- Mathematics Natural Science and Technology Education, University of the Free State, Bloemfontein, South Africa
Due to the freedom afforded natural sciences textbook authors globally and in South Africa, there are verbal and visual misrepresentations of matter and material concepts, constructs, and principles globally, and in South Africa. These misrepresentations may negatively affect the way teachers teach and learners learn natural sciences content, though there are policy frameworks that guide the development of textbooks for secondary school, especially for grade-9. One asks why this is the case when several modes of representation are used in natural sciences textbooks to explain the natural sciences concepts, constructs, and principles aligned to the Curriculum Assessment policy Statements (CAPS) document. The textbooks can use verbal or visual representations or a combination of them. Verbal representation (VeR) of subject matter in textbooks comprises definitions, descriptions, explanations, and step-by-step methods while visual representations (ViR) may include drawings, graphs, and models. The verbal aspects are presented in more detail in textbooks than in curriculum assessment policy statements (CAPS) documents. The ViR (images) are only discussed in the CAPS document and no images are provided. These differences between textbooks and the CAPS document may pose a challenge to natural sciences teachers, especially novice teachers who seek guidance from the textbook rather than analysing the CAPS document to prepare and present a lesson on matter and materials.
1 Introduction
The freedom given to authors of natural sciences textbooks, both globally and in South Africa, has resulted in various verbal and visual misrepresentations of matter and material concepts, constructs, and principles. These misrepresentations may negatively affect the way teachers teach and learners learn natural sciences content, though there are policy frameworks that guide the development of textbooks for secondary school, especially for grade 9. One may wonder why this is so when several modes of representation are used in natural sciences textbooks to explain the concepts, constructs, and principles of the natural sciences as outlined in the curriculum assessment policy statements (CAPS) document. The textbooks can use verbal or visual representations (Sadoski and Paivio, 2001) or a combination of both. Verbal representation (VeR) of subject matter in textbooks comprises definitions, descriptions, explanations, and step-by-step methods (Devetak and Vogrinc, 2013), while visual representations (ViR) may include drawings, graphs, and models (Kapıcı and Savaşcı-Açıkalın, 2015). The verbal aspects are presented in more detail in textbooks than in curriculum assessment policy statements (CAPS) documents. The ViR (images) are only discussed in the CAPS document, and no images are provided. These differences between textbooks and the CAPS document may challenge natural sciences teachers, especially novice teachers who seek guidance from the textbook rather than analyzing the CAPS document to prepare and present a lesson on matter and materials.
Several studies have been conducted on how the combination of VeR and ViR on matter and materials in textbooks impacts the pedagogical process and learner achievement (Bergqvist and Chang Rundgren, 2017). Nonetheless, grade-9 learners in South Africa perform poorly in the knowledge strand of matter and materials (Prinsloo et al., 2016), due to an insufficient understanding of chemical concepts represented in textbooks. Despite the failures, textbooks are often used as a mirror image of the National Curriculum (McDonald and Abd-El-Khalick, 2017), even though they are expected to link between curriculum policy and its implementation (Van den Ham and Heinze, 2018).
2 Conceptual framework
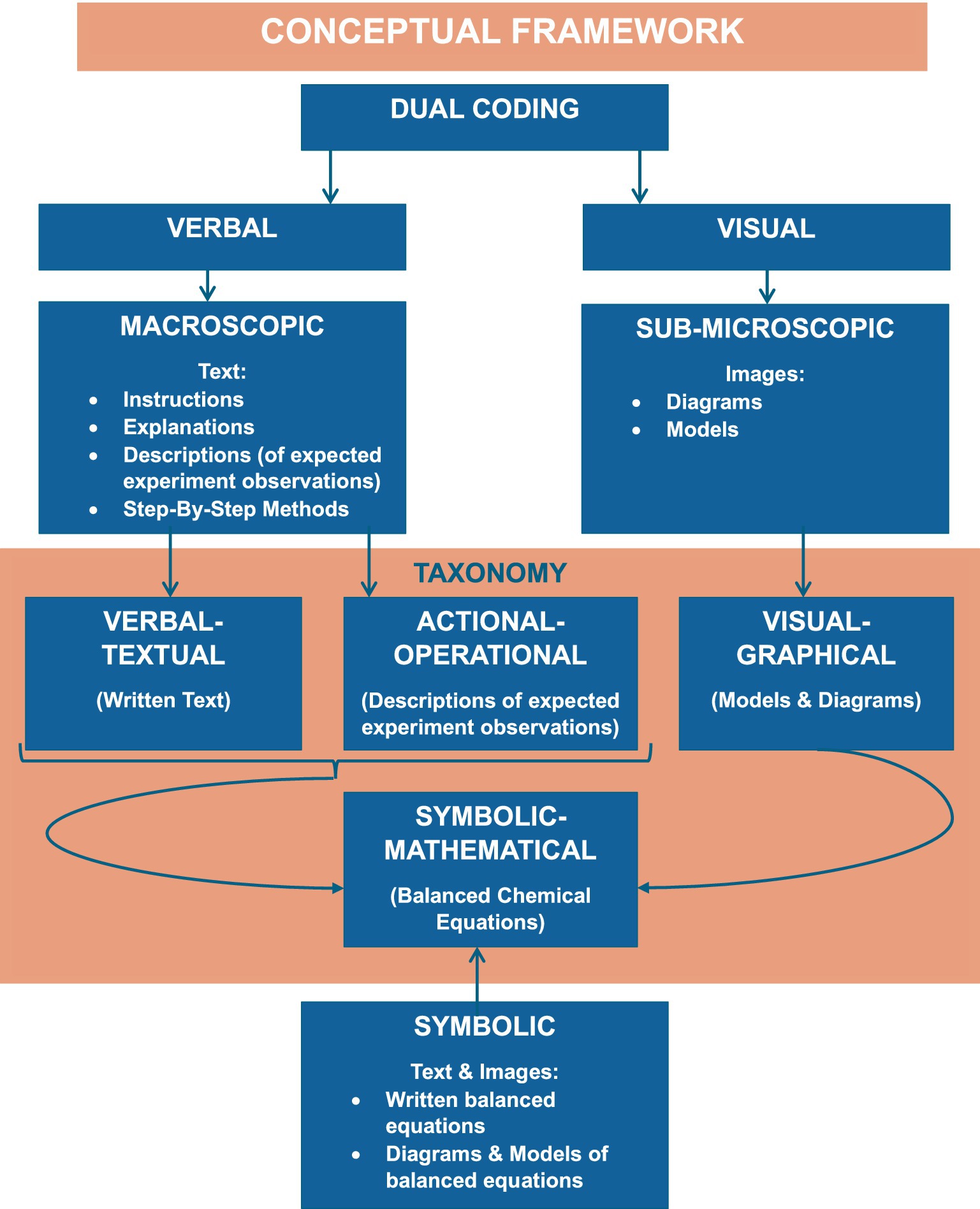
This study used a hybrid theory combining the dual-coding theory by Sadoski and Paivio (2001) and the principles of the chemistry triplet as a combination of multiple, external representations such as macroscopic, sub-microscopic, and symbolic in natural sciences, particularly in chemistry (Johnstone, 1993) that seeks to simplify scientific concepts and phenomena investigated collaboratively. Dual coding involves referential connections that enable performing operations like imaging words and naming pictures or images to words (Paivio and Representations, 1990).
The framework depicted in Figure 1 is structured in such a way that verbal and visual representations (dual coding) are dissected into the chemistry triplet in a similar relationship as exists in the taxonomy of representations. In this study, verbal representations were analyzed on the macroscopic level in terms of instructions, explanations, and descriptions of expected observations. This relates to verbal–textual and actional–operational representations in the taxonomy (Lemke, 1998; Tsui, 2003; Wu and Puntambekar, 2012). Moreover, sub-microscopic representation, in the form of images (diagrams and models) of atoms and compounds, is the level at which visual representation was analyzed, and is congruent with visual–graphical representation (Lemke, 1998; Tsui, 2003; Wu and Puntambekar, 2012). Furthermore, symbolic representation is depicted in the relationship between verbal and visual representations by consolidating text and diagrams in the form of balanced chemical equations. This is a correlation of symbolic–mathematical representation (Lemke, 1998; Tsui, 2003; Wu and Puntambekar, 2012). The three levels of the chemistry triplet speak to one another (Gilbert and Treagust, 2009) and emphasise the uniqueness of and relationship between verbal and visual representations (Sadoski and Paivio, 2001). Hence, this study compared the verbal and visual representations of grade-9 natural sciences concepts, constructs, and principles of matter and materials in three textbooks and the CAPS policy document.
2.1 Literature review
2.1.1 Textbooks
Textbooks are all-pervading teaching and learning resources (Peterson, 2016) whose role is to represent subject matter in a simplified, yet accurate manner (Karásková et al., 2019). The relationship between curriculum policy and curriculum implementation is, in most instances, strengthened by using textbooks for teaching and learning (Van den Ham and Heinze, 2018).
2.1.2 Textbooks and representations
There are different definitions of representations depending on how they are used in teaching. Regarding sense-making, representations are the entities with which all thinking is considered to take place (Gilbert and Treagust, 2009). Churchill (2017: xi) identified the three representations of curriculum knowledge content, namely, declarative, procedural, and conceptual representations, designed for effectiveness within the learning activities. Thirdly, conceptual representations in long-term memory crucially contribute to perception and action, language, and thought (Kiefer and Pulvermüller, 2012). These content representations may be presented verbally, visually, or a combination of both to help make a mental image of the concept presented for decision-making during the instruction.
2.1.3 Verbal representations
Verbal representation in matter and materials is in the form of rules, statements, procedures, and arrangements (Muspratt and Freebody, 2013). For verbal representation in the form of text to be easily understood and represent concepts of interest clearly, it should possess four qualities that enhance learning, namely, simplicity, clear arrangement of text, conciseness, and interest arousal (Opfermann et al., 2017).
2.1.4 Visual representations
Representations of science in the form of iconic or realistic pictures make visualisation and understanding of scientific entities and phenomena easier (Yang and Treagust, 2013; Opfermann et al., 2017). They, Yang and Treagust (2013) further state that the diagrammatic sketches may not be as assistive because they require an individual’s ability to interpret such diagrams. The matter and materials concept uses images that have an inherent communication ability, and each learner gets to understand what the image represents based on how they engage with the image through prior knowledge and experiences (Eilam and Gilbert, 2014). The teacher’s responsibility is to facilitate learning of matter and material concepts and minimise misconceptions. However, without natural sciences subject matter knowledge on how ViR helps learners make sense of matter and materials, they will rely on textbooks and ignore the CAPS document.
2.1.5 Combination of verbal and visual representations
Although there are differences between verbal and visual representations, there is a close link between them (Sadoski and Paivio, 2001). VeR in textbooks is substantially expressed in writing (Anwar and Rahmawati, 2017) and complemented by diagrams that are a simple ViR of intricate concepts and rationalise how these concepts are interlinked (Kembhavi et al., 2016). The CAPS document explains how VeR and ViR could help in making sense of matter and materials concepts. Opfermann et al. (2017) agree that the learning process is enhanced by combining words and pictures rather than by words exclusively. However, the combination of VeR and ViR does not affirm productive learning, it should be carefully assembled (Opfermann et al., 2017). Therefore, the combination of VeR and ViR in the textbook does not remove the teacher’s role in planning for the matter and materials lesson plan and presentation. Even with policy frameworks such as the CAPS document that direct the creation of natural science textbooks, there continue to be ongoing verbal and visual misrepresentations regarding grade-9 natural sciences concepts, constructs, and principles of matter and materials in textbooks utilised in South African schools. Such inaccuracies can adversely affect how educators present these ideas and how students grasp them, possibly leading to poor performance in this area of knowledge. This research focuses on the disparity between the intended curriculum detailed in the CAPS document and the actual content provided in textbooks, intending to identify and analyse these misrepresentations and their effects on teaching and learning. The study aims to compare the verbal and visual representations of grade-9 natural sciences concepts, constructs, and principles of matter and materials in three widely used textbooks with those in the CAPS policy document. The study seeks to identify misrepresentations and assess their potential impact on teaching practices and learner understanding.
2.1.6 Research question
How can verbal and visual representations of grade-9 natural sciences concepts, constructs, and principles of matter and material be compared in three textbooks and the CAPS policy document?
3 Research methodology
This qualitative research approach followed (Creswell and Creswell, 2018) an exploratory research design (Bowen, 2009; Boru, 2018) to purposively select three natural sciences textbooks that were primarily used in schools from a bookstore list in Bloemfontein, Free State, and compared them to a CAPS document. This is because the owner will likely select books based on their perceived popularity or significance rather than random selection. The document analysis schedule was used as a research instrument. The components of matter and materials selected for analysis were the description of compounds, naming, and chemical formulae of compounds, and chemical reactions that align with concepts, constructs, and principles of matter and material. Ethical considerations were observed, and the Research Ethics Committee of the University of the Free State granted permission. Pseudonyms were used to refer to the names of textbooks, for example, textbooks A, B, and C.
4 Results and discussion
Data were analysed using Yin’s (2016) analysis framework to compare three textbooks and the CAPS document following the five stages, namely compiling, disassembling, reassembling, stages of data were conducted before the data was presented to identify the concepts, constructs and principles of matter and materials from the three textbooks and CAPS document interpreting and concluding. In this study, data compiling, disassembling, and reassembling were done before the data was presented. Three elements were looked at during the presentation of data, namely, concepts, constructs, and principles of compounds linked to verbal and visual representations or a combination of both. The results are presented for each textbook and CAPS document. The data are presented in categories and a theme below, to compare verbal and visual representations of grade-9 natural sciences concepts, constructs, and principles of matter and material in three textbooks and the CAPS policy document.
The categories for the theme identified above are presented below by introducing the concepts, constructs, principles of matter, and materials in the CAPS document before presenting them from each textbook.
4.1 The concept of compounds must be explained through descriptions, naming, formulae, and the origin of compounds
In the CAPS policy document, verbal explanations identified were a combination of descriptions of compounds, rules for naming compounds, writing chemical formulae, and instructions for practical work involving compounds. All these aspects were guided by prescriptions and guidelines in the CAPS document for teaching and learning matter and materials in grade-9 natural sciences, on a macroscopic level of compound representation.
In the CAPS document, compounds in grade-9 are referred to as the introduction of compounds in grade-8. Therefore, compounds were described using verbal representations as pure substances made from two or more elements. This description serves as prior knowledge to the knowledge in grade-9 on compounds—which involves assigning chemical names to compounds and making chemical formulae meaningful—is essential to this grade. A compound’s chemical name is specific to that compound and derived from the elements the compound is made of. Moreover, sodium chloride is an example of a chemical name acquired from the two elements that make up the compound. Furthermore, some compounds have common names which are informal names (used in everyday life) that chemists have assigned to compounds such as table salt, water, and ammonia. The description of compounds includes having formulae that support compound names in a symbolic form.
In the CAPS document, verbal prescriptions and guidelines for carrying out practical work on compounds are provided as instructions for creating models, either single compounds or compounds in a chemical reaction. From the previous sentence, the use of experiments is presented abstractly, which would require a visual representation to simplify it. The visually represented experiment allows one to identify and list macroscopic observations during practical activities. In addition, the finding by Demirdöğen (2017) suggests that there is ambiguity in the textual (verbal) content of chemistry textbooks and that the ambiguity causes learners to formulate misconceptions. Furthermore, Lewthwaite (2014) concludes that practical work is the vehicle through which learners experience the tangible, macroscopic aspects of what they learn in chemistry, thus learning better. Nonetheless, if teachers struggle to interpret the verbal representations from the CAPS document well, learners may struggle to make sense of the matter and material concepts.
4.2 Images are necessary for understanding compounds
In the CAPS document, visual representation allows teachers to represent the sub-microscopic level of compounds through drawings and models to guide the learning process of naming compounds and writing down their chemical formulae. In addition, the compounds are included in the chemical reaction process, namely, water, carbon monoxide, carbon dioxide, copper oxide, sodium chloride, and sulphur trioxide. Though the chemical reaction is represented visually, it is still abstract. Hence, it requires the teacher to simplify the naming process using the knowledge acquired in teacher education institutions.
Providing relevant manipulatives is not easy for the teacher since it requires the teacher to be competent to help learners use their senses and mental images in understanding the process of visual chemical representations. In this view, compounds used in school chemistry are listed in the CAPS document and are incorporated into the grade-9 level. The finding by Upahi and Ramnarain (2018) supports this view that proper visual representations of chemical reactions in textbooks are pivotal in simplifying the intricate and abstract concepts of matter and materials. This may, in turn, assist learners to understand these concepts on the relevant levels of chemical representation. In contrast, Eilam and Gilbert (2014) argue that visual representations, such as drawings and models, may obstruct learning, and they highlight this obstruction to learning that takes place if the interpretation of visual representations is incorrect and therefore causes misconceptions. Although the study by Eilam and Gilbert (2014) contrasts with the view by Upahi and Ramnarain (2018) on visual presentation, they agree that an “appropriate” combination of visual and verbal representations must be available during teaching and learning to make the concept of matter and materials easier for learners.
4.3 Chemical reactions must be understood through explanations, images, and symbols
4.3.1 Verbal
4.3.1.1 Why balance chemical equations?
The CAPS document suggests that balancing chemical equations represents the principle of conservation of atoms. In a statement given by CAPS, the principle reads: “no atoms are lost or gained in the reaction, they are simply rearranged” (Department of Basic Education, 2011).
4.3.2 Visual
4.3.2.1 Draw pictures or make models
The CAPS document offers an option of either using drawings or making models of the sub-microscopic level of compounds. The option for drawings and models could be used for naming compounds and writing their chemical formulae. Moreover, drawing pictures or making models to show how products are formed from the rearrangement of atoms when a reaction occurs is essential to learners when they make the metal image of compounds at a microscopic level.
4.3.3 Verbal and visual
4.3.3.1 Use of symbols
According to the CAPS document, the symbolic, verbal, and visual representations are explained according to the different grades and the specific focus of other compounds. The CAPS document shows a verbal explanation about chemical equations having to be balanced, and the explanation states the principle of conservation of atoms. Furthermore, visual representations of chemical equations are explained using drawings or models of reactants and products. Moreover, the compounds involved in chemical reactions, such as polyatomic ions, highlight the hydroxide (−OH) and carbonate (−CO3) ions, which use symbols and numbers to support explanations and drawings or models. In the explanation, learners are expected to know the word equation, which represents an oxidation or combustion reaction between copper and oxygen to produce copper oxide. The inference is that verbal and visual explanations occur in a chemical reaction at the macroscopic level to assist learners in understanding chemical compounds. The identified chemical reaction, explained verbally and represented visually (through models or drawings), is represented symbolically by balancing the chemical equation to understand that matter (made up of atoms) can neither be created nor destroyed. In support of the finding, Lemke (1998), Tsui (2003), and Wu and Puntambekar (2012) identified that the three levels of chemistry triplet speak to one another. In contrast, Gilbert and Treagust (2009) emphasised the uniqueness of and relationship between verbal and visual representations. The following section looked at the three textbooks, A, B, and C, and how the verbal and visual representations of matter and material are compared to the CAPS documents.
4.4 The relationship between compound names and formulae must be explained
4.4.1 What is in a name?
Textbook A explains how names give identity to compounds, specific to each compound. Based on textbook A, the name of a chemical compound comes from the elements the compound is made up of. In addition, the number of atoms of each component of a compound influences the chemical name of the compound. The textbook differentiates between chemical and common names of compounds. Furthermore, two examples highlighted in textbook A include chemical names, carbon monoxide (CO) and carbon dioxide (CO2). Although both compounds are made from the same elements (carbon and oxygen), the number of atoms of each element is unique for each compound. In the two examples in Textbook A, one carbon atom and one oxygen atom represent carbon monoxide (CO) while one carbon atom and two oxygen atoms represent carbon dioxide (CO2). In addition, some compounds have common names, such as water. Whereas learners know the name water, they might not associate it with chemistry but know that it represents a specific substance. Another common name that Textbook A gives is the compound, ammonia.
4.4.2 Explanation for writing chemical formulae
In textbook A, a chemical formula indicates elements in a compound and a specific ratio for specific compounds. Examples of compounds are used to explain chemical formulae in textbook A. In the explanation, sodium chloride (NaCl) and carbon dioxide (CO2) are examples. It explained that in NaCl, each atom of sodium (Na) will have one atom of chlorine (Cl). Therefore, the ratio of atoms in sodium chloride is 1:1. Carbon dioxide (CO2) consists of one carbon atom and two oxygen atoms. The subscript indicates two oxygen atoms, and the ratio of carbon to oxygen is 1:2. In contrast, the CAPS document requires learners to know the chemical names of compounds based on component ‘elements’ and ‘common names’ of some compounds commonly encountered from day to day. In this view, mastering the naming of compounds cannot be achieved without the understanding and knowledge to formulate chemical formulae. The formulation and writing down of chemical formulae of compounds requires explaining the elements that compounds are made of and the ratio in which the atoms of those elements are found in each compound. These component elements and their ratios assist learners with the naming of compounds. The verbal explanations of chemical formulae and models are necessary for learners to understand the observations of compounds as teachers conduct practical work.
4.5 Creating models of the invisible particles of compounds helps with visualisation
4.5.1 Pictures of models
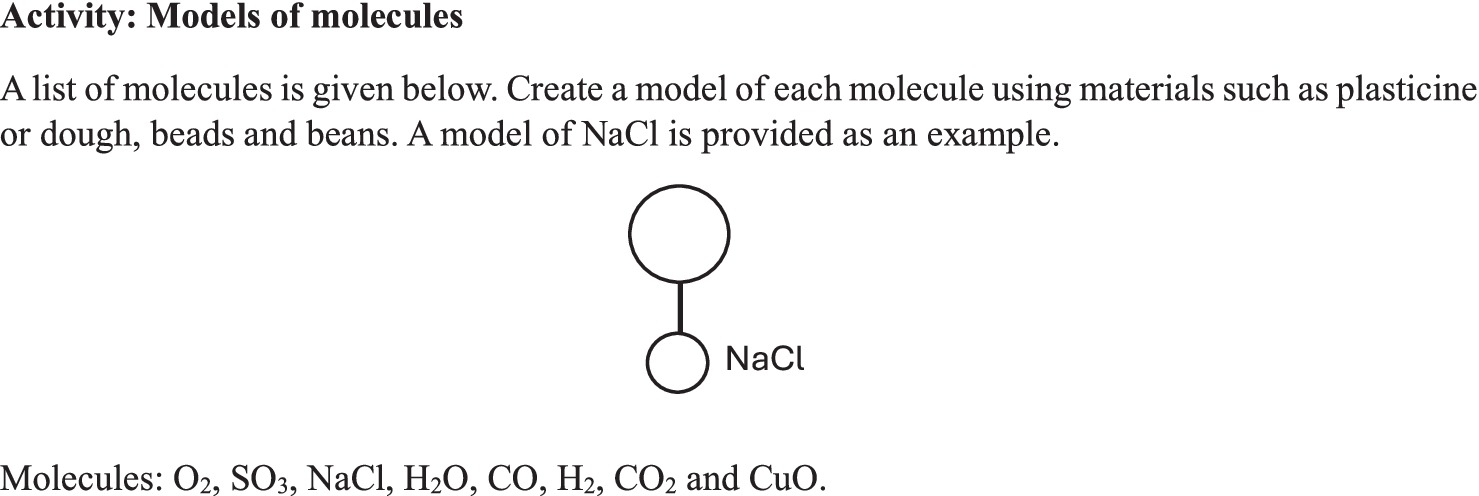
The practical activity in Figure 2 was obtained from Textbook A. Through this activity, the textbook gives verbal instructions and explanations of how practical work is to be carried out and what learners are to experience through observation. This is a verbal representation of compounds (and one element) through a macroscopic experience. Although the explanations and steps for the macroscopic observations are verbal, the models also represent the sub-microscopic level of representation. This suggests that, if used in isolation, verbal explanation of chemical compounds on the molecular level would be inadequate. Because molecules are invisible to the naked eye, images (models or drawings) are necessary to help learners make sense of the explanations provided by the teacher and the textbook.
In this view, images in science textbooks bring abstract concepts ‘to life’ and foster the understanding of such concepts (Sujak and Daniel, 2017). The sub-microscopic aspects of matter and materials that can only be understood through imagination are simplified through drawings and models (Gilbert and Treagust, 2009). Mayeem et al. (2018) argue that learners tend to struggle with differentiating between mental models and physical models, assuming one is a direct replica of the other. Mayeem et al. (2018) further provide examples of how learners’ conceptions create misconceptions that affect how they name compounds and write down chemical formulae. Examples of misconceptions in this regard are seeing atoms as minute spheres or balls and molecules as ball-and-stick structures. Abstract concepts of compounds without the use of images to explain them contribute to misconceptions and confusion. Therefore, it is imperative that learners are assisted by their teachers to differentiate between the models that are presented by the teacher in the textbook and the models they are to imagine.
4.6 Symbols of chemical reactions must be understood through explanations and images
4.6.1 Symbolism
4.6.1.1 Symbols of individual compounds
Textbook A gives verbal explanations and models for the principle of conservation of atoms. This principle is also represented in Textbook A in symbol form. Examples used by the textbook to illustrate this principle are C + O2 → CO2 and 2H2 + O2 → 2H2O. An activity about symbols of individual compounds, making models of the reactants, and rearranging them to display the formation of products are provided in Textbook A.
4.6.1.2 Explanation and use of symbols of compounds in chemical reactions
Textbook A explains that no atoms are lost or gained in a chemical reaction. Hence, chemical equations are balanced. Furthermore, chemical equations are symbols of the elements and compounds involved. The symbols and the numbers that accompany them are there to illustrate that ‘no atoms are lost or gained in a chemical reaction; they are simply rearranged’. Understanding compounds using a symbolic representation of their formation, which is mathematical, was not considered. Having focussed on teaching learners to name and write chemical formulae in the beginning, the textbook completes the content on compounds by then focussing on those chemical formulae and how they interact in a chemical equation. Learners get to see, on a symbolic level, how the names and chemical formulae that they have learned can interact and produce other names and formulae. They get to see that chemical names, chemical formulae, images of elements and compounds, and representations of chemical reactions through chemical equations, all work together. Gilbert and Treagust (2009) comprehend that symbolic representation uses symbols to represent chemical phenomena and may be used in conjunction with both the macroscopic and the sub-microscopic levels of representation. In addition, symbolic representation assists the macroscopic level with quantitative representation, while giving qualitative support to a sub-microscopic representation of physical and chemical changes in chemical reactions (Gilbert and Treagust, 2009). This suggests that although the relationship between verbal explanations and images associated with those explanations is essential, the relationship would fall short when attempting to illustrate or contextualise the conservation of atoms during a chemical reaction. Moreover, verbal explanations and images must be supported by symbolic representations of what occurs in a chemical reaction. Learners need to understand that atoms can never be lost during a chemical reaction, although different substances form in the process.
4.7 Compounds are explained by connecting elements that compounds are made of, to reactions that form those compounds
4.7.1 What is a compound?
In Textbook B, compounds were defined as ‘pure substances formed by a chemical reaction between two or more different elements’. The book explains that compounds receive their names from the names of the elements they are made of. It discusses four steps for naming compounds, see below:
Step 1: Identify elements in the compound.
Step 2: Write down the name of the metal first.
Step 3: If the compound contains only two elements, name the second element and change the ending to ‘-ide’.
Step 4: If the compound contains three elements, one of which is oxygen, name the element that is not oxygen and change the ending to ‘-ate’.
The steps show that there are exceptions to the rules, and one exception discussed is ending a name with ‘-ide’ even though a compound is made of three elements. The example in textbook B is for the polyatomic ion, hydroxide (−OH), found in sodium hydroxide (NaOH).
Prefixes are also discussed for the naming of compounds, explaining that they indicate the number of atoms a specific compound contains. The examples given are carbon monoxide (CO), carbon dioxide (CO2), sulphur trioxide (SO3), and carbon tetrachloride (CCl4). Textbook B also provides common names of some compounds, such as water, ammonia, and vinegar.
4.7.2 Is that all there is to chemical reactions?
Textbook B refers to grade-8 work by mentioning that in grade 8, learners learn that ‘chemical substances can break apart and join together in a chemical reaction’. Also, reacting substances are called reactants, and those formed are called products. Data from Textbook B suggests that emphasis should be placed simultaneously on naming chemical compounds and writing down formulae. Based on their study, Mayeem et al. (2018) agree with the approach used in Textbook B, with reservations. They argue that learners may learn prescribed steps (like those in Textbook B) even without the ability to make sense of the steps they have learned. This suggests that although naming chemical compounds and writing chemical formulae support one another, the pedagogical factor plays a significant role in teaching the concepts, constructs, and principles. A teacher who lacks content and pedagogical knowledge may fail to explain the chemical reaction for learners’ understanding.
4.8 The concept of compounds must be practiced
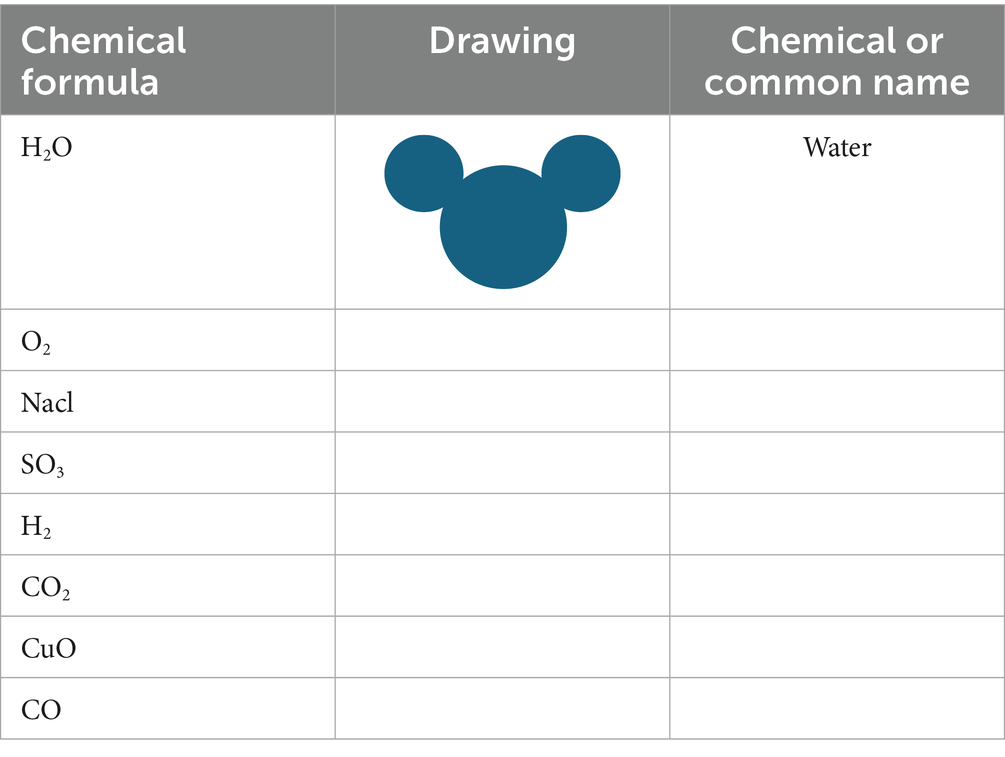
4.8.1 Draw your creation
An activity in Textbook B requires learners to make models of several compounds, then name them and draw picture diagrams of their created models. See Table 1 extract.
From Table 1, compounds can be named based on patterns and steps. It is therefore necessary for learners to practice naming compounds by drawing pictures. Seeing images in the table can assist learners in visualising the sub-microscopic level of chemical compounds. This helps draw pictures of what they visualise in their minds. It furthermore allows teachers to see whether relevant learning of compounds has occurred. Demirdöğen (2017) agrees that learners misinterpret and misunderstand concepts given by chemistry textbooks due to the lack of clarity in the verbal content of the textbooks. They further state that verbal representation of chemistry concepts in textbooks needs to be supported by other forms of representation to assist learners in understanding chemistry better.
In this view, images, whether pictures or models, enhance learners’ understanding of chemistry due to the abstract nature of chemistry and its concepts. Adoption of multiple representations by science textbooks, particularly in matter and materials, should be carefully thought through in line with the content and context of the topic or lesson to accommodate a variety of learners (Savinainen et al., 2015; Eilam and Gilbert, 2014). These representations should be descriptive and depictive (Schnotz, 2005, in Opfermann et al., 2017).
4.9 Chemical equations can be balanced if the symbols of compounds are known
4.9.1 Verbal
4.9.1.1 Balancing chemical equations
The purpose of balancing chemical equations is explained in Textbook B by referring to the atoms involved. The textbook explains that initial atoms react by rearranging into new combinations, and the products that form.
4.9.2 Visual
4.9.2.1 Drawings and models
Drawings are used by Textbook B to explain steps for balancing chemical equations. The textbook describes the process of balancing through seven steps. Activities for balancing follow the steps discussed. In the content, the textbook also states that models can be used to represent chemical reactions, although it does not show any models at that stage.
4.9.3 Verbal and visual collaboration
4.9.3.1 Symbolising explanations and models
From the text and activities in Textbook B, the explanations of chemical reactions and balancing equations are symbolised through chemical formulae. The models used to illustrate the rearrangement of atoms to form compounds are also represented in symbol form. Balancing a chemical reaction is better understood when corresponding images of the sub-microscopic aspects are present. Therefore, balancing chemical equations and employing drawings or models cannot be mutually exclusive. Balancing chemical equations also requires verbal explanations to aid the understanding of symbolic representations. Learners need to be provided with a combination of verbal, visual, and symbolic representations to make sense of the concepts of chemical compounds at different levels of chemical representation. This study agrees with Treagust and Tsui (2013) that verbal and visual representations complement each other’s function to improve the quality of learning and the learning process. Moreover, multiple levels of chemical representation in science textbooks are essential because they speak to different aspects of the teaching and learning process (Kapıcı and Savaşcı-Açıkalın, 2015). This suggests that the appropriate use of verbal and visual representations on the three levels of chemical representation ensures that learners’ acquisition of chemistry knowledge is more holistic. It allows learners to learn, understand, and remember chemistry concepts, constructs, and principles such that they can apply them when tasked to do so.
4.10 Explaining the concept of compounds by referring to grade-8 and using the grade-10 physical sciences curriculum
4.10.1 Grade-9 learners already know about the basics of compounds
The chapter on matter and materials in Textbook C began with a three-page revision of elements on the periodic table, taught in grade-8. It then continued directly to naming compounds related to grade-10 physical sciences.
4.10.2 Advanced naming of compounds
Textbook C explained the naming of compounds briefly as follows. ‘The name of an element changes at the end of a name where it forms part of a compound’. It then listed examples of name changes. Three examples are oxygen becomes oxide, sulphur becomes sulphide, and chlorine becomes chloride. One sentence mentions four examples of compounds in which such name changes exist. Examples of compounds were sodium chloride, iron sulphide, lithium bromide, and magnesium oxide. Textbook C continued to explain the naming of various oxides. That is: one oxygen is monoxide, and two oxygens are dioxide. The list is given up to five oxygens, which make a pentoxide. Another explanation for naming compounds is binding ratios and balancing numbers allocated according to the periodic table of elements. The textbook mentions that more detail will be given in grade-10, but for grade-9, eight valence numbers are explained.
The second-last part of naming in Textbook C involves polyatomic ions: their names, formulae, and binding possibilities. It is accompanied by an activity involving 40 compounds, 22 containing polyatomic ions. The last part of naming compounds is that of common names. A table of chemical formulae, chemical names, and common names is provided. Examples of the compounds in the table are sodium chloride, sodium hydroxide, and potassium hydroxide. An activity of 50 marks followed the common names of compounds. In the activity, 14 marks are allocated to binding possibilities, 20 marks to polyatomic compounds, and 16 marks to common names.
Textbook C struggles to keep up with the CAPS document when explaining compounds, as it partially addresses the prescribed content and affords much attention to chemical bonds, which form part of the physical sciences content in grade 10. Textbook C neglects several concepts and constructs, although it manages to provide additional information about compounds. Textbook C fails to adhere to CAPS prescriptions and guidelines and is therefore limited in representing the curriculum’s intent. In contrast, Stern and Roseman (2001) and Bhatti et al. (2017) argue that natural sciences textbooks should be drafted in such a way that the textual content correlates with the prescriptions and guidelines of the curriculum document to ensure that the outcomes that the curriculum set out to reach are achieved. Likewise, in a study, Polikoff (2015) asserts that textbooks must address all the content specified in the prescriptions and guidelines at the relevant grade levels and have no content beyond the scope provided by the curriculum document.
Based on the verbal content provided by Textbook C, explanations may produce misconceptions because they accompany incomplete basic content whose gaps the explanations cannot fill. The exclusion of the concept of what compounds are seems to imply that learners ought to already know chemical compounds beyond the prescriptions of the curriculum document. However, the limited verbal content may cause difficulties in understanding compounds on the sub-microscopic and symbolic levels, even if images or chemical equations are provided. Moreover, the focus given by Textbook C on grade-10 physical sciences content while leaving out critical grade-9 content defies the intent of the curriculum.
4.11 Images can miss the mark
4.11.1 Drawings – so unrelated
Data from Textbook C contains only three drawings of two elements and one compound, namely sodium (Na), chlorine (Cl), and water (H2O), respectively. The drawings explain binding ratios and binding possibilities for creating chemical formulae. The way the drawings are presented is misleading. For example, the binding ratios and possibilities of sodium and chlorine do not assist in explaining how the chemical formula of water, H2O, came to be. The scientific principle of atom conservation is also misrepresented by the drawings and not supported by the accompanying text. This study agrees with Bergqvist and Chang Rundgren (2017) that visual representations of the sub-microscopic level of chemistry in some senior-phase textbooks made it challenging for learners to comprehend chemical concepts and constructs. Nonetheless, Bergqvist and Chang Rundgren (2017) state that science teachers depend on textbooks for planning, curriculum content, and pedagogical guidance, despite the limitations in some textbooks.
4.12 Drawings of chemical equations gone wrong
4.12.1 Chemical symbols need context
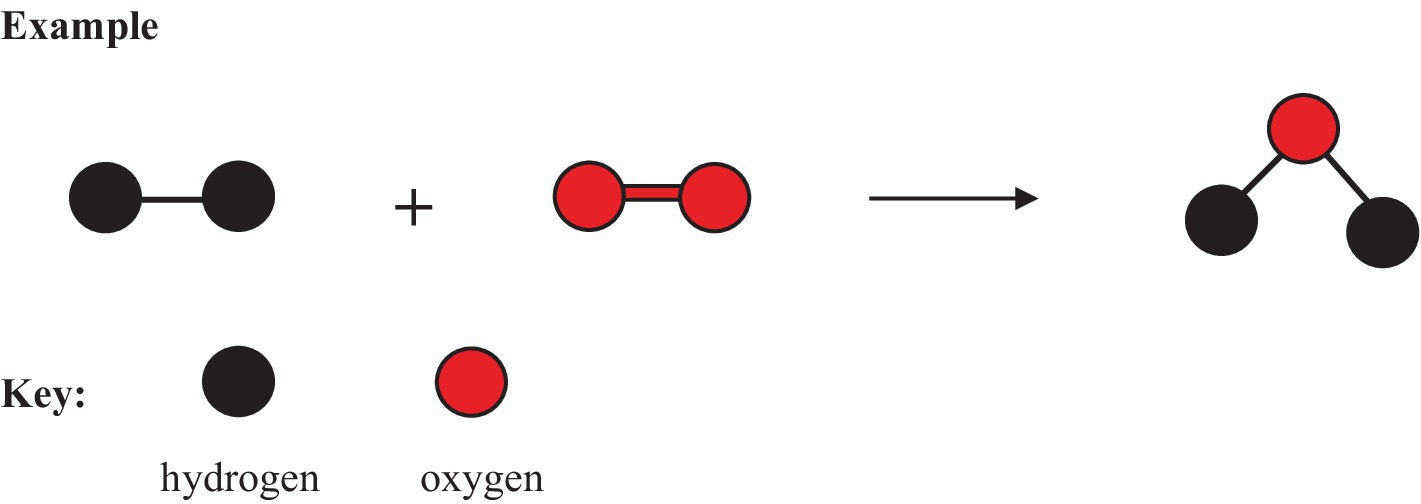
A myriad of chemical symbols representing compound formulae are given in Textbook C. Some are mentioned and others are referred to in the discussion above. Images such as drawings and models are necessary for understanding symbols used to balance chemical equations. Images of two reactants and their product are provided in Textbook C to illustrate why chemical equations should be balanced. The excerpt below shows the balancing of a chemical equation for the reaction between hydrogen (H2) and oxygen (O2) to form water (H2O) using drawings.
In Figure 3, the drawings and the explanations do not support the principle of conservation of atoms. In Textbook C, an attempt is made to represent chemical reactions verbally and visually using explanations and drawings, respectively. However, the verbal explanations in Textbook C do not provide the purpose for balancing chemical equations. Moreover, the explanation and drawings are both scientifically incorrect and misrepresent the principle of atom conservation, which governs the balancing of equations. The explanation and drawings do not reflect in any way on balancing equations. Where symbols are given, they do not correlate to signify the balancing of equations. The symbols are unrelated and can therefore not be combined to form an equation that could be balanced. No assessment activities are found in Textbook C for balancing chemical equations.
Verbal representations in textbooks clarify aspects that must be addressed in the content, without which visual representation will make no sense (Sadoski and Paivio, 2001). Complete understanding of chemical concepts, constructs, and principles at any grade level cannot be achieved without blending verbal, visual, and verbal and visual representations on the macroscopic, sub-microscopic, and symbolic levels (Khine and Khine, 2013). Misrepresentation of scientific knowledge and inaccuracies in some science textbooks influence learners’ ideas about science and scientific concepts (Devetak and Vogrinc, 2013).
5 Conclusion
In this study, we conclude that there are discrepancies in verbal and visual representations of natural sciences matter, and material concepts, constructs, and principles in the selected textbooks are not clearly outlined as represented in the CAPS policy document. Some textbooks struggle to present verbal and visual representations to help explain abstract chemical reaction concepts, constructs, and principles as represented in the CAPS document. Despite this, many booksellers individually and in groups continue to develop verbal and nonverbal natural sciences textbooks that do not align with the verbal and visual representations on the CAPS document. One asks if continuing with the misalignment of matter and material concepts, constructs, and principles is worth it. The arguments may warrant changing the development of science textbooks.
It is therefore fair to claim that the visual and verbal representations in three selected South African natural science textbooks need to be reworked focussing on concepts, constructs, and principles of natural science to meet the requirements of the CAPS policy document. This could help natural sciences teachers make sense of and explain the concepts, constructs, and principles of compounds of matter and material when teaching grade-9 learners.
Author contributions
SM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MR: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors acknowledge the university of the Free State for paying the page fees for the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anwar, R. B., and Rahmawati, D. (2017). Symbolic and verbal representation process in solving mathematics problems based on Polya’s stages. Int. Educ. Stud. 10, 20–28. doi: 10.5539/ies.v10n10p20
Bergqvist, A., and Chang Rundgren, S. N. (2017). The influence of textbooks on teachers’ knowledge of chemical bonding representations relative to students’ difficulties understanding. Res. Sci. Technol. Educ. 35, 215–237. doi: 10.1080/02635143.2017.1295934
Bhatti, A. J., Khurshid, K., and Ahmad, G. (2017). Curriculum Alignment: An Analysis of the Textbook Content. Pak. J. Soc. Sci, 37, 30–43.
Boru, T.. (2018). Chapter five research design and methodology. 5.1. Introduction. PhD Thesis, UNISA.
Bowen, G. (2009). Document analysis as a qualitative research method article. Qual. Res. J. 9, 27–40. doi: 10.3316/QRJ0902027
Creswell, J. W., and Creswell, J. D. (2018). Research design: Qualitative, quantitative, and mixed methods approaches. 5th Edn. California: SAGE.
Demirdöğen, B. (2017). Examination of chemical representations in Turkish high school chemistry textbooks. J. Balt. Sci. Educ. 16, 472–499. doi: 10.33225/jbse/17.16.472
Department of Basic Education (2011) Curriculum and assessment policy statement (CAPS): natural sciences senior phase final ISBN: 978–1–4315-0528-9. Available online at: http://www.thutong.doe.gov.za
Devetak, I., and Vogrinc, J. (2013). “The criteria for evaluating the quality of the science textbooks” in Critical analysis of science textbooks: Evaluating instructional effectiveness. ed. M. S. Khine (Dordrecht: Springer), 3–16.
Eilam, B. and & Gilbert, J. K., “The significance of visual Representations in the teaching of science. In Science teachers’ use of visual Representations. Ed. B. Eilam and J. K. Gilbert Cham: Springer, (2014), 3–28.
Gilbert, J. K., and Treagust, D. (2009). “Introduction: macro, submicro-and symbolic representations and the relationship between them: key models in chemical education” in Multiple Representations in chemical education. eds. J. K. Gilbert and D. Treagust (Dordrecht, The Netherlands: Springer), 4–6.
Johnstone, A. H. (1993). The development of chemistry teaching: a changing response to changing demand. J. Chem. Educ. 70, 701–705. doi: 10.1021/ed070p701
Karásková, N., Doležal, R., Maltsevskaya, N., and Kolář, K. (2019). Didactic capacity of selected Czech and Russian organic chemistry textbooks. Chemistry-Didactics-Ecology-Metrology – Sciendo, 24, 61–76. [online]. doi: 10.2478/cdem-2019-0005
Kapıcı, H. Ö., and Savaşcı-Açıkalın, F. (2015). Examination of visuals about the particulate nature of matter in Turkish middle school science textbooks. Chem. Educ. Res. Pract. 16, 518–536. doi: 10.1039/c5rp00032g
Kembhavi, A., Salvato, M., Kolve, E., Seo, M., Hajishirzi, H., and Farhadi, A., (2016) “A diagram is worth a dozen images,” in Computer vision – ECCV 2016, ECCV 2016: Cham; conference proceeding ed, Bastian Leibe, Jiri Matas, Nicu Sebe, and max welling, computer vision – ECCV 2016. ECCV 2016: Lecture notes in computer science, vol 9908. Springer, Cham.
Khine, M. S., The criteria for evaluating the quality of the science textbooks. In critical analysis of science textbooks: Evaluating instructional effectiveness. Khine, M. S., Dordrecht: Springer, (2013), 303–310.
Kiefer, M., and Pulvermüller, F. (2012). Conceptual representations in mind and brain: theoretical developments, current evidence, and future directions. Cortex 48, 805–825. doi: 10.1016/j.cortex.2011.04.006
Lemke, J. L. (1998). “Multiplying meaning: visual and verbal semiotics in scientific text” in Reading science: Critical and functional perspectives on discourses of science. eds. J. R. Martin and R. Vell (New York: Routledge), 87–113.
Lewthwaite, B. (2014). Thinking about practical work in chemistry: teachers' considerations of selected practices for the macroscopic experience. Chem. Educ. Res. Pract. 15, 35–46. doi: 10.1039/C3RP00122A
Mayeem, B. P., Naa, A. M., and Adjei, A. (2018). Enhancing senior high school students understanding of chemical formulae and nomenclature of inorganic compounds by the use of improvised conceptual models. J. Educ. Pract. 9, 22–45.
McDonald, C. V., and Abd-El-Khalick, F. (2017). Representations of nature of science in school science textbooks. New York: Routledge.
Muspratt, S., and Freebody, P. (2013). “Understanding the disciplines of science: Analysing the language of science textbooks” in Critical analysis of science textbooks: Evaluating instructional effectiveness. ed. M. S. Khine (Dordrecht, Netherlands: Springer), 33–59.
Opfermann, M., Schmeck, A., and Fischer, H. E. (2017). “Multiple Representations in physics and science education – why should we use them?” in Multiple Representations in physics education. eds. D. F. Treagust, R. Duit, and H. E. Fischer (Gewerbestrasse, Cham: Springer), 13.
Paivio, A., and Representations, M. (1990). A dual coding approach. New York: Oxford University Press, 53–95.
Peterson, M. O. (2016). Schemes for Integrating Text and Image in the Science Textbook: Effects on Comprehension and Situational Interest. International Journal of Environmental & Science Education, 11, 1365–1385.
Polikoff, M. S. (2015). How well aligned are textbooks to the common Core standards in mathematics? Am. Educ. Res. J. 52, 1185–1211. doi: 10.3102/0002831215584435
Prinsloo, C. H., Harvey, J., Mosimege, M., Beku, U., Juan, A., Hannan, S., et al. (2016). TIMSS item diagnostic report: South Africa grade 9 science [online]. Pretoria: Department of Basic Education.
Sadoski, M., and Paivio, A. (2001). Imagery and text: A dual coding theory of reading and writing. London: Lawrence Erlbaum Associates.
Savinainen, A., Mäkynen, A., Nieminen, P., and Viiri, J. (2015). The effect of using a visual representation tool in a teaching-learning sequence for teaching Newton's third law. Res. Sci. Educ. 47, 119–135. doi: 10.1007/s11165-015-9492-8
Stern, L., and Roseman, J. E. (2001). Textbook alignment. Sci. Teach. 47, 47–70. doi: 10.1002/tea.20305
Sujak, K. B., and Daniel, E. G. S. (2017). Understanding of macroscopic, microscopic and symbolic Representations among form four students in solving stoichiometric problems. Malaysian Online J. Educ. Sci. 5, 83–96.
Treagust, D. A., and Tsui, C.-Y. (2013). “Introduction to multiple Representations: their importance in biology and biological education” in Multiple Representations in biological education. eds. D. A. Treagust and C.-Y. Tsui (Perth: Springer), 3–18.
Tsui, C.-Y. (2003). Teaching and learning genetics with multiple representations, (Doctoral thesis, Curtin University of Technology, 2003). Bentley, Perth, Western Australia.
Upahi, J. E., and Ramnarain, U. (2018). Representations of chemical phenomena in secondary school chemistry textbooks. Chem. Educ. Res. Pract. 20, 146–159. doi: 10.1039/C8RP00191J
van den Ham, A.-K., and Heinze, A. (2018). Does the textbook matter? Longitudinal effects of textbook choice on primary school students’ achievement in mathematics. Stud. Educ. Eval. 59, 133–140. doi: 10.1016/j.stueduc.2018.07.005
Wu, H.-K., and Puntambekar, S. (2012). Pedagogical affordances of multiple external Representations in scientific processes. J. Sci. Educ. Technol. 21, 754–767. doi: 10.1007/s10956-011-9363-7
Yang, L., and Treagust, D. (2013). “Content analysis of diagrams in secondary school science textbooks” in Critical analysis of science textbooks: Evaluating instructional effectiveness. ed. M. S. Khine (Dordrecht, Netherlands: Springer), 287–300.
Keywords: natural sciences, limitations in textbooks, multiple representations, poor performance, verbal and visual representations
Citation: Mofolo SBO and Rabaza M (2025) Comparing verbal and visual representations of grade-9 natural sciences concepts, constructs, and principles of matter and materials in three textbooks and the CAPS policy document. Front. Educ. 10:1570536. doi: 10.3389/feduc.2025.1570536
Edited by:
Stephen Woodcock, University of Technology Sydney, AustraliaReviewed by:
Fred Kofi Boateng, University of Ghana, GhanaOscar Eybers, University of Pretoria, South Africa
Copyright © 2025 Mofolo and Rabaza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Msebenzi Rabaza, cmFiYXphbUB1ZnMuYWMuemE=
 Serapelo Boipelo Oreeditse Mofolo
Serapelo Boipelo Oreeditse Mofolo Msebenzi Rabaza
Msebenzi Rabaza