- 1Zhejiang Institute of Hydraulics and Estuary (Zhejiang Institute of Marine Planning and Design), Hangzhou, China
- 2Zhejiang Provincial Key Laboratory of Estuary and Coast, Hangzhou, China
- 3Department of Geography and Spatial Information, Ningbo University, Ningbo, China
- 4Zhejiang Collaborative Innovation Center for Land and Marine Spatial Utilization and Governance Research, Ningbo, China
- 5Zhejiang Zhoushan Island Ecosystem Observation and Research Station, Ministry of Natural Resource, Zhoushan, China
- 6Institute of Digital Agriculture, Zhejiang Academy of Agricultural Sciences, Hangzhou, China
Globally, saltmarsh reclamation results in significant losses of coastal wetlands. However, the impacts on above- and below-ground biodiversity and the underlying mechanisms remain poorly understood. We hypothesized that saltmarsh reclamation differently affects plant and soil macrofaunal communities by regulating soil properties through elevation. To test this, we surveyed 36 plots in Sheyang County, eastern China and used t-tests, redundancy analysis, and structural equation modeling to examine differences and the direct/indirect effects of elevation and soil physico-chemical properties on plant and soil macrofaunal diversity. Results showed that plant species richness in reclaimed areas was significantly higher than that in saltmarshes, whereas the total and average biomass of soil macrofauna exhibited an inverse pattern. Plant species richness positively associated with elevation but negatively correlated with soil available phosphorus (AP) and electrical conductivity (EC). The total and average biomass of soil macrofauna positively correlated with soil ammonium nitrogen (AN), total nitrogen (TN), total potassium (TK), and inversely related to total phosphorus (TP), elevation, mud content (SMC). Elevation indirectly increased plant species richness via soil water content (SWC), total carbon (TC), AN, and nitrate nitrogen (NN), but decreased it through bulk density (BD). For soil macrofauna, elevation indirectly reduced total biomass via SWC, TC, and AN, while indirectly increasing it through available potassium (AK). These findings elucidate the mechanisms driving above- and below-ground biodiversity changes following saltmarsh reclamation, providing a comprehensive understanding of these ecological alterations.
1 Introduction
Coastal wetlands play crucial roles in preserving biodiversity, purifying water, offering flood defense, sequestering carbon, fostering tourism, and providing recreational opportunities (Barbier et al., 2008; Temmerman et al., 2013). Yet, land reclamation has emerged as a pivotal factor resulting in the global decline of coastal wetlands, precipitating habitat destruction, biodiversity loss, and ecosystem functions degradation (Gedan et al., 2009; Sun et al., 2015; Sengupta et al., 2023). Globally, approximately 78% of major coastal cities have reclaimed a cumulative of 2.53 × 105 ha of additional land during the 21st Century, with particular prominence in East Asia (Sengupta et al., 2023). China ranks among the countries with the largest coastal wetland reclaimed area in the world. Between 1950 and 2014, China witnessed the loss of 8.01 × 106 ha (58%) of its coastal wetlands, with reclamation and infrastructure construction accounting for 70–82% of the total disappearance (Sun et al., 2015). Despite numerous studies have investigated the alterations in biodiversity patterns resulting from coastal wetland reclamation (Yu et al., 2016; Wang et al., 2021; Ge et al., 2021b), the specific mechanisms driving changes in above- and below-ground biodiversity remain unclear.
Coastal saltmarshes are wetland ecosystems covered by salt-tolerant herbaceous or low-shrub vegetation at the land and sea interface (Silliman, 2014). Saltmarshes are among the most productive vegetation types within coastal wetlands (Loreau et al., 2001; Lotze et al., 2006; Bauer et al., 2013; Tilman et al., 2017). Although saltmarsh reclamation has delivered considerable social and economic benefits to coastal areas, it has simultaneously precipitated the degradation of saltmarsh ecosystems by disrupting hydrological conditions and soil properties (Sengupta et al., 2023). Consequently, biodiversity patterns have been compelled to adapt to these altered environments (Liu et al., 2016; Day et al., 2021). Typically, in coastal wetlands, soil moisture and salinity are the primary factors governing the directions and processes of saltmarsh evolution (Zhang et al., 2013a; Pan et al., 2018). When saltmarshes are converted into aquaculture ponds, the increased elevation of pond embankment, resulting in decreased seawater, soil moisture, pH, salinity, as well as increased soil bulk density and nutrient levels (Cui et al., 2016; Xie et al., 2017; Cao et al., 2018). Moreover, saltmarsh reclamation can lead to significant alterations in land use types and landscape dynamics within coastal zones, thereby exerting a substantial influence on the ecological balance and biodiversity in these regions (Goeldner, 1999; Wu et al., 2018). However, additional research is imperative to comprehensively understand the implications of the variations of elevation and soil properties on both above- and below-ground biodiversity.
Saltmarsh reclamation exerts a profound influence on the composition and diversity of plant communities by modifying the elevation and soil properties (Cui et al., 2016; Xie et al., 2017; Cao et al., 2018). Elevation serves as a critical environmental constraint within coastal wetlands. Higher elevations can reduce tidal currents, flooding frequency, and salinity levels (Silvestri et al., 2005), which in turn influences the spatial distribution of saltmarshes (Balke et al., 2016). Furthermore, alterations in elevation can lead to variations in soil physical-chemical properties, reflecting the effects of seawater leaching over a spatio-temporal sequence (Walker et al., 2010). After reclamation, due to the decreased seawater inundation, saltmarshes with few species experienced significant reduction. Consequently, the soil has transitioned towards drier conditions and improved in quality, facilitating the rapid expansion of xerophytic plant communities (Li et al., 2014; Yu et al., 2016). Moreover, certain plant species can establish themselves in these new habitats through seed dispersal (Visscher et al., 2023). Notably, weeds exhibit remarkable adaptability to their surroundings and are typically widely distributed (Marshall et al., 2003), thereby contributing to increased plant species richness. Research has shown that soil organic carbon, texture and nutrients mainly influence the occurrence and establishment of plants (Gaston et al., 2001; Korres et al., 2017). However, the specific mechanisms by which saltmarsh reclamation impacts plant communities through altering soil properties have not been extensively investigated.
In addition to plant communities, soil macrofauna, as a key component of underground biodiversity, are also significantly affected by land reclamation. Soil macrofauna is an integral component of coastal saltmarsh ecosystems, actively engaging in processes such as energy flow, decomposition, and nutrient cycling (Ricardo et al., 2012; Lv et al., 2019; Xue et al., 2019). Extensive research indicates that saltmarsh reclamation can significantly alter the composition and diversity of soil macrofaunal communities (Salgado et al., 2007; Ryu et al., 2011; Ricardo et al., 2012). Additionally, soil macrofaunal communities are known to exhibit strong correlations with various soil properties (Chao et al., 2012; Ge et al., 2021b), and their assemblages are closely linked to the specific combinations of abiotic factors present in tidal flats (Ryu et al., 2011). The elevation, serving as a comprehensive indicator of environmental conditions, tends to increase with saltmarsh reclamation, thereby substantially affecting the composition, diversity, and biomass of macrofaunal communities (Salgado et al., 2007). The collective influence of soil properties, including moisture, organic matter content, nutrient availability, and grain size, shapes the distribution and diversity of soil macrofaunal communities (Ayuke, 2010; Zhang et al., 2021a; Li et al., 2022). Particularly, soil nutrients are frequently affected by transportation through water flow and human activities associated with reclamation efforts (Bannert et al., 2011; Xie et al., 2017). Current research on coastal wetland reclamation has not thoroughly examined the specific case of saltmarshes being converted into aquaculture ponds. Furthermore, it has not investigated the subsequent alterations in both above- and below-ground biodiversity over several decades, nor has it explored the intricate mechanisms underlying these transformations. Additionally, there is a limited body of research addressing the direct and indirect effects of elevation and soil properties on both plant and soil macrofaunal communities following saltmarsh reclamation.
Jiangsu Province, encompassing a quarter of China’s total coastal wetlands (Xie et al., 2012), featured extensive saltmarshes over 300 km2 by the year 2000 (Sun et al., 2023). These saltmarshes were primarily characterized by invasive Spartina alterniflora (S. alterniflora), and native Phragmites australis (P. australis) and Suaeda spp (Ge et al., 2015; Tian et al., 2016). The cumulative loss of saltmarshes induced by reclamation from 2001 to 2020, reached 249.6 km2 (Sun et al., 2023). We formulate the hypothesis that the transformation of saltmarshes into aquaculture ponds will change land use types and landscape patterns, which, in turn, will alter elevation. This increased elevation can significantly mitigate the impacts of tidal flooding, thereby reducing soil leaching. These changes in environmental conditions directly influence soil physico-chemical properties, including fertility, acidity, alkalinity, and permeability. Consequently, these soil properties directly affect plant and soil macrofaunal communities, while elevation indirectly influences them through these soil properties. To validate this hypothesis, we conducted an investigation across 36 plots distributed in two regions within Sheyang coastal wetland, which encompass both saltmarshes and reclaimed areas (Figure 1). Prior to reclamation, these regions were predominantly saltmarsh ecosystems (Supplementary Figure 1). The landward sides were subsequently transformed into aquaculture ponds, experiencing similar levels of human disturbance. Our aim to address three key questions: 1) What are the impacts of saltmarsh reclamation on plant and soil macrofaunal communities? 2) How do soil physico-chemical properties change in response to saltmarsh reclamation? 3) How does elevation indirectly influence plant and soil macrofaunal communities by regulating soil physico-chemical properties post-saltmarsh reclamation? Our research aims to provide valuable theoretical insights for predicting variations in both plant and soil macrofauna biodiversity resulting from saltmarsh reclamation.
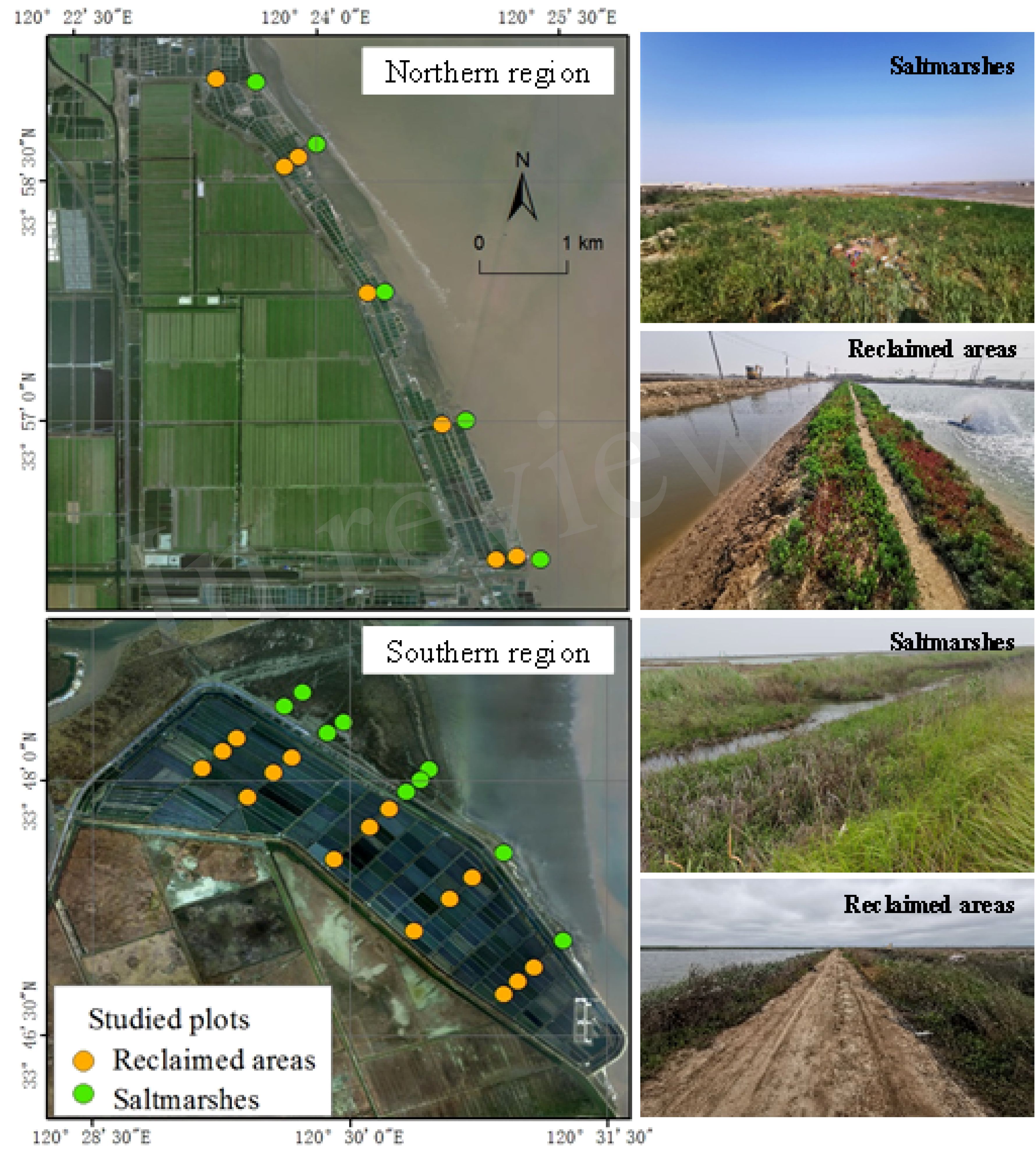
Figure 1. The geographical distribution of the 36 studied plots across the northern and southern regions of Sheyang, Jiangsu Province, eastern China, encompassing both saltmarshes and reclaimed areas.
2 Materials and methods
2.1 Study site
Sheyang County, located within the coordinates 33°31′12″N to 34°07′15″N and 119°55′48″E to 120°34′47″E, is situated in Jiangsu Province, eastern China (Figure 1). This area experiences a typical subtropical monsoon climate, characterized by cool summers and warm winters. The mean annual temperature is 14 °C, with a mean annual precipitation of 1,000 mm. Over recent years, sediment deposition in mudflat provides suitable space for saltmarshes plants to grow. The dominant saltmarsh species were P. australis and Suaeda spp. before the invasion of Spartina alterniflora, which has gradually replaced the native saltmarsh species. Reclamation activities have been conducted in the vicinity of the Sheyang estuary to create aquaculture ponds. The southern region of the estuary was reclaimed in 2005, resulting in an enclosed area of approximately 6.1 km2. The northern region was reclaimed in 2006, covering about 1.5 km2. The construction of pond embankment directly resulted in the isolation of saltmarshes and a reduction in mudflats. The schematic processes of the samplings, measurements, statistical analysis, and mechanism interpretation of our study were showed in Figure 2.
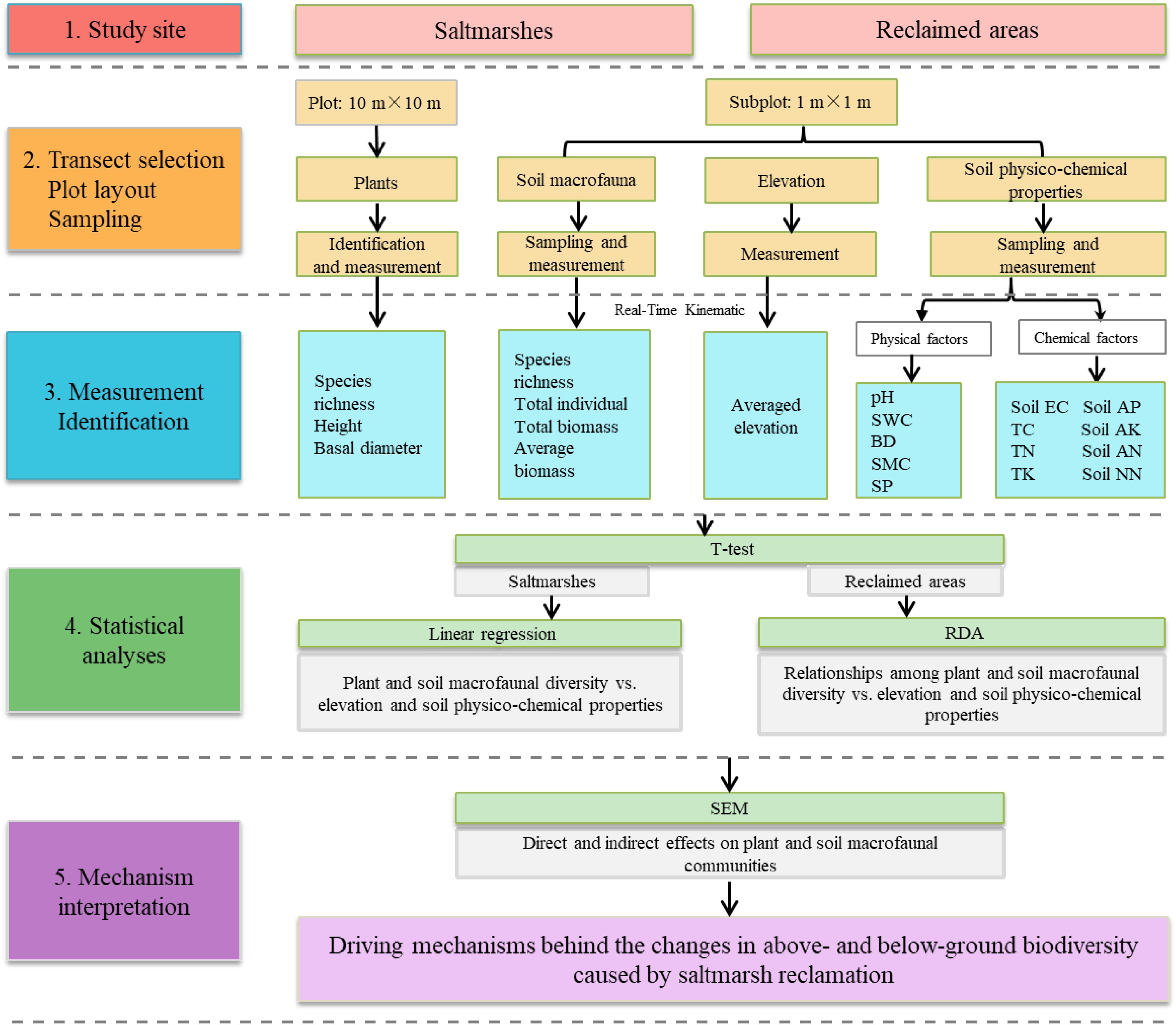
Figure 2. Schematic representation of the study sites, sampling methods, measurements, statistical analysis, and interpretation of mechanism.
2.2 Transect selection, plot layout, and sampling
From May to June 2023, we conducted a comprehensive survey and established 10 transects in the northern and southern regions of the Sheyang estuary (Figure 1). We selected these regions to expand the number of plots, given their comparable reclaimed history and the presence of saltmarshes and reclaimed areas. Our analysis of these measured variables revealed no significant differences between the northern and southern regions (Supplementary Table 5). In general, numerous studies have shown that random or stratified-random sampling methods are commonly used in biodiversity assessments (Singh and Mangat, 1996; Martino et al., 2018). In our study, we selected the transect sampling method because it captures subtle variations in both flora and fauna from saltmarshes to reclaimed areas, while also incorporating elements of randomness and representativeness (Ge et al., 2019, 2021b). These transects were strategically positioned to run perpendicular to the coastlines, with spacing intervals varying from 0.5 to 1.9 km. Within each transect, we randomly selected 3 to 6 replicate sites in both saltmarsh and reclaimed areas, ensuring a separation distance of 30 to 500 m between them. In total, 36 sampling sites were identified (Supplementary Table 1). At each site, we delineated plant plots of approximately 10 m × 10 m. The number of sampling plots in the southern and northern regions is indeed directly proportional to their respective areas (Supplementary Table 1; Supplementary Figure 3). We meticulously identified all existing plant species, documenting their names, dominance-aggregation, coverage, height, and basal diameter. Additionally, we calculated the dominance of plant species using the following equation (Supplementary Table 3): dominance = (relative height + relative coverage + relative frequency)/3.
To assess elevation, soil physico-chemical properties, and soil macrofauna, we randomly established three 1 m × 1 m subplots within each plot. Due to the minimal variations in slope, the consistent slope types in both saltmarshes and reclaimed areas, and the uniform environmental factors across these two regions, we did not consider slope and slope type in our study. We individually measured the elevation of each subplot using Real-Time Kinematic (RTK) technology and subsequently computed the average for each plot. Soil samples were taken using a metallic corer (20 cm depth and 7.5 cm in diameter) from the 0–20 cm soil layer (immediately below the litter layer) within each subplot and then combined. Soil sampling was collected on sunny days with no recorded rain in the preceding three days. For macrofauna collection, we randomly gathered quantitative soil samples measuring 25 cm × 25 cm × 30 cm near the center of each subplot and combined them. Soil macrofauna found on the sample surface were picked up using forceps within 48 hours and preserved in 75% alcohol (Wong et al., 2016). Soil macrofauna was collected using a 2 mm seawater sieve, preserved in 75% alcohol, and stored in plastic bottles. The samples were then transported to the lab for detailed identification, enumeration, and comprehensive analysis of the soil macrofaunal community’s abundance, species diversity, and biomass.
2.3 Soil macrofauna identification
The examination of soil macrofauna was conducted by Qingdao Guomao Environmental Testing Co., Ltd., a professional testing agency, following the implementation standards GB 17378-2007 and GB/T 12763-2007. They used a ZOOM645 stereomicroscope and a BM2000 biological microscope for the identification of soil macrofauna, followed by precise weighing. Before weighing, the specimens were placed on absorbent paper to eliminate surface moisture, and any tubes, hermit crab shells, body camouflage, or other attachments to macrofauna were carefully removed. Refining the identification of species to the lowest taxonomic unit, typically at the genus or species level. The weighing procedure was carried out using an electronic balance boasting a sensitivity of 0.0001 g (JA2003). For tube-dwelling macrofauna, the tubes were first removed before weighing. Hermit crabs were weighed without their shells, while mollusks were generally weighed with their shells, which were then dried. For large or numerous mollusks, the shells and flesh were weighed separately. Filter paper was used to remove surface moisture from the macrofauna. Additionally, separate measurements of flesh and ash weight were obtained for shellfish. Counting methods included considering only the head of easily breakable annelids or segmented organisms, and excluding dead shells for mollusca. In instances where large sample sizes were involved, a portion of the sample was weighted for conversion.
2.4 Soil physico-chemical properties measurement
For each sampling plot, we comprehensively measured 14 key soil physico-chemical properties, including soil pH (pH), soil water content (SWC), bulk density (BD), soil mud content (SMC), soil porosity (SP), soil electrical conductivity (soil EC), total carbon (TC), total nitrogen (TN), total phosphorus contents (TP), total potassium (TK), soil available phosphorus (soil AP), soil available potassium (soil AK), soil ammonium nitrogen (soil AN), and soil nitrate nitrogen (soil NN). These edaphic parameters are widely recognized as crucial factors influencing the distribution patterns of plants and soil macrofauna within coastal wetlands (Ge et al., 2021b). Soil pH was determined using a pH meter. SWC was assessed by comparing soil quality before and after drying. BD was determined using the ring knife method. SMC was measured post-washing with water and subsequent drying. SP was calculated based on bulk density and specific gravity, with specific gravity measured using a pycnometer. Soil EC was measured at a minimum of five locations within each plot using a portable WET Sensor (WET-2, England). TC and TN were analyzed using inductively coupled plasma optical emission spectrometry (Spectro Analytical Instruments) following pressure digestion (HNO3 digestion). TP and TK were determined using the phosphorus vanadium molybdate yellow colorimetric method and flame photometer method after high-temperature digestion (NaOH digestion). Soil AP was assessed using the sodium bicarbonate extracted-molybdenum antimony colorimetric method (Spark, Tecan, Switzerland). Soil AK was detected using the ammonium acetate extraction flame photometer method (XP, BWB, England). Soil AN and Soil NN were measured using the indophenol blue colorimetric method and dual-wavelength ultraviolet spectrophotometry following KCl extraction.
2.5 Statistical analyses
Initially, we utilized linear regression to investigate the impact of elevation on soil physico-chemical properties. Simultaneously, we conducted principal components analysis (PCA) on elevation and soil physico-chemical properties to identify significant correlations among them. Subsequently, we employed t- test to assess the differences of plant and soil macrofaunal diversity, elevation, and soil physico-chemical properties between saltmarshes and reclaimed areas. Furthermore, we performed redundancy analysis (RDA) to examine the relationships among plant and soil macrofaunal diversity, elevation, and soil physico-chemical properties, aiming to identify potential factors influencing plant and soil macrofaunal communities. RDA was performed using the “vegan” package in R software.
Finally, we used structural equation modeling (SEM) to disentangle causal pathways through which elevation and soil physico-chemical properties directly and indirectly affect plant and soil macrofaunal diversity. SEM was fitted using the “sem” package in R software, considering all possible pathways among the explanatory variables, simplifying initial models based on selected models by eliminating nonsignificant pathways. For SEM, we estimated direct and indirect effects as standardized path coefficients, enabling comparisons among predictor variables. The fit of SEM was evaluated using Chi-square (χ2), Comparative fit index (CFI), Tucker–Lewis index (TLI) and Root mean square error of approximation (RMSEA). A satisfactory SEM was deemed when P > 0.05 for the χ2 test, CFI > 0.9, TLI > 0.9, and RMSEA < 0.05.
Before conducting statistical analyses, we assessed the normality of elevation and soil physico-chemical properties using Shapiro-Wilk test (Supplementary Table 2). To ensure symmetry and linearity, numerical predictors underwent log-transformed. Statistical significance was determined at P < 0.05. All statistical analyses were conducted in R version 4.3.1 (R Core Team, 2021).
3 Results
3.1 Variations in the composition of plant and soil macrofaunal communities between saltmarshes and reclaimed areas
In total, 17 plant species were identified across saltmarshes and reclaimed areas, belonging to 15 genera and 11 families (Supplementary Table 3). Notably, Spartina alterniflora was the sole species detected in the saltmarshes. The reclaimed areas harbored 17 plant species, with the most prevalent ones being Phragmites australis, Suaeda, Artemisia capillaris, Avena fatua, and Euphorbia esula. A t-test indicated a statistically significant increase in plant species richness in the reclaimed areas compared with the saltmarshes (t = 8.40, P < 0.0001). However, no statistically significant differences were observed in plant height (t = 0.01, P = 0.99) and basal diameter (t = 0.87, P = 0.39) between saltmarshes and reclaimed areas (Table 1; Figure 3A).
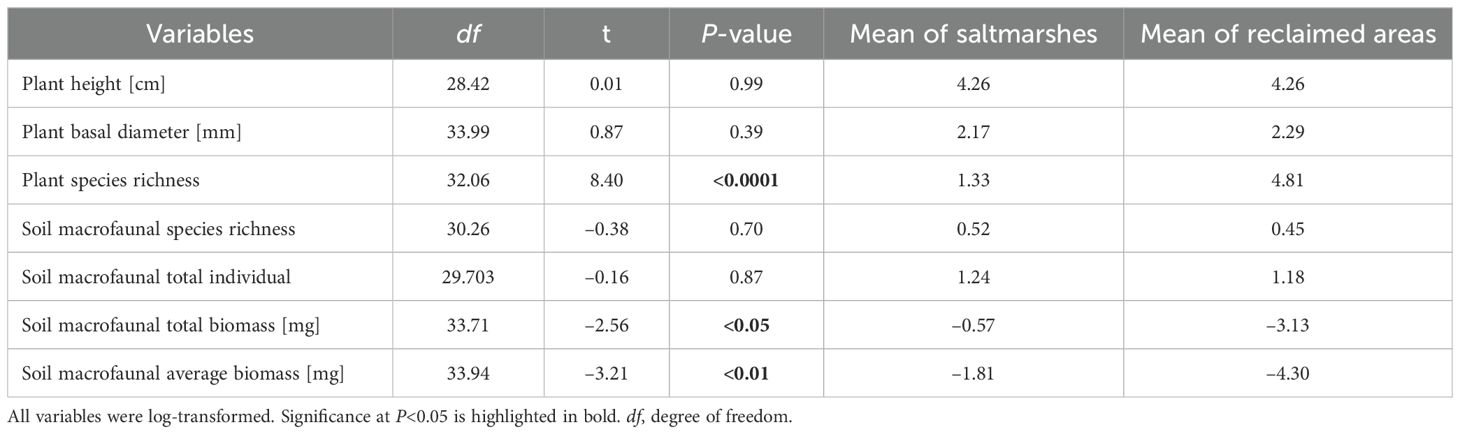
Table 1. The t-test of plant and soil macrofaunal diversity between saltmarshes and reclaimed areas.
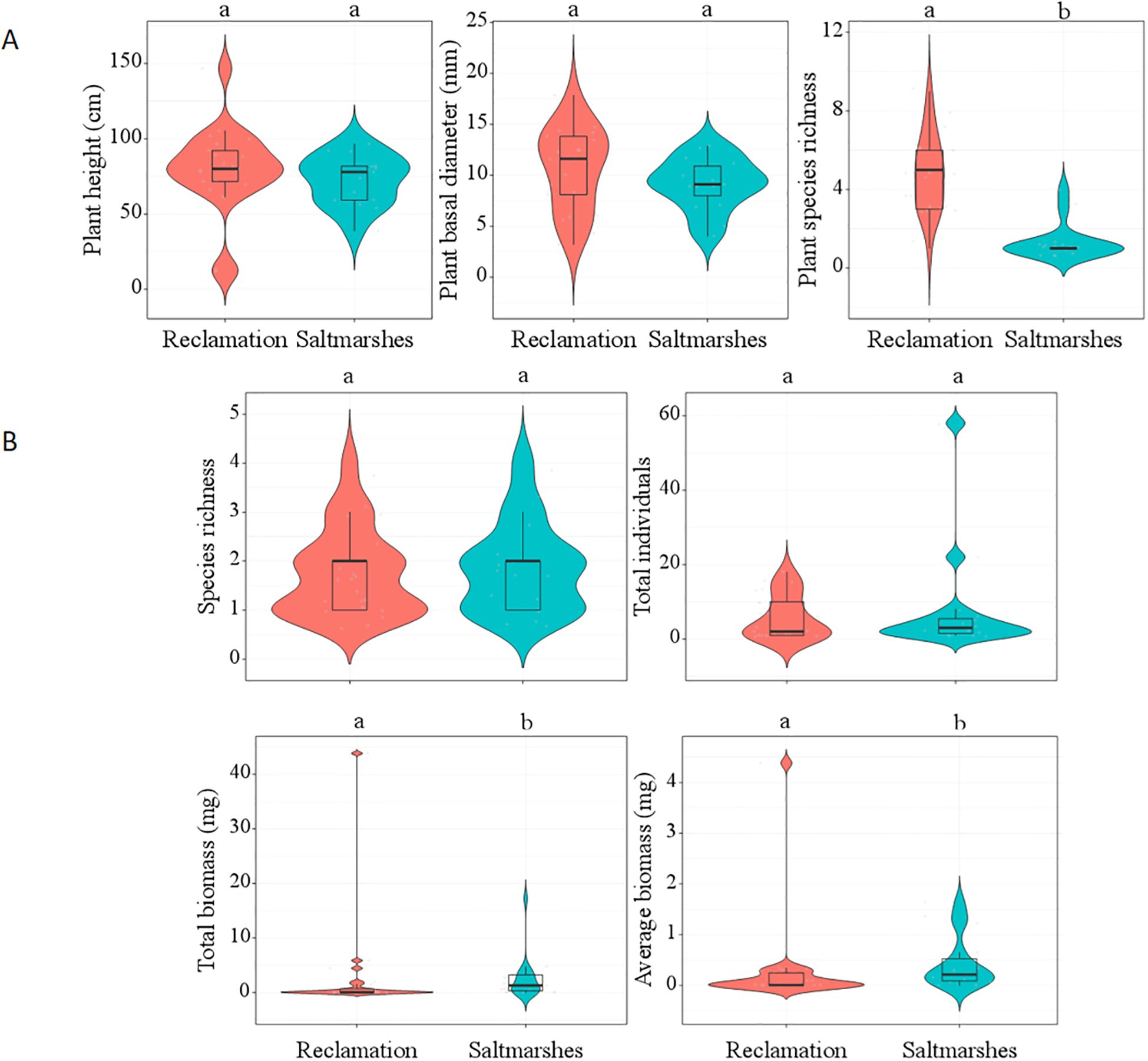
Figure 3. The t-test of plant diversity and soil macrofaunal diversity between saltmarshes and reclaimed areas. Different letters indicate significant differences between saltmarshes and reclaimed areas (P < 0.05). (A) Plant diversity; (B) soil macrofaunal diversity.
In total, 33 soil macrofaunal species were identified across saltmarshes and reclaimed areas, belonging to 4 phyla, 30 genera and 32 families (Supplementary Table 4). In saltmarshes, a total of 180 soil macrofaunal individuals were gathered, which were categorized into 23 species, 3 phyla, 21 families, and 21 genera. The phyla represented were Mollusca (10 species, accounting for 43%), Annelida (7 species, 30%), and Arthropoda (6 species, 26%). The dominant species (individuals ≥ 5%) were Assiminea latericea, Cerithidea largillierti, Umbonium thomasi, Neanthes japonica, Orchomene breviceps, accounting for 76% of the total individuals. Frequently encountered species (number of plots occurring ≥6) were Neanthes japonica, Assiminea latericea, Cerithidea largillierti, Potamocorbula laevis. The average weight of soil macrofauna ranged from 0.001 to 1.40 g, with the three heaviest species being Glauconome primeana, Cerithidea largillierti, and Umbonium thomasi (Supplementary Table 4).
In reclaimed areas, a total of 123 soil macrofaunal individuals were collected, representing 15 species, 3 phyla, 14 families, and 13 genera (Supplementary Table 4). The phyla present were Arthropoda (9 species, 69%), Mollusca (4 species, 31%), and Annelida (2 species, 13%). The dominant species were Neanthes succinea, Assiminea latericea, Porcellio sp., Corophium sinense, which collectively accounted for 75.6% of the individuals. The species most frequently encountered were Assiminea latericea and Porcellio sp. The average biomass of soil macrofauna ranged from 0.001 to 8.56 g, with the two heaviest species being Scapharca subcrenata and Macrophthalmus japonicus. The t-test revealed that the total and average biomass of soil macrofauna in reclaimed areas were significantly lower compared to those in saltmarshes (t = –2.56, P = 0.02; t = –3.21, P < 0.01). Soil macrofaunal total biomass in reclaimed area reduced to 1%. Nonetheless, there were no significant differences in the total individual (t = –0.16, P = 0.87) and species richness (t = 0.70, P = 0.52) between saltmarshes and reclaimed areas (Table 1; Figure 3B).
3.2 Variations in elevation and soil physico-chemical properties between saltmarshes and reclaimed areas
The t-test showed that SWC, SP, soil EC, TC, soil AK, and soil AN were significantly higher in saltmarshes compared with reclaimed areas (P < 0.05), while elevation (E) and BD were significantly lower in saltmarshes than that in reclaimed areas (P < 0.05). No significant differences were observed in soil pH, SMC, TN, TP, TK, soil AP, and soil NN between saltmarshes and reclaimed areas (P > 0.05) (Table 2). The first two PCA axes accounted for 59.3% of the variance in elevation and soil physico-chemical properties (Figure 4). PC1 represented the axe of soil salinity, nutrition and moisture, characterized by higher SWC, SP, soil EC, TC, TN, soil AK, and soil AN, along with lower soil pH and BD. PC2 indicated the axe related to soil phosphorus, with higher TP and soil AP (Supplementary Table 6). The PCA biplot depicted that with increasing elevation, soil pH and BD increased, whereas SP, SWC, soil AK, soil EC, and soil AN decreased (Figure 4; Supplementary Figure 2; Supplementary Figure 6).
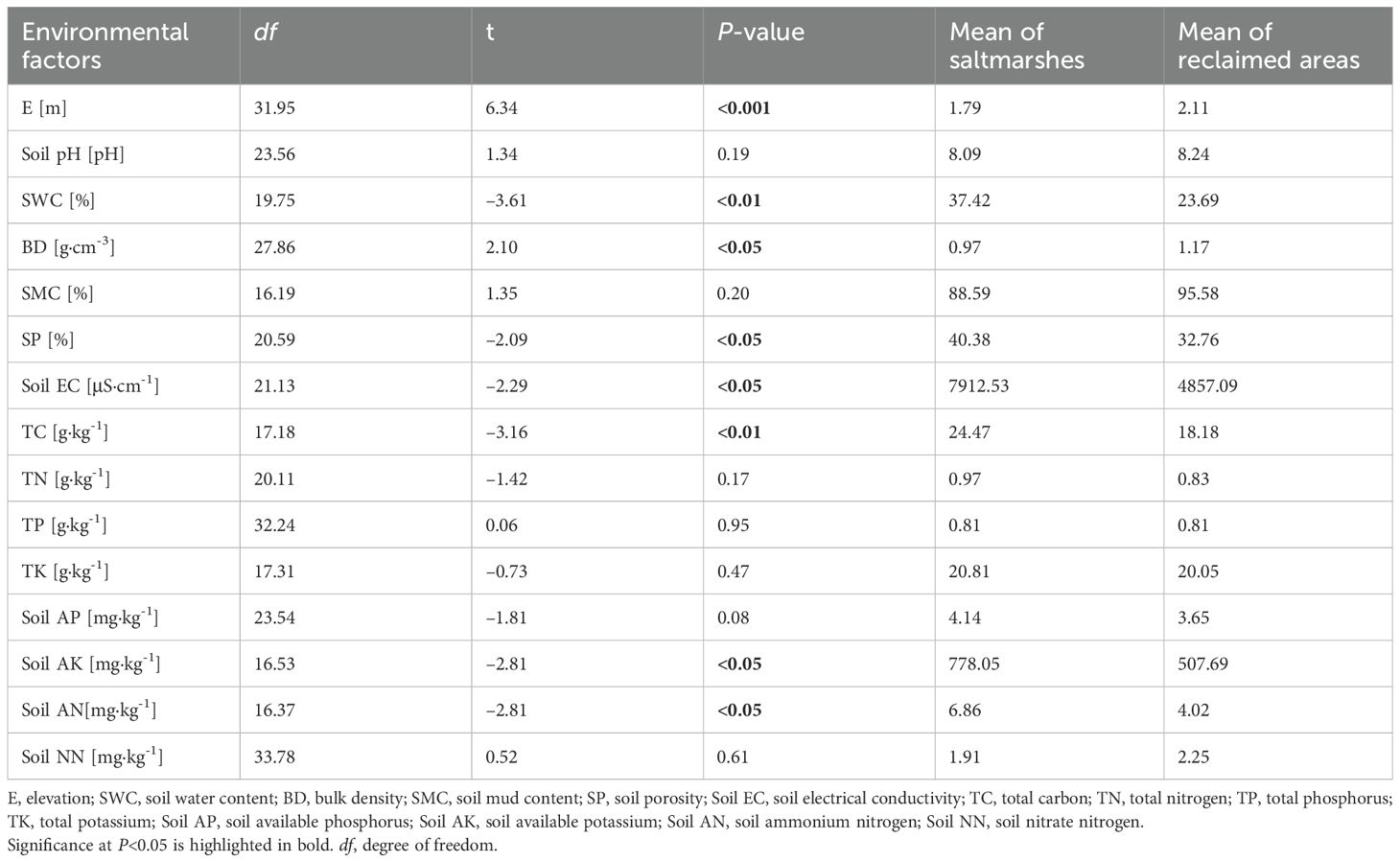
Table 2. The t-test of elevation and soil physico-chemical properties between saltmarshes and reclaimed areas.
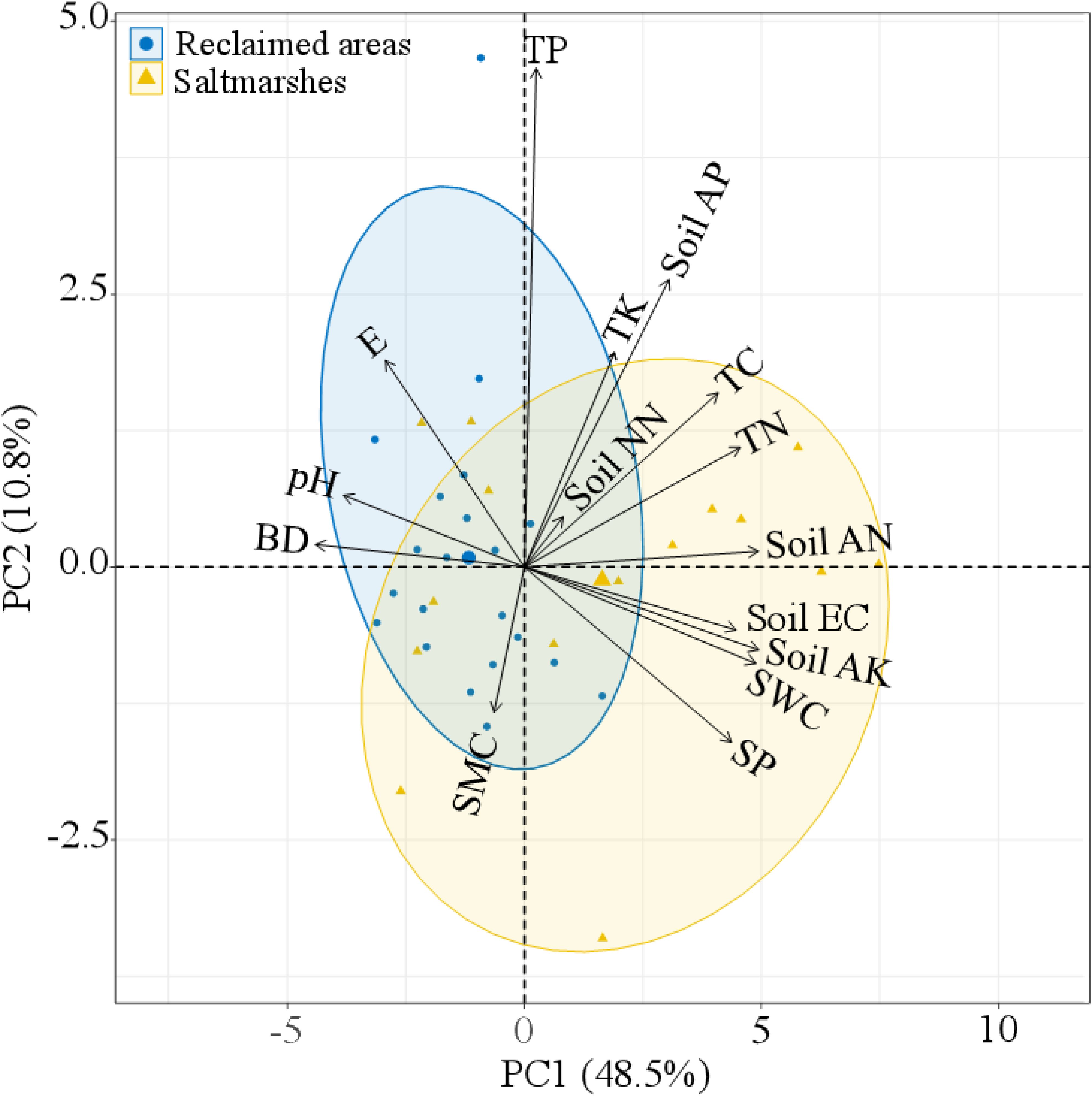
Figure 4. PCA biplot of elevation and soil physico-chemical properties in saltmarshes and reclaimed areas. E, elevation; SWC, soil water content; BD, bulk density; SMC, soil mud content; SP, soil porosity; Soil EC, soil electrical conductivity; TC, total carbon; TN, total nitrogen; TP, total phosphorus; TK, total potassium; Soil AP, soil available phosphorus; Soil AK, soil available potassium; Soil AN, soil ammonium nitrogen; Soil NN, soil nitrate nitrogen. The yellow ellipse represents saltmarshes, while the blue ellipse denotes reclaimed areas.
3.3 Effects of elevation and soil physico-chemical properties on plant and soil macrofaunal communities
The RDA elucidated the intricate relationships among plant and soil macrofaunal communities, elevation, and soil physico-chemical properties in saltmarshes and reclaimed areas (Figure 5). The eigenvectors of RDA1 and RDA2 explained 43.45% and 22.71% of the variance in plant and soil macrofaunal communities, respectively. Plant species richness increased with E, and decreased with soil AP and soil EC. Plant basal diameter increased with soil AP. Plant height increased with soil AN, TK, TN, SP, SWC, and TC. The species richness, total individual, total biomass, and average biomass of soil macrofauna increased with soil AN, TN, TK, SP, SWC, TC, soil NN, soil AK, and soil EC, but decreased with TP, E, SWC, pH, and BD (Figure 5).
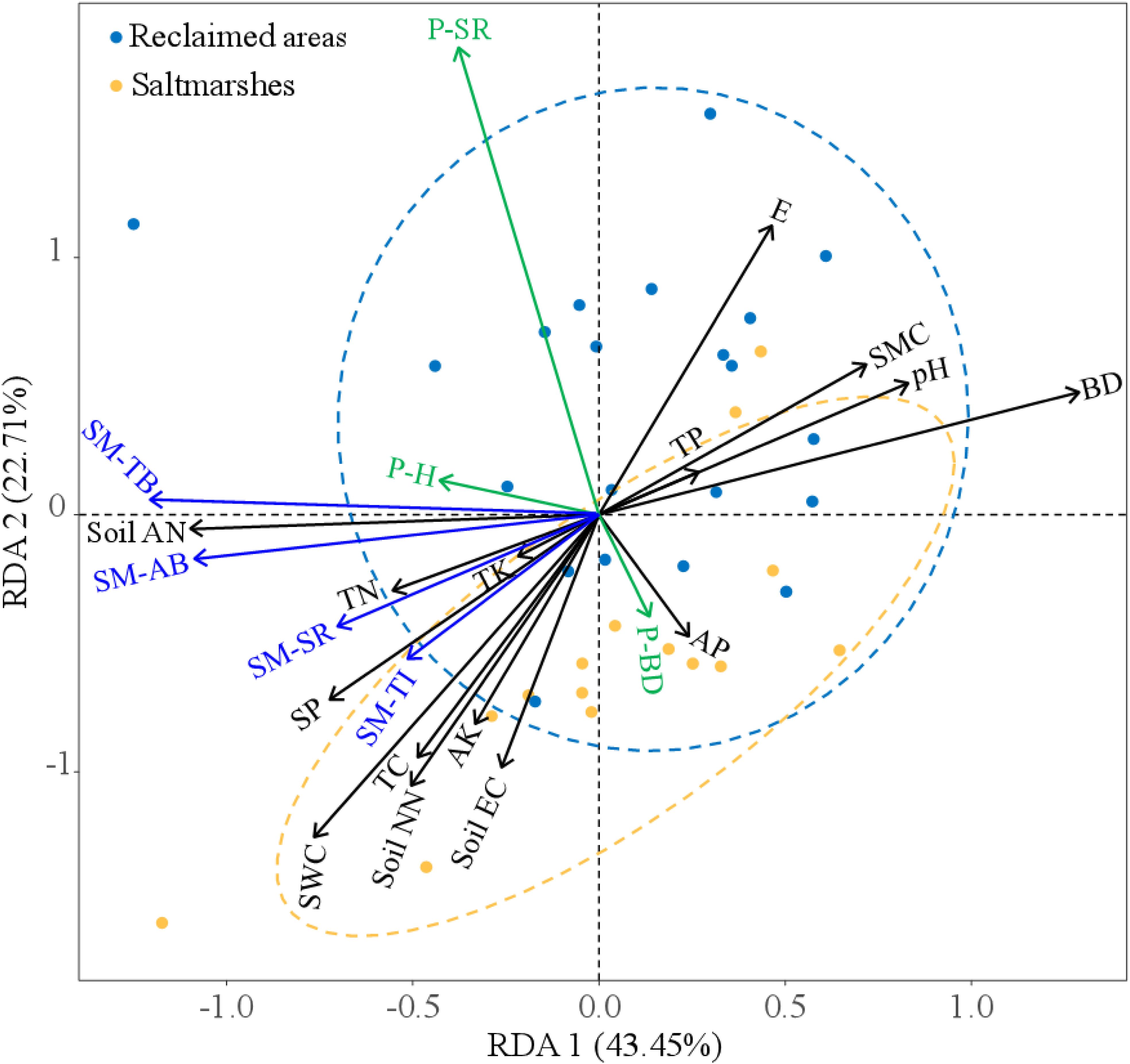
Figure 5. RDA biplot illustrating the relationships among soil properties (black), plant diversity (green), and soil macrofaunal diversity (blue) in saltmarshes and reclaimed areas. P-H, plant height; P-BD, plant basal diameter; P-SR, plant species richness; SM-SR, soil macrofaunal species richness; SM-TI, soil macrofaunal total individual; SM-TB, soil macrofaunal total biomass; SM-AB, soil macrofaunal average biomass. The introduction of abbreviated soil physico-chemical properties can be found in Figure 4.
3.4 Elevation affects plant species richness and soil macrofaunal biomass by regulating soil physico-chemical properties
The best-fitted SEMs demonstrated a strong fit to the data, explaining 96%, 80%, and 75% of the variations in plant species richness, total and average biomass of soil macrofauna, respectively (Figure 6). Although elevation did not directly impact plant species richness and soil macrofaunal biomass, it exerted indirect effects by regulating soil physico-chemical properties (Figure 6; Supplementary Table 7). Specifically, elevation positively influenced plant species richness indirectly through SWC, TC, soil AN, and soil NN, while it negatively affected plant species richness via BD. Elevation indirectly decreased soil macrofaunal total biomass by affecting SWC, TC, and soil AN, but it indirectly increased soil macrofaunal total biomass through soil AK. Additionally, elevation indirectly reduced the average biomass of soil macrofauna by affecting TC and soil AN (Supplementary Table 7).
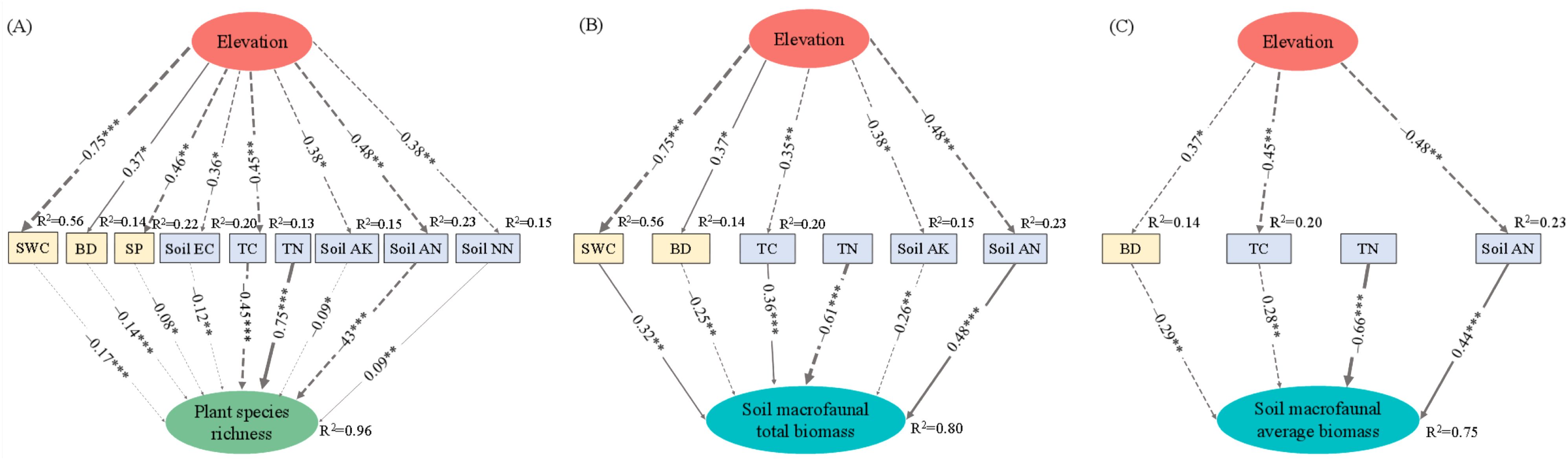
Figure 6. The best-fitted structural equation model explains that elevation indirectly influences plant species richness and soil macrofaunal biomass through soil physico-chemical properties. Notes: Solid and dashed lines represent significant positive and negative paths, respectively (*P < 0.05; **P < 0.01; ***P < 0.001). The standardized regression coefficients are shown for each path, and the width of lines reflects the relative values of standardized path coefficient. R2 indicates the total variation in a dependent variable that is explained by the combined independent variables. All variables were log-transformed. An introduction to the abbreviated soil physico-chemical properties is shown in Figure 4. (A) Plant species richness; (B) Soil macrofaunal total biomass; (C) Soil macrofaunal average biomass.
4 Discussion
4.1 Effects of saltmarsh reclamation on plant and soil macrofaunal communities
Our findings indicate that saltmarsh reclamation significantly increased plant species richness while reducing soil macrofaunal biomass within coastal wetlands. The transformation of saltmarshes into aquaculture ponds primarily influenced land use types and landscape patterns, notably by raising elevation, which altered hydrological conditions and ecological processes (Portnoy and Giblin, 1997; Huang et al., 2021; Naser, 2022). Additionally, saltmarsh reclamation has resulted in long-term changes to soil physico-chemical properties (Li et al., 2014; Xie et al., 2017; Ge et al., 2021a). Specifically, with the increase in terrain elevation, BD and freshwater rose, while SWC, SP, soil EC, TC, soil AN, soil NN, and seawater decreased (Supplementary Figure 6). These changes are attributed to reduced seawater leaching effects, relatively stable salinity and prolonged destabilization (Walker et al., 2010; Chan et al., 2016). Additionally, it is part of the physical-biological feedbacks within ecogeomorphology (Murray et al., 2008). For example, plant growth can significantly modify sediment dynamics (Horstman et al., 2015); conversely, plant growth is also constrained by factors such as sedimentation rate, inundation duration, and salinity, which are influenced by morphological changes (Lovelock et al., 2015).
Saltmarsh reclamation indirectly promotes the establishment and growth of 17 plant species that are tolerant to salt and water, while partially inhibiting the competitive species S. alterniflora. Previous studies have also indicated that saltmarsh reclamation mainly contributes to shifts in plant diversity within coastal wetlands (Li et al., 2014; Allan et al., 2015; Cao et al., 2018) and facilitates the introduction of non-native plant species through various invasive pathways (Zhang and Shao, 2013b). S. alterniflora is the only species left in saltmarshes. About 20 years ago, the dominant saltmarsh species were P. australis and Suaeda spp. The suitable habitat for P. australis and Suaeda spp. has been reclaimed, and S. alterniflora is highly competitive in lower tidal flat, so S. alterniflora has gradually replaced the native saltmarsh species (Netto and Lana, 1999). Soil physico-chemical properties can directly affect plant diversity. For example, plant species richness positively correlated with TN, and negatively correlated with SWC, BD, TC, soil AP, soil EC, and soil AN (Figure 5; Supplementary Figure 4). Especially, optimal salinity levels can promote plant growth, whereas exceeding the tolerance threshold may hinder it (Coletti et al., 2017; Parihar et al., 2015). In reclaimed areas, lower soil EC and SWC often lead to the proliferation of more salt-sensitive and drought-resistant plants (Supplementary Table 1). Additionally, seed germination and seedling growth are influenced by hydrological dynamics, flooding frequency, and sedimentary processes, all of which can impede successful plant establishment (Bouma et al., 2014; Coletti et al., 2017). These results can be explained by plant-soil feedback, where plants alter soil biological and abiotic properties in their growth environment, and the changed soils subsequently affect the fitness of plants (Bever, 1994).
Saltmarsh reclamation has led to an decrease in both the total and average biomass of soil macrofauna. This change is attributed to the presence of distinct soil macrofaunal species in both saltmarshes and reclaimed areas, each exhibiting different biomass levels (Supplementary Table 4). Soil macrofaunal biomass positively correlated with SWC, TC, and soil AN, while showing negative correlations with BD, TN, and soil AK (Figure 5; Supplementary Figure 5). These correlations highlight how soil properties can directly influence the survival and reproduction of soil macrofauna (Ge et al., 2021a). Studies have shown that sediment characteristics, tidal levels, salinity, and elevation collectively impact the establishment and persistence of macrofaunal communities in saltmarshes (Ysebaert and Herman, 2002; Chao et al., 2012; Xue et al., 2019). Following saltmarsh reclamation, many soil macrofaunal communities were lost due to their inability to adapt to the new environments characterized by drier conditions and lower salinity (Wang et al., 2021). Changes in food sources, breeding habitats, and shelters for soil macrofauna also influenced their composition and biodiversity (Ge et al., 2021b; Wang et al., 2010). Conversely, several studies have indicated that reclamation negatively impacts the species richness, abundance, biomass, diversity, and community succession of soil macrofauna (Koo et al., 2008; Wu et al., 2021; Naser, 2022). We observed a reduction in the abundance of Gastropoda, Bivalvia, and Polychaeta groups with limited mobility, while highly active Malacostraca increased in reclaimed areas. Additionally, Insecta larvae were found in reclaimed areas but not in saltmarshes. This aligns with findings that reclamation alters community structure, significantly decreasing the number of larger crustaceans, which are gradually replaced by mollusks and annelids (Huang et al., 2021).
Soil moisture (SWC), nutrients (TN, TK, TC, TP, soil AN, soil NN, and soil AK), salinity (soil EC and pH), and soil physical structure (BD and SP) significantly influence soil macrofaunal communities. These soil properties are also related to the growth, reproduction, and distribution of soil macrofauna (Pandey and Thiruchitrambalam, 2019; Ge et al., 2021b). Soil nutrients are essential for primary productivity and thus play a critical role in food availability for macrofaunal communities (Ge et al., 2021b). Similarly, studies have shown that soil macrofauna are significantly affected by a combination of salinity, soil particle size, and elevation (Ysebaert and Herman, 2002), as well as by soil moisture (Ge et al., 2021a) and clay content (Li et al., 2020). Soil macrofaunal diversity positively correlated with salinity, pH, and larger soil particle diameter, while negatively correlating with TN and TP (Huang et al., 2021). We also found that certain phyla, such as Chaetognatha, Annelida, Mollusca, and Arthropoda, are better adapted to high-salinity soils near the coast.
4.2 Saltmarsh reclamation indirectly influences plant and soil macrofaunal communities by regulating soil properties through elevation
Saltmarsh reclamation plays a pivotal role in shaping both plant and soil macrofaunal communities by regulating soil properties through changes in elevation. The SEMs revealed that soil AN, soil AK, SWC, and TC negatively correlated with elevation, while BD positively correlated with it. These factors, acting as environmental filters, influencing plant and soil macrofaunal diversity. This suggested that elevation-driven changes in soil compaction and hydrology act as habitat filters, selectively excluding taxa with specific physiological tolerances. Elevation is often regarded as a comprehensive indicator of regional environmental conditions (Yuan and Lu, 2003), as it affects sediment composition, flooding conditions, and soil physico-chemical properties along the elevation gradient (Zhang et al., 2021b). The influence of seawater and tidal effects on saltmarshes and soil macrofauna diminishes with increasing elevation (Salgado et al., 2007; Ge et al., 2021b). Many studies investigating the relationship between soil macrofauna and environmental factors frequently utilize elevation gradients, which have long been recognized as critical determinants of ecosystem dynamics and biodiversity (Carey et al., 2015; Yang et al., 2016).
Plant communities in coastal wetlands display a zonal distribution along the elevation gradient. Erosion caused by seawater decreases with rising elevation, leading to diminished environmental stressors and creating more favorable habitats for plant growth, thereby increasing plant abundance (Boorman, 2003). Elevation and plant distribution represent a typical bidirectional feedback process. On one hand, changes in elevation significantly influence the growth and succession of plants, leading to variations in inundation duration and frequency, which affect their long-term adaptation. Consequently, different plant species thrive within their optimal elevation ranges (Keddy, 2010). On the other hand, plant growth can promote sediment accumulation, ultimately resulting in an increase in elevation. Meanwhile, plant distribution is primarily influenced by biotic factors such as competition and environmental filtering (Lambers et al., 2019). Furthermore, soil macrofaunal diversity transitions from saltmarshes to reclaimed areas, characterized by a shift from swimming benthic organisms to burrowing and attached species (Yuan and Lu, 2003).
Soil moisture, nutrients, and salinity significantly impact plant and soil macrofaunal communities, particularly through alterations in elevation during saltmarsh reclamation. Research has shown that salinity and elevation collectively affect soil macrofauna (Ysebaert and Herman, 2002). Additionally, changes in elevation influence flooding duration and soil properties, leading to zonation in the composition and abundance of soil macrofauna in Gulf of Mexico and Brazilian saltmarshes (Netto and Lana, 1999; Mouton and Felder, 2007; Compton et al., 2013; Dewenter et al., 2023). Saltmarshes at lower elevations near seawater often exhibit higher diversity and density of soil macrofauna, while both abundance and diversity tend to decrease with increasing elevation (Zhang et al., 2021b). To effectively mitigate the impacts of saltmarsh reclamation, a comprehensive approach to assessment and management throughout the reclamation processes is essential. This should include a thorough consideration of both above- and below-ground organisms, as well as soil physico-chemical properties. Meanwhile, slope and slope types may significantly impact biodiversity during saltmarsh reclamation (Khan et al., 2013; Huang et al., 2015). Future studies should take these factors into account. Additionally, rising sea levels driven by climate change could alter land use types and landscape patterns in coastal wetlands. As a result, these changes may lead to modifications in elevation and soil properties, subsequently affecting both above- and below-ground biodiversity (Vo et al., 2014).
It should be noted that our findings reflect the ecological conditions within the first two decades following reclamation. Longer-term effects of saltmarsh conversion, particularly those involving successional changes beyond 20 years, require further investigation. In our study, while the number of sampling plots may not be sufficient to fully capture the overall patterns of plant and soil macrofaunal diversity, our results are robust enough to detect several statistically significant trends. This suggests that our selected plots are adequate for identifying the “reclamation effects.” Consequently, our findings highlight the significant role that elevation and soil physico-chemical properties play in influencing both above- and below-ground biodiversity. Further in-depth research is needed to determine whether our conceptual insights can be broadly applied to other coastal ecosystems.
5 Conclusions
Saltmarsh reclamation has led to notable alterations in the elevation and soil physico-chemical properties of coastal wetlands, profoundly affecting both above- and below-ground biodiversity in plant and soil macrofaunal communities. Notably, saltmarsh reclamation has enhanced plant species richness from 1 to 17, resulting in a 99% decrease in soil macrofaunal total biomass. by regulating soil properties through elevated changes. Specifically, soil moisture, nutrients, and salinity are the primary factors influencing plant species richness and soil macrofaunal biomass. Elevation had an indirect positive effect on plant species richness via SWC, TC, soil AN, and soil NN, and an indirect negative effect through BD. Furthermore, elevation indirectly decreased soil macrofaunal total biomass through SWC, TC, and soil AN, but indirectly increased it through soil AK. Our study highlights the underlying mechanisms driving variations in above- and below-ground biodiversity resulting from saltmarsh reclamation. Future studies should establish long-term monitoring of both plant and soil macrofaunal community succession following reclamation and compare the effects of different reclamation methods. Besides, implementing effective management strategies, such as elevation control, preserve tidal creeks in reclaimed areas, establishing priority conservation zones for soil macrofauna in low-elevation areas should be emphasized. These findings can be applied to investigate the impacts of coastal wetland reclamation on both above- and below-ground biodiversity, particularly regarding how variations in land use and landscape interact with elevation on a larger scale.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
GZ: Writing – original draft, Funding acquisition, Conceptualization, Investigation. SW: Writing – original draft, Funding acquisition, Conceptualization, Investigation. MX: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. HH: Writing – original draft, Methodology, Investigation, Writing – review & editing. JH: Conceptualization, Writing – review & editing, Investigation. JG: Writing – review & editing, Methodology, Investigation. ZC: Investigation, Methodology, Writing – review & editing. JZ: Writing – review & editing, Conceptualization, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key R & D Program of China (2022YFC3106201), Zhejiang Basic Public Welfare Research Program (LZJWZ22E090005), National Natural Science Foundation of China (42301081 and 42076178), Natural Science Foundation of Zhejiang Province of China (LQ24C030006), The Open Funding of Zhejiang Collaborative Innovation Center for Land and Marine Spatial Utilization and Governance Research (LHGTXT-2025-005), The funding of Zhejiang Zhoushan Island Ecosystem Observation and Research station, Ministry of Natural Resource (2025002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1633852/full#supplementary-material
References
Allan E., Manning P., Alt F., Binkenstein J., Blaser S., Blüthgen N., et al. (2015). Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843. doi: 10.1111/ele.12469
Ayuke F. O. (2010). Soil macrofauna functional groups and their effects on soil structure, as related to agricultural management practices across agroecological zones of Sub-Saharan Africa (Wageningen, Netherlands: Wageningen University and Research).
Balke T., Stock M., Jensen K., Bouma T. J., and Kleyer M. (2016). A global analysis of the seaward salt marsh extent: The importance of tidal range. Water Resour. Res. 52, 3775–3786. doi: 10.1002/2015WR018318
Bannert A., Kleineidam K., Wissing L., Mueller-Niggemann C., Vogelsang V., Welzl G., et al. (2011). Changes in diversity and functional gene abundances of microbial communities involved in nitrogen fixation, nitrification, and denitrification in a tidal wetland versus paddy soils cultivated for different time periods. Appl. Environ. Microbiol. 77, 6109–6116. doi: 10.1128/aem.01751-10
Barbier E. B., Koch E. W., Silliman B. R., Hacker S. D., Wolanski E., Primavera J., et al. (2008). Coastal ecosystem-based management with nonlinear ecological functions and values. Science 319, 321–323. doi: 10.1126/science.1150349
Bauer J. E., Cai W. J., Raymond P. A., Bianchi T. S., Hopkinson C. S., and Regnier P. A. G. (2013). The changing carbon cycle of the coastal ocean. Nature. 504, 61–70. doi: 10.1038/nature12857
Bever J. D. (1994). Feeback between plants and their soil communities in an old field community. Ecology 75, 1965–1977. doi: 10.2307/1941601
Boorman L. A. (2003). Salt marsh review: an overview of coastal saltmarshes, their dynamic and sensitivity characteristics for conservation and management. Peterborough.: JNCC. Rep. 334, 6–7. Available online at: https://hub.jncc.gov.uk/assets/4c1a28e7-de13-4ff5-b7c8-088e879e5a1a (Accessed February 1, 2003).
Bouma T. J., van Belzen J., Balke T., Zhu Z., Airoldi L., Blight A. J., et al. (2014). Identifying knowledge gaps hampering application of intertidal habitats in coastal protection: Opportunities & steps to take. Coast. Eng. 87, 147–157. doi: 10.1016/j.coastaleng.2013.11.014
Cao H. B., Zhu Z. C., Balke T., Zhang L., and Bouma T. J. (2018). Effects of sediment disturbance regimes on Spartina seedling establishment: Implications for salt marsh creation and restoration. Limnol. Oceanogr. 63, 647–659. doi: 10.1002/lno.10657
Carey J. C., Raposa K. B., Wigand C., and Warren R. S. (2015). Contrasting decadal-scale changes in elevation and vegetation in two long island sound salt marshes. Estuar. Coast. 40, 651–661. doi: 10.1007/s12237-015-0059-8
Chan A. K. Y., Xu W. Z., Liu X. S., Cheung S. G., and Shin P. K. S. (2016). Sediment characteristics and benthic ecological status in contrasting marine environments of subtropical Hong Kong. Mar. pollut. Bull. 103, 360–370. doi: 10.1016/j.marpolbul.2015.12.032
Chao M., Shi Y., Quan W., Shen X., An C., Yuan Q., et al. (2012). Distribution of benthic macroinvertebrates in relation to environmental variables across the Yangtze River Estuary, China. J. Coast. Res. 284, 1008–1019. doi: 10.2112/JCOASTRES-D-11-00194.1
Coletti J. Z., Vogwill R., and Hipsey M. R. (2017). Water management can reinforce plant competition in salt-affected semi-arid wetlands. J. Hydrol. 552, 121–140. doi: 10.1016/j.jhydrol.2017.05.002
Compton T. J., Holthuijsen S., Koolhaas A., Dekinga A., ten Horn J., Smith J., et al. (2013). Distinctly variable mudscapes: Distribution gradients of intertidal macrofauna across the Dutch Wadden Sea. J. Sea. Res. 82, 103–116. doi: 10.1016/j.seares.2013.02.002
Cui X. C., Hu J. L., Wang J. H., Yang J. S., and Lin X. G. (2016). Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 98, 140–149. doi: 10.1016/j.apsoil.2015.10.008
Day J. W., Conner W. H., DeLaune R. D., Hopkinson C. S., Hunter R. G., Shaffer G. P., et al. (2021). A review of 50 years of study of hydrology, wetland dynamics, aquatic metabolism, water quality and trophic status, and nutrient biogeochemistry in the Barataria Basin, Mississippi Delta-system functioning, human impacts and restoration approaches. Water 13, 642. doi: 10.3390/W13050642
Dewenter J., Yong J., Schupp P. J., Lõhmus K., Kröncke I., Moorthi S., et al. (2023). Abundance, biomass and species richness of macrozoobenthos along an intertidal elevation gradient. Ecol. Evol. 13, e10815. doi: 10.1002/ECE3.10815
Gaston L. A., Locke M. A., Zablotowicz R. M., and Reddy K. N. (2001). Spatial variability of soil properties and weed populations in the Mississippi Delta. Soil Sci. Soc Am. J. 65, 449–459. doi: 10.2136/sssaj2001.652449x
Ge B., Cui J., Zhang D., Liu Q., Jiang S., Tang B., et al. (2019). Succession of soil macro-faunal biodiversity in forests converted from croplands after long-term coastal reclamation. Soil Till. Res. 186, 165–171. doi: 10.1016/j.still.2018.10.015
Ge B., Yang R., Yang L., Jiang S., and Tang B. (2021a). Changes in soil macrofaunal communities along soil age gradient under centuries of cultivation after coastal reclamation. Catena 200, 105170. doi: 10.1016/j.catena.2021.105170
Ge B., Zhou J., Yang R., Jiang S., Yang L., and Tang B. (2021b). Lower land use intensity promoted soil macrofaunal biodiversity on a reclaimed coast after land use conversion. Agr. Ecosyst. Environ. 306, 107208. doi: 10.1016/j.agee.2020.107208
Ge Z. M., Zhang L. Q., and Yuan L. (2015). Spatiotemporal dynamics of salt marsh vegetation regulated by plant invasion and abiotic processes in the Yangtze Estuary: observations with a modeling approach. Estuar. Coast. 381, 310–324. doi: 10.1007/s12237-014-9804-7
Gedan K. B., Silliman B. R., and Bertness M. D. (2009). Centuries of human-driven change in salt marsh ecosystems. Annu. Rev. Mar. Sci. 1, 117–141. doi: 10.1146/annurev.marine.010908.163930
Goeldner L. (1999). The German Wadden Sea coast: reclamation and environmental protection. J. Coast. Conserv. 5, 23–30. doi: 10.1007/BF02802736
Horstman E. M., Dohmen-Janssen C. M., Bouma T. J., and Hulscher S. J. M. H. (2015). Tidal-scale flow routing and sedimentation in mangrove forests: Combining field data and numerical modelling. Geomorphology 228, 244–262. doi: 10.1016/j.geomorph.2014.08.011
Huang Y., Li Y., Chen Q., Huang Y., Tian J., Cai M., et al. (2021). Effects of reclamation methods and habitats on macrobenthic communities and ecological health in estuarine coastal wetlands. Mar. pollut. Bull. 168, 112420. doi: 10.1016/j.marpolbul.2021.112420
Huang Y. M., Liu D., and An S. S. (2015). Effects of slope aspect on soil nitrogen and microbial properties in the Chinese Loess region. Catena 125, 135–145. doi: 10.1016/j.catena.2014.09.010
Keddy P. A. (2010). Wetland ecology: principles and conservation (London, United Kingdom: Cambridge university press).
Khan F., Hayat Z., Ahmad W., Ramzan M., Sharif M., Mian A. M., et al. (2013). Effect of slope position on physico-chemical properties of eroded soil. Soil Environ. 32, 22–28. Available at: https://www.researchgate.net/publication/259295824. (Accessed December 15, 2013).
Koo B. J., Shin S. H., Woo H. J., Kim E. S., and Je J. G. (2008). Changes in macrobenthic community structure on Gunsan tidal flat after the closing of the Saemangeum 4th dyke. Ocean. Polar. Res. 30, 497–507. doi: 10.4217/OPR.2008.30.4.497
Korres N. E., Norsworthy J. K., Brye K. R., Skinner V. Jr., and Mauromoustakos A. (2017). Relationships between soil properties and the occurrence of the most agronomically important weed species in the field margins of eastern Arkansas-Implications for weed management in field margins. Weed. Res. 57, 159–171. doi: 10.1111/wre.12249
Lambers H., Oliveira R. S., Lambers H., and Oliveira R. S. (2019). Biotic influences: interactions among plants. Plant Physiol. Ecol., 615–648. doi: 10.1007/978-3-030-29639-1_16
Li S. W., Li F., Song X. K., and Zhang M. L. (2020). The influence of water-sediment regulation on macrobenthic community structures in the Huanghe River (Yellow River) Estuary during 2012-2016. Acta Oceanol. Sin. 39, 120–128. doi: 10.1007/s13131-020-1664-3
Li Y. A., Pang F. G., Zang Y., Zhang Y., Song Y. C., and Mu F. H. (2022). Spatial-temporal heterogeneity of meiofauna in Sanya Bay beach and its influencing factors. Trans. Oceanol. Limnol. 44, 139–148. doi: 10.13984/j.cnki.cn37-1141.2022.03.019
Li J., Pu L., Zhu M., Zhang J., Li P., Dai X., et al. (2014). Evolution of soil properties following reclamation in coastal areas: A review. Geoderma 226-227, 130–139. doi: 10.1016/j.geoderma.2014.02.003
Liu J. G., Yang W., and Li S. X. (2016). Framing ecosystem services in the telecoupled Anthropocene. Front. Ecol. Environ. 14, 27–36. doi: 10.1002/16-0188.1
Loreau M., Naeem S., Inchausti P., Bengtsson J., Grime J. P., Hector A., et al. (2001). Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. doi: 10.1126/science.1064088
Lotze H. K., Lenihan H. S., Bourque B. J., Bradbury R. H., Cooke R. G., Kay M. C., et al. (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809. doi: 10.1126/science.1128035
Lovelock C. E., Cahoon D. R., Friess D. A., Guntenspergen G. R., Krauss K. W., Reef R., et al. (2015). The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 526, 559–563. doi: 10.1038/nature15538
Lv W., Zhou W., and Zhao Y. (2019). Effect of freshwater inflow on self-restoration of macrobenthic diversity in seaward intertidal wetlands influenced by reclamation projects in the Yangtze estuary, China. Mar. pollut. Bull. 138, 177–186. doi: 10.1016/j.marpolbul.2018.11.044
Marshall E. J. P., Brown V. K., Boatman N. D., Lutman P. J. W., Squire G. R., and Ward L. K. (2003). The role of weeds in supporting biological diversity within crop fields. Weed. Res. 43, 77–89. doi: 10.1046/j.1365-3180.2003.00326.x
Martino L., Luengo D., and Míguez J. (2018). Independent random sampling methods (Cham: Springer International Publishing), 65–113. doi: 10.1007/978-3-319-72634-2
Mouton E. C. and Felder D. L. (2007). Burrow distributions and population estimates for the fiddler crabsUca spinicarpa andUca longisignalis in a Gulf of Mexico salt marsh. Estuaries 19, 51–61. doi: 10.2307/1352651
Murray A. B., Knaapen M. A. F., Tal M., and Kirwan M. L. (2008). Biomorphodynamics: Physical-biological feedbacks that shape landscapes. Water Resour. 44, W11301. doi: 10.1029/2007WR006410
Naser H. A. (2022). Community structures of benthic macrofauna in reclaimed and natural intertidal areas in Bahrain, Arabian Gulf. J. Mar. Sci. Eng. 10, 945. doi: 10.3390/JMSE10070945
Netto S. A. and Lana P. C. (1999). The role of above- and below-ground components of Spartina alterniflora (Loisel) and detritus biomass in structuring macrobenthic associations of Paranaguá Bay (SE, Brazil). Hydrobiologia 400, 167–177. doi: 10.1023/A:1003753001411
Pan G. H., Liu Y., Fu Q., Gao J., Zhao Y. Q., Cao X., et al. (2018). Effects of landuse change of coastal tidal flat on the bio-available phosphorus in soils. Environ. Chem. 37, 2378–2386. doi: 10.7524/j.issn.0254-6108.2018011901
Pandey V. and Thiruchitrambalam G. (2019). Spatial and temporal variability of sandy intertidal macrobenthic communities and their relationship with environmental factors in a tropical island. Estuar. Coast. Shelf. Sci. 224, 73–83. doi: 10.1016/j.ecss.2019.04.045
Parihar P., Singh S., Singh R., Singh V. P., and Prasad S. M. (2015). Effect of salinity stress on plants and its tolerance strategies: a review. Environ. Sci. pollut. R. 22, 4056–4075. doi: 10.1007/s11356-014-3739-1
Portnoy J. W. and Giblin A. E. (1997). Effects of historic tidal restrictions on salt marsh sediment chemistry. Biogeochemistry 36, 275–303. doi: 10.1023/A:1005715520988
R Core Team. (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Ricardo S. C., Gustavo M., Carlos H. S. C., Tatiana M. B. C., Ludmila B. G., and Felipe M. (2012). Effects of environmental gradients on sandy beach macrofauna of a semi-enclosed bay. Mar. Ecol. 33, 106–116. doi: 10.1111/j.1439-0485.2011.00457.x
Ryu J., Khim J. S., Choi J. W., Shin H. C., An S., Park J., et al. (2011). Environmentally associated spatial changes of a macrozoobenthic community in the Saemangeum tidal flat, Korea. J. Sea. Res. 65, 390–400. doi: 10.1016/j.seares.2011.03.003
Salgado J. P., Cabral H. N., and Costa M. J. (2007). Spatial and temporal distribution patterns of the macrozoobenthos assemblage in the salt marshes of Tejo estuary (Portugal). Hydrobiologia 587, 225–239. doi: 10.1007/s10750-007-0685-7
Sengupta D., Choi Y. R., Tian B., Brown S., Meadows M., Hackney C. R., et al. (2023). Mapping 21st century global coastal land reclamation. Earths. Future 11, e2022EF002927. doi: 10.1029/2022EF002927
Silvestri S., Deffna A., and Marani M. (2005). Tidal regime, salinity and salt marsh plant zonation. Estuar. Coast. Shelf. Sci. 62, 119–130. doi: 10.1016/j.ecss.2004.08.010
Sun C., Li J., Liu Y., Zhao S., Zheng J., and Zhang S. (2023). Tracking annual changes in the distribution and composition of saltmarsh vegetation on the Jiangsu coast of China using Landsat time series–based phenological parameters. Remote Sens. Environ. 284, 113370. doi: 10.1016/j.rse.2022.113370
Sun Z. G., Sun W. G., Tong C., Zeng C. S., Yu X., and Mou X. J. (2015). China’s coastal wetlands: Conservation history, implementation efforts, existing issues and strategies for future improvement. Environ. Int. 79, 25–41. doi: 10.1016/j.envint.2015.02.017
Temmerman S., Meire P., Bouma T. J., Herman Peter M. J., Ysebaert T., and De Vriend H. J. (2013). Ecosystem-based coastal defence in the face of global change. Nature 504, 79–83. doi: 10.1038/nature12859
Tian B., Wu W. T., Yang Z. Q., and Zhou Y. X. (2016). Drivers, trends, and potential impacts of long-term coastal reclamation in China from 1985 to 2010. Esuar. Coast. Shelf. S. 170, 83–90. doi: 10.1016/j.ecss.2016.01.006
Tilman D., Clark M., Williams D. R., Kimmel K., Polasky S., and Packer C. (2017). Future threats to biodiversity and pathways to their prevention. Nature 546, 73–81. doi: 10.1038/nature22900
Visscher A. M., Wellstein C., Vanek S., Bricca A., Meza K., Huaraca J., et al. (2023). Drivers of growth and establishment of the invasive plant Rumex acetosella within Andean fallow systems. Agr. Ecosyst. Environ. 351, 108446. doi: 10.1016/j.agee.2023.108446
Vo P. T., Ngo H. H., Guo W., Zhou J. L., Nguyen P. D., Listowski A., et al. (2014). A mini-review on the impacts of climate change on wastewater reclamation and reuse. Sci. Total. Environ. 494, 9–17. doi: 10.1016/j.scitotenv.2014.06.090
Walker L. R., Wardle D. A., Bardgett R. D., and Clarkson B. D. (2010). The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 98, 725–736. doi: 10.1111/j.1365-2745.2010.01664.x
Wang X., Chen W. Q., Zhang L. P., Jin D., and Lu C. Y. (2010). Estimating the ecosystem service losses from proposed land reclamation projects: A case study in Xiamen. Ecol. Econ. 69, 2549–2556. doi: 10.1016/j.ecolecon.2010.07.031
Wang S. K., Sheng Q., Zhao F., Zhang T. T., and Zhuang P. (2021). Variable effects on benthic community from diking to eradicate invasive plants in the Yangtze Estuary salt marsh. Front. Mar. 8. doi: 10.3389/FMARS.2021.706353
Wong M. K., Tsukamoto J., Yusuyin Y., Tanaka S., Iwasaki K., and Tan N. P. (2016). Comparison of soil macroinvertebrate communities in Malaysian oil palm plantations with secondary forest from the viewpoint of litter decomposition. For. Ecol. Manage. 381, 63–73. doi: 10.1016/j.foreco.2016.09.011
Wu H. Y., Fu S. F., Wu J., Cai X. Q., and Chen Q. H. (2021). Spatiotemporal variation of benthic biodiversity under persistent and extreme human disturbances in the Xiamen sea area, China. Ocean. Coast. Manage. 207, 105556. doi: 10.1016/j.ocecoaman.2021.105556
Wu W., Yang Z., Tian B., Huang Y., Zhou Y., and Zhang T. (2018). Impacts of coastal reclamation on wetlands: Loss, resilience, and sustainable management. Estuar. Coast. Shelf. Sci. 210, 153–161. doi: 10.1016/j.ecss.2018.06.013
Xie X. F., Pu L. J., Wang Q. Q., Zhu M. G., Xu Y., and Zhang M. (2017). Response of soil physicochemical properties and enzyme activities to long-term reclamation of coastal saline soil, Eastern China. Sci. Total. Environ. 607-608, 1419–1427. doi: 10.1016/j.scitotenv.2017.05.185
Xie Z., Xu L., Duan X., and Xu X. (2012). Analysis of boundary adjustments and land use policy change-A case study of Tianjin Palaeo coast and Wetland National Natural Reserve, China. Ocean. Coast. Manage. 56, 56–63. doi: 10.1016/j.ocecoaman.2012.02.001
Xue J., Yang J., Wang Q., Aronson R. B., and Wu H. (2019). Community structure of benthic macroinvertebrates in reclaimed and natural tidal flats of the Yangtze River estuary. Aquaculture Fisheries. 4, 205–213. doi: 10.1016/j.aaf.2019.04.001
Yang W., Sun T., and Yang Z. (2016). Effect of activities associated with coastal reclamation on the macrobenthos community in coastal wetlands of the Yellow River Delta, China: A literature review and systematic assessment. Ocean. Coast. Manage. 129, 1–9. doi: 10.1016/j.ocecoaman.2016.04.018
Ysebaert T. and Herman P. M. (2002). Spatial and temporal variation in benthic macrofauna and relationships with environmental variables in an estuarine, intertidal soft-sediment environment. Mar. Ecol. Prog. Ser. 244, 105–124. doi: 10.3354/meps244105
Yu J., Zhan C., Li Y., Zhou D., Fu Y., Chu X., et al. (2016). Distribution of carbon, nitrogen and phosphorus in coastal wetland soil related land use in the Modern Yellow River Delta. Sci. Rep. 6, 37940. doi: 10.1038/srep37940
Yuan X. Z. and Lu J. J. (2003). Micro-topographical element-structure and spatial distribution of meiofauna on the tidal flat. Chin. J. Eco. 22, 124–126. doi: 10.13292/j.1000-4890.2003.0156
Zhang H. B., Liu H. Y., Li Y. F., An J., Xue X. Y., and Hou M. X. (2013a). Spatial variation of soil moisture/salinity and the relationship with vegetation under natural conditions in Yancheng coastal wetland. Env. Sci. 34, 540–546. doi: 10.13227/j.hjkx.2013.02.019
Zhang L. and Shao H. (2013b). Direct plant-plant facilitation in coastal wetlands: A review. Estuar. Coast. Shelf. S. 119, 1–6. doi: 10.1016/j.ecss.2013.01.002
Zhang H., Sun T., Cao H., Zhang Y., Yang W., Shao D., et al. (2021a). Movement of mud snails affects population dynamics, primary production and landscape heterogeneity in tidal flat ecosystems. Landsc. Ecol. 36, 3493–3506. doi: 10.1007/S10980-021-01322-7
Zhang X., Zhang Z., Wang W., Fang W., Chiang Y., Liu X., et al. (2021b). Vegetation successions of coastal wetlands in southern Laizhou Bay, Bohai Sea, northern China, influenced by the changes in relative surface elevation and soil salinity. J. Environ. Manage. 293, 112964. doi: 10.1016/j.jenvman.2021.112964
Keywords: above- and below-ground biodiversity, aquaculture ponds, coastal wetland, invasive Spartina alterniflora, plant colonization, structural equation modeling
Citation: Zhang G, Wang S, Xu M, Hu H, Huang J, Gan J, Chen Z and Zeng J (2025) Saltmarsh reclamation enhances plant species richness and reduces soil macrofaunal biomass by regulating soil properties through elevation. Front. Ecol. Evol. 13:1633852. doi: 10.3389/fevo.2025.1633852
Received: 30 May 2025; Accepted: 18 June 2025;
Published: 04 July 2025.
Edited by:
Wujian Xiong, Mianyang Normal University, ChinaReviewed by:
Hang Ci, East China Normal University, ChinaSufeng Pan, Wenzhou Vocational College of Science and Technology, China
Copyright © 2025 Zhang, Wang, Xu, Hu, Huang, Gan, Chen and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingshan Xu, eHVtczAxMjNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Guangzhi Zhang
Guangzhi Zhang Shanshan Wang1,2†
Shanshan Wang1,2† Mingshan Xu
Mingshan Xu Jian Zeng
Jian Zeng