- Department of Biology, York University, Toronto, ON, Canada
Foundational shrub species can support vertebrate communities within desert ecosystems. These shrubs provide thermal refuge to aid in temperature amelioration and to escape predation. Within Southern California, USA, harsh abiotic conditions influence the frequency of these shrub-animal interactions. We tested the hypothesis that increasing shrub density will positively influence local vertebrate communities across a variety of arid ecosystems within Southern California. We used a combination of camera trapping and temperature pendants across a 2-year field study to assess the effects of shrub density and near-surface air temperature on vertebrate community composition. Sites were established across Southern California, each consisting of four 20 m radius microsites, with shrub densities ranging from 0 to 14 individuals. Increasing shrub densities significantly increased the frequency of observation and richness of local vertebrate communities. Relatively higher near-surface air temperatures (NSAT) significantly decreased vertebrate observations, richness, and evenness. Sites with relatively higher annual aridity negatively influenced vertebrate species observations and richness, but could be offset by increasing shrub densities. While shrub encroachment in many ecosystems may have negative impacts on species biodiversity, our findings suggest that increasing densities of foundational shrub species positively influences vertebrate community measurements and composition across varying arid ecosystems. Understanding how these foundational shrub species can be used to assess vertebrate communities can provide key insight into vertebrate-shrub interactions and how these densities can shape the biodiversity of an ecosystem.
Introduction
Within the last decade, Southern California has experienced increasingly harsh climatic conditions, with a combination of record low rainfall and temperature highs resulting in extended drought events (Mann and Gleick, 2015). With increasing anthropogenic changes, animal species have become dependent on both inter- and intraspecific interactions to reduce potential adverse effects (Dangles et al., 2018; Rahman and Candolin, 2022). To reduce harsh abiotic conditions, animals will associate with foundational species, defined as species that play a strong role in structuring an ecological community and define an ecosystem through physically modifying the environment and creating habitats (Westphal et al., 2018; Ellison, 2019). Foundational species often exhibit facilitative associations with animals, where one interacting species benefits while the other is unaffected (Callaway and D’Antonio, 1991; Noble et al., 2016; Lortie et al., 2016, Lortie et al., 2018; Dangles et al., 2018; Ellison, 2019; Lucero et al., 2020, Lucero et al., 2022). Foundational species define ecosystems by shaping the biodiversity of associating species and manage ecosystem processes through erosion control, biodiversity support, and microclimatic regulation (Ellison, 2019; Lortie et al., 2021). Within Southern California deserts, shrubs act as foundational species, providing benefits to both plant and animal communities (Lortie et al., 2016; Zuliani et al., 2021). Vertebrate species are reliant on foundational shrub species (Pugnaire et al., 1996; Braun et al., 2021; Zuliani et al., 2023a) as they are used to escape predation (Filazzola et al., 2017; Salido and Vicente, 2019), thermoregulate (Ivey et al., 2020; Gaudenti et al., 2021; Zuliani et al., 2023b), and as a food source (Lortie et al., 2020). Further understanding the facilitative associations between foundational shrubs and vertebrate species can provide key insight into how environmental resources are used both by vertebrate individuals and communities.
Given that foundational shrubs positively influence local animal communities, increasing the number of shrubs available should provide more opportunities for facilitative associations. In terms of plant-animal interactions, the density of shrubs can influence the net outcome of animal interactions while also influencing the local community composition (Springer et al., 2003; Zuliani et al., 2021). As shrub density increases within an ecosystem, vertebrate species are given more opportunities to benefit from the facilitative associations, such as having more areas to thermoregulate (Milling et al., 2018; Zuliani et al., 2023b). For instance, the federally endangered species Gambelia sila, the Blunt-Nosed Leopard Lizard, uses shrubs within the desert of Southern California to thermoregulate, and has been predicted to have higher abundances as shrub densities increase (Ivey et al., 2020, Ivey et al., 2022; Zuliani et al., 2023b). While shrub cover is more commonly measured when assessing plant communities (Lortie et al., 2020), it can be used in tandem with shrub density to predict animal abundances (Van Auken, 2009; Zuliani et al., 2023a).
While foundational shrubs can positively influence animal communities, desert and grassland ecosystems globally are experiencing shrub encroachment, a phenomenon where woody shrub species increase in density, contributing to significant changes in vegetation cover (Van Auken, 2009; Losapio et al., 2024). Shrub encroachment has complex and sometimes contradictory effects on ecosystem health and biodiversity with traditional perspectives viewing shrub encroachment negatively, as it can increase desertification and reduce diversity in desert and grassland ecosystems (Van Auken, 2009; McCleery et al., 2018). For example, high densities of woody shrubs can negatively affect avian species distribution and diversity (Andersen and Steidl, 2019). However, recent studies have revealed that increasing shrub cover can also benefit wildlife, though these effects are highly context and species dependent (Stanton et al., 2021). In North American arid ecosystems, studies have documented positive relationships between increasing shrub densities and associated species (Stanton et al., 2018; De Souza et al., 2022). Vertebrate species that associate with foundational shrubs generally benefit from encroachment through increased abundance and richness (Zuliani et al., 2023a; Owen et al., 2024). Some Southern California ecosystems, such as the Carrizo Plain National Monument, exemplify these benefits as encroachment can reconvert arid grasslands back into a shrubland (Browning et al., 2008). Increases in shrub density and cover, particularly of foundational species, can promote facilitative associations that positively influence vertebrate abundance and richness (Whitford, 1997; Eldridge and Soliveres, 2014; Schooley et al., 2018; Zuliani et al., 2024). Understanding how changing shrub densities influence animal community provides valuable insights for assessing community composition and informing conservation practices.
Desert ecosystems in Southern California are experiencing higher frequencies of mega-droughts (Reynolds et al., 1999; Kogan and Guo, 2015; Gols et al., 2021). As temperatures increase within deserts, vertebrate associations change to promote activities that are more suitable for thermoregulation and reduce abiotic stressors (Moore et al., 2018). High near-surface air temperatures (NSAT) will influence species at both an individual and community level (Newbold, 2018). These increasing temperatures will cause a significant decline in vertebrate communities by reducing seasonal migration patterns and behavior (Newbold, 2018; Riddell et al., 2021). However, the effects increasing temperatures have on vertebrates are species dependent and can be mediated through ecosystem resources, such as the presence of burrows and shrubs (Pike and Mitchell, 2013; Riddell et al., 2021; Zuliani et al., 2021). Within desert environments, high temperatures are associated with reduction in both humidity and precipitation (Walker and Landau, 2018). Under extreme abiotic conditions, such as high temperatures and significantly low precipitation, the regions within southern California are classified as arid or semi-arid (Abella et al., 2012; Marengo and Bernasconi, 2015). As global temperatures increase, vertebrate species will continue to depend on ecosystem resources, including shrubs, to mitigate harsh abiotic conditions.
As the frequency of these mega-drought events increase in Southern California, ecosystems will become more arid (Germano et al., 2011; Abella et al., 2012; Marengo and Bernasconi, 2015). Increasing aridification of dryland ecosystems is causing a dynamic shift in the structure of ecosystems as plant and animal communities adjust to new conditions (Hacker and Gaines, 1997; James and Tallis, 2019). Across Southern California, there is an increasing aridity gradient in dryland ecosystems, suggesting that local species utilize ecosystem resources differently to reduce harsh abiotic conditions (Welles and Funk, 2021). As these ecosystems become more arid, the negative impacts on plant and animal communities become more prevalent (Lucero et al., 2020). Within animal communities, aridity can have negative impacts on their associations, becoming more reliant on ecosystem resources, such as shrubs, which are more readily available at higher densities (Tews et al., 2004; Zuliani et al., 2021). Furthering the knowledge of how variations in aridity influence local animal species, and how these species utilize ecosystem resources to ameliorate these stressors, can increase our understanding of how these conditions alter environmental processes and community associations.
Determining the effects of shrub density on vertebrate community composition can further the current understanding of facilitative associations and provide insight on how this ecosystem resource is used in dryland ecosystems. Here, we conducted a 2-year study at six different sites across Southern California. We tested the hypothesis that increasing shrub density will positively influence vertebrate communities within Southern California. In this study, we tested the following predictions:
1) Shrub Density at Microsite Level
a. Increasing shrub density will increase the total frequency of observations, richness, and evenness of vertebrate species at the microsite level.
b. The effects of increasing shrub densities at microsite level will positively influence vertebrate community composition.
2) Temperature at Microsite Level
a. Increasing near-surface air temperature will decrease the total observations, richness, and evenness of vertebrate communities.
3) Shrub density & Aridity at Site Level
a. The positive effects of site-level estimates of shrub density on animal communities will increase with increasing relative aridity between sites.
Methods
Study sites
Our study was conducted across arid ecosystems in Southern California for 30-days between May and June 2022 and 2023. Six study sites were divided within the Carrizo Plain National Monument (35.11566, -119.62069), the Cuyama Valley (34.848726, -119.48312), and the Mojave Desert (35.851515, -116.18671; Figure 1). At each study site, four microsites were established, approximately 100 m away from each other, ranging from no shrubs to relatively high densities (the densest shrub patches within a site) (n = 24). To establish each microsite, a random centroid was selected to act as the middle of the 20 m radius microsite. To calculate shrub density, the number of shrubs within the 20 m radius was counted. Shrub cover was measured for each shrub within a microsite by taking the longest dimensional width of the shrub, the perpendicular length, and the height to the highest living tissue (Filazzola et al., 2017; Zuliani et al., 2021). Shrub canopy cover was then estimated by calculating the volume of the shrub individual using the formula of a sphere (Zuliani et al., 2021).
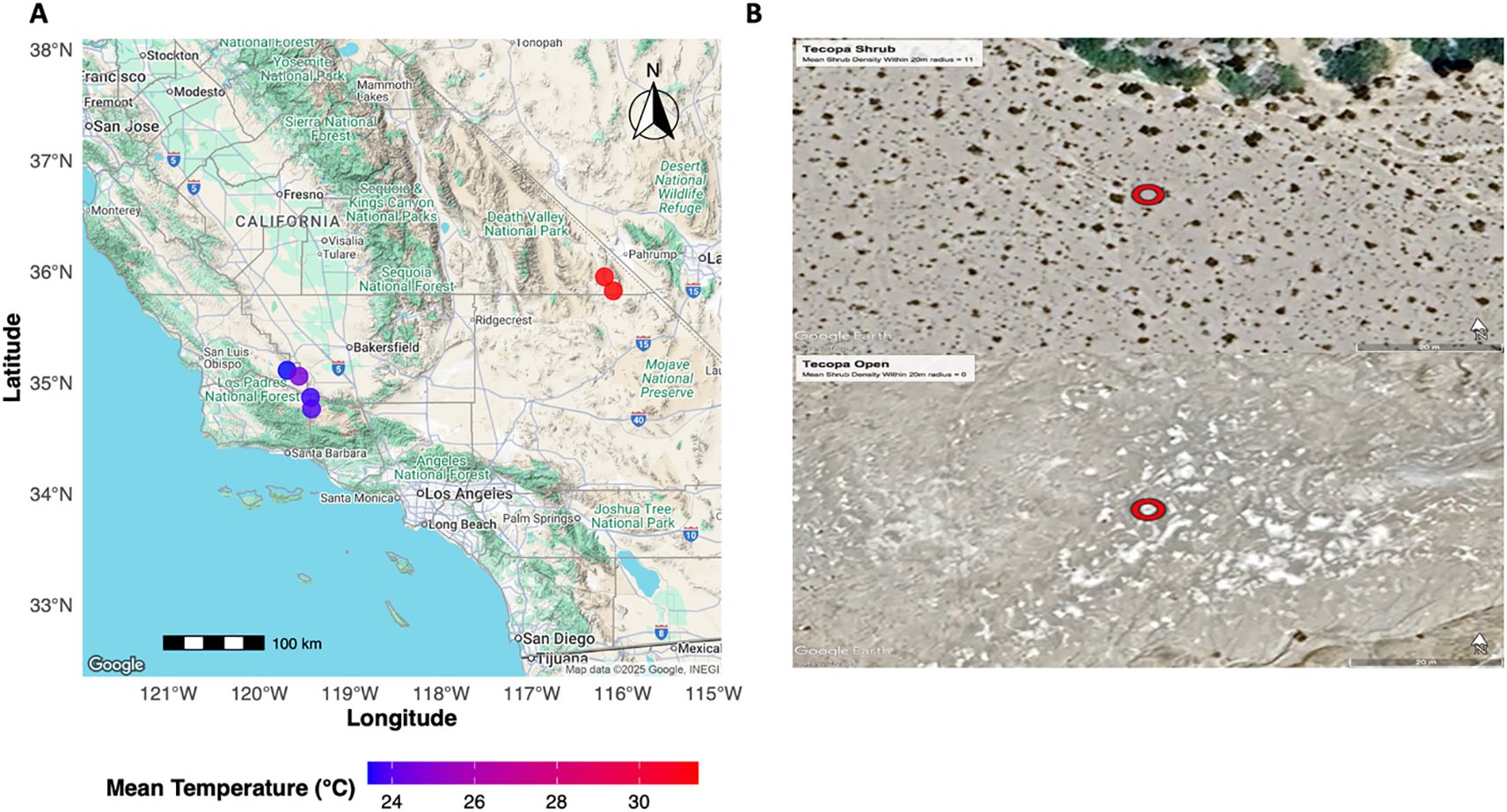
Figure 1. A map (A) of study sites sampled across Southern California. Average temperatures were recorded per study site and displayed using a blue-red color gradient. Images (B) of high and low shrub densities were included to better illustrate the contrast between sample sites. Centroids of the sites are indicated by red open circles. Sites were selected based on the presence of the foundational shrub species Ephedra californica. The map was generated utilizing the R package ggmaps and images of sites were taken via satellite imagery from Google Earth TM.
Study species
Ephedra californica is one of the most common native woody plant species within Southern California. Ephedra californica typically grows 0.25–1 meter in height with a similar spread within a 5–10-year period, forming dense, rounded canopies with distinctive jointed, photosynthetic stems that lack true leaves (Sawyer et al., 2009; Whitford and Steinberger, 2020; Braun et al., 2021). Ephedra californica is a flowering shrub species with a blooming season from March to May, is typically found in sandy soils and the well-developed cryptogram layer of the Mojave Desert (Sawyer et al., 2009; Braun et al., 2021). This is a vital foundation species in California desert ecosystems as it is one of the most common facilitative species in these regions and is at the base of interactions with both plant and animal communities (Lortie et al., 2018; Filazzola et al., 2017). Ephedra californica is resilient and can survive severe abiotic stressors, such as drought, extreme heat, and lack of nutrition, while also surviving mechanical damage, such as branch breaking or herbivory (Lortie et al., 2018). Ephedra californica is used by several species of vertebrates including the Blunt-Nosed Leopard Lizard, Giant Kangaroo Rat, and San Joaquin Jack Rabbits (Prugh and Brashares, 2010; Noble et al., 2016). One frequently observed species at our study sites was Dipodomys heermanni (Heermann’s Kangaroo Rat). This is a small nocturnal rodent species present in relatively high abundances within Southern California and is known for creating interconnecting burrows throughout these arid ecosystems (Prugh and Brashares, 2010). This species consumes the seeds found underneath shrub canopies, resulting in them showing high associations to foundational shrub species. Lepus californicus (Black-tailed Jackrabbit), is a large species active both during the day and at night, is frequently found in the Carrizo Plain and Cuyama Valley, and is commonly detected using camera traps. It typically consumes vegetation in open areas and under shrub canopies (Johnson and Anderson, 1984).
Camera trapping
VIKERI Model A1 camera traps were used to sample animal communities at each site during the day and night (Noble et al., 2016). No flash was emitted by cameras to ensure that there were no disturbances to interacting animal species. Two camera traps were deployed on 20 cm stakes driven in the ground, facing each other at the edge of each 20 m radius microsite. A total of 48 camera traps were deployed across all microsites (6 sites, 4 microsites, 2 cameras per site). Each camera trap was set to medium sensitivity with a 1-minute delay to minimize the number of misfired photos taken from background activity (Tourani et al., 2020; Zuliani et al., 2021; Mashintonio et al., 2022; Leorna and Brinkman, 2024). Camera traps were checked approximately every four to five days to ensure proper function for the 30-day field study between May and June 2022 and 2023. Camera locations were not food-baited. The images were saved on 24 GB SD cards as Joint Photographic Export Group (JPEG) files and examined during data extraction. Each photo was taken as a new species instance with photo ID, site, year, date, camera number, shrub density, presence or absence of animal, species of animal, and camera trap timestamp all recorded. Independent photos were defined as when the individual animal was not observed at the same position within the 1-minute lag-time (Lepard et al., 2018; Zuliani et al., 2021). Camera trap rate of capture was calculated per year by taking the difference between the number of new species instances, with the total number of observations for the 30-day duration (Noble et al., 2016; Zuliani et al., 2021). All data taken from camera traps each year were then combined into one density datasheet.
Species validation
Identification of vertebrate individuals to the species level via camera traps is challenging as the exact physiological characteristics can be difficult to distinguish (Dorning and Harris, 2019). For example, differentiating between Dipodomys merriami and Dipodomys microps is dependent on coloration and variations in length (Sjoberg et al., 1984; Siciliano-Martina et al., 2023). Vertebrate species observations through camera trap photos were validated using a combination of Wildlife Insight (Vélez et al., 2023) and the iNaturalist application (Unger et al., 2021; iNaturalist). Combining both applications for species validation provided the most plausible identification for images with species present and were further validated by individual observers. Unknown animals detected in images were labeled as ‘unknowns’ and were later excluded from the community composition data. Images were saved as JPEG files on hard drives for data analysis.
Microclimatic microsite level measures
Near-surface air temperature for each site was recorded using OMEGA USB loggers, suspended approximately 20 cm above ground on a wooden stake (Ashcroft, 2018; Terando et al., 2018). At shrub density microsites, two loggers were placed under random shrub canopies, while at open microsites two loggers were placed next to camera traps. These loggers remained within the shrub and open microsites for the duration of the field experiment. Hourly near-surface air temperatures were logged (°C) and used to calculate the daily means.
Site-level climate measures
The annual aridity of each region was estimated utilizing the De Martonne Aridity Index equation AI = P/(MAT + 10), where P is the total annual precipitation in mm, and MAT is the Mean Annual Temperature in °C (Zomer et al., 2008; Gebremedhin et al., 2018; Rafiq et al., 2023). Site-level temperature and precipitation data were collected and compiled from local weather stations within 1–5 km of the study sites (Zuliani et al., 2024).
Statistical analysis
All statistical analyses were conducted in R version. 4.3.1 (R Core Development Team, 2024). A Pearson’s correlation test was used to determine the relationship between shrub density and cover and to test if density could be used as a proxy for shrub cover. Density and cover were significantly correlated (r = 0.749, p-value < 0.001) and had moderate to high degree of multicollinearity in GLMs (Supplementary Table S1). Therefore, only density was used for GLMs. A Pearson’s correlation test was used to test for a relationship between temperature and humidity and to determine if temperature could be used as a proxy for humidity in the GLMs. Temperature and humidity at microsites were significantly correlated (Supplementary Figure S1; r = -0.91, p-value < 0.001). Thus, temperature was used in GLMs to represent the microclimate of microsites. The number of individual animal observations (henceforth termed “observations”), species richness, and evenness of vertebrate species was tested at the fine-scale to determine the influence of shrub density within a 20-meter radius and near-surface air temperature. Second degree general linear mixed models (GLMMs) were used to examine these relationships in Southern California for each year independently, with shrub density and near-surface air temperature set as factors and nested within site. Collinearity between shrub density and cover for observations, richness, and evenness was tested in each model using the function ‘check_collinearity’ from the performance R package (Lüdecke et al., 2021). Multiple linear and non-linear models for observations, richness, and evenness, were tested with the models yielding the lowest AIC scores being used for all statistical analyses (Supplementary Table S2; Portet, 2020). Total animal observations and richness were treated as a Poisson distribution, while evenness was treated as a Gaussian distribution. Site-level analysis was conducted utilizing a second degree general linear mixed model with total shrub density and site level aridity as factors. Site-level statistical analyses were conducted with observations and richness treated as a Poisson distribution, while evenness was treated as a Gaussian distribution. All site-level models had shrub density and aridity as covariate. Multivariate analysis of composition was tested using the vegan package (Oksanen et al., 2022). Principle Coordinate Analyses (PCOAs) compared the different vertebrate communities at both shrub and open microsites and to assess whether the composition of the communities varied at increasing shrub density and open microsites within Southern California (Dray et al., 2006).
Results
Shrub density
A total of 250,000 photos was taken, with 2022 yielding 58,000 photos and 2023 yielding 192,000 photos. Models testing vertebrate observations, richness, and evenness showed high collinearity between shrub density and cover (Supplementary Table S1). In 2022, increasing microsite level shrub density positively influenced vertebrate observations, but did not influence richness and evenness (Figure 2; Table 1). In 2023, vertebrate observations and richness significantly increased with increasing microsite level shrub density, while evenness had no effect (Figure 2; Table 1).
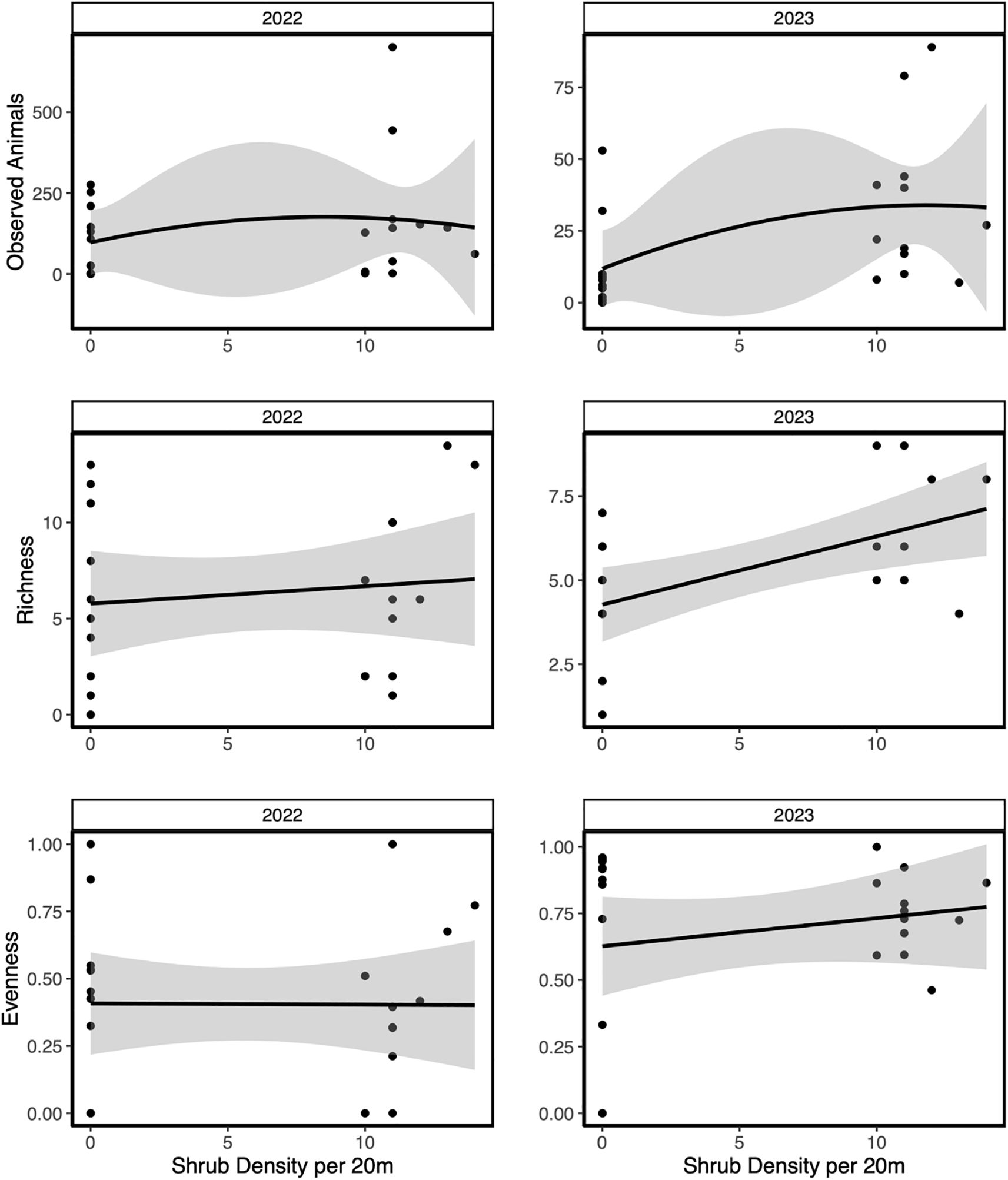
Figure 2. The relative effects of increasing shrub density across arid sites within Southern California. Data were collected from camera traps in 2022 and 2023 field seasons then split by year to display the variation in vertebrate community measurements including Observed animals, Richness, and Evenness. Shaded regions indicate 95% confidence intervals.
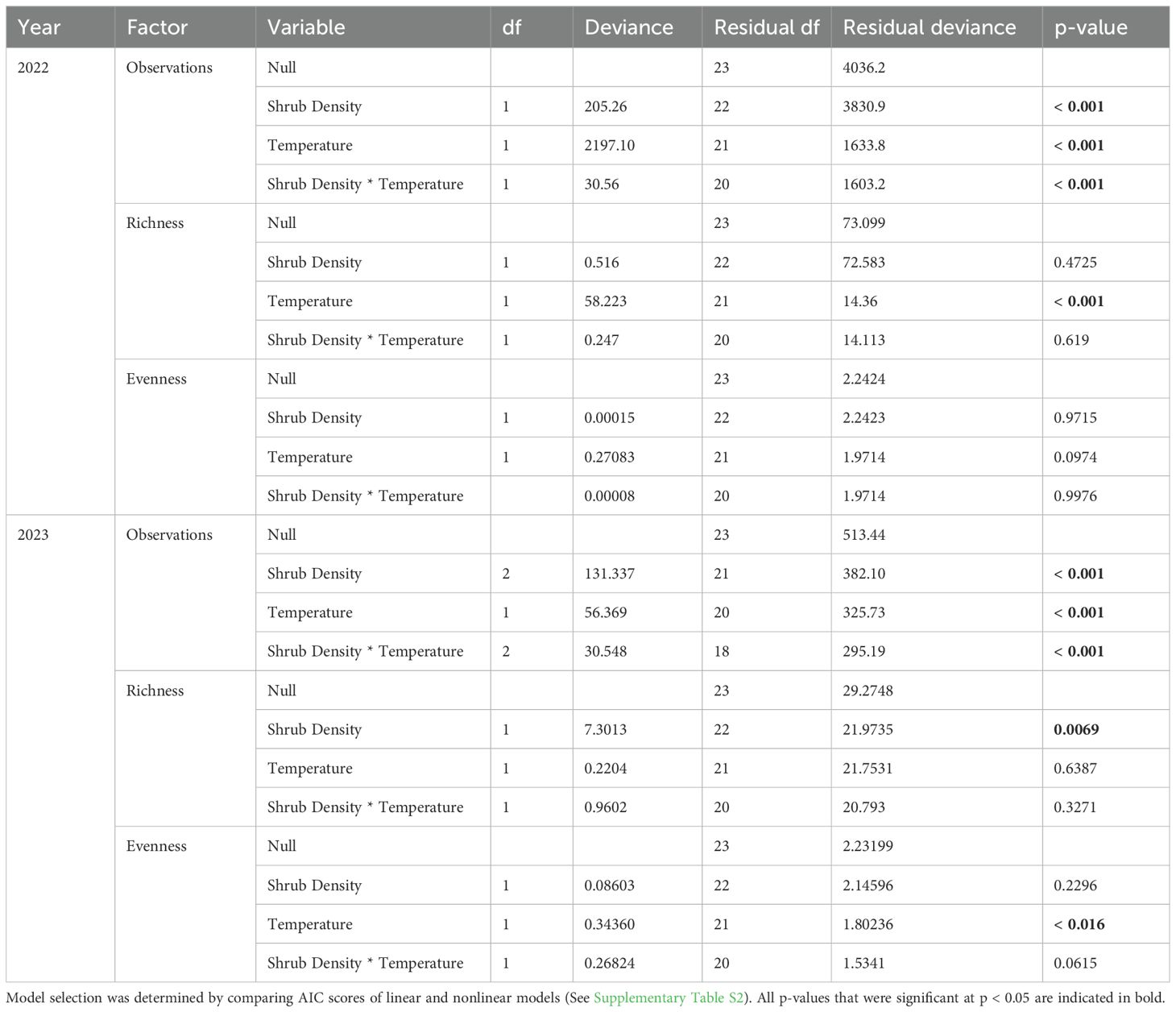
Table 1. Analysis of microsite-level vertebrate observations, richness, and evenness from general linear model for study period with shrub density and logger-temperature as factors.
Near-surface air temperature
In 2022, vertebrate observations and richness significantly decreased with increasing near-surface air temperatures, while evenness was unaffected (Figure 3; Table 1). In 2023, vertebrate observations significantly decreased with increasing near-surface air temperatures, while evenness significantly increased (Figure 3; Table 1).

Figure 3. The relative effects of near-surface air temperature across arid study sites within Southern California. Data were combined from temperature loggers used in 2022 and 2023 field seasons then split by year to display the variation in vertebrate community measurements including Observed Animals, Richness, and Evenness. Shaded regions indicate 95% confidence intervals.
Community contrasts
Dipodomys heermanni was the most observed vertebrate species observed with 2397 observations (Supplementary Table S3; Figure 3). The composition of vertebrate species communities did not significantly vary across shrub and open microsites (Figure 4; PERMANOVA, F2 = 0.2553, R2 = 0.0294, p – value = 0.6184). The composition of vertebrate species communities significantly differed across various sites (Figure 5; PERMANOVA, F2 = 3.736, R2 = 0.0184, p-value = 0.0409) with Carrizo sites having a significantly different community composition than Tecopa sites (Supplementary Figure S3; Observed p-value = 0.0166). Removal of Dipodomys heermanni observations resulted in no significant differences in vertebrate community composition (F2 = 0.975, R2 = 0.0191, p-value = 0.3938).
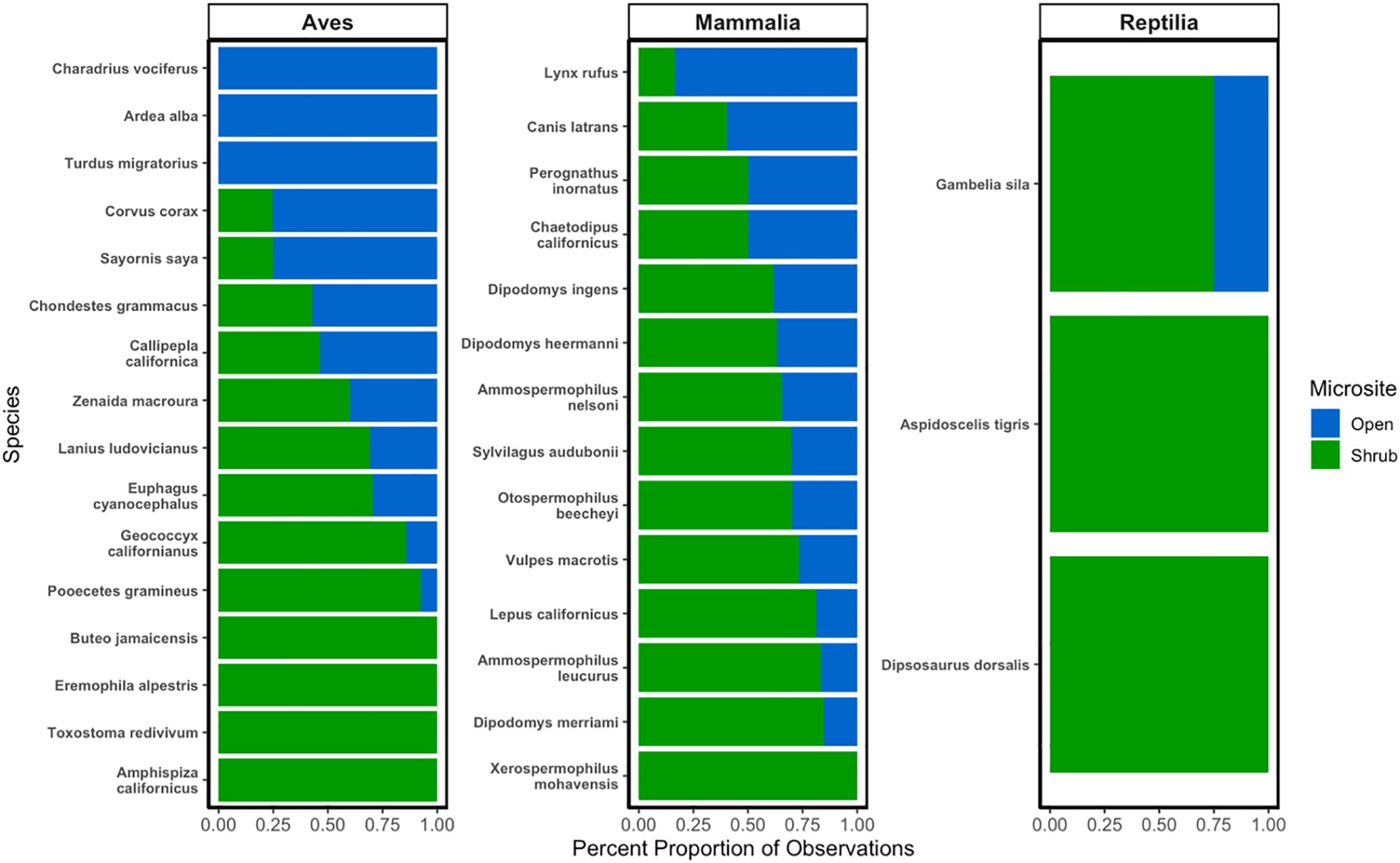
Figure 4. The relative percent proportion of vertebrate species observations across shrub and open microsites across arid ecosystems within Southern California. X-axis displays the percent proportion (%) of individuals at both shrub and open microsites. Y-axis displays the scientific names of each observed individual during both the 2022 and 2023 field seasons. More details on each species can be found in the Supplementary Table S3.
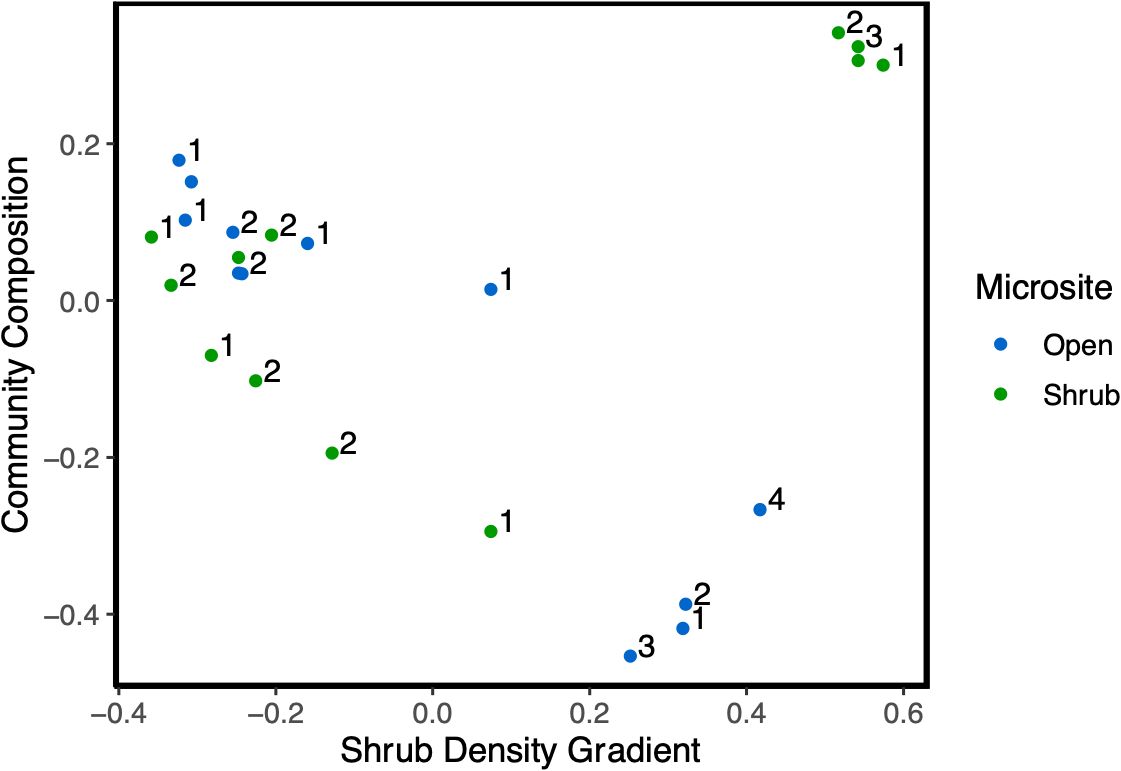
Figure 5. PCOA figure displaying the relative similarity in community composition across a shrub density gradient. Data were combined from camera traps used in 2022 and 2023 field seasons then split by microsite to display the similarities between community compositions.
Site-level shrub density and aridity effects
In 2022, vertebrate observations and evenness significantly increased with higher total site-level shrub density, while in 2023, only vertebrate observations significantly increased (Table 2). In 2022, vertebrate observations and richness increased at higher aridity sites while evenness was unaffected (Figure 6; Table 2). In 2023, only vertebrate observations significantly decreased at higher aridity ecosystems (Figure 6; Table 2).
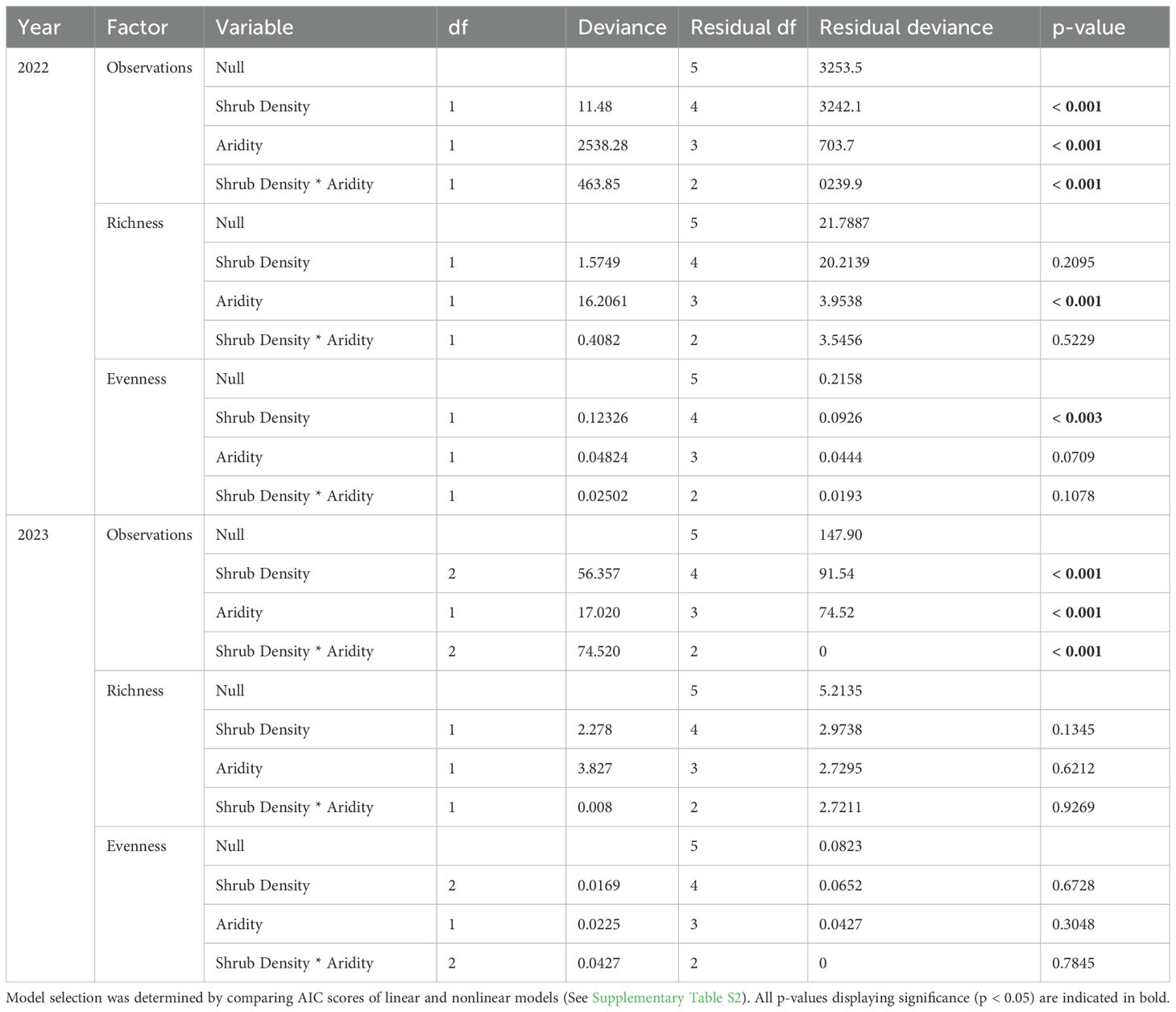
Table 2. Analysis of site-level vertebrate observations, richness, and evenness from general linear mixed model for study period with shrub density and aridity as factors.
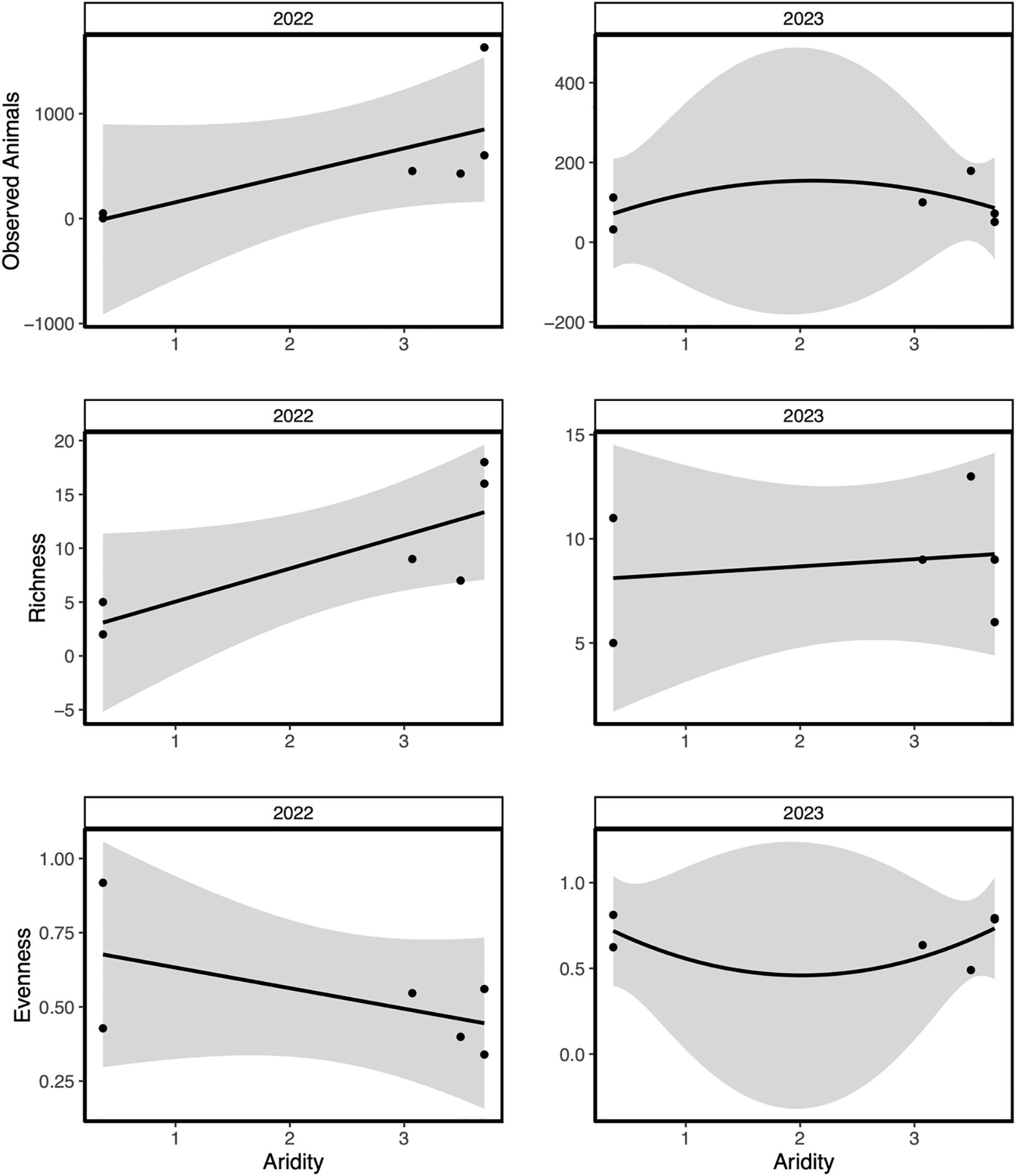
Figure 6. The relative effects of aridity across various arid sites within Southern California. Data were collected from weather stations located near each site. 2022 and 2023 field seasons were split by year to display the variation in vertebrate community measurements including Observed Animals, Richness, and Evenness. Shaded regions indicate a 95% confidence interval.
Discussion
In this study, we examined the effects shrub densities on the vertebrate community composition, individual observations, species richness, and evenness across ecosystems within Southern California. We found support for the hypothesis that increasing shrub density will positively influence vertebrate communities across ecosystems within Southern California. Shrub density positively influenced the observations and richness of vertebrate species. Fine-scale temperatures had a significant negative impact on individual observations and species richness in 2022 and a negative influence on individual observations and evenness in 2023. Sites were significantly more arid in 2022 than in 2023. Community compositions significantly varied between shrubs and open microsites, suggesting that shrubs promote vertebrate communities. Individual vertebrate observations and species richness decreased at sites with lower aridity scores in 2022, while only individual observations decreased at more arid sites in 2023.
In this study, we found that shrub density positively influences animal vertebrate communities. Structural resources, both artificial and natural, are critical in studies that not only observe individual associations, but also measure key structural components of an ecosystem. Globally, foundational shrubs act as benefactor species (Ruttan et al., 2021) through temperature amelioration (Ivey et al., 2020; Zuliani et al., 2023a), production of seeds and other food sources (Lortie et al., 2020), and refuge from predation (Filazzola et al., 2017). However, the effects these foundational shrubs have on vertebrate communities may not have the same influence and can be context-dependent (Stanton et al., 2018). Ephedra californica provides these facilitative effects that are necessary functions for vertebrate species (Westphal et al., 2018; Zuliani et al., 2021). Smaller vertebrate species including Dipodomys heermanni, Gambelia sila, and Ammospermophilus leucurus, utilize these shrubs to reduce predator-prey interactions (Longland and Dimitri, 2021). Species that are reliant on these positive interactions tend to be in locations where ecological resources, including shrub density and cover, are readily accessible and abundant (Zuliani et al., 2023a). For instance, Gambelia sila, the Blunt-nosed Leopard Lizard, is known to use areas of high shrub density and cover to aid in thermoregulation (Ivey et al., 2020; Lortie et al., 2021; Zuliani et al., 2023b). Other desert species, such as Dipodomys ingens (the Giant Kangaroo Rat) and Lepus californicus (the Black-Tailed Jack Rabbit), utilize these shrubs not only for cooling but for foraging and protection from predation (Johnson and Anderson, 1984; Prugh and Brashares, 2010). However, as shrub densities increase, the effects of shrub encroachment may become more prevalent. This increase in shrub density will reconvert these ecosystems into shrublands (Browning 2008), while also enhancing ecosystem functioning through the reduction of desertification and microclimatic buffering (Eldridge and Soliveres, 2014; Filazzola et al., 2017). However, the effects these shrubs have are ecosystem specific, and have varying impacts on local animal communities. Understanding the importance of the direct interaction between these shrubs and local animal communities, across an increasing stress gradient, can provide insight into the local animal community composition and utilization of these shrubs.
The composition of these vertebrate communities, while not different between shrub and open microsites, did differ across sites in Southern California. This suggests that while our study sites have similar community compositions, the variation in their shrub density and temperature directly influences individual associations. Vertebrate species, such as Lepus californicus, while found at all tested sites, were much more frequently observed at Cuyama and the Carrizo Plain National Monument than in Tecopa. This suggests that while some species are able to inhabit multiple ecosystems, sites with more favorable conditions are likely to be more suitable and preferred (Vale and Brito, 2015). Several factors can cause this variation in community composition including elevation, temperature gradient, available vegetation, aridity, shrub cover and density, and water availability (Marengo and Bernasconi, 2015; Welles and Funk, 2021; Ivey et al., 2022; Hillier-Weltman et al., 2025). Previous studies found that kangaroo rat species like Dipodomys heermanni forage more actively in open areas than in woody shrub habitats, which initially appears to contrast with our findings that shrub density increases vertebrate observations (Daly et al., 1992; Upham and Hafner, 2013). However, this difference likely reflects the distinction between foraging behavior and overall habitat use, as our camera traps captured all vertebrate activities including sheltering and thermoregulation rather than just active foraging. Additionally, moonlight can significantly alter the activity and frequency of observations of these nocturnal rodent species (Upham and Hafner, 2013). The presence of moonlight may influence the foraging behavior and observations of kangaroo rat species as they shift their activity from open areas to those with more cover (White and Geluso, 2007). While our study did not measure moonlight, future studies could take this into consideration when assessing nocturnal animal community composition. Directly analyzing the differences in these communities, in combination with multiple factors that can influence community composition, could further explain not only the associations these vertebrate species have with foundational shrubs, but how the increasing aridity of Southern California deserts is altering these ecosystems.
Anthropogenically driven climatic events, specifically drought and temperature extremes, will continue to increase the aridity of ecosystems across Southern California, influencing both vertebrate community measures and composition. In this study, we found that increasing near-surface air temperatures reduced observations, richness, and evenness of vertebrate species. As these high temperatures become more prevalent and extreme, shelter via shrub canopies may be a crucial resource to mitigate these abiotic conditions (Westphal et al., 2018; Ivey et al., 2020; Gaudenti et al., 2021). Previous studies conducted within the Carrizo National Monument showed a higher frequency of shrub-animal association during peak hours of the day when temperatures reached their maximums (Gaudenti et al., 2021; Zuliani et al., 2021; Ivey et al., 2022), suggesting that temperature amelioration is a direct benefit to local vertebrate communities. These shrub canopies generate shade, reducing both near-surface air and soil temperatures within their microclimate, minimizing the risks of overheating (Huxman et al., 2004). The area underneath these shrubs provides more hospitable microclimates for vertebrate communities in an increasingly arid ecosystem. However, these climate extremes are increasing in intensity and are particularly driven by global warming, which is amplifying aridity through rising temperatures and altered precipitation patterns (Dai, 2013). These increasing temperatures and reduced water availability directly contribute to the increased frequency and severity of mega-drought events (Kogan and Guo, 2015; Mann and Gleick, 2015). Furthering the current understanding of how these increasingly harsh climatic events impact vertebrate community measurements and composition can provide substantial insight into both the management of these ecosystems as well as the preservation of these vertebrate communities.
Conclusions
Our study demonstrates that shrub density strongly and consistently influences vertebrate observations and richness across Southern California desert ecosystems, providing compelling evidence for facilitative interactions between foundational shrubs and vertebrate communities. Microsite-level shrub density emerged as a robust predictor of vertebrate activity, with positive effects across both study years despite varying climatic conditions. Conversely, increasing near-surface air temperatures consistently reduced vertebrate observations and richness, emphasizing the critical role of shrub-mediated thermal refugia in these arid systems. These findings have significant implications for desert conservation under accelerating climate change. As temperatures continue to rise and drought events intensify in Southern California, protecting shrub densities will be essential for maintaining vertebrate diversity and community stability. Our results suggest that conservation strategies should prioritize the preservation of foundational shrub species like E. californica, as they serve as key facilitators supporting vertebrate communities through multiple mechanisms including thermal buffering, predator refuge, and resource provisioning during increasingly harsh climatic conditions.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: doi:10.5063/F1B856K9.
Author contributions
MZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. NG: Methodology, Visualization, Writing – review & editing. SM: Conceptualization, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. CL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1648121/full#supplementary-material
References
Abella S. R., Craig D. J., Smith S. D., and Newton A. C. (2012). Identifying native vegetation for reducing exotic species during the restoration of desert ecosystems. Restor. Ecol. 20, 781–787. doi: 10.1111/j.1526-100X.2011.00848.x
Andersen E. M. and Steidl R. J. (2019). Woody plant encroachment restructures bird communities in semiarid grasslands. Biol. Conserv. 240, 108276. doi: 10.1016/j.biocon.2019.108276
Ashcroft M. B. (2018). Which is more biased: Standardized weather stations or microclimatic sensors? Ecol. Evol. 8, 5231–5232. doi: 10.1002/ece3.3965
Braun J., Westphal M., and Lortie C. J. (2021). The shrub Ephedra californica facilitates arthropod communities along a regional desert climatic gradient. Ecosphere 12, e03760. doi: 10.1002/ecs2.3760
Browning D. M., Archer S. R., Asner G. P., McClaran M. P., and Wessman C. A. (2008). Woody plants in grasslands: post-encroachment stand dynamic. Ecol. Appl. 18, 928–944. doi: 10.1890/07-1559.1
Callaway R. M. and D’Antonio C. M. (1991). Shrub facilitation of coast live oak establishment in central california. Madroño38, 158–169. Available online at: http://www.jstor.org/stable/41424857 (Accessed September 27, 2023).
Dai A. (2013). Increasing drought under global warming in observations and models. Nat. Climate Change 3, 52–58. doi: 10.1038/nclimate1633
Daly M., Behrends P. R., Wilson M. I., and Jacobs L. F. (1992). Behavioural modulation of predation risk: Moonlight avoidance and crepuscular compensation in a nocturnal desert rodent, Dipodomys merriami. Anim. Behav. 44, 1–9. doi: 10.1016/S0003-3472(05)80748-1
Dangles O., Herrera M., Carpio C., and Lortie C. J. (2018). Facilitation costs and benefits function simultaneously on stress gradients for animals. Proc. R. Soc. B: Biol. Sci. 285, 20180983. doi: 10.1098/rspb.2018.0983
De Souza G. F., Ferreira M. C., and Munhoz C. B. R. (2022). Decrease in species richness and diversity, and shrub encroachment in Cerrado grasslands: A 20 years study. Appl. Vegetation Sci. 25, e12668. doi: 10.1111/avsc.12668
Dorning J. and Harris S. (2019). The challenges of recognising individuals with few distinguishing features: Identifying red foxes Vulpes vulpes from camera-trap photos. PloS One 14, e0216531. doi: 10.1371/journal.pone.0216531
Dray S., Legendre P., and Peres-Neto P. R. (2006). Spatial modelling: A comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol. Model. 196, 483–493. doi: 10.1016/j.ecolmodel.2006.02.015
Eldridge D. J. and Soliveres S. (2014). Are shrubs really a sign of declining ecosystem function? Disentangling the myths and truths of woody encroachment in Australia. Aust. J. Bot. 62, 594. doi: 10.1071/BT14137
Ellison A. M. (2019). Foundation species, non-trophic interactions, and the value of being common. IScience 13, 254–268. doi: 10.1016/j.isci.2019.02.020
Filazzola A., Westphal M., Powers M., Liczner A. R., (Smith) Woollett D. A., Johnson B., et al. (2017). Non-trophic interactions in deserts: Facilitation, interference, and an endangered lizard species. Basic Appl. Ecol. 20, 51–61. doi: 10.1016/j.baae.2017.01.002
Gaudenti N., Nix E., Maier P., Westphal M. F., and Taylor E. N. (2021). Habitat heterogeneity affects the thermal ecology of an endangered lizard. Ecol. Evol. 11, 14843–14856. doi: 10.1002/ece3.8170
Gebremedhin M. A., Kahsay G. H., and Fanta H. G. (2018). Assessment of spatial distribution of aridity indices in Raya valley, northern Ethiopia. Appl. Water Sci. 8, 217. doi: 10.1007/s13201-018-0868-6
Germano D. J., Rathbun G. B., Saslaw L. R., Cypher B. L., Cypher E. A., and Vredenburgh L. M. (2011). The San Joaquin Desert of California: Ecologically misunderstood and overlooked. Natural Areas J. 31, 138–147. doi: 10.3375/043.031.0206
Gols R., Ojeda-Prieto L. M., Li K., van der Putten W. H., and Harvey J. A. (2021). Within- patch and edge microclimates vary over a growing season and are amplified during a heatwave: Consequences for ectothermic insects. J. Thermal Biol. 99, 103006. doi: 10.1016/j.jtherbio.2021.103006
Hacker S. D. and Gaines S. D. (1997). Some implications of direct positive interactions for community species diversity. Ecology 78, 1990–2003. doi: 10.1890/0012-9658(1997)078[1990:SIODPI]2.0.CO;2
Hillier-Weltman Z., Lortie C., and Zuliani M. (2025). The influence of native shrub density on bird communities in the southern drylands of California, USA. BMC Ecol. Evol. 25, 63. doi: 10.1186/s12862-025-02410-x
Huxman T. E., Snyder K. A., Tissue D., Leffler A. J., Ogle K., Pockman W. T., et al. (2004). Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 141, 254–268. doi: 10.1007/s00442-004-1682-4
iNaturalist. Available online at: https://www.inaturalist.org.
Ivey K. N., Cornwall M., Crowell H., Ghazian N., Nix E., Owen M., et al. (2020). Thermal ecology of the federally endangered blunt-nosed leopard lizard (Gambelia sila). Conserv. Physiol. 8, coaa014. doi: 10.1093/conphys/coaa014
Ivey K. N., Cornwall M. B., Gaudenti N., Maier P. H., Ghazian N., Owen M., et al. (2022). Temperature-based activity estimation accurately predicts surface activity, but not microhabitat use, in the Endangered heliothermic lizard Gambelia sila. Amphib. Reptile Conserv. 16, 25–34.
James R. S. and Tallis J. (2019). The likely effects of thermal climate change on vertebrate skeletal muscle mechanics with possible consequences for animal movement and behaviour. Conserv. Physiol. 7, coz066. doi: 10.1093/conphys/coz066
Johnson R. D. and Anderson J. E. (1984). Diets of black-tailed jack rabbits in relation to population density and vegetation. J. Range Manage. 37, 79–83. doi: 10.2307/3898830
Kogan F. and Guo W. (2015). 2006–2015 mega-drought in the western USA and its monitoring from space data. Geomatics Natural Hazards Risk 6, 651–668. doi: 10.1080/19475705.2015.1079265
Leorna S. and Brinkman T. (2024). Camera trap sampling protocols for open landscapes: The value of time-lapse imagery. Conserv. Sci. Pract. 6, e13094. doi: 10.1111/csp2.13094
Lepard C. C., Moll R. J., Cepek J. D., Lorch P. D., Dennis P. M., Robison T., et al. (2018). The influence of the delay-period setting on camera-trap data storage, wildlife detections and occupancy models. Wildlife Res. 46, 37–53. doi: 10.1071/WR17181
Longland W. S. and Dimitri L. A. (2021). Kangaroo rats: Ecosystem engineers on western rangelands. Rangelands 43, 72–80. doi: 10.1016/j.rala.2020.10.004
Lortie C. J., Braun J., Westphal M., Noble T., Zuliani M., Nix E., et al. (2020). Shrub and vegetation cover predict resource selection use by an endangered species of desert lizard. Sci. Rep. 10, 4884. doi: 10.1038/s41598-020-61880-9
Lortie C. J., Filazzola A., and Sotomayor D. A. (2016). Functional assessment of animal interactions with shrub-facilitation complexes: A formal synthesis and conceptual framework. Funct. Ecol. 30, 41–51. doi: 10.1111/1365-2435.12530
Lortie C. J., Gruber E., Filazzola A., Noble T., and Westphal M. (2018). The Groot Effect: Plant facilitation and desert shrub regrowth following extensive damage. Ecol. Evol. 8, 706–715. doi: 10.1002/ece3.3671
Lortie C. J., Zuliani M., Ghazian N., Haas S., Braun J., Owen M., et al. (2021). Too much of a good thing: Shrub benefactors are less important in higher diversity arid ecosystems. J. Ecol. 109, 1365–2745.13596. doi: 10.1111/1365-2745.13596
Losapio G., De Moraes C. M., Nickels V., Tscheulin T., Zouros N., and Mescher M. C. (2024). The effects of shrub encroachment on arthropod communities depend on grazing history. Global Ecol. Conserv. 50, e02819. doi: 10.1016/j.gecco.2024.e02819
Lucero J. E., Filazzola A., Callaway R. M., Braun J., Ghazian N., Haas S., et al. (2022). Increasing global aridity destabilizes shrub facilitation of exotic but not native plant species. Global Ecol. Conserv. 40, e02345. doi: 10.1016/j.gecco.2022.e02345
Lucero J. E., Seifan M., Callaway R. M., and Lortie C. J. (2020). Positive associations with native shrubs are intense and important for an exotic invader but not the native annual community across an aridity gradient. Diversity Distributions 26, 1177–1197. doi: 10.1111/ddi.13111
Lüdecke D., Ben-Shachar M., Patil I., Waggoner P., and Makowski D. (2021). performance: an R package for assessment, comparison and testing of statistical models. J. Open Source Software 6, 3139. doi: 10.21105/joss.03139
Mann M. E. and Gleick P. H. (2015). Climate change and California drought in the 21st century. Proc. Natl. Acad. Sci. 112, 3858–3859. doi: 10.1073/pnas.1503667112
Marengo J. A. and Bernasconi M. (2015). Regional differences in aridity/drought conditions over Northeast Brazil: Present state and future projections. Climatic Change 129, 103–115. doi: 10.1007/s10584-014-1310-1
Mashintonio A. F., Harris G. M., Stewart D. R., Butler M. J., Sanderson J., and Russell G. (2022). Estimating species richness with camera traps: Modeling the effects of delay period, deployment length, number of sites, and interference imagery. Wildlife Soc. Bull. 46, e1357. doi: 10.1002/wsb.1357
McCleery R., Monadjem A., Baiser B., Fletcher R., Vickers K., and Kruger L. (2018). Animal diversity declines with broad-scale homogenization of canopy cover in African savannas. Biol. Conserv. 226, 54–62. doi: 10.1016/j.biocon.2018.07.020
Milling C. R., Rachlow J. L., Olsoy P. J., Chappell M. A., Johnson T. R., Forbey J. S., et al. (2018). Habitat structure modifies microclimate: An approach for mapping fine-scale thermal refuge. Methods Ecol. Evol. 9, 1648–1657. doi: 10.1111/2041-210X.13008
Moore D., Stow A., and Kearney M. R. (2018). Under the weather?—The direct effects of climate warming on a threatened desert lizard are mediated by their activity phase and burrow system. J. Anim. Ecol. 87, 660–671. doi: 10.1111/1365-2656.12812
Newbold T. (2018). Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B: Biol. Sci. 285, 20180792. doi: 10.1098/rspb.2018.0792
Noble T. J., Lortie C. J., Westphal M., and Butterfield H. S. (2016). A picture is worth a thousand data points: An imagery dataset of paired shrub-open microsites within the Carrizo Plain National Monument. GigaScience 5, 40. doi: 10.1186/s13742-016-0145-2
Oksanen J., Simpson G., Blanchet F., Kindt R., Legendre P., Minchin P., et al. (2022). “vegan: community ecology package,” in R package version 2.6-4. Available online at: https://CRAN.R-project.org/package=vegan.
Owen E., Zuliani M., Goldgisser M., and Lortie C. (2024). The importance of native shrubs on the distribution and diversity of reptiles and amphibians in the central drylands of Southwestern USA. Biodiversity Conserv. 33, 2131–2151. doi: 10.1007/s10531-024-02851-8
Pike D. A. and Mitchell J. C. (2013). Burrow-dwelling ecosystem engineers provide thermal refugia throughout the landscape. Anim. Conserv. 16, 694–703. doi: 10.1111/acv.12049
Portet S. (2020). A primer on model selection using the Akaike Information Criterion. Infect. Dis. Model. 5, 111–128. doi: 10.1016/j.idm.2019.12.010
Prugh L. and Brashares J. (2010). Basking in the moonlight? Effect of illumination on capture success of the endangered giant kangaroo rat. J. Mammalogy 91, 1205–1212. doi: 10.1644/10-MAMM-A-011.1
Pugnaire F. I., Haase P., and Puigdefabregas J. (1996). Facilitation between higher plant species in a semiarid environment. Ecology 77, 1420–1426. doi: 10.2307/2265539
Rafiq M., Cong Li Y., Cheng Y., Rahman G., Zhao Y., and Khan H. U. (2023). Estimation of regional meteorological aridity and drought characteristics in Baluchistan province, Pakistan. PloS One 18, e0293073. doi: 10.1371/journal.pone.0293073
Rahman T. and Candolin U. (2022). Linking animal behavior to ecosystem change in disturbed environments. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.893453
R Core Team (2023). “_R: A language and environment for statistical computing_,” in R foundation for statistical computing(Vienna, Austria). Available online at: https://www.R-project.org/.
Reynolds J. F., Virginia R. A., Kemp P. R., Soyza A. G. D., and Tremmel D. C. (1999). Impact of drought on desert shrubs: effects of seasonality and degree of resource island development. Ecol. Monogr. 69, 69–106. doi: 10.1890/0012-9615(1999)069[0069:IODODS]2.0.CO;2
Riddell E. A., Iknayan K. J., Hargrove L., Tremor S., Patton J. L., Ramirez R., et al. (2021). Exposure to climate change drives stability or collapse of desert mammal and bird communities. Science 371, 633–636. doi: 10.1126/science.abd4605
Ruttan A., Lortie C. J., and Haas S. M. (2021). Shrubs as magnets for pollination: A test of facilitation and reciprocity in a shrub-annual facilitation system. Curr. Res. Insect Sci. 1, 100008. doi: 10.1016/j.cris.2021.100008
Salido C. A. and Vicente N. S. (2019). Sex and refuge distance influence escape decision in a Liolaemus lizard when it is approached by a terrestrial predator. Behaviour 156, 909–925. doi: 10.1163/1568539X-00003546
Sawyer J. O., Keeler-Wolf T., and Evens J. (2009). A manual of California vegetation (Sacaramento, Californica: California Native Plant Society Press). Available online at: http://books.google.com/books?id=y40lAQAAMAAJ.
Schooley R. L., Bestelmeyer B. T., and Campanella A. (2018). Shrub encroachment, productivity pulses, and core-transient dynamics of Chihuahuan Desert rodents. Ecosphere 9, e02330. doi: 10.1002/ecs2.2330
Siciliano-Martina L., Guerra D. A., and Veech J. A. (2023). Forelimb morphology as an adaptation for burrowing in kangaroo rat species (genus Dipodomys) that inhabit different soil substrates. J. Mammalogy 104, 1377–1389. doi: 10.1093/jmammal/gyad092
Sjoberg D. E., Young J. A., McAdoo K., and Evans R. A. (1984). Kangaroo rats. Rangelands Arch. 6, 11–13.
Springer T. L., Dewald C. L., Sims P. L., and Gillen R. L. (2003). How does plant population density affect the forage yield of eastern gamagrass? Crop Sci. 43, 2206–2211. doi: 10.2135/cropsci2003.2206
Stanton R. A., Boone W. W., Soto-Shoender J., Fletcher R. J., Blaum N., and McCleery R. A. (2018). Shrub encroachment and vertebrate diversity: A global meta-analysis. Global Ecol. Biogeography 27, 368–379. doi: 10.1111/geb.12675
Stanton R. A., Fletcher R. J., Sibiya M., Monadjem A., and McCleery R. A. (2021). The effects of shrub encroachment on bird occupancy vary with land use in an African savanna. Anim. Conserv. 24, 194–205. doi: 10.1111/acv.12620
Terando A., Youngsteadt E., Meineke E., and Prado S. (2018). Accurate near surface air temperature measurements are necessary to gauge large-scale ecological responses to global climate change. Ecol. Evol. 8, 5233–5234. doi: 10.1002/ece3.3972
Tews J., Blaum N., and Jeltsch F. (2004). Structural and animal species diversity in arid and semi-arid savannas of the southern Kalahari. Ann. Arid Zone 43, 413. doi: 10.1046/j.0305-0270.2003.00994.x
Tourani M., Brøste E. N., Bakken S., Odden J., and Bischof R. (2020). Sooner, closer, or longer: Detectability of mesocarnivores at camera traps. J. Zoology 312, 259–270. doi: 10.1111/jzo.12828
Unger S., Rollins M., Tietz A., and Dumais H. (2021). iNaturalist as an engaging tool for identifying organisms in outdoor activities. J. Biol. Educ. 55, 537–547. doi: 10.1080/00219266.2020.1739114
Upham N. S. and Hafner J. C. (2013). Do nocturnal rodents in the Great Basin Desert avoid moonlight? J. Mammalogy 94, 59–72. doi: 10.1644/12-MAMM-A-076.1
Vale C. G. and Brito J. C. (2015). Desert-adapted species are vulnerable to climate change: Insights from the warmest region on Earth. Global Ecol. Conserv. 4, 369–379. doi: 10.1016/j.gecco.2015.07.012
Van Auken O. W. (2009). Causes and consequences of woody plant encroachment into western North American grasslands. J. Environ. Manage. 90, 2931–2942. doi: 10.1016/j.jenvman.2009.04.023
Vélez J., McShea W., Shamon H., Castiblanco-Camacho P. J., Tabak M. A., Chalmers C., et al. (2023). An evaluation of platforms for processing camera-trap data using artificial intelligence. Methods Ecol. Evol. 14, 459–477. doi: 10.1111/2041-210X.14044
Walker L. R. and Landau F. H. (2018). “Causes of aridity,” in A natural history of the mojave desert (The University of Arizona Press), 17–22. doi: 10.2307/j.ctt1zxsmnw.7
Welles S. R. and Funk J. L. (2021). Patterns of intraspecific trait variation along an aridity gradient suggest both drought escape and drought tolerance strategies in an invasive herb. Ann. Bot. 127, 461–471. doi: 10.1093/aob/mcaa173
Westphal M. F., Noble T., Butterfield H. S., and Lortie C. J. (2018). A test of desert shrub facilitation via radiotelemetric monitoring of a diurnal lizard. Ecol. Evol. 8, 12153–12162. doi: 10.1002/ece3.4673
White J. A. and Geluso K. (2007). Seasonal differences in onset of surface activity of ord’s kangaroo rat (Dipodomys ordii). J. Mammalogy 88, 234–240. doi: 10.1644/05-MAMM-A-312R3.1
Whitford W. G. (1997). Desertification and animal biodiversity in the desert grasslands of North America. J. Arid Environments 37, 709–720. doi: 10.1006/jare.1997.0313
Whitford W. G. and Steinberger Y. (2020). Herbivory effects on ephedra spp. In the Chihuahuan Desert. Open J. Ecol. 10, 37–44. doi: 10.4236/oje.2020.102003
Zomer R. J., Trabucco A., Bossio D. A., and Verchot L. V. (2008). Climate change mitigation: A spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Agriculture Ecosyst. Environ. 126, 67–80. doi: 10.1016/j.agee.2008.01.014
Zuliani M., Ghazian N., and Lortie C. J. (2021). Shrub density effects on the community structure and composition of a desert animal community. Wildlife Biol. 2021, 1–12. doi: 10.2981/wlb.00774
Zuliani M., Ghazian N., and Lortie C. J. (2023a). A meta-analysis of shrub density as a predictor of animal abundance. Wildlife Biol. 2023, e01042. doi: 10.1002/wlb3.01042
Zuliani M., Ghazian N., and lortie C. J. (2024). Regional aridity of southern california ecosystems 2022/2023 (Version 2). figshare. doi: 10.6084/m9.figshare.25115357.v2
Keywords: aridity, community, density, facilitation, shrub, temperature
Citation: Zuliani M, Ghazian N, MacDonald SE and Lortie CJ (2025) The shrub density effect: unraveling vertebrate community dynamics along an aridity gradient in Southern California. Front. Ecol. Evol. 13:1648121. doi: 10.3389/fevo.2025.1648121
Received: 16 June 2025; Accepted: 26 August 2025;
Published: 10 September 2025.
Edited by:
David Jack Coates, Conservation and Attractions (DBCA), AustraliaReviewed by:
Linda Jane Walters, University of Central Florida, United StatesCharlotte Mills, UNSW Evolution and Ecology Research Centre, Australia
Copyright © 2025 Zuliani, Ghazian, MacDonald and Lortie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Zuliani, enVsaWFuaW1hcmlvOTZAZ21haWwuY29t
 Mario Zuliani
Mario Zuliani Nargol Ghazian
Nargol Ghazian Suzanne E. MacDonald
Suzanne E. MacDonald