- 1Department of Biology, Drake University, Des Moines, IA, United States
- 2Department of Integrative Biology, W.K. Kellogg Biological Station, Michigan State University, Hickory Corners, MI, United States
- 3Ecology, Evolution, and Behavior Program, Michigan State University, East Lansing, MI, United States
Physiological traits may influence the establishment success of non-native species, yet empirical links between physiology and invasiveness remain limited. The American bullfrog (L. catesbeianus) and the green frog (L. clamitans) are closely related species with overlapping native ranges in the eastern United States, but have contrasting invasion histories: bullfrogs have colonized much of the western U.S., while green frogs have not. One hypothesis that could explain this pattern is that invasive species possess greater tolerance to heat stress and enhanced capacity for thermal acclimation. To test this hypothesis, we compared critical thermal maximum (CTMAX) and acclimation capacity in tadpoles of both species from within their native range. We found that the species both exhibit equally high CTMAX. Further, neither species was able to acclimate to a warmer temperature. However, while bullfrogs showed no change in CTMAX after acclimation, green frogs experienced a slight reduction in CTMAX, suggesting that they may be more sensitive to warming than bullfrogs. These results suggest that intrinsic differences in thermal tolerance and plasticity alone do not explain bullfrog invasion success. Other factors—such as competitive dominance, rapid evolutionary shifts, or interacting abiotic and biotic pressures—may facilitate bullfrog persistence in novel, warmer habitats of the western U.S.
Introduction
Invasive species are a leading cause of global biodiversity loss (McKinney and Lockwood, 1999; Mooney and Cleland, 2001). Yet, the proportion of introduced species that become established invasives in new environments is relatively low (Williamson, 1996). Uncovering which traits promote invasiveness can provide insights into why some species become invasive whereas others do not (DeVore et al., 2021). Many ecologically important traits are likely to facilitate invasion, for example, fast life histories that include rapid growth rates and high fecundity (Sakai et al., 2001). More recently, there has been considerable interest in understanding whether greater physiological tolerance to stress promotes invasions (Jarnevich et al., 2018). However, few empirical studies investigate the relationship between invasion success and key physiological traits that play a role in the establishment of non-native species in a new habitat (Kelley, 2014).
In general, the physiological traits associated with invasiveness could permit species to occupy new environments that differ from their native ones, and they may have greater tolerance to stressors such as temperature, desiccation, and disturbance (Olyarnik et al., 2009). For ectotherms, temperature is a particularly important factor influencing the geographic distribution of a species (Bozinovic et al., 2011). Key physiological traits may therefore include broad thermal tolerances that allow a species to exist within a wide thermal range and especially to tolerate high temperatures (Zerebecki and Sorte, 2011), and increased thermal plasticity (acclimation capacity) that allows an organism to recalibrate its thermal threshold after brief exposure to different environmental temperatures (Buckley et al., 2001; Mittan and Zamudio, 2019) enabling function over a wider range of temperatures. Indeed, aquatic ectotherms such as tadpoles may be constantly challenged by high temperatures because their small body size coupled with the high heat capacity of water results in body temperatures that are equal to that of the water (Duarte et al., 2012). However, species with the propensity to become invasive may produce tadpoles with intrinsically higher heat tolerances and acclimation capacities than others.
A powerful approach to testing associations between physiological traits and invasiveness is to compare these traits in closely related species where one is a successful invader and the other is not. The American bullfrog, Lithobates catesbeianus, and the green frog, L. clamitans, are congeners that largely occupy the same native range in the eastern United States (Harding and Mifsud, 2017; Figures 1A, B). Bullfrogs are considered one of the 100 worst invasive species globally (Lowe et al., 2000) and have successfully expanded into new habitats in the western and southwestern United States, among 40 other countries across four continents (Lever, 2003). They were likely introduced in western North America between 1882 and 1904 (Jennings and Hayes, 1985) and have since established vigorous populations in much of the western U.S. (Figure 1 (IUCN, 2020). Some of the places that bullfrogs have become established include areas such as California’s Central Valley and parts of New Mexico and southern Utah in which maximum temperatures are much greater than those in the native range of the two species (Figure 1; Fick and Hijmans, 2017). By contrast, established green frog populations are only documented from a handful of localities outside their native range where they were introduced, including Newfoundland and British Columbia, Canada, and northern Utah and Washington, U.S. (Figure 1 (IUCN, 2020). Although the ecological impacts of these few green frog introductions remain largely unknown, populations in Utah and Washington have been documented since 1966 (Gregoire and Powell, 2023), but have yet to expand in the same capacity as bullfrogs suggesting that green frogs lack traits that promote invasiveness. Previous studies exploring the causes of variation in invasiveness between the two species have focused on differences in their diet (Werner et al., 1995), gut microbiota (Fontaine and Kohl, 2020), susceptibility to toxins (Birdsall et al., 1986) and community interactions (Hecnar and M’Closkey, 1997), but to our knowledge, there is no information on intrinsic thermal physiological differences of the tadpoles of the two species. This is particularly important, as tadpoles are a critical life stage, and potentially more vulnerable to physiological stressors such as temperature due to their small body size and the high thermal conductance of water (Lutterschmidt and Hutchison, 1997; Winter et al., 2016).
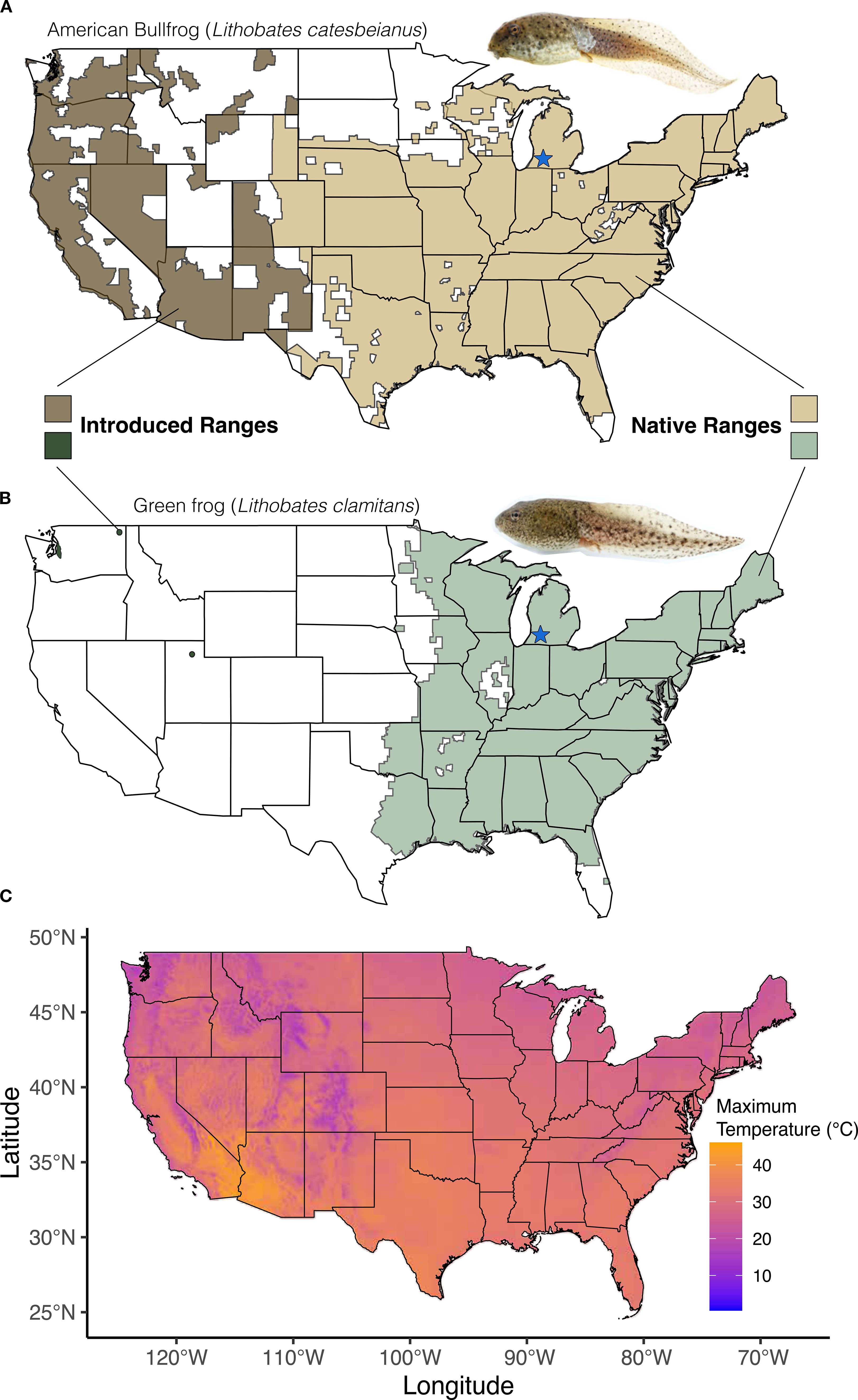
Figure 1. Species range map of bullfrogs and green frogs in the United States. (A) bullfrog native (light brown) and introduced (dark brown) US range. Sampling locality indicated by blue star. (B) green frog native (light green) and introduced (dark green) U.S. range. Sampling locality indicated by blue star. Range data for both species were used from IUCN database, 2022. (C) Maximum temperatures across the U.S. – from 1970-2000 (data from WorldClim 2).
Bullfrogs and green frogs therefore present an opportunity to test predictions about the relationship between physiological traits and invasive ability. Here, we compared physiological traits in bullfrog and green frog tadpoles occurring in a northern part of their native habitat in the eastern U.S. We hypothesized that the critical thermal maximum (CTMAX), i.e., the temperature at which locomotor function ceases (Lutterschmidt and Hutchison, 1997), and capacity to acclimate to higher temperature should differ between bullfrog and green frog tadpoles within their native range. In particular, we predicted that bullfrog tadpoles should have intrinsically higher CTMAX, and a greater capacity to acclimate to higher temperatures. We tested these predictions using wild-caught tadpoles originating from a large semi-natural pond complex in southwestern Michigan.
Materials and methods
Tadpole collection and lab acclimation
In May and June 2022, we collected 77 wild bullfrog and green frog tadpoles (n = 42 bullfrogs and n = 35 green frogs) from the W.K. Kellogg Biological Station Experimental Pond Lab at Michigan State University. This research facility houses 18 experimental ponds that are currently colonized by natural flora and fauna including thriving populations of bullfrogs and green frogs. A logger was placed in one of the ponds to measure the natural temperature regime and inform acclimation temperatures during experiments (Supplementary Figure S1). Using long dip-nets, we collected tadpoles between Gosner stages 25-38 (Gosner, 1960) i.e., individuals in the ‘tadpole’ developmental stage prior to metamorphosis (McDiarmid and Altig, 1999). We then placed two conspecific tadpoles of similar size in rectangular plastic containers (31 x 17 x 9 cm) filled with filtered pond water. Containers were placed within temperature-controlled incubators (Percival Scientific, model I36LLVL) that were held for 2 days at 14°C (for 16 hours) in the day and 10°C (for 8 hours) at night and subjected to a light-dark cycle of 16:8 h L:D, typical of summer months in Michigan.
Critical thermal maxima
To compare heat stress tolerance, we measured the critical thermal maximum (CTMAX), i.e., the temperature at which an organism begins to lose locomotor function (Lutterschmidt and Hutchison, 1997) in 17 bullfrog and 15 green frog tadpoles. Tadpoles were placed in individual mesh containers, partially submerged in a water bath filled with filtered pond water and held at 14°C. The mesh containers confined the tadpoles but allowed them to experience the surrounding water. We then increased the water temperature by 0.3°C per minute, a standard rate for CTMAX experiments in aquatic animals (Dallas and Rivers-Moore, 2012; Gutiérrez-Pesquera et al., 2016) using a custom-built programmable temperature controller connected to a 500 W aquarium-grade titanium heating rod. A water pump was used to ensure homogenization of temperature in the water bath. Approximately every 1-2 minutes, we assessed tadpoles by turning them over onto their backs and watching their response. We defined CTMAX as the temperature at which tadpoles failed to right themselves when turned over (Lutterschmidt and Hutchison, 1997). When tadpoles reached this point, they were rapidly removed from the experimental bath and placed in cool (14°C) pond water to recover. All tadpoles recovered, i.e., resumed normal swimming activity. Following the experiment, we measured the wet mass of each tadpole.
Thermal acclimation capacity
We measured thermal acclimation, which represents reversible plasticity in heat tolerance and can develop over days-to-weeks of sustained exposure to altered environmental temperatures (e.g., Cupp, 1980; Lapwong et al., 2021; Rohr et al., 2018). We placed newly caught tadpoles (15 bullfrogs and 10 green frogs) in an incubator set to reach a maximum of 20°C (for 16 hours per day), and a minimum nighttime temperature of 16°C (held for 8 hours per day) for 6 days. This acclimation temperature was higher than the baseline experiment, but still well within the natural range of variation in the pond temperatures (see Supplementary Figure S1). We chose an acclimation period of 6 days because this period is thought to be long enough to allow physiological changes to take place during thermal acclimation in amphibians (Brattstrom, 1968; Hutchison, 1961). Then, following the same protocol as above, we measured CTMAX.
Statistics
All statistical analyses were performed in R Studio v 2024.04.2 (Posit Team, 2024). To test the prediction that bullfrog tadpoles are more tolerant of heat stress than green frog tadpoles, we performed a linear regression analysis to investigate how CTMAX varies between the two species, while controlling for mass. Mass and Gosner stage were strongly correlated, (r (28) = 0.4, p = 0.03), and Gosner stage had no effect on CTMAX (t = -0.60, p = 0.56). We, therefore, did not include Gosner stage in the analysis. Two data points were dropped from the analysis because the tadpoles did not look healthy at the start of the experiments. Next, to assess acclimation capacity, we conducted a linear regression analysis to examine the effects of acclimation temperature (i.e., 14°C or 20°C) on CTMAX of the two species. Our model evaluated the main effects of acclimation temperature and the interaction between species and acclimation temperature on the response variable, CTMAX, while controlling for mass.
To provide an estimate of acclimation capacity, an acclimation response ratio (ARR, Claussen, 1977) was calculated for each species, using:
where, ARR is the acclimation response ratio, Δ CTMAX is the difference between CTMAX after acclimation to the high temperature and CTMAX after acclimation to the low temperature; and Δ Temperature is the difference between high and the low acclimation temperatures. Higher ARR values indicate greater acclimation capacity. After all experimentation, we euthanized tadpoles by applying a ~1/8th teaspoon of 20% benzocaine cream to their ventral sides until no movement or breathing was detected. All tadpoles were stored in 70% ethanol and assigned a Gosner stage.
Results
Critical thermal maxima
We found no significant difference in CTMAX between the two species (F(1, 27) = 3.82 p = 0.06). Qualitatively, however, green frogs appeared to have lower CTMAX than bullfrogs. Mean CTMAX values were 38.2°C for bullfrogs and 37.5°C for green frogs (Figure 2). The effect of wet mass on CTMAX was also non-significant (F(1, 27) = 2.52; p = 0.12).
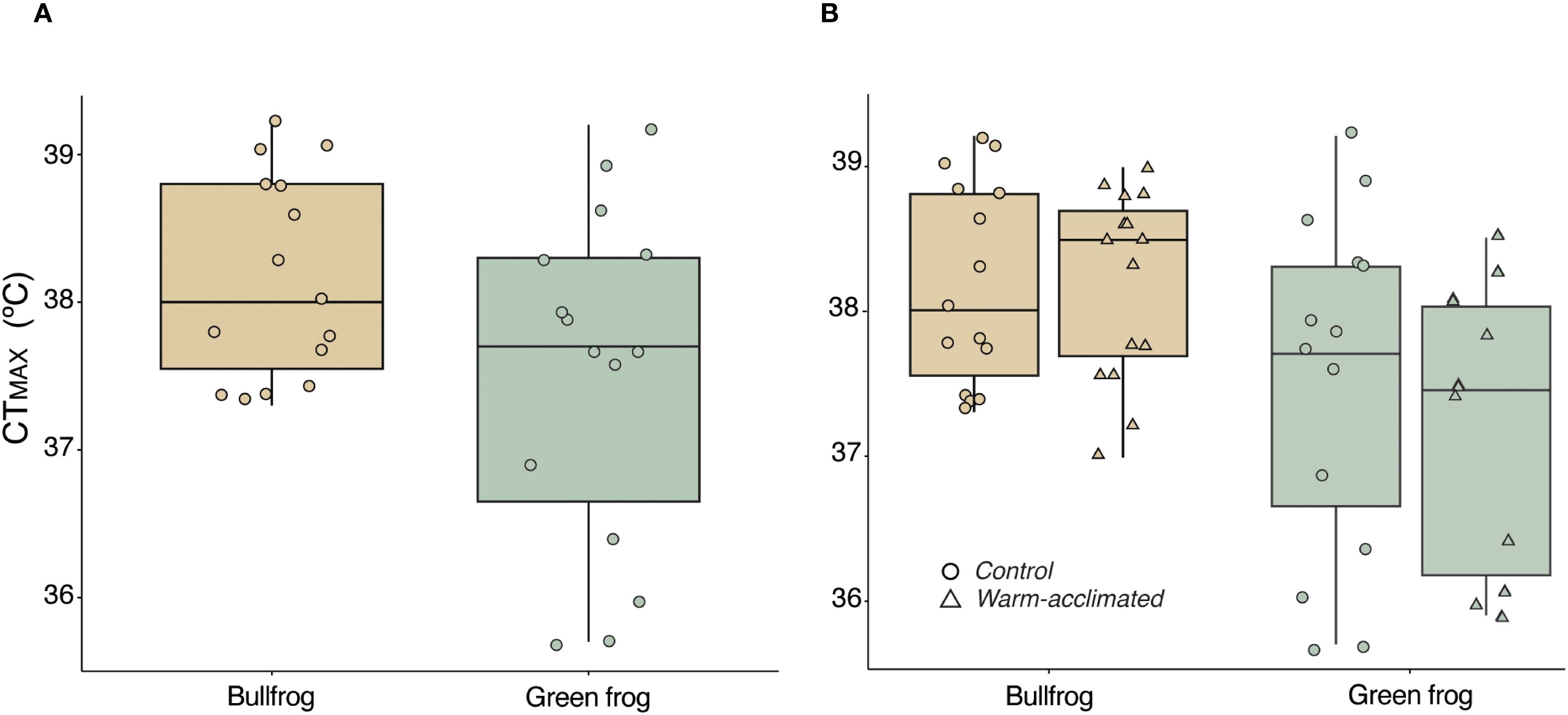
Figure 2. Boxplots from CTMAX experiments on bullfrog and green frog tadpoles. (A) CTMAX of bullfrog and green frog tadpoles after acclimation to 14°C. Green frogs have a qualitatively lower mean CTMAX than bullfrogs. (B) Comparison of CTMAX after cool and warm acclimation. There were no differences in acclimation capacity, however, average green frog CTMAX declined slightly after warm acclimation suggesting that they may be marginally more sensitive to heat stress than bullfrogs.
Thermal acclimation capacity
Our results indicated that the species do not differ in acclimation capacity, i.e., there was no significant interaction between acclimation temperature and species (Figure 2; F(1,50) = 0.46; p = 0.50). However, after acclimation to the warmer temperature, green frogs had significantly lower CTMAX than bullfrogs (F(1, 50) = 11.62; p< 0.01). Mean CTMAX values did not change for bullfrog tadpoles (CTMAX after warm acclimation = 38.2; ARR = 0) but decreased for green frog tadpoles (CTMAX after warm acclimation = 37.2; ARR = -0.03).
Discussion
Researchers are increasingly recognizing that physiological traits, such as tolerance to environmental stress, may predispose some species to invading and colonizing new habitats. Although bullfrogs and green frogs have been frequently studied within an invasive-native species context (e.g., Fontaine and Kohl, 2020), to our knowledge, there is no information on intrinsic thermal physiological differences in the juveniles of the two species.
When subjected to ramping heat-stress experiments after a brief acclimation to a cool, average springtime pond temperature (14°C), bullfrog and green frog tadpoles showed no difference in critical thermal maxima (CTMAX). Overall, both species had high CTMAX values indicating that they can withstand relatively high pond temperature spikes. Indeed, these species occur in exposed, sunlit ponds and can experience maximum temperatures of ~36°C over several consecutive days in the summer (A. Shah, unpubl. data). Their CTMAX are largely comparable to those measured for other temperate tadpoles (Duarte et al., 2012). However, after acclimating to a warmer temperature (20°C), the two species showed significant differences in their CTMAX. These differences were driven by a decrease in CTMAX in green frogs. Decreases in CTMAX after acclimation to warm temperatures may indicate that green frogs are more sensitive to warming (e.g., Shah et al., 2017) and potentially have a lower capacity to acclimate to warmer conditions.
Indeed, during field collections, bullfrog tadpoles substantially outnumbered green frogs in shallow ponds that were several degrees warmer than the deeper ponds in the area (E. VanDenBerg, K. Jaynes, and A. Shah pers. obs.). In light of our results, this observation could be partly explained by the fact that green frog tadpoles are marginally more sensitive to warmer temperatures. However, other factors may also be at play. The higher abundance of bullfrog tadpoles in warmer ponds may additionally result from their ability to outcompete green frog tadpoles. If bullfrogs can perform (i.e., eat, grow, locomote) more effectively than green frogs at warmer temperatures, they may be better able to overtake and displace green frogs in warmer habitats (Mauro et al., 2022). For example, when bullfrogs were removed from an aquatic community, green frog abundance increased significantly, indicating that bullfrogs are superior competitors (Hecnar and M’Closkey, 1997). Testing the interaction between physiological performance and competitive differences in the two species across a range of temperatures was outside the scope of this study, but should be considered as a future research goal (Mauro et al., 2022).
A second potential physiological mechanism that may facilitate bullfrog invasiveness is thermal plasticity or acclimation (Mittan and Zamudio, 2019; Tepolt and Somero, 2014; Xue and Ma, 2020). Acclimation is predicted to enable species to persist across variable spatiotemporal thermal landscapes (Brattstrom and Lawrence, 1962; Franklin et al., 2007; Sørensen et al., 2016) and adaptive plasticity can facilitate successful establishment in novel habitats (Bock et al., 2018; Corl et al., 2018; Mittan and Zamudio, 2019). We expected CTMAX to increase after warm acclimation, particularly in bullfrog tadpoles. However, we found no differences in acclimation capacity between the two species. In fact, neither species showed any acclimation ability; i.e., their CTMAX values remained largely unchanged after acclimation. One possible reason for this lack of plasticity may be that they already possess a relatively high basal tolerance for heat stress (average CTMAX of both species after cool acclimation = 38°C). Some evidence suggests that due to physiological constraints, there may be a trade-off between basal thermal tolerance and the capacity of an organism to further increase its heat tolerance (Stillman, 2003; but see Birrell et al. 2023).
Additionally, acclimation ability can vary across populations (e.g., Cicchino et al., 2023) so, although acclimation capacity is limited in the populations tested in our study, other populations may well be able to acclimate to warmer conditions. For example, bullfrogs living closer to the warmer parts of the native range in North America may have greater acclimation capacity than their counterparts in the cooler parts. Alternatively, longer periods of acclimation to warmer temperatures or investigation of heat shock protein production (Zerebecki and Sorte, 2011) may reveal differences that were not seen in our experiment.
Conclusion
We hypothesized that intrinsic physiological tolerances between two congeners that differ in their invasive ability should vary. Our results revealed no major differences. In addition to being stronger competitors, we surmise that other factors, such as rapid evolution of thermal tolerance or combinations of responses to novel abiotic and biotic conditions may ultimately predispose bullfrog tadpoles to successfully persist in hotter habitats in the western U.S. We note that with only two species (Garland and Adolph, 1994), and measuring traits in only the northern part of their range, we are limited in our ability to make strong conclusions about physiological differences. However, in addition to establishing a first step in measuring these differences, our study also provides a framework for testing other populations and multiple species in the future. Finally, investigating physiological tolerance in regions where bullfrogs were introduced, particularly those where green frogs have also established in the west, would be an important next step in understanding how physiological traits influence invasiveness.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: FigShare, dx.doi.org/10.6084/m9.figshare.30104779.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee (IACUC) at Michigan State University (project PROTO202200152). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EV: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. KJ: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review & editing. AS: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. AAS was supported by a National Science Foundation award (grant award # 2221744). EV was supported by a National Science Foundation Research Experience for Undergraduates (REU) fellowship awarded to Kellogg Biological Station (grant award 1757530).
Acknowledgments
We are grateful to A. Hutchens and N.S. Warner for their assistance during this study. This work was conducted under a Michigan Department of Natural Resources collection permit FSCP04192022140548 and approved by the Michigan State University Institutional Animal Care and Use Committee (IACUC ID: PROTO202200152). We are grateful to H. A. Woods and members of the Fitzpatrick and Shah labs at KBS for providing us with helpful comments on earlier versions of this manuscript. This is Kellogg Biological Station contribution no. 2425.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Chat GPT was used to help with R code syntax to make the map in Figure 1 and for the plots in Figure 2.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1671218/full#supplementary-material
References
Birdsall C. W., Grue C. E., and Anderson A. (1986). Lead concentrations in bullfrog Rana catesbeiana and green frog R. clamitans tadpoles inhabiting highway drainages. Environ. pollut. Ser. A Ecol. Biol. 40, 233–247. doi: 10.1016/0143-1471(86)90098-X
Birrell J. H., Frakes J. I., Shah A. A., and Woods H. A. (2023). Mechanisms underlying thermal breadth differ by species in insects from adjacent but thermally distinct streams–A test of the climate variability hypothesis. J. Thermal Biol. 112, 103435.
Bock D. G., Kantar M. B., Caseys C., Matthey-Doret R., and Rieseberg L. H. (2018). Evolution of invasiveness by genetic accommodation. Nat. Ecol. Evol. 2, 991–999. doi: 10.1038/s41559-018-0553-z
Bozinovic F., Calosi P., and Spicer J. I. (2011). Physiological correlates of geographic range in animals. Annu. Rev. Ecology Evolution Systematics 42, 155–179. doi: 10.1146/annurev-ecolsys-102710-145055
Brattstrom B. H. (1968). Thermal acclimation in Anuran amphibians as a function of latitude and altitude. Comp. Biochem. Physiol. 24, 93–111. doi: 10.1016/0010-406X(68)90961-4
Brattstrom B. H. and Lawrence P. (1962). The rate of thermal acclimation in anuran amphibians. Physiol. Zoology 35, 148–156. doi: 10.1086/physzool.35.2.30152723
Buckley B. A., Owen M. E., and Hofmann G. E. (2001). Adjusting the thermostat: The threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J. Exp. Biol. 204, 3571–3579. doi: 10.1242/jeb.204.20.3571
Cicchino A. S., Shah A. A., Forester B. R., Dunham J. B., Poff N. L., Ghalambor C. K., et al. (2023). Acclimation capacity of critical thermal maximum varies among populations: Consequences for estimates of vulnerability. Ecosphere 14, e4691. doi: 10.1002/ecs2.4691
Claussen D. L. (1977). Thermal acclimation in ambystomatid salamanders. Comp. Biochem. Physiol. – Part A: Physiol. 58, 333–340.
Corl A., Bi K., Luke C., Challa A. S., Stern A. J., Sinervo B., et al. (2018). The genetic basis of adaptation following plastic changes in coloration in a novel environment. Curr. Biol. 28, 2970–2977.e7. doi: 10.1016/j.cub.2018.06.075
Cupp P. V. (1980). Thermal tolerance of five salientian amphibians during development and metamorphosis. Herpetologica 36, 234–244.
Dallas H. F. and Rivers-Moore N. A. (2012). Critical Thermal Maxima of aquatic macroinvertebrates: Towards identifying bioindicators of thermal alteration. Hydrobiologia 679, 61–76. doi: 10.1007/s10750-011-0856-4
DeVore J. L., Shine R., and Ducatez S. (2021). Spatial ecology of cane toads (Rhinella marina) in their native range: A radiotelemetric study from French Guiana. Sci. Rep. 11, 11817. doi: 10.1038/s41598-021-91262-8
Duarte H., Tejedo M., Katzenberger M., Marangoni F., Baldo D., Beltrán J. F., et al. (2012). Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Global Change Biol. 18, 412–421. doi: 10.1111/j.1365-2486.2011.02518.x
Fick S. E. and Hijmans R. J. (2017). WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatology 37, 4302–4315. doi: 10.1002/joc.5086
Fontaine S. S. and Kohl K. D. (2020). Gut microbiota of invasive bullfrog tadpoles responds more rapidly to temperature than a noninvasive congener. Mol. Ecol. 29, 2449–2462. doi: 10.1111/mec.15487
Franklin C. E., Davison W., and Seebacher F. (2007). Antarctic fish can compensate for rising temperatures: Thermal acclimation of cardiac performance in Pagothenia borchgrevinki. J. Exp. Biol. 210, 3068–3074. doi: 10.1242/jeb.003137
Garland T. and Adolph S. C. (1994). Why not to do two-species comparative studies: limitations on inferring adaptation. Physiol. Zoology 67, 797–828. doi: 10.1086/physzool.67.4.30163866
Gosner K. L. (1960). A simplified table for staging anuran embryos and larvae with notes on identification. Herpitologica 16, 183–190.
Gregoire D. and Powell R. (2023). Lithobates clamitans (Latreille in Sonnini de Manoncourt and Latreille 1801) (USGS Nonindigenous Aquatic Species Database).
Gutiérrez-Pesquera L. M., Tejedo M., Olalla-Tárraga M.Á., Duarte H., Nicieza A., and Solé M. (2016). Testing the climate variability hypothesis in thermal tolerance limits of tropical and temperate tadpoles. J. Biogeography 43, 1166–1178. doi: 10.1111/jbi.12700
Harding J. A. and Mifsud D. A. (2017). Amphibians and Reptiles of the Great Lakes Region (Ann Arbor, MI: University of Michigan Press).
Hecnar S. J. and M’Closkey R. T. (1997). Changes in the composition of a ranid frog community following bullfrog extinction. Am. Midland Nat. 137, 145–150. doi: 10.2307/2426763
Hutchison V. H. (1961). Critical thermal maxima in salamanders. Physiol. Zoology 34, 92–125. doi: 10.1086/physzool.34.2.30152688
IUCN (2020). “IUCN Red List of Threatened Species: Aquarana catesbeianus,” in IUCN Red List of Threatened Species.
Jarnevich C. S., Young N. E., Talbert M., and Talbert C. (2018). Forecasting an invasive species’ distribution with global distribution data, local data, and physiological information. Ecosphere 9 (5), e02279. doi: 10.1002/ecs2.2279
Jennings M. R. and Hayes M. P. (1985). Pre-1900 Overharvest of California Red-Legged Frogs (Rana aurora draytonii): The Inducement for Bullfrog (Rana catesbeiana) Introduction. Herpitologica 41, 94–103.
Kelley A. L. (2014). The role thermal physiology plays in species invasion. Conserv. Physiol. 2 (1), cou045. doi: 10.1093/conphys/cou045
Lapwong Y., Dejtaradol A., and Webb J. K. (2021). Plasticity in thermal hardening of the invasive Asian house gecko. Evolutionary Ecol. 35, 631–641. doi: 10.1007/s10682-021-10116-x
Lever C. (2003). Naturalized amphibians and reptiles of the world (Oxford, UK: Oxford University Press).
Lowe S., Browne M., Boudjelas S., and De Poorter M. (2000). 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database (The Invasive Species Specialist Group).
Lutterschmidt W. I. and Hutchison V. H. (1997). The critical thermal maximum: History and critique. Can. J. Zoology 75, 1561–1574. doi: 10.1139/z97-783
Mauro A. A., Shah A. A., Martin P. R., and Ghalambor C. K. (2022). An integrative perspective on the mechanistic basis of context- dependent species interactions. Integr. Comp. Biol. 62, 164–178. doi: 10.1093/icb/icac055
McDiarmid R. and Altig R. (1999). Tadpoles: The biology of anuran larvae. Ed. McDiarmid R. A. R. (Chicago, IL: Chicago University Press).
McKinney M. L. and Lockwood J. L. (1999). Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453. doi: 10.1016/S0169-5347(99)01679-1
Mittan C. S. and Zamudio K. R. (2019). Rapid adaptation to cold in the invasive cane toad Rhinella marina. Conserv. Physiol. 7 (1), coy075. doi: 10.1093/conphys/coy075
Mooney H. A. and Cleland E. E. (2001). The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. 98, 5446–5451. doi: 10.1073/pnas.091093398
Olyarnik S. V., Bracken M. E. S., Byrnes J. E., Hughes A. R., Hultgren K. M., and Stachowicz J. J. (2009). “Ecological factors affecting community invasibility,” in Biological Invasions in Marine Ecosystems. Eds. Rilov G. and Crooks J. A. (Heidelberg: Springer).
Posit Team (2024). RStudio [Computer software] (Posit Software, PBC). Available online at: http://www.posit.co/.
Rohr J. R., Civitello D. J., Cohen J. M., Roznik E. A., Sinervo B., and Dell A. I. (2018). The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 21, 1425–1439. doi: 10.1111/ele.13107
Sakai A. K., Allendorf F. W., Holt J. S., Lodge D. M., Molofsky J., With K. A., et al. (2001). The population biology of invasive species. Annu. Rev. Ecol. Systematics 32, 305–332. doi: 10.1146/annurev.ecolsys.32.081501.114037
Shah A. A., Funk W. C., and Ghalambor C. K. (2017). Thermal acclimation ability varies in temperate and tropical aquatic insects from different elevations. Integr. Comp. Biol. 57, 977–987. doi: 10.1093/icb/icx101
Stillman J. H. (2003). Acclimation capacity underlies susceptibility to climate change. Science 301 (5629), 65–65.
Sørensen J. G., Kristensen T. N., and Overgaard J. (2016). Evolutionary and ecological patterns of thermal acclimation capacity in Drosophila: Is it important for keeping up with climate change? Curr. Opin. Insect Sci. 17, 98–104. doi: 10.1016/j.cois.2016.08.003
Tepolt C. K. and Somero G. N. (2014). Master of all trades: Thermal acclimation and adaptation of cardiac function in a broadly distributed marine invasive species, the European green crab, Carcinus maenas. J. Exp. Biol. 217, 1129–1138. doi: 10.1242/jeb.093849
Werner E. E., Wellborn G. A., and McPeek M. A. (1995). Diet composition in postmetamorphic bullfrogs and green Frogs: Implications for interspecific predation and competition. J. Herpetology 29, 600–607. doi: 10.2307/1564744
Winter M., Fiedler W., Hochachka W. M., Koehncke A., Meiri S., and De la Riva I. (2016). Patterns and biases in climate change research on amphibians and reptiles: A systematic review. R. Soc. Open Sci. 3, 160158. doi: 10.1098/rsos.160158
Xue Q. and Ma C. S. (2020). Aged virgin adults respond to extreme heat events with phenotypic plasticity in an invasive species, Drosophila suzukii. J. Insect Physiol. 121, 104016. doi: 10.1016/j.jinsphys.2020.104016
Keywords: acclimation capacity, bullfrog, critical thermal maximum, green frog, invasive species, tadpole
Citation: VanDenBerg ER, Jaynes KE and Shah AA (2025) Testing differences in thermal tolerance between two amphibians with contrasting invasion abilities. Front. Ecol. Evol. 13:1671218. doi: 10.3389/fevo.2025.1671218
Received: 22 July 2025; Accepted: 04 September 2025;
Published: 22 September 2025.
Edited by:
Lin Zhang, Hubei University of Chinese Medicine, ChinaReviewed by:
Jaime A Collazo, North Carolina State University, United StatesLi Ma, Lishui University, China
Copyright © 2025 VanDenBerg, Jaynes and Shah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Evelyn R. VanDenBerg, ZXZlbHlucnZkYkBnbWFpbC5jb20=; Alisha A. Shah, YWFzaGFoQG1zdS5lZHU=
†ORCID: Evelyn R. VanDenBerg, orcid.org/0009-0000-4846-0098
Kyle E. Jaynes, orcid.org/0000-0002-4188-0468
Alisha A. Shah, orcid.org/0000-0002-8454-7905
 Evelyn R. VanDenBerg1*†
Evelyn R. VanDenBerg1*† Kyle E. Jaynes
Kyle E. Jaynes Alisha A. Shah
Alisha A. Shah