- 1Leni and Peter W. May Department of Orthopaedic Surgery, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 2Raquel and Jaime Gilinski Department of Obstetrics, Gynecology and Reproductive Science, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 3Jack and Lucy Clark Department of Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Functional hypothalamic amenorrhea (FHA) is a reversible neuroendocrine condition prevalent among adolescent female athletes that often results from energy deficiency, reflecting an imbalance between energy intake and expenditure due to factors such as disordered eating, psychological stress, and excessive physical activity. By disrupting hypothalamic-pituitary-ovarian (HPO) axis signaling, FHA in adolescence typically leads to hypoestrogenism and subsequent impairment of bone mineral accrual during a crucial period of skeletal development. This review synthesizes current evidence on the pathophysiology of FHA in relation to bone health, emphasizing the impact of altered estrogen, IGF-1, leptin, and cortisol levels. We further summarize the main risk factors of FHA and examine their effect on reduced bone mineral density (BMD), compromised bone microarchitecture, and increased fracture risk. Studies emphasize the high risk of osteopenia, osteoporosis, and stress fractures in female athletes with FHA. Diagnosis of FHA requires exclusion of organic pathology and a multidisciplinary evaluation of orthopedic, nutritional, endocrinological, psychological, and exercise-related contributors. Evidence-based management prioritizes lifestyle modification, nutritional rehabilitation, and psychological support, with transdermal estrogen therapy as a promising treatment for refractory cases. Ongoing controversies include the limited skeletal benefits of oral contraceptives versus growing evidence for transdermal estrogen, and the paradoxical effects of exercise as both protective and harmful under energy-deficient conditions. Additionally, persistent clinical challenges are highlighted, such as underdiagnosis of menstrual dysfunction and lasting microarchitectural deficits despite weight restoration. Ultimately, early identification and intervention are essential to optimize long-term skeletal and reproductive outcomes for adolescent female athletes affected by FHA.
Introduction
Functional Hypothalamic Amenorrhea (FHA) is the cessation of menses due to suppression or insufficiency of the hypothalamic-pituitary-ovarian (HPO) axis in the absence of any identifiable organic causes (1). FHA results from low energy availability, an imbalance between energy intake and expenditure, which may stem from factors such as anorexia nervosa (AN), excessive exercise, weight loss, or psychological stress (2). Adolescent female athletes are particularly vulnerable to developing FHA due to high physical and psychological demands as well as decreased overall energy availability. In fact, studies indicate that approximately 54% of adolescent athletes under 18 years old experience menstrual dysfunctions such as FHA as opposed to 21% in the equivalent non-athlete population (3).
Adolescence and young adulthood are critical periods for optimizing long-term bone health, underscoring the importance of proper nutrition and healthy hormonal regulation in this time period. Since estrogen plays a crucial role in maintaining bone health, hypoestrogenism in FHA due to HPO axis dysregulation can have a detrimental effect (4). Adequate nutrition is required to maintain energy balance as well as hormonal and long-term bone health in female athletes who have heightened energy demands (5). When FHA occurs during adolescence, it can interfere with peak bone mass acquisition, leading to persistent deficits in bone density which can double the risk of fractures later in life (6). This disruption of skeletal homeostasis is evident through reductions in bone mineral density (BMD). Lu et al. shows that approximately 15% of patients with FHA (average age: 24.64 ± 6.02 years) experience low bone mass, which is correlated with hypoestrogenism and weight loss (7). Defined by the American College of Sports Medicine in 1992, the “female athlete triad” describes this phenomenon, highlighting the interplay between low energy availability and bone health (8). Ultimately, this decrease in BMD increases the risk of osteopenia and osteoporosis, heightening fracture susceptibility (9). The concept of Relative Energy Deficiency in Sport (RED-S), introduced and most recently updated in the 2023 International Olympic Committee (IOC) Consensus Statement, expands upon the Female Athlete Triad by recognizing the broader multisystem consequences of low energy availability across both sexes and all levels of sport participation. RED-S emphasizes that inadequate energy intake relative to expenditure affects not only reproductive and skeletal health, but also cardiovascular, metabolic, and psychological function (10, 11). Given these risks, providers must use multidisciplinary approaches to identify bone health issues in female athletes during key growth periods.
This review aims to define the effects of functional hypothalamic amenorrhea (FHA) on female bone health, emphasizing timely recognition and referral. This review will provide evidence-based recommendations for physicians and healthcare providers to address underlying hormonal disruptions contributing to FHA. Additionally, we include a discussion of current and emerging treatment strategies, highlight gaps in the literature, and identify areas of future research for FHA management in young female athletes.
Pathophysiology
Overview of bone development in adolescent populations–hormonal perspectives
Bone accrual during adolescence is critical, with up to 90% of peak bone mass achieved by the end of puberty (12). Adolescence represents a time with high bone-turnover, where bone formation and resorption levels peak before decreasing to adult levels in late puberty. These metabolic processes throughout puberty are intricately regulated by the balance of gonadal hormones, growth hormones (GH), and insulin-like growth factor-1 (IGF-1) (13).
Estrogen
Estrogens, particularly estradiol, play pivotal roles in regulating bone turnover and growth plate closure (14). Estradiol has a biphasic effect on bone growth, serving distinct functions at different stages of adolescence (15). In early adolescence, it stimulates longitudinal bone growth by upregulating osteoblast activity and promoting osteoclast apoptosis (16). As estradiol levels rise in late adolescence, its role shifts toward promoting epiphyseal plate closure. Estradiol acts directly on the growth plate chondrocytes to accelerate their maturation and increase the expression of estrogen receptor-α (ERα), causing cessation of growth plate cartilage production (17).
The HPO axis is a complex endocrine system that controls the development and regulation of the female reproductive system (18). First, in the hypothalamus, neurosecretory cells secrete gonadotropin-releasing hormone (GnRH) in a pulsatile manner (19). This stimulates gonadotropes in the anterior pituitary gland to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which act on the ovaries to stimulate follicular development, ovulation, corpus luteum formation, and the production of estrogen and progesterone (20). These ovarian hormones then exert feedback control on the hypothalamus and pituitary to regulate the cycle. This process becomes fully active at the time of puberty.
In FHA, low energy availability resulting from inadequate caloric intake relative to energy expenditure, whether due to risk factors such as restrictive eating, high training load, or psychological stress, suppresses GnRH pulsatility, thereby disrupting the HPO axis (21). This disturbance leads to reduced LH and FSH secretion which results in decreased ovarian estrogen and progesterone production, anovulation, and amenorrhea. Estrogen deficiency accelerates bone resorption by impairing osteoblast function and removing estrogen’s inhibitory effect on osteoclasts, creating an imbalance in bone remodeling that favors net bone loss (22). Furthermore, Kisspeptin, an endogenous hormone that stimulates GnRH secretion, is modulated by signals of energy status such as leptin, ghrelin, and insulin (23). In a female athlete with depleted energy stores, Kisspeptin secretion may be reduced, further causing dysregulation of the HPO axis and FHA (24).
Growth hormone
In addition to estrogen, GH also plays a direct role in growth plate maturation (25). IGF-1, primarily stimulated by GH in the liver, enhances osteoblast activity and promotes chondrocyte proliferation and differentiation at the growth plates, leading to longitudinal bone growth (26). Throughout adolescence, GH and IGF-1 work synergistically with estradiol to regulate epiphyseal plate closure (27). However, FHA leads to suppressed GH-IGF signaling, primarily due to reduced hepatic IGF-1 production despite normal or elevated GH levels, indicating GH resistance. This state of GH resistance exacerbates bone loss by impairing osteoblast function and reducing bone matrix production, ultimately compromising peak bone mass acquisition. The concurrent hypoestrogenism caused by FHA further amplifies bone resorption, reducing BMD. This disruption of the GH-IGF axis increases the risk of osteopenia, osteoporosis, and stress fractures, with lasting consequences for skeletal integrity (2).
Leptin, cortisol, and thyroid hormones
Leptin is a hormone released by adipose tissue that regulates energy balance and appetite, and plays a key role in the hypothalamic regulation of reproductive function (28). Low leptin levels signal to the brain that energy stores are insufficient, leading to the suppression of the hypothalamic-pituitary-gonadal axis and reduced estrogen production (21). In female athletes with low body fat, leptin levels are reduced and thus contribute to FHA. Additionally, elevated cortisol and thyroid levels, both commonly seen in FHA due to chronic stress or energy deficits, contribute to bone loss by increasing osteoclastic activity and inhibiting osteoblast function (29). This hormonal excess in FHA leads to lower bone mineral density and an increased risk of fractures (30).
Risk factors for functional hypothalamic amenorrhea
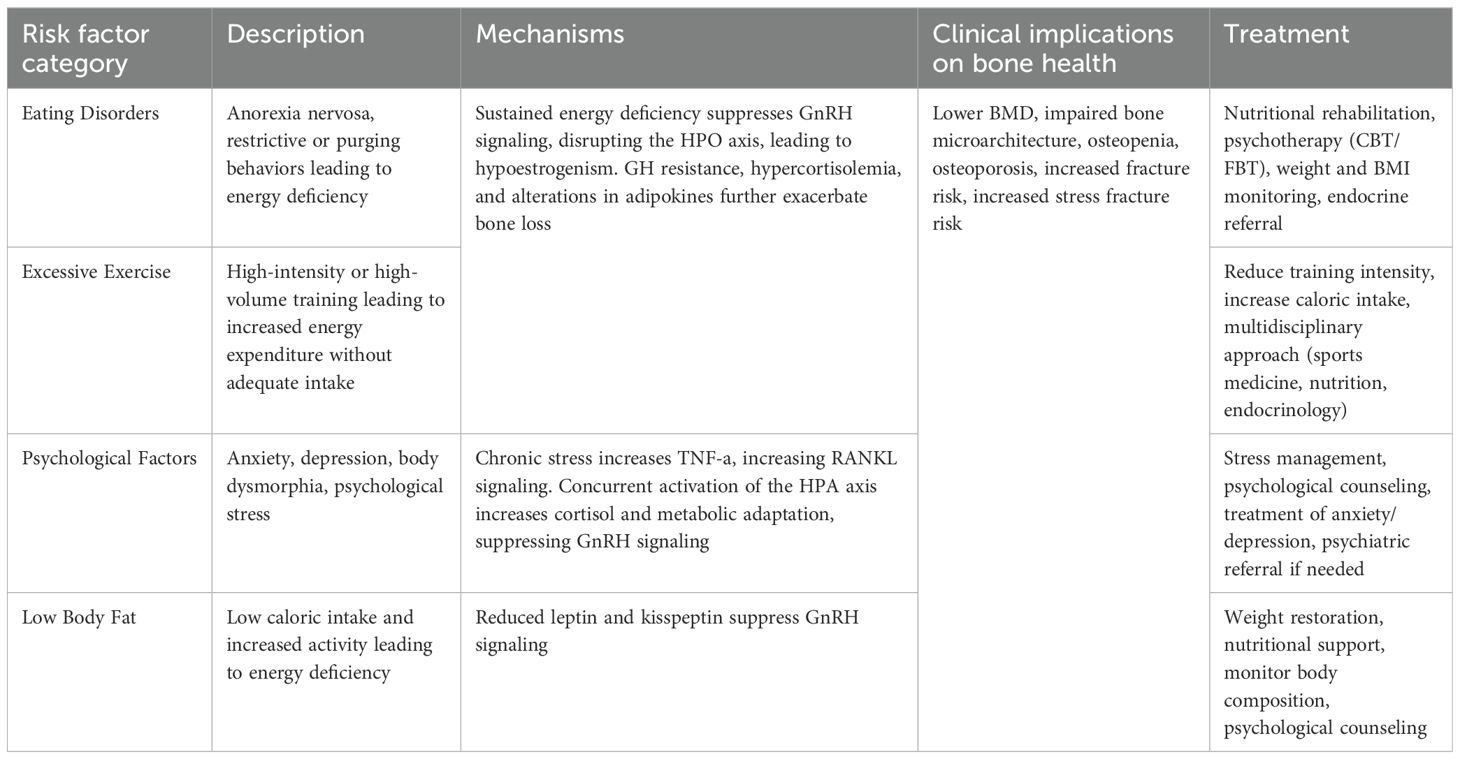
FHA in adolescent females is a multifactorial condition that arises from an intricate interplay of physiological, psychological, and behavioral stressors (Table 1). The primary contributors to FHA include disordered eating patterns, excessive exercise, psychological stress, and low body fat, all of which can have profound implications for bone health and overall well-being (31).
Eating disorders and anorexia nervosa
One of the fundamental risk factors for FHA is an imbalance between energy intake and expenditure; adolescent females who engage in restrictive eating behaviors, such as anorexia nervosa (AN), are at high risk (32). AN is defined by severe restriction of caloric and nutrient intake relative to requirements, intense fear of gaining weight, and distorted body image, leading to chronic energy deficiency and low weight (<18.5 BMI) (33). This sustained caloric and energy deficit induces a survival mechanism whereby the body aims to conserve energy during starvation by shutting down non-essential functions, including reproduction. Therefore, patients with AN are prone to having suppressed GnRH pulsatility and a disrupted HPO axis, thus leading to hypoestrogenism. Additional hormonal changes in AN, such as growth hormone resistance, hypercortisolemia, and alterations in adipokines such as low leptin levels, further exacerbate bone loss (21).
Studies support this by indicating that young women with eating disorders experience significant skeletal deficits, including reduced bone accretion and increased risk of fractures. Faje et al. reported that adolescent females ages 14–22 years old with AN had lower areal bone mineral density at the lumbar spine and hip as well as impaired cortical and trabecular microarchitecture, indicating lower bone strength (34). Moreover, a long-term population-based cohort study of 193 young women who acquired AN during puberty observed a three-fold higher risk of fractures as far as 38 years after diagnosis, and a 57% cumulative incidence risk of any fracture (35).
Furthermore, Kandemir et al. illustrate that both female athletes with AN and those with secondary amenorrhea exhibit lower BMD compared to healthy controls, with more severe bone defects observed in AN (36). The study also highlights that AN patients have greater impairments in trabecular bone microarchitecture, including lower trabecular number and thinner trabeculae. Additionally, AN patients exhibited lower bone formation markers, further indicating diminished overall bone health. Recent research underscores that female athletes with eating disorders should meet established weight and health criteria before resuming full training regimens to mitigate the risk of severe skeletal consequences (37).
Excessive exercise
The number of adolescent girls participating in competitive sports has increased substantially over the past several decades, largely due to the implementation of Title IX in 1972 (38). While this has led to numerous physical and psychological benefits, it has also been accompanied by a rise in cases of the female athlete triad, a syndrome which encompasses one or more interrelated conditions including low energy availability, menstrual dysfunction, and low bone mineral density (BMD) (39–42). In fact, one study by Skorseth et al. showed that among 38 high school distance runners, 36% had low energy availability, 54% had menstrual abnormalities, and 16% had low bone mineral density (BMD) (43).
Low energy availability resulting from high training volumes or intensities without adequate caloric intake plays a central role in disrupting the HPO axis. Exercise alone does not cause FHA; rather, it is the imbalance between energy intake and expenditure that leads to hypothalamic suppression, estrogen deficiency, and menstrual irregularities (44–46). A retrospective study by Ackerman et al. of 175 young athletes ages 14–25 found that athletes with amenorrhea have four times the lifetime fracture risk compared to non-athletes (37). Stress fractures are particularly common in this population, occurring in approximately 32% of athletes with amenorrhea compared to only 5.9% in eumenorrheic athletes, and 0% in non-amenorrheic athletes. Furthermore, bone microarchitecture was more negatively affected in amenorrheic athletes, especially in those who sustained multiple stress fractures highlighting the detrimental combination of excess exercise with amenorrhea.
Recognizing these risks, the American College of Sports Medicine (ACSM) recommends screening for the female athlete triad and other underlying medical issues in any athlete with a history of six months or more of amenorrhea or oligomenorrhea (40). Screening includes a thorough evaluation of menstrual change, disordered eating patterns, cardiac arrhythmias such as bradycardia, weight change, depression, or stress fracture. Early intervention is crucial, as persistent energy deficiency and menstrual suppression during adolescence can have lasting consequences, increasing the risk of osteoporosis and fragility fractures later in life. Given the interplay amongst exercise, energy availability, and skeletal health, a multidisciplinary approach that includes nutrition, endocrinology, and sports medicine is essential for preventing and managing FHA in young female athletes (47).
Psychological factors
Adolescence represents a formative stage for the development of body image and self-perception (47). During this time, individuals may be particularly vulnerable to negative body image, weight-related bullying, and excessive pressure to achieve athletic or academic excellence. These factors have been implicated in the onset and perpetuation of FHA (48).
Psychological distress is often intertwined with maladaptive behaviors such as excessive exercise and restrictive eating, which are frequently employed as coping mechanisms. However, rather than alleviating stress, these behaviors often serve as amplifiers, perpetuating a cycle of anxiety and physiological dysregulation (49). Mood disorders, such as anxiety and depression, are frequently associated with amenorrhea, and behaviors like hyper-exercise and restrictive eating may reflect underlying obsessive or anxious tendencies (50, 51). A meta-analysis conducted by Morrison et al. found that psychological factors associated with FHA include depression and preoccupied attitudes surrounding eating, specifically driven by a desire for thinness (21). Women with FHA demonstrated higher levels of anxiety, sleep disorders, dysfunctional attitudes, and alexithymia compared to women without FHA. Given this interplay, a comprehensive psychological assessment is essential, and in cases where these symptoms are identified, referral to therapeutic and psychiatric care is recommended (52).
Beyond its impact on menstrual function, psychological stress exerts direct effects on skeletal health. Chronic stress is associated with low-grade inflammation, as evidenced by increased levels of proinflammatory markers such as tumor necrosis factor-alpha (TNF-α), which upregulate receptor activator nuclear factor kappa-B ligand (RANKL) signaling and promote bone resorption (21). Additionally, stress-induced hyperactivation of the sympathetic nervous system leads to elevated noradrenaline levels, which negatively impact bone metabolism (53). Osteoclasts and osteoblasts express adrenergic receptors, and studies suggest that stress-induced bone loss can be mitigated with beta-adrenergic antagonists such as propranolol (54). These findings highlight a direct biological link between psychological stress and skeletal deterioration.
Furthermore, the hypothalamic-pituitary-adrenal (HPA) axis plays a pivotal role in the intersection of psychological stress, energy metabolism, and reproductive suppression (55). Psychological stressors, whether external (i.e., academic pressure, familial pressure, athletic demands) or internal (i.e., fear of weight gain, low self-esteem, obsessive thinking, emotional distress, perfectionism), activate the HPA axis, leading to increased cortisol secretion and metabolic adaptations that ultimately suppress GnRH pulsatility (56). Both human and animal studies suggest that energy imbalance sensitizes the HPO axis to psychological stress, heightening its vulnerability to menstrual disturbances (57, 58). Importantly, not only actual stressors but also the perception or anticipation of stress can elicit similar endocrine disruptors, reinforcing the complex bidirectional relationship between psychological and metabolic stress in FHA (59).
Addressing the psychological underpinnings of FHA is crucial not only for restoring menstrual function but also for safeguarding long-term bone health in young female athletes.
Low body fat
Low body fat is an independent yet closely intertwined risk factor for FHA in adolescent females. Adequate fat mass is essential for normal pubertal development and menstrual regulation, as adipose tissue functions not only as a reservoir for energy but also as an active endocrine organ (21). Specifically, adipose tissue produces the hormone leptin, which serves as a gauge for energy reserves and guides the central nervous system to balance food intake and energy expenditure accordingly (60).
Evidence suggests that the minimum level of body fat percentage to initiate menarche is approximately 20%, and between 17-22% is required to maintain normal menstruation (61). Latzer et al. reported that a total body fat percentage of 21.2% had the highest discriminative ability for the resumption of menses in adolescents with FHA, with a sensitivity of 88% and specificity of 85% (62).
In the context of athletics, aesthetic sports (i.e. gymnastics, cheerleading, ballet, figure skating, rhythmic gymnastics, distance running), in which leanness may confer an advantage, have a particularly high prevalence of low body fat and associated exercise-induced amenorrhea (46). A cross-sectional study conducted by Amoruso et al. of 288 female athletes under age 25 found that 41.1% of athletes practicing in aesthetic sports reported menstrual irregularities (63). Compared to athletes of non-aesthetic sports, training for aesthetic sports also significantly increased the risk of developing menstrual irregularities (64, 65). These findings demonstrate that aside from being a byproduct of disordered eating or excessive exercise, low body fat poses as a distinct physiological risk factor that can independently disrupt reproductive function through disordered metabolic and neuroendocrine signaling. The resulting hormonal changes favor bone resorption over formation, leading to reductions in bone mineral density and compromised bone microarchitecture.
Diagnosis
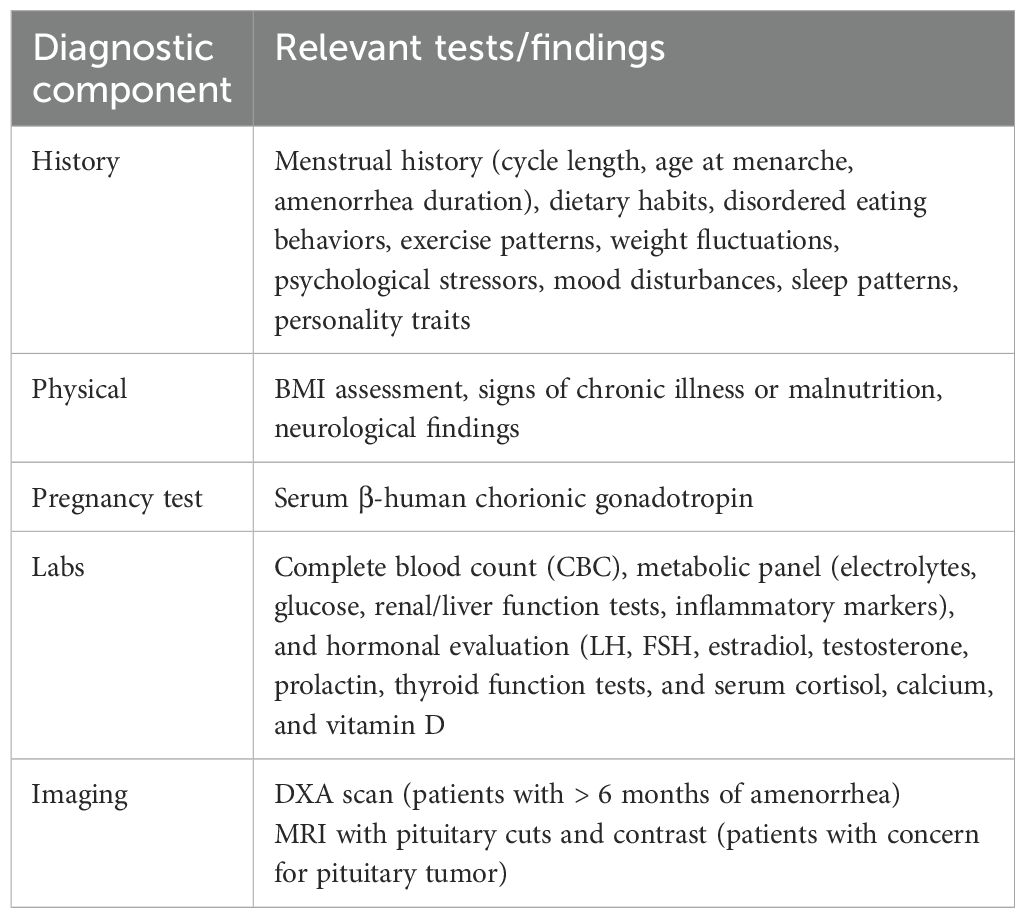
The diagnosis of FHA is one of exclusion, requiring a comprehensive evaluation to rule out anatomic or organic causes of menstrual dysfunction (Table 2). Clinicians should consider FHA in adolescents and women with persistent menstrual cycle intervals exceeding 45 days or those experiencing absent menses for three months or more (47). A thorough clinical assessment begins with a detailed personal history, focusing on dietary habits, disordered eating behaviors, exercise patterns, weight fluctuations, stressors, mood disturbances, sleep patterns, and personality traits such as perfectionism and a high need for social approval. Additionally, a family history of eating disorders, psychiatric diagnoses, reproductive disorders, or menstrual irregularities should be obtained to identify potential genetic or environmental predispositions (1).
A physical examination is essential to assess for organic causes of amenorrhea. Pregnancy must be excluded through a β-human chorionic gonadotropin test. Laboratory tests should include a complete blood count and metabolic panel, including electrolytes, glucose, renal and liver function tests, as well as inflammatory markers (i.e., erythrocyte sedimentation rate or C-reactive protein) to rule out systemic illness (47, 66, 67). Hormonal evaluation should assess levels of LH, FSH, estradiol, testosterone, prolactin, thyroid function tests, and serum cortisol, calcium, and vitamin D to rule out other endocrine imbalances contributing to menstrual dysfunction (40, 68–70).
Given the significant impact of FHA on bone health, clinicians are advised to obtain a baseline BMD measurement via dual-energy X-ray absorptiometry (DXA) in patients with six or more months of active amenorrhea (47, 71, 72). In cases where there is a history or suspicion of severe nutritional deficiency, significant energy deficit, or skeletal fragility, DXA screening may be performed earlier. MRI with pituitary cuts and contrast is recommended in adolescents with presumed FHA and a history of persistent or severe headaches; persistent vomiting that is not self-induced; changes to vision, thirst, or urination; neurologic signs; or any other clinical indicators that suggest imbalances in pituitary hormone in order to identify pituitary or other tumors (73).
In summary, in adolescent female athletes, FHA warrants careful clinical attention, as menstrual irregularities are often overlooked or misinterpreted as benign consequences of intense training. However, persistent amenorrhea reflects underlying energy deficiency and significantly increases the risk of impaired peak bone mass, osteopenia, and stress fractures. Due to the complex interplay between physiological and psychological factors, a multidisciplinary approach involving endocrinology, gynecology, sports medicine, nutrition, and mental health professionals is recommended to create a comprehensive assessment and management plan. It is vital that physicians are prepared to diagnose and comprehensively treat FHA, especially in critical periods of skeletal development.
Treatment
Current standard of care
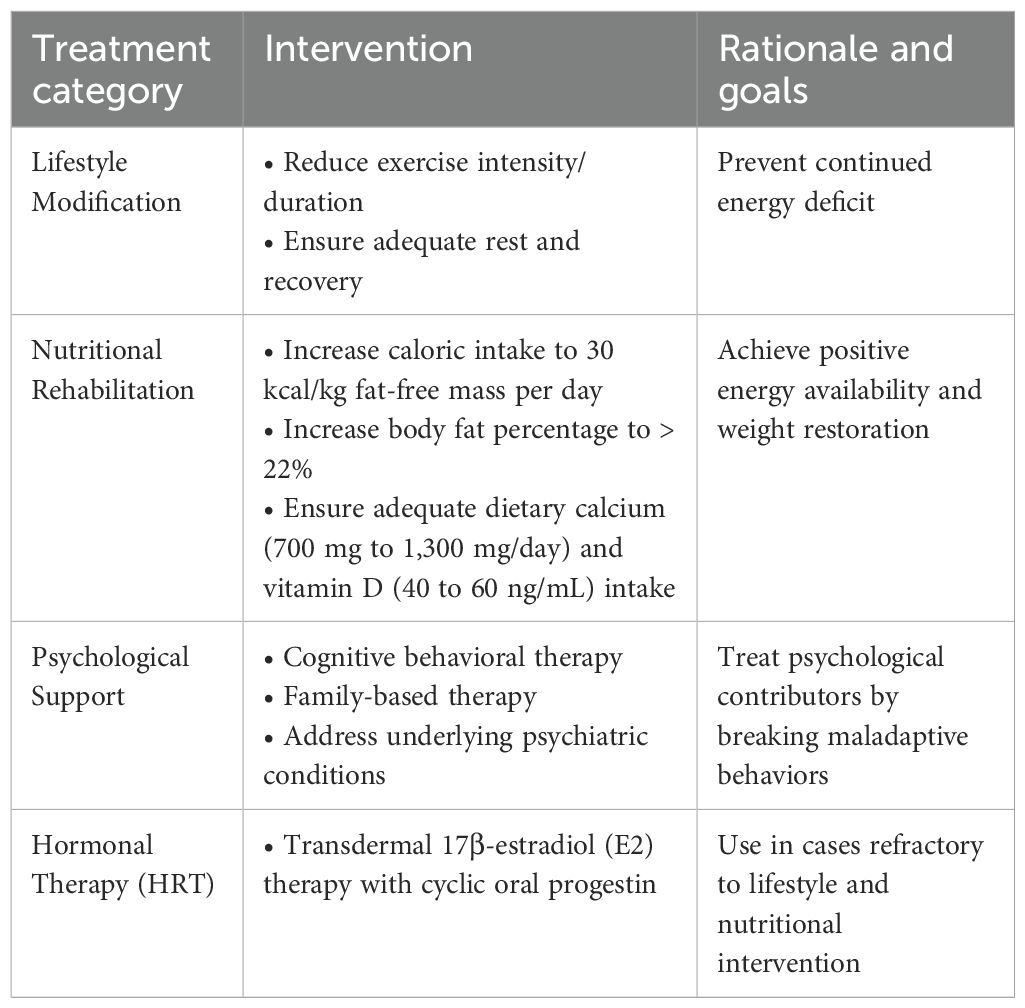
Once the diagnosis of FHA is established, treatment should focus on addressing the underlying causes, such as energy deficiency, excessive exercise, and psychological stressors, rather than simply restoring menstrual cycles with pharmacologic interventions (Table 3) (47).
The cornerstone of FHA management is lifestyle modification. Increasing energy availability through adequate caloric intake and reducing excessive exercise are critical first steps. Nutritional rehabilitation, ideally guided by a dietitian specializing in sports nutrition or eating disorders, is essential to correcting energy deficits. This includes increasing caloric intake to reach an energy availability threshold of 30 kcal/kg fat-free mass per day. An increase in body fat percentage above 22% may be required to restore menstrual function (74). Patients should additionally receive dietary calcium (700 mg to 1,300 mg/day) and vitamin D supplementation (40 to 60 ng/mL) (75–77). A gradual reduction in training intensity, particularly for endurance athletes or those engaging in high-impact sports, may be necessary (1). Weight-bearing exercise has been shown to have beneficial effects on bone accrual and peak bone mass (78). However, research has demonstrated a persistent lack of skeletal gains and deficits to bone microarchitecture in both female athletes with eating disorders and female athletes with normal-weight amenorrhea during adolescence, despite an increase in weight-bearing exercise. Additionally, addressing psychological stressors through psychotherapy and/or medication management is recommended, particularly for patients with high levels of anxiety, depression, or disordered eating behaviors (79). Clinicians should provide comprehensive patient education regarding the recovery process, emphasizing that menstrual irregularities are expected during treatment and do not necessarily indicate failure of intervention or impaired fertility. Patients should also be counseled that ovulation may resume before the return of regular menses, meaning conception is still possible despite irregular cycles (80).
For adolescent patients with refractory FHA, hormone replacement therapy (HRT) may be indicated to address hypoestrogenic states. The American College of Obstetricians and Gynecologists recommends HRT to benefit bone health in cases of FHA that do not resolve despite lifestyle modifications aimed at restoring energy balance (46). Historically, oral contraceptive pills have been prescribed for women and adolescents with FHA to modulate endogenous hormone levels and regulate menstrual cycles; however, most studies have demonstrated limited benefit of this intervention on restoring BMD (81, 82). Instead, the Endocrine Society Clinical Practice Guideline suggests short-term HRT in the form of Transdermal 17β-estradiol (E2) therapy with cyclic oral progestin in adolescents who continue to experience FHA that is refractory to nutritional, psychological, and/or modified exercise intervention (47). E2 therapy treats FHA by replacing the estrogen deficiency that results from suppressed HPO axis activity (83). In a retrospective cohort study of patients with hypogonadism aged 15 to 24, Dural et al. demonstrated that transdermal E2 therapy had a significant benefit on bone mass acquisition at the lumbar spine (84). Unlike oral contraceptives, E2 has been shown to significantly improve bone mineral density while preserving IGF-I secretion (85). These findings support transdermal E2 as an effective strategy to improve bone accrual in this population without suppressing IGF-I.
Future directions
A greater emphasis on precision medicine, described by tailoring interventions based on an individual’s metabolic, genetic, and psychological profile, may enhance recovery outcomes. Digital health tools, including mobile applications and wearable devices, are also being explored to monitor energy balance and menstrual cycle patterns in real-time, offering new ways to personalize treatment and track progress (86, 87).
Emerging pharmacological therapies for FHA refractory to lifestyle modification have also been under recent investigation. For example, Metreleptin is a recombinant human leptin analog that has been shown to restore menstrual function and improve markers of bone metabolism (88). However, limited data and weight loss concerns have precluded its recommendation as a standard therapy. Additionally, Kisspeptin analogs such as MVT-602 represent a promising emerging therapy for FHA, with early-phase trials demonstrating their ability to restore GnRH pulsatility and stimulate gonadotropin secretion. Although not yet available for clinical use, kisspeptin-based treatments may offer a novel, targeted approach to reactivating the reproductive axis while minimizing systemic side effects (89). Ultimately, the future of FHA management lies in a multidisciplinary, individualized approach that prioritizes long-term reproductive, metabolic, and skeletal health.
Discussion and conclusion
FHA in adolescent female athletes poses a major risk to their musculoskeletal health, in both the short and long-term. Recognizing the breadth of risk factors that can predispose a female athlete to FHA is crucial in early detection and prevention. These risk factors include inadequate caloric intake relative to energy expenditure and psychological stressors. Orthopedists and sports medicine physicians must be prepared to diagnose and treat FHA, as adolescent female athletes may present with fractures unknowingly due to hypoestrogenism. From a preventative standpoint, these physicians may play a crucial role in identifying and addressing risk factors for FHA before long-term bone damage ensues. Understanding the interplay of hormones in the HPO axis is key in diagnosing the underlying cause of FHA and preventing a disruption in axis function. Additionally, physicians are encouraged to normalize conversations about menstruation in clinical settings across all fields, and integrate screening tools, such as period tracking apps and sleep monitors, to help athletes surveil patterns over time. Creating a multidisciplinary, athlete-centered care model that fosters open dialogue can facilitate earlier recognition of FHA and reduce long-term skeletal consequences in female athletes (90).
Author contributions
LW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LL: Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CV: Conceptualization, Data curation, Formal Analysis, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BS: Conceptualization, Data curation, Formal Analysis, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SR: Conceptualization, Data curation, Formal Analysis, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saadedine M, Kapoor E, and Shufelt C. Functional hypothalamic amenorrhea: recognition and management of a challenging diagnosis. Mayo Clin Proc. (2023) 98:1376–85. doi: 10.1016/j.mayocp.2023.05.027
2. Behary P and Comninos AN. Bone perspectives in functional hypothalamic amenorrhoea: an update and future avenues. Front Endocrinol (Lausanne). (2022) 13:923791. doi: 10.3389/fendo.2022.923791
3. Hoch AZ, Pajewski NM, Moraski L, Carrera GF, Wilson CR, Hoffmann RG, et al. Prevalence of the female athlete triad in high school athletes and sedentary students. Clin J Sport Med. (2009) 19:421–8. doi: 10.1097/JSM.0b013e3181b8c136
4. Meczekalski B, Katulski K, Czyzyk A, Podfigurna-Stopa A, and Maciejewska-Jeske M. Functional hypothalamic amenorrhea and its influence on women’s health. J endocrinological Invest. (2014) 37:1099–108. doi: 10.1007/s40618-014-0169-3
5. Maya J and Misra M. The female athlete triad: review of current literature. Curr Opin Endocrinol Diabetes Obes. (2022) 29:44–51. doi: 10.1097/MED.0000000000000690
6. Pedreira CC, Maya J, and Misra M. Functional hypothalamic amenorrhea: Impact on bone and neuropsychiatric outcomes. Front Endocrinol (Lausanne). (2022) 13:953180. doi: 10.3389/fendo.2022.953180
7. Lu Y, Lu P, Lin L, Chen H, Zhang F, and Li X. Characteristics of bone mineral density in patients with functional hypothalamic amenorrhoea and its association with reproductive hormones and body composition. Clin Endocrinol (Oxf). (2024) 100:358–65. doi: 10.1111/cen.15016
8. Nazem TG and Ackerman KE. The female athlete triad. Sports Health. (2012) 4:302. doi: 10.1177/1941738112439685
9. Indirli R, Lanzi V, Mantovani G, Arosio M, and Ferrante E. Bone health in functional hypothalamic amenorrhea: What the endocrinologist needs to know. Front Endocrinol (Lausanne). (2022) 13:946695. doi: 10.3389/fendo.2022.946695
10. Mountjoy M, Ackerman KE, Bailey DM, Burke LM, Constantini N, Hackney AC, et al. 2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs). Br J Sports Med. (2023) 57:1073–97. doi: 10.1136/bjsports-2023-106994
11. von Brackel FN, Munzinger R, Bartosik M, Simon A, Barvencik F, Oheim R, et al. Impact of relative energy deficiency in sport (REDs) on bone health in elite athletes: A retrospective analysis. J Cachexia Sarcopenia Muscle. (2025) 16:e70082. doi: 10.1002/jcsm.70082
12. Benjamin RM. Bone health: preventing osteoporosis. Public Health Rep. (2010) 125:368–70. doi: 10.1177/003335491012500302
13. Pérez-López FR, Chedraui P, and Cuadros-López JL. Bone mass gain during puberty and adolescence: deconstructing gender characteristics. Curr Med Chem. (2010) 17:453–66. doi: 10.2174/092986710790226138
14. Syed F and Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. (2005) 328:688–96. doi: 10.1016/j.bbrc.2004.11.097
15. Xu L, Wang Q, Wang Q, Lyytikäinen A, Mikkola T, Völgyi E, et al. Concerted actions of insulin-like growth factor 1, testosterone, and estradiol on peripubertal bone growth: a 7-year longitudinal study. J Bone Miner Res. (2011) 26:2204–11. doi: 10.1002/jbmr.422
16. Wang Q, Nicholson PHF, Suuriniemi M, Lyytikäinen A, Helkala E, Alen M, et al. Relationship of sex hormones to bone geometric properties and mineral density in early pubertal girls. J Clin Endocrinol Metab. (2004) 89:1698–703. doi: 10.1210/jc.2003-031113
17. Eastell R. Role of oestrogen in the regulation of bone turnover at the menarche. J Endocrinol. (2005) 185:223–34. doi: 10.1677/joe.1.06059
18. Sun BZ, Kangarloo T, Adams JM, Sluss PM, Welt CK, Chandler DW, et al. Healthy post-menarchal adolescent girls demonstrate multi-level reproductive axis immaturity. J Clin Endocrinol Metab. (2019) 104:613–23. doi: 10.1210/jc.2018-00595
19. Stamatiades GA and Kaiser UB. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol Cell Endocrinol. (2018) 463:131–41. doi: 10.1016/j.mce.2017.10.015
20. Palermo R. Differential actions of FSH and LH during folliculogenesis. Reprod BioMed Online. (2007) 15:326–37. doi: 10.1016/S1472-6483(10)60347-1
21. Morrison AE, Fleming S, and Levy MJ. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin Endocrinol (Oxf). (2021) 95:229–38. doi: 10.1111/cen.14399
22. Khosla S, Oursler MJ, and Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. (2012) 23:576–81. doi: 10.1016/j.tem.2012.03.008
23. Navarro VM. Metabolic regulation of kisspeptin: the link between energy balance and reproduction. Nat Rev Endocrinol. (2020) 16:389–403. doi: 10.1038/s41574-020-0363-7
24. Kreisman MJ, Tadrousse KS, McCosh RB, and Breen KM. Neuroendocrine basis for disrupted ovarian cyclicity in female mice during chronic undernutrition. Endocrinology. (2021) 162:bqab103. doi: 10.1210/endocr/bqab103
25. Matar M, Al-Shaar L, Maalouf J, Nabulsi M, Arabi A, Choucair M, et al. The relationship between calciotropic hormones, IGF-1, and bone mass across pubertal stages. J Clin Endocrinol Metab. (2016) 101:4860–70. doi: 10.1210/jc.2016-3071
26. Libanati C, Baylink DJ, Lois-Wenzel E, Srinvasan N, and Mohan S. Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab. (1999) 84:2807–14. doi: 10.1210/jc.84.8.2807
27. Yakar S, Werner H, and Rosen CJ. Insulin-like growth factors: actions on the skeleton. J Mol Endocrinol. (2018) 61:T115–37. doi: 10.1530/JME-17-0298
28. Andrico S, Gambera A, Specchia C, Pellegrini C, Falsetti L, and Sartori E. Leptin in functional hypothalamic amenorrhoea. Hum Reprod. (2002) 17:2043–8. doi: 10.1093/humrep/17.8.2043
29. Lindahl MS, Olovsson M, Nyberg S, Thorsen K, Olsson T, and Sundström Poromaa I. Increased cortisol responsivity to adrenocorticotropic hormone and low plasma levels of interleukin-1 receptor antagonist in women with functional hypothalamic amenorrhea. Fertil Steril. (2007) 87:136–42. doi: 10.1016/j.fertnstert.2006.06.029
30. Sanders KM, Kawwass JF, Loucks T, and Berga SL. Heightened cortisol response to exercise challenge in women with functional hypothalamic amenorrhea. Am J Obstet Gynecol. (2018) 218:230.e1–6. doi: 10.1016/j.ajog.2017.11.579
31. Mancini A, Vergani E, Bruno C, Barini A, Silvestrini A, Meucci E, et al. Relationships between thyroid hormones, insulin-like growth factor-1 and antioxidant levels in hypothalamic amenorrhea and impact on bone metabolism. Hormone Metab Res. (2019) 51:303–9. doi: 10.1055/a-0859-4285
32. Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, and Ramos RH. Functional hypothalamic amenorrhea: hypoleptinemia and disordered eating. J Clin Endocrinol Metab. (1999) 84:873–7. doi: 10.1210/jcem.84.3.5551
33. Phillipou A, Schmidt U, Neill E, Miles S, McGorry P, and Eddy KT. Anorexia nervosa-facts, frustrations, and the future. JAMA Psychiatry. (2025) 82:410–7. doi: 10.1001/jamapsychiatry.2025.0812
34. Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, et al. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab. (2013) 98:1923–9. doi: 10.1210/jc.2012-4153
35. Lucas AR, Melton LJ 3rd, Crowson CS, and O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. (1999) 74:972–7. doi: 10.1016/S0025-6196(11)63994-3
36. Kandemir N, Slattery M, Ackerman KE, Tulsiani S, Bose A, Singhal V, et al. Bone parameters in anorexia nervosa and athletic amenorrhea: comparison of two hypothalamic amenorrhea states. J Clin Endocrinol Metab. (2018) 103:2392–402. doi: 10.1210/jc.2018-00338
37. Ackerman KE, Cano SN DE, Nardo Maffazioli G, HM C, Lee H, and Misra M. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci sports Exercise. (2015) 47:1577–86. doi: 10.1249/MSS.0000000000000574
38. History of Title IX (2019). Women’s Sports Foundation. Available online at: https://www.womenssportsfoundation.org/advocacy/history-of-title-ix/ (Accessed June 4, 2025).
39. Brown KA, Dewoolkar AV, Baker N, and Dodich C. The female athlete triad: special considerations for adolescent female athletes. Transl Pediatr. (2017) 6:144–9. doi: 10.21037/tp.2017.04.04
40. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. (2007) 39:1867–82. doi: 10.1249/mss.0b013e318149f111
41. Weiss Kelly AK and Hecht S. Council on sports medicine and fitness. Female Athlete Triad. Pediatr. (2016) 138:e20160922. doi: 10.1542/peds.2016-0922
42. De Souza MJ, Koltun KJ, Etter CV, and Southmayd EA. Current status of the Female Athlete Triad: Update and future directions. Curr Osteoporos Rep. (2017) 15:577–87. doi: 10.1007/s11914-017-0412-x
43. Skorseth P, Segovia N, Hastings K, and Kraus E. Prevalence of female athlete triad risk factors and iron supplementation among high school distance runners: results from a triad risk screening tool. Orthop J Sports Med. (2020) 8:2325967120959725. doi: 10.1177/2325967120959725
44. Maïmoun L, Georgopoulos NA, and Sultan C. Endocrine disorders in adolescent and young female athletes: impact on growth, menstrual cycles, and bone mass acquisition. J Clin Endocrinol Metab. (2014) 99:4037–50. doi: 10.1210/jc.2013-3030
45. Salamunes ACC, Williams NI, and De Souza MJ. Are menstrual disturbances associated with an energy availability threshold? A critical review of the evidence. Appl Physiol Nutr Metab. (2024) 49:584–98. doi: 10.1139/apnm-2023-0418
46. Huhmann K. Menses requires energy: A review of how disordered eating, excessive exercise, and high stress lead to menstrual irregularities. Clin Ther. (2020) 42:401–7. doi: 10.1016/j.clinthera.2020.01.016
47. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102:1413–39. doi: 10.1210/jc.2017-00131
48. Bonazza F, Politi G, Leone D, Vegni E, and Borghi L. Psychological factors in functional hypothalamic amenorrhea: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:981491. doi: 10.3389/fendo.2023.981491
49. Strock NCA, De Souza MJ, and Williams NI. Eating behaviours related to psychological stress are associated with functional hypothalamic amenorrhoea in exercising women. J Sports Sci. (2020) 38:2396–406. doi: 10.1080/02640414.2020.1786297
50. Gendall KA, Joyce PR, Carter FA, McIntosh VV, Jordan J, and Bulik CM. The psychobiology and diagnostic significance of amenorrhea in patients with anorexia nervosa. Fertility sterility. (2006) 85:1531–5. doi: 10.1016/j.fertnstert.2005.10.048
51. Baskaran C, Kumar P, Plessow F, Nimmala S, Ackerman KE, Eddy KT, et al. Depressive and anxiety symptoms, and neural correlates of reward and punishment anticipation in female athletes with amenorrhea. Front Endocrinol. (2023) 14:976050. doi: 10.3389/fendo.2023.976050
52. Deligeoroglou E, Athanasopoulos N, Tsimaris P, Dimopoulos KD, Vrachnis N, and Creatsas G. Evaluation and management of adolescent amenorrhea. Ann N Y Acad Sci. (2010) 1205:23–32. doi: 10.1111/j.1749-6632.2010.05669.x
53. Togari A and Arai M. Pharmacological topics of bone metabolism: the physiological function of the sympathetic nervous system in modulating bone resorption. J Pharmacol Sci. (2008) 106:542–6. doi: 10.1254/jphs.FM0070227
54. Huang J, Wu T, Jiang Y-R, Zheng X-Q, Wang H, Liu H, et al. β-Receptor blocker enhances the anabolic effect of PTH after osteoporotic fracture. Bone Res. (2024) 12:18. doi: 10.1038/s41413-024-00321-z
55. Tsigos C and Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. (2002) 53:865–71. doi: 10.1016/S0022-3999(02)00429-4
56. Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. (2016) 6:603–21. doi: 10.1002/j.2040-4603.2016.tb00694.x
57. Saadedine M, Berga SL, Faubion SS, and Shufelt CL. The silent pandemic of stress: impact on menstrual cycle and ovulation. Stress. (2025) 28:2457767. doi: 10.1080/10253890.2025.2457767
58. Chrousos GP, Torpy DJ, and Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. (1998) 129:229–40. doi: 10.7326/0003-4819-129-3-199808010-00012
59. Schliep KC, Mumford SL, Vladutiu CJ, Ahrens KA, Perkins NJ, Sjaarda LA, et al. Perceived stress, reproductive hormones, and ovulatory function: a prospective cohort study. Epidemiology. (2015) 26:177–84. doi: 10.1097/EDE.0000000000000238
60. Kelesidis T, Kelesidis I, Chou S, and Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Internal Med. (2010) 152:93. doi: 10.7326/0003-4819-152-2-201001190-00008
61. Huang L, Hou JW, Fan HY, Tsai MC, Yang C, Hsu JB, et al. Critical body fat percentage required for puberty onset: the Taiwan Pubertal Longitudinal Study. J endocrinological Invest. (2023) 46:629–36. doi: 10.1007/s40618-022-01970-9
62. Tokatly LI, Kidron-Levy H, Stein D, Levy AE, Yosef G, Ziv-Baran T, et al. Predicting menstrual recovery in adolescents with anorexia nervosa using body fat percent estimated by bioimpedance analysis. J Adolesc Health. (2019) 64:501–7. doi: 10.1016/j.jadohealth.2018.10.008
63. Amoruso I, Fonzo M, Barro A, Scardina C, Titton F, Bertoncello C, et al. Determinants of menstrual dysfunction in the female athlete triad: A cross-sectional study in Italian athletes. Psychol sport Exercise. (2024) 73:102653. doi: 10.1016/j.psychsport.2024.102653
64. Gimunová M, Paulínyová A, Bernaciková M, and Paludo AC. The prevalence of menstrual cycle disorders in female athletes from different sports disciplines: A rapid review. Int J Environ Res Public Health. (2022) 19:14243. doi: 10.3390/ijerph192114243
65. Witkoś J, Luberda E, Błażejewski G, and Strój E. Menstrual cycle disorders as an early symptom of energy deficiency among female physique athletes assessed using the Low Energy Availability in Females Questionnaire (LEAF-Q). PloS One. (2024) 19:e0303703. doi: 10.1371/journal.pone.0303703
66. Fong H-F, Divasta AD, Difabio D, Ringelheim J, Jonas MM, and Gordon CM. Prevalence and predictors of abnormal liver enzymes in young women with anorexia nervosa. J Pediatr. (2008) 153:247–53. doi: 10.1016/j.jpeds.2008.01.036
67. Singhal V, de Lourdes Eguiguren M, Eisenbach L, Clarke H, Slattery M, Eddy K, et al. Body composition, hemodynamic, and biochemical parameters of young female normal-weight oligo-amenorrheic and eumenorrheic athletes and nonathletes. Ann Nutr Metab. (2014) 65:264–71. doi: 10.1159/000366024
68. Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. (2004) 82:266–72. doi: 10.1016/j.fertnstert.2004.02.098
69. Rosner W, Hankinson SE, Sluss PM, Vesper HW, and Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. (2013) 98:1376–87. doi: 10.1210/jc.2012-3780
70. Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, et al. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. (1989) 68:301–8. doi: 10.1210/jcem-68-2-301
71. Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. (2006) 91:2931–7. doi: 10.1210/jc.2005-2818
72. Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. (2014) 17:225–42. doi: 10.1016/j.jocd.2014.01.003
73. Klein DA, Paradise SL, and Reeder RM. Amenorrhea: A systematic approach to diagnosis and management. afp. (2019) 100:39–48.
74. Dobranowska K, Plińska S, and Dobosz A. Dietary and lifestyle management of functional hypothalamic amenorrhea: A comprehensive review. Nutrients. (2024) 16:2967. doi: 10.3390/nu16172967
75. Office of Dietary Supplements, National Institutes of Health. Calcium (2025). Available online at: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/ (Accessed June 9, 2025).
76. Chauhan K, Shahrokhi M, and Huecker MR. Vitamin D. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing (2023).
77. Endocrine Society. Vitamin D for the Prevention of Disease (2024). Available online at: https://www.endocrine.org/clinical-practice-guidelines/vitamin-d-for-prevention-of-disease (Accessed June 9, 2025).
78. Ackerman KE and Misra M. Bone health in adolescent athletes with a focus on female athlete triad. Physician sportsmedicine. (2011) 39:131. doi: 10.3810/psm.2011.02.1871
79. Berga SL, Marcus, Loucks TL, Hlastala S, Ringham R, and Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertility sterility. (2003) 80:976–81. doi: 10.1016/S0015-0282(03)01124-5
80. De Souza MJ, Ricker EA, Mallinson RJ, Allaway HCM, Koltun KJ, Strock NCA, et al. Bone mineral density in response to increased energy intake in exercising women with oligomenorrhea/amenorrhea: the REFUEL randomized controlled trial. Am J Clin Nutr. (2022) 115:1501–12. doi: 10.1093/ajcn/nqac044
81. Cobb KL, Bachrach LK, Sowers M, Nieves J, Greendale GA, Kent KK, et al. The effect of oral contraceptives on bone mass and stress fractures in female runners. Med Sci Sports Exerc. (2007) 39:1464–73. doi: 10.1249/mss.0b013e318074e532
82. Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG, et al. Persistent osteopenia in ballet dancers with amenorrhea and delayed menarche despite hormone therapy: a longitudinal study. Fertility sterility. (2003) 80:398–404. doi: 10.1016/S0015-0282(03)00660-5
83. Genazzani AD, Podfigurna-Stopa A, Czyzyk A, Katulski K, Prati A, Despini G, et al. Short-term estriol administration modulates hypothalamo-pituitary function in patients with functional hypothalamic amenorrhea (FHA). Gynecological Endocrinol. (2016) 32:210–5. doi: 10.3109/09513590.2015.1118452
84. Dural O, Ulusoy HE, Ates Tikiz M, Gurbanova T, Yasa C, Gungor Ugurlucan F, et al. Effects of hormone replacement therapy on low bone mineral density in adolescents and young women with hypogonadism: comparison of oral and transdermal 17 beta-estradiol administration. J Pediatr Adolesc Gynecol. (2022) 35:634–7. doi: 10.1016/j.jpag.2022.05.004
85. Stanosz S, Żochowska E, Safranow K, Sieja K, and Stanosz M. Influence of modified transdermal hormone replacement therapy on the concentrations of hormones, growth factors, and bone mineral density in women with osteopenia. Metabolism. (2009) 58:1–7. doi: 10.1016/j.metabol.2008.07.016
86. Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Mineral Res. (2011) 26:2430–8. doi: 10.1002/jbmr.447
87. Pape J, Herbison AE, and Leeners B. Recovery of menses after functional hypothalamic amenorrhoea: if, when and why. Hum Reprod Update. (2020) 27:130–53. doi: 10.1093/humupd/dmaa032
88. Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci. (2011) 108:6585–90. doi: 10.1073/pnas.1015674108
89. Abbara A, Eng PC, Phylactou M, Clarke SA, Richardson R, Sykes CM, et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest. (2020) 130:6739–53. doi: 10.1172/JCI139681
Keywords: functional hypothalamic amenorrhea (FHA), adolescent athletes, hypoestrogenism, bone density, female athlete triad, relative energy deficiency in sport (RED-S)
Citation: Wong L, Leibner L, Vicioso C, Shah B and Ranade SC (2025) Functional hypothalamic amenorrhea in adolescent athletes impairs bone accrual and increases fracture risk. Front. Endocrinol. 16:1709695. doi: 10.3389/fendo.2025.1709695
Received: 20 September 2025; Accepted: 24 November 2025; Revised: 21 November 2025;
Published: 09 December 2025.
Edited by:
Fátima Baptista, Universidade de Lisboa, PortugalReviewed by:
Alan David Rogol, University of Virginia, United StatesMarek Bolanowski, Wroclaw Medical University, Poland
Copyright © 2025 Wong, Leibner, Vicioso, Shah and Ranade. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laurel Wong, bGF1cmVsLndvbmdAaWNhaG4ubXNzbS5lZHU=
†These authors have contributed equally to this work
‡ORCID: Laurel Wong, orcid.org/0009-0006-9435-6252
 Laurel Wong
Laurel Wong Lily Leibner2†
Lily Leibner2† Camila Vicioso
Camila Vicioso