- 1Department of Horticultural Science, University of Minnesota, Saint Paul, MN, United States
- 2Department of Plant and Microbial Biology, University of Minnesota, Saint Paul, MN, United States
Production of day-neutral strawberries (Fragaria x ananassa) is increasing in the Upper Midwest region of the USA, resulting in an extended strawberry harvest season compared to traditional June-bearing production systems. However, the longer harvest season comes with additional insect pest pressure and the need for novel integrated pest management strategies. Spotted-wing drosophila (Drosophila suzukii; SWD) and tarnished plant bug (Lygus lineolaris; TPB) can decrease strawberry yield and fruit quality. Insecticides are the dominant management strategy for both insect pests; however, fewer effective insecticides are available in organic production systems. Interplanting is an integrated pest management strategy which can provide conservation biological control and may repel or attract species of interest through volatile organic compound (VOCs) emissions. We investigated the effect of interplanting alfalfa and sweet alyssum with day-neutral strawberry plants on fruit yield and quality, SWD infestation and TPB damage, arthropod abundance and diversity. Additionally, we measured the relative abundance of sweet alyssum VOCs, acetophenone and benzaldehyde, in the field because they may be aversive to SWD adults. The interplanting treatment inconsistently affected fruit production; in year one of the study, the control treatment had larger fruit than the other two treatments and, in year two, berries interplanted with sweet alyssum had more marketable yield than the other two treatments. Treatments did not affect SWD infestation or TPB damage. Acetophenone and benzaldehyde VOC production varied in the sweet alyssum plots by time of day and date. The control treatment had less arthropod abundance and diversity compared to the intercrop treatments. Intercropping may provide resources for arthropod communities but may not reduce key pest species such as SWD and TPB in day-neutral strawberries.
1 Introduction
Strawberries are an economically important crop, popular globally for their nutritional content and flavor (Schwieterman et al., 2014; Petran et al., 2017). Research on extending the season and market for strawberries in the Upper Midwest region of the USA has focused on day-neutral strawberry (Fragaria x ananassa; DNS) production, which generates fruit later in the growing season, from mid-summer into fall compared to traditional June-bearing strawberry (JBS) cultivars (Petran et al., 2017; Anderson et al., 2019; Samtani et al., 2019). Although strawberry plants are host to many insect pests (Carroll et al., 2022), two key pests are particularly challenging for production of DNS in the Upper Midwest: tarnished plant bug (Lygus lineolaris; TPB) and spotted-wing drosophila (Drosophila suzukii; SWD), both of which are intensified by the extended growing season (Samtani et al., 2019).
SWD, a non-native vinegar fly, is highly polyphagous, feeding on a variety of fruit crop and non-crop hosts (Bellamy et al., 2013; Asplen et al., 2015; Lee et al., 2015; Little et al., 2020). Adult female SWD damage small and soft fruit (e.g., berries and stone fruit) during oviposition (Atallah et al., 2014). SWD ovipositors are sclerotized and enlarged compared to other drosophilids, which allows for oviposition during fruit ripening and egg or larvae infestation at the time of harvest (Atallah et al., 2014). Further reduction in fruit marketability is caused when SWD larvae feed on and develop within the mesocarp.
In the Upper Midwest, SWD populations peak between mid-August and mid-September which coincides with the peak harvest period of DNS (Anderson et al., 2019; University of Minnesota Extension, 2024). Most of the research conducted on SWD has focused on raspberry and blueberry crops, which have shown greater susceptibility to SWD infestations (Bellamy et al., 2013). In comparison, damage caused by SWD in strawberries has been described as relatively less severe, although recent studies report increased infestation (Goodhue et al., 2011; Ganjisaffar et al., 2023; Gullickson et al., 2024). Despite advances in cultural control techniques, management of SWD is still predominately managed with broad-spectrum chemical controls which have damaging ecological impacts on non-target species, soil, and water quality, and contribute to insecticide resistance (Biondi et al., 2012; Van Timmeren and Isaacs, 2013; Gress and Zalom, 2018; Van Timmeren et al., 2018; Gullickson et al., 2019; Sial et al., 2019; Schöneberg et al., 2020).
In addition to SWD, the native insect pest TPB causes substantial reduction in the quality and marketability of strawberries (Wold and Hutchison, 2003; Dumont and Provost, 2019, 2022; Hagler et al., 2020). TPB nymphs feed on strawberry blossoms with piercing-sucking mouthparts, which damages the flower receptacle leading to misshapen and unmarketable strawberry fruit. TPB is polyphagous, affecting cash crops such as cotton (Smith et al., 2023) and 130 other economically important plant species out of its 328 identified host plants (Young, 1986). Alfalfa (Medicago sativa) is considered one of its main host species (Esquivel and Mowery, 2007; Smith et al., 2023). While exclusion netting proves effective on a small scale, the primary management approach for TPB relies on frequent insecticide applications or using tractor-mounted vacuums to remove nymphs from the field, both of which are broad spectrum, and vacuuming has not been consistently effective at reducing pest damage (Swezey et al., 2007; Chouinard et al., 2017; Lu et al., 2022). Only a limited number of organic chemical insecticides demonstrate efficacy against TPB. In organic settings, pyrethrin or azadirachtin-based insecticides are the most applied (Dumont and Provost, 2019). Moreover, TPB populations have developed resistance and antixenosis to various insecticide classes, necessitating the consideration of additional integrated pest management (IPM) tactics for small to medium-sized farms typical of the Upper Midwest (Smith et al., 2023).
Development of an effective push-pull pest management system for both insect pests could reduce insecticide risks to beneficial insects and be applicable in conventional and organic production systems (Khan et al., 2000; Cook et al., 2007). In DNS production, implementing border- and inter-plantings of attractive plant species could pull pests away from strawberry plants, while aversive plant species could push pests away from the host fruit (Hagler et al., 2020; Nieto et al., 2023; Hetherington et al., 2024). Plants and microbial yeast release volatile organic compounds (VOCs) that attract SWD to fruit crops, a behavior exploited for SWD monitoring with baited traps and attract-and-kill tactics (Cha et al., 2012; Keesey et al., 2015; Bueno et al., 2019). Recent studies have focused on aversive odorants to deter SWD from ovipositing in ripening fruit and for post-harvest protection (Renkema et al., 2016, 2017, 2020; Wallingford et al., 2016a; Bedini et al., 2020; Cha et al., 2020; Gullickson et al., 2020; Renkema and Smith, 2020; Stockton et al., 2021). Although various VOCs have reduced SWD fruit infestation in lab and field settings for raspberries, further optimization is needed due to unpleasant aromas for people (Wallingford et al., 2016a; Cha et al., 2020; Stockton et al., 2021), expense (Wallingford et al., 2016b; Kirkpatrick et al., 2018b), inadequate pest reduction due to no established acceptable threshold for SWD (Ganjisaffar et al., 2023), and lack of effective studies in day-neutral strawberry production systems (Renkema et al., 2020). Sweet alyssum (Lobularia maritima) has been shown to facilitate natural enemies and reduce other pests, and its two main floral VOCs, acetophenone and benzaldehyde, are deterrent to SWD in the laboratory (Begum et al., 2006; Hogg et al., 2011; Renkema and Smith, 2020; Tsuruda et al., 2022). Alfalfa has been investigated for its potential as an attractive sink for TPB in June bearing strawberries (Hetherington et al., 2024), but it remains uncertain if alfalfa would be effective throughout the longer DNS flowering season in the Upper Midwest. These plants also have the potential to provide beneficial insects with floral resources and thereby increase biodiversity (Hogg et al., 2011; Foti et al., 2017). However, the VOC composition of field-grown alfalfa and sweet alyssum, and their effect on pest damage to strawberries, remains unknown.
In this study, we investigated whether interplanting alfalfa and sweet alyssum with DNS plants affected: 1) fruit yield and quality; 2) SWD infestation and TPB damage; and 3) arthropod abundance and diversity. Additionally, we investigated the relative abundance of the acetophenone and benzaldehyde in field-grown sweet alyssum flowers at different times throughout the day and the growing season. We hypothesized that yield and fruit quality would differ among interplanting treatments potentially lower due to competition for resources among the strawberries and interplants or insect pest pressure, or higher if the interplants provided resources for additional biological control or brought in pollinators. Additionally, we hypothesized that DNS interplanted with sweet alyssum would have lower rates of SWD infestation in strawberry fruit due to sweet alyssum flower volatiles, which would deter SWD females from ovipositing in fruit. In year two of our study, we investigated whether sweet alyssum produced deterrent VOCs in the field and the diurnal and seasonal timing of when they were produced. We also hypothesized that TPB populations and damage on strawberry fruit would be lower among the alfalfa treatment relative to the control, due to alfalfa attracting TPB away from the strawberry plants. Finally, we hypothesized that the arthropod communities would be more abundant and diverse in strawberries next to sweet alyssum and alfalfa because of additional plant diversity and floral resources compared to the control.
2 Materials and methods
2.1 Location and plant materials
This research was conducted at the Minnesota Agricultural Experiment Station on the University of Minnesota St. Paul campus on USDA certified organic land (45°0’31.09” N, 93°18’56.313” W) in 2021 and 2023. Composted manure was used as fertilizer prior to planting, followed with fertigation throughout the season (Neptune’s Harvest Fish and Seaweed Fertilizer 2-3-1, Neptune’s Harvest, Gloucester, MA) to supply the plants with 5.6 kg N ha-1 week-1. Bare root day-neutral strawberries (cv. Albion, Nourse Farms, Whately, MA) were planted on 17 May 2021 and 5 May 2023 on plastic mulch covered raised beds over drip irrigation. The two outer rows served as buffer rows to minimize edge effects, and the middle row was used for data collection (Supplementary Figure 1). The data row was a staggered double row with strawberry plants spaced 30.5 cm apart. Treatments consisted of transplanted seedlings for interplanted 1.5 m long sections of alfalfa (cv. Vernal, Johnny’s Selected Seeds, Winslow, ME) or sweet alyssum (cv. Snow Crystals, Park Seed, Greenwood, SC) planted at 6.6 plants m-1, compared to an unplanted control around the strawberry plants. Each treatment plot was replicated 4 times (12 plots total). Plots were spaced 10 m apart to minimize the potential for interference with the volatile treatments based on previous spotted wing drosophila chemical ecology research (Wallingford et al., 2018).
2.2 Strawberry production
All fully ripe strawberry fruit were harvested twice weekly from the data rows. Harvested fruits were placed in labeled plastic clamshells and stored in a cooler until they were brought to the lab to record insect damage, yield (g), the number of fruits, and average fruit weight (g). In 2021, strawberries were harvested beginning on 21 June until 21 October. In 2023, strawberries were harvested from 19 June to 5 October. Harvest for each year concluded when freezing temperatures killed the remaining strawberry flowers.
2.3 Spotted-wing drosophila infestation and tarnished plant bug damage
Baited lures were not used for monitoring and assessing SWD populations in the strawberry plots, as these traps poorly reflect the number of SWD in the field and can bring in additional individuals through spillover (Kirkpatrick et al., 2018a; Leach et al., 2019). A random subsample of 5 marketable fruits per treatment replication was used to determine SWD infestation (proportion of fruit with eggs and number of eggs per fruit) for each harvest date. Individual fruits were visually assessed under a dissecting microscope (8-35x magnification, Leica EZ4W) for SWD eggs as indicated by breathing filaments. Microscopy was used due to increased accuracy at quantifying SWD presence over salt water extraction methods, especially for eggs and first instar larvae (Shaw et al., 2019; Van Timmeren et al., 2021).
TPB populations were sampled once per week throughout the flowering seasons (June – October). Between 10:00 and 14:00, five strawberry flower clusters were randomly selected for each treatment replication and were gently tapped above a white sheet of paper to count the combined number of TPB nymphs and adults. Plot sampling order was assigned randomly. TPB damage was assessed twice per week at harvest when marketable and unmarketable fruit was sorted. USDA number 1 or number 2 standards were used to determine whether fruit met marketability standards (USDA-AMS, 2006). Fruit was considered unmarketable due to TPB if more than approximately 10% of the fruit surface was deformed by TPB feeding.
2.4 Arthropod abundance and diversity
Arthropods in the strawberry plants were sampled weekly from 2 June to 12 October 2021 and every other week in 2023 from 8 June to 4 October. Arthropod specimens were collected using a handheld insect vacuum (Skil® 2810 18V vacuum, BioQuip) for a total of 2 minutes per treatment plot each week. The sampling order was assigned using randomly generated numbers in R. Vacuumed arthropod samples were stored in labeled cannisters that were taken to the lab and frozen at -20°C before pinning and subsequent identification based on morphological characteristics. Vacuumed arthropod samples were identified to a minimum of family level or the most specific taxonomic level when it was feasible to do so. Following identification, arthropods were categorized into functional feeding groups (detrivore/fungivore, herbivore, nectivore/pollinator, omnivore/other, and predator/parasitoid) based on available literature (i.e., Hogg et al., 2011).
2.5 VOC sampling and chemical analysis
To determine whether acetophenone and benzaldehyde were produced by sweet alyssum plants in the treatment plots and the timing of production, disposable research-grade, platinum-catalyzed polydimethylsiloxane (PDMS) discs (5 mm x 1 mm) (Interstate Specialty Products, Sutton, MA) were placed next to sweet alyssum flower clusters (approximately 30–40 individual flowers) to capture volatile organic compounds in the field. PDMS discs for were washed and treated to remove absorbed compounds and were then attached to a paper clip and stored in a sealed glass jar until use (see: Supplementary Material). For each replication, three PDMS discs on paper clips were placed in 10 cm2 Teflon bags (Welch Fluorocarbon Inc., Dover, NH) and bags were placed over flower clusters before sealing with a twist-tie. Sampling dates were 12 July, 23 August, and 14 September, all in 2023. Samples were collected starting at 6:00am, 11:00am, and 5:00pm, and each sampling period lasted for three hours. In each sweet alyssum treatment plot, two separate Teflon sampling bags with a set of three PDMS discs each were placed around flower clusters. At each sampling period, two Teflon sampling bags with three PDMS discs each were also placed in the field, but not around flowers, as negative controls. After three hours, bags were removed from the flower clusters, re-sealed, and placed on dry ice while transporting from the field to the lab. PDMS discs were removed from the paper clip and placed in an amber glass vial along with 150 µL 50:50 dichloromethane:ethyl acetate solvent to extract absorbed compounds. The vials were placed on ice and gently shaken for 1 hour. The extraction solvent was transferred into glass inserts in autosampler vials and the contents were analyzed with gas chromatography-mass spectrometry (GC-MS). GC-MS analysis was performed using an Agilent 7890A GC and 5973 mass selective detector (MSD) single quadrupole instrument with a Gerstel MPS 2 autosampler. Helium was used as the GC carrier gas with a 7.5 min solvent delay. The flow rate was held constant at 1 mL/min. Samples (1 µL) were injected using a Gerstel Multipurpose sampler (MPS), in pulsed splitless mode to an Agilent 30m DB-5MS UI, 0.25 mm I.D., 0.25 µm film thickness capillary GC column. Inlet and interface temperatures were set to 280°C. The initial oven temperature was 70°C and was held for 4 min. Oven temperature was then increased at a rate of 15°C/min to 270°C where it was held for 4 min before returning to initial settings. The quadrupole temperature was set to 150°C and the source temp: 230°C. The MS scan range was 30–220 m/z.
2.6 Statistical analysis
Strawberry production data, i.e., cumulative total and marketable yields (g/plant), were first assessed for normality using the Shapiro-Wilk test and visual inspection of histograms and quantile-quantile plots. Separate non-parametric Kruskal-Wallis tests were initially used to assess the effects of treatment and year on these yields. Data were not pooled because a significant year effect was observed. Instead, each year was analyzed independently for treatment effects. Within-year data satisfied the requirements for a one-way ANOVA, for which yield was the response variable and treatment was the explanatory variable. Tukey’s test was used for post-hoc analyses.
A one-way ANOVA test was used to analyze the mean number of SWD eggs per strawberry for each year independently after significant differences were observed between years, preventing data from being pooled. For the proportion of infested strawberries, only the 2023 data met the necessary requirements, and in that case one-way ANOVA was used to determine treatment effects within each year, otherwise Kruskal-Wallis was used. TPB scouting data were integer counts and were analyzed using a generalized linear model (GLM) with a negative binomial distribution. The TPB count was the response variable, and treatment, year, and a treatment-by-year interaction were included as explanatory variables. The proportion of TPB-damaged fruit at the end of the season was analyzed with a two-way ANOVA to assess the effects of treatment and year.
To analyze arthropod family composition, family count data from each treatment and sampling date were square-root transformed to reduce the influence of highly abundant families. A non-metric multidimensional scaling (NMDS) was used for visualization, with the NMDS performed on a Bray-Curtis dissimilarity matrix. A permutational multivariate analysis of variance (PERMANOVA), with 9,999 permutations, was used to test differences in community composition among treatments, years, and a treatment year interaction. To analyze whether there were any differences in specific functional groups or individual families of interest, generalized linear models with a Poisson distribution followed by a one-way ANOVA were used.
GC-MS data were analyzed using ChemStation (Agilent) and MZmine (v2.53; Pluskal et al., 2010). Benzaldehyde and acetetophenone peaks were identified by comparison of fragmentation patterns with those form the NIST 20 EI database using ChemStation and confirmed by co-injection of commercially obtained standard compounds (Sigma-Aldrich, Milwaukee, WI). Sample peak heights at the specified retention times were baseline corrected (GC-MS peak height of a treatment sample minus the peak height of a negative control) to reduce instrument noise and determine relative abundance of target molecules before statistical analysis. The abundance was relative to the negative control as well as to compare sampling times and dates. Separate Kruskal-Wallis tests were performed to assess the effects of time and date on the baseline corrected peak height of acetophenone and benzaldehyde, with the peak height of acetophenone or benzaldehyde as the response variable and time or date as explanatory variables.
3 Results
3.1 Strawberry production
Year had a significant effect on total yield, marketable yield, and the proportion of marketable fruit in this experiment (Table 1; Figure 1). Cumulative total yield was significantly greater in 2021 than in 2023 (Kruskal-Wallis; X2 = 10.453, df = 1, P = 0.001). Cumulative marketable yield was also significantly greater in 2021 compared to 2023 (Kruskal-Wallis; X2 = 15.87, df = 1, P < 0.001).
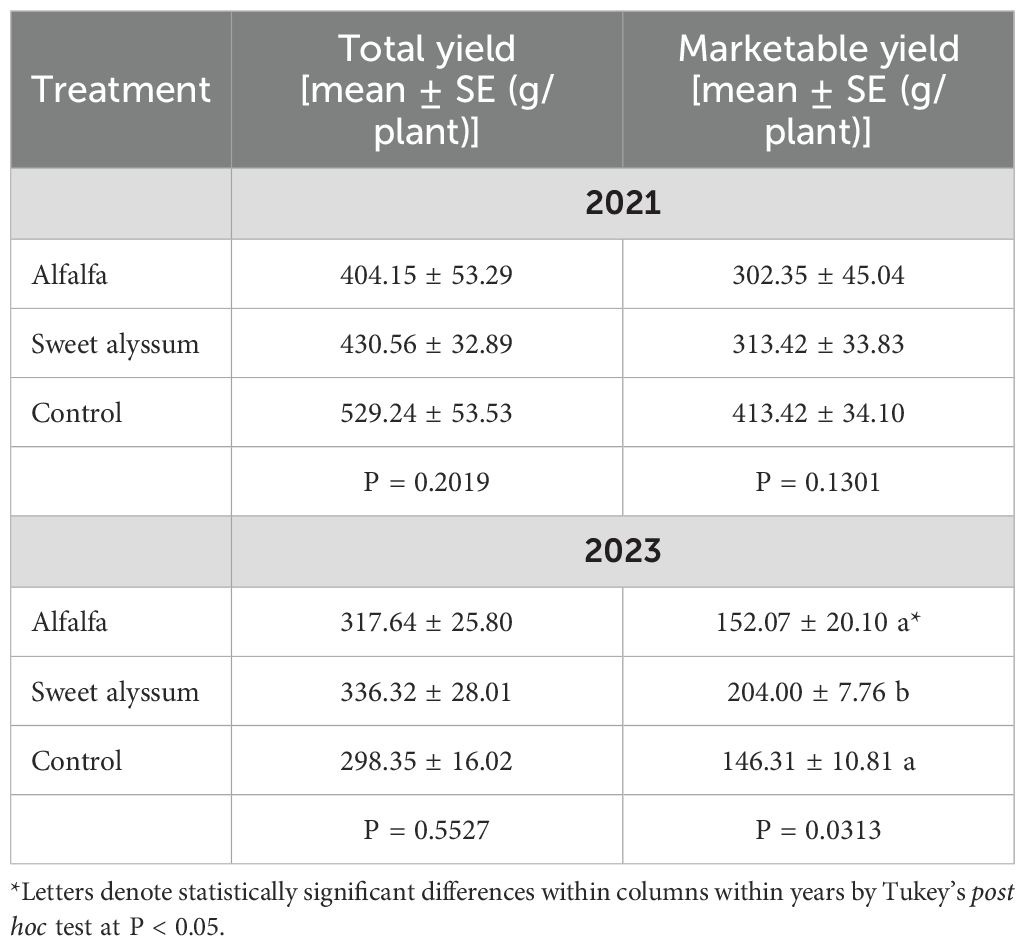
Table 1. Strawberry production (total and marketable yield) among interplanting treatments in 2021 and 2023.
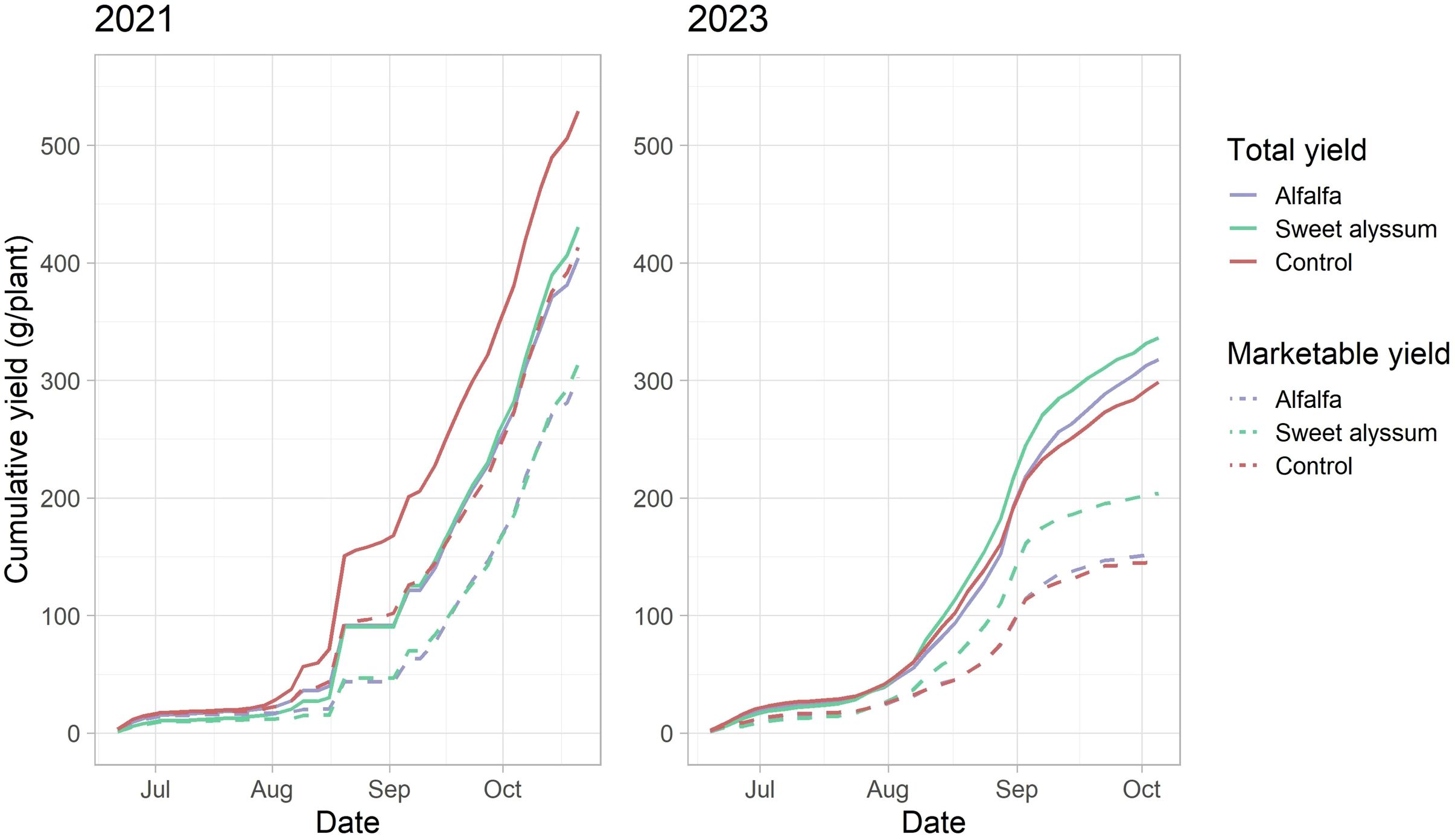
Figure 1. The cumulative total yield (g / plant) (solid lines) and marketable yield (g / plant) (dashed lines) of day-neutral strawberries (Fragaria x ananassa cv. Albion) when grown with two interplanting treatments and a control without an interplant in St. Paul, Minnesota, U.S.A. Both yield measurements were significantly lower in 2023 compared to 2021 (total yield, p < 0.001; marketable yield, p < 0.001). In 2023, the strawberry plants interplanted with sweet alyssum had significantly greater marketable yield (g / plant) than the alfalfa interplanted treatment or control (p = 0.031).
In 2021, there were no significant differences in the cumulative total yield (ANOVA; F = 1.922, df = 2, 9, P = 0.2019), cumulative marketable yield (ANOVA; F = 2.58, df = 2, 9, P = 0.130) among the three treatments (Table 1, Figure 1). In 2023, cumulative total yield (ANOVA; F = 0.6337, df = 2, 9, P = 0.5527) was not different among treatments. However, cumulative marketable yield was significantly different among the three treatments (ANOVA; F = 5.214, df = 2, 9, P = 0.0313); with greater marketable yield in the sweet alyssum interplanted strawberries compared to the alfalfa interplanted or control treatments.
3.2 Spotted-wing drosophila fruit infestation and tarnished plant bug damage
The average number of SWD eggs per strawberry during the peak of the harvest season (1 Aug to end of season, 21 Oct 2021 and 5 Oct 2023) ranged from 0.40 to 1.04 (Table 2). The number of eggs per strawberry was significantly lower in 2021 compared to the 2023 growing season (ANOVA: F = 6.986, df = 1, 18, P = 0.0167). There were no significant differences in the number of SWD eggs in strawberries among the three treatments in either year (ANOVA; 2021: F = 1.488, df = 2, 9, P = 0.276; 2023: F = 1.624, df = 2, 9, P = 0.250).
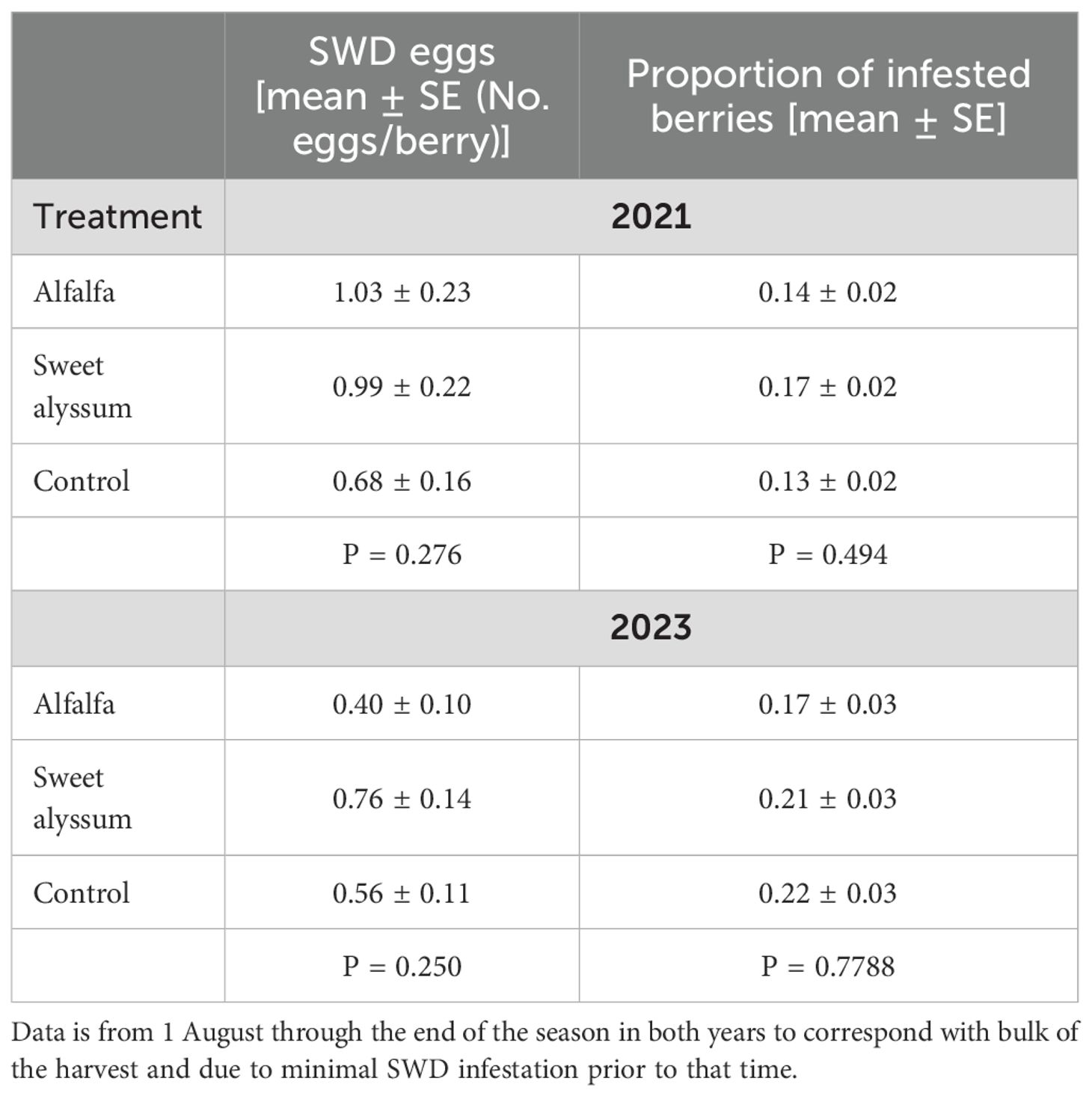
Table 2. Drosophila suzukii (SWD) infestation (number of eggs per berry and proportion of infested berries) in day-neutral strawberries among interplanting treatments in 2021 and 2023.
There were significant differences between years in the proportion of SWD infested strawberries (Kruskal-Wallis; X2 = 4.083, df = 1, P = 0.043), with a greater proportion of strawberries infested in 2023 compared to 2021. However, among treatments, no significant differences in the proportion of SWD infested strawberries were observed in either year (ANOVA; 2021: F = 0.763, df = 2, 9, P = 0.494; 2023: X2 = 0.5, df = 2, P = 0.779) (Table 2).
The average weekly tarnished plant bug nymph counts ranged from 1.47 to 2.36 and were not significantly different among the three treatments in either year (Table 3). Likewise, the proportion of unmarketable fruit due to TPB feeding was not significantly different among the three treatments (Table 3). TPB counts were significantly lower in 2023 than in 2021 (GLM; X2 = 13.774, P = 0.0002), but the proportion of unmarketable fruit was not significantly different between years (GLM; X2 = 2.692, P = 0.101).
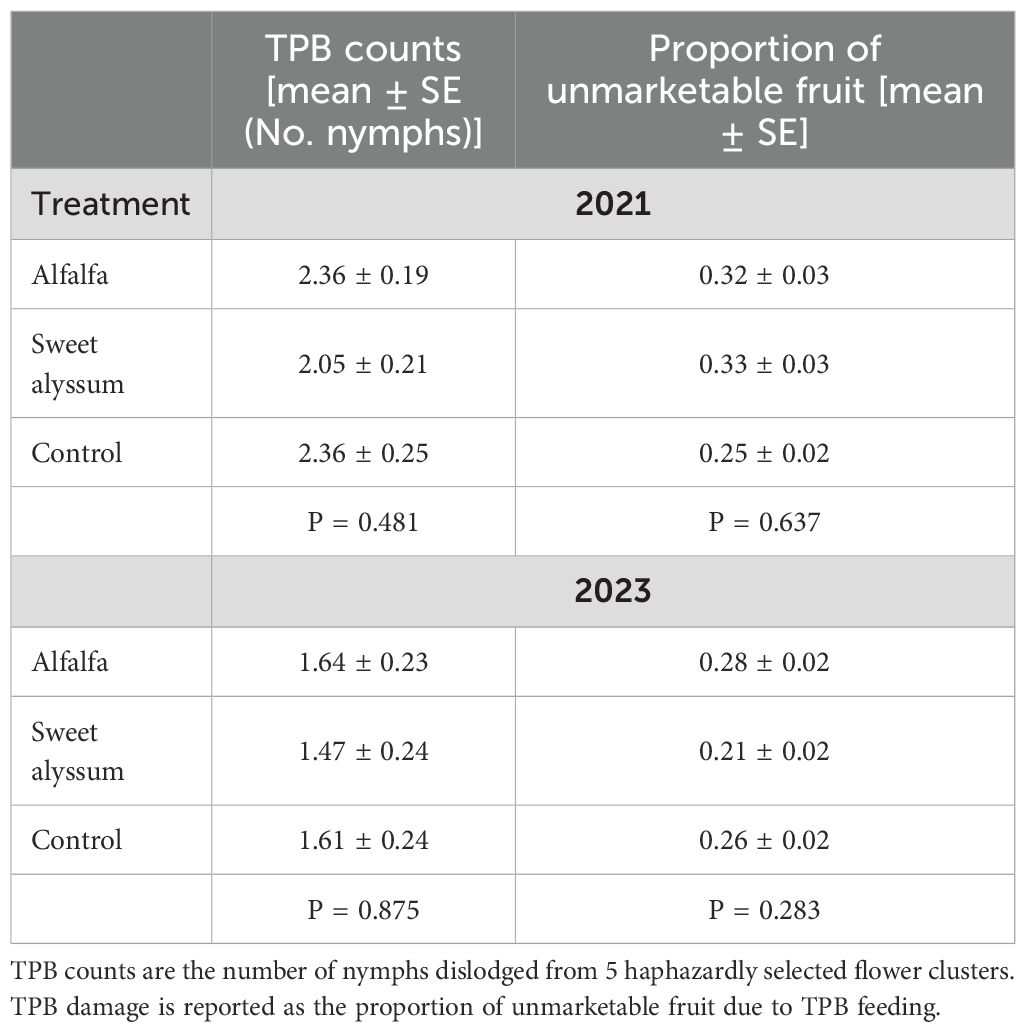
Table 3. Tarnished plant bug (Lygus lineolaris; TPB) counts and damage on day-neutral strawberries among interplanting treatments in 2021 and 2023.
3.3 Arthropod abundance and diversity
The number of sampled arthropod specimens totaled 5949 and was made up of 15 orders (Table 4) and 99 families (Supplementary Table 1). Arthropod family composition was significantly influenced by treatment (PERMANOVA; pseudo-F=1.55, df = 2, 319, P = 0.036) and year (PERMANOVA; pseudo-F=18.67, df = 1, 319, P<0.001). However, there was no significant interaction between treatment and year (PERMANOVA; pseudo-F=0.46, df = 2, 319, P = 0.989), suggesting that the effect of treatment on family composition was consistent between the two years. Pairwise comparisons showed a significant difference in family composition between the control and sweet alyssum treatments (pairwise PERMANOVA; pseudo-F=1.72, df = 1, 212, P = 0.049). No significant differences were found between the alfalfa and control plots (pairwise PERMANOVA; pseudo-F=1.14, df = 1, 215, P = 0.315) and only marginal differences between the alfalfa and sweet alyssum plots (pairwise PERMANOVA; pseudo-F=1.55, df = 1, 217, P = 0.089).
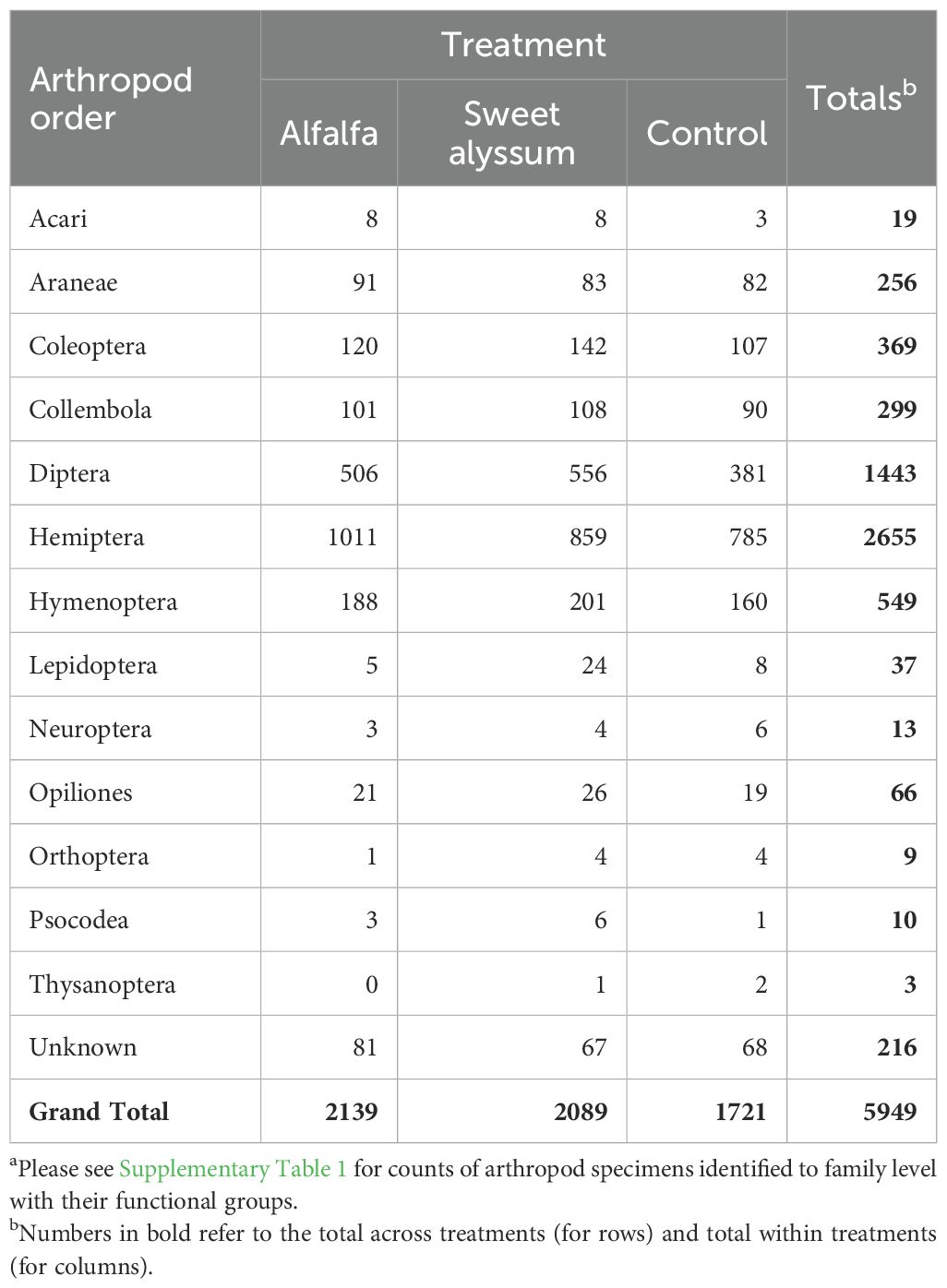
Table 4. Number of arthropod specimens identified to order levela collected from day-neutral strawberry plants among interplanting treatment during vacuum sampling in 2021 and 2023.
The composition of arthropod functional groups (Table 5) was significantly affected by year (PERMANOVA; pseudo-F=20.79, df = 1, 309, P<0.001). However, the effect of treatment (PERMANOVA; pseudo-F=1.35, df = 2, 309, P = 0.233) and the interaction between treatment and year (PERMANOVA; pseudo-F=0.43, df = 2, 309, P = 0.864) were not significant. The control treatment had significantly fewer herbivores compared to the sweet alyssum or alfalfa treatments in 2021, even after accounting for sampling date, but no differences were observed in 2023 (GLM; 2021: X2 = 6.539, df = 2, P = 0.038; 2023: X2 = 3.433, df = 2, P = 0.180). Additionally, there were significantly fewer predators and parasitoids in the control compared to the other two treatments in 2021, and marginally fewer in 2023 (GLM; 2021: X2 = 6.748, df = 2, P = 0.034; 2023: X2 = 5.921, df = 2, P = 0.052). However, there were no significant differences in the number of nectivores and pollinators among the treatments (GLM; 2021: X2 = 0.9003, df = 2, P = 0.638; 2023: X2 = 0.108, df = 2, P = 0.947).
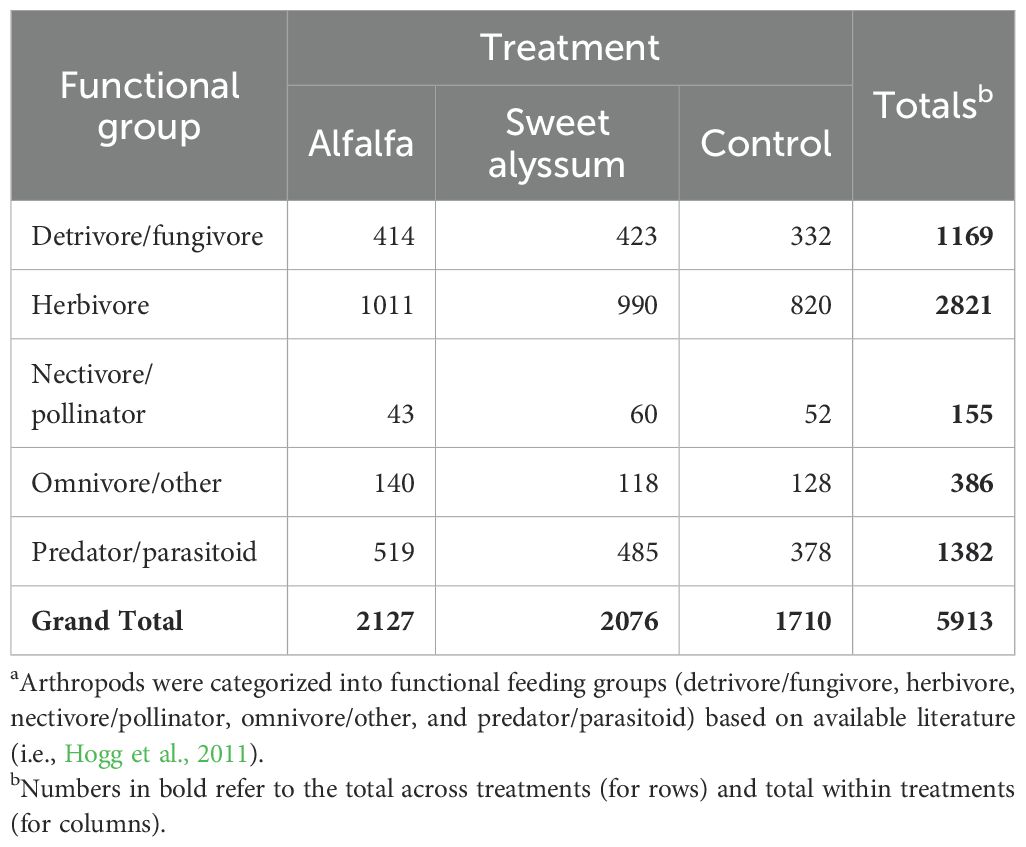
Table 5. Count of arthropod specimens collected from day-neutral strawberry plants among interplanting treatment during vacuum sampling in 2021 and 2023 and categorized in functional groupsa.
Although there were numerical differences, the number of individual specimens collected from treatment groups of specific families of interest did not vary significantly among the three treatments in either year. There were no significant differences among treatments for the pest families of interest Drosophilidae (e.g., SWD) (GLM; X2 = 1.5458 df = 2, P = 0.4617; 2023: X2 = 0.511, df = 2, P = 0.774), and Miridae (e.g., TPB) (GLM; X2 = 2.9152, df = 2, P = 0.2328; 2023: X2 = 0.729, df = 2, P = 0.694). There were also no significant differences for beneficial generalist predators Anthocoridae (e.g., minute pirate bug, Orius insidiosus) (GLM; X2 = 3.0595, df = 2, P = 0.2166; 2023: X2 = 1.516, df = 2, P = 0.469), or Nabidae (e.g., Nabis americoferus) (GLM; 2021: X2 = 0.20683, df = 2, P = 0.9018; 2023: X2 = 0.2258, df = 2, P = 0.8932).
3.4 VOC sampling and chemical analysis
Relative abundances of acetophenone (X2 = 4.826, df = 1, P = 0.028) and benzaldehyde (X2 = 4.264, df = 1, P = 0.039) were significantly greater in sweet alyssum treatments compared to the negative controls. There were significantly different relative abundances of acetophenone among the three sampling times during the day (X2 = 11.303, df = 2, P = 0.004). There was a significantly lower abundance of acetophenone during the evening sampling time (Figure 2A). Likewise, benzaldehyde relative abundances were significantly different among the three sampling times during (X2 = 16.802, df = 2, P = 0.0002). All three time periods were significantly different from each other. Additionally, there were significant differences in relative abundances for both acetophenone (X2 = 36.225, df = 2, P < 0.0001) and benzaldehyde (X2 = 21.505, df = 2, P < 0.0001) among the three sampling dates. For acetophenone, relative abundance was significantly lower on 23 Aug compared to the two other dates (Figure 2B). Benzaldehyde relative abundance was significantly greater on 12 Jul compared to the two other dates.
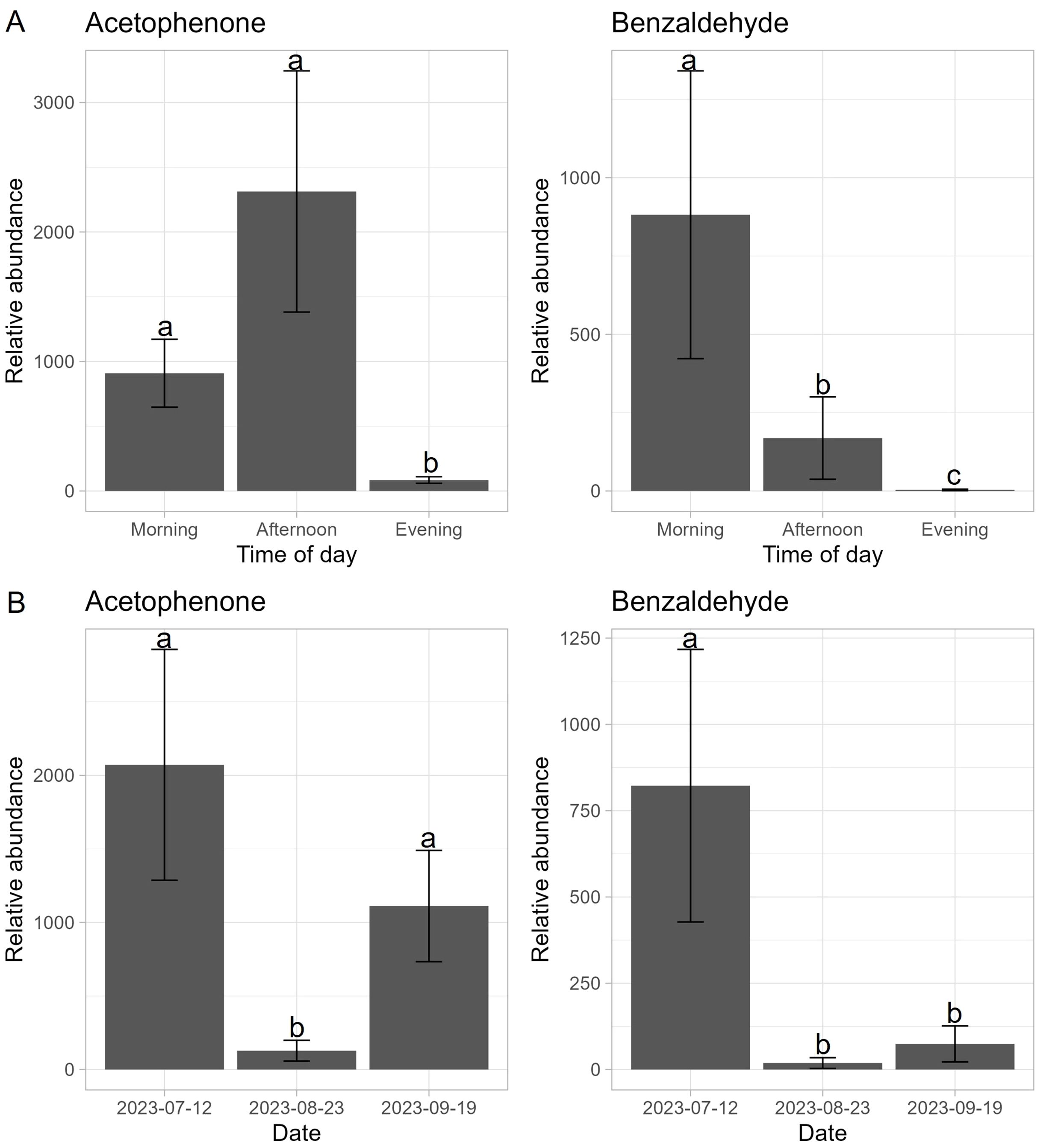
Figure 2. Relative abundances (sample peak height of treatment minus the negative control) of acetophenone and benzaldehyde produced by sweet alyssum (Lobularia maritima) at three different (A) time periods, morning (6:00 am - 9:00 am), afternoon (11:00 am - 2:00 pm), and evening (5:00 pm - 8:00 pm) and (B) separate dates, 12 July, 23 August, and 19 September 2023 in St. Paul, Minnesota. Letters denote significant differences among time periods for each molecule at α=0.05.
4 Discussion
This study investigated the effect of interplanting sweet alyssum and alfalfa on fruit production and insect pest management in day-neutral strawberries. The effect of interplanting treatments on day-neutral strawberry production was variable between the two years of this study; the treatments affected marketable yield in 2023, but did not affect total yield, nor the number of berries produced in either year. The sweet alyssum treatment had greater marketable yield than the other treatments in 2023. We did not observe more pollinators or fewer herbivores in the sweet alyssum compared to the control.
Year affected total and marketable yields, potentially due to seasonal environmental differences. For example, the 2023 growing season ended approximately two weeks earlier than the 2021 season. Another explanation for lower yields in the second year of this study could have been due to fungal pathogens. Although no strawberries were planted in the plot in 2022, strawberry fungal pathogens such as verticillium wilt (Verticillium sp.) may have persisted in the soil and contributed to reduced yields in the second year of the study (Anderson et al., 2019).
Treatments did not affect SWD oviposition or TPB counts in the DNS. For SWD, treatments did not affect egg counts nor the proportion of infested fruit in either year of this study. This is consistent with another study with JBS, in which Tsuruda et al. (2022) concluded that interplanting sweet alyssum alongside JBS plants did not decrease SWD infestation. The authors suggested that sweet alyssum may be more effective for fruit crops which ripen later in the season because it may support late season parasitoid adults as they establish a local population (Tsuruda et al., 2022). However, we did not observe any effect on SWD with a comparatively late season strawberry harvest. Another study with peppermint interplants found a similar lack of treatment effect in the field, although laboratory results were promising (Renkema et al., 2020). Likewise, TPB counts and impact on strawberries was not affected by treatment. Although other studies have found that alfalfa is the preferred host of TPB and perimeter strips functioned as a sink for TPB, we found no evidence of this effect in day-neutral strawberries (Esquivel and Mowery, 2007; Hetherington et al., 2024). Hetherington et al. (2024) suggest spatial separation and a short period of JBS susceptibility would be necessary for alfalfa to act as a net sink, rather than both a sink and source. We conclude that alfalfa is likely not a suitable trap crop for TPB in small-scale day-neutral strawberry production, since TPB can still move from alfalfa into strawberry fields even with some spatial separation.
To understand how sweet alyssum may function as a source of repellent or attractive volatile organic compounds, we collected and analyzed the two main VOCs, acetophenone and benzaldehyde, from the flowers at three different time points on three different days in 2023. Previous studies identified acetophenone and benzaldehyde as main compounds of sweet alyssum flower aroma and that they showed some repellency towards SWD, although benzaldehyde showed no effect on SWD in another study (Wallingford et al., 2016b; Renkema and Smith, 2020). Our study focused on these likely repellent compounds; however, vegetative tissue or trace floral VOCs may have also changed the arthropod communities and behavior. We observed inconsistent production of these SWD repellent compounds in the field, which could explain why other studies have not found them to be successful at limiting SWD infestation either. Compounds peaked in the morning (benzaldehyde) and afternoon (acetophenone) and were negligible in the evening. Since SWD has crepuscular behavior (Jaffe and Guédot, 2019), and sweet alyssum appears to produce the most aroma during mid-day, it follows that there may be some temporal incompatibilities of using repellent intercrops in a push-pull setting. Future studies investigating push-pull scenarios with living repellent plants should consider the concentration and timing of VOC production and not assume that it is constant. It is possible that these volatile compounds are produced earlier in the day to correspond to when diurnal pollinators are most active (Hoballah et al., 2005; Fenske and Imaizumi, 2016). Additionally, relative abundance of these compounds was affected by sampling date. Some days (12 July and 19 September 2023) had high relative abundances and 23 August had low to no measurable abundance, even though there were no apparent differences in the number of flowers in each sampling bag, which further suggests that these treatments may only be effective some of the time. Therefore, although there are likely benefits of interplanting sweet alyssum to support arthropod biodiversity, it is unlikely to be a viable repellent for SWD due to the temporally inconsistent production of the volatiles.
Another objective of this study was to investigate whether the treatments provided a conservation biological control benefit by affecting arthropod abundance and diversity. In the treatments, we observed greater arthropod family diversity at specific sampling times and greater arthropod abundances both on a per sample basis and over the course of the season. Herbivores, predators, and parasitoids were all more abundant in the treatments compared to the control. We observed more predators and parasitoids in the treatments in both years, suggesting that alfalfa and sweet alyssum support beneficial insects from a conservation biological control standpoint. This is consistent with the findings from Lu et al. (2022), where the authors found that increased woody-habitat near the agroecosystem contributed to greater abundances of predators, however, they did not find differences in Lygus populations based on nearby habitat.
Increasing flowering plant diversity in agricultural ecosystems has the potential to support arthropod biodiversity, although beneficial functional groups were not the only ones affected by the treatments (Connelly et al., 2015; Lu et al., 2022). Treatments had more herbivores in 2021, which could have negative effects on strawberry production, although no differences were observed in 2023. Finally, there were no differences in the number of pollinators among the three treatments, so although these treatments increased flower resources and diversity, this likely did not result in more pollination services. Additionally, all treatments were receiving the same fertilizer so the reason for greater yields in the sweet alyssum treatment is unknown. DNS cultivar flowers are self-compatible, and will produce fruit in the absence of pollinators, but bee visits have been shown to increase fruit mass by 35 to 40% (Chagnon et al., 1993; Connelly et al., 2015). The number of individual specimens from specific families of interest did not vary among the treatments, adding to our previous evidence that sweet alyssum did not repel SWD, and alfalfa did not attract TBP. We also failed to observe more individuals from specific generalist predator families such as Anthocoridae and Nabidae. By contrast, a recent study in Minnesota apple found that Anthocoridae was the most abundant generalist predator (Nelson, 2023). For DNS, the effects of treatments appear to be general, and lack evidence for targeting specific taxa, potentially because the pests and some beneficial arthropods are polyphagous, and not specialists to DNS.
5 Conclusion
Interplanting day-neutral strawberries with sweet alyssum or alfalfa increased insect abundance and diversity, particularly for herbivore and predator, and parasitoid families, which may have positive implications for supporting arthropod biodiversity in agroecosystems. However, there was no observed SWD or TPB pest management benefit from these treatments and there were inconsistent effects on fruit production. More research is needed on using living plants to produce aversive and attractive volatiles in situ due to the complex nature of volatile quantity and timing.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MG: Methodology, Writing – review & editing, Investigation, Conceptualization, Data curation, Formal analysis, Writing – original draft, Funding acquisition, Visualization. JS: Methodology, Data curation, Supervision, Investigation, Resources, Writing – review & editing. AH: Project administration, Visualization, Data curation, Methodology, Investigation, Conceptualization, Funding acquisition, Supervision, Writing – review & editing, Resources. MR: Writing – original draft, Resources, Funding acquisition, Project administration, Data curation, Conceptualization, Methodology, Supervision, Writing – review & editing, Investigation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding for this project was provided by the North American Strawberry Growers Association.
Acknowledgments
The authors thank Adam Schacherer, Jay Delacy, Will Pradel, Paige Waddick, and Eric Burkness for their assistance with data collection for these experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1651123/full#supplementary-material
References
Anderson H. C., Rogers M. A., and Hoover E. E. (2019). Low tunnel covering and microclimate, fruit yield, and quality in an organic strawberry production system. Horttechnology 29, 590–598. doi: 10.21273/HORTTECH04319-19
Asplen M. K., Anfora G., Biondi A., Choi D. S., Chu D., Daane K. M., et al. (2015). Invasion biology of spotted wing drosophila (Drosophila suzukii): a global perspective and future priorities. J. Pest Sci. (2004) 88, 469–494. doi: 10.1007/s10340-015-0681-z
Atallah J., Teixeira L., Salazar R., Zaragoza G., and Kopp A. (2014). The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc. R. Soc B Biol. Sci. 281, 1–9. doi: 10.1098/rspb.2013.2840
Bedini S., Cosci F., Tani C., Pierattini E. C., Venturi F., Lucchi A., et al. (2020). Essential oils as post-harvest crop protectants against the fruit fly Drosophila suzukii: bioactivity and organoleptic profile. Insects 11, 1–17. doi: 10.3390/insects11080508
Begum M., Gurr G. M., Wratten S. D., Hedberg P. R., and Nicol H. I. (2006). Using selective food plants to maximize biological control of vineyard pests. J. Appl. Ecol. 43, 547–554. doi: 10.1111/j.1365-2664.2006.01168.x
Bellamy D. E., Sisterson M. S., and Walse S. S. (2013). Quantifying host potentials: indexing postharvest fresh fruits for spotted wing drosophila, Drosophila suzukii. PloS One 8, 1–10. doi: 10.1371/journal.pone.0061227
Biondi A., Mommaerts V., Smagghe G., Viñuela E., Zappalà L., and Desneux N. (2012). The non-target impact of spinosyns on beneficial arthropods. Pest Manage. Sci. 68, 1523–1536. doi: 10.1002/ps.3396
Bueno E., Martin K. R., Raguso R. A., Mcmullen J. G., Hesler S. P., Loeb G. M., et al. (2019). Response of wild spotted wing drosophila (Drosophila suzukii) to microbial volatiles. J. Chem. Ecol. 46, 688–698. doi: 10.1007/s10886-019-01139-4
Carroll J., Pritts M., Loeb G., Weber C., Curtis P., Bihn E., et al. (2022). Production and IPM Guide for Organic Strawberries (Ithaca, NY). Available online at: https://cropandpestguides.cce.cornell.edu/ (Accessed November 11, 2022).
Cha D. H., Adams T., Rogg H., and Landolt P. J. (2012). Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila suzukii. J. Chem. Ecol. 38, 1419–1431. doi: 10.1007/s10886-012-0196-5
Cha D. H., Roh G. H., Hesler S. P., Wallingford A., Stockton D. G., Park S. K., et al. (2020). 2-Pentylfuran: a novel repellent of Drosophila suzukii. Pest Manage. Sci. 77, 1757–1764. doi: 10.1002/ps.6196
Chagnon M., Gingras J., and DeOliveira D. (1993). Complementary aspects of strawberry pollination by honey and indigenous bees (Hymenoptera). J. Econ. Entomol. 86, 416–420. doi: 10.1093/jee/86.2.416
Chouinard G., Veilleux J., Pelletier F., Larose M., Philion V., and Cormier D. (2017). Impact of exclusion netting row covers on arthropod presence and crop damage to ‘Honeycrisp’ apple trees in North America: A five-year study. Crop Prot. 98, 248–254. doi: 10.1016/j.cropro.2017.04.008
Connelly H., Poveda K., and Loeb G. (2015). Landscape simplification decreases wild bee pollination services to strawberry. Agric. Ecosyst. Environ. 211, 51–56. doi: 10.1016/j.agee.2015.05.004
Cook S. M., Khan Z. R., and Pickett J. A. (2007). The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400. doi: 10.1146/annurev.ento.52.110405.091407
Dumont F. and Provost C. (2019). Combining the use of trap crops and insecticide sprays to control the tarnished plant bug (Hemiptera: Miridae) in strawberry (Rosaceae) fields. Can. Entomol. 151, 251–259. doi: 10.4039/tce.2019.7
Dumont F. and Provost C. (2022). Using autumnal trap crops to manage tarnished plant bugs (Lygus lineolaris). Insects 13, 1–10. doi: 10.3390/insects13050441
Esquivel J. F. and Mowery S. V. (2007). Host plants of the tarnished plant bug (Heteroptera: Miridae) in central Texas. Environ. Entomol. 36, 725–730. doi: 10.1093/ee/36.4.725
Fenske M. P. and Imaizumi T. (2016). Circadian rhythms in floral scent emission. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00462
Foti M. C., Rostás M., Peri E., Park K. C., Slimani T., Wratten S. D., et al. (2017). Chemical ecology meets conservation biological control: identifying plant volatiles as predictors of floral resource suitability for an egg parasitoid of stink bugs. J. Pest Sci. (2004) 90, 299–310. doi: 10.1007/s10340-016-0758-3
Ganjisaffar F., Abrieux A., Gress B. E., Chiu J. C., and Zalom F. G. (2023). Drosophila infestations of California strawberries and identification of Drosophila suzukii using a TaqMan assay. Appl. Sci. 13, 1–10. doi: 10.3390/app13158783
Goodhue R. E., Bolda M., Farnsworth D., Williams J. C., and Zalom F. G. (2011). Spotted wing drosophila infestation of California strawberries and raspberries: economic analysis of potential revenue losses and control costs. Pest Manage. Sci. 67, 1396–1402. doi: 10.1002/ps.2259
Gress B. E. and Zalom F. G. (2018). Identification and risk assessment of spinosad resistance in a California population of Drosophila suzukii. Pest Manage. Sci. 75, 1270–1276. doi: 10.1002/ps.5240
Gullickson M. G., Digiacomo G., and Rogers M. A. (2024). Efficacy and economic viability of organic control methods for spotted-wing drosophila in day-neutral strawberry production in the Upper Midwest. Horttechnology 34, 618–628. doi: 10.21273/HORTTECH05461-24
Gullickson M., Hodge C. F., Hegeman A., and Rogers M. (2020). Deterrent effects of essential oils on spotted-wing drosophila (Drosophila suzukii): Implications for organic management in berry crops. Insects 11, 1–12. doi: 10.3390/insects11080536
Gullickson M. G., Rogers M. A., Burkness E. C., and Hutchison W. D. (2019). Efficacy of organic and conventional insecticides for Drosophila suzukii when combined with erythritol, a non-nutritive feeding stimulant. Crop Prot. 125, 1–6. doi: 10.1016/j.cropro.2019.104878
Hagler J. R., Nieto D. J., Machtley S. A., and Swezey S. L. (2020). Predator demographics and dispersal in alfalfa trap-cropped strawberry. Entomol. Exp. Appl. 168, 53–58. doi: 10.1111/eea.12864
Hetherington M. C., Fox M., Johnson M., Lopina A., Mechelke E., Weissner M., et al. (2024). Impact of alfalfa perimeter strips on Lygus lineolaris and beneficial arthropods in June-bearing strawberry fields. J. Pest Sci. (2004) 98, 145–157. doi: 10.1007/s10340-024-01795-w
Hoballah M. E., Stuurman J., Turlings T. C. J., Guerin P. M., Connétable S., and Kuhlemeier C. (2005). The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator Manduca sexta. Planta 222, 141–150. doi: 10.1007/s00425-005-1506-8
Hogg B. N., Bugg R. L., and Daane K. M. (2011). Attractiveness of common insectary and harvestable floral resources to beneficial insects. Biol. Control 56, 76–84. doi: 10.1016/j.biocontrol.2010.09.007
Jaffe B. D. and Guédot C. (2019). Vertical and temporal distribution of spotted-wing drosophila (Drosophila suzukii) and pollinators within cultivated raspberries. Pest Manage. Sci. 75, 2188–2194. doi: 10.1002/ps.5343
Keesey I. W., Knaden M., and Hansson B. S. (2015). Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 41, 121–128. doi: 10.1007/s10886-015-0544-3
Khan Z. R., Pickett J. A., van den Berg J., Wadhams L. J., and Woodcock C. M. (2000). Exploiting chemical ecology and species diversity: stem borer and striga control for maize and sorghum in Africa. Pest Manage. Sci. 56, 957–962. doi: 10.1002/1526-4998(200011)56:11<957::AID-PS236>3.0.CO;2-T
Kirkpatrick D. M., Gut L. J., and Miller J. R. (2018a). Estimating monitoring trap plume reach and trapping area for Drosophila suzukii (Diptera: Drosophilidae) in Michigan tart cherry. J. Econ. Entomol. 111, 1285–1289. doi: 10.1093/jee/toy062
Kirkpatrick D. M., Leach H. L., Xu P., Dong K., Isaacs R., and Gut L. J. (2018b). Comparative antennal and behavioral responses of summer and winter morph Drosophila suzukii (Diptera: Drosophilidae) to ecologically relevant volatiles. Environ. Entomol. 47, 700–706. doi: 10.1093/ee/nvy046
Leach H., Van Timmeren S., Wetzel W., and Isaacs R. (2019). Predicting within- and between-year variation in activity of the invasive spotted wing drosophila (Diptera: Drosophilidae) in a temperate region. Environ. Entomol. 48, 1223–1233. doi: 10.1093/ee/nvz101
Lee J. C., Dreves A. J., Cave A. M., Kawai S., Isaacs R., Miller J. C., et al. (2015). Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann. Entomol. Soc Am. 108, 117–129. doi: 10.1093/aesa/sau014
Little C. M., Chapman T. W., and Hillier N. K. (2020). Plasticity is key to success of Drosophila suzukii (Diptera: Drosophilidae) invasion. J. Insect Sci. 20, 1–8. doi: 10.1093/jisesa/ieaa034
Lu A., Gonthier D. J., Sciligo A. R., Garcia K., Chiba T., Juárez G., et al. (2022). Changes in arthropod communities mediate the effects of landscape composition and farm management on pest control ecosystem services in organically managed strawberry crops. J. Appl. Ecol. 59, 585–597. doi: 10.1111/1365-2664.14076
Nelson S. G. A. (2023). Expanded use of hail netting in Minnesota apple: Impacts on insect pests, fruit production, and natural enemies (University of Minnesota). https://hdl.handle.net/11299/259576 (Accessed May 17, 2024).
Nieto D. J., Hagler J. R., Swezey S. L., Machtley S. A., and Bryer J. A. (2023). Immigration of Lygus spp. (Hemiptera: Miridae) and predaceous natural enemies to trap-cropped organic strawberry. Environ. Entomol. 52, 824–831. doi: 10.1093/ee/nvad085
Petran A., Hoover E., Hayes L., and Poppe S. (2017). Yield and quality characteristics of day-neutral strawberry in the United States Upper Midwest using organic practices. Biol. Agric. Hortic. 33, 73–88. doi: 10.1080/01448765.2016.1188152
Pluskal T., Castillo S., Villar-Briones A., and Orešič M. (2010). MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinf. 11, 1–11. doi: 10.1186/1471-2105-11-395
Renkema J. M., Buitenhuis R., and Hallett R. H. (2017). Reduced Drosophila suzukii infestation in berries using deterrent compounds and laminate polymer flakes. Insects 8, 1–17. doi: 10.3390/insects8040117
Renkema J. M., Frewin A., and Hallett R. H. (2020). Effects of interplanting peppermint (Lamiaceae) in strawberry (Rosaceae) on Drosophila suzukii (Diptera: Drosophilidae) and seed-feeding pests (Hemiptera: Lygaeidae, Miridae, Rhyparochromidae). Can. Entomol. 152, 575–586. doi: 10.4039/tce.2020.34
Renkema J. M. and Smith D. (2020). Effects of sweet alyssum flowers and their volatile compounds on Drosophila suzukii (Matsumura) in the laboratory. J. Appl. Entomol. 144, 968–971. doi: 10.1111/jen.12803
Renkema J. M., Wright D., Buitenhuis R., and Hallett R. H. (2016). Plant essential oils and potassium metabisulfite as repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 6, 1–10. doi: 10.1038/srep21432
Samtani J. B., Rom C. R., Friedrich H., Fennimore S. A., Finn C. E., Petran A., et al. (2019). The status and future of the strawberry industry in the United States. Horttechnology 29, 11–24. doi: 10.21273/horttech04135-18
Schöneberg T., Arsenault-Benoit A., Taylor C. M., Butler B. R., Dalton D. T., Walton V. M., et al. (2020). Pruning of small fruit crops can affect habitat suitability for Drosophila suzukii. Agric. Ecosyst. Environ. 294, 106860. doi: 10.1016/j.agee.2020.106860
Schwieterman M. L., Colquhoun T. A., Jaworski E. A., Bartoshuk L. M., Gilbert J. L., Tieman D. M., et al. (2014). Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PloS One 9, 1–12. doi: 10.1371/journal.pone.0088446
Shaw B., Cannon M. F. L., Buss D. S., Cross J. V., Brain P., and Fountain M. T. (2019). Comparison of extraction methods for quantifying Drosophila suzukii (Diptera: Drosophilidae) larvae in soft- and stone-fruits. Crop Prot. 124, 1–5. doi: 10.1016/j.cropro.2019.104868
Sial A. A., Roubos C. R., Gautam B. K., Fanning P. D., Van Timmeren S., Spies J., et al. (2019). Evaluation of organic insecticides for management of spotted-wing drosophila (Drosophila suzukii) in berry crops. J. Appl. Entomol. 143, 593–608. doi: 10.1111/jen.12629
Smith J., Crow W. D., Catchot A. L., Gore J., Cook D. R., Musser F., et al. (2023). Evaluating efficacy and chemical concentrations of commonly used insecticides targeting tarnished plant bug in Mid-South cotton. J. Cotton Sci. 27, 74–80. doi: 10.56454/HKRJ8091
Stockton D. G., Wallingford A. K., Cha D. H., and Loeb G. M. (2021). Automated aerosol puffers effectively deliver 1-OCTEN-3-OL, an oviposition antagonist useful against spotted-wing drosophila. Pest Manage. Sci. 77, 389–396. doi: 10.1002/ps.6028
Swezey S. L., Nieto D. J., and Bryer J. A. (2007). Control of western tarnished plant bug Lygus hesperus Knight (Hemiptera: Miridae) in California organic strawberries using alfalfa trap crops and tractor-mounted vacuums. Environ. Entomol. 36, 1457–1465. doi: 10.1093/ee/36.6.1457
Tsuruda M., Girod P., Clausen M., and Carrillo J. (2022). Aromatic border plants in early season berries do not increase parasitism of spotted wing drosophila, Drosophila suzukii. Pest Manage. Sci. 79, 134–139. doi: 10.1002/ps.7182
University of Minnesota Extension (2024). Historical SWD Trap Data (FruitEdge). Available online at: https://fruitedge.umn.edu/historical-swd-trap-data (Accessed March 19, 2024).
USDA-AMS (2006). United States Standards for Grades of Strawberries. 1. Available online at: https://www.federalregister.gov/documents/2006/01/24/E6-781/united-states-standards-for-grades-of-strawberries (Accessed January 25, 2024).
Van Timmeren S., Davis A. R., and Isaacs R. (2021). Optimization of larval sampling method for monitoring Drosophila suzukii (Diptera: Drosophilidae) in blueberries. J. Econ. Entomol. 114, 1–11. doi: 10.1093/jee/toab096
Van Timmeren S. and Isaacs R. (2013). Control of spotted wing drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot. 54, 126–133. doi: 10.1016/j.cropro.2013.08.003
Van Timmeren S., Mota-Sanchez D., Wise J. C., and Isaacs R. (2018). Baseline susceptibility of spotted wing drosophila (Drosophila suzukii) to four key insecticide classes. Pest Manage. Sci. 74, 78–87. doi: 10.1002/ps.4702
Wallingford A. K., Cha D. H., and Loeb G. M. (2018). Evaluating a push–pull strategy for management of Drosophila suzukii Matsumura in red raspberry. Pest Manage. Sci. 74, 120–125. doi: 10.1002/ps.4666
Wallingford A. K., Connelly H. L., Brind’Amour G. D., Boucher M. T., Mafra-Neto A., and Loeb G. M. (2016a). Field evaluation of an oviposition deterrent for management of spotted-wing drosophila, Drosophila suzukii, and potential nontarget effects. J. Econ. Entomol. 109, 1779–1784. doi: 10.1093/jee/tow116
Wallingford A. K., Hesler S. P., Cha D. H., and Loeb G. M. (2016b). Behavioral response of spotted-wing drosophila, Drosophila suzukii Matsumura, to aversive odors and a potential oviposition deterrent in the field. Pest Manage. Sci. 72, 701–706. doi: 10.1002/ps.4040
Wold S. J. and Hutchison W. D. (2003). Phenology of Lygus lineolaris (Hemiptera: Miridae) in Minnesota June-bearing strawberries: Comparison of sampling methods and habitats. J. Econ. Entomol. 96, 1814–1820. doi: 10.1093/jee/96.6.1814
Keywords: Drosophila suzukii, Lygus lineolaris, chemical ecology, biodiversity, integrated pest management, behavior modification, conservation biological control
Citation: Gullickson MG, Suresh J, Hegeman AD and Rogers MA (2025) Ecological effects of interplanted sweet alyssum and alfalfa in an organic day-neutral strawberry production system. Front. Ecol. Evol. 13:1651123. doi: 10.3389/fevo.2025.1651123
Received: 20 June 2025; Accepted: 22 October 2025;
Published: 12 November 2025.
Edited by:
Sergio Angeli, Free University of Bozen-Bolzano, ItalyReviewed by:
Stefan Dötterl, University of Salzburg, AustriaMokhtar Abdulsattar Arif, Ministry of Agriculture, Iraq
Copyright © 2025 Gullickson, Suresh, Hegeman and Rogers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew G. Gullickson, Z3VsbGkxMzlAdW1uLmVkdQ==
 Matthew G. Gullickson
Matthew G. Gullickson Jayanti Suresh1
Jayanti Suresh1 Adrian D. Hegeman
Adrian D. Hegeman Mary A. Rogers
Mary A. Rogers