical conductivity; SOM, soil organic matter; WC, soil water content; PR, plant richness; GC, grass coverage. BS, body size; Cep, cephalic setae present; Amp, large amphid; Cla, clavate/conicocylindrical tail; Rou, rounded tail; Con:, conical tail; Lon, long tail; Al, algivore; Ba, bacterivore; Fu, fungivore; He, herbivore; Pr, predator; Om, omnivore.
4 Discussion
4.1 Taxonomic shifts in soil nematode communities
Our results demonstrate that nematode richness and abundance both decreased significantly as the tidal flat was converted to dry land, which was accompanied by the loss of marine species. This finding is consistent with previous work by Wu et al. (2005), which found that dike construction eliminated aquatic nematodes. Tidal flat reclamation often causes dramatic changes in environmental conditions, including salinization, loss of soil moisture and fertility, and the degradation of vegetation communities. The lowest levels of soil organic matter and water content, along with the highest measurements of EC, were observed in newly reclaimed land in the 1-year stage and contributed to nematode abundance and richness in this area, which was lower than in all other reclamation stages. We found that nematodes were able to rapidly colonize reclaimed land, despite harsh conditions, as evidenced by the presence of 30 genera in the 1-year stage. This resilience can likely be attributed to their excellent capacity for passive dispersal and their ability to endure extreme conditions through cryptobiosis (Crowe and Cooper, 1971).
Following the initial reclamation stage, nematode abundance and richness increased with increasing age, similar to the successional patterns commonly observed in other soil microorganisms (Wang et al., 2020) and macrofauna (Ge et al., 2019). LMM analysis revealed that the increase in nematode diversity was primarily associated with increasing resource availability (soil organic matter, grass coverage, and plant richness) along the chronosequence. Soil fertility and vegetation are often considered the main controls on soil faunal community characteristics at the local scale (Bokhorst et al., 2017; Laliberte et al., 2017). Higher soil organic matter is typically an indicator of greater resource inputs and can sustain higher soil biodiversity. Plants serve as food sources for soil fauna and also offer diverse habitats for soil organisms (Wardle et al., 2004b). A global meta-analysis of long-term soil chronosequence studies also identified vegetation coverage as a key factor driving belowground biodiversity in low-productivity ecosystems (Delgado-Baquerizo et al., 2019). Here, vegetation types changed because of both natural succession and human activity, transitioning from bare land and communities dominated by the pioneer halophytic plant T. chinensis community to grasslands and finally secondary forests. This variation in vegetation type offers distinct microhabitats and food resources, thereby influencing soil faunal diversity and community composition (Yang et al., 2021).
The chronosequence examined here spanned restoration ages up to approximately 50 years, with the ecosystem characterized by nutrient accumulation and biodiversity recovery across stages. However, long-term chronosequence studies in other ecosystems have illuminated the potential for degradation in later stages (Bokhorst et al., 2017; Lei et al., 2015; Wardle et al., 2004b). For instance, in a 120-year-old chronosequence in the Hailuogou Glacier forefield in China, the nematode community exhibited retrogressive characteristics, including reduced phosphorus bioavailability and significant declines in nematode density (Lei et al., 2015). As of yet, however, such degradation has not been reported in reclaimed coastal areas.
4.2 Functional shifts in soil nematode communities
Our study revealed that nematode functional diversity decreased from the tidal flat stage to the 1-year stage, but we also measured subsequent significant increases with increasing reclamation age. This change in functional diversity may be due to environmental filtering or biotic interactions (Mouillot et al., 2013). In early reclamation stages, resource limitations (low plant diversity and soil organic matter), environmental stresses (high bulk density, pH, and EC), and dispersal limitation selected for species with particular traits and constrained functional diversity, whereas higher plant diversity and soil quality in later stages provided more potential ecological niches and enabled the coexistence of species with diverse functional traits. Moreover, the more frequent biotic interactions in later stages driven by higher nematode abundance and diversity led to trait divergence to reduce niche overlap and competition between organisms (Kunstler et al., 2016), thereby increasing functional diversity.
We also found that the functional composition of nematode communities changed significantly along the chronosequence, highlighting the potential importance of trade-offs in functional traits and variation in ecological strategies at different stages reclamation (Roff and Fairbairn, 2007). The proportion of nematodes with cephalic setae and large amphids was significantly higher in the tidal flat stage than in other stages. Cephalic setae and amphids are considered essential sensory organs for aquatic species, but their functionality is reduced in terrestrial environments (Zullini and Semprucci, 2020). Consequently, these features are generally reduced or absent in soil-dwelling species (Zullini and Semprucci, 2020). CCA analysis also indicated that nematodes with cephalic setae and large amphids preferred higher soil water content. We did not detect significant differences in cephalic setae or amphid from the 1-year to the 50-year sites, suggesting that these traits are not highly plastic in response to changing environmental conditions in terrestrial habitats. Body size is a fundamental trait that is correlated with many physiological and ecological characteristics, such as metabolic rate, vagility, and trophic position (Bie et al., 2012; Brose et al., 2006; Brown et al., 2004). We expected that increasing resource availability and soil pore space with increasing reclamation age would support large-bodied nematodes in later stages. However, we did not observe significant changes in body size, potentially due to functional trait trade-offs. Nematodes may adapt via other functional traits, such as feeding habit or reproductive strategy, which could be more advantageous for resource acquisition than an increase in body size.
Thistle and Sherman (1985) suggested that nematode tail morphology could be important for locomotion and reproduction. Here, we observed a shift in tail shape along the chronosequence, from clavate/conicocylindrical to conical, then to long, and finally to rounded tails. As the habitat transitioned from waterlogged, unstable sediment to drier, more structured soils, nematodes adjusted their tail morphology to optimize locomotion, stability, and ecological interactions within the context of a changing environment. Clavate/conicocylindrical tail shape is often associated with aquatic or semi-aquatic environments, where nematodes need to maintain buoyancy and stability in waterlogged sediments (Franzo and Del Negro, 2019; Semprucci et al., 2022). Broader, cylindrical tails may help them navigate in unstable, fine-grained tidal sediments. Currently, there is limited evidence in the literature regarding the role of nematode morphology and its underlying drivers in terrestrial environments. We assume that early reclamation may require greater motility for dispersal and colonization, favoring long or tapered tails that facilitate more efficient movement through soils with varying structure (Ptatscheck and Traunspurger, 2020; Schratzberger et al., 2007). In later stages, as soil conditions stabilize and nematodes establish permanent niches, selection may favor shorter tails and more sessile lifestyles that optimize resource exploitation rather than dispersal, such as those seen in root-associated nematodes.
We observed pronounced responses of life-history strategy and feeding habit to reclamation stage. As reclamation progressed, the relative abundance of bacterivores and r-strategists declined, whereas that of fungivores, herbivores, and K-strategists increased. Nematodes classified as r-strategists are generally considered pioneer taxa during ecosystem succession due to their higher dispersal ability, fecundity, and resilience in harsh environments. Consistent with our findings, Darby et al. (2007) and Guerrieri et al. (2024) observed higher proportions of r-strategists in the early stages of soil crust and glacial forefield ecosystems. Enrichment opportunists (c-p 1) can respond quickly to increases in food resources, rapidly coming to dominate nematode communities. However, they tend to be replaced by higher groups in later stages of succession or recovery (de Goede et al., 1993). In this study, the proportion of enrichment opportunists peaked in the 5-year grasslands, which were characterized by rapid resource turnover, before being displaced by persisters.
Food resources play a critical role in determining the distribution of soil fauna (Ingimarsdóttir et al., 2014), with resource type modulating the trophic structure of soil faunal communities across reclamation stages. Ge et al. (2019) found that the abundance and richness of herbivorous and detritivorous soil macrofauna increased with reclamation age, while those of omnivorous and predatory taxa were unaffected. Significant changes in the trophic structure of soil faunal communities have also been observed in other soil chronosequences (Laliberte et al., 2017; Lei et al., 2015). Our study revealed that bacterivores dominated early reclamation stages but that their relative abundance decreased significantly in later stages as the abundance of fungivores and herbivores increased significantly. This change is indicative of a shift from bacterial to fungal and root channels during reclamation (Wardle et al., 2004b). No significant change was observed in the relative abundance of omnivorous nematodes, which may be attributed to the availability of alternative food sources for these nematodes (e.g., algae, protozoa) (Bilgrami et al., 1991; Hunt et al., 1987). Work by Laliberte et al. (2017) in a soil chronosequence also revealed more intense responses among nematodes at high trophic levels compared to responses among nematodes at low trophic levels. These shifts in the trophic structure of nematode communities may influence key soil ecosystem functions, including nutrient cycling efficiency, organic matter decomposition and overall soil food web stability (Laliberte et al., 2017; Wardle et al., 2004b).
5 Conclusion
This study provides novel insight into the taxonomic and functional shifts of soil nematode communities during coastal reclamation at Hengsha Island. Our findings demonstrated that both taxonomic and functional diversity declined following tidal flat reclamation but that they gradually recovered over time. Shifts in functional traits were associated with changes in soil and vegetation conditions, reflecting environmental filtering and successional processes. These findings contribute to deeper understanding of how soil biodiversity responds to land use change, particularly through the lens of functional traits. Incorporating functional traits into soil monitoring may improve assessments of ecosystem recovery and guide future restoration efforts. Future research should focus on the long-term persistence of nematode communities and their functional roles in nutrient cycling and soil ecosystem stability under ongoing environmental change.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
SP: Funding acquisition, Visualization, Formal analysis, Data curation, Methodology, Conceptualization, Writing – review & editing, Software, Investigation, Writing – original draft. JL: Project administration, Writing – review & editing, Resources. HW: Writing – review & editing, Investigation. YC: Supervision, Writing – review & editing, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Shanghai Municipal Commission of Science and Technology (17DZ1202800), Wenzhou Science and Technology Bureau (N2024004), Cangnan Industrial Research Institute of Modern Agriculture (2023CNYJY13) and Wenzhou Vocational College of Science and Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1606331/full#supplementary-material
References
Andriuzzi W. S., Franco A. L. C., Ankrom K. E., Cui S., de Tomasel C. M., Guan P., et al. (2020). Body size structure of soil fauna along geographic and temporal gradients of precipitation in grasslands. Soil Biol. Biochem. 140, 107638. doi: 10.1016/j.soilbio.2019.107638
Bardgett R. D. and van der Putten W. H. (2014). Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. doi: 10.1038/nature13855
Bie T., Meester L., Brendonck L., Martens K., Goddeeris B., Ercken D., et al. (2012). Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecol. Lett. 15, 740–747. doi: 10.1111/j.1461-0248.2012.01794.x
Bilgrami A. L., Khan Z., and Jairajpuri M. S. (1991). Observations on the predation ability of Aporcelaimellus Nivalis (Altherr 1952) heyns 1966 (Nematoda: dorylaimida). Nematologica 37, 333–342.
Bokhorst S., Kardol P., Bellingham P. J., Kooyman R. M., Richardson S. J., Schmidt S., et al. (2017). Responses of communities of soil organisms and plants to soil aging at two contrasting long-term chronosequences. Soil Biol. Biochem. 106, 69–79. doi: 10.1016/j.soilbio.2016.12.014
Bongers T. (1990). The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83, 14–19. doi: 10.1007/BF00324627
Bongers T. and Bongers M. (1998). Functional diversity of nematodes. Appl. Soil Ecol. 10, 239–251. doi: 10.1016/S0929-1393(98)00123-1
Bongers T. and Ferris H. (1999). Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 14, 224. doi: 10.1016/S0169-5347(98)01583-3
Brose U., Jonsson T., Berlow E. L., Warren P., Banasek-Richter C., Bersier L.-F., et al. (2006). Consumer resource body-size relationships in natural food webs. Ecology 87, 2411–2417. doi: 10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2
Brousseau P.-M., Gravel D., and Handa I. T. (2018). On the development of a predictive functional trait approach for studying terrestrial arthropods. J. Anim. Ecol. 87, 1209–1220. doi: 10.1111/1365-2656.12834
Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., and West G. B. (2004). Toward a metabolic theory of ecology. Ecology 85, 1771–1789. doi: 10.1890/03-9000
Crowe J. H. and Cooper A. F. (1971). Cryptobiosis. Sci. Am. 225, 30–37. doi: 10.1038/scientificamerican1271-30
Cui X., Hu J., Wang J., Yang J., and Lin X. (2016). Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 98, 140–149. doi: 10.1016/j.apsoil.2015.10.008
Darby B. J., Neher D. A., and Belnap J. (2007). Soil nematode communities are ecologically more mature beneath late- than early-successional stage biological soil crusts. Appl. Soil Ecol. 35, 203–212. doi: 10.1016/j.apsoil.2006.04.006
De Deyn G. B., Raaijmakers C. E., Zoomer H. R., Berg M. P., de Ruiter P. C., Verhoef H. A., et al. (2003). Soil invertebrate fauna enhances grassland succession and diversity. Nature 422, 711–713. doi: 10.1038/nature01548
De Deyn G. B. and van der Putten W. H. (2005). Linking aboveground and belowground diversity. Trends Ecol. Evol. 20, 625–633. doi: 10.1016/j.tree.2005.08.009
de Goede R. G. M, G., Bongers T., and Ettema C. H. (1993). Graphical presentation and interpretation of nematode community structure: c-p triangles. Med. Fac. Landbouww. Univ. Gent. 58, 743–750.
Delgado-Baquerizo M., Bardgett R. D., Vitousek P. M., Maestre F. T., Williams M. A., Eldridge D. J., et al. (2019). Changes in belowground biodiversity during ecosystem development. Proc. Natl. Acad. Sci. U. S. A 116, 6891. doi: 10.1073/pnas.1818400116
Edwards K. F. and Stachowicz J. J. (2010). Multivariate trade-offs, succession, and phenological differentiation in a guild of colonial invertebrates. Ecology 91, 3146–3152. doi: 10.1890/10-0440.1
Eisenhauer N., Bender S. F., Calderón-Sanou I., De Vries F. T., Lembrechts J. J., Thuiller W., et al. (2022). Frontiers in soil ecology—Insights from the World Biodiversity Forum 2022. J. Sustain. Agric. Environ. 1, 245–261. doi: 10.1002/sae2.12031
Ellers J., Berg M. P., Dias A. T. C., Fontana S., Ooms A., and Moretti M. (2018). Diversity in form and function: Vertical distribution of soil fauna mediates multidimensional trait variation. J. Anim. Ecol. 87, 933–944. doi: 10.1111/1365-2656.12838
Feng Y., Sun T., Zhu M. S., Qi M., Yang W., and Shao D. D. (2018). Salt marsh vegetation distribution patterns along groundwater table and salinity gradients in yellow river estuary under the influence of land reclamation. Ecol. Indic. 92, 82–90. doi: 10.1016/j.ecolind.2017.09.027
Ficetola G. F., Marta S., Guerrieri A., Gobbi M., Ambrosini R., Fontaneto D., et al. (2021). Dynamics of ecological communities following current retreat of glaciers. Annu. Rev. Ecol. Evol. Syst. 52, 405–426. doi: 10.1146/annurev-ecolsys-010521-040017
Franzo A. and Del Negro P. (2019). Functional diversity of free-living nematodes in river lagoons: can biological traits analysis (BTA) integrate traditional taxonomic-based approaches as a monitoring tool? Mar. Environ. Res. 145, 164–176. doi: 10.1016/j.marenvres.2019.02.015
Fu Q. L., Ding N. F., Liu C., Lin Y. C., and Guo B. (2014). Soil development under different cropping systems in a reclaimed coastal soil chronosequence. Geoderma 230, 50–57. doi: 10.1016/j.geoderma.2014.03.026
Garnier E., Cortez J., Billès G., Navas M.-L., Roumet C., Debussche M., et al. (2004). Plant functional markers capture ecosystem properties during secondary succession. Ecology 85, 2630–2637. doi: 10.1890/03-0799
Ge B., Cui J., Zhang D., Liu Q., Jiang S., Tang B., et al. (2019). Succession of soil macro-faunal biodiversity in forests converted from croplands after long-term coastal reclamation. Soil Tillage Res. 186, 165–171. doi: 10.1016/j.still.2018.10.015
Geisen S., Wall D. H., and van der Putten W. H. (2019). Challenges and opportunities for soil biodiversity in the Anthropocene. Curr. Biol. 29, R1036–R1044. doi: 10.1016/j.cub.2019.08.007
Gobbi M., Ballarin F., Brambilla M., Compostella C., Isaia M., Losapio G., et al. (2017). Life in harsh environments: carabid and spider trait types and functional diversity on a debris-covered glacier and along its foreland. Ecol. Entomol. 42, 838–848. doi: 10.1111/een.12456
Guerrieri A., Cantera I., Marta S., Bonin A., Carteron A., Ambrosini R., et al. (2024). Local climate modulates the development of soil nematode communities after glacier retreat. Global Change Biol. 30, e17057. doi: 10.1111/gcb.17057
Háněl L. (2010). An outline of soil nematode succession on abandoned fields in South Bohemia. Appl. Soil Ecol. 46, 355–371. doi: 10.1016/j.apsoil.2010.10.005
Hunt H. W., Coleman D. C., Ingham E. R., Ingham R. E., and Morley C. R. (1987). The detrital food web in a shortgrass prairie. Biol. Fertility Soils 3, 57–68. doi: 10.1007/BF00260580
Ingimarsdóttir M., Michelsen A., Ripa J., and Hedlund K. (2014). Food sources of early colonising arthropods: The importance of allochthonous input. Pedobiologia 57, 21–26. doi: 10.1016/j.pedobi.2013.09.004
Kunstler G., Falster D., Coomes D. A., Hui F., Kooyman R. M., Laughlin D. C., et al. (2016). Plant functional traits have globally consistent effects on competition. Nature 529, 204–207. doi: 10.1038/nature16476
Laliberte E., Kardol P., Didham R. K., Teste F. P., Turner B. L., and Wardle D. A. (2017). Soil fertility shapes belowground food webs across a regional climate gradient. Ecol. Lett. 20, 1273–1284. doi: 10.1111/ele.12823
Laliberté E. and Legendre P. (2010). A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. doi: 10.1890/08-2244.1
Lei Y., Zhou J., Xiao H., Duan B., Wu Y., Korpelainen H., et al. (2015). Soil nematode assemblages as bioindicators of primary succession along a 120-year-old chronosequence on the Hailuogou Glacier forefield, SW China. Soil Biol. Biochem. 88, 362–371. doi: 10.1016/j.soilbio.2015.06.013
Li J., Pu L., Zhu M., Zhang J., Li P., Dai X., et al. (2014). Evolution of soil properties following reclamation in coastal areas: A review. Geoderma 226, 130–139. doi: 10.1016/j.geoderma.2014.02.003
Li X. and Zhang C. (2021). Effect of natural and artificial afforestation reclamation on soil properties and vegetation in coastal saline silt soils. Catena 198, 105066. doi: 10.1016/j.catena.2020.105066
Liu T., Guo R., Ran W., Whalen J. K., and Li H. (2015). Body size is a sensitive trait-based indicator of soil nematode community response to fertilization in rice and wheat agroecosystems. Soil Biol. Biochem. 88, 275–281. doi: 10.1016/j.soilbio.2015.05.027
Liu X., Liu Q., Zhang Y., Hua E., and Zhang Z. (2018). Effects of Yellow Sea Cold Water Mass on marine nematodes based on biological trait analysis. Mar. Environ. Res. 141, 167–185. doi: 10.1016/j.marenvres.2018.08.013
Lu Q., Liu T., Wang N., Dou Z., Wang K., and Zuo Y. (2020). A review of soil nematodes as biological indicators for the assessment of soil health. Front. Agric. Sci. Eng. 7, 275–281. doi: 10.15302/J-FASE-2020327
Ma T., Li X., Bai J., and Cui B. (2019). Habitat modification in relation to coastal reclamation and its impacts on waterbirds along China’s coast. Glob. Ecol. Conserv. 17, e00585. doi: 10.1016/j.gecco.2019.e00585
Maaß S., Caruso T., and Rillig M. C. (2015). Functional role of microarthropods in soil aggregation. Pedobiologia 58, 59–63. doi: 10.1016/j.pedobi.2015.03.001
Martínantón M., Negro V., Campo J. M. D., Lópezgutiérrez J. S., and Esteban M. D. (2016). Review of coastal land reclamation situation in the world. J. Coast. Res. 75, 667–671. doi: 10.2112/SI75-133.1
Mason N. W. H., De Bello F., Mouillot D., Pavoine S., and Dray S. (2013). A guide for using functional diversity indices to reveal changes in assembly processes along ecological gradients. J. Veg. Sci. 24, 794–806. doi: 10.1111/jvs.12013
Mason N. W. H., Mouillot D., Lee W. G., and Wilson J. B. (2005). Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111, 112–118. doi: 10.1111/j.0030-1299.2005.13886.x
McGill B., Enquist B., Weiher E., and Westoby M. (2006). Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. doi: 10.1016/j.tree.2006.02.002
Mills A. A. S. and Adl M. S. (2011). Changes in nematode abundances and body length in response to management intensive grazing in a low-input temperate pasture. Soil Biol. Biochem. 43, 150–158. doi: 10.1016/j.soilbio.2010.09.027
Min M. M. and Kim J. H. (2000). Plant succession and interaction between soil and plants after land reclamation on the west coast of Korea. J. Plant Biol. 43, 41–47. doi: 10.1007/BF03031035
Mouillot D., Graham N. A., Villeger S., Mason N. W., and Bellwood D. R. (2013). A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. doi: 10.1016/j.tree.2012.10.004
Mulder C. and Maas R. (2017). Unifying the functional diversity in natural and cultivated soils using the overall body-mass distribution of nematodes. BMC Ecol. 17, 1–14. doi: 10.1186/s12898-017-0145-9
Osler G. H. R. and Martin S. (2007). Toward a complete soil C and N cycle: incorporating the soil fauna. Ecology 88, 1611–1621. doi: 10.1890/06-1357.1
Pan F., Li N., Zou W., Han X., and McLaughlin N. B. (2016). Soil nematode community structure and metabolic footprint in the early pedogenesis of a Mollisol. Eur. J. Soil Biol. 77, 17–25. doi: 10.1016/j.ejsobi.2016.09.004
Pan S., Liang J., Wu H., Wei L., and Cai Y. (2022). Effect of urban greening and afforestation on soil microarthropod communities in coastal reclaimed land: Insights from functional traits. Appl. Soil Ecol. 173, 104391. doi: 10.1016/j.apsoil.2022.104391
Pey B., Nahmani J., Auclerc A., Capowiez Y., Cluzeau D., Cortet J., et al. (2014). Current use of and future needs for soil invertebrate functional traits in community ecology. Basic Appl. Ecol. 15, 194–206. doi: 10.1016/j.baae.2014.03.007
Ptatscheck C. and Traunspurger W. (2020). The ability to get everywhere: dispersal modes of free-living, aquatic nematodes. Hydrobiologia 847, 3519–3547. doi: 10.1007/s10750-020-04373-0
R Core Team (2022). R: A language and environment for statistical computing. Available online at: http://www.R-project.org (Accessed December 25, 2024).
Roff D. A. and Fairbairn D. J. (2007). The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447. doi: 10.1111/j.1420-9101.2006.01255.x
Schratzberger M., Warr K., and Rogers S. I. (2007). Functional diversity of nematode communities in the southwestern North Sea. Mar. Environ. Res. 63, 368–389. doi: 10.1016/j.marenvres.2006.10.006
Sechi V., De Goede R. G. M., Rutgers M., Brussaard L., and Mulder C. (2018). Functional diversity in nematode communities across terrestrial ecosystems. Basic Appl. Ecol. 30, 76–86. doi: 10.1016/j.baae.2018.05.004
Semprucci F., Cesaroni L., Guidi L., and Balsamo M. (2018). Do the morphological and functional traits of free-living marine nematodes mirror taxonomical diversity? Mar. Environ. Res. 135, 114–122. doi: 10.1016/j.marenvres.2018.02.001
Semprucci F., Grassi E., and Balsamo M. (2022). Simple is the best: an alternative method for the analysis of free-living nematode assemblage structure. Water 14, 1114. doi: 10.3390/w14071114
Sengupta D., Chen R., Meadows M. E., and Banerjee A. (2020). Gaining or losing ground? Tracking Asia’s hunger for ‘new’ coastal land in the era of sea level rise. Sci. Total Environ. 732, 139290. doi: 10.1016/j.scitotenv.2020.139290
Sroczyńska K., Chainho P., Vieira S., and Adão H. (2021). What makes a better indicator? Taxonomic vs functional response of nematodes to estuarine gradient. Ecol. Indic. 121, 107113. doi: 10.1016/j.ecolind.2020.107113
Sun Y., Li X., He Y., Jia Y., Ma Z., Guo W., et al. (2011). Impact factors on distribution and characteristics of natural plant community in reclamation zones of Changjiang River estuary. Chin. Geogr. Sci. 22, 154–166. doi: 10.1007/s11769-011-0475-z
Symonds M. R. E. and Moussalli A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 65, 13–21. doi: 10.1007/s00265-010-1037-6
Thistle D., Lambs J., and Sherman K. (1995). Nematode tail-shape groups respond to environmental differences in the deep sea. Vie Milieu/Life Environ. 45, 107–115.
Thistle D. and Sherman K. M. (1985). The nematode fauna of a deep-sea site exposed to strong near-bottom currents. Deep Sea Res. Part A. Oceanographic Res. Papers 32, 1077–1088. doi: 10.1016/0198-0149(85)90063-9
Tian B., Wu W., Yang Z., and Zhou Y. (2016). Drivers, trends, and potential impacts of long-term coastal reclamation in China from 1985 to 2010. Estuar. Coast. Shelf Sci. 170, 83–90. doi: 10.1016/j.ecss.2016.01.006
van den Hoogen J., Geisen S., Routh D., Ferris H., Traunspurger W., Wardle D. A., et al. (2019). Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198. doi: 10.1038/s41586-019-1418-6
Violle C., Navas M.-L., Vile D., Kazakou E., Fortunel C., Hummel I., et al. (2007). Let the concept of trait be functional! Oikos 116, 882–892. doi: 10.1111/j.0030-1299.2007.15559.x
Walker L. R., Wardle D. A., Bardgett R. D., and Clarkson B. D. (2010). The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 98, 725–736. doi: 10.1111/j.1365-2745.2010.01664.x
Wang W., Liu H., Li Y., Su J. J. O., and Management C. (2014). Development and management of land reclamation in China. Ocean Coast. Manage. 102, 415–425. doi: 10.1016/j.ocecoaman.2014.03.009
Wang X., Yang J., Xie X., Chen X., Pu L., and Zhang X. (2020). Soil microbial succession with soil development since costal reclamation. Catena 187, 104393. doi: 10.1016/j.catena.2019.104393
Wardle D. A., Bardgett R. D., Klironomos J. N., Setala H., van der Putten W. H., and Wall D. H. (2004a). Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633. doi: 10.1126/science.1094875
Wardle D. A., Walker L. R., and Bardgett R. D. (2004b). Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305, 509–513. doi: 10.1126/science.1098778
Wu J. H., Fu C. Z., Chen S. S., and Chen J. K. (2002). Soil faunal response to land use: effect of estuarine tideland reclamation on nematode communities. Appl. Soil Ecol. 21, 131–147. doi: 10.1016/S0929-1393(02)00065-3
Wu J., Fu C., Lu F., and Chen J. (2005). Changes in free-living nematode community structure in relation to progressive land reclamation at an intertidal marsh. Appl. Soil Ecol. 29, 47–58. doi: 10.1016/j.apsoil.2004.09.003
Xing W., Cheng X., Xiong J., Yuan H., and Yu M. (2020). Variations in soil biological properties in poplar plantations along coastal reclamation stages. Appl. Soil Ecol. 154, 103649. doi: 10.1016/j.apsoil.2020.103649
Xue J., Yang J., Wang Q., Aronson R. B., and Wu H. (2019). Community structure of benthic macroinvertebrates in reclaimed and natural tidal flats of the Yangtze River estuary. Aquacult. Fish. 4, 205–213. doi: 10.1016/j.aaf.2019.04.001
Yang X., Shao M., Li T., Gan M., and Chen M. (2021). Community characteristics and distribution patterns of soil fauna after vegetation restoration in the northern Loess Plateau. Ecol. Indic. 122, 107236. doi: 10.1016/j.ecolind.2020.107236
Yeates G. W., Bongers T., De Goede R. G., Freckman D. W., and Georgieva S. S. (1993). Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 25, 315–331.
Young H. S., McCauley D. J., Galetti M., and Dirzo R. (2016). Patterns, causes, and consequences of Anthropocene defaunation. Annu. Rev. Ecol. Evol. Syst. 47, 333–358. doi: 10.1146/annurev-ecolsys-112414-054142
Zhang A., Chen S., Chen J., Cui H., Jiang X., Xiao S., et al. (2023). Shrub and precipitation interactions shape functional diversity of nematode communities on the Qinghai–Tibet Plateau. Global Change Biol. 29, 2746–2758. doi: 10.1111/gcb.16638
Zhang C., Wright I. J., Nielsen U. N., Geisen S., and Liu M. (2024). Linking nematodes and ecosystem function: a trait-based framework. Trends Ecol. Evol. 39, 644–653. doi: 10.1016/j.tree.2024.02.002
Zhang H., Yin A., Yang X., Wu P., Fan M., Wu J., et al. (2019). Changes in surface soil organic/inorganic carbon concentrations and their driving forces in reclaimed coastal tidal flats. Geoderma 352, 150–159. doi: 10.1016/j.geoderma.2019.06.003
Zhao J., Xun R., He X., Zhang W., Fu W., and Wang K. (2015). Size spectra of soil nematode assemblages under different land use types. Soil Biol. Biochem. 85, 130–136. doi: 10.1016/j.soilbio.2015.02.035
Keywords: coastal reclamation, soil nematode, functional diversity, functional composition, soil chronosequence
Citation: Pan S, Liang J, Wu H and Cai Y (2025) Taxonomic and functional shifts in soil nematode communities following estuarine island reclamation. Front. Ecol. Evol. 13:1606331. doi: 10.3389/fevo.2025.1606331
Received: 05 April 2025; Accepted: 09 May 2025;
Published: 02 June 2025.
Edited by:
Jingyang Li, Chongqing Three Gorges University, ChinaReviewed by:
Mingshan Xu, Zhejiang Institute of Hydraulics & Estuary, ChinaAnna Yang, Ningbo Forestry Development Center, China
Copyright © 2025 Pan, Liang, Wu and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongli Cai, eWxjYWkyMDIwQHNqdHUuZWR1LmNu
 Sufeng Pan1,2
Sufeng Pan1,2 Yongli Cai
Yongli Cai