- 1Guangdong Provincial Key Laboratory of Silviculture, Protection and Utilization, Guangdong Academy of Forestry, Guangzhou, China
- 2College of Horticulture and Landscape Architecture, Zhongkai University of Agriculture and Engineering, Guangzhou, China
Camphora officinarum Nees, is a significant economic tree indigenous to southern China. Despites of previous efforts, ecological niches of C. officinarum remain unclear with few sampling and neglectance of chemotypes. In the present study, new efforts have been made to investigate the integrated C. officinarum and its four chemotypes based on 546 occurances using utilizing MaxEnt model from 2021 to 2080.The findings indicate that highly suitable regions for the integrated C. officinarum are projected to diminish between 2021 and 2080, resulting in habitat fragmentation. The climatic variables influencing this are the annual mean precipitation (bio12), the minimum temperature of the coldest month (bio6), the mean precipitation during the warmest month (bio18), and the annual mean temperature (bio1). Furthermore, the regions highly suitable for each chemotype of C. officinarum are anticipated to undergo shifts and diversification from 2021 to 2080, with the primary factors driving these changes being the maximum precipitation in the driest month (bio14) and elevation. The study proposes conservation strategies at the end, furnishing a foundation for conservation and domestication of C. officinarum in South China.
1 Introduction
The phenomenon of global climate change has been scientifically established and exerts significant influence on the global environment. An increasing number of ecologists and conservation biologists have started researching to determine the effects of this phenomenon on specific regions and species (Jarvie and Svenning, 2018; Picazo et al., 2020; Abbass et al., 2022; Dahunsi et al., 2023; Yuan et al., 2024). As the research advances, it has become increasingly evident that the potential ramifications of climate change on natural ecosystems could result in substantial diminutions of plant diversity (Muluneh and Security, 2021; Weiskopf et al., 2024). Over the past century, the global temperature has experienced an increase of 1.5°C with difference evidence, with a notably accelerated rise in the most recent three decades (Hoegh-Guldberg et al., 2019). The persistent escalation of global temperatures has led to alterations in the distribution of plant species, as they progressively shift their ranges towards higher latitudes and altitudes in response to the warming climate (Kelly and Goulden, 2008; Boisvert-Marsh et al., 2014; Freeman et al., 2018). The emergence of plant chemotypes is a result of plants producing distinct secondary metabolites as a reaction to both biological and abiotic stresses. The later are primarily composed of environmental elements such as light, temperature, and precipitation (Boncan et al., 2020; Jin et al., 2025). These environmental pressures induce variations in plant secondary metabolites, which subsequently affect their distribution patterns (Verma and Shukla, 2015; Mahajan et al., 2020). Through the examination of the impacts of historical, contemporary, and projected climatic alterations on the dispersion of plant species, it is possible to enhance comprehension of the mechanisms that underpin the development of species distribution patterns, thereby facilitating the conservation and management of plant resources.
Phytoecological research is crucial for understanding plant community composition, interspecies interactions, environmental impacts on plants, and their adaptive responses, playing a key role in botanical conservation. The conceptual framework of ecological niches frequently serves as the foundational principle for species distribution models (SDMs) (Cassini, 2011; McInerny and Etienne, 2012). Species distribution models offer an essential methodology for accurately simulating the distribution of plant species and potential shifts in their habitats, finding extensive application in ecological research, plant conservation initiatives, and evolutionary analyses (Phillips et al., 2006; Townsend et al., 2007). Predictive models for potential habitats encompass Genetic Algorithm for Rule-set Production (GARP), Maximum Entropy Modeling (MaxEnt), Climate-based Ecological Niche Mode (CLIMEX), and Bioclimatic Envelope Model (Bioclim). Each model possesses unique merits, with MaxEnt being the most prevalent species distribution model. The MaxEnt model is a species distribution model grounded in the principle of maximum entropy. It operates by examining environmental variables associated with species distribution data to ascertain the distribution probability that maximizes conditional entropy, thus forecasting potential species habitats (Phillips et al., 2006). Studies reveal that the MaxEnt model outperforms other species distribution models in predictive accuracy and stability, while also being user-friendly, computationally efficient, and fewer samples (Elith et al., 2006, 2011). This model has been broadly employed to project potential habitat distributions for invasive alien species, ecosystems, and endangered protected species in the recent years (Zhang et al., 2021; Zhao et al., 2021; Gao et al., 2022; Zhao et al., 2022; Guo et al., 2023; Hosseini et al., 2024).
Camphora officinarum Nees, commonly referred to as fragrant camphor, is a member of the Camphora genus within the Lauraceae family (Yang et al., 2022). It is predominantly indigenous to provinces situated south of the Yangtze River basin, encompassing broad regions of Southern China. The species is acknowledged for its substantial economic importance for both timber and essential oil usages. The wood of the camphor tree is valued for its hardness, fine texture, appealing grain patterns, and unique scent, making it a sought-after material for construction, furniture-making, and sculptural purposes (Zhou and Yan, 2016). Essential oil of camphor trees is extensively utilized in the cosmetics, food additive, insect repellent, pharmaceutical, and related industries (Li et al., 2021; Lee et al., 2022). The essential oils derived from the camphor tree exhibit a considerable degree of endogenous variability, resulting in the identification of over ten distinct chemical types, characterized by their primary chemical constituents, including linalool-type, eucalyptol-type, borneol-type, artemisia-ketone type, camphor-type (Stubbs et al., 2004; Jiang et al., 2014, 2022). Previous studies on camphor trees have unveiled considerable disparities in essential oil content and composition across different cultivar sources of Cinnamomum camphora (=Camphora officinarum) (Zhang et al., 2023). Research has demonstrated that the synthesis and accumulation of plant secondary metabolites are intricately linked to environmental conditions, encompassing factors such as light, temperature, and water (Verma and Shukla, 2015; Boncan et al., 2020; Hou et al., 2025). A few previous studies projected the prospective distribution zones of 75~182 C. officinarum samplings using MaxEnt models, indicating that the distribution zones are progressively shifting towards northern latitudes and higher elevations (Zhang et al., 2019; Meng et al., 2021; Li et al., 2023). Despite these efforts in ecological modeling of C. officinarum, the generated MaxEnt models face two major limitations: (1) they fail to account for environmental adaptation differences among distinct essential oil chemical types, which may significantly influence species’ climate response strategies; (2) conflicting research findings regarding the dominant environmental factor affecting camphor tree distribution (temperature vs precipitation), which likely stem from noise caused by sample chemotype mixing. The objective of this study is to examine the distinct ecological niches of four chemotypes (linalool, artemisia ketone, eucalyptol, and camphor) of C. officinarum under projected warming conditions from 2021 to 2080. Additionally, the climatic factors influencing potential shifts in the optimal growth conditions for each chemotype will be explored. These new insights offer theoretical support for scientific conservation, selective cultivation, and domestication of C. officinarum.
2 Materials and methods
2.1 Sample collection and chemotype determination of C. officinarum
Surveys for sample collection of C. officinarum were performed across ten provinces/regions in Southern China, encompassing Fujian Province, Guangdong Province, Guangxi Zhuang Autonomous Region, Hubei Province, Hunan Province, Jiangsu Province, Jiangxi Province, Sichuan Province and Zhejiang Province between 2022 and 2023. Utilizing GPS tracking, 546 individuals were sampled with latitude and longitude data for natural camphor forests (Supplementary Table S1). Furthermore, we gathered mature, disease-free camphor tree leaves (at least 4 grams per sample) during these surveys. These samples underwent two concurrent tests for subsequent chemical profile analysis and essential oil content determination.
To determine the chemotype of C. officinarum for each sample, fresh camphor tree leaves were meticulously gathered from the field, subsequently cleansed, dehydrated, and measured to 4 grams per sample. The specimens were submerged in liquid nitrogen and subsequently pulverized into a fine powder with a mesh size of 200. This powder was then immersed in a solvent mixture comprising n-hexane, ethanol, and ethyl acetate in a ratio of 2:1:1, with a solvent-to-sample ratio of 1:2. The resultant mixture was subjected to ultrasonic treatment at a frequency of 20 kHz for a duration of 20 minutes, followed by incubation at a temperature of 56°C for 30 minutes. Centrifugation of the mixture yielded an upper layer that was subsequently analyzed using gas chromatography-mass spectrometry (GC-MS, QP2010 Ultra, Shimadzu Corporation, Japan). The chromatography column employed was a SH Rxi 5Sil MS quartz capillary column, measuring 30 meters in length, 0.25 millimeters in diameter, and 0.25 micrometers in thickness. The gas chromatography conditions were as follows: the initial column temperature was set at 70°C, which was then increased to 160°C at a rate of 2°C per minute, held for 2 minutes, and subsequently ramped up to 220°C at a rate of 10°C per minute for a duration of 5 minutes. The auto-sampler was utilized with an injection volume of 0.5 μL, employing a split injection method with a 1:100 split ratio at a temperature of 230°C. The helium carrier gas was maintained at a flow rate of 30 mL/min, with a constant flow rate of 1.19 ml/min through the column. The mass spectrometry parameters included an ion source temperature of 200°C, an interface temperature of 250°C, and a scan range of m/z 50-500. The essential oil compounds derived from the camphor tree samples were then collected for further analysis. The species distribution data compiled frequently manifests substantial geographical heterogeneity (Syfert et al., 2013), as certain areas may have dense populations, while others are sparsely populated due to a variety of factors. Such disparities in distribution can result in an overemphasis on environmental variables within the model, thus impacting its precision (Boria et al., 2014; Fourcade et al., 2014). A preliminary filtering of the collected camphor tree latitude and longitude data was implemented, eliminating records that were missing, erroneous, or duplicated. The geographic coordinates were subsequently converted to decimal format and organized into tables by species name, longitude, and latitude in chronological order, which were then saved as CSV files. Utilizing ENMTools5.26 (Warren et al., 2021), the preliminary screening results were subjected to a deconvolution process with a 50-kilometer refinement distance (Low et al., 2021). This procedure culminated in 276 entries (50.5% of the 546 samples) for valid camphor tree distribution records for the purposes of MaxEnt model predictions.
2.2 Ecological niche modeling of four chemotypes of C. officinarum
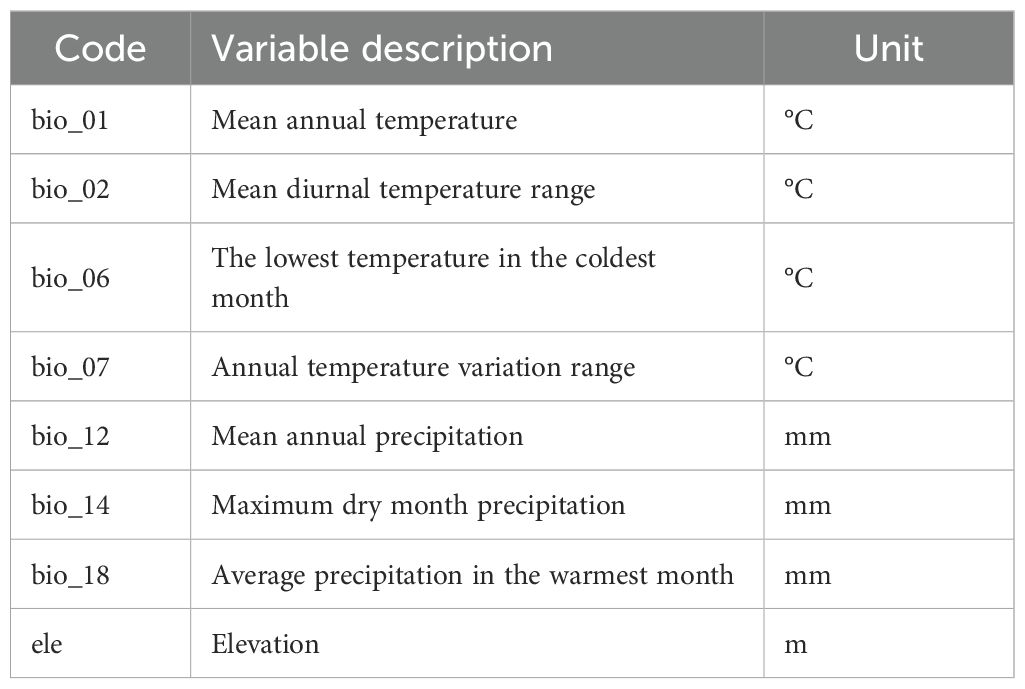
In the present study, nineteen bioclimatic variables (bio1-bio19) and elevation (ele) were collected as the environmental parameters for the modeling process. The climatic data were derived from the World Meteorological Organization’s Worldclim database (v2.1, http://www.worldclim.org). Passed and future projections for the SSP2-4.5 scenario across three distinct periods—2021–2040, 2041–2060, and 2061–2080—were derived from an ensemble of 35 CMIP6 global climate models, which were downscaled to a resolution of 2.5 arc-minutes. The Global Climate Model (GCM) data employed encompassed the BCC-CSM2-MR climate system model from the Beijing Climate Center (BCC), which adeptly captures regional climate patterns. It is noteworthy that the BCC-CSM2-MR simulation exhibited a high correlation coefficient of 0.86 with observational data, underscoring its precision and dependability (Liu and Chen, 2024; Zhang et al., 2025).
The 20 variable datasets were subjected to rasterization through the application of the SDM Toolx tool using the ArcGIS software v.10.8 (Environmental Systems Research Institute, https://www.esri.com/) and subsequently transformed into an ASC II format that is compatible with the MaxEnt modeling framework (Childs, 2004). In light of the potential for overlapping correlations within environmental climate data, which could precipitate redundant modeling phenomena and thereby diminish model accuracy, a preliminary screening of climate environmental factors was conducted to refine the precision of the model. The climate variables extracted were subjected to a correlation analysis employing ENMTools version 5.26, with the retention of factors exhibiting correlations exceeding 0.8 (Zheng et al., 2024; Sun and Deng, 2025; Zhao et al., 2025), indicating a greater ecological significance. A total of eight environmental factors (bio1, bio2, bio6, bio7, bio12, bio14, bio18 and ele) were ultimately selected for the construction of the model (Supplementary Figure S1 and Table 1).
In the foundational configuration, random sampling was implemented. During the computational phase, the distribution points were established at 25% for the test set and 75% for the training set, with 10 iterations executed to guarantee precision. A total of 1,000 iterations were conducted (Kong et al., 2021). The map data was compiled in logistic format, with predictions visualized through images and response curves. The significance of climate variables was evaluated using the jackknife method (Miller, 1974). The precision of the model was assessed via Receiver Operating Characteristics (ROC) curve analysis, with the Area Under the Curve (AUC) calculated as a performance metric. AUC values range from 0 to 1.0, values closer to 1 indicate greater reliability, while those ≤0.6 suggest unreliable predictions (Lobo et al., 2008). Other parameters were maintained at their default settings. Subsequently, the MaxEnt software was utilized to input the csv file containing camphor tree distribution data and filtered environmental factors for model execution.
Given the significant variations in environmental adaptability among different plant species, there are no stringent criteria for classifying model-predicted suitable habitats. From the outcomes of 10 repeated MaxEnt model executions, the mean value was chosen as the predictive outcome. The predictive result files were transformed into raster files using ArcGIS software v10.8 (https://www.esri.com/) and subsequently classified into five suitability zones through manual grading, with values ranging from 0 to 1. The distribution probabilities (P values) of camphor trees and various chemical types were categorized into five levels: (1) Unsuitable zone (P ≤ 0.1), (2) Low suitability zone (0.1 ≤ P ≤ 0.3), (3) Marginal suitability zone (0.3 ≤ P ≤ 0.5), (4) Suitable zone (0.5 ≤ P ≤ 0.7), and (5) High suitability zone (0.7 ≤ P ≤ 1.0). Utilizing ArcGIS software, the graded files were reclassified by selecting the optimal five suitability zones. The layer was then clipped using China’s vector map to compute the actual area of each suitability zone based on their respective grades.
2.3 Climatic determinants of varied chemotypes of C. officinarum
Utilizing the outcomes of the Jackknife method test and the contribution rates derived from the MaxEnt model, the principal environmental determinants that influence the adaptability zones of camphor trees were ascertained (Phillips et al., 2017). When the cumulative contribution rate of environmental determinants surpasses 80%, and subsequent determinants contribute less than 5%, the latter are deemed non-dominant, whereas the former are considered dominant (Phillips et al., 2006). The Jackknife method (Miller, 1974; Wu, 1986) evaluates how environmental factors affect species distribution predictions. Results show two gain types: regularized training gain and test gain, each with three values (excluding, including, or using all variables). A higher score when a variable is included alone indicates a stronger influence on species distribution.
3 Results
3.1 Sample collection and chemotype determination of C. officinarum
The chemotypes of the 546 samples of C. officinarum were determined using GC-MS (Supplementary Table S1), and four chemotypes (artemisia ketone, camphor, eucalyptol, and linalool) were determined based on 107 compounds (Supplementary Table S2). The four major chemotypes possess 470 samples, of which 213 samples for artemisia ketone-type, 117 for eucalyptol-type, 71 for camphor-type, and 69 for camphor-type. The data was further processed by converting it into a tabular format with regard to chemotype, longitude, and latitude coordinates. Using ENMTools5.26, the preliminary distribution data underwent deconvolution. The final dataset for subsequent analysis comprised 218 entries (46.4% of the 470 samples) of overall resource distribution across four chemical types: 64 artemisia ketone-type, 54 eucalyptol-type, 35 camphor-type, and 31 camphor-type entries.
A scatter plot of linear regression analysis shows the geographical distribution of various chemical types with 95% confidence intervals (Figure 1). The plot shows a considerable differences (P < 0.05) between artemisia ketone-type and camphor-type camphor trees with regard to geographic distribution. Artemisia ketone-type trees are mainly in the 25–26 degrees latitude range, while camphor-type trees are mostly between 29–31 degrees. Both types have little latitude variation but significant longitudinal differences. To investigate the provincial disparities in the distribution of chemical compound types in camphor trees, a plot was drawn to reveal inter-provincial variations in the distribution of chemical types (Figure 2). Linalool-type is predominantly distributed in Guangdong, Guangxi, Jiangxi, Hubei, Hunan, Fujian, and Zhejiang. Guangxi exhibits the highest distribution proportion (96%). Artemisia ketone-type is distributed in Guangdong, Jiangxi, Hubei, Hunan, Fujian, Zhejiang, Jiangsu, Guizhou, and Sichuan. The eucalyptol type is mainly distributed in Hubei (41%), Zhejiang (40%) and Jiangxi (39%) The camphor type is distributed in Guizhou (46%), Hubei (36%) and Zhejiang (33%) These results clearly reflect the geographical variations in the four chemotypes of C. officinarum.
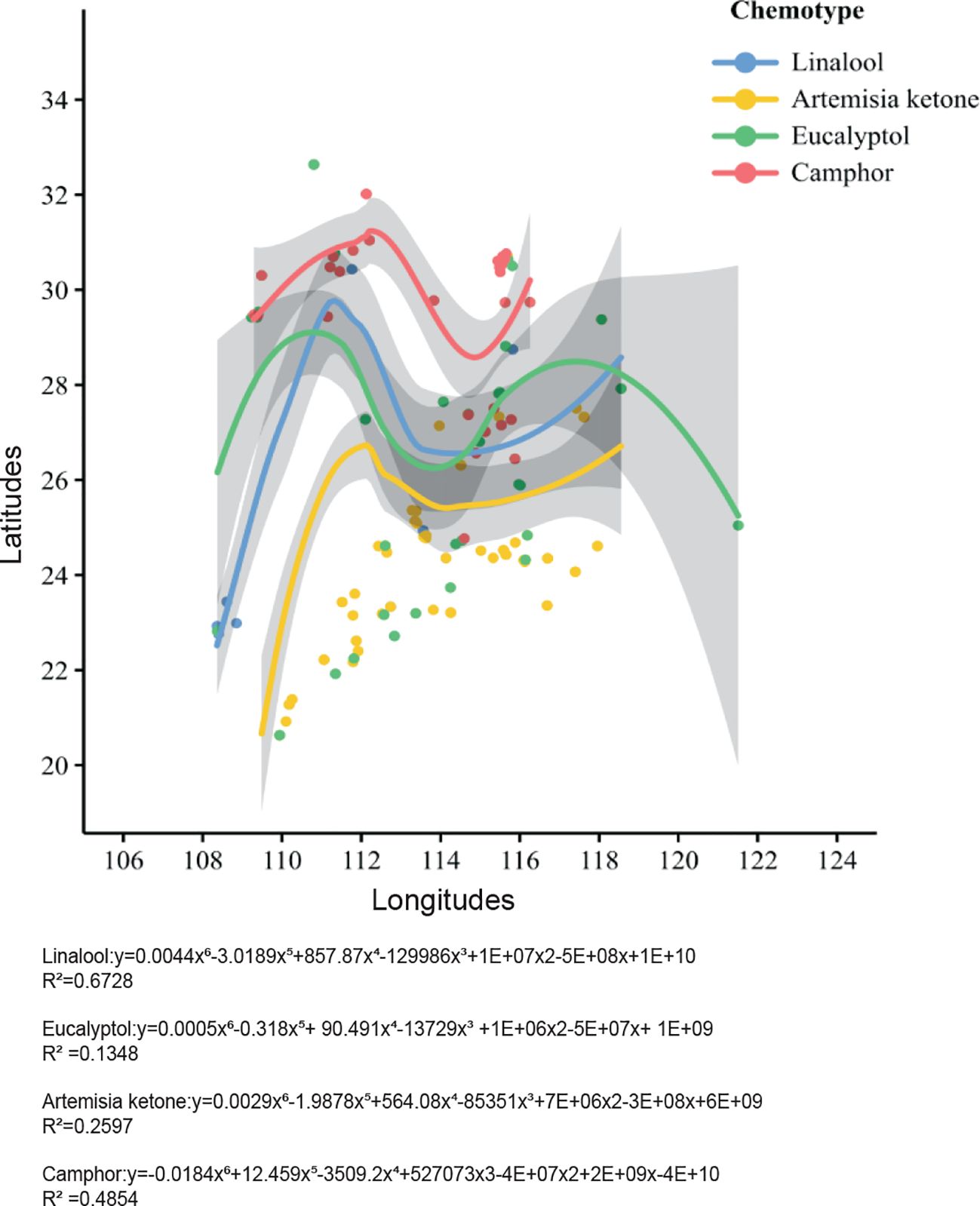
Figure 1. Latitude and longitude scatter plot of distribution of the four chemotypes of C. officinarum.
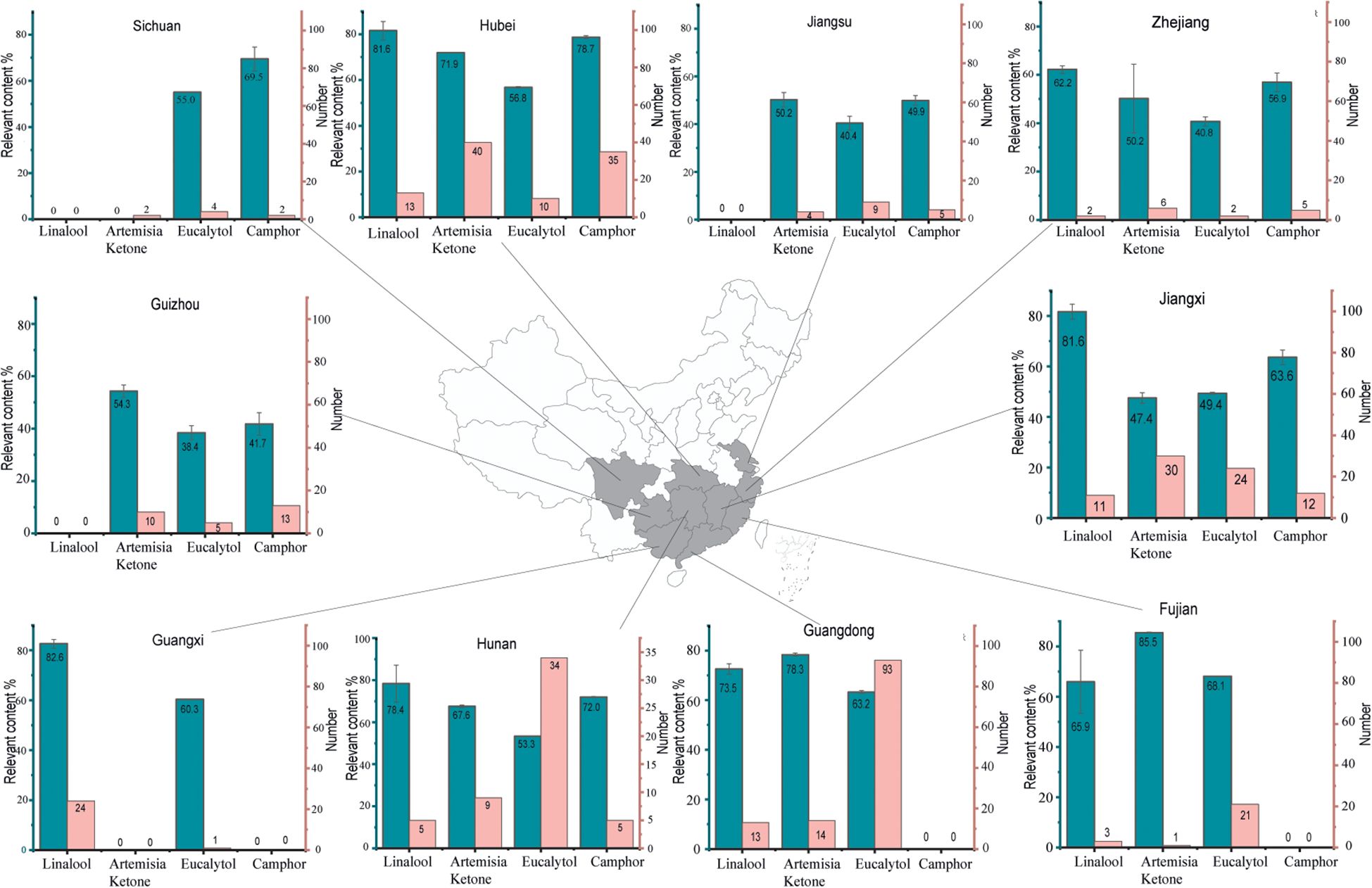
Figure 2. Analysis of the chemical composition and relative abundance in 546 leaf samples from C. officinarum in Southern China. The central map delineates the geographical extent of the sampling areas, while the peripheral bar graphs illustrate the relative content of the primary chemical constituents (%) represented by cyan bars (left y-axis), and the number of detected individuals by pink bars (right y-axis) across the four identified chemotypes.
With respect to essential oil composition, camphor-type camphor trees exhibit higher relative concentrations in Guangxi, Jiangxi, Hunan, and Hubei provinces, while displaying lower levels in Guangdong, Fujian, and Zhejiang. Artemisia ketone-type camphor trees demonstrate elevated concentrations in Fujian and Guangdong provinces, whereas they are relatively scarce in Hubei, Hunan, Guizhou, Jiangsu, Zhejiang, and Jiangxi. Eucalyptol-type camphor trees display higher concentrations in Fujian, Guangdong, Sichuan, and Guangxi provinces, but their presence is notably reduced in Jiangxi, Hunan, Zhejiang, Jiangsu, and Guizhou. Camphor-type camphor trees show higher levels in Hunan, Zhejiang, and Sichuan provinces, while remaining comparatively scarce in Jiangxi, Zhejiang, Jiangsu, and Guizhou.
3.2 Ecological niche modeling of integrated C. officinarum and its four chemotypes
Under current climatic conditions (from 2021 to 2040), suitable regions for integrated C. officinarum are predominantly located south of the Qinling-Huaihe Line, with high-adaptation zones concentrated south of the Yangtze River (Figure 3). The highly suitable regions of integrated C. officinarum covers 1.2361 million km² (12.88% of China’s land area), concentrated in Sichuan, Guizhou, Guangxi, Guangdong, Fujian, Jiangxi, Zhejiang, Jiangsu, and Taiwan, with partial presence in Hubei, Anhui, and southern Xizang. The suitable region spans 1.0714 million km² (11.16% of China’s land area), primarily located in Shandong, Henan, and Yunnan. In the future from 2041 to 2080, the high-adaptation zone is projected to undergo gradual contraction and fragmentation. Despite these spatial shifts, the core highly suitable and suitable regions south of the Yangtze gradually shrink and fragment from 2041 to 2080, alongside the northward movement of low-adaptation zones in integrated C. officinarum (Figure 3).
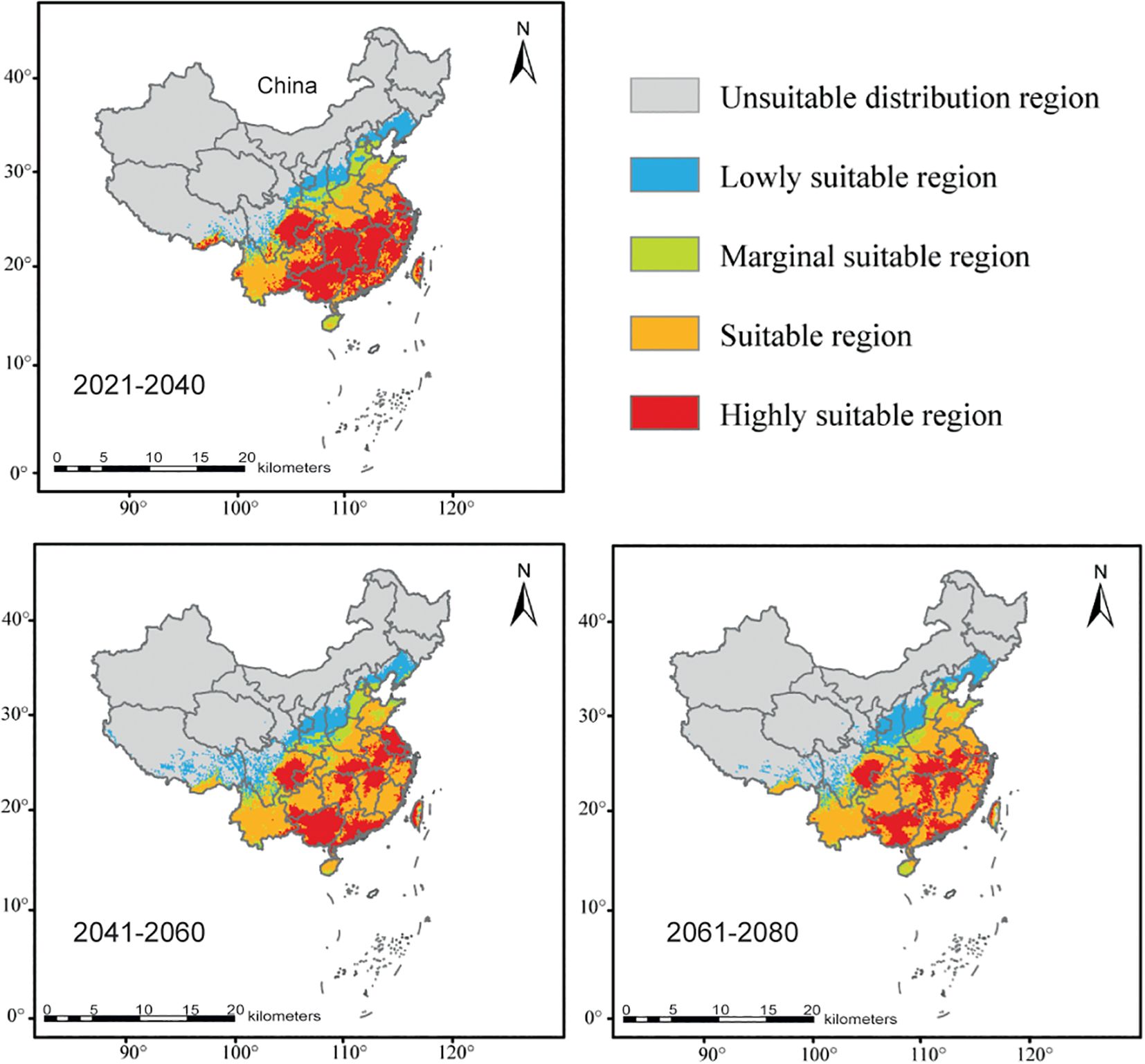
Figure 3. Predicted potential suitable areas for integrated C. officinarum in Southern China under three climate scenarios: 2021–2040, 2041–2060, and 2061–2080. These areas are categorized into five distinct suitability levels: unsuitable (gray), low suitability (blue), marginal suitability (green), suitable (orange), and highly suitable (red).
The MaxEnt model of all four types of C. officinarum (linalool, artemisia ketone, eucalyptol, and camphor) consistently achieved AUC values exceeding 0.9 across 10 training iterations. All four chemotypes currently exhibit varied distributions of highly suitable zones from 2021 to 2040 (Figure 4): the highly suitable region of the linalool-type concentrated in Guangdong; the highly suitable region of artemisia ketone-type concentrated in partial Jiangxi and Guangdong; the highly suitable region of eucalyptol-type concentrated in Jiangxi and partial Guangdong; the highly suitable region of camphor-type concentrated in partial Jiangxi and Hubei. Projected changes of ecological niches among the four chemotypes lead to considerable different suitable zones from 2021 to 2080 (Figure 4). Highly suitable region of the linalool-type shifted from partial Jiangxi to Guangxi, with total suitable habitat area increased from 371,670 km² to 380,000 km². Highly suitable region of the artemisia ketone-type within Guangdong contracted gradually from 349,170 km² to 230,280 km². Highly suitable region of the eucalyptol-type diminished in Guangdong by 2080.Highly suitable region of the camphor-type expanded to encompass Jiangxi, Hunan, and Hubei, with total suitable habitat area increased from 211,400 km² to 222,500 km². These results indicate that climate change significantly influence the distribution patterns of integrated C. officinarum and its four chemotypes from 2021 to 2080.
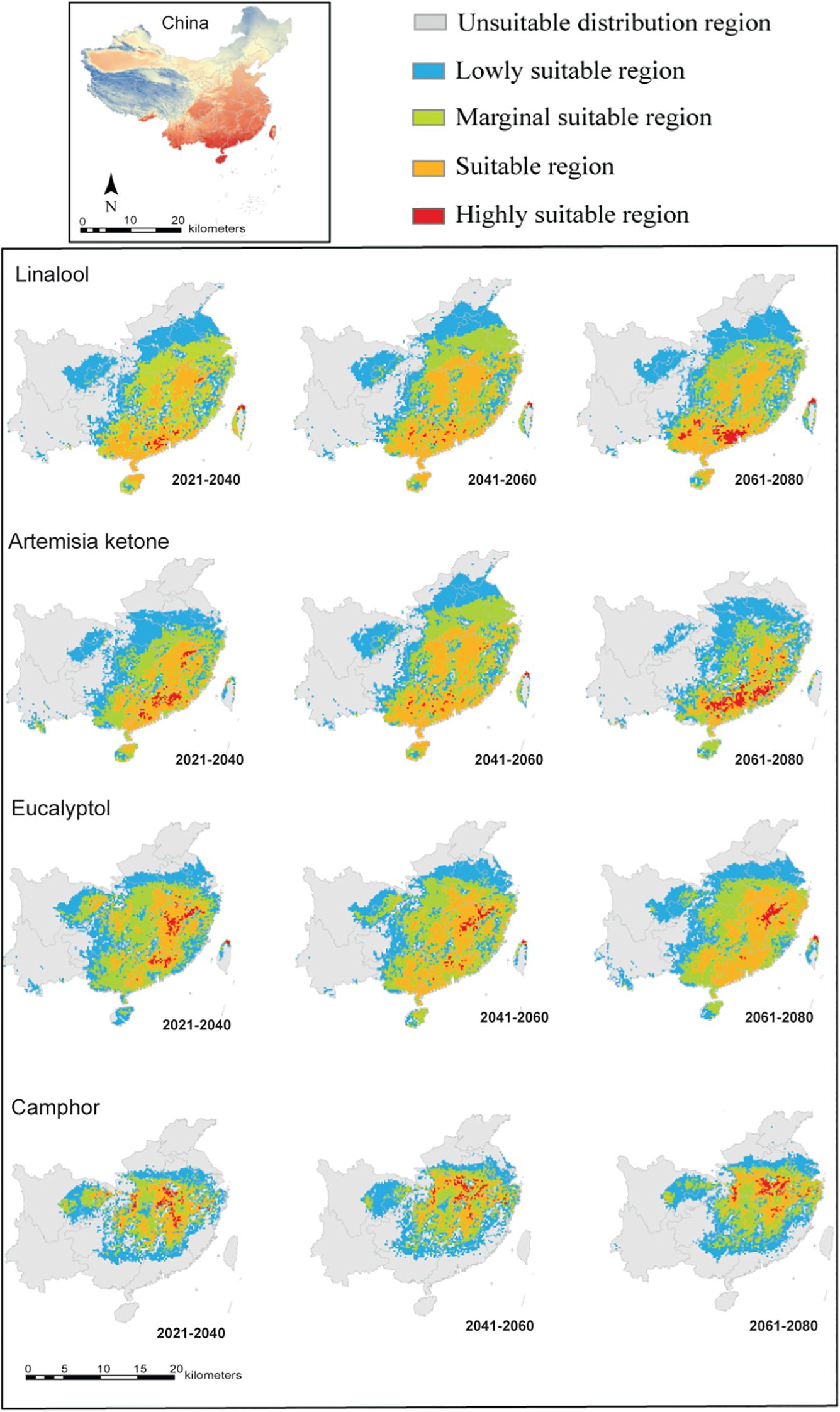
Figure 4. Predicted potential suitable habitats for four chemical types of C. officinarum in Southern China under future climate scenarios (2021–2080). The areas are categorized into five distinct suitability levels: unsuitable (gray), low suitability (blue), marginal suitability (green), suitable (orange), and highly suitable (red).
3.3 Climatic determinants of integrated C. officinarum and its four chemotypes
With respect to integrated C. officinarum, the annual average precipitation (bio12) contributed 0% from 2021 to 2040, whereas the ratio increases to 35.5% from 2041 to 2080 (Figure 5). Conversely, the minimum temperature of the coldest month (bio6) accounted for 20.8% from 2021 to 2040, which is expected to decrease to 16.5% from 2041 to 2080. Additionally, the contribution of average precipitation in the warmest month (bio18) accounts for 15.9% from 2021 to 2040, is projected to increase to 20.4% from 2041 to 2060, and subsequently decrease to 16.4% from 2061 to 2080. In contrast, the annual average temperature (bio1) contributed 19.4% from 2021 to 2040, is expected to decrease to 18.2% from 2041 to 2060, and increase to 20.4% from 2061 to 2080 (Figure 5).
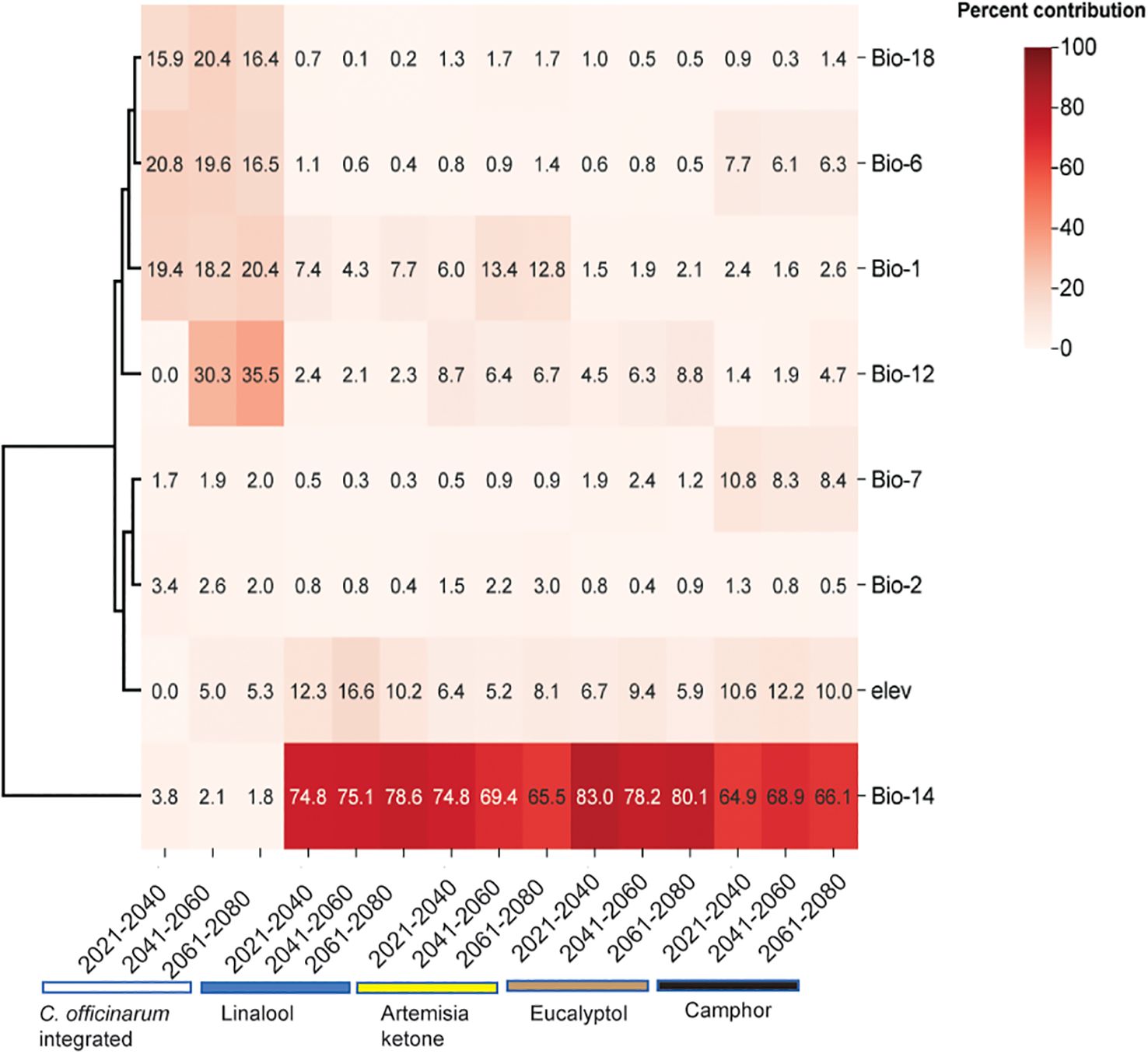
Figure 5. Heat map of environmental factor contribution rates of integrated C. officinarum and the four chemotypes. Each line represents different climatic factors: bio1 (annual mean temperature), bio6 (min temperature of coldest month), bio12 (annual precipitation), bio18 (precipitation of warmest quarter), bio14 (precipitation of driest month), bio7 (temperature annual range) and ele (elevation).
The response curves show major environmental factors affecting the distribution of integrated camphor trees and its four chemotypes. The mean annual precipitation (bio12) of 1000–2400 mm is most suitable for camphor tree growth, with an optimal peak at about 1500 mm (Figure 6A). As rainfall increases, the adaptability probability gradually declines and drops below 0.5 when over 2400 mm. The lowest temperature in the coldest month (bio6) of -3°C-9°C is favorable (Figure 6B). The probability peaks at 3°C, decreases as temperature rises, drops below 0.5 above 9°C, and stabilizes at 23°C. Integrative camphor trees grow well when the average precipitation in the warmest month (bio18) exceeds 400 mm. The probability peaks at 500 mm of precipitation, then gradually decreases and stabilizes at 0.5 or higher when precipitation reaches 3000 mm (Figure 6C). The mean annual temperature (bio1) of 13-20°C is ideal, peaking at 17°C and falling below 0.5 above 21°C (Figure 6D).
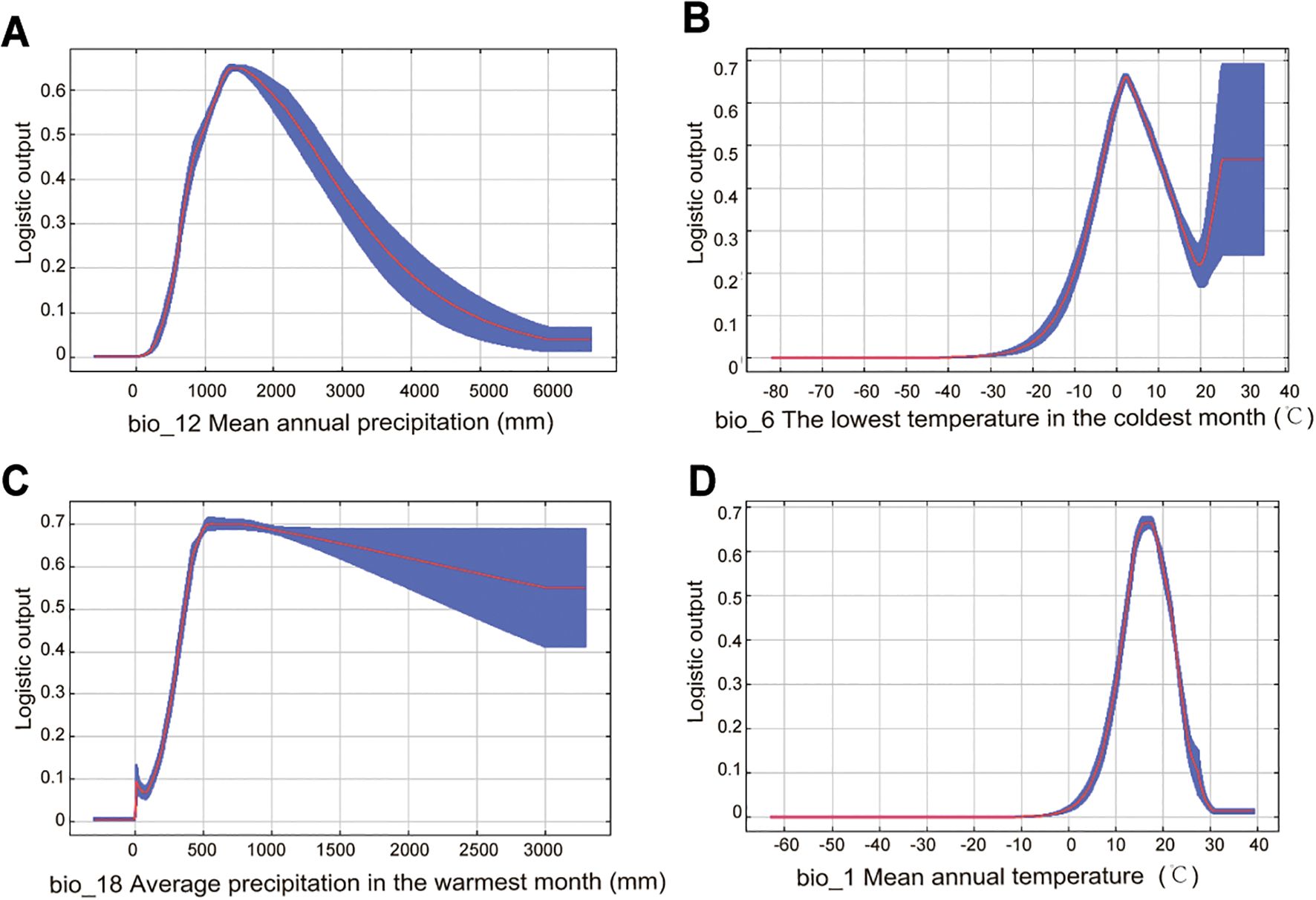
Figure 6. Response curves illustrating the distribution probability in relation to key environmental factors for integrated C. officinarum. Each panel depicts the predicted probability of existence (y-axis) across gradients of essential bioclimatic variables (x-axis), arranged in the following order: bio12 (annual precipitation, A), bio6 (minimum temperature of the coldest month, B), bio18 (precipitation during the warmest quarter, C), and bio1 (annual mean temperature, D).
Different from the environmental factors of integrative C. officinarum, the driest month precipitation (bio14) was the dominant factor (64.9–83% contribution), followed by elevation (ele, 5.2–12.3%) and annual temperature (bio1, 1.5–13.4%)(Figure 5). The linalool-type chemotype thrives, when the maximum driest month precipitation (bio14) exceeds 20 mm (Figure 7A) peaking at 180 mm probably stabilizes at 0.9. The linalool-type grows best at 0–400 meters altitude, peaking around 100 meters (Figure 7B) and declining sharply above 400 meters. The artemisia ketone-type grows well when bio 14 is 21–64 mm, peaking at 26 mm (Figure 7C). It grows well at altitudes of 0–400 meters, peaking around 100 meters and vanishing above 400 meters (Figure 7D). The eucalyptol-type requires bio14 above 20 mm, peaking at 180 mm (Figure 7E). The optimal altitude of the eucalyptol-type is 0–500 meters, peaking around 100 meters. (Figure 7F). The camphor-type grows well when bio14 reaches 450 mm, declining after 600 mm (Figure 7G). The optimal altitude is 0–400 meters, peaking around 100 meters and negligible above 400 meters (Figure 7H).
4 Discussion
Wild camphor trees exhibit significant biochemical diversity, serving as a vital genetic resource for cultivating high-quality essential oil germplasm of C. officinarum. However, global climate change threatens their natural habitats and distribution patterns, complicating conservation efforts. Using the MaxEnt model and climatic data, this study simulates optimal distribution areas, evaluates current and future spatial ranges, and identifies key climatic drivers. The findings offer critical insights for targeted cultivation strategies and evidence-based conservation of camphor trees and their four chemotypes.
4.1 Shifted distribution patterns of integrated C. officinarum from 2021 to 2080
Previous research indicates that the integrated camphor trees currently exhibit a broad range of suitable habitats in South China (Zhang et al., 2019; Meng et al., 2021; Li et al., 2023). This region is characterized by a subtropical monsoon climate, which features hot and humid summers alongside mild and drier winters (Qiu et al., 2011). The suitable habitat range for C. officinarum is predicted to expand based on 181 occurrences from the periods between 2025 and 2085 (Zhang et al., 2019). Another study, which analyzed 182 occurrences, predicted that the suitable regions for C. officinarum are expected to expand northward and to higher elevations by 2050 and 2070 (Meng et al., 2021). However, the current study, which included a larger sample size of 546 samples, has revealed that suitable areas for integrated C. officinarum are likely to experience significant contraction and fragmentation between 2021 and 2080 due to rising temperatures associated with global climate change (Figure 3). These findings underscore the need for adaptive conservation strategies of climate-resilient integrated camphor trees.
4.2 Climatic suitability for integrated C. officinarum
Elevated temperatures compromise the energy accumulation of camphor trees, leading to insufficient energy reserves for growth, development, and population proliferation in Lauraceae (Tan et al., 2023). Furthermore, increasing temperatures elevate levels of reactive oxygen species (ROS), causing oxidative damage that hinders plant growth (Petrov et al., 2015; Ma et al., 2019). In the present study, the MaxEnt model indicates that camphor trees experience optimal growth conditions at an annual average temperature range of 13-20°C, with a probability exceeding 50%. However, since the global temperature has risen by 1.5°C with different evidence over the past century (Hoegh-Guldberg et al., 2019), such climatic alterations will inevitably modify distribution patterns of camphor trees. We found these favorable conditions for integrated C. officinarum are to be diminish, disappearing entirely at annual average temperatures of 30°C (Figure 6). Except for temperature, precipitation also plays a crucial role in the distribution of integrated camphor trees. Annual rainfall between 1000–2500 mm fosters optimal growth conditions (with a probability exceeding 50%), but these conditions significantly decrease with higher rainfall. Excessive precipitation elevates soil moisture, disrupting the water balance, reducing soil oxygen levels, and impairing plant metabolism (Heikkinen et al., 2012). Environmental factors, particularly climatic conditions, are central to the historical distribution and future pattern changes of integrated C. officinarum.
4.3 Chemotype-specific habitat adaptations
The four chemotypes of C. officinarum (linalool, artemisia ketone, eucalyptol, and camphor) exert markedly distinct influences on their optimal habitats, thereby indicating a robust correlation between secondary metabolisms and geographical distribution. This result is congruent with that in a series of previous studies. For examples, an analysis of essential oils derived from 617 specimens of Melaleuca alternifolia revealed their classification into six chemotypes, each exhibiting distinct regional distribution patterns across various areas (Homer et al., 2000). Similarly, the essential oils from Leptospermum scoparium were categorized into three chemotypes, which also demonstrated clear regional distribution patterns in New Zealand (Perry et al., 1997). In the case of Scutellaria baicalensis (Chinese skullcap), a majority of chemical components exhibited negative correlations with latitude (Guo et al., 2013). Environmental factors can significantly impact the oil yield and essential oil profile of Cinnamomum camphora (also known as C. officinarum) (Zhang et al., 2023). Our results indicate that the distribution of the four chemotypes of C. officinarum and variations in the proportions of dominant chemical content in South China (Figures 1, 2), suggesting the presence of habitat adaptation in C. officinarum.
4.4 Precipitation and terpenoid synthesis
Our results of show that the driest month precipitation (bio14) as the primary climatic factors influences the distribution of the four chemotypes of camphor trees (Figure 5). This phenomenon may be correlated with pollination mechanisms: linalool serves as the primary source of aromatic compounds in plants, and its unique scent plays a pivotal role in plant-insect interactions (Reisenman et al., 2010). Elevated precipitation levels significantly diminish pollen viability, resulting in pollination failure (Mao and Huang, 2009). Research also substantiates that water stress influences the synthesis of linalool, as shortages in carbon substrates and ATP severely constrain monoterpene production (Bertin and Staudt, 1996), thereby indicating that drought conditions affect linalool synthesis. The artemisia ketone-type and camphor-type of C. officinarum probably exhibit a degree of drought tolerance. Previous studies show that drought stress triggers reactive free radical accumulation in plants, prompting the synthesis of antioxidant-rich compounds like flavonoids, terpenes, and phenols (Caser et al., 2019; Dehghanian et al., 2022). Under drought stress, the synthesis of indole alkaloids in Vinca rosea thioflavones increases (Liu et al., 2017). Of these, the artemisia ketone-type demonstrates the highest probability of occurrence when precipitation reaches 25 mm during the driest month, with this probability declining as precipitation increases (Figure 7). Therefore, precipitation might impact secondary metabolites in C. officinarum, and further determines their chemotypes.
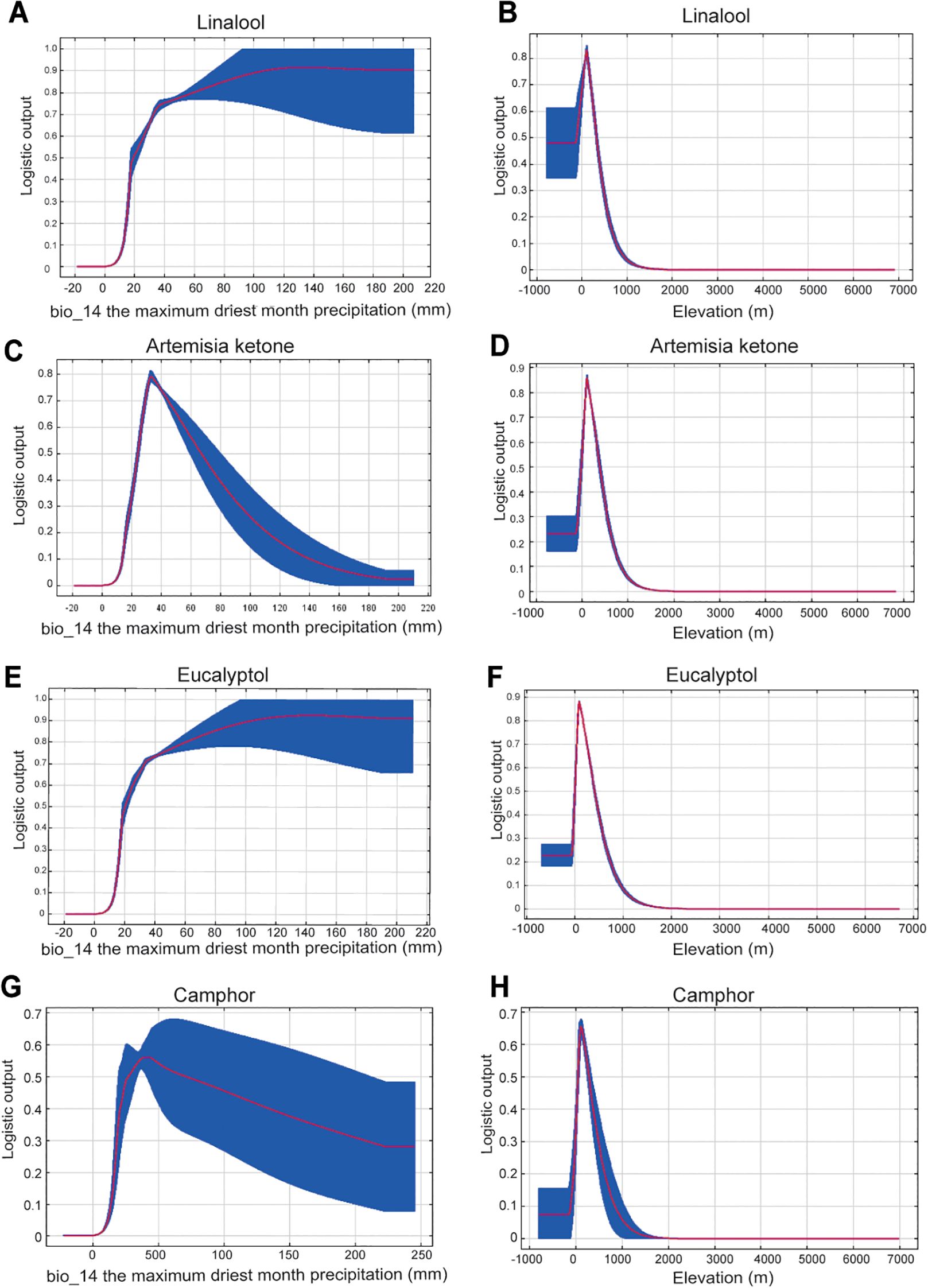
Figure 7. Response curves of distribution probability in relation to key environmental factors for the four chemotypes of C. officinarum. Each panel illustrates the predicted probability of existence (y-axis) across gradients of critical bioclimatic variables (x-axis), with the following order: (A, B) linalool chemotype with bio14 (precipitation of the driest month) and elevation (ele); (C, D) artemisia ketone-type with bio14 and ele; (E, F) eucalyptol-type with bio14 and ele; (G, H) camphor-type with bio14 and ele.
4.5 Temperature effects on terpenoid biosynthesis
Beyond precipitation factors, our results show that eucalyptol-type cultivation is better suited for warmer regions, while artemisia ketone-type and camphor-type show thermotolerance: Artemisia ketone-type thrives at mean annual temperatures above 25°C, and camphor-type favors environments with annual temperature variations exceeding 31°C (Figure 6). In leaves of C. camphora (also known as C. officinarum), a previous study has shown that terpinene and β-pinene have been verified to improve thermotolerance (Tian et al., 2020). In other anigosperms, the medicinal plant Mahonia bodinieri shows enhanced biosynthesis and alkaloid accumulation with increased light exposure (Kong et al., 2016). Studies on baicalin, a flavonoid secondary metabolite from Erigeron breviscapus, indicate that flavonoids offer photoprotective advantages, and intense sunlight exposure greatly boosts its concentration (Zhou et al., 2016). Camellia japonica exhibits upregulated expression of genes associated with unsaturated fatty acid and jasmonic acid biosynthesis under cold stress (Li et al., 2016). Studies on Dendrobium officinale indicate that both temperature and light conditions regulate the content of its secondary metabolites (Yuan et al., 2020). Higher temperatures enhance the volatilization of terpenoids in the leaves of Ocimum basilicum by decreasing the linalool content (Tursun and Telci, 2020). Therefore, beyond precipitation, temperature might also influence the biosynthetic processes of terpenoids in C. officinarum, and further determines their chemotypes.
4.6 Recommendations for resource conservation
Taking into account the results shown above, a comprehensive conservation strategy for C. officinarum is proposed: first, to protect historically highly suitable areas of C. officinarum such as Jiangxi, Guangdong and Guangxi to ensure their health and stability of natural population. Second, given the potential shifts of their highly suitable regions from 2021 to 2080, ecological corridors should be strategically planned such as Fujian and Hunan. It aims to address population isolation resulting from fragmented distribution and to promote genetic exchange. Third, conservation strategy should also consider different scenarios for each chemotypes of C. officinarum. For examples, the linalool-type prefers the suitable areas in Guangdong and Guangxi; while camphor-type prefers suitable regions such as Hubei and Hunan from 2041 to 2080s. Fourth, through international cooperation, we can collectively confront climate challenges and prevent extreme weather from further diminishing the suitable habitats for C. officinarum, not only within China but also for surrounding Asian countries.
5 Conclusions
The present study investigates the ecological niches of integrated C. officinarum and its four chemotypes based on 546 occurrences using MaXent model in South China. Our results indicate that the highly suitable habitat for integrated C. officinarum is expected to shrink between 2021 and 2080, resulting in habitat fragmentation. The climatic determinants include annual average precipitation (bio12), the minimum temperature of the coldest month (bio6), average precipitation in the warmest month (bio18), and the annual average temperature (bio1). The highly suitable regions for each chemotype of C. officinarum are expected to shift and differentiate from 2021 to 2080. The determining factors for these shifts are the maximum precipitation during the driest month (bio14) and elevation. International collaboration remains crucial for the sustainable utilization of camphor tree resources in the face of climate change from 2021 to 2080.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
CH: Supervision, Writing – original draft, Writing – review & editing, Resources, Formal analysis. YJ: Methodology, Software, Visualization, Writing – original draft, Resources. BH: Resources, Project administration, Writing – original draft. JC: Resources, Writing – original draft, Methodology. MW: Resources, Writing – original draft. YZ: Writing – original draft, Software. BL: Investigation, Conceptualization, Writing – original draft. HL: Software, Writing – original draft, Resources. YL: Writing – original draft, Resources, Methodology, Supervision, Validation. QZ: Funding acquisition, Investigation, Writing – original draft, Resources, Validation, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This investigation was supported by the Technology Program from Forestry Administration of Guangdong Province (2022KJCX006 to Qian Zhang) and Guangdong Provincial Key Laboratory of Silviculture, Protection and Utilization (SPU 2025–02 to Chen Hou).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1676177/full#supplementary-material
References
Abbass K., Qasim M. Z., Song H., Murshed M., Mahmood H., and Younis I. (2022). A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. pollut. Res. 29, 42539–42559. doi: 10.1007/s11356-022-19718-6
Bertin N. and Staudt M. (1996). Effect of water stress on monoterpene emissions from young potted holm oak (Quercus ilex L.) trees. Oecologia 107, 456–462. doi: 10.1007/BF00333935
Boisvert-Marsh L., Périé C., and de Blois S. (2014). Shifting with climate? Evidence for recent changes in tree species distribution at high latitudes. Ecosphere 5, 1–33. doi: 10.1890/ES14-00111.1
Boncan D. A. T., Tsang S. S., Li C., Lee I. H., Lam H., Chan T., et al. (2020). Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 21, 7382. doi: 10.3390/ijms21197382
Boria R. A., Olson L. E., Goodman S. M., and Anderson R. P. (2014). Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275, 73–77. doi: 10.1016/j.ecolmodel.2013.12.012
Caser M., Chitarra W., D’Angiolillo F., Perrone I., Demasi S., Lovisolo C., et al. (2019). Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 129, 85–96. doi: 10.1016/j.indcrop.2018.11.068
Cassini M. H. (2011). Ecological principles of species distribution models: the habitat matching rule. J. Biogeogr. 38, 2057–2065. doi: 10.1111/j.1365-2699.2011.02552.x
Dahunsi A. M., Oyikeke T. S., Abdulfatai M. A., and Afolabi L. A. (2023). Spatio-temporal assessment of the impacts of the trends in physical and biogeochemical parameters on the primary production of the Gulf of Guinea. Heliyon 9, e13047. doi: 10.1016/j.heliyon.2023.e13047
Dehghanian Z., Habibi K., Dehghanian M., Aliyar S., Lajayer B. A., Astatkie T., et al. (2022). Reinforcing the bulwark: unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 8, e09094. doi: 10.1016/j.heliyon.2022.e09094
Elith J., Graham C. H., Anderson R. P., Dudík M., Ferrier S., Guisan A., et al. (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29, 129–151. doi: 10.1111/J.2006.0906-7590.04596.X
Elith J., Phillips S. J., Hastie T., Dudík M., Chee Y. E., and Yates C. J. (2011). A statistical explanation of MaxEnt for ecologists. Diversity Distrib. 17, 43–57. doi: 10.1111/j.1472-4642.2010.00725.x
Fourcade Y., Engler J. O., Rödder D., and Secondi J. (2014). Mapping species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias. PloS One 9, e97122. doi: 10.1371/journal.pone.0097122
Freeman B. G., Lee-Yaw J. A., Sunday J. M., and Hargreaves A. L. (2018). Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Global Ecol. Biogeogr. 27, 1268–1276. doi: 10.1111/geb.12774
Gao X., Liu J., and Huang Z. (2022). The impact of climate change on the distribution of rare and endangered tree Firmiana kwangsiensis using the Maxent modeling. Ecol. Evol. Dev. 12, e9165. doi: 10.1002/ece3.9165
Guo L., Wang S., Zhang J., Yang G., Zhao M., Ma W., et al. (2013). Effects of ecological factors on secondary metabolites and inorganic elements of Scutellaria baicalensis and analysis of geoherblism. Sci. China Life Sci. 56, 1047–1056. doi: 10.1007/s11427-013-4562-5
Guo Y., Zhang S., Tang S., Pan J., Ren L., Tian X., et al. (2023). Analysis of the prediction of the suitable distribution of Polygonatum kingianum under different climatic conditions based on the MaxEnt model. Front. Earth Sci. 11, 1111878. doi: 10.3389/feart.2023.1111878
Heikkinen R. K., Marmion M., and Luoto M. (2012). Does the interpolation accuracy of species distribution models come at the expense of transferability? Ecography 35, 276–288. doi: 10.1111/j.1600-0587.2011.06999.x
Hoegh-Guldberg O., Jacob D., Taylor M., Guillén Bolaños T., Bindi M., Brown S., et al. (2019). The human imperative of stabilizing global climate change at 1.5 C. Science 365, eaaw6974. doi: 10.1126/science.aaw6974
Homer L. E., Leach D. N., Lea D., Lee L. S., Henry R. J., and Baverstock P. R. (2000). Natural variation in the essential oil content of Melaleuca alternifolia Cheel (Myrtaceae). Biochem. Syst. Ecol. Evol. 28, 367–382. doi: 10.1016/S0305-1978(99)00071-X
Hosseini N., Ghorbanpour M., and Mostafavi H. (2024). Habitat potential modelling and the effect of climate change on the current and future distribution of three Thymus species in Iran using MaxEnt. Sci. Rep. 14, 3641. doi: 10.1038/s41598-024-53405-5
Hou C., Cai Y., Yao J., Xie P., He B., Lian H., et al. (2025). Decoding the chemodiversity blueprint: chromosome-scale genome assembly unveils photosynthesis-terpenoid coordination in Cinnamomum burmanni through genomic and miRNA regulatory networks. Plant Sci. 360, 112733. doi: 10.1016/j.plantsci.2025.112733
Jarvie S. and Svenning J. C. (2018). Using species distribution modelling to determine opportunities for trophic rewilding under future scenarios of climate change. Philos. Transct. R. Soc. B 373, 20170446. doi: 10.1098/rstb.2017.0446
Jiang R., Chen X., Liao X., Peng D., Han X., Zhu C., et al. (2022). A chromosome-level genome of the camphor tree and the underlying genetic and climatic factors for its top-geoherbalism. Front. Plant Sci. 13, 827890. doi: 10.3389/fpls.2022.827890
Jiang X., Wu Y., Xiao F., Xiong Z., and Xu H. (2014). Transcriptome analysis for leaves of five chemical types in Cinnamomum camphora. Hereditas 36, 58–68.
Jin W., Yang Z., Xu K., Liu Q., Luo Q., Li L., et al. (2025). A Comprehensive review of plant volatile terpenoids, elucidating interactions with surroundings, systematic synthesis, regulation, and targeted engineering production. Biology 14, 466. doi: 10.3390/biology14050466
Kelly A. E. and Goulden M. L. (2008). Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. 105, 11823–11826. doi: 10.1073/pnas.0802891105
Kong D.-X., Li Y.-Q., Wang M.-L., Bai M., Zou R., Tang H., et al. (2016). Effects of light intensity on leaf photosynthetic characteristics, chloroplast structure, and alkaloid content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiol. Plant. 38, 120. doi: 10.1007/s11738-016-2147-1
Kong F., Tang L., He H., Yang F., Tao J., and Wang W. (2021). Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. pollut. Res. 28, 34655–34663. doi: 10.1007/s11356-021-13121-3
Lee S.-H., Kim D.-S., Park S.-H., and Park H. (2022). Phytochemistry and applications of Cinnamomum camphora essential oils. Molecules 27, 2695. doi: 10.3390/molecules27092695
Li Q., Ji N., Wang Z.-g., Lu C.-x., and Lin H. (2021). Four solvent extraction of Cinnamomum camphora xylem and analysis of the anti-fungal activity of the extractives. Wood Res. 66, 666–677. doi: 10.37763/wr.1336-4561/66.4.666677
Li Q., Lei S., Du K., Li L., Pang X., Wang Z., et al. (2016). RNA-seq based transcriptomic analysis uncovers α-linolenic acid and jasmonic acid biosynthesis pathways respond to cold acclimation in Camellia japonica. Sci. Rep. 6, 36463. doi: 10.1038/srep36463
Li D., Lin H.-Y., Wang X., Bi B., Gao Y., Shao L., et al. (2023). Genome and whole-genome resequencing of Cinnamomum camphora elucidate its dominance in subtropical urban landscapes. BMC Biol. 21, 192. doi: 10.1186/s12915-023-01692-1
Liu Y. and Chen L. (2024). Predicting the impact of climate change on Corylus pecies distribution in China: Integrating climatic, topographic, and anthropogenic factors. Ecol. Evol. 14, e70528. doi: 10.1002/ece3.70528
Liu Y., Meng Q., Duan X., Zhang Z., and Li D. (2017). Effects of PEG-induced drought stress on regulation of indole alkaloid biosynthesis in Catharanthus roseus. J. Plant Interact. 12, 87–91. doi: 10.1080/17429145.2017.1293852
Lobo J. M., Jiménez-Valverde A., and Real R. (2008). AUC: a misleading measure of the performance of predictive distribution models. Global Ecol. Biogeogr. 17, 145–151. doi: 10.1111/j.1466-8238.2007.00358.x
Low B. W., Zeng Y., Tan H. H., and Yeo D. C. (2021). Predictor complexity and feature selection affect Maxent model transferability: Evidence from global freshwater invasive species. Diversity Distrib. 27, 497–511. doi: 10.1111/ddi.13211
Ma Y., Wang B., Zhang R., Gao Y., Zhang X., Li Y., et al. (2019). Initial simulated acid rain impacts reactive oxygen species metabolism and photosynthetic abilities in Cinnamonum camphora undergoing high temperature. Ind. Crops Prod. 135, 352–361. doi: 10.1016/j.indcrop.2019.04.050
Mahajan M., Kuiry R., and Pal P. K. (2020). Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromatic. Plants 18, 100255. doi: 10.1016/j.jarmap.2020.100255
Mao Y. Y. and Huang S. Q. (2009). Pollen resistance to water in 80 angiosperm species: flower structures protect rain-susceptible pollen. New Phytol. 183, 892–899. doi: 10.1111/j.1469-8137.2009.02925.x
McInerny G. J. and Etienne R. S. (2012). Ditch the niche–is the niche a useful concept in ecology or species distribution modelling? J. Biogeogr. 39, 2096–2102. doi: 10.1111/jbi.12033
Meng J., Li M., Guo J., Zhao D., and Tao J. (2021). Predicting suitable environments and potential occurrences for Cinnamomum camphora (Linn.) Presl. Forests 12, 1126. doi: 10.3390/f12081126
Muluneh M. G. J. A. and Security F. (2021). Impact of climate change on biodiversity and food security: a global perspective—a review article. Agric. Food Secur. 10, 1–25. doi: 10.1186/s40066-021-00318-5
Perry N. B., Brennan N. J., Van Klink J. W., Harris W., Douglas M. H., McGimpsey J. A., et al. (1997). Essential oils from New Zealand manuka and kanuka: chemotaxonomy of Leptospermum. Phytochemistry 44, 1485–1494. doi: 10.1016/S0031-9422(96)00743-1
Petrov V., Hille J., Mueller-Roeber B., and Gechev T. S. (2015). ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 6, 69. doi: 10.3389/fpls.2015.00069
Phillips S. J., Anderson R. P., Dudík M., Schapire R. E., and Blair M. E. (2017). Opening the black box: An open-source release of Maxent. Ecography 40, 887–893. doi: 10.1111/ecog.03049
Phillips S. J., Anderson R. P., and Schapire R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259. doi: 10.1016/j.ecolmodel.2005.03.026
Picazo F., Vilmi A., Aalto J., Soininen J., Casamayor E. O., Liu Y., et al. (2020). Climate mediates continental scale patterns of stream microbial functional diversity. Microbiome 8, 92. doi: 10.1186/s40168-020-00873-2
Qiu Y.-X., Fu C.-X., and Comes H. P. (2011). Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol. Phylogenet. Evol. 59, 225–244. doi: 10.1016/j.ympev.2011.01.012
Reisenman C. E., Riffell J. A., Bernays E. A., and Hildebrand J. G. (2010). Antagonistic effects of floral scent in an insect–plant interaction. Proc. R. Soc. B: Biol. Sci. 277, 2371–2379. doi: 10.1098/rspb.2010.0163
Stubbs B. J., Specht A., and Brushett D. (2004). The essential oil of Cinnamomum camphora (L.) Nees and Eberm.—variation in oil composition throughout the tree in two chemotypes from eastern Australia. J. Essential. Oil Res. 16, 9–14. doi: 10.1080/10412905.2004.9698636
Sun S. and Deng Z. (2025). Analysis of a potentially suitable habitat for Solanum aculeatissimum in Southwest China under climate change scenarios. Plants 14, 1979. doi: 10.3390/plants14131979
Syfert M. M., Smith M. J., and Coomes D. A. (2013). The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PloS One 8, e55158. doi: 10.1371/annotation/35be5dff-7709-4029-8cfa-f1357e5001f5
Tan C., Ferguson D. K., Tang Z., and Yang Y. (2023). Distribution and conservation of the lauraceae in China. Global Ecol. Conserv. Genet. 46, e02566. doi: 10.1016/j.gecco.2023.e02566
Tian Z., Luo Q., Li Y., Zuo Z. J. I. C., and Products (2020). Terpinene and β-pinene acting as signaling molecules to improve Cinnamomum camphora thermotolerance. Ind. Crops Prod. 154, 112641. doi: 10.1016/j.indcrop.2020.112641
Townsend P. A., Papeş M., and Eaton M. (2007). Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography 30, 550–560. doi: 10.1111/j.0906-7590.2007.05102.x
Tursun A. O. and Telci I. (2020). The effects of carbon dioxide and temperature on essential oil composition of purple basil (Ocimum basilicum L.). J. Essential. Oil Bearing. Plants 23, 255–265. doi: 10.1080/0972060X.2020.1741452
Verma N. and Shukla S. (2015). Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromatic. Plants 2, 105–113. doi: 10.1016/j.jarmap.2015.09.002
Warren D. L., Matzke N. J., Cardillo M., Baumgartner J. B., Beaumont L. J., Turelli M., et al. (2021). ENMTools 1.0: an R package for comparative ecological biogeography. Ecography 44, 504–511. doi: 10.1111/ecog.05485
Weiskopf S. R., Isbell F., Arce-Plata M. I., Di Marco M., Harfoot M., Johnson J., et al. (2024). Biodiversity loss reduces global terrestrial carbon storage. Nat. Commun. 15, 4354. doi: 10.1038/s41467-024-47872-7
Wu C.-F. J. (1986). Jackknife, bootstrap and other resampling methods in regression analysis. Ann. Stat 14, 1261–1295. doi: 10.1214/aos/1176350142
Yang Z., Liu B., Yang Y., and Ferguson D. K. (2022). Phylogeny and taxonomy of Cinnamomum (Lauraceae). Ecol. Evol. 12, e9378. doi: 10.1002/ece3.9378
Yuan X., Li S., Chen J., Yu H., Yang T., Wang C., et al. (2024). Impacts of global climate change on agricultural production: a comprehensive review. Agronomy 14, 1360. doi: 10.3390/agronomy14071360
Yuan Y., Tang X., Jia Z., Li C., Ma J., and Zhang J. (2020). The effects of ecological factors on the main medicinal components of Dendrobium officinale under different cultivation modes. Forests 11, 94. doi: 10.3390/f11010094
Zhang L., Jing Z., Li Z., Liu Y., and Fang S. (2019). Predictive modeling of suitable habitats for Cinnamomum Camphora (L.) presl using maxent model under climate change in China. Int. J. Environ. Res. Public Health 16, 3185. doi: 10.3390/ijerph16173185
Zhang Y., Tang J., Ren G., Zhao K., and Wang X. (2021). Global potential distribution prediction of Xanthium italicum based on Maxent model. Sci. Rep. 11, 16545. doi: 10.1038/s41598-021-96041-z
Zhang L., Yang C., Wang P., Xie G., and Wang W. (2025). Assessing the potential global distribution of Monochamus sutor (Coleoptera: Cerambycidae) under the influence of climate change and human activities based on Maximum Entropy model. J. Econ. Entomol. 118, 1174–1187. doi: 10.1093/jee/toaf093
Zhang T., Zheng Y., Fu C., Yang H., Liu X., Qiu F., et al. (2023). Chemical variation and environmental influence on essential oil of Cinnamomum camphora. Molecules 28, 973. doi: 10.3390/molecules28030973
Zhao G., Cui X., Sun J., Li T., Wang Q., Ye X., et al. (2021). Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 132, 108256. doi: 10.1016/j.ecolind.2021.108256
Zhao Y., Liu J., Zhang Z., Zhao Y., Cui D., Zhou Y., et al. (2025). Northward expanding variation of neo-Chinese-style landscape influenced by bamboos in China under climate change based on MaxEnt Model. Forests 16, 428. doi: 10.3390/f16030428
Zhao Z., Xiao N., Shen M., and Li J. (2022). Comparison between optimized MaxEnt and random forest modeling in predicting potential distribution: A case study with Quasipaa boulengeri in China. Sci. Total. Environ. 842, 156867. doi: 10.1016/j.scitotenv.2022.156867
Zheng H., Mao X., Lin Y., Fu K., Qi Z., and Wu Y. (2024). Reconstructing the biological invasion of noxious invasive weed Parthenium hysterophorus and invasion risk assessment in China. Front. Plant Sci. 15, 1430576. doi: 10.3389/fpls.2024.1430576
Zhou R., Su W., Zhang G., Zhang Y., and Guo X. (2016). Relationship between flavonoids and photoprotection in shade-developed Erigeron breviscapus transferred to sunlight. Photosynthetica 54, 201–209. doi: 10.1007/s11099-016-0074-4
Keywords: biogeography, essential oil, species distribution model, adaptive area change, resource-oriented conservation
Citation: Hou C, Jiang Y, He B, Chen J, Wang M, Zhong Y, Li B, Lian H, Li Y and Zhang Q (2025) Climate-driven redistribution of essential oil chemotypes in Camphora officinarum: MaxEnt-based habitat projections and conservation priorities for Southern China (2021–2080). Front. Ecol. Evol. 13:1676177. doi: 10.3389/fevo.2025.1676177
Received: 31 July 2025; Accepted: 10 October 2025;
Published: 31 October 2025.
Edited by:
Adam Kleczkowski, University of Strathclyde, United KingdomReviewed by:
Tinyiko Cavin Shivambu, University of South Africa, South AfricaLeroy Soria-Díaz, Universidad Autónoma de Tamaulipas, Mexico
Copyright © 2025 Hou, Jiang, He, Chen, Wang, Zhong, Li, Lian, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongquan Li, eW9uZ3F1YW5saUB6aGt1LmVkdS5jbg==; Qian Zhang, emhhbmdxNzYxMEBzaW5vZ2FmLmNu
†These authors have contributed equally to this work
 Chen Hou
Chen Hou Yingchao Jiang2†
Yingchao Jiang2† Yongquan Li
Yongquan Li Qian Zhang
Qian Zhang