- 1Department of Neuroscience, Brown University, Providence, RI, United States
- 2Carney Institute for Brain Science, Brown University, Providence, RI, United States
- 3Department of Cognitive and Psychological Sciences, Brown University, Providence, RI, United States
Big brown bats (Eptesicus fuscus) have a diverse vocal repertoire. We tested the hypothesis that frequency-modulated bouts (FMBs) are male-specific calls produced during food competition. Seven pairs of bats (male-male, male-female, female-female) competed in the laboratory to capture a food item. Within this restricted behavioral context, we identified six common social call types, broadly classified as aggressive in previous literature. Female-female pairs produced significantly fewer calls than the pairs containing males, and their social calls were longer in duration. FMB calls were absent in all female-female pairs but were present, in varying numbers, in pairs containing males. FMBs recorded in the laboratory resembled those recorded from big brown bats foraging in the wild. Results support the hypothesis that FMBs are produced in foraging interactions in both the laboratory and the field, and confirm previous reports of less robust vocal interactions between female bats.
1 Introduction
Many bat species (Chiroptera) are highly social, living in communal roosts and gathering in groups at foraging sites. Vocalizations are important in mediating these social behaviors (Gillam and Fenton, 2016; Chaverri et al., 2018). A variety of social calls have been identified in several species, including courtship calls, pup isolation calls, contact calls, aggressive calls, food defense calls, and distress calls (Pfalzer and Kusch, 2003; Gillam and Fenton, 2016). Some of these vocalizations are so acoustically complex that they have been described as songs (Saccopteryx bilineata, Behr and von Helversen, 2004; Tadarida brasiliensis; Bohn et al., 2009). Although echolocation calls operate for guiding orientation and for prey detection, this acoustic sensing system also plays a role in social interactions. Bats listen in on the echolocation pulses of other bats (Griffin, 1958; Balcombe and Fenton, 1988; Dechmann et al., 2009; Übernickel et al., 2013) to find appropriate foraging areas or roosting sites. Echolocation calls also provide information about the sex, age, and identity of the sender—cues that are important in social interactions (Masters et al., 1995; Voigt-Heucke et al., 2010; Jones and Siemers, 2011; Knörnschild et al., 2012; Yovel et al., 2009).
Big brown bats (Eptesicus fuscus; Kurta and Baker, 1990) roost in colonies that can include dozens to hundreds of individuals (Hayes and Wiles, 2013). They hunt for prey alone and in groups (Simmons et al., 2001) by broadcasting short, frequency-modulated (FM) echolocation pulses made up of two harmonics (the first harmonic sweeping downward from 50 kHz to 22 kHz; the second harmonic sweeping downward from 90 kHz to 45 kHz) and listening for returning echoes (Griffin, 1958). Big brown bats actively adjust the amplitude, duration, lowest frequency, and rate of emission of these pulses during insect hunting or when facing a challenging perceptual task (Simmons et al., 1979; Surlykke and Moss, 2000; Hiryu et al., 2010; Kothari et al., 2014; Wheeler et al., 2016; Fry et al., 2024). They also produce a variety of non-echolocation social calls in different behavioral contexts, including flying, foraging, and roosting (Gadziola et al., 2012; Wright et al., 2013, 2014; Springall et al., 2019; Montoya et al., 2022; Salles et al., 2024). Acoustic properties and functional significance of this diverse social call repertoire are understudied compared to those of their echolocation.
A major challenge in identifying and comparing social calls within this species is linking the acoustic features of these vocalizations to the behavioral contexts in which they are produced. Gadziola et al. (2012) analyzed social calls emitted by captive roosting bats vocalizing spontaneously while undisturbed by an experimenter, tested in an intruder paradigm, or probed with a tactile stimulus. The researchers identified 18 different “syllable” types, varying in acoustic complexity and broadly categorized as aggressive or appeasement. None of these syllable types was labeled as sex-specific. Wright et al. (2013, 2014) recorded social calls from pairs of flying bats competing in the laboratory to acquire a food item. Of a total of seven call types, they identified a particular call, called a frequency-modulated bout (FMB), produced exclusively by male bats as a food defense call. Springall et al. (2019) identified seven social calls emitted by big brown bats flying in their natural habitat, some of which appeared acoustically similar to calls identified in laboratory studies. Based on their review of the literature, Montoya et al. (2022) concluded that there was only partial overlap in social calls produced by roosting and flying bats. Salles et al. (2024) identified 12 different call types associated with locomotion, feeding, aggression, grooming, and mating, emitted by pairs of bats freely interacting in a small enclosure. In contrast to the results of Wright et al. (2013, 2014), these researchers observed that both male and female bats produced FMB calls in this setting. Differences in the results of these studies could stem from variations in experimental design and behavioral context, or individual differences between the samples of bats tested.
In this experiment, we examine the hypothesized sex-specificity of FMB calls by testing interactions of pairs of bats in a competitive feeding paradigm in a controlled laboratory environment. Pairs of bats of either sex competed to capture a tethered food item while their behavioral interactions and vocalizations were recorded. We hypothesized that FMBs would be produced only by pairs of bats containing males. Bats were also tested individually to confirm that FMBs are not triggered solely by the presence of food. We analyzed a set of field recordings from wild, free-flying big brown bats to assess whether these calls are produced in natural foraging interactions or are an artifact of laboratory testing. Finally, we compared the acoustic structure of other social calls emitted in the competitive feeding scenario to those observed in other behavioral contexts to evaluate the diversity of the big brown bat’s vocal repertoire.
2 Methods
2.1 Animals
Six wild-caught adult big brown bats (two males, Jorge and Moose; four females, Bebe, Eva, Exie, and Freyja) participated in the experiment. Because the bats were wild-caught, their ages are unknown; the two males were captured in 2019 and 2022, three females were captured in 2021 and 2022, and the remaining female was captured in 2015. Three other bats (one male, two females) began individual training, but would not participate in paired testing. Numbers of bats available for this experiment were limited due to strict regulations on numbers of wild captures imposed by the state of Rhode Island scientific collector permits. Upon entering laboratory care, the bats were vaccinated against rabies and implanted with subcutaneous microchips (Trovan ID-100A RFID transponder, Trovan LID-573 microchip reader) for individual identification. They were housed in groups of 2–3 in a temperature- and humidity-controlled Biohazard Level 2 colony room (22-24°C and 40-60% relative humidity) on a reverse light cycle (12 hours dark, 12 hours light). All bats had free access to water and were fed rationed amounts of vitamin-enriched live mealworms (Tenebrio larvae), once daily in their home enclosures, during experiments as a food reward, or after experiments if they declined to participate in testing. All laboratory personnel were vaccinated against rabies, with antibody levels tested every other year, and wore full personal protective equipment when handling bats (Fenton et al., 2024).
2.2 Apparatus and equipment
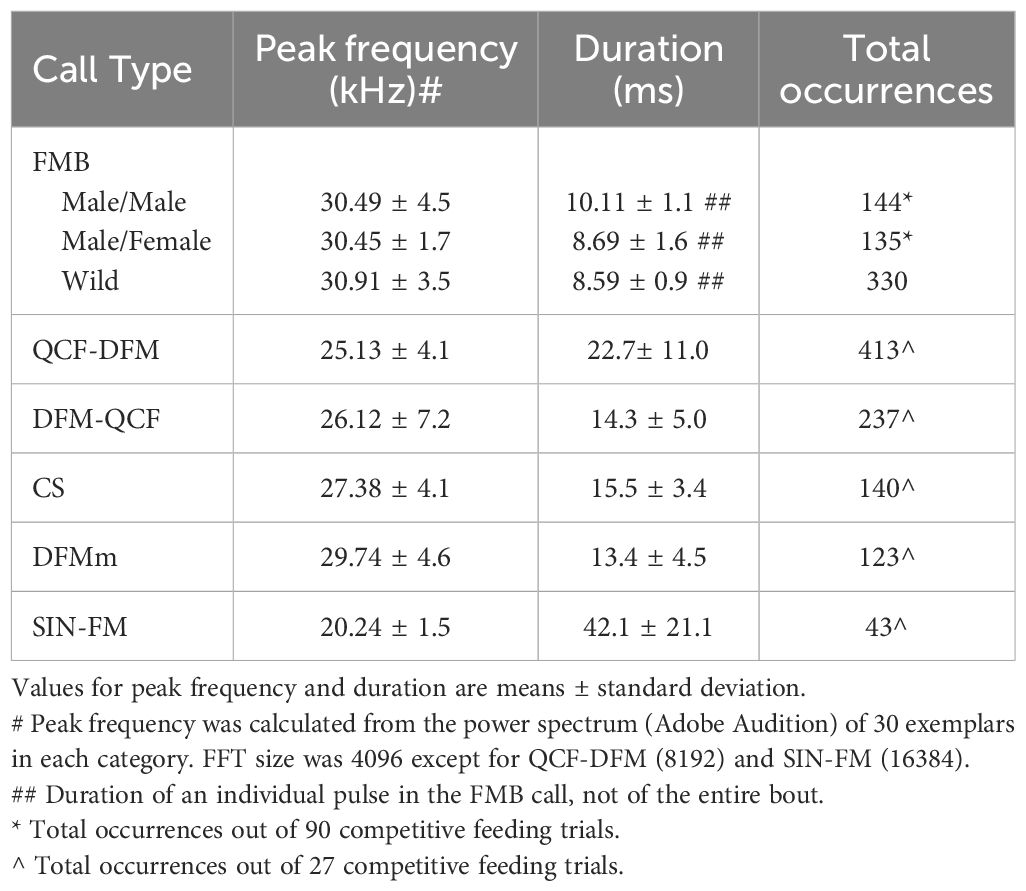
Training and experimental trials took place in a Biohazard Level 2 flight room (6.28 m x 4.23 m x 2.72 m, Figure 1), which was carpeted and lined with acoustic foam to reduce reverberations. Bats were tested on a custom-built padded steel platform (22 cm x 25.5 cm) fixed to a tripod positioned 108 cm above the floor. Mealworm prey were tethered to a string attached to the ceiling 142 cm from the center of the platform.
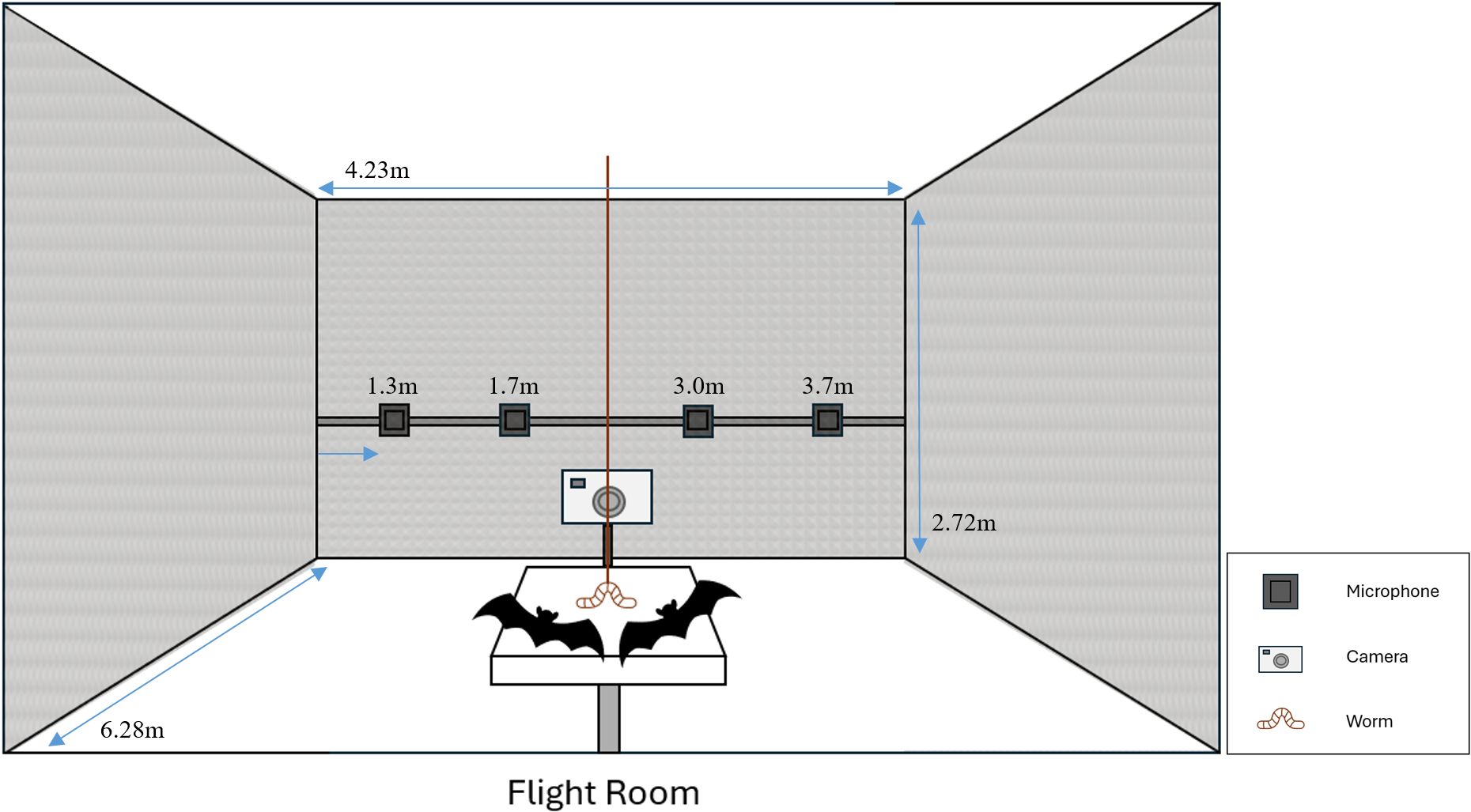
Figure 1. Diagram of the flight room (blue double-sided arrows: 6.28 m X 4.23 m X 2.72 m) and experimental platform (not to scale). Four MEMS microphones to record bat vocalizations were mounted on the back wall, 4.9 m away from the platform’s position, at the designated horizontal positions (1.3, 1.7, 3.0, and 3.9 m from the left wall, indicated by the blue right-facing arrow). The video camera recorded bat interactions. The bats competed to capture the dangling mealworm.
We collected three types of data: observations, video recordings, and audio recordings. Observations involved noting in real time how the bats interacted during trials and which individual bat captured the mealworm. Video recordings of bat interactions were taken with an Aurora Sport Night Vision camera (SIONYX, Beverly MA, USA) mounted on a tripod positioned level with and 1 meter away from the platform. Acoustic recordings of bat vocalizations were captured by four MEMS microphones (Dodotronic Momimic; Castel Gandolfo, IT) attached to the back wall of the flight room. Microphone outputs were connected to an eight-channel ZOOM recorder (ZOOM F8, 192 kHz sampling rate). These recordings were saved as.wav files and processed using Raven Pro (Cornell Lab of Ornithology) and Adobe Audition 2024 software.
2.3 Procedure
Bats were initially trained one at a time to catch a mealworm suspended by a string above the middle of the platform (Figure 1). During these training sessions, an individual bat was released by an experimenter from the front center of the platform and allotted 20 sec to capture the mealworm. The mealworm was replaced after each trial. Bats were not fed before training, so presumably they were motivated to capture the food. Individual bats were tested for 10 trials per day over a total of 14 days. All bats received their full daily mealworm allotment either during or after training. By the end of the training period, all six bats had successfully captured the mealworm, with an 80% success rate. The other three bats typically flew off the platform rather than attempt to capture the mealworm, and were excluded from paired testing.
We then devised a competitive foraging scenario where two bats competed simultaneously for the suspended mealworm. Bats were tested in pairs (one male-male pair, three male-female pairs, three female-female pairs; Table 1) with the constraint that no individual bat was tested more than once in any pair group on any testing day. In each trial, the two bats were released simultaneously by two experimenters from opposite front corners of the platform. The bats were not restrained in any way and were free to approach and interact with each other, fly off the platform, or capture the mealworm. Each bat pair was tested for 10 trials per testing day, with their release points switching halfway (five on the left and five on the right) to control for any side bias. If neither bat moved from the starting position within 120 sec, the trial was stopped and the data discarded. All bats received their full daily mealworm allotment either during or after training. Up to four experimenters participated: two to simultaneously release the bats and monitor their behavior; one to control the video and audio recordings; and one to handle and replace the mealworm after each trial.

Table 1. Numbers of echolocation calls, non-FMB social calls, and FMB calls produced by each bat pair in a sample of 90 competitive feeding trials.
2.4 Acoustic analyses
We identified all social and FMB calls occurring in the entire database (total of 270 trials: 30 male-male, 120 male-female, 120 female-female). Because only two males were willing to participate, only 30 trials (3 days of testing, 10 trials each day) were available for this pair group. To statistically compare data from all three pair groups, we selected a sample of 30 trials from the male-female pair group (10 trials from each pair) and a sample of 30 trials from the female-female pair group (10 trials from each pair), for a total subsample of 90 trials (Table 1). Within the male-female and female-female pair groups, we selected testing days with the highest level of calling activity and the highest rates of successful mealworm capture, and then randomly selected individual trials from these days. We counted numbers of echolocation calls, social calls, and FMB calls in these 90 trials.
Sound files were processed manually in Raven Pro (v. 1.6; Cornell Laboratory of Ornithology) using selection tables and then classified into echolocation pulses and social calls. Echolocation pulses were identified based on established criteria (two-harmonic FM downsweeps, durations < 5 ms) for big brown bats (Simmons et al., 1979; Surlykke and Moss, 2000; Gadziola et al., 2012; Wright et al., 2013). Calls that did not meet the criteria for echolocation calls were categorized as social calls. Within the category of social calls, we identified and analyzed all FMB calls using the criteria outlined by Wright et al. (2013, 2014). These manual classifications were performed independently by two of the authors and cross-validated (reliability coefficient for echolocation and FMB calls > 0.99).
To quantify the acoustic features of non-FMB social calls, we chose a set of 27 trials, with three trials chosen from each bat pair listed in Table 1. Within each bat pair, we selected specific audio files randomly, with the constraint that social (non-FMB) calls were present in that file. All social calls identified within these 27 trials were re-analyzed in Adobe Audition (2025) by computing spectrograms and power spectra, and measuring call duration and peak frequency. Classifications of social calls into specific types were performed independently by two authors (initial reliability coefficient, 0.49). Due to this low reliability, classifications were cross-validated through discussions and group analyses by three of the authors. Our goal was to compare calls with those described in previous laboratory studies (Gadziola et al., 2012; Wright et al., 2013, 2014; Salles et al., 2024).
Statistical analyses were performed in R Studio (RStudio Team, 2020) and SPSS v. 25 (IBM, Armonk, NY). Illustrations were made in CorelDRAW (Alludo, Ottawa ON) or SigmaPlot v. 16 (Grafiti, Palo Alto CA).
2.5 Nomenclature
We aimed to compare social calls in our experimental context to those recorded by other investigators in other laboratory studies (Gadziola et al., 2012; Wright et al., 2013, 2014; Salles et al., 2024). However, the methods and criteria used to categorize and label big brown bat social calls differ between published studies, and example illustrations of spectrogram shapes in those studies often do not display the extent of any inter-individual or contextual variability that may occur. We focused on identifying calls that seemed most consistent with these previously established classifications based on published spectrograms and tables of acoustic parameters. We adopted a strategy of “lumping” rather than “splitting” call types because of the considerable individual variability in our sample. Call types that were uncommon in our sample, even if mentioned in previous work, were not analyzed.
2.6 Field recordings
To explore whether FMB calls were part of the repertoire of wild bats or were an artifact of captivity, we recorded vocalizations of big brown bats foraging at a known, established site at a park in Providence, RI. Recordings were made over a period of 42 min on March 29, 2023, using an Echo Meter Touch 2 Pro for iOS (Wildlife Acoustics, Inc.) at a sampling rate of 384 kHz. Bats were not captured due to regulatory restrictions, and so the bat’s sex could not be determined. Audio files were analyzed using Adobe Audition. Species identification was confirmed using Kaleidoscope Pro software (Wildlife Acoustics).
3 Results
Bats recorded during individual training sessions remained still on the platform, crawled towards the tethered mealworm to capture and then eat it, or flew off the platform. We did not observe any grooming behaviors. Behaviors observed in real-time by the paired bats in the competitive feeding context, later validated by viewing saved videos, included crawling on the platform towards either the other bat or the mealworm, remaining still, and self-grooming. Occasionally, one or both bats flew off the platform; these trials were not used for acoustic analysis if these flights occurred early in the trial, before either of the two bats approached each other or the mealworm. Overt aggressive interactions (i.e., using wings to push the other bat) were rare; however, bats often approached each other, seemingly directing more attention to their conspecific than to the tethered food item. We did not observe any social grooming or mating behaviors.
Individual bats emitted single echolocation pulses at relatively fixed intervals. It was only when the bat began to approach the tethered mealworm, in preparation for capture, that echolocation pulses were produced at a faster rate. Paired bats tended to produce echolocation pulses as single or as double pulses, with the successful bat’s emission rates increasing as it approached the tethered prey.
In the total sample of 270 competitive feeding trials, male-female pairs participated in 120 trials and female-female pairs participated in 120 trials. Within these 120 trials, male-female pairs produced a total of 2,378 non-FMB (range 8-798) social calls and 346 FMBs (range 0-173). In the same number of trials, female-female pairs produced a total of 490 non-FMB (range 5-124) social calls and 0 FMBs. Differences in call number by pair group were analyzed using linear mixed models with bat as a random factor. Results (Supplementary Table 1A) showed a statistically significant effect of pair group on the number of social (P = 0.011) and FMB (P = 0.019) calls.
To compare social call production between the three pair groups, we analyzed the sample of 90 competitive feeding trials with equal numbers of trials for each group (Table 1). Bat pairs in these groups produced echolocation pulses (n = 19,679) and social calls (n = 2,799, sum of non-FMB social calls and FMBs). Male-male pairs produced more non-FMB social calls (n = 1,230) than male-female (n = 1,128) or female-female (n = 162) pairs; male-male pairs also produced more FMB calls (n = 144) than male-female pairs (n = 135) and female-female pairs (n = 0). Differences in social and FMB call production were analyzed using linear mixed models with bat as a random factor. Results (Supplementary Table 1B) showed that male–male and male–female pairs produced significantly more social (P = 0.0002, P = 0.009) and FMB (P = 0.021; P = 0.016) calls than female–female pairs. Examination of data from the three pairs within each bat group revealed considerable variability; for example, one male-female pair and one female-female pair were responsible for most of the social call emission within their respective pair group.
3.1 Social call classification
In the subsample of 27 trials, we analyzed all social calls that occurred commonly (Table 2); calls that appeared infrequently (< 5% of all social calls) were noted but were not analyzed fully. Although we observed mealworm capture and eating, we omitted from analysis any feeding buzzes, which are typically produced in the final stages of prey capture (Simmons et al., 1979; Surlykke and Moss, 2000). Common calls were categorized into groups based on acoustic properties described in at least one previous analysis of laboratory social behavior in big brown bats. These six call types (spectrograms in Figure 2) are (1) FMB (frequency modulated bouts, individual downward FM pulses clustered in groups of 3-5; Wright et al., 2013, 2014; Salles et al., 2024); (2) DFM-QCF (downward FM sweep transitioning to a low quasi-constant frequency portion of varying duration; Gadziola et al., 2012); (3) QCF-DFM (a low quasi-constant frequency portion transitioning into a downward FM sweep of varying duration; Gadziola et al., 2012); (4) CS (chevron-shaped; short duration upward FM followed by downward FM; Wright et al., 2013; Salles et al., 2024); (5) DFMm (downward FM of longer duration than echolocation pulses with no or minimal quasi-constant frequency portion); and (6) SIN-FM (long duration upward and downward FM oscillations; Gadziola et al., 2012). Five of these call types were categorized as aggressive in at least one previous study. The DFMm call was observed by Gadziola et al. (2012) in both aggressive and appeasement contexts. We observed very few calls similar to those classified as appeasement calls (Gadziola et al., 2012; Salles et al., 2024).

Figure 2. Spectrograms of six types of social calls most commonly produced in the laboratory competitive feeding context, calculated using Adobe Audition (sampling rate 192 kHz, 24-bit) and compiled in CorelDRAW. (A) DFM-QCF call of one bat interspersed with echolocation (echo) pulses from the other bat. (B) Series of calls from one interaction, showing, in order, a CS call, echolocation pulse (not labeled), DFMm, echo, DFMm, FMB bout, and echolocation (echo). (C) DFMm call by one bat. (D) SIN-FM call followed by a noisy burst (not classified) and a second SIN-FM call. (E) QCF-DFM followed by echolocation (not labeled), an unclassified call, CS, echolocation (not labeled), and QCF-DFM. (F) DCF-QCF call by one bat. (G) QCF-DFM call by one bat. Scale bars apply to all calls within that column.
Durations of social calls in the sample of 90 competitive feeding trials were log-transformed and compared across the three bat groups using one-way analysis of variance. There were significant differences between pair groups [F(2, 2577) = 96.5, Bonferroni-corrected two-tailed P < 0.001, η2 = 0.07; Figure 3]. Post-hoc comparisons showed that call duration differed between male-male and male-female and between male-male and female-female groups (Bonferroni-corrected P < 0.001), and between male-female and female-female groups (Bonferroni-corrected P = 0.007). Female-female pairs produced social calls with longer durations than pairs containing males.

Figure 3. Histograms of (non-FMB) social call durations in 90 competitive feeding trials. Pair groups correspond to those listed in Table 1. The legend P1, P2, P3 applies to the specific pairs in the three groups; for the male-male data, the legend corresponds to the three sets of trials for that pair. Durations include those for calls that were uncommon in our database and thus were not classified. One male-female pair (red bars) produced few social calls of short duration. Social call durations differ significantly across pair group, and are longer in female-female pairs.
3.2 Frequency-modulated bouts
FMBs are emitted in regular bouts of 3–5 calls (Table 3) resembling echolocation pulses in shape and bandwidth and are often followed by echolocation pulses (Figures 2, 4). Each individual call in the bout is longer in duration than echolocation pulses (Table 2, Figure 5). Within a bout, individual calls are emitted at intervals between 14–17 ms. In the competitive feeding trials (Figures 4A, B), FMB calls were produced by the male-male pair and by the male-female pairs (~10% of all social calls for each pair group). But there was considerable variability between the three male-female pairs (values of 85%, 6%, and 0%) even within the same behavioral context.
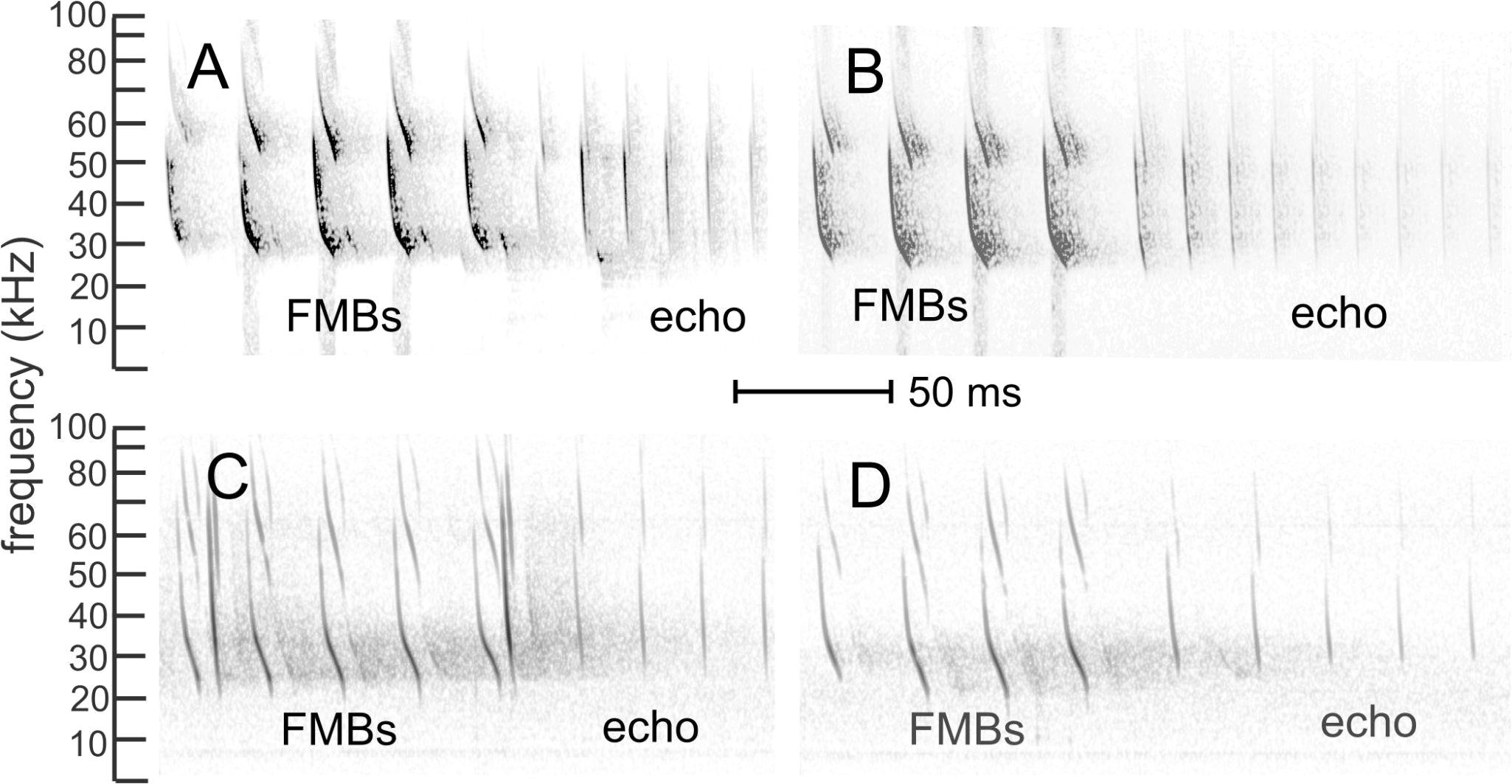
Figure 4. FMB calls and echolocation pulses in two different contexts. (A) Laboratory recording of a five bout FMB (FMBs) followed by a series of echolocation pulses (echo). (B) Laboratory recording of a four bout FMB followed by a series of echolocation pulses. (C) Field recording of a five bout FMB followed by a series of echolocation pulses. A second bat’s echolocation pulses overlap the FMB bout. (D) Field recording of a four bout FMB followed by a series of echolocation pulses. The time scale is the same for all spectrograms.
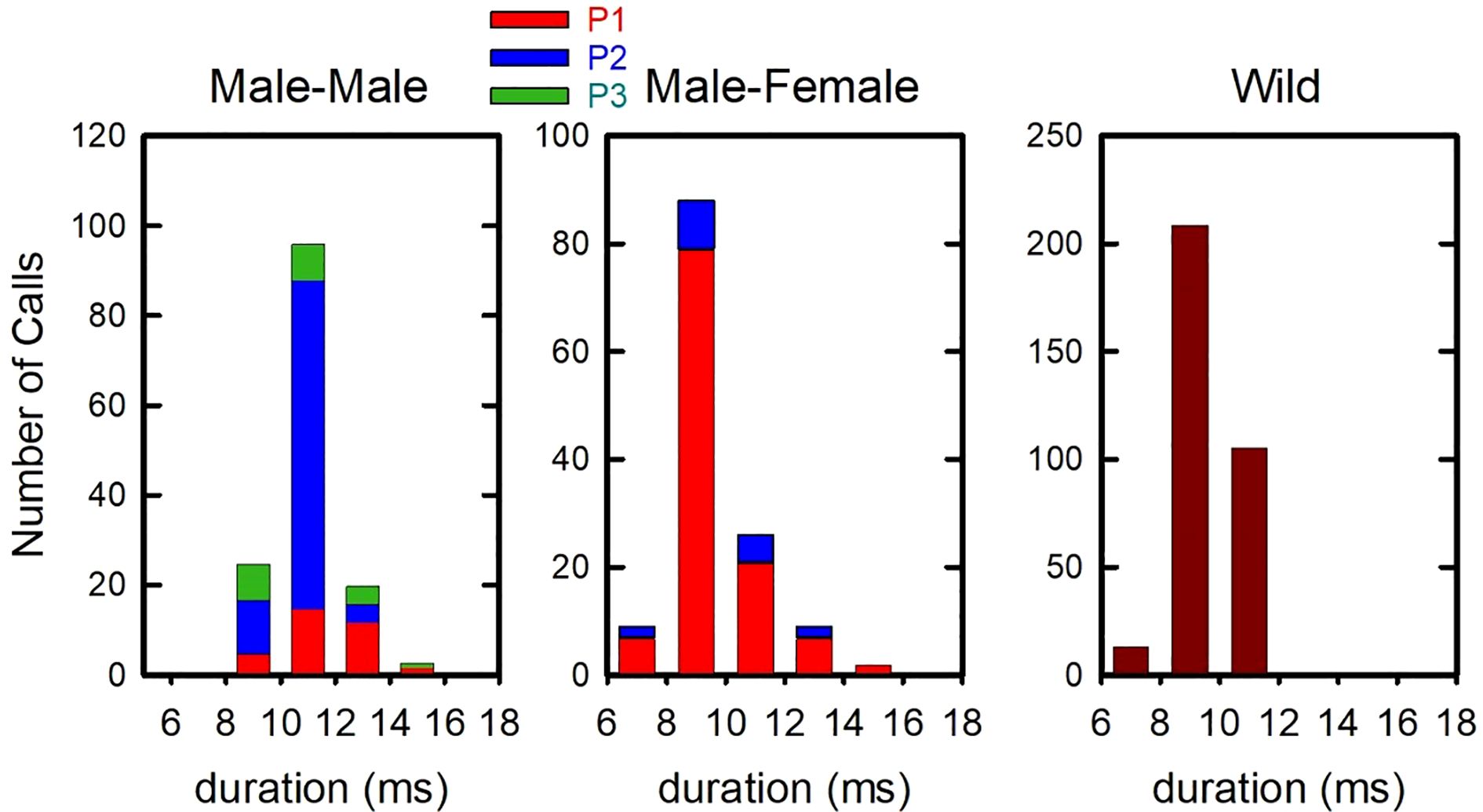
Figure 5. Histograms of durations of FMB calls produced by male-male pairs (left) and male-female pairs (middle) in the laboratory, and by free-flying wild bats at a known foraging site (right).
We recorded vocalizations of free-flying big brown bats at a known group foraging site. Big brown bat echolocation calls were identified based on published criteria for this species (Simmons et al., 1979, 2001; Surlykke and Moss, 2000) and verified using Kaleidoscope Pro software (recognition accuracy 84%). Only one other species (Lasiurus cinereus) was present at the foraging site, recognized at 98% accuracy. Within the 42 min recording time, we identified in the big brown bat vocalizations 330 FMB calls, all organized into bouts of 3–5 individual pulses.
We compared durations of FMB calls produced by male-male pairs, male-female pairs, and wild bats. Call durations were log-transformed and analyzed with one-way analysis of variance. There was a significant group effect [F(2,606) = 63.96, Bonferroni-corrected two-tailed P < 0.001, η2 = 0.174]. Results of pairwise comparisons showed that male-male pairs produced significantly longer FMB calls than either the male-female pair or the bats foraging in the wild (Figure 5).
4 Discussion
Big brown bats are gregarious animals whose repertoire of social calls in different contexts has been understudied. The purpose of our work was to examine the role of one social call, the FMB, in interactions between pairs of big brown bats tested in a competitive laboratory feeding context. We compared FMBs produced in this context with those emitted by bats flying in the wild. We identified and classified five other social calls with acoustic properties similar to social calls described in other laboratory studies. Our data emphasize both the variety of the big brown bat’s social calls and the significant individual differences in vocal interactions among conspecifics.
4.1 Variability in vocal interactions
Our results show that paired bats in the competitive feeding context emitted more echolocation than social calls, which aligns with the use of echolocation for spatial orientation (Simmons et al., 1979; Surlykke and Moss, 2000). Similarly, 71% of all calls emitted by interacting big brown bats in the laboratory study by Salles et al. (2024) were echolocation pulses. The high number of echolocation calls in both studies suggests that these sounds may also play a role in social recognition of conspecifics, as previously suggested for this species (Masters et al., 1995). The amount of calling varied across bat pairs and among individual bats within those pairs. Female-female pairs produced fewer vocalizations, whether echolocation or social, than pairs with males. Wright et al. (2013, 2014) similarly reported that bat pairs containing male bats produced more social calls than female-female pairs. FMB calls were produced only in pairs containing male bats and were absent in all female-female pairs; this finding confirms results of Wright et al. (2013, 2014) in free-flying bats. FMBs with spectral features similar to those recorded in the laboratory were also emitted by bats foraging in the wild, indicating that the FMB call is not limited to artificial laboratory environments.
There was considerable individual variability in calling within bat pairs. For example, in the total sample of 120 trials, the number of non-FMB social calls produced by male-female pairs ranged from 8 to 798, and the number of FMBs ranged from 0 to 173. Similar variability in social call production (range of 5 to 124) was observed in female-female pairs. These data suggest that social call production in the same behavioral context was influenced by the interaction of the identity of both the sender and the receiver; a bat modified its calling behavior depending on the identity of the conspecific. Because these social calls were categorized as aggressive in previous work (Gadziola et al., 2012; Wright et al., 2013, 2014; Salles et al., 2024), the dominant or submissive status of each individual could have influenced calling behavior. Data from the Asian particolored bat (Vespertilio sinensis) indicate that social calls provide information about the “quality” (health) and dominance status of the caller (Luo et al., 2017; Zhao et al., 2018). Extensions of that study to big brown bats will provide valuable insight into the kind of information transmitted by different social calls.
An interesting finding in our study is the absence of any calls defined in previous literature as appeasement calls. Gadziola et al. (2012) observed DFMm calls in both aggressive and appeasement contexts, and so this call cannot be classified as having solely an appeasement function. Those researchers stated that appeasement calls were difficult to evoke in their experimental setup, and occurred primarily during lengthy recording sessions. Anecdotally, some of our bat pairs (two of the three female/female pairs) tended to remain silent and motionless during testing. Silence, rather than emitting appeasement calls, may serve as a strategy to avoid aggressive interactions, similar to how silence is used to prevent sonar jamming (Chiu et al., 2008).
4.2 Sex specificity of FMBs
We identified FMB calls with acoustic features similar to those described in previous work (Wright et al, 2013, 2014; Salles et al., 2024). In our experiment, FMB calls were emitted only in a social interaction; individual bats given the opportunity to capture a tethered mealworm did not emit FMBs, only echolocation calls. Further, FMBs were specific to interactions involving at least one male bat; no FMBs were recorded during any female-female interactions. Because we cannot reliably identify which bat in a pair is producing a particular vocalization, especially in trials with very high calling rates, we cannot determine for certain if all FMBs in male/female pairs were emitted by the male rather than the female bat. The one male bat that did not emit FMBs in the presence of a female might have emitted those calls in the presence of another male. As suggested above, both the individual bat and the identity of the conspecific likely influence the types of vocal interactions that can occur.
Wright et al. (2013, 2014) reported that FMBs were produced exclusively by flying male big brown bats competing in a laboratory flight room to catch a tethered mealworm. In contrast, Salles et al. (2024) reported that female bats crawling in a restricted enclosure produced more FMBs than males. These researchers suggested that the sex-specific nature of FMBs is limited to flight contexts. Our results with female bats are consistent with those of Wright et al. (2013, 2014). Although our study and that of Salles et al. (2024) involved bats crawling rather than flying, other differences in experimental conditions could have affected the outcomes. For instance, our bats were not confined and could fly away from the experimental setting at will. We only had four females willing to participate, whereas Salles et al. (2024) tested six females. Due to these limitations and our difficulty in reliably identifying which bat was vocalizing at any given time, we cannot dismiss the possibility that female bats may produce FMBs under certain social situations. We observed calls from female-female pairs with similar bandwidth and downsweep shape as FMB calls recorded from male-male and male-female pairs, but these calls lacked the characteristic FMB bout structure and were longer in duration, so they were not classified as such.
Wright et al. (2013) described the FMB call as serving a food defense function, and Wright et al. (2014) highlighted its role in food claiming. Salles et al. (2024) classified the FMB as an aggressive or territorial call. Gadziola et al. (2012) did not identify FMB calls; however, in their study, bats were not tested during competition for food. In our paradigm, FMBs were produced before either bat in the pair approached the tethered mealworm. We suggest that food claiming better describes the function of the call rather than food defense, and that FMBs are best categorized within a broad group of aggressive calls.
Our data suggest that FMBs emitted in the laboratory context are similar in structure to those recorded from free-flying bats at a known group foraging site, indicating that FMBs are not an artifact of captivity. Because regulatory restrictions prevented us from capturing these bats, we do not know their sex. Springall et al. (2019), in their study of foraging big brown bats, identified a “complex call” with a spectrogram shape, bout structure, and bout duration similar to what we term an FMB. The complex call was recorded from two bats approaching a common prey item, supporting the idea that this call, like the FMB, is involved in food claiming.
4.3 Social call classification
We identified six common call types in our database, which are acoustically similar to those broadly classified as aggressive calls in previous laboratory (Gadziola et al., 2012; Wright et al., 2013, 2014; Salles et al., 2024) and field studies (Springall et al., 2019) with big brown bats. Because we relied on time-consuming, non-automated identification of calls, we only analyzed calls that appeared frequently in our database; calls that occurred rarely were not included. Therefore, we do not provide a complete description of all social calls recorded in our experimental setting, and our results likely underestimate the diversity of the big brown bat’s social repertoire, even within a limited context.
Comparing social call structures and usage across different studies is complex. First, various tasks and contexts—such as flying in natural environments, flying in a laboratory flight room, crawling, competing for food in a constrained laboratory setting, and responding to intruders—have been used to elicit social vocalizations. Montoya et al. (2022) noted that roosting and flying bats produce different types of social calls with limited overlap. While similarities across these contexts can be observed, differences may affect how the bats themselves perceive their environment and, consequently, the vocalizations they produce.
Second, all studies conducted so far have tested a limited number of animals across a small range of tasks. In the wild, big brown bats fly and forage in groups (Simmons et al., 2001; Springall et al., 2019) and can roost in colonies consisting of hundreds of individuals (Hayes and Wiles, 2013). Due to smaller sample sizes in controlled environments and restricted time spans in which bats are willing to participate in experiments, fully understanding individual variability in social call production and use remains difficult. That is, it is not clear which call types are stereotyped and which are graded. Differences in acoustic parameters among individuals have only been documented for some big brown social calls (Wright et al., 2013), while the overall extent and importance of individual variability in other calls in the repertoire remains unknown. Studies on Brazilian free-tailed bats (Bohn et al., 2009) have highlighted significant individual differences in song features, such as the number of syllables within phrases and the order of phrases. Challenges in classification may result from these individual differences.
Few earlier studies on big brown bats examined the acoustic context of social calling and instead focused on acoustic features of isolated social calls. Gadziola et al. (2012) provided some examples of call sequences to highlight transitions between syllables and how these might change with context. Analyzing transitions between syllables can be difficult, especially when more than one bat is vocally active, because even automatic classifiers are trained on individual calls (syllables) and may misidentify sequences. Here, we present some calling sequences to show sequences of call types and transitions between them. Analysis of call sequences in combination with individual variability and task demands suggests that individuals may alter the parameters of their social calls, similar to how they modify echolocation calls in response to environmental demands (Surlykke and Moss, 2000; Simmons et al., 2001). We do not know if call modifications are intentional changes made to communicate information or if they result from production limitations. The extent of individual call modification might explain the differences in correspondence between our study and previous research, making this a promising area for further investigation.
Finally, using different naming conventions and criteria for classifying calls introduces variability and error. Gadziola et al. (2012); Wright et al. (2013), and Springall et al. (2019) used discriminant function analysis combined with visual examination of time-frequency structure to categorize social calls, but their classifications and naming conventions are not fully consistent with each other. Different numbers of social call types, ranging from six in Wright et al. (2013) to 18 in Gadziola et al. (2012), have been identified. This may reflect the varying social contexts experienced by the bats, as discussed earlier, or it may instead be due to differences in criteria for classification. Establishing a consistent system of nomenclature is essential for further analysis of bat social call repertoires. It is also vital to supplement acoustic feature distinctions with those related to behavioral context (Pfalzer and Kusch, 2003).
4.4 Limitations and future directions
There are several limitations to our study, but also multiple avenues for future research. To classify calls, we relied on examining published spectrograms and tables of acoustic parameters and compared these to our own acoustic analyses. The challenge is that most literature provides mainly isolated examples of spectrograms rather than sequences of calls. Our visualization and analysis of call series reveal vocal variability even within the same individual, along with gradual transitions between call types. We improved the reliability of our manual analysis by having all social calls in the selected subset of trials analyzed and classified independently by two of the authors, then reviewed together by three authors. To make our classifications as consistent as possible with those published previously, we adopted a strategy of “lumping” rather than “splitting” call types.
Although automated classifiers (i.e., Kaleidoscope Pro; Roemer et al., 2021; Tabak et al., 2022; Nunez-Mir et al., 2025) provide advantages in speed and reliability over human visual classification, there is no widely accepted, freely available, and easy-to-use automated method for bat call classification with high accuracy for both echolocation and social calls. Even for echolocation calls, the accuracy of currently-available classifiers varies considerably across algorithms and species (Rydell et al., 2017). Classifiers are trained on isolated examples of calls and tested with other calls presented out of sequence or disconnected, which can decrease their accuracy in identifying vocal transitions between different types of social calls. The classification accuracy of BattyKoda (Nunez-Mir et al., 2025), which handles both echolocation and social calls, ranged broadly from 21% to 90% depending on call type. Finally, the output of a machine learning algorithm may not reflect the bat’s own perception of similarities and differences between calls.
Other limitations of our study include the small sample size and the inability to identify which bat in a pair vocalized at any specific time during a trial. Of the nine bats we recorded in individual trials, only six were willing to participate in the paired trials. We cannot determine why some bats were unwilling to participate, as food reinforcement was consistent for all individuals. During paired trials, bats were free to move on the platform and often turned their bodies and heads to face each other, which kept their mouths out of camera view. Additionally, even when their mouths were open and visible as a sign of vocalization, both bats frequently had open mouths — especially when approaching each other — suggesting they were vocalizing at the same time. Amplitude of calling was used where appropriate to identify individuals, such as when one bat stayed still on the platform while the other moved, although this was not always reliable.
Finally, we were unable to evaluate either the communicative intent of the caller or the perception of the receiver. Playback experiments with individual bats (Luo et al., 2017; Zhao et al., 2018; Guo et al., 2021; Fry et al., 2024) or pairs of bats in settings that allow multiple response options can reveal how the bat, rather than the experimenter or the algorithm, classifies calls within the vocal repertoire.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Audio files from the 27 selected competitive feeding trials are available in the Brown Digital Repository https://doi.org/10.26300/vtgm-4x49.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee, Brown University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RF: Writing – original draft, Investigation, Formal analysis, Conceptualization, Data curation. ES: Methodology, Investigation, Formal analysis, Writing – original draft. JS: Writing – review & editing, Resources, Visualization, Funding acquisition, Formal analysis, Validation, Methodology, Supervision, Project administration. AS: Supervision, Funding acquisition, Writing – review & editing, Writing – original draft, Project administration, Formal analysis, Resources, Validation, Investigation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the Office of Naval Research (awards #N00014-17-1-2736, N00014-24-1-2665) to J.A.S. and A.M.S.
Acknowledgments
We thank Cara Kaminski for assistance in data collection, and Jamie Trost for statistical advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2025.1690988/full#supplementary-material
References
Balcombe J. and Fenton M. B. (1988). Eavesdropping by bats – the influence of echolocation call design and foraging strategy. Ethology 79, 158–166. doi: 10.1111/j.1439-0310.1988.tb00708.x
Behr O. and von Helversen O. (2004). Bat serenades – complex courtship songs of the sacwinged bat (Saccopteryx bilineata). Behav. Ecol. Sociobiol. 56, 106–115. doi: 10.1007/s00265-004-0768-7
Bohn K. M., Schmidt-French B., Schwartz C., Smotherman M., and Pollak G. D. (2009). Versatility and stereotypy of free-tailed bat songs. PloS One 4, e6746. doi: 10.1371/journal.pone.0006746
Chaverri G., Ancillotto L., and Russo D. (2018). Social communication in bats. Biol. Rev. 93, 1938–1954. doi: 10.1111/brv.12427
Chiu C., Xian W., and Moss C. F. (2008). Flying in silence: Echolocating bats cease vocalizing to avoid sonar jamming. Proc. Natl. Acad. Sci. U.S.A. 105, 13116–13121. doi: 10.1073/pnas.0804408105
Dechmann D. K. N., Heucke S. L., Giuggioli L., Safi K., Voight C. C., and Wikelski M. (2009). Experimental evidence for group hunting via eavesdropping in echolocating bats. Proc. R. Soc B. 276, 2721–2728. doi: 10.1098/rspb.2009.0473
Fenton M. B., Faure P. A., Bernard E., Becker D. J., Jackson A. C., Kingston T., et al. (2024). Bat handlers, bat bites, and rabies: vaccination and serological testing of humans at risk. Facets 9, 1–11. doi: 10.1139/facets-2024-0056
Fry R. N., Tuninetti A., Simmons J. A., and Simmons A. M. (2024). Manipulating environmental clutter reveals dynamic active sensing strategies in big brown bats. Anim. Behav. Cogn. 11, 61–78. doi: 10.26451/abc.11.01.04.2024
Gadziola M. A., Grimsley J. M. S., Faure P. A., and Wenstrup J. J. (2012). Social vocalizations of big brown bats vary with behavioral context. PloS One 7, e44550. doi: 10.1371/journal.pone.0044550
Gillam E. and Fenton M. B. (2016). “Roles of acoustic social communication in the lives of bats,” in Bat Bioacoustics. Eds. Fenton M. B., Grinnell A. D., Popper A. N., and Fay R. R. (Springer, New York), 117–140.
Guo D., Ding J., Liu H., Zhou L., Feng J., Luo B., et al. (2021). Social calls influence the foraging behavior in wild big-footed myotis. Front. Zool. 18, 3. doi: 10.1186/s12983-020-00384-8
Hayes G. and Wiles G. J. (2013). “Big Brown Bat (Eptesicus fuscus),” in State of Washington Bat Conservation Plan (Olympia WA: Washington Department of Fish and Wildlife). Available online at: https://wdfw.wa.gov/species-habitats/species/eptesicus-fuscus#desc-range (Accessed August 1, 2025).
Hiryu S., Bates M. E., Simmons J. A., and Riquimaroux H. (2010). FM echolocating bats shift frequencies to avoid broadcast-echo ambiguity in clutter. Proc. Natl. Acad. Sci. USA 107, 7048–7053. doi: 10.1073/pnas.1000429107
Jones G. and Siemers B. M. (2011). The communicative potential of bat echolocation pulses. J. Comp. Physiol. A 197, 447–457. doi: 10.1007/s00359-010-0565-x
Kaleidoscope Pro Wildlife Acoustics. Available online at: https://www.wildlifeacoustics.com/products/kaleidoscope/automatic-bat-identification (Accessed September 12, 2023).
Knörnschild M., Jung K., Nagy M., Metz M., and Kalko E. (2012). Bat echolocation calls facilitate social communication. Proc. R. Soc B. 279, 4827–4835. doi: 10.1098/rspb.2012.1995
Kothari N. B., Wohlgemuth M. J., Hulgard K., Surlykke A., and Moss C. F. (2014). Timing matters: Sonar call groups facilitate target localization in bats. Front. Physiol. 5, 1–13. doi: 10.3389/fphys.2014.00168
Luo B., Lu G., Chen K., Guo D., Huang X., Liu Y., et al. (2017). Social calls honestly signal female competitive ability in Asian particoloured bats. Anim. Behav. 127, 101–108. doi: 10.1016/j.anbehav.2017.03.012
Masters W., Raver K. A. S., and Kazial K. A. (1995). Sonar signals of big brown bats, Eptesicus fuscus, contain information about individual identity, age and family affiliation. Anim. Behav. 50, 1243–1260. doi: 10.1016/0003-3472(95)80041-7
Montoya J., Lee Y., and Salles A. (2022). Social communication in big brown bats. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.903107
Nunez-Mir G. C., Boergens K. N., Montoya J. C., ter Hofstede H., and Salles A. (2025). BattyCoda: A novel open-source software for bat call annotation and classification. Ecol. Inform. 89, 103195. doi: 10.1016/j.ecoinf.2025.103195
Pfalzer G. and Kusch J. (2003). Structure and variability of bat social calls: implications for specificity and individual recognition. J. Zool. 261, 21–33. doi: 10.1017/S0952836903003935
Roemer C., Julien J.-F., Ahoudji P. P., Chassot J.-M., Genta M., Colombo R., et al. (2021). An automatic classifier of bat sonotypes around the world. MEE 12, 2432–2444. doi: 10.1111/2041-210X.13721
RStudio Team (2020). RStudio: Integrated development for R (Boston, MA: RStudio, PBC). Available online at: http://www.rstudio.com/ (Accessed June 17, 2025).
Rydell J., Nyman S., Eklof J., Jones G., and Russo D. (2017). Testing the performances of automated identification of bat echolocation calls: A request for prudence. Ecol. Inform. 78, 416–420. doi: 10.1016/j.ecolind.2017.03.023
Salles A., Loscalzo E., Montoya J., Mendoza R., Boergens K. M., and Moss C. F. (2024). Auditory processing of communication calls in interacting bats. iScience 27, 109872. doi: 10.1016/j.isci.2024.109872
Simmons J. A., Eastman K. M., Horowitz S. S., O’Farrell M. J., and Lee D. N. (2001). Versatility of biosonar in the big brown bat, Eptesicus fuscus. ARLO 2, 43–48. doi: 10.1007/s00359-016-1105-0
Simmons J. A., Fenton M. B., and O’Farrell M. J. (1979). Echolocation and pursuit of prey by bats. Science 203, 16–21. doi: 10.1126/science.758674
Springall B. T., Li H., and Kalcounis-Rueppell M. C. (2019). The in-flight social calls of insectivorous bats: species specific behaviors and contexts of social call production. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00441
Surlykke A. and Moss C. F. (2000). Echolocation behavior of big brown bats, Eptesicus fuscus, in the field and the laboratory. J. Acoust. Soc Am. 108, 2419–2429. doi: 10.1121/1.1315295
Tabak M. A., Murray K. L., Reed A. M., Lombardi J. A., and Bay K. J. (2022). Automated classification of bat echolocation call recordings with artificial intelligence. Ecol. Inform. 68, 101526. doi: 10.1016/j.ecoinf.2021.101526
Übernickel K., Tschapka M., and Kalko E. K. V. (2013). Selective eavesdropping behaviour in three neotropical bat species. Ethology 119, 66–76. doi: 10.1111/eth.12038
Voigt-Heucke S. L., Taborsky M., and Dechmann D. K. N. (2010). A dual function of echolocation: bats use echolocation calls to identify familiar and unfamiliar individuals. Anim. Behav. 80, 59–67. doi: 10.1016/j.anbehav.2010.03.025
Wheeler A. R., Fulton K. A., Gaudette J. E., Simmons R. A., Matsuo I., and Simmons J. A. (2016). Echolocating big brown bats, Eptesicus fuscus, modulate pulse intervals to overcome range ambiguity in cluttered surroundings. Front. Behav. Neuro. 10, 125. doi: 10.3389/fnbeh.2016.00125
Wright G. S., Chiu C., Xian W., Moss C. F., and Wilkinson G. S. (2013). Social calls of flying big brown bats (Eptesicus fuscus). Front. Physiol. 4. doi: 10.3389/fphys.2013.00214
Wright G. S., Chiu C., Xian W., Wilkinson G. S., and Moss C. F. (2014). Social calls predict foraging success in big brown bats. Curr. Biol. 24, 885–889. doi: 10.1016/j.cub.2014.02.058
Yovel Y., Melcon M. L., Franz M. O., Denzinger A., and Schnitzler H.-U. (2009). The voice of bats: how greater mouse-eared bats recognize individuals based on their echolocation calls. PloS Comp. Biol. 5, e1000400. doi: 10.1371/journal.pcbi.1000400
Keywords: bats, bioacoustics, Chiroptera, social communication, vocalizations
Citation: Fry RN, Suong E, Simmons JA and Simmons AM (2025) Social calls of big brown bats in a competitive feeding context. Front. Ecol. Evol. 13:1690988. doi: 10.3389/fevo.2025.1690988
Received: 22 August 2025; Accepted: 30 October 2025;
Published: 20 November 2025.
Edited by:
Eric Schuppe, University of California, San Francisco, United StatesReviewed by:
Daniel J. Tobiansky, St. Mary’s College of Maryland, United StatesBrock Fenton, Western University, Canada
Copyright © 2025 Fry, Suong, Simmons and Simmons. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Megela Simmons, QW5kcmVhX1NpbW1vbnNAYnJvd24uZWR1
 Reese N. Fry
Reese N. Fry Emily Suong1
Emily Suong1 James A. Simmons
James A. Simmons Andrea Megela Simmons
Andrea Megela Simmons