- 1State Key Laboratory of Water Cycle and Water Security in River Basin, China Institute of Water Resources and Hydropower Research, Beijing, China
- 2College of Ecology, Resources and Environment, Dezhou University, Dezhou, China
- 3Department of Water Ecology and Environment, China Institute of Water Resources and Hydropower Research, Beijing, China
Identifying keystone species and investigating their ecological regulatory role will help to prioritize important species and gain a better understanding of community stability mechanisms. In this study, macroinvertebrates were sampled from Chishui River (CSH, an undammed river) and Heishui River (HSH, a dammed river) in April 2018, 2019, and 2020. Macroinvertebrate networks were constructed to identify keystone species and reveal their ecological regulatory role on community stability. The results showed that the loss of high-connectivity species had a greater impact on macroinvertebrate community stability than the loss of high-biomass and high-density species, which indicated that high-connectivity species with low abundance were keystone species. Moreover, these were primarily composed of less abundant species. The functional feeding traits of keystone species were dominated by predators in the undammed river and prey (i.e., collector-gatherers) in the dammed river, which suggested that the construction of dams transformed the functional feeding groups of keystone species. In addition, after the loss of keystone species, the decline rates of the half of the robustness (R50), survival area (SA), and network connectivity robustness (CR) in the dam-constructed reaches were higher than those in other reaches. This result demonstrated that the construction of dams may reduce the resistance of macroinvertebrate communities to keystone species loss. This study provides an important scientific basis for conserving aquatic life and maintaining the structural and functional stability of river ecosystems.
1 Introduction
Ecosystem worldwide are being reshaped because of species losses and gains, brought on by anthropogenic disturbances, non-indigenous species and climate change (Ståhl et al., 2025). It is increasingly important to understand the influence of species losses on ecosystem stability to elucidate the ongoing dramatic decline of biodiversity. The loss of a single species may have ripple effect on the community, causing more species to be affected or even go locally extinct (Estrada, 2007; Wang and Tang, 2019; Wang et al., 2024). Keystone species play a decisive role in the stability of ecosystems, as they can affect the structure and function of ecosystems in a manner disproportionate to their abundance, and a small change in them may have a larger effect on the structure and function of communities (Libralato et al., 2006; Ortiz et al., 2017; Power et al., 1996). Therefore, identifying and protecting keystone species can help to maintain the community and even increase biodiversity. To date, most studies on keystone species have focused on microbial communities (Banerjee et al., 2018; Chao et al., 2024), while empirical evidence about keystone species of macroinvertebrates in river ecosystems remains largely unknown but has been suggested to be as important for understanding ecosystem stability (Brown and Lawson, 2010; Encalada et al., 2010; Simeone et al., 2021). For instance, mussel filtration and biodeposition may be associated with habitat quality for other invertebrates by reducing phytoplankton density in the water column and increasing inputs of sediment organic matter, which indicates that freshwater mussels play an important role in sustaining ecosystem function (Simeone et al., 2021). Thus, identification of keystone species can increase predict ability of the persistence of species-rich communities and improve the efficiency of management efforts for species with high extinction risk (Zhu et al., 2024).
Keystone species correlated with other species through interspecific feeding relationships, competitive relationships, and symbiotic relationships (Wang et al., 2024). Based on this attribute, a quantitative description of the closeness of connections between species is crucial for identifying keystone species. In a community, some species have high centrality (central positions) in the community and connect to most other species, while many other species have low centrality (peripheral positions). In general, the top ranked species by centrality (high-connectivity species) are often considered as the keystone species in the community (Mello et al., 2015; Zhu et al., 2024). The positional importance of these central species in a community is illustrated by a faster breakdown of the whole network structure and stability when species are selectively removed on the order of higher to lower centrality than when species are randomly removed from a community (Albert et al., 2000; Dunne et al., 2002; Stouffer et al., 2012; Zhu et al., 2024). For the organisms’ symbioses, a growing number of studies make efforts to explore the underlying drivers of species specialization (Su et al., 2024; Wang et al., 2024). Many studies demonstrated that the centrality was regulated by abundance (Hervías-Parejo et al., 2020; Zhu et al., 2024), functional traits (Gómez and Perfectti, 2012), and phylogenetic relatedness (Dallas et al., 2019). By analyzing benthic fishes and macroinvertebrates, studies have illustrated that central species can be regulated by abundances (Zhu et al., 2024) and feeding relationships (Su et al., 2024). Moreover, besides the central species, the dominant species also play an important role in the resistance and recovery of grassland communities when facing environmental disturbance (Wang et al., 2025). However, the keystone species that maintain macroinvertebrate communities and their characteristics remain unclear, necessitating further investigation.
Ecological network analysis, a systems-level approach useful for integrating many layers of data, has proven to be an effective method for generalizing interspecific interaction relationships (Robinson et al., 2022). This type of mechanistic approach linking community composition and ecosystem functioning is central to enhancing our understanding of interspecific relationships (Chao et al., 2024; Wang et al., 2024). Ecological networks could represent the flow of energy through a network of interacting species, where nodes represent species and links represent trophic links and energy flow (Chao et al., 2024). Nodes can be removed to simulate species losses in the natural ecosystems (Keyes et al., 2021; Wang and Tang, 2019). Network robustness analysis is an effective approach to studying stability in interspecific relationships-the effect of species losses on the ability of communities to resist secondary extinctions (Dunne et al., 2002; Landi et al., 2018; Ståhl et al., 2025). Network properties are often used as proxies to gain insight into the ecological robustness (Dunne et al., 2002; Ståhl et al., 2025). Connectance is an important topological parameter, which is also central in the ongoing debate on the relationship between diversity, complexity and stability (Landi et al., 2018). Although previous studies have shown that connectance is positively correlated with robustness (Dunne et al., 2002; Bellingeri and Cassi, 2018), the relationship between the two remains controversial, and more recent studies have found no clear patterns between them (Canning and Death, 2018). Interaction strengths between species were found better at predicting robustness, relative ascendency and centrality takes the distribution of links into account (Canning and Death, 2018; Banerjee et al., 2018). These metrics are based on the position of species in the trophic structure and reflect the degree of functional redundancy in the ecosystem. Removing species with strong connectivity may reduce network functioning (Bellingeri et al., 2019).
Rivers are recognized as highly dynamic ecosystems that exhibit considerable variations in morphology, substrate, and velocity. These variations collectively contribute to the diverse habitats populated by aquatic organisms (Szoszkiewicz et al., 2018; Zheng and Yin, 2023; Li et al., 2024). However, the construction of dams has significantly hindered the longitudinal continuity of rivers, resulting in low-heterogeneity habitats for various aquatic organisms (e.g. fishes and macroinvertebrates). In rivers, macroinvertebrates are important aquatic organisms. Their high diversity and wide range of life-history traits and ecological requirements have rendered them extensively utilized as efficacious indicators for the changes in river ecosystems (Liu et al., 2021; Zhang et al., 2018; Ma et al., 2024). Furthermore, the drift behavior of macroinvertebrates constitutes a significant biological adaptation mechanism, which could effectively facilitate the expansion of population distribution and enhance the complexity of interspecific relationships (Shi et al., 2020). However, these drift behaviors could be impeded by the construction of dams, leading to changes in composition and structure of macroinvertebrates, as well as alterations in keystone species. These changes could subsequently lead to the fragmentation of the biological communities and decrease the complexity of interspecific interactions. Moreover, the macroinvertebrate community acts as a pivotal intermediary in the energy flow of the aquatic food webs (Encalada et al., 2010; Del Campo et al., 2025), exerting a direct influence on both the foundational components of the food chain (e.g. attached algae) and the predators (e.g. fish) through intricate predator-prey interactions. For example, in the waters of the Changdao Archipelago, Alpheus japonicus and Oratosquilla oratoria were identified as keystone species, loss of these species led to a significant decrease in the number and robustness of feeding relationships within food web (Su et al., 2024). Moreover, in the South Carolina (USA) stream, changes in heterogeneous habitats affected the keystone species dynamics, and these keystone species acted as a community-wide perturbation in low-heterogeneity habitats (Brown and Lawson, 2010). Thus, the change of macroinvertebrate composition and structure under the influence of dams can ultimately affect the stability of river ecosystems.
The main objective of this study is to quantify how the stability of river ecosystems may be influenced and predicted by keystone species extinction. To address our main objective, we used the collected quantitative data on macroinvertebrate communities to construct ecological networks in undammed and dammed rivers. We asked the following questions: 1) how does network robustness differ with the removal of different species, 2) which species best predict the robustness in these macroinvertebrate community networks, 3) how does macroinvertebrate network robustness differ in undammed and dammed river? We focused on deletion sequences ordered from highest to lowest biomass, density, and connectivity, for which we hypothesize: 1) that the removal of high-biomass and high-density species has less impact on network robustness than that of high-connectivity species; 2) that high-connectivity species may best predict the network robustness of macroinvertebrate communities; 3) that macroinvertebrate networks in dammed rivers are less robust than those in undammed river. This study will help us understand the stability of riverine macroinvertebrate communities to species loss and other dam-related disturbances.
2 Materials and methods
2.1 Study area
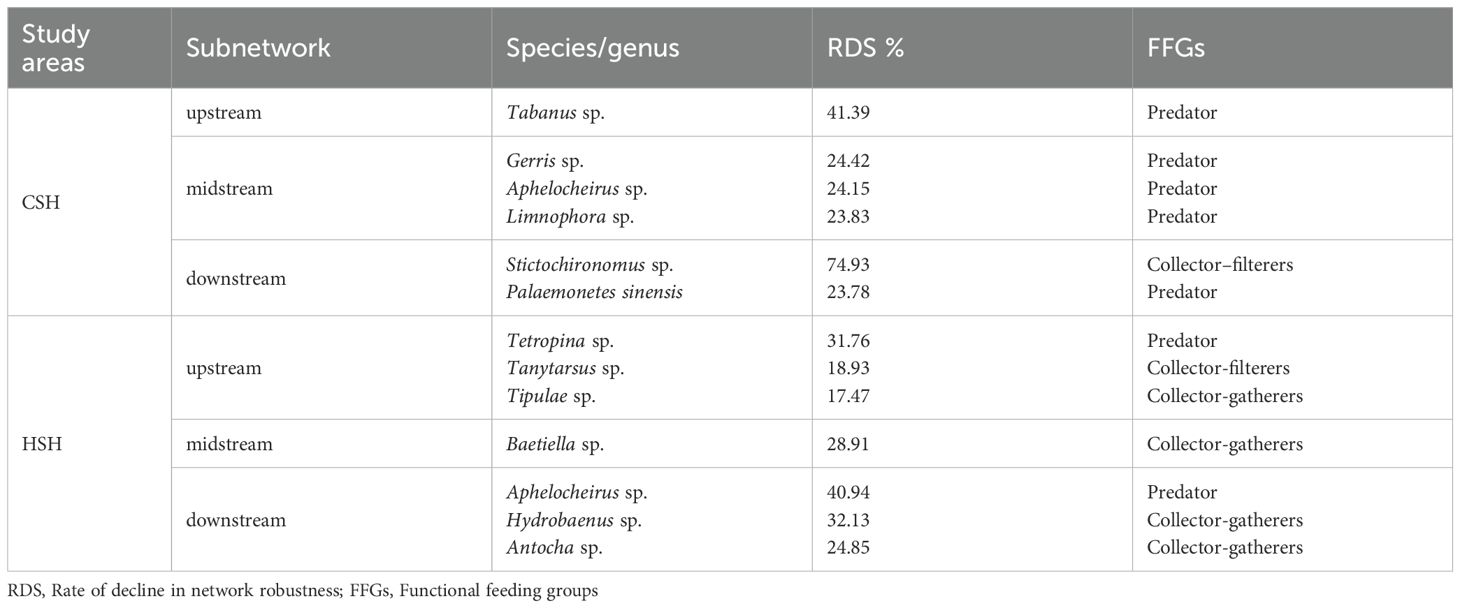
This study was conducted in the Chishui River (CSH) and Heishui River (HSH), located in the subtropical monsoon region in southwestern China. CSH (27°20′-28°50′N, 104°45′-106°51′E) is an undammed river in the upper Yangtze River, which is less disturbed by human activities. Thus, CSH is an important part of the Rare and Endemic Fishes Nature Reserve of the Yangtze River (Jiang et al., 2017). The drainage area and mainstream length of CSH are 20440 km2 and 436.5 km, respectively. Under the influence of monsoon climate, approximately 60% of the annual precipitation occurs from June to September, and approximately 4% occurs from December to January (Chi et al., 2017; Liu et al., 2008). The altitude of the head stream ranges from 1000 m to 1760 m, and the altitude of mid-downstream ranges from 500 m to 1000 m. The annual precipitation of the headstream and mid-downstream is approximately 900 ~ 1000 mm and 1000 ~ 1500 mm, respectively (Chi et al., 2017; Jiang et al., 2011). The complex of surface geology, climate and landscape conditions, as well as the diversity of habitat types provides diverse refuge for macroinvertebrates (Jiang et al., 2017).
HSH (26°48′N-28°7′, 102°20′-102°53′E) is a tributary of the Jingshajiang River, located within the Baihetan Reservoir (Song et al., 2018; Liu et al., 2008; Lv et al., 2020). The basin area is 3591 km2, with a total length of 173.3 km. The natural fall between the upstream and downstream reaches is 1931m (Song et al., 2018; Liu et al., 2008; Lv et al., 2020). The data from Ningnan Hydrological Station (2007~2015) indicated that the measured average discharge of HSH was 68.31 m3/s and the average runoff depth was 701.87 mm. Approximately 72.66% and 27.34% of runoff occur in the wet season and the dry season, respectively (Song et al., 2018; Liu et al., 2008; Lv et al., 2020). Four cascade hydropower stations have been built in the midstream reaches of HSH due to the high availability of hydropower resources (Song et al., 2018; Liu et al., 2008). Moreover, its unique geographical location and habitat suitability have made HSH an alternative habitat for fish in the downstream of Jinsha River, and a hotspot area for lotic biodiversity conservation (Lv et al., 2020).
2.2 Field sampling
Macroinvertebrates were investigated from 23 sites at CSH and 23 sites at HSH, respectively (Figure 1). Sample collection was conducted in April 2018, 2019, and 2020. A Surber sampler (30×30 cm, 500 μm mesh) was used to collect macroinvertebrates. We took three replicate quantitative samples at each sampling site. Samples were taken at the center of rivers and close to the riverbank in the upstream reaches, and samples were taken at easily accessible sites (most at the banks of the river) at mid-downstream reaches. All sampling sites encompassed the most representative benthic microhabitats, including riffles, pools, and the area covered by aquatic macrophytes (including lotic and lentic) (Qu et al., 2019; Zhang et al., 2018).
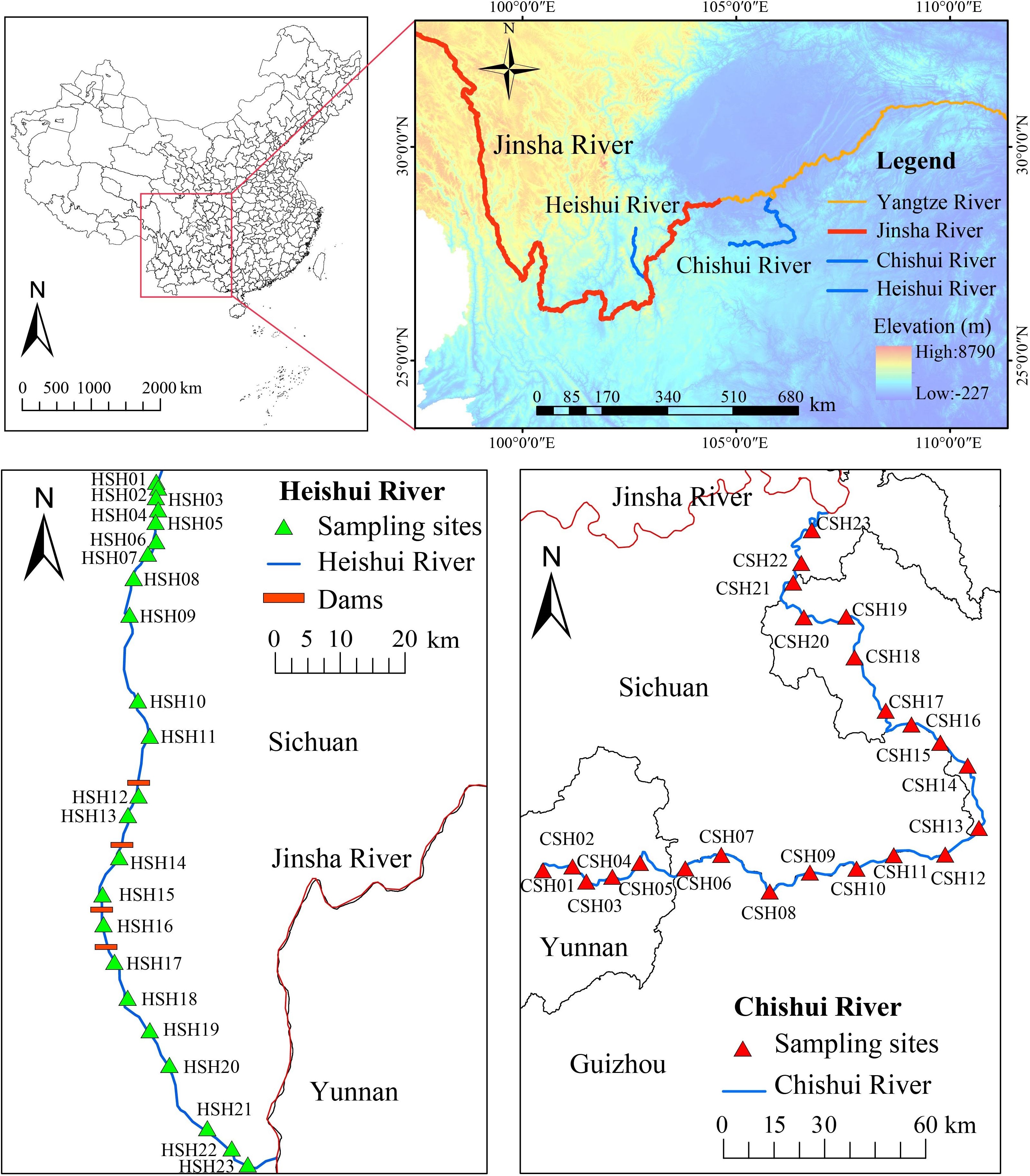
Figure 1. Study area position, watershed location and sample sites. The map was created in ArcGIS 10.2 (http://desktop.arcgis.com/en/arcmap/).
We disturbed the substrate using a shovel to ensure that macroinvertebrates could float and be directed into the net, and used the 500 μm mesh sieve to filter and rinse the samples. Three replicates from each sampling site were mixed into one biological sample that was stored in a 500 ml jar. In the laboratory, biological samples were identified to genus according to previous references (Dudgeon, 2000; Zhou et al., 2003), and the biomass (i.e., the weight of individuals) and density (i.e., the number of species per unit space) were recorded for each taxon.
Physical habitat conditions and water chemistry factors were measured at each sampling position. The latitude, longitude, and elevation above sea level were measured using a handheld global positioning system (Trimble Juno SA GPS, Trimble Navigation Ltd., Sunnyvale, CA, USA). Water depth (WD) and velocity were obtained by a digital velocity meter (Global Water flow probe FP201, Global Water Instrumentation, Gold River, CA, USA) placed in each sampling site with three replicates. A multiparameter water quality probe (YSI-Pro Plus, YSI Inc., Yellow Spring, OH, USA) was used to measure water temperature, dissolved oxygen (DO), pH, conductivity, and total dissolved solids in situ. The habitat score (HS) was measured to reflect the physical habitat quality of each river (Qu et al., 2019). In HSH, ten key aspects were considered including substrate composition, in-stream habitat complexity, range of combined WD and velocity, bank stability, channel sinuosity, water quantity, visual inspection of water cleanliness, riparian plant biodiversity, habitat environmental stress, and land use types. According to the standard scoring scheme, scoring for each aspect ranged from 0 to 20, which represents low to high habitat quality (Qu et al., 2019).
2.3 Determine highly-connectivity species
High-connectivity species were determined using three metrics of centrality, including degree centrality (DC), closeness centrality (CC), and betweenness centrality (BC). The centrality degree of species i (DCi) is the number of other nodes connected to node i (Dablander and Hinne, 2019; Freeman, 1978). Species that show a high value for DCi are hubs (i.e., connected to many other species). Closeness centrality (CC) quantifies the minimum number of steps from one node to all other nodes. High CCi values identify species that spread their impact more rapidly to other species when disturbed. Betweenness centrality (BC) indicates the frequency with which a species is on the shortest path for each pair of other species. The higher the BCi value, the greater the ability of a species to control information exchange and energy flow in the food web (Freeman, 1978; Wang et al., 2008). Banerjee (Banerjee et al., 2018) concluded that the species i with high DCi, high CCi, and low BCi was a high-connectivity species (85% accuracy). To date, this method has been widely used to identify high-connectivity species (Liu et al., 2021).
2.4 Robustness
To measure the robustness of the ecological network, we calculated network connectivity robustness (CR), the half of robustness (R50) and survival area (SA). CR was used as the primary indicator to assess the vulnerabilities of macroinvertebrate communities to the species loss. Moreover, the cascading effects of species loss were also considered in this study, R50 and SA were used to reflect the risks of secondary extinctions caused by species loss (Zhao et al., 2016). CR indicates the closeness of connections of remaining species after the loss of a species. The lower CR values indicate weaker interspecific relationships of the remaining species caused by the loss of a species, resulting in the reduction of interspecific energy flow. Thus, CR could reflect the functional stability of ecosystem based on the influence of species loss on interspecific energy flow. CR was calculated based on the relative efficiency ratio, which was computed as the ratio of the species number in the largest connected subnetwork to the remaining species number after the loss of a species (Du et al., 2010; Hastings et al., 2016; Wang and Tang, 2019). The calculation formula is as follows:
Where C is the number of species in the largest connected subnetwork within the remaining network after species removal, N is the number of species in the initial network, Nr is the number of removed species.
In addition, R50 refers to the number of species which have to be removed to make secondary extinctions reach 50% (Zhao et al., 2016). The higher R50 is, the lower the risk of secondary extinction induced by the primary species loss. Furthermore, the secondary extinction and the area below the curve (SA) were used to quantify the tolerance of a system to the species loss. The higher SA values, the lower the impact of species loss on community stability. SA was calculated as follows:
Where S is the number of species, p is the number of removed species, Nr is the number of species that ultimately survive after species removal and secondary extinction.
2.5 Simulation of species removal
Macroinvertebrate interspecific relationships were summarized as ecological networks. We applied spearman rank correlation since regression analysis is unsuitable for simulation studies with large data sets (White et al., 2014; Ståhl et al., 2025). Significant correlations (p < 0.05 adjusted by False Discovery Rate correction) were used to construct the network. In the constructed macroinvertebrates’ network, we simulated the species removal process and analyzed the impacts of species removal on the entire community. Only one species was removed from the networks at a time. We assumed that after a certain species was removed, its closely related species (those associated only with the removed species) would be affected and disappear. Thus, besides the actively removed species, other affected species were also removed simultaneously. Then, a new ecological network was constructed through the analysis of interspecific relationships among the remaining species, and its network robustness was also calculated. Then, species were removed sequentially (from highest to lowest biomass, highest to lowest density, highest to lowest connectivity, and in randomized order), and the aforementioned analysis were conducted to determine the impact of species removal on the entire community. The species removal steps are as follows: (1) Macroinvertebrate interspecific interactions were considered by computing pairwise Spearman’s correlation coefficients within the network. The relationship between species i and j (|aij|) was recorded as 1 when those species showed statistically significant (p < 0.05) correlation; (2) The number of connections (Di) of nodes i was calculated; (3) The centrality characteristics of species i were determined by calculating the DCi, CCi, and BCi, and identified the central species (high-connectivity species); (4) Species were removed in descending order based on their connections, density, biomass, as well as removing species randomly; (5) After each species removal, the row and column of the matrix where the species was located were set to 0; (6) The connections in the remaining network, the species secondarily removed due to the active species removal, and the remaining species were counted; (7) The CR, R50, and SA were calculated after each species removal to reflect the impact of species removal on the entire communities; (8) Steps (4) – (7) were repeated until all species were removed.
2.6 Network metrics
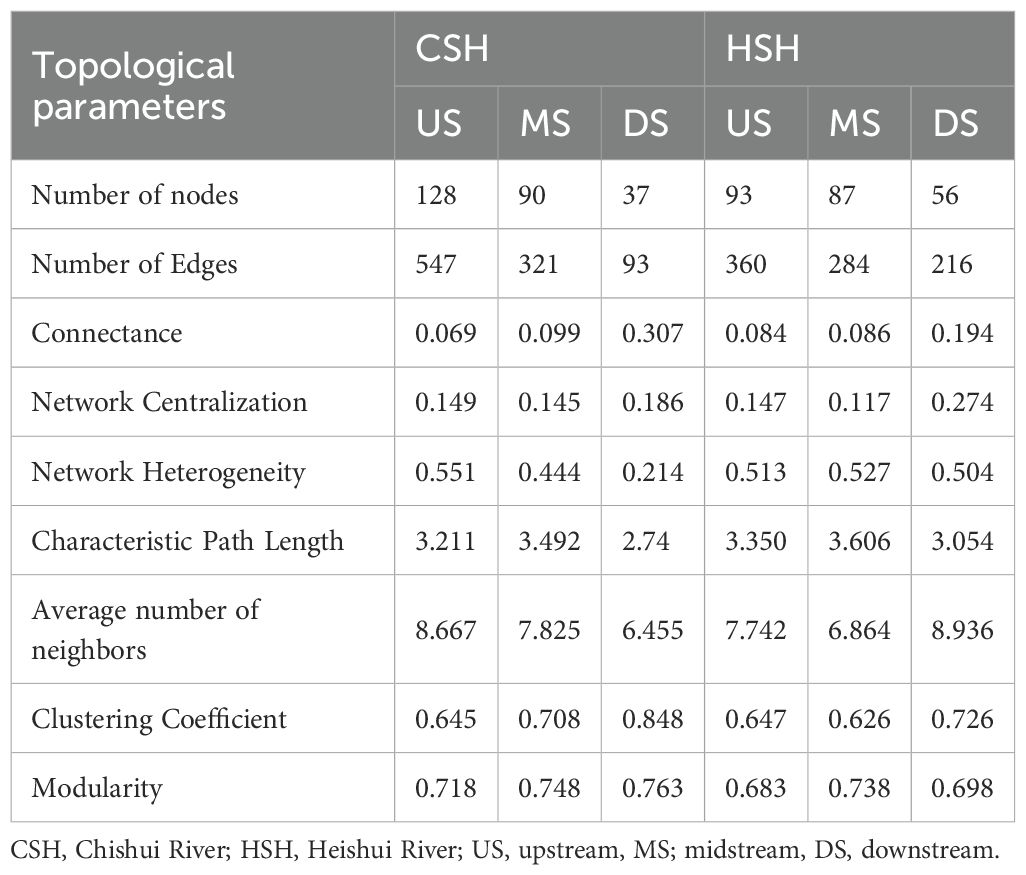
Networks were analyzed in Cytoscape software (version 3.6.1) with the edge weighted spring-embedded layout. Several topological and node/edge metrics, including connectance, clustering coefficient, average number of neighbors, characteristic path length, network heterogeneity and centrality, were calculated using Analyzer plugin within Cytoscape. Connectance is a network property that is calculated from the number of species and the number of links. It describes the proportion of realized links out of all possible links in a network, and is often used as a measure of network complexity. The clustering coefficient was calculated to represent trends of nodes clustering in the network. The characteristic path length is the number of steps along the shortest path for all possible pairs of species, with a decrease in the characteristic path length possibly indicating a faster spread of disturbances in the network. Network heterogeneity quantifies the diversity of connections between nodes in networks, even with different topologies. Average number of neighbors calculated the average connectivity of a node in the network. The modularity value was calculated using the Cluster Maker plugin in Cytoscape (Newman, 2006).
3 Results
3.1 Macroinvertebrate ecological networks and connectedness
In the undammed river (CSH), the macroinvertebrate network in the upstream was constituted by 128 nodes and 547 edges, while that in the midstream was composed of 90 nodes and 321 edges, and that in the downstream was composed of 37 nodes and 93 edges (Table 1; Figure 2). The physical factors (flow, water depth, elevation, and habitat score) and nutrient factors (TP, TN, NO3-, and PO4-) dominate the community composition and interspecific relationships in the upstream and midstream of CSH, while nutrient factors (TP, TN, NO3-, and PO4-) were prominent factors influencing community interspecific relationships in the downstream (Figure 2).
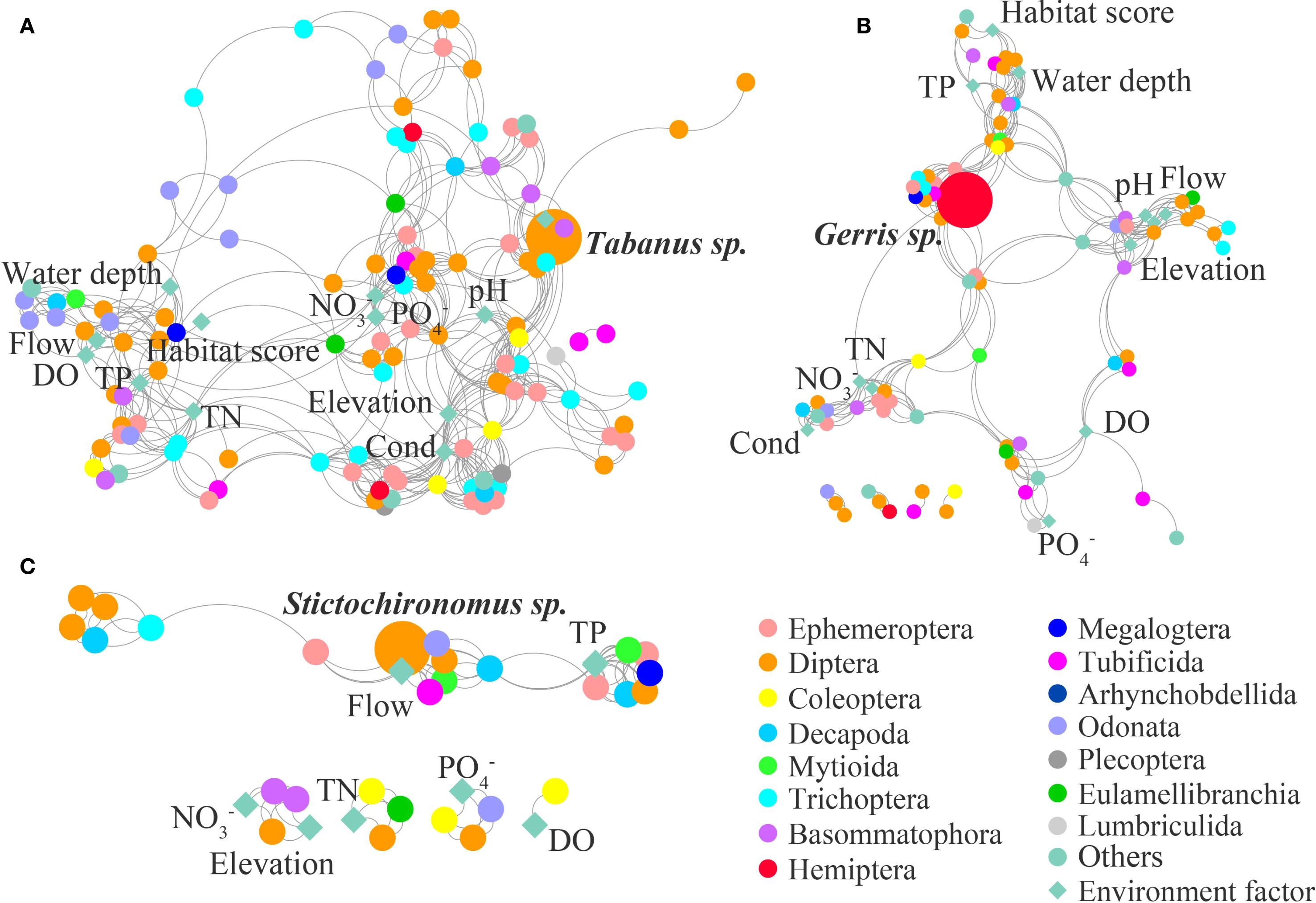
Figure 2. Macroinvertebrate ecological network in the upstream (A), midstream (B), and downstream (C) of CSH.
In the dammed river (HSH), the macroinvertebrate network in the upstream consisted of 93 nodes and 360 edges, while that in the midstream was constituted by 87 nodes and 284 edges, and that in the downstream consisted of 56 nodes and 216 edges (Table 1; Figure 3). The physical factors (flow, water depth, elevation, Cond, pH and habitat score) and nutrient factors (TP, TN, NO3-, and PO4-) dominate the community composition and interspecific relationships in the upstream and midstream of the HSH, while nutrient factors (TP, TN, NO3-, and PO4-) were prominent factors influencing community interspecific relationships in the downstream (Figure 3).
Figure 3. Macroinvertebrate ecological network in the upstream (A), midstream (B), and downstream (C) of HSH.
Both in the undammed river and dammed river, the number of nodes and edges decreased from upstream to downstream. Macroinvertebrate community biomass, density, richness, and diversity also decreased from upstream to downstream in the two rivers (Supplementary Tables 1, 2). Clustering coefficient were higher in downstream (CSH: 0.848, HSH: 0.726) than in upstream (0.645 and 0.647) and midstream (0.708 and 0.626) in the two rivers (Table 1), which indicated that the macroinvertebrate communities exhibited stronger species clustering and closer interspecific relationships downstream. Regarding the modularity of the network, the values of modularity were higher in the downstream (0.763) of the undammed river, while the values of modularity were higher in the midstream (dam-constructed reaches) (0.738) of the dammed river. The results revealed that the macroinvertebrate community was fragmented into several small subcommunities with close interspecific connections in these reaches, indicating that the macroinvertebrate communities exhibited a high degree of dispersion.
3.2 Identify keystone species of macroinvertebrates
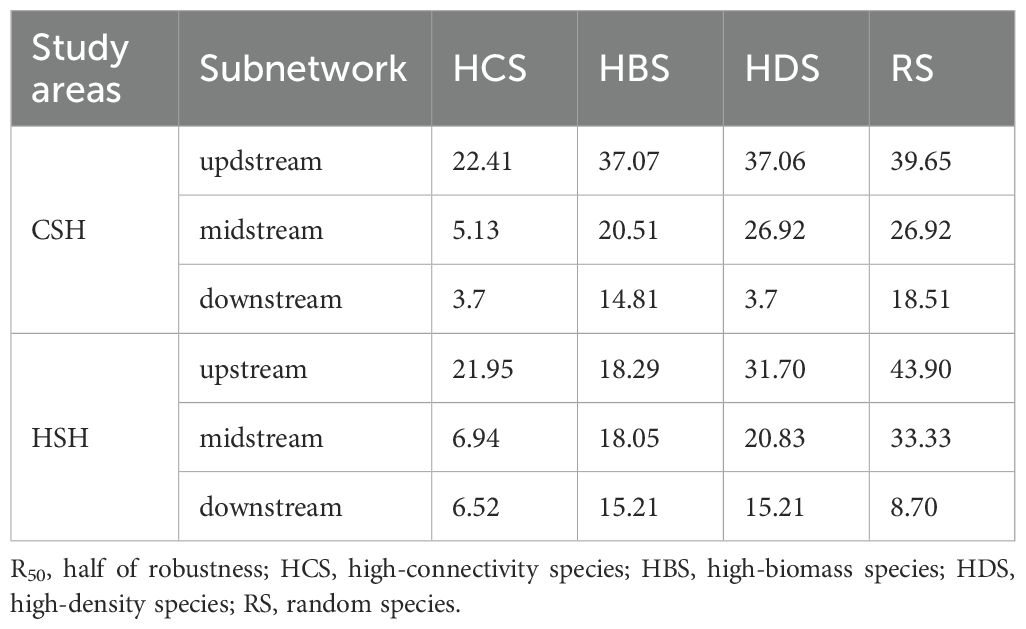
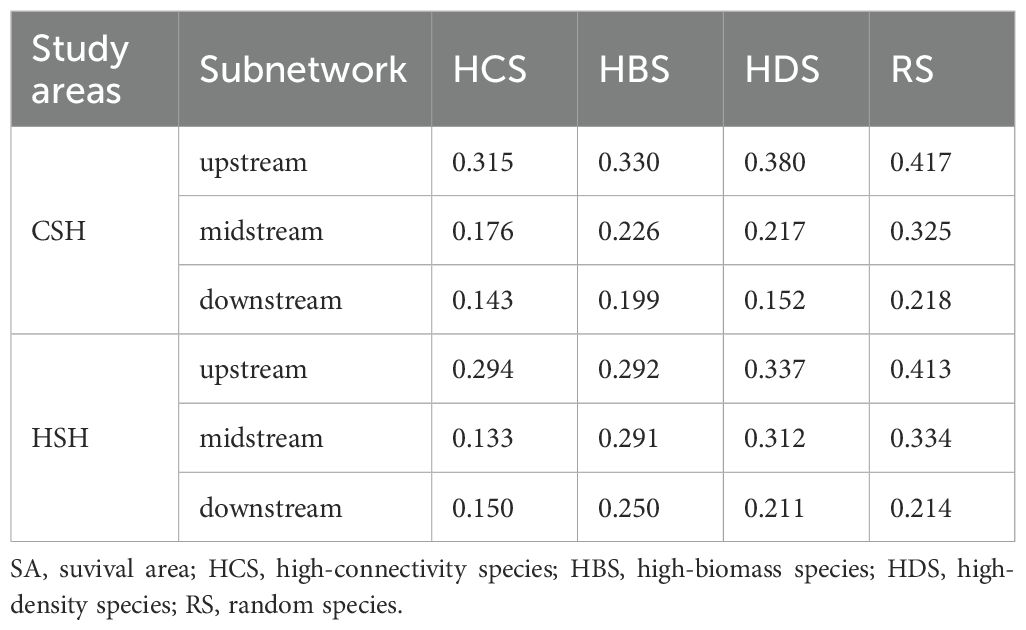
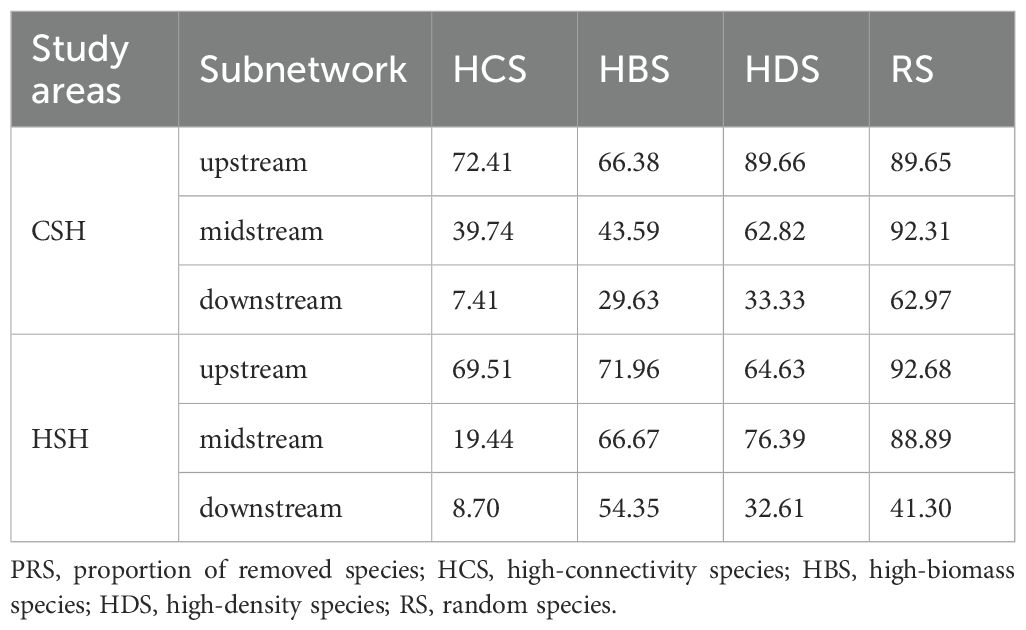
In the undammed river, the values of R50 (upstream: 22.41%, midstream:5.13%, downstream: 3.7%) when removing high-connectivity species were lower than those when removing other species (Figure 4; Table 2). In the dammed river, the values of R50 (midstream:6.94%, downstream: 6.52%) when removing high-connectivity species were also lower than those when removing other species. In addition, when removing high-connectivity species, the SA values (upstream: 0.315, midstream: 0.176, and downstream: 0.143) were also lower than the SA values of high-biomass species (0.330, 0.226, and 0.199) and those of high-density species (0.380, 0.217, and 0.152) (Figure 5; Table 3). From the perspective of community functional stability based on interspecific energy flow, when CR decreased to its lowest values in undammed rivers, the decrease in community functional stability caused by the removal of high-connectivity species was lower in the midstream (39.74%) and downstream (7.41%) than that caused by the removal of high-biomass species (midstream: 43.59%, downstream: 29.63%) and high-density species (62.82% and 33.33%) (Table 4; Figure 6). Moreover, when CR decreased to its lowest values in dammed rivers, the removal rates of high-connectivity species (midstream: 19.44%, downstream: 8.7%) were also lower than those of high-biomass species and high-density species. The result of R50 and SA indicated that the loss of high-connectivity species was more likely to trigger the cascading effects compared to other species. Furthermore, the removal of high-connectivity species also has a more important impact on macroinvertebrate community functional stability. Therefore, the high-connectivity species could be identified as keystone species in the two rivers, which play a more crucial role than dominant species in maintaining the structure and functional stability of macroinvertebrate communities.
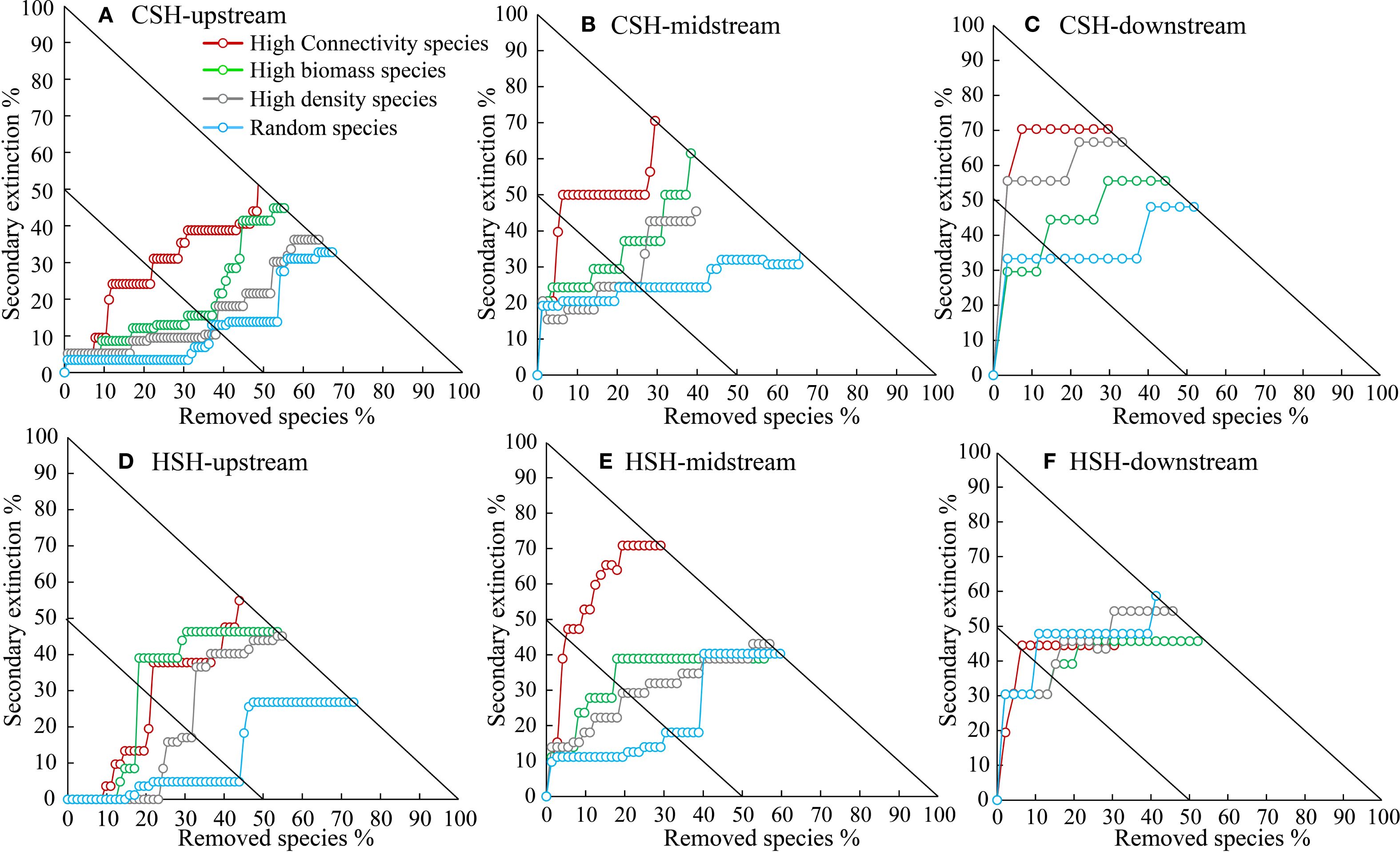
Figure 4. Effects of species loss on half of robustness (R50) of the macroinvertebrate community in the upstream (A), midstream (B), and downstream (C) of CSH, and in the upstream (D), midstream (E), and downstream (F) of HSH.
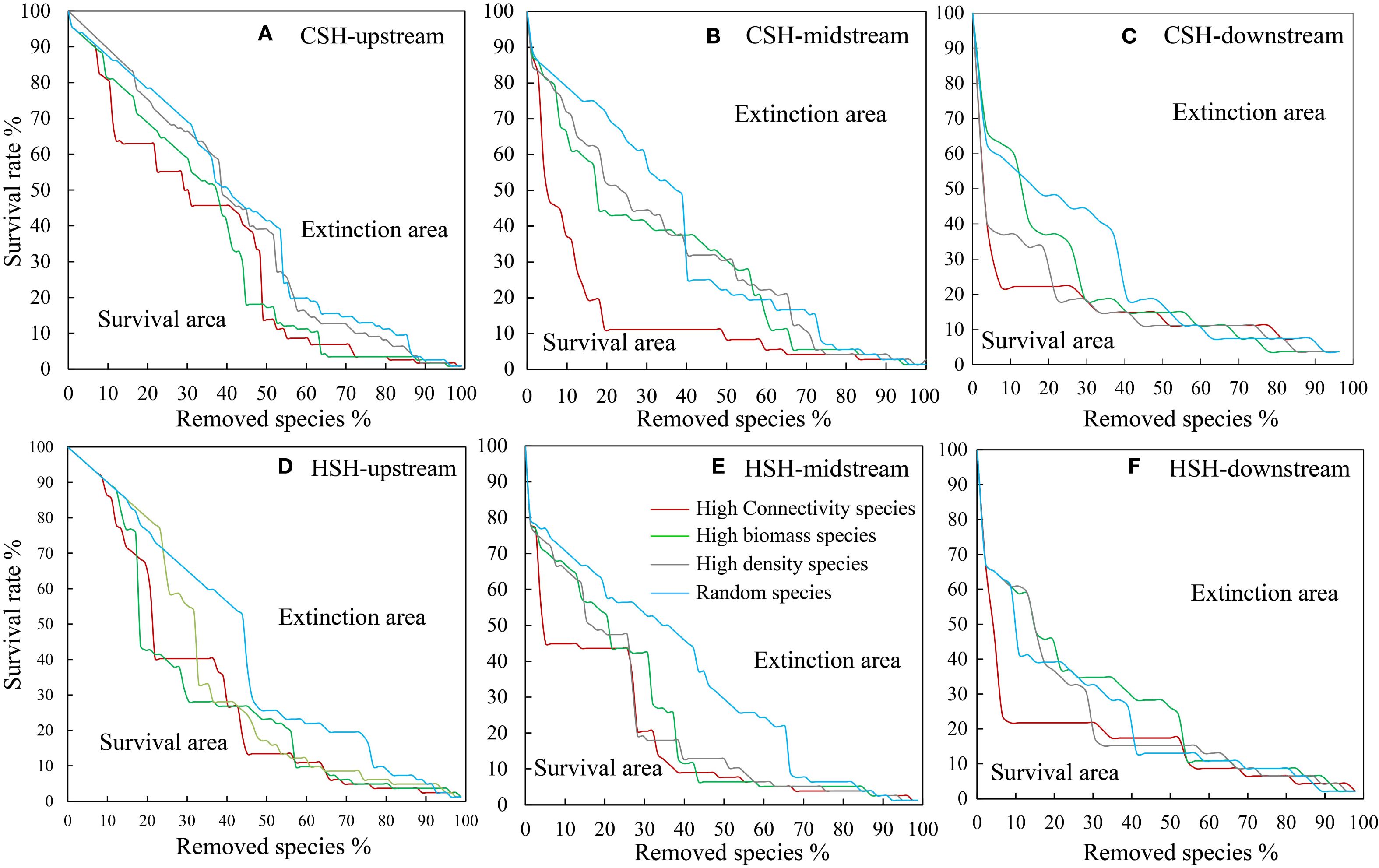
Figure 5. Effects of species loss on the species survival area (SA) of the macroinvertebrate community in the upstream (A), midstream (B), and downstream (C) of CSH, and in the upstream (D), midstream (E), and downstream (F) of HSH.
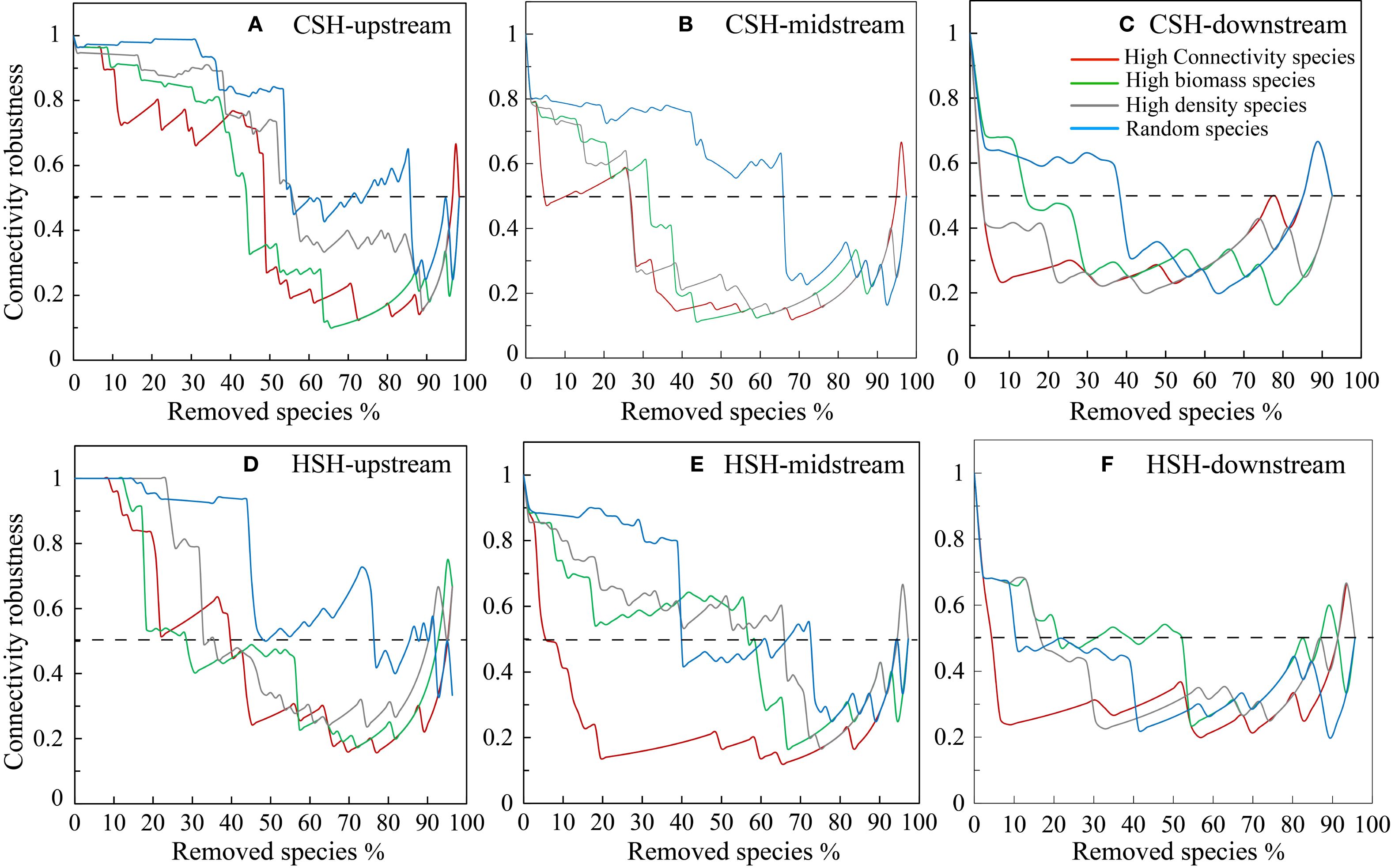
Figure 6. Effects of species loss on network connectivity robustness (CR) (dashed line indicating 50% of CR) in the upstream (A), midstream (B), and downstream (C) of CSH, and in the upstream (D), midstream (E), and downstream (F) of HSH.
3.3 Community composition and functional feeding groups of keystone species
In CSH, the top five keystone species in upstream were affiliated with Coleoptera (Psephenidae spp.: 0.5%, Elmididae sp.: 6.20%, and Stenelmis sp.: 2.97%), and those in downstream were affiliated with Diptera (Stictochironomus sp.: 30.53%, Chironomus sp.: 10%, and Cricotopus sp.: 8.95%). Moreover, in midstream, diverse taxa such as Hemiptera, Ephemeroptera, Arhynchobdellida, Diptera, and Decapoda constituted the top five keystone species (Supplementary Table 3). In HSH, the top five keystone species in upstream were affiliated with Diptera (Paramerina sp.: 3.27%, Cricotopus sp.: 4.49%, and Diamesa sp.: 0.23%), those in midstream were mainly affiliated with Ephemeroptera (Heptagenia sp.: 2.68%, Baetiella sp.: 0.51%, and Serratella sp.: 0.06%), and those in downstream were mainly affiliated with Diptera (Antocha sp.: 3.39% and Chironomidae pupa: 1.21%) (Supplementary Table 4).
The composition of keystone species functional feeding groups in CSH and HSH differs. The diversity of functional groups was lower in dammed reaches than in other reaches (Figure 7). In addition, in CSH, Tabanus sp. (41.39%) dominated keystone species in upstream, Gerris sp. (24.42%), Aphelocheirus sp. (24.15%), and Limnophora sp. (23.83%) were the dominant ones in midstream, as well as Stictochironomus sp. (74.93%) played the dominant role in downstream. We concluded that keystone species dominated by predators caused the decline rates of CR exceeding 15% in naturally undisturbed rivers (Table 5). in the undammed rivers. In HSH, Tetropina sp. (31.76%), Tanytarsus sp. (18.93%), Tipula sp. (17.47%) dominated keystone species in upstream, Baetiella sp. (28.91%) was the dominant one in midstream, as well as Aphelocheirus sp. (40.94%), Hydrobaenus sp. (32.13%), and Antocha sp. (24.85%) played the dominant role in downstream (Table 5). The result showed that the construction of dams led to the change in the structure of keystone species, as keystone species shifted from being primarily represented by predators in the undammed river (CSH) to being mainly collector-gatherers in the dam-constructed reaches of HSH. Therefore, the construction of dams reduced the resistance of macroinvertebrate communities to keystone species loss by changing the structure and function of keystone species, as well as decreasing the functional diversity of these species.
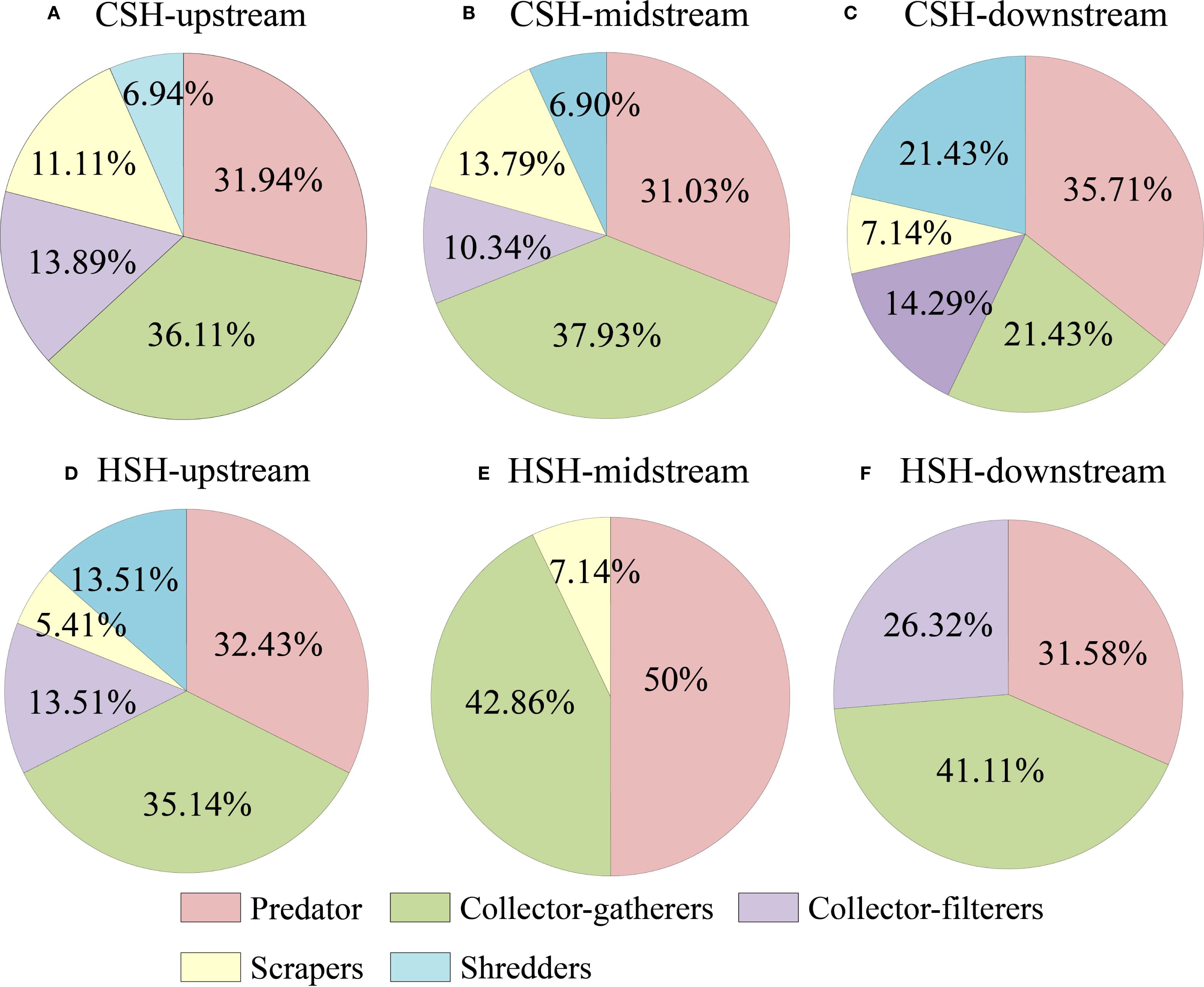
Figure 7. Functional feeding groups of keystone species in the upstream (A), midstream (B), and downstream (C) of CSH, and in the upstream (D), midstream (E), and downstream (F) of HSH.
3.4 Influence of dam construction on macroinvertebrate community stability
Comparative analyses were conducted on the stability of macroinvertebrate communities to the loss of keystone species in different river reaches. In undammed river (CSH), the values of R50, SA, and CR when keystone species were removed were lower in the downstream than in other reaches (TableS 2–4). Thus, the R50, SA, and CR values caused by the disappearance of keystone species decreased successively from the upstream to the downstream, which was consistent with the changes in habitat scores of undammed rivers (Supplementary Table 1). However, in dammed river (HSH), the loss of keystone species led to a more rapid decline in SA in the dam-constructed reaches (midstream) than downstream (Table 3). Furthermore, in the dam-constructed reaches, the removal of keystone species led to a faster decrease in CR. Precisely, when the removal rate reached 19.44%, the community function stability dropped to its lowest point. Therefore, compared with naturally undammed rivers, the construction of dams led to an increased sensitivity of macroinvertebrate communities to keystone species loss, resulting in a reduction in the structure and functional stability of dammed river ecosystems.
4 Discussion
4.1 Keystone species of macroinvertebrate communities
According to the change of R50, SA, and CR under four species loss scenarios, the high-connectivity species have greater impacts on network robustness than other species. Thus, the high-connectivity species could be keystone species of macroinvertebrates in both CSH and HSH. Several previous studies also proved that high-connectivity species play an important role in mediating the microbiome composition and functions, and these species were identified as the microbial keystone species (Chao et al., 2024; Wang et al., 2024). Generally, high-connectivity species are at the central positions in a community and connect to most other species. Due to their extensive connections with other species, the extinction of high-connectivity species may have a ripple effect on the community. Their loss may affect most other species and result in a faster breakdown of the entire community’s structure and function (Dunne et al., 2002; Stouffer et al., 2012; Wang et al., 2024). Like in previous studies focusing on microbial taxa, high-connectivity species were identified as keystone species also in our study, focusing on macroinvertebrate communities in both undammed and dammed riverine reaches. Moreover, several studies have shown that the abundance determines the positional importance of plant species (Zhu et al., 2024), while this study found different results. Based on the analysis of the relative abundance of keystone species, our research found that less abundant species tended to occupy central positions in both undammed and dammed rivers (Supplementary Tables 3, 4). In general, biomes are composed of a few abundant taxa and a large proportion of rare taxa. Abundant taxa mostly contribute to biomass production, energy flow, and nutrient cycling, whereas low-abundant taxa predominantly contribute to species richness. Low-abundant taxa exhibit greater ecological redundancy and could drive changes in biomes under disturbed environments (Xue et al., 2018). Moreover, previous studies have also found that hub species of crop fungal communities belong to rare taxa, which determine the stability of crop fungal communities and ecosystem functions (Xiong et al., 2021). Consistent with these research findings, our results suggest that high-connectivity species with low abundance are keystone species that play a central role in sustaining the stability of macroinvertebrate communities.
The central placement of the keystone species in the macroinvertebrate communities was affected by their biological functional traits. For instance, through their filtration and biodeposition processes, freshwater mussels reduce phytoplankton density in the water column and increase inputs of sediment organic matter, which affects the habitat quality of other invertebrates and thereby influences the functioning of ecosystem processes (Simeone et al., 2021). This study explored the functional feeding traits of keystone species that led to the greatest decline of CR. This result showed that predators predominated in the keystone species in upstream, midstream, and downstream of CSH. However, collector–gatherers dominated the keystone species in HSH, especially in the midstream (dam-constructed reaches) (Table 5). Top predators were generally considered as the keystone species (Ge et al., 2004; Paine, 1969). In surface freshwaters, predators are located at high trophic levels in food webs and feed on other small macroinvertebrates like Chironomidae larvae and Oligochaetes (Wang et al., 2024; Zheng and Yin, 2023; Li et al., 2024). There are specialist predators and generalist predators in river ecosystems. Generally, specialist predators feed on specific types of prey, while generalist predators can feed on many different types of prey (Hilker and Lewis, 2010). This study found that Tabanus sp., Gerris sp., Aphelocheirus sp., Limnophora sp., and Palaemonetes sinensis are all generalist predators, and they can feed on other small macroinvertebrates like Chironomid larvae and other small Mollusca in the undammed river. Moreover, based on the centrality of keystone species, predators can achieve top-down effects on the functional stability of the macroinvertebrate community through relatively shorter feeding pathways. Thus, the shorter feeding relationship pathways of these generalist predators with a wider range of other prey led them to be the keystone species in the undammed river (CSH). In general, these predator–prey systems are primarily found in environments with a predominantly unidirectional flow such as naturally undisturbed streams and rivers (Hilker and Lewis, 2010). Alterations of natural flow regimes (e.g., due to the construction of dams) could significantly affect the habitat available, as well as their foraging, reproductive, and competitive behavior of organisms (Hilker and Lewis, 2010). Our research showed that the construction of dams changed the composition of functional feeding groups of keystone species. Collector–gatherers dominated the keystone species in the dammed river (Table 5), which determined also a functional shift of keystone species from being primarily predators to mostly representing preys. The construction of dams intercepts the river channel, reduces the flow velocity and habitat homogeneity, and increases the stability of the substrate. Changes in river hydrological conditions will lead to the accumulation of fine sediment and organic debris in front of the dam, which provides favorable habitat conditions for collector-gatherers (Encalada et al., 2010; Li et al., 2024; Del Campo et al., 2025). However, other small macroinvertebrates adapted to flowing water environment were unable to survive in this habitat and thus ceased to exist, which further led to changes in the food sources and population distribution of predators in the dammed river. Since the predators tend to be specialists (feeding on the species that can adapt to the dammed habitat), they cannot persist without the prey. Consequently, the prey (collector–gatherers) became a keystone species in the dammed river.
4.2 The effect of keystone species on macroinvertebrate community stability
Comparative analyses were conducted to reveal the different impacts of keystone species on ecosystem stability between the undammed river (CSH) and dammed river (HSH), as well as among the upstream, midstream, and downstream reaches. The results showed that the resistance of the macroinvertebrate community in HSH to the loss of keystone species was lower than that in CSH. Moreover, the macroinvertebrate community in downstream reaches of undammed river (CSH) was more sensitive to the loss of keystone species than in the upstream and midstream sections. Differently, macroinvertebrate community in the midstream (dam-constructed reaches) reaches of dammed river (HSH) was more sensitive to the loss of keystone species than in other reaches of the same river. The role of keystone species in maintaining community stability could be influenced by the complexity of interspecific relationships, which is mainly measured by a series of network topological metrics including network connectance and clustering coefficient (Liu et al., 2021; Wang et al., 2024). Connectance is often used to express the complexity of ecological networks, and to assess network robustness to perturbations (e.g. secondary extinctions) (Dunne et al., 2002; Ståhl et al., 2025). Clustering coefficient is used to express the degree of tightness in interspecific relationships. In this study, the low complexity of macroinvertebrate interspecific relationships in downstream of CSH was revealed by low species richness and a low number of linkages. Based on higher network clustering coefficient, we also found that the closest interspecific interactions occurred in the downstream reaches of both rivers. These low complexity and high tightness of interspecific relationships in the downstream led to the low resistance of the macroinvertebrate community to the loss of keystone species and reduced the stability of communities. Combined with the influences of environmental factors, we found that both physical factors (flow, water depth, elevation, and habitat score) and nutrient factors (TP, TN, NO3-, and PO4-) dominate the community composition and interspecific relationships in the upstream and midstream of the undammed river (CSH), while nutrient factors (TP, TN, NO3-, and PO4-) were prominent factors influencing community interspecific relationships in the downstream (Figure 2). The upstream and midstream of CSH are located in mountainous areas, with less interference from human activities. However, many towns and cities (e.g., Maotai Town and Chishui City) were built along the downstream of CSH, where human activities are relatively concentrated. Human activities could lead to amounts of nutrients flowing into the river and influence the interspecific relationships, and further decrease the stability of macroinvertebrate communities.
Unlike undammed rivers, the community stability of the dam-constructed reaches (midstream) in dammed rivers was more sensitive to the loss of keystone species (Figures 4, 6). This result is consistent with previous studies that the changes in heterogeneous habitats under the influence of dams could affect the keystone species dynamics, and low-heterogeneity habitats could increase the regulatory role of keystone species on community-wide perturbation (Brown and Lawson, 2010). Many small subcommunities with close interspecific connections were clustered in the dam-constructed reaches (midstream) based on the higher network modularity, which indicated that there was a high degree of aggregation of macroinvertebrate communities. Network modularity reflects the distribution characteristics of small aggregated communities, which represent synergistic and competitive interactions as well as niche differentiation (Newman, 2006). The closeness of interspecific connections is strong within a subcommunity, while it is weak between different subcommunities. In general, connector species between different subcommunities are responsible for interspecific fluxes, and when these subcommunities are poorly connected or not connected at all, the loss of these species would probably have a large impact on the overall flux structure of the communities. Several studies also found that the disappearance of keystone species could cause the break-up of modules and networks (Banerjee et al., 2018). Thus, when faced with the loss of keystone species, macroinvertebrate communities in dam-constructed reaches (midstream) with a higher degree of dispersion are more likely to be affected and collapse. In river ecosystems, the drift behavior of macroinvertebrates is an important biological adaptation mechanism, which can effectively expand population distribution and enhance the complexity of interspecific relationships (Shi et al., 2020). The construction of dams has blocked the longitudinal drift of macroinvertebrates, resulting in patchy habitat availability for macroinvertebrates. The drift behavior of macroinvertebrates can be blocked by dams, which could lead to the fragmentation of small aggregated subcommunities. This because some species, those preferring low-flow velocity environments and exhibiting a high degree of interspecific similarity in ecological niches and functions, dominate such communities (Dominguez-Garcia and Kefi, 2024; Estrada, 2007; Ge, 2019). Moreover, dams can also block the longitudinal drift of organic matter and reduce the abundance of species that feed on organic debris (Liu et al., 2021). The reduction of a specific species will lead to the decrease of predators’ food sources and the increase in the similarity of predators’ feeding habits. This will decrease the complexity of interspecific relationships and thus reduce the resistance of the macroinvertebrate community to the loss of keystone species. Our study confirmed that the construction of dams could cause the break-up of macroinvertebrate communities, and the fragmentation of communities may further reduce their resistance to the loss of keystone species, thereby resulting in the reduction of ecosystem stability.
Network analysis is a systems-level approach for integrating many layers of data. Organisms’ data can be used to identify interspecific interactions, these interactions can then be used to build network to examine identify keystone species, and clusters of potentially interacting species that can be associated with their ecological traits (Robinson et al., 2022; Chao et al., 2024; Wang et al., 2024). Although this study has certain limitations in constructing ecological networks (e.g., not specifying the type of interspecific relationship; not disentangling co-occurrences due to potential interaction from co-occurrences due to stochasticity or common responses to environmental factors), generalizing the relationships between organisms and using these relationships to study the role of key species are the key focuses of this research. Based on the results of the generalized analysis of interspecific relationships, we further analyzed and discussed the functional traits of keystone species, aiming to elucidate the ecological role of these keystone species from the perspective of real interspecific relationships. Thus, using species interactions to build networks can facilitate macroinvertebrate community dynamics assessments by visualizing the overall structural and functional relationships within a system.
5 Conclusions
The present study analyzed the changes in macroinvertebrate community stability following the loss of different species, with the aim of identifying keystone species in undammed and dammed rivers. In particular, we explored the effect of dam construction on macroinvertebrate community stability. The results showed that the high-connectivity species with low abundance were keystone species, because the loss of these species had a greater impact on community stability compared to other species (high-biomass species and high-density species). The functional feeding traits of keystone species were explored in this study. We found that predators dominated keystone species in the undammed river (CSH), while prey (collector-gatherers) dominated keystone species in the dammed river (HSH). Our findings suggested that the construction of dams could transform the functional feeding groups of keystone species from predators to prey. Furthermore, the impact of keystone species loss on half of robustness (R50), survival area (SA), and network connectivity robustness (CR) was greater in dam-constructed reaches (HSH) than in undammed reaches. This result demonstrated that the construction of dams reduced the resistance of macroinvertebrate communities to the loss of keystone species, indicating that dam construction could cause notable changes of interspecific relationships and ultimately the break-up of macroinvertebrate communities. Community fragmentation may further reduce their resistance to keystone species loss, thereby decreasing ecosystem stability.
These findings provide valuable recommendations for developing strategies to protect biodiversity and restore the function and stability of dammed river ecosystems.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. MZ: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. WP: Funding acquisition, Resources, Supervision, Visualization, Writing – review & editing. XQ: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Key R&D Program of China (2021YFC3200102, 2023YFC3205901), the National Natural Science Foundation of China (32501457), and the Open Research Fund of State Key Laboratory of Simulation and Regulation of Water Cycle in River Basin (China Institute of Water Resources and Hydropower Research) (IWHR-SKL-KF202304).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1640255/full#supplementary-material
References
Albert R., Jeong H., and Barabási A. L. (2000). Error and attack tolerance of complex networks. Nature. 406, 378–382. doi: 10.1038/35019019
Banerjee S., Schlaeppi K., and van der Heijden M. G. A. (2018). Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 16, 567–576. doi: 10.1038/s41579-018-0024-1
Bellingeri M. and Cassi D. (2018). Robustness of weighted networks. Physica A Stat. Mech. Appl. 489, 47–55.
Bellingeri M., Bevacqua D., and Cassi D. (2019). The heterogeneity in link weights may decrease the robustness of real-world complex weighted networks. Sci. Rep. 9, 10692.
Brown B. L. and Lawson R. L. (2010). Habitat heterogeneity and activity of an omnivorous ecosystem engineer control stream community dynamics. Ecology. 91, 1799–1810. doi: 10.1890/09-0350.1
Canning A. D. and Death R. G. (2018). Relative ascendency predicts food web robustness. Ecol. Res. 33, 873–878. doi: 10.1007/s11284-018-1585-1
Chao H., Cai A., Heimburger B., Wu Y., Zhao D., Sun M., et al. (2024). Keystone taxa enhance the stability of soil bacterial communities and multifunctionality under steelworks disturbance. J. Environ. Management. 356, 120664. doi: 10.1016/j.jenvman.2024.120664
Chi S., Gong Y., Wang H., Zheng J., Hu J., Hu J., et al. (2017). A pilot macroinvertebrate-based multimetric index (MMI-CS) for assessing the ecological status of the Chishui River basin, China. Ecol. Indicators. 83, 84–95. doi: 10.1016/j.ecolind.2017.07.045
Dablander F. and Hinne M. (2019). Node centrality measures are a poor substitute for causal inference. Sci. Rep. 9, 6846. doi: 10.1038/s41598-019-43033-9
Dallas T. A., Han B. A., Nunn C. L., Park A. W., Stephens P. R., and Drake J. M. (2019). Host traits associated with species roles in parasite sharing networks. Oikos. 128, 23–32. doi: 10.1111/oik.05602
Del Campo R., Blackman R. C., Martini J., Fuß T., Bistarelli L. T., Gessner M. O., et al. (2025). Functional macroinvertebrate diversity stabilizes decomposition among leaf litter resources across a river network. Ecol. Monographs. 95, e70010. doi: 10.1002/ecm.70010
Dominguez-Garcia V. and Kefi S. (2024). The structure and robustness of ecological networks with two interaction types. PloS Comput. Biol. 20, e1011770. doi: 10.1371/journal.pcbi.1011770
Du W., Cai M., and Du H. (2010). Study on indices of network structure robustness and their application. J. Xi'an Jiaotong University. 44, 93–97. doi: CNKI:SUN:XAJT.0.2010-04-021
Dudgeon D. (2000). The ecology of tropical Asian river and streams in Relation to biodiersity conservation (China: Hong Kong University Press, Hong Kong).
Dunne J. A., Williams R. J., and Martinez N. D. (2002). Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol. Letters. 5, 558–567. doi: 10.1046/j.1461-0248.2002.00354.x
Encalada A. C., Calles J., Ferreira V., Canhoto C. M., and Graça M. A. (2010). Riparian land use and the relationship between the benthos and litter decomposition in tropical montane streams. Freshw. Biol. 55, 1719–1733. doi: 10.1111/j.1365-2427.2010.02406.x
Estrada E. (2007). Food webs robustness to biodiversity loss: the roles of connectance, expansibility and degree distribution. J. Theor. Biol. 244, 296–307. doi: 10.1016/j.jtbi.2006.08.002
Freeman L. C. (1978). Centrality in social networks conceptual clarification. Soc. Networks. 1, 215–239. doi: 10.1016/0378-8733(78)90021-7
Ge J. (2019). Research on the theory and application of flow-ecology relationship in a dam-river: a case study of Shaying River. China Institute of Water Resources and Hydropower Research.
Ge B., Bao Y., and Zheng X. (2004). A review on key species study in ecology. Chin. J. Ecology. 23, 102–106. doi: CNKI:SUN:STXZ.0.2004-06-021
Gómez J. M. and Perfectti F. (2012). Fitness Consequences of centrality in mutualistic individual-based networks. Proc. R. Soc. B: Biol. Sci. 279, 1754–1760. doi: 10.1098/rspb.2011.2244
Hastings A., McCann K. S., and de Ruiter P. C. (2016). Introduction to the special issue: theory of food webs. Theor. Ecology. 9, 1–2. doi: 10.1007/s12080-016-0292-1
Hervías-Parejo S., Tur C., Heleno R., Nogales M., Timóteo S., and Traveset A. (2020). Species functional traits and abundance as drivers of multiplex ecological networks: first empirical quantification of inter-layer edge weights. Proc. R. Soc. B: Biol. Sci. 287, 20202127. doi: 10.1098/rspb.2020.2127
Hilker F. M. and Lewis M. A. (2010). Predator-prey systems in streams and rivers. Theor. Ecology. 3, 175–193. doi: 10.1007/s12080-009-0062-4
Jiang X., Xiong J., and Xie Z. (2017). Longitudinal and seasonal patterns of macroinvertebrate communities in a large undammed river system in Southwest China. Quaternary Int. 440, 1–12. doi: 10.1016/j.quaint.2016.07.016
Jiang X., Xiong J., Xie Z., and Chen Y. (2011). Longitudinal patterns of macroinvertebrate functional feeding groups in a Chinese river system: A test for river continuum concept (RCC). Quaternary Int. 244, 289–295. doi: 10.1016/j.quaint.2010.08.015
Keyes A. A., McLaughlin J. P., Barner A. K., and Dee L. E. (2021). An ecological network approach to predict ecosystem service vulnerability to species losses. Nat. Commun. 12, 1–11. doi: 10.1038/s41467-021-21824-x
Landi P., Minoarivelo H. O., Brännström Å., Hui C., and Dieckmann U. (2018). Complexity and stability of ecological networks: a review of the theory. Population Ecol. 60, 319–345. doi: 10.1007/s10144-018-0628-3
Li J. X., Ma M. D., Wang L. Y., Jin Y. J., Liu Y. M., Yin X. W., et al. (2024). Ecological drivers of taxonomic, functional, and phylogenetic beta diversity of macroinvertebrates in Wei River Basin of northwest China. Front. Ecol. Evolution. 12, 1410915. doi: 10.3389/fevo.2024.1410915
Libralato S., Christensen V., and Pauly D. (2006). A method for identifying keystone species in food web models. Ecol. Modelling. 195, 153–171. doi: 10.1016/j.ecolmodel.2005.11.029
Liu Y., Fan N., An S., Bai X., Liu F., Xu Z., et al. (2008). Characteristics of water isotopes and hydrograph separation during the wet season in the Heishui River, China. J. Hydrology. 353, 314–321. doi: 10.1016/j.jhydrol.2008.02.017
Liu Y., Zhang M., Peng W., Qu X., Zhang Y., Du L., et al. (2021). Phylogenetic and functional diversity could be better indicators of macroinvertebrate community stability. Ecol. Indicators. 129, 107892. doi: 10.1016/j.ecolind.2021.107892
Lv Y., Xie Y., Wang S., Qu X., and Zhang M. (2020). Studies about Heishui River as an alternative habitat for aquatic biodiversity protection based on macroinvertebrate community similarity. China Environ. Science. 40, 2647–2657. doi: 10.19674/j.cnki.issn1000-6923.2020.0297
Ma J. X., Yin X. W., Liu G., and Song J. X. (2024). Intensification of human land use decreases taxonomic, Functional, and phylogenetic diversity of macroinvertebrate community in Weihe River basin, China. Diversity Basel. 16, 513. doi: 10.3390/d16090513
Mello M. A. R., Rodrigues F. A., Costa L. D. F., Kissling W. D., Şekercioglu Ç.H., Marquitti F. M. D., et al. (2015). Keystone species in seed dispersal networks are mainly determined by dietary specialization. Oikos. 124, 1031–1039. doi: 10.1111/oik.01613
Newman M. E. J. (2006). Modularity and community structure in networks. Proc. Natl. Acad. Sci. 103, 8577–8582. doi: 10.1073/pnas.0601602103
Ortiz M., Hermosillo-Nuñez B., González J., Rodríguez-Zaragoza F., Gómez I., and Jordán F. (2017). Quantifying keystone species complexes: Ecosystem-based conservation management in the King George Island (Antarctic Peninsula). Ecol. Indicators. 81, 453–460. doi: 10.1016/j.ecolind.2017.06.016
Paine R. T. (1969). A nnote on trophic complexity and community stability. Am. Naturalist. 103, 91–93. doi: 10.1086/282586
Power M. E., Dietrich W. E., and Finlay J. C. (1996). Dams and downstream aquatic biodiversity: potential food web consequences of hydrologic and geomorphic change. Environ. Management. 20, 887–895. doi: 10.1007/BF01205969
Qu X., Peng W., Liu Y., Zhang M., Ren Z., Wu N., et al. (2019). Networks and ordination analyses reveal the stream community structures of fish, macroinvertebrate and benthic algae, and their responses to nutrient enrichment. Ecol. Indicators. 101, 501–511. doi: 10.1016/j.ecolind.2019.01.030
Robinson G. V., Porter T. M., Maitland V. G., Wright M. T. G., and Hajibabaei M. (2022). Multi-marker metabarcoding resolves subtle variations in freshwater condition: Bioindicators, ecological traits, and trophic interactions. Ecol. Indicators. 145, 109603. doi: 10.1016/j.ecolind.2022.109603
Shi J., Du Y., Zhang Y., Zhang M., Cai Q., and Qu X. (2020). Effects of multiple runoff dams on diel drift mechanisms of macroinvertebrate. Res. Environ. Sci. 33, 1894–1900. doi: 10.13198/j.issn.1001-6929.2020.05.27
Simeone D., Tagliaro C. H., and Beasley C. R. (2021). Filter and deposit: a potential role of freshwater mussels in ecosystem functioning associated with enhanced macroinvertebrate assemblage structure in a Neotropical river. Hydrobiologia. 848, 4211–4223. doi: 10.1007/s10750-021-04633-7
Song Y., Cheng B., and Hu W. (2018). Quantitative analysis of conservation priority for fish species in Heishui River. J. Hydroecology. 39, 65–72. doi: 10.15928/j.1674-3075.2018.06.010
Ståhl P. P. G., Riikka P. D., Zhang L., Nordström M. C., and Kortsch S. (2025). Food web robustness depends on the network type and threshold for extinction. Oikos. 2025, e11139. doi: 10.1111/oik.11139
Stouffer D. B., Sales-Pardo M., Sirer M. I., and Bascompte J. (2012). Evolutionary conservation of species’ roles in food webs. Science. 335, 1489–1492. doi: 10.1126/science.1216556
Su C., Zhang Q., Zhao Y., Li F., Wei C., and Shan X. (2024). Diversity and keystone species of the benthic fish and invertebrate community in the waters of the Changdao Archipelago. Prog. Fishery Sci. 45, 82–95. doi: 10.19663/j.issn2095-9869.20230713001
Szoszkiewicz K., Jusik S., Lewin I., Czerniawska-Kusza I., Kupiec J. M., and Szostak M. (2018). Macrophyte and macroinvertebrate patterns in unimpacted mountain rivers of two European ecoregions. Hydrobiologia. 808, 327–342. doi: 10.1007/s10750-017-3435-5
Wang Z., Dong X., Boeck H. J., Dong G., Jiang S., Chen J., et al. (2025). Dominant species rather than plant biodiversity shape grassland resistance and recovery in response to heatwaves and mowing. J. Ecol. doi: 10.1111/1365-2745.70138
Wang H., Hernandez J. M., and Van Mieghem P. (2008). Betweenness centrality in a weighted network. Phys. Rev. E. 77, 046105. doi: 10.1103/PhysRevE.77.046105
Wang Y., Kindong R., Gao C., and Wang J. (2024). Identification of keystone species in ecological communities in the East China Sea. Fishes. 8, 224. doi: 10.3390/fishes8050224
Wang F. and Tang Y. (2019). Determination of key species in the food web and their impact on the robustness. Biodiversity Science. 27, 1132–1137. doi: 10.17520/biods.2019208
White J. W., Rassweiler A., Samhouri J. F., Stier A. C., and White C. (2014). Ecologists should not use statistical significance tests to interpret simulation model results. Oikos 123, 385–388. doi: 10.1111/j.1600-0706.2013.01073.x
Xiong C., He J.-Z., Singh B. K., Zhu Y.-G., Wang J.-T., Li P.-P., et al. (2021). Rare taxa maintain the stability of crop mycobiomes and ecosystem functions. Environ. Microbiol. 24, 1907–1924. doi: 10.1111/1462-2920.15262
Xue Y., Chen H., Yang J. R., Liu M., Huang B., and Yang J. (2018). Distinct patterns and processes of abundant and rare eukaryotic plankton communities following a reservoir cyanobacterial bloom. ISME J. 12, 2263–2277. doi: 10.1038/s41396-018-0159-0
Zhang M., Munoz-Mas R., Martinez-Capel F., Qu X., Zhang H., Peng W., et al. (2018). Determining the macroinvertebrate community indicators and relevant environmental predictors of the Hun-Tai River Basin (Northeast China): A study based on community patterning. Sci. Total Environment. 634, 749–759. doi: 10.1016/j.scitotenv.2018.04.021
Zhao L., Zhang H., O'Gorman E. J., Tian W., Ma A., Moore J. C., et al. (2016). Weighting and indirect effects identify keystone species in food webs. Ecol. Letters. 19, 1032–1040. doi: 10.1111/ele.12638
Zheng B. and Yin X. W. (2023). Assembly mechanism of macroinvertebrate metacommunities and ecological factors of multiple aspects of beta diversity in a boreal river basin, China. Front. Ecol. Evol. 11, 1131403. doi: 10.3389/fevo.2023.1131403
Zhou C., Gui H., and Zhou K. (2003). Larval key to families of Ephemeroptera from China (insecta). J. Nanjing Normal University. Natural Science. 26, 65–68. doi: CNKI:SUN:NJSF.0.2003-03-010
Keywords: keystone species, high-connectivity species, macroinvertebrates, community stability, river ecosystem
Citation: Liu Y, Zhang M, Peng W and Qu X (2025) High-connectivity species drive macroinvertebrate community stability in river ecosystems. Front. Ecol. Evol. 13:1640255. doi: 10.3389/fevo.2025.1640255
Received: 03 June 2025; Accepted: 09 September 2025;
Published: 30 September 2025.
Edited by:
Francesco Cerasoli, University of L’Aquila, ItalyReviewed by:
Xuwang Yin, Dalian Ocean University, ChinaRaphael Ligeiro, Federal University of Pará, Brazil
Copyright © 2025 Liu, Zhang, Peng and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhang, emhhbmdtaW5AaXdoci5jb20=
 Yang Liu
Yang Liu Min Zhang
Min Zhang Wenqi Peng1,3
Wenqi Peng1,3 Xiaodong Qu
Xiaodong Qu