- 1Acoustics Research Institute, Austrian Academy of Sciences, Vienna, Austria
- 2Institute for Globally Distributed Open Research and Education (IGDORE), Gruenau im Almtal, Austria
Introduction: Predator recognition is essential for prey survival, yet, whether responses are shaped by evolutionary predispositions or by ecological experience remains debated.
Methods: We tested vigilance responses of fifty-one free-ranging Southern giraffes (Giraffa giraffa) to controlled playbacks of lion roar-grunt sequences in two South African populations: a predator-naïve population in a reserve without lions and a predator-experienced population in a reserve where lions were reintroduced five years ago.
Results: Both populations oriented rapidly to lion calls, suggesting that acoustic features of lion vocalizations act as generalized danger cues. However, predator-experienced giraffes sustained vigilance ten times longer (mean ± SD: 513.34 ± 421.34 s, N = 24) compared to predator-naïve giraffes (49.06 ± 46.26 s, N = 27). Vigilance responses during lion playbacks, in general, were higher in the predator-experienced population, whereas responses to control calls did not differ between sites.
Discussion: These findings indicate that while immediate orientation likely reflects evolved sensitivity to acoustically harsh predator cues, the persistence of vigilance is shaped by ecological experience. Our study demonstrates that predator reintroduction can rapidly recalibrate prey risk perception, highlighting the dynamic interplay between evolved predispositions and learning in shaping antipredator responses.
1 Introduction
Predation is a fundamental ecological force that shapes the behavior, physiology, and cognition of prey species (Lima and Dill, 1990; Brown and Chivers, 2005). For large herbivores, the ability to detect predators early is essential for survival and shapes many behavioral trade-offs, including the balance between time spent foraging and vigilance (Beauchamp, 2015). Across African savannas, resource gradients and predation risk jointly shape the spatial distribution, habitat use, and associations of herbivores. In particular, lions (Panthera leo) influence herbivore habitat selection and temporal activity patterns through both long-term and short-term predation risk (Valeix et al., 2009a, 2009; Creel et al., 2014; Anderson et al., 2016). Such spatial overlap among prey species, and shared exposure to common predators, creates opportunities for interspecific information transfer and the evolution of generalized responses to threat cues (Magrath et al., 2015; Goodale et al., 2020).
Prey species rely on different sensory cues to assess predation risk. When visual information is constrained by distance, habitat structure, or time of day, acoustic cues often become particularly important (Templeton et al., 2005; Hettena et al., 2014). These cues include both direct predator vocalizations and heterospecific alarm calls. While predator calls may provide reliable information on the type and proximity of a threat, heterospecific alarms can expand the information available to prey by effectively increasing the number of individuals scanning for danger (Magrath et al., 2015, 2020). Large African ungulates, for instance, are known to eavesdrop on alarm calls of other species, with response strength varying according to the reliability of the caller as an indicator of shared predators (Palmer and Gross, 2018). Responses to such calls may bring immediate benefits, such as increased vigilance or rapid escape, but can also have longer-term advantages, including enhanced foraging opportunities and the associative learning of predator identities (Magrath et al., 2015). The effectiveness of these cues often depends on their structural features, such as harshness, chaotic elements, or non-tonality, which can signal urgency or arousal (Morton, 1977; Briefer, 2012). Such findings illustrate that ungulates discriminate acoustic cues based on their ecological relevance, a prerequisite for adaptive predator recognition across sensory modalities.
A central question in this context is whether predator recognition is innate or shaped by learning, or both. Threat sensitivity emerges through experience, allowing individuals to fine-tune their responses to ecologically relevant dangers. For instance, harbor seals (Phoca vitulina) respond strongly to unfamiliar fish-eating killer whale (Orcinus orca) calls but ignore calls from local, non-threatening killer whales (Deecke et al., 2002). Rodents acquire recognition of raptor calls through associative learning (Kindermann et al., 2009), and burrowing bettongs (Bettongia lesueur) develop visual predator recognition only after repeated exposure (Steindler et al., 2020). These findings suggest that learned predator recognition allows animals to calibrate their responses to local threat conditions, helping to avoid unnecessary responses while maintaining appropriate vigilance. In ungulates, exposure to lions enhances responsiveness to lion roars (Makin et al., 2019), and primates are more likely to recognize predator cues when threats are ecologically relevant (Sánchez-Vidal et al., 2024). These findings support the idea that predator recognition in long-lived species involves both evolved predispositions and learned components. Moreover, studies show that predator-naïve populations may lose or attenuate antipredator responses over time, as demonstrated by Vancouver Island marmots (Marmota vancouverensis) that lost predator discrimination in captivity after just a few generations (Dixon-MacCallum et al., 2021). In contrast, other species retain antipredator responses for millennia, such as California ground squirrels (Spermophilus beecheyi), which maintain rattlesnake recognition even after more than 70,000 years of isolation (Blumstein, 2006). Such variation highlights how ecological context and evolutionary history interact to determine whether antipredator responses degrade or persist. These dynamics can be further understood within a landscape-of-fear framework, where spatial and temporal variation in risk modulates the trade-offs between foraging and vigilance (Creel et al., 2014; Laundré et al., 2014). Understanding how these processes operate in large herbivores is particularly important, as many ungulates now persist in landscapes where predator distributions are changing through recolonization and reintroduction (Hunter et al., 2007; Hayward and Somers, 2009; Chapron et al., 2014).
Recent studies further demonstrate that the return of large carnivores, such as lions, can reshape prey demography, habitat use, and risk perception (Annear et al., 2023). These questions are particularly relevant for ungulates, which provide valuable systems for examining how predator recognition interacts with ecological experience. Among them, giraffes (Giraffa spp.) represent an ideal model species. Although often assumed to rely primarily on visual cues due to their height and visual range (Coimbra et al., 2013; Williams, 2016), giraffes remain vulnerable to lion predation, with both calves and adults targeted (Hayward and Kerley, 2005). Their vigilance behavior is influenced by social context (Cameron and du Toit, 2005; Marealle et al., 2020), but their use of acoustic cues for predator detection remains largely unexplored. A recent study, however, demonstrated that giraffes responded more strongly to heterospecific alarm calls from red-billed oxpeckers (Buphagus erythrorhynchus) by sustaining vigilance for longer durations in lion-inhabited areas compared to areas without lions, with responses modulated by the acoustic harshness of the calls (Baotic and Szipl, 2025). These findings indicate that giraffes incorporate heterospecific acoustic cues into their risk assessment and that prior predator exposure enhances responsiveness to alarm calls. The study also showed that oxpecker alarm calls are acoustically harsh, suggesting that call structure interacts with ecological experience to shape vigilance behavior. This raises the question of whether such experience-dependent modulation extends to direct predator cues, such as lion roars, which are the focus of the present study.
Because acoustic cues convey information not only through context but also through their structural properties, understanding the acoustic features that underlie such responses is critical. Nonlinear phenomena (including subharmonics, biphonation, and deterministic chaos) in animal vocalizations are commonly associated with heightened arousal and are frequently observed in alarm signals across taxa (Wilden et al., 1998). A recent review highlights that nonlinear phenomena (NLP) are widespread in vertebrate vocal repertoires and are thought to have evolved to enhance signal salience, attract attention, prevent habituation, and convey arousal, threat, or distress through perceptual harshness (Massenet et al., 2025). One way to quantify this harshness is through the ‘harmonic-to-noise ratio’ (HNR), which measures the relative contribution of harmonic versus noisy components in a signal. Lower HNR values often result from the presence of NLP and indicate reduced harmonic structure and increased noisiness, thereby producing acoustically harsher sounds that are more attention-grabbing (Riede et al., 2005). However, detectability alone does not determine behavioral responses; cue interpretation is modulated by prior experience and ecological context (Blumstein, 2006; Fardell et al., 2020). These dynamics are consistent with theoretical frameworks that describe antipredator behavior as a function of both evolved sensitivity to threat-related cues and individual learning history (Sih et al., 2010). Such processes are especially pronounced in ecosystems undergoing predator restoration, where the return of large carnivores like lions alters prey experience, learning opportunities, and risk perception (Hunter et al., 2007; Hayward and Somers, 2009; Palmer et al., 2022; Annear et al., 2023).
To examine how acoustic signal structure and ecological experience shape predator recognition, we conducted a playback experiment on two free-ranging giraffe populations in South Africa: one in a lion-free reserve (naïve) and one in a reserve with recently introduced lions (experienced). Individuals were presented with lion roar-grunt playback sequences (a biologically relevant predator cue), and dove coo calls (neutral control). We quantified behavioral responses, particularly vigilance, and analyzed stimulus structure focusing on HNR to assess whether acoustic harshness influences perceived threat. Based on this framework, we formulated two non-mutually exclusive hypotheses: (1) giraffes exhibit a predisposed sensitivity to predator vocalizations even in the absence of exposure, and (2) ecological experience shapes the magnitude of antipredator responses. We predicted that if predator vocalizations are inherently salient, giraffes in both populations would show immediate orienting responses and engage in vigilance. Conversely, if ecological experience is key, predator-experienced individuals would sustain vigilance for longer durations, especially in response to acoustically harsh (low-HNR) stimuli.
This study builds on previous research on predator recognition in ungulates, which has primarily focused on whether animals detect predator cues, typically characterizing responses as present or absent (e.g. Makin et al., 2019). In contrast, we compare populations that differ in long-term exposure to predators and quantify not just detection, but the magnitude and duration of antipredator behavior. By integrating ecological context with acoustic signal structure, we provide a more nuanced understanding of how predator recognition can persist, degrade, or intensify depending on risk exposure. Through the integration of acoustic, ecological, and behavioral perspectives, our study offers new insight into the mechanisms underlying antipredator plasticity and predator recognition in giraffes.
2 Material and methods
2.1 Study sites and subjects
This study was conducted between July and September 2024 at two private reserves in South Africa’s Limpopo Province, chosen to represent contrasting predator exposure histories in wild Southern giraffes (Giraffa giraffa). Lapalala Wilderness (LW; 48,000 ha) inhabits a resident lion population introduced between 2019 and 2020, which had grown to 15 individuals by the time of this study. In contrast, Mogalakwena River Reserve (MRR; 1,500 ha) remains free of large predators and thus provides a predator-naïve comparison group. Both study sites represent comparable dry-season savanna habitats, with similar vegetation structure and visibility, minimizing potential confounds related to habitat differences. To localize and approach giraffes for testing, site-specific methods were employed. At MRR, individuals were followed on foot by a single habituated observer (AB), using slow and non-invasive approaches to prevent disturbance. Giraffes at this site were well accustomed to human presence due to a long-term behavioral monitoring project conducted prior to this study. At LW, giraffes were located using a DJI Mini 4 Pro drone flown at altitudes between 40 and 70 meters, after which they were approached by vehicle. Giraffes at LW were habituated to vehicle presence through regular exposure to park management and monitoring activities and have shown no disturbance-related behaviors in previous playback experiments (Baotic and Szipl, 2025). Drone use was limited to pre-trial localization and never coincided with playback experiments or behavioral recording. In both reserves, giraffes were only tested once they were stationary, feeding calmly, and showed no signs of alertness, ensuring that vigilance responses reflected stimulus perception rather than observer disturbance. Evidence of active predation pressure at LW is supported by giraffe carcasses with confirmed signs of lion predation (Annear et al., 2023). Importantly, MRR is not bordered by reserves or farms containing lions, ensuring that giraffes at this site had no opportunity for exposure to lion vocalizations or predation risk. We focused exclusively on adult giraffes of both sexes. Individuals were identified based on their unique coat patterns (Shorrocks and Croft, 2009; Miele et al., 2021) and classified as adults following morphological and developmental criteria established by Muller (2018). A total of 27 individuals were included at MRR (13 females, 14 males) and 24 at LW (12 females, 12 males).
2.2 Acoustic stimuli
We used two categories of auditory stimuli in playback trials: lion roar-grunt calls (Figure 1A), and ring-necked dove (Streptopelia capicola) coo calls (Figure 1B). Lion vocalizations served as biologically relevant predator cues (Grinnell and McComb, 2001), while dove calls acted as a non-threatening control (Slabbekoorn et al., 1999). Dove calls were selected due to their function as neutral conspecific contact signals and absence of alarm-related associations in the literature. Playback stimuli were compiled into standardized packages, each containing two playback sequences. Within each sequence, lion or dove calls were presented as temporally naturalistic bouts, with inter-call intervals matched across packages. Dove calls were sourced from a previous playback experiment conducted by the authors of this study (Baotic and Szipl, 2025). Lion calls were sourced from existing archives and collaborators (see acknowledgements), ensuring a diverse representation of male and female vocalizations. Lion recordings were visually inspected for signal clarity and absence of audio artifacts (no overlap with other audio signal or friction sounds) by using spectrograms in Audacity version 3.3 (Audacity Team, 2023). Only sequences with an intact acoustic structure and no interfering background signals were retained. To minimize ambient noise in the playback signals, we applied the ‘noise reduction’ effect in Audacity. Noise profile filtering has been shown to effectively reduce background noise while preserving the acoustic properties of vocalizations (Baker and Logue, 2007; Benedict et al., 2021). A one-second noise profile was created before the onset of a call, followed by a 4–10 dB noise reduction (depending on the recording’s acoustic characteristics), using a sensitivity setting of 6.0 and three frequency smoothing bands. To further enhance sound clarity, additional spectral content filtering was performed in Praat version 6.4.27 (Boersma and Weenink, 2025). A high-pass filter was applied to remove excess low-frequency background noise below 450 Hz for dove calls, and below 30 Hz for lion calls. A low-pass filter was used to remove upper ambient frequencies, cutting off above 1.3 kHz in dove calls and above 20 kHz in lion calls. To ensure consistent peak amplitude across all sequences, while avoiding distortion due to excessive loudness (clipping effect), we normalized the audio files to -1.0 dB peak amplitude using Audacity. To ensure consistent stimuli conditions, the sound pressure level (SPL) of each playback sequence was standardized to 85 dB (Z-weighted) using an NTI AL1 SPL meter with an NTI MiniSPL microphone.
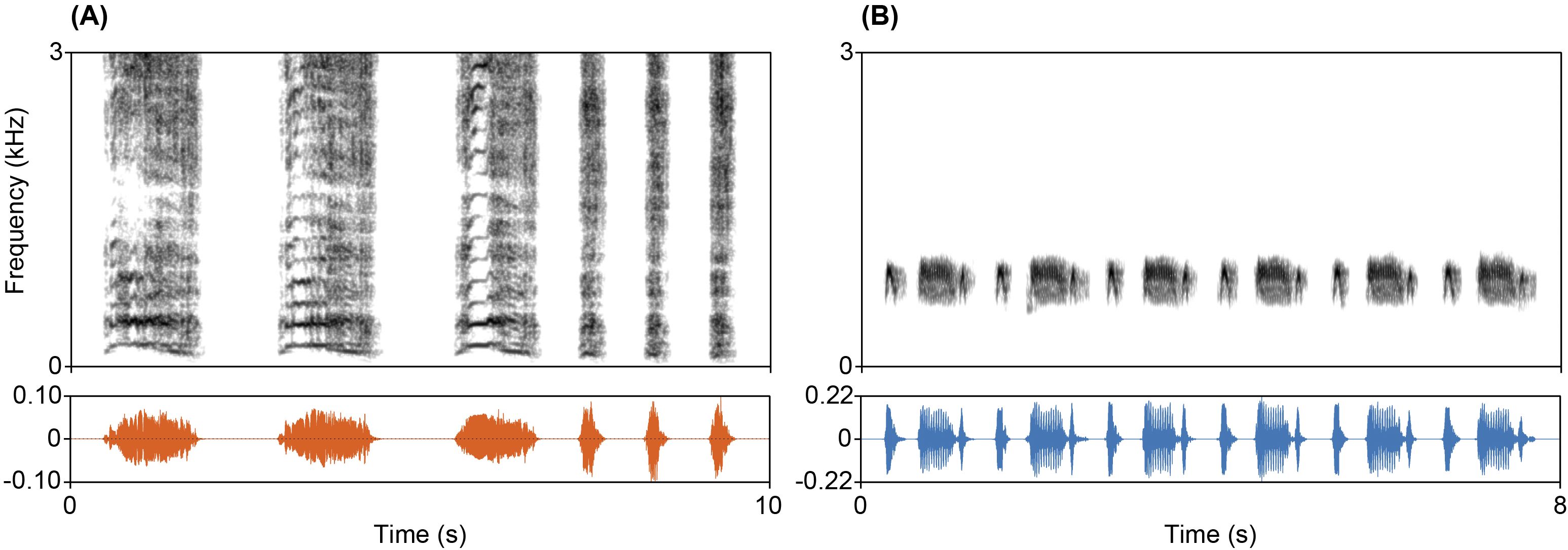
Figure 1. Spectrograms and oscillograms of acoustic stimuli used in the playback experiment. (A) Lion (Panthera leo) vocalization sequence (recorded by Timmy Moser), consisting of three full roars followed by three grunts. The mean harmonic-to-noise ratio (HNR) of this stimulus was 5.3 dB. (B) Ring-necked dove (Streptopelia capicola) stimulus composed of six coo calls with a mean HNR of 19.6 dB. Spectrograms were generated in Praat using a fast Fourier transform (FFT) method with a dynamic range of 55.0 dB. Window length was set to 0.08 s for the lion stimulus and 0.03 s for the dove stimulus.
Lion and dove single calls served as templates to create 27 playback packages, each containing two sequences - one for each species. Lion sequences contained three ‘roar’ and three ‘grunt’ calls, and dove sequences contained six ‘coo’ calls. A total of 84 lion roars (1.6 ± 0.3 s) and 84 lion grunts (0.6 ± 0.2 s) from fifteen individuals and 126 dove perch-coo calls (1.1 ± 0.05 s) were used, with values given as mean ± standard deviation (SD). The calls per sequence, as well as the order of sequences within each package, were randomly assigned to control for order effects.
2.3 Playback experiment procedure
Propagation tests, using a JBL-EON Compact loudspeaker (37.5 Hz to 20 kHz frequency response) connected via Bluetooth to an Apple iPhone 14 Pro, confirmed that spectral properties of playback stimuli remained intact at distances up to 150 meters. Lion and dove stimuli were re-recorded using a Sound Devices MixPre-3 II (frequency response: 10 Hz to 80 kHz) and an omnidirectional Neumann KM 183 MT microphone, saved as *.wav files (44.1 kHz, 16-bit). Spectrograms of lion vocalizations are provided in Supplementary Figure S1 and for dove calls in Supplementary Figure S2 in the Supplementary File 1.
Vigilance behavior was recorded as the primary response variable. Given the established role of risk assessment and predator detection in African ungulates (Creel et al., 2014), we identified vigilance behavior as the primary response variable. Both predation risk and social factors shape vigilance in giraffes (Cameron and du Toit, 2005). To isolate responses to predator cues and avoid conspecific influences, only one giraffe at a time was tested, with no other individuals nearby. This ensured that vigilance was attributable solely to lion calls, controlling for collective vigilance effects.
Playback trials were conducted between 09:30 and 17:40, i.e. after sunrise and before sunset, to avoid the crepuscular periods when lions are typically most active and to ensure consistent daylight conditions. Trials were carried out under calm weather conditions to prevent scattering of the sound stimuli due to wind gusts. At MRR, giraffes were followed on foot during their feeding activities and approached slowly to prevent premature disturbance, allowing them to habituate to the observer (Anton Baotic). Due to LW’s vast size, we used a DJI Mini 4 Pro drone to localize giraffes. Once located, the giraffes were followed by vehicle prior to trial initiation.
Individual giraffes were observed until they displayed relaxed feeding behavior, defined as continuous browsing with no visible signs of alertness. Trials were initiated only when the focal animal was stationary and no other conspecifics were within the visible vicinity, minimizing the influence of social facilitation or group vigilance. To ensure that the measured behavior reflected responses to playback stimuli alone, we confirmed that no vehicles from reserve staff were present in the area.
The JBL-EON Compact speaker was hidden behind vegetation to prevent stimulus association with the observer and speaker, respectively, and positioned to face the giraffe. Once the animal had engaged in uninterrupted feeding for a minimum of 10 seconds, we initiated the playback sequence (lion or dove). Each sequence followed a three-phase structure: (1) baseline (pre-stimulus behavior), (2) response (during stimulus), and (3) post-response (resumption of feeding for 10 seconds after playback). When individuals moved after the first sequence (i.e., before fully completing the experiment), the observer followed and resumed trials once the animal returned to calm foraging. Each of the 51 individuals included in the dataset was tested twice, once with a lion playback and once with a dove playback, within the same day, ensuring no day-to-day variation within individuals. We further documented the distance between the speaker and the giraffe using a Nikon Prostaff 1000 laser rangefinder (range: 20–133 meter). The experimental setup is illustrated in Figure 2.
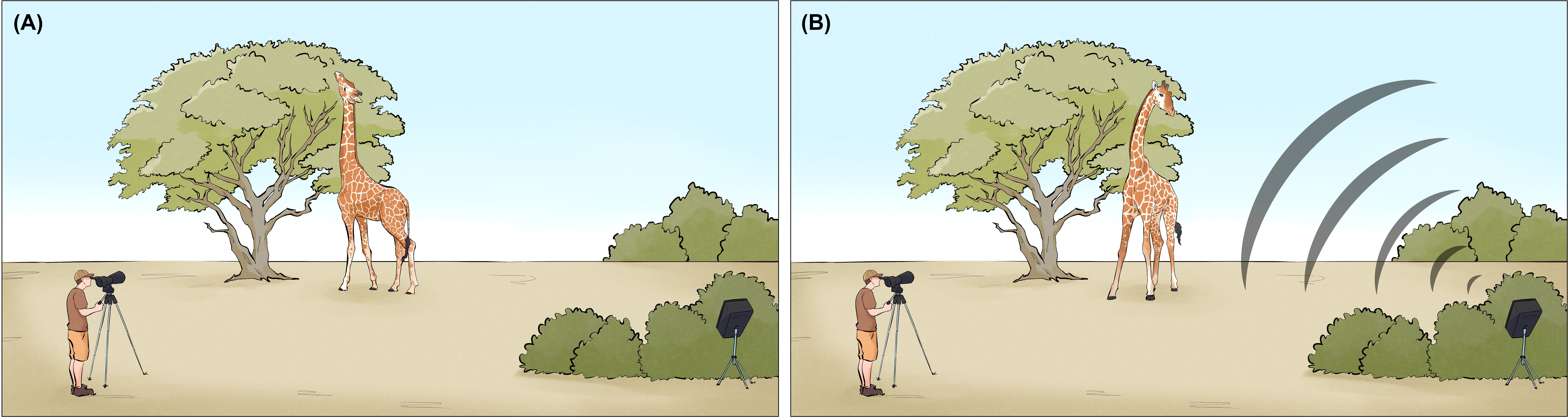
Figure 2. Experimental setup of a playback. (A) The focal giraffe feeds stationary at a tree, (B) and turns toward the hidden speaker responding to a lion roar-grunt sequence. Figure reproduced from Baotic and Szipl (2025), BMC Biology, under the terms of the Creative Commons Attribution 4.0 International License (CC BY).
2.4 Behavioral data collection and coding
Behavioral responses were recorded using a Sony FDR-AX53 video camera. Videos were later coded frame-by-frame (20 ms resolution) using Solomon Coder 17.03 for Windows. We used continuous sampling and recorded the duration (in seconds) of predefined behavioral categories including ‘Feeding’ (mouth attached to foliage and feeding continuously for ≥10 s), ‘Not feeding’ (cessation of feeding), ‘Turn to’ (orienting toward the sound source), ‘Scanning’ (stationary vigilant posture with head/neck raised, screening/monitoring environment), ‘Approach-Investigation’ (cautious movement toward the sound source), ‘Displacement’ (walking continuously in the opposite direction of the loudspeaker for ≥10 s), and five ear postures indicative of attentional state (illustrated in Figure 3). We note that ‘Approach-Investigation’ is considered a vigilance response in giraffes, reflecting cautious investigative movements toward a novel or potentially threatening stimulus rather than attraction (Langman, 1977). Behavioral definitions followed an ethogram previously established and validated in (Baotic and Szipl, 2025), with full behavioral definitions provided in Table 1. If the focal giraffe exhibited displacement behavior, the playback trial was considered complete. If the focal giraffe showed displacement behavior by walking continuously in the opposite direction of the loudspeaker for at least 10 seconds, the playback trial was considered complete.
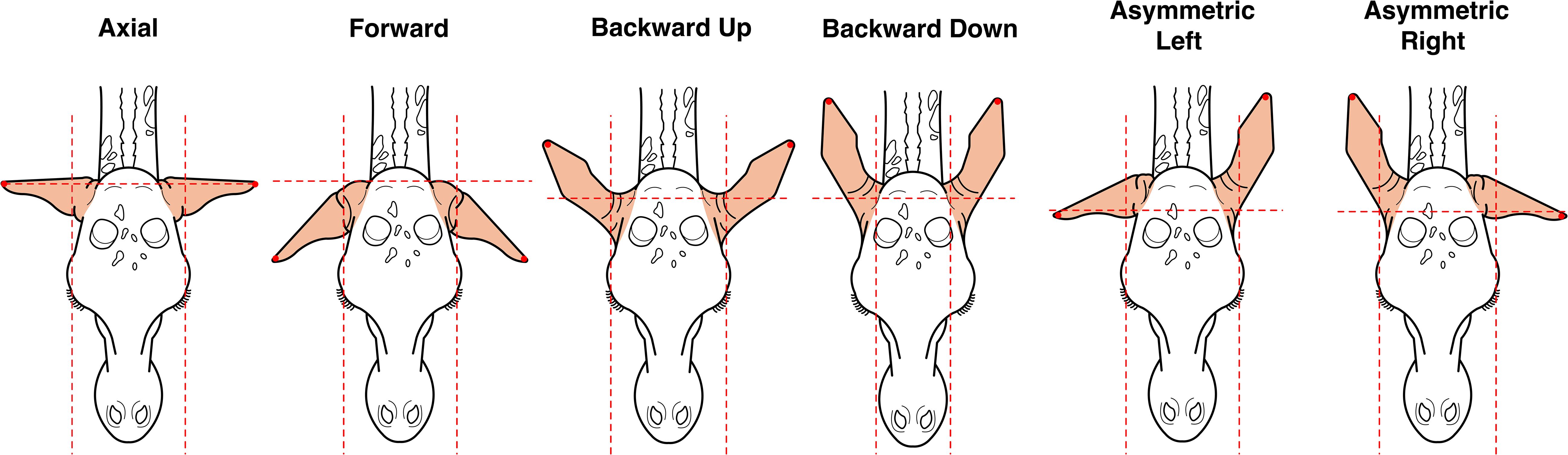
Figure 3. Illustrations of giraffe ear postures used for behavioral classification. Axial: Both ears extended laterally, forming an approximate 90° angle relative to the head-to-tail axis. Forward: Ears angled forward, deviating more than 30° above the horizontal plane, directed toward the giraffe’s front. Backward-Up: Ears angled backward and elevated above the neck line, exceeding 30° from the horizontal. Backward-Down: Ears directed rearward and downward, positioned at or below the level of the neck. Asymmetric Left/Right: One ear points backward while the other ear remains either forward or laterally extended (axial), creating a clear left–right asymmetry. Figure reproduced from Baotic and Szipl (2025), BMC Biology, under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0).
In addition to behavioral durations, we measured latencies to ‘Not feeding’ and to orient toward the speaker (‘Turn to’ and ‘Scanning’). If a behavior did not occur within 20 seconds of stimulus onset, the maximum value of 20 seconds was assigned by the coding software. These censored values indicate the absence of a measurable response and were excluded from the calculation of mean latencies.
Inter-rater reliability was not reassessed in the current study, as all behavioral categories and coding procedures followed the validated protocol established by Baotic and Szipl (2025).
2.5 Acoustic characterization of stimuli
To quantify the acoustic structure of the playback stimuli, we analyzed both call durations and signal tonality using Praat (version 6.4.27). For each playback package, we calculated the average harmonic-to-noise ratio (HNR) across all included calls, providing a proxy for the relative contribution of harmonic versus noisy components in the signal (Boersma and Weenink, 2025). HNR has previously been shown to correlate with perceptual judgments of roughness and vocal harshness in non-human animal vocalizations (Riede et al., 2005), making it a relevant measure for evaluating acoustic salience. HNR values were derived using the ‘To Harmonicity (cc)’ function with the following settings: a time step of 0.001, a silence threshold of 0.1, and one period per window. We adjusted the minimum pitch floor to match species-specific vocal properties, 450 Hz for dove calls and 30 Hz for lion calls. Call durations were obtained using the ‘Get total duration’ command. The resulting mean HNR values served as a continuous acoustic predictor variable in our statistical analyses, enabling us to examine whether variation in signal structure influenced behavioral responses.
2.6 Statistical analysis
We conducted a principal component analysis (PCA) to reduce the number of behavioral responses coded from the video recordings of the response phases into a smaller set of components. The PCA yielded one main principal component (PC; eigenvalue >1.0) that explained 92.6% of the total variance. This component reflected the duration of vigilance-related behaviors, with higher scores indicating longer vigilance responses. The PC was driven by the durations of ‘Not feeding’, ‘Ears forward’, ‘Ears asymmetric, ‘Vigilance’ (this variable comprised not feeding, turning toward the sound source, approach-investigation and scanning), and ‘Total response time’, with only ‘Ears forward’ and ‘Ears asymmetric’ among the ear postures contributing meaningfully (Table 2). This PC captured both initial reactions to stimuli (not feeding and turning toward the sound source, ears forward) and sustained alert behaviors (scanning, approach-investigation, total response duration). Therefore, we termed this component the ‘vigilance behavior’ component. Behavioral categories with eigenvalues below 1.0 that loaded onto separate PCs were discarded. The overall Kaiser-Meyer-Olkin measure of sampling adequacy (MSA) was 0.82, with estimates ranging from 0.77 to 0.94, confirming the data was suitable for PCA. We extracted regression scores of the PC for further analyses. All behavioral categories had positive loadings on the PC (cp. Table 2). Thus, higher positive regression scores translate to higher values of the behavioral categories summarized in this PC, and can be interpreted as longer durations of vigilance behaviors.
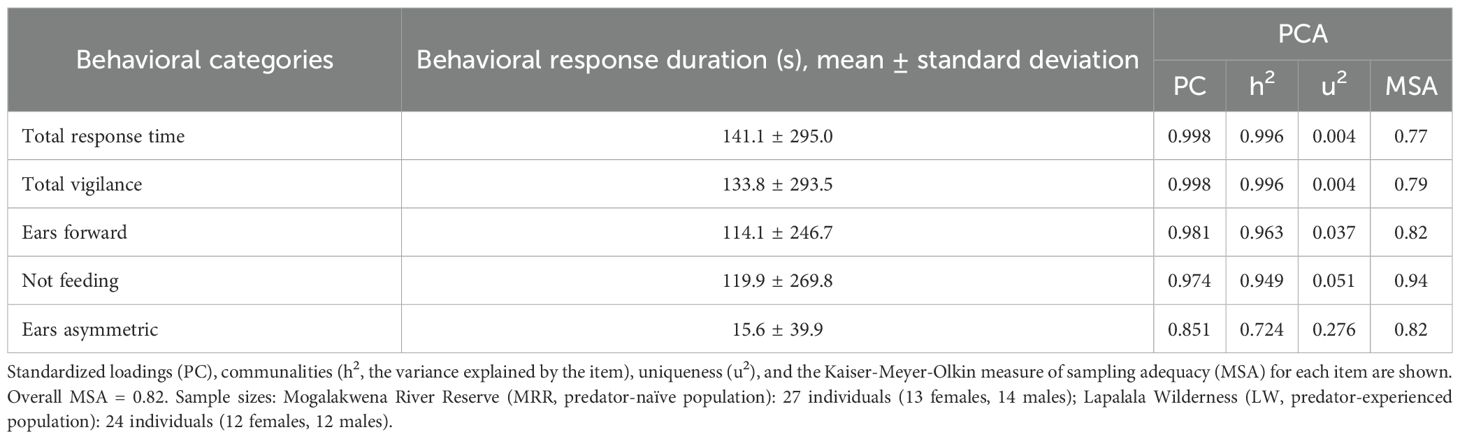
Table 2. Behavioral responses to playbacks (in seconds) were summarized using principal component analysis (PCA) with ‘varimax’ rotation.
We applied Linear Mixed Models (LMMs) with a Gaussian distribution and an identity link function to assess the effects of the different stimuli and various individuals and environmental factors on the ‘vigilance behavior’ component. We first assessed multicollinearity between fixed effects (Zuur et al., 2009) and detected high collinearity between stimulus type and mean HNR of the stimuli. Thus, we run two separate LMMs, fitted by maximum likelihood, either including stimulus type or mean HNR as a fixed effect. The identity of each test individual (giraffe ID) was included as a random effect to account for repeated measures of the same individuals when testing different stimuli. The first LMM examined the effects of stimulus type and reserve on the ‘vigilance behavior’ component, with giraffe ID as a random intercept. The full model included stimulus type, reserve, sex, playback order, and the interaction of stimulus type and reserve. The second LMM investigated the effects of acoustic-related features of the stimuli on the ‘vigilance behavior’ component. The full model contained the fixed effects HNR, reserve, sex, stimulus order, and the HNR × reserve interaction. Likelihood Ratio Tests (LRT) were used to compare the null models containing only the random effect and the intercept with full models (either containing stimulus type or mean HNR).
To ensure that vigilance responses were not affected by potential confounding variables, we tested for the effects of both playback distance and time of day. An additional LMM including distance (in meters) as a covariate and its interaction with reserve, while retaining the stimulus type × reserve interaction and giraffe ID as a random effect, confirmed that vigilance responses were not confounded by variation in playback distance (see Supplementary Table S4 in Supplementary File 1). Likewise, a Spearman rank correlation between vigilance component scores and time of day, calculated for lion trials only, revealed no significant relationship (ρ = –0.19, N = 51, p = 0.17). Time of day for each playback was extracted from the video time stamps and converted to decimal hours (e.g., 14:32 = 14.53 h) using the formula hour + minute/60 + second/3600 in Excel. As neither distance nor time of day influenced vigilance behavior, these factors were not included as covariates in the final models.
To investigate whether response speed to lion playbacks differed between lion-experienced and lion-naïve giraffes, we analyzed the latencies of vigilance-related behaviors following lion roar-grunt sequences. Because latency values were only available for trials in which a behavioral response occurred within 20s of playback onset, trials without a detectable response were excluded from the latency dataset. We selected the behavioral responses ‘Not feeding’, ‘Scanning’, and ‘Turn to’, which represent immediate vigilance responses and also contributed to the vigilance behavior component derived from the PCA. As sample sizes varied among behaviors and were in some cases too small to reliably fit mixed models, we used non-parametric pair-wise Mann-Whitney U tests to compare latencies between reserves.
Statistical analyses were conducted in R Studio Version 2024.12.1 + 563 (RStudio Team, 2025), with R Version 4.4.3 (R Core Team, 2025), with following packages: ‘psych’ (Revelle, 2024) for PCA, ‘lme4’ (Bates et al., 2015) for LMMs, ‘emmeans’ (Lenth, 2025) to conduct pairwise comparisons of interaction terms (post hoc tests, with Tukey-adjusted p values). The dataset generated and analyzed during this study is included in this article and its Supplementary File 2.
3 Results
3.1 Behavioral responses to stimulus type
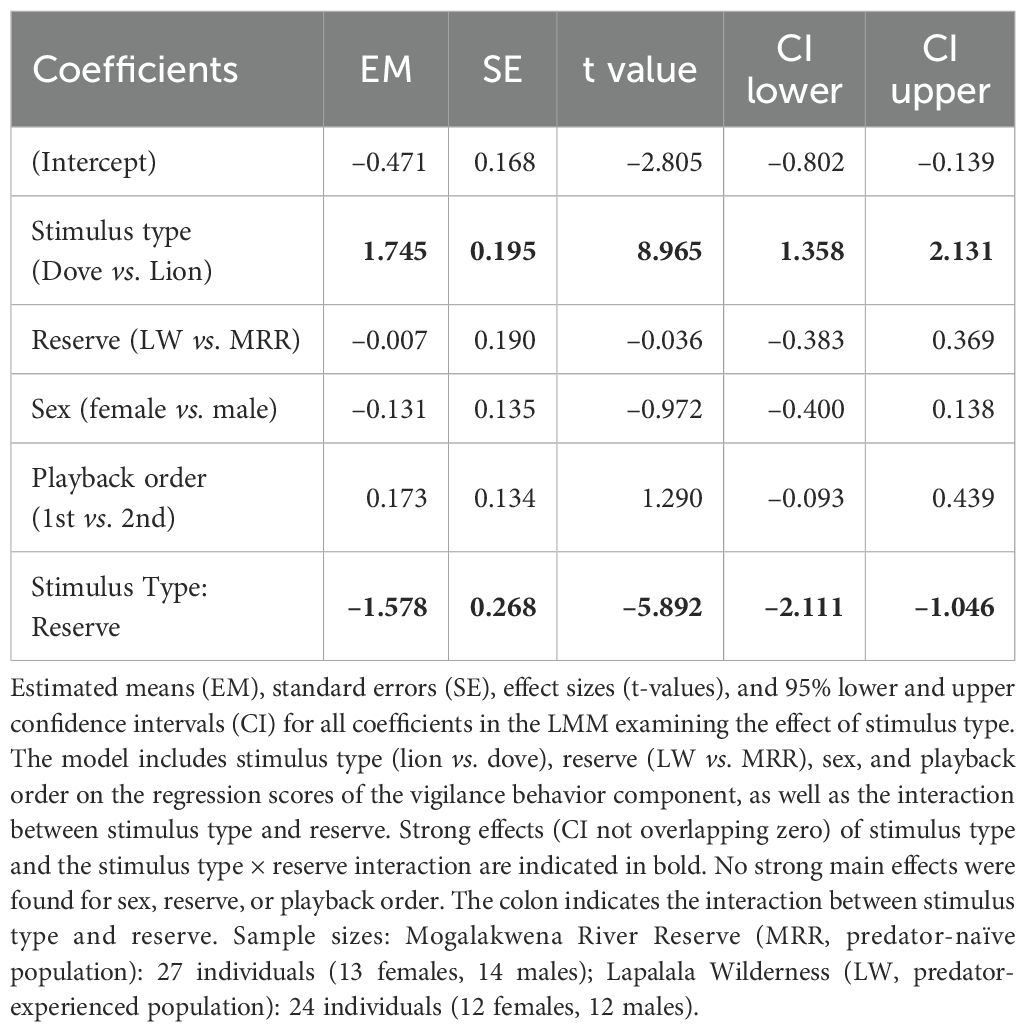
To examine how giraffes respond to predator cues, we fitted a LMM to PC scores representing the ‘vigilance behavior’ component, with descriptive statistics of the underlying behavioral measures provided in Supplementary Table S1. The model included fixed effects for stimulus type (lion vs. dove), reserve (LW vs. MRR), sex, playback order, and the interaction between stimulus type and reserve. The model significantly outperformed the null model containing only the random intercept (Likelihood ratio test: χ²(5) = 78.513, p < 0.0001).
There was a strong interaction between stimulus type and reserve (β = –1.578, SE = 0.268, t = –5.892, 95% CI = [–2.111, –1.046]), indicating that the effect of lion versus dove stimuli on vigilance behavior differed markedly between sites. Overall, giraffes showed stronger vigilance responses to lion than to dove calls (β = 1.745, SE = 0.195, t = 8.965, 95% CI = [1.358, 2.131]), an effect that was strongest among predator-experienced individuals. No meaningful effects were found for sex, order, or reserve alone. For a tabular overview of all coefficients, see Table 3.
Pairwise comparisons of estimated marginal means (Tukey-adjusted) confirmed that vigilance responses to lion calls were significantly stronger at the predator-experienced reserve (LW) than at the predator-naïve site (MRR; β = 1.585, SE = 0.196, t = 8.088, p < 0.0001), whereas responses to dove calls did not differ between reserves (Table 4). As shown in Figure 4A, vigilance-related PC scores were clearly elevated during lion trials at LW, while individuals at MRR (predator-naïve) showed little differentiation between the two stimuli.
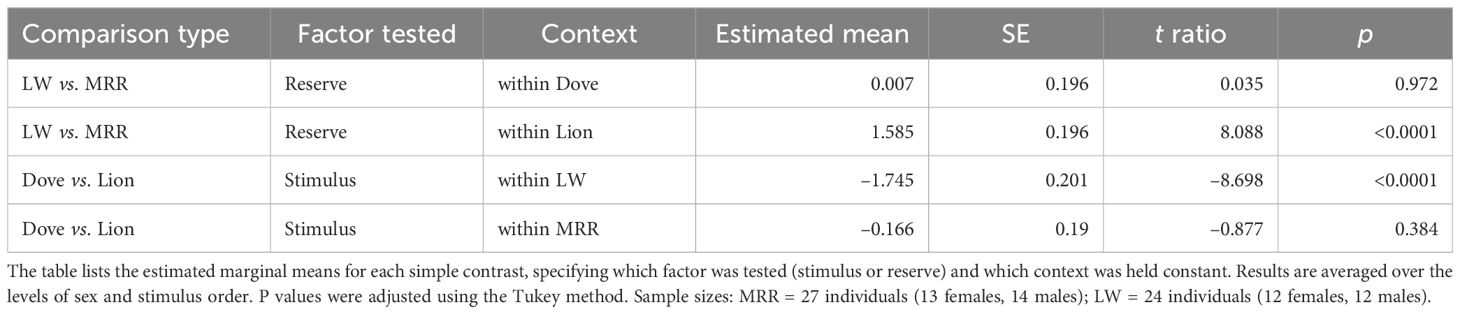
Table 4. Simple pairwise contrasts derived from the LMM for the interaction between stimulus type (Dove vs. Lion) and reserve (Lapalala Wilderness, LW vs. Mogalakwena River Reserve, MRR).
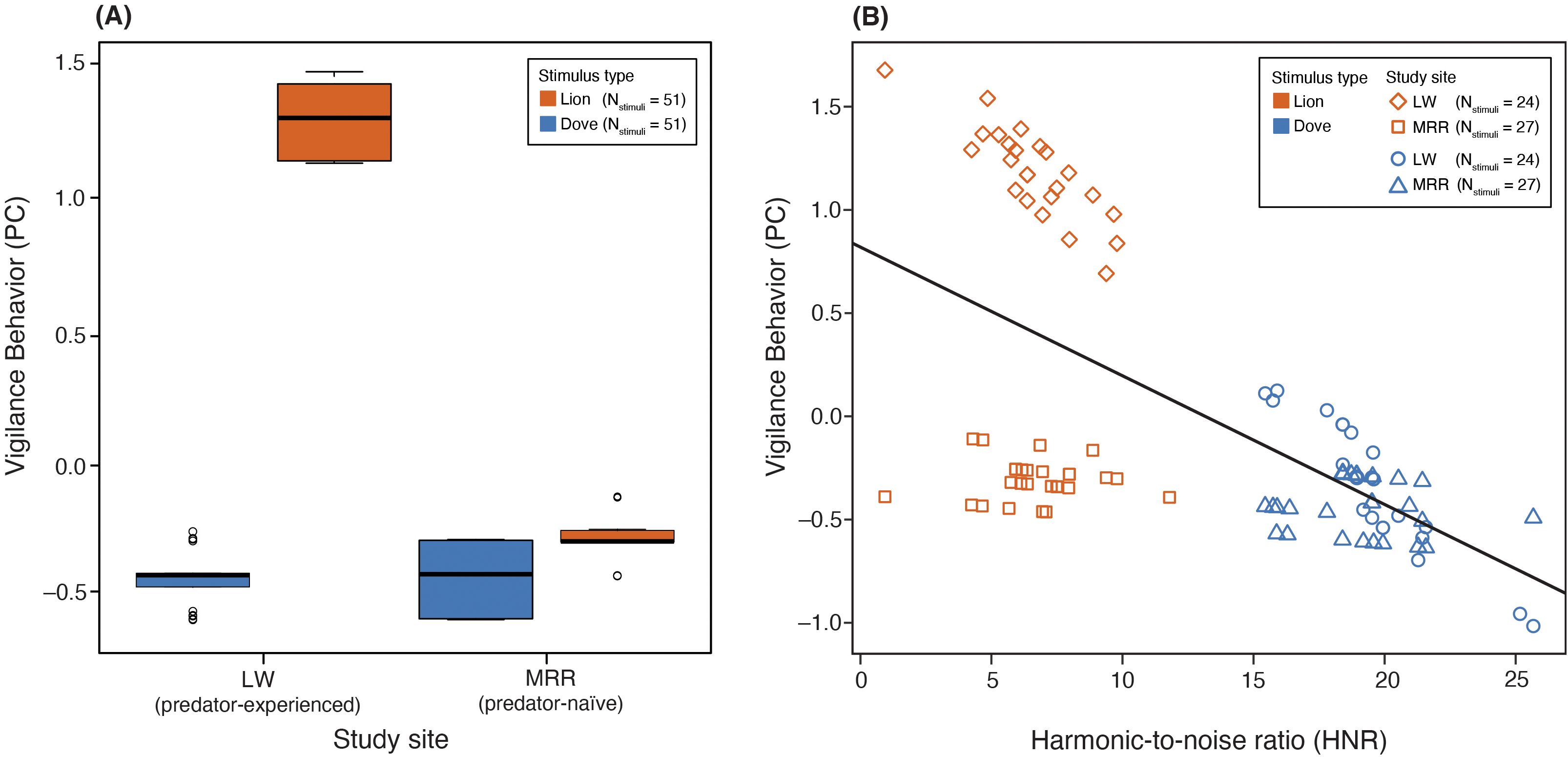
Figure 4. Vigilance responses of giraffes to playback stimuli across study sites. (A) PC scores derived from a principal component analysis of vigilance-related behaviors, comparing responses to lion roars and dove coos at two reserves: Lapalala Wilderness (LW, predator-experienced population) and Mogalakwena River Reserve (MRR, predator-naïve population). Higher PC scores indicate stronger vigilance responses. (B) Relationship between acoustic harshness (harmonic-to-noise ratio, HNR) and vigilance behavior (PC scores) across both stimulus types and study sites. Lower HNR values (i.e., harsher, noisier calls) were associated with higher PC scores, indicating stronger behavioral responses. Giraffes from Lapalala Wilderness (LW) showed generally higher PC responses across the HNR gradient than those from Mogalakwena River Reserve (MRR), suggesting experience-dependent modulation of cue sensitivity. Regression line: y = -0.06142x + 0.79626.
Representative video samples from both study sites demonstrate the differential responses at MRR, a predator-naïve male giraffe briefly orients toward the lion roar-grunt playback and shows no response to the dove coo call (Video S1), whereas at LW, a predator-experienced male giraffe sustains vigilance for a longer duration when exposed to the lion stimulus, also showing no response to the dove stimulus (Video S2; see also Supplementary File 1 for DOIs).
3.2 Influence of harmonic-to-noise ratio and ecological context
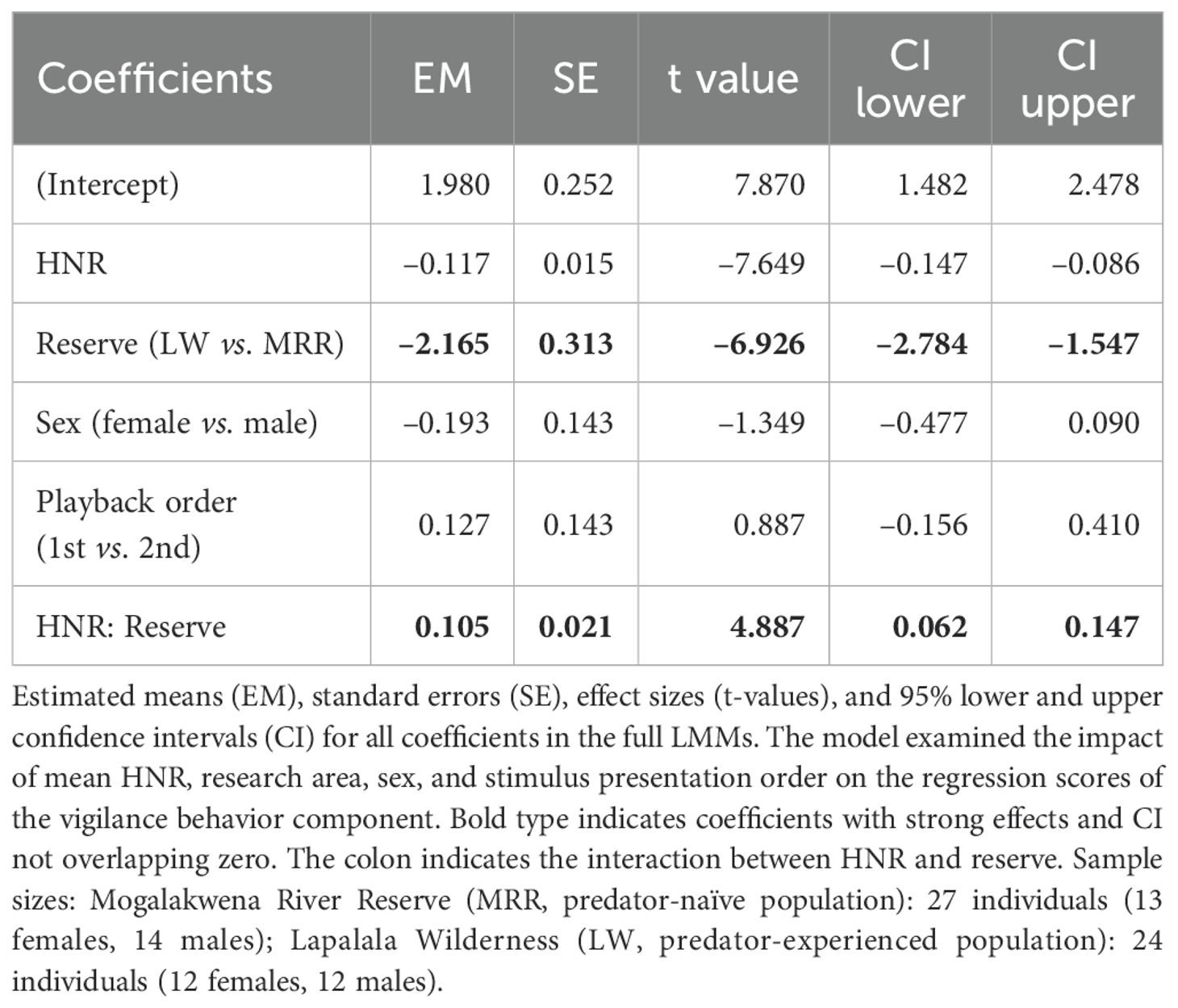
The second LMM assessed whether acoustic features of the stimuli, particularly the harmonic-to-noise ratio (HNR), influenced giraffe vigilance behavior, as represented by PC regression scores. The full model included HNR, reserve (LW vs. MRR), sex, stimulus order, and the HNR × reserve interaction as fixed effects, with giraffe ID as a random factor. Compared to the null model, the full model explained significantly more variation in vigilance behavior (LRT: χ²(5) = 65.818, p < 0.0001).
Analysis of fixed effects showed a pronounced negative main effect of HNR (β = –0.117, SE = 0.015, t = –7.649, CI = [–0.15, –0.09]), indicating that giraffes showed stronger vigilance responses to acoustically harsher stimuli. In our dataset, lion roar-grunt sequences had substantially lower HNR values (HNR = 6.6 ± 2.0) than dove coo calls (HNR = 19.3 ± 2.3), confirming that low HNR was associated with the predator stimulus. Giraffes at LW also exhibited greater vigilance compared to those at MRR (β = –2.165, SE = 0.313, t = –6.926, CI = [–2.78, –1.55]). Crucially, there was an interaction between HNR and reserve (β = –1.105, SE = 0.021, t = –4.887, CI = [–0.06, –0.15]) revealing that the effect of acoustic harshness varied by location (pairwise contrast: β = 0.807, SE = 0.147, t = 5.478, p < 0.0001). Sex and playback order had no influence on the vigilance behavior. For a tabular overview of all coefficients, see Table 5.
To further examine whether HNR effects simply reflected species identity, we tested for a relationship between HNR values of lion calls and giraffe vigilance responses (PC scores) within lion trials only (N = 51). This correlation was not significant (Spearman’s ρ = 0.013, p = 0.929), indicating that variation in HNR among lion calls did not predict vigilance responses. Thus, the effect of HNR on response strength primarily reflects the broad contrast between lion and dove calls rather than within-species variation.
Predator-experienced giraffes were more sensitive to changes in HNR, with calls of lower HNR eliciting stronger vigilance (reflected by higher regression scores of the PC) than in predator-naïve individuals (Figure 4B). These results suggest that both call structure and ecological context potentially shape antipredator responses in giraffes. Vigilance increased most strongly to stimuli with low harmonic-to-noise ratios, which in this case were lion roars. This pattern was especially pronounced in predator-experienced individuals, highlighting a potential role of experience or habitat-associated risk in modulating acoustic threat assessment.
3.3 Response latencies to lion playback stimuli
Mann-Whitney U tests revealed no significant differences in response latencies between the two reserves for any of the behaviors (Not feeding: U = 234.5, NLW=24, NMRR=26, p = 0.135; Scanning: U = 61.0, NLW=10, NMRR=8, p = 0.068; Turn to speaker: U = 240.5 NLW=24, NMRR=26, p = 0.168). These findings indicate that giraffes respond rapidly to predator sounds regardless of recent exposure to predators. This supports the idea that initial sensitivity to lion calls may be partly instinctive, although experience likely shapes the strength and duration of subsequent responses. For a tabular overview of the latency measures, see Supplementary Table S2 for LW and Supplementary Table S3 for MRR in the Supplementary File 1.
4 Discussion
Our study demonstrates that ecological experience strongly shapes how giraffes respond to predator vocalizations. Giraffes in a reserve where lions were reintroduced five years ago exhibited stronger and more persistent vigilance responses to lion roars than individuals from a predator-free reserve. While both populations oriented toward the lion calls, only predator-experienced giraffes maintained elevated vigilance, indicating that experience drives sustained antipredator responses. Importantly, the main effect of reserve was negligible, showing that the difference was not due to general reactivity between sites but was specifically driven by stronger responses to lion calls at the predator-experienced site. This is consistent with findings across other African ungulates, where impala (Aepyceros melampus) and wildebeest (Connochaetes taurinus) showed elevated vigilance under predation pressure (Hunter and Skinner, 1998), and zebra (Equus quagga), tsessebe (Damaliscus lunatus), wildebeest, and impala exhibited proactive antipredator responses to predator activity, with the strongest effects in impala and zebra (Bennitt et al., 2024).
Beyond the role of ecological experience, our results also point to the influence of acoustic structure. Giraffes at LW were more sensitive to changes in HNR, with lower HNR calls eliciting stronger vigilance than at MRR. Acoustic structure therefore modulated vigilance: vocalizations with lower HNR (which is typical of lion roars and grunts) elicited stronger responses, particularly in predator-experienced individuals. This suggests that acoustic harshness enhances salience and influences response strength when paired with ecological relevance. Our findings thus align with broader evidence that prey species use acoustic structure to evaluate predation risk (Hettena et al., 2014). This aligns with findings from other species: marmots respond more strongly to alarm calls with added noise (Blumstein and Récapet, 2009), and vigilance increases under uncertainty in high-risk contexts (Bell et al., 2009). Our results suggest a dual mechanism: a predisposed sensitivity to acoustically salient predator cues (as both populations responded equally fast), and an experience-based modulation of response persistence. This pattern is consistent with prior findings in a variety of taxa where predator recognition and response intensity are shaped by prior exposure (e.g. Deecke et al., 2002; Kindermann et al., 2009; Makin et al., 2019; Steindler et al., 2020; Sánchez-Vidal et al., 2024).
Giraffes across both sites oriented rapidly to the lion playback, suggesting that certain acoustic features act as generalized danger cues. Nonlinear phenomena are widespread in mammalian vocal repertoires and are often associated with perceptual harshness, heightened salience, and signals of arousal or threat (Wilden et al., 1998; Riede et al., 2005; Massenet et al., 2025). While detailed analyses of nonlinear phenomena in lion roars are scarce, lion vocalizations are characterized by low fundamental frequencies, dense formant spacing, and noisy segments that contribute to their harsh acoustic profile (Frey and Gebler, 2010). Moreover, lions preferentially roar under atmospheric conditions that enhance sound propagation, suggesting that the calls are adapted for long-distance detectability (Wijers et al., 2021). In our dataset, lion roars were also consistently associated with low HNR, further contributing to their perceptual harshness compared to control calls. Importantly, we found no evidence that variation in HNR within lion calls predicted vigilance responses. This supports the interpretation that HNR effects are not artifacts of within-species variation but instead reflect the broad acoustic contrast between lion roars (low HNR) and dove coos (high HNR). These features make lion roars particularly difficult to ignore. While our focus is on prey responses, it is worth noting that lion roars also function in intraspecific communication, especially in territorial defense and male-male competition (McComb et al., 1994; Grinnell and McComb, 2001; Grinnell, 2002), contexts in which high detectability is advantageous. Together, these features suggest that the structure of lion vocalizations acts as a generalized cue of danger in giraffes that are capable of triggering immediate vigilance responses, even in predator-naïve individuals. Such acoustic properties likely explain why giraffes across both populations responded rapidly to lion calls. However, while orientation latencies were uniformly short, vigilance duration varied substantially between populations.
Predator-experienced giraffes maintained heightened vigilance for longer. This suggests that although the onset response may reflect evolved perceptual tuning to harsh sound structures, the persistence of antipredator behavior is shaped by ecological experience. Since lions were recently introduced to LW, with confirmed giraffe predation events (Annear et al., 2023), giraffes in this reserve may have developed heightened sensitivity to lion roars through direct predator exposure. Similar decoupling of response onset and duration has been observed in other taxa. For instance, New Holland honeyeaters (Phylidonyris novaehollandiae) initiate flight within milliseconds of hearing an alarm call but adjust hiding duration based on call structure and perceived urgency (McLachlan and Magrath, 2020). Likewise, yellow-bellied marmots (Marmota flaviventris) responded more intensely to manipulated conspecific alarm calls containing added noisy elements than to normal calls. The added nonlinear element increased attentional engagement and reduced foraging (Blumstein and Récapet, 2009), which is consistent with broader evidence that noisy features enhance salience and delay habituation (Massenet et al., 2025). Comparable plasticity is seen in large mammals: moose (Alces alces), rapidly reacquired predator-specific responses after recolonization by wolves (Berger et al., 2001), and predator-experienced caribou (Rangifer tarandus caribou) display stronger and spatially specific avoidance than naïve conspecifics, further illustrating how ecological experience shapes the persistence and intensity of antipredator responses (Derguy et al., 2025). Together, these findings, alongside our results, align with theoretical frameworks suggesting that antipredator behavior integrates evolved sensitivity to acoustic salience with experience-based risk assessment (Lima and Dill, 1990; Stankowich and Blumstein, 2005; Fardell et al., 2020). Our results in giraffes provide a complementary example of this interplay, showing how immediate orientation to harsh acoustic cues is likely underpinned by evolved perceptual tuning, whereas the persistence of vigilance depends on ecological experience. Such behavioral plasticity is particularly relevant in rewilding contexts, where antipredator responses can degrade in predator-free environments and require reacquisition after predator return (Blumstein, 2006; Palmer et al., 2022). Comparable dynamics have recently been documented in African systems, where the reintroduction of lions has altered prey demography, vigilance, and habitat use (Annear et al., 2023). Our results also highlight how landscapes of fear are reconfigured following predator reintroduction, as prey reacquire appropriate risk responses and recalibrate vigilance behavior (Creel et al., 2014; Laundré et al., 2014).
While our study provides new insight into giraffe risk perception, several limitations warrant consideration. HNR was a useful proxy for acoustic harshness, but it does not capture all relevant features. The study also focused solely on adult individuals; including age or developmental stage could yield a more complete picture of antipredator plasticity. Although we cannot entirely exclude potential influences such as habitat structure, observer presence, or drone use, we consider these unlikely to explain the observed population-level differences. These factors were minimized through site-specific habituation protocols, pre-trial-only drone use, and structurally similar open savanna habitats across sites (see Methods for details). Potential confounding factors such as the time of day and the distance between the speaker and the focal giraffe were statistically tested and found to have no influence on vigilance responses (see Supplementary Table S4 and related analyses). This confirms that the observed population differences were not driven by experimental or contextual variation.
Future studies should examine additional acoustic parameters, such as specific nonlinear components or frequency modulation. Longitudinal or repeated-exposure studies could reveal how predator recognition develops, stabilizes, or declines over time. Future work should integrate behavioral, physiological, and cognitive measures. Playback studies in managed populations, combined with stress hormone assays or memory and discrimination tests, could reveal how giraffes encode, retain information on, and respond to predator cues. Moreover, it remains to be investigated how predator experience is acquired in giraffes, for example, whether through individual learning, repeated encounters, or social transmission from more experienced group members. In a conservation context, such work could inform predator conditioning protocols prior to reintroduction (Blumstein et al., 2019), ensuring that naïve giraffes can reacquire functional antipredator behaviors in changing landscapes of fear.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by Animal Research Ethics Committee of the University of the Free State (UFS-AED2022/0045/23). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
AB: Methodology, Visualization, Investigation, Data curation, Software, Conceptualization, Writing – original draft, Resources, Writing – review & editing, Validation, Project administration, Supervision, Funding acquisition, Formal analysis. GS: Formal analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded in whole or in part by the Austrian Science Fund (FWF) [grant-doi 10.55776/P36120]. For open access purposes, the author has applied a CC BY public copyright license to any author-accepted manuscript version arising from this submission.
Acknowledgments
We sincerely thank the management and staff of Mogalakwena River Reserve and Lapalala Wilderness for granting research access and supporting our fieldwork. We are especially grateful for their logistical assistance and warm hospitality throughout data collection. We thank the Mogalakwena Research Centre for generously sharing their giraffe ID-kit, which greatly facilitated individual identification, and the Lapalala Wilderness team for their continued support in the field. We also thank Timmy Moser (www.instagram.com/mosertimmy), Sebastian Dunn (https://dunnaudio.com), the Borror Laboratory of Bioacoustics (Borror Laboratory of Bioacoustics, 2024), Lisa Treiber (Treiber, 2023), and Lion Mountain Media (www.lionmountain.co.za) for kindly providing acoustic recordings of lion roar sequences. Finally, we thank the management teams of both reserves for permitting the use of the drone to assist in localizing giraffes during our research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1634218/full#supplementary-material
References
Anderson T. M., White S., Davis B., Erhardt R., Palmer M., Swanson A., et al. (2016). The spatial distribution of African savannah herbivores: species associations and habitat occupancy in a landscape context. Philos. Trans. R. Soc. B: Biol. Sci. 371, 20150314. doi: 10.1098/rstb.2015.0314
Annear E., Minnie L., Andrew K., and Kerley G. I. H. (2023). Can smaller predators expand their prey base through killing juveniles? The influence of prey demography and season on prey selection for cheetahs and lions. Oecologia 201, 649–660. doi: 10.1007/s00442-023-05335-8
Audacity Team (2023). Audacity: Free Audio Editor and Recorder [Computer program]. Available online at: https://www.audacityteam.org/ (Accessed April 24, 2023).
Baker M. C. and Logue D. M. (2007). A comparison of three noise reduction procedures applied to bird vocal signals. J. Field Ornithology 78, 240–253. doi: 10.1111/j.1557-9263.2007.00109.x
Baotic A. and Szipl G. (2025). Predator experience enhances giraffe vigilance to oxpecker alarm calls. BMC Biol. 23 (1), 304. doi: 10.1186/s12915-025-02395-5
Bates D., Mächler M., Bolker B., and Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Beauchamp G. (2015). “Vigilance, alarm calling, pursuit deterrence, and predation inspection,” in Escaping from Predators: An Integrative View of Escape Decisions. Eds. Blumstein D. T. and Cooper J. W. E. (Cambridge University Press, Cambridge), 265–286.
Bell M. B. V., Radford A. N., Rose R., Wade H. M., and Ridley A. R. (2009). The value of constant surveillance in a risky environment. Proc. R. Soc. B: Biol. Sci. 276, 2997–3005. doi: 10.1098/rspb.2009.0276
Benedict L., Hardt B., and Dargis L. (2021). Form and function predict acoustic transmission properties of the songs of male and female canyon wrens. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.722967
Bennitt E., Bartlam-Brooks H. L. A., Hubel T. Y., Jordan N. R., McNutt J. W., and Wilson A. M. (2024). Proactive cursorial and ambush predation risk avoidance in four African herbivore species. Ecol. Evol. 14, e11529. doi: 10.1002/ece3.11529
Berger J., Swenson J. E., and Persson I.-L. (2001). Recolonizing carnivores and naïve prey: conservation lessons from Pleistocene extinctions. Science 291, 1036–1039. doi: 10.1126/science.1056466
Blumstein D. T. (2006). The multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethology 112, 209–217. doi: 10.1111/j.1439-0310.2006.01209.x
Blumstein D. T., Letnic M., and Moseby K. E. (2019). In situ predator conditioning of naïve prey prior to reintroduction. Philos. Trans. R. Soc. B: Biol. Sci. 374, 20180058. doi: 10.1098/rstb.2018.0058
Blumstein D. T. and Récapet C. (2009). The sound of arousal: The addition of novel non-linearities Increases responsiveness in marmot alarm calls. Ethology 115, 1074–1081. doi: 10.1111/j.1439-0310.2009.01691.x
Boersma P. and Weenink D. (2025). Praat: doing phonetics by computer [Computer program]. Available online at: http://www.praat.org/ (Accessed January 27, 2025).
Borror Laboratory of Bioacoustics (2024). (Columbus, OH: The Ohio State University). CC BY-NC-ND 4.0.
Briefer E. F. (2012). Vocal expression of emotions in mammals: mechanisms of production and evidence. J. Zoology 288, 1–20. doi: 10.1111/j.1469-7998.2012.00920.x
Brown G. E. and Chivers D. P. (2005). “Learning as an Adaptive Response to Predation,” in Ecology Of Predator-Prey Interactions. Eds. Barbosa P. and Castellanos I. (Oxford University Press).
Cameron E. Z. and du Toit J. T. (2005). Social influences on vigilance behaviour in giraffes, Giraffa camelopardalis. Anim. Behav. 69, 1337–1344. doi: 10.1016/j.anbehav.2004.08.015
Chapron G., Kaczensky P., Linnell J. D. C., von Arx M., Huber D., Andrén H., et al. (2014). Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517–1519. doi: 10.1126/science.1257553
Coimbra J. P., Hart N. S., Collin S. P., and Manger P. R. (2013). Scene from above: Retinal ganglion cell topography and spatial resolving power in the giraffe (Giraffa camelopardalis). J. Comp. Neurol. 521, 2042–2057. doi: 10.1002/cne.23271
Creel S., Schuette P., and Christianson D. (2014). Effects of predation risk on group size, vigilance, and foraging behavior in an African ungulate community. Behav. Ecol. 25, 773–784. doi: 10.1093/beheco/aru050
Deecke V. B., Slater P. J. B., and Ford J. K. B. (2002). Selective habituation shapes acoustic predator recognition in harbour seals. Nature 420, 171–173. doi: 10.1038/nature01030
Derguy L., Leblond M., and St-Laurent M.-H. (2025). Living in fear: How experience shapes caribou responses to predation risk. Ecosphere 16, e70155. doi: 10.1002/ecs2.70155
Dixon-MacCallum G. P., Rich J. L., Lloyd N., Blumstein D. T., and Moehrenschlager A. (2021). Loss of predator discrimination by critically endangered Vancouver island marmots within five generations of breeding for release. Front. Conserv. Sci. 2. doi: 10.3389/fcosc.2021.718562
Fardell L. L., Pavey C. R., and Dickman C. R. (2020). Fear and stressing in predator–prey ecology: considering the twin stressors of predators and people on mammals. PeerJ 8, e9104. doi: 10.7717/peerj.9104
Frey R. and Gebler A. (2010). “Mechanisms and evolution of roaring-like vocalization in mammals,” in Handbook of Behavioral Neuroscience. Ed. Stefan M. B. (Elsevier), 439–450.
Goodale E., Sridhar H., Sieving K. E., Bangal P., Colorado Z. G. J., Farine D. R., et al. (2020). Mixed company: a framework for understanding the composition and organization of mixed-species animal groups. Biol. Rev. 95, 889–910. doi: 10.1111/brv.12591
Grinnell J. (2002). Modes of cooperation during territorial defense by African lions. Hum. Nat. 13, 85–104. doi: 10.1007/s12110-002-1015-4
Grinnell J. and McComb K. (2001). Roaring and social communication in African lions: the limitations imposed by listeners. Anim. Behav. 62, 93–98. doi: 10.1006/anbe.2001.1735
Hayward M. W. and Kerley G. I. H. (2005). Prey preferences of the lion (Panthera leo). J. Zoology 267, 309–322. doi: 10.1017/S0952836905007508
Hayward M. W. and Somers M. J. (2009). “Reintroduction of Top-Order Predators: Using Science to Restore One of the Drivers of Biodiversity,” in Reintroduction of Top-Order Predators, 1–9.
Hettena A. M., Munoz N., and Blumstein D. T. (2014). Prey responses to predator's sounds: a review and empirical study. Ethology 120, 427–452. doi: 10.1111/eth.12219
Hunter L. T. B., Pretorius K., Carlisle L. C., Rickelton M., Walker C., Slotow R., et al. (2007). Restoring lions Panthera leo to northern KwaZulu-Natal, South Africa: short-term biological and technical success but equivocal long-term conservation. Oryx 41, 196–204. doi: 10.1017/S003060530700172X
Hunter L. T. B. and Skinner J. D. (1998). Vigilance behaviour in African ungulates: The role of predation pressure. Behaviour 135, 195–211. doi: 10.1163/156853998793066320
Kindermann T., Siemers B., and Fendt M. (2009). Innate or learned acoustic recognition of avian predators in rodents? J. Exp. Biol. 212, 506–513. doi: 10.1242/jeb.024174
Langman V. A. (1977). Cow-calf relationships in giraffe (Giraffa camelopardalis giraffa). Z. für Tierpsychologie 43, 264–286. doi: 10.1111/j.1439-0310.1977.tb00074.x
Laundré J. W., Hernández L., Medina P. L., Campanella A., López-Portillo J., González-Romero A., et al. (2014). The landscape of fear: the missing link to understand top-down and bottom-up controls of prey abundance? Ecology 95, 1141–1152. doi: 10.1890/13-1083.1
Lenth R. (2025). Emmeans: Estimated Marginal Means, Aka Least-Squares Means (R package). Available online at: https://CRAN.R-project.org/package=emmeans (Accessed March 3, 2025).
Lima S. L. and Dill L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zoology 68, 619–640. doi: 10.1139/z90-092
Magrath R. D., Haff T. M., Fallow P. M., and Radford A. N. (2015). Eavesdropping on heterospecific alarm calls: from mechanisms to consequences. Biol. Rev. 90, 560–586. doi: 10.1111/brv.12122
Magrath R. D., Haff T. M., and Igic B. (2020). “Interspecific communication: Gaining information from heterospecific alarm calls,” in Coding Strategies in Vertebrate Acoustic Communication. Eds. Aubin T. and Mathevon N. (Springer International Publishing, Cham), 287–314.
Makin D. F., Chamaillé-Jammes S., and Shrader A. M. (2019). Alarm calls or predator calls: which elicit stronger responses in ungulate communities living with and without lions? Oecologia 190, 25–35. doi: 10.1007/s00442-019-04391-3
Marealle W., Holmern T., and Røskaft E. (2020). Factors affecting group size and vigilance behaviour of Maasai giraffe (Giraffa camelopardalis tippelskirchi) on the Serengeti-Ngorongoro ecosystem, Tanzania. East Afr. J. Environ. Natural Resour. 2, 14–23. doi: 10.37284/eajenr.2.1.133
Massenet M., Mathevon N., Anikin A., Briefer E. F., Fitch W. T., and Reby D. (2025). Nonlinear phenomena in vertebrate vocalizations: mechanisms and communicative functions. Philos. Trans. R. Soc. B: Biol. Sci. 380, 20240002. doi: 10.1098/rstb.2024.0002
McComb K., Packer C., and Pusey A. (1994). Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim. Behav. 47, 379–387. doi: 10.1006/anbe.1994.1052
McLachlan J. R. and Magrath R. D. (2020). Speedy revelations: how alarm calls can convey rapid, reliable information about urgent danger. Proc. R. Soc. B: Biol. Sci. 287, 20192772. doi: 10.1098/rspb.2019.2772
Miele V., Dussert G., Spataro B., Chamaillé-Jammes S., Allainé D., and Bonenfant C. (2021). Revisiting animal photo-identification using deep metric learning and network analysis. Methods Ecol. Evol. 12 (5), 863–873. doi: 10.1111/2041-210X.13577
Morton E. S. (1977). On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 111, 855–869. doi: 10.1086/283219
Muller Z. (2018). Population structure of giraffes is affected by management in the Great Rift Valley, Kenya. PloS One 13, e0189678. doi: 10.1371/journal.pone.0189678
Palmer M. S., Gaynor K. M., Becker J. A., Abraham J. O., Mumma M. A., and Pringle R. M. (2022). Dynamic landscapes of fear: understanding spatiotemporal risk. Trends Ecol. Evol. 37, 911–925. doi: 10.1016/j.tree.2022.06.007
Palmer M. S. and Gross A. (2018). Eavesdropping in an African large mammal community: antipredator responses vary according to signaller reliability. Anim. Behav. 137, 1–9. doi: 10.1016/j.anbehav.2017.12.018
Revelle W. (2024). psych: procedures for psychological, psychometric, and personality research. R package version 2.4.12, Northwestern University, Evanston. Available online at: https://CRAN.r-project.org/package=psych (Accessed March 3, 2025)
Riede T., Mitchell B. R., Tokuda I., and Owren M. J. (2005). Characterizing noise in nonhuman vocalizations: Acoustic analysis and human perception of barks by coyotes and dogs. J. Acoustical Soc. America 118, 514–522. doi: 10.1121/1.1928748
RStudio Team (2025). RStudio: Integrated Development Environment for R. Version 2024.12.1 + 563. (Accessed March 3, 2025).
R Core Team (2025). R: A language and environment for statistical computing 4.4.3. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/ (Accessed March 3, 2025).
Sánchez-Vidal R. O., Rangel-Negrín A., Briseño-Jaramillo M., Sosa-López J. R., and Dias P. A. D. (2024). Acoustic recognition of predators by mantled howler monkeys (Alouatta palliata): a playback experiment with naïve and experienced subjects. Am. J. Biol. Anthropology 185, e25013. doi: 10.1002/ajpa.25013
Shorrocks B. and Croft D. P. (2009). Necks and networks: a preliminary study of population structure in the reticulated giraffe (Giraffa camelopardalis reticulata de Winston). Afr. J. Ecol. 47, 374–381. doi: 10.1111/j.1365-2028.2008.00984.x
Sih A., Bolnick D. I., Luttbeg B., Orrock J. L., Peacor S. D., Pintor L. M., et al. (2010). Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119, 610–621. doi: 10.1111/j.1600-0706.2009.18039.x
Slabbekoorn H., Kort S. d., and Cate C. T. (1999). Comparative analysis of perch-coo vocalizations in Streptopelia doves. Auk 116, 737–748. doi: 10.2307/4089334
Stankowich T. and Blumstein D. T. (2005). Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B: Biol. Sci. 272, 2627–2634. doi: 10.1098/rspb.2005.3251
Steindler L. A., Blumstein D. T., West R., Moseby K. E., and Letnic M. (2020). Exposure to a novel predator induces visual predator recognition by naïve prey. Behav. Ecol. Sociobiology 74, 102. doi: 10.1007/s00265-020-02884-3
Templeton C. N., Greene E., and Davis K. (2005). Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937. doi: 10.1126/science.1108841
Treiber L. (2023). Vocal individuality of female African lions (Panthera leo) (Austria: Department of Behavioral and Cognitive Biology, University of Vienna). [Master’s thesis].
Valeix M., Fritz H., Loveridge A. J., Davidson Z., Hunt J. E., Murindagomo F., et al. (2009a). Does the risk of encountering lions influence African herbivore behaviour at waterholes? Behav. Ecol. Sociobiology 63, 1483–1494. doi: 10.1007/s00265-009-0760-3
Valeix M., Loveridge A. J., Chamaillé-Jammes S., Davidson Z., Murindagomo F., Fritz H., et al. (2009b). Behavioral adjustments of African herbivores to predation risk by lions: Spatiotemporal variations influence habitat use. Ecology 90, 23–30. doi: 10.1890/08-0606.1
Wijers M., Trethowan P., du Preez B., Chamaillé-Jammes S., Loveridge A. J., Macdonald D. W., et al. (2021). The influence of spatial features and atmospheric conditions on African lion vocal behaviour. Anim. Behav. 174, 63–76. doi: 10.1016/j.anbehav.2021.01.027
Wilden I., Herzel H., Peters G., and Tembrock G. (1998). Subharmonics, biphontion and deterministic chaos in mammal vocalization. Bioacoustics 9, 171–176. doi: 10.1080/09524622.1998.9753394
Williams E. M. (2016). Giraffe stature and neck elongation: vigilance as an evolutionary mechanism. Biology 5 (3), 35. doi: 10.3390/biology5030035
Keywords: giraffe, lion, predator-prey dynamics, behavioral ecology, landscape of fear, bioacoustics, playback, vigilance
Citation: Baotic A and Szipl G (2025) Learning to fear: predator recognition in giraffes is shaped by evolved sensitivity and ecological experience. Front. Ecol. Evol. 13:1634218. doi: 10.3389/fevo.2025.1634218
Received: 23 May 2025; Accepted: 13 October 2025;
Published: 03 November 2025.
Edited by:
Donald Price, University of Nevada, United StatesReviewed by:
Aisha C. Bründl, Game and Wildlife Conservation Trust (GWCT), United KingdomJuliana M. Berbert, Federal University of ABC, Brazil
Copyright © 2025 Baotic and Szipl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anton Baotic, Y29udGFjdEBhbnRvbmJhb3RpYy5jb20=
 Anton Baotic
Anton Baotic Georgine Szipl2
Georgine Szipl2